ABSTRACT
Introduction and objectives: Transcatheter aortic valve implantation (TAVI) is established as the standard of care for patients across all surgical risk profiles, with expanding indications in younger and lower-risk populations. A substantial proportion of patients eligible for TAVI have preexisting cardiac implantable electronic devices (CIED). Temporary transvenous right ventricular (RV) pacing is routinely used during TAVI to facilitate procedural safety but carries inherent risks, including RV perforation, cardiac tamponade, and lead dislodgement.
Methods: Alternative pacing strategies, such as left ventricular pacing via the valve delivery guidewire, have been proposed to reduce procedural complications. In patients with a preexisting CIED, leveraging the implanted device for procedural pacing represents a rational and potentially safer option. However, its adoption remains limited, largely due to unfamiliarity with device-specific programming.
Results: This article provides a detailed, step-by-step practical guide for programming rapid ventricular pacing using the most widely encountered CIED platforms: Biotronik, Medtronic, Abbott/St. Jude, Sorin, and Boston Scientific. The specific programming pathways for each manufacturer are summarized to facilitate safe, effective, and reproducible implementation during TAVI.
Conclusions: Use of CIED-based pacing during TAVI is a feasible and safe alternative to temporary RV pacing, with the potential to reduce procedural complications, such as cardiac tamponade and hemorrhage. Widespread adoption of this strategy requires enhanced operator familiarity with the programming of different devices. Given the high ventricular rates involved, these maneuvers should be performed in a monitored setting with immediate availability of resuscitation and defibrillation capabilities.
Keywords: TAVI. Pacing. Cardiac implantable electronic devices. Ventricular perforation.
RESUMEN
Introducción y objetivos: El implante percutáneo de válvula aórtica (TAVI) se ha establecido como el estándar de atención para pacientes de todos los perfiles de riesgo quirúrgico. Una proporción significativa de los candidatos a TAVI son portadores de dispositivos cardiacos implantables (DCI). La estimulación ventricular derecha transvenosa temporal es una práctica habitual, pero conlleva riesgos como perforación ventricular, taponamiento y desplazamiento del electrodo.
Métodos: Para reducir las complicaciones se han propuesto estrategias de estimulación alternativas, como la estimulación ventricular izquierda a través de la guía de liberación de la válvula; sin embargo, en los pacientes con un DCI preexistente, aprovechar dicho dispositivo para la estimulación durante el procedimiento representa una opción racional y potencialmente más segura. No obstante, su utilización sigue siendo limitada, principalmente debido al desconocimiento de la programación específica de cada dispositivo.
Resultados: Este artículo ofrece una guía práctica detallada, paso a paso, para la programación de la estimulación ventricular rápida utilizando las plataformas de DCI más comunes: Biotronik, Medtronic, Abbott/St. Jude, Sorin y Boston Scientific. Se resumen las rutas de programación específicas de cada fabricante para facilitar una implementación segura, eficaz y reproducible.
Conclusiones: La estimulación basada en DCI durante el TAVI es una alternativa factible y segura, con el potencial de reducir complicaciones tales como el taponamiento cardiaco y el sangrado. Su adopción generalizada requiere que los operadores estén familiarizados con la programación de los distintos dispositivos. Estas maniobras deben realizarse en un entorno monitorizado y con disponibilidad inmediata de medios de reanimación y desfibrilación.
Palabras clave: TAVI. Marcapasos. Dispositivos cardiacos implantables. Perforación ventricular.
Abbreviations
CIED: cardiac implantable electronic device. ICD: implantable cardioverter defibrillator. NIPS: non-invasive programmed stimulation. RV: right ventricular. TAVI: transcatheter aortic valve implantation.
INTRODUCTION
Transcatheter aortic valve implantation (TAVI) has emerged as the preferred therapeutic option across the full spectrum of surgical risk, and more recently its indications have expanded to include younger patients and those at low surgical risk.1-4 Patients undergoing TAVI are typically elderly and burdened with significant cardiovascular comorbidities, with a considerable proportion (8.7% to 23.1%) having preexisting cardiac implantable electronic devices (CIED).5-6
Temporary transvenous pacing is a well-established and integral component of TAVI, facilitating procedural success by reducing cardiac contractility during balloon aortic valvuloplasty and valve deployment. The target pacing rate varies according to the valve type: approximately 120 bpm for self-expanding valves and approximately 180 bpm for balloon-expandable valves.7 In the 2 scenarios, the objective is to mitigate the risk of micro- or macro-dislodgement or embolization during implantation.
Notwithstanding its utility, temporary right ventricular (RV) pacing is associated with potential complications, including myocardial injury, RV dysfunction, and perforation, which may culminate in cardiac tamponade.8-10 Furthermore, lead dislodgement and pacing failure have been reported among patients undergoing temporary pacing for a variety of indications, with incidence rates of approximately 4.6% and 9.5%, respectively. Although these figures are not specific to TAVI, they highlight that such complications are not rare and may result in ineffective pacing or sensing, thereby increasing the risk of critical intraoperative events, such as valve embolization or migration.11
To address these limitations, alternative pacing strategies have been proposed. Among them, left ventricular pacing via the valve delivery guidewire offers several advantages, such as elimination of the need for venous access, reduction of the risk of RV perforation, and potential reduction in procedural duration.12,13
In patients with preexisting CIED, use of the permanent device for procedural pacing appears to be a rational strategy to minimize complications.
The present article aims to address this unmet need by providing a concise and practical step-by-step guide for the use of the most widely encountered CIED programming consoles, thereby promoting safer and more efficient pacing management during TAVI.
METHODS
This step-by-step guide for pacing patients with preexisting CIED during TAVI is derived from the routine clinical practice of our high-volume TAVI center, developed in close collaboration with our Electrophysiology Unit and in full compliance with the usage recommendations provided by our industry partners.
RESULTS
Biotronik (Germany)
Not all pacemaker models support electrophysiological testing. However, this limitation can be overcome by using the threshold measurement section. Select “Test”, then “Pacing Threshold”, and choose “Manual” (figure 1A,B). First, select the pacing mode (eg, VVI), then set the desired pacing rate (up to 200 bpm) and ensure the output is set to the maximum (7.5V) to guarantee proper capture. Once all parameters are configured, press the “Start” button to begin stimulation, which will continue until manually stopped. If the device supports rapid pacing, such as non-Invasive programmed stimulation (NIPS), overdrive pacing can be performed using the VVI or V00 mode (figure 1C). Select the desired mode and basic rate, then press the “Start Backup Program” button.
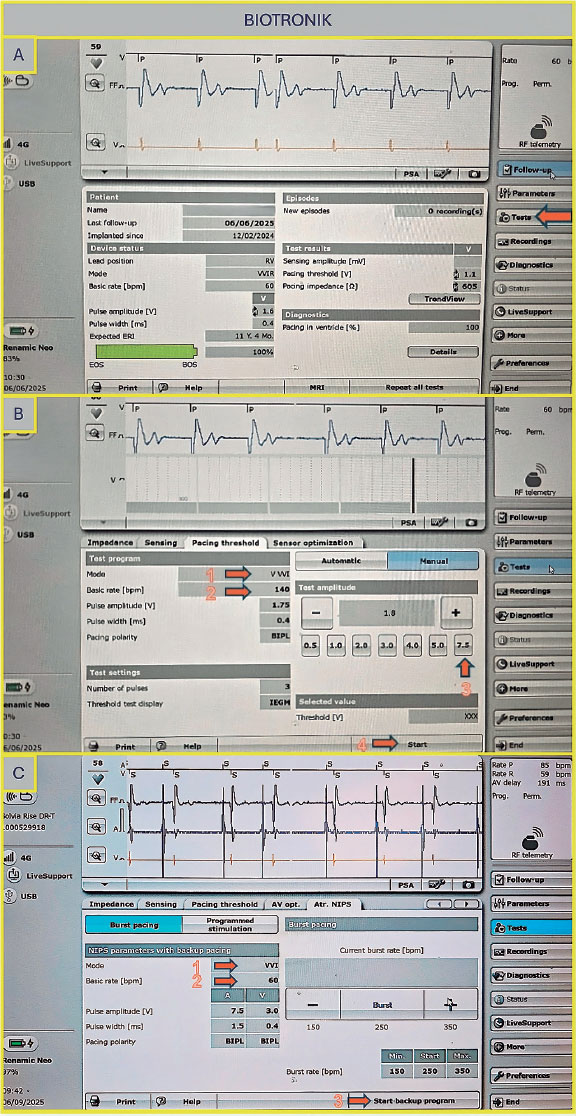
Figure 1. A: main screen of the Biotronik console. Select “Test” as indicated by the arrow to proceed to the pacing tests. B: to proceed to the pacing tests, select “Manual,” then set the following parameters: pacing mode (arrow 1), basic rate (arrow 2), amplitude (arrow 3), and press “Start” to initiate pacing (arrow 4). C: configure the NIPS parameters by selecting VVI mode (arrow 1), setting the basic rate (arrow 2), and pressing “Start” to begin pacing (arrow 3). NIPS: Non-Invasive Programmed Stimulation.
Medtronic (United States)
Once on the main screen (figure 2A), press the “Test” tab, then navigate to “Electrophysiology Study” and select either “Fixed Burst” or “Burst” (figure 2B). The interface will prompt you to choose the chamber in which pacing will be applied — select “Right Ventricular”. Adjust the interval to the desired value (as the interval decreases, the frequency increases — for example, 200 bpm corresponds to 300 ms). Once all parameters are set, press and hold the “Press and Hold” button to begin stimulation; release it to stop.
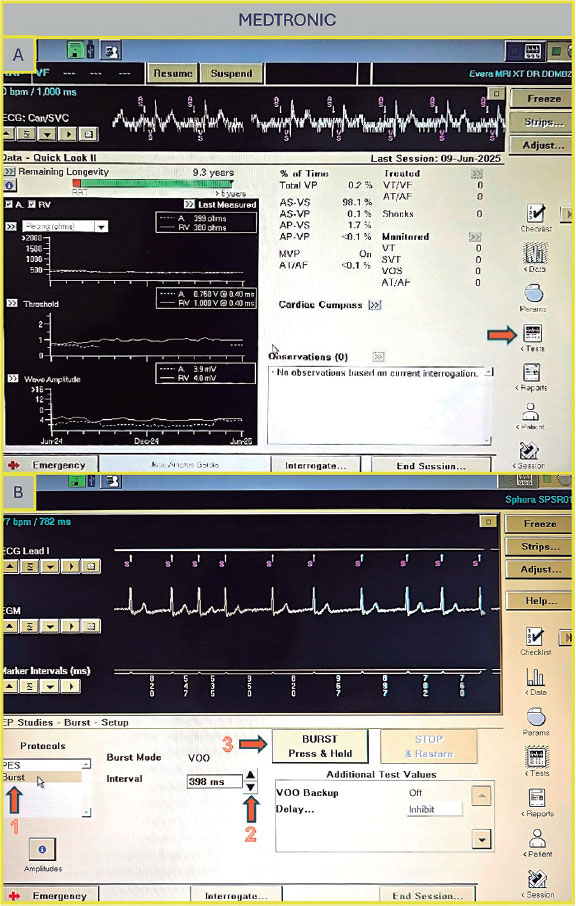
Figure 2. A: main screen of the Medtronic console. Press “Tests” (arrow) to access the electrophysiology study screen. B: select “Fixed Burst” or “Burst” (arrow 1), adjust the interval as needed (arrow 2), and press and hold the “Burst” button (arrow 3) to deliver pacing.
Abbott/St Jude (United States)
Once on the main screen (figure 3A), press “Test”. There are 2 options to perform rapid ventricular pacing:
- –NIPS (figure 3B): Click “Ventricular NIPS”, then select “Burst”, set the “Pulse Amplitude” (the output should be high to ensure capture), and adjust the pacing rate. In this case, the S1–S1 interval corresponds to the cycle length of the stimuli applied and must be set according to the desired heart rate. For example, 200 bpm corresponds to an S1–S1 interval of 300 ms.
- –Temporary pacing (figure 3C): Select the pacing mode (in this case, VVI), the desired rate (up to 170 bpm), and the maximum output to ensure capture. Once all parameters are set, press “Start Temporary” to begin pacing. Stimulation will continue until manually stopped.
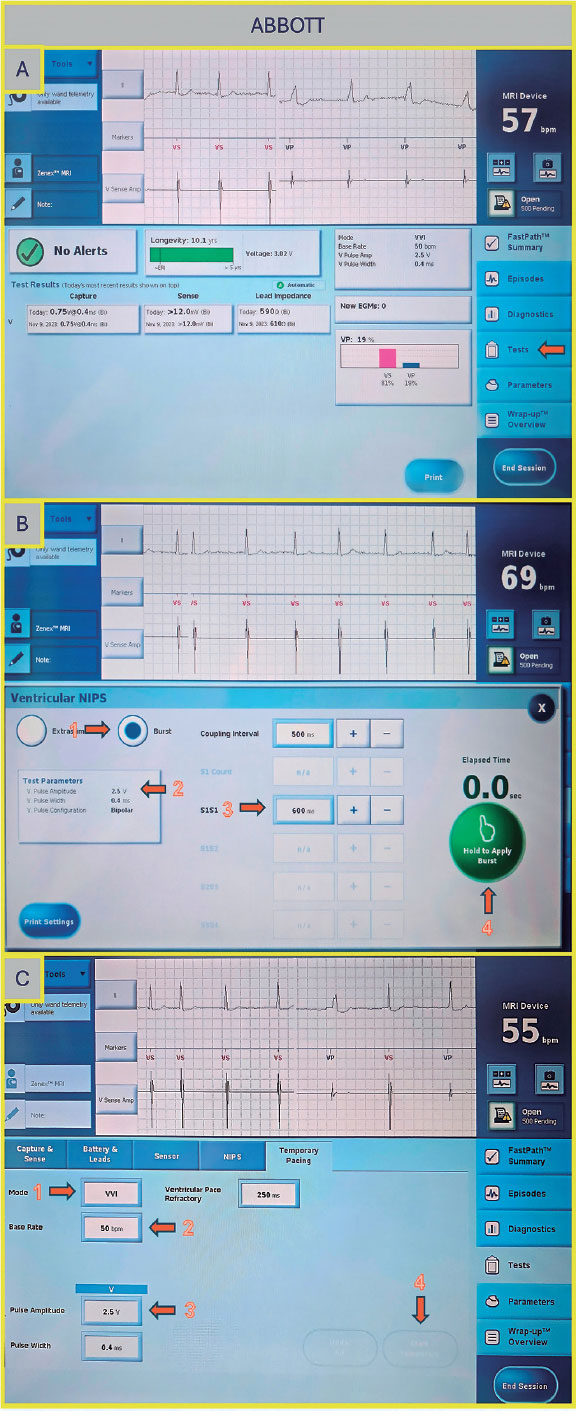
Figure 3. A: main screen of the Abbott/St. Jude console. Press “Test” to proceed to the electrophysiology study screen. B: ventricular NIPS screen. Select “Burst” (arrow 1), set the amplitude (arrow 2), adjust the S1–S1 interval (arrow 3), and press the green button to deliver pacing (arrow 4). C: temporary pacing screen. Select the pacing mode (arrow 1), desired rate (arrow 2), and pulse amplitude (arrow 3), then press the “Start Temporary” button (arrow 4).
Sorin (Italy)
Select ”Tests”, then choose ”NIPS”. Under ”Mode”, select either ventricular pacing or ventricular burst, depending on the desired function. Next, set the ”Basic Period” (figure 4A,B) by choosing the corresponding rate in milliseconds (for example, 200 bpm corresponds to 300 ms). Once all parameters are set, press ”Start NIPS” to initiate the test.
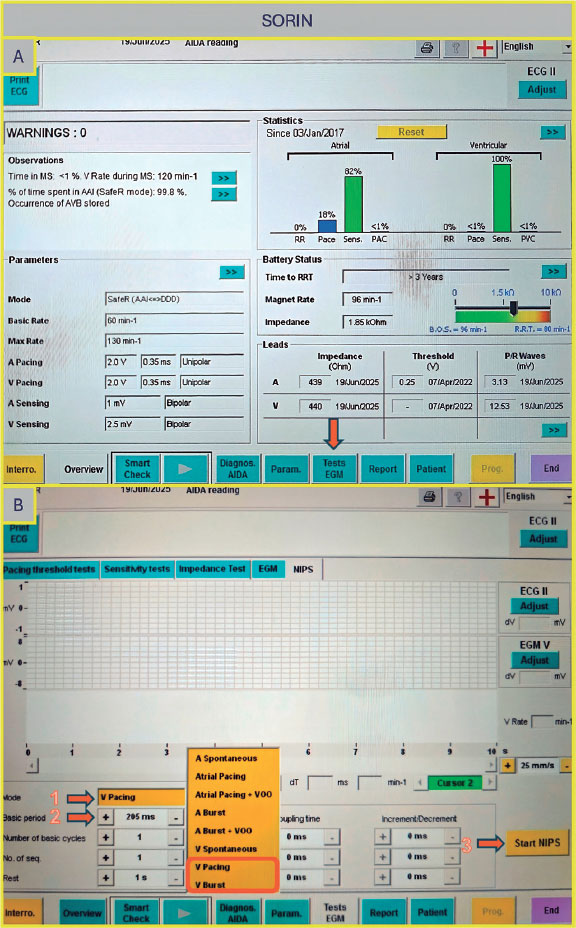
Figure 4. A: main screen of the Sorin console. Select “Tests EGM” (arrow) to access pacing options. B: “Tests EGM” screen. Select the pacing mode (arrow 1), the interval period (arrow 2), and then press the “Start NIPS” button (arrow 3).
Boston Scientific (United States)
Once on the main screen (figure 5A), select “Test”, then choose “Temp Brady” (figure 5B) From there, select VVI mode and set the desired heart rate under “Lower Rate Limit.” As in previous cases, ensure that the maximum output is selected to guarantee proper capture. After all parameters have been configured, press the ”Start” button to begin.
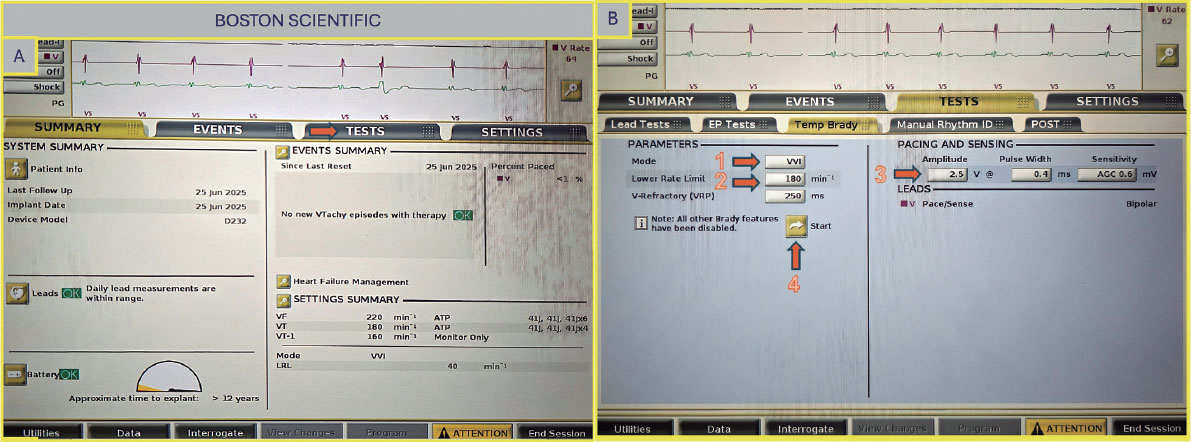
Figure 5. A: main screen of the Boston Scientific console. Select “Test” (arrow) to proceed to the pacing setup. B: select VVI mode (arrow 1), set the lower rate limit (arrow 2) and amplitude (arrow 3), then press the “Start” button to begin pacing (arrow 4).
DISCUSSION
While earlier permanent pacing devices had limitations in achieving instantaneous burst pacing —such as ramping up the ventricular rate instead of immediate increase and requiring time to reset the function—contemporary devices have largely overcome these issues.14 Most current models allow rapid burst pacing up to 180 bpm with immediate start and stop, using electrophysiology program mode settings or whenever electrophysiology program is not available (especially in some pacemakers) via a threshold test with maximum output and maximum duration. In cases where this is not possible or does not ensure adequate rapid pacing, the placement of a temporary pacing lead should be considered. Table 1 lists several of the most widely used CIED in our routine clinical practice and specifies whether they provide the capability for rapid temporary pacing or electrophysiological testing). It is always required to ensure that any changes in device programming are reversed and individually optimized; therefore, verification of the device settings at the beginning of the procedure is recommended, and any changes or modifications at the end should be avoided. In patients with implantable cardioverter defibrillator or cardiac resynchronization therapy-defibrillator devices, tachyarrhythmia therapies should be deactivated before the procedure to avoid inappropriate shocks. Of note, some temporary pacing algorithms have a programmed duration limit, so it is essential to verify in advance that high-rate temporary pacing can be maintained for the entire time required for prosthesis deployment, as this may vary depending on the device and pacing mode used.
| Manufacturer | VVI-Pacemaker (EP study available yes/no) | DDD-Pacemaker (EP study available yes/no) | VVI-Defibrillator (EP study available yes/no) | DDD-Defibrillator (EP study available yes/no) | CRT-Pacemaker (EP study available yes/no) | CRT-Defibrillator (EP study available yes/no) |
|---|---|---|---|---|---|---|
| Biotronik | Ecuro SR (No) Enticos 4 SR (Yes) Edora 8 SR T (No) Evia SR T (Yes) Amvia Edge SR-T (Yes) Amvia Sky SR-T (Yes) |
Amvia Sky DR-T (No) Amvia Edge DR-T (Yes) Edora 8 DR-T (No) Evity 8 DR T (No) Ecuro DR (No) Effecta DR (Yes) Enticos 4 DR (Yes) Evia DR (Yes) Solvia Rise DR-T (no) |
Intica Neo 5 VRT (Yes) Iforia 3 VR-T (Yes) |
Iforia 3 DR-T (Yes) Inlexa 3 DR-T (Yes) Intica 5/7 (Yes) |
Edora 8 HF-T (Yes) Evia HF-T (Yes) Rivacor HF-T (Yes) Etrinsa 8 HF-T (Yes) |
Intica HF-T (Yes) Rivacor HF-T QP (Yes) |
| Boston Scientific | Essentio SR (Yes) Advantio SR (Yes) Accolade SR (Yes) |
Essentio DR (Yes) Advantio DR (Yes) Accolade DR (Yes) Ingenio (Yes) |
Charisma EL ICD (Yes) Inogen VR (Yes) Punctua NE ICD (Yes) Vigilant EL VR (Yes) Resonate VR (Yes) Autogen VR (Yes) Energen VR (Yes) |
Vigilant EL DR (Yes) Resonate (Yes) Energen (Yes) |
Intua (Yes) Visionist CRT-P (Yes) Valitude CRT-P (Yes) Invive (Yes) Accolate CRT-P (Yes) Incepta CRT-P (Yes) Essentio CRT-P (Yes) Resonate X4 CRT-P (Yes) |
Charisma CRT-D (Yes) Inogen CRT-D (Yes) Origen CRT-D (Yes) |
| Medtronic | Micra VR (No) Attesta SR (Yes) Sphera SR (Yes) Advisa SR (Yes) Ensura SR (Yes) Azure SR (Yes) |
Azure DR (Yes) Adapta DR (Yes) Advisa DR (Yes) Attesta DR (Yes) Ensura DR (Yes) Sphera DR (Yes) Sensia DR (Yes) Relia DR (Yes) |
Evera S VR (Yes) Maximo II VR (Yes/No) Protecta VR (Yes) Visia AF (Yes) Cobalt XT (Yes) |
Evera DR (Yes) Protecta DR (Yes) |
Serena CRT-P (Yes) Percepta CRT-P (Yes) |
Brava CRT-D (Yes) Compia CRT-D (Yes) Cobalt CRT-D (Yes) Claria CRT-D (Yes) |
| Sorin | Reply SR (Yes) Kora SR (Yes) |
Reply DR (Yes) Kora DR (Yes) Vega DR (Yes) |
Resiliant (Yes) Platinum SR (Yes) Intensia VR (Yes) Paradym SR (Yes) |
Platinum DR (Yes) Intensia DR (Yes) Paradym DR (Yes) |
Platinum CRT-P (Yes) Kora CRT-P (Yes) Luna CRT-P (Yes) Resiliant CRT-P (Yes) |
Platinum CRT-D (Yes) Paradym CRT-D (Yes) |
| Abbott/ St. Jude | Endurity Core (Yes) Assurity SR (Yes) Zephyr XL SR (Yes) |
Assurity DR (Yes) Accent DR (Yes) Endurity (Yes) Sustain XL DR (No) Verity ADx XL DR (No) |
Ellipse VR (Yes) Assura VR (Yes) Fortify Assura SR (Yes) Ellipse VR (Yes) Gallant VR (Yes) Neutrino (Yes) Entrant (Yes) |
Ellipse DR (Yes) Assura DR (Yes) Fortify Assura DR (Yes) Ellipse DR (Yes) Gallant DR (Yes) Neutrino DR (Yes) |
Quadra Allure CRT-P (Yes) Endurity CRT-P (Yes) Entrant CRT-P (Yes) Gallant CRT-P (Yes) |
Quadra Assura CRT-D (Yes) Unify Assura (Yes) CRT-D Fortify Assura CRT-D (Yes) Ellipse HF CRT-D (Yes) Entrant HF CRT-D (Yes) |
|
CRT, cardiac resynchronization therapy; CRT-P, cardiac resynchronization therapy pacemaker; CRT-D, cardiac resynchronization therapy defibrillator; DDD, dual-chamber pacing, dual-chamber sensing, and dual response to sensing; DR, dual chamber-rate; EP, electrophysiological; HF, heart failure; ICD, implantable cardioverter-defibrillators; SR, single rate; VVI, ventricular pacing, ventricular sensing, and inhibited response to a sensed event. |
||||||
Given the high ventricular rates required during this process and the deactivation of therapies in patients with implantable cardioverter defibrillator or cardiac resynchronization therapy-defibrillator devices, these maneuvers should be undertaken in a controlled environment with continuous monitoring, immediate availability of cardiopulmonary resuscitation, and access to external defibrillation equipment.
Currently, temporary RV pacing remains a common clinical practice during TAVI in this patient subgroup despite growing evidence that pacing via an implanted CIED is safe and feasible and may reduce pacemaker-related complications, such as cardiac tamponade and hemorrhage vs temporary RV pacing.9,15 However, certain challenges may arise, mainly of an organizational nature. During TAVI, a manufacturer-specific CIED programmer must be available, and the operator responsible for pacing should be adequately trained on how to operate the device programming functions. Because programming options vary by manufacturer and device type, operators must have thorough and specialized knowledge. In addition, the presence of trained nurses with specific expertise is essential to support device programming and ensure procedural safety. These devices can deliver rapid ventricular pacing through anti-tachycardia pacing functions but programming them often requires adjusting more complex parameters. Our proposed strategy is simpler and more practical, especially for professionals less experienced with advanced device programming.
CONCLUSIONS
In patients undergoing TAVI with preexisting cardiac implantable electronic devices, leveraging the implanted device for procedural pacing is a feasible and potentially safer alternative to temporary RV pacing. This strategy may reduce the risk of complications such as perforation, lead dislodgement, and bleeding. However, its broader adoption is hindered by the variability in device programming interfaces and limited operator familiarity. The step-by-step instructions provided in this guide aim to facilitate the practical implementation of this approach across the most widely encountered CIED platforms, ultimately promoting safer, more efficient, and complication-free TAVI procedures.
FUNDING
None declared.
ETHICAL CONSIDERATIONS
This is a step-by-step practical guide article; therefore, ethical committee approval was deemed unnecessary. No informed consent was required. Potential gender bias has been considered and excluded.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
Artificial intelligence has not been used in the development of this paper.
AUTHORS’ CONTRIBUTIONS
F. Pensotti and L.J. Garnacho elaborated the manuscript under the supervision of I.J. Amat-Santos that revised and drafted the manuscript. All the remaining authors conducted a critical review. All authors approved the final version.
CONFLICTS OF INTEREST
None declared.
WHAT IS KNOWN ABOUT THE TOPIC?
- Despite the fact that a substantial proportion of patients undergoing TAVI have a preexisting CIED, temporary pacing through these devices is rarely used. Instead, operators often prefer temporary RV pacing, which, however, carries inherent risks.
WHAT DOES THIS STUDY ADD?
- The step-by-step instructions of this guide are designed to help implement this approach on the most common CIED platforms, thereby ensuring safer, more efficient, and complication-free TAVI.
REFERENCES
1. Mack MJ, Leon MB, Smith CR, et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1):a randomised controlled trial. Lancet. 2015;385:2477-2484.
2. Leon MB, Smith CR, Mack MJ, et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med. 2016;374: 1609-1620.
3. Mack MJ, Leon MB, Thourani VH, et al. Transcatheter Aortic-Valve Replacement in Low-Risk Patients at Five Years. N Engl J Med. 2023;389: 1949-1960.
4. Forrest JK, Deeb GM, Yakubov SJ, et al. 4-Year Outcomes of Patients With Aortic Stenosis in the Evolut Low Risk Trial. J Am Coll Cardiol. 2023;82: 2163-2165.
5. Tichelbäcker T, Bergau L, Puls M, et al. Insights into permanent pacemaker implantation following TAVR in a real-world cohort. PLoS One. 2018; 13:0204503.
6. Wasim D, Ali AM, Bleie Ø, et al. Prevalence and predictors of permanent pacemaker implantation in patients with aortic stenosis undergoing transcatheter aortic valve implantation:a prospective cohort study. BMJ Open. 2025;15:093073.
7. Abdel-Wahab M, Mehilli J, Frerker C, et al. Comparison of Balloon-Expandable vs Self-expandable Valves in Patients Undergoing Transcatheter Aortic Valve Replacement:The CHOICE Randomized Clinical Trial. JAMA. 2014;311:1503.
8. Axell RG, White PA, Giblett JP, et al. Rapid Pacing–Induced Right Ventricular Dysfunction Is Evident After Balloon-Expandable Transfemoral Aortic Valve Replacement. J Am Coll Cardiol. 2017;69:903-904.
9. Feldt K, Dalén M, Meduri CU, et al. Reducing cardiac tamponade caused by temporary pacemaker perforation in transcatheter aortic valve replacement. Int J Cardiol. 2023;377:26-32.
10. Barbash IM, Dvir D, Ben-Dor I, et al. Prevalence and Effect of Myocardial Injury After Transcatheter Aortic Valve Replacement. Am J Cardiol. 2013; 111:1337-1343.
11. Tjong FVY, de Ruijter UW, Beurskens NEG, et al. A comprehensive scoping review on transvenous temporary pacing therapy. Neth Heart J. 2019;27: 462-473.
12. Hilling?Smith R, Cockburn J, Dooley M, et al Rapid pacing using the 0.035?in. Retrograde left ventricular support wire in 208 cases of transcatheter aortic valve implantation and balloon aortic valvuloplasty. Cathet Cardio Intervent. 2017;89:783-786.
13. Savvoulidis P, Mechery A, Lawton E, et al. Comparison of left ventricular with right ventricular rapid pacing on tamponade during TAVI. Int J Cardiol. 2022;360:46-52.
14. DAS MK, Dandamudi G, Steiner HA. Modern pacemakers:hope or hype?Pacing Clin Electrophysiol. 2009;32:1207-1221.
15. Haum M, Steffen J, Sadoni S, et al. Pacing Using Cardiac Implantable Electric Device During TAVR:10-Year Experience of a High-Volume Center. JACC Cardiovasc Interv. 2024;17:1020-1028.














