ABSTRACT
Introduction and objectives: To compare the effects of drug-coated balloon (DCB) vs drug-eluting stent (DES) in patients presenting with de novo large vessel coronary artery disease (CAD).
Methods: We conducted a systematic research of randomized controlled trials comparing DCB vs DES in patients with de novo large vessel CAD. Data were pooled by meta-analysis using a random-effects model. The prespecified primary endpoint was target lesion revascularization (TLR).
Results: A total of 7 trials enrolling 2961 patients were included. The use of DCB vs DES was associated with a similar risk of TLR (OR, 1.21; 95%CI, 0.44-3.30; I2 = 48%), all-cause mortality (OR, 1.56; 95%CI, 0.94- 2.57; I2 = 0%), cardiac death (OR, 1.65; 95%CI, 0.90-3.05; I2=0%), myocardial infarction (OR, 0.97; 95%CI, 0.58-1.61; I2 = 0%), major adverse cardiovascular adverse (OR, 1.19; 95%CI, 0.74-1.90; I2 = 13.5%) and late lumen loss (standardized mean difference [SMD], −0.35; 95%CI, −0.74 to 0.04; I2 = 81.4%). However, the DCB was associated with a higher risk of target vessel revascularization (OR, 2.47; 95%CI, 1.52-4.03; I2 = 0%) and smaller minimal lumen diameter during late follow-up (SMD, −0.36; 95%CI, −0.56 to −0.15; I2 = 34.5%). Nevertheless, prediction intervals included the value of no difference for both outcomes.
Conclusions: In patients with de novo large vessel CAD the use of DCB vs DES is associated with a similar risk of TLR. However, the DES achieves better late angiographic results.
Keywords: Drug-coated balloon. Drug-eluting stent. Coronary artery disease.
RESUMEN
Introducción y objetivos: Comparar los efectos del balón farmacoactivo (BFA) frente al stent farmacoactivo (SFA) en pacientes con enfermedad arterial coronaria (EAC) de vaso grande de novo.
Métodos: Se realizó una búsqueda sistemática de ensayos clínicos aleatorizados comparando BFA frente a SFA en pacientes con EAC de vaso grande de novo. Los datos se agruparon mediante un metanálisis de efectos aleatorios. El objetivo primario fue la necesidad de revascularización de la lesión diana (RLD).
Resultados: Se incluyeron 7 ensayos con 2.961 pacientes. El uso de BFA, en comparación con SFA, se asoció con un riesgo similar de RLD (OR = 1,21; IC95%, 0,44-3,30; I2 = 48%), muerte por todas las causas (OR = 1,56; IC95%, 0,94-2,57; I2 = 0%), muerte de causa cardiovascular (OR = 1,65; IC95%, 0,90-3,05; I2 = 0%), infarto de miocardio (OR = 0,97; IC95%, 0,58-1,61; I2 = 0%), acontecimientos adversos cardiacos mayores (OR = 1,19; IC95%, 0,74-1,90; I2 = 13,5%) y pérdida luminal tardía (DME = −0,35; IC95%, −0,74 a 0.04; I2 = 81,4%). Sin embargo, el BFA se asoció a un mayor riesgo de revascularización del vaso diana (OR = 2,47; IC95%, 1,52-4,03; I2 = 0%) y a un menor diámetro luminal mínimo en el seguimiento (DME: −0,36; IC95%, −0,56 a −0,15; I2 = 34,5%), aunque los intervalos de predicción incluyeron el valor nulo para ambos resultados.
Conclusiones: En los pacientes con EAC de vaso grande de novo, el BFA comparado con el SFA se asoció a un riesgo similar de RLD, obteniendo el SFA mejores resultados angiográficos.
Palabras clave: Balón farmacoactivo. Stent farmacoactivo. Enfermedad arterial coronaria.
Abbreviations
CAD: coronary artery disease. DCB: drug-coated balloon. DES: drug-eluting stent. MI: myocardial infarction. MLD: minimum lumen diameter. TLR: target lesion revascularization.
INTRODUCTION
Drug-eluting stents (DES) remain the standard of treatment for patients undergoing percutaneous coronary intervention (PCI).1,2 However, DES are associated with a gradually and permanent increased risk of adverse events, particularly due to late stent thrombosis and in-stent restenosis, with a 2% incidence rate per year with no plateau observed.1 This risk is even higher when complex and long lesions are treated.3 In recent years, drug-coated balloons (DCB) have emerged as a potential alternative treatment option to DES. Following adequate lesion preparation, unlike traditional stents, DCBs can release an antiproliferative drug into the vessel wall without leaving behind a permanent metal scaffold. Notably, permanent scaffolding can distort and constrain the coronary vessel, thus impairing vasomotion and adaptive remodelling, while also promoting chronic inflammation.4 DCB-PCI is a well-established treatment for in-stent restenosis and small-vessel coronary artery disease (CAD).5,6 However, its role in de novo large vessel CAD remains controversial. In a recent randomized clinical trial (RCT) with patients undergoing de novo CAD revascularization, a strategy of DCB-PCI did not achieve non-inferiority vs DES in terms of device-oriented composite endpoint driven by higher rates of target lesion revascularization (TLR).7 Contrary to prior published research, our findings did not support similar clinical outcomes for DCB vs DES in patients with de novo large vessel CAD.8,9 A recent meta-analysis of 15 studies compared DCB-PCI or hybrid angioplasty vs DES-PCI in patients with vessels > 2.75 mm in diameter showing no significant differences in the clinical endpoints of TLR, cardiac death, and MI.10 However, 14 of the 15 included studies were non-RCT, and the recent previously reported RCT was not included. Nevertheless, individual non-inferiority studies often lack the statistical power needed to definitively compare these technologies, underscoring the need for a systematic appraisal of treatment effects and evidence quality. Therefore, we conducted a systematic review and meta-analysis of available RCT to provide a comprehensive and quantitative assessment of evidence on the efficacy of DCB vs the current-generation DES in de novo large vessel CAD in terms of adverse events at longest available follow-up.
METHODS
Search strategy and selection criteria
We conducted a meta-analysis of RCT according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2009 guidelines.11 Two reviewers independently identified the relevant studies through an electronic search across the MEDLINE and Embase databases (from inception to October 2024). In addition, we employed backward snowballing (eg, reference review from identified articles and pertinent reviews). No language, publication date or publication status restrictions were imposed. This study is registered with PROSPERO and the search strategy is available in the supplementary data.
Study selection
Two reviewers independently assessed trial eligibility based on titles, abstracts, and full-text reports. Discrepancies in study selection were discussed and resolved with a third investigator. Eligible studies needed to meet the following pre-specified criteria: a) RCT comparing PCI with DCB and PCI with DES; b) study population including patients with de novo large vessel CAD (eg, defined as vessel diameter ≥ 2.5 mm);12 c) availability of clinical outcome data (without restriction as to follow-up time). Exclusion criteria were a) lack of a randomized design; b) studies including patients undergoing treatment for in-stent restenosis; c) studies including patients with de novo small vessel CAD; d) lack of any clinical outcome data.
A reference vessel diameter ≥ 2.5 mm was established as the cut-off value to define large vessel based on a recent proposed standardized definition.12
Data extraction
Three investigators (J. Llau García, S. Huélamo Montoro and J. A. Sorolla Romero) independently assessed studies for possible inclusion, with the senior investigator (J. Sanz-Sánchez) resolving discrepancies. Non-relevant articles were excluded based on title and abstract. The same investigators independently extracted data on study design, measurements, patient characteristics, and outcomes using a standardized data-extraction form. Data extraction conflicts were discussed and resolved with the senior investigator.
Data on authors, year of publication, inclusion and exclusion criteria, sample size, patients’ baseline patients, endpoint definitions, effect estimates, and follow-up time were collected.
Endpoints
The prespecified primary endpoint was TLR. Secondary clinical endpoints were all-cause mortality, cardiac death, myocardial infarction (MI), target vessel revascularization (TVR) and major adverse cardiovascular events (MACE). Secondary angiographic endpoints were minimum lumen diameter (MLD) and late lumen loss (LLL). Each endpoint was assessed according to the definitions reported in the original study protocols, as summarized in table 1 of the supplementary data. All the endpoints were assessed at the maximum follow-up available.
Table 1. Main features of included studies
| Study | Year of publication | No. of patients | Type of Device | Reference vessel diameter (mean ± SD) (mm) | Multicenter | Clinical follow up (months) | Angiographic follow-up (months) | |
|---|---|---|---|---|---|---|---|---|
| DCB | DES | |||||||
| REC-CAGEFREE I7 | 2024 | 1133 | 1139 | Paclitaxel-DCB Sirolimus-DES |
3.00 ± 0.55 | YES | 24 | NO |
| Nishiyama et al.13 | 2016 | 30 | 30 | Paclitaxel-DCB Everolimus-DES |
2.80 ± 0.63 | NO | 8 | 8 |
| Xue Yu et al.8 | 2022 | 85 | 85 | Paclitaxel-DCB Everolimus-DES |
2.89 ± 0.33 | NO | 12 | 9 |
| REVELATION9 | 2019 | 60 | 60 | Paclitaxel-DCB Sirolimus and everolimus DES |
3.24 ± 0.50 | NO | 24 | 9 |
| Gobic et al.15 | 2017 | 38 | 37 | Paclitaxel-DCB Sirolimus-DES |
> 2.50 | NO | 6 | 6 |
| Hao et al.16 | 2021 | 38 | 42 | Paclitaxel-DCB NA |
> 2.50 | NO | 12 | 12 |
| Wang et al.14 | 2022 | 92 | 92 | Paclitaxel-DCB Sirolimus-DES |
3.37 ± 0.52 | NO | 12 | 9 |
|
DCB, drug-coated balloon; DES, drug-eluting stent; NA, not available. |
||||||||
Risk of bias
The risk of bias in each study was assessed using the revised Cochrane risk of bias tool (RoB 2.0).11 Three investigators (J. Llau García, S. Huélamo Montoro and J. A. Sorolla Romero) independently assessed 5 domains of bias in RCT: a) randomization process, b) deviations from intended interventions, c) missing outcome data, d) outcome measurement, and e) selection of reported results (table 2 of the supplementary data).
Table 2. Baseline clinical characteristics of included patients
| Study | Age (years) | Male (%) | Diabetes (%) | Smoking (%) | Hypertension (%) | LVEF (%) | Clinical Presentation (CCS/ACS) (%) | Multivessel (%) | Complex lesion (%) |
|---|---|---|---|---|---|---|---|---|---|
| REC-CAGEFREE I7 | 62 | 69.3 | 27.3 | 45 | 60.1 | 60 | 44.9/55.3 | 4.8 | 0 |
| Nishiyama et al.13 | 69 | 73.3 | 41.6 | 60 | 83.3 | NA | 0/100 | NA | 36 |
| Xue Yu et al.8 | 63.3 | 69.3 | 24.1 | 54 | 63.9 | > 40 | 11.1/88.9 | 84 | 44.1 |
| REVELATION9 | 57 | 87 | 10 | 60 | 31 | 57.6 | 0/100 | 71.6 | N/A |
| Gobic et al.15 | 57.4 | 87 | 10 | 49.5 | 33.4 | 50.2 | 0/100 | NA | N/A |
| Hao et al.16 | 57.5 | 78.5 | 31.5 | 29.5 | 24 | 46 | 0/100 | NA | N/A |
| Wang et al.14 | 49.5 | 93.5 | 81.6 | 81.5 | 71.8 | NA | 0/100 | NA | N/A |
|
ACS, acute coronary syndrome; CCS, chronic coronary syndrome; NA, not available. |
|||||||||
Statistical analysis
Odds ratios (OR) and 95% confidence intervals (95%CI) were calculated using the DerSimonian and Laird random-effects model, with the estimate of heterogeneity being obtained from the Mantel-Haenszel method. The presence of heterogeneity among studies was evaluated with the Cochran Q chi-square test, with P ≤ .10 being considered of statistical significance, and using the I2 test to evaluate inconsistency. A value of 0% indicates no observed heterogeneity, and values of ≤ 25%, ≤ 50%, > 50% indicate low, moderate, and high heterogeneity, respectively. Prediction intervals (95%) in addition to conventional 95%CI around ORs were calculated to assess residual uncertainty. Publication bias and the small study effect were assessed for all outcomes, using funnel plots. The presence of publication bias was investigated using Harbord and Egger tests and visual estimation with funnel plots. We performed a sensitivity analysis by removing one study at a time to confirm that the findings, when compared with DES, were not driven by any single study. To account for different lengths of follow-up across studies, another sensitivity analysis was performed using the Poisson regression model with random intervention effects to calculate inverse-variance weighted averages of study-specific log stratified incidence rate ratios (IRRs). Results were displayed as IRRs, which are exponential ratios of the regression model. Additionally, random-effect meta-regression analyses were performed to assess the impact of the following variables on treatment effect with respect to the primary endpoint: eg, percentage of patients with acute coronary syndrome (ACS), percentage of patients with diabetes mellitus, mean reference vessel diameter and follow-up duration. The statistical level of significance was 2-tailed P < .05. Stata version 18.0 (StataCorp LP, College Station, United States), was used for statistical analyses.
RESULTS
Search results
Figure 1 illustrates the PRISMA study search and selection process. A total of 7 RCT were identified and included in this analysis. The main features of included studies are shown in table 1.
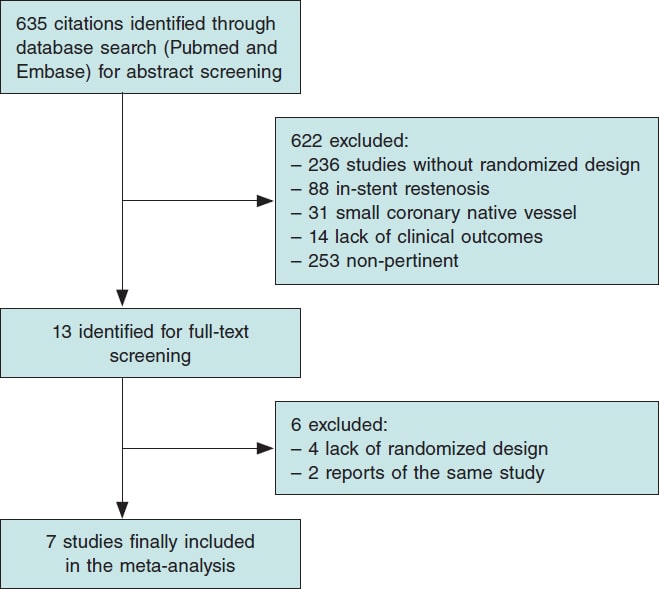
Figure 1. Flow diagram of the search for studies included in the meta-analysis according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement.
All studies had a non-inferiority design. A clinical primary endpoint was selected in 1 study,7 and an invasive functional endpoint was selected in another trial,9 while angiographic primary endpoints were prespecified in the remaining studies.8,13-16 The mean clinical and angiographic follow-up were 21.5 months and 8.9 months respectively. A total of 4 studies were conducted in the context of ACS9,14-17 and 1 study in the context of chronic coronary syndrome (CCS).13 Finally, 2 studies enrolled both ACS and CCS patients.7,8 A total of 3 trials enrolled patients treated with second-generation DES (Firebird 2.0 [Microport, China], Xience Xpedition [Abbott Vascular, United States], Orsiro [Biotronik, Germany]),7,9,13 and 2 studies enrolled patients treated with third-generation DES (Biomine [Meril Life Sciences, India], Cordimax [Rientech, China]).14,15 One trial enrolled patients treated with second and third-generation DES (Xience Xpedition [Abbott Vascular, United States], Resolute Integrity, [Medtronic, United States], Firehawk, [MicroPort, China]).8 All studies included patients who underwent paclitaxel-DCB-PCI ([Pantera Lux, Biotronik, Germany],9,14 [SeQuent Please, B Braun, Germany],7,8,13,15 [Bingo DCB, Yinyi Biotech,China]),16 and none with sirolimus-DCB-PCI.
Baseline characteristics
A total of 2961 patients were included, 1476 of whom received DCB and 1485, DES for de novo large vessel CAD. The patients main baseline characteristics are shown in table 2.
Publication bias and asymmetry
Funnel-plot distributions of the pre-specified outcomes indicate absence of publication bias for all the outcomes (figures 1-8 of the supplementary data).
Risk of bias assessment
Table 2 of the supplementary data illustrates the results of the risk of bias assessment with the RoB 2.0 tool. One trial was considered at low overall risk of bias,7 5 raised some concerns8,9,13,14,16 and 1 presented a high overall risk of bias.15
Outcomes
Clinical outcomes
DCB use compared with DES was associated with a similar risk of TLR (OR, 1.21; 95%CI, 0.44-3.30; I2 = 48%), all-cause mortality (OR, 1.56; 95%CI, 0.94- 2.57; I2 = 0%), cardiac death (OR, 1.65; 95%CI, 0.90-3.05; I2 = 0%), MI (OR, 0.97; 95%CI, 0.58-1.61; I2 = 0%) and MACE (OR, 1.19; 95%CI, 0.74-1.90; I2 = 13.5%). However, DCB was associated with a higher risk of TVR (OR, 2.47; 95%CI, 1.52- 4.03; I2 = 0%) (figure 2, figure 3 and figures 9-10 of the supplementary data).
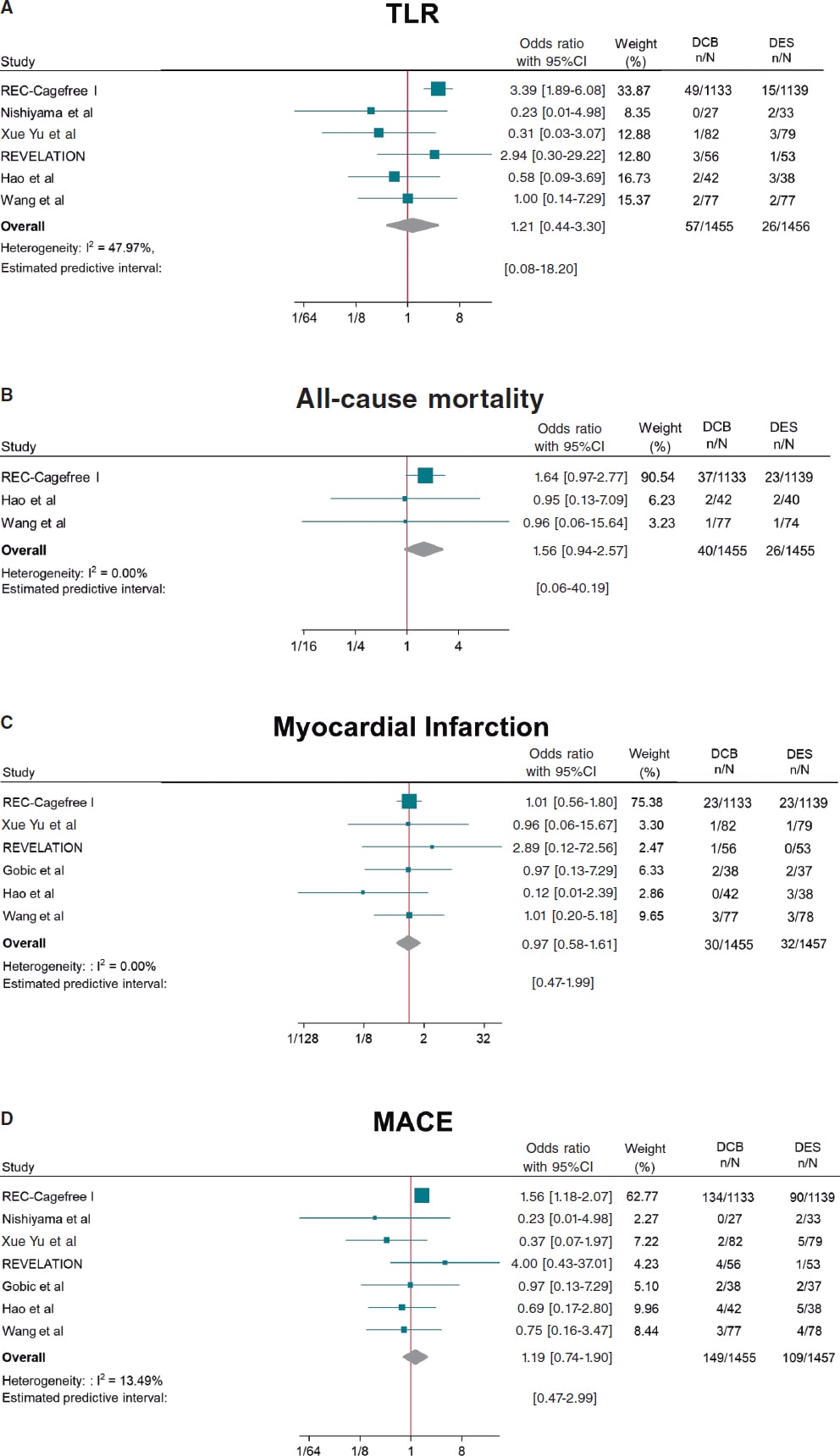
Figure 2. Forest plot reporting trial-specific and summary ORs with 95%CIs for the endpoint of A) target lesion revascularization; B) all-cause mortality; C) myocardial infarction; D) MACE. 95%CI, 95% confidence interval; DCB, drug-coated balloon; DES, drug-eluting stents; MACE, major adverse cardiovascular events; OR, odds ratio. References: REC-Cagefree I.,7 Nishiyama et al.,13 Xue Yu et al.,8 REVELATION,9 Hao et al.,16 Wang et al.,14 and Gobic et al.15
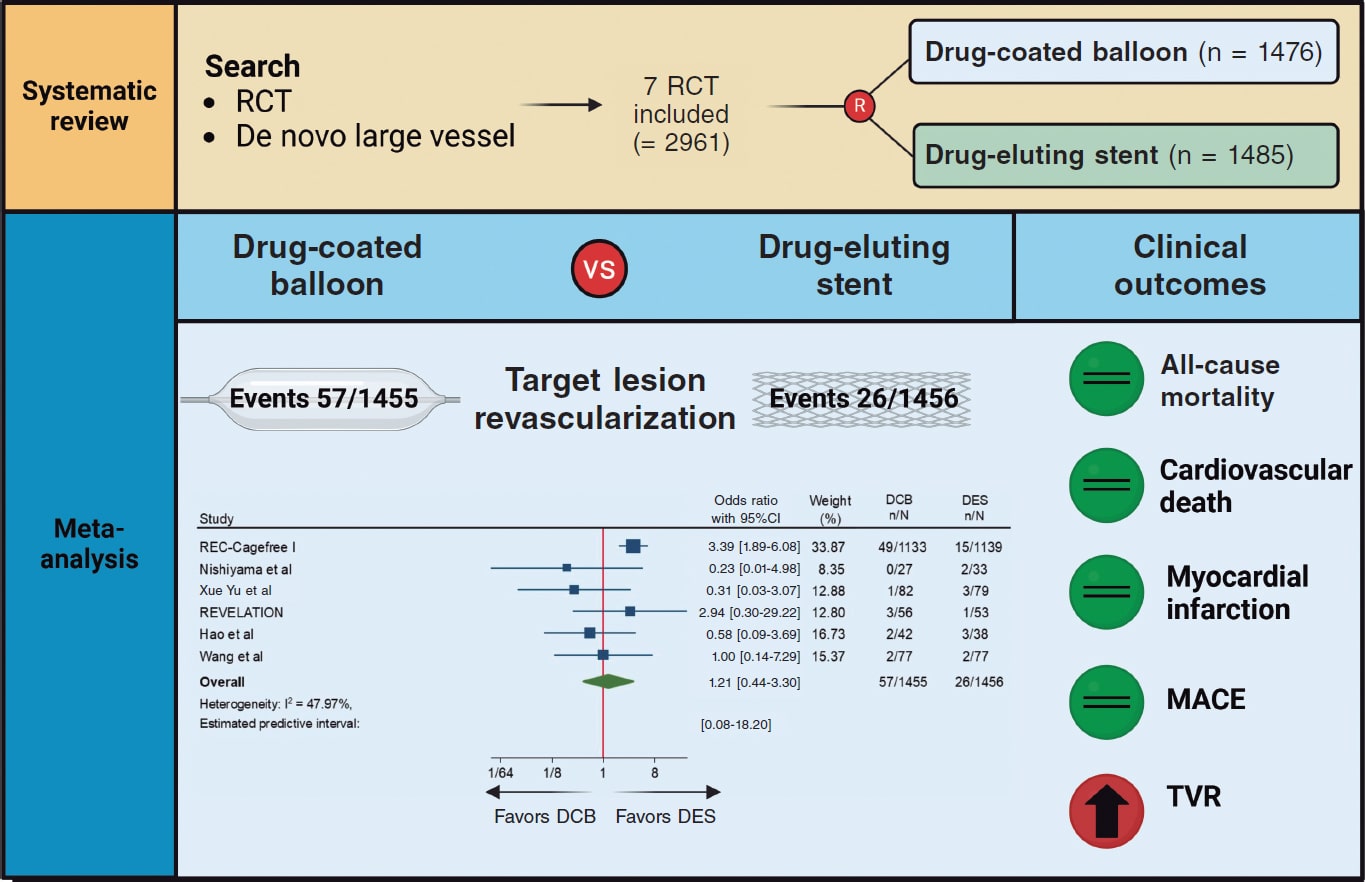
Figure 3. Central Illustration. DCB, drug-coated balloon; DES, drug-eluting stent; RCT, randomized clinical trial; TVR, target vessel revascularization. References: REC-Cagefree I.,7 Nishiyama et al.,13 Xue Yu et al.,8 REVELATION,9 Hao et al.,16 and Wang et al.14
Angiographic outcomes
Compared with DES, DCB use yielded significant smaller MLD (SMD, −0.36; 95%CI, −0.56 to −0.15; I2 = 34.5%) and similar risk of LLL (SMD, −0.35; 95%CI, −0.74 to 0.04; I2 = 81.4%) at follow-up (figure 4).
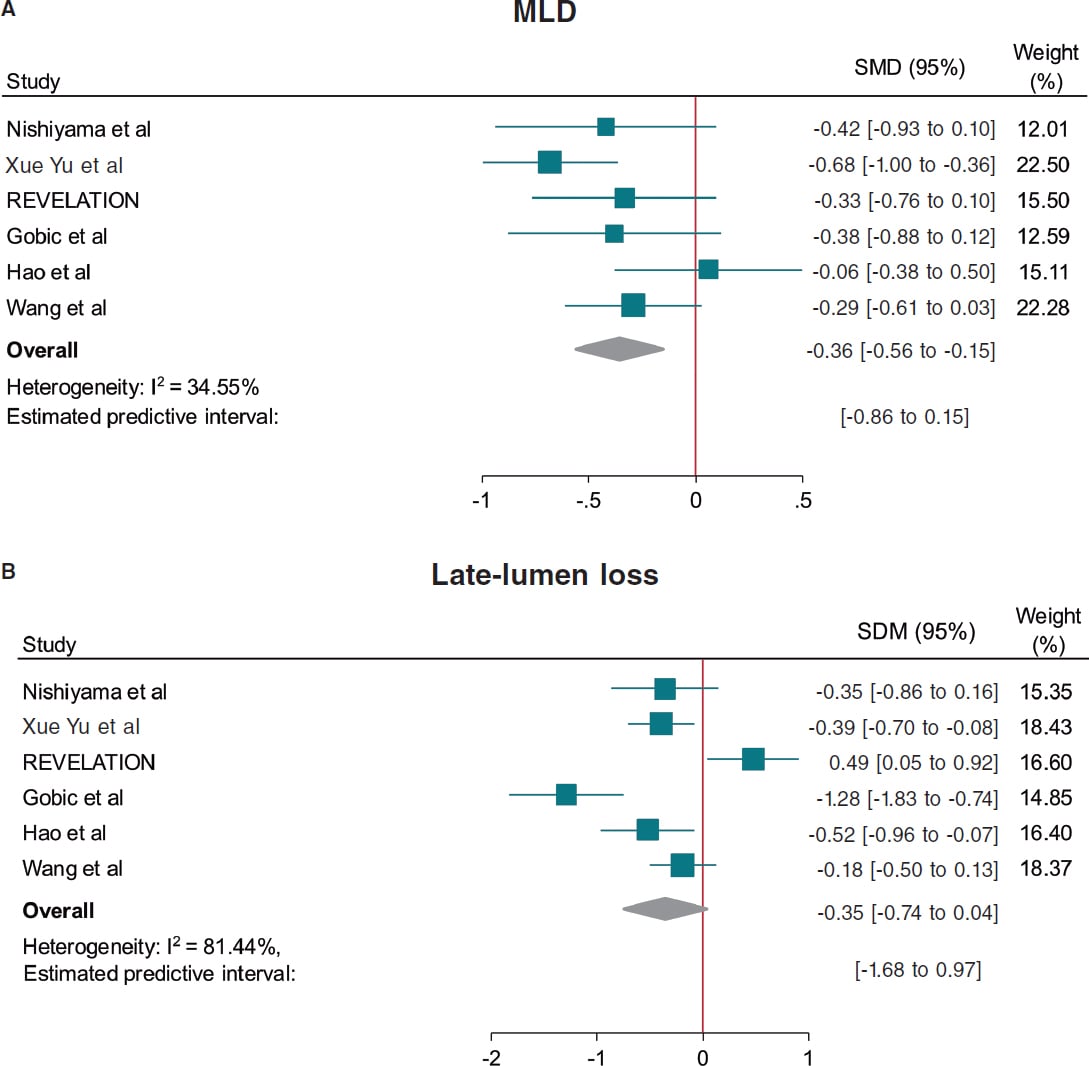
Figure 4. Forest plot reporting trial-specific and summary ORs with 95%CIs for the endpoint of A: minimum lumen diameter, and B: late-lumen loss. 95%CI, 95% confidence interval; DCB, drug-coated balloon; DES, drug-eluting stents; MACE, major adverse cardiovascular events; MLD, minimum lumen diameter; SMD, standardized mean difference; OR, odds ratio. References: Nishiyama et al.,13 Xue Yu et al.,8 REVELATION,9 Gobic et al.,15 Hao et al.,16 and Wang et al.14.
Prediction intervals were consistent with CI for all the outcomes except for TVR and MLD, which included the value of no difference.
Sensitivity analysis
A leave-one-out pooled analysis by iteratively removing one study at a time was performed for all endpoints. Treatment effects were consistent with the main analysis for TLR, all-cause mortality, cardiac death, MI and MLD. The risk of TVR was no longer significantly higher among patients undergoing DCB when removing the CAGEFREE I trial,7 and the risk of LLL was significantly lower among patients undergoing DCB-PCI when removing the REVELATION trial.9 However, an increased risk of MACE was observed among patients undergoing DCB-PCI when removing the study by Xue Yu et al.18 (tables 3-10 of the supplementary data). A sensitivity analysis using estimated IRRs was performed to account for varying follow-up lengths, confirming that our main analysis findings remained unchanged (table 11 of the supplementary data).
Random effect meta-regression analysis found no significant impact of the proportion of patients presenting with ACS (P = .882), diabetes mellitus (P = .641), mean reference vessel diameter (P = .985) and follow-up duration (P = .951) on treatment effect with respect to the primary endpoint.
DISCUSSION
This meta-analysis provides a comprehensive and updated quantitative analysis of available evidence on the comparison of DCB vs DES in de novo large vessel CAD, including data from 2961 patients enrolled in 7 RCT. The main findings of the study are:
a) The use of DCB was associated with a similar risk of clinical events vs DES except for TVR. However, data for this outcome was only available in 3 of the 7 included studies and the increased risk in patients undergoing DCB-PCI was not significant when the CAGEFREE I trial was removed. In addition, prediction intervals were not consistent with the CI. Therefore, the results of this outcome should be interpreted with caution.
b) The effect of DCB on the risk of TLR was not affected by the proportion of patients presenting with ACS or diabetes, as well as the mean reference vessel diameter or follow-up duration as assessed by meta-regression analysis.
c) DCB was associated with lower MLD at angiographic follow-up, but with similar LLL vs DES.
DES are the standard of treatment for patients undergoing PCI. However, complications such as stent thrombosis and in-stent restenosis still occur with rates estimated at 0.7-1% and 5-10% at the 10-year follow-up respectively.19,20 Therefore, in recent years there has been a growing concern for developing strategies to reduce stent-related adverse events. In this context, DCBs have emerged as a potential treatment alternative based on a “leaving nothing behind” strategy. Nevertheless, data of patients presenting with de novo large CAD is scarce and conflicting. The CAGEFREE I is the only available clinically powered RCT that included 2272 patients undergoing de novo non-complex CAD revascularization across 40 centers in China. A strategy of DCB-PCI did not achieve non-inferiority vs DES in terms of device-oriented composite endpoint driven by higher rates of TLR in the DCB-PCI group (3.1% vs 1.2%, P = .002). On the other hand, in single-center RCT conducted by Nishiyama et al. with 60 patients with CCS undergoing elective PCI a trend toward lower rates of TLR in the DCB-PCI group (0% vs 6.1%, P = .193) was shown at the 8-mont follow-up.13 Similarly, in a RCT including 170 patients undergoing PCI for de novo large CAD lower rates of TLR at the 12-month follow-up were found in patients undergoing DCB-PCI (1.6% vs 3.4%, P = .306).14 In our analysis when pooling data from all available RCT, the risk of TLR was similar among patients undergoing DCB-PCI or DES-PCI. Notably, since this result was obtained with a moderate heterogeneity (I2 ≈ 50%), it should be interpreted with caution regarding its general applicability. These findings remained unvaried at the leave-one-out analysis. In addition, prediction intervals were consistent with CI around ORs showing lack of residual uncertainty. Previous studies have shown that in-stent restenosis after DES is not a benign phenomenon, presenting as an ACS in about 70% of the cases, with 5-10% of these resulting in MI.21 We could speculate that the lack of permanent scaffold with DCB vs DES may predispose to a less aggressive pattern of restenosis and not increase the risk of thrombotic vessel closure beyond 3 months when vessel healing after DCB-PCI has occurred.22
Notably, 5 of the 7 studies included in this meta-analysis enrolled patients presenting with ACS. A total of 34% of the patients included in the CAGEFREE study presented with ACS, with 16% being STEMI cases.7 Four other studies only included STEMI patients.7,9,14-16 Although the performance of DCB in the STEMI scenario is unknown, its use in clinical practice is increasing.23 Culprit lesion plaques in STEMI patients are usually soft and adequate plaque modification can be easily achieved through DCB-PCI (< 30% residual stenosis and low grade of dissection).23 Moreover, the ruptured lipid rich plaque can potentially be an ideal reservoir for effective paclitaxel uptake.24 On the other hand, DCBs carry specific risks for STEMI patients, such as acute recoil and culprit lesion closure, because they don’t provide vessel scaffolding.
In our study, the proportion of patients presenting with ACS had no impact on treatment effects on the meta-regression analysis. Nevertheless, further RCT with adequate sample size are needed to obtain more solid evidence in this field. Of note, complex lesions (eg, severe calcification and bifurcations with planned two-stent technique) were excluded from the studies that included patients presenting with CCS.7,8 Therefore, our findings might not be generalized to this population.
The better angiographic surrogate outcomes with DES-PCI vs DCB-PCI found in our meta-analysis after pooling data from 6 studies can be explained by the absence of a metal scaffold to expand the vessel lumen and the acute recoil following balloon angioplasty. This justifies the lower MLD achieved after DCB-PCI vs DES-PCI. While our analysis did not show significant differences regarding LLL during follow-up, the value of LLL was lower among patients undergoing DCB-PCI when excluding the REVELATION trial.9,17 This study showed extremely low LLL in both DCB and DES groups vs other available evidence from RCT.15,16 The presence of positive vessel remodeling with a late lumen enlargement after the use of DCB evaluated by intracoronary imaging modalities has been evidenced in multiple studies, and seems to be associated with small vessel disease, fibrous and layered plaques and a post-PCI medial dissection arc > 90°.25,26,27 However, evidence of this phenomenon in patients with large vessel CAD is less known.22 It should, therefore, be noted that all studies in this meta-analysis used paclitaxel-DCB. While the evidence comparing sirolimus and paclitaxel-DCB is scarce, 2 recent RCT have shown better angiographic results with the lipophilic component. In the first one, with 121 patients with the novo small vessel CAD, sirolimus-DCB failed to achieve non-inferiority for net-lumen gain at 6 months.28 In the second study, with 70 patients, the 2 devices showed similar results of LLL at 6 months, although patients treated with paclitaxel-DCB had more frequent late luminal enlargement.29 Due to the small sample size and although there is not enough evidence to evaluate differences across clinical endpoints, we cannot assume that there is a class effect across all DCBs. There are larger ongoing RCT to evaluate the outcomes of sirolimus DCB vs DES in large vessels that will provide evidence in this field.30,31
Limitations
The results of our investigation should be interpreted in light of some limitations. First, this is a study-level meta-analysis providing average treatment effects. The lack of patient-level data from the included studies prevents us from assessing the impact of baseline clinical, angiographic and procedural characteristics on treatment effects. Second, minor differences in definition were present for some endpoints (eg, MACE), limiting the reliability of effect estimates. Third, one study which accounted for approximately 75% of all patients included did not included angiographic follow-up,7 thus limiting the evaluation of DCB and DES on angiographic outcomes. Fourth, the clinical follow-up varied from 6 to 24 months. Ideally, outcomes such as TLR should be compared at uniform follow-up across studies (eg, at 1 year), which was not consistently possible in the current analysis. Nonetheless, these differences in follow-up duration were accounted with the IRRs, as detailed in the Methods section. However, longer follow-ups are needed to establish the safety and efficacy profile of DCB vs DES throughout time. Fifth, the definition of large vessel is inconsistent across trials, which might be a source of bias. Finally, the limited number of studies and patients, and the small event rate for some endpoints, such as all-cause mortality may reduce the power for detecting significant differences across groups.
CONCLUSIONS
This meta-analysis provides the most updated quantitative evidence on the use of DCB vs DES for the treatment of de novo large vessel CAD in both CCS and ACS. DCB-PCI is associated with similar TLR and LLL at mid-term follow-up representing an appealing treatment option for patients with large vessel CAD.
FUNDING
None declared.
ETHICAL CONSIDERATIONS
Ethics approval was deemed unnecesary for this meta-analysis as all data were collected and synthesized from previous studies. Additionally, no informed consent was required as there were no patients involved in our work. The meta-analysis of RCT was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2009 guidelines. We confirm that sex/gender biases have been taken into consideration.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
No artificial intelligence has been used in the preparation of this article.
AUTHORS’ CONTRIBUTIONS
J. Llau García, S. Huelamo Montoro and J.A. Sorolla Romero participated in literature research and study selection. J.A. Sorolla Romero, L. Novelli and J. Sanz Sánchez contributed to the conception, design, drafting and revision of the article. P. Rubio, J.L. Díez Gil, L. Martínez-Dolz, I.J. Amat Santos, B. Cortese, F. Alfonso, and H.M. Garcia-Garcia contributed to the critical revision of the intellectual content of the article.
CONFLICTS OF INTEREST
F. Alfonso is an associate editor of REC: Interventional Cardiology; the journal’s editorial procedure to ensure impartial handling of the manuscript has been followed. The authors declared no relevant relationships with the contents of this paper.
WHAT IS KNOWN ABOUT THE TOPIC?
- DCB are a well-established treatment for patients with small-vessel CAD.
- Available published evidence of patients with de novo large vessel CAD is scarce and shows conflicting results.
WHAT DOES THIS STUDY ADD?
- In this meta-analysis including data from 2961 patients enrolled in 7 RCT, DCB showed similar risk of clinical events at follow-up vs DES in the treatment of de novo large vessel CAD.
- The use of DCB might be considered as an alternative option to DES in patients undergoing PCI for non-complex de novo large vessel CAD.
REFERENCES
1. Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87-165.
2. Lawton JS, Tamis-Holland JE, Bangalore S, et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization:Executive Summary:A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e4-e17.
3. Kong MG, Han JK, Kang JH, et al. Clinical outcomes of long stenting in the drug-eluting stent era:patient-level pooled analysis from the GRAND-DES registry. EuroIntervention J Eur Collab Work Group Interv Cardiol Eur Soc Cardiol. 2021;16:1318-1325.
4. Kawai T, Watanabe T, Yamada T, et al. Coronary vasomotion after treatment with drug-coated balloons or drug-eluting stents:a prospective, open-label, single-centre randomised trial. EuroIntervention. 2022;18:e140?e148.
5. Jeger RV, Eccleshall S, Wan Ahmad WA, et al. Drug-Coated Balloons for Coronary Artery Disease:Third Report of the International DCB Consensus Group. JACC Cardiovasc Interv. 2020;13:1391-1402.
6. Sanz Sánchez J, Chiarito M, Cortese B, et al. Drug-Coated balloons vs drug-eluting stents for the treatment of small coronary artery disease:A meta-analysis of randomized trials. Catheter Cardiovasc Interv Off J Soc Card Angiogr Interv. 2021;98:66-75.
7. Gao C, He X, Ouyang F, et al. Drug-coated balloon angioplasty with rescue stenting versus intended stenting for the treatment of patients with de novo coronary artery lesions (REC-CAGEFREE I):an open-label, randomised, non-inferiority trial. Lancet Lond Engl. 2024;404:1040-1050.
8. Yu X, Wang X, Ji F, et al. A Non-inferiority, Randomized Clinical Trial Comparing Paclitaxel-Coated Balloon Versus New-Generation Drug-Eluting Stents on Angiographic Outcomes for Coronary De Novo Lesions. Cardiovasc Drugs Ther. 2022;36:655-664.
9. Vos NS, Fagel ND, Amoroso G, et al. Paclitaxel-Coated Balloon Angioplasty Versus Drug-Eluting Stent in Acute Myocardial Infarction:The REVELATION Randomized Trial. JACC Cardiovasc Interv. 2019;12:1691-1699.
10. Gobbi C, Giangiacomi F, Merinopoulos I, et al. Drug coated balloon angioplasty for de novo coronary lesions in large vessels:a systematic review and meta-analysis. Sci Rep. 2025;15:4921.
11. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses:the PRISMA statement. BMJ. 2009;339:b2535.
12. Sanz-Sánchez J, Chiarito M, Gill GS, et al. Small Vessel Coronary Artery Disease:Rationale for Standardized Definition and Critical Appraisal of the Literature. J Soc Cardiovasc Angiogr Interv. 2022;1:100403.
13. Nishiyama N, Komatsu T, Kuroyanagi T, et al. Clinical value of drug-coated balloon angioplasty for de novo lesions in patients with coronary artery disease. Int J Cardiol. 2016;222:113-118.
14. Wang Z, Yin Y, Li J, et al. New Ultrasound-Controlled Paclitaxel Releasing Balloon vs. Asymmetric Drug-Eluting Stent in Primary ST-Segment Elevation Myocardial Infarction-A Prospective Randomized Trial. Circ J Off J Jpn Circ Soc. 2022;86:642-650.
15. Gobic´D, Tomulic´V, Lulic´D, et al. Drug-Coated Balloon Versus Drug-Eluting Stent in Primary Percutaneous Coronary Intervention:A Feasibility Study. Am J Med Sci. 2017;354:553-560.
16. Hao X, Huang D, Wang Z, Zhang J, Liu H, Lu Y. Study on the safety and effectiveness of drug-coated balloons in patients with acute myocardial infarction. J Cardiothorac Surg. 2021;16:178.
17. Niehe SR, Vos NS, Van Der Schaaf RJ, et al. Two-Year Clinical Outcomes of the REVELATION Study:Sustained Safety and Feasibility of Paclitaxel-Coated Balloon Angioplasty Versus Drug-Eluting Stent in Acute Myocardial Infarction. J Invasive Cardiol. 2022;34:E39-E42.
18. Xue W, Ma J, Yu X, et al. Analysis of the incidence and influencing factors associated with binary restenosis of target lesions after drug-coated balloon angioplasty for patients with in-stent restenosis. BMC Cardiovasc Disord. 2022;22:493.
19. Coughlan JJ, Maeng M, Räber L, et al. Ten-year patterns of stent thrombosis after percutaneous coronary intervention with new- versus early-generation drug-eluting stents:insights from the DECADE cooperation. Rev Esp Cardiol. 2022;75:894-902.
20. Madhavan MV, Redfors B, Ali ZA, Prasad M, et al. Long-Term Outcomes After Revascularization for Stable Ischemic Heart Disease:An Individual Patient-Level Pooled Analysis of 19 Randomized Coronary Stent Trials. Circ Cardiovasc Interv.2020;13:e008565.
21. Buchanan KD, Torguson R, Rogers T, et al. In-Stent Restenosis of Drug-Eluting Stents Compared With a Matched Group of Patients With De Novo Coronary Artery Stenosis. Am J Cardiol. 2018;121:1512-1518.
22. Antonio Sorolla Romero J, Calderón AT, Tschischke JPV, Luis Díez Gil J, Garcia-Garcia HM, Sánchez JS. Coronary plaque modification and impact on the microcirculation territory after drug-coated balloon angioplasty:the PLAMI study. Rev Esp Cardiol. 2025;78:481-482.
23. Merinopoulos I, Gunawardena T, Corballis N, et al. Assessment of Paclitaxel Drug-Coated Balloon Only Angioplasty in STEMI. JACC Cardiovasc Interv. 2023;16:771-779.
24. Maranhão RC, Tavares ER, Padoveze AF, Valduga CJ, Rodrigues DG, Pereira MD. Paclitaxel associated with cholesterol-rich nanoemulsions promotes atherosclerosis regression in the rabbit. Atherosclerosis. 2008;197:959-966.
25. Kleber FX, Schulz A, Waliszewski M, et al. Local paclitaxel induces late lumen enlargement in coronary arteries after balloon angioplasty. Clin Res Cardiol Off J Ger Card Soc. 2015;104:217-225.
26. Alfonso F, Rivero F. Late lumen enlargement after drug-coated balloon therapy:turning foes into friends. EuroIntervention J Eur Collab Work Group Interv Cardiol Eur Soc Cardiol. 2024;20:523-525.
27. Yamamoto T, Sawada T, Uzu K, Takaya T, Kawai H, Yasaka Y. Possible mechanism of late lumen enlargement after treatment for de novo coronary lesions with drug-coated balloon. Int J Cardiol. 2020;321:30-37.
28. Ninomiya K, Serruys PW, Colombo A, et al. A Prospective Randomized Trial Comparing Sirolimus-Coated Balloon With Paclitaxel-Coated Balloon in De Novo Small Vessels. JACC Cardiovasc Interv. 2023;16:2884-2896.
29. Ahmad WAW, Nuruddin AA, Abdul KMASK, et al. Treatment of Coronary De Novo Lesions by a Sirolimus- or Paclitaxel-Coated Balloon. JACC Cardiovasc Interv. 2022;15:770-779.
30. Spaulding C, Krackhardt F, Bogaerts K, et al. Comparing a strategy of sirolimus-eluting balloon treatment to drug-eluting stent implantation in de novo coronary lesions in all-comers:Design and rationale of the SELUTION DeNovo Trial. Am Heart J. 2023;258:77-84.
31. Greco A, Sciahbasi A, Abizaid A, et al. Sirolimus-coated balloon versus everolimus-eluting stent in de novo coronary artery disease:Rationale and design of the TRANSFORM II randomized clinical trial. Catheter Cardiovasc Interv Off J Soc Card Angiogr Interv. 2022;100:544-552.










