ABSTRACT
Introduction and objectives: Transcatheter treatment for tricuspid regurgitation (TR) has grown exponentially in recent years. The Edwards EVOQUE system (Edwards Lifesciences, United States), which obtained CE marking in 2023, is a transcatheter tricuspid valve designed to address severe cases of TR that are not amenable to repair using other transcatheter techniques. This study presents the initial experience at our center with the implantation of the EVOQUE tricuspid valve in 10 patients with symptomatic TR. Our objective was to analyze the initial clinical and 30-day imaging follow-up.
Methods: We conducted a prospective, single-center observational study. A total of 10 patients with severe TR who underwent EVOQUE tricuspid valve implantation were included. The 30-day early outcomes were evaluated using clinical parameters and echocardiographic findings. We assessed the reduction of TR, right ventricular function, and potential postoperative complications.
Results: Ten patients with symptomatic severe TR were included. The median follow-up was 80 days, and the patients' mean age, 77.2 years. The mean TRISCORE was 4.6, and the mean EuroSCORE II, 3.9%. Pacemaker dependance was observed in 40% of patients. The rate of procedural success was 100% according to the Tricuspid Valve Academic Research Consortium criteria. The maximum transprosthetic gradient was 4.8 mmHg. Significant complications included 1 case of severe mitral regurgitation following implantation, 1 complete atrioventricular block, 2 transient right ventricular failures, and 2 prosthetic valve thromboses. The median length of stay was 7 days.
Conclusions: The early outcomes of the EVOQUE tricuspid valve implantation are promising, with significant TR reduction and few complications. Further studies are needed to confirm the safety and efficacy profile of the device in the routine clinical practice.
Keywords: Tricuspid regurgitation. EVOQUE tricuspid valve. Transcatheter implantation. Right ventricular failure. Atrioventricular block. Prosthetic valve thrombosis.
RESUMEN
Introducción y objetivos: El tratamiento percutáneo de la insuficiencia tricuspídea (IT) ha supuesto una revolución en el abordaje de estos pacientes en los últimos años. El sistema EVOQUE (Edwards Lifesciences, EE.UU.), que obtuvo el marcado CE 2023, es una prótesis tricúspide percutánea diseñada para tratar casos de IT grave que no son reparables mediante otras técnicas percutáneas. Este trabajo presenta la experiencia inicial en nuestro centro con el implante de prótesis EVOQUE en 10 pacientes con IT sintomática. Nuestro objetivo fue analizar el seguimiento inicial a 30 días, clínico y por imagen.
Métodos: Estudio observacional prospectivo unicéntrico. Se incluyeron 10 pacientes seleccionados con IT grave a los que se implantó una prótesis EVOQUE. La evolución clínica inicial se evaluó mediante parámetros clínicos y ecocardiográficos a los 30 días. Se valoraron la reducción de la IT, la función ventricular derecha y las posibles complicaciones posoperatorias.
Resultados: Se incluyeron 10 pacientes con IT grave sintomática. La mediana de seguimiento fue de 80 días y la edad media de los pacientes fue de 77,2 años; TRISCORE medio 4,6; EuroSCORE II medio 3,9%. Un 40% de los pacientes eran portadores de marcapasos. El éxito del procedimiento fue del 100% según los criterios del >Tricuspid Valve Academic Research Consortium. Se produjo una reducción marcada de la IT en todos los casos, quedando IT leve o mínima tras el procedimiento. El gradiente transprotésico medio fue de 2,5 mmHg. Como complicaciones destacaron 1 caso de insuficiencia mitral grave posimplante, 1 bloqueo auriculoventricular completo, 2 fallos ventriculares derechos transitorios y 2 trombosis de prótesis. La mediana de estancia hospitalaria fue de 7 días.
Conclusiones: El implante de prótesis tricúspide EVOQUE ofrece resultados iniciales prometedores, con una reducción prácticamente completa de la IT y pocas complicaciones. Son necesarios más estudios para confirmar la seguridad y eficacia del dispositivo en la práctica clínica.
Palabras clave: Insuficiencia tricuspídea. Prótesis EVOQUE. Implante percutáneo. Fallo ventricular derecho. Bloqueo auriculoventricular. Trombosis de prótesis.
Abbreviations
AVB: atrioventricular block. CT: computed tomography. TR: tricuspid regurgitation.
INTRODUCTION
Severe tricuspid regurgitation (TR) is associated with a poor prognosis.1 Although the optimal timing for an isolated tricuspid valve procedure remains uncertain, in the late stages of the disease natural progression, patients already exhibit signs of right heart failure and respond poorly to diuretic treatment.2 In these advanced stages, any intervention may be futile.
Initial treatment involves diuretic drugs, with surgery being reserved only for selected cases. Furthermore, surgical outcomes are still modest, with high mortality rates associated with isolated tricuspid valve procedures.3 In this context, the European Society of Cardiology clinical practice guidelines on the management of cardiovascular diseases suggest that transcatheter techniques might play a role in the TR of selected patients.4
In recent years, there has been an exponential growth in transcatheter treatments for TR. The most widespread tricuspid repair techniques are percutaneous edge-to-edge repair and annuloplasty.5,6 In patients with complex tricuspid anatomies and those in more advanced stages of the disease, characterized by a severely dilated tricuspid annulus and large coaptation gaps, edge-to-edge repair and annuloplasty offer suboptimal results.7 For these patients, the alternative could be orthotopic transcatheter tricuspid valve replacement.
The EVOQUE transcatheter tricuspid valve replacement system (Edwards Lifesciences, United States) obtained the CE marking in 2023. The first implantation results of an EVOQUE tricuspid valve in Spain have recently been published.8
We present the first Spanish series of patients with severe symptomatic TR undergoing transcatheter implantation of the EVOQUE valve in the tricuspid position. The objective is to communicate our initial experience in terms of TR reduction, short-term follow-up, and postoperative complications, and provide scientific evidence on this innovative technology.
METHODS
We conducted a prospective, single-center observational study including 10 consecutive patients who underwent EVOQUE valve implantation to treat their symptomatic TR. Echocardiographic parameters and the 30-day clinical outcomes were evaluated. Transthoracic echocardiography was performed before discharge, and a follow-up computed tomography (CT) was performed 1 month following the procedure.
Patients had, at least, severe TR, quantified according to the Hahn and Zamorano classification:7 0, absent or minimal; 1, mild; 2, moderate; 3, severe; 4, massive; and 5, torrential. All patients showed signs or symptoms of TR or had been hospitalized for heart failure despite optimal medical therapy. In addition, all were eligible for valve replacement with the EVOQUE system. The indication for the intervention was established by the heart team, in full compliance with the European Society of Cardiology clinical practice guidelines on the management of valvular heart disease.4
Patients with severely depressed right ventricular systolic function, with anatomies that prevented correct device placement, and those with a life expectancies < 12 months were excluded. CT images were analyzed as part of the screening process by the operators and the Edwards EVOQUE team.
Preoperative analysis
Preoperative CT was performed to assess the feasibility of valve implantation. The patients’ treatment rejection rate was 17%.
The EVOQUE valve
The EVOQUE valve is a self-expanding nitinol tri-leaflet bovine pericardial valve with an anchoring system that can extend between the chordae tendineae of the subvalvular apparatus to capture the free edge of the native tricuspid leaflets (septal, anterior, and posterior) with an intra-annular skirt. The 28-Fr delivery system outer diameter has been designed for transfemoral venous access, and includes a primary flexion handle, a secondary flexion handle, and a depth handle to facilitate alignment and positioning of the device inside the native valve. There are 3 sizes are available, with outer diameters of 44 mm, 48 mm, 52 mm, and 56 mm.9
Implantation procedure
The procedure is monitored with fluoroscopy and intraoperative transesophageal echocardiography, relying heavily on live 3D imaging to ensure correct valve position and trajectory. A pre-shaped support guidewire (Safari type, Boston Scientific, United States) is placed inside a deflectable sheath (9-14-Fr) in the right ventricle, across the tricuspid annulus. The device is advanced through the delivery catheter and positioned across the tricuspid annulus, where it is deployed from the ventricular side, while placing the anchors underneath the tricuspid leaflets and above the papillary muscles. Since, at this point, the delivery system is still recapturable, a comprehensive assessment of anatomy and position can be performed. During device deployment, the 9 anchors are identified, ensuring that the leaflets are above them in systole and the papillary muscle heads are below.
Afterwards, leaflet “capture” occurs, which is monitored by 3D transesophageal echocardiography, while making the necessary trajectory and height adjustments. Continuous retraction of the system results in shortening of the valve ventricular portion. After confirming satisfactory leaflet capture, the valve ventricular portion is fully expanded, and the valve atrial portion is released from the system. Minimal changes in device position upon release may be due to further device shortening, tension release, and anchor coupling with annular tissue. After releasing the valve, the device stability, residual central and paravalvular regurgitation, valve orifice area, diastolic gradients, and hemodynamics are all confirmed by echocardiography and fluoroscopy.
Regarding antithrombotic regimen, the established protocol was to maintain the patient’s pre-existing anticoagulation (all patients except for 1 had an indication for chronic anticoagulation), without antiplatelet therapy. The only patient without baseline anticoagulation received acetylsalicylic acid.
Statistical analysis
Statistical analysis for descriptive studies was performed using Student’s t-test for mean comparison. The Wilcoxon signed-rank test was used for TR comparison before the procedure and after EVOQUE valve implantation. SPSS version 22.0 was used for the analysis.
Ethical aspects
The study protocol fully complies with the principles set forth in the Declaration of Helsinki and was approved by the hospital local ethics committee.
RESULTS
The patients’ baseline clinical characteristics are shown in table 1. Their mean age was 77.2 years, and 60% were women (n = 6). The severity of TR was categorized as severe in 30% of cases, massive in 30%, and torrential in 40%. The patients’ mean TRISCORE score was 4.6, indicating low-to-moderate risk. According to the 4A classification10 (asthenia, anorexia, ascites, and edema), most patients fell within the A1 and A3 categories. Regarding baseline rhythm, 80% of patients were in atrial fibrillation. One patient had a pre-existing right bundle branch block and developed complete atrioventricular block (AVB) after valve implantation, requiring permanent pacemaker implantation in the coronary sinus. A total of 40% of the patients were pacemaker carriers, which added complexity to the procedures, while 30% had previous hospitalizations for right heart failure symptoms. The mean B-type natriuretic peptide value of the patients was 221 pg/mL.
Table 1. Baseline clinical characteristics of the patients (n = 10)
| Characteristic | Characteristic |
|---|---|
| Age, years | 77.7 ± 6.8 |
| Female sex | 6 (60.0%) |
| Hypertension | 7 (70.0%) |
| Dyslipidemia | 2 (20.0%) |
| Diabetes mellitus | 2 (20.0%) |
| Peripheral arterial disease | 0 (0.0%) |
| Dialysis | 1 (10.0%) |
| Atrial fibrillation | 8 (80.0%) |
| Previous myocardial infarction | 2 (20.0%) |
| Previous valvular surgery | 5 (50.0%) |
| Pacemaker | 4 (40.0%) |
| Right bundle branch block | 1 (10.0%) |
| Left bundle branch block | 0 (0.0%) |
| STS score (%; mortality for mitral valve replacement) | 7.7 ± 5.7 |
| EuroSCORE-II (%) | 3.9 ± 2.3 |
| TRISCORE (points) | 4.6 ± 2.2 |
| 4A classification | 1.7 ± 1.2 |
| Previous hospitalizations for right heart failure | 3 (30.0%) |
| Physical examination | |
| NYHA functional class II | 9 (90.0%) |
| NYHA functional class III | 1 (10.0%) |
| Edema | 9 (90.0%) |
| Ascites | 3 (30.0%) |
| Treatment | |
| Furosemide | 8 (80.0%) |
| Furosemide dose (mg) | 55.0 ± 20.7 |
| Mineralocorticoid receptor antagonist | 6 (60.0%) |
| Mineralocorticoid receptor antagonist dose (mg) | 45.8 ± 29.2 |
| Lab test results | |
| Creatinine (mg/dL) | 1.6 ± 1.7 |
| eGFR (mL/min/m²) | 52.0 ± 19.5 |
| Hemoglobin (mg/dL) | 13.3 ± 1.7 |
| AST (IU/L) | 29.8 ± 5.5 |
| ALT (IU/L) | 20.5 ± 5.4 |
| GGT (IU/L) | 201.9 ± 159.7 |
| Alkaline phosphatase (IU/L) | 163.1 ± 84.3 |
| BNP (pg/mL) | 221.0 ± 239.0 |
| Echocardiogram | |
| Tricuspid regurgitation severity | |
| Severe (grade III) | 3 (30.0%) |
| Massive (grade IV) | 3 (30.0%) |
| Torrential (grade V) | 4 (40.0%) |
| Etiology | |
| Functional, atrial | 4 (40.0%) |
| Functional, ventricular | 4 (40.0%) |
| Rheumatic | 1 (10.0%) |
| Valve prolapse | 1 (10.0%) |
| RV ejection fraction determined by 3D echocardiography (%) | 49.0 ± 7.1 |
| RV longitudinal strain | −19.8 ± 6.6 |
| TAPSE (mm) | 19.7 ± 4.4 |
| Vena contracta area (cm²) | 9.0 ± 0.9 |
| 3D tricuspid ERO area (cm²) | 0.86 ± 0.4 |
| Right heart catheterization | |
| Systolic pulmonary artery pressure (mmHg) | 41.7 ± 10.5 |
| Mean pulmonary artery pressure (mmHg) | 27.1 ± 6.0 |
| Pulmonary capillary wedge pressure (mmHg) | 18.4 ± 6.0 |
| Right atrial pressure (mmHg) | 17.9 ± 6.7 |
| RV end-diastolic pressure (mmHg) | 13.0 ± 5.8 |
| Pulmonary vascular resistance (WU) | 2.2 ± 0.7 |
| Procedural data | |
| 44 mm valve | 2 (20.0%) |
| 48 mm valve | 3 (30.0%) |
| 52 mm valve | 5 (50.0%) |
| None or trivial residual TR | 7 (70.0%) |
| Mild residual TR | 3 (30.0%) |
| At least moderate residual TR | 0 (0.0%) |
| Prosthetic gradient after implantation | 2.6 ± 1.1 |
| Procedural time (min) | 130 ± 31.5 |
|
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BNP, B-type natriuretic peptide; 3D, three-dimensional; eGFR, estimated glomerular filtration rate; ERO, effective regurgitant orifice; GGT, gamma-glutamyltransferase; NYHA, New York Heart Association; RV, right ventricle; STS, Society of Thoracic Surgeons; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation.
|
|
Right heart catheterization for baseline pressure measurement was performed in 70% of the patients. Significant pulmonary hypertension was present in 5 of the 7 patients who underwent the procedure (71%). Pulmonary hypertension was post-capillary in all cases, with elevated pulmonary capillary wedge pressure.
The baseline echocardiography categorized the TR as severe, massive, or torrential in all patients, based on standard severity parameters. Regarding the assessment of right ventricular function, the usual estimation difficulties were observed, and tricuspid annular plane systolic excursion, right ventricular strain, and ejection fraction by 3D echocardiography were evaluated. Information is shown in
Requiring no transseptal puncture, the implantation procedure is performed under general anesthesia with transesophageal echocardiography guidance. The median duration of the procedures was 129.5 minutes. Figure 1 illustrates different moments of the intervention.
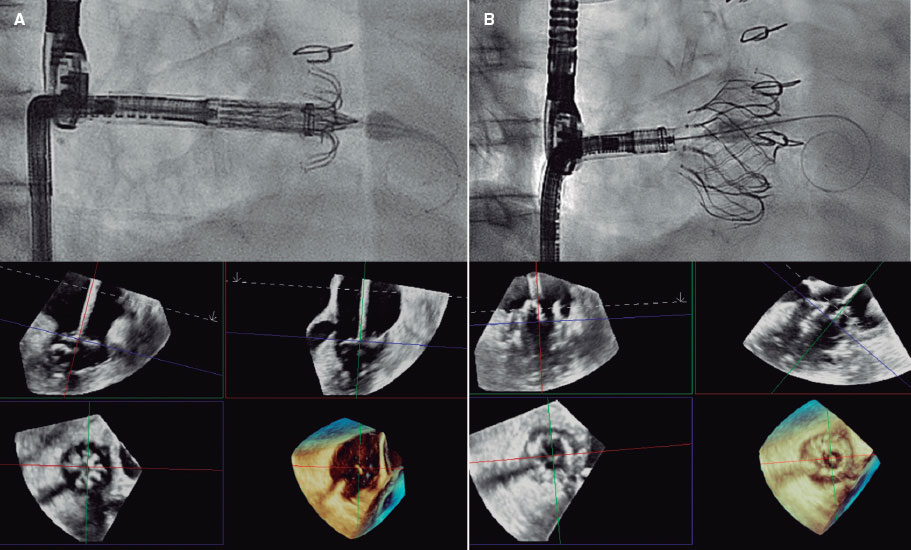
Figure 1. EVOQUE valve implantation (Edwards Lifesciences, United States). A: each anchor and its capture position with the leaflet can be assessed using 3D echocardiography. B: optimal final results.
Results, in terms of TR reduction, were excellent in all patients, with residual TR being at most mild and without paravalvular leaks (figure 2). The rate of procedural success was 100% according to the Tricuspid Valve Academic Research Consortium criteria.11 Transprosthetic gradient was 4.8 mmHg in the worst-case scenario, with a mean transtricuspid gradient in the 10 patients of 2.5 mmHg.
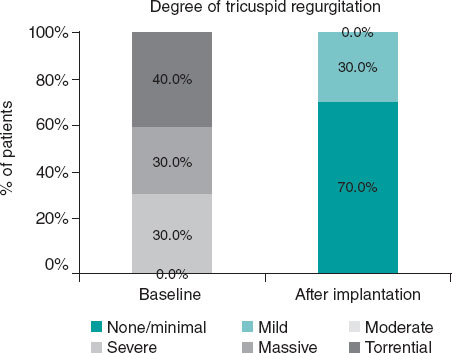
Figure 2. Near-complete reduction of tricuspid regurgitation (TR) after EVOQUE valve (Edwards Lifesciences, United States) implantation, immediately and at the follow-up. The Wilcoxon signed-rank test was performed for paired data (baseline vs post-implantation TR).
Early (30-day) complications after the intervention are shown in
Table 2. Early complications and initial progression
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | Patient 9 | Patient 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Early complications | Hematuria Severe mitral regurgitation | None | Right ventricular failure | Right ventricular failure Complete AV block | None | None | None | None | Prosthetic valve thrombosis | None |
| Treatment at discharge | ||||||||||
| Antithrombotic treatment | Acenocoumarol | Acenocoumarol | Edoxaban | Acenocoumarol | Aspirin | Acenocoumarol | Rivaroxaban | Rivaroxaban | Acenocoumarol | Acenocoumarol |
| Furosemide (mg) | 80 | Patient on hemodialysis | 80 | 40 | 0 | 160 | 40 | 40 | 40 | 40 |
| Mineralocorticoid receptor antagonist (mg) | 25 | Patient on hemodialysis | 25 | 0 | 0 | 25 | 25 | 25 | 25 | 50 |
| Length of stay (days) | 19 | 3 | 14 | 10 | 5 | 7 | 10 | 3 | 7 | 3 |
AV, atrioventricular. |
||||||||||
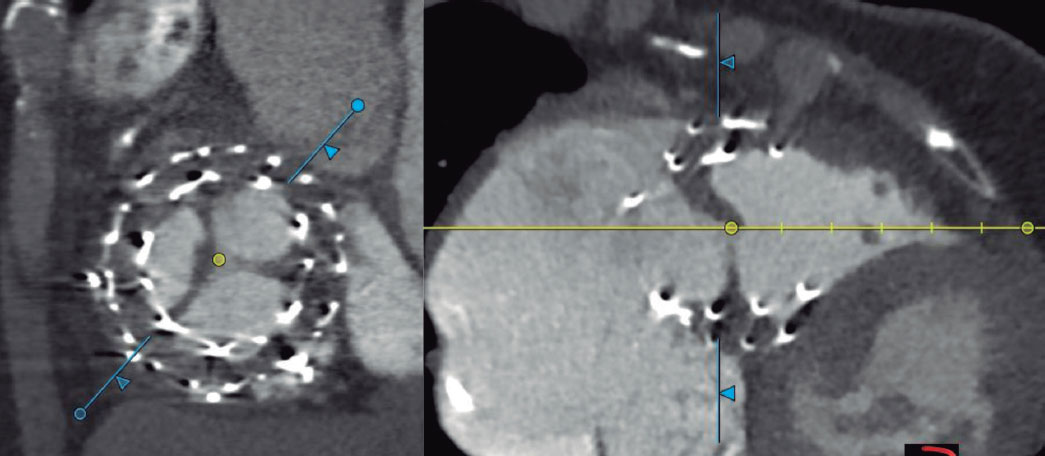
Figure 3. Anterior leaflet thrombosis of the EVOQUE valve (Edwards Lifesciences, United States).
DISCUSSION
The initial experience in Spain with the transcatheter implantation of the EVOQUE tricuspid valve shows excellent results in terms of TR reduction, well beyond the transcatheter treatments available so far for tricuspid valve repair. However, it is important to compare these data with those of the TRISCEND12 and TRISCEND II13 clinical trials. The former12 analyzed 176 patients with, at least, moderate TR treated with the EVOQUE valve. Significant and sustained TR reduction, increased cardiac output, and improved survival were reported, with low readmission rates and clinical and quality of life improvement. More recently, the results of the TRISCEND II trial13,14 have been published on 392 patients randomized to receive the EVOQUE valve plus optimal medical therapy or optimal medical therapy alone. TR reduction was notable in all cases. In our series, the clinical outcomes of all patients were mild or less residual TR following implantation, without significant paravalvular leaks. Similarly, the TRISCEND II study13 also showed a significant and sustained TR reduction, indicating that the EVOQUE valve is effective. Regarding valve-induced atrioventricular conduction disturbances, in our series, 1 patient (10%) with pre-existing right bundle branch block developed complete AVB requiring the implantation of a single-chamber pacemaker with coronary sinus pacing. In the TRISCEND II study,13 17.4% of patients from the intervention group also required permanent pacing after implantation. This finding highlights the need for prolonged monitoring, as clear predictors of AVB development have not been identified. One patient from our series had severe mitral regurgitation after implantation, which resolved with levosimendan. This type of complication was not specifically reported in the TRISCEND II, suggesting that it may be a rare finding. Mitral regurgitation is a dynamic condition which depends on each patient’s hemodynamic status. The patient who presented it had moderate mitral regurgitation prior to the procedure. Patients with severe mitral regurgitation were excluded from the TRISCEND study.13
Comprehensive assessment of right ventricular function prior to implantation is essential. In our series, 2 patients (20%) presented transient right ventricular failure, which resolved with optimal medical therapy. Acute right ventricular failure is not specifically mentioned in the TRISCEND II trial,13 which excluded patients with pre-existing severe right ventricular failure and severe pulmonary hypertension.
Another relevant aspect is antithrombotic treatment after valve implantation. In our series, 1 patient presented EVOQUE valve thrombosis on the control CT performed 1 month after the procedure; no thrombus was visualized on the pre-discharge echocardiography, and the transprosthetic gradient was normal (2 mmHg). This patient was discharged on rivaroxaban 20 mg, which she was already on. The other patient with prosthetic valve thrombosis was on acenocoumarol due to a mechanical heart valve, with an INR (International Normalized Ratio) between 2.5 and 3.5. In the 2 cases, anticoagulation therapy with low molecular weight heparin was prescribed. In the first case, thrombosis persisted on the transesophageal echocardiography performed 1 week later; the second case is still pending follow-up echocardiography. Of note, the TRISCEND II study13 recommended anticoagulation with warfarin (INR, 2.5-3.5) or a different anticoagulant, and antiplatelet therapy with acetylsalicylic acid for 6 months. In our center, the protocol requires maintaining only the patient’s pre-existing anticoagulation (all of whom had an indication for chronic anticoagulation), without antiplatelet therapy. The only patient without baseline anticoagulation received acetylsalicylic acid. On the other hand, in our center, the 2 cases of prosthetic valve thrombosis were diagnosed by CT, which was not performed following the TRISCEND II study protocol criteria13, meaning subclinical thrombosis could not be ruled out. Whether this has any relevance at the follow-up in terms of early valve degeneration is something that will have to be studied in the future. It is considered necessary to perform a CT in these patients undergoing novel transcatheter treatments to anatomically assess the results more accurately and rule out any complications that may go unnoticed with other imaging modalities.
The trade-off of antithrombotic treatment is an increased risk of bleeding. The TRISCEND II study13 reported a rate of major bleeding of 15.4% in the intervention group; in our series, it was 10%, as only 1 patient exhibited overt hematuria requiring transfusion, who was on acenocoumarol without antiplatelet therapy. Although the rate of major bleeding was slightly lower in our series than in the TRISCEND II study,13 we did have 1 case of prosthetic valve thrombosis. Therefore, more evidence is needed to make solid recommendations on the antithrombotic regimen that optimizes the risk/benefit ratio.
Hemodynamic result after valve implantation was optimal in terms of TR reduction. In our series, the maximum transprosthetic gradient was 4.8 mmHg, which is consistent with the low values reported in the TRISCEND II,13 indicating adequate hemodynamic valvular function in the 2 cohorts, which supports the ability of the EVOQUE valve to provide efficient valvular performance with low flow resistance. Of note, in the case of prosthetic valve thrombosis diagnosed by CT, the transtricuspid gradient by echocardiography was 2 mmHg, which highlights the utility of CT in these patients’ initial follow-up, as subclinical thrombosis could otherwise go unnoticed.
The TRISCEND II trial13 observed a reduction in hospital readmission rates and an improvement in clinical parameters. Although quality of life was not directly measured in our series, the significant reduction in TR suggests a relevant clinical benefit that could be reflected in future follow-ups.
CONCLUSIONS
EVOQUE valve implantation is effective in reducing TR and offers an acceptable safety profile in patients with severe TR. Careful patient selection and close monitoring are essential, particularly in those with pre-existing cardiac rhythm disturbances or compromised right ventricular function. Further real-world studies are needed to confirm the long-term safety and efficacy profile of this valve.
FUNDING
None declared.
ETHICAL CONSIDERATIONS
This work has been approved by >Hospital Ramón y Cajal Ethics Committee. Informed consent was obtained from all the patients. Possible sex and gender variables have been taken into consideration in full compliance with the SAGER guidelines.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
Artificial intelligence was not used.
AUTHORS’ CONTRIBUTIONS
A. Sánchez-Recalde conceived and designed this work and supervised the drafting of the manuscript. A. Pardo Sanz was responsible for manuscript drafting, data management, and image design. A. González and A. García were involved in the study of patients and clinical outcomes, and manuscript revision. L.M. Domínguez and J. Alfredo Salinas assisted with image and table design. L. Salido collaborated drafting and revising the manuscript. C. Fernández-Golfín and J.L. Zamorano were responsible for supervising the entire process and final version of the article.
CONFLICTS OF INTEREST
None declared.
WHAT IS KNOWN ABOUT THE TOPIC?
- Significant tricuspid regurgitation (TR) is associated with high morbidity and mortality.
- Medical or surgical treatment options are not optimal, and TR surgery is high-risk, which has driven the development of transcatheter treatments.
- The Edwards EVOQUE valve system has demonstrated, in studies such as the TRISCEND and TRISCEND II, a significant and sustained reduction in TR, improving the patients’ cardiac function and quality of life.
- The most frequently reported complications are right ventricular failure, major bleeding, and the need for pacemaker implantation
- The hemodynamic function of the EVOQUE valve has been shown to be adequate, with low transprosthetic gradients and minimal paravalvular leaks.
WHAT DOES THIS STUDY ADD?
- The clinical experience, at our center, with transcatheter implantation of the EVOQUE valve in 10 patients with, at least, severe symptomatic TR is presented, providing relevant real-world data on its safety and efficacy profile.
- A uniform reduction of TR to mild or less in all patients is demonstrated, with an adequate transprosthetic gradient and no significant paravalvular leaks.
- Notable complications include transient right ventricular failure, development of complete atrioventricular block, bleeding, and the possibility of prosthetic valve thrombosis.
- Adjusting the antithrombotic regimen will be necessary after future studies with longer follow-up.
- The utility of computed tomography to rule out thrombosis and other early complications is highlighted.
- The need to continue accumulating real-world clinical practice data to confirm the safety and impact of the EVOQUE valve in different populations and contexts is emphasized.
REFERENCES
1. Nishiura N, Kitai T, Okada T, et al. Long-Term Clinical Outcomes in Patients With Severe Tricuspid Regurgitation. J Am Heart Assoc. 2023;12:025751.
2. Messika-Zeitoun D, Chan V, Labinaz M, Burwash IG, Dreyfus J. Intervention for Tricuspid Valve Regurgitation:Timing Is Key, and Earlier Is Better Than Later. Can J Cardiol. 2024;40:182-184.
3. Scotti A, Sturla M, Granada JF, et al. Outcomes of isolated tricuspid valve replacement:a systematic review and meta-analysis of 5,316 patients from 35 studies. EuroIntervention. 2022;18:840-851.
4. Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease:Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J.2022;43:561-632.
5. Nickenig G, Kowalski M, Hausleiter J, et al. Transcatheter Treatment of Severe Tricuspid Regurgitation With the Edge-to-Edge MitraClip Technique. Circulation. 2017;135:1802-1814.
6. Pardo Sanz A, Gómez JLZ, Tahoces LS, et al. Long-term outcomes of percutaneous tricuspid annuloplasty with Cardioband device. Eur Heart J Cardiovasc Imaging. 2022;23:979-988.
7. Hahn RT, Zamorano JL. The need for a new tricuspid regurgitation grading scheme. Eur Heart J Cardiovasc Imaging.2017;18:1342-1343.
8. Li C-HP, Asmarats L, Santaló-Corcoy M. Initial experience on percutaneous tricuspid valve replacement using the EVOQUE prosthesis. Rev Esp Cardiol. 2025;78:74-75.
9. Hahn RT, Makkar R, Makar M, et al. EVOQUE Tricuspid Valve Replacement System:State-of-the-Art Screening and Intraprocedural Guidance. JACC Cardiovasc Interv. 2024;17:2093-2112.
10. González-Gómez A, Fernández-Golfín C, Hinojar R, et al. The 4A classification for patients with tricuspid regurgitation. Rev Esp Cardiol. 2023;76:845-851.
11. Hahn RT, Lawlor MK, Davidson CJ, et al. Tricuspid Valve Academic Research Consortium Definitions for Tricuspid Regurgitation and Trial Endpoints. Ann Thorac Surg. 2023;116:908-932.
12. Kodali S, Hahn RT, Makkar R, et al. Transfemoral tricuspid valve replacement and one-year outcomes:the TRISCEND study. Eur Heart J. 2023;44:4862-4873.
13. Hahn RT, Makkar R, Thourani VH, et al. Transcatheter Valve Replacement in Severe Tricuspid Regurgitation. N Engl J Med. 2025;392:115-126.
14. Grayburn PA, Kodali SK, Hahn RT, et al. TRISCEND II:Novel Randomized Trial Design for Transcatheter Tricuspid Valve Replacement. Am J Cardiol. 2024;225:171-177.










