To the Editor,
Orbital atherectomy (OA) is a technique designed to treat non-dilatable calcified lesions in coronary arteries using the Diamondback 360 device (Abbott, United States), which consists of a 1.25 mm diamond-coated crown that enables bidirectional lesion treatment through a dual mechanism by combining centrifugal force (creating elliptical orbits) and surface abrasion to modify the calcified plaque.1
Intracoronary imaging is recommended if severe calcified lesions are suspected; several characteristics guide the use of plaque modification techniques. In summary, the “rule of 5” can be adopted: lesions in which calcium occupies > 50% of the circumference, extends > 5 mm longitudinally, and has > 0.5 mm thickness require advanced calcium modification techniques. A wide range of techniques currently exist for treating calcified lesions, including atherectomy systems such as rotational atherectomy (RA) and OA, specialized balloons (intravascular lithotripsy, cutting balloons, scoring balloons, high-pressure balloons, etc.), as well as excimer laser coronary atherectomy (ELCA). Each has advantages and disadvantages. In cases of deep calcium layers, OA or intravascular lithotripsy (IVL) balloons may be preferred, while for superficial calcium, RA and OA are generally used.2
The recently published ROLLER-COASTR study compared RA, IVL, and ELCA in calcified coronary disease based on stent expansion assessed by optical coherence tomography (OCT). In this study, IVL was non-inferior to RA in terms of stent expansion. ELCA, on the other hand, did not reach this threshold (RA, 86.4% ± 14.1%; IVL, 85.6% ± 13.3%; ELCA, 80.3% ± 13.3%). The minimum stent area, procedural success rate, and complication rates were comparable across all 3 groups.3
“Uncrossable” lesions are those in which a low-profile balloon (≤ 1 mm) or microcatheter cannot be advanced, thus precluding the advancement of intravascular ultrasound or OCT catheters due to poor crossing profile. Currently, there is no established recommendation for using OA in uncrossable lesions.
Among potential strategies, ELCA has been recommended for these types of lesions due to its ability to modify lesion tissue, which subsequently allows advancement of angioplasty equipment. However, severe calcification has been associated with ELCA failure.4
When comparing atherectomy techniques, studies using OCT to assess safety and efficacy profile have shown differences between RA and OA. Thus, patients treated with RA have shown a larger treated area (1.34 mm2 vs 0.83 mm2; P = .004) and greater stent expansion (99.5% vs 90.6%), with no differences in clinical events at the 8-month follow-up.5
One case report describes a successful angioplasty for a chronic total occlusion in the right coronary artery using OA. This was achieved after other techniques, specifically ELCA and RA had failed.6
We present our 4-year experience with uncrossable lesions at the Hemodynamics Department of Hospital Universitario Lucus Augusti (Lugo, Spain). During this time, a total of 114 OAs were performed on patients with severely calcified lesions, 6 of which were uncrossable (5.3%). The OA clinical database was approved by the Galician Health Service ethics committee. The processing of informed consent was deemed unnecessary. The clinical and technical characteristics of the lesions are shown in table 1.
Table 1. Clinical and technical characteristics of orbital atherectomy cases in uncrossable lesions
| Case | Sex | Age (years) | Clinical context | Coronary artery | Primary ViperWire passagea | Device that failed to cross | Number of cycles | High speed use (120 000 rpm) | Prior atherectomy technique | Devices used after orbital atherectomy | Number of stents | Complications | Other |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 74 | NSTEACS | LAD | Yes | 0.85 mm SC balloon | 4 | No | No | NC balloon | 2 | None | – |
| 2 | Male | 68 | CCS | RCA | No | 1.9-Fr microcatheter | 8 | Yes | No | Cutting balloon NC balloon | 3 | Distal dissection with preserved blood flow after balloon angioplasty | ISR (stents implanted > 1 year) |
| 3 | Female | 82 | STEACS | LMCA-LAD | Yes | 1 mm SC balloon | 5 | No | No | NC balloon | 2 | None | Cardiogenic shock with IABP placement |
| 4 | Male | 73 | NSTEACS | RCA | Yes | 1 mm SC balloon | 3 | Yes | No | Cutting balloon, NC balloon | 1 | None | – |
| 5 | Male | 78 | NSTEACS | RCA | Yes | 1 mm SC balloon | 10 | Yes | Yes (ELCA)b | Cutting balloon NC balloon | 2 | None | – |
| 6 | Male | 57 | CCS | RCA | No | 1.9-Fr microcatheter | 7 | Yes | No | NC balloon | 3 | Slow flow resolved after the administration of intracoronary epinephrine | Guidewire tip perforation without pericardial effusion, later resolved |
|
CCS, chronic coronary syndrome; ELCA, excimer laser coronary atherectomy; IABP, intra-aortic balloon pump; ISR, in-stent restenosis; LAD, left anterior descending coronary artery; LMCA-LAD, left main coronary artery-left anterior descending coronary artery; NC, non-compliant; NSTEACS, non-ST-segment elevation acute coronary syndrome; RCA, right coronary artery; SC, semi-compliant; STEACS, ST-segment elevation acute coronary syndrome. aViperWire (Abbott, United States) is the specific guidewire for the orbital atherectomy (OA) device. In cases where the ViperWire did not cross initially, a microcatheter mounted over an intracoronary guidewire was advanced towards the uncrossable area. Since it could not be advanced further, it was later exchanged, and the OA guidewire eventually crossed from that point. bThe bias introduced by the use of ELCA should be considered (7 pulses in total —starting with 30 mJ and 25 Hz— and 5 pulses at a maximum energy peak of 80 > and 80 Hz); although ELCA failed to cross the lesion, it modified the plaque tissue favoring the subsequent success of OA. |
|||||||||||||
Regarding OA execution, crown movement should be slow, with subtle 1 mm-to-3 mm advancements unlike the “pecking” technique used with the olive-shaped burr when performing RA. Moreover, crown advancement should always be controlled, avoiding forceful pushing that could cause verticalization when encountering an uncrossable lesion, potentially leading to uncontrolled orbit. This situation, along with maintaining the crown in the same position for long periods of time can result in complications, such as dissection, perforation, etc. Such complex uncrossable lesions should be treated in cath labs with extensive OA experience and the capacity to switch to other techniques, such as RA, ELCA, etc. when needed.
In all our cases, OA was successful, with the crown crossing the lesion and allowing subsequent angioplasty without major complications. Figure 1 illustrates the angiographic images of each case.
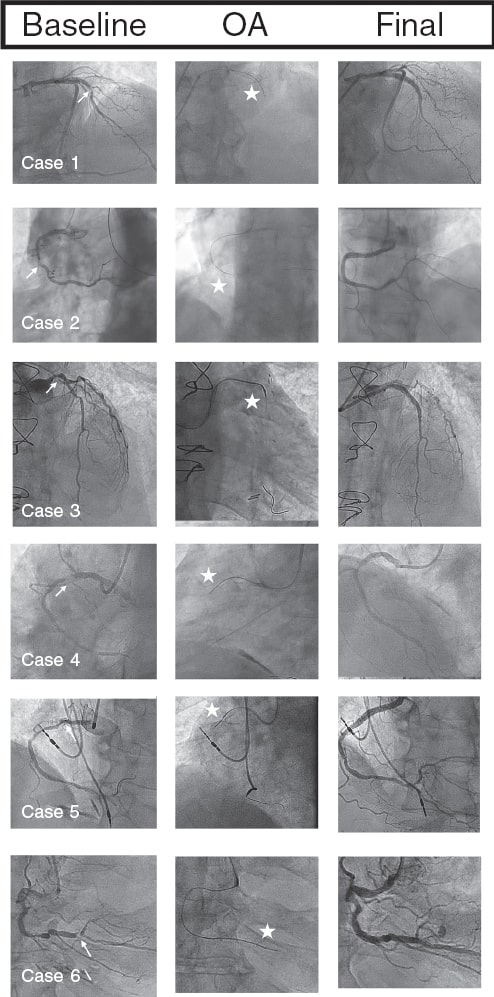
Figure 1. Baseline coronary angiography, orbital atherectomy (OA), and final coronary angiography. The arrows point to the uncrossable lesion, and the stars indicate the OA crown.
Even though this is a small case series, our findings suggest that OA could be a valuable technique for treating uncrossable coronary lesions, especially when performed by an experienced heart team. However, larger prospective studies are still needed to confirm this initial hypothesis.
FUNDING
None declared.
ETHICAL CONSIDERATIONS
The OA clinical database was approved by Hospital Universitario Lucus Augusti Ethics Committee of the Galician Health Service. The processing of informed consent was deemed unnecessary due to the design of the study. Sex and gender variables were considered in accordance with SAGER guidelines.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
No artificial intelligence was used in the preparation of this article.
AUTHORS’ CONTRIBUTIONS
J. Bayón prepared the text and figures. R.A. Mori-Junco reviewed the text. M. Juskova and M. Abellás-Sequeiros participated in the literature search. C. González-Juanatey conducted the final review of the article. All authors gave final approval.
CONFLICTS OF INTEREST
J. Bayón is a proctor for orbital atherectomy with the Abbott Diamondback 360. The remaining authors declared no conflicts of interest whatsoever.
REFERENCES
1. Shlofmitz E, Shlofmitz R, Lee MS. Orbital Atherectomy:A Comprehensive Review. Interv Cardiol Clin. 2019;8:161-171.
2. Jurado-Román A, Gomez-Menchero A, Gonzalo N, et al. Plaque modification techniques to treat calcified coronary lesions. Position paper from the ACI-SEC. REC Interv Cardiol. 2023;5:46-61.
3. Jurado-Román A, Gómez-Menchero A, Rivero-Santana B, et al. Rotational Atherectomy, Lithotripsy, or Laser for Calcified Coronary Stenosis:The ROLLER COASTR-EPIC22 Trial. J Am Coll Cardiol Intv. 2025;18:606-618.
4. Ojeda S, Azzalini L, Suárez de Lezo J, et al. Excimer laser coronary atherectomy for uncrossable coronary lesions. A multicenter registry. Catheter Cardiovasc Interv. 2021;98:1241-1249.
5. Okamoto N, Egami Y, Nohara H, et al. Direct Comparison of Rotacional vs Orbitacional Atherectomy for Calcified Lesions Guided by Optical Coherence Tomography. JACC Cardiovasc Interv. 2023;16:2125-2136.
6. Helal A, Ehtisham J, Shaukat N. Overcoming Uncrossable Calcified RCA Using Orbital Atherectomy After Failure of Rotational Atherectomy. Catheter Cardiovasc Interv. 2025;105:1265-1268.










