Original article
Optical coherence tomography for the diagnosis and management of stent thrombosis
Tomografía de coherencia óptica en el diagnóstico y el tratamiento de la trombosis del stent
aServicio de Cardiología, Hospital Universitario Central de Asturias, Oviedo, Asturias, Spain
bServicio de Cardiología, Hospital Universitario de La Princesa, Universidad Autónoma de Madrid, IIS-IP, Madrid, Spain cCentro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), Instituto de Salud Carlos III, Madrid, Spain dServicio de Cardiología, Hospital Universitario de Cabueñes, Gijón, Spain
ABSTRACT
Introduction and objectives: Excimer laser coronary atherectomy (ELCA) is increasingly used in complex percutaneous coronary interventions (PCI), particularly in cases of “balloon failure,” which includes both uncrossable and undilatable coronary artery lesions. Although these 2 scenarios represent distinct technical and clinical challenges, they are usually evaluated using the same safety and efficacy endpoints. As a result, there is a lack of specific evidence on the safety and efficacy profile of ELCA in each of these situations. Furthermore, the role of intracoronary imaging in optimizing ELCA use remains insufficiently defined.
Methods: This will be an investigator-initiated, multicenter, single-arm, open-label, prospective observational study. Patients with an indication for PCI and undilatable (non-compliant balloon dilatation < 80% at burst pressure) or uncrossable (uncrossable with a “small-profile balloon” with adequate support, left to the operator’s discretion) coronary artery lesions treated with ELCA will be included. Intravascular imaging will be highly advised and analyzed in a core laboratory. Device success, angiographical success, procedural success, clinical success and related complications will be evaluated. Patients will be postoperatively followed for 1 year and clinical events will be recorded.
Conclusions: The LUDICO study will be a multicentre, prospective study of ELCA therapy in uncrossable or undilatable coronary lesions. The study aims to evaluate the safety and efficacy profile of ELCA in these lesions as well as the clinical results at the 1 year follow-up in this setting. (ClinicalTrials.gov: NCT07206082).
Keywords: Percutaneous coronary intervention. Excimer laser coronary atherectomy. Intravascular imaging. Optical coherence tomography. Complex coronary intervention.
RESUMEN
Introducción y objetivos: La aterectomía coronaria con láser de excímeros (ELCA) se utiliza cada vez más en intervenciones coronarias percutáneas (ICP) complejas, en particular en caso de «fallo del balón», que incluye tanto lesiones coronarias no cruzables como no dilatables. Aunque estos 2 escenarios representan desafíos técnicos y clínicos distintos, con frecuencia se han evaluado utilizando los mismos criterios de efectividad y seguridad. Como resultado, existe una falta de evidencia específica sobre la seguridad y la efectividad de la ELCA en cada una de estas situaciones. Además, el papel de la imagen intracoronaria en la optimización del uso de la ELCA sigue estando insuficientemente descrito.
Métodos: Se trata de un estudio observacional prospectivo, abierto, multicéntrico e iniciado por los investigadores. Se incluirán pacientes con indicación de ICP y lesiones coronarias no dilatables (dilatación con balón no distensible < 80% a presión de ruptura) o no cruzables (no cruzables con un balón de bajo perfil y adecuado soporte, a criterio del operador) tratados con ELCA. Se recomendará el uso de imagen intravascular, que se analizará en un laboratorio central. Se evaluarán el éxito del dispositivo, el éxito angiográfico, el éxito del procedimiento, el éxito clínico y las complicaciones asociadas. Se seguirá a los pacientes durante 1 año tras el procedimiento y se registrarán los eventos clínicos.
Conclusiones: El estudio LUDICO será un estudio prospectivo y multicéntrico sobre el uso de ELCA en lesiones coronarias no cruzables o no dilatables. Su objetivo es evaluar la efectividad y la seguridad de la ELCA en estas situaciones, así como los resultados clínicos durante un seguimiento de 1 año. (ClinicalTrials.gov: NCT07206082).
Palabras clave: Intervención coronaria percutánea. Aterectomía coronaria con láser de excímeros. Imagen intravascular. Tomografía de coherencia óptica. Intervención coronaria compleja.
Abbreviations
ELCA: excimer laser coronary angioplasty. IVUS: intravascular ultrasound. OCT: optical coherence tomography. PCI: percutaneous coronary intervention. RA: rotational atherectomy.
INTRODUCTION
Excimer laser coronary atherectomy (ELCA) has been applied since the 1980s in multiple anatomical and clinical settings, with several studies supporting its safety and efficacy profile.1,2 Common indications include in-stent restenoses, stent underexpansion, calcified coronary lesions, saphenous vein graft stenoses, thrombotic lesions, bifurcations, and chronic total coronary occlusions.3-14 In practice, however, ELCA is predominantly used in the setting of balloon failure–specifically uncrossable and undilatable coronary artery lesions. However, historical studies have typically applied a uniform definition of device success across both lesion types, potentially overlooking important nuances that could influence outcomes and therapeutic decision-making.
Furthermore, despite growing recognition of the value of intracoronary imaging in optimizing complex percutaneous coronary intervention (PCI),15 prior ELCA studies have largely underutilized this tool, limiting insight into the mechanisms of success or failure in balloon-resistant lesions.
The safety and efficacy profile of coronary laser in undilatable and uncrossable lesions (LUDICO) study is a real-world, observational study designed to evaluate the use of ELCA specifically in cases of balloon failure. The study has 2 primary objectives: a) to refine the definition of ELCA procedural success based on the type of balloon failure encountered—distinguishing between uncrossable and undilatable lesions—, and b) to emphasize the critical role of intracoronary imaging in guiding ELCA and interpreting procedural outcomes. By addressing these critical gaps, the study aims to provide a more precise and and clinically meaningful framework for the contemporary use of ELCA in complex coronary interventions.
METHODS
Study design and population
This is a prospective, multicentre, observational study including consecutive patients undergoing ELCA in undilatable (expansion < 80% of the distal vessel diameter after inflation of a 1:1 non-compliant balloon at 18 atm) and uncrossable coronary artery lesions (uncrossable after using a small-profile balloon with adequate support left to the operator’s discretion). At least 15 national centers will be contacted to participate in the study. Participant centers will be required to have experience with ELCA and complex PCI, with a minimum of > 5 prior ELCA cases performed. Inclusion and exclusion criteria are described in table 1. This study was conducted in full compliance with the STROBE guidelines for observational studies.16 The study protocol was registered in ClinicalTrials.gov (NCT07206082).
Table 1. Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| Patients > 18 | Patients with known allergies to ASA, clopidogrel, prasugrel, or ticagrelor |
| Patients with either stable coronary artery disease or acute coronary syndromes as the clinical presentation | Patients unable to provide informed consent, either personally or through a legal representative |
| Patients with severe coronary lesions (> 70% by visual estimation) in native vessels or coronary bypass grafts | Patients with clinical or hemodynamic instability defined as: sustained hypotension (SBP ≤ 90 mmHg for ≥ 30 minutes or use of pharmacological, or mechanical support to maintain an SBP ≥ 90 mmHg) or evidence of end‐organ hypoperfusion including urine output of < 30 mL/h, cool extremities, altered mental status, or serum lactate > 2.0 mmol/L |
| “Uncrossable” coronary lesions (eg, lesions that cannot be crossed with a 0.7:1 balloon after successful guidewire passage) or “Undilatable” lesions (eg, those in which balloon dilation with a 1:1 non-compliant balloon at 18 atm results in < 80% expansion relative to the distal reference vessel diameter; this group includes both de novo lesions and in-stent restenosis or underexpanded stents) |
Patients with significant comorbidities and a life expectancy of < 1 year |
ASA, acetylsalicylic acid; SBP, systolic blood pressure. |
Procedure
PCI will be performed in accordance with current clinical practice guidelines on coronary revascularization.15,17
In uncrossable lesions, following successful guidewire passage and failed balloon crossing, ELCA will be performed (as described in the following section). PCI will be completed with optional predilatation at the operator’s discretion, followed by stenting or drugcoated balloon implantation. Intravascular imaging [preferably with optical coherence tomography (OCT)] will be recommended after laser application to characterize the lesion substrate and evaluate the effect of the laser and at the end of the procedure.
In undilatable lesions, if balloon dilation is inadequate, an initial intracoronary imaging assessment will be conducted. Afterwards, laser atherectomy will be performed, followed by a second intracoronary imaging assessment to evaluate the effects of ELCA on the lesion. PCI will, then, be completed with balloon dilation and stenting or drug-coated balloon implantation, at the operator’s discretion. A third intracoronary imaging pullback will be performed to assess the final procedural outcome (figure 1).
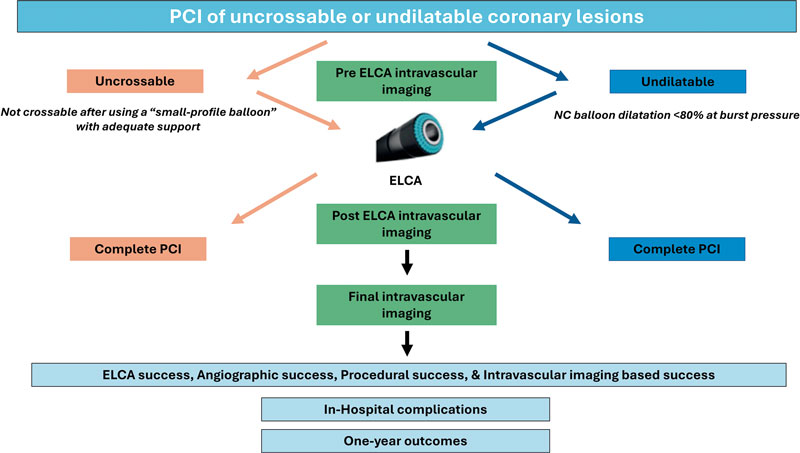
Figure 1. Central illustration. LUDICO study flowchart. ELCA, excimer laser coronary atherectomy; NC, non-compliant; PCI, percutaneous coronary intervention.
Laser atherectomy technique
ELCA procedure will be performed using the Spectranetics CVX300 (Spectranetics, United States) and the latest generation Philips Laser System Excimer (Philips, United States) System, which is based on pulsed xenon‐chlorine laser catheters capable of delivering excimer energy (wavelength, 308 nm; pulse length, 185 ns) from 30 mJ/mm2 to 80 mJ/mm2 (fluencies) at pulse repetition rates of 25 Hz to 80 Hz.
The ELCA technique will be performed according to current recommendations.18 The choice of laser catheter size will be left to the operator’s discretion, selecting among the available rapid-exchange concentric probes (0.9 mm, 1.4 mm, 1.7 mm, or 2.0 mm). The selection of fluence, and repetition rate will be left to the operator’s discretion. A saline infusion technique will be recommended, although application of laser with blood or contrast will be recommended in resistant lesions. In the event of unsuccessful initial therapy, additional plaque modification techniques may be employed at the operator’s discretion and will be thoroughly recorded and described.
Clinical definitions and follow-up
Laser success will be defined differently for uncrossable and for undilatable lesions. For the former, laser success will be defined as the ability of the laser catheter to cross the lesion. Laser success will also be considered in cases where the laser catheter cannot cross the lesion but proximal laser application permits subsequent balloon crossing. For the latter, laser success will be defined as successful balloon dilation (sized 1:1 to the vessel diameter), with adequate expansion (> 80% in 2 orthogonal projections) following laser therapy without the need for other plaque modification technique.
Angiographic success will be defined as Thrombolysis in Myocardial Infarction (TIMI) grade-3 final flow and a percent diameter stenosis < 20%. Procedural success will be defined as angiographic success without severe procedural complications (death, coronary perforation, abrupt vessel closure, flow-limiting dissection). Intracoronary imaging-based success will be defined as a stent expansion ≥ 80% (OCT or intravascular ultrasound [IVUS]) or a minimal stent area (MSA) ≥ 4.5 mm2 in OCT or ≥ 5.5 mm2 in IVUS.
Intracoronary imaging
Intracoronary imaging will aim to describe the lesion characteristics and identify potential predictors of adequate stent expansion and procedural result. Therefore, intracoronary imaging will be highly recommended and the advised imaging modality will be OCT as its better spatial resolution vs IVUS allows better tissue characterization, plaque modification assessment and visualization of stent failure etiologies.19 A baseline intracoronary imaging evaluation is recommended, when possible, to describe the lesion characteristics and identify potential predictors of ELCA success or failure. Additionally, a second intracoronary imaging run is strongly advised immediately after laser therapy. This second run aims to describe the effect of ELCA in the coronary plaque. Evaluating and characterizing changes in the coronary plaque might help guide the optimal ELCA result and allow appropriate adjustment of therapy settings (fluence, repetition rate and infusion characteristics). Finally, a postoperative intravascular imaging run is strongly recommended once the final angiographic result is achieved. All intracoronary imaging data will be analyzed by a core laboratory. In the baseline intracoronary imaging run, lesion characteristics will be described as follows: minimum lumen area (MLA), minimum and maximum lumen diameter, lesion length, calcification angle, calcification thickness. In the post-ELCA imaging run the following parameters will be evaluated: MLA, number of calcium fractures and characteristics, presence of dissection, including its angle and length. In the final imaging run, MSA, stent apposition and dissections will be described. In both OCT and IVUS assessments, a dual-reference approach will be used: the proximal and distal reference lumen diameters will be identified, and MSA will be divided by each of these diameters separately. The final stent expansion index will be calculated as the mean of the 2 resulting values. Second, the tapered mode is only available in OCT: reference lumen profile is estimated based on the distal and proximal reference frame mean diameter and side branch mean diameter in between. With stent lengths > 50 mm, the dual method is preferred. With stent lengths < 50 mm the tapered method is often used. If the dual method is used, the stent expansion percentage of both segments will be recorded with the lower value of the two measurements used for analysis. The main variables to be evaluated by intravascular imaging are summarized and graphically shown in figure 2.
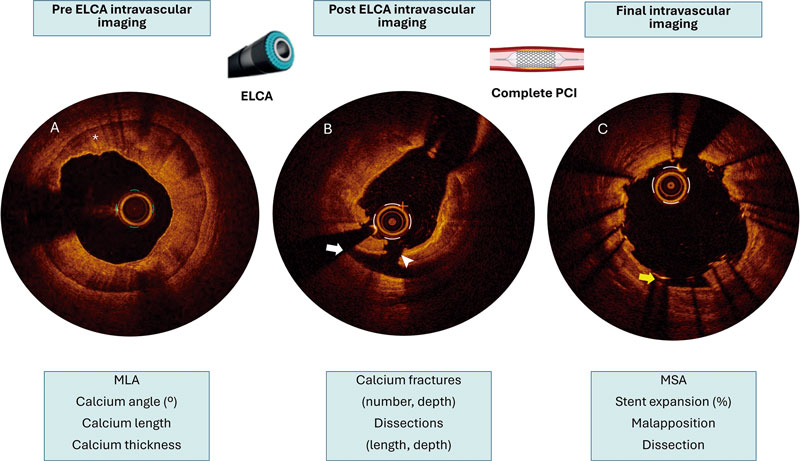
Figure 2. Example of the advised intracoronary imaging assessment in LUDICO study. A: baseline optical coherence tomography (OCT) image of a severely calcified lesion. The asterisk points to a calcium arc of 360° with a maximum thickness of 0.9 mm. B: OCT image after ELCA with contrast media. White arrow points to a dissection. The white arrowhead points to a deep calcium fracture. C: results after stenting. The yellow arrow points to a small area of malapposition. ELCA, excimer laser coronary atherectomy; MLA, minimal lumen area; MSA, minimal stent area; PCI, percutaneous coronary intervention.
Follow-up
Follow-up will be conducted at 3 different timeframes:a) after PCI; procedural success and complications will be thoroughly documented, and all patients will be evaluated for any postoperative events, such as chest pain, heart failure, bleeding, or ischemic events; b) at hospital discharge, documenting clinical status, complications and antiplatelet therapy; and c) 1 year after the index PCI; clinical events and antiplatelet therapy will be recorded.
The primary endpoint at the follow-up will be the composite endpoint of major adverse cardiovascular events, defined as the occurrence of cardiac death, target vessel-related acute myocardial infarction, target vessel revascularization, or definite/probable stent thrombosis. Secondary efficacy endpoints will include all-cause mortality, cardiac death, non-fatal myocardial infarction, target lesion revascularization, and target vessel revascularization. Secondary safety endpoints will include stroke and bleeding events (classified according to the Bleeding Academic Research Consortium [BARC] criteria). Endpoint definitions are shown in table 2.
Table 2. Procedural and clinical definitions
| Procedural definitions | |
|---|---|
| ELCA success | Uncrossable: defined as the ability of the laser catheter to cross the lesion or allow subsequent crossing with a predilatation balloon following laser application |
| Undilatable: defined as successful balloon dilation with adequate expansion following laser therapy | |
| Angiographic success | Defined adequate stent implantation and expansion, with residual stenosis < 20% and TIMI grade-3 flow, without crossover to another plaque modification technique |
| Procedural success | Angiographic success without severe procedural complications (death, coronary perforation, abrupt vessel closure, flow-limiting dissection) |
| Imaging based success | Defined as a stent expansion ≥ 80% (OCT or IVUS) or a MSA ≥ 4.5 mm2 in OCT or ≥ 5.5 mm2 in IVUS |
| Severely calcified coronary lesion | Angiographically: opacification in both sides of the artery before contrast administration |
| Intracoronary imaging: > 180° calcium arc or calcium thickness > 5 mm | |
| Clinical definitions | |
| MACE | Defined as the occurrence of cardiac death, target vessel-related acute MI, target vessel revascularization, or definite/probable stent thrombosis |
| Cardiac death | According to ARC definitions:31
|
| Non-fatal MI | Third universal definition of MI.32 In addition, procedure-related myocardial infarction—defined as a troponin elevation > 5 times the upper limit of normal in patients with previously normal troponin levels, or a ≥ 20% increase in patients with previously elevated troponin levels, along with electrocardiographic changes or new areas of myocardial necrosis detected by imaging—was included |
| Stent thrombosis | According to ARC criteria:
|
| Stroke | New neurological focal deficit with imaging confirmation and assessed by a neurologist |
| TLR | New coronary artery lesion in the previously treated coronary lesion including 5 mm proximal and distal to the implanted stent |
| TVR | New coronary artery lesion in the previously treated coronary vessel |
| Hemorrhage | According to BARC classification33 |
|
ARC, Academic Research Consortium; BARC, Bleeding Academic Research Consortium; ECG, electrocardiogram; ELCA, excimer laser coronary atherectomy; IVUS, intravascular ultrasound; MACE, major adverse cardiovascular events; MI, myocardial infarction; MSA, minimal stent area; OCT, optical coherence tomography; STEMI, ST-segment elevation myocardial infarction; TIMI, Thrombolysis in Myocardial Infarction; TLR, target lesion revascularization; TVR, target vessel revascularization. |
|
Sample size estimation
The planned sample size of 230 patients was determined based on expected device success rates reported in prior studies of ELCA for undilatable and uncrossable lesions. Assuming a conservative laser success rate of 80%, a cohort of 230 patients would yield a 95% confidence interval with a precision of approximately ± 5% (estimated range, 74.8%–85.2%), which is considered adequate for reliably estimating procedural efficacy in the routine clinical practice. Moreover, this sample size ensures sufficient statistical power to support multivariable analyses of predictors of both intraoperative and follow-up outcomes. With an anticipated 40–50 events, the study would allow the inclusion of approximately 4 to 5 covariates in multivariable regression models while maintaining acceptable model stability. Based on the expected procedural volume at each participant center and the required sample size, the recruitment period is 2 to 3 years.
Statistical analysis
Quantitative variables following a normal distribution will be expressed as mean ± standard deviation. Those not following a normal distribution will be reported using the median and minimum and maximum values. Qualitative variables will be expressed as absolute numbers and frequencies.
A significance level of 0.5 will be considered, and 95% confidence intervals will be calculated for the primary outcome variables. Normality of the data will be assessed using the Kolmogorov-Smirnov test. Based on the distribution, appropriate statistical tests will be applied to compare relevant variables. For comparisons of means, the Student t test for independent samples will be used, or the non-parametric Mann-Whitney U test in case of dichotomous qualitative variables. For comparisons involving non-dichotomous qualitative variables, ANOVA or the non-parametric Kruskal-Wallis test will be employed. For bivariate analysis of qualitative variables, the chi-square test or Fisher’s exact test will be used.
Multivariate analysis will be conducted using forward stepwise Cox regression analysis. Event-free survival curves will be constructed using the Kaplan-Meier method. Variables will be considered potential risk predictors in the multivariate model if they demonstrate a statistically significant association in the univariate analysis or show a trend toward significance. All statistical analyses will be conducted using Stata 16.1 (StataCorp, United States).
Ethical considerations
This study was conducted in full compliance with the principles outlined in the Declaration of Helsinki and with the International Council for Harmonization (ICH) Good Clinical Practice guidelines, including the most recent ICH E6 (R3) update. Before enrollment, patients or their legal representatives must be fully informed about the nature of the study and must provide written informed consent. The study protocol was approved by the Institutional Review Board at each participant center.
DISCUSSION
The LUDICO study will be a multicenter study to assess the safety, efficacy, and clinical outcomes of ELCA specifically in undilatable or uncrossable coronary artery lesions with lesion-specific endpoints and preferential use of intravascular imaging. We believe that this real-life approach will provide valuable insights into the 2 main clinical scenarios in which ELCA is currently used.
Three recent large registries confirmed ELCA to be a safe technique with an assumable rate of complications.20-22 However, these studies analyzed the overall procedural performance but failed to describe the lesion specific characteristics or intravascular imaging data. The findings of studies reporting balloon failure scenarios5,10-12,23,24 are summarized in figure 3. The LAVA multicenter registry set the main contemporary clinical indications for ELCA.12 This registry analysed ELCA use in 130 lesions and stratified them in 3 scenarios: uncrossable, undilatable and thrombotic. The LAVA and other studies analyzing ELCA has shown good performance of ELCA in balloon-failure, with lower rates of ELCA success in uncrossable vs undilatable lesions. However, one significant limitation is present in these studies: situations of balloonfailure include undilatable, uncrossable, or lesions with both components. In the routine clinical practice, these 2 situations are distinct; however, ELCA success has often been defined uniformly, potentially confounding the real efficacy of the device. Consequently, the LUDICO study aims to address this issue by specifically defining 2 endpoints based on the type of balloon failure, uncrossable or undilatable.
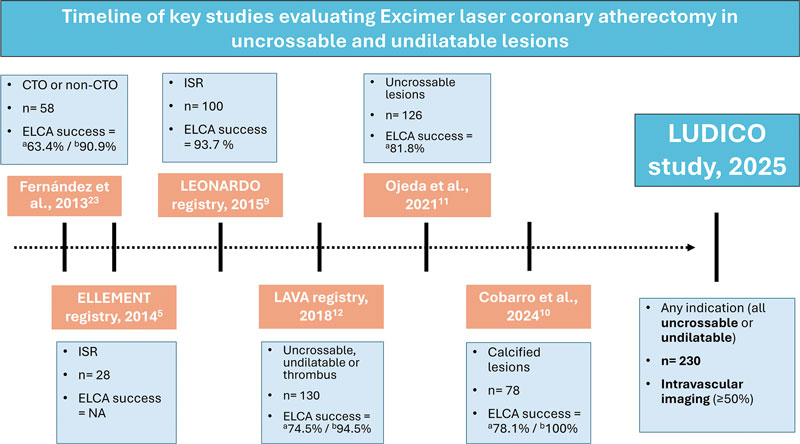
Figure 3. Timeline of key studies evaluating ELCA in uncrossable and undilatable lesions. CTO, chronic total coronary occlusion; ELCA, excimer laser coronary atherectomy; NA, not available; ISR, in-stent restenosis. a Uncrossable lesions. b Undilatable lesions.
Nonetheless, the definition of ELCA success in uncrossable lesions might be ambiguous in some cases. For instance, cases in which neither the ELCA catheter nor subsequent balloons are able to cross the lesion should not be considered procedurals failures if a microcatheter can subsequently cross and enable successful completion of the procedure using the RASER technique—a combination of ELCA and rotational atherectomy (RA). However, to simplify the endpoint, we have considered this situation a crossover to RA. In contrast, for undilatable lesions, the definition of ELCA success is less prone to interpretation; however, clearly defining what constitutes an undilatable lesion remains essential. This highlights the importance of a compliance test —that is, performing an initial balloon dilatation to objectively demonstrate that the lesion cannot be adequately expanded. Such a test is critical to identify lesions that are likely to benefit from plaque modification techniques, including ELCA. Arguably, the results of some randomized controlled trials in plaque modification devices (such as ECLIPSE25 using orbital atherectomy and ROLLERCOASTR7 using ELCA, intravascular lithotripsy and RA) may have been influenced by the absence of “compliance test”, potentially including coronary lesions in which plaque modification would not have been necessary after balloon testing, thereby reducing the differences across groups. Additionally, the recent CRATER trial showed that a total of 20.9% of patients in bailout RA group required crossover to RA because of balloon failure,26 which highlights the high frequency of this situation and underscores the importance of its prompt identification to select the most appropriate plaque modification technique such as ELCA.
RA is the most extensively studied strategy for managing uncrossable coronary lesions, supported by wide clinical experience and robust evidence.7,26,27 However, RA presents important limitations in specific scenarios where ELCA may offer clear advantages —such as in-stent restenosis or bifurcation lesions requiring side branch protection—given the risk of scaffold damage or distal embolization of debris.28 Orbital atherectomy, although less studied in uncrossable lesions,29,30 shares similar drawbacks due to its ablative mechanism. By contrast, ELCA is compatible with 6-Fr catheters, can be used over any standard guidewire, and has a less demanding learning curve.18 Of note, while RA demonstrates limited efficacy against deep calcium, ELCA can affect both superficial and deep calcification.4 Collectively, these features position ELCA as a uniquely valuable tool among plaque-modification techniques. Its capacity to safely treat in-stent restenosis, thrombotic lesions, uncrossable lesions, and bifurcations requiring side branch protection underscores advantages not readily attainable with RA or orbital atherectomy, thereby reinforcing ELCA as a superior alternative in selected complex PCI scenarios.
In conclusion, the use of intravascular imaging has been limited in most of the studies that have evaluated ELCA in balloon-failure, particularly those focused on uncrossable lesions. Additionally, none of these studies have described the findings of intravascular imaging before and after ELCA and identified potential predictors of success. In fact, the effect of ELCA in intravascular imaging remains an open question as there is a paucity of studies that have evaluated it and have been limited to in-stent restenosis.4 Therefore, one of the aims of the LUDICO study is to evaluate the effects of ELCA by intravascular imaging (preferably by OCT, due to its better spatial resolution) and identify potential predictors of ELCA success or failure and its effect on the coronary plaque. We hypothesize that recognizing potential predictors in intravascular imaging could help operators guide the procedures and identify the anatomical characteristics that best predict a favourable outcome with ELCA, thereby optimizing patient selection and procedural planning.
Limitations
First, this multicentre prospective study will be conducted in a single country, which may limit the generalizability of its findings to other settings. However, these high-volume centres, with wide experience in complex PCI comply with the international recommendations and their practice is comparable to other similar centres. Second, because of to the nonblinded study design, selection bias may have occurred, whereby certain lesions, such as extremely calcified or highly complex, were preferentially treated with alternative techniques or revascularization strategies. Additionally, there will not be a control group to assess the efficacy of the ELCA therapy vs other therapies. Finally, although intracoronary imaging will be highly recommended, we foresee that the baseline evaluation will be limited to just a few cases. In fact, by definition, uncrossable lesions will rarely have a baseline evaluation. Besides, in the event of the patient having kidney disease, OCT runs could be avoided, conducting to less OCT runs, or even to the absence of intravascular imaging.
CONCLUSIONS
The LUDICO study will be a multicenter, prospective study of ELCA therapy in uncrossable or undilatable coronary artery lesions with specific success definitions for each indication. The study aims to evaluate the safety and efficacy profile of ELCA and the clinical outcomes during the follow-up. The OCT evaluation will provide insights into the effect of ELCA in this subset of coronary lesions.
FUNDING
The LUDICO study was supported by a non-restricted grant from Biomenco.
ETHICAL CONSIDERATIONS
The study was conducted in full compliance with the principles outlined in the Declaration of Helsinki. Institutional Ethics Committee approval was obtained (institutional approval number: 5502), and all participants gave their written informed consent prior to enrolment. The confidentiality and anonymity of participants were strictly preserved throughout the study. Sex and gender considerations were addressed following the recommendations of the SAGER guidelines to ensure accurate and equitable reporting.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
Artificial intelligence assisted technologies were used exclusively to support language editing and improvement of style. No artificial intelligence tools were employed to generate, analyse, or interpret the data. The authors take full responsibility for the integrity, accuracy, and originality of the manuscript content.
AUTHORS’ CONTRIBUTIONS
A. Jurado-Román and J. Zubiaur contributed to the study equally and share first authorship. A. Jurado-Román is responsible of the study conception and design. J. Zubiaur, A. Jurado-Román, and M. Basile were involved in the draft manuscript preparation. All authors reviewed the results and approved the final version of the manuscript.
CONFLICTS OF INTEREST
R. Moreno is associate editor of REC: Interventional Cardiology; the journal’s editorial procedure to ensure impartial handling of the manuscript has been followed; moreover, he has received consulting fees and honoraria/speaker fees from Abbott vascular, Boston Scientific, Medtronic, Terumo, and Biotronik. A. Jurado-Román reported receiving consulting fees from Boston Scientific and Philips; honoraria/speaker fees from Abbott, Boston Scientific, Shockwave Medical, World Medica, and Philips; and serves as a proctor for Abbott, Boston Scientific, World Medica, and Philips. G. Galeote has received honoraria/speaker fees from Meril, Boston Scientific, Abbott SMT, and Biomenco. A. Gonzálvez-García has received honoraria from Abbott. J. Suárez de Lezo has received honoraria/ speaker fees from Abbott and Philips. F. Hidalgo has received honoraria/speaker fees from Philips. M. Basile reported receiving consulting fees and speaking fees from Iberhospitex. B. Garcia del Blanco disclosed his role as a proctor for Edwards Lifescienses and his participation on the Advisory Board of Iberhospitex. All other authors declared no conflicts of interest whatsoever.
WHAT IS KNOWN ABOUT THE TOPIC?
- ELCA has demonstrated its usefulness across several challenging lesion subsets, including in-stent restenosis, stent underexpansion, calcified plaques, saphenous vein graft disease, thrombotic lesions, bifurcations, and chronic total coronary occlusions.
- However, in real-world practice, its main indication remains balloon failure, particularly in lesions that are either uncrossable or undilatable.
- Despite this, most earlier studies applied a uniform definition of device success for these distinct scenarios, potentially missing clinically relevant nuances that may affect outcomes and guide treatment strategies.
WHAT DOES THIS STUDY ADD?
- The LUDICO study is designed as a multicenter investigation to evaluate the safety, efficacy, and clinical outcomes of ELCA specifically in undilatable or uncrossable coronary artery lesions, incorporating individualized endpoints for each subset and emphasizing the use of intravascular imaging.
- This real-world strategy is expected to yield meaningful insights into the 2 primary clinical situations in which ELCA is currently employed: uncrossable and undilatable coronary artery lesions.
REFERENCES
1. Choy DS. History of lasers in medicine. Thorac Cardiovasc Surg. 1988;36 Suppl 2:114–117.
2. Köster R, Kähler J, Brockhoff C, Münzel T, Meinertz T. Laser coronary angioplasty: history, present and future. Am J Cardiovasc Drugs. 2002;2:197–207.
3. Bilodeau L, Fretz EB, Taeymans Y, Koolen J, Taylor K, Hilton DJ. Novel use of a high-energy excimer laser catheter for calcified and complex coronary artery lesions. Catheter Cardiovasc Interv. 2004;62:155–161.
4. Lee T, Shlofmitz RA, Song L, et al. The effectiveness of excimer laser angioplasty to treat coronary in-stent restenosis with peri-stent calcium as assessed by optical coherence tomography. EuroIntervention. 2019;15:e279–288.
5. Latib A, Takagi K, Chizzola G, et al. Excimer Laser LEsion Modification to Expand Non-dilatable sTents: The ELLEMENT Registry. Cardiovasc Revasc Med. 2014;15:8–12.
6. Dörr M, Vogelgesang D, Hummel A, et al. Excimer laser thrombus elimination for prevention of distal embolization and no-reflow in patients with acute ST elevation myocardial infarction: results from the randomized LaserAMI study. Int J Cardiol. 2007;116:20–26.
7. Jurado-Román A, Gómez MA, Rivero-Santana B, et al. Rotational Atherectomy, Lithotripsy, or Laser for Calcified Coronary Stenosis. JACC: Cardiovasc Interv. 2025;18:606–618.
8. Giugliano GR, Falcone MW, Mego D, et al. A prospective multicenter registry of laser therapy for degenerated saphenous vein graft stenosis: the COronary graft Results following Atherectomy with Laser (CORAL) trial. Cardiovasc Revasc Med. 2012;13:84–89.
9. Ambrosini V, Sorropago G, Laurenzano E, et al. Early outcome of high energy Laser (Excimer) facilitated coronary angioplasty ON hARD and complex calcified and balloOn-resistant coronary lesions: LEONARDO Study. Cardiovasc Revasc Med. 2015;16:141–146.
10. Cobarro L, Jurado-Román A, Tébar-Márquez D, et al. Excimer laser coronary atherectomy in severely calcified lesions: time to bust the myth. REC Interv Cardiol. 2023;6:33–40.
11. Ojeda S, Azzalini L, Suárez de Lezo J, et al. Excimer laser coronary atherectomy for uncrossable coronary lesions. A multicenter registry. Catheter Cardiovasc Interv. 2021;98:1241–1249.
12. Karacsonyi M, Armstrong EJ, Huu Tam D, et al. Contemporary Use of Laser During Percutaneous Coronary Interventions: Insights from the Laser Veterans Affairs (LAVA) Multicenter Registry. J Invasive Cardiol. 2018;30:195–201.
13. Tomasello SD, Rochira C, Mazzapicchi A, et al. Clinical Outcomes of Percutaneous Coronary Intervention Using Excimer Laser Coronary Atherectomy for Complex Coronary Lesions: The ACCELERATE Registry. Catheter Cardiovasc Interv. 2025;106:1630–1638.
14. Basile M, Gómez-Menchero A, Rivero-Santana B, et al. Rotational Atherectomy, Lithotripsy, or Laser for Calcified Coronary Stenosis: One-Year Outcomes From the ROLLER COASTER-EPIC22 Trial. Cath Cardiovasc Interv. 2025;106:702–710.
15. Vrints C, Felicita Andreotti F, Koskinas KC, et al. 2024 ESC Guidelines for the management of chronic coronary syndromes. Eur Heart J. 2024;45:3415–3537.
16. Cuschieri S. The STROBE guidelines. Saudi J Anaesth. 2019;13(Suppl 1):S31–S34.
17. Byrne RA, Rossello X, Coughlan JJ, et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J Acute Cardiovasc Care. 2024;13:55–161.
18. Rawlins J, Din JN, Talwar S, O’Kane P. Coronary Intervention with the Excimer Laser: Review of the Technology and Outcome Data. Interv Cardiol. 2016;11:27–32.
19. Nagaraja V, Kalra A, Puri R. When to use intravascular ultrasound or optical coherence tomography during percutaneous coronary intervention? Cardiovasc Diag Ther. 2020;10:1429444–1421444.
20. Sintek M, Coverstone E, Bach R, et al. Excimer Laser Coronary Angioplasty in Coronary Lesions: Use and Safety From the NCDR/CATH PCI Registry. Circ Cardiovasc Interv. 2021;14:e010061.
21. Protty MB, Gallagher S, Farooq V, et al. Combined use of rotational and excimer lASER coronary atherectomy (RASER) during complex coronary angioplasty—An analysis of cases (2006–2016) from the British Cardiovascular Intervention Society database. Cath Cardiovasc Interv. 2021;97:E911–E918.
22. Hinton J, Tuffs C, Varma R, et al. An analysis of long-term clinical outcome following the use of excimer laser coronary atherectomy in a large UK PCI center. Catheter Cardiovasc Interv. 2024;104:27–33.
23. Fernandez JP, Hobson AR, McKenzie D, et al. Beyond the balloon: excimer coronary laser atherectomy used alone or in combination with rotational atherectomy in the treatment of chronic total occlusions, non-crossable and non-expansible coronary lesions. EuroIntervention. 2013;9:243–250.
24. Ambrosini V, Sorropago G, Laurenzano E, et al. Early outcome of high energy Laser (Excimer) facilitated coronary angioplasty ON hARD and complex calcified and balloOn-resistant coronary lesions: LEONARDO Study. Cardiovasc Revasc Med. 2015;16:141–146.
25. Kirtane AJ, Généreux P, Lewis B, et al. Orbital atherectomy versus balloon angioplasty before drug-eluting stent implantation in severely calcified lesions eligible for both treatment strategies (ECLIPSE): a multicentre, open-label, randomised trial. Lancet. 2025;405:1240–1251.
26. Galeote G, Zubiaur J, Jurado-Román A, et al. Coronary Rotational Atherectomy Elective Versus Bailout in Patients With Severely Calcified Lesions and Chronic Renal Failure (CRATER) Trial. Catheter Cardiovasc Interv. 2025;106:1702–1712.
27. Abdel-Wahab M, Toelg R, Byrne RA, et al. High-Speed Rotational Atherectomy Versus Modified Balloons Prior to Drug-Eluting Stent Implantation in Severely Calcified Coronary Lesions. Circ Cardiovasc Interv. 2018;11:e007415.
28. Rivero-Santana B, Galán C, Pérez-Martínez C, et al. ELLIS Study: Comparative Analysis of Excimer Laser Coronary Angioplasty and Intravascular Lithotripsy on Drug-Eluting Stent as Assessed by Scanning Electron Microscopy. Circ Cardiovasc Interv. 2024;17:e014505.
29. Helal A, Ehtisham J, Shaukat N. Overcoming Uncrossable Calcified RCA Using Orbital Atherectomy After Failure of Rotational Atherectomy. Catheter Cardiovasc Interv. 2025;105:1265–1268.
30. Bayón J, Mori-Junco RA, Jusková M, Abellas-Sequeiros M, González-Juanatey C. Feasibility and safety of orbital atherectomy in uncrossable lesions. REC: Interv Cardiol. 2025;7:269–271.
31. Cutlip DE, Windecker S, Mehran R, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–2351.
32. Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Eur Heart J. 2012;33:2551–2567.
33. Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736–2747.
ABSTRACT
Introduction and objectives: Manual thrombectomy (MT) during primary percutaneous coronary intervention (PCI) aims to reduce thrombus burden. Our study evaluates the outcomes and predictors of successful MT.
Methods: The Hunted registry is a retrospective, single-center cohort study including patients who underwent MT during PCI using the Hunter catheter from July 2020 through February 2022. MT success was defined as an angiographic reduction to a Thrombolysis in Myocardial Infarction (TIMI) thrombus grade of ≤ 2, with clinical follow-up for major adverse cardiovascular events.
Results: Among 750 patients with acute myocardial infarction who underwent PCI, 401 (53%) received MT. The mean age of treated patients was 62 years (80% men). MT was effective in 327 patients (81.55%). Predictors of successful MT included larger vessel diameter (P < .001), high thrombus burden (TIMI grade ≥ 4 flow; P < .001), and non-circumflex target vessels (P < .001). Device-related complications occurred in 17 patients (4.3%). At follow-up, major adverse events occurred in 8.98% of patients at 1 year and in 9.97% at 2 years.
Conclusions: In patients with ST-segment elevation myocardial infarction undergoing PCI, MT with the Hunter catheter in selected cases with high thrombus burden (TIMI grade ≥ 4 flow), non-circumflex target vessels, and vessel diameters > 2.5 mm, is a safe and effective technique with a low rate of complications.
Keywords: ST-segment elevation myocardial infarction. Percutaneous coronary intervention. Manual thrombectomy. Thrombus burden.
RESUMEN
Introducción y objetivos: La trombectomía manual (TM) en la intervención coronaria percutánea primaria (ICPp) intenta reducir la carga trombótica. Este estudio evalúa los resultados y los factores predictores de éxito de la TM.
Métodos: El registro Hunted es un estudio de cohortes retrospectivo, unicéntrico, de pacientes tratados con TM en ICPp utilizando el catéter Hunter, desde julio de 2020 hasta febrero de 2022. El éxito de la TM se definió como una disminución angiográfica a grado ≤ 2 en la escala Thrombolysis in Myocardial Infarction (TIMI), con seguimiento clínico de eventos cardiovasculares adversos mayores.
Resultados: De los 750 pacientes con infarto agudo de miocardio tratados con ICPp, en 401 (53%) se realizó TM. Los pacientes tratados tenían una edad media de 62 años y el 80% eran varones. La TM fue efectiva en 327 (81,55%) pacientes. Los predictores de TM efectiva fueron un mayor diámetro del vaso (p < 0,001), una alta carga de trombo (TIMI ≥ 4; p < 0,001) y un vaso diferente de la circunfleja (p < 0,001). Se presentaron complicaciones relacionadas con el dispositivo en 17 pacientes (4,3%). En el seguimiento, el 8,98% presentaron eventos mayores a 1 año y el 9,97% a 2 años.
Conclusiones: En los pacientes con infarto de miocardio con elevación del segmento ST sometidos a ICPp, la estrategia de TM con catéter Hunter, en casos seleccionados con alta carga trombótica (escala TIMI ≥ 4), otros vasos que no fueran la circunfleja y diámetros > 2,5 mm, es una técnica eficaz y segura con una baja tasa de complicaciones.
Palabras clave: Infarto de miocardio con elevación del segmento ST. Intervención coronaria percutánea primaria. Trombectomía manual. Carga trombótica.
Abbreviations
MACE: major adverse cardiovascular events. MT: manual thrombectomy. PCI: primary coronary intervention. STEMI: ST-segment elevation myocardial infarction. TIMI: Thrombolysis in Myocardial Infarction.
INTRODUCTION
In patients with ST-segment elevation myocardial infarction (STEMI), the treatment of choice is percutaneous coronary intervention (PCI) performed within the appropriate time window and by experienced operators. PCI has been shown to reduce mortality, reinfarction, and stroke compared with fibrinolysis.1 Among other factors, this benefit may be attributed to greater epicardial reperfusion and higher TIMI (Thrombolysis in Myocardial Infarction) flow grades and myocardial blush grades achieved with PCI in the culprit artery, all of which are known to influence survival.2,3
PCI have distinctive characteristics, including the presence of a high thrombus burden and performance in patients in a markedly thrombogenic state. To reduce the local thrombus burden in the culprit artery, manual thrombectomy (MT) has been widely used during PCI to reduce thrombus load, prevent distal embolization, and improve final myocardial perfusion.3,4 Despite its initial widespread adoption, routine use of MT in all patients undergoing PCI is no longer recommended, as randomized clinical trials have not demonstrated consistent clinical benefit.3-7
MT should be reserved for patients in whom it is most likely to provide benefit. To optimize the efficacy of PCI, the American8 and European9 clinical practice guidelines recommend MT in high-risk patients with a moderate-to-high thrombus burden who present with short ischemia times. Currently, however, there are no clearly defined criteria to precisely identify patients or thrombotic lesions that would derive the greatest benefit from MT.
The aim of our study was to evaluate the results of MT performed with the Hunter catheter (IHT–Iberhospitex SA, Barcelona, Spain), which has a high extraction capacity, in selected patients with STEMI undergoing PCI, and analyze the angiographic patterns associated with successful MT in our center.
METHODS
Study design and population
The Hunted registry is a single-center, observational, retrospective study. We included all patients diagnosed with STEMI who underwent PCI and in whom MT was performed using the Hunter thrombus aspiration catheter. This device was the first-choice catheter for MT in our center during the study period. The decision to perform MT was always left to the discretion of the operator performing the PCI, following homogeneous criteria among operators, subjectively based on angiographical evidence of a large angiographically visible thrombus. MT was not recommended in coronary vessels with a diameter < 2 mm or for the extraction of chronic thrombi or atherosclerotic plaques. The study period ranged from July 2020 through February 2022. Patients in whom MT was performed using a catheter other than the Hunter device were excluded.
The primary objective of the study was to evaluate the success of thrombus aspiration during PCI in patients with STEMI. Effective MT was defined as an angiographical reduction in thrombus burden, achieving a TIMI thrombus grade ≤ 2 (thrombus dimension < 50% of the vessel diameter). Moreover, angiographic factors associated with effective MT were assessed.
Secondary objectives included describing the clinical and angiographic characteristics of the patients and evaluating major adverse cardiovascular events (MACE) during hospitalization and follow-up.
MACE were defined as a composite endpoint of cardiovascular and noncardiovascular death, stroke, and acute myocardial infarction. Two follow-up time points were established at 1 and 2 years after PCI. Total ischemia time was defined as the interval, in minutes, between symptom onset and reperfusion, defined as passage of the intracoronary guidewire, in accordance with clinical practice guidelines.
Thrombus burden was graded according to the TIMI thrombus scale,10 which includes 5 grades: grade 1, possible thrombus; grade 2, thrombus dimension < 50% of the vessel diameter; grade 3, thrombus dimension 0.5 to 2.0 vessel diameters; grade 4, thrombus dimension > 2.0 vessel diameters; and grade 5, total vessel occlusion by thrombus. Grades 4 and 5 were considered high thrombus burden.
Successful PCI was defined as achievement of TIMI grade 3 flow with residual percent diameter stenosis < 20%, without device-related complications or intraoperative MACE. For study purposes, patients were categorized into 2 groups according to whether MT was effective or not. Furthermore, these groups were compared to identify angiographic parameters that could predict MT success.
Data for the variables included in the Hunted registry were collected using a dedicated electronic case report form. Retrospective angiographic analysis was performed exclusively by 3 operators. All measures were taken to ensure confidentiality and protection of patient health record information. The study protocol fully complied with international recommendations for clinical research outlined in the Declaration of Helsinki and was approved by the hospital Ethics and Research Committee.
Characteristics of the Hunter device and aspiration technique
The Hunter thrombus aspiration catheter is a 140 cm rapid-exchange aspiration catheter compatible with a 6-Fr guiding catheter. Its tip has a slightly conical, low-profile, atraumatic design. The effective distal aspiration area measures 0.95 mm2, and it can aspirate up to 1.92 mL per second, one of the highest capacities available. The distal segment is coated with a hydrophilic surface to facilitate device navigability.
The standard thrombectomy technique used in our center consisted of advancing the Hunter catheter to a segment proximal to the culprit lesion. Aspiration was always initiated proximal to the culprit lesion. The catheter was then slowly advanced across the lesion under continuous aspiration to reach the distal segment, while continuous filling of the syringe was observed. If filling stopped, the catheter was withdrawn until aspiration resumed or removed completely if aspiration could not be reestablished. Continuous aspiration until removal from the guiding catheter was mandatory, as was thorough subsequent flushing of the guiding catheter, to prevent embolization of residual thrombotic material.
All retrieved material from the catheter and aspiration syringe was subsequently filtered using the filters provided with the device packaging. The procedure was repeated as many times as deemed necessary by the operator until the desired reduction in thrombus burden was achieved.
Statistical analysis
Qualitative variables are expressed as frequencies and percentages, and the quantitative ones as mean and standard deviation when normally distributed, and as median and interquartile range when distribution was nonnormal.
Qualitative variables were compared using the chi-square test, with odds ratios (OR) and 95% confidence intervals (95%CI) calculated. Quantitative ones were compared using the Student t test or nonparametric tests, as appropriate.
In univariate analysis, each variable was individually assessed for its association with effective MT. Variables showing a statistically significant association were included in the multivariate analysis. Multiple regression models were used to control for potential confounders and determine the independent effect of each variable on the outcome.
Survival curves were analyzed using the Kaplan–Meier method, and survival-related parameters were evaluated using Cox proportional hazards analysis. A 2-sided P value < .05 was considered statistically significant.
Statistical analyses were performed using STATA version 15 (StataCorp, United States).
RESULTS
During the study period, a total of 750 PCI were performed in patients diagnosed with STEMI. MT using the Hunter catheter was performed in 401 patients (53.47%). The clinical characteristics of the patients, STEMI features, and PCI are shown in table 1.
Table 1. Clinical characteristics of patients and procedural variables
| Clinical characteristics | (n = 401) |
|---|---|
| Age, years | 62.38 ± 12.43 |
| Male sex | 319 (80) |
| Current/former smoker | 163 (40.65) / 35 (8.73) |
| Hypertension | 207 (51.62) |
| Dyslipidemia | 186 (46.38) |
| Diabetes mellitus | 86 (21.45) |
| Kidney failure | 7 (1.75) |
| Previous stroke | 16 (3.99) |
| Peripheral vascular disease | 8 (2.00) |
| Previous AMI | 48 (11.97) |
| Previous PCI | 50 (12.47) |
| Prior CABG | 5 (1.25) |
| Infarct location | |
| Anterior | 166 (41.50) |
| Inferior | 207 (51.75) |
| Lateral | 27 (6.75) |
| Killip-Kimball classification | |
| I | 338 (84.71) |
| II | 18 (4.51) |
| III | 8 (2.01) |
| IV | 35 (8.77) |
| Procedural variables | |
| Radial access | 385 (96.01) |
| No. of diseased vessels* | |
| 1 | 233 (58.10) |
| 2 | 111 (27.68) |
| 3 | 57 (14.21) |
| Infarct-related artery | |
| Right coronary artery | 183 (45.64) |
| Left anterior descending coronary artery | 166 (41.40) |
| Left circumflex artery | 48 (11.97) |
| Venous graft | 1 (0.25) |
| Left main coronary artery | 3 (0.75) |
| No. of stents implanted | |
| 0 | 34 (8.47) |
| 1 | 288 (71.82) |
| 2 | 60 (14.96) |
| 3 | 19 (4.74) |
| Drug-eluting stent (n = 367) | 362 (98.64) |
| Total ischemic time | |
| < 90 min | 43 (10.72) |
| 91-180 min | 167 (41.65) |
| 181-270 min | 82 (20.45) |
| 271-360 min | 42 (10.47) |
| > 360 min | 67 (16.71) |
|
AMI, acute myocardial infarction; CABG, coronary artery bypass grafting; PCI, percutaneous coronary intervention. |
|
Angiographic analysis of the culprit lesion and flow in the infarct-related artery is shown in table 2. Initial TIMI grade flow in the infarct-related artery, prior to intracoronary guidewire passage was 0 in 83% of cases. A high thrombus burden was observed in 87.5% of patients, corresponding to TIMI thrombus grades 4 or 5; 53.4% had grade 5 and 34.2% had grade 4. Only 50 patients (12.5%) had a TIMI grade < 4 flow, defined as a low thrombus burden.
Table 2. Angiographic analysis of patients treated with percutaneous coronary intervention and manual thrombectomy
| Angiographic variables | |
|---|---|
| Vessel diameter | 3.25 [3.00-3.50] |
| 2.0-2.5 mm | 61 (15.21) |
| 2.6-3.0 mm | 122 (30.42) |
| 3.1-3.5 mm | 124 (30.92) |
| 3.6-4.0 mm | 66 (16.46) |
| > 4.0 mm | 28 (6.98) |
| Lesion length, mm | 16 [15-20] |
| AHA/ACC lesion type | |
| B1 | 60 (14.96) |
| B2 | 161 (40.15) |
| C | 180 (44.89) |
| Thrombus burden grade (TIMI) | |
| 1 | 1 (0.25) |
| 2 | 5 (1.25) |
| 3 | 44 (10.97) |
| 4 | 137 (34.16) |
| 5 | 214 (53.37) |
| Pre-PCI IRA TIMI flow grade | |
| 0 | 332 (82.79) |
| 1 | 18 (4.49) |
| 2 | 24 (5.98) |
| 3 | 27 (6.74) |
| Post-PCI IRA TIMI flow grade | |
| 0 | 2 (0.50); |
| 1 | 3 (0.75); |
| 2 | 10 (2.49); |
| 3 | 386 (96.26) |
|
AHA/ACC, American Heart Association/American College of Cardiology; IRA, infarct-related artery; PCI, percutaneous coronary intervention; TIMI, Thrombolysis in Myocardial Infarction. |
|
According to the predefined criteria, effective MT was achieved in 327 of 401 patients (81.5%). Thrombectomy was considered ineffective in the 6 patients who had initial TIMI thrombus grades 1 and 2. Final post-PCI coronary flow was TIMI grade < 3 flow in 15 patients (3.74%). Overall, the PCI was successful in approximately 97% of cases. Device-related complications were recorded in 17 patients (4.24%): severe arrhythmias (ventricular fibrillation or ventricular tachycardia) occurring during reperfusion in 10 patients; severe no-reflow due to distal thrombus migration that could not be successfully treated in 4 patients; and coronary dissection after passage of the MT catheter, which was successfully treated with stenting in 3 patients. There were no cases of perioperative stroke due to migration of aspirated thrombus.
Comparative analysis of angiographic factors and ischemia time between patients with effective and noneffective MT is shown in table 3. MT was effective more frequently in patients with culprit coronary vessels > 3 mm in diameter (112 [34%] vs 12 mm [16%]; P < .001) and in those with a TIMI grade ≥ 4 thrombus burden (297 [91%] vs 54 [73%]; P < .001). Among the 61 patients with vessels < 2.5 mm, MT was ineffective in 36 (59.01%) vs 38 of 340 patients (11.2%) with vessels > 2.5 mm (P < .001). There were no statistically significant differences in ischemia time in relation to MT success.
Table 3. Comparison of angiographic and procedural characteristics between patients with effective and noneffective manual thrombectomy
| Procedural variables | Effective MT (n = 327) | Noneffective MT (n = 74) | P |
|---|---|---|---|
| Infarct-related artery | .008 | ||
| Right coronary artery | 153 (46.79) | 30 (40.54) | |
| Left anterior descending coronary artery | 140 (42.81) | 26 (35.14) | |
| Left circumflex artery | 30 (9.17) | 18 (24.32) | |
| Saphenous vein graft | 1 (0.31) | – | |
| Left main coronary artery | 3 (0.92) | – | |
| AHA/ACC classification of the culprit lesion | .004 | ||
| B1 | 51 (15.60) | 9 (12.16) | |
| B2 | 142 (43.42) | 19 (25.68) | |
| C | 134 (40.98) | 46 (62.16) | |
| Reference diameter of the culprit lesion | < .001 | ||
| 2.0 mm-2.5 mm | 25 (7.65) | 36 (48.69) | |
| 2.6 mm-3.0 mm | 101 (30.89) | 21 (28.38) | |
| 3.1 mm-3.5 mm | 112 (34.25) | 12 (16.22) | |
| 3.6 mm-4.0 mm | 64 (19.57) | 2 (2.70) | |
| > 4.0 mm | 25 (7.64) | 3 (4.10) | |
| Thrombus burden grade (TIMI) | < .001 | ||
| Low thrombus burden (TIMI < 4) | 30 (9.18) | 20 (27.03) | |
| High thrombus burden (TIMI ≥ 4) | 297 (90.82) | 54 (72.97) | |
| Pre-PCI TIMI grade flow | .031 | ||
| TIMI grade 0-1 flow | 291 (88.99) | 59 (79.73) | |
| TIMI grade 2-3 flow | 36 (11.01) | 15 (20.27) | |
| Post-PCI TIMI grade flow | .61 | ||
| TIMI grade 0-1 flow | 2 (0.61) | 3 (4.05) | |
| TIMI grade 2 flow | 8 (2.45) | 1 (1.35) | |
| TIMI grade 3 flow | 317 (96.94) | 70 (94.59) | |
| Time from symptom onset to reperfusion | .79 | ||
| ≤ 90 min | 37 (11.31) | 6 (8.11) | |
| 91-180 min | 139 (42.51) | 28 (37.84) | |
| 181-270 min | 68 (20.80) | 14 (18.92) | |
| 271-360 min | 33 (10.09) | 9 (12.16) | |
| > 360 min | 50 (15.29) | 17 (22.97) | |
| Final procedural success | 317 (96.94) | 70 (94.59) | .61 |
|
AHA/ACC, American Heart Association/American College of Cardiology; MT, manual thrombectomy; PCI, percutaneous coronary intervention; TIMI, Thrombolysis in Myocardial Infarction. |
|||
The 1- and 2-year follow-up was completed in 100% of included patients. MACE occurred in 32 patients (7.98%) at 30 days, 36 patients (8.98%) at 1 year, and 40 patients (9.97%) at 2 years (table 4). Kaplan–Meier curves for event-free survival during follow-up and cardiovascular death according to effective vs noneffective MT are shown in figure 1 and figure 2, respectively. Individual components of MACE at 1 year are shown in figure 3.
Table 4. Incidence rate of the composite endpoint of major adverse cardiovascular events during follow-up
| Cardiovascular events at follow-up | n (%) |
|---|---|
| At 30 days | 32 (7.98) |
| At 1 year | 36 (8.98) |
| At 2 years | 40 (9.97) |
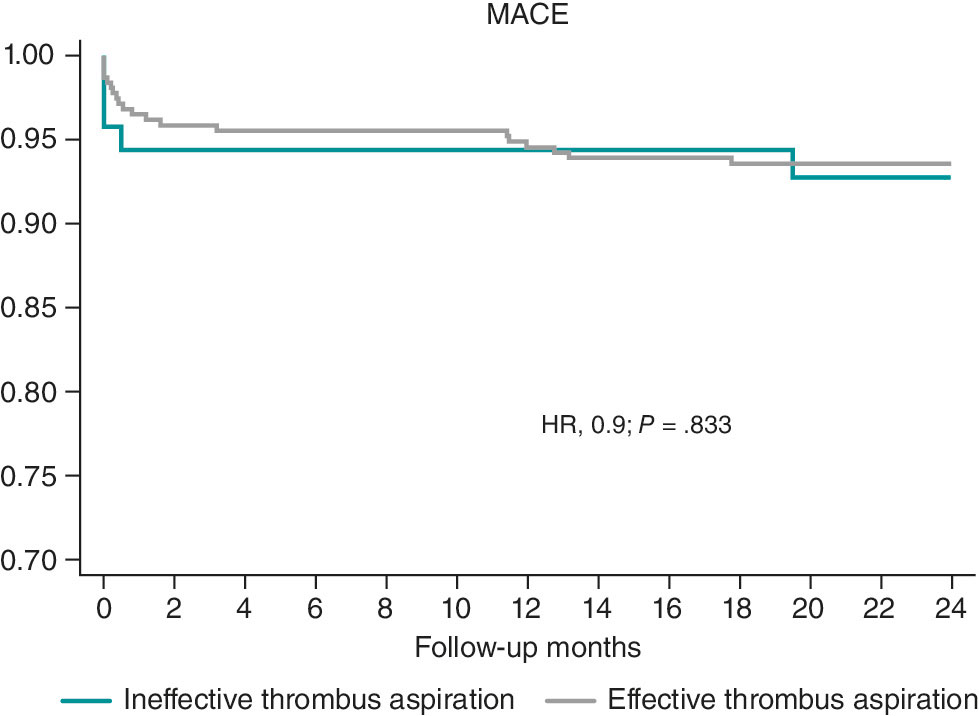
Figure 1. Kaplan–Meier curves for survival free from major adverse cardiovascular events (MACE). HR, hazard ratio.
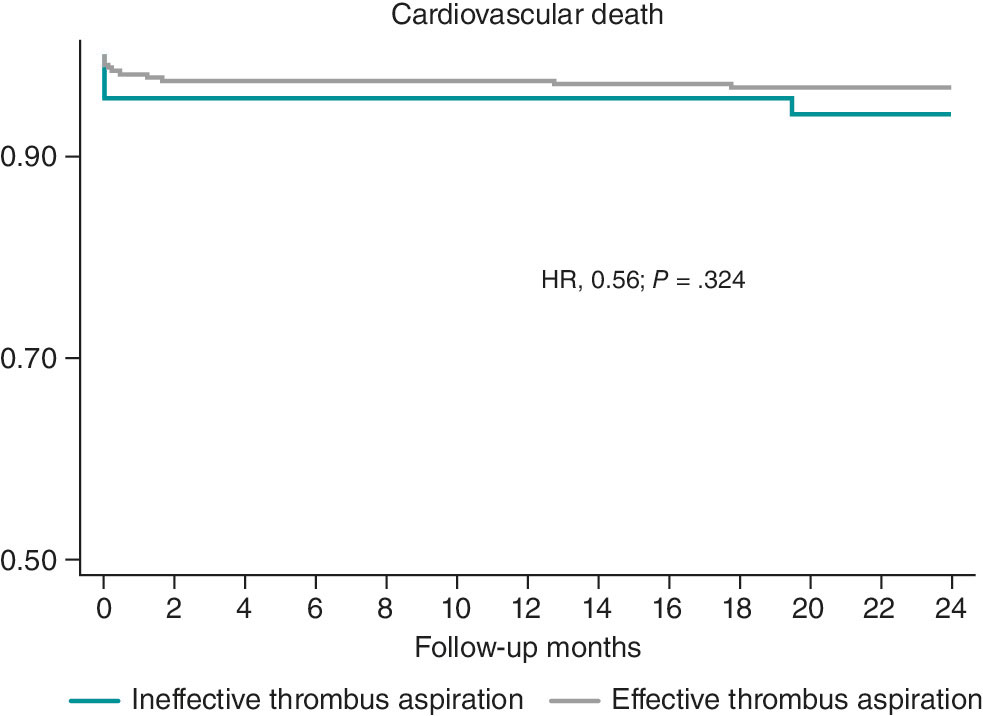
Figure 2. Kaplan–Meier survival curves for cardiovascular death. HR, hazard ratio.
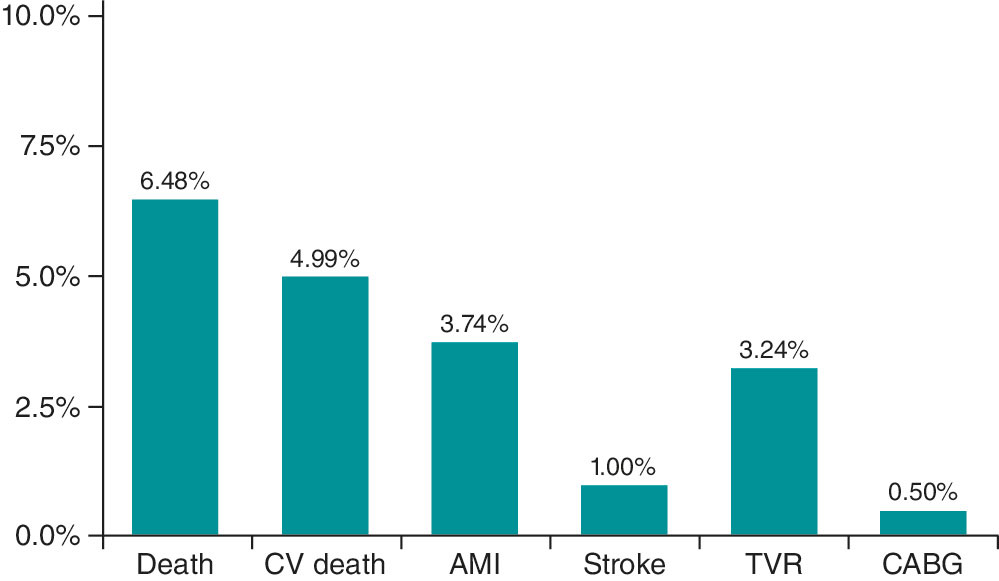
Figure 3. Individual components of major adverse cardiovascular events at the 1-year follow-up. AMI, acute myocardial infarction; CABG, coronary artery bypass grafting; CV, cardiovascular; TVR, target vessel revascularization.
DISCUSSION
In selected patients, MT using the Hunter catheter is a safe and effective strategy to reduce angiographically assessed thrombus burden during PCI.
Current clinical practice guidelines do not recommend routine MT during PCI but suggest considering it in patients with a high thrombus burden, based on individual assessment and operator experience.8,9 In our series, MT was performed in 53.5% of patients with STEMI treated with PCI, a higher proportion than reported in other countries.11-13 Only 12% of patients undergoing MT did not have a TIMI grade 4–5 thrombus burden on angiographic analysis.
In this selected population with high thrombus burden, 88% had TIMI grade ≥ 4 flow, which is similar to the 79% observed in the TOTAL trial,14 with favorable results in both cases. In contrast, only 33% of patients from the TASTE trial7 had a high thrombus burden.
A high thrombus burden appears to be a key determinant of achieving effective MT and may be associated with better clinical outcomes. In our cohort, although effective MT was achieved in 82% of cases, it was not significantly correlated with final procedural success (97% vs 95%; P = .67), likely due to the small sample size of the 2 groups. MT efficacy was higher in vessels with high thrombus burdens (TIMI grade ≥ 4 flow; P < .001), which is consistent with a meta-analysis showing less cardiovascular death in patients with STEMI undergoing PCI with MT in the high thrombus burden subgroup vs PCI alone (2.5% vs 3.2%; hazard ratio [HR], 0.81; 95%CI, 0.65–0.98; P = .03).15 These findings reinforce the concept that appropriate patient selection is crucial to benefit from MT. Moreover, the same meta-analysis reported a higher risk of stroke (0.9% vs 0.5% in the PCI-alone group),15 a complication that may be related to the TM technique used.
Strict adherence to proper technique is essential to minimize complications and maximize success. In our series, emphasis was placed on following a standardized and rigorous technique, as described in the Methods section, resulting in a low complication rate (4.3%). Many of these complications were not directly related to MT per se but rather to reperfusion, such as ventricular arrhythmias. MT-related stroke is a potential complication; in the TOTAL trial,16 the stroke rate was 0.7%, twice that observed in the PCI-alone group, whereas in the large real-world SCAAR registry, there was no increase in the incidence rate of stroke across the groups,17 which are findings more consistent with our results and possibly related to differences in MT technique.
To identify angiographic predictors of MT success, we compared the characteristics of patients with effective and noneffective MT. The former had significantly larger culprit vessel diameters; 48% of patients with noneffective MT had vessels measuring 2.5 mm. Although MT is generally discouraged in vessels < 2 mm, larger vessels may harbor greater thrombus burden and thus derive greater benefit from MT with the Hunter catheter, which has demonstrated higher in vitro aspiration capacity compared with other devices. Therefore, vessel size is a critical differentiating factor: in vessels < 2.5 mm, MT was ineffective in 59% of cases, a significantly higher proportion than the 11.2% of noneffective MT observed in larger vessels. The other major difference between groups was thrombus burden, as effective MT was achieved more frequently in patients with higher thrombus loads (TIMI grade ≥ 3 flow).15 Furthermore, this factor represents a major difference among randomized clinical trials, in which the proportion of patients with high thrombus burden varied substantially.15 More complex lesions, such as American Heart Association/American College of Cardiology type C lesions, those associated with calcification in addition to thrombus, long lesions, and left circumflex artery lesions were associated with higher rates of noneffective MT. These differences should be considered when selecting appropriate candidates for MT with the Hunter catheter, favoring patients with vessel diameters > 2.5 mm, abundant thrombus burden (TIMI grade ≥ 4 flow), and culprit arteries other than the left circumflex one.
Studies have shown that total ischemia time, which we believe may determine differences in thrombus composition,18 may influence the efficacy of thrombus aspiration.19 In our series, there were no differences between the effective and noneffective MT groups with respect to infarction duration.
When assessing whether effective MT impacted the outcome of the PCI, slightly different procedural success rates were observed: 97% for effective MT vs 95% for noneffective MT (P = .61). Statistical significance was not reached, possibly due to the small sample size. Only 4 patients experienced no-reflow that could not be resolved, with no differences across groups and without demonstrating that MT could prevent distal embolization, as suggested in former studies.17-19
Short- and long-term clinical outcomes in patients with STEMI who required MT were favorable, with a low 1-year cardiovascular death rate of 3.5%, comparable to that reported in randomized clinical trials. In the TASTE trial,7 the 30-day mortality rate was 2.4% in the MT group and 2.9% in the PCI-alone group (HR, 0.84; 95%CI, 0.70–1.01; P = .06). In the TAPAS trial,20 the 30-day all-cause mortality rate was 2.1% in the MT group vs 4.0% in the conventional PCI group, reaching statistical significance at the 1-year follow-up (P = .07). Large registries have reported mortality rates similar to those observed in our series, with 2.8% vs 3.0% in the Swedish registry21 and comparable findings in the Japanese registry.22 The overall mortality rate observed in a meta-analysis with aggregated data from published MT studies was 3.7%.15 When selecting patients with a high thrombus burden (TIMI grade ≥ 3 flow in the meta-analysis subgroup), the cardiovascular death rate was 2.5% (170 of 6872 patients) in the MT group vs 3.1% (205 of 6599 patients) in the PCI-alone group (HR, 0.8; 95%CI, 0.65–0.98; P = .03).15 Proper selection of this subgroup of patients with a high thrombus burden is, therefore, crucial to maximize the therapeutic benefit.
Limitations
As a single-center, observational, retrospective registry, this study has inherent limitations related to its design. First, because it reflects the experience of a single center—albeit with more than 15 years of experience in PCI—operator homogeneity may limit extrapolation of the results. The decision to perform MT was always left to the discretion of the operator, and no control group without MT was available for patient comparison. Because of the retrospective design of the analysis, we could not determine the cause of ineffective MT in all patients, which is why this variable could not be included in the analysis. This study should not be interpreted as an evaluation of thrombus aspiration in general, but rather as an assessment of outcomes in a selected population treated with the Hunter device; these selection criteria represent the primary contribution of this work to current scientific knowledge. The study did not incorporate systematic criteria to address sex- and gender-related variables during methodological development or result analysis. Although angiographic analysis was not performed by an independent core laboratory, it was conducted by 3 experienced analysts. In this analysis, MT success, procedural success, and baseline thrombus burden were defined using the TIMI scale.
CONCLUSIONS
In patients with STEMI undergoing PCI, selective use of MT with the Hunter catheter in cases with high thrombus burden (TIMI grade ≥ 4 flow), non-circumflex culprit vessels, and vessel diameters > 2.5 mm is a safe and effective strategy associated with a low complication rate. Further studies are needed to assess the impact of this strategy on PCI outcomes, MACE, and stroke.
FUNDING
This project was supported by IHT-Iberhospitex S.A. (Lliçà de Vall, Barcelona, Spain). As sponsor, the company collaborated in the study design but had no role in data collection, analysis, or interpretation. Manuscript preparation and the decision to submit for publication were entirely independent of the sponsor and performed by the research team.
ETHICAL CONSIDERATIONS
The study was approved by Hospital Universitari Germans Trias i Pujol Ethics Committee (Barcelona, Spain) (CEIC code: PI-22-281) and conducted in full compliance with the principles outlined in the Declaration of Helsinki. SAGER guidelines were not applied to address gender bias.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
No artificial intelligence tools were used in the preparation of this manuscript.
AUTHORS’ CONTRIBUTIONS
D.G. Borraz-Noriega: angiographic analysis, data review, and manuscript drafting. J.F. Andrés-Cordón: angiographic analysis, data review, and statistical analysis. E. Cañedo: data collection and clinical follow-up. F. Panchano-Castro: angiographic analysis and data review. M. Trichilo: data collection and clinical follow-up. V. Vilalta: data collection and critical manuscript review. O. Rodríguez-Leor: data collection and critical manuscript review. E. Fernández-Nofrerias: critical manuscript review. I. Santos-Pardo: critical manuscript review. X. Carrillo: study design, overall supervision, and manuscript drafting. All authors reviewed and approved the final version.
CONFLICTS OF INTEREST
None declared.
WHAT IS KNOWN ABOUT THIS TOPIC?
- Routine use of manual thrombectomy has not demonstrated clear benefit. Current clinical practice guidelines recommend individualized use in selected patients with high thrombus burden. Clear angiographic criteria for identifying patients most likely to benefit from thrombectomy are lacking.
WHAT DOES THIS STUDY ADD?
- The Hunted registry provides specific evidence on thrombectomy performed with the Hunter catheter during PCI.
- It identifies angiographic predictors of thrombectomy success (vessel diameter > 2.5 mm, TIMI ≥ 4 thrombus burden, non-circumflex coronary arteries).
- The Hunter catheter, with its larger effective aspiration area, along with proper technique, demonstrates a low complication rate and favorable clinical outcomes.
REFERENCES
1. Keeley EC, Boura JA, Grines CL, et al. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361:13-20.
2. Chesebro JH, Knatterud G, Roberts R, et al. Thrombolysis in Myocardial Infarction (TIMI) Trial, Phase I: A comparison between intravenous tissue plasminogen activator and intravenous streptokinase. Clinical findings through hospital discharge. Circulation. 1987;76:142-154.
3. Moens AL, Claeys MJ, Timmermans JP, et al. Myocardial ischemia/reperfusion-injury, a clinical view on a complex pathophysiological process. Int J Cardiol. 2005;100:179-190.
4. Bhindi R, Kajander OA, Jolly SS, et al. Culprit lesion thrombus burden after manual thrombectomy or percutaneous coronary intervention-alone in ST-segment elevation myocardial infarction: the OTC sub-study of the TOTAL trial. Eur Heart J. 2015;36:1892-1900.
5. Sim DS, Jeong MH, Ahn Y, et al. Korea Acute Myocardial Infarction Registry (KAMIR) Investigators. Manual thrombus aspiration during primary percutaneous coronary intervention: Impact of total ischemic time. J Cardiol. 2016;27:753-758.
6. Vlaar PJ, Svilaas T, van der Horst IC, et al. Cardiac death and reinfarction after 1 year in the Thrombus Aspiration during Percutaneous coronary intervention in Acute myocardial infarction Study (TAPAS): a 1-year follow-up study. Lancet. 2008;371:1915-1920.
7. Lagerqvist B, Fröbert O, Olivecrona GK, et al. Outcomes 1 Year after Thrombus Aspiration for Myocardial Infarction. N Engl J Med. 2014;371:1111-1120.
8. Levine GN, Bates ER, Blankenship JC, et al. ACC/AHA/SCAI Focused Update on Primary Percutaneous Coronary Intervention for Patients with ST-Elevation Myocardial Infarction: An Update of the 2011 ACCF/AHA/SCAI and the 2013 ACCF/AHA Guidelines. J Am Coll Cardiol. 2016;67:1235-1250.
9. Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119-177.
10. Sianos G, Papafaklis MI, Serruys PW. Angiographic thrombus burden classification in patients with ST-segment elevation myocardial infarction treated with percutaneous coronary intervention. J Invasive Cardiol. 2010;22:6B-14B.
11. Freixa X, Jurado-Román A, Cid B, et al. Spanish cardiac catheterization and coronary intervention registry. 31st official report of the Interventional Cardiology Association of the Spanish Society of Cardiology (1990-2021). Rev Esp Cardiol. 2022;75:1040-1049.
12. Kimura K, Kimura T, Ishihisa M, et al. JCS 2018 Guideline on diagnosis and treatment of acute coronary syndrome. Circulation. 2019;83:1085-1196.
13. Qu Y-Y, Zhang X-G, Ju C-W, et al. Age-Related Utilization of Thrombus Aspiration in Patients with ST-Segment Elevation Myocardial Infarction: Findings From the Improving Care for Cardiovascular Disease in China Project. Front Cardiovasc Med. 2022;9:791007.
14. Svilaas T, Vlaar PJ, van der Horst IC, et al. Thrombus aspiration during primary percutaneous coronary intervention. N Engl J Med. 2008;358:557-567.
15. Jolly SS, James S, Džavík V, et al. Thrombus Aspiration in ST-Segment–Elevation Myocardial Infarction, An Individual Patient Meta-Analysis: Thrombectomy Trialists Collaboration. Circulation. 2017;135:143-152.
16. Jolly SS, Cairns JA, Yusuf S, et al. Randomized trial of primary PCI with or without routine manual thrombectomy. N Engl J Med. 2015;372:1389-1398.
17. Angeras O, Haraldsson I, Redfors B, et al. Impact of Thrombus Aspiration on Mortality, Stent Thrombosis, and Stroke in Patients with ST-Segment–Elevation Myocardial Infarction: A Report From the Swedish Coronary Angiography and Angioplasty Registry. J Am Heart Assoc. 2018;7:e007680.
18. Carol A, Bernet M, Curós A, et al. Thrombus age, clinical presentation, and reperfusion grade in myocardial infarction. Cardiovasc Pathol. 2014;23:126-130.
19. Sim DS, Jeong MH, Ahn Y, et al. Manual thrombus aspiration during primary percutaneous coronary intervention: Impact of total ischemic time. J Cardiol. 2017;69:428-435.
20. Vlaar PJ, Svilaas T, van der Horst IC, et al. Cardiac death and reinfarction after 1 year in the Thrombus Aspiration during Percutaneous coronary intervention in Acute myocardial infarction Study (TAPAS): a 1-year follow-up study. Lancet. 2008;371:1915-1920.
21. Fröbert, O, Lagerqvist B, Olivecrona GK, et al. Thrombus aspiration during ST-segment elevation myocardial infarction. N Engl J Med. 2013;369:1587-1597.
22. Inohara T, Kohsaka S, Yamaji K, et al. Use of Thrombus Aspiration for Patients with Acute Coronary Syndrome: Insights from the Nationwide J-PCI Registry. J Am Heart Assoc. 2022;11:e025728.
ABSTRACT
Introduction and objectives: Although acute myocardial infarction (AMI) remains a leading cause of death in Mexico, the impact of out-of-hours presentation on mortality remains understudied. The aim of this study was to evaluate the association between out-of-hours admissions (nights, weekends, holidays) and 30-day mortality in patients with AMI in Mexican hospitals, with a focus on the role of catheterization laboratories (cath lab).
Methods: We conducted a retrospective cohort study to analyze emergency admissions from December 2014 through August 2023. Admissions were classified as out-of-hours or during-hours and were stratified by hospital type (with or without cath lab). Cox regression models adjusted for sociodemographic, health, and temporal variables were used to analyze mortality risks.
Results: The study included a total of 29 131 cases: 4515 in percutaneous coronary intervention (PCI)-capable centers (group 1) and 24 616 in non-PCI-capable centers (group 2). Admissions outside regular hours accounted for 46.7% in group 1 and 53.6% in group 2. Adjusted analysis showed that although the presence of a cath lab was protective (HR, 0.25; 95%CI, 0.23-0.28), admissions outside regular hours increased the risk of mortality in both groups (group 1: HR, 1.25; 95%CI, 1.04-1.50; group 2: HR, 1.16; 95%CI, 1.11-1.22). Although overnight shifts increased the risk of death in both groups, weekends and holidays increased such risk only in non-PCI-capable centers.
Conclusions: Out-of-hours admissions were associated with higher mortality, and unlike in developed countries, the presence of a cath lab did not improve out-of-hours outcomes.
Keywords: Acute myocardial infarction. Out-of-hours. Mexico.
RESUMEN
Introducción y objetivos: El infarto agudo de miocardio (IAM) sigue siendo una causa principal de muerte en México, pero el impacto de su presentación fuera de horario sobre la mortalidad está poco investigado. El objetivo de este estudio fue evaluar la asociación entre las admisiones fuera de horario (noches, fines de semana y festivos) y la mortalidad a 30 días en pacientes con IAM en hospitales mexicanos, con énfasis en el papel de las salas de hemodinámica (SH).
Métodos: Se realizó un estudio de cohorte retrospectivo que analizó las admisiones en urgencias desde diciembre de 2014 hasta agosto de 2023. Las admisiones se clasificaron como fuera o dentro de horario, y se estratificaron según el tipo de hospital (con o sin SH). Se emplearon modelos de regresión de Cox ajustados por variables sociodemográficas, de salud y temporales para analizar el riesgo de mortalidad.
Resultados: El estudio incluyó 29.131 casos: 4.515 en hospitales con SH (grupo 1) y 24.616 en hospitales sin esta infraestructura (grupo 2). Las admisiones fuera de horario representaron el 46,7% en el grupo 1 y el 53,6% en el grupo 2. El análisis ajustado mostró que, aunque la presencia de una SH fue protectora (HR = 0,25; IC95%, 0,23-0,28), las admisiones fuera de horario aumentaron el riesgo de mortalidad en ambos grupos (grupo 1: HR = 1.25; IC95%, 1,04-1,50; grupo 2: HR = 1,16; IC95%, 1,11-1,22). Los turnos nocturnos también incrementaron el riesgo de muerte en ambos grupos, pero los fines de semana y festivos solo lo hicieron en los hospitales sin SH.
Conclusiones: Las admisiones fuera de horario se asociaron con mayor mortalidad y, a diferencia de los países desarrollados, la presencia de una SH no mejoró los resultados fuera de horario.
Palabras clave: Infarto agudo de miocardio. Fuera de horario. México.
Abbreviations
AMI: acute myocardial infarction. Cath lab: catetherization laboratory. PCI: percutaneous coronary intervention.
INTRODUCTION
Acute myocardial infarction (AMI) remains a leading cause of morbidity and mortality worldwide, despite advances in prevention and treatment.1-3 Evidence suggests that patients with AMI admitted outside working hours–nights, weekends, and holidays–experience worse outcomes,4-6 likely due to treatment delays,7 limited specialist availability, and operational constraints.4,8,9 While this association is well-documented in high-income settings, its impact in low- and middle-income countries such as Mexico, where health care resources are often constrained, remains less understood.10-12
Among Organisation for Economic Co-operation and Development (OECD) countries, Mexico reports the highest AMI mortality rate (23.7%), far exceeding the OECD average of 7%.3,12 Throughout the past century, the Mexican public health system has evolved from fragmentation to institutional organization, with key institutions such as the Mexican Social Security Institute (IMSS) (est. 1943) and the Institute for Social Security and Services for State Workers or Civil Service Social Security and Services Institute (ISSSTE) serving formal and public-sector workers, respectively.13,14 To address coverage gaps for the uninsured, programs such as Seguro Popular (2003), Institute of Health for Welfare (INSABI) (2020), and IMSS-BIENESTAR (2023) were established. In recent years, more than 53 million uninsured individuals, about 42% of the population, receive care through the Mexican Ministry of Health (SSA) across nearly 12 000 health centers and more than 680 hospitals.13
Despite system reforms, the management of AMI in Mexico remains hindered by limited access to specialized services, fragmented insurance coverage, and an underperforming emergency response system. These issues, exacerbated by socioeconomic disparities and insufficient preventive care, contribute to delays and reduced quality of treatment.15,16 The main aim of the current study was to evaluate the association between out-of-hours presentation and 30-day AMI-related mortality in Mexican emergency departments.
METHODS
Study design, guidelines, and data source
We conducted a retrospective cohort study in full compliance with the Guidelines for Strengthening the Reporting of Observational Studies in Epidemiology (STROBE).17 The present study used information from the public database of emergency department admissions from December 2014 through August 2023 in Mexico, published and validated by the General Directorate of Health (DGIS).18 This database compiles anonymous information from the SSA hospital registry (Seguro Popular/INSABI/IMSS-BIENESTAR programs) nationwide. Additional information on individual treatments (eg, percutaneous coronary intervention [PCI], thrombolysis, or door-to-balloon time), patient comorbidities, and delays in patient arrival was not available in the database.
Participants and sample size
The study included cases diagnosed with AMI (International Classification of Diseases [ICD-10, I21.0 to I21.9]), with lengths of stay of ≤ 30 days, classified as a qualified emergency, aged ≥ 18 years, and treated in a SSA hospital. Cases without complete information or not treated in the emergency department (referred to another health care unit, voluntarily discharged, or referred to outpatient care) were excluded from the analysis.
Definition of predictors and outcome
Sociodemographic (sex and age), time-related variables (admission and discharge dates), health care-related variables (type of emergency, discharge status, bed type, and AMI type according to ICD-10), and catheterization laboratories (cath labs) characteristics were collected. Several variables were grouped for analysis, including region of residence, death (yes/no), and length of stay (admission-to-discharge interval). The main predictor, out-of-hours, was defined as admissions during overnight shifts (19:00-6:59 h), weekends, or official holidays (per Article 74 of the Mexican Federal Labor Law).19 Furthermore, the COVID-19 pandemic (from 23 March 2020 to 9 May 2023)20 was considered. Hospitals were classified as PCI-capable and non-PCI-capable centers based on the SSA equipment database; and accurate and up-to-date information on PCI-capable centers was obtained; detailed definitions are provided in table S1.
Statistical analysis
Quantitative variables were expressed as mean ± standard deviation (SD), and categorical variables as frequencies and percentages. The primary sample was divided into 2 groups to observe the differences between PCI-capable and non-PCI-capable centers, and all analyses were systematically performed in each group. The chi-square and Student t tests evaluated the difference between the out-hours and on-hours groups. Univariate and multivariate Cox regression analyses were performed with proportional hazards adjusted for age, sex, year of admission, type of emergency, type of bed, territorial regions, type of AMI, admission during the COVID-19 pandemic, and characteristics of the cath lab. Moreover, Kaplan-Meier survival curves were constructed to estimate and visualize the cumulative mortality rate according to out-of-hours admission components. Statistical significance was set at P < .05. Confidence intervals (CI) were set at 95% (95%CI). Descriptive and analytical methods were performed in SPSS, version 25.0 (IBM, United States) and R software version 4.2.0 (R Foundation for Statistical Computing, Austria).
Ethical considerations
The study was based on data from the public emergency income database published by the DGIS.4 Accordingly, the confidentiality of the subjects is governed by the Mexican standard NOM-012-SSA3-2012,21 and the study is classified as risk-free research based on the principles of the General Law of Research for Health.22 The research was approved by the Ethics Committee of the Women’s Hospital, SSA (No. 202403-47).
RESULTS
General characteristics
The final dataset included a total of 29 131 cases: 4515 from PCI-capable centers (group 1) and 24 616 from non-PCI-capable centers (group 2) (figure 1). Out-of-hours admissions accounted for 46.7% in group 1 and 53.6% in group 2, with night admissions being the most frequent and holiday admissions the least common (table S2). Among PCI-capable centers, 32.2% provided nightshift coverage, while 53.1% offered weekend availability. Out-of-hours admissions were associated with a higher proportion of male patients, longer lengths of stay, greater use of shock beds, higher mortality, more pronounced regional differences, and a greater impact of the COVID-19 pandemic (table S3). Moreover, in PCI-capable centers, out-of-hours admissions were associated with a lower daily average number of procedures and fewer professionals per shift (table S4).
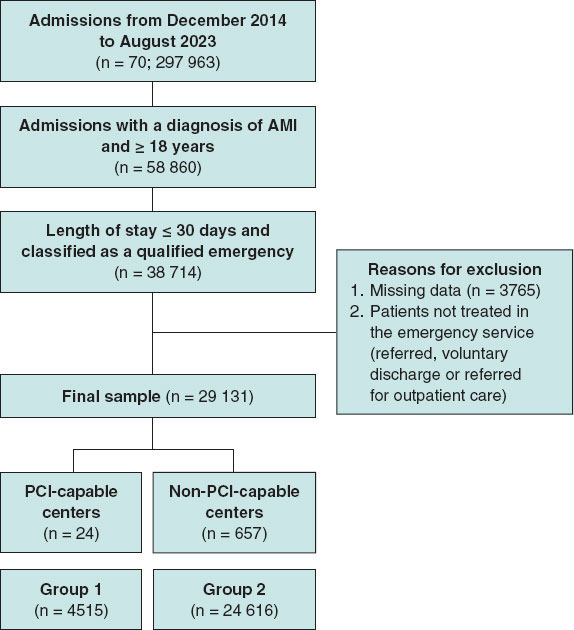
Figure 1. Flowchart of filtering process. Patients with AMI (ICD-10 I21.0-I21.9), aged ≥ 18 years, hospitalized ≤ 30 days in Ministry of Health (SSA) emergency services were included. Cases with incomplete data or not treated in emergency care were excluded. The final cohort was categorized as PCI-capable and non-PCI-capable centers, identified through percutaneous coronary intervention or cardiac catheterization records. AMI, acute myocardial infarction; PCI, percutaneous coronary intervention.
Association between out-of-hours care and the risk of death
Univariate analysis showed that the presence of a cath lab was protective (HR, 0.22; 95%CI, 0.204-0.246). Out-of-hours, overnight shifts, weekend admissions, and the COVID-19 pandemic were associated with higher mortality in both groups. The risks from out-of-hours (HR, 1.46; 95%CI, 1.21-1.73 vs HR, 1.18; 95%CI, 1.11-1.23) and night-shift admissions (HR, 1.18; 95%CI, 1.12-1.23 vs HR, 1.13; 95%CI, 1.08-1.18) were more pronounced in PCI-capable centers, and holidays increased mortality only in non-PCI-capable centers (HR, 1.21; 95%CI, 1.03-1.41) (figure 2). Among cath lab characteristics, higher mean procedure volume (HR, 0.888; 95%CI, 0.840-0.938), greater staffing (HR, 0.800; 95%CI, 0.760-0.842), and availability during afternoon (HR, 0.515; 95%CI, 0.427-0.620), night (HR, 0.290; 95%CI, 0.233-0.361), and weekend shifts (HR, 0.317; 95%CI, 0.264-0.380) were associated with reduced mortality.
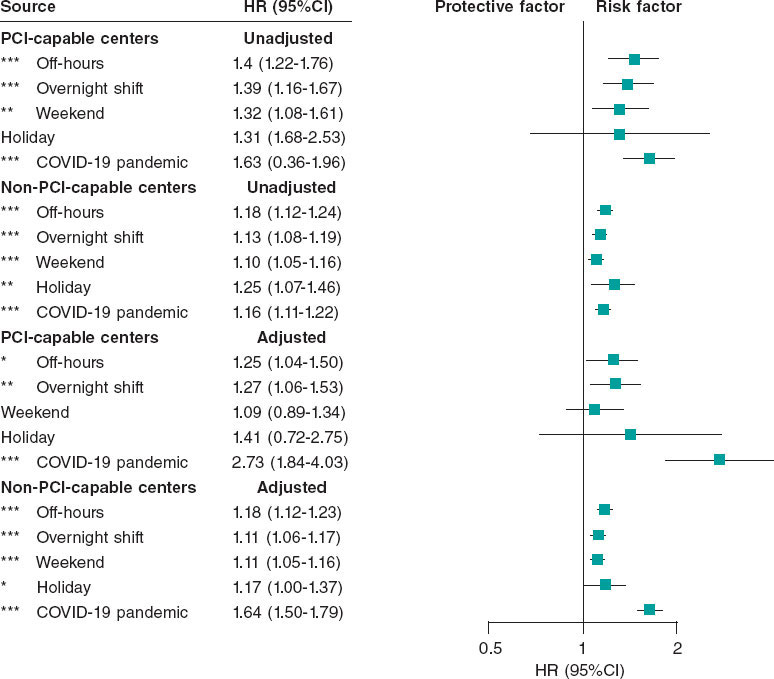
Figure 2. Mortality risk associated with out-of-hours admissions for acute myocardial infarction: analysis stratified by hospital type (PCI-capable and non-PCI- capable centers). The graph shows results from unadjusted and adjusted Cox regression models. Adjustments included age, sex, emergency type, bed type, region, year of admission, AMI type, and COVID-19 period. For PCI-capable centers, additional adjustments were made for cath lab availability by shift, angiography type, and number of specialists per shift. Points represent mortality HRs with horizontal lines for 95%CI. Results are shown separately for PCI-capable and non-PCI-capable centers. HR > 1 indicates increased mortality risk; HR < 1 indicates reduced risk. *P < .05; ** P < .01; *** P < .001. 95%CI, 95% confidence interval; COVID-19, coronavirus disease 2019; HR, hazard ratio; PCI, percutaneous coronary intervention.
After adjusting for potential confounders, the presence of a cath lab remained protective (HR, 0.25; 95%CI, 0.23-0.28), whereas out-of-hours, night-shift admissions, and the COVID-19 pandemic continued to be associated with increased mortality in both groups. Adjusted mortality risk from out-of-hours (HR, 1.25; 95%CI, 1.04-1.50 vs HR, 1.18; 95%CI, 1.12-1.23) and night-shift admissions (HR, 1.27; 95%CI, 1.06-1.53 vs HR, 1.11; 95%CI, 1.06-1.17) persisted, while weekend and holiday effects remained significant only in non-PCI-capable centers (figure 2). Regarding cath lab characteristics, only nightshift availability (HR, 0.149; 95%CI, 0.089-0.248) and mean health care staff per shift (HR, 0.770; 95%CI, 0.710-0.834) were significantly associated with reduced mortality. Kaplan-Meier survival curves are shown in figure 3.
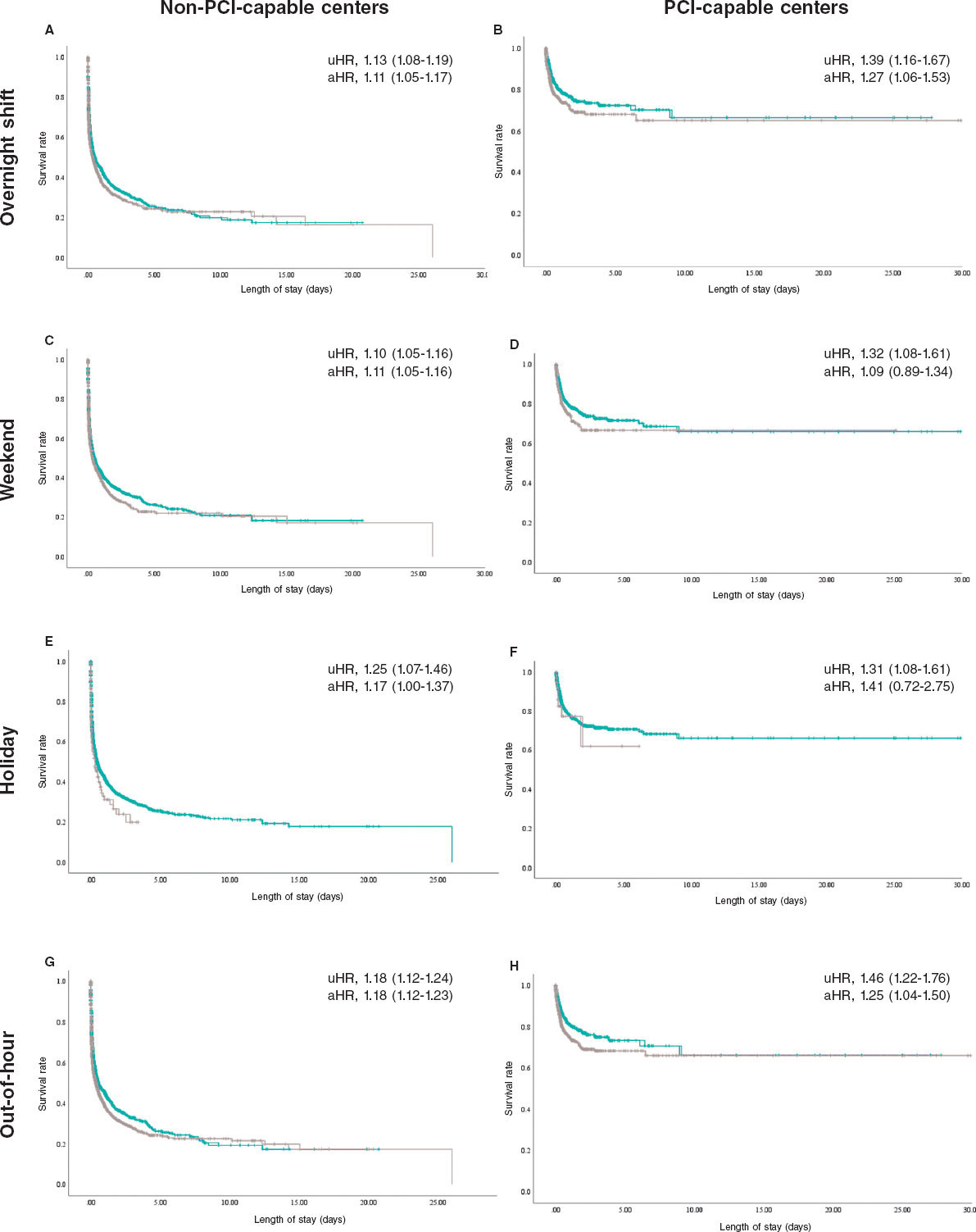
Figure 3. Kaplan–Meier analysis of mortality of out-of-hours acute myocardial infarctions by hospital type. Each column represents cath lab availability, while each row represents the components of out-of-hours admission. Each panel (A-H) shows the unadjusted (uHR) and adjusted mortality risk (aHR) with corresponding 95% confidence intervals.
DISCUSSION
Major findings
This nationwide study examined AMI admissions across a large number of Mexican hospitals, comparing PCI-capable and non- PCI-capable centers. Out-of-hours admissions were common and associated with higher mortality, longer lengths of stay, greater shock bed use, and a higher proportion of male patients. While the presence of a cath lab appeared generally protective after adjustment, out-of-hours and nightshift admissions showed a modest increase in mortality in both groups. Although weekend and holiday effects were more evident in non-PCI-capable centers, these results should be interpreted with caution given the absence of detailed clinical data, including reperfusion strategies, ST-segment elevation myocardial infarction/non-ST-segment elevation myocardial infarction (STEMI/NSTEMI) classification, and comorbidities.
A key finding is that PCI-capable centers did not fully eliminate excess mortality during out-of-hours periods. Advanced interventional capabilities may mitigate some risk during weekends and holidays; however, challenges remain during overnight shifts, as only 32.2% of studied centers offered cath lab availability during overnight shifts. Out-of-hours admissions were associated with fewer procedures, lower staffing, and limited specialized personnel compared with regular hours, which is consistent with former studies4,8,9 (table S4). Additional factors likely contributing to poorer outcomes include reduced staffing, delays in care, limited access to specialized personnel, and the impact of human factors such as fatigue and sleep deprivation during nightshifts, despite the availability of advanced cardiac interventions.4,8,9 Longer door-to-balloon times, limited 24/7 cath lab coverage, and the relatively low number of cath labs per capita in Mexico further exacerbate these challenges. Moreover, the increased out-of-hours risk from PCI-capable centers may reflect their role as referral centers, where longer patient arrival times and procedural delays are more frequent.16
Pre-hospital and COVID-19 challenges in the management of AMI
Several institutions in Mexico have implemented programs to standardize the management of AMI, such as the IMSS and SOCIME national “Code Infarction” (2015) and ISSSTE “AsISSSTE Infarto” (2018).23,24 In contrast, SSA-affiliated institutions (Seguro Popular/INSABI/IMSS-BIENESTAR) follow more varied protocols, leading to disparities in care. Consequently, heterogeneity in the management of AMI management across institutions likely exacerbates challenges in pre-hospital care. Regulated, by NOM-034-SSA3-2013 and coordinated via Medical Emergency Regulatory Centers,25 the pre-hospital system faces challenges such as poor inter-institutional coordination, insufficient ambulance equipment, and personnel shortages, especially in rural areas. These factors increase response times and complicate AMI emergency management.26 Former studies report prolonged door-to-balloon times (up to 648 minutes) due to traffic, fragmented health care, and delayed diagnosis, with weekend and nighttime admissions independently predicting treatment delays over 12 hours. While this study does not examine treatment or pre-hospital delays, other research highlights these as major issues in Mexico. Araiza-Garaygordobil et al.16 reported a mean door-to-balloon time of 648 minutes in a Mexico City referral hospital, 3 times longer compared with developed countries, due to traffic, health care fragmentation and delayed primary diagnosis. Baños-González et al.15 found that patient delays, often from symptom unawareness or limited resources, led to late arrivals (> 12 hours), with 60% being transported by ambulance. Weekend and nighttime admissions independently predicted delays > 12 hours, with a mean 11-hour wait for first medical contact.
The COVID-19 pandemic was associated with an increased AMI mortality in both hospital groups, with a more pronounced effect in PCI-capable centers. Globally, the pandemic disrupted health care delivery, reducing hospitalizations and PCI procedures, partly due to lower referral rates and patients’ reluctance to seek care over concerns of viral exposure. Consequently, delays from symptom onset to first medical contact and prolonged door-to-balloon times occurred, both of which are known to adversely affect AMI outcomes.27 In Mexico, Rodríguez-González et al.28 reported a 51% decline in STEMI diagnoses in hospitals during early 2020, accompanied by longer arrival times and a 4.9%-6.8% increase in STEMI-related mortality. In other countries, such as Spain, PCI rates decreased by 10.1% during 2020.29 A recent meta-analysis reported a significant reduction in the number of PCI (IRR, 0.72; 95%CI, 0.67-0.77), I² = 92.5%) and an increase in time from symptom onset to first medical contact by a mean 69.4 minutes ([11-127], I² = 99.4%) during the COVID-19 pandemic. However, no significant change was observed in door-to-balloon times (3.33 minutes [0.32-6.98]; I² = 94.2%).30
Although detailed clinical data were not available in our study, the impact of treatment on AMI mortality has been extensively investigated in Mexico. The nationwide RENASCA cohort31 (21 826 patients, 2014–2017) reported high prevalence of diabetes (48%), hypertension (60.5%), smoking (46.8%), dyslipidemia (35.3%), and metabolic syndrome (39.1%) relative to multinational registries.31,32 Patients with STEMI (14.9%) experienced higher cardiovascular mortality vs patients with NSTEMI (7.6%), reflecting referral patterns and more severe in-hospital complications, including cardiogenic shock and arrhythmias. Implementation of the IMSS “Code Infarction” program reduced patients without reperfusion from 65.2% to 28.6%, increased fibrinolysis to 40.1%, and PCI to 31.3%.33 By comparison, Spain’s well-structured “Code Infarction” protocol achieved a primary PCI rate of 87%, median door-to-balloon time of 193 minutes, and minimal pre-hospital delays, with 55.3% of patients receiving timely care and most remaining delays attributable to initial diagnosis (18.5%).34
Global trends in out-of-hours mortality: a comparison across regions
Compared with previous meta-analyses, which often lack data from developing regions such as Latin America, our findings highlight important disparities. Sorita et al.4 reported higher short-term mortality rates and delayed PCI in patients with STEMI admitted outside regular hours, especially outside North America. Wang et al.5 found a slight increase in short-term mortality but no effect on long-term outcomes or PCI risk. Other analyses showed no significant differences in mortality or door-to-balloon times.35 Studies from developing countries such as Indonesia36,37 reported no mortality differences by admission time, while in Iraq,38 resource shortages and conflict led to higher out-of-hours mortality and limited reperfusion access. Latin American studies from Brazil39-41 and Argentina42 generally found no outcome differences by admission timing. Differences with our results are likely due to hospital infrastructure and staffing; Mexican public hospitals face substantial limitations after hours vs tertiary referral centers with 24/7 cath lab availability. Additionally, socioeconomic barriers and low awareness of AMI symptoms delay care, and the lack of standardized protocols further increases mortality risk.
Strategies to improve the out-of-hours management of AMI
The findings highlight several areas for improvement within the Mexican health care system, particularly regarding non-PCI-capable centers. All 3 components were associated with higher mortality, underscoring the need for enhanced pre-hospital and in-hospital management. Efforts should focus on early diagnosis, including increased availability of electrocardiography and the implementation of telemedicine platforms to allow cardiologists to promptly assess and guide treatment. Expanding access to thrombolytic therapy and ensuring experienced personnel are available during out-of-hours shifts can help reduce treatment delays. Furthermore, improving coordination across health centers through clear referral pathways, effective communication strategies, and standardized protocols, especially in hospitals serving uninsured patients, can streamline care and reduce variability in management. In PCI- capable centers, mortality was particularly elevated during overnight shifts, likely due to reduced cath lab availability within these hours. Despite existing protocols and optimized door-to-balloon times, strengthening nighttime staffing and training remains essential. This may include targeted training and incentives to encourage health care professionals to work during these shifts, thereby ensuring that high-quality care is maintained at all times.
Strengths and limitations
The present study is one of the largest cohorts to date examining the association between AMI-related mortality and time of presentation in Mexico. It provides valuable insights into the potential impact of out-of-hours admissions on outcomes, underscoring the importance of optimizing staffing, patient flow, and timely access to interventions such as PCI. Nevertheless, the findings should be interpreted with caution. The retrospective design, absence of detailed treatment information (eg, PCI, thrombolysis, door-to-balloon times), lack of comorbidity data, and inability to differentiate between STEMI and NSTEMI limit the depth and precision of the analyses. Furthermore, the study does not capture key factors such as patient-level delays, cath lab utilization patterns, or disparities in infrastructure and resource availability. Future research should integrate more comprehensive clinical and procedural data and explore the influence of provider fatigue, variability in care standards, and treatment delays to better delineate gaps in care.
CONCLUSIONS
Out-of-hours presentations were associated with high mortality rates, and contrary to findings in developed countries, the presence of a cath lab did not improve out-of-hours outcomes, which suggests that factors beyond cath lab availability, including systemic inefficiencies, resource limitations, and health infrastructure deficiencies, may profoundly affect patient outcomes in Mexico.
FUNDING
None declared.
ETHICAL CONSIDERATIONS
Ethical approval for this study was granted by the Ethics Committee of the Women’s Hospital, SSA (Approval No. 202403-47). The research used publicly available emergency income data published by the DGIS, for which subject confidentiality is governed by the Mexican standard NOM-012-SSA3-2012. In accordance with the General Health Research Law, the study is classified as risk-free research. Sex variable was defined as according to the definition of the Sex and Gender Equity in Research (SAGER) guidelines (table S1).
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
No generative artificial intelligence (AI) tools were used in the conception, design, data collection and analysis of this manuscript. All content was produced entirely by the authors.
AUTHORS’ CONTRIBUTIONS
D. Arriaga-Izabal contributed to the conceptualization, methodology, data curation, data analysis, investigation, and writing of the original draft. F. Morales-Lazcano was responsible for investigation, data curation, and drafting the original manuscript. A. Canizalez-Román provided supervision, project administration, and validation, and participated in the review and editing of the manuscript. All authors read and approved the final version of the manuscript.
CONFLICTS OF INTEREST
None declared.
WHAT IS KNOWN ABOUT THE TOPIC?
- Out-of-hours admissions (overnight shifts, weekends, holidays) are associated with higher AMI mortality, primarily due to treatment delays and reduced specialist availability.
WHAT DOES THIS STUDY ADD?
- Out-of-hours admissions in Mexican hospitals were associated with higher 30-day mortality rates, irrespective of cath lab availability. Although cath labs were generally protective, they did not fully mitigate risk during nights, weekends, or holidays, with increased mortality mainly observed in non-PCI-capable centers. The study is limited by the absence of detailed clinical data, including AMI type (STEMI/NSTEMI), reperfusion strategy, door-to-balloon times, and comorbidities, restricting conclusions regarding the precise impact of specialized care. Systemic factors, including staffing limitations and regional disparities, likely contribute to these outcomes, underscoring the need for improved out-of-hours AMI care.
REFERENCES
1. Ogita M, Suwa S, Ebina H, et al. Off-hours presentation does not affect in-hospital mortality of Japanese patients with acute myocardial infarction:J-MINUET substudy. J Cardiol. 2017;70:553-558.
2. Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 2010:a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380: 2095-2128.
3. Organization for Economic Co-operation and Development (OECD). Health at a Glance 2023:OECD Indicators. 2023. Available at: https://doi.org/ 10.1787/7a7afb35-en. Accessed 25 Jan 2025.
4. Sorita A, Ahmed A, Starr SR, et al. Off-hour presentation and outcomes in patients with acute myocardial infarction:systematic review and meta-analysis. BMJ. 2014;348:7393.
5. Wang B, Zhang Y, Wang X, Hu T, Li J, Geng J. Off-hours presentation is associated with short-term mortality but not with long-term mortality in patients with ST-segment elevation myocardial infarction:A meta-analysis. PLOS ONE. 2017;12:0189572.
6. Yu YY, Zhao BW, Ma L, Dai XC. Association Between Out-of-Hour Admission and Short- and Long-Term Mortality in Acute Myocardial Infarction:A Systematic Review and Meta-Analysis. Front Cardiovasc Med. 2021;8 752675.
7. Magid DJ, Wang Y, Herrin J, et al. Relationship between time of day, day of week, timeliness of reperfusion, and in-hospital mortality for patients with acute ST-segment elevation myocardial infarction. JAMA. 2005;294:803-812.
8. Needleman J, Buerhaus P, Pankratz VS, Leibson CL, Stevens SR, Harris M. Nurse staffing and inpatient hospital mortality. N Engl J Med. 2011;364:1037-1045.
9. Musy SN, Endrich O, Leichtle AB, Griffiths P, Nakas CT, Simon M. The association between nurse staffing and inpatient mortality:A shift-level retrospective longitudinal study. Int J Nurs Stud. 2021;120:103950.
10. Albuquerque GO de, Szuster E, Corrêa LCT, et al. Análise dos resultados do atendimento ao paciente com infarto agudo do miocárdio com supradesnivelamento do segmento ST nos períodos diurno e noturno. Rev Bras Cardiol Invasiva. 2009;17:52-57.
11. Cardoso O, Quadros A, Voltolini I, et al. Angioplastia Primária no Infarto Agudo do Miocárdio:Existe Diferença de Resultados entre as Angioplastias Realizadas Dentro e Fora do Horário de Rotina?Rev Bras Cardiol Invasiva. 2010;18.
12. Pérez-Cuevas R, Contreras-Sánchez SE, Doubova SV, et al. Gaps between supply and demand of acute myocardial infarction treatment in Mexico. Salud Pública México. 2020;62:540-549.
13. Robledo Z. La transformación del sistema de salud mexicano. Salud Pública México. 2024;66:767-773.
14. Gómez Dantés O, Sesma S, Becerril VM, Knaul FM, Arreola H, Frenk J. Sistema de salud de México. Salud Publica Mex. 2011;53 Suppl 2:s220-32.
15. Baños-González MA, Henne-Otero OL, Torres-Hernández ME, et al. Factores asociados con retraso en la terapia de reperfusión en infarto agudo de miocardio con elevación del segmento ST (IMCEST) en un hospital del sureste mexicano. Gac Médica México. 2016;152:495-502.
16. Araiza-Garaygordobil D, González-Pacheco H, Sierra-Fernández C, et al. Pre-hospital delay of patients with ST-elevation myocardial infarction in Mexico City. Arch Cardiol Mex. 2019;89:174-176.
17. Cuschieri S. The STROBE guidelines. Saudi J Anaesth. 2019;13(Suppl 1): S31-S34.
18. Dirección General de información en Salud [DGIS]. Datos Abiertos de Urgencias. 2023. Available at: https://rb.gy/lb0tw. Accessed 25 Jan 2025.
19. Procuraduría Federal de la Defensa del Trabajo. ¿Sabes Cuáles Son Los Días de Descanso Obligatorio Para Este 2024?. 2023. Available at: https://www.gob.mx/profedet/articulos/sabes-cuales-son-los-dias-de-descanso-obligatorio-para-este-2024. Accessed 25 Jan 2025.
20. Secretaría de Salud. México pone fin a la emergencia sanitaria por COVID-19. 2023. Available at: https://www.gob.mx/salud/prensa/mexico-pone-fin-a-la-emergencia-sanitaria-por-covid-19-secretaria-de-salud. Accessed 25 Jan 2025.
21. Diario Oficial de la Federación [DOF]. NORMA Oficial Mexicana NOM-012-SSA3-2012, Que establece los criterios para la ejecución de proyectos de investigación para la salud en seres humanos. 2013. Available at: https://platiica.economia.gob.mx/normalizacion/nom-012-ssa3-2012/. Accessed 25 Jan 2025.
22. Diario Oficial de la Federación [DOF]. NORMA Oficial Mexicana NOM-012-SSA3-2012, Que establece los criterios para la ejecución de proyectos de investigación para la salud en seres humanos. 2013. Available at: https://platiica.economia.gob.mx/normalizacion/nom-012-ssa3-2012/. Accessed 25 Jan 2025.
23. Robledo-Aburto ZA, Duque-Molina C, Lara-Saldaña GJ, Borrayo-Sánchez G, Avilés-Hernández R, Reyna-Sevilla A. Protocolo de atención Código Infarto, hacia la federalización de IMSS-Bienestar. Rev Médica Inst Mex Seguro Soc. 2022;60(Suppl 2):S49-S53.
24. Institute for Social Security and Services for State Workers or Civil Service Social Security and Services Institute (ISSSTE). Con Asissste Infarto disminuye mortalidad por problemas cardiovasculares. .mx. Available at: https://www.gob.mx/issste/prensa/con-asissste-infarto-disminuye-mortalidad-por-problemas-cardiovasculares. Accessed 21 Jan, 2025.
25. Diario Oficial de la Federación [DOF]. NOM-034-SSA3-2013, Regulación de los servicios de salud. Atención médica prehospitalaria. 2013. Available at: https://platiica.economia.gob.mx/normalizacion/nom-034-ssa3-2013/. Accessed 26 2025 Jan.
26. Ministry of Health (SSA). Modelo de Atención Médica Prehospitalaria. 2017. Available at: https://www.gob.mx/cms/uploads/attachment/file/250824/MODELO_DE_ATENCION_MEDICA_PREHOSPITALARIA.pdf. Accessed 21 2025 Jan
27. Sharma A, Razuk V, Nicolas J, Beerkens F, Dangas GD. Two years into the COVID-19 pandemic:implications for the cardiac catheterization laboratory and its current practices. J Transcat Interv. 2022;30 eA20220003.
28. Rodríguez-González EF, Briceño-Gómez EE, Gómez-Cruz EJ, Rivas-Hernández Z, Chacón-Sánchez J, Cabrera-Rayo A. ¿A dónde se fueron los infartos de miocardio durante la pandemia por COVID-19 en México?Med Interna México. 2023;39:538-540.
29. Romaguera R, Ojeda S, Cruz-González I, Moreno R. Spanish Cardiac Catheterization and Coronary Intervention Registry. 30th Official Report of the Interventional Cardiology Association of the Spanish Society of Cardiology (1990-2020) in the year of the COVID-19 pandemic. Rev Esp Cardiol. 2021;74:1095-1105.
30. Nadarajah R, Wu J, Hurdus B, et al. The collateral damage of COVID-19 to cardiovascular services:a meta-analysis. Eur Heart J. 2022;43: 3164-3178.
31. Borrayo-Sánchez G, Rosas-Peralta M, Ramírez-Arias E, et al. STEMI and NSTEMI:Real-world Study in Mexico (RENASCA). Arch Med Res. 2018; 49:609-619.
32. Tang EW, Wong CK, Herbison P. Global Registry of Acute Coronary Events (GRACE) hospital discharge risk score accurately predicts long-term mortality post acute coronary syndrome. Am Heart J. 2007;153:29-35.
33. Martinez-Sanchez C, Borrayo G, Carrillo J, Juarez U, Quintanilla J, Jerjes-Sanchez C. Clinical management and hospital outcomes of acute coronary syndrome patients in Mexico:The Third National Registry of Acute Coronary Syndromes (RENASICA III). Arch Cardiol México. 2016;86:221-232.
34. Rodríguez-Leor O, Cid-Álvarez AB, Pérez de Prado A, et al. Analysis of the management of ST-segment elevation myocardial infarction in Spain. Results from the ACI-SEC Infarction Code Registry. Rev Esp Cardiol. 2022; 75:669-680.
35. Wang X, Yan J, Su Q, Sun Y, Yang H, Li L. Is there an association between time of admission and in-hospital mortality in patients with non-ST-elevation myocardial infarction?A meta-analysis. Sci Rep. 2015;5:14409.
36. Dharma S, Dakota I, Sukmawan R, Andriantoro H, Siswanto BB, Rao SV. Two-year mortality of primary angioplasty for acute myocardial infarction during regular working hours versus off-hours. Cardiovasc Revascularization Med Mol Interv. 2018;19(7 Pt B):826-830.
37. Javanshir E, Ramandi ED, Ghaffari S, et al. Association Between Off-hour Presentations and In-hospital Mortality for Patients with Acute ST-Elevation Myocardial Infarction Treated with Primary Percutaneous Coronary Intervention. J Saudi Heart Assoc. 2020;32:242-247.
38. Al-Asadi JN, Kadhim FN. Day of admission and risk of myocardial infarction mortality in a cardiac care unit in Basrah, Iraq. Niger J Clin Pract. 2014;17:579-584.
39. Machado GP, Araujo GN de, Mariani S, et al. On- vs. -hours admission of patients with ST-elevation acute myocardial infarction undergoing percutaneous coronary interventions:data from a tertiary university brazilian hospital. Clin Biomed Res. 2018;38:30-34.
40. Evangelista PA, Barreto SM, Guerra HL. Hospital admission and hospital death associated to ischemic heart diseases at the National Health System (SUS). Arq Bras Cardiol. 2008;90:130-138.
41. Barbosa R, Cesar F, Bayerl D, et al. Acute Myocardial Infarction and Primary Percutaneous Coronary Intervention at Night Time. Int J Cardiovasc Sci. 2018;2018;31:513-519.
42. Rosende A, Mariani JA, Abreu MD, Gagliardi JA, Doval HC, Tajer CD. Distribución de la frecuencia de síndrome coronario agudo acorde al día de la semana. Análisis del Registro Epi-Cardio. Rev Argent Cardiol. 2015; 83:1-10.
ABSTRACT
Introduction and objectives: To evaluate the impact of bleeding on the risk-benefit balance of coronary revascularization prior to transfemoral transcatheter aortic valve implantation (TF-TAVI).
Methods: We conducted a retrospective analysis of the patients who underwent TF-TAVI at our center between 2008 and 2018 to evalute the management of coronary artery disease (percutaneous revascularization vs no revascularization). Subsequently, the rate of major bleeding —defined according to the Bleeding Academic Research Consortium (BARC) criteria (type 3-5)— and major adverse cardiovascular events (MACE) was compared between the 2 groups over a mean 60-month follow-up period.
Results: A total of 379 patients were included. The overall rate of major bleeding was 21.4%, higher in revascularized patients but without reaching statistical significance. The rate of major bleeding between coronary angiography and TF-TAVI implantation was 5.5% and significantly higher in revascularized patients (12.0% vs 3.5%; P = .07). During the hospitalization for TF-TAVI and throughout follow-up, the rate of major bleeding was 6.1% and 9.6%, respectively, with no significant inter-group differences. There were no significant differences either in the 5-year rate of MACE.
Conclusions: In our patient cohort, pre-TF-TAVI preoperative coronary revascularization was associated with an initially higher bleeding risk; however, no statistically significant differences were observed in major bleeding or MACE at the 5-year follow-up. These findings support the need to generate high-quality clinical evidence to demonstrate the net clinical benefit of coronary revascularization in this context.
Keywords: Transcatheter aortic valve implantation. Coronary revascularization. Bleeding.
RESUMEN
Introducción y objetivos: Valorar el impacto del sangrado en la relación riesgo-beneficio de la revascularización coronaria previa al implante percutáneo de válvula aórtica por vía transfemoral (TAVI-TF).
Métodos: Se realizó un análisis retrospectivo de los pacientes tratados con TAVI-TF en nuestro centro entre los años 2008 y 2018, y se identificó la actuación sobre su enfermedad coronaria (revascularización percutánea frente a no revascularización). Posteriormente, se comparó entre ambos grupos la incidencia de sangrado mayor, definido por los criterios del Bleeding Academic Research Consortium (BARC) (tipos 3-5), y de eventos cardiovasculares adversos mayores (MACE) isquémicos durante un seguimiento medio de 60 meses.
Resultados: Se incluyeron 379 pacientes. La incidencia total de sangrado mayor fue del 21,4%, más alta en los pacientes con revascularización, pero sin alcanzar la significación estadística. La incidencia global de sangrado mayor entre la coronariografía diagnóstica y el TAVI fue del 5,5%, y resultó significativamente más alta en los pacientes revascularizados (12,0% frente a 3,5%; p = 0,07). Durante el ingreso para el TAVI-TF y el seguimiento posterior de 60 meses, la incidencia global de sangrado mayor fue del 6,1% y del 9,6%, respectivamente, sin diferencias significativas entre ambos grupos. Tampoco hubo diferencias en la incidencia de MACE a los 5 años de seguimiento.
Conclusiones: En nuestra cohorte de pacientes, la revascularización coronaria previa al TAVI-TF conlleva un aumento inicial del riesgo de sangrado, sin diferencias estadísticamente significativas en sangrado mayor ni en MACE en el seguimiento a 5 años. Estos hallazgos apoyan la necesidad de generar una evidencia clínica de calidad que demuestre un beneficio clínico neto de la revascularización en este contexto.
Palabras clave: Implante percutáneo de válvula aórtica. Revascularización coronaria. Sangrados.
Abreviaturas
MACE: major adverse cardiovascular events. TF-TAVI: transfemoral transcatheter aortic valve implantation.
INTRODUCTION
Currently, transfemoral transcatheter aortic valve implantation (TF-TAVI) is the treatment of choice for most patients with severe aortic stenosis, particularly those with high surgical risk or advanced age.1 Several clinical trials have demonstrated comparable clinical outcomes between TF-TAVI and surgical aortic valve replacement.2-4 Major and minor bleeding remain one of the most frequent procedural complications and are associated with higher morbidity and mortality rates.5 Although, in recent years, improvements in materials (reduction in caliber required for valve implantation) and increasing operator experience have substantially reduced perioperative bleeding rates, such rates remain significantly high. One of the main risk factors for bleeding is the requirement for perioperative dual antiplatelet therapy,6 most widely necessary when coronary revascularization is performed along with TAVI.
In addition, the high prevalence of coronary artery disease in patients undergoing TF-TAVI, reported in up to 80% of cases in published series, along with current clinical practice guideline recommendations to revascularize all ≥ 70% proximal coronary stenoses, results in a high rate of revascularization.1
Our group recently published data showing that systematic, complete revascularization in patients undergoing TF-TAVI does not provide prognostic benefit in terms of mortality or major adverse cardiovascular events (MACE) (a composite of death, myocardial infarction, stroke, and heart failure-related hospitalization.)7 Given the high rate of bleeding events in these patients, it is of substantial clinical interest to evaluate whether revascularization may confer an increased bleeding risk and assess its clinical impact.6
METHODS
We conducted a retrospective study based on the historical cohort of patients who underwent TF-TAVI at our center from 2008 through 2018. Study information was drawn from the local database (Géminis) and supplemented by electronic health record review to document follow-up events. The study was approved by the Clinical Research Ethics Committee of A Coruña-Ferrol (Spain). The primary endpoint of the study was to compare the rate of major bleeding—defined according to the Bleeding Academic Research Consortium (BARC) criteria (types 3–5)—occurring after diagnostic coronary angiography, during the index hospitalization for TAVI, and during follow-up. Additional endpoints included the rate of MACE and the composite endpoint of MACE plus major bleeding over the same period of time, comparing patients who underwent percutaneous coronary revascularization with those managed conservatively.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation, and qualitative variables as proportions. We used the Student t test and analysis of variance (ANOVA) with first-order polynomial contrast for continuous variables. For categorical variables, we used the chi-square test for linear trend or Fisher’s exact test as appropriate.
We conducted survival analyses using the Cox proportional hazards model to determine whether an association existed between coronary revascularization and patient prognosis in terms of mortality, MACE, major bleeding (BARC 3–5), and a composite of MACE and major bleeding. Results were expressed as age- and sex-adjusted survival curves.
Statistical analysis was performed with SPSS 26.0 (IBM, USA) and R version 4.1.3 (R Foundation for Statistical Computing, Austria). Statistical significance was set at P < .05 for all comparisons.
RESULTS
A total of 379 patients who underwent TF-TAVI between 2008 and 2018 were included. Four patients were lost to follow-up, leaving 375 patients for the statistical analysis. Table 1 illustrates the patients’ baseline characteristics, with a mean age of 83.1 years and predominance of the female sex and intermediate surgical risk (Society of Thoracic Surgeons score, 4.3%). Although most baseline characteristics were well balanced between patients with and without revascularization, the latter had a slightly higher surgical risk (4.5% vs 3.5%) and a higher proportion of women (61.3% vs 47.8%).
Table 1. Baseline characteristics of the patients
| Variable | Nonrevascularized | Revascularized | Total | P |
|---|---|---|---|---|
| Age, years | 84 (5.5) | 82 (6.7) | 83.1 (5.9) | .015 |
| Female sex | 176 (61.3%) | 44 (47.8%) | 220 (58.0%) | .022 |
| Diabetes | 83 (28.9%) | 36 (39.1%) | 119 (31.4%) | .066 |
| Hypertension | 219 (76.3%) | 73 (79.3%) | 292 (77.0%) | .546 |
| Hypercholesterolemia | 165 (57.5%) | 65 (70.7%) | 230 (60.6%) | .025 |
| Body mass index | 29.6 (5.2) | 28.3 (5.5) | 29.3 (5.3) | .032 |
| Baseline hemoglobin (mg/dL) | 12.1 (1.6) | 11.8 (1.8) | 12.1 (1.7) | .160 |
| Creatinine clearance (mL/min) | 55.7 (20.9) | 53.6 (21.4) | 55.2 (21.0) | .420 |
| STS score | 4.5% (2.5) | 3.5% (3.8) | 4.3% (2.9) | .002 |
| EuroSCORE I | 13.3% (7.8) | 8.6% (5.2) | 12.2% (7.5) | .001 |
| EuroSCORE II | 4.4% (3.4) | 2.6% (1.9) | 4.0% (3.2) | .001 |
| Baseline LVEF | 59.7% (13.2) | 56.9% (13.5) | 59.0% (13.3) | .083 |
| Baseline Max PG (mmHg) | 80.6 (25.0) | 79.1 (22.2) | 80.3 (24.4) | .602 |
| Baseline Mean PG (mmHg) | 47.1 (15.4) | 47.2 (14.0) | 47.1 (15.0) | .952 |
| Baseline aortic regurgitation | .384 | |||
| Grade 0 | 69 (24.2%) | 23 (25.6%) | 92 (24.3%) | |
| Grade 1 | 151 (53.0%) | 54 (60.0%) | 205 (54.0%) | |
| Grade 2 | 46 (16.1%) | 11 (12.2%) | 57 (15.0%) | |
| Grade 3 | 15 (5.3%) | 1 (1.1%) | 16 (4.2%) | |
| Grade 4 | 4 (1.4%) | 1 (1.1%) | 5 (1.3%) | |
| Angina symptoms | 45 (15.7%) | 23 (25.0%) | 68 (17.9%) | .043 |
| NYHA class | .278 | |||
| 0 | 1 (0.3%) | 1 (1.1%) | 2 (0.5%) | |
| 1 | 41 (14.3%) | 19 (20.7%) | 60 (15.8%) | |
| 3 | 221 (77.0%) | 62 (67.4%) | 283 (74.7%) | |
| 4 | 24 (8.4%) | 10 (10.9%) | 34 (8.9%) | |
| Prior AMI | 32 (11.3%) | 21 (23.1%) | 53 (14.0%) | .005 |
| Prior CABG | 18 (6.3%) | 6 (6.6%) | 24 (6.3%) | .931 |
| Prior PCI | 24 (8.5%) | 75 (82.4%) | 99 (26.1%) | .001 |
| Stroke | 26 (9.2%) | 8 (8.9%) | 34 (9.0%) | .932 |
| Liver disease | 8 (2.8%) | 1 (1.1%) | 9 (2.4%) | .351 |
| COPD | 39 (13.7%) | 7 (7.7%) | 46 (12.1%) | .126 |
| Peripheral arterial disease | 9 (3.1%) | 5 (5.4%) | 14 (3.7%) | .309 |
| VKA therapy | 83 (29.2%) | 21 (23.1%) | 104 (27.4%) | .254 |
| DOAC therapy | 11 (3.9%) | 3 (3.3%) | 14 (3.7%) | .801 |
|
AMI, myocardial infarction; CABG, coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; DOAC, direct oral anticoagulants; LVEF, left ventricular ejection fraction; Max PG, maximum pressure gradient; Mean PG, mean pressure gradient; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; STS, Society of Thoracic Surgeons; VKA, vitamin K antagonist. |
||||
Regarding symptoms, most patients were in New York Heart Association functional class III (74.7%). Among revascularized patients, 25.0% (23 patients) reported angina symptoms vs only 15.7% (41 patients) from the nonrevascularized group. Regarding anticoagulant therapy, 29.2% (83 patients) from the nonrevascularized group were on vitamin K antagonists and 3.9% (11 patients) on direct oral anticoagulants. These rates were slightly lower among revascularized patients, with 23.1% (21 patients) on vitamin K antagonists and 3.3% (3 patients) on direct oral anticoagulants (table 1).
Percutaneous revascularization and bleeding
Of the patients undergoing TF-TAVI, 92 (24.3%) underwent coronary revascularization, which in our center was always performed before valve replacement, with a median interval of 88 days (46–162) between revascularization and TAVI. Consequently, most revascularized patients were on dual antiplatelet therapy when they underwent TAVI. The decision to revascularize was made in a multidisciplinary meeting according to contemporary clinical practice guidelines (2008–2018). The prevailing strategy was to revascularize most coronary lesions, and the clinical criterion remained unchanged throughout the study period.
Clinical outcomes during follow-up are shown in table 2. There were no statistically significant differences in the time elapsed between diagnostic catheterization and TAVI between the 2 groups (median of 118 days for revascularized patients and 123 days for the nonrevascularized ones; P = .835). Figure 1 illustrates that the overall rate of major bleeding was 21.4% (81/375), with a rate of 28.3% (26/92) for revascularized patients and 19.0% (55/283) for the nonrevascularized ones, without reaching statistical significance (P = .074).
Table 2. Clinical outcomes during follow-up according to revascularization status
| Clinical outcome | Nonrevascularized n (%) | Revascularized n (%) | Total, n (%) | P |
|---|---|---|---|---|
| Major bleeding (BARC 3-5) | 55 (19.0%) | 26 (28.3%) | 81 (21.4%) | .074 |
| MACE at 60 months | 160 (55.7%) | 49 (53.2%) | 209 (55.1%) | .082 |
| Pre-TAVI MACE | 58 (20.4%) | 25 (27.2%) | 83 (22.1%) | NS |
| Mortality at 60 months | 125 (43.7%) | 36 (39.0%) | 161 (42.2%) | NS |
| AMI at 60 months | 8 (2.8%) | 10 (11.5%) | 18 (4.8%) | .001 |
| Revascularization at 60 months | 3 (1.0%) | 6 (6.5%) | 9 (2.4%) | .003 |
| Composite endpoint | 174 (60.6%) | 61 (66.3%) | 235 (62.0%) | .139 |
|
AMI, myocardial infarction; BARC, Bleeding Academic Research Consortium; MACE, major adverse cardiovascular events; NS, not significant; TAVI, transcatheter aortic valve implantation. |
||||
Figure 1. Central illustration. Overall rate of major bleeding across follow-up periods. BARC, Bleeding Academic Research Consortium; TAVI, transcatheter aortic valve implantation.
Table S1 illustrates the bleeding events classified by BARC criteria according to revascularization status during follow-up. The overall rate of major bleeding (BARC 3–5) between coronary angiography and TF-TAVI was 5.5% (21/375) and was significantly higher among revascularized patients (12.0% vs 3.5%; P = .007). During the index hospitalization for TF-TAVI, the overall rate of major bleeding was 6.1% (23/375), with no statistically significant differences between the 2 groups (8.7% vs 5.3%; P = .31). During post-TAVI follow-up, the overall rate of major bleeding was 9.6% (36/375), with no significant differences between the groups either (7.6% vs 10.2%; P = .545) (figure 1).
After a mean follow-up of 60 months, 55.1% (209/379) of patients experienced MACE: 55.7% in the nonrevascularized group (160 patients), and 53.2% in the revascularized group (49 patients). There were no statistically significant differences (P = .082) (figure 2). The overall mortality rate at 60 months was 42.2% (161/378): 43.7% in nonrevascularized patients (125 patients), and 39.0% in revascularized ones (36 patients). Again, no significant differences were reported (P = .380) (figure 3).
Figure 2. Kaplan–Meier curve for MACE-free survival by revascularization status.

Figure 3. Kaplan–Meier curve for overall survival by revascularization status.
The revascularized group exhibited higher rates of myocardial infarction (11.5% vs 2.8%; P = .001) and repeat revascularization (6.5% vs 1.0%; P = .003).
After a mean 60-month follow-up, the composite endpoint (MACE or major bleeding) occurred in 62.0% (235 patients) of the total sample, without significant differences across the groups (66.3% nonrevascularized vs 60.6% revascularized; P = .139) (figure 4).
Figure 4. Kaplan–Meier curve for combined event–free survival (ischemic or hemorrhagic) by revascularization status.
DISCUSSION
There is currently no definitive clinical evidence on the optimal management of coronary artery disease in patients scheduled for TAVI. Clinical guidelines recommend percutaneous revascularization in all patients undergoing TF-TAVI with percent diameter stenoses ≥ 70% in the target vessel proximal segments, with a Class IIa recommendation. However, to this date, only 2 randomized clinical trials have assessed the benefit of pre-TAVI revascularization, with conflicting results.8,9
Major bleeding remains one of the most frequent and prognostically relevant complications after TF-TAVI. In fact, in the PARTNER 2 trial conducted with intermediate-risk patients, major bleeding was reported in up to 15.2% of patients 1 year after TAVI.³ Despite this, evidence on the impact of pre-TAVI coronary revascularization on bleeding risk is scarce.
In the ACTIVATION trial,8 a total of 235 patients scheduled for TF-TAVI who had significant coronary artery disease were randomized to undergo percutaneous revascularization (n = 119) or receive optimal medical therapy (n = 116). Outcomes in the 2 groups were evaluated according to a composite primary endpoint of all-cause mortality and hospitalization. At 1 year, noninferiority of the strategy of adding percutaneous revascularization to TF-TAVI could not be demonstrated in patients who did not undergo revascularization; however, higher bleeding rates were observed in the intervention group.
The results of the NOTION-3 trial9 have been recently published. In this study, 452 patients scheduled to undergo TF-TAVI with significant coronary artery disease were randomized to receive either an invasive or a conservative strategy. In this case, the decision to revascularize was guided by the severity of stenosis as assessed by fractional flow reserve. Outcomes were evaluated according to a composite primary endpoint of all-cause mortality, myocardial infarction, and emergency revascularization. At 2 years, patients who had been revascularized showed a significant reduction in the risk of MACE compared with the conservative strategy (26.0% vs 36.0%), driven primarily by a higher rate of unplanned revascularization and without an effect on mortality. However, this risk reduction was accompanied by a higher rate of bleeding events (28.0% vs 20.0%). A major limitation of this trial is its open-label design and the fact that it did not exclude patients with angina, which may have contributed to the increased rate of unplanned revascularization.
In our study, there were no statistically significant differences in MACE or major bleeding at 60 months between revascularized and nonrevascularized patients. However, a higher rate of major bleeding occurred among the former during the time elapsed between diagnostic coronary angiography and TAVI, which is consistent with former studies demonstrating higher bleeding rates in patients on dual antiplatelet therapy.8,9 Furthermore, this group exhibited higher rates of myocardial infarction and subsequent revascularization. Although these findings were expected, they should be interpreted with caution because, despite statistically significant differences, the small number of events limits statistical power to draw definitive conclusions. A reasonable strategy may be selective revascularization aimed at symptom control, particularly in patients with angina.
Our study has several important limitations. The most significant one is that it is a single-center, observational, nonrandomized, retrospective analysis, which results in multiple sources of selection bias. First, a biological selection bias exists because the study includes patients who self-selected by surviving to a mean age of 83 years with sufficient biological status to be considered eligible for TF-TAVI. Second, a clinical selection bias is present regarding which patients were selected for TAVI, as the retrospective design makes it impossible to standardize the criteria originally used to determine candidacy. Finally, our cohort only includes patients in whom the procedure was ultimately performed—not those who initially underwent evaluation for TAVI—which means that some patients who underwent diagnostic cardiac catheterization (with or without revascularization) may not have proceeded to TAVI and are therefore not included. The proportion of such patients and the reasons for not completing the procedure are unknown. Although the causes may be diverse, given the procedural risks of coronary interventions and the frequent presence of complex coronary artery disease in this population, it is plausible that some candidates did not undergo TAVI because of revascularization-related complications; however, this cannot be demonstrated with our data and remains speculative. Moreover, this is a single-center study with a limited sample size, which may restrict the external validity of the findings and the statistical power to detect inter-group differences.
CONCLUSIONS
In our cohort, pre-TF-TAVI systematic coronary revascularization was associated with an increased early risk of major bleeding, specifically between diagnostic catheterization and valve implantation. There were no statistically significant differences in long-term major bleeding, MACE, or the composite endpoint between revascularized and nonrevascularized patients. These findings, together with recent evidence indicating that revascularization of stable coronary disease does not clearly improve prognosis,10 reinforce the need for high-quality clinical evidence to define the clinical impact of pre-TAVI systematic coronary revascularization.
FUNDING
None declared.
ETHICAL CONSIDERATIONS
The study was approved by the Clinical Research Ethics Committee of A Coruña-Ferrol. Informed consent was not required due to the retrospective design of the study and use of a preexisting clinical database. SAGER guidelines were followed to minimize potential sex-related bias.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
No artificial intelligence tools were used in the preparation of this article.
AUTHORS’ CONTRIBUTIONS
C. Vidau Getán contributed to data collection and manuscript drafting. D. López Vázquez was the main reviewer and contributed to refinement of statistical analysis. X. Flores Ríos conceived the study and conducted the initial statistical analysis. M. González Montes and G. González Barbeito participated in data collection. The remaining coauthors reviewed the final version of the manuscript. All authors gave their final approval.
CONFLICTS OF INTEREST
None declared.
WHAT IS KNOWN ABOUT THE TOPIC?
- Clinical practice guidelines recommend percutaneous revascularization in TAVI candidates with percent diameter stenoses ≥ 70% in the target vessel proximal segments.
- No high-quality evidence demonstrates a clinical benefit of systematic pre-TAVI coronary revascularization.
- Two clinical trials have been conducted in this patient population, with inconsistent results regarding the benefits observed in terms of ischemic events, and with a higher rate of bleeding events in revascularized patients.
WHAT DOES THIS STUDY ADD?
- Revascularized patients showed higher rates of early major bleeding (between diagnostic catheterization and TAVI), without significant long-term differences in ischemic or hemorrhagic events.
- Results support the need for robust evidence to clarify the clinical impact of systematic pre-TAVI coronary revascularization.
REFERENCES
1. Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2022;43:561-632.
2. Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus Surgical Aortic-Valve Replacement in High-Risk Patients. N Engl J Med.2011;364:2187-2198.
3. Leon MB, Smith CR, Mack MJ, et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med. 2016;374:1609-1620.
4. Reardon MJ, Mieghem NMV, Popma JJ, et al. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med. 2017;376:1321-1331.
5. Moat N, Brecker S. Transfemoral TAVI is superior to SAVR in elderly high-risk patients with symptomatic severe aortic stenosis! Eur Heart J.2016;37:3513-3514.
6. Avvedimento M, Nuche J, Farjat-Pasos JI, Rodés-Cabau J. Bleeding Events After Transcatheter Aortic Valve Replacement:JACC State-of-the-Art Review. J Am Coll Cardiol. 2023;81:684-702.
7. Vázquez DJL, López GA, Guzmán MQ, et al. Prognostic impact of coronary lesions and its revascularization in a 5-year follow-up after the TAVI procedure. Catheter Cardiovasc Interv. 2023;102:513-520.
8. Patterson T, Clayton T, Dodd M, et al. ACTIVATION (PercutAneous Coronary inTervention prIor to transcatheter aortic VAlve implantaTION):A Randomized Clinical Trial. JACC Cardiovasc Interv. 2021;14:1965- 1974.
9. Lønborg J, Jabbari R, Sabbah M, et al. PCI in Patients Undergoing Transcatheter Aortic-Valve Implantation. N Engl J Med. 2024;391:2189-2200.
10. Maron DJ, Hochman JS, Reynolds HR, et al. ISCHEMIA Research Group. Initial Invasive or Conservative Strategy for Stable Coronary Disease. N Engl J Med.2020;382:1395-1407.
ABSTRACT
Introduction and objectives: Mitral regurgitation is one of the most common heart valve diseases. Valve replacement surgery is a guideline-recommended option; however, in a significant proportion of patients, this option is not feasible. In such cases, mitral transcatheter edge-to-edge repair (M-TEER) is a potential therapeutic alternative. Nevertheless, the results of a randomized clinical trial have shown divergent results. Recently, the results of the RESHAPE-HF2 trial were published, providing additional insights. The objective of this work is to evaluate whether there are any differences between performing M-TEER and keeping patients under guideline-directed medical therapy (GDMT).
Methods: We conducted a meta-analysis following the PRISMA guidelines. We searched for studies across the PubMed, Embase, and Cochrane databases until February 2025. We establish the following inclusion criteria: patients with secondary mitral regurgitation, studies comparing M-TEER plus GDMT vs GDMT alone, and who reported hospitalization due to heart failure (HF) or mortality.
Results: A total of 3 randomized clinical trials meet the inclusion criteria, including a total of 1423 patients: 704 received M-TEER and 719, GDMT alone. M-TEER was associated with a reduced risk of HF-related hospitalization with a risk ratio (RR) of 0.71 (95%CI, 0.56-0.90; P = .004). We did not find any differences in all-cause mortality with a RR of 0.80 (95%CI, 0.63-1.02; P = .07).
Conclusions: In this meta-analysis, M-TEER plus GDMT shows a lower risk of HF-related hospitalization vs GDMT alone. We did not find any differences in the risk of all-cause mortality or cardiac death.
Registered at PROSPERO: CRD42025645047.
Keywords: Mitral regurgitation. Mitral transcatheter edge-to-edge repair. Heart failure. M-TEER.
RESUMEN
Introducción y objetivos: La regurgitación mitral es una de las valvulopatías cardiacas más comunes. La cirugía de reemplazo valvular es una opción recomendada por las guías clínicas. Sin embargo, en un porcentaje significativo de pacientes, esta opción no es viable. En estos casos, la reparación mitral percutánea de borde a borde (M-TEER) es una posible alternativa terapéutica. No obstante, los resultados de los ensayos clínicos aleatorizados han mostrado resultados divergentes. Recientemente se han publicado los resultados del estudio RESHAPE-HF2, que aportan información adicional sobre este tema. El objetivo de este trabajo fue evaluar si existen diferencias entre realizar M-TEER o mantener a los pacientes bajo tratamiento médico según las guías clínicas (TMSG).
Métodos: Se realizó un metanálisis siguiendo las guías PRISMA. Se buscaron estudios en las bases de datos PubMed, Embase y Cochrane hasta febrero de 2025. Se establecieron los siguientes criterios de inclusión: pacientes con insuficiencia mitral secundaria, estudios que comparaban M-TEER más TMSG frente a solo TMSG, y que indicaran hospitalización por insuficiencia cardiaca o mortalidad.
Resultados: Tres ensayos clínicos aleatorizados cumplieron los criterios de inclusión, con un total de 1.423 pacientes, de los que 704 se trataron con M-TEER y 719 recibieron solo TMSG. La M-TEER se asoció con una reducción del riesgo de hospitalización por insuficiencia cardiaca con una razón de riesgo de 0,71 (IC95%, 0,56-0,90; p = 0,004). No se encontraron diferencias en cuanto a muerte por cualquier causa, con una razón de riesgo de 0,80 (IC95%, 0,63-1,02; p = 0,07).
Conclusiones: En este metanálisis, la M-TEER, en combinación con el TMSG, mostró una reducción del riesgo de hospitalización por insuficiencia cardiaca en comparación con el TMSG solo. No se hallaron diferencias en el riesgo de muerte por cualquier causa (muerte de causa cardiovascular o infarto agudo de miocardio).
Registrado en PROSPERO: CRD42025645047.
Palabras clave: Regurgitación mitral. Reparación percutánea de borde a borde. Insuficiencia cardiaca. M-TEER.
Abreviaturas
MR: mitral regurgitation. M-TEER: mitral transcatheter edge-to-edge repair. GDMT: guideline-direct medical therapy. HF: heart failure. NYHA: New York Heart Association.
INTRODUCTION
Mitral regurgitation (MR) is a prevalent valvular heart disease associated with significant morbidity and mortality, particularly in patients with heart failure (HF).1 Traditional management strategies include optimal medical therapy to treat symptoms and surgery for definitive correction.2 However, current clinical practice guidelines only recommend mitral valve repair if the patient is undergoing another intervention such as coronary artery bypass graft or aortic valve replacement; nonetheless, many patients are at a high risk for surgery due to comorbid conditions, for this reason, alternative therapeutic options is required.3
Mitral transcatheter edge-to-edge repair (M-TEER) has emerged as a minimally invasive option for patients with symptomatic mitral regurgitation who are ineligible for surgery.4 While M-TEER has shown promising results, its comparative efficacy vs guideline- directed medical therapy (GDMT) is still a matter of discussion.5
This meta-analysis aims to assess the impact of M-TEER plus GDMT vs GDMT alone on major clinical outcomes in patients with secondary MR. The primary endpoint evaluated was HF-related hospitalization. Secondary endpoints included all-cause mortality, cardiac death, improvement in New York Heart Association (NYHA) functional class (FC), and the incidence rate of stroke and myocardial infarction. These endpoints were selected for their clinical relevance and potential to inform therapeutic decision-making in this high-risk patient population.
METHODS
We conducted this meta-analysis in full compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement guidelines.6 All the stages of this study were performed following the Cochrane Handbook for Systematic Reviews of Interventions, version 6.3.7 The protocol for this meta-analysis was registered prospectively in PROSPERO on 10 February 2025 with protocol ID CRD42025645047.
Search strategy
We conducted a systematic search across 3 electronic databases (PubMed, EMBASE, and COCHRANE) from inception until February 2025. Search terms included combinations of the following keywords: “transcatheter”, “secondary”, “mitral valve”, “replacement”, regurgitation”, and “insufficiency”. These keywords were combined using Boolean operators AND, OR. The reference lists of eligible studies and previous reviews were checked to identify additional valuable articles.
Criteria of the included studies
Studies were considered for inclusion in the meta-analysis based on the following criteria: a) randomized clinical trials (RCT) or observational cohort studies in Spanish or English that b) compared transcatheter mitral valve replacement vs optimal medical therapy in adult patients with mitral valve regurgitation due to secondary causes; and c) studies that reported outcomes of interest such as the index HF-related hospitalization, HF-related readmissions or cardiac death, and all-cause mortality.
Studies were excluded if they were non-original articles such as systematic reviews, letters, abstracts, meta-analyses, case reports, or case series. Furthermore, studies were excluded if mitral regurgitation was due to primary causes or if prior surgical repair had been performed.
Although observational cohort studies were eligible, we only identified 1 cohort study that did not meet the inclusion criteria during full-text screening. As a result, only RCTs were ultimately included in the meta-analysis.
Data extraction
To ensure accuracy, we used Zotero to eliminate duplicate references. We screened each paper based on title and abstract as a first step, followed by a full-text review as the second step. Two authors (D.A. Navarro Martínez, and D. Paulino-González) independently screened each paper, with any disagreements being resolved by a third author (A.L. García Loera). In addition, references of the included studies were reviewed and added if they met our eligibility criteria. Data extraction was conducted using Excel spreadsheets capturing the following information: a) baseline characteristics of the studied population, including baseline medication, echocardiographic baseline characteristics, and comorbidities; b) summary of the characteristics of the included studies; c) outcome measures; and d) quality assessment domains.
Primary and secondary endpoints
The primary endpoints established for this meta-analysis included: a) all-cause mortality (hazard ratio [HR]), defined as all-cause mortality, assessed using time-to-event analysis,8 and b) HF-related hospitalization, defined as hospital admission for worsening HF following the intervention (M-TEER or initiation of optimal medical therapy).9
Secondary endpoints included a) stroke, defined as an episode of neurological dysfunction caused by focal cerebral, spinal, or retinal infarction;10 and b) myocardial infarction, defined according to the Fourth Universal Definition.11
All endpoints were assessed as dichotomous categorical variables, which were reported in percentages. The risk ratio (RR) with its corresponding 95% confidence interval (95%CI) was calculated.
Assessment of heterogeneity
To assess heterogeneity, we used Cochrane’s Q-statistic with a significance level of P < .05. Additionally, the I²-statistic was used to quantify the proportion of variability due to heterogeneity, with values > 50% being considered indicative of high heterogeneity.12
Statistical analyses
To assess dichotomous data, we evaluated event frequencies and totals from each study group to calculate the RR and its 95%CI. Moreover, we obtained the HR and the 95%CI to calculate the standard error (SE).
The variables analyzed in this study were based on data reported in the original studies of the intention-to-treat principle. We implemented a random-effects model using the DerSimonian-Laird13 method to account for variability and allow comparison across studies. Given the limitations and strengths of this method, we conducted a sensitivity analysis using the Hartung-Knapp-Sidik-Jonkman method. Forest plots were utilized as a visual representation of the estimated outcomes.
Considering the differences in the populations included in the clinical trials, we conducted an additional exploratory analysis that focused on the RESHAPE-HF214 and COAPT15 trials for the primary endpoints by removing MITRA-FR16 from the analysis.
All statistical analyses, including the calculation of RR and SE, were conducted using RevMan V.5.4.1 software.
RESULTS
The initial database search yielded a total of 164 potentially relevant articles. After removing duplicates, a total of 129 articles finally remained for title and abstract screening. Following this initial screening, 8 articles were identified as potentially eligible for full-text review. Finally, after a detailed evaluation of the full texts under the predefined inclusion and exclusion criteria, 3 studies were included. A summary of the study selection process is shown in the PRISMA flowchart figure 1.
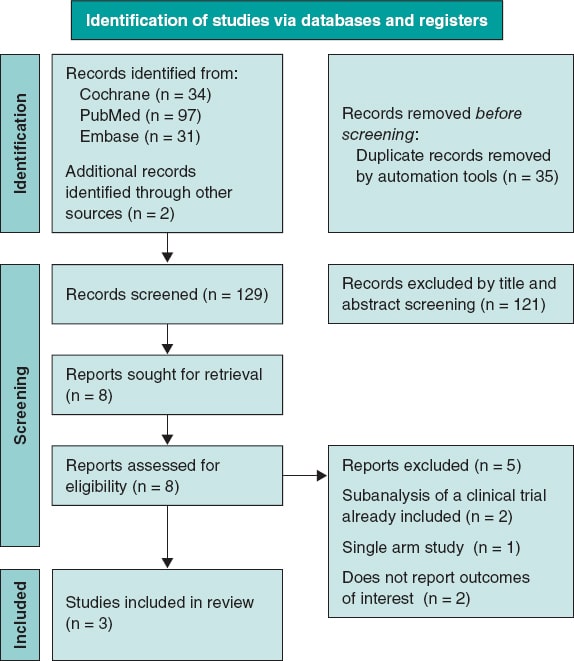
Figure 1. PRISMA 2020 flow diagram for new systematic reviews which included searches of databases and registers only.
Our analysis included 3 RCTs.14-16 We consulted the extended report of the 2-year outcomes of the MITRA-FR trial17 and 1 additional article on the RESAHPE-HF2 to obtain more data regarding hospitalization.18 All included studies compared the use of M-TEER plus GDMT vs GDMT alone. To standardize the outcomes, the median follow-up was set at 24 months; only the NYHA FC was evaluated with 1-year results. All 3 included studies used the MitraClip (Abbott, United States) device.
Clinical baseline characteristics of the patients
We obtained a total population of 1423 patients; among them 704 received M-TEER and 719, GDMT alone. In the RESHAPE-HF214 the device group showed the following characteristics: mean age was 70 years old; left ventricular ejection fraction (LVEF), 32%; median left ventricular end-diastolic volume (LVEDV), 200 mL; median effective regurgitation orifice (EROA), 0.23 cm2; median N-terminal pro-B-type natriuretic peptide (NT-proBNP), 2651; and brain natriuretic peptide (BNP), 556. In the COAPT15 trial, median age was 71 years old; LVEF, 31%; LVEDV, 194 mL; median EROA, 0.41 cm2; median NT-proBNP, 5174; and BNP, 1014. Finally in the MITRA-FR trial16, mean age was 71 years old; LVEF, 33%; LVEDV, 136.2 mL; median EROA, 0.31 cm2; median NT-proBNP, 3407; and BNP, 765. Regarding etiology, all trials enrolled mixed ischemic and non-ischemic etiology populations. Patient and study baseline characteristics, including comorbidities, drugs, and echocardiographic data, are shown in table 1, table 2, and table 3.
Table 1. Baseline characteristics of patients
| Reference | Groups | N | Age | AF or flutter | Diabetes | Previous MI | Nonischemic etiology | Ischemic etiology | 6-min walk distance | Beta- blocker | ACEI | ARB | ARNI | SGLT2i | MRA | Diuretics | Oral anti- coagulants | BNP | NT-proBNP | EuroSCORE II |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RESHAPE-HF214 | M-TEER | 250 | 70.0 ± 10.4 | 118 (47.2) | 91 (36.4) | 144 (57.6) | 88 (35.2) | – | 300 (220-382) | 238 (95.2) | 142 (56.8) | 51 (20.4) | 40 (16.0) | 24 (9.6) | 200 (80.0) | 239 (95.6) | 163 (65.2) | 556 (312-1018) | 2651 (1630-4918) | 5.3 (2.7-8.9) |
| Optimal medical therapy | 255 | 69.4 ± 10.7 | 125 (49.0) | 85 (33.3) | 135 (52.9) | 88 (34.5) | – | 310 (200-378) | 246 (96.5) | 142 (55.7) | 45 (17.6) | 28 (11.0) | 22 (8.6) | 215 (84.3) | 243 (95.3) | 152 (59.6) | 406 (231-874) | 2816 (1306-5496) | 5.3 (2.9-9.0) | |
| COAPT15 | M-TEER | 302 | 71.7 ± 11.8 | 173 (57.3) | 106 (35.1) | 156 (51.7) | 118 (39.1) | 184 (60.9) | 261.3 ± 125.3 | 275 (91.1) | 138 (45.7) | 66 (21.9) | 13 (4.3) | NR | 153 (50.7) | 270 (89.4) | 140 (46.4) | 1014.8 ± 1086.0 | 5174.3 ± 6566.6 | NR |
| Optimal medical therapy | 312 | 72.8 ± 10.5 | 166 (53.2) | 123 (39.4) | 160 (51.3) | 123 (39.4) | 189 (60.6) | 246.4 ± 127.1 | 280 (89.7) | 115 (36.9) | 72 (23.1) | 9 (2.9) | NR | 155 (49.7) | 277 (88.8) | 125 (40.1) | 1017.1 ± 1212.8 | 5943.9 ± 8437.6 | NR | |
| MITRA-FR16 | M-TEER | 152 | 70.1 ± 10.1 | 49/142 (34.5) | 50 (32.9) | 75 (49.3) | 57 (37.5) | 95 (62.5) | 307 (212-387) | 134 (88.2) | 111 (73.0) | 111 (73.0) | 14/140 (10) | NR | 86 (56.6) | 151 (99.3) | 93 (61.2) | NR | NR | 7.33 ± 6.29 |
| Optimal medical therapy | 152 | 70.6 ± 9.9 | 48/147 (32.7) | 39 (25.7) | 52 (34.2) | 66 (43.7) | 85 (56.3) | 335 (210-410) | 138 (90.8) | 113 (74.3) | 113 (74.3) | 17/140 (12.1) | NR | 80 (53.0) | 149 (98.0) | 93 (61.2) | NR | NR | 6.57 ± 5.24 | |
|
ACEI, angiotensin-converting enzyme inhibitors; AF, atrial fibrillation; ARB, angiotensin receptor blockers; ARNI, angiotensin receptor-neprilysin inhibitors; BNP, B-type natriuretic peptide; M-TEER, mitral transcatheter edge-to-edge repair; MI, myocardial infarction; MRA, mineralocorticoid receptor antagonists; NR, not reported; NT-proBNP, N-terminal pro-B-type natriuretic peptide; SGLT2i, sodium-glucose cotransporter 2 inhibitors. |
||||||||||||||||||||
Table 2. Summary of included studies
| Authors and year | Study design | Device | Population size | Compared interventions | Mean follow-up | Key findings |
|---|---|---|---|---|---|---|
| Anker et al. RESHAPE-HF2 202414 | RCT | MitraClip | 505 | M-TEER Optimal medical therapy | 24 months |
|
| Stone et al. COAP 201815 | RCT | MitraClip | 614 | M-TEER Optimal medical therapy | 24 months |
|
| Obadia et al. MITRA - FR 201816 | RCT | MitraClip | 304 | M-TEER Optimal medical therapy | 24 months |
|
|
95%CI, 95% confidence interval; HF, heart failure; HR, hazard ratio; M-TEER, mitral transcatheter edge-to-edge repair; OR, odds ratio. |
||||||
Table 3. Echocardiographic baseline characteristics
| Baseline characteristics | RESHAPE-HF214 | COAPT15 | MITRA-FR16 | |||
|---|---|---|---|---|---|---|
| Device group | Control group | Device group | Control group | Device group | Control group | |
| Severity MR Grade 3+, n,% (n) | 141 (56.4) (250) | 141(55.3) (255) | 148 (49.0) (302) | 172 (55.3) (312) | – | – |
| Severity MR Grade 4+, n,% (n) | 109 (43.6) (250) | 114 (44.7) (255) | 154 (51.0) (302) | 139 (44.7) (312) | – | – |
| LVEF, % (n) | 32 (26-37) (250) | 31 (25-37) (255) | 31.3 ± 9.1 (302) | 31.3 ± 9.6 (312) | 33.3 ± 6.5 | 32.9 ± 6.7 |
| LVEDV, mL (n) | 137 (100-173) (250) | 140 (104-176) (255) | 135.5 ± 56.1 (302) | 134.3 ± 60.3 (312) | – | – |
| LVEDV, mL (n) | 200 (153-249) (250) | 206 (158-250) (255) | 194.4 ± 69.2 (302) | 191.0 ± 72.9 (312) | 136.2 ± 37.4 | 134.5 ± 33.1 |
| LVESD, cm (n) | 5.8 (5.3-6.5) (250) | 5.9 (5.3-6.4) (255) | 5.3 ± 0.9 (302) | 5.3 ± 0.9 (312) | – | – |
| LVEDD, cm (n) | 6.9 (6.3-7.6) (250) | 6.8 (6.4-7.5) (255) | 6.2 ± 0.7 | 6.2 ± 0.8 | – | – |
| EROA, cm2 (n) | 0.23 (0.20-0.30) (250) | 0.23 (0.19-0.29) (255) | 0.41 ± 0.15 | 0.40 ± 0.15 | 0.31 ± 10* | 0.31 ± 11* |
| Regurgitant volume, mL (n) | 35.4 (28.9-43.9) (250) | 35.6 (28.2-42.5) (255) | – | – | 45 ± 13 | 45 ± 14 |
|
EROA, effective regurgitant orifice area; LV, left ventricular; LVEDD, left ventricular end-diastolic dimension; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESD, left ventricular end-systolic dimension; MR, mitral regurgitation. RESHAPE-HF2 data express median and interquartile range [IQR] in the COAPT trial. MITRA-FR data express median ± standard deviation. * This data was originally expressed in mm2 and has been converted to standardized meditation. |
||||||
Risk of bias assessment
To evaluate the quality of the included randomized controlled trials, we used the Risk of Bias 2 (RoB2) tool from the Cochrane Handbook of Systematic Reviews of Interventions 6.3.7 This methodology allowed us to systematically assess the methodological quality of each study, thereby strengthening the validity of our findings.
Of the 3 studies, 1 had a low risk of bias,14 while the other 2 raised some concerns.15,16 The studies with some concerns were limited by factors in their methodologies, as they were open-label studies (figure 2).
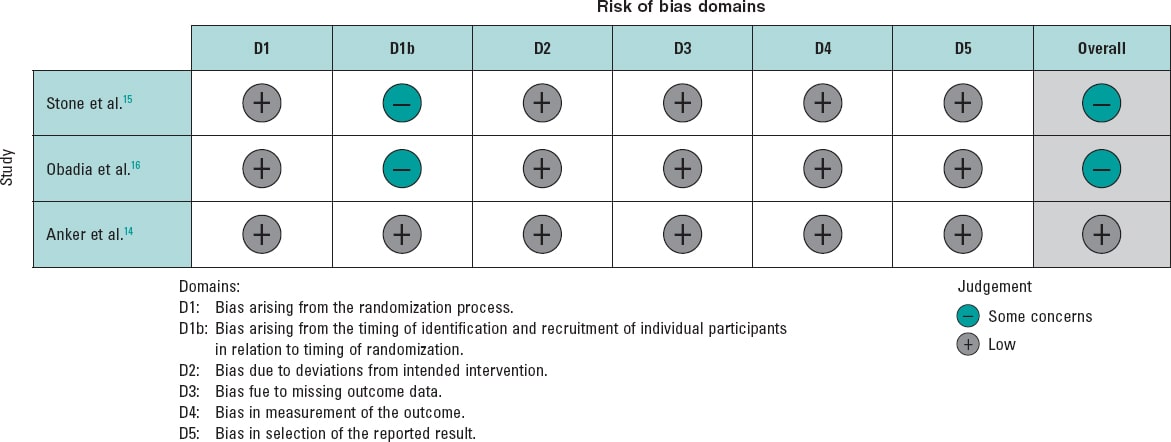
Figure 2. Risk of bias for each included randomized clinical trial. The bibliographical references mentioned in this figure correspond to Stone et al.,14 Obadia et al.,15 and Anker et al.16.
Outcomes
HF-related hospitalization
The analysis of this outcome showed a significant reduction, with a RR of 0.71 (95%CI, 0.56-0.90; P = .004). However, substantial heterogeneity was observed, with an I² value of 79%. Considering the differences in the MITRA-FR study population, we conducted an exploratory analysis excluding this trial. Results still demonstrated a significant reduction in the risk of hospitalization, with a RR of 0.63 (95%CI, 0.55-0.72, P < .00001), along with a marked improvement in heterogeneity (I² = 0%). Of note, this finding is exploratory and should be interpreted with caution.
The analysis, including all studies, is shown in figure 3A, and the exploratory analyses are shown in figure 3B. Furthermore, we analyzed HR for this endpoint and, given the limited number of studies, we conducted a sensitivity analysis using the Hartung-Knapp-Sidik-Jonkman (HKSJ) method; all these analyses are provided in the supplementary data (figures S1-S4).
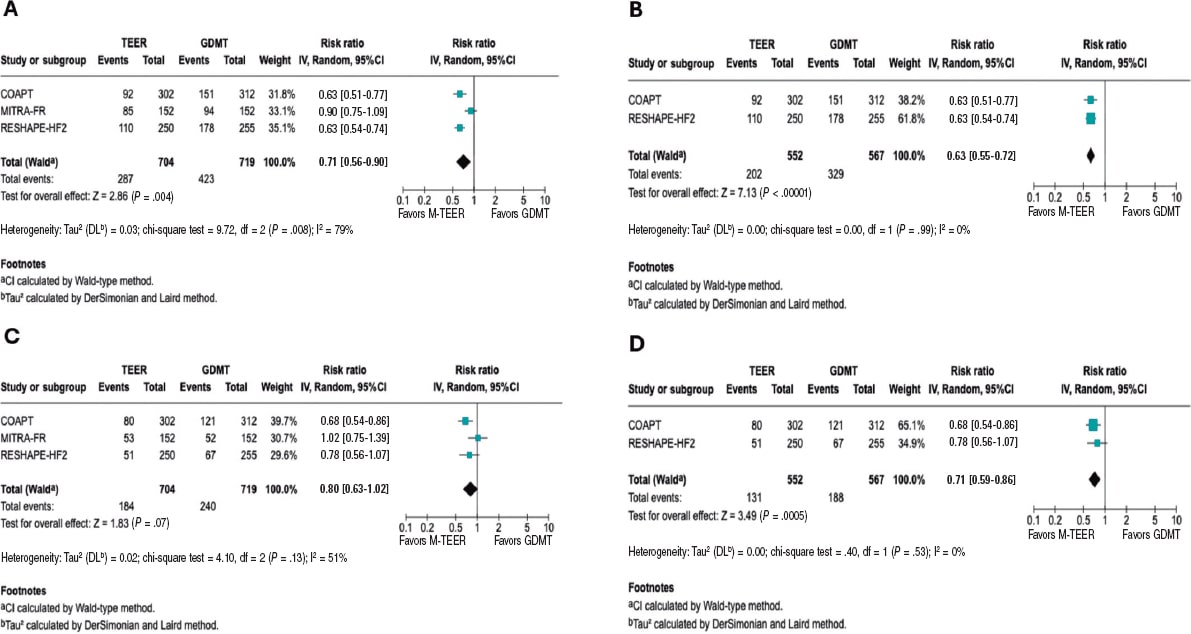
Figure 3. Forest plot of risk ratios (RR). Lines denote the 95% confidence intervals (95%CI) for each trial. A: forest plot of RR for HF related hospitalization. B: forest plot of exploratory RR for HF-related hospitalization. C: forest plot of RR for all-cause mortality. D: forest plot of exploratory RR for all-cause mortality. 95%CI, 95% confidence interval; GDMT, guideline directed medical therapy; M-TEER, mitral transcatheter edge-to-edge repair. The bibliographical references mentioned in this figure correspond to Stone et al.,14 Obadia et al.,15 and Anker et al.16.
All-cause mortality
The RR for all-cause mortality was 0.80 (95%CI, 0.63–1.02; P = .07). However, substantial heterogeneity was observed across the studies (I² = 56%). In the exploratory analysis, a significant reduction in mortality was found, with an RR of 0.71 (95%CI, 0.59–0.86; P = .0005) and no heterogeneity across the studies (I² = 0%). The analyses, including all studies, are shown in figure 3C, and the exploratory analysis in figure 3D.
Both the HR analysis and the sensitivity analysis are provided in the supplementary data (figures S5-S8).
Cardiac death
The RR for death from cardiovascular causes was 0.81 (95%CI, 0.62–1.06, P = .12), with low to moderate heterogeneity (I² = 48%). In the exploratory analysis, a significant reduction in cardiac death was observed, with an RR of 0.74 (95%CI, 0.55–1.00; P = .05) and low heterogeneity (I² = 41%). The analyses, including all studies, are shown in figure 4A, while the exploratory analysis, in figure 4B. The sensitivity analysis and HR results are provided in the supplementary data (figures S9-S12).
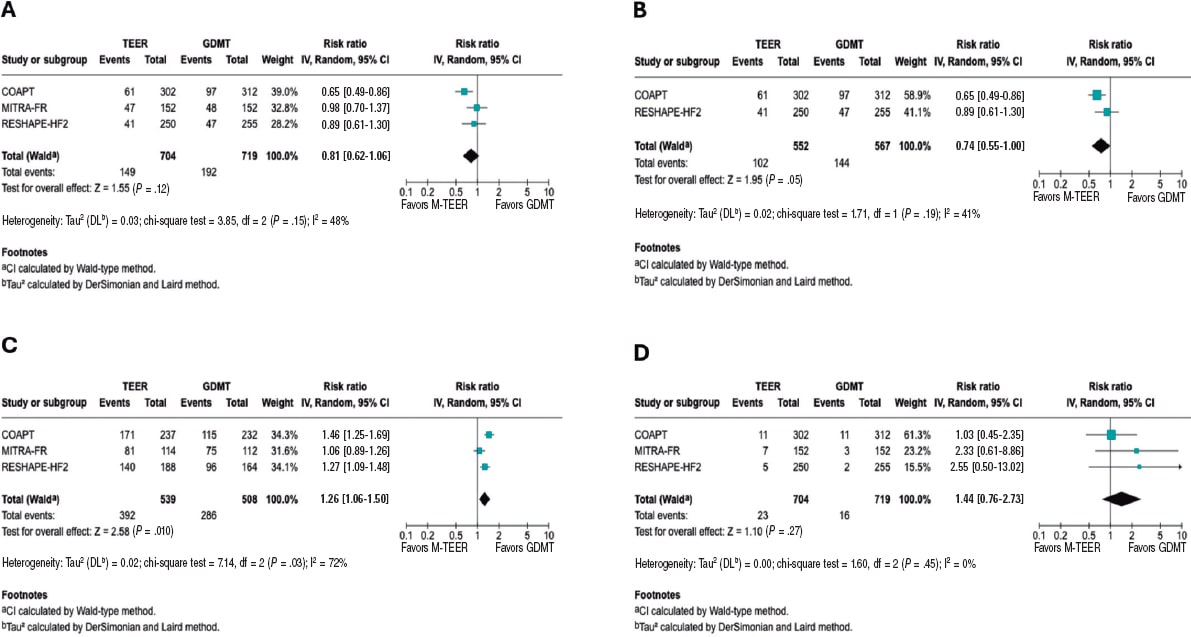
Figure 4. Forest plot of risk ratio (RR). A: forest plot of RR for cardiac death. B: forest plot of exploratory RR for cardiac death. C: forest plot of RR of NYHA FC I/II at 1 year. D: forest plot of RR for stroke. 95%CI, 95% confidence interval; GDMT, guideline directed medical therapy; M-TEER, mitral transcatheter edge-to-edge repair. The bibliographical references mentioned in this figure correspond to Stone et al.,14 Obadia et al.,15 and Anker et al16. Lines denote the 95%CI for each trial.
NYHA FC
In this analysis of the NYHA FC, we observed that patients undergoing M-TEER are more likely to be found in NYHA FC I/II at 12 months, with a RR 1.26 (95%CI, 1.06–1.50; P = .010), respectively. Of note, the high heterogeneity (I² = 72%). Furthermore, it is also essential to note that the NYHA FC is a subjective classification, which is why the findings of this outcome should be interpreted with caution. These results are shown in figure 4C.
Stroke
There were no statistical differences between M-TEER and optimal medical therapy regarding stroke, with a RR of 1.44 (95%CI, 0.76 - 2.73, P = .27) without any heterogeneity being reported across the studies (I² = 0%). These results are shown in figure 4D.
Myocardial Infarction
Regarding the risk of myocardial infarction, our analysis demonstrated no statistical difference between the M-TEER and optimal medical therapy groups (RR, 0.83; 95%CI, 0.43-1.61; P = .58). No heterogeneity among the studies was observed (I² = 0%); this finding is shown in supplementary data (figure S13).
Exploratory analysis by severity
We conducted an exploratory analysis stratified by the baseline grade of mitral regurgitation. Data on the composite endpoint of all-cause death or HF-related hospitalization were available according to MR grade. For patients with MR 3+, the RR was 0.75 (95%CI, 0.56-1.00; P = .05; I² = 12%). For those with severe MR 4+, data were available only from the RESHAPE-HF2 and COAPT trials, yielding a RR of 0.55 (95%CI, 0.42-0.73; P = .0001; I² = 0%). This finding is shown in the supplementary data (figure S14 to figure S15).
DISCUSSION
Our meta-analysis provides an evaluation of 3 major RCTs, COAPT15, MITRA-FR16, and RESHAPE-HF214, evaluating M-TEER plus GDMT vs GDMT alone in patients with secondary MR. In patients with persistent symptoms despite adequate GDMT and who do not meet the criteria for definitive surgical replacement, mitral M-TEER has emerged as a promising alternative. In this updated meta-analysis, no statistically significant effect on all-cause mortality was found; however, the marked between-trial heterogeneity suggests that M-TEER provides meaningful clinical benefit when used in appropriately selected patients.
Our study showed that heterogeneity of results was mainly driven by MITRA-FR. COAPT and RESHAPE-HF2 have multiple similar differences compared with MITRA-FR, as evidenced in trial cohort baseline characteristics, methods, and outcome directions. This is better portrayed by an exploratory analysis comparing COAPT and RESHAPE-HF2 results, showing a 37% reduction in the risk of HF-related hospitalization risk and a 32% reduction in cardiac death (figure 3). The overall neutral mortality rate resulting from our study may be driven by heterogeneity across trials rather than a lack of intrinsic therapeutic benefit.
Among the main differences across trials, the MITRA-FR trial had broader inclusion of patients with tricuspid regurgitation, advanced LV remodeling, greater dilation, and smaller EROA vs the other 2 trials. Another key source of heterogeneity was baseline MR severity. COAPT and RESHAPE-HF2 primarily enrolled patients with grade 3+ and 4+ MR, whereas MITRA-FR is only focused on grade 4+, which may contribute to the observed outcome differences. In our exploratory analysis by MR grade, data showed consistent trends favoring M-TEER; however, these findings should be interpreted with caution due to limited subgroup data.
As proposed by Paul et al.,19 one strategy to evaluate secondary MR is to consider the proportion between EROA and LVEDV. In the MITRA-FR trial, many patients had smaller EROA with markedly dilated ventricles, a phenotype in which GDMT may have more impact than valve proceduren. In the COAPT and RESHAPE-HF2 trials, a greater proportion of patients had large EROA relative to the LVEDV,20 making MR a primary driver of symptoms and outcomes, and, therefore, with potential for greater benefit from M-TEER.
In the trial-level subgroup analyses, the COAPT15 and RESHAPE-HF214 trials reported no significant interaction between treatment assignment and ischemic vs non-ischemic etiology, which indicates consistency of benefit across etiologies. Similarly, the MITRA-FR16 did not identify significant heterogeneity regarding etiology across prespecified subgroups. This suggests that ischemic vs non-ischemic alone should not be used to decide the treatment option in secondary MR. Instead, clinical decisions should prioritize other factors, such as the MR severity, the degree of LV dysfunction, symptom burden, and response to optimal medical therapy.21
Furthermore, differences between trials in procedural success are noteworthy, defined as achieving MR ≤ 2+. This was substantially lower in the MITRA-FR (75.6% MR ≤ 2+ at discharge) vs the COAPT (94.8% MR ≤ 2+ at 12 months) and the RESHAPE-HF2 (90.4% MR ≤ 2+ at 12 months). Multiple reasons could have influenced such differences; for instance, operator experience requirement in MITRA-FR (≥ 5 prior MitraClip cases) vs the high-volume and more experienced centers from the other trials. Similarly, post-approval data from the U.S. SSTS/ACC TVT Registry22 demonstrated > 90% MR reduction to ≤ 2+ and survival rates consistent with trial outcomes, underscoring the reproducibility of clinical benefit in experienced centers. Furthermore, device technology is a main contributor to these differences, from first-generation clips in MITRA-FR, to second-generation in COAPT, to the fourth-generation in RESHAPE-HF2 with independent leaflet grasping and wider arm options, potentially explaining discrepant clinical results regarding MR durability and outcomes. Lastly, GDMT implementation differed across trials. MITRA-FR was conducted before the widespread use of ARNI and SGLT2 inhibitors; COAPT was conducted before the adoption of SGLT2 inhibitors; and RESHAPE-HF2 reflects the contemporary use of quadruple therapy.
Despite the differences in patient phenotypes across the trials, in many developing countries, access to transcatheter valve procedures remains limited, primarily due to their high cost and the need for specialized infrastructure.23,24 The pronounced disparities in access to cutting-edge technologies, coupled with the centralization of these procedures in a few urban centers or high-specialty hospitals, a phenomenon observed even in high-income countries such as the United States, leave a substantial portion of the population without viable treatment options.25 Consequently, the most impactful and broadly deployable intervention in these regions remains rigorous GDMT optimization and provider education. Nevertheless, patient phenotyping should still be performed to identify those who may potentially have an outsized benefit from future M-TEER referrals.
By integrating trial-level population characteristics with clinical outcomes, our work bridges the gap between isolated trial findings and real-world patient selection, thus offering a potential framework for future prospective studies and for refining guideline criteria. While the results from our study must be interpreted with caution, they provide hypothesis-generating evidence supporting the concept that patient selection, particularly considering the balance between EROA and LVEDV and GDMT optimization, may be critical to optimizing the benefit of M-TEER in secondary MR. This approach moves beyond the broad application of current guideline recommendations and points toward a more individualized strategy in which anatomical and functional parameters guide intervention.
Limitations
The primary limitation of this study lies in the heterogeneity of the populations enrolled in the randomized controlled trials, as evidenced by the I2 observed in the analyses, which may influence the overall results. Second, differences in MR severity across trials represent a key limitation. While the COAPT and RESHAPE-HF2 trials mainly included patients with MR grade 3+ or 4, MITRA-FR enrolled a more severe grade, which may partly explain divergent results. Lastly, an individual patient-level meta-analysis could not be conducted due to lack of data; this level of analysis would further increase statistical power, especially in subgroup analyses, enhancing the robustness and generalizability of the findings.
CONCLUSIONS
In this meta-analysis, M-TEER plus GDMT shows a lower risk of HF-related hospitalization vs GDMT alone. We did not find any differences in the risk of all-cause mortality, cardiac death, stroke, or myocardial infarction.
FUNDING
None declared.
ETHICAL CONSIDERATIONS
This meta-analysis was performed using data from previously published studies. As it is based entirely on secondary data, no new data were collected from human or animal participants; on the other hand, the use of SAGER guidelines was not applicable in this study. All included studies were approved from the relevant center ethics committees. The authors confirm that all data utilized were publicly accessible, and no confidential information was used without proper authorization.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
During the preparation of this work, the authors used ChatGPT 4o to review the document’s syntax and grammar. After using this tool/service, the authors reviewed and edited the content as needed and take full responsibility for the content of the published article.
AUTHORS’ CONTRIBUTIONS
D. Paulino-González: conceptualization; formal analysis, writing, review, and editing. A.L. García-Loera: methodology, investigation, writing, review, and editing. D.A. Navarro-Martínez: methodology, formal analysis. M.A. Pardiño-Vega: writing, review, and editing, supervision. K.P. Zúñiga-Montaño: investigation, writing, review, and editing.
CONFLICTS OF INTEREST
None declared.
WHAT IS KNOWN ABOUT THE TOPIC?
- Mitral regurgitation is a prevalent condition that negatively affects the patients’ quality of life. M-TEER-has emerged as an alternative therapeutic option for patients who are ineligible for surgery. However, its benefit remains unclear vs GDMT, as clinical trials evaluating this procedure have reported disparate results, with benefit in primary outcomes observed in the COAPT trial and in the recently published RHESAPE-HF2 trial, but not in the MITRA-FR trial; discrepancy in the results requires further study.
WHAT DOES THIS STUDY ADD?
- This meta-analysis provides a comprehensive evaluation of the evidence comparing M-TEER plus GDMT vs GDMT alone in secondary mitral regurgitation. Our findings confirm that M-TEER is associated with a significant reduction in HF-related hospitalizations. Of note, by examining trial populations in detail, we highlight how patient selection influences outcomes: in studies enrolling patients with less ventricular dilation and lower biomarker levels of congestion (such as the COAPT and RESHAPE-HF2 trials), M-TEER was associated with additional benefits in cardiac death and all-cause mortality. These results underscore the potential role of anatomical and functional parameters, such as the balance between EROA and LVEDV, in identifying patients most likely to benefit from this procedure.
REFERENCES
1. Chehab O, Roberts-Thomson R, Ng Yin Ling C, et al. Secondary mitral regurgitation:pathophysiology, proportionality and prognosis. Heart. 2020;106:716-723.
2. Sengodan P, Younes A, Shah N, Maraey A, Chitwood WR Jr, Movahed A. Contemporary review of the evolution of various treatment modalities for mitral regurgitation. Expert Rev Cardiovasc Ther. 2024;22:639-651.
3. Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease:A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e1159-e1195.
4. Itabashi Y, Kobayashi S, Mizutani Y, Torikai K, Taguchi I. Treatment of secondary mitral regurgitation by transcatheter edge-to-edge repair using MitraClip. J Med Ultrason (2001). 2022;49:389-403.
5. Barnes C, Sharma H, Gamble J, Dawkins S. Management of secondary mitral regurgitation:from drugs to devices. Heart. 2024;110:1099-1106.
6. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement:an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
7. Sterne JAC, Savovic´J, Page MJ, et al. RoB 2:a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898.
8. Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure:A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines [published correction appears in Circulation. 2022;145:e1033.] [published correction appears in Circulation. 2022;146:e185.] [published correction appears in Circulation. 2023;147:e674]. Circulation. 2022;145:e895-e1032.
9. Abraham WT, Psotka MA, Fiuzat M, et al. Standardized Definitions for Evaluation of Heart Failure Therapies:Scientific Expert Panel From the Heart Failure Collaboratory and Academic Research Consortium. JACC Heart Fail. 2020;8:961-972.
10. Sacco RL, Kasner SE, Broderick JP, et al. An updated definition of stroke for the 21st century:a statement for healthcare professionals from the American Heart Association/American Stroke Association [published correction appears in Stroke. 2019;50:e239]. Stroke.2013;44:2064-2089.
11. Thygesen K, Alpert JS, Jaffe AS, et al. Fourth Universal Definition of Myocardial Infarction (2018). Glob Heart. 2018;13:305-338.
12. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560.
13. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177-188.
14. Anker SD, Friede T, von Bardeleben RS, et al. Transcatheter Valve Repair in Heart Failure with Moderate to Severe Mitral Regurgitation. N Engl J Med.2024;391:1799-1809.
15. Stone GW, Lindenfeld J, Abraham WT, et al. Transcatheter Mitral-Valve Repair in Patients with Heart Failure. N Engl J Med. 2018;379:2307-2318.
16. Obadia JF, Messika-Zeitoun D, Leurent G, et al. Percutaneous Repair or Medical Treatment for Secondary Mitral Regurgitation. N Engl J Med.2018;379:2297-2306.
17. Iung B, Armoiry X, Vahanian A, et al. Percutaneous repair or medical treatment for secondary mitral regurgitation:outcomes at 2 years. Eur J Heart Fail. 2019;21:1619-1627.
18. Ponikowski P, Friede T, von Bardeleben RS, et al. Hospitalization of Symptomatic Patients With Heart Failure and Moderate to Severe Functional Mitral Regurgitation Treated With MitraClip:Insights From RESHAPE-HF2. J Am Coll Cardiol. 2024;84:2347-2363.
19. Grayburn PA, Sannino A, Packer M. Proportionate and Disproportionate Functional Mitral Regurgitation:A New Conceptual Framework That Reconciles the Results of the MITRA-FR and COAPT Trials. JACC Cardiovasc Imaging. 2019;12:353-362.
20. Anker SD, Friede T, von Bardeleben RS. Percutaneous repair of moderate-to-severe or severe functional mitral regurgitation in patients with symptomatic heart failure:Baseline characteristics of patients in the RESHAPE-HF2 trial and comparison to COAPT and MITRA-FR trials. Eur J Heart Fail. 2024;26:1608-1615.
21. Nappi F, Singh SSA, Bellomo F.Exploring the Operative Strategy for Secondary Mitral Regurgitation:A Systematic Review. Biomed Res Int.2021;2021:3466813.
22. American College of Cardiology. STS/ACC TVT Registry Analysis Finds TMVr Safe and Effective in Real-World Setting. ACC. 2023 Mar 5. Available at: https://www.acc.org/Latest-in-Cardiology/Articles/2023/03/01/22/45/Sun-1215pm-sts-acc-tvt-acc-2023. Accessed 1 Jul 2025.
23. Bernardi FLM, Ribeiro HB, Nombela-Franco L, et al. Recent Developments and Current Status of Transcatheter Aortic Valve Replacement Practice in Latin America - the WRITTEN LATAM Study. Arq Bras Cardiol. 2022;118:1085-1096.
24. Bana A. TAVR-present, future, and challenges in developing countries. Indian J Thorac Cardiovasc Surg. 2019;35:473-484.
25. Steitieh D, Zaidi A, Xu S, et al. Racial Disparities in Access to High-Volume Mitral Valve Transcatheter Edge-to-Edge Repair Centers. J Soc Cardiovasc Angiogr Interv. 2022;1:100398.
- VerifyNow in acute coronary syndrome: design of the EPIC17-VERONICA trial to optimize platelet inhibition
- DEB or DES for the treatment of calcified nodules after intravascular lithotripsy: the DEBSCAN-IVL trial design
- Transcatheter aortic valve implantation without immediate cardiac surgery backup. A single-center retrospective analysis
- Optical coherence tomography for the diagnosis and management of stent thrombosis
Editorials
Expanding the role of drug-coated balloons in native large coronary artery disease
aDepartment of Biomedical Sciences, Humanitas University, Pieve Emanuele, Milan, Italy
bCardio Center, IRCCS Humanitas Research Hospital, Rozzano, Milan, Italy
cDivision of Cardiology, Montefiore Medical Center, Bronx, New York, United States
Original articles
Editorials
The role of angiography-derived physiological assessment techniques in the post-FAVOR III Europe era?
aServicio de Cardiología, Hospital General Universitario Gregorio Marañón, Instituto de Investigación Sanitaria Gregorio Marañón, Universidad Complutense de Madrid, Madrid, Spain
bCentro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), Instituto de Salud Carlos III, Madrid, Spain
Interviews
An interview with Camino Bañuelos
aServicio de Cardiología, Hospital Clínico San Carlos, Instituto de Investigación Sanitaria del Hospital Clínico San Carlos (IdISSC), Madrid, Spain
bServicio de Cardiología, Hospital Clínico Universitario de Santiago de Compostela, Santiago de Compostela, A Coruña, Spain










