ABSTRACT
Introduction and objectives: Coronary artery disease and mental health disorders are often coexistent. Selective serotonin reuptake inhibitors (SSRIs) are often used in this context but have been associated with an increased risk of bleeding due to platelet dysfunction. Previous studies have assessed this risk in patients treated with clopidogrel-based dual antiplatelet therapy (DAPT) with contradictory results. However, there is no data regarding the use of SSRIs and potent P2Y12 inhibitors or triple antithrombotic therapy after percutaneous coronary intervention (PCI). The purpose of this study was to assess the impact of SSRIs on bleeding outcomes after PCI in patients treated with clopidogrel, prasugrel or ticagrelor-based DAPT or triple antithrombotic therapy.
Methods: Retrospective study including all patients undergoing PCI at a high-volume center during 2018. Patients on SSRIs were propensity-score-matched on a 1:1 ratio with patients naive to SSRIs adjusting for the baseline differences. The primary endpoint was major bleeding (BARC type 3 or 5 bleeding) at the 1-year follow-up. Secondary endpoints were a composite of major/non-major clinically relevant bleeding (BARC type 2, 3 or 5 bleeding), and a composite of major adverse cardiovascular events.
Results: Out of a total of 1063 patients treated with PCI during the study period, 1002 met the selection criteria, and 139 (13.9%) were on SSRIs. The latter had a higher bleeding risk before matching [PRECISE-DAPT, 16 [10-24] vs 13 [9-21]; P = .040]. No differences were reported in major bleeding (2.9% vs 2.9%, P = .991), major/non-major clinically relevant bleeding (2.9% vs 7.2%, P = .120) or in major adverse cardiovascular events (7.9% vs 7.9%, P = .979) in patients treated with SSRIs.
Conclusions: The use of SSRIs was frequent in patients treated with PCI, and although it was a marker of a higher bleeding risk at baseline, this was not associated with an additional bleeding liability.
Keywords: Bleeding. Coronary artery disease. Percutaneous coronary intervention. Selective serotonin reuptake inhibitors. Antithrombotic therapy.
RESUMEN
Introducción y objetivos: La cardiopatía isquémica y la enfermedad mental coexisten a menudo. Los inhibidores selectivos de la recaptación de serotonina (ISRS) se utilizan con frecuencia en este contexto, pero se han asociado con un incremento en el riesgo hemorrágico. Los estudios previos han evaluado este fenómeno en pacientes tratados con clopidogrel, con resultados contradictorios. No hay datos sobre el uso de ISRS e inhibidores del P2Y12 potentes o triple terapia antitrombótica. El objetivo de este estudio fue examinar el impacto de los ISRS en los eventos hemorrágicos en pacientes tratados con doble (incluyendo clopidogrel, prasugrel o ticagrelor) o triple terapia antitrombótica tras una intervención coronaria percutánea (ICP).
Métodos: Estudio retrospectivo en el que se incluyeron todos los pacientes tratados con ICP en un centro de alto volumen durante 2018. Los pacientes en tratamiento con ISRS fueron emparejados mediante puntaje de propensión con pacientes sin ISRS. El objetivo primario fue el sangrado mayor al año de seguimiento (BARC 3 o 5). Los objetivos secundarios fueron un combinado de sangrado mayor o menor clínicamente relevante (BARC 2, 3 o 5) y un combinado de eventos cardiovasculares adversos mayores.
Resultados: De los 1.063 pacientes tratados con ICP durante el periodo del estudio, 1.002 cumplieron los criterios de selección y 139 (13,9%) recibían ISRS. Los pacientes con ISRS tenían un mayor riesgo de sangrado antes del emparejamiento (PRECISE-DAPT: 16 [10-24] frente a 13 [9-21]; p = 0,040). No hubo diferencias en el objetivo primario (2,9% frente a 2,9%; p = 0,991) ni en los objetivos secundarios de sangrado mayor o menor clínicamente relevante (2,9 frente a 7,2%; p = 0,120) y eventos cardiovasculares adversos mayores (7,9 frente a 7,9%; p = 0,979).
Conclusiones: El uso de ISRS fue frecuente en los pacientes tratados con ICP, y aunque fue un marcador de riesgo hemorrágico basal, no se asoció con un mayor riesgo de sangrado en el seguimiento.
Palabras clave: Sangrado. Enfermedad coronaria. Intervencionismo coronario percutáneo. Inhibidores selectivos de la recaptación de serotonina. Terapia antitrombótica.
Abbreviations
DAPT: dual antiplatelet therapy. PCI: percutaneous coronary intervention. SSRIs: selective serotonin reuptake inhibitors.
INTRODUCTION
Coronary artery disease and mental health disorders frequently coexist and have a bidirectional relationship.1,2 Patients with mental health disorders have an increased risk of coronary artery disease and, inversely, it is not rare for patients to experience symptoms of depression or anxiety after a cardiac event.3 Moreover, depression in patients with CHD is associated with a poor adherence to treatment, unhealthy lifestyle habits, and a poor prognosis.4-8
Selective serotonin reuptake inhibitors (SSRIs) are often prescribed as first-line agents to treat depression and anxiety,9,10 but have a potential for increased bleeding risk due to the concomitant inhibitory effect on the platelet serotonin reuptake transporter (5-HTT).11 Platelet 5-HTT inhibition has been associated with a reduced platelet activation and aggregation, and with a prolonged bleeding time.12,13 On the other hand, some studies have linked SSRI-related bleeding risk to older age, comorbidities or polypharmacy.14,15
Bleeding risk due to antithrombotic therapy is a major concern following percutaneous coronary intervention (PCI) as hemorrhagic events are prognostically unfavorable as recurrent ischemic events.16,17 While bleeding risk depends on multiple clinical and laboratory features,18,19 the identification of potential modifiable factors is key to optimize the balance between ischemic and bleeding risk.20 Prior studies have evaluated the bleeding risk of patients with a concomitant treatment of SSRIs and dual antiplatelet therapy (DAPT) plus aspirin and clopidogrel with contradictory results.21-23 However, the impact of SSRIs plus therapy with more potent P2Y12 inhibitors (eg, ticagrelor or prasugrel) or triple antithrombotic therapy with DAPT plus an oral anticoagulant (OAC) has never been explored. In this study we tried to compare the 1-year risk of bleeding after PCI and concomitant guideline-recommended antithrombotic therapy (including clopidogrel, ticagrelor or prasugrel-based DAPT and triple antithrombotic therapy) in patients with or without prescribed SSRIs.
METHODS
Study design and setting
Retrospective study including all consecutive patients discharged after PCI performed at a single center during 2018. Those treated with SSRIs were propensity score-matched (PSM) to a control group to compare bleeding outcomes at the 1-year follow-up. Antithrombotic treatment was decided by the clinical cardiologist in accordance with the current clinical practice guidelines.24 This study was conducted according to the Declaration of Helsinki and was approved by the local clinical research ethics committee. Written informed consent was obtained from all patients before the PCI.
Population
All patients discharged after the PCI performed during the study period were eligible. Those treated at discharge with single antiplatelet therapy, DAPT were excluded—not including acetylsalicylic acid—as well as those anticoagulated with low-molecular-weight heparin for other reasons. Patients with missing information at the follow-up were also excluded. Clinical and procedural data, treatment at discharge, and outcomes during the first year were reviewed through electronic health records. Patients were treated with SSRIs if their list of prescriptions at discharge included one of the following: citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine or sertraline.
Endpoints
The primary safety endpoint was major bleeding at the 1-year follow-up. Secondary endpoints were a composite of major or non-major clinically relevant bleeding, and a composite of major adverse cardiovascular events (MACE). Major bleeding was defined as a bleeding event type 3 or 5 according to the Bleeding Academic Research Consortium (BARC). Major/non-major clinically relevant bleeding was defined as BARC type 2, 3 or 5 bleeding event.25 MACE was defined as a composite outcome of cardiovascular death, non-fatal myocardial infarction or unplanned revascularization. Events were independently adjudicated by 2 cardiologists who were unaware of the SSRIs group.
Statistical analysis
Categorical variables were expressed as counts (percentages), and the continuous ones as mean ± standard deviation or median [interquartile range] according to their distribution assessed using the Shapiro-Wilk test. P values were obtained using the chi-square test or the Mann-Whitney U test, as appropriate. PSM was conducted to account for the confounding biases.26 Logistic regression was used to determine the probability of being treated with SSRIs and included the following confounding variables potentially associated with SSRIs treatment and the primary endpoint:27 age, sex, prior relevant bleeding, hypertension, cancer, past medical history of hematologic disease or anemia, liver disease, creatinine clearance, treatment with potent P2Y12 inhibitors or concomitant OAC. The nearest neighbor matching method with no replacement, and a caliper width of 0.1 were used in the PSM on a 1:1 ratio. Propensity score histograms and standardized mean differences before and after the PSM were used to evaluate the balance of the groups regarding the covariates.28 Time-to-event analyses were conducted using the Kaplan-Meier and Cox proportional hazards methods. To determine major bleeding predictors in the unmatched cohort, a multivariate Cox regression model was conducted that used a purposeful selection model and prioritized parsimony. Clinical meaningful variables and those showing P values < 0.2 in the univariate analysis were included. Statistical analyses were performed using SPSS software (version 24; IBM Corp., United States) and R software (version 4.0.3; R Foundation for Statistical Computing, Austria). Matching was performed using the MatchIt R package (Ho, Imai, King, & Stuart, 2011) while covariate balance was assessed using the Cobalt R package (Greifer, 2021).
RESULTS
Baseline clinical characteristics
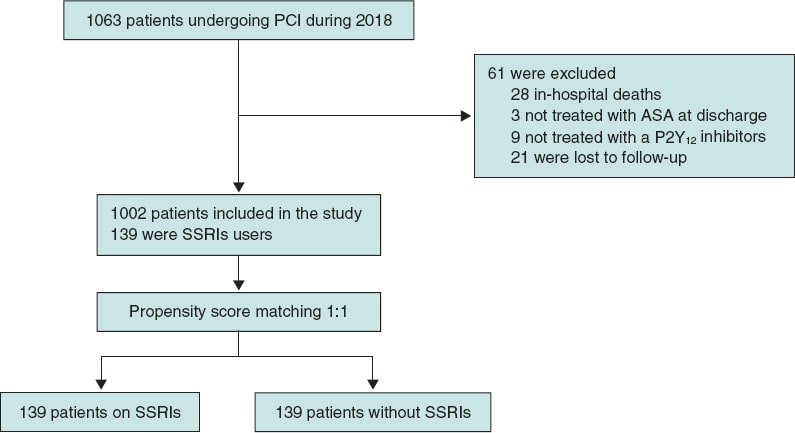
A total of 1063 patients were treated with PCI during the study period, 1002 of whom met the selection criteria and were included in the analysis. A total of 139 patients (13.9%) were treated with SSRIs at discharge (figure 1). Median age was 66 years (58-75), and 745 patients (74.4%) were male with a median PRECISE-DAPT score of 13 [9-22]. Regarding antithrombotic therapy, 684 patients (68.3%) were treated with potent P2Y12 inhibitors and 102 (10.2%) were concomitantly treated with OAC. The baseline clinical characteristics of the overall population and the unmatched groups are shown on table 1. Patients from the SSRIs group were more likely to be women. They also had a more extensive past medical history of hypertension, diabetes mellitus, cancer, significant bleeding, and hematologic disease or anemia. Both the HAS-BLED and the PRECISE-DAPT bleeding risk scores were higher in the SSRIs group.
Figure 1. Patient flowchart. PCI, percutaneous coronary intervention; SSRIs, selective serotonin reuptake inhibitors.
Table 1. Baseline clinical characteristics of the overall population, and SSRIs/non-SSRIs users before matching
| Variable | Overall (N = 1002) | SSRI (N = 139) | Non-SSRI (N = 863) | P |
|---|---|---|---|---|
| Age, years | 66 [58-75] | 67 [60-76] | 66 [57-75] | .530 |
| Sex, male | 745 (74.4) | 76 (54.7) | 669 (77.5) | .001* |
| BMI | 28.7 [25.9-31.8] | 30.0 [25.8-32.0] | 28.6 [25.9-31.7] | .067 |
| Hypertension | 688 (68.7) | 112 (80.6) | 576 (66.7) | .001* |
| Diabetes mellitus | 370 (36.9) | 64 (46.0) | 306 (35.5) | .017* |
| Hyperlipidemia | 525 (52.4) | 83 (59.7) | 442 (51.2) | .059 |
| Smoking (current or former) | 260 (25.9) | 34 (24.5) | 226 (26.2) | .709 |
| Previous revascularization | 248 (24.8) | 41 (29.5) | 207 (24.0) | .174 |
| COPD | 67 (6.7) | 10 (7.2) | 57 (6.6) | .740 |
| Chronic kidney disease | 115 (11.5) | 17 (12.2) | 98 (11.4) | .774 |
| Cancer | 98 (9.8) | 20 (14.4) | 78 (9.0) | .044* |
| Liver disease | 37 (3.7) | 8 (5.8) | 29 (3.4) | .166 |
| Hematologic disease or anemia | 99 (9.9) | 25 (18) | 74 (8.6) | .001* |
| Previous relevant bleeding | 31 (3.1) | 9 (6.5) | 22 (2.5) | .010* |
| Atrial fibrillation | 87 (8.7) | 11 (7.9) | 76 (8.8) | .871 |
| Oral anticoagulant | 102 (10.2) | 9 (6.5) | 93 (10.8) | .119 |
| Potent P2Y12 inhibitors | 684 (68.3) | 90 (64.7) | 594 (68.8) | .323 |
| Ticagrelor, no. (%) | 660 (65.9) | 86 (61.8) | 574 (66.5) | .543 |
| Prasugrel | 24 (2.4) | 4 (2.9) | 20 (2.3) | .543 |
| DAPT duration (months) | 8 [6-12] | 6 [6-12] | 8 [6-12] | .440 |
| PRECISE-DAPT | 13 [9-22] | 16 [10-24] | 13 [9-21] | .040* |
| PRECISE-DAPT ≥ 25 | 195 [19.5] | 34 [24.5] | 161 [18.7] | .109 |
| HAS-BLED | 2 (2-3) | 3 (2-3) | 2 (2-3) | .034* |
| Creatinine clearance, mL/min/1.73 m2 | 100 [82.3-124.1] | 94.8 [72.9-125.2] | 100 [82.7-124.1] | .154 |
| Clinical presentation | ||||
| CCS | 441 (44.0) | 66 (47.5) | 375 (43.5) | .375 |
| ACS | 561 (56.0) | 73 (52.5) | 488 (56.5) | |
|
ACS, acute coronary syndrome; BMI, body mass index (kg/m2); CCS, chronic coronary syndrome; COPD, chronic obstructive pulmonary disease; DAPT, dual antiplatelet therapy; SSRI, selective serotonin reuptake inhibitors. Data are expressed as no. (%), mean ± standard deviation or median [interquartile range]. * Indicates a statistically significant difference with P values < .05. |
||||
Unmatched analysis
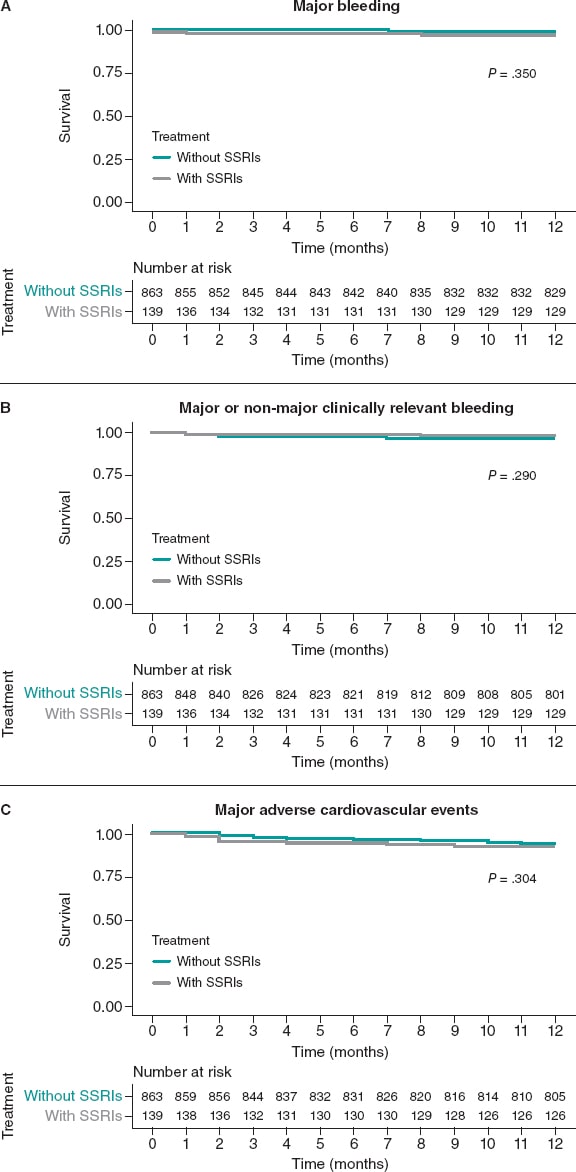
In the overall population there were a total of 19 major bleeding events at the 1-year follow-up: 4 (2.9%) in the SSRIs group, and 15 (1.7%) in the unmatched non-SSRIs group (P = .350). Of these, 4 (21.1%) were fatal, 10 (52.6%) GI bleedings, 4 (21.1%) intracranial bleedings while the remaining ones occurred in other locations.
The multivariable Cox model identified the following independent predictors for the primary endpoint of major bleeding: PRECISE-DAPT score ≥ 25, and concomitant anticoagulation. Table 2 shows the univariable and multivariable Cox predictors for the primary endpoint.
Table 2. Univariable and multivariable Cox predictors for major bleeding
| Variable | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| HR (95%CI) | P | HR (95%CI) | P | |
| Age, years | 1.06 (1.02-1.11) | .008 | ||
| Sex, male | 0.47 (0.19-1.18) | .107 | ||
| BMI | 0.98 (0.89-1.09) | .756 | ||
| Hypertension | 0.99 (0.38-2.61) | .989 | ||
| Diabetes mellitus | 1.91 (0.78-4.79) | .160 | ||
| Hyperlipidemia | 0.82 (0.33-2.01) | .664 | ||
| Chronic kidney disease | 3.67 (1.39-9.66) | .008 | ||
| Cancer | 2.47 (0.82-7.46) | .107 | ||
| Liver disease | 1.48 (0.19-11.05) | .705 | ||
| Hematologic disease or anemia | 2.47 (0.82-7.46) | .107 | ||
| Previous relevant bleeding | 3.91 (0.90-16.91) | .068 | ||
| Atrial fibrillation | 5.45 (2.05-14.53) | .001 | ||
| Oral anticoagulant | 8.22 (3.34-20.23) | .001 | 6.99 (2.78-17.64) | .001 |
| Potent P2Y12 inhibitors | 0.16 (0.06-0.45) | .001 | ||
| PRECISE-DAPT ≥ 25 | 4.77 (1.94-11.75) | .001 | 3.59 (1.44-8.98) | .006 |
| HAS-BLED | 1.69 (1.17-2.43) | .005 | ||
| Creatinine clearance | 0.98 (0.97-0.99) | .024 | ||
| SSRI | 1.68 (0.56-5.07) | .356 | 1.95 (0.64-5.93) | .241 |
|
95%CI, 95% confidence interval; BMI, body mass index (kg/m2); HR, hazard ratio; SSRI, selective serotonin reuptake inhibitors. |
||||
The major/non-major clinically relevant bleeding endpoint occurred in 4 patients (2.9%) from the SSRIs group, and in 43 patients (4.9%) from the unmatched no-SSRIs group (P = .290). The rate of MACE was similar in both groups: 11 events (7.9%) in the SSRIs group and 50 events (5.8%) in the non-SSRIs group.
The Kaplan-Meier curves and the associated risk tables for each endpoint of the unmatched cohorts are shown on figure 2.
Figure 2. Kaplan-Meier curves for the primary bleeding outcome (A), the secondary composite bleeding (B), and the ischemic outcomes (C). Unmatched cohort. SSRIs, selective serotonin reuptake inhibitors.
Propensity score matching analysis
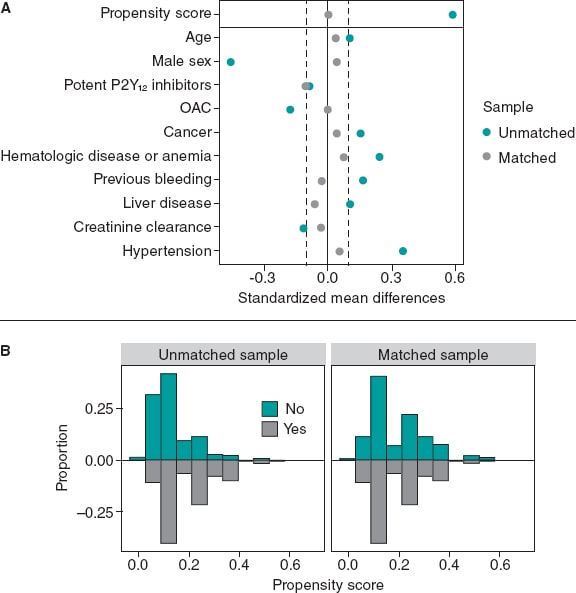
The variables used in the PSM, the standardized mean differences, and the Propensity score distributions of the unmatched and matched samples are shown on figure 3. PSM resulted in an excellent balance of covariates with standardized mean differences ≤ 10% in all variables included in the Propensity score. There was also a very good balance across the other baseline characteristics and bleeding risk scores except for diabetes mellitus and hyperlipemia that were more prevalent in the SSRIs group (table 3).
Figure 3. Variables used in the propensity score matching analysis and their standardized differences (A), and the propensity score distributions (B) of the unmatched and matched samples. OAC, oral anticoagulant.
Table 3. Baseline clinical characteristics of SSRIs/non-SSRIs users after matching
| Variable | SSRI (N = 139) | Non-SSRI (N = 139) | P |
|---|---|---|---|
| Chronic obstructive pulmonary disease | |||
| Age, years | 68 [60-76] | 67 [58-75] | .757 |
| Sex, male | 76 (54.7) | 73 (52.5) | .810 |
| BMI | 30.0 [25.8-32.0] | 28.4 [25.3-32.4] | .143 |
| Hypertension | 112 (80.6) | 109 (78.4) | .656 |
| Diabetes mellitus | 64 (46.0) | 48 (34.5) | .050 |
| Hyperlipidemia | 83 (59.7) | 67 (48.2) | .045 |
| Smoking (current or former) | 34 (24.5) | 28 (20.1) | .330 |
| Previous revascularization | 41 (29.5) | 30 (21.6) | .153 |
| COPD | 10 (7.2) | 9 (6.5) | .816 |
| Chronic kidney disease | 17 (12.2) | 19 (13.7) | .721 |
| Cancer | 20 (14.4) | 18 (12.9) | .727 |
| Liver disease | 8 (5.8) | 10 (7.2) | .626 |
| Hematologic disease or anemia | 25 (18) | 21 (15.1) | .519 |
| Previous relevant bleeding | 9 (6.5) | 10 (7.2) | .812 |
| Atrial fibrillation | 11 (7.9) | 11 (7.9) | 1.000 |
| Oral anticoagulant | 9 (6.5) | 9 (6.5) | 1.000 |
| Potent P2Y12 inhibitors | 90 (64.7) | 97 (69.8) | .371 |
| Ticagrelor | 86 (61.8) | 91 (65.5) | .749 |
| Prasugrel | 4 (2.9) | 6 (4.3) | .749 |
| DAPT duration, months | 6 [6-12] | 6 [6-12] | .810 |
| PRECISE-DAPT | 16 [10-24] | 15 [10-24] | .863 |
| PRECISE-DAPT ≥ 25 | 34 (24.5) | 32 (23.0) | .778 |
| HAS-BLED | 3 [2-3] | 3 [2-3] | .560 |
| Creatinine clearance, | |||
| mL/min/1.73 m2 | 94.8 [72.9-125.2] | 100 [82.7-114.0] | .747 |
| Clinical presentation | |||
| CCS | 66 (47.5) 73 (52.5) |
63 (45.3) 76 (54.7) |
.718 |
| ACS | |||
|
ACS, acute coronary syndrome; BMI, body mass index (kg/m2); CCS, chronic coronary syndrome; COPD, chronic obstructive pulmonary disease; DAPT, dual antiplatelet therapy; SSRI, selective serotonin reuptake inhibitors. Data are expressed as no. (%), mean ± standard deviation or median [interquartile range]. |
|||
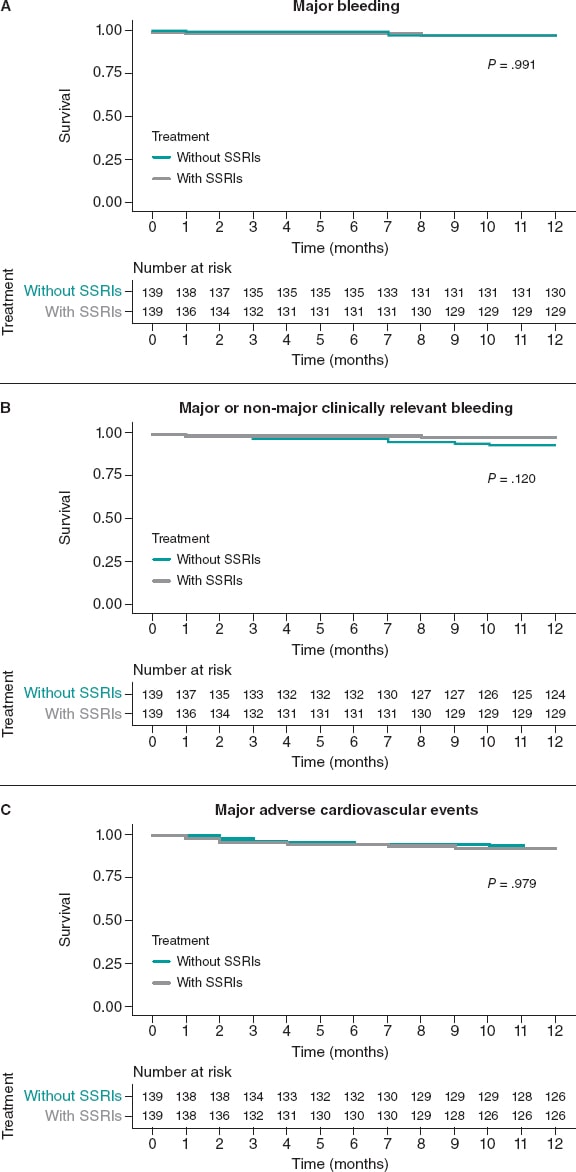
The rate of major bleeding at the 1-year follow-up was 2.9% for both patients on SSRIs and the matched SSRIs non-users (HR, 1.01; 95%CI, 0.25-4.03; P = .991). There were no non-major clinically relevant bleedings in the SSRIs group and 6 (4.3%) among SSRIs non-users (HR, 0.39; 95%CI, 0.16-1.27; P = .120). No differences in MACE were reported between the SSRI and the non-SSRIs groups (HR, 1.01; 95%CI, 0.44-2.33; P = .979) (figure 4).
Figure 4. Kaplan-Meier curves for the primary bleeding outcome (A), the secondary composite bleeding (B), and the ischemic outcomes (C). Matched cohorts. SSRIs, selective serotonin reuptake inhibitors.
DISCUSSION
The main findings of this study can be summarized as follows: a) the use of SSRIs was frequent among patients undergoing PCI; b) patients prescribed with SSRIs had a higher baseline bleeding risk; c) despite the imbalance reported in the baseline characteristics, after adjustment SSRIs users were not associated with a significant excess of major or clinically relevant bleeding at the 1-year follow-up.
There is a strict correlation between coronary artery disease and mental health disorders. In our study up to 13.9% of patients treated with PCI were prescribed SSRIs. This group has more comorbidities and bleeding risk factors with the potential to complicate the clinical decision-making process regarding antithrombotic therapy selection. Importantly, whether SSRIs trigger a higher bleeding risk through a biological effect on platelet 5-HTT receptors or are a marker of a higher bleeding risk through concomitant comorbidities has been the matter of discussion in prior studies.
Labos et al.21 reported an increased risk of bleeding in patients taking both SSRIs and acetylsalicylic acid or clopidogrel-based DAPT after myocardial infarction. On the contrary, Lasella et al.22 assessed the impact of SSRI therapy on patients on DAPT after PCI finding no excessive bleedings in patients on SSRIs. Interestingly, they reported a lower risk of MACE in patients on SSRIs compared to those on mirtazapine, but a higher risk compared to patients on either one of the 2 antidepressants. This may be explained by a protective effect of SSRIs on MACE29 that could be exceeded by the unfavorable effect of mental health disorders on cardiovascular events.30 Another interpretation could be associated with the pharmacokinetics of clopidogrel since it is a prodrug that requires enzymatic conversion into its active metabolite by cytochrome P450 (CYP).31 Bykov et al.23 reported an increased risk of ischemic events in patients on clopidogrel and a CYP2C19-inhibiting SSRI compared to those on noninhibiting SSRIs. No differences were found regarding major bleeding. The study did not include a group of patients without SSRI treatment.
We should mention that none of the aforementioned studies included patients treated with potent P2Y12 inhibitors, which is currently the standard of care of patients with ACS. To our knowledge, this is the first study to assess the impact of SSRIs on a cohort of patients treated with potent P2Y12 inhibitors prasugrel or ticagrelor. In our population, two thirds of the patients were treated with potent P2Y12 inhibitors, which is more consistent with the antiplatelet strategies recommended by the current clinical practice guidelines.32,33 In this clinical setting, despite the imbalances reported in the baseline bleeding risk in an unadjusted analysis, we found no differences regarding major or clinically relevant bleeding events among patients on SSRIs and the matched group without a SSRI prescription. Hence, while the prescription of SSRIs can be a marker of a higher risk population with more comorbidities and risk factors, this may not translate into an independent predictor of bleeding events after accounting for the potential confounders. This is consistent with prior evidence in the medical literature. In the study conducted by Labos et al.21 patients on SSRI had a more significant past medical history of hypertension, renal failure, anemia or other hematologic disease, and non-GI bleeding. Lasella et al.22 reported that SSRIs users were more likely to have diabetes, hypertension, dyslipidemia, COPD, and chronic kidney disease.
Our findings are clinically relevant for different reasons. Although SSRIs have been associated with a potential for an increased bleeding risk, a direct translation into an excess of adverse events has not been confirmed yet. Our data provide reassurance on the relative safety profile of potent antithrombotic therapies in association with SSRIs, which did not substantially increase the risk of bleeding during the first year after PCI when the treatment decision-making process is based on a thorough evaluation of the features of bleeding and ischemic risk.
Our study also included a proportion of patients treated with concomitant antiplatelet and OAC therapy (~10%), which is consistent with the current standard practice.34 The impact of SSRIs on bleeding outcomes in patients with AF treated with OAC has also been examined in the past. Various authors have reported a higher risk of major bleeding in patients concurrently treated with SSRIs and warfarin.35,36 On the contrary, Quinn et al.37 did not find a significantly increased risk of bleeding among patients from the ROCKET AF trial assigned to warfarin or rivaroxaban who were also on SSRIs. However, there was a modest but non-statistically significant higher risk of major bleeding in the warfarin group. Since SSRIs are CYP2C9 inhibitors, an increase of warfarin plasma concentrations could explain these findings.38 This reaffirms the importance of non-vitamin k antagonists to reduce the risk of bleeding also in this population given the need for multiple antithrombotic agents after the PCI and the higher baseline bleeding risk reported.39
Limitations
The current study has several limitations. First, its retrospective observational design, and the relatively small size of the sample limits our ability to provide definitive conclusions due to the residual possibility of type-2 errors. Secondly, despite the PSM resulted in a good balance between the selected potential confounders and the other baseline characteristics, the presence of residual confounding factors cannot be completely ruled out. For example, some variables associated with bleeding like the presence of diabetes mellitus or peripheral arterial disease were not included in the propensity score model. Yet similar findings were observed in the adjusted and unadjusted analyses. Thirdly, the classification of SSRI users was based on treatment at discharge without accounting for treatment adherence or discontinuation.
CONCLUSIONS
In this real-world study, a combination of SSRIs and potent antithrombotic therapies was frequently prescribed after PCI. Although the prescription of SSRIs was associated with a higher baseline bleeding risk in the unadjusted analysis this was not the case with an excess of major or clinically relevant bleeding reported at the follow-up.
FUNDING
None reported.
AUTHORS’ CONTRIBUTIONS
R. González-Manzanares, and S. Ojeda conceived and designed the study. R. González-Manzanares, M. Ruiz-Moreno, C. Fernández-Avilés, L. Carmona-Artime, G. Flores-Vergara, and F. Costa collected analyzed data and interpreted the results. R. González-Manzanares, M. Ruiz-Moreno, S. Ojeda, and F. Hidalgo drafted the manuscript and completed the critical revisions. S. Ojeda, F. Hidalgo, G. Flores-Vergara, F. Costa, J. Suárez de Lezo, and M. Pan reviewed and revised the manuscript, and approved its final version before submission. All authors gave their final approval to the version that would eventually be published.
CONFLICTS OF INTEREST
S. Ojeda is an associate editor of REC: Interventional Cardiology. The journal’s editorial procedure to ensure impartial handling of the manuscript has been followed. S. Ojeda, and M. Pan declared having received honoraria for lectures given for Abbott, Boston, World Medical, and Terumo. J Suárez de Lezo declared having received honoraria for lectures given for Abbott. The remaining authors declared no conflicts of interest whatsoever.
WHAT IS KNOWN ABOUT THE TOPIC?
- Coronary artery disease and mental health disorders frequently coexist. The combination of SSRIs and potent antithrombotic therapies is common.
- Bleeding events after PCI worsen prognosis same as recurrent ischemic events.
- SSRIs have been potentially associated with an increased risk of bleeding. Data regarding the concomitant use of SSRIs and potent antithrombotic therapies is scarce and inconclusive.
WHAT DOES THIS STUDY ADD?
- This is the first study to assess the impact of SSRIs on the bleeding outcomes in the current PCI practice using potent P2Y12 inhibitors or triple antithrombotic therapy.
- SSRIs users have a higher bleeding risk profile.
- The use of SSRIs was not associated with a higher risk of major bleeding after adjusting for the potential confounders.
REFERENCES
1. De Hert M, Detraux J, Vancampfort D. The intriguing relationship between coronary heart disease and mental disorders. Dialogues Clin Neurosci. 2018;20:31-40.
2. Jha MK, Qamar A, Vaduganathan M, Charney DS, Murrough JW. Screening and Management of Depression in Patients With Cardiovascular Disease:JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;73:1827-1845.
3. Lane D, Carroll D, Ring C, Beevers DG, Lip GY. The prevalence and persistence of depression and anxiety following myocardial infarction. Br J Health Psychol. 2002;7:11-21.
4. Gehi A, Haas D, Pipkin S, Whooley MA. Depression and medication adherence in outpatients with coronary heart disease:findings from the Heart and Soul Study. Arch Intern Med. 2005;165:2508-2513.
5. Sin NL, Kumar AD, Gehi AK, Whooley MA. Direction of Association Between Depressive Symptoms and Lifestyle Behaviors in Patients with Coronary Heart Disease:the Heart and Soul Study. Ann Behav Med. 2016;50:523-532.
6. Meijer A, Conradi HJ, Bos EH, et al. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events:a meta-analysis of 25 years of research. Gen Hosp Psychiatry. 2011;33:203-216.
7. Carney RM, Blumenthal JA, Freedland KE, et al. Depression and late mortality after myocardial infarction in the Enhancing Recovery in Coronary Heart Disease (ENRICHD) study. Psychosom Med. 2004;66:466-474.
8. Valgimigli M, Garcia-Garcia HM, Vrijens B, et al. Standardized classification and framework for reporting, interpreting, and analysing medication non-adherence in cardiovascular clinical trials:a consensus report from the Non-adherence Academic Research Consortium (NARC) [published correction appears in Eur Heart J. 2019;40:2774]. Eur Heart J. 2019;40:2070-2085.
9. Ghaffari Darab M, Hedayati A, Khorasani E, Bayati M, Keshavarz K. Selective serotonin reuptake inhibitors in major depression disorder treatment:an umbrella review on systematic reviews. Int J Psychiatry Clin Pract. 2020;24:357-370.
10. Bandelow B. Current and Novel Psychopharmacological Drugs for Anxiety Disorders. Adv Exp Med Biol. 2020;1191:347-365.
11. Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med. 2009;60:355-366.
12. Abdelmalik N, RuhéHG, Barwari K, et al. Effect of the selective serotonin reuptake inhibitor paroxetine on platelet function is modified by a SLC6A4 serotonin transporter polymorphism. J Thromb Haemost. 2008;6:2168-2174.
13. De Abajo FJ. Effects of selective serotonin reuptake inhibitors on platelet function:mechanisms, clinical outcomes and implications for use in elderly patients. Drugs Aging. 2011;28:345-367.
14. Hougardy DM, Egberts TC, van der Graaf F, Brenninkmeijer VJ, Derijks LJ. Serotonin transporter polymorphism and bleeding time during SSRI therapy. Br J Clin Pharmacol. 2008;65:761-766.
15. Yuan Y, Tsoi K, Hunt RH. Selective serotonin reuptake inhibitors and risk of upper GI bleeding:confusion or confounding?Am J Med. 2006;119:719-727.
16. Valgimigli M, Costa F, Lokhnygina et al. Trade-off of myocardial infarction vs. bleeding types on mortality after acute coronary syndrome:lessons from the Thrombin Receptor Antagonist for Clinical Event Reduction in Acute Coronary Syndrome (TRACER) randomized trial. Eur. Heart J. 2017;38:804-810.
17. Mehran R, Pocock SJ, Stone GW, et al. Associations of major bleeding and myocardial infarction with the incidence and timing of mortality in patients presenting with non-ST-elevation acute coronary syndromes:a risk model from the ACUITY trial. Eur Heart J. 2009;30:1457-1466.
18. Mehran R, Pocock SJ, Nikolsky E, et al. A risk score to predict bleeding in patients with acute coronary syndromes. J Am Coll Cardiol. 2010;55:2556-2566.
19. Costa F, van Klaveren D, James S, et al. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score:a pooled analysis of individual-patient datasets from clinical trials. Lancet. 2017;389:1025-1034.
20. Kang DO, An H, Park GU, et al. Cardiovascular and Bleeding Risks Associated With Nonsteroidal Anti-Inflammatory Drugs After Myocardial Infarction. J Am Coll Cardiol. 2020;76:518-529.
21. Labos C, Dasgupta K, Nedjar H, Turecki G, Rahme E. Risk of bleeding associated with combined use of selective serotonin reuptake inhibitors and antiplatelet therapy following acute myocardial infarction. CMAJ. 2011;183:1835-1843.
22. Iasella CJ, Kreider MS, Huang L, Coons JC, Stevenson JM. Effect of Selective Serotonin Reuptake Inhibitors on Cardiovascular Outcomes After Percutaneous Coronary Intervention:A Retrospective Cohort Study. Clin Drug Investig. 2019;39:543-551.
23. Bykov K, Schneeweiss S, Donneyong MM, Dong YH, Choudhry NK, Gagne JJ. Impact of an Interaction Between Clopidogrel and Selective Serotonin Reuptake Inhibitors. Am J Cardiol. 2017;119:651-657.
24. Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS:The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2018;39:213-260.
25. Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials:a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736-2747.
26. Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res. 2011;46:399-424.
27. Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Stürmer T. Variable selection for propensity score models. Am J Epidemiol. 2006;163:1149-1156.
28. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083-3107.
29. Fernandes N, Prada L, Rosa MM, et al. The impact of SSRIs on mortality and cardiovascular events in patients with coronary artery disease and depression:systematic review and meta-analysis. Clin Res Cardiol. 2021;110:183-193.
30. Zhang WY, Nan N, Song XT, Tian JF, Yang XY. Impact of depression on clinical outcomes following percutaneous coronary intervention:a systematic review and meta-analysis. BMJ Open. 2019;9:e026445.
31. Price MJ, Tantry US, Gurbel PA. The influence of CYP2C19 polymorphisms on the pharmacokinetics, pharmacodynamics, and clinical effectiveness of P2Y(12) inhibitors. Rev Cardiovasc Med. 2011;12:1-12.
32. Patel A, Goodman SG, Tan M, et al. Contemporary use of guideline-based higher potency P2Y12 receptor inhibitor therapy in patients with moderate-to-high risk non-ST-segment elevation myocardial infarction:Results from the Canadian ACS reflective II cross-sectional study. Clin Cardiol. 2021;44:839-847.
33. De Luca L, Zeymer U, Claeys MJ, et al. Comparison of P2Y12 receptor inhibitors in patients with ST-elevation myocardial infarction in clinical practice:a propensity score analysis of five contemporary European registries. Eur Heart J Cardiovasc Pharmacother. 2021;7:94-103.
34. Costa F, Garcia-Ruiz V, Licordari R, Fimiani L. The High Bleeding Risk Patient with Coronary Artery Disease. Cardiol Clin. 2020;38:481-490.
35. Quinn GR, Singer DE, Chang Y, et al. Effect of selective serotonin reuptake inhibitors on bleeding risk in patients with atrial fibrillation taking warfarin. Am J Cardiol. 2014;114:583-586.
36. Schelleman H, Brensinger CM, Bilker WB, Hennessy S. Antidepressant-warfarin interaction and associated gastrointestinal bleeding risk in a case-control study [published correction appears in PLoS One. 2015;10:e0121926]. PLoS One. 2011;6:e21447.
37. Quinn GR, Hellkamp AS, Hankey GJ, et al. Selective Serotonin Reuptake Inhibitors and Bleeding Risk in Anticoagulated Patients With Atrial Fibrillation:An Analysis From the ROCKET AF Trial. J Am Heart Assoc. 2018;7(15):e00∳.
38. Sansone RA, Sansone LA. Warfarin and Antidepressants:Happiness without Hemorrhaging. Psychiatry (Edgmont). 2009;6:24-29.
39. Costa F, Valgimigli M, Steg PG, et al. Antithrombotic therapy according to baseline bleeding risk in patients with atrial fibrillation undergoing percutaneous coronary intervention:applying the PRECISE-DAPT score in RE-DUAL PCI [published online ahead of print, 2020 Dec 1]. Eur Heart J Cardiovasc Pharmacother. 2020;pvaa135.