Article
Ischemic heart disease and acute cardiac care
REC Interv Cardiol. 2019;1:21-25
Access to side branches with a sharply angulated origin: usefulness of a specific wire for chronic occlusions
Acceso a ramas laterales con origen muy angulado: utilidad de una guía específica de oclusión crónica
Servicio de Cardiología, Hospital de Cabueñes, Gijón, Asturias, España
ABSTRACT
Introduction and objectives: Thrombus removal in patients with ST-segment elevation myocardial infarction (STEMI) undergoing percutaneous coronary intervention (PCI) can be challenging in the presence of a large thrombus burden. Excimer laser coronary angioplasty (ELCA) is an adjuvant device capable of vaporizing thrombus. This study aimed to evaluate the safety and efficacy profile of ELCA in PCI.
Methods: Patients with STEMI undergoing PCI with concomitant use of ELCA for thrombus removal were retrospectively identified at our center. Data were collected on the device efficacy and its contribution to overall procedural success. Additionally, ELCA-related complications and major adverse cardiovascular events were recorded at a 2-year follow-up.
Results: ELCA was used in 130 STEMI patients, 124 (95.4%) of whom had a large thrombus burden. TIMI grade flow improved significantly after ELCA: before laser application, TIMI grade-0 flow was reported in 79 (60.8%) cases and TIMI grade-1 flow in 32 (24.6%) cases. After ELCA, TIMI grade-2 and 3 flows were achieved in 45 (34.6%) and 66 (50.8%) cases, respectively (P < .001). Technical and procedural success were achieved in 128 (98.5%) and 124 (95.4%) cases, respectively. The complications included 1 death at the cath lab (0.8%), 1 coronary perforation (0.8%), and 3 distal embolizations (2.3%). At the 2-years follow-up, major adverse cardiovascular events occurred in 18.3% of the population.
Conclusions: In the context of STEMI, ELCA seems to be an effective device for thrombus dissolution, with adequate technical and procedural success rates. In the present cohort, ELCA use was associated with a low complication rate and favorable long-term outcomes.
Keywords: Acute coronary syndrome. Thrombectomy. Excimer laser coronary angioplasty.
RESUMEN
Introducción y objetivos: La eliminación de trombos durante la intervención coronaria percutánea primaria (ICPp) en el infarto agudo de miocardio con elevación del segmento ST (IAMCEST) es un desafío en presencia de una carga trombótica elevada. La angioplastia coronaria con láser de excímeros (ELCA) es una técnica complementaria que permite vaporizar el trombo. Este estudio evaluó la eficacia y la seguridad de la ELCA en el contexto de la ICPp.
Métodos: Análisis retrospectivo unicéntrico de pacientes con IAMCEST sometidos a ICPp con ELCA. Se evaluaron la eficacia en la disolución del trombo, la mejoría del flujo, el éxito del procedimiento, las complicaciones asociadas y los acontecimientos cardiovasculares adversos mayores durante un seguimiento de 2 años.
Resultados: Se realizó ELCA en 130 pacientes con IAMCEST, de los cuales 124 (95,4%) tenían carga trombótica elevada. El flujo TIMI mejoró significativamente tras la ELCA: previamente era 0 en 79 casos (60,8%) y 1 en 32 casos (24,6%), y se lograron flujos TIMI 2 y 3 en 45 casos (34,6%) y 66 casos (50,8%), respectivamente (p < 0,001). Las tasas de éxito técnico y del procedimiento fueron del 98,5% y el 95,4%, respectivamente. Las complicaciones incluyeron 1 muerte intraprocedimiento (0,8%), 1 perforación coronaria (0,8%) y 3 embolizaciones distales (2,3%). A los 2 años, la tasa de acontecimientos cardiovasculares adversos mayores fue del 18,3%.
Conclusiones: La ELCA parece ser una técnica eficaz y segura en el IAMCEST para la disolución del trombo, con altas tasas de éxito técnico y procedimental, baja incidencia de complicaciones y resultados favorables a largo plazo.
Palabras clave: Síndrome coronario agudo. Trombectomía. Angioplastia coronaria con láser de excímeros.
Abbreviations
ELCA: excimer laser coronary angioplasty. LTB: large thrombus burden. MACE: major adverse cardiovascular events. PCI: percutaneous coronary intervention. STEMI: ST-segment elevation myocardial infarction. TIMI: Thrombolysis in Myocardial Infarction.
INTRODUCTION
In patients with ST-segment elevation myocardial infarction (STEMI), percutaneous coronary intervention (PCI) is the preferred reperfusion strategy, as long as it can be performed within 120 minutes of the electrocardiogram-based diagnosis.1 Many patients with STEMI present with thrombotic occlusion of the infarct-related artery. Therefore, the use of devices aimed at reducing thrombus burden is a reasonable consideration to minimize distal embolization and no-reflow. Persistent no-reflow in patients with STEMI undergoing PCI is associated with the worst in-hospital outcomes and increased long-term mortality.2
While early studies on manual thrombus aspiration suggested benefits in terms of improved myocardial blush grades and ST-segment elevation resolution,3 larger trials comparing manual thrombus aspiration with PCI alone showed no significant reduction in cardiovascular death, recurrent myocardial infarction, cardiogenic shock, or a New York Heart Association FC IV heart failure within 180 days.4 Consequently, routine aspiration thrombectomy is no longer recommended in patients with STEMI.5
Thrombus removal, particularly when dealing with a large thrombus burden (LTB) in the context of STEMI, remains a critical and sometimes challenging aspect of PCI. Excimer laser coronary angioplasty (ELCA Coronary Laser Atherectomy Catheter, Koninklijke Philips N.V., The Netherlands) is a well-established adjuvant therapy for coronary interventions. ELCA uses xenon-chloride gas as the lasing medium to produce UV light energy, which is delivered to the target site through an optical fiber. This energy has the ability to ablate inorganic material through photochemical, photothermal, and photomechanical mechanisms.6,7 The microparticles released during laser ablation measure < 10 µm and are absorbed by the reticuloendothelial system, theoretically reducing the risk of microvasculature obstruction.8 These unique characteristics of ELCA have facilitated its use as an adjuvant therapy in patients with STEMI to ablate and remove thrombus.
Although ELCA is part of the therapeutic armamentarium in some PCI-capable centers, literature data is limited on its safety and efficacy profile in this specific scenario. The aim of this study was to evaluate the contribution of ELCA, focusing on its safety and efficacy profile as an adjuvant therapy in patients with STEMI undergoing PCI in our center.
METHODS
Data from all patients undergoing PCI with the simultaneous use of ELCA as an adjuvant technique were retrospectively recorded in a dedicated database after each procedure, starting from the introduction of the device in our center. ELCA procedures were performed by 5 interventional cardiologists with dedicated training in the use of the device.
This study was approved by Parque Sanitario Pere Virgili ethics committee (Barcelona, Spain) (reference No.: CEIM 003/2025). For the purposes of this study, we selected the subgroup of patients with STEMI who underwent PCI in which ELCA was used to facilitate thrombus removal.
Thrombus burden was assessed using the thrombus grading classification9 as defined by the Thrombolysis in Myocardial Infarction (TIMI) study group, ranging from 0 to 5. A LTB was defined as a thrombus score ≥ 3. According to our internal protocol, ELCA was considered in STEMI patients in the presence of angiographic evidence of LTB, defined as TIMI thrombus grade ≥ 3, particularly if TIMI grade-0–1 flow or, poor visualization of the distal vessel, or as a bailout strategy after unsuccessful manual thrombectomy. Clinical variables were meticulously refined, and follow-up details were obtained through a thorough review of the patients’ health records. Following coronary angiography and successful guidewire crossing of the culprit lesion, ELCA was left at the operator’s discretion. It was used either as a primary device for thrombus removal or as a bailout strategy when manual thrombus aspiration did not improve TIMI grade flow. The selection of catheter size was mainly based on the target vessel diameter and on the characteristics of the vessel and the lesion; a 0.9 mm ELCA catheter is usually used in tortuous anatomies due to its better navigability and in small-caliber vessels, whereas a 1.4 mm catheter is used in selected cases involving larger proximal vessels with straight segments. Catheter size (0.9 mm or 1.4 mm) was selected based on vessel diameter and lesion characteristics. Laser fluence (45-60 mJ/mm²) and pulse repetition rate (25-40 Hz) were chosen as per manufacturer’s recommendations.
Before laser application, the target vessel was flushed with saline solution to prevent interaction between the laser and blood or contrast medium. In all cases, continuous saline infusion was administered during laser delivery to avoid coronary artery wall heating. Laser energy was delivered using an ‘on-off’ technique, consisting of 10-s laser activation cycles interspersed with 5-s pauses. The laser catheter was advanced at a rate of approximately 1 mm/s over a 0.014-in coronary guidewire through the target lesion, following the manufacturer’s recommendations.7,10 After 2–3 laser catheter passes, a follow-up coronary angiography was performed to evaluate the efficacy of laser application and assess the feasibility of stent implantation. TIMI grade flow was recorded after the ELCA procedure (Post-ELCA TIMI grade flow) and once the PCI would have been completed (final TIMI grade flow). Technical success was defined as the ability to advance the laser catheter through the entire target lesion and deliver laser energy successfully. Procedural success was defined as achieving a final TIMI grade ≥ 2 flow without any major cath lab-related complications, such as death, coronary perforation, or emergency bypass surgery after PCI completion. All procedural complications, including death, coronary perforation,11 emergency bypass surgery, distal embolization, ventricular arrhythmia, and no-reflow were carefully documented and reported. Follow-up was conducted via retrospective review of health records, and major adverse cardiovascular events (MACE) defined as a composite endpoint of all-cause mortality, new myocardial infarction, and target lesion revascularization were recorded at the follow-up.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation for normally distributed data or as the median (interquartile range) for non-normally distributed data. Inter-group comparisons were performed using an unpaired Student’s t-test for normally distributed variables and the Mann–Whitney U test for non-normally distributed variables. Categorical variables are expressed as counts and percentages and were analyzed using the chi-square test or Fisher’s exact test, as appropriate.
The composite endpoint of MACE was analyzed as time-to-event data at the follow-up. Kaplan–Meier survival analysis was performed to estimate the event-free survival rates. All statistical analyses were conducted using SPSS Statistics (version 23.0, IBM Corp., United States). A 2-tailed P value < .05 was considered statistically significant.
RESULTS
Between July 2015 and August 2024, a total of 130 PCI s were performed in patients with STEMI using ELCA as an adjuvant therapy for thrombus removal. The patients’ mean age was 61.8 ± 11.7 years, with 18 (13.8%) being women and 18 (13.8%) diagnosed with diabetes mellitus. ELCA was employed as the primary device for thrombus dissolution in 66 cases (50.8%) and as a bailout strategy in 64 cases (49.2%). Within the bailout group, manual thrombus aspiration was performed in 47 cases (36.2%), balloon dilation in 6 cases (4.6%), and thrombus debulking using the dotter effect in 11 cases (8.5%).
In the overall cohort, 124 patients (95.4%) presented with culprit lesions with a LTB. Before laser energy application, TIMI grade-0 flow was reported in 79 (60.8%) cases TIMI grade-1 flow in 32 (24.6%). After ELCA, TIMI grade-2 and 3 flows were achieved in 45 (34.6%) and 66 (50.8%) cases, respectively; P < .001 (figure 1).

Figure 1. TIMI grade flow distribution before and after ELCA application. Stacked bar graph showing the distribution of TIMI grade 0-3 flows at 3 different time points: initial angiography, post-ELCA, and final angiographic result after PCI. A marked improvement in coronary flow is observed following ELCA, with a progressive increase in TIMI grade-3 flow from 6.2% to 74.6%. ELCA, excimer laser coronary angioplasty; TIMI, Thrombolysis in Myocardial Infarction.
Technical success was achieved in 128 (98.5%) cases, and procedural success in 124 (95.4%) (table 1). Procedural success was significantly higher when ELCA was used as the initial strategy vs when it was used as the bailout strategy (100% vs 90.6%; P = .013). However, procedural time was significantly longer in the bailout vs the initial strategy group (69.81 vs 48.50 min, respectively) (table 2).
Table 1. Baseline characteristics of patients
| Variable (n = 130) | Value |
|---|---|
| Age, yr | 61.8 ± 11.7 |
| Female | 18 (13.8) |
| Hypertension | 59 (45,4%) |
| Hypercholesterolemia | 57 (43,8%) |
| Tobacco use | 78 (60%) |
| Diabetes mellitus | 18 (13.8) |
| Killip classification | |
| I | 98 (75.4) |
| II | 18 (13.8) |
| III | 3 (2.3) |
| IV | 11 (8.5) |
| Radial access | 118 (90,7%) |
| Femoral access | 12 (9,3%) |
| Lesion localization | |
| LMCA | 3 (2,3%) |
| LAD | 55 (42,3%) |
| LCX | 8 (6,2%) |
| RCA | 64 (49,2 %) |
| Primary device | 66 (50.8) |
| Bailout strategy | 64 (49.2) |
| Large thrombus burden | 124 (95.4) |
| Laser catheter size, Fr | |
| 0.9 | 114 (87.7) |
| 1.4 | 16 (12.3%) |
| Procedural time, min | 60 (43–86) |
| Fluoroscopy time, min | 22.2 ±12.2 |
| Laser frequency, Hz | 31 ± 10.4 |
| Laser fluency, mJ/mm2 | 46.5 ± 9.17 |
| Laser delivery time, s | 125.9 ± 83.4 |
| Technical success | 128 (98.5) |
| Procedural success | 124 (95.4) |
|
LAD: left anterior descending coronary artery; LCX: left circumflex artery; LMCA: left main coronary artery; RCA: right coronary artery. Categorical data are presented as absolute value and percentage, n (%); and continuous variables as mean ± standard deviation or first and third quartiles. |
|
Table 2. Difference in variables between the initial and bailout strategy groups
| Variable | ELCA as the initial strategy (n = 66) | ELCA as the bailout strategy (n = 64) | P-value |
|---|---|---|---|
| Complications | 8 (12.1%) | 3 (4.7%) | .100 |
| Large thrombus burden | 64 (97%) | 60 (93.8%) | .440 |
| Technical success | 65 (98.5%) | 63 (98.4%) | 1.000 |
| Procedural success | 66 (100%) | 58 (90.6%) | .013 |
| Procedural time, median | 48.50 (38.83–66.61) | 69.81 (55.36–101) | < .001 |
|
ELCA, excimer laser coronary angioplasty. Categorical data are presented as absolute value and percentage, n (%); and continuous variables as mean ± standard deviation or first and third quartiles. |
|||
One case of type IV coronary perforation, according to the modified Ellis classification, occurred in an octogenarian patient with an ecstatic and tortuous right coronary artery. Perforation sealing was achieved with the implantation of a covered stent. One cath lab-related death occurred in a patient with an uncrossable mid-segment of a left anterior descending coronary artery lesion and initial TIMI grade-3 flow. Following balloon dilation and partial advancement of the laser probe, complete vessel occlusion and suspected left main coronary artery dissection resulted in cardiac arrest and cath lab-related death.
Other procedural complications included distal embolization in 3 (2.3%) cases and slow flow or no-reflow in 4 (3.1%). Among the slow/no-reflow cases, 1 occurred after laser application, and 3 following stent implantation and/or post-dilation. All were successfully managed with optimal medical therapy, achieving final TIMI grade-2 flow. One episode of ventricular arrhythmia occurred during saline washout of the target vessel, requiring electrical cardioversion. Additionally, 1 case of stent thrombosis (0.8%) occurred intraoperatively (figure 2).
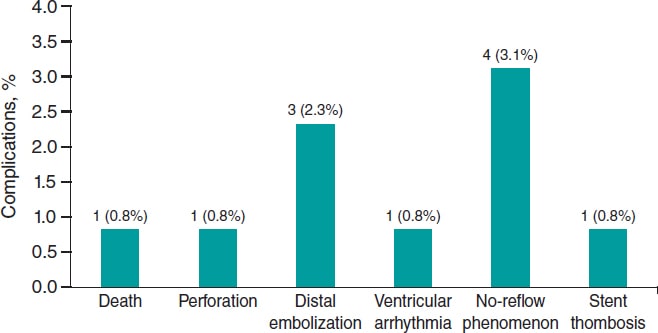
Figure 2. ELCA-related procedural complications. Bar chart showing the frequency and percentage of major complications during or immediately after ELCA. The most common was no-reflow (3.1%), followed by distal embolization (2.3%). Other events (death, perforation, ventricular arrhythmia, and stent thrombosis) were rare (0.8% each). ELCA, excimer laser coronary angioplasty.
Long-term follow-up data were missing for 6 patients (4.6%). At the 2-year follow-up, the event-free rate for combined MACE was 0.80 (95%CI, 0.73–0.88) as determined by the Kaplan–Meier estimator (table 3 and figure 3).
Table 3. List of adverse clinical events
| Patient No. | Event | Date |
|---|---|---|
| 6 | Death | 1 |
| 13 | Death | 493 |
| 15 | Death | 148 |
| 23 | Death | 11 |
| 33 | Death | 170 |
| 36 | Death | 4 |
| 43 | New myocardial infarction associated with TLR | 39 |
| 50 | New myocardial infarction | 213 |
| 61 | Death | 16 |
| 77 | Death | 1 |
| 83 | New myocardial infarction associated with TLR | 119 |
| 84 | Death | 4 |
| 92 | Death | 1 |
| 98 | Death | 0 |
| 101 | Death | 37 |
| 110 | Death | 0 |
| 113 | Death | 12 |
| 118 | Death | 253 |
| 121 | Death | 139 |
| 124 | New myocardial infarction associated with TLR | 291 |
| 128 | Death | 10 |
|
TLR, target lesion revascularization. Lost to follow-up: 6 patients (4.6%). |
||
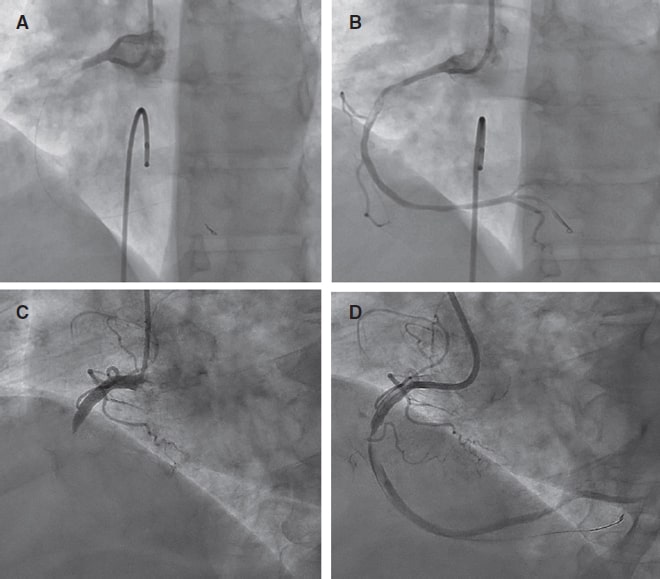
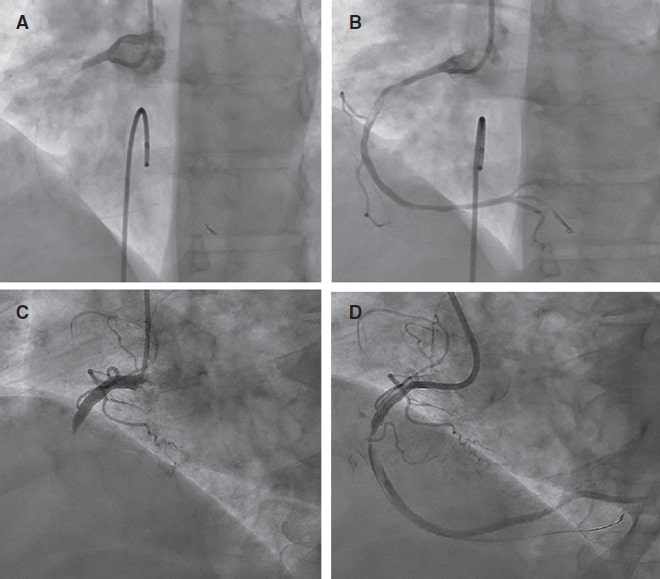
Figure 3. Pre- and post-ELCA findings in 2 typical cases of right coronary artery with large thrombus burden. ELCA, excimer laser coronary angioplasty.
DISCUSSION
The main finding of this single-center study is that coronary laser angioplasty is a feasible, safe, and effective adjuvant therapy in the context of PCI (videos 1-4 of the supplementary data), demonstrating a low rate of complications and an acceptable long-term rate of MACE.
Data on the use of ELCA in acute myocardial infarction remain limited, with most evidence coming from non-randomized clinical trials. The CARMEL trial,12 the largest multicenter study to date, evaluated the safety, feasibility, and acute outcomes of ELCA in patients with acute myocardial infarction within 24 h of symptom onset requiring urgent PCI. TIMI grade flow significantly improved after laser application, increasing from 1.2 to 2.8, with an overall procedural success rate of 91% and a low distal embolization rate of 2%, even though 65% of cases had a LTB. In our study, 95.4% of the patients had culprit lesions with a LTB, and laser delivery significantly improved the mean TIMI grade flow from 0.6 to 2.29, with a comparable distal embolization rate of 2.3%.
Arai et al.13 retrospectively analyzed 113 consecutive acute coronary syndrome cases undergoing PCI comparing an ELCA group (n = 48) with a thrombus aspiration group (n = 50). They found that ELCA was associated with a significantly shorter door-to-reperfusion time, a better myocardial blush grade, and fewer MACE vs thrombus aspiration. These favorable outcomes are likely attributable to ELCA’s ability to vaporize thrombi through acoustic shockwave propagation and dissolution mechanisms,12 as well as its capacity to suppress platelet aggregation kinetics (a phenomenon known as the ‘stunned platelet’ effect).14
Reperfusion injury to the coronary microcirculation is a critical concern during PCI in STEMI patients. While manual thrombus aspiration can reduce the rate of no-reflow in patients with a LTB, residual thrombi and decreased coronary flow following thrombectomy have been associated with a higher risk of no-reflow.15 In a study of 812 patients with STEMI and a LTB undergoing PCI, Jeon et al.16 reported that 34.4% experienced failed thrombus aspiration, defined as no thrombus retrieval, remnant thrombus grade ≥ 2, or distal embolization. This failure was associated with an increased risk of impaired myocardial perfusion and microvascular obstruction.
ELCA’s ability to vaporize thrombi (with a low rate of distal embolization) and mitigate platelet activation, key cofactors in myocardial reperfusion damage,17 can potentially reduce this undesirable effect. Although the direct impact of ELCA on coronary microcirculation in PCI has not been well documented, evidence from smaller studies suggests potential benefits. For example, Ambrosini et al.18 investigated ELCA in 66 patients with acute myocardial infarction and complete thrombotic occlusion of the infarcted related artery, demonstrating excellent acute coronary and myocardial reperfusion outcomes (as assessed by the myocardial blush score and the corrected TIMI frame count), as well as a low rate of long-term left ventricular remodeling (8%). The significant improvement in mean TIMI grade flow observed immediately after ELCA application in our cohort may indirectly suggest a protective effect of this technique on coronary microcirculation. However, the lack of large studies comparing ELCA with conventional STEMI treatment limits the ability to definitively confirm the benefits of coronary laser therapy in this setting. Shibata et al.19 explored the impact of ELCA on myocardial salvage using nuclear scintigraphy in 72 STEMI patients and an onset-to-balloon time < 6 h, comparing ELCA (n = 32) and non-ELCA (n = 40) groups. Their findings indicated a trend towards a higher myocardial salvage index in the ELCA vs the non-ELCA group (57.6% vs 45.6%).
Limitations
This study has several limitations. It is a retrospective analysis, which inherently introduces biases related to data collection, interpretation and application of inclusion and exclusion criteria. Besides, the absence of a comparative group limits the ability to establish the definitive clinical benefit of ELCA and its potential superiority over other strategies in the context of STEMI patients undergoing PCI. Furthermore, while the significant improvement of TIMI grade flow observed after laser application suggests potential benefits for coronary microcirculation, we did not directly assess this effect or thrombus burden reduction since post-ELCA thrombus grading was not systematically recorded. Unfortunately, in our retrospective database, PCI details (segmental analysis of coronary arteries and classification), the use of intravascular imaging modalities, dual antiplatelet therapy regimens (aspirin in addition to a potent P2Y12 inhibitor, or clopidogrel when prasugrel or ticagrelor were contraindicated, was routinely prescribed following current guidelines recommendations) or post-PCI echocardiography or cardiac magnetic resonance parameters were not systematically collected (unavailable in the health reports we revised) and follow-up data were missing for 4.6% of patients, all of which limited our ability to assess their potential impact on clinical outcomes. Last, our findings represent the experience of a single center, the percentage of women and patients with diabetes is relatively low, and procedures were performed by 5 trained operators, which may limit the external validity of the results.
CONCLUSIONS
ELCA seems to be an effective device for thrombus dissolution in the STEMI scenario, with excellent technical and procedural success rates. Besides, a low complication rate and favorable long-term outcomes with an acceptable event-free survival rate was observed in the present cohort.
DATA AVAILABILITY
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
FUNDING
None declared.
ETHICAL CONSIDERATIONS
This study was approved by the center Ethics Committee (waiving the need for informed consent due to the retrospective nature of the investigation) in full compliance with national legislation and the principles set forth in the Declaration of Helsinki. Sex was reported as per biological attributes (SAGER guidelines).
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
The authors state that no generative artificial intelligence technologies were used in the preparation or revision of this article.
AUTHORS’ CONTRIBUTIONS
A. Pernigotti and M. Mohandes were responsible for the conceptualization and study design and contributed equally as co-first authors. M. Mohandes, A. Pernigotti, R. Bejarano, H. Coimbra, F. Fernández, C. Moreno, M. Torres, J. Guarinos were involved in data collection and statistical analysis. M. Mohandes, A. Pernigotti, and J.L. Ferreiro were involved in manuscript drafting and critical revision and were responsible for the supervision and final approval. All authors have accepted responsibility for the entire content of this manuscript and consented to its submission to the journal. Each author reviewed all results and approved the final version of the manuscript.
CONFLICTS OF INTEREST
The authors declared no conflicts of interest related to this manuscript. J.L. Ferreiro declared having received speaker’s fees from Eli Lilly Co, Daiichi Sankyo, Inc., AstraZeneca, Pfizer, Abbott, Boehringer Ingelheim, Bristol-Myers Squibb, Rovi, Terumo and Ferrer; consulting fees from AstraZeneca, Eli Lilly Co., Ferrer, Boston Scientific, Pfizer, Boehringer Ingelheim, Daiichi Sankyo, Inc., Bristol-Myers Squibb and Biotronik; and research grants from AstraZeneca, not related to this manuscript.
WHAT IS KNOWN ABOUT THE TOPIC?
- ELCA is a specialized technique used as adjuvant therapy during PCI for STEMI, particularly in patients with LTB.
- Although former studies have shown that ELCA can improve coronary flow and potentially reduce thrombotic material, data in the setting of acute myocardial infarction remain limited.
- ELCA is mostly used in high-volume centers by experienced operators, and standardized criteria for use in STEMI patients are not consistently reported in the literature.
WHAT DOES THIS STUDY ADD?
- This is one of the largest retrospective single-center series (130 patients) ever reported on the use of ELCA in STEMI patients with angiographically defined LTB.
- The study shows a high rate of technical and procedural success, significant improvement in TIMI flow, low rate of complication, and acceptable long-term outcomes.
- It provides detailed information on operator training, device selection, and laser settings, contributing to transparency and reproducibility.
- It also identifies current limitations in data reporting (eg, lack of systematic thrombus grading or dual antiplatelet therapy regimen documentation), underscoring the need for standardization in future studies.
SUPPLEMENTARY DATA
Vídeo 1. Mohandes M. DOI: 10.24875/RECICE.M25000537
Vídeo 2. Mohandes M. DOI: 10.24875/RECICE.M25000537
Vídeo 3. Mohandes M. DOI: 10.24875/RECICE.M25000537
Vídeo 4. Mohandes M. DOI: 10.24875/RECICE.M25000537
REFERENCES
1. Byrne RA, Rossello X, Coughlan JJ, et al. 2023 ESC guidelines for the management of acute coronary syndromes. Eur Heart J. 2023;44:3720-3826.
2. Kim MC, Cho JY, Jeong HC, et al. Long-term clinical outcomes of transient and persistent no reflow phenomena following percutaneous coronary intervention in patients with acute myocardial infarction. Korean Circ J. 2016;46:490-498.
3. Sardella G, Mancone M, Bucciarelli-Ducci C, et al. Thrombus aspiration during primary percutaneous coronary intervention improves myocardial reperfusion and reduces infarct size:the EXPIRA prospective, randomized trial. J Am Coll Cardiol. 2009;53:309-315.
4. Jolly SS, Cairns JA, Yusuf S, et al. Randomized trial of primary PCI with or without routine manual thrombectomy. N Engl J Med. 2015;372:1389-1398.
5. Lawton JS, Tamis-Holland JE, Bangalore S, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization:Executive summary. Circulation. 2022;145:e4-e17.
6. Grundfest WS, Litvack F, Forrester JS, et al. Laser ablation of human atherosclerotic plaque without adjacent tissue injury. J Am Coll Cardiol. 1985;5:929-933.
7. Mohandes M, Fernández L, Rojas S, et al. Safety and efficacy of coronary laser ablation as an adjuvant therapy in percutaneous coronary intervention:a single-centre experience. Coron Artery Dis. 2021;32:241-246.
8. Rawlins J, Din JN, Talwar S, O'Kane P. Coronary intervention with the excimer laser:review of the technology and outcome data. Interv Cardiol Rev. 2016;11:27-32.
9. Gibson CM, de Lemos JA, Murphy SA, et al. Combination therapy with abciximab reduces angiographically evident thrombus in acute myocardial infarction:a TIMI 14 substudy. Circulation. 2001;103:2550-2554.
10. Topaz O, Das T, Dahm J, et al. Excimer laser revascularisation:current indications, applications and techniques. Lasers Med Sci. 2001;16:72-77.
11. Ellis SG, Ajluni S, Arnold AZ, et al. Increased coronary perforation in the new device era. Incidence, classification, management, and outcome. Circulation. 1994;90:2725-2730.
12. Topaz O, Ebersole D, Das T, et al. Excimer laser angioplasty in acute myocardial infarction (the CARMEL multicenter trial). Am J Cardiol. 2004;93:694-701.
13. Arai T, Tsuchiyama T, Inagaki D, et al. Benefits of excimer laser coronary angioplasty over thrombus aspiration therapy for patients with acute coronary syndrome and thrombolysis in myocardial infarction flow grade 0. Lasers Med Sci. 2022;38:13.
14. Topaz O, Minisi AJ, Bernardo NL, et al. Alterations of platelet aggregation kinetics with ultraviolet laser emission:the “stunned platelet“phenomenon. Thromb Haemost. 2001;86:1087-1093.
15. Ahn SG, Choi HH, Lee JH, et al. The impact of initial and residual thrombus burden on the no-reflow phenomenon in patients with ST-segment elevation myocardial infarction. Coron Artery Dis. 2015;26:245-253.
16. Jeon HS, Kim YI, Lee JH, et al. Failed thrombus aspiration and reduced myocardial perfusion in patients with STEMI and large thrombus burden. JACC Cardiovasc Interv. 2024;17:2216-2225.
17. Rezkalla SH, Kloner RA. No-reflow phenomenon. Circulation. 2002;105:656-662.
18. Ambrosini V, Cioppa A, Salemme L, et al. Excimer laser in acute myocardial infarction:single centre experience on 66 patients. Int J Cardiol. 2008;127:98-102.
19. Shibata N, Takagi K, Morishima I, et al. The impact of the excimer laser on myocardial salvage in ST-elevation acute myocardial infarction via nuclear scintigraphy. Int J Cardiovasc Imaging. 2020;36:161-170.

ABSTRACT
Introduction and objectives: To compare the effects of drug-coated balloon (DCB) vs drug-eluting stent (DES) in patients presenting with de novo large vessel coronary artery disease (CAD).
Methods: We conducted a systematic research of randomized controlled trials comparing DCB vs DES in patients with de novo large vessel CAD. Data were pooled by meta-analysis using a random-effects model. The prespecified primary endpoint was target lesion revascularization (TLR).
Results: A total of 7 trials enrolling 2961 patients were included. The use of DCB vs DES was associated with a similar risk of TLR (OR, 1.21; 95%CI, 0.44-3.30; I2 = 48%), all-cause mortality (OR, 1.56; 95%CI, 0.94- 2.57; I2 = 0%), cardiac death (OR, 1.65; 95%CI, 0.90-3.05; I2=0%), myocardial infarction (OR, 0.97; 95%CI, 0.58-1.61; I2 = 0%), major adverse cardiovascular adverse (OR, 1.19; 95%CI, 0.74-1.90; I2 = 13.5%) and late lumen loss (standardized mean difference [SMD], −0.35; 95%CI, −0.74 to 0.04; I2 = 81.4%). However, the DCB was associated with a higher risk of target vessel revascularization (OR, 2.47; 95%CI, 1.52-4.03; I2 = 0%) and smaller minimal lumen diameter during late follow-up (SMD, −0.36; 95%CI, −0.56 to −0.15; I2 = 34.5%). Nevertheless, prediction intervals included the value of no difference for both outcomes.
Conclusions: In patients with de novo large vessel CAD the use of DCB vs DES is associated with a similar risk of TLR. However, the DES achieves better late angiographic results.
Keywords: Drug-coated balloon. Drug-eluting stent. Coronary artery disease.
RESUMEN
Introducción y objetivos: Comparar los efectos del balón farmacoactivo (BFA) frente al stent farmacoactivo (SFA) en pacientes con enfermedad arterial coronaria (EAC) de vaso grande de novo.
Métodos: Se realizó una búsqueda sistemática de ensayos clínicos aleatorizados comparando BFA frente a SFA en pacientes con EAC de vaso grande de novo. Los datos se agruparon mediante un metanálisis de efectos aleatorios. El objetivo primario fue la necesidad de revascularización de la lesión diana (RLD).
Resultados: Se incluyeron 7 ensayos con 2.961 pacientes. El uso de BFA, en comparación con SFA, se asoció con un riesgo similar de RLD (OR = 1,21; IC95%, 0,44-3,30; I2 = 48%), muerte por todas las causas (OR = 1,56; IC95%, 0,94-2,57; I2 = 0%), muerte de causa cardiovascular (OR = 1,65; IC95%, 0,90-3,05; I2 = 0%), infarto de miocardio (OR = 0,97; IC95%, 0,58-1,61; I2 = 0%), acontecimientos adversos cardiacos mayores (OR = 1,19; IC95%, 0,74-1,90; I2 = 13,5%) y pérdida luminal tardía (DME = −0,35; IC95%, −0,74 a 0.04; I2 = 81,4%). Sin embargo, el BFA se asoció a un mayor riesgo de revascularización del vaso diana (OR = 2,47; IC95%, 1,52-4,03; I2 = 0%) y a un menor diámetro luminal mínimo en el seguimiento (DME: −0,36; IC95%, −0,56 a −0,15; I2 = 34,5%), aunque los intervalos de predicción incluyeron el valor nulo para ambos resultados.
Conclusiones: En los pacientes con EAC de vaso grande de novo, el BFA comparado con el SFA se asoció a un riesgo similar de RLD, obteniendo el SFA mejores resultados angiográficos.
Palabras clave: Balón farmacoactivo. Stent farmacoactivo. Enfermedad arterial coronaria.
Abbreviations
CAD: coronary artery disease. DCB: drug-coated balloon. DES: drug-eluting stent. MI: myocardial infarction. MLD: minimum lumen diameter. TLR: target lesion revascularization.
INTRODUCTION
Drug-eluting stents (DES) remain the standard of treatment for patients undergoing percutaneous coronary intervention (PCI).1,2 However, DES are associated with a gradually and permanent increased risk of adverse events, particularly due to late stent thrombosis and in-stent restenosis, with a 2% incidence rate per year with no plateau observed.1 This risk is even higher when complex and long lesions are treated.3 In recent years, drug-coated balloons (DCB) have emerged as a potential alternative treatment option to DES. Following adequate lesion preparation, unlike traditional stents, DCBs can release an antiproliferative drug into the vessel wall without leaving behind a permanent metal scaffold. Notably, permanent scaffolding can distort and constrain the coronary vessel, thus impairing vasomotion and adaptive remodelling, while also promoting chronic inflammation.4 DCB-PCI is a well-established treatment for in-stent restenosis and small-vessel coronary artery disease (CAD).5,6 However, its role in de novo large vessel CAD remains controversial. In a recent randomized clinical trial (RCT) with patients undergoing de novo CAD revascularization, a strategy of DCB-PCI did not achieve non-inferiority vs DES in terms of device-oriented composite endpoint driven by higher rates of target lesion revascularization (TLR).7 Contrary to prior published research, our findings did not support similar clinical outcomes for DCB vs DES in patients with de novo large vessel CAD.8,9 A recent meta-analysis of 15 studies compared DCB-PCI or hybrid angioplasty vs DES-PCI in patients with vessels > 2.75 mm in diameter showing no significant differences in the clinical endpoints of TLR, cardiac death, and MI.10 However, 14 of the 15 included studies were non-RCT, and the recent previously reported RCT was not included. Nevertheless, individual non-inferiority studies often lack the statistical power needed to definitively compare these technologies, underscoring the need for a systematic appraisal of treatment effects and evidence quality. Therefore, we conducted a systematic review and meta-analysis of available RCT to provide a comprehensive and quantitative assessment of evidence on the efficacy of DCB vs the current-generation DES in de novo large vessel CAD in terms of adverse events at longest available follow-up.
METHODS
Search strategy and selection criteria
We conducted a meta-analysis of RCT according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2009 guidelines.11 Two reviewers independently identified the relevant studies through an electronic search across the MEDLINE and Embase databases (from inception to October 2024). In addition, we employed backward snowballing (eg, reference review from identified articles and pertinent reviews). No language, publication date or publication status restrictions were imposed. This study is registered with PROSPERO and the search strategy is available in the supplementary data.
Study selection
Two reviewers independently assessed trial eligibility based on titles, abstracts, and full-text reports. Discrepancies in study selection were discussed and resolved with a third investigator. Eligible studies needed to meet the following pre-specified criteria: a) RCT comparing PCI with DCB and PCI with DES; b) study population including patients with de novo large vessel CAD (eg, defined as vessel diameter ≥ 2.5 mm);12 c) availability of clinical outcome data (without restriction as to follow-up time). Exclusion criteria were a) lack of a randomized design; b) studies including patients undergoing treatment for in-stent restenosis; c) studies including patients with de novo small vessel CAD; d) lack of any clinical outcome data.
A reference vessel diameter ≥ 2.5 mm was established as the cut-off value to define large vessel based on a recent proposed standardized definition.12
Data extraction
Three investigators (J. Llau García, S. Huélamo Montoro and J. A. Sorolla Romero) independently assessed studies for possible inclusion, with the senior investigator (J. Sanz-Sánchez) resolving discrepancies. Non-relevant articles were excluded based on title and abstract. The same investigators independently extracted data on study design, measurements, patient characteristics, and outcomes using a standardized data-extraction form. Data extraction conflicts were discussed and resolved with the senior investigator.
Data on authors, year of publication, inclusion and exclusion criteria, sample size, patients’ baseline patients, endpoint definitions, effect estimates, and follow-up time were collected.
Endpoints
The prespecified primary endpoint was TLR. Secondary clinical endpoints were all-cause mortality, cardiac death, myocardial infarction (MI), target vessel revascularization (TVR) and major adverse cardiovascular events (MACE). Secondary angiographic endpoints were minimum lumen diameter (MLD) and late lumen loss (LLL). Each endpoint was assessed according to the definitions reported in the original study protocols, as summarized in table 1 of the supplementary data. All the endpoints were assessed at the maximum follow-up available.
Table 1. Main features of included studies
| Study | Year of publication | No. of patients | Type of Device | Reference vessel diameter (mean ± SD) (mm) | Multicenter | Clinical follow up (months) | Angiographic follow-up (months) | |
|---|---|---|---|---|---|---|---|---|
| DCB | DES | |||||||
| REC-CAGEFREE I7 | 2024 | 1133 | 1139 | Paclitaxel-DCB Sirolimus-DES |
3.00 ± 0.55 | YES | 24 | NO |
| Nishiyama et al.13 | 2016 | 30 | 30 | Paclitaxel-DCB Everolimus-DES |
2.80 ± 0.63 | NO | 8 | 8 |
| Xue Yu et al.8 | 2022 | 85 | 85 | Paclitaxel-DCB Everolimus-DES |
2.89 ± 0.33 | NO | 12 | 9 |
| REVELATION9 | 2019 | 60 | 60 | Paclitaxel-DCB Sirolimus and everolimus DES |
3.24 ± 0.50 | NO | 24 | 9 |
| Gobic et al.15 | 2017 | 38 | 37 | Paclitaxel-DCB Sirolimus-DES |
> 2.50 | NO | 6 | 6 |
| Hao et al.16 | 2021 | 38 | 42 | Paclitaxel-DCB NA |
> 2.50 | NO | 12 | 12 |
| Wang et al.14 | 2022 | 92 | 92 | Paclitaxel-DCB Sirolimus-DES |
3.37 ± 0.52 | NO | 12 | 9 |
|
DCB, drug-coated balloon; DES, drug-eluting stent; NA, not available. |
||||||||
Risk of bias
The risk of bias in each study was assessed using the revised Cochrane risk of bias tool (RoB 2.0).11 Three investigators (J. Llau García, S. Huélamo Montoro and J. A. Sorolla Romero) independently assessed 5 domains of bias in RCT: a) randomization process, b) deviations from intended interventions, c) missing outcome data, d) outcome measurement, and e) selection of reported results (table 2 of the supplementary data).
Table 2. Baseline clinical characteristics of included patients
| Study | Age (years) | Male (%) | Diabetes (%) | Smoking (%) | Hypertension (%) | LVEF (%) | Clinical Presentation (CCS/ACS) (%) | Multivessel (%) | Complex lesion (%) |
|---|---|---|---|---|---|---|---|---|---|
| REC-CAGEFREE I7 | 62 | 69.3 | 27.3 | 45 | 60.1 | 60 | 44.9/55.3 | 4.8 | 0 |
| Nishiyama et al.13 | 69 | 73.3 | 41.6 | 60 | 83.3 | NA | 0/100 | NA | 36 |
| Xue Yu et al.8 | 63.3 | 69.3 | 24.1 | 54 | 63.9 | > 40 | 11.1/88.9 | 84 | 44.1 |
| REVELATION9 | 57 | 87 | 10 | 60 | 31 | 57.6 | 0/100 | 71.6 | N/A |
| Gobic et al.15 | 57.4 | 87 | 10 | 49.5 | 33.4 | 50.2 | 0/100 | NA | N/A |
| Hao et al.16 | 57.5 | 78.5 | 31.5 | 29.5 | 24 | 46 | 0/100 | NA | N/A |
| Wang et al.14 | 49.5 | 93.5 | 81.6 | 81.5 | 71.8 | NA | 0/100 | NA | N/A |
|
ACS, acute coronary syndrome; CCS, chronic coronary syndrome; NA, not available. |
|||||||||
Statistical analysis
Odds ratios (OR) and 95% confidence intervals (95%CI) were calculated using the DerSimonian and Laird random-effects model, with the estimate of heterogeneity being obtained from the Mantel-Haenszel method. The presence of heterogeneity among studies was evaluated with the Cochran Q chi-square test, with P ≤ .10 being considered of statistical significance, and using the I2 test to evaluate inconsistency. A value of 0% indicates no observed heterogeneity, and values of ≤ 25%, ≤ 50%, > 50% indicate low, moderate, and high heterogeneity, respectively. Prediction intervals (95%) in addition to conventional 95%CI around ORs were calculated to assess residual uncertainty. Publication bias and the small study effect were assessed for all outcomes, using funnel plots. The presence of publication bias was investigated using Harbord and Egger tests and visual estimation with funnel plots. We performed a sensitivity analysis by removing one study at a time to confirm that the findings, when compared with DES, were not driven by any single study. To account for different lengths of follow-up across studies, another sensitivity analysis was performed using the Poisson regression model with random intervention effects to calculate inverse-variance weighted averages of study-specific log stratified incidence rate ratios (IRRs). Results were displayed as IRRs, which are exponential ratios of the regression model. Additionally, random-effect meta-regression analyses were performed to assess the impact of the following variables on treatment effect with respect to the primary endpoint: eg, percentage of patients with acute coronary syndrome (ACS), percentage of patients with diabetes mellitus, mean reference vessel diameter and follow-up duration. The statistical level of significance was 2-tailed P < .05. Stata version 18.0 (StataCorp LP, College Station, United States), was used for statistical analyses.
RESULTS
Search results
Figure 1 illustrates the PRISMA study search and selection process. A total of 7 RCT were identified and included in this analysis. The main features of included studies are shown in table 1.
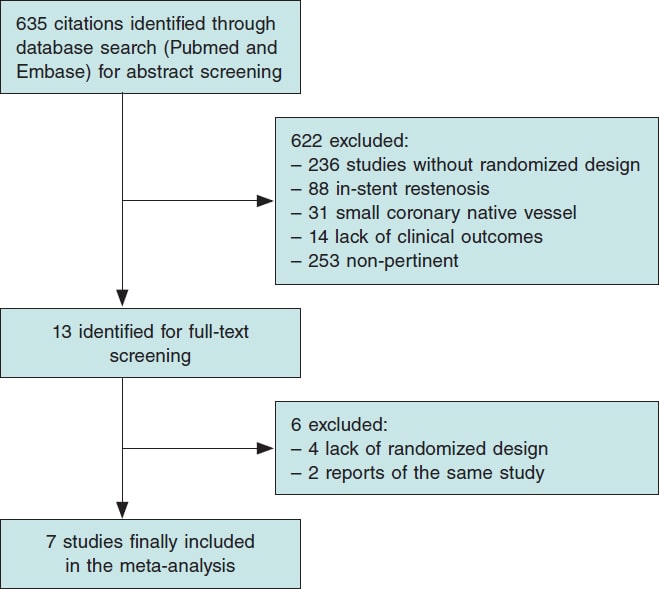
Figure 1. Flow diagram of the search for studies included in the meta-analysis according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement.
All studies had a non-inferiority design. A clinical primary endpoint was selected in 1 study,7 and an invasive functional endpoint was selected in another trial,9 while angiographic primary endpoints were prespecified in the remaining studies.8,13-16 The mean clinical and angiographic follow-up were 21.5 months and 8.9 months respectively. A total of 4 studies were conducted in the context of ACS9,14-17 and 1 study in the context of chronic coronary syndrome (CCS).13 Finally, 2 studies enrolled both ACS and CCS patients.7,8 A total of 3 trials enrolled patients treated with second-generation DES (Firebird 2.0 [Microport, China], Xience Xpedition [Abbott Vascular, United States], Orsiro [Biotronik, Germany]),7,9,13 and 2 studies enrolled patients treated with third-generation DES (Biomine [Meril Life Sciences, India], Cordimax [Rientech, China]).14,15 One trial enrolled patients treated with second and third-generation DES (Xience Xpedition [Abbott Vascular, United States], Resolute Integrity, [Medtronic, United States], Firehawk, [MicroPort, China]).8 All studies included patients who underwent paclitaxel-DCB-PCI ([Pantera Lux, Biotronik, Germany],9,14 [SeQuent Please, B Braun, Germany],7,8,13,15 [Bingo DCB, Yinyi Biotech,China]),16 and none with sirolimus-DCB-PCI.
Baseline characteristics
A total of 2961 patients were included, 1476 of whom received DCB and 1485, DES for de novo large vessel CAD. The patients main baseline characteristics are shown in table 2.
Publication bias and asymmetry
Funnel-plot distributions of the pre-specified outcomes indicate absence of publication bias for all the outcomes (figures 1-8 of the supplementary data).
Risk of bias assessment
Table 2 of the supplementary data illustrates the results of the risk of bias assessment with the RoB 2.0 tool. One trial was considered at low overall risk of bias,7 5 raised some concerns8,9,13,14,16 and 1 presented a high overall risk of bias.15
Outcomes
Clinical outcomes
DCB use compared with DES was associated with a similar risk of TLR (OR, 1.21; 95%CI, 0.44-3.30; I2 = 48%), all-cause mortality (OR, 1.56; 95%CI, 0.94- 2.57; I2 = 0%), cardiac death (OR, 1.65; 95%CI, 0.90-3.05; I2 = 0%), MI (OR, 0.97; 95%CI, 0.58-1.61; I2 = 0%) and MACE (OR, 1.19; 95%CI, 0.74-1.90; I2 = 13.5%). However, DCB was associated with a higher risk of TVR (OR, 2.47; 95%CI, 1.52- 4.03; I2 = 0%) (figure 2, figure 3 and figures 9-10 of the supplementary data).
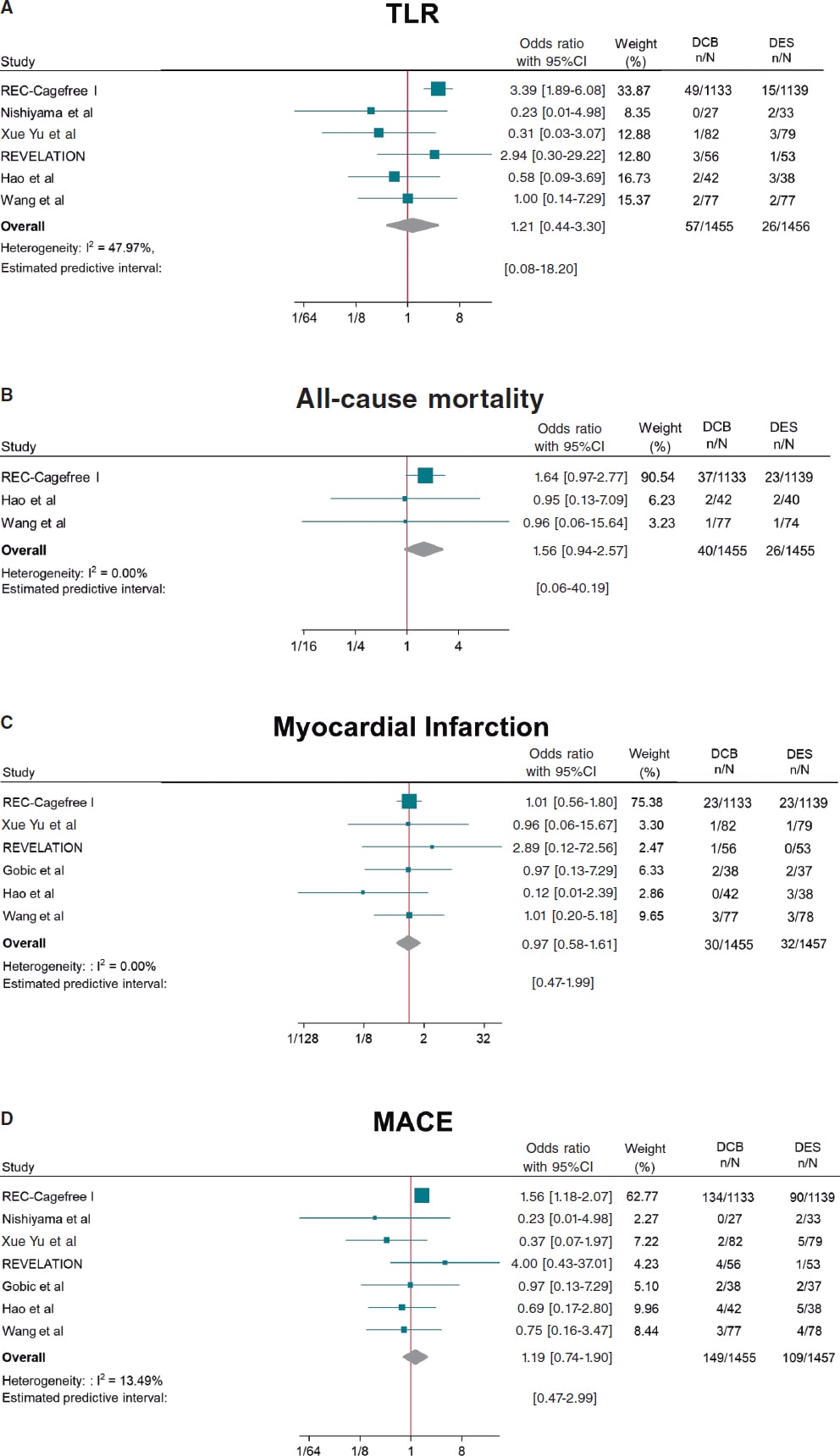
Figure 2. Forest plot reporting trial-specific and summary ORs with 95%CIs for the endpoint of A) target lesion revascularization; B) all-cause mortality; C) myocardial infarction; D) MACE. 95%CI, 95% confidence interval; DCB, drug-coated balloon; DES, drug-eluting stents; MACE, major adverse cardiovascular events; OR, odds ratio. References: REC-Cagefree I.,7 Nishiyama et al.,13 Xue Yu et al.,8 REVELATION,9 Hao et al.,16 Wang et al.,14 and Gobic et al.15
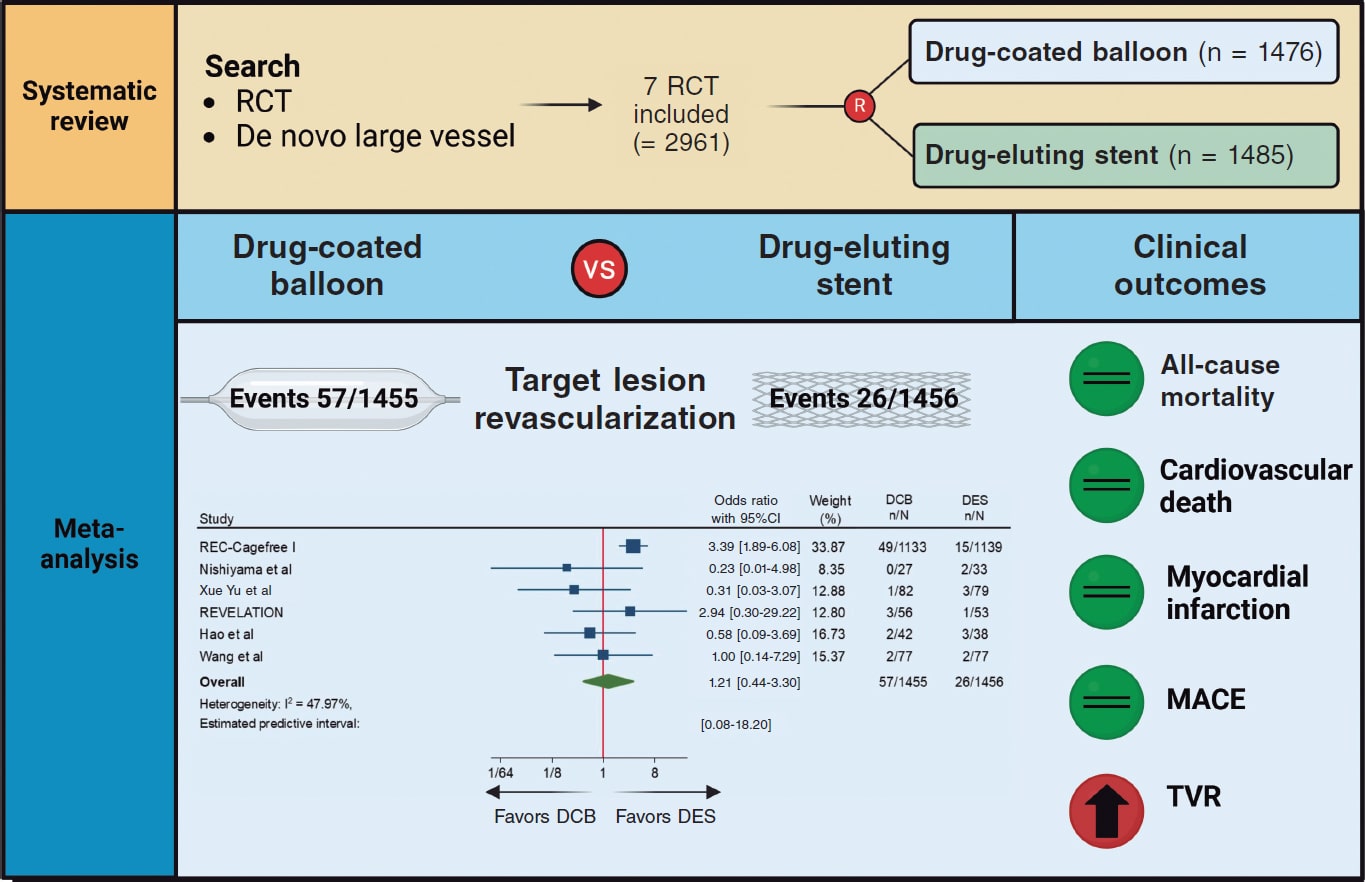
Figure 3. Central Illustration. DCB, drug-coated balloon; DES, drug-eluting stent; RCT, randomized clinical trial; TVR, target vessel revascularization. References: REC-Cagefree I.,7 Nishiyama et al.,13 Xue Yu et al.,8 REVELATION,9 Hao et al.,16 and Wang et al.14
Angiographic outcomes
Compared with DES, DCB use yielded significant smaller MLD (SMD, −0.36; 95%CI, −0.56 to −0.15; I2 = 34.5%) and similar risk of LLL (SMD, −0.35; 95%CI, −0.74 to 0.04; I2 = 81.4%) at follow-up (figure 4).
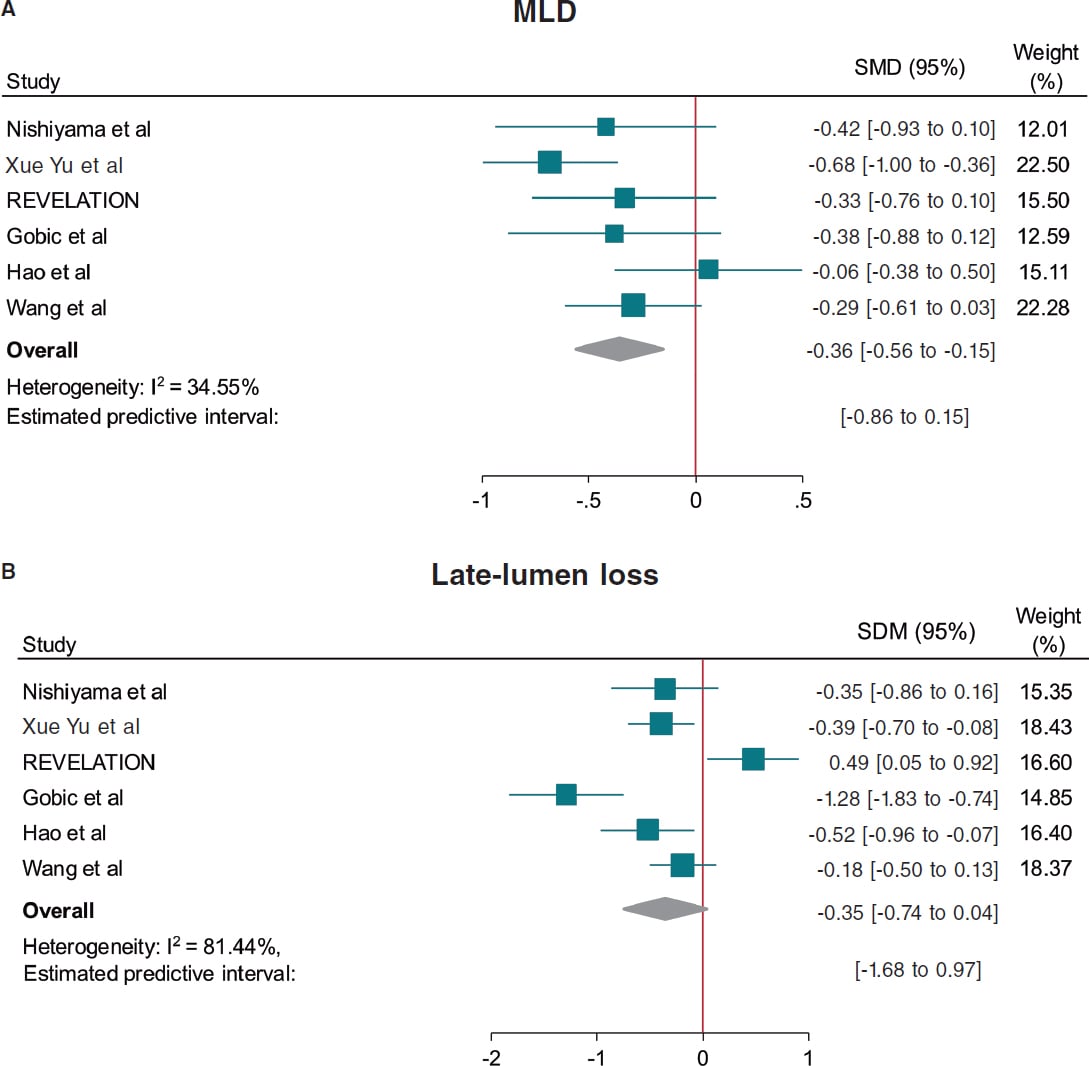
Figure 4. Forest plot reporting trial-specific and summary ORs with 95%CIs for the endpoint of A: minimum lumen diameter, and B: late-lumen loss. 95%CI, 95% confidence interval; DCB, drug-coated balloon; DES, drug-eluting stents; MACE, major adverse cardiovascular events; MLD, minimum lumen diameter; SMD, standardized mean difference; OR, odds ratio. References: Nishiyama et al.,13 Xue Yu et al.,8 REVELATION,9 Gobic et al.,15 Hao et al.,16 and Wang et al.14.
Prediction intervals were consistent with CI for all the outcomes except for TVR and MLD, which included the value of no difference.
Sensitivity analysis
A leave-one-out pooled analysis by iteratively removing one study at a time was performed for all endpoints. Treatment effects were consistent with the main analysis for TLR, all-cause mortality, cardiac death, MI and MLD. The risk of TVR was no longer significantly higher among patients undergoing DCB when removing the CAGEFREE I trial,7 and the risk of LLL was significantly lower among patients undergoing DCB-PCI when removing the REVELATION trial.9 However, an increased risk of MACE was observed among patients undergoing DCB-PCI when removing the study by Xue Yu et al.18 (tables 3-10 of the supplementary data). A sensitivity analysis using estimated IRRs was performed to account for varying follow-up lengths, confirming that our main analysis findings remained unchanged (table 11 of the supplementary data).
Random effect meta-regression analysis found no significant impact of the proportion of patients presenting with ACS (P = .882), diabetes mellitus (P = .641), mean reference vessel diameter (P = .985) and follow-up duration (P = .951) on treatment effect with respect to the primary endpoint.
DISCUSSION
This meta-analysis provides a comprehensive and updated quantitative analysis of available evidence on the comparison of DCB vs DES in de novo large vessel CAD, including data from 2961 patients enrolled in 7 RCT. The main findings of the study are:
a) The use of DCB was associated with a similar risk of clinical events vs DES except for TVR. However, data for this outcome was only available in 3 of the 7 included studies and the increased risk in patients undergoing DCB-PCI was not significant when the CAGEFREE I trial was removed. In addition, prediction intervals were not consistent with the CI. Therefore, the results of this outcome should be interpreted with caution.
b) The effect of DCB on the risk of TLR was not affected by the proportion of patients presenting with ACS or diabetes, as well as the mean reference vessel diameter or follow-up duration as assessed by meta-regression analysis.
c) DCB was associated with lower MLD at angiographic follow-up, but with similar LLL vs DES.
DES are the standard of treatment for patients undergoing PCI. However, complications such as stent thrombosis and in-stent restenosis still occur with rates estimated at 0.7-1% and 5-10% at the 10-year follow-up respectively.19,20 Therefore, in recent years there has been a growing concern for developing strategies to reduce stent-related adverse events. In this context, DCBs have emerged as a potential treatment alternative based on a “leaving nothing behind” strategy. Nevertheless, data of patients presenting with de novo large CAD is scarce and conflicting. The CAGEFREE I is the only available clinically powered RCT that included 2272 patients undergoing de novo non-complex CAD revascularization across 40 centers in China. A strategy of DCB-PCI did not achieve non-inferiority vs DES in terms of device-oriented composite endpoint driven by higher rates of TLR in the DCB-PCI group (3.1% vs 1.2%, P = .002). On the other hand, in single-center RCT conducted by Nishiyama et al. with 60 patients with CCS undergoing elective PCI a trend toward lower rates of TLR in the DCB-PCI group (0% vs 6.1%, P = .193) was shown at the 8-mont follow-up.13 Similarly, in a RCT including 170 patients undergoing PCI for de novo large CAD lower rates of TLR at the 12-month follow-up were found in patients undergoing DCB-PCI (1.6% vs 3.4%, P = .306).14 In our analysis when pooling data from all available RCT, the risk of TLR was similar among patients undergoing DCB-PCI or DES-PCI. Notably, since this result was obtained with a moderate heterogeneity (I2 ≈ 50%), it should be interpreted with caution regarding its general applicability. These findings remained unvaried at the leave-one-out analysis. In addition, prediction intervals were consistent with CI around ORs showing lack of residual uncertainty. Previous studies have shown that in-stent restenosis after DES is not a benign phenomenon, presenting as an ACS in about 70% of the cases, with 5-10% of these resulting in MI.21 We could speculate that the lack of permanent scaffold with DCB vs DES may predispose to a less aggressive pattern of restenosis and not increase the risk of thrombotic vessel closure beyond 3 months when vessel healing after DCB-PCI has occurred.22
Notably, 5 of the 7 studies included in this meta-analysis enrolled patients presenting with ACS. A total of 34% of the patients included in the CAGEFREE study presented with ACS, with 16% being STEMI cases.7 Four other studies only included STEMI patients.7,9,14-16 Although the performance of DCB in the STEMI scenario is unknown, its use in clinical practice is increasing.23 Culprit lesion plaques in STEMI patients are usually soft and adequate plaque modification can be easily achieved through DCB-PCI (< 30% residual stenosis and low grade of dissection).23 Moreover, the ruptured lipid rich plaque can potentially be an ideal reservoir for effective paclitaxel uptake.24 On the other hand, DCBs carry specific risks for STEMI patients, such as acute recoil and culprit lesion closure, because they don’t provide vessel scaffolding.
In our study, the proportion of patients presenting with ACS had no impact on treatment effects on the meta-regression analysis. Nevertheless, further RCT with adequate sample size are needed to obtain more solid evidence in this field. Of note, complex lesions (eg, severe calcification and bifurcations with planned two-stent technique) were excluded from the studies that included patients presenting with CCS.7,8 Therefore, our findings might not be generalized to this population.
The better angiographic surrogate outcomes with DES-PCI vs DCB-PCI found in our meta-analysis after pooling data from 6 studies can be explained by the absence of a metal scaffold to expand the vessel lumen and the acute recoil following balloon angioplasty. This justifies the lower MLD achieved after DCB-PCI vs DES-PCI. While our analysis did not show significant differences regarding LLL during follow-up, the value of LLL was lower among patients undergoing DCB-PCI when excluding the REVELATION trial.9,17 This study showed extremely low LLL in both DCB and DES groups vs other available evidence from RCT.15,16 The presence of positive vessel remodeling with a late lumen enlargement after the use of DCB evaluated by intracoronary imaging modalities has been evidenced in multiple studies, and seems to be associated with small vessel disease, fibrous and layered plaques and a post-PCI medial dissection arc > 90°.25,26,27 However, evidence of this phenomenon in patients with large vessel CAD is less known.22 It should, therefore, be noted that all studies in this meta-analysis used paclitaxel-DCB. While the evidence comparing sirolimus and paclitaxel-DCB is scarce, 2 recent RCT have shown better angiographic results with the lipophilic component. In the first one, with 121 patients with the novo small vessel CAD, sirolimus-DCB failed to achieve non-inferiority for net-lumen gain at 6 months.28 In the second study, with 70 patients, the 2 devices showed similar results of LLL at 6 months, although patients treated with paclitaxel-DCB had more frequent late luminal enlargement.29 Due to the small sample size and although there is not enough evidence to evaluate differences across clinical endpoints, we cannot assume that there is a class effect across all DCBs. There are larger ongoing RCT to evaluate the outcomes of sirolimus DCB vs DES in large vessels that will provide evidence in this field.30,31
Limitations
The results of our investigation should be interpreted in light of some limitations. First, this is a study-level meta-analysis providing average treatment effects. The lack of patient-level data from the included studies prevents us from assessing the impact of baseline clinical, angiographic and procedural characteristics on treatment effects. Second, minor differences in definition were present for some endpoints (eg, MACE), limiting the reliability of effect estimates. Third, one study which accounted for approximately 75% of all patients included did not included angiographic follow-up,7 thus limiting the evaluation of DCB and DES on angiographic outcomes. Fourth, the clinical follow-up varied from 6 to 24 months. Ideally, outcomes such as TLR should be compared at uniform follow-up across studies (eg, at 1 year), which was not consistently possible in the current analysis. Nonetheless, these differences in follow-up duration were accounted with the IRRs, as detailed in the Methods section. However, longer follow-ups are needed to establish the safety and efficacy profile of DCB vs DES throughout time. Fifth, the definition of large vessel is inconsistent across trials, which might be a source of bias. Finally, the limited number of studies and patients, and the small event rate for some endpoints, such as all-cause mortality may reduce the power for detecting significant differences across groups.
CONCLUSIONS
This meta-analysis provides the most updated quantitative evidence on the use of DCB vs DES for the treatment of de novo large vessel CAD in both CCS and ACS. DCB-PCI is associated with similar TLR and LLL at mid-term follow-up representing an appealing treatment option for patients with large vessel CAD.
FUNDING
None declared.
ETHICAL CONSIDERATIONS
Ethics approval was deemed unnecesary for this meta-analysis as all data were collected and synthesized from previous studies. Additionally, no informed consent was required as there were no patients involved in our work. The meta-analysis of RCT was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2009 guidelines. We confirm that sex/gender biases have been taken into consideration.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
No artificial intelligence has been used in the preparation of this article.
AUTHORS’ CONTRIBUTIONS
J. Llau García, S. Huelamo Montoro and J.A. Sorolla Romero participated in literature research and study selection. J.A. Sorolla Romero, L. Novelli and J. Sanz Sánchez contributed to the conception, design, drafting and revision of the article. P. Rubio, J.L. Díez Gil, L. Martínez-Dolz, I.J. Amat Santos, B. Cortese, F. Alfonso, and H.M. Garcia-Garcia contributed to the critical revision of the intellectual content of the article.
CONFLICTS OF INTEREST
F. Alfonso is an associate editor of REC: Interventional Cardiology; the journal’s editorial procedure to ensure impartial handling of the manuscript has been followed. The authors declared no relevant relationships with the contents of this paper.
WHAT IS KNOWN ABOUT THE TOPIC?
- DCB are a well-established treatment for patients with small-vessel CAD.
- Available published evidence of patients with de novo large vessel CAD is scarce and shows conflicting results.
WHAT DOES THIS STUDY ADD?
- In this meta-analysis including data from 2961 patients enrolled in 7 RCT, DCB showed similar risk of clinical events at follow-up vs DES in the treatment of de novo large vessel CAD.
- The use of DCB might be considered as an alternative option to DES in patients undergoing PCI for non-complex de novo large vessel CAD.
REFERENCES
1. Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87-165.
2. Lawton JS, Tamis-Holland JE, Bangalore S, et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization:Executive Summary:A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e4-e17.
3. Kong MG, Han JK, Kang JH, et al. Clinical outcomes of long stenting in the drug-eluting stent era:patient-level pooled analysis from the GRAND-DES registry. EuroIntervention J Eur Collab Work Group Interv Cardiol Eur Soc Cardiol. 2021;16:1318-1325.
4. Kawai T, Watanabe T, Yamada T, et al. Coronary vasomotion after treatment with drug-coated balloons or drug-eluting stents:a prospective, open-label, single-centre randomised trial. EuroIntervention. 2022;18:e140?e148.
5. Jeger RV, Eccleshall S, Wan Ahmad WA, et al. Drug-Coated Balloons for Coronary Artery Disease:Third Report of the International DCB Consensus Group. JACC Cardiovasc Interv. 2020;13:1391-1402.
6. Sanz Sánchez J, Chiarito M, Cortese B, et al. Drug-Coated balloons vs drug-eluting stents for the treatment of small coronary artery disease:A meta-analysis of randomized trials. Catheter Cardiovasc Interv Off J Soc Card Angiogr Interv. 2021;98:66-75.
7. Gao C, He X, Ouyang F, et al. Drug-coated balloon angioplasty with rescue stenting versus intended stenting for the treatment of patients with de novo coronary artery lesions (REC-CAGEFREE I):an open-label, randomised, non-inferiority trial. Lancet Lond Engl. 2024;404:1040-1050.
8. Yu X, Wang X, Ji F, et al. A Non-inferiority, Randomized Clinical Trial Comparing Paclitaxel-Coated Balloon Versus New-Generation Drug-Eluting Stents on Angiographic Outcomes for Coronary De Novo Lesions. Cardiovasc Drugs Ther. 2022;36:655-664.
9. Vos NS, Fagel ND, Amoroso G, et al. Paclitaxel-Coated Balloon Angioplasty Versus Drug-Eluting Stent in Acute Myocardial Infarction:The REVELATION Randomized Trial. JACC Cardiovasc Interv. 2019;12:1691-1699.
10. Gobbi C, Giangiacomi F, Merinopoulos I, et al. Drug coated balloon angioplasty for de novo coronary lesions in large vessels:a systematic review and meta-analysis. Sci Rep. 2025;15:4921.
11. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses:the PRISMA statement. BMJ. 2009;339:b2535.
12. Sanz-Sánchez J, Chiarito M, Gill GS, et al. Small Vessel Coronary Artery Disease:Rationale for Standardized Definition and Critical Appraisal of the Literature. J Soc Cardiovasc Angiogr Interv. 2022;1:100403.
13. Nishiyama N, Komatsu T, Kuroyanagi T, et al. Clinical value of drug-coated balloon angioplasty for de novo lesions in patients with coronary artery disease. Int J Cardiol. 2016;222:113-118.
14. Wang Z, Yin Y, Li J, et al. New Ultrasound-Controlled Paclitaxel Releasing Balloon vs. Asymmetric Drug-Eluting Stent in Primary ST-Segment Elevation Myocardial Infarction-A Prospective Randomized Trial. Circ J Off J Jpn Circ Soc. 2022;86:642-650.
15. Gobic´D, Tomulic´V, Lulic´D, et al. Drug-Coated Balloon Versus Drug-Eluting Stent in Primary Percutaneous Coronary Intervention:A Feasibility Study. Am J Med Sci. 2017;354:553-560.
16. Hao X, Huang D, Wang Z, Zhang J, Liu H, Lu Y. Study on the safety and effectiveness of drug-coated balloons in patients with acute myocardial infarction. J Cardiothorac Surg. 2021;16:178.
17. Niehe SR, Vos NS, Van Der Schaaf RJ, et al. Two-Year Clinical Outcomes of the REVELATION Study:Sustained Safety and Feasibility of Paclitaxel-Coated Balloon Angioplasty Versus Drug-Eluting Stent in Acute Myocardial Infarction. J Invasive Cardiol. 2022;34:E39-E42.
18. Xue W, Ma J, Yu X, et al. Analysis of the incidence and influencing factors associated with binary restenosis of target lesions after drug-coated balloon angioplasty for patients with in-stent restenosis. BMC Cardiovasc Disord. 2022;22:493.
19. Coughlan JJ, Maeng M, Räber L, et al. Ten-year patterns of stent thrombosis after percutaneous coronary intervention with new- versus early-generation drug-eluting stents:insights from the DECADE cooperation. Rev Esp Cardiol. 2022;75:894-902.
20. Madhavan MV, Redfors B, Ali ZA, Prasad M, et al. Long-Term Outcomes After Revascularization for Stable Ischemic Heart Disease:An Individual Patient-Level Pooled Analysis of 19 Randomized Coronary Stent Trials. Circ Cardiovasc Interv.2020;13:e008565.
21. Buchanan KD, Torguson R, Rogers T, et al. In-Stent Restenosis of Drug-Eluting Stents Compared With a Matched Group of Patients With De Novo Coronary Artery Stenosis. Am J Cardiol. 2018;121:1512-1518.
22. Antonio Sorolla Romero J, Calderón AT, Tschischke JPV, Luis Díez Gil J, Garcia-Garcia HM, Sánchez JS. Coronary plaque modification and impact on the microcirculation territory after drug-coated balloon angioplasty:the PLAMI study. Rev Esp Cardiol. 2025;78:481-482.
23. Merinopoulos I, Gunawardena T, Corballis N, et al. Assessment of Paclitaxel Drug-Coated Balloon Only Angioplasty in STEMI. JACC Cardiovasc Interv. 2023;16:771-779.
24. Maranhão RC, Tavares ER, Padoveze AF, Valduga CJ, Rodrigues DG, Pereira MD. Paclitaxel associated with cholesterol-rich nanoemulsions promotes atherosclerosis regression in the rabbit. Atherosclerosis. 2008;197:959-966.
25. Kleber FX, Schulz A, Waliszewski M, et al. Local paclitaxel induces late lumen enlargement in coronary arteries after balloon angioplasty. Clin Res Cardiol Off J Ger Card Soc. 2015;104:217-225.
26. Alfonso F, Rivero F. Late lumen enlargement after drug-coated balloon therapy:turning foes into friends. EuroIntervention J Eur Collab Work Group Interv Cardiol Eur Soc Cardiol. 2024;20:523-525.
27. Yamamoto T, Sawada T, Uzu K, Takaya T, Kawai H, Yasaka Y. Possible mechanism of late lumen enlargement after treatment for de novo coronary lesions with drug-coated balloon. Int J Cardiol. 2020;321:30-37.
28. Ninomiya K, Serruys PW, Colombo A, et al. A Prospective Randomized Trial Comparing Sirolimus-Coated Balloon With Paclitaxel-Coated Balloon in De Novo Small Vessels. JACC Cardiovasc Interv. 2023;16:2884-2896.
29. Ahmad WAW, Nuruddin AA, Abdul KMASK, et al. Treatment of Coronary De Novo Lesions by a Sirolimus- or Paclitaxel-Coated Balloon. JACC Cardiovasc Interv. 2022;15:770-779.
30. Spaulding C, Krackhardt F, Bogaerts K, et al. Comparing a strategy of sirolimus-eluting balloon treatment to drug-eluting stent implantation in de novo coronary lesions in all-comers:Design and rationale of the SELUTION DeNovo Trial. Am Heart J. 2023;258:77-84.
31. Greco A, Sciahbasi A, Abizaid A, et al. Sirolimus-coated balloon versus everolimus-eluting stent in de novo coronary artery disease:Rationale and design of the TRANSFORM II randomized clinical trial. Catheter Cardiovasc Interv Off J Soc Card Angiogr Interv. 2022;100:544-552.

ABSTRACT
Introduction and objectives: The early administration of unfractionated heparin (UFH) for ST-segment elevation myocardial infarction (STEMI) is still a matter of discussion, and clinical practice guidelines leave the timing of administration prior to angioplasty at the physician’s discretion.
Methods: We conducted a systematic search across PubMed/Cochrane databases for studies comparing pre-treatment with UFH with a comparative untreated group (non-UFH) of patients with STEMI undergoing primary angioplasty and including TIMI flow and 30-day mortality targets from June 2024 through September 2024. We conducted a randomized meta-analysis and assessed the risk of publication bias to detect asymmetry in the included studies.
Results: We included a total of 7 studies published from 2002 through 2022 (6 retrospective trials and 1 substudy of a randomized trial) for a total of 36 831 patients: 17 751 in the UFH pre-treatment group and 19 080 in the non-UFH control group. A total of 6202 patients (31.6%) on UFH had TIMI grade-II/III flow vs 5106 (23.0%) on non-NFH while 490 (3.9%) on UFH died within 30 days vs 673 (5.1%) on non-NFH. Meta-analysis demonstrated a higher probability of TIMI grade-II/III flow (HR, 1.35; 95%CI, 1.25-1.45; P < .0001) and a lower 30-day mortality rate in patients on UFH pretreatment (HR, 0.80; 95%CI, 0.72-0.90; P = .0002), with no differences being reported in bleeding complications (HR, 0.87; 95%CI, 0.72-1.05; P = .150).
Conclusions: Meta-analysis of studies shows that pretreatment with UFH in STEMI patients undergoing primary angioplasty is associated with a higher probability of TIMI grade-II/III flow and a lower risk of early mortality.
Meta-analysis registered in PROSPERO (CRD420250655362).
Keywords: Meta-analysis. Unfractionated heparin. ST-segment elevation myocardial infarction. Prognosis. Pre-treatment. Acute myocardial infarction.
RESUMEN
Introducción y objetivos: La administración temprana de heparina no fraccionada (HNF) en el infarto agudo de miocardio con elevación del segmento ST (IAMCEST) está sujeta a controversia, por lo que las guías de práctica clínica dejan a criterio médico el momento de su administración antes de la angioplastia.
Métodos: Entre junio y septiembre de 2024 se realizó una búsqueda sistemática en PubMed y Cochrane de estudios que comparasen el pretratamiento con HNF con un grupo control no tratado (no-HNF) en pacientes con IAMCEST tratados con angioplastia primaria e incluyesen los objetivos de flujo TIMI y la mortalidad a 30 días. Se llevó a cabo un metanálisis aleatorizado, en el que se evaluó el riesgo de sesgo de publicación para detectar asimetría en los estudios incluidos.
Resultados: Se incluyeron 7 estudios publicados entre 2002 y 2022, de los cuales 6 eran retrospectivos y 1 subestudio de un ensayo aleatorizado, con 36.831 pacientes: 17.751 el grupo de pretratamiento con HNF y 19.080 el grupo control no-HNF. Un total de 6.202 (31,6%) con HNF tuvieron flujo TIMI II/III, frente a 5.106 (23,0%) de los no-HNF, y 490 (3,9%) con HNF fallecieron en 30 días, frente a 673 (5,1%) de los no-HNF. El metanálisis demostró mayor probabilidad de flujo TIMI II/III (HR = 1,35; IC95%, 1,25-1,45; p < 0,0001) y menor mortalidad en los pacientes que recibieron pretratamiento con HNF (HR = 0,80; IC95%, 0,72-0,90; p = 0,0002), sin diferencias en complicaciones hemorrágicas (HR = 0,87; IC95%, 0,72-1,05; p = 0,150).
Conclusiones: El metanálisis muestra que el pretratamiento con HNF en pacientes con IAMCEST y angioplastia primaria se asocia a una mayor probabilidad de flujo TIMI II/III y un menor riesgo de mortalidad precoz.
Metanálisis registrado en PROSPERO (CRD420250655362).
Palabras clave: Metanálisis. Heparina no fraccionada. Infarto con elevación del segmento ST. Pronóstico. Pretratamiento. Infarto agudo de miocardio.
Abbreviations
PCI: percutaneous coronary intervention. STEMI: ST-segment elevation myocardial infarction. TIMI: Thrombolysis in Myocardial Infarction. UFH: unfractionated heparin.
INTRODUCTION
The implementation of STEMI Code protocols, involving emergency and cardiology services, has led to improved care for ST-segment elevation myocardial infarction (STEMI) and lower morbidity and mortality rates.1 Correct diagnosis of STEMI, pretreatment with antiplatelet agents, and the organization of rapid and direct transfer to a center with an PCI-capable center for primary angioplasty are quality standards in the management of STEMI.1
Parenteral anticoagulation is generally recommended in acute coronary syndrome at the time of diagnosis.1 In STEMI, the use of unfractionated heparin (UFH) during primary angioplasty is recommended to prevent coronary thrombosis and device-related complications, but its use should be discontinued after the procedure.2
The precise role of UFH pretreatment at the time of first medical contact for patients diagnosed with STEMI remains to be fully defined. The 2023 clinical practice guidelines of the European Society of Cardiology1 allow attending physicians to decide when to administer UFH during treatment, as there is no solid evidence supporting its early use. This flexibility is based on the absence of conclusive data on the benefits of UFH at this stage of treatment.1,3
The most recent data published on the implementation of STEMI Code protocols and care networks in Spain reveal heterogeneity in response times and transport among the different autonomous communities (AC).4,5 This heterogeneity is also observed in the administration of anticoagulant and antiplatelet pretreatment across the 17 AC. A review of STEMI Code protocols as of December 2024 shows that in 6 of the 17 (35.3%) AC, pretreatment with UFH is recommended, representing coverage for 43.1% of the Spanish population (table 1 of the supplementary data). All AC, except for one, include dual antiplatelet therapy as pretreatment at the first medical contact in their protocols. Differences in the standardization of UFH use in STEMI Code protocols reflect the lack of evidence and concrete recommendations on this topic.
Table 1. Demographic data of populations and characteristics of the selected studies
| Study | Design | Country | n | n (%) | Age, years | Concomitant antiplatelets, drug, and dosis | Male sex | Door-to-balloon time | UFH dosis | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UFH | Non-UFH | UFH | Non-UFH | UFH | Non-UFH | UFH | Non-UFH | UFH | Non-UFH | UFH | Non-UFH | ||||
| Fabris et al. (2022)10 | Retrospective | Italy | 537 | 237 (44.1%) | 300 (55.9%) | 66.0 ± 12.5 | 67.0 ± 12.0 | ASA: 237, 250 mg (100%) Ticagrelor: 237, 280 mg (100%) | ASA: 300, 250 mg (100%) PCI-capable center: UFH + ticagrelor or prasugrel or clopidogrel | 176 (74.3%) | 226 (75.3%) | 77 | 74 | 70 IU/kg | 70 IU/kg |
| Emilsson et al. (2022)6 | Retrospective | Sweden | 22 376 | 11 188 (50.0%) | 11 188 (50.0%) | 67.0 ± 12.0 | 67.0 ± 12.0 | ASA: 226, NR (2%) Clopidogrel: 221, NR (22%) Ticagrelor: 4642, NR (41%), Prasugrel: 574, NR (5.1%) | ASA: 170, NR (1.5%) Clopidogrel: 28, NR (0.3%) Ticagrelor: 4486, N (40%) Prasugrel: 608, NR (5.4%) | 7877 (70%) | 7992 (71%) | 276 ± 244 | 290 ± 270 | NR | NR |
| Bloom et al. (2021)7 | Retrospective | Australia | 2746 | 1373 (50.0%) | 1373 (50.0%) | 63.0 ± 12.4 | 63.2 ± 12.7 | ASA: 1327, NR (96.6%) Ticagrelor: 944, NR (68.8%) | ASA: 1326, NR (96.6%) Ticagrelor: 975, NR (70.9%) | 1099 (80.0%) | 1081 (78.7%) | 48 ± 23 | 64 ± 47 | 4000 + 1000 IU/h in ambulance | 4000 IU |
| McGinley et al. (2020)8 | Retrospective | Scotland | 1000 | 437 (43.7%) | 563 (56.3%) | 63.7 ± NR | 63.7 ± NR | ASA: 437, NR (100%) Clopidogrel: 437, NR (100%) | ASA: NR Clopidogrel: NR | 304 (69.6%) | 390 (69.3%) | NR | NR | 5000 IU | 5000 IU |
| Karlsson et al. (2019)11 | Subanálisis de ensayo clínico aleatorizado | Sweden | 7144 | 2898 (40.6%) | 4246 (59.4%) | 66.0 ± 11.5 | 66.0 ± 11.6 | ASA: 2817, NR (97.2%), Clopidogrel: 1662, NR (57.4%), Ticagrelor: 731, NR (25.2%) Prasugrel: 243, NR (8.4%) | ASA: 3636, NR (85.6%) Clopidogrel: 2489, NR (58.6%) Ticagrelor: 578, NR (13.6%) Prasugrel: 338, NR (8%) | 2169 (74.8%) | 3177 (74.8%) | 185 (125–320) | 181 (118–327) | NR | NR |
| Giralt et al. (2015)9 | Retrospective | Spain | 1326 | 758 (57.2%) | 568 (42.8%) | 61.3 ± 12.8 | 63.4 ± 12.8 | ASA: 744, NR (98.2%) Clopidogrel: 742, NR (97.9%) | ASA: 531, NR (93.5%) Clopidogrel: 503, NR (88.6%) | 618 (81.5%) | 434 (76.4%) | 107 (86–133) | 105 (83–140) | 5000 IU | 5000 IU |
| Zijlstra et al. (2002)12 | Retrospective | The Netherlands | 1702 | 860 (50.5%) | 842 (49.5%) | 59.0 ± 11.0 | 61.0 ± 11.0 | ASA: 860, NR (100%) | ASA: 842, NR (100%) | 696 (80.9%) | 665 (79.0%) | 81 ± 43 | 26 ± 39 | NR | NR |
|
ASA, acetylsalicylic acid; IU, international units; NR, not reported; UFH, unfractionated heparin. |
|||||||||||||||
The aim of this study is to perform a systematic review and meta-analysis of existing studies on UFH pretreatment in the context of primary angioplasty as a reperfusion treatment for STEMI in terms of TIMI (Thrombolysis in Myocardial Infarction) grade 2-3 flow at the start of the procedure and early 30-day in-hospital mortality.
The meta-analysis follows PRISMA guidelines to ensure transparency and quality (table 2 of the supplementary data).
Table 2. Events from the studies
| Study | n | n (%) | Open artery: TIMI grade 2-3 flow | Major bleeding | 30-day mortality | ||||
|---|---|---|---|---|---|---|---|---|---|
| UFH | Non-UFH | UFH | Non-UFH | UFH | Non-UFH | UFH | Non-UFH | ||
| Fabris et al. (2022)10 | 537 | 237 (44.1%) | 300 (55.9%) | 113 (47.7%) | 87 (29.0%) | 5 (2.1%) | 6 (2.0%) | 23 (9.7%) | 28 (9.3%) |
| Emilsson et al. (2022)6 | 22 376 | 11 188 (50.0%) | 11 188 (50.0%) | 4233 (37.8%) | 3263 (29.2%) | 199 (17.8%) | 199 (17.8%) | 313 (2.8%) | 395 (3.5%) |
| Bloom et al. (2021)7 | 2746 | 1373 (50.0%) | 1373 (50.0%) | 178 (13.0%) | 128 (9.3%) | 19 (1.4%) | 26 (1.9%) | 41 (3.0%) | 48 (3.5%) |
| McGinley et al. (2020)8 | 1000 | 437 (43.7%) | 563 (56.3%) | 111 (25.4%) | 136 (24.2%) | 6 (1.4%) | 5 (0.9%) | 11 (2.5%) | 47 (8.3%) |
| Karlsson et al. (2019)11 | 7144 | 2898 (40.6%) | 4246 (59.4%) | 1075 (37.1%) | 1204 (28.3%) | 47 (1.6%) | 92 (2.2%) | 76 (2.6%) | 131 (3.1%) |
| Giralt et al. (2015)9 | 1326 | 758 (57.2%) | 568 (42.8%) | 229 (30.2%) | 120 (21.1%) | 9 (1.2%) | 6 (1.1%) | NR | NR |
| Zijlstra et al. (2002)12 | 1702 | 860 (50.5%) | 842 (49.5%) | 263 (30.6%) | 168 (20.0%) | 43 (5.0%) | 59 (7.0%) | 26 (3.0%) | 24 (2.9%) |
| Total | 36 831 | 17 751 (48.0%) | 19 080 (51.9%) | 6202 (31.6%) | 5106 (23.0%) | 328 (4.3%) | 393 (4.7%) | 490 (3.9%) | 673 (5.1%) |
|
NR, not reported; TIMI: Thrombolysis in Myocardial Infarction; UFH, unfractionated heparin. |
|||||||||
METHODS
Literature search
We conducted a systematic search of scientific literature across Medline-PubMed and the Cochrane Controlled Register of Trials (CENTRAL) from May through September 2024. We accessed several observational studies and clinical trials comparing UFH pretreatment in the ambulance vs no pretreatment in patients diagnosed with STEMI treated with primary angioplasty. A lower date limit was set to 2002, and no language restrictions were applied. Studies were selected if they included information on initial TIMI grade flow, 30-day early mortality, and major bleeding complications. In the article by Emilsson et al.6 data from the propensity score cohort, which provides better adjustment, were used, and the same population was used in the study by Bloom et al.7. The references of the selected studies were analyzed to obtain additional articles via cross-referencing. Both the search and article selection methodology are shown in figure 1.
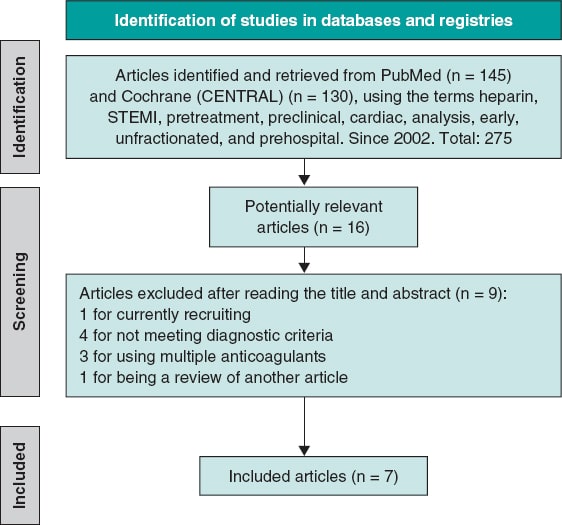
Figure 1. Flowchart of the literature search. STEMI, ST-segment elevation myocardial infarction.
The PRISMA guidelines were followed (table 2 of the supplementary data), and the meta-analysis was registered in PROSPERO (CRD420250655362).
Inclusion criteria
We included observational, retrospective, and clinical trials analyzing the use of UFH as pretreatment in patients diagnosed with STEMI, administered at the first medical contact, in the ambulance or at a non-PCI-capable center prior to arrival at the destination hospital where the percutaneous coronary intervention (PCI) would be performed along with a control group of patients without UFH pretreatment. Moreover, studies had to provide information on initial TIMI grade flow, the 30-day mortality rate, and major bleeding complications. Only studies with a population of at least 500 individuals were included.
Exclusion criteria
We excluded repeated series from the same group, studies prior to 2002, those on pretreatment with anticoagulants other than UFH, studies without a control comparator group, or those that included patients diagnosed with non-ST-segment elevation acute coronary syndrome, angina, or another cardiac event different from STEMI. Studies with less than 500 individuals were also excluded.
Selection of publications
The article search was conducted across Medline-PubMed and CENTRAL databases using the following terms: “preclinical,” “cardiac,” “heparin,” “analysis, early,” “unfractionated,” “STEMI,” “prehospital,” and “pretreatment.” A total of 275 relevant articles were identified, from which, after initial exclusion based on inclusion and exclusion criteria, 16 were selected, and 7 finally remained6-12 (6 of them conducted in European populations and 1 in Australia.7) All were observational and retrospective studies, except for the one conducted by Karlsson et al.11, which is a subanalysis of a randomized clinical trial.
Primary and secondary endpoints
The primary endpoint was to analyze the association of UFH pretreatment with the presence of initial TIMI grade 2-3 flow in the infarct-related artery on diagnostic coronary angiography, prior to the start of PCI, in patients for whom PCI was indicated. The secondary endpoints were to analyze the association of UFH pretreatment with 30-day mortality in STEMI patients with an indication for PCI, and the presence of major bleeding complications13 (clinically significant bleeding events, such as bleeding requiring medical intervention or blood transfusion, or resulting in a significant hemoglobin decrease).
Data collection and management
Abstracts and methods sections of selected publications were systematically reviewed to ensure they met the inclusion criteria. Disagreements were resolved by consensus. Measures were taken to avoid duplication of articles.
Risk of bias
The risk of bias in the studies included in the meta-analysis was assessed using a combination of robust statistical approaches and widely recognized visual methods. The Harbord test (table 3 of the supplementary data), specifically designed to detect publication bias in meta-analyses reporting risk ratios (RR), was used. Additionally, funnel plots were analyzed, allowing a visual assessment of asymmetry in the distribution of study effects. The funnel plots for each variable are included in figures 1-3 of the supplementary data.
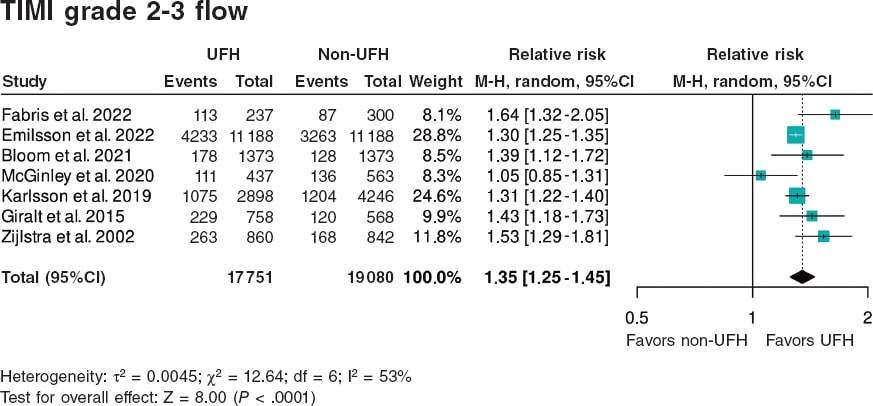
Figura 2. Forest plot of the prevalence of TIMI grade 2-3 flow. References cited in this figure: Fabris et al.10, Emilsson et. al.6, Bloom et al.7, McGinley et al.8, Karlsson et al.11, Giralt et al.9, and Zijlstra et al.12. 95%C, 95% confidence interval; M-H, Mantel-Haenszel’s method; UFH, unfractionated heparin.
In this meta-analysis, the variables of interest include TIMI grade 2-3 flow, 30-day mortality, and major bleeding. Furthermore, their potential bias was analyzed using the above-mentioned statistical procedures.
Statistical analysis
For statistical analysis, version 4.2.1 of R was used, using the metabin function from the meta package to synthesize the results of studies comparing UFH pretreatment vs UFH administered in the cath lab. Binary event data and totals for each group were analyzed using the RR model, with the inverse variance method, which weights studies based on precision, giving more weight to those with lower variance. A forest plot was generated using a random-effects model; the graph shows point estimates and corresponding 95% confidence intervals (95%CI) for each individual study, along with an overall RR estimate. The graph design followed the RevMan format, with customized labels indicating the groups of interest (UFH and non-UFH) and direction of effects (favors UFH and favors non-UFH), improving clinical interpretation. Heterogeneity analysis included calculation of the I2 value, which quantifies the proportion of variability among studies not attributable to chance, due to observed heterogeneity. As indicated by the τ2 value from the Harbord test analysis (table 3 of the supplementary data), the random-effects model is most appropriate in this situation.
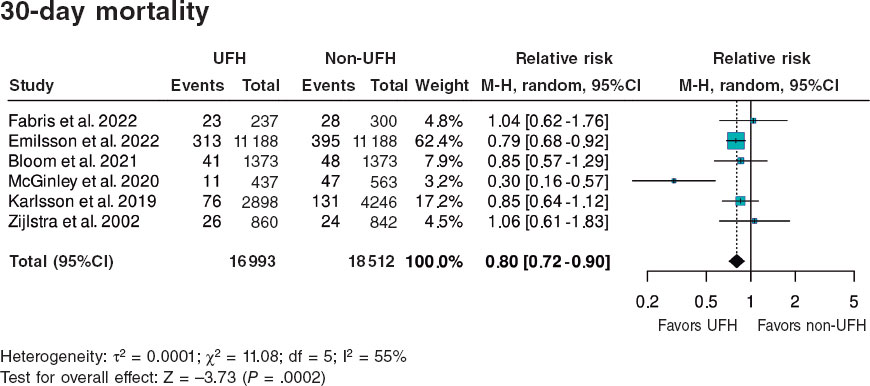
Figure 3. Forest plot of the prevalence of 30-day mortality. References cited in this figure: Fabris et al.,10 Emilsson et al.,6 Bloom et al.,7 McGinley et al.,8 Karlsson et al.,11 Giralt et al.,9 and Zijlstra et al.12. 95%C, 95% confidence interval; M-H, Mantel-Haenszel’s method; UFH, unfractionated heparin.
RESULTS
A total of 36 831 patients were included, of whom 17 751 (48.2%) received pretreatment with UFH and 19 080 (51.8%) did not, the latter representing the control group for comparison. Of note, data from the article by Emilsson et al.6 were obtained from the propensity score-matched cohort, which provides better adjustment; therefore, the study population was 22 376 rather than 41 631 patients. Additionally, this adjusted cohort was used in the study by Bloom et al.7 with 2746 instead of 4720 patients. The data shown in table 1 correspond to the propensity score-matched cohort and include a summary of the demographics and characteristics of each study. A substantial portion of the population was male (70–80% of the total), with mean ages ranging from 60 to 67 years. Only 5 of the 7 studies reported the UFH dose, which ranged from 4000 to 5000 IU.
Primary endpoint
A total of 6202 (31.6%) patients pretreated with UFH achieved TIMI grade 2-3 flow vs 5106 (23.0%) from the non-pretreated group (table 2).
The meta-analysis of data from the 7 studies6-12 demonstrated a significant increase in the likelihood of TIMI grade 2-3 flow, with a hazard ratio (HR) of 1.35 (95%CI, 1.25–1.45; P < .0001) (figure 2). Although all studies showed statistically significant differences, except for the one by McGinley et al.8, heterogeneity in the magnitude of association was high (I2 = 53%).
Secondary endpoints
A total of 490 (3.9%) patients pretreated with UFH died within 30 days vs 673 (5.1%) from the non-pretreated group (table 2). The meta-analysis of data from the 7 included studies6-12 showed a reduction in 30-day mortality in patients who received pretreatment with UFH (HR, 0.80; 95%CI, 0.72–0.90; P = .0002) vs those who did not. Two studies showed a significantly lower mortality rate in the UFH group individually, including the one with the largest sample size6 (65.8% of patients included in the meta-analysis). Heterogeneity in the magnitude of association was high (I2 = 55%) (figure 3).
Regarding the association between UFH pretreatment and the rate of hemorrhagic complications, there were no significant associations in the meta-analysis of the 7 studies6-12 (HR, 0.87; 95%CI, 0.72–1.05; P = .1502). None of the studies showed a significant association, either beneficial or harmful, regarding bleeding complications. Heterogeneity among the studies was low (I2 = 0%) (figure 4).
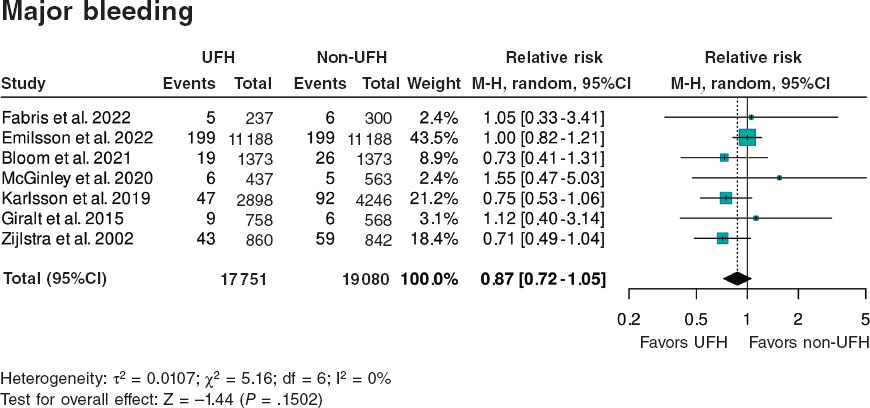
Figure 4. Forest plot of the prevalence of major bleeding. References cited in this figure: Fabris et al.,10 Emilsson et al.,6 Bloom et al.,7 McGinley et al.,8 Karlsson et al.,11 Giralt et al.,9 and Zijlstra et al.12. 95%C, 95% confidence interval; M-H, Mantel-Haenszel’s method; UFH, unfractionated heparin.
DISCUSSION
This meta-analysis demonstrates that for STEMI patients, administering UFH as pretreatment at the point of first medical contact is more beneficial than doing so at the PCI-capable center.
The observed benefit consists of a higher percentage of initial TIMI grade 2-3 flow (absolute increase of 8.6% and relative increase od 37.4%) and a lower 30-day mortality rate (absolute reduction of 1.2% and relative reduction of 23.5%). While most studies individually indicated a benefit of UFH pretreatment for open arteries, only 2 of the 7 studies primarily supported a mortality benefit. In terms of safety, pretreatment at first medical contact had a neutral effect on the risk of bleeding complications, and results were homogeneous across all 7 studies.
A recently published meta-analysis of 14 studies14 with different inclusion criteria and endpoints, incorporated studies on UFH and on a mix of other anticoagulants, such as low molecular weight heparin (enoxaparin), bivalirudin, and fondaparinux, along with additional events like cardiogenic shock, in-hospital mortality, and 1-year mortality. Both analyses showed consistent results on the benefit of pretreatment on open artery rates and early mortality, although they differed in the rate of bleeding complication. The referenced meta-analysis14 found a beneficial association between pretreatment with UFH or fractionated heparin in the reduction of bleeding complications. Moreover, the meta-analysis also analyzed the impact of pretreatment on the percentage of patients with cardiogenic shock, showing a positive association between pretreatment with UFH or fractionated heparin and this outcome. Of note, the heterogeneity in design, timing, and concomitant antiplatelet therapy among the studies included in the 2 meta-analyses. Notably, a small observational study conducted in Spain reported benefits from UFH pretreatment.15
Arguments for and against UFH pretreatment in STEMI
UFH is inexpensive, accessible, administered intravenously, and widely used in health care centers and ambulances. UFH is a necessary drug to prevent arterial and catheter thrombosis during PCI.
Beyond the potentially beneficial effects on open artery rates demonstrated in the meta-analysis, the use of UFH as pretreatment at the first medical contact does not seem to influence PCI per se. The rate of arterial puncture-related complications in anticoagulated patients is low when radial access is used; according to recent studies on the STEMI Code in Spain, 90% of angioplasties in STEMI patients are performed via radial access.4,5 There is still no information available on the potential impact of pretreatment on thrombus burden, no-reflow, ST-segment resolution, or infarct size, and no current studies have been designed to address these specific issues3 (table 4 of the supplementary data).
Clinical practice guidelines do not make definitive recommendations either due to the absence of adequately randomized trials evaluating the value of UFH pretreatment—not because there is evidence of no effect.1,3
In some patients misdiagnosed with STEMI (eg, those with aortic syndrome, pericarditis, myocarditis, NSTEMI, transient ST-segment elevation, Takotsubo syndrome, pulmonary embolism, pneumothorax, thoracic or esophageal disease, or musculoskeletal chest pain), pretreatment with UFH and antiplatelets may be harmful.
Notably, recent studies on the STEMI Code in Spain4-5 report that in 16.6% of all STEMI Code activations, the diagnosis of STEMI could not be confirmed, and in 3.6% of cases, no angioplasty was performed.
These misdiagnoses were not analyzed in the included meta-analyses. The design of the studies, most of which were retrospective, complicates the identification of risks associated with UFH use in these contexts. Theoretically, UFH pretreatment might be more beneficial in patients with recent thrombus formation, shorter times from symptom onset to first medical contact, and longer transfers to the PCI-capable center. However, in this meta-analysis, the review of study characteristics does not allow conclusions in this regard. There was no evident relationship between time metrics and the use of UFH pretreatment and the rates of open arteries or short-term mortality.
Need for randomized clinical trials
Clinical practice guidelines have become key reference documents for organizing health care delivery.1,2 These guidelines, developed by international experts who thoroughly review the scientific evidence to support their recommendations, are not always implemented in the routine clinical practice. One example, relevant to our field, is the use of antiplatelet pretreatment at the first medical contact. One year after the publication of the European Society of Cardiology guidelines on the management of acute coronary syndrome,1 which assigned a class IIb recommendation and level B evidence to dual antiplatelet therapy (DAPT) in STEMI, nearly all hospitals in Spain continued to administer DAPT at the first medical contact (table 1 of the supplementary data).
The current recommendation against antiplatelet pretreatment, similarly to the indication on pretreatment with UFH, is based on the absence of evidence demonstrating a benefit from DAPT and concerns on the potential bleeding risk associated with the use of long half-life antiplatelet agents in patients who may require emergency revascularization surgery.16-18 However, the need for emergency surgery is extremely rare in STEMI cases. Implementation of the clinical practice guidelines and reducing pretreatment to a single antiplatelet agent has been adopted in other European countries with preliminary positive results (EuroPCR 2024 presentation).19 In countries such as Denmark and Germany, and certain regions of Italy, STEMI care protocols include the administration of a single antiplatelet agent (acetylsalicylic acid) along with UFH.20 Despite the absence of a clear recommendation regarding UFH in the guidelines, it can be inferred that protocol developers trust in the theoretically beneficial effect of UFH, complementing antiplatelet therapy at the time of the first medical contact.
Study limitations
This study has inherent limitations related to its design. Observational studies, especially retrospective ones, are susceptible to various biases, including selection and confounding, as well as uncontrolled factors that may affect internal validity. However, several measures were taken to mitigate these biases and provide a robust interpretation of the findings, including a detailed sensitivity analysis to assess result consistency. Moreover, heterogeneity was explored using the Harbord test, which yielded high p-values for publication bias, indicating no significant evidence of such bias influencing our results. Despite this, heterogeneity remains an inherent challenge in meta-analyses that include observational studies with varying designs and quality. This variability was documented using measures such as τ2 and considered in interpreting the findings.
Despite these limitations, the results remain valuable and should be interpreted with caution.
Another limitation is the heterogeneity in the number of patients included in the selected studies, the times for care and transfer, the doses of UFH administered, the definition of hemorrhagic complications, and the concomitant antiplatelet regimens used, all of which may have introduced bias.
CONCLUSIONS
The meta-analysis of retrospective studies and one clinical trial shows that pretreatment with UFH in patients with STEMI undergoing a primary angioplasty is associated with an increase in the initial TIMI grade 2-3 flow and a lower early mortality rate (figure 5).
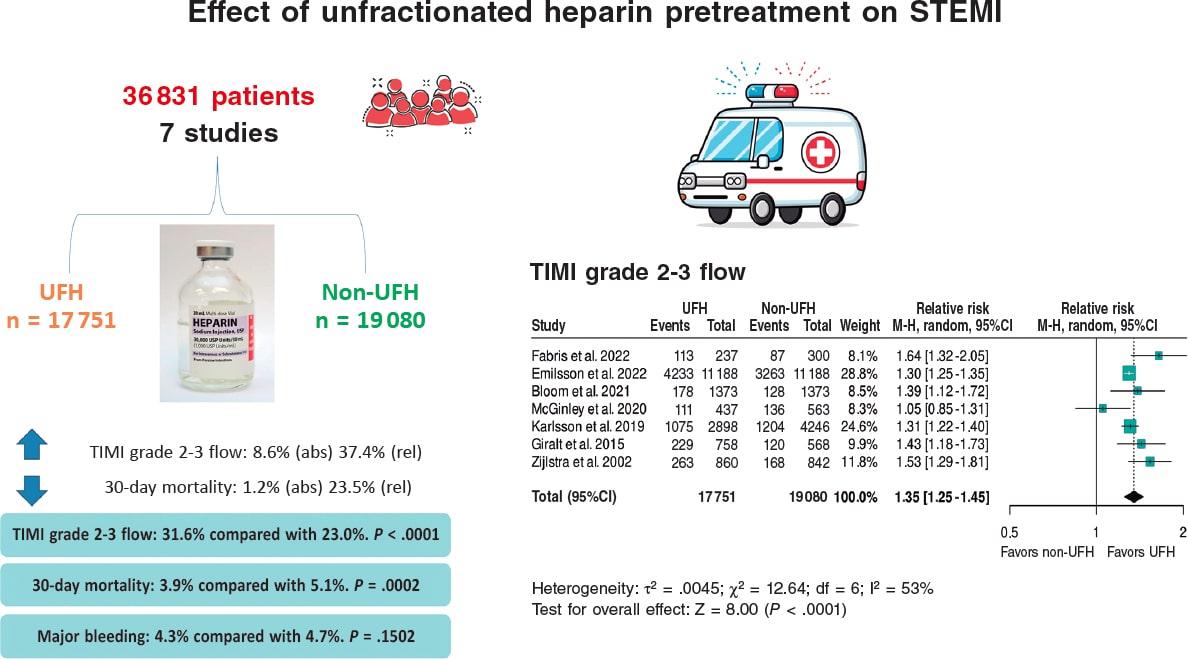
Figura 5. Effect of unfractionated heparin pretreatment in patients with ST-segment elevation myocardial infarction (STEMI). Forest plot of the prevalence of TIMI grade 2-3 flow. References cited in this figure: Fabris et al.10, Emilsson et al.6, Bloom et al.7, McGinley et al.8, Karlsson et al.11, Giralt et al.9, and Zijlstra et al.12. 95% CI: 95% confidence interval, M-H: Mantel-Haenszel’s method, UFH: unfractionated heparin.
Specifically designed clinical trials are needed to establish the impact of early UFH administration, and current clinical practice guidelines should provide clearer recommendations on the optimal timing of UFH pretreatment in STEMI patients.
FUNDING
This work was funded by CIBERCV CB16/11/00385.
ETHICAL CONSIDERATIONS
Ethical considerations are not applicable to a meta-analysis, as no direct clinical data from individuals are collected and therefore ethics committee evaluation is deemed unnecessary. A subgroup analysis by sex was not performed because it would result in a loss of statistical power, and both women and men were represented. There is no prior evidence or data suggesting that men and women respond differently to IV heparin anticoagulant treatment.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
Artificial intelligence was not used.
AUTHORS’ CONTRIBUTIONS
M. Roldán Medina and A. Riquelme Pérez equally contributed to various phases of the study: study conception and design, data acquisition, analysis, and interpretation. Additionally, M. Roldán Medina contributed to drafting the original manuscript and editing and reviewing the final version, while A. Riquelme López was responsible for the final revision of the article.
R. López-Palop and P. Carrillo contributed to data acquisition, analysis, and interpretation, and to the final text review and editing. J. Lacunza participated in data acquisition, analysis, and interpretation. R. Valdesuso contributed to data acquisition, analysis, and interpretation.
J. García de Lara was involved in data acquisition, analysis, interpretation, and final text review and editing. J. Hurtado-Martínez contributed to data acquisition, analysis, and interpretation. J.M. Durán contributed to data acquisition, analysis, and interpretation. E. Pinar-Bermúdez participated in data acquisition, analysis, and interpretation. J.R. Gimeno and D. Pascual-
CONFLICTS OF INTEREST
None declared.
ACKNOWLEDGMENTS
The authors would like to thank all colleagues from the Cardiology Department at Hospital Virgen de la Arrixaca, IMIB, and Universidad de Murcia whose collaboration made this study possible.
WHAT IS KNOWN ABOUT THE TOPIC?
- The early administration of unfractionated heparin (UFH) in ST-segment elevation myocardial infarction (STEMI) is controversial. Current clinical practice guidelines leave the timing of its administration before primary angioplasty to the physician’s discretion and do not provide clear recommendations on UFH pretreatment in STEMI patients prior to their arrival at the PCI-capable center.
WHAT DOES THIS STUDY ADD?
- Our meta-analysis and systematic review of studies on the safety and efficacy profile of UFH pretreatment in STEMI patients, compared with control patients who did not receive such pretreatment, demonstrates that pretreatment with UFH was associated with an increased TIMI grade 2-3 flow, a lower 30-day mortality rate, and fewer major bleeding events.
REFERENCES
1. Byrne RA, Rossello X, Coughlan JJ, et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J Acute Cardiovasc Care. 2024;13:55-161. Erratum in:Eur Heart J Acute Cardiovasc Care. 2024;13:455
2. Lawton JS, Tamis-Holland JE, Sripal Bangalore, et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization.J Am Coll Cardiol. 2022;79:197-215.
3. Collet J, Zeitouni M. Heparin pretreatment in STEMI:is earlier always better?EuroIntervention. 2022;18:697-699.
4. Rodríguez-Leor O, Cid-Álvarez AB, Moreno R, et al. Regional differences in STEMI care in Spain. Data from the ACI-SEC Infarction Code Registry. REC Interv Cardiol. 2023;5:118-128.
5. Rodríguez-Leor O, Cid-Álvarez AB, Pérez de Prado A, et al. Analysis of the management of ST-segment elevation myocardial infarction in Spain. Results from the ACI-SEC Infarction Code Registry. Rev Esp Cardiol. 2022;75:669-680.
6. Emilsson O, Bergman S, Mohammad M, et al. Pretreatment with heparin in patients with ST-segment elevation myocardial infarction:a report from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR). EuroIntervention. 2022;18:709-718.
7. Bloom J, Andrew E, Nehme Z, et al. Pre-hospital heparin use for ST-elevation myocardial infarction is safe and improves angiographic outcomes. Eur Heart J Acute Cardiovasc Care. 2021;10:1140-1147.
8. McGinley C, Mordi I, Kell P, et al. Prehospital Administration of Unfractionated Heparin in ST-Segment Elevation Myocardial Infarction Is Associated With Improved Long-Term Survival. J Cardiovasc Pharmacol. 2020;76:159-163.
9. Giralt T, Carrillo X, Rodriguez-Leor O, et al. Time-dependent effects of unfractionated heparin in patients with ST-elevation myocardial infarction transferred for primary angioplasty. Int J Cardiol. 2015;198:70,-74.
10. Fabris E, Menzio S, Gregorio C, et al. Effect of prehospital treatment in STEMI patients undergoing primary PCI. Catheter Cardiovasc Interv. 2022;99:1500-1508.
11. Karlsson S, Andell P, Mohammad M, et al. Editor's Choice —Heparin pre-treatment in patients with ST-segment elevation myocardial infarction and the risk of intracoronary thrombus and total vessel occlusion. Insights from the TASTE trial. Eur Heart J Acute Cardiovasc Care. 2019;8:15-23.
12. Zijlstra F, Ernst N, de BoerMJ, et al. Influence of prehospital administration of aspirin and heparin on initial patency of the infarct-related artery in patients with acute ST elevation myocardial infarction. J Am Coll Cardiol. 2002;39:1733-1737.
13. Mehran R, Rao S, Bhatt D, et al. Standardized bleeding definitions for cardiovascular clinical trials:a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736-2747.
14. Costa G, Resende B, Oliveiros B, Gonçalves L, Teixeira R. Heparin pretreatment in ST segment elevation myocardial infarction:a systematic review and meta-analysis. Coron Artery Dis. 2025;36:28-38.
15. Ariza A, Ferreiro JL, Sánchez-Salado JC, Lorente V, Gómez-Hospital JA, Cequier A. Early Anticoagulation May Improve Preprocedural Patency of the Infarct-related Artery in Primary Percutaneous Coronary Intervention. Rev Esp Cardiol. 2013;66:148-150.
16. Koul S, Smith J, Götberg M, et al. No Benefit of Ticagrelor Pretreatment Compared With Treatment During Percutaneous Coronary Intervention in Patients With ST-Segment-Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. Circ Cardiovasc Interv. 2018;11:e005528.
17. Russo RG, Wikler D, Rahimi K, Danaei G. Self-Administration of Aspirin After Chest Pain for the Prevention of Premature Cardiovascular Mortality in the United States:A Population-Based Analysis. J Am Heart Assoc. 2024;13:e032778.
18. Chen ZM, Jiang LX, Chen YP, et al. Addition of clopidogrel to aspirin in 45 852 patients with acute myocardial infarction:randomised placebo-controlled trial. Lancet. 2005;366:1607-1621.
19. Siller J, Angiolillo D, De Luca L, Rymer J, Thim T, Zeymer U. Real-world practice adoption of latest guidelines for the acute management of ACS patients. En:Paris 2024. EuroPCR Course 2024. 35th edition;2024 May 21-24;Paris, France. Available at:https://www.pcronline.com/Courses/EuroPCR. Consulted 1 Mar 2025.
20. De Luca L, Maggioni A, Cavallini C, et al. Clinical profile and management of patients with acute myocardial infarction admitted to cardiac care units:The EYESHOT-2 registry. Int J Cardiol. 2025;418:132601.

ABSTRACT
Introduction and objectives: Assessment and treatment of intermediate coronary lesions, defined as those which represent 30%-90% of the vessel lumen, remains a clinical challenge. Physiological evaluation techniques, such as fractional flow reserve (FFR), non-adenosine-based methods, such as instantaneous wave-free ratio or resting full-cycle ratio, and angiography-derived physiological assessment techniques (ADPAT) have transformed the diagnostic landscape. This meta-analysis aimed to systematically review and compare the diagnostic performance of ADPAT and FFR evaluating intermediate coronary lesions.
Methods: We conducted a systematic review of comparative research on FFR and ADPAT from January through February 2024.
Results: A total of 27 studies were finally included in the meta-analysis for a total of 4818 patients and 5440 vessels. Overall, a strong correlation between the different ADPAT and FFR was observed (r = 0.83; 95%CI, 0.80-0.85), with a mean ADPAT value of 0.82; 95%CI, 0.81-0.83 and a mean FFR of 0.83; 95%CI, 0.82-0.85. The summary area under the curve for predicting significant FFR (≤ 0.80) was excellent at 0.947. The overall sensitivity rate was 85% (95%CI, 81-87) with a specificity rate of 93% (95%CI, 91-94). The positive predictive value was 86% (95%CI, 83-88) with a total negative predictive value of 92% (95%CI, 91-94).
Conclusions: ADPAT show good correlation and concordance with FFR for intermediate coronary lesion evaluation. However, due to unfavorable outcomes observed in the FAVOR III Europe trial1 with quantitative flow ratio-guided revascularization, its clinical role should be reconsidered and potentially limited to scenarios where invasive assessment or adenosine use is not feasible. Further evaluation is warranted to confirm its diagnostic performance in broader clinical contexts.
Registered at PROSPERO: CRD420251042828.
Keywords: Clinical research. Fractional flow reserve. Angiographic/fluoroscopic. Meta-analysis.
RESUMEN
Introducción y objetivos: La evaluación y el tratamiento de las lesiones coronarias intermedias, definidas como aquellas que comprometen entre el 30 y el 90% de la luz del vaso, continúan representando un desafío clínico. Las técnicas de evaluación fisiológica (como la reserva fraccional de flujo [RFF]), los métodos que no requieren adenosina (como el índice instantáneo libre de ondas o el índice de ciclo completo en reposo) y las técnicas de evaluación fisiológica derivadas de la angiografía (ADPAT) han transformado el panorama diagnóstico. Este metanálisis tuvo como objetivo revisar sistemáticamente y comparar el rendimiento diagnóstico de las ADPAT frente a la RFF en la evaluación de lesiones coronarias intermedias.
Métodos: Entre enero y febrero de 2024 se realizó una revisión sistemática de investigaciones comparativas entre RFF y ADPAT.
Resultados: Se incluyeron 27 estudios en el metanálisis, con un total de 4.818 pacientes y 5.440 vasos. En general, se observó una fuerte correlación entre las distintas ADPAT y la RFF (r = 0,83; IC95%, 0,80-0,85), con un valor medio de ADPAT de 0,82 (IC95%, 0,81-0,83) y un valor medio de FFR de 0,83 (IC95%, 0,82-0,85). El área bajo la curva resumen para predecir una RFF significativa (≤ 0,80) fue excelente, con un valor de 0,947. La sensibilidad global fue del 85% (IC95%, 81-87) y la especificidad fue del 93% (IC95%, 91-94). El valor predictivo positivo fue del 86% (IC95%, 83-88) y el valor predictivo negativo total fue del 92% (IC95%, 91-94).
Conclusiones: Las ADPAT muestran una buena correlación y concordancia con la RFF en la evaluación de lesiones coronarias intermedias. Sin embargo, debido a los resultados desfavorables observados en el estudio FAVOR III Europe1 con la revascularización guiada por el índice cuantitativo de flujo, su papel clínico se debe reconsiderar y posiblemente limitar a escenarios en los que no sea factible realizar una evaluación invasiva ni utilizar adenosina. Se requiere una evaluación adicional para confirmar su rendimiento diagnóstico en contextos clínicos más amplios.
Registrado en PROSPERO: CRD420251042828.
Palabras clave: Investigación clínica. Reserva fraccional de flujo. Angiografía/fluoroscopia. Metanálisis.
Abbreviations
ADPAT: angiography-derived physiological assessment techniques. AUC: area under the curve. FFR: fractional flow reserve. QFR: quantitative flow ratio. uFR: Murray law-based quantitative flow reserve.
INTRODUCTION
Assessment and treatment of intermediate coronary lesions (those where percent diameter stenosis accounts for 30%-90% of the vessel lumen) remains a clinical challenge.1 Over the past 10 years this field has undergone significant changes, primarily due to theoretical and technological advances in physiological evaluation techniques.2,3
Prior to the existence of these techniques, the assessment of intermediate lesions was based on the degree of relative narrowing of the vessel lumen vs healthy segments, being this reduction subjectively determined by the operator, without knowledge of its physiological repercussion.2 The development of pressure guidewire methods, along with their validation and proven prognostic significance (particularly in the context of chronic coronary syndrome) from the late 1990s to the early 2000s,4 has led to substantial progress in intermediate lesions evaluation, which has enabled a more accurate classification based on their clinical relevance.5
The initial method developed, and still considered the gold standard, is fractional flow reserve (FFR).5 This technique estimates blood flow across a coronary lesion by measuring pressure differences.6 To make this estimation between pressure and flow, maximal coronary vessel hyperemia, primarily achieved through adenosine infusion, is necessary.6 FFR is defined as significant if flow difference across the lesion is > 20% (FFR ≤ 0.80).6 Beyond merely identifying which lesions benefit from revascularization, FFR has shown improved survival vs revascularization based on relative narrowing assessment. Furthermore, it has allowed lesion exclusion where revascularization is deemed unnecessary, thus reducing stent implantation rates and any potential complications associated with both this procedure and antiplatelet therapy.7
Despite the clear benefits of using intracoronary physiology, the need for invasive pressure guidewires, IV adenosine (with its potential complications), the time required, and even the outright rejection by interventional cardiologist may have led to a lower than expected adoption.8 These limitations triggered the appearance of non-adenosine-based methods, such as the instantaneous wave-free ratio (iFR) or resting full-cycle ratio.9,10 These methods use a specific moment of the cardiac cycle (for example the iFR uses the diastolic wave-free period) where microvascular resistances are minimal, allowing correlation between pressures and flow without the use of adenosine.11,12 However, despite eliminating this limitation, the use of pressure guidewires is still a barrier.8
Simultaneously with the development of these adenosine-free techniques, angiography-derived physiological assessment techniques (ADPAT) emerged, enabling the physiological evaluation of coronary lesions without the need for a guidewire or adenosine. These techniques, initially derived from those used in coronary lesion assessment in computational tomography,13 are based on the computational evaluation of lesions through fluid dynamics in coronary angiography. Since then, multiple options have emerged including QFR, Murray law-based quantitative flow ratio (uFR), vessel fractional flow reserve (vFRR), fractional flow reserve derived from routine coronary angiography (FFRangio) and coronary angiography-derived fractional flow reserve (CaFFR). All of them have been validated and compared with the gold standard FFR in prospective direct comparative studies of diagnostic accuracy.14-20
The aim of this article was to provide a review of the different validation studies of ADPAT vs FFR and offer a meta-analysis on the accuracy of each option, both collectively and individually.
METHODS
Literature search strategy
We conducted a systematic review of comparative research on FFR and ADPAT from January through February 2024. The PubMed database was used to search for articles on concordance, agreement, and diagnostic accuracy. Multiple searches were conducted using the following algorithm: FFR/FFR permuted with each mainly commercialized tool (QFR, uFR, vFRR, FFRangio and CaFFR) while trying to avoid CT and articles developed mainly in acute coronary syndrome through the commands “NOT (CT) NOT (“acute coronary syndrome”)”. Date range was limited from January 2012 through December 2023. PRISMA statement guidelines were followed, and the review was prospectively registered in the International Prospective Register of Systematic Reviews (PROSPERO) with registration No. CRD420251042828.
Eligible criteria
A total of 4580 terms were identified through the entire search process. These terms and their combinations were carefully selected by 2 different operators to refine the search for articles comparing the main ADPAT from the main commercial vs FFR. Articles involving coronary computed tomography angiography and those where comparisons were mainly drawn within the context of acute coronary syndrome were also excluded by the operators. Based on these criteria, an initial pool of studies was established.
A total of 15 studies were subsequently excluded based on prespecified criteria, including those that specified the presence of patients with concurrent or treated aortic stenosis, had more than 25% of patients diagnosed with atrial fibrillation, or involved angiography- derived physiological assessments for coronary lesions conducted within the first 29 days of acute myocardial infarction (either on the culprit lesion or non-culprit lesions).
In cases where the time elapsed from myocardial infarction to angiography-derived evaluation was nonspecific; articles were also excluded if more than 30% of patients had undergone coronary angiography due to acute myocardial infarction.
Furthermore, studies specifying the presence of 10% or more patients with prior surgical revascularization were excluded, as were those where the comparison between angiography-based physiological assessment methods and FFR was conducted on mammary artery grafts, radial artery grafts, or saphenous vein grafts.
After applying the selection criteria, a total of 29 articles were initially chosen for analysis. However, 2 articles (FAST [virtual FFR])21 and Ai et al.22 were subsequently excluded because they did not provide or calculate sensitivity and specificity data from their analyses. Consequently, the final analysis included 27 articles.
Two articles were divided and included as different items in the analysis as they showed 2 different analyzed cohorts on their studies: Smit et al.,23 where QFR was compared with the FFR in 2 cohorts: 1 with diabetes mellitus and the other without the disease; Zuo et al.24 divided patients in 2 cohorts based on whether the vessel was severely calcified or not. The uFR was compared with the FFR in each group. Each cohort was included in our analysis. Finally, the study by Emori et al.25 “Diagnostic accuracy of quantitative flow ratio for assessing myocardial ischemia in prior myocardial infarction,” presented 2 distinct cohorts based on the presence of prior myocardial infarction (≥ 30 days from coronary angiography). Although one cohort depicted an acute coronary syndrome scenario, it fulfilled our inclusion criteria, leading to the inclusion of both cohorts in the final analysis.
Statistical and methodologic analysis
The homogeneity across studies was contrasted using the QH statistic. Regarding the low sensitivity of this test, P < .10 values were considered significant. To overcome this limitation, the I2 statistic was estimated as well, which measures the proportion of the total variation of the studies, explained by the heterogeneity and its 95% confidence interval (95%CI). A random effects model was used for all cases using the pooled method of DerSimonian Laird. If heterogeneity was present, meta-regression analyses were conducted to explore the sources of heterogeneity (figure 1 of the supplementary data). The presence of publication bias was tested using the Deek funnelplot (figure 2 of the supplementary data).

Figure 1. Selected articles flowchart and exclusion criteria. ADPAT, angiography-derived physiological assessment techniques; AMI, acute myocardial infarction.

Figure 2. Summary receiver operating characteristic (SROC) curves and Q* index for subgroup analyses of software-derived coronary angiography-derived fractional flow reserve (caFFR); FFR, fractional flow reserve; QFR, quantitative flow ratio; uFR, Murray law-based quantitative flow reserve; VFAI, vessel fractional anatomy index; vFFR, vessel fractional flow reserve.
From the reported values of sensitivity, specificity, negative predictive value, positive predictive value, accuracy, and the number of vessels assessed, all 2 × 2 tables for the 0.8 cutoff point of the tests were constructed. Subsequently, pooled estimates for sensitivity, specificity, positive predictive value, negative predictive value, positive likelihood ratio, and negative likelihood ratio were derived from these data.26
The confidence intervals of sensitivity and specificity were calculated using the F distribution method to compute the exact confidence limits for the binomial proportion (x/n). The summary receive operator curve (SROC) was also calculated from which we drew all the points of sensitivity and 1-specificity and adjusted the weighted regression curve using Moses’ Model. Spearman correlation coefficient between sensitivity and specificity was used to assessed constant diagnostic odds ratio (positive likelihood ratio and negative likelihood ratio) employing a symmetric SROC.27 The area under curve (AUC) was computed by numeric integration of the curve equation using the trapezoidal method. Additionally, we applied the bootstrap methods for estimated AUC of multiple SROC. We provided the resultant bootstrap P values and 95%CI of the AUC for pairwise comparisons of the different methods (table 1 of the supplementary data). Furthermore, we provided an influence diagnostic method based on the AUC by performing leave-one-study-out analyses (table 2 of the supplementary data). Pearson correlation coefficients were transformed into Fisher’s z-values to calculate variance and we performed a meta-analysis and calculated the 95%CI (figure 3 of the supplementary data). Fagan’s Nomogram (figure 4 of the supplementary data) was used to graphically estimate how the result from a diagnostic test altered the probability of a patient having a disease. We assessed applicability and risk of bias based on the modified version of the QUADAS-2 tool28 (figure 5A,B of the supplementary data). All analyses were conducted using R Statistical Software (v4.2.0; R Core Team 2022) and performed using dmetatools R package (1.1.1; Noma H 2023), mada R package (0.5.11; Sousa-Pinto 2022) and TeachingDemos R package (2.13; Greg Snow 2024).
Table 1. Patients’ baseline characteristics
| Patients’ baseline characteristics (n = 4818) | |
|---|---|
| Characteristics (cohorts where this data is available) | (± 95%CI) or % |
| Mean age (26) | 66.4 ± 1.3 |
| Male (26) | 3318 (68.9%) |
| Mean BMI (kg/m2) (17) | 26 ± 0.8 |
| Hypertension (25) | 3189 (66.2%) |
| Diabetes (25) | 1263 (26.2%) |
| Dyslipidemia (21) | 2438 (50.6%) |
| Mean LVEF (%) (10) | 59.6 ± 3.3 |
| Prior or current smoker (23) | 1406 (29.2%) |
| Prior MI (20) | 566 (11.7%) |
| Prior PCI (20) | 1314 (27.3%) |
| Prior CABG (13) | 47 (1%) |
|
BMI, body mass index; CABG, coronary artery bypass grafting; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PCI, percutaneous coronary intervention. Data are expressed as mean value and standard deviation across the studies. |
|
Table 2. Indications for cardiac catheterization
| Indication for cardiac catheterization | (%) |
|---|---|
| Silent isquemia | 323 (6.8) |
| Stable angina | 2483 (51.5) |
| Acute coronary syndrome | 1475 (30.6) |
| Unstable angina | 1142 (23.7) |
| AMI | 333 (6.9) |
| NSTEMI | 204 (4.2) |
| STEMI | 13 (0.3) |
| MI subtype not specified | 116 (2.4) |
| Others | 127 (2.6) |
|
AMI, acute myocardial infarction; MI, myocardial infarction; NSTEMI, non-ST-segment elevation myocardial infarction; STEMI, ST-segment elevation myocardial infarction. |
|

Figure 3. Forest plots and summary statistics for sensitivity and specificity estimates from a meta-analysis of FFR across different indices, using a random-effects model. 95%CI, 95% confidence interval; caFFR, coronary angiography–derived fractional flow reserve; FFR, fractional flow reserve; QFR, quantitative flow ratio; uFR, Murray law-based quantitative flow reserve; VFAI, vessel fractional anatomy index; vFFR, vessel fractional flow reserve. Xu et al.,16 2017; Fearon et al.,36 2019; Yuasa et al.,33 2023; Morris et al.,39 2013; Westra et al.,29 2018; Echavarría-Pinto et al.,31 2022; Stähli et al.,34 2019; Omori et al.,35 2019; Westra et al.,17 2018; Li et al.,18 2020; Pellicano et al.,14 2017; Emori et al.,25 2018; Tu et al.,15 2014; Zuo et al.,24 2024; Tu et al.,19 2021; Omori et al.,42 2023; Hrakesh et al.,32 2020; Kornowski et al.,37 2016; Masdjedi et al.,20 2022; Tröbs et al.,38 2016; Yazaki et al.,30 2017; Smit et al.23 2019; Daemen et al.,43 2022; and Papafaklis et al.,41 2014.

Figure 4. Forest plots and summary statistics for positive predictive value (PPV) and negative predictive value (NPV) estimates from a meta-analysis of FFR across different indices, using a random-effects model. 95%CI, 95% confidence interval; caFFR, coronary angiography–derived fractional flow reserve; FFR, fractional flow reserve; QFR, quantitative flow ratio; uFR, Murray law-based quantitative flow reserve; VFAI, vessel fractional anatomy index; vFFR, vessel fractional flow reserve. Xu et al.,16 2017; Fearon et al.,36 2019; Yuasa et al.,33 2023; Morris et al.,39 2013; Westra et al.,29 2018; Echavarría-Pinto et al.,31 2022; Stähli et al.,34 2019; Omori et al.,35 2019; Westra et al.,17 2018; Li et al.,18 2020; Pellicano et al.,14 2017; Emori et al.,25 2018; Tu et al.,15 2014; Zuo et al.,24 2024; Tu et al.,19 2021; Omori et al.,42 2023; Hrakesh et al.,32 2020; Kornowski et al.,37 2016; Masdjedi et al.,20 2022; Tröbs et al.,38 2016; Yazaki et al.,30 2017; Smit et al.,23 2019; Daemen et al.,43 2022; and Papafaklis et al.,41 2014.
RESULTS
Finally, a total of 27 articles were suitable for inclusion, as illustrated in figure 1. From these articles, a total of 4818 patients and 5440 vessels were added to the analysis. The population characteristics and mean cardiovascular risk factors are detailed in table 1 highlighting the existence of 3189 (66.18%) patients with hypertension, 2438 (50.6%) with dyslipidemia, and 1263 (26.2%) with diabetes. Notably, most patients included in the study were men (68.86% of the sample).
Thirteen of the selected articles were prospective in design. The most extensively studied vessel was the left anterior descending coronary artery (2921; 53.69%), followed by the right coronary artery (1075; 19.61%) and the left circumflex artery (772; 14.2%). Additionally, 89 left main coronary arteries were analyzed, accounting for 1.6% of all vessels. Angiography was primarily performed for stable angina (2483; 51.53%). Of note, while 1475 (30.61%) angiographies were prompted by acute coronary syndrome, only 333 (6.9% of the total) were performed in the context of acute myocardial infarction with or without ST-segment elevation, and the remaining 1142 in the context of unstable angina. Indications for cardiac catheterization are shown in table 2. The left anterior descending coronary artery was the most frequently studied vessel, accounting for 2921 patients (53.7% of the total studies). Proportions for other vessels are available in table 3.
Table 3. Number of studies per vessel performed across the different studies
| Vessel characteristics (n = 5440) | (%) |
|---|---|
| Left main coronary artery | 89 (1.7) |
| Left anterior descending coronary artery | 2921 (53.7) |
| Diagonal branch | 52 (1) |
| Ramus intermedius | 54 (1) |
| Left circumflex artery | 772 (14.2) |
| Obtuse marginal branch | 108 (2) |
| Right coronary artery | 1075 (19.8) |
| Posterolateral branch | 7 (0.1) |
| Interventricular branch | 8 (0.15) |
The QFR15-17,23,25,29-34 (QAngio XA 3D QFR, Medis Medical Imaging System; The Netherlands) was the most widely used software with a total of 13 patient cohorts from 11 articles, comprising 1987 patients and 2315 vessels, which accounts for 41.2% and 42.6% of the total, respectively. The correlation between QFR and FFR was excellent, showing an r = 0.82 (95%CI, 0.77-0.877). The overall sensitivity rate of QFR was 84% (95%CI, 80-88) with a specificity rate of 90% (95%CI, 87-93). The positive predictive value was 81% (95%CI, 77-84) with a total negative predictive value of 92% (95%CI, 90-94). The AUC for this technique was 0.937.
The second most analyzed technique, with a total of 5 articles, was FFRangio14,35-38 (Cathworks FFRangio, Israel), where this technology was employed in 696 patients and 841 vessels (14.4% and 15.45% of the total, respectively). The overall sensitivity rate of FFRangio was 90% (95%CI, 83-94) with a specificity rate of 95% (95%CI, 91-97). The positive predictive value was 90% (95%CI, 85-93) with a total negative predictive value of 94% (95%CI, 91-96).
vFFR (Pie Medical Imaging, The Netherlands) on the other hand, had an excellent correlation with FFR across the 3 included studies,20,39,40 contributing 647 patients and 663 vessels to the analysis (representing 13.42% of patients and 11.96% of vessels). The mean sensitivity and specificity rates were 82% (95%CI, 72-89) and 0.94% (95%CI, 89-97), respectively. The summary positive predictive value was 89% (95%CI, 82-93), and the summary negative predictive value, 91% (95%CI, 86-94).
Following its recent validation in 2022, the uFR (AngioPlus, Pulse Medical Imaging Technology, China) is supported by only 2 articles,19,24 one of which includes 2 cohorts based on vessel calcification. The uFR had a sensitivity rate of 80% (95%CI, 69-87) and a specificity rate of 0.94 (95%CI, 89-97). The summary positive predictive value was 85% (95%CI, 79-90), and the summary negative predictive value, 91% (95%CI, 87-94).
Only 1 article of CaFFR (Flashangio, Rainmed Ltd., China) was included.18
The analysis included 2 non-commercialized tools, VFAI41 and AngioFFR,42 which were not individually evaluated. Both were compared to FFR only once.
Overall, a strong correlation between the different ADPAT and FFR was observed (r = 0.83, 95%CI, 0.80-0.85), with a mean ADPAT value of 0.82 (95%CI, 0.81-0.83) (all the ADPAT set a value ≤ 0.80 for lesion significance) and a mean FFR of 0.83 (95%CI, 0.82-0.85).
The summary AUC for predicting significant FFR (≤ 0.80) was excellent at 0.947. The SROC for the different ADPAT is shown in figure 2.
The overall sensitivity rate was 85% (95%CI, 81-87) with a specificity rate of 93% (95%CI, 91-94). The positive predictive value was 86% (95%CI, 83-88) with a total negative predictive value of 92% (95%CI, 91-94). The main commercially available ADPAT values of sensibility, specificity, positive predictive value and negative predictive value are shown in figure 3 and figure 4.
DISCUSSION
Key findings
Our key findings were: a) ADPAT emerge as a reliable and practical method for assessing the physiological significance of intermediate coronary lesions, which is consistent with previous literature.44-46 ADPAT consistently demonstrates agreement with the current gold standard (FFR) regarding mean values and lesion classification, without vasodilator medication or pressure guidance; b) By summarizing the diagnostic capabilities of each ADPAT from the included studies, we were able to perform the first direct comparison of various angiography-based methods for evaluating coronary lesions. We presented the main commercially available options and their respective diagnostic accuracies relative to FFR. Additionally, an overview of these techniques was provided; c) We also included innovative methods, such as uFR, based on Murray’s Law, while offering a unique approach by using a single projection to estimate lesion significance, potentially overcoming a significant limitation of current techniques, which often require specific projections and a certain quality image.
The overall results confirmed that different ADPAT serve as an appropriate method for evaluating intermediate coronary lesions, as they demonstrated a strong correlation with FFR. This correlation extended to sensitivity, specificity, and predictive values as illustrated in figure 4. Notably, the studies exhibited homogeneity without significant discrepancies in their weighting within the analysis, as observed through the resampling techniques employed.
In comparative analysis, while ADPAT exhibit adequate sensitivity and positive predictive values regarding lesion significance, their specificity and negative predictive value exceed 90%. This high specificity allows ADPAT to more accurately identify physiologically non-significant lesions, thereby avoiding unnecessary revascularization.
From a technical standpoint, it was notable that these results were primarily obtained from assessments of the left anterior descending coronary artery (53.6%), with only 1 dedicated study on the left main coronary artery. Despite this, left main coronary arteries contributed a significant proportion (1.66%) to the overall analysis, showcasing proficient classification of significant lesions (AUC = 0.82) and indicating the feasibility of applying tools in this context.
QFR was the most frequently included tool in the analysis, representing 13 out of 27 cohorts. Despite multiple validations vs the FFR in diverse contexts, most studies align closely, demonstrating a correlation between QFR and FFR.
Comparing results across different tools, minimal differences were observed, with FFRangio and CaFFR showing slightly superior overall results vs other methods. However, it’s important to note that the results of the CaFFR are based solely on validation articles, and when considering only validation studies, results among tools are very similar.
Although QFR is frequently studied, its results might require more robust validation because there are limited articles on FFRangio, especially on chronic coronary syndrome in patient groups like those with left main disease or diabetes.
While ADPAT have been validated vs the FFR in various clinical scenarios, such as severe aortic stenosis, atrial fibrillation, or non-culprit coronary lesions in acute coronary syndrome, the inclusion of these scenarios in our analysis could potentially bias the results due to variations in study characteristics and the unique features of each disease affecting lesion assessment.
The limitation of this study stems from including a large proportion of pivotal studies for each analyzed tool, which were not performed under real-world clinical conditions. Consequently, the applicability of their results may be restricted, as demonstrated by a recent study from independent laboratories comparing the 5 main non-hyperemic indices with FFR under real-life conditions.47
Although the study demonstrated a good correlation between the indices and FFR, the levels of diagnostic accuracy reported in the pivotal studies were not achieved.
In this regard, QFR has been recently evaluated vs the FFR in the FAVOR III Europe trial,1 which included 2000 patients who were randomized (1:1) to QFR-guided or FFR-guided treatment of intermediate lesions. The results showed that the QFR-guided group had higher rates of mortality, myocardial infarction, and unplanned revascularization at 12 months.
Although these findings may initially seem discouraging, they do not contradict the results of our study, in which non-hyperemic indices demonstrated superior performance over conventional angiography in the functional classification of lesions. Therefore, their use remains valuable in clinical scenarios where invasive assessment with a pressure guidewire or the use of adenosine is not feasible or contraindicated.
Of note, while QFR is currently the most widely used non-hyperemic index, it is the only one that has been evaluated in clinical trials with hard clinical endpoints vs FFR. Other tools with promising results are still to be investigated in this context.
CONCLUSIONS
Substantial correlations and concordances have been demonstrated between ADPAT and FFR. These techniques have also shown accurate categorization of lesions deemed significant by the current gold standard (FFR). However, the results of the FAVOR III Europe study1 indicate that QFR–guided revascularization, compared with FFR-guided revascularization, is associated with higher rates of mortality, myocardial infarction, and unplanned revascularization. Therefore, the current role of ADPAT requires re-evaluation.
In this context, the use of QFR may be most appropriate when invasive assessment using a pressure guidewire is not feasible or when adenosine is contraindicated. Additionally, due to the unique characteristics of other clinical scenarios, further reviews are warranted to evaluate the diagnostic accuracy of this index.
FUNDING
C. Cortés-Villar has been beneficiary of Río Hortega CM22/00168 and Miguel Servet CP24/00128 grants from Instituto de Salud Carlos III. This work has been partially funded by Gerencia Regional de Salud de Castilla y León with grant number GRS 3157/A1/2024.
ETHICAL CONSIDERATIONS
The present study was conducted in full compliance with the clinical practice guidelines set forth in the Declaration of Helsinki for clinical research and was approved by the ethics committees of the reference hospital (Hospital Clínico Universitario de Valladolid) and other participant centers. Possible sex- and gender-related biases were also taken into consideration according to the SAGER recommendations.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
No artificial intelligence was used in the writing of this text.
AUTHORS’ CONTRIBUTIONS
J. Ruiz-Ruiz and C. Cortés-Villar participated in the study design, data analysis, manuscript drafting, and critical revision. C. Fernández-Cordón and M. García-Gómez contributed to data collection and results analysis. A. Lozano-Ibáñez and D. Carnicero-Martínez contributed to data gathering. S. Blasco-Turrión and M. Carrasco-Moraleja contributed to the statistical analysis. J.A. San Román-Calvar and I.J. Amat-Santos performed the final review and approved the version for publication.
CONFLICTS OF INTEREST
None declared.
REFERENCES
1. Andersen BK, Sejr-Hansen M, Maillard L, et al. Quantitative flow ratio versus fractional flow reserve for coronary revascularisation guidance (FAVOR III Europe):a multicentre, randomised, non-inferiority trial. Lancet. 2024;404:1835-1846.
2. Patil CV, Beyar R. Intermediate coronary artery stenosis:Evidence-based decisions in interventions to avoid the oculostenotic reflex. Int J Cardiovasc Intervent. 2000;3:195-206.
3. Gould KL, Lipscomb K, Hamilton GW. Physiologic basis for assessing critical coronary stenosis. Instantaneous flow response and regional distribution during coronary hyperemia as measures of coronary flow reserve. Am J Cardiol. 1974;33:87-94.
4. De Bruyne B, Paulus WJ, Vantrimpont PJ, Sys SU, Heyndrickx GR, Pijls NHJ. Transstenotic coronary pressure gradient measurement in humans:In vitro and in vivo evaluation of a new pressure monitoring angioplasty guide wire. J Am Coll Cardiol. 1993;22:119-126.
5. Sousa-Uva M, Neumann FJ, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur J Cardio-thoracic Surg. 2019;55:4-90.
6. Tonino PAL, De Bruyne B, Pijls NHJ, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2011;365:213-224.
7. De Bruyne B, Pijls NHJ, Kalesan B, et al. Fractional Flow Reserve–Guided PCI versus Medical Therapy in Stable Coronary Disease. N Engl J Med. 2012;367:991-1001.
8. Johnson NP, Koo BK. Coronary Psychology:Do You Believe?JACC Cardiovasc Interv. 2018;11:1492-1494.
9. De Waard GA, Di Mario C, Lerman A, Serruys PW, Van Royen N. Instantaneous wave-free ratio to guide coronary revascularisation:Physiological framework, validation and differences from fractional flow reserve. EuroIntervention. 2017;13:450-458.
10. Svanerud J, Ahn JM, Jeremias A, et al. Validation of a novel non-hyperaemic index of coronary artery stenosis severity:The Resting Full-cycle Ratio (VALIDATE RFR) study. EuroIntervention. 2018;14:806-814.
11. Davies JE, Sen S, Dehbi H-M, et al. Use of the Instantaneous Wave-free Ratio or Fractional Flow Reserve in PCI. N Engl J Med. 2017;376:1824-1834.
12. Götberg M, Christiansen EH, Gudmundsdottir IJ, et al. Instantaneous Wave-free Ratio versus Fractional Flow Reserve to Guide PCI. N Engl J Med. 2017;376:1813-1823.
13. Min JK, Leipsic J, Pencina MJ, et al. Diagnostic accuracy of fractional flow reserve from anatomic CT angiography. Jama. 2012;308:1237-1245.
14. Pellicano M, Lavi I, De Bruyne B, et al. Validation study of image-based fractional flow reserve during coronary angiography. Circ Cardiovasc Interv. 2017;10:E005259.
15. Tu S, Barbato E, Köszegi Z, et al. Fractional flow reserve calculation from 3-dimensional quantitative coronary angiography and TIMI frame count:A fast computer model to quantify the functional significance of moderately obstructed coronary arteries. JACC Cardiovasc Interv. 2014;7:768-777.
16. Xu B, Tu S, Qiao S, et al. Diagnostic Accuracy of Angiography-Based Quantitative Flow Ratio Measurements for Online Assessment of Coronary Stenosis. J Am Coll Cardiol. 2017;70:3077-3087.
17. Westra J, Andersen BK, Campo G, et al. Diagnostic performance of in-procedure angiography-derived quantitative flow reserve compared to pressure-derived fractional flow reserve:The FAVOR II Europe-Japan study. J Am Heart Assoc. 2018;7:e009603.
18. Li J, Gong Y, Wang W, et al. Accuracy of Computational Pressure-Fluid Dynamics applied to Coronary Angiography to Derive Fractional Flow Reserve –FLASH FFR. Cardiovasc Res. 2020;116:1349-1356.
19. Tu S, Ding D, Chang Y, Li C, Wijns W, Xu B. Diagnostic accuracy of quantitative flow ratio for assessment of coronary stenosis significance from a single angiographic view:A novel method based on bifurcation fractal law. Catheter Cardiovasc Interv. 2021;97(S2):1040-1047.
20. Masdjedi K, Tanaka N, Van Belle E, et al. Vessel fractional flow reserve (vFFR) for the assessment of stenosis severity:the FAST II study. EuroIntervention. 2022;17:1498-1505.
21. Masdjedi K, van Zandvoort LJC, Balbi MM, et al. Validation of a three-dimensional quantitative coronary angiography-based software to calculate fractional flow reserve:The FAST study. EuroIntervention. 2021;16:591-599.
22. Ai H, Zheng N, Li L, et al. Agreement of Angiography-Derived and Wire-Based Fractional Flow Reserves in Percutaneous Coronary Intervention. Front Cardiovasc Med. 2021;8:654392.
23. Smit JM, El Mahdiui M, van Rosendael AR, et al. Comparison of Diagnostic Performance of Quantitative Flow Ratio in Patients With Versus Without Diabetes Mellitus. Am J Cardiol. 2019;123:1722-1728.
24. Zuo W, Sun R, Xu Y, et al. Impact of calcification on Murray law-based quantitative flow ratio for physiological assessment of intermediate coronary stenoses. Cardiol J. 2024;31:205-214.
25. Emori H, Kubo T, Kameyama T, et al. Diagnostic accuracy of quantitative flow ratio for assessing myocardial ischemia in prior myocardial infarction. Circ J. 2018;82:807-814.
26. Deeks JJ. Systematic reviews of evaluations of diagnostic and screening tests. Bmj. 2001;323:157.
27. Walter SD. Properties of the summary receiver operating characteristic (SROC) curve for diagnostic test data. Stat Med. 2002;21:1237-1256.
28. Whiting PF, Reitsma JB, Leeflang MMG, et al. QUADAS-2:a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529-536.
29. Westra J, Tu S, Winther S, et al. Evaluation of Coronary Artery Stenosis by Quantitative Flow Ratio during Invasive Coronary Angiography:The WIFI II Study (Wire-Free Functional Imaging II). Circ Cardiovasc Imaging. 2018;11:1-8.
30. Yazaki K, Otsuka M, Kataoka S, et al. Applicability of 3-dimensional quantitative coronary angiography-derived computed fractional flow reserve for intermediate coronary stenosis. Circ J. 2017;81:988-992.
31. Echavarría-Pinto M, Van de Hoef TP, Pacheco-Beltran N, et al. Diagnostic agreement of quantitative flow ratio with fractional flow reserve in a Latin-American population. Int J Cardiovasc Imaging. 2022;38:1423-1430.
32. Hrakesh O, Hay M, Lim RY, et al. Comparison of diagnostic performance between quantitative flow ratio, non-hyperemic pressure indices and fractional flow reserve. Cardiovasc Diagn Ther. 2020;10:442-452.
33. Yuasa S, Lauri FM, Mejia-Renteria H, et al. Angiography-derived functional assessment of left main coronary stenoses. Catheter Cardiovasc Interv. 2023;101:1045-1052.
34. Stähli BE, Erbay A, Steiner J, et al. Comparison of resting distal to aortic coronary pressure with angiography-based quantitative flow ratio. Int J Cardiol. 2019;279:12-17.
35. Omori H, Witberg G, Kawase Y, et al. Angiogram based fractional flow reserve in patients with dual/triple vessel coronary artery disease. Int J Cardiol. 2019;283:17-22.
36. Fearon WF, Achenbach S, Engstrom T, et al. Accuracy of Fractional Flow Reserve Derived from Coronary Angiography. Circulation. 2019;139:477-484.
37. Kornowski R, Lavi I, Pellicano M, et al. Fractional Flow Reserve Derived From Routine Coronary Angiograms. J Am Coll Cardiol. 2016;68:2235-2237.
38. Tröbs M, Achenbach S, Röther J, et al. Comparison of Fractional Flow Reserve Based on Computational Fluid Dynamics Modeling Using Coronary Angiographic Vessel Morphology Versus Invasively Measured Fractional Flow Reserve. Am J Cardiol. 2016;117:29-35.
39. Morris PD, Ryan D, Morton AC, et al. Virtual fractional flow reserve from coronary angiography:Modeling the significance of coronary lesions. Results from the VIRTU-1 (VIRTUal fractional flow reserve from coronary angiography) study. JACC Cardiovasc Interv. 2013;6:149-157.
40. Neleman T, Masdjedi K, Van Zandvoort LJC, et al. Extended Validation of Novel 3D Quantitative Coronary Angiography-Based Software to Calculate vFFR:The FAST EXTEND Study. JACC Cardiovasc Imaging. 2021;14:504-506.
41. Papafaklis MI, Muramatsu T, Ishibashi Y, et al. Fast virtual functional assessment of intermediate coronary lesions using routine angiographic data and blood flow simulation in humans:Comparison with pressure wire - fractional flow reserve. EuroIntervention. 2014;10:574-583.
42. Omori H, Kawase Y, Mizukami T, et al. Diagnostic Accuracy of Artificial Intelligence-Based Angiography-Derived Fractional Flow Reserve Using Pressure Wire-Based Fractional Flow Reserve as a Reference. Circ J. 2023;87:783-790.
43. Scoccia A, Tomaniak M, Neleman T, Groenland FTW, Plantes ACZ des, Daemen J. Angiography-Based Fractional Flow Reserve:State of the Art. Curr Cardiol Rep. 2022;24:667-678.
44. Cortés C, Carrasco-Moraleja M, Aparisi A, et al. Quantitative flow ratio—Meta-analysis and systematic review. Catheter Cardiovasc Interv. 2021;97:807-814.
45. Faria D, Hennessey B, Shabbir A, et al. Functional coronary angiography for the assessment of the epicardial vessels and the microcirculation. EuroIntervention. 2023;19:203-221.
46. Leone AM, Campo G, Gallo F, et al. Adenosine-Free Indexes vs. Fractional Flow Reserve for Functional Assessment of Coronary Stenoses:Systematic Review and Meta-Analysis. Int J Cardiol. 2020;299:93-99.
47. Ninomiya K, Serruys PW, Kotoku N, et al. Anonymous Comparison of Various Angiography-Derived Fractional Flow Reserve Software With Pressure-Derived Physiological Assessment. JACC Cardiovasc Interv. 2023;16:1778-1790.
ABSTRACT
Introduction and objectives: Several tools have been implemented to assess the functional significance of coronary lesions. Their reliability in the management of acute coronary syndrome (ACS) might be affected by alterations in the acute phase that go beyond the affected area. Our main objective was to evaluate the reliability of invasive physiological indices for non-culprit lesions (NCL) in patients with ACS.
Methods: We conducted a systematic review across ClinicalTrials.gov, Embase, Google Scholar, PubMed, and Web of Science from inception through 5 December 2024. Additionally, a citation analysis and web searches were conducted.
Results: A total of 20 articles, with 4379 patients were included in the analysis. The main study design is a cohort study. The following methods were compared between acute and staged interventions: a) angiography-derived; b) hyperemic; and c) non-hyperemic indices. A significant difference in fractional flow reserve, instantaneous wave-free ratio, and quantitative flow ratio was found in one or more articles. There were no articles reporting any important changes in the Murray law-based quantitative flow ratio, resting distal-to-aortic coronary pressure ratio, or vessel fractional flow reserve. However, these indices rely on retrospective and/or limited data. All significant variations were observed in cohorts of ST-segment elevation myocardial infarction. Unlike quantitative flow ratio, the fractional flow reserve and instantaneous wave-free ratio demonstrated consistent directions of change towards lower and higher values, respectively. Prospective cohorts and randomized controlled trials including non-ST-segment elevation acute coronary syndrome did not prove the existence of significant differences between acute and follow-up fractional flow reserve.
Conclusions: Physiological methods lack complete reliability for evaluating NCL during acute ST-segment elevation myocardial infarction. However, considering directions of change, fractional flow reserve is suitable for guiding the revascularization of acute positive NCL. Conversely, instantaneous wave-free ratio can be used to defer the revascularization of negative NCL. In non-ST-segment elevation acute coronary syndrome, fractional flow reserve is appropriate for assessing NCL within the acute phase.
Keywords: Fractional flow reserve. Instantaneous wave-free ratio. Quantitative flow ratio.
RESUMEN
Introducción y objetivos: Se han implementado varias herramientas para evaluar la importancia funcional de las lesiones coronarias. Su fiabilidad en el síndrome coronario agudo (SCA) podría verse afectada por perturbaciones en la fase aguda que se extienden más allá de la zona afectada. Nuestro objetivo principal fue evaluar la fiabilidad de los índices fisiológicos invasivos para las lesiones no culpables (LNC) en pacientes con SCA.
Métodos: Se realizó una revisión sistemática en ClinicalTrials.gov, Embase, Google Scholar, PubMed y Web of Science, desde el inicio hasta el 06/12/2024. Además, se hizo un análisis de citas y búsquedas en la web.
Resultados: Se incluyeron en el análisis 20 estudios, que abarcaban 4.379 pacientes. El principal diseño de estudio es el de cohorte. Se compararon los siguientes métodos entre procedimientos agudos y diferidos: a) índices derivados de la angiografía; b) índices hiperémicos; y c) índices no hiperémicos. En uno o más artículos se hallaron diferencias significativas en la reserva fraccional de flujo, el índice diastólico instantáneo sin ondas y el cociente de flujo cuantitativo. Ningún artículo informó de cambios importantes en el cociente de flujo cuantitativo basado en la ley de Murray, el cociente de presión coronaria distal-aórtica en reposo o la reserva fraccional de flujo del vaso. Sin embargo, estos estudios se basan en datos retrospectivos o limitados. Todas las variaciones significativas se observaron en cohortes de pacientes con infarto de miocardio con elevación del segmento ST. A diferencia del cociente de flujo cuantitativo, la reserva fraccional de flujo y el índice diastólico instantáneo sin ondas mostraron direcciones de cambio coherentes, hacia valores más bajos y más altos, respectivamente. Las cohortes prospectivas y los ensayos controlados aleatorizados que incluyeron pacientes con infarto de miocardio sin elevación del segmento ST no encontraron diferencias importantes entre la reserva fraccional de flujo aguda y la diferida.
Conclusiones: Los métodos fisiológicos no tienen una total fiabilidad para evaluar la gravedad de las LNC durante el infarto agudo de miocardio con elevación del segmento ST. Sin embargo, teniendo en cuenta las direcciones del cambio, la reserva fraccional de flujo es adecuada para guiar la revascularización de una LNC positiva en la fase aguda. Por el contrario, el índice diastólico instantáneo sin ondas se puede utilizar para aplazar la revascularización de una LNC con valoración negativa. En el SCA sin elevación del segmento ST, la reserva fraccional de flujo es adecuada para evaluar una LNC en la fase aguda.
Palabras clave: Reserva fraccional de flujo. Indice diastolico instantaneo sin ondas. Cociente de flujo cuantitativo.
Abbreviations
ACS: acute coronary syndrome. FFR: fractional flow reserve. iFR: instantaneous wave-free ratio. NCL: non-culprit lesions. QFR: quantitative flow ratio.
INTRODUCTION
The optimal strategy and timing of complete revascularization in patients with ST-segment elevation myocardial infarction (STEMI) and multivessel coronary artery disease remains unclear, and current recommendations are controversial.1 According to 2023 European Society of Cardiology (ESC) guidelines, complete revascularization, based solely on angiographic severity, is recommended in “stable” STEMI patients.2 Conversely, the 2023 Asia-Pacific Expert Consensus Document suggested a treatment strategy of non-culprit lesions (NCL) based on angiographic severity and invasive physiological assessment with fractional flow reserve (FFR) or non-hyperemic pressure ratios for patients with STEMI.3
FFR and non-hyperemic pressure ratios may be inaccurate in acute coronary syndrome (ACS), as hyperemic flow may be reduced due to microcirculatory dysfunction, while the resting flow may be higher due to neurohumoral compensatory mechanisms.4
Angiography-derived indices are additional physiological tools. They need ≥ 1 angiographic projections plus frame count analysis and/or aortic pressure that may also be different in the acute setting.
Furthermore, drugs such as hypolipidemic agents may promote plaque regression, potentially impacting the results of physiological assessment after a few months into therapy.5
Our main objective was to evaluate the changes in invasive physiological measurements of NCL between the acute and staged phases of ACS.
Secondly, we aimed to evaluate the effects of different therapies on physiological measurements.
METHODS
Eligibility criteria
We included studies that evaluated the physiology of NCL during acute and staged interventions for ACS. Studies conducted on assessments following percutaneous coronary interventions of non-culprit vessels, or with patients with chronic coronary syndrome were excluded.
Case reports, conference abstracts, commentaries, editorials, and reviews were excluded as well. An initial protocol was registered in PROSPERO with registration No. CRD42024574683.
Search strategy, and study selection
We conducted the search across ClinicalTrials.gov, Embase (via Ovid), Google Scholar, PubMed, and Web of Science from inception through 26 April 2024 (initial search). We used the “Review articles” filter in Google Scholar and the “Topic” field in Web of Science. No language restrictions were applied.
Duplicates were removed using Deduplicator (SR-Accelerator) software. Title/abstract and full text screening was conducted independently by 2 authors using Rayyan software.
Back in July, 2 authors conducted a backward and forward citation analysis of the included articles using Citationchaser software.
The search strings were repeated in 6 December 2024 (in Embase, sources with invalid date limits were excluded). Simultaneously, we looked into any online conference news on imaging modalities and physiological measurements.6 Additionally, we looked into the “Slide Library” section using the “2024” filter on another web page.7
Finally, we manually reviewed the references of the articles included after the initial search.
All discrepancies were resolved by consensus.
Selection process was recorded in sufficient detail to complete a Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) flow diagram.8
Data extraction
The following data were extracted from each article: a) study characteristics; b) population characteristics; c) type of physiological index(es); d) follow-up duration; e) primary endpoint.
The primary endpoint was the variation between acute and staged indices regarding statistical significance, mean difference (MD), and disagreement on revascularization decision.
One author extracted the data, and another one checked it independently. We contacted the authors of eligible studies when clarifications were needed.
Risk of bias assessment
Risk of bias was assessed using the Joanna Briggs Institute (JBI) critical appraisal tools,9-11 as appropriate.
Two authors independently assessed the risk of bias for each study. We used red for high, yellow for moderate, and green for low risk of bias based on positive answers being ≤ 49%, 50%-69%, or ≥ 70%.
Data synthesis
We conducted a descriptive synthesis of the evidence. Results from data extraction were shown in separate tables based on risk of bias, or bubble charts. Some data were rounded to the nearest integer (age, diameter of stenosis of NCL, and follow-up) or 2 decimal places (MD).
Unless otherwise specified, P values < .05 were considered statistically significant. When MDs were unreported, they were estimated by calculating the difference between staged and acute mean values. When required, a formula for estimating the means was applied.12
In bubble charts, the size of the bubbles represents the number of patients or lesions if the former was not reported. Acute−/staged+ disagreement indicates an acute value above the threshold, with the staged value below the revascularization cut-off. Acute+/staged− disagreement represents the opposite.
RESULTS
Characteristics of the articles, participants, and indices
Results of the search and selection processes are shown in figure 1. Extracted data are shown in table 1 and table 2.

Figure 1. PRISMA flow diagram. PRISMA, preferred reporting items for systematic reviews and meta-analyses.
Table 1. Extracted data of studies with low risk of bias
| First author | Patients (No.) | Age (years) | STEMI (%) | PDS of NCL (%) | Type of index | Follow-up (days) | Comparison across measurements | |
|---|---|---|---|---|---|---|---|---|
| P-value | Mean difference (staged−acute value) | |||||||
| Bär13 | 94a | 59 ± 10 | 53 | 37 ± 8 | cQFR | 365 | NR | 0.00 |
| 99b | 58 ± 8 | 54 | 37 ± 8 | NR | − 0.01 | |||
| Cortés14 | 88 | 68 ± 11 | 100 | 59 ± 12 | cQFR | 6 ± 4 | S | + 0.06 |
| Erbay15 | 321 | 66 [58-76] | 50.5c | 47 [36-57] | cQFR | 49 [42-58] | NS | + 0.01 |
| Hou16 | 2256 | 64 ± 6 | 100 | 65 ± 9 | muQFR | (7-45) | NS | 0.00 |
| Huang17 | 92 | 65 ± 10 | 100 | (30-80) | vFFR | 15 [3-30] | NS | 0.00 |
| Kirigaya18 | 50 | 63 ± 11 | 100 | 46 ± 13 | cQFR | 14 ± 5 | NS | + 0.01 |
| Mensink19 | 150d | 64 ± 9 | 35.3 | NR | FFR | 84 | NR | 0.00 |
| Musto20 | 50 | 68 ± 11 | 100 | 58 ± 12 | FFR | 6 ± 2 | NS | 0.00 |
| iFR | NS | 0.00 | ||||||
| Ntalianis21 | 101 | 63 ± 12 | 74.2 | 56 ± 14 | FFR | 35 ± 4 | NS | 0.00 |
| Sejr-hansen22 | NRe | NR | 100 | 56 [48-66] | cQFR | 13 [7-31] | NS | − 0.02 |
| iFR | S | + 0.02 | ||||||
| Shukla23 | 31 | 56 ± 8 | 100 | 78 ± 9 | FFR | 18 ± 4 | S | − 0.01 |
| Thim24 | 120 | 66 ± 11 | 100 | 50 [41-59] | iFR | 16 [5-32] | S | + 0.03 |
| Van der Hoeven25 | 73 | 61 ± 10 | 100 | 55 ± 13 | FFR | 31 ± 6 | S | − 0.03 |
| iFR | NS | + 0.01 | ||||||
| Resting Pd/Pa | NS | + 0.01 | ||||||
| Wang26 | 70 | 62 | 100 | NR | QFR | 30 | NS | − 0.01 |
| FFR | S | − 0.03 | ||||||
| Zhao27 | 102f | 66 ± 6 | 100 | 64 ± 5 | cQFR | 365 | NR | + 0.01 |
| 253g | 65 ± 6 | 64 ± 6 | NR | − 0.01 | ||||
|
cQFR, contrast quantitative flow ratio; FFR, fractional flow reserve; iFR, instantaneous wave-free ratio; muQFR, Murray law-based QFR; NCL, non-culprit lesions; NR, not reported; NS, non-significant; Pd/Pa, distal-to-aortic coronary pressure ratio; QFR, quantitative flow ratio; PDS, percent diameter stenosis; S, significant; STEMI, ST-segment elevation myocardial infarction; vFFR, vessel fractional flow reserve. Data are expressed as mean, mean ± standard deviation or median [interquartile range] or (range) (age, PDS of NCL, follow-up). a Statin + alirocumab subgroup. b Statin + placebo subgroup. c Percentage of ST-segment elevation acute coronary syndrome. d Overall population (statin + evolocumab or placebo subgroups). e No. of lesions analyzed: 70. f Statin + evolocumab subgroup. g Statin monotherapy subgroup. |
||||||||
A total of 20 articles were included13-32 (1 article in the form of a conference presentation).19 Publication years went from 2010 through 2024. The total number of reported patients was 4379.
In every publication, the patients are predominantly men and non-diabetic. The main clinical presentation was STEMI, except for 3 studies.19,29,31
The following methods were assessed: a) angiography-derived: Murray law-based quantitative flow ratio (muQFR), quantitative flow ratio (QFR), vessel FFR (vFFR); b) hyperemic (FFR); and c) non-hyperemic indices: instantaneous wave-free ratio (iFR), resting distal-to-aortic coronary pressure ratio (Pd/Pa). When reported, the FFR was obtained using adenosine.
Reported patients for each index are as follows: 2340 (muQFR), 1187 (QFR), 710 (FFR), 243 (iFR), 92 (vFFR), and 73 (resting Pd/Pa).
Risk of bias
The studies mainly used an observational (cohort) design. Cohort studies on angiography-derived methods were retrospective, except for 1 article on QFR.28 Those on FFR and non-hyperemic indices were prospective, except for 2 substudies.22,26
QFR was also evaluated by 1 quasi-experimental study27 and 1 randomized controlled trial.13
Finally, the FFR was assessed by 2 randomized controlled trials, in samples with predominance of non-ST-segment elevation myocardial infarction (NSTEMI).19,31,33
Results are shown in table 1 of the supplementary data, table 2 of the supplementary data, and table 3 of the supplementary data. There were no studies with high risk of bias.
Table 2. Data drawn from studies with moderate risk of bias
| First author | Patients (No.) | Age (years) | STEMI (%) | PDS of NCL (%) | Type of index | Follow-up (days) | Comparison across measurements | |
|---|---|---|---|---|---|---|---|---|
| P-value | Mean difference (staged−acute value) | |||||||
| Barauskas28 | 79 | NR | 100 | (35-75) | QFR | ≥ 91 | NSa | − 0.02 |
| Jo29 | 115 | 60 ± 12 | 32.2 | NR | FFR | 182 | NS | − 0.01 |
| Li30 | 84 | 60 ± 11 | 100 | (50-90) | muQFR | 8 ± 2 | NS | 0.00 |
| Park31 | 60b | 57 ± 11 | 30 | NR | FFR | 182 | NS | − 0.02 |
| 60c | 59 ± 10 | 33.3 | NR | NS | − 0.01 | |||
| Spitaleri32 | 31 | 64 ± 12 | 100 | 59 ± 13 | cQFR | (3-4) | NS | 0.00 |
|
cQFR, contrast quantitative flow ratio; FFR, fractional flow reserve; muQFR, Murray law-based QFR; NCL, non-culprit lesions; NS, non-significant; NR, not reported; PDS, percent diameter stenosis; QFR, quantitative flow ratio; STEMI, ST-segment elevation myocardial infarction. Data are expressed as ≥ lower limit or mean or mean ± standard deviation or (range) (age, PDS of NCL, follow-up). a Level of significance was set at P < .001. b Ticagrelor subgroup. c Clopidogrel subgroup. |
||||||||
Primary endpoint
Statistical significance
There were no articles on relevant changes in muQFR,16,30 resting Pd/Pa,25 and vFFR17 at the follow-up.
A significant difference in FFR, iFR, and QFR was found in 3, 2, and 1 article(s),14,22-26 respectively. In 1 study, the difference in QFR was non-significant, with a significance threshold of .001.28
These variations were seen in cohorts of STEMI patients.14,22-26 Studies including non-ST-segment elevation acute coronary syndrome (NSTEACS) did not show any relevant differences regarding the QFR15 or the FFR.19,21,29,31
A total of 4 articles20,22,25,26 evaluated > 1 method. The iFR and FFR were both stable in the study by Musto et al.,20 while the iFR was more stable than the FFR in a different article.25 The QFR was compared to both the FFR26 and the iFR.22 Unlike these indices, the QFR did not show any significant changes in staged phases.22,26
Mean differences
The most valued indices showed varying results. muQFR had MD values close to 0 in both studies.16,30
QFR variations were observed at both lower22,26,28 and higher values.14,15,18 Conversely, the FFR and the iFR varied towards smaller and greater values, respectively.22-26,29,31 Their MDs ranged from − 0.02 to + 0.06 (QFR), − 0.03 to 0.00 (FFR), and 0.00 to + 0.03 (iFR).14,19-21,24,25,28 MD values of 0.01 were observed more often.
In STEMI patients, the MDs of the FFR, the iFR, and the QFR were close to 0 only in studies with mean follow-ups of < 1 week.20,32 In studies including NSTEACS, the FFR MDs were close to 0 after longer mean follow-ups (> 1 month).19,21 Furthermore, Ntalianis et al. showed a greater stability of FFR in patients with NSTEMI (MD, 0.00) vs those with STEMI (MD, − 0.02).21
Disagreement
Disagreement in the indication for revascularization is shown in figure 2. MDs of 0.01 resulted in variable disagreements: 5%-18%.15,18,23,25

Figure 2. Disagreement between acute and staged values in the indication for PCI. B, Barauskas; C, Cortés; E, Erbay; FFR, fractional flow reserve; H, Huang; iFR, instantaneous wave-free ratio; K, Kirigaya; L, Li; muQFR, Murray law-based QFR; N, Ntalianis; PCI, percutaneous coronary intervention; QFR, quantitative flow ratio; SE, Sejr-Hansen; SH, Shukla; SP, Spitaleri; T, Thim; V, van der Hoeven; vFFR, vessel fractional flow reserve.
Unlike the QFR, the FFR and the iFR consistently showed a higher frequency of one type of disagreement: acute−/staged+ for FFR,21,23,25 and acute+/staged− for iFR.24,25
Secondary endpoint
A total of 4 studies compared the effects of different drugs on the physiological parameters.13,19,27,31
Ticagrelor (which can increase the levels of adenosine) was compared to clopidogrel and no significant differences were found in the FFR of non-culprit vessels after 6 months of treatment.31
Another 3 studies compared a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor (eg, alirocumab or evolocumab) plus high-intensity statin (HIST) (eg, rosuvastatin 20 mg/day) vs statin-only therapy.13,19,27
In a nonrandomized study, the QFR values were significantly higher in the evolucumab group at 12 months.27 However, in 2 randomized studies, no significant differences were observed across the 2 treatment groups in the QFR at 12 months or in the FFR at 3 months, respectively.13,19
DISCUSSION
The main findings of this systematic review are these: firstly, in STEMI patients, the muQFR, resting Pd/Pa, and vFFR indices remained relatively stable in retrospective and/or small studies. The FFR, iFR, and QFR showed variability between acute and staged phases. Secondly, the FFR did not change significantly in prospective cohorts or randomized controlled trials including NSTEACS. Thirdly, the QFR was more stable than both the FFR and the iFR in direct comparisons, although only the FFR and the iFR exhibited consistent directions of change. Fourthly, PCSK9 inhibitors added to HIST did not influence physiological measurements compared with HIST in randomized controlled trials.
The muQFR demonstrated stability in a large sample of patients. This index is based on a single angiographic view, unlike other angiography-based methods that require 2 angiographic projections. This characteristic might reduce observer variability and enhance reliability. Future prospective and comparative studies are needed to confirm the validity of this method.
Although low variations for FFR, iFR, and QFR were observed in cohorts of STEMI patients,20,32 these studies were limited by short-term follow-ups. Thim et al. found a non-significant change in the iFR with 5-day follow-ups, whereas there were significant changes with ≥ 5 day follow-ups.24 Therefore, physiological disarrangements initiated at the acute moment of STEMI might still exist if a staged procedure is conducted close to the index event.24,25
Angiographic, hemodynamic, and microcirculatory variables may alter acute physiologic assessment and account for the higher reliability of the FFR in NSTEACS vs STEMI.
In patients with microvascular dysfunction, epicardial blood flow cannot increase sufficiently during maximal hyperemia, thus causing a reduced pressure gradient across the stenotic lesion,29 and higher FFR values.
In STEMI patients, microcirculatory indices (coronary flow reserve and index of microcirculatory resistance) were significantly worse during the acute phase, along with a higher FFR.25 Conversely, studies including NSTEACS did not show any significant differences in the coronary flow reserve and/or index of microcirculatory resistance at the follow-up.21,29,31
Furthermore, STEMI patients showed greater acute angiographic severity, along with lower QFR or iFR values,14,22 which may be attributed to vasoconstriction typically occurring during the acute phase.
Consequently, the FFR seems more reliable in NSTEACS vs STEMI due to reduced acute microcirculatory impairment and/or vasoconstriction.
Literature trials support the use of the FFR in NCL of NSTEMI during the acute phase (eg, within the index hospitalization).34,35 In contrast, acute FFR-guided complete revascularization did not show any significant benefits in terms of death or myocardial infarction in STEMI patients.36-39
The higher stability of QFR when directly compared to the FFR or the iFR was limited to a small number of patients in post-hoc substudies.22,26 A MD of 0.01 sometimes led to non-trivial disagreement on revascularization decision,25 likely due to baseline values being near the cut-off. Therefore, it is essential to have an index which remains stable or demonstrates consistent changes, such as the FFR and the iFR. Similarly, these indices demonstrated a greater frequency of a specific type of disagreement (methodological variations–wire positioning–may explain the less frequent cases of disagreement).24
Therefore, the FFR and the iFR could be considered in the acute STEMI as an alternative to delayed assessments,25 considering that the FFR tends to decrease and the iFR tends to increase. The FFR could guide the revascularization of positive lesions (FFR ≤ 0.80).25 In patients with a FFR > 0.80, acute iFR assessment can be used to delay the revascularization of negative NCL (iFR > 0.89).24 In the remaining cases (iFR ≤ 0.89), some authors suggested a staged reevaluation.24 At least 5 days after the index procedure should go by. This was the minimum time needed to observe the initial resolution of acute physiological disturbances.24
Finally, when plaques are correctly identified as functionally negative, they may still be vulnerable and associated with adverse events. NCL exhibiting thin-cap fibroatheromas as defined by optical coherence tomography, and having a muQFR ≤ 0.80, showed the highest event rate,40 which suggests that imaging can offer additional prognostic information.
PCSK9 inhibitors have shown minimal impact on coronary physiology, despite greatly reducing low-density lipoprotein-cholesterol (LDL-C) levels. A large treatment effect on HIST only,19 minor flow limitation at baseline, and microvascular compensation may account for this finding.13
However, combining alirocumab with HIST resulted in a greater increase in cap thickness of fibroatheromas vs statin monotherapy as assessed by optical coherence tomography.41 Moreover, lower LDL-C levels after an ACS are associated with the occurrence of fewer cardiovascular events.2 Therefore, PCSK9 inhibitor treatment is recommended in patients who do not reach their LDL-C target despite maximum tolerated statin and ezetimibe therapy.2
Limitations
The wide variety of indices to assess coronary physiology has led to a lack of evidence on some of them; similarly, few studies made direct comparisons among such indices.
Our evaluations are mainly based on observational studies with a very different follow-ups.
Angiography-based methods frequently exhibited bias due to their retrospective analysis. Some patients were excluded because of the poor quality of angiographies or anatomic issues, such as ostial lesion or severe vascular tortuosity. Some angiographies were not obtained optimally according to the specific acquisition guide.
CONCLUSIONS
The assessment of functional indices for NCL during the initial procedure for STEMI is not absolutely reliable. This evidence is due to potential variability of the FFR, the iFR, and the QFR outside the acute phase. Although variation was not significant for muQFR, resting Pd/Pa, and vFFR, retrospective and/or limited data limit the generalizability of these findings.
Both the FFR and the iFR showed consistent directions of change. Therefore, during an acute STEMI, the FFR can guide the revascularization of positive NCL, while the iFR can help defer revascularization of negative NCL. A negative FFR with a positive iFR should be reevaluated.
The FFR shows robust data supporting its use in NLC of NSTEMI during the acute phase, meaning that it is a more reliable index for initial ACS procedures.
DATA AVAILABILITY
Search string for Google Scholar: “acute coronary syndrome”|”myocardial infarction” “fractional flow reserve”|FFR| “hyperemic ind”|”resting ind”|iFR|”instantaneous wave-free ratio”| “angiography-based ind”|”angiography-derived ind”|QFR|”quantitative flow ratio”|OFR staged|repeated|later. The remaining search strings are available upon request.
FUNDING
None declared.
ETHICAL CONSIDERATIONS
Ethical committee and patient’s informed consent: not applicable. We followed the SAGER guidelines with respect to possible sex/gender bias.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
Microsoft Copilot was used to help edit the English version of the text.
AUTHORS’ CONTRIBUTIONS
F. Vergni designed the work. F. Vergni, S. Buscarini, L. Ciurlanti, and F.L. Gurgoglione contributed to data acquisition (screening, and/or extraction). F. Vergni, and L. Ciurlanti conducted the critical appraisal. F. Vergni, and S. Buscarini contributed to data interpretation. F. Vergni, and F.L. Gurgoglione drafted, edited and reviewed the work. F. Vergni, S. Buscarini, L. Ciurlanti, F.L. Gurgoglione, F. Pellone, and M. Luzi approved the final version for publication.
CONFLICTS OF INTEREST
None declared.
WHAT IS KNOWN ABOUT THE TOPIC?
- The role of physiological assessment of NCL in patients with ACS is still under discussion because its reliability might be flawed due to alterations of both the hyperemic and resting flow in the acute phase.
WHAT DOES THIS STUDY ADD?
- In NSTEACS, it is appropriate to use the FFR for the acute evaluation of NCL. Regarding STEMI, a hybrid approach with both acute FFR and iFR can be considered, with delayed reassessment for doubtful NCL.
REFERENCES
1. Benatti G, Gragnano F, Vignali L, CalabròP, Gurgoglione FL, Niccoli G. Timing and modality of complete revascularization in patients presenting with ST-segment elevation myocardial infarction and multivessel coronary artery disease. Int J Cardiol. 2023;380:6-11.
2. Byrne RA, Rossello X, Coughlan JJ, et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J. 2023;44:3720-3826. Published correction appears in Eur Heart J. 2024;45:1145.
3. Koo BK, Hwang D, Park S, et al. Practical Application of Coronary Physiologic Assessment:Asia-Pacific Expert Consensus Document:Part 2. JACC Asia. 2023;3:825-842.
4. Mejía-Rentería H, Lee JM, van der Hoeven NW, et al. Coronary Microcirculation Downstream Non-Infarct-Related Arteries in the Subacute Phase of Myocardial Infarction:Implications for Physiology-Guided Revascularization. J Am Heart Assoc. 2019;8:011534.
5. Takenaka H, Okamura T, Miyazaki Y, et al. Serial changes in the quantitative flow ratio in patients with intermediate residual stenosis after percutaneous coronary intervention. Heart Vessels. 2022;37:363-373.
6. Cardiovascular Research Foundation. Conference News. Available at: https://www.tctmd.com. Accessed 30 Mar 2025.
7. Clinical Trial Results. Slide Library. Available at: https://clinicaltrialresults.org. Accessed 30 Mar 2025.
8. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement:an updated guideline for reporting systematic reviews. BMJ. 2021;372:71.
9. Moola S, Munn Z, Tufanaru C, et al. Chapter 7:systematic reviews of etiology and risk. In:Aromataris E, Munn Z, editors. JBI Manual for Evidence Synthesis. Adelaide:JBI;2020.
10. Barker TH, Habibi N, Aromataris E, et al. The revised JBI critical appraisal tool for the assessment of risk of bias for quasi-experimental studies. JBI Evid Synth. 2024;22:378-388.
11. Barker TH, Stone JC, Sears K, et al. The revised JBI critical appraisal tool for the assessment of risk of bias for randomized controlled trials. JBI Evid Synth. 2023;21:494-506.
12. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135.
13. Bär S, Kavaliauskaite R, Otsuka T, et al. Impact of alirocumab on plaque regression and haemodynamics of non-culprit arteries in patients with acute myocardial infarction:a prespecified substudy of the PACMAN-AMI trial. EuroIntervention. 2023;19:286-296.
14. Cortés C, Rodríguez-Gabella T, Gutiérrez H, et al. Quantitative flow ratio in myocardial infarction for the evaluation of non-infarct-related arteries. The QIMERA pilot study. REC Interv Cardiol. 2019;1:13-20.
15. Erbay A, Penzel L, Abdelwahed YS, et al. Feasibility and diagnostic reliability of quantitative flow ratio in the assessment of non-culprit lesions in acute coronary syndrome. Int J Cardiovasc Imaging. 2021;37:1815-1823.
16. Hou S, Zhu X, Zhao Q, et al. Quantitative flow ratio-guided staged percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction. Heliyon. 2024;10:39335.
17. Huang J, Groenland FTW, Scoccia A, et al. Acute-setting vs. -setting vessel fractional flow reserve of intermediate non-culprit lesions in patients with ST-segment elevation myocardial infarction (FAST STAGED study). Int J Cardiol Heart Vasc. 2023;45:101192.
18. Kirigaya H, Okada K, Hibi K, et al. Diagnostic performance and limitation of quantitative flow ratio for functional assessment of intermediate coronary stenosis. J Cardiol. 2021;77:492-499.
19. Mensink F, Los J, van Geuns RJ. Functional and morphological changes of significant non-culprit coronary artery stenosis by extensive LDL-C reduction with PCSK9 inhibitors. Results of the randomized, placebo-controlled FITTER trial. In:ESC Congress 2024;2024 30 August - 2 September;London, United Kingdom. Available at: https://clinicaltrialresults.org/slide-library/.
20. Musto C, De Felice F, Rigattieri S, et al. Instantaneous wave-free ratio and fractional flow reserve for the assessment of nonculprit lesions during the index procedure in patients with ST-segment elevation myocardial infarction:The WAVE study. Am Heart J. 2017;193:63-69.
21. Ntalianis A, Sels JW, Davidavicius G, et al. Fractional flow reserve for the assessment of nonculprit coronary artery stenoses in patients with acute myocardial infarction. JACC Cardiovasc Interv. 2010;3:1274-1281.
22. Sejr-Hansen M, Westra J, Thim T, et al. Quantitative flow ratio for immediate assessment of nonculprit lesions in patients with ST-segment elevation myocardial infarction-An iSTemI substudy. Catheter Cardiovasc Interv. 2019;94:686-692.
23. Shukla A, Dwivedi SK, Chandra S, et al. Reliability of Fractional Flow Reserve in Non-Infarct-Related Arteries in ST-Segment Elevation Myocardial Infarction Patients Undergoing a Pharmaco-Invasive Approach. Cureus. 2024;16:52668.
24. Thim T, Götberg M, Fröbert O, et al. Nonculprit Stenosis Evaluation Using Instantaneous Wave-Free Ratio in Patients With ST-Segment Elevation Myocardial Infarction. JACC Cardiovasc Interv. 2017;10:2528-2535.
25. der Hoeven NW, Janssens GN, de Waard GA, et al. Temporal Changes in Coronary Hyperemic and Resting Hemodynamic Indices in Nonculprit Vessels of Patients With ST-Segment Elevation Myocardial Infarction. JAMA Cardiol. 2019;4:736-744.
26. Wang L, Travieso A, van der Hoeven N, et al. Improved Nonculprit Stenosis Assessment in Patients With ST-Segment Elevation Myocardial Infarction Using Quantitative Flow Ratio. JACC Cardiovasc Interv. 2023;16:1828-1830.
27. Zhao Q, Sun S, Zhou F, Yue J, Luo X, Qu X. The Inhibition of Evolocumab on Non-Infarct-Related Artery Disease in Patients with ST-Elevation Myocardial Infarction. Int J Gen Med. 2023;16:2771-2781.
28. Barauskas M, Žiubryte·G, Jodka N, Unikas R. Quantitative Flow Ratio for Assessment of Non-Culprit Coronary Artery Lesions During Percutaneous Coronary Intervention (PCI) in 79 Patients Diagnosed with ST-Elevation Myocardial Infarction (STemI):A Study from a Single Center in Lithuania. Med Sci Monit. 2023;29:939360.
29. Jo YS, Moon H, Park K. Different Microcirculation Response Between Culprit and Non-Culprit Vessels in Patients With Acute Coronary Syndrome. J Am Heart Assoc. 2020;9:015507.
30. Li X, Mi L, Duan J, Tao L, Xu X, Wang G. Murray law-based quantitative flow ratio for assessment of nonculprit lesions in patients with ST-segment elevation myocardial infarction. Cardiol J. 2024;31:522-527.
31. Park K, Cho YR, Park JS, Park TH, Kim MH, Kim YD. Comparison of the Effects of Ticagrelor and Clopidogrel on Microvascular Dysfunction in Patients With Acute Coronary Syndrome Using Invasive Physiologic Indices. Circ Cardiovasc Interv. 2019;12:008105.
32. Spitaleri G, Tebaldi M, Biscaglia S, et al. Quantitative Flow Ratio Identifies Nonculprit Coronary Lesions Requiring Revascularization in Patients With ST-Segment-Elevation Myocardial Infarction and Multivessel Disease. Circ Cardiovasc Interv. 2018;11:006023.
33. Mensink FB, Los J, Oemrawsingh RM, et al. Functional and morphological improvement of significant non-culprit coronary artery stenosis by LDL-C reduction with a PCSK9 antibody:Rationale and design of the randomized FITTER trial. Heliyon. 2024;10:38077.
34. Biscaglia S, Guiducci V, Escaned J, et al. Complete or Culprit-Only PCI in Older Patients with Myocardial Infarction. N Engl J Med. 2023;389:889-898.
35. Lee JM, Kim HK, Park KH, et al. Fractional flow reserve versus angiography-guided strategy in acute myocardial infarction with multivessel disease:a randomized trial. Eur Heart J. 2023;44:473-484.
36. Böhm F, Mogensen B, Engstrøm T, et al. FFR-Guided Complete or Culprit-Only PCI in Patients with Myocardial Infarction. N Engl J Med. 2024;390:1481-1492.
37. Smits PC, Abdel-Wahab M, Neumann FJ, et al. Fractional Flow Reserve-Guided Multivessel Angioplasty in Myocardial Infarction. N Engl J Med. 2017;376:1234-1244.
38. Engstrøm T, Kelbæk H, Helqvist S, et al. Complete revascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3—PRIMULTI):an open-label, randomised controlled trial. Lancet. 2015;386:665-671.
39. Puymirat E, Cayla G, Simon T, et al. Multivessel PCI Guided by FFR or Angiography for Myocardial Infarction. N Engl J Med. 2021;385:297-308.
40. Xu X, Fang C, Jiang S, et al. Functional or anatomical assessment of non-culprit lesions in acute myocardial infarction. EuroIntervention. 2025;21:217-228.
41. Räber L, Ueki Y, Otsuka T, et al. Effect of Alirocumab Added to High-Intensity Statin Therapy on Coronary Atherosclerosis in Patients With Acute Myocardial Infarction:The PACMAN-AMI Randomized Clinical Trial. JAMA. 2022;327:1771-1781.
- Prognostic value of global plaque volume calculated from the 3D reconstruction of the coronary tree in patients without significant coronary artery disease. A multicenter study
- Long-term prognostic impact of the left anterior descending coronary artery as the STEMI-related culprit vessel: subanalysis of the EXAMINATION-EXTEND trial
- New scoring balloon to treat moderate-to-severe calcified coronary lesions. The first-in-man Naviscore study
- The ultrathin-strut everolimus-eluting stent in a real-world population: the Everythin multicenter registry
Editorials
Expanding the role of drug-coated balloons in native large coronary artery disease
aDepartment of Biomedical Sciences, Humanitas University, Pieve Emanuele, Milan, Italy
bCardio Center, IRCCS Humanitas Research Hospital, Rozzano, Milan, Italy
cDivision of Cardiology, Montefiore Medical Center, Bronx, New York, United States
Original articles
Editorials
The role of angiography-derived physiological assessment techniques in the post-FAVOR III Europe era?
aServicio de Cardiología, Hospital General Universitario Gregorio Marañón, Instituto de Investigación Sanitaria Gregorio Marañón, Universidad Complutense de Madrid, Madrid, Spain
bCentro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), Instituto de Salud Carlos III, Madrid, Spain
Interviews
An interview with Camino Bañuelos
aServicio de Cardiología, Hospital Clínico San Carlos, Instituto de Investigación Sanitaria del Hospital Clínico San Carlos (IdISSC), Madrid, Spain
bServicio de Cardiología, Hospital Clínico Universitario de Santiago de Compostela, Santiago de Compostela, A Coruña, Spain








