Original article
Angiography-derived index versus fractional flow reserve for intermediate coronary lesions: a meta-analysis review
Índice derivado de la angiografía frente a reserva fraccional de flujo en lesiones coronarias intermedias. Revisión de metanálisis
aDepartamento de Cardiología, Hospital Clínico Universitario de Valladolid, Valladolid, Spain bCentro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), Instituto de Salud Carlos III, Madrid, Spain
ABSTRACT
Introduction and objectives: Mitral regurgitation is one of the most common heart valve diseases. Valve replacement surgery is a guideline-recommended option; however, in a significant proportion of patients, this option is not feasible. In such cases, mitral transcatheter edge-to-edge repair (M-TEER) is a potential therapeutic alternative. Nevertheless, the results of a randomized clinical trial have shown divergent results. Recently, the results of the RESHAPE-HF2 trial were published, providing additional insights. The objective of this work is to evaluate whether there are any differences between performing M-TEER and keeping patients under guideline-directed medical therapy (GDMT).
Methods: We conducted a meta-analysis following the PRISMA guidelines. We searched for studies across the PubMed, Embase, and Cochrane databases until February 2025. We establish the following inclusion criteria: patients with secondary mitral regurgitation, studies comparing M-TEER plus GDMT vs GDMT alone, and who reported hospitalization due to heart failure (HF) or mortality.
Results: A total of 3 randomized clinical trials meet the inclusion criteria, including a total of 1423 patients: 704 received M-TEER and 719, GDMT alone. M-TEER was associated with a reduced risk of HF-related hospitalization with a risk ratio (RR) of 0.71 (95%CI, 0.56-0.90; P = .004). We did not find any differences in all-cause mortality with a RR of 0.80 (95%CI, 0.63-1.02; P = .07).
Conclusions: In this meta-analysis, M-TEER plus GDMT shows a lower risk of HF-related hospitalization vs GDMT alone. We did not find any differences in the risk of all-cause mortality or cardiac death.
Registered at PROSPERO: CRD42025645047.
Keywords: Mitral regurgitation. Mitral transcatheter edge-to-edge repair. Heart failure. M-TEER.
RESUMEN
Introducción y objetivos: La regurgitación mitral es una de las valvulopatías cardiacas más comunes. La cirugía de reemplazo valvular es una opción recomendada por las guías clínicas. Sin embargo, en un porcentaje significativo de pacientes, esta opción no es viable. En estos casos, la reparación mitral percutánea de borde a borde (M-TEER) es una posible alternativa terapéutica. No obstante, los resultados de los ensayos clínicos aleatorizados han mostrado resultados divergentes. Recientemente se han publicado los resultados del estudio RESHAPE-HF2, que aportan información adicional sobre este tema. El objetivo de este trabajo fue evaluar si existen diferencias entre realizar M-TEER o mantener a los pacientes bajo tratamiento médico según las guías clínicas (TMSG).
Métodos: Se realizó un metanálisis siguiendo las guías PRISMA. Se buscaron estudios en las bases de datos PubMed, Embase y Cochrane hasta febrero de 2025. Se establecieron los siguientes criterios de inclusión: pacientes con insuficiencia mitral secundaria, estudios que comparaban M-TEER más TMSG frente a solo TMSG, y que indicaran hospitalización por insuficiencia cardiaca o mortalidad.
Resultados: Tres ensayos clínicos aleatorizados cumplieron los criterios de inclusión, con un total de 1.423 pacientes, de los que 704 se trataron con M-TEER y 719 recibieron solo TMSG. La M-TEER se asoció con una reducción del riesgo de hospitalización por insuficiencia cardiaca con una razón de riesgo de 0,71 (IC95%, 0,56-0,90; p = 0,004). No se encontraron diferencias en cuanto a muerte por cualquier causa, con una razón de riesgo de 0,80 (IC95%, 0,63-1,02; p = 0,07).
Conclusiones: En este metanálisis, la M-TEER, en combinación con el TMSG, mostró una reducción del riesgo de hospitalización por insuficiencia cardiaca en comparación con el TMSG solo. No se hallaron diferencias en el riesgo de muerte por cualquier causa (muerte de causa cardiovascular o infarto agudo de miocardio).
Registrado en PROSPERO: CRD42025645047.
Palabras clave: Regurgitación mitral. Reparación percutánea de borde a borde. Insuficiencia cardiaca. M-TEER.
Abreviaturas
MR: mitral regurgitation. M-TEER: mitral transcatheter edge-to-edge repair. GDMT: guideline-direct medical therapy. HF: heart failure. NYHA: New York Heart Association.
INTRODUCTION
Mitral regurgitation (MR) is a prevalent valvular heart disease associated with significant morbidity and mortality, particularly in patients with heart failure (HF).1 Traditional management strategies include optimal medical therapy to treat symptoms and surgery for definitive correction.2 However, current clinical practice guidelines only recommend mitral valve repair if the patient is undergoing another intervention such as coronary artery bypass graft or aortic valve replacement; nonetheless, many patients are at a high risk for surgery due to comorbid conditions, for this reason, alternative therapeutic options is required.3
Mitral transcatheter edge-to-edge repair (M-TEER) has emerged as a minimally invasive option for patients with symptomatic mitral regurgitation who are ineligible for surgery.4 While M-TEER has shown promising results, its comparative efficacy vs guideline- directed medical therapy (GDMT) is still a matter of discussion.5
This meta-analysis aims to assess the impact of M-TEER plus GDMT vs GDMT alone on major clinical outcomes in patients with secondary MR. The primary endpoint evaluated was HF-related hospitalization. Secondary endpoints included all-cause mortality, cardiac death, improvement in New York Heart Association (NYHA) functional class (FC), and the incidence rate of stroke and myocardial infarction. These endpoints were selected for their clinical relevance and potential to inform therapeutic decision-making in this high-risk patient population.
METHODS
We conducted this meta-analysis in full compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement guidelines.6 All the stages of this study were performed following the Cochrane Handbook for Systematic Reviews of Interventions, version 6.3.7 The protocol for this meta-analysis was registered prospectively in PROSPERO on 10 February 2025 with protocol ID CRD42025645047.
Search strategy
We conducted a systematic search across 3 electronic databases (PubMed, EMBASE, and COCHRANE) from inception until February 2025. Search terms included combinations of the following keywords: “transcatheter”, “secondary”, “mitral valve”, “replacement”, regurgitation”, and “insufficiency”. These keywords were combined using Boolean operators AND, OR. The reference lists of eligible studies and previous reviews were checked to identify additional valuable articles.
Criteria of the included studies
Studies were considered for inclusion in the meta-analysis based on the following criteria: a) randomized clinical trials (RCT) or observational cohort studies in Spanish or English that b) compared transcatheter mitral valve replacement vs optimal medical therapy in adult patients with mitral valve regurgitation due to secondary causes; and c) studies that reported outcomes of interest such as the index HF-related hospitalization, HF-related readmissions or cardiac death, and all-cause mortality.
Studies were excluded if they were non-original articles such as systematic reviews, letters, abstracts, meta-analyses, case reports, or case series. Furthermore, studies were excluded if mitral regurgitation was due to primary causes or if prior surgical repair had been performed.
Although observational cohort studies were eligible, we only identified 1 cohort study that did not meet the inclusion criteria during full-text screening. As a result, only RCTs were ultimately included in the meta-analysis.
Data extraction
To ensure accuracy, we used Zotero to eliminate duplicate references. We screened each paper based on title and abstract as a first step, followed by a full-text review as the second step. Two authors (D.A. Navarro Martínez, and D. Paulino-González) independently screened each paper, with any disagreements being resolved by a third author (A.L. García Loera). In addition, references of the included studies were reviewed and added if they met our eligibility criteria. Data extraction was conducted using Excel spreadsheets capturing the following information: a) baseline characteristics of the studied population, including baseline medication, echocardiographic baseline characteristics, and comorbidities; b) summary of the characteristics of the included studies; c) outcome measures; and d) quality assessment domains.
Primary and secondary endpoints
The primary endpoints established for this meta-analysis included: a) all-cause mortality (hazard ratio [HR]), defined as all-cause mortality, assessed using time-to-event analysis,8 and b) HF-related hospitalization, defined as hospital admission for worsening HF following the intervention (M-TEER or initiation of optimal medical therapy).9
Secondary endpoints included a) stroke, defined as an episode of neurological dysfunction caused by focal cerebral, spinal, or retinal infarction;10 and b) myocardial infarction, defined according to the Fourth Universal Definition.11
All endpoints were assessed as dichotomous categorical variables, which were reported in percentages. The risk ratio (RR) with its corresponding 95% confidence interval (95%CI) was calculated.
Assessment of heterogeneity
To assess heterogeneity, we used Cochrane’s Q-statistic with a significance level of P < .05. Additionally, the I²-statistic was used to quantify the proportion of variability due to heterogeneity, with values > 50% being considered indicative of high heterogeneity.12
Statistical analyses
To assess dichotomous data, we evaluated event frequencies and totals from each study group to calculate the RR and its 95%CI. Moreover, we obtained the HR and the 95%CI to calculate the standard error (SE).
The variables analyzed in this study were based on data reported in the original studies of the intention-to-treat principle. We implemented a random-effects model using the DerSimonian-Laird13 method to account for variability and allow comparison across studies. Given the limitations and strengths of this method, we conducted a sensitivity analysis using the Hartung-Knapp-Sidik-Jonkman method. Forest plots were utilized as a visual representation of the estimated outcomes.
Considering the differences in the populations included in the clinical trials, we conducted an additional exploratory analysis that focused on the RESHAPE-HF214 and COAPT15 trials for the primary endpoints by removing MITRA-FR16 from the analysis.
All statistical analyses, including the calculation of RR and SE, were conducted using RevMan V.5.4.1 software.
RESULTS
The initial database search yielded a total of 164 potentially relevant articles. After removing duplicates, a total of 129 articles finally remained for title and abstract screening. Following this initial screening, 8 articles were identified as potentially eligible for full-text review. Finally, after a detailed evaluation of the full texts under the predefined inclusion and exclusion criteria, 3 studies were included. A summary of the study selection process is shown in the PRISMA flowchart figure 1.
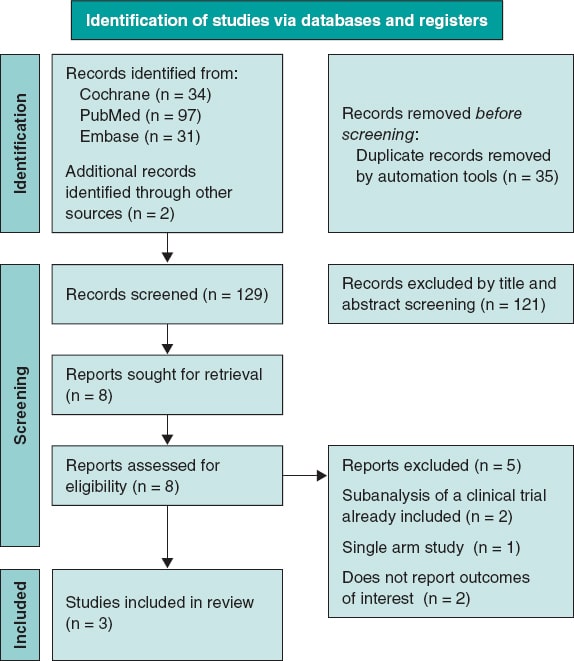
Figure 1. PRISMA 2020 flow diagram for new systematic reviews which included searches of databases and registers only.
Our analysis included 3 RCTs.14-16 We consulted the extended report of the 2-year outcomes of the MITRA-FR trial17 and 1 additional article on the RESAHPE-HF2 to obtain more data regarding hospitalization.18 All included studies compared the use of M-TEER plus GDMT vs GDMT alone. To standardize the outcomes, the median follow-up was set at 24 months; only the NYHA FC was evaluated with 1-year results. All 3 included studies used the MitraClip (Abbott, United States) device.
Clinical baseline characteristics of the patients
We obtained a total population of 1423 patients; among them 704 received M-TEER and 719, GDMT alone. In the RESHAPE-HF214 the device group showed the following characteristics: mean age was 70 years old; left ventricular ejection fraction (LVEF), 32%; median left ventricular end-diastolic volume (LVEDV), 200 mL; median effective regurgitation orifice (EROA), 0.23 cm2; median N-terminal pro-B-type natriuretic peptide (NT-proBNP), 2651; and brain natriuretic peptide (BNP), 556. In the COAPT15 trial, median age was 71 years old; LVEF, 31%; LVEDV, 194 mL; median EROA, 0.41 cm2; median NT-proBNP, 5174; and BNP, 1014. Finally in the MITRA-FR trial16, mean age was 71 years old; LVEF, 33%; LVEDV, 136.2 mL; median EROA, 0.31 cm2; median NT-proBNP, 3407; and BNP, 765. Regarding etiology, all trials enrolled mixed ischemic and non-ischemic etiology populations. Patient and study baseline characteristics, including comorbidities, drugs, and echocardiographic data, are shown in table 1, table 2, and table 3.
Table 1. Baseline characteristics of patients
| Reference | Groups | N | Age | AF or flutter | Diabetes | Previous MI | Nonischemic etiology | Ischemic etiology | 6-min walk distance | Beta- blocker | ACEI | ARB | ARNI | SGLT2i | MRA | Diuretics | Oral anti- coagulants | BNP | NT-proBNP | EuroSCORE II |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RESHAPE-HF214 | M-TEER | 250 | 70.0 ± 10.4 | 118 (47.2) | 91 (36.4) | 144 (57.6) | 88 (35.2) | – | 300 (220-382) | 238 (95.2) | 142 (56.8) | 51 (20.4) | 40 (16.0) | 24 (9.6) | 200 (80.0) | 239 (95.6) | 163 (65.2) | 556 (312-1018) | 2651 (1630-4918) | 5.3 (2.7-8.9) |
| Optimal medical therapy | 255 | 69.4 ± 10.7 | 125 (49.0) | 85 (33.3) | 135 (52.9) | 88 (34.5) | – | 310 (200-378) | 246 (96.5) | 142 (55.7) | 45 (17.6) | 28 (11.0) | 22 (8.6) | 215 (84.3) | 243 (95.3) | 152 (59.6) | 406 (231-874) | 2816 (1306-5496) | 5.3 (2.9-9.0) | |
| COAPT15 | M-TEER | 302 | 71.7 ± 11.8 | 173 (57.3) | 106 (35.1) | 156 (51.7) | 118 (39.1) | 184 (60.9) | 261.3 ± 125.3 | 275 (91.1) | 138 (45.7) | 66 (21.9) | 13 (4.3) | NR | 153 (50.7) | 270 (89.4) | 140 (46.4) | 1014.8 ± 1086.0 | 5174.3 ± 6566.6 | NR |
| Optimal medical therapy | 312 | 72.8 ± 10.5 | 166 (53.2) | 123 (39.4) | 160 (51.3) | 123 (39.4) | 189 (60.6) | 246.4 ± 127.1 | 280 (89.7) | 115 (36.9) | 72 (23.1) | 9 (2.9) | NR | 155 (49.7) | 277 (88.8) | 125 (40.1) | 1017.1 ± 1212.8 | 5943.9 ± 8437.6 | NR | |
| MITRA-FR16 | M-TEER | 152 | 70.1 ± 10.1 | 49/142 (34.5) | 50 (32.9) | 75 (49.3) | 57 (37.5) | 95 (62.5) | 307 (212-387) | 134 (88.2) | 111 (73.0) | 111 (73.0) | 14/140 (10) | NR | 86 (56.6) | 151 (99.3) | 93 (61.2) | NR | NR | 7.33 ± 6.29 |
| Optimal medical therapy | 152 | 70.6 ± 9.9 | 48/147 (32.7) | 39 (25.7) | 52 (34.2) | 66 (43.7) | 85 (56.3) | 335 (210-410) | 138 (90.8) | 113 (74.3) | 113 (74.3) | 17/140 (12.1) | NR | 80 (53.0) | 149 (98.0) | 93 (61.2) | NR | NR | 6.57 ± 5.24 | |
|
ACEI, angiotensin-converting enzyme inhibitors; AF, atrial fibrillation; ARB, angiotensin receptor blockers; ARNI, angiotensin receptor-neprilysin inhibitors; BNP, B-type natriuretic peptide; M-TEER, mitral transcatheter edge-to-edge repair; MI, myocardial infarction; MRA, mineralocorticoid receptor antagonists; NR, not reported; NT-proBNP, N-terminal pro-B-type natriuretic peptide; SGLT2i, sodium-glucose cotransporter 2 inhibitors. |
||||||||||||||||||||
Table 2. Summary of included studies
| Authors and year | Study design | Device | Population size | Compared interventions | Mean follow-up | Key findings |
|---|---|---|---|---|---|---|
| Anker et al. RESHAPE-HF2 202414 | RCT | MitraClip | 505 | M-TEER Optimal medical therapy | 24 months |
|
| Stone et al. COAP 201815 | RCT | MitraClip | 614 | M-TEER Optimal medical therapy | 24 months |
|
| Obadia et al. MITRA - FR 201816 | RCT | MitraClip | 304 | M-TEER Optimal medical therapy | 24 months |
|
|
95%CI, 95% confidence interval; HF, heart failure; HR, hazard ratio; M-TEER, mitral transcatheter edge-to-edge repair; OR, odds ratio. |
||||||
Table 3. Echocardiographic baseline characteristics
| Baseline characteristics | RESHAPE-HF214 | COAPT15 | MITRA-FR16 | |||
|---|---|---|---|---|---|---|
| Device group | Control group | Device group | Control group | Device group | Control group | |
| Severity MR Grade 3+, n,% (n) | 141 (56.4) (250) | 141(55.3) (255) | 148 (49.0) (302) | 172 (55.3) (312) | – | – |
| Severity MR Grade 4+, n,% (n) | 109 (43.6) (250) | 114 (44.7) (255) | 154 (51.0) (302) | 139 (44.7) (312) | – | – |
| LVEF, % (n) | 32 (26-37) (250) | 31 (25-37) (255) | 31.3 ± 9.1 (302) | 31.3 ± 9.6 (312) | 33.3 ± 6.5 | 32.9 ± 6.7 |
| LVEDV, mL (n) | 137 (100-173) (250) | 140 (104-176) (255) | 135.5 ± 56.1 (302) | 134.3 ± 60.3 (312) | – | – |
| LVEDV, mL (n) | 200 (153-249) (250) | 206 (158-250) (255) | 194.4 ± 69.2 (302) | 191.0 ± 72.9 (312) | 136.2 ± 37.4 | 134.5 ± 33.1 |
| LVESD, cm (n) | 5.8 (5.3-6.5) (250) | 5.9 (5.3-6.4) (255) | 5.3 ± 0.9 (302) | 5.3 ± 0.9 (312) | – | – |
| LVEDD, cm (n) | 6.9 (6.3-7.6) (250) | 6.8 (6.4-7.5) (255) | 6.2 ± 0.7 | 6.2 ± 0.8 | – | – |
| EROA, cm2 (n) | 0.23 (0.20-0.30) (250) | 0.23 (0.19-0.29) (255) | 0.41 ± 0.15 | 0.40 ± 0.15 | 0.31 ± 10* | 0.31 ± 11* |
| Regurgitant volume, mL (n) | 35.4 (28.9-43.9) (250) | 35.6 (28.2-42.5) (255) | – | – | 45 ± 13 | 45 ± 14 |
|
EROA, effective regurgitant orifice area; LV, left ventricular; LVEDD, left ventricular end-diastolic dimension; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESD, left ventricular end-systolic dimension; MR, mitral regurgitation. RESHAPE-HF2 data express median and interquartile range [IQR] in the COAPT trial. MITRA-FR data express median ± standard deviation. * This data was originally expressed in mm2 and has been converted to standardized meditation. |
||||||
Risk of bias assessment
To evaluate the quality of the included randomized controlled trials, we used the Risk of Bias 2 (RoB2) tool from the Cochrane Handbook of Systematic Reviews of Interventions 6.3.7 This methodology allowed us to systematically assess the methodological quality of each study, thereby strengthening the validity of our findings.
Of the 3 studies, 1 had a low risk of bias,14 while the other 2 raised some concerns.15,16 The studies with some concerns were limited by factors in their methodologies, as they were open-label studies (figure 2).
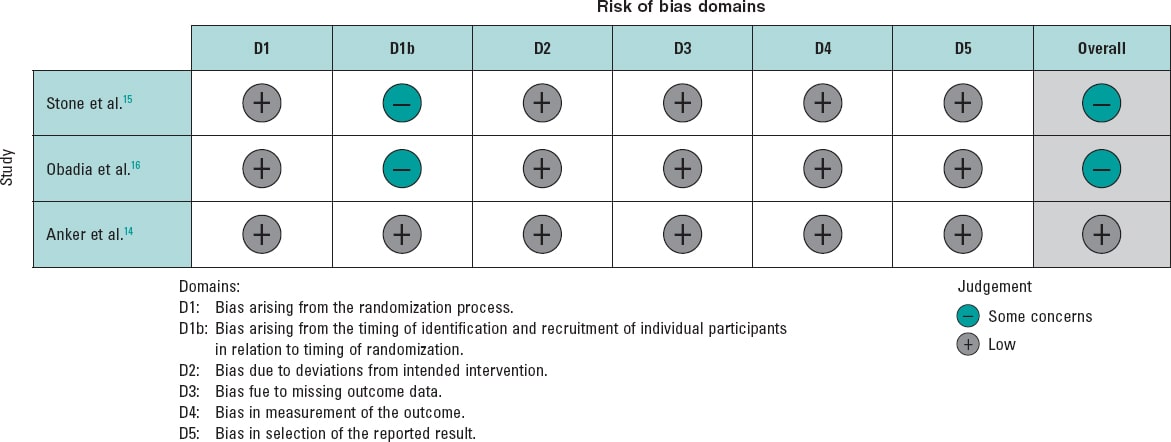
Figure 2. Risk of bias for each included randomized clinical trial. The bibliographical references mentioned in this figure correspond to Stone et al.,14 Obadia et al.,15 and Anker et al.16.
Outcomes
HF-related hospitalization
The analysis of this outcome showed a significant reduction, with a RR of 0.71 (95%CI, 0.56-0.90; P = .004). However, substantial heterogeneity was observed, with an I² value of 79%. Considering the differences in the MITRA-FR study population, we conducted an exploratory analysis excluding this trial. Results still demonstrated a significant reduction in the risk of hospitalization, with a RR of 0.63 (95%CI, 0.55-0.72, P < .00001), along with a marked improvement in heterogeneity (I² = 0%). Of note, this finding is exploratory and should be interpreted with caution.
The analysis, including all studies, is shown in figure 3A, and the exploratory analyses are shown in figure 3B. Furthermore, we analyzed HR for this endpoint and, given the limited number of studies, we conducted a sensitivity analysis using the Hartung-Knapp-Sidik-Jonkman (HKSJ) method; all these analyses are provided in the supplementary data (figures S1-S4).
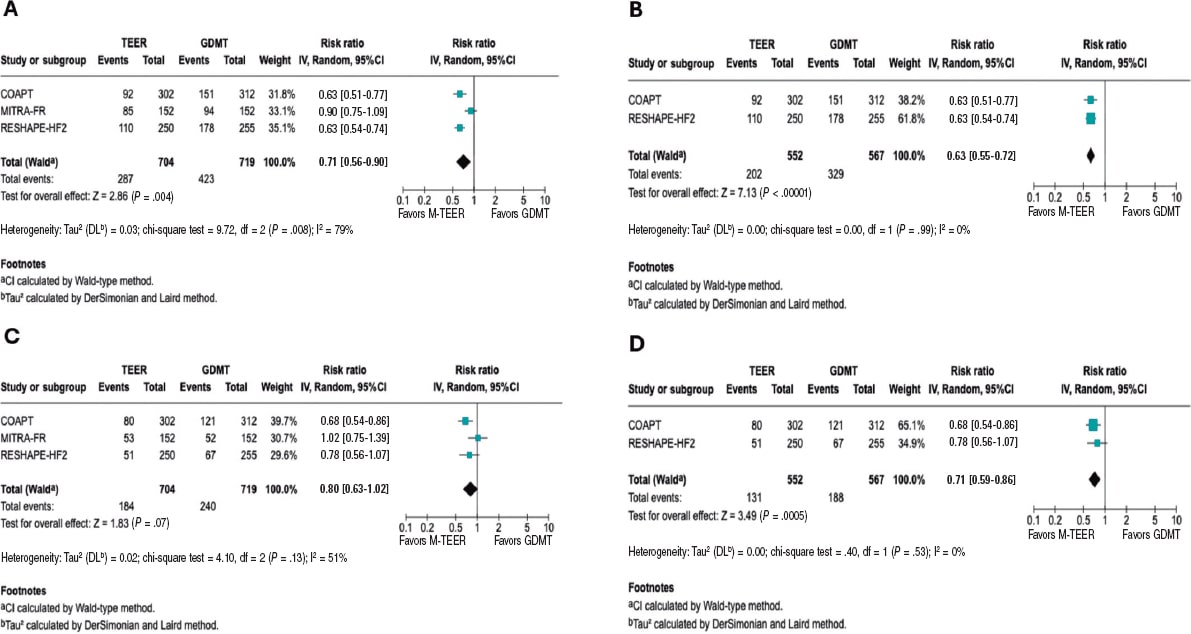
Figure 3. Forest plot of risk ratios (RR). Lines denote the 95% confidence intervals (95%CI) for each trial. A: forest plot of RR for HF related hospitalization. B: forest plot of exploratory RR for HF-related hospitalization. C: forest plot of RR for all-cause mortality. D: forest plot of exploratory RR for all-cause mortality. 95%CI, 95% confidence interval; GDMT, guideline directed medical therapy; M-TEER, mitral transcatheter edge-to-edge repair. The bibliographical references mentioned in this figure correspond to Stone et al.,14 Obadia et al.,15 and Anker et al.16.
All-cause mortality
The RR for all-cause mortality was 0.80 (95%CI, 0.63–1.02; P = .07). However, substantial heterogeneity was observed across the studies (I² = 56%). In the exploratory analysis, a significant reduction in mortality was found, with an RR of 0.71 (95%CI, 0.59–0.86; P = .0005) and no heterogeneity across the studies (I² = 0%). The analyses, including all studies, are shown in figure 3C, and the exploratory analysis in figure 3D.
Both the HR analysis and the sensitivity analysis are provided in the supplementary data (figures S5-S8).
Cardiac death
The RR for death from cardiovascular causes was 0.81 (95%CI, 0.62–1.06, P = .12), with low to moderate heterogeneity (I² = 48%). In the exploratory analysis, a significant reduction in cardiac death was observed, with an RR of 0.74 (95%CI, 0.55–1.00; P = .05) and low heterogeneity (I² = 41%). The analyses, including all studies, are shown in figure 4A, while the exploratory analysis, in figure 4B. The sensitivity analysis and HR results are provided in the supplementary data (figures S9-S12).
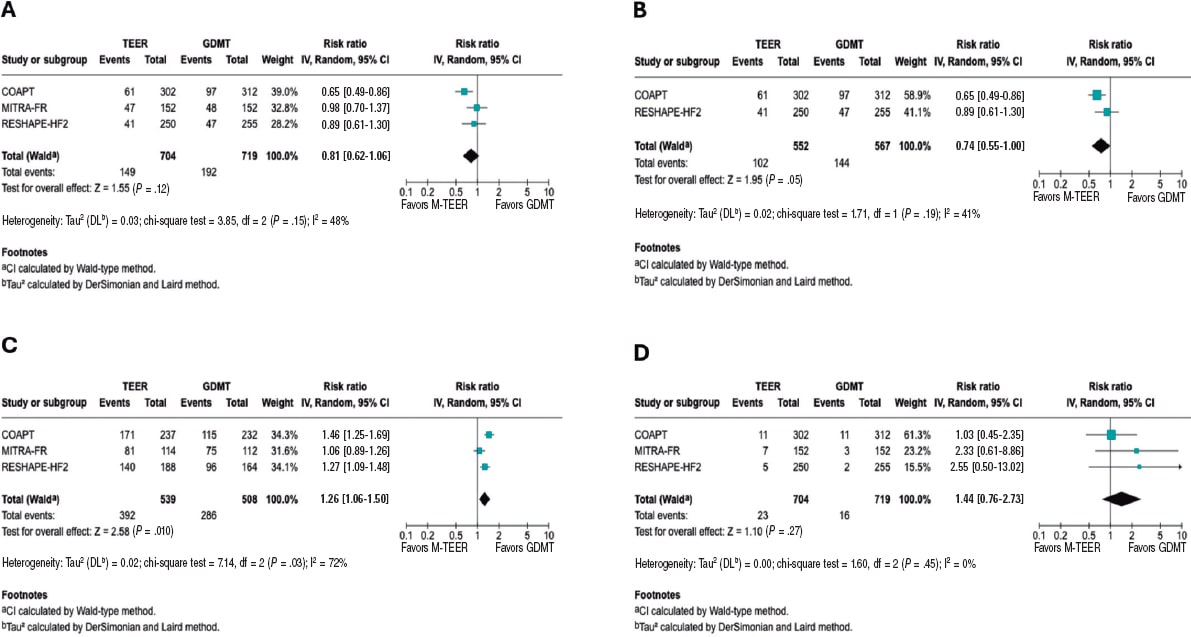
Figure 4. Forest plot of risk ratio (RR). A: forest plot of RR for cardiac death. B: forest plot of exploratory RR for cardiac death. C: forest plot of RR of NYHA FC I/II at 1 year. D: forest plot of RR for stroke. 95%CI, 95% confidence interval; GDMT, guideline directed medical therapy; M-TEER, mitral transcatheter edge-to-edge repair. The bibliographical references mentioned in this figure correspond to Stone et al.,14 Obadia et al.,15 and Anker et al16. Lines denote the 95%CI for each trial.
NYHA FC
In this analysis of the NYHA FC, we observed that patients undergoing M-TEER are more likely to be found in NYHA FC I/II at 12 months, with a RR 1.26 (95%CI, 1.06–1.50; P = .010), respectively. Of note, the high heterogeneity (I² = 72%). Furthermore, it is also essential to note that the NYHA FC is a subjective classification, which is why the findings of this outcome should be interpreted with caution. These results are shown in figure 4C.
Stroke
There were no statistical differences between M-TEER and optimal medical therapy regarding stroke, with a RR of 1.44 (95%CI, 0.76 - 2.73, P = .27) without any heterogeneity being reported across the studies (I² = 0%). These results are shown in figure 4D.
Myocardial Infarction
Regarding the risk of myocardial infarction, our analysis demonstrated no statistical difference between the M-TEER and optimal medical therapy groups (RR, 0.83; 95%CI, 0.43-1.61; P = .58). No heterogeneity among the studies was observed (I² = 0%); this finding is shown in supplementary data (figure S13).
Exploratory analysis by severity
We conducted an exploratory analysis stratified by the baseline grade of mitral regurgitation. Data on the composite endpoint of all-cause death or HF-related hospitalization were available according to MR grade. For patients with MR 3+, the RR was 0.75 (95%CI, 0.56-1.00; P = .05; I² = 12%). For those with severe MR 4+, data were available only from the RESHAPE-HF2 and COAPT trials, yielding a RR of 0.55 (95%CI, 0.42-0.73; P = .0001; I² = 0%). This finding is shown in the supplementary data (figure S14 to figure S15).
DISCUSSION
Our meta-analysis provides an evaluation of 3 major RCTs, COAPT15, MITRA-FR16, and RESHAPE-HF214, evaluating M-TEER plus GDMT vs GDMT alone in patients with secondary MR. In patients with persistent symptoms despite adequate GDMT and who do not meet the criteria for definitive surgical replacement, mitral M-TEER has emerged as a promising alternative. In this updated meta-analysis, no statistically significant effect on all-cause mortality was found; however, the marked between-trial heterogeneity suggests that M-TEER provides meaningful clinical benefit when used in appropriately selected patients.
Our study showed that heterogeneity of results was mainly driven by MITRA-FR. COAPT and RESHAPE-HF2 have multiple similar differences compared with MITRA-FR, as evidenced in trial cohort baseline characteristics, methods, and outcome directions. This is better portrayed by an exploratory analysis comparing COAPT and RESHAPE-HF2 results, showing a 37% reduction in the risk of HF-related hospitalization risk and a 32% reduction in cardiac death (figure 3). The overall neutral mortality rate resulting from our study may be driven by heterogeneity across trials rather than a lack of intrinsic therapeutic benefit.
Among the main differences across trials, the MITRA-FR trial had broader inclusion of patients with tricuspid regurgitation, advanced LV remodeling, greater dilation, and smaller EROA vs the other 2 trials. Another key source of heterogeneity was baseline MR severity. COAPT and RESHAPE-HF2 primarily enrolled patients with grade 3+ and 4+ MR, whereas MITRA-FR is only focused on grade 4+, which may contribute to the observed outcome differences. In our exploratory analysis by MR grade, data showed consistent trends favoring M-TEER; however, these findings should be interpreted with caution due to limited subgroup data.
As proposed by Paul et al.,19 one strategy to evaluate secondary MR is to consider the proportion between EROA and LVEDV. In the MITRA-FR trial, many patients had smaller EROA with markedly dilated ventricles, a phenotype in which GDMT may have more impact than valve proceduren. In the COAPT and RESHAPE-HF2 trials, a greater proportion of patients had large EROA relative to the LVEDV,20 making MR a primary driver of symptoms and outcomes, and, therefore, with potential for greater benefit from M-TEER.
In the trial-level subgroup analyses, the COAPT15 and RESHAPE-HF214 trials reported no significant interaction between treatment assignment and ischemic vs non-ischemic etiology, which indicates consistency of benefit across etiologies. Similarly, the MITRA-FR16 did not identify significant heterogeneity regarding etiology across prespecified subgroups. This suggests that ischemic vs non-ischemic alone should not be used to decide the treatment option in secondary MR. Instead, clinical decisions should prioritize other factors, such as the MR severity, the degree of LV dysfunction, symptom burden, and response to optimal medical therapy.21
Furthermore, differences between trials in procedural success are noteworthy, defined as achieving MR ≤ 2+. This was substantially lower in the MITRA-FR (75.6% MR ≤ 2+ at discharge) vs the COAPT (94.8% MR ≤ 2+ at 12 months) and the RESHAPE-HF2 (90.4% MR ≤ 2+ at 12 months). Multiple reasons could have influenced such differences; for instance, operator experience requirement in MITRA-FR (≥ 5 prior MitraClip cases) vs the high-volume and more experienced centers from the other trials. Similarly, post-approval data from the U.S. SSTS/ACC TVT Registry22 demonstrated > 90% MR reduction to ≤ 2+ and survival rates consistent with trial outcomes, underscoring the reproducibility of clinical benefit in experienced centers. Furthermore, device technology is a main contributor to these differences, from first-generation clips in MITRA-FR, to second-generation in COAPT, to the fourth-generation in RESHAPE-HF2 with independent leaflet grasping and wider arm options, potentially explaining discrepant clinical results regarding MR durability and outcomes. Lastly, GDMT implementation differed across trials. MITRA-FR was conducted before the widespread use of ARNI and SGLT2 inhibitors; COAPT was conducted before the adoption of SGLT2 inhibitors; and RESHAPE-HF2 reflects the contemporary use of quadruple therapy.
Despite the differences in patient phenotypes across the trials, in many developing countries, access to transcatheter valve procedures remains limited, primarily due to their high cost and the need for specialized infrastructure.23,24 The pronounced disparities in access to cutting-edge technologies, coupled with the centralization of these procedures in a few urban centers or high-specialty hospitals, a phenomenon observed even in high-income countries such as the United States, leave a substantial portion of the population without viable treatment options.25 Consequently, the most impactful and broadly deployable intervention in these regions remains rigorous GDMT optimization and provider education. Nevertheless, patient phenotyping should still be performed to identify those who may potentially have an outsized benefit from future M-TEER referrals.
By integrating trial-level population characteristics with clinical outcomes, our work bridges the gap between isolated trial findings and real-world patient selection, thus offering a potential framework for future prospective studies and for refining guideline criteria. While the results from our study must be interpreted with caution, they provide hypothesis-generating evidence supporting the concept that patient selection, particularly considering the balance between EROA and LVEDV and GDMT optimization, may be critical to optimizing the benefit of M-TEER in secondary MR. This approach moves beyond the broad application of current guideline recommendations and points toward a more individualized strategy in which anatomical and functional parameters guide intervention.
Limitations
The primary limitation of this study lies in the heterogeneity of the populations enrolled in the randomized controlled trials, as evidenced by the I2 observed in the analyses, which may influence the overall results. Second, differences in MR severity across trials represent a key limitation. While the COAPT and RESHAPE-HF2 trials mainly included patients with MR grade 3+ or 4, MITRA-FR enrolled a more severe grade, which may partly explain divergent results. Lastly, an individual patient-level meta-analysis could not be conducted due to lack of data; this level of analysis would further increase statistical power, especially in subgroup analyses, enhancing the robustness and generalizability of the findings.
CONCLUSIONS
In this meta-analysis, M-TEER plus GDMT shows a lower risk of HF-related hospitalization vs GDMT alone. We did not find any differences in the risk of all-cause mortality, cardiac death, stroke, or myocardial infarction.
FUNDING
None declared.
ETHICAL CONSIDERATIONS
This meta-analysis was performed using data from previously published studies. As it is based entirely on secondary data, no new data were collected from human or animal participants; on the other hand, the use of SAGER guidelines was not applicable in this study. All included studies were approved from the relevant center ethics committees. The authors confirm that all data utilized were publicly accessible, and no confidential information was used without proper authorization.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
During the preparation of this work, the authors used ChatGPT 4o to review the document’s syntax and grammar. After using this tool/service, the authors reviewed and edited the content as needed and take full responsibility for the content of the published article.
AUTHORS’ CONTRIBUTIONS
D. Paulino-González: conceptualization; formal analysis, writing, review, and editing. A.L. García-Loera: methodology, investigation, writing, review, and editing. D.A. Navarro-Martínez: methodology, formal analysis. M.A. Pardiño-Vega: writing, review, and editing, supervision. K.P. Zúñiga-Montaño: investigation, writing, review, and editing.
CONFLICTS OF INTEREST
None declared.
WHAT IS KNOWN ABOUT THE TOPIC?
- Mitral regurgitation is a prevalent condition that negatively affects the patients’ quality of life. M-TEER-has emerged as an alternative therapeutic option for patients who are ineligible for surgery. However, its benefit remains unclear vs GDMT, as clinical trials evaluating this procedure have reported disparate results, with benefit in primary outcomes observed in the COAPT trial and in the recently published RHESAPE-HF2 trial, but not in the MITRA-FR trial; discrepancy in the results requires further study.
WHAT DOES THIS STUDY ADD?
- This meta-analysis provides a comprehensive evaluation of the evidence comparing M-TEER plus GDMT vs GDMT alone in secondary mitral regurgitation. Our findings confirm that M-TEER is associated with a significant reduction in HF-related hospitalizations. Of note, by examining trial populations in detail, we highlight how patient selection influences outcomes: in studies enrolling patients with less ventricular dilation and lower biomarker levels of congestion (such as the COAPT and RESHAPE-HF2 trials), M-TEER was associated with additional benefits in cardiac death and all-cause mortality. These results underscore the potential role of anatomical and functional parameters, such as the balance between EROA and LVEDV, in identifying patients most likely to benefit from this procedure.
REFERENCES
1. Chehab O, Roberts-Thomson R, Ng Yin Ling C, et al. Secondary mitral regurgitation:pathophysiology, proportionality and prognosis. Heart. 2020;106:716-723.
2. Sengodan P, Younes A, Shah N, Maraey A, Chitwood WR Jr, Movahed A. Contemporary review of the evolution of various treatment modalities for mitral regurgitation. Expert Rev Cardiovasc Ther. 2024;22:639-651.
3. Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease:A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e1159-e1195.
4. Itabashi Y, Kobayashi S, Mizutani Y, Torikai K, Taguchi I. Treatment of secondary mitral regurgitation by transcatheter edge-to-edge repair using MitraClip. J Med Ultrason (2001). 2022;49:389-403.
5. Barnes C, Sharma H, Gamble J, Dawkins S. Management of secondary mitral regurgitation:from drugs to devices. Heart. 2024;110:1099-1106.
6. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement:an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
7. Sterne JAC, Savovic´J, Page MJ, et al. RoB 2:a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898.
8. Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure:A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines [published correction appears in Circulation. 2022;145:e1033.] [published correction appears in Circulation. 2022;146:e185.] [published correction appears in Circulation. 2023;147:e674]. Circulation. 2022;145:e895-e1032.
9. Abraham WT, Psotka MA, Fiuzat M, et al. Standardized Definitions for Evaluation of Heart Failure Therapies:Scientific Expert Panel From the Heart Failure Collaboratory and Academic Research Consortium. JACC Heart Fail. 2020;8:961-972.
10. Sacco RL, Kasner SE, Broderick JP, et al. An updated definition of stroke for the 21st century:a statement for healthcare professionals from the American Heart Association/American Stroke Association [published correction appears in Stroke. 2019;50:e239]. Stroke.2013;44:2064-2089.
11. Thygesen K, Alpert JS, Jaffe AS, et al. Fourth Universal Definition of Myocardial Infarction (2018). Glob Heart. 2018;13:305-338.
12. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560.
13. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177-188.
14. Anker SD, Friede T, von Bardeleben RS, et al. Transcatheter Valve Repair in Heart Failure with Moderate to Severe Mitral Regurgitation. N Engl J Med.2024;391:1799-1809.
15. Stone GW, Lindenfeld J, Abraham WT, et al. Transcatheter Mitral-Valve Repair in Patients with Heart Failure. N Engl J Med. 2018;379:2307-2318.
16. Obadia JF, Messika-Zeitoun D, Leurent G, et al. Percutaneous Repair or Medical Treatment for Secondary Mitral Regurgitation. N Engl J Med.2018;379:2297-2306.
17. Iung B, Armoiry X, Vahanian A, et al. Percutaneous repair or medical treatment for secondary mitral regurgitation:outcomes at 2 years. Eur J Heart Fail. 2019;21:1619-1627.
18. Ponikowski P, Friede T, von Bardeleben RS, et al. Hospitalization of Symptomatic Patients With Heart Failure and Moderate to Severe Functional Mitral Regurgitation Treated With MitraClip:Insights From RESHAPE-HF2. J Am Coll Cardiol. 2024;84:2347-2363.
19. Grayburn PA, Sannino A, Packer M. Proportionate and Disproportionate Functional Mitral Regurgitation:A New Conceptual Framework That Reconciles the Results of the MITRA-FR and COAPT Trials. JACC Cardiovasc Imaging. 2019;12:353-362.
20. Anker SD, Friede T, von Bardeleben RS. Percutaneous repair of moderate-to-severe or severe functional mitral regurgitation in patients with symptomatic heart failure:Baseline characteristics of patients in the RESHAPE-HF2 trial and comparison to COAPT and MITRA-FR trials. Eur J Heart Fail. 2024;26:1608-1615.
21. Nappi F, Singh SSA, Bellomo F.Exploring the Operative Strategy for Secondary Mitral Regurgitation:A Systematic Review. Biomed Res Int.2021;2021:3466813.
22. American College of Cardiology. STS/ACC TVT Registry Analysis Finds TMVr Safe and Effective in Real-World Setting. ACC. 2023 Mar 5. Available at: https://www.acc.org/Latest-in-Cardiology/Articles/2023/03/01/22/45/Sun-1215pm-sts-acc-tvt-acc-2023. Accessed 1 Jul 2025.
23. Bernardi FLM, Ribeiro HB, Nombela-Franco L, et al. Recent Developments and Current Status of Transcatheter Aortic Valve Replacement Practice in Latin America - the WRITTEN LATAM Study. Arq Bras Cardiol. 2022;118:1085-1096.
24. Bana A. TAVR-present, future, and challenges in developing countries. Indian J Thorac Cardiovasc Surg. 2019;35:473-484.
25. Steitieh D, Zaidi A, Xu S, et al. Racial Disparities in Access to High-Volume Mitral Valve Transcatheter Edge-to-Edge Repair Centers. J Soc Cardiovasc Angiogr Interv. 2022;1:100398.
ABSTRACT
Introduction and objectives: De-escalation from prasugrel and ticagrelor to clopidogrel in patients undergoing percutaneous coronary intervention after acute coronary syndrome (ACS) is a strategy aimed at reducing bleeding. This study evaluates whether VerifyNow (Werfen, Spain)–guided de-escalation, based on platelet aggregation measurement, provides a therapeutic benefit in ACS management.
Methods: This ongoing multicenter, prospective, randomized 1:1 trial will enroll 634 patients with ACS who underwent revascularization with a sirolimus-eluting stent and were discharged on dual antiplatelet therapy with ticagrelor or prasugrel. Only those patients with a very low platelet reactivity level (platelet reactivity units ≤ 30) based on VerifyNow 1 month after discharge will be included. The primary endpoint is a composite of cardiovascular death, nonfatal acute myocardial infarction, nonfatal stroke, and bleeding at 1-year follow-up.
Results: The EPIC17-VERONICA study (NCT04654052) will reveal the efficacy profile of the de-escalation strategy, based on the VerifyNow platelet aggregation test, and determine the role of this device in the selection of patients who are eligible to benefit from this strategy.
Conclusions: This study will determine whether platelet function testing provide clinical benefit in the management of patients with ACS.
Keywords: Acute coronary syndrome. Antiplatelet therapy. Platelet function test. Bleeding.
RESUMEN
Introducción y objetivos: La desescalada desde prasugrel y ticagrelor a clopidogrel en pacientes tras intervencionismo coronario percutáneo por síndrome coronario agudo (SCA) constituye una de las estrategias para intentar disminuir las hemorragias. El objetivo de este estudio es averiguar si dicha desescalada guiada por la prueba de agregación plaquetaria VerifyNow (Werfen, España) tiene un efecto beneficioso en el tratamiento del SCA.
Métodos: Estudio multicéntrico, prospectivo y aleatorizado 1:1, en curso. Se incluirán 634 pacientes con SCA y revascularización con stent de sirolimus que sean dados de alta con doble terapia antiagregante con ticagrelor o prasugrel. Solo se incluirán aquellos con un nivel de reactividad plaquetaria muy bajo (unidades de reactividad plaquetaria ≤ 30) basado en VerifyNow al mes del alta. El objetivo primario es un combinado de muerte por causa cardiovascular, infarto agudo de miocardio no fatal, accidente cerebrovascular no fatal y sangrado en un seguimiento a 1 año.
Resultados: El estudio EPIC17-VERONICA (NCT04654052) permitirá averiguar la eficacia de la estrategia de desescalada basada en la prueba de agregación plaquetaria VerifyNow, además de conocer el papel de este dispositivo en la selección de los pacientes candidatos a beneficiarse de esta estrategia.
Conclusiones: Este estudio determinará si las pruebas de función plaquetaria aportan beneficio en el tratamiento tras el SCA.
Palabras clave: Síndrome coronario agudo. Terapia antiagregante. Prueba de función plaquetaria. Sangrado.
Abbreviations
ACS: acute coronary syndrome. PCI: percutaneous coronary intervention. PRU: platelet reactivity units.
INTRODUCTION
Following percutaneous coronary intervention (PCI) in patients with acute coronary syndrome (ACS), a 12-month regimen of dual antiplatelet therapy with a P2Y12 receptor inhibitor and acetylsalicylic acid is recommended, regardless of the type of stent implanted, except when contraindicated.1 Although prasugrel and ticagrelor are preferred over clopidogrel in this setting, there is ongoing debate regarding the potency and duration of dual antiplatelet therapy. This controversy stems from the fact that most patients concurrently face 2 opposing and potentially fatal risks—ischemic and hemorrhagic—which must be carefully balanced on an individual basis.
The introduction of stents with reduced thrombogenicity, together with evidence that thrombotic risk is highest during the first few months after PCI while hemorrhagic risk remains relatively constant throughout time, has led to research efforts focused on minimizing bleeding complications. These strategies include shortening dual antiplatelet therapy, using P2Y12 inhibitors as monotherapy, and implementing de-escalation strategies.2,3
De-escalation consists of switching from prasugrel or ticagrelor to clopidogrel and can be guided (using genetic or platelet function testing) or unguided. Because this strategy may increase ischemic events, it is not recommended within the first month after PCI.1
In the TOPIC trial,4 the unguided de-escalation strategy initiated 1 month after ACS significantly reduced hemorrhagic events (Bleeding Academic Research Consortium [BARC] grade ≥ 2 bleeding events) at 1 year without increasing the ischemic ones. In the TROPICAL-ACS trial,5 the platelet function testing–guided de-escalation from prasugrel to clopidogrel 2 weeks after revascularization was noninferior to standard therapy, showing a trend toward fewer hemorrhages at 12 months and a similar rate of thrombotic events.1,2,6 In the TALOS-AMI trial,7 12-month event rates were lower, primarily because of fewer hemorrhagic events among patients who underwent unguided de-escalation 1 month after ACS. Table 1 summarizes these studies.
Table 1. De-escalation clinical trials in patients with acute coronary syndrome
| TOPIC (2017)4 | TROPICAL-ACS (2018)5 | TALOS-AMI (2021)12 | |
|---|---|---|---|
| Population | n = 645 | n = 2610 | n = 2697 |
| Design | Open-label, single-center, randomized, superiority trial | Open-label, multicenter, randomized, noninferiority trial | Open-label, multicenter, randomized, noninferiority trial |
| Strategy | Standard therapy vs unguided de-escalation | Standard therapy vs platelet function testing–guided therapy (Multiplate device) | Standard therapy vs unguided de-escalation |
| Control group | Continued dual antiplatelet therapy with acetylsalicylic acid and ticagrelor or prasugrel | Continued dual antiplatelet therapy with acetylsalicylic acid and prasugrel | Continued dual antiplatelet therapy with acetylsalicylic acid and ticagrelor |
| Experimental group | De-escalation to acetylsalicylic acid and clopidogrel | 1-week regimen of prasugrel, followed by 1-week regimen of clopidogrel and either prasugrel or clopidogrel from day 14 onward, according to platelet function testing results | De-escalation to acetylsalicylic acid and clopidogrel |
| Time from revascularization to de-escalation | 1 month | 2 weeks | 1 month |
| Follow-up | 1 year | 1 year | 1 year |
| Primary endpoint | Cardiac death, emergency revascularization, stroke, or BARC ≥ 2 bleeding events | Cardiac death, myocardial infarction, stroke, or BARC ≥ 2 bleeding events | Cardiac death, myocardial infarction, stroke, or BARC ≥ 2 bleeding events |
| Results | 13.4% in experimental group vs 26.3% in control group (HR, 0.48; 95%CI, 0.34–0.68; P < .01) | 7.3% in experimental group vs 9.0% in control group (HR, 0.81; 95%CI, 0.62–1.06; P = .0004) | 4.6% in experimental group vs 8.2% in control group (HR, 0.55; 95%CI, 0.42–0.76; P < .0001) |
|
95%CI, 95% confidence interval; BARC, Bleeding Academic Research Consortium; HR, hazard ratio. |
|||
After the positive results of the TOPIC trial, the VerifyNow to optimise platelet inhibition in coronary acute syndrome (EPIC17-VERONICA) trial (ClinicalTrials.gov: NCT04654052) aims to further refine this strategy by only applying de-escalation to patients with excessive antiplatelet effects from prasugrel or ticagrelor after the first month who are at theoretical risk of hemorrhage based on the VerifyNow platelet aggregation test (Werfen, Spain). Thus, patients demonstrating an adequate pharmacologic response will continue prasugrel or ticagrelor therapy for 1 year, whereas those with very low platelet reactivity after a 1-month regimen of dual antiplatelet therapy with these agents constitute the target population of this study.
METHODS
Design
We are conducting a multicenter, prospective, randomized clinical trial at 16 Spanish centers. Based on the results of the platelet aggregation test for P2Y12 inhibition (platelet reactivity units [PRU]) using the VerifyNow system, patients with very low platelet reactivity (PRU ≤ 30) are randomized in a 1:1 ratio to either continue treatment with ticagrelor or prasugrel, or to de-escalate to clopidogrel. Patients with PRU > 30 are not randomized. The study flowchart is shown in figure 1.
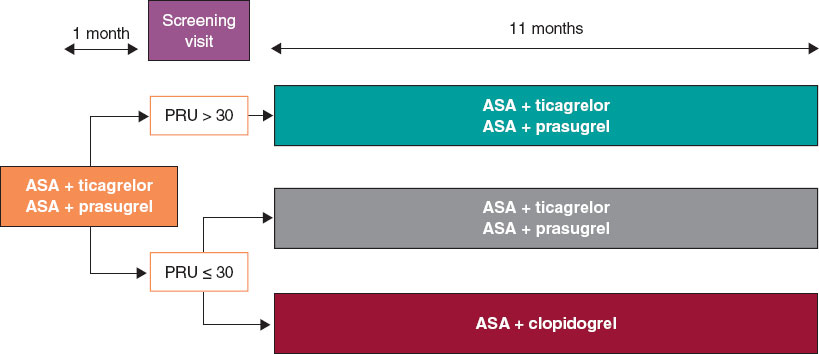
Figure 1. Study flowchart. AAS, acetylsalicylic acid; PRU, platelet reactivity units.
The study is being conducted in full compliance with the principles outlined in the Declaration of Helsinki and has been approved by the central ethics committee (Comité del Bierzo, León, Spain) and endorsed by the ethics committees of all participant centers. The appendix lists the participant centers and principal investigators.
The study sponsor (Fundación para la Educación en Procedimientos de Intervencionismo en Cardiología [EPIC]) is fully responsible, together with the principal investigators, for data management and confidentiality.
Population
Inclusion and exclusion criteria
Table 2 summarizes the inclusion and exclusion criteria. Briefly, all patients with ACS undergoing PCI with a sirolimus-eluting stent and a bioresorbable polymer during hospitalization and discharged on dual antiplatelet therapy with acetylsalicylic acid and ticagrelor or prasugrel are eligible for inclusion.
Table 2. Inclusion and exclusion criteria
| Inclusion criteria |
|---|
| Patients > 18 years |
| Patients with acute coronary syndrome undergoing percutaneous revascularization with a sirolimus-eluting stent with a bioresorbable polymer and discharged on dual antiplatelet therapy with acetylsalicylic acid and ticagrelor or prasugrel |
| Signed informed consent |
| Exclusion criteria |
| History of intracranial hemorrhage |
| Contraindication to acetylsalicylic acid, clopidogrel, prasugrel, or ticagrelor |
| Major ischemic or bleeding events during the first month of antiplatelet therapy |
| Thrombocytopenia < 50 000/µL |
| Permanent oral anticoagulation |
| Pregnancy or breastfeeding |
| Inability to complete the 1-year follow-up |
| Life expectancy < 24 months |
Written informed consent must be obtained before the platelet aggregation tes is performed.
Study protocol and randomization
Eligible patients are scheduled for P2Y12 receptor inhibition testing with the VerifyNow system between 30 and 40 days after hospital discharge. Measurements are obtained at least 6 hours after the administration of the last P2Y12 inhibitor dose. Patients with PRU ≤ 30 (very low platelet reactivity) are randomized in a 1:1 ratio using an electronic system to either continue their current treatment or de-escalate to clopidogrel, 75 mg once daily. De-escalation is preceded by a loading dose of 600 mg administered 24 hours after the last dose of ticagrelor or 75 mg 24 hours after the last dose of prasugrel, in accordance with the 2017 European Society of Cardiology clinical practice guidelines.8
The remaining patients with PRU > 30 are not randomized, and their dual antiplatelet therapy remains unchanged from discharge.
Clinical follow-up
Patients in the 2 randomized groups undergo telephone follow-up to monitor clinical events at 2, 5, 8, and 11 months after enrollment, corresponding to 3, 6, 9, and 12 months after hospital discharge.
For patients with PRU > 30 on the 1-month VerifyNow platelet aggregation test who are not randomized, only baseline characteristics are recorded, and no further follow-up is conducted.
Protocol of the VerifyNow platelet aggregation test
The VerifyNow system determines platelet activity by measuring in vitro aggregation in a blood sample exposed to specific agonists. This optical detection instrument (figure 2), which operates on a turbidimetric principle, uses single-use cartridges. In this study, PRUTest-specific kits are employed. (Werfen, Spain) to assess platelet aggregation while on P2Y12 receptor inhibitor therapy (ticagrelor, prasugrel, and clopidogrel). Each PRUTest kit contains lyophilized microbeads coated with fibrinogen, platelet activators, and a buffered solution. The test is based on the ability of activated platelets to bind fibrinogen-coated microbeads. Light transmission increases as activated platelets bind to and aggregate with the fibrinogen-coated microspheres. The kit measures this change in the optical signal and reports the results in PRU units (figure 3).

Figure 2. VerifyNow system. Reproduced with permission from Werfen.
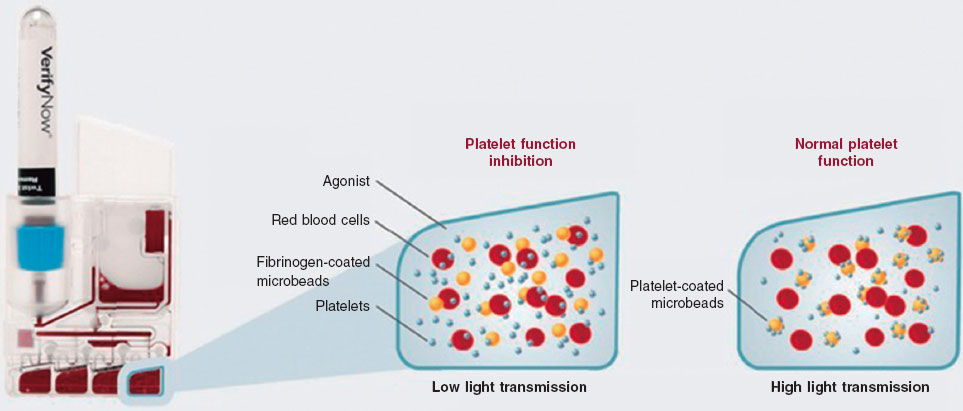
Figure 3. Performance of the VerifyNow system based on light transmission aggregometry. Light transmission increases as activated platelets bind and aggregate to the fibrinogen-coated microbeads in the kit. Therefore, high light transmission (corresponding to elevated platelet reactivity unit [PRU] values) indicates normal platelet function, whereas low light transmission (decreased PRU values) reflects platelet inhibition induced by the tested drugs.
An antiplatelet effect of the drug is considered present with PRU ≤ 180 (figure 4). Only patients with PRU ≤ 30 are randomized, as these are considered to have very low platelet reactivity while on antiplatelet therapy.
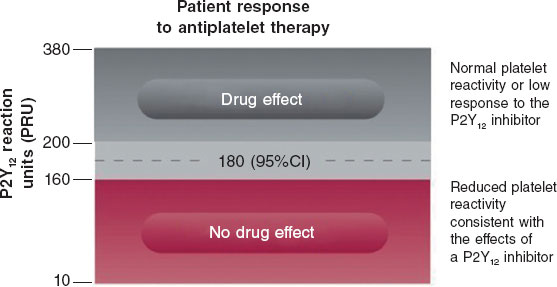
Figure 4. Reference levels for platelet reactivity units (PRU). 95%CI, 95% confidence interval.
Endpoints
The primary endpoint of the study is to compare the efficacy of de-escalation from ticagrelor or prasugrel to clopidogrel in patients undergoing PCI in the ACS setting, using the VerifyNow platelet aggregation test vs standard dual antiplatelet therapy at the 1-year follow-up. The rate of net adverse cardiovascular events is the primary endpoint of the study, defined as a composite of cardiac death, nonfatal myocardial infarction, nonfatal stroke, and hemorrhage (defined as Bleeding Academic Research Consortium [BARC] grade ≥ 2 bleeding events). The BARC scale is shown in table S1.
Furthermore, the study aims to compare several secondary endpoints (table 3), such as the occurrence of ischemic events during follow-up: cardiac death and all-cause mortality, acute myocardial infarction, stroke, stent thrombosis, and need for emergency revascularization. Moreover, the hemorrhage rate (defined as BARC grade ≥ 2 bleeding events) will be compared. The definitions of all study endpoints are shown in table S2.
Table 3. Endpoints of the study
| Primary endpoint |
|---|
| To compare the percentage of net adverse cardiovascular events between the 2 subgroups of patients with low platelet reactivity (PRU ≤ 30) who were randomized to de-escalation to clopidogrel vs standard therapy |
| Secondary endpoints |
| To compare the rate of cardiac death between the 2 randomized patient subgroups |
| To compare the rate of all-cause mortality between the 2 randomized patient subgroups |
| To compare the rate of acute myocardial infarction between the 2 randomized patient subgroups |
| To compare the rate of stroke between the 2 randomized patient subgroups |
| To compare the rate of stent thrombosis between the 2 randomized patient subgroups |
| To compare the rate of emergency revascularization between the 2 randomized patient subgroups |
| To compare the rate of bleeding events (defined as BARC ≥ 2) between the 2 randomized patient subgroups |
|
BARC, Bleeding Academic Research Consortium; PRU, platelet reactivity units. |
Statistics
Sample size calculation
Sample size was calculated for the randomized clinical trial cohort. The total number of patients (including those not randomized with PRU > 30) will depend on the total required to reach the estimated sample size for the randomized clinical trial.
We estimate a smaller difference in event rates across the groups than that observed in the TOPIC trial,4 specifically, 14% in the de-escalation group vs 22% in the standard therapy group. Assuming a significance level of 0.05, a power of 80%, a 2-tailed P-value and a 10% loss to follow-up, a total of 634 randomized patients (317 per group) will be required.
Statistical analysis plan
Quantitative variables will be expressed as mean and standard deviation if normally distributed, or as median and interquartile range otherwise. Categorical variables will be expressed as absolute values and percentages. Study data will be analyzed using one-way analysis of variance (ANOVA) for continuous variables, and Fisher’s exact or chi-square tests for categorical variables, as appropriate. Nonparametric tests will be used for variables that are not normally distributed or cannot be normalized. For the main outcome measure, Kaplan-Meier survival curves with log-rank statistics will be presented for prespecified criteria, and multivariable Cox regression will be performed to adjust for known risk factors and potential confounders. Hazard ratios and 95% confidence intervals will be reported for all statistically significant variables.
Intention-to-treat (according to randomization assignment) and per-protocol analyses (in case of crossover) will be conducted. The former will serve as the study primary analysis.
DISCUSSION
The EPIC17-VERONICA trial aims to demonstrate the efficacy of a VerifyNow platelet aggregation test-guided de-escalation strategy in reducing hemorrhagic events without increasing ischemic events in patients with ACS who have undergone percutaneous revascularization and exhibit very low platelet reactivity after the first month of treatment with prasugrel or ticagrelor.
The initial lack of expected results from platelet function testing to identify patients at risk for thrombotic events while on clopidogrel in the GRAVITAS,9 TRIGGER-PCI,10 and ARCTIC11 trials relegated its use to a class IIb recommendation in the European Society of Cardiology antiplatelet guidelines for determining the optimal timing of cardiac surgery after ACS.8 However, the 1-year results of the large-scale multicenter ADAPT-DES trial12 with 8500 PCI patients demonstrated that platelet reactivity assessed with the VerifyNow platelet aggregation test is an independent predictor of bleeding events.
In the TOPIC4 and TALOS-AMI7 trials, the unguided de-escalation strategy significantly reduced bleeding events without increasing ischemic events. In the TROPICAL-ACS5 trial, this platelet aggregation test–guided de-escalation strategy showed a trend toward fewer hemorrhages, with a similar rate of thrombotic complications.
The EPIC17-VERONICA study further seeks to improve the application of this de-escalation strategy by using the VerifyNow platelet aggregation test to identify patients with very low platelet reactivity (PRU ≤ 30) as those most likely to benefit from de-escalation.
CONCLUSIONS
The EPIC17-VERONICA trial has been designed to investigate the efficacy of de-escalating from the most potent antiplatelet agents (ticagrelor and prasugrel) to clopidogrel after the first month of therapy in patients with ACS and very low platelet reactivity, aiming to reduce bleeding events without increasing ischemic complications. Furthermore, it will provide evidence on the clinical utility of the VerifyNow platelet aggregation test for patient selection.
FUNDING
None declared.
ETHICAL CONSIDERATIONS
The study is being conducted in full compliance with the principles outlined in the Declaration of Helsinki on clinical research and has been approved by the central ethics committee (Comité del Bierzo, León, Spain) and endorsed by the ethics committees of all participant centers. Written informed consent is required prior to performing ant platelet aggregation measurements. Sex and gender bias considerations have been addressed.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
No artificial intelligence was used in the preparation of this manuscript.
AUTHORS’ CONTRIBUTIONS
C. Garilleti Cámara and I. Lozano Martínez-Luengas drafted the manuscript; the remaining authors critically revised the document and approved the final version.
CONFLICTS OF INTEREST
J.M. de la Torre Hernández is Editor-in-Chief of REC: Interventional Cardiology; A. Pérez de Prado is Associate Editor of REC: Interventional Cardiology. In both cases, the journal’s editorial procedure to ensure impartial handling of the manuscript has been followed. The remaining authors declared no conflicts of interest whatsoever.
WHAT IS KNOWN ABOUT THE TOPIC?
- De-escalation from the most potent antiplatelet agents to clopidogrel is one of the strategies used to reduce hemorrhage after percutaneous revascularization in acute coronary syndrome. This de-escalation can be performed guided or unguided by genetic or platelet function testing.
WHAT DOES THIS STUDY ADD?
- The VERONICA trial is the first to use the VerifyNow platelet aggregation test to select patients eligible for de-escalation.
REFERENCES
1. Byrne RA, Rossello X, Coughlan JJ, et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J. 2023;44:3720-3826.
2. Angiolillo DA, Galli M, Collet JP, Kastrati A, O’Donoghue MO. Antiplatelet therapy after percutaneous coronary intervention. EuroIntervention. 2022; 17:e1371-e1396.
3. Angiolillo DJ. The Evolution of Antiplatelet Therapy in the Treatment of Acute Coronary Syndromes. Drugs. 2012;72:2087-2116.
4. Cuisset T, Deharo P, Quilici J, et al. Benefit of switching dual antiplatelet therapy after acute coronary syndrome: the TOPIC (timing of platelet inhibition after acute coronary syndrome) randomized study. Eur Heart J. 2017;38:3070-3078.
5. Sibbing D, Aradi D, Jacobshagen C, et al. Guided de-escalation of antiplatelet treatment in patients with acute coronary syndrome undergoing percutaneous coronary intervention (TROPICAL-ACS): a randomised, open-label, multicentre trial. Lancet. 2017;390:1747-1757.
6. Gorog DA, Ferreiro JL, Ahrens I, et al. De-escalation or abbreviation of dual antiplatelet therapy in acute coronary syndromes and percutaneous coronary intervention: a Consensus Statement from an international expert panel on coronary thrombosis. Nat Rev Cardiol. 2023;20:830-844.
7. Kim CJ, Park MW, Kim MC, et al. Unguided de-escalation from ticagrelor to clopidogrel in stabilised patients with acute myocardial infarction undergoing percutaneous coronary intervention (TALOS-AMI): an investigator-initiated, open-label, multicentre, non-inferiority, randomised trial. Lancet. 2021;398:1305-1316.
8. Valgimigli A del G de TM, Bueno H, Byrne RA, et al. Actualización ESC 2017 sobre el tratamiento antiagregante plaquetario doble en la enfermedad coronaria, desarrollada en colaboración con la EACTS. Rev Esp Cardiol. 2018;71:42.e1-42.e58.
9. Price MJ, Berger PB, Teirstein PS, et al. Standard- vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial. JAMA. 2011;305:1097-105. Erratum in: JAMA. 2011;305;2174. Stillablower, Michael E [corrected to Stillabower, Michael E]. PMID: 21406646.
10. Trenk D, Stone GW, Gawaz M, et al. A Randomized Trial of Prasugrel Versus Clopidogrel in Patients With High Platelet Reactivity on Clopidogrel After Elective Percutaneous Coronary Intervention With Implantation of Drug-Eluting Stents. J Am Coll Cardiol. 2012;59:2159-2164.
11. Collet JP, Cuisset T, Rangé G, et al. Bedside Monitoring to Adjust Antiplatelet Therapy for Coronary Stenting. N Engl J Med. 2012;367:2100-2109.
12. Sibbing D, Schulz S, Braun S, et al. Antiplatelet effects of clopidogrel and bleeding in patients undergoing coronary stent placement. J Thromb Haemost. 2010;8:250-256.
ABSTRACT
Introduction and objectives: Calcified coronary nodules (CN) are among the most challenging lesions for percutaneous coronary intervention, as drug-eluting stents (DES) frequently result in suboptimal expansion, malapposition, and recurrent adverse events. Although intravascular lithotripsy (IVL) provides effective plaque modification, the optimal definitive strategy remains unclear. Drug-eluting balloons (DEB) have demonstrated potential in the treatment of complex lesions in which stent implantation may be less desirable. This trial aims to compare the safety and efficacy profile of DEB vs DES after IVL in patients with CN.
Methods: We conducted a retrospective, investigator-initiated, multicenter, non-inferiority, randomized clinical trial.
Results: A total of 128 patients with de novo CN confirmed by intracoronary imaging in vessels measuring 2.5 mm to 4.0 mm in diameter will be enrolled across 10 high-volume percutaneous coronary intervention centers. After lesion preparation with IVL, patients will be randomized on a 1:1 ratio to receive a DEB or a DES. The co-primary endpoints are late lumen loss and net luminal gain at 9 ± 1 months of angiographic follow-up, both assessed by an independent core laboratory. Secondary endpoints include procedural, angiographic, and clinical outcomes, adjudicated by a blinded clinical events committee. Clinical follow-up will be conducted at 1 month, 1 year, and 2 years.
Conclusions: The DEBSCAN-IVL trial will provide the first randomized evidence comparing DEB and DES after IVL for CN.
Registered at ClinicalTrials.gov: NCT06657833.
Keywords: Calcified nodule. Intravascular lithotripsy. Drug-eluting balloons. Drug-eluting stents. Complex percutaneous coronary intervention.
RESUMEN
Introducción y objetivos: Los nódulos coronarios calcificados (NC) se encuentran entre las lesiones más desafiantes para la intervención coronaria percutánea, ya que los stents farmacoactivos (SFA) con frecuencia presentan expansión subóptima, mala aposición y eventos adversos recurrentes. La litotricia intravascular (LIV) permite una modificación eficaz de la placa, pero la estrategia definitiva óptima sigue sin estar clara. Los balones farmacoactivos (BFA) han mostrado resultados prometedores en lesiones complejas en las que la implantación de stents podría ser menos favorable. Este ensayo tiene como objetivo comparar la seguridad y la eficacia del BFA frente al SFA después de la LIV en pacientes con NC.
Métodos: Ensayo clínico prospectivo, por iniciativa del investigador, multicéntrico, de no inferioridad y aleatorizado.
Resultados: Un total de 128 pacientes con NC de novo confirmados mediante imagen intracoronaria en vasos de 2,5-4,0 mm de diámetro serán incluidos en 10 centros de intervencionismo coronario percutáneo de alto volumen. Tras la preparación de la lesión con LIV, los pacientes serán aleatorizados 1:1 para ser tratados con BFA o SFA. Los criterios de valoración coprimarios son la pérdida luminal tardía y la ganancia luminal neta en el seguimiento angiográfico a 9 ± 1 meses, evaluadas por un laboratorio central independiente. Los criterios secundarios incluyen resultados procedimentales, angiográficos y clínicos, adjudicados por un comité de eventos clínicos enmascarado. El seguimiento clínico se realizará a 1 mes, 1 año y 2 años.
Conclusiones: El ensayo DEBSCAN-IVL proporcionará la primera evidencia de comparación de BFA y SFA aleatorizados después de IVL en NC.
Registrado en ClinicalTrials.gov: NCT06657833.
Palabras clave: Nódulo calcificado. Litotricia intravascular. Balón farmacoactivo. Stent farmacoactivo. Intervención coronaria percutánea compleja.
Abreviaturas
CN: calcified coronary nodule. DEB: drug-eluting balloon. DES: drug-eluting stent. IVL: intravascular lithotripsy. OCT: optical coherence tomography. PCI: percutaneous coronary intervention.
INTRODUCTION
Calcified coronary nodules (CN) represent the most complex type of calcified lesion for percutaneous coronary intervention (PCI), as they are associated with worse angiographic and clinical outcomes after drug-eluting stent (DES) implantation.1-8
Intravascular lithotripsy (IVL) has shown favorable results in this context.9 However, stent implantation after IVL may not always be the best treatment option due to suboptimal stent expansion and severe malapposition in a non-negligible percentage of patients which, along with possible nodule protrusion through the stent struts, may be associated with an increased need for new target lesion revascularization (TLR), and a higher rate of major adverse cardiovascular events (MACE).10-12
Drug-eluting balloons (DEB) have demonstrated to be a safe and effective alternative to DES in various settings, especially in those in which stenting is associated with worse outcomes, such as small vessel disease and in-stent restenosis.13 Therefore, their use has increased exponentially in recent years and has expanded to other lesion types.14
In the specific setting of calcified lesions, there are some data on the safety and efficacy profile of DEB after an adequate plaque modification.15-19 Moreover, in this setting, DEB have shown similar clinical outcomes with favorable late lumen loss rate compared with DES.20-23
Despite the increasing use of DEB in calcified lesions, evidence on the safety and efficacy profile of CN treatment is lacking. In this setting, where the risj of suboptimal stent expansion and apposition—and the consequent likelihood of MACE— is higher,24 a leave-nothing-behind strategy using DEB following optimal plaque modification technique may be a more appealing approach.Therefore, our aim is to compare the safety and efficacy profile of the use of DEB or DES after IVL in CN within the context of a randomized controlled trial.
METHODS
Patients and study design
The DEBSCAN-IVL trial is an investigator-initiated, multicenter, open-label, prospective, randomized, controlled clinical trial including 10 high-volume centers.
Patients will be randomized to receive a DEB or a DES after optimal treatment with IVL if they meet all the inclusion criteria and have no exclusion criteria. Inclusion criteria are age ≥ 18 years with a clinical indication for PCI (presenting with chronic or acute coronary syndromes) in a CN-induced de novo severe coronary lesion (confirmed via intracoronary imaging) in vessels with a reference diameter between 2.5 mm and 4.0 mm. Patients who meet at least 1 of the following conditions will be excluded: inability to provide oral and written informed consent or unwillingness to return for systematic angiographic follow-up; pregnant or breastfeeding patients; cardiogenic shock or cardiac arrest at the time of the index procedure; inability to maintain dual antiplatelet therapy for at least 1 month; life expectancy < 1 year; index lesion located at the left main coronary artery or in an aorto-ostial location; target lesion previously treated with stents or DEB or with high thrombus burden at the time of PCI (Thrombolysis In Myocardial Infarction [TIMI] thrombus grade ≥ 3).
Patients who meet all the inclusion criteria and none of the exclusion criteria will be treated with IVL and randomized to receive final therapy with DEB or DES. Randomization will occur via a web-based system. The complete inclusion and exclusion criteria are shown in table 1, and the study flowchart in figure 1.
Table 1. Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
Patients must meet all inclusion criteria:
|
Patients must not meet any criteria:
|
|
DEB, drug-eluting balloon; IVUS, intravascular ultrasound; OCT, optical coherence tomography; PCI, percutaneous coronary intervention; TIMI, Thrombolysis in Myocardial Infarction. |
|
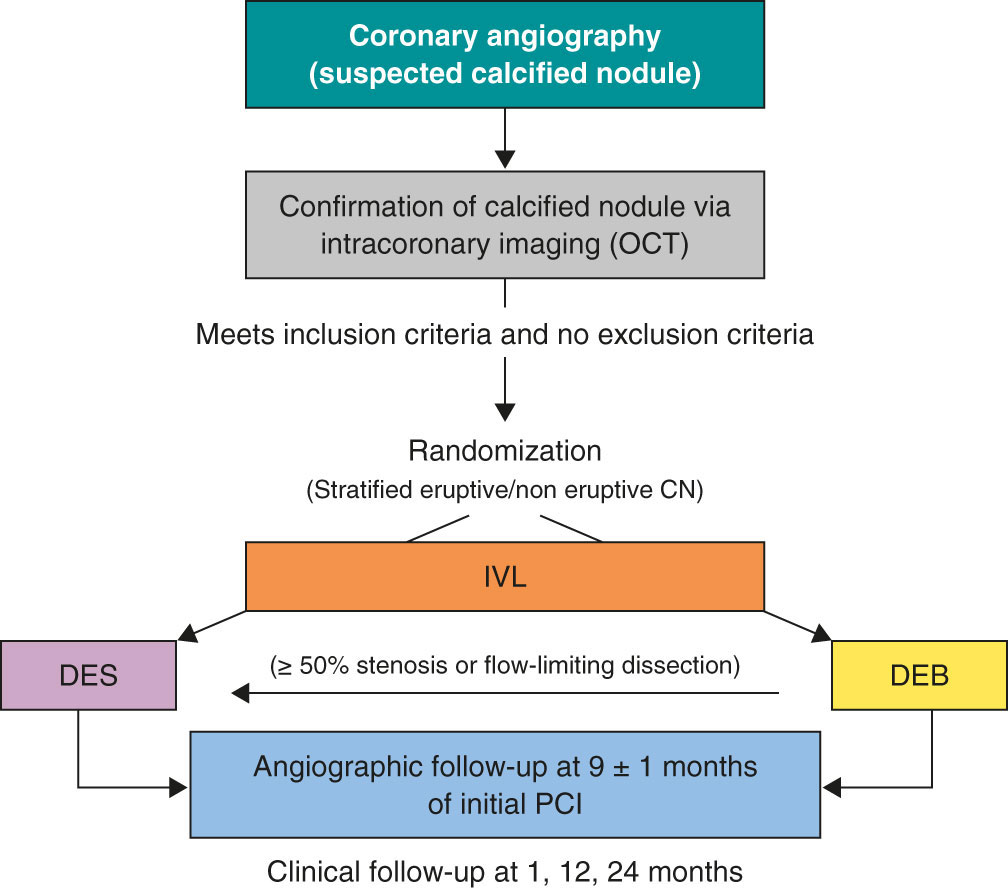
Figure 1. Central illustration. Study design flowchart. CN, calcified coronary nodule; DEB, drug-eluting balloons; DES, drug-eluting stents; IVL, intravascular lithotripsy; OCT, optical coherence tomography; PCI, percutaneous coronary intervention.
Primary and secondary endpoints
The endpoint of this study is to evaluate and compare the safety and efficacy profile of DEB or DES as final treatment strategies for CN previously modified by IVL.
Co-primary endpoints will be the late lumen loss (LLL) and net luminal gain at 9 ± 1 months of angiographic follow-up, as assessed by an independent core laboratory, with a non-inferiority hypothesis between the 2 groups. LLL is defined as the difference between postoperative and follow-up minimal lumen diameter, whereas net gain is defined as the difference between follow-up and preoperative minimal lumen diameter, according to the latest Drug Coated Balloon Academic Research Consortium Consensus Document.25
Secondary endpoints of the study will include procedural, angiographic and clinical outcomes. Procedural endpoints will include the rate of crossover between treatment groups, angiographic success (defined as final TIMI grade-3 flow and a residual final percent diameter stenosis < 30% in the DEB group or < 20% in the DES group), device success (defined as angiographic success without crossover between treatment group), procedural success (defined as angiographic success without the occurrence of severe procedural complications, including cardiac death, target vessel perioperative myocardial infarction [MI], need for new clinically driven TLR, stent thrombosis [ST], stroke, flow-limiting dissection or vessel perforation). Angiographic endpoints will include the minimal lumen diameter measured immediately after the intervention and at the time of angiographic follow-up, the residual percent diameter stenosis at both timeframes, and the rate of binary restenosis, defined as a luminal diameter reduction o≥ 50% during follow-up.25 Secondary endpoints will include procedural adverse events (such as dissection, perforation, acute vessel occlusion, slow flow or no-reflow, and intraoperative thrombosis), major hemorrhagic events (classified as Bleeding Academic Research Consortium [BARC] type ≥ 3),26 and hemodynamic instability (requiring unplanned administration of vasopressors, inotropes, or ventricular support devices), cardiac death, target lesion-related MI (TL-MI), need for TLR, and ST, and MACE (defined as a composite of cardiac death, TL-MI, and TLR). TLR and ST are defined according to the Academic Research Consortium criteria.27 MACE and its components will be assessed during the index hospitalization and at 6-month, 1-year, and 2-year follow-up visits. Detailed endpoints definitions are shown in appendix S1.
Primary outcome assessment will be conducted by a central independent core laboratory. All medical data will be anonymized and stored, and confidentiality will be protected at any time in full compliance with the current legislation. The clinical events committee (CEC) and the independent core laboratory will be blinded to the treatment group. Secondary outcomes will be assessed via centralized angiographic analysis and structured clinical follow-up, either in person or via telephone, at scheduled time points.
Devices
- – IVL: Shockwave Balloon (Shockwave Medical, United States).
- – Optical coherence tomography (OCT) or intracoronary ultrasound (IVUS) system, based on availability at each participating center.
- – DEB: paclitaxel-eluting balloon (Pantera Lux, Biotronik, Switzerland)
- – DES: new-generation zotarolimus eluting stent (Onyx Frontier, Medtronic, United States).
Procedure
When a CN is suspected on coronary angiography, intracoronary imaging—preferably OCT, with IVUS as an alternative—will be performed to confirm the diagnosis. After confirmation of a CN in the target lesion, patients will be randomized on a 1:1 ratio to receive a DEB or a DES. Randomization will be stratified to ensure a balanced distribution of eruptive and non-eruptive nodules across both treatment groups. A CN (figure 2) will be defined as a calcified segment with an accumulation of protruding nodular calcification (small calcium deposits) with disruption of the fibrous cap (eruptive CN) or an intact thick fibrous cap (non-eruptive CN).28-30
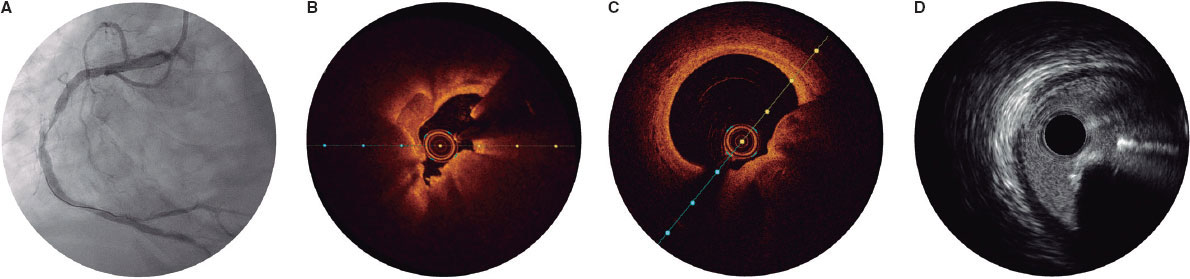
Figure 2. Calcified nodule appearance on angiography (A), optical coherence tomography (eruptive [B] and non-eruptive [C]) and intravascular ultrasound (D).
All patients will be treated with IVL, using a balloon sized 1:1 to the vessel reference diameter. A minimum of 80 pulses per lesion is recommended. If the IVL balloon cannot cross the lesion, predilation with smaller balloons is permitted. Additionally, the use of adjuvant techniques such as rotational atherectomy or excimer laser coronary atherectomy will be allowed only when deemed necessary to facilitate IVL balloon crossing. Postdilation with a non-compliant balloon after IVL is recommended before proceeding with the final assigned treatment modality.
Once optimal lesion preparation has been achieved, defined as > 80% balloon expansion in 2 orthogonal projections with a balloon sized 1:1 to the vessel, patients will receive a DEB or a DES, according to their initial randomization. If a patient randomized to the DEB group experiences a flow-limiting dissection or exhibits a percent diameter stenosis > 50%, conversion to DES implantation will be permitted at the operator’s discretion. Similarly, any crossover from DES to DEB will be documented, along with the reasons for these procedural decisions.
It is recommended that the DEB reach the target lesion within 2 minutes, as drug loss may occur during transit.13 Thus, operators need to anticipate difficulties in reaching the target lesion (proximal coronary disease or tortuosity) and ensure optimal support prior to using the DEB. If difficulties in reaching the target lesion are anticipated, the use of guide extension catheters is recommended. The recommended DEB inflation time is 60 seconds.
The PCI will be performed according to current European Society of Cardiology (ESC) guidelines, including perio- and postoperative antithrombotic management.31,32 Patients should ideally receive dual antiplatelet therapy at least 2 to 4 hours prior to the PCI to ensure optimal platelet inhibition. In cases where this is not feasible, administration of IV antiplatelet agents, such as acetylsalicylic acid with or without cangrelor, immediately before the procedure is recommended.
Intracoronary imaging with either OCT or IVUS (the same imaging modality that was initially used) is recommended at the end of the procedure.
Angiographic analysis
Quantitative coronary imaging and intracoronary analysis of baseline and follow-up angiographies will be conducted by an independent central laboratory (Barcicore, Spain). At least 2 well-selected orthogonal views—free of foreshortening and side-branch overlap—focused on the target lesion are required after intracoronary nitroglycerine administration. These views should be obtained before treatment, after the intervention, and during follow-up angiography to ensure consistent angulation and enable accurate, reproducible measurements.
Follow-up
Post-PCI antithrombotic therapy will abide by the latest ESC clinical practice guidelines, considering the individual ischemic and bleeding risk profile of each patient.31,32 Regardless of the assigned treatment group (DEB or DES), a 6-month regimen of dual antiplatelet therapy (aspirin and clopidogrel) is recommended in patients with stable coronary artery disease, and a 12-month regimen of dual antiplatelet therapy (preferably using prasugrel or ticagrelor as a P2Y12 inhibitor) in patients with acute coronary syndrome. For patients requiring chronic oral anticoagulation, the choice and duration of antithrombotic therapy will follow current guideline recommendations, with triple therapy (oral anticoagulant + aspirin + clopidogrel) limited to 1 month, whenever feasible. Electrocardiogram and troponin assessment will be performed 24 hours after the PCI. All patients will be discharged with a scheduled angiographic follow-up at 9 ± 1 months. OCT is recommended during this follow-up, especially if angiography suggests progression of coronary artery disease in the target lesion. In cases where angiography or intracoronary imaging indicates disease progression, but the percent diameter stenosis is < 90%, revascularization should be guided by ischemia and confirmed with a pressure guidewire. Clinical follow-up visits are scheduled at 12 and 24 months. Schedule of visits and data assessment throughout the study are shown in table S1.
Statistical analysis
The primary endpoint analysis will be performed by lesion and by intention-to-treat with a 1-sided Student t test with an alfa of 0.05 between the DES and the DEB group. A per-protocol analysis, including crossover cases, will also be conducted for sensitivity purposes. If the hypothesis of non-inferiority is confirmed, a superiority 2-sided analysis will be performed. Clinical endpoints will be analyzed on a per-patient basis.
Quantitative variables will be expressed as mean ± standard deviation if normally distributed, and as median with minimum and maximum values if they do not follow a normal distribution. Normality will be assessed using the Kolmogorov-Smirnov test. Qualitative variables will be described by their absolute values and frequencies, and will be expressed as absolute counts and percentages. A P < .05 will be considered statistically significant, and 95% confidence intervals (95%CI) will be reported for all main analyses. For comparisons of continuous variables between the 2 groups, the Student t test will be used if normality is confirmed, or the Mann-Whitney U test if non-parametric. For comparisons across > 2 groups, the ANOVA test or the Kruskal-Wallis test will be applied, as appropriate. Associations across categorical variables will be analyzed using the chi-square test or Fisher’s exact test when expected frequencies are small. Correlations between continuous variables will be explored using Pearson’s or Spearman’s correlation coefficient, depending on their distribution.
A multivariate analysis will be conducted using Cox proportional hazards regression with forward stepwise selection, including variables that are significantly associated with outcomes (or show a trend) in the univariate analysis. Kaplan-Meier curves will be generated for event-free survival, and differences will be assessed using the log-rank test.
Prespecified subgroup analysis
Subgroup analysis will be performed according to the following prespecified categories: type of calcified nodule (eruptive vs non-eruptive), age (< 75 vs ≥ 75 years), sex (male vs female), presence of diabetes mellitus (yes vs no), location of the calcified nodule within a true bifurcation lesion involving a side branch ≥ 2.5 mm (yes vs no), and clinical presentation (acute coronary syndrome vs chronic coronary syndrome). In addition, a prespecified OCT subgroup analysis will be performed in patients with available OCT imaging at both the end of the procedure and follow, including assessments of minimal lumen area (or minimal stent area in stented segments) and minimal lumen diameter.
Sample size calculation
The hypothesis is that DEB-PCI for CN is not inferior to state-of-the-art DES-PCI in terms of LLL and net luminal gain at the lesion. The sample size calculation was based on an expected LLL of 0.20 mm in the DES group, with a non-inferiority margin (delta) of 0.30 mm, a significance level (alpha) of 5%, and a statistical power of 80%. The estimate of LLL in the control group was derived from previous studies evaluating the same DES platform.33-35 Assuming a 20% attrition rate for angiographic follow-up, 64 patients per group (128 patients in total) will be required to provide adequate statistical power. The study is not powered for clinical endpoints, which will be considered exploratory and hypothesis-generating.
Organization and ethical concerns
The study protocol has been approved by the local ethics committees of all participant centers. Written informed consent will be obtained from all patients prior to enrollment. The DEBSCAN-IVL trial is an investigator-initiated study conducted in full compliance with Good Clinical Practice guidelines applicable to interventional and epidemiological research. The rights, safety, and well-being of all participants will be protected full compliance with the principles set forth in the Declaration of Helsinki, applicable EU legislation, and local legal requirements. Participant data will be handled confidentially and anonymously. The trial is registered at ClinicalTrials.gov (NCT06657833). The sponsor of the study is Fundación EPIC. The study is supported by unrestricted research grants from Fundación EPIC, Shockwave Medical, Biotronik, and Medtronic.
The steering committee serves as the primary decision-making body of the trial and bears full responsibility for its scientific and clinical conduct. A clinical events committee (CEC), composed of independent interventional cardiologists not participating in the study and blinded to treatment allocation, will adjudicate all clinical events and endpoints. The CEC will operate according to pre-specified definitions outlined in the study protocol and will remain blinded to the overall trial outcomes.
DISCUSSION
CN represent the most complex type of calcified lesion for PCI, as they are associated with the worst angiographic and clinical outcomes after DES implantation.1-8 Three main factors may contribute to these unfavorable results: the nature of the nodule per se, the plaque modification technique used, and the final revascularization strategy (DES or DEB). Although our understanding of the origin and behavior of calcified nodules has grown, it remains unclear which lesions are likely to respond favorably to PCI, and which are not. Eruptive CN, for instance, may be more amenable to initial modification, yet paradoxically, they have also been associated with higher rates of adverse clinical events during follow-up.29,36
Regarding plaque modification techniques, current evidence is limited. Rotational atherectomy (RA), while commonly used, is constrained by wire bias and frequently requires large burr sizes.2 Although orbital atherectomy might overcome some of these limitations, randomized data comparing it with other advanced plaque modification techniques are lacking.37 Balloon-based techniques, in contrast, may fail to cross severely stenotic nodular lesions but have the advantage of avoiding the wire bias inherent to atherectomy.
However, conventional or scoring/cutting balloons often prove insufficient to fully modify the depth of nodular calcium, and very high-pressure special balloons carry the risk of overstretching the usually normal opposite vessel wall causing perforation. In this context, IVL has emerged as a promising alternative, offering the most robust evidence to date for nodular plaque modification.29,38
Traditionally, stent implantation has been the standard definitive treatment for CN.23 However, stenting in nodular lesions frequently leads to suboptimal expansion and incomplete apposition, particularly at the shoulders of the nodule. Moreover, In these patients, TLR is often driven not by classic in-stent restenosis, but by late protrusion of the calcified nodule through the stent struts.10,11,39 These limitations have generated interest in a “leave nothing behind” strategy after effective plaque modification.
DEB have demonstrated to be safe and effective in various settings, particularly small vessel disease and in-stent restenosis, where DES implantation may be less favorable.13 Therefore, their use has grown significantly in recent years.14 In the context of calcified lesions, there are concerns that adequate drug-uptake may be compromised, but preliminary evidence suggests DEB may offer good outcomes after adequate plaque preparation.15-17 For instance, Ito et al.18 evaluated a total of 81 patients with de novo lesions treated with DEB, including 46 with calcified lesions. While LLL and restenosis appeared slightly higher in the calcified group, these differences were not statistically significant and did not translate into worse clinical outcomes at 2 years. Notably, 82% of these lesions were pre-treated with RA. Similarly, Nagai et al. reported a TLR rate of 16.3% in 190 severely calcified lesions treated with RA followed by DEB.19 Rissanen et al. found MACE rates of 14% and 20% at 12 and 24 months, respectively, in 82 complex de novo calcified lesions treated with DEB after RA and balloon predilation, with very low rates of clinically driven TLR.20 Furthermore, favorable findings have been reported by Shiraishi et al., including a subset of calcified nodules.16
Comparative studies have further explored DEB vs DES in calcified lesions. Ueno et al.21 conducted a single-center cohort study comparing the clinical outcomes of 166 severe calcified lesions treated with either DEB or DES after RA at a median follow-up of 3 years. The TLR rates were similar across the groups (15.6% vs 16.3%; P = .99), while LLL was significantly lower in the DEB group (0.09 mm vs 0.52 mm; P =.009). Iwasaki et al.22 compared 194 patients with de novo calcified lesions in non-small vessels the RA + DEB vs RA + DES strategies. There were no significant differences at 1 year in terms of MACE, cardiac death, myocardial infarction, TLR or hemorrhage.
Despite this data on the performance of DEB in calcified lesions, evidence on the safety and efficacy profile in the CN setting is lacking. However, given the high likelihood of suboptimal stent expansion and malapposition in this setting, which may lead to increased MACE risk,24 a metal-free strategy using DEB following optimal plaque modification seems to be an attractive and feasible approach.
Intracoronary imaging-guided PCI has been consistently associated with improved procedural outcomes and a reduction in major adverse cardiovascular events, including mortality, particularly in complex lesions.40 Intracoronary imaging plays a pivotal role in this context. Compared with conventional angiography, it provides a far more accurate assessment of coronary disease severity and plaque morphology.1 This is particularly relevant in calcified and complex lesions, where procedural planning and outcomes are significantly impacted by the detailed anatomical insights obtained. OCT, in particular, offers superior spatial resolution compared to IVUS, allowing for precise quantification of the calcium burden.28,41
In the case of CN, OCT enables accurate assessment of the plaque substrate and procedural results, including stent expansion and apposition, or in DEB-treated lesions, the extent of plaque modification.
The DEBSCAN-IVL trial will be comparing the safety and efficacy profile of DEB vs DES after lesion preparation with IVL in patients with CN, assessing both angiographic and clinical outcomes. Moreover, the trial will provide valuable information on the underlying plaque morphology and the response to different PCI strategies following the systematic use of intracoronary imaging. The central hypothesis of the study is that a DEB strategy, after IVL-based plaque modification in calcified nodules, is not inferior to DES implantation in terms of LLL and net gain, while potentially reducing the risk of long-term adverse events through improved biocompatibility and vessel healing. In addition, the analysis will be stratified according to nodule morphology, specifically differentiating eruptive vs non-eruptive CN, 2 entities that are thought to have distinct biological behavior and potentially different response to plaque modification and PCI.6,7,29,36 This stratified analysis may provide novel insights into the prognostic and therapeutic implications of nodule subtype and guide future individualized interventional strategies.
CONCLUSIONS
The DEBSCAN-IVL trial is an investigator-initiated, multicenter, open-label, prospective, randomized, controlled clinical trial designed to compare the safety and efficacy profile of the use of DEB or DES after IVL in CN. The co-primary endpoints are LLL and net gain at 9 ± 1 months of angiographic follow-up. The findings are expected to inform clinical decision-making and support a more individualized approach on the management of this specific type of calcified coronary disease.
DATA AVAILABILITY
This manuscript refers to the protocol of a study, therefore there is not available data related to this manuscript.
FUNDING
The DEBSCAN-IVL study was supported by non-restricted grants from Shockwave, Biotronik and Medtronic.
ETHICAL CONSIDERATIONS
The study was conducted in full compliance with the principles set forth in the Declaration of Helsinki. Institutional Ethics Committee approval was obtained, and all participants gave their written informed consent prior to enrollment. The confidentiality and anonymity of participants were strictly preserved throughout the study. Sex and gender considerations were addressed following the recommendations of the SAGER guidelines to ensure accurate and equitable reporting.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
Artificial intelligence assisted technologies were used exclusively to support language editing and improvement of style. No artificial intelligence tools were employed to generate, analyze, or interpret the data. The authors take full responsibility for the integrity, accuracy, and originality of the manuscript content.
AUTHORS’ CONTRIBUTIONS
A. Jurado-Román, M. Basile and R. Moreno drafted the manuscript. The remaining authors performed a critical review, and all authors approved the final version for publication.
CONFLICTS OF INTEREST
A. Jurado-Román is a proctor for Abbott, Boston Scientific, World Medica, and Philips; has received consulting fees from Boston Scientific and Philips; and has received speaker fees from Abbott, Boston Scientific, Shockwave Medical, Philips, and World Medica. J.M. Montero-Cabezas received a research grant from Shockwave Medical and speaker fees from Abiomed, Boston Scientific, and Penumbra Inc. A. Pérez de Prado reports receiving institutional research grants from Abbott and Shockwave Medical and speaker honoraria and consulting fees from iVascular, Boston Scientific, Terumo, B. Braun, and Abbott Vascular. I.J. Amat-Santos is proctor for Boston Scientific. A. Pérez de Prado, F. Alfonso and R. Moreno are associate editors of REC: Interventional Cardiology; the journal’s editorial procedure to ensure impartial handling of the manuscript has been followed. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
WHAT IS KNOWN ABOUT THE TOPIC?
- CN are among the most complex calcified lesions for PCI, as DES often result in suboptimal expansion, malapposition, and long-term adverse events. IVL is an effective and safe technique for modifying nodular calcium. DEB have proven effective in complex lesions such as small vessel disease and in-stent restenosis, suggesting potential utility where stent implantation might be suboptimal. However, robust evidence on the safety and efficacy of DEB specifically for CN after IVL is currently lacking.
WHAT DOES THIS STUDY ADD?
- The DEBSCAN-IVL trial will be the first randomized study to compare DEB and DES after IVL in patients with CN. It will evaluate angiographic endpoints such as late lumen loss and net luminal gain, as well as procedural and clinical outcomes. The study is expected to provide crucial insights into whether a “leave-nothing-behind“ approach with DEB can achieve comparable efficacy to DES while potentially improving vessel healing and reducing longterm complications in this challenging patient population.
REFERENCES
1. Jurado-Román A, Gómez-Menchero A, Gonzalo N, et al. Plaque modification techniques to treat calcified coronary lesions. Position paper from the ACI-SEC. REC Interv Cardiol. 2023;5:46-61.
2. Morofuji T, Kuramitsu S, Shinozaki T, et al. Clinical impact of calcified nodule in patients with heavily calcified lesions requiring rotational atherectomy. Catheter Cardiovasc Interv. 2021;97:10-19.
3. Lee T, Mintz GS, Matsumura M, et al. Prevalence, Predictors, and Clinical Presentation of a Calcified Nodule as Assessed by Optical Coherence Tomography. JACC Cardiovasc Imaging. 2017;10:883-891.
4. Lei F, Yin Y, Liu X, et al. Clinical Outcomes of Different Calcified Culprit Plaques in Patients with Acute Coronary Syndrome. J Clin Med. 2022;11:4018.
5. Mintz GS. Intravascular Imaging of Coronary Calcification and Its Clinical Implications. JACC Cardiovasc Imaging. 2015;8:461-471.
6. Torii S, Sato Y, Otsuka F, et al. Eruptive Calcified Nodules as a Potential Mechanism of Acute Coronary Thrombosis and Sudden Death. J Am Coll Cardiol. 2021;77:1599-1611.
7. Sato T, Matsumura M, Yamamoto K, et al. Impact of Eruptive vs Noneruptive Calcified Nodule Morphology on Acute and Long-Term Outcomes After Stenting. JACC Cardiovasc Interv. 2023;16:1024-1035.
8. Nagata T, Minami Y, Katsura A, et al. Optical coherence tomography factors for adverse events in patients with severe coronary calcification. Int J Cardiol. 2023;376:28-34.
9. Ali ZA, Nef H, Escaned J, et al. Safety and Effectiveness of Coronary Intravascular Lithotripsy for Treatment of Severely Calcified Coronary Stenoses:The Disrupt CAD II Study. Circ Cardiovasc Interv. 2019;12:e008434.
10. Kaihara T, Higuma T, Kotoku N, et al. Calcified Nodule Protruding Into the Lumen Through Stent Struts:An In Vivo OCT Analysis. Cardiovasc Revasc Med. 2020;21:116-118.
11. Kawai K, Akahori H, Imanaka T, et al. Coronary restenosis of in-stent protruding bump with rapid progression:Optical frequency domain imaging and angioscopic observation. J Cardiol Cases. 2019;19:12-14.
12. Isodono K, Fujii K, Fujimoto T, et al. The frequency and clinical characteristics of in-stent restenosis due to calcified nodule development after coronary stent implantation. Int J Cardiovasc Imaging. 2021;37:15-23.
13. Jeger RV, Eccleshall S, Wan Ahmad WA, et al. Drug-Coated Balloons for Coronary Artery Disease. JACC Cardiovasc Interv. 2020;13:1391-1402.
14. Jurado-Román A, Freixa X, Cid B, et al. Spanish cardiac catheterization and coronary intervention registry. 32nd official report of the Interventional Cardiology Association of the Spanish Society of Cardiology (1990-2022). Rev Esp Cardiol. 2023;76:1021-1031.
15. Basavarajaiah S, Loku Waduge BH, Watkin R, Athukorala S. Is a high calcific burden an indication, or a contraindication for Drug Coated Balloon?Rev Cardiovasc Med. 2021;22:1087-1093.
16. Shiraishi J, Kataoka E, Ozawa T, et al. Angiographic and Clinical Outcomes After Stent-less Coronary Intervention Using Rotational Atherectomy and Drug-Coated Balloon in Patients with De Novo Lesions. Cardiovasc Revasc Med. 2020;21:647-653.
17. Ho HH, Lee JH, Khoo DZL, Hpone KKS, Li KFC. Shockwave intravascular lithotripsy and drug-coated balloon angioplasty in calcified coronary arteries:preliminary experience in two cases. J Geriatr Cardiol. 2021;18:689-691.
18. Ito R, Ueno K, Yoshida T, et al. Outcomes after drug?coated balloon treatment for patients with calcified coronary lesions. J Intervent Cardiol. 2018;31:436-441.
19. Nagai T, Mizobuchi M, Funatsu A, Kobayashi T, Nakamura S. Acute and mid-term outcomes of drug-coated balloon following rotational atherectomy. Cardiovasc Interv Ther. 2020;35:242-249.
20. Rissanen TT, Uskela S, Siljander A, et al. Percutaneous Coronary Intervention of Complex Calcified Lesions With Drug?Coated Balloon After Rotational Atherectomy. J Intervent Cardiol. 2017;30:139-146.
21. Ueno K, Morita N, Kojima Y, et al. Safety and Long-Term Efficacy of Drug-Coated Balloon Angioplasty following Rotational Atherectomy for Severely Calcified Coronary Lesions Compared with New Generation Drug-Eluting Stents. J Intervent Cardiol. 2019;2019:1-10.
22. Iwasaki Y, Koike J, Ko T, et al. Comparison of drug-eluting stents vs drug-coated balloon after rotational atherectomy for severely calcified lesions of nonsmall vessels. Heart Vessels. 2021;36:189-199.
23. Rivero-Santana B, Jurado-Roman A, Galeote G, et al. Drug-eluting balloons in Calcified Coronary Lesions:A Meta-Analysis of Clinical and Angiographic Outcomes. J Clin Med. 2024;13:2779.
24. Muramatsu T, Kozuma K, Tanabe K, et al. Clinical expert consensus document on drug-coated balloon for coronary artery disease from the Japanese Association of Cardiovascular Intervention and Therapeutics. Cardiovasc Interv Ther. 2023;38:166-176.
25. Fezzi S, Scheller B, Cortese B, et al. Definitions and standardized endpoints for the use of drug-coated balloon in coronary artery disease:consensus document of the Drug Coated Balloon Academic Research Consortium. EuroIntervention. 2025;21:e1116-e1136.
26. Mehran R, Rao SV, Bhatt DL, et al. Standardized Bleeding Definitions for Cardiovascular Clinical Trials:A Consensus Report From the Bleeding Academic Research Consortium. Circulation. 2011;123:2736-2747.
27. Garcia-Garcia HM, McFadden EP, Farb A, et al. Standardized End Point Definitions for Coronary Intervention Trials:The Academic Research Consortium-2 Consensus Document. Circulation. 2018;137:2635-2650.
28. Johnson TW, Räber L, Di Mario C, et al. Clinical use of intracoronary imaging. Part 2:acute coronary syndromes, ambiguous coronary angiography findings, and guiding interventional decision-making:an expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. Eur Heart J. 2019;40:2566-2584.
29. Fernández-Cordón C, Brilakis ES, García-Gómez M, et al. Calcified nodules in the coronary arteries:systematic review on incidence and percutaneous coronary intervention outcomes. Rev Esp Cardiol. 2025;78:977-991.
30. Guagliumi G, Pellegrini D, Maehara A, Mintz GS. All calcified nodules are made equal and require the same approach:pros and cons. EuroIntervention. 2023;19:e110-e112.
31. Byrne RA, Rossello X, Coughlan JJ, et al. 2023 ESC Guidelines for the management of acute coronary syndromes:Developed by the task force on the management of acute coronary syndromes of the European Society of Cardiology (ESC). Eur Heart J. 2023;44:3720-3826.
32. Vrints C, Andreotti F, Koskinas KC, et al. 2024 ESC Guidelines for the management of chronic coronary syndromes:Developed by the task force for the management of chronic coronary syndromes of the European Society of Cardiology (ESC) Endorsed by the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2024;45:3415-3537.
33. Latib A, Colombo A, Castriota F, et al. A Randomized Multicenter Study Comparing a Paclitaxel Drug-eluting balloon With a Paclitaxel-Eluting Stent in Small Coronary Vessels. J Am Coll Cardiol. 2012;60:2473-2480.
34. Cannon LA, Simon DI, Kereiakes D, et al. The XIENCE nanoTM everolimus eluting coronary stent system for the treatment of small coronary arteries:The SPIRIT small vessel trial. Catheter Cardiovasc Interv. 2012;80:546-553.
35. Cortese B, Di Palma G, Guimaraes MG, et al. Drug-Coated Balloon Versus Drug-Eluting Stent for Small Coronary Vessel Disease. JACC Cardiovasc Interv. 2020;13:2840-2849.
36. Prati F, Gatto L, Fabbiocchi F, et al. Clinical outcomes of calcified nodules detected by optical coherence tomography:a sub-analysis of the CLIMA study. EuroIntervention. 2020;16:380-386.
37. Shin D, Dakroub A, Singh M, et al. Debulking Effect of Orbital Atherectomy for Calcified Nodule Assessed by Optical Coherence Tomography. Circ Cardiovasc Interv. 2024;17.
38. Ali ZA, Kereiakes D, Hill J, et al. Safety and Effectiveness of Coronary Intravascular Lithotripsy for Treatment of Calcified Nodules. JACC Cardiovasc Interv. 2023;16(9):1122-1124.
39. Madhavan MV, Alsaloum M, Maehara A, et al. Recurrent Calcified Nodule Protrusion Through Stent Struts After Percutaneous Coronary Intervention of the RCA. JACC Cardiovasc Interv. 2023;16:2463-2465.
40. Stone GW, Christiansen EH, Ali ZA, et al. Intravascular imaging-guided coronary drug-eluting stent implantation:an updated network meta-analysis. The Lancet. 2024;403:824-837.
41. Amabile N, RangéG, Landolff Q, et al. OCT vs Angiography for Guidance of Percutaneous Coronary Intervention of Calcified Lesions:The CALIPSO Randomized Clinical Trial. JAMA Cardiol. 2025;10:666.
ABSTRACT
Introduction and objectives: Transcatheter aortic valve implantation (TAVI) is traditionally performed with on-site cardiac surgery (CS) backup. However, procedural advances enabled TAVI to be performed safely without immediate CS backup. This study describes our single-center experience with TAVI performed in a center without on-site CS backup.
Methods: We conducted a retrospective analysis of the first 300 patients undergoing TAVI without on-site CS backup between 2020 and 2024. The primary endpoint was 30-day mortality. Secondary endpoints included procedural and in-hospital mortality, stroke, emergency cardiac surgery (ECS), vascular complications, major hemorrhage, and pacemaker implantation. Outcomes were compared with those from the Portuguese national TAVI registry.
Results: The cohort mean age was 82 ± 5 years (54% women). The median STS risk score was 3.8 [IQR, 2.3–6.6], with 17% high-risk patients (STS > 8). Most procedures were elective (83%). Transfemoral access was used in 99% of cases, and self-expandable valves were implanted in 95%. The 30-day mortality rate was 3.7% (n = 11), while stroke occurred in 2.7% (n = 8). The procedural survival rate was 99% (n = 298). No cases of ECS occurred (n = 0), coronary obstruction, TAVI-in-TAVI deployment as a bailout, or valve embolization were reported. Pericardial tamponade occurred in 0.7% of cases (n = 2). Major hemorrhage and vascular complications occurred in 8%, and pacemaker implantation in 20%. The 1-year mortality rate was 12%, with 4% attributed to cardiovascular causes; among survivors, and 91% reported symptomatic improvement. There were no significant differences in outcomes vs the results from the TAVI national registry.
Conclusions: TAVI was safely and effectively performed without on-site CS, including emergency and complex cases. The non-ECS rate and outcomes comparable to national benchmarks support the feasibility of TAVI in selected non-CS centers. In this context, expanding TAVI access may reduce waiting times and improve the management of severe aortic stenosis while maintaining high procedural quality.
Keywords: Transcatheter aortic valve implantation. TAVI. Severe aortic stenosis. Cardiac surgery backup.
RESUMEN
Introducción y objetivos: El implante percutáneo de válvula aórtica (TAVI) se realiza tradicionalmente con el apoyo de cirugía cardiaca mínimamente invasiva (CCMI) en el mismo centro. Sin embargo, los avances en los procedimientos han permitido realizar TAVI de forma segura sin cirugía cardiaca inmediata. Este estudio describe la experiencia de nuestro centro en el TAVI sin CCMI.
Métodos: Análisis retrospectivo de los primeros 300 pacientes a quienes se realizó TAVI sin CCMI entre 2020 y 2024. El objetivo principal fue la mortalidad a los 30 días. Los objetivos secundarios fueron la mortalidad intraprocedimiento y la mortalidad hospitalaria, el accidente cerebrovascular, la cirugía cardiaca de urgencia (CCU), las complicaciones vasculares, la hemorragia grave y el implante de marcapasos. Los resultados se compararon con el registro nacional portugués de TAVI.
Resultados: La edad media de la cohorte fue de 82 ± 5 años y el 54% eran mujeres. La mediana de la puntuación de riesgo STS fue de 3,8 [IQR: 2,3-6,6], con el 17% de pacientes de alto riesgo (STS > 8). La mayoría de las intervenciones fueron electivas (83%). Se utilizó el acceso transfemoral en el 99% de los casos y se implantaron válvulas autoexpandibles en el 95% de ellos. La tasa de mortalidad a los 30 días fue del 3,7 % (n = 11). Se produjeron accidentes cerebrovasculares en el 2,7% (n = 8). La tasa de supervivencia al procedimiento fue del 99% (n = 298). No se precisó CCU en ningún paciente y no hubo casos de obstrucción coronaria, necesidad de TAVI en TAVI como medida de rescate ni embolización valvular. Dos pacientes presentaron taponamiento pericárdico (0,7%). Se produjeron hemorragias graves y complicaciones vasculares en el 8% de los pacientes, y se implantó marcapasos en el 20%. Al año, la tasa de mortalidad fue del 12%, el 4% por causas cardiovasculares. El 91% de los supervivientes presentaron una mejora de los síntomas. No hubo diferencias significativas en los resultados en comparación con los del registro nacional de TAVI.
Conclusiones: El TAVI se realizó de forma segura y eficaz sin CCMI, incluso en casos urgentes y complejos. La no necesidad de CCU y los resultados comparables a los referentes nacionales respaldan la viabilidad del TAVI en centros seleccionados sin cirugía cardiaca. Ampliar el acceso al TAVI en este contexto puede reducir los tiempos de espera y mejorar la atención de la estenosis aórtica grave, al tiempo que se mantiene una alta calidad del procedimiento.
Palabras clave: Implante percutáneo de válvula aórtica. TAVI. Estenosis aórtica grave. Cirugía cardiaca mínimamente invasiva.
Abbreviations
AS: aortic stenosis. CS: cardiac surgery. ECS: emergency cardiac surgery. TAVI: transcatheter aortic valve implantation.
INTRODUCTION
Aortic stenosis (AS) is the most common primary valvular heart disease requiring intervention.1 Its prevalence is estimated at 3–5% in individuals older than 75 years,2,3 and it is expected to increase due to longer life expectancy, growing awareness, and improved diagnostic accuracy.4 The mortality rate of untreated severe symptomatic AS reaches 10-20% within the first year and 45% at 4 years.2,5
Transcatheter aortic valve implantation (TAVI) is a well-established, less invasive alternative to surgical aortic valve replacement for patients with severe symptomatic AS.1,6 Initially reserved for high-risk patients, TAVI indications have expanded to intermediate-risk and older lower-risk patients.1,6 Improvements in device technology, procedural techniques, and operator expertise have led to fewer complications and enhanced overall safety.3 The increasing prevalence of AS and the expansion of TAVI indications highlight the need to increase procedural capacity to meet current and future clinical demands and ensure timely access to treatment.7
Current clinical practice guidelines recommend that TAVI must be performed exclusively at centers with on-site cardiac surgery (CS) backup,1,6 as surgical backup provides a safety net in complications requiring emergency cardiac surgery (ECS).8 Nonetheless, the rate of ECS has significantly decreased to 0.5-1% of TAVI,9 and the outcomes of ECS remain poor,9 with a 54% survival rate at the index event and only 22% at 1 year,10 raising concerns about the actual benefits of mandatory surgical backup.
TAVI availability remains variable, with regional disparities due to the centralized distribution of CS centers.4,11 As a result, access is often limited in regions without tertiary CS centers, leading to prolonged waiting periods associated with a worse prognosis.3 TAVI waiting list mortality rate reaches 18%, highlighting the need for timely intervention.12 Expanding TAVI to centers without on-site CS will improve access, increase procedures, reduce health care inequalities, and alleviate surgical centers, allowing them to focus on higher-risk procedures.3,13 The limited number of eligible centers constrains the national procedural volume, preventing the health system’s ability to meet the population’s growing TAVI needs.4,14
This study aims to describe our experience with TAVI in a center without on-site CS and compare outcomes to the national benchmark of centers with surgical backup.
METHODS
Study population
We conducted a retrospective, single-center cohort study including the first 300 consecutive patients who underwent TAVI at our center, Hospital Espírito Santo de Évora (Portugal), between 2020 and 2024. This study was conducted in a hospital without an on-site CS department. Patients were identified through the institutional structural heart procedure registry. The study was approved by the center ethics committee, informed consent was obtained from all participants, and the study was conducted in full compliance with the Declaration of Helsinki.
Data collection
Clinical, echocardiographic, laboratory, and procedural data were obtained from electronic health records, including imaging modalities, procedural documentation, and discharge summaries. Baseline characteristics included demographic, clinical, and echocardiographic parameters, and procedural information such as access route and valve type.
Endpoints
The primary endpoint was the 30-day all-cause mortality rate. The secondary endpoints were need for ECS, in-hospital mortality, stroke, 1-year all-cause mortality rate, 1-year cardiac death, vascular complications, major hemorrhage, and permanent pacemaker implantation. Outcomes were defined according to the Valve Academic Research Consortium-3 (VARC-3) criteria.15 ECS was defined as any unplanned cardiac surgical conversion to open surgery required to manage a life-threatening complication occurring during or shortly after the procedure, performed before the patient leaves the procedural environment.
In addition, outcomes were compared with the most recent Portuguese national TAVI registry,16 which exclusively includes centers with on-site CS, to provide a benchmark for procedural safety and efficacy profile.
Follow-up
Follow-up was performed through clinic visits at 3 and 12 months, complemented by telephone contact and review of electronic health records when in-person visits were not possible. Symptomatic improvement was evaluated based on changes in New York Heart Association (NYHA) functional class.
Statistical analysis
Categorical variables were expressed as frequencies and percentages and compared using the chi-square test or Fisher’s exact test, as appropriate. Continuous variables were assessed for normality using the Shapiro–Wilk test. Normally distributed data were expressed as mean ± standard deviation (SD) and compared using the Student t test. Non-normally distributed variables were expressed as the median and interquartile range (IQR) and compared using the Mann–Whitney U test. Statistical significance was set at a 2-tailed P-value < .05. All statistical analyses were performed using Stata version 18.0 (StataCorp, United States).
RESULTS
Baseline characteristics
The first consecutive 300 patients undergoing TAVI between 2020 and 2024 were included. The cohort mean age was 82 ± 5 years (62–101), and 54% (n = 161) were women. The median Society of Thoracic Surgery (STS) risk score was 3.8 [IQR, 2.3–6.6], with 17% (n = 51) classified as high-risk (STS > 8). Prior hospitalization for symptomatic AS occurred in 21% (n = 64). Seven patients (2%) had undergone previous surgical aortic valve replacement and were treated with the valve-in-valve procedure. Low-flow low-gradient severe AS was observed in 10% (n = 31) and bicuspid aortic valve in 6% (n = 18). The baseline characteristics of the included patients are summarized in table 1 and table 2. The Portuguese National TAVI registry included 2346 patients. Compared with our cohort, the national registry included more patients with NYHA FC > II (68% vs 51%; P < .01) and COPD (22% vs 12%; P < .01), whereas our center had a higher prevalence of chronic kidney disease (50% vs 38%; P < .01). The baseline characteristics of the Portuguese National TAVI Registry and a comparison with our cohort are shown in table 3.
Table 1. Baseline characteristics and comorbidities
| Baseline characteristics | Values |
|---|---|
| Age, years | 82 ± 5 [62-101] |
| Female sex, % (n) | 54 (161) |
| STS score, % | 3.75; IQR [2.29-6.55] |
| Low risk (STS < 4), % (n) | 52 (156) |
| Intermediate risk (STS 4-8) %, (n) | 31 (93) |
| High risk (STS > 8) % (n) | 17 (51) |
| EuroSCORE, % | 2.23 IQR [2.29-6.55] |
| Prior hospitalization due to AS, % (n) | 21 (64) |
| Hypertension, % (n) | 86 (258) |
| Diabetes mellitus, % (n) | 35 (104) |
| Dyslipidemia, % (n) | 71 (214) |
| eGFR < 60 mL/min/1.73m² | 50 (50) |
| AF/flutter, % (n) | 22 (65) |
| Pacemaker, % (n) | 15 (46) |
| CAD, % (n) | 21 (63) |
| Transthoracic echocardiogram | |
| Mean transaortic gradient (mmHg) | 48 ± 14 |
| Peak transaortic velocity (m/s) | 4.3 ± 0.7 |
| AVA (cm2) | 0.74 ± 0.2 |
| LVEF (%) | 57 ± 12 |
| LVEF < 40%, % (n) | 12 (36) |
| LF/LG AS, % (n) | 10 (31) |
| SPAP (mmHg) | 38 ± 14 |
| Significant aortic regurgitation, % (n)* | 24 (71) |
| Significant mitral regurgitation, % (n)* | 27 (83) |
| CCTA | |
| Aortic annulus perimeter, mm | 74 ± 9 |
| Aortic annulus area, cm2 | 4.3 ± 0.9 |
| Aortic annulus diameter derived from perimeter, mm | 23.3 ± 3.3 |
| Aortic valve calcium score, UA | 2912 ± 1572 |
| Femoral artery min diameter, mm | 7.1 ± 1.3 |
| Bicuspid aortic valve, % (n) | 6 (18) |
|
Data is expressed as number (n) and standard deviation [ST]. Baseline characteristics, prevalence, and comorbidities, including transthoracic echocardiography and coronary computed tomography angiography (CCTA) findings from the total population. AF, atrial fibrillation; AS, aortic stenosis; AVA, aortic valve area; CAD, coronary artery disease; CCTA, coronary computed tomography angiography; eGFR, estimated glomerular filtration rate; LF/LG, low-flow low-gradient; LVEF, left ventricular ejection fraction; SAVR, surgical aortic valve replacement; SPAP, systolic pulmonary arterial pressure; STS, Society of Thoracic Surgeons. * Significant valvular heart disease was defined as > grade 2. |
|
Table 2. Baseline characteristics comparison between our cohort and the Portuguese TAVI registry
| Baseline characteristics | Our center (n = 300) | National registry (n = 2346) | P value |
|---|---|---|---|
| Age, years | 82 ± 5 | 81 ± 7 | .6 |
| Female sex, % | 54 | 53 | .8 |
| STS risk score, % [IQR] | 3.8 [2.3-6.6] | 4.7 [3.0-7.1] | .7 |
| EuroSCORE II risk, % | 2.3 [1.6-4.0] | 4.3 [2.5-7.1] | .3 |
| NYHA class > 2, % | 51 | 68 | < .01 |
| DM, % | 35 | 33 | .5 |
| COPD, % | 12 | 22 | < .01 |
| GRF < 60 mL/kg/m2, % | 50 | 38 | < .01 |
| AF, % | 22 | 25 | .3 |
| PCI, % | 14 | 23 | < .01 |
| Stroke, % | 8 | 12 | .06 |
| TTE | |||
| Mean gradient (mmHg) | 48 ± 14 | 49 ± 16 | .8 |
| AVA (cm2) | 0.72 ± 0.20 | 0.64 ± 0.20 | .7 |
| LVEF < 50, % | 21 | 28 | .08 |
|
Baseline characteristics comparison between our cohort and the National TAVI registry.16 AF, atrial fibrillation; AVA, aortic valve area; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; GFR, glomerular filtration rate; IQR, interquartile range; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; STS, Society of Thoracic Surgeons; TTE, transthoracic echocardiography. |
|||
Table 3. Clinical context and procedural characteristics
| Clinical context | Values |
|---|---|
| Elective procedure, % (n) | 83 (248) |
| Admitted prior to procedure, % (n) | 17 (52) |
| Days until TAVI (if admitted), (days) | 12 ± 8 |
| Cardiogenic shock, % (n) | 5 (15) |
| Invasive mechanical ventilation, % (n) | 1.7 (5) |
| Non-invasive mechanical ventilation, % (n) | 2.7 (8) |
| Significant coronary artery disease, % (n) | 11 (33) |
| Pre-TAVI PCI, % (n) | 7 (21) |
| Serum creatinine (mg/dL) | 1.05 [0.86-1.41] |
| Hemoglobin (g/dL) | 12.2 ± 1.9 |
| NT-proBNP (pg/mL) | 1865 [292-4250] |
| Evaluation time (days) | 15 [3-54] |
| Waiting time (days) | 59 [22-122] |
| Patient origin | |
| Our hospital area, % (n) | 62 (185) |
| Our area of influence, % (n) | 17 (53) |
| Outside our area of influence, % (n) | 21 (63) |
| Procedural characteristics | |
| Femoral access, % (n) | 99 (299) |
| Secondary access | |
| Radial, % (n) | 10 (29) |
| Femoral, % (n) | 90 (271) |
| Pre-dilation, % (n) | 58 (175) |
| Valve type | |
| Self-expandable valves, % (n) | 95 (286) |
| Evolut, % (n) | 91 (260/286) |
| Acurate, % (n) | 2 (6/286) |
| Navitor, % (n) | 7 (20/286) |
| Balloon-expandable valves, % (n) | 5 (14) |
| Myval, % (n) | 100 (14/14) |
| Valve size (mm) | 27.5 ± 3.0 |
| Post-dilation, % (n) | 38 (113) |
| Fluoroscopy time (min) | 26 [21-33] |
| Contrast volume (mL) | 216 [173-263] |
|
Clinical context characteristics of the population and procedural characteristics. Data is expressed as percentage and number (n) unless otherwise indicated. LAD, left anterior descending coronary artery; LCx, left circumflex artery; PCI, percutaneous coronary intervention; RCA, right coronary artery; TAVI, transcatheter aortic valve implantation. |
|
Procedural characteristics
Most of our procedures were elective (83%; n = 249), while 17% (n = 51) were performed urgently following unplanned hospital admission for symptomatic severe AS. Cardiogenic shock was found in 5% (n = 15), and 4% (n = 12) required ventilatory support (table 3). Transfemoral access was used in 99% of cases (n = 298); the remaining 2 were performed via transcarotid and through an aortofemoral bypass graft route, both with surgical exposure by the vascular surgery team. Self-expandable valves were used in 95% of cases (n = 286), predominantly the Evolut family (Medtronic, United States) in 91%, n = 259, followed by Navitor (Abbott, United States) in 7% (n = 20) and Acurate (Boston Scientific, United States) in 2% (n = 6). The balloon-expandable valve Myval (Meril, India) was used in 5% (n = 14) (table 4).
Table 4. Procedural and follow-up outcomes
| Procedural outcomes | Values |
|---|---|
| Procedural death, % (n) | 0.7 (2) |
| In hospital death, % (n) | 2 (6) |
| Stroke, % (n) | 2.7 (8) |
| Emergency cardiac surgery, % (n) | 0 (0) |
| Major bleeding, % (n) | 8 (25) |
| Vascular complication, % (n) | 8 (24) |
| Acute kidney injury, % (n) | 6 (18) |
| Tamponade, % (n) | 0.7 (2) |
| Length of ICU stay (days) | 2 [2-3] |
| Length of stay (days) | 3 [2-6] |
| In elective patients (days) | 3 [2-5] |
| Follow-up outcomes | |
| 1 month | |
| 30-day mortality, % (n) | 3.7 (11) |
| Permanent pacemaker implantation, % (n) | 20 (61) |
| 1 year | |
| 1-year mortality, % (n) | 12.4 (27/217) |
| Cardiac death, % (n) | 4 (8/203) |
| Hospital readmission, % (n) | 17 (51/300) |
| Symptomatic improvement, % (n) | 91 (246/269) |
|
Procedural and follow-up outcomes by VARC-3 Criteria.15 Data with %, (n) are expressed as percentages and number. The variables with (days) are expressed as number of days and IQR. Major bleeding is defined as VARC-3 type 2-3: overt bleeding requiring medical intervention, hospitalization, or transfusion ≥ 1 unit of blood. Emergency cardiac surgery is any unplanned cardiac surgery needed to manage life-threatening complications during or shortly after the procedure, performed before the patient leaves the procedural setting. Vascular complications are arterial or venous injury, dissection, stenosis, ischemia, thrombosis, pseudoaneurysm, hematoma, distal embolization, or closure device failure related to access sites requiring intervention or resulting in clinical sequelae. Acute kidney injury is defined according to KDIGO criteria as an increase in serum creatinine ≥ 0.3 mg/dL within 48 hours or ≥ 1.5 times baseline within 7 days. |
|
Procedural and 1-month outcomes
The primary endpoint, 30-day mortality rate, was 3.7% (n = 11), while in-hospital mortality was 2% (n = 6). Survival at the end of the procedure was achieved in 99% of patients (n = 298). No patient required ECS. Two patients (0.7%) underwent transcathether pericardiocentesis for cardiac tamponade, one due to a self-contained left ventricular guidewire perforation that did not require ECS, and the other of undetermined cause, which persisted and ultimately required delayed exploratory cardiac surgery, resulting in postoperative death. No cases of aortic valvular annulus rupture, coronary obstruction, TAVI-in-TAVI deployment, or valve embolization occurred. Stroke occurred in 2.7% (n = 8), 1.6% of which (n = 5) were disabling, while major bleeding and vascular complications were each observed in 8%. Pacemaker implantation was required in 20% (n = 61) (table 4). A detailed description of procedural and early causes of death is shown in table S1.
Follow-up outcomes
The 1-year all-cause mortality rate was 12% (n = 36), and cardiac death, 4% (n = 12). A detailed description of the causes of death is provided in table S1. Readmission occurred in 17% (n = 51), including 32 cardiovascular and 19 non-cardiovascular events. A detailed description of the causes of readmission is provided in table S1. Among surviving patients with available data, 91% reported symptomatic improvement, assessed by the NYHA functional class (table 4).
National TAVI registry comparison
Compared with the Portuguese TAVI national registry results available16 (table 5), our center demonstrated a non-statistically significant lower 30-day mortality rate (3.7% vs 4.8%; OR, 0.8; 95%CI, 0.44–1.47; P = .5) and similar 1-year mortality rates (12% vs 11%; OR, 1.0; 95%CI, 0.76–1.47; P = .8) (figure 1). The rate of ECS was equivalent in both groups (0% vs 0.4%; P = .5), as were the rates of vascular complications (8% vs 6.8%; P = .4) and major bleeding (8.3% vs 13.3%; P = .2). Our stroke rate was numerically lower (2.7% vs 4.6%; P = .1), as was the rate of acute kidney injury (6% vs 4.2%; P = .5). Pacemaker implantation rates were similar (20% vs 19%; P = .7) (figure 2). However, 1-year hospitalization was more frequent in our cohort (17% vs 9.6%; P = .03).
Table 5. Outcomes comparison between our center and the national registry
| Outcome measure | Our center (n = 300) | National registry (n = 2346) | Odds ratio 95%CI | P value |
|---|---|---|---|---|
| 30-day mortality, % (n) | 3.7 (11) | 4.8 (110/2297) | 0.8 [0.4-1.4] | .79 |
| 1-year mortality, % (n) | 12 (36) | 11 (194/1706) | 1.1 [0.6-1.5] | .86 |
| Tamponade, % (n) | 0.7 (2) | 1.0 (8/775) | 0.6 [0.2-2] | .73 |
| Coronary obstruction, % (n) | 0 (0) | 1.8 (14/772) | NE | .09 |
| Emergency cardiac surgery, % (n) | 0 (0) | 0.4 (4/954) | 0.8 [0.2-6.8] | .35 |
| TAVI-in-TAVI, % (n) | 0 (0) | 1.1 (8/725) | NE | .09 |
| Vascular complication, % (n) | 8 (24) | 7 (120/1766) | 1.2 [0.9-1.6] | .43 |
| Major hemorrhage, % (n) | 8 (25) | 13 (273/2054) | 0.6 [0.4-0.9] | .02 |
| Stroke, % (n) | 2.7 (8) | 4.6 (88/1893) | 0.6 [0.3-1.2] | .14 |
| AKI, % (n) | 6 (18) | 4.2 (79/1892) | 1.5 [0.9-2.1] | .46 |
| Pacemaker implantation, % (n) | 20 (60) | 19.0 (374/1964) | 1.1 [0.9-1.3] | .69 |
| Hospital readmission, % (n) | 17 (51) | 10 (98/1017) | 1.9 [1.3-2.8] | .03 |
|
Procedural and clinical outcomes comparison between our cohort and the TAVI National Registry.16 Data is expressed as percentages. Acute kidney injury (AKI) is defined according to KDIGO criteria as an increase in serum creatinine ≥ 0.3 mg/dL within 48 hours or ≥ 1.5 times baseline levels within 7 days. 95%CI, 95% confidence interval; NE, not estimable; TAVI, transcatheter aortic valve implantation. |
||||
Figure 1. Forest plot comparing the odds ratios (OR) of primary endpoints between our center without on-site cardiac surgery (CS) backup and the national Portuguese TAVI registry16 (all centers with on-site CS backup).
Figure 2. Forest plot of procedural outcomes comparing our center without on-site CS backup with the national Portuguese TAVI registry16 (centers with on-site CS backup).
DISCUSSION
This single-center study is the first national experience of TAVI in a non-on-site CS backup center. Our results suggest that this model is feasible and safe, with outcomes comparable to those reported by national and international series, including centers with surgical backup. Our outcomes were similar across key endpoints vs the National Portuguese TAVI registry,16 which includes only centers with surgical backup. Although no patients from our series required ECS, and 1 patient underwent delayed surgical intervention due to persistent pericardial effusion, the procedure was unsuccessful. This observation is consistent with other reports indicating that outcomes of emergency conversion after TAVI are generally poor, even in centers with surgical backup.8,10
TAVI safety and efficacy profile have improved significantly through careful procedural planning, the involvement of a multidisciplinary heart team (including cardiac surgery), and growing operator experience supported by technological advances. As a result, the need for immediate surgical backup has become increasingly less relevant. Although the complications that require surgical intervention remain rare, they are associated with high morbidity and mortality despite surgical management. Within this context, our results support the feasibility and suggest the non-inferiority of performing TAVI without on-site CS, reinforcing its applicability across various clinical scenarios, including younger individuals and those with multivalvular or coronary artery disease.
Our program reflects the contemporary TAVI landscape, including a heterogeneous and high-risk population with a significant proportion of emergency and unstable cases, including hospitalized patients and those in cardiogenic shock. In addition, we treated patients with complex anatomical and clinical characteristics such as valve-in-valve procedures, bicuspid aortic valves, reduced LVEF, and pulmonary hypertension. This all-comers profile mirrors the real-world spectrum that structured TAVI programs must address today, extending beyond elective transfemoral procedures for native AS.
Of note, our median waiting time for the procedure was short (59 days [IQR, 22-122]), and 20% of patients were referred from outside our direct hospital catchment area. This suggests that our center has become a regional reference for TAVI despite the lack of on-site CS, which reflects both the accessibility of our program and the trust placed in our heart team’s expertise. Importantly, many of these patients were referred because traditional TAVI centers could not meet procedural demand promptly, highlighting our role in addressing unmet clinical needs within the region.
Our findings align closely with results from countries where TAVI is performed without on-site CS, including Spain,11 Germany,17 and Austria.18 In Spain, the multicenter registry reported a conversion rate to open-heart surgery of 0.3% in centers without on-site CS backup.11 The German AQUA registry, which included more than 17 000 patients, found no significant differences in outcomes between centers with and without CS, with a 30-day mortality rate of 3.8% in hospitals with visiting CS vs 4.2% in those with CS backup, with emergency surgery rates of 0.3% and 0.7%, respectively17. Similarly, a study from Austria has shown favorable outcomes in centers without on-site surgery, with no significant differences in in-hospital mortality or surgical conversion rates.18 Our outcomes are consistent with these findings, with a 30-day mortality rate of 3.7% and no cases of ECS. Consistent with previous experiences from other countries, our results demonstrate equivalent and non-inferior results compared with centers that have on-site surgical backup.
Importantly, our study reflects a more recent era, with procedures performed in lower-risk patients, using the latest-generation devices, by more experienced operators, and following more precise preoperative planning with advanced CT imaging modalities. In addition, unlike earlier studies where visiting surgeons were present, our center performed all procedures without on-site surgical support, demonstrating the feasibility of a fully independent model. This study provides contemporary real-world evidence that TAVI can be safely and effectively performed in selected patients in centers without on-site CS backup, supporting broader access while maintaining quality standards.
Our study carries important policy implications. In the context of rising TAVI demand and resource constraints in high-volume surgical centers, decentralizing care to centers without on-site CS backup may enhance access without compromising patient outcomes. Our data supports the expansion of TAVI programs under carefully controlled conditions: standardized protocols, well-trained interventional teams, strong referral networks, and access to surgical support within a structured regional pathway. Regulatory agencies may consider revisiting existing requirements for on-site surgery, fostering a model where expertise guides the safe implementation of structural heart procedures while ensuring timely access to defined surgical centers for protocoled backup in case of delayed surgical needs.
These findings highlight the safety and viability of expanding TAVI programs to selected centers without surgical backup. With rigorous patient selection, experienced operators, and standardized procedural pathways, excellent outcomes can be achieved without immediate surgical support. Our experience supports a more inclusive structural heart care model that delivers timely and effective therapy to a broader patient population without compromising safety or efficacy.
Limitations
While these results are encouraging, several limitations must be acknowledged. First, this was a single-center, retrospective study, and although data collection was comprehensive, the potential for unmeasured confounding remains. Second, long-term follow-up beyond 1 year was unavailable, limiting conclusions on valve durability and late complications. Third, patient selection and procedural planning were guided by a highly experienced heart team, which may not be generalizable to all centers without surgical backup. Finally, although the baseline characteristics of our cohort and those of the Portuguese national registry (table 3) appear to be broadly comparable, result comparisons should be interpreted with caution, as no statistical adjustment was performed, the analysis was retrospective, and patients in the registry generally had a less favorable clinical profile.
CONCLUSIONS
Our study demonstrates that TAVI can be safely and effectively performed in centers without on-site CS backup, even in a heterogeneous, all-comers population. Outcomes appear broadly comparable and support the non-inferiority of this approach relative to centers with on-site CS. The risk of ECS was very low, and its incremental benefit may be limited, while CS centers remain few and frequently overburdened. These findings suggest that, with careful case planning and growing operator experience, expanding TAVI programs to selected non-CS centers is a safe and feasible strategy to address the growing demand and improve access for patients with severe AS. Randomized controlled trials are needed to confirm these results and guide broader implementation.
DATA AVAILABILITY
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
FUNDING
None declared.
ETHICAL CONSIDERATIONS
This study was approved by Hospital Espírito Santo de Évora ethics committee, ULSAC. All procedures were performed in full compliance with the ethical standards of the center research committee and with the Declaration of Helsinki. Informed consent was obtained from all individual participants included in the study. This study was conducted in full compliance with the SAGER guidelines. Sex and gender considerations were addressed appropriately, and any potential sex- or gender-related differences were assessed and reported where relevant.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
No artificial intelligence tools were used in the preparation of this manuscript.
AUTHORS’ CONTRIBUTIONS
A. Rocha de Almeida: conceptualization, methodology, data curation, formal analysis, investigation, original draft writing and review and editing of the final draft. R. Fernandes, Â. Bento, D. Neves, D. Brás, G. Mendes were in charge of original draft writing and review and editing of the final draft. R. Rocha, M. Paralta Figueiredo and R. Viana were in charge of data curation, reviewed and edited the final draft. R. Louro and Á. Laranjeira Santos were in charge of review and editing of the final draft. L. Patrício was in charge of conceptualization, supervision, review and editing of the final draft and validation. All authors read and approved the final draft.
CONFLICTS OF INTEREST
None declared.
WHAT IS KNOWN ABOUT THE TOPIC?
- TAVI programs are recommended to be established in centers with on-site CS, as some complications may require emergency surgery.
- However, the rate of post-TACI ECS is consistently low, and the added clinical benefit of having immediate surgical backup is limited in contemporary practice.
WHAT DOES THIS STUDY ADD?
- This is the first national study to assess TAVI outcomes in a center without immediate on-site CS backup.
- Among 300 consecutive patients, 30-day mortality rate was comparable to national and international cohorts, and the need for ECS was 0% (n = 0).
- These findings support the safety and feasibility of performing TAVI in selected centers without on-site CS backup.
REFERENCES
1. Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2022;43:561-632.
2. Généreux P, Sharma RP, Cubeddu RJ, et al. The Mortality Burden of Untreated Aortic Stenosis. J Am Coll Cardiol. 2023;82:2101-2109.
3. Compagnone M, Dall'Ara G, Grotti S, et al. Transcatheter Aortic Valve Replacement Without On-Site Cardiac Surgery. JACC Cardiovasc Interv. 2023;16:3026-3030.
4. Ali N, Faour A, Rawlins J, et al. 'Valve for Life':tackling the deficit in transcatheter treatment of heart valve disease in the UK. Open Heart. 2021;8:001547.
5. Coisne A, Montaigne D, Aghezzaf S, et al. Association of Mortality With Aortic Stenosis Severity in Outpatients. JAMA Cardiol. 2021;6:1424.
6. Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease:A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143:72-227.
7. Elbaz-Greener G, Masih S, Fang J, et al. Temporal Trends and Clinical Consequences of Wait Times for Transcatheter Aortic Valve Replacement. Circulation. 2018;138:483-493.
8. Aarts HM, van Nieuwkerk AC, Hemelrijk KI, et al. Surgical Bailout in Patients Undergoing Transfemoral Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv. 2025;18:89-99.
9. Carroll JD, Mack MJ, Vemulapalli S, et al. STS-ACC TVT Registry of Transcatheter Aortic Valve Replacement. J Am Coll Cardiol. 2020;76: 2492-2516.
10. Eggebrecht H, Vaquerizo B, Moris C, et al. Incidence and outcomes of emergent cardiac surgery during transfemoral transcatheter aortic valve implantation (TAVI):insights from the European Registry on Emergent Cardiac Surgery during TAVI (EuRECS-TAVI). Eur Heart J. 2018;39: 676-684.
11. Roa Garrido J, Jimenez Mazuecos J, Sigismondi A, et al. Transfemoral TAVR at Hospitals Without On-Site Cardiac Surgery Department in Spain. JACC Cardiovasc Interv. 2019;12:896-898.
12. Rocha de Almeida A, Carias de Sousa M, Magro C, et al. CO 92. Telemonitoring Aortic Valvular Intervention Waiting Lista Patients Prognostic Value. Rev Port Cardiol. 2023;42:S3-S85.
13. Kobo O, Saada M, Roguin A. Can Transcatheter Aortic Valve Implantation (TAVI) Be Performed at Institutions Without On-Site Cardiac Surgery Departments?Cardiovasc Revasc Med. 2022;41:159-165.
14. Iannopollo G, Cocco M, Leone A, et al. Transcatheter aortic?valve implantation with or without on?site cardiac surgery:The TRACS trial. Am Heart J. 2025;280:7-17.
15. Généreux P, Piazza N, Alu MC, et al. Valve Academic Research Consortium 3:Updated Endpoint Definitions for Aortic Valve Clinical Research. J Am Coll Cardiol. 2021;77:2717-2746.
16. Guerreiro C, Ferreira PC, Teles RC, et al. Short and long-term clinical impact of transcatheter aortic valve implantation in Portugal according to different access routes:Data from the Portuguese National Registry of TAVI. Rev Port Cardiol. 2020;39:705-717.
17. Eggebrecht H, Bestehorn M, Haude M, et al. Outcomes of transfemoral transcatheter aortic valve implantation at hospitals with and without on-site cardiac surgery department:insights from the prospective German aortic valve replacement quality assurance registry (AQUA) in 17 919 patients. Eur Heart J. 2016;37:2240-2248.
18. Florian E, David Z, K FM, et al. Impact of On-Site Cardiac Surgery on Clinical Outcomes After Transfemoral Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv. 2018;11:2160-2167.
ABSTRACT
Introduction and objectives: Stent thrombosis (ST) is a rare but potentially fatal complication of percutaneous coronary interventions. With its high spatial resolution, optical coherence tomography (OCT) allows identification of underlying mechanical causes of stent thrombosis that may be overlooked by conventional angiography.
Methods: We conducted a prospective, single-center registry that consecutively included patients with a definitive diagnosis of ST who underwent OCT at the acute presentation. The presence of underlying mechanical abnormalities was assessed, and their likelihood as the primary cause of ST was determined. In-hospital and follow-up prognosis was evaluated.
Results: A total of 105 patients were included in the final analysis. Mechanical abnormalities were identified by the OCT in 87% of cases and deemed the most probable cause of ST in 77.1%. Findings varied significantly by timing of stent thrombosis: in acute cases, no mechanical abnormality was most common (41.8%); in subacute cases, stent underexpansion predominated (47.8%); in late cases, malapposition was most frequent (30.8%); and in very late cases, neoatherosclerosis was the leading cause (52%). However, no significant differences were found in relation to the type of stent involved. In all cases, treatment was tailored to correct the detected abnormality, with a new stent being implanted in 52% of patients. There were no OCT-related complications.
Conclusions: OCT is a safe and valuable tool in the assessment of ST as it allows the identification of distinct causative mechanisms according to timing of ST and helps optimize the management of patients experiencing this rare but serious complication.
Keywords: Optical coherence tomography. Stent thrombosis. Intracoronary imaging.
RESUMEN
Introducción y objetivos: La trombosis del stent (TS) es una complicación infrecuente del intervencionismo coronario, pero potencialmente letal. Su fisiopatología es multifactorial y en algunos casos no está bien esclarecida. La tomografía de coherencia óptica (OCT) ofrece una alta resolución espacial y permite identificar causas mecánicas subyacentes relacionadas con la TS que escapan a la angiografía convencional.
Métodos: Registro prospectivo unicéntrico que incluyó consecutivamente pacientes con diagnóstico de TS definitiva a los que se realizó OCT en el momento agudo. Se evaluó la presencia de anomalías mecánicas subyacentes y se estableció si podían considerarse la causa más probable de la TS. Se evaluaron el pronóstico intrahospitalario y la evolución clínica durante el seguimiento.
Resultados: Se incluyeron 105 pacientes. Se identificaron anomalías mecánicas por OCT en el 87% de los casos, y finalmente se establecieron como causa más probable de la TS en el 77,1%. Los hallazgos difirieron de manera significativa en función de la temporalidad de las TS: en las agudas, lo más frecuente fue no encontrar ninguna anomalía mecánica (41,8%); en las subagudas, predominó la infraexpansión del stent (47,8%); en las tardías, la mala aposición (30,8%), y en las muy tardías, la neoateroesclerosis (52%). En cambio, no se encontraron diferencias en los hallazgos de OCT en función del tipo de stent trombosado. En todos los casos se realizó un tratamiento dirigido a corregir la anomalía detectada, con implante de nuevo stent en el 52% de los pacientes. No se detectaron complicaciones relacionadas con la técnica de OCT.
Conclusiones: La OCT es una herramienta segura y útil en el estudio de la TS. Permite identificar mecanismos causales específicos en función de la temporalidad y optimizar el tratamiento de los pacientes que sufren esta rara, pero grave, complicación.
Palabras clave: Tomografía de coherencia óptica. Trombosis del stent. Imagen intracoronaria.
Abbreviations
OCT: optical coherence tomography. ST: stent thrombosis.
INTRODUCTION
Technological advances in coronary intervention, particularly the development of new-generation drug-eluting stents, have transformed the treatment of ischemic heart disease by effectively reducing restenosis and improving clinical outcomes for the patients.1 Nonetheless, stent thrombosis (ST) remains a rare but devastating complication, with an associated mortality rate of up to 40%.2 Its pathophysiological mechanisms are complex and multifactorial, involving patient-related factors, implantation technique, stent type, and the natural progression of the disease.3
Traditionally, coronary angiography has been the tool of choice for assessing potential complications after stent implantation. However, its inability to adequately visualize the interaction between the stent and the vessel wall limits its diagnostic utility. In this context, optical coherence tomography (OCT), owing to its excellent spatial resolution, enables in vivo identification of underlying mechanical abnormalities that may be responsible for or contribute to ST.
The primary endpoint of this study was to evaluate the role of OCT in characterizing the mechanisms involved in ST, analyzing its feasibility, safety profile, therapeutic implications, and potential to optimize revascularization strategies in this complex clinical setting. Preliminary findings from this study were reported previously,4 and the final results are presented herein.
METHODS
Study population
We conducted a prospective, single-center registry that consecutively included patients with a diagnosis of definite ST, as defined by Academic Research Consortium criteria, from October 2013 through December 2022 at Hospital Universitario de La Princesa (Madrid, Spain). Throughout this period, stents were implanted in 6881 patients. Patients with severe hemodynamic instability or lesions inaccessible with the OCT catheter were excluded.
We applied a systematic protocol that included OCT acquisition before and after treatment. If antegrade flow was not restored after crossing the lesion with the guidewire, thrombus aspiration was recommended; furthermore, when TIMI grade 0–1 flow persisted, inflation of a balloon < 2 mm in diameter at low pressure was recommended to avoid distortion of the underlying lesion. The final treatment strategy for ST was left to the operator’s discretion.
ST was classified based on the interval since implantation as acute (< 24 h), subacute (24 h to 30 d), late (30 d to 1 y), or very late (> 1 y). Stents were categorized as bare-metal, first-generation drug-eluting, new-generation drug-eluting, or bioresorbable stents.
We performed prospective clinical follow-up to assess in-hospital mortality and, after discharge, a composite of cardiac death, recurrent myocardial infarction, recurrent ST, or repeat culprit vessel revascularization.
The study protocol was approved by Hospital Universitario de La Princesa Ethics Committee, and all patients gave their prior written informed consent before being included in the study.
OCT acquisition and analysis
OCT systems available at the time (Dragonfly, St. Jude Medical, and OPTIS AptiVue, Abbott) were used. Images were acquired with a nonocclusive technique and a contrast volume of 15 mL at a rate of 5 mL/s for the left coronary system and 12 mL at a rate of 4 mL/s for the right coronary artery. The analyzed segment included the stent and 10 mm adjacent to its edges. Poor-quality studies due to insufficient flushing or artefacts were excluded. Additionally, we performed a cross-sectional morphometric analysis, including minimum lumen area, minimum and maximum stent area, reference areas and diameters, stent expansion index (minimum stent area / mean reference area ×100), thrombus burden (length and area), and malapposition (axial distance from the strut surface to the lumen border greater than the strut thickness [significant if > 200 µm]).5 Struts directly exposed to the vessel lumen were classified as uncovered. Neoatherosclerosis was defined as neointimal changes including lipidic, fibro-lipidic, or calcified tissue. Plaque rupture was assessed too. The presence of edge dissection (separation of vascular tissue extending into the media, ≥ 2 mm in length and > 60°)6 or stent edge disease (ruptured lipid plaque adjacent to the stent edge, due to incomplete coverage or disease progression)7,8 was evaluated.
Primary endpoint: mechanical abnormalities and dominant finding
We analyzed the presence of mechanical abnormalities and identified a dominant finding for each case. When multiple abnormalities coexisted, we defined the dominant finding as the one prevailing in the area with the greatest thrombus burden.9 Potentially causative mechanical abnormalities included edge dissection, underexpansion, severe malapposition, edge disease progression, neoatherosclerosis, and uncovered struts (for late and very late ST). Furthermore, we recorded the absence of mechanical abnormalities. The dominant finding was, then, assessed as the potential main cause of ST, after excluding other possible causes, such as inadequate antiplatelet therapy or prothrombotic clinical conditions. Predictors of neoatherosclerosis and plaque rupture in very late ST were also studied.
Statistical analysis
Statistical analysis was performed using R software. Continuous variables were expressed as mean (SD) or median (interquartile range, P25–P75) and compared using the Student t test or Wilcoxon test. For comparisons across more than 2 groups, ANOVA or Kruskal–Wallis tests were used. Categorical variables were compared using the chi-square test or Fisher’s exact test. Logistic regression models were applied to identify possible predictors of in-hospital mortality, and Cox regression models were used for post discharge events. Survival analyses were performed with Kaplan–Meier curves and log-rank tests. Statistical significance was defined as P < .05.
RESULTS
Clinical and angiographic characteristics
A total of 142 patients with a diagnosis of definite ST were registered, 105 of whom were included in the final analysis (figure 1). A total of 17 cases of acute ST (16%), 23 cases of subacute ST (22%), 13 cases of late ST (12%), and 52 cases of very late ST (50%) were reported. The baseline patient characteristics based on the ST timing (acute/subacute vs late/very late) are shown in table 1. The most common presentation was ST-segment elevation myocardial infarction (81.9%), and the most frequently affected vessel was the left anterior descending coronary artery (42%). Most thrombosed devices were new-generation drug-eluting stents (53.3%), followed by bare-metal stents (28.6%), first-generation drug-eluting stents (13.3%), and bioresorbable scaffolds (4.8%). Bare-metal stents were significantly more common in late/very late ST, whereas new- generation drug-eluting stents and bioresorbable scaffolds were more common in acute/subacute ST. For ST treatment, drug-eluting stents and drug-coated balloons were used significantly more often in late and very late ST, whereas glycoprotein IIb/IIIa inhibitors and balloon optimization were more frequently employed in acute and subacute ST.
Table 1. Baseline, angiographic, and procedural characteristics of the study population
| Variables | Overall 105 (%) | Acute/Subacute ST n = 40 (%) | Late/Very late ST n = 65 (%) | P |
|---|---|---|---|---|
| Age, years | 65.8 ± 11.8 | 49 | 82 | – |
| Male sex | 89 (84.8) | 30 (76.9) | 59 (89.4) | .150 |
| Risk factors | ||||
| Smoking | 47 (44.8) | 16 (41) | 31 (47) | .697 |
| Hypertension | 68 (64.8) | 25 (64.1) | 43 (65.1) | 1.0 |
| Dyslipidemia | 69 (65.7) | 19 (48.7) | 50 (75.7) | .009 |
| Diabetes mellitus | 28 (26.7) | 10 (25.6) | 18 (27.8) | 1.0 |
| Previous treatment | < .001 | |||
| Dual antiplatelet therapy | 45 (40.9) | 32 (80) | 13 (20) | |
| Single antiplatelet therapy | 53 (50.5) | 7 (18) | 46 (69.7) | – |
| None | 5 (3.8) | 0 | 5 (7.6) | – |
| Clinical presentation | < .001 | |||
| STEMI | 83 (81.9) | 34 (85) | 49 (75.3) | |
| Killip-Kimball class IV | 14 (13.3) | 5 (12.8) | 9 (13.6) | – |
| Stents analyzed | 105 | 40 | 65 | < .001 |
| Bare-metal stent | 30 (28.6) | 6 (15.4) | 24 (36.4) | – |
| First-generation DES | 14 (13.3) | 1 (2.6) | 13 (19.7) | – |
| New-generation DES | 56 (53.3) | 29 (72.5) | 27 (41.5) | – |
| Bioresorbable scaffold | 5 (4.8) | 4 (10.3) | 1 (1.5) | – |
| Type of treatment | < 0.001 | |||
| Conservative | 6 (5.7) | 2 (5.3) | 2 (3.1) | – |
| Standard balloon (SC/NC) | 24 (22.9) | 13 (34.2) | 11 (16.9) | – |
| Drug-coated balloon | 4 (3.8) | 0 | 4 (6.1) | – |
| Drug-eluting stent | 52 (49.5) | 12 (30) | 40 (61.5) | – |
| Bioresorbable stent | 3 (2.9) | 0 | 3 (4.6) | – |
| NC balloon + GP IIb/IIIa inhibitor | 12 (11.4) | 10 (26.3) | 2 (3.1) | – |
|
DES, drug-eluting stent; NC, non-compliant; SC, semi-compliant; ST, stent thrombosis; STEMI: ST-segment elevation myocardial infarction. |
||||
Figure 1. Flowchart of patient inclusion in the study. OCT, optical coherence tomography.
OCT analysis
No complications related to the OCT technique were observed. Morphometric and stent–vessel wall interaction data are shown in table 2. A total of 24 834 cross-sections were evaluated, of which 1453 (5.8%) could not be analyzed because of abundant residual thrombus.
Table 2. Morphometric analysis and stent–vessel wall interaction according to type of stent thrombosis
| Variables | Acute ST (n = 17) | Subacute ST (n = 23) | Late ST (n = 13) | Very late ST (n = 52) | P |
|---|---|---|---|---|---|
| Thrombus | |||||
| Fr with thrombus per stent, % | 68.8 ± 24 | 61.7 ± 21 | 37.1 ± 27.9 | 42.6 ± 24.3 | < .001 |
| Maximum thrombus area, mm2 | 4.38 ± 1.72 | 3.12 ± 1.82 | 2.5 ± 1.6 | 2.0 ± 1.4 | < .001 |
| Maximum length, mm | 22.9 ± 30 | 15.35 ± 19 | 8.4 ± 5.8 | 7.9 ± 4.7 | < .001 |
| Malapposition | |||||
| Fr with malapposition per stent, % | 13.3 ± 14 | 9.3 ± 9 | 11.4 ± 11.5 | 2.3 ± 5.9 | < .001 |
| Maximum area, mm2 | 0.48 ± 0.54 | 0.61 ± 0.7 | 1.29 ± 1.5 | 0.42 ± 1.21 | < .001 |
| Maximum strut length, mm | 3.11 ± 3.99 | 1.92 ± 2.12 | 2.26 ± 2.3 | 0.6 ± 1.31 | < .001 |
| Stents with at least 1 Fr with malapposition, % | 13 (76.5) | 17 (73.9) | 10 (76.9) | 10 (19.2) | < .001 |
| Coverage | |||||
| Fr with uncovered struts, % | 88.2 ± 27.5 | 77.9 ± 30.6 | 21.3 ± 28.5 | 3.37 ± 11.42 | < .001 |
| Maximum length of uncovered struts, mm | 19.3 ± 11.6 | 16.1 ± 8.5 | 5.1 ± 8.6 | 1.6 ± 5.3 | < .001 |
| Stents with at least 1 uncovered Fr, % | 17 (100) | 22 (95.6) | 10 (76.9) | 15 (28.8) | < .001 |
| Neoatherosclerosis | |||||
| Fr with neoatherosclerosis per stent, n | 0 | 0 | 3.1 ± 2.9 | 41.1 ± 47.8 | < .001 |
| Stents with at least 1 Fr with neoatherosclerosis, n | 0 | 0 | 3 (23) | 78 | < .001 |
| Expansion | |||||
| Mean reference area, mm2 | 7.74 ± 2.15 | 5.76 ± 2.41 | 7.46 ± 2.29 | 6.19 ± 2.09 | .012 |
| Minimum stent area, mm2 | 6.98 ± 2.06 | 4.35 ± 1.53 | 6.09 ± 2.03 | 5.6 ± 1.89 | < .001 |
| Expansion index, % | 91.78 ± 21 | 86.9 ± 42.6 | 84 ± 19.9 | 95.21 ± 32.4 | 0.48 |
| Expansion index < 80%, n (%) | 6 (35.3) | 12 (52.1) | 5 (38.5) | 17 (33.3) | 0.1 |
|
Fr, frames; ST, stent thrombosis. |
|||||
There were no differences in underexpansion rates based on the type of thrombosis, and only a minimum stent area < 4.5 mm2 was observed in subacute ST. The number of uncovered struts decreased significantly over time (although 30% of stents still exhibited uncovered struts at the 1-year follow-up), as did thrombus burden and malapposition (figure 2). Additionally, we analyzed these findings by stent type (table 3), showing that uncovered or malapposed struts were more frequent in drug-eluting stents than in bare-metal stents.
Table 3. Morphometric analysis according to stent type
| Variables | Bare-metal stent (n = 30) | First-generation DES (n = 14) | New-generation DES (n = 56) | Bioresorbable scaffold (n = 5) | P |
|---|---|---|---|---|---|
| No. of Fr with uncovered struts | 29.4 ± 64 | 27.8 ± 87 | 130.6 ± 128.6 | 121.8 ± 89.4 | < .001 |
| Stents with at least 1 uncovered strut, % | 10 (33) | 6 (42) | 42 (75) | 5 (100) | < .001 |
| No. of frames with thrombus per stent | 82.3 ± 44 | 100.9 ± 51 | 134.4 ± 83.2 | 73.4 ± 59.7 | .022 |
| Stents with at least 1 malapposed strut, % | 6 (20) | 5 (35.7) | 36 (64.3) | 3 (60) | < .001 |
| Stents with expansion < 80%, % | 20.7 | 0.5 | 44.6 | 0.4 | .104 |
| Stents with neoatherosclerosis, % | 17 (56.7) | 7 (50) | 16 (28.6) | 0 | .013 |
|
DES, drug-eluting stent; Fr, frames. |
|||||
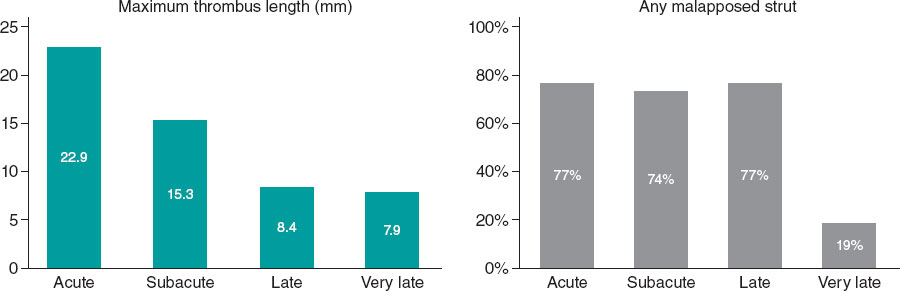
Figure 2. Morphometric findings over time.
Primary endpoint
Underlying mechanical abnormalities were identified in 91 of 105 patients (86.7%). Of these, 10 patients also had documented nonadherence to antiplatelet therapy in the days preceding ST; therefore, a mechanical abnormality was considered the most likely single cause in 81 patients (77.1%). The global distribution of dominant findings is shown in figure 3, and representative examples of ST cases in figure 4.
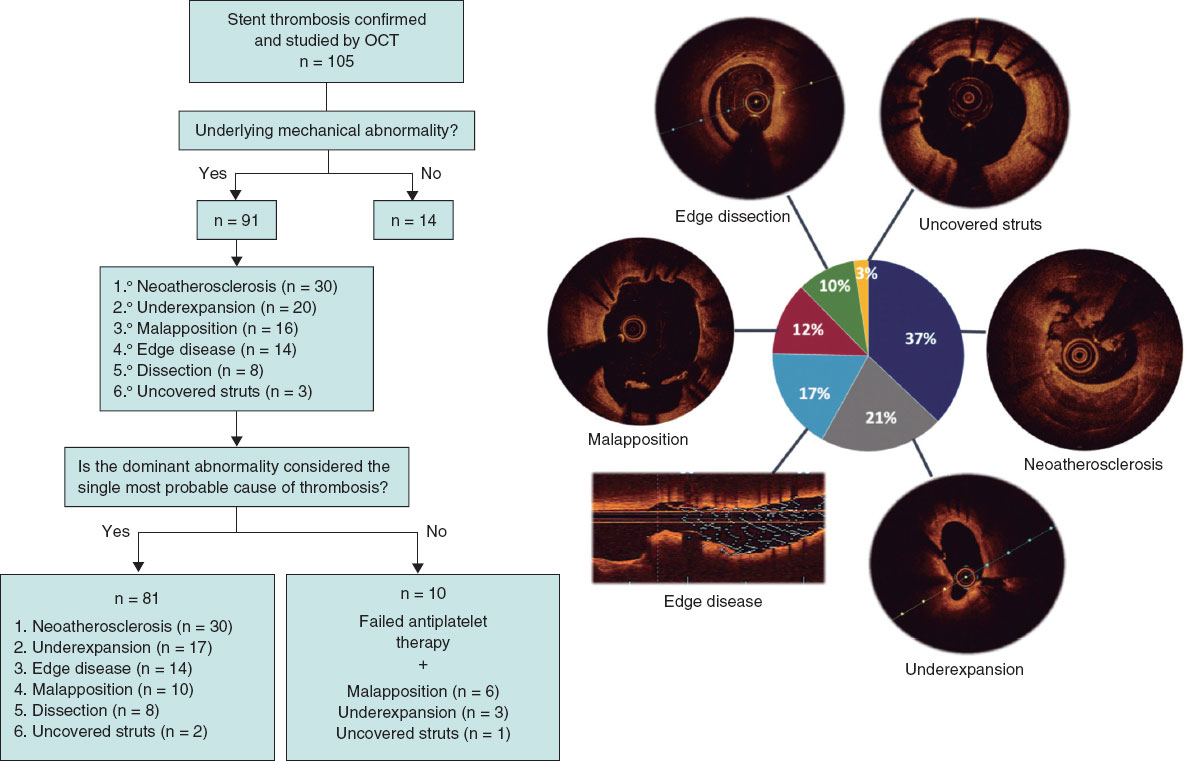
Figure 3. Central illustration. Assignment of the dominant finding and most likely cause of stent thrombosis. OCT, optical coherence tomography.
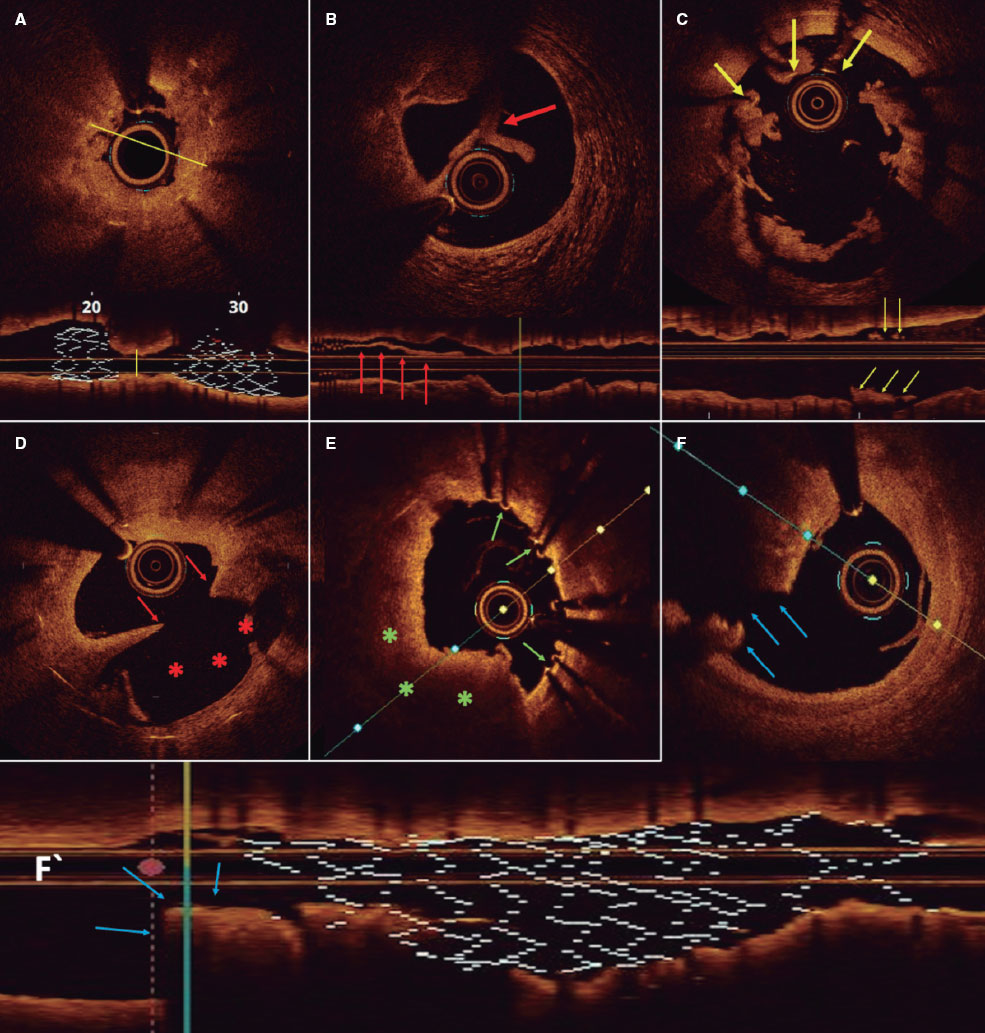
Figure 4. Representative optical coherence tomography findings. A: longitudinal and cross-sectional views of a stent with marked underexpansion (line indicates minimum diameter). B: stent-edge dissection. Arrow indicates separated tissue flap from the vessel wall. C: malapposition. Arrows indicate struts associated with thrombus that are not in contact with the vessel wall. D: neoatherosclerosis with plaque rupture. Arrows indicate intimal discontinuity, and asterisks show the evacuated plaque cavity. E: uncovered struts (arrows). F and F‘: cross-sectional and longitudinal views of a stent with distal edge disease and plaque rupture (arrows).
Representative optical coherence tomography findings. A: longitudinal and cross-sectional views of a stent with marked underexpansion (line indicates minimum diameter). B: stent-edge dissection. Arrow indicates separated tissue flap from the vessel wall. C: malapposition. Arrows indicate struts associated with thrombus that are not in contact with the vessel wall. D: neoatherosclerosis with plaque rupture. Arrows indicate intimal discontinuity, and asterisks show the evacuated plaque cavity. E: uncovered struts (arrows). F and F’: cross-sectional and longitudinal views of a stent with distal edge disease and plaque rupture (arrows).
The dominant finding varied significantly by ST timing (P < .001) (table 4). In acute ST, the most frequent result was no identifiable abnormality (41.2%), and the dominant finding was stent-edge dissection (23.5%); in subacute ST, it was underexpansion (47.8%); in late ST, malapposition; and in very late ST, neoatherosclerosis (52%). However, there were no significant differences by stent type (P = .07): in bare-metal and first-generation drug-eluting stents, the most common abnormality was neoatherosclerosis (46.7% and 35.7%, respectively), whereas in new-generation drug-eluting stents, underexpansion predominated (26.8%). In very late ST, neoatherosclerosis was the most common finding in both bare-metal and drug-eluting stents (56.7% vs 50%; P = .45), regardless of whether thrombosis occurred within 5 years or later after stent implantation. Five cases of bioresorbable scaffold thrombosis were recorded whose specific findings have already been reported previously.10
Table 4. Dominant findings according to timing of stent thrombosis and stent type
| Dominant finding | Acute ST (n = 17) | Subacute ST (n = 23) | Late ST (n = 13) | Very late ST (n = 52) |
|---|---|---|---|---|
| Edge dissection | 4 (23.5) | 4 (17.4) | 0 | 0 |
| Underexpansion | 2 (11.8) | 11 (47.8) | 1 (7.7) | 6 (11.5) |
| Malapposition | 3 (17.6) | 3 (13.0) | 4 (30.8) | 6 (11.5) |
| Neoatherosclerosis | 0 | 0 | 3 (23.1) | 27 (52.0) |
| No finding | 7 (41.8) | 3 (13.0) | 1 (7.7) | 3 (5.7) |
| Edge disease | 1 (5.9) | 2 (8.7) | 2 (15.4) | 9 (17.3) |
| Uncovered struts | 0 | 0 | 2 (15.4) | 1 (2.0) |
| Dominant finding | Bare-metal stent (n = 30) | First-generation DES (n = 14) | New-generation DES (n = 56) | Bioresorbable scaffold (n = 5) |
| Edge dissection | 2 (6.7) | 0 | 5 (8.9) | 1 (20.0) |
| Underexpansion | 2 (6.7) | 2 (14.2) | 15 (26.8) | 1 (20.0) |
| Malapposition | 5 (16.7) | 3 (21.4) | 8 (14.3) | 0 |
| Neoatherosclerosis | 14 (46.7) | 5 (35.7) | 11 (19.6) | 0 |
| No finding | 1 (3.3) | 2 (14.3) | 9 (16.1) | 2 (40.0) |
| Edge disease | 6 (20.0) | 1 (7.1) | 6 (10.7) | 1 (20.0) |
| Uncovered struts | 0 | 1 (7.1) | 2 (3.6) | 0 |
|
DES, drug-eluting stent; ST, stent thrombosis. |
||||
Neoatherosclerosis and plaque rupture
Neoatherosclerosis was identified in 40 patients, regardless of whether it was the dominant finding. There were no differences in baseline characteristics between patients with and without neoatherosclerosis, although those with neoatherosclerosis had larger minimum stent areas, smaller minimum lumen areas, and fewer uncovered and malapposed struts (table 1 of the supplementary data). Minimum lumen area was the only factor associated with a higher risk of neoatherosclerosis (odds ratio [OR], 0.39; 95% confidence interval [CI], 0.16–0.75; P = .013).
Plaque rupture was reported in 16 patients with neoatherosclerosis. There were no differences in baseline characteristics or in the composition of neoatherosclerosis between patients with and without plaque rupture (table 2 of the supplementary data). Despite the limited sample size, minimum lumen area was identified as a protective factor against plaque rupture (OR, 0.22; 95%CI, 0.04–0.7; P = .035). The presence of neovessels or calcium inside the stent was not associated with plaque rupture, nor was stent type.
Clinical follow-up
The in-hospital mortality rate for the overall cohort was 9.2%, with no significant differences being reported between patients who did and did not undergo OCT (8.5% vs 11%; P = .7). The main predictors of mortality were chronic kidney disease (OR, 9.56; 95%CI, 2.28–41.14; P = .002) and Killip-Kimball class III/IV status (OR, 14.8; 95%CI, 3.38–79.6; P = .001).
A total of 28 composite endpoints were recorded at the follow-up (median, 2143 days [15–2906]). Event-free survival rates at 1 and 5 years were 83.3% and 73.1%, respectively. There were 13 deaths at the follow-up. Estimated survival rates were 96% and 86.7% at 1 and 5 years, respectively (figure 1 of the supplementary data).
DISCUSSION
ST remains a serious complication with a high mortality rate.11 Understanding its pathophysiology is essential for improving prevention, diagnosis, and eventually treatment.12 In this context, OCT provides crucial real-time information, allowing for much more precise diagnosis and individualized treatment.
This study represents the largest national series of ST cases evaluated with OCT. The main findings are 1) the use of OCT in the acute phase of ST is safe and feasible in experienced centers; 2) OCT identified mechanical abnormalities in 86.7% of ST cases; 3) the dominant finding varied according to the timing of ST (in acute ST, no mechanical abnormality was most common, while underexpansion predominated in subacute ST, malapposition in late ST, and neoatherosclerosis in very late ST); 4) OCT enabled treatment guidance based on the dominant finding and might reduce the need for additional stenting; and 5) in-hospital mortality rate was low (9.2%), with Killip-Kimball class IV and chronic kidney disease being the main predictors.
An adequate-quality OCT study was achieved in 74% of patients, which is a rate considerably higher than that reported by the main European registries.9,13 Although thrombus can limit visibility, image quality can be improved with thrombus aspiration or the use of catheter extension systems. Unlike other studies in which OCT was performed in a procedure separate from the index ST, our study conducted OCT during the acute event, representing a key methodological strength.
OCT demonstrated a remarkable ability to detect mechanical abnormalities (86.7%). In this setting, the information provided by angiography is insufficient. In the PESTO trial,13 angiography identified the cause of ST in only 12% of cases, whereas OCT did so in more than 90% of the cases. The CLI-OPCI trial14 was the first to demonstrate that OCT provides information on immediate stent outcomes not appreciable on angiography, prompting additional interventions in up to one-third of patients.
Although some findings are consistent with the PRESTIGE study,9 our analysis provides relevant nuances. In particular, in acute ST, most cases showed no evident mechanical abnormalities (unlike our preliminary results, in which malapposition predominated). This finding reinforces the role of antithrombotic treatment and prothrombotic states as key factors, supported by the greater thrombus burden observed in early vs late ST. Unlike previous studies, our results emphasize the utility of OCT not only in identifying mechanical causes but also in avoiding unnecessary interventions, thereby underscoring the importance of optimal medical therapy. In addition, in our study, uncovered struts were not considered a cause of acute or subacute ST, based on the assumption that no stent is covered by neointima within the first 30 days.
The association between acute malapposition and ST remains controversial.15-17 A possible explanation is that although malapposition is almost ubiquitous after stent implantation,18,19 its relationship with thrombosis is difficult to establish because of the low probability of a later event.
Underexpansion, however, is established as one of the most important predictors of ST. In the CLI-OPCI study,20 a minimum lumen area > 4.5 mm2 was identified as a threshold for discriminating events during follow-up. This cutoff value is not achievable in small vessels and does not apply to left main lesions. Relative expansion6,21 may thus be a more appropriate measure, although its superiority over absolute expansion in predicting events has not yet been established.
In late ST, malapposition was the most common finding. Most cases of nonsevere acute malapposition resolve during follow-up, although up to 30% may persist.18 One study22 reported substantially lower rates of malapposition (8%) than those observed in previous large registries,9,13 likely because the index implantation was image-guided; this also supported that 75% of cases represented acquired malapposition.
Neoatherosclerosis was detected in most late and very late ST, irrespective of stent type, unlike other studies in which it predominated in drug-eluting stents. This may be explained by the longer interval between implantation and thrombosis in bare-metal stents, which is consistent with the observation that follow-up duration is the most important predictor of neoatherosclerosis.23 The use of OCT avoided unnecessary new stenting in 48% of patients, a higher rate compared with other ST series without image guidance24 and even higher than in the PRESTIGE registry.9
Limitations
The present study has several limitations: 1) the absence of a control group precludes predictive evaluation of certain findings such as malapposition, and superiority of OCT over non–intracoronary image-guided interventions cannot be definitively established; 2) no core laboratory was used for OCT analysis, which may limit external reproducibility; 3) selection bias exists due to exclusion of the most severe patients and those with complex anatomy, which may account for favorable outcomes and could influence the prevalence of some mechanisms; 4) ST is a multifactorial process, and the dominant finding cannot be considered the definitive cause; 5) the presence of thrombus hampers evaluation of underlying structures; 6) the index stent implantation was not image-guided, preventing differentiation between acute and acquired malapposition; and 7) serial OCT studies were not performed in many patients, which means that correction of the detected abnormality could not be assessed.
CONCLUSIONS
OCT is a safe, feasible, and highly useful tool for the treatment of ST. It allows identification of the most likely cause of the event in most cases—which varies according to the timing of thrombosis—and enables individualized treatment by addressing the underlying abnormality.
FUNDING
None declared.
ETHICAL CONSIDERATIONS
This study was approved by Hospital Universitario de La Princesa Ethics Committee. All procedures conformed to the ethical principles of the Declaration of Helsinki. This study followed the SAGER guidelines. Written informed consent for publication was obtained and archived.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
No artificial intelligence tools were used in the preparation of this manuscript.
AUTHORS’ CONTRIBUTIONS
All authors contributed equally to the conception, design, and analysis of the study, as well as to drafting and revising the manuscript. Furthermore, all authors approved the final version and are responsible for its content.
CONFLICTS OF INTEREST
F. Alfonso is Associate Editor of REC: Interventional Cardiology; the journal’s editorial procedure to ensure impartial handling of the manuscript has been followed. The remaining authors declared no conflicts of interest whatsoever.
WHAT IS KNOWN ABOUT THE TOPIC?
- ST is a rare but clinically significant complication, characterized by high mortality, recurrence, and a complex, multifactorial pathophysiology. A thorough understanding of its underlying mechanisms is essential to guide appropriate preventive and therapeutic strategies.
WHAT DOES THIS STUDY ADD?
- This study is the largest national series of consecutive ST cases evaluated with OCT and demonstrates the ability of this imaging modality to detect underlying mechanical abnormalities potentially involved in the pathogenesis of this serious complication.
- Mechanical abnormalities associated with ST vary significantly according to timing of presentation.
- Improving stent implantation technique could reduce the rate of ST by addressing causes such as malapposition and underexpansion.
- The use of OCT during ST treatment allows procedures to be guided and optimized.
REFERENCES
1. Kastrati A, Mehilli J, Pache J, et al. Analysis of 14 Trials Comparing Sirolimus-Eluting Stents with Bare-Metal Stents. N Engl J Med. 2007;356: 1030-1039.
2. Alfonso F. The “Vulnerable“Stent. Why So Dreadful?J Am Coll Cardiol. 2008;51:2403-2406.
3. Park KW, Hwang SJ, Kwon DA, et al. Characteristics and predictors of drug-eluting stent thrombosis:Results from the multicenter Korea stent thrombosis (KoST) registry. Circ J. 2011;75:1626-1632.
4. Cuesta J, Rivero F, Bastante T, et al. Optical Coherence Tomography Findings in Patients With Stent Thrombosis. Rev Esp Cardiol. 2017;70: 1050-1058.
5. Prati F, Kodama T, Romagnoli E, et al. Suboptimal stent deployment is associated with subacute stent thrombosis:Optical coherence tomography insights from a multicenter matched study. From the CLI Foundation investigators:The CLI-THRO study. Am Heart J. 2015;169:249-256.
6. Räber L, Mintz GS, Koskinas KC, et al. Clinical use of intracoronary imaging. Part 1:Guidance and optimization of coronary interventions. An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. Eur Heart J. 2018;39:3281-3300.
7. Alfonso F, Fernandez-Viña F, Medina M, Hernandez R. Neoatherosclerosis:The Missing Link Between Very Late Stent Thrombosis and Very Late In-Stent Restenosis. J Am Coll Cardiol. 2013;61:155.
8. Joner M, Koppara T, Byrne RA, et al. Neoatherosclerosis in Patients With Coronary Stent Thrombosis:Findings From Optical Coherence Tomography Imaging (A Report of the PRESTIGE Consortium). JACC Cardiovasc Interv. 2018;11:1340-1350.
9. Adriaenssens T, Joner M, Godschalk TC, et al. Optical Coherence Tomography Findings in Patients With Coronary Stent Thrombosis:A Report of the PRESTIGE Consortium (Prevention of Late Stent Thrombosis by an Interdisciplinary Global European Effort). Circulation. 2017;136: 1007-1021.
10. Cuesta J, García-Guimaraes M, Basante T, Rivero F, Antuña P, Alfonso F. Bioresorbable Vascular Scaffold Thrombosis:Clinical and Optical Coherence Tomography Findings. Rev Esp Cardiol. 2019;72:90-91.
11. la Torre-Hernández JM, Alfonso F, Hernández F, et al. Drug-Eluting Stent Thrombosis. J Am Coll Cardiol. 2008;51:986-990.
12. Alfonso F, Dutary J, Paulo M, et al. Combined use of optical coherence tomography and intravascular ultrasound imaging in patients undergoing coronary interventions for stent thrombosis. Heart. 2012;98:1213-1220.
13. Souteyrand G, Amabile N, Mangin L, et al. Mechanisms of stent thrombosis analysed by optical coherence tomography:insights from the national PESTO French registry. Eur Heart J. 2016;37:1208-1216.
14. Prati F, Di Vito L, Biondi-Zoccai G, et al. Angiography alone versus angiography plus optical coherence tomography to guide decision-making during percutaneous coronary intervention:the Centro per la Lotta contro l'Infarto-Optimisation of Percutaneous Coronary Intervention (CLI-OPCI) study. EuroIntervention. 2012;8:823-829.
15. Ng JCK, Lian SS, Zhong L, Collet C, Foin N, Ang HY. Stent malapposition generates stent thrombosis:Insights from a thrombosis model. Int J Cardiol. 2022;353:43-45.
16. Prati F, Romagnoli E, La Manna A, et al. Long-term consequences of optical coherence tomography findings during percutaneous coronary intervention:the Centro Per La Lotta Contro L'infarto – Optimization Of Percutaneous Coronary Intervention (CLI-OPCI) LATE study. EuroIntervention. 2018;14:443-451.
17. Romagnoli E, Gatto L, La Manna A, et al. Role of residual acute stent malapposition in percutaneous coronary interventions. Catheter Cardiovasc Interv. 2017;90:566-575.
18. Shimamura K, Kubo T, Akasaka T, et al. Outcomes of everolimus-eluting stent incomplete stent apposition:a serial optical coherence tomography analysis. Eur Heart J Cardiovasc Imaging. 2015;16:23-28.
19. Ali ZA, Maehara A, Généreux P, et al. Optical coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation (ILUMIEN III:OPTIMIZE PCI):a randomised controlled trial. Lancet. 2016;388:2618-2628.
20. Prati F, Romagnoli E, Burzotta F, et al. Clinical Impact of OCT Findings During PCI:The CLI-OPCI II Study. JACC Cardiovasc Imaging. 2015;8:1297-1305.
21. Ali ZA, Landmesser U, Maehara A, et al. Optical Coherence Tomography– Guided versus Angiography-Guided PCI. N Engl J Med. 2023;389:1466-1476.
22. Mori H, Sekimoto T, Arai T, et al. Mechanisms of Very Late Stent Thrombosis in Japanese Patients as Assessed by Optical Coherence Tomography. Can J Cardiol. 2024;40:696-704.
23. Otsuka F, Byrne RA, Yahagi K, et al. Neoatherosclerosis:Overview of histopathologic findings and implications for intravascular imaging assessment. Eur Heart J. 2015;36:2147-2159.
24. Armstrong EJ, Feldman DN, Wang TY, et al. Clinical Presentation, Management, and Outcomes of Angiographically Documented Early, Late, and Very Late Stent Thrombosis. JACC Cardiovasc Interv. 2012;5:131-140.
* Corresponding author.
- Access to transcatheter aortic valve implantation: interregional variability and expert evaluation
- Safety and efficacy profile of excimer laser coronary angioplasty for thrombus removal in STEMI
- Drug-coated balloons vs drug-eluting stents for the treatment of large native coronary artery disease. Meta-analysis of randomized controlled trials
- Heart block after transcatheter septal defect closure in infants under 10 kg: clinical outcomes and management options
Special articles
Original articles
Editorials
Original articles
Editorials
Post-TAVI management of frail patients: outcomes beyond implantation
Unidad de Hemodinámica y Cardiología Intervencionista, Servicio de Cardiología, Hospital General Universitario de Elche, Elche, Alicante, Spain
Original articles
Debate
Debate: Does the distal radial approach offer added value over the conventional radial approach?
Yes, it does
Servicio de Cardiología, Hospital Universitario Sant Joan d’Alacant, Alicante, Spain
No, it does not
Unidad de Cardiología Intervencionista, Servicio de Cardiología, Hospital Universitario Galdakao, Galdakao, Vizcaya, España














