ABSTRACT
Introduction and objectives: The Interventional Cardiology Association of the Spanish Society of Cardiology (ACI-SEC) and the Interventional Working Group of the Spanish Society of Pediatric Cardiology (GTH-SECPCC) present their annual activity report for 2022.
Methods: All Spanish centers with catheterization laboratories and interventional activity in congenital heart diseases were invited to participate. Data were collected online and analyzed by an external company, together with the members of the ACI-SEC and the GTH-SECPCC.
Results: A total of 22 centers participated (19 public and 3 private). Interventional data on adult congenital diseases contributed by another 99 hospitals to the Registry of Cardiac Catheterization and Interventional Cardiology of the ACI-SEC in 2022 were incorporated into the analysis. A total of 1141 diagnostic studies (4.3% more than in 2021) and 2508 interventional catheterizations (61.5% more than in 2020) were registered. The most frequent procedures were atrial septal defect closure (1135 cases), percutaneous closure of patent ductus arteriosus (262 cases), and pulmonary branch artery angioplasty (234 cases). The most significant increases in volume were related to balloon aortic valvuloplasty (48.9%), atrial septal defect closure (45.2%), and ventricular septal defect closure (40.7%). Interventional procedures were successful in 97.6%, with major procedural complications occurring in 1.4% and in-hospital mortality in 0.2%.
Conclusions: This report is the third publication of the Spanish Cardiac Catheterization in Congenital Heart Diseases Registry. Both diagnostic and interventional procedures substantially increased, particularly in balloon aortic valvuloplasty, atrial septal defect closure, and ventricular septal defect closure. Most interventional techniques continue to demonstrate excellent safety and effectiveness outcomes.
Keywords: Atrial septal defect closure. Cardiac catheterization. Congenital heart disease. Percutaneous valve implantation.
RESUMEN
Introducción y objetivos: La Asociación de Cardiología Intervencionista de la Sociedad Española de Cardiología (ACI-SEC) y el Grupo de Trabajo de Hemodinámica de la Sociedad Española de Cardiología Pediátrica y Cardiopatías Congénitas (GTH-SECPCC) presentan su informe anual de actividad hemodinámica en cardiopatías congénitas correspondiente al año 2022.
Métodos: Se invitó a participar a los centros españoles con laboratorio de hemodinámica y actividad intervencionista en cardiopatías congénitas. La recogida de datos se realizó mediante un cuestionario telemático. Una empresa externa analizó los resultados, que fueron revisados por miembros de la ACI-SEC y el GTH-SECPCC.
Resultados: Participaron en el registro 22 centros (19 públicos y 3 privados). Se incorporaron al análisis los datos de intervencionismo en cardiopatías congénitas del adulto aportados por otros 99 hospitales al Registro de Hemodinámica y Cardiología Intervencionista de la ACI-SEC del año 2022. Se registraron 1.141 estudios diagnósticos (un 4,3% más que en 2021) y 2.508 cateterismos intervencionistas (un 61,5% más que en 2021). Las técnicas con mayor casuística fueron el cierre de defectos interauriculares (1.135 casos), el cierre de ductus arterioso (262 casos) y la angioplastia de ramas pulmonares (234 casos). El incremento más significativo se comunicó en la valvuloplastia aórtica (48,9%), el cierre de defectos interauriculares (45,2%) y el cierre de comunicación interventricular (40,7%). La tasa de éxito en los procedimientos intervencionistas fue del 97,6%, con una tasa de complicaciones mayores del 1,4 % y una mortalidad intrahospitalaria del 0,2%.
Conclusiones: El presente trabajo es la tercera publicación del Registro Español de Intervencionismo en Cardiopatías Congénitas. Se ha comunicado un aumento muy significativo de la mayoría de los procedimientos terapéuticos, destacando el incremento de la valvuloplastia aórtica, del cierre de defectos interauriculares y del cierre de comunicación interventricular. Todas las técnicas intervencionistas han reportado excelentes datos de seguridad y eficacia.
Palabras clave: Cardiopatías congénitas. Cateterismo cardiaco. Cierre de comunicación interauricular. Implante percutáneo de válvula aórtica.
INTRODUCTION
The collaborative effort between the Interventional Cardiology Association of the Spanish Society of Cardiology (ACI-SEC) and the Interventional Working Group of the Spanish Society of Pediatric Cardiology (GTH-SECPCC), which was initiated in 2019, allowed the reactivation of a Spanish registry of cardiac catheterizations and interventional cardiology in patients with congenital heart diseases. The results of this collaboration have been published in the first 2 reports of the activity conducted from 2020 to 2021.1,2 The main weakness highlighted in both reports is the inadequate estimation of interventional procedures performed in patients older than 18 years. Despite being highly representative of pediatric activity, the number of participating centers, did not seem sufficient to accurately reflect the activity carried out in adult congenital heart diseases in Spain.3,4
This article analyzes the current report, focusing on the activity conducted in 2022, and aims to consolidate the objective of reliably measuring the scope of interventional procedures to treat congenital heart diseases in all age groups. The results of this report were made public at the XXXIV ACI-SEC Congress held in Santander, Spain on June 7th, 2022.
METHODS
The data presented come from a retrospective, voluntary, unaudited, and annually updated registry. This year, a substantial and coordinated change has been made to the section on interventional procedures for the treatment of congenital heart diseases of the ACI-SEC Spanish Registry of Cardiac Catheterization and Interventional Cardiology to standardize data from the 2 registries and facilitate their incorporation into the study of its interventional activity in patients older than 18 years.5
All hospitals already participating in the ACI-SEC Spanish Registry of Cardiac Catheterization and Interventional Cardiology were invited to participate, as well as all pediatric hospitals represented in the GTH-SECPCC. Data were collected by the investigator of each participating hospital through the official website of the ACI-SEC.6
The registry results were managed and cleaned by an external company (Tride, Madrid, Spain), and were subsequently reviewed and compared with those obtained in previous years by members of the GTH-SECPCC and the ACI-SEC board. If the data were discordant, the center in question was contacted for clarification and error minimization.
Due to the methodological characteristics of the study and the fact that it was purely an activity registry, there was no requirement for approval from an ethics committee or processing of informed consent forms.
RESULTS
Resources and infrastructure
Twenty-two hospitals participated (6 more than in 2021), 19 from the publicly-funded health sector and 3 from the private sector (appendix 1 of the supplementary data). Data on cardiac catheterizations in adult congenital heart diseases from 2022 were provided by another 99 hospitals to the ACI-SEC Spanish Registry of Cardiac Catheterization and Interventional Cardiology of the and were included in the analysis (appendix 2 of the supplementary data).
Thirty-four cath labs with interventional activity for congenital heart diseases were included in the registry, of which 7 (20.8%) are pediatric cardiac cath labs exclusively; 9 of them with biplane image-guided systems and 14 with the possibility of implementing rotational angiography. The median number of monthly days dedicated by each hospital to interventional procedures for congenital heart disease was 6 [3-17] days vs 7 days in 2021. Fifteen (68.1%) of these hospitals have round-the-clock catheterization services, even for pediatric patients.
Data on medical staffing revealed that 67 interventional cardiologists with full-time dedication to the specialty were registered, of which 37 (55.3%) treated adults and 30 (44.7%) pediatric patients.
Diagnostic procedures
A total of 1141 diagnostic studies were reported, representing a 4.3% increase compared with the previous year. Age distribution was as follows: 37 (3.2%) cardiac catheterizations were performed in infants younger than 1 month, 127 (11.1%) in patients aged from 1 month to 1 year, 578 (50.7%) in patients from 1 to 18 years, and 399 (35.5%) in patients older than 18 years.
Sixty cardiac catheterizations (5.4%) were classified as emergency procedures. Regarding morbidity, 7 (0.6%) cases of serious complications were reported: 4 arrhythmias (2 with severe hemodynamic instability and cardiac arrest), 1 vascular event, and 1 cardiac tamponade; there was 1 procedure-related death.
Interventional procedures
The activity reported in this section increased by 61.5% compared with the previous year. In all, 2508 therapeutic catheterizations were reported and grouped into 13 categories with the following age distribution: 3 procedures (0.1%) were performed in the fetal period, 163 (6.4%) in infants younger than 1 month, 208 (8.3%) in patients aged from 1 month to 1 year, 754 (30.1%) in patients aged from 1 to 18 years, and 1380 (55%) in patients older than 18 years, of which 903 were added by incorporating data from the ACI-SEC Spanish Registry of Cardiac Catheterization and Interventional Cardiology of the (table 1 and table 2).
Table 1. Number of interventional procedures and distribution by age groups
| Variable |
Total |
Fetal |
< 1 month |
1 month to 1 year |
1 to 18 years |
> 18 years |
| Interventional procedures |
2508 |
3 (0.1) |
163 (6.4) |
208 (8.3) |
754 (30.1) |
1380 (55.0) |
| Congenital aortic valvuloplasty |
67 |
2 (3.0) |
9 (13.4) |
14 (20.9) |
22 (32.8) |
20 (29.9) |
| Congenital pulmonary valvuloplasty |
138 |
1 (0.7) |
34 (24.6) |
39 (28.3) |
34 (24.6) |
30 (21.7) |
| Congenital mitral valvuloplasty |
0 |
- |
0 |
0 |
0 |
0 |
| Pulmonary angioplasty |
135 |
- |
0 |
7 (5.2) |
75 (55.6) |
53 (39.3) |
| Pulmonary branch angioplasty |
234 |
- |
2 (0.9) |
45 (19.2) |
136 (58.1) |
51 (21.8) |
| Aortic angioplasty |
126 |
- |
3 (2.4) |
28 (22.2) |
40 (31.7) |
55 (43.7) |
| Other angioplasty procedures |
100 |
- |
26 (26.0) |
22 (22.0) |
37 (37.0) |
15 (15.0) |
| Atrial septal defect/patent foramen ovale closure |
1135 |
- |
- |
2 (0.2)a |
130 (11.5) |
1003 (88.4) |
| Patent ductus arteriosus closure |
262 |
24 (9.2)b |
17 (6.5)b |
30 (11.5)b |
147 (56.1) |
44 (16.8) |
| Ventricular septal defect closure |
38 |
- |
- |
1 (2.6)a |
23 (60.5) |
14 (36.8) |
| Other occlusions |
91 |
- |
2 (2.2) |
8 (8.8) |
39 (42.9) |
42 (46.2) |
| Foreign body removal |
23 |
- |
3 (13.0) |
0 |
18 (78.3) |
2 (8.7) |
| Atrial septostomy |
72 |
- |
43 (59.7) |
12 (16.7) |
17 (23.6) |
0 |
| Transcatheter aortic valve implantation |
87 |
- |
- |
- |
36 (41.4)c |
51 (58.6) |
|
a In this case, infants younger than 1 month and from 1 month to 1 year are not shown separately and consequently the value corresponds to infants younger than 1 year.
b In patent ductus arteriosus closure, groups are premature (fetal), < 6 months (< 1 month), and 6 months to 1 year (1 month to 1 year).
c Reported as participants younger than 18 years and consequently the value corresponds to participants younger than 18 years.
Data are expressed as n (%).
|
Table 2. Number of interventional catheterizations performed in patients older than 18 years and distribution according to the source registry
| Variable |
> 18 years |
| |
Total |
RICCa |
RHCIb |
| Interventional procedures |
1380 |
477 |
903 |
| Congenital aortic valvuloplasty |
20 |
19 |
1 |
| Congenital pulmonary valvuloplasty |
30 |
12 |
18 |
| Congenital mitral valvuloplasty |
0 |
0 |
0 |
| Pulmonary angioplasty |
53 |
21 |
32 |
| Pulmonary branch angioplasty |
51 |
26 |
25 |
| Aortic angioplasty |
55 |
33 |
22 |
| Other angioplasty procedures |
15 |
10 |
5 |
| Atrial septal defect/patent foramen ovale closure |
1003 |
221 |
782 |
| Patent ductus arteriosus closure |
44 |
11 |
33 |
| Ventricular septal defect closure |
14 |
4 |
10 |
| Other occlusions |
42 |
18 |
24 |
| Foreign body removal |
2 |
2 |
0 |
| Atrial septostomy |
0 |
0 |
0 |
| Transcatheter aortic valve implantation |
51 |
51 |
0 |
|
a Data provided by the 22 centers participating in ACI-SEC Spanish Cardiac Catheterization in Congenital Heart Diseases Registry (RICC) and the GTH-SECPCC (2022).
b Data provided by the 96 centers participating in the 2022 ACI-SEC Spanish Registry of Cardiac Catheterization and Interventional Cardiology Registry (RHCI).
Data are expressed as n.
|
A total of 148 cardiac catheterizations were classified as urgent (9.7% of all procedures performed with this reported datum). The number of interventional procedures reported by each center was distributed as follows: 5 hospitals (21.7%) reported more than 150 catheterizations, 3 (13%) between 75 and 150 interventions, and 8 (47.1%) less than 75 catheterizations. The overall effectiveness of the various interventional techniques used was 97.6%, with most centers reporting effectiveness of more than 95% (table 3).
Table 3. Summary of reported efficacy of interventional procedures
| Interventional procedures |
n |
Cases with success/inefficacy data |
Success |
Inefficacy |
| Congenital aortic valvuloplasty |
67 |
46 (68) |
43 (93.5) |
3 (6.5) |
| Congenital pulmonary valvuloplasty |
138 |
118 (85) |
117 (99.2) |
1 (0.8) |
| Congenital mitral valvuloplasty |
0 |
- |
- |
- |
| Pulmonary angioplasty |
135 |
95 (70) |
90 (94.7) |
5 (5.3) |
| Pulmonary branch angioplasty |
234 |
205 (87.6) |
199 (97.1) |
6 (2.9) |
| Aortic angioplasty |
126 |
108 (85.7) |
106 (98.1) |
2 (1.9) |
| Other angioplasty procedures |
100 |
95 (95) |
91 (95.8) |
4 (4.2) |
| Atrial septal defect/patent foramen ovale closure |
1135 |
1024 (90.2) |
1003 (97.9) |
21 (2.1) |
| Patent ductus arteriosus closure |
262 |
251 (95.8) |
248 (98.8) |
3 (1.2) |
| Ventricular septal defect closure |
38 |
30 (78.9) |
29 (96.7) |
1 (3.3) |
| Other occlusions |
91 |
66 (72.5) |
65 (98.5) |
1 (1.5) |
| Foreign body removal |
23 |
23 (100) |
22 (95.7) |
1 (4.3) |
| Atrial septostomy |
72 |
72 (100) |
70 (97.2) |
2 (2.8) |
| Transcatheter aortic valve implantation |
87 |
87 (100) |
84 (96.6) |
3 (3.4) |
| Total |
2508 |
2220 (88.5) |
2167 (97.6) |
53 (2.4) |
|
Data are expressed as n.
|
Percutaneous valvuloplasty procedures
Sixty-seven aortic valvuloplasty procedures were reported to treat congenital aortic stenosis (a 48.9% increase compared with 2021), including 2 fetal valvuloplasty procedures. Forty-two (62.6%) of these procedures were performed in patients older than 1 year, of which 20 (29.9%) were older than 18 years. Previously untreated native valves were dilated in 70% of cases.
In all, 138 pulmonary valvuloplasty procedures were reported, including 1 fetal valvuloplasty, representing a 32.7% increase compared with the previous year. Technical data were reported in 104 cases (85%): 95 (90%) were native valves; 7 (4.8%) were imperforate valves; and in 2 cases (1.9%), the procedure was associated with ductal stenting.
Lastly, there were no cases of mitral valvuloplasty that year.
Percutaneous angioplasty procedures
A total of 135 right ventricular outflow tract dilatations were reported (a 25% increase compared with 2021). Technical and anatomical data were reported for 96 (72.7%) procedures: surgical conduit angioplasty was performed in 62% of procedures and native tract angioplasty in the remaining 38%. Stent implantation was performed in 51% of cases, conventional balloon dilation in 43%, and cutting balloon in 5%.
There were 234 pulmonary branch angioplasty procedures. Technical data were obtained from 205 (87.6%) interventions: proximal branches were dilated in 191 interventions (93.1%) and peripheral arteries (lobar-segmental) in the remaining procedures. Stent implantation was performed in 102 (49.7%) catheterizations, conventional balloon dilation in 98 (47.8%), and cutting balloon dilation in 5 (2.4%).
Of 126 aortic angioplasty procedures, anatomical data were reported for 104 (82.5%) procedures: 70 (67.3%) were reinterventions and 34 (32.6%) were treatments on native aortas. The dilation substrate was the aortic arch/isthmus in all cases except for 1 angioplasty of the ascending aorta. The distribution of the technique used was as follows: conventional balloon angioplasty in 29%, implantation of uncovered stents in 18.5%, implantation of covered stents in 37.9%, and redilatation with a previously implanted stent balloon in 14.5%.
A further 100 catheterizations were reported in the category of “other angioplasty procedures,” representing a decrease in their frequency by 9.1% compared with the previous year. The anatomical substrate of the angioplasty was reported in 73 cases, highlighting patent ductus arteriosus dilation in 25 cases, systemic veins in 16, Fontan conduits in 10, and surgical fistulas in 8. Fifty-five percent of the procedures were associated with stent implantation.
Shunt closure and other occlusive procedures
There were 1135 atrial septal defect closures: 782 (68.8%) came from the incorporation of data from the ACI-SEC Spanish Registry of Cardiac Catheterization and Interventional Cardiology of the same year (table 2). Consequently, the volume of patients older than 18 years who underwent this technique was 83.8% overall. The predominant anatomical substrate of the defect was patent foramen ovale, with 705 (62.1%) cases. A total of 72.1% of atrial septal defects (ASD) were classified as complex, and the remaining ASD as simple. Data on procedure guidance were reported in 348 cases (28.3%): transesophageal echocardiography was used in 80.4%, intracardiac echocardiography in 12.6%, and angiographic measurements with balloon in 6.8%.
Patent ductus arteriosus closure accounted for 262 catheterizations. More than half of all procedures (56.1%) were performed in patients aged 1 to 18 years, while 9.2% were performed in premature infants (24 cases). The route of choice was antegrade venous access in 70% of closures. Occlusive devices were used in 88.4% of cases and controlled-release coil devices in the remainder.
Thirty-eight catheterizations for ventricular septal defect (VSD) closures were reported, increasing their frequency by 40.7% compared with the previous year. Data on the anatomical substrate of the VSD were reported in 28 (73.6%) cases, with the following distribution: 20 (71.4%) perimembranous, 6 (21.4%) muscular, and 2 (7.1%) postoperative. Occlusive devices were used in 89.2% of cases and coil-type occluders in the remainder. Two devices were implanted via a hybrid approach and the remaining devices via transcatheter access (93.3%).
Ninety-one catheterizations fell within the category “various occlusive procedures”. Data on the type of occlusion were reported in 65 (71.4%) cases, with closure of systemic-to-pulmonary collateral vessels in 40 (61.5%) cases, venous collaterals in 13 (20%), coronary fistulas in 3 (4.6%), and Fontan fenestrations in 2 (3%). The most widely used material was coil-type occluders (38.8%), followed by occlusive devices (36.1%), and particles as the only material or in combination with others (25%).
Atrial septostomy
Seventy-two atrial septostomy procedures were reported (a 33.3% increase compared with the previous year). Echocardiography was used for imaging guidance in 22.5% of cases, fluoroscopy in 28%, and a combination of the 2 imaging modalities in 49.2%. Forty-nine (68%) interventions were balloon atrial septoplasty procedures (Rashkind). There were also 7 procedures with radiofrequency-guided septal perforation, 7 with needle perforation, and 15 with septal stent implantation.
Percutaneous valve implantations
Eighty-seven procedures were reported, of which 51 (58.6%) were performed in patients older than 18 years. The hybrid approach was used in 2 cases, while the fully percutaneous approach was used in the remaining cases. The pulmonary position was predominant (96.5%), with 2 successful valve implantations being performed in the tricuspid position and 1 in the mitral position. The anatomical substrate of implantation in the pulmonary position had the following distribution: 33 in the surgical conduit, 31 in the native tract, followed by 20 valve-in-valve procedures.
Complications
Morbidity and mortality data were reported for 2401 interventional procedures, with 35 serious adverse events (table 4), including 6 deaths, which translated into a rate of major complication of 1.4% and a mortality rate of 0.2%. The categories associated with higher morbidity rates were percutaneous valve implantation (8%), other angioplasty procedures (6%), and VSD closure (5.2%). The most common complications were device embolizations (8 cases): 4 in ASD closures, 2 in patent ductus arteriosus closures, and 2 stents implanted in the setting of pulmonary angioplasty procedures; surgical removal of the embolized valve was required in only 1 case of ASD closure. Less frequent were vascular complications (6 cases), 3 of them being associated with pulmonary angioplasty procedures. There were 4 cases of severe arrhythmias, including 2 cases of cardiac arrest requiring bailout extracorporeal membrane oxygenation.
Table 4. Distribution of major complications and reported deaths in various interventional procedures
| Procedure |
n |
Major complications |
Deaths |
| Congenital aortic valvuloplasty |
67 |
3 (6.5)
– 1 severe aortic regurgitation
– 1 unspecified
– 1 death |
1 |
| Valvuloplastia pulmonar congénita |
138a (111) |
2 (1.8)
– 1 tricuspid valve rupture
– 1 unspecified |
0 |
| Valvuloplastia mitral congénita |
0 |
0 |
0 |
| Angioplastia pulmonar |
135b (102) |
1 (0.9)
– 1 unspecified |
0 |
| Angioplastia ramas pulmonares |
227c (202) |
6 (2.9)
– 3 vascular dissections
– 1 pulmonary hemorrhage
– 2 stent embolizations |
0 |
| Angioplastia aórtica |
124d (102) |
3 (2.9)
– 2 vascular dissections
– 1 death |
1 |
| Otras angioplastias |
100 |
6 (6)
– 1 coronary thrombosis
– 1 CPR-ECMO – 1 vascular dissection
– 1 neurological event
– 2 deaths |
2 |
| Cierre de comunicación interauricular/foramen oval |
1135 |
5 (0.4)
– 4 embolizations (1 required surgery)
– 1 neurological event |
0 |
| Cierre de conducto |
262 |
3 (1.1)
– 2 embolizations not requiring surgery
– 1 death |
1 |
| Cierre de comunicación interventricular |
38 |
2 (5.2)
– 1 atrioventricular block
– 1 CPR-ECMO |
0 |
| Otras oclusiones |
91 |
0 |
0 |
| Retirada de cuerpo extraño |
23 |
0 |
0 |
| Atrioseptostomía |
72 |
1 (1.3)
– 1 unspecified |
0 |
| Implantación de válvula percutánea |
87 |
4 (8.0)
– 1 vascular dissection
– 1 pulmonary duct dissection
– 1 ventricular tachycardia
– 1 death |
1 |
| Total |
2508e (2401) |
35 (1.4) |
6 (0.2) |
|
CPR, cardiopulmonary resuscitation; ECMO, extracorporeal membrane oxygenation.
a Percentages calculated based on 111 reported cases.
b Percentages calculated based on 102 reported cases.
c Percentages calculated based on 202 reported cases.
d Percentages calculated based on 102 reported cases.
e Percentages calculated based on 2411 reported cases.
Data are expressed as n (%).
|
DISCUSSION
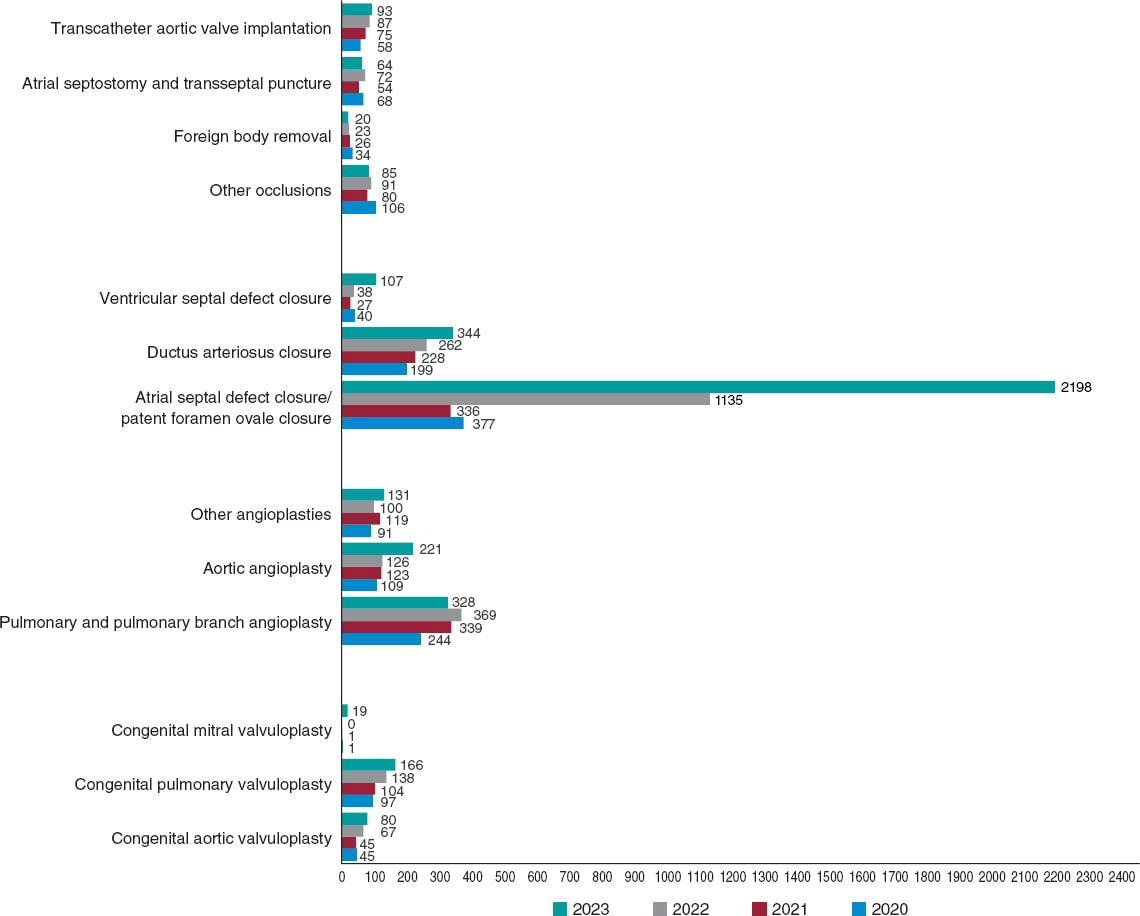
To date, one of the main weaknesses of this registry has been its limitations in adequately assessing the interventional activity carried out in the context of adult congenital heart disease. For this reason, and as the most significant novel addition to this report, we included data from 99 hospitals reporting their activities in adult congenital heart disease to the 2022 Spanish Registry of Cardiac Catheterization and Interventional Cardiology in the analysis of the various interventional categories. This has resulted in a significant increase in catheterization volume, totaling 3649 procedures (1002 more than in 2021). Their comparison with the activity conducted in previous years and the significant increase in registered procedures should be analyzed considering this methodological difference, and taking into account the increase in participating centers, 6 more than in 2021 (figure 1).
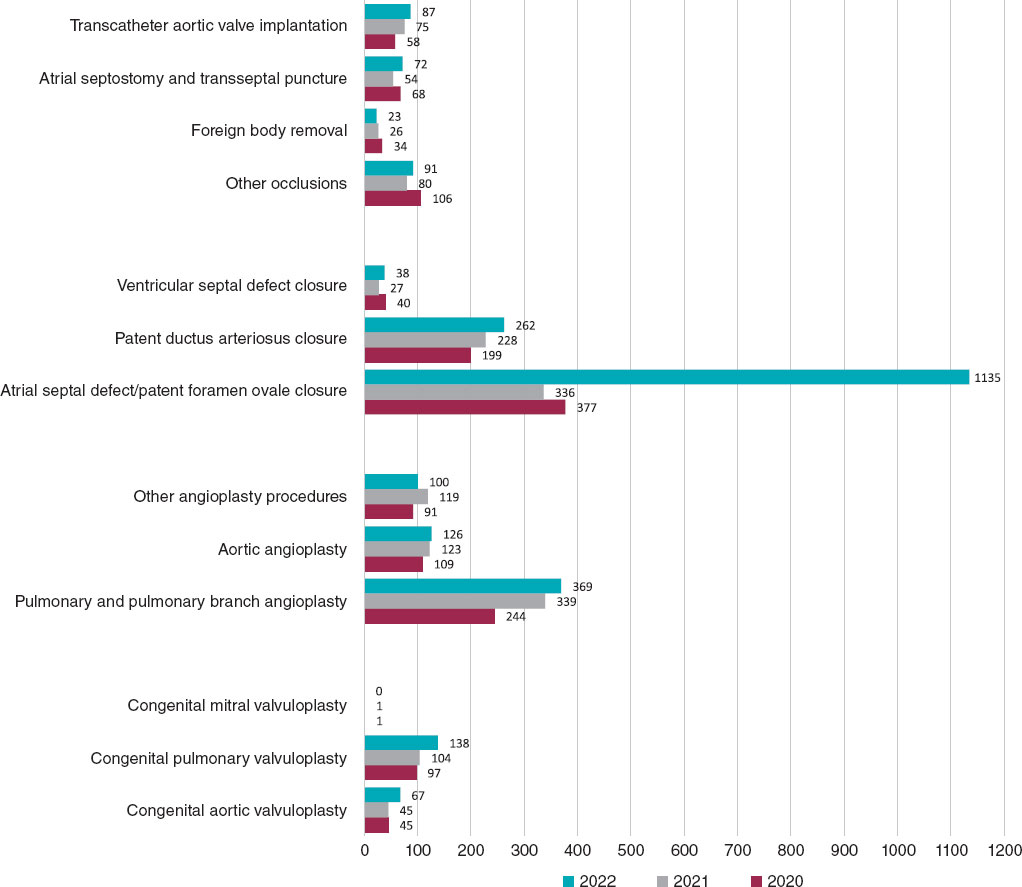
Figure 1. Comparison of the number of interventional procedures performed in 2020, 2021, and 2022.
The total number of registered interventional procedures was 2508, with notable increases in techniques such as ASD closure, aortic and pulmonary valvuloplasty, atrial septostomy, and VSD closure. A total of 55% of cardiac catheterizations were performed in patients older than 18 years (compared with 32% in 2021), demonstrating an improvement in the representation of interventional procedures for adult congenital heart diseases. Once again, in the pediatric setting, we noted that fetal interventional activity in Spain is very limited, with only 3 reported cases (2 aortic valvuloplasty procedures and 1 pulmonary valvuloplasty procedure), despite evidence of its value and effectiveness in these and other prenatal scenarios, such as pulmonary atresia with intact ventricular septum and hypoplastic left heart syndrome.7
The reported data on the effectiveness of various interventional techniques yielded an overall success rate of 97.6% (compared with 95% in 2021) and a mortality rate of 0.2% (the same as in 2021), with 6 procedure-related deaths. These results are consistent with those reported from most international studies to date.8,9 The rate of serious adverse events of 1.4% is the lowest reported so far (2% in 2020 and 2.7% in 2022), with a decrease in the frequency of all types of complications reported. Device embolizations continue to account for the highest number of cases, amounting to 22.5% overall, followed by vascular complications (20% overall).
The volume of valvuloplasty procedures has significantly increased with respect to 2021: a 48.9% increase in aortic valvuloplasty and a 32.7% increase in pulmonary valvuloplasty. For the first time, most cases involving one of these 2 techniques involved patients older than 1 year. In aortic valvuloplasty, the rate of serious events (6.5%) decreased compared with the previous year (11.1%), although with 1 associated death. The report shows that pulmonary valvuloplasty has become established as one of the techniques with the best results, with a 99.2% efficacy rate and a 1.8% complication rate. These data support the value of pulmonary valvuloplasty as the technique of choice in congenital pulmonary valve stenosis in our setting. However, its mid- and long-term outcomes may be influenced by unspecified anatomical and genetic factors.10
Both in pulmonary angioplasty procedures (of native tract or ducts) and pulmonary branch angioplasty procedures, stent implantation has surpassed conventional balloon dilation as the technique of choice, which has again reduced the use of cutting balloons. The most widely performed aortic angioplasty procedures continue to be aortic arch and isthmus dilatation, which are performed in almost all patients; of note, in this context, the increase in covered stent implantation, which, for the first time, has surpassed other dilation techniques. This increase could be explained by the intention to improve the safety of the procedure by reducing damage to the aortic wall in certain scenarios.11 Furthermore, the availability of covered stents with lower implantation profiles has facilitated their use in pediatric patients of increasingly lower weight and younger age.12
ASD closure remained the most widely performed interventional technique in the registry (45.2% of all interventional catheterizations). The inclusion of patent foramen ovale closure as a procedure within this category and its classification as a congenital heart disease may be controversial but can be reevaluated in future reports. Its rarity in the pediatric setting contrasts with its increasing application in adults, confirming the maturity of the technique and the widespread acceptance of the scientific evidence supporting its use.13 Transesophageal echocardiography guidance remains the usual imaging modality for ASD closure; both intracardiac echocardiography and balloon sizing of the defect are infrequent.
A notable finding was the increasing use of patent ductus arteriosus closure in the group of premature newborns (9.4% overall), as well as confirmation of the preference for the transcatheter option over surgery for these pediatric patients in our setting.14 Antegrade venous access and the use of occlusive devices remain widespread procedures in a consolidated technique that has one of the best effectiveness rates in the registry (98.9%).
The reported data on the safety and efficacy of VSD closure show substantial improvement compared with previous reports: the major complication rate decreased from 18% in 2021 to 5.2% in 2022, while the success rate increased from 77.3% in 2021 to 96.7% in 2022. These figures reflect a change in trend, which could be related to the introduction of new closure devices, and the adoption of technical changes facilitating their approach.15-17 All of this would facilitate the widespread use of the procedure, whose frequency has increased significantly by up to 40.7% compared with the previous year. The increase in the number of cases registered in patients older than 18 years was notable, reaching 38% overall (compared with 22% in 2021).
A 16% volume increase and a significant improvement in the reported safety and efficacy data of transcatheter aortic valve implantation were also reported, of which approximately 60% were performed in patients older than 18 years. There was a decrease in the tricuspid position as the anatomical substrate for implantation (from 10 cases in 2021 down to only 2 cases in 2022), at a time when transcatheter aortic valve implantation has reached an unprecedented growth as a structural heart procedure in Spain.5 Access to new valves—especially self-expanding valves—and the continuous publication of scientific evidence endorsing the results of this technique, continue to enhance the expectations of the percutaneous management of patients with right ventricular outflow tract dysfunction in all anatomical scenarios.18,19
Limitations
The characteristics of this registry may be weakened by its retrospective, voluntary, and unaudited design. Expanding the collected data on certain techniques of special interest would help improve its quality and should be considered in future reports.
CONCLUSIONS
The main finding of this report is the significant increase in the number of interventional procedures recorded compared with previous years, which was closely related to the increase in participating centers. There has been significant growth in aortic valvuloplasty, ASD closure, and VSD closure procedures. The data obtained provide a realistic overview of interventional activity in congenital heart diseases in Spain among all age groups. The reported safety and efficacy results demonstrate the consolidation of most techniques in our setting and are consistent with those published in other international studies.
The incorporation of a greater number of centers with interventional activity in congenital heart diseases into the registry will optimize the quality and reliability of the information generated.
FUNDING
None declared.
ETHICAL CONSIDERATIONS
Due to the methodological characteristics of the study and its nature as solely an activity registry, there was no requirement for approval from ethics committees or signing of informed consent forms.
The characteristics of the present work exclude the consideration of possible variables of sex and gender.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
No artificial intelligence tools were used in the preparation of this article.
AUTHORS’ CONTRIBUTIONS
All authors contributed substantially to data collection and the critical review of this work. F. Ballesteros Tejerizo and F. Coserría Sánchez wrote the article.
CONFLICTS OF INTEREST
S. Ojeda Pineda is an associate editor of REC: Interventional Cardiology; the journal’s editorial procedure to ensure the impartial processing of the manuscript has been followed. The remaining authors declare no conflicts of interest.
WHAT IS KNOWN ABOUT THE TOPIC?
- Cardiac catheterization remains an indispensable procedure in the management of patients with congenital heart diseases.
- The existence of a national registry of pediatric percutaneous procedures and adult congenital heart diseases is essential to understand the current panorama of interventional cardiology in Spain and generate valuable information for professionals, patients, and families.
- The continuity of this registry allows understanding the level of implementation and results of various techniques, as well as their variation over time.
WHAT DOES THIS STUDY ADD?
- Some methodological changes and the gradual increase in the number of centers participating in the registry have enabled the collection of more realistic information on interventional activity for congenital heart diseases among all age groups in Spain.
- A highly significant increase in interventional procedures performed in 2022 was reported, with ASD and VSD closure and aortic valvuloplasty procedures being the techniques experiencing the greatest growth.
- The most widely performed procedures continue to be ASD closure, patent ductus arteriosus closure, and pulmonary artery branch angioplasty.
- The most frequent procedure-related adverse events were device embolizations and vascular complications.
SUPPLEMENTARY DATA

REFERENCES
1. Ballesteros Tejerizo F, Coserría Sánchez F, Romaguera R, et al. Spanish Cardiac Catheterization in Congenital Heart Diseases Registry. First Official Report from ACI-SEC and GTH-SECPCC (2020). REC Interv Cardiol. 2022;4:173-180.
2. Ballesteros Tejerizo F, Coserría Sánchez F, Freixa X, et al. Spanish cardiac catheterization in congenital heart diseases registry. Second official report from the ACI-SEC and the GTH-SECPCC (2021). REC Interv Cardiol. 2023;5:185-192.
3. Romaguera R, Ojeda S, Cruz-González I, et al. Spanish Cardiac Catheterization and Coronary Intervention Registry. 30th Official Report of the Interventional Cardiology Association of the Spanish Society of Cardiology (1990-2020) in the year of the COVID-19 pandemic. Rev Esp Cardiol. 2021;74:1096-1106.
4. Freixa X, Jurado-Roman A, Cid B, et al. Spanish cardiac catheterization and coronary intervention registry. 31st Official Report of the Interventional Cardiology Association of the Spanish Society of Cardiology (1990-2021). Rev Esp Cardiol. 2022;75:1040-1049.
5. Jurado-Román A, Freixa X, Cid B, et al. Spanish cardiac catheterization and coronary intervention registry. 32nd Official Report of the Interventional Cardiology Association of the Spanish Society of Cardiology (1990-2022). Rev Esp Cardiol. 2023;76:1021-1031.
6. Asociación de Cardiología Intervencionista de la Sociedad Española de Cardiología. Registro de Actividad ACI-SEC. Available at:http://www.registroactividadacisec.es. Accessed 21 Jun 2023.
7. Friedman KG, Tworetzky W. Fetal cardiac interventions:Where do we stand?Arch Cardiovasc Dis. 2020;113:121-128.
8. Kevin D, Wei Du, Fleming GA, et al. Validation and refinement of the catheterization RISK score for pediatrics (CRISP score):An analysis from the congenital cardiac interventional study consortium. Catheter Cardiovasc Interv. 2019;93:97-104.
9. Quinn BP, Ye M, Gauvreau K, et al. Procedural Risk in Congenital Cardiac Catheterization (PREDIC3T). J Am Heart Assoc. 2022;11:022832.
10. Hansen RL, Naimi I, Wang H, et al. Long-term outcomes up to 25 years following balloon pulmonary valvuloplasty:a multicenter study. Congenit Heart Dis. 2019;14:1037-1045.
11. Stassen J, De Meester P, Troost E, et al. Covered stent placement for treatment of coarctation of the aorta:immediate and long-term results. Acta Cardiol. 2021;76:464-472.
12. Al Balushi A, Pascall E, Jones MI, Qureshi S, Butera G. Initial experience with a novel ePTFE-covered balloon expandable stent in patients with near-atretic or severe aortic coarctation and small femoral arterial access. Cardiol Young. 2021;31:224-228.
13. Saver JL, Carroll JD, Thaler DE, et al.;RESPECT Investigators. Long-Term Outcomes of Patent Foramen Ovale Closure or Medical Therapy after Stroke. N Engl J Med. 2017;377:1022-1032.
14. Rodríguez-Ogando A, Ballesteros Tejerizo F, Blanco Bravo D, et al. Transcatheter Occlusion of Patent Ductus Arteriosus in Preterm Infants Weighing Less Than 2 kg With the Amplatzer Duct Occluder II Additional Sizes Device. Rev Esp Cardiol. 2018;71:861-876.
15. Alvarez-Fuente M, Carrasco JI, Insa B, et al. Percutaneous closure of ventricular septal defect with the KONAR-MF device. REC Interv Cardiol. 2022;4:181-185.
16. Rasines Rodri?guez A, Aristoy Zabaleta MM, Abelleira Pardeiro, et al. Retrograde closure of perimembranous ventricular septal defects. A paradigm shift. REC Interv Cardiol. 2023;5:73-75.
17. Nistor IA, Mesa Rubio D, Pan Álvarez-Ossorio M. Percutaneous VSD closure with the KONAR-MF occluder:fusion helps. Rev Esp Cardiol. 2023;77:106.
18. A?lvarez-Fuente M, Toledano M, Hernández I, et al. Initial experience with the new percutaneous pulmonary self-expandable Venus P-valve. REC Interv Cardiol. 2023;5:263-269.
19. Hascoët S, Bentham JR, Giugno L, et al. Outcomes of transcatheter pulmonary SAPIEN 3 implantation:an international registry. Eur Heart J. 2024;45:198-210.
* Corresponding author.
E-mail address: (F. Ballesteros Tejerizo).
 @luciotpadilla
@luciotpadilla