Original article
REC Interv Cardiol. 2025;7:6-14
Percutaneous treatment of the left main coronary artery in older adults. Impact of frailty on mid-term results
Tratamiento percutáneo del tronco coronario en ancianos. Impacto de la fragilidad en los resultados a medio plazo
aServicio de Cardiología, Hospital Universitario Reina Sofía, Cordoba, Spain bInstituto Maimónides de Investigación Biomédica de (IMIBIC), Cordoba, Spain cCentro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), Spain dDepartamento de Medicina, Universidad de Córdoba, Cordoba, Spain ◊These authors contributed equally as senior authors.

ABSTRACT
Introduction and objectives: Ultrathin-strut stents (UTS) represent a significant advancement in percutaneous coronary intervention. This study aimed to evaluate the safety and short- to mid-term outcomes of stenting with the thinnest struts on the market (50 μm) using a biodegradable everolimus-eluting polymer (Evermine 50) in real-world patients with coronary artery disease.
Methods: A single-arm, multicenter, prospective study was conducted in real-world patients. A total of 161 patients with de novo lesions who received at least 1 UTS stent were enrolled. The primary safety endpoint was the occurrence of major adverse cardiovascular events, defined as cardiac death, target-vessel myocardial infarction, or the need for revascularization of the target lesion at 12 months. The incidence of stent thrombosis at 12 months was also analyzed.
Results: The study included 161 patients with a mean age of 64 ± 14 years; 79% were male, 34% had diabetes, and 66% had hypertension. The most common indication for intervention was non-ST-segment elevation myocardial infarction (42%), followed by ST-segment elevation myocardial infarction (22%). The procedural success rate was 100%. At 12 months of follow-up, the incidence of MACE was 2.5%, and the definite stent thrombosis rate was 1.3%.
Conclusions: The use of the 50 μm UTS stent with a biodegradable everolimus-eluting polymer demonstrated a favorable safety profile and good clinical outcomes in unselected patients at 1 year of follow-up.
Keywords: Coronary artery disease. Percutaneous coronary intervention. Ultrathin struts.
RESUMEN
Introducción y objetivos: Los stents de struts ultrafinos (SUF) constituyen una mejora en el campo del intervencionismo coronario percutáneo. El objetivo de este estudio fue evaluar la seguridad y los resultados a corto y medio plazo del stent con los struts más finos del mercado (50 μm), con polímero biodegradable y liberador de everolimus (Evermine 50), en pacientes del mundo real con enfermedad coronaria.
Métodos: Se diseñó un estudio prospectivo, multicéntrico, de un solo grupo, en pacientes del mundo real. Se incluyeron 161 pacientes con lesiones de novo en los que se implantó al menos 1 stent de SUF. La variable principal de seguridad fueron los eventos adversos cardiovasculares mayores, compuesto de muerte cardiaca, infarto de miocardio atribuido al vaso diana y necesidad de revascularización de la lesión diana a los 12 meses de seguimiento. También se analizó la incidencia de trombosis del stent a los 12 meses del procedimiento.
Resultados: De los 161 pacientes incluidos (edad media 64 ± 14 años; 79% varones), el 34% eran diabéticos y el 66% eran hipertensos. La indicación más frecuente fue infarto sin elevación del segmento ST (42%), con un 22% de casos en contexto de infarto con elevación del segmento ST. El porcentaje de éxito del procedimiento fue del 100%. A los 12 meses de seguimiento, la incidencia de eventos adversos cardiovasculares mayores fue del 2,5%, con una tasa de trombosis del stent definitiva del 1,3%.
Conclusiones: El uso de stent con SUF de 50 μm, con polímero biodegradable y liberador de everolimus en pacientes no seleccionados mostró unos buenos resultados clínicos, así como un buen perfil de seguridad a 1 año de seguimiento.
Palabras clave: Enfermedad coronaria. Intervencionismo coronario percutaneo. Strut ultrafino.
Abbreviations
MACE: major adverse cardiovascular events. MI: myocardial infarction. PCI: percutaneous coronary intervention. ST: stent thrombosis. STEMI: ST-segment elevation myocardial infarction. UTS: ultra-thin strut.
INTRODUCTION
Percutaneous coronary intervention (PCI) has grown exponentially along with the technological evolution associated with this procedure. The continuous advancement of technology has enabled the development of stents with thinner struts, which offer a series of advantages over stents with thicker struts. One of the advantages of these new stents is the improved device profile—with increased flexibility—providing better navigability and greater lesion crossing capability. On the other hand, ultra-thin struts (UTS) cause fewer disturbances to normal laminar blood flow at target lesion level, due to the reduced protrusion of material into the vascular lumen. This seems to be associated with a lower degree of platelet activation and muscle cell proliferation,—the processes involved in stent failure—in terms of stent thrombosis (ST) and in-stent restenosis.1,2 In lesions located in small caliber vessels (≤ 2.5 mm), the use of UTS could provide additional advantages due to a higher ratio between the size of the struts and the lesion luminal area.3 Furthermore, UTS stents seem to be associated with less acute damage to the vascular endothelium during stent deployment. This reduced initial aggression could diminish the barotrauma-related inflammatory response and, therefore, prevent in-stent restenosis and promote faster device endothelialization.4,5 Studies have indicated that the use of UTS stents could be associated with lower rates of in-stent restenosis and a reduced need for new revascularizations.6,7
The Evermine 50 EES stent (Meril Life Sciences, India) is a UTS (50 μm) stent with CE marking consisting of a cobalt-chromium alloy platform with an everolimus-eluting biodegradable polymer. The aim of this study was to evaluate the 1-year safety and efficacy outcomes after UTS stent deployment in real-world patients with coronary artery disease.
METHODS
We conducted a prospective, non-randomized, multicenter study with patients who underwent UTS stent deployment at 4 different Spanish hospitals (data from the Everythin Registry). To be included in the study, patients had to be older than 18 years, with available coronary angiographies in the context of chronic or acute coronary syndrome, and have, at least, 1 target lesion with a 2 mm up to 4.5 mm reference vessel diameter on visual estimation. Overlapping stents was ill-advised and, if necessary, the overlap length should be ≤ 2 mm. PCI in multiple vessels and lesions during the same surgical act was allowed, and deferred procedures within the first 90 days since the initial procedure were also accepted. In these cases, any further procedures were not coded as an event—i.e. need for new revascularization—but as scheduled procedures. Only 1 case—1 target lesion treated with UTS stent deployment—was counted per patient. Deploying the study UTS stent was not mandatory in any of the other treated lesions, only in the target lesion/vessel.
The study followed the privacy policy of each research center, including regulations for the appropriate use of data from patient research. The study was approved by the Ethics Committee for Drug Research of the coordinating center. Moreover, the study was conducted in full compliance with the terms set forth in the Declaration of Helsinki. All patients signed specific informed consent forms prior to being included in the study.
Study device and procedure
The Evermine 50 EES (Meril Life Sciences, India) is a UTS (50 μm) stent with a cobalt-chromium platform coated with a biodegradable polymer composed of poly-L-lactic acid and poly(lactic-co-glycolic) acid. The Evermine stent—which has a hybrid design with an open cell in its central part and a closed cell at the edges—releases everolimus (1.25 μg/mm²) as the antiproliferative drug. The stent has received the corresponding CE marking and is available in several lengths from 8 mm up to 48 mm with diameters ranging from 2 mm up to 4.5 mm. The main features of the Evermine 50 EES device are illustrated in figure 1.

Figure 1. A: illustrative image of the Evermine 50 stent (Meril Life Sciences, India). B: description of the main characteristics of the stent. C: comparison of the study stent strut thickness vs major competing next-generation stents. PLGA, poly(lactic-co-glycolic acid); PLLA, poly-L-lactic acid (Images courtesy of Meril Life Sciences. Images reproduced with permission from Meril Life Sciences or its affiliates.)
PCI was performed following each center routine practice within the recommendations outlined in the clinical practice guidelines.8 The PSP algorithm (predilation, sizing [stent size selection], and postdilation) was recommended for optimal device implantation. The study protocol recommended postdilation, especially in cases where any degree of underexpansion was identified immediately after device implantation. Although the study protocol recommended the use of intravascular imaging modalities to guide the procedure, this was left to the operator’s discretion. All patients received a 300 mg loading dose of acetylsalicylic acid prior to the intervention followed by a loading dose of a second antiplatelet agent—clopidogrel, prasugrel, or ticagrelor—after the PCI, which was maintained for 3 up to 12 months and left to the discretion of the responsible investigator of the center.
Endpoints and definitions
The primary endpoint of the study was the occurrence of major adverse cardiovascular events (MACE) at 12 months. MACE were defined as the composite of cardiac death, non-fatal target vessel myocardial infarction (MI), or the need for target lesion revascularization. Secondary endpoints included each individual component of the composite endpoint, the overall mortality and ST (both definite and probable) according to the definitions of the Academic Research Consortium9 12 months after implantation. Additionally, the rates of device and procedural success were taken into consideration. Device success was defined as the deployment of the study stent in the target lesion with a final percent diameter residual stenosis < 30% by visual estimation. Procedural success was defined as the success of the device without any in-hospital complications, including death, MI, and target lesion revascularization.
Statistical analysis
Quantitative variables are expressed as mean and standard deviation or as median and interquartile range [IQR], depending on their distribution. Categorical variables are expressed as number and percentage. All analyses were performed using the statistical tool STATA 12 (StataCorp LLC, United States).
RESULTS
Demographic and baseline clinical characteristics
A total of 161 patients were included in the study from November 2020 through April 2022 whose demographic data and clinical characteristics are shown in table 1. The mean age was 64 ± 14 years, and 79% were male. A total of 66% of the patients were hypertensive; 53% had dyslipidemia; 34%, diabetes mellitus, and 59% a history of smoking. A total of 20% of the patients had experienced a prior MI, and 22% a previous PCI. The most common indication for the intervention was the diagnosis of non-ST-segment elevation acute myocardial infarction (42%), followed by ST-segment elevation myocardial infarction (STEMI) (22%) and chronic coronary syndrome (21%).
Table 1. Baseline characteristics of the study population
| Basal characteristics | Patients (n = 161) |
|---|---|
| Age (years) ± SD | 64 ± 14 |
| Male, n (%) | 126 (79) |
| BMI (kg/m²) | 28 ± 3.5 |
| Hypertension, n (%) | 106 (66) |
| Dyslipidemia, n (%) | 86 (53) |
| Diabetes mellitus, n (%) | 55 (34) |
| Smoking status, n (%) | |
| Non-smoker | 65 (40) |
| Former smoker | 49 (30) |
| Current smoker | 47 (29) |
| Previous AMI, n (%) | 33 (20) |
| Previous stroke, n (%) | 2 (1.2) |
| Atrial fibrillation, n (%) | 7 (4.3) |
| Peripheral vascular disease, n (%) | 10 (6.2) |
| Previous coronary angioplasty, n (%) | 36 (22) |
| Previous coronary artery bypass grafting, n (%) | 4 (2.5) |
| COPD, n (%) | 13 (8) |
| Chronic kidney disease, n (%) | 14 (9) |
| Glomerular filtration rate (mL/min/1.73 m²) | 61 ± 10 |
| Left ventricular function, (%) | 55 ± 11 |
| Indication for coronary angiography, n (%) | |
| Chronic coronary syndrome | 34 (21) |
| Unstable angina | 24 (15) |
| NSTEMI | 67 (42) |
| STEMI | 36 (22) |
|
AMI, acute myocardial infarction; BMI, body mass index; COPD, chronic obstructive pulmonary disease; NSTEMI, non-ST-segment elevation acute myocardial infarction; SD, standard deviation; STEMI, ST-segment elevation myocardial infarction. |
|
Angiographic and procedural characteristics
The lesion angiographic characteristics, and the results of the intervention are shown in table 2. Most patients had significant single-vessel disease (71%), being the presence of 2 or 3-vessel disease far less common (20% and 9%, respectively). The most widely treated vessel was the left anterior descending coronary artery (54%), followed by the right coronary artery (27%) and the left circumflex artery (17%). The target lesion median percent diameter stenosis by visual estimation was 90% [IQR, 75-99]. A total of 29% of the target lesions showed some degree of calcification on angiography. Intracoronary imaging modalities (7% optical coherence tomography) were used to guide the PCI in 11% of the cases. The mean number of stents deployed per lesion was 1.04 ± 0.22, with a median stent diameter of 3.0 mm [IQR 2.75-3.5] and a median stent length of 19 mm [IQR 19-24]. Pre- and postdilation were performed in 71% and 39% of the cases, respectively. The device and procedural success rates were 100%, without any procedure-related complications being reported in patients treated during the inpatient period.
Table 2. Angiographic, procedural and clinical follow-up characteristics of the cohort
| Angiographic and procedural characteristics | Patients (n = 161) |
|---|---|
| Radial access, n (%) | 158 (98) |
| Diseased vessels, n (%) | |
| 1-vessel disease | 114 (71) |
| 2-vessel disease | 32 (20) |
| 3-vessel disease | 15 (9) |
| Target lesion location, n (%) | |
| Left main coronary artery | 3 (1.8) |
| Proximal left anterior descending coronary artery | 37 (23) |
| Mid left anterior descending coronary artery | 40 (24.8) |
| Distal left anterior descending coronary artery | 10 (6.2) |
| Proximal left circumflex artery | 10 (6.2) |
| Mid left circumflex artery | 11 (6.8) |
| Distal left circumflex artery | 6 (3.7) |
| Proximal right coronary artery | 13 (8) |
| Mid right coronary artery | 18 (11.2) |
| Distal right coronary artery | 13 (8) |
| Bifurcation lesions, n (%) | 12 (7.5) |
| Calcified lesions, n (%) | 46 (29) |
| Visual percent diameter stenosis, median [IQR] | 90 [75-99] |
| Predilation, n (%) | 114 (71) |
| Postdilation, n (%) | 63 (39) |
| Intracoronary imaging modalities, n (%) | 18 (11) |
| Optical coherence tomography | 11 (7) |
| Intravascular ultrasound | 7 (4) |
| No. of stents deployed, mean ± SD | 1.04 ± 0.22 |
| Stent diameter (mm), median [IQR] | 3.0 [2.75-3.5] |
| Stent length (mm), median [IQR] | 19 [19-24] |
| Device success, n (%) | 161 (100) |
| Procedural success, n (%) | 161 (100) |
| Antiplatelet therapy after PCI, n (%) | |
| Acetylsalicylic acid | 161 (100) |
| Clopidogrel | 78 (48) |
| Ticagrelor | 68 (42) |
| Prasugrel | 15 (9) |
| Clinical follow-up | |
| 12-month follow-up, n (%) | 158 (98) |
| MACE, n (%) | 4 (2.5) |
| Cardiac death | 1 (0.6) |
| Target vessel MI | 2 (1.3) |
| Target lesion revascularization | 2 (1.3) |
| Overall mortality, n (%) | 3 (1.9) |
| Stent thrombosis, n (%) | |
| Definite | 2 (1.3) |
| Probable | 1 (0.6) |
|
IQR, interquartile range; MACE, major adverse cardiovascular events; MI, myocardial infarction; PCI, percutaneous coronary intervention; SD, standard deviation. |
|
Clinical outcomes at the follow-up
The 12-month follow-up was completed in 158 patients (98%). One year after implantation, 4 patients exhibited MACE (2.5%), and 3 patients died (1.9%). The cause of death was cardiac in 1 patient (due to a probable ST 7 days after the procedure) and non-cardiac in the remaining 2 (one due to lung neoplasm and the other to multiple organ failure). There were 2 non-fatal MIs (1.3%), both due to late definite ST (1 occurred 8 months after stent deployment and was associated with the study UTS stent, while the other one occurred 9 months after deployment due to a different thrombosed non-UTS stent implanted in a lesion of the target lesion same vessel. Only 2 patients required target lesion revascularization at the follow-up (1 due to ST and the other one due to in-stent restenosis).
DISCUSSION
The present study prospectively and multicentrically evaluates the safety and efficacy profile of implanting an UTS stent in a real-world population. Its main findings are that the UTS stent demonstrated a high procedural success rate, without in-hospital complications, acceptable midterm clinical outcomes, and a 2.5% rate of MACE 12 months after implantation.
The baseline characteristics of the study population are similar to the ones reported in previous studies that analyzed various stent technologies in patients with atherosclerotic coronary artery disease.10-12 However, it is noteworthy that in this study, 79% of cases were performed in the context of an acute coronary syndrome, including 22% of patients diagnosed with STEMI. In acute coronary syndrome—especially STEMI—there are factors associated with poorer outcomes of the implanted device, both in the short and long term. Firstly, the state of generalized vasoconstriction of the coronary tree and high thrombotic burden can complicate the appropriate selection of the size of the stent, thus leading to the implantation of smaller devices in relation to the actual size of the vessel, a mechanism involved in ST and in-stent restenosis. Furthermore, in the context of acute lesions, there is a higher risk of embolization and no-reflow or slow-flow phenomena, which can sometimes condition suboptimal final outcomes in terms of distal coronary flow, involving a greater risk of further ST. In our study, no ST occurred in patients with an early diagnosis of STEMI. Although it is worth mentioning that the results of the study stent were good—even in demanding contexts such as STEMI—the absolute number of STEMI patients included was low, meaning that data should be contrasted in larger series.
UTS stents provide better navigability, flexibility, and conformity to the vessel geometry. However, there may be doubts on whether the presence of UTS can lead to a reduction of the stent radial strength, which could have further implications for treating more unfavorable lesions, such as calcified lesions. Although, in the present study, 29% of the treated lesions showed some degree of calcification on angiography, the success rate of the stent reached 100%. This demonstrates the good performance of this UTS stent across different scenarios, achieving excellent radial strength even in the most challenging situations, such as calcified coronary lesions. These results are especially relevant in the specific context of the study, where, despite the recommendation for systematic postdilation, the final rate of stent postdilation was relatively low (39%).
Previous studies have consistently shown good clinical follow-up results for UTS stents with low rates of ST.13-15 The reason for this low rate of ST would be strut thickness per se, which would favor early neointimal coverage, thereby reducing the risk of ST (especially late and very ST).4 In the specific case of the study device (Evermine 50 EES), Patted et al.13 described the 6-month follow-up results of 251 patients. In this single-center, prospective experience, the authors describe a 6-month rate of MACE of 0.8%, with no ST at the follow-up. Regarding differences with respect to our series, nearly one-third of the cases were procedures in asymptomatic patients or with silent ischemia. Additionally, the rate of postdilation (57%) was higher than that of our cohort, which may have influenced the ST outcomes. The same group retrospectively described the results of 171 patients treated with the Evermine 50 EES stent,16 with 2-year rates of procedural success and MACE of 100% (same as in our study) and 2.4%, respectively. Again, the authors noted the absence of definite or probable ST at the follow-up. In this single-center cohort, the rate of stent postdilation was not reported, which may have implications for the prevention of MACE, especially ST. A meta-analysis that analyzed various types of UTS stents found no significant differences in the likelihood of stent failure, including ST across different stents with struts < 70 μm.17 In the present study, although the 1-year rate of definite ST after stent deployment was 1.3%, only 1 of these STs was attributed to the study device. The rate of ST is similar to that of other real-world experiences with second and third-generation stents,18-20 which confirms the good performance of the Evermine 50 EES in unselected real-world patients.
Limitations
The main limitations of the study are the relatively low number of patients included, and the absence of a comparator group. Furthermore, although the events reported at the follow-up were reviewed by the principal investigator of the coordinating center based on the case reports submitted by each principal investigator from the collaborating centers, these events were not allocated by an independent event adjudication committee. The fact that, in our cohort, few intracoronary imaging modalities were used to guide the PCI—reflecting real clinical practice—could be interpreted as a limitation of the study.
CONCLUSIONS
With data from a prospective, multicentric study of real-world patients, the PCIs performed with a 50 μm UTS stent, with a biodegradable polymer and everolimus elution had good clinical outcomes and a favorable safety profile at the 12-month follow-up.
FUNDING
None declared.
ETHICAL CONSIDERATIONS
The study was approved by the Drug Research Ethics Committee of the coordinating center. The study was conducted in full compliance with the terms outlined in the Declaration of Helsinki. All patients signed specific informed consent forms prior to the intervention and before being included in the study.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
No artificial intelligence was used for this work.
AUTHORS’ CONTRIBUTIONS
J. Casanova-Sandoval and M. García-Guimarães participated in the conception and design of the study, analysis and interpretation of results, and drafting the manuscript. G. Miñana Escrivà, E. Bosch-Peligero, J.F. Muñoz-Camacho, D. Fernández-Rodríguez, K. Rivera, A. Fernández-Cisnal, and D. Valcárcel-Paz participated in data acquisition and critically reviewed the content of the manuscript. All authors gave their final approval for the publication of the latest draft of the manuscript.
CONFLICTS OF INTEREST
None declared.
WHAT IS KNOWN ABOUT THE TOPIC?
- The use of UTS stents may be associated, through various mechanisms, with better clinical outcomes compared with thicker-strut stents. Previous studies suggest that UTS stents are associated with less stent failure, preventing in-stent restenosis and ST.
WHAT DOES THIS STUDY ADD?
- In this prospective, multicentric study of real-world patients, the use of a 50 μm UTS stent with a biodegradable polymer and everolimus elution was associated with good clinical outcomes, and a favorable safety profile at the 12-month clinical follow-up.
REFERENCES
1. Duraiswamy N, Schoephoerster RT, Moreno MR, Moore JE. Stented artery flow patterns and their effects on the artery wall. Annu Rev Fluid Mech. 2007;39:357-382.
2. Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium:Pathophysiological basis and clinical perspectives. Physiol Rev. 2011;91:327-387.
3. Buiten RA, Ploumen EH, Zocca P, et al. Outcomes in Patients Treated with Thin-Strut, Very Thin-Strut, or Ultrathin-Strut Drug-Eluting Stents in Small Coronary Vessels:A Prespecified Analysis of the Randomized BIO-RESORT Trial. JAMA Cardiology. 2019;4:659-669.
4. Miura T, Ueki Y, Senda K, et al. Early vascular response of ultra-thin bioresorbable polymer sirolimus-eluting stents assessed by optical frequency domain imaging:the EVALUATION study. Cardiovasc Interv Ther. 2021;36:281-288.
5. Otaegui Irurueta I, González Sucarrats S, Barrón Molina JL, et al. Can an ultrathin strut stent design and a polymer free, proendothelializing probucol matrix coating improve early strut healing?The FRIENDLY-OCT trial. An intra-patient randomized study with OCT, evaluating early strut coverage of a novel probucol coated polymer-free and ultra-thin strut sirolimus-eluting stent compared to a biodegradable polymer sirolimus-eluting stent. Int J Cardiol. 2022;360:13-20.
6. Kastrati A, Mehilli J, Dirschinger J, et al. Intracoronary stenting and angiographic results:Strut thickness effect on restenosis outcome (ISAR-STEREO) trial. Circulation. 2001;103:2816-2821.
7. Farb A, Weber DK, Kolodgie FD, Burke AP, Virmani R. Morphological predictors of restenosis after coronary stenting in humans. Circulation. 2002;105:2974-2980.
8. Neumann F-J, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87-165.
9. Garcia-Garcia HM, McFadden EP, Farb A, et al. Standardized End Point Definitions for Coronary Intervention Trials. Eur Heart J. 2018;39:2192-2207.
10. Nakamura M, Kadota K, Nakagawa Y, et al. Ultrathin, Biodegradable-Polymer Sirolimus-Eluting Stent vs Thin, Durable-Polymer Everolimus-Eluting Stent. JACC Cardiovasc Interv. 2022;15:1324-1334.
11. de la Torre Hernández JM, Ocaranza Sanchez R, Santas Alvarez M, et al. Comparison of One-Year Outcomes Between the ihtDEStiny BD Stent and the Durable-Polymer Everolimus- and Zotarolimus-Eluting Stents:A Propensity-Score-Matched Analysis. Cardiovasc Revasc Med. 2021;31:1-6.
12. De Silva K, Li Kam Wa ME, Wells T, et al. The everolimus eluting Synergy MegatronTM drug-eluting stent platform:Early outcomes from the European Synergy MegatronTM Implanters'Registry. Catheter Cardiovasc Interv. 2023;102:1222-1228.
13. Patted SV, Patted AS, Turiya PK, Thakkar AS. Clinical Outcomes of World's Thinnest (50 µm) Strut Biodegradable Polymer Coated Everolimus-Eluting Coronary Stent System in Real-World Patients. Cardiol Res. 2018;9:370-377.
14. Araujo GN, Machado GP, Moura M, et al. Real-World Assessment of an Ultrathin Strut, Sirolimus-Eluting Stent in Patients with ST-Elevation Myocardial Infarction Submitted to Primary Percutaneous Coronary Intervention (INSTEMI Registry). Arq Bras Cardiol. 2023;120:e20220594.
15. Kasturi S, Polasa S, Sowdagar MA, et al. Safety and Clinical Performance of Biodegradable Polymer-Coated Ultra-Thin Everolimus-Eluting Stents in “Real-World“Patients:A Multicenter Registry (PERFORM-EVER). Anatol J Cardiol. 2022;26:619-628.
16. Patted SV, Thakkar AS. Clinical outcomes of ultrathin strut biodegradable polymer-coated everolimus-eluting stent in patients with coronary artery disease. ARYA Atheroscler. 2020;16:130-135.
17. Marengo G, Bruno F, Scudeler L, et al. Comparison Among Ultra-Thin Coronary Stents:A Network Meta-Analysis. Am J Cardiol. 2024;216:9-18.
18. Park S, Rha S-W, Choi BG, et al. Efficacy and Safety of Sirolimus-Eluting Stent with Biodegradable Polymer UltimasterTM in Unselected Korean Population:A Multicenter, Prospective, Observational Study From Korean Multicenter Ultimaster Registry. Korean Circ J. 2024;54:339-350.
19. Yu HY, Ahn J, Choi BG, et al. Three-Year Clinical Outcomes With the Cilotax Dual Drug-Eluting Stent vs Everolimus-Eluting Stents in Patients With Acute Myocardial Infarction. Texas Hear Inst J. 2024;51:e238271.
20. Nakao S, Ishihara T, Tsujimura T, et al. Two-year real world clinical outcomes after intravascular imaging device guided percutaneous coronary intervention with ultrathin-strut biodegradable-polymer sirolimus-eluting stent. Int J Cardiol. 2024;399:131686.

ABSTRACT
Introduction and objectives: Transcatheter aortic valve implantation (TAVI) for pure aortic regurgitation is challenging due to inadequate device anchoring and increased risks of device embolization and paravalvular regurgitation (PVR). This study aimed to review the safety and efficacy of TAVI for aortic regurgitation with devices specifically designed for this indication.
Methods: A comprehensive search of PubMed, Web of Science, Cochrane Library, and major conference archives up to April 2024 identified 143 unique results based on predefined criteria.
Results: Fifteen studies (n = 788 patients) were included, with J-Valve used in 357 patients and JenaValve in 431. Men represented 51% of the cohort, with a mean age of 74.7 ± 8.8 years and an STS-PROM score of 5.8 ± 4.9%. Transapical and transfemoral access routes were used in 62.7% and 37.3% of patients, respectively. Overall, procedural success was achieved in 95.9% of cases; surgical conversion was required in 1.8%, device migration/embolization occurred in 3.2%, and a second valve (in-valve) was required in 2.0% of patients. At 30 days, 95.5% of patients were alive, and device success was reported in 93.3% of cases. Mild PVR was observed in 18.0% of patients, moderate-to-severe PVR in 1.7%, and permanent pacemaker implantation (PPI) was required in 13.0%. In studies focusing on transfemoral procedures (all using JenaValve), the pooled estimates showed a procedural success rate of 97.8% (95%CI, 94.4-100), device success of 97.0% (95%CI, 94.8-99.2), 30-day mortality of 1.96% (95%CI, 0.20-3.72), moderate-to-severe PVR of 0.47% (95%CI, 0.00-1.47), and PPI requirement of 18.7% (95%CI, 13.9-23.4)
Conclusions: This systematic review of relatively small observational studies demonstrates the safety and favorable early outcomes of TAVI using J-Valve and JenaValve in patients with pure aortic regurgitation, especially when the transfemoral approach is used. Nevertheless, the need for PPI remains frequent.
Keywords: Aortic regurgitation. Transcatheter aortic valve implantation. Outcome. Systematic review. J-Valve. JenaValve.
RESUMEN
Introducción y objetivos: El implante percutáneo de válvula aórtica (TAVI) para la insuficiencia aórtica pura es un reto debido al anclaje inadecuado del dispositivo y al mayor riesgo de su embolización y de fuga periprotésica (FPP). Nuestro objetivo fue revisar la seguridad y la eficacia del TAVI para la insuficiencia aórtica con dispositivos dedicados a esta indicación.
Métodos: Una búsqueda exhaustiva mediante criterios predefinidos en PubMed, Web of Science y Cochrane Library, así como en los principales archivos de congresos hasta abril de 2024, identificó 143 resultados únicos.
Resultados: Se incluyeron 15 estudios (n = 788 pacientes), en los que se utilizó J-Valve en 357 pacientes y JenaValve en 431. El 51% eran varones, la edad media fue de 74,7 ± 8,8 años y la puntuación STS-PROM fue de 5,8 ± 4,9%. Se utilizaron accesos transapicales y transfemorales en el 62,7 y el 37,3% de los casos respectivamente. En general, la intervención fue satisfactoria en el 95,9% de los casos; se requirió conversión quirúrgica en el 1,8%, se produjo migración/embolización del dispositivo en el 3,2% y fue necesaria una segunda válvula (in-valve) en el 2%. A los 30 días, el 95,5% de los pacientes estaban vivos y el éxito del dispositivo se alcanzó en el 93,3%. Se observó una FPP leve en el 18,0% y una FPP moderada-grave en el 1,7%, mientras que en el 13,0% fue necesario implantar un marcapasos permanente. En los estudios de intervenciones transfemorales (todas con JenaValve), la estimación conjunta del éxito de la intervención fue del 97,8% (IC95%, 94,4-100), del éxito del dispositivo fue del 97,0% (IC95%, 94,8-99,2), de la mortalidad a 30 días fue del 1,96% (IC95%, 0,20-3,72), de la FPP moderada-grave fue del 0,47% (IC95%, 0,0-1,47) y del implante de marcapasos permanente fue del 18,7% (IC95%, 13,9-23,4).
Conclusiones: Esta revisión sistemática de estudios observacionales relativamente pequeños demuestra la seguridad y los resultados precoces favorables del TAVI con J-Valve y JenaValve en pacientes con insuficiencia aórtica pura, en especial cuando se utiliza el abordaje transfemoral. No obstante, la necesidad de marcapasos permanente sigue siendo frecuente.
Palabras clave: Insuficiencia aórtica. Válvula aórtica percutánea. Resultados. Revisión sistemática. J-Valve. JenaValve.
Abbreviations
AoR: aortic regurgitation. NYHA: New York Heart Association. PPI: permanent pacemaker implantation. PVR: paravalvular regurgitation. TAVI: transcatheter aortic valve implantation.
INTRODUCTION
Aortic regurgitation (AR) results from abnormalities in the valve cusps or the structures supporting them (ie, the aortic root and annulus).1 The prevalence of AR increases with age, affecting 2% of people older than 70 years.2,3 Patients with severe AR have impaired functional capacity and increased mortality compared with the general population.2,4
If left untreated, severe AR leads to left ventricular dysfunction and heart failure in approximately 50% of patients.2 Although surgical aortic valve replacement is the recommended treatment for symptomatic severe AR,5 many elderly patients with this condition are refused surgery due to high operative risk.6
Since the introduction of transcatheter aortic valve implantation (TAVI) in 2002, it has demonstrated good safety and efficacy in various patient groups and several anatomical contexts.7-13 However, due to the high stroke volume, the lack of aortic annular calcification, and the frequent dilatation of the aortic root/annulus, TAVI for pure native AR is associated with an increased risk of adverse events including device dislocation and paravalvular regurgitation (PVR).14 The J-Valve(J.C. Medical, United States) and the JenaValve (JenaValve Technology GmbH, United States) are dedicated, next-generation, self-expanding transcatheter valves designed to address the challenges associated with native pure AR.15,16
To date, the evidence on the safety and efficacy of these technologies in native pure AR is limited. We conducted a systematic review of the current data on the safety and efficacy of TAVI using the J-Valve or JenaValve in patients with native pure AR.
METHODS
This systematic review and associated meta-analysis were conducted in accordance with the standards outlined in the PRISMA statement and the Cochrane Handbook for Systematic Reviews of Interventions (version 5.1.0).17,18 The study protocol was prospectively registered (PROSPERO registration number: CRD42023460306).
Data collection
We included studies that involved a minimum of 10 patients who underwent TAVI with the J-Valve or JenaValve for native pure or predominant AR. Studies were excluded if they involved mixed aortic valve disease (moderate to severe stenosis and regurgitation) or prior aortic valve replacement (valve-in-valve procedures).
Information sources, search strategy, and study selection
Three online databases (PubMed, Web of Science, and Cochrane Library) were searched up to March 2024 using the following search terms: ((aortic valve insufficiency OR aortic regurgitation OR regurgitant aortic valve OR aortic incompetency OR incompetent aortic valve OR NAVR OR noncalcific aortic valve) AND (transcatheter aortic valve replacement OR transcatheter aortic valve OR transfemoral aortic valve OR transaortic aortic valve OR transapical aortic valve OR transcutaneous aortic valve OR percutaneous aortic valve OR TAVI OR TAVR) AND (J-Valve OR JenaValve)). Additional relevant studies were identified through a manual search of secondary sources, including references of initially identified articles, reviews, commentaries, and archives of major cardiology conferences.
Endnote software (Clarivate Analytics, United States) was used to remove duplicates. The retrieved references were screened in 2 steps: first, all authors independently screened the titles and abstracts to determine their relevance, and second, the full-text articles of the identified abstracts were reviewed for final eligibility in the quantitative analysis. The Rayyan website was used in the selection process.19 For overlapping study populations, the most recent publication was chosen for inclusion.
Data extraction and outcomes
The data were extracted into a standardized data extraction sheet, which included: a) study characteristics, b) the patients’ baseline characteristics, c) echocardiographic and computed tomographic data, d) procedural data, and e) short-term clinical outcomes.
The main endpoints of the current investigation were device success, procedural success, and 30-day all-cause mortality. Additional outcomes of interest included bleeding, vascular complications, stroke, permanent pacemaker implantation (PPI), and PVR within 30 days.
Assessing the risk of bias
The quality of the retrieved studies was evaluated according to the Cochrane Handbook for Systematic Reviews of Interventions (version 5.1.0, updated March 2011). The risk of bias was assessed using appropriate tools based on the study design: the National Institutes of Health (NIH) tool for single-arm observational studies, the Newcastle-Ottawa Scale (NOS) for comparative observational studies, and the NIH tool for case-series studies. The individual studies were classified as ‘Low risk’ or ‘Good,’ ‘High risk’ or ‘Poor,’ and ‘Unclear risk’ or ‘Fair’ of bias.
Assessment of heterogeneity
The statistical heterogeneity among the studies was assessed using the chi-square test, specifically the Cochrane Q test. The chi-square statistic, known as Cochrane Q, was used to compute the I-squared value using the following formula: I2 = ([Q – df] / Q) × 100%. Significant heterogeneity was defined as a chi-square P value < .1. An I-squared value equal to or more than 40% was considered indicative of a significant level of heterogeneity.
Quantitative analysis
The DerSimonian and Laird meta-analysis approach was used to obtain the pooled effect size for all outcomes. Proportions and 95% confidence intervals (95%CI) were computed using R software (version 4.3.1 for Windows) and the Meta package.
A random-effects model, which gives relatively higher weight to smaller studies to account for heterogeneity, was used when heterogeneity was deemed significant. A fixed-effects model was chosen when heterogeneity was lower. Consequently, the predicted effects in our meta-analysis are conservative estimates that account for potential inconsistencies.
Certainty assessment
A certainty evaluation was performed using sensitivity analysis (leave-one-out meta-analysis) to test the robustness of the evidence. This analysis was conducted using R software (version 4.3.1 for Windows) with the Meta package and Metainf function. Sensitivity analyses were was run in several scenarios for each outcome in the meta-analysis, eliminating one study in each scenario, to ensure that the overall effect size was not dependent on any single study.
RESULTS
Literature search
Our search identified 143 results after duplicates were removed. Following title and abstract screening, 29 articles were selected for full-text review. Of these, 15 studies6,14,20-32 were included in the systematic review, with 5 studies of transfemoral TAVI being included in the quantitative meta-analysis. No further articles were included after manually searching the references of the included studies. The selection process is illustrated in a PRISMA flow diagram (figure 1). According to the NIH and NOS scales for quality assessment, the overall quality of the included studies was rated as good for all investigations, as shown in the supplementary data.
Figure 1. PRISMA flow diagram of the study.
Patient and procedural characteristics
Overall, 788 patients underwent TAVI for native pure or predominant AR (J-Valve, 357 patients; JenaValve, 431 patients). Most J-Valve procedures were performed in China, while most JenaValve procedures were conducted in Europe and North America. The average surgical risk was elevated but showed significant variability, with Log EuroSCORE at 22.8 ± 12.3, EuroSCORE II at 7.1 ± 6.6, and Society of Thoracic Surgeons - Predicted Risk of Mortality (STS-PROM) at 5.9 ± 4.7.
The mean age was 73.6 ± 7.3 years for J-Valve recipients and 75.9 ± 10.0 years for JenaValve recipients. Males comprised 61.9% of J-Valve recipients and 42.0% of JenaValve recipients. The body mass index (BMI) was 22.6 ± 3.0 for J-Valve recipients and 25.3 ± 5.7 for JenaValve recipients. The STS-PROM score was 6.7 ± 5.9 for J-Valve recipients and 4.4 ± 3.5 for JenaValve recipients. Most patients had severe symptoms, with New York Heart Association (NYHA) class III/IV dyspnea present in 75.9% of J-Valve recipients and 57.3% of JenaValve recipients. Demographic, clinical, echocardiographic, and computed tomography data from the individual studies are summarized in table 1 and table 2.
Most J-Valve implantations were performed via the transapical approach (92.4%), whereas JenaValve implantations were transapical in 36.7% of cases and transfemoral in 63.3%. The annulus diameter was 26.0 ± 2.4 mm for J-Valve and 25.6 ± 2.3 mm for JenaValve. The device size was 27.2 ± 1.9 mm for J-Valve and 26.1 ± 0.2 mm for JenaValve. The most frequently used device size was 27 mm. Further procedural data from the individual studies are summarized in table 3.
Table 1. Baseline characteristics of patients included in 15 unique studies
| Study ID | Countries | Recruitment | Device | Approach | Patient n | Male | Age | BMI (kg/m2) | EuroSCORE I | EuroSCORE II | STS-PROM | NYHA III/IV | HTN |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Garcia et al.20 2023 | USA, Canada | May 2018 - Oct 2022 | J-Valve | TFa | 27 | 16 (59) | 79.3 ± 9.6 | - | - | - | 4.1 ± 2.0 | 26 (96.3) | 24 (89) |
| Kong et al.21 2022 | China | Sept 2016 - Sept 2022 | J-Valve | TA | 69 | 52 (75.4) | 71.5 ± 7.9 | 22.70 ± 3.15 | - | - | 3.8 ± 3.9 | 53 (76.8) | 48 (69.6) |
| Liu et al.b22 2022 | China | March 2014 - June 2019 | J-Valve | TA | 161 | 119 (73.9) | 72.5 ± 6.2 | - | - | - | 9.9 ± 5.7 | 157 (98.1) | 107 (66.5) |
| Huan Liu et al.23 2020 | China | May 2014 - October 2018 | J-Valve | TA | 47 | 34 (72.3) | 73.7 ± 7.9 | 22.6 ± 2.9 | 24.3 ± 5.1 | - | 35 (74.5) | 31 (66.0) | |
| W. Liu et al.24 2019 | China | June 2017 - December 2018 | J-Valve | TA | 53 | - | 76.4 ± 5.2 | - | - | - | 6.3 ± 1.8 | - | - |
| Vahl et al.32 2024 | USA (20 sites) | June 8, 2018 - Aug 29, 2022 | JenaValve | TF | 180 | 95 (53) | 75.5 ± 10.8 | 25.3 ± 6.1 | - | - | 4.1 ± 3.4 | 122 (68) | 149 (83) |
| Adamet al.25 2023 | Germany (6 centers) | Sept 2021 - July 2022 | JenaValve | TF | 58 | 37 (63.8) | 76.5 ± 9.0 | 26.19 ± 4.36 | - | 6.10 ± 6.60 | 4.2 ± 4.3 | 43 (74) | 53 (91) |
| Baumbach et al.26 2023 | UK | - | JenaValve | TF | 12 | 7 (58) | 83.3 ± 6.7 | - | - | - | 4.6 [4.1-6.6] | 11 (92) | 8 (67) |
| Ranard et al.27 2022 | USA | July 2018 - March 2020 | JenaValve | TF | 11 | - | 77.6 ± 8.9 | - | - | - | - | - | - |
| Baldus et al.28 2019 | Germany and Netherlands (7 centers) | - | JenaValve | TF | 12 | 4 (33.3) | 75 ± 7.2 | - | - | - | 3.5 ± 2.1 | 8 (67) | - |
| Silaschi et al.29 2018 | Germany (15 center) | 2012 - 2015 | JenaValve | TA | 30 | 12 (40.0) | 74.4 ± 9.3 | - | 17.7 ± 14.8 | 6.9 ± 6.5 | 4.9 ± 3.5 | 27 (90) | 24 (80.0) |
| Sawaya et al.14 2017 | Europe, North America, and Asia Middle East (18 center) | July 2007 - Sept 2016 | JenaValvec | TA | 23/146 | - | - | - | - | - | - | - | - |
| Yoon et al.6 2017 | Europe, North America, and Asia | Sept 2007 - Feb 2017 | JenaValved | TAe | 64/212 | - | - | - | - | - | - | - | - |
| Seiffert et al.30 2014 | 9 centers, Germany | April 2012 - October 2013 | JenaValve | TA | 31 | 20 (64.5) | 73.8 ± 9.1 | 24.0 ± 4.5 | 23.6 ± 14.5 | 9.3 ± 6.4 | 5.4 ± 3.6 | 28 (90.3) | 26 (83.9) |
| Schlingloff et al.31 2014 | Hamburg, Germany | December 2012 - Sept 2013 | JenaValve | TA | 10 | 6 (60) | 79.1 ± 9.3 | - | 28.3 ± 17.1 | - | 7.0 ± 1.0 | 9 (90) | - |
| Garcia et al.20 2023 | 5 (19) | 7 (26) | 12 (44) | 4 (15) | NA | 3 (11) | 4 (15) | - | - | 4 (15) | 13 (48) | 4 (15) | |
| Kong et al.21 2022 | 9 (13.0) | 14 (20.3) | 18 (26.1) | 7 (10.1) | 5 (7.2) | 2 (2.9) | 6 (8.7) | - | 19 (27.5) | 0 | 4 (5.8) | 1(1.4) | |
| Liu et al.b22 2022 | 24 (14.9%) | 50 (31.1) | 36 (22.4)f | - | 34 (21.1) | 5 (3.1) | 51 (31.7) | 53 (32.9) | 52 (32.3) | - | 4 (2.5) | - | |
| Huan Liu et al.23 2020 | 4 (8.5) | 9 (19.1) | 9 (19.1) | 10 (21.3) | - | 1 (2.1) | 15 (31.9) | - | 11 (23.4) | 0 (0) | 2 (4.3) | 2 (4.3) | |
| W. Liu et al.24 2019 | - | - | - | - | - | - | - | - | - | - | - | - | |
| Vahl et al.32 2024 | 26 (14) | 32 (18) | 72 (40) | 21 (12) | 58 (33) | 30 (16) | 19 (11) | - | - | - | 37 (23) | 20 (12) | |
| Adamet al.25 2023 | 14 (24) | 9 (16) | 34 (59) | 7 (12) | - | 7 (12) | 8 (14) | - | 25 (43) | 5 (8.6) | 17 (29) | - | |
| Baumbach et al.26 2023 | 1 (8) | 2 (17) | 7 (58) | - | 4 (33) | - | 2 (17) | - | - | - | 2 (17) | ||
| Ranard et al27 2022 | - | - | - | - | - | - | - | - | - | - | - | - | |
| Baldus et al.28 2019 | - | - | 5 (42) | - | - | - | - | 3 (25) | - | - | 2 (17) | - | |
| Silaschi et al.29 2018 | 5 (16.7) | 5 (16.7) | 9 (30.0) | 3 (10.0) | 11 (36.7) | 4 (13.3) | 2 (6.7) | 10 (33.3) | 14 (46.7) | 1 (3.3) | 8 (26.7) | 5 (16.7) | |
| Sawaya et al.14 2017 | - | - | - | - | - | - | - | - | - | - | - | - | |
| Yoon et al.6 2017 | - | - | - | - | - | - | - | - | - | - | - | - | |
| Seiffert et al.30 2014 | 4 (12.9) | 9 (29.0) | 6 (19.3) | 6 (19.3) | - | 3 (9.7) | 6 (19.3) | 6 (20) | 20 (64.5) | 11 (35.5) | 10 (32.2) | 7 (22.6) | |
| Schlingloff et al.31 2014 | - | - | - | - | - | - | - | - | - | - | - | - | |
|
AF, atrial fibrillation; AS, aortic stenosis; BMI, body mass index; CABG, coronary artery bypass grafting; CAD, coronary artery disease; COPD, chronic (obstructive) pulmonary disease; DM, diabetes mellitus; EuroSCORE, European System for Cardiac Operative Risk Evaluation; HTN, hypertension; MI, myocardial infarction; NYHA, New York Heart Association; PVD, peripheral vascular disease; PCI, percutaneous coronary intervention; STS-PROM, Society of Thoracic Surgeons Predicted Risk Of Mortality; TA, transapical; TF, transfemoral. The data are presented as mean ± standard deviation, median [IQR], or No. (%). a F in 21. Other access: 1 carotid, 4 subclavian, 1 transcaval. b Liu et al. 22 (2022) included 29 (18.0%) patients with concomitant mild AS and 1 patient (0.6%) with bioprosthetic valve failure. c Sawaya et al. 14 (2017) included different devices; the number of JenaValve recipients was 23. d Yoon et al. 6 (2017) included different devices, but number of JenaValve patients was 64. e Yoon et al. 6 (2017) included 63 transapical implantations. f Atrial fibrillation/flutter. |
|||||||||||||
Table 2. Echocardiographic and computed tomographic data
| Study ID | LVEF (%) | LVEDD (mm) | MR, ≥ moderate | Aortic regurgitation grade | Bicuspid AV | Ascending aorta diameter | Aortic annulus diameter | Aortic annulus perimeter | |
|---|---|---|---|---|---|---|---|---|---|
| Moderate | Severe | ||||||||
| Garcia et al.20 2023 | 54 [37–60] | 55 ± 90 | - | 5 (19) | 22 (81) | 1 (4) | - | 25.6 ± 3 | 81 ± 10.5 |
| Kong et al.21 2022 | 50.8 ± 12.4 | - | - | 69 (100) | - | - | - | - | |
| Liu et al.b22 2022 | 52.3 ± 12.8 | 65.1 ± 9.3 | - | - | 161 (100) | 13 (8.1) | 41.4 ± 5.2 | 26.2 ± 2.4 | - |
| Huan Liu et al.23 2020 | 52.3 ± 12.4 | 59.2 ± 8.4 | 5 (10.6) | 0 | 47 (100) | 3 (6.4) | 40.1 ± 4.9 | 27.1 ± 2.2a | - |
| W. Liu et al.24 2019 | - | - | - | 0 | 53 (100) | - | - | - | - |
| Vahl et al.32 2024 | 53.8 ± 11.4 | - | - | 5 (3) | 116 (64) | - | 37·3 ± 5·0 | - | 79·1 ± 6·1 |
| Adamet al.25 2023 | - | - | 25 (43.1)b | 2 (3.4) | 56 (96.6)c | - | - | - | 80.3 ± 9.7 |
| Baumbach et al.26 2023 | 47 [39–56] | 60 [59–66] | - | - | 12 (100) | - | - | 27 × 24d | - |
| Ranard et al27 2022 | 44.6 ± 10.4 | 64 ± 8 | - | 11 (100) | - | - | - | - | |
| Baldus et al.28 2019 | 53.0 ± 8.5 | - | 10 (83) | - | 12 (100) | - | - | 25 ± 2.3 | - |
| Silaschi et al.29 2018 | 49.6 ± 13.3 | - | 15 (50) | 1 (3.3) | 29 (96.7) | - | - | 24.3 ± 1.9 | - |
| Sawaya et al.14 2017 | - | - | - | - | - | - | - | - | - |
| Yoon et al.6 2017 | - | - | - | - | - | - | - | - | - |
| Seiffert et al.30 2014 | 46.8 ± 16.1 | - | 8 (25.8) | 1 (3.2) | 30 (96.8) | - | 36.6 ± 7.0 | 24.7 ± 1.5 | - |
| Schlingloff et al.31 2014 | 48.2 ± 15.8 | 62 ± 2.2 | 3 (30) | - | 10 (100) | - | - | - | - |
|
AR, aortic regurgitation; Bicuspid AV, bicuspid aortic valve; LVEF, left ventricular ejection fraction; LVEDD, left ventricular end-diastolic diameter; MR, mitral regurgitation. The data are presented as mean ± standard deviation, No. (%), or median [IQR]. a Perimeter-derived diameter. b Including mild to moderate MR. c Including moderately-severe and severe AR. d Data presented as median. |
|||||||||
Table 3. Procedural characteristics
| Study ID | Device | Access | Valve prosthesis size (mm) | Average prosthesis size, mm | BPostD | ||||
|---|---|---|---|---|---|---|---|---|---|
| 21 mm | 23 mm | 25 mm | 27 mm | 29 mm | |||||
| Garcia et al.20 2023 | J-Valve | TFa | - | - | - | - | - | 26.9 ± 1.8 | 0 (0) |
| Kong et al.21 2022 | J-Valve | TA | - | - | - | - | 59 (85.9) | 29c | - |
| Liu et al.b22 2022 | J-Valve | TA | 4 (2.5) | 15 (9.3) | 35 (21.7) | 64 (39.75) | 43 (26.7) | 26.6 ± 2.0 | - |
| Huan Liu et al.23 2020 | J-Valve | TA | - | 1 (2.1) | 7 (14.9) | 26 (55.3) | 13 (27.7) | 27.2 ± 1.4 | 0 (0) |
| W. Liu et al.24 2019 | J-Valve | TA | - | - | - | - | - | - | - |
| Vahl et al.32 2024 | JenaValve | TF | - | 40 (23) | 35 (20) | 102 (58) | - | 25.7 ± 1.6 | 7 (4) |
| Adamet al.25 2023 | JenaValve | TF | - | 4 (6.9) | 16 (27.6) | 38 (65.5) | - | 26.2 ± 1.2 | 2 (3.4) |
| Baumbach et al.26 2023 | JenaValve | TF | - | - | 3 (25) | 9 (75) | - | 26.5 ± 0.9 | - |
| Ranard et al27 2022 | JenaValve | TF | - | - | - | - | - | - | - |
| Baldus et al.28 2019 | JenaValve | TF | - | 2 (16.7) | 2 (16.7) | 8 (66.7) | - | 26 ± 1.6 | 0 (0) |
| Silaschi et al.29 2018 | JenaValve | TA | - | 4 (13.3) | 11 (36.7) | 15 (50.0) | - | 25.7 ± 1.4 | 1 (3.3) |
| Sawaya et al.14 2017 | JenaValve | TA | - | - | - | - | - | - | - |
| Yoon et al.6 2017 | JenaValve | TAb | - | - | - | - | - | - | - |
| Seiffert et al.30 2014 | JenaValve | TA | - | 4 (12.9) | 7 (22.6) | 20 (64.5) | - | 26.3 ± 1.5 | 2 (6.4) |
| Schlingloff et al.31 2014 | JenaValve | TA | - | 1 (10) | 2 (20) | 7(70) | - | 26.2 ± 1.4 | - |
|
BPostD, balloon postdilatation; TA, transapical; TF, transfemoral. The data are presented as mean ± standard deviation or No. (%). a Transfemoral in 21. Other access: 1 carotid, 4 subclavian, 1 transcaval. b Transapical in 63/64. c Data presented as mean. |
|||||||||
In-hospital outcomes
Overall, in-hospital outcomes were favorable. Procedural success was achieved in 95.9% (n = 518/540). Surgical conversion was required in 1.8% (n = 12/678), device migration or embolization occurred in 3.2% (n = 17/540), and a second valve (in-valve) was required in 2.0% (n = 13/651). Only 1 patient (out of 502) experienced coronary obstruction, and no patients developed annular rupture (among 449). Details of in-hospital outcomes from the individual studies are summarized in table 4.
| Study ID | Procedural success | Conversion to surgery | Coronary obstruction | Annulus rupture | Device migration/embolization | Need for second valve | Bleeding, major or life-threatening | Vascular and access-related complications | Acute kidney injury | In-hospital mortality | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Event | Total | Event | Total | Event | Total | Event | Total | Event | Total | Event | Total | Event | Total | Event | Total | Event | Total | Event | Total | |
| Garcia et al.20 2023 | 22 (81) | 27 | 2 (7) | 27 | - | - | - | - | 3 (11.1) | 27 | 3 (11.1) | 27 | - | - | 5(18.5) | 27 | - | - | 1 (3.7) | - |
| Kong et al.21 2022 | 67 (98.5) | 68 | 1 (1.4) | 69 | - | - | - | - | 1(1.4) | 68 | - | - | 5 (7.4) | 68 | - | - | - | - | 0 (0) | 68 |
| Liu et al.b22 2022 | - | - | 4 (2.5) | 161 | 1 (0.6) | 161 | 0 (0) | 161 | 4 (2.5) | 161 | 0 (0) | 161 | 1 (0.6) | 161 | - | - | - | - | 3 (1.9) | 161 |
| Huan Liu et al.23 2020 | 46 (97.9) | 47 | 0 (0) | 47 | 0 (0) | 47 | 0 (0) | 47 | 1(2.1) | - | 1 (2.1) | 47 | 0 | 47 | 0 (0) | 47 | 8(17.0) | 47 | - | - |
| W. Liu et al.24 2019 | 51 (96.2) | 53 | 2 (3.8) | 53 | 0 (0) | 53 | - | - | 2 (3.8) | 53 | 1 (1.9) | 53 | 5 (14.3) | 53 | - | - | - | - | - | - |
| Vahl et al.32 2024 | 171 (95) | 180 | 1 (< 1) | 180 | 0 (0) | 180 | 0 (0) | 180 | 4(2.2) | 180 | 1 (< 1) | 180 | 8 (4) | 180 | 7 (4) | 180 | 2 (1) | 180 | 0 (0) | 180 |
| Adamet al.25 2023 | 58 (100) | 58 | 0 (0) | 58 | - | - | - | - | 0 (0) | 58 | 0 (0) | 58 | 0 (0) | 58 | 4 (6.9) | 58 | 7 (12) | 58 | 0 (0) | 58 |
| Baumbach et al.26 2023 | 12 (100) | 12 | - | - | - | - | - | - | - | - | - | - | 1 (8.3) | 12 | 5(41.7) | 12 | 1 (8.3) | 12 | - | - |
| Ranard et al.27 2022 | 11 (100) | 11 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Baldus et al.28 2019 | 11 (92) | 12 | 1 (8.3) | 12 | - | - | - | - | - | - | - | - | - | - | 1 (8.3) | 12 | - | - | 0 (0) | 12 |
| Silaschi et al.29.2018 | 29 (96.7) | 30 | 1 (3.7) | 27 | 0 (0) | 30 | 0 (0) | 30 | 1 (3.3) | 30 | 0 | 30 | 1 (3.3) | 30 | 1 (3.3) | 30 | 0 (0) | 30 | - | - |
| Sawaya et al.14 2017 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 2(8.7) | 23 | - | - | - | - |
| Yoon et al.6 2017 | - | - | - | - | - | - | - | - | - | - | 6 (9.4) | 64 | 5 (7.8) | 64 | 1 (1.6) | 64 | 4 (9.4) | 47 | - | - |
| Seiffert et al.30 2014 | 30 (96.8) | 31 | 0 (0) | 31 | 0 (0) | 31 | 0 (0) | 31 | 1 (3.2) | 31 | 1 (3.2) | 31 | 3 (9.7) | 31 | 4 (13) | 31 | 7 (22.5) | 31 | - | - |
| Schlingloff et al.31 2014 | 10 (100) | 10 | 0 (0) | 10 | - | - | - | - | - | - | - | - | 0 (0) | 10 | - | - | - | - | 0 (0) | 10 |
|
The data are presented as No (%). |
||||||||||||||||||||
Thirty-day outcomes
At 30 days, 95.5% of patients were alive (n = 716/750), and device success was achieved in 93.3% (n = 498/534). Mild PVR was observed in 18.0% (n = 86/478), while moderate-to-severe PVR occurred in 1.7% (n = 12/703; including 10 patients with J-Valve and 2 patients with JenaValve). PPI was required in 13.0% (n = 86/711; with 25 patients receiving J-Valve and 61 receiving JenaValve). Further 30-day outcomes from the individual studies are summarized in table 5.
| Study ID | Device success | 30-day all-cause mortality | 30-day Stroke | 30-day PPI | 30-day mild PVR | 30-day PVR ≥ moderate | 30-day EOA (cm2) | 30-day mean AVPG | 30-day repeat procedure for valve-related dysfunction | NYHA class III/ IV | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Event | Total | Event | Total | Event | Total | Event | Total | Event | Total | Event | Total | Event | Total | Event | Total | |||
| Garcia et al.20 2023 | - | - | 1 (4) | 24 | 1 (4) | 24 | 3 (13) | 24 | 8 (33) | 24 | 0 (0) | 24 | 2.1 ± 0.6 | 7 ± 4 | - | - | 3 (12) | 24 |
| Kong et al.21 2022 | - | - | 1 (1.5) | 68 | 2 (2.9) | 68 | 5 (7.5) | 67 | 19 (28) | 68 | 4 (5.9) | 68 | - | - | - | - | 7 (10) | 68 |
| Liu et al.b22 2022 | 153 (95.0) | 161 | 3 (1.9) | 161 | 1 (0.6) | 161 | 13 (8.3) | 155 | - | - | 4 (1.9) | 161 | - | 8.5 ± 2.9 | 1 (0.6) | 161 | 1 (0.6) | 161 |
| Huan Liu et al.23 2020 | - | - | 1 (2.1) | 47 | 0 (0) | 47 | 2 (4.3) | 46 | 14(30.4) | 47 | 1 (2.1) | 47 | - | 7.9 ± 2.4 | 0 (0) | 47 | 2 (4.5) | 44 |
| W. Liu et al.24 2019 | - | - | 5 (9.2) | 53 | 0 (0) | 53 | 2 (5.7) | 53 | 3 (5.6) | 53 | 1 (1.9) | 53 | - | - | - | - | - | - |
| Vahl et al.32 2024 | 174 (96.7)b | 180 | 4 (2) | 180 | 4 (2) | 180 | 36 (24) | 180a | 31 (19) | 180 | 1 (0.6) | 180 | 2.8 ± 0.6e | 3·9 ± 1·6 | - | - | 16 (9) | 180 |
| Adamet al.25 2023 | 47 (98) | 48 | 1 (1.7) | 58 | 0 (0) | 57 | 10 (19.6) | 51 | 2 (4.1) | 49 | 0 (0) | 49 | 2.65 ± 0.6c | 4.5 ± 2.0 | - | - | 4 (7.7) | 52 |
| Baumbach et al.26 2023 | - | - | 0 (0) | 12 | - | - | 2 (17) | 12 | 3 (33) | 12 | 0 | 12 | - | - | - | - | 3 (25) | 12 |
| Ranard et al.27 2022 | - | - | - | - | - | - | - | - | 0 (0) | 11 | 0 (0) | 11 | 2.7 ± 0.4 | 4.1 ± 1.7 | - | - | - | - |
| Baldus et al.28 2019 | - | - | 0 (0) | 12 | 0 (0) | 12 | 1 (8.3) | 12 | 2 (20) | 10 | 0 (0) | 10 | 2.4 ± 0.5 | 4.3 ± 1.7 | - | - | 0 (0) | 9 |
| Silaschi et al.29 2018 | 24 (88.9) | 27 | 3 (10.0) | 30 | 1 (3.3) | 30 | 1 (3.8) | 26 | 4 (15.4) | 26 | 0 (0) | 26 | - | 11.4 ± 3.7d | 1 (3.3) | 30 | 11 (41) | 27 |
| Sawaya et al.14 2017 | 18 (78.2) | 23 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Yoon et al.6 2017 | 52 (82.8) | 64 | 8 (12.5) | 64 | 5 (7.8) | 64 | 7 (15.8) | 47 | - | - | 1 (1.6) | 64 | - | - | - | - | - | - |
| Seiffert et al.30 2014 | 30 (96.8) | 31 | 4 (12.9) | 31 | 0 (0) | 31 | 2 (71.4) | 28 | - | - | - | - | - | 7.9 ± 4.0d | - | - | 4 (15.3) | 26 |
| Schlingloff et al.31 2014 | - | - | 3 (30) | 10 | - | - | 2 (20) | 10 | 0 (0) | 6 | 0 (0) | 6 | - | 7.2 ± 4.3 | - | - | 0 (0) | 10 |
|
AVPG, aortic valve pressure gradient; EOA, effective orifice area; NYHA, New York Heart Association; PPI, permanent pacemaker; PVR, prosthetic valve regurgitation. The data are presented as No. (%). a 30 patients had a previous pacemaker. b Data of device success reported in the abstract presented in TCT 2023. Makkar et al.33 2023. c Assessed at discharge. d Immediate postprocedural measurement. e Data of EOA mentioned in the abstract published in JAAC. Reference: Hamid et al.342024. |
||||||||||||||||||
Quantitative analysis of the outcomes of transfemoral TAVI for aortic regurgitation
A meta-analysis of 5 studies25-28,32 of transfemoral TAVI for AR (all with the JenaValve) included 273 patients (mean age, 77.6 years; 52.4% male). Pooled estimates were as follows: procedural success was 97.8% (95%CI, 94.4%-100%, I2 = 43%, P value = .13) (figure 2A), conversion to surgery was 0.49% (95%CI, 0.0%-1.5%, I2 = 0%, P value = .56) (figure 2B), device migration/embolization was 1.2% (95%CI, 0.0-3.3%, I2 = 47%, P value = .17) (figure 2C), and the need for a second valve was 0.46% (95%CI, 0.0%-1.44%, I2 = 0%, P value = .67) (figure 2D). Further details of in-hospital outcomes are summarized in table 6 and in the supplementary data.
Figure 2. A. Forest plot of procedural success of TF JenaValve. The bibliographical references mentioned in this figure correspond to: Adam et al.25 2023, Baumbach et al.26 2023, Ranard et al.27 2022, Baldus et al.28 2019, Vahl et al.32 2024; B. Forest plot of conversion to surgery TF JenaValve. The bibliographical references mentioned in this figure correspond to: Adam et al.25 2023, Baldus et al.28 2019, Vahl et al.32 2024; C. Forest plot of device migration/embolization TF JenaValve. The bibliographical references mentioned in this figure correspond to: Adam et al.25 2023, Vahl et al.32 2024; D. Forest plot of need for a second valve TF JenaValve. The bibliographical references mentioned in this figure correspond to: Adam et al.25 2023, Vahl et al.32 2024. 95%CI, 95% confidence interval.
Table 6. Quantitative analysis of in-hospital outcomes of transfemoral transcatheter aortic valve implantation for aortic regurgitation
| Variables | Reporting studies (n) | Total patients (n) | Proportion with the endpoint (95%CI) | Heterogeneity |
|---|---|---|---|---|
| Procedural success | 5 | 273 | 0.9782 (0.9438-1.000) | I2 = 43%, P = .13 |
| Device success | 2 | 228 | 0.9704 (0.9484-0.9924) | I2 = 0%, P = .61 |
| Conversion to surgery | 3 | 250 | 0.0049 (0.0000-0.0147) | I2 = 0%, P = .56 |
| Device migration/ embolization | 2 | 238 | 0.0116 (0.0000-0.0334) | I2 = 47%, P = .17 |
| Need for a second Valve | 2 | 238 | 0.0046 (0.0000-0.0144) | I2 = 0%, P = .67 |
| Bleeding, major or life-threatening | 3 | 250 | 0.0249 (0.0000-0.0656) | I2 = 66%, P = .05 |
| Vascular complications | 4 | 262 | 0.0572 (0.0174-0.0969) | I2 = 61%, P = .05 |
| Acute kidney injury | 3 | 250 | 0.0592 (0.000-0.1386) | I2 = 72%, P = .03 |
| In-hospital mortality | 3 | 250 | 0.0000 (0.0000-0.0073) | I2 = 0%, P = 1.00 |
|
95%CI, 95% confidence interval. |
||||
At 30 days, the pooled estimate of device success was 97.0% (95%CI, 94.8%-99.2%, I2 = 0%, P value = .61) (figure 3A), and the pooled estimate of all-cause mortality was 2.0% (95%CI, 0.2%-3.7%, I2 = 0%, P value = .95) (figure 3B). The rate of PPI was 18.7% (95%CI, 13.9%-23.4%, I2 = 0%, P value = .58) (figure 3C). Mild PVR rate was 10.6% (95%CI, 1.7%-19.4%, I2 = 75%, P < .01) (figure 4A) with statistically significant heterogeneity resolved by omitting Vahl et al.32 yielding a rate of 4.7% (95%CI, 0.0%-9.5%, I2 = 38%) (supplementary data), while the rate of moderate-severe PVR was 0.47% (95%CI, 0.0%-1.47%, I2 = 0%, P- = 1.00) (figure 4B). Further 30-day outcomes are summarized in table 7 and in the supplementary data.
Figure 3. A. Forest plot of device success TF JenaValve. The bibliographical references mentioned in this figure correspond to: Adam et al.25 2023, Makkar et al.33 2023; B. Forest plot of 30-day all-cause mortality TF JenaValve. The bibliographical references mentioned in this figure correspond to: Adam et al.25 2023, Baumbach et al.26 2023, Baldus et al.28 2019, Vahl et al.32 2024; C. Forest plot of 30-day permanent pacemaker implantation TF JenaValve. The bibliographical references mentioned in this figure correspond to: Adam et al.25 2023, Baumbach et al.26 2023, Baldus et al.28 2019, Vahl et al.32 2024.
Figure 4. A. Forest plot of 30-day of mild prosthetic valve regurgitation TF JenaValve. The bibliographical references mentioned in this figure correspond to: Adam et al.25 2023, Baumbach et al.26 2023, Ranard et al.27 2022, Baldus et al.28 2019, Vahl et al.32 2024; B. Forest plot of 30-day of greater than mild prosthetic valve regurgitation TF JenaValve. The bibliographical references mentioned in this figure correspond to: Adam et al.25 2023, Baumbach et al.26 2023, Ranard et al.27 2022, Baldus et al.28 2019, Vahl et al.32 2024.
Table 7. Quantitative analysis of 30-day outcomes of transfemoral transcatheter aortic valve implantation for aortic regurgitation
| Variables | Reporting studies (n) | Total patients (n) | Proportion with the endpoint (95%CI) | Heterogeneity |
|---|---|---|---|---|
| 30-day all-cause mortality | 4 | 262 | 0.0196 (0.0020-0.0372) | I2 = 0%, P = .95 |
| 30-day stroke | 3 | 250 | 0.0112 (0.0000-0.0316) | I2= 0%, P = .38 |
| 30-day PPM implantation | 4 | 255 | 0.1867 (0.1391-0.2344) | I2 = 0%, P = .58 |
| 30-day mild PVR | 5 | 262 | 0.1056 (0.0168-0.1944) | I2 = 75%, P < .01 |
| 30-day moderate PVR | 5 | 262 | 0.0047 (0.0000-0.0147) | I2 = 0%, P = 1.00 |
|
95%CI, 95% confidence interval; PPM, permanent pacemaker; PVR, prosthetic valve regurgitation. |
||||
DISCUSSION
In this study, we included data from 788 patients who underwent TAVI using 1 of the 2 dedicated devices specifically designed for use in pure/predominant AR: the J-Valve and the JenaValve (figure 5). Studies published up to April 2024 were included, providing a contemporary and comprehensive analysis of published data in this field to date. Overall, 357 patients received the J-Valve (in 5 studies), while 431 received the JenaValve (in 10 studies). These patients were generally at increased surgical risk. J-Valve recipients were predominantly Chinese, tended to be slightly younger, had a smaller BMI, wand showed a clear male predominance compared with JenaValve recipients.
Figure 5. Central illustration. Features of the contemporary generations of 2 TAVI systems dedicated to aortic regurgitation.
The use of the 2 technologies (J-Valve and JenaValve) was influenced by their geographical availability, leading to differences between the populations treated with each device. Moreover, as mentioned earlier, the 2 groups differed in age, sex, and STS-PROM scores. Additionally, most of the transfemoral implantations involved the JenaValve, while the vast majority of J-Valve implantations were transapical. Consequently, direct statistical comparison between the 2 devices and the 2 access routes was deemed inappropriate. For similar reasons, we avoided pooling data from all JenaValve procedures (mixing transapical and transfemoral implantations) and from all transapical procedures (mixing J-Valves and JenaValves). This approach minimized the risk of drawing invalid conclusions by mixing heterogeneous data or comparing outcomes without accounting for important independent confounders. Patients receiving the JenaValve via the transfemoral approach constituted a homogeneous subgroup, allowing for pooled/quantitative analysis. The findings of this latter analysis are particularly important, as transfemoral access currently dominates the TAVI field.
Our systematic review combines prospective and retrospective studies, which share common limitations such as small sample sizes and nonrandomized designs. Therefore, the findings should be regarded as preliminary and require validation in larger randomized studies. From the available data, our major observations can be summarized as follows: first, TAVI using AR-dedicated devices demonstrated a high success rate with a reassuring early safety profile. Second, the rates of surgical conversion, device dislocation, and second valve implantation were low (2%-3%). Third, both dedicated devices effectively eliminated or reduced AR, with only 1% to 2% of patients having ≥ moderate residual AR. Fourth, the results of transfemoral TAVI for AR using the JenaValve were particularly encouraging, although the PPI rate was still relatively high. Taken together, these initial findings suggest that transcatheter treatment of AR, especially through transfemoral access, may be a safe and effective alternative to surgery in appropriately selected patients.
Treating AR with TAVI using the first/older generations of transcatheter heart valves has been associated with suboptimal results.35,36 However, subsequent studies showed that next/newer generation transcatheter heart valves can improve outcomes, bringing them closer to those achieved in patients with AS.13 With the introduction of dedicated devices, several key outcomes have shown further improvement, yielding very high procedural and device success rates and low rates of conversion to surgery, device migration or embolization, the need for a second valve, and PVR. Although annular injury is a concern given the frequent association of AR with aortopathy, no cases of annular rupture were reported with the 2 self-expanding dedicated devices. We also observed low rates of acute kidney injury, bleeding, vascular complications, and in-hospital mortality. Whether this low rate of early complications will translate into improved long-term clinical outcomes remains to be determined and should be explored in longitudinal prospective studies.
A major challenge associated with TAVI for native pure/predominant AR is the risk of device migration/embolization and paravalvular leakage. This risk arises from the absence of calcification in the landing zone, the large size of the aortic annulus, and the high stroke volume in AR patients. The design of the 2 AR-dedicated TAVI devices aims is to mitigate this risk (figure 5).
The JenaValve device features an natomically-oriented design with ‘supporting arms’ that can be positioned in the sinuses of the aortic root, ensuring precise placement of the valve stent. Additionally, the fixation of the oriented device to the native valve leaflet through clip attachment provides an extra axial expansion force, enabling secure fixation even in the absence of leaflet calcifications.37
The J-Valve device is characterized by its U-shaped grasper that captures the aortic valve leaflets, achieving ‘axial’ fixation, which complements the ‘radial’ fixation, which is less reliable in the absence of calcification. Furthermore, the dual-phase release mechanism of this device (the graspers are initially released, followed by the valve) can aid in precise placement of the graspers prior to valve deployment and decrease the likelihood of damage to the native valve.38
Our data suggest that these innovative designs are associated with very low rates of device dislocation and paravalvular leakage, which in turn results in low rates of second valve requirement and surgical conversion. Importantly, these benefits did not come at the expense of increased risk of annular injury or coronary obstruction. However, a relatively high rate of PPI was observed with JenaValve, reaching nearly 19% in 5 studies of its updated transfemoral version. This may reflect a tendency for a relatively deeper implantation, a common issue with early experience of nearly all TAVI systems that tends to improve over time and typically portends a decline in PPI rates.39-42
While the current review includes preliminary single-arm, observational, small-scale studies, several randomized trials are have been conducted on J-Valve and JenaValve.43-47 While the results of these trials are pending, our data suggest a positive outcome.
In the currently available data, there is a dominance of transapical access procedures among J-Valve implantations. However, with the trend toward more minimalistic TAVI procedures, the transapical approach may only be a precursor, with the transfemoral approach expected to eventually become the standard, as already observed with the JenaValve. The most recent data, presented in 2023, on transfemoral J-Valve procedures (from the compassionate use experience in North America) is particularly reassuring.20
Study limitations
The scope of our investigation was restricted to observational studies, abstracts, and conference presentations;, none of which were randomized controlled trials. This inherently limits the quality of the evidence produced. Additionally, the present findings may have been influenced by publication bias favoring TAVI for native pure or predominant AR, which was mitigated by our. However, we sought to mitigate this bias through an exhaustive review of the available literature and the meticulous exclusion of overlapping or duplicate data. The total patient population remained relatively small, and follow-up was restricted to 30-day outcomes, so the findings should be interpreted with these limitations in mind.
CONCLUSIONS
This systematic review provides a comprehensive and up-to-date analysis of data on TAVI with dedicated devices for native pure/predominant AR. The initial experience discussed in the present review demonstrates the safety and favorable early outcomes of TAVI using J-Valve and JenaValve in patients with pure/predominant AR, especially when the transfemoral approach is used. Nevertheless, PPI requirement remains frequent.
FUNDING
None.
ETHICAL CONSIDERATIONS
The present article is a literature review and, as such, ethics approval was not required. The study did not involve patient recruitment or access to disaggregated information on individuals and therefore informed consent was not required. Possible sex/gender biases have been taken into account in the preparation of this article.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
No artificial intelligence was used in the preparation of this article.
AUTHORS’ CONTRIBUTIONS
A. Hassan, M. Abdelshafy, and R.A. Diab performed the literature review, data analysis, and initial manuscript drafting. H. Wienemann, M. Adam, S. García, and M. Saad critically reviewed the manuscript. M. Abdelghani conceived the idea, designed and supervised data collection and analysis, and finalized the manuscript.
CONFLICTS OF INTEREST
M. Adam reports personal fees and speaker honoraria from Abbott, Boston Scientific, Edwards Lifesciences, JenaValve, and Medtronic. S. Garcia reports institutional grants from J.C. Medical and JenaValve. All other authors have no conflict of interest to report.
WHAT IS KNOWN ABOUT THE TOPIC?
- The off-label use of the next-generation nondedicated TAVI devices to treat pure AR is associated with an increased risk of device embolization and PVR.
WHAT DOES THIS STUDY ADD?
- TAVI for AR with devices specifically designed for this indication (J-Valve and JenaValve) shows favorable early safety and efficacy, especially when the transfemoral approach is used. Nevertheless, the need for PPI remains frequent.
REFERENCES
1. Flint N, Wunderlich NC, Shmueli H, Ben-Zekry S, Siegel RJ, Beigel R. Aortic Regurgitation. Curr Cardiol Rep. 2019;21:65.
2. Maurer G. Aortic regurgitation. Heart. 2006;92:994-1000.
3. Singh JP, Evans JC, Levy D, et al. Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham Heart Study). Am J Cardiol. 1999;83:897-902.
4. Dujardin KS, Enriquez-Sarano M, Schaff HV, Bailey KR, Seward JB, Tajik AJ. Mortality and morbidity of aortic regurgitation in clinical practice. A long-term follow-up study. Circulation. 1999;99:1851-1857.
5. Bonow RO, Carabello BA, Chatterjee K, et al. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease:a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease):endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2008;118:523-661.
6. Yoon S-H, Schmidt T, Bleiziffer S, et al. Transcatheter Aortic Valve Replacement in Pure Native Aortic Valve Regurgitation. J Am Coll Cardiol. 2017;70:2752-2763.
7. Adams DH, Popma JJ, Reardon MJ, et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis.N Engl J Med. 2014;370:1790-1798.
8. Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187-2198.
9. Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597-1607.
10. Reardon MJ, Van Mieghem NM, Popma JJ, et al. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med. 2017;376:1321-1331.
11. Leon MB, Smith CR, Mack MJ, et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med. 2016;374:1609-1620.
12. Dvir D, Webb JG, Bleiziffer S, et al. Transcatheter aortic valve implantation in failed bioprosthetic surgical valves. JAMA. 2014;312:162-170.
13. Yoon S-H, Bleiziffer S, De Backer O, et al. Outcomes in transcatheter aortic valve replacement for bicuspid versus tricuspid aortic valve stenosis. J Am Coll Cardiol. 2017;69:2579-2589.
14. Sawaya FJ, Deutsch M-A, Seiffert M, et al. Safety and efficacy of transcatheter aortic valve replacement in the treatment of pure aortic regurgitation in native valves and failing surgical bioprostheses:results from an international registry study. JACC Cardiovasc Interv. 2017;10:1048-1056.
15. Zhu D, Chen Y, Zhang J, Hu J, Guo Y. Transapical implantation of a new second-generation transcatheter heart valve in patients with pure aortic regurgitation:a preliminary report. Interact Cardiovasc Thorac Surg. 2015;20:860-862.
16. Treede H, Rastan A, Ferrari M, Ensminger S, Figulla H-R, Mohr F-W. JenaValve. EuroIntervention. 2012;8 Suppl Q:Q88-93.
17. Page M, McKenzie J, Bossuyt P, et al. The PRISMA 2020 statement:an updated guideline for reporting systematic reviews. BMJ. 2021;372:71.
18. Cochrane Handbook for Systematic Reviews of Interventions(5.1.0). Available at: https://handbook-5-1.cochrane.org. Accessed 19 July 2024.
19. Systematic review screening tool/software. Available at: https://www.rayyan.ai/. Accessed 19 July 2024.
20. Garcia S, Ye J, Webb J, et al. Transcatheter treatment of native aortic valve regurgitation:the north american experience with a novel device. JACC Cardiovasc Interv. 2023;16:1953-1960.
21. Kong M, Hong Z, Liu X, Zhu X, Wang J, Dong A. 30-Day Outcomes after Surgical or Transapical Aortic Valve Replacement in Symptomatic Aortic Regurgitation. J Cardiovasc Dev Dis. 2022;9:224.
22. Liu L, Peng Y, Shi J, Qian H, Guo Y. Initial experience with repositionable J-Valve for severe aortic regurgitation:a single-center experience. J Cardiovasc Surg (Torino). 2022;63:521-528.
23. Liu H, Liu S, Lu Y, et al. Transapical transcatheter aortic valve implantation for predominant aortic regurgitation with a self-expandable valve. J Thorac Dis. 2020;12:538-549.
24. Liu W, Zhou YJ, Zhang HB, Meng XU, Gao YN. P1852The clinical experience of J valve transapical transcatheter aortic valve replacement system in high-risk patients with severe pure aortic regurgitation. Eur Heart J. 2019;40(Supplement_1):ehz748.0603.
25. Adam M, Tamm AR, Wienemann H, et al. Transcatheter Aortic Valve Replacement for Isolated Aortic Regurgitation Using a New Self-Expanding TAVR System. JACC Cardiovasc Interv. 2023;16:1965-1973.
26. Baumbach A, Patel KP, Kennon S, et al. A heart valve dedicated for aortic regurgitation:Review of technology and early clinical experience with the transfemoral Trilogy system. Catheter Cardiovasc Interv. 2023;102:766-771.
27. Ranard LS, Gogia S, Hamid N, et al. Left ventricular remodeling after jenavalve trilogy transcatheter aortic valve replacement in patients with pure aortic regurgitation. J Am Coll Cardiol. 2022;79:647.
28. Baldus S, Treede H, Kempfert J, Kim WK, Rudolph T and Bleiziffer S, Mieghem NV. A Feasibility Study to Assess Safety and Effectiveness of the JenaValve Transfemoral TAVR System in the Treatment of Patients With Severe Aortic Regurgitation. Euro PCR 2019. Paris, France.
29. Silaschi M, Conradi L, Wendler O, S et al. The JUPITER registry:One-year outcomes of transapical aortic valve implantation using a second generation transcatheter heart valve for aortic regurgitation. Catheter Cardiovasc Interv. 2018;91:1345-1351.
30. Seiffert M, Bader R, Kappert U, et al. Initial German experience with transapical implantation of a second-generation transcatheter heart valve for the treatment of aortic regurgitation. JACC Cardiovasc Interv. 2014;7:1168-1174.
31. Schlingloff F, Schäfer U, Frerker C, Schmoeckel M, Bader R. Transcatheter aortic valve implantation of a second-generation valve for pure aortic regurgitation:procedural outcome, haemodynamic data and follow-up. Interact Cardiovasc Thorac Surg. 2014;19:388-393.
32. Vahl TP, Thourani VH, Makkar RR, et al. Transcatheter aortic valve implantation in patients with high-risk symptomatic native aortic regurgitation (ALIGN-AR):a prospective, multicentre, single-arm study. Lancet. 2024;403:1451-1459.
33. Makkar R. Valve Hemodynamics and Device Performance:A New Era in Safety and Technical Success for TAVR in AR [video]. TCT 2023. Available at: https://www.crfconnect.com/episode/valve-hemodynamics-and-device-performance-a-new-era-in-safety-and-technical-success-for-tavr-in-ar-1700.
34. Hamid, N, Vahl, T, Thourani, V. al. Hemodynamic performance of the JenaValve Trilogy™system from the ALIGN-AR trial - the first dedicated transcatheter aortic valve for aortic regurgitation. J Am Coll Cardiol. 2024;83(Suppl 13):803.
35. Ullah W, Suleiman A-RM, Osman H, et al. Trends and outcomes of transcatheter aortic valve implantation in aortic insufficiency:A nationwide readmission database analysis. Curr Probl Cardiol. 202449(1 Pt A):102012.
36. Haddad A, Arwani R, Altayar O, Sawas T, Murad MH, de Marchena E. Transcatheter aortic valve replacement in patients with pure native aortic valve regurgitation:A systematic review and meta-analysis. Clin Cardiol. 2019;42:159-166.
37. Seiffert M, Diemert P, Koschyk D, et al. Transapical implantation of a second-generation transcatheter heart valve in patients with noncalcified aortic regurgitation. JACC Cardiovasc Interv. 2013;6:590-597.
38. Zhu D, Hu J, Meng W, Guo Y. Successful transcatheter aortic valve implantation for pure aortic regurgitation using a new second generation self-expanding J-Valve(TM) system - the first in-man implantation. Heart Lung Circ. 2015;24:411-414.
39. Sammour Y, Banerjee K, Kumar A, et al. Systematic Approach to High Implantation of SAPIEN-3 Valve Achieves a Lower Rate of Conduction Abnormalities Including Pacemaker Implantation. Circ Cardiovasc Interv. 2021;14:009407.
40. Eliav R, Elitzur Y, Planer D, et al. Predictors for permanent pacemaker implantation following transcatheter aortic valve implantation:trends over the past decade. J Interv Card Electrophysiol. 2021;62:299-307.
41. Vora AN, Gada H, Manandhar P, et al. National variability in pacemaker implantation rate following TAVR:insights from the STS/ACC TVT registry. JACC Cardiovasc Interv. 2024;17:391-401.
42. Mauri V, Abdel-Wahab M, Bleiziffer S, et al. Temporal trends of TAVI treatment characteristics in high volume centers in Germany 2013-2020. Clin Res Cardiol. 2022;111:881-888.
43. J-Valve Compassionate Use. Available at: https://clinicaltrials.gov/study/NCT03876964. Accessed 15 July 2024.
44. Clinical Trial in China. Available at: https://clinicaltrials.gov/study/NCT05580952?tab=results. Accessed 15 July 2024.
45. Safety &Efficacy of the J-Valve Ausper System in Patients With Severe Aortic Stenosis and/or Aortic Regurgitation. Available at: https://clinicaltrials.gov/study/NCT03025971. Accessed 15 July 2024.
46. THE ALIGN-AR EFS TRIAL:JenaValve Pericardial TAVR Aortic Regurgitation Study. Available at: https://clinicaltrials.gov/study/NCT02732704. Accessed 15 July 2024.
47. JUPITER Study:Transapical Aortic Valve Implantation for Aortic Regurgitation (JUPITER). Available at: https://clinicaltrials.gov/study/NCT01598844. Accessed 15 July 2024.

ABSTRACT
Introduction and objectives: The use of transradial access for percutaneous coronary procedures has increased due to its advantages over the femoral approach. However, this benefit comes at the expense of a higher rate of radial artery occlusion (RAO). Our objective was to assess the incidence and predictors of RAO following transradial catheterization. Additionally, we studied anatomic variations of the radial artery (RA).
Methods: This prospective study enrolled 427 patients who underwent coronary angiography or angioplasty via transradial access. The forearm arteries were evaluated by ultrasound. If RAO was present, follow-up ultrasound examinations were performed at 1 and 3 months postprocedure.
Results: Our study population included 288 men (67.4%) and 139 women (32.6%). The mean age was 61.9 ± 11.1 years. RAO occurred in 48 patients (11.24%), and spontaneous recanalization was observed within 3 months in 15 patients (32.6%). On multivariate analysis, independent predictors of RAO were younger age (OR, 0.642; 95%CI, 0.480-0.858; P = .031), low periprocedural systolic blood pressure (OR, 0.598; 95%CI, 0.415-0.862; P = .007), a small radial diameter (OR, 0.371; 95%CI, 0.323-0.618; P = .031), insufficient anticoagulation (OR, 0.287; 95%CI, 0.163-0.505; P < .001), occlusive hemostasis (OR, 0.128; 95%CI, 0.047-0.353; P < .001), and long duration of hemostasis. The overall incidence of RA anatomic variations was 14.8% (n = 63). Among these, 40 patients (63.5%) had a high radial origin, 18 (28.6%) had extreme RA tortuosity, and 5 (7.9%) had a complete radioulnar loop.
Conclusions: The main modifiable predictors of RAO are insufficient heparinization and occlusive hemostasis. Preventive strategies should focus primarily on these 2 predictive factors to reduce the risk of RAO.
Keywords: Anatomic variations. Cardiac catheterization. Doppler ultrasound. Percutaneous coronary intervention. Predictors. Radial artery occlusion. Transradial access.
RESUMEN
Introducción y objetivos: El acceso transradial para procedimientos coronarios percutáneos ha crecido en popularidad debido a sus ventajas sobre el abordaje femoral. Sin embargo, este beneficio se ve ensombrecido por una mayor tasa de oclusión de la arteria radial (OAR). Nuestro objetivo fue evaluar la incidencia y los factores predictivos de OAR tras el cateterismo transradial. También se estudiaron las variaciones anatómicas de la arteria radial (AR).
Métodos: En este estudio prospectivo participaron 427 pacientes a los que se había realizado angiografía coronaria o angioplastia mediante acceso transradial. Se realizó una evaluación ecográfica de las arterias del antebrazo. En caso de OAR, se llevó a cabo otro control ecográfico al mes y a los 3 meses de la intervención.
Resultados: La población de estudio incluyó a 288 varones (67,4%) y 139 mujeres (32,6%). La edad media fue de 61,9 ± 11,1 años. La OAR se produjo en 48 pacientes (11,24%), de los cuales en 15 (32,6%) se produjo recanalización espontánea en el plazo de 3 meses. En el análisis multivariante, la edad más joven (OR = 0,642; IC95%, 0,480-0,858; p = 0,031), la presión arterial sistólica periprocedimiento baja (OR = 0,598; IC95%, 0,415-0,862; p = 0,007), el diámetro radial pequeño (OR = 0,371; IC95%, 0,323-0,618; p = 0,031), la anticoagulación insuficiente (OR = 0,287; IC95%, 0,163-0,505; p < 0,001), la hemostasia oclusiva (OR = 0,128; IC95%, 0,047-0,353; p < 0,001) y la larga duración de la hemostasia aparecieron como predictores independientes de OAR. La incidencia global de variaciones anatómicas de la AR fue del 14,8% (n = 63). Entre estos pacientes, 40 (63,5%) tenían un origen radial alto, 18 (28,6%) presentaban una tortuosidad extrema de la AR y 5 (7,9%) tenían un asa radiocubital completa.
Conclusiones: La heparinización insuficiente y la hemostasia oclusiva son los principales predictores de OAR modificables. La estrategia preventiva debe centrarse principalmente en estos 2 factores predictivos.
Palabras clave: Variaciones anatómicas. Cateterismo cardiaco. Ecografía Doppler. Intervención coronaria percutánea. Predictores. Oclusión de la arteria radial. Acceso transradial.
Abbreviations
RA: radial artery. RAO: radial artery occlusion.
INTRODUCTION
The use of the transradial approach for coronary interventions has become increasingly widespread in interventional cardiology due to its numerous advantages.1 As a result, current guidelines recommend it as the first-line approach.2
However, the benefits of this technique are tempered by the risk of radial artery occlusion (RAO), with reported rates ranging from 5% to 30%.3,4 The aim of this study was to assess the incidence and predictors of RAO following transradial catheterization using Doppler ultrasound for evaluation.
METHODS
Patient population
This longitudinal, single-center prospective study was conducted in the cardiology department of the Military Central Hospital in Algiers. After applying exclusion criteria (hemodynamic instability and ST-segment elevation myocardial infarction), we included 427 consecutive patients undergoing transradial coronary procedures between January 2019 and March 2020. The study adhered to the principles of the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practices and was approved by the local ethics committee. All patients provided written informed consent.
Radial artery cannulation and retrograde radial arteriography
After radial artery (RA) puncture, a radial hydrophilic sheath (Radiofocus II, TERUMO Medical, Japan, or Prelude, MERIT Medical, United States) was introduced. An antispastic cocktail was then administered into the RA through the sheath, consisting of a saline solution, a vasodilator (1 mL of nicardipine), and a bolus of unfractionated heparin, which was administered either intravenously or directly into the RA as part of the spasmolytic cocktail, depending on the operator’s preference. In patients on vitamin K antagonists, these medications were not discontinued prior to the procedure.
Retrograde radial arteriography was performed by injecting a mixture of 4 mL of contrast and 4 mL of isotonic saline through the sheath. Radiographic images were then obtained in an anteroposterior projection.
Transradial coronary procedure
The standard approach was conventional right radial access. For coronary angiography, 5-French (Fr) hydrophilic sheaths and catheters were usually used. If the patient required revascularization, an ad hoc percutaneous coronary intervention was performed, using 6-Fr guiding catheters after exchanging the sheath from 5-Fr to 6-Fr. The usual dose of heparin is 5000 IU (2500 IU for oral anticoagulation with a vitamin K antagonist).
Hemostasis procedure
At the end of the procedure, the sheath was removed, and hemostasis was achieved using a hemostatic compression device (TR BAND, TERUMO Medical, Japan). A reverse Barbeau test5 was systematically performed. The hemostasis device was removed by nurses in the hospitalization unit. No standardized protocol for the duration of hemostasis was followed.
Assessment of postprocedural radial artery patency
Radial Doppler assessments were conducted before and after each transradial procedure. To evaluate RAO, pulsed Doppler was performed bilaterally on the radial and ulnar arteries. Normal arterial flow was indicated by a biphasic or triphasic signal, reflecting good perfusion. In cases of RAO, 2 additional ultrasonographic examinations were performed at 1 and 3 months, following the same protocol. Artery patency was assessed by an independent operator.
Classifications and definitions
RAO was defined as the absence of anterograde flow in the RA on ultrasound (figure 1). The location of the radial occlusion was identified using color and pulsed Doppler. We delineated 3 anatomical territories: the distal third, extending from the radial styloid to approximately 7 to 10 cm proximally; the proximal third, from the elbow folds to approximately 7 to 10 cm distally; and the middle third, located between the previous 2 regions (middle part of the forearm).
Figure 1. Radial artery with occlusion in the distal third. Pulsed Doppler flow targets a stop flow indicating radial occlusion.
The type of hemostasis, whether occlusive or patent, was assessed: patent hemostasis was indicated by the presence of a plethysmographic signal in the RA during the reverse Barbeau test,5 which involves compression of the ulnar artery. The operator did not intervene during this process but simply recorded whether the artery remained patent or not.
The internal luminal diameter of the RA was defined as the distance between the leading edges of the intima-lumen interface on the superficial wall and the lumen-intima interface on the deep wall.6
The R/S ratio (radial/sheath) was calculated by dividing the luminal diameter of the RA by the external diameter of the sheath (Radiofocus II: 5-Fr = 2.29 mm, 6-Fr = 2.62 mm, 7-Fr = 2.97 mm; Prelude: 5-Fr = 2.52 mm, 6-Fr = 2.83 mm). This ratio was categorized qualitatively as < 1 or ≥ 1.
RA anatomical variations of clinical relevance were classified according to definitions provided in the literature.7,8 A high origin (high bifurcation) of the RA (figure 2) was defined with reference to the intercondylar line of the humerus. A radioulnar loop was characterized by the presence of a complete 360° loop of the RA, while radial tortuosity was identified by a curvature greater than 45°.
Figure 2. Anatomic variations of the radial artery. A: high origin of the radial artery. The radial and ulnar arteries separate at the level of the middle third of the humerus (arrow). B: radioulnar loop was defined as a complete 360° loop of the radial artery distal to the bifurcation of the brachial artery (arrow).
A blood pressure profile was obtained on the same side as the radial access. Forearm hematomas were classified according to the “EASY” study9: type I: < 5 cm in diameter; type II: < 10 cm; type III: > 10 cm but not extending to the elbow; type IV: extending beyond the elbow; type V: resulting in an ischemic lesion.
Statistical analysis
The statistical analysis was performed using IBM SPSS Software version 25. Parameters of interest are reported with their 95% confidence intervals (95%CI). For all tests, a significance threshold of 5% was retained. All tests were performed bilaterally. The following tests were used to compare groups: the chi-square test was used to compare 2 qualitative variables; the Student t-test or analysis of variance was used to compare a quantitative variable with a qualitative variable, with the Fisher test being applied when variances were unequal; and logistic regression was used to identify predictors of RAO.
RESULTS
Clinical and procedural characteristics of the study population
During the study period, 441 patients were screened. Of these, transradial access failed in 14 patients, who were excluded from the study, resulting in an eligible sample of 427 patients (mean age 61.9 ± 11.1 years, 67.4% male). Among the patients, 260 had hypertension (60.9%), and nearly half had diabetes (48.9%).
Table 1 summarizes the procedural data. The sheaths used were mainly 6-Fr (83.6%), and heparin was injected intra-arterially in 63.5% of patients. The mean heparin dose was 5669 ± 1394 IU, with a higher dose given when percutaneous coronary intervention was performed (4940 ± 339 IU vs 7491 ± 1368 IU; P < .001).
| Procedural characteristics | Patients N (%) |
|---|---|
| Indication | |
| CCS | 227 (53.2%) |
| ACS (NSTEMI) | 200 (46.8%) |
| Type of procedure | |
| Diagnostic angiography | 305 (71.4%) |
| PCI | 122 (28.6%) |
| Previous radial procedures | 68 (15.9%) |
| Right radial access | 410 (96.0%) |
| Puncture attempts | |
| 1 attempt | 258 (60.4%) |
| 2 attempts | 99 (23.2%) |
| ≥ 3 attempts | 70 (16.4%) |
| Sheath size | |
| 5-Fr | 68 (15.9%) |
| 6-Fr | 357 (83.6%) |
| 7-Fr | 2 (0.5%) |
| Heparin administration | |
| Intra-arterial | 271(63.5%) |
| Intravenous | 156 (36.5%) |
| Heparin dose (IU) | 5669 ± 1394 |
| Angiography | 4940 ± 339 |
| PCI | 7491 ± 1368 |
| Catheter diameter | |
| 5-Fr | 300 (70.3%) |
| 6-Fr | 125 (29.3%) |
| 7-Fr | 2 (0.5%) |
| Number of catheters used | |
| 1 | 43 (10.1%) |
| 2 | 271 (63.5%) |
| ≥ 3 | 113 (26.4%) |
| Fluoroscopy time (min) | 11.22 ± 12.09 |
| Radiation dose (mGy) | 564 ± 538 |
| Contrast amount (mL) | 98.97 ± 54.09 |
| Procedure time (min) | 39.16 ± 34.6 |
| Angiography | 21.63 ± 9.98 |
| PCI | 82.99 ± 35.39 |
| Coronary lesions | |
| Normal coronaries | 134 (31.4%) |
| 1 vessel disease | 131 (30.7%) |
| 2 vessel disease | 87 (20.4%) |
| 3 vessel disease | 75 (17.6%) |
|
ACS, acute coronary syndrome; CCS, chronic coronary syndrome; Fr, French; IU, international unit; NSTEMI, non–ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention. |
|
Incidence and characteristics of radial artery occlusion
RAO occurred in 48 patients (11.24%). Of these, 89.6% were asymptomatic, and the radial pulse remained palpable in 14 patients (29.2%). At 1 month, 2 patients were lost to follow-up. Among the remaining 46 patients, spontaneous recanalization occurred in 13 patients (28.3%). At the 3-month follow-up, the recanalization rate increased to 32.6% (15 cases).
The site of RAO was the distal third in 7 patients (14.6%), the middle third in 21 patients (43.8%), and the proximal third in 20 patients (41.7%).
Predictors of radial artery occlusion
Patients with RAO were significantly younger (table 2). The mean periprocedural systolic blood pressure in the RAO group was significantly lower (138.04 mmHg ± 21.92, vs 145.84 mmHg ± 21.10; P = .017). Type A Barbeau test was associated with a higher risk of RAO compared with types B and C, and patients with occlusion had a smaller RA diameter (2.34 mm ± 0.40 vs 2.61 mm ± 0.37; P < .001) (figure 3).
Table 2. Comparison of patients with and without RAO
| Clinical data | Procedural data | ||||||
|---|---|---|---|---|---|---|---|
| Non-RAO (n= 379) | RAO (n= 48) | P | Non-RAO (n= 379) | RAO (n= 48) | P | ||
| Age | 62.6 ± 10.6 | 56.4 ± 14.0 | < .001* | Previous TRA | 61 (16.0%) | 7 (14.5) | .63 |
| Female sex | 122 (32.1%) | 17 (35.4%) | .65 | Diagnostic angiography | 266 (70.1%) | 39 (81.2%) | .11 |
| Hypertension | 237 (62.5%) | 23 (47%) | .051 | ≥ 2 puncture attempts | 147 (38.7%) | 22 (45.8%) | .41 |
| Diabetes | 182 (48%) | 27 (56%) | .28 | IV heparin | 136 (35.8%) | 20 (41.6%) | .43 |
| Dyslipidemia | 44 (11.6%) | 6 (12.5%) | .85 | Heparin dose (IU) | 5754 ± 1378 | 5007 ± 1352 | < .001* |
| Smoking | 75 (19.7%) | 9 (18.7%) | .86 | Spasm | 60 (15.8%) | 12 (25.0%) | .11 |
| BMI ≥ 30 kg/m2 | 123 (32.4%) | 16 (33.3%) | .90 | Procedure time (min) | 39.85 ± 34.56 | 33.73 ± 34.78 | .249 |
| Mean PSBP (mm Hg) | 145.84 ± 21.10 | 138.04 ± 21.92 | .017* | Number of catheters | 2.30 ± 0.88 | 2.21 ± 0.92 | .75 |
| Barbeau test type A | 99 (26.1%) | 20 (41.6%) | .044* | Occlusive hemostasis | 241 (63.5%) | 42 (87.5%) | .001* |
| RAD (mm) | 2.61 ± 0.37 | 2.34 ± 0.40 | < .001* | Hemostasis duration (h) | 4.29 ± 1.22 | 5.15 ± 1.41 | .006* |
| APT | 336 (88%) | 41 (85%) | .51 | ||||
| VKA (INR ≥ 2) | 16 (4.2%) | 5 (10.4%) | .061 | ||||
| MVCD | 149 (39.3%) | 13 (27.1%) | .20 | ||||
|
APT, antiplatelet therapy; BMI, body mass index; INR, international normalized ratio; IV, intravenous; IU, international unit; MVCD, multivessel coronary disease = ≥ 2 lesions ; PSBP, periprocedural systolic blood pressure; RAD , radial artery diameter; RAO, radial artery occlusion; TRA , transradial access; VKA, vitamin K antagonist. * Statistically significant. |
|||||||
Figure 3. Radial artery diameter as a predictor of occlusion. A: the radial diameter is significantly smaller if there is RAO. B: less than 2.5 mm, the risk of occlusion becomes greater.
RAO procedural factors are listed in table 2. An R/S ratio < 1 was found in 35 patients in the RAO group vs 153 patients in the non-RAO group (72.9% vs 40.3%, P < .001). The mean heparin dose was significantly lower in patients with RAO (5007 ± 1352IU vs 5754 ± 1378 IU; P < .001), and the dose adjusted to weight was also significantly lower in the RAO group (62.31 ± 17.82 IU/kg vs 75.73 ± 22.57 IU/kg; P < .001). In addition, the RAO rate decreased significantly when the heparin dose exceeded 70 IU/kg.
Forty-two patients in the RAO group had occlusive hemostasis vs 241 in the non-RAO group (87.5% vs 63.5%; P = .001). Surprisingly, two-thirds of our patients (283 [66.3%]) had occlusive hemostasis. The mean duration of hemostasis was longer if there was RAO (5.15 h ± 1.41 vs 4.29 h ± 1.22; P < .001).
On multivariate logistic regression analysis (figure 4), the following factors were independent predictors of RAO: young age (odds ratio [OR], 0.642; 95%CI, 0.480-0.858; P = .031), low periprocedural systolic blood pressure (OR, 0.598; 95%CI, 0.415-0.862; P = .007), type A Barbeau test (OR, 0.441; 95%CI, 0.198-0.981; P = .045), small RA diameter (OR, 0.371; 95%CI, 0.323-0.618; P = .031), insufficient anticoagulation (OR, 0.287; 95%CI, 0.163-0.505; P < .001), occlusive hemostasis (OR, 0.128; 95%CI, 0.047-0.353; P < .001), and a long hemostasis duration (OR, 1.786; 95%CI, 1.428-2.039; P < .001).
Figure 4. Independent factors predictive of radial artery occlusion. Multiple logistic regression analysis revealed that the independent factors predictive of radial occlusion were young age, low periprocedural systolic blood pressure, type A Barbeau test, small radial artery diameter, insufficient anticoagulation, occlusive hemostasis, and long hemostasis duration. 95%CI, 95% confidence interval; PSBP, periprocedural systolic blood pressure; RAO, radial artery occlusion.
Anatomic variations of the radial artery
The mean radial diameter was 2.58 mm ± 0.39, and the diameter was larger in men (2.69 mm ± 0.37 vs 2.36 mm ± 0.31; P < .001) and smaller in patients with diabetes (2.53 mm ± 0.38 vs 2.64 mm ± 0.38; P = .003). The mean radial diameter was significantly larger than the mean ulnar diameter (2.58 mm ± 0.39 vs 2.22 mm ± 0.43; P < .001).
Radial anatomical variations affected 63 patients (14.8%). The most common variation was a high origin of the RA, observed in 63.5% of cases (40 patients), followed by radial tortuosity in 28.6% (18 patients), radioulnar loop in 7.9% (5 patients). Anatomical variations were more frequent in women (23% vs 10.8%; P = .001) and in older patients, with a mean age of 66.3 years ± 10.2 vs 61.2 years ± 11.2 in those without variations (P = .001).
Periprocedural complications
Radial spasm occurred in 72 patients (16.9%). This complication was more frequent in women (29% vs 10.1%; P < .001), patients with diabetes (22.5% vs 11.5%; P = .002), and when 6-Fr catheters were used (14% vs 24%; P = .035). Forearm hematoma occurred in 25 patients (5.85%). According to the EASY classification,9 most hematomas were type I (17 patients, 68%), followed by type II (6 patients, 24%), with type III occurring in only 2 patients (8%).
DISCUSSION
The rate of RAO remains relatively high in some institutions.10,11 In the PROPHET study, the acute incidence of RAO (12%) was almost halved in 28 days (7%).3 Recanalization occurs as a result of activation of primary fibrinolysis.12 In the present study, the rate of radial recanalization at 3 months was 32.6%. The only predictor of recanalization was radial diameter: the larger the diameter, the higher the rate of spontaneous recanalization.
Zankl et al.13 found that RAO was located in the distal third of the forearm in 49% of patients, in the distal and middle third in 13.7%, and in the entire forearm (proximal third) in 37.3%. Dissections of the media also occur in the proximal RA, likely due to catheter progression or manipulation without protection of the sheath.14 In our opinion, this would explain the location of RAO in the proximal part of the artery.
Among our patients with RAO, 29.2% had a radial pulse. According to Uhlemann et al.,4 in 19.5% of patients with RAO on Doppler, the RA pulse was still palpable. This was likely due to retrograde filling of the RA by collaterals. Therefore, the diagnosis of RAO should be confirmed using a more objective method, such as Doppler ultrasound.
Young age is a predictor of RAO, possibly due to higher sympathetic reactivity in younger individuals, which increases their risk of spasm. However, this characteristic does not influence the rate of recanalization, likely because prolonged radial spasm leads to the formation of a permanent intra-arterial thrombus.
Low mean systolic blood pressure was also a predictor of radial occlusion. We speculate that hypertension and arterial stiffness may prevent complete interruption of flow during compression, thereby helping to maintain radial patency.15
There was a higher incidence of occlusion with type A Barbeau test. We believe that in cases with well-developed ulnar circulation, the ulnar artery generates a competitive retrograde flow that opposes the radial flow, promoting occlusion and hindering recanalization.
The likelihood of developing RAO is related to the size of the sheath,16 or more precisely, the R/S ratio.17 A prospective registry showed that 5-Fr sheaths reduced the rate of RAO by up to 55% compared with 6-Fr.4
A study by Pancholy et al.,18 demonstrated that intravenous heparin is as effective as intra-arterial heparin in reducing the incidence of RAO, suggesting that the systemic effect of heparin is more important than its local effect. A recently published meta-analysis identified higher heparin doses as the most significant measure for decreasing RAO.12 This results is in line with our finding that a dose of less than 70 IU/kg seems to promote the occurrence of RAO. The high prevalence of RAO and the benefit of higher doses of unfractionated heparin (≥ 50 IU/kg) in this setting were also highlighted by a meta-analysis of 112 studies.19 In a randomized superiority trial comparing high-dose (100 IU/kg) and standard-dose (50 IU/kg) heparin, the RAO rate was significantly lower in the high-dose group.20 Recent evidence suggests that a small dose of rivaroxaban, given orally after a transradial procedure, may decrease the occurrence of RAO at 1 month.21,22
Using the reverse Barbeau test, Sanmartin et al.23 found that 60% of patients had an absence of radial flow during compression. These observations led to the concept of nonocclusive hemostasis (patent hemostasis). In the PROPHET study,3 RAO was significantly less frequent in the group that underwent nonocclusive hemostasis than in the control group.
The duration of hemostatic compression has been studied in large, randomized trials.24-26 The authors concluded that compression duration was a strong predictor of RAO.
In a meta-analysis by Rashid et al.,27 the incidence of RAO after diagnostic coronary angiography was notably higher compared with percutaneous coronary intervention, possibly due to the use of higher anticoagulation doses during interventions.12 However, opposite findings have been reported by other studies.
In our sample, the mean radial diameter was 2.58 mm ± 0.39 and was significantly larger in men. Velasco et al.28 reported a mean arterial diameter of 2.22 ± 0.35 mm, while a Polish study found a mean diameter of 2.17 ± 0.53 mm for the right RA and 2.25 ± 0.43 mm for the left RA.29 The ulnar artery is also used in interventional cardiology,30 although there is no consensus on its size compared with the RA.
Autopsy studies of arterial anatomic variations of the upper extremity have reported frequencies between 4% and 18.5%.8 In the literature, the most frequent anatomic variation of the RA is high bifurcation. Yoo et al.31 reported a 2.4% incidence of high radial origin in 1191 Korean patients. Tortuosity of the RA frequently affects patients with high radial origin, possibly due to the elongated course of the RA predisposing it to tortuosity, which is considered one of the most common causes of procedural failure, along with radial spasm.32
Radioulnar loop is the most common cause of procedural failure with experienced operators.33 Angiographic evaluation of the radioulnar anastomosis is mandatory in such cases, as there is often a negotiable anastomosis between the radial and ulnar arteries.
In our study, radial spasm was the leading cause of procedural failure, occurring in 50% of the 14 patients who experienced such failures. Ruiz-Salmerón et al.34 found that RA anatomic variations were strongly associated with radial spasm in a multivariate analysis. The relationship between radial spasm and anatomic variations is mainly explained by the strong correlation with high radial origin and the radioulnar loop.
Study limitations
Since this study is a prospective registry and not a randomized trial, selection bias cannot be excluded. Our study represents a single-center experience with a limited number of patients, despite being one of the largest prospective registries of vascular ultrasound in radial catheterization to date. Among the other limitations of the study, we note the lack of standardized protocols for both heparin use and compression.
CONCLUSIONS
With the increasing number of transradial procedures and the greater age of patients undergoing these interventions, leading to more complex procedures, it is essential to maintain the patency of the RA for future access. Although predictors of RAO after cardiac catheterization have been identified, implementing preventive measures in practice remains a challenge. The main modifiable predictors associated with the risk of RAO are insufficient heparinization and occlusive hemostasis. Therefore, preventive strategies should primarily focus on addressing these 2 factors.
FUNDING
None.
ETHICAL CONSIDERATIONS
The study was conducted in accordance with the provisions of the Declaration of Helsinki and with the International Conference on Harmonization Good Clinical Practices and was approved by the local ethics committee. All patients included in the study provided written informed consent, which is archived and available. Our study population included both sexes. Gender had no influence on the occurrence of radial occlusion.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
No artificial intelligence software was used in the preparation of this study.
AUTHORS’ CONTRIBUTIONS
All authors meet the criteria for authorship as defined by the International Committee of Medical Journal Editors. M.S. Lounes, A. Meftah, C. Belhadi, K. Allal, H. Boulaam, A. Sayah, I. Hafidi, and E. Tebache contributed to the acquisition and analysis of data for this article. M.S. Lounes, A. Bedjaoui, A. Allali, and S. Benkhedda were responsible for the study design and the writing of the article. M.S. Lounes, A. Allali, and S. Benkhedda contributed to writing and critical revision of the content. All authors have read and approved the final version of the article and agree to be accountable for all aspects of the work, including the accuracy and integrity of all its parts.
CONFLICTS OF INTEREST
None.
WHAT IS KNOWN ABOUT THE TOPIC?
- Despite recommendations on the prevention of RAO in interventional cardiology, its incidence remains relatively high in some centers.
- Spontaneous recanalization of the artery may occur during follow-up.
- Permanent occlusion of the radial artery prevents any possibility of its further use (interventional procedures, dialysis, etc.)
WHAT DOES THIS STUDY ADD?
- RAO is not limited to the distal part of the artery and can affect the entire length of the vessel.
- Diagnosis of RAO should be confirmed using Doppler ultrasound, which remains the gold standard.
- The 2 independent modifiable predictors of RAO are the anticoagulation protocol and hemostasis technique.
- Anatomic variations of the RA may impact the procedure. A high origin of the RA is the most frequent, followed by radial tortuosities. After radial spasm, the radioulnar loop is the most common cause of procedural failure with experienced operators.
REFERENCES
1. Cruden NL, Teh CH, Starkey IR, Newby DE. Reduced vascular complications and length of stay with transradial rescue angioplasty for acute myocardial infarction. Catheter Cardiovasc Interv. 2007;70:670-675.
2. Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87-165.
3. Pancholy SB, Coppola J, Patel T, Roke-Thomas M. Prevention of radial artery occlusion-patent hemostasis evaluation trial (PROPHET study):a randomized comparison of traditional versus patency documented hemostasis after transradial catheterization. Catheter Cardiovasc Interv. 2008;72:335-340.
4. Uhlemann M, Möbius-Winkler S, Mende M, et al. The Leipzig prospective vascular ultrasound registry in radial artery catheterization:impact of sheath size on vascular complications. JACC Cardiovasc Interv.2012;5:36-43.
5. Da Silva RL, Britto PF, Joaquim RM, et al. Clinical accuracy of reverse Barbeau test in the diagnosis of radial artery occlusion after transradial catheterization. J Transcat Intervent. 2021;29:eA20200037.
6. Costa F, van Leeuwen MA, Daemen J, et al. The Rotterdam Radial Access Research:Ultrasound-Based Radial Artery Evaluation for Diagnostic and Therapeutic Coronary Procedures. Circ Cardiovasc Interv. 2016;9:003129.
7. Uglietta JP, Kadir S. Arteriographic study of variant arterial anatomy of the upper extremities. Cardiovasc Intervent Radiol. 1989;12:145-148.
8. Rodríguez-Niedenführ M, Vázquez T, Nearn L, et al. Variations of the arterial pattern in the upper limb revisited:a morphological and statistical study, with a review of the literature. J Anat. 2001;199:547-566.
9. Bertrand OF. Acute forearm muscle swelling post transradial catheterization and compartment syndrome:prevention is better than treatment!Catheter Cardiovasc Interv. 2010;75:366-368.
10. Sadaka MA, Etman W, Ahmed W, et al. Incidence and predictors of radial artery occlusion after transradial coronary catheterization. Egypt Heart J. 2019;71:12.
11. Dousi M, Sotirakou K, Fatsi A. The Use of Acetylsalicylic Acid As A Measure of Prevention of Radial Artery Occlusion in Patients Who Perform Coronary Angiography with Tra Technique. J Radiol Clin Imaging. 2020;3:13-21.
12. Rashid M, Kwok CS, Pancholy S, et al. Radial Artery Occlusion After Transradial Interventions:A Systematic Review and Meta-Analysis. J Am Heart Assoc. 2016;5:002686.
13. Zankl AR, Andrassy M, Volz C, et al. Radial artery thrombosis following transradial coronary angiography:incidence and rationale for treatment of symptomatic patients with low-molecular-weight heparins. Clin Res Cardiol. 2010;99:841-847.
14. Bi XL, Fu XH, Gu XS, et al. Influence of Puncture Site on Radial Artery Occlusion After Transradial Coronary Intervention. Chin Med J (Engl). 2016;129:898-902.
15. Buturak A, Gorgulu S, Norgaz T, et al. The long-term incidence and predictors of radial artery occlusion following a transradial coronary procedure. Cardiol J. 2014;21:350-356.
16. Dahm JB, Vogelgesang D, Hummel A, et al. A randomized trial of 5 vs. 6 French transradial percutaneous coronary interventions. Catheter Cardiovasc Interv. 2002;57:172-176.
17. Saito S, Ikei H, Hosokawa G, Tanaka S. Influence of the ratio between radial artery inner diameter and sheath outer diameter on radial artery flow after transradial coronary intervention. Catheter Cardiovasc Interv.1999;46:173-178.
18. Pancholy SB. Comparison of the effect of intra-arterial versus intravenous heparin on radial artery occlusion after transradial catheterization. Am J Cardiol. 2009;104:1083-1085.
19. Hahalis GN, Aznaouridis K, Tsigkas G, et al. Radial Artery and Ulnar Artery Occlusions Following Coronary Procedures and the Impact of Anticoagulation:ARTEMIS Systematic Review and Meta-Analysis. J Am Heart Assoc. 2017;6:005430.
20. Hahalis GN, Leopoulou M, Tsigkas G, et al. Multicenter Randomized Evaluation of High Versus Standard Heparin Dose on Incident Radial Arterial Occlusion After Transradial Coronary Angiography:The SPIRIT OF ARTEMIS Study. JACC Cardiovasc Interv. 2018;11:2241-2250.
21. Liang D, Lin Q, Zhu Q, et al. Short-Term Postoperative Use of Rivaroxaban to Prevent Radial Artery Occlusion After Transradial Coronary Procedure:The RESTORE Randomized Trial. Circ Cardiovasc Interv. 2022;15:011555.
22. Hammami R, Abid S, Jihen J, et al. Prevention of radial artery occlusion with rivaroxaban after trans-radial access coronary procedures:The RIVARAD multicentric randomized trial. Front Cardiovasc Med. 2023;10:1160459.
23. Sanmartin M, Gomez M, Rumoroso JR, et al. Interruption of blood flow during compression and radial artery occlusion after transradial catheterization. Catheter Cardiovasc Interv. 2007;70:185-189.
24. Politi L, Aprile A, Paganelli C, et al. Randomized clinical trial on short-time compression with Kaolin-filled pad:a new strategy to avoid early bleeding and subacute radial artery occlusion after percutaneous coronary intervention. J Interv Cardiol. 2011;24:65-72.
25. Dharma S, Kedev S, Patel T, et al. A novel approach to reduce radial artery occlusion after transradial catheterization:postprocedural/prehemostasis intra-arterial nitroglycerin. Catheter Cardiovasc Interv.2015;85:818-825.
26. Aminian A, Saito S, Takahashi A, et al. Impact of sheath size and hemostasis time on radial artery patency after transradial coronary angiography and intervention in Japanese and non-Japanese patients:A substudy from RAP and BEAT randomized multicenter trial. Catheter Cardiovasc Interv. 2018;92:844-851.
27. Sinha SK, Jha MJ, Mishra V, et al. Radial Artery Occlusion. Incidence, Predictors and Long-term outcome after TRAnsradial Catheterization:clinico-Doppler ultrasound-based study (RAIL-TRAC study). Acta Cardiol. 2017;72:318-327.
28. Velasco A, Ono C, Nugent K, et al. Ultrasonic evaluation of the radial artery diameter in a local population from Texas. J Invasive Cardiol. 2012;24:339-341.
29. Peruga JP, Peruga JZ, Kasprzak JD, et al. Ultrasound evaluation of forearm arteries in patients undergoing percutaneous coronary intervention via radial artery access:results of one-year follow-up. Kardiologia Polska. 2015;73:502-510.
30. Knebel AV, Cardoso CO, Correa Rodrigues LH, et al. Safety and feasibility of transulnar cardiac catheterization. Tex Heart Inst J. 2008;35:268-272.
31. Yoo BS, Yoon J, Ko JY, et al. Anatomical consideration of the radial artery for transradial coronary procedures:arterial diameter, branching anomaly and vessel tortuosity. Int J Cardiol. 2005;101:421-427.
32. Pristipino C, Roncella A, Trani C, et al. Identifying factors that predict the choice and success rate of radial artery catheterisation in contemporary real world cardiology practice:a sub-analysis of the PREVAIL study data. EuroIntervention. 2010;6:240-246.
33. Louvard Y, Lefèvre T. Loops and transradial approach in coronary diagnosis and intervention. Catheter Cardiovasc Interv. 2000;51:250-252.
34. Ruiz-Salmerón RJ, Mora R, Vélez-Gimón M, et al. Radial artery spasm in transradial cardiac catheterization. Assessment of factors related to its occurrence, and of its consequences during follow-up. Rev Esp Cardiol. 2005;58:504-511.
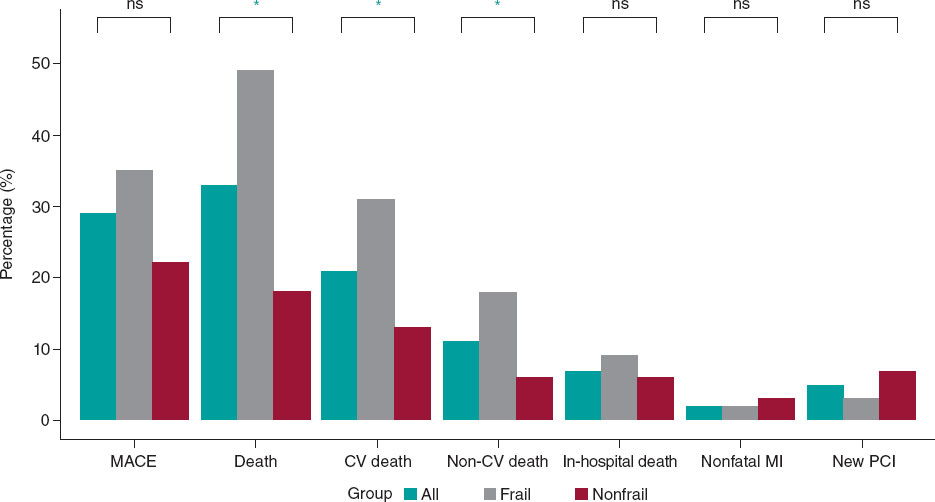
ABSTRACT
Introduction and objectives: In elderly and frail patients, there is limited evidence on the therapeutic management of left main coronary artery (LM) disease. The objective of this study was to evaluate mid-term clinical outcomes in older adults undergoing percutaneous coronary intervention (PCI) of LM.
Methods: We conducted a retrospective study including all older patients (≥ 75 years) undergoing LM-PCI at a high-volume center between 2017 and 2021. The primary endpoint was a composite of major adverse cardiovascular events (MACE). Patients were grouped according to the presence of frailty based on the FRAIL scale. Inverse probability of treatment weighting was used to account for clinical differences between the 2 groups.
Results: A total of 140 patients were included in the study (median age 80 [78-84]; 36% women). Of them, 49% met the criteria for frailty. After a median follow-up of 19 [5-35] months, 40 MACE (29%) were recorded. The all-cause death rate was 32%. There were no differences in the risk of MACE between frailty groups, but patients with frailty had an increased risk of all-cause mortality (HRadj, 1.95 [1.02-3.75]; P = .046).
Conclusions: LM-PCI in older adults with multiple associated comorbidities could be considered a feasible option in this special population. The rate of MACE at follow-up was acceptable. Frailty was associated with a worse prognosis in terms of all-cause mortality at follow-up.
Keywords: Coronary artery disease. Left main coronary artery. Percutaneous coronary intervention. Elderly. Frailty.
RESUMEN
Introducción y objetivos: La evidencia sobre el abordaje terapéutico de la enfermedad del tronco coronario izquierdo (TCI) en pacientes ancianos y frágiles es limitada. El objetivo de este estudio fue evaluar los resultados clínicos a medio plazo en ancianos que recibieron una intervención coronaria percutánea (ICP) del TCI.
Métodos: Estudio retrospectivo en el que se incluyeron todos los pacientes ancianos (≥ 75 años) tratados con ICP del TCI en un centro de alto volumen entre 2017 y 2021. El objetivo principal fue un compuesto de eventos adversos cardiovasculares mayores (MACE). Los pacientes fueron agrupados en función de su fragilidad según la escala FRAIL. Se utilizó la ponderación de probabilidad inversa de tratamiento para tener en cuenta las diferencias clínicas entre los 2 grupos.
Resultados: Se incluyeron 140 pacientes (mediana de edad: 80 años [78-84]; 36% mujeres), de los cuales el 49% cumplían los criterios de fragilidad. Tras una mediana de seguimiento de 19 meses (5-35) se registraron 40 MACE (29%). La tasa de mortalidad por todas las causas fue del 32%. No se observaron diferencias en el riesgo de MACE entre los grupos, aunque los pacientes frágiles presentaron una mayor mortalidad por todas las causas (HRa = 1,95 [1,02-3,75]; p = 0,046).
Conclusiones: La ICP del TCI en pacientes ancianos con comorbilidad podría considerarse una opción factible en esta población especial. La tasa de MACE en el seguimiento resulta aceptable. La fragilidad se asoció con un peor pronóstico en términos de mortalidad por todas las causas durante el seguimiento.
Palabras clave: Enfermedad arterial coronaria. Tronco coronario izquierdo. Intervención coronaria percutánea. Paciente anciano. Fragilidad.
Abbreviations
CABG: coronary artery bypass grafting. LM: left main coronary artery. PCI: percutaneous coronary intervention.
INTRODUCTION
The left main coronary artery (LM) supplies 84% of the blood flow to the left ventricle in patients with right dominance,1 making LM disease the coronary lesion with the worst prognosis. The prevalence of this disease is not negligible, as it is found in 4.8% of coronary angiograms,2 highlighting the prognostic importance of these lesions. Conservative treatment is a rarely a feasible option due to the high rate of cardiac adverse events during short-term follow-up, with a mortality rate exceeding 50%.3
Coronary artery bypass grafting (CABG) has traditionally been the most widely accepted revascularization strategy.4 In recent years, there have been significant pharmacological and technological improvements in percutaneous revascularization techniques, such as drug-eluting stents and intracoronary diagnostic techniques.5 These improvements, together with comparative studies, have prompted discussion on the various alternatives.6 Presently, the choice of revascularization strategy should be based on the complexity of the coronary anatomy and surgical risk.7
However, evidence is limited in older adults who are scarcely represented in classic studies. Furthermore, in these patients, frailty is a frequent and unstudied characteristic that can influence their prognosis. In this special population, CABG is usually ruled out due to high-surgical risk. On the other hand, percutaneous coronary intervention (PCI) could be a potential therapeutic option, although with little evidence to date.8 Consequently, we postulated that PCI of the LM might be feasible and safe in older patients, with a low incidence of associated complications and an acceptable rate of major adverse cardiac events (MACE) during follow-up.
METHODS
Study design
We conducted a retrospective, single-center study of older patients diagnosed with LM disease who underwent PCI. The study aimed to evaluate mid-term clinical outcomes and examine the prognostic significance of frailty in these patients. The study protocol was approved by the local clinical research ethics committee according to institutional and good clinical practice guidelines. Recruitment took place from January 2017 to December 2021 at Hospital Universitario Reina Sofía (Cordoba, Spain). Patients were eligible if they were aged ≥ 75 years at the time of LM disease diagnosis, and PCI was chosen as the treatment after deliberation by heart team discussion, or due to instability requiring emergent revascularization. Exclusion criteria consisted of end-stage chronic diseases, patients under palliative care, contraindications to dual antiplatelet therapy, and incomplete follow-up data. Included patients were grouped according to frailty status, determined by the FRAIL scale, with patients scoring 3 or more points considered frail.9 Definitions are shown in the supplementary data.
Outcomes
The main objective of the study was to describe mid-term clinical outcomes in older patients undergoing LM-PCI. We also aimed to compare clinical events according to the presence of frailty. The primary endpoint was a composite of MACE, defined as a composite of cardiovascular death (including death of uncertain cause), nonfatal myocardial infarction, the need for new revascularization, and stroke. Secondary outcomes were the individual components of MACE and all-cause mortality.
Angiographic analysis
Quantitative analysis of the coronary arteries was performed using the validated CAAS system (Pie Medica Imaging, the Netherlands). The basal anatomy of the LM bifurcation with the anterior descending artery and the circumflex artery was classified according to the Medina classification.10 The measurements analyzed included the reference diameter of the LM and its percentage of stenosis. The complexity of the coronary anatomy was studied using the SYNTAX scale.6
Statistical analysis
Categorical data are presented as counts (percentages), while continuous data are expressed as mean ± standard deviation or median [interquartile range]. Between-group comparisons were performed using the chi-square test or the Fisher exact test for categorical variables and the Student t-test or the Mann-Whitney U test for continuous variables. Kaplan-Meier curves and Cox regression models were used to analyze clinical events according to frailty. Inverse probability of treatment weighting (IPTW) was used to account for clinical differences between the 2 groups.11 Propensity scores were calculated using a logistic regression model that included the following covariates: age, sex, left ventricular ejection fraction, atrial fibrillation, chronic kidney disease, anemia, and chronic obstructive pulmonary disease. Standardized mean differences before and after weighting were used to evaluate the balance of the groups regarding the covariates. A difference of < 10% was considered to indicate a satisfactory balance. The distributions of the propensity scores before and after weighting were plotted to assess the degree of overlap between the 2 groups. Confidence intervals for the IPTW coefficients were obtained using robust sandwich-type variance estimators (figure 1 of the supplementary data).12 All tests were 2-tailed and significance was set at P < .05. Statistical analyses were performed using SPSS software (V 24; IBM Corp., United States) and R software (V4.0.3; R Foundation for Statistical Computing, Austria).
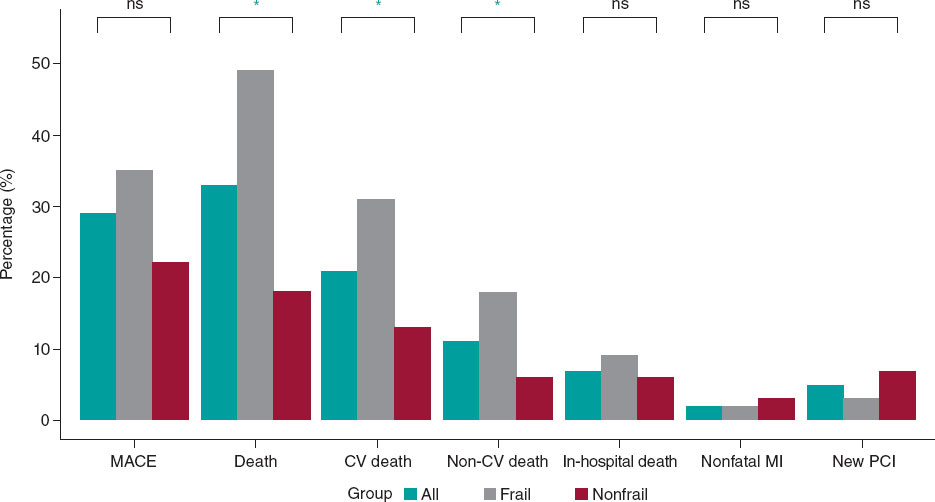
Figure 1. Main events to follow-up. CV, cardiovascular; MACE, mayor adverse cardiovascular events; MI, myocardial infarction; NS, nonsignificant; PCI, percutaneous coronary intervention. * P < .005.
RESULTS
During the study period, our hospital treated 437 patients with significant LM lesions percutaneously. Of them, a total of 140 patients met the inclusion criteria and were included in the analysis (figure 2 of the supplementary data).
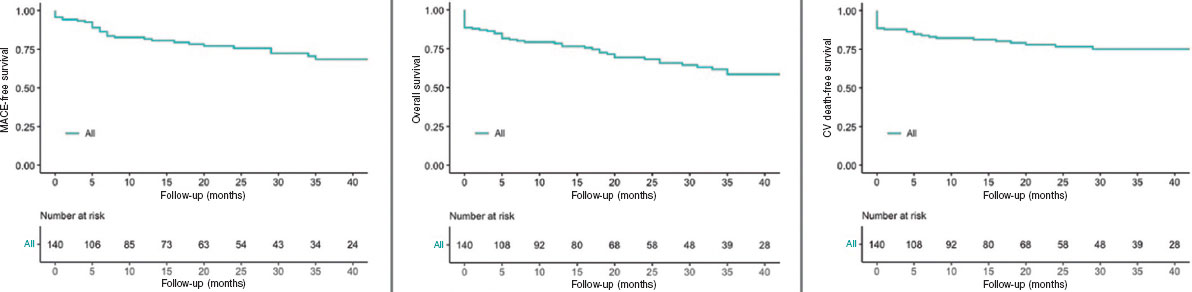
Figure 2. Kaplan-Meier Curves of the primary outcome and mortality. CV, cardiovascular; MACE, major adverse cardiovascular events.
Baseline characteristics
The baseline clinical characteristics, clinical presentation and antithrombotic treatment administered are detailed in table 1. The median age of the patients was 80 [78-84] years and 36% (51 patients) were women. Most of the patients had a history of hypertension (84%, 118 patients) and 58% (81 patients) were diabetic. More than a third of the patient cohort had a previous personal history of ischemic heart disease (37%, 52 patients) and 33% (46 patients) had chronic kidney disease. Among noncardiovascular comorbidities, active cancer was present in 11 patients (8%) and prior blood transfusions had been required in 16 patients (11%). The mean EuroSCORE II was 3.07 [1.96-5.7] to assess surgical risk. Forty-eight patients (34%) had left ventricular systolic dysfunction at the time of revascularization.
Table 1. Patients’ baseline characteristics
| Characteristics | Total n = 140 | Nonfrail n = 72 (51) | Frail n = 68 (49) | P |
|---|---|---|---|---|
| Baseline clinical characteristics | ||||
| Age, years | 80 [78-84] | 80 [77-84] | 80 [78-84] | .090 |
| Female sex | 51 (36) | 18 (25) | 33 (49) | .004 |
| Hypertension | 118 (84) | 61 (85) | 57 (84) | .884 |
| Diabetes | 81 (58) | 36 (50) | 45 (66) | .053 |
| Hypercholesterolemia | 112 (80) | 56 (78) | 56 (82) | .999 |
| Smoking history | 7 (5) | 5 (7) | 2 (3) | .442 |
| Previous ischemic heart disease | 52 (37) | 31 (43) | 21 (31) | .136 |
| Chronic kidney disease | 46 (33) | 22 (33) | 24 (39) | .481 |
| Atrial fibrillation | 22 (16) | 7 (10) | 15 (22) | .041 |
| Peripheral artery disease | 20 (14) | 14 (20) | 6 (9) | .073 |
| COPD | 17 (12) | 6 (8) | 11 (16) | .156 |
| Previous stroke | 16 (11) | 10 (14) | 6 (9) | .073 |
| Valve disease | 15 (11) | 7 (7) | 10 (15) | .114 |
| Anemia | 29 (21) | 10 (14) | 19 (28) | .040 |
| Active cancer | 11 (8) | 7 (10) | 4 (6) | .399 |
| Liver disease | 4 (3) | 3 (4) | 1 (2) | .339 |
| Previous blood transfusions | 16 (11) | 5 (7) | 11 (16) | .086 |
| Recent surgery or trauma | 38 (27) | 19 (26) | 19 (28) | .836 |
| EuroScore II | 3.07 [1.96-5.7] | 2.76 [1.83-4.18] | 3.80 [2.04-7.85] | .010 |
| Glomerular filtration rate (mL/min) | 71.4 [48.4-87.3] | 76.71 [51.01-87.51] | 61.40 [41.40-81.85] | .072 |
| Creatinine (mg/dL) | 1.02 [0.87-1.30] | 1.00 [0.80-1.85] | 1.03 [0.90-1.50] | .109 |
| Hemoglobin (mg/dL) (mean, ±SD) | 12.6 (± 2) | 13.02 (± 2) | 12.16 (± 1.9) | .017 |
| Hematocrit | 38.6 [34.6-43.0] | 39.6 [36.0-44.7] | 36.6 [33.9-42.1] | .031 |
| Platelets (× 109/L) | 208 [171-246] | 211 [182-244] | 196 [160-250] | .340 |
| Hs-cTnI (ng/L) | 954 [40-7352] | 2250 [30-10 000] | 650 [40-5600] | .245 |
| LVEF | 60 [39-67] | 60 [45-68] | 58 [35-63] | .245 |
| LV systolic dysfunction | 48 (34) | 20 (32) | 28 (46) | .106 |
| Clinical presentation | ||||
| Acute coronary syndrome | 85 (61) | 45 (63) | 40 (59) | .656 |
| NSTEMI | 61 (44) | 28 (39) | 33 (49) | .250 |
| STEMI | 9 (6) | 6 (8) | 3 (4) | .495 |
| Unstable angina | 15 (11) | 11 (15) | 4 (6) | .101 |
| Chronic coronary syndrome | 55 (39) | 27 (38) | 28 (41) | .656 |
| Antiplatelet therapy | ||||
| Dual antiplatelet therapy | 104 (74) | 57 (79) | 47 (69) | .174 |
| Aspirin + clopidogrel | 61 (43) | 31 (43) | 30 (44) | .899 |
| Aspirin + ticagrelor | 43 (31) | 26 (36) | 17 (25) | .154 |
| Triple antiplatelet therapy | ||||
| Aspirin + clopidogrel + anticoagulant | 36 (26) | 15 (2) | 21 (31) | .174 |
|
COPD, chronic obstructive pulmonary disease; Hs-cTnI, high sensitivity cardiac troponin I; LV, left ventricle; LVEF, left ventricular ejection fraction; NSTEMI, non-ST-elevation myocardial infarction; STEMI, ST-segment elevation myocardial infarction. Data are expressed as No. (%), mean ± standard deviation or median [interquartile range]. |
||||
The most common clinical presentation was acute coronary syndrome (85 patients, 61% of cases). Among these, onset consisted of ST-segment elevation myocardial infarction (STEMI) in 9 patients (6%), non-ST-segment elevation myocardial infarction in 61 patients (44%), and unstable angina in 15 patients (10%). The remaining patients (55, 39%) presented with chronic coronary syndrome.
A total of 104 patients (74%) were discharged with dual antiplatelet therapy. The main combination was aspirin and clopidogrel (61 patients, 43%). In 36 patients (26%), initial triple therapy (anticoagulation and dual antiplatelet therapy) was chosen due to concurrent conditions requiring chronic oral anticoagulation.
Based on the FRAIL scale, almost half of the patients (68 patients, 49%) met clinical criteria for frailty at the time of revascularization. The baseline characteristics of frail and nonfrail patients are shown in table 1. No statistically significant differences were found in terms of age, main cardiovascular risk factors or noncardiovascular comorbidities between the 2 groups. However, compared with nonfrail patients, those with frailty were more likely to be female (49% vs 25%; P = .004), to have atrial fibrillation (22% vs 10%; P = .041), a higher EuroSCORE level (3.80 vs 2.76; P = .010), and anemia (28% vs 14%; P = .040), and consequently a lower hematocrit and hemoglobin value (36.6% vs 39.6%; P = .031 and 12.16 mg/dL vs 13.02 mg/dL; P = .017, respectively).
Angiographic and procedural characteristics
Angiographic and procedural data are shown in table 2. The arterial access of choice was radial access (81% of procedures, 113 patients). A median SYNTAX score of 21 [15-29.5] was observed in 96 patients (68%) with multivessel disease, and 62 patients (44%) had a SYNTAX score > 22. The most common angiographic involvement of the LM was the distal segment (61%, 86 patients), while the most common plaque distribution according to the Medina classification was “1,1,1” (35 patients, 41% of LM bifurcation lesions). The strategy of choice for the treatment of the bifurcation was the provisional stent strategy (85% of LM bifurcation lesions, 73 patients), while the upfront 2-stent strategy was used in only 13 patients (15% of the LM bifurcation lesions). The mean diameter of the LM was 4.1 [± 3.5-4.5] mm with a mean angiographic stenosis of 62% (± 7). In 59 patients (42%), the procedure was guided using intravascular imaging techniques (58 patients using intracoronary ultrasound and 1 patient using coherence tomography). Coronary physiology was used in 5 patients (4%) to guide the need for revascularization or to check the result after percutaneous treatment. In 7 (5%) patients, mechanical support was required, either due to cardiogenic shock, or as a preventive measure in high-risk angioplasty (5 patients with an intra-aortic balloon pump and 2 with an Impella CP device [Abiomed, United States]). Intraprocedural complications occurred in 8 patients (6%), including a major complication in 4 patients (3 intraprocedural deaths and 1 cardiogenic shock), and a minor complication in 4 patients (1 coronary dissection with Thrombolysis in Myocardial Infarction (TIMI) grade 3 distal flow, 1 pseudoaneurysm, and 2 bleeding events from the femoral access resolved by stent implantation). The LM diameter was larger in patients with frailty than in those without (4 mm [4-4.5] vs 3.5 mm [3.5-4.5]; P = .023), a paradoxical finding since the percentage of women was higher in the group with frailty percentage of women. However, this information did not seem to be clinically relevant. No other clinically relevant differences were found between the 2 groups (table 2).
Table 2. Patients’ angiographic and procedural characteristics
| Characteristics | Total n = 140 | Nonfrail n = 72 (51) | Frail n = 68 (49) | P |
|---|---|---|---|---|
| Angiographic characteristics | ||||
| Multivessel disease | 96 (68) | 50 (69) | 46 (68) | .819 |
| SYNTAX score | 21 [15-29,5] | 21 [17-28.5] | 21.5 [14-30.6] | .752 |
| SYNTAX score > 22 | 62 (44) | 25 (39) | 31 (46) | .463 |
| LM diameter (mm) | 4 [3.5-4.5] | 3.5 [3.5-4.5] | 4 [4-4.5] | .023 |
| LM stenosis | 62 (± 7) | 64 (± 6) | 61 (± 5) | .342 |
| LM bifurcation | 86 (61) | 39 (54) | 47 (69) | .069 |
| Medina (1,1,1) | 35 (41) | 20 (51) | 15 (32) | .690 |
| Medina (1,1,0) | 33 (39) | 10 (26) | 23 (49) | .027 |
| Medina (1,0,1) | 8 (9) | 3 (8) | 5 (11) | .724 |
| Medina (0,1,1) | 3 (3) | 2 (5) | 1 (2) | .588 |
| Medina (1,0,0) | 4 (5) | 1 (3) | 3 (6) | .623 |
| Medina (0,1,0) | 0 (0) | 0 (0) | 0 (0) | - |
| Medina (0,0,1) | 3 (3) | 3 (8) | 0 (0) | .089 |
| Intracoronary diagnostic technique | ||||
| Intravascular imaging | 59 (42) | 28 (39) | 31 (46) | .422 |
| IVUS | 58 (41) | 28 (39) | 30 (44) | .530 |
| OCT | 0 (0) | 0 (0) | 1 (2) | .486 |
| Intracoronary physiology test | 5 (4) | 4 (6) | 1 (2) | .367 |
| Procedure characteristics | ||||
| Radial access | 113 (81) | 60 (83) | 53 (78) | .253 |
| Contrast (mL) | 200 [160-255] | 215 [150-259] | 200 [160-250] | .553 |
| Temporary pacemakers | 6 (4) | 3 (4) | 3 (4) | 1.000 |
| LV assist devices | 7 (5) | 4 (6) | 3 (4) | 1.000 |
| Intra-aortic balloon pump | 5 (4) | 4 (6) | 1 (2) | .367 |
| Impella | 2 (1) | 0 (0) | 2 (3) | .239 |
| One-stent bifurcation technique | 73 (85) | 34 (87) | 39 (83) | .588 |
| Stent MB + kissing | 20 (27) | 12 (35) | 7 (18) | .077 |
| Two-stent bifurcation technique | 13 (15) | 5 (13) | 8 (17) | .636 |
| T stenting | 3 (23) | 2 (40) | 1 (12.5) | .498 |
| TAP | 2 (15) | 0 (0) | 2 (25) | .498 |
| Culotte | 5 (39) | 1 (20) | 4 (50) | .371 |
| DK-Crush | 2 (15) | 1 (20) | 1 (12.5) | 1.000 |
| SKS | 1 (8) | 1 (20) | 0 (0) | .413 |
| MB stent diameter (mm) | 3.5 [3-3.5] | 3.5 [3-3.5] | 3.5 [3-3.5] | .877 |
| MB stent length (mm) | 18 [15-18] | 18 [15-18] | 18 [15-18] | .896 |
| SB stent diameter (mm) | 3.5 [3-3.5] | 3.25 [2.8-3.5] | 3.5 [3-3.6] | .371 |
| SB stent length (mm) | 15 [12-18] | 15.5 [15-21] | 15 [11-18] | .342 |
| Complications | ||||
| Intraprocedural complications | 8 (6) | 6 (8) | 2 (3) | .157 |
| Major | 4 (3) | 3 (4) | 1 (2) | .356 |
| Minor | 4 (3) | 3 (4) | 1 (2) | .356 |
|
DK, double kissing; IVUS, intravascular ultrasound; LM, left main; LV, left ventricle; MB, main branch; OCT, optical coherence tomography; SB, side branch; SKS, simultaneous kissing stents. TAP, T and small protrusion. Data are expressed as No. (%), mean ± standard deviation or median [interquartile range]. |
||||
Clinical results at follow-up
After a median follow-up of 19 months [5-35], a total of 40 (29%) MACE were recorded: 3 (2%) patients had a nonfatal myocardial infarction, 7 (5%) patients required repeat revascularization (3 for restenosis of the LM, and 4 in a different vessel), and 30 patients (21%) died of cardiac and/or uncertain causes. No strokes were reported during follow-up. Sixteen patients (11%) died of noncardiac causes during follow-up.
Clinical outcomes are presented in figure 1 and figure 2. No independent predictor of MACE was identified. The independent predictors of all-cause mortality were left ventricular ejection fraction (hazard ratio [HR], 0.90 [0.96-0.99]; P = .014), chronic kidney disease (HR, 2.26 [1.16-4.42]; P = .017), and particularly the presence of frailty (HR, 2.42 [1.17-5.02]; P = .018) (table 1 of the supplementary data). The primary endpoint of MACE occurred in 24 (35%) patients in the frail group and in 16 (22%) patients in the nonfrail group (HR, 1.61 [0.79-3.28]; P = .193). Frail patients had an increased risk of cardiovascular mortality: 21 (31%) vs 9 (13%); HR, 2.64 (1.21-5.77); P = .015. All-cause mortality was also more frequent in the frail group: 33 (49%) vs 13 (18%); HR, 2.94 (1.55-5.59); P = .001). The events during follow-up are presented in table 2 of the supplementary data. After IPTW adjustment, only the difference in all-cause mortality remained significant (HR, 1.95 [1.02-3.75]; P = .046). Survival analysis of the weighted population is shown in figure 3.
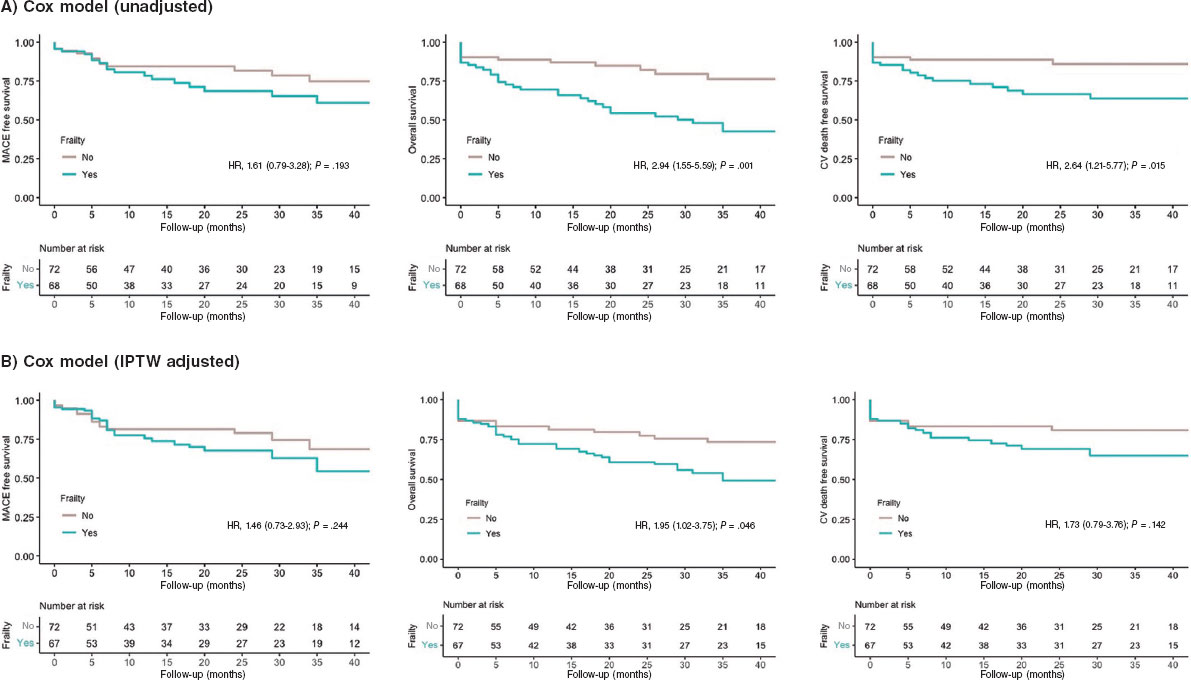
Figure 3. Kaplan-Meier Curves of the secondary outcomes. CV, cardiovascular; IPTW, inverse probability of treatment weighting; MACE, major adverse cardiovascular events.
DISCUSSION
The present study describes the feasibility of LM-PCI in a cohort of older patients. The main results were as follows: a) the rate of MACE at mid-term follow-up was 29%, mainly driven by cardiovascular and/or uncertain cause death; b) a high percentage of frailty was found in our population (49%); c) frail patients had a 2-fold increased risk of all-cause mortality during follow-up (HR, 1.95 [1.02-3.75]; P = .046) (figure 4).
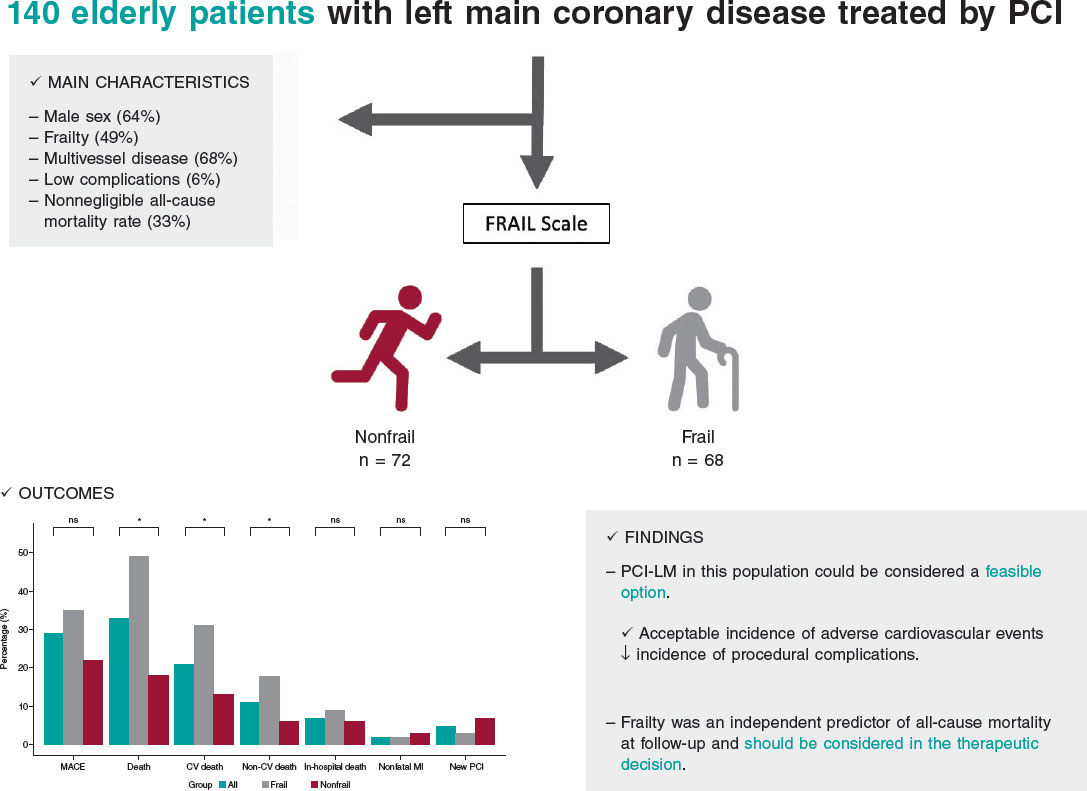
Figure 4. Central illustration. Results of percutaneous treatment of LM in elderly patients and impact of frailty. CV, cardiovascular; LM, left main coronary artery; MACE, major adverse cardiovascular events; MI, myocardial infarction; NS, non-significant; PCI, percutaneous coronary intervention.
The treatment of LM disease has traditionally been surgical, given the complexity involved and significant prognostic impact.13 However, the marked advances in interventional cardiology in recent decades have modified the approach.14,15 Contrasting evidence from clinical trials and meta-analyses shows that percutaneous treatment has similar results to surgical approaches in terms of mortality, acute myocardial infarction, and stroke at 5 years of follow-up.16 This shift has is reflected in the evolving recommendations in clinical practice guidelines, and the current European revascularization guidelines assign a grade of recommendation IA to both surgical and percutaneous strategies for the treatment of LM disease when the anatomy is not complex (SYNTAX < 22), and a class IIa recommendation for cases of intermediate complexity (SYNTAX 23-32).7
Nevertheless, the population analyzed in the study has specific clinical characteristics, and is not usually represented in large clinical trials (older patients and those with frailty and a high burden of associated comorbidities). These variables are not systematically included in surgical risk scores but are generally taken into account in routine clinical practice and often influence heart team decisions on the treatment strategy.17 Therefore, because this particular patient cohort is often excluded from research, there are no conclusive data on the benefit of percutaneous revascularization.
Our results are in line with those of previous registries in terms of MACE and all-cause mortality, as well as the association between age and a marked incidence of mortality due to noncardiac causes during follow-up. However, unlike earlier studies, we observed no differences in cardiovascular mortality, despite these patients having a more complex coronary anatomy than younger patients.18 In this regard, our study cohort had a median SYNTAX score of 21, and 44% of the patients had a score above 22. Like previous studies, this SYNTAX index score was not associated with a higher probability of cardiac events during follow-up in this special population.
In the present study, rates of acute myocardial infarction and new revascularization of the target lesion were lower than in other cohorts. Although it is difficult to make direct comparisons, we postulate that the use of new-generation drug-eluting stents and a higher proportion of revascularization guided by intracoronary diagnostic techniques may have influenced this finding. However, the use of intracoronary imaging techniques in our study was relatively low (42%) considering their benefit in patients with complex coronary lesions.19
In recent years, there has been growing interest in understanding the impact of comorbidities and frailty in older patients with cardiovascular disease.20,21 Several studies have compared invasive strategies with conservative approaches in older patients, demonstrating benefits for revascularization.22,23 However, the MOSCA-FRAIL trial compared both strategies in frail patients and observed that an invasive strategy did not confer additional benefit compared with conservative management of these patients, despite a fairly low percentage of LM disease.24 In our study, we observed a 2-fold increase in the risk of all-cause mortality in patients with frailty, suggesting the need to add systematic evaluation of frailty in older patients undergoing LM-PCI. Such assessment can aid in selecting the optimal therapeutic strategy, taking into account the likelihood of mortality during follow-up, irrespective of the application of an invasive strategy in coronary disease. These results, moreover, are consistent with other cardiovascular diseases with significant prevalence and mortality, such as heart failure.25
Study limitations
The present study has several limitations. First, it has the limitations inherent to its observational and retrospective design. Although the sample size is relatively small, it represents the largest study specifically focused on LM-PCI in older patients and analyses associated comorbidities and their impact on cardiovascular adverse events. Second, the absence of a control group receiving conservative treatment hinders the ability to draw more robust conclusions on the safety and efficacy of LM-PCI in these patients. In addition, the selection of cutoff points (age ≥ 75 years) to define this cohort of older patients was arbitrarily based on the exclusion criteria of the main clinical trials previously published. A high percentage of patients with frailty may not have undergone revascularization and would therefore have been excluded from the study. Regarding the prognostic significance of frailty, although we used IPTW to reduce confounding bias, we cannot rule out the possibility of residual confounding due to unmeasured covariables. Furthermore, there are no data on bleeding events during follow-up, which is an important concern given the impact of antiplatelet therapy in these patients. Finally, the percentage of intracoronary imaging use was lower than expected.
CONCLUSIONS
In real-life patients with advanced age and multiple associated comorbidities, percutaneous treatment of LM could be considered a feasible option, with an acceptable incidence of adverse cardiovascular events during follow-up and a low incidence of complications associated with the procedure. Frailty was an independent predictor of all-cause mortality during follow-up. When weighing the risks of LM-PCI in older patients, frailty should be taken into account in the therapeutic decision-making process.
FUNDING
None.
ETHICAL CONSIDERATIONS
The study protocol was approved by the Local Clinical Research Ethics Committee according to institutional and Good Clinical Practice guidelines. All patients signed the informed consent for publication. The authors confirm that sex and gender variables have been considered in accordance with the SAGER guidelines.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
No artificial intelligence was used in the preparation of the study.
AUTHORS’ CONTRIBUTIONS
I. Gallo, M. Alvarado and J. Perea contributed to data collection. R. González-Manzanares performed the statistical analysis. J. Suárez de Lezo and M. Romero contributed to the interpretation of the results. I. Gallo and F. Hidalgo wrote the manuscript. S. Ojeda and M. Pan reviewed the manuscript.
CONFLICTS OF INTEREST
S. Ojeda is associate editor of REC: Interventional Cardiology. The journal’s editorial procedure to ensure impartial processing of the manuscript has been followed. S. Ojeda has received consulting fees from Medtronic and Edwards and speaker fees from Philips, World Medical and Boston Scientific and is holder of a research grant (PI21/00949) from the Spanish Ministry of Science and Innovation (Instituto de Salud Carlos III). M. Pan has received speaker fees from Abbott, Boston Scientific, World Medical and Philips and holds a research grant (PI21/00949) from the Spanish Ministry of Science and Innovation (Instituto de Salud Carlos III). The remaining authors declare no conflicts of interest.
WHAT IS KNOWN ABOUT THE TOPIC?
- Coronary artery disease is closely related to age and the aging process.
- The prognosis of LM disease is uncertain and, due to due to advances in interventional cardiology in recent years, there is a need for further evidence on treatment options.
- Frailty is associated with a worse prognosis in various diseases.
WHAT DOES THIS STUDY ADD?
- LM-PCI in older adults is a feasible option in high-volume centers.
- Frailty is prevalent in older patients with LM disease and is associated with increased all-cause mortality.
REFERENCES
1. Collet C, Capodanno D, Onuma Y, et al. Left main coronary artery disease:pathophysiology, diagnosis, and treatment. Nat Rev Cardiol.2018;15:321-331.
2. Giannoglou GD, Antoniadis AP, Chatzizisis YS, et al. Prevalence of narrowing >or=50% of the left main coronary artery among 17,300 patients having coronary angiography. Am J Cardiol. 2006;98:1202-1205.
3. Ramadan R, Boden WE, Kinlay S. Management of Left Main Coronary Artery Disease. J Am Heart Assoc. 2018;7:008151.
4. Yusuf S, Zucker D, Peduzzi P, et al. Effect of coronary artery bypass graft surgery on survival:overview of 10-year results from randomized trials by the Coronary Artery Bypass Graft Surgery Trialists Collaboration. Lancet. 1994;344:563-570.
5. Suárez de Lezo J, Medina A, Pan M, et al. Rapamycin-eluting stents for the treatment of unprotected left main coronary disease. Am Heart J. 2004;148:481-485.
6. Thuijs DJFM, Kappetein AP, Serruys PW, et al. Percutaneous coronary intervention versus coronary artery bypass grafting in patients with three-vessel or left main coronary artery disease:10-year follow-up of the multicentre randomised controlled SYNTAX trial.Lancet. 2019;394:1325-1334.
7. Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87-165.
8. Ono M, Serruys PW, Hara H, et al. 10-Year Follow-Up After Revascularization in Elderly Patients With Complex Coronary Artery Disease. J Am Coll Cardiol. 2021;77:2761-2773.
9. Abellan van Kan G, Rolland Y, Bergman H, et al. The I.A.N.A. Task Force on frailty assessment of older people in clinical practice. J Nutr Health Aging. 2008;12:29-37.
10. Medina A, Suárez de Lezo J, Pan M. Una clasificación simple de las lesiones coronarias en bifurcación [A new classification of coronary bifurcation lesions]. Rev Esp Cardiol. 2006;59:183.
11. Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW. the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34:3661-3679.
12. Austin PC. Variance estimation when using inverse probability of treatment weighting (IPTW. survival analysis. Stat Med.2016;35:5642-5655.
13. Baydoun H, Jabbar A, Nakhle A, et al. Revascularization of Left Main Coronary Artery. Cardiovasc Revasc Med.2019;20:1014-1049.
14. Stone GW, Sabik JF, Serruys PW, et al. Everolimus-Eluting Stents or Bypass Surgery for Left Main Coronary Artery Disease. N Engl J Med. 2016;375:2223-2235.
15. Mäkikallio T, Holm NR, Lindsay M, et al. Percutaneous coronary angioplasty versus coronary artery bypass grafting in treatment of unprotected left main stenosis (NOBLE):a prospective, randomised, open-label, non-inferiority trial. Lancet. 2016;388:2743-2752.
16. Sabatine MS, Bergmark BA, Murphy SA, et al. Percutaneous coronary intervention with drug-eluting stents versus coronary artery bypass grafting in left main coronary artery disease:an individual patient data meta-analysis. Lancet. 2021;398:2247-2257.
17. Gach O, Louis O, Martinez C, et al. Predictors of early and late outcome of percutaneous coronary intervention in octogenarians. Acta Cardiol. 2003;58:289-294.
18. Gómez-Hospital JA, Gomez-Lara J, Rondan J, et al. Long-term follow-up after percutaneous treatment of the unprotected left main stenosis in high-risk patients not suitable for bypass surgery. Rev Esp Cardiol. 2012;65:530-537.
19. Lee JM, Choi KH, Song YB, et al. Intravascular Imaging-Guided or Angiography-Guided Complex PCI. N Engl J Med. 2023;388:1668-1679.
20. Díez-Villanueva P, Arizá-SoléA, Vidán MT et al. Recomendaciones de la Sección de Cardiología Geriátrica de la Sociedad Española de Cardiología para la valoración de la fragilidad en el anciano con cardiopatía. Rev Esp Cardiol. 2019;72:63–71.
21. Pernias V, García Acuña JM, Raposeiras-Roubín S, et al. Influencia de las comorbilidades en la decisión del tratamiento invasivo en ancianos con SCASEST. REC Interv Cardiol. 2021;3:15-20.
22. Kaura A, Sterne JAC, Trickey A, et al. Invasive versus non-invasive management of older patients with non-ST elevation myocardial infarction (SENIOR-NSTEMI):a cohort study based on routine clinical data. Lancet. 2020;396:623-634.
23. Tegn N, Abdelnoor M, Aaberge L, et al. Invasive versus conservative strategy in patients aged 80 years or older with non-ST-elevation myocardial infarction or unstable angina pectoris (After Eighty study):an open-label randomised controlled trial. Lancet.2016;387:1057-1065.
24. Sanchis J, Bueno H, Miñana G, et al. Effect of Routine Invasive vs Conservative Strategy in Older Adults With Frailty and Non-ST-Segment Elevation Acute Myocardial Infarction:A Randomized Clinical Trial. JAMA Intern Med. 2023;183:407-415.
25. Jiménez-Méndez C, Díez-Villanueva P, Bonanad C, et al. Frailty and prognosis of older patients with chronic heart failure. Rev Esp Cardiol. 2022;75:1011-1019.
* Corresponding author.
@NachoGalloFer;
@FranJHidalgo;
@rafaelglezm;
@MarcoA1788;
@PereaJorge5;
@cardiojsl;
@OjedaOjeda18;
@MPAOSS;
@Cardio_HURS
Editor's page
Original articles
Editorials
Original articles
Editorials
Post-TAVI management of frail patients: outcomes beyond implantation
Unidad de Hemodinámica y Cardiología Intervencionista, Servicio de Cardiología, Hospital General Universitario de Elche, Elche, Alicante, Spain
Original articles
Debate
Debate: Does the distal radial approach offer added value over the conventional radial approach?
Yes, it does
Servicio de Cardiología, Hospital Universitario Sant Joan d’Alacant, Alicante, Spain
No, it does not
Unidad de Cardiología Intervencionista, Servicio de Cardiología, Hospital Universitario Galdakao, Galdakao, Vizcaya, España