ABSTRACT
Introduction and objectives: Most patients with acute pulmonary embolism (PE) receive anticoagulation only. Reperfusion is required in high-risk and a minority of intermediate-risk PE patients. Systemic thrombolysis (ST) is the first-line reperfusion therapy, but due to contraindications and major bleeding concerns, the use of catheter-directed therapies (CDT) is increasing as a suitable alternative. The objective of the present study was to detect predictors of the use of CDT compared with other therapies in patients with acute PE.
Methods: This registry included consecutive intermediate- and high-risk PE patients in 2 tertiary centers with a 24/7 PE response team from 2014 to 2022. The patients were grouped according to the primary treatment: anticoagulation only, CDT, or ST. We evaluated predictors of treatment assignment and safety endpoints.
Results: A total of 274 patients were included. Of them, 112 received anticoagulation only, 96 received ST as the primary treatment, and 66 underwent CDT first. Comorbidities were higher in the CDT group than in the other 2 groups. Patients undergoing ST/CDT had higher PE severity parameters at hospital admission. On multivariable analysis, independent predictors for the use of CDT were Charlson Comorbidity Index (OR, 1.29; 95%CI, 1.05-1.59), recent surgery (OR, 11.07; 95%CI, 3.07-39.87), and bilateral central PE (OR, 2.42; 95%CI, 1.10-5.32). Analysis of early safety outcomes showed that intracranial bleeding occurred only in the ST group (1.8% of patients).
Conclusions: This contemporary registry used CDT as the primary treatment in 24% of intermediate- and high-risk patients, mainly in comorbid and postsurgical patients. CDT was a safe and effective alternative to medical therapy in selected patients.
Keywords: Catheter-directed therapies. Pulmonary embolism. Systemic thrombolysis. Anticoagulation. Local thrombolysis.
RESUMEN
Introducción y objetivos: La mayoría de los pacientes con embolia pulmonar (EP) aguda reciben únicamente anticoagulación. La reperfusión es necesaria en los pacientes con EP de alto riesgo y en una minoría de pacientes con EP de riesgo intermedio-alto. La trombólisis sistémica (TS) es el tratamiento de reperfusión de primera línea, pero debido a las contraindicaciones y a la preocupación por las hemorragias graves, las terapias dirigidas por catéter (TDC) están surgiendo como una alternativa adecuada. El objetivo del presente estudio fue detectar predictores del uso de TDC con respecto a otras terapias en pacientes con EP aguda.
Métodos: Este registro incluyó pacientes consecutivos con EP de riesgo intermedio y alto en dos centros terciarios, con un equipo de respuesta a la EP, desde 2014 hasta 2022. Los pacientes se agruparon según la terapia inicial: solo anticoagulación, TDC o TS; y se evaluaron los predictores de selección de terapia y variables de seguridad.
Resultados: Se incluyó a un total de 274 pacientes. De ellos, 112 recibieron solo anticoagulación, 96 recibieron TS como tratamiento primario y 66 fueron sometidos a TDC en un primer momento. Las comorbilidades fueron mayores en el grupo TDC que en los otros dos. Los pacientes sometidos a TS o TDC presentaban mayores parámetros de gravedad de la EP al ingreso hospitalario. Tras el análisis multivariable, el índice de comorbilidad de Charlson (OR = 1,29; IC95%, 1,05-1,59), la cirugía reciente (OR = 11,07; IC95%, 3,07-39,87) y la EP central bilateral (OR = 2,42; IC95%, 1,10-5,32) siguieron siendo predictores independientes del uso de TDC. En cuanto a los resultados precoces de seguridad, sólo se produjeron hemorragias intracraneales en el grupo TS (1,8% de los pacientes).
Conclusiones: Este registro contemporáneo utilizó TDC como terapia inicial en el 24% de los pacientes de riesgo intermedio y alto, principalmente en pacientes comórbidos y posquirúrgicos. La TDC fue una alternativa segura y eficaz al tratamiento médico en pacientes seleccionados.
Palabras clave: Terapia dirigida por catéter. Intervencionismo dirigido por catéter. Embolia pulmonar. Trombólisis sistémica. Anticoagulación. Trombólisis local.
Abbreviation
AC: anticoagulation alone. CDT: catheter-directed therapies. HR: high risk. IHR: intermediate-high risk. PE: pulmonary embolism. ST: systemic thrombolysis.
INTRODUCTION
Pulmonary embolism (PE) is the third leading cause of cardiovascular death and the first avoidable cause of death in hospitalized patients.1 According to the European Society of Cardiology (ESC) guidelines, the treatment of PE is based on patient risk assessment.2 Reperfusion therapy with systemic thrombolysis (ST) is indicated as the first-line therapy in patients with high-risk (HR) PE and in those with intermediate-high risk (IHR) PE who deteriorate on anticoagulant drugs.2 However, ST is underused because of contraindications in roughly 30% of patients and even in those with HR-PE and no formal contraindications.3-5 Moreover, this therapy carries a significant risk of major bleeding (≈10%-15%), especially in patients with advanced age, recent surgery, or active cancer.3
Catheter-directed therapies (CDT) have emerged as an alternative to ST for reperfusion in patients with acute PE.6-10 These techniques may improve surrogate right parameters of ventricular failure and clinical outcomes with lower bleeding rates. In a meta-analysis of observational studies comparing catheter-directed thrombolysis vs ST, the risk of in-hospital death and intracranial hemorrhage was reduced in patients undergoing percutaneous intervention. 11 The current ESC guidelines state that CDT should be considered in patients with HR-PE an unsuccessful attempt at thrombolysis or a contraindication to this treatment, and as a rescue treatment for IHR-PE patients with clinical deterioration.2 However, the penetration of interventional therapies is increasing, showing a discrepancy between guideline recommendations and clinical practice.
There is currently scarce evidence in the literature on the contemporary choice of reperfusion therapy, the parameters leading physicians to select one reperfusion therapy over the others, and the target population who may derive the greatest benefit from CDT. Therefore, the main objective of the present study was to identify the clinical factors associated with the choice of CDT as PE therapy in a contemporary cohort of patients with acute PE.
METHODS
Study design
This study was based on an ambispective multicenter registry that included consecutive patients with intermediate-risk (IR) and HR-PE, evaluated by local Pulmonary Embolism Response Teams (PERT), classified according to ESC guidelines,2 and treated with CDT.12 Two tertiary care centers also recruited patients evaluated by the PERT and treated medically, as previously reported in a single-center experience.13 This study analyzed all consecutive patients evaluated by the local PERT in these 2 hospitals from 2014 to 2022.
The inclusion criteria were patients aged more than 18 years with a confirmed diagnosis of acute IR- or HR-PE (by computed tomography or transthoracic echocardiogram plus clinical suspicion in unstable patients unable to undergo computed tomography). We excluded patients with an uncertain diagnosis of PE, those with > 7 days from symptom onset to diagnosis, and those with low-risk PE according to ESC guidelines.2 The registry was observational, with no recommendation on PE management. Thus, treatment was established according to the criteria of the treating physicians, and the use of CDT was chosen according to availability and the decision of the PERT. The reporting of this study adheres to the Strengthening The Reporting of Observational studies in Epidemiology (STROBE) guideline for cohort studies.14
Data collection and variable definitions
A secure web-based database stored anonymized data in both centers. Data were self-reported by local investigators from digital clinical records and included vital signs and laboratory values. Initial admission to the cardiac intensive care unit included more granular data with recording of hourly clinical vital signs, shock parameters at admission, and worsening during cardiac intensive care unit admission and subsequently after reperfusion (if the patient underwent reperfusion). After hospital discharge, structured follow-up was conducted with visits at 1-month, 3- to 6-months, and 12-months. However, 30-day follow-up results are included in this study. The right ventricle/left ventricle ratio was mainly derived from computed tomography except in patients with no baseline computed tomography due to instability. Bilateral central PE was diagnosed when a thrombus was detected in both main pulmonary arteries by computed tomography or angiography. PE risk was stratified according to ESC guidelines.2 In all patients, we calculated the shock index, defined by the heart rate to systolic blood pressure ratio, Pulmonary Embolism Severity Index score,15 Bova score,16 and Charlson Comorbidity Index.17 For most patients who underwent CDT, hemodynamic parameters (such as systolic and mean pulmonary artery pressure) were measured invasively, with a catheter placed in the pulmonary artery.
Pulmonary embolism therapies
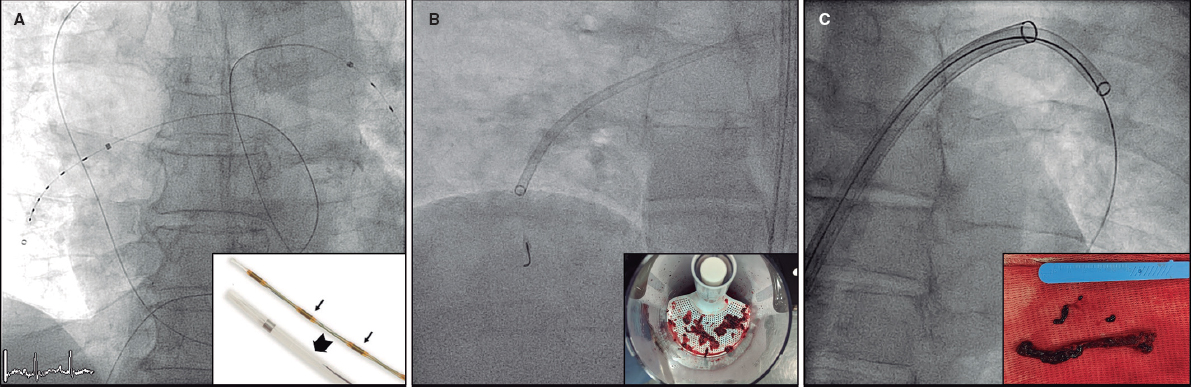
Parenteral anticoagulation was started immediately after PE diagnosis. ST was given through a peripheral vein following the doses recommended in the ESC guidelines.2 CDT included: a) catheter-directed thrombolysis using a multiperforated catheter(s) inserted into the pulmonary artery and left for 6 to 24 hours to infuse low-dose thrombolytics (usually alteplase 0.25 mg/kg or the tenecteplase equivalent); b) mechanical thrombus fragmentation; c) thrombus aspiration using either nondedicated catheters (usually 8-Fr coronary guiding catheters) or dedicated catheters (Indigo 8-Fr [Penumbra, United States], Nautilus 10-Fr [iVascular, Spain], or FlowTriever 24-Fr [Inari medical, United States]); or d) a combination of them. The dose of fibrinolytic therapy (both for ST and catheter-directed thrombolysis) was decided by the treating physician. See figure 1 for an illustration of different CDT options.
Figure 1. Catheter-directed therapies used in the study. Representative images of catheter-directed therapies. A: ultrasound-assisted thrombolysis, EKOS system (Boston Scientific, United States). B: percutaneous thrombectomy with Indigo system (Penumbra, United States). C: large-bore thrombus aspiration, FlowTriever catheter (Inari, United States).
Objectives
The main endpoint of the present study was to detect predictors of the use of CDT in patients with IR- or HR-PE requiring reperfusion therapy. Another endpoint was to compare the characteristics of the patients who received the different therapies for acute PE: anticoagulation alone (AC), ST, or CDT. If more than 1 reperfusion therapy was used, the patients were grouped according to the first administered therapy. The analysis focused on identifying variables associated with the choice of different therapies by the treating physician. Thirty-day all-cause mortality was reported as a safety outcome. We also analyzed in-hospital adverse events, such as bleeding events according to the International Society of Thrombosis and Hemostasis classification18 and acute kidney injury. In patients undergoing CDT, we also recorded procedural results (eg, hemodynamic improvement).
Ethics and funding
The registry protocol was approved by the clinical research ethics committee at Hospital Clínico San Carlos as the central committee for the registry, following local research regulations (code 18/010-E). All prospectively included patients signed an informed consent form. An informed consent waiver was granted from the ethics research committee for patients recruited retrospectively. The investigation was an academic, unfunded, investigator-initiated study.
Statistical analysis
Categorical variables are presented as numbers and percentages, and continuous variables as mean ± standard deviation (SD) or median [interquartile range (IQR)], as appropriate. Group comparisons (AC, CDT, and ST) for continuous variables were performed using the ANOVA and chi-square tests for categorical variables. Comparisons between groups were performed with the Student t-test or Wilcoxon test, as appropriate, for continuous variables and the chi-square test for categorical variables. The predictors for using the different reperfusion techniques (ie, CDT or ST) were determined using a logistic regression analysis. The univariate analysis included baseline and clinical variables at PE diagnosis. Variables with P values < .10 in the univariable analysis were included in the multivariable model. Paired t-tests were used to analyze the change in hemodynamic parameters after transcatheter procedures. Missing values for covariates, if any, were not imputed. Statistical analyses were performed using Stata 16 (StataCorp, College Station, United States).
RESULTS
Baseline characteristics and risk stratification
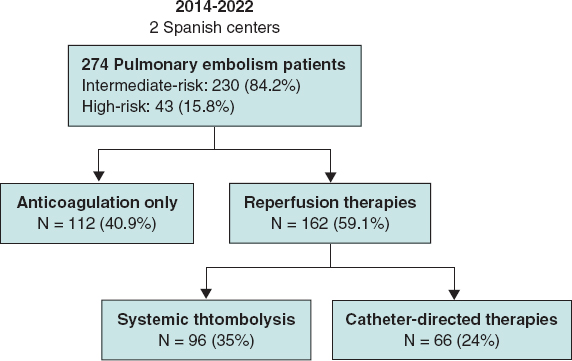
From 2014 to 2022, a total of 274 patients were included in the registry (9.5% with intermediate-low risk, 74.7% with IHR, and 15.8% with HR-PE) (figure 2). Of them, 112 patients (40.9%) received AC only: 57% low molecular weight heparin and 43% unfractionated heparin. The remaining 162 patients (59.1%) underwent reperfusion therapy: 35% received ST as the primary treatment and 24% underwent CDT first. Of the ST group, all the patients received alteplase as fibrinolytic treatment and 5 patients underwent rescue CDT after unsuccessful ST. Notably, 58% of IHR-PE patients in our cohort received reperfusion therapies.
Figure 2. Study patients and selected therapy.
Patients’ baseline characteristics according to the treatment strategy are shown in table 1. The study was well balanced regarding gender (52% men); however, there were more men in the AC group than in the ST group (58.0% vs 42.7%, P = .027). Patients in the AC and CDT groups were significantly older than those in the ST group (65.9 ± 16.2 and 62.3 ± 14.7 vs 57.4 ± 16.6 years, respectively, P < .001). Regarding comorbidities, previous cancer was more common among patients in the CDT group than in those in the ST group. The Charlson Comorbidity Index was higher in the CDT group than in the other 2 groups. Among precipitating factors for PE, a history of recent surgery was more frequent in patients in the CDT group than in the other 2 groups, while a recent hospital admission was more frequent in the AC and CDT groups than in the ST group.
Table 1. Baseline characteristics
| Total | AC | ST | CDT | P | ||||
|---|---|---|---|---|---|---|---|---|
| N = 274 | N = 112 | N = 96 | N = 66 | Global | AC vs ST | AC vs CDT | ST vs CDT | |
| Male sex | 142 (51.8%) | 65 (58.0%) | 41 (42.7%) | 36 (54.5%) | .077 | .027 | .650 | .138 |
| Age, years | 62.1 (16.4) | 65.9 (16.2) | 57.4 (16.6) | 62.3 (14.7) | < .001 | < .001 | .136 | .056 |
| Body mass index (kg/m2) | 29.4 (6.7) | 29.4 (6.0) | 29.7 (8.8) | 29.2 (5.0) | .921 | .765 | .890 | .724 |
| Obesity | 133 (48.5%) | 52 (46.4%) | 54 (56.3%) | 27 (40.9%) | .136 | .167 | .533 | .078 |
| Prior venous thromboembolism | 53 (19.4%) | 20 (17.9%) | 22 (23.2%) | 11 (16.7%) | .511 | .345 | .840 | .316 |
| Previous cancer | 42 (15.3%) | 15 (13.4%) | 11 (11.5%) | 16 (24.2%) | .065 | .674 | .065 | .032 |
| Hypertension | 135 (49.5%) | 55 (49.1%) | 46 (47.9%) | 34 (52.3%) | .857 | .864 | .681 | .585 |
| Diabetes mellitus | 51 (18.7%) | 18 (16.1%) | 19 (19.8%) | 14 (21.5%) | .628 | .484 | .362 | .788 |
| Heart failure | 14 (5.1%) | 8 (7.1%) | 3 (3.1%) | 3 (4.5%) | .411 | .197 | .487 | .638 |
| Chronic kidney disease | 20 (7.3%) | 10 (8.9%) | 4 (4.2%) | 6 (9.1%) | .342 | .172 | .971 | .201 |
| Charlson Comorbidity Index | 1.0 (1.6) | 0.8 (1.4) | 0.9 (1.5) | 1.5 (1.8) | .026 | .676 | .010 | .043 |
| Recent surgery | 35 (12.8%) | 12 (10.8%) | 4 (4.2%) | 19 (28.8%) | < .001 | .074 | .002 | <.001 |
| Recent immobilization | 48 (17.5%) | 14 (12.5%) | 17 (17.7%) | 17 (25.8%) | .080 | .293 | .024 | .216 |
| Recent hospital admission | 28 (10.3%) | 14 (12.6%) | 4 (4.2%) | 10 (15.2%) | .044 | .032 | .633 | .014 |
|
AC, anticoagulation; CDT, catheter-directed therapies; ST, systemic thrombolysis. Data are shown as mean (SD) for continuous variables and No. (%) for categorical variables. P values denote the significance of the differences between the groups for continuous variables analyzed by the ANOVA test and Student t-test, as appropriate. The chi-square test was used to assess the significance of between-group differences for categorical variables. Obesity was defined as body mass index ≥ 30 kg/m2. Statistically significant values are highlighted in bold letters. |
||||||||
Clinical and risk stratification parameters at hospital admission are shown in table 2. Patients who received reperfusion therapies, either with CDT or ST, had higher severity parameters than those in the AC group (eg, shock index, right ventricular involvement, or lactate levels). The Pulmonary Embolism Severity Index score, which incorporates comorbidities and PE severity parameters, was higher in CDT patients than in the other 2 groups (P < .001).
Table 2. Risk stratification parameters at hospital admission
| Total | AC | ST | CDT | P | ||||
|---|---|---|---|---|---|---|---|---|
| N = 274 | N = 112 | N = 96 | N = 66 | Global | AC vs ST | AC vs CDT | ST vs CDT | |
| Systolic blood pressure, mmHga | 118.7 (25.3) | 126.8 (23.1) | 114.5 (25.9) | 110.8 (24.6) | < .001 | < .001 | < .001 | .359 |
| Heart rate, bpm | 106.9 (18.8) | 99.5 (19.7) | 112.9 (16.3) | 110.9 (16.2) | < .001 | < .001 | < .001 | .459 |
| Shock Index | 0.96 (0.36) | 0.82 (0.28) | 1.06 (0.39) | 1.07 (0.35) | < .001 | < .001 | < .001 | .953 |
| Respiratory failure | 71 (28.9%) | 28 (26.4%) | 29 (34.9%) | 14 (24.6%) | .314 | .205 | .796 | .191 |
| Syncope | 57 (20.8%) | 23 (20.5%) | 18 (18.8%) | 16 (24.2%) | .696 | .747 | .564 | .399 |
| Deep vein thrombosis | 74 (27.6%) | 34 (30.6%) | 23 (24.5%) | 17 (27.0%) | .612 | .326 | .612 | .723 |
| Right ventricular involvement | 249 (94.0%) | 93 (87.7%) | 94 (98.9%) | 62 (96.9%) | .002 | .002 | .042 | .346 |
| Bilateral pulmonary embolism | 175 (63.9%) | 70 (62.5%) | 57 (59.4%) | 48 (72.7%) | .204 | .645 | .163 | .080 |
| Lactate, mmol/L | 2.9 (2.9) | 2.2 (2.0) | 3.7 (3.8) | 3.0 (2.6) | .006 | .002 | .039 | .315 |
| Elevated troponin levels | 209 (86.0%) | 85 (83.3%) | 73 (89.0%) | 51 (86.4%) | .539 | .271 | .600 | .642 |
| Elevated NT-proBNP levels | 167 (78.4%) | 74 (77.9%) | 57 (78.1%) | 36 (80.0%) | .958 | .977 | .777 | .804 |
| High-risk PEb | 43 (15.8%) | 8 (7.1%) | 18 (18.8%) | 17 (26.2%) | .002 | .012 | < .001 | .264 |
| PESI score | 105.1 (35.1) | 97.6 (29.3) | 104.9 (36.1) | 118.2 (39.4) | < .001 | .109 | < .001 | .028 |
| Bova score | 4.7 (1.5) | 4.2 (1.5) | 5.1 (1.4) | 5.0 (1.5) | < .001 | < .001 | .002 | .526 |
|
AC, anticoagulation; CDT, catheter-directed therapies; PE, pulmonary embolism; PESI, pulmonary embolism severity index; ST, systemic thrombolysis. Statistically significant values are highlighted in bold. Data are shown as mean ± standard deviation for continuous variables and No. (%) for categorical variables. P values denote the significance of the differences between the groups for continuous variables analyzed by the ANOVA test and Student t-test, as appropriate. The chi-square test tested the significance of between-group differences for categorical variables. aThis variable reflects systolic blood pressure at hospital admission, but some of these patients were under vasopressors, and others were stable on admission and later deteriorated hemodynamically. bAs defined by the European Society of Cardiology guidelines. |
||||||||
Reperfusion therapies
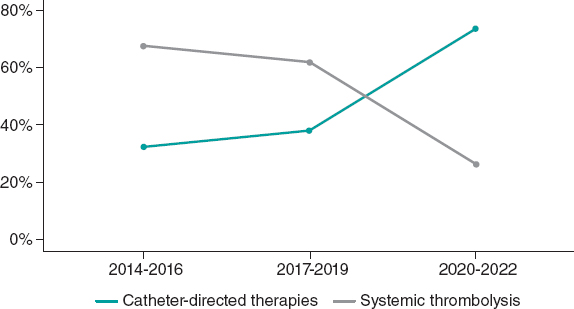
Figure 3 shows the trend in the choice between the 2 primary reperfusion therapies over time. There was a progressive increase in the use of CDT and a consequent decrease in the use of ST. The variables that might have led the treating physicians to choose between the 2 reperfusion therapies are shown in table 3. In the univariate analysis, the variables associated with the choice of CDT instead of ST were those reflecting comorbidities, such as older age, previous cancer, and the Charlson Comorbidity Index. Recent surgery and hospital admission were also associated with the choice of CDT. After multivariable analysis in this cohort of patients with acute PE, the only independent predictors of the choice of CDT over ST were the Charlson Comorbidity Index and recent surgery. In addition, this analysis showed that the presence of bilateral central PE was associated with the treating physician’s choice of CDT instead of ST.
Figure 3. Choice of reperfusion therapy over the years.
Table 3. Univariate and multivariable predictors of the choice of CDT over ST or AC as a first-line therapy in acute pulmonary embolism
| Univariable | Multivariable | |||
|---|---|---|---|---|
| Variables | OR (95%CI) | P | OR (95%CI) | P |
| Male sex | 1.61 (0.86-3.03) | .139 | ||
| Age (per year) | 1.02 (1.00-1.04) | .058* | ||
| Body mass index (per kg/m2) | 0.99 (0.94-1.04) | .722 | ||
| Prior venous thromboembolism | 0.66 (0.30-1.48) | .317 | ||
| Previous cancer | 2.47 (1.06-5.75) | .035* | ||
| Hypertension | 1.19 (0.63-2.24) | .585 | ||
| Diabetes mellitus | 1.11 (0.51-2.42) | .788 | ||
| Heart failure | 1.48 (0.29-7.55) | .640 | ||
| Chronic kidney disease | 2.30 (0.62-8.49) | .211 | ||
| Recent surgery | 9.30 (2.99-28.90) | < .001 | 11.07 (3.07-39.87) | < .001 |
| Recent immobilization | 1.61 (0.75-3.45) | .219 | ||
| Recent hospital admission | 4.11 (1.23-13.72) | .022 | 1.25 (0.29-5.43) | .767 |
| Systolic blood pressure (per mmHg) | 0.99 (0.98-1.01) | .357 | ||
| Heart rate (per bpm) | 0.99 (0.97-1.01) | .457 | ||
| Respiratory failure | 0.61 (0.29-1.29) | .193 | ||
| Syncope | 1.39 (0.65-2.97) | .400 | ||
| Deep vein thrombosis | 1.14 (0.55-2.36) | .723 | ||
| Right ventricular involvement | 0.33 (0.03-3.72) | .369 | ||
| Bilateral central pulmonary embolism | 1.82 (0.93-3.59) | .082 | 2.42 (1.10-5.32) | .028 |
| Lactate (per mmol/L) | 0.94 (0.83-1.06) | .317 | ||
| Elevated troponin levels | 1.00 (1.00-1.00) | .312 | ||
| Elevated NT-proBNP levels | 1.12 (0.45-2.81) | .804 | ||
| Charlson Comorbidity Index | 1.21 (1.00-1.47) | .048 | 1.29 (1.05-1.59) | .018 |
|
OR, ods ratio; 95%CI, 95% confidence interval. *Age and previous cancer were not included in the multivariable model despite being significant in the univariate analysis to avoid problems of collinearity because they are included in the Charlson Comorbidity Index. |
||||
Procedural characteristics in the CDT group are displayed in table 4. The median treatment delay from diagnosis of acute PE to percutaneous treatment was 6.0 [interquartile range [IQR], 3.5-19.0] hours and the mean procedure length was 89.0 ± 44.4 minutes. Catheter-directed thrombolysis was used in 35 patients (53.0%), and the most frequently used thrombolytic drug was alteplase (71.4%), with a mean dose of 16.7 ± 7.2 mg. The median bolus dose in patients treated with alteplase was 4 [IQR, 2.9-6.3] mg and the median perfusion time of the remaining dose was 16.0 [IQR, 12.0-20.0] hours. In all patients treated with tenecteplase, the drug was administered as a bolus. Thrombus aspiration was performed in 42 patients (63.6%). The most commonly used aspiration devices were coronary catheters (42.9%), followed by FlowTriever catheter (Inari Medical, United States) (38.1%). A combined thrombolysis plus aspiration technique was performed in 11 patients. Systolic pulmonary artery pressure decreased from 57.9 ± 15.4 to 47.6 ± 12.6 mmHg (mean: −10.3 ± 11.3 mmHg, P < .001) after the percutaneous procedure, while the mean pulmonary artery pressure decreased from 35.0 ± 9.1 to 28.6 ± 8.8 mmHg (mean: −6.4 ± 6.8 mmHg, P < .001). Systolic blood pressure significantly increased after the procedure from 127.8 ± 23.4 to 138.8 ± 22.0 mmHg (mean: +11.0 ± 24.5 mmHg, P = .028).
Table 4. Procedural characteristics in the catheter-directed therapies group
| Patients with percutaneous intervention (N = 66) | |
|---|---|
| Therapy delay, hours* | 6.0 [3.3-19.0] |
| Procedure length, minutes | 89.0 (44.4) |
| Vascular access | |
| Femoral | 64 (97.0%) |
| Brachial | 2 (3.0%) |
| Maximum sheath diameter, French | 8.0 [6.0-20.0] |
| Catheter-directed thrombolysis | 35 (53.0%) |
| Thrombolytic drug | |
| Alteplase | 25 (71.4%) |
| Tenecteplase | 10 (28.6%) |
| Drug dose | |
| Alteplase, mg | 16.7 (7.2) |
| Tenecteplase, units | 3737.5 (1947.8) |
| Ultrasound-assisted | 2 (5.7%) |
| Thrombus aspiration | 42 (63.6%) |
| Catheter | |
| Coronary catheters | 18 (42.9%) |
| FlowTriever | 16 (38.1%) |
| Indigo | 6 (14.3%) |
| Nautilus | 2 (4.8%) |
| sPAP change, mmHg | −10.3 (11.3) |
| mPAP change, mmHg | −6.4 (6.8) |
| sBP change, mmHg | +11.0 (24.5) |
| mBP change, mmHg | +5.3 (17.6) |
|
mBP, mean blood pressure; mPAP, mean pulmonary artery pressure; rTPA, alteplase; sBP, systolic blood pressure; sPAP, systolic pulmonary artery pressure; TNK, tenecteplase. Data are shown as mean ± standard deviation or median [interquartile range] for continuous variables, as appropriate, and No. (%) for categorical variables. * Therapy delay was defined as the time that elapsed between diagnosis of pulmonary embolism and the procedure. |
|
Safety outcomes
Early clinical outcomes and in-hospital events according to the treatment strategy are shown in table 5. The median length of hospitalization was 8 [IQR, 6.0-13.0] days. In-hospital major bleeding, as defined by the International Society of Thrombosis and Hemostasis, occurred in 7 patients (7.3%) in the ST group and in 9 patients (13.6%) in the CDT group. Intracranial bleeding occurred in 5 patients, all of them in the ST group, during hospital admission. Vascular access complications, including minor and major events, were found in 6 (10.6%) of the patients who underwent CDT. Of note, 5 of these patients received catheter-directed thrombolysis (4 with alteplase and 1 with tenecteplase) and the tenecteplase-treated patient underwent aspiration with a nonspecific catheter. One of the vascular complications was a hematoma related to extracorporeal membrane oxygenation implantation and which, therefore, bore no direct relationship with the CDT procedure. The remaining events were 1 incident of femoral access bleeding leading to hypovolemic shock and eventual death (a local thrombolysis CDT case), 2 hematomas requiring transfusion, and another 2 hematomas not requiring transfusion. The incidence of 30-day all-cause mortality was 4.6%, 10.4% and 15.9% for the AC, ST and CDT groups, respectively (P = .045). Twenty-two patients died due to hemodynamic or respiratory deterioration related to PE, 2 patients died from anoxic encephalopathy (both in the CDT group), and 1 patient died from severe intracranial bleeding (ST group).
Table 5. Early safety outcomes in patients with acute pulmonary embolism
| Total | AC | ST | CDT | P | ||||
|---|---|---|---|---|---|---|---|---|
| N = 274 | N = 112 | N = 96 | N = 66 | Global | AC vs ST | AC vs CDT | ST vs CDT | |
| Admission length, days | 8.0 (6.0-13.0) | 7.0 (6.0-11.0) | 9.0 (6.0-12.5) | 10.0 (6.0-23.0) | .132 | 0.394 | .052 | .178 |
| In-hospital events | ||||||||
| Major bleeding* | 18 (6.6%) | 2 (1.8%) | 7 (7.3%) | 9 (13.6%) | .008 | 0.052 | .002 | .184 |
| Intracranial bleeding | 5 (1.8%) | 0 (0.0%) | 5 (5.2%) | 0 (0.0%) | .009 | 0.014 | - | .060 |
| Acute kidney injury | 22 (8.0%) | 11 (9.8%) | 9 (9.4%) | 2 (3.0%) | .228 | 0.913 | .093 | .115 |
| Vascular access complication | - | - | - | 6 (10.6%) | - | - | - | - |
| 30-day all-cause death | 25 (9.3%) | 5 (4.6%) | 10 (10.4%) | 10 (15.9%) | .045 | 0.110 | .011 | .310 |
|
AC, anticoagulation; CDT, catheter-directed therapies; ST, systemic thrombolysis. Data are shown as median [interquartile range] for continuous variables and No. (%) for categorical variables. *As defined by the International Society of Thrombosis and Hemostasis. |
||||||||
DISCUSSION
The present study explores the clinical characteristics, risk profile and outcomes of patients with IR and HR-PE in 2 tertiary care referral centers with a 24/7 PERT team. The main findings were as follows: a) in this contemporary PE cohort, the factors associated with the choice of CDT over ST in the multivariable analysis were a higher Charlson Comorbidity Index, a history of recent surgery, and a proximal, bilateral PE; b) the choice of CDT as reperfusion therapy has increased; and c) CDT significantly improves hemodynamic parameters, suggesting that the effectiveness of the treatment is preserved in this comorbid population; nonetheless, the risk of complications is not negligible and should be considered in decision-making.
To our knowledge, this is the first study that focuses on the parameters associated with treating physicians’ choice between the available treatment strategies in patients with acute IR and HR-PE. As expected, patients undergoing reperfusion had worse hemodynamic status and more frequently had right ventricular impairment or higher lactate levels. ST was more frequently used in patients with fewer comorbidities (eg, younger age, recent surgery, or hospital admission), which is in agreement with previous studies.3,5 In contrast, CDT was chosen for patients with a greater number of comorbidities and probably with a higher bleeding risk (recent surgery). However, there were no differences in age, sex or previous comorbidities between the group of patients treated with AC and those who underwent CDT, with only PE severity as a driver for CDT reperfusion.
Catheter-directed therapies as an increasingly chosen option
In the last 10 years, CDT has emerged as a promising alternative to ST, but randomized studies vs standard medical therapy are lacking. The PE landscape currently has 2 scenarios on the opposite side of the innovation curve. On the one side, the early adopters (United States scenario) are using CDT with a very low threshold as an elective therapy for submassive PE (including the entire IR spectrum) despite the lack of randomized evidence or strong guideline recommendations. Conversely, awareness of CDT and its availability might be relatively low in late-adopter countries and nonacademic nontertiary centers, leading to inequalities in patients’ access to advanced therapies for PE.
The rise in CDT treatments is due to the growing market and the promising results of early studies showing nearly immediate improvement in right ventricular function and hemodynamic status compared with conservative treatment,7,10,19,20 with very low bleeding risk.21,22 The variety of techniques (figure 1) might add some heterogeneity but discussion of the various CDTs is beyond the scope of this manuscript.
The significant number of patients treated with reperfusion in our cohort (59% of IHR-PE patients and 81% of HR-PE patients) may reflect that PERTs are currently activated only for a higher-risk segment of patients, but also reflects the optimal accessibility to reperfusion when ST and CDT are available together.
Systemic thrombolysis vs catheter-directed therapies
ST is the treatment of choice for patients with hemodynamic instability and PE-related cardiopulmonary arrest, although the mortality benefit is mainly based on a small clinical trial (n = 8) that was prematurely terminated.23 Risk factors for PE are age, multiple comorbidities and especially past or active cancer,24 which also confer an exceedingly high bleeding risk,25 especially when treated with ST. Previous studies have shown that major bleeding occurs in ≈10% to 15% of acute PE patients treated with ST, while intracranial bleeding events occur in around 1.5% to 2% of this patient population.3,4,26,27 It is probably for this reason that this treatment is not frequently applied in older patients with previous comorbidities, as shown in the present study and other previous publications.3-5 Thus, managing older, comorbid and oncologic patients with ongoing acute PE remains a real challenge for clinicians, and in this particular scenario, CDT may be a safe and effective option for PE treatment. In fact, the multivariable analysis performed in our study showed that increasing comorbidities was an independent factor for the use of CDT over ST as the preferred reperfusion therapy. These results suggest a new choice for this group of highly vulnerable patients who would not otherwise be treated with reperfusion and therefore would have a higher mortality risk due to the conservative approach.3 However, these results should be interpreted with caution because of the low percentage of patients treated with ST in the present study (35.0%) and the low percentage of HR-PE patients included (15.8%). Furthermore, given the large time period covered by the study, a significant percentage of IHR-PE patients undergoing ST were included. Following the publication of the PEITHO trial28 and the emergence of specific catheters for PE treatment, the administration of ST in IHR-PE patients became less frequent, even in those with worse progress within this subgroup. Therefore, it is likely that our study population does not accurately represent patients in current clinical practice.
Postsurgical patients are especially complex because surgery is a risk factor for PE and is a formal contraindication for ST. Percutaneous thrombectomy has shown a low incidence of major bleeding in single-arm studies and seems a good alternative in these patients.8,9,29 However, to use these devices, the thrombus must be in the proximal segment of the main pulmonary arteries. Indeed, bilateral central PE was an independent variable that prompted the choice of CDT in our study.
Anticoagulation vs catheter-directed therapies
Anticoagulation only is recommended for low-risk and stable IR-PE patients.2 ST in IR-PE decreased the risk of hemodynamic decompensation but at a high cost of bleeding,28 and consequently reperfusion therapies are intended for patients with hemodynamic deterioration.2 Nonetheless, the irruption of transcatheter therapies, especially large-bore aspiration devices, could provide the advantages of pulmonary reperfusion observed in the PEITHO trial28 without the worrisome adverse effects (mainly bleeding events). Our study shows that the use of CDTs has clearly increased in recent years but they were still being reasonably reserved for the higher-risk PE spectrum. Ongoing large clinical trials, such as PEERLESS (NCT: 05111613), HI-PEITHO (NCT: 04790370), and PE-TRACT (NCT: 05591118), will definitely clarify the indication for CDT in patients with acute IHR-PE.
Early safety outcomes in patients with acute pulmonary embolism
Our study showed an incidence of 30-day all-cause mortality of 9.3%, which is lower than that in other observational studies.21,30 However, the cited studies included only patients undergoing reperfusion therapies (either CDT or ST) and the present study also included patients undergoing conservative management, who can be expected to have lower severity and therefore better prognosis. In contrast to the findings of other published literature,19,21,31 the incidence of in-hospital major bleeding and early all-cause death was relatively high in the CDT group in our cohort. These results can be explained by 2 main reasons: first, patients in the CDT group in our cohort were older and had more comorbidities, with 30% having a formal contraindication for ST; and second, the CDT group included almost 50% of patients receiving thrombolytic drugs, which are associated with a higher risk of bleeding than thrombus aspiration alone. Furthermore, among the group of patients who underwent catheter-guided thrombolysis, tenecteplase was used in 28.6%, with this drug demonstrating a high incidence of major bleeding in the PEITHO trial.28 Finally, the vascular access used in the vast majority of patients in the present study was femoral (97.0%), with an incidence of vascular complications of 10.6% (all of them occurring in patients undergoing catheter-directed thrombolysis or aspiration with a nonspecific catheter). Previous studies have shown a low incidence of major bleeding when catheter-directed thrombolysis is performed through brachial access.32 However, specific devices, especially large-bore aspiration devices, can currently only be used via femoral access due to their large caliber. In addition, there were no intracranial bleeding events in patients undergoing CDT in our cohort.
On the other hand, our study showed a significant hemodynamic improvement in patients who underwent CDT, in accordance with previous studies.7-10,33 This benefit is important, but the futility of interventional treatments must be considered in very old and comorbid patients, balancing cost-effectiveness and clinical judgment.34 More data are needed to establish the risk-benefit balance of CDT compared with anticoagulation and ST in older patients or patients with a high comorbidity burden.
Limitations
Several limitations should be considered when interpreting the results of this study. Due to its observational nature, the presence of unmeasured confounders could have influenced the conclusions of the study. The total number of patients admitted with PE in the study period in the 2 centers is unknown, and consequently a survival bias should be acknowledged. The percentage of intermediate-low risk patients included was relatively low, suggesting that PERT activation was selected for the most severe patients. Thus, a selection bias may have occurred in this study. Specific devices for the percutaneous treatment of PE were not initially available at the beginning of this study, and were incorporated as they became available (first specific devices in 2018). This was a registry with self-reported data without external monitoring, and consequently local investigators are responsible for the integrity of the data.
CONCLUSIONS
The results of this study show that the factors associated with the choice of CDT on multivariable analysis were a higher Charlson comorbidity index, a history of recent surgery, and proximal, bilateral PE. The choice of CDT over ST as reperfusion therapy increased during the study period. CDT was an effective option for older, comorbid patients with PE, but the management of acute PE patients is challenging and should be individualized.
FUNDING
This research did not receive a specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
ETHICAL CONSIDERATIONS
The registry protocol was approved by the clinical research ethics committee at Hospital Clínico San Carlos as the central committee for the registry, following local research regulations (code 18/010-E). All prospectively included patients signed an informed consent form. An informed consent waiver was granted from the ethics research committee for patients recruited retrospectively. Sex but not gender data were included in the database design in 2018.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
No artificial intelligence was used in the preparation of this manuscript.
AUTHORS’ CONTRIBUTIONS
C. Real designed the study outline, performed the statistical analysis, and drafted the article. C. Ferrera designed the study outline and drafted the article. M.E. Vázquez-Álvarez participated in data collection and data interpretation, M. Huanca, F.J. Noriega, E. Gutiérrez-Ibañes, A.M. Mañas-Hernández, N. Ramos-López, M. Juárez, P. Jiménez-Quevedo, J. Elízaga, and A. Viana-Tejedor participated in data collection and critically revised the manuscript. P. Salinas designed the protocol, database and study outline, coordinated the data analysis and interpretation, and critically revised the manuscript. All authors gave final approval of the version to be published.
CONFLICTS OF INTEREST
The authors report no conflicts of interest with respect to the content of this manuscript.
WHAT IS KNOWN ABOUT THE TOPIC?
- Catheter-directed therapies (CDT) have emerged as a safe and effective reperfusion therapy in patients with acute pulmonary embolism (PE). According to ESC guidelines, these therapies should be considered in patients with HR-PE and failed thrombolysis or a contraindication to this therapy and as a rescue treatment for IHR-PE patients with clinical deterioration. However, several studies aiming to establish the indication for these therapies in a broader spectrum of patients have been published in recent years. Furthermore, reperfusion therapy with systemic thrombolysis (ST) is known to be underused due to concerns about bleeding, and consequently CDT may be a feasible option in this profile of patients who would otherwise go untreated.
WHAT DOES THIS STUDY ADD?
- In clinical practice in two tertiary centers, the factors associated with the choice of CDT over ST were comorbidities, a history of recent surgery, and proximal, bilateral PE. However, the risk profile of patients treated with the 2 therapies was similar in each risk stratum. Therefore, we conclude that CDT could be a safe and effective alternative in patients requiring reperfusion therapy.
ACKNOWLEDGMENTS
The authors thank María Beneito-Durá for providing statistical advice.
REFERENCES
1. Götzinger F, Lauder L, Sharp ASP, et al. Interventional therapies for pulmonary embolism. Nat Rev Cardiol. 2023 Oct;20:670-684.
2. Konstantinides S V, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41:543-603.
3. Keller K, Hobohm L, Ebner M, et al. Trends in thrombolytic treatment and outcomes of acute pulmonary embolism in Germany. Eur Heart J. 2020;41:522-529.
4. Jiménez D, Bikdeli B, Barrios D, et al. Epidemiology, patterns of care and mortality for patients with hemodynamically unstable acute symptomatic pulmonary embolism. Int J Cardiol. 2018;269:327-333.
5. Stein PD, Matta F. Thrombolytic Therapy in Unstable Patients with Acute Pulmonary Embolism:Saves Lives but Underused. Am J Med. 2012;125:465-470.
6. Pruszczyk P, Klok FK, Kucher N, et al. Percutaneous treatment options for acute pulmonary embolism:a clinical consensus statement by the ESC Working Group on Pulmonary Circulation and Right Ventricular Function and the European Association of Percutaneous Cardiovascular Interventions. EuroIntervention. 2022;18:e623-e638.
7. Kucher N, Boekstegers P, Müller OJ, et al. Randomized, Controlled Trial of Ultrasound-Assisted Catheter-Directed Thrombolysis for Acute Intermediate-Risk Pulmonary Embolism. Circulation. 2014;129:479-486.
8. Tu T, Toma C, Tapson VF, et al. A Prospective, Single-Arm, Multicenter Trial of Catheter-Directed Mechanical Thrombectomy for Intermediate-Risk Acute Pulmonary Embolism. JACC Cardiovasc Interv. 2019;12:859-869.
9. Sista AK, Horowitz JM, Tapson VF, et al. Indigo Aspiration System for Treatment of Pulmonary Embolism. JACC Cardiovasc Interv. 2021;14:319-329.
10. Kroupa J, Buk M, Weichet J, et al. A pilot randomised trial of catheter-directed thrombolysis or standard anticoagulation for patients with intermediate-high risk acute pulmonary embolism. EuroIntervention. 2022;18:e639-e646.
11. Pasha AK, Siddiqui MU, Siddiqui MD, et al. Catheter directed compared to systemically delivered thrombolysis for pulmonary embolism:a systematic review and meta-analysis. J Thromb Thrombolysis. 2022;53:454-466.
12. Salinas P, Vázquez-Álvarez M-E, Salvatella N, et al. Catheter-directed therapy for acute pulmonary embolism:results of a multicenter national registry. Rev Esp Cardiol. 2024;77:138-147.
13. Ramos-López N, Ferrera C, Luque T, et al. Impact of a pulmonary embolism response team initiative on hospital mortality of patients with bilateral pulmonary embolism. Med Clin. 2023;160:469-475.
14. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement:guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344-349.
15. Aujesky D, Obrosky DS, Stone RA, et al. Derivation and Validation of a Prognostic Model for Pulmonary Embolism. Am J Respir Crit Care Med. 2005;172:1041-1046.
16. Bova C, Sanchez O, Prandoni P, et al. Identification of intermediate-risk patients with acute symptomatic pulmonary embolism. Eur Respir J. 2014;44:694-703.
17. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies:Development and validation. J Chronic Dis. 1987;40:373-383.
18. Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non?surgical patients. J Thromb Haemost. 2005;3:692-694.
19. Ismayl M, Machanahalli Balakrishna A, et al. Meta-Analysis Comparing Catheter-Directed Thrombolysis Versus Systemic Anticoagulation Alone for Submassive Pulmonary Embolism. Am J Cardiol. 2022;178:154-162.
20. Kuo WT, Banerjee A, Kim PS, et al. Pulmonary Embolism Response to Fragmentation, Embolectomy, and Catheter Thrombolysis (PERFECT). Chest. 2015;148:667-673.
21. Beyer SE, Shanafelt C, Pinto DS, et al. Utilization and Outcomes of Thrombolytic Therapy for Acute Pulmonary Embolism. Chest. 2020;157:645-653.
22. Pei DT, Liu J, Yaqoob M, et al. Meta-Analysis of Catheter Directed Ultrasound-Assisted Thrombolysis in Pulmonary Embolism. Am J Cardiol. 2019;124:1470-1477.
23. Jerjes-Sanchez C, Ramírez-Rivera A, de Lourdes García M, et al. Streptokinase and heparin versus heparin alone in massive pulmonary embolism:A randomized controlled trial. J Thromb Thrombolysis. 1995;2:227-229.
24. Blom JW. Malignancies, Prothrombotic Mutations, and the Risk of Venous Thrombosis. JAMA. 2005;293:715.
25. de Winter MA, Dorresteijn JAN, Ageno W, et al. Estimating Bleeding Risk in Patients with Cancer-Associated Thrombosis:Evaluation of Existing Risk Scores and Development of a New Risk Score. Thromb Haemost. 2022;122:818-829.
26. Marti C, John G, Konstantinides S, et al. Systemic thrombolytic therapy for acute pulmonary embolism:a systematic review and meta-analysis. Eur Heart J. 2015;36:605-614.
27. Chatterjee S, Chakraborty A, Weinberg I, et al. Thrombolysis for Pulmonary Embolism and Risk of All-Cause Mortality, Major Bleeding, and Intracranial Hemorrhage. JAMA. 2014;311:2414.
28. Meyer G, Vicaut E, Danays T, et al. Fibrinolysis for Patients with Intermediate-Risk Pulmonary Embolism. N Engl J Med. 2014;370:1402-1411.
29. Toma C, Jaber WA, Weinberg MD, et al. Acute outcomes for the full US cohort of the FLASH mechanical thrombectomy registry in pulmonary embolism. EuroIntervention. 2023;18:1201-1212.
30. Hobohm L, Schmidt FP, Gori T, et al. In-hospital outcomes of catheter-directed thrombolysis in patients with pulmonary embolism. Eur Hear J Acute Cardiovasc Care. 2021;10:258-264.
31. Stein PD, Matta F, Hughes MJ. Catheter-Directed Thrombolysis in Submassive Pulmonary Embolism and Acute Cor Pulmonale. Am J Cardiol. 2020;131:109-114.
32. Portero-Portaz JJ, Córdoba-Soriano JG, Gallardo-López A, Gutiérrez-Díez A, Melehi El-Assali D, Jiménez-Mazuecos JM. Resultados de la terapia dirigida por catéter en la tromboembolia pulmonar aguda. Rev Esp Cardiol. 2020;73:953-954.
33. Piazza G, Hohlfelder B, Jaff MR, et al. A Prospective, Single-Arm, Multicenter Trial of Ultrasound-Facilitated, Catheter-Directed, Low-Dose TFibrinolysis for Acute Massive and Submassive Pulmonary Embolism. JACC Cardiovasc Interv. 2015;8:1382-1392.
34. Lindman BR, Alexander KP, O'Gara PT, Afilalo J. Futility, Benefit, and Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv. 2014;7:707-716.