ABSTRACT
Introduction and objectives: Drug-eluting balloons (DEB) are an established treatment option for in-stent restenosis (ISR). This study aimed to assess the safety and efficacy of a novel DEB in patients with ISR.
Methods: This prospective, single-center study enrolled a consecutive cohort of patients diagnosed with ISR who underwent coronary angioplasty with a new second-generation paclitaxel-eluting balloon. The 3 main endpoints were myocardial infarction, target lesion revascularization, and target vessel revascularization. Baseline variables were collected, including patient and procedure characteristics. Follow-up data were collected through medical records or telephone contact.
Results: The study included 160 consecutive patients with 206 treated lesions (mean age, 71.4 ± 14.9 years, 15.5% women) undergoing percutaneous coronary intervention with DEB for ISR. A total of 53.3% of patients had acute coronary syndrome. The average diameter of the treated vessel was 3.10 ± 0.7 mm. The DEB used had a mean diameter of 3.1 ± 0.6 mm and a mean length of 23.1 ± 6.8 mm. Predilatation was performed in 98% of the lesions, and a noncompliant balloon was used in 80%. Intracoronary imaging was used in 24% of cases. At the end of the procedure, 98.5% of patients had Thrombolysis in Myocardial Infarction flow grade 3, residual stenosis was > 30% in 3.4%, and dissection occurred in 1.4%. Bail-out stenting was required in 4.8% of patients. Mortality was nil during follow-up (maximum 768 days). The incidence of myocardial infarction, target lesion revascularization, and target vessel revascularization were 5.4% (95%CI, 0.69-10.1), 8.4% (95%CI, 0-17.8), and 14.2% (95%CI, 3.61-24.78), respectively.
Conclusions: In this cohort of patients with ISR treated with DEB, we observed a low rate of adverse events in both the short- and mid-term. These results support the safety and efficacy of this new generation of DEB for treating ISR.
Keywords: In-stent restenosis. Drug-eluting balloon. Paclitaxel.
RESUMEN
Introducción y objetivos: El balón farmacoactivo (BFA) es un tratamiento establecido para tratar la reestenosis intrastent (RIS). El objetivo de este estudio fue valorar la eficacia y la seguridad de un nuevo BFA en pacientes con RIS.
Métodos: Cohorte prospectiva, unicéntrica y consecutiva de pacientes con RIS tratados con angioplastia coronaria con un nuevo balón liberador de paclitaxel de segunda generación. Los 3 eventos principales del estudio fueron infarto de miocardio, revascularización de la lesión diana y revascularización del vaso diana. Se recogieron variables basales, incluidas las características del paciente y del procedimiento. Los datos referentes al seguimiento se obtuvieron de registros médicos o por contacto telefónico.
Resultados: Se incluyeron 160 pacientes consecutivos con 206 lesiones tratadas (71,4 ± 14,9 años, el 15,5% mujeres) que fueron tratados con una intervención coronaria percutánea con BFA debido a RIS. El 53,3% de los pacientes presentaban síndrome coronario agudo. El diámetro medio del vaso tratado fue de 3,1 ± 0,7 mm. El diámetro y la longitud del BFA empleado fueron de 3,1 ± 0,6 mm y 23,1 ± 6,8, respectivamente. El 98% de las lesiones se predilataron y en el 80% se empleó un balón no distensible. El 24% de las angioplastias fueron guiadas por imagen intracoronaria. El 98,5% de los pacientes presentaban un flujo Thrombolysis in Myocardial Infarction de grado 3 al final de la angioplastia. Hubo estenosis residual > 30% en el 3,4%, y el 1,4% presentaron disección. El 4,8% de los pacientes requirieron stent de rescate. Al finalizar el seguimiento (máximo 768 días), ningún paciente había fallecido. Las incidencias de infarto de miocardio, de revascularización de la lesión diana y de revascularización del vaso diana fueron del 5,4% (IC95%, 0,69-10,1), el 8,4% (IC95%, 0-17,8) y el 14,2% (IC95%, 3,61-24,78), respectivamente.
Conclusiones: En esta cohorte de pacientes con RIS tratados con BFA se observa una baja tasa de eventos clínicos adversos, tanto a corto como a mediano plazo. Estos resultados respaldan la eficacia y la seguridad de esta nueva generación de BFA para pacientes con RIS.
Palabras clave: Reestenosis intrastent. Balón farmacoactivo. Paclitaxel.
Abbreviations
DEB: drug-eluting balloon. ISR: in-stent restenosis. TLR: target lesion revascularization. TVR: target vessel revascularization.
INTRODUCTION
Patients with coronary in-stent restenosis (ISR) represent a clinical challenge.1 Evidence indicates that these patients are at increased risk of recurrent symptoms, myocardial infarction, and repeated coronary revascularizations.2 The use of drug-eluting balloons (DEB) is a novel alternative therapeutic strategy in patients with ISR.1,3,4 The effect of DEBs in coronary angioplasty is based on the rapid and uniform transfer of antiproliferative drugs into the vessel wall using a single balloon through a lipophilic matrix without the need for permanent implants.5
Over time, new DEB technologies are developed and launched onto the market. The Essential Pro (iVascular, Spain) is a paclitaxel-eluting balloon catheter with advancements to enhance catheter pushability and drug delivery. We believe it is essential to report outcomes from real-world settings. In this study, we report our findings on the safety and efficacy of this new DEB in patients with ISR.
METHODS
Design and population
This prospective, single-center study included a cohort of consecutive patients undergoing DEB angioplasty with the Essential Pro. The center treating these patients performs more than 1500 percutaneous coronary interventions per year. The 2 inclusion criteria for this analysis were: a) use of an Essential Pro DEB and b) its application for ISR treatment. ISR was defined as stenosis more than 50% within the stented segment, and treatment was indicated according to the treating physician’s judgment.6 The use of the Essential Pro DEB was prioritized during the study period to treat all eligible patients for DEB angioplasty, while other DEB devices were rarely used due to inventory constraints. There were no exclusion criteria. Patients may have undergone stent coronary angioplasty of other lesions in the same or a different setting.
Drug-eluting balloon characteristics
The Essential Pro is a paclitaxel-eluting balloon with a uniform 3 μg/mm2 eluting formulation, consisting of paclitaxel (80%) and a biocompatible amphiphilic excipient (20%).7 The balloon incorporates the proprietary TransferTech technology (iVascular, Spain), which is based on the ultrasonic deposition of nanodrops, followed by a dry-off process, resulting in a homogeneous microcrystalline drug coating. This allows more uniform and complete treatment of the vessel with the antiproliferative drug. The microcrystalline structure, coupled with the lipophilic nature of both paclitaxel and the excipient, facilitates drug transfer within 45 to 60 seconds. The Essential Pro balloon has been designed with a smooth transition and a very low tip profile of 0.016 inches, enhancing flexibility, trackability, and device crossability. The balloon is compatible with 5-Fr sheaths in all available diameters.
Procedures
All procedures and decisions in this study reflect real-world clinical practice. Therefore, clinical indications, the use and selection of DEBs, procedural steps, and medical treatments were decided by treating physicians without following any specific guidelines. All coronary angiograms performed during follow-up were part of routine clinical practice and were assessed by our research team when available. Baseline and follow-up data were collected in a single anonymized dedicated database. Procedural aspects, as well as both baseline and follow-up angiograms, were independently evaluated by 3 different interventional cardiologists. Physicians were trained to consult senior staff if they had doubts when assessing angiograms or clinical records. Follow-up was conducted using clinical records, and patients with no on-site clinical visits during follow-up were contacted by telephone following standard clinical practice in our institution. This study was approved by our local institutional review board and patients provided consent for the use of their anonymized information for research purposes before inclusion. This was an investigator-initiated study with no sponsoring or funding.
Outcome definitions
Device delivery was defined as successful DEB insufflation in the affected coronary segment. Procedural, angiographic, and other standard outcomes were defined according to the Second Academic Research Consortium criteria.8 Cardiovascular mortality was defined as any death without a clear noncardiovascular cause. Acute myocardial infarction was defined as any myocardial infarction meeting the fourth version of the Universal Myocardial Infarction Criteria.9 Target lesion revascularization (TLR) was defined as any revascularization within or 5 mm beyond the treated segment.8 Target vessel revascularization (TVR) was defined as revascularization of the index treated vessel.8 Coronary-related hospitalization was defined as a new hospitalization in which a coronary origin was suspected as the main reason for admission. The 3 main efficacy outcomes were myocardial infarction, TLR, and TVR.
Statistical analysis
Categorical variables are presented as percentages, and continuous variables as mean standard deviation (SD) when appropriate. Since the same patient may receive more than 1 DEB (for the same or different territory), the denominator for balloon-specific variables was based on the total DEBs used (such as treated vessel, vessel diameter, DEB diameter, and length), while the denominator of patient-level variables (such as age, sex, or clinical outcomes) was each single individual. Clinical outcomes during follow-up are presented at 30 days, 1 year, and total follow-up. The Kaplan-Meier method was used for estimating both the total follow-up risk and generating survival curves. Data were analyzed using IBM SPSS Statistics 25.
RESULTS
From December 2020 to June 2023, 290 patients with 352 coronary lesions were treated with DEB. Among them, 160 patients (206 lesions) underwent DEB angioplasty due to ISR. Out of the 160 patients receiving DEB for ISR, 46 patients (29%) received more than 1 DEB angioplasty for ISR, either during the same procedure or staged to a different lesion.
The patients’ baseline characteristics are summarized in table 1. The mean age was 71.4 ± 14.9 years, 15.5% were women, and 35.5% had diabetes. Clinical presentation was stable angina in 29.7%, unstable angina in 30.5%, non–ST-segment elevation myocardial infarction in 9.9%, ST-segment elevation myocardial infarction in 12.9%, and 16.7% were asymptomatic.
Table 1. Baseline characteristics
| Patient characteristics | |
| Age, y | 71.4 (14.9) |
| Sex women | 20 (15.5) |
| BMI, kg/m2 | 29.2 (10.5) |
| Hypertension | 115 (87.7) |
| Active smoking | 8 (6.1) |
| Diabetes mellitus | 46 (35.3) |
| Previous MI | 67 (51.5) |
| Previous CABG | 26 (20) |
| Reduced LVEF (< 30%) | 10 (7.6) |
| Laboratory parameters | |
| Hemoglobin, g/dL | 13.9 (1.5) |
| GFR, mL/min/1.73 m2 | 82.9 (25.4) |
| Active medication | |
| Aspirin | 110 (84.6) |
| Clopidogrel | 75 (57.6) |
| Ticagrelor | 3 (2.3) |
| Prasugrel | 2 (1.5) |
| Anticoagulation | 20 (15.2) |
| Clinical presentation | |
| Silent ischemia | 22 (16.7) |
| Stable angina | 39 (29.7) |
| Unstable angina | 40 (30.5) |
| NSTEMI | 13 (9.9) |
| STEMI | 17 (12.9) |
|
BMI, body mass index; CABG, coronary artery bypass grafting; GFR, glomerular filtration rate; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NSTEMI, non–ST-segment elevation myocardial infarction; STEMI, ST-segment elevation myocardial infarction. Data are expressed as No. (%). |
|
Procedural characteristics are detailed in table 2. The most commonly treated vessel was the left anterior descending artery (48.7%), followed by the left circumflex (30.7%), and the right coronary artery (17%). Bifurcation was present in 10.7%. Lesion preparation was performed in 98.2% of cases (80% with a noncompliant balloon). Intracoronary imaging was used in 24% of patients. None of the patients underwent rotational atherectomy, and 2.4% underwent balloon lithotripsy before DEB delivery. The mean vessel diameter was 3.1 ± 0.65 mm. The mean DEB diameter was 3.1 ± 0.6 mm, and the mean length was 23.1 ± 6.8 mm. Device delivery was successful in 100% of cases (figure 1). The final angiographic assessment revealed a final dissection in 1.4%, Thrombolysis in Myocardial Infarction flow less than 3 in 1.5%, and residual stenosis more than 30% in 3.4%. Bail-out stenting was needed in 4.8%.
Table 2. Characteristics of the treated lesion
| Treated vessel | |
| LAD | 100 (48.7) |
| LCx | 63 (30.7) |
| Right coronary artery | 35 (17) |
| Left main coronary artery | 5 (2.4) |
| Graft | 2 (0.9) |
| Anatomical characteristics | |
| Bifurcation lesion | 22 (10.7) |
| Vessel diameter, mm | 3.1 (0.65) |
| Procedural characteristics | |
| IVUS-guided PCI | 51 (24) |
| Lesion predilatation | 202 (98) |
| Predilatation with NC balloon | 165 (80) |
| Intravascular lithotripsy | 5 (2.4) |
| DEB diameter, mm | 3.1 (0.6) |
| DEB length, mm | 23.1 (6.8) |
| Result after DEB PCI | |
| Vessel dissection | 3 (1.4) |
| TIMI flow 3 | 203 (98.5) |
| Residual stenosis > 30% | 194 (3.4) |
| Bail-out stenting | 10 (4.8) |
|
DEB, drug-eluting balloon; IVUS, intravascular ultrasound; LAD, left anterior descending artery; LCx, left circumflex artery; NC, noncompliant; PCI, percutaneous coronary intervention; RCA, right coronary artery; TIMI, Thrombolysis in Myocardial Infarction. Data are expressed as No. (%). |
|
Figure 1. Central illustration. Main findings on the safety and efficacy of the Essential Pro drug-eluting balloon in patients with in-stent restenosis. Kaplan-Meier shows freedom from TLR. MI, myocardial infarction; TLR, target lesion revascularization; TVR, target vessel revascularization.
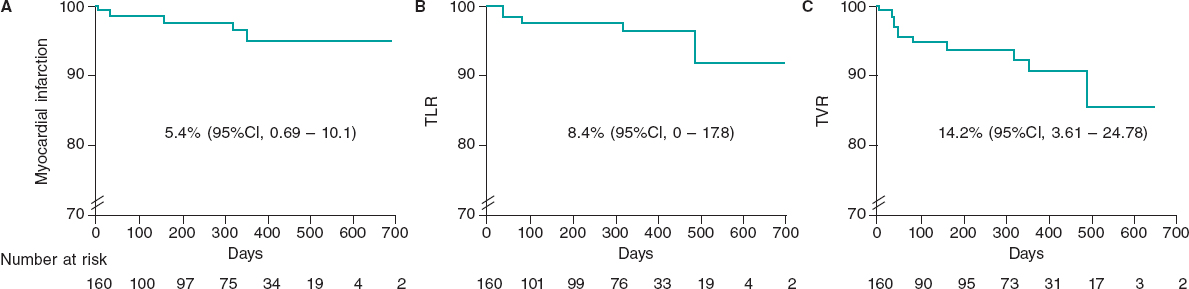
After discharge, 93.3% of the patients were successfully contacted. The median follow-up was 361 days, including censored patients, with a maximum of 768 days. At 30 days of follow-up, there were no deaths or TLR, there was 1 myocardial infarction (0.6%), TVR occurred in 0.6%, and 6 patients were readmitted to hospital due to a coronary syndrome (4.1%). At the 1-year follow-up, mortality was 0%, myocardial infarction occurred in 3.4%, TLR in 2.5%, TVR in 6.3%, and coronary-related rehospitalizations in 11.8%. At 18 months, the TLR rate was 4.3%. When all available follow-up was included (figure 2), mortality was 0%, myocardial infarction occurred in 5.4% (95% confidence interval [95%CI], 0.69-10.1), TLR in 8.4% (95%CI, 0-17.8), and TVR in 14.2% (95%CI, 3.61-24.78). During follow-up, none of the patients underwent surgical revascularization.
Figure 2. Survival curves of key clinical outcomes. Kaplan-Meier estimates for survival free from myocardial infarction (A), target lesion revascularization (B), and target vessel revascularization (C) in days. 95%CI, 95% confidence interval; TLR, target lesion revascularization; TVR, target vessel revascularization.
DISCUSSION
This is the first study to describe a real-world experience with the Essential Pro DEB for the treatment of ISR. In this cohort, all attempts at DEB delivery were successful, and less than 1 in 20 patients required bail-out stenting. The use of this new-generation DEB catheter was associated with high efficacy and a low incidence of adverse clinical outcomes during follow-up.
Patients with ISR are at higher risk of recurrent events than those undergoing non-ISR angioplasty.10 The annual rate of ISR requiring TLR is around 2%,3 representing up to 11% of all percutaneous coronary interventions performed in the United States.11,12 Notably, 52% of patients presenting with symptomatic ISR have unstable angina, and up to 27% have an acute myocardial infarction.12 Therefore, ISR poses a significant clinical challenge due to both its frequency and severity. The use of DEB in the ISR scenario avoids the addition of extra stent layers, which may have detrimental effects in the long term.
The use of DEB in ISR poses certain challenges. DEB platforms commonly have lower lesion crossability than regular coronary balloon catheters. DEBs also have larger profiles than conventional balloons making it difficult to cross the lesion and requiring aggressive maneuvers that could lead to a loss of coating drug during delivery.13 However, in our study, all attempted DEB deployments were successful. This high success rate may be due to improvements in second-generation DEBs, as well as better lesion evaluation and lesion preparation.
In the present study, TLR occurred in 2.5% of the patients and TVR in 6.3% at 1 year, while TLR occurred in 4.3% at 18 months. This event rate may seem low when compared with a prior systematic review of randomized and observational studies, which reported a TVR rate after DEB treatment of 11.3% with a calculated weighted mean follow-up of 18 months.14 In a recent investigational device exemption randomized trial for a paclitaxel-coated balloon in ISR, the rate of TLR at 1 year was 13%.15 However, prior evidence stems from diverse settings, designs, and populations, making it difficult to draw strong conclusions.
The rate of TLR with the previous generation of the Essential Pro DEB in a smaller cohort (n = 31) was 10% at 6 months.16 While this rate may seem higher than that reported in our study, the small number of events (n = 3) makes comparisons challenging.
Limitations
This study has some limitations. First, it was based on a real-world cohort involving different operators from the same center, which does not follow specific protocols. Only a quarter of the patients underwent angioplasty assessment guided by intracoronary imaging. The lack of sponsorship to cover intracoronary imaging costs and its limited use reflects the usual clinical practice of this center. During the performance of this study, few patients with ISR were treated with other DEB catheters due to the lack of specific DEB sizes in stock. Since this situation was rare and was unrelated to clinical or medical coverage characteristics, it is unlikely to introduce significant bias. Since this was a substudy of a larger DEB cohort, some variables specific to ISR, such as the time from prior stent implantation or the type of stent used, were not available.
Second, there were no dedicated follow-up visits for this study. Although most of these patients were followed up by local cardiologists who maintained regular medical records, some required telephone contact for follow-up. Third, angiographic assessment was not duplicated, and no core lab was available. Finally, the number of events was low despite consecutive enrollment from late 2020, impacting the precision of Kaplan-Meier estimates for key clinical outcomes. Some limitations are related to real-world practice settings, which, on the other hand, enhance external validity with less selection bias compared with other more controlled designs.
CONCLUSIONS
Among patients with ISR, the Essential Pro DEB catheter had a high delivery rate and a low incidence of adverse clinical outcomes during follow-up. These results further underscore the safety and efficacy of this new-generation DEB for patients with ISR.
FUNDING
This work received no industry sponsoring or funding.
ETHICAL CONSIDERATIONS
This study was approved by our local institutional review board at the Instituto Cardiovascular de Buenos Aires, and patients provided written informed consent to use their anonymized information for research purposes before their inclusion. Possible sex/gender biases have been considered in the preparation of this paper.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
No artificial intelligence tool was used in the preparation of this study.
AUTHORS’ CONTRIBUTIONS
L. Padilla conceived and oversaw all the process. F. Liberman, J. Tello, P. Rosas, P. Spaletra, G. Pedernera, P. Mascolo, S. Ordoñez, P. Santilli, and A. Candiello collected data and analyzed coronary angiograms. F. Cura and J. Belardi provided senior advice. P. Lamelas performed the statistical analysis and generated the first draft of the manuscript.
CONFLICTS OF INTEREST
L. Padilla has received proctoring and consulting honoraria from Terumo and Boston Scientific. P. Spaletra has received honoraria from Boston Scientific. F. Cura has received honoraria from Medtronic, Boston Scientific, Terumo, and Meril. P. Lamelas has received proctoring and consulting honoraria from Medtronic, Boston Scientific, Meril, Microport. The remaining authors have no conflicts of interest to declare.
WHAT IS KNOWN ABOUT THE TOPIC?
- Patients with ISR are at high risk of recurrent events and are commonly treated with DEB. New or newer generation DEBs are frequently launched onto the market. It is important to report the real-world safety and efficacy of interventional devices. The Essential Pro is a secondgeneration paclitaxel-eluting balloon. Enhancements of this DEB include improvements in forward pushability, crossover capacity, and drug delivery capabilities.
WHAT DOES THIS STUDY ADD?
- Using this new-generation DEB, all attempts at treating ISR (n = 206) were successful. Intravascular ultrasound was used in 24%. The incidence of adverse events, from the procedure to mid-term follow-up, was infrequent and probably lower than that previously reported. These realworld results emphasize the safety and efficacy of this novel generation DEB for patients with ISR.
REFERENCES
1. Giacoppo D, Saucedo J, Scheller B. Coronary Drug-Coated Balloons for De Novo and In-Stent Restenosis Indications. J Soc Cardiovasc Angiogr Interv. 2023. https://doi.org/10.1016/j.jscai.2023.100625.
2. Pleva L, Kukla P, Hlinomaz O. Treatment of coronary in-stent restenosis:A systematic review. J Geriatr Cardiol. 2018;15:173-184.
3. Giustino G, Colombo A, Camaj A, et al. Coronary In-Stent Restenosis:JACC State-of-the-Art Review. J Am Coll Cardiol. 2022;80:348-372.
4. Indermuehle A, Bahl R, Lansky AJ, et al. Drug-eluting balloon angioplasty for in-stent restenosis:A systematic review and meta-analysis of randomised controlled trials. Heart. 2012;99:327-333.
5. Jeger R V., Eccleshall S, Wan Ahmad WA, et al. Drug-Coated Balloons for Coronary Artery Disease:Third Report of the International DCB Consensus Group. JACC Cardiovasc Interv. 2020;13:1391-1402.
6. Klein LW, Nathan S, Maehara A, et al. SCAI Expert Consensus Statement on Management of In-Stent Restenosis and Stent Thrombosis. J Soc Cardiovasc Angiogr Interv. 2023;2:100971.
7. Pérez de Prado A, Pérez-Martínez C, Cuellas Ramón C, et al. Safety and Efficacy of Different Paclitaxel-eluting Balloons in a Porcine Model. Rev Esp Cardiol. 2014;67:456-462.
8. Garcia-Garcia HM, McFadden EP, Farb A, et al. Standardized end point definitions for coronary intervention trials:The academic research consortium 2 consensus document. Circulation. 2018;137:2635-2650.
9. Domienik-Karlowicz J, Kupczyn´ska K, Michalski B, et al. Fourth universal definition of myocardial infarction. Selected messages from the european society of cardiology document and lessons learned from the new guidelines on st-segment elevation myocardial infarction and non-st-segment elevation-acute coronary syndrome. Cardiol J. 2021;28:195-201.
10. Steinberg DH, Pinto Slottow TL, Buch AN, et al. Impact of In-Stent Restenosis on Death and Myocardial Infarction. Am J Cardiol. 2007;100:1109-13.
11. Madhavan M V, Kirtane AJ, Redfors B, et al. Stent-Related Adverse Events >1 Year After Percutaneous Coronary Intervention. J Am Coll Cardiol. 2020;75:590-604.
12. Moussa ID, Mohananey D, Saucedo J, et al. Trends and Outcomes of Restenosis After Coronary Stent Implantation in the United States. J Am Coll Cardiol. 2020;76:1521-1531.
13. Yoshida R, Ishii H, Morishima I, et al. Impact of adjunctive use of guide extension catheter on midterm outcome of drug-coated balloon angioplasty. EuroIntervention. 2019;15:688-691.
14. Cui KY, Lyu SZ, Zhang M, Song XT, Yuan F, Xu F. Drug-Eluting Balloon versus New-Generation Drug-Eluting Stent for the Treatment of In-Stent Restenosis:An Updated Systematic Review and Meta-Analysis. Chin Med J (Engl). 2018;131:600-607.
15. Yeh RW, Shlofmitz R, Moses J, et al. Paclitaxel-Coated Balloon vs Uncoated Balloon for Coronary In-Stent Restenosis:The AGENT IDE Randomized Clinical Trial. JAMA. 2024;331:1015-1024.
16. de la Torre Hernández JM, Garcia Camarero T, Lozano Ruiz-Poveda F, et al. Angiography and Optical Coherence Tomography Assessment of the Drug-Coated Balloon ESSENTIAL for the Treatment of In-Stent Restenosis. Cardiovasc Revasc Med. 2020;21:508-513.