ABSTRACT
Coronary obstruction (CO) is a rare but potentially fatal complication of transcatheter aortic valve implantation (TAVI). The present article aims to summarize the evidence on CO risk factors and provide an overview of preventive strategies. We performed a comprehensive literature review focused on these items. The analysis included studies addressing patient-specific characteristics, procedural aspects, and the effectiveness of various prevention techniques in mitigating CO risk. Specific risk factors for CO, which can be assessed by evaluating patient characteristics using computed tomography, are described. Procedural factors associated with an increased risk of CO are discussed. Preventive techniques, including the chimney stent and bioprosthetic aortic scallop intentional laceration to prevent iatrogenic coronary artery obstruction (BASILICA), are also described, highlighting the advantages and disadvantages of each method. The present review also provides an overview of emerging dedicated devices designed to address this complication. In conclusion, identifying patients at risk for CO is crucial for optimizing TAVI outcomes. Comprehensive imaging assessment and appropriate preventive strategies, such as the BASILICA technique, can mitigate the risk of CO and improve patient outcomes. Further research is needed to validate emerging dedicated devices.
Keywords: Transcatheter aortic valve replacement. Coronary artery obstruction. Coronary protection techniques.
RESUMEN
La obstrucción de las arterias coronarias (OC) es una complicación rara, pero potencialmente fatal, del implante percutáneo de válvula aórtica (TAVI). El objetivo de esta revisión es resumir la evidencia sobre los factores de riesgo de OC y las estrategias preventivas. Se realizó una revisión integral de la literatura centrada en estos aspectos. El análisis consideró estudios que abordaran las características del paciente, los factores procedimentales y la efectividad de diferentes técnicas preventivas para reducir el riesgo de OC. Se describen los factores relacionados con el paciente y del procedimiento que condicionan un mayor riesgo de OC. A lo largo del texto se detallan las técnicas para disminuir el riesgo de OC, incluidos el stent en chimenea y la técnica BASILICA. Además, se aporta una descripción general de los dispositivos diseñados para abordar esta complicación. En conclusión, la identificación de los factores de riesgo de OC es crucial para optimizar los resultados del TAVI. La evaluación exhaustiva mediante imagen multimodal, junto a estrategias preventivas apropiadas, como la técnica BASILICA, pueden mitigar el riesgo de OC y mejorar los resultados. Aún se requiere más investigación para validar los dispositivos emergentes.
Palabras clave: Implante percutáneo de válvula aórtica. Obstrucción de arterias coronarias. Técnicas de protección coronaria.
Abbreviations BSV: biological surgical valve. CO: coronary obstruction. SAVR: surgical aortic valve replacement. SOV: sinus of Valsalva. STJ: sinotubular junction. TAVI: transcatheter aortic valve implantation. THV: transcatheter heart valve. VTC: valve to coronary distance.
INTRODUCTION
Transcatheter aortic valve implantation (TAVI) has evolved rapidly, achieving substantial safety and efficacy.1,2 However, complications such as conduction disturbances, access site-related complications, and coronary obstruction (CO), remain concerning due to their morbidity and mortality. CO is a rare (0.5-8%) but potentially lethal complication during TAVI.3-5 The reported in-hospital to 30-day mortality rate associated with this event is about 30% to 50%.6-8 CO can occur in an acute setting during valve implantation, before the patient has left the operating room, or it can be delayed, occurring after the patient left operating room following a successful TAVI. Delayed CO can be classified as early (0-7 days) or late (> 7 days).9
There are 2 main mechanisms of CO. The first is direct obstruction by displacement of a native or degenerated prosthetic leaflet caused by the transcatheter heart valve (THV). This is most common in patients with low coronary takeoff, accompanied by a narrow sinus of Valsalva (SOV).4 The second mechanism involves indirect obstruction wherein the leaflet is also displaced, occluding the sinotubular junction (STJ), with consequent sinus sequestration. This is more frequent with a low and narrow STJ. Most COs occur at the level of the coronary ostium (92%) and primarily on the left coronary artery (78%).4 Other causes of CO include embolization and direct obstruction of the coronary ostia by the TAVI prosthesis.3-5,7,10-12
After a thorough assessment, high-risk anatomical characteristics could favor surgical aortic valve replacement (SAVR). Nonetheless, if the surgical risk is prohibitive, it is necessary to proceed with TAVI. In such situations, coronary artery protection techniques are essential to enhance safety and minimize risks.13,14
The present review aims to summarize and analyze the predictors of CO, as well as the current techniques and strategies used to prevent this complication in the setting of TAVI procedures.
ASSOCIATED FACTORS IN CORONARY ARTERY OBSTRUCTION AFTER TAVI
Meticulous planning of TAVI and a comprehensive understanding of the underlying mechanisms that predispose to complications are imperative to improve outcomes. Computed tomography (CT) is crucial in evaluating TAVI candidates, including estimating possible complications.15 The main predictors of TAVI-related CO are summarized in table 1.
Table 1. TAVI-related coronary artery occlusion-associated factors
| Predictors | Commentary |
|---|---|
| Anatomical factors | Coronary ostia height < 12 mm (< 10 mm: maximum risk)a |
| Sinus of Valsalva diameter < 30 mma | |
| Cusp height > coronary height | |
| Low STJ height and narrow STJ diameter | |
| VTC ≤ 4 mm | |
| Culprit leaflet calcification > 600 mm3 | |
| Valve-in-valve TAVI | VTC ≤ 4 mma,b |
| Stentless BSV or stented BSV with externally mounted leafletsb | |
| Female sex | Probably related to smaller anatomy in women |
| THV and procedural factors | Balloon-expandable valves associated with a higher rate of acute CO |
| Self-expanding valves associated with delayed CO | |
| Extended sealing cuff | |
| High implantation | |
|
BSV, biological surgical valve; CO, coronary obstruction; STJ, sinotubular junction; TAVI, transcatheter aortic valve implantation; THV, transcatheter heart valve; VTC, valve-to- coronary distance. aEstimated using computed tomography. bValve-in-valve TAVI itself has been associated with higher risk; however, these factors increase the probability of CO. |
|
Anatomical factors contributing to CO in patients with native aortic valves
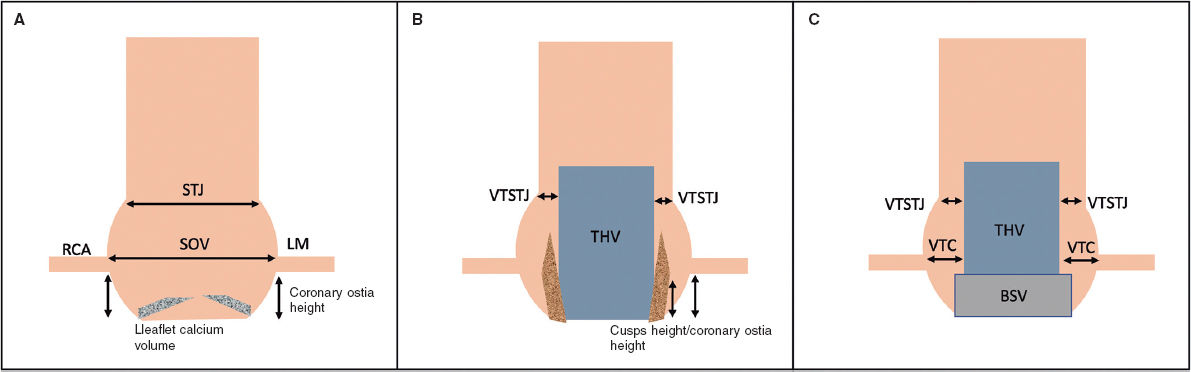
The main predictor is a low coronary ostia height, measured by CT from the plane of the aortic annulus. A previous expert consensus suggested a cutoff height of < 10 mm as indicative of maximum risk.16,17 However, data from a multicenter registry found that about 80% of the patients with CO had a left main (LM) coronary ostium height < 12 mm (mean height of 11 mm).3 Furthermore, Ribeiro et al. reported that approximately 60% of the patients with CO had a coronary ostia height > 10 mm, suggesting that the cutoff should be increased to 12 mm.18 The right coronary artery (RCA) ostium was affected only in 11% of all cases of CO in a previous registry.3 This is due to the higher takeoff of this artery compared with LM in most cases,19 underscoring the importance of these measures.
Another risk factor is a narrow aortic root with a SOV diameter < 30 mm.7,11 The valve-to-coronary (VTC) distance is the distance from the coronary ostia to the anticipated final position of the displaced bioprosthetic leaflets after TAVI.15 To calculate VTC using CT, a virtual cylinder representing the THV is used and the horizontal distance between this cylinder and the coronary ostia is measured.15 If the VTC is > 6 mm, the risk of CO is low; between 4 and 6 mm, the risk is borderline; and at < 4 mm, the risk is maximum.7,15 However, VTC measurements are not 100% specific. This might be related to the differences between the estimated and observed VTC that have been described by Tzimas et al.20
The relationship between aortic cusp height and coronary height is a relatively novel criterion. Cusp height is the vertical distance from the annular plane to the top of the cusp commissural attachment. This measurement is likely more reproducible than leaflet length.
The risk of indirect CO by sinus sequestration is higher when the annular diameter is larger than the STJ diameter, and the cusp height is higher than the STJ height.21 Similarly to VTC, the virtual distance from the THV to the STJ (VTSTJ) distance should be calculated. Figure 1 shows a schematic representation of the measures related to a predictive value for CO.
Figure 1. Schematic representation of the aortic root and CO predictors. A: native aortic valve. Coronary ostia height and the width of the SOV are predictors for CO. Leaflet calcium volume could also influence outcomes in this setting. B: displaced cusps of a native aortic valve displaced by a THV are represented in this figure. A narrower VTSTJ and a greater height of the cusps in relation to the height of the coronary ostia have been related to the risk of CO. C: aortic root with a BSV. ViV TAVI is a risk factor per se for CO; however, this is increased with shorter VTC distances. On the other hand, a tight STJ has been suggested as another factor contributing to the risk of CO. BSV, biological surgical valve; LM, left main; RCA, right coronary artery; SOV, sinus of Valsalva; STJ, sinotubular junction; THV, transcatheter heart valve; VTC, valve to coronary; VTSTJ, virtual distance from the THV to the STJ.
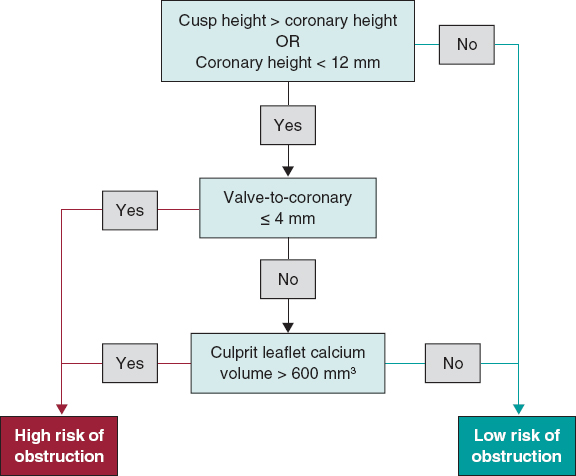
Khan et al. have developed a predictive algorithm to assess the risk of CO.4 The algorithm considers cusp height greater than coronary height and either VTC ≤ 4 mm or culprit leaflet calcium volume > 600 mm3. The model exhibited excellent performance in predicting LM and RCA ostia obstruction. Figure 2 shows a flowchart for assessing the risk of CO in patients with native aortic valves.
Figure 2. Evaluation of coronary obstruction risk in patients undergoing TAVI for native aortic valves.
Patient characteristics associated with CO
Female sex has been associated with a higher incidence of CO. Approximately 80% of the patients in the CO registries are women.18 This association is likely due to the anatomical differences between the sexes. Women tend to have a smaller aortic root, smaller SOV dimensions, and a lower coronary ostia height.19
Regarding patient history, prior coronary artery bypass has been associated with a lower incidence of symptomatic CO due to the “protective effect” of providing alternative blood flow.18 However, graft patency should always be evaluated before the TAVI procedure.22
Procedural factors affecting CO
THV type may be related to outcomes. Balloon-expandable valves are associated with a higher risk of acute CO than self-expandable valves.11,18 This difference could partly be explained by the frame characteristics and the implantation mechanism.18 However, a later registry assessing delayed CO showed that self-expandable valves were associated with higher rates of this complication than balloon-expandable valves. This is likely because self-expandable valves are nitinol-based and continue to expand after initial deployment.9 Other factors that could contribute to CO in this setting are flow stagnation and device micromigration. Jabbour et al. have postulated that endothelialization and thrombus embolization could be implicated in late delayed CO.9
Bioprosthetic surgical valves and valve-in-valve TAVI
TAVI have become a new alternative to SAVR in patients with a failed biological surgical valve (BSV) and high or prohibitive perioperative risk.1,2 Valve-in-valve (ViV) TAVI accounts for approximately 5% of all TAVI procedures in the United States.23 The CO rate is 4- to 6-fold higher in ViV procedures than in native valves.9 The higher CO risk is probably related to the supra-annular design of most BSVs, lowering coronary ostia height, while valve suturing draws the coronaries closer, with a consequent reduction in sinus width.24
Comprehensive preprocedural report must be obtained.23 The details of the previous intervention must be investigated, including the exact model and size of the BSV.25 This differentiation is crucial because stentless (eg, Freedom [Sorin Biomedica, Italy], Toronto SPV [St Jude Medical, United States], Freestyle [Medtronic, United States]), and stented valves with externally mounted leaflets (eg, Mitroflow [Sorin Biomedica, Italy], Trifecta [St Jude Medical, United States]) have a higher risk of CO.25 Ribeiro et al. have reported a significantly higher incidence of CO in patients with stentless valves (3.7%) and stented vales with externally mounted leaflets (6.4%), compared with those with stented valves with internally mounted leaflets (0.7%). Furthermore, in the same registry, the presence of these types of valves was demonstrated to be an independent predictor for CO.7
The VTC distance estimated by CT is one of the most accurate predictors of CO following a ViV TAVI.7,26 The coronary ostia height and the mean diameter of the SOV must be considered.3,7,15 Another potential anatomical risk factor for CO in a ViV procedure is a narrow STJ, as well as supra-annular position and high leaflet profile of the BSV.27,28
Redo-TAVI. Implications for coronary artery obstruction
The current trend in the treatment of aortic disease suggests that shortly, patients with longer life expectancies will undergo TAVI instead of SAVR.29 Thus, redo-TAVI will probably play a central role in treating patients with failed THV. However, data on predictors to avoid complications in this setting are still scarce.
In some of the first registries and systematic reviews assessing redo-TAVI or TAVI-in-TAVI, researchers reported very low rates of periprocedural complications, ranging from zero to only 0.9% of CO.30-32 This is likely due to the careful evaluation of the anatomy with knowledge of the predictive factors discussed above, ruling out patients at higher risk and leading to selection bias.
Redo-TAVI procedures could be related to CO risk and impaired coronary access.33 The implantation of a second THV overlaps the stent frames of the 2 prostheses, with possible compression of the leaflets of the first THV, creating a covered cylinder up to the edge of the leaflets.34 Overlapping of the stent frame and loss of free flow can impair both coronary flow and the possibility of cannulation.
In patients undergoing TAVI-in-TAVI, the STJ is critical in accessing the coronary arteries and acts as an anatomical bottleneck: a higher and broader STJ will leave more space between the first THV and the aortic wall and, therefore, easier access to coronary ostia and a lower probability of flow impairment.34 The height of the leaflets of the first THV implanted also could affect access and flow. Previous THV with supra-annular leaflets and THV with high implantation could lead to a higher risk of interaction with the STJ and impairment of the flow in the case of a second THV.34,35 Therefore, it was suggested that the VTSTJ should be calculated, especially in TAVI-in-TAVI and ViV TAVI.36
Tarantini et al. suggested an algorithm to predict the risk of CO and the feasibility of future coronary access. These authors considered CT evaluation of the coronary ostia height in relation to the first THV, a distance of 2 mm from the THV to the aortic wall, and confirmation of feasible coronary cannulation with the prior valve in place. If the coronary ostia are below the risk plane of the prior THV, the distance to the aortic wall is < 2 mm, and coronary cannulation is not possible, then TAVI-in-TAVI is considered unfeasible.33,37 The width of the aortic root again shows its importance in the risk of CO in this setting.
Redondo et al. have also highlighted another aspect to consider in the planning and execution of a TAVI-in-TAVI procedure: the alignment of the commissural posts of the previous THV with the actual localization of the coronary ostia. If a patient with a previous TAVI has a high risk of CO, intentional laceration of the bioprosthetic or native aortic scallop can be applied to prevent iatrogenic coronary artery obstruction during the TAVI (BASILICA) technique and mitigate the risk. This strategy, which consists of lacerating the previous leaflet to allow normal coronary flow and will be more fully described below, can be ineffective if there is inadequate alignment of the coronary ostia in relation to the commissural posts of the first THV. This can be caused by an eccentric location of the of the coronary ostia.38
STRATEGIES TO PREVENT CORONARY ARTERY OBSTRUCTION AFTER TAVI
As we have repeatedly emphasized, the first and most crucial step for preventing periprocedural TAVI complications is an exhaustive imaging evaluation and adequate planning. If CO is considered highly likely to occur, a risk reassessment could favor SAVR. An excessive surgical risk that mandates continuing with the transcatheter strategy requires coronary protection techniques.22
Coronary wire protection
This is the simplest protection technique in the setting of TAVI with a high risk of CO and was one of the first protective strategies reported. The technique involves placing a 0.014-inch coronary guidewire in one or both arteries through guiding catheters after crossing the aortic valve with the stiff wire. Depending on the operator’s preferences, an angioplasty balloon ranging from 2.5 mm to 3.5 mm in diameter is advanced through the coronary wire to prepare a dilatation if there is a sudden occlusion.14,39,40 If acute CO occurs, the coronary wire is be used to perform an ostial angioplasty with a balloon or the implantation of a stent to recover coronary flow.
The safety and feasibility of this technique have been demonstrated in previous reports.13,14 However, there is a need for more evidence from randomized clinical trials, which may hinder the generalizability of the effectiveness of this approach. In addition, the absence of standardized procedural guidelines can contribute to variability in its application and outcomes. Despite these challenges, the most significant concern remains the persistent risk of occlusion even after the wire has been removed, as demonstrated in the Spanish Society of Cardiology registry.5
Chimney/snorkel stent technique
The chimney stent technique is a strategy involving the placement of a coronary guidewire with an undeployed stent in one or both coronary arteries, implanting the stent if CO occurs, so that it protrudes outside and above the coronary ostium, resembling a “chimney” or a “snorkel.” First reported by Chakravarty et al., this strategy was initially used to treat an anticipated acute CO of the LM coronary artery in a patient with a degenerated BSV.41 Several cases reports have shown its effectiveness and safety.42,43
Clinical follow-up has found acceptable mid-term outcomes (follow-up time of 612 days, interquartile range: 405-842 days) in a registry, with only 1 case of stent failure and 1 case of possible late stent thrombosis.44 Longer follow-up results are required to respond to concerns about stent-related outcomes. Difficult coronary re-access through the “snorkel” is to be expected, which raises doubts if there are subsequent coronary complications. Potential mechanisms for eventual stent failure include persistent turbulent flow across the THV and the stent, galvanic corrosion, and local inflammatory processes.10
Procedural details
The chimney technique involves a series of critical steps. These steps, which may vary slightly across different cath-labs, are based on existing literature and experience. As with any complex procedure, it must be performed by an experienced interventional team. Figure 3 shows an example of a real case using the chimney/snorkel technique to protect a patient at high risk of CO.
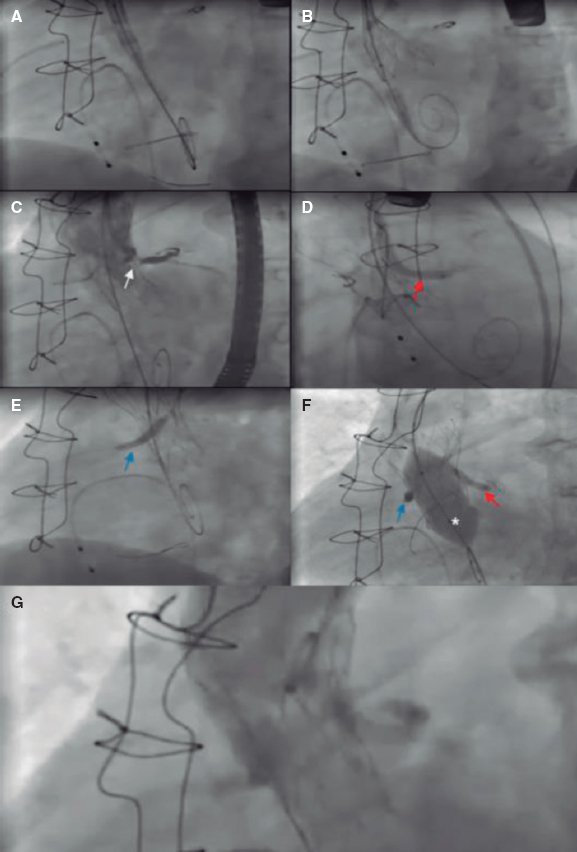
Figure 3. Main steps of a valve-in-valve (Freedom valve [Sorin Biomedica, Italy]) transcatheter aortic valve implantation in which a bilateral chimney technique is used to protect both coronary arteries. A: before valve implantation, undeployed drug-eluting stents were positioned in the right coronary artery (3.5 × 28 mm) and the LM (4 × 33 mm) in preparation for percutaneous coronary intervention in case of acute coronary obstruction. B: then a Portico valve (Abbott Vascular, United States) is advanced, and deployment is started. C: during valve deployment, contrast injections were performed to assess coronary ostia patency. The moment of the occlusion of the LM ostium is observed (white arrow). D: given the acute coronary obstruction, the stent of the LM is implanted (red arrow). E: posteriorly, due to the high risk, the stent of the right coronary artery is also implanted (blue arrow). F: postdilatation with a valvuloplasty balloon (*) was chosen to improve the expansion of the transcatheter heart valve. To avoid compression of the stents, the balloons of the stents were inflated at the same time as the aortic balloon. G: final angiographic follow-up shows both ostia patency and the absence of aortic regurgitation. LM, left main.
First step: patient assessment
-
– A thorough preprocedural evaluation is crucial. The procedure should be performed after the patient is discussed in a Heart Team composed of clinicians, interventional cardiologists, and cardiac surgeons with sufficient expertise.
Second step: vascular accesses
-
– Obtain radial access for the secondary access (Pigtail catheter). When protecting both coronaries, guiding catheters may be used for contrast injections to direct THV implantation and assess ostia patency.
-
– Common femoral artery access for THV implantation or alternative access if needed.
Use the contralateral femoral artery to access a guiding catheter for coronary protection. Ideally, a 7-Fr catheter (Extra back-up [EBU] or Judkins left [JL] for the LM, and Judkins right [JR] for the RCA).
-
– Obtain venous access for the pacemaker, if required.
Third step: preparation of coronary protection and THV deployment
-
– Cross the aortic valve and position the TAVI guidewire in the left ventricle (LV).
-
– Position the 0.014-inch coronary guidewire in the artery at risk.
-
– Advance stents over the coronary guidewires, ensuring they are long enough to anchor and protrude above the THV leaflets. A guiding catheter extension may be used to protect the stent from interacting with the THV.
-
– Perform valvuloplasty, if needed, and assess coronary flow during the process.8,42
-
– Advance the THV through the LV wire and deploy it, monitoring coronary flow using contrast injections.
Fourth step: stent deployment and postprocedure evaluation
-
– If the coronary flow is affected during THV implantation, pull up the undeployed stents protruding into the aorta, and deploy them.
-
– Maintain a low threshold for stent implantation, as recrossing the THV structure can be challenging.
-
– Consider flaring the proximal segment of the stent with a balloon to improve the possibility of reaccessing the coronary arteries.
-
– Perform postdilatation if needed, using a “kissing balloon” technique to avoid coronary stent crushing.43
-
– Conduct a final echocardiographic and angiographic evaluation to confirm successful results before ending the procedure.
Postprocedural treatment
The optimal antiplatelet therapy for these patients is uncertain. Maintaining dual antiplatelet therapy (aspirin plus clopidogrel) for at least 6 months is generally recommended. However, in the elderly population with comorbidities, bleeding risk should be considered. For patients on anticoagulants, triple therapy may be used for 1 week, followed by dual therapy (clopidogrel plus anticoagulant) for 3 to 6 months before continuing with the anticoagulant alone. More evidence is needed to determine the best strategy in these cases.
The BASILICA technique
The BASILICA technique is another strategy suggested to prevent CO. This strategy was developed as a pre-emptive measure before THV implantation, lacerating the leaflets to prevent their compression against the coronary ostia, which could lead to acute occlusion.24,45 Creating a “triangle of flow” facilitates blood flow to the coronary artery.46 BASILICA was designated as an alternative to stent-based techniques, which may have limitations such as potential extrinsic compression, unknown long-term thrombosis risk, and challenges in coronary access.10
Khan et al. reported 30-day outcomes for 30 patients who underwent BASILICA, with no CO reported and successful procedures in 28 patients. Safety outcomes, including major cardiovascular complications, stroke, kidney injury, and death, were reported in 70% of patients but were unrelated to BASILICA.36 Recently, we demonstrated that this procedure can be performed with a very low risk of major cardiac adverse events and a high success rate in patients with native and prosthetic aortic valves.47 Hemodynamic instability after valve laceration was rare and resolved after THV implantation. The unsuccessful procedures were probably due to significant calcification of the leaflets, avoiding their perforation before splitting.45 One-year follow-up results indicated no additional strokes or myocardial infarctions, with only 2 more deaths.48 Kitamura et al. reported even better results, with no major vascular complications, need for mechanical circulatory support, stroke, or mortality at 30 days.49 The applicability of the BASILICA technique to failed THV is limited due to the design of some THV types and commissural alignment. Benchtop models found that leaflet splitting was effective in older generation Sapien XT valves but was less effective in the newer Sapien 3 (Edwards Lifesciences, United States) and EVOLUT (Medtronic, United States) valves.50 Furthermore, even in the case of a feasible laceration, the new THV commissures might align unfavorably. In addition, positioning the new THV skirt too high may obstruct the lacerated leaflet.
Contraindications have yet to be clearly defined, but the technique may be ineffective in cases with extremely narrow SOV, eccentric coronary ostia, or highly calcified cusps. Additionally, it should be avoided in cases of endocarditis or valve thrombosis.46 Regarding eccentricity, this could be one of the most important obstacles for an effective protection of the coronary ostia, especially in patients undergoing a TAVI-in-TAVI procedure, as Redondo et al. have suggested in a previous publication. In these cases, if the coronary ostia are located in an eccentric position within the SOV, the laceration will probably not be aligned with the ostia, suppressing its efficacy.38
Procedural details
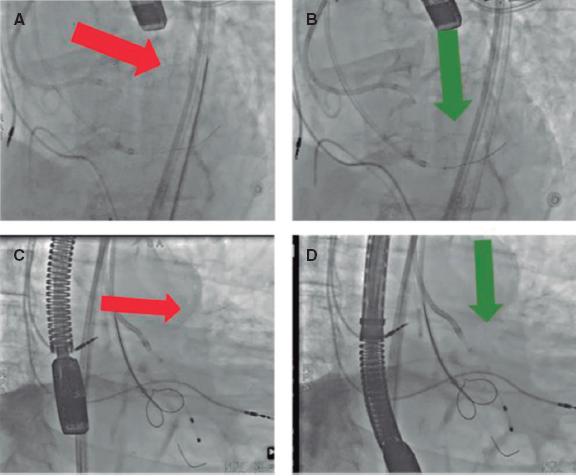
The procedure should be performed with transesophageal echocardiographic (TEE) guidance to ensure the best outcomes and facilitate the approach, and general anesthesia is mandatory. Some operators prefer the use of intracardiac echocardiography and in these cases general anesthesia is not necessary. Figure 4 shows a ViV TAVI procedure using the BASILICA technique to protect the LM due to the high risk of occlusion.
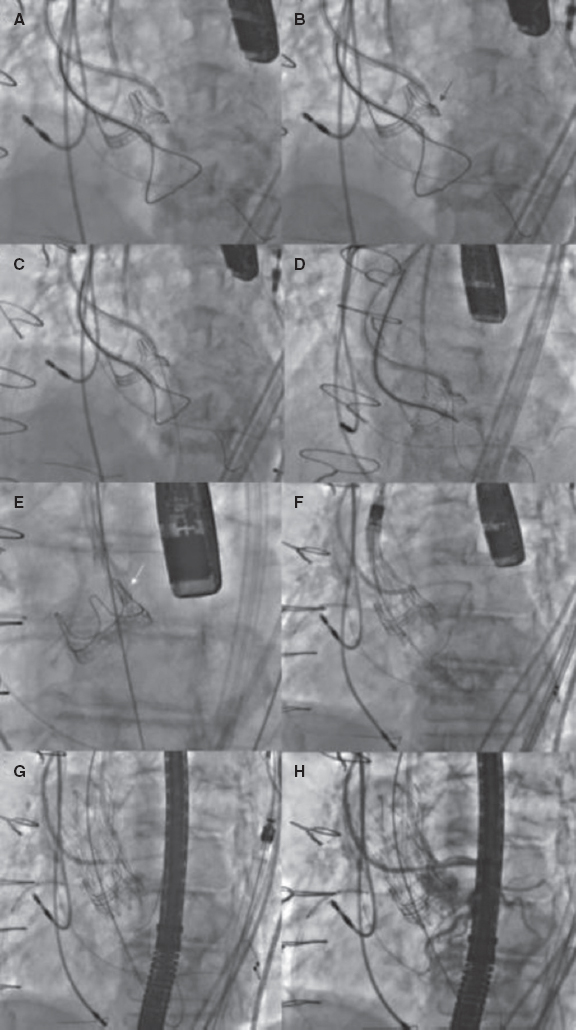
Figure 4. Main steps of a valve-in-valve transcatheter aortic valve implantation with BASILICA technique. A: first, a guiding catheter JR (8-Fr) was placed in the left ventricle with a snare, and a pigtail in the ascending aorta for an aortogram. A diagnostic JR (5-Fr) inside a guiding catheter AL 3 (8-Fr) was placed above the aortic prosthesis leaflet with a Finecross 130 microcatheter and an Astato XS 20 guidewire (Asahi Intecc, United States) inside. B: once the optimal perforation spot in the left cusp was identified using echography and angiography and the guidewire was correctly positioned, it was electrified, and the leaflet was perforated (red arrow). C: then, the wire was trapped with the snare placed in the left ventricular outflow tract, and it was pulled inside the guide catheter JR (D, E) before the externalization of the wire; a “V-shape” was performed in the middle part of the wire. Then, it was advanced, and when the “V-shape” contacted the leaflet (E, white arrow), the wire was electrified again while it was pulled at both ends, lacerating the leaflet. F, G: a self-expandable transcatheter heart valve was implanted, and coronary patency was finally confirmed (H). BASILICA, bioprosthetic aortic scallop intentional laceration to prevent iatrogenic coronary artery obstruction; JR, Judkins right.
First step: patient assessment
-
– Careful assessment must always be conducted for patients undergoing a TAVI procedure. Procedural planning must involve multi-imaging assessment, with CT images playing a central role.
Second step: vascular accesses
-
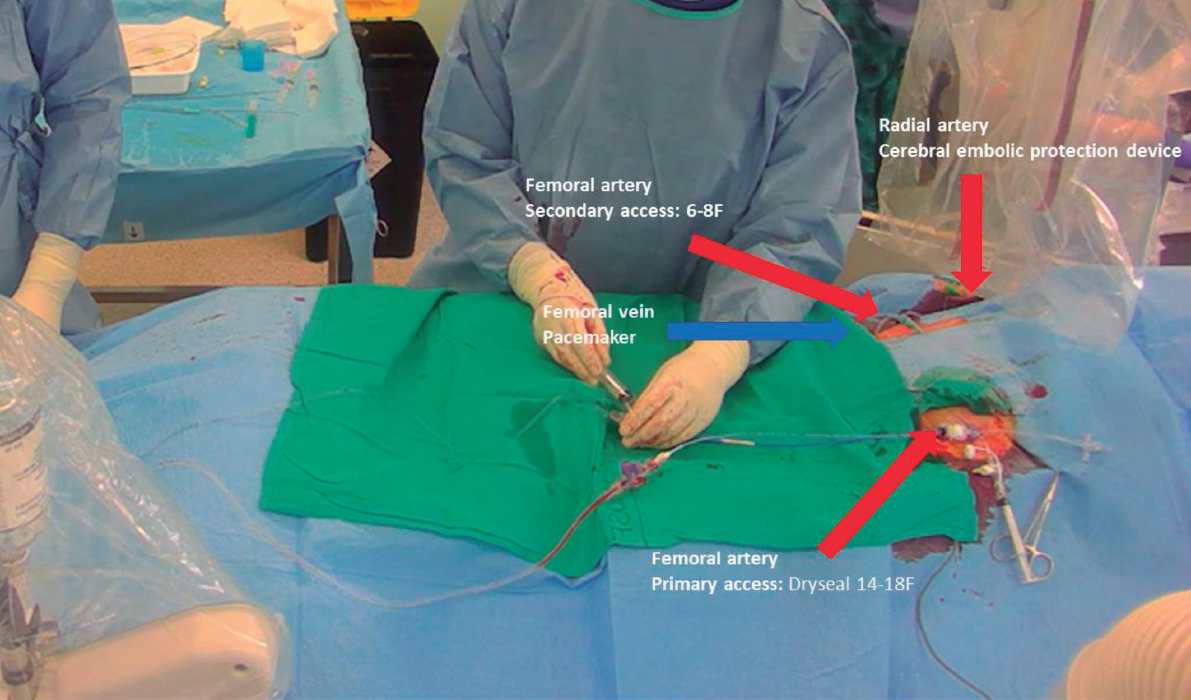
– Initially, at least 3 arterial accesses are needed for this technique (figure 5).24
-
– A 14-Fr sheath (at least) is used for the primary access. A Dryseal sheath (GORE, United States) is recommended as it can accommodate 2 guiding catheters and maintain hemostasis. One guiding catheter (7-8F) is used to perforate the leaflet, and the other is a pigtail placed in the LV.
-
– If the iliofemoral anatomy is complex, we recommend using a femoral sheath that can be deployed to advance the THV. By doing this, the interventional cardiologist can ensure that the THV advances smoothly after lacerating the leaflet.
-
– The second access is placed in the contralateral common femoral artery to insert a catheter which is used to position a snare in the LV.
-
– The third access is inserted in the radial artery to place a cerebral embolic protection device (Sentinel [Boston Scientific, United States]).
-
– If needed, venous access should be obtained to implant a temporary pacemaker.
Figure 5. Patient setup with 3 arterial accesses —right radial for cerebral embolic protection device and 2 femoral— and 1 venous access for temporary pacemaker.
Third step: leaflet perforation
-
– The aortic valve should be crossed, and a 6-Fr multipurpose (MP) guiding catheter is placed in the LV outflow tract (LVOT). Using the MP, a goose neck snare with the size of the LVOT (20-30 mm) is positioned in the LVOT. Parallel to the snare, using the same MP catheter, a 0.018 wire is placed into the LV, reaching the apex; this wire allows the snare to be redirected into the LV if it is pulled out. Instead of an MP, a 6-Fr JR could be used, depending on the angulation of the anatomy.
-
– Subsequently, different catheters should be chosen, ideally, a 7-8F, depending on the cusp that needs to be lacerated. To approach the left cusp, an Amplatz left (AL) 3 is the first option; however, depending on the aortic root anatomy, an AL1, AL2, AL4, EBU 3.5, and 4, can also be used. For the right cusp, an MP is usually used, or a JR if the aorta is angulated.
-
– To perforate the left cusp, a diagnostic long 5-Fr catheter is typically needed inside the 8-Fr catheter (mother-and-child). The first option is a 125 cm diagnostic internal mammary or JR 4 catheter.
-
– With a telescope of devices, a 300 cm wire (suggested: Astato XS 20 300 cm [Asahi Intecc, United States]) with a microcatheter, both inside the 5-Fr internal mammary and the 8-Fr guiding catheter.
-
– The telescope of devices is oriented toward the base of the target cusp, with the correct orientation to avoid undesired perforations guided by fluoroscopy and TEE. The target leaflet should be projected in 2 fluoroscopic angles, “front view” and “side view”. These projections, estimated using CT assessment, help achieve an accurate approach to the leaflet. Contrast injections can further assist in estimating the spatial relationship of the valve (figure 6).
-
– Once an optimal position of the “telescope” with a correct “attack angle” is achieved, leaflet perforation is attempted. The catheters and wire complex are propped, and the microcatheter is brought closer to the leaflet. The wire is then electrified to perform perforation.
-
– To electrify the wire, its back is scraped about 1 to 3 cm with a scalpel blade until the metal part is exposed, then connected to an electric pencil with a mosquito clamp. The electrosurgical generator is set to “pure cut” mode, and the power is set according to the leaflet; 30 watts for porcine, 50 watts for bovine or native, and 70 watts for severely calcified leaflets. Electrification should be brief (less than a second) and stopped immediately after the wire crosses the leaflet.46
-
– After perforation, the 300 cm wire is positioned in the LVOT, attempting to cross it through the snare. Snaring should be performed high in the LVOT to prevent mitral valve injury. Once snaring is achieved, the 300 cm wire is pulled inside the snare guide without externalizing the wire.
-
– TEE guidance should be used to ensure that the wire is not entangled with the mitral apparatus.
Figure 6. Catheter orientation for leaflet perforation. A: side view with off axis direction. B: side view with correct direction. C: en-face view with off axis direction. D: en- face view with correct direction.
Fourth step: THV preparation
-
– After perforating the leaflet and before performing the laceration, prepare the THV to ensure it is ready for prompt implantation once the leaflet has been modified, as leaflet perforation can be time-consuming. The valve cannot remain crimped for an extended period, which could increase the risk of THV damage.
-
– Once the leaflet has been perforated, the aortic valve should be recrossed to position a pigtail catheter from the arterial main access to proceed quickly with THV implantation if there is hemodynamic instability after the leaflet laceration.
Fifth step: leaflet laceration
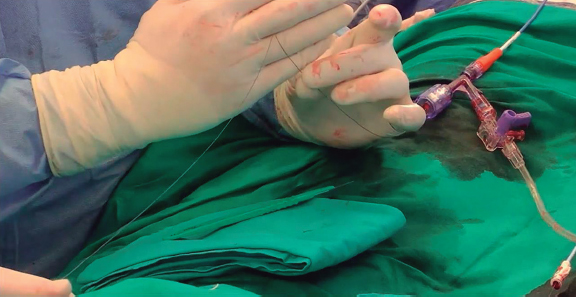
-
– Before the externalization of the 300-cm wire, a “V-shape” must be created in the middle part of the wire. To create this V-shape, the wire must be kinked and denuded with a scalpel blade of about 10 mm in the kinked part (figure 7). Then, the wire is advanced until the V-shape is in contact with the leaflet.
-
– The microcatheter position is fixed with a torque device to identify the “flying V”.
-
– Once the V-shape is in the correct place, the wire is pulled at both ends, coinciding with a new electrification of the wire with the pencil connected in the same place as that used for perforation. The power to be applied is higher this time and varies depending on the type of leaflet; 50 watts for a porcine valve, 70 watts for a bovine or native valve, and 100 watts for a severely calcified leaflet.
-
– Dextrose solution injection in each guide catheter may be performed simultaneously with the laceration. However, if the dextrose is not used, the catheters should be flushed before laceration to remove all blood content.
-
– To avoid hemodynamic instability caused by prolonged laceration of a leaflet without THV implantation, both leaflets must be addressed simultaneously to protect both coronary ostia, if needed. This requires additional vascular accesses, such as using a 14- to 18 Fr sheath in 1 femoral artery for 1 leaflet and double access with 2 sheaths (6-8 Fr) in the other femoral artery or using another large sheath (14-18F) in the other femoral, but with increased bleeding and vascular risk.
Figure 7. Astato XS 20 (Asahi Intecc, United States) with flying V for leaflet laceration.
Sixth step: THV implantation and postdilatation
-
– The THV should be implanted promptly after laceration. The catheters used for laceration are removed, and the pigtail placed in the LV is then used to advance the stiff wire for the THV implantation. The height of implantation should be balanced between the risk of high gradients with a low position and the risk of CO with implantation that is too high. Too high implantation can result in the skirt covering the “triangle of flow”.46 Recommendations for each kind of THV should be followed, attempting to keep the lower range of recommended depth, eg, for an EVOLUT Pro+ valve, (Medtronic, United States) 3 mm deep using cusp overlap projection. This is of particular importance in supra-annular THV.
-
– Operators should be highly cautious with postdilatation and BSV ring fracture in BASILICA procedures as they can increase the risk of CO.
-
– If the risk of CO is considered too high, operators can protect the coronary arteries with guidewires and undeployed stents at their discretion.24
-
– After THV is implanted, the patency of the coronary ostia must be checked with intra-aortic injection (preferred instead of selective injection). In addition, a TEE assessment would help to check the hemodynamic results and the absence of other potential complications.
-
– Like other TAVI, the procedure should conclude with proper hemostasis and checking of the accesses.
Splitting devices
The BASILICA technique has yielded promising results but is a complex procedure that requires a highly skilled team. The ShortCut (Pi-Cardia, Israel) was designed to simplify the laceration and splitting of the leaflets.51 Initially intended for BSV, the devices comprise a handle, delivery system, and distal unit, introduced through a 16-Fr sheath to the common femoral artery. TEE guides its positioning, and it acts on the leaflet mechanically.51
Dvir et al. reported the findings of the preclinical and first-in-human experience using this device. These authors tested the device in 8 patients with failed BSV. In all patients, the TAVI procedure was successful without CO. They did not report any neurological events, and the patients were discharged with good clinical status.51 although the initial results are promising, an evidence gap remains. The results of larger registries or even trials comparing it with the BASILICA technique could confirm the usefulness of this device in the future.
UNICORN procedure
The undermining iatrogenic coronary obstruction with radiofrequency needle (UNICORN) procedure is a novel technique aiming to address the CO risk in patients undergoing a TAVI-in-TAVI procedure. The first-in-man experience using this new strategy was reported by Chan et al. These authors used a coronary guidewire inside a telescoping system composed of a 7-Fr Amplatz left-1 guide catheter (Cordis, United States) and a 135-cm Navicross support catheter (Terumo, Japan) to traverse a prosthetic leaflet with the help of a radiofrequency impulse.52 Once the leaflet was perforated, successive dilatations of the fenestration with balloons of increasing caliber were performed. The last step allowed a balloon-expandable valve to be advanced through the perforated leaflet and subsequently deploy the transcatheter valve.52
The implantation of the balloon-expandable valve through the fenestration finishes the laceration and entrapment of the previous leaflet, minimizing the risk of leaflet recoil obstructing the coronary ostium or embolization.52 The first experience was successful and demonstrated the feasibility of this strategy; however, more data on long-term outcomes are needed.
CONCLUSIONS
To optimize outcomes in TAVI procedures, it is essential to identify patients at risk of CO. These patients can be best identified by a structured evaluation that includes specific CT measurements, such as cusp and coronary height, VTC distance, calcium volume, and other anatomical and procedural risk features. Coupled with appropriate preventive procedures, such as the BASILICA technique, this comprehensive patient assessment can mitigate the risk of CO. However, further research is needed to validate the different strategies and emerging dedicated devices that aim to prevent this complication. As TAVI procedures continue to expand, identifying and managing the risk of CO will remain an essential consideration for optimizing outcomes and improving patient safety.
FUNDING
This research received no external funding.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
No artificial intelligence was used.
CONFLICTS OF INTEREST
A. Regueiro is a consultant for Abbott Vascular, Meril Life, and OpSens. X. Freixa is a consultant for Abbott Vascular outside the submitted work. M. Sabaté is a consultant for Abbott Vascular and iVascular outside the submitted work. None of the other authors have any conflict of interest to disclose.
ACKNOWLEDGMENTS
F. Spione has been supported by a research grant provided by the Cardiopath PhD program.
REFERENCES
1. Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease:Executive Summary:A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143:e35-e71.
2. Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2022;43:561-632.
3. Ribeiro HB, Webb JG, Makkar RR, et al. Predictive factors, management, and clinical outcomes of coronary obstruction following transcatheter aortic valve implantation:insights from a large multicenter registry. J Am Coll Cardiol. 2013;62:1552-1562.
4. Khan JM, Kamioka N, Lisko JC, et al. Coronary Obstruction From TAVR in Native Aortic Stenosis:Development and Validation of Multivariate Prediction Model. JACC Cardiovasc Interv. 2023;16:415-425.
5. Ojeda S, González-Manzanares R, Jimenez-Quevedo P, et al. Coronary Obstruction after Transcatheter Aortic Valve Implantation. Insights from the Spanish TAVI Registry. JACC Cardiovasc Interv. 2023;16:1208-1217.
6. Holmes DR, Jr., Nishimura RA, Grover FL, et al. Annual Outcomes With Transcatheter Valve Therapy:From the STS/ACC TVT Registry. J Am Coll Cardiol. 2015;66:2813-2823.
7. Ribeiro HB, Rodés-Cabau J, Blanke P, et al. Incidence, predictors, and clinical outcomes of coronary obstruction following transcatheter aortic valve replacement for degenerative bioprosthetic surgical valves:insights from the VIVID registry. Eur Heart J. 2018;39:687-695.
8. Akinseye OA, Jha SK, Ibebuogu UN. Clinical outcomes of coronary occlusion following transcatheter aortic valve replacement:A systematic review. Cardiovasc Revasc Med. 2018;19:229-236.
9. Jabbour RJ, Tanaka A, Finkelstein A, et al. Delayed Coronary Obstruction After Transcatheter Aortic Valve Replacement. J Am Coll Cardiol. 2018;71:1513-1524.
10. Lederman RJ, Babaliaros VC, Rogers T, et al. Preventing Coronary Obstruction During Transcatheter Aortic Valve Replacement:From Computed Tomography to BASILICA. JACC Cardiovasc Interv. 2019;12:1197-1216.
11. Arai T, Lefèvre T, Hovasse T, et al. Incidence and predictors of coronary obstruction following transcatheter aortic valve implantation in the real world. Catheter Cardiovasc Interv. 2017;90:1192-1197.
12. Dvir D, Webb J, Brecker S, et al. Transcatheter aortic valve replacement for degenerative bioprosthetic surgical valves:results from the global valve-in-valve registry. Circulation. 2012;126:2335-2344.
13. Abramowitz Y, Chakravarty T, Jilaihawi H, et al. Clinical impact of coronary protection during transcatheter aortic valve implantation:first reported series of patients. EuroIntervention. 2015;11:572-581.
14. Yamamoto M, Shimura T, Kano S, et al. Impact of preparatory coronary protection in patients at high anatomical risk of acute coronary obstruction during transcatheter aortic valve implantation. Int J Cardiol. 2016;217:58-63.
15. Blanke P, Soon J, Dvir D, et al. Computed tomography assessment for transcatheter aortic valve in valve implantation:The vancouver approach to predict anatomical risk for coronary obstruction and other considerations. J Cardiovasc Comput Tomogr. 2016;10:491-499.
16. Achenbach S, Delgado V, Hausleiter J, Schoenhagen P, Min JK, Leipsic JA. SCCT expert consensus document on computed tomography imaging before transcatheter aortic valve implantation (TAVI)/transcatheter aortic valve replacement (TAVR). J Cardiovasc Comput Tomogr. 2012;6:366-80.
17. Holmes DR, Jr., Mack MJ, Kaul S, et al. 2012 ACCF/AATS/SCAI/STS expert consensus document on transcatheter aortic valve replacement. J Am Coll Cardiol. 2012;59:1200-1254.
18. Ribeiro HB, Nombela-Franco L, Urena M, et al. Coronary obstruction following transcatheter aortic valve implantation:a systematic review. JACC Cardiovasc Interv. 2013;6:452-461.
19. Buellesfeld L, Stortecky S, Kalesan B, et al. Aortic root dimensions among patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2013;6:72-83.
20. Tzimas G, Akodad M, Meier D, et al. Predicted vs Observed Valve to Coronary Distance in Valve-in-Valve TAVR:A Computed Tomography Study. JACC Cardiovasc Interv. 2023;16:2021-2030.
21. Pilgrim T, Tomii D. Predicting Coronary Obstruction After TAVR:Better Safe Than Sorry. JACC Cardiovasc Interv. 2023;16:426-428.
22. Fetahovic T, Hayman S, Cox S, Cole C, Rafter T, Camuglia A. The Prophylactic Chimney Snorkel Technique for the Prevention of Acute Coronary Occlusion in High Risk for Coronary Obstruction Transcatheter Aortic Valve Replacement/Implantation Cases. Heart Lung Circ. 2019;28:e126-e130.
23. Vemulapalli S, Carroll JD, Mack MJ, et al. Procedural Volume and Outcomes for Transcatheter Aortic-Valve Replacement. N Engl J Med. 2019;380:2541-2550.
24. Khan JM, Dvir D, Greenbaum AB, et al. Transcatheter Laceration of Aortic Leaflets to Prevent Coronary Obstruction During Transcatheter Aortic Valve Replacement:Concept to First-in- Human. JACC Cardiovasc Interv. 2018;11:677-689.
25. Bapat V. Technical pitfalls and tips for the valve-in-valve procedure. Ann Cardiothorac Surg. 2017;6:541-552.
26. Barbanti M. Avoiding Coronary Occlusion and Root Rupture in TAVI - The Role of Pre- procedural Imaging and Prosthesis Selection. Interv Cardiol. 2015;10:94-97.
27. Dvir D, Leipsic J, Blanke P, et al. Coronary obstruction in transcatheter aortic valve-in- valve implantation:preprocedural evaluation, device selection, protection, and treatment. Circ Cardiovasc Interv. 2015;8:e002079.
28. Valvo R, Costa G, Barbanti M. How to Avoid Coronary Occlusion During TAVR Valve-in- Valve Procedures. Front Cardiovasc Med. 2019;6:168.
29. Mack MJ, Leon MB, Thourani VH, et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N Engl J Med. 2019;380:1695-1705.
30. Landes U, Webb JG, De Backer O, et al. Repeat Transcatheter Aortic Valve Replacement for Transcatheter Prosthesis Dysfunction. J Am Coll Cardiol. 2020;75:1882-1893.
31. Gallo M, Fovino LN, Blitzer D, et al. Transcatheter aortic valve replacement for structural degeneration of previously implanted transcatheter valves (TAVR-in-TAVR):a systematic review. Eur J Cardiothorac Surg. 2022;61:967-976.
32. Barbanti M, Webb JG, Tamburino C, et al. Outcomes of Redo Transcatheter Aortic Valve Replacement for the Treatment of Postprocedural and Late Occurrence of Paravalvular Regurgitation and Transcatheter Valve Failure. Circ Cardiovasc Interv. 2016;9:e003930.
33. Tarantini G, Fabris T, Nai Fovino L. TAVR-in-TAVR and coronary access:importance of preprocedural planning. EuroIntervention. 2020;16:e129-e132.
34. Buzzatti N, Romano V, De Backer O, et al. Coronary Access After Repeated Transcatheter Aortic Valve Implantation:A Glimpse Into the Future. JACC Cardiovasc Imaging. 2020;13:508-515.
35. Percy ED, Harloff MT, Hirji S, et al. Nationally Representative Repeat Transcatheter Aortic Valve Replacement Outcomes:Report From the Centers for Medicare and Medicaid Services. JACC Cardiovasc Interv. 2021;14:1717-1726.
36. Komatsu I, Mackensen GB, Aldea GS, Reisman M, Dvir D. Bioprosthetic or native aortic scallop intentional laceration to prevent iatrogenic coronary artery obstruction. Part 1:how to evaluate patients for BASILICA. EuroIntervention. 2019;15:47-54.
37. Nai Fovino L, Scotti A, Massussi M, et al. Coronary Angiography After Transcatheter Aortic Valve Replacement (TAVR) to Evaluate the Risk of Coronary Access Impairment After TAVR-in-TAVR. J Am Heart Assoc. 2020;9:e016446.
38. Redondo A, Baladrón Zorita C, Tchétché D, et al. Commissural Versus Coronary Optimized Alignment During Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv. 2022;15:135-146.
39. Rodés-Cabau J, Webb JG, Cheung A, et al. Transcatheter aortic valve implantation for the treatment of severe symptomatic aortic stenosis in patients at very high or prohibitive surgical risk:acute and late outcomes of the multicenter Canadian experience. J Am Coll Cardiol. 2010;55:1080-1090.
40. Thomas M, Schymik G, Walther T, et al. Thirty-day results of the SAPIEN aortic Bioprosthesis European Outcome (SOURCE) Registry:A European registry of transcatheter aortic valve implantation using the Edwards SAPIEN valve. Circulation. 2010;122:62-69.
41. Chakravarty T, Jilaihawi H, Nakamura M, et al. Pre-emptive positioning of a coronary stent in the left anterior descending artery for left main protection:a prerequisite for transcatheter aortic valve-in-valve implantation for failing stentless bioprostheses?Catheter Cardiovasc Interv. 2013;82:E630-E636.
42. González LF, Mata RB, Roman KG-S, Villa JA. Emergent Chimney Stent to Treat Left Main Occlusion Following Valve-In-Valve Transfemoral Aortic Implantation Chimney Stent Following Valve-In-Valve TAVI. J Cardiovasc Thorac Surg.2018;3:1-2.
43. Spaziano M, Akodad M, Hovasse T, Lefèvre T, Bouvier E, Chevalier B. Simultaneous TAVR and Left Main “Chimney“Stenting in a Patient With Low Left Main Height. JACC Cardiovasc Interv. Oct 23 2017;10:e185-e187.
44. Mercanti F, Rosseel L, Neylon A, et al. Chimney Stenting for Coronary Occlusion During TAVR:Insights From the Chimney Registry. JACC Cardiovasc Interv. 2020;13:751-761.
45. Khan JM, Greenbaum AB, Babaliaros VC, et al. The BASILICA Trial:Prospective Multicenter Investigation of Intentional Leaflet Laceration to Prevent TAVR Coronary Obstruction. JACC Cardiovasc Interv. 2019;12:1240-1252.
46. Komatsu I, Mackensen GB, Aldea GS, Reisman M, Dvir D. Bioprosthetic or native aortic scallop intentional laceration to prevent iatrogenic coronary artery obstruction. Part 2:how to perform BASILICA. EuroIntervention. 2019;15:55-66.
47. Cepas-Guillén P, Gabani R, Giménez-Milà M, Sanchis L, Freixa X, Regueiro A. Safety and efficacy of the BASILICA technique in patients at high risk of coronary obstruction undergoing TAVI. Rev Esp Cardiol. 2024;77:181-183.
48. Khan JM, Greenbaum AB, Babaliaros VC, et al. BASILICA Trial:One-Year Outcomes of Transcatheter Electrosurgical Leaflet Laceration to Prevent TAVR Coronary Obstruction. Circ Cardiovasc Interv. 2021;14:e010238.
49. Kitamura M, Majunke N, Holzhey D, et al. Systematic use of intentional leaflet laceration to prevent TAVI-induced coronary obstruction:feasibility and early clinical outcomes of the BASILICA technique. EuroIntervention. 2020;16:682-690.
50. Khan JM, Bruce CG, Babaliaros VC, Greenbaum AB, Rogers T, Lederman RJ. TAVR Roulette:Caution Regarding BASILICA Laceration for TAVR-in-TAVR. JACC Cardiovasc Interv. 2020;13:787-789.
51. Dvir D, Leon MB, Abdel-Wahab M, et al. First-in-Human Dedicated Leaflet Splitting Device for Prevention of Coronary Obstruction in Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv. 2023;16:94-102.
52. Chan KE, Tai-Leung Chan D, Lam CS, et al. First-in-Human Undermining Iatrogenic Coronary Obstruction With Radiofrequency Needle (UNICORN) Procedure During Valve-in-Valve Transcatheter Aortic Valve Replacement. Circ Cardiovasc Interv. 2022;15:928-931.
* Corresponding author.
E-mail address: (A. Regueiro).
@anderregueiro; @victorArevalos; @freixa_xavier; @sbrugaletta; @lsanchisruiz; @fraspio; @hospitalclinic