ABSTRACT
A substantial number of patients undergoing coronary angiography for angina or ischemia in noninvasive tests have coronary arteries without lesions or with nonsignificant stenosis. Many of these patients have nonobstructive myocardial ischemia (INOCA/ANOCA), which is an entity with prognostic importance that significantly affects patients’ quality of life. The absence of a proper diagnosis leads to inappropriate medical treatment, repeat diagnostic tests, and greater use of social and health resources. An adequate diagnostic strategy is required for individualized treatment that improves symptoms and quality of life. In this document from the SEC-Clinical Cardiology Association, SEC Interventional Cardiology Association, SEC-Ischemic Heart Disease and Acute Cardiac Care Association, and SEC-Cardiovascular Imaging Association of the Spanish Society of Cardiology, we provide simple and practical algorithms, with the aim of facilitating the early diagnosis and most appropriate treatment for patients with ANOCA.
Keywords: ANOCA. INOCA. Microvascular dysfunction. Vasospastic angina.
RESUMEN
Un número importante de aquellos pacientes en quienes se realiza coronariografía por angina o isquemia presentan en pruebas no invasivas arterias coronarias sin lesiones o con estenosis no significativas. Muchos de estos pacientes tienen isquemia miocárdica de causa no obstructiva (INOCA/ANOCA), una condición con importancia pronóstica que afecta de manera considerable la calidad de vida. La ausencia de un diagnóstico que haga posible un tratamiento médico efectivo acarrea la repetición de pruebas diagnósticas y un mayor uso de recursos sociosanitarios. Es necesaria una estrategia diagnóstica adecuada para poder realizar un tratamiento personalizado, que mejore los síntomas y la calidad de vida. En este documento de la SEC-Asociación de Cardiología Clínica, SEC Asociación de Cardiología Intervencionista, SEC-Asociación de Cardiopatía Isquémica y Cuidados Agudos Cardiovasculares, y SEC-Asociación de Imagen Cardiaca, se establecen unos algoritmos sencillos y prácticos con el objetivo de facilitar el diagnóstico precoz y el tratamiento más adecuado de los pacientes con ANOCA.
Palabras clave: ANOCA. INOCA. Disfunción microvascular. Angina vasoespástica.
Abbreviations ANOCA: angina with nonobstructive coronary arteries. CFR: coronary flow reserve. IMR: index of microcirculatory resistance. INOCA: ischemia with nonobstructive coronary artery disease. PET: positron emission tomography. SEC: Sociedad Española de Cardiología.
INTRODUCTION
Angina pectoris affects more than 100 million persons worldwide.1-5 According to the OFRECE study, the prevalence of angina in Spain is around 2.6%, indicating that there are more than 270 000 affected individuals.4 A significant number of stable patients referred for coronary angiography due to angina or a positive ischemia test do not have obstructive coronary artery disease.1 Many of these patients have ANOCA (angina with nonobstructive coronary arteries), or INOCA (ischemia with nonobstructive coronary artery disease) of nonobstructive origin. These 2 entities are manifestations of the same disease, which is why the recommendations provided by this document are applicable to both.
Angina pectoris is more prevalent among women (50%-70%) than men (30%-50%), although its true prevalence remains unknown.1-5 In these patients, angina or ischemia is produced by coronary vascular dysfunction due to vasomotor disorders of the epicardial vessels or arterioles, and/or coronary microvascular dysfunction.6-8
An important point is that, currently, angina pectoris is significantly underdiagnosed, and consequently many patients suffer its consequences without receiving potentially effective treatment. The reasons for this lack of diagnosis and treatment are various. First, there is the inertia associated with the paradigm that has dominated the diagnostic approach to patients with angina for decades focused on identifying coronary artery stenosis rather than vasomotor or coronary microvascular disorders. Additionally, patients with angina without coronary artery stenosis have generally been considered low-risk patients with poor response to conventional antianginal medical therapy.9 Second, and partly related to the previous point, many noninvasive techniques are based on identifying the regional ischemia that is characteristic of coronary artery stenosis (dysregulated contraction or isotope uptake during exertion or stress), making them less sensitive and specific for the detection of nonobstructive ischemia. Third, most cardiologists have not had access to the invasive techniques that provide objective evidence of vascular dysfunction in their patients. These intracoronary techniques have been considered the sole domain of interventional cardiologists, who do not usually play a key role in the management and follow-up of patients with INOCA. These barriers prevent the valuable information provided by invasive techniques from being used in the clinical management of these patients. Finally, patients with ANOCA/INOCA often have extracardiac diseases and conditions that require a multidisciplinary approach, complicating follow-up for specialized cardiologists.
In 2019, the European Society of Cardiology guidelines on the diagnosis and management of patients with chronic coronary syndrome represented a significant advance in the recognition of microvascular angina and the value of specific diagnostic techniques. Therefore, in the diagnostic approach in patients with suspected coronary microvascular angina, the guidelines indicate that coronary flow reserve (CFR) and microcirculatory resistance should be measured through pressure-guided techniques in patients with persistent symptoms but angiographically normal coronary arteries, or moderate stenosis and a normal fractional flow reserve (recommendation IIaB). Even the remaining recommendations, such as the administration of intracoronary acetylcholine during coronary angiography, or the use of transthoracic Doppler echocardiography of the anterior descending artery, cardiac magnetic resonance (CMR), or positron emission tomography (PET) for the noninvasive evaluation of CFR, have a lower level of recommendation (IIbB). In patients with suspected vasospastic angina, the guidelines recommend intracoronary provocation testing to identify coronary artery spasm (recommendation IIaB).10
However, over the past few years, numerous studies have been conducted in patients with ANOCA to assess the efficacy profile of new invasive diagnostic tests for their specific diagnosis, as well as randomized clinical trials assessing symptomatic improvement with individualized therapies. These trials consistently suggest that individualized and multidisciplinary approaches to these patients help to relieve symptoms, reduce the number of medical visits and prescribed therapies, and lower the costs associated with this syndrome.11-13
OBJECTIVES OF THIS DOCUMENT
This document is endorsed by the Clinical Cardiology Association, and the Interventional Cardiology Association, Ischemic Heart Disease and Acute Cardiac Care Association, and Cardiovascular Imaging Association of the Spanish Society of Cardiology (SEC) and aims to:
-
Review the various causes of ANOCA syndrome and current methods for its diagnosis and individualized treatment.
-
Propose a diagnostic and treatment algorithm for the approach to these patients in compliance with the clinical practice guidelines of the European Society of Cardiology on the management chronic coronary syndrome and the latest evidence.
-
Encourage various health care entities to create multidisciplinary pathways for the diagnosis, treatment, and targeted follow-up of these patients.
This document was drafted based on the interpretation of the latest scientific evidence, with an eminently practical focus so that the recommendations can be effectively applied in our setting. Each Association of the SEC provided scientific evidence and their view of their respective fields. Afterward, through consensus, they all created a single document including practical recommendations. The selection of the members that would eventually draft the document was left to the presidents of these Associations and was based on their clinical experience and expertise in the field.
IMPORTANCE OF ANOCA IN ROUTINE CLINICAL PRACTICE
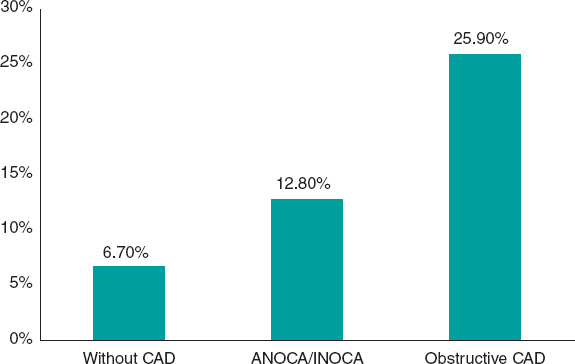
While it has been acknowledged for decades that angina without coronary artery lesions could constitute a separate nosological entity (initially called syndrome X), routine clinical practice has paid little attention to affected patients, primarily due to the widespread notion that their prognosis is good.14 However, numerous subsequent studies in which the diagnosis of ANOCA was based on objective evidence of coronary vascular dysfunction, unlike that of syndrome X, consistently showed that nonobstructive ischemia has a significant prognostic impact. The risk of adverse coronary events in these patients is largely determined by factors such as plaque burden, demonstration of myocardial ischemia, microvascular dysfunction, and the presence of vasospasm or coronary endothelial dysfunction. For example, a study of 917 women with signs or symptoms of myocardial ischemia showed that the composite endpoint of myocardial infarction or cardiac death occurred in 6.5% of women without coronary artery disease, 12.8% of those with nonobstructive atherosclerosis, and 25.9% of those with obstructive coronary artery disease at 10 years of follow-up (figure 1).15 A meta-analysis of 54 studies and 35 039 patients confirmed an increased risk of nonfatal myocardial infarction and death, with an incidence rate of 0.98 per 100 person-years in patients with ANOCA at 5 years of follow-up. The risk was higher in individuals with confirmed ischemia (vs those without ischemia) and patients with nonobstructive coronary artery disease (vs those with normal coronary arteries).16
Figure 1. Risk of myocardial infarction or cardiovascular death at 10 years of follow-up in a cohort of women.15 ANOCA/INOCA, angina/ischemia with nonobstructive coronary arteries; CAD, coronary artery disease.
Similarly, even in the presence of angiographically normal coronary arteries, microvascular dysfunction demonstrated by a reduced CFR has proven to be a powerful determinant of the risk of death and myocardial infarction in these patients.17 Additionally, more cardiovascular complications, including stroke and heart failure,18 have also been reported in these individuals, along with a higher prevalence of small vessel cerebral disease.19 In conclusion, patients with coronary microvascular dysfunction, identified by an impaired CFR, have a higher risk of major cardiovascular events.20
Intracoronary acetylcholine provocation testing also allows coronary risk stratification. An abnormal response to intracoronary acetylcholine indicates vasomotor disorders due to endothelial dysfunction or smooth muscle cell hyperreactivity. In addition to causing vasospastic angina, coronary vasomotor disorders are associated with a higher long-term risk of cardiovascular events in patients with angina, especially when associated with increased coronary microcirculation.13,21 Even moderate vasoconstrictor responses to acetylcholine can be predictive of a worse prognosis in this context.20
Additionally, patients with ANOCA often show persistent symptoms, partly due to the lack of an early diagnosis, thus leading to treatment delay. This is associated with a higher number of unnecessary diagnostic tests to rule out obstructive coronary artery disease, visits to the emergency room, hospital admissions, anxiety, impaired quality of life, episodes of sick leave, and higher direct and indirect health care costs.16,22,23
Diagnosing INOCA is essential to provide effective therapies to control angina symptoms. The CorMicA trial (Coronary microvascular angina) included 151 patients with ANOCA who underwent cardiac catheterization and invasive functional assessment (CFR determination, index of microcirculatory resistance, and fractional flow reserve) followed by acetylcholine vasoreactivity testing.11 The patients were randomized to reveal their specific endotype, which would guide treatment based on the results (intervention group), vs standard treatment, which would be administered blind to the test results (control group). Targeted therapy was individualized based on the endotypes documented in the invasive study (vasospastic angina: smoking cessation, long-acting calcium channel blockers, long-acting nitrates, and lifestyle changes; microvascular angina: beta-blockers, lifestyle changes, possible angiotensin-converting enzyme inhibitors and statins; noncardiac chest pain: withdrawal of antianginal treatment). Targeted therapy was significantly associated with an improved angina-related quality of life at 6 months (measured using the Seattle Angina Questionnaire), disease perception, and treatment satisfaction, although no differences were reported in the risk of major adverse cardiovascular events. More antianginal drugs were prescribed in the intervention group (87.8% vs 48.7%; P < .001). While these results are very interesting, it is important to note that this was a single study with a limited number of patients.
ENDOTYPES OF PATIENTS WITH ANOCA
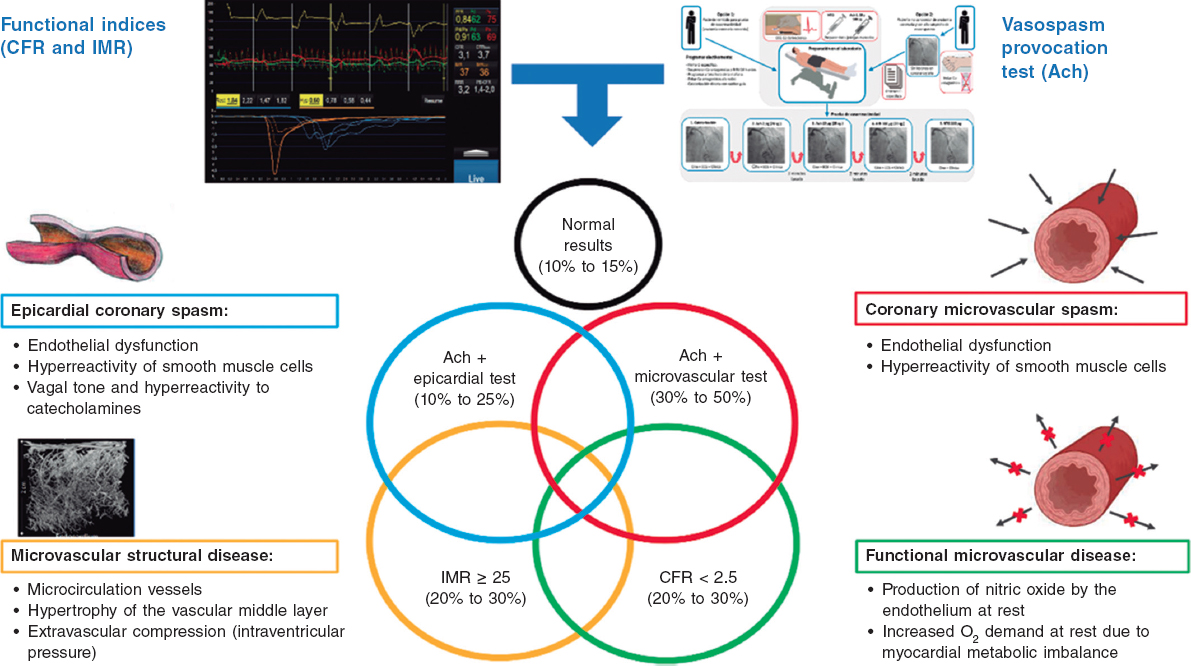
The specific causes of ANOCA are not yet fully described, and are likely multifactorial in most patients. Figure 2 illustrates the specific causes discovered so far and the pathophysiological mechanisms involved in their genesis. Of note, specific diagnostic techniques often do not allow us to differentiate among the various pathophysiological mechanisms. In fact, in many patients, these mechanisms overlap. Four pathophysiological mechanisms causing ANOCA have been described to date:
Figure 2. Possible results of an invasive functional study in a patient with ANOCA. Specific causes discovered to date with the pathophysiological mechanisms involved in their genesis. Ach, acetylcholine; ANOCA, angina with nonobstructive coronary arteries; CFR, coronary flow reserve; IMR, index of microcirculatory resistance. (Figure self-developed from Meeder et al.,1 Jansen et al.,3 Kunadian et al.,7 Kunadian et al.,34 and Hokimoto et al.35.)
-
Microvascular dysfunction due to structural changes to the microcirculation. The density of microvessels in patients with hypertensive cardiomyopathy is lower than that in patients without this condition.24 Remodeling of the coronary microcirculation has also been described, including arteriolar medial layer hypertrophy and induration in patients with hypertension, added to other cardiovascular risk factors, vascular infiltration by amyloid in cardiac amyloidosis, and reduced luminal caliber due to extrinsic compression in cases of ventricular hypertrophy or increased left intraventricular pressure.3,7,25 These changes reduce microcirculatory conductance, resulting in increased microvascular resistances (index of microcirculatory resistance [IMR] ≥ 25). Elevated IMR values are associated with older age and left ventricular hypertrophy, with no clear difference between the sexes.26,27
-
Functional microvascular disease. An increase in resting coronary blood flow, leading to reduced CFR levels has been reported, especially in women with few risk factors and no objectively observable structural heart disease.28 Although coronary flow is usually preserved at maximum hyperemia, many of these patients have a low exercise capacity. These patients may have an imbalance in oxygen availability (due to increased demand), with endothelial involvement being the main mechanism (due to increased nitric oxide synthesis).29 In addition, these patients tend to have a greater number of associated ischemic abnormalities in organs such as the kidneys, retina, and central nervous system, suggesting systemic involvement.30
-
Microvascular dysfunction due to microcirculatory spasm. Microvascular dysfunction due to vasospasm is more common in women with cardiovascular risk factors, with endothelial dysfunction likely playing a significant role. It is a common finding in larger and medium-sized arterioles and manifests as paradoxical vasoconstriction in response to increased myocardial oxygen demand, which becomes apparent after intracoronary of acetylcholine administration.3,7,19,31
-
Epicardial spasm. Epicardial spasm is not usually associated with traditional risk factors, except for smoking. This type of vasospasm is believed to be caused by 2 main mechanisms: endothelial dysfunction and smooth muscle cell hyperreactivity. These 2 mechanisms respond differently to stimuli from the autonomic nervous system, depending on whether the stimuli are from the sympathetic system (such as exercise or a cold stimulation test), or whether the stimuli are from the parasympathetic system and provoke an exacerbated response (eg, nocturnal spasms).19,32
CLINICAL CHARACTERISTICS OF PATIENTS WITH ANOCA
The first step in identifying patients with ANOCA is diagnostic suspicion. Patients with microvascular angina often report angina-like chest pain, typically on exertion, but it can also occur at rest. ANOCA is more common in women, and affected individuals generally show poor response to short-acting nitrates. In some cases, instead of angina, patients may have angina equivalents such as exertional dyspnea or atypical symptoms such as nausea, vomiting, dizziness, or fatigue. In microvascular spasm, which is also more common in women, unstable angina can occur with a variable response to nitrates.1-3
Regarding angina due to coronary vasomotor disorders, the spectrum and clinical signs of these disorders are much more varied than the pattern of Prinzmetal’s angina, which is a highly specific case of vasomotor disorder caused by an occlusive spasm of an epicardial vessel. However, this disorder is not representative of much more common substrates such as nonocclusive diffuse spasm and arteriolar or microvascular spasm. For example, in vasomotor disorders due to endothelial dysfunction, the dominant symptom is exertional angina, whereas in vasomotor disorders triggered by smooth muscle cell hyperreactivity of coronary vessels (such as in Prinzmetal’s angina), angina tends to occur at rest or becomes unstable, especially at night. Nevertheless, it can also be associated with exertional chest pain and be triggered by specific stimuli such as stress, cold, or an increase in vasoconstrictor humoral factors. Angina can also be associated with other conditions such as migraines or Raynaud’s phenomenon. Some anticancer drugs, such as 5-fluorouracil and capecitabine, among others, are known to be associated with vasospastic angina.33 Similarly, the initial clinical manifestation of epicardial spasm can be myocardial infarction with nonobstructive coronary arteries (MINOCA).19 This condition is often associated with smoking, unlike other traditional risk factors such as hypertension, diabetes mellitus, and dyslipidemia.19,32
NONINVASIVE DIAGNOSTIC APPROACH IN PATIENTS WITH ANOCA
The diagnostic approach to patients with ANOCA falls within the diagnostic process of chronic coronary syndrome as recommended by the current clinical practice guidelines and is initially noninvasive.10 However, it is important to note that the available scientific evidence—sometimes scarce—has already been analyzed, and consequently some statements are based not only on clinical trials but also on consensus among the authors of the document.
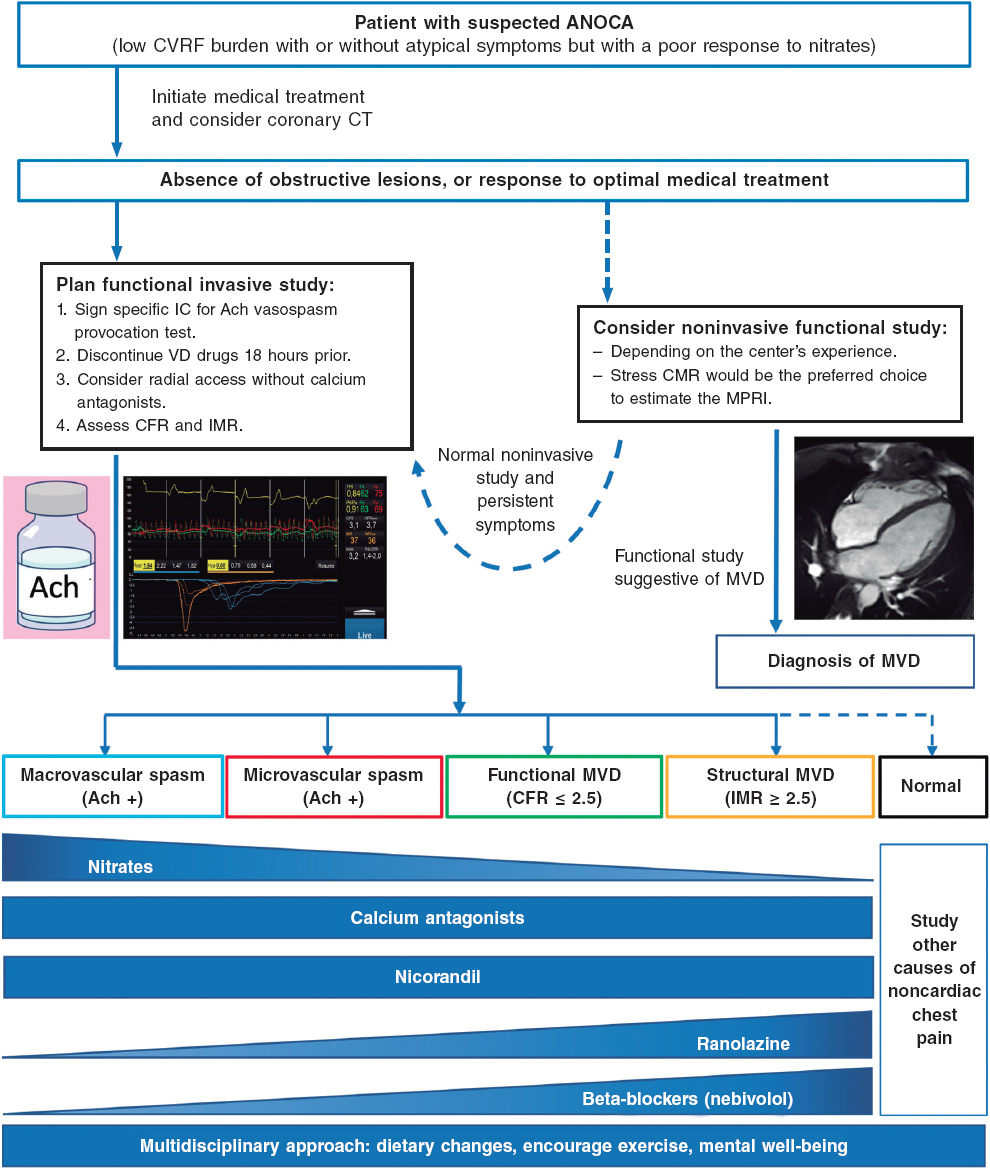
After angina is suspected, the patient should be referred to the cardiology unit for basic symptom examination, including an electrocardiogram, echocardiogram, a complete blood count, and clinical response to initial antianginal treatment. A noninvasive strategy is advised for most patients with nonlimiting symptoms and a low or intermediate pretest risk of obstructive coronary artery. This strategy involves noninvasive imaging modalities, including functional studies, based on surrogates of myocardial blood flow and CFR, and/or anatomical studies, mainly coronary computed tomography.3 The diagnostic tests performed will depend, among other factors, on the patient’s exercise tolerance and the availability and experience of each center (figure 3).1,3,7,34,35
Figure 3. Diagnostic approach to patients with suspected ANOCA or INOCA. Ach, acetylcholine; ANOCA, angina with nonobstructive coronary arteries; IC, informed consent; CFR, coronary flow reserve; CT, computed tomography; CVRF, cardiovascular risk factors; IMR, index of microcirculatory resistance; INOCA, ischemia with nonobstructive coronary arteries; MPRI, myocardial perfusion reserve index; MRI, magnetic resonance imaging; MVD, microvascular dysfunction; VD, vasodilator. (Figure self-developed from Meeder et al.,1 Perera et al.,2 Jansen et al.,3 Kunadian et al.,7 Ang and Berry,31 Kunadian et al.,34 and Hokimoto et al.35.)
Of note, in many patients with ANOCA, noninvasive imaging modalities for detecting ischemia have low sensitivity for the diagnosis of most endotypes, especially those associated with coronary vasomotor disorders. In a registry of patients studied with noninvasive ischemia detection tests and invasive functional tests (considered the reference standard for diagnosis), only 50% of those with a low CFR showed abnormalities in the noninvasive imaging tests.36 In fact, no noninvasive stress test can reliably detect the presence of microvascular spasms or coronary endothelial dysfunction and a negative stress test does not exclude the presence of vasomotor coronary dysfunction, especially in symptomatic patients.7 The reasons for the low sensitivity of these techniques are diverse. However, an important reason is that they rely on visualizing regional differences among myocardial segments (nonuniform tracer uptake in single-photon emission computed tomography, differences in myocardial segment mobility in stress echocardiography). Given the characteristics of microvascular angina, in which ischemia can be widespread, it is difficult to find regional defects in noninvasive tests. Moreover, patients with vasospasms usually test negative in stress tests based on comparison between rest and hyperemia. Therefore, it is important to note that ANOCA should always be suspected in patients with suggestive chest pain and a normal coronary computed tomography scan, or without obstructive coronary artery disease (< 50% reduction in diameter), and in patients who test negative on noninvasive imaging modalities for ischemia detection. Currently, no imaging modality allows the direct anatomical visualization of coronary microcirculation in vivo in humans, which is why its evaluation relies on measuring parameters that reflect functional status, such as myocardial blood flow and myocardial flow reserve.7
However, certain ANOCA endotypes with low CFR and a high suspicion of microvascular angina can be diagnosed noninvasively through various imaging modalities such as PET, transthoracic Doppler echocardiography, contrast-enhanced transthoracic echocardiography, and CMR. CFR is defined as an increased flow between the resting state and maximum hyperemia. CFR values < 2 to 2.5 are considered pathological.1
PET allows determination of myocardial blood flow at rest and during hyperemia in absolute terms, which facilitates the calculation of CFR. Although PET is considered the reference noninvasive imaging modality and correlates well with invasive study (CFR < 2 is associated with a worse prognosis regardless of the severity of coronary artery disease),37 its availability is highly limited in our setting,3,38 due to its high cost and the need for specific cyclotron-produced radiation-emitting radiotracers, such as oxygen-15-labeled water, nitrogen-13-labeled ammonia, or rubidium-82, a potassium analog.
Transthoracic Doppler echocardiography allows for the measurement of baseline and hyperemic blood flow velocity (after adenosine administration) using pulsed-wave Doppler. CFR < 2.5 is considered diagnostic of microvascular dysfunction. However, this imaging modality requires highly trained personnel and can only be used in the left anterior descending coronary artery.3,39 On the other hand, contrast-enhanced transthoracic echocardiography using microbubbles allows estimation of myocardial perfusion flow based on its degree of opacification. The latter imaging modality has shown good correlation with PET, although there may be significant interobserver variability, thus requiring further validation in studies.40
Finally, CMR can determine myocardial perfusion using stress and contrast agents (gadolinium) to calculate the myocardial perfusion reserve index, which is a surrogate parameter of CFR. This imaging modality is more widely available than PET, and has less interobserver variability than echocardiographic studies, making it the most suitable imaging modality for the study microvascular dysfunction in our setting. However, CMR is still pending validation in the remaining ANOCA endotypes.3,41 Hyperemia or coronary vasodilation can be achieved through adenosine infusion, or the administration of a single bolus of regadenoson, and stress vs resting perfusion can be compared quantitatively. The diagnostic ability of stress CMR in microvascular dysfunction was demonstrated 2 decades ago.42 A myocardial perfusion reserve index < 1.84 has shown sensitivity and specificity rates of 73% and 74%, respectively, to predict abnormalities in invasive coronary physiology studies, with an area under the ROC curve of 0.78.41 A quantitative assessment of stress perfusion studies showed an even stronger correlation with invasive studies in a series of 65 patients (50 with stable angina, 46% of whom had no coronary artery lesions, and 15 healthy volunteers) to distinguish multivessel disease from microvascular dysfunction, with an area under the ROC curve of 0.94 (P < .001) for the absolute quantification of myocardial flow during stress < 1.82 mL/g/min.43 In this study, myocardial flow during stress correlated better with invasive measurements than with myocardial flow reserve. Additionally, its prognostic capability has also been demonstrated. In a series of 218 patients with angina and coronary arteries without epicardial lesions,44, a myocardial perfusion reserve index ≤ 1.47 was associated with a 3-fold higher risk of major cardiovascular events compared with patients with values > 1.47 (hazard ratio, 3.14; 95% confidence interval, 1.58-6.25; P = .001). In another series of 395 patients, myocardial perfusion reserve improved the prognostic value vs the baseline model (age, sex, and late enhancement) of the primary endpoint defined as a composite of cardiac death, nonfatal myocardial infarction, aborted sudden death, or late revascularization, at 460 days of follow-up. Moreover, this study confirmed that quantitative perfusion (defined as > 10% ischemic myocardium) was superior to qualitative perfusion (defined as perfusion defects in > 2 segments) in the assessment of ischemia.45 Rahman et al.46 also demonstrated that high-resolution CMR techniques using fully quantitative perfusion were properly accurate and outperformed visual assessment in detecting microvascular dysfunction.
Unfortunately, some of the tests that could help in the noninvasive functional diagnosis of patients with ANOCA/INOCA are not available in routine clinical practice in many centers in Spain, thus limiting the diagnostic approach in these patients.
Table 1 shows the diagnostic criteria for ANOCA, while figure 3 illustrates the complete diagnostic algorithm proposed for patients with ANOCA, specifying the initial strategy, when to schedule invasive studies, and the possible therapies based on the specific endotype.
Table 1. Diagnostic criteria for ANOCA
| Endotype | Physiopathology | Criteria | Comments |
|---|---|---|---|
| Microvascular angina | Coronary microvascular dysfunction | Myocardial ischemia symptoms | • Exertional or resting angina • Angina equivalent (exertional dyspnea) |
| Evidence of myocardial ischemia | • Positive ischemia detection test | ||
| Absence of obstructive coronary artery disease | • FFR > 0.80 or stenosis < 50% • Confirmed by coronary CT or coronary angiography |
||
| Impaired coronary microvascular function | • Adenosine test: CFR ≤ 2.0 (2.5 according to the method), IMR ≥ 25, HMR ≥ 1.9 • Microvascular spasm (spontaneous or acetylcholine test): angina, EKG changes, without epicardial spasm (lumen reduction < 90%) |
||
| Vasospastic angina | Epicardial spasm | Symptoms | • Angina, more at rest, especially nocturnal • Reduced exercise tolerance, especially in the morning • Response to nitrates and calcium antagonists |
| EKG changes | • ST-segment changes (elevation/depression) ≥ 1 mV • New negative U waves |
||
| Absence of obstructive coronary artery disease | • FFR > 0.80 or stenosis < 50% • Confirmed by coronary CT or coronary angiography |
||
| Coronary spasm | • Vasoconstriction > 90% with angina and spontaneous EKG changes, or after provocation test (acetylcholine) | ||
| Preserved coronary microvascular function | • Adenosine test: CFR > 2.0 (2.5 according to the method), IMR < 25, HMR < 1.9 | ||
| Mixed | Microvascular angina and epicardial spasm | Absence of obstructive coronary artery disease | • FFR > 0.80 or stenosis < 50% • Confirmed by coronary CT or coronary angiography |
| Microvascular angina | • Microvascular dysfunction • Adenosine test: CFR ≤ 2.0 (2.5 according to the method); IMR ≥ 25, HMR ≥ 1.9 |
||
| Coronary spasm | • Angina + EKG changes + epicardial vasoconstriction (> 90%) | ||
| Noncardiac chest Pain | None | Absence of obstructive coronary artery disease | • FFR > 0.80 or stenosis < 50% • Confirmed by coronary CT or coronary angiography |
| Normal functional tests | • Adenosine test: CFR > 2.0 (2.5 according to the method), IMR < 25, HMR < 1.9 • Negative acetylcholine test |
||
|
ANOCA, angina with nonobstructive coronary arteries; CFR, coronary flow reserve; CT, coronary computed tomography; EKG, electrocardiogram; FFR, fractional flow reserve; HMR, hyperemic microvascular resistance; IMR, index of microvascular resistance. Table based on data from Meeder et al.,1 Perera et al.,2 Jansen et al.,3 Kunadian et al.,7 Mejia-Renteria et al.,19 Ong et al.,25 Ang and Berry,31 Kunadian et al.,34 and Hokimoto et al.35. |
|||
INVASIVE DIAGNOSTIC APPROACH IN PATIENTS WITH ANOCA
Although these are very safe procedures, there are risks involved in the invasive assessment of patients with suspected ANOCA. Therefore, it is of paramount importance that the health professionals involved should have specific training in performing and interpreting various tests. Adequate pathways should also be implemented. Currently, the use of 2 functional tests is advised, consisting of a vasospasm provocation test with intracoronary acetylcholine infusion and a microvascular function test using a pressure-temperature sensor-tipped wire at rest and during maximum pharmacological hyperemia.7,11,34,35
Vasospasm provocation testing with intracoronary acetylcholine is advised. Since the technical data sheet of acetylcholine does not include its intracoronary use, the pharmacy department of the medical center must be contacted for prior authorization. In most cases, patients must provide their prior written informed consent for the off-label use of the drug.47 This test has demonstrated high sensitivity and specificity rates (around 90% and 100%, respectively, depending on the patient’s characteristics) for diagnosing micro- and macrovascular vasospastic angina, with very few complications.47,48 Before the test is conducted, the use of long-acting vasodilator drugs should be avoided. A minimum of 18 hours without oral or topical vasodilator agents is advised to avoid false negatives. Although the use of beta-blockers may increase vasoconstriction after acetylcholine infusion, their discontinuation before the test is not advised if these drugs are deemed necessary. In procedures performed via the radial route, the use of calcium antagonists should also be avoided.47 Essentially, the test involves the infusion of increasing acetylcholine doses while simultaneously assessing the reproduction of the patient’s symptoms, changes in the 12-lead electrocardiogram, and the presence of spasms in the epicardial arteries > 90% of their baseline diameter. The Spanish Society of Cardiology Working Group on Cardiac Catheterization and Interventional Cardiology recently published a technical document on the performance and interpretation of this test.47
Microvascular function can be assessed using intracoronary Doppler, or pressure-temperature sensor-tipped wires. However, the only currently available guidewires are pressure-temperature sensor-tipped wires (Pressurewire X, Abbott, United States), which use the thermodilution method. Coronary thermodilution allows coronary flow values to be obtained at rest and during maximum hyperemia after the infusion of any microcirculation vasodilator agent (usually adenosine or its derivatives). These values are obtained after the infusion of 3 mL of physiological saline solution through the guide catheter and by measuring the transit time of this solution between the proximal segment of the artery and the distal segment, where the distal guidewire thermistor is located, both at rest and during maximum hyperemia. By obtaining flow data at rest and during maximum hyperemia, the CFR can be calculated, which under normal conditions should be > 2.5. CFR values ≤ 2.5 are considered diagnostic of microvascular dysfunction. Since the pressure of microcirculation perfusion (measured in the distal segment of the artery where the guidewire is located) can be obtained while performing the test during maximum hyperemia, the minimum microcirculation resistance (IMR) can be estimated. In studies performed in healthy patients, a cutoff value of 25 has been established. IMR values ≥ 25 are also indicative of microvascular dysfunction.7,34,35
There is another promising method in the invasive diagnosis of patients with ANOCA. Using the same pressure guidewire and a dedicated microcatheter (RayFlow, Hexacath, France), absolute coronary flow values (in mL/min) and absolute microcirculation resistances (in Wood units) can be obtained.49 Since these are absolute values, they partly depend on the perfusion territory of the artery and the studied segment. Currently, research is underway to develop an indexed approach using this method.50
THERAPEUTIC APPROACH IN PATIENTS WITH ANOCA
General approach
In patients with ANOCA, treatment should focus on relieving symptoms and improving the risk profile, quality of life, and prognosis. In this regard, early diagnosis, identification of the pathophysiological mechanisms involved, and early initiation of treatment tailored to the INOCA endotype are key to achieving therapeutic success.1,3,7,25,31,34,35,51-54 However, currently available studies of specific medical treatment for this condition are small, with heterogeneous methodologies and variable results, which makes it difficult to establish robust recommendations for the therapeutic management of these patients.
Lifestyle changes and control of cardiovascular risk factors
First, given the impact of cardiovascular risk factors on the development of coronary microvascular dysfunction and epicardial spasm, effective control of these risk factors is essential, including lifestyle changes (weight loss, physical exercise, smoking cessation, stress reduction), and appropriate pharmacological therapies.10 To reduce the risk of coronary vasospasm, it is important to avoid triggering factors such as smoking and the use of certain drugs (cocaine and amphetamine).10
Statins are beneficial not only due to their effect on lipid profile, but also due to their positive effect on endothelial function and in preventing the development of coronary spasms.55,56 Renin-angiotensin-aldosterone system inhibitors are beneficial to reduce blood pressure and improve endothelial function. In fact, these drugs have been reported to have positive effects on both coronary microvascular dysfunction and epicardial coronary vasospasm.55-57 The role of aspirin in patients without known cardiovascular disease is controversial.55,56 In the Japanese guidelines, aspirin is not advised in the absence of angiographically confirmed stenosis in patients with vasospasm (class IIIB indication).35
Antianginal treatment
Antianginal treatment is crucial for symptom relief. Preferential use of drugs that reduce myocardial oxygen consumption is advised in patients with a structural endotype of INOCA (microvascular dysfunction), such as beta-blockers or calcium channel blockers (ivabradine may also be considered in certain cases), along with other drugs such as ranolazine, trimetazidine, and nicorandil. On the other hand, calcium channel blockers, nitrates, nicorandil, or a combination of these, are advised in patients with a vasomotor endotype of INOCA (whether epicardial or microvascular spasm) (table 2).1,3,7,25,31,34,35,51-54
Table 2. Therapeutic approach for patients with ANOCA or INOCA
| General treatment | |||
|---|---|---|---|
| Lifestyle changes | • Mediterranean diet • Physical exercise • Weight control • Stress reduction |
||
| Cardiovascular risk factor control | • Hypertension • Dyslipidemia • Diabetes • Smoking cessation |
||
| Aspirin | • With previous CVD • Without previous CVD, its use is controversial |
||
| ACEI or ARA II | • Blood pressure reduction • Improvement in endothelial function: possible benefit in microvascular coronary dysfunction and coronary vasospasm |
||
| Statins | • Reduction in total cholesterol and LDL • Improvement in endothelial function • Possible benefit in vasospastic angina |
||
| Anti-anginal drugs | Microvascular angina | Beta-blockers | • Decreased myocardial oxygen consumption* |
| Calcium antagonists | • Decreased myocardial oxygen consumption • Vascular smooth muscle relaxation |
||
| Ranolazine | • Improvement in microvascular perfusion reserve | ||
| Trimetazidine | • Increased cellular tolerance to ischemia | ||
| Vasospastic angina | Calcium antagonists | • Decreased myocardial oxygen consumption • Decreased coronary spasm via relaxation of vascular smooth muscle |
|
| Nitrates | • Decreased myocardial oxygen consumption • Decreased coronary spasm via relaxation of vascular smooth muscle |
||
| Nicorandil | • Coronary vasodilator effect | ||
| Microvascular angina + vasospastic angina | Calcium antagonists, nitrates, ranolazine, trimetazidine, nicorandil | ||
|
* Consider the use of nebivolol due to its antioxidant properties through nitric oxide. ACEI, angiotensin-converting enzyme inhibitors; ANOCA, angina with nonobstructive coronary arteries; ARA II, angiotensin II receptor antagonists; CVD, cardiovascular disease; INOCA, ischemia with nonobstructive coronary arteries; LDL, low-density lipoproteins. Table based on data from Meeder et al.,1 Jansen et al.,3 Kunadian et al.,7 Kobayashi et al.,26 Ang and Berry,31 Kunadian et al.,34 Hokimoto et al.,35 Beltrame et al.,51 Mehta et al.,52 Seitz et al.,53 and Abouelnour et al.54. |
|||
There is some evidence on nebivolol compared with other beta-blockers, due to its potential vasodilatory effect that targets the production of nitric oxide.58 A beneficial effect of carvedilol has also been suggested by improving endothelium-dependent dilation.59 A randomized clinical trial of 81 patients demonstrated the benefit of ranolazine treatment in relieving symptoms in patients with CFR values < 2.5.60 Diltiazem treatment shows no benefits in improving symptoms, quality of life, or coronary microvascular function in the randomized EDIT-CMD trial of 73 patients with ANOCA in a 6-week course of treatment, although there was a reduction in induced epicardial vasospasms.12 Finally, there are promising potential benefits associated with drugs that have new therapeutic targets, such as cilostazol, a phosphodiesterase 3 inhibitor that targets coronary vasospasm,61 or zibotentan, a selective endothelin A antagonist with benefits on microcirculation and endothelial dysfunction,62 or fasudil, a rho-kinase enzyme inhibitor capable of reducing the IMR in patients with a positive vasospasm provocation test and elevated IMR.13
Treatment for resistant angina
The use of drugs such as low-dose tricyclic antidepressants (which modulate norepinephrine uptake and have anticholinergic effects, which can induce analgesia), or neurostimulators that block the transfer of pain at the spinal cord has been proposed in patients with resistant angina, and even coronary interventions in the case of vasospastic angina refractory to medical therapy.51
Patient follow-up
The follow-up of these patients should be coordinated between primary care physicians and cardiologists, and once symptoms are under control, follow-up should preferably be conducted in primary care units, with referrals to cardiology if there is decompensation. In addition, given the particularities of ANOCA, it is essential to inform patients about their disease and its implications. A multidisciplinary approach is necessary since other health professionals, such as psychologists, internists, and pain clinics, may sometimes be required.
Future lines of research
Finally, ongoing clinical trials are currently exploring whether intensive treatment of coronary atherosclerosis with high-intensity statins, renin-angiotensin-aldosterone system inhibitors, and low doses of aspirin improves angina and ischemia. The WARRIOR trial (NCT03417388) is studying whether such treatment improves outcomes, and the MINOCA-BAT trial (NCT03686696) is investigating whether the combined use of beta-blockers and renin-angiotensin-aldosterone system inhibitors reduces major cardiovascular clinical events.
CONCLUSIONS
Patients with suspected ANOCA exhibit a wide array of presentations that can currently be diagnosed and treated with effective individualized therapies. It is important for clinical cardiologists to become familiar with the various abnormalities in patients with ANOCA, and the currently available diagnostic and therapeutic tools. Invasive diagnostic tests constitute a new option requiring specific training for their correct performance and interpretation, as well as CMR with adenosine or regadenoson for myocardial perfusion calculation. In conclusion, specific actions need to be taken by all health centers to create diagnostic and therapeutic protocols for the management of these patients.
FUNDING
None declared.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
Artificial intelligence has not been used in the preparation of this document.
AUTHORS’ CONTRIBUTIONS
All authors contributed equally to the conception, literature search, development, drafting, reading, and final approval of the manuscript. C. Escobar served as the consensus coordinator.
CONFLICTS OF INTEREST
J. Escaned, a recipient of the Intensification of Research Activity Project INT22/00088 from Instituto de Salud Carlos III, declared speaker’s fees for his involvement in educational activities for Abbott and Philips. C. Escobar, J.M. Gámez, and V. Barrios declared lecture fees from Menarini. The remaining authors declared no conflicts of interest whatsoever.
REFERENCES
1. Meeder JG, Hartzema-Meijer MJ, Jansen TPJ, Konst RE, Damman P, Elias-Smale SE. Outpatient Management of Patients With Angina With No Obstructive Coronary Arteries:How to Come to a Proper Diagnosis and Therapy. Front Cardiovasc Med. 2021;8:716319.
2. Perera D, Berry C, Hoole SP, et al. Invasive coronary physiology in patients with angina and non-obstructive coronary artery disease:a consensus document from the coronary microvascular dysfunction workstream of the British Heart Foundation/National Institute for Health Research Partnership. Heart. 2022;109:88-95.
3. Jansen TPJ, Konst RE, Elias-Smale SE, et al. Assessing Microvascular Dysfunction in Angina With Unobstructed Coronary Arteries:JACC Review Topic of the Week. J Am Coll Cardiol. 2021;78:1471-1479.
4. Alonso JJ, Muñiz J, Gómez-Doblas JC, et al. Prevalencia de angina estable en España. Resultados del estudio OFRECE. Rev Esp Cardiol. 2015;68:691-699.
5. Douglas PS, Hoffmann U, Patel MR, et al. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med. 2015;372:1291-1300.
6. Rahman H, Ryan M, Lumley M, et al. Coronary microvascular dysfunction is associated with myocardial ischemia and abnormal coronary perfusion during exercise. Circulation. 2019;140:1805-1816.
7. Kunadian V, Chieffo A, Camici PG, et al. An EAPCI expert consensus document on ischaemia with non-obstructive coronary arteries in collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology and Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. Eur Heart J. 2020;41:3504-3520.
8. Taqueti VR. Coronary microvascular dysfunction in vasospastic angina:provocative role for the microcirculation in macrovessel disease prognosis. J Am Coll Cardiol. 2019;74:2361-2364.
9. Luu JM, Wei J, Shufelt CL, et al. Clinical Practice Variations in the Management of Ischemia With No Obstructive Coronary Artery Disease. J Am Heart Assoc. 2022;11:e022573.
10. Knuuti J, Wijns W, Saraste A, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407-477.
11. Ford TJ, Stanley B, Good R, et al. Stratified medical therapy using invasive coronary function testing in angina:the CorMicA trial. J Am Coll Cardiol. 2018;72(23 Pt A):2841-2855.
12. Jansen TPJ, Konst RE, de Vos A, et al. Efficacy of Diltiazem to Improve Coronary Vasomotor Dysfunction in ANOCA:The EDIT-CMD Randomized Clinical Trial. JACC Cardiovasc Imaging. 2022;15:1473-1484.
13. Suda A, Takahashi J, Hao K, et al. Coronary Functional Abnormalities in Patients With Angina and Nonobstructive Coronary Artery Disease. J Am Coll Cardiol. 2019;74:2350-2360.
14. Kaski JC, Collins P, Nihoyannopoulos P, Maseri A, Poole-Wilson PA, Rosano GM. Cardiac syndrome X:clinical characteristics and left ven-tricular function:long-term follow-up study. J Am Coll Cardiol. 1995;25:807-814.
15. Sharaf B, Wood T, Shaw L, et al. Adverse outcomes among women presenting with signs and symptoms of ischemia and no obstructive coronary artery disease:findings from the national heart, lung, and blood institute-sponsored women's ischemia syndrome evaluation (WISE) angiographic core laboratory. Am Heart J. 2013;166:134-141.
16. Radico F, Zimarino M, Fulgenzi F, et al. Determinants of long-term clinical outcomes in patients with angina but without obstructive coronary artery disease:a systematic review and meta-analysis. Eur Heart J. 2018;39:2135-2146.
17. Pepine CJ, Anderson RD, Sharaf BL, et al. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia:results from the National Heart, Lung and Blood Institute WISE (Women's Ischemia Syndrome Evaluation) study. J Am Coll Cardiol. 2010;55:2825-2832.
18. Patel S, Fung M, Liang Z, Butalia S, Anderson TJ. Temporal Trends of the Prevalence of Angina With No Obstructive Coronary Artery Disease (ANOCA). Can J Cardiol. 2023;39:63-70.
19. Mejia-Renteria H, Travieso A, Matías-Guiu JA, et al. Coronary microvascular dysfunction is associated with impaired cognitive function:the Cerebral-Coronary Connection study (C3 study). Eur Heart J. 2023;44:113-125.
20. Boerhout CKM, de Waard GA, Lee JM, et al. Prognostic value of structural and functional coronary microvascular dysfunction in patients with non-obstructive coronary artery disease;from the multicentre international ILIAS registry. EuroIntervention. 2022;18:719-728.
21. Grigorian-Shamagian L, Oteo JF, Gutiérrez-Barrios A, et al. Endothelial dysfunction in patients with angina and non-obstructed coronary arteries is associated with an increased risk of mayor cardiovascular events. Results of the Spanish ENDOCOR registry. Int J Cardiol. 2023;370:18-25.
22. Jespersen L, Abildstrøm SZ, Hvelplund A, Prescott E. Persistent angina:highly prevalent and associated with long-term anxiety, depression, low physical functioning, and quality of life in stable angina pectoris. Clin Res Cardiol. 2013;102:571-581.
23. Schumann CL, Mathew RC, Dean JL, et al. Functional and Economic Impact of INOCA and Influence of Coronary Microvascular Dysfunction. JACC Cardiovasc Imaging. 2021;14:1369-1379.
24. Nadruz W. Myocardial remodeling in hypertension. J Hum Hypertens. 2015;29:1-6.
25. Ong P, Camici PG, Beltrame JF, et al.;Coronary Vasomotion Disorders International Study Group (COVADIS). International standardization of diagnostic criteria for microvascular angina. Int J Cardiol. 2018;250:16-20.
26. Kobayashi Y, Fearon WF, Honda Y, et al. Effect of Sex Differences on Invasive Measures of Coronary Microvascular Dysfunction in Patients With Angina in the Absence of Obstructive Coronary Artery Disease. JACC Cardiovasc Interv. 2015;8:1433-1441.
27. Chung JH, Lee KE, Lee JM, et al. Effect of Sex Difference of Coronary Microvascular Dysfunction on Long-Term Outcomes in Deferred Lesions. JACC Cardiovasc Interv. 2020;13:1669-1679.
28. Nardone M, McCarthy M, Ardern CI, et al. Concurrently Low Coronary Flow Reserve and Low Index of Microvascular Resistance Are Associated With Elevated Resting Coronary Flow in Patients With Chest Pain and Nonobstructive Coronary Arteries. Circ Cardiovasc Interv. 2022;15:e011323.
29. Rahman H, Demir OM, Khan F, et al. Physiological Stratification of Patients With Angina Due to Coronary Microvascular Dysfunction. J Am Coll Cardiol. 2020;75:2538-2549.
30. Beltrame JF, Crea F, Kaski JC, et al. International standardization of diagnostic criteria for vasospastic angina. Eur Heart J. 2017;38:2565-2568.
31. Ang DTY, Berry C. What an Interventionalist Needs to Know About INOCA. Interv Cardiol. 2021;16:e32.
32. Lanza GA, Careri G, Crea F. Mechanisms of coronary artery spasm. Circulation. 2011;124:1774-1782.
33. Matsumoto T, Saito Y, Saito K, et al. Relation Between Cancer and Vasospastic Angina. Adv Ther. 2021;38:4344-4353.
34. Kunadian V, Chieffo A, Camici PG, et al. An EAPCI Expert Consensus Document on Ischaemia with Non-Obstructive Coronary Arteries in Collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology &Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. EuroIntervention. 2021;16:1049-1069.
35. Hokimoto S, Kaikita K, Yasuda S, et al. JCS/CVIT/JCC 2023 Guideline Focused Update on Diagnosis and Treatment of Vasospastic Angina (Coronary Spastic Angina) and Coronary Microvascular Dysfunction. Circ J. 2023;87:879-936.
36. Lee SH, Shin D, Lee JM, et al. Clinical Relevance of Ischemia with Nonobstructive Coronary Arteries According to Coronary Microvascular Dysfunction. J Am Heart Assoc. 2022;11:e025171.
37. Ziadi MC, Dekemp RA, Williams KA, et al. Impaired myocardial flow reserve on rubidium-82 positron emission tomography imaging predicts adverse outcomes in patients assessed for myocardial ischemia. J Am Coll Cardiol. 2011;58;740-748.
38. Driessen RS, Raijmakers PG, Stuijfzand WJ, Knaapen P. Myocardial perfusion imaging with PET. Int J Cardiovasc Imaging. 2017;33:1021-1031.
39. Michelsen MM, Mygind ND, Pena A, et al. Transthoracic Doppler echocardiography compared with positron emission tomography for assessment of coronary microvascular dysfunction:the iPOWER study. Int J Cardiol. 2017;228:435-443.
40. Vogel R, Indermühle A, Reinhardt J, et al. The quantification of absolute myocardial perfusion in humans by contrast echocardiography:algorithm and validation. J Am Coll Cardiol. 2005;45:754-762.
41. Thomson LE, Wei J, Agarwal M, et al. Cardiac magnetic resonance myocardial perfusion reserve index is reduced in women with coronary microvascular dysfunction. A National Heart, Lung, and Blood Institute–sponsored study from the Women's Ischemia Syndrome Evaluation. Circ Cardiovasc Imaging. 2015;8:e002481.
42. Panting JR, Gatehouse PD, Yang GZ, et al. Abnormal subendocardial perfusion in cardiac syndrome X detected by cardiovascular magnetic resonance imaging. N Engl J Med. 2002;346:1948-1953.
43. Kotecha T, Martinez-Naharro A, Boldrini M, et al. Automated Pixel-Wise Quantitative Myocardial Perfusion Mapping by CMR to Detect Obstructive Coronary Artery Disease and Coronary Microvascular Dysfunction:Validation Against Invasive Coronary Physiology. JACC Cardiovasc Imaging. 2019;12:1958-1969.
44. Zhou W, Lee JCY, Leung ST, et al. Long-Term Prognosis of Patients With Coronary Microvascular Disease Using Stress Perfusion Cardiac Magnetic Resonance. JACC Cardiovasc Imaging. 2021;14:602-611.
45. Sammut EC, Villa ADM, Di Giovine G, et al. Prognostic Value of Quantitative Stress Perfusion Cardiac Magnetic Resonance. JACC Cardiovasc Imaging. 2018;11:686-694.
46. Rahman H, Scannell CM, Demir OM, et al. High-Resolution Cardiac Magnetic Resonance Imaging Techniques for the Identification of Coronary Microvascular Dysfunction. JACC Cardiovasc Imaging. 2021;14:978-986.
47. Gutiérrez E, Gómez-Lara J, Escaned J, et al. Assessment of the endothelial function and spasm provocation test performed by intracoronary infusion of acetylcholine. Technical report from the ACI-SEC. REC Interv Cardiol. 2021;3:286-296.
48. Montone RA, Rinaldi R, Del Buono MG, et al. Safety and prognostic relevance of acetylcholine testing in patients with stable myocardial ischaemia or myocardial infarction and non-obstructive coronary arteries. EuroIntervention. 2022;18:e666-e676.
49. Rivero F, Gutiérrez-Barrios A, Gomez-Lara J, et al. Coronary microvascular dysfunction assessed by continuous intracoronary thermodilution:A comparative study with index of microvascular resistance. Int J Cardiol. 2021;333:1-7.
50. de Vos A, Jansen TPJ, van't Veer M, et al. Microvascular Resistance Reserve to Assess Microvascular Dysfunction in ANOCA Patients. JACC Cardiovasc Interv. 2023;16:470-481.
51. Beltrame JF, Tavella R, Jones D, Zeitz C. Management of ischaemia with non-obstructive coronary arteries (INOCA). BMJ. 2021;375:e060602.
52. Mehta PK, Huang J, Levit RD, Malas W, Waheed N, Bairey Merz CN. Ischemia and no obstructive coronary arteries (INOCA):A narrative review. Atherosclerosis. 2022;363:8-21.
53. Seitz A, Martínez Pereyra V, Sechtem U, Ong P. Update on coronary artery spasm 2022 –A narrative review. Int J Cardiol. 2022;359:1-6.
54. Abouelnour A, Gori T. Vasomotor Dysfunction in Patients with Ischemia and Non-Obstructive Coronary Artery Disease:Current Diagnostic and Therapeutic Strategies. Biomedicines. 2021;9:1774.
55. Ong P, Athanasiadis A, Sechtem U. Treatment of Angina Pectoris Associated with Coronary Microvascular Dysfunction. Cardiovasc Drugs Ther. 2016;30:351-356.
56. Picard F, Sayah N, Spagnoli V, Adjedj J, Varenne O. Vasospastic angina:A literature review of current evidence. Arch Cardiovasc Dis. 2019;112:44-55.
57. Choi BG, Jeon SY, Rha SW, et al. Impact of Renin-Angiotensin System Inhibitors on Long-Term Clinical Outcomes of Patients With Coronary Artery Spasm. J Am Heart Assoc. 2016;5:e003217.
58. Erdamar H, Sen N, Tavil Y, et al. The effect of nebivolol treatment on oxidative stress and antioxidant status in patients with cardiac syndrome-X. Coron Artery Dis. 2009;20:238-244.
59. Matsuda Y, Akita H, Terashima M, et al. Carvedilol improves endothelium-dependent dilatation in patients with coronary artery disease. Am Heart J. 2000;140:753-759.
60. Rambarat CA, Elgendy IY, Handberg EM, et al. Late sodium channel blockade improves angina and myocardial perfusion in patients with severe coronary microvascular dysfunction:Women's Ischemia Syndrome Evaluation-Coronary Vascular Dysfunction ancillary study. Int J Cardiol. 2019;276:8-13.
61. Shin ES, Lee JH, Yoo SY, et al. A randomised, multicentre, double blind, placebo controlled trial to evaluate the efficacy and safety of cilostazol in patients with vasospastic angina. Heart. 2014;100:1531-1536.
62. Morrow AJ, Ford TJ, Mangion K, et al. Rationale and design of the Medical Research Council's Precision Medicine with Zibotentan in Microvascular Angina (PRIZE) trial. Am Heart J. 2020;229:70-80.
* Corresponding author.
@JEscaned; @AntoniCarolRuiz; @S_Raposeiras; @jmgamez3; @rfreixap; @Ana_Viana_T; @clinica_sec; @AgudosSEC