ABSTRACT
Atrial functional mitral regurgitation (AFMR) has recently been the focus of numerous original articles and reviews. This entity has been highlighted by population aging, the increasing prevalence of heart failure with preserved ejection fraction and atrial fibrillation, and the advent of the transcatheter techniques for mitral valve repair. AFMR is phenotype of mitral regurgitation that presents specific diagnostic challenges and current guidelines do not provide strong recommendations for its management. Cumulative data show that the outcomes of patients with severe AFMR are poor if left untreated. However, new heart failure therapies and minimally invasive techniques may have a positive impact on the outcomes of these patients.
Keywords: Atrial functional mitral regurgitation. Diagnosis. Echocardiography. Edge-to-edge repair. Transcatheter mitral valve repair.
RESUMEN
La insuficiencia mitral funcional auricular (IMFA) ha sido recientemente el foco de atención de muchos artículos y revisiones originales. El envejecimiento de la población, el aumento de la prevalencia de insuficiencia cardiaca con fracción de eyección preservada y fibrilación auricular así como el desarrollo de las técnicas transcatéter para la reparación de la válvula mitral han resaltado la IMFA, un fenotipo de insuficiencia mitral que presenta desafíos diagnósticos específicos y para el cual actualmente las guías no tienen recomendaciones sólidas para su tratamiento. Existe una importante acumulación de datos que sugieren que el pronóstico de los pacientes con IMFA grave es malo si no se tratan. Sin embargo, las nuevas terapias para la insuficiencia cardiaca y las técnicas mínimamente invasivas pueden tener un impacto positivo en el prognóstico de esos pacientes
Palabras clave: Insuficiencia mitral funcional atrial. Diagnóstico. Ecocardiografía. Reparación de borde a borde. Reparación mitral transcatéter.
Abbreviations AFMR: atrial functional mitral regurgitation.
The triad proposed by Prof. Carpentier is frequently used to characterize the mechanism of mitral regurgitation: etiology, lesion and dysfunction.1 Fibroelastic deficiency, myxomatous disease, rheumatic heart disease and endocarditis are etiologies that directly cause lesions of the mitral valve, such as chorda rupture, excessive leaflet tissue, thickening and calcification of the leaflets, and subvalvular apparatus, and leaflet perforation. Consequently, the resulting mitral regurgitation has been classified as organic or primary mitral regurgitation. Ischemic heart disease, dilated cardiomyopathy and atrial fibrillation are etiologies that lead to mitral annulus dilatation and leaflet movement restriction. These lesions are considered secondary to the remodeling process of the left ventricle and atrium. Consequently, the resulting mitral regurgitation is considered secondary or functional. The approach to surgical repair has differed between primary and secondary mitral regurgitation. In primary mitral regurgitation, repair techniques have involved resection of the redundant scallop of the mitral leaflet, implantation of neochordae, the use of a pericardial patch (in leaflet perforation), and mitral annuloplasty to restore normal coaptation. In secondary mitral regurgitation, restrictive mitral annuloplasty is the mainstay technique.
The advent of transcatheter mitral valve repair techniques have underscored the importance of the assessment of the etiology of mitral regurgitation and characterization of the mitral valve apparatus, particularly focusing on mitral valve area, the leaflet length and motion, coaptation depth and length and location of the largest vena contract of the regurgitant jet.2 These factors are key to select the patients with mitral regurgitation in whom a transcatheter edge-to-edge repair will be successful. Current guidelines outline the mitral valve characteristics that define the ideal, the challenging and the prohibitive anatomy of the mitral valve for successful transcatheter edge-to-edge mitral valve repair.3 For patients with primary mitral regurgitation and anatomically suitable mitral valve apparatus who have high surgical risk or are deemed inoperable, transcatheter edge-to-edge mitral valve repair may be considered (class IIb). In patients with secondary mitral regurgitation and anatomically suitable mitral valve apparatus who have high surgical risk or are deemed inoperable and in whom coronary revascularization is not needed, transcatheter edge-to-edge mitral valve repair has a class IIa recommendation. In this last clinical scenario, there are currently many patients who have secondary mitral regurgitation due to atrial and mitral annulus dilatation and these patients are different from patients in whom the mitral regurgitation is caused by left ventricular dilatation and dysfunction. The surgical risk of patients with atrial functional mitral regurgitation (AFMR) is usually lower than the patients with ventricular functional mitral regurgitation and the evidence supporting the use of surgical vs transcatheter mitral valve repair remains elusive.
AFMR occurs in the setting of permanent atrial fibrillation or heart failure with preserved ejection fraction fraction4-6 and is characterized by mitral annular dilatation, dysfunction, and the loss of atrial synchrony.7 In patients with heart failure and preserved ejection fraction, left ventricular remodeling, characterized by concentric hypertrophy and increased stiffness, results in elevated left ventricular filling pressures that are transmitted to the left atrium. In response to these increased pressures, the left atrium undergoes dilatation as a compensatory mechanism to buffer the increased pressures and prevent their transmission to the pulmonary circulation. However, chronic left atrial remodeling leads to atrial dysfunction and mitral annulus dilatation, contributing to the failure of leaflet coaptation.7
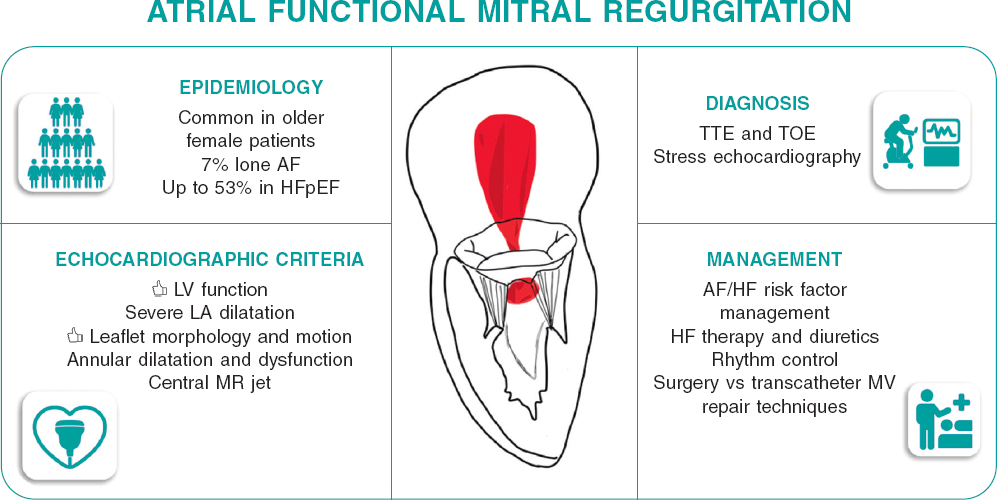
The frequency of AFMR among patients with atrial fibrillation is reported to be up to 7%, while this figure can rise to 53% in patients with heart failure and preserved ejection fraction.8 Furthermore, data from the large National Echocardiographic Database of Australia Registry reported frequencies of significant AFMR of 8% among patients with atrial fibrillation and no underlying structural heart disease, 28% in patients with long-standing atrial fibrillation, and 20% in those with heart failure and preserved ejection fraction.9
Based on various series, it is known that AFMR most commonly affects elderly female patients with a history of atrial fibrillation and arterial hypertension.10 It is noteworthy that atrial fibrillation and heart failure with preserved ejection fraction often coexist, leading to greater remodeling, more symptoms, and worse clinical outcomes.11 The diagnosis of AFMR is mainly performed with transthoracic and transesophageal echocardiography.8 The main echocardiographic characteristics of AFMR include normal morphology and movement of the mitral leaflets with impaired coaptation due to mitral annulus dilatation, often with varying grades of calcification. Grading AFMR can be challenging as it is a dysfunction that depends on the patients’ loading conditions. Additionally, the presence of atrial fibrillation adds complexity to AFMR grading due to beat-to-beat variability. It is crucial to consider the role of exercise echocardiography, which can reveal the presence of symptoms and detect severe AFMR during peak exercise. The induction of significant tricuspid regurgitation and pulmonary hypertension is also common during exercise. Exercise echocardiography may serve as the second step before other imaging techniques, such as cardiac magnetic resonance, to identify patients with severe AFMR.
The clinical implications of AFMR have been recently described.8 The prognosis of severe AFMR under medical therapy is similar to that of left ventricular functional mitral regurgitation. Compared with patients with primary mitral regurgitation, AFMR was associated with worse survival and more heart failure hospitalizations. Importantly, patients with AFMR are less frequently referred to surgical mitral valve repair or replacement than patients with left ventricular functional mitral regurgitation or primary mitral regurgitation.12 This is probably related to the pathophysiology of AFMR: guidelines recommend first to prescribe optimal medical therapy (in this case for heart failure with preserved ejection fraction) and achieve rhythm control (if atrial fibrillation is present) prior to intervention.3 The evidence on the survival benefit of isolated surgical mitral valve repair for AFMR is scarce. Surgical mitral valve repair using complete rigid ring annuloplasty has shown a low reoperation rate and low recurrence of mitral regurgitation at 5 years of follow-up.13
Based on a large registry, machine-learning has identified 4 clusters of patients with mitral regurgitation undergoing transcatheter edge-to-edge mitral valve repair and with disparate clinical outcomes.14 Patients in cluster 1 (isolated mitral regurgitation), characterized by dilated left atrium, preserved left ventricular ejection fraction, and 60% in atrial fibrillation showed the best survival while patients with cluster 4 (biatrial dilatation), characterized by extremely dilated left and right atria, left ventricular ejection fraction at the lower limit of normality and all of them in atrial fibrillation had the poorest outcomes.14 These results were confirmed in an external cohort. However, the exact lesion of the mitral valve leading to mitral regurgitation remains unknown. Therefore, there may be patients with primary mitral regurgitation. Currently, there are no randomized clinical trials comparing the outcomes of surgical repair vs transcatheter edge-to-edge mitral valve repair for patients with AFMR.
The field of AFMR will attract significant attention since the prevalence of heart failure with preserved ejection fraction and atrial fibrillation, the main underlying pathophysiological etiologies of AFMR, will increase along with the aging of the population. New effective therapies such as sodium-glucose cotransporter-2 inhibitors,15 glucagon-like peptide-1 agonist,16 and early atrial fibrillation ablation techniques17 may have an impact on the prevalence of AFMR. However, new trials focused on AFMR will be needed and, before then, we probably need to enhance the focus on this entity that has been largely neglected and considered as a bystander of other diseases. Precise diagnosis and characterization of AFMR are needed (figure 1). In addition, large registries reporting on the outcomes of AFMR under medical therapy and treated with surgical and transcatheter mitral valve repair techniques are needed to further delineate and design new randomized trials that will refine guideline recommendations. Therefore, establishing AFMR as a new entity was an unmet clinical need to provide the optimal, personalized treatment for each patient with mitral regurgitation.
Figure 1. Characterization and management of atrial functional mitral regurgitation. AF, atrial fibrillation; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; LA, left atrial; MR, mitral regurgitation; MV, mitral valve; TOE, transesophageal echocardiography; TTE, transthoracic echocardiography.
FUNDING
None.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
No artificial intelligence-based tools have been used to draft this manuscript or to generate the figure.
AUTHORS’ CONTRIBUTIONS
The authors confirm contribution to the paper as follows. Article conception and design: V. Delgado, S. Danojevic, M. De Raffele and L. Niro. Methodology: V. Delgado, S. Danojevic. Validation, M. De Raffele and L. Niro. Literature search: S. Danojevic, M. De Raffele and L. Niro. Writing—original draft preparation: S. Danojevic. Writing—review and editing: V. Delgado, M. De Raffele and L. Niro. Supervision: V. Delgado. All authors have read and agreed to the published version of the manuscript.
CONFLICTS OF INTEREST
V. Delgado has received speaker fees from Edwards Lifesciences, GE Healthcare, Novartis and Philips; consulting fees from Novo Nordisk, MSD, and Edwards Lifesciences. The remaining authors have no conflicts of interest.
REFERENCES
1. Carpentier A. Cardiac valve surgery--The “French correction”. J Thorac Cardiovasc Surg. 1983;86:323-37.
2. Mauri L, Garg P, Massaro JM, et al. The EVEREST II Trial:Design and rationale for a randomized study of the evalve mitraclip system compared with mitral valve surgery for mitral regurgitation. Am Heart J. 2010;160:23-29.
3. Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2022;43:561-632.
4. Zoghbi WA, Levine RA, Flachskampf F, et al. Atrial Functional Mitral Regurgitation:A JACC:Cardiovascular Imaging Expert Panel Viewpoint. JACC Cardiovasc Imaging. 2022;15:1870-1882.
5. Levine RA, Schwammenthal E. Ischemic mitral regurgitation on the threshold of a solution:From paradoxes to unifying concepts. Circulation. 2005;112:745-758.
6. Ennezat PV, Maréchaux S, Pibarot P, Le Jemtel TH. Secondary mitral regurgitation in heart failure with reduced or preserved left ventricular ejection fraction. Cardiology. 2013;125:110-117.
7. Gertz ZM, Raina A, Saghy L, et al. Evidence of atrial functional mitral regurgitation due to atrial fibrillation:Reversal with arrhythmia control. J Am Coll Cardiol. 2011;58:1474-81.
8. Deferm S, Bertrand PB, Verbrugge FH, et al. Atrial Functional Mitral Regurgitation:JACC Review Topic of the Week. J Am Coll Cardiol. 2019;73:2465-2476.
9. Moonen A, Ng MKC, Playford D, Strange G, Scalia GM, Celermajer DS. Atrial functional mitral regurgitation:Prevalence, characteristics and outcomes from the National Echo Database of Australia. Open Heart. 2023;10:e002180.
10. Dziadzko V, Dziadzko M, Medina-Inojosa JR, et al. Causes and mechanisms of isolated mitral regurgitation in the community:Clinical context and outcome. Eur Heart J. 2019;40:2194-2202.
11. Lam CSP, Rienstra M, Tay WT, et al. Atrial Fibrillation in Heart Failure With Preserved Ejection Fraction:Association With Exercise Capacity, Left Ventricular Filling Pressures, Natriuretic Peptides, and Left Atrial Volume. JACC Heart Fail. 2017;5:92-98.
12. Mesi O, Gad MM, Crane AD, et al. Severe Atrial Functional Mitral Regurgitation:Clinical and Echocardiographic Characteristics, Management and Outcomes. JACC Cardiovasc Imaging. 2021;14:797-808.
13. Wagner CM, Brescia AA, Watt TMF, et al. Surgical strategy and outcomes for atrial functional mitral regurgitation:All functional mitral regurgitation is not the same!J Thorac Cardiovasc Surg. 2022;167(2):647-655.
14. Trenkwalder T, Lachmann M, Stolz L, et al. Machine learning identifies pathophysiologically and prognostically informative phenotypes among patients with mitral regurgitation undergoing transcatheter edge-to-edge repair. Eur Heart J Cardiovasc Imaging. 2023;24:574-587.
15. Gulsin GS, Graham-Brown MPM, Squire IB, Davies MJ, McCann GP. Benefits of sodium glucose cotransporter 2 inhibitors across the spectrum of cardiovascular diseases. Heart. 2022;108:16-21.
16. Kosiborod MN, Abildstrøm SZ, Borlaug BA, et al. Semaglutide in Patients with Heart Failure with Preserved Ejection Fraction and Obesity. N Engl J Med. 2023;389:1069-1084.
17. Yamauchi R, Morishima I, Okumura K, et al. Association Between Catheter Ablation for Nonparoxysmal Atrial Fibrillation and Functional Mitral Regurgitation in Patients With Heart Failure With Preserved Ejection Fraction. Am J Cardiol. 2023;207:192-201.
* Corresponding author.
@VDelgadoGarcia; @icorcat; @suzidanojevic; @Doc_Niro; @MartinaRaffele