Article
Ischemic heart disease and acute cardiac care
REC Interv Cardiol. 2019;1:21-25
Access to side branches with a sharply angulated origin: usefulness of a specific wire for chronic occlusions
Acceso a ramas laterales con origen muy angulado: utilidad de una guía específica de oclusión crónica
Servicio de Cardiología, Hospital de Cabueñes, Gijón, Asturias, España

ABSTRACT
Introduction and objectives: The objective of this study was to describe our experience with coronary physiology assessment using the instantaneous wave-free ratio (iFR) and/or a Syncvision-guided iFR-pullback study [Syncvision version 4.1.0.5, Philips Volcano, Belgium] in all-comer patients.
Methods: Consecutive patients undergoing coronary physiology assessment with the iFR (and/or a Syncvision-guided iFR-pullback study) at our center between January 2017 and December 2019 were included. The iFR cut-off value was 0.89. The primary endpoint was a composite of cardiac death, myocardial infarction, probable or definitive stent thrombosis, and target lesion revascularization.
Results: A total of 277 patients with 433 lesions evaluated were included. The mean age was 65 ± 10 years and 74% were men. Personal history of diabetes mellitus was present in 41% of patients. Clinical presentation was stable angina in 160 patients (58%), and acute coronary syndrome in 117 patients (42%). iFRs > 0.89 were obtained in 266 lesions (61.4%) on which the PCI was postponed. The remaining lesions were revascularized. The Syncvision software was used to guide the iFR-pullback study in 155 lesions (36%) and the decision-making process, mainly in long, diffuse or sequential lesions (91 lesions, 58.7%), and intermediate lesions (52 lesions, 33.5%). After a median follow-up of 18 months, the primary endpoint occurred in 17 patients (6.1%) without differences regarding the baseline iFR (≤ 0.89 or > 0.89) (4.2% vs 3.8%; P = .9) or the clinical presentation (stable angina or acute coronary syndrome) (4.4% vs 8.5%; P = .1)
Conclusions: The use of coronary physiology assessment with the iFR and the Syncvision-guided iFR-pullback study in the routine daily practice and in all-comer patients seems safe with a low percentage of major adverse cardiovascular events at the mid-term follow-up.
Keywords: Physiological assessment. All-comer patients. Syncvision-guided iFR-pullback study.
RESUMEN
Introducción y objetivos: El propósito del estudio fue describir nuestra experiencia con el uso del índice diastólico instantáneo sin ondas (iFR) para la evaluación fisiológica coronaria o el uso del software Syncvision/iFR (Syncvision versión 4.1.0.5, Philips Volcano, Bélgica) en todo tipo de pacientes.
Métodos: Se incluyeron todos los pacientes consecutivos a quienes, entre enero de 2017 y diciembre de 2019, se realizó en nuestro centro una evaluación fisiológica coronaria con iFR o con Syncvision/iFR. El valor de corte establecido para el iFR fue 0,89. El objetivo primario fue un compuesto de muerte cardiaca, infarto de miocardio, trombosis de stent probable o definitiva y nueva revascularización de la lesión evaluada.
Resultados: Se incluyeron 277 pacientes con 433 lesiones evaluadas. La edad media fue de 65 ± 10 años y el 74% eran varones. El 41% tenía antecedente de diabetes mellitus. La presentación clínica fue angina estable en 160 pacientes (58%) y síndrome coronario agudo en 117 pacientes (42%). Se obtuvo un iFR > 0,89 en 266 lesiones (61,4%), en las cuales la intervención coronaria percutánea fue diferida. Las lesiones restantes se revascularizaron. El software Syncvision/iFR se usó en 155 lesiones (36%) para guiar la toma de decisiones, principalmente lesiones largas, difusas o secuenciales (91 lesiones, 58,7%) y lesiones intermedias (52 lesiones, 33,5%). Tras un periodo de seguimiento de 18 meses, el objetivo primario se observó en 17 pacientes (6,1%), sin diferencias en función del iFR basal (≤ 0,89 o > 0,89) (4,2 frente a 3,8%; p = 0,9) ni de la presentación clínica (angina estable o síndrome coronario agudo) (4,4 frente a 8,5%; p = 0,1).
Conclusiones: La evaluación fisiológica coronaria con iFR y el software Syncvision/iFR en la práctica diaria y en todo tipo de pacientes parece ser segura, con un bajo porcentaje de eventos cardiacos adversos mayores a medio plazo.
Palabras clave: Evaluacion fisiologica. Todo tipo de pacientes. Software Syncvision/iFR.
Abbreviations
iFR: instantaneous wave-free ratio. PCI: percutaneous coronary intervention. MACE: major adverse cardiovascular events.
INTRODUCTION
Physiological assessment using the fractional flow reserve (FFR) or the instantaneous wave-free ratio (iFR) is strongly recommended by the European guidelines to the guide percutaneous coronary intervention (PCI) decision-making process to treat intermediate coronary stenosis (indication I, level of evidence A) and multivessel disease (indication IIa, level of evidence B).1-7
The established cut-off values based on landmark trials to safely postpone treatment of a coronary lesion are FFRs > 0.80 and iFRs > 0.89.2-7 Unlike the FFR, the new iFR resting index allows us to analyze the physiological significance of each segment in the presence of coronary arteries with several lesions. Syncvision is a new software that analyzes the specific contribution of each coronary segment allowing us to predict physiological improvement after percutaneous treatment.8,9 It’s not necessary to use any vasodilators either, thus reducing any potential side effects.3,4
However, the evidence supporting the use of coronary physiology assessment with both indices and the use of the Syncvision software in other type of lesions and other clinical scenarios is scarce.8-10 For this reason, it is not quite clear whether the same cut-off value established in the landmark trials should be used; or if safety, utility, and efficacy will be the same.
The objective of this study is to describe our experience with coronary physiology assessment using the iFR (and/or the Syncvision- guided iFR-pullback study) in all-comer patients undergoing invasive coronary angiography.
METHODS
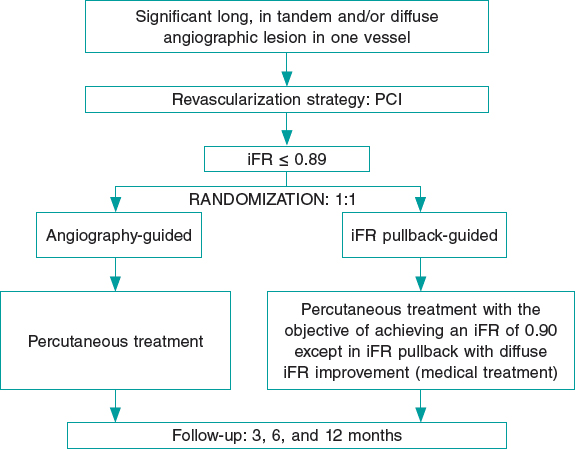
We performed a single-center retrospective study including all patients who underwent functional assessments (using the iFR) and/or the Syncvision software at our center between January 2017 and December 2019 on a PCI decision-making process. The cut-off value to consider the need for revascularization was the same one established by the landmark clinical trials (iFR ≤ 0.89).3,4 The pressure guidewires used for the functional assessment were the Volcano Verrata, and the Volcano Verrata Plus (Philips Volcano, Belgium). The use of the Syncvision software to guide the iFR study as well as the lesions assessed were left to the operator’s discretion.
All subjects included in the study gave their informed consent to undergo the procedure and for data analysis and publication. Additionally, the study received the proper ethical oversight and was approved by our center ethics committee.
Inclusion and exclusion criteria
Patients with the following criteria were included: a) consecutive patients in whom an invasive coronary angiography was performed due to stable or unstable symptoms or silent ischemia; b) presence of, at least, a lesion or vessel physiologically assessed with the iFR during the index procedure. The following exclusion criteria were stablished: a) impossibility to understand the informed consent during the index procedure; b) written informed consent to use data for research purposes not provided.
Lesion classification
The lesions physiologically assessed were classified based on their angiographic characteristics and/or clinical setting: a) intermediate lesions: lesions with a 40% to 80% angiographic stenosis as seen on the quantitative coronary angiography (QCA); b) sequential or diffuse coronary lesions: presence of, at least, 2 sequential lesions or a coronary segment with diffuse disease (coronary vessel with multiple plaques in most of the epicardial territory) with a total length of 25 mm; c) bifurcation lesions: presence of a coronary stenosis at bifurcation level with a side branch size large enough to be protected; d) in-stent restenosis: presence of focal or diffuse in-stent restenosis with a a 40% to 80% angiographic stenosis as seen on the QCA; e) coronary bypass lesion, defined as, at least, a lesion in the coronary artery bypass grafting or native vessel presenting with proximal total occlusion.
Endpoints
The primary endpoint of the study was the rate of major adverse cardiovascular events (MACE) at the follow-up. The MACE were defined as a composite of cardiac death, myocardial infarction (MI), definitive or probable stent thrombosis, and new target lesion revascularization (TLR). All deaths were considered cardiovascular unless unequivocal non-cardiac causes would be established. Myocardial infarction included spontaneous ST-segment elevation MI or non-ST-segment elevation acute myocardial infarction. The TLR was defined as a new revascularization of a baseline physiologically negative lesion at the follow-up or as a repeat revascularization of a baseline physiologically positive lesion percutaneously treated during the index procedure.
The secondary endpoints established were: a) analysis of the primary endpoint components separately; b) rate of MACE based on the clinical setting (stable angina or acute coronary syndrome), non-ST-segment elevation acute myocardial infarction (NSTEMI), and ST segment elevation myocardial infarction (STEMI); c) rate of MACE based on the baseline iFR; d) to determine the type of lesions where the Syncvision software was used for the iFR-pullback study.
Follow-up
The patients’ follow-up was performed through phone calls, hospital record reviews or outpatient visits.
Quantitative coronary measurements
Quantitative coronary measurements were performed using a validated system (CAAS system, Pied Medica Imaging, The Netherlands). These were the measurements analyzed: reference vessel diameter, minimum lumen diameter, percent diameter stenosis, and lesion length. All measurements were performed at baseline and after the PCI.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation, and the Student t test was used to establish comparisons. The categorical variables were expressed as frequency and percentage, and compared using the chi-square test. The univariate analysis was performed with the following covariates: age, male sex, current smoking status, dyslipidemia, left ventricular ejection fraction, acute coronary syndrome, multivessel disease, clopidogrel, ticagrelor, right coronary artery as the study vessel, other vessels analyzed, and baseline iFRs ≤ 0.89. Results were reported using odds ratios (OR), and two-sided 95% confidence intervals. In all the cases, P values < .05 were considered statistically significant. The statistical analysis was performed using the IBM-SPSS statistical software package (version 24.0 for Macintosh, SPSS Corp., United States).
RESULTS
The study flowchart is shown on figure 1. During the study period, a total of 2951 patients underwent coronary angiography at our center. The iFR-based physiological assessment was performed in 277 patients (9.4%) with 433 lesions. The baseline clinical data are shown on table 1. The mean age was 65 ± 10 years, and 74% of the patients (204) were men. The prevalence of comorbidities was high (diabetes mellitus, 41%; previous MI, 32%; peripheral arterial disease, 4%; cerebrovascular disease, 6%; chronic kidney disease, 13%). The clinical presentation included stable angina in 160 patients (58%), NSTEMI in 91 patients (33%), and STEMI in 26 patients (9%).
Table 1. Baseline clinical data
| Patients | Total (N = 277) | Stable angina (N = 160) | ACS (N = 117) | P |
|---|---|---|---|---|
| Age, years | 65 ± 10 | 65 ± 10 | 64 ± 11 | .071 |
| Sex, male, N (%) | 204 (74) | 116 (72) | 94 (80) | .112 |
| Hypertension, N (%) | 175 (63) | 101 (63) | 77 (66) | .645 |
| Diabetes mellitus, N (%) | 114 (41) | 58 (36) | 52 (44) | .169 |
| Dyslipidemia, N (%) | 157 (57) | 101 (63) | 58 (50) | .024 |
| Current smoker, N (%) | 72 (26) | 29 (18) | 42 (36) | .001 |
| Previous myocardial infarction, N (%) | 89 (32) | 53 (33) | 37 (32) | .792 |
| Previous revascularization, N (%) | 94 (34) | 50 (31) | 32 (27) | .518 |
| Percutaneous, N (%) | 80 (85) | 50 (31) | 30 (26) | .336 |
| Surgical, N (%) | 14 (15) | 8 (16) | 6 (19) | .095 |
| Atrial fibrillation, N (%) | 39 (14) | 19 (12) | 13 (11) | .844 |
| Heart failure, N (%) | 8 (3) | 7 (4) | 2 (2) | .216 |
| Prior ACE, N (%) | 17 (6) | 11 (7) | 9 (8) | .795 |
| Peripheral arterial disease, N (%) | 11 (4) | 7 (4) | 5 (4) | .967 |
| Previous bleeding, N (%) | 3 (1) | 2 (1) | 2 (2) | .752 |
| Chronic kidney disease, N (%) | 36 (13) | 19 (12) | 17 (15) | .486 |
| Hemoglobin, g/dL | 13.96 ± 1.7 | 13.87 ± 1.8 | 14.13 ± 1.8 | .365 |
| Creatinine, g/dL | 0.98 ± 0.47 | 1 ± 0.63 | 1 ± 0.37 | .584 |
| Left fentricular ejection fraction, % | 59 ± 15 | 57 ± 16 | 60 ± 13 | .098 |
|
ACE, acute cerebrovascular event; ACS, acute coronary syndrome. Data are expressed as number (N) and percentage (%). |
||||
Angiographic and procedural data
Angiographic and procedural data are shown on table 2. Radial access was the access of choice in most of the cases (392 lesions, 91%). A total of 186 patients (67%) showed angiographic multivessel disease. Regarding the angiographic Syntax I score, 232 patients (84%) had Syntax scores < 22, 41 patients (15%) between 22 and 32, and only 4 patients (1%) > 32 without any differences being reported between stable and unstable patients. The vessel most frequently analyzed was the left anterior descending coronary artery (180, 42%) followed by the right coronary artery (99, 23%). The left main coronary artery was evaluated in 23 patients (5%).
Table 2. Angiographic and procedural data
| Patients | Total (N = 277) | Stable angina (N = 160) | ACS (N = 117) | P |
|---|---|---|---|---|
| Radial access, N (%) | 251 (90) | 147 (92) | 104 (89) | .329 |
| Multivessel disease, N (%) | 165 (59) | 84 (52) | 81 (69) | .004 |
| Syntax score | 11 ± 8 | 10 ± 8 | 12 ± 8 | .885 |
| Low risk (< 22) | 45 (16) | 25 (16) | 20 (17) | .184 |
| Intermediate risk (22-32) | 6 (2) | 1 (1) | 5 (4) | .066 |
| High risk (> 32) | 1 (1) | 1 (1) | 0 | .331 |
| Acetylsalicylic acid, N (%) | 245 (88) | 142 (88) | 103 (88) | .740 |
| P2Y12 inhibitor, N (%) | 195 (71) | 98 (61) | 97 (83) | |
| Clopidogrel | 63 (23) | 40 (25) | 23 (20) | .011 |
| Ticagrelor | 127 (46) | 56 (35) | 71 (61) | .019 |
| Prasugrel | 65 (2) | 2 (1) | 3 (3) | .642 |
| Vessel analyzed, N (%) | ||||
| LAD | 121 (44) | 66 (41) | 55 (47) | .318 |
| LCx | 40 (14) | 26 (16) | 14 (12) | .327 |
| RCA | 75 (27) | 50 (31) | 25 (21) | .072 |
| LMCA | 15 (5) | 8 (5) | 7 (6) | .712 |
| Other | 27 (10) | 11 (7) | 16 (14) | .057 |
| Reference vessel diameter (mm) | 3.3 ± 3 | 3.3 ± 3 | 3.3 ± 3 | .971 |
| Vessel stenosis (%) | 49 ± 16 | 49 ± 17 | 49 ± 16 | .816 |
| Vessel minimal lumen diameter (mm) | 1.6 ± 0.6 | 1.5 ± 0.6 | 1.5 ± 0.5 | .203 |
| Vessel lesion length (mm) | 21 ± 12 | 21 ± 13 | 20 ± 11 | .174 |
| Vessel stent diameter (mm) | 2.8 ± 0.4 | 2.8 ± 0.4 | 2.8 ± 0.4 | .581 |
| Type of stent implanted (%) | ||||
| DES | 100 | |||
| BMS | 0 | |||
| Other | 0 | |||
| Immediate angiographic optimal result (%) | 100 | |||
| Contrast used (mL) | 142 ± 91 | 151 ± 110 | 164 ± 72 | .166 |
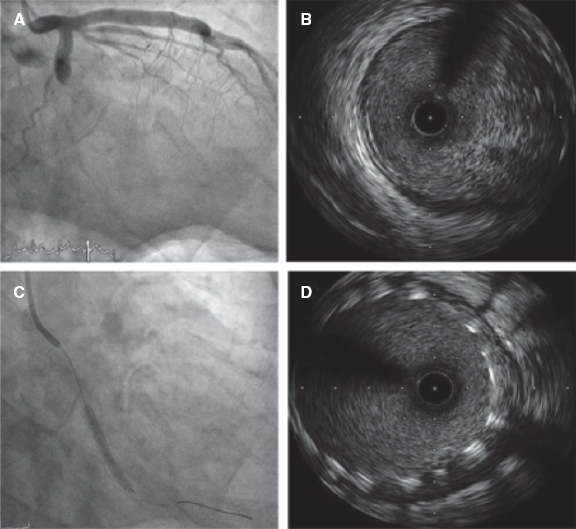
| Intracoronary imaging, N (%) | 6 (2) | 6 (4) | 0 | .034 |
| Procedural complications, N (%) | 3 (1) | 2 (1) | 1 (1) | .754 |
| Baseline iFR | 0.88 ± 0.12 | 0.89 ± 0.12 | 0.86 ± 0.14 | .097 |
| Final iFR | 0.93 ± 0.04 | 0.93 ± 0.04 | 0.93 ± 0.04 | .951 |
| Syncvision-guided iFR-pullback study, N (%) | 155 lesions (36) | 94 lesions (36) | 61 lesions (35) | .4 |
| Lesions evaluated | Total (N = 433) | Stable angina (N = 258) | ACS (N = 175) | P |
| Angiographically moderate lesions, N (%) | 244 (56.4) | 149 (58) | 95 (54) | .475 |
| Sequential/diffuse coronary lesions, N (%) | 118 (27.3) | 64 (25) | 53 (30) | .208 |
| Bifurcation lesions, N (%) | 51 (11.8) | 31 (12) | 20 (11) | .853 |
| In-stent restenosis, N (%) | 15 (3.5) | 11 (4.3) | 4 (2.3) | .269 |
| Coronary artery bypass grafting, N (%) | 2 (0.5) | 0 (0) | 2 (1.1) | .085 |
| Other lesions, N (%) | 3 (0.75) | 2 (0.8) | 1 (0.6) | .802 |
|
ACS, acute coronary syndrome; BMS, bare metal stent; DES, drug-eluting stent; iFR, instantaneous wave-free ratio; LAD, left anterior descending coronary artery; LCx, left circumflex artery; LMCA, left main coronary artery; RCA, right coronary artery. Data are expressed as number (N) and percentage (%). |
||||
The mean reference diameter was 3.3 mm ± 3 mm with a mean vessel stenosis of 49% ± 16%, and a mean lesion length of 21 mm ± 12 mm. The mean diameter of the stent implanted was 2.8 ± 0.4. All the stents implanted were drug-eluting stents (100%). Intracoronary imaging was used in 14 patients (3%).
The instantaneous wave-free ratio was obtained in 433 lesions, with a baseline value of 0.89 ± 0.12. The physiological assessment results after the PCI were obtained in 129 lesions (29.8%) with a final iFR of 0.93 ± 0.04.
The lesions physiologically assessed are shown on table 2. The most common type of lesions undergoing physiological assessment were angiographically moderate lesions (244, 56.4%) followed by sequential and diffuse lesions (118, 27.3%). Physiological assessment was used in 51 bifurcation lesions (11.8%) basically to guide the intervention over the side branch while using a provisional stenting strategy.
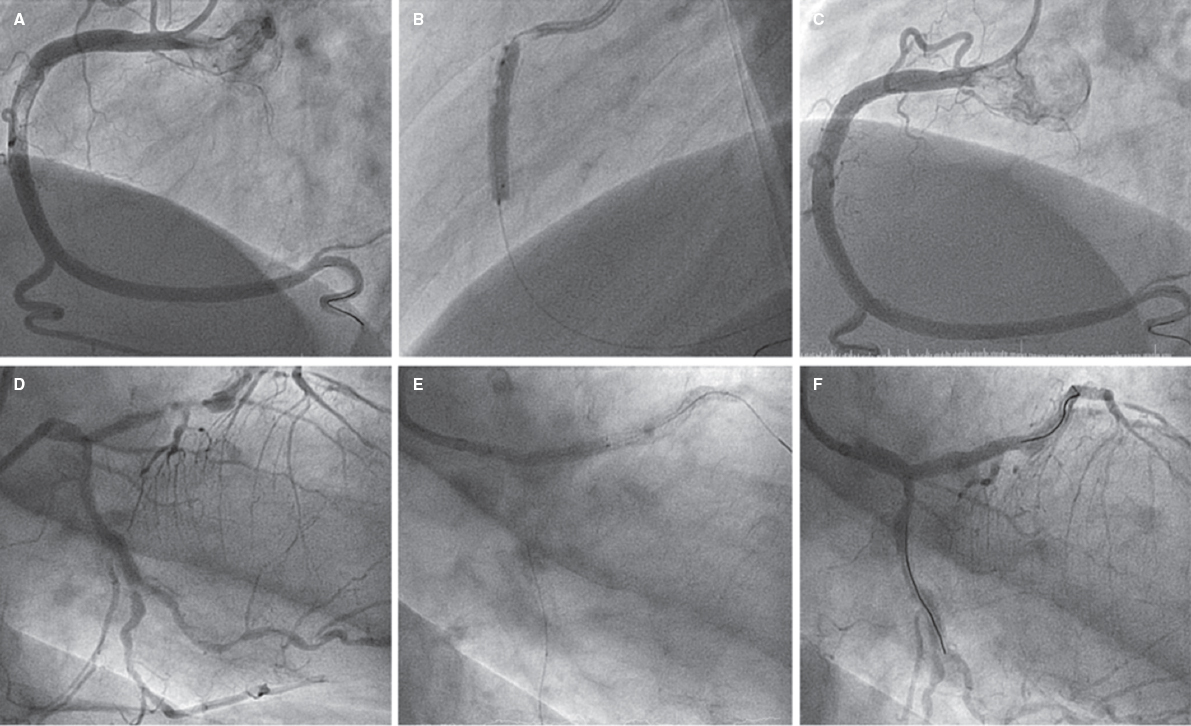
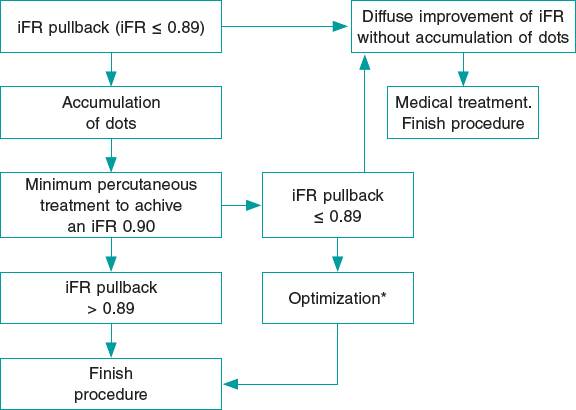
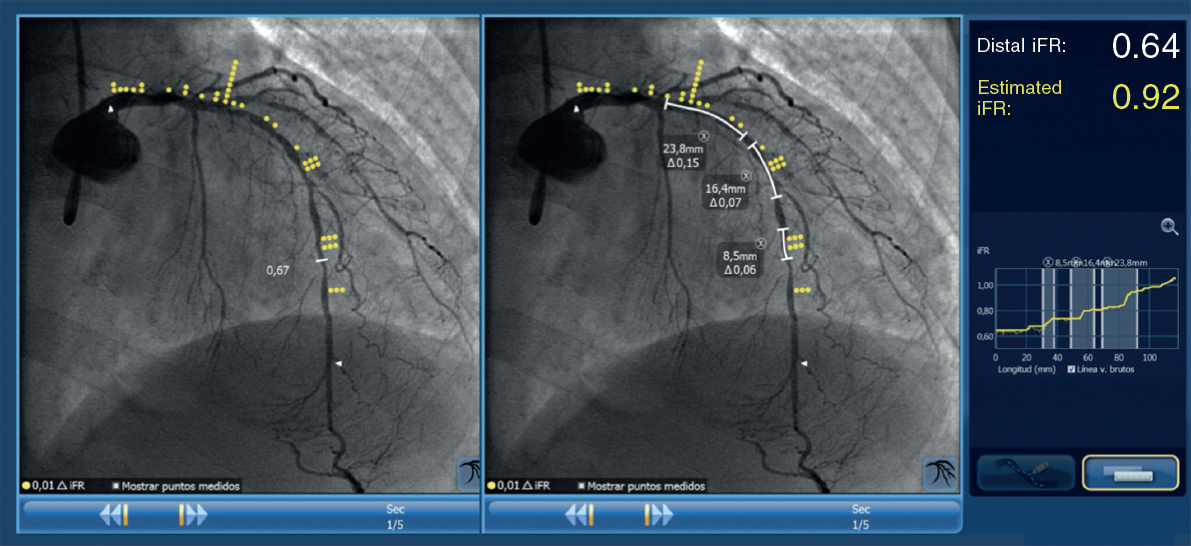
The Syncvision software for the iFR-pullback study was used in 155 lesions to guide the decision-making process (35.8%). Sequential and diffuse coronary lesions were the most common lesions analyzed by the iFR-pullback study (91 vessels, 58.7%, figure 2) followed by angiographically moderate lesions (52 vessels, 33.5%). This software was used in 5 bifurcation lesions (3.2%) to establish a baseline physiological classification or confirm an optimal physiological result after the PCI in both branches. The remaining lesions assessed by the iFR-pullback study were 6 focal or diffuse in-stent restenoses (3.9%) and 1 saphenous vein bypass graft with diffuse disease (0.6%).
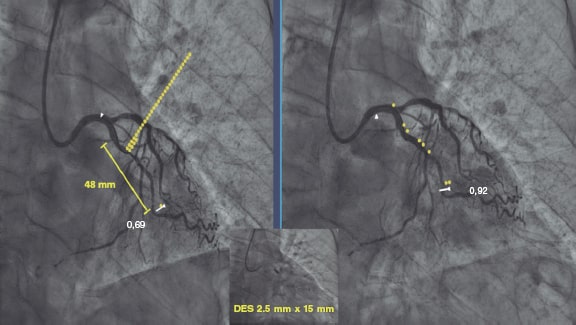
Figure 2. Images of iFR-coregistration with the Syncvision software from a left circumflex artery with diffuse disease in its middle segment (48 mm of lesion length). The baseline distal iFR was 0.69. The Syncvision-guided iFR-pullback study demonstrated physiological significance only in the proximal segment. Direct implantation of a 2.5 mm × 15 mm DES was performed with a final iFR of 0.92. The stent length reduction regarding the angiographic lesion was 33 mm.
Follow-up
Follow-up data were available for 274 out of 277 patients (99%). After a mean 18 ± 10-month follow-up, 17 patients (6.1 %) presented with a major adverse cardiovascular events (table 3), 7 patients (2.5 %) with TLR, 2 of them over a lesion treated during the index procedure (0.7%) and 5 (1.8%) due to disease progression of a baseline physiologically negative lesion; 6 patients (2.2 %) suffered from acute myocardial infarction (1 patient due to acute stent thrombosis, another to a new lesion not evaluated at the index procedure, another to a baseline physiologically non-significant lesion, and the remaining 3 patients due to failed previously revascularized lesions); also, 4 patients (1.4%) presented with unclear or cardiac death. There were no differences regarding MACE between baseline physiologically negative and positive lesions (table 3).
Table 3. Rate of major adverse cardiovascular events at the follow-up based on the clinical presentation
| MACE (277 patients, 433 lesions) | iFR ≤ 0.89 (N = 167 lesions) | iFR > 0.89 (N = 266 lesions) | P | Stable angina (N = 160) | ACS (N = 117) | P | |
|---|---|---|---|---|---|---|---|
| Overall, N (%) | 17 (6.1) | 7 (4.2) | 10 (3.8) | .9 | 7 (4.4) | 10 (8.5) | .1 |
| Unclear or cardiac death, N (%) | 4 (1.4) | 2 (1.2) | 2 (0.8) | .2 | 3 (1.9) | 1 (0.8) | .9 |
| Myocardial infarction, N (%) | 6 (2.2) | 1 (0.6) | 5 (1.9) | .46 | 1 (0.6) | 5 (4.3) | < .05 |
| Target lesion revascularization, N (%) | 7 (2.5) | 4 (2.4) | 3 (1.1) | .09 | 3 (1.9) | 4 (3.4) | .2 |
|
ACS, acute coronary syndrome; iFR, instantaneous wave-free ratio; MACE, major adverse cardiovascular events. Data are expressed as number (N) and percentage (%). |
|||||||
Based on their clinical signs, patients who presented with ACS had an increased rate of new myocardial infarction at the follow-up (5.3% vs 0.6%; P < .05), although no differences were found regarding unclear or cardiac death (0.9% vs 1.8%; P = .9) and the overall MACE (8.5% vs 4.4%; OR, 2.056, 0.759-5.572; P = .156 (table 3).
Finally, we performed a univariate analysis and found no risk or protective factors for MACE in this cohort of patients (table 4).
Table 4. Univariate analysis of the different variables with potential impact in the rate of major adverse cardiovascular events between groups
| Variable | Univariate analysis | |
|---|---|---|
| OR (95%CI) | P | |
| Age | 1.01 (0.97-1.06) | .608 |
| Male | 2.54 (0.57-11.40) | .224 |
| Current smoker | 1.23 (0.42-3.60) | .713 |
| Dyslipidemia | 1.39 (0.50-3.87) | .531 |
| Left ventricular ejection fraction (%) | 0.99 (0.95-1.04) | .684 |
| Acute coronary syndrome | 2.06 (0.76-5.57) | .156 |
| Multivessel disease | 0.90 (0.33-2.45) | .842 |
| Clopidogrel | 0.75 (0.23-2.44) | .623 |
| Ticagrelor | 1.52 (0.46-4.96) | .490 |
| Right coronary artery as examined vessel | 1.52 (0.54-4.26) | .428 |
| Other vessel analyzed | 1.26 (0.27-5.82) | .769 |
| Baseline iFR ≤ 0.89 | 1.43 (0.88-2.32) | .152 |
|
95%CI, confidence interval; iFR, instantaneous wave-free ratio; OR, odds ratio. |
||
DISCUSSION
This study tried to describe our experience using the physiological assessment and the Syncvision software in all-comer patients who underwent percutaneous coronary evaluations. The main findings of our study are: a) the use of the iFR in lesions of all-comer patients with the same cut-off values than established in the main trials showed a low percentage of MACE at the mid-term follow-up (6.1%); b) patients who presented with acute coronary syndrome showed an increased rate of myocardial infarction at the mid-term follow-up, and a trend towards a higher rate of MACE (OR, 2.056, 0.759-5.572; P = .156); c) The Syncvision-guided iFR-pullback study provided additional information to guide the PCI decision-making process, especially in complex lesions like sequential lesions and diffuse coronary artery disease.
The fractional flow reserve was the first physiological index that demonstrated its utility, safety, and efficacy guiding the revascularization decision-making process.2,5-7 To obtain it, the use of a hyperemic agent to reduce vascular resistance is mandatory. Adenosine is the most commonly used drug, but it presents a series of side effects and contraindications.3,4,11,12 The more recent resting index (the instantaneous wave-free ratio) has demonstrated similar utility, safety, and efficacy to the FFR.3,4 Furthermore, it has 2 main advantages: first, it is not necessary to use vasodilators, thus reducing side effects, contraindications for use, and procedural time; secondly, it allows us to assess the contribution of each lesion when the vessel presents several lesions, with the specific Syncvision-guided iFR-pullback study.8,9
For these reasons, the coronary physiology assessment is already the routine practice at the cath lab for the assessment of intermediate lesions,2-5 and multivessel disease.6,7 The main clinical setting included in these studies was stable angina. Patients with NSTEMI could be included if the lesion evaluated was identified as a non-culprit lesion. However, patients with STEMI, left main coronary artery lesions, and coronary artery bypass grafting lesions were not represented in the trials; also, the percentage of bifurcation lesions and sequential or diffuse coronary lesions is tiny. The cut-off value for the FFR and the iFR is well defined in those trials, being safe to postpone a lesion with a FFR > 0.80 or an iFR > 0.89. However, information is scarce on the utility and efficacy of physiological assessment and the same cut-off values in other types of lesions and clinical presentations.13 A multicenter registry that used the iFR to guide revascularization in patients with left main coronary artery stenosis has just been published. Using a cut-off value of 0.89, the authors conclude that postponing a left main coronary artery lesion with a iFR > 0.89 seems to be safe.10
Our study results suggest that the use of physiological assessment and the Syncvision software to guide the PCI decision-making process in all-comer patients with the same cut-off values as established by the landmark trials seems useful and safe regardless of the lesion and clinical presentation undergoing evaluation. Also, the MACE rates are similar to those reported by the landmark trials with selected lesions and patients.3,4 The iFR was the index used more often. The reasons are the faster and more comfortable use,3,4 and the possibility of lesion assessment with the Syncvision software.8,9
An important point of the study was to evaluate the rate of MACE based on the clinical presentation. Although no significant differences in the overall rate of MACE were found, patients who presented with acute coronary syndrome showed a significantly higher rate of MI at the follow-up, and a trend towards a higher rate of overall MACE. We think that this absence of statistical significance could be associated with a lack of statistical power.
A type of lesion included in the study was bifurcation lesions. Physiological assessment was used mainly to guide the side branch results during a provisional stenting strategy, thus keeping the pressure wire jailed as previously described.14,15 However, another interesting use of the iFR-pullback study with the Syncvision software was to stablish the baseline physiological contribution of every segment included in the most accepted classification.16
Finally, the Syncvision-guided iFR-pullback study was used in 155 lesions (36%). The main type of lesions where this software was used were diffuse and tandem lesions. This software can predict the physiological contribution of each lesion or coronary segment, which is why we believe that it is a very useful tool to avoid treating lesions without any physiological contribution and probably without clinical benefits. That is why this software seems to reduce the total stent length implanted regarding angiographically-guided revascularization with potential benefits at long-term follow-up.17,18 A clinical trial is currently in the recruitment phase to demonstrate the efficacy of this software reducing the length of the stent implanted in this type of lesions without detriment to the adverse events.19
In our experience, the key aspects to properly perform this technique are: a) a perfect aortic pressure curve allows the accurate detection of diastole through the software; b) passing the pressure sensor as distally as possible; c) finding a projection where the artery can be seen completely and with the least foreshortening possible; d) withdrawing the pressure guidewire very slowly so that the software can perfectly recognize the length of each arterial segment; e) checking that there is not drift when the pressure guidewire reaches the coronary ostium (iFR different to 1 ± 0.02) to avoid erroneous results; f) performing the coronary angiography in the same position as the guidewire withdrawal without any modifications to the height of the table or the C-arm, and with a higher flow and volume of contrast to facilitate the software recognition of all the lesions. The main problem when using this technique is the presence of lesions with complicated wiring. The pressure wire has a hydrophilic non-polymeric coating that is useful in most lesions. However, it may be very challenging to reach the distal part of the artery in very complex lesions (calcified, angled lesions…), and our experience with previous normalization, wire disconnection, the microcatheter exchange technique, and reconnection is very limited, but still there is a significant level of drift.
Limitations
The study presents several limitations. It is a retrospective, single-center analysis with a low number of patients and lesions. Therefore, the results should be interpreted with caution, although it could be a hypothesis-generating study for future larger scale randomized clinical trials.
CONCLUSIONS
The use of coronary physiology assessment using the iFR and the Syncvision-guided iFR-pullback study in the routine daily practice and in all-comer patients seems safe with a low percentage of MACE at the mid-term follow-up. The Syncvision-guided iFR-pullback study provides additional information to guide the PCI decision-making process.
FUNDING
The study has not had funding.
AUTHORS’ CONTRIBUTION
F.J. Hidalgo-Lesmes prepared the main draft of the manuscript. S. Ojeda-Pineda participated in the drafting of the manuscript. C. Pericet-Rodríguez, R. González-Manzanares, A. Fernández-Ruiz, and M.G. Flores-Vergara all contributed to the analysis and interpretation of data. A. Luque-Moreno, J. Suárez de Lezo, and F. Mazuelos-Bellido participated in the conception and design of the study. M.A. Romero-Moreno, and J.M. Segura Saint-Gerons revised the manuscript critically for important intellectual content. M. Pan Álvarez-Ossorio approved the final version of the manuscript.
CONFLICTS OF INTEREST
F.J. Hidalgo-Lesmes received minor fees from Philips Volcano Europe unrelated to the manuscript; S. Ojeda-Pineda received minor fees from Terumo and Philips Volcano Europe unrelated to the manuscript; M. Pan Álvarez-Ossorio received minor fees from Terumo, Abbott Vascular, and Philips Volcano Europe unrelated to the manuscript. The remaining authors declared no conflicts of interest.
WHAT IS KNOWN ABOUT THE TOPIC?
- Physiological assessments with the iFR are strongly recommended by the European guidelines on coronary revascularization to guide the PCI decision-making process in intermediate coronary stenosis.
- However, the evidence supporting the use of coronary physiology assessment, and the new Syncvision-iFR software in other type of lesions and clinical settings is scarce.
WHAT DOES THIS STUDY ADD?
- This study describes our experience with the iFR and the Syncvision-iFR software in all-comer patients and demonstrates an acceptable percentage of MACE at the mid-term follow-up.
- Furthermore, the study shows that the Syncvision-guided iFR-pullback study provides additional information to guide the PCI decision-making process, particularly in complex lesions like sequential lesions and diffuse coronary artery disease.
REFERENCES
1. Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87-165.
2. Pijls NHJ, van Schaardenburgh P, Manoharan G, et al. Percutaneous Coronary Intervention of Functionally Nonsignificant Stenosis. 5-Year Follow-Up of the DEFER Study. J Am Coll Cardiol. 2007;49:2105-2111.
3. Davies JE, Sen S, Dehbi H-M, et al. Use of the Instantaneous Wave-free Ratio or Fractional Flow Reserve in PCI. N Engl J Med. 2017;376:1824-1834.
4. Götberg M, Christiansen EH, Gudmundsdottir IJ, et al. Instantaneous Wave-free Ratio versus Fractional Flow Reserve to Guide PCI. N Engl J Med. 2017;376:1813-1823.
5. Pijls NHJ, de Bruyne B, Peels K, et al. Measurement of Fractional Flow Reserve to Assess the Functional Severity of Coronary-Artery Stenoses. N Engl J Med. 1996;334:1703-1708.
6. Tonino AL, Bruyne B De, Pijls NHJ, et al. Fractional Flow Reserve versus Angiography for Guiding Percutaneous Coronary Intervention Pim. N Engl J Med. 2015:687-696.
7. Van Nunen LX, Zimmermann FM, Tonino PAL, et al. Fractional flow reserve versus angiography for guidance of PCI in patients with multivessel coronary artery disease (FAME):5-year follow-up of a randomised controlled trial. Lancet. 2015;386:1853-1860.
8. Nijjer SS, Sen S, Petraco R, Mayet J, Francis DP, Davies JER. The Instantaneous wave-Free Ratio (iFR) pullback:A novel innovation using baseline physiology to optimise coronary angioplasty in tandem lesions. Cardiovasc Revasc Med. 2015;16:167-171.
9. Nijjer SS, Sen S, Petraco R, et al. Pre-angioplasty instantaneous wave-free ratio pullback provides virtual intervention and predicts hemodynamic outcome for serial lesions and diffuse coronary artery disease. JACC Cardiovasc Interv. 2014;7:1386-1396.
10. Warisawa T, Cook CM, Rajkumar C, et al. Safety of Revascularization Deferral of Left Main Stenosis Based on Instantaneous Wave-Free Ratio Evaluation. JACC Cardiovasc Interv. 2020;13:1655-1664.
11. Gili S, Barbero U, Errigo D, et al. Intracoronary versus intravenous adenosine to assess fractional flow reserve:A systematic review and meta-analysis. J Cardiovasc Med. 2018;19:274-283.
12. Patel HR, Shah P, Bajaj S, Virk H, Bikkina M, Shamoon F. Intracoronary adenosine-induced ventricular arrhythmias during fractional flow reserve (FFR) measurement:case series and literature review. Cardiovasc Interv Ther. 2017;32:374-380.
13. Ihdayhid AR, Koh JS, Ramzy J, et al. The Role of Fractional Flow Reserve and Instantaneous Wave-Free Ratio Measurements in Patients with Acute Coronary Syndrome. Curr Cardiol Rep. 2019;21.
14. Burzotta F, Lassen JF, Banning AP, et al. Percutaneous coronary intervention in left main coronary artery disease:The 13th consensus document from the European Bifurcation Club. EuroIntervention. 2018;14:112-120.
15. Hidalgo F, Pan M, Ojeda S, et al. Feasibility and Efficacy of the Jailed Pressure Wire Technique for Coronary Bifurcation Lesions. JACC Cardiovasc Interv. 2019;12:109-111.
16. Medina A, Suárez de Lezo J, Pan M. A New Classification of Coronary Bifurcation Lesions. Rev Esp Cardiol. 2006;59:183.
17. Mauri L, O'Malley AJ, Popma JJ, et al. Comparison of thrombosis and restenosis risk from stent length of sirolimus-eluting stents versus bare metal stents. Am J Cardiol. 2005;95:1140-1145.
18. Kikuta Y, Cook CM, Sharp ASP, et al. Pre-Angioplasty Instantaneous Wave-Free Ratio Pullback Predicts Hemodynamic Outcome In Humans With Coronary Artery Disease:Primary Results of the International Multicenter iFR GRADIENT Registry. JACC Cardiovasc Interv. 2018;11:757-767.
19. Hidalgo F, Ojeda S, de Lezo JS, et al. Usefulness of a co-registration strategy with iFR in long and/or diffuse coronary lesions (iLARDI):study protocol. REC Interv Cardiol. 2021;3:190-195.
Abstract
Introduction and objectives: Patients with a low post-percutaneous coronary intervention (PCI) fractional flow reserve (FFR) are at a higher risk for future adverse cardiac events. The objective of the current study was to assess specific patient and procedural predictors of post-PCI FFR.
Methods: The FFR-SEARCH study is a prospective single-center registry of 1000 consecutive all-comer patients who underwent FFR measurements after an angiographically successful PCI with a dedicated microcatheter. Mixed effects models were used to search for independent predictors of post-PCI FFR.
Results: The mean post-PCI distal coronary pressure divided by the aortic pressure (Pd/Pa) was 0.96 ± 0.04 and the mean post-PCI FFR, 0.91 ± 0.07. After adjusting for the independent predictors of post-PCI FFR, the left anterior descending coronary artery as the measured vessel was the strongest predictor of post-PCI FFR (adjusted β = -0.063; 95%CI, -0.070 to -0.056; P < .0001) followed by the postprocedural minimum lumen diameter (adjusted β = 0.039; 95%CI, 0.015-0.065; P = .002). Additionally, male sex, in-stent restenosis, chronic total coronary occlusions, and pre- and post-dilatation were negatively associated with postprocedural FFR. Conversely, type A lesions, thrombus-containing lesions, postprocedural percent stenosis, and stent diameter were positively associated with postprocedural FFR. The R2 for the complete model was 53%.
Conclusions: Multiple independent patient and vessel related predictors of postprocedural FFR were identified, including sex, the left anterior descending coronary artery as the measured vessel, and postprocedural minimum lumen diameter.
Keywords: Percutaneous coronary intervention. Post-PCI FFR. Predictors.
RESUMEN
Introducción y objetivos: Los pacientes con una reserva fraccional de flujo (FFR) posintervención coronaria percutánea (ICP) baja tienen mayor riesgo de futuros eventos cardiacos adversos. El objetivo del presente estudio fue evaluar predictores específicos de pacientes y procedimientos de FFR tras una ICP.
Métodos: El estudio FFR-SEARCH es un registro prospectivo de un solo centro que incluyó 1.000 pacientes consecutivos que se sometieron a una evaluación de la FFR tras una ICP con éxito angiográfico utilizando un microcatéter específico. Se utilizaron modelos de efectos mixtos para buscar predictores independientes de FFR tras la ICP.
Resultados: La media de presión distal dividida entre la presión aórtica tras la ICP fue de 0,96 ± 0,04, y la media de la FFR tras la ICP fue de 0,91 ± 0,07. Tras ajustar por predictores independientes de FFR tras la ICP, la arteria descendente anterior izquierda como vaso medido fue el predictor más fuerte (β ajustado = −0,063; IC95%, −0,070 a −0,056; p < 0,0001), seguida del diámetro luminal mínimo posprocedimiento (β ajustado = 0,039; IC95%, 0,015 a 0,065; p = 0,002). Además, el sexo masculino, la reestenosis del stent, las oclusiones totales crónicas y la pre- y posdilatación se correlacionaron negativamente con la FFR posprocedimiento. Por el contrario, las lesiones de tipo A, las lesiones con trombos, el porcentaje de estenosis posprocedimiento y el diámetro del stent se correlacionaron positivamente con la FFR posprocedimiento. El R2 para el modelo completo fue del 53%.
Conclusiones: Se identificaron diversos predictores independientes relacionados con los pacientes y con los vasos para la FFR posprocedimiento, incluyendo el sexo, la arteria descendente anterior izquierda como vaso medido y el diámetro luminal mínimo posprocedimiento.
Palabras clave: Intervención coronaria percutánea. FFR post-ICP. Predictores.
Abbreviations:
FFR: fractional flow reserve. LAD: left anterior descending coronary artery. MLD: minimum luminal diameter. PCI: percutaneous coronary intervention.
INTRODUCTION
The limitations of an accurate assessment of the hemodynamic significance of coronary artery lesions through angiographic guidance alone are well-known.1 Instead, the fractional flow reserve (FFR) has proven to be a useful technique to address the coronary physiology and the hemodynamic significance of coronary segments before and after performing an intervention.2-4 Also, measuring FFR post-stenting has proven to be a strong and independent predictor of major adverse cardiovascular events at the 2-year follow-up.3-5
While FFR primarily takes into account the relative luminal narrowing and the amount of viable myocardium perfused by a specific vessel, several factors have been shown to impact the FFR values prior to performing a percutaneous coronary intervention (PCI). Therefore, longer lesion length, high syntax scores, calcifications, and tortuosity are associated with significantly lower FFR values. Conversely, the presence of microvascular dysfunction, chronic kidney disease and female gender have been associated with higher FFR values.6-11
At the present time, there is lack of data on independent predictors of post-PCI FFR. Therefore, the objective of the present study was to assess the patient and procedural characteristics associated with low post-PCI FFR in an all-comer patient population.
METHODS
The FFR-SEARCH study is a prospective single-center registry that assessed the routine distal pressure divided by the aortic pressure (Pd/Pa) and FFR values of all consecutive patients after an angiographically successful PCI. The primary endpoint was to study the impact of post-PCI FFR on the rate of major adverse cardiovascular event at the 2-year follow-up. Accordingly, no further actions were taken to improve post-PCI FFR. The study was performed in full compliance with the Declaration of Helsinki. The study protocol was approved by the local ethics committee. All patients gave their written informed consent to undergo the procedure. Also, anonymous datasets for research purposes were used in compliance with the Dutch Medical Research Act. A total of 1512 patients treated between March 2016 and May 2017 at the Erasmus Medical Center were eligible to enter our study. A total of 504 of these patients were excluded due to hemodynamic instability (156), a rather small distal outflow (129), the operator’s decision not to proceed with post-PCI hemodynamic assessment (148) or other reasons (79). A total of 1000 patients were included in the study. The microcatheter could not cross the treated lesion in 28 patients, technical issues with the catheter prevented post-PCI assessments in 11 patients, and in 2 patients the post-PCI FFR measurements had to be aborted prematurely due to adenosine intolerance. This left 959 patients whose post-PCI FFR values were measured in at least 1 angiographically successfully treated lesion.
Quantitative coronary angiography
The preprocedural lesion type was defined according to the ACC/AHA guidelines12 and divided into 4 categories: A, B1, B2, and C. Comprehensive quantitative coronary angiography analyses were performed pre- and post-stent implantation in all the treated lesions. An angiographic view with minimal foreshortening of the lesion and minimal overlapping with other vessels was selected. Similar angiographic views were used pre- and post-stent implantation. Measurements included pre- and postprocedural percent diameter stenosis, reference vessel diameter, lesion length, and minimum luminal diameter (MLD). In case of a total occlusion in patients presenting with ST-segment elevation myocardial infarction (STEMI) or chronic total coronary occlusion (CTO), the MLD was considered zero and the percent diameter stenosis, 100%. The reference vessel diameter and the lesion length were measured from the first angiographic view with restored flow. All measurements were taken using CAAS for Windows, version 2.11.2 (Pie Medical Imaging, The Netherlands).
Fractional flow reserve measurements
All FFR measurements were acquired using the Navvus RXi system (ACIST Medical Systems, United States), a dedicated FFR microcatheter with optical pressure sensor technology.13,14 Measurements were performed after an intracoronary bolus of nitrates (200 µg). The catheter was advanced while mounted over the previously used guidewire approximately 20 mm distal to the most distal border of the stent. The FFR was defined as the mean distal coronary artery pressure divided by the mean aortic pressure during maximum hyperemia achieved by the continuous IV infusion of adenosine at a rate of 140 µg/kg/min via the antecubital vein. In this study no vessels were assessed using intracoronary adenosine.
Statistical analysis
At baseline, the categorical variables were expressed as counts (percentage) and the continuous ones as mean ± standard deviation. To assess the independent predictors of post-PCI FFR, all the patient and vessel characteristics were primarily assessed through an univariate test using a mixed effects model (LME-model) with a random effect for the patients and a fixed effect for the post-PCI FFR. All variables were subsequently inserted in a multivariate LME-model using the enter method that resulted in all the significant independent predictors of post-PCI FFR values. A forest plot was developed to depict all variables with the corresponding 95% confidence intervals (95%CI). Beta (β) values show the average increase or decrease of the FFR values in the case of dichotomous variables or the increment per unit increase in the case of continuous variables. Statistical analyses were performed using the statistical software package R (version 3.5.1, packages: Hmisc, lme4 and nlme, RStudio Team, United States).
RESULTS
Demographic characteristics
The mean age was 64.6 ± 11.8 years and 72.5% were males. In 959 patients, at least, 1 lesion was measured with an overall 1165 successfully treated and measured lesions. The patient demographics and baseline characteristics are shown on table 1. Up to 70% of the patients presented with an acute coronary syndrome, and 18% had confirmed thrombus as seen on the angiography. Intravascular imaging modalities were used in 9.6% of the patients to guide the procedure. Overall, 1.4 ± 0.6 lesions were treated per patient and in 1.2 ± 0.5 lesions per patient the post-PCI FFR was successfully assessed. The average overall stent length per vessel was 29 mm ± 17 mm with an average stent diameter of 3.2 mm ± 0.5 mm.
Table 1. Baseline patient and vessel characteristics
| Variable | Total FFR-SEARCH registry |
|---|---|
| Patient characteristics | (N = 1000) |
| Age | 64.6 ± 11.8 |
| Sex, male | 725 (73) |
| Hypertension | 515 (52) |
| Hypercholesterolemia | 451 (45) |
| Diabetes | 191 (19) |
| Smoking history | 499 (50) |
| Previous stroke | 77 (8) |
| Peripheral arterial disease | 76 (8) |
| Previous myocardial infarction | 203 (20) |
| Previous PCI | 264 (26) |
| Previous CABG | 57 (6) |
| Indication for PCI | |
| Stable angina | 304 (30) |
| NSTEMI | 367 (37) |
| STEMI | 329 (33) |
| Vessel characteristics | (N = 1165) |
| Lesion type | |
| A | 125 (11) |
| B1 | 233 (20) |
| B2 | 379 (33) |
| C | 428 (37) |
| LAD | 593 (51) |
| Bifurcation | 138 (12) |
| Calcified | 402 (35) |
| In-stent restenosis | 39 (3) |
| Thrombus | 214 (18) |
| Stent thrombosis | 14 (1) |
| Ostial | 97 (8) |
| CTO | 42 (4) |
| Stenosis pre procedural | 69 ± 22 |
| Reference diameter pre procedural (mm) | 2.6 ± 0.6 |
| Length pre procedural (cm) | 21 ± 11 |
| MLD pre (mm) | 0.9 ± 0.6 |
| Predilatation | 769 (66) |
| Postdilatation | 691 (59) |
| Stenosis post procedural | 44 ± 13 |
| Reference diameter post procedural (mm) | 2.7 ± 0.5 |
| Length post procedural (cm) | 24 ± 13 |
| MLD post procedural (mm) | 2.6 ± 0.5 |
| Number of stents | 1.4 ± 0.6 |
| Stent length (cm) | 29 ± 17 |
| Stent diameter (mm) | 3.2 ± 0.5 |
| Mean post-PCI Pd/Pa | 0.96 ± 0.04 |
| Mean post-PCI FFR | 0.91 ± 0.07 |
|
CABG, coronary artery bypass graft; CTO, chronic total coronary occlusion; FFR, fractional flow reserve; LAD, left anterior descending artery; MLD, minimum luminal diameter; NSTEMI, non-ST segment elevation acute myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction; Pd/Pa, ratio of mean distal coronary artery pressure to mean aortic pressure; Values are expressed as mean ± standard deviation or no. (%). |
|
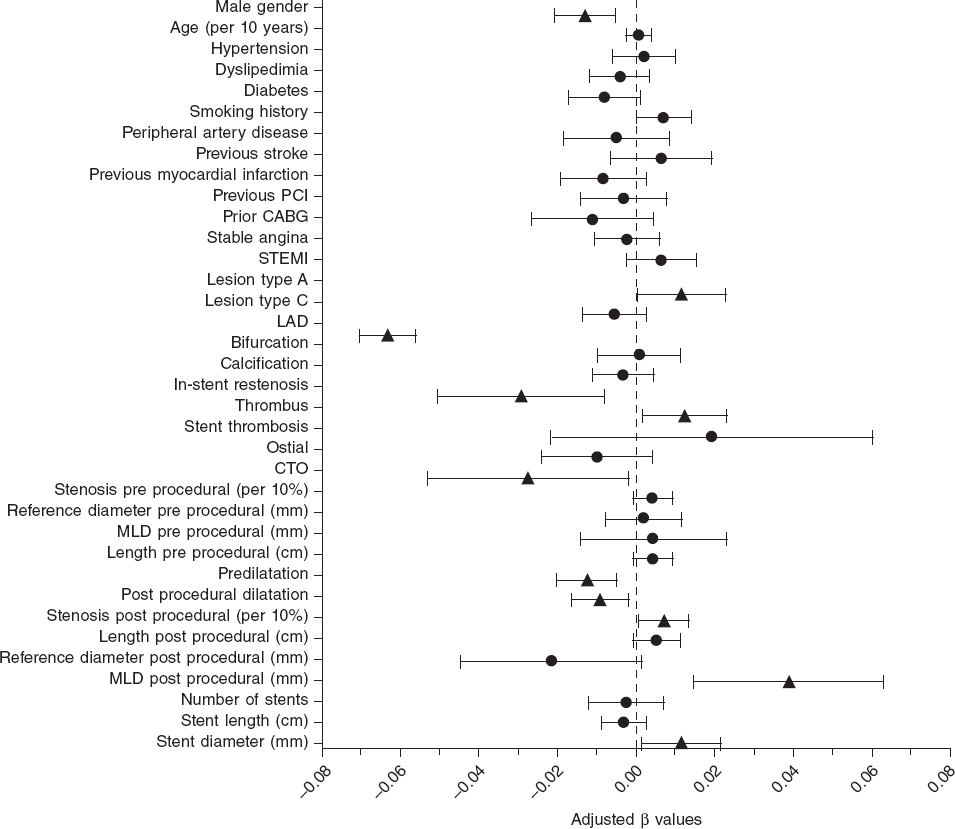
The mean post-PCI FFR was 0.91 ± 0.07 and 7.7% of vessels had a post-PCI FFR ≤ 0.80. In the LME-model and after adjusting for independent predictors of post-PCI FFR, the left anterior descending coronary artery (LAD) as the measured vessel was the strongest predictor of post-PCI FFR (adjusted β = -0.063; 95%CI, -0.070 to -0.056; P < .0001) followed by the postprocedural MLD (adjusted β = 0.039; 95%CI, 0.015-0.065]; P = .002). Additionally, male sex, in-stent restenosis, CTO, and pre- and post-dilatation were negatively correlated with postprocedural FFR. Conversely, type A lesions, thrombus-containing lesions, postprocedural percent diameter stenosis, and stent diameter were positively correlated with postprocedural FFR. The R2 for the entire model was 53%. Figure 1 shows all significant and non-significant adjusted predictors included in the LME-model. Table 2 shows all adjusted and unadjusted predictors with corresponding β values and 95%CI. The most important predictors are shown on figure 2.
Figure 1. Forest plot of independent predictors of post-PCI FFR. Adjusted beta values with 95% confidence intervals. Triangles indicate significant predictors while circles are indicative of non-significant predictors in the multivariate generalized mixed model to predict post-PCI FFR. ACS, acute coronary syndrome; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction; LAD, left anterior descending coronary artery; CTO, chronic total coronary occlusion; MLD, minimum lumen diameter.
Table 2. Predictors for post-PCI FFR
| Variable | Unadjusted | Adjusted | ||
|---|---|---|---|---|
| P | β(95%CI) | P | β(95%CI) | |
| Patient characteristics | ||||
| Male sex | .214 | -0.006 (-0.015 – 0.003) | .001 | -0.013 (-0.021 – -0.005) |
| Age (per 10 years) | .976 | 0.000 (-0.03 – 0.03) | .724 | 0.001 (-0.002 – 0.003) |
| Hypertension | .013 | -0.010 (-0.018 – -0.002) | .610 | 0.002 (-0.006 – 0.010) |
| Hypercholesterolemia | < .001 | -0.019 (-0.027 – -0.011) | .287 | -0.004 (-0.012 – 0.004) |
| Diabetes | < .001 | 0.018 (0.008 – 0.042) | .081 | -0.008 (-0.017 – 0.001) |
| Smoking history | .007 | 0.020 (0.010 – 0.019) | .054 | 0.007 (-0.0001 – 0.014) |
| Previous stroke | .831 | -0.002 (-0.017 – 0.013) | .342 | 0.006 (-0.0007 – 0.019) |
| Peripheral arterial disease | .022 | -0.017 (-0.032 – -0.003) | .460 | -0.005 (-0.018 – 0.008) |
| Previous myocardial infarction | .002 | -0.016 (-0.026 – -0.006) | .137 | -0.008 (-0.019 – 0.003) |
| Previous PCI | < .001 | -0.016 (-0.025 – -0.007) | .569 | -0.032 (-0.014 – 0.008) |
| Previous CABG | .896 | -0.001 (-0.019 – 0.017) | .166 | -0.011 (-0.014 – 0.004) |
| Indication for PCI | ||||
| Stable angina | < .001 | -0.025 (-0.034 – -0.016) | .563 | -0.002 (-0.011 – 0.005) |
| STEMI | < .001 | 0.032 (0.025 – 0.041) | .171 | 0.006 (-0.003 – 0.015) |
| Vessel characteristics | ||||
| Lesion type | ||||
| A | <.001 | 0.022 (0.009 – 0.035) | .040 | 0.012 (0.0005 – 0.023) |
| C | .045 | -0.008 (-0.016 – -0.0002) | .172 | -0.006 (-0.014 – 0.002) |
| LAD | <.001 | -0.070 ( -0.077 – -0.064) | <.001 | -0.063 (-0.070 – -0.056) |
| Bifurcation | < .001 | -0.024 (-0.036 – - 0.012) | .883 | 0.001 (-0.010 – 0.011) |
| Calcified | < .001 | -0.025 (-0.033 – -0.017) | .409 | -0.003 (-0.011 – 0.005) |
| In-stent restenosis | .006 | -0.031 (-0.053 – -0.009) | .007 | -0.029 (-0.051 – -0.008) |
| Thrombus | < .001 | 0.031 (0.021 – 0.042) | .026 | 0.012 (-0.001 – 0.023) |
| Stent thrombosis | .920 | 0.002 (-0.034 – 0.038) | .362 | 0.019 (-0.022 – 0.060) |
| Ostial | .181 | -0.010 (-0.024 – 0.005) | .165 | -0.010 (-0.024 – 0.004) |
| CTO | .002 | -0.034 (-0.056 – -0.013) | .036 | -0.027 (-0.053 – -0.002) |
| Stenosis pre procedural (per 10%) | <.001 | 0.007 (0.005 – 0.009) | .105 | 0.004 (-0.0009 – 0.009) |
| Reference diameter pre procedural (mm) | <.001 | 0.030 (0.023 – 0.037) | .704 | 0.002 (-0.008 – 0.011) |
| Length pre procedural (cm) | .900 | -0.00002 (-0.004 – 0.003) | .101 | 0.004 (0.0008 – 0.009) |
| MLD pre procedural (mm) | <.001 | -0.015 (-0.022 – -0.008) | .638 | 0.004 (-0.014 – 0.023) |
| Predilatation | <.001 | -0.019 (-.027 – -0.011) | .002 | -0.012 (-0.020 – -0.005) |
| Postdilatation | <.001 | 0.027 (-0.035 – -0.019) | .015 | -0.009 (-0.016 – -0.002) |
| Stenosis post procedural (per 10%) | .077 | 0.003 (-0.0003 – 0.006) | .029 | 0.01 (0.0007 – 0.01) |
| Reference diameter post procedural (mm) | <.001 | 0.035 (0.027 – 0.042) | .067 | -0.022 (-0.045 – 0.002) |
| Length post procedural (cm) | .312 | -0.002 (-0.005 – 0.001) | .086 | 0.001 (-0.0007 – 0.001) |
| MLD post procedural (mm) | <.001 | 0.032 (0.024 – 0.040) | .002 | 0.039 (0.015 – 0.063) |
| Number of stents | <.001 | -0.012 (-0.018 – -0.006) | .620 | -0.002 (-0.012 – 0.007) |
| Stent length (cm) | <.001 | 0.019 (0.009 – 0.041) | .286 | -0.003 (-0.009 – 0.002) |
| Stent diameter (mm) | <.001 | 0.033 (0.025 – 0.042) | .026 | 0.012 (0.001 – 0.022) |
|
Beta (β) values are indicative of the average increase or decrease of the FFR values in cases of dichotomous variables or the increment per unit increase in cases of continuous variables. 95%CI, 95% confidence interval; CABG, coronary artery bypass graft; CTO, chronic total coronary occlusion; FFR, fractional flow reserve; LAD, left anterior descending coronary artery; MLD, minimum lumen diameter; STEMI, ST-segment elevation myocardial infarction. |
||||
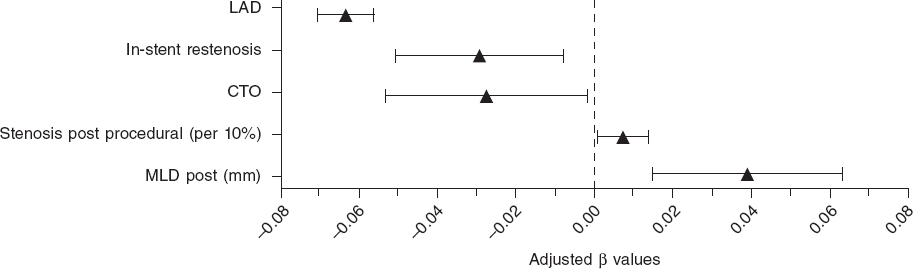
Figure 2. Forest plot of most important predictors of post-PCI FFR. Adjusted beta values with 95% confidence intervals. The figure includes all significant predictors from the multivariate generalized mixed model predicting post-PCI FFR except for categorical variables with beta values < 0.02. LAD, left anterior descending coronary artery; CTO, chronic total coronary occlusion; MLD, minimum lumen diameter.
DISCUSSION
This study is the largest report to this day of predictors of post-PCI FFR. Based on data derived from the FFR-SEARCH registry, we could identify several patient and procedural predictors of post-PCI FFR. These predictors will bring more in-depth interpretations of post-PCI FFR values to be able to identify correctly which vessels are prone to future events. At first, male gender appeared to be negatively correlated with postprocedural FFR. This finding is consistent with the findings of former studies that focused on the impact of gender on pre-PCI FFR measurements.6,11,15,16 Compared to females, males are known to have a lower prevalence of microvascular dysfunction.8,17 The concept of FFR is based on drug-induced maximal hyperemia to minimize microvascular resistance. Microvascular dysfunction may hamper this vasodilator response and consequently result in a dampened flow response and high FFR.15 Subsequently, on average, males have larger myocardial masses and myocardial perfusion territories compared to females.18,19 The importance of the latter is illustrated by the second and strongest predictor of post-PCI FFR in this study, the FFR measurements in the LAD. FFR values are associated with the myocardial mass and the outflow territory of the measured vessel. As such, the LAD—the vessel with the largest perfusion area—has previously been associated with lower pre- and postprocedural FFR values.20-22
The diameters of the stents implanted in the RCA are larger, on average, but the outflow territory of the LAD is even larger.23 This discrepancy between luminal dimensions and myocardial mass may explain why the optimal improvement of the FFR measurements in the LAD is difficult to achieve.23
Thirdly, larger stent diameters and larger post-PCI MLDs were associated with higher post-PCI FFR values. However, higher postprocedural percent stenosis was also associated with higher post-PCI FFR values. While these findings may seem contradictory, post procedural percent stenosis was not associated with post-PCI physiology in the DEFINE PCI study either.24
In the intravascular ultrasound substudy of the FFR-SEARCH registry, van Zandvoort et al. showed that evident signs of residual luminal narrowing including focal lesions, underexpansion, and malapposition were present in a significant amount of vessels with post-PCI FFR values ≤ 0.85. These findings were not readily apparent on the comprehensive quantitative coronary angiography.25 Percent diameter stenosis was 20% in the cohort of patients with post-PCI FFR values ≤ 0.85 and > 0.85.26
Together with the latter predictors of post-PCI FFR we identified several others. A dedicated analysis of 26 CTOs recently showed that postprocedural FFR values are typically low initially; however they seem to increase at the 4-month follow-up. The initially low post-PCI FFR values is thought to be due to the microvascular dysfunction of the recently opened vessel, a phenomenon that improves after several months.27 In-stent restenosis and pre- and postdilatation were associated with lower post-PCI FFR values. A finding that is consistent with former studies that showed that, in general, complex lesions are associated with lower post-PCI FFR values.20,21,26,28
Also, it was interesting to see the impact of clinical presentation on post-PCI FFR values in the study population in which most patients presented with acute coronary syndrome. Contrary to former studies that questioned the validity of invasive hyperemic physiological indices in patients with acute coronary syndrome, we could not confirm the impact of clinical presentation on post-PCI FFR values. However, the identification of a thrombus, that often occurs after a ruptured plaque in patients with acute coronary syndrome, was associated with significantly higher FFR values. Despite the restoration of epicardial flow by the PCI, a relatively large number of patients with STEMI have abnormal myocardial perfusion at the end of the procedure.29 This phenomenon is thought to be related to microvascular obstruction due to distal embolization (reperfusion injury) and tissue inflammation due to myocyte necrosis.30,31 The latter may explain the significantly higher post-PCI FFR values reported in patients presenting with thrombus-containing lesions compared to those without such lesions. Conversely, our findings also show that in patients without thrombus-containing lesions the post-PCI FFR may be a valuable diagnostic tool for the identification of patients at a high risk of future adverse cardiac events.
Limitations
This study was conducted with the Navvus microcatheter, a dedicated rapid exchange microcatheter with a mean diameter of 0.022 in that proved its utility in a slight but significant underestimation of the FFR compared to conventional 0.014 in pressure guidewires.32 That is why we cannot directly extrapolate the current findings to wire-based FFR devices.14 Based on the study protocol, no further action was taken in the presence of low post-PCI FFR values. The Target FFR and FFR REACT studies (NCT03259815 and NTR6711) will provide further information on post-PCI FFR and the potential of further actions to improve post-PCI FFR and clinical outcomes.33,34 These studies should also focus on the trade-off of potential benefits and harm when performing additional interventions in order to improve the final FFR values.
CONCLUSIONS
In this substudy of the FFR-SEARCH registry, the largest real-world post-PCI FFR registry conducted to this day, we identified sex, LAD vessels, postprocedural MLD, and several other independent predictors of postprocedural FFR.
FUNDING
The FFR SEARCH study was conducted with institutional support from ACIST Medical Inc.
AUTHORS' CONTRIBUTION
Conception and design: L.J.C. van Zandvoort, N.M. van Mieghem, and J. Daemen. Data aquisition: L.J.C. van Zandvoort, K. Masdjedi, J. Wilschut, W. Den Dekker, R. Diletti, F. Zijlstra, N.M. van Mieghem, and J. Daemen. Statistical analysis and manuscript writing: L.J.C. van Zandvoort and J. Daemen. Providing criticial feedback to the manuscript and approving the final content: L.J.C. van Zandvoort, K. Masdjedi, T. Neleman, M.N Tovar Forero, J. Wilschut, W. Den Dekker, R. Diletti, F. Zijlstra, N.M. van Mieghem, and J. Daemen.
CONFLICTS OF INTEREST
L.J.C. van Zandvoort received institutional research support from Acist medical Inc. J. Daemen received institutional research support from Pie Medical, ACIST Medical Inc., PulseCath, Medtronic, Boston Scientific, Abbott Vascular, Pie Medical and speaker and consultancy fees from PulseCath, Medtronic, ReCor Medical, ACIST Medical Inc. and Pie Medical. The remaining authors declared no conflicts of interest.
WHAT IS KNOWN ABOUT THE TOPIC?
- FFR has proven to be a useful technique to address coronary physiology and the hemodynamic significance of coronary segments pre- and post-intervention.
- Also, the FFR post-stenting has proven to be a strong and independent predictor of major adverse cardiovascular events at the 2-year follow-up.
- Unfortunately, at present, there is lack of data on independent predictors of post PCI FFR.
WHAT DOES THIS STUDY ADD?
- This study is the largest report to this day on predictors of post-PCI FFR.
- Based on data from the FFR-SEARCH registry, we could identify several patient and procedural predictors of post-PCI FFR.
- The main predictors included sex, LAD vessels, and postprocedural lumen dimensions. These predictors will help us interpret post-PCI FFR values and identify correctly the vessels that are prone to future events.
REFERENCES
1. Topol EJ, Nissen SE. Our preoccupation with coronary luminology. The dissociation between clinical and angiographic findings in ischemic heart disease. Circulation. 1995;92:2333-2342.
2. De Bruyne B, Fearon WF, Pijls NHJ, et al. Fractional flow reserve-guided PCI for stable coronary artery disease. N Engl J Med. 2014;1533-4406.
3. Wolfrum M, Fahrni G, de Maria GL, et al. Impact of impaired fractional flow reserve after coronary interventions on outcomes:a systematic review and meta-analysis. BMC Cardiovasc Disord. 2016;16:177.
4. Rimac G, Fearon WF, De Bruyne B, et al. Clinical value of post-percutaneous coronary intervention fractional flow reserve value:A systematic review and meta-analysis. Am Heart J. 2017;183:1-9.
5. Kasula S, Agarwal SK, Hacioglu Y, et al. Clinical and prognostic value of poststenting fractional flow reserve in acute coronary syndromes. Heart. 2016;102:1988-1994.
6. Sareen N, Baber U, Kezbor S, et al. Clinical and angiographic predictors of haemodynamically significant angiographic lesions:development and validation of a risk score to predict positive fractional flow reserve. EuroIntervention. 2017;12:e2228-e2235.
7. Baranauskas A, Peace A, Kibarskis A, et al. FFR result post PCI is suboptimal in long diffuse coronary artery disease. EuroIntervention. 2016;12:1473-1480.
8. Crystal GJ, Klein LW. Fractional flow reserve:physiological basis, advantages and limitations, and potential gender differences. Curr Cardiol Rev. 2015;11:209-219.
9. Ahmadi A, Leipsic J, Ovrehus KA, et al. Lesion-Specific and Vessel-Related Determinants of Fractional Flow Reserve Beyond Coronary Artery Stenosis. JACC Cardiovasc Imaging. 2018;11:521-530.
10. Tebaldi M, Biscaglia S, Fineschi M, et al. Fractional Flow Reserve Evaluation and Chronic Kidney Disease:Analysis From a Multicenter Italian Registry (the FREAK Study). Catheter Cardiovasc Interv. 2016;88:555-562.
11. Fineschi M, Guerrieri G, Orphal D, et al. The impact of gender on fractional flow reserve measurements. EuroIntervention. 2013;9:360-366.
12. Ryan TJ, Faxon DP, Gunnar RM, et al. Guidelines for percutaneous transluminal coronary angioplasty. A report of the American College of Cardiology/American Heart Association Task Force on Assessment of Diagnostic and Therapeutic Cardiovascular Procedures (Subcommittee on Percutaneous Transluminal Coronary Angioplasty). Circulation. 1988;78:486-502.
13. Diletti R, Van Mieghem NM, Valgimigli M, et al. Rapid exchange ultra-thin microcatheter using fibre-optic sensing technology for measurement of intracoronary fractional flow reserve. EuroIntervention. 2015;11:428-432.
14. Menon M, Jaffe W, Watson T, Webster M. Assessment of coronary fractional flow reserve using a monorail pressure catheter:the first-in-human ACCESS-NZ trial. EuroIntervention. 2015;11:257-263.
15. van de Hoef TP, Meuwissen M, Escaned J, et al. Fractional flow reserve as a surrogate for inducible myocardial ischaemia. Nat Rev Cardiol. 2013;10:439-452.
16. Kim HS, Tonino PA, De Bruyne B, et al. The impact of sex differences on fractional flow reserve-guided percutaneous coronary intervention:a FAME (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation) substudy. JACC Cardiovasc Interv. 2012;5:1037-1042.
17. Reis SE, Holubkov R, Lee JS, et al. Coronary flow velocity response to adenosine characterizes coronary microvascular function in women with chest pain and no obstructive coronary disease. Results from the pilot phase of the Women's Ischemia Syndrome Evaluation (WISE) study. J Am Coll Cardiol. 1999;33:1469-1475.
18. Iqbal MB, Shah N, Khan M, Wallis W. Reduction in myocardial perfusion territory and its effect on the physiological severity of a coronary stenosis. Circ Cardiovasc Interv. 2010;3:89-90.
19. Lin FY, Devereux RB, Roman MJ, et al. Cardiac chamber volumes, function, and mass as determined by 64-multidetector row computed tomography:mean values among healthy adults free of hypertension and obesity. JACC Cardiovasc Imaging. 2008;1:782-786.
20. Nam CW, Hur SH, Cho YK, et al. Relation of fractional flow reserve after drug-eluting stent implantation to one-year outcomes. Am J Cardiol. 2011;107:1763-1767.
21. Doh JH, Nam CW, Koo BK, et al. Clinical Relevance of Poststent Fractional Flow Reserve After Drug-Eluting Stent Implantation. J Invasive Cardiol. 2015;27:346-351.
22. Agarwal SK, Kasula S, Hacioglu Y, Ahmed Z, Uretsky BF, Hakeem A. Utilizing Post-Intervention Fractional Flow Reserve to Optimize Acute Results and the Relationship to Long-Term Outcomes. JACC Cardiovasc Interv. 2016;9:1022-1031.
23. Kimura Y, Tanaka N, Okura H, et al. Characterization of real-world patients with low fractional flow reserve immediately after drug-eluting stents implantation. Cardiovasc Interv Ther. 2016;31:29-37.
24. Jeremias A, Davies JE, Maehara A, et al. Blinded Physiological Assessment of Residual Ischemia After Successful Angiographic Percutaneous Coronary Intervention:The DEFINE PCI Study. JACC:Cardiovasc Interv. 2019;12:1991-2001.
25. van Zandvoort LJC, Masdjedi K, Witberg K, et al. Explanation of Postprocedural Fractional Flow Reserve Below 0.85. Circ Cardiovasc Interv. 2019;12:e007030.
26. van Zandvoort LJC, Witberg K, Ligthart J, et al. Explanation of post procedural fractional flow reserve below 0.85:a comprehensive ultrasound analysis of the FFR Search registry. In Cardiovascular Research Technologies (CRT) Conference 2018 March 3-6;Washingtong DC, United States. 2018.
27. Karamasis GV, Kalogeropoulos AS, Mohdnazri SR, et al. Serial Fractional Flow Reserve Measurements Post Coronary Chronic Total Occlusion Percutaneous Coronary Intervention. Circ Cardiovasc Interv. 2018;11:e006941.
28. Pijls NH, Klauss V, Siebert U, et al. Coronary pressure measurement after stenting predicts adverse events at follow-up:a multicenter registry. Circulation. 2002;105:2950-2954.
29. Stone GW, Webb J, Cox DA, et al. Distal microcirculatory protection during percutaneous coronary intervention in acute ST-segment elevation myocardial infarction:a randomized controlled trial. JAMA. 2005;293:1063-1072.
30. Shah NR, Al-Lamee R, Davies J. Fractional flow reserve in acute coronary syndromes:A review. Int J Cardiol Heart Vasc. 2014;5:20-25.
31. Cuculi F, De Maria GL, Meier P, et al. Impact of microvascular obstruction on the assessment of coronary flow reserve, index of microcirculatory resistance, and fractional flow reserve after ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2014;64:1894-904.
32. Pouillot C, Fournier S, Glasenapp J, et al. Pressure wire versus microcatheter for FFR measurement:a head-to-head comparison. EuroIntervention. 2018;13:e1850-e1856.
33. van Zandvoort LJC, Masdjedi K, Tovar Forero MN, et al. Fractional flow reserve guided percutaneous coronary intervention optimization directed by high-definition intravascular ultrasound versus standard of care:Rationale and study design of the prospective randomized FFR-REACT trial. Am Heart J. 2019;213:66-72.
34. Collison D, McClure JD, Berry C, Oldroyd KG. A randomized controlled trial of a physiology-guided percutaneous coronary intervention optimization strategy:Rationale and design of the TARGET FFR study. Clin Cardiol. 2020;43:414-422.
ABSTRACT
Introduction and objectives: The presence of comorbidities in elderly patients with non-ST-segment elevation acute coronary syndrome worsens its prognosis. The objective of the study was to analyze the impact of the burden of comorbidities in the decision of using invasive management in these patients.
Methods: A total of 7211 patients > 70 years old from 11 Spanish registries were included. Individual data were analyzed in a common database. We assessed the presence of 6 comorbidities and their association with coronary angiography during admission.
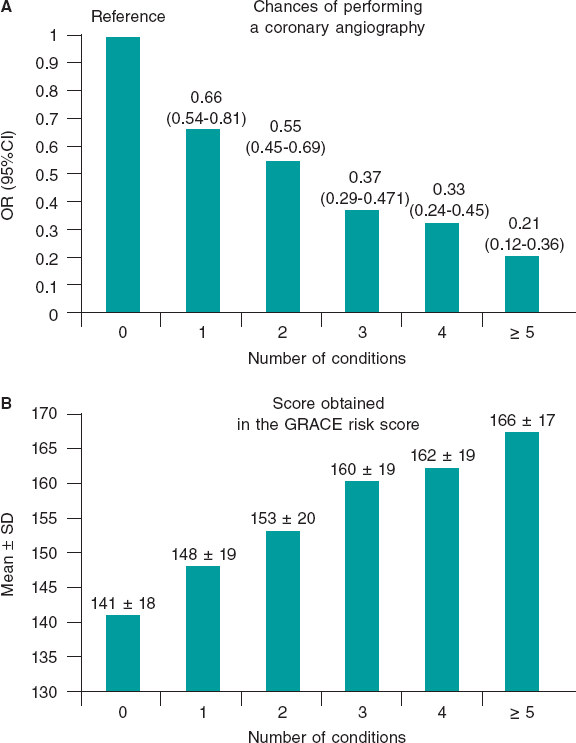
Results: The mean age was 79 ± 6 years and the mean CRACE score was 150 ± 21 points. A total of 1179 patients (16%) were treated conservatively. The presence of each comorbidity was associated with less invasive management (adjusted for predictive clinical variables): cerebrovascular disease (OR, 0.78; 95%CI, 0.64-0.95; P = .01), anemia (OR, 0.64; 95%CI, 0.54-0.76; P < .0001), chronic kidney disease (OR, 0.65; 95%CI, 0.56-0.75; P < .0001), peripheral arterial disease (OR, 0.79; 95%CI, 0.65-0.96; P = .02), chronic lung disease (OR, 0.85; IC95%, 0.71-0.99; P = .05), and diabetes mellitus (OR, 0.85; 95%CI, 0.74-0.98; P < .03). The increase in the number of comorbidities (comorbidity burden) was associated with a reduction in coronary angiographies after adjusting for the GRACE score: 1 comorbidity (OR, 0.66; 95%CI, 0.54-0.81), 2 comorbidities (OR, 0.55; 95%CI, 0.45-0.69), 3 comorbidities (OR, 0.37; 95%CI, 0.29-0.47), 4 comorbidities (OR, 0.33; 95%CI, 0.24-0.45), ≥ 5 comorbidities (OR, 0.21; 95%CI, 0.12-0.36); all P values < .0001 compared to 0.
Conclusions: The number of coronary angiographies performed drops as the number of comorbidities increases in elderly patients with non-ST-segment elevation acute coronary syndrome. More studies are still needed to know what the best management of these patients should be.
Keywords: Comorbidities. Elderly. Acute coronary syndrome. Coronary angiography.
Resumen
Introducción y objetivos: La comorbilidad en ancianos con síndrome coronario agudo sin elevación del segmento ST empeora el pronóstico. El objetivo fue analizar la influencia de la carga de comorbilidad en la decisión del tratamiento invasivo en ancianos con SCASEST.
Métodos: Se incluyeron 7.211 pacientes mayores de 70 años procedentes de 11 registros españoles. Los datos se analizaron en una base de datos conjunta. Se evaluó la presencia de 6 enfermedades simultáneas y su asociación con la realización de coronariografía durante el ingreso.
Resultados: La edad media fue de 79 ± 6 años y la puntuación GRACE media fue de 150 ± 21 puntos. Fueron tratados de manera conservadora 1.179 pacientes (16%). La presencia de cada enfermedad se asoció con un menor abordaje invasivo (ajustado por variables clínicas predictivas): enfermedad cerebrovascular (odds ratio [OR] = 0,78; intervalo de confianza del 95% [IC95%], 0,64-0,95; p = 0,01), anemia (OR = 0,64; IC95%, 0,54-0,76; p < 0,0001), insuficiencia renal (OR = 0,65; IC95%, 0,56-0,75; p < 0,0001), arteriopatía periférica (OR = 0,79; IC95%, 0,65-0,96; p = 0,02), enfermedad pulmonar crónica (OR = 0,85; IC95%, 0,71-0,99; p = 0,05) y diabetes mellitus (OR = 0,85; IC95%, 0,74-0,98; p = 0,03). Asimismo, el aumento del número de enfermedades (carga de comorbilidad) se asoció con menor realización de coronariografías, ajustado por la escala GRACE: 1 enfermedad (OR = 0,66; IC95%, 0,54-0,81); 2 (OR = 0,55; IC95%, 0,45-0,69); 3 (OR = 0,37; IC95%, 0,29-0,47); 4 (OR = 0,33; IC95%, 0,24-0,45); ≥ 5 (OR = 0,21; IC95%, 0,12-0,36); todos p < 0,0001, en comparación con ninguna enfermedad.
Conclusiones: Conforme aumenta la comorbilidad disminuye la realización de coronariografías en ancianos con síndrome coronario agudo sin elevación del segmento ST. Se necesitan estudios que investiguen la mejor estrategia diagnóstico-terapéutica en estos pacientes.
Palabras clave: Comorbilidad. Ancianos. Síndrome coronario agudo. Coronariografía.
Abbreviations:
ACS: acute coronary syndrome. DM: diabetes mellitus. NSTEACS: non-ST-segment elevation acute coronary syndrome.
INTRODUCTION
Population ageing leads to an increase in the number of elderly patients who suffer non-ST-segment elevation acute coronary syndrome (NSTEACS). This population group, that has been misrepresented in large studies, has a great comorbidity burden that increases with age1 and an important impact on prognosis.2-4 The ideal therapeutic strategy for the management of these patients is still unknown. The benefit of an invasive strategy in elderly patients with NSTEACS and comorbidities is still unclear.5-9 In general, elderly patients with comorbidities undergo fewer coronary angiographies despite their worse prognosis.10 This clinical practice —apparently in contrast with the recommendations published in the clinical practice guidelines11— seems to be based on the perception of a scarce benefit due to the worse intrinsic prognosis associated with comorbidities.
In this study the data of 11 Spanish NSTEACS registries were collected to set up a common database with over 7000 elderly patients with NSTEACS. In this preliminary analysis, the objective was to study the impact of comorbidities on the decision to go with invasive approach.
METHODS
Study design
The study was conducted from 11 cohorts of Spanish registries of patients with NSTEACS (annex).2,12-20 All cases were included in a single database of patients with chest pain and a diagnosis of NSTEACS, > 70 years of age and with, at least, a 1-year follow-up.
ANNEX. Registries included in the study.
| Hospital Clínico Universitario, Valencia2 |
| Hospital Universitario Joan XXIII, Tarragona12 |
| Hospital Universitario de Bellvitge, Barcelona13 |
| Hospital Ramón y Cajal, Madrid14 |
| Hospital Universitario de San Juan, Alicante15 |
| LONGEVO multicenter registry16 |
| ACHILLES multicenter registry17 |
| Hospital Álvaro Cunqueiro, Vigo18 |
| Hospital Clínico Universitario, Santiago de Compostela19 |
| Hospital Universitario Vall d’Hebron, Barcelona20 |
| Hospital Universitario de La Princesa, Madrid* |
|
* Unpublished data. |
The anthropometric and social-demographic data, main cardiovascular risk factors, and analytical and hemodynamic data at admission or during hospitalization were registered.
Patients were treated according to each center routine clinical practice and the decision to treat the NSTEACS invasively, with or without a coronary angiography, was left to the discretion of the treating physician. The 6-month mortality GRACE risk score was determined in all the patients.21
A total of 6 conditions that proved to have a higher prognostic impact on elderly patients hospitalized due to acute coronary syndrome (ACS) in a previous study were included:22 renal failure (glomerular filtration rate < 60mL/min/1.73m2), anemia (hemoglobin levels < 11 g/dL), diabetes mellitus (DM), cerebrovascular disease, peripheral arterial disease, and chronic pulmonary disease.
Endpoints
The study primary endpoint was to assess how the presence of comorbidities impacted the decision to perform a coronary angiography during admission.
Statistical analysis
Categorical variables were expressed as absolute values (percentages) and compared using the unpaired Student t test or the ANOVA. The continuous ones were expressed as mean ± standard deviation and compared using the chi-square test.
Initially, the correlation between each disease and the performance of a coronary angiography through univariable analysis were assessed. Then, a first binary logistics regression model was conducted including the 6 conditions and the clinical variables associated with the performance of the coronary angiography in the univariable analysis. The odds ratio (OR) and the 95% confidence intervals (95%CI) were estimated. Afterwards, patients were classified according to their comorbidity burden, defined by the number of concomitant conditions (from 0 to 6). A second logistics regression model was conducted where comorbidity burden was adjusted for the predictive clinical variables in the previous analysis. Finally, a third logistics regression model was conducted where the comorbidity burden was adjusted based on the GRACE risk score. Differences were considered statistically significant with P values < .05
RESULTS
A total of 7211 patients with a mean age of 79 ± 6 years were included; 62% were males. Table 1 shows the population baseline characteristics. The prevalence of comorbidities was DM in 2874 patients (40%), chronic kidney disease in 3070 patients (42.6%), anemia in 1025 (14.2%), peripheral arterial disease in 1006 (14%), chronic pulmonary disease in 1161 (16%), and previous stroke in 831 (11.5%).
Table 1. Differences in the baseline characteristics based on the therapeutic approach
| All N = 7211 | Conservative approach N = 1 179 (16) | Invasive approach N = 6 032 (84) | P | |
|---|---|---|---|---|
| Age (years) | 79 ± 6 | 82 ± 6 | 78 ± 5 | .001 |
| Males | 4 441 (61.6) | 597 (50.6) | 3 844 (63.7) | .0001 |
| Smoking | 621 (8.6) | 72 (6.1) | 549 (9.1) | .0001 |
| Hypertension | 5 723 (79.4) | 943 (80) | 4 780 (79.2) | .58 |
| Dyslipidemia | 4 262 (59) | 609 (51.7) | 3 653 (60.6) | .0001 |
| Previous myocardial infarction | 1 682 (23.3) | 371 (31.7) | 1 308 (21.7) | .0001 |
| Pervious percutaneous coronary intervention | 1 334 (19) | 175 (14.8) | 1 159 (19.2) | .0001 |
| Previous coronary surgery | 573 (7.9) | 104 (8.8) | 469 (7.8) | .24 |
| Previous heart failure | 641 (8.9) | 198 (16.8) | 443 (7.3) | .0001 |
| Killip ≥ 2 | 1 889 (26.2) | 463 (39.3) | 1 426 (23.6) | .0001 |
| ST-segment depression | 2 638 (36.6) | 396 (33.6) | 2 242 (37.2) | .02 |
| High troponin levels | 5 319 (73.7) | 920 (78) | 4 399 (73) | .001 |
| Left ventricular ejection fraction (%) | 54 ± 11 | 54 ± 12 | 55 ± 11 | .03 |
| GRACE | 150 ± 21 | 159 ± 21 | 147 ± 19 | .0001 |
| Comorbidities | ||||
| Anemia | 1 025 (14.2) | 273 (23.2) | 752 (12.5) | .0001 |
| Peripheral arterial disease | 1 006 (14) | 196 (16.6) | 810 (13.4) | .04 |
| Chronic pulmonary disease | 1 161 (16.1) | 210 (17.8) | 951 (15.8) | .08 |
| Diabetes mellitus | 2 874 (39.9) | 522 (44.3) | 2 352 (39) | .0001 |
| Cerebrovascular disease | 831 (11.5) | 186 (15.8) | 645 (10.7) | .0001 |
| Chronic kidney disease | 3 070 (42.6) | 716 (60.7) | 2 354 (39) | .0001 |
|
Data are expressed as no. (%) or mean ± standard deviation. |
||||
During admission 6032 patients (84%) underwent a coronary angiography. A total of 4339 patients (60%) were revascularized: 3848 (53%) of them through percutaneous coronary intervention and 491 (7%) through surgery. Patients on conservative management (1179, 16%) were predominantly women with higher scores in the GRACE score, and a past medical history of infarction or heart failure. Conversely, smoking and high levels of troponins or ST-segment depressions on the electrocardiogram performed at admission and a previous percutaneous coronary intervention were associated with a higher invasive approach (table 1). The GRACE risk score was lower in patients who underwent catheterization (147 ± 19 vs 159 ± 21; P = .0001).
The presence of each of the 6 conditions studied was associated with fewer coronary angiographies performed: chronic kidney disease, 60.7% vs 39% (P = .0001); anemia, 23.2% vs 12.5% (P = .0001); DM, 44.3% vs 39% (P = .0001); cerebrovascular disease, 15.8% vs 10.7% (P = .0001); peripheral arterial disease, 16.6% vs 13.4% (p = .04); and chronic pulmonary disease, 17.8% vs 15.8% (P = .08) (table 1).
In the multivariable analysis adjusted for the main cardiovascular risk factors and clinical variables that were statistically significant in the univariable analysis, the 6 conditions associated with a lower probability of an indication for coronary angiography were: cerebrovascular disease, OR, 0.78 (IC95%, 0.64-0.95; P = .01); anemia, OR, 0.64 (IC95%, 0.54-0.76; P < .0001); chronic kidney disease, OR, 0.65 (IC95%, 0.56-0.75; P < .0001); peripheral arterial disease, OR, 0.79 (IC95%, 0.65-0.96; P = .02); chronic pulmonary disease, OR, 0.85 (IC95%, 0.71-0.99; P = .05); and DM, OR, 0.85 (IC95%, 0.74-0.98; P = .03). Table 2 shows the clinical variables associated with the indication for coronary angiography.
Table 2. Results: multivariable analysis for the indication of a coronary angiography
| Variable | OR | 95%CI | P | |
|---|---|---|---|---|
| Age (years) | 0.89 | 0.88-0.91 | .0001 | |
| Males | 1.48 | 1.28-1.71 | .0001 | |
| Dyslipidemia | 1.44 | 1.26-1.66 | .0001 | |
| Previous myocardial infarction | 0.46 | 0.39-0.54 | .0001 | |
| Previous heart failure | 0.68 | 0.56-0.84 | .0001 | |
| Previous percutaneous coronary intervention | 1.91 | 1.55-2.34 | .0001 | |
| Killip ≥ 2 | 0.68 | 0.56-0.80 | .0001 | |
| ST-segment depression | 1.44 | 1.25-1.66 | .0001 | |
| Left ventricular ejection fraction (by 5%) | 0.98 | 0.98-0.99 | .001 | |
| Anemia | 0.64 | 0.54-0.76 | .0001 | |
| Peripheral artery disease | 0.79 | 0.65-0.96 | .02 | |
| Chronic pulmonary disease | 0.85 | 0.71-0.99 | .05 | |
| Diabetes mellitus | 0.85 | 0.74-0.98 | .03 | |
| Cerebrovascular disease | 0.78 | 0.64-0.95 | .01 | |
| Chronic kidney disease | 0.65 | 0.56-0.75 | .0001 | |
|
95%CI: 95% confidence interval; OR: odds ratio. |
||||
Comorbidity burden was defined as the number of present conditions (from 0 to 6). This was their distribution: 0 conditions, N = 1891 (26%); 1 condition, N = 2413 (33.5%); 2 conditions, N = 1638 (22.7%); 3 conditions, N = 879 (12.2%); 4 conditions, N = 314 (4.4%); and 5 or 6 conditions, N = 76 (1.1%). The analysis of the comorbidity burden adjusted for the clinical variables associated with the indication for coronary angiography showed a negative correlation between the number of conditions and the probability to perform a coronary angiography: 1 condition, OR, 0.66 (95%CI, 0.54-0.81); 2 conditions, OR, 0.55 (95%CI, 0.45-0.69); 3 conditions, OR, 0.37 (95%CI, 0.29-0.46); 4 conditions, OR, 0.32 (95%CI, 0.23-0.45); and 5 or 6 conditions, OR, 0.21 (95%CI, 0.12-0.37); All P values < .0001 compared to no condition.
With more conditions, higher GRACE risk scores (table 3). The negative correlation between the comorbidity burden and the performance of the coronary angiography was kept after adjusting for the GRACE risk score. Figure 1 shows that with more conditions, the probability to perform a coronary angiography increased too (figure 1A) despite the higher risk posed by higher GRACE risk scores (figure 1B, table 3).
Table 3. Distribution of comorbidity burden and the score obtained in the GRACE risk score (P < .0001 for the tendency)
| Conditions | N = 7 211 | GRACE risk score |
|---|---|---|
| 0 | 1891 (26) | 141 ± 18 |
| 1 | 2413 (33.5) | 148 ± 19 |
| 2 | 1638 (22.7) | 153 ± 20 |
| 3 | 879 (12.2) | 160 ± 19 |
| 4 | 314 (4.4) | 162 ± 19 |
| ≥ 5 | 76 (1.1) | 166 ± 17 |
|
Data are expressed as no. (%) or mean ± standard deviation. |
||
Figure 1. A: chances of undergoing a coronary angiography based on the number of concomitant conditions. The odds ratio (OR) with a 95% confidence interval (95%IC) can be seen. Analysis adjusted for the GRACE risk score. B: Representation of the correlation between comorbidity burden and the GRACE risk score. The mean ± standard deviation (SD) of the GRACE risk score can be seen.
DISCUSSION
The main findings of our study were: a) the 6 conditions studied (cerebrovascular disease, anemia, chronic kidney disease, peripheral arterial disease, chronic pulmonary disease, and DM) were independently associated with a lower probability to use the invasive approach; b) with higher comorbidity burdens, considered as the number of concomitant conditions, lower chances of performing coronary angiographies.
There is a high prevalence of comorbidities in elderly patients with NSTEACS that greatly impacts prognosis in the short and mid-term.2,4 The Charlson index is the most commonly used tool to assess comorbidities.23,24 However, the analysis of the 6 conditions studied (chronic kidney disease, anemia, DM, cerebrovascular disease, peripheral arterial disease, and chronic pulmonary disease) has proven to be a useful risk stratification tool and have great predictive discriminatory capabilities that are similar to the Charlson index.22
Comorbidity burden is very important for the in-hospital management of NSTEACS.2,3,6,9,10 Although the optimal therapeutic strategy for the management of elderly patients with NSTEACS is still unknown, several studies show certain benefits with revascularization.5,7,8,25-30
Our study shows that with higher comorbidity burdens, lower chances of undergoing coronary angiographies. This may be due to the fact that comorbidities are seen as contraindications for the invasive approach.10 However, the risk of suffering an acute myocardial infarction according to the GRACE risk score increases parallel to the number of concomitant conditions. Actually, these may be the patients who would benefit the most from an invasive approach.31,32
The presence of each one of these 6 conditions was independently associated with fewer invasive approaches. On the one hand, cerebrovascular disease and peripheral arterial disease are responsible for a greater spread of atherosclerotic disease.33 Anemia has proven to be a powerful predictor of mortality in the ACS setting;34-36 we used the 11 g/dL threshold as the cut-off value that had the greatest impact on mortality in former studies.34 Its specific weight in the decision to administer conservative treatment may be justified by its clear association with the occurrence of hemorrhagic events in the ACS setting.37 Chronic kidney disease is an expression of a greater spread of cardiovascular disease and is independently associated with more mortality after an ACS. There is a linear correlation between the risk of death due to cardiovascular causes and lower glomerular filtration rates.17,38 DM is a powerful predictor of mortality, and not only due to cardiovascular causes. There is a clear correlation between DM and major adverse cardiovascular events, and these are patients at very high risk.39 Chronic pulmonary disease is associated with a worse short-term prognosis after an acute myocardial infarction. Also, in the management of NSTEACS it is associated with diagnostic delays, fewer invasive approaches, and a lower use of drugs for secondary prevention purposes.40
In the multivariable analysis, age, previous acute myocardial infarctions, previous heart failure, Killip class ≥ 2 at admission, and a reduced ejection fraction were associated with fewer invasive approaches. Elderly patients receive fewer evidence-based therapies. The older the age, the lower the rate of performing coronary angiographies.41 On top of age, a past medical history of infarction, heart failure, a reduced ejection fraction, and scores ≥ 2 in the Killip classification are important aspects in the prognosis of ACS that, in general, translate into a worse ventricular function. Paradoxically, our findings suggest that the higher the risk, the lower the chances of performing a coronary angiography. Actually, these findings are consistent with former studies published.10,42 It is possible that the perception of fewer benefits from revascularization or higher risk in the revascularization procedures may explain these results.2 On the other hand, male sex, dyslipidemia, previous percutaneous coronary interventions, and ST-segment depressions at admission were associated with more invasive approaches. Several studies suggest that women undergo fewer invasive approaches compared to men despite the mortality benefits seen.43 Previous angioplasties, ST-segment depressions, and dyslipidemia are probably interpreted as ischemic risk factors, which may explain their association with a higher frequency of invasive approaches.20,28,44
Limitations
The main limitation of our study is that it is an observational registry with its corresponding selection bias and differences in the management of patients depending on the different centers involved. On the other hand, although the multivariable model was adjusted for percutaneous coronary intervention or previous coronary surgeries, it was not adjusted for previous coronary angiographies. It is possible that the previous knowledge of the coronary anatomy impacted the decision to perform fewer coronary angiographies in patients at higher risk.
CONCLUSIONS
The presence of comorbidities greatly impacts the therapeutic decision in elderly patients with ACS. With more conditions, higher GRACE risk scores, and lower chances of indicating a coronary angiography
This paradox of higher-risk and more conservative treatment justifies conducting new studies to determine the benefits of the invasive strategy in elderly patients with NSTEACS and comorbidities to establish the best therapeutic decision.
FUNDING
This article was funded by a grant from the Carlos III Health Institute: CIBERCV 16/11/00420, Madrid, Spain.
CONFLICTS OF INTEREST
J. Sanchis is an associate editor of REC: Interventional Cardiology; the journal’s editorial procedure to ensure impartial handling of the manuscript has been followed. J. Núñez has received funding from Novartis, Vitor Pharma, and Boehringer Ingelheim, and a grant from Astra Zeneca and Vitor Pharma. J.A. Barrabés has received funding for the educational activities conducted for AstraZeneca, and for his job as consultor for Bayer. The remaining authors did not declare any conflicts of interest whatsoever.
WHAT IS KNOWN ABOUT THE TOPIC?
- Elderly patients with NSTEACS have a higher comorbidity burden. Concomitant conditions are associated with worse prognosis. Elderly patients with comorbidities undergo fewer coronary angiographies despite their worse prognosis, which is in sharp contrast with the recommendations published in the clinical practice guidelines.
WHAT DOES THIS STUDY ADD?
- This analysis of a multicenter registry shows the correlation between comorbidity burden and invasive therapeutic approach in elderly patients with NSTEACS. With more concomitant conditions, higher GRACE risk scores, but lower chances of indicating a coronary angiography.
REFERENCES
1. Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education:a cross-sectional study. Lancet. 2012;380:37-43.
2. Sanchis J, Núñez J, BodíV, Núñez E, García-Alvarez A, Bonanad C, et al. Influence of comorbid conditions on one-year outcomes in non-ST-segment elevation acute coronary syndrome. Mayo Clin Proc. 2011;86:291-296.
3. Chirinos JA, Veerani A, Zambrano JP, Schob A, Perez G, Mendez AJ, et al. Evaluation of comorbidity scores to predict all-cause mortality in patients with established coronary artery disease. Int J Cardiol. 2007;117:97-102.
4. Sanchis J, Bonanad C, Ruiz V, Fernández J, García-Blas S, Mainar L, et al. Frailty and other geriatric conditions for risk stratification of older patients with acute coronary syndrome. Am Heart J. 2014;168:784-791.
5. Bardaji A, Barrabés JA, Ribera A, et al. Revascularization in older adult patients with non-ST-segment elevation acute coronary syndrome:effect and impact on 6-month mortality [published online ahead of print, 2019 May 14]. Eur Heart J Acute Cardiovasc Care. 2019;2048∖19849922.
6. Chuang AM, Hancock DG, Halabi A, et al. Invasive management of acute coronary syndrome:Interaction with competing risks. Int J Cardiol. 2018; 269:13-18.
7. Tegn N, Abdelnoor M, Aaberge L, et al. Invasive versus conservative strategy in patients aged 80 years or older with non-ST-elevation myocardial infarction or unstable angina pectoris (After Eighty study):an open-label randomized controlled trial. Lancet. 2016;387:1057-1065.
8. Sanchis J, Núñez E, Barrabés JA, et al. Randomized comparison between the invasive and conservative strategies in comorbid elderly patients with non-ST elevation myocardial infarction. Eur J Intern Med. 2016;35:89-94.
9. Palau P, Núñez J, Sanchis J, et al. Differential prognostic effect of revascularization according to a simple comorbidity index in high-risk non-ST- segment elevation acute coronary syndrome. Clin Cardiol. 2012;35:237-243.
10. Savonitto S, Morici N, De Servi S. Treatment of acute coronary syndromes in the elderly and in patients with comorbidities. Rev Esp Cardiol. 2014;67:564-573.
11. Roffi M, Patrono C, Collet JP, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation:Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:267-315.
12. Camprubi M, Cabrera S, Sans J, et al. Body Mass Index and Hospital Mortality in Patients with Acute Coronary Syndrome Receiving Care in a University Hospital. J Obes. 2012;2012:287939.
13. Ariza-SoléA, Sánchez-Salado JC, Lorente V, et al. Is it possible to separate ischemic and bleeding risk in patients with non-ST segment elevation acute coronary syndromes?Int J Cardiol. 2014;171:448-450.
14. Alonso Salinas GL, Sanmartín Fernández M, Pascual Izco M, et al. Frailty predicts major bleeding within 30 days in elderly patients with Acute Coronary Syndrome. Int J Cardiol. 2016;222:590-593.
15. Cordero A, López-Palop R, Carrillo P, et al. Prevalence and Postdischarge Incidence of Malignancies in Patients With Acute Coronary Syndrome. Rev Esp Cardiol. 2018;71:267-273.
16. Alegre O, Formiga F, López-Palop R, et al. An Easy Assessment of Frailty at Baseline Independently Predicts Prognosis in Very Elderly Patients With Acute Coronary Syndromes. J Am Med Dir Assoc. 2018;19:296-303.
17. Rivera-Caravaca JM, Ruiz-Nodar JM, Tello-Montoliu A, et al. Disparities in the Estimation of Glomerular Filtration Rate According to Cockcroft-Gault, Modification of Diet in Renal Disease-4, and Chronic Kidney Disease Epidemiology Collaboration Equations and Relation With Outcomes in Patients With Acute Coronary Syndrome. J Am Heart Assoc. 2018;7:e008725.
18. Abu-Assi E, Raposeiras-Roubin S, Cobas-Paz R, et al. Assessing the performance of the PRECISE-DAPT and PARIS risk scores for predicting one-year out-of-hospital bleeding in acute coronary syndrome patients. EuroIntervention. 2018;13:1914-1922.
19. Álvarez Álvarez B, Abou Jokh Casas C, Cordero A, et al. Early revascularization and long-term mortality in high-risk patients with non-ST-elevation myocardial infarction. The CARDIOCHUS-HUSJ registry. Rev Esp Cardiol. 2020;73:35-42.
20. MilàL, Barrabés JA, Lidón RM, et al. Prior adherence to recommended lipid control targets in patients admitted for acute coronary syndrome. Rev Esp Cardiol. 2019;73:376-382.
21. Eagle KA, Lim MJ, Dabbous OH, et al. A validated prediction model for all forms of acute coronary syndrome:estimating the risk of 6-month postdischarge death in an international registry. JAMA. 2004:291:2727-2733.
22. Sanchis J, Soler M, Núñez J, et al. Comorbidity assessment for mortality risk stratification in elderly patients with acute coronary syndrome. Eur J Intern Med. 2019;62:48-53.
23. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies:development and validation. J Chronic Dis. 1987;40:373-383.
24. Núñez J, Núñez E, Fácila L, et al. Prognostic value of Charlson comorbidity index at 30 days and 1 year after acute myocardial infarction. Rev Esp Cardiol. 2004;57:842-849.
25. De Servi S, Cavallini C, Dellavalle A, et al. Non-ST-elevation acute coronary syndrome in the elderly:treatment strategies and 30-day outcome. Am Heart J. 2004;147:830-836.
26. Sillano D, Resmini C, Meliga E, et al. Retrospective multicenter observational study of the interventional management of coronary disease in the very elderly:the NINETY. Catheter Cardiovasc Interv. 2013;82:414-421.
27. Kolte D, Khera S, Palaniswamy C, et al. Early invasive versus initial conservative treatment strategies in octogenarians with UA/NSTEMI. Am J Med. 2013;126:1076-1083.
28. Núñez J, Ruiz V, Bonanad C, et al. Percutaneous coronary intervention and recurrent hospitalizations in elderly patients with non ST-segment acute coronary syndrome:The role of frailty. Int J Cardiol. 2017;228:456-458.
29. LlaóI, Ariza-SoléA, Sanchis J, et al. Invasive strategy and frailty in very elderly patients with acute coronary syndromes. EuroIntervention. 2018;14:e336-342.
30. Sanchis J, Ariza-SoléA, Abu-Assi E, et al. Invasive Versus Conservative Strategy in Frail Patients With NSTEMI:The MOSCA-FRAIL Clinical Trial Study Design. Rev Esp Cardiol. 2019;72:154-159.
31. de Groot V, Beckerman H, Lankhorst GJ, et al. How to measure comorbidity. A critical review of available methods. J Clin Epidemiol. 2003;56:221-229.
32. Park JY, Kim MH, Bae EJ, et al. Comorbidities can predict mortality of kidney transplant recipients:comparison with the Charlson comorbidity index. Transplant Proc. 2018;50:1068-1073.
33. Chirinos JA, Veerani A, Zambrano JP, et al. Evaluation of comorbidity scores to predict all-cause mortality in patients with established coronary artery disease. Int J Cardiol. 2007;117:97-102.
34. Lawler PR, Filion KB, Dourian T, et al. Anemia and mortality in acute coronary syndromes:a systematic review and meta-analysis. Am Heart J. 2013;165:143-153.
35. Ford I, Bezlyak V, Stott DJ, et al. Reduced glomerular filtration rate and its association with clinical outcome in older patients at risk of vascular events:secondary analysis. PLoS Med. 2009;6:e16.
36. Sabatine MS, Morrow DA, Giugliano RP, et al. Association of hemoglobin levels with clinical outcomes in acute coronary syndromes. Circulation. 2005;111:2042-2049.
37. Vicente-Ibarra N, Marín F, Pernias-Escrig V, et al. Impact of anemia as risk factor for major bleeding and mortality in patients with acute coronary syndrome. Eur J Intern Med. 2019;61:48-53.
38. Goldenberg I, Subirana I, Boyko V, et al. Relation between renal function and outcomes in patients with non-ST-segment elevation acute coronary syndrome:real-world data from the European Public Health Outcome Research and Indicators Collection Project. Arch Intern Med. 2010;170:888-895.
39. Rao Kondapally Seshasai S, Kaptoge S, Thompson A, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death [published correction appears in N Engl J Med. 2011;364:1281]. N Engl J Med. 2011;364:829-841.
40. Rothnie KJ, Smeeth L, Herrett E, et al. Closing the mortality gap after a myocardial infarction in people with and without chronic obstructive pulmonary disease. Heart. 2015;101:1103-1110.
41. Avezum A, Makdisse M, Spencer F, et al. Impact of age on management and outcome of acute coronary syndrome:observations from the Global Registry of Acute Coronary Events (GRACE). Am Heart J. 2005;149:67-73.
42. Itzahki Ben Zadok O, Ben-Gal T, Abelow A, et al. Temporal Trends in the Characteristics, Management and Outcomes of Patients With Acute Coronary Syndrome According to Their Killip Class. Am J Cardiol. 2019;124:1862-1868.
43. Mehta LS, Beckie TM, DeVon HA, et al. Acute Myocardial Infarction in Women:A Scientific Statement From the American Heart Association. Circulation. 2016;133:916-947.
44. Yudi MB, Clark DJ, Farouque O, et al. Trends and predictors of recurrent acute coronary syndrome hospitalizations and unplanned revascularization after index acute myocardial infarction treated with percutaneous coronary intervention. Am Heart J. 2019;212:134-143.

ABSTRACT
Introduction and objectives: The STENTYS Xposition S stent (STENTYS S.A, Paris, France) is the only self-apposing sirolimus- eluting stent available in the market. The stent features make it useful to treat challenging lesions with proximal-distal different vessel diameter, ectasia, high thrombus burden, bifurcation lesions including the left main coronary artery or vein grafts. We describe our own experience with the use of this stent and evaluate its efficacy and safety profile.
Methods: We included all consecutive patients treated with the STENTYS Xposition S from January 2018 to October 2019. All coronary lesions were quantified using QCA (quantitative coronary angiography).
Results: A total of 62 lesions in 50 patients were treated with the STENTYS Xposition S. The median age of the patients was 66 years (49-92). The most common clinical presentation was ST-segment elevation acute coronary syndrome in 23 patients (46%). Ectasia and significant vessel diameter variance were the most common scenario in 72.6% of cases and bifurcation in the remaining 27.4% (2 of them in the left main coronary artery). Pre-dilatation was performed in 32 lesions (51.6%) and post-dilatation in 37 (59.7%). Angiographic success was achieved in all patients except for 1. At the median 373-day follow-up (256-439), 1 patient had an acute myocardial infarction 3 months after the percutaneous intervention and 1 patient died due to cardiac failure during admission. There were no cases of definitive stent thrombosis or target lesion revascularization.
Conclusions: The STENTYS Xposition S self-apposing stent showed good angiographic and clinical outcomes in our real-world experience.
Keywords: Self-apposing stent. Coronary lesion. Major adverse cardiovascular events.
RESUMEN
Introducción y objetivos: El stent STENTYS Xposition S (STENTYS S.A., París, Francia) es el único stent autoexpandible liberador de sirolimus disponible en el mercado. Sus características hacen que resulte útil en lesiones que presentan gran diferencia del diámetro del vaso proximal-distal, ectasia, alta carga de trombo o que se encuentren en bifurcaciones e injertos venosos. Describimos nuestra experiencia con el uso de este tipo de stent, evaluando su seguridad y eficacia.
Métodos: Se incluyeron todos los pacientes consecutivos tratados con STENTYS desde enero de 2018 hasta octubre de 2019. Todas las lesiones coronarias fueron cuantificadas por angiografía coronaria cuantitativa.
Resultados: Se trataron con STENTYS Xposition S 62 lesiones en 50 pacientes. La mediana de edad de los pacientes fue de 66 años (49-92). La clínica de presentación más frecuente fue el síndrome coronario agudo con elevación del segmento ST en 23 pacientes (46%). La ectasia coronaria y la gran diferencia en los diámetros proximal y distal a la lesión fue la indicación más frecuente para el uso de este tipo de stent, en el 72,6% de los casos, seguida del intervencionismo sobre bifurcación en el 27,4% de los pacientes (2 de ellos en el tronco coronario izquierdo). Se realizó predilatación en 32 lesiones (51,6%) y posdilatación en 37 (59,7%). Se logró el éxito angiográfico en todos los pacientes excepto en 1. Tras una mediana de seguimiento de 373 días (256-439), 1 paciente presentó infarto agudo de miocardio a los 3 meses y 1 paciente falleció durante el ingreso por insuficiencia cardiaca. No hubo ningún caso de trombosis definitiva del stent ni de revascularización de la lesión tratada.
Conclusiones: En nuestra experiencia de la vida real, el stent STENTYS Xposition S demostró un buen resultado angiográfico y clínico.
Palabras clave: Stent autoexpandible. Lesión coronaria. Eventos cardiovasculares adversos mayores.
Abbreviations
LMCA: left main coronary artery. MACE: major adverse cardiovascular events.
INTRODUCTION
The STENTYS Xposition S (STENTYS S.A., Paris, France) is a sirolimus-eluting self-expanding nitinol stent designed to adapt its size to the vessel diameter and facilitate its complete apposition when exerting chronic strength towards the outside. It has long been confirmed that one of the most important factors of stent thrombosis is the incorrect apposition of the stent.1 The characteristics of this stent make it especially useful to revascularize acute coronary syndromes (ACS), especially ST-segment elevation acute coronary syndromes with lesions with high thrombotic load. Also, a potential benefit in ectatic coronary vessels and lesions with great proximal and the distal diameter mismatch has been confirmed, bifurcations (left main coronary artery [LMCA] included), and venous grafts.
The objective of this study was to assess the benefit of this stent in the routine clinical practice by analyzing the type of lesions this stent is used with and the immediate angiographic results and at the clinical follow-up.
METHODS
A cohort of consecutive patients treated with the STENTYS Xposition S stent was analyzed from January 2018 through October 2019 in a tertiary hospital where over 1000 percutaneous coronary interventions are performed each year. All coronary lesions were quantified using a quantitative coronary angiography. Lesions in vessels with changes in size (ectasia or proximal-distal diameter mismatch of the lesion), in a bifurcation, in the presence of a high thrombotic load or in a venous graft were analyzed. The interventional strategy to be followed, imaging modalities included, was left to the operator’s criterion. The clinical and follow-up data were obtained from the electronical clinical records of the healthcare system of our autonomous community. All events were defined in a standard way according to the Academic Research Consortium-2 (ARC-2) consensus document.2
Patients' informed consent was obtained to the interventional procedure and, subsequently, verbal informed consent was given during the follow-up.
The data analysis was conducted using the IBM SPSS 20.0 statistical software package. Continuous variables were expressed as mean ± standard deviation or median with interquartile range depending on whether they followed a normal distribution or not, respectively. Qualitative variables were expressed as relative percentage. The cumulative incidence of events at the follow-up was estimated.
RESULTS
From January 2018 through September 2019, 1692 percutaneous coronary interventions with stent implantation were performed. The STENTYS Xposition S stent was used in 50 patients (62 lesions). The patients’ median age was 66 years [49-92]. Eighty-eight per cent of the patients were males. Table 1 shows the clinical characteristic of patients and coronary lesions. The most common clinical presentation was ST-segment elevation acute coronary syndrome in 23 patients (46%) followed by non-ST-segment elevation acute coronary syndrome in 22 patients (44%), and stable angina in 5 patients (10%). According to the classification established by the American College of Cardiology/American Heart Association the most common type of lesion was B1 lesion (38.7%). The right coronary artery was the most frequently treated vessel in 33 patients (53.2%).
Table 1 Clinical characteristics of patients and angiographic characteristics of the lesions
| Patients (N) | 50 |
| Age (years) | 66.6 (49-92) |
| Males | 44 (88%) |
| Arterial hypertension | 33 (66%) |
| Body mass index (kg/m2) | 27.9 ± 4.9 |
| Dyslipidemia | 32 (64%) |
| Diabetes mellitus | 12 (24%) |
| Smoking | 27 (54%) |
| Family history of ischemic heart disease | 3 (6%) |
| Peripheral vasculopathy | 3 (6%) |
| Atrial fibrillation | 6 (12%) |
| Chronic pulmonary disease | 6 (12%) |
| Kidney disease | 6 (12%) |
| Stable angina pectoris | 5 (10%) |
| NSTEACS | 22 (44%) |
| STEACS | 23 (46%) |
| Lesions (N) | 62 |
| Lesion length (mm) | 14.56 ± 3.64 |
| Reference diameter (mm) | 4.1 ± 0.8 |
| Percent stenosis. QCA (%) | 70.08 ± 17 |
| Location of the lesion | |
| Left main coronary artery | 3 (4.8) |
| Left anterior descending coronary artery | 11 (17.7) |
| Left circumflex artery | 15 (24.2) |
| Right coronary artery | 33 (53.2) |
| Classification of the lesion | |
| A | 0 |
| B1 | 24 (38.8) |
| B2 | 19 (30.6) |
| C | 19 (30.6) |
| Indication for STENTYS | |
| Ectasia. Proximal-distal diameter mismatch | 45 (72.6) |
| Bifurcation | 17 (27.4) |
| Provisional stenting technique | 15 (88.2) |
| Double stent technique | 2 (11.8) |
|
NSTEACS, non-ST-segment elevation acute coronary syndrome; QCA, quantitative coronary angiography; STEACS, ST-segment elevation acute coronary syndrome. Kidney damage: glomerular filtration rate < 60 mL/min/1.73 m2. Data are expressed as N (%) o mean ± standard deviation. |
|
Ectasia and great proximal-distal diameter mistmatch at the lesion were the main indication for the use of this stent, in 72.6% of the lesions, with a mean vessel reference diameter of 4.1 mm ± 0.8 mm. A certain size was required to use this type of stent. The percutaneous coronary interventional on a bifurcation was the second most common indication, in 27.4% of the patients (2 of them on the LMCA). The most common type of bifurcation according to the Medina classification was 1-1-0, in 9 cases (52.9%). The secondary branch was damaged in 17% of the patients. The provisional stenting technique was the most widely used in 15 cases (88.2% of bifurcations) re-crossing to the secondary branch in 9 of them (60%). The dilatation of the secondary branch only occurred in 7 patients and only in the other 2 stents were implanted: one in a 0-1-1 bifurcation according to the Medina classification (minicrash technique) and the other in a 1-1-1 bifurcation according to this classification (TAP technique [T-and protrusion technique]). In both cases the STENTYS Xposition S stent was implanted in the main vessel and a non-self-apposing stent in the secondary branch (figure 1).
Figure 1. A: lesion with significant thrombotic load in the mid right coronary artery, which remains after thrombus aspiration. B: 3.5-4.5 mm × 27 mm Xposition S direct stent implantation. C: final angiographic result. D: significant stenosis in distal left main coronary artery. E: 3-3.5 mm × 27 mm Xposition S stent implantation from the proximal left main coronary artery to the left anterior descending coronary artery. F: angiographic result after postdilatation.
A high thrombotic load (Thrombolysis in Myocardial Infarction flow grade 4-5) was seen in 8 lesions. All of them in ectatic coronary vessels or with proximal-distal caliber mismatch. No case of venous graft treated with STENTYS was reported.
Predilatation occurred in 32 lesions (51.6%) and postdilatation in 37 (59.7%). The criterion used for postdilatation was angiography guided visual underexpansion. Intravascular ultrasound was performed in 15 patients (30%) before the implant. It was also used in 2 patients to optimize the percutaneous coronary intervention given the persistent stent underexpansion seen on the angiography. In both cases the minimum lumen area was > 5.5 mm2 with stent expansion > 80% and lack of incomplete apposition (defined as a strut separation of > 0.4 mm axial and 1 mm longitudinal) (figure 2). The optical coherence tomography was performed in a patient with ST-segment elevation acute coronary syndrome before and after the implant. It revealed a high thrombotic load with lack of immediate stent malapposition.
Figure 2. A: acute thrombotic occlusion in left circumflex artery with Thrombolysis in Myocardial Infarction flow grade 0. B: the intravascular ultrasound shows a great deal of thrombus in the lesions despite thrombus aspiration. C: implantation of 2 3.5-4.5 mm × 27 mm Xposition S overlapping stents. D: the intravascular ultrasound performed after stent implantation confirms the good angiographic results and lack of stent malapposition.
Angiographic success was achieved (with the stent properly implanted, a residual lesion ≤ 10%, and Thrombolysis in Myocardial Infarction flow grade 3) in all patients but 1, in whom stent implantation failed in a severely calcified LMCA lesion. In this case, predilatation was first attempted using a conventional balloon and then a cutting balloon on the LMCA severe distal lesion. A 3.3-4.5 mm × 22 mm STENTYS Xposition S stent was implanted with stent loss during retrieval, which remained braced to the guide catheter. Afterwards, a balloon-expandable drug-eluting stent was successfully implanted. The un-crimped stent was retrieved by crossing a guidewire from the femoral access through the stent distal struts. It was finally captured with a snare.
The median score obtained in the PRECISE-DAPT risk calculator (Predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy) was 16.5 (7-25), and the median score obtained in the DAPT index (Dual antiplatelet therapy) was 1.15 (−2-4). Ticagrelor was the most commonly used P2Y12 inhibitor (58.1%). A 12-month course of dual antiplatelet therapy was prescribed in 48 patients (96%).
After a median follow-up of 373 days (256-439), 1 patient had an acute myocardial infarction 3 months after the intervention. However, the coronary angiography did not reveal coronary artery disease progression but confirmed the good results of the previous intervention. An 84-year-old woman died at admission due to heart failure. Three patients died of non-cardiac causes: 1 due to septic shock at admission, the other patient died 6 months after the percutaneous coronary intervention due to high-grade lymphoma, and the third one 4 months after the percutaneous coronary intervention due to lung cancer. No cases of definitive stent thrombosis or revascularization of the treated lesion were reported. No bleeding was seen either at the follow-up.
DISCUSSION
This type of stent is not widely used in our setting and we believe 2 are the reasons why. The first one is the need for a learning curve to know how to handle this implant. In former iterations of the device, the delivery system had some technical limitations like the jumping phenomenon that could occur right when the sheath was being released due to the elastic properties of nitinol. Unlike its predecessor (STENTYS sirolimus DES), the stent of the new STENTYS Xposition S system, is mounted over a semicompliant balloon and covered by a 0.0032 in-thick sheath. The reason for balloon inflation is not to dilate the stent, but to rupture the external sheath from the distal to the proximal border to allow a proper vessel-wall stent apposition. This has reduced the complexity of the release mechanism.3 However, we should remember that after the implant, the retrieval of both the balloon and the device sheath should be conducted with care by separating the guide catheter from the ostium to avoid deep intubation. The other reason that may explain why this stent is still not widely used can the augmented profile of the device and its rigidity, which both reduce its navigational and crossing capabilities compared to balloon-expandable stents.
Due to the characteristics of the stent and the experienced gained using it, the clinical settings where it can be useful are: ectatic vessel, since the stent reaches 6.5 mm of diameter with the device L size; proximal and distal diameter mismatch due to its adaptative capabilities to the vessel caliber; lesions with high thrombotic load, since this stent self-expanding capabilities facilitate its expansion until it reaches the vessel wall if thrombus reabsorption occurs, which avoids late stent malapposition; and bifurcations with ostial damage and 30º to 70° angles. The stent z-shaped mesh and the presence of small interconnectors facilitate re-crossing the lateral branch and disconnecting the struts without having to use the final kissing balloon technique. Thanks to its self-expanding capabilities, the unconnected struts cover the lateral branch ostium making the double stent technique unnecessary on many occasions.
In the studies published on former iterations of the device, the self-expanding stent proved superior to the balloon-expandable stent regarding better apposition. The randomized APOSSITION II clinical trial,4 conducted among patients with acute myocardial infarction, showed a lower rate of stent malapposition (defined as > 5% of struts per patient as seen on the optical coherence tomography) 3 days after the primary percutaneous coronary intervention. The APOSSITION IV clinical trial,5 also conducted among patients with acute myocardial infarction, showed a significantly lower percentage of stent malapposition at the 4-month follow-up in patients treated with self-expanding stents compared to patients treated with balloon-expandable stents (0.07% vs 1.16%; P = .002). However, no inter-group differences were found at the 9-month follow-up (0.43% vs 0.28%; P = .55) or in the rate of major adverse cardiovascular events (MACE). The clinical repercussions of this improvement in the early apposition of the stent has not been studied thoroughly. The APOSSITTION III trial6 showed that the use of STENTYS BMS in the percutaneous coronary intervention setting was associated with acceptable cardiovascular results at the 2-year follow-up, an overall rate of MACE of 11.2%, and a rate of stent thrombosis of 3.3%. We should mention that this study revealed a significant reduction of adverse events after the systematic implementation of a standard protocol (predilatation, implantation, postdilatation). The data available support the hypothesis of the need for mild postdilatation to avoid early complications probably because the stent does not have enough radial strength to achieve a proper expansion in rigid often calcified lesions, especially when predilatation is not fully effective. Therefore, postdilatation would avoid the incomplete expansion of the stent, which may increase the risk of stent thrombosis.7
Our study with the STENTYS Xposition S stent reached angiographic success in 98.4% of the cases, although we should remember that, from the anatomical point of view, they were not complex lesions (only 30% were type C lesion). Stent implantation failed in 1 severely calcified LMCA lesion; it is precisely in this type of lesions where its use is ill-advised, especially if predilatation is not effective.8
Regarding its use in bifurcations the studies published to this day have also discussed a former iteration of this device with good results. In the observational, multicenter, and prospective OPEN II trial,9 the rate of MACE at the 12-month follow-up was 13% (10.1% at 6 months). This rate of events was basically due to the need for revascularization of the treated lesion, while the rate of stent thrombosis at the 12-month follow-up was 1%. We should also mention that the kissing balloon technique was only used in 21.7% of the patients. Also, there were no significant differences in the rate of MACE between patients in whom the kissing balloon technique was used and those in whom it was not used.9
To this day, the only study published on the new STENTYS Xposition S model is the TRUNC, a prospective and multicenter study that assessed the efficacy and safety profile of this type of stent in the LMCA. Angiographic success was achieved in 96.6% of the patients and the overall rate of MACE was 8.3% at the 12-month follow-up, basically due to revascularization of the lesion treated in 5.4%.10 Here we should mention the preliminary results reported by the SIZING (Worldwide every-day practice registry assessing the Xposition S self-apposing stent in challenging lesions with vessel diameter variance) and WIN (World-wide registry to assess the STENTYS Xposition S for revascularization of coronary arteries in routine clinical practice) registries. Both registries confirm the safety and efficacy profile of the current iteration of the stent in the routine clinical practice.
Limitations
Our study has several limitations. Because of its retrospective, single-center nature and the limited number of cases involved, we cannot draw definitive conclusions on the device safety and efficacy profile. No intracoronary imaging modality was performed systematically to guide the implant, which may have been useful, especially the optical coherence tomography. However, we believe that this study is relevant due to the scarce evidence available on the last iteration of this stent.
CONCLUSIONS
In our series of lesions located in ectatic vessels or with proximal-distal diameter mismatch and in bifurcations, the STENTYS Xposition S stent is a good therapeutic alternative that achieves good immediate angiographic results and good mid-term clinical results.
FUNDING
No funding to declare.
AUTHORS' CONTRIBUTIONS
Data collection: A. Pérez Guerrero, I. Caballero Jambrina. Data analysis: A. Pérez Guerrero, G. Fuertes Ferré, I. Caballero Jambrina, G. Galache Osuna, M.C. Gracia Ferrer. Analysis and interpretation of data: A. Pérez Guerrero, G. Fuertes Ferré, J. Sánchez-Rubio, G. Galache Osuna, M.C. Gracia Ferrer. Critical review of manuscript: J.A. Diarte de Miguel, M.R. Ortas Nadal.
CONFLICTS OF INTEREST
None declared.
WHAT IS KNOWN ABOUT THE TOPIC?
- Balloon expandable stents can have limitation in certain scenarios like in the revascularization of lesions with significant proximal-distal diameter mismatch, high thrombotic loads, and situations of bifurcations or in venous grafts. In these situations, the STENTYS Xposition S self-expanding stent can be especially useful.
WHAT DOES THIS STUDY ADD?
- This type of stent is not widely used in our specialty. We described the experience of our own center with the STENTYS Xposition S stent. Despite the greater difficulty when trying to advance it and the complexity involved in its delivery, the rate of successful implantation was high. We should not forget that this type of stent is recommended in non-complex or non-calcified anatomical lesions. In general, predilatation is recommended to prepare the lesion and postdilatation to secure the proper expansion of the stent since the stent lacks the necessary radial strength. In our series of patients, the STENTYS Xposition S stent was safe and with a low rate of adverse cardiovascular adverse events at the 1-year follow-up.
REFERENCES
1. Cook S, Eshtehardi P, Kalesan B, et al. Impact of incomplete stent apposition on long-term clinical outcome after drug-eluting stent implantation. Eur Heart J. 2012;33:1334-1343.
2. Garcia-Garcia HM, McFadden EP, Farb A, et al. Standardized End Point Definitions for Coronary Intervention Trials:The Academic Research Consortium-2 Consensus Document. Circulation. 2018;137:2635-2650.
3. Lu H, IJsselmuiden AJ, Grundeken MJ, et al. First-in-man evaluation of the novel balloon delivery system STENTYS Xposition S for the self-apposing coronary artery stent:impact on longitudinal geographic miss during stenting. EuroIntervention. 2016;11:1341-1345.
4. Van Geuns R-J, Tamburino C, Fajadet J, et al. Self-expanding versus balloon-expandable stents in acute myocardial infarction:Results from the APPOSITION II study. Self-expanding stents in ST-segment elevation myocardial infarction. J Am Coll Cardiol Intv. 2012;5:1209-1219.
5. Van Geuns RJ, Yetgin T, La Manna A, et al. STENTYS self-apposing sirolimus-eluting stent in ST-segment elevation myocardial infarction:results from the randomised APPOSITION IV trial. EuroIntervention. 2016;11:1267-1274.
6. Koch KT, Grundeken MJ, Vos NS, et al. One-year clinical outcomes of the STENTYS Self-Apposing(R) coronary stent in patients presenting with ST-segment elevation myocardial infarction:results from the APPOSITION III registry. EuroIntervention. 2015;11:264-271.
7. Sato T, Kameyama T, Noto T, Nozawa T, Inoue H. Impact of preinterventional plaque composition and eccentricity on late-acquired incomplete stent apposition after sirolimus- eluting stent implantation:an intravascular ultrasound radiofrequency analysis. Coron Artery Dis. 2012;23:432-437.
8. Verheye S, Ramcharitar S, Grube E, et al. Six-month clinical and angiographic results of the STENTYS R self-apposing stent in bifurcation lesions. EuroIntervention. 2011;7:580-587.
9. Naber CK, Pyxaras SA, Nef H, et al. Final results of a self-apposing paclitaxel-eluting stent for the percutaneous treatment of de novo lesions in native bifurcated coronary arteries study. EuroIntervention. 2016;12:356-358.
10. Tamburino C, Briguori C, Jessurun GA, et al. TCT-329 prospective evaluation of drug eluting selfapposing stent for the treatment of unprotected left main coronary artery disease:1-year results of the TRUNC study. J Am Coll Cardiol. 2018;72:134-135.
ABSTRACT
Introduction and objectives: patients with long, sequential and diffuse coronary lesions who undergo a percutaneous coronary intervention remain at a high risk of suffering cardiovascular events despite the improved safety and efficacy of the new drug-eluting stents. The objective of this study was to analyze the utility of SyncVision/iFR-guided revascularization (SyncVision version 4.1.0.5, Philips Volcano, Belgium) in this type of lesions.
Methods: Randomized, multicenter, controlled, and open-label trial designed to compare SyncVision/iFR-guided and angiography-guided revascularizations in patients with long, sequential or diffuse significant angiographic coronary stenosis (ClinicalTrials.gov identifier: NCT04283734). A total of 100 patients will be randomized (1:1, no stratification). The primary endpoint is the average length of the stent implanted. The secondary endpoint is a composite of cardiac death, myocardial infarction, definitive or probable stent thrombosis, new target lesion revascularization or new target lesion failure; and the presence of residual ischemia as seen on single-photon emission computed tomography at the 6-month follow-up. Patients will be followed for 12 months after the procedure.
Results: The trial is currently in the recruitment phase, and it has already recruited the first 7 patients. We expect to complete the recruitment phase by February 2021 and the follow-up by February 2022.
Conclusions: The iLARDI study is the first randomized trial to assess the potential utility of SyncVision-guided revascularization in long, sequential and diffuse coronary lesions.
Keywords: Diffuse coronary artery disease. Long coronary artery disease. Instantaneous wave-free ratio. SyncVision software.
RESUMEN
Introducción y objetivos: Los pacientes con lesiones coronarias largas, secuenciales o difusas tratadas percutáneamente continúan presentando un riesgo alto de eventos cardiovasculares a pesar de la mejoría de la seguridad y de la eficacia de los nuevos stents liberadores de fármacos. El objetivo de este estudio es analizar la utilidad del software SyncVision/iFR (SyncVision versión 4.1.0.5, Philips Volcano, Bélgica) para guiar la revascularización en este tipo de lesiones.
Métodos: Estudio aleatorizado, multicéntrico, controlado y abierto para comparar la revascularización guiada por SyncVision/iFR respecto a la revascularización guiada por angiografía en pacientes con lesiones coronarias largas, secuenciales o difusas (identificador de ClinicalTrials.gov: NCT04283734). Se incluirá a 100 pacientes (aleatorización 1:1 no estratificada). El objetivo primario es la longitud total del stent implantado. Como objetivo secundario se ha establecido un combinado de muerte cardiaca, infarto de miocardio, trombosis definitiva o probable del stent, nueva revascularización de la lesión tratada en el procedimiento basal o nueva revascularización de la lesión analizada en el procedimiento basal, y la presencia de isquemia residual evaluada por tomografía computarizada por emisión de fotón simple a los 6 meses de seguimiento. El tiempo de seguimiento será de 12 meses tras el procedimiento índice.
Resultados: El estudio se encuentra actualmente en fase de reclutamiento, con los primeros 7 pacientes ya incluidos. Esperamos completar el reclutamiento en febrero de 2021 y el seguimiento en febrero de 2022.
Conclusiones: El estudio iLARDI es el primer estudio aleatorizado para la evaluación de la potencial utilidad de la revascularización guiada por SyncVision en lesiones coronarias largas, secuenciales y difusas.
Palabras clave: Lesiones coronarias difusas. Lesiones coronarias largas. Relación en el periodo instantáneo libre de ondas. Software SyncVision.
Abbreviations:
PCI: percutaneous coronary intervention. iFR: instantaneous wave-free ratio. MACE: major adverse cardiovascular events.
INTRODUCTION
The physiological assessment of coronary lesions is a routine practice in contemporary cath labs and is strongly recommended by the European guidelines to guide the percutaneous coronary intervention (PCI) decision-making process.1 Unlike fractional flow reserve, the new instantaneous wave-free ratio (iFR) index allows us to analyze the physiological significance of each lesion and each coronary segment.2-5 This has led to the creation of the new and specific SyncVision software package (SyncVision version 4.1.0.5, Philips Volcano, Belgium), that shows the functional compromise of each lesion and predicts the expected iFR improvement after percutaneous treatment.3,4
Few observational studies published have analyzed the reduction in the length of the stent implanted compared to angiography-guided revascularization in long and diffuse coronary lesions.4,5 However, this reduction could be detrimental to the complete coverage of the plaque in this type of lesions, which has proven to be a predictor of major adverse cardiovascular events at the follow-up.6
The objective of our study is to analyze the utility of the iFR and SyncVision software to guide the PCI decision-making process in long, sequential, and diffuse coronary lesions.
METHODS
We have designed a multicenter, randomized, controlled, and open-label trial to compare SyncVision/iFR-guided revascularization to angiography-guided revascularization in patients with long, sequential or diffuse significant angiographic coronary lesions (ClinicalTrials.gov identifier: NCT04283734). All the variables that will be analyzed during the study are shown on table 1.
Table 1. Variables that will be analyzed during the study
| Nº | Variable | Expressed as |
|---|---|---|
| Personal medical history | ||
| 1 | Sex (men/women) | no. (%) |
| 2 | Age (years) | no. ± SD |
| 3 | Hypertension | no. (%) |
| 4 | Diabetes mellitus | no. (%) |
| 5 | Dyslipidemia | no. (%) |
| 6 | Former smoker | no. (%) |
| 7 | Previous ischemic cardiomyopathy | no. (%) |
| 8 | Previous revascularization | no. (%) |
| 9 | Atrial fibrillation | no. (%) |
| 10 | Heart failure | no. (%) |
| 11 | Previous stroke | no. (%) |
| 12 | Peripheral artery disease | no. (%) |
| 13 | Previous significant bleeding | no. (%) |
| 14 | Basal hemoglobin levels (mg/dL) | no. ± SD |
| 15 | Basal creatinine levels (mg/dL) | no. ± SD |
| 16 | Left ventricular ejection fraction (%) | no. ± SD |
| 17 | Clinical presentation (stable angina/NSTEMI/STEMI) | no. (%) |
| 18 | Baseline ultra-sensitive troponin levels (ng/L) | no. ± SD |
| Procedural data | ||
| 19 | Arterial access (radial/femoral/other) | no. (%) |
| 20 | P2Y12 inhibitor preload | no. (%) |
| 21 | IIb/IIIa inhibitor use during the procedure | no. (%) |
| 22 | Multivessel disease | no. (%) |
| 23 | Syntax score | no. ± SD |
| 24 | Randomized vessel (LAD/LCx/RCA/other) | no. (%) |
| 25 | Vessel lesion length (mm) | no. ± SD |
| 26 | Vessel reference diameter (mm) | no. ± SD |
| 27 | Vessel stenosis (%) | no. ± SD |
| 28 | Total stent length as seen on the angiography (mm) | no. ± SD |
| 29 | Total length of the stent implanted (mm) | no. ± SD |
| 30 | Differences between stent length estimated and implanted (mm) | no. ± SD |
| 31 | Stent diameter (mm) | no. ± SD |
| 32 | Optimal angiographic result (final TIMI III flow, absence of dissections and residual stenosis < 20%) | no. (%) |
| 33 | Contrast (milliliters) | no. ± SD |
| 34 | Use of intracoronary imaging | no. (%) |
| 35 | Use of rotablation | no. (%) |
| 36 | Procedural complications (no reflow/ dissection/acute vessel closure/perforation/other) | no. (%) |
| 37 | Baseline iFR in the intervention group | no. ± SD |
| 38 | Diffuse improvement of iFR by SyncVision | no. (%) |
| 39 | Estimated stent length to achieve an iFR > 0.89 (mm) | no. ± SD |
| 40 | Final iFR in the intervention group | no. ± SD |
| 41 | Need to implant an additional stent | no. (%) |
| Hospitalization data | ||
| 42 | Bleeding complications | no. (%) |
| 43 | Ultra-sensitive troponin peak levels (ng/L) | no. ± SD |
| 44 | Periprocedural myocardial infarction | no. (%) |
| 45 | In-hospital death | no. (%) |
| 46 | In-hospital stroke | no. (%) |
| 47 | In-hospital stent thrombosis | no. (%) |
| Pharmacological treatment at discharge | ||
| 48 | Aspirin | no. (%) |
| 49 | P2Y12 Inhibitor (no/clopidogrel/ticagrelor/prasugrel) | no. (%) |
| 50 | Anticoagulation (no/acenocumarol/rivaroxaban/ dabigatran/apixaban/edoxaban) | no. (%) |
| 51 | Beta-blockers | no. (%) |
| 52 | ACEI/ARB/ARNI | no. (%) |
| 53 | Calcium antagonists | no. (%) |
| 54 | Other anti-ischemic drugs | no. (%) |
| Follow-up visits (after 3, 6, and 12 months) | ||
| 55 | Bleeding complications | no. (%) |
| 56 | Dual antiplatelet therapy | no. (%) |
| 57 | Anticoagulation (no/acenocumarol/rivaroxaban/ dabigatran/apixaban/edoxaban) | no. (%) |
| 58 | Probable or definitive stent thrombosis | no. (%) |
| 59 | Spontaneous myocardial infarction | no. (%) |
| 60 | New target lesion revascularization | no. (%) |
| 61 | New target vessel revascularization | no. (%) |
| 62 | Revascularization of other vessel | no. (%) |
| 63 | Death | no. (%) |
| 64 | Cause of death (cardiac/non cardiac) | no. (%) |
| 65 | Stroke | no. (%) |
| 66 | Beta-blockers | no. (%) |
| 67 | ACEI/ARB/ARNI | no. (%) |
| 68 | Calcium antagonists | no. (%) |
| 69 | Other anti-ischemic drugs | no. (%) |
| 70 | Residual angina (I/II/III/IV) | no. (%) |
| 71 | Withdrawal from the study | no. (%) |
| 72 | Lost to follow-up | no. (%) |
|
ACEI, angiotensin-converting-enzyme inhibitors; ARB, angiotensin receptor blockers; ARNI, angiotensin receptor blocker and neprilysin inhibitor; LAD, left anterior descending coronary artery; LCx, left circunflex artery; RCA, right coronary artery; SD, standard deviation; TIMI, Thrombolysis in Myocardial Infarction. NSTEMI, non-ST-segment elevation myocardial infarction; STEMI, ST-segment elevation myocardial infarction. |
||
Additionally, the study has received the proper ethical oversight and has been approved by the Ethical Comitee of Córdoba.
Inclusion and exclusion criteria
Patients with the following criteria are being included: a) patients > 18 years old who require percutaneous coronary treatment due to ischemia (silent, stable angina or acute coronary syndrome); b) presence of a vessel with sequential lesions separated by < 10 mm from each other with a total lesion length > 25 mm and a percent diameter stenosis > 60% (as seen on the quantitative coronary angiography assessment) in, at least, 1 segment; or a coronary segment > 30 mm with diffuse disease, and a percent diameter stenosis > 60% (as seen on the quantitative coronary angiography assessment) in, at least, 1 region; c) baseline iFR ≤ 0.89 distal to a potentially randomizable lesion.
We have stablished the following exclusion criteria: a) patients with acute coronary syndrome with non-optimal results in the culprit vessel (final Thrombolysis in Myocardial Infarction (TIMI) flow grade < III, non-reflow phenomenon during treatment, residual coronary dissection, lost or compromise of a major side branch); b) patients with acute coronary syndrome and left ventricular ejection fraction < 45%; c) life expectancy < 12 months; d) patients with severe aortic stenosis; e) contraindication for dual antiplatelet therapy for at least 12 months; f) presence of significant thrombocytopenia (< 10 x 109/L); g) patients with an indication for bypass surgery according to the heart team; h) pregnancy; i) inability to understand the informed consent.
Endpoints
The study primary endpoint is the reduction of the average length of the stent implanted in the SyncVision-guided group measured in millimeters (mm) compared to the angiography-guided group. The study secondary endpoint is a composite of cardiac death, myocardial infarction, definitive or probable stent thrombosis, new target lesion revascularization or new target lesion failure (major adverse cardiovascular events [MACE]); and the assessment of residual ischemia through single-photon emission computed tomography at the 6-month follow-up.
Procedure
After the diagnostic phase, the use of intracoronary vasodilators is mandatory to exclude possible coronary spasms. Lesions will be assessed by 2 expert operators (prior to randomization) to determine the coronary segment to treat when the revascularization is angiography-guided based on current routine clinical practice. Afterwards, the iFR will be determined at baseline. If the obtained iFR is ≤ 0.89, patients will be randomized to the angiography-guided revascularization group (the control group) or to the iFR pullback-guided revascularization group using the SyncVision software (figure 1). Intracoronary imaging can be used in both groups based on the operator’s criteria to optimize the angiographic result.
Figure 1. Summary of randomization, treatment targets, and follow-up of the iLARDI study. iFR, instantaneous wave-free ratio; MACE, major adverse cardiovascular events; PCI, percutaneous coronary intervention.
In the intervention group, a pressure wire (Verrata pressure guidewire, Philips Volcano, Belgium) will be inserted trough a guide catheter towards the vessel ostium to normalize the pressure between the aortic and the vessel ostium. Secondly, the pressure wire will be advanced distally to the lesion. Under stable hemodynamic conditions (without the administration of vasodilators), we will determine the baseline iFR. Afterwards, the wire will be removed under continuous fluoroscopy, and in the same projection. If the iFR at the vessel ostium is 1 ± 0.02, the absence of drift will be confirmed and an angiogram in the same angiographic position will be performed. The SyncVision software can recognize the vessel analyzed and identify the physiological contribution of every lesion and every segment, predicting the improvement of the iFR after treatment. The iFR improvement is depicted as yellow dots. Each yellow dot represents an iFR improvement of 0.01 if that zone was percutaneously treated. The accumulation of many yellow dots suggests that the contribution of that lesion to physiological compromise is high. After performing the physiological assessment of each lesion, the operator would have to treat the minimum segment needed to achieve an iFR of 0.90. Cases without an accumulation of dots have been considered as physiological diffuse disease (defined as the presence of < 20% of the total number of dots) in the coronary segment physiologically assessed. Those cases will be medically treated due to the theoretical absence of benefit of the percutaneous treatment (figure 2 and figure 3).
Figure 2. Flowchart of technical treatment details of patients randomized to the intervention group.
* We consider as optimization the postdilatation of the previous stented area if an in-stent accumulation of yellow dots is seen; or the percutaneous treatment of a new segment with physiological compromise not seen in the baseline iFR-pullback study. iFR, instantaneous wave-free ratio.
Figure 3. Image of iFR co-registration using the SyncVision software in a patient included in the study and randomized to the intervention group with a diffuse lesion in the left anterior descending coronary artery, and the physiological contribution of every segment. The estimated length of the stent to achieve an iFR > 0.89 is 50.6 mm.
Follow-up
Patients will be followed either through phone calls or physical examination at the 3, 6 and 12-month follow-up. At the 6-month follow-up a stress single-photon emission computed tomography (physiological or pharmacological) will be performed in all patients. The composite of cardiovascular death, definitive or probably stent thrombosis, new target lesion failure or new target lesion revascularization will be considered as MACE.
Quantitative coronary measurements
Quantitative coronary measurements will be performed using a validated system (CAAS system, Pie Medica Imaging, Netherlands). The measurements analyzed will be the vessel reference diameter, the vessel minimal lumen diameter, and the percentage of stenosis. All measurements will be taken at baseline and after the PCI.
Statistical analysis
Regarding the statistical analysis, quantitative variables will be expressed as mean ± standard deviation and qualitative variables as absolute numbers and percentages. To determine the relationship among quantitative variables, we will be using the paired Student t test for paired data. To determine the relationship among the qualitative ones, we will use the chi-squared test. In all cases, differences will be considered significant with P values < .05. We will be using the IBM SPSS Statistics software package (version 24.0 for Macintosh, SPSS Corp., United States). To calculate the sample size, we have performed a retrospective analysis of the last 20 patients who were treated at our centre and showed a sequential or diffuse lesion in the coronary vessel analyzed from the iFR-pullback study. The mean length of the stent implanted was 43 ± 9 mm and the reduction of stent length was 12 ± 8 mm on the angiographic analysis. With these data, we have stablished an expected length reduction of 15 mm. The calculated sample size to achieve the primary endpoint with an 80% confidence level and a 5% margin of error was 100 patients.
RESULTS
The recruitment of patients started back in February 2020. After 1 month, we have included the first 7 patients. We expect to complete the recruitment by February 2021 and the follow-up by February 2022.
DISCUSSION
To our knowledge, this randomized study is, the first one to assess the potential benefits of using the SyncVision software in long, sequential or diffuse coronary lesions. Currently, the study is in the recruitment phase and the first patients have already been recruited.
The iFR has proven to be useful in the PCI guide decision-making process.7,8 However, the evidence supporting the use of SyncVision is scarce and controversial in long, sequential or diffuse lesions. On the one hand, the software allows us to know the coronary segments with the highest physiological compromise. This allows us to revascularize only those segments that immediately improve the physiological result with a potential reduction of the length of the stent implanted, which happens to be a predictor of MACE at the follow-up.9 On the other hand, it’s possible that even if we obtain a good immediate physiological result and a reduction of the stent length implanted we won’t be fully covering the plaque in some lesions or coronary segments, which has also proven to be a predictor of MACE.6
A limitation of the study is the sample size, enough to achieve the primary endpoint, but probably inadequate to see differences in MACE. However, we think that it can provide an early insight on the utility of iFR pullback study to guide the PCI decision-making process in this type of lesion. Also, it can be a hypothesis-generator study for future larger-scale studies to show benefits in terms of clinical events reduction.
For these reasons, we believe that the iLARDI is an interesting study that will shows us the potential benefit of SyncVision to guide the PCI decision-making process in long, sequential or diffuse coronary lesions. We intend to complete the results by February 2022.
CONCLUSIONS
The iLARDI study is the first randomized trial to assess the potential utility of SyncVision-guided revascularization in long, sequential and diffuse coronary lesions.
FUNDING
Funds from the Plan Andaluz de Investigación, Desarrollo e Innovación (PAIDI) have been used to pay for the liability insurance associated with clinical research.
AUTHORS' CONTRIBUTION
All the authors have participated in the study and in the manuscript:
F. Hidalgo has participated has mainly drafted of the manuscript and has participated in the conception and design of the study. R. González has also participated in the conception and design of the study, and in the analysis and interpretation of data. S. Ojeda has mainly participated in the conception, design of the study and revision of the manuscript. C. Pericet has participated in the conception and design of the study. A. Lostalo has also collaborated in the analysis and interpretation of data. J. Segura has also revised it critically for important intellectual content. N. Paredes and J.C. Elizalde have also contributed in the analysis and interpretation of data. A. Luque has participated in the draft of the manuscript. F. Mazuelos has also contributed in the analysis and interpretation of data. J. Suárez de Lezo and M. Romero have revised it critically for important intellectual content. M. Pan has done the final approval of the manuscript submitted.
CONFLICTS OF INTEREST
F. Hidalgo, S. Ojeda, and J. Segura received personal fees from Philips Volcano. M. Pan received minor fees from Abbott, Philips Volcano, and Terumo. The remaining authors declared no conflicts of interest whatsoever.
WHAT IS KNOWN ABOUT THE TOPIC?
- The physiological assessment of coronary lesions is a routine practice in the cath lab. The iFR and the SyncVision software allow us to know what is the individual contribution of every coronary lesion and contribute in the PCI decision-making process. However, to our knowledge, no randomized studies have been published on the utility of their use in long, sequential and diffuse coronary lesions.
WHAT DOES THIS STUDY ADD?
- The iLARDI study will show the potential utility of SyncVision/iFR-guided revascularizations in this type of lesions (long, sequential and diffuse coronary lesions) regarding the reduction of the stent length and the potential reduction of major adverse cardiovascular events at the follow-up.
REFERENCES
1. Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87-165.
2. Kim H-L, Koo B-K, Nam C-W, et al. Clinical and physiological outcomes of fractional flow reserve guided percutaneous coronary intervention in patients with serial stenosis within one coronary artery. JACC Cardiovasc Interv. 2012;5:1013?1018.
3. Nijjer SS, Sen S, Petraco R et al. The Instantaneous Wave-Free Ratio (iFR) pullback:a novel innovation using baseline physiology to optimise coronary angioplasty in tandem lesions. Cardiovasc Revasc Med. 2015;16:167-171.
4. Nijjer SS, Sen S, Petraco R et al. Pre-Angioplasty Instantaneous Wave-Free Ratio Pullback Provides Virtual Intervention and Predicts Hemodynamic Outcome for Serial Lesions and Diffuse Coronary Artery Disease. JACC Cardiovasc Interv. 2014;7:1386-1396.
5. Kikuta Y, Cook CM, Sharp ASP et al. Pre-Angioplasty Instantaneous Wave-Free Ratio Pullback Predicts Hemodynamic Outcome In Humans With Coronary Artery Disease. Primary Results of the International Mul-ticenter iFR GRADIENT Registry. JACC Cardiovasc Interv. 2018;11:757-767.
6. Costa MA, Angiolillo DJ, Tannenbaum M et al. Impact of Stent Deployment Procedural Factors on Long-Term Effectiveness and Safety of Sirolimus-Eluting Stents (Final Results of the Multicenter Prospective STLLR Trial). Am J Cardiol. 2008;101:1704-1711.
7. Davies JE, Sen S, Dehbi HM, Al-Lamee R, Petraco R, Nijjer SS et al. Use of instantaneous wave-free ratio or fractional flow reserve in PCI. N Engl J Med. 2017;376:1824-1834.
8. Gotberg M, Crhistiansen EH, Gudmundsdottir IJ, et al. Instantaneous wave-free ratio versus fractional flow reserve to guide PCI. N Engl J Med. 2017;376:1813?1823.
9. Coner A, Cicek D, Akinci S, et al. Mid-term clinical outcomes of new generation drug-eluting stents for treatment of diffuse coronary artery ||aadisease. Turk Kardiyol Dern Ars. 2018;46:659-666.
- Rotational atherectomy for the management of bifurcation lesions: a pilot randomized study
- Microalbuminuria predicts contrast-induced nephropathy in patients with acute coronary syndrome
- Use of subcutaneous nitroglycerin to facilitate transradial access in coronary procedures (NiSAR Study)
- Pharmacoinvasive strategy as reperfusion treatment in non-capable primary percutaneous coronary intervention areas
Editorials
Expanding the role of drug-coated balloons in native large coronary artery disease
aDepartment of Biomedical Sciences, Humanitas University, Pieve Emanuele, Milan, Italy
bCardio Center, IRCCS Humanitas Research Hospital, Rozzano, Milan, Italy
cDivision of Cardiology, Montefiore Medical Center, Bronx, New York, United States
Original articles
Editorials
The role of angiography-derived physiological assessment techniques in the post-FAVOR III Europe era?
aServicio de Cardiología, Hospital General Universitario Gregorio Marañón, Instituto de Investigación Sanitaria Gregorio Marañón, Universidad Complutense de Madrid, Madrid, Spain
bCentro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), Instituto de Salud Carlos III, Madrid, Spain
Interviews
An interview with Camino Bañuelos
aServicio de Cardiología, Hospital Clínico San Carlos, Instituto de Investigación Sanitaria del Hospital Clínico San Carlos (IdISSC), Madrid, Spain
bServicio de Cardiología, Hospital Clínico Universitario de Santiago de Compostela, Santiago de Compostela, A Coruña, Spain