Article
Ischemic heart disease and acute cardiac care
REC Interv Cardiol. 2019;1:21-25
Access to side branches with a sharply angulated origin: usefulness of a specific wire for chronic occlusions
Acceso a ramas laterales con origen muy angulado: utilidad de una guía específica de oclusión crónica
Servicio de Cardiología, Hospital de Cabueñes, Gijón, Asturias, España
ABSTRACT
Introduction and objectives: When using radial access established as the approach of choice to perform coronary angiographies it is important to avoid radial spasm as it is the leading cause of access failure. This study aims to determine whether a topical anesthetic cream reduces the rate of radial spasm, as well as the increased gain with the use of different vasodilators.
Methods: Randomized, double-blind, and single-center clinical trial. Patients will be randomized to receive the anesthetic cream vs placebo, and 4 types of different vasodilator cocktails will be used in each group. The presence—or not—of radial spam and caliper gain will be analyzed.
Conclusions: Demonstrating the efficacy of the anesthetic cream, and different vasodilators to reduce radial spam would have a significant clinical impact, and justify its systematic use when performing coronary angiographies.
Registered at The Spanish Agency of Medicines and Medical Devices (AEMPS) EudraCT number: 2017-000321-12.
Keywords: Radial spasm. Anesthetic cream. Vasodilators. Coronary angiography. Luminal diameter.
RESUMEN
Introducción y objetivos: Con el abordaje radial establecido como técnica de elección para la coronariografía, es importante evitar el espasmo radial como principal causa de fallo en el acceso intravascular. En este estudio se pretende demostrar si la anestesia tópica en crema disminuye la incidencia de espasmo radial, así como conocer la ganancia de calibre con el uso de diferentes vasodilatadores.
Métodos: Ensayo clínico aleatorizado doble ciego en un solo centro. Los pacientes se aleatorizarán para recibir crema anestésica o placebo, y se utilizarán 4 tipos de cócteles vasodilatadores en cada grupo. Se analizará la presencia o no de espasmo radial y la ganancia de calibre como objetivos primarios.
Conclusiones: La demostración de la eficacia de la crema anestésica y de los diferentes vasodilatadores en la disminución del espasmo radial tendría un impacto clínico importante y justificaría su uso sistemático en la coronariografía.
Registrado en la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) con n.º EudraCT: 2017-000321-12.
Palabras clave: Espasmo radial. Crema anestésica. Vasodilatadores. Coronariografía. Diámetro luminal.
Abbreviations
MLD: mean luminal diameter. RS: radial spasm. TA: topical anesthesia.
INTRODUCTION
Radial approach for cardiac catheterizations has become the most widely used across the world. In Spain it represents up to 75% of all the procedures performed and, in some centers, up to 91.1%.1 Compared to traditional femoral approach, this access has clearly proven its superiority from the safety standpoint of the procedures.2
Arterial canalization failure is often due to radial spasm (RS), and it can occur in up to 10% of all attempts. Also, it is associated with feminine sex, young age, low weight3 or deficits of certain enzymes that act on the endothelium.4 The special histological characteristics of this artery—with a high density of alpha-adrenergic receptors and smooth muscle cells—make it more prone to spasm.5
On the other hand, pain during lumbar puncture contributes to arterial canalization failure due to a higher frequency of appearance of spasm, vasovagal reaction with hypotension and discomfort for patient and operator, and the patient’s possible hemodynamic instability. Similarly, several patients complain of discomfort. As a matter of fact, the arterial puncture is described by many patients as the main moment of discomfort.5
Former studies have reported on the greater success achieved with isolated punctures for arterial gas analysis in the radial artery with the use of anesthesia injected around the puncture site. Also, more comfort and less pain have been reported by the patients.6 However, for many professionals injected anesthesia is ill-advised due to the pain caused by the injection. Also, because there are times that pain leads discomfort, and eventually RS.7 Despite of all this, the use of injected anesthesia is a common thing in procedures performed via radial access.
On the other hand, in the pediatric population as well as in different anatomical locations or in skin surgery, the use of topical anesthesia (TA) in the form of gel, cream or ointment has proven to minimize the pain associated with venous or arterial punctures, and some procedures too.8 The use of this type of anesthetic agents has not been properly studied in the cardiac catheterization setting. However, it could minimize the rate of RS, reduce pain when using this access, and improve the patient’s perception.
Together with TA, the use of different vasodilator drug combinations with unfractionated heparin (the so-called «radial cocktail»)—after successful arterial access—has proven to reduce the rates or arterial spasm and radial occlusion after the procedure.9-12 In particular drugs like verapamil, nitroglycerin, nitroprusside, nicorandil, isosorbide dinitrate or phentolamine in different doses have been compared with one another and also with placebo with heterogeneous results with arterial spams having been reported in 4% to 12% of the cases. Verapamil in doses of 5 mg and nitroglycerin 200 µg have yielded the best results so far. However, to this date, no comparison studies between the 2 drugs at these doses have ever been drawn or randomized for this matter.13 Therefore, it has not been fully established which is the best drug combination to prevent spasm and radial occlusion.
At our center, the current radial puncture procedure includes the use of injected anesthesia around the puncture site plus a cocktail of 5000 IU of unfractionated heparin, and 2.5 mg of verapamil. The rate of RS in our cath lab is around 10% of all punctures performed. In some patients, other drugs commonly available in our setting are often used—at the operator’s criterion—like nitroprusside, nitroglycerin or high doses of verapamil.
The objective of this study is to demonstrate whether the administration of topical anesthesia reduces the rate of RS and improves the patient’s perception regardless of the vasodilator used. Also, to compare arterial caliber gain with different vasodilators.
METHODS
Study design
Double-blind randomized clinical trial conducted at a single center to analyze the rate of RS in patients treated with TA in cream with lidocaine 25 mg/g + prilocaine 25 mg/g (Emla) in topical solution compared to placebo, as well as the effect of vasodilators (table 1) (verapamil 2.5 mg or 5 mg, nitroglycerin 200 µg, nitroprusside 150 µg) in the arterial caliber while attempting vascular access to perform diagnostic transradial cardiac catheterization.
Table 1. Inclusion and exclusion criteria of the E-RADIAL study
| Composition of the radial cocktail | Type of dilution |
|---|---|
| Cocktail #1 (verapamil 2.5 mg): | 12.5 mg of verapamil are diluted in 95 mL of FSS at 0.9%. A total of 20 mL are loaded in the syringe and fully administered. |
| Cocktail #2 (verapamil 5 mg): | 25 mg of verapamil are diluted in 90 mL of FSS at 0.9%. A total of 20 mL are loaded in the syringe and fully administered. |
| Cocktail #3 (nitroglycerin 0.2 mg): | 5 mg of nitroglycerin are diluted in 95 mL of FSS at 0.9%. A total of 4 mL of this solution are loaded in a 20 mL-syringe that is completed with FSS at 0.9%. The entire load of the syringe is administered. |
| Cocktail #4 (nitroprusside 0.150 mg): | 50 mg are diluted in 10 mL of FSS at 0.9% followed by the extraction of 1 mL of this solution that is diluted again in 100 mL of FSS at 0.9%. A total of 3 mL of the latter solution are loaded in a 20 mL-syringe that is completed with FSS at 0.9%. The entire load of the syringe is administered. |
|
FSS, physiological saline solution. |
|
Study population
The study will be conducted entirely at Unidad de Hemodinámica y Cardiología Intervencionista of Complejo Hospitalario Universitario de Albacete, Spain. All consecutive patients treated with diagnostic cardiac catheterization via radial access from November 2020 until completing the sample estimated will be included. Patients will need to meet the inclusion criteria and none of the exclusion ones (table 2).
Table 2. Inclusion and exclusion criteria of the E-RADIAL study
| Inclusion criteria | Exclusion criteria |
|---|---|
| Age > 18 years | Allergy or intolerance to any of the drugs used in the study. |
| Informed consent signing | Baseline systolic arterial blood pressure < 90 mmHg. |
| Elective diagnostic cardiac catheterization with intended radial access |
Impossibility to understand the study or give the corresponding informed consent. |
| Introductor 5 French |
Ethical aspects
The study has been approved by the center ethics committee, and a favorable resolution was obtained. The study has been registered by Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) with registration No. EudraCT: 2017-000321-12. The study will observe the principles established in the Declaration of Helsinki. Also, written informed consent will be obtained from all the patients before joining the study.
Study endpoints
Primary endpoints
– Study the rate of RS using a topical anesthetic cream before radial puncture.
– Study radial artery caliber gain using different vasodilators.
Secondary endpoints
– Study the rate of radial-radial, and radial-femoral crossing with each strategy.
– Study the rate of vasovagal reactions requiring treatment in each group.
– Study parameters associated with pain during radial artery canalization using pain assessment analogue scales.
– Subjective assessment of pain and comfort by the patient using pain assessment analogue scales, and dedicated tests.
– Subjective assessment of the difficulty involved in the puncture and perception of RS by the operator using dedicated tests.
Study development
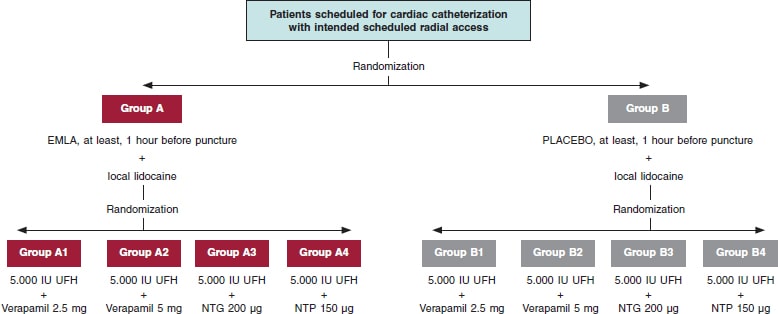
The administration of TA/placebo plus cocktail (table 1) will be fully randomized (figure 1). Both the patient and the treating interventional cardiology will be blind to the group they’ll be assigned to. If certain circumstances or complications occur, and if deemed necessary, the chain of secrecy can be broken only if investigators abide, and only under strict clinical judgement.
Figure 1. Flowchart of patients from the E-RADIAL study. NTG, nitroglycerin; NTP, nitroprusside; UFH, unfractionated heparin.
Placebo with cream of similar color, consistency, and characteristics to Emla will be prepared, and they both will be marked with letters A (Emla) and B (placebo). Both placebo and the TA will be prepared by personnel from the hospital pharmacy unit. The nursing team in charge of the patients while waiting for cardiac catheterization at the cath lab will randomize each patient, and the only blind element of the study. TA or placebo will be administered in both wrists and, at least, 1 hour before the procedure.
Prior to puncture, 25 mg of subcutaneous local anesthesia will be injected into the puncture area (mepivacaine at 2%). Another 1-2 minutes will need to pass before it starts to work.
Different cocktails (table 1) will be prepared at the dilution often used at Complejo Hospitalario Universitario de Albacete cath lab in 100 mL-jars of physiological saline solution (NaCl at 0.9%). Each jar will be marked with an alphanumeric code and its content will remain blind to everyone but the nursing team in charge of randomization.
Variable quantification during puncture
After monitoring the patient, arterial blood pressure will be determined invasively, as well as the baseline heart rate before administering the cocktail that will be used just after the introduction of hydrophilic guidewire (Radiofocus 5-Fr, Terumo, Japan). Similarly, arterial blood pressure will be recorded 2 minutes after the cocktail administration, as well as the maximum heart rate during puncture.
All vagal data that can occur and any other complications associated with access will be written down. The crossing rate to other accesses will also be studied prioritizing homolateral (cubital, distal radial) or contralateral access. Unless the operator specifies otherwise, femoral access will be set aside as the third go-to option.
Radial spasm determination and caliber gain quantification
RS will be defined as yes/no—both qualitative and dichotomically—and considered as sudden, transient, and abrupt narrowing of the radial artery during puncture. It will be clinically determined by, at least, 1 of the following events: loss of pulse during puncture, pain in the upper limb during catheter manipulation or entrapment. Its presence can also be determined through the angiography if spasm is seen during contrast injection.
Caliber gain will be determined through quantitative analysis of the radial artery luminogram. Therefore, an angiography will be immediately performed after the insertion of the introducer sheath plus another one 2 minutes after the injection of the antispasmodic cocktail. The radial artery caliber will be measured in the segment located between the tip of the arterial introducer sheath—2 cm away from it—and the location where it meets the humeral artery. Measurements will be acquired through computerized quantitative analysis (Xcelera, Philips, United States) after previous calibration of the arterial introducer sheath in the same segment before and after the cocktail injection to determine the mean luminal diameter (MLD).
Caliber gain will be estimated in percentage according to the following formula:
Caliber gain = 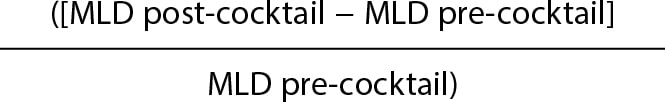 × 100
× 100
Postoperative patient assessment
The patient will be asked to give his opinion on the radial puncture through the pain qualitative analogue scale, and the comfort scale consisting of 4 questions (annex of the supplementary data).
Similarly, the interventional cardiologist will give his evaluation through a survey including 2 questions (annex of the supplementary data), the difficulties found while performing the puncture, and how the procedure was accomplished via the access used.
Statistical analysis
The analysis will be conducted using the SPSS statistical software package for Windows v 21.0.
In descriptive statistics frequencies and percentages will be used to express discrete variables while mean, median, mode, standard deviation, and ranges will be used to express continuous variables. The rate of spams and other study components will be described through frequencies and percentages. The statistical analysis of the main variables will be conducted by intention-to-treat analysis. The chi-square test will be used to study differences among proportions while the continuous variables will be analyzed using the Student t test if normally distributed or else non-parametric tests if not normally distributed. In the presence of non-homogeneous distribution of confounding variables between the groups that will be analyzed, a logistic regression analysis will be conducted that should collect those clinically significant and non-homogeneously distributed parameters.
It is our will to conduct an intermediate analysis after which the study will move on or not (existence of a significant difference in the primary endpoint of RS > 7,5% between both groups).
Estimate of the sample size
According to former studies, it is estimated that the proportion of patients who will have RS in the control group will be 10%3,5 being the criterion of clinical effectiveness the reduction of this percentage off by 50%, which is why it will be necessary to have a minimum sample of 668 patients.
This volume of patients will allow us to confirm the statistical significance of the variations described in radial artery vasodilation with different types of vasodilators.
DISCUSSION
Currently, the arterial approach via radial access is used in 91.1%1 of all diagnostic and therapeutic coronary angiographies performed. In particular, the rates of bleeding complications have dropped thus contributing to the patients’ comfort. This access has facilitated the implementation of safe coronary angiography and outpatient angioplasty programs even in complex settings.14-16
Hand in hand with this and assuming pain hypothesis and adrenergic discharge are caused by puncture and risk factors for RS, different strategies have come up to contribute to the proper administration of anesthesia promoting patients’ comfort, and looking to reduce the rate of RS. As it happens in other places, at our center the use of subcutaneously injected anesthesia is the common practice since the direct correlation between less RS and proper anesthetic release in the punction area has already been confirmed.5 This study paves the way for a possible change in the routine clinical practice that could be associated—or not—with TA in cream pharmaceutical form. The medical literature includes different and very heterogeneous studies that, whether randomized or not, have tried to assess the utility of this type of creams. However, all of them include small samples (usually less than 100 patients), which makes it difficult to extrapolate the results.
We have a few examples of injected anesthesia vs a composite of TA plus injected anesthesia with favorable results from the latter.17,18 As far as we know, the heterogeneity of designs, and the small sample sizes make us question studies like these.
Although subcutaneous anesthesia—often with lidocaine—has proven to improve pain at the puncture site and reduce the rate of RS compared to TA there is a huge controversy regarding the active principles and drug combination that should be used, the specific action times of these drugs or which are the best pharmaceutical forms. However, it seems that the cream/ointment formulation, and the lidocaine/prilocaine combination (Emla type) yield the best results of all.18
Assuming that this type of formulation is the most widely studied and looking to achieve an adequate design with a representative sample, the E-RADIAL trial (Effectiveness in preventing radial spasm of different vasodilators and topic local anesthesia during transradial cardiac catheterization) has just been started. Although it is not the first trial to propose this hypothesis, it is the first one indeed to confirm it on a double-blind randomized clinical trial and compare it to different radial cocktails and a wide sample size.
This vasolidator comparison is a particularly new approach of our trial. There is some controversy on the use, or not, of such drugs: although some centers in our country do not use vasodilators on a routine basis, it seems to be proven that, overall, its use promotes arterial dilatation and, therefore, the navigability of catheters with lower rates of spasm.9,13 Currently, no such thing as head-on comparisons of cocktails have been drawn in trials to assess their efficacy and safety profile.19 Therefore, we designed our study taking into consideration that a comparison can be drawn among these different drugs in quantitative terms using MLD gain.
Although not part of our study primary endpoints we assume that—with radial access clearly established in the routine clinical practice of cath labs—the operator’s experience, his learning curve or even the rotating fellow/resident’s learning curve can have an impact on the rate of success of puncture, RS, as well as on other complications. This can be an interesting aspect we could discuss. As far as we know both in the current medical literature and good practice recommendations regarding the radial access20—although with limitations depending on the study analyzed—it seems reasonable to assume that the threshold to overtake the learning curve would be at around 30-5021 cases for conventional diagnostic coronary angiography, and > 100-200 cases for complex coronary anatomies22,23 or even in the ST-segment elevation acute coronary syndrome setting. In the E-RADIAL study, all operators widely exceed the number of cases recommended for this curve in diagnostic coronary angiography. Even so, while collecting data for the E-RADIAL we’ll have the possibility to know the identity of the operator who will perform the puncture, his years of experience using radial access, and whether a resident or a novel interventional cardiology (< 2 years of experience) was involved. Also, we will try to know descriptively the rate of puncture success, and whether any RS differences or other complications occurred.
The design of this clinical trial used 4 types of radial cocktail (table 1) from the ones most widely used ones in today’s clinical practice. However, this is also a controversial issue. On the one hand, some centers don’t use vasodilators systematically after radial puncture. On the other hand, choosing one over the other at the cath labs where they’re used is often based on the good clinical results obtained empirically in the routine clinical practice. Unlike the use of heparin to prevent radial occlusion, evidence is scarce regarding benefits from vasodilators, and no homogeneous head-on comparisons have been drawn among different drug cocktails. Verapamil in doses of 5 mg, and nitroglycerin in doses of 200 µg have yielded the best results so far. However, to this date, they have never been compared to one another at these doses or in a randomized way.13 Certain clinical features of the patients can turn the use of these cocktails into a controversial issue. As an example of this, in patients with very severe left ventricular dysfunction or severe aortic stenosis the use of these drugs can trigger significant adverse reactions, mainly hypotension or significant hemodynamic changes. Although, in theory, overall, these drugs are contraindicated in these clinical settings, the dose used, slow infusion, and other factors like the patients’ clinical stability, the existence—or not—of associated heart failure or different comorbidities can turn the use of these drugs into a safe practice. In its design the E-RADIAL study includes a head-on comparison of cocktails and some of the aforementioned drugs and doses. Therefore, it is an opportunity to know what the clinical implication of these drugs really is regarding adverse events.
One of the possible weaknesses or aspects that should be discussed in this trial is pain assessment and quantification. A reproducible design was attempted while assuming the difficulties posed by individual subjectivity. Therefore, following in the footsteps of former studies and registries, we decided to use the most standardized method available to this date in the medical literature: analogue scales.
Another possible weakness or cofounding factor in the study design is the systematic use of sodium heparin via arterial access as standard prevention against radial occlusion.20 According to the drug label24 the heparin-induced cardiac tamponade solution is often an acid solution with a pH between 5.0 and 7.5. The mean arterial pH is between the traditional values of 7.35 and 7.45, and could be partially altered when in contact with heparin solutions thus favoring, through different mechanisms, the development of RS, something not clearly established to this date. To solve this possible bias, the IV—not intraarterial use—of heparin was selected. Although evidence is certainly scarce and heterogeneous the IV use of heparin does not seem to increase the rate of radial occlusion, which is more associated with the heparin dose used and factors like compression time, type of material or size of the radial introducer sheath used that are well established as predictors of radial occlusion.25,26
CONCLUSIONS
The E-RADIAL study is the first randomized clinical trial to assess, on the one hand, the implications of less RS due to topical anesthesia and, on the other, arterial caliber gain with the use of different vasodilators.
FUNDING
None whatsoever.
AUTHORS’ CONTRIBUTIONS
J. J. Portero-Portaz: idea, methodology, validation, formal analysis, drafting of the original project; J. G. Córdoba-Soriano: idea, methodology, review and edition of the manuscript; A. Gutiérrez-Díez: idea, methodology, validation, formal analysis, review and edition of the manuscript; A. Gallardo-López, and D. Melehi El-Assali: idea, methodology, review and edition of the manuscript; L. Expósito-Calamardo, and A. Prieto-Lobato: research, review, and edition of the manuscript; E. García-Martínez, S. Ruiz-Sánchez, M. R. Ortiz Navarro, and E. Riquelme-Bravo: methodology, review, and edition of the manuscript; J. Jiménez-Mazuecos: idea, methodology, review and edition of the manuscript.
CONFLICTS OF INTEREST
Authors declared having no affiliation or participation in any organization or entity with any financial or non-financial interest in the topic at stake or in the materials discussed in this manuscript.
ACKNOWLEDGEMENTS
We wish to thank the nursing personnel of our unit for their work, dedication, and availability during the entire study.
WHAT IS KNOWN ABOUT THE TOPIC?
- RS is the leading cause of access failure in diagnostic or therapeutic coronary angiographies.
- The use of injected local anesthesia is standardized and reduces the rate of RS.
- There is no consensus on the use or non-use of vasodilators, which depends on the characteristics and routine clinical practice of each center.
WHAT DOES THIS STUDY ADD?
- The E-RADIAL study can pave the way to systematization in the use of other type of anesthesia.
- It will provide relevant information on the effectiveness of different vasodilators through head-on comparisons of the most widely used agents.
REFERENCES
1. Romaguera R, Ojeda S, Cruz-González I, Moreno R. Registro Español de Hemodinámica y Cardiología Intervencionista. XXX Informe Oficial de la Asociación de Cardiología Intervencionista de la Sociedad Española de Cardiología (1990-2020) en el año de la pandemia de la COVID-19. Rev. Esp Cardiol. 2021;74:1095-1105.
2. Rao SV, Turi ZG, Wong SC, Brener SJ, Stone GW. Radial versus femoral access. J Am Coll Cardiol. 2013;62(17 Suppl):S11-20.
3. Dandekar VK, Vidovich MI, Shroff AR. Complications of transradial catheterization. Cardiovasc Revasc Med. 2012;13:39-50.
4. Kocayigit I, Cakar MA, Kahyaog˘lu B, Aksoy MNM, Tatli E, Akdemir R. The relationship between serum asymmetric dimethylarginine levels and radial artery spasm. Anatol J Cardiol. 2020;23:228-232.
5. Ho HH, Jafary FH, Ong PJ. Radial artery spasm during transradial cardiac catheterization and percutaneous coronary intervention: incidence, predisposing factors, prevention, and management. Cardiovasc Revasc Med. 2012;
13:193-195.
6. Hudson TL, Dukes SF, Reilly K. Use of local anesthesia for arterial punctures. Am J Crit Care. 2006;15:595-599.
7. France JE, Beech FJ, Jakeman N, Benger JR. Anaesthesia for arterial puncture in the emergency department: a randomized trial of subcutaneous lidocaine, ethyl chloride or nothing. Eur J Emerg Med. 2008;15:218-220.
8. Tran NQ, Pretto JJ, Worsnop CJ. A randomized controlled trial of the effectiveness of topical amethocaine in reducing pain during arterial puncture. Chest. 2002;122:1357-1360.
9. Boyer N, Beyer A, Gupta V, et al. The effects of intra-arterial vasodilators on radial artery size and spasm: implications for contemporary use of trans-radial access for coronary angiography and percutaneous coronary intervention. Cardiovasc Revasc Med. 2013;14:321-324.
10. Ruiz-Salmerón RJ, Mora R, Vélez-Gimón M, et al. Espasmo radial en el cateterismo cardíaco transradial. Análisis de los factores asociados con su aparición y de sus consecuencias tras el procedimiento. Rev Esp Cardiol. 2005;58:504-511.
11. Majure DT, Hallaux M, Yeghiazarians Y, Boyle AJ. Topical nitroglycerin and lidocaine locally vasodilate the radial artery without affecting systemic blood pressure: a dose-finding phase I study. J Crit Care. 2012;27:532.e9-13.
12. Beyer AT, Ng R, Singh A, et al. Topical nitroglycerin and lidocaine to dilate the radial artery prior to transradial cardiac catheterization: a randomized, placebo-controlled, double-blind clinical trial: the PRE-DILATE Study. Int J Cardiol. 2013;168:2575-2578.
13. Kwok CS, Rashid M, Fraser D, Nolan J, Mamas M. Intra-arterial vasodilators to prevent radial artery spasm: a systematic review and pooled analysis of clinical studies. Cardiovasc Revasc Med. 2015;16:484-490.
14. Córdoba-Soriano JG, Rivera-Juárez A, Gutiérrez-Díez A, et al. The Feasibility and Safety of Ambulatory Percutaneous Coronary Interventions in Complex Lesions. Cardiovasc Revasc Med. 2019;20:875-882.
15. Córdoba-Soriano JG, Jiménez-Mazuecos J, Rivera Juárez A, et al. Safety and Feasibility of Outpatient Percutaneous Coronary Intervention in Selected Patients: A Spanish Multicenter Registry. Rev Esp Cardiol. 2017;70:535-542.
16. Gallego-Sánchez G, Gallardo-López A, Córdoba-Soriano JG, et al. Safety of transradial diagnostic cardiac catheterization in patients under oral anticoagulant therapy. J Cardiol. 2017;69:561-564.
17. Tatlı E, Adem Yılmaztepe M, Gökhan Vural M, et al. Cutaneous analgesia before transradial access for coronary intervention to prevent radial artery spasm. Perfusion. 2018;33:110-114.
18. Youn YJ, Kim WT, Lee JW, et al. Eutectic mixture of local anesthesia cream can reduce both the radial pain and sympathetic response during transradial coronary angiography. Korean Circ J. 2011;41:726-732.
19. Shehab A, Bhagavathula AS, Kaes AA, et al. Effect of Vasodilatory Medications on Blood Pressure in Patients Undergoing Transradial Coronary Angiography: A Comparative Study. Heart Views. 2020;21:75-79.
20. Shroff AR, Gulati R, Drachman DE, et al. SCAI expert consensus statement update on best practices for transradial angiography and intervention. Catheter Cardiovasc Interv. 2020;95:245-252.
21. Hess CN, Peterson ED, Neely ML, et al. The learning curve for transradial percutaneous coronary intervention among operators in the United States: a study from the National Cardiovascular Data Registry. Circulation. 2014;
129:2277-2286.
22. Azzalini L, Ly HQ. Letter by Azzalini and Ly regarding article, “The learning curve for transradial percutaneous coronary intervention among operators in the United States: a study from the National Cardiovascular Data Registry”. Circulation. 2015;131:e357.
23. Hamon M, Pristipino C, Di Mario C, et al. European Association of Percutaneous Cardiovascular Interventions; Working Group on Acute Cardiac Care of the European Society of Cardiology; Working Group on Thrombosis on the European Society of Cardiology. Consensus document on the radial approach in percutaneous cardiovascular interventions: position paper by the European Association of Percutaneous Cardiovascular Interventions and Working Groups on Acute Cardiac Care** and Thrombosis of the European Society of Cardiology.EuroIntervention. 2013; 8:1242–1251.
24. Ficha técnica de la heparina sódica. Agencia Española de Medicamentos y Productos Sanitarios. Available online: https://cima.aemps.es/cima/dochtml/ft/56029/FT_56029.html. Accessed 20 Nov 2021.
25. Pancholy SB. Comparison of the effect of intra-arterial versus intravenous heparin on radial artery occlusion after transradial catheterization. Am J Cardiol. 2009;104:1083-1085.
26. Rashid M, Kwok CS, Pancholy S, et al. Radial Artery Occlusion After Transradial Interventions: A Systematic Review and Meta-Analysis. J Am Heart Assoc. 201625;5:e002686.
ABSTRACT
Introduction and objectives: Noncompliant balloon postdilatation of coronary stents improves clinical results. Regular noncompliant balloons (RegNC) have less crossability and a tapered-tip that can complicate successful stent postdilatation. The mechanical conditions of a new spherical tip non-compliant balloon (SphNC) could facilitate stent postdilatation. We tried to evaluate the effectiveness of a new SphNC in the routine percutaneous coronary intervention (PCI) practice.
Methods: Prospective multicenter technical registry to assess the effectiveness of a new SphNC for stent postdilatation with 2 study arms: use of SphNC as the first choice or as the secondary choice after RegNC failure. The primary endpoint was technical success defined as advancing the SphNC across the stent segment. Secondary endpoints were angiographic success defined as technical success and residual stenosis < 30% with final TIMI grade-3 flow, and procedural success defined as angiographic success without mechanical stent complications or any perioperative major adverse cardiovascular events.
Results: The SphNC was used in 263 lesions (177 lesions as first choice, and 86 after RegNC failure) in 250 procedures. The use of the complex technique to advance the SphNC was low (9.9%). Technical, angiographic, and procedural success rates were 98.9%, 98.3%, and 98.3%, respectively, as the first choice, and 98.8%, 97.7%, and 96.5%, respectively, after RegNC failure. SphNC had similar size (3.39 mm ± 0.6 mm vs 3.34 mm ± 0.6 mm; P = nonsignificant), and shorter lengths (11 mm ± 2 mm vs 12 mm ± 3 mm; P = .005) compared to RegNC. No stent-related mechanical complications were reported.
Conclusions: SphNC for coronary stent postdilatation in the routine PCI clinical practice has a very high technical success rate as the first choice (98.9%), as well as in cases of RegNC failure (98.8% with low complex technique requirements, and a safe profile).
Keywords: Complex PCI. Stent postdilatation. Tapered-tip balloon. Spherical tip balloon.
RESUMEN
Introducción y objetivos: La posdilatación de stents coronarios con balones no distensibles mejora los resultados clínicos. Los balones no distensibles normales (RegNC) presentan peor navegabilidad y tienen una punta cónica que puede dificultar la posdilatación exitosa. Las condiciones mecánicas de un nuevo balón no distensible con punta esférica (EsfNC) podrían facilitar la posdilatación del stent. Evaluamos la efectividad del EsfNC en la posdilatación coronaria para la intervención coronaria percutánea en la práctica clínica habitual.
Métodos: Registro técnico prospectivo y multicéntrico para evaluar la efectividad de un nuevo EsfNC en posdilatación coronaria, con 2 grupos de estudio: uso de EsfNC como primera opción o uso de EsfNC ante el fracaso de RegNC. El evento primario fue el éxito técnico, definido como conseguir avanzar el EsfNC hasta el segmento que posdilatar dentro del stent. Los eventos secundarios fueron el éxito angiográfico, definido como éxito técnico junto con estenosis residual < 30% con flujo final TIMI 3, y el éxito del procedimiento, definido como éxito angiográfico sin complicación mecánica del stent ni eventos cardiovasculares mayores periprocedimiento.
Resultados: Se usó EsfNC en 263 lesiones (en 177 como primera opción y en 86 tras el fracaso de RegNC), en 250 procedimientos. Se usaron técnicas complejas para avanzar el EsfNC en el 9,9% de los procedimientos. Los porcentajes de éxito técnico, angiográfico y de procedimiento fueron del 98,9%, el 98,3% y el 98,3% como primera opción, y del 98,8%, el 97,7% y el 96,5% tras fracaso de RegNC, respectivamente. Los EsfNC tuvieron similar calibre (3,39 ± 0,6 frente a 3,34 ± 0,6 mm; p = no significativo) y longitud más corta (11 ± 2 frente a 12 ± 3 mm; p = 0,005) que los RegNC. No se comunicaron complicaciones mecánicas del stent.
Conclusiones: La posdilatación coronaria con EsfNC para la intervención coronaria percutánea en la práctica clínica habitual muestra un porcentaje muy alto de éxito técnico, tanto en primera opción (98,9%) como en casos de fracaso de RegNC (98,8%), con baja necesidad de técnicas complejas y buen perfil de seguridad.
Palabras clave: Intervención coronaria percutánea compleja. Posdilatación coronaria. Balón no distensible. Balón no distensible punta esférica.
Abbreviations
NC: noncompliant balloon. PCI: percutaneous coronary intervention. RegNC: regular noncompliant balloon. SphNC: spherical tip noncompliant balloon.
INTRODUCTION
Optimal stenting is crucial in the long-term clinical outcomes while proper stent expansion and apposition reduce the risk of thrombosis and restenosis.1 Coronary stent postdilatation increases luminal area while reducing stent strut malapposition.2,3
Unlike semicompliant balloons, noncompliant (NC) balloon postdilatation allows uniform dilatation at higher pressures, which reduces the risk of damage to the vessel wall (edge dissection or coronary perforation),4 and is associated with greater stent expansion and a lower rate of target lesion revascularization.5 Therefore, postdilatation using NC balloons is a common strategy to increase the luminal area of underexpanded stents or increase the stent proximal caliber in long lesions or in bifurcation techniques like the proximal optimization technique (POT) or the conventional kissing-balloon technique in a safe and predictable way.6,7
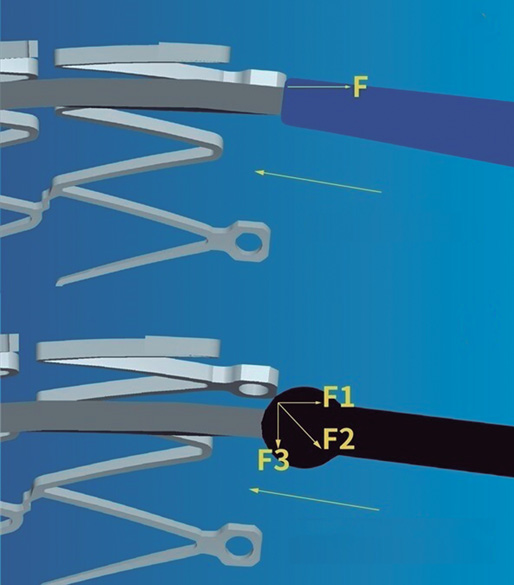
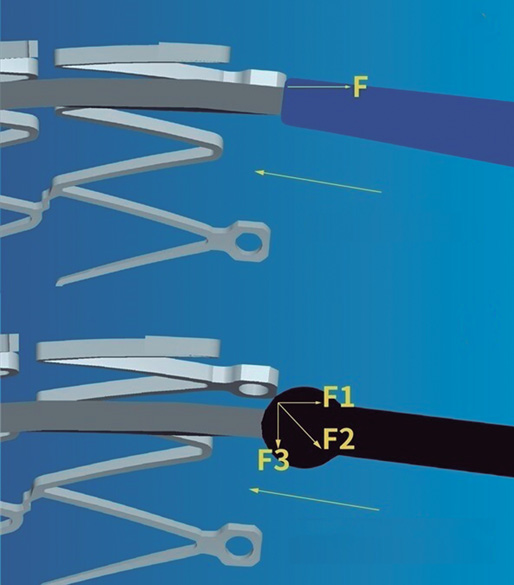
The navigability of NC balloons is more limited, a significant setback in cases of coronary tortuosity, calcified lesions or proximal stent edge malapposition. Regular noncompliant balloons (RegNC) include a cone-shaped tip that can collide with the struts or with the proximal stent edge, thus conditioning a force vector opposed to the push force that can potentially interfere with its advancement (figure 1A), and eventually lead to mechanical stent failure in cases of inadequate coaxiality. In these cases, the use of complex techniques (buddy-wire, buddy-balloon, anchoring...) or specific devices (guide catheter extension systems) are often needed to advance the balloon, which increases the cost of the procedure.
Figure 1. A: the cone-shaped tip of a regular noncompliant balloon can collide with the stent struts thus conditioning a force (F) vector opposed to the push force that can potentially interfere with its advancement. B: the spherical tip contributes to decomposing and reducing the resistance force vector opposed to the push vector, thus facilitating the balloon advancement towards the inside of the stent. Courtesy of APT Medical, China.
The cone-shaped tip has been replaced by a spherical tip in a new NC balloon (NC Conqueror Spherical tip, APT Medical, China) (SphNC). Despite its greater crossing profile (0.039 in), the spherical tip contributes to decomposing and reducing the resistance force vector opposed to the push vector (figure 1B), facilitating the advancement of the balloon until reaching the inside of the stent and the post-dilatable segment.
Figure 2. Actual appearance of the spherical tip noncompliant balloon used in the study. Courtesy of APT Medical, China.
Our objective is to assess the effectiveness of this new SphNC in coronary postdilatation during percutaneous coronary intervention (PCI) in the routine clinical practice.
METHODS
The RECONQUISTHA trial is a prospective and multicenter technical registry conducted in 16 high volume PCI-capable centers (> 500 PCIs/year)8 designed to assess the effectiveness of SphNC in coronary postdilatation during PCI in the routine clinical practice. Since it is a technical registry that used no personal or clinical data on a device approved with CE marking no ethics committee approval or informed consent forms were required.
Inclusion and exclusion criteria
The only inclusion criterion was the indication for coronary postdilatation with the SphNC according to the operator (long, calcified, ostial lesion, bifurcation and angiographic or stent balloon underexpansion). The exclusion criteria were the use of a ≤ 5-Fr guide catheter, vessel size < 2mm or > 5mm, jailed branch postdilatation without previous opening of the stent struts of the main vessel towards such branch or finding 1 of the following scenarios before postdilatation: mechanical stent failure, stent edge dissection ≥ C, coronary perforation or TIMI grade ≤ 2 flow in the main vessel or lateral branch.
Procedure
All lesions were treated with stenting according to the operator’s criterion and according to the routine clinical practice (arterial access, guide catheter caliber, predilatation or plaque modification, and intracoronary imaging). Also, they should be treated with standard antithrombotic treatment (dual antiplatelet therapy with acetylsalicylic acid, and P2Y12 receptor inhibitors prior to the PCI plus weight-adjusted unfractionated heparin at doses of 100 IU/kg with further boluses to achieve activated coagulation times between 250 s and 300 s).
The use of the SphNC was considered in 2 different clinical settings that categorized the lesions into 2 study groups: use of SphNC as first-line treatment, and use of SphNC as a second choice after failed RegNC advancement. Complex techniques like the buddy-wire, buddy-balloon, anchoring or guide catheter extension system were allowed to advance both the RegNC and the SphNC. In cases where the second choice after failed RegNCn advancement was used despite the use of a complex technique, the same complex technique with the SphNC was advised too.
The spherical tip noncompliant balloon
The NC Conqueror Spherical tip balloon (APT Medical, China) is a rapid exchange balloon catheter for percutaneous coronary interventions that is compatible with a 0.014 in intracoronary guidewire. This device has a distinctive tungsten radiopaque spherical tip (0.039 in crossing profile) designed to minimize resistance while advancing the balloon towards the inside of the stent (figure 2). It is available in calibers ranging from 2 mm to 5 mm in intervals of 0.25 mm to 0.5 mm, and lengths of 6 mm, 8 mm, 12 mm, 15 mm, 20 mm, and 30 mm. Nominal pressure stands at 12 atm, and rated pressure burst at around 20 atm (18 atm in 4.5 mm to 5 mm calibers). The device has the CE marking.
Definition of endpoints
The study primary endpoint was technical success defined as the successful advancement of the SphNC until reaching the stent post-dilatable segment. Secondary endpoints were angiographic success—defined as technical success with residual stenosis < 30% with final TIMI grade-3 flow—and procedural success defined as angiographic success without mechanical stent failure or perioperative major adverse cardiovascular events like myocardial infarction—based on the criteria established by the Academic Research Consortium [ARC]-29—stroke, coronary perforation, need for emergency heart surgery or death.
The hypothesis was to consider the study positive if technical success was achieved in > 80% of the lesions regarding the use of the SphNC as the go-to option (according to data published on technical success rates with postdilatation balloons10), and in > 30% regarding the use of the SphNC after failed RegNC advancement (random criterion based on the success of the new balloon in 1 out of 3 cases of failed RegNC advancement).
Data curation
The characteristics of the lesion and the PCI, the indication for postdilatation, any information on the devices used, and quantitative and procedural angiographic results were collected prospectively. Data was introduced in an anonymized electronic database specifically designed for the purpose of the study. Lesions were categorized based on the classification established by the American College of Cardiology and the American Heart Association (ACC/AHA).11 Coronary calcification was defined as moderate whenever coronary radiopacities would be found prior to the injection of contrast or severe whenever these radiopacities would damage both sides of the arterial lumen.12 Coronary tortuosity was defined as moderate if ≥ 3 consecutive curvatures between 45° and 90° were found during diastole or severe if any previous curvatures between 90° and 180° would be found or that encompassed the lesion.13 Angulation inside the lesion was measured as the angle between the start and the end of stenosis. The presence of ostial stenosis > 50% in the branch lateral to the lesion or the need to place the protection guidewire in the lateral branch was considered bifurcation. The stent suboptimal expansion was defined as residual stenosis ≥ 10% on the coronary quantitative angiography after the PCI. Residual stenosis ≥ 30% was considered stent underexpansion (the use of intracoronary imaging was not mandatory). Mechanical stent failure was defined as longitudinal stent deformation or fracture. The patients’ personal or clinical data were not collected.
Statistical analysis
In each of the study groups the overall and individual data were analyzed (SphNC as the go-to option, and as the second choice after failed RegNC advancement). Data was expressed as percentages regarding the categorical variables or as mean and standard deviation regarding the continuous ones. Categorical variables were compared using the chi-square test (or Fisher’s exact test when appropriate). Continuous variables were compared using the Student t test. P values < .05 were considered statistically significant.
RESULTS
From February through June 2021, the SphNC was used in 263 lesions (in 177 lesions as the go-to option, and in 86 lesions after failed RegNC advancement) in a total of 250 procedures. All the lesions were treated with state-of-the-art drug-eluting stents. The characteristics of the lesions and the PCIs, and the immediate angiographic results—both overall and with the use of the SphNC as the first choice or after failed RegNC advancement—are shown on table 1 and table 2. A total of 9.9% of the lesions required complex techniques to move the SphNC forward. Lesions in the failed RegNC group were more unfavorable with a lower rate of direct stenting, greater tortuosity and angulation inside the lesion, more need for cutting balloon during predilatation, shorter SphNC length, and a higher rate of angiographic data of suboptimal stent expansion.
Table 1. Characteristics of the lesions
| Total (N = 263) |
SphNC as the go-to option (N = 177) |
SphNC after failed RegNC (N = 86) |
P* | Total (N = 263) |
SphNC as the go-to option (N = 177) |
SphNC after failed RegNC (N = 86) |
P* | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Target vessel | .27 | AHA classification | .1 | ||||||||
| LAD | 40.7% (107) | 41.8% (74) | 38.4% (33) | A | 2.7% (7) | 4% (7) | 0% (0) | ||||
| LCx | 20.5% (54) | 17.5% (31) | 26.7% (23) | B1 | 23.6% (62) | 26% (46) | 18.6% (16) | ||||
| RCA | 30% (79) | 31.1% (55) | 27.9% (24) | B2 | 45.2% (119) | 44.1% (78) | 47.7% (41) | ||||
| LMCA | 7.6% (20) | 8.5% (15) | 5.8% (5) | C | 26.4% (57) | 26% (46) | 33.7% (29) | ||||
| CABG | 1.2% (3) | 1.1% (2) | 1.2% (1) | Baseline TIMI flow | .65 | ||||||
| Location | .98 | 0 | 21.7% (57) | 20.9% (37) | 23.3% (20) | ||||||
| Proximal | 42.6% (112) | 42.9% (76) | 41.9% (36) | 1 | 1.1% (3) | 1.7% (3) | 0% (0) | ||||
| Medial | 43.3% (114) | 42.9% (76) | 44.2% (38) | 2 | 8.4% (22) | 8.5% (15) | 8.1% (7) | ||||
| Distal | 14.1% (37) | 14.1% (25) | 12% (12) | 3 | 68.8% (181) | 68.9% (122) | 68.6% (59) | ||||
| Calcification | .54 | Bifurcation | 29.7% (78) | 27.7% (49) | 33.7% (29) | .31 | |||||
| Moderate | 39.5% (104) | 41.2% (73) | 36% (31) | 2 stents | 7.6% (20) | 5.6% (10) | 11.6% (10) | .16 | |||
| Severe | 18.6% (49) | 16.9% (30) | 22.1% (19) | Ostial | 11.1% (24) | 13% (23) | 8.1% (7) | .24 | |||
| Tortuosity | <.001 | CTO | 5.7% (15) | 7.9% (14) | 1.2% (1) | .03 | |||||
| Moderate | 35.7% (94) | 35% (62) | 37.2% (32) | STEMI | 16.3% (43) | 13.6% (24) | 22.1% (19) | .08 | |||
| Severe | 6.1% (16) | 1.7% (3) | 15.1% (13) | Lesion on the QCA | |||||||
| Lesion angulation | <.001 | MLD (mm) | 1.01 ± 1.04 | 1.05 ± 1.06 | 0.9 ± 1 | .27 | |||||
| <30 ° | 62.4% (164) | 70.1% (124) | 46.5% (40) | VRD (mm) | 3.34 ± 0.62 | 3.3 ± 0.59 | 3.44 ± 0.65 | .09 | |||
| 30º-70 ° | 30.8% (81) | 26% (46) | 40.7% (35) | Percent diameter stenosis (%) | 83 ± 17 | 82 ± 17 | 84 ± 16 | .48 | |||
| > 70 ° | 6.8% (18) | 4% (7) | 12.8% (11) | Stenotic area (%) | 88 ± 13 | 87 ± 14 | 89 ± 12 | .13 | |||
|
AHA, American Heart Association; CABG, coronary artery bypass graft; CTO, chronic total coronary occlusion; LAD, left anterior descending coronary artery; LCx, left circumflex artery; LMCA, left main coronary artery; MLD, minimal lumen diameter; QCA, quantitative coronary angiography; RCA, right coronary artery; RegNC, cone-shaped tip regular noncompliant balloon; SphNC, spherical tip noncompliant balloon; STEMI, ST-segment elevation myocardial infarction; TIMI, Thrombolysis in Myocardial Infarction; VRD, vessel reference diameter. * P between SphNC groups as the go-to option, and SphNC after failed RegNC. |
|||||||||||
Table 2. Characteristics of percutaneous coronary intervention and angiographic outcomes
| Total (N = 263) |
SphNC as the go-to option (N = 177) |
SphNC after failed RegNC (N = 86) |
P* | Total (N = 263) |
SphNC as the go-to option (N = 177) |
SphNC after failed RegNC (N = 86) |
P* | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Plaque modification | Kissing balloon | 1.9% (5) | 2.3% (4) | 1.2% (1) | ||||||
| Noncompliant balloon | 44.9% (118) | 47.5% (84) | 39.5% (34) | .45 | Other | 1.5% (4) | 1.1% (2) | 2.3% (2) | ||
| Scoring balloon | 12.5% (33) | 9% (16) | 19.8% (17) | .14 | SphNC | |||||
| Cutting balloon | 4.9% (13) | 2.3% (4) | 10.5% (9) | .01 | Caliber (mm) | 3.36 ± 0.55 | 3.34 ± 0.53 | 3.39 ± 0.6 | .5 | |
| Lithotripsy balloon | 1.9% (5) | 1.7% (3) | 2.3% (2) | .66 | Length (mm) | 12 ± 3 | 13 ± 2 | 11 ± 2 | <.001 | |
| Rotational atherectomy | 1.9% (5) | 1.1% (2) | 3.5% (3) | .33 | Atm | 18 ± 3 | 18 ± 2 | 18 ± 3 | .09 | |
| Direct stenting | 14.1% (37) | 16.9% (30) | 8.1% (7) | .05 | Complex technique | .52 | ||||
| Stent | Guide catheter extension system | 7.6% (20) | 7.9% (14) | 7% (6) | ||||||
| Caliber (mm) | 3.07 ± 0.52 | 3.04 ± 0.49 | 3.12 ± 0.57 | .3 | Buddy-wire | 1.9% (5) | 1.1% (2) | 3.5% (3) | ||
| Length (mm) | 27 ± 11 | 27 ± 10 | 27 ± 11 | .95 | Anchoring | 0.4% (1) | 0.6% (1) | 0% (0) | ||
| Atm | 14 ± 2 | 15 ± 2 | 14 ± 2 | .02 | Intracoronary imaging | 9.5% (27) | 9.1% (16) | 10.4% (9) | .51 | |
| Number of stents in the lesion | .47 | QCA after PCI | ||||||||
| 1 | 81.4% (214) | 81.4% (144) | 81.4% (70) | MLD (mm) | 3.23 ± 0.58 | 3.19 ± 0.56 | 3.29 ± 0.61 | .21 | ||
| 2 | 13.7% (36) | 12.4% (22) | 16.3% (14) | Percent diameter stenosis (%) | 4 ± 5 | 3 ± 5 | 4 ± 5 | .18 | ||
| 3 | 5% (13) | 6.2% (11) | 2.3% (2) | Stenotic area (%) | 6 ± 8 | 5 ± 8 | 7 ± 8 | .05 | ||
| Overall stent length (mm) | 32 ± 18 | 32 ± 18 | 32 ± 16 | .96 | Stent expansion | .03 | ||||
| Postdilatation indication | .29 | Optimal | 97.7% (257) | 99.4% (176) | 94.2% (81) | |||||
| Long lesion | 39.9% (105) | 44.6% (79) | 30.2% (26) | Suboptimal | 1.9% (5) | 0.6% (1) | 4.7% (4) | |||
| Suboptimal expansion | 30.8% (81) | 28.2% (50) | 36% (31) | Underexpansion | 0.4% (1) | 0% (0) | 1.2% (1) | |||
| POT | 16% (42) | 13% (23) | 22.1% (19) | Final TIMI grade-3 flow | 99.6% (262) | 99.4% (176) | 100% (86) | 1 | ||
| Calcified lesion | 6.8% (18) | 7.3% (13) | 5.8% (5) | |||||||
| Aorto-ostial lesion | 3% (8) | 3.4% (6) | 2.3% (2) | |||||||
|
Atm, balloon inflation atmospheres; MLD, minimal lumen diameter; PCI, percutaneous coronary intervention; POT, proximal optimization technique in bifurcation; QCA, quantitative coronary angiography; RegNC, cone-shaped tip regular noncompliant balloon; SphNC, spherical tip noncompliant balloon; TIMI, Thrombolysis in Myocardial Infarction. * P between SphNC groups as the go-to option, and SphNC after failed RegNC. |
||||||||||
The overall rates of technical, angiographic, and procedural success were very high and similar between both groups (table 3).
Table 3. Rates of primary and secondary endpoints
| Total (N = 63) | SphNC as the go-top option (N = 177) | SphNC after failed RegNC (N = 86) | P* | |
|---|---|---|---|---|
| Primary endpoint | ||||
| Technical success | 98.9% (260) | 98.9% (175) | 98.8% (85) | 1 |
| Secondary endpoint | ||||
| Angiographic success | 98.1% (258) | 98.3% (174) | 97.7% (84) | .66 |
| Procedural success | 97.7% (257) | 98.3% (174) | 96.5% (83) | .33 |
| Mechanical stent failure | 0% (0) | 0% (0) | 0% (0) | N/A |
| Perioperative complications | 0.4% (1) | 0% (0) | 1.2% (1) | .32 |
|
N/A, non-applicable; RegNC: cone-shaped tip regular noncompliant balloon; SphNC, spherical tip noncompliant balloon. * P between SphNC groups as the go-to option, and SphNC after failed RegNC. |
||||
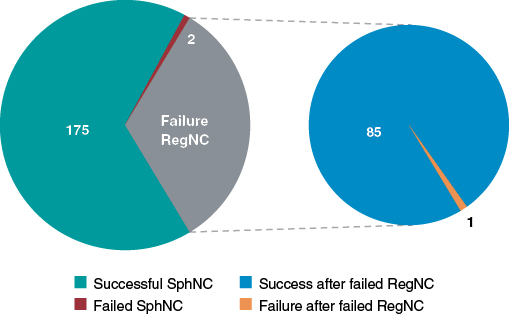
The rate of technical success in the same lesion where the RegNC had failed was very high (98.8%): only 1 SphNC failed too (figure 3). The SphNC and the RegNC had a similar mean caliber (3.39 ± 0.6 mm vs 3.34 ± 0.6 mm; P = .06) while the SphNC had a shorter mean length (11 ± 2 mm vs 12 ± 3 mm; P = .005). The length of the SphNC was shorter, similar or longer in 36%, 46.5%, and 17.5% of the lesions, respectively. The same complex techniques were used to advance the RegNC and the SphNC in 7 lesions (the guide catheter extension system and the buddy-wire technique were used in 6 and 1 cases, respectively). The buddy-wire technique was used in 2 lesions to advance the SphNC, but not previously with the RegNC. In 1 lesion where the RegNC could not be advanced despite anchoring, the SphNC was moved forward without the need for a complex technique. Both the RegNC and the SphNC had been previously used on 3 and 9 occasions, respectively for predilatation purposes.
The description of failed primary and secondary endpoints with the SphNC is shown on table 4. In 2 of the 3 cases without technical success regarding the SphNC, a shorter RegNC was eventually advanced. No instances of mechanical stent failure were reported. One proximal fracture of the catheter hypotube was reported due to excessive resistance during push in 1 SphNC. Nonetheless, the device could be retrieved uneventfully. Only 1 major adverse cardiovascular event was reported: 1 distal branch perforation due to an angioplasty guidewire unrelated with the use of the SphNC that occurred while unsuccessfully trying to advance the RegNC. However, according to the definitions of the study protocol, it was adjudicated as lack of procedural success.
Table 4. Description of cases of failed spherical tip noncompliant balloon
| Case of failed SphNC | Failed event | Use of SphNC | Postdilatation indication | Success of other NC balloons | Complex technique | Complication |
|---|---|---|---|---|---|---|
| Lesion #46 | Technical success | Go-to option | Suboptimal expansion | Yes | No | No |
| Lesion #71 | Technical success | Go-to option | Long lesion | No | No | No |
| Lesion #83 | Technical success | Failed RegNC | POT | Yes | No | Hypotube rupture |
| Lesion #84 | Angiographic success (QCA) | Failed RegNC | Suboptimal expansion | N/A | Guide catheter extension system | No |
| Lesion #224 | Angiographic success (TIMI flow) | Go-to option | Suboptimal expansion | N/A | No | No |
| Lesion #258 | Procedural success | Failed RegNC | POT | N/A | No | Coronary perforation |
|
NA, non-applicable; POT, proximal optimization technique in bifurcation; QCA, quantitative coronary angiography; RegNC, cone-shaped tip regular noncompliant balloon; SphNC, spherical tip noncompliant balloon; TIMI, Thrombolysis in Myocardial Infarction. |
||||||
Figure 3. Overall technical success and failure of the spherical tip noncompliant balloon (SphNC), as well as in cases of failed regular noncompliant balloon (RegNC); section chart on the right.
DISCUSSION
As far as we know, it is the first time that a clinical trial—the REPIC02-RECONQUISTHA—reports on the most extensive experience using SphNC for coronary stent postdilatation. The registry included 16 high volume PCI-capable centers and collected data from 263 lesions where SphNCs were used at the operator’s discretion both as the go-to and second choice after failed RegNC advancement in the same lesion. Based on the initial hypothesis, the study can be considered positive; findings can be summarized as follows: a) very high rates of technical, angiographic and procedural success defined, respectively, as the capacity to move forward towards the inside of the stent and reach an adequate expansion without mechanical stent failure or periprocedural complications; b) very high rates of technical, angiographic and procedural success in the same lesions where the RegNC failed; and c) lower need for complex techniques to achieve technical success.
Over the last few years, the arrival of new techniques and modern devices has facilitated the performance of successful PCIs on more complex lesions in the routine clinical practice. Although the operators were not specifically encouraged to include complex lesions in the study, our data show this reality where over 70% of the lesions were type B2/C, and nearly 50% showed significant calcification, tortuosity or angulation inside the lesion. These characteristics reduce the success of the PCI14,15 and can eventually lead to stent malapposition16 and underexpansion17 or difficulties advancing the devices until reaching such stents, which means that the availability of effective and safe postdilatation balloons is essential to perform successful PCIs.
There is no data in the medical literature to compare or discuss our findings. Our study can be considered positive as it exceeded 80% of the success anticipated in the initial hypothesis. Despite the complexity of the lesions reported, the overall and subgroup outcomes of use of the SphNCs as the go-to option are nothing new since they can be expected in the assessment of any NC balloons (rate of success and proper stent expansion > 90%)10 since it is rare to find difficulties or impossibilities if complex techniques are used to advance these devices. However, these maneuvers can lead to severe complications like mechanical stent deformation.18,19 The lack of mechanical stent failure in our series places the SphNC as an effective and safe device for coronary postdilatation.
After the first 200 procedures, the percentage of cases where the SphNC was used in lesions where the RegNC would have failed was low. Since focus was on assessing the SphNC performance in this context, only inclusions in this subgroup were allowed later on. As already mentioned, the rate of RegNC failure is rare, and the rhythm of inclusion of the next 50 procedures was a slower. We designed this study group considering that the sequential use of a SphNC in the same lesion where a RegNC had failed would show the potential benefit of this new device. The SphNC achieved technical success in 98.8% of the lesions in this subgroup and validated its superiority in the exact same lesions where the RegNC had failed, which can be considered the most valuable piece of information from our study. The mean SphNC length was shorter compared to the RegNC (a 1 mm difference, which is statistically significant due to similar and narrow standard deviations, yet of uncertain practical significance). However, the operators used SphNCs and RegNCs of similar length in most of the lesions. In this study subgroup, lesions were more unfavorable, which may explain the rate of failure with RegNCs, the discretely low rate of angiographic and procedural success reported, and the presence of the complications described (fracture of the SphNC hypotube or coronary perforation).
It has been reported that the tortuosity and angulation seen until the lesion are predictors of failed PCI or perioperative complications.14,15,20 In our series, their prevalence was high—around 40%—and up to 50% in lesions where the RegNC failed. Several complex techniques for the management of these anatomies have been described,21 but they increase procedural time and cost. Eddin et al.22 determined that tortuosity and angulation were the main predictors for the use of a guide catheter extension system. Also, angulations > 45° proximal to the lesion predict its use with a 73% sensitivity and a 74% specificity. In different series, tortuosity and angulation justify the use of a guide catheter extension system in 22% to 43% of the cases.18 Despite the significant tortuosity and angulation of our series, the need for a guide catheter extension system or any other kind of complex technique to advance the SphNC was low (< 8% and 10% respectively), which is why this device emerges as a useful tool in the coronary tortuosity setting with potential to reduce procedural costs.
The study design was moderately ambitious since we hypothesized that if the SphNC were successful in 1 out of 3 lesions where the RegNC had failed this outcome would have been good enough for the new device. The fact that it exceeded the success rate of 30% proposed in the hypothesis makes us think of the results as positive. To better understand these outcomes, 5 videos have been provided as supplementary data including examples of failed RegNCs and succesful SphNCs in the same lesion.
Limitations
Despite its prospective design, the study has several limitations. The indication of postdilatation with SphNC based only on the operator’s criterion may have conditioned selection biases, thus preventing the inclusion of very unfavorable lesions. The study design does not allow us to assess the superiority of the SphNC over the RegNC regarding the lower need for complex techniques, mechanical stent failure or better angiographic and procedural outcomes. The use of intracoronary imaging was low, and a more comprehensive assessment of stent expansion with imaging techniques could have changed the data of the PCI final outcomes and, consequently, the secondary endpoints. The SphNC recrossing after first inflation was anecdotal and is, therefore, ill-advised. We should mention that our results cannot be extrapolated to coronary predilatation because the device has not been tested prior to stenting. Finally, the lack of follow-up to monitor the patients’ clinical course does not allow us to assess the clinical impact derived from the use of SphNC.
CONCLUSIONS
Coronary postdilatation with the SphNC during PCI in the routine clinical practice has a very high rate of technical success both as first choice (98.9%), and in cases of failed RegNC advancement (98.8%) with a lower need for complex techniques, and a good safety profile.
FUNDING
RECONQUISTHA is an investigator-initiated trial promoted and developed by Fundación EPIC, Spain as the clinical research organization sponsored by IZASA Medical, Spain. All the authors received research grants for their participation in the study.
AUTHORS’ CONTRIBUTIONS
J. A. Linares Vicente: design, data curation, analysis, and interpretation, and manuscript drafting. A. Pérez de Prado, and J. R. Rumoroso Cuevas: design, data curation, manuscript drafting, and critical review of its content. K. García San Román, F. Lozano Ruiz-Póveda, G. Veiga Fernández, A. Gómez Menchero, G. Moreno Terribas, G. Miñana Escrivà, J. Sánchez Gila, C. Arellano Serrano, G. Martín Cáceres, P. Bazal Chacón, P. Martín Lorenzo, F. Rebollal Leal, and J. Moreu Burgos: data curation, critical review of the content of the manuscript, and final approval.
CONFLICTS OF INTEREST
A. Pérez de Prado is an associate editor of REC: Interventional Cardiology; the journal’s editorial procedure to ensure impartial handling of the manuscript has been followed. J. A. Linares has received lecture fees from IZASA Medical, Spain.
WHAT IS KNOWN ABOUT THE TOPIC?
- Coronary stent postdilatation with NC balloons is associated with better clinical outcomes. The complexity of PCIs in the routine clinical practice is on the rise. The navigability of RegNCs is limited, and their cone-shaped tip can complicate moving forward inside of the stent. Therefore, success could be limited in in complex lesions.
WHAT DOES THIS STUDY ADD?
- In the routine clinical practice, coronary postdilatation using SphNC while performing a PCI has a very high rate of technical success even in complex clinical settings where the RegNC has failed (especially in coronary tortuosity), a lower need for complex techniques, and a good safety profile. Therefore, it could be considered as the go-to option for coronary postdilatation when performing complex PCIs.
SUPPLEMENTARY DATA
Vídeo 1. Linares Vicente J.A. DOI: 10.24875/RECICE.M22000289
Vídeo 2. Linares Vicente J.A. DOI: 10.24875/RECICE.M22000289
Vídeo 3. Linares Vicente J.A. DOI: 10.24875/RECICE.M22000289
Vídeo 4. Linares Vicente J.A. DOI: 10.24875/RECICE.M22000289
Vídeo 5. Linares Vicente J.A. DOI: 10.24875/RECICE.M22000289
REFERENCES
1. Takano Y, Yeatman LA, Higgins JR, et al. Optimizing stent expansion with new stent delivery systems. J Am Coll Cardiol. 2001;38:1622-1627.
2. Romagnoli E, Sangiorgi GM, Cosgrave J, Guillet E, Colombo A. Drug-Eluting Stenting. The Case for Post-Dilation. JACC: Cardiovasc Interv. 2008;
1:22-31.
3. Brodie BR, Cooper C, Jones M, Fitzgerald P, Cummins F. Is adjunctive balloon postdilatation necessary after coronary stent deployment? Final results from the POSTIT trial. Catheter Cardiovasc Interv. 2003;59:184-192.
4. Seth A, Gupta S, Singh VP, Kumar V. Expert Opinion: Optimising stent deployment in contemporary practice: The role of intracoronary imaging and non-compliant balloons. Interv Cardiol. 2017;12:81-84.
5. Pasceri V, Pelliccia F, Pristipino C, et al. Clinical effects of routine postdilatation of drug-eluting stents. Catheter Cardiovasc Interv. 2014;83:898-904.
6. Mylotte D, Hovasse T, Ziani A, et al. Non-compliant balloons for final kissing inflation in coronary bifurcation lesions treated with provisional side branch stenting: A pilot study. EuroIntervention. 2012;7:1162-1169.
7. Park TK, Lee JH, Song YB, et al. Impact of non-compliant balloons on long-term clinical outcomes in coronary bifurcation lesions: Results from the COBIS (COronary BIfurcation Stent) II registry. EuroIntervention. 2016;12:456-464.
8. Moreno R, Ojeda S, Romaguera R, et al. Actualización de las recomendaciones sobre requisitos y equipamiento en cardiología intervencionista. REC Interv Cardiol. 2021;3:33-44.
9. Garcia-Garcia HM, McFadden EP, Farb A, et al. Standardized End Point Definitions for Coronary Intervention Trials: The Academic Research Consortium-2 Consensus Document. Circulation. 2018;137:2635-2650.
10. Secco GG, Buettner A, Parisi R, et al. Clinical Experience with Very High-Pressure Dilatation for Resistant Coronary Lesions. Cardiovasc Revasc Med. 2019;20:1083-1087.
11. Ryan TJ, Faxon DP, Gunnar RM, et al. Guidelines for percutaneous transluminal coronary angioplasty. A report of the American College of Cardiology/American Heart Association Task Force on Assessment of Diagnostic and Therapeutic Cardiovascular Procedures (Subcommittee on Percutaneous Transluminal Coronary Angioplasty). Circulation. 1988;78:486-502.
12. Madhavan MV, Tarigopula M, Mintz GS, Maehara A, Stone GW, Généreux P. Coronary Artery Calcification. J Am Coll Cardiol. 2014;63:1703-1714.
13. Jakob M, Spasojevic D, Krogmann ON, Wiher H, Hug R, Hess OM. Tortuosity of coronary arteries in chronic pressure and volume overload. Cathet Cardiovasc Diag. 1996;38:25-31.
14. Ellis SG, Vandormael MG, Cowley MJ, et al. Coronary morphologic and clinical determinants of procedural outcome with angioplasty for multivessel coronary disease. Implications for patient selection. Multivessel Angioplasty Prognosis Study Group. Circulation. 1990;82:1193-1202.
15. Moushmoush B, Kramer B, Hsieh AM, Klein LW. Does the AHA/ACC task force grading system predict outcome in multivessel coronary angioplasty? Cathet Cardiovasc Diag. 1992;27:97-105.
16. Wang B, Mintz GS, Witzenbichler B, et al. Predictors and Long‐Term Clinical Impact of Acute Stent Malapposition: An Assessment of Dual Antiplatelet Therapy With Drug‐Eluting Stents (ADAPT‐DES) Intravascular Ultrasound Substudy. J Am Heart Assoc. 2016;5:e004438.
17. Komaki S, Ishii M, Ikebe S, et al. Association between coronary artery calcium score and stent expansion in percutaneous coronary intervention. Int J Cardiol. 2021;334:31-36.
18. Fabris E, Kennedy MW, di Mario C, et al. Guide extension, unmissable tool in the armamentarium of modern interventional cardiology. A comprehensive review. Int J Cardiol. 2016;222:141-147.
19. Arnous S, Shakhshir N, Wiper A, et al. Incidence and mechanisms of longitudinal stent deformation associated with Biomatrix, Resolute, Element, and Xience stents: Angiographic and case-by-case review of 1,800 PCIs. Catheter Cardiovasc Interv. 2015;86:1002-11.
20. Ellis SG, Topol EJ. Results of percutaneous transluminal coronary angioplasty of high-risk angulated stenoses. Am J Cardiol. 1990;66:932-7.
21. Saeed B, Banerjee S, Brilakis ES. Percutaneous Coronary Intervention in Tortuous Coronary Arteries: Associated Complications and Strategies to Improve Success. J Interv Cardiol. 2008;21:504-511.
22. Eddin MJ, Armstrong EJ, Javed U, Rogers JH. Transradial interventions with the GuideLiner catheter: Role of proximal vessel angulation. Cardiovasc Revasc Med. 2013;14:275-279.

ABSTRACT
Introduction and objectives: Systemic coronary artery embolism is one of the mechanisms of acute myocardial infarction of nonatherosclerotic origin. However, the epidemiological, clinical, and angiographic profile of this entity has not been properly established yet. Our objective was to describe the clinical characteristics, angiographic features, and prognosis of acute coronary syndromes (ACS) due to systemic embolism (ACS-E), compare them to those due to coronary atherosclerosis (ACS-A), and identify predictive clinical factors of ACS-E.
Methods: All consecutive patients with ACS—admitted to a tertiary hospital from 2003 through 2018—were classified as ACS-E (n = 40) or ACS-A (n = 4989), and prospectively recruited on a multipurpose database.
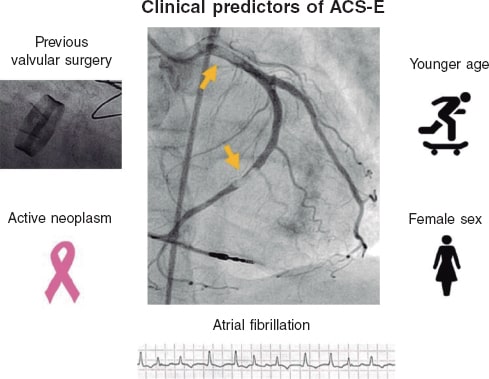
Results: Patients with ACS-E were younger (27.5% vs 9.6% were < 45 years old, P < .001), more often women (42.5% vs 22.5%, P = .003), and had higher rates of atrial fibrillation (AF) (40.0% vs 5.3%, P < .001), previous stroke (15.0% vs 3.6%, P < .001), active neoplasms (17.5% vs 6.9%, P =.009), and previous valvular surgery (12.5% vs 0.5%, P < .001). Also, a higher proportion of them were on warfarin (27.5% vs 2.9%, P < .001). The most frequent culprit vessel was the left anterior descending coronary artery in both groups. A percutaneous coronary intervention was attempted in all patients with ACS-A, and in 75.0% of those with ACS-E (P < .001) being successful in 99.1% and 80.0%, respectively. The in-hospital all-cause mortality rate was 15.0% regarding ACS-E, and 4.0% in the control group (P < .001). A multivariate analysis was performed to study the independent predictors of ACS-E, identify AF, previous valvular surgery, and active neoplasms, younger age, and female sex.
Conclusions: ACS-E and ACS-A have different clinical and angiographic characteristics. Atrial fibrillation, previous valvular surgery, active neoplasms, younger age, and female sex were all independent predictors of ACS-E.
Keywords: Coronary artery embolism. Atrial fibrillation. Acute coronary syndrome. Myocardial infarction.
RESUMEN
Introducción y objetivos: La embolia coronaria de origen sistémico representa uno de los mecanismos de infarto agudo de miocardio de causa no aterosclerótica. Sin embargo, el perfil epidemiológico, clínico y angiográfico de esta entidad no ha sido aún bien definido. Nuestro objetivo fue describir las características clínicas y angiográficas y el pronóstico de los síndromes coronarios agudos (SCA) de origen embólico (SCA-E), compararlos con aquellos debidos a aterosclerosis (SCA-A) e identificar predictores clínicos de SCA-E.
Métodos: Todos los pacientes con SCA atendidos en un hospital terciario entre 2003 y 2018 se clasificaron en SCA-E (n = 40) o SCA-A (n = 4.989) e incluidos de forma prospectiva en un registro multipropósito.
Resultados: Entre los pacientes con SCA-E existía mayor proporción de jóvenes (27,5 frente a 9,6% tenían menos de 45 años, p < 0,001), mujeres (42,5 frente a 22,5%, p = 0,003), fibrilación auricular (FA) (40,0 frente a 5,3%, p < 0,001), neoplasias activas (17,5 frente a 6,9%, p = 0,009), cirugía valvular previa (12,5 frente a 0,5%, p < 0,001) y una mayor proporción de los mismos se encontraba en tratamiento con warfarina (27,5 frente a 2,9%, p < 0,001). El vaso responsable con mayor frecuencia fue la descendente anterior en ambos grupos. En todos los pacientes con SCA-A se llevó a cabo una intervención coronaria percutánea, frente al 75,0% de los pacientes con SCA-E (p < 0,001), la cual se completó con éxito en el 99,1% y el 80,0% de los casos, respectivamente. La mortalidad por todas las causas en el grupo de SCA-E fue del 15,0% frente al 4,0% en el grupo control (p < 0,001). Se llevó a cabo un análisis multivariante para estudiar predictores independientes de SCA-E, identificando la FA, la cirugía valvular previa, la presencia de una neoplasia activa, una menor edad y el sexo femenino.
Conclusiones: Los SCA-E y los SCA-A presentan características clínicas y angiográficas diferentes. La FA, la cirugía valvular previa, la presencia de una neoplasia activa, ser más joven y el sexo femenino son predictores independientes de SCA-E.
Palabras clave: Embolia coronaria. Fibrilacion auricular. Sindrome coronario agudo. Infarto de miocardio.
Abbreviations
ACS: acute coronary syndrome. ACS-A: acute coronary syndrome due to atherosclerosis. ACS-E: acute coronary syndrome due to systemic embolism. AF: atrial fibrillation. AMI: acute myocardial infarction. STEMI: ST-segment elevation myocardial infarction.
INTRODUCTION
Systemic coronary artery embolism is one of the mechanisms of acute myocardial infarction (AMI) of non-atherosclerotic origin and represents 3% to 14% of all acute coronary syndromes (ACS) reported, according to angiographic and autopsy studies. However, the real prevalence of this entity remains unknown due to the uncertainty of its diagnosis in the acute setting.1,2
Atrial fibrillation (AF), cardiomyopathies, valvular heart disease, malignancies, and infective endocarditis have previously been associated with ACS due to systemic embolism (ACS-E).1,3 Nevertheless, the epidemiological, clinical, and angiographic profile of this entity has not been properly established yet.
Our objective was to describe the clinical characteristics, angiographic features, therapeutic management, and prognosis of ACS-E, compare it to ACS due to coronary atherosclerosis (ACS-A), and identify predictive clinical factors of ACS-E.
METHODS
Study population
All consecutive patients with ACS—admitted to a tertiary hospital from January 2003 through December 2018—were evaluated, classified as ACS-E or ACS-A, and prospectively recruited on a multipurpose database. The protocol was approved by the local ethics committee (internal code 22/137-E), and patients’ informed consent was waived because it involved only the analysis of data obtained during standard clinical practice.
AMI was defined as elevated cardiac troponin levels (myocardial injury) with clinical evidence of acute myocardial ischemia including symptoms, new ischemic electrocardiographic changes, development of pathological Q waves on the electrocardiogram, new regional wall motion abnormalities in a pattern consistent with ischemic aetiology, and/or angiographic identification of a coronary thrombus.4 All patients underwent a thorough diagnostic work-up including detailed clinical histories and physical examinations, serial electrocardiograms, blood tests, transthoracic echocardiographies, and invasive coronary angiographies. Intracoronary imaging techniques like optical coherence tomography or intravascular ultrasound were left to the operator’s discretion.
Diagnosis of ACS-E was achieved according to the angiographic evidence of coronary artery thrombosis without atherosclerotic components, concomitant multi-site coronary artery embolism or concomitant systemic embolization excluding left ventricular thrombus due to AMI.1 Only emboli of principal coronary arteries were considered. Patients with the following angiographic findings were systematically excluded: a) presence of atherosclerosis at culprit lesion level, b) evidence of > 25% coronary artery stenosis outside the culprit lesion, c) plaque rupture or coronary erosion at culprit lesion level found on the intravascular imaging, d) coronary artery ectasia, and e) other causes of non-atherosclerotic AMI (vasospasm, spontaneous coronary artery dissection).
Angiographic evaluation of the culprit site was performed by 2 expert operators with an intention to rule out a) the presence of thrombus (defined as noncalcified filling defect outlined by contrast media), b) presence of angiographic stenosis, and c) signs of atherosclerosis (eg, vessel wall calcification). The rest of the angiogram was assessed looking for angiographic stenosis or atherosclerosis.
Clinical events
Epidemiological data, clinical features, angiographic characteristics, management, and outcomes were prospectively collected as patients were recruited and retrospectively analysed. The long-term follow-up of ACS-E was performed by monitoring any recurrences of systemic emboli (including cardiogenic stroke), and the occurrence of major adverse cardiovascular and cerebrovascular events including cardiac death, myocardial infarction, new percutaneous coronary intervention (PCI), hospitalization due to heart failure or stroke more than 30 days after admission due to ACS-E.
In the present study, we first performed a detailed description of the episodes of ACS-E followed by a comparison to ACS-A to identify clinical peculiarities, and predictors.
Statistical analysis
Quantitative variables were expressed as median and interquartile range [IQR] or mean and standard deviation. The assessment of normality and equality of variances for continuous data was performed using the Shapiro-Wilk test and the Levene test, respectively. Thereafter, continuous variables were compared using the Student t test, the Fisher-Pittman permutation test or the median test when appropriate. Categorical variables were expressed as frequencies and percentages.
Variables in which statistically significant differences were seen in the univariate model and those clinically relevant were introduced in a multivariate analysis using stepwise logistic regression to identify clinical predictors of ACS-E.
All tests were 2-sided, and differences were considered statistically significant with P values < .05. Statistical analyses were performed using Stata/IC12.1 statistical software package (StataCorp, College Station, Texas, United States).
RESULTS
During the study period, a total of 5029 patients with ACS were included. After applying the previously described diagnostic criteria, 40 patients (0.8%) were classified as ACS-E and 4989 (99.2%) as ACS-A.
Acute coronary syndrome due to systemic embolism population
Regarding patients with ACS-E, 17 were women (42.5%), and the population’s mean age was 60.3 years old. A total of 2 patients (5.0%) had a past medical history of exertional angina, 4 (10.0%) carried a prosthetic valve, and 2 (5.0%) and 1 (2.5%) had non-corrected severe mitral regurgitation, and severe aortic stenosis, respectively. The mean left ventricular ejection fraction was 55.0% ± 12.3%, and 16 patients (40.0%) had any form of AF. Also, 1 patient (2.5%) was diagnosed with infective endocarditis in the aortic valve right after being admitted due to ACS. Regarding other medical conditions, 7 patients (17.5%) had active neoplasms, and 3 (7.5%) chronic kidney disease. Information associated with other baseline characteristics is shown on table 1 of the supplementary data.
Table 1. Baseline epidemiological and clinical characteristics
| ACS-A N = 4989 | ACS-E N = 40 | P | |
|---|---|---|---|
| Age (years) | 63.0 ± 13.4 | 60.3 ± 18.7 | .129 |
| Age < 45 years | 480 (9.6) | 11 (27.5) | < .001 |
| Age > 80 years | 559 (11.2) | 9 (22.5) | .025 |
| Female sex | 1120 (22.5) | 17 (42.5) | .003 |
| Diabetes | 1087 (21.8) | 4 (10.0) | .070 |
| Hypertension | 2632 (52.8) | 16 (40.0) | .108 |
| Dyslipidemia | 2192 (43.9) | 11 (27.5) | .037 |
| Smoking | 3101 (62.2) | 22 (55.0) | .353 |
| BMI | 27.6 ± 4.1 | 27.1 ± 4.2 | .424 |
| Chronic kidney failure | 239 (4.8) | 3 (7.5) | .425 |
| Peripheral vascular disease | 241 (4.8) | 1 (2.5) | .493 |
| Stroke | 181 (3.6) | 6 (15.0) | < .001 |
| Active neoplasm | 343 (6.9) | 7 (17.5) | .009 |
| AF | 262 (5.3) | 16 (40.0) | < .001 |
| Treatment with warfarin | 143 (2.9) | 11 (27.5) | < .001 |
| Previous valvular surgery | 25 (0.5) | 5 (12.5) | < .001 |
| Past medical history of angina | 1698 (34.0) | 2 (5.0) | < .001 |
|
ACS-A, acute coronary syndrome due to atherosclerosis; ACS-E, acute coronary syndrome due to systemic embolism; AF, atrial fibrillation; BMI, body mass index; NSTEMI, non-ST-elevation acute myocardial infarction; STEMI, ST-segment elevation myocardial infarction. |
|||
A total of 32 patients (80.0%) had ST-segment elevation myocardial infarction (STEMI) 3 of whom received fibrinolytic therapy, undergoing bailout PCI in 2 of the cases. A total of 28 patients (70.0%) underwent a primary PCI and the remaining 12 (30.0%) were catheterized in another scenario. The most frequent culprit vessel was the left anterior descending coronary artery (LAD) that accounted for 13 (32.5%) of the cases followed by the right coronary artery (n = 10; 25.0%), and the left circumflex artery (n = 9; 22.5%). Besides, the proximal (n = 12; 30.0%) and medium (n = 12; 30.0%) segments of the vessels were the ones most often compromised (table 2 of the supplementary data).
| ACS-A N = 4989 | ACS-E N = 40 | P | |
|---|---|---|---|
| Culprit lesions | |||
| LMCA | 113 (2.3%) | 1 (2.5%) | .921 |
| LAD | 2274 (45.6%) | 15 (37.5%) | .108 |
| Cx | 1064 (21.3%) | 11 (27.5%) | .344 |
| RCA | 1902 (38.1%) | 10 (25.0%) | .125 |
| Number of vessels with moderate lesions (> 50%) | 1.6 ± 0.0 | 0.8 ± 0.1 | < .001 |
| Number of vessels with severe lesions (> 70%) | 1.3 ± 0.0 | 0.8 ± 0.1 | < .001 |
|
ACS-A, acute coronary syndrome due to atherosclerosis; ACS-E, acute coronary syndrome due to systemic embolism; Cx, circumflex coronary artery; LAD, left anterior descending coronary artery; LMCA, left main coronary artery; PCI, percutaneous coronary intervention; RCA, right coronary artery. |
|||
On the coronary angiography, 25 patients (62.5%) showed TIMI grade-0 flow (Thrombolysis in Myocardial Infarction) before crossing the wire. Twenty-nine cases (72.5%) received thrombus aspiration therapy and 7 underwent balloon angioplasty. None of the patients were treated with stenting, but TIMI grade-3 flow was observed in 32 cases (77.5%) after the PCI (table 3 of the supplementary data). Regarding intracoronary imaging during the PCI, pre- and postoperative optical coherence tomography and intravascular ultrasound were performed in 3 (7.5%) and 1 (2.5%) patients, respectively. Antithrombotic treatment at presentation and after PCI is shown on table 4 of the supplementary data.
Table 3. Complications during PCI, hospitalization, and follow-up in patients with ACS-E
| During PCI | |
| Cardiac arrest | 3 (7.5) |
| Slow flow/no reflow | 8 (20.0) |
| Perforation | 1 (2.5) |
| Embolizationa | 15 (37.5) |
| Coronary dissection | 0 (0) |
| Coronary perforation | 1 (2.5) |
| Cardiac tamponade | 0 (0) |
| During admission | |
| Vascular complicationsb | 2 (5.0) |
| Heart failure | 12 (30.0) |
| Arrhythmic complicationsc | 7 (17.5) |
| Extracardiac complicationsd | 9 (22.5) |
| Death | 6 (15.0) |
| At the follow-up | |
| MACCE | 13 (38.2) |
| AMI | 4 (11.8) |
| New PCI | 2 (5.9) |
| Stroke | 2 (5.9) |
| Hospitalization | 11 (32.4) |
| Heart failure | 11 (32.4) |
| NYHA | |
| I | 24 (70.6) |
| II | 6 (17.6) |
| III | 1 (2.9) |
| IV | 4 (11.8) |
| Systemic embolism | 0 (0) |
| Pulmonary embolism | 1 (2.9) |
| Deathe | 12 (35.3) |
|
AMI, acute myocardial infarction; PCI, Percutaneous coronary intervention; MACCE, major adverse cardiovascular and cerebrovascular events; NYHA, New York Heart Association Functional Classification. a In 2 cases, embolization of thrombotic material reached a different vessel from the culprit one. b 1 case of femoral pseudoaneurysm and radial pseudoaneurysm, respectively were treated with conservative measures. c 4 cases of bradyarrhythmia and 3 cases of tachyarrhythmia. d 8 cases of infection and 1 case of stroke coexisting with subarachnoid haemorrhage. e Due to heart failure in 5 cases, ventricular arrythmia in the AMI setting in 1 case and multiorgan failure due to advanced pulmonary neoplasm in a different case. In the remaining the patients, the cause of death could not be identified. |
|
Table 4. Multivariate analysis to identify clinical predictors of acute coronary syndrome due to systemic embolism
| Variables* | Adjusted OR (95%CI) | P |
|---|---|---|
| Age (years) | 0.95 (0.92-0.97) | < .001 |
| Female sex | 2.80 (1.37-5.65) | .007 |
| Dyslipidemia | 0.45 (0.22-0.93) | .024 |
| Active neoplasm | 3.37 (1.33-8.54) | .019 |
| Previous valvular surgery | 4.28 (1.19-15.5) | .038 |
| Past medical history of angina | 0.17 (0.05-0.55) | < .001 |
| AF | 16.10 (7.23-35.9) | < .001 |
|
95%CI, 95% confidence interval; AF, atrial fibrillation; OR, odds ratio. * Variables from the univariate model introduced in the analysis included: age, female sex, diabetes, dyslipidemia, stroke, active neoplasm, previous valvular surgery, past medical history of angina, AF, and chronic treatment with oral anticoagulants. |
||
Comparison between acute coronary syndrome due to systemic embolism and acute coronary syndrome due to atherosclerosis
Baseline characteristics
Compared to ACS-A, there was a significantly higher proportion of patients under 45 and over 80 years old in the ACS-E group. Besides, a higher proportion of women was observed (42.5% vs 22.5%; P = .003). Among these patients, cardiovascular risk factors were less prevalent compared to those with ACS-A, although statistically significant differences were only seen regarding dyslipidemia. A significantly higher proportion of patients with ACS-E had active neoplasms, AF, previous strokes, and had undergone heart valve surgery. Also, 27.5% of the patients from the ACS-E group were on warfarin (P < .001) at presentation whereas the patients with ACS-A often had a past medical history of angina (34.0% vs 5.0%; P < .001). The inter-group differences regarding other medical conditions are also shown on table 1.
Clinical and angiographic characteristics and outcomes
Regarding the episode of ACS, no differences were seen regarding the presentation as STEMI between both groups (ACS-E, 80.0% vs ACS-A, 67.0%; P = .082). However, patients with ACS-A showed significantly longer times between the diagnosis of ACS and the performance of a coronary angiography (1.16 ± 0.8 hours vs 0.81 ± 0.5 hours; P = .003). No differences were seen regarding the rate of cardiogenic shock.
The presence of other moderate or severe stenoses, apart from the culprit one, was significantly more frequent among patients with ACS-A (table 2). PCI was attempted in all the patients with ACS-A and in 75.0% of those with ACS-E (P < .001) being successful in 99.1% and 80.0%, respectively. Conversely, adjuvant treatment with GP IIb/IIIa inhibitors was used in 55.0% of the patients with ACS-E and 36.0% of the patients from the ACS-A group (P = .020).
Complications during PCI and hospitalization in the ACS-E group are shown on table 3 including death that occurred in 5 patients (12.5%) due to heart failure/cardiogenic shock and anoxic encephalopathy after cardiac arrest in another case. A control coronary angiography was performed in 14 cases (40.0%) with persistence of culprit vessel compromise in 2 (14.3%). The median follow-up after the episode was 5.8 ± 4.8 years. Three days after emergency thrombus aspiration due to acute LAD occlusion, a 51-year-old woman with acute myeloid leukemia presented a recurrent ACS-E with new compromise of both the LAD and a marginal branch. No recurrent emboli in other systemic territories were identified in any of the cases. However, major adverse cardiovascular and cerebrovascular events at the follow-up occurred in 13 patients with ACS-E (38.2%) while death occurred in 12 patients (35.3%) being attributed to cardiac causes in 6 cases (50.0%) (table 3). The overall major adverse cardiovascular and cerebrovascular events-free survival during hospitalization and at the follow-up was estimated using Kaplan-Meier curves (figure 1).
Figure 1. MACCE-free survival during admission and at the 4-year follow-up in patients with ACS-E. ACS-E, acute coronary syndrome due to systemic embolism, FU, follow-up; IQR, interquartile range; MACCE, major adverse cardiovascular and cerebrovascular events.
The in-hospital all-cause mortality rate was 15.0% in the ACS-E group and 4.0% in the control group (P < .001).
Predictors of acute coronary syndrome due to systemic embolism
To determine the clinical predictors of ACS-E, a multivariate analysis was performed including those variables with statistically significant differences in the univariate model and those considered clinically relevant. Therefore, younger age, female sex, an active neoplasm, previous heart valve surgery, and a past medical history of AF were identified as independent predictive factors for ACS-E (table 4, figure 2).
Figure 2. Independent clinical predictors of ACS-E. ACS-E, acute coronary syndrome due to systemic embolism.
DISCUSSION
The main findings of our study include a) the prevalence of ACS-E in patients admitted due to AMI was low (0.8%); b) the in-hospital mortality rate was higher among patients with ACS-E as compared to ACS of atherosclerotic origin; and c) being younger, female sex, an active neoplasm, previous heart valve surgery, and AF were identified as ACS-E predictors.
Systemic coronary artery embolism is one of the underlying mechanisms of AMI of non-atherosclerotic cause.4 First autopsy studies reported a prevalence of coronary emboli in patients with AMI of 13%5 although subsequent studies conducted at the clinical setting described a frequency of around 3%.1 The low prevalence seen in our series (0.8%) may be associated with strict diagnostic criteria excluding patients with coronary artery stenosis > 25% outside the culprit lesion, and emboli due to secondary coronary arteries. However, the real occurrence of ACS-E remains unknown since the early presentation can be indistinguishable from an ACS-A.6
Also, the limited rate of coronary artery emboli reported compared to other vascular territories may also be associated with intrinsic anatomical and physiological characteristics like aortic caliber differences, the acute angle at which the coronary arteries originate at the sinus of Valsalva,7 and the position of the coronary ostia behind the valve cusps during systole.3,8
Although some series comparing ACS-A and ACS-E have not described gender differences when focusing on STEMI,2 in our study, the proportion of women was significantly higher among ACS-E (43% vs 22%; P = .003). Similarly, Shibata et al. reported rates of 40% vs 29% (P = .087).1 Besides, according to the aforementioned authors, a lower prevalence of traditional cardiovascular risk factors was seen within the embolic group of our cohort, although statistically significant differences were only noticed regarding dyslipidemia (27.5% vs 43.9%; P = .037).
Regarding the compromise of coronary arteries, the LAD was the most commonly affected vessel in both the ACS-E and the ACS-A (table 3). Similarly, a previous autopsy study had shown that coronary emboli are up to 4 times more common in the LAD compared to the right coronary artery, and in the LAD compared to the the left circumflex artery.5 Also, in a recent systematic review including 129 case reports and case series of coronary emboli, Lacey et al. described that the LAD was the most frequently affected vessel (45.3%).6 However, such differences in the distribution of culprit coronary vessels may be explained by bias associated with the fact that arteries with larger territories are more likely to be involved in autopsies1 and case reports.
On the interventional treatment used, in our study, 30 patients (75.0%) from the ACS-E group underwent thrombus aspiration followed by balloon angioplasty in 8 cases. None of the patients from this group were treated with stenting. Similarly, Shibata et al. performed initial thrombus aspiration in 96.6% of embolic patients undergoing PCI followed by balloon angioplasty in 14.3% of the cases and stenting in 17.9%.1 Thrombus aspiration has proven to be a feasible option to treat AMI with angiographic evidence of thrombus including cases associated with coronary emboli.9 However, these devices may be less useful to aspirate large thrombi due to the smaller diameter of the lumen of the inner catheter.10 Besides, in specific situations like small arteries or distal coronary occlusions, simple wire manipulation added to antithrombotic drugs (including glycoprotein IIb/IIIa inhibitors, which were more frequently used in the ACS-E group) may be the preferred option to achieve reperfusion.2
At the follow-up after an episode of ACS-E (5.8 ± 4.8 years) in our series, the major adverse cardiovascular and cerebrovascular events occurred in 37.1% of the patients. However, no recurrences of systemic emboli were documented in accordance with other previous series.2 The in-hospital all-cause mortality rate was significantly higher among patients with ACS-E (15% vs 4%; P < .001) mainly due to cardiovascular causes. Shibata et al. reported no differences in the 30-day mortality rate, but significantly higher cardiovascular and all-cause mortality rates in ACS-E compared to ACS-A.1 Similarly, Popovic et al. observed that 64% of all deaths reported at the follow-up after an episode of STEMI due to coronary embolism were due to cardiac causes.2
Finally, after multivariate analysis, AF, previous heart valve surgery, active neoplasm, female sex, and younger age were identified as clinical predictors of ACS-E. AF has been described as the most frequent condition predisposing to coronary artery embolism being present in 40.0% of ACS-E in our study and in up to 73% in other current series.1,3 However, early studies reported that valvular heart disease, especially rheumatic, and infective endocarditis represented the most common causes of coronary artery embolism.5,11 This disparity may be associated with the advances made in antibiotic therapy implementation over the last few decades, and the remarkable increase of AF prevalence parallel to the gradual aging of the population.1,2,12,13 Furthermore, it has been reported that the risk of AMI associated with AF is significantly higher in women14,15 and patients without coronary artery disease.15-18
On the other hand, it is fully recognized that patients with active neoplasms are at a significantly higher risk of developing thrombotic events, both venous and arterial.19 The pathogenesis of cancer-associated coagulopathy is complex including a multifactorial interaction among the patient’s comorbidities, the specific malignancy, and treatment with several chemotherapeutic agents or immunomodulatory drugs that often lead to hypercoagulability, platelet activation, and endothelial injury.20 Besides, it has also been described that malignancy is associated with a higher risk of developing AF following interactions at the pathophysiological level.21,22 In our series, 17.5% of the patients presented active neoplasms in accordance with Popovic et al.2 who reported a prevalence of 15.1% notably higher than the one reported by Shibata et al.1 and Lacey et al.6 of 10% and 1.4%, respectively.
Limitations
The present study presents several limitations. First, being a retrospective study may have resulted in a certain degree of bias. Secondly, applying strict diagnostic criteria which excluded patients with ≥ 25% coronary artery stenosis outside the culprit lesion may have omitted cases of ACS-E in patients with concomitant coronary artery disease. Thirdly, in contrast with all previous reports on this matter, only emboli of major coronary arteries were considered, which possibly resulted in a lower number of ACS-E being diagnosed. Finally, including patients over a long period of time may explain some differences in treatment modalities, and the low use of intracoronary imaging seen in our series.
CONCLUSIONS
ACS-E and ACS-A have different clinical and angiographic characteristics. Female sex, younger age, past medical history of active neoplasms, previous valvular surgery, and AF were all independent predictors of ACS-E. Patients with ACS-E had a higher in-hospital mortality rate mainly due to cardiovascular causes.
FUNDING
None whatsoever.
AUTHORS’ CONTRIBUTIONS
All authors contributed to the study conception and design. Material preparation and data collection were prepared by A. Jerónimo, A. Travieso, A. McInerney, B. Hennessey, and L. Marroquín. Statistical analysis was conducted by A. Jerónimo, M.J. Pérez- Vyzcaino, and N. Gonzalo. The manuscript first draft was written by A. Jerónimo, and N. Gonzalo, and all authors commented on previous versions of the manuscript. All authors read and approved the manuscript final version.
CONFLICTS OF INTEREST
None reported.
WHAT IS KNOWN ABOUT THE TOPIC?
- According to angiographic studies and autopsies, systemic coronary artery embolism is representative of 3% to 14% of all ACSs. However, the real prevalence of this entity remains unknown due to uncertainty of its diagnosis in the acute setting. AF, infective endocarditis, valvular heart disease, and malignancies have been associated with ACS-E, but the clinical and angiographic profile of this entity has not been properly established to this date.
WHAT DOES THIS STUDY ADD?
- Our study describes the epidemiological, clinical, and angiographic characteristics of patients with ACS-E comparing them to ACS-A and admitted to a single centre during the same period of time. On this regard, patients with ACS-E were younger compared to those with ACS-A, female in a higher proportion, and more often had AF, previous stroke, previous valvular surgery, and active neoplasms. The left anterior descending coronary artery was the most common culprit vessel in both groups, but patients with ACS-A presented with a significantly higher proportion of other significant stenoses. On the therapeutic approach regarding the PCI, thrombus aspiration was the most frequent strategy in ACS-E without stenting in any of the cases. Besides, the in-hospital all-cause mortality rate was significantly higher among patients with ACS-E mainly due to cardiovascular causes. A younger age, female sex, active neoplasms, previous valvular surgery, and a past medical history of AF were identified as independent clinical predictors of ACS-E.
REFERENCES
1. Shibata T, Kawakami S, Noguchi T, et al. Prevalence, Clinical Features, and Prognosis of Acute Myocardial Infarction Attributable to Coronary Artery Embolism. Circulation. 2015;132:241–250.
2. Popovic B, Agrinier N, Bouchahda N, et al. Coronary Embolism Among ST-Segment-Elevation Myocardial Infarction Patients:Mechanisms and Manegement. Circ Cardiovasc Interv. 2018;11:e005587.
3. Kolodgie FD, Virmani R, Finn A V, Romero ME. Embolic Myocardial Infarction as a Consequence of Atrial Fibrillation:A Prevailing Disease of the Future. Circulation. 2015;132:223–226.
4. Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction (2018). Eur Heart J. 2019;40:237-269.
5. Prizel KR, Hutchins GM, Bulkley BH. Coronary artery embolism and myocardial infarction. Ann Intern Med. 1978;88:155-161.
6. Lacey MJ, Raza S, Rehman H, Puri R, Bhatt DL, Kalra A. Coronary embolism:A systematic review. Cardiovasc Revasc Med. 2020;21:367-374.
7. Cheng JT, Cahill WJ, Foley EF. Coronary embolism. J Am Med Assoc. 1953;153:211-213.
8. Cheng TO. Coronary embolism. Int J Cardiol. 2009;136:1-3.
9. Kotooka N, Otsuka Y, Yasuda S, Morii I, Kawamura A, Miyazaki S. Three cases of acute myocardial infarction due to coronary embolism:treatment using a thrombus aspiration device. Jpn Heart J. 2004;45:861-866.
10. Stoel MG, von Birgelen C, Zijlstra F. Aspiration of embolized thrombus during primary percutaneous coronary intervention. Catheter Cardiovasc Interv. 2009;73:781-786.
11. Charles RG, Epstein EJ. Diagnosis of coronary embolism:A review. J R Soc Med. 1983;76:863-869.
12. Roxas CJ, Weekes AJ. Acute myocardial infarction caused by coronary embolism from infective endocarditis. J Emerg Med. 2011;40:509-514.
13. Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS):The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373-498.
14. Soliman EZ, Safford MM, Muntner P, et al. Atrial fibrillation and the risk of myocardial infarction. JAMA Intern Med. 2014;174:107-114.
15. Emdin CA, Wong CX, Hsiao AJ, et al. Atrial fibrillation as risk factor for cardiovascular disease and death in women compared with men:systematic review and meta-analysis of cohort studies. BMJ. 2016;532:h7013.
16. Ruddox V, Sandven I, Munkhaugen J et al. Atrial fibrillation and the risk for myocardial infarction, all-cause mortality and heart failure:A systematic review and meta-analysis. Eur J Prev Cardiol. 2017;24:1555-1566.
17. Guo XY, Li N, Du X, et al. Atrial fibrillation is associated with an increased risk of myocardial infarction:insights from a meta-analysis. Atherosclerosis. 2016;254:1–7.
18. Bayturan O, Puri R, Tuzcu EM, et al. Atrial fibrillation, progression of coronary atherosclerosis and myocardial infarction. Eur J Prev Cardiol. 2017;24:373–381.
19. Falanga A, Schieppati F, Russo D. Cancer Tissue Procoagulant Mechanisms and the Hypercoagulable State of Patients with Cancer. Semin Thromb Hemost. 2015;41:756-764.
20. Aronson D, Brenner B. Arterial thrombosis and cancer. Thromb Res. 2018;164 Suppl 1:S23-S28.
21. Liu F, Xu Z, Luo J, et al. Effectiveness and Safety of DOACs vs. VKAs in AF Patients With Cancer:Evidence From Randomized Clinical Trials and Observational Studies. Front Cardiovasc Med. 20215;8:766377.
22. Chu G, Versteeg HH, Verschoor AJ, et al. Atrial fibrillation and cancer - An unexplored field in cardiovascular oncology. Blood Rev. 2019;35:59-67.
ABSTRACT
Introduction and objectives: During the lockdown due to the pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a decrease in the number of admissions due to acute coronary syndrome (ACS) was observed. The objective of our study was to evaluate the impact lockdown had on the incidence, morbidity and mortality, and management of ACS.
Methods: A retrospective and multicenter study was conducted including patients admitted due to ACS from February 14 through June 24, 2020. Patients with acute myocardial infarction and coronary arteries without significant lesions were excluded. The following groups were established based on the period of admission: a) 1 month before lockdown; b) during lockdown; and c) 1 month after lockdown. The differences in mortality seen among the 3 groups were evaluated, as well as the temporal differences reported between symptom onset and the first medical contact (FMC).
Results: a total of 634 patients were included (group a, 205; group b, 303, and group c, 126). A 41% decrease in the number of admissions due to ACS was observed during the first month of lockdown compared to the previous month, as well as diagnostic delay during this same period (group a, 66 minutes (45-180), group b, 120 minutes (60-240), and group c, 120 minutes (60-240), P = .007). However, a higher mortality rate during confinement was not reported (RR, 1.26; 95%CI, 0.53-2.97; P = .60).
Conclusions: During lockdown, a remarkable decrease in the number of admissions due to ACS was observed, and although there was an increase in the time elapsed from symptom onset to the FCM in this period in patients with STEMI, the mortality rate was similar in the 3 groups studied.
Keywords: COVID-19. SARS-CoV-2. Acute coronary syndrome. Pandemic. Revascularization. Lockdown.
RESUMEN
Introducción y objetivos: Durante el confinamiento por la pandemia provocada por el coronavirus del síndrome respiratorio agudo grave de tipo 2 (SARS-CoV-2) se observó un descenso en los ingresos por síndrome coronario agudo (SCA). El objetivo de este estudio fue evaluar el impacto del confinamiento en la incidencia, la morbimortalidad y el tratamiento del SCA.
Métodos: Estudio retrospectivo y multicéntrico, en el que se incluyeron los pacientes ingresados por SCA entre el 14 de febrero y el 24 de junio de 2020. Se excluyeron los pacientes con infarto agudo de miocardio y coronarias sin lesiones significativas. Se establecieron 3 grupos en función del periodo de ingreso: a) 1 mes antes del confinamiento; b) durante el confinamiento; y c) 1 mes después del confinamiento. Se evaluaron las diferencias en la mortalidad entre los 3 grupos, así como las diferencias temporales entre el inicio de los síntomas y el primer contacto médico.
Resultados: Se incluyeron 634 pacientes (grupo A: 205; grupo B: 303; grupo C: 126). Se observó un descenso del 41% en los ingresos por SCA durante el primer mes del confinamiento respecto al mes previo, así como un retraso en el diagnóstico durante este mismo periodo: grupo A, 66 minutos (45-180); grupo B, 120 minutos (60-240); grupo C, 120 minutos (60-240) (p = 0,007). Sin embargo, no hubo mayor mortalidad durante el confinamiento (riesgo relativo, 1.26; intervalo de confianza del 95%, 0.53-2.97; p = 0,60).
Conclusiones: Durante el confinamiento se produjo un marcado descenso en los ingresos por SCA y, a pesar de que se dilató el tiempo desde el inicio de los síntomas hasta el primer contacto médico en este periodo en los pacientes con SCA con elevación del segmento ST, la mortalidad fue similar en los 3 grupos estudiados.
Palabras clave: COVID-19. SARS-CoV-2. Síndrome coronario agudo. Pandemia. Revascularización. Confinamiento.
Abbreviations
ACS: acute coronary syndrome. SARS-CoV-2: severe acute respiratory syndrome coronavirus 2. STEMI: ST-segment elevation myocardial infarction.
INTRODUCTION
By the end of December 2019, The People’s Republic of China reported the World Health Organization on the first cases detected of an unknown pneumonia caused by a new type of coronavirus in the City of Wuhan, China.1,2 Since then, the disease caused by this virus has spread rapidly bringing the healthcare systems of several countries to the point of collapse ultimately triggering dramatic preventive measures by the health authorities.
The pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has had a tremendous social, economic, and health impact across the world. Again and again, the healthcare setting has sustained several organizational and care changes that have triggered significant variations in the management therapeutic approach of the remaining diseases.3-5 Some studies have reported a lower number of admissions due to cardiovascular diseases, which has had a significant impact on morbidity and mortality alike.6-8
Pressure to the healthcare system due to COVID-19, the lockdown, and the lower demand for assistance are some of the reasons that may account for these changes. The objective of this study is to assess the rate of acute coronary syndrome (ACS) across the different stages of the pandemic in Spain, as well as the impact it has had on morbidity, mortality, and therapeutic management.
METHODS
Retrospective, observational, and multicenter study including data from patients admitted to 4 tertiary care centers of our country from 3 autonomous communities due to ACS from February 14, 2020 through June 24, 2020. Patients with ST-segment elevation acute coronary syndrome (STEACS), and non-ST-segment elevation acute coronary syndrome and were included. Patients with acute myocardial infarction and without significant lesions in coronary arteries were excluded. Patients were categorized into 3 groups based on the length of hospital stay: group A, from February 14 through March 14, 2020 (1 month before the lockdown); group B, from March 15 through May 24, 2020 (during the lockdown), and group C, from May 25 through June 24, 2020 (1 month after the stay-at-home lockdown). The patients’ baseline characteristics, acute complications, and cardiovascular events reported at the follow-up like all-cause mortality, cardiac death, stroke, reinfarction, stent thrombosis, and need for rehospitalization were recorded. In patients with STEACS the times elapsed between symptom onset and the first medical contact (FMC), and between electrocardiographic diagnosis until reperfusion were recorded. Clinical follow-up was completed back in July 25, 2020. Data curation was approved by the local ethics committee of each participant center.
The study primary endpoint was to assess the differences reported in all-cause mortality after 30 days since the onset of the acute coronary event among the 3 study groups. The study secondary endpoint was to analyze the differences reported in a composite of cardiac death, stroke, admission due to new ACS, stent thrombosis, and need for new revascularization. Complications reported after infarction at the follow-up, a high left ventricular ejection fraction, and revascularization times (from symptom onset until the first medical contact, and from diagnosis until reperfusion) were also studied in a secondary analysis and compared among the 3 groups.
Statistical analysis
Categorical variables were expressed as number and percentage using brackets and compared using the chi-square test or Fisher’s exact test, when appropriate. Continuous variables were expressed as mean and standard deviation or median and interquartile range in cases without a normal distribution. The Shapiro-Wilk test was used to assess the normal distribution of continuous variables that were compared using the analysis of variance (ANOVA) for independent samples or Kruskall-Wallis H test based on their normal distribution looking for differences among the 3 groups. Survival was studied using the Kaplan-Meier curves, and differences were assessed using the log-rank test. Cox proportional hazards regression analysis was used to assess the impact of group B (lockdown period) in the overall mortality of the patients. All estimates were performed using the statistical software package STATA version 15.1. P values < .05 were considered statistically significant.
RESULTS
A total of 634 patients were included from February 14, 2020 through June 24, 2020. Of these, 205 were patients from group A, 303 from group B, and 126 from group C with a median follow-up of 98 days (63-137 days). The number of admissions due to ACS was 120, 138, and 151 within the first, second, and third months since the state of alarm declared. This lowered the rate of admissions due to ACS by 41%, 33%, and 26%, respectively compared to the rates reported 1 month before the lockdown for the same 30-day period (figure 1).
Figure 1. Absolute number of patients admitted due to acute coronary syndrome, expressed in weeks and categorized into group A, B, and C.
A total of 356 (56.2%) from the overall number of patients were admitted due to STEACS, and 278 (43.8%) due to non-ST-segment elevation acute coronary syndrome. The cohort baseline characteristics are shown on table 1. Patients admitted during the lockdown (group B) were younger (P = .012) and had lower levels of hypertension and dyslipidemia. On the other hand, these patients’ past medical history showed less ischemic heart disease, and coronary revascularization (P < .001).
Table 1. Baseline characteristics, diagnosis at admission, and treatment
| Variable | Total (N = 634) | Group A (N = 205) | Group B (N = 303) | Group C (N = 126) | P |
|---|---|---|---|---|---|
| Age | 66.3 ±12.6 | 67.4 ±11.6 | 64.8 ±12.7 | 68.2 ±13.6 | .012 |
| Sex, male | 494 (77.9) | 158 (77.1) | 241 (79.5) | 95 (75.4) | .603 |
| AHT | 400 (63.1) | 143 (69.8) | 176 (58.1) | 81 (64.3) | .027 |
| DM | 191 (30.1) | 71 (35.1) | 89 (29.4) | 30 (23.8) | .086 |
| DL | 368 (58.0) | 137 (66.8) | 164 (54.1) | 67 (53.2) | .008 |
| Smoking | 364 (57.4) | 124 (60.5) | 182 (60.1) | 58 (46.0) | .015 |
| PVD | 36 (5.7) | 15 (7.3) | 16 (5.3) | 5 (4.0) | .405 |
| Stroke | 37 (5.8) | 11 (5.4) | 16 (5.3) | 110 (7.9) | .531 |
| CKD (GF < 60) | 30 (4.7) | 18 (8.8) | 7 (2.3) | 5 (4.0) | .003 |
| COPD | 45 (7.1) | 14 (6.8) | 22 (7.3) | 9 (7.1) | .981 |
| AF | 40 (6.3) | 16 (7.8) | 16 (5.3) | 8 (6.4) | .517 |
| IHD | 150 (23.7) | 79 (38.5) | 46 (15.2) | 25 (19.8) | < .001 |
| AMI | 103 (16.3) | 52 (25.4) | 31 (10.2) | 20 (15.9) | < .001 |
| PCI | 117 (18.5) | 60 (29.3) | 36 (11.9) | 21 (16.7) | < .001 |
| CABG | 23 (3.6) | 12 (5.9) | 7 (2.3) | 4 (3.2) | .112 |
| Diagnoses | |||||
| UA | 83 (13.1) | 36 (17.6) | 27 (8.9) | 20 (15.9) | .003 |
| NSTEMI | 195 (30.8) | 67 (32.7) | 83 (27.4) | 45 (35.7) | .003 |
| STEACS | 356 (56.2) | 102 (49.8) | 193 (63.7) | 61 (48.4) | .003 |
| GRACE | 120.1 ±35.6 | 118.4 ±35.4 | 119.1 ±34.6 | 124.8 ±38.3 | .264 |
| CRUSADE | 31.4 ±13.8 | 34.1 ±15.2 | 30.4 ±13.3 | 29.7 ±11.8 | .001 |
| Cardiac catheterization | 616 (97.5) | 198 (96.6) | 295 (97.7) | 123 (98.4) | .565 |
| Emergency | 375 (59.5) | 112 (54.9) | 190 (63.1) | 73 (58.4) | .447 |
| Deferred | 242 (38.4) | 87 (42.7) | 105 (34.9) | 50 (40.0) | .447 |
| Fibrinolysis | 29 (5.1) | 10 (5.7) | 13 (4.5) | 6 (6.1) | .652 |
| PCI | 534 (94.3) | 165 (93.2) | 276 (95.2) | 93 (94.0) | .652 |
| CABG | 29 (4.6) | 11 (5.4) | 8 (2.7) | 10 (8.1) | .045 |
| LMCA or 3-vessel disease | 136 (21.5) | 52 (25.4) | 55 (18.6) | 29 (23.0) | .135 |
| CABG (LMCA or 3-vessels) | 22 (16.3) | 9 (17.7) | 3 (5.5) | 10 (34.5) | .003 |
| Conservative treatment | 3 (0.5) | 2 (1.1) | 1 (0.3) | 0 (0) | .652 |
| Complete revascularization | 456 (75.6) | 138 (74.6) | 223 (76.1) | 95 (76.0) | .926 |
| LVEF at discharge | 49.2 ±11.1 | 49.7 ±11.6 | 48.6 ±11.2 | 49.9 ±10.0 | .421 |
|
AF, atrial fibrillation; AHT, arterial hypertension; AMI, acute myocardial infarction; CABG, coronary artery bypass graft; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DL, dyslipidemia; DM, diabetes mellitus; GF, glomerular filtration; HT, arterial hypertension; IHD, ischemic heart disease; LMCA, left main coronary artery; LVEF, left ventricular ejection fraction; NSTEMI, non-ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention; PVD, peripheral vascular disease; STEACS, ST-segment elevation acute coronary syndrome; UA, unstable angina. Data are expressed as no. (%) or mean ± standard deviation. |
|||||
A diagnostic coronary angiography was performed on 97.1% of the cohort without any differences being reported regarding percutaneous coronary intervention throughout the different periods studied (P = .652); however, a significant reduction in the number of surgical coronary revascularizations performed during the lockdown was reported (group A, 5.4%; group B, 2.7%; group C, 8.1%; P = .045) including the subgroup of patients with left main coronary artery disease or 3-vessel disease (P = .003) (table 1).
A total of 36 deaths were reported, 22 of which were due to cardiovascular causes. No statistically significant differences were reported in the all-cause mortality rate after 30 days among the 3 groups (P = .327). According to a Cox regression analysis, being in the lockdown group (group B) was not associated with a higher all-cause mortality rate (P = .60). No survival differences were reported either among the 3 groups (figure 2).
Figure 2. Kaplan-Meier survival curves for all-cause mortality in groups A (February 14-March 14), B (March 15-May 24), and C (May 25-June 24).
No significant differences were reported at the follow-up in a composite of cardiac death, stroke, readmission due to new ACS, stent thrombosis, and new revascularization (P = .120). The remaining clinical events at the follow-up are shown on table 2 and the in-hospital events on table 3.
Table 2. Clinical events at the follow-up
| Variable | Total (N = 634) | Group A (N = 205) | Group B (N = 303) | Group C (N = 126) | P |
|---|---|---|---|---|---|
| All-cause mortality | 36 (5.7) | 15 (7.3) | 13 (4.3) | 8 (6.4) | .327 |
| Cardiac death | 22 (64.7) | 7 (50) | 9 (75) | 6 (75) | .427 |
| Stroke | 20 (3.2) | 9 (4.4) | 8 (2.6) | 3 (2.4) | .551 |
| Re-AMI | 4 (0.7) | 1 (0.5) | 2 (0.7) | 1 (0.8) | 1.000 |
| Stent thrombosis | 12 (2.0) | 8 (4.1) | 1 (0.3) | 3 (2.4) | .006 |
| New revascularization | 6 (1.0) | 4 (2.0) | 2 (0.7) | 0 (0) | .259 |
| CV death + stroke + Re-AMI + stent thrombosis + new revascularization | 57 (9.0) | 24 (11.7) | 20 (6.6) | 13 (10.3) | .120 |
|
CV, cardiovascular; Re-AMI, new acute myocardial infarction. Data are expressed as no. (%). |
|||||
| Variable | Total (N = 634) | Group A (N = 205) | Group B (N = 303) | Group C (N = 126) | P |
|---|---|---|---|---|---|
| Inotropic agents | 53 (8.5) | 17 (8.4) | 27 (9.0) | 9 (7.2) | .836 |
| PM at admission | 12 (1.9) | 4 (2.0) | 8 (2.7) | 0 (0) | .188 |
| IABP | 11 (1.7) | 7 (3.4) | 4 (1.3) | 0 (0) | .048 |
| OTI | 41 (6.5) | 15 (7.3) | 21 (7.0) | 5 (4.0) | .444 |
| NIMV | 18 (2.9) | 6 (2.9) | 7 (2.3) | 5 (4.0) | .604 |
| RRT | 10 (1.6) | 6 (3) | 3 (1.0) | 1 (0.8) | .192 |
| AVB | 20 (3.2) | 7 (3.4) | 12 (4.0) | 1 (0.8) | .227 |
| SMVT | 18 (2.9) | 6 (2.9) | 9 (3.0) | 3 (2.4) | 1.000 |
| VF | 29 (4.6) | 12 (5.9) | 12 (4.0) | 5 (4.0) | .582 |
| AF at admission | 42 (6.7) | 11 (5.4) | 23 (7.6) | 8 (6.4) | .597 |
| BARC bleeding type > 3 | 16 (2.5) | 2 (1.0) | 9 (3.0) | 5 (4.0) | .161 |
| Infection | 57 (9.0) | 12 (6.0) | 28 (10.1) | 17 (11.0) | .184 |
| ARDS | 12 (1.9) | 1 (0.5) | 7 (2.5) | 4 (2.6) | .208 |
| Mechanical complications | 10 (1.6) | 3 (1.5) | 6 (2.0) | 1 (0.8) | .774 |
| Killip III or IV | 62 (9.8) | 20 (9.8) | 31 (10.3) | 11 (8.8) | .898 |
|
AF, atrial fibrillation; ARDS, acute respiratory distress syndrome; AVB, atrioventricular block; BARC, Bleeding Academic Research Consortium; IABP, intra-aortic balloon pump; NIMV, non-invasive mechanical ventilation; OTI, orotracheal intubation; PM, pacemaker; RRT, renal replacement therapy; SMVT, sustained monomorphic ventricular tachycardia; VF, ventricular fibrillation. Data are expressed as no. (%). |
|||||
Regarding delay times, significant differences were reported among the different groups with longer times elapsed between symptom onset and the first medical contact during (group B) and after lockdown (group C) compared to the previous period (group A): group A, 66 min (45-180), group B, 120 min (60-240), group C, 120 min (60-240); P = .007). The time elapsed between symptom onset until the first medical contact was similar in groups B and C (P = .7102). Finally, the time elapsed between diagnosis and reperfusion was shorter in patients from group C (P = .025) compared to the remaining cohort (table 4).
Table 4. Times between symptom onset and the first medical contact, and between electrocardiographic diagnosis and reperfusion (guidewire passage), in minutes, in the cohort of patients with ST-segment elevation acute coronary syndrome
| Variable | Total | Grupo A | Grupo B | Grupo C | p |
|---|---|---|---|---|---|
| Symptom onset-first medical contact (N = 332) | 120 [60-240] | 66 [45-180] (N = 97) | 120 [60-240] (N = 180) | 120 [60-240] (N = 55) | .007 |
| Diagnosis-reperfusion (N = 322) | 120 [60-180] | 120 [60-186] (N = 93) | 120 [60-225] (N = 176) | 60 [60-120] (N = 53) | .025 |
|
Data are expressed as median [interquartile range]. |
|||||
DISCUSSION
The main findings from this study were a lower number of admissions due to ACS within the first few months of lockdown, and longer periods of time elapsed between symptom onset and the first medical contact in patients with STEACS that did not translate into higher morbidity and mortality rates.
Lower rate of acute coronary syndrome
Former studies have reported less activity at the cath lab due to fewer admissions due to ACS during the pandemic, especially in the STEACS setting.7,9-11 Our findings confirm this trend with a significant 41% drop within the first 30 days compared to the previous month. This reduction was kept in the remaining time during and after lockdown; however, as the isolation measures were being lifted and the rate of cases of COVID-19 dropped, a gradual increase in the number of admissions due to ACS was confirmed. One of the contributing factors may have been the intense pressure put to the healthcare system within the first few months of lockdown with the corresponding underdiagnosis of ACS and fewer admissions reported.12 Another hypothesis that may justified the lower rate of ACS during this time is the higher number of out-of-hospital sudden deaths reported. Although reported by other authors in the past, this was not cause for analysis in our study.13-16
Times elapsed among symptom onset, the first medical contact, and revascularization in patients with ST-segment elevation myocardial infarction, and association with adverse events
During the lockdown (group B) patients with STEACS were admitted more often (P = .003). The time elapsed between symptom onset and the first medical contact was significantly longer during this time compared to other times, which is consistent with the peak number of cases reported (similar findings to those reported by former studies);17 however, this delay did not increase the rates of mechanical complications or mortality. This can be explained because patients admitted during the lockdown (group B) were younger and had fewer comorbidities.18,19 Data suggests that elderly patients with more serious past medical histories and associated comorbidities may have delayed or even postponed indefinitely their access to the healthcare system over fears of getting infected.20,21
Rodríguez-Leor et al.22 reported time delays between symptom onset and the first medical contact, and similar times between diagnosis and reperfusion. This delay was associated with a higher mortality rate during the pandemic (7.5% vs 5.1%), which contradicts our findings. The lack of a direct association between time delays until diagnosis and the appearance of adverse events is not easy to explain. However, a plausible hypothesis can be the higher number of out-of-hospital sudden deaths reported due to mechanical complications or malignant arrhythmias followed by the corresponding selection bias since this study included hospitalized patients only.
Therapeutic strategies: percutaneous coronary intervention and surgical revascularization
No differences were found regarding the percutaneous invasive management of patients with ACS before, during or after lockdown. This data is consistent with most studies published on the management of ACS during the pandemic.12,22
However, we should mention the significant decrease of myocardial revascularization procedures despite the non-negligible number of patients with left main coronary artery disease or 3-vessel disease. A total of 17.7% of these patients were treated with myocardial revascularization 1 month before the lockdown, only 5.5% during the lockdown, and 34.5% the following month. Although some registries confirm the lower number of coronary artery bypass grafts performed,23 this tendency has not been confirmed in other studies.18,23
The fact that fewer myocardial revascularization procedures were performed during the lockdown can be explained by the overall tendency to delay any surgical acts as much as possible during these months, something already hypothesized in other studies.24
Limitations
This study has some limitations associated with the analysis of multicenter and observational data. Also, the study short follow-up period may have prevented the finding of potential consequences or differential events among the study groups. The lack of information on cases of ACS treated during the pandemic that never really made it to tertiary care centers also casts a shadow over the conclusions that can be drawn.
CONCLUSIONS
Significantly fewer admissions due to ACS were reported during the lockdown. Also, although time between symptom onset and the first medical contact was longer during this period in patients with STEACS, the mortality rate was similar among the 3 study groups.
FUNDING
None reported.
AUTHORS’ CONTRIBUTIONS
J. Echarte-Morales: clinical data mining, manuscript drafting, project design, and management of the study. C. Minguito-Carazo: data analysis, manuscript drafting and revision process. PL Cepas-Guillén, V. Vallejo García, ID. Poveda Pinedo, A. Salazar Rodríguez, E. Arbas Redondo, J. Guzmán Bofarull, and D. Tebar Márquez: data mining and manuscript revision process. E. Sánchez Muñoz: data mining. E. Martínez Gómez: data mining, manuscript drafting and revision process. T. Benito-González: statistical counselling, and manuscript revision process. M. López Benito, A. Viana Tejedor, I. Cruz-González, PL Sánchez Fernández, M. Sabaté, and F. Fernández-Vázquez: project organization. Authors submitting this manuscript accept full responsibility for its content as defined by the International Committee of Medical Journal Editors (ICMJE).
CONFLICTS OF INTEREST
None whatsoever.
WHAT IS KNOWN ABOUT THE TOPIC?
- Admissions due to STEACS decreased during the lockdown.
- More mechanical complications were reported during the pandemic due to delayed treatments.
WHAT DOES THIS STUDY ADD?
- Unlike former studies that mainly focused on patients with STEACS, this study includes patients admitted before, during, and 1 month after lockdown with a diagnosis of ACS (including STEACS and non-ST-segment elevation acute coronary syndrome).
- Fewer myocardial revascularization procedures were performed during the lockdown despite the growing number of patients with left main coronary artery disease or 3-vessel disease.
- Although time between symptom onset and the first medical contact was longer in the group of patients with STEACS, the mortality rate was similar before, during, and after lockdown, as it happened with mechanical complications.
REFERENCES
1. WHO. World Health Organization. Pneumonia of Unknown Cause –China. 2020. Available online:https://www.who.int/emergencies/disease-outbreak-news/item/2020-DON229. Accessed 20 Sep 2021.
2. Zhu N, Zhang D, Wang W, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382:727-733.
3. Morelli N, Rota E, Terracciano C, et al. The Baffling Case of Ischemic Stroke Disappearance from the Casualty Department in the COVID-19 Era. Eur Neurol. 2020;83:213-215.
4. Babu N, Kohli P, Mishra C, et al. To evaluate the effect of COVID-19 pandemic and national lockdown on patient care at a tertiary-care ophthalmology institute. Indian J Ophthalmol. 2020;68:1540-1544.
5. De Vincentiis L, Carr RA, Mariani MP, Ferrara G. Cancer diagnostic rates during the 2020 âlockdown', due to COVID-19 pandemic, compared with the 2018-2019:An audit study from cellular pathology. J Clin Pathol. 2021;74:187-189.
6. De Rosa S, Spaccarotella C, Basso C, et al. Reduction of hospitalizations for myocardial infarction in Italy in the COVID-19 era. Eur Heart J. 2020;41:2083-2088.
7. Rodríguez-Leor O, Cid-Álvarez B, Ojeda S, et al. Impacto de la pandemia de COVID-19 sobre la actividad asistencial en cardiología intervencionista en España. REC Interv Cardiol. 2020;2:82-89.
8. Romaguera R, Cruz-González I, Jurado-Román A, et al. Consideraciones sobre el abordaje invasivo de la cardiopatía isquémica y estructural durante el brote de coronavirus COVID-19. REC Interv Cardiol. 2020;2:112-117.
9. Garcia S, Albaghdadi MS, Meraj PM, et al. Reduction in ST-Segment Elevation Cardiac Catheterization Laboratory Activations in the United States During COVID-19 Pandemic. J Am Coll Cardiol. 2020;75:2871-2872.
10. Metzler B, Siostrzonek P, Binder RK, Bauer A, Reinstadler SJ. Decline of acute coronary syndrome admissions in Austria since the outbreak of COVID-19:The pandemic response causes cardiac collateral damage. Eur Heart J. 2020;41:1852-1853.
11. Pessoa-Amorim G, Camm CF, Gajendragadkar P, et al. Admission of patients with STEMI since the outbreak of the COVID-19 pandemic:A survey by the european society of cardiology. Eur Heart J Qual Care Clin Outcomes. 2020;6:210-216.
12. Salinas P, Travieso-González A, Vergara-Uzcategui CE, Macaya F, Núñez-Gil IJ, Fernández-Ortiz A. Relación temporal entre ingresos por síndrome coronario agudo con tratamiento invasivo y confinamiento durante la pandemia de COVID-19. REC Interv Cardiol. 2020;2:307-309
13. Laura E. Wong;MD;PhD;Jessica E. Hawkins;MSEd;Simone Langness;Karen L. Murrell;Patricia Iris;MD &Amanda Sammann;MPH. Where Are All the Patients?Addressing Covid-19 Fear to Encourage Sick Patients to Seek Emergency Care. NEJM Catal. 2020. Available online:https://catalyst.nejm.org/doi/full/10.1056/CAT.20.0193. Accessed 20 Sep 2021.
14. Lai PH, Lancet EA, Weiden MD, et al. Characteristics Associated with Out-of-Hospital Cardiac Arrests and Resuscitations during the Novel Coronavirus Disease 2019 Pandemic in New York City. JAMA Cardiol. 2020;5:1154-1163.
15. Marijon E, Karam N, Jost D, et al. Out-of-hospital cardiac arrest during the COVID-19 pandemic in Paris, France:a population-based, observational study. Lancet Public Health. 2020;5:e437-e443.
16. Baldi E, Sechi GM, Mare C, et al. Out-of-Hospital Cardiac Arrest during the Covid-19 Outbreak in Italy. N Engl J Med. 2020;383:496-498.
17. Tam CCF, Cheung KS, Lam S, et al. Impact of Coronavirus Disease 2019 (COVID-19) Outbreak on ST-Segment-Elevation Myocardial Infarction Care in Hong Kong, China. Circ Cardiovasc Qual Outcomes. 2020;13:e006631.
18. Gluckman TJ, Wilson MA, Chiu ST, et al. Case Rates, Treatment Approaches, and Outcomes in Acute Myocardial Infarction during the Coronavirus Disease 2019 Pandemic. JAMA Cardiol. 2020;5:1419-1424.
19. Wu J, Mamas M, Rashid M, et al. Patient response, treatments, and mortality for acute myocardial infarction during the COVID-19 pandemic. Eur Heart J Qual Care Clin Outcomes. 2021;7:238-246.
20. Franchini S, Spessot M, Landoni G, et al. Stranger months:How SARS-CoV-2, fear of contagion, and lockdown measures impacted attendance and clinical activity during February and March 2020 at an urban Emergency Department in Milan. Disaster Med Public Health Prep. 2020;15(5):e33-e42.
21. Baldi E, Savastano S. Fear of contagion:One of the most devious enemies to fight during COVID-19 pandemic. Disaster Med Public Health Prep. 2021;15:e8-e9.
22. Rodríguez-Leor O, Cid-Álvarez B, Pérez de Prado A, et al. Impact of COVID-19 on ST-segment elevation myocardial infarction care. The Spanish experience. Rev Esp Cardiol. 2020;73:994-1002.
23. Mafham MM, Spata E, Goldacre R, et al. COVID-19 pandemic and admission rates for and management of acute coronary syndromes in England. Lancet. 2020;39:381-389.
24. Álvarez Gallego M, Gortázar de las Casas S, Pascual Migueláñez I, et al. SARS-CoV-2 pandemic on the activity and professionals of a General Surgery and Digestive Surgery Service in a tertiary hospital. Cir Esp. 2020;98:320-327.
ABSTRACT
Introduction and objectives: Coronary artery disease and mental health disorders are often coexistent. Selective serotonin reuptake inhibitors (SSRIs) are often used in this context but have been associated with an increased risk of bleeding due to platelet dysfunction. Previous studies have assessed this risk in patients treated with clopidogrel-based dual antiplatelet therapy (DAPT) with contradictory results. However, there is no data regarding the use of SSRIs and potent P2Y12 inhibitors or triple antithrombotic therapy after percutaneous coronary intervention (PCI). The purpose of this study was to assess the impact of SSRIs on bleeding outcomes after PCI in patients treated with clopidogrel, prasugrel or ticagrelor-based DAPT or triple antithrombotic therapy.
Methods: Retrospective study including all patients undergoing PCI at a high-volume center during 2018. Patients on SSRIs were propensity-score-matched on a 1:1 ratio with patients naive to SSRIs adjusting for the baseline differences. The primary endpoint was major bleeding (BARC type 3 or 5 bleeding) at the 1-year follow-up. Secondary endpoints were a composite of major/non-major clinically relevant bleeding (BARC type 2, 3 or 5 bleeding), and a composite of major adverse cardiovascular events.
Results: Out of a total of 1063 patients treated with PCI during the study period, 1002 met the selection criteria, and 139 (13.9%) were on SSRIs. The latter had a higher bleeding risk before matching [PRECISE-DAPT, 16 [10-24] vs 13 [9-21]; P = .040]. No differences were reported in major bleeding (2.9% vs 2.9%, P = .991), major/non-major clinically relevant bleeding (2.9% vs 7.2%, P = .120) or in major adverse cardiovascular events (7.9% vs 7.9%, P = .979) in patients treated with SSRIs.
Conclusions: The use of SSRIs was frequent in patients treated with PCI, and although it was a marker of a higher bleeding risk at baseline, this was not associated with an additional bleeding liability.
Keywords: Bleeding. Coronary artery disease. Percutaneous coronary intervention. Selective serotonin reuptake inhibitors. Antithrombotic therapy.
RESUMEN
Introducción y objetivos: La cardiopatía isquémica y la enfermedad mental coexisten a menudo. Los inhibidores selectivos de la recaptación de serotonina (ISRS) se utilizan con frecuencia en este contexto, pero se han asociado con un incremento en el riesgo hemorrágico. Los estudios previos han evaluado este fenómeno en pacientes tratados con clopidogrel, con resultados contradictorios. No hay datos sobre el uso de ISRS e inhibidores del P2Y12 potentes o triple terapia antitrombótica. El objetivo de este estudio fue examinar el impacto de los ISRS en los eventos hemorrágicos en pacientes tratados con doble (incluyendo clopidogrel, prasugrel o ticagrelor) o triple terapia antitrombótica tras una intervención coronaria percutánea (ICP).
Métodos: Estudio retrospectivo en el que se incluyeron todos los pacientes tratados con ICP en un centro de alto volumen durante 2018. Los pacientes en tratamiento con ISRS fueron emparejados mediante puntaje de propensión con pacientes sin ISRS. El objetivo primario fue el sangrado mayor al año de seguimiento (BARC 3 o 5). Los objetivos secundarios fueron un combinado de sangrado mayor o menor clínicamente relevante (BARC 2, 3 o 5) y un combinado de eventos cardiovasculares adversos mayores.
Resultados: De los 1.063 pacientes tratados con ICP durante el periodo del estudio, 1.002 cumplieron los criterios de selección y 139 (13,9%) recibían ISRS. Los pacientes con ISRS tenían un mayor riesgo de sangrado antes del emparejamiento (PRECISE-DAPT: 16 [10-24] frente a 13 [9-21]; p = 0,040). No hubo diferencias en el objetivo primario (2,9% frente a 2,9%; p = 0,991) ni en los objetivos secundarios de sangrado mayor o menor clínicamente relevante (2,9 frente a 7,2%; p = 0,120) y eventos cardiovasculares adversos mayores (7,9 frente a 7,9%; p = 0,979).
Conclusiones: El uso de ISRS fue frecuente en los pacientes tratados con ICP, y aunque fue un marcador de riesgo hemorrágico basal, no se asoció con un mayor riesgo de sangrado en el seguimiento.
Palabras clave: Sangrado. Enfermedad coronaria. Intervencionismo coronario percutáneo. Inhibidores selectivos de la recaptación de serotonina. Terapia antitrombótica.
Abbreviations
DAPT: dual antiplatelet therapy. PCI: percutaneous coronary intervention. SSRIs: selective serotonin reuptake inhibitors.
INTRODUCTION
Coronary artery disease and mental health disorders frequently coexist and have a bidirectional relationship.1,2 Patients with mental health disorders have an increased risk of coronary artery disease and, inversely, it is not rare for patients to experience symptoms of depression or anxiety after a cardiac event.3 Moreover, depression in patients with CHD is associated with a poor adherence to treatment, unhealthy lifestyle habits, and a poor prognosis.4-8
Selective serotonin reuptake inhibitors (SSRIs) are often prescribed as first-line agents to treat depression and anxiety,9,10 but have a potential for increased bleeding risk due to the concomitant inhibitory effect on the platelet serotonin reuptake transporter (5-HTT).11 Platelet 5-HTT inhibition has been associated with a reduced platelet activation and aggregation, and with a prolonged bleeding time.12,13 On the other hand, some studies have linked SSRI-related bleeding risk to older age, comorbidities or polypharmacy.14,15
Bleeding risk due to antithrombotic therapy is a major concern following percutaneous coronary intervention (PCI) as hemorrhagic events are prognostically unfavorable as recurrent ischemic events.16,17 While bleeding risk depends on multiple clinical and laboratory features,18,19 the identification of potential modifiable factors is key to optimize the balance between ischemic and bleeding risk.20 Prior studies have evaluated the bleeding risk of patients with a concomitant treatment of SSRIs and dual antiplatelet therapy (DAPT) plus aspirin and clopidogrel with contradictory results.21-23 However, the impact of SSRIs plus therapy with more potent P2Y12 inhibitors (eg, ticagrelor or prasugrel) or triple antithrombotic therapy with DAPT plus an oral anticoagulant (OAC) has never been explored. In this study we tried to compare the 1-year risk of bleeding after PCI and concomitant guideline-recommended antithrombotic therapy (including clopidogrel, ticagrelor or prasugrel-based DAPT and triple antithrombotic therapy) in patients with or without prescribed SSRIs.
METHODS
Study design and setting
Retrospective study including all consecutive patients discharged after PCI performed at a single center during 2018. Those treated with SSRIs were propensity score-matched (PSM) to a control group to compare bleeding outcomes at the 1-year follow-up. Antithrombotic treatment was decided by the clinical cardiologist in accordance with the current clinical practice guidelines.24 This study was conducted according to the Declaration of Helsinki and was approved by the local clinical research ethics committee. Written informed consent was obtained from all patients before the PCI.
Population
All patients discharged after the PCI performed during the study period were eligible. Those treated at discharge with single antiplatelet therapy, DAPT were excluded—not including acetylsalicylic acid—as well as those anticoagulated with low-molecular-weight heparin for other reasons. Patients with missing information at the follow-up were also excluded. Clinical and procedural data, treatment at discharge, and outcomes during the first year were reviewed through electronic health records. Patients were treated with SSRIs if their list of prescriptions at discharge included one of the following: citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine or sertraline.
Endpoints
The primary safety endpoint was major bleeding at the 1-year follow-up. Secondary endpoints were a composite of major or non-major clinically relevant bleeding, and a composite of major adverse cardiovascular events (MACE). Major bleeding was defined as a bleeding event type 3 or 5 according to the Bleeding Academic Research Consortium (BARC). Major/non-major clinically relevant bleeding was defined as BARC type 2, 3 or 5 bleeding event.25 MACE was defined as a composite outcome of cardiovascular death, non-fatal myocardial infarction or unplanned revascularization. Events were independently adjudicated by 2 cardiologists who were unaware of the SSRIs group.
Statistical analysis
Categorical variables were expressed as counts (percentages), and the continuous ones as mean ± standard deviation or median [interquartile range] according to their distribution assessed using the Shapiro-Wilk test. P values were obtained using the chi-square test or the Mann-Whitney U test, as appropriate. PSM was conducted to account for the confounding biases.26 Logistic regression was used to determine the probability of being treated with SSRIs and included the following confounding variables potentially associated with SSRIs treatment and the primary endpoint:27 age, sex, prior relevant bleeding, hypertension, cancer, past medical history of hematologic disease or anemia, liver disease, creatinine clearance, treatment with potent P2Y12 inhibitors or concomitant OAC. The nearest neighbor matching method with no replacement, and a caliper width of 0.1 were used in the PSM on a 1:1 ratio. Propensity score histograms and standardized mean differences before and after the PSM were used to evaluate the balance of the groups regarding the covariates.28 Time-to-event analyses were conducted using the Kaplan-Meier and Cox proportional hazards methods. To determine major bleeding predictors in the unmatched cohort, a multivariate Cox regression model was conducted that used a purposeful selection model and prioritized parsimony. Clinical meaningful variables and those showing P values < 0.2 in the univariate analysis were included. Statistical analyses were performed using SPSS software (version 24; IBM Corp., United States) and R software (version 4.0.3; R Foundation for Statistical Computing, Austria). Matching was performed using the MatchIt R package (Ho, Imai, King, & Stuart, 2011) while covariate balance was assessed using the Cobalt R package (Greifer, 2021).
RESULTS
Baseline clinical characteristics
A total of 1063 patients were treated with PCI during the study period, 1002 of whom met the selection criteria and were included in the analysis. A total of 139 patients (13.9%) were treated with SSRIs at discharge (figure 1). Median age was 66 years (58-75), and 745 patients (74.4%) were male with a median PRECISE-DAPT score of 13 [9-22]. Regarding antithrombotic therapy, 684 patients (68.3%) were treated with potent P2Y12 inhibitors and 102 (10.2%) were concomitantly treated with OAC. The baseline clinical characteristics of the overall population and the unmatched groups are shown on table 1. Patients from the SSRIs group were more likely to be women. They also had a more extensive past medical history of hypertension, diabetes mellitus, cancer, significant bleeding, and hematologic disease or anemia. Both the HAS-BLED and the PRECISE-DAPT bleeding risk scores were higher in the SSRIs group.
Figure 1. Patient flowchart. PCI, percutaneous coronary intervention; SSRIs, selective serotonin reuptake inhibitors.
Table 1. Baseline clinical characteristics of the overall population, and SSRIs/non-SSRIs users before matching
| Variable | Overall (N = 1002) | SSRI (N = 139) | Non-SSRI (N = 863) | P |
|---|---|---|---|---|
| Age, years | 66 [58-75] | 67 [60-76] | 66 [57-75] | .530 |
| Sex, male | 745 (74.4) | 76 (54.7) | 669 (77.5) | .001* |
| BMI | 28.7 [25.9-31.8] | 30.0 [25.8-32.0] | 28.6 [25.9-31.7] | .067 |
| Hypertension | 688 (68.7) | 112 (80.6) | 576 (66.7) | .001* |
| Diabetes mellitus | 370 (36.9) | 64 (46.0) | 306 (35.5) | .017* |
| Hyperlipidemia | 525 (52.4) | 83 (59.7) | 442 (51.2) | .059 |
| Smoking (current or former) | 260 (25.9) | 34 (24.5) | 226 (26.2) | .709 |
| Previous revascularization | 248 (24.8) | 41 (29.5) | 207 (24.0) | .174 |
| COPD | 67 (6.7) | 10 (7.2) | 57 (6.6) | .740 |
| Chronic kidney disease | 115 (11.5) | 17 (12.2) | 98 (11.4) | .774 |
| Cancer | 98 (9.8) | 20 (14.4) | 78 (9.0) | .044* |
| Liver disease | 37 (3.7) | 8 (5.8) | 29 (3.4) | .166 |
| Hematologic disease or anemia | 99 (9.9) | 25 (18) | 74 (8.6) | .001* |
| Previous relevant bleeding | 31 (3.1) | 9 (6.5) | 22 (2.5) | .010* |
| Atrial fibrillation | 87 (8.7) | 11 (7.9) | 76 (8.8) | .871 |
| Oral anticoagulant | 102 (10.2) | 9 (6.5) | 93 (10.8) | .119 |
| Potent P2Y12 inhibitors | 684 (68.3) | 90 (64.7) | 594 (68.8) | .323 |
| Ticagrelor, no. (%) | 660 (65.9) | 86 (61.8) | 574 (66.5) | .543 |
| Prasugrel | 24 (2.4) | 4 (2.9) | 20 (2.3) | .543 |
| DAPT duration (months) | 8 [6-12] | 6 [6-12] | 8 [6-12] | .440 |
| PRECISE-DAPT | 13 [9-22] | 16 [10-24] | 13 [9-21] | .040* |
| PRECISE-DAPT ≥ 25 | 195 [19.5] | 34 [24.5] | 161 [18.7] | .109 |
| HAS-BLED | 2 (2-3) | 3 (2-3) | 2 (2-3) | .034* |
| Creatinine clearance, mL/min/1.73 m2 | 100 [82.3-124.1] | 94.8 [72.9-125.2] | 100 [82.7-124.1] | .154 |
| Clinical presentation | ||||
| CCS | 441 (44.0) | 66 (47.5) | 375 (43.5) | .375 |
| ACS | 561 (56.0) | 73 (52.5) | 488 (56.5) | |
|
ACS, acute coronary syndrome; BMI, body mass index (kg/m2); CCS, chronic coronary syndrome; COPD, chronic obstructive pulmonary disease; DAPT, dual antiplatelet therapy; SSRI, selective serotonin reuptake inhibitors. Data are expressed as no. (%), mean ± standard deviation or median [interquartile range]. * Indicates a statistically significant difference with P values < .05. |
||||
Unmatched analysis
In the overall population there were a total of 19 major bleeding events at the 1-year follow-up: 4 (2.9%) in the SSRIs group, and 15 (1.7%) in the unmatched non-SSRIs group (P = .350). Of these, 4 (21.1%) were fatal, 10 (52.6%) GI bleedings, 4 (21.1%) intracranial bleedings while the remaining ones occurred in other locations.
The multivariable Cox model identified the following independent predictors for the primary endpoint of major bleeding: PRECISE-DAPT score ≥ 25, and concomitant anticoagulation. Table 2 shows the univariable and multivariable Cox predictors for the primary endpoint.
Table 2. Univariable and multivariable Cox predictors for major bleeding
| Variable | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| HR (95%CI) | P | HR (95%CI) | P | |
| Age, years | 1.06 (1.02-1.11) | .008 | ||
| Sex, male | 0.47 (0.19-1.18) | .107 | ||
| BMI | 0.98 (0.89-1.09) | .756 | ||
| Hypertension | 0.99 (0.38-2.61) | .989 | ||
| Diabetes mellitus | 1.91 (0.78-4.79) | .160 | ||
| Hyperlipidemia | 0.82 (0.33-2.01) | .664 | ||
| Chronic kidney disease | 3.67 (1.39-9.66) | .008 | ||
| Cancer | 2.47 (0.82-7.46) | .107 | ||
| Liver disease | 1.48 (0.19-11.05) | .705 | ||
| Hematologic disease or anemia | 2.47 (0.82-7.46) | .107 | ||
| Previous relevant bleeding | 3.91 (0.90-16.91) | .068 | ||
| Atrial fibrillation | 5.45 (2.05-14.53) | .001 | ||
| Oral anticoagulant | 8.22 (3.34-20.23) | .001 | 6.99 (2.78-17.64) | .001 |
| Potent P2Y12 inhibitors | 0.16 (0.06-0.45) | .001 | ||
| PRECISE-DAPT ≥ 25 | 4.77 (1.94-11.75) | .001 | 3.59 (1.44-8.98) | .006 |
| HAS-BLED | 1.69 (1.17-2.43) | .005 | ||
| Creatinine clearance | 0.98 (0.97-0.99) | .024 | ||
| SSRI | 1.68 (0.56-5.07) | .356 | 1.95 (0.64-5.93) | .241 |
|
95%CI, 95% confidence interval; BMI, body mass index (kg/m2); HR, hazard ratio; SSRI, selective serotonin reuptake inhibitors. |
||||
The major/non-major clinically relevant bleeding endpoint occurred in 4 patients (2.9%) from the SSRIs group, and in 43 patients (4.9%) from the unmatched no-SSRIs group (P = .290). The rate of MACE was similar in both groups: 11 events (7.9%) in the SSRIs group and 50 events (5.8%) in the non-SSRIs group.
The Kaplan-Meier curves and the associated risk tables for each endpoint of the unmatched cohorts are shown on figure 2.
Figure 2. Kaplan-Meier curves for the primary bleeding outcome (A), the secondary composite bleeding (B), and the ischemic outcomes (C). Unmatched cohort. SSRIs, selective serotonin reuptake inhibitors.
Propensity score matching analysis
The variables used in the PSM, the standardized mean differences, and the Propensity score distributions of the unmatched and matched samples are shown on figure 3. PSM resulted in an excellent balance of covariates with standardized mean differences ≤ 10% in all variables included in the Propensity score. There was also a very good balance across the other baseline characteristics and bleeding risk scores except for diabetes mellitus and hyperlipemia that were more prevalent in the SSRIs group (table 3).
Figure 3. Variables used in the propensity score matching analysis and their standardized differences (A), and the propensity score distributions (B) of the unmatched and matched samples. OAC, oral anticoagulant.
Table 3. Baseline clinical characteristics of SSRIs/non-SSRIs users after matching
| Variable | SSRI (N = 139) | Non-SSRI (N = 139) | P |
|---|---|---|---|
| Chronic obstructive pulmonary disease | |||
| Age, years | 68 [60-76] | 67 [58-75] | .757 |
| Sex, male | 76 (54.7) | 73 (52.5) | .810 |
| BMI | 30.0 [25.8-32.0] | 28.4 [25.3-32.4] | .143 |
| Hypertension | 112 (80.6) | 109 (78.4) | .656 |
| Diabetes mellitus | 64 (46.0) | 48 (34.5) | .050 |
| Hyperlipidemia | 83 (59.7) | 67 (48.2) | .045 |
| Smoking (current or former) | 34 (24.5) | 28 (20.1) | .330 |
| Previous revascularization | 41 (29.5) | 30 (21.6) | .153 |
| COPD | 10 (7.2) | 9 (6.5) | .816 |
| Chronic kidney disease | 17 (12.2) | 19 (13.7) | .721 |
| Cancer | 20 (14.4) | 18 (12.9) | .727 |
| Liver disease | 8 (5.8) | 10 (7.2) | .626 |
| Hematologic disease or anemia | 25 (18) | 21 (15.1) | .519 |
| Previous relevant bleeding | 9 (6.5) | 10 (7.2) | .812 |
| Atrial fibrillation | 11 (7.9) | 11 (7.9) | 1.000 |
| Oral anticoagulant | 9 (6.5) | 9 (6.5) | 1.000 |
| Potent P2Y12 inhibitors | 90 (64.7) | 97 (69.8) | .371 |
| Ticagrelor | 86 (61.8) | 91 (65.5) | .749 |
| Prasugrel | 4 (2.9) | 6 (4.3) | .749 |
| DAPT duration, months | 6 [6-12] | 6 [6-12] | .810 |
| PRECISE-DAPT | 16 [10-24] | 15 [10-24] | .863 |
| PRECISE-DAPT ≥ 25 | 34 (24.5) | 32 (23.0) | .778 |
| HAS-BLED | 3 [2-3] | 3 [2-3] | .560 |
| Creatinine clearance, | |||
| mL/min/1.73 m2 | 94.8 [72.9-125.2] | 100 [82.7-114.0] | .747 |
| Clinical presentation | |||
| CCS | 66 (47.5) 73 (52.5) |
63 (45.3) 76 (54.7) |
.718 |
| ACS | |||
|
ACS, acute coronary syndrome; BMI, body mass index (kg/m2); CCS, chronic coronary syndrome; COPD, chronic obstructive pulmonary disease; DAPT, dual antiplatelet therapy; SSRI, selective serotonin reuptake inhibitors. Data are expressed as no. (%), mean ± standard deviation or median [interquartile range]. |
|||
The rate of major bleeding at the 1-year follow-up was 2.9% for both patients on SSRIs and the matched SSRIs non-users (HR, 1.01; 95%CI, 0.25-4.03; P = .991). There were no non-major clinically relevant bleedings in the SSRIs group and 6 (4.3%) among SSRIs non-users (HR, 0.39; 95%CI, 0.16-1.27; P = .120). No differences in MACE were reported between the SSRI and the non-SSRIs groups (HR, 1.01; 95%CI, 0.44-2.33; P = .979) (figure 4).
Figure 4. Kaplan-Meier curves for the primary bleeding outcome (A), the secondary composite bleeding (B), and the ischemic outcomes (C). Matched cohorts. SSRIs, selective serotonin reuptake inhibitors.
DISCUSSION
The main findings of this study can be summarized as follows: a) the use of SSRIs was frequent among patients undergoing PCI; b) patients prescribed with SSRIs had a higher baseline bleeding risk; c) despite the imbalance reported in the baseline characteristics, after adjustment SSRIs users were not associated with a significant excess of major or clinically relevant bleeding at the 1-year follow-up.
There is a strict correlation between coronary artery disease and mental health disorders. In our study up to 13.9% of patients treated with PCI were prescribed SSRIs. This group has more comorbidities and bleeding risk factors with the potential to complicate the clinical decision-making process regarding antithrombotic therapy selection. Importantly, whether SSRIs trigger a higher bleeding risk through a biological effect on platelet 5-HTT receptors or are a marker of a higher bleeding risk through concomitant comorbidities has been the matter of discussion in prior studies.
Labos et al.21 reported an increased risk of bleeding in patients taking both SSRIs and acetylsalicylic acid or clopidogrel-based DAPT after myocardial infarction. On the contrary, Lasella et al.22 assessed the impact of SSRI therapy on patients on DAPT after PCI finding no excessive bleedings in patients on SSRIs. Interestingly, they reported a lower risk of MACE in patients on SSRIs compared to those on mirtazapine, but a higher risk compared to patients on either one of the 2 antidepressants. This may be explained by a protective effect of SSRIs on MACE29 that could be exceeded by the unfavorable effect of mental health disorders on cardiovascular events.30 Another interpretation could be associated with the pharmacokinetics of clopidogrel since it is a prodrug that requires enzymatic conversion into its active metabolite by cytochrome P450 (CYP).31 Bykov et al.23 reported an increased risk of ischemic events in patients on clopidogrel and a CYP2C19-inhibiting SSRI compared to those on noninhibiting SSRIs. No differences were found regarding major bleeding. The study did not include a group of patients without SSRI treatment.
We should mention that none of the aforementioned studies included patients treated with potent P2Y12 inhibitors, which is currently the standard of care of patients with ACS. To our knowledge, this is the first study to assess the impact of SSRIs on a cohort of patients treated with potent P2Y12 inhibitors prasugrel or ticagrelor. In our population, two thirds of the patients were treated with potent P2Y12 inhibitors, which is more consistent with the antiplatelet strategies recommended by the current clinical practice guidelines.32,33 In this clinical setting, despite the imbalances reported in the baseline bleeding risk in an unadjusted analysis, we found no differences regarding major or clinically relevant bleeding events among patients on SSRIs and the matched group without a SSRI prescription. Hence, while the prescription of SSRIs can be a marker of a higher risk population with more comorbidities and risk factors, this may not translate into an independent predictor of bleeding events after accounting for the potential confounders. This is consistent with prior evidence in the medical literature. In the study conducted by Labos et al.21 patients on SSRI had a more significant past medical history of hypertension, renal failure, anemia or other hematologic disease, and non-GI bleeding. Lasella et al.22 reported that SSRIs users were more likely to have diabetes, hypertension, dyslipidemia, COPD, and chronic kidney disease.
Our findings are clinically relevant for different reasons. Although SSRIs have been associated with a potential for an increased bleeding risk, a direct translation into an excess of adverse events has not been confirmed yet. Our data provide reassurance on the relative safety profile of potent antithrombotic therapies in association with SSRIs, which did not substantially increase the risk of bleeding during the first year after PCI when the treatment decision-making process is based on a thorough evaluation of the features of bleeding and ischemic risk.
Our study also included a proportion of patients treated with concomitant antiplatelet and OAC therapy (~10%), which is consistent with the current standard practice.34 The impact of SSRIs on bleeding outcomes in patients with AF treated with OAC has also been examined in the past. Various authors have reported a higher risk of major bleeding in patients concurrently treated with SSRIs and warfarin.35,36 On the contrary, Quinn et al.37 did not find a significantly increased risk of bleeding among patients from the ROCKET AF trial assigned to warfarin or rivaroxaban who were also on SSRIs. However, there was a modest but non-statistically significant higher risk of major bleeding in the warfarin group. Since SSRIs are CYP2C9 inhibitors, an increase of warfarin plasma concentrations could explain these findings.38 This reaffirms the importance of non-vitamin k antagonists to reduce the risk of bleeding also in this population given the need for multiple antithrombotic agents after the PCI and the higher baseline bleeding risk reported.39
Limitations
The current study has several limitations. First, its retrospective observational design, and the relatively small size of the sample limits our ability to provide definitive conclusions due to the residual possibility of type-2 errors. Secondly, despite the PSM resulted in a good balance between the selected potential confounders and the other baseline characteristics, the presence of residual confounding factors cannot be completely ruled out. For example, some variables associated with bleeding like the presence of diabetes mellitus or peripheral arterial disease were not included in the propensity score model. Yet similar findings were observed in the adjusted and unadjusted analyses. Thirdly, the classification of SSRI users was based on treatment at discharge without accounting for treatment adherence or discontinuation.
CONCLUSIONS
In this real-world study, a combination of SSRIs and potent antithrombotic therapies was frequently prescribed after PCI. Although the prescription of SSRIs was associated with a higher baseline bleeding risk in the unadjusted analysis this was not the case with an excess of major or clinically relevant bleeding reported at the follow-up.
FUNDING
None reported.
AUTHORS’ CONTRIBUTIONS
R. González-Manzanares, and S. Ojeda conceived and designed the study. R. González-Manzanares, M. Ruiz-Moreno, C. Fernández-Avilés, L. Carmona-Artime, G. Flores-Vergara, and F. Costa collected analyzed data and interpreted the results. R. González-Manzanares, M. Ruiz-Moreno, S. Ojeda, and F. Hidalgo drafted the manuscript and completed the critical revisions. S. Ojeda, F. Hidalgo, G. Flores-Vergara, F. Costa, J. Suárez de Lezo, and M. Pan reviewed and revised the manuscript, and approved its final version before submission. All authors gave their final approval to the version that would eventually be published.
CONFLICTS OF INTEREST
S. Ojeda is an associate editor of REC: Interventional Cardiology. The journal’s editorial procedure to ensure impartial handling of the manuscript has been followed. S. Ojeda, and M. Pan declared having received honoraria for lectures given for Abbott, Boston, World Medical, and Terumo. J Suárez de Lezo declared having received honoraria for lectures given for Abbott. The remaining authors declared no conflicts of interest whatsoever.
WHAT IS KNOWN ABOUT THE TOPIC?
- Coronary artery disease and mental health disorders frequently coexist. The combination of SSRIs and potent antithrombotic therapies is common.
- Bleeding events after PCI worsen prognosis same as recurrent ischemic events.
- SSRIs have been potentially associated with an increased risk of bleeding. Data regarding the concomitant use of SSRIs and potent antithrombotic therapies is scarce and inconclusive.
WHAT DOES THIS STUDY ADD?
- This is the first study to assess the impact of SSRIs on the bleeding outcomes in the current PCI practice using potent P2Y12 inhibitors or triple antithrombotic therapy.
- SSRIs users have a higher bleeding risk profile.
- The use of SSRIs was not associated with a higher risk of major bleeding after adjusting for the potential confounders.
REFERENCES
1. De Hert M, Detraux J, Vancampfort D. The intriguing relationship between coronary heart disease and mental disorders. Dialogues Clin Neurosci. 2018;20:31-40.
2. Jha MK, Qamar A, Vaduganathan M, Charney DS, Murrough JW. Screening and Management of Depression in Patients With Cardiovascular Disease:JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;73:1827-1845.
3. Lane D, Carroll D, Ring C, Beevers DG, Lip GY. The prevalence and persistence of depression and anxiety following myocardial infarction. Br J Health Psychol. 2002;7:11-21.
4. Gehi A, Haas D, Pipkin S, Whooley MA. Depression and medication adherence in outpatients with coronary heart disease:findings from the Heart and Soul Study. Arch Intern Med. 2005;165:2508-2513.
5. Sin NL, Kumar AD, Gehi AK, Whooley MA. Direction of Association Between Depressive Symptoms and Lifestyle Behaviors in Patients with Coronary Heart Disease:the Heart and Soul Study. Ann Behav Med. 2016;50:523-532.
6. Meijer A, Conradi HJ, Bos EH, et al. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events:a meta-analysis of 25 years of research. Gen Hosp Psychiatry. 2011;33:203-216.
7. Carney RM, Blumenthal JA, Freedland KE, et al. Depression and late mortality after myocardial infarction in the Enhancing Recovery in Coronary Heart Disease (ENRICHD) study. Psychosom Med. 2004;66:466-474.
8. Valgimigli M, Garcia-Garcia HM, Vrijens B, et al. Standardized classification and framework for reporting, interpreting, and analysing medication non-adherence in cardiovascular clinical trials:a consensus report from the Non-adherence Academic Research Consortium (NARC) [published correction appears in Eur Heart J. 2019;40:2774]. Eur Heart J. 2019;40:2070-2085.
9. Ghaffari Darab M, Hedayati A, Khorasani E, Bayati M, Keshavarz K. Selective serotonin reuptake inhibitors in major depression disorder treatment:an umbrella review on systematic reviews. Int J Psychiatry Clin Pract. 2020;24:357-370.
10. Bandelow B. Current and Novel Psychopharmacological Drugs for Anxiety Disorders. Adv Exp Med Biol. 2020;1191:347-365.
11. Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med. 2009;60:355-366.
12. Abdelmalik N, RuhéHG, Barwari K, et al. Effect of the selective serotonin reuptake inhibitor paroxetine on platelet function is modified by a SLC6A4 serotonin transporter polymorphism. J Thromb Haemost. 2008;6:2168-2174.
13. De Abajo FJ. Effects of selective serotonin reuptake inhibitors on platelet function:mechanisms, clinical outcomes and implications for use in elderly patients. Drugs Aging. 2011;28:345-367.
14. Hougardy DM, Egberts TC, van der Graaf F, Brenninkmeijer VJ, Derijks LJ. Serotonin transporter polymorphism and bleeding time during SSRI therapy. Br J Clin Pharmacol. 2008;65:761-766.
15. Yuan Y, Tsoi K, Hunt RH. Selective serotonin reuptake inhibitors and risk of upper GI bleeding:confusion or confounding?Am J Med. 2006;119:719-727.
16. Valgimigli M, Costa F, Lokhnygina et al. Trade-off of myocardial infarction vs. bleeding types on mortality after acute coronary syndrome:lessons from the Thrombin Receptor Antagonist for Clinical Event Reduction in Acute Coronary Syndrome (TRACER) randomized trial. Eur. Heart J. 2017;38:804-810.
17. Mehran R, Pocock SJ, Stone GW, et al. Associations of major bleeding and myocardial infarction with the incidence and timing of mortality in patients presenting with non-ST-elevation acute coronary syndromes:a risk model from the ACUITY trial. Eur Heart J. 2009;30:1457-1466.
18. Mehran R, Pocock SJ, Nikolsky E, et al. A risk score to predict bleeding in patients with acute coronary syndromes. J Am Coll Cardiol. 2010;55:2556-2566.
19. Costa F, van Klaveren D, James S, et al. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score:a pooled analysis of individual-patient datasets from clinical trials. Lancet. 2017;389:1025-1034.
20. Kang DO, An H, Park GU, et al. Cardiovascular and Bleeding Risks Associated With Nonsteroidal Anti-Inflammatory Drugs After Myocardial Infarction. J Am Coll Cardiol. 2020;76:518-529.
21. Labos C, Dasgupta K, Nedjar H, Turecki G, Rahme E. Risk of bleeding associated with combined use of selective serotonin reuptake inhibitors and antiplatelet therapy following acute myocardial infarction. CMAJ. 2011;183:1835-1843.
22. Iasella CJ, Kreider MS, Huang L, Coons JC, Stevenson JM. Effect of Selective Serotonin Reuptake Inhibitors on Cardiovascular Outcomes After Percutaneous Coronary Intervention:A Retrospective Cohort Study. Clin Drug Investig. 2019;39:543-551.
23. Bykov K, Schneeweiss S, Donneyong MM, Dong YH, Choudhry NK, Gagne JJ. Impact of an Interaction Between Clopidogrel and Selective Serotonin Reuptake Inhibitors. Am J Cardiol. 2017;119:651-657.
24. Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS:The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2018;39:213-260.
25. Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials:a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736-2747.
26. Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res. 2011;46:399-424.
27. Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Stürmer T. Variable selection for propensity score models. Am J Epidemiol. 2006;163:1149-1156.
28. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083-3107.
29. Fernandes N, Prada L, Rosa MM, et al. The impact of SSRIs on mortality and cardiovascular events in patients with coronary artery disease and depression:systematic review and meta-analysis. Clin Res Cardiol. 2021;110:183-193.
30. Zhang WY, Nan N, Song XT, Tian JF, Yang XY. Impact of depression on clinical outcomes following percutaneous coronary intervention:a systematic review and meta-analysis. BMJ Open. 2019;9:e026445.
31. Price MJ, Tantry US, Gurbel PA. The influence of CYP2C19 polymorphisms on the pharmacokinetics, pharmacodynamics, and clinical effectiveness of P2Y(12) inhibitors. Rev Cardiovasc Med. 2011;12:1-12.
32. Patel A, Goodman SG, Tan M, et al. Contemporary use of guideline-based higher potency P2Y12 receptor inhibitor therapy in patients with moderate-to-high risk non-ST-segment elevation myocardial infarction:Results from the Canadian ACS reflective II cross-sectional study. Clin Cardiol. 2021;44:839-847.
33. De Luca L, Zeymer U, Claeys MJ, et al. Comparison of P2Y12 receptor inhibitors in patients with ST-elevation myocardial infarction in clinical practice:a propensity score analysis of five contemporary European registries. Eur Heart J Cardiovasc Pharmacother. 2021;7:94-103.
34. Costa F, Garcia-Ruiz V, Licordari R, Fimiani L. The High Bleeding Risk Patient with Coronary Artery Disease. Cardiol Clin. 2020;38:481-490.
35. Quinn GR, Singer DE, Chang Y, et al. Effect of selective serotonin reuptake inhibitors on bleeding risk in patients with atrial fibrillation taking warfarin. Am J Cardiol. 2014;114:583-586.
36. Schelleman H, Brensinger CM, Bilker WB, Hennessy S. Antidepressant-warfarin interaction and associated gastrointestinal bleeding risk in a case-control study [published correction appears in PLoS One. 2015;10:e0121926]. PLoS One. 2011;6:e21447.
37. Quinn GR, Hellkamp AS, Hankey GJ, et al. Selective Serotonin Reuptake Inhibitors and Bleeding Risk in Anticoagulated Patients With Atrial Fibrillation:An Analysis From the ROCKET AF Trial. J Am Heart Assoc. 2018;7(15):e00∳.
38. Sansone RA, Sansone LA. Warfarin and Antidepressants:Happiness without Hemorrhaging. Psychiatry (Edgmont). 2009;6:24-29.
39. Costa F, Valgimigli M, Steg PG, et al. Antithrombotic therapy according to baseline bleeding risk in patients with atrial fibrillation undergoing percutaneous coronary intervention:applying the PRECISE-DAPT score in RE-DUAL PCI [published online ahead of print, 2020 Dec 1]. Eur Heart J Cardiovasc Pharmacother. 2020;pvaa135.
- Clinical follow-up of long nontapered sirolimus-eluting coronary stent in real-world patients with de novo lesions. The Billar registry
- Stent-grafts versus drug-eluting stents in arterial aneurysms, insights from the International Coronary Artery Aneurysm Registry (CAAR)
- Diabetes mellitus and long-term safety of FFR and iFR-based coronary revascularization deferral
- Deflation speed of the stent delivery system and primary angioplasty results: a randomized study
Editorials
Expanding the role of drug-coated balloons in native large coronary artery disease
aDepartment of Biomedical Sciences, Humanitas University, Pieve Emanuele, Milan, Italy
bCardio Center, IRCCS Humanitas Research Hospital, Rozzano, Milan, Italy
cDivision of Cardiology, Montefiore Medical Center, Bronx, New York, United States
Original articles
Editorials
The role of angiography-derived physiological assessment techniques in the post-FAVOR III Europe era?
aServicio de Cardiología, Hospital General Universitario Gregorio Marañón, Instituto de Investigación Sanitaria Gregorio Marañón, Universidad Complutense de Madrid, Madrid, Spain
bCentro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), Instituto de Salud Carlos III, Madrid, Spain
Interviews
An interview with Camino Bañuelos
aServicio de Cardiología, Hospital Clínico San Carlos, Instituto de Investigación Sanitaria del Hospital Clínico San Carlos (IdISSC), Madrid, Spain
bServicio de Cardiología, Hospital Clínico Universitario de Santiago de Compostela, Santiago de Compostela, A Coruña, Spain