Article
Ischemic heart disease and acute cardiac care
REC Interv Cardiol. 2019;1:21-25
Access to side branches with a sharply angulated origin: usefulness of a specific wire for chronic occlusions
Acceso a ramas laterales con origen muy angulado: utilidad de una guía específica de oclusión crónica
Servicio de Cardiología, Hospital de Cabueñes, Gijón, Asturias, España
ABSTRACT
Introduction and objectives: Delayed vascular healing may induce late stent thrombosis. Optical coherence tomography (OCT) is useful to evaluate endothelial coverage. The objective of this study was to compare stent coverage and apposition in non-complex coronary artery lesions treated with durable polymer-coated everolimus-eluting stents (durable-polymer EES) vs biodegradable polymer-coated everolimus-eluting stents (biodegradable-polymer EES) vs polymer-free biolimus-eluting stents (BES) 1 and 6 months after stent implantation.
Methods: Prospective, multicenter, non-randomized study that compared the 3 types of DES. Follow-up angiography and OCT were performed 1 and 6 months later. The primary endpoint was the rate of uncovered struts as assessed by the OCT at 1 month.
Results: A total of 104 patients with de novo non-complex coronary artery lesions were enrolled. A total of 44 patients were treated with polymer-free BES, 35 with biodegradable-polymer EES, and 25 with durable-polymer EES. A high rate of uncovered struts was found at 1 month with no significant differences reported among the stents (80.2%, polymer-free BES; 88.1%, biodegradable-polymer EES; 82.5%, durable-polymer EES; P = .209). Coverage improved after 6 months in the 3 groups without significant differences being reported (97%, 95%, and 93.7%, respectively; P = .172).
Conclusions: In patients with de novo non-complex coronary artery lesions treated with durable vs biodegradable vs polymer-free DES, strut coverage and apposition were suboptimal at 1 month with significant improvement at 6 months.
Keywords: Optical coherence tomography. Drug-eluting stents. Endothelization. Apposition. Restenosis.
RESUMEN
Introducción y objetivos: A pesar del desarrollo de los stents farmacoactivos, el retraso en la endotelización puede causar trombosis tardía. La tomografía de coherencia óptica puede evaluar la cobertura intimal. El objetivo de este estudio fue comparar la cobertura y la aposición en lesiones coronarias no complejas de 3 tipos de stent: stent de everolimus con polímero persistente, stent de everolimus con polímero bioabsorbible y stent de biolimus sin polímero, a 1 y 6 meses del implante.
Métodos: Se diseñó un estudio prospectivo, multicéntrico, no aleatorizado, que comparó 3 stents farmacoactivos. Se realizaron angiografía y tomografía de coherencia óptica a 1 o 6 meses. El objetivo primario fue comparar la cobertura.
Resultados: Se incluyeron 104 pacientes con lesiones coronarias de novo no complejas. Se implantó stent sin polímero a 44 pacientes, stent con polímero bioabsorbible a 35 pacientes y stent con polímero persistente a 25 pacientes. Al mes, se observó una alta tasa de struts no cubiertos, sin diferencias significativas entre los grupos (80,2% sin polímero, 88,1% con polímero bioabsorbible y 82,5% con polímero persistente; p = 0,209). La cobertura mejoró a los 6 meses en los 3 stents, sin diferencias significativas entre ellos (97, 95 y 93,7%, respectivamente; p = 0,172).
Conclusiones: En los pacientes con lesiones coronarias no complejas tratados con stent con polímero persistente, con polímero bioabsorbible o sin polímero, la cobertura y la aposición fueron subóptimas a 1 mes del implante, con mejoría significativa a los 6 meses.
Palabras clave: Tomografía de coherencia óptica. Stent farmacoactivo. Endotelización. Aposición. Reestenosis.
Abbreviations
DAPT: dual antiplatelet therapy. DES: drug-eluting stents. OCT: optical coherence tomography. PCI: percutaneous coronary intervention. QCA: quantitative coronary angiography.
INTRODUCTION
Uncovered stent struts is one of the key predictors of stent thrombosis,1,2 and dual antiplatelet therapy (DAPT) has shown to reduce its risk.3 However, DAPT increases the risk of hemorrhage, and nearly one third of the patients treated with percutaneous coronary intervention (PCI) are considered at high bleeding risk. Given the desire for earlier discontinuation of DAPT to reduce the risk of bleeding complications, healing of the stents at earlier time points is desirable.
Drug-eluting stents (DES) significantly reduce neointimal hyperplasia and restenosis compared to bare-metal stents (BMS). However, the main concern regarding first-generation DES was late stent thrombosis due to lack of endothelization of stent struts.1 Therefore, a new generation of DES was developed based on improved thinner metal platforms, new drugs (alternative antiproliferative -limus analogues),4-6 and more biocompatible polymers.7 The evolution of DES moved towards DES with biodegradable polymers.8-10 The comparative studies between DES with biodegradable polymers and BMS showed a lower rate of cardiac death, target vessel-related myocardial reinfarction, and revascularization at 1 year.10 Compared to biodegradable polymer DES, those with durable polymer were noninferior regarding acute coronary syndromes with respect to all-cause mortality, nonfatal myocardial infarction, and revascularization.11 Furthermore, the most recent advance to overcome stent thrombosis has been the polymer-free DES. This type of DES was initially designed to reduce the risk of stent thrombosis in patients with high bleeding risk who could only take short courses of DAPT. It was compared to BMS showing a better efficacy and safety profile.13 It has recently been compared to DES in large clinical trials, especially in patients with high bleeding risk and need for shorter DAPT courses. In these studies, the use of polymer-based zotarolimus-eluting stent was noninferior to polymer-free DES14, and non-measurable differences in device-oriented composite primary endpoints were found.15
However, despite these large clinical trials, there is scarce information on the difference between the characteristics of arterial healing among different types of latest generation DES. Optical coherence tomography (OCT) is a widely used high-resolution intracoronary imaging modality to assess vascular response after stent implantation, thus detecting stent strut coverage and its apposition to the vessel wall.16,17 Stent strut coverage as studied by the OCT is considered a valuable surrogate marker for vessel healing after DES implantation.
The objective of this study was to compare durable polymer-coated everolimus-eluting stents (durable-polymer EES) vs biodegradable polymer-coated everolimus-eluting stents (biodegradable-polymer EES) vs polymer-free BES using stent strut coverage as assessed by the optical coherence tomography (OCT) as a surrogate marker to evaluate short-term arterial healing.
METHODS
Patient population and data collection
This was a prospective, multicenter, non-randomized study that compared 3 different types of DES: a) the durable polymer-coated everolimus-eluting stent Xience DES (Abbott, United States); b) the biodegradable polymer-coated everolimus-eluting stent Synergy DES (Boston Scientific, United States), and c) the polymer-free BES Biofreedom DES (Biosensors International Ltd, Singapore). The study was conducted at 4 Spanish teaching hospitals.
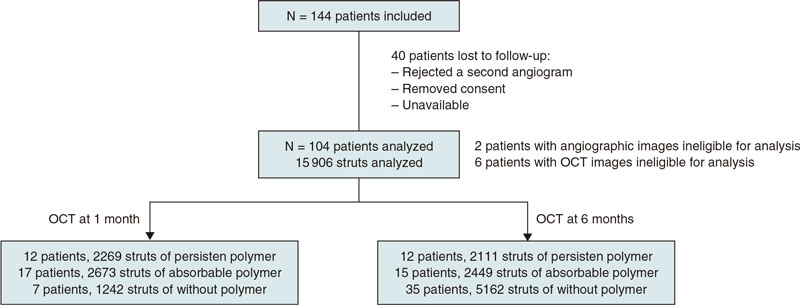
A total of 144 patients were consecutively recruited from January 2018 through December 2019. Patients were eligible if they had been admitted due to stable coronary artery disease or acute coronary syndrome without cardiogenic shock. The medical team selected the type of stent that should be implanted. The detailed study flowchart is shown on figure 1. Inclusion criteria were a) de novo lesions; b) ≥ 1 target lesions in the same or different coronary artery; c) no need for stent overlapping, and a 10 mm minimal distance between the stents; d) stent length between 8 mm and 30 mm; e) use of stents with diameters ≥ 2.5 mm.
Figure 1. Flowchart of patient inclusion.
Exclusion criteria were a) complex lesions including ostial lesions, chronic total coronary occlusions, calcified lesions requiring calcium modification techniques, and bifurcations requiring the kissing balloon technique; b) target lesions in small vessels (< 2.5 mm) and long lesions (> 30 mm) requiring small diameter stents (2.25 mm) or overlapping stents; c) diabetic patients; d) very tortuous arteries that anticipated the impossibility of access with the OCT catheter for follow-up purposes; and e) complications during index procedure. Patients with diabetes mellitus were excluded from the study because of their pro-inflammatory status that facilitates both stent thrombosis and restenosis.18
Once recruited, patients were consecutively assigned to a 1- or 6-month OCT follow-up group. The baseline characteristics, angiographic and procedural data, follow-up data, and outcome data were prospectively collected by the study coordinators. Clinical data at follow-up were obtained from the clinical records. This study was performed following the principles established by the Declaration of Helsinki, ISO14155, and the clinical practice guidelines. The study protocol was approved by the Institutional Ethics Committee (IEC) and the hospital research committee. Informed consent was obtained from all the patients.
Percutaneous coronary intervention, angiographic analysis, and optical coherence tomography
In the index procedure stents were implanted according to the standard approach. Patients were medically treated following the European guidelines on the management of chronic ischemic heart disease or acute coronary syndrome.19
Regarding the initial angiographic analysis, 2 orthogonal projections without coronary guidewire were obtained after finishing the index procedure. These same projections were acquired at follow-up. The off-line analysis of the angiographic images (quantitative coronary angiography [QCA]) was performed in an independent core lab (Barcelona Cardiac Imaging Core-Lab [BARCICORElab]), following their standard protocol. They used a dedicated software (CAAS, version 5.9; Pie Medical BV, The Netherlands). Methods used in this core lab have been previously reported20.
The follow-up angiography was performed at 1-or-6-month follow-up. Angiographic and OCT images were obtained from each patient. The Dragonfly frequency domain OCT C7-XR system (St. Jude Medical, United States) was used. This analysis was performed at the same independent core lab with a dedicated software (St. Jude Medical). Further information can be found on the supplementary data. The struts were classified as non-covered if their surface was totally or partially exposed to the lumen, and without any tissue coverage above its high-density scaffold. Stent strut apposition was defined as the perpendicular distance between the luminal edge of the strut and the vascular wall. Incomplete apposition was considered when distance was higher compared to the total strut thickness considering the addition of strut plus polymer. Intimal hyperplasia was measured as the perpendicular distance between the luminal surface of the stent strut and the luminal surface of the neointima.
Endpoints
The study primary endpoint was the percentage of uncovered struts among durable-polymer EES vs biodegradable-polymer EES vs polymer-free BES as seen on the OCT at 1 month.
The study secondary endpoint was to compare the coverage and apposition of these 3 different types of DES on the OCT 1 vs 6 months after implantation. In addition, we evaluated the intimal hyperplasia in the 3 stent groups over time.
Statistical analysis
Continuous variables were expressed as mean and standard deviation except when they did not follow normal distribution, in which case they were expressed as median and 25th-75th percentile. Categorical variables were expressed as frequency and percentage. The analysis of the clinical differences was performed using the chi-square test or Fisher’s exact test for qualitative variables. Comparison among quantitative variables was performed using the 1-way ANOVA test. Generalized estimating equations, considering the clustering nature of the OCT data, were used to conduct analyses at strut level. All probability values were two-sided, P values < .05 were considered statistically significant. Statistical analysis was performed using the SPSS software package, version 22.0 (SPSS, United States). The sample size estimate is shown on the supplementary data.
RESULTS
Baseline clinical characteristics
A total of 104 patients from 4 different hospitals were included in the study; 44 patients were treated with a polymer-free BES, 35 with a biodegradable-polymer EES, and 25 patients with a durable-polymer EES. Of these, 37 patients underwent follow-up angiography and OCT 1 month after DES implantation, and 67 patients after 6 months. Mean age was 57 years; most patients were man (11% women). The inter-group baseline clinical characteristics are shown on table 1 according to the type of stent implanted. We observed a statistically significant difference in the left ventricular ejection fraction that was slightly lower in patients who received a polymer-free BES (54% vs 60%). The number of patients who needed postdilatation was higher in the durable-polymer EES group (68%) especially compared to the polymer-free BES group (38%).
Table 1. Baseline clinical, lesion, and procedural characteristics
| Polymer-free BES (N = 44) |
Biodegradable-polymer EES (N = 35) |
Durable-polymer EES (N = 25) |
P | |
|---|---|---|---|---|
| Age | 57 ± 8 | 61 ± 9 | 59 ± 10 | .094 |
| Women | 3 (7) | 4 (11) | 4 (16) | .482 |
| Dyslipidemia | 24 (55) | 19 (54) | 14 (56) | .990 |
| Hypertension | 17 (39) | 14 (40) | 13 (52) | .527 |
| Family history of ischemic heart disease | 10 (23) | 4 (11) | 6 (24) | .353 |
| Smoker | 26 (59) | 13 (37) | 9 (36) | .076 |
| LVEF % | 54 ± 9 | 60 ± 9 | 60 ± 8 | .006 |
| Chronic kidney disease (creatinine > 1.5 mg/dL) | 0 (0) | 1 (3) | 0 (0) | .370 |
| Previous MI | 2 (5) | 7 (20) | 4 (12) | .102 |
| Previous PCI | 2 (5) | 6 (17) | 4 (16) | .159 |
| Previous CABG | 1 (2) | 0 (0) | 1 (4) | .526 |
| Target lesion location | .101 | |||
| Left anterior descending coronary artery | 18 (41) | 10 (29) | 11 (44) | |
| Left circumflex artery | 10 (23) | 8 (23) | 19 (40) | |
| Right coronary artery | 15 (34) | 13 (37) | 4 (16) | |
| Secondary artery (diagonal, posterolateral, posterior descending) | 1 (2) | 4 (11) | 0 (0) | |
| Stent length, mm | 18.6 ± 5 | 18.8 ± 6 | 19.5 ± 6 | .769 |
| Stent diameter, mm | 3.4 ± 0.8 | 3.1 ± 0.5 | 3.1 ± 0.4 | .053 |
| Predilatation, % | 19 (43) | 16 (73) | 13 (53) | .076 |
| Postdilatation, % | 16 (38) | 12 (57) | 17 (68) | .05 |
|
Data are expressed as no. (%) or mean ± standard deviation. |
||||
Procedural and lesion characteristics
Procedural characteristics based on the type of stent implanted are shown on table 1. We found no significant differences in stent diameter or length in the 3 stent groups. The left anterior descending coronary artery was the most treated of all whereas secondary arteries were scarcely included in this study.
Angiographic analysis
The lesion angiographic characteristics are shown on table 2 and table 1 of the supplementary data. There were 2 patients with angiographic images with insufficient quality for analysis, both from the biodegradable-polymer EES group. There were no significant differences in the lesions before or after the PCI. After 1 month, no significant differences were reported regarding lumen loss or percent diameter stenosis. No differences were reported among the 3 stent groups at 6 months.
Table 2. Angiographic analysis
| Polymer-free BES (N = 44) | Biodegradable-polymer EES (N = 35) | Durable-polymer EES (N = 25) | P | |
|---|---|---|---|---|
| 1-month follow-up | (N = 7) | (N = 16) | (N = 12) | |
| Stent length, mm | 17.85 ± 4.32 | 19.24 ± 5.63 | 19.39 ± 4.41 | .788 |
| Reference lumen diameter, mm | 2.93 ± 0.60 | 2.80 ± 0.53 | 2.77 ± 0.55 | .827 |
| Minimal lumen diameter, mm | 2.75 ± 0.46 | 2.65 ± 0.50 | 2.51 ± 0.49 | .586 |
| Late lumen loss, mm | 0.03 ± 0.09 | 0.04 ± 0.10 | 0.03 ± 0.08 | .965 |
| Percentage diameter stenosis, % | 5.57 ± 6.27 | 6.50 ± 7.14 | 8.67 ± 9.27 | .658 |
| 6-month follow-up | (n = 37) | (n = 17) | (n = 13) | |
| Stent length, mm | 18.99 ± 4.92 | 20.03 ± 6.55 | 18.13 ± 4.95 | .627 |
| Reference lumen diameter, mm | 2.75 ± 0.57 | 2.79 ± 0.50 | 2.65 ± 0.34 | .757 |
| Minimal lumen diameter, mm | 2.54 ± 0.45 | 2.34 ± 0.41 | 2.39 ± 0.37 | .213 |
| Late lumen loss, mm | 0.19 ± 0.25 | 0.28 ± 0.24 | 0.20 ± 0.18 | .368 |
| Percentage diameter stenosis, % | 5.77 ± 15.30 | 15.18 ± 12.92 | 10.08 ± 7.40 | .065 |
|
Data are expressed as no. (%) or mean ± standard deviation. |
||||
OCT outcomes
OCT results are shown on table 3, and table 2 in the supplementary data. There were 6 patients with OCT images with insufficient quality for analysis, 2 from the polymer-free BES, 3 from the biodegradable-polymer EES group, and 1 from the durable-polymer EES group. Stent strut coverage and apposition were analyzed, as well as neointimal hyperplasia. Overall, 15 906 struts were examined. Of these, 4380 struts were from durable-polymer EES; 5122 from biodegradable-polymer EES; and 6404 from polymer-free BES; 6184 and 9722 struts were analyzed at 1 and 6 months, respectively.
Table 3. Optical coherence tomography analysis
| Polymer-free BES | Biodegradable-polymer EES | Durable-polymer EES | Pa | Pb | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Follow-up | 1-month (N = 7) |
6-months (N = 35) |
P | 1-month (N = 17) |
6-months (N = 15) |
P | 1-month (N = 12) |
6-months (N = 12) |
P | ||
| Strut level analysis | (N = 1242) | (N = 5162) | (N = 2673) | (N = 2449) | (N = 2269) | (N = 2111) | |||||
| Uncovered struts, n | 238 (19.2) | 154 (3.0) | < .001 | 318 (11.9) | 123 (5.0) | .007 | 396 (17.5) | 133 (6.3) | .001 | .209 | .172 |
| Malapposed struts, n | 66 (5.3) | 13 (0.3) | < .001 | 101 (3.8) | 21(0.9) | .001 | 138 (6.1) | 35 (1.7) | .029 | .497 | .071 |
| Neointimal thickness, µm | 50.7 ± 41.9 | 138.1 ± 102.9 | < .001 | 59.9 ± 45.1 | 88.3 ± 83.9 | .005 | 48.9 ± 38.1 | 85.5 ± 68.6 | < .001 | .083 | < .001 |
|
Data are expressed as no. or mean ± standard deviation. Categorical variables were estimated using the chi-square test; quantitative variables were estimated with 1-way ANOVA test, and strut level analyses were conducted using generalized estimating equations considering the clustering nature of OCT data. |
|||||||||||
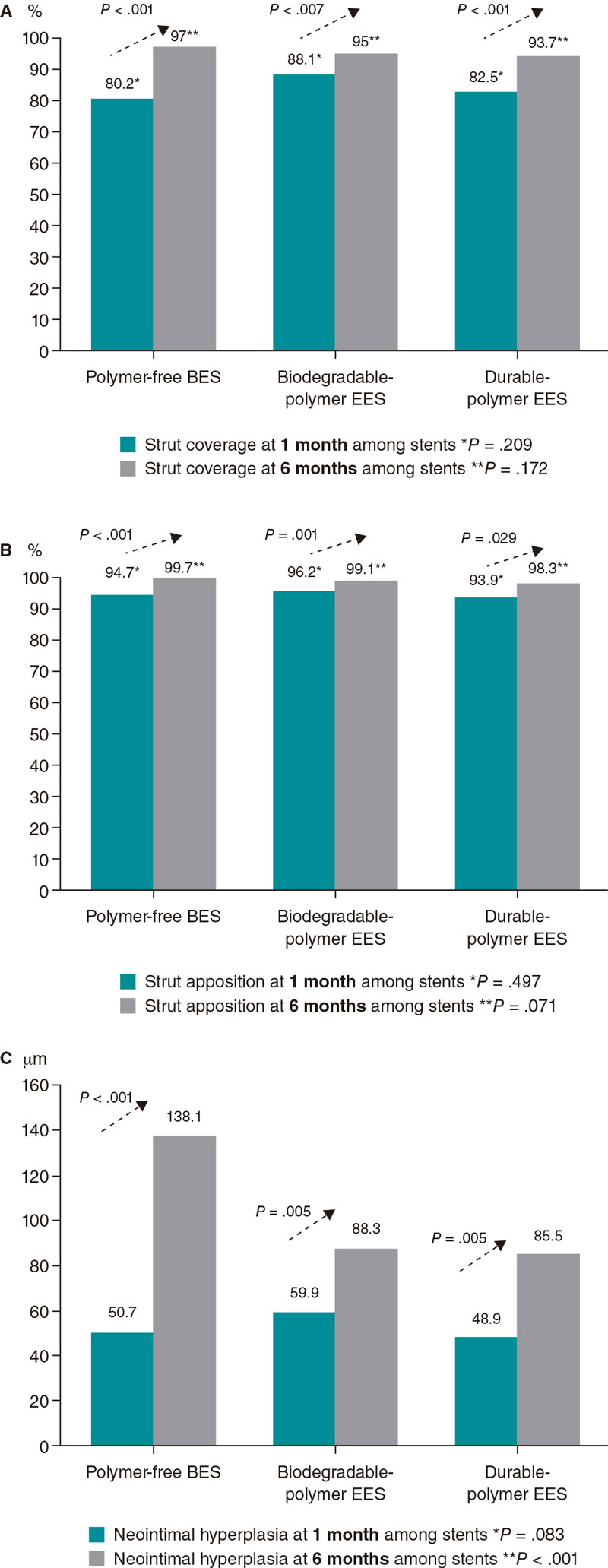
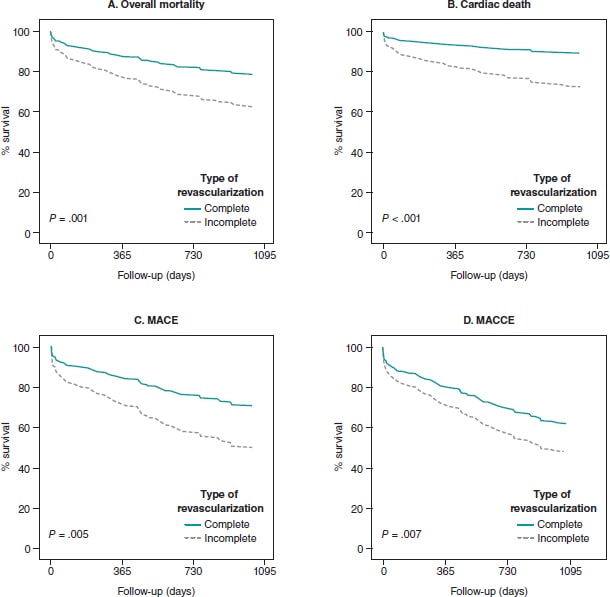
A high rate of uncovered struts was found among stents 1 month after implantation, with no significant differences (P = .209). We observed ≥ 5% of uncovered struts in > 80% of the patients. There was better coverage in the 3 stent groups at 6 months compared to 1 month (P < .001 polymer-free BES; P = .007 biodegradable-polymer EES; P = .001 durable-polymer EES). No statistically significant differences were reported in strut coverage at 6 months among the different stents (P = .172) (figure 2, figure 3A).
Figure 2. Endpoint comparison at 1 and 6 months. A: percentage of struts covered at 1 and 6 months; B: percentage of struts apposed at 1 and 6 months; C: neointimal hyperplasia at 1 and 6 months. BES, biolimus-eluting stent; EES, everolimus-eluting stent.
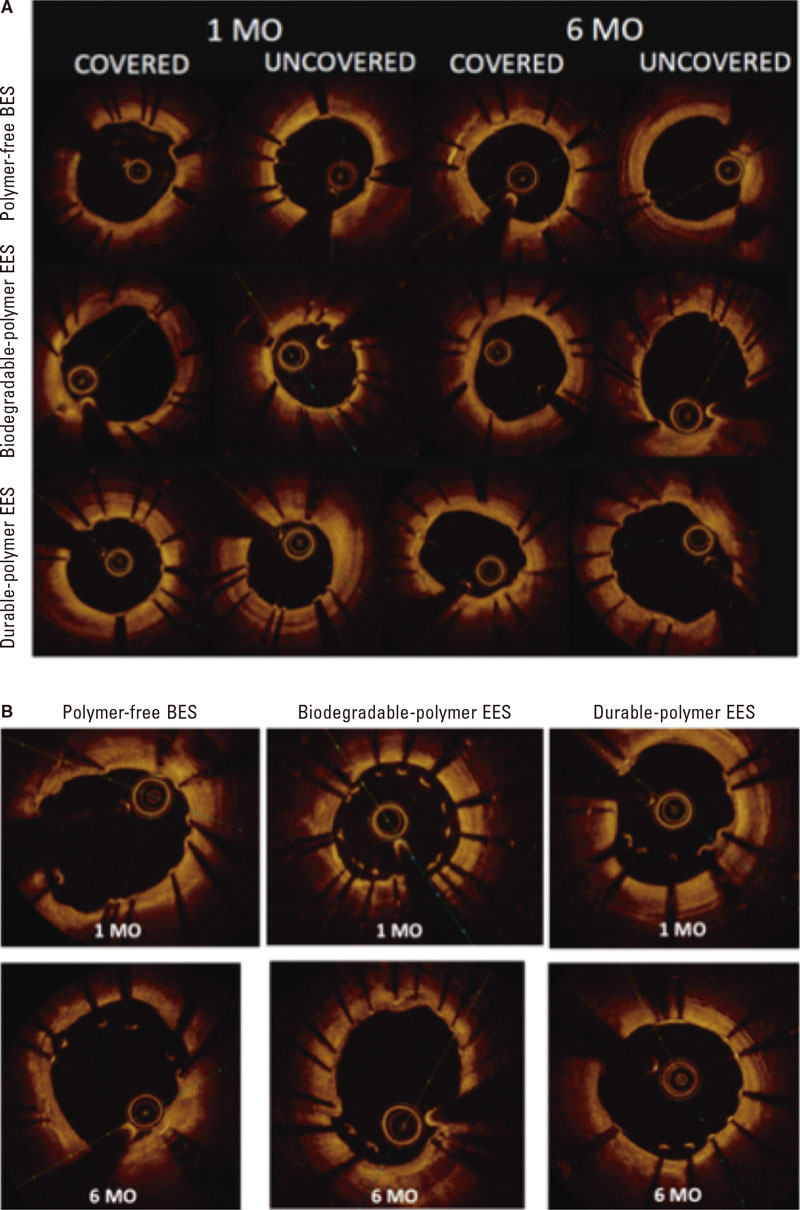
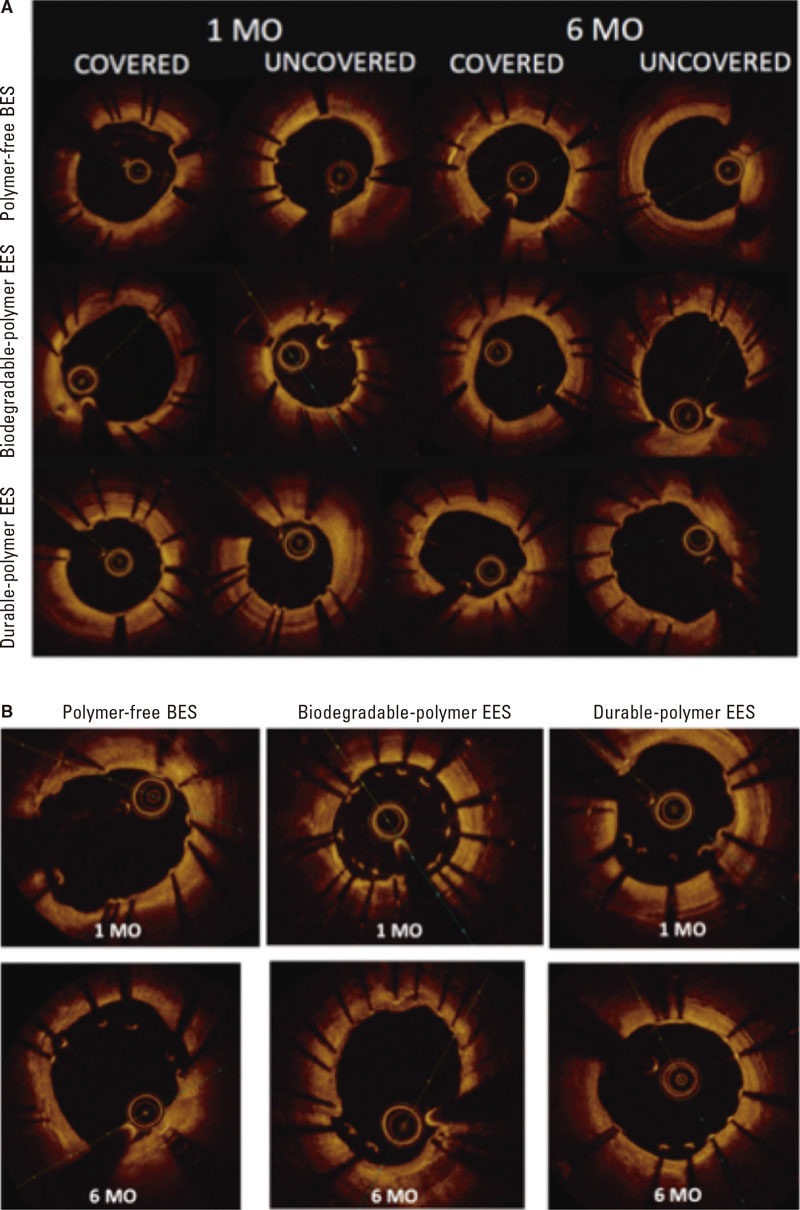
Figure 3. Optical coherence tomography. A: optical coherence tomography showing covered and uncovered stent struts at 1 and 6 months; B: optical coherence tomography showing malapposed stent struts at 1 and 6 months. BES, biolimus-eluting stent; EES, everolimus-eluting stent; MO, months.
Regarding strut apposition to the artery walls, no significant differences were reported among the 3 stents after 1 month (P = .497). We observed ≥ 5% of malapposed struts in 29% to 30% of the patients with no differences among stents. No significant differences were reported among the stents after 6 months either. The rate of apposition was higher at 6 months compared to 1 month in all stent groups (P < .001 polymer-free BES; P = .001 biodegradable-polymer EES; P = .029 durable-polymer EES) (figure 2, figure 3B).
When we analyzed neointimal hyperplasia, no significant differences were found at 1 month among the 3 stent groups (P = .083). At 6 months, we found higher hyperplasia in the polymer-free BES compared to the durable-polymer EES (P < .001). We found more hyperplasia at 6 months compared to 1 month in all groups (P < .001 polymer-free BES; P < .001 biodegradable-polymer EES; P = .005 durable-polymer EES; figure 2).
DISCUSSION
The main findings of this prospective multicenter registry are: a) in non-diabetic patients with de novo non-complex coronary lesions treated with durable vs biodegradable vs polymer-free DES, strut coverage was similar and low (≥ 5% of uncovered struts in > 80% of patients) at 1 month; b) there was a similar high rate of malapposed stent struts (4% to 6%) at 1 month; c) intimal coverage and apposition improved significantly at 6 months; d) polymer-free BES had higher intimal hyperplasia at 6 months.
OCT findings suggest that, in non-diabetic patients with non-complex coronary lesions treated with 3 latest generation DES, there is a similar high rate of uncovered and malapposed struts at 1 month. It is after 6 months when we could see better coverage and apposition.
Several small-scale OCT studies have been performed to compare the coverage and apposition of stents with permanent and absorbable polymers.21-24 Conclusions of these studies differ with most of them stating that the absorbable polymer stent has better coverage than the permanent polymer, and 1 of them concluding the opposite.23 One of the studies found coverage to be sufficient after 3 months,22 whereas another stated that coverage improved at 12 months.24 In this study, we found no significant differences in stent strut coverage or apposition between permanent and absorbable polymer at 1 or 6 months on the OCT analysis (figure 2). Differences in stent strut coverage at follow-up may in part be explained by the stent platform, the polymers used to control drug release, and the antiproliferative drug itself. The stents of the study had a similar drug (-limus analogue) but different polymeric features (durable vs biodegradable vs polymer-free), and different platform thickness (the polymer-free BES had a thicker platform). Probably within the first month the drug effect is most important, and it was similar among the study stents (antiproliferative -limus analogues). However, over time (between 1 and 6 months), other stent features such as platform thickness or polymeric features might play a role that could explain the differences seen regarding coverage at 3 months in other studies.22,23
Accordingly, other studies have analyzed other types of polymer-free stents different to the one from our study.25,26 They found that coverage was achieved in a high percentage at 3 to 9 months, reaching conclusions that were similar to our study. One of the studies26 performed an OCT at 1, 3, and 9 months, demonstrating higher strut coverage over time, which is consistent with our results. Only 1 study analyzed the Biolimus A9 polymer-free stent with OCT without comparing it to other stents.27 It was a prospective single-center single-armed study that examined strut coverage of the Biolimus A9 polymer-free stent at 1, 2, 3, 4, 5, and 9 months. Researchers found that coverage was fast and improved over time with the stent remaining safe and effective. These results are similar to ours in the sense that coverage was significantly better at 6 months. However, we also compared polymer-free stents to other polymer-based stents, something that, to the best of our knowledge, has not been tested before.
One of the limitations of extrapolating the clinical safety of the stents and the degree of intimal coverage as seen on the OCT is that there is no consensus on the cut-off value of coverage that would allow safe discontinuation of DAPT. Few studies1,28 have tried to decide on a percentage of coverage, with the only in-vivo study estimating that > 5.9% of uncovered struts was an independent risk factor for stent thrombosis.28 However, these studies were limited in the number of patients included, and only some stents were tested. Larger studies are needed to decide on a security threshold for strut coverage that would make it safe to stop DAPT without increasing the risk of stent thrombosis. Therefore, the rate of coverage at 1 month (80% to 88%) in our study seems insufficient, reaching a very high percentage after 6 months (94% to 97%) in the 3 types of stents.
Finally, our study shows that intimal hyperplasia was significantly higher in polymer-free stents at 6 months. Polymer-free Biolimus A9 has a stainless steel and thicker platform, which has been associated with more intimal hyperplasia and in-stent restenosis in previous studies.27 The other 2 types of DES have a cobalt-chromium platform which has largely substituted stainless steel to provide sufficient strength and visibility with thinner struts of around 70-90 μm, resulting in lower rates of angiographic and clinical restenosis.29 Thus, inflammatory response to this thicker stent platform (130-140 μm) could be in part responsible for this finding.
Study limitations
This was an OCT-based study; unfortunately, it was not powered to assess clinical outcomes. Our study was non-randomized. However, we minimized the confounding factors through selected inclusion/exclusion criteria for patients and lesions (non-diabetic patients with non-complex coronary lesions). We analyzed the differences among the groups and no significant differences were found, except in the left ventricular ejection fraction, which was significantly lower in the group of polymer-free stents. It has not been described in the medical literature whether a lower left ventricular ejection fraction has any correlation with stent thrombosis. This study included selected non-diabetic patients with simple coronary artery lesions. Therefore, the conclusions cannot be extrapolated to other groups with different characteristics.
The distribution of patients who underwent the follow-up angiography in the polymer-free DES group at 1 or 6 months was uneven. More patients rejected the follow-up angiography at 1 month in the polymer-free DES group, which may account for this difference. Finally, the complexity of the analysis of 3 different groups in 2 different moments of time caused a disgregation of cases. This led to a small N in each group, with the potential biases associated.
CONCLUSIONS
In non-diabetic patients, a significantly high percentage of uncovered struts was detected at 1 month with OCT in latest generation DES regardless of the polymeric features of the stent (durable vs biodegradable vs polymer-free stent) in the non-complex coronary artery lesion setting. Our OCT findings do not support improved short-term healing characteristics of stents with biodegradable polymer or polymer-free based -limus elution compared to current generation of durable polymer DES.
FUNDING
This study received funding from Abbott Vascular and Biosensors International. Funders were not involved in the study design, collection, analysis, interpretation of data, drafting of this article or decision to submit it for publication.
AUTHORS’ CONTRIBUTIONS
A. Calvo-Fernández, R. Elosua, and B. Vaquerizo designed the study. J. Gómez-Lara, Héctor Cubero-Gallego, Helena Tizón-Marcos, N. Salvatella, A. Negrete, R. Millán, J.L. Díez, J.M. de la Torre Hernández, and B. Vaquerizo participated in the recruitment of patients and database contribution. A. Calvo-Fernández, C. Ivern, A. Sánchez-Carpintero, and B. Vaquerizo managed the database. A. Calvo-Fernández, X. Durán, N. Farré, and B. Vaquerizo conducted the data analysis and statistics. A. Calvo-Fernández, R. Elosua, N. Farré, H. Cubero-Gallego, and B. Vaquerizo drafted the paper with feedback from all the authors.
CONFLICTS OF INTEREST
J.M. de la Torre Hernandez is an associate editor of REC: Interventional Cardiology; the journal’s editorial procedure to ensure impartial handling of the manuscript has been followed; he has received grants and research support from Abbott Medical, Biosensors, Bristol Myers Squibb, Amgen; honoraria or consultation fees from Boston Scientific, Medtronic, Biotronik, Astra Zeneca, Daiichi-Sankyo. N. Salvatella has received teaching honoraria from Abbott Vascular, and consultation fees from Boston Scientific. The remaining authors declared no other competing interests.
WHAT IS KNOWN ABOUT THE TOPIC?
- Current DES with biodegradable polymer coating or the new polymer-free biolimus stent have been proposed as the optimal solution to the problem of delayed coronary artery healing characteristics seen with first-generation durable polymer-coated DES.
WHAT DOES THIS STUDY ADD?
- This multicenter registry compared 3 types of DES with similar drug elution (-limus analogue), but different stent polymeric features (durable vs biodegradable vs polymer free).
- Using the percentage of uncovered struts at short term assessed by optical coherence tomography as a surrogate endpoint, healing characteristics at 1 month were similar among stents and insufficient after 1 month.
- Our findings do not support a preferential use of stents with biodegradable polymer-based or polymer-free stents to reduce the time of dual antiplatelet therapy at 1 month.
REFERENCES
1. Finn AV, Joner M, Nakazawa G, et al. Pathological Correlates of Late Drug-Eluting Stent Thrombosis: Strut Coverage as a Marker of Endothelization. Circulation. 2007;115:2435-2441.
2. Guagliumi G, Sirbu V, Musumeci G, et al. Examination of the In Vivo Mechanisms of Late Drug-Eluting Stent Thrombosis. J Am Coll Cardiol Intv. 2012;5:12-20.
3. Magnani G, Valgimigli M. Dual Antiplatelet Therapy After Drug-eluting Stent Implantation. Interv Cardiol. 2016;11:51-53.
4. Kelly CR, Teirstein PS, Meredith IT, et al. Long-Term Safety and Efficacy of Platinum Chromium Everolimus-Eluting Stents in Coronary Artery Disease: 5-Year Results From the PLATINUM Trial. J Am Coll Cardiol Intv. 2017;10:2392-2400.
5. Maeng M, Tilsted HH, Jensen LO, et al. Differential clinical outcomes after 1 year versus 5 years in a randomised comparison of zotarolimus-eluting and sirolimus-eluting coronary stents (the SORT OUT III study): a multicentre, open-label, randomised superiority trial. Lancet. 2014;383:2047-2056.
6. Jakobsen L, Christiansen EH, Maeng M, et al. Final five-year outcomes after implantation of biodegradable polymer-coated biolimus-eluting stents versus durable polymer-coated sirolimus-eluting stents. EuroIntervention. 2017;13:1336-1344.
7. Sarno G, Lagerqyist B, Fröbert O, et al. Lower risk of stent thrombosis and restenosis with unrestricted use of ‘new-generation’ drug-eluting stents: a report from the nationwide Swedish Coronary Angiography and Angioplasty Registry (SCAAR). Eur Heart J. 2021;33:606-613.
8. von Birgelen C, Kok MM, van der Heijden LC, et al. Very thin strut biodegradable polymer everolimus-eluting and sirolimus-eluting stents versus durable polymer zotarolimus-eluting stents in allcomers with coronary artery disease (BIO-RESORT): a three-arm, randomised, non-inferiority trial. Lancet. 2016;388:2607-2617.
9. Raungaard B, Jensen LO, Tilsted HH, et al. Zotarolimus-eluting durable-polymer-coated stent versus a biolimus-eluting biodegradable-polymer-coated stent in unselected patients undergoing percutaneous coronary intervention (SORT OUT VI): a randomised non-inferiority trial. Lancet. 2015;385:1527-1535.
10. Jensen LO, Thayssen P, Maeng M, et al. Randomized Comparison of a Biodegradable Polymer Ultrathin Strut Sirolimus-Eluting Stent With a Biodegradable Polymer Biolimus-Eluting Stent in Patients Treated With Percutaneous Coronary Intervention. Circ Cardiovasc Interv. 2019;9:e003610.
11. Räber L, Kelbæk H, Ostojic M, et al. Effect of biolimus-eluting stents with biodegradable polymer vs bare-metal stents on cardiovascular events among patients with acute myocardial infarction: the COMFORTABLE AMI randomized trial. JAMA. 2012;308:777-787.
12. Kim HS, Kang J, Hwang D, et al, HOST-REDUCE-POLYTECH-ACS Trial Investigators. Durable Polymer Versus Biodegradable Polymer Drug-Eluting Stents After Percutaneous Coronary Intervention in Patients with Acute Coronary Syndrome: The HOST-REDUCE-POLYTECH-ACS Trial. Circulation. 2021;143:1081-1091.
13. Urban P, Meredith IT, Abizaid A, et al. Polymer-free Drug-Coated Coronary Stents in Patients at High Bleeding Risk. N Engl J Med. 2015;373:2038-2047.
14. Windecker S, Latib A, Kedhi E, et al; ONYX-ONE Investigators. Polymer-based or Polymer-free Stents in Patients at High Bleeding Risk. N Engl J Med. 2020;382:1208-1218.
15. Kufner S, Ernst M, Cassese S, et al; ISAR-TEST-5 Investigators. 10-Year Outcomes From a Randomized Trial of Polymer-Free Versus Durable Polymer Drug-Eluting Coronary Stents. J Am Coll Cardiol. 2020;76:146-158.
16. Lee KS, Lee JZ, Hsu CH, et al. Temporal Trends in Strut-Level Optical Coherence Tomography Evaluation of Coronary Stent Coverage: A Systematic Review and Meta-Analysis. Catheter Cardiovasc Interv. 2016;88:1083-1093.
17. Tearney GJ, Regar E, Akasaka T, et al. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J Am Coll Cardiol. 2012;59:1058-1072.
18. Yuan, J. Xu GM. Early and Late Stent Thrombosis in Patients with Versus Without Diabetes Mellitus Following Percutaneous Coronary Intervention with Drug-Eluting Stents: A Systematic Review and Meta-Analysis. Am J Cardiovasc Drugs. 2018;18:483-492.
19. Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87-165.
20. Gomez-Lara J, Teruel L, Homs S, et al. Lumen enlargement of the coronary segments located distal to chronic total occlusions successfully treated with drug-eluting stents at follow-up. EuroIntervention. 2014;9:1181-1188.
21. Teeuwen K, Spoormans E, Bennett J, et al. Optical coherence tomography findings: insights from the “randomised multicentre trial investigating angiographic outcomes of hybrid sirolimus-eluting stents with biodegradable polymer compared with everolimus-eluting stents with durable polymer in chronic total occlusions” (PRISON IV) trial. EuroIntervention. 2017;13:e522-e530.
22. Kretov E, Naryshkin I, Baystrukov V, et al. Three-months optical coherence tomography analysis of a biodegradable polymer, sirolimus-eluting stent. J Interv Cardiol. 2018;31:442-449.
23. Adriaenssens T, Ughi GJ, Dubois C, et al. STACCATO (Assessment of Stent sTrut Apposition and Coverage in Coronary ArTeries with Optical coherence tomography in patients with STEMI, NSTEMI and stable/unstable angina undergoing everolimus vs biolimus A9-eluting stent implantation): a randomised controlled trial. EuroIntervention. 2016;11:e1619-e1626.
24. Kim BK, Hong MK, Shin DH, et al. Optical coherence tomography analysis of strut coverage in biolimus- and sirolimus-eluting stents: 3-Month and 12-month serial follow-up. Int J Cardiol. 2013;168:4617-4623.
25. Suwannasom P, Onuma Y, Benit E, et al. Evaluation of vascular healing of polymer-free sirolimus-eluting stents in native coronary artery stenosis: a serial follow-up at three and six months with optical coherence tomography imaging. EuroIntervention. 2016;12:e574-e583.
26. Worthley SG, Abizaid A, Kirtane AJ, et al. First-in-Human Evaluation of a Novel Polymer-Free Drug-Filled Stent. JACC Cardiovasc Interv. 2017;10:147-156.
27. Lee SWL, Tam FCC, Chan KKW, et al. Establishment of healing profile and neointimal transformation in the new polymer-free biolimus A9-coated coronary stent by longitudinal sequential optical coherence tomography assessments: the EGO-BIOFREEDOM study. EuroIntervention. 2018;14:780-788.
28. Won H, Shin DH, Kim BK, et al. Optical coherence tomography derived cut-off value of uncovered stent struts to predict adverse clinical outcomes after drug-eluting stent implantation. Int J Cardiovasc Imaging. 2013;29:1255-1263.
29. Pache J, Kastrati A, Mehilli J, et al. Intracoronary stenting and angiographic results: Strut thickness effect on restenosis outcome (ISAR-STEREO-2) trial. J Am Coll Cardiol. 2003;41:1283-1288.
ABSTRACT
Introduction and objectives: The optimal time to perform a diagnostic coronary angiography in patients admitted due to non-ST-segment elevation acute coronary syndrome (NSTEACS) and start pretreatment with dual antiplatelet therapy is controversial. Our study aims to identify the current diagnostic and therapeutic approach, and clinical progression of patients with NSTEACS in our country.
Methods: The IMPACT-TIMING-GO trial (Impact of time of intervention in patients with myocardial infarction with non-ST segment elevation. Management and outcomes) is a national, observational, prospective, and multicenter registry that will include consecutive patients from 24 Spanish centers with a clinical diagnosis of NSTEACS treated with diagnostic coronary angiography and with present unstable or causal atherosclerotic coronary artery disease. The study primary endpoint is to assess the level of compliance to clinical practice guidelines in patients admitted due to NSTEACS undergoing coronary angiography in Spain, describe the use of antithrombotic treatment prior to cardiac catheterization, and register the time elapsed until it is performed. Major adverse cardiovascular events will also be described like all-cause mortality, non-fatal myocardial infarction and non-fatal stroke, and the rate of major bleeding according to the BARC (Bleeding Academic Research Consortium) scale at 1- and 3-year follow-up.
Results: This study will provide more information on the impact of different early management strategies in patients admitted with NSTEACS in Spain, and the degree of implementation of current recommendations into the routine clinical practice. It will also provide information on these patients’ baseline and clinical characteristics.
Conclusions: This is the first prospective study conducted in Spain that will be reporting on the early therapeutic strategies—both pharmacological and interventional—implemented in our country in patients with NSTEACS after the publication of the 2020 European guidelines, and on the clinical short- and long-term outcomes of these patients.
Keywords: Acute coronary syndrome. Acute myocardial infarction. Non-ST-segment elevation acute coronary syndrome. Dual antiplatelet therapy. Pretreatment. Early invasive strategy. ESC guidelines. Diabetes mellitus. Hemorrhage. Revascularization.
RESUMEN
Introducción y objetivos: El momento óptimo para la realización de un cateterismo diagnóstico en pacientes con síndrome coronario agudo sin elevación del segmento ST (SCASEST) y la necesidad de pretratamiento con doble antiagregación son motivo de controversia. Este estudio pretende conocer el abordaje diagnóstico y terapéutico actual, así como la evolución clínica de los pacientes con SCASEST en España.
Métodos: El estudio IMPACT of Time of Intervention in patients with Myocardial Infarction with Non-ST seGment elevation. ManaGement and Outcomes (IMPACT-TIMING-GO) es un registro nacional observacional, prospectivo y multicéntrico, que incluirá pacientes consecutivos con diagnóstico de SCASEST tratados con coronariografía diagnóstica y que presenten enfermedad coronaria aterosclerótica inestable o causal en 24 centros españoles. El objetivo primario del estudio es conocer el grado de cumplimiento de las recomendaciones de las guías de práctica clínica en pacientes que ingresan por SCASEST tratados con coronariografía en España, describir el uso del tratamiento antitrombótico antes del cateterismo y determinar el tiempo hasta este en la práctica clínica real. Se describirán también los eventos adversos cardiovasculares mayores: mortalidad por cualquier causa, infarto no fatal e ictus no fatal, y también la incidencia de hemorragia mayor según la escala BARC (Bleeding Academic Research Consortium) durante el seguimiento a 1 y 3 años.
Resultados: Este registro permitirá mejorar el conocimiento en relación con el abordaje terapéutico inicial en pacientes que ingresan por SCASEST en España. Contribuirá a conocer sus características basales y su evolución clínica, así como el grado de adherencia y cumplimiento de las recomendaciones de las que se dispone actualmente.
Conclusiones: Se trata del primer estudio prospectivo realizado en España que permitirá conocer las estrategias terapéuticas iniciales, tanto farmacológicas como intervencionistas, que se realizan en nuestro país en pacientes con SCASEST tras la publicación de las guías europeas de 2020, y la evolución clínica de estos pacientes a corto y largo plazo.
Palabras clave: Síndrome coronario agudo. Infarto agudo de miocardio. Síndrome coronario agudo sin elevación del segmento ST. Doble antiagregación plaquetaria. Pretratamiento. Coronariografía precoz. Guía ESC. Diabetes mellitus. Hemorragia. Revascularización.
Abbreviations
IMPACT-TIMING-GO: Impact of Time of Intervention in patients with Myocardial Infarction with non-ST segment elevation ManaGement and Outcomes. SCA: síndrome coronario agudo. SCASEST: síndrome coronario agudo sin elevación del segmento ST.
INTRODUCTION
Ischemic heart disease is the leading cause of mortality in developed countries.1 The rate of acute coronary syndrome (ACS), specially non-ST-segment elevation ACS (NSTEACS), has increased over the last few years, in part, due to the ageing of the population.2-3 Given the underlying pathophysiology4 patients receive specific antithrombotic treatment, and invasive approach is used in most of the cases.1-3 The new guidelines published by the European Society of Cardiology (ESC) on the management of NSTEACS1 include changes compared to the guidelines published back in 2016. The most significant ones include antithrombotic treatment, the revascularization strategy, and several controversial innovations.
In the guidelines published in 2020, early cardiac catheterization within the first 24 hours after admission was advised (level of evidence IA) in patients diagnosed with acute myocardial infarction with GRACE scores (Global Registry of Acute Coronary Events) > 140 or dynamic electrocardiographic changes suggestive of ischemia.1 Also, the previous window of recommendation of 0 to 72 hours for moderate risk patients is now gone.4 On the other hand, the systematic use of pretreatment at admission with an P2Y12 inhibitor antiplatelet drug (ticagrelor, prasugrel or clopidogrel) in patients to be treated with an early invasive strategy is now ill-advised.1
The objective of the IMPACT registry (Time of intervention in patients with myocardial infarction with non-ST segment elevation, management and outcomes [IMPACT-TIMING-GO]) is to get the big picture on the current treatment of NSTEACS, in Spain, in association with catheterization times, use of pretreatment in these patients, and describe the possible prognostic implications of the different strategies used in real life.
METHODS
Study design and population
This is an observational, prospective, multicenter, and nationwide registry that will include all consecutive patients admitted with a diagnosis of NSTEACS to the different participant centers, treated with diagnostic coronary angiography, and with unstable or causal atherosclerotic disease regardless of further treatment administered by the heart team. The baseline characteristics of the patients included, and their clinical progression regarding in-hospital events will be studied. Patients will undergo a 1-and-3-year clinical follow-up period.
This registry has been promoted by the Spanish Society of Cardiology Young Cardiologists Working Group with scientific support from the Spanish Society of Cardiology Research Agency. Also, it has been approved by different Research Ethics Committees with drugs from all the participant hospitals. Finally, it has been designed according to the STROBE checklist for observational studies.
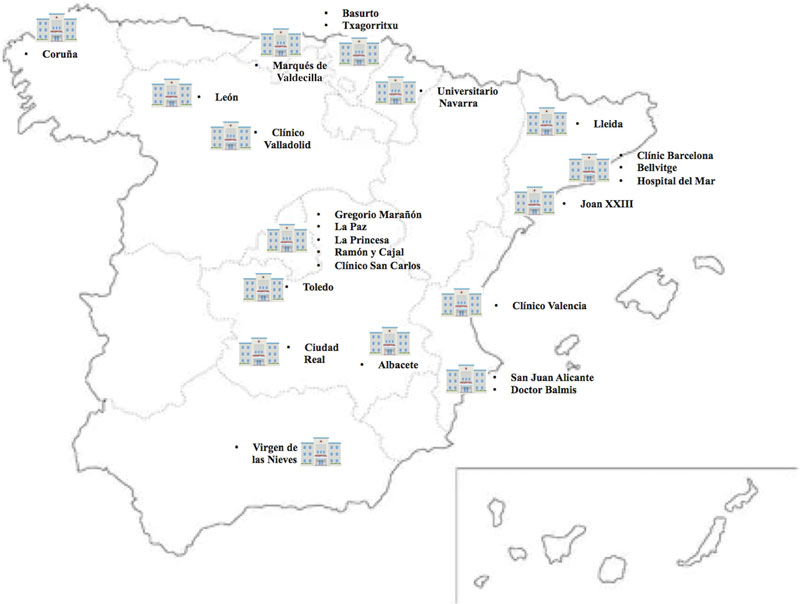
The list of centers that will eventually participate in the registry is shown on figure 1. Inclusion and exclusion criteria are shown on table 1. The presence of elevated markers of myocardial damage or electrocardiographic changes is not mandatory. Patients with a clinical diagnosis of unstable angina can be included as long as coronary angiography confirms the clinical diagnosis.
Figure 1. Map with the Spanish participant centers in the IMPACT-TIMING-GO registry.
Table 1. Inclusion and exclusion criteria of the IMPACT-TIMING-GO registry
| Inclusion criteria |
|---|
| NSTEACS with in-hospital invasive treatment regardless of when it is performed. |
| Evidence of causal or unstable atherosclerotic disease. |
| Age ≥ 18 years. |
| Capacity to give informed consent. |
| Exclusion criteria |
| Minors and those who withdraw their consent to be included or followed at any time during the study. |
| Assessment of myocardial damage markers associated with type 2 myocardial infarction. |
| Patients without any signs of coronary artery disease including those with myocarditis, Prinzmetal angina, takotsubo syndrome or MINOCA. |
| Patients diagnosed with spontaneous coronary artery dissection. |
| Patients with complete left bundle branch block or pacemaker rhythm on the electrocardiogram performed at admission. |
| Patients with a valve heart disease eligible for surgery. |
| Patients with a known past medical history of diffuse coronary artery disease noneligible for revascularization. |
| Patients with known or confirmed allergy to some antiplatelet drug. |
|
IMPACT-TIMING-GO, IMPACT of time of intervention in patients with myocardial infarction with non-ST segment elevation. Management and outcomes; MINOCA, Myocardial infarction with non-obstructive coronary artery disease; NSTEACS, non-ST-segment elevation acute coronary syndrome. |
Endpoints
The study primary endpoint is to know the degree of compliance of the recommendations included in the clinical practice guide-lines in patients admitted due to NSTEACS treated with coronary angiography, in Spain, describe the use of antithrombotic treatment before cardiac catheterization, and the time elapsed until it is performed in the real-world clinical practice.
The secondary endpoints are:
- – To describe the baseline, clinical, and epidemiological characteristics of the study population.
- – To study the rates of cardiovascular mortality, new revascularization, stent thrombosis, and hospitalizations due to heart failure during admission and at the 1-and-3-year follow-up.
- – To describe major cardiovascular adverse events of all-cause mortality, non-fatal stroke, non-fatal infarction, and the rate of major bleeding grades 3, 4, and 5 according to the BARC scale (Bleeding Academic Research consortium.5) Data will be analyzed during admission and at the 1-and-3-year follow-up.
- – To know the medical treatment at discharge and at follow-up of patients discharged in Spain after NSTEACS.
- – To know the degree of control of the different cardiovascular risk factors associated with the endpoints defined in the ESC guidelines 2021 on prevention of cardiovascular disease in the routine clinical practice.6
Data curation and definitions
Data will be collected prospectively by trained medical investigators from each participant center in a specific standard form. Demographic data, the baseline clinical characteristics, and all analytical, electrocardiographic, and echocardiographic data will be included as well.
Similarly, data on disease progression and the in-hospital stay, indication for coronary angiography and when it is be performed, type of treatment received (conservative, stent implantation or revascularization surgery), and the in-hospital complications occurred (hemorrhages and severity, heart failure or shock, reinfarction, stroke, confusional state, mechanical and arrhythmic complications, infectious complications requiring antibiotic therapy, and mortality causes) will be collected. Finally, the medical treatment at hospital discharge and level of compliance of the current recommendations based on the clinical practice guidelines will be studied too.
The definitions of the variables are shown on table 2.7-8
Table 2. Definitions of target variables
| Variable | Definition |
|---|---|
| All-cause mortality | All deaths regardless of their cause. |
| Cardiovascular death | All deaths of vascular causes both cardiac (heart failure/shock; malignant arrhythmias; myocardial infarction) and non-coronary vascular including cerebrovascular disease, pulmonary embolism, aneurysms/aortic dissections, acute ischemia of lower limbs, etc.
All sudden deaths of unknown causes will be adjudicated as cardiovascular death. |
| Non-cardiac death | All deaths that do not meet the previous definition like deaths due to infections, cancer, pulmonary diseases, accidents, suicide or trauma. |
| Myocardial infarction | It is defined based on the criteria established in the 4th and current Universal definition.4 Therefore, patients with type 2 infarction, extracardiac causes or without elevated markers of myocardial damage were excluded. |
| Stroke/Transient ischemic attack | New-onset neurological, focal or global deficit due to ischemia or hemorrhage, and as long as it is part of diagnostic judgement at hospital discharge. |
| Stent thrombosis | Defined based on the Academic Research Consortium of randomized clinical trials with stents.7 |
| New revascularization | All unscheduled revascularizations performed after hospital discharge, whether surgical or percutaneous, including target vessel failure and target lesion failure. |
| Admission due to heart failure | Unscheduled hospital admission > 24 hours with a primary diagnosis of heart failure based on the current defintion.8 |
Follow-up
Clinical follow-up to detect events will be conducted by medical investigators through on-site visits, health record reviews or phone calls with the patient, family members or treating physician at 1 and 3 years. Clinical variables, functional class, and additional variables (analytical, electrocardiographic, and echocardiographic, and treatment received) will be included. The overall mortality rate and its causes, need of emergency hospitalization (duration > 24 hours) and its causes, and the rates of non-fatal infarction and stroke will be collected as well. All deaths due to myocardial infarction, sudden death or heart failure will be considered cardiovascular deaths.
Sample size estimate
Taking the events seen in previous studies with a population of similar characteristics as the reference,9-14 a sample size of 800 patients will be enough to know the baseline characteristics of the study population, and the therapeutic approach currently used in Spain in our routine clinical practice. Patients lost to follow-up will be handled by multiple imputation.
Statistical analysis
Categorical variables will be expressed as number and percentage. Quantitative variables will be expressed as mean ± standard deviation. Quantitative variables with normal distribution will be expressed as median and interquartile range [25%-75%]. The normal distribution of quantitative variables will be assessed using the Kolmogorov-Smirnoff test. Regarding the reference variables, Student t test will be used to compare quantitative variables, and the chi-square test or Fisher’s exact test, if applicable, to compare categorical variables. Statistical analysis will be performed using the SPSS statistical software version 22.0 (IBM Corp., Armonk, United States).
Specific studies on subgroups of special interest will be conducted: feminine sex, patients ≥ 75 years, those with GRACE scores > 140, diabetic patients, those with a past medical history of renal failure, with an indication for chronic oral anticoagulation, with multivessel disease, acute myocardial infarction, ventricular dysfunction according to the current clinical practice guidelines and based on the day of admission (holiday vs working day), and patients who require transfer to tertiary centers to receive a coronary angiography.
Ethical principles
Inclusion in the study will not imply changes to the patients’ treatment. Instead, it will follow the routine clinical practice and the recommendations set forth by the current clinical practice guidelines. Therefore, antithrombotic treatment and additional examinations including the need for a coronary angiography and the time it is performed will all be decided by the heart team based on the routine clinical practice. Coronary angiography, vascular access, antithrombotic treatment during the procedure, and the material and devices used will all be decided by the treating operator in charge of the case. All patients will sign a written informed consent form before being included in the study that will be conducted in full compliance with the Declaration of Helsinki. This study will also observe all legal regulations applicable to this type of studies and follow the good clinical practice rules while being conducted.
DISCUSSION
The IMPACT-TIMING-GO registry will give us information on the current real-world management of patients with NSTEACS with invasive treatment and causal coronary artery disease, which will allow us to assess the degree of implementation of the current recommendations of ESC guidelines 2020 on cardiac catheterizations performed within the first 24 hours and no pretreatment with P2Y12 inhibitors. Similarly, different prognostic differences that early invasive treatment and no pretreatment could have in the real life of patients diagnosed with NSTEACS could be suggested.
Despite the clinical practice guidelines recommendations on the invasive treatment of patients with NSTEACS, the main clinical trials published to this date have been unable to demonstrate any clear benefits from systematic early invasive treatment.9-14 The VERDICT trial,9 published in 2018, randomized 2147 patients with NSTEACS on a 1:1 ratio to receive early (< 12 hours) or delayed (48 to 72 hours) cardiac catheterization. No significant differences were found in the composite endpoint of major cardiovascular events at 4-year follow-up. However, in the subgroup of patients with GRACE scores > 140 statistically significant differences were seen favorable to the early strategy regarding major adverse cardiovascular events (hazard ratio, 0.81; 95% confidence interval, 0.67-1.01; P = .023). Consistent with this, the TIMACS clinical trial10 published in 2008 of 3031 patients with NSTEACS found no differences in the primary endpoint when early invasive strategy (< 24 hours) and delayed approach (> 36 hours) were compared, except for, once again, in patients with GRACE scores > 140. Other randomized clinical trials with fewer patients show contradictory results11-14 some without significant differences.15 Also, in many cases, the results favorable to the early strategy are associated with refractory ischemia, not with hard endpoints like cardiovascular mortality or non-fatal myocardial infarction. In Spain, evidence on the management of NSTEACS is prior to the current clinical practice guidelines,16-17 and the most recent registry is retrospective, which is suggestive of a possible mortality benefit in patients with GRACE scores > 140.18 Over the last 2 decades, in our country, the use of an invasive strategy in patients with NSTEACS has increased significantly from 20% in the MASCARA registry in 200516 up to 80% in the DIOCLES study from 2012.17 However, evidence is scarce on catheterization times, our capacity to adapt to current recommendations (the median time of the DIOCLES trial was 3 days), the possible impact this time reduction can have, and on the consequencies from not starting antiplatelet pretreatment in patients who don’t meet the times recommended.
On the other hand, the current formal recommendation from the clinical practice guidelines of not pretreating systematically with a P2Y12 inhibitor (level of recommendation IIIA1) patients on early invasive treatment is mainly based on 3 clinical trials and their meta-analysis.19 In the ACCOAST trial, pretreatment with prasugrel did not reduce thrombotic events in patients with NSTEMI. However, cardiac surgery-related and potentially fatal hemorrhages increased.20 We should mention that the median time elapsed since the prasugrel loading dose until the coronary angiography was performed was 4 hours. In the ISAR-REACT 5 trial published in 2019, a non-pretreatment strategy with prasugrel in patients with ACS vs pretreatment with ticagrelor proved superior regarding the primary endpoint of thrombotic events with a tendency towards fewer hemorrhagic events.21 We should mention that the intrinsic effect of the drug used should not be obviated or else the fact that the median time elapsed since randomization until the prasugrel loading dose was received in the non-pretreatment group was 60 minutes. Finally, the first study that compared 2 different pretreatment strategies vs the intraoperative administration of ticagrelor did not show any clear benefits regarding thrombosis or a deleterious effect of pretreatment regarding bleeding.22 Once again, the median time elapsed until the cardiac catheterization was performed was < 24 hours since hospital admission (23 hours). Surprisingly, clinical practice guidelines leave the door opened to a weak level of recommendation (IIbC) regarding pretreatment of patients in whom early catheterization < 24 hours is not possible.1
In conclusion, current recommendations on early invasive treatment and no antiplatelet pretreatment in patients with NSTEACS are controversial and can also be difficult to implement in the routine clinical practice in our setting. The ultimate objective of the IMPACT-TIMING-GO registry is to shed light on the current management of NSTEACS in Spain. After the impact that the COVID-19 pandemic has had on the general structure of the healthcare system and the drop in the number of interventional procedures performed in 2020,23 we should expect to see pre-pandemic numbers in 2022 and cath labs and cardiac surgery intensive care units going back to normal. Therefore, moment seems ripe to conduct a real-world registry.
CONCLUSIONS
The IMPACT-TIMING-GO registry is the first prospective study ever conducted in Spain that will be giving us information on the early therapeutic strategies—both pharmacological and interventional—performed in our country in patients with NSTEACS after the publication of the ESC guidelines 2020, and the impact of these and other measures indicated in these patients at follow-up.
FUNDING
This unfunded study has been promoted by the Spanish Society of Cardiology Young Cardiologists Working Group with scientific endorsement from the Spanish Society of Cardiology.
AUTHORS’ CONTRIBUTIONS
Study design, data curation and review, statistical analysis, and manuscript drafting: P. Díez-Villanueva, F. Díez-Delhoyo, and M.T. López-LLuva. All the authors participated in the manuscript review and approval process.
CONFLICTS OF INTEREST
None reported.
ACKNOWLEDGEMENTS
We wish to thank the Spanish Society of Cardiology Young Cardiologists Working Group for their drive to engage the youth in medical research.
WHAT IS KNOWN ABOUT THE TOPIC?
- The management of patients with NSTEACS includes dual antiplatelet therapy with a P2Y12 inhibitor and, in most cases, invasive approach through cardiac catheterization for further revascularization. The current ESC clinical practice guidelines recommend early invasive approach (<24 hours) and no pretreatment systemically though both aspects are still controversial.
- The degree of implementation of such recommendations in the routine clinical practice, in Spain, is still unknown.
WHAT DOES THIS STUDY ADD?
- This study will improve our knowledge on early therapeutic approach, and its prognostic impact in patients admitted due to NSTEACS in Spain.
- Also, it will bring us information on the characteristics and clinical evolution of these patients in association with the recommendations and therapeutic targets we have today.
REFERENCES
1. Collet JP, Thiele H, Barbato E, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42:1289-1367.
2. Díez-Villanueva P, Méndez CJ, Alfonso F. Non-ST elevation acute coronary syndrome in the elderly. J Geriatr Cardiol JGC. 2020;17:9-15.
3. Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC Guideline for the management of patients with non-ST-elevation acute coronary syndromes: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:2354-2394.
4. Roffi M, Patrono C, Collet JP, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:267-315.
5. Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736-2747.
6. Visseren FLJ, Mach F, Smulders YM, et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice: Developed by the Task Force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies With the special contribution of the European Association of Preventive Cardiology (EAPC). Rev Esp Cardiol. 2022;75:429.
7. Garcia-Garcia HM, McFadden EP, Farb A, et al. Standardized End Point Definitions for Coronary Intervention Trials: The Academic Research Consortium-2 Consensus Document. Circulation. 2018;137:2635-2650.
8. McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599-3726.
9. Kofoed KF, Kelbæk H, Hansen PR, et al. Early Versus Standard Care Invasive Examination and Treatment of Patients With Non-ST-Segment Elevation Acute Coronary Syndrome. Circulation. 2018;138:2741-2750.
10. Mehta SR, Granger CB, Boden WE, et al. Early versus delayed invasive intervention in acute coronary syndromes. N Engl J Med. 2009;360:2165-2175.
11. Thiele H, Rach J, Klein N, et al. Optimal timing of invasive angiography in stable non-ST-elevation myocardial infarction: the Leipzig Immediate versus early and late PercutaneouS coronary Intervention triAl in NSTEMI (LIPSIA-NSTEMI Trial). Eur Heart J. 2012;33:2035-2043.
12. Milosevic A, Vasiljevic-Pokrajcic Z, Milasinovic D, et al. Immediate Versus Delayed Invasive Intervention for Non-STEMI Patients: The RIDDLE-NSTEMI Study. JACC Cardiovasc Interv. 2016;9:541-549.
13. Montalescot G, Cayla G, Collet JP, et al. Immediate vs delayed intervention for acute coronary syndromes: a randomized clinical trial. JAMA. 2009;302:947-954.
14. Lemesle G, Laine M, Pankert M, et al. Optimal Timing of Intervention in NSTE-ACS Without Pre-Treatment: The EARLY Randomized Trial. JACC Cardiovasc Interv. 2020;13:907-917.
15. Janssens GN, van der Hoeven NW, Lemkes JS, et al. 1-Year Outcomes of Delayed Versus Immediate Intervention in Patients With Transient ST-Segment Elevation Myocardial Infarction. JACC Cardiovasc Interv. 2019;12:2272-2282.
16. Ferreira-González I, Permanyer-Miralda G, Marrugat J, et al. MASCARA (Manejo del Síndrome Coronario Agudo. Registro Actualizado) study. General findings. Rev Esp Cardiol. 2008;61:803-816.
17. Barrabés JA, Bardají A, Jiménez-Candil J, et al. Prognosis and management of acute coronary syndrome in Spain in 2012: the DIOCLES study. Rev Esp Cardiol. 2015;68:98-106.
18. Álvarez Álvarez B, Abou Jokh Casas C, Cordero A, et al. Early revascularization and long-term mortality in high-risk patients with non-ST-elevation myocardial infarction. The CARDIOCHUS-HUSJ registry. Rev Esp Cardiol. 2020;73:35-42.
19. Dawson LP, Chen D, Dagan M, et al. Assessment of Pretreatment With Oral P2Y12 Inhibitors and Cardiovascular and Bleeding Outcomes in Patients With Non-ST Elevation Acute Coronary Syndromes: A Systematic Review and Meta-analysis. JAMA Netw Open. 2021;4:e2134322.
20. Montalescot G, Bolognese L, Dudek D, et al. Pretreatment with prasugrel in non-ST-segment elevation acute coronary syndromes. N Engl J Med. 2013;369:999-1010.
21. Schüpke S, Neumann FJ, Menichelli M, et al. Ticagrelor or Prasugrel in Patients with Acute Coronary Syndromes. N Engl J Med. 2019;381:1524-1534.
22. Tarantini G, Mojoli M, Varbella F, et al. Timing of Oral P2Y12 Inhibitor Administration in Non-ST Elevation Acute Coronary Syndrome. J Am Coll Cardiol. 2020;76:2450-2459.
23. Romaguera R, Ojeda S, Cruz-González I, collaborators of the ACI-SEC, REGISTRY COLLABORATORS. Spanish Cardiac Catheterization and Coronary Intervention Registry. 30th Official Report of the Interventional Cardiology Association of the Spanish Society of Cardiology (1990-2020) in the year of the COVID-19 pandemic. Rev Esp Cardiol. 2021;74:1095-1105.

ABSTRACT
Introduction and objectives: There are few data on the utility of drug-coated balloons (DCB) for the side branch treatment of bifurcated lesions. Our objective was to determine the long-term effectiveness of such device in this scenario.
Methods: Retrospective-prospective registry of all such lesions treated with DCB (paclitaxel coating) at our unit from 2018 until present day with clinical follow-up including a record of adverse events.
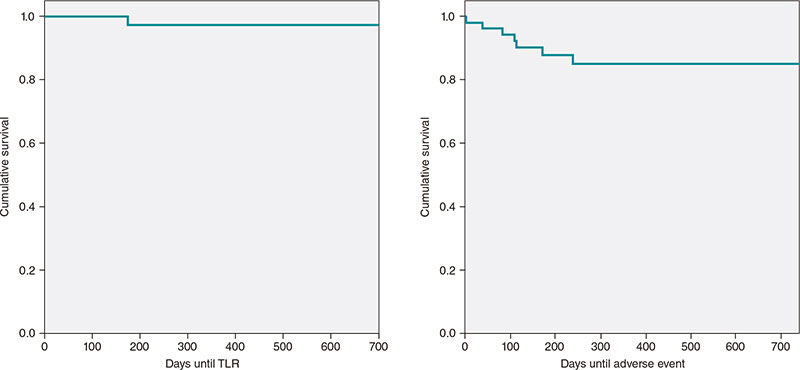
Results: A total of 56 lesions from 55 patients were included. The main demographic characteristics were mean age, 66.2 ± 11.3; and/or women, 27.3%; hypertension, 67.3%; dyslipidemia, 83.6%, and diabetes, 32.7%. The most common causes according to the coronary angiography were non-ST segment elevation acute coronary syndrome and stable angina. The main characteristics of the lesions were the location (circumflex-obtuse marginal, 19.6%; left anterior descending-diagonal, 64.3%; left main-circumflex, 8.9%; posterior descending-posterolateral trunk, 7.1%. The Medina classification was 1-1-1 37.5% of the times, and 1-1-0, 19.6% of the times. The rate of in-stent restenotic lesions was 32.1%. Procedural characteristics: radial access, 100%; side branch (SB) and main branch (MB) predilatation, 83.9% and 58.9%, respectively; MB stenting, 71.4%; POT technique, 35.7%; final kissing, 48.2%; optical coherence tomography/intravascular ultrasound, 7.1%. Procedural success was achieved in 98.2% of the cases. The median follow-up he all-cause mortality, myocardial infarction and lesion thrombosis, and target lesion revascularization rates were .7%, 0%, and 3.6%, respectively.
Conclusions: SB treatment with DCB in selected bifurcation lesions is safe and highly effective with a long-term success rate of 96.4%. Very large studies are still required to compare this strategy to SB conservative approach, and determine its optimal treatment.
Keywords: Drug-coated balloon. Bifurcation lesions. Follow-up study. Side branch.
RESUMEN
Introducción y objetivos: Hay pocos datos acerca de la utilidad del balón farmacoactivo (BFA) para el tratamiento de la rama lateral de las lesiones en bifurcación. El objetivo fue determinar la efectividad a largo plazo de dicho dispositivo en este escenario.
Métodos: Registro retrospectivo-prospectivo de todas las lesiones de este tipo tratadas con BFA recubierto de paclitaxel en nuestra unidad desde 2018 hasta la actualidad. Se realizó un seguimiento clínico con registro de eventos adversos.
Resultados: Se incluyeron 56 lesiones de 55 pacientes. Principales características demográficas: edad media 66,2 ± 11,3 años, 27,3% mujeres, 67,3% hipertensión arterial, 83,6% dislipemia y 32,7% diabetes. Las indicaciones más frecuentes para el cateterismo fueron síndrome coronario agudo sin elevación del ST y angina estable. Características de las lesiones tratadas: localización circunfleja-obtusa marginal 19,6%, descendente anterior-diagonal 64,3%, tronco común-circunfleja 8,9% y descendente posterior-tronco posterolateral 7,1%. Según la clasificación de Medina, el tipo más frecuente fue el 1,1,1 con el 37,3%, seguido del 1,1,0 con el 19,6%. Las lesiones tipo reestenosis en el interior del stent fueron del 32,1%. Características principales del procedimiento: acceso radial 100%, predilatación de rama lateral 83,9% y de rama principal 58,9%, stent en rama principal 71,4%, técnica POT 35,7%, kissing final 48,2% y tomografía de coherencia óptica/ecocardiografía intravascular 7,1%. Se logró el éxito del procedimiento en el 98,2%. Con un seguimiento medio de 12 meses, se registraron una incidencia de muerte por cualquier causa del 3,7%, trombosis lesional o infarto 0%, y revascularización de la lesión diana del 3,6%.
Conclusiones: El tratamiento con BFA de la rama lateral en lesiones bifurcadas seleccionadas es seguro y presenta una alta efectividad, con una tasa de éxito a largo plazo del 96,4%. Serían necesarios estudios muy amplios que permitieran comparar dicha estrategia con el abordaje conservador de la rama lateral y determinar cuál es su tratamiento óptimo.
Palabras clave: Balón farmacoactivo. Lesiones en bifurcación. Estudio de seguimiento. Rama lateral.
Abbreviations
DCB: drug-coated balloon. ISR: in-stent restenosis. MB: main branch. SB: side branch.
INTRODUCTION
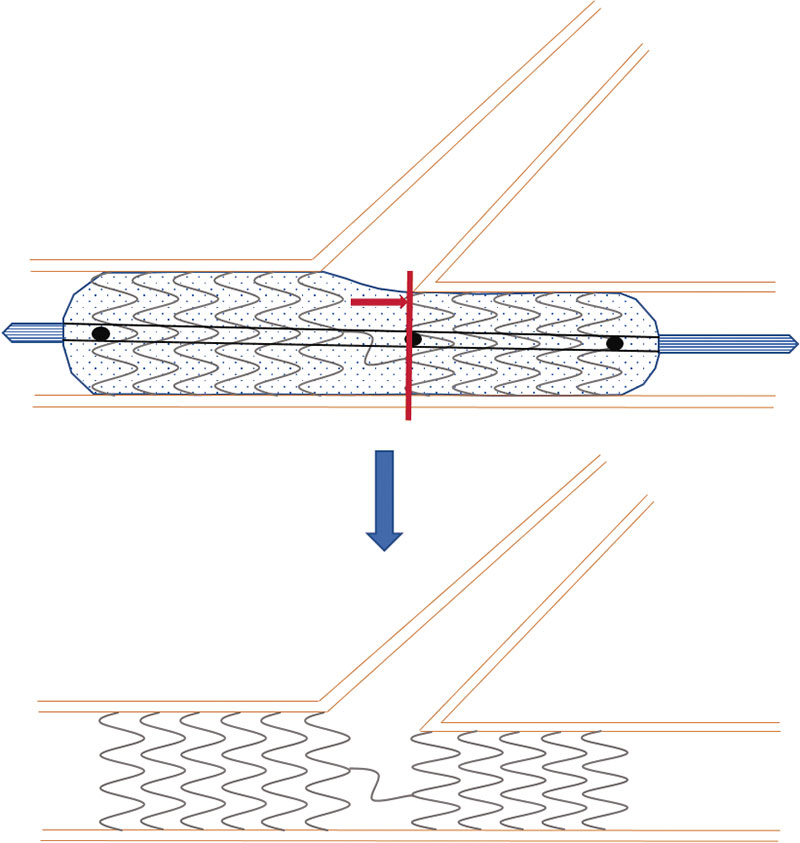
Coronary bifurcation lesions are still challenging for interventional cardiologists. The complexity surrounding such lesions regarding their anatomical, functional, and even clinical aspects truly complicates the management of this entity despite its high incidence rate that can be up to 20% of all the lesions that are treated at a cath lab on a routine basis. The relentless publication of articles on such lesions over the last few decades, the creation of specific study groups like the European Bifurcation Club, and the periodic publication of consensus documents for the management of this entity shows, without a doubt, that this scenario is in constant change and has not been solved today yet. One of the most controversial aspects is the importance of the side branch (SB) regarding the long-term prognosis of such lesions. Drug-coated balloon (DCB) is part of the therapeutic armamentarium of interventional cardiologists to treat coronary bifurcation lesions. Its utility for the management of certain anatomical settings like in-stent restenosis (ISR) type of lesions has already been demonstrated. However, its effectiveness to treat the SB is much less evident with scarce studies available in the medical literature. The theoretical advances posed by this device to treat the SB would be the administration of antiproliferative drugs into the ostium mainly, the lack of distortion of its original anatomy, and the minimization of strut deformation at carina level.1
This article presents a registry with the results obtained in our unit with the management of SB with DCB with a longer than usual clinical follow-up in this type of studies.
METHODS
This was a single-center, prospective-retrospective registry started back in 2019 of all coronary bifurcation lesions where the SB was treated with paclitaxel-coated DCB from October 2018 through March 2022. The device used was the SeQuent Please NEO (Braun, Germany), a paclitaxel-iopromide coated polymer-free balloon using Paccocath technology. Inclusion criteria were the presence of coronary bifurcation lesions with 1 compromised SB of, at least, 2 mm in diameter through visual angiographic estimate regardless of the aprioristic presence of a diseased SB or the appearance of carina displacement or slow flow after treating the main branch (MB). Also, the operator should consider the DCB approach of clinical and prognostic interest. Patient recruitment in the registry was on the rise: 4 patients in 2018, another 4 in 2019, 9 patients in 2020, 31 in 2021, and finally 7 within the first 3 months of 2022. No exclusion criteria were established. Approach strategy consisted of an early provisional stenting or DCB technique to treat the MB when damaged. Further management of SB with DCB was left to the operator’s criterion if, after treating the MB, significant damage done to the SB would require stenting in such branch. In that case, the patient would not be included, and the SB would not be eligible for treatment with a DCB. If, after preparing the lesion, the operator would actually consider using the DCB option, that would be the time to include the patient in the study. The rate of procedural failure—defined as the impossibility to cross the lesion with the DCB once it was used or unsatisfactory angiographic outcomes after balloon inflation involving SB stenting. The protocol for using the DCB—based on the recommendations established on the use of such devices—consisted of SB predilatation with non-compliant or scoring balloons in a 0.8-1 vessel/balloon diameter ratio, use of the device if an acceptable angiographic result with TIMI grade-3 flow was achieved, lack of significant dissection, and residual stenosis < 30%. If other lesions different from the one that triggered the inclusion in the registry needed revascularization, this was scheduled for a second surgical act. The study design followed a per protocol analysis to estimate the benefits of the technique described compared to the routine clinical practice including cases with successful DCB treatment at the follow-up and excluding those with acute device failure or impossibility to use the device once opened for being unable to cross the lesion. The lack of dissection after DCB that required stenting with residual stenosis < 50%, and final TIMI grade-3 flow was considered as procedural success. Device failure, on the other hand, was considered as an impossible DCB inflation once used or the need for stenting the SB with unsatisfactory DCB results. Different clinical variables from the patient were analyzed, as well as the lesion anatomy, and the procedural intervention per se. Retrospective clinical follow-up of patients successfully treated with the DCB was conducted. Follow-up went on for a maximum of 2 years after the procedure, and prospectively since the registry started back in 2019 until present time. This follow-up was conducted through phone calls or by checking the patients’ electronic health records. The ARC-2 definitions2 were used to collect the adverse clinical events including a composite endpoint of all-cause mortality, cardiac death, myocardial infarction, device thrombosis, clinically driven target lesion failure and revascularization, target vessel failure outside the target lesion, and revascularization of other lesions occurred at follow-up. All patients signed their written informed consent forms, and the study was approved by our center research ethics committee.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation. Categorical variables are expressed as frequency and percentage. Also, actuarial curves of adverse event-free survival using the Kaplan-Meier method were built, specifically target lesion failure-free and adverse event-free curves (all-cause mortality, target lesion revascularization, target vessel failure, and revascularization of other lesions).
RESULTS
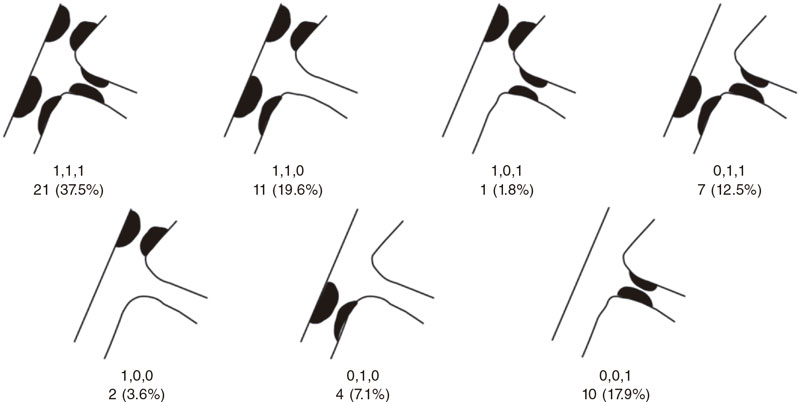
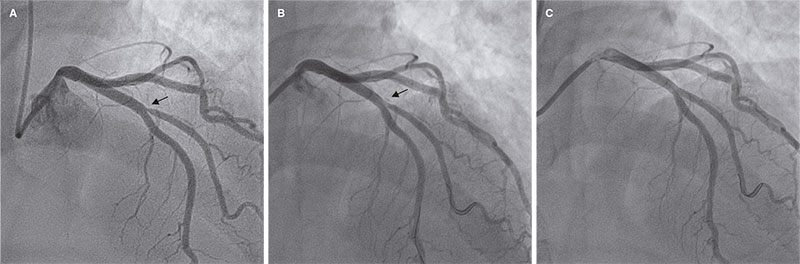
A total of 55 patients and 56 lesions were included since 2 different bifurcations found in 1 of the patients were treated in the same procedure. The patient/lesion flowchart included in the study is shown on figure 1. The patients’ clinical characteristics are shown on table 1. Vascular access was radial in 100% of the cases using a 6-Fr introducer sheath also in all of them. Table 2 shows the anatomical characteristics of target lesions. Figure 2 shows a schematic representation of the type of lesion according to the Medina classification. Table 3 shows the variables associated with the procedure. We should mention that all the clinical and anatomical data shown here, the patients’ high-risk profile with high prevalence of cardiovascular risk factors, and the large number of ISR-type of lesions reached 32.1% of the sample. The rate of lesions included with damage to 2 or 3 different bifurcation segments was 71.4% (40 out of 56). Regarding procedural factors the high rate of procedural success was significant (low rate of acute device failure with only 1 case of a type A dissection image after DCB inflation without damage to the distal flow and > 30% residual stenosis). Therefore, because of lesion location at ostium level, and possible damage to the MB (the left anterior descending coronary artery in this case), the operator decided to perform drug-eluting stent implantation for sealing purposes (figure 3). In all the remaining procedures, the acute result of the DCB was successful. In our series, the scarce use of intracoronary imaging modalities (only 7.1%) was also remarkable.
Figure 1. Flowchart of patients/lesions included in the study.
Figure 2. Number of lesions based on the type of bifurcation damage according to the Medina classification.
Figure 3. Only case of acute device failure. A: diagonal branch ostial lesion prior to the intervention (arrow); B: suboptimal outcome after drug-coated balloon (arrow); C: final outcome after stenting the side branch.
Table 1. Patients’ clinical characteristics
| N | 55 |
| Age | 66.2 ± 11.3 years [range, 45-91] |
| Sex | |
| Men | 40 (72.7%) |
| Women | 15 (27.3%) |
| Hypertension | 37 (67.3%) |
| Dyslipidemia | 46 (83.6%) |
| Smoking | 17 (30.9%) |
| Diabetes | 18 (32.7%) |
| Previous PTA | 28 (50.9%) |
| Previous coronary artery bypass graft | 1 (1.8%) |
| Indication for coronary angiography | |
| NSTEACS | 20 (36.4%) |
| STEACS | 9 (16.4%) |
| Stable angina | 20 (36.4%) |
| Other | 6 (10.9%) |
|
NSTEACS, non-ST-segment elevation acute coronary syndrome; PTA, percutaneous transluminal angioplasty; STEACS, ST-segment elevation acute coronary syndrome. |
|
Table 2. Anatomical characteristics of the lesions
| N | 56 |
| Diseased vessel | |
| LMCA-LCx | 5 (8.9%) |
| LAD-diagonal | 36 (64.3%) |
| LCx-OMA | 11 (19.6%) |
| PDA-PLT | 4 (7.1%) |
| ISR-type of lesion | 18 (32.1%) |
|
ISR, in-stent restenosis; LAD, left anterior descending coronary artery; LCx, left circumflex artery; LMCA, left main coronary artery; OMA, obtuse marginal artery; PDA, posterior descending artery; PLT, posterolateral trunk. |
|
Table 3. Procedural characteristics
| N | 56 |
| Predilatation | |
| SB | 47 (83.9%) |
| MB | 33 (58.9%) |
| MB treatment | |
| Stent | 40 (71.4%) |
| DCB | 4 (7.1%) |
| DCB diameter for the SB (mm) | |
| 2 | 20 (35.7%) |
| 2.25 | 4 (7.1%) |
| 2.5 | 23 (41.1%) |
| 3 | 8 (14.3%) |
| 3.5 | 1 (1.8%) |
| Postdilatation | |
| MB | 36 (64.3%) |
| POT | 20 (35.7%) |
| SB | 17 (30.4%) |
| Final kissing balloon | 27 (48.2%) |
| OCT/IVUS | 4 (7.1%) |
| Procedural success | 55 (98.2%) |
|
DCB, drug-coated balloon; IVUS, intravascular ultrasound; MB, main branch; OCT, optical coherence tomography; POT, proximal optimization technique; SB, side branch. |
|
The rate of adverse events at follow-up is shown on table 4. After a median follow-up of 12 months (377 ± 244 days; range, 64-734 days) only 2 clinically driven target lesion revascularizations (3.6%) were reported. Both were performed due to in-stent lesions that did not reach the target lesion proximal or distal borders. The first one was performed in a case of ISR of the SB in a very small vessel without acute ischemia whose new revascularization was performed late, more specifically, 23 months after the index procedure (figure 4). The second one was performed 6 months after the procedure—also without acute ischemic signs—but with ISR in the main vessel while the SB remained patent without significant restenosis (figure 5). Both cases were treated with drug-eluting stent implantation. Two deaths were reported: 1 cardiac death due to advanced left ventricular dysfunction in an 80-year-old woman who, after percutaneous coronary intervention, was implanted with a transfemoral aortic valve and a definitive pacemaker, with poor disease progression that eventually led to her death. The other death was septic shock related. No admissions due to acute myocardial infarction or episodes of target lesion thrombosis (both probable and definitive) were reported. No cases of target vessel failure outside the target lesion were reported either. A total of 5 revascularizations of other lesions (9.3%) were performed—all of them scheduled—but none due to acute coronary syndrome. The Kaplan-Meier curves showing target lesion revascularization-free and adverse event-free survival are shown on figure 6.
Table 4. Rate of adverse cardiovascular events at follow-up
| N | 54/55 |
| Follow-up days | 377 ± 244 [range, 79-734] |
| All-cause mortality | 2/54 (3,7%) |
| Cardiac death | 1/54 (1,8%) |
| Myocardial infarction/target lesion device thrombosis | 0/55 (0%) |
| Target lesion revascularization | 2/55 (3,6%) |
| Target vessel failure outside the target lesion | 0/55 (0%) |
| Revascularization of other lesions outside the target vessel | 5/54 (9.3%) |
Figure 1. First case of target lesion failure due to late restenosis. A: early in-stent restenosis type of lesion in obtuse marginal artery (arrow); B: final outcomes after drug-coated balloon; C: new in-stent restenosis in the side branch at 23 months (arrow).
Figure 5. Second case of target lesion failure. A: early obtuse marginal artery bifurcation lesion with distal left circumflex artery (arrow); B: outcomes after stenting the main branch, and drug-coated balloon implantation into the side branch; C: 6-month follow-up with restenosis at main branch level (arrow).
Figure 6. Kaplan-Meier curve of actuarial target lesion revascularization (TLR)-free survival and composite adverse events-free survival (all-cause mortality, TLR, revascularization of other lesions).
DISCUSSION
The latest document of the European Bifurcation Club on the utility of DCBs to treat SBs in coronary bifurcation lesions pays little attention to it due to the lack of large enough clinical trials to be conclusive.3 Despite the huge amount of medical literature available on the management of coronary bifurcation lesions, the actual significance of the SB and its involvement in target lesion failure has not been properly explained to this date. A study conducted by Oh et al.4 conclude that treating the SB in 1089 patients with bifurcation lesions at left anterior descending coronary artery-diagonal branch level was associated with a lower—yet not statistically significant—rate of target vessel failure. However, this difference was statistically significant when the subgroup studied included low-risk patients. On the other hand, a different clinical trial that studied factors associated with failed revascularizations of the left main coronary artery bifurcation found that the presence of MB stent struts inside the SB ostium was one of them5 suggestive that the use of intracoronary imaging modalities like intracoronary ultrasound or optical coherence tomography could improve results, at least, on such location, by telling us what patients would benefit from specifically treating the SB.
The strongest evidence available to this date leans towards the utility of DCB to treat ISR-type of lesions without a word dedicated to the SB. Very few studies have focused on the effectiveness of DCB to treat the SB. Such document advocates for treating coronary bifurcation lesions with the provisional stenting strategy according to the latest clinical practice guidelines drafted by the European Cardiology Society followed by treating the SB with a DCB. The first clinical trials on this regard were published back in 2011 like the DEBIUT,6 BABILON,7 DEBSIDE,8 the study conducted by Herrador et al.,9 the PEPCAD V,10 and the PEPCAD-BIF11 clinical trials. These studies showed contradictory—yet overall satisfactory—data regarding the effectiveness of DCB. These studies presented better quantitative angiographic parameters regarding restenosis or late lumen loss. However, not in every one of them this was associated with a lower rate of revascularization. As a matter of fact, there were doubts around the possibility of a higher rate of late thrombosis suggested by some of these trials. The recently published BEYOND clinical trial conducted by Jing et al.12 compared the use of a conventional balloon vs DCB to treat the SB with a 9-month angiographic follow-up. This trial found that the DCB was associated with better results regarding less late lumen loss. However, such an improvement did not translate into a lower rate of clinical adverse events since surprisingly enough no new revascularizations were reported in any of the 2 groups. A recent meta-analysis13 that included 10 studies on the effect of DCB on the SB concluded that such technique improved the angiographic outcomes significantly. However, this did not translate either into statistically significant clinical outcomes (target lesion failure mainly) basically due, according to the authors, to the low rate of this adverse event reported, and the fact that the study was statistically underpowered due to its small sample size. In a different study published in 2022,14 the management of coronary bifurcation lesions of left main coronary artery using 2 strategies was compared: double stenting for the MB and the SB vs 1 stent into the MB, and 1 DCB into the SB. They found controversial results at follow-up between both groups in different angiographic parameters with similar rates of restenosis and adverse events. However, the group treated with DCB significantly improved all the parameters associated with the SB (left circumflex artery, in this study)—as opposed to those associated with the MB (left main coronary artery-left anterior descending coronary artery)—with less late lumen loss (0.43 vs -0.17; P < .001), less luminal narrowing (16.7 vs 32.1; P = .002), and greater minimal lumen diameter (2.4 vs 1.8; P = .0031). Still, the rate of restenosis in the left circumflex artery (SB in this study) was 4 times higher in the double stenting group compared to the DCB group (30.4% vs 7.7%) although this difference was not statistically significant (P = .09). This could be indicative of greater superiority of the DCB if studies with larger samples would be conducted. Another recent study published in 202115 randomized 219 true de novo coronary bifurcation lesions where the SB was treated with conventional balloon vs DCB. At 12-month clinical and angiographic follow-up, significant improvements were reported in both the angiographic (less late lumen loss and greater minimal lumen diameter) and clinical parameters with a lower rate of major adverse cardiovascular events being reported. This improvement, however, did not translate into significant reductions regarding new revascularizations or target vessel failure.
The rate of target lesion failure requiring new revascularization was 3.6%, a figure that is consistent with most former studies. However, the range found goes from a surprising 0% up to a whopping 22%. However, we should mention the truly unfavorable clinical and anatomical profile of our sample since in most clinical trials, ISR-type of lesions, left main coronary artery disease or ST-segment elevation acute coronary syndrome—all allowed in our registry—were considered exclusion criteria regarding.
Out of the only 2 cases reported of target lesion failure requiring new revascularization, 1 occurred in a patient with an ISR-type of lesion. This occurred precisely in the SB while in former studies7—as already explained—the main incidence rate of failure occurred in the MB, not in the SB. The exclusion of patients with ISR would account for this difference. In our sample this type of lesions were 32.7% of all the lesions included. This added to the high rate (30.6%) of Medina 1,1,1 coronary bifurcation lesions (the one with the greatest complexity of all bifurcations) demonstrates the truly unfavorable profile of our sample. As a matter of fact, the rate of lesions included with damage to, at least, 2 segments of 1 bifurcation according to the Medina classification reached 71.4%. Very few studies have been conducted on this subgroup of patients. One of the most significant ones is the one conducted by Harada et al.16 that included 177 patients with ISR-type of lesions both in the MB and the SB treated with DCB. The latter was used in 80.6% of the SBs. The rate of binary restenosis was 24% at 6-to-8-month angiographic follow-up while the 1-year rate of new target lesion revascularization was 22%.
Limitations
Our study main limitation is the lack of a control group with lesions of similar characteristics, which would have allowed us to compare both groups and determine exactly the impact DCB has on the prognosis of patients. Similarly, the lack of angiographic follow-up does not discard the possibility of device failure. However, this would probably occur in the SB, not the MB, since it is in the latter where target lesion failure occurs according to the BABILON clinical trial.7 Another study limitation we should take into consideration is the elevated presence of small SB with a rate of use, in our sample, of DCB sizes < 2.25 mm of 43.7%. This would make target lesion failure go clinically inadvertently in some of these cases. Finally, we should mention that this study is limited by the relatively small number of patients included. Also, because due to its observational nature, no selection biases can be excluded.
CONCLUSIONS
The findings presented here show the experience of a single center with a very low rate of acute procedural complications, and a low rate of long-term adverse events despite dealing very high-risk profile lesions and patients with a 3.6% rate of target lesion failure reported. It is crucial to select the right type of lesions that can benefit from such therapy (basically the lack of a large plaque burden in the SB), a very refined technique of lesion preparation, and a greater use of tools to guide the angioplasty like intracoronary ultrasound or optical coherence tomography, preferably in ISR-type of lesions whose clinical progression is more unfavorable compared to that of de novo lesions. Despite the low rate of adverse events reported since no comparison with a control group was made, no definitive conclusions can be drawn on the advantages of DCBs in this clinical setting. We can only say that both in the «real-world» and the routine clinical practice described here, such strategy yields good long-term results without prejudice to other strategies may have given better or worse results regarding effectiveness. Randomized clinical trials are needed with enough statistical power and large enough samples to corroborate the promising data obtained from former studies to confirm or discard the superiority of DCB in the management of the SB in coronary bifurcation lesions. Until that time, the DCB can be considered a therapeutic tool that can be tremendously useful to improve the long-term results obtained in this type of complex lesions.
FUNDING
None whatsoever.
AUTHORS’ CONTRIBUTIONS
J. Valencia drafted the manuscript. J. Valencia, F. Torrez-Mezcua, and M. Herrero-Brocal participated in data curation, and in the clinical follow-up of the patients. J. Valencia, J. Pineda, P. Bordes, F. Torres-Saura, and J.M. Ruiz-Nodar participated in patient recruitment and in the manuscript critical review process. All the authors gave their final approval to the manuscript.
CONFLICTS OF INTEREST
None reported.
WHAT IS KNOWN ABOUT THE TOPIC?
- To this date, there is a limited number of studies that have analyzed the role of DCB to treat the SB of coronary bifurcation lesions. Although such role seems beneficial regarding the improvement of the parameters analyzed, this still has not translated into a clear significant improvement of clinical parameters like target lesion/vessel failure or need for new revascularizations. On the other hand, the exact relevance of the SB and the role it plays in the short- and long-term prognosis of coronary bifurcation lesions remains unknown.
WHAT DOES THIS STUDY ADD?
- Our registry provides the experience of a single large volume center treating this type of lesions with a long follow-up. Also, it represents the «real-world» setting, that has been considered cut off from large randomized clinical trials too many times. The favorable results obtained in our study in a very unfavorable clinical and anatomical setting can situate the DCB as an extremely useful tool to improve the long-term results of percutaneous coronary interventions performed on coronary bifurcation lesions at our cath labs.
REFERENCES
1. Sawaya FJ, Lefèvre T, Chevalier B, et al. Contemporary Approach to Coronary Bifurcation Lesion Treatment. JACC Cardiovasc Interv. 2016;9:1861-1878.
2. Spitzer A, McFadden E, Vranckx P, et al. Critical Appraisal of Contemporary Clinical Endpoint Definitions in Coronary Intervention Trials: A Guidance Document. JACC: Cardiovasc Interv. 2019;12:805-819.
3. Jeger RV, Eccleshall S, Wan Ahmad WA, et al. Drug-Coated Balloons for Coronary Artery Disease: Third Report of the International DCB Consensus Group. JACC Cardiovasc Interv. 2020;13:1391-1402.
4. Oh GC, Park KW, Kang J, et al. Association of Side-Branch Treatment and Patient Factors in Left Anterior Descending Artery True Bifurcation Lesions: Analysis from the GRAND-DES Pooled Registry. J Interv Cardiol. 2020. https://doi.org/10.1155/2020/8858642.
5. Mori H, Torii S, Harari E, et al. Pathological mechanisms of left main stent failure. Int J Cardiol. 2018;263:9-16.
6. Stella PR, Belkacemi A, Dubois C, et al. A multicenter randomized comparison of drug-eluting balloon plus bare-metal stent versus bare-metal stent versus drug-eluting stent in bifurcation lesions treated with a single-stenting technique: six-month angiographic and 12-month clinical results of the drug-eluting balloon in bifurcations trial. Catheter Cardiovasc Interv. 2012;80:1138-1146.
7. López Mínguez JR, Nogales Asensio JM, Doncel Vecino LJ, et al. A prospective randomised study of the paclitaxel-coated balloon catheter in bifurcated coronary lesions (BABILON trial): 24-month clinical and angiographic results. EuroIntervention. 2014;10:50-7.
8. Berland J, Lefèvre T, Brenot P, et al. DANUBIO — a new drug-eluting balloon for the treatment of side branches in bifurcation lesions: six-month angiographic follow-up results of the DEBSIDE trial. EuroIntervention. 2015;11:868-876.
9. Herrador JA, Fernandez JC, Guzman M, Aragon V. Drug-eluting vs. conventional balloon for side branch dilation in coronary bifurcations treated by provisional T stenting. J Interv Cardiol. 2013;26:454-462.
10. Mathey DG, Wendig I, Boxberger M, Bonaventura K, Kleber FX. Treatment of bifurcation lesions with a drug-eluting balloon: the PEPCAD V (Paclitaxel Eluting PTCA Balloon in Coronary Artery Disease) trial. EuroIntervention. 2011;7 Suppl K:K61-65.
11. Kleber FX, Rittger H, Ludwig J, et al. Drug eluting balloons as stand alone procedure for coronary bifurcational lesions: results of the randomized multicenter PEPCAD-BIF trial. Clin Res Cardiol. 2016;105:613-621.
12. Jing QM, Zhao X, Han YL, et al. A drug-eluting Balloon for the trEatment of coronarY bifurcatiON lesions in the side branch: a prospective multicenter ranDomized (BEYOND) clinical trial in China. Chin Med J. 2020;133:899-908.
13. Zheng Y, Li J, Wang L, et al. Effect of Drug-Coated Balloon in Side Branch Protection for de novo Coronary Bifurcation Lesions: A Systematic Review and Meta-Analysis. Front Cardiovasc Med. 2021;8:758560.
14. Liu H, Tao H, Han X, et al. Improved Outcomes of Combined Main Branch Stenting and Side Branch Drug-Coated Balloon versus Two-Stent Strategy in Patients with Left Main Bifurcation Lesions. J Interv Cardiol. 2022. https://doi.org/10.1155/2022/8250057.
15. Li Y, Mao Q, Liu H, Zhou D, Zhao J. Effect of Paclitaxel-Coated Balloon Angioplasty on Side Branch Lesion and Cardiovascular Outcomes in Patients with De Novo True Coronary Bifurcation Lesions Undergoing Percutaneous Coronary Intervention. Cardiovasc Drugs Ther. 2021. https://doi.org/10.1007/s10557-021-07225-8.
16. Harada Y, Colleran R, Pinieck S, et al. Angiographic and clinical outcomes of patients treated with drug-coated balloon angioplasty for in-stent restenosis after coronary bifurcation stenting with a two-stent technique. EuroIntervention. 2017;12:2132-2139.
Abstract
Introduction and objectives: To describe the efficacy of the BIOSS LIM C dedicated sirolimus-eluting stent to treat coronary bifurcation lesions, and impact on the bifurcation angle and carina through quantitative coronary angiography.
Methods: Observational prospective study including 124 patients with bifurcation lesions treated with a BIOSS LIM C dedicated sirolimus-eluting stent excluding restenotic lesions and those without main vessel involvement.
Results: The stent was successfully deployed in 121 patients (97.6%) while in 18 (14.5%) double stenting was used. The quantitative coronary analysis has shown proper stent expansion with a mean residual stenosis of 18% in the proximal segment, nearly 0% in the distal segment, and 21% in the side branch. The angiographic results of double stenting showed higher mean diameters (2.12 ± 0.30 vs 1.60 ± 0.42; P < .001), and lower residual stenosis (18.36 ± 9.94 vs 28.49 ± 14.19%, P < .01). Distortion imposed on the bifurcation angulation was minimal with an absolute reduction of 5 degrees (52.8 ± 18.4 vs 47.5 ± 17.2; P = .001).
Conclusions: The dedicated BIOSS LIM C stent has had a very high success rate to treat coronary bifurcation lesions. Angiographic results are good with a remarkably low impact on the native bifurcation angulation, and excellent results from double stenting. We think this can be a very useful device to treat coronary bifurcation lesions with the advantage of easing out the bailout deployment of a second stent into the side branch.
Keywords: Dedicated stent. Bifurcation lesion. BIOSS LIM C sirolimus-eluting stent.
RESUMEN
Introducción y objetivos: El estudio EPIC03-BIOSS se llevó a cabo para describir la eficacia del stent farmacoactivo dedicado BIOSS LIM C en el tratamiento de las lesiones en bifurcación, así como la modificación inducida sobre la lesión en bifurcación por angiografía cuantitativa automatizada.
Métodos: Estudio observacional prospectivo en el que se incluyeron 124 pacientes con lesión en bifurcación tratados con stent farmacoactivo BIOSS LIM C, excluidas las lesiones por reestenosis y aquellas en las que no había afección del vaso principal.
Resultados: El stent se implantó con éxito en 121 pacientes (97,6%); en 18 (14,5%) se utilizó una técnica de 2 stents. El análisis por angiografía cuantitativa automatizada mostró una estenosis residual media del 18% en el segmento proximal, de prácticamente el 0% en el segmento distal y del 21% en la rama lateral. Los resultados angiográficos para la técnica de doble stent muestran unos diámetros (2,12 ± 0,30 frente a 1,60 ± 0,42 mm; p < 0,001) y estenosis residuales (18,36 ± 9,94 frente a 28,49 ± 14,19%; p < 0,01) significativamente mejores. La distorsión sobre la angulación nativa del vaso resultó mínima, con una reducción absoluta de unos 5° (52,8 ± 18,4 frente a 47,5 ± 17,2°; p = 0,001).
Conclusiones: El stent BIOSS LIM C consigue una elevada tasa de éxito para el tratamiento de las lesiones en bifurcación. Los resultados angiográficos son buenos, destacando la escasa distorsión sobre la angulación nativa del vaso y los excelentes resultados. Consideramos que puede ser un buen dispositivo para el tratamiento de las bifurcaciones, la ventaja de poder facilitar la implantación no prevista de un segundo stent.
Palabras clave: Stent dedicado. Lesión en bifurcación. Stent liberador de sirolimus BIOSS LIM C.
Abbreviations
MB: main branch; OCT: optical coherence tomography; POT: proximal optimization technique; QCA: quantitative coronary angiography: SB: side branch.
INTRODUCTION
Percutaneous treatment of coronary bifurcation lesions can represent up to 20% of all coronary lesions treated.1 The definition of bifurcation lesion given by the European Bifurcation Club2 includes those that effect a relevant side branch (SB) whether by its angiographic diameter or the myocardium at risk associated with such SB.
The pseudo-fractal anatomy of coronary bifurcation lesions3 involves a significant caliber difference between proximal and distal segments. The study of the structural limits of tubular stents has favored the development of treatment techniques4,5 designed to optimize implantation in an anatomy that is not completely cylindrical with good angiographic and clinical results.6-10 Also, a progressive escalated strategy has been agreed based on parameters of damage to the SB from the provisional stenting technique to the complex double stenting one.
Although specific platforms have been designed to treat coronary bifurcation lesions, these have been limited for expert operator use only. On the one hand, dedicated stents can be categorized into stents designed to treat the main vessel by securing proper access to the SB, (Nile Croco & Pax, Minvasys, France, the Multi-Link Frontier, Abbott Vascular Devices, United States or the TAXUS Petal, Boston Scientific, United States). On the other hand, stents designed to treat the SB first to later complete the main branch (MB) with a tubular stent (Tryton Side Branch stent, Tryton Medical, United States; Sideguard, Cappella Inc, United States). However, because of the discrete results reported or their complexity, they have not become entirely popular.
The BIOSS LIM C stent11 (Balton, Poland) is a 70 µm ultra-thin-strut chrome-cobalt platform with a sirolimus-eluting biodegradable polymer of polylactic acid. It is a dedicated stent for bifurcations that consists of 2 segments of different size linked by 2 long connective struts (figure 1). Goal is to keep the pseudo-fractal correlation between the proximal and distal portions of the SB and facilitate the technique to access the SB or the provisional stenting technique. Former studies have given good results with successful implantation rates of 100%, and target lesion revascularization rates from 6.8% to 9.8%.12-14
Figure 1. Image of the BIOSS stent design. Note the structure in 2 bodies with central space for the side branch.
The objective of this study is to describe immediate angiographic results assessed through quantitative coronary angiography (QCA) in terms of deformation of the lesion native angulation and expansion, especially at the level of the polygon of confluence and at the origin of the side branch.
METHODS
Patients
This is a prospective, multicenter registry started by independent investigators that included patients with ischemic heart disease referred for percutaneous coronary revascularization of a bifurcated lesion considered as a lesion with distal branches with a minimum diameter of 2 mm.
Patients with lesions damaging the bifurcation on the SB only were excluded. Also, patients with restenosis, complete total coronary occlusions, a contraindication for dual antiplatelet therapy, cardiogenic shock, minors or patients who gave their express rejection to be included.
Study was conducted according to the Declaration of Helsinki and the study protocol was approved by the different ethics committees of participant centers. The specific written informed consent was obtained from all the patients included in the study.
Procedure
Stenting was performed following the implantation recommendations of every device (figure 2) by implanting and performing the final control in the angiographic view with better deployment of bifurcation. Anticoagulation with sodium heparin or low-molecular weight heparin was administered according to the usual standards of every cath lab. Specific treatment of each coronary bifurcation lesion was left to the operator’s criterion. Predilatation of both branches, the provisional stenting technique or the early double stenting technique were allowed whenever, at least, 1 dedicated stent from the study was used.
Figure 2. Scheme of the positioning of the BIOSS stent for correct implantation. Central marker needs to be adjusted to the carina of bifurcation, and the ostium of the distal main branch.
Procedural success was defined as the implantation of a BIOSS LIM C stent into the bifurcation lesion with residual stenosis on visual estimate < 30% in the MB and 50% in the SB.
Clinical follow-up
Telephone or on-site follow-up was conducted at 30 days and 12 months. Patients were surveyed on adverse cardiovascular events of death, myocardial infarction, stroke, stent thrombosis, need for new revascularization or bleeding.
Angiographic analysis
Angiographic analysis was conducted independently by an imaging lab (BARCICORE-Lab, Spain) using a specific dedicated software for bifurcations (QAngio XA 7.3, The Netherlands) with which all angiographic measurements were acquired including the measurements of bifurcation angulation. Two analysts selected the images before and after implantation without the intracoronary guidewire and in the same view (< 10º of difference). Angiographic analysis was conducted in end-diastole following the lab internal protocols.
Software used allows us to measure the 3 segments of a bifurcation simultaneously (proximal MB, distal MB, and SB) and obtain individual results from all the segments including the polygon of confluence of bifurcation. All analyses were conducted taking the proximal and distal borders of the stent deployed as the reference framework. When the double stenting technique was used, the distal border of the stent implanted into the SB was used as the analysis distal limit. In case of single-stent implantation, only the proximal 5 mm of the SB were used. Figure 3 shows an example of angiographic analysis.
Figure 3. Quantitative coronary angiography showing the percentage diameter stenosis (PDS) at the proximal main vessel (1), distal main vessel (2), and 5 mm proximal to the side branch (3) before (A) and after the procedure (B). Also, it measures changes to the bifurcation angle between the distal branches. Image C shows the limits of each segment (1, 2, and 3), and the borders of the polygon of confluence.
Statistical analysis
All quantitative data are expressed as mean ± standard deviation (SD) while qualitative data are expressed as number (percentage). For the quantitative angiographic analysis (QCA) between angiographic values before and after implantation, Student t test was used for paired data (quantitative data) while the McNemar test (qualitative data) was used when appropriate. For comparison purposes between the cohorts treated with provisional stenting and double stenting, Student t test or the Mann-Whitney U test (quantitative data) were used. Also, the chi-square test or Fisher’s exact test (qualitative data) were used, when appropriate. P values ≤ .05 were considered statistically significant. Statistical analyses were conducted using the statistical software package SPSS version 20.
RESULTS
Baseline clinical data
From August 2018 through February 2021 a total of 124 patients were included in the study (figure 4). The demographic data of patients are shown on table 1. We should mention the rates of patients with diabetes [26.9% (32/124)], and acute coronary syndrome [52,8% (66/124)], 12,8% (16/124) of whom had ongoing ST-segment elevation. No significant differences were reported in the baseline clinical characteristics between the single-stent and the double stenting cohorts.
Table 1. Description of population
| Total | |
|---|---|
| Baseline demographics | 124 |
| Feminine sex | 23 (18.47%) |
| Age | 65.48 (11.09) |
| Arterial hypertension | 79 (63.2%) |
| Dyslipidemia | 72 (57.6%) |
| Diabetes Mellitus | 32 (25.6%) |
| On insulin | 8 (6.4%) |
| Current smoker | 39 (31.2%) |
| Chronic kidney disease | 10 (8%) |
| Peripheral vasculopathy | 8 (6.4%) |
| Baseline treatment | |
| Acetylsalicylic acid | 81 (64.8%) |
| Clopidogrel | 34 (27.2%) |
| Ticagrelor | 18 (14.4%) |
| Prasugrel | 2 (0.6%) |
| Oral anticoagulant drugs | 9 (7.2%) |
| Vitamin K inhibitor | 1 (0.8%) |
| Direct-acting oral anticoagulants | 8 (6.4%) |
| Indication | |
| Stable angina | 37 (29.6%) |
| Silent ischemia | 12 (9.6%) |
| Ventricular dysfunction | 3 (2.4%) |
| STEACS | 6 (4.8%) |
| NSTEACS/unstable angina | 16 (12.8%) |
| NSTEACS/myocardial infarction | 34 (27.2%) |
| STEACS | 16 (12.8%) |
| AMI | 25 (20%) |
| Previous CABG | 4 (3.2%) |
| Previous PCI | 29 (23.2%) |
| Ejection fraction (%) | 54.31 (12.29) |
| Atrial fibrillation | 9 (7.2%) |
| Heart failure | 16 (12.8%) |
|
AMI, acute myocardial infarction; CABG, coronary artery bypass graft; NSTEACS, non-ST-segment elevation acute coronary syndrome; PCI, percutaneous coronary intervention; STEACS, ST-segment elevation acute coronary syndrome. |
|
Figure 4. Flowchart of the EPIC03-BIOSS study. QCA, quantitative coronary angiography.
Angiographic and procedural data
Angiographic and procedural data are shown on table 2 and table 3. Most procedures were performed via radial access (120, 96.8%) and the angiography revealed the presence of 3-vessel coronary artery disease in 19 patients (15.3%). In 10 cases (8%) the target lesion was found in the left main coronary artery. Regarding complexity, in 55 cases (44.3%) the lesions treated fell into the B2/C categories of the American Heart Association/American College of Cardiology while 32 lesions (25.8%) were categorized as moderate or severe.
Table 2. Angiographic data on visual estimate
| Total | Provisional | Complex | P | |
|---|---|---|---|---|
| Radial access | 120 (96%) | 110 (97.3%) | 10 (90.9%) | .314 |
| LMCA lesion | 11 (8.8%) | 10 (8.8%) | 1 (9.1%) | .830 |
| Proximal LAD | 77 (61.6%) | 71 (62.8%) | 6 (54.5%) | .746 |
| Coronary artery disease | .524 | |||
| 1 vessel | 55 (44%) | 51 (46.4%) | 4 (36.4%) | |
| 2 vessels | 47 (37.6%) | 43 (39.1%) | 4 (36.4%) | |
| 3 vessels | 19 (15.2%) | 16 (14.5%) | 3 (27.3%) | |
| Right dominance | 109 (87.2%) | 99 (87.6%) | 10 (90.9%) | .773 |
| Damaged bifurcation | .693 | |||
| LMCA-LAD/LCX | 10 (8%) | 8 (7.3%) | 2 (18.2%) | |
| LAD/Diagonal | 71 (56.8%) | 53 (57.5%) | 4 (54.6%) | |
| LCX/OMA | 28 (22.4%) | 25 (22.2%) | 3 (27.3%) | |
| RCA/PL | 15 (12.0%) | 15 (13.2%) | 0 (0%) | |
| Medina classification | .001 | |||
| 100 | 8 (6.4%) | 8 (7.3%) | 0 (0%) | |
| 010 | 18 (14.4%) | 18 (15.9%) | 0 (0%) | |
| 001 | 0 (0%) | 0 (0%) | 0 (0%) | |
| 110 | 38 (30.4%) | 38 (33.6%) | 0 (0%) | |
| 101 | 5 (4%) | 5 (4.4%) | 0 (0%) | |
| 011 | 10 (8%) | 6 (5.3%) | 4 (36.4%) | |
| 111 | 44 (35.2%) | 38 (33.6%) | 6 (54.5%) | |
| True | 59 (47.2%) | 49 (43.3%) | 10 (100%) | |
| Calcification (moderate or severe) | 32 (25.6%) | 28 (24.8%) | 4 (36.4%) | .542 |
| Proximal tortuosity | 25 (20%) | 20 (17.7%) | 5 (45.5%) | .04 |
| Thrombus | 15 (20%) | 14 (12.4%) | 1 (0.9%) | 1.000 |
| Type of lesion | .362 | |||
| A | 3 (2.4%) | 3 (2.7%) | 0 (0%) | |
| B1 | 37 (29.6%) | 31 (27.4%) | 6 (54.5%) | |
| B2 | 45 (36%) | 43 (38.1%) | 2 (18.2%) | |
| C | 10 (8%) | 10 (8.8%) | 0 (0.0%) | |
|
LAD, left anterior descending coronary artery; LCX, left circumflex artery; LMCA, left main coronary artery; OMA, obtuse marginal artery; PL, posterolateral; RCA, right coronary artery. |
||||
Table 3. Procedural description and follow-up
| Total | Provisional | Complex | P | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Procedure | 124 | 106 (85.5%) | 18 (14.5%) | |||||||
| Early strategy | 124 | 113 (90.4%) | 11 (9.6%) | |||||||
| Guide catheter | .289 | |||||||||
| 6-Fr | 113 (90.4%) | 104 (92%) | 9 (81.8%) | |||||||
| 7-Fr | 10 (8%) | 8 (7.1%) | 2 (18.2%) | |||||||
| 8-Fr | 1 (0.8%) | 1 (0.9%) | 0 (0%) | |||||||
| SB predilatation | 100 (80%) | 90 (79.6%) | 10 (90.9%) | .690 | ||||||
| Rotational atherectomy | 0 (0%) | 0 (0%) | 0 | 1.000 | ||||||
| Stenting | 121 (97.6%) | 111 (98.2%) | 10 (90.9%) | .314 | ||||||
| Length of stent | 19.73 (3.23) | 19.57 (3.21) | 21.45 (3.04) | .064 | ||||||
| POT | 33 (26.6%) | 30 (26.5%) | 3 (27.3%) | .800 | ||||||
| SB dilatation | 47 (37.9%) | 43 (38.1%) | 4 (36.4%) | .449 | ||||||
| Kissing after stenting the MB | 21 (44.7%) | 21 (48.8%) | 0 (0%) | |||||||
| SB dilatation only | 26 (55.3%) | 22 (51.2%) | 4 (100%) | |||||||
| Additional stenting | 17 (13.7%) | 16 (14.2%) | 1 (9.1%) | |||||||
| Stent into the SB | 18 (14.5%) | 9 (8%) | 9 (81.8%) | .000 | ||||||
| Kissing after stenting the SB | 16 (88.9%) | 7 (77.8%) | 9 (100%) | |||||||
| Imaging modalities | 11 (8.9%) | 8 (7.1%) | 3 (27.3%) | .023 | ||||||
| IVUS | 9 (7.3%) | 7 (6.2%) | 2 (18.2%) | |||||||
| OCT | 2 (1.6%) | 1 (0.9%) | 1 (9.1%) | |||||||
| Complications | 1 (0.8%) | 1 (0.9%) | 0 | |||||||
| Occlusion of the SB | 1 (0.8%) | 1 (0.9%) | 0 (0%) | |||||||
| Success (lack of stenosis ≥ 50%) | 114 (91.2%) | 104 (92%) | 10 (90.9%) | .338 | ||||||
| TIMI-flow grade at MB | .638 | |||||||||
| 3 | 114 (91.9%) | 103 (91.2%) | 11 (100%) | |||||||
| 2 | 0 (0%) | 0 (0%) | 0 (0%) | |||||||
| 1 | 3 (2.4%) | 3 (2.7%) | 0 (0%) | |||||||
| TIMI-flow grade at SB | .638 | |||||||||
| 3 | 114 (91.9%) | 103 (91.2%) | 11 (100%) | |||||||
| 2 | 0 (0%) | 0 (0%) | 0 (0%) | |||||||
| 1 | 3 (2.4%) | 3 (2.7%) | 0 (0%) | |||||||
| MB stenosis | 3.27% (6.14) | 3.24% (6.25) | 3.57% (4.76) | .892 | ||||||
| SB stenosis | 14.74% (19.94) | 15.55% (20.45) | 4.29% (4.5) | .211 | ||||||
| ≥ 50% stenosis of MB | 0 (0%) | 0 (0%) | 0 (0%) | 1.000 | ||||||
| ≥ 50% stenosis of SB | 7 (7.1%) | 7 (7.7%) | 0 (0%) | .585 | ||||||
| ≥ 30% stenosis of SB | 22 (22.4%) | 22 (24.2%) | 0 (0%) | .158 | ||||||
| 12-month follow-up | 110 (88.7%) | 92 (86.8%) | 18 (100%) | |||||||
| Death | 0 (0%) | 0 (0%) | 0 (0%) | |||||||
| Stent related AMI | 2 (1.8%) | 1 (1.1%) | 1 (5.5%) | .223 | ||||||
| Re-PCI | 6 (5.4%) | 5 (5.4%) | 1 (5.5%) | .555 | ||||||
| Thrombosis | 1 (0.9%) | 0 (0%) | 1 (5.5%) | .115 | ||||||
|
AMI, acute myocardial infarction; IVUS, intravascular ultrasound; OCT optical coherence tomography; POT, proximal optimization therapy; Re-PCI, re-percutaneous coronary intervention; SB, side branch; MB, main branch; TIMI, Thrombolysis in Myocardial Infarction. |
||||||||||
When we analyzed the differences between the cohorts treated with the provisional stenting technique and the double stenting one (table 2 and table 3) we saw that in the latter the rate of true bifurcations, proximal tortuosity, and use of imaging modalities was significantly higher.
During the procedure (table 3) the BIOSS LIM C stent was successfully implanted in 121 patients (97.6%). In the 3 cases when the stent was not implanted, the lesions showed moderate or severe calcification and proximal tortuosity. One case out of the 121 treated with stenting became complicated with SB occlusion following a dissection that could not be revascularized. In 30 (26.5%) out of the 113 cases (90.4%) where the early provisional stenting strategy was used, the POT (proximal optimization technique) was used. In 43 (38.1%) it was necessary to dilate the SB through kissing-balloon or simple dilatation (table 3). Finally, in 9 cases (7.2%) initially treated with the single-stent strategy, a second stent was needed in the SB. The double stenting strategy was initially adopted in 11 patients (9.6%). However, after SB dilatation and stent implantation into the MB the implantation of a second stent was deemed as unnecessary in 2 of them (18.2%). Therefore, 18 patients (14.5%) were eventually treated with the double stenting technique. The rates of predilatation, rotational atherectomy, use of POT, and successful implantation were similar in both cohorts.
Quantitative angiography of bifurcation
The angiographic images of 92 patients were available (table 4). Mean residual stenosis of 18% was seen in the proximal segment and nearly 0% in the MB distal segment. In the SB, the postoperative mean residual stenosis was 21% with significant residual stenosis in 5% of all patients treated with the provisional stenting technique.
Table 4. Quantitative coronary angiography of bifurcation in the entire cohort. Comparison between simple and double stent
| N = 92 lesions | Entire population (N = 92) | 1-stent technique (N = 75) | 2-stent technique (N = 17) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Pre | Post | P | Pre | Post | P | Pre | Post | P |
| Minimum lumen diameter, mm | 0.97 ± 0.48 | 1.70 ± 0.44 | < .001 | 0.99 ± 0.48 | 1.60 ± 0.42 | < .001 | 0.85 ± 0.47 | 2.12 ± 0.30 | < .001 |
| Maximum percentage diameter stenosis, % | 62.78 ± 17.70 | 26.62 ± 14.03 | < .001 | 61.63 ± 17.92 | 28.49 ± 14.19 | < .001 | 67.86 ± 16.25 | 18.36 ± 9.94 | < .001 |
| Carinal angle, degrees (º) | 52.8 ± 18.4 | 47.5 ± 17.2 | .001 | 52.3 ± 13.4 | 46.4 ± 18.1 | .002 | 55.3 ± 13.3 | 51.9 ± 12.5 | .161 |
| Proximal main branch | |||||||||
| Length, mm | 11.15 ± 5.28 | 10.86 ± 5.22 | .154 | 11.56 ± 5.59 | 11.21 ± 5.53 | .155 | 9.06 ± 2.50 | 8.97 ± 2.69 | .776 |
| Reference lumen diameter, mm | 3.83 ± 1.13 | 3.88 ± 0.80 | .674 | 3.65 ± 0.98 | 3.84 ± 0.71 | .089 | 4.75 ± 1.42 | 4.10 ± 1.12 | .066 |
| Minimum lumen diameter, mm | 1.63 ± 0.85 | 2.96 ± 0.62 | < .001 | 1.60 ± 0.77 | 2.85 ± 0.46 | < .001 | 1.78 ± 1.15 | 3.50 ± 0.93 | < .001 |
| Percentage diameter stenosis, % | 55.36 ± 20.81 | 18.09 ± 10.34 | < .001 | 54.12 ± 20.78 | 19.44 ± 10.01 | < .001 | 61.16 ± 20.58 | 11.77 ± 9.78 | < .001 |
| Binary stenosis (SD ≥ 50%), N (%) | 58 (63.0) | 0 | < .001 | 45 (60.0) | 0 | < .001 | 13 (76.5) | 0 | < .001 |
| Distal main branch (BIOSS) | |||||||||
| Length, mm | 10.35 ± 5.36 | 9.96 ± 5.46 | .190 | 10.61 ± 5.69 | 10.28 ± 5.76 | .105 | 9.13 ± 3.24 | 8.66 ± 3.43 | .082 |
| Reference lumen diameter, mm | 2.33 ± 0.45 | 2.34 ± 0.42 | .797 | 2.29 ± 0.45 | 2.32 ± 0.42 | .547 | 2.49 ± 0.39 | 2.42 ± 0.40 | .409 |
| Minimum lumen diameter, mm | 1.19 ± 0.56 | 2.28 ± 0.36 | < .001 | 1.24 ± 0.56 | 2.27 ± 0.37 | < .001 | 0.99 ± 0.53 | 2.34 ± 0.30 | < .001 |
| Percentage diameter stenosis, % | 48.43 ± 23.07 | 0.12 ± 15.00 | < .001 | 46.67 ± 22.98 | -0.62 ± 15.06 | < .001 | 60.61 ± 19.77 | 3.40 ± 14.74 | < .001 |
| Binary stenosis (SD ≥ 50%), N (%) | 43 (46.7) | 0 | < .001 | 31 (41.3) | 0 | < .001 | 12 (70.6) | 0 | < .001 |
| Side branch | |||||||||
| Length, mm | 6.32 ± 3.81 | 6.27 ± 3.47 | .689 | 5.20 ± 0.99 | 5.21 ± 0.89 | .905 | 11.58 ± 6.88 | 11.25 ± 6.08 | .549 |
| Reference lumen diameter, mm | 2.18 ± 0.48 | 2.22 ± 0.49 | .199 | 2.12 ± 0.46 | 2.16 ± 0.42 | .268 | 2.42 ± 0.51 | 2.49 ± 0.71 | .536 |
| Minimum lumen diameter, mm | 1.41 ± 0.64 | 1.75 ± 0.52 | < .001 | 1.46 ± 0.57 | 1.62 ± 0.43 | .022 | 1.19 ± 0.88 | 2.31 ± 0.50 | < .001 |
| Percentage diameter stenosis, % | 34.16 ± 27.52 | 20.94 ± 19.14 | < .001 | 30.06 ± 25.34 | 24.35 ± 17.13 | .070 | 52.24 ± 30.19 | 5.88 ± 20.77 | < .001 |
| Binary stenosis (SD ≥ 50%), N (%) | 22 (23.9) | 5 (5.4) | < .001 | 13 (17.3) | 5 (6.6) | .044 | 9 (52.9) | 0 | < .001 |
|
PDS, percentage diameter stenosis. |
|||||||||
Comparing patients treated with the single-stent technique or the double stenting technique (table 4) revealed that, at proximal segment level, expansion results are better with the double stenting technique with residual stenosis of 11% (vs 19% with the single-stent) although starting from greater baseline reference diameters (3.65 ± 0.98 vs 4.75 ± 1.42). The double stenting technique showed excellent results on the SB with a significantly greater minimum lumen diameter (2.31 ± 0.50 vs 1.62 ± 0.43, P = .01), and minimum residual stenosis of 5.9% (vs 24.3% with the provisional stenting technique) with special attention to the lack of stenosis > 50%. Results were similar in the distal segment.
Quantitative angiography of the polygon of confluence
At the polygon of confluence (table 5) results show residual stenoses of 17.15% ± 10.96% at the polygon core, and 19.21% ± 20.56% for the ostium of the SB. When the provisional stenting and double stenting techniques were compared (table 5), data from the QCA show better minimum lumen diameters for the double stenting technique in the bifurcation core and the ostium of the SB, and almost identical for the ostium of the distal segment.
Table 5. Quantitative coronary angiography. Polygon of confluence. Comparative between simple and double stent
| N = 92 lesions | Provisional technique (N = 75) | Double stenting technique (N = 17) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | P | Pre | Post | P | Pre | Post | P | |
| Bifurcation core | |||||||||
| Reference lumen diameter, mm | 4.04 ± 1.13 | 3.97 ± 0.80 | .531 | 3.83 ± 0.97 | 3.88 ± 0.69 | .618 | 4.82 ± 1.34 | 4.35 ± 1.10 | .095 |
| Minimum lumen diameter, mm | 2.09 ± 0.94 | 3.28 ± 0.78 | < .001 | 1.87 ± 0.72 | 2.93 ± 0.49 | < .001 | 2.25 ± 0.98 | 3.63 ± 0.90 | < .001 |
| Percentage diameter stenosis, % | 48.49 ± 17.94 | 17.15 ± 10.96 | < .001 | 47.57 ± 18.23 | 18.76 ± 10.68 | < .001 | 52.39 ± 15.70 | 10.31 ± 9.63 | < .001 |
| Binary stenosis (SD ≥ 50%), N (%) | 46 (50.0) | 0 | < .001 | 35 (46.7) | 0 | < .001 | 11 (64.7) | 0 | .001 |
| Distal main branch ostium (BIOSS) | |||||||||
| Reference lumen diameter, mm | 2.34 ± 0.45 | 2.35 ± 0.43 | .842 | 2.29 ± 0.45 | 2.32 ± 0.42 | .544 | 2.55 ± 0.40 | 2.48 ± 0.43 | .406 |
| Minimum lumen diameter, mm | 1.38 ± 0.53 | 2.40 ± 0.37 | < .001 | 1.40 ± 0.50 | 2.39 ± 0.36 | < .001 | 1.32 ± 0.67 | 2.45 ± 0.41 | < .001 |
| Percentage diameter stenosis, % | 40.59 ± 21.36 | -3.67 ± 15.54 | < .001 | 38.61 ± 20.61 | -4.57 ± 15.76 | < .001 | 48.95 ± 23.07 | 0.13 ± 14.37 | < .001 |
| Binary stenosis (SD ≥ 50%), N (%) | 34 (37.0) | 0 | < .001 | 23 (30.7) | 0 | < .001 | 11 (64.7) | 0 | .001 |
| Side branch ostium | |||||||||
| Reference lumen diameter, mm | 2.21 ± 0.50 | 2.24 ± 0.51 | .280 | 2.14 ± 0.46 | 2.17 ± 0.41 | .375 | 2.51 ± 0.56 | 2.57 ± 0.72 | .553 |
| Minimum lumen diameter, mm | 1.56 ± 0.52 | 1.80 ± 0.55 | < .001 | 1.58 ± 0.47 | 1.65 ± 0.45 | .219 | 1.47 ± 0.71 | 2.40 ± 0.52 | < .001 |
| Percentage diameter stenosis, % | 28.21 ± 21.77 | 19.21 ± 20.56 | .004 | 24.91 ± 20.30 | 23.13 ± 17.97 | .501 | 42.19 ± 22.82 | 2.58 ± 22.99 | < .001 |
| Binary stenosis (SD ≥ 50%), N (%) | 16 (17.4) | 5 (5.4) | .012 | 9 (12.0) | 5 (6.7) | .267 | 7 (41.2) | 0 | .016 |
|
PDS, percentage diameter stenosis. |
|||||||||
Bifurcation angle (table 4) showed a slight change after stenting with a statistically significant reduction from 52.8 ± 18.4 º down to 47.5 ± 17.2º (P = .001). In the double stenting cohort, angulation modification was similar—in absolute terms—with a reduction of some 4º. However, this difference was not statistically significant (table 4). No significant correlation between the degree of angulation modification and the SB residual stenosis was reported (P = .86).
Clinical outcomes
Only 1 procedural complication was reported consisting of the occlusion of the SB in a patient treated with the provisional stenting technique that could not be solved. No major clinical events, cases of stent thrombosis or target lesion revascularization were reported at 30 days.
One year after implantation, 110 patients (88.7%) were contacted. A case of definitive device thrombosis (0.91%) was reported at 1-year follow-up especially in a case of double stenting treated with primary angioplasty. This case added to other 5 cases of new target lesion revascularization due to restenosis (4.54%) reveal a 12-month rate of target lesion failure of 5.45%. Two of these restenoses were found in the ostium of the SB and the remaining 3 were in the main vessel. No deaths and 3 infarctions (2.72%) were reported all of them associated with the device in relation to stent thrombosis and, in the other 2 cases, due to restenosis with minimum mobilization of troponin.
DISCUSSION
The study main findings are: a) the BIOSS LIM C dedicated stent has a high rate of success at 30 days in patients with complex coronary bifurcation lesions; b) such device is basically used with the provisional stenting strategy and is associated with a very reduced need for stenting in the SB; c) immediate angiographic outcomes show the proper behavior from the stent in the 3 bifurcation segments, as well as in the polygon of confluence where the contact surface between the stent and the artery is minimum.
Demographic data confirm that this is a non-selected population with a prevalence of risk factors, comorbidities, heart disease, and anatomical characteristics of the lesions we see in the routine clinical practice of any cath lab these days.
The device had a high rate of implantation success that was consistent with the easiness of its design being successfully implanted in > 97% of the cases. Success rate is similar to that reported in most studies with tubular stents used in bifurcations, something unreported in previous series of dedicated stents (Axxess, Frontier, and Nile studies). For example, the Frontier stent15 had a rate of restenosis of nearly 29.9%. The Nile stent16,17 was successfully used in tortuous arteries and distal segments with acceptable results with a rate of target lesion revascularization of 8.4%. However, it required distribution of angiographic stenosis focused on the carina. The self-expandable Axxess stent18 showed favorable results with a 1-year rare of cardiovascular events and restenosis of 7.7% and 6.4%, respectively. Nonetheless, it only treated the polygon of confluence and the segment immediately proximal to the ostia of the branches. Also, it was limited to certain angles and lengths needing, on many occasions, the use of additional stents.
In 14.5% of the cases, the double stenting technique was used. This is a dedicated stent in such a way that, when crossing the SB, the lack of struts in the polygon of confluence facilitates its advance without requiring previous opening. Results from the study support just how easy it is to use it with the double stenting technique. In all the cases where it was used, a second stent was successfully implanted into the SB. In 6 (66%) out of the 9 cases where the double stenting strategy was planned, the SB stent was directly implanted without dilatation. This data, though very limited, could signal a possible advantage of the BIOSS LIM C dedicated stent to facilitate access of a second stent to the SB when necessary. On the other hand, angiographic results after implantation are particularly remarkable for the double stenting technique: both the minimum lumen diameters and the residual stenosis of the proximal segments and the SB are better compared to those seen in the cohort where the provisional stenting technique was used.
If procedural data are analyzed, something that calls our attention is the SB predilatation in 47 cases where the SB was not damaged significantly. The protocol did not define the obligation to predilate the SB. According to the investigator’s criterion in each case, the observation of a lesion that did not reach a 50% stenosis was still considered to pose risk of carina displacement.
Another aspect we should mention is the strikingly low use of the POT reported in 33 cases (26%), and similarly in both cohorts. On this regard, we should mention that the device is designed with a proximal segment of a greater caliber in such a way that with simple inflation this proximal postdilatation is already incorporated. The POLBOS I19 and II14 clinical trials showed that the use of POT improved the rate of target lesion revascularization. Also, a tendency towards less late lumen loss was reported. However, POT was used at a rate of 37%, which is similar to that of our cohort.
One of the main study endpoints was to assess the potential disadvantage of design in 2 stent segments. This design provides a space for the polygon of confluence—of 0.9 mm to 1.5 mm—between both segments linked by 2 connectors. In this space, the metal-to-artery ratio is significantly lower, which may contribute to a relatively systematic underexpansion.
Results of the provisional cohort on the polygon of confluence show that mean residual stenosis is 19% at the core of the polygon and 23% in the ostium of the SB. These data suggest a certain impact in the angiographic results of this relative lack of scaffold between both segments that we believe could be the cause for a certain degree of underexpansion at the polygon of confluence.
Another study primary endpoint was to see the degree of damage in the bifurcation native angulation. Godino et al.20 analyzed changes to the bifurcation angle after angioplasty using the 1 or 2-stent technique in a cohort of 215 patients. They described a mean reduction of around 10º of the angle in the left main coronary artery, and 7º in the remaining bifurcations with the double stenting technique. However, with the provisional stenting technique no significant differences were reported. In our study, the results seen in the overall population show a statistically significant change—although not very relevant numerically—of the bifurcation angle that went from 53º to 47º. Contrary to what was reported by Godino et al.20, in our study, in the 2-stent cohort, changes to the bifurcation angle were not significant with mean reductions of 3º. However, in the provisional stenting cohort, a significant reduction of the angle was seen of 6º. In any case, we consider that this just has simple statistical significance; it seems very unlikely that a 5º variation of the bifurcation angle can be seen through visual estimate, and much less that any clinical disadvantages can occur.
Overall, the follow-up results were good and consistent with what was described in former studies.14,19 There was a significant loss of cases for the QCA since 92 cases were available only. Another study limitation is that regarding the analysis of the double stenting technique. Although the QCA data suggest good results for the double stenting technique, the study was not designed to draw comparisons between the provisional and the double stenting techniques. Also, the information collected is limited, and the number of patients treated with the double stenting technique is not enough to reach any conclusions.
An additional limitation we would like to mention is the low use of imaging modalities. Probably in coronary bifurcation lesions its systematic use can be beneficial.
In conclusion, we believe that this study conducted at several centers with different operators reveals how relatively easy it is to use the BIOSS LIM C stent to treat coronary bifurcation lesions. We’re pretty sure this is significant enough to simplify the double stenting approach where the scarce distortion overlapping the bifurcation native angulation called our attention. On the other hand, the weak spot would be the feeble metal scaffold that remains in the polygon of confluence probably due to a certain degree of underexpansion. However, the good clinical outcomes reported at 1-year follow-up are indicative that such design is not really a problem.
Limitations
This was a prospective observational study, which means that any comparisons among the routine techniques used to treat coronary bifurcation lesions can be limited. The informed consent from all the patients was not obtained. Although information from 88% of the cohort was obtained, losses to follow-up were slightly higher than they should have been.
CONCLUSIONS
The BIOSS LIM C dedicated stent works well to treat coronary bifurcation lesions. Angiographically, the stent has a space at the polygon of confluence to facilitate access to the SB. This is associated with a lower metal-to-artery ratio conditioning residual stenosis of around 20%. However, such residual stenosis does not necessarily trigger more events at 1 year and, at the end, this carinal design allows easy access to the SB in case a second stent would be needed and with excellent results. Finally, the stent-induced distortion on the angle of the carina is limited, around 5º.
FUNDING
The study was funded with a graft from Fundación EPIC, which was unconditionally funded by the LOGSA group.
AUTHORS’ CONTRIBUTIONS
As the study lead co-investigators, B. García del Blanco, and A. Pérez de Prado drafted the protocol, managed funds, directed the project, recruited the patients, wrote part of the article, and made their contributions to the overall drafting of the article. J. Gómez-Lara conducted the angiographic analysis at the core lab, the statistical analysis, drafted part of the article, and made his contributions to the article overall draft. I. In his capacity of sub-investigator, Otaegui Irurueta recruited patients by performing procedures and protocol follow-ups, entered the data required in the data curation notebook, depurated and finalized the data entered in the database, was involved in statistical analysis, drafted part of the article, participated in the article overall draft by compiling all the sections drafted from the remaining authors, and responded to the corrections requested by reviewers. M.A. Carmona Ramírez dealt with all regulatory actions needed to start the study and include the different participant centers both with the Spanish Agency of Medicines and Medical Devices and the different centers and ethics committees. As study sub-investigators, the remaining authors recruited patients, performed the procedures and follow-ups according to protocol, filled out the data curation notebook, and responded to all the questions asked.
CONFLICTS OF INTEREST
A. Pérez de Prado is an associate editor of REC: Interventional Cardiology; the journal’s editorial procedure to ensure impartial handling of the manuscript has been followed. He has received research grants from the following research sponsors (Fundación EPIC): Abbott, Biosensors, Biotronik, Bristol-Myers-Squibb, Boston Scientific, Cardiva, iVascular, Shockwave Ltd, Terumo, Volcano Philips; also, fees for his theoretical or practical proctoring for Braun, Boston Scientific, and Terumo. The remaining authors declared no conflicts of interest whatsoever.
WHAT IS KNOWN ABOUT THE TOPIC?
- Dedicated stents facilitate better adaptation to fractal anatomy of coronary bifurcation lesions with less bifurcation angle distortion and access to the side branch. The BIOSS LIM C stent has shown favorable results in randomized clinical trials compared to second-generation tubular stents.
WHAT DOES THIS STUDY ADD?
- In this study, the angiographic pattern of coronary bifurcation lesions with the implantation of BIOSS LIM C dedicated stent is shown. Also, it shows the feasibility of its systematic use—with a high rate of success and scarce damage to bifurcation angulation—can have. Residual underexpansion at the polygon of confluence is acceptable for the provisional stenting technique despite a reduced metal-to-artery ratio, and excellent for the double stenting technique.
REFERENCES
1. Ojeda S, Romaguera R, Cruz-González I, Moreno R. Spanish Cardiac Catheterization and Coronary Intervention Registry. 29th Official Report of the Interventional Cardiology Association of the Spanish Society of Cardiology (1990-2019). Rev Esp Cardiol. 2020;73:927-936.
2. Burzotta F, Lassen JF, Lefèbre T, et al. Percutaneous coronary intervention for bifurcation coronary lesions: the 15th consensus document from the European Bifurcation Club. EuroIntervention. 2021;16:1307-1317.
3. Huo Y, Kassab GS. Scaling laws of coronary circulation in health and disease. J Biomech. 2016;49:2531-2539.
4. Foin N, Sen S, Allegria E, et al. Maximal expansion capacity with current DES platforms: a critical factor for stent selection in the treatment of left main bifurcations? EuroIntervention. 2013;8:1315-1325.
5. Finet G, Derimay F, Motreff P, et al. Comparative Analysis of Sequential Proximal Optimizing Technique Versus Kissing Balloon Inflation Technique in Provisional Bifurcation Stenting: Fractal Coronary Bifurcation Bench Test. JACC Cardiovasc Interv. 2015;8:1308-1317.
6. Maeng M, Hom NR, Erglis A, et al. Long-term results after simple versus complex stenting of coronary artery bifurcation lesions: Nordic Bifurcation Study 5-year follow-up results. J Am Coll Cardiol. 2013;62:30-34.
7. Hildick-Smith D, Behan MW, Lassen JF, et al. The EBC TWO Study (European Bifurcation Coronary TWO): A Randomized Comparison of Provisional T-Stenting Versus a Systematic 2 Stent Culotte Strategy in Large Caliber True Bifurcations. Circ Cardiovasc Interv. 2016;9:e003643.
8. Ferenc M, Gick M, Kienzle R-P, et al. Randomized trial on routine vs. provisional T-stenting in the treatment of de novo coronary bifurcation lesions. Eur Heart J. 2008;29:2859.
9. Ferenc M, Ayoub M, Büttner HJ, et al. Long-term outcomes of routine versus provisional T-stenting for de novo coronary bifurcation lesions: five-year results of the Bifurcations Bad Krozingen I study. EuroIntervention. 2015;11:856-859.
10. Erglis A, Kumsars I, Niemelä M, et al. Randomized comparison of coronary bifurcation stenting with the crush versus the culotte technique using sirolimus eluting stents: the Nordic stent technique study. Circ Cardiovasc Interv. 2009;2:27-34.
11. Gil RJ, Bil J, Kern A, Pawłowski T. First-in-man study of dedicated bifurcation cobalt-chromium sirolimus-eluting stent BIOSS LIM C® - Three-month results. Kardiol Pol. 2018;76:464-470.
12. Gil RJ, Bil J, Grundeken MJ, et al. Long-term effectiveness and safety of the sirolimus-eluting BiOSS LIM® dedicated bifurcation stent in the treatment of distal left main stenosis: an international registry. EuroIntervention. 2016;12:1246-1254.
13. Gil RJ, Bil J, Vassiliev D, Garcia LAI. First-in-man study of dedicated bifurcation sirolimus-eluting stent: 12-month results of BiOSS LIM® Registry. J Interv Cardiol. 2015;28:51-60.
14. Gil RJ, Bil J, Grundeken MJ, et al. Regular drug-eluting stents versus the dedicated coronary bifurcation sirolimus-eluting BiOSS LIM® stent: the randomised, multicentre, open-label, controlled POLBOS II trial. EuroIntervention. 2016;12:e1404-e1412.
15. Lefèvre T, Ormiston J, Guagliumi G, et al. The Frontier stent registry: safety and feasibility of a novel dedicated stent for the treatment of bifurcation coronary artery lesions. J Am Coll Cardiol. 2005;46:592-598.
16. Costa RA, Abizaid A, Abizaid AS, et al. Procedural and early clinical outcomes of patients with de novo coronary bifurcation lesions treated with the novel Nile PAX dedicated bifurcation polymer-free paclitaxel coated stents: results from the prospective, multicentre, non-randomised BIPAX clinical trial. EuroIntervention. 2012;7:1301-1309.
17. TCT-50: Complex Coronary Bifurcation Lesions Treated with the Novel Polymer-Free Dedicated Bifurcation Paclitaxel-Eluting Stent (Nile Pax): 9-Month Clinical and Angiographic Results of the Prospective, Multicenter BIPAX Clinical Trial. J Am Coll Cardiol. 2011;58:B15.
18. Verheye S, Agostoni P, Dubois CL, et al. 9-Month clinical, angiographic, and intravascular ultrasound results of a prospective evaluation of the Axxess self-expanding biolimus A9-eluting stent in coronary bifurcation lesions: the DIVERGE (Drug-Eluting Stent Intervention for Treating Side Branches Effectively) study. J Am Coll Cardiol. 2009;53:1031-1039.
19. Gil RJ, Bil J, Džavík V, et al. Regular Drug-Eluting Stent vs Dedicated Coronary Bifurcation BiOSS Expert Stent: Multicenter Open-Label Randomized Controlled POLBOS I Trial. Can J Cardiol. 2015;31:671-678.
20. Godino C, Al-Lamee R, C La Rosa C, et al. Coronary left main and non-left main bifurcation angles: how are the angles modified by different bifurcation stenting techniques? J Interv Cardiol. 2010;23:382-393.
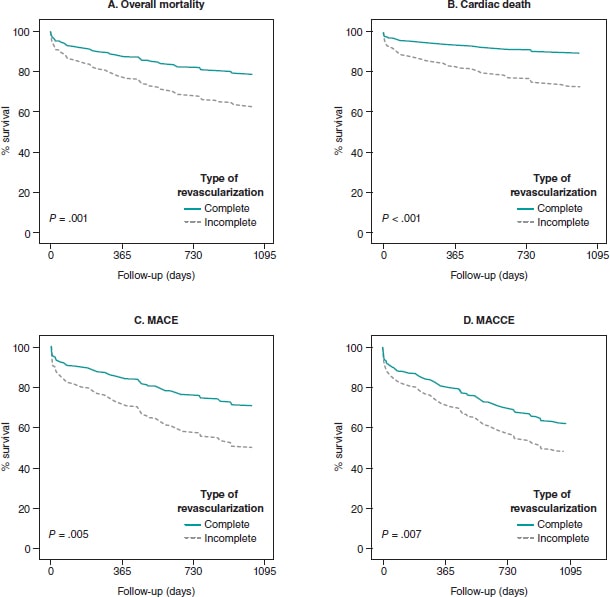
ABSTRACT
Introduction and objectives: The need for complete coronary artery revascularization after acute coronary syndrome in diabetic patients with multivessel coronary artery disease was discussed, even more so if they reflect the routine clinical practice (“real world”). Therefore, the objective of this study is to analyze cardiovascular complications in diabetics with and without complete revascularization included in clinical trials and in the routine clinical practice.
Methods: This was a single-center retrospective study of diabetic patients with multivessel coronary artery disease. We analyzed 733 diabetic patients: 299 (40.8%) with compatible criteria to be included in clinical trials, and 434 real-world patients (59.2%).
Results: Real-world patients make up 59.2% of the sample. They are characterized by a higher percentage of risk factors, older mean age, and more comorbidities. Diabetics with multivessel coronary artery disease included in the trials have a lower risk of overall mortality (HR, 0.30; 95%CI, 0.16-0.57; P < .001), cardiac death (HR, 0.33; 95%CI, 0.15-0.71; P = .03), and major adverse cardiovascular events (HR, 0.58; 95%CI, 0.38-0.86; P = .008). On the other hand, receiving complete revascularization reduces the risk of cardiac death (HR, 0.32; 95%CI, 0.13-0.83; P = .019), and major adverse cardiovascular events (HR, 0.50; 95%CI, 0.29-0.89; P = .017) in real-world diabetic patients.
Conclusions: It is suggested that fully revascularizing real-world patients would improve survival prognosis. In addition, diabetics included in clinical trials present fewer complications compared to those not included.
Keywords: Diabetes. Revascularization. Real world. Multivessel coronary artery disease.
RESUMEN
Introducción y objetivos: Se debate la necesidad de realizar revascularización coronaria completa tras un síndrome coronario agudo en pacientes diabéticos con enfermedad coronaria multivaso, y más aún si estos son reflejo de los pacientes de la práctica clínica habitual (mundo real). Por ello, el objetivo de este trabajo es analizar las complicaciones cardiovasculares en pacientes diabéticos con y sin revascularización completa incluibles en ensayos clínicos como de la práctica clínica habitual.
Métodos: Estudio unicéntrico retrospectivo de pacientes diabéticos con enfermedad coronaria multivaso. Se analizaron 733 pacientes diabéticos: 299 (40,8%) con criterios compatibles de inclusión de ensayos clínicos y 434 (59,2%) del mundo real.
Resultados: Los pacientes del mundo real constituyen el 59,2% de la muestra. Se caracterizan por presentar mayor porcentaje de factores de riesgo, mayor edad media y comorbilidad. Los diabéticos con enfermedad coronaria multivaso incluibles en ensayos tienen menor riesgo de mortalidad total (HR = 0,30; IC95%, 0,16-0,57; p < 0,001), de mortalidad de causa cardiaca (HR = 0,33; IC95%, 0,15-0,71; p = 0,03) y de sufrir eventos cardiovasculares adversos mayores (HR = 0,58; IC95%, 0,38-0,86; p = 0,008). Por otro lado, recibir revascularización completa desciende el riesgo de mortalidad de causa cardiaca (HR = 0,32; IC95%, 0,13-0,83; p = 0,019) y de eventos cardiacos adversos mayores (HR = 0,50; IC95%, 0,29-0,89; p = 0,017) en los pacientes diabéticos del mundo real.
Conclusiones: Se sugiere que revascularizar completamente a los pacientes del mundo real mejoraría el pronóstico en cuanto a supervivencia. Asimismo, los diabéticos incluibles en ensayos clínicos presentan menos complicaciones que los diabéticos no incluibles.
Palabras clave: Diabetes. Revascularización. Mundo real. Enfermedad coronaria multivaso.
Abbreviations
CR: complete revascularization. IR: incomplete revascularization. MACCE: major adverse cardiovascular and cerebrovascular events. MACE: major adverse cardiovascular events. MCAD: multivessel coronary artery disease.
INTRODUCTION
Cardiovascular diseases—the most prominent of which is ischemic heart disease (IHD)—are a problem of global health and responsible for 1 out of every 3 premature deaths worldwide.1 In Spain, IHD is thought to increase health spending and morbidity due to the ageing of the population, and the greater number of survivors.2
Diabetes mellitus is closely associated with ischemic heart disease. It makes patients—most of them elderly patients—have a very high cardiovascular risk.3 Hypoglycemia and hyperinsulinemia are both associated with a higher risk of developing multivessel coronary artery disease (MCAD).4 In the long-term, this leads to a grim prognosis and more cardiovascular death in these diabetic patients.5
Due to the high incidence rate of MCAD, several studies have been conducted to see what type of revascularization is the most suitable one for the profile of these patients. It has been suggested that anatomic complete revascularization (CR) is associated with a lower rate of major adverse cardiovascular events (MACE)—a composite endpoint of death, non-fatal myocardial infarction, and need for new revascularization—compared to anatomic incomplete revascularization (IR).6 As a matter of fact, it has been reported that the risk of MACE increases significantly when performing IR with minimal residual disease in coronary vessels.7 Therefore, the treatment recommended is to perform anatomic CR. When this is not feasible, functional CR—currently widely used—is advised.
On the one hand, in patients with stable angina refractory to conservative treatment or non-ST-segment elevation acute coronary syndrome (NSTEACS), single-stage CR is advised through surgical coronary revascularization or percutaneous coronary intervention.8 On the other hand, in patients with ST-segment elevation acute coronary syndrome (STEACS) staged CR has been suggested by treating, first of all, the culprit coronary artery causing the clinical signs, and then the remaining stenotic arteries.9 However, in the routine clinical practice, it has been observed that patients with greater comorbidities and worse prognosis are often treated with IR, which worsens even more their clinical evolution since the survival associated with cardiac death, and MACE is lower.10
However, one of the main problems we face is passing scientific knowledge from clinical trials on to the routine clinical practice. It has been reported that most of the patients from cardiology units meet some of the exclusion criteria posed by these clinical trials. Such patients are highly heterogeneous, older, and have several cardiovascular risk factors and concomitant diseases, which worsens their prognosis. Therefore, the findings from clinical trials should be used with caution in the overall population.11,12
Thus, the objective of this study was to analyze whether there are significant differences regarding mortality and cardiovascular events between patients treated with CR or IR, which is why patients with clinical trial inclusion criteria and patients with characteristics from the routine clinical practice (real world) were included.
METHODS
This was a retrospective, single-center study that used data anonymization and included 733 diabetic patients with MCAD treated with coronary angiography from January 1, 2012 through December 31, 2014. Participants were divided into 2 groups based on whether or not they met the FREEDOM clinical trial inclusion criteria.13 In this study, those who met these criteria were considered participants eligible for clinical trials while the rest (the non-eligible ones) were categorized as patients from the routine clinical practice or real-world patients.
Due to data anonymization, it was not necessary to request any approval from the ethics committee since it had already been obtained by Chueca González et al.10 who used the same patient selection. The different informed consents authorize us to treat data to conduct this study.
Study population
Patients over 18 years old with an indication for revascularization—both percutaneous coronary intervention and surgery—due to acute coronary syndromes (STEACS, NSTEACS or unstable angina), refractory stable angina, refractory heart failure, valvular heart disease, cardiac arrest, new revascularization or cardiogenic shock were included in the study Patients with previous cardiac surgeries due to coronary artery disease and valvular heart disease, as well as patients with valvular heart diseases plus a surgical indication were excluded from the study.
The following were categorized as real-world patients (non-FREEDOM): those with STEACS within the 72 previous hours, those with a past medical history of percutaneous transluminal angioplasty, stroke or major bleeding within the previous 6 months, those with functional class III or IV according to the New York Heart Association, in-stent restenosis, known dementia or dependency, at least moderate, according to the Barthel index, and finally those with an extracardiac disease (chronic obstructive pulmonary disease, hepatopathies, chronic kidney disease) bringing survival under 5 years. All the criteria developed have been described previously.10
Definitions
When coronary angiography was performed, patients were considered diabetic if they had already been diagnosed as such in their health record or if they were on hypoglycemic treatments like diet, oral antidiabetic drugs or insulin.
MCAD consists of the existence of ≥ 70% luminal stenosis in 2 or more epicardial vessels covering, at least, 2 or more different coronary artery territories. Also, such lesions are prone to revascularization via angioplasty and surgery according to the clinical practice guidelines and the criterion of interventional cardiologists and cardiac surgeons.
Coronary lesions were treated with anatomic CR. Epicardial vessels > 2 mm in caliber with > 70% stenosis were treated regardless of whether the areas compromised were viable or necrotic.
The indication to perform the coronary angiography was given based on the patient’s clinical course that led to his admission. Acute coronary syndromes with and without ST-segment elevation according to the third universal definition of infarction were included.14 Also, this test was performed on other diseases like unstable angina, refractory stable angina to medical treatment, and other (decompensated heart failure, cardiogenic shock, cardiac arrest, ventricular arrhythmias).
Left ventricular ejection fraction was categorized as dichotomic with values ≥ 40% or < 40%.
To assess the stage of kidney failure the glomerular filtration rate was estimated using the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) equation. It was defined as reduced with glomerular filtration rates < 45 mL/min/1.73 m3, which is consistent with stage 3B chronic kidney disease.
Regarding the variables that should be measure at the follow-up, events such as death, non-fatal myocardial infarction, and new revascularization were categorized as MACE. On the other hand, major adverse cardiovascular and cerebrovascular events (MACCE) were considered a composite endpoint of death, non-fatal myocardial infarction, new revascularization, and stroke.
Statistical analysis
All statistical analyses were conducted using the statistical software package SPSS version 21.0 for Windows. Quantitative variables were studied using the Student t test for independent samples, and expressed as mean ± standard deviation. Cualitative variables were compared using the chi-square test and expressed as percentages and absolute numbers. Also, the odds ratios (OR) were obtained too. Multivariate analysis was conducted through survival analysis using Cox regression method. Also, survival charts and hazard ratios (HR) with their corresponding 95% confidence intervals were obtained for the significant covariables of the univariate analysis. P values ≤ .05 were considered statistically significant.
RESULTS
A total of 733 diabetic patients met the inclusion criteria to participate in the study. A total of 299 of these patients (40.8%) had criteria that were compatible with the clinical trials, and 434 (59.2%) with the real world. The presence of CR was less common in both groups compared to participants treated with IR. Among the patients in whom CR was achieved, this type of revascularization turned out to be more common in those with criteria from the FREEDOM trial (43.5%).
Patients’ baseline characteristics
Multiple parameters collected at admission were analyzed to perform coronary angiography (table 1).
Table 1. Patients’ baseline characteristics based on compliance of the FREEDOM criteria
| Total | CR in patients from CT | IR in patients from CT | P | Total | CR in patients from the RCP | IR in patients from the RCP | P | |
|---|---|---|---|---|---|---|---|---|
| N | 40.8 (299) | 43.5% (130) | 56.5% (169) | < .001 | 59.2% (434) | 28.3% (123) | 71.7% (311) | < .001 |
| Age | 66.2 ± 9 | 64.1 ± 9.1 | 68 ± 8.4 | < .001 | 69.8 ± 9.6 | 67.5 ± 9.9 | 70.7 ± 9.3 | .002 |
| + 80 years | 6 (18) | 16.7 (3) | 83.3 (15) | .10 | 15.4 (67) | 17.9 (12) | 82.1 (55) | .364 |
| Woman | 30.1 (90) | 40 (36) | 60 (54) | .45 | 31.1 (135) | 28.1 (38) | 71.9 (97) | .95 |
| 3 vessels | 44.8 (134) | 30.6 (41) | 69.4 (93) | < .001 | 54.6 (171) | 35.3 (30) | 61.8 (141) | < .001 |
| Indication | .42 | .01 | ||||||
| STEACS | 2(6) | 16.7 (1) | 83.3 (5) | 32.5 (141) | 39 (55) | 61 (86) | ||
| NSTEACS | 35.5 (106) | 40.6 (43) | 59.4 (63) | 32 (139) | 19.4 (27) | 80.6 (112) | ||
| Unstable angina | 28.4 (85) | 47.1 (40) | 52.9 (45) | 15.7 (57) | 27.9 (19) | 72.1 (49) | ||
| Stable angina | 28.1 (84) | 47.6 (40) | 52.4 (44) | 13.1 (57) | 29.8 (17) | 70.2 (40) | ||
| LVEF < 40% | 14.4 (43) | 32.6 (14) | 67.4 (29) | .14 | 29.8 (129) | 21. (28) | 78.3 (101) | .048 |
| Hypertension | 84.3 (252) | 42.9 (108) | 57.1 (144) | .63 | 84.1 (365) | 26 (95) | 74 (270) | .046 |
| Dyslipidemia | 66.9 (200) | 45 (90) | 55 (110) | .46 | 61.1 (265) | 25.7 (68) | 74.3 (197) | .127 |
| Obesity | 28.1 (84) | 52.4 (44) | 47.6 (40) | .07 | 27.9 (121) | 27.3 (33) | 72.7 (88) | .813 |
| Tobacco use history | 46.5 (139) | 51.1 (71) | 48.9 (68) | .019 | 43.5 (189) | 29.1 (55) | 70.9 (134) | .078 |
| Treatment of DM | 43.5 (130) | 56.5 (169) | .67 | 28.3 (123) | 71.7 (311) | .83 | ||
| Diet | 7.7 (23) | 52.2 (12) | 47.8 (11) | 31.8 (7) | 68.2 (15) | |||
| Oral antidiabetic drugs | 65.9 (197) | 43.1 (85) | 56.9 (112) | 27.3 (73) | 72.7 (194) | |||
| Insulin | 26.4 (79) | 41.8 (33) | 58.2 (46) | 29.7 (43) | 70.3 (102) | |||
| Previous infarction | 12.7 (38) | 28.9 (11) | 71.1 (27) | .053 | 17.3 (75) | 32 (24) | 68 (51) | .439 |
| Heart failure | 4.3 (13) | 23.1 (3) | 76.9 (10) | .129 | 9.2 (40) | 7.5 (3) | 92.5 (37) | .002 |
| Peripheral arterial disease | 8.4 (25) | 32 (8) | 68 (17) | .227 | 13.8 (60) | 15 (9) | 85 (51) | .014 |
| Stroke | 6.4 (19) | 31.6 (6) | 68.4 (13) | .28 | 9.4 (41) | 22 (9) | 78 (32) | .34 |
| COPD | 13.7 (41) | 46.3 (19) | 53.7 (22) | .691 | 18.7 (81) | 21 (17) | 79 (64) | .103 |
| GFR < 45 | 7.4 (22) | 18.2 (4) | 81.8 (18) | .013 | 13.8 (60) | 16.7 (10) | 83.3 (50) | .031 |
| Previous PCI | 17.1 (51) | 35.3 (18) | 64.7 (33) | .195 | 22.1 (96) | 25 (24) | 75 (72) | .41 |
| EuroSCORE II | 2.27±2.27 | 1.84 ± 1.62 | 2.59 ± 2.63 | .004 | 7.57 ± 11.2 | 5.71 ± 7.98 | 8.39 ± 12.24 | .025 |
|
COPD, chronic obstructive pulmonary disease; CR, complete revascularization; CT, clinical trials; DM, diabetes mellitus; GFR, glomerular filtration rate; IR, incomplete revascularization; LVEF, left ventricular ejection fraction; NSTEACS, non-ST-segment elevation acute coronary syndrome; PCI, percutaneous coronary intervention; RCP, routine clinical practice; STEACS, ST-segment elevation acute coronary syndrome. Data are expressed as no. (%). |
||||||||
Differences were found when patients were compared based on their clinical characteristics (patients from clinical trials and from the real world) and type of revascularization received. The most significant differences were found in real-world patients. On the one hand, mean age (69.8 years) was older with more patients > 80 years compared to the group with clinical trial criteria (mean, 66.2 years). On the other hand, in this group there was a higher incidence rate of coronary artery disease with damage to 3 vessels (54.6%) compared to patients from clinical trials (44.8%). Regarding the indication of coronary angiography, a higher rate of acute coronary syndromes—both STEACS and NSTEACS—in such subgroup was reported. They were eventually treated with IR (61% and 80.6%; P < .001) more often. However, patients with characteristics from clinical trials had NSTEACS, and unstable and stable angina as the main indications for coronary angiography. In these patients, CR was achieved more often compared to real-world diabetics.
Major events at 30-day and 35-month follow-up
When it comes to patients with clinical trial inclusion criteria, achieving CR suggested the occurrence of fewer major events—both overall mortality and cardiovascular system-related mortality—especially at 35-month follow-up without any significant differences being reported (table 2).
Table 2. Major events at 30-day and 35-month follow-up in patients with criteria from the FREEDOM clinical trial
| Event | 30 days | P | 35 months | P | ||
|---|---|---|---|---|---|---|
| CR | IR | CR | IR | |||
| Mortality | 0.4 (1) | 0.4 (1) | .863 | 2.8 (5) | 6.7 (12) | .285 |
| Cardiac death | 0 | 0.4 (1) | .375 | 1.1 (2) | 4.5 (8) | .154 |
| Acute myocardial infarction | 0.4 (1) | 0.7 (2) | .715 | 2.4 (4) | 5.4 (9) | .359 |
| Stroke | 0 | 0.7 (2) | .209 | 1.2 (2) | 1.9 (3) | .883 |
| MACE | 0.7 (2) | 1.5 (4) | .601 | 9.7 (19) | 17.9 (35) | .348 |
| MACCE | 0.7 (2) | 2.2 (6) | .277 | 11.2 (22) | 18.9 (37) | .451 |
|
CR, complete revascularization; IR, incomplete revascularization; MACE, major adverse cardiovascular events (death, non-fatal myocardial infarction, and need for new revascularization); MACCE, major adverse cardiovascular and cerebrovascular events (death, non-fatal myocardial infarction, need for new revascularization and stroke). Data are expressed as no. (%). |
||||||
The same tendency was seen in real-world patients. Therefore, CR reduced the risk of overall mortality (OR, 0.84; 95%CI, 0.74-0.95; P = .006), cardiac death (OR, 0.81; 95%CI, 0.73-0.91; P = .002), and MACE (OR, 0.84; 95%CI, 0.74-0.96; P = .012) at 35-month follow-up (table 3). In the survival analysis, the same tendency was found in the said subgroup of participants at 35-month follow-up. CR reduced the risk of cardiac death (HR, 0.35; 95%CI, 0.13-0.90; P = .029), and MACE (HR, 0.5; 95%CI, 0.28-0.89; P = .019). Similarly, EuroSCORE-II > 5 increased the risk of cardiac death (HR, 2.74; 95%CI, 1.11-6.75; P = .028). Finally, a higher risk of MACE (HR, 2.08; 95%CI, 1.03-4.23; P = .042), and MACCE (HR, 2.36; 95%CI, 1.13-4.95; P = .023) was reported (figure 1).
Table 3. Major events at 30-day and 35-month follow-up in patients from the routine clinical practice
| Event | 30 days | P | 35 months | P | ||
|---|---|---|---|---|---|---|
| CR | IR | CR | IR | |||
| Mortality | 1.8 (6) | 8.5 (29) | .257 | 5.5 (14) | 38.4 (98) | .006 |
| Cardiac death | 1.5 (5) | 8.1 (27) | .195 | 3.2 (8) | 31.2 (78) | .002 |
| Acute myocardial infarction | 0.7 (3) | 1.7 (7) | .911 | 7.7 (21) | 17.2 (47) | .626 |
| Stroke | 0 | 0.3 (1) | .522 | 1.5 (3) | 4.9 (10) | .650 |
| MACE | 1.7 (5) | 7.9 (24) | .225 | 6.5 (15) | 40 (92) | .012 |
| MACCE | 1.7 (5) | 7.9 (24) | .225 | 8.3% (19) | 41.3 (95) | .089 |
|
CR, complete revascularization; IR, incomplete revascularization; MACE, major adverse cardiovascular events (death, non-fatal myocardial infarction, and need for new revascularization; MACCE, major adverse cardiovascular and cerebrovascular events (death, non-fatal myocardial infarction, need for new revascularization and stroke).Data are expressed as no. (%). |
||||||
Figure 1. Evolution of survival in diabetic patients from the routine clinical practice with multivessel coronary artery disease. MACE, major adverse cardiovascular events (death, non-fatal myocardial infarction, and need for new revascularization); MACCE, major adverse cardiovascular and cerebrovascular events (death, non-fatal myocardial infarction, need for new revascularization and stroke).
When survival was analyzed in the 4 groups of patients (figure 2), those with FREEDOM clinical trial inclusion criteria had a lower risk of overall mortality (HR, 0.30; 95%CI, 0.16-0.57; P < .001), cardiac death (HR, 0.33; 95%CI, 0.15-0.71; P = .03), MACE (HR, 0.58; 95%CI, 0.38-0.86; P = .008), and MACCE (HR, 0.59; 95%CI, 0.40-0.89; P = .01). On the one hand, achieving CR lowered the risk of cardiac death (HR, 0.32; 95%CI, 0.13-0.83; P = .019), and MACE (HR, 0.50; 95%CI, 0.29-0.89; P = .017). On the other hand, EuroSCORE > 5 when coronary angiography was performed was associated with higher rates of overall mortality (HR, 2.16; 95%CI, 1.06-4.41; P = .034), cardiac death (HR, 3.48; 95%CI, 1.49-8.16; P = .004), MACE (HR, 2.18; 95%CI, 1.26-3.78; P = .005), and MACCE (HR, 2; 95%CI, 1.18-3.40; P = .011). It was reported that if the patient had heart failure, this increased the risk of MACE (HR, 2.44; 95%CI, 1.25-4.74; P = .009), and MACCE (HR, 2.77; 95%CI, 1.39-5.53; P = .004). On the remaining variables, a lower overall mortality rate was confirmed if the patient was a non-smoker (HR, 0.46; 95%CI, 0.24-0.89; P = .02), and a higher risk of MACCE was reported if he had hypertension (HR, 1.50; 95%CI, 1.01-2.24; P = .049) or 3-vessel disease (HR, 1.44; 95%CI, 1.06-1.97; P = .022).
Figure 2. Evolution of survival in diabetic patients con multivessel coronary artery disease. CT, clinical trials; CR, complete revascularization; IR, incomplete revascularization; MACCE, major adverse cardiovascular and cerebrovascular events (death, non-fatal myocardial infarction, need for new revascularization and stroke); MACE, major adverse cardiovascular events (death, non-fatal myocardial infarction, and need for new revascularization); RCP, routine clinical practice.
Type of procedure received
Regarding the therapies received, we found a higher rate of percutaneous coronary interventions (80.8%) performed in all the study subgroups. Real-world patients were treated with coronary artery bypass graft more often compared to patients from clinical trials. Finally, conservative treatment was more common (9%) in real-world patients with IR (table 4).
Table 4. Type of procedures performed per group of patients
| Treatment | CR in patients from CT | IR in patients from CT | CR in patients from the RCP | IR in patients from the RCP | Total |
|---|---|---|---|---|---|
| Conservative | 0 | 4.1 (7) | 0 | 9 (28) | 4.8 (35) |
| PCI | 78.5 (102) | 87 (147) | 76.4 (94) | 80.1 (249) | 80.8 (592) |
| CABG | 21.5 (28) | 8.9 (15) | 23.6 (29) | 10.9 (34) | 14.5 (106) |
|
CABG, coronary artery bypass graft; CR, complete revascularization; CT, clinical trials; IR, incomplete revascularization; PCI, percutaneous coronary intervention; RCP, routine clinical practice.Data are expressed as no. (%). |
|||||
DISCUSSION
The main conclusions of this study are: a) although patients potentially eligible for clinical trials receive CR more often no significant differences have been found regarding survival or adverse cardiovascular events; b) real-world patients are treated with IR more often. In these patients, less mortality has been suggested, both overall and cardiac, as well as fewer MACE at 35-month follow-up have been reported if CR is achieved; c) patients with FREEDOM clinical trial criteria have higher survival rates compared to real-world diabetics; d) most patients are treated with percutaneous coronary intervention.
One of the main problems when analyzing the repercussions of CR is the lack of clinical trials with patients similar to those found in the routine clinical practice or the real word. This complicates the extrapolation of results to the overall population since, in most studies, homogeneous participants can be found often with a better clinical profile.11,12 This reality would explain why CR does not improve the survival rate of such patients who are younger and have fewer comorbidities and cardiovascular risk factors, while in diabetics from the routine clinical practice CR does provide improvements because these are older patients with more MCAD and comorbidities (reduced ejection fraction, arterial hypertension, heart failure, peripheral arterial disease, and chronic kidney disease with, at least, a 3B stage). However, the current scientific evidence available recommends performing a therapeutic effort to achieve CR. It has been suggested that it improves survival in both overall mortality and cardiac death in real-world patients, and avoids IR that is an independent predictor of mortality (HR, 2.46; 95%CI, 1.46-4.13; P = .001).10 On the other hand, The Complete Trial9 confirmed less cardiac death and fewer new reinfarctions (HR, 0.74; 95%CI, 0.60-0.91; P = .004) when CR was performed in patients with STEACS compared to patients in whom only the culprit vessel was treated. Finally, a recent metanalysis confirmed that CR also reduces the overall mortality rate (RR, 0.73; 95%CI, 0.66-0.81), the need for new revascularizations (RR, 0.77; 95%CI, 0.66-0.88), and the occurrence of new myocardial infarctions (RR, 0.74; 95%CI, 0.64-0.85).15
In the first place, this study included diabetic patients who would not be eligible for clinical trials because they are a too heterogeneous population whose characteristics and comorbidities resemble those of real-world patients too much. Secondly, the study deals with a discussed topic these days because cardiovascular diseases, ischemic heart disease among them, are the leading cause of death in developed countries. Diabetes is especially associated with it most often leading to MCAD. Therefore, it is necessary to assess whether CR provides benefits regarding survival always focusing on the patient and his clinical-functional status because, at times, there is controversy on whether to treat all lesions or only the culprit lesions causing the problem.
Finally, if CR provides the benefits suggested in this and other studies, it could improve the survival of diabetic patients and their quality of life and that of their relatives. Also, it would reduce the health spending and the years of life lost or the disability-adjusted years of life by reducing mortality, the need for reinterventions to treat new infarctions and strokes, as well as the need for new revascularizations.
Limitations
This study was conducted from a statistical analysis of a database already used by Chueca González et al.10. It was a retrospective, single-center registry, which limits the possibilities of establishing causality and extrapolating the results to the overall population. Similarly, since the population was designed for a different study, statistical power was probably lost since the size of the sample and the characteristics of the participants included are different from the ones a study like the present one would require.
Another aspect we should mention is the technology of the stents currently used compared to those used during this study recruitment process. Since they appeared, different types and generations of stents associated with different antiproliferative drugs have been manufactured. This has improved secondary survival and minimized the occurrence of in-stent stenosis. Therefore, the study results could be different compared to those obtained today.
Finally, the definitions of CR vary based on the study. Some recommend it in the presence of > 50% occlusions of luminal diameter. Others with occlusions > 70%. In some cases, only coronary vessels with minimum diameters of 2 mm are considered. In other cases, these diameters need to be 1.5 mm. Specifically, this study only considered vessels with stenosis > 70% with minimum calibers of 2 mm.
CONCLUSIONS
This study suggests that diabetics eligible for clinical trials have fewer complications compared to non-eligible diabetic patients. Also, this suggests that real-world diabetics have worse prognosis in case of MCAD. Under this circumstance, it is suggested that achieving CR would improve their long-term survival.
In conclusion, further studies, and clinical trials including real-world patients are advised. Also, they would need to include updated diagnostic criteria and new therapeutic techniques—both pharmacological and interventional—to obtain new evidence to guide us on the therapeutic effort needed to treat patients who require coronary artery revascularization whether through angioplasty or surgery.
FUNDING
This study was funded by the Cardiovascular Biomedical Research Center Network (CB16/11/00360), Instituto de Salud Carlos III. Also, it has been co-funded by the European Regional Development Fund.
The statistical analysis of this manuscript was funded by the Chair of Advanced Therapies in Cardiovascular Diseases at Universidad de Málaga, Spain (CIF Q-2918001-E).
AUTHORS’ CONTRIBUTIONS
F. Puyol-Ruiz: study design, data curation, and drafting of the manuscript. M. Jiménez-Navarro: study design, data curation, and critical review of the manuscript. EM. Chueca-González, F. Carrasco-Chinchilla, J. L. López-Benítez, J. H. Alonso-Briales, J. M. Melero- Tejedor, and J. M. Hernández-García: data curation.
CONFLICTS OF INTEREST
None reported.
ACKNOWLEDGEMENTS
We wish to thank María Jiménez Salva for her constant support and collaboration while drafting this manuscript.
WHAT IS KNOWN ABOUT THE TOPIC?
- Due to the ageing of the population, health spending and morbidity due to ischemic heart disease and diabetes are expected to grow.
- Diabetes increases the number of cases of MCAD, which is associated with a worse prognosis.
- Results from clinical trials should be applied to real-world patients with caution.
WHAT DOES THIS STUDY ADD?
- More than half of diabetic patients with MCAD had exclusion criteria to participate in the FREEDOM clinical trial
- Real-world diabetics have a worse prognosis against MCAD.
- Anatomic CR reduces the risk of cardiac death and MACE at 35-month follow-up.
- More studies and clinical trials are needed with real-world patients.
REFERENCES
1. Roth GA, Mensah GA, Johnson CO, et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019: Update From the GBD 2019 Study. J Am Coll Cardiol. 2020;76:2982-3021.
2. Dégano IR, Elosua R, Marrugat J. Epidemiología del síndrome coronario agudo en España: estimación del número de casos y la tendencia de 2005 a 2049. Rev Esp Cardiol. 2013;66:472-481.
3. Ruiz-García A, Arranz-Martínez E, García-Álvarez JC, et al. Prevalence of diabetes mellitus in Spanish primary care setting and its association with cardiovascular risk factors and cardiovascular diseases. SIMETAP-DM study. Clin Investig Arterioscler. 2020;32:15-26.
4. Adeva-Andany MM, Funcasta-Calderón R, Fernández-Fernández C, Ameneiros-Rodríguez E, Domínguez-Montero A. Subclinical vascular disease in patients with diabetes is associated with insulin resistance. Diabetes Metab Syndr Clin Res Rev. 2019;13:2198-2206.
5. Burgess S, Juergens CP, Yang W, et al. Cardiac mortality, diabetes mellitus, and multivessel disease in ST elevation myocardial infarction. Int J Cardiol. 2021;323:13-18.
6. Hosoyama K, Maeda K, Saiki Y. What does complete revascularization mean in 2021? – Definitions, implications, and biases. Curr Opin Cardiol. 2021;36:748-754.
7. Burgess SN, French JK, Nguyen TL, et al. The impact of incomplete revascularization on early and late outcomes in ST-elevation myocardial infarction. Am Heart J. 2018;205:31-41.
8. Gaba P, Gersh BJ, Ali ZA, Moses JW, Stone GW. Complete versus incomplete coronary revascularization: definitions, assessment and outcomes. Nat Rev Cardiol. 2021;18:155-168.
9. Mehta SR, Wood DA, Storey RF, et al. Complete Revascularization with Multivessel PCI for Myocardial Infarction. N Engl J Med. 2019;381:1411-1421.
10. Chueca González EM, Carrasco Chinchilla F, López Benítez JL, et al. Enfermedad coronaria multivaso en el paciente diabético en la vida real: ¿eficacia o efectividad? REC CardioClinics. 2019;54:81-90.
11. Wasilewski J, Polon´ski L, Lekston A, et al. Who is eligible for randomized trials? A comparison between the exclusion criteria defined by the ISCHEMIA trial and 3102 real-world patients with stable coronary artery disease undergoing stent implantation in a single cardiology center. Trials. 2015;16:1-7.
12. Laursen PN, Holmvang L, Lønborg J, et al. Comparison between patients included in randomized controlled trials of ischemic heart disease and real-world data. A nationwide study. Am Heart J. 2018;204:128-138.
13. Goel SS, Shishehbor MH. Strategies for multivessel revascularization in patients with diabetes. Cardiol Rev. 2013;29:2375-2384.
14. Thygesen K, Alpert JS, Jaffe AS, et al. Documento de consenso de expertos. Tercera definición universal del infarto de miocardio. Rev Esp Cardiol. 2013;66:1-15.
15. Zimarino M, Ricci F, Romanello M, Di Nicola M, Corazzini A, De Caterina R. Complete myocardial revascularization confers a larger clinical benefit when performed with state-of-the-art techniques in high-risk patients with multivessel coronary artery disease: A meta-analysis of randomized and observational studies. Catheter Cardiovasc Interv. 2016;87:3-12.
- Rationale and study design on the effectiveness of vasodilators and topical local anesthetics to prevent radial spasm. The E-RADIAL trial
- Effectiveness of spherical tip noncompliant balloon for stent postdilatation: the REPIC02-RECONQUISTHA study
- Clinical predictors and angiographic features of acute myocardial infarction due to systemic embolism
- Incidence, morbidity and mortality, and management of acute coronary syndrome during the time of COVID-19 lockdown
Editorials
Expanding the role of drug-coated balloons in native large coronary artery disease
aDepartment of Biomedical Sciences, Humanitas University, Pieve Emanuele, Milan, Italy
bCardio Center, IRCCS Humanitas Research Hospital, Rozzano, Milan, Italy
cDivision of Cardiology, Montefiore Medical Center, Bronx, New York, United States
Original articles
Editorials
The role of angiography-derived physiological assessment techniques in the post-FAVOR III Europe era?
aServicio de Cardiología, Hospital General Universitario Gregorio Marañón, Instituto de Investigación Sanitaria Gregorio Marañón, Universidad Complutense de Madrid, Madrid, Spain
bCentro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), Instituto de Salud Carlos III, Madrid, Spain
Interviews
An interview with Camino Bañuelos
aServicio de Cardiología, Hospital Clínico San Carlos, Instituto de Investigación Sanitaria del Hospital Clínico San Carlos (IdISSC), Madrid, Spain
bServicio de Cardiología, Hospital Clínico Universitario de Santiago de Compostela, Santiago de Compostela, A Coruña, Spain