Article
Ischemic heart disease and acute cardiac care
REC Interv Cardiol. 2019;1:21-25
Access to side branches with a sharply angulated origin: usefulness of a specific wire for chronic occlusions
Acceso a ramas laterales con origen muy angulado: utilidad de una guía específica de oclusión crónica
Servicio de Cardiología, Hospital de Cabueñes, Gijón, Asturias, España

ABSTRACT
Introduction and objectives: The use of transradial access for percutaneous coronary procedures has increased due to its advantages over the femoral approach. However, this benefit comes at the expense of a higher rate of radial artery occlusion (RAO). Our objective was to assess the incidence and predictors of RAO following transradial catheterization. Additionally, we studied anatomic variations of the radial artery (RA).
Methods: This prospective study enrolled 427 patients who underwent coronary angiography or angioplasty via transradial access. The forearm arteries were evaluated by ultrasound. If RAO was present, follow-up ultrasound examinations were performed at 1 and 3 months postprocedure.
Results: Our study population included 288 men (67.4%) and 139 women (32.6%). The mean age was 61.9 ± 11.1 years. RAO occurred in 48 patients (11.24%), and spontaneous recanalization was observed within 3 months in 15 patients (32.6%). On multivariate analysis, independent predictors of RAO were younger age (OR, 0.642; 95%CI, 0.480-0.858; P = .031), low periprocedural systolic blood pressure (OR, 0.598; 95%CI, 0.415-0.862; P = .007), a small radial diameter (OR, 0.371; 95%CI, 0.323-0.618; P = .031), insufficient anticoagulation (OR, 0.287; 95%CI, 0.163-0.505; P < .001), occlusive hemostasis (OR, 0.128; 95%CI, 0.047-0.353; P < .001), and long duration of hemostasis. The overall incidence of RA anatomic variations was 14.8% (n = 63). Among these, 40 patients (63.5%) had a high radial origin, 18 (28.6%) had extreme RA tortuosity, and 5 (7.9%) had a complete radioulnar loop.
Conclusions: The main modifiable predictors of RAO are insufficient heparinization and occlusive hemostasis. Preventive strategies should focus primarily on these 2 predictive factors to reduce the risk of RAO.
Keywords: Anatomic variations. Cardiac catheterization. Doppler ultrasound. Percutaneous coronary intervention. Predictors. Radial artery occlusion. Transradial access.
RESUMEN
Introducción y objetivos: El acceso transradial para procedimientos coronarios percutáneos ha crecido en popularidad debido a sus ventajas sobre el abordaje femoral. Sin embargo, este beneficio se ve ensombrecido por una mayor tasa de oclusión de la arteria radial (OAR). Nuestro objetivo fue evaluar la incidencia y los factores predictivos de OAR tras el cateterismo transradial. También se estudiaron las variaciones anatómicas de la arteria radial (AR).
Métodos: En este estudio prospectivo participaron 427 pacientes a los que se había realizado angiografía coronaria o angioplastia mediante acceso transradial. Se realizó una evaluación ecográfica de las arterias del antebrazo. En caso de OAR, se llevó a cabo otro control ecográfico al mes y a los 3 meses de la intervención.
Resultados: La población de estudio incluyó a 288 varones (67,4%) y 139 mujeres (32,6%). La edad media fue de 61,9 ± 11,1 años. La OAR se produjo en 48 pacientes (11,24%), de los cuales en 15 (32,6%) se produjo recanalización espontánea en el plazo de 3 meses. En el análisis multivariante, la edad más joven (OR = 0,642; IC95%, 0,480-0,858; p = 0,031), la presión arterial sistólica periprocedimiento baja (OR = 0,598; IC95%, 0,415-0,862; p = 0,007), el diámetro radial pequeño (OR = 0,371; IC95%, 0,323-0,618; p = 0,031), la anticoagulación insuficiente (OR = 0,287; IC95%, 0,163-0,505; p < 0,001), la hemostasia oclusiva (OR = 0,128; IC95%, 0,047-0,353; p < 0,001) y la larga duración de la hemostasia aparecieron como predictores independientes de OAR. La incidencia global de variaciones anatómicas de la AR fue del 14,8% (n = 63). Entre estos pacientes, 40 (63,5%) tenían un origen radial alto, 18 (28,6%) presentaban una tortuosidad extrema de la AR y 5 (7,9%) tenían un asa radiocubital completa.
Conclusiones: La heparinización insuficiente y la hemostasia oclusiva son los principales predictores de OAR modificables. La estrategia preventiva debe centrarse principalmente en estos 2 factores predictivos.
Palabras clave: Variaciones anatómicas. Cateterismo cardiaco. Ecografía Doppler. Intervención coronaria percutánea. Predictores. Oclusión de la arteria radial. Acceso transradial.
Abbreviations
RA: radial artery. RAO: radial artery occlusion.
INTRODUCTION
The use of the transradial approach for coronary interventions has become increasingly widespread in interventional cardiology due to its numerous advantages.1 As a result, current guidelines recommend it as the first-line approach.2
However, the benefits of this technique are tempered by the risk of radial artery occlusion (RAO), with reported rates ranging from 5% to 30%.3,4 The aim of this study was to assess the incidence and predictors of RAO following transradial catheterization using Doppler ultrasound for evaluation.
METHODS
Patient population
This longitudinal, single-center prospective study was conducted in the cardiology department of the Military Central Hospital in Algiers. After applying exclusion criteria (hemodynamic instability and ST-segment elevation myocardial infarction), we included 427 consecutive patients undergoing transradial coronary procedures between January 2019 and March 2020. The study adhered to the principles of the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practices and was approved by the local ethics committee. All patients provided written informed consent.
Radial artery cannulation and retrograde radial arteriography
After radial artery (RA) puncture, a radial hydrophilic sheath (Radiofocus II, TERUMO Medical, Japan, or Prelude, MERIT Medical, United States) was introduced. An antispastic cocktail was then administered into the RA through the sheath, consisting of a saline solution, a vasodilator (1 mL of nicardipine), and a bolus of unfractionated heparin, which was administered either intravenously or directly into the RA as part of the spasmolytic cocktail, depending on the operator’s preference. In patients on vitamin K antagonists, these medications were not discontinued prior to the procedure.
Retrograde radial arteriography was performed by injecting a mixture of 4 mL of contrast and 4 mL of isotonic saline through the sheath. Radiographic images were then obtained in an anteroposterior projection.
Transradial coronary procedure
The standard approach was conventional right radial access. For coronary angiography, 5-French (Fr) hydrophilic sheaths and catheters were usually used. If the patient required revascularization, an ad hoc percutaneous coronary intervention was performed, using 6-Fr guiding catheters after exchanging the sheath from 5-Fr to 6-Fr. The usual dose of heparin is 5000 IU (2500 IU for oral anticoagulation with a vitamin K antagonist).
Hemostasis procedure
At the end of the procedure, the sheath was removed, and hemostasis was achieved using a hemostatic compression device (TR BAND, TERUMO Medical, Japan). A reverse Barbeau test5 was systematically performed. The hemostasis device was removed by nurses in the hospitalization unit. No standardized protocol for the duration of hemostasis was followed.
Assessment of postprocedural radial artery patency
Radial Doppler assessments were conducted before and after each transradial procedure. To evaluate RAO, pulsed Doppler was performed bilaterally on the radial and ulnar arteries. Normal arterial flow was indicated by a biphasic or triphasic signal, reflecting good perfusion. In cases of RAO, 2 additional ultrasonographic examinations were performed at 1 and 3 months, following the same protocol. Artery patency was assessed by an independent operator.
Classifications and definitions
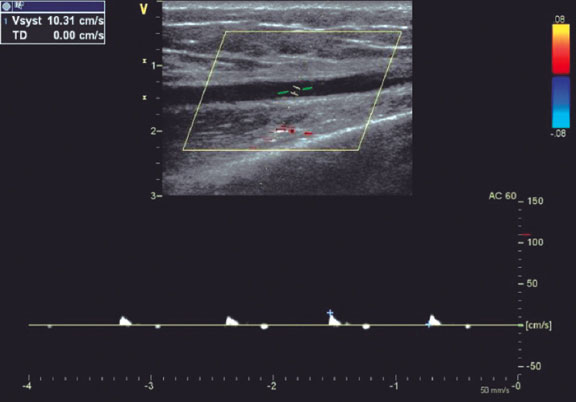
RAO was defined as the absence of anterograde flow in the RA on ultrasound (figure 1). The location of the radial occlusion was identified using color and pulsed Doppler. We delineated 3 anatomical territories: the distal third, extending from the radial styloid to approximately 7 to 10 cm proximally; the proximal third, from the elbow folds to approximately 7 to 10 cm distally; and the middle third, located between the previous 2 regions (middle part of the forearm).
Figure 1. Radial artery with occlusion in the distal third. Pulsed Doppler flow targets a stop flow indicating radial occlusion.
The type of hemostasis, whether occlusive or patent, was assessed: patent hemostasis was indicated by the presence of a plethysmographic signal in the RA during the reverse Barbeau test,5 which involves compression of the ulnar artery. The operator did not intervene during this process but simply recorded whether the artery remained patent or not.
The internal luminal diameter of the RA was defined as the distance between the leading edges of the intima-lumen interface on the superficial wall and the lumen-intima interface on the deep wall.6
The R/S ratio (radial/sheath) was calculated by dividing the luminal diameter of the RA by the external diameter of the sheath (Radiofocus II: 5-Fr = 2.29 mm, 6-Fr = 2.62 mm, 7-Fr = 2.97 mm; Prelude: 5-Fr = 2.52 mm, 6-Fr = 2.83 mm). This ratio was categorized qualitatively as < 1 or ≥ 1.
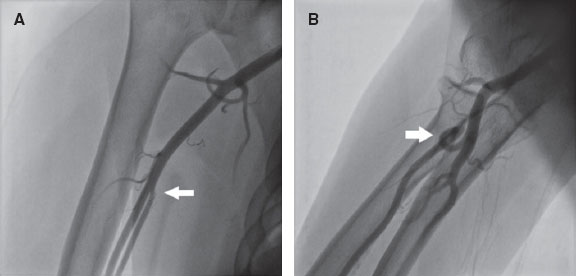
RA anatomical variations of clinical relevance were classified according to definitions provided in the literature.7,8 A high origin (high bifurcation) of the RA (figure 2) was defined with reference to the intercondylar line of the humerus. A radioulnar loop was characterized by the presence of a complete 360° loop of the RA, while radial tortuosity was identified by a curvature greater than 45°.
Figure 2. Anatomic variations of the radial artery. A: high origin of the radial artery. The radial and ulnar arteries separate at the level of the middle third of the humerus (arrow). B: radioulnar loop was defined as a complete 360° loop of the radial artery distal to the bifurcation of the brachial artery (arrow).
A blood pressure profile was obtained on the same side as the radial access. Forearm hematomas were classified according to the “EASY” study9: type I: < 5 cm in diameter; type II: < 10 cm; type III: > 10 cm but not extending to the elbow; type IV: extending beyond the elbow; type V: resulting in an ischemic lesion.
Statistical analysis
The statistical analysis was performed using IBM SPSS Software version 25. Parameters of interest are reported with their 95% confidence intervals (95%CI). For all tests, a significance threshold of 5% was retained. All tests were performed bilaterally. The following tests were used to compare groups: the chi-square test was used to compare 2 qualitative variables; the Student t-test or analysis of variance was used to compare a quantitative variable with a qualitative variable, with the Fisher test being applied when variances were unequal; and logistic regression was used to identify predictors of RAO.
RESULTS
Clinical and procedural characteristics of the study population
During the study period, 441 patients were screened. Of these, transradial access failed in 14 patients, who were excluded from the study, resulting in an eligible sample of 427 patients (mean age 61.9 ± 11.1 years, 67.4% male). Among the patients, 260 had hypertension (60.9%), and nearly half had diabetes (48.9%).
Table 1 summarizes the procedural data. The sheaths used were mainly 6-Fr (83.6%), and heparin was injected intra-arterially in 63.5% of patients. The mean heparin dose was 5669 ± 1394 IU, with a higher dose given when percutaneous coronary intervention was performed (4940 ± 339 IU vs 7491 ± 1368 IU; P < .001).
| Procedural characteristics | Patients N (%) |
|---|---|
| Indication | |
| CCS | 227 (53.2%) |
| ACS (NSTEMI) | 200 (46.8%) |
| Type of procedure | |
| Diagnostic angiography | 305 (71.4%) |
| PCI | 122 (28.6%) |
| Previous radial procedures | 68 (15.9%) |
| Right radial access | 410 (96.0%) |
| Puncture attempts | |
| 1 attempt | 258 (60.4%) |
| 2 attempts | 99 (23.2%) |
| ≥ 3 attempts | 70 (16.4%) |
| Sheath size | |
| 5-Fr | 68 (15.9%) |
| 6-Fr | 357 (83.6%) |
| 7-Fr | 2 (0.5%) |
| Heparin administration | |
| Intra-arterial | 271(63.5%) |
| Intravenous | 156 (36.5%) |
| Heparin dose (IU) | 5669 ± 1394 |
| Angiography | 4940 ± 339 |
| PCI | 7491 ± 1368 |
| Catheter diameter | |
| 5-Fr | 300 (70.3%) |
| 6-Fr | 125 (29.3%) |
| 7-Fr | 2 (0.5%) |
| Number of catheters used | |
| 1 | 43 (10.1%) |
| 2 | 271 (63.5%) |
| ≥ 3 | 113 (26.4%) |
| Fluoroscopy time (min) | 11.22 ± 12.09 |
| Radiation dose (mGy) | 564 ± 538 |
| Contrast amount (mL) | 98.97 ± 54.09 |
| Procedure time (min) | 39.16 ± 34.6 |
| Angiography | 21.63 ± 9.98 |
| PCI | 82.99 ± 35.39 |
| Coronary lesions | |
| Normal coronaries | 134 (31.4%) |
| 1 vessel disease | 131 (30.7%) |
| 2 vessel disease | 87 (20.4%) |
| 3 vessel disease | 75 (17.6%) |
|
ACS, acute coronary syndrome; CCS, chronic coronary syndrome; Fr, French; IU, international unit; NSTEMI, non–ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention. |
|
Incidence and characteristics of radial artery occlusion
RAO occurred in 48 patients (11.24%). Of these, 89.6% were asymptomatic, and the radial pulse remained palpable in 14 patients (29.2%). At 1 month, 2 patients were lost to follow-up. Among the remaining 46 patients, spontaneous recanalization occurred in 13 patients (28.3%). At the 3-month follow-up, the recanalization rate increased to 32.6% (15 cases).
The site of RAO was the distal third in 7 patients (14.6%), the middle third in 21 patients (43.8%), and the proximal third in 20 patients (41.7%).
Predictors of radial artery occlusion
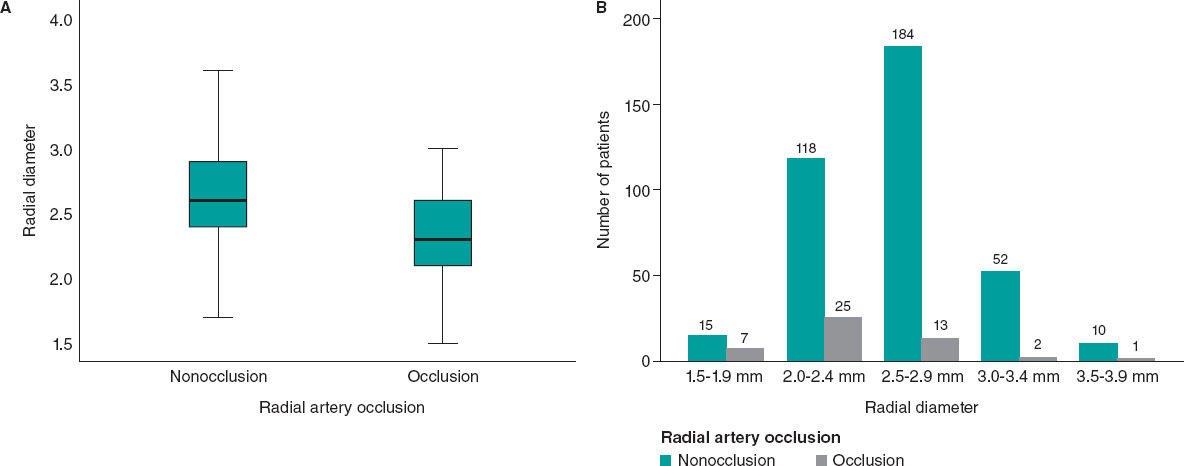
Patients with RAO were significantly younger (table 2). The mean periprocedural systolic blood pressure in the RAO group was significantly lower (138.04 mmHg ± 21.92, vs 145.84 mmHg ± 21.10; P = .017). Type A Barbeau test was associated with a higher risk of RAO compared with types B and C, and patients with occlusion had a smaller RA diameter (2.34 mm ± 0.40 vs 2.61 mm ± 0.37; P < .001) (figure 3).
Table 2. Comparison of patients with and without RAO
| Clinical data | Procedural data | ||||||
|---|---|---|---|---|---|---|---|
| Non-RAO (n= 379) | RAO (n= 48) | P | Non-RAO (n= 379) | RAO (n= 48) | P | ||
| Age | 62.6 ± 10.6 | 56.4 ± 14.0 | < .001* | Previous TRA | 61 (16.0%) | 7 (14.5) | .63 |
| Female sex | 122 (32.1%) | 17 (35.4%) | .65 | Diagnostic angiography | 266 (70.1%) | 39 (81.2%) | .11 |
| Hypertension | 237 (62.5%) | 23 (47%) | .051 | ≥ 2 puncture attempts | 147 (38.7%) | 22 (45.8%) | .41 |
| Diabetes | 182 (48%) | 27 (56%) | .28 | IV heparin | 136 (35.8%) | 20 (41.6%) | .43 |
| Dyslipidemia | 44 (11.6%) | 6 (12.5%) | .85 | Heparin dose (IU) | 5754 ± 1378 | 5007 ± 1352 | < .001* |
| Smoking | 75 (19.7%) | 9 (18.7%) | .86 | Spasm | 60 (15.8%) | 12 (25.0%) | .11 |
| BMI ≥ 30 kg/m2 | 123 (32.4%) | 16 (33.3%) | .90 | Procedure time (min) | 39.85 ± 34.56 | 33.73 ± 34.78 | .249 |
| Mean PSBP (mm Hg) | 145.84 ± 21.10 | 138.04 ± 21.92 | .017* | Number of catheters | 2.30 ± 0.88 | 2.21 ± 0.92 | .75 |
| Barbeau test type A | 99 (26.1%) | 20 (41.6%) | .044* | Occlusive hemostasis | 241 (63.5%) | 42 (87.5%) | .001* |
| RAD (mm) | 2.61 ± 0.37 | 2.34 ± 0.40 | < .001* | Hemostasis duration (h) | 4.29 ± 1.22 | 5.15 ± 1.41 | .006* |
| APT | 336 (88%) | 41 (85%) | .51 | ||||
| VKA (INR ≥ 2) | 16 (4.2%) | 5 (10.4%) | .061 | ||||
| MVCD | 149 (39.3%) | 13 (27.1%) | .20 | ||||
|
APT, antiplatelet therapy; BMI, body mass index; INR, international normalized ratio; IV, intravenous; IU, international unit; MVCD, multivessel coronary disease = ≥ 2 lesions ; PSBP, periprocedural systolic blood pressure; RAD , radial artery diameter; RAO, radial artery occlusion; TRA , transradial access; VKA, vitamin K antagonist. * Statistically significant. |
|||||||
Figure 3. Radial artery diameter as a predictor of occlusion. A: the radial diameter is significantly smaller if there is RAO. B: less than 2.5 mm, the risk of occlusion becomes greater.
RAO procedural factors are listed in table 2. An R/S ratio < 1 was found in 35 patients in the RAO group vs 153 patients in the non-RAO group (72.9% vs 40.3%, P < .001). The mean heparin dose was significantly lower in patients with RAO (5007 ± 1352IU vs 5754 ± 1378 IU; P < .001), and the dose adjusted to weight was also significantly lower in the RAO group (62.31 ± 17.82 IU/kg vs 75.73 ± 22.57 IU/kg; P < .001). In addition, the RAO rate decreased significantly when the heparin dose exceeded 70 IU/kg.
Forty-two patients in the RAO group had occlusive hemostasis vs 241 in the non-RAO group (87.5% vs 63.5%; P = .001). Surprisingly, two-thirds of our patients (283 [66.3%]) had occlusive hemostasis. The mean duration of hemostasis was longer if there was RAO (5.15 h ± 1.41 vs 4.29 h ± 1.22; P < .001).
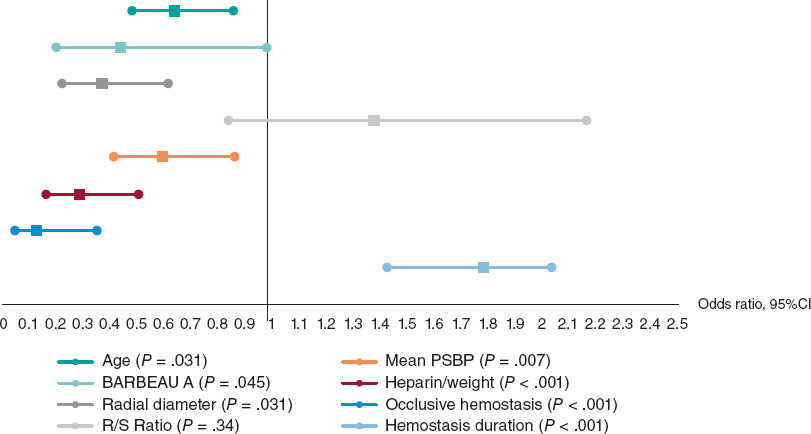
On multivariate logistic regression analysis (figure 4), the following factors were independent predictors of RAO: young age (odds ratio [OR], 0.642; 95%CI, 0.480-0.858; P = .031), low periprocedural systolic blood pressure (OR, 0.598; 95%CI, 0.415-0.862; P = .007), type A Barbeau test (OR, 0.441; 95%CI, 0.198-0.981; P = .045), small RA diameter (OR, 0.371; 95%CI, 0.323-0.618; P = .031), insufficient anticoagulation (OR, 0.287; 95%CI, 0.163-0.505; P < .001), occlusive hemostasis (OR, 0.128; 95%CI, 0.047-0.353; P < .001), and a long hemostasis duration (OR, 1.786; 95%CI, 1.428-2.039; P < .001).
Figure 4. Independent factors predictive of radial artery occlusion. Multiple logistic regression analysis revealed that the independent factors predictive of radial occlusion were young age, low periprocedural systolic blood pressure, type A Barbeau test, small radial artery diameter, insufficient anticoagulation, occlusive hemostasis, and long hemostasis duration. 95%CI, 95% confidence interval; PSBP, periprocedural systolic blood pressure; RAO, radial artery occlusion.
Anatomic variations of the radial artery
The mean radial diameter was 2.58 mm ± 0.39, and the diameter was larger in men (2.69 mm ± 0.37 vs 2.36 mm ± 0.31; P < .001) and smaller in patients with diabetes (2.53 mm ± 0.38 vs 2.64 mm ± 0.38; P = .003). The mean radial diameter was significantly larger than the mean ulnar diameter (2.58 mm ± 0.39 vs 2.22 mm ± 0.43; P < .001).
Radial anatomical variations affected 63 patients (14.8%). The most common variation was a high origin of the RA, observed in 63.5% of cases (40 patients), followed by radial tortuosity in 28.6% (18 patients), radioulnar loop in 7.9% (5 patients). Anatomical variations were more frequent in women (23% vs 10.8%; P = .001) and in older patients, with a mean age of 66.3 years ± 10.2 vs 61.2 years ± 11.2 in those without variations (P = .001).
Periprocedural complications
Radial spasm occurred in 72 patients (16.9%). This complication was more frequent in women (29% vs 10.1%; P < .001), patients with diabetes (22.5% vs 11.5%; P = .002), and when 6-Fr catheters were used (14% vs 24%; P = .035). Forearm hematoma occurred in 25 patients (5.85%). According to the EASY classification,9 most hematomas were type I (17 patients, 68%), followed by type II (6 patients, 24%), with type III occurring in only 2 patients (8%).
DISCUSSION
The rate of RAO remains relatively high in some institutions.10,11 In the PROPHET study, the acute incidence of RAO (12%) was almost halved in 28 days (7%).3 Recanalization occurs as a result of activation of primary fibrinolysis.12 In the present study, the rate of radial recanalization at 3 months was 32.6%. The only predictor of recanalization was radial diameter: the larger the diameter, the higher the rate of spontaneous recanalization.
Zankl et al.13 found that RAO was located in the distal third of the forearm in 49% of patients, in the distal and middle third in 13.7%, and in the entire forearm (proximal third) in 37.3%. Dissections of the media also occur in the proximal RA, likely due to catheter progression or manipulation without protection of the sheath.14 In our opinion, this would explain the location of RAO in the proximal part of the artery.
Among our patients with RAO, 29.2% had a radial pulse. According to Uhlemann et al.,4 in 19.5% of patients with RAO on Doppler, the RA pulse was still palpable. This was likely due to retrograde filling of the RA by collaterals. Therefore, the diagnosis of RAO should be confirmed using a more objective method, such as Doppler ultrasound.
Young age is a predictor of RAO, possibly due to higher sympathetic reactivity in younger individuals, which increases their risk of spasm. However, this characteristic does not influence the rate of recanalization, likely because prolonged radial spasm leads to the formation of a permanent intra-arterial thrombus.
Low mean systolic blood pressure was also a predictor of radial occlusion. We speculate that hypertension and arterial stiffness may prevent complete interruption of flow during compression, thereby helping to maintain radial patency.15
There was a higher incidence of occlusion with type A Barbeau test. We believe that in cases with well-developed ulnar circulation, the ulnar artery generates a competitive retrograde flow that opposes the radial flow, promoting occlusion and hindering recanalization.
The likelihood of developing RAO is related to the size of the sheath,16 or more precisely, the R/S ratio.17 A prospective registry showed that 5-Fr sheaths reduced the rate of RAO by up to 55% compared with 6-Fr.4
A study by Pancholy et al.,18 demonstrated that intravenous heparin is as effective as intra-arterial heparin in reducing the incidence of RAO, suggesting that the systemic effect of heparin is more important than its local effect. A recently published meta-analysis identified higher heparin doses as the most significant measure for decreasing RAO.12 This results is in line with our finding that a dose of less than 70 IU/kg seems to promote the occurrence of RAO. The high prevalence of RAO and the benefit of higher doses of unfractionated heparin (≥ 50 IU/kg) in this setting were also highlighted by a meta-analysis of 112 studies.19 In a randomized superiority trial comparing high-dose (100 IU/kg) and standard-dose (50 IU/kg) heparin, the RAO rate was significantly lower in the high-dose group.20 Recent evidence suggests that a small dose of rivaroxaban, given orally after a transradial procedure, may decrease the occurrence of RAO at 1 month.21,22
Using the reverse Barbeau test, Sanmartin et al.23 found that 60% of patients had an absence of radial flow during compression. These observations led to the concept of nonocclusive hemostasis (patent hemostasis). In the PROPHET study,3 RAO was significantly less frequent in the group that underwent nonocclusive hemostasis than in the control group.
The duration of hemostatic compression has been studied in large, randomized trials.24-26 The authors concluded that compression duration was a strong predictor of RAO.
In a meta-analysis by Rashid et al.,27 the incidence of RAO after diagnostic coronary angiography was notably higher compared with percutaneous coronary intervention, possibly due to the use of higher anticoagulation doses during interventions.12 However, opposite findings have been reported by other studies.
In our sample, the mean radial diameter was 2.58 mm ± 0.39 and was significantly larger in men. Velasco et al.28 reported a mean arterial diameter of 2.22 ± 0.35 mm, while a Polish study found a mean diameter of 2.17 ± 0.53 mm for the right RA and 2.25 ± 0.43 mm for the left RA.29 The ulnar artery is also used in interventional cardiology,30 although there is no consensus on its size compared with the RA.
Autopsy studies of arterial anatomic variations of the upper extremity have reported frequencies between 4% and 18.5%.8 In the literature, the most frequent anatomic variation of the RA is high bifurcation. Yoo et al.31 reported a 2.4% incidence of high radial origin in 1191 Korean patients. Tortuosity of the RA frequently affects patients with high radial origin, possibly due to the elongated course of the RA predisposing it to tortuosity, which is considered one of the most common causes of procedural failure, along with radial spasm.32
Radioulnar loop is the most common cause of procedural failure with experienced operators.33 Angiographic evaluation of the radioulnar anastomosis is mandatory in such cases, as there is often a negotiable anastomosis between the radial and ulnar arteries.
In our study, radial spasm was the leading cause of procedural failure, occurring in 50% of the 14 patients who experienced such failures. Ruiz-Salmerón et al.34 found that RA anatomic variations were strongly associated with radial spasm in a multivariate analysis. The relationship between radial spasm and anatomic variations is mainly explained by the strong correlation with high radial origin and the radioulnar loop.
Study limitations
Since this study is a prospective registry and not a randomized trial, selection bias cannot be excluded. Our study represents a single-center experience with a limited number of patients, despite being one of the largest prospective registries of vascular ultrasound in radial catheterization to date. Among the other limitations of the study, we note the lack of standardized protocols for both heparin use and compression.
CONCLUSIONS
With the increasing number of transradial procedures and the greater age of patients undergoing these interventions, leading to more complex procedures, it is essential to maintain the patency of the RA for future access. Although predictors of RAO after cardiac catheterization have been identified, implementing preventive measures in practice remains a challenge. The main modifiable predictors associated with the risk of RAO are insufficient heparinization and occlusive hemostasis. Therefore, preventive strategies should primarily focus on addressing these 2 factors.
FUNDING
None.
ETHICAL CONSIDERATIONS
The study was conducted in accordance with the provisions of the Declaration of Helsinki and with the International Conference on Harmonization Good Clinical Practices and was approved by the local ethics committee. All patients included in the study provided written informed consent, which is archived and available. Our study population included both sexes. Gender had no influence on the occurrence of radial occlusion.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
No artificial intelligence software was used in the preparation of this study.
AUTHORS’ CONTRIBUTIONS
All authors meet the criteria for authorship as defined by the International Committee of Medical Journal Editors. M.S. Lounes, A. Meftah, C. Belhadi, K. Allal, H. Boulaam, A. Sayah, I. Hafidi, and E. Tebache contributed to the acquisition and analysis of data for this article. M.S. Lounes, A. Bedjaoui, A. Allali, and S. Benkhedda were responsible for the study design and the writing of the article. M.S. Lounes, A. Allali, and S. Benkhedda contributed to writing and critical revision of the content. All authors have read and approved the final version of the article and agree to be accountable for all aspects of the work, including the accuracy and integrity of all its parts.
CONFLICTS OF INTEREST
None.
WHAT IS KNOWN ABOUT THE TOPIC?
- Despite recommendations on the prevention of RAO in interventional cardiology, its incidence remains relatively high in some centers.
- Spontaneous recanalization of the artery may occur during follow-up.
- Permanent occlusion of the radial artery prevents any possibility of its further use (interventional procedures, dialysis, etc.)
WHAT DOES THIS STUDY ADD?
- RAO is not limited to the distal part of the artery and can affect the entire length of the vessel.
- Diagnosis of RAO should be confirmed using Doppler ultrasound, which remains the gold standard.
- The 2 independent modifiable predictors of RAO are the anticoagulation protocol and hemostasis technique.
- Anatomic variations of the RA may impact the procedure. A high origin of the RA is the most frequent, followed by radial tortuosities. After radial spasm, the radioulnar loop is the most common cause of procedural failure with experienced operators.
REFERENCES
1. Cruden NL, Teh CH, Starkey IR, Newby DE. Reduced vascular complications and length of stay with transradial rescue angioplasty for acute myocardial infarction. Catheter Cardiovasc Interv. 2007;70:670-675.
2. Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87-165.
3. Pancholy SB, Coppola J, Patel T, Roke-Thomas M. Prevention of radial artery occlusion-patent hemostasis evaluation trial (PROPHET study):a randomized comparison of traditional versus patency documented hemostasis after transradial catheterization. Catheter Cardiovasc Interv. 2008;72:335-340.
4. Uhlemann M, Möbius-Winkler S, Mende M, et al. The Leipzig prospective vascular ultrasound registry in radial artery catheterization:impact of sheath size on vascular complications. JACC Cardiovasc Interv.2012;5:36-43.
5. Da Silva RL, Britto PF, Joaquim RM, et al. Clinical accuracy of reverse Barbeau test in the diagnosis of radial artery occlusion after transradial catheterization. J Transcat Intervent. 2021;29:eA20200037.
6. Costa F, van Leeuwen MA, Daemen J, et al. The Rotterdam Radial Access Research:Ultrasound-Based Radial Artery Evaluation for Diagnostic and Therapeutic Coronary Procedures. Circ Cardiovasc Interv. 2016;9:003129.
7. Uglietta JP, Kadir S. Arteriographic study of variant arterial anatomy of the upper extremities. Cardiovasc Intervent Radiol. 1989;12:145-148.
8. Rodríguez-Niedenführ M, Vázquez T, Nearn L, et al. Variations of the arterial pattern in the upper limb revisited:a morphological and statistical study, with a review of the literature. J Anat. 2001;199:547-566.
9. Bertrand OF. Acute forearm muscle swelling post transradial catheterization and compartment syndrome:prevention is better than treatment!Catheter Cardiovasc Interv. 2010;75:366-368.
10. Sadaka MA, Etman W, Ahmed W, et al. Incidence and predictors of radial artery occlusion after transradial coronary catheterization. Egypt Heart J. 2019;71:12.
11. Dousi M, Sotirakou K, Fatsi A. The Use of Acetylsalicylic Acid As A Measure of Prevention of Radial Artery Occlusion in Patients Who Perform Coronary Angiography with Tra Technique. J Radiol Clin Imaging. 2020;3:13-21.
12. Rashid M, Kwok CS, Pancholy S, et al. Radial Artery Occlusion After Transradial Interventions:A Systematic Review and Meta-Analysis. J Am Heart Assoc. 2016;5:002686.
13. Zankl AR, Andrassy M, Volz C, et al. Radial artery thrombosis following transradial coronary angiography:incidence and rationale for treatment of symptomatic patients with low-molecular-weight heparins. Clin Res Cardiol. 2010;99:841-847.
14. Bi XL, Fu XH, Gu XS, et al. Influence of Puncture Site on Radial Artery Occlusion After Transradial Coronary Intervention. Chin Med J (Engl). 2016;129:898-902.
15. Buturak A, Gorgulu S, Norgaz T, et al. The long-term incidence and predictors of radial artery occlusion following a transradial coronary procedure. Cardiol J. 2014;21:350-356.
16. Dahm JB, Vogelgesang D, Hummel A, et al. A randomized trial of 5 vs. 6 French transradial percutaneous coronary interventions. Catheter Cardiovasc Interv. 2002;57:172-176.
17. Saito S, Ikei H, Hosokawa G, Tanaka S. Influence of the ratio between radial artery inner diameter and sheath outer diameter on radial artery flow after transradial coronary intervention. Catheter Cardiovasc Interv.1999;46:173-178.
18. Pancholy SB. Comparison of the effect of intra-arterial versus intravenous heparin on radial artery occlusion after transradial catheterization. Am J Cardiol. 2009;104:1083-1085.
19. Hahalis GN, Aznaouridis K, Tsigkas G, et al. Radial Artery and Ulnar Artery Occlusions Following Coronary Procedures and the Impact of Anticoagulation:ARTEMIS Systematic Review and Meta-Analysis. J Am Heart Assoc. 2017;6:005430.
20. Hahalis GN, Leopoulou M, Tsigkas G, et al. Multicenter Randomized Evaluation of High Versus Standard Heparin Dose on Incident Radial Arterial Occlusion After Transradial Coronary Angiography:The SPIRIT OF ARTEMIS Study. JACC Cardiovasc Interv. 2018;11:2241-2250.
21. Liang D, Lin Q, Zhu Q, et al. Short-Term Postoperative Use of Rivaroxaban to Prevent Radial Artery Occlusion After Transradial Coronary Procedure:The RESTORE Randomized Trial. Circ Cardiovasc Interv. 2022;15:011555.
22. Hammami R, Abid S, Jihen J, et al. Prevention of radial artery occlusion with rivaroxaban after trans-radial access coronary procedures:The RIVARAD multicentric randomized trial. Front Cardiovasc Med. 2023;10:1160459.
23. Sanmartin M, Gomez M, Rumoroso JR, et al. Interruption of blood flow during compression and radial artery occlusion after transradial catheterization. Catheter Cardiovasc Interv. 2007;70:185-189.
24. Politi L, Aprile A, Paganelli C, et al. Randomized clinical trial on short-time compression with Kaolin-filled pad:a new strategy to avoid early bleeding and subacute radial artery occlusion after percutaneous coronary intervention. J Interv Cardiol. 2011;24:65-72.
25. Dharma S, Kedev S, Patel T, et al. A novel approach to reduce radial artery occlusion after transradial catheterization:postprocedural/prehemostasis intra-arterial nitroglycerin. Catheter Cardiovasc Interv.2015;85:818-825.
26. Aminian A, Saito S, Takahashi A, et al. Impact of sheath size and hemostasis time on radial artery patency after transradial coronary angiography and intervention in Japanese and non-Japanese patients:A substudy from RAP and BEAT randomized multicenter trial. Catheter Cardiovasc Interv. 2018;92:844-851.
27. Sinha SK, Jha MJ, Mishra V, et al. Radial Artery Occlusion. Incidence, Predictors and Long-term outcome after TRAnsradial Catheterization:clinico-Doppler ultrasound-based study (RAIL-TRAC study). Acta Cardiol. 2017;72:318-327.
28. Velasco A, Ono C, Nugent K, et al. Ultrasonic evaluation of the radial artery diameter in a local population from Texas. J Invasive Cardiol. 2012;24:339-341.
29. Peruga JP, Peruga JZ, Kasprzak JD, et al. Ultrasound evaluation of forearm arteries in patients undergoing percutaneous coronary intervention via radial artery access:results of one-year follow-up. Kardiologia Polska. 2015;73:502-510.
30. Knebel AV, Cardoso CO, Correa Rodrigues LH, et al. Safety and feasibility of transulnar cardiac catheterization. Tex Heart Inst J. 2008;35:268-272.
31. Yoo BS, Yoon J, Ko JY, et al. Anatomical consideration of the radial artery for transradial coronary procedures:arterial diameter, branching anomaly and vessel tortuosity. Int J Cardiol. 2005;101:421-427.
32. Pristipino C, Roncella A, Trani C, et al. Identifying factors that predict the choice and success rate of radial artery catheterisation in contemporary real world cardiology practice:a sub-analysis of the PREVAIL study data. EuroIntervention. 2010;6:240-246.
33. Louvard Y, Lefèvre T. Loops and transradial approach in coronary diagnosis and intervention. Catheter Cardiovasc Interv. 2000;51:250-252.
34. Ruiz-Salmerón RJ, Mora R, Vélez-Gimón M, et al. Radial artery spasm in transradial cardiac catheterization. Assessment of factors related to its occurrence, and of its consequences during follow-up. Rev Esp Cardiol. 2005;58:504-511.
ABSTRACT
Introduction and objectives: Distal radial access (DRA) for coronary procedures is currently recognized as an alternative to conventional transradial access, with documented advantages primarily related to access-related complications. However, widespread adoption of DRA as the default approach remains limited. Therefore, this prospective cohort study aimed to present our initial experience with DRA for coronary procedures in any clinical settings.
Methods: From August 2020 to November 2023, we included 1000 DRA procedures (943 patients) conducted at a single center. The study enrolled a diverse patient population. We recommended pre- and postprocedural ultrasound evaluations of the radial artery course, with ultrasound-guided DRA puncture. The primary endpoint was DRA success, while secondary endpoints included coronary procedure success, DRA performance metrics, and the incidence of access-related complications.
Results: The DRA success rate was 97.4% (n = 974), with coronary procedure success at 96.9% (n = 969). The median DRA time was 40 [interquartile range, 30-60] seconds. Diagnostic procedures accounted for 64% (n = 644) of cases, while 36% (n = 356) involved percutaneous coronary intervention (PCI), including primary PCI in 13% (n = 128). Pre-procedure ultrasound evaluation and ultrasound-guided DRA were performed in 83% (n = 830) and 85% (n = 848) of cases, respectively. Access-related complications occurred in 2.9% (n = 29).
Conclusions: This study shows the safety and feasibility of DRA for coronary procedures, particularly when performed under ultrasound guidance in a diverse patient population. High rates of successful access and coronary procedure outcomes were observed, together with a low incidence of access-related complications. The study was registered on ClinicalTrials.gov (NTC06165406).
Keywords: Vascular access. Distal radial artery. Coronary angiography. Percutaneous transluminal coronary angioplasty. Doppler ultrasound. Access-related complications.
RESUMEN
Introducción y objetivos: Actualmente, el acceso radial distal (ARD) para procedimientos coronarios es una alternativa al acceso radial convencional, con algunas ventajas descritas principalmente en términos de complicaciones relacionadas con el acceso. A pesar de la evidencia, pocos centros han establecido el ARD como acceso sistemático para procedimientos coronarios. El objetivo de esta cohorte prospectiva es presentar la experiencia inicial en nuestro centro con el ARD en pacientes con indicación de procedimientos coronarios en cualquier escenario clínico.
Métodos: Se incluyeron 1.000 procedimientos de ARD (943 pacientes) realizados en un único centro de agosto de 2020 a noviembre de 2023. El estudio fue realizado con pacientes en cualquier escenario clínico. Se recomendó la valoración por ultrasonido del trayecto de la arteria radial antes y después del procedimiento, así como la punción ecoguiada. El objetivo principal fue el éxito del ARD. Como objetivos secundarios se consideraron el éxito del procedimiento coronario, el desempeño del ARD y las complicaciones relacionadas con el acceso.
Resultados: El éxito del ARD fue del 97,4% (n = 974) y el éxito del procedimiento coronario fue del 96,9% (n = 969). El tiempo de acceso del ARD fue de 40 segundos [rango intercuartílico, 30-60]. Se realizaron procedimientos diagnósticos en el 64% (n = 644) e intervencionismo coronario percutáneo (ICP) en el 36% (n = 356), incluyendo ICP primario en el 13% (n = 128) de los pacientes. La valoración por ultrasonido antes del procedimiento se llevó a cabo en el 83% (n = 830) y la punción ecoguiada en el 85% (n = 848). La incidencia de complicaciones relacionadas con el acceso fue del 2,9% (n = 29).
Conclusiones: Este estudio muestra la viabilidad y la seguridad del ARD principalmente guiado por ultrasonido para los procedimientos coronarios en cualquier escenario clínico, con un alto porcentaje de éxito del acceso y de éxito del procedimiento, además de una baja incidencia de complicaciones relacionadas con el acceso. El estudio fue registrado en ClinicalTrials.gov (NTC06165406).
Palabras clave: Acceso vascular. Arteria radial distal. Coronariografía. Angioplastia coronaria transluminal percutánea. Ultrasonido Doppler. Complicaciones relacionadas con el acceso.
Abbreviations
CAG: coronary angiography. DRA: distal radial access. DRart: distal radial artery. PRart: proximal radial artery. TRA: transradial access.
INTRODUCTION
Currently, distal radial access (DRA) in the anatomical snuffbox for both noncoronary and coronary procedures is gaining popularity. Since its introduction by Babunashvili et al.,1 in 2011, several observational studies have validated the feasibility and safety of DRA,2-4 comparing it with conventional transradial access (TRA). DRA has shown advantages such as a lower incidence of radial artery occlusion (RAO) and shorter hemostasis time, with minimal access-related complications.5,6 The usefulness of ultrasound to guide DRA and evaluate access-related complications has also been described.7,8 Recent randomized trials comparing DRA with TRA have reported conflicting results regarding RAO incidence, crossover rates, and access times.9-11 Nevertheless, meta-analyses consistently support the benefits of DRA, albeit with a higher crossover rate.12-13 One of the limitations of most studies on DRA is the restricted inclusion of patients in emergent situations or complex percutaneous coronary interventions (PCI), such as ST-segment elevation myocardial infarction (STEMI); therefore, the feasibility of the approach in this context is somewhat scarce.2,9-11,14 Despite current evidence, the use of DRA as the default access for coronary procedures is still not widely implemented in most centers. Hence, this prospective single-center cohort aimed to present the experience of our first 1000 DRA in patients undergoing coronary procedures in any clinical settings.
METHODS
Population and study design
The Distal Radial Access for Diagnostic and Interventional Coronary Procedures in an all-comer population (DISTAL) registry is a prospective observational investigation aiming to assess the performance of DRA and compare clinical and procedural characteristics in a diverse population undergoing coronary procedures. This interim analysis presents our initial experience with DRA conducted at a single center. All DRA procedures performed by 4 experienced operators, previously proficient in TRA, were included in the study from August 2020 to November 2023.
This study was approved by the Ethics Committee of our institution (CEIC-2804) and was conducted following the principles of the Declaration of Helsinki. All patients gave their informed written consent before the procedure.
Inclusion and exclusion criteria
The study included patients aged 18 years and older undergoing diagnostic or therapeutic coronary procedures using DRA in any clinical setting. Patients with an unsuitable distal radial artery (DRart) assessed by ultrasound (non-permeable or diameter <1 .8 mm) were excluded, as were patients with no palpable pulse of DRart with such unsuitability characteristics. Additional exclusion criteria encompassed participation in other clinical trials, known allergy to iodinated contrast, inability to provide informed consent, and women of childbearing age without a negative pregnancy test. While the Barbeau test was recommended, it was not mandatory for inclusion.15
Endpoints
The primary endpoint was the success of DRA and the main secondary endpoint was the success of the coronary procedure. Other secondary endpoints included DRA procedure time, total procedure duration, the incidence of radial artery spasm, exposure to ionizing radiation, patient comfort levels, hemostasis time, access-related complications, and the impact of ultrasound guidance on DRA performance. Detailed definitions of these endpoints are provided in the supplementary data.
Distal radial access technique
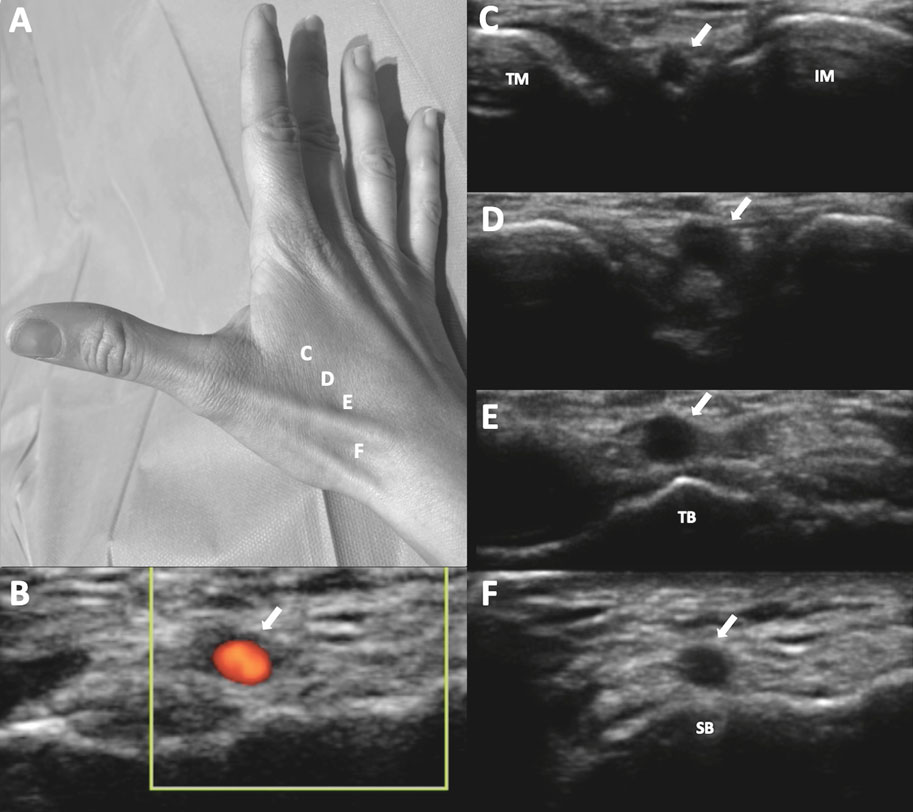
The DRA technique has been previously described,2,4,16-18 and is explained in detail in the supplementary data. Key aspects of interest included patient selection, the decision to use ultrasound-guided puncture19 (figure 1) vs blind with palpation puncture at the discretion of the operator, patient positioning for right (r) or left (l) DRA, the puncture technique itself, and the hemostasis procedure (figure 2).
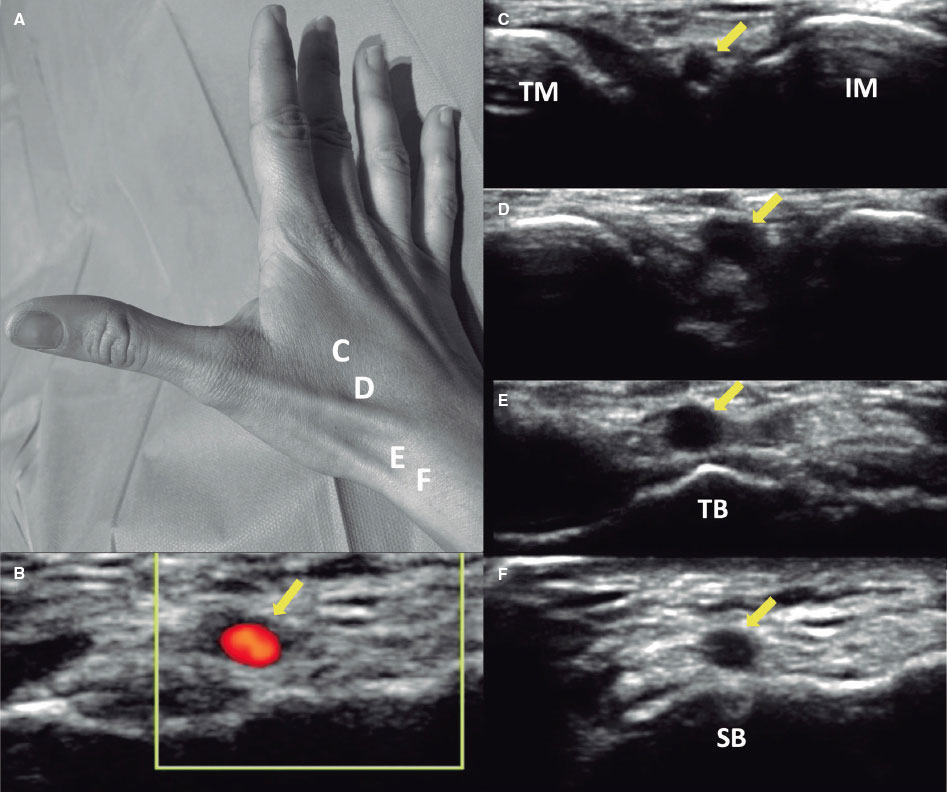
Figure 1. A: markers for ultrasound positioning in the anatomical snuffbox. B: patency of the distal radial artery (DRart) confirmed by color Doppler ultrasound. C-D: course of DRart between the metacarpal bones. E-F: recommended puncture sites of the DRart on a surface bone. IM, index metacarpal; SB, scaphoid bone; TB, trapezium bone; TM, thumb metacarpal.
Figure 2. Distal radial access (DRA) technique. Position of the hand for A) right DRA and B) left DRA. C: ultrasound-guided DRA technique. D: blind with palpation DRA puncture. E: final position of the introducer sheaths on the right and left DRA. F: hemostasis devices in DRA.
Statistical analysis
Sample size and statistical power calculations were performed using the GRANMO calculator.20 A sample size of 1000 procedures was determined to provide a statistical power greater than 99% to detect a difference of 3% or more in the proportion of DRA success (primary endpoint) at our center, assuming an alpha risk of 1%. This calculation was based on a reference proportion from previous medical literature estimated around 95%.11,18,21
Categorical variables are presented as counts (percentages), while continuous variables were assessed for normal distribution using the Kolmogorov-Smirnov test. Normally distributed variables are expressed as mean (standard deviation), and nonnormally distributed variables as median [interquartile range].
To evaluate the impact of the learning curve, comparisons were made among quartiles of the study period for variables including access failure, DRA time, total procedure time, and access-related complications. Analysis of variance or the Kruskal-Wallis test was used depending on the normality of the variable. Logistic regression analysis (logit command) was used with the first quartile as the reference to compare percentages among quartiles.
Statistical analyses were conducted using SPSS Statistics 20.0 software (IBM, United States) and STATA 12 (StataCorp, College Station, United States). A p-value < 0.05 was considered statistically significant for all tests.
RESULTS
From August 2020 to November 2023, a total of 1000 DRA procedures (943 patients) were performed. Table 1 shows the patients’ baseline clinical characteristics. The mean age was 68 years, and 29% of the patients were women. A total of 47% of the procedures were performed on an outpatient basis. In 35% of cases, the indication was acute coronary syndrome (13% STEMI).
Table 1. Baseline clinical characteristics
| Clinical characteristics | n = 1000 |
|---|---|
| Age, (years), mean (SD) | 68.1 (11.7) |
| Female, n (%) | 289 (28.9) |
| Weight, (kg), mean (SD) | 78.0 (14.8) |
| Height, (cm), mean (SD) | 167.9 (8.1) |
| Body mass index, (kg/m2), mean (SD) | 28.0 (4.5) |
| Hypertension, n (%) | 735 (73.5) |
| Dyslipidemia, n (%) | 578 (57.8) |
| Diabetes mellitus, n (%) | 353 (35.3) |
| Current smoker, n (%) | 246 (24.6) |
| Family history of premature coronary heart disease, n (%) | 54 (5.4) |
| Previous peripheral artery disease, n (%) | 50 (0.5) |
| Previous stroke, n (%) | 41 (4.1) |
| Previous heart failure, n (%) | 252 (25.2) |
| GFR (mL/minute/1.73m2), mean (SD) | 72.4 (20.0) |
| Dialysis, n (%) | 27 (2.7) |
| Left ventricular ejection fraction, mean (SD) | 52.6 (16.2) |
| Atrial fibrillation, n (%) | 170 (17.0) |
| OAC | |
| Acenocoumarin, n (%) | 170 (17.0) |
| Direct OAC, n (%) | 81 (8.1) |
| Previous CAG, n (%) | 251 (25.1) |
| Previous CABG, n (%) | 43 (4.3) |
| Previous PCI, n (%) | 218 (21.8) |
| Previous ischemic heart disease | |
| Previous STEMI, n (%) | 133 (13.3) |
| Previous NSTEMI, n (%) | 69 (6.9) |
| Previous CCS, n (%) | 53 (5.3) |
| CAG indication | |
| Chronic coronary syndrome, n (%) | 207 (20.7) |
| STEMI, n (%) | 128 (12.8) |
| NSTEMI, n (%) | 224 (22.4) |
| Staged PCI, n (%) | 60 (6.0) |
| Diagnostic, n (%) | 381 (38.1) |
| Preoperative CAG in patients with VHD, n (%) | 183 (18.3) |
| Dilated cardiomyopathy, n (%) | 158 (15.8) |
| Ventricular tachycardia, n (%) | 24 (2.4) |
| Others, n (%) | 16 (1.6) |
| Outpatient coronary arteriography, n (%) | 470 (47) |
|
CABG, coronary artery bypass grafting; CAG, coronary angiography; CCS, chronic coronary syndrome; GFR, glomerular filtration rate; NSTEMI, non−ST-segment elevation myocardial infarction; OAC, oral anticoagulation; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction; VHD, valvular heart disease. Data are expressed as No. (%) or mean ± standard deviation. |
|
Table 2 presents the characteristics of the radial artery and the DRA procedure. High rates of preprocedure ultrasound evaluation and ultrasound-guided technique for DRA were noted (83% and 85%, respectively). Notably, the percentage of coronary procedures showing insufficient catheter length due to DRA was low (3.7%).
Table 2. Characteristics of the DRA procedure
| Procedure characteristics | n = 1000 |
|---|---|
| Preprocedure characteristics | |
| Arterial pulse strength scale | |
| Absent, n (%) | 12 (1.2) |
| Weak, n (%) | 167 (16.7) |
| Normal, n (%) | 652 (65.2) |
| Strong, n (%) | 169 (16.9) |
| Radial artery preprocedure ultrasound evaluation, n (%) | 830 (83.0) |
| Arterial tortuosity | |
| Radial, n (%) | 23 (2.3) |
| Subclavian, n (%) | 62 (6.2) |
| Calcified radial artery, n (%) | 26 (2.6) |
| Distal radial artery size, mm (SD) | 2.3 (0.3) |
| Proximal radial artery size, mm (SD) | 2.5 (0.4) |
| Depth of the distal radial artery, mm (SD) | 3.8 (1.0) |
| DRA technique | |
| CAG by the same DRA, n (%) | 57 (5.7) |
| Ultrasound-guided access, n (%) | 848 (84.8) |
| DRA side | |
| Right DRA, n (%) | 627 (62.7) |
| Left DRA, n (%) | 373 (37.3) |
| Introducer size | |
| 5 French, n (%) | 256 (25.6) |
| 6 French, n (%) | 744 (74.4) |
| Introducer sheath type | |
| Prelude Ideal (Merit Medical) Introducer Kit, n (%) | 950 (95.0) |
| Radifocus Introducer II Kit A (Terumo Corporation), n (%) | 50 (5.0) |
| Short length of the radial catheter | 37 (3.7) |
| Postprocedure arterial patency evaluation, n (%) | 907 (90.7) |
| Postprocedure puncture site bleeding, n (%) | 55 (5.5) |
|
CAG, coronary angiography; DRA, distal radial access. Data are expressed as No. (%) or mean ± standard deviation. |
|
Table 3 summarizes the characteristics of coronary procedures, including the extent of coronary artery disease, types of procedures, and features of patients who underwent PCI. In general, 64% of the procedures were only diagnostic, while 36% included PCI.
Table 3. Characteristics of the coronary procedure
| Procedure characteristics | n = 1000 |
|---|---|
| Coronary disease extent | |
| One vessel, n (%) | 285 (28.5) |
| Two vessels, n (%) | 174 (17.4) |
| Three vessels, n (%) | 176 (17.6) |
| LMCAD, n (%) | 55 (5.5) |
| Coronary bypass graft, n (%) | 27 (2.7) |
| Characteristics of the coronary procedure | |
| Type of coronary procedures | |
| Diagnostic, n (%) | 644 (64.4) |
| PCI, n (%) | 356 (35.6) |
| Ambulatory PCI, n (%) | 90 (9.0) |
| PCI culprit lesion | |
| LMCAD, n (%) | 9 (0.9) |
| Left anterior descending artery, n (%) | 164 (16.4) |
| Circumflex coronary artery, n (%) | 95 (9.5) |
| Right coronary artery, n (%) | 100 (10.0) |
| Coronary bypass graft | 2 (0.2) |
| Specific techniques | |
| Wire-based intracoronary physiological assessment, n (%) | 57 (5.7) |
| Optical coherence tomography, n (%) | 21 (2.1) |
| Intravascular ultrasound, n (%) | 30 (3.0) |
| Guide catheter extension system, n (%) | 15 (1.5) |
| Rotational atherectomy, n (%) | 16 (1.6) |
| Cutting balloon, n (%) | 34 (3.4) |
| Intracoronary lithotripsy, n (%) | 8 (8.0) |
| Thrombus aspiration, n (%) | 81 (8.1) |
| Intracoronary perfusion catheter, n (%) | 7 (0.7) |
| Special PCI procedures | |
| Complex bifurcation, n (%) | 60 (6.0) |
| Chronic total occlusion, n (%) | 16 (1.6) |
| Volume of contrast, (mL), mean (SD) | 85.0 (53.1) |
| Heparin dose, (IU), median [IQR] | 5000 (3000-8500) |
|
LMCAD, left main coronary artery disease; PCI, percutaneous coronary intervention. |
|
Table 4 depicts the clinical endpoints. The DRA success rate was 97.4% and the coronary procedure success rate was 96.9%. The median access time was 40 (interquartile range [IQR], 30-60) seconds, and 4% of patients experienced radial artery spasm. The overall rate of access-related complications was low (2.9%).
Table 4. Clinical endpoints
| Clinical endpoints | n = 1000 |
|---|---|
| Primary endpoint | |
| DRA success, n (%) | 974 (97.4) |
| Coronary procedure success by DRA, n (%) | 969 (96.9) |
| Secondary endpoints | |
| Access time, (sec), median [IQR] | 40 (30-60) |
| Procedure time, (min), median [IQR] | 29.0 [17.3-45.0] |
| Radial artery spasm, n (%) | 44 (4.4) |
| DAP, (Gy.m2), median [IQR] | 32.7 [19.2-63.0] |
| Fluoroscopy time (min), median [IQR] | 4.6 [2.5-10.0] |
| VAS patient comfort for access, mean (SD) | 2.2 (0.6) |
| VAS patient comfort for hemostasis, mean (SD) | 2.1 (0.4) |
| Hemostasis time, (hour), mean, (SD) | 2.9 (1.1) |
| Access-related complications (all), n (%) | 29 (2.9) |
| Radial artery occlusion, n (%) | 10 (1.0) |
| Hematoma, n (%) | |
| Type I-a, n (%) | 11 (1.1) |
| Type I-b, n (%) | 1 (0.1) |
| Type II, n (%) | 1 (0.1) |
| Type III, n (%) | 1 (0.1) |
| Type IV, n (%) | 0 (0) |
| Radial pseudoaneurysm, n (%) | 0 (0) |
| Radial dissection, n (%) | 5 (0.5) |
| Arteriovenous fistula, n (%) | 0 (0) |
|
DAP, dose-area product; DRA, distal radial access; VAS, visual analog scale. Data are expressed as No. (%), mean ± standard deviation, or median [interquartile range]. |
|
Combined preprocedure ultrasound evaluation and ultrasound-guided puncture were performed in 82.8% of cases, with successful DRA achieved in 97.7% compared with 95.9% in those who did not undergo ultrasound guidance (P = .183). Based on the strength of the arterial pulse—absent, weak, normal, and strong—ultrasound-guided puncture was performed in 100%, 91%, 89.7%, and 45.5% of cases, respectively. Access time was longer with ultrasound-guided puncture than with nonultrasound-guided puncture (40 s [30-70] vs 35 s [30-45]; P < .001). The success of DRA in relation to the use of ultrasound-guided technique among all strengths of arterial pulse is detailed in table 1 of the supplementary data.
Arterial patency after removal of the hemostatic device was assessed in 907 patients (90.7%), revealing RAO in only 1% (n = 10).
In the quartile analysis, a shift in the selection of DRA side was observed, with lDRA initially more commonly used, shifting to rDRA as the preferred access in later quartiles (figure 3A). DRA failure rates were low in all quartiles but decreased significantly from the third quartile onwards (figure 3B). Access time decreased significantly from the second quartile onwards and remained stable thereafter (figure 3C). However, no significant differences were found in total procedure duration between quartiles (figure 3D).
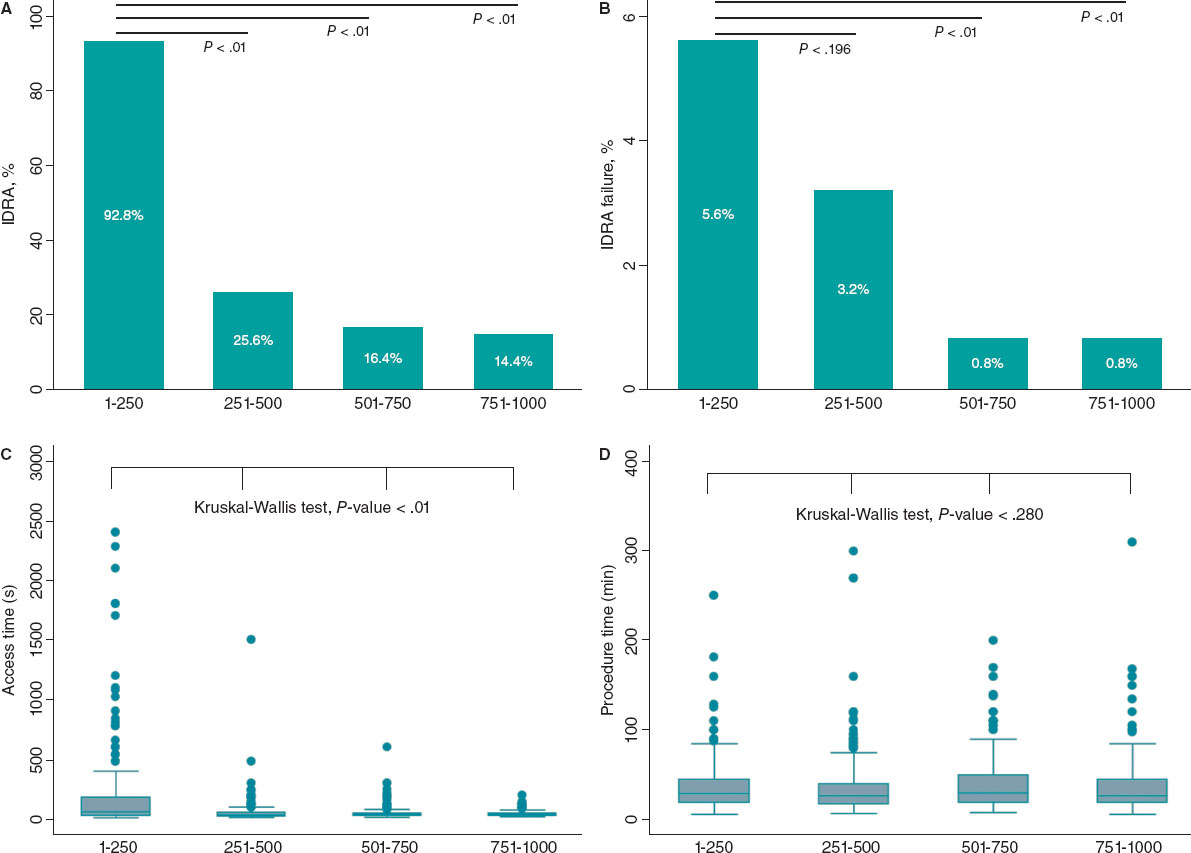
Figure 3. Stratified analysis by quartiles of patients over the study period. A: use of left vs right distal radial access (DRA). B: DRA access failure rate by quartile. C: DRA access time in seconds. D: total procedural time in minutes.
DISCUSSION
Using data from a large prospective registry of patients who underwent DRA for coronary procedures, with high use of ultrasound-guided techniques, our study showed that DRA achieves high rates of access and procedural success, coupled with a low incidence of access-related complications in an all-comer population.
The usefulness of ultrasound in the distal radial access technique
Understanding the anatomy of the anatomical snuffbox is crucial for successful DRA, and ultrasound serves as a valuable tool in achieving this, offering demonstrated advantages.5,16,17,22 In our study, preprocedure ultrasound evaluation and ultrasound-guided DRA techniques were used in most patients. In addition to assessing arterial diameters and evaluating calcification and tortuosity, ultrasound enabled us to exclude patients with unsuitable distal radial arteries. Overall, we found no significant differences between ultrasound-guided and nonultrasound-guided DRA, although the former was associated with longer access times. However, the role of ultrasound is particularly noteworthy in cases of weak or absent arterial pulses, which are often underrepresented in prior studies. The presence of a suboptimal arterial pulse can stem from various factors, including small DRart, hypotension, collateral blood supply, or depth of DRart.11 In our study, most patients with weak pulses underwent ultrasound-guided puncture, with a favorable trend toward successful access in those who did. However, in patients with normal to strong pulses, no differences in DRA success were found, and even prolongation of access time was observed with its use. Therefore, in this type of pulse, an ultrasound-guided puncture is probably not necessary.
Feasibility, safety, and technical issues in distal radial access
This study corroborates the previously reported advantages of DRA,3,9,10,12,13,18 such as a low rate of RAO, acceptable access time, short hemostasis time, and adequate patient comfort.
Furthermore, the absence of an increased risk of hand dysfunction after DRA has been demonstrated,23 even compared with TRA at 12 months of follow-up, documented by Al-Azizi et al.24 Here, we focus on controversial issues that may have hampered wider adoption of this technique, and our results may provide additional support for DRA.
High success rates of DRA in coronary procedures have been reported in numerous studies.2-4,17,18,25 In addition, recent clinical trials and meta-analyzes describe a higher crossover rate compared with TRA.9-13
In contrast to our results, trials comparing DRA with TRA have reported lower access success and longer puncture times.9-11 Conversely, our study demonstrates remarkably high success rates for DRA and coronary procedures, as well as shorter access time, consistent with registries in which DRA is the default approach among experienced operators, as shown by the largest registries published to date, the DISTRACTION and KODRA studies.2-4,18,21
The KODRA trial included 4977 DRA procedures from a Korean registry.21 The authors reported a DRA success rate of 94.4%, with a crossover rate of 6.7%. In contrast to our work, the use of ultrasound-guided puncture in KODRA was low (6.4%). Additionally, the authors found predictors of DRA failure, such as the presence of a weak pulse and limited operator experience (less than 100 cases).
The equivalence of rDRA and lDRA has previously been demonstrated, and contemporary studies use mainly rDRA.9-11,17 As in the first registries, which suggested a potential advantage of lDRA, we started our experience with lDRA but, based on operator comfort and preference, the use of the rDRA increased over time.
Although the feasibility and benefits of DRA over TRA in STEMI have been observed, the literature on the topic remains scarce.2,9-11 In our registry, all attempted DRA procedures in patients with STEMI were successful. However, the first DRA in STEMI was performed after the operators had surpassed the learning curve for the technique (up to case 320). Similarly, the use of DRA for complex PCI has been previously described.22,26,27 In our cohort, all complex PCI procedures were performed without crossover.
The puncture site in DRA, situated 5 cm distal to TRA, may lead to an inadequate catheter length in specific contexts (such as tall patients, dilated aorta, subclavian artery tortuosity, and the need for retrograde access to PCI for chronic total occlusions).28 We found a low incidence of short catheter length during DRA procedures, with only 1 crossover due to severe tortuosity of the subclavian artery.
DRA-related complications have been consistently reported to be low.2,9-11,18 Similarly, we found a very low rate of complications, the most common being type I-a hematoma. In our study, the incidence of in-hospital RAO was 1%.
The number of DRA procedures to overcome the learning curve and maintain a success rate above 94% is around 150 to 200.2,8 However, in our early experience, we achieved this percentage after the first 20 cases per operator.17 In this study, operators navigated the learning curve in the first quartile; however, success significantly improved to more than 99% in the last 2 quartiles, probably because DRA became the default access for coronary procedures among operators.
Limitations
First, this study was an interim analysis of the leading participating site and coordinator of the DISTAL registry (NTC06165406), conducted because substantial enrollment from other sites was lacking. Although the data cannot be fully extrapolated to other centers, recalculation of the sample size was considered sufficient to evaluate the results.
Second, patient enrollment was not consecutive because the decision to use DRA was at the operators’ discretion. Only one-third of coronary procedures during the study period used this approach. However, we included all patients in whom operators intended to use DRA in any clinical setting were included, with only 21 patients excluded due to DRart ≤1.8mm. Third, this was a descriptive cohort of DRA, without a comparison control group. Fourth, the scale used to assess the arterial pulse is subjective. However, this scale is widely used in routine clinical practice and has been used in multiple DRA studies. Finally, radial artery patency was not evaluated in 9.7% of the patients before discharge, and no evaluation was conducted at 1 month; therefore, the in-hospital rate of radial artery occlusion may be underestimated and no mid-term data are available on the patency of the DRart.
CONCLUSIONS
This study shows the safety and feasibility of DRA primarily guided by ultrasound for coronary procedures in an all-comer population, with high rates of both access and procedural success, in addition to a very low rate of access-related complications.
FUNDING
None declared.
ETHICAL CONSIDERATIONS
This study was approved by the Ethics Committee of our institution (CEIC-2804) and was conducted following the principles of the Declaration of Helsinki. All patients gave their informed written consent before the procedure.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
Not used.
AUTHORS’ CONTRIBUTIONS
K. Rivera and D. Fernández-Rodríguez conceived and designed the study. K. Rivera, D. Fernández-Rodríguez, M. García-Guimarães, J. Casanova-Sandoval, and J. L. Ferreiro analyzed data, and drafted the manuscript. All authors contributed to the treatment of patients, data acquisition and mining, and review and approval of the final version of the manuscript.
CONFLICTS OF INTEREST
J. L. Ferreiro reports a) honoraria for lectures from Eli Lilly Co, Daiichi Sankyio, Inc, AstraZeneca, Pfizer, Abbott, Boehringer Ingelheim, Bistol-Myers Squibb, Rovi, Terumo and Ferrer; b) consulting fees from AstraZeneca, Eli Lilly Co, Ferrer, Boston Scientific, Pfizer, Boehringer Ingelheim, Daiichi Sankyo, Inc, Bristol-Myers Squibb and Biotronik; c) research grants from AstraZeneca. The remaining authors have no conflicts of interest to declare.
WHAT IS KNOWN ABOUT THE TOPIC?
- Previous studies have demonstrated the safety and feasibility and safety DRA. Compared with TRA, DRA has several advantages, despite the high prevalence of crossover and controversial incidence of radial artery occlusion.
WHAT DOES THIS STUDY ADD?
- The results of this cohort show the safety and feasibility of DRA in an all-comer population throughout the spectrum of DRart pulses. Our study demonstrates that preprocedure ultrasound evaluation and the ultrasound-guided DRA technique help to achieve a low crossover rate, which is especially useful in patients with an unfavorable arterial pulse. According to our observations, DRA in urgent/emergent procedures and complex PCI is feasible and safe once the learning curve has been overcome and the operator is familiar with the technique.
REFERENCES
1. Babunashvili A, Dundua D. Recanalization and reuse of early occluded radial artery within 6 days after previous transradial diagnostic procedure. Catheter Cardiovasc Interv. 2011;77:530-536.
2. Lee JW, Park SW, Son JW, Ahn SG, Lee SH. Real-world experience of the left distal transradial approach for coronary angiography and percutaneous coronary intervention:A prospective observational study (LeDRA). EuroIntervention. 2018;14:e995-e1003.
3. Oliveira MDP, Navarro EC, Kiemeneij F. Distal transradial access as default approach for coronary angiography and interventions. Cardiovasc Diagn Ther. 2019;9:513-519.
4. Kiemeneij F. Left distal transradial access in the anatomical snuffbox for coronary angiography (ldTRA) and interventions (ldTRI). EuroIntervention. 2017;13:851-857.
5. Sgueglia GA, Di Giorgio A, Gaspardone A, Babunashvili A. Anatomic Basis and Physiological Rationale of Distal Radial Artery Access for Percutaneous Coronary and Endovascular Procedures. JACC Cardiovasc Interv. 2018;11:2113-2119.
6. Lu H, Wu D, Chen X. Comparison of Distal Transradial Access in Anatomic Snuffbox Versus Transradial Access for Coronary Angiography. Heart Surg Forum. 2020;23:E407-E410.
7. Ghose T, Kachru R, Dey J, Khan WU, et al. Safety and Feasibility of Ultrasound-Guided Access for Coronary Interventions through Distal Left Radial Route. J Interv Cardiol. 2022;2022:2141524.
8. Roh JW, Kim Y, Lee OH, et al. The learning curve of the distal radial access for coronary intervention. Sci Rep. 2021;11:13217.
9. Tsigkas G, Papageorgiou A, Moulias A, et al. Distal or Traditional Transradial Access Site for Coronary Procedures:A Single-Center, Randomized Study. JACC Cardiovasc Interv. 2022;15:22-32.
10. Aminian A, Sgueglia GA, Wiemer M, et al. Distal Versus Conventional Radial Access for Coronary Angiography and Intervention:The DISCO RADIAL Trial. JACC Cardiovasc Interv. 2022;15:1191-1201.
11. Kozin´ski Ł, Orzałkiewicz Z, Da˛browska-Kugacka A. Feasibility and Safety of the Routine Distal Transradial Approach in the Anatomical Snuffbox for Coronary Procedures:The ANTARES Randomized Trial. J Clin Med. 2023;12:7608.
12. Ferrante G, Condello F, Rao SV, et al. Distal vs Conventional Radial Access for Coronary Angiography and/or Intervention:A Meta-Analysis of Randomized Trials. JACC Cardiovasc Interv. 2022;15:2297-2311.
13. Barbarawi M, Barbarawi O, Jailani M, Al-Abdouh A, Mhanna M, Robinson P. Traditional versus distal radial access for coronary angiography:A meta-Analysis of randomized controlled trials. Coron Artery Dis. 2023;34:274-280.
14. Erdem K, Kurtogˇlu E, Küçük MA, Ilgenli TF, Kizmaz M. Distal transradial versus conventional transradial access in acute coronary syndrome. Turk Kardiyoloji Dernegi Arsivi. 2021;49:257-265.
15. Valgimigli M, Campo G, Penzo C, Tebaldi M, Biscaglia S, Ferrari R. Transradial coronary catheterization and intervention across the whole spectrum of allen test results. J Am Coll Cardiol. 2014;63:1833-1841.
16. Sgueglia GA, Lee BK, Cho BR, et al. Distal Radial Access:Consensus Report of the First Korea-Europe Transradial Intervention Meeting. JACC Cardiovasc Interv. 2021;14:892-906.
17. Rivera K, Fernández-Rodríguez D, Casanova-Sandoval J, et al. Comparison between the Right and Left Distal Radial Access for Patients Undergoing Coronary Procedures:A Propensity Score Matching Analysis. J Interv Cardiol. 2022;2022:7932114.
18. Oliveira MD, Navarro EC, Caixeta A. Distal transradial access for coronary procedures:A prospective cohort of 3,683 all-comers patients from the DISTRACTION registry. Cardiovasc Diagn Ther. 2022;12:208-219.
19. Hadjivassiliou A, Kiemeneij F, Nathan S, Klass D. Ultrasound-guided access to the distal radial artery at the anatomical snuffbox for catheter-based vascular interventions:A technical guide. EuroIntervention. 2021;16:1342-1348.
20. Calculadora de tamaño muestral GRANMO. Available at:https://www.imim.cat/media/upload/arxius/granmo/granmo_v704.html. Accessed 25 Mar 2024.
21. Lee JW, Kim Y, Lee BK, et al. Distal Radial Access for Coronary Procedures in a Large Prospective Multicenter Registry:The KODRA Trial. JACC Cardiovasc Interv. 2024;17:329-340.
22. Zong B, Liu Y, Han B, Feng CG. Safety and feasibility of a 7F thin-walled sheath via distal transradial artery access for complex coronary intervention. Front Cardiovasc Med. 2022;9:959197.
23. Sgueglia GA, Hassan A, Harb S, et al. International Hand Function Study Following Distal Radial Access:The RATATOUILLE Study. JACC Cardiovasc Interv. 2022;15:1205-1215.
24. Al-Azizi K, Moubarak G, Dib C, et al. Distal Versus Proximal Radial Artery Access for Cardiac Catheterization:1-Year Outcomes. Am J Cardiol. 2024;220:102-110.
25. Rivera K, Fernández-Rodríguez D, Bullones J, et al. Impact of sex differences on the feasibility and safety of distal radial access for coronary procedures:a multicenter prospective observational study. Coron Artery Dis. 2024;35(5):360-367.
26. Rivera K, Fernández-Rodríguez D, García-Guimarães M, Ramírez Martínez T, Casanova-Sandoval J. Intravascular ultrasound-guided percutaneous exclusion of a complicated coronary artery aneurysm presenting as ST-segment elevation myocardial infarction. Coron Artery Dis. 2023;34:527-528.
27. Nikolakopoulos I, Patel T, Jefferson BK, et al. Distal Radial Access in Chronic Total Occlusion Percutaneous Coronary Intervention:Insights From the PROGRESS-CTO Registry. J Invasive Cardiol. 2021;33:E717-E722.
28. Davies RE, Gilchrist IC. Back hand approach to radial access:The snuff box approach. Cardiovasc Revasc Med. 2018;19:324-326.
ABSTRACT
Introduction and objectives: Drug-eluting balloons (DEB) are an established treatment option for in-stent restenosis (ISR). This study aimed to assess the safety and efficacy of a novel DEB in patients with ISR.
Methods: This prospective, single-center study enrolled a consecutive cohort of patients diagnosed with ISR who underwent coronary angioplasty with a new second-generation paclitaxel-eluting balloon. The 3 main endpoints were myocardial infarction, target lesion revascularization, and target vessel revascularization. Baseline variables were collected, including patient and procedure characteristics. Follow-up data were collected through medical records or telephone contact.
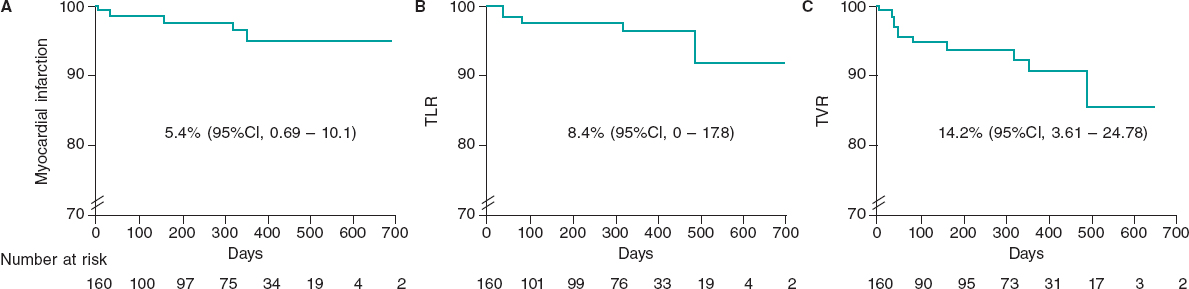
Results: The study included 160 consecutive patients with 206 treated lesions (mean age, 71.4 ± 14.9 years, 15.5% women) undergoing percutaneous coronary intervention with DEB for ISR. A total of 53.3% of patients had acute coronary syndrome. The average diameter of the treated vessel was 3.10 ± 0.7 mm. The DEB used had a mean diameter of 3.1 ± 0.6 mm and a mean length of 23.1 ± 6.8 mm. Predilatation was performed in 98% of the lesions, and a noncompliant balloon was used in 80%. Intracoronary imaging was used in 24% of cases. At the end of the procedure, 98.5% of patients had Thrombolysis in Myocardial Infarction flow grade 3, residual stenosis was > 30% in 3.4%, and dissection occurred in 1.4%. Bail-out stenting was required in 4.8% of patients. Mortality was nil during follow-up (maximum 768 days). The incidence of myocardial infarction, target lesion revascularization, and target vessel revascularization were 5.4% (95%CI, 0.69-10.1), 8.4% (95%CI, 0-17.8), and 14.2% (95%CI, 3.61-24.78), respectively.
Conclusions: In this cohort of patients with ISR treated with DEB, we observed a low rate of adverse events in both the short- and mid-term. These results support the safety and efficacy of this new generation of DEB for treating ISR.
Keywords: In-stent restenosis. Drug-eluting balloon. Paclitaxel.
RESUMEN
Introducción y objetivos: El balón farmacoactivo (BFA) es un tratamiento establecido para tratar la reestenosis intrastent (RIS). El objetivo de este estudio fue valorar la eficacia y la seguridad de un nuevo BFA en pacientes con RIS.
Métodos: Cohorte prospectiva, unicéntrica y consecutiva de pacientes con RIS tratados con angioplastia coronaria con un nuevo balón liberador de paclitaxel de segunda generación. Los 3 eventos principales del estudio fueron infarto de miocardio, revascularización de la lesión diana y revascularización del vaso diana. Se recogieron variables basales, incluidas las características del paciente y del procedimiento. Los datos referentes al seguimiento se obtuvieron de registros médicos o por contacto telefónico.
Resultados: Se incluyeron 160 pacientes consecutivos con 206 lesiones tratadas (71,4 ± 14,9 años, el 15,5% mujeres) que fueron tratados con una intervención coronaria percutánea con BFA debido a RIS. El 53,3% de los pacientes presentaban síndrome coronario agudo. El diámetro medio del vaso tratado fue de 3,1 ± 0,7 mm. El diámetro y la longitud del BFA empleado fueron de 3,1 ± 0,6 mm y 23,1 ± 6,8, respectivamente. El 98% de las lesiones se predilataron y en el 80% se empleó un balón no distensible. El 24% de las angioplastias fueron guiadas por imagen intracoronaria. El 98,5% de los pacientes presentaban un flujo Thrombolysis in Myocardial Infarction de grado 3 al final de la angioplastia. Hubo estenosis residual > 30% en el 3,4%, y el 1,4% presentaron disección. El 4,8% de los pacientes requirieron stent de rescate. Al finalizar el seguimiento (máximo 768 días), ningún paciente había fallecido. Las incidencias de infarto de miocardio, de revascularización de la lesión diana y de revascularización del vaso diana fueron del 5,4% (IC95%, 0,69-10,1), el 8,4% (IC95%, 0-17,8) y el 14,2% (IC95%, 3,61-24,78), respectivamente.
Conclusiones: En esta cohorte de pacientes con RIS tratados con BFA se observa una baja tasa de eventos clínicos adversos, tanto a corto como a mediano plazo. Estos resultados respaldan la eficacia y la seguridad de esta nueva generación de BFA para pacientes con RIS.
Palabras clave: Reestenosis intrastent. Balón farmacoactivo. Paclitaxel.
Abbreviations
DEB: drug-eluting balloon. ISR: in-stent restenosis. TLR: target lesion revascularization. TVR: target vessel revascularization.
INTRODUCTION
Patients with coronary in-stent restenosis (ISR) represent a clinical challenge.1 Evidence indicates that these patients are at increased risk of recurrent symptoms, myocardial infarction, and repeated coronary revascularizations.2 The use of drug-eluting balloons (DEB) is a novel alternative therapeutic strategy in patients with ISR.1,3,4 The effect of DEBs in coronary angioplasty is based on the rapid and uniform transfer of antiproliferative drugs into the vessel wall using a single balloon through a lipophilic matrix without the need for permanent implants.5
Over time, new DEB technologies are developed and launched onto the market. The Essential Pro (iVascular, Spain) is a paclitaxel-eluting balloon catheter with advancements to enhance catheter pushability and drug delivery. We believe it is essential to report outcomes from real-world settings. In this study, we report our findings on the safety and efficacy of this new DEB in patients with ISR.
METHODS
Design and population
This prospective, single-center study included a cohort of consecutive patients undergoing DEB angioplasty with the Essential Pro. The center treating these patients performs more than 1500 percutaneous coronary interventions per year. The 2 inclusion criteria for this analysis were: a) use of an Essential Pro DEB and b) its application for ISR treatment. ISR was defined as stenosis more than 50% within the stented segment, and treatment was indicated according to the treating physician’s judgment.6 The use of the Essential Pro DEB was prioritized during the study period to treat all eligible patients for DEB angioplasty, while other DEB devices were rarely used due to inventory constraints. There were no exclusion criteria. Patients may have undergone stent coronary angioplasty of other lesions in the same or a different setting.
Drug-eluting balloon characteristics
The Essential Pro is a paclitaxel-eluting balloon with a uniform 3 μg/mm2 eluting formulation, consisting of paclitaxel (80%) and a biocompatible amphiphilic excipient (20%).7 The balloon incorporates the proprietary TransferTech technology (iVascular, Spain), which is based on the ultrasonic deposition of nanodrops, followed by a dry-off process, resulting in a homogeneous microcrystalline drug coating. This allows more uniform and complete treatment of the vessel with the antiproliferative drug. The microcrystalline structure, coupled with the lipophilic nature of both paclitaxel and the excipient, facilitates drug transfer within 45 to 60 seconds. The Essential Pro balloon has been designed with a smooth transition and a very low tip profile of 0.016 inches, enhancing flexibility, trackability, and device crossability. The balloon is compatible with 5-Fr sheaths in all available diameters.
Procedures
All procedures and decisions in this study reflect real-world clinical practice. Therefore, clinical indications, the use and selection of DEBs, procedural steps, and medical treatments were decided by treating physicians without following any specific guidelines. All coronary angiograms performed during follow-up were part of routine clinical practice and were assessed by our research team when available. Baseline and follow-up data were collected in a single anonymized dedicated database. Procedural aspects, as well as both baseline and follow-up angiograms, were independently evaluated by 3 different interventional cardiologists. Physicians were trained to consult senior staff if they had doubts when assessing angiograms or clinical records. Follow-up was conducted using clinical records, and patients with no on-site clinical visits during follow-up were contacted by telephone following standard clinical practice in our institution. This study was approved by our local institutional review board and patients provided consent for the use of their anonymized information for research purposes before inclusion. This was an investigator-initiated study with no sponsoring or funding.
Outcome definitions
Device delivery was defined as successful DEB insufflation in the affected coronary segment. Procedural, angiographic, and other standard outcomes were defined according to the Second Academic Research Consortium criteria.8 Cardiovascular mortality was defined as any death without a clear noncardiovascular cause. Acute myocardial infarction was defined as any myocardial infarction meeting the fourth version of the Universal Myocardial Infarction Criteria.9 Target lesion revascularization (TLR) was defined as any revascularization within or 5 mm beyond the treated segment.8 Target vessel revascularization (TVR) was defined as revascularization of the index treated vessel.8 Coronary-related hospitalization was defined as a new hospitalization in which a coronary origin was suspected as the main reason for admission. The 3 main efficacy outcomes were myocardial infarction, TLR, and TVR.
Statistical analysis
Categorical variables are presented as percentages, and continuous variables as mean standard deviation (SD) when appropriate. Since the same patient may receive more than 1 DEB (for the same or different territory), the denominator for balloon-specific variables was based on the total DEBs used (such as treated vessel, vessel diameter, DEB diameter, and length), while the denominator of patient-level variables (such as age, sex, or clinical outcomes) was each single individual. Clinical outcomes during follow-up are presented at 30 days, 1 year, and total follow-up. The Kaplan-Meier method was used for estimating both the total follow-up risk and generating survival curves. Data were analyzed using IBM SPSS Statistics 25.
RESULTS
From December 2020 to June 2023, 290 patients with 352 coronary lesions were treated with DEB. Among them, 160 patients (206 lesions) underwent DEB angioplasty due to ISR. Out of the 160 patients receiving DEB for ISR, 46 patients (29%) received more than 1 DEB angioplasty for ISR, either during the same procedure or staged to a different lesion.
The patients’ baseline characteristics are summarized in table 1. The mean age was 71.4 ± 14.9 years, 15.5% were women, and 35.5% had diabetes. Clinical presentation was stable angina in 29.7%, unstable angina in 30.5%, non–ST-segment elevation myocardial infarction in 9.9%, ST-segment elevation myocardial infarction in 12.9%, and 16.7% were asymptomatic.
Table 1. Baseline characteristics
| Patient characteristics | |
| Age, y | 71.4 (14.9) |
| Sex women | 20 (15.5) |
| BMI, kg/m2 | 29.2 (10.5) |
| Hypertension | 115 (87.7) |
| Active smoking | 8 (6.1) |
| Diabetes mellitus | 46 (35.3) |
| Previous MI | 67 (51.5) |
| Previous CABG | 26 (20) |
| Reduced LVEF (< 30%) | 10 (7.6) |
| Laboratory parameters | |
| Hemoglobin, g/dL | 13.9 (1.5) |
| GFR, mL/min/1.73 m2 | 82.9 (25.4) |
| Active medication | |
| Aspirin | 110 (84.6) |
| Clopidogrel | 75 (57.6) |
| Ticagrelor | 3 (2.3) |
| Prasugrel | 2 (1.5) |
| Anticoagulation | 20 (15.2) |
| Clinical presentation | |
| Silent ischemia | 22 (16.7) |
| Stable angina | 39 (29.7) |
| Unstable angina | 40 (30.5) |
| NSTEMI | 13 (9.9) |
| STEMI | 17 (12.9) |
|
BMI, body mass index; CABG, coronary artery bypass grafting; GFR, glomerular filtration rate; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NSTEMI, non–ST-segment elevation myocardial infarction; STEMI, ST-segment elevation myocardial infarction. Data are expressed as No. (%). |
|
Procedural characteristics are detailed in table 2. The most commonly treated vessel was the left anterior descending artery (48.7%), followed by the left circumflex (30.7%), and the right coronary artery (17%). Bifurcation was present in 10.7%. Lesion preparation was performed in 98.2% of cases (80% with a noncompliant balloon). Intracoronary imaging was used in 24% of patients. None of the patients underwent rotational atherectomy, and 2.4% underwent balloon lithotripsy before DEB delivery. The mean vessel diameter was 3.1 ± 0.65 mm. The mean DEB diameter was 3.1 ± 0.6 mm, and the mean length was 23.1 ± 6.8 mm. Device delivery was successful in 100% of cases (figure 1). The final angiographic assessment revealed a final dissection in 1.4%, Thrombolysis in Myocardial Infarction flow less than 3 in 1.5%, and residual stenosis more than 30% in 3.4%. Bail-out stenting was needed in 4.8%.
Table 2. Characteristics of the treated lesion
| Treated vessel | |
| LAD | 100 (48.7) |
| LCx | 63 (30.7) |
| Right coronary artery | 35 (17) |
| Left main coronary artery | 5 (2.4) |
| Graft | 2 (0.9) |
| Anatomical characteristics | |
| Bifurcation lesion | 22 (10.7) |
| Vessel diameter, mm | 3.1 (0.65) |
| Procedural characteristics | |
| IVUS-guided PCI | 51 (24) |
| Lesion predilatation | 202 (98) |
| Predilatation with NC balloon | 165 (80) |
| Intravascular lithotripsy | 5 (2.4) |
| DEB diameter, mm | 3.1 (0.6) |
| DEB length, mm | 23.1 (6.8) |
| Result after DEB PCI | |
| Vessel dissection | 3 (1.4) |
| TIMI flow 3 | 203 (98.5) |
| Residual stenosis > 30% | 194 (3.4) |
| Bail-out stenting | 10 (4.8) |
|
DEB, drug-eluting balloon; IVUS, intravascular ultrasound; LAD, left anterior descending artery; LCx, left circumflex artery; NC, noncompliant; PCI, percutaneous coronary intervention; RCA, right coronary artery; TIMI, Thrombolysis in Myocardial Infarction. Data are expressed as No. (%). |
|
Figure 1. Central illustration. Main findings on the safety and efficacy of the Essential Pro drug-eluting balloon in patients with in-stent restenosis. Kaplan-Meier shows freedom from TLR. MI, myocardial infarction; TLR, target lesion revascularization; TVR, target vessel revascularization.
After discharge, 93.3% of the patients were successfully contacted. The median follow-up was 361 days, including censored patients, with a maximum of 768 days. At 30 days of follow-up, there were no deaths or TLR, there was 1 myocardial infarction (0.6%), TVR occurred in 0.6%, and 6 patients were readmitted to hospital due to a coronary syndrome (4.1%). At the 1-year follow-up, mortality was 0%, myocardial infarction occurred in 3.4%, TLR in 2.5%, TVR in 6.3%, and coronary-related rehospitalizations in 11.8%. At 18 months, the TLR rate was 4.3%. When all available follow-up was included (figure 2), mortality was 0%, myocardial infarction occurred in 5.4% (95% confidence interval [95%CI], 0.69-10.1), TLR in 8.4% (95%CI, 0-17.8), and TVR in 14.2% (95%CI, 3.61-24.78). During follow-up, none of the patients underwent surgical revascularization.
Figure 2. Survival curves of key clinical outcomes. Kaplan-Meier estimates for survival free from myocardial infarction (A), target lesion revascularization (B), and target vessel revascularization (C) in days. 95%CI, 95% confidence interval; TLR, target lesion revascularization; TVR, target vessel revascularization.
DISCUSSION
This is the first study to describe a real-world experience with the Essential Pro DEB for the treatment of ISR. In this cohort, all attempts at DEB delivery were successful, and less than 1 in 20 patients required bail-out stenting. The use of this new-generation DEB catheter was associated with high efficacy and a low incidence of adverse clinical outcomes during follow-up.
Patients with ISR are at higher risk of recurrent events than those undergoing non-ISR angioplasty.10 The annual rate of ISR requiring TLR is around 2%,3 representing up to 11% of all percutaneous coronary interventions performed in the United States.11,12 Notably, 52% of patients presenting with symptomatic ISR have unstable angina, and up to 27% have an acute myocardial infarction.12 Therefore, ISR poses a significant clinical challenge due to both its frequency and severity. The use of DEB in the ISR scenario avoids the addition of extra stent layers, which may have detrimental effects in the long term.
The use of DEB in ISR poses certain challenges. DEB platforms commonly have lower lesion crossability than regular coronary balloon catheters. DEBs also have larger profiles than conventional balloons making it difficult to cross the lesion and requiring aggressive maneuvers that could lead to a loss of coating drug during delivery.13 However, in our study, all attempted DEB deployments were successful. This high success rate may be due to improvements in second-generation DEBs, as well as better lesion evaluation and lesion preparation.
In the present study, TLR occurred in 2.5% of the patients and TVR in 6.3% at 1 year, while TLR occurred in 4.3% at 18 months. This event rate may seem low when compared with a prior systematic review of randomized and observational studies, which reported a TVR rate after DEB treatment of 11.3% with a calculated weighted mean follow-up of 18 months.14 In a recent investigational device exemption randomized trial for a paclitaxel-coated balloon in ISR, the rate of TLR at 1 year was 13%.15 However, prior evidence stems from diverse settings, designs, and populations, making it difficult to draw strong conclusions.
The rate of TLR with the previous generation of the Essential Pro DEB in a smaller cohort (n = 31) was 10% at 6 months.16 While this rate may seem higher than that reported in our study, the small number of events (n = 3) makes comparisons challenging.
Limitations
This study has some limitations. First, it was based on a real-world cohort involving different operators from the same center, which does not follow specific protocols. Only a quarter of the patients underwent angioplasty assessment guided by intracoronary imaging. The lack of sponsorship to cover intracoronary imaging costs and its limited use reflects the usual clinical practice of this center. During the performance of this study, few patients with ISR were treated with other DEB catheters due to the lack of specific DEB sizes in stock. Since this situation was rare and was unrelated to clinical or medical coverage characteristics, it is unlikely to introduce significant bias. Since this was a substudy of a larger DEB cohort, some variables specific to ISR, such as the time from prior stent implantation or the type of stent used, were not available.
Second, there were no dedicated follow-up visits for this study. Although most of these patients were followed up by local cardiologists who maintained regular medical records, some required telephone contact for follow-up. Third, angiographic assessment was not duplicated, and no core lab was available. Finally, the number of events was low despite consecutive enrollment from late 2020, impacting the precision of Kaplan-Meier estimates for key clinical outcomes. Some limitations are related to real-world practice settings, which, on the other hand, enhance external validity with less selection bias compared with other more controlled designs.
CONCLUSIONS
Among patients with ISR, the Essential Pro DEB catheter had a high delivery rate and a low incidence of adverse clinical outcomes during follow-up. These results further underscore the safety and efficacy of this new-generation DEB for patients with ISR.
FUNDING
This work received no industry sponsoring or funding.
ETHICAL CONSIDERATIONS
This study was approved by our local institutional review board at the Instituto Cardiovascular de Buenos Aires, and patients provided written informed consent to use their anonymized information for research purposes before their inclusion. Possible sex/gender biases have been considered in the preparation of this paper.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
No artificial intelligence tool was used in the preparation of this study.
AUTHORS’ CONTRIBUTIONS
L. Padilla conceived and oversaw all the process. F. Liberman, J. Tello, P. Rosas, P. Spaletra, G. Pedernera, P. Mascolo, S. Ordoñez, P. Santilli, and A. Candiello collected data and analyzed coronary angiograms. F. Cura and J. Belardi provided senior advice. P. Lamelas performed the statistical analysis and generated the first draft of the manuscript.
CONFLICTS OF INTEREST
L. Padilla has received proctoring and consulting honoraria from Terumo and Boston Scientific. P. Spaletra has received honoraria from Boston Scientific. F. Cura has received honoraria from Medtronic, Boston Scientific, Terumo, and Meril. P. Lamelas has received proctoring and consulting honoraria from Medtronic, Boston Scientific, Meril, Microport. The remaining authors have no conflicts of interest to declare.
WHAT IS KNOWN ABOUT THE TOPIC?
- Patients with ISR are at high risk of recurrent events and are commonly treated with DEB. New or newer generation DEBs are frequently launched onto the market. It is important to report the real-world safety and efficacy of interventional devices. The Essential Pro is a secondgeneration paclitaxel-eluting balloon. Enhancements of this DEB include improvements in forward pushability, crossover capacity, and drug delivery capabilities.
WHAT DOES THIS STUDY ADD?
- Using this new-generation DEB, all attempts at treating ISR (n = 206) were successful. Intravascular ultrasound was used in 24%. The incidence of adverse events, from the procedure to mid-term follow-up, was infrequent and probably lower than that previously reported. These realworld results emphasize the safety and efficacy of this novel generation DEB for patients with ISR.
REFERENCES
1. Giacoppo D, Saucedo J, Scheller B. Coronary Drug-Coated Balloons for De Novo and In-Stent Restenosis Indications. J Soc Cardiovasc Angiogr Interv. 2023. https://doi.org/10.1016/j.jscai.2023.100625.
2. Pleva L, Kukla P, Hlinomaz O. Treatment of coronary in-stent restenosis:A systematic review. J Geriatr Cardiol. 2018;15:173-184.
3. Giustino G, Colombo A, Camaj A, et al. Coronary In-Stent Restenosis:JACC State-of-the-Art Review. J Am Coll Cardiol. 2022;80:348-372.
4. Indermuehle A, Bahl R, Lansky AJ, et al. Drug-eluting balloon angioplasty for in-stent restenosis:A systematic review and meta-analysis of randomised controlled trials. Heart. 2012;99:327-333.
5. Jeger R V., Eccleshall S, Wan Ahmad WA, et al. Drug-Coated Balloons for Coronary Artery Disease:Third Report of the International DCB Consensus Group. JACC Cardiovasc Interv. 2020;13:1391-1402.
6. Klein LW, Nathan S, Maehara A, et al. SCAI Expert Consensus Statement on Management of In-Stent Restenosis and Stent Thrombosis. J Soc Cardiovasc Angiogr Interv. 2023;2:100971.
7. Pérez de Prado A, Pérez-Martínez C, Cuellas Ramón C, et al. Safety and Efficacy of Different Paclitaxel-eluting Balloons in a Porcine Model. Rev Esp Cardiol. 2014;67:456-462.
8. Garcia-Garcia HM, McFadden EP, Farb A, et al. Standardized end point definitions for coronary intervention trials:The academic research consortium 2 consensus document. Circulation. 2018;137:2635-2650.
9. Domienik-Karlowicz J, Kupczyn´ska K, Michalski B, et al. Fourth universal definition of myocardial infarction. Selected messages from the european society of cardiology document and lessons learned from the new guidelines on st-segment elevation myocardial infarction and non-st-segment elevation-acute coronary syndrome. Cardiol J. 2021;28:195-201.
10. Steinberg DH, Pinto Slottow TL, Buch AN, et al. Impact of In-Stent Restenosis on Death and Myocardial Infarction. Am J Cardiol. 2007;100:1109-13.
11. Madhavan M V, Kirtane AJ, Redfors B, et al. Stent-Related Adverse Events >1 Year After Percutaneous Coronary Intervention. J Am Coll Cardiol. 2020;75:590-604.
12. Moussa ID, Mohananey D, Saucedo J, et al. Trends and Outcomes of Restenosis After Coronary Stent Implantation in the United States. J Am Coll Cardiol. 2020;76:1521-1531.
13. Yoshida R, Ishii H, Morishima I, et al. Impact of adjunctive use of guide extension catheter on midterm outcome of drug-coated balloon angioplasty. EuroIntervention. 2019;15:688-691.
14. Cui KY, Lyu SZ, Zhang M, Song XT, Yuan F, Xu F. Drug-Eluting Balloon versus New-Generation Drug-Eluting Stent for the Treatment of In-Stent Restenosis:An Updated Systematic Review and Meta-Analysis. Chin Med J (Engl). 2018;131:600-607.
15. Yeh RW, Shlofmitz R, Moses J, et al. Paclitaxel-Coated Balloon vs Uncoated Balloon for Coronary In-Stent Restenosis:The AGENT IDE Randomized Clinical Trial. JAMA. 2024;331:1015-1024.
16. de la Torre Hernández JM, Garcia Camarero T, Lozano Ruiz-Poveda F, et al. Angiography and Optical Coherence Tomography Assessment of the Drug-Coated Balloon ESSENTIAL for the Treatment of In-Stent Restenosis. Cardiovasc Revasc Med. 2020;21:508-513.
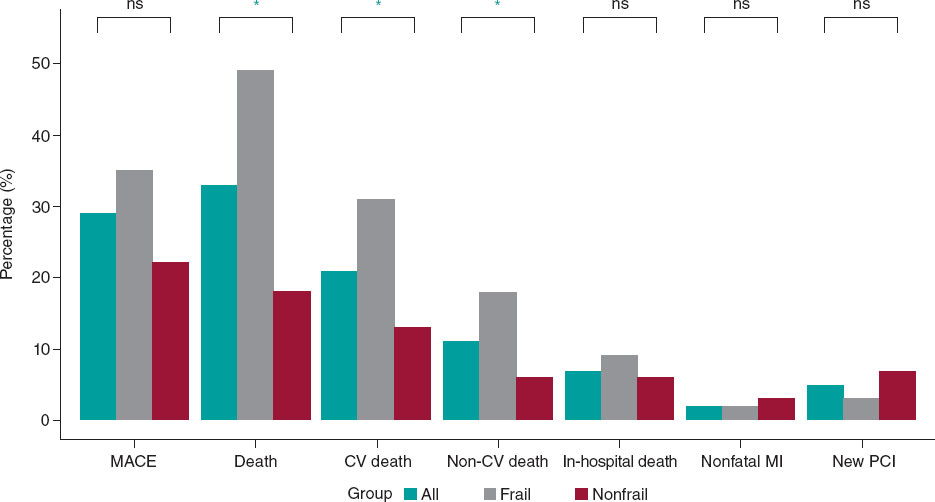
ABSTRACT
Introduction and objectives: In elderly and frail patients, there is limited evidence on the therapeutic management of left main coronary artery (LM) disease. The objective of this study was to evaluate mid-term clinical outcomes in older adults undergoing percutaneous coronary intervention (PCI) of LM.
Methods: We conducted a retrospective study including all older patients (≥ 75 years) undergoing LM-PCI at a high-volume center between 2017 and 2021. The primary endpoint was a composite of major adverse cardiovascular events (MACE). Patients were grouped according to the presence of frailty based on the FRAIL scale. Inverse probability of treatment weighting was used to account for clinical differences between the 2 groups.
Results: A total of 140 patients were included in the study (median age 80 [78-84]; 36% women). Of them, 49% met the criteria for frailty. After a median follow-up of 19 [5-35] months, 40 MACE (29%) were recorded. The all-cause death rate was 32%. There were no differences in the risk of MACE between frailty groups, but patients with frailty had an increased risk of all-cause mortality (HRadj, 1.95 [1.02-3.75]; P = .046).
Conclusions: LM-PCI in older adults with multiple associated comorbidities could be considered a feasible option in this special population. The rate of MACE at follow-up was acceptable. Frailty was associated with a worse prognosis in terms of all-cause mortality at follow-up.
Keywords: Coronary artery disease. Left main coronary artery. Percutaneous coronary intervention. Elderly. Frailty.
RESUMEN
Introducción y objetivos: La evidencia sobre el abordaje terapéutico de la enfermedad del tronco coronario izquierdo (TCI) en pacientes ancianos y frágiles es limitada. El objetivo de este estudio fue evaluar los resultados clínicos a medio plazo en ancianos que recibieron una intervención coronaria percutánea (ICP) del TCI.
Métodos: Estudio retrospectivo en el que se incluyeron todos los pacientes ancianos (≥ 75 años) tratados con ICP del TCI en un centro de alto volumen entre 2017 y 2021. El objetivo principal fue un compuesto de eventos adversos cardiovasculares mayores (MACE). Los pacientes fueron agrupados en función de su fragilidad según la escala FRAIL. Se utilizó la ponderación de probabilidad inversa de tratamiento para tener en cuenta las diferencias clínicas entre los 2 grupos.
Resultados: Se incluyeron 140 pacientes (mediana de edad: 80 años [78-84]; 36% mujeres), de los cuales el 49% cumplían los criterios de fragilidad. Tras una mediana de seguimiento de 19 meses (5-35) se registraron 40 MACE (29%). La tasa de mortalidad por todas las causas fue del 32%. No se observaron diferencias en el riesgo de MACE entre los grupos, aunque los pacientes frágiles presentaron una mayor mortalidad por todas las causas (HRa = 1,95 [1,02-3,75]; p = 0,046).
Conclusiones: La ICP del TCI en pacientes ancianos con comorbilidad podría considerarse una opción factible en esta población especial. La tasa de MACE en el seguimiento resulta aceptable. La fragilidad se asoció con un peor pronóstico en términos de mortalidad por todas las causas durante el seguimiento.
Palabras clave: Enfermedad arterial coronaria. Tronco coronario izquierdo. Intervención coronaria percutánea. Paciente anciano. Fragilidad.
Abbreviations
CABG: coronary artery bypass grafting. LM: left main coronary artery. PCI: percutaneous coronary intervention.
INTRODUCTION
The left main coronary artery (LM) supplies 84% of the blood flow to the left ventricle in patients with right dominance,1 making LM disease the coronary lesion with the worst prognosis. The prevalence of this disease is not negligible, as it is found in 4.8% of coronary angiograms,2 highlighting the prognostic importance of these lesions. Conservative treatment is a rarely a feasible option due to the high rate of cardiac adverse events during short-term follow-up, with a mortality rate exceeding 50%.3
Coronary artery bypass grafting (CABG) has traditionally been the most widely accepted revascularization strategy.4 In recent years, there have been significant pharmacological and technological improvements in percutaneous revascularization techniques, such as drug-eluting stents and intracoronary diagnostic techniques.5 These improvements, together with comparative studies, have prompted discussion on the various alternatives.6 Presently, the choice of revascularization strategy should be based on the complexity of the coronary anatomy and surgical risk.7
However, evidence is limited in older adults who are scarcely represented in classic studies. Furthermore, in these patients, frailty is a frequent and unstudied characteristic that can influence their prognosis. In this special population, CABG is usually ruled out due to high-surgical risk. On the other hand, percutaneous coronary intervention (PCI) could be a potential therapeutic option, although with little evidence to date.8 Consequently, we postulated that PCI of the LM might be feasible and safe in older patients, with a low incidence of associated complications and an acceptable rate of major adverse cardiac events (MACE) during follow-up.
METHODS
Study design
We conducted a retrospective, single-center study of older patients diagnosed with LM disease who underwent PCI. The study aimed to evaluate mid-term clinical outcomes and examine the prognostic significance of frailty in these patients. The study protocol was approved by the local clinical research ethics committee according to institutional and good clinical practice guidelines. Recruitment took place from January 2017 to December 2021 at Hospital Universitario Reina Sofía (Cordoba, Spain). Patients were eligible if they were aged ≥ 75 years at the time of LM disease diagnosis, and PCI was chosen as the treatment after deliberation by heart team discussion, or due to instability requiring emergent revascularization. Exclusion criteria consisted of end-stage chronic diseases, patients under palliative care, contraindications to dual antiplatelet therapy, and incomplete follow-up data. Included patients were grouped according to frailty status, determined by the FRAIL scale, with patients scoring 3 or more points considered frail.9 Definitions are shown in the supplementary data.
Outcomes
The main objective of the study was to describe mid-term clinical outcomes in older patients undergoing LM-PCI. We also aimed to compare clinical events according to the presence of frailty. The primary endpoint was a composite of MACE, defined as a composite of cardiovascular death (including death of uncertain cause), nonfatal myocardial infarction, the need for new revascularization, and stroke. Secondary outcomes were the individual components of MACE and all-cause mortality.
Angiographic analysis
Quantitative analysis of the coronary arteries was performed using the validated CAAS system (Pie Medica Imaging, the Netherlands). The basal anatomy of the LM bifurcation with the anterior descending artery and the circumflex artery was classified according to the Medina classification.10 The measurements analyzed included the reference diameter of the LM and its percentage of stenosis. The complexity of the coronary anatomy was studied using the SYNTAX scale.6
Statistical analysis
Categorical data are presented as counts (percentages), while continuous data are expressed as mean ± standard deviation or median [interquartile range]. Between-group comparisons were performed using the chi-square test or the Fisher exact test for categorical variables and the Student t-test or the Mann-Whitney U test for continuous variables. Kaplan-Meier curves and Cox regression models were used to analyze clinical events according to frailty. Inverse probability of treatment weighting (IPTW) was used to account for clinical differences between the 2 groups.11 Propensity scores were calculated using a logistic regression model that included the following covariates: age, sex, left ventricular ejection fraction, atrial fibrillation, chronic kidney disease, anemia, and chronic obstructive pulmonary disease. Standardized mean differences before and after weighting were used to evaluate the balance of the groups regarding the covariates. A difference of < 10% was considered to indicate a satisfactory balance. The distributions of the propensity scores before and after weighting were plotted to assess the degree of overlap between the 2 groups. Confidence intervals for the IPTW coefficients were obtained using robust sandwich-type variance estimators (figure 1 of the supplementary data).12 All tests were 2-tailed and significance was set at P < .05. Statistical analyses were performed using SPSS software (V 24; IBM Corp., United States) and R software (V4.0.3; R Foundation for Statistical Computing, Austria).
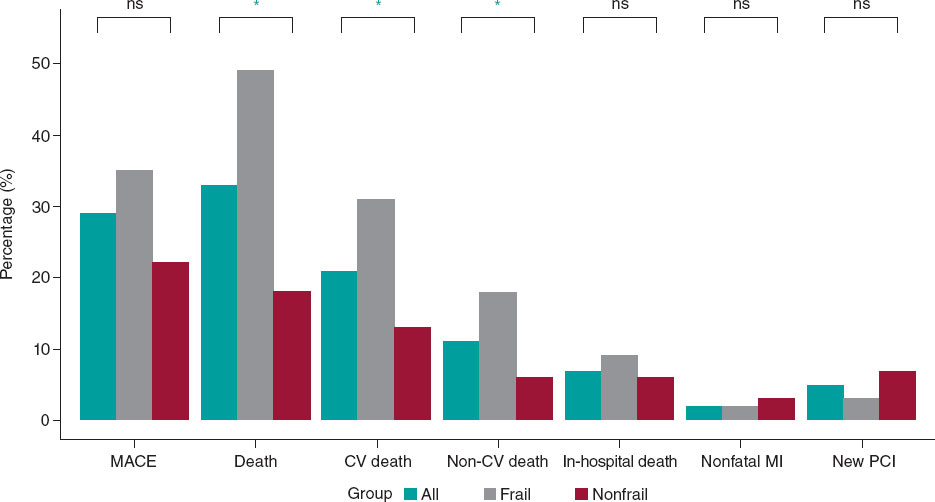
Figure 1. Main events to follow-up. CV, cardiovascular; MACE, mayor adverse cardiovascular events; MI, myocardial infarction; NS, nonsignificant; PCI, percutaneous coronary intervention. * P < .005.
RESULTS
During the study period, our hospital treated 437 patients with significant LM lesions percutaneously. Of them, a total of 140 patients met the inclusion criteria and were included in the analysis (figure 2 of the supplementary data).
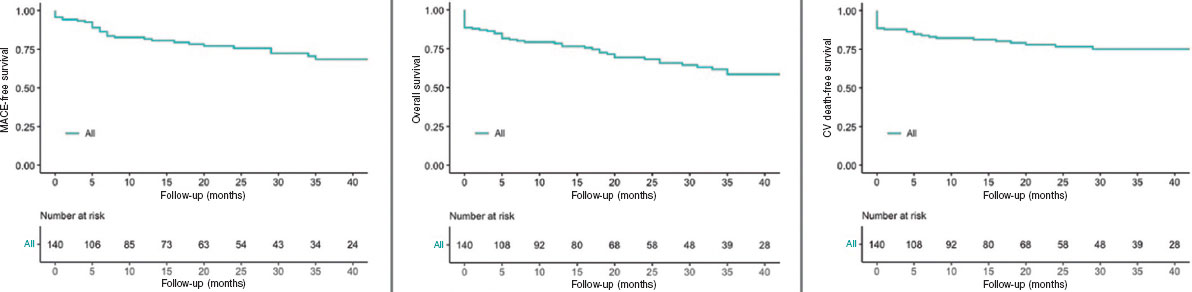
Figure 2. Kaplan-Meier Curves of the primary outcome and mortality. CV, cardiovascular; MACE, major adverse cardiovascular events.
Baseline characteristics
The baseline clinical characteristics, clinical presentation and antithrombotic treatment administered are detailed in table 1. The median age of the patients was 80 [78-84] years and 36% (51 patients) were women. Most of the patients had a history of hypertension (84%, 118 patients) and 58% (81 patients) were diabetic. More than a third of the patient cohort had a previous personal history of ischemic heart disease (37%, 52 patients) and 33% (46 patients) had chronic kidney disease. Among noncardiovascular comorbidities, active cancer was present in 11 patients (8%) and prior blood transfusions had been required in 16 patients (11%). The mean EuroSCORE II was 3.07 [1.96-5.7] to assess surgical risk. Forty-eight patients (34%) had left ventricular systolic dysfunction at the time of revascularization.
Table 1. Patients’ baseline characteristics
| Characteristics | Total n = 140 | Nonfrail n = 72 (51) | Frail n = 68 (49) | P |
|---|---|---|---|---|
| Baseline clinical characteristics | ||||
| Age, years | 80 [78-84] | 80 [77-84] | 80 [78-84] | .090 |
| Female sex | 51 (36) | 18 (25) | 33 (49) | .004 |
| Hypertension | 118 (84) | 61 (85) | 57 (84) | .884 |
| Diabetes | 81 (58) | 36 (50) | 45 (66) | .053 |
| Hypercholesterolemia | 112 (80) | 56 (78) | 56 (82) | .999 |
| Smoking history | 7 (5) | 5 (7) | 2 (3) | .442 |
| Previous ischemic heart disease | 52 (37) | 31 (43) | 21 (31) | .136 |
| Chronic kidney disease | 46 (33) | 22 (33) | 24 (39) | .481 |
| Atrial fibrillation | 22 (16) | 7 (10) | 15 (22) | .041 |
| Peripheral artery disease | 20 (14) | 14 (20) | 6 (9) | .073 |
| COPD | 17 (12) | 6 (8) | 11 (16) | .156 |
| Previous stroke | 16 (11) | 10 (14) | 6 (9) | .073 |
| Valve disease | 15 (11) | 7 (7) | 10 (15) | .114 |
| Anemia | 29 (21) | 10 (14) | 19 (28) | .040 |
| Active cancer | 11 (8) | 7 (10) | 4 (6) | .399 |
| Liver disease | 4 (3) | 3 (4) | 1 (2) | .339 |
| Previous blood transfusions | 16 (11) | 5 (7) | 11 (16) | .086 |
| Recent surgery or trauma | 38 (27) | 19 (26) | 19 (28) | .836 |
| EuroScore II | 3.07 [1.96-5.7] | 2.76 [1.83-4.18] | 3.80 [2.04-7.85] | .010 |
| Glomerular filtration rate (mL/min) | 71.4 [48.4-87.3] | 76.71 [51.01-87.51] | 61.40 [41.40-81.85] | .072 |
| Creatinine (mg/dL) | 1.02 [0.87-1.30] | 1.00 [0.80-1.85] | 1.03 [0.90-1.50] | .109 |
| Hemoglobin (mg/dL) (mean, ±SD) | 12.6 (± 2) | 13.02 (± 2) | 12.16 (± 1.9) | .017 |
| Hematocrit | 38.6 [34.6-43.0] | 39.6 [36.0-44.7] | 36.6 [33.9-42.1] | .031 |
| Platelets (× 109/L) | 208 [171-246] | 211 [182-244] | 196 [160-250] | .340 |
| Hs-cTnI (ng/L) | 954 [40-7352] | 2250 [30-10 000] | 650 [40-5600] | .245 |
| LVEF | 60 [39-67] | 60 [45-68] | 58 [35-63] | .245 |
| LV systolic dysfunction | 48 (34) | 20 (32) | 28 (46) | .106 |
| Clinical presentation | ||||
| Acute coronary syndrome | 85 (61) | 45 (63) | 40 (59) | .656 |
| NSTEMI | 61 (44) | 28 (39) | 33 (49) | .250 |
| STEMI | 9 (6) | 6 (8) | 3 (4) | .495 |
| Unstable angina | 15 (11) | 11 (15) | 4 (6) | .101 |
| Chronic coronary syndrome | 55 (39) | 27 (38) | 28 (41) | .656 |
| Antiplatelet therapy | ||||
| Dual antiplatelet therapy | 104 (74) | 57 (79) | 47 (69) | .174 |
| Aspirin + clopidogrel | 61 (43) | 31 (43) | 30 (44) | .899 |
| Aspirin + ticagrelor | 43 (31) | 26 (36) | 17 (25) | .154 |
| Triple antiplatelet therapy | ||||
| Aspirin + clopidogrel + anticoagulant | 36 (26) | 15 (2) | 21 (31) | .174 |
|
COPD, chronic obstructive pulmonary disease; Hs-cTnI, high sensitivity cardiac troponin I; LV, left ventricle; LVEF, left ventricular ejection fraction; NSTEMI, non-ST-elevation myocardial infarction; STEMI, ST-segment elevation myocardial infarction. Data are expressed as No. (%), mean ± standard deviation or median [interquartile range]. |
||||
The most common clinical presentation was acute coronary syndrome (85 patients, 61% of cases). Among these, onset consisted of ST-segment elevation myocardial infarction (STEMI) in 9 patients (6%), non-ST-segment elevation myocardial infarction in 61 patients (44%), and unstable angina in 15 patients (10%). The remaining patients (55, 39%) presented with chronic coronary syndrome.
A total of 104 patients (74%) were discharged with dual antiplatelet therapy. The main combination was aspirin and clopidogrel (61 patients, 43%). In 36 patients (26%), initial triple therapy (anticoagulation and dual antiplatelet therapy) was chosen due to concurrent conditions requiring chronic oral anticoagulation.
Based on the FRAIL scale, almost half of the patients (68 patients, 49%) met clinical criteria for frailty at the time of revascularization. The baseline characteristics of frail and nonfrail patients are shown in table 1. No statistically significant differences were found in terms of age, main cardiovascular risk factors or noncardiovascular comorbidities between the 2 groups. However, compared with nonfrail patients, those with frailty were more likely to be female (49% vs 25%; P = .004), to have atrial fibrillation (22% vs 10%; P = .041), a higher EuroSCORE level (3.80 vs 2.76; P = .010), and anemia (28% vs 14%; P = .040), and consequently a lower hematocrit and hemoglobin value (36.6% vs 39.6%; P = .031 and 12.16 mg/dL vs 13.02 mg/dL; P = .017, respectively).
Angiographic and procedural characteristics
Angiographic and procedural data are shown in table 2. The arterial access of choice was radial access (81% of procedures, 113 patients). A median SYNTAX score of 21 [15-29.5] was observed in 96 patients (68%) with multivessel disease, and 62 patients (44%) had a SYNTAX score > 22. The most common angiographic involvement of the LM was the distal segment (61%, 86 patients), while the most common plaque distribution according to the Medina classification was “1,1,1” (35 patients, 41% of LM bifurcation lesions). The strategy of choice for the treatment of the bifurcation was the provisional stent strategy (85% of LM bifurcation lesions, 73 patients), while the upfront 2-stent strategy was used in only 13 patients (15% of the LM bifurcation lesions). The mean diameter of the LM was 4.1 [± 3.5-4.5] mm with a mean angiographic stenosis of 62% (± 7). In 59 patients (42%), the procedure was guided using intravascular imaging techniques (58 patients using intracoronary ultrasound and 1 patient using coherence tomography). Coronary physiology was used in 5 patients (4%) to guide the need for revascularization or to check the result after percutaneous treatment. In 7 (5%) patients, mechanical support was required, either due to cardiogenic shock, or as a preventive measure in high-risk angioplasty (5 patients with an intra-aortic balloon pump and 2 with an Impella CP device [Abiomed, United States]). Intraprocedural complications occurred in 8 patients (6%), including a major complication in 4 patients (3 intraprocedural deaths and 1 cardiogenic shock), and a minor complication in 4 patients (1 coronary dissection with Thrombolysis in Myocardial Infarction (TIMI) grade 3 distal flow, 1 pseudoaneurysm, and 2 bleeding events from the femoral access resolved by stent implantation). The LM diameter was larger in patients with frailty than in those without (4 mm [4-4.5] vs 3.5 mm [3.5-4.5]; P = .023), a paradoxical finding since the percentage of women was higher in the group with frailty percentage of women. However, this information did not seem to be clinically relevant. No other clinically relevant differences were found between the 2 groups (table 2).
Table 2. Patients’ angiographic and procedural characteristics
| Characteristics | Total n = 140 | Nonfrail n = 72 (51) | Frail n = 68 (49) | P |
|---|---|---|---|---|
| Angiographic characteristics | ||||
| Multivessel disease | 96 (68) | 50 (69) | 46 (68) | .819 |
| SYNTAX score | 21 [15-29,5] | 21 [17-28.5] | 21.5 [14-30.6] | .752 |
| SYNTAX score > 22 | 62 (44) | 25 (39) | 31 (46) | .463 |
| LM diameter (mm) | 4 [3.5-4.5] | 3.5 [3.5-4.5] | 4 [4-4.5] | .023 |
| LM stenosis | 62 (± 7) | 64 (± 6) | 61 (± 5) | .342 |
| LM bifurcation | 86 (61) | 39 (54) | 47 (69) | .069 |
| Medina (1,1,1) | 35 (41) | 20 (51) | 15 (32) | .690 |
| Medina (1,1,0) | 33 (39) | 10 (26) | 23 (49) | .027 |
| Medina (1,0,1) | 8 (9) | 3 (8) | 5 (11) | .724 |
| Medina (0,1,1) | 3 (3) | 2 (5) | 1 (2) | .588 |
| Medina (1,0,0) | 4 (5) | 1 (3) | 3 (6) | .623 |
| Medina (0,1,0) | 0 (0) | 0 (0) | 0 (0) | - |
| Medina (0,0,1) | 3 (3) | 3 (8) | 0 (0) | .089 |
| Intracoronary diagnostic technique | ||||
| Intravascular imaging | 59 (42) | 28 (39) | 31 (46) | .422 |
| IVUS | 58 (41) | 28 (39) | 30 (44) | .530 |
| OCT | 0 (0) | 0 (0) | 1 (2) | .486 |
| Intracoronary physiology test | 5 (4) | 4 (6) | 1 (2) | .367 |
| Procedure characteristics | ||||
| Radial access | 113 (81) | 60 (83) | 53 (78) | .253 |
| Contrast (mL) | 200 [160-255] | 215 [150-259] | 200 [160-250] | .553 |
| Temporary pacemakers | 6 (4) | 3 (4) | 3 (4) | 1.000 |
| LV assist devices | 7 (5) | 4 (6) | 3 (4) | 1.000 |
| Intra-aortic balloon pump | 5 (4) | 4 (6) | 1 (2) | .367 |
| Impella | 2 (1) | 0 (0) | 2 (3) | .239 |
| One-stent bifurcation technique | 73 (85) | 34 (87) | 39 (83) | .588 |
| Stent MB + kissing | 20 (27) | 12 (35) | 7 (18) | .077 |
| Two-stent bifurcation technique | 13 (15) | 5 (13) | 8 (17) | .636 |
| T stenting | 3 (23) | 2 (40) | 1 (12.5) | .498 |
| TAP | 2 (15) | 0 (0) | 2 (25) | .498 |
| Culotte | 5 (39) | 1 (20) | 4 (50) | .371 |
| DK-Crush | 2 (15) | 1 (20) | 1 (12.5) | 1.000 |
| SKS | 1 (8) | 1 (20) | 0 (0) | .413 |
| MB stent diameter (mm) | 3.5 [3-3.5] | 3.5 [3-3.5] | 3.5 [3-3.5] | .877 |
| MB stent length (mm) | 18 [15-18] | 18 [15-18] | 18 [15-18] | .896 |
| SB stent diameter (mm) | 3.5 [3-3.5] | 3.25 [2.8-3.5] | 3.5 [3-3.6] | .371 |
| SB stent length (mm) | 15 [12-18] | 15.5 [15-21] | 15 [11-18] | .342 |
| Complications | ||||
| Intraprocedural complications | 8 (6) | 6 (8) | 2 (3) | .157 |
| Major | 4 (3) | 3 (4) | 1 (2) | .356 |
| Minor | 4 (3) | 3 (4) | 1 (2) | .356 |
|
DK, double kissing; IVUS, intravascular ultrasound; LM, left main; LV, left ventricle; MB, main branch; OCT, optical coherence tomography; SB, side branch; SKS, simultaneous kissing stents. TAP, T and small protrusion. Data are expressed as No. (%), mean ± standard deviation or median [interquartile range]. |
||||
Clinical results at follow-up
After a median follow-up of 19 months [5-35], a total of 40 (29%) MACE were recorded: 3 (2%) patients had a nonfatal myocardial infarction, 7 (5%) patients required repeat revascularization (3 for restenosis of the LM, and 4 in a different vessel), and 30 patients (21%) died of cardiac and/or uncertain causes. No strokes were reported during follow-up. Sixteen patients (11%) died of noncardiac causes during follow-up.
Clinical outcomes are presented in figure 1 and figure 2. No independent predictor of MACE was identified. The independent predictors of all-cause mortality were left ventricular ejection fraction (hazard ratio [HR], 0.90 [0.96-0.99]; P = .014), chronic kidney disease (HR, 2.26 [1.16-4.42]; P = .017), and particularly the presence of frailty (HR, 2.42 [1.17-5.02]; P = .018) (table 1 of the supplementary data). The primary endpoint of MACE occurred in 24 (35%) patients in the frail group and in 16 (22%) patients in the nonfrail group (HR, 1.61 [0.79-3.28]; P = .193). Frail patients had an increased risk of cardiovascular mortality: 21 (31%) vs 9 (13%); HR, 2.64 (1.21-5.77); P = .015. All-cause mortality was also more frequent in the frail group: 33 (49%) vs 13 (18%); HR, 2.94 (1.55-5.59); P = .001). The events during follow-up are presented in table 2 of the supplementary data. After IPTW adjustment, only the difference in all-cause mortality remained significant (HR, 1.95 [1.02-3.75]; P = .046). Survival analysis of the weighted population is shown in figure 3.
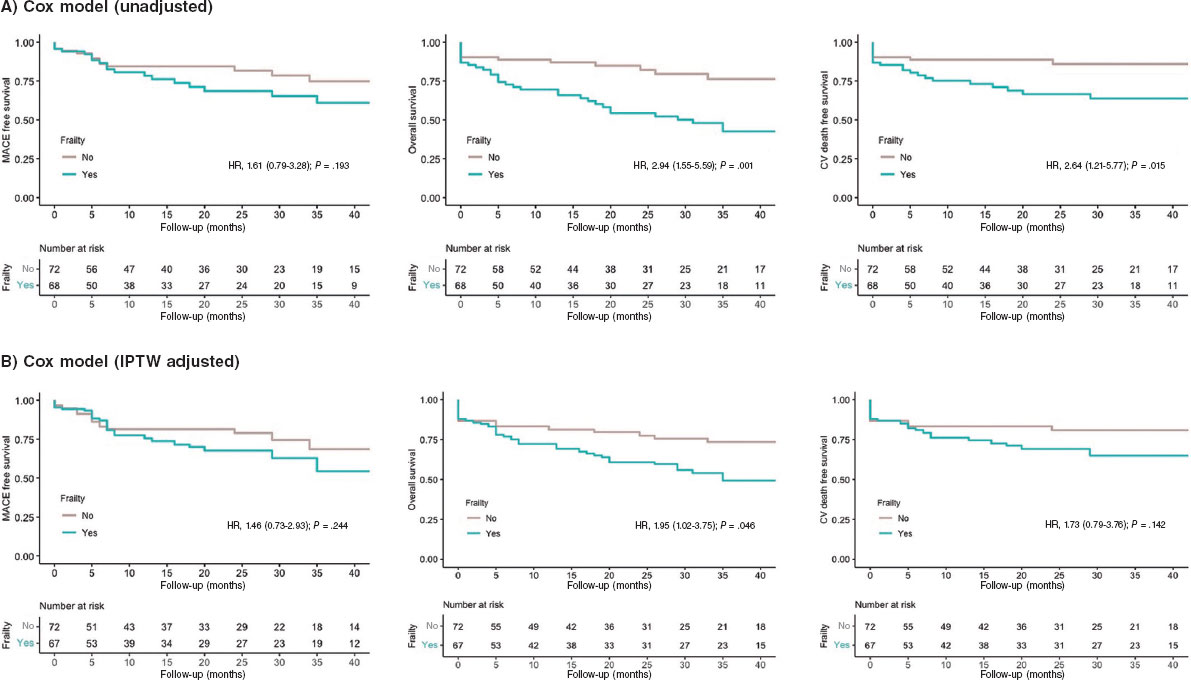
Figure 3. Kaplan-Meier Curves of the secondary outcomes. CV, cardiovascular; IPTW, inverse probability of treatment weighting; MACE, major adverse cardiovascular events.
DISCUSSION
The present study describes the feasibility of LM-PCI in a cohort of older patients. The main results were as follows: a) the rate of MACE at mid-term follow-up was 29%, mainly driven by cardiovascular and/or uncertain cause death; b) a high percentage of frailty was found in our population (49%); c) frail patients had a 2-fold increased risk of all-cause mortality during follow-up (HR, 1.95 [1.02-3.75]; P = .046) (figure 4).
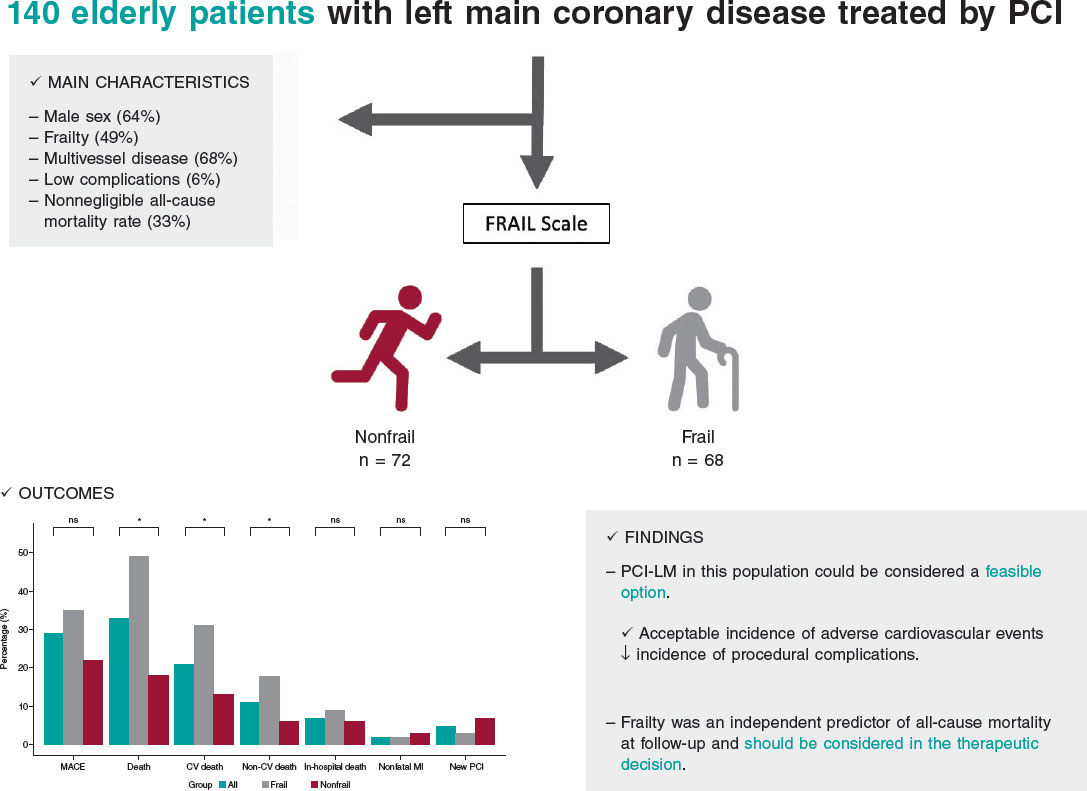
Figure 4. Central illustration. Results of percutaneous treatment of LM in elderly patients and impact of frailty. CV, cardiovascular; LM, left main coronary artery; MACE, major adverse cardiovascular events; MI, myocardial infarction; NS, non-significant; PCI, percutaneous coronary intervention.
The treatment of LM disease has traditionally been surgical, given the complexity involved and significant prognostic impact.13 However, the marked advances in interventional cardiology in recent decades have modified the approach.14,15 Contrasting evidence from clinical trials and meta-analyses shows that percutaneous treatment has similar results to surgical approaches in terms of mortality, acute myocardial infarction, and stroke at 5 years of follow-up.16 This shift has is reflected in the evolving recommendations in clinical practice guidelines, and the current European revascularization guidelines assign a grade of recommendation IA to both surgical and percutaneous strategies for the treatment of LM disease when the anatomy is not complex (SYNTAX < 22), and a class IIa recommendation for cases of intermediate complexity (SYNTAX 23-32).7
Nevertheless, the population analyzed in the study has specific clinical characteristics, and is not usually represented in large clinical trials (older patients and those with frailty and a high burden of associated comorbidities). These variables are not systematically included in surgical risk scores but are generally taken into account in routine clinical practice and often influence heart team decisions on the treatment strategy.17 Therefore, because this particular patient cohort is often excluded from research, there are no conclusive data on the benefit of percutaneous revascularization.
Our results are in line with those of previous registries in terms of MACE and all-cause mortality, as well as the association between age and a marked incidence of mortality due to noncardiac causes during follow-up. However, unlike earlier studies, we observed no differences in cardiovascular mortality, despite these patients having a more complex coronary anatomy than younger patients.18 In this regard, our study cohort had a median SYNTAX score of 21, and 44% of the patients had a score above 22. Like previous studies, this SYNTAX index score was not associated with a higher probability of cardiac events during follow-up in this special population.
In the present study, rates of acute myocardial infarction and new revascularization of the target lesion were lower than in other cohorts. Although it is difficult to make direct comparisons, we postulate that the use of new-generation drug-eluting stents and a higher proportion of revascularization guided by intracoronary diagnostic techniques may have influenced this finding. However, the use of intracoronary imaging techniques in our study was relatively low (42%) considering their benefit in patients with complex coronary lesions.19
In recent years, there has been growing interest in understanding the impact of comorbidities and frailty in older patients with cardiovascular disease.20,21 Several studies have compared invasive strategies with conservative approaches in older patients, demonstrating benefits for revascularization.22,23 However, the MOSCA-FRAIL trial compared both strategies in frail patients and observed that an invasive strategy did not confer additional benefit compared with conservative management of these patients, despite a fairly low percentage of LM disease.24 In our study, we observed a 2-fold increase in the risk of all-cause mortality in patients with frailty, suggesting the need to add systematic evaluation of frailty in older patients undergoing LM-PCI. Such assessment can aid in selecting the optimal therapeutic strategy, taking into account the likelihood of mortality during follow-up, irrespective of the application of an invasive strategy in coronary disease. These results, moreover, are consistent with other cardiovascular diseases with significant prevalence and mortality, such as heart failure.25
Study limitations
The present study has several limitations. First, it has the limitations inherent to its observational and retrospective design. Although the sample size is relatively small, it represents the largest study specifically focused on LM-PCI in older patients and analyses associated comorbidities and their impact on cardiovascular adverse events. Second, the absence of a control group receiving conservative treatment hinders the ability to draw more robust conclusions on the safety and efficacy of LM-PCI in these patients. In addition, the selection of cutoff points (age ≥ 75 years) to define this cohort of older patients was arbitrarily based on the exclusion criteria of the main clinical trials previously published. A high percentage of patients with frailty may not have undergone revascularization and would therefore have been excluded from the study. Regarding the prognostic significance of frailty, although we used IPTW to reduce confounding bias, we cannot rule out the possibility of residual confounding due to unmeasured covariables. Furthermore, there are no data on bleeding events during follow-up, which is an important concern given the impact of antiplatelet therapy in these patients. Finally, the percentage of intracoronary imaging use was lower than expected.
CONCLUSIONS
In real-life patients with advanced age and multiple associated comorbidities, percutaneous treatment of LM could be considered a feasible option, with an acceptable incidence of adverse cardiovascular events during follow-up and a low incidence of complications associated with the procedure. Frailty was an independent predictor of all-cause mortality during follow-up. When weighing the risks of LM-PCI in older patients, frailty should be taken into account in the therapeutic decision-making process.
FUNDING
None.
ETHICAL CONSIDERATIONS
The study protocol was approved by the Local Clinical Research Ethics Committee according to institutional and Good Clinical Practice guidelines. All patients signed the informed consent for publication. The authors confirm that sex and gender variables have been considered in accordance with the SAGER guidelines.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
No artificial intelligence was used in the preparation of the study.
AUTHORS’ CONTRIBUTIONS
I. Gallo, M. Alvarado and J. Perea contributed to data collection. R. González-Manzanares performed the statistical analysis. J. Suárez de Lezo and M. Romero contributed to the interpretation of the results. I. Gallo and F. Hidalgo wrote the manuscript. S. Ojeda and M. Pan reviewed the manuscript.
CONFLICTS OF INTEREST
S. Ojeda is associate editor of REC: Interventional Cardiology. The journal’s editorial procedure to ensure impartial processing of the manuscript has been followed. S. Ojeda has received consulting fees from Medtronic and Edwards and speaker fees from Philips, World Medical and Boston Scientific and is holder of a research grant (PI21/00949) from the Spanish Ministry of Science and Innovation (Instituto de Salud Carlos III). M. Pan has received speaker fees from Abbott, Boston Scientific, World Medical and Philips and holds a research grant (PI21/00949) from the Spanish Ministry of Science and Innovation (Instituto de Salud Carlos III). The remaining authors declare no conflicts of interest.
WHAT IS KNOWN ABOUT THE TOPIC?
- Coronary artery disease is closely related to age and the aging process.
- The prognosis of LM disease is uncertain and, due to due to advances in interventional cardiology in recent years, there is a need for further evidence on treatment options.
- Frailty is associated with a worse prognosis in various diseases.
WHAT DOES THIS STUDY ADD?
- LM-PCI in older adults is a feasible option in high-volume centers.
- Frailty is prevalent in older patients with LM disease and is associated with increased all-cause mortality.
REFERENCES
1. Collet C, Capodanno D, Onuma Y, et al. Left main coronary artery disease:pathophysiology, diagnosis, and treatment. Nat Rev Cardiol.2018;15:321-331.
2. Giannoglou GD, Antoniadis AP, Chatzizisis YS, et al. Prevalence of narrowing >or=50% of the left main coronary artery among 17,300 patients having coronary angiography. Am J Cardiol. 2006;98:1202-1205.
3. Ramadan R, Boden WE, Kinlay S. Management of Left Main Coronary Artery Disease. J Am Heart Assoc. 2018;7:008151.
4. Yusuf S, Zucker D, Peduzzi P, et al. Effect of coronary artery bypass graft surgery on survival:overview of 10-year results from randomized trials by the Coronary Artery Bypass Graft Surgery Trialists Collaboration. Lancet. 1994;344:563-570.
5. Suárez de Lezo J, Medina A, Pan M, et al. Rapamycin-eluting stents for the treatment of unprotected left main coronary disease. Am Heart J. 2004;148:481-485.
6. Thuijs DJFM, Kappetein AP, Serruys PW, et al. Percutaneous coronary intervention versus coronary artery bypass grafting in patients with three-vessel or left main coronary artery disease:10-year follow-up of the multicentre randomised controlled SYNTAX trial.Lancet. 2019;394:1325-1334.
7. Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87-165.
8. Ono M, Serruys PW, Hara H, et al. 10-Year Follow-Up After Revascularization in Elderly Patients With Complex Coronary Artery Disease. J Am Coll Cardiol. 2021;77:2761-2773.
9. Abellan van Kan G, Rolland Y, Bergman H, et al. The I.A.N.A. Task Force on frailty assessment of older people in clinical practice. J Nutr Health Aging. 2008;12:29-37.
10. Medina A, Suárez de Lezo J, Pan M. Una clasificación simple de las lesiones coronarias en bifurcación [A new classification of coronary bifurcation lesions]. Rev Esp Cardiol. 2006;59:183.
11. Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW. the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34:3661-3679.
12. Austin PC. Variance estimation when using inverse probability of treatment weighting (IPTW. survival analysis. Stat Med.2016;35:5642-5655.
13. Baydoun H, Jabbar A, Nakhle A, et al. Revascularization of Left Main Coronary Artery. Cardiovasc Revasc Med.2019;20:1014-1049.
14. Stone GW, Sabik JF, Serruys PW, et al. Everolimus-Eluting Stents or Bypass Surgery for Left Main Coronary Artery Disease. N Engl J Med. 2016;375:2223-2235.
15. Mäkikallio T, Holm NR, Lindsay M, et al. Percutaneous coronary angioplasty versus coronary artery bypass grafting in treatment of unprotected left main stenosis (NOBLE):a prospective, randomised, open-label, non-inferiority trial. Lancet. 2016;388:2743-2752.
16. Sabatine MS, Bergmark BA, Murphy SA, et al. Percutaneous coronary intervention with drug-eluting stents versus coronary artery bypass grafting in left main coronary artery disease:an individual patient data meta-analysis. Lancet. 2021;398:2247-2257.
17. Gach O, Louis O, Martinez C, et al. Predictors of early and late outcome of percutaneous coronary intervention in octogenarians. Acta Cardiol. 2003;58:289-294.
18. Gómez-Hospital JA, Gomez-Lara J, Rondan J, et al. Long-term follow-up after percutaneous treatment of the unprotected left main stenosis in high-risk patients not suitable for bypass surgery. Rev Esp Cardiol. 2012;65:530-537.
19. Lee JM, Choi KH, Song YB, et al. Intravascular Imaging-Guided or Angiography-Guided Complex PCI. N Engl J Med. 2023;388:1668-1679.
20. Díez-Villanueva P, Arizá-SoléA, Vidán MT et al. Recomendaciones de la Sección de Cardiología Geriátrica de la Sociedad Española de Cardiología para la valoración de la fragilidad en el anciano con cardiopatía. Rev Esp Cardiol. 2019;72:63–71.
21. Pernias V, García Acuña JM, Raposeiras-Roubín S, et al. Influencia de las comorbilidades en la decisión del tratamiento invasivo en ancianos con SCASEST. REC Interv Cardiol. 2021;3:15-20.
22. Kaura A, Sterne JAC, Trickey A, et al. Invasive versus non-invasive management of older patients with non-ST elevation myocardial infarction (SENIOR-NSTEMI):a cohort study based on routine clinical data. Lancet. 2020;396:623-634.
23. Tegn N, Abdelnoor M, Aaberge L, et al. Invasive versus conservative strategy in patients aged 80 years or older with non-ST-elevation myocardial infarction or unstable angina pectoris (After Eighty study):an open-label randomised controlled trial. Lancet.2016;387:1057-1065.
24. Sanchis J, Bueno H, Miñana G, et al. Effect of Routine Invasive vs Conservative Strategy in Older Adults With Frailty and Non-ST-Segment Elevation Acute Myocardial Infarction:A Randomized Clinical Trial. JAMA Intern Med. 2023;183:407-415.
25. Jiménez-Méndez C, Díez-Villanueva P, Bonanad C, et al. Frailty and prognosis of older patients with chronic heart failure. Rev Esp Cardiol. 2022;75:1011-1019.
* Corresponding author.
@NachoGalloFer;
@FranJHidalgo;
@rafaelglezm;
@MarcoA1788;
@PereaJorge5;
@cardiojsl;
@OjedaOjeda18;
@MPAOSS;
@Cardio_HURS
ABSTRACT
Introduction and objectives: Functional assessment of coronary stenosis severity with the piezo-electric sensor pressure wire has shown a discrepancy of up to 20% between hyperemic and nonhyperemic indexes. No data are available with fiber-optic pressure wires. The aim of this study was to evaluate the incidence and factors related to the diagnostic discordance between these indexes with a fiber-optic pressure wire. Secondary aims were to assess diagnostic reproducibility in 2 consecutive measurements of fractional flow reserve (FFR) and diastolic pressure ratio (dPR) and evaluate the drift rate.
Methods: We conducted a prospective, observational multicenter study in patients undergoing functional assessment with a fiber-optic pressure wire. We took 2 consecutive measurements of the dPR (cutoff point 0.89) and FFR (cut-off point 0.80) in each lesion analyzed. The diagnostic correlation between 2 measurements with the same technique and between the 2 techniques (dPR and FFR) was assessed. Clinical and angiographic factors associated with discordance (FFR−/dPR+ and FFR+/dPR−) between the 2 techniques were analyzed.
Results: We included 428 cases of stenosis (361 patients). Diagnostic reproducibility was 95.8% for the dPR, with a correlation coefficient between the 2 measurements (dPR1 and dPR2) of 0.974 (P < .0001). For FFR, the diagnostic reproducibility was 94.9% with a correlation coefficient (FFR1 and FFR2) of 0.942 (P < .0001). The diagnostic discordance was 18.2% (FFR+/dPR− 8.2% and FFR−/dPR+ 10%). Among the variables analyzed, the factors significantly associated with FFR−/dPR+ discordance in the multivariate analysis were hypertension and intracoronary adenosine. The only factors significantly associated with FFR+/dPR− discordance were age < 75 years and stenosis > 60%. The drift rate was 5.7%.
Conclusions: Although FFR and dPR measurements with a fiber-optic pressure wire have excellent reproducibility and a low drift rate, the discordance rate remains similar to those in previous studies with a piezo-electric pressure wire. FFR−/dPR+ discordance is associated with intracoronary adenosine and hypertension. FFR+/dPR− discordance is related to age < 75 years old and stenosis > 60%.
Keywords: Coronary physiology. Fractional flow reserve. Nonhyperemic index. Discordance. Drift.
RESUMEN
Introducción y objetivos: La valoración funcional de las estenosis coronarias con guías de presión de sensor piezoeléctrico ha mostrado hasta un 20% de discordancia entre los índices hiperémico y no hiperémico. No hay datos disponibles con guía de presión de sensor óptico. El objetivo del estudio es evaluar la incidencia y los factores relacionados con la discordancia diagnóstica entre estos índices con guía de presión de sensor óptico. Como objetivos secundarios se evaluó la reproducibilidad diagnóstica en dos determinaciones consecutivas de la reserva fraccional de flujo (RFF) y la diastolic pressure ratio (dPR). También se evaluó la tasa de drift.
Métodos: Estudio observacional, prospectivo, multicéntrico, en pacientes a quienes se realiza una valoración funcional con guía de presión de sensor óptico. Se hicieron dos mediciones consecutivas de dPR (umbral 0,89) y RFF (umbral 0,80) en cada lesión analizada. Se valoró la correlación diagnóstica entre dos mediciones con la misma técnica y entre ambas técnicas (dPR y RFF). Se analizaron factores clínicos y angiográficos asociados a la discordancia (RFF−/dPR+ y RFF+/dPR−) entre ambas técnicas.
Resultados: Se incluyeron 428 estenosis (361 pacientes). La reproducibilidad diagnóstica fue del 95,8% para dPR, con un coeficiente de correlación entre ambas mediciones (dPR1 y dPR2) de 0,974 (p < 0,0001). Para RFF la reproducibilidad diagnóstica fue del 94,9%, con un coeficiente de correlación (RFF1 y RFF2) de 0,942 (p < 0,0001). La discordancia diagnóstica fue del 18,2% (RFF+/dPR− 8,2% y RFF−/dPR+ 10%). Entre las variables analizadas, en el análisis multivariado, la hipertensión arterial y la administración intracoronaria de adenosina se asociaron de manera significativa con la discordancia RFF−/dPR+. Solo la edad < 75 años y la estenosis > 60% se asociaron de manera significativa con la discordancia RFF+/dPR−. La tasa de drift fue del 5,7%.
Conclusiones: Aunque las mediciones de RFF y dPR con guía de presión de sensor óptico tienen una excelente reproducibilidad y una baja incidencia de drift, la tasa de discordancia permanece similar a la de estudios previos con guía de presión de sensor piezoeléctrico. La adenosina intracoronaria y la hipertensión arterial se asocian con la discordancia RFF−/dPR+. La edad < 75 años y la estenosis > 60% se asocian a discordancia RFF+/dPR−.
Palabras clave: Fisiología coronaria. Reserva fraccional de flujo. Índice no hiperémico. Discordancia. Drift.
Abbreviations
dPR: diastolic pressure ratio. FFR: fractional flow reserve. FOSW: fiber-optic sensor wire. iFR: instantaneous wave-free ratio: PPSW: piezoelectric pressure sensor wire.
INTRODUCTION
Fractional flow reserve (FFR) measurement is an invasive procedure performed during coronary angiography to determine the functional significance of coronary stenoses.
In recent years, the instantaneous wave-free ratio (iFR) resting index has been developed to assess the functional significance of coronary stenoses without the need for adenosine administration. The optimal iFR cutoff value—equivalent to 0.80 in FFR—was initially established at 0.89.1 In 2017, 2 clinical studies comparing FFR with iFR found no significant differences in clinical outcomes at follow-up.2-3 After the publication of these 2 studies, the European Society of Cardiology guidelines on myocardial revascularization4 assigned resting indices the same grade of recommendation as FFR for the functional assessment of coronary lesions.
Despite the validation of these 2 techniques in clinical trials and their inclusion in clinical practice guidelines, up to 20% discordance has been reported between iFR+/FFR− or iFR−/FFR+5 Several clinical factors, such as diabetes,6 and anatomical factors, such as lesion location in the left main or proximal left anterior descending coronary arteries, have been identified in association with this discordance.7
Previous studies comparing FFR with iFR using a piezoelectric pressure sensor wire (PPSW) calculated the mean distal-to-aortic pressure ratio beginning 25% into diastole and ending 5 ms before end diastole.1
Recently, a new resting index—the diastolic pressure ratio (dPR)—has been developed to calculate the mean distal-to-aortic pressure ratio over the entire diastolic phase (from the lowest point of the dicrotic notch up to 50 ms before the onset of the upstroke of the next beat)8 using a fiber-optic sensor wire (FOSW).
A study that compared the values of different resting indices (iFR, dPR, dPR25-75, dPRmid, iFRmatlab, iFR50ms, and iFR100ms) revealed that all were numerically identical,8 meaning that the results obtained with the iFR can be extrapolated to other resting indices.
To date, no study has compared the agreement between dPR and FFR measured using a FOSW. One advantage of the FOSW over the PPSW is the lower loss of mean pressure matching in the wire compared with the measurement obtained in the guide catheter (drift).9 Although various iFR studies state that drifts < ± 0.02 are considered acceptable, the drifts reported with the FOSW were even lower at < ± 0.01.10
The diagnostic reproducibility of PPSW decreases significantly when close to the threshold value of 0.80 and is approximately 80% when measurements are < 0.77 or > 0.83, and around 90% with values < 0.76 or > 0.84.11 Since the FOSW is less sensitive to changes in humidity and temperature, greater reproducibility of results can be expected when the measurement is repeated.
Considering that most discordant measurements have been associated with cutoff values, the better reproducibility of measurements and practically nonexistent drift of the FOSW can more accurately determine FFR and dPR measurements and reduce discrepancies.
METHODS
Study design
In this prospective, observational, and multicenter registry of consecutive coronary stenoses, we conducted a study with FOSW based on our routine clinical practice.
We included consecutive patients with clinical signs and coronary angiography findings suggesting the need for a functional study with a pressure wire. We excluded patients with cardiogenic shock, heart failure, severe anemia (hemoglobin < 10 mg/dL), heart rate < 50 or > 100 bpm, baseline systolic blood pressure < 90 mmHg or > 160 mmHg, severe coronary artery lesions in distal segments, and contraindications for the administration of adenosine.
Objective
The aim of this study was to evaluate the incidence and factors related to diagnostic discrepancies between these indices using the FOSW. Secondary aims consisted of assessing the diagnostic reproducibility of FOSW in 2 consecutive measurements of FFR and dPR and evaluating the drift rate.
Procedure
The study was approved by the Drugs Research Ethics Committee of the Basque Country (internal code PS 2019039). All patients received information on the study and were asked to sign a written informed consent form prior to their participation in the study.
We performed coronary angiography using standard methods, with visual estimation of severity after intracoronary nitroglycerin administration. We included lesions with up to 50% to 75% percent diameter stenosis and collected data on the reference luminal diameter, minimum luminal diameter, lesion length, calcification, and vessel tortuosity for each studied lesion.
We performed 2 consecutive measurements of dPR (threshold, 0.89) and FFR (threshold, 0.80) for each studied lesion and analyzed the clinical and angiographic factors to determine their correlation with discordance (FFR−/dPR+ and FFR+/dPR−). We took dPR1 and FFR1 as reference values for discrepancy analysis.
We conducted the FOSW functional study with 5-, 6-, or 7-Fr guide catheters without side holes, using an OptoWire (Opsens Medical, Canada). After advancing the wire toward the tip of the guide catheter, we removed the introducer sheath and flushed the system with saline solution to prevent damping of the pressure wire resulting in equal pressure of the wire and the guide catheter at the tip of the catheter. After advancing the pressure wire distally, we administered 200 μg of intracoronary nitroglycerin before taking any measurements. We took the 2 dPR measurements after waiting the necessary time to obtain confirmation of a stable baseline distal-to-aortic coronary pressure ratio (Pd/Pa).
Subsequently, we took 2 different FFR measurements. Hyperemia was induced according to standard practice in each center (through intracoronary or IV adenosine infusion). If intracoronary adenosine was infused, for the second measurement, we waited until the baseline heart rate, blood pressure, and Pd/Pa were regained and then infused the same dose of adenosine. If IV adenosine was infused, the infusion was stopped until baseline heart rate, blood pressure, and Pd/Pa were regained, and then we infused adenosine at the same rate.
We evaluated the presence of drift upon removal of the pressure wire from the guide catheter. Drift was defined as a difference in Pd/Pa of at least ± 0.02 upon removal of the pressure wire from the guide catheter. In the presence of significant drift, measurements were repeated.
Cutoff values
The cutoff value was ≤ 0.80 for FFR and ≤ 0.89 for dPR.10 We categorized all studied vessels based on dPR and FFR values into 4 groups: concordant positive group (FFR ≤ 0.80 and dPR ≤ 0.89), concordant negative group (FFR > 0.80 and dPR > 0.89), discordant FFR+/dPR− group (FFR ≤ 0.80 and dPR > 0.89), and discordant FFR−/dPR+ group (FFR > 0.80 and dPR ≤ 0.89).
Statistical analysis
Continuous variables are expressed as mean and standard deviation, while categorical variables are expressed as percentages. We measured the association between continuous variables using Pearson’s correlation coefficient. To determine differences in variables in the FFR/dPR concordance groups we used ANOVA (for continuous variables) and the chi-square test (for categorical variables). We used the chi-square test to assess how each variable impacted FFR−/dPR+ and FFR+/dPR− discrepancies, and a multiple logistic regression model with backward elimination to determine the factors impacting FFR−/dPR+ and FFR+/dPR− discrepancies. On univariate analysis, we included variables with P < .1 in the logistic regression analysis and excluded those with a total n < 10. The analysis was conducted using SPSS software (version 20.1) and R (version 4.0.4).
RESULTS
We included a total of 428 stenoses in 361 patients. Table 1 and table 2 show the patients’ baseline characteristics, clinical presentation, and procedural characteristics.
Table 1. Patients’ baseline characteristics
| N = 361 | |
|---|---|
| Age (years) | 65.80 ± 10.5 |
| Male sex | 76.9 |
| Hypertension | 63.3 |
| Diabetes mellitus | 31 |
| Hypercholesterolemia | 60.4 |
| Active/former smoker | 19.7/40.5 |
| Previous acute coronary syndrome | 30.5 |
| Atrial fibrillation | 14.7 |
| Heart failure/dysfunction | 15.4 |
| Peripheral artery disease | 10 |
| Valvular heart disease, previous bypass, stroke | < 6 |
|
Data are expressed as No. (%) mean ± standard deviation. |
|
Table 2. Clinical presentation and procedural characteristics
| N = 361 | |
|---|---|
| Clinical presentation | N = 361 |
| Chest pain | 45.8 |
| Acute coronary syndrome | 23.1 |
| Unstable angina | 7.1 |
| Left ventricular dysfunction | 9.9 |
| Others | 14.2 |
| Procedural characteristics | |
| Baseline systolic blood pressure (mmHg) | 132 ± 24 |
| Systolic blood pressure during hyperemia (mmHg) | 125 ± 25 |
| Baseline heart rate (bpm) | 70 ± 12 |
| Heart rate during hyperemia (bpm) | 69 ± 15 |
| Reference luminal diameter (mm) | 3.09 ± 0.53 |
| Stenosis (%) | 54 ± 8 |
| Lesion length (mm) | 17.9 ± 12.2 |
| IV/intracoronary adenosine | 33/67 |
| Catheter size (5-Fr/6-Fr) | 17.5/81 |
| Drift ≥ ± 0.02 | 5.7 |
| dPR | 0.90 ± 0.08 |
| FFR | 0.83 ± 0.08 |
|
dPR, diastolic pressure ratio; FFR, fractional flow reserve. Data are expressed as No. (%) mean ± standard deviation. |
|
Sixty-seven percent of the patients received intracoronary adenosine; the mean doses of intracoronary adenosine administered were 324 μg (standard deviation [SD] ± 152) via the right coronary artery and 442 μg (SD ± 234) via the left coronary artery.
The medians of dPR measurements were 0.90 and 0.90 (SD ± 0.08) for the first and second measurements, with positivity rates of 27.4% and 27.9%, respectively. For FFR, the medians were 0.83 and 0.83 (SD ± 0.08) for the first and second measurements, with positivity rates of 28.1% and 30%, respectively.
The most widely studied vessel was the left anterior descending coronary artery (63%), followed by the left circumflex (20%) and right coronary arteries (16%).
The left anterior descending coronary artery showed a higher positivity rate (dPR+, 35.3%; FFR, 34%) than the left circumflex (dPR, 11.9%; FFR, 20.5%) and right coronary arteries (dPR, 15.9%; FFR, 17.4%).
Diagnostic reproducibility was 95.8% for dPR, with a correlation coefficient between the 2 measurements (dPR1 and dPR2) of 0.974 (P < .0001) and a mean difference of 0.019 (max, 0.12; min, −0.17). For dPR values < 0.86 or > 0.92, diagnostic reproducibility was 99.6%, decreasing to 90.7% when values were ≥ 0.86 or ≤ 0.92. For FFR, diagnostic reproducibility was 94.9%, with a correlation coefficient (FFR1 and FFR2) of 0.942 (P < .0001) and a mean difference of 0.029 (max, 0.14; min, −0.18) (figure 1). Values < 0.77 or > 0.83 showed a diagnostic reproducibility of 98.6%, decreasing to 86.4% when these values were ≥ 0.77 or ≤ 0.83.
Figure 1. Correlation coefficient and histogram of the differences between the 2 dPR and FFR measurements. dPR, diastolic pressure ratio; FFR, fractional flow reserve; SD, standard deviation.
The diagnostic concordance (figure 2) between FFR and dPR was 82%, with a correlation coefficient of 0.721 (P < .0001), while diagnostic discordance was 18.2% (FFR+/dPR–, 8.2% and FFR–/dPR+, 10.0%). In the FFR+/dPR– discordant group, FFR was 0.76 ± 0.04 and dPR, 0.93 ± 0.03. In the FFR–/dPR+ discordant group, FFR was 0.84 ± 0.03 and dPR, 0.86 ± 0.03.
Figure 2. Distribution of lesions according to FFR and dPR, with the rate of concordant and discordant measurements. dPR, diastolic pressure ratio; FFR, fractional flow reserve.
Out of the 75 discordant results reported, the measurements at the cutoff value (7 stenoses with FFR 0.80 and 18 stenoses with dPR 0.89) showed a discordance rate of 72%, which decreased as it moved away from the cutoff value (figure 3).
Figure 3. Probability of diagnostic discordance between FFR and dPR. The probability of discordance is close to 50% around the FFR cutoff point of 0.80 and decreases as it moves away from this point. Empirical model (bar chart) and model proposed by Petraco et al.11 (in grey). dPR, diastolic pressure ratio; FFR, fractional flow reserve.
Table 1 of the supplementary data illustrates the association between clinical and anatomical characteristics and the extent of agreement between FFR and dPR.
Out of all the variables analyzed in the multivariate analysis, hypertension (odds ratio [OR], 3.48, 95% confidence interval [95%CI], 1.01-11.98; P = .043) and intracoronary adenosine (OR, 7.04; 95%CI, 1.63-30.3; P = .001) were significantly associated with FFR–/dPR+ discordance. Age younger than 75 years (OR, 4.52; 95%CI, 1.03-20; P = .016) and percent diameter stenosis > 60% (OR, 6.69; 95%CI, 2.79-16; P < .001) were significantly associated with FFR+/dPR– discordance (table 3).
Table 3. Univariate analysis and multivariate logistic regression of variables associated with discordance
| Variables | FFR+/dPR− | FFR−/dPR+ | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate logistic regression | Univariate analysis | Multivariate logistic regression | |||||
| OR (95%CI) | P | OR (95%CI) | P | OR (95%CI) | P | OR (95%CI) | P | |
| Age < 75 years | 9.5 vs 3.3 | .039 | 4.52 (1.03-20) | .016 | 7.1 vs 8.9 | .347 | ||
| Female sex | 5.7 vs 8.9 | .231 | 11.4 vs 6.2 | .079 | ||||
| Hypertension | 7.2 vs 10.4 | .178 | 10 vs 2.2 | .002 | 3.48 (1.01-11.98) | .043 | ||
| Diabetes mellitus | 7.3 vs 8.6 | .406 | 11.7 vs 5.3 | .018 | 2.11 (0.95-4.69) | .064 | ||
| Dyslipidemia | 7.5 vs 9.5 | .304 | 7.1 vs 6.7 | .525 | ||||
| HF/LV dysfunction | 4.4 vs 9.0 | .154 | 11.8 vs 6.7 | .118 | ||||
| Valvular heart disease | 7.7 vs 8.3 | .635 | 19.2 vs 6.8 | .037 | ||||
| Coronary calcification | 6.7 vs 8.6 | .387 | 5.3 vs 8 | .302 | ||||
| Moderate/severe tortuosity | 7.8 vs 8.3 | .516 | 10.6 vs 5.7 | .054 | ||||
| Left main coronary artery | 0 vs 7.9 | .612 | 50 vs 6.9 | .007 | ||||
| Left anterior descending coronary artery | 7.9 vs 7.6 | .531 | 9.4 vs 4.4 | .041 | ||||
| Right coronary artery | 10.8 vs 7.1 | .176 | 2.4 vs 8.8 | .029 | ||||
| Left circumflex artery | 4.3 vs 8.5 | .179 | 2.9 vs 8.4 | .081 | ||||
| RLD > 3 mm | 6.6 vs 13.3 | .049 | 7.5 vs 8 | .518 | ||||
| Length > 20 mm | 12.5 vs 5.4 | .010 | 7.8 vs 7.3 | .492 | ||||
| Stenosis > 60% | 16 vs 3 | < .001 | 6.69 (2.79-16) | < .001 | 5.8 vs 8.3 | .227 | ||
| Heart rate > 80 bpm | 8.4 vs 8 | .527 | 10.8 vs 6.8 | .155 | ||||
| Intracoronary adenosine | 7.4 vs 8.5 | .713 | 13 vs 3.8 | .004 | 7.04 (1.63-30.3) | .001 | ||
|
95%CI, 95% confidence interval; dPR, diastolic pressure ratio; FFR, fractional flow reserve; HF, heart failure; LV, left ventricle; OR, odds ratio; RLD, reference luminal diameter. Data are expresed in %. |
||||||||
The drift rate was 5.7%.
DISCUSSION
We present the results of the first study conducted with a FOSW capable of measuring the diagnostic variability of 2 consecutive determinations of nonhyperemic and hyperemic indices, as well as the diagnostic discordance between the 2 techniques.
Previous discordance studies between the 2 indices with PPSW revealed discordance rates ranging from 12% to 22%,12,13 largely depending on the proximity of the values to the cutoff point. In a study by Lee et al.,12 the mean iFR and FFR values were 0.95 ± 0.10 and 0.87 ± 0.11, respectively, with a discordance rate of 12%, while in a study by Warisawa et al.,13 the mean iFR and FFR values were 0.89 ± 0.05 and 0.80 ± 0.03, respectively, with a discordance rate of 22%. In our study, the discordance rate was 18.2%, with a mean dPR of 0.90 (SD ± 0.08) and a mean FFR of 0.83 (SD ± 0.08), which is a slightly lower discordance rate than that reported by previous studies on PPSW and mean iFR and FFR values close to the cutoff point, which may be indicative of the accuracy of measurements obtained with FOSW.
The main findings of this study were the excellent diagnostic reproducibility of the FOSW, the clinical and anatomical variables related to FFR/dPR discordance, and the low drift rate reported in the measurements.
Diagnostic reproducibility with the fiber-optic sensor wire
Diagnostic reproducibility with the FOSW was excellent, with a variation between 2 consecutive measurements < 0.02 for dPR and < 0.03 for FFR. This accuracy in measurement confers excellent diagnostic reproducibility. These data are better than those previously reported with PPSW.11
Clinical and anatomical variables associated with FFR/dPR discordance
For FFR+/dPR− discordance, in the multivariate analysis, only age younger than 75 years and percent diameter stenosis > 60% were significantly associated with FFR+/dPR− discordance. This discordance in participants younger than 75 years could be explained by a slower baseline flow and a greater coronary flow reserve in younger patients with preserved microvascular function.14,15 Although discordance due to a higher percent diameter stenosis has already been described in previous studies,15,16 such discordance requires a preserved coronary flow reserve.6 When arterial flow velocity significantly increases during hyperemia, the pressure gradient does so too, decreasing distal coronary pressure during hyperemia substantially compared with baseline values, resulting in a low FFR value.
For FFR−/dPR+ discordance, in the multivariate analysis, the associated variables were hypertension and the administration of intracoronary adenosine. Although hypertension has not been associated with FFR−/dPR+ discordance in previous studies, it is known that patients with hypertension and left ventricular hypertrophy have a reduced coronary flow reserve17 and a possible lack of vasodilatory response to adenosine due to an increased left ventricular end-diastolic pressure. These 2 factors could play a key role in the association between hypertension and FFR−/dPR+ discordance.
Although IV adenosine is the most widely studied route of administration to achieve maximum hyperemia, intracoronary adenosine at doses > 300 μg may be equally or more effective in achieving maximum hyperemia18 and with fewer adverse events.19 In our study, the FFR−/dPR+ discordance reported when intracoronary adenosine was used could be a result of a failure to achieve adequate hyperemia.
These variables related to discordance demonstrate that dPR and FFR measure different aspects of coronary circulation, which may be affected differently in distinct patients or myocardial territories, leading to discordant FFR values and nonhyperemic indices.20
Drift in the fiber-optic pressure wire
The incidence of drift in clinical studies of pressure wires is not well known, and the drift considered acceptable has varied over the years. Previously, FFR measurement was repeated when drift was > 5 mmHg,21 while in more recent studies, drift > 3 mmHg has been considered significant. When FFR is between 0.77 and 0.82, drift ≤ 3 mmHg can reclassify 18.7% of stenoses,22 and this reclassification may be higher when a nonhyperemic diastolic or whole-cycle index is used.23 In the CONTRAST trial analysis of the PPSW, the drift rate (Pd/Pa ± 0.03) was 17.5%,24 while a more recent study comparing drift between FOSW and PPSW revealed a significantly lower rate with the FOSW (4.8% vs 26.7%; P = .02).9 In our study, the drift rate was 5.7%, which is consistent with other studies on FOSW, and much lower than that reported with PPSW, facilitating the use of pressure wire in routine clinical practice.
Limitations
Our study has several limitations. Both the severity and length of coronary lesions were quantified by the operator’s visual estimation at the time of the procedure, and since this was a study without a core laboratory, we cannot rule out the possibility that some of the discrepancies found were due to technical problems in determining the indices. Since the study was based on our routine clinical practice, most patients received intracoronary adenosine, and the protocol did not specify the intracoronary infusion comprehensively, which may have resulted in the lower hyperemia reported in some patients.
Target lesion revascularization was based on dPR or FFR values according to the operators’ decision. Patient selection for pressure guidance evaluation was also left to the treating physician’s discretion, which may have resulted in biases. However, our intention was to study dPR and FFR indices under real-world conditions.
CONCLUSIONS
Although FFR and dPR measurements with FOSW have excellent reproducibility and a low incidence of drift, the discordance rate remains similar to that reported by previous studies with PPSW, and largely depends on the proximity of values to the cutoff point. Intracoronary adenosine and hypertension, which imply a lack of hyperemia or increased microvascular resistance, are associated with FFR−/dPR+ discordance. Age younger than 75 years and the severity of stenosis, which may be associated with a preserved coronary flow reserve, are related to FFR+/dPR− discordance.
FUNDING
This study received no funding.
ETHICAL CONSIDERATIONS
This study was approved by the Drugs Research Ethics Committee of the Basque Country (internal code PS 2019039) for its implementation. All patients received a patient information sheet about the study and signed an informed consent form before enrollment. The study took into consideration sex and gender variables before drafting this article.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
No artificial intelligence has been used.
AUTHORS’ CONTRIBUTIONS
M. Sádaba Sagredo drafted the protocol, included patients as the lead investigator of his center, and drafted the manuscript. A. Subinas Elorriaga and A. Quirós contributed to the statistical analysis and drafting of the manuscript. The remaining authors are lead investigators of the READI EPIC-14 trial in their respective centers and contributed to patient inclusion and article review.
CONFLICTS OF INTEREST
None declared.
WHAT IS KNOWN ABOUT THE TOPIC?
- Determination of fractional flow reserve (FFR) is a widely used technique to establish the functional significance of coronary stenoses. In recent years, resting indices have been developed to assess the functional significance of coronary stenoses without the need for adenosine administration. The optimal cutoff value—equivalent to 0.80 in FFR—has been established at 0.89. Despite its validation in clinical trials and endorsement in clinical practice guidelines, discordant results are obtained in up to 20% of the cases between the 2 techniques.
WHAT DOES THIS STUDY ADD?
- Studies on discordance between hyperemic and nonhyperemic indices are conducted with piezoelectric pressure sensor wires. Fiber-optic sensor wires are not sensitive to temperature or humidity changes, making measurements more reproducible and drift rates very low.
- No previous studies have compared the concordance between hyperemic and nonhyperemic indices with the use of a fiber-optic sensor wire. –Despite the low diagnostic variability of diastolic pressure ratio (dPR) and FFR (4.2% for dPR and 5.1% for FFR) in 2 consecutive measurements, and a similarly low drift rate (5.7%), the discrepancy between the 2 indices remains similar to that reported by previous studies (18.2%), indicating that discrepancies are more related to clinical and anatomical variables and proximity to the cutoff value than to the pressure wire used.
SUPPLEMENTARY DATA
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.24875/ RECIC.M24000448.
ACKNOWLEDGEMENTS
We wish to thank M.ª Ángeles Carmona for her support in data collection and patient inclusion.
REFERENCES
1. Escaned J, Echavarría-Pinto M, Garcia-Garcia HM, et al. Prospective Assessment of the Diagnostic Accuracy of Instantaneous Wave-Free Ratio to Assess Coronary Stenosis Relevance:Results of ADVISE II International, Multicenter Study (ADenosine Vasodilator Independent Stenosis Evaluation II). JACC Cardiovasc Interv. 2015;8:824-833.
2. Davies JE, Sen S, Dehbi HM, et al. Use of the Instantaneous Wave-free Ratio or Fractional Flow Reserve in PCI. N Engl J Med. 2017;376:1824-1834.
3. Götberg M, Christiansen EH, Gudmundsdottir IJ, et al. Instantaneous Wave-free Ratio versus Fractional Flow Reserve to Guide PCI. N Engl J Med. 2017;376:1813-1823.
4. Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87-165.
5. Jeremias A, Maehara A, Généreux P, et al. Multicenter Core Laboratory Comparison of the Instantaneous Wave-Free Ratio and Resting Pd/Pa With Fractional Flow Reserve. The RESOLVE Study. J Am Coll Cardiol. 2014;63:1253-1261.
6. Cook CM, Jeremias A, Petraco R, et al. Fractional Flow Reserve/Instantaneous Wave-Free Ratio Discordance in Angiographically Intermediate Coronary Stenoses:An Analysis Using Doppler-Derived Coronary Flow Measurements. JACC Cardiovasc Interv. 2017;10:2514-2524.
7. Kobayashi Y, Johnson NP, Berry C, et al. The Influence of Lesion Location on the Diagnostic Accuracy of Adenosine-Free Coronary Pressure Wire Measurements. JACC Cardiovasc Interv. 2016;9:2390-2399.
8. Van't Veer M, Pijls N, Hennigan B, et al. Comparison of Different Diastolic Resting Indexes to iFR. Are They All Equal?J Am Coll Cardiol. 2017;70:3088-3096.
9. Haddad K, Potter B, Matteau A, et al. Assessing the Accuracy of a Second-Generation Optical Sensor Pressure Wire in a Wire-to-Wire Comparison (The ACCURACY Study). Cardiovasc Revasc Med. 2022;35:51-56.
10. Kern M. Comparing FFR Tools:New Wires and a Pressure Microcatheter. Cath Lab Digest. 2016;24(5).
11. Petraco R, Sen S, Echavarria-Pinto M, et al. Fractional Flow Reserve-Guided Revascularization. Practical Implications of a Diagnostic Gray Zone and Measurements Variability on Clinical Decisions. JACC Cardiovasc Interv. 2013;6:222-225.
12. Lee JM, Shin E-S, Nam C-W, et al. Discrepancy Between Fractional Flow Reserve and Instantaneous Wave-free Ratio:Clinical and Angiographic Characteristics. Int J Cardiol. 2017;245:63-68.
13. Warisawa T, Cook C, Howard JP, et al. Physiological Pattern of Disease Assessed by Pressure-Wire Pullback Has an Influence on Fractional Flow Reserve/Instantaneous Wave-Free Ratio Discordance. Insights From the Multicenter AJIP Registry. Circ Cardiovasc Interv. 2019;12:e007494.
14. Lim HS, Pim AL, De Bruyne B, et al. The impact of age on fractional flow reserve-guided percutaneous coronary intervention:A FAME (Fractional Flow Reserve versus Angiography for Multivessel Evaluation) trial substudy. Int J Cardiol. 2014;177:66-70.
15. Dérimay F, Johnson NP, Zimmermann FM, et al. Predictive factors of discordance between the instantaneous wave-free ratio and fractional flow reserve. Catheter Cardiovasc Interv. 2019;94:356-363.
16. Wienemann H, Meyer A, Mauri V, et al. Comparison of Resting Full-Cycle Ratio and Fractional Flow Reserve in a German Real-World Cohort. Front Cardiovasc Med. 2021;8:744181.
17. Schäfer S, Kelm, Mingers S, Strauer BE. Left ventricular remodeling impairs coronary flow reserve in hypertensive patients. J Hypertens. 2002;20:1431-1437.
18. Lopez-Palop R, Carrillo P, Frutos A, et al. Comparison of effectiveness of high-dose intracoronary adenosine versus intravenous administration on the assessment of fractional flow reserve in patients with coronary heart disease. Am J Cardiol. 2013;111:1277-1283.
19. Abo-Aly M, Lolay G, Adams C, et al. Comparison of Intracoronary versus Intravenous Adenosine-induced Maximal Hyperemia for Fractional Flow Reserve Measurement:A Systematic Review and Meta-analysis. Catheter Cardiovasc Interv. 2019;94:714-721.
20. Nijjer SS, De Waard GA, Sen S, et al. Coronary pressure and flow relationships in humans:phasic analysis of normal and pathological vessels and the implications for stenosis assessment:a report from the Iberian–Dutch–English (IDEAL) collaborators. Eur Heart J. 2016;37:2069-2080.
21. Vranckx P, Cutlip DE, McFadden EP, Kern MJ, Mehran R, Muller O. Coronary Pressure–Derived Fractional Flow Reserve Measurements. Recommendations for Standardization, Recording, and Reporting as a Core Laboratory Technique. Proposals for Integration in Clinical Trials. Circ Cardiovasc Interv. 2012;5:312-317.
22. Wakasa N, Kuromochi T, Mihashi N, et al. Impact of Pressure Signal Drift on Fractional Flow Reserve-Based Decision-Making for Patients With Intermediate Coronary Artery Stenosis. Circ J. 2016;80:1812-1819.
23. Cook C, Ahmad Y, Shun-Shin MJ, et al. Quantification of the Effect of Pressure Wire Drift on the Diagnostic Performance of Fractional Flow Reserve, Instantaneous Wave-Free Ratio, and Whole-Cycle Pd/Pa. Circ Cardiovasc Interv. 2016;9:e002988.
24. Matsumura M, Johnson N, Fearon WF, et al. Accuracy of Fractional Flow Reserve Measurements in Clinical Practice:Observations From a Core Laboratory Analysis. JACC Cardiovasc Interv. 2017;10:1392-1401.
- Plaque modification and impact on the microcirculation territory after drug-coated balloon angioplasty. The PLAMI study design
- On- vs off-hours primary percutaneous coronary intervention: a single-center 5-year experience
- Angina or ischemia with no obstructed coronary arteries: a specific diagnostic and therapeutic protocol
- Excimer laser coronary atherectomy in severely calcified lesions: time to bust the myth
Editorials
Expanding the role of drug-coated balloons in native large coronary artery disease
aDepartment of Biomedical Sciences, Humanitas University, Pieve Emanuele, Milan, Italy
bCardio Center, IRCCS Humanitas Research Hospital, Rozzano, Milan, Italy
cDivision of Cardiology, Montefiore Medical Center, Bronx, New York, United States
Original articles
Editorials
The role of angiography-derived physiological assessment techniques in the post-FAVOR III Europe era?
aServicio de Cardiología, Hospital General Universitario Gregorio Marañón, Instituto de Investigación Sanitaria Gregorio Marañón, Universidad Complutense de Madrid, Madrid, Spain
bCentro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), Instituto de Salud Carlos III, Madrid, Spain
Interviews
An interview with Camino Bañuelos
aServicio de Cardiología, Hospital Clínico San Carlos, Instituto de Investigación Sanitaria del Hospital Clínico San Carlos (IdISSC), Madrid, Spain
bServicio de Cardiología, Hospital Clínico Universitario de Santiago de Compostela, Santiago de Compostela, A Coruña, Spain