Article
Ischemic heart disease and acute cardiac care
REC Interv Cardiol. 2019;1:21-25
Access to side branches with a sharply angulated origin: usefulness of a specific wire for chronic occlusions
Acceso a ramas laterales con origen muy angulado: utilidad de una guía específica de oclusión crónica
Servicio de Cardiología, Hospital de Cabueñes, Gijón, Asturias, España
ABSTRACT
Introduction and objectives: Coronary lesions with stent overlapping are associated with higher neointimal proliferation that leads to more restenosis. Furthermore, the tapering of coronary arteries is a major challenge when treating long coronary lesions. This study attempted to assess the safety and clinical level of performance of long nontapered sirolimus-eluting coronary stent systems (> 36 mm) to treat long and diffused de novo coronary lesions in real-world scenarios.
Methods: This was a prospective, non-randomized, multicentre study that included 696 consecutive patients treated with the long nontapered BioMime sirolimus-eluting coronary stent system in long and diffused de novo coronary lesions. The safety endpoint was major adverse cardiovascular events defined as a composite of cardiac death, myocardial infarction, clinically driven target lesion revascularization, stent thrombosis, and major bleeding at the 12-month follow-up.
Results: Of a total of 696 patients, 38.79% were diabetic. The mean age of all the patients was 64.6 ± 14 years, and 80% were males. The indication for revascularization was acute coronary syndrome in 63.1%. A total of 899 lesions were identified out of which 742 were successfully treated with long BioMime stents (37 mm, 40 mm, 44 mm, and 48 mm). The cumulative incidence of major adverse cardiovascular events was 8.1% at the 12-month follow-up including cardiac death (2.09%), myocardial infarction (1.34%), and total stent thrombosis (0.5%).
Conclusions: This study confirms the safety and good performance of long nontapered BioMime coronary stents to treat de novo coronary stenosis. Therefore, it can be considered a safe and effective treatment for long and diffused de novo coronary lesions in the routine clinical practice.
Keywords: Coronary angioplasty. Drug-eluting stent. Nontapered stents.
RESUMEN
Introducción y objetivos: Las lesiones coronarias largas y difusas, cuando se tratan percutáneamente, requieren a menudo superposición de los stents, que se asocia a una mayor tasa de reestenosis. Por otro lado, el adelgazamiento progresivo de las arterias dificulta el tratamiento de las lesiones largas. En este estudio se analizan la seguridad y la eficacia clínica de los stents liberadores de sirolimus largos no cónicos (> 36 mm) para el tratamiento de lesiones largas de novo en un escenario real.
Métodos: Estudio prospectivo, no aleatorizado, multicéntrico, con 696 pacientes consecutivos con implantación de stent BioMime largo no cónico para el tratamiento de lesiones coronarias de novo largas y difusas. El criterio de valoración de seguridad fueron los eventos adversos cardiovasculares mayores en el seguimiento, definidos como la combinación de muerte cardiaca, infarto de miocardio, necesidad de nueva revascularización en la misma lesión guiada por la clínica, trombosis del stent o hemorragia mayor a los 12 meses.
Resultados: De los 696 pacientes incluidos, el 38,79% eran diabéticos. La edad media fue de 64,6 ± 14 años y el 80% eran varones. La indicación de revascularización fue un síndrome coronario agudo en el 63,1%. Se identificaron 899 lesiones, de las que 742 se trataron con éxito con stents BioMime (37-40-44-48 mm). La incidencia acumulada de eventos adversos cardiovasculares mayores fue del 8,1% a los 12 meses, con un 2,09% de muertes de causa cardiaca, un 1,34% de infartos de miocardio y un 0,5% de trombosis del stent.
Conclusiones: El presente estudio confirma la seguridad y el buen perfil clínico a 12 meses del stent BioMime largo no cónico para el tratamiento de lesiones coronarias de novo largas y difusas, por lo que debe considerarse un tratamiento seguro y eficaz para este tipo de lesiones en la práctica clínica habitual.
Palabras clave: Angioplastia coronaria. Stents farmacoactivos. Stents largos no cónicos.
Abbreviations
CAD: coronary artery disease. DES: drug-eluting stent. MACE: major adverse cardiovascular events. PCI: percutaneous coronary intervention. SES: sirolimus-eluting stent. ST: stent thrombosis.
INTRODUCTION
The most widely used strategy to treat coronary artery disease (CAD) is percutaneous coronary intervention (PCI) with stent implantation, particularly with the current generation of drug-eluting coronary stents (DES), since their distinctive features improve the clinical outcomes of PCI.1 However, the treatment of long and diffused coronary lesions remains challenging, especially in long lesions in tapered coronary arteries where variations in vessel diameter may require the implantation of > 1 stent per lesion.2,3
The use of either multiple stents or a single long stent are the most common treatment strategies for long and diffused lesions in tapered arteries. Both approaches may be associated with clinical failure due to the potential risk of mechanical mismatch of the stent size.1,4,5 Multiple short overlapping stents with variable diameters are often implanted to adequately match the size of long tapered lesions. Because of potential discrepancies regarding diameters when using long nontapered stents, a proximal optimization technique may be used to reconstruct the vessel natural geometry. However, this solution does not come without problems such as stent fracture due to vessel rigidity, restenosis due to a higher vascular injury, delayed healing, very late stent thrombosis (ST), vessel aneurysm, side branch jailing, higher treatment cost, overuse of antirestenotic drugs, and increased exposure to radiation and contrast media, and death or myocardial infarction.6,7
A single long BioMime (Meril Life Sciences Pvt. Ltd., India), an ultrathin biodegradable polymer coated sirolimus-eluting coronary stent (SES) system, is often enough to treat long and diffused lesions. Thus, the local arterial walls can be saved from overexposure to drug/metal avoiding any potential associated adverse events at the follow-up like delayed healing, perioperative myocardial infarction (MI), risk of target lesion revascularization, and very late ST. The aim of this study was to evaluate the safety and level of performance of the long nontapered BioMime SES system (37 mm, 40 mm, 44 mm, 48 mm) in consecutive real-world patients with long and diffused de novo coronary lesions.
METHODS
Study design and population
This was a prospective, non-randomized, multicentre study that included a total of 696 consecutive patients (aged ≥ 18 years) from 14 clinical centers across Spain. All the study investigators are listed in the appendix of this article.
All consecutive patients included had been treated of long and diffuse de novo coronary lesions through the implantation of, at least, 1 long nontapered BioMime system (37 mm, 40 mm, 44 mm, 48 mm). The study was conducted in observance of the privacy policy of each research center including its rules and regulations for the appropriate use of data in patient-oriented research. This study was also conducted in observance of the Declaration of Helsinki, and approved by the ethics committee. Written informed consents were obtained from all the participants before the procedure.
Study device and procedure
The BioMime is a biodegradable polymer coated SES system with different lengths available to treat long and diffused coronary lesions. It uses an ultra-thin strut (65 µm), and a cobalt-chromium platform that has a unique hybrid design of open and closed cells with uniformly thin coating (2 µm) of bioabsorbable polymers, PLLA (poly-L-lactic acid), and PLGA (poly-lactic-co-glycolic acid). The stent elutes sirolimus (1.25 µg/mm2) between 30 and 40 days after implantation. The currently available long lengths of BioMime are 37 mm, 40 mm, 44 mm, and 48 mm. The device is CE marked.
The PCI was performed according to the standard treatment guidelines and followed by each participant center. Predilatation and postdilatation were left to the operator’s discretion though postdilatation was recommended per protocol.
Preoperatively, a 300 mg loading dose of aspirin plus a second anti-platelet agent (clopidogrel, ticagrelor, or prasugrel according to the clinical settings and operator’s preference) were administered in all the consecutive patients included.
Postoperatively, all patients were administered a 12-month course of dual antiplatelet therapy plus aspirin (75 mg to 100 mg once a day) indefinitely beyond the first year. A 1.6- and 12-month clinical follow-up was conducted after the index procedure, as required, and based on symptoms.
Endpoints and definitions
The safety endpoints were the occurrence of major adverse cardiovascular events (MACE) at the 1-, 6-, and 12-month follow-up after the index procedure. MACE was defined as a composite of cardiac death, target vessel myocardial infarction, clinically driven target lesion revascularization, ST, and major bleeding.
MI was defined as the development of new pathological Q waves on the electrocardiogram or elevated creatinine kinase (CK) levels ≥ 2 times the upper limit of normal with elevated CK-MB levels in the absence of new pathological Q waves or new ischemic symptoms (eg, chest pain or shortness of breath).8 Cardiac death was defined as any deaths resulting from AMI, sudden cardiac death, heart failure mortality or stroke. Clinically driven target lesion revascularization was defined as a new PCI performed on the target lesion or coronary artery bypass graft of the lesion in the previously treated segment or within the 5 mm proximal or distal to the stent site or edge of DES inflation. ST was classified based on the definitions established by the Academic Research Consortium.9 Moderate-to-severe bleeding events were defined according to the GUSTO (Global Use of Strategies to Open Occluded Arteries) criteria. Procedural success was defined as a successful PCI without in-hospital major clinical complications including death, MI, and clinically driven target lesion revascularization. Device success was defined as the deployment of the study stent at the intended target lesion attaining final residual stenosis < 30% of the target lesion estimated both angiographically and through visual estimation.
Statistical analysis
Since there is no intervention, to study this cohort of patients we thought that the best method was to perform a descriptive analysis for an objective, comprehensive, and informative study of data. A a descriptive statistical analysis of the relevant variables was performed after collecting data. All statistical analyses were performed using the SPSS statistical software platform. Measures of central tendency such as means summarize the level of performance of a group of scores while measures of variability describe the spread of scores among the participants. Both are important to understand the behavior of this cohort. One provides information on the level of performance, and the other tells us how consistent that performance is. Categorical data were expressed as frequency and percentages. No further models were conducted as the idea of this paper was to describe a group of patients, not to compare groups or search for significant inter-group differences.
RESULTS
Baseline demographic and clinical characteristics
The data of 696 consecutive patients (742 BioMime stents implanted, 157 different stents) were collected in the study that mostly included males (80.1%). The baseline demographic and clinical characteristics of patients are shown on table 1. The patients’ mean age was 64.6 ± 14 years. Conventional risk factors for CAD in the study population were diabetes mellitus (39%), hypertension (67.2%), dyslipidemia (64.8%), and active smoking (26.44%). The clinical status at admission is shown on table 1. Most patients (63.39%) had acute coronary syndrome.
Table 1. Baseline demographic and clinical characteristics
| Patients | N = 696 |
|---|---|
| Patients, demographics | |
| Age, years | 64.6 ± 14 |
| Male | 556 (80.1) |
| Baseline past medical history | |
| Diabetes mellitus | 271 (38.79) |
| Hypertension | 466 (66.80) |
| Dyslipidemia | 452 (64.80) |
| Active smoker | 180 (26.44) |
| Previous CABG | 57 (8.54) |
| Previous PCI | 223 (32.07) |
| Vascular peripheral disease | 69 (10.64) |
| Previous MI | 181 (25.63) |
| Cardiac status at the index procedure | |
| Stable angina | 254 (36.49) |
| Unstable angina | 29 (4.16) |
| STEMI | 227 (32.61) |
| NSTEMI | 186 (26.72) |
| Left ventricular ejection fraction < 30% | 181 (26) |
|
CABG, coronary artery bypass graft; NSTEMI, non-ST-elevation acute myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction. Data are expressed as no. (%) or mean ± standard deviation. |
|
Lesion and procedural characteristics
Out of a total of 899 lesions identified in 696 consecutive patients, 742 long and diffused de novo type C coronary lesions (1.07 lesions per patient) were successfully treated with long BioMime stents. No other stents were needed to treat the lesion initially handled with a long BioMime device. A total of 157 other lesions were treated with 157 different stents. Therefore, no overlapping was needed in any of the lesions treated with a long BioMime device. A total of 40% of the patients had 1-vessel disease, 37% 2-vessel disease and 23% of the patients had 3-vessel disease. The left anterior descending coronary artery followed by the right coronary artery were the main arteries treated. In 3.8% of the cases BioMime implantation involved the left main coronary artery. The mean length of the implanted BioMime SES system was 43.8 mm along with an average diameter of 3.1 mm. The immediate procedural and device success rates were 99.7% and 100%, respectively. The procedural variables are shown on table 2 and table 3.
Table 2. Lesion and procedural characteristics
| Patients | N = 696 |
|---|---|
| Total no. of lesions treated with the BioMime Morph SES system | 742 |
| Total no. of lesions treated with other stents | 157 |
| BioMime target lesion location | |
| Left anterior descending coronary artery | |
| Proximal LAD | 146 (21.40) |
| Mid LAD | 216 (30.80) |
| Distal LAD | 28 (4.50) |
| Diagonal | 11 (1.60) |
| Right coronary artery | |
| Proximal RCA | 174 (25.10) |
| Mid RCA | 257 (36.80) |
| Distal RCA | 97 (14.10) |
| Left circumflex artery | |
| Proximal LCX | 56 (8.20) |
| Mid LCX | 90 (12.90) |
| Distal LCX | 28 (4.10) |
| Left main coronary artery | 26 (3.80) |
| Diseased vessel | 1.84 ± 0.78 |
|
LAD, left anterior descending coronary artery; LCX, left circumflex artery; RCA, right coronary artery; SES, sirolimus-eluting stent. Data are expressed as no. (%). |
|
Table 3. BioMime sirolimus-eluting stent system characteristics
| Stent lenght (mm) | |
| 37 | 100 |
| 40 | 189 |
| 44 | 128 |
| 48 | 325 |
| Average stent length (mm) | 43.80 |
| Stent diameter (mm) | |
| 2.25 | 42 |
| 2.5 | 153 |
| 2.75 | 84 |
| 3 | 263 |
| 3.5 | 185 |
| 4 | 13 |
| 4.5 | 2 |
| Maximum pressure | |
| Predilatation | 298 (86) |
| Postdilatation | 376 (54) |
| Maximum pressure | 14.6 ± 3.2 |
| Average stent diamenter used (mm) | 3.1 |
|
Data are expressed as no. (%). |
|
Clinical outcomes at follow-up
Clinical follow-up was completed in 96.12% of the patients included at the 12-month follow-up. A total of 3.88% out of 696 patients were lost to follow-up after 12 months.
The cumulative incidence of MACE at the 1-, 6-, and 12-month follow-up was 2.2%, 6.6%, and 8.1%, respectively. The individual MACE at the follow-up are shown on table 4. The rates of cardiac death were 0.59% and 2.09% after 1 month and 1 year, respectively.
Table 4. MACE at the follow-up
| % of patients | MACE | |
|---|---|---|
| Follow-up | ||
| 1 month | 682 (97.99) | 13 (2.2) |
| 6 to 9 months | 675 (97.27) | 44 (6.57) |
| 12 months | 668 (96.12) | 53 (8.1) |
| MACE | ||
| Bleeding at 1-M | 20 (0.29) | |
| Death at 1-M | 41 (0.59) | |
| MI at 1-M | 41 (0.59) | |
| Bleeding at 12-M | 5 (0.75) | |
| Death at 12-M | 13 (2.09) | |
| MI at 12-M | 9 (1.34) | |
| Total ST at 12-M | 3 (0.50) | |
|
MACE, major adverse cardiovascular events; M, month; MI, myocardial infarction; ST, stent thrombosis. Data are expressed as no. (%). |
||
DISCUSSION
In the current study, the long nontapered BioMime SES system proved its safety and level of performance in consecutive real-world patients with long and diffused de novo coronary lesions. Despite the all-comers inclusion criteria defining a high-risk population, and the anatomical need for a long stent, procedural (99.7%) and device (100%) success were achieved and the clinical follow-up was quite favorable.
Studies have shown that the dimensions of coronary arteries taper naturally along with their length. They observed that 23% of the arteries had ≥ 1 mm taper and 19% arteries a 0.5 mm to 0.99 mm taper.10 Stent sizing is critical for a successful PCI regarding the treatment of long tapered lesions. Stent oversizing (stents that are larger in diameter compared to the healthy artery) may induce pathological stress on the arterial wall, aneurysm formation, late ST, and even late perforations. Stent undersizing, on the other hand, (stents that are smaller in diameter compared to the healthy artery) may lead to ST due to stent malapposition.11 Consistent with this, tapered stents were developed to potentially minimize clinical failure and maximize clinical benefits in these patients. This fact may be due to the specific design of the BioMime stents.
Ultrathin struts facilitate navegability, flexibility, and conformability of the vessel geometry while maintaining an excellent radial force. In addition, the open cell design throughout the entire body of the stent favors a less stiff device that follows more closely the tapered contour of the artery resulting in less arterial wall stress. Compliant stents should be considered for tapered artery applications, perhaps even to avoid the need for tapered stents, at least up to 48 mm length, as shown in our data.12-16
The use of long coronary stents (≥ 30 mm), but not as long as the lesions treated in this registry, to treat long and diffuse native vessel disease, saphenous vein graft disease, and long coronary dissections is associated with a reasonable procedural success rate and acceptable early and intermediate-term clinical outcomes.17 The treatment of very long CAD showed similar target lesion faliure at the 2-year follow-up for single DESs compared to overlapped DESs.18 Our results suggest that both strategies are reasonable therapeutic options for patients with diffuse CAD. However, DES overlap occurs in > 10% of the patients treated with PCI in the routine clinical practice, and has been associated with impaired angiographic and long-term clinical outcomes including death or myocardial infarction.19 In addition, the development of risk areas for malapposition with a single stent is significantly lower compared to overlapping stents. In cases where stent overlap cannot be avoided, deployment strategies should be optimized or new stent designs considered to reduce the risk of restenosis.20 A single stent strategy is often more cost-effectiveness, and involves the administration of fewer contrast and fewer balloons. New designs of very long stents allow us not only to treat increasingly complex lesions, but also to simplify the procedure, and reduce the number of stents used with very favorable results, at least, similar to those obtained with overlapping stents.21 Former studies have confirmed the safety and level of performance of the BioMime Morph, a very long tapered stent (60 mm) that can be considered the treatment of choice for very long and diffused tapered de novo coronary lesions in the routine clinical practice.22 However, in long lesions treated with single stents of up to 48 mm in length, our results suggest that nontapered stents give very good clinical results.
Limitations
One limitation may be the follow-up period that may not be enough to determine the long-term safety and level of performance of long BioMime SES system in patients with long and diffused de novo coronary lesions.
CONCLUSIONS
This study confirmed the favorable procedural and device success, and the optimal safety outcomes reported at the follow up, of the long nontapered BioMime SES system, up to 48 mm length, in real-world patients with long and diffused de novo coronary lesions.
FUNDING
The current study was partially funded by Palex Medical, and Meril (data collection, web design, and ethical committee).
AUTHORS’ CONTRIBUTIONS
E. Domingo contributed to the study design, database completion, clinical follow-up, data analysis, and manuscript writing. J. Guindo contributed to the study design. R. Calviño Santos, J. Antoni Gomez, X. Carrillo, J. Sánchez, L. Andraka, A. Torres, J. Casanova-Sandoval, R. Ocaranza Sanchez, J. León Jiménez, J.F. Muñoz, R. Trillo Nouche, and M. Fuertes contributed to the database completion, and clinical follow-up. I. Otaegui contributed to the database completion, data analysis, and clinical follow-up. B. García del Balnco contributed to the study design, data analysis, and manuscript writing.
CONFLICTS OF INTEREST
None reported.
APPENDIX 1: STUDY INVESTIGATORS
-
Gerard Marti Aguasca. Hospital Universitario Vall d’Hebron, Servicio de Cardiología.
-
Vicenç Serra García. Hospital Universitario Vall d’Hebron, Servi- cio de Cardiología.
-
Bernat Serra Creus. Hospital Universitario Vall d’Hebron, Ser- vicio de Cardiología.
-
Neus Bellera Gotarda. Hospital Universitario Vall d’Hebron, Servi- cio de Cardiología.
-
Jorge Salgado Fernández. Complejo Hospitalario Universitario A Coruña, Servicio de Cardiología.
-
Montserrat Gracida Blancas. Hospital Universitari de Bellvitge, Servicio de Cardiología.
-
Lara Fuentes Castillo. Hospital Universitari de Bellvitge, Servicio de Cardiología.
-
Eduard Fernández-Nofrerias, Hospital Germans Trias i Pujol, Servicio de Cardiología.
-
Oriol Rodríguez-Leor. Hospital Germans Trias i Pujol, Servicio de Cardiología.
-
Omar Abdul Jawad Altisent. Hospital Germans Trias i Pujol, Servicio de Cardiología.
-
Gabriel Galache. Hospital Universitario Miguel Servet, Servicio de Cardiología.
-
Rosario Hortas. Hospital Universitario Miguel Servet, Servicio de Cardiología.
-
Eduard Bosch. Parc Taulí Hospital Universitari, Servicio de Cardiología.
-
Daniel Valcarcel. Parc Taulí Hospital Universitari, Servicio de Cardiología.
-
Maite Alfageme. HUA – Txagorritxu, Servicio de Cardiología.
-
Merche Sanz. HUA – Txagorritxu, Servicio de Cardiología.
-
Melisa Santás Álvarez. Hospital Lucus Augusti, Servicio de Cardiología.
-
Diego López Otero. Hospital Clínico Universitario de Santiago – CHUS, Servicio de Cardiología.
-
Juan Carlos Sanmartin Pena. Hospital Clínico Universitario de Santiago -CHUS, Servicio de Cardiología.
-
Ana Belén Cid Álvarez. Hospital Clínico Universitario de Santiago -CHUS, Servicio de Cardiología.
REFERENCES
1. Tan CK, Tin ZL, Arshad MKM, et al. Treatment with 48-mm everolimus eluting stents:Procedural safety and 12-month patient outcome. Herz. 2019;44:419-424.
2. Roach MR, MacLean NF. The importance of taper proximal and distal to Y-bifurcations in arteries. Front Med Biol Eng. 1993;5:127-133.
3. Zubaid M, Buller C, Mancini GB. Normal angiographic tapering of the coronary arteries. Can J Cardiol. 2002;18:973-980.
4. Sgueglia GA, Belloni F, Summaria F, et al. One-year follow-up of patients treated with new-generation polymer-based 38 mm everolimus-eluting stent:the P38 study. Catheter Cardiovasc Interv. 2015;85:218-224.
5. Timmins LH, Meyer CA, Moreno MR, Moore JE, Jr. Mechanical modeling of stents deployed in tapered arteries. Ann Biomed Eng. 2008;36:2042-2050.
6. Ellis SG, Holmes DR. Strategic approaches in coronary intervention. 2006:Lippincott Williams &Wilkins. P. 299-304.
7. Raber L, Juni P, Loffel L, et al. Impact of stent overlap on angiographic and long-term clinical outcome in patients undergoing drug-eluting stent implantation. J Am Coll Cardiol. 2010;55:1178-1188.
8. Mendis S, Thygesen K, Kuulasmaa K, et al. World Health Organization definition of myocardial infarction:2008- 09 revision. Int J Epidemiol. 2011;40:139-146.
9. Cutlip DE, Windecker S, Mehran R, et al. Clinical end points in coronary stent trials:a case for standardized definitions. Circulation. 2007;115:2344-2351.
10. Banka VS, Baker HA, 3rd, Vemuri DN, Voci G, Maniet AR. Effectiveness of decremental diameter balloon catheters (tapered balloon). Am J Cardiol. 1992;69:188-193.
11. Kitahara H, Okada K, Kimura T, et al. Impact of stent size selection on acute and long-term outcomes after drug-eluting stent implantation in de novo coronary lesions. Circ Cardiovasc Interv. 2017;10:e004795.
12. Sinha SK, Mahrotra A, Abhishekh NK, et al. Acute stent loss and its retrieval of a long, tapering morph stent in a tortuous, calcified lesion. Cardiol Res. 2018;9:63-67.
13. Zivelonghi C, van Kuijk JP, Nijenhuis V, et al. First report of the use of long-tapered sirolimus-eluting coronary stent for the treatment of chronic total occlusions with the hybrid algorithm. Catheter Cardiovasc Interv. 201;5:1-9.
14. Matchin YG, Atanesyan RV, Kononets EN, Danilov NM, Bubnov DS, Ageev FT. The first experience of using very long stents covered with sirolimus (4060 mm) in the treatment of patients with extensive and diffuse lesions of the coronary arteries. Kardiologiia. 2017;57:19-26.
15. Timmins LH, Meyer CA, Moreno MR, Moore Jr. JE. Mechanical modeling of stents deployed in tapered arteries. Ann Biomed Eng. 2008;36:2042-2050.
16. Xiang Shen, Yong-Quan Deng, Song Ji, Zhong-Min Xie. Flexibility behavior of coronary stents:the role of linker investigated with numerical simulation. J Mech Med Biol. 2017. https://doi.org/10.1142/S0219519417501123.
17. Mushahwar SS, Pyatt JR, Lowe R, Morrison WL, Perry RA, Ramsdale DA. Clinical outcomes of long coronary stents:a single-center experience. Int J Cardiovasc Intervent. 2001;4:29-33.
18. Sim HW, Thong EH, Loh PH, et al. Treating very long coronary artery lesions in the contemporary drug-eluting-stent era:single long 48 mm stent versus two overlapping stents showed comparable clinical results. Cardiovasc Revasc Med. 2020;21:1115-1118.
19. Peter Jüni RL, Löffel L, Wandel S, et al. Impact of Stent Overlap on Angiographic and Long-Term Clinical Outcome in Patients Undergoing Drug-Eluting Stent Implantation. J Am Coll Cardiol. 2010;55:1178–1188.
20. Lagache M, Coppel R, Finet G, et al. Impact of Malapposed and Overlapping Stents on Hemodynamics:A 2D Parametric Computational Fluid Dynamics Study. Mathematics. 2021;9:795.
21. Jurado-Román A, Abellán-Huerta J, Antonio Requena J, et al. Comparison of Clinical Outcomes Between Very Long Stents and Overlapping Stents for the Treatment of Diffuse Coronary Disease in Real Clinical Practice. Cardiovasc Revasc Med. 2019;20:681-686.
22. Patted SV, Jain RK, Jiwani PA, et al. Clinical Outcomes of Novel Long-Tapered Sirolimus Eluting Coronary Stent System in Real-World Patients With Long Diffused De Novo Coronary Lesions. Cardiol Res. 2018;9:350-357.
ABSTRACT
Introduction and objectives: Coronary artery aneurysms are a complex situation. Our main objective is to describe the frequency of use of covered stents (grafts) for their management, as well as to characterize their long-term results compared to drug-eluting stents.
Methods: Ambispective observational study with data from the International Coronary Artery Aneurysm Registry (CAAR) (NCT-02563626). Only patients who received a stent-graft or a drug-eluting stent where the aneurysm occurred were selected.
Results: A total of 17 patients received, at least, 1 stent-graft while 196 received 1 drug-eluting in the aneurysmal vessel. Male predominance, a higher rate of dyslipidemia, a past medical history of coronary artery disease, previously revascularized coronary artery disease, and giant aneurysms were reported in the stent-graft cohort. The independent predictive variables of the composite endpoint of all-cause mortality, heart failure, unstable angina, reinfarction, stroke, systemic embolism, bleeding or any aneurysmal complications at the median follow-up of 38 months were suggestive of the existence of connective tissue diseases (HR, 5.94; 95%CI, 1.82-19.37), left ventricular dysfunction ≤ 55% (HR, 1.84; 95%CI, 1.09-3.1), and an acute indication for heart catheterization (HR, 2.98; 95%CI, 1.39-6.3). The use of stent-grafts was not associated with the occurrence of more composite endpoints (23.5% vs 29.6%; P = .598).
Conclusions: The use of stent-grafts to treat coronary aneurysms is feasible and safe in the long-term. Randomized clinical trials are needed to decide what the best treatment is for these complex lesions.
Keywords: Coronary aneurysm. Registry. Stent. Stent graft. Angioplasty.
RESUMEN
Introducción y objetivos: Los aneurismas coronarios son una situación compleja. Planteamos como objetivo principal describir la frecuencia de utilización de stents recubiertos (grafts) para su tratamiento y caracterizar sus resultados a largo plazo en comparación con stents farmacoactivos.
Métodos: Estudio observacional ambispectivo, con información procedente del Registro Internacional de Aneurismas Coronarios (CAAR) (NCT-02563626). Se seleccionaron los pacientes que recibieron un stent-graft o un stent farmacoactivo en la zona del aneurisma.
Resultados: Un total de 17 pacientes recibieron al menos un stent-graft y 196 un stent farmacoactivo en la zona aneurismática. Se observa un predominio del sexo masculino y una mayor frecuencia de dislipemia, antecedentes de coronariopatía, enfermedad coronaria revascularizada previamente y aneurismas gigantes en la cohorte de stent-graft. Como variables independientes predictoras del desarrollo del evento combinado (muerte por cualquier causa, insuficiencia cardiaca, angina inestable, reinfarto, ictus, embolia sistémica, sangrado o cualquier complicación en el aneurisma), tras una mediana de seguimiento de 38 meses, destacaron la existencia de conectivopatías (hazard ratio [HR] = 5,94; intervalo de confianza del 95% [IC95%], 1,82-19,37), la disfunción del ventrículo izquierdo ≤ 55% (HR = 1,84; IC95%, 1,09-3,1) y la indicación aguda del cateterismo índice (HR = 2,98; IC95%, 1,39-6,3). El uso de stent-grafts comparado con el de stents farmacoactivos no se asoció al desarrollo de más eventos combinados (23,5 frente a 29,6%; p = 0,598).
Conclusiones: El uso de stents recubiertos en aneurismas coronarios es factible y seguro a largo plazo. Se necesitan estudios clínicos aleatorizados para decidir el mejor tratamiento de este tipo de lesiones complejas.
Palabras clave: Aneurismas coronarios. Registro. Resultados. Stent. Stent-graft. Angioplastia.
Abbreviations
LVEF: Left ventricular ejection fraction.
INTRODUCTION
The first descriptions of a coronary aneurysm were reported by Morgagni back in 1761, and the first series of 21 patients were reported in 1929.1-4 Since then, a variable incidence rate—between 0.3% and 12%—has been reported in several series following the implementation of imaging modalities and coronary angiography.5 The overall incidence rate reported in a cohort of over 436 000 contemporary coronary angiographies from an international registry is 0.35%.5 Same as it happens with the clinical presentation and profile, treatment varies significantly.5,6 Still, revascularization is often required here.6 Over the last few years, some of the alternatives available propose the use of stent-grafts for the exclusion of coronary aneurysms.5-14
These devices—initially developed for other indications15 such as coronary perforations—have proven useful and safe in the short-term, and in cases and series previously published.7-10,12
The main goal of this paper is to describe the frequency of use of this type of stents for the management of coronary aneurysms and characterize its long-term results using patients with drug-eluting stents as the control group since they have had good results in this context.5
METHODS
This paper uses data curated from the International Coronary Artery Aneurysm Registry (CAAR) (NCT-02563626).16 Using a methodology already published, this ambispective registry included data from adult patients (≥ 18 years) who underwent a coronary angiography for whatever reason in 32 hospitals from 9 different countries.5 Coronary aneurysm was defined as a focal dilatation (< 1/3 of the vessel) 1.5 times larger compared to the vessel diameter in a healthy adjacent segment; the giant aneurysm was defined as a dilatation 4 times larger compared to the reference diameter.16 Investigators were advised to collect a consecutive case series in specific closed periods of time. Both the clinical and the procedural variables were collected, as well as the events occurred during the index hospital stay considered as that moment when it was first reported that the patient had, at least, 1 coronary aneurysm. Then, after validating which patients were eligible, the clinical follow-up was performed with information from the health records collected via medical consultations or phone calls. As stated in former reports, the protocol was initially approved by the coordinating center ethics committee and then by the centers that required it. Data were collected anonymously, and patients gave their informed consent to all the study procedures. Clinical decisions were always made by the treating physician of every patient without any influence from the study protocol whatsoever. The analysis of this study only included patients who received a stent-grafts or drug-eluting stents in an aneurysmal area.
The study primary endpoint was to describe the real-life use of stent-grafts to treat coronary aneurysms. Secondary endpoints were to determine the occurrence of events at the long-term follow-up. Similarly, another secondary endpoint was to conduct a comparison with patients who received drug-eluting stents in the aneurysmal area. If both types of stents were implanted, the patient from the stent-graft group was considered. Similarly, the analyses were conducted individually in each patient.
Statistical analysis
The statistical package SPSS v24.0 (IBM-SPSS, United States) was used to conduct the statistical analysis. Data are expressed as mean ± standard deviation or as median and interquartile range, when appropriate. Categorical variables were expressed as percentages. Inter-group comparisons were made using the chi-square test with qualitative variables. On the other hand, the Student t test, Mann-Whitney U test or Wilcoxon test were used, when appropriate, with continuous variables. The long-term event-free survival curves for the different analyses and groups were obtained using the Kaplan-Meier method. In them, the inter-group comparisons were performed using the log-rank test.
Based on the principle of parsimony, multivariable models were used in which, to avoid ann excess of variables in the analysis, only those with P values ≤ .10 were included in the univariate study that will be further explained later. Both the hazard ratio (HR) and the confidence intervals were estimated at 95% (95%CI) based on a Cox logistic regression model with backward elimination (Wald). Two-tailed P values < .05 were considered statistically significant.
RESULTS
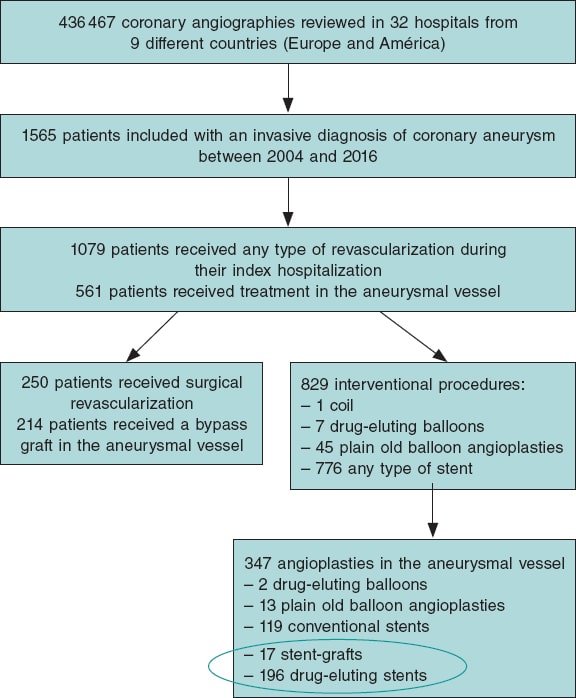
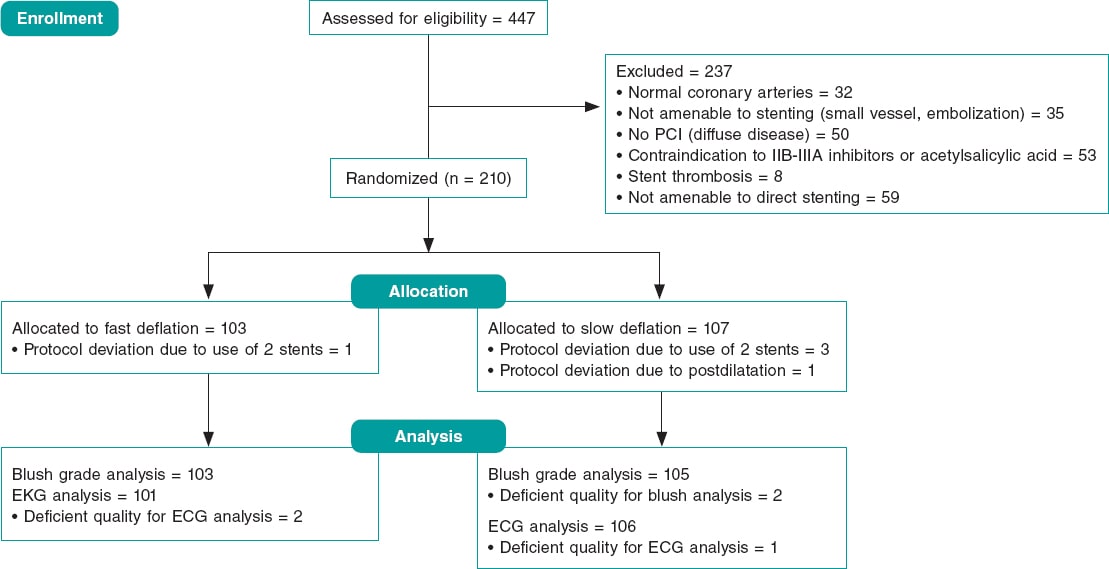
Out of a total of 1565 patients eventually considered in the global registry, 250 were referred for coronary artery surgery and 829 to receive some type of percutaneous revascularization.5 A total of 17 of these patients received, at least, 1 stent-graft to treat their coronary aneurysm. Also, 196 patients received a drug-eluting stent in the aneurysmal area. Therefore, the 17 and 196 patients mentioned before were included in the subsequent analyses of this study. Figure 1 shows the flow of patients.
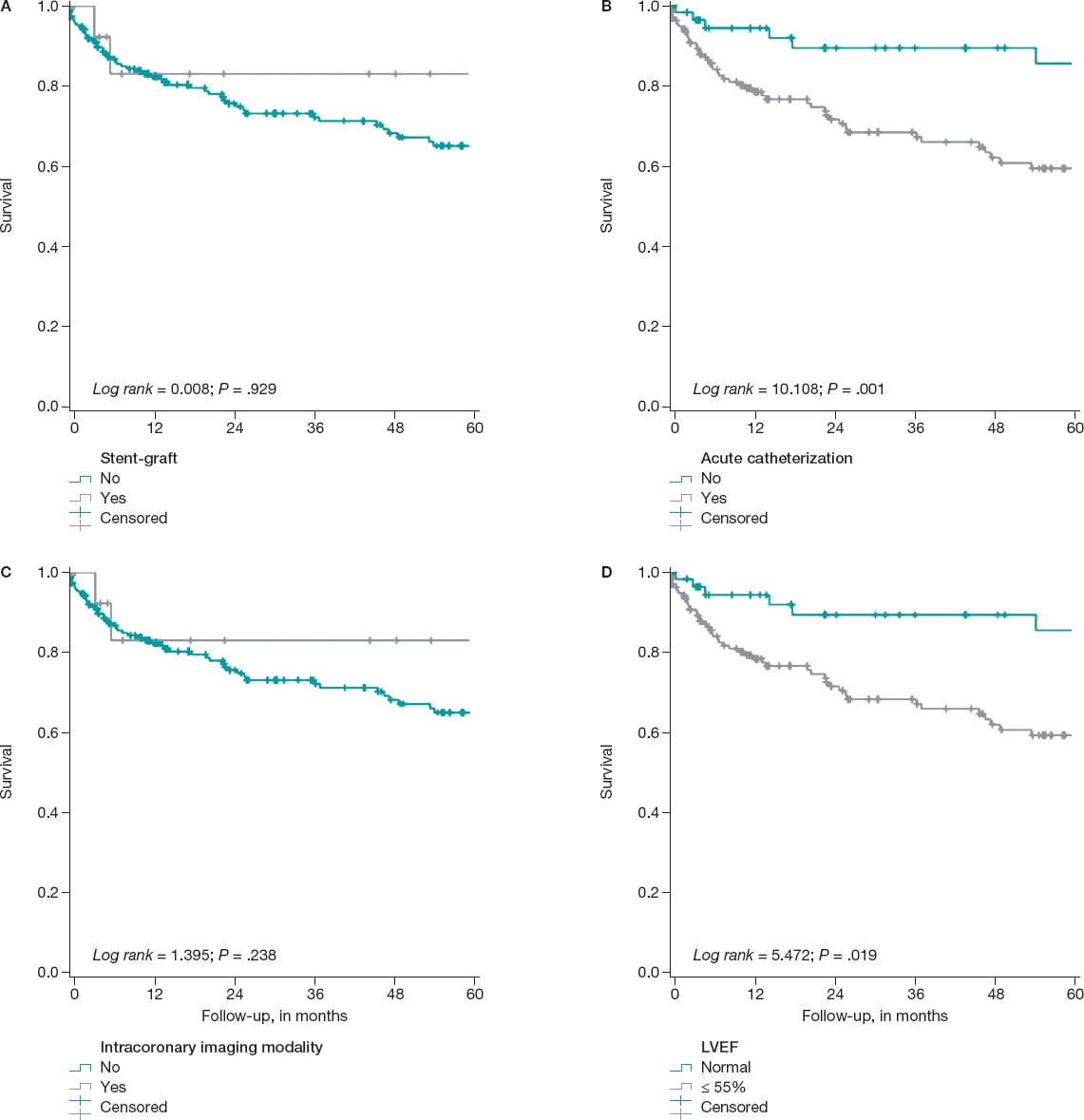
Figure 1. Flow of the registry patients. The devices encircled in an oval were analyzed in this study. In the stent-graft group it was studied whether patients received a device of this type regardless of other devices.
Approximately, 8% of the patients specifically treated in the aneurysmal area received a stent-graft. Table 1 shows the clinical and angiographic characteristics, and the long-term events of both patients who received stent-grafts and those who received drug-eluting stents. Males were predominant and often showed signs of dyslipidemia, previous coronary arteriopathy, coronary artery disease with previous revascularization, and giant aneurysms in the cohort implanted with stent-grafts. The frequency and type of complications reported at the long-term follow-up with an overall median follow-up of 38 months are shown on table 1. No statistically significant differences were seen at the follow-up regarding the clinical events. A composite event rate of major adverse cardiovascular events (MACE) of 29.6% was reported in patients treated with drug-eluting stents compared to 23.5% in those treated with stent-grafts. Individually, the most common event reported in the group implanted with stent-grafts was unstable angina (11.8%). In the group treated with drug-eluting stents, the most common event was unstable angina (10.2%) and death (10.2%). Every individual event is shown on table 1.
Table 1. Overall characteristics of patients treated with stent-grafts compared to those treated with drug-eluting stents as first-line therapy for the management of coronary aneurysms
| Patients | Stent-graft (N = 17) | Drug-eluting stent (N = 196) | P |
|---|---|---|---|
| Clinical characteristics | |||
| Age, years | 61.47 ± 13.8 | 63.84 ± 12.8 | .467 |
| Sex, male | 16 (94.1) | 146 (74.5) | .069 |
| Arterial hypertension | 11 (64.7) | 142 (72.4) | .496 |
| Dyslipidemia | 15 (88.2) | 119 (60.7) | .024 |
| Diabetes | 3 (17.6) | 58 (29.6) | .296 |
| Smoking habit | .218 | ||
| Active smoker | 10 (58.8) | 82 (41.8) | |
| Former smoker | 3 (17.6) | 25 (12.8) | |
| Family history of coronary arteriopathy | 7 (41.2) | 14 (7.1) | < .001 |
| Kidney disease (CrCl < 30) | 1 (5.9) | 14 (7.1) | .846 |
| Peripheral vasculopathy | 1 (5.9) | 18 (9.2) | .647 |
| Aortopathy – aneurysms | 1 (5.9) | 6 (3.1) | .531 |
| Atrial fibrillation | 1 (5.9) | 7 (3.6) | .631 |
| Connective tissue disease | 0 | 3 (1.5) | .607 |
| LVEF | 56.8 ± 6.1 | 55.6 ± 11.4 | .657 |
| Previous revascularization | 8 (47.0) | 41 (20.9) | .014 |
| Angiographic characteristics | |||
| Right dominance | 14 (82.4) | 166 (84.7) | .641 |
| Serious coronary stenoses | 15 (88.2) | .132 | |
| 1 vessel disease | 4 (23.5) | 62 (31.6) | |
| 2-vessel disease | 6 (35.3) | 68 (34.7) | |
| 3-vessel disease | 5 (29.4) | 62 (31.6) | |
| Location of the aneurysma | |||
| Left main coronary artery | 0 | 3 (1.5) | .607 |
| LAD | 7 (41.2) | 125 (63.8) | .066 |
| LCX | 4 (23.5) | 49 (25) | .893 |
| RCA | 6 (35.3) | 53 (27.0) | .466 |
| Type of aneurysmb | .450 | ||
| Fusiform | 5 (29.4) | 85 (43.8) | |
| Saccular | 12 (70.6) | 107 (55.2) | |
| Giant aneurysm | 3 (17,6) | 5 (2,6) | .02 |
| Number of aneurysms per patient | .940 | ||
| 1 | 15 (88.2) | 155 (79.1.2) | |
| 2 | 2 (6.3) | 30 (15.3) | |
| 3 | 0 | 6 (3.1) | |
| 4 or more | 0 | 5 (2.5) | |
| Indication for catheterization, acute | 11 (64.7) | 144 (73.5) | .436 |
| Indication for catheterization | .179 | ||
| STEACS | 6 (35.3) | 49 (25.0) | |
| NSTEACS | 4 (23.5) | 91 (46.4) | |
| Heart failure | 1 (5.9) | 2 (1) | |
| Stable angina | 6 (35.3) | 32 (16.3) | |
| Other | 0 | 22 (11.2) | |
| Type of stent | – | ||
| Aneugraft | 4 (23.5) | ||
| Jostent-graftmaster | 11 (64.7) | ||
| Papyrus | 1 (5.9) | ||
| Undetermined stent-graft | 1 (5.9) | ||
| ABSORB | 2 (1.0) | ||
| ACTIVE | 28 (14.3) | ||
| BIOFREEDOM | 1 (0.5) | ||
| BIOMATRIX | 4 (2.0) | ||
| COMBO | 2 (1.0) | ||
| COROFLEX | 1 (0.5) | ||
| CRE8 | 8 (4.1) | ||
| CYPHER | 3 (1.5) | ||
| GENOUS | 1 (0.5) | ||
| JANUS | 2 (1.0) | ||
| NO ESPECIF | 8 (4.1) | ||
| ONYX | 1 (0.5) | ||
| ORSIRO | 3 (1.5) | ||
| PROMUS | 20 (10.2) | ||
| RESOLUTE | 23 (11.7) | ||
| STENTYS | 6 (3.1) | ||
| SYNERGY | 12 (6.1) | ||
| XIENCE | 47 (24.0) | ||
| TAXUS | 22 (11.2) | ||
| YUKON | 2 (1.0) | ||
| Size of the stent-graft, medians | |||
| Diameter | 3.5 (3.5-4.0) | 3.5 (3.0-3.75) | .336 |
| Length | 18.0 (16.0-26.0) | 20.0 (15.0-28.0) | .014 |
| Intracoronary imaging modalities | |||
| IVUS | 5 (29.4) | 19 (9.7) | .014 |
| OCT | 1 (5.9) | 7 (3.6) | .631 |
| Any or both | 6 (35.3) | 26 (13.3) | .015 |
| Follow-up | |||
| Median follow-up, months | 29.9 (2.33-51.54) | 46.95 (11.92-76.75) | .093 |
| Dual antiplatelet therapy at discharge | 17 (100) | 193 (99.5) | .767 |
| Duration of dual antiplatelet therapy, median | 12.0 (11.0-12.0) | 12 (12.0-12.0) | .372 |
| Oral anticoagulation/new indication | 2/0 | 9/0 | |
| Adverse events | |||
| Heart failure | 0 | 3 (1.5) | .607 |
| Unstable angina | 2 (11.8) | 20 (10.2) | .839 |
| Reinfarction | 1 (5.9) | 16 (8.2) | .739 |
| Clinically relevant bleeding | 1 (5.9) | 8 (4.1) | .723 |
| Embolism | 0 | 1 (0.5) | .768 |
| Stroke | 0 | 2 (1) | .676 |
| Dead | 0 | 20 (10.2) | .166 |
| All of the above or complicated aneurysm (MACE) | 4 (23.5) | 58 (29.6) | .598 |
| Coronary angiography at the follow-up | 8 (47.0) | 61 (31.1) | .187 |
| Control | 3 (17.6) | 16 (8.2) | |
| Stable angina | 3 (17.6) | 6 (3.1) | |
| NSTEACS | 2 (11.8) | 25 (12.8) | |
| STEACS | 0 | 6 (3.1) | |
| Other | 0 | 8 (4.0) | |
| Aneurysmal complications on the angiographyc | |||
| Growth | 0 | 7 (11.5) | .312 |
| New aneurysms | 0 | 3 (4.9) | .521 |
| Thrombosis | 0 | 6 (9.8) | .353 |
| In-stent restenosis | 1 (12.5) | 0 | .005 |
|
Cr, creatinine; IVUS, intravascular ultrasound; LAD, left anterior descending coronary artery; LCX, left circumflex artery; LVEF, left ventricular ejection fraction; MACE, major adverse cardiovascular events; NSTEACS, non-ST-segment elevation acute coronary syndrome; OCT, optical coherence tomography; RCA, right coronary artery; STEACS, ST-segment elevation acute coronary syndrome. Data are expressed as no. (%) or mean ± standard deviation. a There are more aneurysms than patients because the same patient can have several aneurysms. b Aneurysm was categorized as mixed (fusiform and saccular) in 2 patients. c Statistics is performed on a lower N, only in those with a coronary angiography at the follow-up. |
|||
Coronary angiographies at the follow-up became available for 69 patients (32.4%). Eight of them were performed in the group with stent-grafts and only 1 confirmed failed stent implantation due to in-stent restenosis. In the group treated with drug-eluting stents, the aneurysm grew bigger or new aneurysms appeared in over 15% of the patients with follow-up coronary angiographies available. The rate of thrombosis in this selected group reached 9.8%. Table 2 provides an overall comparison between patients with the composite endpoint of MACE and those without it.
Table 2. Clinical and angiographic characteristics of patients depending on whether they showed, at least, 1 major adverse cardiovascular event at the follow-upa
| Patients | Without events (N = 151) | Some MACE (N = 62) | P |
|---|---|---|---|
| Clinical characteristics | |||
| Age, years | 62.99 ± 12.37 | 65.29 ± 13.93 | .234 |
| Sex, make | 115 (76.2) | 47 (75.8) | .956 |
| Arterial hypertension | 107 (70.9) | 456 (74.2) | .623 |
| Dyslipidemia | 93 (61.6) | 41 (66.1) | .533 |
| Diabetes | 39 (25.8) | 22 (35.5) | .157 |
| Smoking habit | .808 | ||
| Active smoker | 64 (42.4) | 28 (30.4) | |
| Former smoker | 19 (12.6) | 9 (14.5) | |
| Family history of coronary arteriopathy | 17 (11.3) | 4 (6.5) | .285 |
| Kidney disease (CrCl < 30) | 8 (5.3) | 7 (11.3) | .120 |
| Peripheral vasculopathy | 9 (6.0) | 10 (16.1) | .018 |
| Aortopathy – aneurysms | 3 (2.0) | 4 (6.5) | .097 |
| Atrial fibrillation | 5 (3.3) | 3 (4.8) | .594 |
| Connective tissue disease | 0 | 3 (4.8) | .006 |
| LVEF | 56.62 ± 9.74 | 53.67 ± 13.44 | .080 |
| Previous revascularization | 36 (23.8) | 13 (21.0) | .651 |
| Angiographic characteristics | |||
| Right dominance | 127 (84.1) | 53 (85.5) | .237 |
| Serious coronary stenoses | 147 (97.4) | 60 (96.8) | .817 |
| 1 vessel disease | 47 (31.1) | 19 (30.6) | |
| 2-vessel disease | 52 (34.4) | 22 (35.5) | |
| 3-vessel disease | 48 (31.8) | 19 (30.6) | |
| Location of the aneurysmb | .429 | ||
| Left main coronary artery | 3 (2.0) | 0 | |
| LAD | 88 (58.3) | 44 (71) | |
| LCX | 41 (27.2) | 12 (19.4) | |
| RCA | 41 (27.2) | 18 (29.0) | |
| Type of aneurysmc | .676 | ||
| Fusiform | 62 (41.1) | 28 (45.2) | |
| Saccular | 86 (57.0) | 33 (53.2) | |
| Giant aneurysm | 4 (2.6) | 4 (6.5) | .185 |
| Number of aneurysms per patient | |||
| 1 | 122 (80.8) | 48 (77.4) | |
| 2 | 20 (13.2) | 12 (19.4) | |
| 3 | 6 (4.0) | 0 | |
| 4 or more | 3 (2.0) | 2 (3.2) | |
| Indication for catheterization, acute | 101 (66.9) | 54 (87.1) | .002 |
| Indication for catheterization | .053 | ||
| STEACS | 38 (25.1) | 17 (27.4) | |
| NSTEACS | 61 (40.4) | 34 (54.8) | |
| Heart failure | 2 (1.3) | 1 (1.6) | |
| Stable angina | 33 (21.8) | 5 (8.1) | |
| Other | 17 (11.2) | 5 (8.1) | |
| Type of stent | .598 | ||
| Stent-graft | 13 (8.6) | 4 (6.5) | |
| Drug-eluting stent | 138 (91.4) | 58 (93.5) | |
| Size of the stent-graft, medians | |||
| Diameter | 3.38 (3.0-4.0) | 3.28 (3.0-3.5) | .521 |
| Length | 22.00 (15.0-28.0) | 21.74 (15.0-25.0) | .843 |
| Intracoronary imaging modalities | |||
| IVUS | 17 (11.3) | 7 (11.3) | .995 |
| OCT | 8 (5.3) | 0 | .065 |
| Median follow-up, months | 34.0 (12.0-76.0) | 46.93 (18.75-79.75) | .646 |
|
CD: coronaria derecha; CX: circunfleja; Cr: creatinina; DA: descendente anterior; FEVI: fracción de eyección del ventrículo izquierdo; IVUS: ecocardiografía intravascular; MACE: eventos adversos cardiovasculares mayores; OCT: tomografía de coherencia óptica; SCACEST: síndrome coronario agudo con elevación del segmento ST; SCASEST: síndrome coronario agudo sin elevación del segmento ST. Los datos se expresan como n (%) o media ± desviación estándar. a Se consideró como MACE el combinado de muerte de cualquier causa, ingreso por insuficiencia cardiaca, angina inestable, reinfarto, ictus, embolia sistémica, sangrado que precisó atención médica o cualquier complicación del aneurisma (crecimiento, nuevo aneurisma, reestenosis o trombosis). b Hay más aneurismas que pacientes, porque cada enfermo puede presentar varios. c En varios pacientes (3 y 1, respectivamente) el aneurisma fue considerado mixto. |
|||
The multivariate analysis on the occurrence of MACE included in the model the use of stent-grafts. On the other hand, the univariate analysis included variables with P values ≤ .10. All of them are shown on table 2 including the presence or not, of peripheral vasculopathy (on therapy), previous diagnosis of aneurysm (in a territory different from the coronary one), diagnosed connective tissue disease, left ventricular ejection fraction, use of intracoronary imaging modalities (optical coherence tomography or intravascular ultrasound), and acute indication to perform index catheterization.
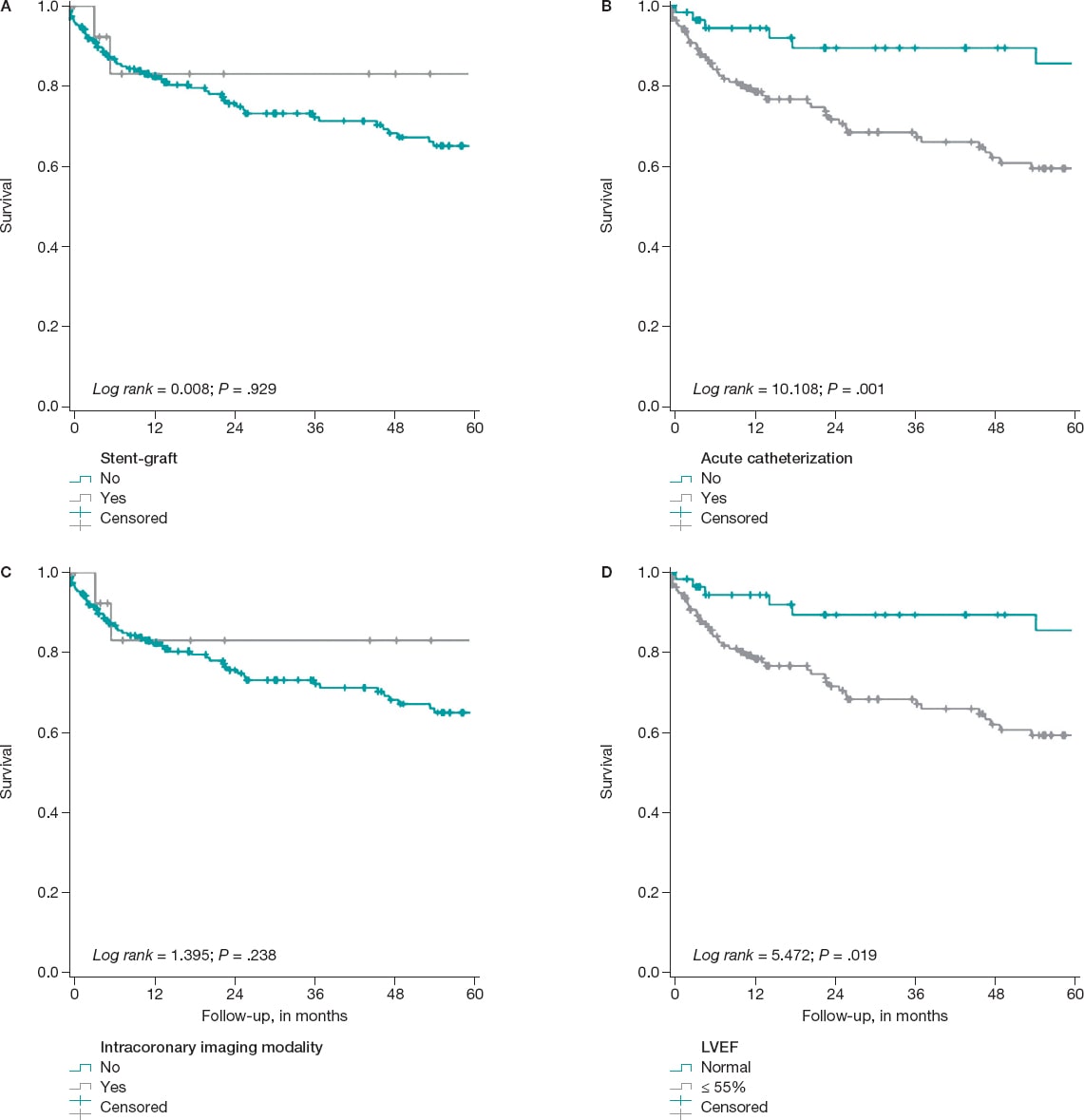
It was confirmed that the following variables remain in the model as independent predictors of the development of the composite endpoint: the existence of connective tissue disease (HR, 5.94; 95%CI, 1.82-19.37), left ventricular dysfunction—below 55%—(HR, 1.84; 95%CI, 1.09-3.1), and the acute indication for index catheterization (HR, 2.98; 95%CI, 1.39-6.3) (figure 2). The use of intracoronary imaging modalities—more common in the cohort implanted with stent-grafts—reached differences that were not statistically significant in the multivariate analysis. It was not a discriminator either regardless of the use of stent-grafts or drug-eluting stents (table 1, table 2, and figure 2).
Figure 2. Kaplan Meier survival curves free of the composite MACE event. A: on the use, or not of the stent-graft for the management of the aneurysm. B: based on whether the indication for index catheterization was acute (acute coronary syndrome, heart failure, etc.). C: regarding the use, during the angioplasty, of any of these intracoronary imaging modalities (intravascular ultrasound, optical coherence tomography or both), D: stratification based on the left ventricular ejection fraction (LVEF) when the angioplasty was performed.
DISCUSSION
This analysis is one of the largest series of coronary aneurysms published including data from real-life patients. It compares 2 of the most widely used therapeutic strategies in this context,5 and its main findings are:
a) The most widely used revascularization method in patients with coronary aneurysms was percutaneous.
b) The exclusion technique, that is, the use of stent-grafts, was used in a relatively lower number of cases (8%).
c) The clinical profile of patients treated with drug-eluting stents was similar compared to patients treated with stent-grafts. However, the presence of giant aneurysms is more common in the latter group. Also, it is probably one of the factors that operators pay most attention to when choosing one stent over the other.
d) An acute indication for the index catheterization and the presence of ventricular dysfunction, at that particular moment, are independent factors of poor prognosis in the study cohort.
e) In the long-term, a similar safety and efficacy profile can be seen in both arms of treatment making stent-grafts a reasonable alternative in selected cases with coronary aneurysms.
The specific treatment of patients with coronary aneurysms has not been well-defined yet to the point that it is not even quoted by the international clinical guidelines on revascularization.5 Over the last few years, several series and registries have been published trying to shed light on this issue.5,6,8,11 Generally speaking, coronary aneurysm is a rare coronary comorbidity. Nonetheless, the average interventional cardiologist sees 1 or several cases each year in his cath lab.7,16 As a matter of fact, in our own experience its estimated that its incidence rate is around 0.35% according to over 430 000 coronary angiographies performed,5 and around 1% according to a recent Chinese series of a little over 11 000 coronary angiographies.17 For this reason, it is important to have clinical data available to guide the management of this entity.7
Also, the coronary aneurysm is a clear marker of anatomic complexity and in adult patients it is suggestive of extensive coronary artery disease, and possibly, poor prognosis compared to milder forms of coronary arteriopathy.7 In previous analyses, the use of drug-eluting stents in patients with coronary aneurysms has been proposed as a therapeutic option clearly superior to conventional stents.5 That is why—as it happens with the rest of patients with ischemic heart disease—this type of platforms is widely recommended for patients with coronary aneurysms. Similarly, the use of an intense and thorough antithrombotic therapy is probably associated with fewer evolutionary complications, which is really reasonable considering the already mentioned high ischemic risk of these patients.11,18
The use of stent-grafts has been proposed as an alternative that can restore the anatomy of the blood vessel. Although the early design of these stents originally served other purposes, the data supporting the feasibility of their use with a high rate of success are extensive.8 In our series, the stent most widely used was the classically designed Jostent Graftmaster coronary stent graft system (Abbott Vascular, United States) (nearly 65%). It is composed of a PTFE layer between 2 stainless-steel stents that may have influenced the results. As a matter of fact, in our setting, Jurado-Román et al.15 conducted a multicenter registry on a certain state-of-the-art stent-graft. They proved that, in several real-life indications, the rate of events is reasonable (MACE, 7.1% at an average 22 months). However, the rate of stent thrombosis was slightly higher (3%) compared to the rate reported by drug-eluting stents in common uses.
The use of intracoronary imaging modalities to perform angioplasties in patients with coronary aneurysms possibly has prognostic implications as it happens in other complex clinical situations (diagnostic doubts, left main coronary artery, bifurcations). In this series, although they were more widely used in the group with stent-grafts implanted, no statistically significant differences were seen on the development of MACE (figure 2). This possibly has to do with the size of the study sample. Also, a tendency was seen towards fewer events in the group of patients with procedures optimized through intracoronary imaging guidance whether intravascular ultrasound or optical coherence tomography.
Limitations
This study has limitation associated with the particular design of the study. Also, a relatively small number of participants was included, which may have complicated the detection of differences in the analyses due to the lack of statistical power. The decision to implant stent-grafts or drug-eluting stents was entirely left to each patient’s medical team, which may have been associated with a certain degree of heterogeneity in the protocols that could have also been more dynamic in time. At the very complete follow-up from the clinical standpoint, control angiographies became available for a limited number of patients only (32%) who met the criterion set by the treating physicians. This may have underestimated the rate of complications, especially the subclinical ones, or be associated with selection biases in both groups.
However, this study is an approach to real-life clinical practice for a relatively rare heart disease on which there is little information available. It also includes a long-term clinical follow-up.
CONCLUSIONS
Stents-grafts can be used to treat coronary aneurysms and are safe in the long-term. Randomized clinical trials are needed to decide what the best treatment is for this type of complex coronary lesions.
FUNDING
None.
AUTHORS’ CONTRIBUTIONS
I. J. Núñez-Gil, CAAR coordinator: study design, data analysis, and draft writing. E. Cerrato, M. Bollati, L. Nombela-Franco, and A. Fernández-Ortiz: study design. E. Cerrato, M. Bollati, B. Terol, E. Alfonso-Rodríguez, S. J. Camacho-Freire, P. A. Villablanca, I. J. Amat-Santos, J.M. de la Torre-Hernández, I. Pascual, C. Liebetrau, B. Camacho, M. Pavani, R. A. Latini, F.Varbella, V. A. Jiménez Díaz, D. Piraino, MM, F. Alfonso, J. Antonio Linares, J. M. Jiménez-Mazuecos, J. Palazuelos- Molinero, and I. Lozano: data mining and recruitment. E. Cerrato, M. Bollati, B. Terol, L. Nombela-Franco, E. Alfonso-Rodríguez, S. J. Camacho-Freire, P. A. Villablanca, I. J. Amat-Santos J.M. de la Torre-Hernández, I. Pascual, C. Liebetrau, B. Camacho, M. Pavani, R. A. Latini, F.Varbella, V. A. Jiménez Díaz, Davide Piraino, M. Mancone, F. Alfonso, J. A. Linares, J. M. Jiménez-Mazuecos, J. Palazuelos- Molinero, IÍ. Lozano, and A. Fernández-Ortiz: reading and critical review of the manuscript.
CONFLICTS OF INTEREST
J. M. de la Torre Hernández is the editor-in-chief of REC: Interventional Cardiology, and F. Alfonso is an associate editor of this journal. The journal’s editorial procedure to ensure impartial handling of the manuscript has been followed. No other conflicts of interest have been declared whatsoever.
WHAT IS KNOWN ABOUT THE TOPIC?
- Coronary aneurysms are a complex entity whose incidence rate is between 0.3 and 12% in the different series already published.
- Treatment, like the presentation and the clinical profile, is varied. However, revascularization is often required.
- In this sense, over the last few years, some of the alternatives available propose the use of stent-grafts for the exclusion of coronary aneurysms.
WHAT DOES THIS STUDY ADD?
- The main goal of this paper was to describe the frequency of use of this type of stents to treat coronary aneurysms and then characterize its long-term results.
- From a total of 829 patients with coronary aneurysms treated with some type of percutaneous revascularization, data on the use of stent-grafts and drug-eluting stents was collected in 17 and 196 patients, respectively.
- It seems obvious that patients treated with stent-grafts for the management of coronary aneurysms have a high ischemic load, often complex anatomies, and even more often giant aneurysms.
- The use of stent-grafts for the management of coronary aneurysms is feasible and safe in the long-term. However, randomized clinical trials are still needed to decide what the best therapy is for this type of complex coronary lesions.
REFERENCES
1. Bourgon A. Biblioth Med. 1812;37:183. Citado por Scott DH. Aneurysm of the coronary arteries. Am Heart J. 1948;36:403-421.
2. Packard M, Wechsler H. Aneurysms of coronary arteries. Arch Intern Med. 1929;43:1-14.
3. Swaye PS, Fisher LD, Litwin P, et al. Aneurysmal coronary artery disease. Circulation. 1983;67:134-138.
4. Cohen P, O'Gara PT. Coronary artery aneurysms:a review of the natural history, pathophysiology, and management. Cardiol Rev. 2008;16:301-304.
5. Núñez-Gil IJ, Cerrato E, Bollati M, et al. Coronary artery aneurysms, insights from the international coronary artery aneurysm registry (CAAR). Int J Cardiol. 2020;299:49-55.
6. Núñez-Gil IJ, Terol B, Feltes G, et al. Coronary aneurysms in the acute patient:Incidence, characterization and long-term management results. Cardiovasc Revasc Med. 2018;19(5 Pt B):589-596.
7. Kawsara A, Núñez Gil IJ, Alqahtani F, Moreland J, Rihal CS, Alkhouli M. Management of Coronary Artery Aneurysms. JACC Cardiovasc Interv. 2018;11:1211-1223.
8. Will M, Kwok CS, Nagaraja V, et al. Outcomes of patients who undergo elective covered stent treatment for coronary artery aneurysms. Cardiovasc Revasc Med. 2021:S1553-8389(21)00264-5.
9. Núñez-Gil IJ, Alberca PM, Gonzalo N, Nombela-Franco L, Salinas P, Fernández-Ortiz A. Giant coronary aneurysm culprit of an acute coronary syndrome. Rev Port Cardiol (Engl Ed). 2018;37:203.e1-203.e5.
10. Cha JJ, Kook H, Hong SJ, et al. Successful Long-term Patency of a Complicated Coronary Aneurysm at a Prior Coronary Branch Stent Treated with a Stent-graft and Dedicated Bifurcation Stent. Korean Circ J. 2021;51:551-553.
11. Khubber S, Chana R, Meenakshisundaram C, et al. Coronary artery aneurysms:outcomes following medical, percutaneous interventional and surgical management. Open Heart. 2021;8:e001440.
12. Arbas-Redondo E, Jurado-Román A, Jiménez-Valero S, Galeote-García G, Gonzálvez-García A, Moreno-Gómez R. Acquired coronary aneurysm after stent implantation at a bifurcation excluded with a Papyrus covered stent subsequently fenestrated. Cardiovasc Interv Ther. 2022;37:215-216.
13. Della Rosa F, Molina-Martin de Nicolas J, Bonfils L, Fajadet J. Symptomatic giant coronary artery aneurysm treated with covered stents. Coron Artery Dis. 2020;31:658-659.
14. Tehrani S, Faircloth M, Chua TP, Rathore S. Percutaneous coronary intervention in coronary artery aneurysms;technical aspects. Report of case series and literature review. Cardiovasc Revasc Med. 2021;28S:243-248.
15. Jurado-Román A, Rodríguez O, Amat I, et al. Clinical outcomes after implantation of polyurethane-covered cobalt-chromium stents. Insights from the Papyrus-Spain registry. Cardiovasc Revasc Med. 2021;29:22-28.
16. Núñez-Gil IJ, Nombela-Franco L, Bagur R, et al. Rationale and design of a multicenter, international and collaborative Coronary Artery Aneurysm Registry (CAAR). Clin Cardiol. 2017;40:580-585.
17. Jiang X, Zhou P, Wen C, et al. Coronary Anomalies in 11,267 Southwest Chinese Patients Determined by Angiography. Biomed Res Int. 2021;2021:6693784.
18. D'Ascenzo F, Saglietto A, Ramakrishna H, et al. Usefulness of oral anticoagulation in patients with coronary aneurysms:Insights from the CAAR registry. Catheter Cardiovasc Interv. 2021;98(5):864-871.
ABSTRACT
Introduction and objectives: The safety of physiology-based revascularization in patients with diabetes mellitus has been scarcely investigated. Our objective was to determine the safety of deferring revascularization based on the fractional flow reserve (FFR) or the instantaneous wave-free ratio (iFR) in diabetic patients.
Methods: Single-center, retrospective analysis of patients with intermediate coronary stenoses in whom revascularization was deferred based on FFR > 0.80 or iFR > 0.89 values. The long-term rate of major adverse cardiovascular events, a composite of all-cause mortality, myocardial infarction, and target vessel revascularization (TVR), was assessed in diabetic and non-diabetic patients at the follow-up. The rate of TVR based on the type of physiological index used to defer the lesion was also evaluated.
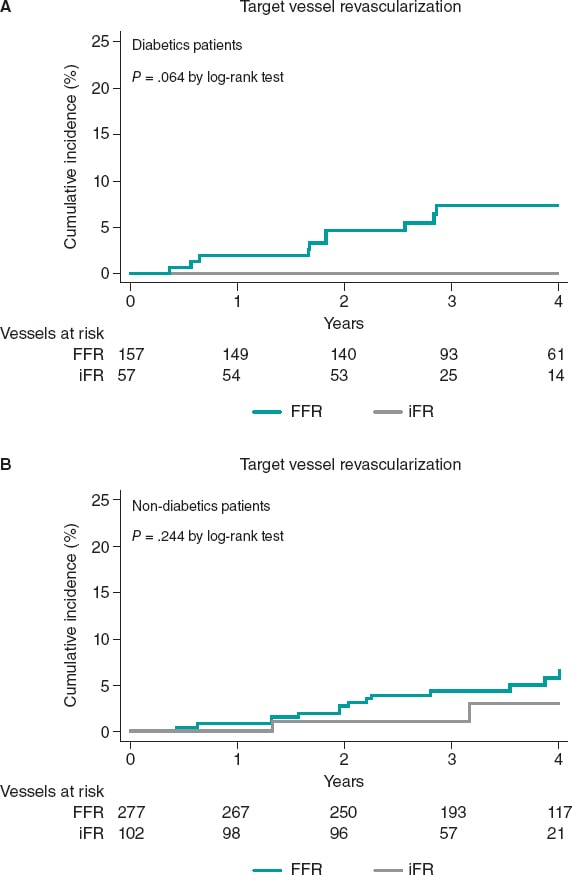
Results: We evaluated 164 diabetic (214 vessels) and 280 non-diabetic patients (379 vessels). No significant differences in the rate of major adverse cardiovascular events was seen between diabetic and non-diabetic patients (20.1% vs 13.2%; P = .245) at a median follow-up of 43 months. All-cause mortality and cardiac death were not statistically different between both groups in the adjusted analysis (P > .05). A trend towards a higher rate of myocardial infarction was seen in diabetic patients (6.7% vs 2.9%; P = .063). However, the rate of target vessel myocardial infarction was similar in both groups (P = .874). Overall, TVR was similar in diabetics and non-diabetics (4.7% vs 4.2%; P = .814); however, when analyzed based on the physiological index, numerically, diabetics had a higher rate of TVR when the FFR was used in the decision-making process compared to when the iFR was used (6.4% vs 0.0%; P = .064).
Conclusions: Deferring the revascularization of intermediate stenoses in patients with DM based on the FFR or the iFR is safe regarding the risk of TVR or target vessel myocardial infarction, with a rate of events at the long-term follow-up similar to that seen in non-diabetic patients.
Keywords: Fractional flow reserve. Instantaneous wave-free ratio. iFR. Diabetes mellitus.
RESUMEN
Introducción y objetivos: La seguridad de la revascularización fisiológica en pacientes diabéticos ha sido poco investigada. El objetivo fue determinar la seguridad de diferir la revascularización basándose en la reserva fraccional de flujo (FFR) o en el índice instantáneo libre de ondas (iFR) en pacientes con diabetes mellitus.
Métodos: Análisis retrospectivo, unicéntrico, de pacientes con estenosis coronarias intermedias en quienes se había diferido la revascularización en función de unos valores de FFR > 0,80 o de iFR > 0,89. Se analizó la incidencia a largo plazo de eventos cardiovasculares adversos mayores, una combinación de muerte por cualquier causa, infarto miocárdico y revascularización del vaso diana (RVD) en pacientes con y sin diabetes. También se evaluó la incidencia de RVD según el tipo de índice fisiológico utilizado para diferir la revascularización.
Resultados: Se evaluaron 164 pacientes diabéticos (214 vasos) y 280 pacientes no diabéticos (379 vasos), con una mediana de seguimiento de 43 meses. No se observaron diferencias significativas en los eventos cardiovasculares adversos mayores entre pacientes con y sin diabetes mellitus (20,1 frente a 13,2%; p = 0,245). La mortalidad por cualquier causa y de causa cardiaca no fue estadísticamente diferente entre ambos grupos en el análisis ajustado (p > 0,05). Se observó una tendencia a una mayor incidencia de infarto de miocardio en los pacientes con diabetes mellitus (6,7 frente a 2,9%; p = 0,063), pero el infarto relacionado con el vaso diana fue similar en ambos grupos (p = 0,906). En general, la RVD fue similar en diabéticos y no diabéticos (4,7 frente a 4,2%; p = 0,787); sin embargo, cuando se analizó según el índice fisiológico, los diabéticos tuvieron una mayor tasa numérica de RVD cuando se utilizó la FFR en la toma de decisiones en comparación con el iFR (6,4 frente a 0,0%; p = 0,064).
Conclusiones: Diferir la revascularización de estenosis intermedias en pacientes con diabetes mellitus según la FFR o el iFR es seguro en términos de RVD e infarto relacionado con el vaso diana, con una tasa de eventos en el seguimiento a largo plazo similar a la observada en pacientes sin diabetes mellitus.
Palabras clave: Reserva fraccional de flujo. Indice instantaneo libre de ondas. iFR. Diabetes mellitus.
Abbreviations
DM: diabetes mellitus. FFR: fractional flow reserve. iFR: instantaneous wave-free ratio. MACE: major adverse cardiovascular events. TVR: target vessel revascularization.
INTRODUCTION
Physiological evaluation has a class IA recommendation to guide coronary revascularization in the current clinical practice guidelines.1 Fractional flow reserve (FFR) and instantaneous wave-free ratio (iFR) have proven to be safe tools to guide revascularization therapy in several clinical scenarios.2-4
The results of the DEFER trial at the 15-year follow-up showed the long-term safety of FFR to defer therapy in functionally non-significant stenosis.5 Afterwards, the DEFINE-FLAIR and the iFR-SWEDEHEART trials proved the non-inferiority of the iFR compared to the FFR to guide revascularization of moderate stenosis at the 1-year follow-up.3,6 The utility of physiological guidance to guide revascularization in multivessel disease has been confirmed in the FAME and the SYNTAX II clinical trials.7,8
However, the prognostic value of pressure guidewire assessment in certain high-risk groups has not been firmly established yet. A pooled analysis of the DEFINE-FLAIR and the iFR-SWEDEHEART trials found a higher rate of events in patients with acute coronary syndrome in whom revascularization of non-culprit vessels was deferred based on the FFR or the iFR compared to stable patients.3,6 Patients with diabetes mellitus (DM) are a high-risk group with a well-known higher burden of cardiovascular disease and worse prognosis including more extensive atherosclerosis, more prevalence of multivessel disease, and a faster disease progression compared to non-diabetic patients.9-11 The special characteristics of the extent and spread of atherosclerosis in patients with DM raises concerns on the safety surrounding deferring revascularization in this population. Our objective was to evaluate the safety of revascularization deferral based on pressure guidewire interrogation in diabetic patients at the long-term follow-up.
METHODS
Study population
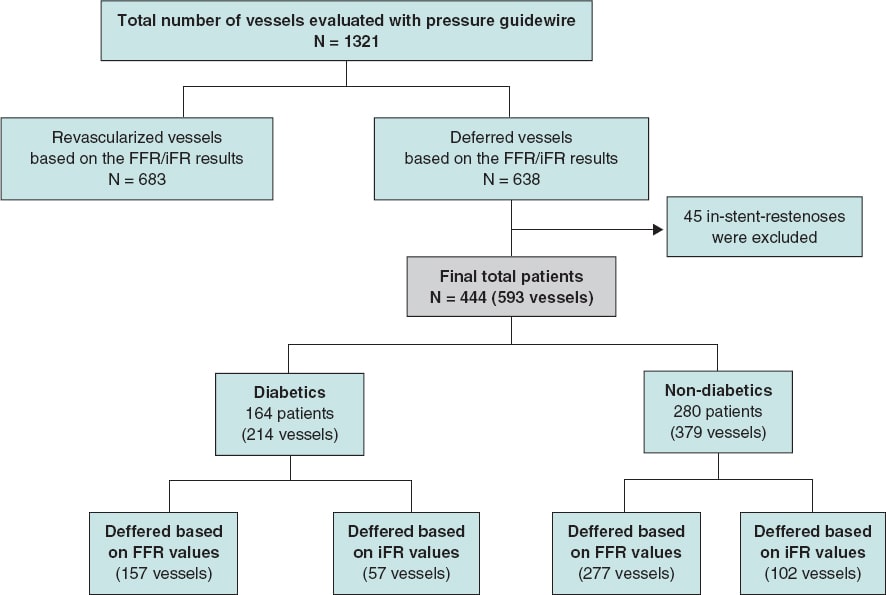
This is a single center, retrospective, and open-label trial. The study population was recruited from a total of 1321 consecutive patients with coronary artery disease in whom the iFR or the FFR indices were used to determine the need for coronary revascularization from January 2012 through December 2016. In 444 patients (34%) the revascularization of ≥ 1 lesions was deferred based on FFR values > 0.80 or iFR values > 0.89. Patients with stable angina and acute coronary syndrome (with non-culprit stenosis interrogated with pressure guidewires) were included in the study. For the analysis, the overall population was divided into 2 groups: DM and non-DM. The DM group was defined based on their past personal history included in their medical records. The study flow chart depicting patient selection is shown on figure 1.
Figure 1. Study flow chart. iFR, instantaneous wave-free ratio; FFR, fractional flow reserve.
This retrospective, cohort study was conducted according to the principles established by the Declaration of Helsinki. Both the informed consent and the research committee assessment were spared due to the retrospective nature of the study; each patient included in the database was encrypted and de-identified to protect everyone’s privacy.
The physiological procedure
Pressure guidewire assessment was performed using a commercial guidewire (Verrata, Philips Healthcare, United States; PressureWire [Certus. Aeris, X] St. Jude Medical, United States) and the standard technique previously reported.3,12 As a standard practice, an intracoronary bolus of nitrates (200 mcg) was administered before the FFR or iFR measurements. The cases submitted for FFR assessment received IV adenosine at a rate of 140 µg/kg/min. The cut-off values to defer revascularization were FFR > 0.80 or iFR > 0.89. The presence of significant drift was discarded by placing the sensor of the pressure guidewire on the tip of the guiding catheter at the end of the physiological measurements acquisition.
In patients with stable angina, the physiological evaluation was performed as part of the same procedure, and all intermediate stenoses were assessed. In patients with acute coronary syndrome, interrogation with the pressure guidewire was performed at a staged procedure in non-culprit vessels only.
Endpoints
The primary endpoint was the 4-year risk of major adverse cardiovascular events (MACE) defined as a composite endpoint of all-cause mortality, myocardial infarction or unplanned target vessel revascularization (TVR). The secondary endpoints were a) the individual components of MACE, b) the rate of target vessel myocardial infarction, and c) the rate of unplanned TVR based on the physiological index used (FFR or iFR)
Statistical analysis
Continuous variables were expressed as mean ± standard deviation (SD). Discrete variables are summarized as frequency (percentages). Under baseline conditions, group comparisons were made using the Student t test or the Mann Whittney U test for continuous variables and Pearson’s chi-square test for discrete data.
Time-to-event analysis was performed using the Kaplan-Meier method, and group comparison was performed using the Mantel-Cox (log-rank) test. For the primary and secondary endpoint comparison between diabetics and non-diabetics, a Cox Proportional hazards model was used to estimate hazard ratios (HR). The adjusted analysis was performed based on age, sex, hypertension, dyslipidemia, smoking habit, chronic kidney disease, previous stroke, previous percutaneous coronary intervention, and coronary artery bypass graft surgery.
All probability values were 2-sided with 95% confidence intervals (95%CI). P values < .05 were considered statistically significant. The SPSS version 23.0 (IBM Corp, Armonk, NY, United States) and STATA version 15 (Stata Corp, College Station, TX, United States) statistical packages were used for statistical analyses.
RESULTS
Baseline clinical and angiographic characteristics
The baseline clinical characteristics are shown on table 1. In the overall study population, mean age was 68.4 years, and 39.6% were patients with DM (164 patients). As expected, patients with DM had more cardiovascular risk factors and comorbidities compared to patients without DM. There were no significant differences in the clinical presentation between both study groups (P > .05). Most patients received optimal medical treatment at the hospital discharge without significant differences between DM and non-DM patients (P > .05).
Table 1. Baseline characteristics
| Total (N = 444) | Diabetics (N = 164) | Non-diabetics (N = 280) | P | |
|---|---|---|---|---|
| Clinical characteristics, N (%) | ||||
| Sex | .026 | |||
| Male | 340 (76.5) | 116 (70.7) | 224 (80.0) | |
| Female | 104 (23.4) | 48 (29.3) | 56 (20.0) | |
| Age (year) | 68.41 | 70.02 | 67.46 | .003 |
| Arterial hypertension | 321 (72.3) | 138 (84.1) | 183 (65.4) | <.001 |
| Hyperlipidemia | 287 (64.6) | 124 (75.6) | 163 (58.2) | <.001 |
| Current smoker | 253 (57.0) | 85 (51.8) | 168 (60.0) | .093 |
| Chronic kidney disease | 41 (9.2) | 29 (17.7) | 12 (4.3) | .000 |
| COPD | 30 (6.8) | 9 (5.5) | 21 (7.5) | .415 |
| Previous cerebrovascular disease | 21 (4.7) | 12 (7.3) | 9 (3.2) | .049 |
| Peripheral vascular disease | 38 (8.6) | 18 (11.0) | 20 (7.1) | .164 |
| Previous AMI | 47 (10.6) | 17 (10.4) | 30 (10.7) | .908 |
| Previous PCI | 220 (49.5) | 70 (42.7) | 150 (53.6) | .027 |
| Previous CABG | 13 (2.9) | 9 (5.5) | 4 (1.4) | .019 |
| Clinical presentation, N (%) | ||||
| Myocardial infarction | 148 (33.3) | 46 (28.0) | 102 (36.4) | .302 |
| Unstable angina | 89 (20.0) | 33 (20.1) | 56 (20.0) | |
| Stable angina | 112 (25.2) | 44 (26.8) | 68 (24.3) | |
| Silent ischemia | 46 (10.4) | 22 (13.4) | 24 (8.6) | |
| Other | 49 (11.0) | 19 (11.6) | 30 (10.7) | |
| Therapy at discharge, N (%) | ||||
| Aspirina | 408 (93.8) | 150 (94.3) | 258 (93.5) | .720 |
| Clopidogrela | 165 (37.9) | 53 (33.3) | 112 (40.6) | .134 |
| Prasugrela | 22 (5.1) | 9 (5.7) | 13 (4.7) | .663 |
| Ticagrelora | 78 (17.9) | 30 (18.9) | 48 (17.4) | .699 |
| DAPT | 332 (56.3) | 111 (52.6) | 221 (58.3) | .181 |
| Statinsb | 396 (93.2) | 148 (93.1) | 248 (93.2) | .952 |
| Beta-blockersb | 334 (78.6) | 121 (76.1) | 213 (80.1) | .334 |
| ACEIb | 324 (76.2) | 126 (79.2) | 198 (74.4) | .260 |
| Acenocoumarola | 41 (9.4) | 18 (11.3) | 23 (8.3) | .304 |
| Insulin | 53 (8.9) | 53 (24.8) | ||
|
ACEI, angiotensin converting enzyme inhibitors; AMI, acute myocardial infarction; CABG, coronary artery bypass grafting;; COPD, chronic obstructive pulmonary disease; PCI, percutaneous coronary intervention. a n = 435. b n = 425. |
||||
Characteristics of vessels with deferred revascularization
On average, deferred revascularization was performed in vessels with stenoses of intermediate severity (percent diameter stenosis, 59.73% ± 9.2%). The most frequently interrogated artery was the left anterior descending coronary artery (43.2%). In most patients, only 1 vessel was deferred (72.7%). Nevertheless, in about 4% of patients, revascularization was deferred in 3 vessels within the same procedure.
In our study population, revascularization deferral was based more frequently in the FFR (434 vessels, 73.2%) compared to the iFR (159 vessels, 26.8%). The same ratio applied to patients with DM: revascularization was deferred in 157 vessels (73.4%) based on FFR values compared to 57 vessels (26.6%) based on iFR values. The mean FFR and iFR values of the overall population were 0.87 ± 0.46 and 0.94 ± 0.41, respectively without any significant differences between diabetic and non-diabetic patients (table 2).
Table 2. Characteristics of the deferred arteries
| Total (N = 593) | Diabetics (N = 214) | Non-diabetics (N = 379) | P | |
|---|---|---|---|---|
| Deferred vessel | ||||
| LMCA | 25 (4.2) | 8 (3.7) | 17 (4.5) | .664 |
| LAD | 256 (43.2) | 90 (42.1) | 166 (43.8) | .681 |
| LCX | 173 (29.2) | 59 (27.6) | 114 (30.1) | .519 |
| RCA | 138 (23.3) | 57 (26.6) | 81 (21.4) | .145 |
| Number of deferred vessels per patient* | ||||
| 1 vessel | 323 (72.7) | 122 (74.4) | 201 (71.8) | .475 |
| 2 vessels | 98 (22.1) | 35 (21.3) | 63 (22.5) | |
| 3 vessels | 19 (4.3) | 7 (4.3) | 12 (4.3) | |
| 4 vessels | 4 (0.9) | 4 (1.4) | 0 (0.0) | |
| Coronary physiological parameters | ||||
| Mean FFR | 0.87 ± 0.46 | 0.86 ± 0.41 | 0.87 ± 0.48 | .387 |
| Mean iFR | 0.94 ± 0.41 | 0.94 ± 0.43 | 0.95 ± 0.40 | .091 |
| Deferred based on FFR values | 434 (73.2) | 157 (73.4) | 277 (73.1) | .942 |
| Deferred based on iFR values | 159 (26.8) | 57 (26.6) | 102 (26.9) | .942 |
|
FFR, fractional flow reserve; iFR, instantaneous wave-free ratio; LAD, left anterior descending coronary artery; LCX, left circumflex artery; LMCA, left main coronary artery; RCA, right coronary artery. * n = 444. |
||||
Clinical outcomes at the long-term follow-up based on the presence of diabetes
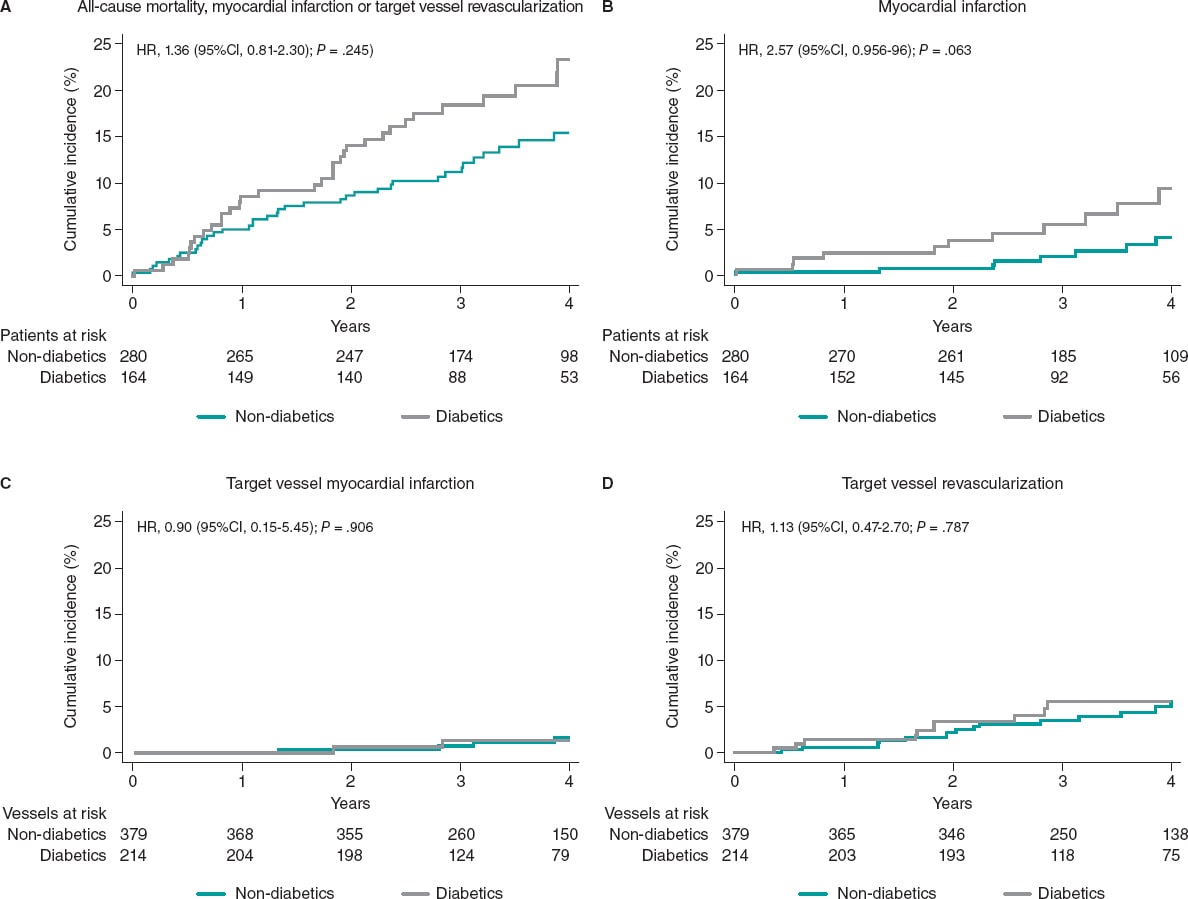
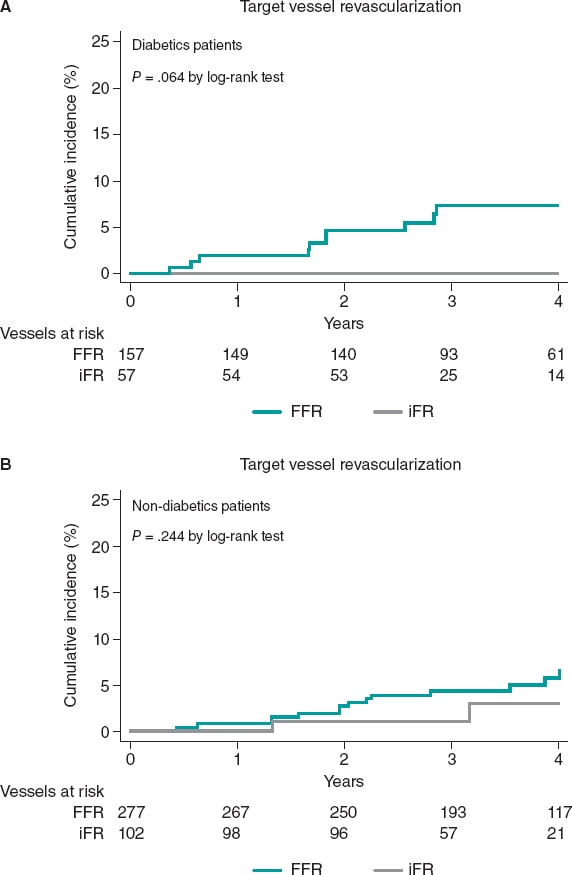
The median follow-up was 43 months [interquartile range, 31.1-55.8] without any differences being reported between DM and non-DM patients. The clinical outcomes are shown on table 3. Diabetic patients had higher rates of MACE (33 [20.1%] vs 37 [13.2%] in non-DM patients) although this difference did not reach statistical significance in the adjusted analysis (HR, 0.98, 95%CI; 0.46-2.11, P = .964). The all-cause mortality rate was higher in diabetics (18 [10.8%] vs 15 [5.3%] in non-diabetics), but the rates of cardiovascular death were not statistically different in either group (3.1% vs 2.1%). A trend towards a higher rate of myocardial infarction was seen in patients with DM (6.7% vs 2.9%; P = .063), yet target vessel myocardial infarction was similar in both groups (HR, 0,87; 95%CI, 0.15-4.89, P = .906). Similar rates of unplanned revascularization and TVR were seen between diabetics and non-diabetics (figure 2 and table 3).
Table 3. Clinical events at the 4-year follow-up based on the presence of diabetes
| Diabetics (N = 164) |
Non-diabetics (N = 280) |
Unadjusted analysis | Fully adjusted analysis* | |||
|---|---|---|---|---|---|---|
| HR (95%CI) | P | HR (95%CI) | P | |||
| MACE | 33 (20.1) | 37 (13.2) | 1.58 (0.99-2.53) | .058 | 0.98 (0.46-2.11) | .964 |
| All-cause mortality | 18 (10.8) | 15 (5.3) | 2.10 (1.06-4.17) | .034 | 2.01 (0.92-4.40) | .079 |
| Cardiovascular death | 5 (3.1) | 6 (2.1) | 1.45 (0.44-4.74) | .543 | 0.72 (0.19- 2.76) | .641 |
| Myocardial infarction | 11 (6.7) | 8 (2.9) | 2.54 (1.02-6.32) | .045 | 2.56 (0.95- 6.91) | .063 |
| Unplanned revascularization | 17 (10.4) | 20 (7.1) | 1.53 (0.80-2.93) | .195 | 1.55 (0.77- 3.10 | .219 |
| Diabetic vessels (N = 214) |
Non-diabetic vessels (N = 379) |
Unadjusted analysis | Fully adjusted analysis* | |||
| HR (95%CI) | P | HR (95%CI) | P | |||
| Target vessel myocardial infarction | 2 (0.9) | 4 (1.1) | 0.96 (0.18-5.23) | .971 | 0.87 (0.15-4.89) | .874 |
| Target vessel revascularization | 10 (4.7) | 16 (4.2) | 1.15 (0.52-2.54) | .767 | 1.14 (0.38-3.42) | .814 |
|
95%CI, 95% confidence interval; HR, hazard ratio; MACE, mayor adverse cardiovascular events (all-cause mortality, myocardial infarction, target vessel revascularization). * HR and P values are obtained after adjusting the model with different baseline variables (age, hypertension, dyslipidemia, smoking habit, chronic kidney disease, previous percutaneous coronary intervention, and previous coronary artery bypass grafting). |
||||||
Figure 2. Clinical outcomes in diabetic and non-diabetic patients at the 4-year follow-up. 95%CI, 95% confidence interval; HR, hazard ratio.
Clinical outcomes at the long-term follow-up based on the physiological index used to defer revascularization
In patients with DM the physiological index used (FFR or iFR) that led to revascularization deferral was not associated with a significant difference in the rate of MACE (P = .688) or with significant differences in all-cause mortality, cardiovascular death, myocardial infarction or unplanned revascularization. Similar rates of target vessel myocardial infarction were seen in patients deferred with both techniques (DM or non-DM) (table 4).
Table 4. Clinical outcomes at the 4-year follow-up based on the technique to defer revascularization
| Patients deferred based on FFR values (N = 347) |
Patients deferred based on iFR values (N = 97) |
P (log-rank) |
|
|---|---|---|---|
| MACE | 59 (17.0) | 11 (11.3) | .288 |
| Diabetics | 27 (21.1) | 6 (16.7) | .688 |
| Non-diabetics | 32 (14.6) | 5 (8.2) | .277 |
| All-cause mortality | 25 (7.2) | 8 (8.3) | .574 |
| Diabetics | 13 (10.2) | 5 (13.9) | .417 |
| Non-diabetics | 12 (5.5) | 3 (4.9) | .972 |
| Cardiovascular mortality | 8 (2.3) | 3 (3.1) | .593 |
| Diabetics | 4 (3.1) | 1 (2.8) | .964 |
| Non-diabetics | 4 (1.8) | 2 (3.3) | .436 |
| Myocardial infarction | 16 (4.6) | 3 (3.1) | .762 |
| Diabetics | 10 (7.8) | 1 (2.8) | .396 |
| Non-diabetics | 6 (2.7) | 2 (3.3) | .596 |
| Unplanned revascularization | 33 (9.5) | 4 (4.1) | .133 |
| Diabetics | 16 (12.5) | 1 (2.8) | .112 |
| Non-diabetics | 17 (7.8) | 3 (4.9) | .542 |
| Patients deferred based on FFR values (N = 434) |
Patients deferred based on iFR values (N = 159) |
P (log-rank) |
|
| Target vessel myocardial infarction | 4 (0.9) | 2 (1.3) | .527 |
| Diabetics | 2 (1.3) | 0 (0.0) | .433 |
| Non-diabetics | 2 (0.7) | 2 (2.0) | .172 |
| Target vessel revascularization | 24 (5.5) | 2 (1.3) | .037 |
| Diabetics | 10 (6.4) | 0 (0.0) | .064 |
| Non-diabetics | 14 (5.1) | 2 (2.0) | .244 |
|
iFR, instantaneous wave-free ratio; FFR, fractional flow reserve; MACE, mayor adverse cardiovascular events (all cause-mortality, myocardial infarction, target vessel revascularization). |
|||
The rate of TVR was significantly higher in patients deferred based on FFR values compared to patients deferred based on iFR values (24 [5.5%] vs 2 [1.3%], P = .037). This result was mainly driven by a significant trend towards a higher rate of TVR in patients with DM deferred based on FFR values compared to diabetic patients deferred based on iFR values (10 [6.4%] vs 0 [0%]), a result that did not achieve statistical significance (P = .065). This trend towards a lower rate of TVR in iFR-deferred vessels was not seen in non-DM patients (14 [5.1%] vs 2 [2.0%], P = .244) (figure 3).
Figure 3. Target vessel revascularization based on the technique used to defer revascularization at the 4-years follow-up. iFR, instantaneous wavefree ratio; FFR, fractional flow reserve.
DISCUSSION
The main findings of this study are: a) patients with DM had high rates of MACE. However, deferring the revascularization of intermediate stenoses in patients with DM based on the physiological assessment results with pressure guidewires is safe regarding the risk of TVR or target vessel myocardial infarction with a similar rate of events at the long-term follow-up compared to that seen in non-diabetic patients; b) both the FFR and the iFR can be used safely to defer intermediate stenosis in diabetic patients. c) there was a trend towards a higher rate of TVR in diabetic patients deffered based on FFR values.
Clinical outcomes based on the presence of diabetes mellitus
The use of coronary physiology to guide revascularization improves patient outcomes compared to angiographic assessment.3,6,7,13 Currently both the FFR and the iFR have a class IA recommendation in the clinical practice guidelines regarding revascularization for the functional assessment of coronary stenoses.1
Diabetic patients are a high-risk population with a more aggressive and accelerated atherosclerosis compared to non-diabetic patients. In the PARADIGM (Progression of atherosclerotic plaque determined by computed tomographic angiography imaging) study, the presence of DM was an independent risk factor for plaque progression.14 In a pooled analysis of 5 intravascular ultrasound trials, Nicholls SJ et al. found that patients with DM had a greater percent atheroma volume and a more rapid progression.15
Around 25% of the patients enrolled in pivotal studies that proved the effectiveness of the FFR and the iFR had DM.3,4,6,16,17 The safety of physiology-guided revascularization deferral in the DM setting has not been specifically assessed in randomized clinical trials. On the other hand, the results of the few non-randomized studies that have evaluated physiology-guided management in diabetics show conflicting results.18,19
Domínguez-Franco et al. analyzed the prognostic safety of the FFR in diabetics. Although, their results are consistent with ours in the sense that no differences were found in the TVR at the long-term follow-up after revascularization deferral in DM vs non-DM patients, the applicability and strength of that study is limited by its small sample size (136 patients, 144 lesions). Also, the use of a FFR cut-off value for the decision-making process was 0.75 while in contemporary practice cut-off values of 0.80 are often used.18
Recently, Kennedy et al. analyzed 250 patients (128 DM, and 122 non-DM patients) and found that DM was associated with a higher rate of failed deferred stenoses (18.1% vs 7.5%, P ≤ .01, Cox regression-adjusted (HR, 3.65; 95%CI, 1.40-9.53, P < .01), and target lesion revascularization of the deferred lesion (17.2% vs 7.5%; HR, 3.52; 95%CI, 1.34-9.30; P = .01). Nevertheless, and consistent with our results, no significant differences in the rate of target vessel myocardial infarction were seen (6.1% vs 2.0%; HR, 3.34; 95%CI, 0.64-17.30; P = 0.15).19 The TLR reported in the former study is much higher than the one seen in our population and the one reported by former studies (eg, in the FAME study the 2-year rate of TLR was 3.2% in FFR-negative lesions).2 These differences can be associated with the characteristics of concomitant medical therapy, which was not specified and may affect the evolution of patients with DM critically. In our study population most patients received optimal medical therapy with over 93% receiving statins while the former study did not specify the medical treatment used. Another important factor can be the percentage of insulin-treated patients with DM (42% in the former study vs 24% in our population). In a different study the same authors found that insulin therapy was a predictive factor of deferred lesion failure in patients with FFR values > 0.80.20 Differences in the risk profile of the populations may, therefore, explain the different results obtained. Our study, with a larger sample size, proves that compared to non-diabetics deferring the revascularization of intermediate stenoses in diabetic patients is safe, and with no differences in TVR at the follow-up. Another study with similar results was the one conducted by Van Belle et al. who saw that the FFR is an important tool to redefine the severity of stenosis in patients with DM with good results at 1 year in deferred patients (HR, 0.77; 95%CI, 0.47-1.25; P = .29; reclassified vs non-reclassified patients with DM).21
Clinical outcomes based on the physiological index used to defer revascularization
Our results suggest that both the FFR and the iFR can be used safely to defer intermediate stenoses in patients with DM. Our findings regarding the low rates of MACE at the follow-up are consistent with the sub-analysis of patients with DM from the DEFINE-FLAIR trial compared to the 1-year follow-up.22 Interestingly enough, we found a trend towards a higher rate of TVR in diabetics deffered with the FFR compared to the iFR. This can be associated with the presence of microvascular dysfunction in diabetic patients, and with the better correlation of iFR with indices that assess microcirculation like coronary flow reserve (CFR). One study evaluated the performance of the iFR and the FFR against invasive CFR in 216 stenoses to find a significantly stronger correlation and a higher diagnostic performance for the iFR (iFR area under the ROC curve, 0.82 vs FFR area under the ROC curve, 0.72; P < .001, for a coronary flow velocity reserve of 2).23 Cook et al. evaluated 567 vessels with sensor-tipped pressure and Doppler ultrasound guidewires and found that with discordant FFR–/iFR+ , the hyperemic flow velocity and the CFR were similar to the FFR+/iFR+ group (P > .05). However, with discordant FFR+/iFR–, the hyperemic flow velocity and the CFR were similar to both the FFR–/iFR– and the coronary unobstructed groups (P > .05).24 These findings may potentially explain the lower performance of FFR in the presence of microvascular dysfunction, and the tendency we found towards a higher TVR in diabetic patients deferred with FFR. Interestingly enough, this tendency was not found in non-DM patients, which supports the hypothesis of microvascular compromise as one of the potential causes for the differences observed between the 2 indices.
Limitations
This study has several limitations. First, this is a single-center observational, retrospective, non-randomized study. The results should be analyzed with caution and can only be interpreted as hypothesis-generating given the small sample size that limits the study statistical power. There were more patients evaluated with the FFR compared to the iFR, which may have influenced the results. Neither clinicians nor patients were blinded to the physiological results, which may have influenced future decisions on revascularization. Most patients had a single deferred vessel, meaning that extrapolation of this data to patients with multivessel disease is complex. Finally, microvascular dysfunction was not evaluated in this population, and the actual impact on the results cannot be determined.
CONCLUSIONS
Deferring the revascularization of intermediate stenoses in patients with DM based on the FFR or the iFR is safe regarding the risk of TVR or target vessel myocardial infarction, with a similar rate of these events at the long-term follow-up compared to the rate seen in non-diabetic patients.
FUNDING
None whatsoever.
AUTHORS’ CONTRIBUTIONS
A.F. Castro-Mejía, and A. Travieso-González contributed to the study idea, design, acquisition, analysis, and interpretation of data, and writing of the article, M.J. Pérez-Vizcayno contributed to both the analysis and interpretation of data, H. Mejía-Rentería, I.J. Núñez-Gil, P. Salinas, L. Nombela-Franco, P. Jiménez-Quevedo, A. Fernández-Ortiz, and C. Macaya contributed to the writing of the article, and made a critical review of its intellectual content. J. Escaned, and N. Gonzalo contributed to the writing of the article, made a critical review of its intellectual content, and gave their final approval to the version that would eventually be published.
CONFLICTS OF INTEREST
I.J Núñez-Gil is a consultant for Aztraseneca. P. Salinas received speaker fees from Boston Scientific, Terumo, Alvinedica, and Biomenco. L. Nombela-Franco has served as a proctor for Abbott, and received speaker fees from Edwards Lifesciences Inc. A. Fernández-Ortiz is a speaker at the educational events of Medtronic, Biotronik, Biosensor, and Bayer. J. Escaned is a speaker and consultant for Abbott, Boston Scientific, and Philips, and received personal fees from Philips Volcano, Boston Scientific, and Abbott/St. Jude Medical outside the submitted work. N. Gonzalo is a speaker at educational events for Abbott, and Boston Scientific. The remaining authors declared no conflicts of interest.
WHAT IS KNOWN ABOUT THE TOPIC?
- The FFR and the iFR have proven to be safe tools to guide revascularization treatment in several clinical scenarios at the long-term follow-up. However, the safety of physiology-based revascularization in diabetics, who have a high-risk of cardiovascular events, has been scarcely investigated.
WHAT DOES THIS STUDY ADD?
- Deferring the revascularization of intermediate stenosis in diabetic patients based on the results of physiological evaluation with pressure guidewires is safe, and has a low rate of secondary events being the deferred vessel similar to those seen in non-diabetic patients at the longterm follow-up.
REFERENCES
1. Neumann F-J, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2018;40:87-165.
2. Pijls NH, Fearon WF, Tonino PA, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention in patients with multivessel coronary artery disease:2-year follow-up of the FAME (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation) study. J Am Coll Cardiol. 2010;56:177-184.
3. Davies JE, Sen S, Dehbi HM, et al. Use of the Instantaneous Wave-free Ratio or Fractional Flow Reserve in PCI. New Engl J Med. 2017;376:1824-1834.
4. Escaned J, Ryan N, Mejia-Renteria H, et al. Safety of the Deferral of Coronary Revascularization on the Basis of Instantaneous Wave-Free Ratio and Fractional Flow Reserve Measurements in Stable Coronary Artery Disease and Acute Coronary Syndromes. JACC Cardiovasc Interv. 2018;11:1437-1449.
5. Zimmermann FM, Ferrara A, Johnson NP, et al. Deferral vs performance of percutaneous coronary intervention of functionally non-significant coronary stenosis:15-year follow-up of the DEFER trial. Eur Heart J. 2015;36:3182-3188.
6. Gotberg M, Christiansen EH, Gudmundsdottir IJ, et al. Instantaneous Wave-free Ratio versus Fractional Flow Reserve to Guide PCI. New Engl J Med. 2017;376:1813-1823.
7. Escaned J, Collet C, Ryan N, et al. Clinical outcomes of state-of-the-art percutaneous coronary revascularization in patients with de novo three vessel disease:1-year results of the SYNTAX II study. Eur Heart J. 2017;38:3124-3134.
8. van Nunen LX, Zimmermann FM, Tonino PA, et al. Fractional flow reserve versus angiography for guidance of PCI in patients with multivessel coronary artery disease (FAME):5-year follow-up of a randomised controlled trial. Lancet. 2015;386:853-1860.
9. Norhammar A, Malmberg K, Diderholm E, et al. Diabetes mellitus:the major risk factor in unstable coronary artery disease even after consideration of the extent of coronary artery disease and benefits of revascularization. J Am Coll Cardiol. 2004;43:585-591.
10. Castro Mejia A, Ortega Armas M, Lopez Ferreo L. Factores de riesgo en pacientes con cardiopatía isquémica angiográficamente severa:diferencias según sexo. Rev Cuba Cardiol Cir Cardiovasc. 2015;21:7p.
11. Esper RB, Farkouh ME, Ribeiro EE, et al. SYNTAX Score in Patients With Diabetes Undergoing Coronary Revascularization in the FREEDOM Trial. J Am Coll Cardiol. 2018;72:2826-2837.
12. Jeremias A, Kirtane AJ, Stone GW. A Test in Context. Fractional Flow Reserve:Accuracy, Prognostic Implications, and Limitations. J Am Coll Cardiol. 2017;69:2748-2758.
13. Tonino PA, De Bruyne B, Pijls NH, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. New Engl J Med. 2009;360:213-224.
14. Kim U, Leipsic JA, Sellers SL, et al. Natural History of Diabetic Coronary Atherosclerosis by Quantitative Measurement of Serial Coronary Computed Tomographic Angiography. Results of the PARADIGM Study (Progression of Atherosclerotic Plaque Determined by Computed Tomographic Angiography Imaging). J Am Coll Cardiol Img. 2018:11:1461-1471.
15. Nicholls SJ, Tuzcu EM, Kalidindi S, et al. Effect of Diabetes on Progression of Coronary Atherosclerosis and Arterial Remodeling:A Pooled Analysis of 5 Intravascular Ultrasound Trials. J Am Coll Cardiol.2008;52:255-262.
16. Xaplanteris P, Fournier S, Pijls NHJ, et al. Five-Year Outcomes with PCI Guided by Fractional Flow Reserve. New Engl J Med. 2018;379:250-259.
17. De Bruyne B, Fearon WF, Pijls NH, et al. Fractional flow reserve-guided PCI for stable coronary artery disease. New Engl J Med. 2014;371:1208-1217.
18. Domínguez-Franco AJ, Jiménez-Navarro MF, Muñoz-García AJ, et al. Pronóstico a largo plazo de diferir la intervención coronaria en diabéticos sobre la base de la reserva fraccional de flujo. Rev Esp Cardiol. 2008;61:352-359.
19. Kennedy MW, Kaplan E, Hermanides RS, et al. Clinical outcomes of deferred revascularisation using fractional flow reserve in patients with and without diabetes mellitus. Cardiovasc Diabetol. 2016;15:100.
20. Kennedy MW, Fabris E, Hermanides RS, et al. Factors associated with deferred lesion failure following fractional flow reserve assessment in patients with diabetes mellitus. Catheter Cardiovasc Interv.2017;90:1077-1083.
21. Van Belle E, Cosenza A, Baptista SB, et al. Usefulness of Routine Fractional Flow Reserve for Clinical Management of Coronary Artery Disease in Patients With Diabetes. JAMA Cardiol. 2020;5:272-281.
22. Lee JM, Choi KH, Koo BK, et al. Comparison of Major Adverse Cardiac Events Between Instantaneous Wave-Free Ratio and Fractional Flow Reserve-Guided Strategy in Patients With or Without Type 2 Diabetes:A Secondary Analysis of a Randomized Clinical Trial. JAMA Cardiol. 2019;4:857-864.
23. Petraco R, van de Hoef TP, Nijjer S, et al. Baseline instantaneous wave-free ratio as a pressure-only estimation of underlying coronary flow reserve:results of the JUSTIFY-CFR Study (Joined Coronary Pressure and Flow Analysis to Determine Diagnostic Characteristics of Basal and Hyperemic Indices of Functional Lesion Severity-Coronary Flow Reserve). Circ Cardiovasc Interv. 2014;7:492-502.
24. Cook CM, Jeremias A, Petraco R, et al. Fractional Flow Reserve/Instantaneous Wave-Free Ratio Discordance in Angiographically Intermediate Coronary Stenoses:An Analysis Using Doppler-Derived Coronary Flow Measurements. JACC Cardiovasc Interv. 2017;10:2514-2524.
ABSTRACT
Introduction and objectives: Distal embolization and no-reflow are common complications in primary angioplasty and the information available on the role played by the deflation speed of the stent delivery system is scarce. Our aim is to analyze how the deflation speed of the stent delivery system impacts the results of primary angioplasty.
Methods: From December 2016 through February 2019, all consecutive patients with ST-segment elevation myocardial infarction undergoing urgent coronary angiography at our institution and who were eligible for thrombectomy, IIB-IIIA inhibitors, and direct stenting were randomized in a 1:1 ratio to rapid (group 1, n = 103) or slow deflation of the stent delivery system, at 1 atm/second, (group 2, n = 107). Pre- and postdilatation was not allowed per protocol. The primary outcomes were myocardial blush ≥ 2 and ST-segment resolution ≥ 70% while the size of myocardial damage, the ejection fraction both at discharge and at the 12-month follow-up, and the overall and 12-month cardiovascular mortality rates were the secondary outcomes.
Results: The study was stopped prematurely with 50% of the estimated sample size due to futility. Myocardial blush ≥ 2 occurred in 77 (74.7%) vs 79 (75.2%) of the patients, P = .93, and ST-segment resolution ≥ 70% occurred in 54 (53.9%) vs 59 (55.5%) of the patients, P = .75 in groups 1 and 2, respectively without any differences being reported in any of the secondary endpoints.
Conclusions: In our series, the deflation speed of the stent delivery system in primary angioplasty did not modify the myocardial blush ≥ 2, the ST-segment resolution ≥ 70% or impacted the clinical outcomes, the size of myocardial infarction according to the biomarkers or the ejection fraction.
Keywords: Primary angioplasty. ST-segment-elevation myocardial infarction. No-reflow. ST-segment resolution. Myocardial blush.
RESUMEN
Introducción y objetivos: La embolización distal y el fenómeno de no-reflow son complicaciones frecuentes de la angioplastia primaria. La información disponible sobre la influencia de la velocidad de desinflado del sistema de liberación del stent es escasa. Nuestro objetivo es analizar la influencia de este factor en los resultados de la angioplastia primaria.
Métodos: Entre diciembre de 2016 y febrero de 2019, todos los pacientes consecutivos con infarto de miocardio con elevación del segmento ST sometidos a coronariografía urgente en nuestro centro y que eran susceptibles de trombectomía, inhibidores de IIB-IIIA e implante directo de stent fueron aleatorizados 1:1 a un desinflado rápido del sistema de liberación (grupo 1, n = 103) o a un desinflado lento a 1 atm/s (grupo 2, n = 107). Por protocolo, no se permitió la predilatación previa ni posterior. Los objetivos primarios fueron el grado de blush miocárdico ≥ 2 y la resolución del segmento ST ≥ 70%. Los objetivos secundarios fueron el tamaño del infarto, la fracción de eyección al alta y a los 12 meses, y las mortalidades total y cardiovascular a los 12 meses.
Resultados: El estudio se detuvo prematuramente con el 50% del tamaño muestral calculado por futilidad. Se encontró blush ≥ 2 en 77 (74,7%) frente a 79 (75,2%) pacientes (p = 0,93) y resolución del segmento ST ≥ 70% en 54 (53,9%) frente a 59 (55,5%) pacientes (p = 0,75) en los grupos 1 y 2, respectivamente, sin diferencias en ninguno de los objetivos secundarios.
Conclusiones: En nuestra serie, la velocidad de desinflado del sistema de liberación del stent en la angioplastia primaria no modificó el blush miocárdico ni la resolución del segmento ST, y tampoco demostró tener influencia en los resultados clínicos, el tamaño del infarto según los biomarcadores ni la fracción de eyección.
Palabras clave: Angioplastia primaria. Infarto con elevacion del segmento ST. No-reflow. Resolucion del segmento ST. Blush miocardico.
Abbreviations
MB: myocardial blush. pPCI: primary percutaneous coronary intervention. STR: ST-segment resolution. STEMI: ST-segment elevation myocardial infarction. TIMI: Thrombolysis in Myocardial Infarction.
INTRODUCTION
Distal embolization and slow coronary flow often limit the success of primary percutaneous coronary angioplasty (pPCI). In 25% to 50% of the cases, despite satisfactory flow restoration, poor microvascular reperfusion can be seen, which leads to worse prognoses.1 This is a field of ongoing discussion because strategies that initially showed positive results have later been questioned like direct stenting,2 thrombus aspiration,3,4 and the administration of beta-blockers,5 and IIB-IIIA inhibitors.6
It has been confirmed that aggressive balloon dilatation with a high balloon-to-artery ratio may favor the presence of no-reflow and it has been speculated that the deflation speed of the stent delivery system may impact the results too, although the information available on this regard is scarce.
No-reflow may be due to different pathophysiological factors such as distal embolization, ischemia-reperfusion injury, and the susceptibility of coronary microcirculation to injury.7,8 Rapid stent balloon deflation may trigger the so-called siphon effect and rapid changes in coronary hemodynamics that can be associated with distal embolization, and microcirculatory dysfunction.9 As part of a published report, the investigators built an in vitro experimental study and combined it with a computer model to eventually find that the wall shear stress due to the different balloon deflation strategies used triggered differences in the flow final velocity as well.
Our objective is to analyze the impact of the deflation speed of the stent delivery system on myocardial blush (MB), and the ST-segment resolution (STR) in the acute phase, as well as the prognosis and ejection fraction at the 12-month follow-up.
METHODS
Patients
A randomized, parallel, single-center study was conducted with a 24-hour program of pPCI including 440 000 patients. Recruitment was carried out by convenience sampling and eligible patients were all consecutive subjects with ST-segment elevation myocardial infarction (STEMI) referred to receive a pPCI who had a culprit lesion eligible for direct stenting. Patients should have ST-segment elevations ≥ 0.1 mV in 2 contiguous leads or new left bundle branch block.
Exclusion criteria were contraindications to acetylsalicylic acid, clopidogrel or IIB-IIIA inhibitors, impossibility to complete the follow-up, life expectancy < 12 months, lesion not amenable to direct stenting, culprit lesions located at grafts or in-stent thrombosis, and previous oral anticoagulation.
After performing the coronary angiography, the patients who met the inclusion criteria and had no exclusion criteria gave their initial oral consent and were allocated by simple randomization through a computer-generated list that would create individual codes. These codes were inserted one by one in identical envelopes—prepared by personnel not involved in the study—that were thick enough so the codes could not be seen. All patients were asked to confirm their participation by giving their written informed consent within 24 hours. The study protocol was designed in full compliance with the ethical guidelines of the 1975 Declaration of Helsinki as shown in a prior approval granted by the center human research committee.
Parallel groups were created by a) direct stenting with fast deflation of the stent delivery system after 20 seconds of balloon inflation (group 1), or b) direct stenting with slow deflation at 1 atm/second after the same period of inflation (group 2).
Procedure
Patients and outcome evaluators were blind to the procedure. To minimize variability and any potential confounders the protocol was strict and included the administration of 250 mg of acetylsalicylic acid followed by 600 mg of clopidogrel at the first medical contact (according to the myocardial infarction protocol of our unit), 70 mg/kg of IV heparin, and IV abciximab or tirofiban at the beginning of the procedure for a 12-hour administration course. Manual thrombectomy and posdilatation of the stent were performed systematically, but implantation of a second stent was not allowed per protocol. Intention-to-treat and per protocol analyses were performed. The former dictated the main analysis. The volume of contrast per injection was 6 mL administered for 3 seconds into the left main coronary artery followed by 4 mL administered for 2 seconds into the right coronary artery using the ACIST device (ACIST Medical Systems Inc., United States). Intracoronary nitroglycerine (100 µg to 200 µg) was administered before the final injection to assess MB. Myocardial blush was studied in the right anterior oblique 20-degree projection with 20-degree caudal angulation, and in the left anterior oblique 45-degree projection with 20-degree cranial angulation regarding the left main coronary artery, and in the anteroposterior projection with 20-degree cranial angulation regarding the right coronary artery. Recordings were acquired at 30 images/second without image magnification with a prolonged duration until the venous phase of the myocardial circulation was completed.
Within the first 30 minutes upon arrival to the coronary care unit, patients underwent a 12-lead electrocardiogram and blood samples were obtained for troponin I assessment 6 and 24 hours after the procedure, as well as additional measurements until a reduction in the levels reported was confirmed.
Optimal medical management according to guidelines was recommended with statins, beta-blockers, or renin-angiotensin system blockers. Also, dual antiplatelet therapy was indicated for 12 months. Switching to ticagrelor during admission was also recommended in the absence of significant risk of bleeding.
Outcomes
The 2 primary endpoints were how the deflation speed of the stent delivery system impacted MB at the end of the procedure, and the STR. The final MB was analyzed blindly by an external core laboratory in a different region and the variable analyzed was the percentage of MB grade ≥ 2 vs < 2 between both groups by visual assessment. Two interventional cardiologists with > 10 years of experience grading MBs10 were involved in the evaluation and, in case of disagreement, a third opinion was requested. The STR was analyzed by evaluators not involved in the study who were blind to the procedure. The J-point was manually identified with respect to the nearest 0.5 mm in all leads except in the aVR lead. Using the TP segment as the isoelectric baseline interval, the extent of the ST-segment elevation with respect to the nearest 0.05 mV was measured 80 ms after the J-point. The STR was estimated by a reduction in the sum of the ST-segment elevation in all leads except in the aVR from the baseline ECG compared to the ECG performed upon arrival at the coronary care unit. The variable was a binary outcome, the ≥ 70% resolution of the sum of millimeters of ST-elevation between both recordings.
The secondary endpoints were: a) size of the myocardial damage comparing the maximum levels of troponin I; b) ejection fraction at discharge; c) ejection fraction at 12 months; d) all-cause mortality rate at 12 months; and e) 12-month cardiovascular mortality rate.
Definitions
Angiographic thrombus burden was defined according to Sianos’ classification11 while collateral supply was defined according to Rentrop classification.12
Quantitative coronary angiography
The Medis Suite XA system (Medis Medical Imaging, Israel) was used for the analysis according to the experts’ standards.13 Lesion length was measured once the vessel flow had been restored after thrombectomy. The diameter parameters were taken at the end of the procedure after the stent was deployed due to the difficulties reported while performing analyses in thrombotic vessels. The following data were used: reference vessel diameter (the average lumen diameter assumed without atherosclerotic disease), minimal lumen diameter, postoperative stenosis, and the stent-to-artery ratio.
Sample size calculations
Based on a primary endpoint of STR of 50% in the control group14,15 and an increase up to 62.5% in the procedural group following, the principle of minimum clinically significant difference between treatments of 25%,16 and a dropout rate of 10%, 420 patients, 210 per group, were needed.
Interim analysis
Given the uncertainty of the results and the lack of data available on the medical literature, an interim futility analysis was planned after recruiting 50% of the sample size.
Statistical analysis
Quantitative variables with normal distribution were expressed as means and standard deviation, and those without a normal distribution as median and interquartile range. Categorical variables were expressed as absolute values and percentages. The mean comparison was carried out using the Student t test in normal distribution or the Mann-Whitney U test when that assumption was not met. The chi-square test or Fisher’s exact test were used to compare proportions. Two-tailed tests were used to analyze all studies. P values ≤ .05 were considered statistically significant. A logistic regression analysis was performed to adjust for possible imbalances and measure how the deflation speed rate of the stent delivery system impacted each of the 2 primary endpoints. The variables that met the 2 criteria of a reasonable association with the outcomes and P values < .20 in the univariate analysis were tested in the multivariate analysis. The calculations were performed using the SPSS 27.0.0.0 statistical software (IBM Corp, United States).
RESULTS
Baseline
From December 2016 through February 2019 a total of 447 patients were referred to our cath lab with a diagnosis of STEMI (figure 1, flow diagram). A total of 237 (53%) were not eligible for randomization and the remaining 210 (47%) were allocated to fast (103, 49%) or slow balloon deflation (107, 51%). The initially calculated sample size was 420 patients but, after an interim analysis with 50% of the sample recruited, the study was terminated early due to futility. There was 1 protocol violation in the first group and 4 in the second group. The intention-to-treat analysis is seen in this section and the per protocol analysis on tables 3 to 5 of the supplementary data. The baseline and procedural characteristics of the study cohort are shown on table 1 and table 2. There were no statistical differences between both groups although, despite the randomization process, there was a non-significant trend towards a larger vessel diameter in the slow deflation group. All cases were performed with 6-Fr guiding catheters.
Figure 1. Study flow-chart. ECG, electrocardiogram; PCI, percutaneous coronary intervention.
Table 1. Baseline clinical characteristics
| Fast deflation N = 103 | Slow deflation N = 107 | P | |
|---|---|---|---|
| Age | 59.73 (10.56) | 59.33 (10.71) | .78 |
| Sex (female) | 26 (25.2) | 20 (18.7) | .25 |
| Diabetes | 14 (13.6) | 21 (19.6) | .24 |
| Hypertension | 40 (38.9) | 48 (44.8) | .37 |
| Hypercholesterolemia | 37 (35.9) | 45 (42.1) | .36 |
| Smoking | 65 (63.1) | 71 (66.3) | .62 |
| Previous myocardial infarction | 4 (3.9) | 6 (5.6) | .75 |
| Previous percutaneous coronary intervention | 3 (2.9) | 4 (3.7) | 1.00 |
| Previous coronary artery bypass graft | 0 (0) | 1 (0.1) | 1.00 |
| Previous stroke | 1 (0.1) | 0 (0.0) | .49 |
| Creatinine clearance levels < 60 mL/min | 14 (13.6) | 22 (20.5) | .18 |
| Blood pressure at admission | 123.4 (30.8) | 129.6 (28) | .13 |
| Shock | 4 (3.9) | 1 (0.09) | .21 |
| Radial access | 103 (100) | 105 (98.1) | .50 |
| Number of diseased vessels | 1.38 (0.61) | 1.45(0.66) | .42 |
| Total ischemic time | 192 (125-295) | 169 (120-260) | .21 |
| First medical visit to balloon time | 87 (66-130) | 80 (65-114) | .22 |
| ST elevation before procedure (mm) | 11.40 (6.74) | 12.63 (8.06) | .24 |
|
Quantitative variables with normal distribution are expressed as means and standard deviation (SD), variables with non-normal distribution as median and interquartile range, and categorical variables are expressed as absolute values and percentages. |
|||
Table 2. Characteristics of the procedure
| Fast deflation N = 103 | Slow deflation N = 107 | P | |
|---|---|---|---|
| Vessel | .60 | ||
| Left anterior descending coronary artert | 44 (42.7) | 40 (37.4) | |
| Left circumflex artery | 13 (12.6) | 18 (16.8) | |
| Right coronary artery | 46 (44.7) | 49 (45.8) | |
| Preoperative TIMI ≥ grade 2 flowa | 10 (9.7) | 17 (15.9) | .21 |
| Rentrop ≥ 2 | 15 (14.6) | 19 (17.8) | .53 |
| Thrombus grade score ≥ 4 | 46 (44.6) | 50 (46.7) | .76 |
| Drug-eluting stent | 100 (97.1) | 101 (94.4) | .50 |
| Percent diameter stenosis | 99.28 (3.43) | 98.89 (6.48) | .58 |
| RVDb | 2.74 (0.42) | 2.86 (0.47) | .07 |
| Lesion length | 14.07 (5.94) | 13.44 (4.71) | .39 |
| Stent diameter | 3.23 (0.47) | 3.32 (0.57) | .17 |
| Maximum inflation pressure | 14.68 (1.48) | 14.77 (1.69) | .67 |
| MLDc | 2.89 (0.38) | 3.00 (0.49) | .06 |
| Minimum lumen diameter | 2.63 (0.39) | 2.67 (0.48) | .48 |
| Postoperative stenosis | 8.92 (4.75) | 11.20 (6.25) | .01 |
| Stent-to-artery ratio | 1.05 (0.07) | 1.05 (0.08) | .95 |
|
Quantitative variables with normal distribution are expressed as means and standard deviation (SD), variables with non-normal distribution as median and interquartile range, and categorical variables are expressed as absolute values and percentages. a TIMI, Thrombolysis in Myocardial Infarction risk score. b RVD, reference vessel diameter after the procedure. c MLD, maximum lumen diameter after the procedure. |
|||
| Fast deflation N = 103 | Slow deflation N = 107 | P | |
|---|---|---|---|
| Myocardial blush ≥ 2 | 77 (74.7) | 79 (75.2) | .93 |
| Postoperative ST-segment elevation (mm) | 4.26 (5.19) | 4.03 (4.69) | .73 |
| ST-segment elevation resolution (mm) | 7.03 (6.99) | 8.56 (8.11) | .15 |
| Percentage of resolution (%) | 64.97 (33.35) | 65.40 (34.69) | .92 |
| Resolution ≥ 70 % | 54 (53.4) | 59 (55.6) | .75 |
| TIMI grade flow after the procedure | .38 | ||
| 0 | 1 | 0 | |
| 1 | 0 | 1 | |
| 2 | 5 | 9 | |
| 3 | 97 | 97 | |
| Maximum troponin-I levels | 47.84 (14-129) | 72 (29.7-144.75) | .14 |
| Ejection fraction at discharge | 53.9 (8.58) | 54.62 (8.71) | .55 |
| Ejection fraction at 12 months | 57.43 (8.20) | 57.75 (6.48) | .76 |
| In-hospital mortality rate | 1 (0.9) | 2 (1.8) | 1.00 |
| Overall mortality rate at 12 months | 3 (2.9) | 3 (2.8) | 1.00 |
| Cardiovascular mortality rate at 12 months | 2 (1.9) | 3 (2.8) | 1.00 |
| Myocardial infarction | 1 (0.9) | 1 (0.9) | 1.00 |
| Target vessel revascularization | 0 | 1 (0.9) | 1.00 |
|
Quantitative variables with normal distribution are expressed as means and standard deviation (SD), variables with non-normal distribution as median and interquartile range, and categorical variables are expressed as absolute values and percentages. TIMI, Thrombolysis in Myocardial Infarction risk score. |
|||
Table 4. Predictors of myocardial blush ≥ 2 and ST-segment resolution
| OR | 95%CI | P | |
|---|---|---|---|
| Predictors of myocardial blush ≥ 2 | |||
| Systolic blood pressure at admission | 1.02 | 1.02-1.03 | .011 |
| Creatinine clearance levels <60 mL/min | 0.29 | 0.13-0.66 | .003 |
| Postoperative maximum lumen diameter | 3.08 | 1.24-7.63 | .015 |
| Hypertension | 0.52 | 0.26-1.06 | .074 |
| Predictors of ST-segment resolution | |||
| Diabetes | 0.16 | 0.06-0.43 | < .001 |
| Previous myocardial infarction | 13.54 | 1.47-124.91 | .022 |
| Left anterior descending coronary artery | 0.46 | 0.24-0.91 | .025 |
| Preoperative TIMI grade flow ≥ 2 | 3.95 | 1.36-11.46 | .011 |
| Postoperative TIMI grade 3 flow | 7.10 | 1.76-28.68 | .006 |
| Rentrop grade ≥ 2 collateral circulation | 0.31 | 0.13-0.75 | .010 |
|
Quantitative variables with normal distribution are expressed as means and standard deviation (SD), variables with non-normal distribution as median and interquartile range, and categorical variables are expressed as absolute values and percentages. 95%CI, 95% confidence interval; OR, odds ratio; TIMI, Thrombolysis in Myocardial Infarction risk score. |
|||
Endpoints
The primary endpoint, MB grade ≥ 2compared to < 2, occurred in 77 (74.7%) vs 79 (75.2%), P = .93, of the patients, and STR ≥ 70% in 54 (53.9%) vs 59 (55.5%), P = .75, of the patients from the rapid and slow deflation groups, respectively. Also, there were no differences in any of the secondary endpoints regarding the size of myocardial damage, the ejection fraction at discharge, the ejection fraction at 12 months, the overall mortality rate at 12 months or in the cardiovascular mortality rate at 12 months (table 3).
Predictors of myocardial blush
The univariate analysis was performed with the variables shown on table 1 of the supplementary data. The variables age, creatinine clearance levels < 60 mL/min, postoperative maximum lumen diameter, past medical history of hypertension, systolic blood pressure at admission, Rentrop grade ≥ 2 collateral circulation, and the first medical contact to balloon time were tested using a logistic regression model. Systolic blood pressure at admission, creatinine clearance levels < 60 mL/min, and the postoperative maximum lumen diameter were predictors of blush ≥ 2 while in the final model hypertension remained with P values = .074 (table 4). The predictive power was moderate with an area under the ROC curve of 0.71 (0.63-0.80) (figure 2).
Figure 2. Receiver operating characteristic curve of the logistic regression model for myocardial blush prediction.
Predictors of ST-segment resolution ≥ 70%
The univariate analysis was performed with the variables listed on table 2 of the supplementary data. The variables tested in the multivariate analysis were sex, diabetes, hypercholesterolemia, smoking, shock, left anterior descending coronary artery, previous myocardial infarction, preoperative TIMI grade ≥ 2 flow, postoperative TIMI grade 3 flow, and Rentrop grade ≥ 2 collateral circulation, number of millimeters of ST elevation before the procedure, and creatinine clearance levels < 60 mL/min. The logistic regression model included diabetes, previous myocardial infarction, left anterior descending coronary artery, preoperative TIMI grade ≥ 2 flow, postoperative TIMI grade 3 flow, and Rentrop grade ≥ 2 collateral circulation as predictors of ST-segment resolution ≥ 70% (table 4). The area under the ROC curve was 0.75 (0.68-0.82) (figure 3).
Figure 3. Receiver operating characteristic curve of the logistic regression model for ST-segment resolution.
Per protocol analysis
Protocol deviation was seen in 5 patients. In the rapid deflation group 2 stents were needed in 1 patient. In the slow deflation group 3 patients received 2 stents followed by 1 postdilatation (figure 1). Tables 3, 4 and 5 of the supplementary data show the per protocol analysis without any significant differences compared to the intention-to-treat analysis.
Missing values
In 2 patients from the slow deflation group, the quality of the angiogram did not allow us to perform a proper analysis. Regarding the electrocardiogram, suboptimal quality was recorded in 2 patients from the rapid deflation group and in 1 patient from the slow deflation group. All of them may be considered as missing values completely at random, which means that the randomization balance was never affected.
DISCUSSION
In this randomized study we assessed how the deflation speed of the stent delivery system impacted myocardial blush ≥ 2, and ST-segment resolution ≥ 70%. The most important findings are: a) the study was stopped with 50% of the predefined sample sized due to futility and neither MB nor STR were modified by the intervention; b) no differences were seen in the size of myocardial damage, ejection fraction at 12 months and discharge or in the all-cause and 12-month cardiovascular mortality rates; c) systolic blood pressure at admission, creatinine clearance levels < 60 mL/min, and postoperative maximum lumen diameter played a role in MB while the past medical history of hypertension would have probably been included in the final model if the sample size would have been larger; and d) STR was influenced by diabetes, previous myocardial infarction, left anterior descending coronary artery, preoperative TIMI grade ≥ 2 flow, postoperative TIMI grade 3 flow, and Rentrop grade ≥ 2 collateral circulation.
The data available on the medical literature on this research topic is significantly scarce and, to our knowledge, only 1 group has provided information. Gu et al.17 also studied the association of balloon deflation during stent deployment with coronary flow and clinical outcomes regarding pPCI in a series of 211 patients. They found that slow deflation led to favorable coronary flow and infarct size compared to conventional rapid deflation. These contradictory results may be justified by the remarkable differences seen between both cohorts. Former studies have reported on the role of balloon inflation,18 thrombectomy,19,20 and IIB-IIIA inhibition21 in the management of MB. In our series, we designed a strict protocol to control these potential confounders, which is why pre- and postdilatation was not allowed, and both thrombus aspiration and IIB-IIIA inhibitors were essential components of the procedure. The study conducted by Gu et al. allowed both pre- and postdilatation while the use of thrombectomy, and IIB-IIIA inhibitors was left to the operator’s discretion. Indeed, predilatation was performed in > 80% of the patients from both groups, postdilatation in roughly 40%, thrombus aspiration in only 20%, and IIB-IIIA inhibitors were administered in 70% of the patients. Undoubtedly, the approach conducted by Gu et al. favored external validity although, in our opinion, the influence of these 4 factors may have influenced the results deeply, mainly when no adjustment was performed through a multivariate analysis. Finally, although closely related, the TIMI frame count and MB are not the same endpoint, and the ST-segment resolution was not assessed in the study conducted by Gu et al. Regarding the clinical endpoints, no differences were seen between the 2 strategies in any of the 2 studies.
As we mentioned, we were not able to show that the deflation speed of the stent delivery system impacted MB. In the multivariate analysis performed, blood pressure levels at admission, creatinine clearance levels, and the postoperative maximum lumen diameter were all predictors of MB while a past medical history of hypertension would have probably reached statistical significance with a larger sample size. Former reports have underlined how blood pressure impacts MB during the procedure.22 Also, patients with hypertension due to an increased microvascular resistance have shown an impaired flow.22 In addition, it has been reported that the adverse event of renal function regarding cardiovascular events may be mediated by an increased microvascular resistance.23 Time to treatment has impacted MB in previous studies.24 In our cohort, there were significant differences in the univariate analysis, but in the last step of the multivariate analysis it was removed from the final model, although it would have probably been present with a larger sample size. However, in the comparison of our series with the aforementioned study, we tested the vessel size as a predictor of MB while this variable was not analyzed in Luca’s study, but it had played a role in previous cohorts.25
Consistent with this, the deflation speed of the stent delivery system did not seem to play a role in STR. We found up to 6 factors that proved its impact on the ST-segment resolution, most of them already described in former studies. As it leads to a lower ST-segment elevation, collateral circulation reduces the impact of pPCI in STR.26 Anterior infarctions with culprit lesion in the left anterior descending coronary artery also led to lower ST-segment recoveryies in previous cohorts.27-29 This was also seen with preoperative TIMI grade < 2 flow, and final TIMI grade flow < 3,14,27,28,30 and diabetes.14,28 In our series, previous myocardial infarction was a predictor of STR, although we found no explanation for this finding.
Limitations
The study was stopped in the interim analysis based on the criterion of futility. However, we do not expect the results of primary endpoints to have been any different with the whole sample size. We could have probably found more predictors and a higher predictive power of the MB and STR models, but this was not the endpoint of our study. The risk profile of the patients was low because the inclusion criteria of direct stenting, use of IIB-IIIA inhibitors, and thrombectomy focused the study on lesions more frequently associated with younger patients with a low bleeding risk and less calcification, which are features associated with better outcomes. This limits the external validity of the study because, as shown on figure 1, roughly 50% of the patients were ineligible to enter the study. This may have also played a role in the lack of differences seen between the study groups. However, as we have already explained, the purpose of our study was to avoid any confounders. Clopidogrel was the P2Y12 inhibitor at the first medical visit according to the protocol of the regional myocardial infarction network of our area. This may also limit the external validity of the results. Myocardial blush was visually assessed and, although it was performed by 2 experienced operators, certain degree of subjectivity cannot be ruled out. The predictive power for both MB and STR was low, but it has also occurred in former series28 being the concordance between those factors described as moderate.31 Finally, we could not find any explanations for the role of previous myocardial infarction predicting STR as this factor was not present in former series.
CONCLUSIONS
In our series, the deflation speed of the stent delivery system in primary angioplasty did not change myocardial blush or ST-segment resolution and no influence was seen on the clinical outcomes, size of myocardial infarction assessed by biomarkers, and ejection fraction at discharge and after 12 months.
FUNDING
The study has been supported by a research grant from Abbott Laboratories.
AUTHORS’ CONTRIBUTIONS
B. Vega, J. M. Vegas, J. Rondan, E. Segovia, and Í. Lozano: design, data mining, manuscript drafting, and manuscript revision. A. Pérez de Prado, C. Cuellas-Ramon, M. López-Benito, T. Benito-González, and F. Hernández-Vázquez: blush measurements, and manuscript revision.
CONFLICTS OF INTEREST
The authors declared no conflict of interests whatsoever.
WHAT IS KNOWN ABOUT THE TOPIC?
- Distal embolization and slow coronary flow frequently reduce the success of primary angioplasty.
- Several interventions have been tested but it is a field of ongoing debate because the strategies that showed positive results at the beginning have now been questioned such as direct stenting, thrombus aspiration, and use of beta-blockers and IIB-IIIA inhibitors.
- It has been demonstrated that aggressive balloon dilatation with a high balloon to artery ratio may favor the presence of no-reflow. Also, it has been speculated that the deflation speed of the stent delivery system may impact the results, although the information available on this regard is scarce.
WHAT DOES THIS STUDY ADD?
- Our objective is to analyze how the deflation speed of the stent delivery system impacts myocardial blush, ST-segment resolution in the acute phase, prognosis, and the ejection fraction at 12months.
- The study was prematurely stopped due to futility because the speed of deflation of the stent delivery system did not change the primary outcomes or impacted the size of the infarction, prognosis or the ejection fraction at 12 months whatsoever.
REFERENCES
1. Ito H, Tomooka T, Sakai N, et al. Lack of myocardial perfusion immediately after successful thrombolysis. A predictor of poor recovery of left ventricular function in anterior myocardial infarction. Circulation. 1992;85:1699-1705.
2. Mahmoud KD, Jolly SS, James S, et al. Clinical impact of direct stenting and interaction with thrombus aspiration in patients with ST-segment elevation myocardial infarction undergoing percutaneous coronary intervention:Thrombectomy Trialists Collaboration. Eur Heart J. 2018;39:2472-2479.
3. Frobert O, Lagerqvist B, Olivecrona GK, et al. Thrombus aspiration during ST-segment elevation myocardial infarction. N Engl J Med. 2013;369:1587-1597.
4. Jolly SS, Cairns JA, Yusuf S, et al. Outcomes after thrombus aspiration for ST elevation myocardial infarction:1-year follow-up of the prospective randomised TOTAL trial. Lancet. 2016;387:127-135.
5. Roolvink V, Ibanez B, Ottervanger JP, et al. Early Intravenous Beta-Blockers in Patients With ST-Segment Elevation Myocardial Infarction Before Primary Percutaneous Coronary Intervention. J Am Coll Cardiol. 2016;67:2705-2715.
6. Ellis SG, Tendera M, de Belder MA, et al. Facilitated PCI in patients with ST-elevation myocardial infarction. N Engl J Med. 2008;358:2205-2217.
7. Konijnenberg LSF, Damman P, Duncker DJ, et al. Pathophysiology and diagnosis of coronary microvascular dysfunction in ST-elevation myocardial infarction. Cardiovasc Res. 2020;116:787-805.
8. Dong M, Mu N, Guo F, et al. The beneficial effects of postconditioning on no-reflow phenomenon after percutaneous coronary intervention in patients with ST-elevation acute myocardial infarction. J Thromb Thrombolysis. 2014;38:208-214.
9. Li R, Zijlstra JG, Kamps JA, van Meurs M, Molema G. Abrupt reflow enhances cytokine-induced proinflammatory activation of endothelial cells during simulated shock and resuscitation. Shock. 2014;42:356-364.
10. Perez de Prado A, Fernandez-Vazquez F, Cuellas-Ramon JC, Iglesias-Garriz I. Coronary clearance frame count:a new index of microvascular perfusion. J Thromb Thrombolysis. 2005;19:97-100.
11. Sianos G, Papafaklis MI, Daemen J, et al. Angiographic stent thrombosis after routine use of drug-eluting stents in ST-segment elevation myocardial infarction:the importance of thrombus burden. J Am Coll Cardiol. 2007;50:573-583.
12. Rentrop KP, Cohen M, Blanke H, Phillips RA. Changes in collateral channel filling immediately after controlled coronary artery occlusion by an angioplasty balloon in human subjects. J Am Coll Cardiol. 1985;5:587-592.
13. Suzuki N, Asano T, Nakazawa G, et al. Clinical expert consensus document on quantitative coronary angiography from the Japanese Association of Cardiovascular Intervention and Therapeutics. Cardiovasc Interv Ther. 2020;35:105-116.
14. Farkouh ME, Reiffel J, Dressler O, et al. Relationship between ST-segment recovery and clinical outcomes after primary percutaneous coronary intervention:the HORIZONS-AMI ECG substudy report. Circ Cardiovasc Interv. 2013;6:216-223.
15. Fabris E, van 't Hof A, Hamm CW, et al. Clinical impact and predictors of complete ST segment resolution after primary percutaneous coronary intervention:A subanalysis of the ATLANTIC Trial. Eur Heart J Acute Cardiovasc Care. 2019;8:208-217.
16. Fregni F. Sample Size Calculation. Clinical Thinking in Clinical Research:Applied Theory and Practice Using Case Studies. New York:Oxford University Press. 2018:225-242.
17. Gu J, Zhuo Y, Liu TJ, et al. Balloon Deflation Strategy during Primary Percutaneous Coronary Intervention in Acute ST-Segment Elevation Myocardial Infarction:A Randomized Controlled Clinical Trial and Numerical Simulation-Based Analysis. Cardiol Res Pract. 2020;2020:4826073.
18. Loubeyre C, Morice MC, Lefevre T, Piechaud JF, Louvard Y, Dumas P. A randomized comparison of direct stenting with conventional stent implantation in selected patients with acute myocardial infarction. J Am Coll Cardiol. 2002;39:15-21.
19. Lemesle G, Sudre A, Bouallal R, et al. Impact of thrombus aspiration use and direct stenting on final myocardial blush score in patients presenting with ST-elevation myocardial infarction. Cardiovasc Revasc Med. 2010;11:149-154.
20. Sardella G, Mancone M, Nguyen BL, et al. The effect of thrombectomy on myocardial blush in primary angioplasty:the Randomized Evaluation of Thrombus Aspiration by two thrombectomy devices in acute Myocardial Infarction (RETAMI) trial. Catheter Cardiovasc Interv. 2008;71:84-91.
21. G DEL, Bellandi F, Huber K, et al. Early glycoprotein IIb-IIIa inhibitors in primary angioplasty-abciximab long-term results (EGYPT-ALT) cooperation:individual patient's data meta-analysis. J Thromb Haemost. 2011;9:2361-2370.
22. Marra MP, Corbetti F, Cacciavillani L, et al. Relationship between myocardial blush grades, staining, and severe microvascular damage after primary percutaneous coronary intervention a study performed with contrast-enhanced magnetic resonance in a large consecutive series of patients. Am Heart J. 2010;159:1124-1132.
23. Bajaj NS, Singh A, Zhou W, et al. Coronary Microvascular Dysfunction, Left Ventricular Remodeling, and Clinical Outcomes in Patients With Chronic Kidney Impairment. Circulation. 2020;141:21-33.
24. De Luca G, van 't Hof AW, de Boer MJ, et al. Time-to-treatment significantly affects the extent of ST-segment resolution and myocardial blush in patients with acute myocardial infarction treated by primary angioplasty. Eur Heart J. 2004;25:1009-1013.
25. Ng VG, Lansky AJ, Toro S, et al. Prognostic utility of myocardial blush grade after PCI in patients with NSTE-ACS:Analysis from the ACUITY trial. Catheter Cardiovasc Interv. 2016;88:215-224.
26. Bottner RK, Morea CJ, Green CR, Renzi RH, Kent KM, Krucoff MW. Quantitation of ischemia during total coronary occlusion with computer-assisted high resolution ST-segment monitoring:effect of collateral flow. J Electrocardiol. 1987;20 Suppl:104-106.
27. Brodie BR, Stuckey TD, Hansen C, et al. Relation between electrocardiographic ST-segment resolution and early and late outcomes after primary percutaneous coronary intervention for acute myocardial infarction. Am J Cardiol. 2005;95:343-348.
28. Verouden NJ, Haeck JD, Kuijt WJ, et al. Clinical and angiographic predictors of ST-segment recovery after primary percutaneous coronary intervention. Am J Cardiol. 2010;105:1692-1697.
29. Lefevre T, Garcia E, Reimers B, et al. X-sizer for thrombectomy in acute myocardial infarction improves ST-segment resolution:results of the X-sizer in AMI for negligible embolization and optimal ST resolution (X AMINE ST) trial. J Am Coll Cardiol. 2005;46:246-252.
30. De Luca G, Ernst N, van 't Hof AW, et al. Preprocedural Thrombolysis in Myocardial Infarction (TIMI) flow significantly affects the extent of ST-segment resolution and myocardial blush in patients with acute anterior myocardial infarction treated by primary angioplasty. Am Heart J. 2005;150:827-831.
31. Brener SJ, Dizon JM, Mehran R, et al. Complementary prognostic utility of myocardial blush grade and ST-segment resolution after primary percutaneous coronary intervention:analysis from the HORIZONS-AMI trial. Am Heart J. 2013;166:676-683.

ABSTRACT
Introduction and objectives: Patients with left main coronary artery (LMCA) stenosis have been excluded from the trials that support the non-inferiority of the instantaneous wave-free ratio (iFR) compared to the fractional flow reserve (FFR) in the decision-making process of coronary revascularization. This study proposes to prospectively assess the concordance between the two indices in LMCA lesions and to validate the iFR cut-off value of 0.89 for clinical use.
Methods: National, prospective, and observational multicenter registry of 300 consecutive patients with intermediate lesions in the LMCA (angiographic stenosis, 25% to 60%. A pressure gudiewire study and determination of the RFF and the iFR will be performed: in the event of a negative concordant result (FFR > 0.80/iFR > 0.89), no treatment will be performed; in case of a positive concordant result (FFR ≤ 0.80/iFR ≤ 0.89), revascularization will be performed; In the event of a discordant result (FFR> 0.80/iFR ≤ 0.89 or FFR ≤ 0.80/iFR> 0.89), an intravascular echocardiography will be performed and revascularization will be delayed if the minimum lumen area is > 6 mm2. The primary clinical endpoint will be a composite of cardiovascular death, LMCA lesion-related non-fatal infarction or need for revascularization of the LMCA lesion at 12 months.
Conclusions: Confirm that an iFR-guided decision-making process in patients with intermediate LMCA stenosis is clinically safe and would have a significant clinical impact. Also, justify its systematic use when prescribing treatment in these potentially high-risk patients.
Registered at ClinicalTrials.gov ( Identifier: NCT03767621).
Keywords: iFR. FFR. Left main coronary artery.
RESUMEN
Introducción y objetivos: Los pacientes con estenosis en el tronco coronario izquierdo (TCI) han sido excluidos de los ensayos que apoyan la no inferioridad del cociente de presiones en el índice diastólico instantáneo sin ondas (iFR) respecto a la reserva fraccional de flujo (RFF) en la toma de decisiones sobre revascularización coronaria. El presente estudio propone valorar de manera prospectiva la concordancia entre los dos índices en lesiones del TCI y validar el valor de corte del iFR de 0,89 para su uso clínico.
Métodos: Registro multicéntrico nacional, prospectivo, observacional, con la inclusión de 300 pacientes consecutivos con lesiones intermedias (estenosis angiográfica 25-60%) en el TCI. Se realizará un estudio con guía de presión y determinación de RFF e iFR. En caso de resultado concordante negativo (RFF > 0,80 / iFR > 0,89), no se realizará tratamiento; en caso de resultado concordante positivo (RFF ≤ 0,80 / iFR ≤ 0,89), se realizará revascularización; en caso de resultado discordante (RFF > 0,80 / iFR ≤ 0,89 o RFF ≤ 0,80 / iFR > 0,89), se realizará estudio con ecocardiografía intravascular y se considerará diferir la revascularización si el área luminal mínima es > 6 mm2. El criterio de valoración clínico primario será la incidencia del combinado de muerte cardiovascular, infarto no mortal relacionado con la lesión del TCI o necesidad de revascularización de la lesión del TCI a los 12 meses.
Conclusiones: La demostración de la seguridad clínica en la toma de decisiones del iFR en pacientes con lesiones intermedias en el TCI tendría un impacto clínico importante y justificaría su uso sistemático para la decisión del tratamiento en estos pacientes de potencial alto riesgo.
Registrado en ClinicalTrials.gov (identificador: NCT03767621).
Palabras clave: iFR. RFF. Tronco coronario izquierdo.
Abbreviations
MLA: minimum lumen area. FFR: fractional flow reserve. iFR: instantaneous wave-free ratio. IVUS: intravascular ultrasound. LMCA: left main coronary artery.
INTRODUCTION
Assessing functional severity of coronary stenoses at left main coronary artery (LMCA) level through coronary angiography has serious limitations.1 To treat angiographically intermediate stenoses (25% to 60% diameter) the use of invasive (ultrasound or optical coherence tomography) or functional imaging modalities (determining fractional flow reserve [FFR] to indicate the need for revascularization) has been proposed.2 Patients with LMCA stenosis have traditionally been excluded from randomized clinical trials that assessed the prognostic capabilities of the functional assessment of coronary stenoses through the use of FFR.3-5 The use of FFR to assess LMCA stenoses is backed by a limited number of non-randomized clinical trials that confirmed that FFR values > 0.80 is associated with a low risk of events if no revascularization is performed in patients with intermediate LMCA stenoses.6
The instantaneous wave-free ratio (iFR) is a new, easier-to-use, and cost-effective invasive index to assess the coronary function compared to FFR since there is no need to induce maximum coronary hyperemia to estimate it.7 Although a non-inferior prognostic value of iFR compared to the FFR has recently been confirmed in patients with intermediate lesions in 2 large trials, the presence of LMCA lesions was largely anecdotal or inexistent in both indices.8,9 However, a non-randomized clinical trial has been published with a similar design to those previously conducted with the FFR that provides encouraging data on the value of iFR in the decision-making process regarding the LMCA. However, in such trial, the FFR—the most widely used index to assess intermediate LMCA stenoses—was not determined at the same time, which means that the results of this registry cannot be put into context.10 Also, there are signs that the location of the LMCA lesion is a predictor of worse concordance between both indices.11
Proving the clinical safety of iFR in patients with intermediate LMCA lesions would have a major clinical impact and justify its systematic use in the decision-making process regarding the management of these high-risk patients.
The objective of this study is to assess the concordance between 2 physiological indices—the FFR and the iFR—in the assessment of intermediate LMCA lesions. Also, to validate prospectively the clinical safety profile of a revascularization strategy based on an iFR cut-off value of 0.89.
METHODS
Study design
National, prospective, observational, and multicenter registry including 300 consecutive patients with intermediate LMCA lesions (25% to 60% angiographic stenosis). A study will be conducted in all patients using intracoronary guidewire pressures. Also, both the FFR and the iFR values will be determined distal to the LMCA. Per protocol it is advised that the indication for revascularization should be decided based on the result of the iFR in such a way that:
-
– In patients with iFR and FFR values in the LMCA lesion > 0.89 and > 0.80, respectively clinical follow-up without LMCA lesion revascularization is indicated. In the presence of other lesions outside the LMCA with percutaneous revascularization criteria, the revascularization of these other lesions is indicated.
-
– In patients with iFR and FFR values in the LMCA lesion ≤ 0.89 and ≤ 0.80, respectively the revascularization of the LMCA lesion is indicated (percutaneous through a drug-eluting stent or surgical). In the presence of other lesions outside the LMCA with revascularization criteria (whether percutaneous or surgical), the revascularization of these other lesions is indicated.
-
– In case of discrepancy between the FFR and the iFR (positive vs negative or vice versa with 2 or more points above or below the respective cut-off value) an intravascular ultrasound (IVUS) should be performed to decide whether to indicate revascularization or not; with minimum lumen areas (MLA) > 6 mm2 revascularization is ill-advised.
Patients whose management is not consistent with what the iFR value recommends will not be addressed for the strategy safety analysis, and clinical outcomes will be assessed separately.
Figure 1 shows the decision-making algorithm based on FFR and iFR results. IVUS is indicated in controversial cases, and recommended in the remaining cases to determine the correlation between the MLA and the iFR.
Figure 1. Decision-making algorithm based on the FFR and iFR results. DES, drug-eluting stent; FFR, fractional flow reserve; iFR, instantaneous wave-free ratio; IV, intravenous; IVUS, intravascular ultrasound; LMCA, left main coronary artery; MACE, major adverse cardiovascular events, MLA, minimum lumen area.
In patients eligible for percutaneous treatment, IVUS is highly recommended, and its utility will be assessed prospectively during the planning and optimization of the procedure.
Clinical follow-up is advised from 12 months to 5 years to determine the prognostic primary endpoint by assessing a composite endpoint of cardiovascular death, LMCA lesion-related non-fatal infarction or need for LMCA revascularization at the 12-months and 5-year follow-up.
Notifications
The study has been approved by the reference ethics committee and notified to the local ethics committee of all participant centers. The study has been registered in Clinicaltrials.gov with registration number NCT03767621. Devices with CE marking have only been used, and only for the indications already approved. The study observes the principles established by the Declaration of Helsinki. All patients gave their prior written informed consent to participate in the study.
Study population
Patients with suspected or confirmed ischemic heart disease on whom a coronary angiography is performed that detects intermediate angiographic LMCA stenoses (between 25% and 60%). Also, patients in whom intracoronary pressure guidewires are used to determine the iFR and the FFR in the LMCA lesion to decide on the indication for myocardial revascularization—whether percutaneous with a DES or surgical—based on the indication considered more appropriate.
Inclusion and exclusion criteria are shown on table 1. In cases of severe lesions at left anterior descending coronary artery or left circumflex artery level, the patient will not be included in the study unless the LMCA lesion is assessed after the percutaneous treatment of these lesions while taking into account that, if the LMCA lesion is significant, treatment will be percutaneous.
Table 1. Inclusion and exclusion criteria of the iLITRO-EPIC-07 trial
| Inclusion criteria |
| Patients with intermediate LMCA lesions (25% to 60% angiographic stenosis on visual estimations) eligible for a pressure guidewire study to determine the iFR |
| Patients aged ≥ 18 years |
| Patients capable of giving their informed consent |
| Exclusion criteria |
| Patients with an indication for coronary artery bypass graft regardless of the significance of the LMCA lesion |
| Patients with LMCA lesions showing ulceration, dissection or thrombus |
| Patients with lesions in a previously non-dysfunctional arterial or venous graft in the territory irrigated by the LMCA (protected LMCA) |
| Patients with acute coronary syndrome with potentially culprit lesion in the LMCA |
| Patients incapable of giving their informed consent |
|
iFR, instantaneous wave-free ratio; LMCA, left main coronary artery. |
Study endpoints
The iLITRO-EPIC 07 trial has 2 primary endpoints:
-
1) To establish concordance before indicating revascularization between 2 invasive functional assessment indices through intracoronary pressure guidewire in intermediate LMCA lesions with FFR and iFR cut-off values ≥ 0.80 (with IV adenosine) and ≥ 0.89 to delay treatment.
-
2) To validate prospectively the safety profile associated with the decision-making process regarding the revascularization of intermediate LMCA stenoses based on an iFR cut-off value of 0.89 measured using an intracoronary pressure guidewire to decide whether to revascularize or not based on the number of patients with delayed LMCA revascularization of the composite endpoint of cardiovascular death, LMCA lesion-related non-fatal infarction or need for LMCA revascularization at the 12-month follow-up.
Secondary endpoints are to determine the correlation between the iFR value in these lesions and the MLA determined by the IVUS and the utility of IVUS for the planning and optimization of LMCA lesions (table 2).
Table 2. Secondary endpoints of the iLITRO-EPIC-07 trial
| Correlation between the assessment obtained through pressure guidewire (iFR) and the minimum lumen area measured through IVUS |
| Role of IVUS in the planning of treatment in the subgroup of patients treated with percutaneous therapy |
| Role of IVUS in the optimization of treatment in the subgroup of patients treated with percutaneous therapy |
| All-cause mortality at 12 months and 5 years |
| Cardiovascular death at 12 months and 5 years |
| Non-fatal infarction at 12 months and 5 years |
| LMCA lesion-related non-fatal infarction at 12 months and 5 years |
| Revascularization at 12 months and 5 years |
| Myocardial infarction associated with the revascularization of the LMCA (whether percutaneous or surgical) |
| Thrombosis of 1 or several stents in the LMCA at 12 months and 5 years |
| Restenosis of 1 or several stents in the LMCA at 12 months and 5 years |
| New target lesion revascularization in the LMCA (whether percutaneous or surgical) at 12 months and 5 years |
|
iFR, instantaneous wave-free ratio; LMCA, left main coronary artery; IVUS: intravascular ultrasound. |
Study procedure
Figure 2 shows the procedure methodology on a flowchart.
Figure 2. Study protocol and procedures. ACS, acute coronary syndrome; CABG, coronary artery bypass graft; FFR, fractional flow reserve; iFR, instantaneous wave-free ratio; IVUS, intravascular ultrasound; LAD, left anterior descending coronary artery; LCX, left circumflex artery; LMCA, left main coronary artery; MLA, minimum lumen area; PCI, percutaneous coronary intervention; QCA, quantitative coronary angiography.
Protocol to perform a study using a pressure guidewire
The patient is eligible for functional assessment in the presence of intermediate LMCA stenoses with visual estimations on the coronary angiography between 25% and 60%.
After catheterization using a guide catheter, at least, 200 µg of intracoronary nitroglycerin should be administered to keep coronary reactivity under control. Afterwards, the intracoronary guidewire should be advanced with the sensor placed in the ostium of the guide catheter; also, pressure curves should be brought back to normal for 5 to 10 heart beats. if the lesion has an ostial location, normalization will occur by removing the guide catheter from the coronary artery and placing the guidewire into the aorta. Afterwards, the guidewire should be removed from the catheter, and coronary catheterization performed to advance the guidewire.
The pressure guidewire should be advanced until, at least, 3 times the diameter of the vessel beyond the most distal stenosis to be able to measure the iFR according to the standard protocol.
After measuring the iFR, the guidewire should be removed with pressure curve monitorization until the inside of the guide catheter. At this point, the presence of the pressure calibration loss phenomenon (drifting) should be discarded. In case of overt drift (Pd/Pa measured on the catheter tip < 0.98 or > 1.02) measures should be taken again.
Afterwards, the FFR will be determined during hyperemia through the administration of adenosine in continuous IV infusion at doses ≥ 140 µg/kg/min for, at least, 2 minutes or an IV bolus of 0.4 mg of regadenoson.
After measuring the FFR, the guidewire should be removed with pressure curve monitorization until the inside of the guide catheter. At this point, the presence of drifting should be discarded. In case of overt drift (Pd/Pa measured on the catheter tip < 0.98 or > 1.02) measures should be taken again.
In case of discrepancy between the results of the FFR and the iFR (FFR ≤ 0.80 with iFR ≥ 0.90 or FFR ≥ 0.81 with iFR ≤ 0.89) IVUS will be performed, and the MLA determined. Revascularization will be indicated with MLAs < 6 mm2 based on the results from the LITRO trial.12
Protocol to conduct IVUS studies
IVUS studies will be mandatory if the FFR and the iFR disagree. In patients eligible for percutaneous treatment of their LMCA lesions, the IVUS is highly recommended to guide the procedure. In the remaining patients (when iFR-guided medical therapy or surgical revascularization is decided) the IVUS is recommended to establish the correlation between the iFR value and the MLA measured on the LMCA whenever possible. The IVUS system used can be mechanical or rotational with resolutions between 20 MHz and 60 MHz.
An 0.014 in intracoronary guidewire will be advanced to perform the IVUS study (it can be the same pressure guidewire used to determine the iFR) towards the left anterior descending or left circumflex coronary arteries. After the administration of 200 µg of intracoronary nitroglycerin, the IVUS catheter will be advanced distal to the LMCA bifurcation. Afterwards, the catheter will be manual or automatically removed until the ascending aorta. It is essential that the guide catheter should remain outside the coronary artery to study the left main coronary artery entirely including its ostial region. The catheter will be placed in the left anterior descending coronary artery (preferably) or left circumflex artery or both (to conduct 2 studies with MLA determination from these positions and eventually pick the one with the lowest values).
In cases of catheter backward jump, even on manual mode (with calcified angulation) it is recommended to move the catheter forward from the aorta to acquire images of the region of interest that had not been properly assessed.
Technical aspects of the assessment of left main coronary artery lesions through fractional flow reserve
The study of LMCA lesions using pressure guidewires has some particularities that should be addressed when conducting the study.
Location of the lesion
A total of 3 different possible lesion locations can be anatomically distinguished on the LMCA depending on whether there is damage to the ostium, body or distal portion (bifurcation). The location of the lesion inside the LMCA has implications when conducting the study with the pressure guidewire. When the lesion is found in the ostium or the body, catheterization should be coaxial. Non-coaxial catheterization involves contact of the catheter lumen with the vessel wall to the extent that it can dampen the aortic pressure and artificially elevate the value of the FFR. For this reason, non-selective catheterization is advised when equalizing or normalizing the catheter and guidewire pressures when the latter is placed distal to the lesion to measure the FFR during maximum hyperemia. When the lesion is found in the LMCA distal portion and there is damage to its origin and main branches, both the distal LMCA and each one of its branches should be treated as 1 functional unit regardless of the degree of damage to these branches. To estimate the FFR, measurements are taken from the left anterior descending and left circumflex coronary arteries. The LMCA lesion is considered functionally significantly when the measurements of either one of the 2 main vessels is < 0.80.
Induction of hyperemia
In the assessment of LMCA lesions the use of an intracoronary bolus of adenosine is ill-advised because, since the non-selective catheterization of the left coronary artery is required, part of the drugs administered never reach this coronary artery, which is why the induction of hyperemia can be suboptimal. For this reason, the IV administration of drugs whether adenosine (infusions of 140 µg/kg/min for, at least, 2 minutes) or regadenoson (doses of 0.4 mg in IV bolus) is advised.13
Presence of left anterior descending or left circumflex coronary artery lesions
The presence of 1 isolated LMCA lesion is not rare. A series of all-comers treated with diagnostic coronary angiography proved that, in patients with damage to the LMCA, only 9% had 1 single LMCA lesion, 17% had 1 LMCA lesion plus damage to 1 vessel, 35% had 1 LMCA lesion plus damage to 2 vessels, and 38% had LMCA disease plus damage to 3 vessels.14
Statistical analysis
Demographic, clinical, hemodynamic, and procedural data will be presented for the entire group. Continuous variables will be expressed as mean, and standard deviation (or if the distribution of the values do not follow a normal, as median, and interquartile range). Categorical variables will be expressed as frequencies and percentages. The data obtained will be studied using the unilateral analysis of variance (ANOVA) for the continuous variables, and Fisher’s exact test or the chi-square test for the categorical variables, when appropriate. When appropriate, non-parametric tests will be used with variables without a normal distribution or when normalization is not possible. The Kaplan-Meier survival curves will be presented for the previously specified criteria. The concordance analyses will be conducted using Cohen’s kappa coefficient. Also, sensitivity, specificity, positive and negative predictive values, and the area under the receiver operating characteristic (ROC) curve will be estimated.
Data curation and monitorization
Clinical, angiographic, physiological, and IVUS data will all be saved in a safe electronic CRD managed by Fundación EPIC, the promotor of the study. Clinical data at both the 12-month and 5-year follow-up, as well as the presence of cardiovascular events at the follow-up will also be saved in the same electronic CRD.
DISCUSSION
The iLITRO-EPIC 07 trial has a double primary endpoint: on the one hand, to establish the concordance between 2 intracoronary physiological indices, the FFR and the iFR, when assessing the severity of intermediate LMCA lesions; on the other hand, to study the use of a predetermined iFR value to indicate the revascularization of intermediate LMCA lesions with an up to 5-year clinical follow-up.
Left main coronary artery disease. Implications for the interventional cardiologist
Significant LMCA disease, understood as a stenosis in its greater diameter > 50%, is associated with a poor mid-term prognosis. Studies prior to coronary revascularization confirmed survival rates < 40% at the 4-year follow-up after diagnosis.15
The limitations of the angiographic assessment of the severity of LMCA lesions are well established.16-18 Before suggesting revascularization in a patient with LMCA lesions, in particular ostial lesions, it is important to know whether the lesion really needs to be revascularized, that is, whether it is hemodynamically significant. LMCA stenoses are found in between 4% to 9% of all diagnostic coronary angiographies.1 Due to their anatomical location, catheter-induced artifacts or to the severity of distal lesions, among other factors, interpreting LMCA lesions is associated with the highest intra- and inter-observer variability compared to lesions found in other parts of the coronary tree.16 When stenoses ≥ 50% were found in the CASS registry,19 a second observer confirmed that the stenosis was not significant in 19% of the cases.
Several former studies have confirmed that the prognosis of patients with functionally insignificant LMCA lesions is favorable.6 Also, that the surgical revascularization of hemodynamically insignificant lesions is associated with a high rate of early graft failure.20
The LITRO trial, led by the Spanish Society of Cardiology Working Group on Intracoronary Diagnostic Techniques, was a multicenter and prospective study. It proved that, in patients with angiographically intermediate LMCA lesions, the presence of a MLA ≥ 6 mm2 measured on the IVUS allows us to delay revascularization in a safely manner.12
Evidence to guide the revascularization of the left main coronary artery through functional assessment
To this date, no definitive data on the prognostic value of iFR measurements in intermediate LMCA stenoses have been published. The presence of a significant stenosis (> 70%) on the coronary angiography was an exclusion criteria in the DEFER, FAME, and FAME II clinical trials, as well as in the DEFINE FLAIR trial. Only the IFR SWEDEHEART trial included 30 patients with significant LMCA stenoses (1.6% of all the patients included).3-5,8,9 An observational and retrospective study of 314 patients confirmed that delaying the revascularization of the LMCA using a iFR cut-off value of 0.89 as the guide was safe at the 30-month clinical follow-up.10 However, in this observational registry the FFR, a widely validated index in the LMCA, was not obtained at the same time. This means that the results reported by this registry cannot be put into context and the concordance between both indices cannot be analyzed either.
The data available that support the use of the FFR in LMCA lesions come from several studies shown on table 1. The cut-off values used in these studies go from 0.75 to 0.80. In the study that has included, to this date, the highest number of patients with intermediate angiographic lesions, 213, only patients with FFR values < 0.80 were treated. However, in patients with higher values a conservative manage was used. No differences in the mortality or severe cardiovascular event rates were reported at the 5-year follow-up.6 Therefore, the reference FFR value for LMCA lesions, as well as the remaining lesions, is < 0.80.
A metanalysis that included data from 8 landmark studies found no differences in the primary endpoint of death, non-fatal myocardial infarction or revascularization. However, the need for revascularization was greater in the group on medical therapy: whether this was primarily due to the revascularization of the LMCA is still under discussion.21
A recent study that assessed the correlation between the FFR and the iFR values based on the location of the lesion studied revealed that such correlation was weaker when the lesion was found on the LMCA or in the proximal left anterior descending coronary artery compared to other locations. This was attributed to a greater amount of vessel-dependent myocardium in these proximal lesions. Taking the FFR value and an iFR cut-off value ≥ 0.89 as a reference, both the false positives (21.9%) and the false negatives (26.7%) were more evident when the lesion was found on the LMCA or the proximal left anterior descending coronary atery.11 Some studies have suggested that resting indices like the iFR could provide better measurements of coronary flow during hyperemia compared to the FFR.22,23 This means that using the FFR as the gold standard could be questionable in this setting. Also, the scientific evidence available indicates that the discrepancies seen between the iFR and the FFR are not associated with a worse prognosis.24 This means that the present study could clarify whether the iFR is associated with a weaker indication for revascularization in intermediate LMCA lesions with the exact same clinical safety compared to the FFR.
CONCLUSIONS
The iLITRO-EPIC 07 trial is the first prospective study to assess the concordance between the FFR and the iFR in intermediate LMCA lesions. Also, that it is safe to guide the indication for revascularization based on an iFR cut-off value of 0.89.
FUNDING
The promoter of the study, Fundación EPIC, has received an institutional research grant from Phillips Volcano (The Netherlands) to pay for the design and maintenance costs of the electronic CRD. Philips Volcano has not been involved in the design of the study or protocol whatsoever. Philips Volcano has not been involved in the development of the study whatsoever including recruitment, follow-up, data curation, result analysis and interpretation, writing or final approval of both the protocol and this manuscript. The authors are solely responsible for the study design, writing, edition, and final version of the manuscript.
AUTHORS’ CONTRIBUTIONS
All the authors are lead investigators of the iLITRO-EPIC07 trial at their corresponding working centers, collaborated in the writing of the study protocol, and in the recruitment of the patients. The manuscript was written by O. Rodríguez-Leor, J.M. de la Torre-Hernández, and A. Pérez de Prado; the remaining authors reviewed the manuscript.
CONFLICTs OF INTEREST
A. Pérez de Prado declared to have received fees from iVascular, Boston Scientific, Terumo, B. Braun, and Abbott Vascular. José M. de la Torre Hernández is the editor-in-chief of REC: Interventional Cardiology. F. Alfonso, and J. Sanchis are associate editors of REC: Interventional Cardiology; the journal’s editorial procedure to ensure impartial handling of the manuscript has been followed.
WHAT IS KNOWN ABOUT THE TOPIC?
- In intermediate LMCA stenoses (25% to 60% diameter) the use of invasive (ultrasound or optical coherence tomography) or functional imaging modalities (by measuring the FFR) has been proposed to eventually indicate the need for revascularization. Patients with LMCA stenoses were excluded from randomized clinical trials that assessed the prognostic capabilities of the functional assessment using the FFR.3 However, its use has been backed by several non-randomized clinical trials that confirmed that values > 0.80 are indicative of a low risk of events if revascularization is eventually spared. The iFR is a new physiological index that does not require hyperemia to be determined, which simplifies the whole process. There are still no data on the concordance between both indices in LMCA lesions or the safety of this new index in the assessment of these patients.
WHAT DOES THIS STUDY ADD?
- The iLITRO-EPIC07 trial is an attempt to prospectively assess the concordance between the FFR and the iFR, as well as the safety profile of an iFR-guided revascularization strategy.
REFERENCES
1. Lindstaedt M, Spiecker M, Perings C, et al. How good are experienced interventional cardiologist at predicting the functional significance of intermediate or equivocal left main coronary stenosis?Int J Cardiol. 2007;120:254-261.
2. Windecker S, Kohl P, Alfonso F, et al. 2014 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2014;35:2541-2619.
3. Pijls NH, van Schaardenburgh P, Manoharan G, et al. Percutaneous coronary intervention of functionally nonsignificant stenosis:5-year follow-up of the DEFER Study. J Am Coll Cardiol. 2007;49:2105-2111.
4. Tonino PA, De Bruyne B, Pijls NH, et al. Fractional flow reserve vs. angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360:213-224.
5. De Bruyne B, Pijls NH, Kalesan B, et al. Fractional flow reserve-guided PCI vs. medical therapy in stable coronary disease. N Engl J Med. 2012;367:991-1001.
6. Hamilos M, Muller O, Cuisset T, et al. Long-term clinical outcome after fractional flow reserve-guided treatment in patients with angiographically equivocal left main coronary artery stenosis. Circulation. 2009;120:1505-1512.
7. Sen S, Escaned J, Malik IS, et al. Development and validation of a new adenosine-independent index of stenosis severity from coronary wave-intensity analysis:results of the ADVISE (ADenosine Vasodilator Independent Stenosis Evaluation) study. J Am Coll Cardiol. 2012;59:1392-1402.
8. Davies JE, Sen S, Dehbi HM, et al. Use of the instantaneous wave-free ratio or fractional flow reserve in PCI. N Engl J Med. 2017;376:1824-1834.
9. Götberg M, Christiansen EH, Gudmundsdottir IJ, et al. iFRSWEDEHEART Investigators. Instantaneous free-wave ratio versus fractional flow reserve to guide PCI. N Engl J Med. 2017;376:1813-1823.
10. Warisawa T, Cook CM, Rajkumar C, et al. Safety of Revascularization Deferral of Left Main Stenosis Based on Instantaneous Wave-Free Ratio Evaluation. JACC Cardiovasc Interv. 2020;13:1655-1664.
11. Kobayashi Y, Johnoson NP, Berry C, et al. The influence of lesion location on the diagnostic accuracy of adenosine-free coronary pressure wire measurements. J Am Coll Cardiol Interv. 2016;9:2390-2399.
12. de la Torre Hernandez JM, Hernandez F, Alfonso F, et al. Prospective application of pre-defined intravascular ultrasound criteria for assessment of intermediate left main coronary artery lesions results from the multicenter LITRO study. J Am Coll Cardiol. 2011;58:351-358.
13. Nair PK, Marroquin OC, Mulukutla SR, et al. Clinical utility of regadenoson for assessing fractional flow reserve. JACC Cardiovasc Interv. 2011;1085-1092.
14. Ragosta M, Dee S, Sarembock IJ, et al. Prevalence of unfavorable angiographic characteristics for percutaneous intervention in patients with unprotected left main coronary artery disease. Catheter Cardiovasc Interv. 2006;68:357-362.
15. Cameron A, Kemp HG Jr, Fisher LD, et al. Left main coronary artery stenosis:angiographic determination. Circulation. 1983;68:484-489.
16. Fisher LD, Judkins MP, Lesperance J, et al. Reproducibility of coronary arteriographic reading in the coronary artery study (CASS). Catheter Cardiovasc Diagn. 1982;8:565-575.
17. Arnett EN, Isner JM, Redwood DR, et al. Coronary artery narrowing in coronary heart disease:comparison of cineangiographic and necropsy findings. Ann Intern Med. 1979;91:350-356.
18. Lenzen MJ, Boersma E, Bertrand ME, et al. Management and outcome of patients with established coronary artery disease:the Euro Heart Survey on coronary revascularization. Eur Heart J. 2005;26:1169-1179.
19. Kandzari DE, Colombo A, Park SJ, et al. Revascularization for unprotected left main disease:evolution of the evidence basis to redefine treatments standards. J Am Coll Cardiol. 2009;54:1576-1588.
20. Botman CJ, Schonberger J, Koolen S, et al. Does stenosis severity of native vessels influence bypass graft patency?A prospective FFR-guided study. Ann Thorac Surg. 2007;83:2093-2097.
21. Mallidi J, Atreya AR, Cook J, et al. Long term outcomes following fractional flow reserve guided treatment of angiographically ambiguous left main coronary artery disease:a meta-analysis of prospective cohort studies. Catheter Cardiovasc Interv. 2015;86:12-18.
22. Petraco R, van de Hoef TP, Nijjer S, et al. Baseline instantaneous wave-free ratio as a pressure-only estimation of underlying coronary flow reserve:results of the JUSTIFY-CFR Study (Joined Coronary Pressure and Flow Analysis to Determine Diagnostic Characteristics of Basal and Hyperemic Indices of Functional Lesion Severity Coronary Flow Reserve). Circ Cardiovasc Interv. 2014;7:492-502.
23. Nijjer SS, de Waard GA, Sen S, et al. Coronary pressure and flow relationships in humans:phasic analysis of normal and pathological vessels and the implications for stenosis assessment:a report from the Iberian-Dutch-English (IDEAL) collaborators. Eur Heart J. 2016;37:2069-2080.
24. Lee JM, Shin ES, Nam CW, et al. Clinical outcomes according to fractional flow reserve or instantaneous wave-free ratio in deferred lesions. JACC Cardiovasc Interv. 2017;10:2502-2510.
- Comparison of quantitative calcium parameters between optical coherence tomography and invasive coronary angiography
- 5-year results of cutting or scoring balloon before drug-eluting balloon to treat in-stent restenosis
- Real-world registry of the durable Angiolite fluoroacrylate polymer-based sirolimus-eluting stent: the EPIC02 – RANGO study
- Long-term (> 12 months) single-center registry of Magmaris implantation in the acute coronary syndrome setting
Editorials
Expanding the role of drug-coated balloons in native large coronary artery disease
aDepartment of Biomedical Sciences, Humanitas University, Pieve Emanuele, Milan, Italy
bCardio Center, IRCCS Humanitas Research Hospital, Rozzano, Milan, Italy
cDivision of Cardiology, Montefiore Medical Center, Bronx, New York, United States
Original articles
Editorials
The role of angiography-derived physiological assessment techniques in the post-FAVOR III Europe era?
aServicio de Cardiología, Hospital General Universitario Gregorio Marañón, Instituto de Investigación Sanitaria Gregorio Marañón, Universidad Complutense de Madrid, Madrid, Spain
bCentro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), Instituto de Salud Carlos III, Madrid, Spain
Interviews
An interview with Camino Bañuelos
aServicio de Cardiología, Hospital Clínico San Carlos, Instituto de Investigación Sanitaria del Hospital Clínico San Carlos (IdISSC), Madrid, Spain
bServicio de Cardiología, Hospital Clínico Universitario de Santiago de Compostela, Santiago de Compostela, A Coruña, Spain