Article
Ischemic heart disease and acute cardiac care
REC Interv Cardiol. 2019;1:21-25
Access to side branches with a sharply angulated origin: usefulness of a specific wire for chronic occlusions
Acceso a ramas laterales con origen muy angulado: utilidad de una guía específica de oclusión crónica
Servicio de Cardiología, Hospital de Cabueñes, Gijón, Asturias, España
ABSTRACT
Introduction and objectives: Spontaneous coronary artery dissection (SCAD) is a rare but increasingly recognized cause for acute coronary syndrome. The optimal management and treatment of SCAD is still unknown.
Methods: Data analysis of a prospective protocol including centralized care management of a consecutive series of patients with SCAD diagnosed between January 2010 and December 2018. Major adverse cardiovascular events included all-cause mortality, new myocardial infarction, coronary revascularization, ventricular arrhythmia, heart failure or stroke.
Results: A total of 33 consecutive patients were included (41 lesions). Intravascular imaging modalities were used to confirm the diagnosis in 42% patients. None of the patient showed images of thrombus formation in the true lumen. Conservative treatment was the initial approach in most of the cases (82%). No deaths were reported during the index admission, but 15% experienced major adverse cardiovascular events. The coronary computed tomography angiography performed in 58% of patients during the admission identified SCADs in 79% of the patients. Most of the patients managed with conservative treatment received only 1 antiplatelet agent for a limited period of time (17 months [9-35]). During a median clinical follow-up of 33 months [13-49], 82% of patients did not have any adverse events. The angiographic surveillance obtained in 48% of patients at the 6-month follow-up confirmed the complete healing of the SCAD image in 86% of the patients. The screening for extracoronary vascular findings (97% of patients) resulted in a high prevalence of abnormalities (59%).
Conclusions: The unrestricted use of intravascular imaging modalities showed no thrombus in the true lumen of patients with SCAD. In patients managed with conservative treatment, a limited course of antiplatelet monotherapy is safe and provides good clinical outcomes. Performing a coronary computed tomography angiography in the acute phase of SCAD is useful at the follow-up. The screening for extracoronary vascular findings confirmed a high prevalence of abnormalities.
Keywords: Spontaneous coronary artery dissection. Coronary artery disease. Acute coronary syndrome. Optical coherence tomography. Fibromuscular dysplasia.
RESUMEN
Introducción y objetivos: La disección coronaria espontánea (DCE) constituye una causa infrecuente, pero cada vez más reconocida, de síndrome coronario agudo. La actitud diagnóstico-terapéutica idónea sigue sin esclarecerse.
Métodos: Análisis del seguimiento prospectivo y centralizado de una serie de pacientes consecutivos diagnosticados de DCE desde enero de 2010 hasta diciembre de 2018. Se definió evento cardiovascular adverso mayor como la aparición de muerte de cualquier causa, reinfarto no mortal, revascularización no planificada, arritmia ventricular, insuficiencia cardiaca o ictus.
Resultados: Se incluyó a 33 pacientes con DCE (41 lesiones). En el 42% se realizó un estudio con imagen intracoronaria para confirmar el diagnóstico, sin identificar trombo en la luz verdadera en ninguno de ellos. En la mayoría de los casos (82%) se eligió un tratamiento médico conservador. Ningún paciente falleció durante el ingreso, pero el 15% presentó algún evento mayor. En el momento agudo se realizó tomografía computarizada coronaria al 58% de los pacientes y se identificó la DCE en el 79% de los casos. La mayoría de los pacientes con tratamiento conservador recibieron antiagregación simple un tiempo limitado (17 meses [9-35]). Con una mediana de seguimiento de 33 meses [13-49], el 82% no sufrió ningún evento adverso. Al 48% se les realizó control angiográfico a los 6 meses, que mostró resolución en el 86% de los casos. El cribado de anomalías vasculares extracoronarias se realizó en el 97% de los pacientes y se hallaron alteraciones en el 59%, incluyendo 3 pacientes con aneurisma intracraneal.
Conclusiones: En esta serie, con una amplia utilización de imagen intracoronaria, no se ha identificado trombo en la luz verdadera en ningún caso de DCE. En los pacientes tratados de forma conservadora, la monoterapia antiagregante es segura y se asocia a buenos resultados clínicos. La tomografía computarizada coronaria durante el ingreso es útil en el seguimiento. El cribado sistemático de anomalías vasculares extracoronarias revela una alta prevalencia de alteraciones.
Palabras clave: Disección coronaria espontánea. Enfermedad coronaria. Síndrome coronario agudo. Tomografía de coherencia óptica. Displasia fibromuscular.
Abbreviations ACS: acute coronary syndrome. EVA: extracoronary vascular abnormalities. FMD: fibromuscular dysplasia. PCI: percutaneous coronary intervention. SCAD: spontaneous coronary artery dissection.
INTRODUCTION
Spontaneous coronary artery dissection (SCAD) is a rare cause of acute coronary syndrome (SCA). However, especially in women, it has been identified as the underlying pathophysiological mechanism in a growing percentage of cases. SCAD is defined as the separation of the coronary artery wall layers not associated with trauma, iatrogenesis, atherosclerosis or extension of an aortic dissection.1 Clinical signs are myocardial ischemia and are due to the coronary flow limitation that alters the arterial parietal structures.
The first description ever reported by Pretty2 back in 1931 was followed by the description of isolated cases and small series for years. However, we have recently seen a significant increase of information on SCADs lately. Nowadays, clinical profile, diagnostic and therapeutic approach, and prognosis can be found in the SCAD and they vary significantly compared to atherosclerosis—the most common cause of ACS.3,4 Even the European Society of Cardiology5 and the American Heart Association6 have recently published 2 consensus documents on this disease.
In light of the growing evidence and in an attempt to enrich it, back in 2010 our center started a specific program of diagnosis and follow-up of patients with SCAD. The results and conclusions are presented here.
METHODS
All cases of SCAD were collected prospectively since 2010. Diagnosis, treatment, and follow-up were centralized and unified according to the scientific evidence available at the time. Given the length of the study period (9 years) and the extensive medical literature available on this issue over the years, new aspects in the assessment of patients (such as fibromuscular dysplasia [FMD]) have been introduced gradually. This protocol and the data collection book were approved by our center ethics committee and registered in a validated repository (NCT03607981). The patients’ informed consents were obtained in all cases.
Clinical information and follow-up
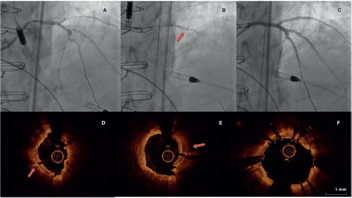
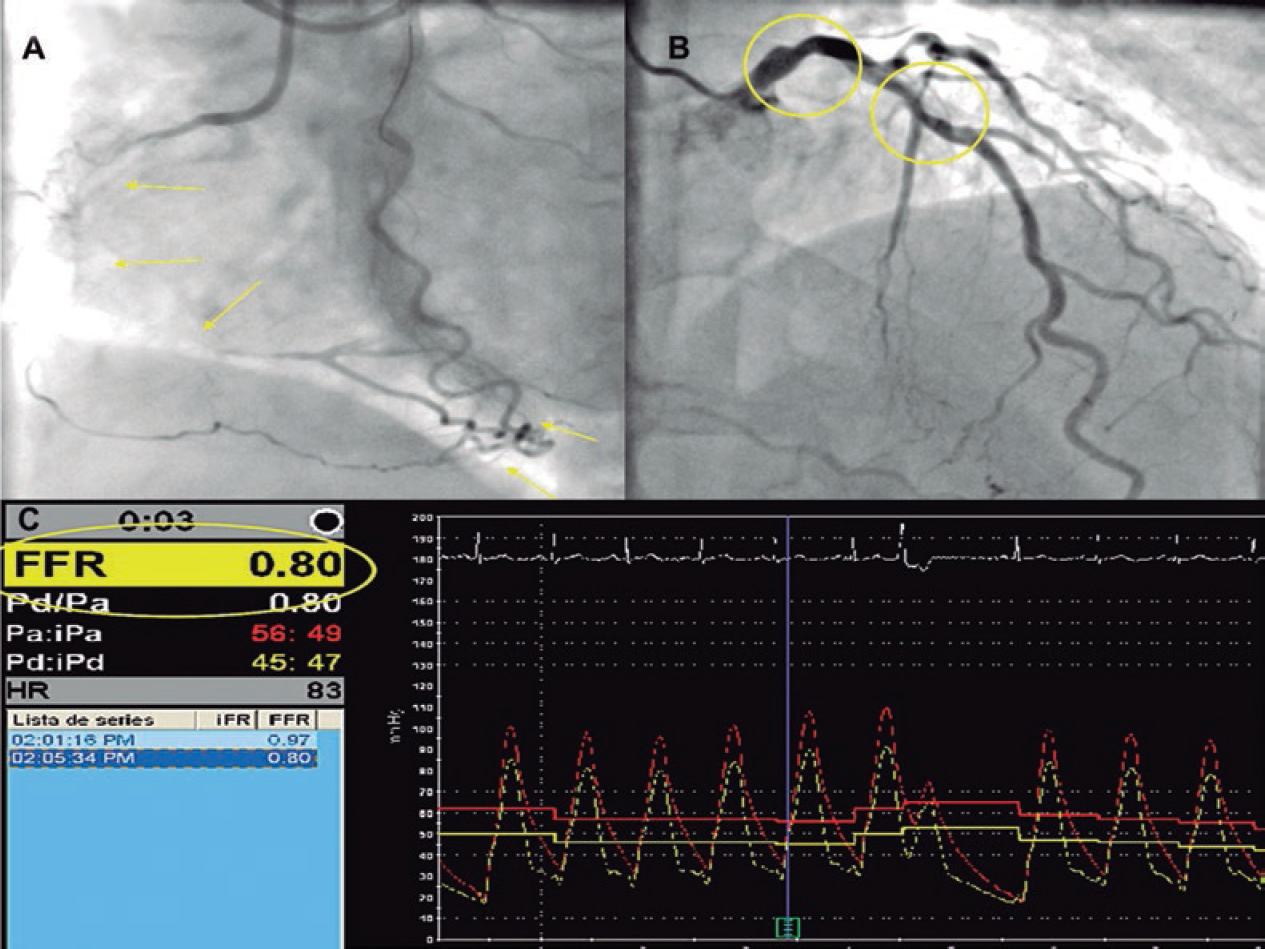
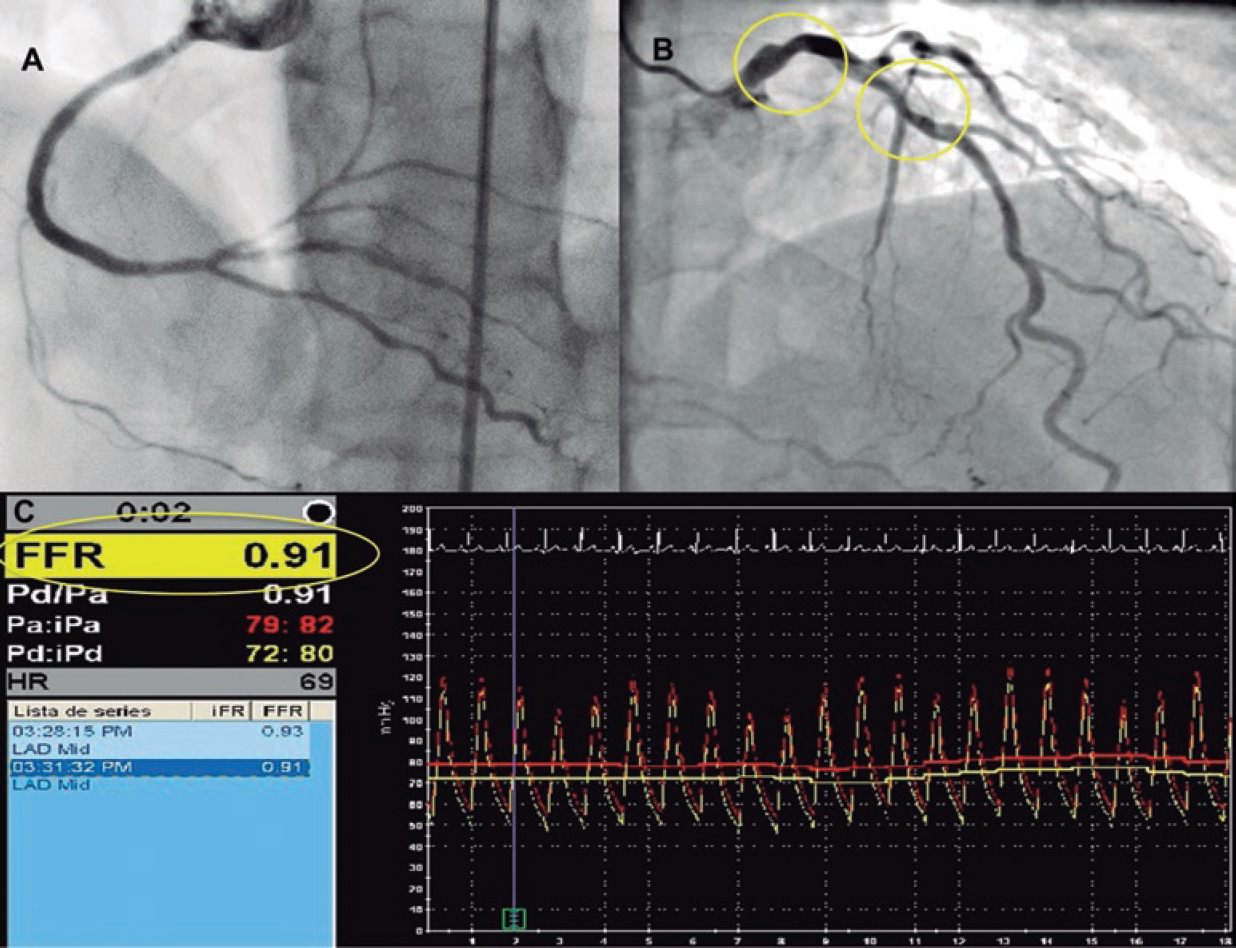
The demographic characteristics, the patients’ personal past medical histories, data at admission, and disease progression were collected in the clinical history at admission and follow-up in a SCAD monographic review (T. Bastante). The coronary angiography and intravascular imaging studies were analyzed by 3 expert interventional cardiologists (T. Bastante, M. García-Guimaraes, and F. Alfonso) and the final diagnosis of SCAD was only established if they all agreed unanimously. The use of intracoronary imaging modalities (intravascular ultrasound [IVUS] or optical coherence tomography [OCT]) was left to the operator’s discretion. However, it was recommended in cases of suspicious diagnosis (especially type 2 and 3 SCADs according to Saw angiographic classification7) or need to perform PCI as long as the segment under study was accessible and in a not overly tortuous artery. When the OCT was used, the intracoronary image was classified as double lumen when the separation of the arterial layers originated true and false lumens, both with lack of refraction due to complete contrast washout. Intramural hematoma was defined as the separation of arterial layers occupied by moderately refracting material with an attenuation consistent with intraparietal bleeding without complete contrast washout. Both the IVUS and the OCT tried to identify the communication between the false and the true lumen and the presence of thrombotic material in the latter (figure 1 shows typical examples). The initial recommended treatment was a wait-and-see conservative approach and the PCI was only performed in cases of clinical instability or symptom persistence. During admission, and as long as it was possible, a coronary computed tomography (CT) scan was performed for a better characterization of coronary lesions. This information was used during follow-up as a comparative pattern in a new coronary CT scan to confirm the healing of the SCAD or in the reappearance of symptoms for reevaluation purposes. Patients with a diagnosis suggestive, but not definitive, of SCAD were scheduled to receive a control coronary angiography within the following months.
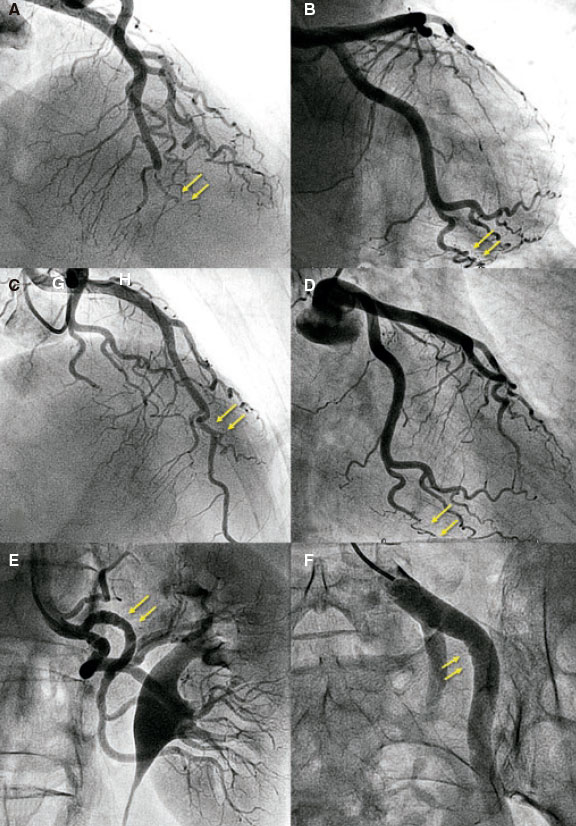
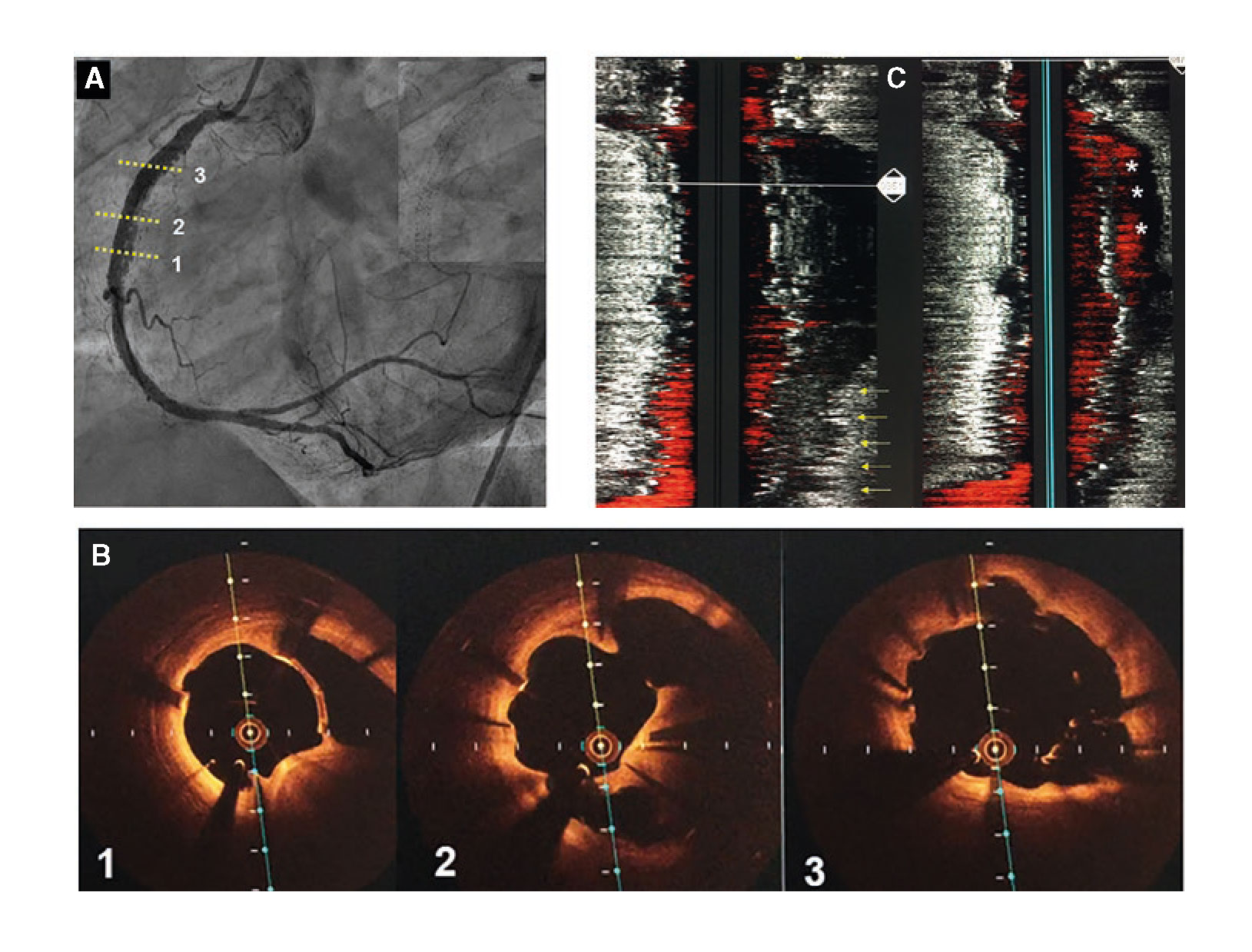
Figure 1. Images of angiography (A-C) and optical coherence tomography (OCT) (D-I). A: type 1 spontaneous coronary dissection (SCAD) in the medial portion of the left anterior descending coronary artery. The arrows point to the characteristic imaging of double lumen with linear intraluminal filling defect outlined by contrast (video 1 of the supplementary data). B: type 2 SCAD in distal left anterior descending coronary artery and diagonal branch. The arrows point to the sudden loss of vessel caliber with length > 20 mm (video 2 of the supplementary data). C: type 3 SCAD in obtuse marginal artery. The arrows point to focal stenosis with length < 20 mm similar to an atherosclerotic lesion (video 3 of the supplementary data). D-F: OCT images showing the double lumen morphology (TL, true lumen; FL, false lumen). Note the unusual image of subintimal calcium displaced with the flap (++). G-H: morphology of intramural hematoma (IMH). I: entry (arrow) with partially thrombosed false lumen. The asterisk (*) shows guidewire artifact.
Definitions
In order to classify the angiographic patterns of SCAD, the aforementioned specific classification developed by Saw et al.7 was used (figure 1 shows examples of this). Two different criteria of success were established for cases where a PCI was required. In the first place, conventional procedural success was defined as a final TIMI flow grade 2-3 (Thrombolysis in Myocardial Infarction) with residual stenosis < 30% after stent/scaffold implantation or < 50% after simple balloon angioplasty. Secondly, the PCI-SCAD was considered successful with flow improvements ≥ 1 grade in the TIMI score and a final TIMI flow grade of 2-3.8 Major cardiovascular adverse events (MACE) at the follow-up included all-cause mortality, reinfarction, unscheduled revascularization, ventricular arrhythmia, heart failure, and stroke.
Screening of extracoronary vascular abnormalities
Since 2013 and as long as it was possible, a selective angiography of both renal and iliac arteries during the diagnostic coronary angiography was performed. Also, 3 to 6 months after the event, the study was completed using the angio-CT scan to examine the floor of the middle cranial fossa up to the femoral arteries (modification of the protocol published by Liang et al.9) including intracranial vessels, supra-aortic trunks, the aorta, and mesenteric, renal, and iliac branches. FMD was defined as the presence of focal narrowing separated by dilatation areas with the traditional «pearl necklace» appearance (multifocal shape) or the presence of tubular focal lesions (unifocal shape). Aneurysms were defined as dilatations > 50% with respect to the caliber of the normal, adjacent arterial segment. Dissection was defined as a double lumen morphology in the arterial segment. The screening of extracoronary vascular abnormalities (EVA) was considered complete when the intracranial territories, supra-aortic trunks, the aorta, and the splanchnic, renal, and iliac territories all had been examined (using angiography, angio-CT scan or both).
Statistical analysis
Quantitative variables were expressed as mean ± standard deviation or median [interquartile range] according to their distribution. Categorical variables were expressed as numbers (percentage). The analysis was conducted using the STATA 12 statistical software package (StataCorp LLC, United States).
RESULTS
Between January 2010 and December 2018 our center performed 12 951 diagnostic coronary angiographies that identified 37 SCADs (41 lesions) in 33 patients (0.28%). Prevalence among the coronary angiographies performed due to ACS (4185) was 1%, although prevalence among women in this context rose to 3%. If the percentage of patients with a final diagnosis of SCAD in the group of women with ACS under 50 is analyzed, prevalence rose to 12.5%. There are more diagnoses over the years from 1 or 2 patients per year initially to 5-7 annual patients over the last period (figure 1 of the supplementary data).
The baseline characteristics of the patients included in the study are shown on table 1. Most (97%) were middle-aged women (56 ± 12 years). Only 7 women (21%) had no traditional cardiovascular risk factors. Five patients (15%) had a personal past medical history of ischemic heart disease, 2 of them with a confirmed diagnosis of SCAD. A study conducted a posteriori confirmed that the remaining 3 patients showed clinical signs consistent with an initially misdiagnosed SCAD (ACS with coronary arteries interpreted as normal, 1 of them in the peripartum).
Table 1. Baseline characteristics of the patients
| n = 33 | |
|---|---|
| Women | 32 (97) |
| Age (years) | 56 ± 12 |
| Race | |
| Caucasian | 28 (85) |
| Other | 5 (15) |
| Cardiovascular risk factors | |
| Smoking habit | |
| Current smoker | 9 (27) |
| Former smoker | 7 (21) |
| Hypertension | 12 (36) |
| Hypercholesterolemia | 14 (42) |
| Diabetes | 2 (6) |
| Family history of ischemic heart disease | 4 (12) |
| Family history of SCAD | 2 (6) |
| Relevant findings | |
| Previous diagnosis of ischemic heart disease | 5 (15) |
| Confirmed diagnosis of previous SCAD | 2 (6) |
| Chronic inflammatory disease | 3 (9) |
| Depressive disorder | 5 (15) |
| Anxiety disorder | 9 (27) |
| History of hypothyroidism | 11 (33) |
| Gynecological/obstetric past medical history | n = 32 |
| Menopause | 24 (75) |
| Menopause age (years) | 49 ± 4 |
| Hormone replacement therapy | 2 (7) |
| Oral hormonal contraceptive | 1 (3) |
| Intrauterine device | 1 (3) |
| Nulliparous | 3 (9) |
| Multiparous | 18 (44) |
| History of miscarriage | 3 (9) |
|
SCAD, spontaneous coronary artery dissection. Data are expressed as no. (%) or mean ± standard deviation. |
|
Table 2 shows the characteristics at hospital admission and during the angiographic assessment. All patients presented with myocardial infarction, most of them (73%) with non-ST-elevation acute myocardial infarction. There was a trigger factor in one third of the cases; the most common was emotional stress (21%) followed by intense physical exercise (9%). Presentation at the peripartum was rare (1 patient only). The artery most frequently compromised was the left anterior descending coronary artery (51%). Eighteen percent of the patients had multivessel disease. Intracoronary imaging modalities (IVUS or OCT) were used in 42% of the cases, mostly OCT (33%). Sixteen lesions in 14 patients were assessed. Those assessed through the OCT confirmed the presence of fenestration between the false and the true lumen in 7 lesions (58%). There were no images consistent with thrombi in the true lumen in any of the cases assessed using intracoronary imaging modalities.
Table 2. Characteristics of the patients at hospital admission and in the angiographic assessment
| n = 33 | |
|---|---|
| Clinical diagnosis at admission | |
| STEMI | 9 (27) |
| NSTEMI | 24 (73) |
| Event-triggering factors | 11 (33) |
| Intense physical exercise | 3 (9) |
| Emotional stress | 7 (21) |
| Peripartum | 1 (3) |
| Angiographic characteristics | n = 33 (41 lesions) |
| Access | |
| Radial | 29 (88) |
| Femoral | 4 (12) |
| Diseased vessel | |
| Left anterior descending coronary artery | 21 (51) |
| Circumflex artery | 10 (24) |
| Right coronary artery | 10 (24) |
| Diseased segment | |
| Proximal | 10 (24) |
| Medial | 11 (27) |
| Distal | 20 (49) |
| Secondary branches | 18 (44) |
| Multivessel disease | 6 (18) |
| Multi-segment disease | 13 (32) |
| Saw et al. angiographic classification7 | |
| Type 1 | 6 (15) |
| Type 2 | 32 (78) |
| Type 3 | 3 (7) |
| Percentage of stenosis (visual estimate) | 77 ± 24 |
| Length of the lesion (mm) | 41 ± 28 |
| Initial TIMI flow grade | |
| 0 | 5 (12) |
| 1 | 5 (12) |
| 2 | 1 (2) |
| 3 | 3 (73) |
| Intracoronary imaging modality | n = 14 (16 lesions) |
| IVUS | 4 lesiones |
| Fenestration | 0 |
| Thrombus | 0 |
| OCT | 12 lesions |
| Double lumen | 7 (58) |
| Intramural hematoma | 2 (16) |
| Both | 3 (25) |
| Fenestration | 7 (58) |
| Thrombus | 0 |
|
CT, computed tomography; IVUS, intravascular ultrasound; NSTEMI, non-ST-elevation acute myocardial infarction; OCT, optical coherence tomography; STEMI, ST-segment elevation myocardial infarction; TIMI, Thrombolysis in Myocardial Infarction. Data are expressed as no. (%) or mean ± standard deviation. |
|
Table 3 shows treatment and the in-hospital disease progression. Initial conservative treatment was the first option in most cases (82%). Only 6 patients were treated with PCI as the initial strategy, 4 of them due to progressive flow worsening with the injections of contrast. The PCI conventional success was reported in 50% of the cases, and the PCI-SCAD success in 67% of the cases. One iatrogenic dissection was reported in the left main coronary artery.
Table 3. Management and in-hospital disease progression of patients
| n = 33 | |
|---|---|
| Initial treatment | |
| Conservative | 27 (82) |
| PCI | 6 (18) |
| PTCA-balloon | 2 (6) |
| Bare-metal stent | 2 (6) |
| Drug-eluting stent | 1 (3) |
| Bioresorbable vascular scaffold device | 1 (3) |
| Results from the PCI group | n = 6 |
| Conventional success | 3 (50) |
| PCI-SCAD success | 4 (67) |
| In-hospital disease progression | n = 33 |
| Peak troponin T levels (ng/mL) | 378 [132-1705] |
| Peak creatine kinase levels (U/L) | 403 [169-1181] |
| Left ventricular dysfunction [LVEF < 50%] | 5 (17) |
| Segmental abnormalities on the TTE | 17 (52) |
| MACE | 5 (15) |
| Death | 0 |
| Reinfarction | 0 |
| New coronary angiography | 4 (12) |
| Unplanned revascularization | 3 (9) |
| PCI group (n = 6) | 2 (33) |
| Conservative management group (n = 27) | 1 (4) |
| Ventricular tachycardia/fibrillation | 2 (6) |
| Heart failure | 1 (3) |
| Hospital stay (days) | 4 [3-7] |
| Coronary CT scan at admission | n = 19 (58) |
| SCAD visible on the coronary CT scan | 15 (79) |
| Treatment at hospital discharge | n = 33 |
| ASA | 31 (94) |
| Clopidogrel | 9 (27) |
| Ticagrelor | 5 (15) |
| Prasugrel | 0 |
| Dual antiplatelet therapy | 14 (42) |
| Anticoagulation | 2 (6) |
| Beta-blockers | 28 (85) |
| ACEI/ARA II | 21 (64) |
| Statins | 25 (76) |
| Nitrates | 3 (9) |
| Calcium antagonists | 3 (9) |
|
ACEI, angiotensin-converting enzyme inhibitors; ARA-II, angiontensin II receptor antagonist; ASA, acetylsalicylic acid; CT, coronary tomography; LVEF, left ventricular ejection fraction; MACE, major cardiovascular adverse events; PCI, percutaneous coronary intervention; PTCA, percutaneous transluminal coronary angioplasty; SCAD, spontaneous coronary artery dissection; TTE, transthoracic echocardiography. Data are expressed as no. (%) or mean ± standard deviation or median [interquartile range]. |
|
During in-hospital disease progression no patient died or suffered any reinfarctions. However, a new coronary angiography was required in 4 patients with symptoms. Except for the patient with a left main coronary artery iatrogenic dissection initially treated with conservative treatment no case was due to failed initial conservative treatment. The remaining 3 patients had acute stent thrombosis, SCAD of a vessel other than the index, and progression of the SCAD adjacent to the segment treated with the stent. Overall, the rate of in-hospital MACE was 15% and events focused on patients who required PCI. Acetylsalicylic acid (ASA) was prescribed to 94% of the patients at hospital discharge and dual antiplatelet therapy to 14 patients only (42%) of whom 7 required PCI. Fifty-eight percent of the patients received coronary CT scans during admission and images consistent with SCAD were found in 79% of the cases.
Table 4 shows out-of-hospital disease progression. Median follow-up was 33 months [13-49], the overall rate of events was 18%. Two deaths were reported, 1 due to cardiovascular causes (sudden death 6 years after the SCAD) and the other due to non-cardiovascular causes (sepsis in the abdominal postoperative). Only 1 patient required a new revascularization due to restenosis of the stent implanted to treat the SCAD. Three out of the 4 patients (12%) with SCAD relapse had suffered events prior to the index event that were compatible with SCAD; that is, each one of them had presented with, at least, 3 events. Except for 1 recurrence at the 7-month follow-up, most events occurred more than 2 years after the index event (figure 2). Regarding pharmacological treatment, ASA was kept for a median 17 months [9-35] after the event and the second antiplatelet drug was withdrawn early in most of the patients. Of the patients who received conservative treatment, only 25% were still on dual antiplatelet therapy 6 months after the event (median 0 months [0-6]). In those patients who required PCI, dual antiplatelet therapy was keep for a median 5 months [1-7].
Table 4. Out-of-hospital disease progression and follow-up of the patients
| n = 33 | |
|---|---|
| Follow-up time (months) | 33 [13-49] |
| MACE | 6 (18) |
| Death | 2 (6) |
| New AMI | 3 (9) |
| Recurrence | 4 (12) |
| New revascularization | 1 (3) |
| Heart failure | 1 (3) |
| Stroke | 1 (3) |
| Time on ASA (months) | 17 [9-35] |
| Time on dual antiplatelet therapy (months) | |
| Conservative treatment group | 0 [0-6] |
| PCI group | 5 [1-7] |
| Control SCAD | n = 16 (48) |
| Coronary CT scan | 9 |
| Planned | 6 |
| Due to symptoms | 3 |
| Invasive coronary angiography | 11 |
| Planned | 3 |
| Due to symptoms | 8 |
| Screening of EVA | N = 32 (97) |
| Type of screening | |
| CT scan | 18 (56) |
| Angiography | 5 (16) |
| Angiography + CT scan | 9 (28) |
| Complete screening | 28 (88) |
| EVA data | 19 (59) |
| Type of EVA | |
| Fibromuscular dysplasia | 15 (47) |
| Aneurysm | 5 (15) |
| Other | 1 (3) |
| Location of EVA | |
| Renal arteries | 9 (28) |
| Iliac arteries | 7 (22) |
| Supra-aortic trunks | 5 (16) |
| Intracranial | 3 (9) |
| Other | 5 (16) |
|
AMI, acute myocardial infarction; ASA, acetylsalicylic acid; CT, computed tomography; EVA, extracoronary vascular abnormalities; MACE, major cardiovascular adverse events; PCI, percutaneous coronary intervention; SCAD, spontaneous coronary artery dissection. Data are expressed as no. (%) or mean ± standard deviation or median [interquartile range]. |
|
Angiography control was performed in 48% of the patients, in 9 of them using coronary CT scan and invasive coronary angiography in 11 patients. The coronary CT scan was performed in 3 patients in the context of a new episode of chest pain. After comparing it with the previous CT scan performed at the index event, the new SCAD was discarded (figure 3). However, most coronary angiographies were performed in the context of a new cardiac event; only 3 patients received a planned control coronary angiography. Out of the 16 patients on angiographic control, imaging improved with restitutio ad integrum in 75% of them. Six months after the SCAD, the documented rate of resolution rose to 86%.
Figure 2. Fifty-five-year old woman with non-ST-elevation acute myocardial infarction (NSTEMI). She had experienced 2 previous acute myocardial infarctions with «normal coronary arteries» according to the coronary angiography. A: sudden caliber loss with tapering until the occlusion in the medial-distal portion of the left anterior descending coronary artery compatible with spontaneous coronary artery dissection (SCAD); conservative treatment. B: tortuous obtuse marginal artery without evident abnormalities. Two years later new hospitalization due to NSTEMI. C: caliber and flow recovery in the anterior descending coronary artery. D: obtuse marginal artery with sudden caliber loss and tapering (compare to B) compatible with SCAD; conservative treatment. E-F: selective angiographies of left renal and left iliac arteries with fibromuscular dysplasia.
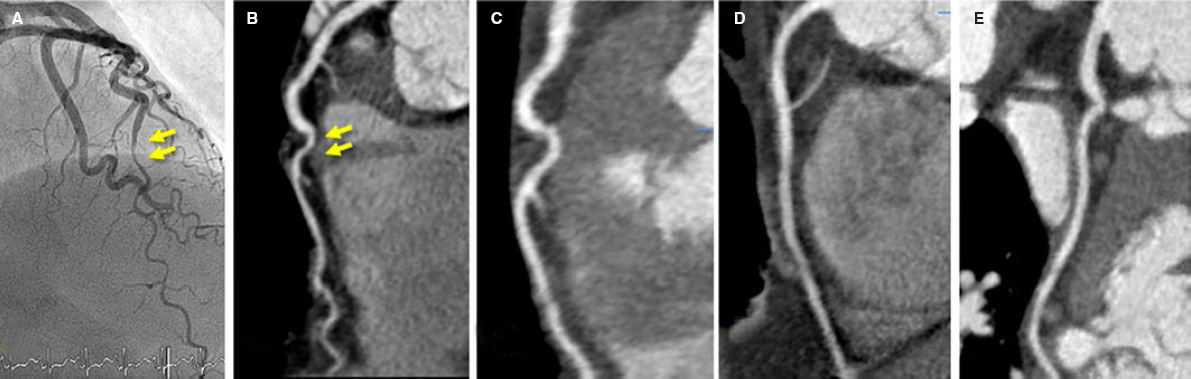
Figure 3. Fifty-three-year old woman with non-ST-elevation acute myocardial infarction. After a tortuous segment, the sudden caliber loss of the left anterior descending coronary artery with vessel tapering can be seen (arrows) on the coronary angiography (A) and coronary computed tomography (CT) scan (B) compatible with a spontaneous coronary artery dissection (SCAD) at the medial-distal portion of the left anterior descending coronary artery. Four years later, the patient presents to the ER with prolonged chest pain without alterations on the ECG or high markers of myocardial damage. The coronary CT scan shows the coronary arteries without images indicative of SCAD. C: left anterior descending coronary artery. D: right coronary artery. E: circumflex artery.
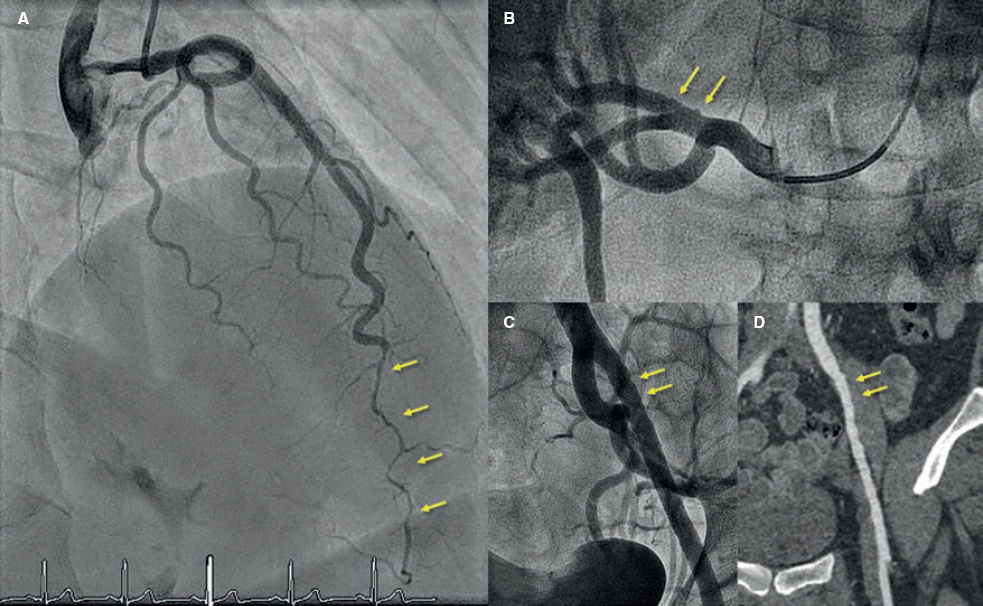
Figure 4. Forty-five-year old woman with non-ST-elevation acute myocardial infarction. The coronary angiography (A) shows a spontaneous coronary artery dissection at the medial-distal portion of the left anterior descending coronary artery (arrows). During catheterization the selective coronary angiography performed on the right renal (B) and left iliac arteries (C) shows wall irregularities compatibles with fibromuscular dysplasia (arrows). D: coronary computed tomography angiography at the follow-up with findings compatible with fibromuscular dysplasia on the left external iliac artery (arrows); note the greater sensitivity of coronary angiography (C) for the detection of subtle parietal abnormalities compared to coronary computed tomography angiography (D).
The screening of EVA was performed in 97% of the patients (full screening in 88%). Fifty-nine percent of the patients showed abnormalities that went up to 61% when the screening of EVA was complete. The abnormality most commonly found was FMD (47%) followed by arterial aneurysms (in 5 patients, 3 of which were intracranial aneurysms). The renal and iliac arteries were the most commonly compromised arteries of all: half of the patients studied showed abnormalities in either one of these arteries (examples in figure 2 and figure 4). After the study, the stroke team indicated the closure of the 3 intracranial aneurysms.
DISCUSSION
This study prospectively reports on the results of a current series of patients with SCAD with an updated and systematized diagnostic-therapeutic process and prolonged clinical follow-up. The clinical profile is consistent with what it is known about this disease:3,8,10 middle-aged woman with risk factors and low concomitance of chronic inflammatory disorders, autoimmune diseases or collagen diseases. Both the presentation and the angiographic characteristics were consistent with what has already been described: non-ST-elevation acute myocardial infarction that damaged the medial-distal segments and secondary branches predominantly with a higher incidence reported on the left anterior descending coronary artery. The most common Saw angiographic classification was type 2. Comparatively, in this series, the use of intracoronary imaging modalities was superior to other larger and recent series (42% vs 7.6% and 13% in the series of Saw et al.10 and Tweet et al.,8 respectively); this brings high reliability in the inequivocal diagnosis of SCAD. The most important conclusions of intracoronary imaging are: a) when OCT was the imaging modality used, the fenestration of both lumens could be identified in half of the lesions; b) the presence of mixed patterns (double lumen and intramural hematoma) within the same lesion is not an uncommon finding, which supports the evolutionary theory between both patterns; and c) lastly and probably the most important conclusion of all, intraluminal thrombi were not found in any of the lesions studied.
As it has already been described, an initial wait-and-see conservative approach with no interventions seems to bring good results to patients with SCAD.8,10,11 The rate of in-hospital MACE was low (15%). No deaths were reported, and bailout revascularizations were not necessary in any of the patients who received conservative treatment, except for 1 case due to iatrogenic dissection of left main coronary artery during the initial catheterization. Also, during the patients’ initial disease progression, they already showed preserved left ventricular ejection fraction. Similarly, out-of-hospital disease progression was good: 2 deaths were reported (1 due to non-cardiovascular causes) at the 2.7-year median follow-up, and 12% had a new episode of SCAD. These are similar data to those described in a Canadian series12 (10.4% at the 3.1-year median follow-up) and significantly lower to the rate of recurrence of 27% at the 2.3-years of median follow-up reported by Mayo Clinic.8
Unlike the atherosclerosis related ACS, in SCAD the ideal antithrombotic therapy has not been totally established. It seems logical to avoid aggressiveness, especially when 1 of the most plausible etiopathogenic theories is intraparietal bleeding of vasa vasorum as the initial event.13,14 Therefore, given the lack of intraluminal thrombus in a high percentage of patients studied with IVUS and OCT in this series a low-intensity antithrombotic therapy was used. ASA was kept for an average 1.5 years and the second antiplatelet drug was only indicated at hospital discharge in patients who required PCI and for the shortest period of time possible. The satisfactory disease progression reported with rates of out-of-hospital events consistent with those reported in other large series (from 10% to 20%)1 and the low rate of recurrence suggest that low-intensity antithrombotic therapy can be an excellent option for these patients.
There is very little information on the value of coronary CT scan during SCAD related hospitalizations. It was performed in 58% of patients from this series and SCAD was identified in three fourths of the cases. A more extensive analysis of these findings has been recently published by our group.15 The current study shows that this information was very useful in the follow-up of 3 patients to discard new episodes of SCAD and avoid the coronary angiography and associated risks for the patients (3.4% of iatrogenic dissections in patients with SCAD).16 However, in one fourth of the patients the SCAD could not be identified in the acute phase not even with the previous coronary angiography as guidance. Therefore, the value of coronary CT scan as an early diagnostic imaging modality is limited in this context.
Back in 2012 the association between SCAD and FMD17 was described for the first time, and later studies only not confirmed the high prevalence of this association but also of other EVA (aneurysms, dissections, and thrombosis).18-20 In the European consensus document recently published the screening of EVA is recommended in patients with SCAD.5 To our knowledge and up to this day this study shows the results of the most complete screening of FMD and other EVA. With a study in 97% of the patients—complete in 88%—the great presence of EVA (60%) confirms this interesting association. The need to conduct these studies may be put into question since most findings are associated with discrete and typical parietal abnormalities of FMD that do not lead necessarily to significant functional disorders. As a matter of fact, after the long follow-up of patients and despite the high prevalence of EVA, the extracardiac arterial events reported were only 1 stroke. However, there are 3 reasons to support the screening: a) in case of suspicious diagnosis, it may be the key to confirm the diagnosis of SCAD;21 b) knowing arterial parietal structural alterations can be useful for the diagnosis and treatment of future extracardiac events; and c) the finding of intracranial aneurysms is not negligible (9% in our series, but up to 14% in the Canadian series16) and it is relevant due to the risk of intracranial bleeding and secondary morbimortality. As a matter of fact, in 3 of our patients a percutaneous coronary intervention was indicated to seal the intracranial aneurysm.
Limitations
The main limitations of this study are the small size of the sample and the fact that it focused on a single center only. However, this study has a long follow-up with a unified treatment given the centralization of the patients.
CONCLUSIONS
In our center the centralization and protocolization of patients with SCAD systematized both treatment and the performance of additional tests. Intracoronary imaging allows us to confirm diagnosis in angiographically suspicious cases without showing any thrombi in the true lumen whatsoever. A low-intensity antithrombotic strategy with ASA only and for a limited period of time seems to give good results in the management of SCADs with conservative treatment. The high rate of spontaneous resolution of SCAD was confirmed in the 6-month images. Over half of the patients with SCADs show some EVA. Performing a coronary CT scan in the acute phase was useful, comparatively speaking, in new events and scheduled controls.
CONFLICTS OF INTEREST
F. Alfonso is an associate editor of REC: Interventional Cardiology; the editorial protocol of the journal was observed to guarantee an impartial manuscript handling.
WHAT IS KNOWN ABOUT THE TOPIC?
- SCAD is a rare disease more commonly regarded as the cause of ACS, especially in women.
- The pathophysiological substrate and prognosis are different from common atherosclerosis as well as the management recommended.
- To this day, the information on SCADs comes from many retrospective series since no randomized, controlled clinical trials have been conducted yet.
WHAT DOES THIS STUDY ADD?
- This was a prospective study with a fairly long follow-up that collected data on a specific diagnostic, therapeutic, centralized, and updated approach based on the new scientific evidence available on the management of SCAD.
- The study presented the results of an almost universal screening of ECA with a high percentage of patients with unequivocal diagnosis of SCAD (thanks to the common use of intravascular imaging modalities) and angiographic control during disease progression.
- Treatment with a very low-intensity antithrombotic strategy (antiplatelet therapy with ASA only and not indefinitely) is safe with excellent results during disease progression.
SUPPLEMENTARY DATA
Video 1. Bastante T. DOI: 10.24875/RECICE.M20000096
Video 2. Bastante T. DOI: 10.24875/RECICE.M20000096
Video 3. Bastante T. DOI: 10.24875/RECICE.M20000096
REFERENCES
1. Saw J, Mancini GBJ, Humphries KH. Contemporary Review on Spontaneous Coronary Artery Dissection. J Am Coll Cardiol. 2016;68:297-312.
2. Pretty H. Dissecting aneurysms of coronary artery in woman aged 42:rupture. BMJ. 1931;1:667.
3. Alfonso F, Bastante T. Spontaneous coronary artery dissection novel diagnostic insights from large series of patients. Circ Cardiovasc Interv. 2014;7:638-641.
4. Bastante T, Cuesta J, García-Guimaraes M, et al. Current management of spontaneous coronary artery dissection. Expert Rev Cardiovasc Ther. 2017;15:619-628.
5. Adlam D, Alfonso F, Maas A, et al. European Society of Cardiology, acute cardiovascular care association, SCAD study group:a position paper on spontaneous coronary artery dissection. Eur Heart J. 2018;39:3353-3368.
6. Hayes SN, Kim CESH, Saw J, et al. Spontaneous Coronary Artery Dissection:Current State of the Science:A Scientific Statement from the American Heart Association. Circulation. 2018;137:e523-e557.
7. Saw J. Coronary angiogram classification of spontaneous coronary artery dissection. Catheter Cardiovasc Interv. 2014;84:1115-1122.
8. Tweet MS, Eleid MF, Best PJM, et al. Spontaneous coronary artery dissection:Revascularization versus conservative therapy. Circ Cardiovasc Interv. 2014;7:777-786.
9. Liang JJ, Prasad M, Tweet MS, et al. A novel application of CT angiography to detect extracoronary vascular abnormalities in patients with spontaneous coronary artery dissection. J Cardiovasc Comput Tomogr. 2014;8:189-197.
10. Saw J, Starovoytov A, Humphries K, et al. Canadian spontaneous coronary artery dissection cohort study:in-hospital and 30-day outcomes. Eur Heart J. 2019;40:1188-1197.
11. Alfonso F, Paulo M, Lennie V, et al. Spontaneous coronary artery dissection:long-term follow-up of a large series of patients prospectively managed with a “conservative“ therapeutic strategy. JACC Cardiol Intv. 2012;5:1062-1070.
12. Saw J, Humphries K, Aymong E, et al. Spontaneous coronary artery dissection:Clinical outcomes and risk of recurrence. J Am Coll Cardiol. 2017;70:1148-1158.
13. Waterbury TM, Tweet MS, Hayes SN, et al. Early natural history of spontaneous coronary artery dissection. Circ Cardiovasc Interv. 2018;11:e006772.
14. Jackson R, Al-Hussaini A, Joseph S, et al. Spontaneous coronary artery dissection. Patophisiological insights from optical coherence tomography. JACC Cardiovasc Imaging. 2019;12:2475-2488.
15. Pozo-Osinalde E, García-Guimaraes M, Bastante T, et al. Characteristic findings of acute spontaneous coronary artery dissection by cardiac computed tomography. Coron Artery Dis. 2019. https://doi.org/10.1097/MCA.0000000000000819
16. Prakash R, Starovoytov A, Heydari M, et al. Catheter-Induced iatrogenic coronary artery dissection in patients with spontaneous coronary artery dissection. J Am Coll Cardiol Intv. 2016;9:1851-1852.
17. Saw J, Poulter R, Fung A, et al. Spontaneous coronary artery dissection in patients with fibromuscular dysplasia:a case series. Circ Cardiovasc Interv. 2012;5:134-137.
18. Saw J, Aymong E, Sedlak T, et al. Spontaneous coronary artery dissection:association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv. 2014;7:645-655.
19. Bastante T, Rivero F, Cuesta J, et al. Association of spontaneous coronary artery dissection with fibromuscular dysplasia. Rev Esp Cardiol. 2015;68:719-720.
20. Prasad M, Tweet MS, Hayes SN, et al. Prevalence of extracoronary vascular abnormalities and fibromuscular dysplasia in patients with spontaneous coronary artery dissection. Am J Cardiol. 2015;115:1672-1677.
21. Bastante T, García-Guimaraes M, Rivero F, et al. Isolated septal branch lesion as the only diagnostic clue for spontaneous coronary artery dissection. Coron Artery Dis. 2020;31:98-99.
ABSTRACT
Introduction and objectives: Chronic total coronary occlusion (CTO) is often a complex entity to deal with through a percutaneous coronary intervention, and the clinical benefits of successful recanalization still remain uncertain. Most registries feature data in limited time periods and do not reflect the impact that specific dedicated programs have on recanalization. Our study evaluates the results of a CTO program on a long-term period of time.
Methods: All patients’ CTOs treated with percutaneous coronary interventions at our center from 2002 through 2017 were prospectively included in the registry. The clinical, angiographic and procedural data were collected, and clinical follow-up was conducted. Three consecutive periods of time were considered for the analysis of temporal trends.
Results: Atotal of 424 CTOs (408 patients) were included. In 339 patients (80%) the procedure was successful. The rate of success increased over time, from 57% in 2002-2006 to 87% in 2012-2017 (P = .001). The most important independent predictor of procedural failure was lesion tortuosity. After a median follow-up of 39.7 months, the rates of major adverse cardiovascular events and cardiovascular mortality in success vs failed groups were 13.9% vs 24.7% (P = .015) and 3.6% vs 14.1% (P = .001), respectively. These were the independent predictors of cardiovascular mortality: chronic kidney disease, left anterior descending artery occlusion, and procedural failure.
Conclusions: Our series shows a high rate of success in CTO recanalization, which has increased over the last few years due to greater expertise and improved program-specific technical advances. Several angiographic and procedural variables have been identified as predictors of failure. Successful procedures, especially on the left anterior descendent coronary artery, were associated with lower rates of cardiovascular mortality.
Keywords: Chronic total coronary occlusion. Percutaneous coronary intervention. Ischemic heart disease.
RESUMEN
Introducción y objetivos: La oclusión total coronaria crónica (OTC) es generalmente compleja de abordar con intervencionismo percutáneo y el beneficio clínico de su recanalización sigue siendo incierto. La mayoría de los registros aportan datos limitados en el tiempo y no reflejan el impacto de un programa específico para su tratamiento. Nuestro estudio evalúa los resultados de un programa de OTC a largo plazo.
Métodos: Se incluyeron de forma prospectiva todos los pacientes tratados con un intento de revascularización percutánea de una OTC entre los años 2002 y 2017. Se obtuvieron datos clínicos, angiográficos, intraprocedimiento y del seguimiento. Se consideraron 3 periodos temporales consecutivos para el análisis.
Resultados: Se incluyeron 408 pacientes (424 OTC). La desobstrucción fue exitosa en 339 lesiones (80%). El éxito se incrementó con el tiempo, de un 57% en 2002-2006 a un 87% en 2012-2017 (p = 0,001). El predictor independiente más potente de procedimiento fallido fue la tortuosidad intralesional. Tras una mediana de seguimiento de 39,7 meses, las tasas de eventos adversos cardiacos mayores y de muerte cardiaca en los grupos de éxito y fracaso fueron del 13,9 frente al 24,7% (p = 0,015) y del 3,6 frente al 14,1% (p = 0,001), respectivamente. Los predictores independientes de mortalidad cardiaca fueron la insuficiencia renal crónica, la oclusión de la arteria descendente anterior y el fallo del procedimiento.
Conclusiones: Nuestra serie muestra unas tasas elevadas de éxito en la recanalización de una OTC, incrementada en los últimos años debido a la experiencia y al desarrollo técnico del programa. Se han identificado numerosas variables clínicas y angiográficas como predictoras de fallo del procedimiento. El éxito en el procedimiento, en especial en la arteria descendente anterior, se asoció con una menor mortalidad cardiaca.
Palabras clave: Oclusión total crónica. Intervención coronaria percutánea. Cardiopatía isquémica.
Abbreviations ACS: acute coronary syndrome. CABG: coronary artery bypass graft. CTO: chronic total coronary occlusion. LAD: left anterior descending coronary artery. MACE: major adverse cardiovascular events. PCI: percutaneous coronary intervention.
INTRODUCTION
Percutaneous coronary interventions (PCI) of chronic total coronary occlusions (CTO) represent up to 12% of all PCIs performed.1 The reason to perform the percutaneous recanalization of a CTO is to improve clinical symptoms which, ultimately, has potential survival benefits as suggested by some observational studies.2-4 However, the clinical benefits of successful recanalization remain undefined and to this day accepting that opening CTOs saves lives, despite the favorable consistent results from several contemporary registries, is still not supported by randomized clinical trials.5
Given the complexity of these procedures, a specific program with dedicated CTO-trained operators is encouraged. Also, most of the published registries and randomized clinical trials are performed in highly skilled centers and feature results in limited periods of time usually on specific devices, but not long-term results.2-5
We present the results of a specific PCI program for CTO lesions, starting with the introduction of drug-eluting stents from 2002 through 2017. The profile of patients and lesions, procedural data, results, and long-term clinical outcomes have been analyzed during the time frame of the program.
METHODS
This prospective registry conducted in a single center with an active PCI program for CTOs started back in 2002. It included 1 single operator who would progressively develop proper skills.
All consecutive patients treated of their CTOs, at least once, through percutaneous recanalization during the period 2002–2017 were included. Clinical data, angiographic characteristics, and procedural features were collected. The patients gave their informed consent and the study was approved by the local review board.
The indication for the recanalization of the CTO was the presence of angina, confirmation of ischemia through provocation tests or viable myocardium assessed through magnetic resonance imaging since 2004 when this diagnostic imaging modality became available at our center. No angiographic exclusion criteria were applied. Therefore, long occlusions, severely calcified lesions, and ostial locations were included if clinically indicated. Patients with an indication for coronary artery bypass graft (CABG) were excluded.
CTOs diagnosed in the setting of an ST-segment elevation acute coronary syndrome were scheduled for intervention that was performed at least 4 weeks after the index procedure. In cases of non-ST-segment elevation, CTOs were approached during the initial catheterization or in a subsequent staged procedure at the operator’s discretion. Also, in 28 out of the 101 cases of CTOs diagnosed in the context of an ACS, the ad-hoc desobstruction of the CTO was attempted.
Most CTOs were performed by the lead operator who focused their experience on trying to improve the rate of success for the benefit of the patient.
For the analysis of temporal trends regarding techniques and results, patients were classified into 3 consecutive periods of time: 2002-2006, 2007-2011, and 2012-2017. Also, the entire cohort was divided into 2 groups regarding success or failure in the recanalization of the CTO. Follow-up data were obtained from hospital records and the contact kept with the patients and the information provided were prospectively included in a database. No routine angiographic follow-up assessment was conducted.
Procedures were performed according to standard practices through the femoral or radial approach. Antithrombotic therapy consisted of unfractionated heparin (100 U/Kg) with additional administration when appropriate, to achieve activated clotting times of 250 seconds or 300 seconds using the antegrade and retrograde approaches, respectively. Aspirin 100 mg was administered orally prior to the PCI. Before stent implantation patients received perioperatively 300 mg to 600 mg of clopidogrel followed by a daily administration of 75 mg for the prescribed period of dual antiplatelet therapy.
CTOs were defined as coronary obstructions with TIMI flow grade 0 of at least 3 months duration.
Procedural success was defined as achieving residual post-PCI stenosis < 30% associated with TIMI flow 2–3.
Mortality was considered cardiovascular unless an evident non-cardiac cause was identified. Myocardial infarction was defined according to the Third Universal Definition established by the European Society of Cardiology and the American College of Cardio- logy Foundation. Target lesion revascularization was defined as a repeated PCI on the target lesion or CABG on the target vessel following ischemia-driven restenosis. Target vessel revascularization was defined as repeated PCI or CABG on any segments of the target vessel. Major adverse cardiovascular events (MACE) were defined as cardiovascular death, myocardial infarction or need for surgical or percutaneous target vessel revascularization. Stent thrombosis was defined according to the Academic Research Consortium criteria.
The angiographic characteristics expected to be predictive of procedural success were classified according to the recommendations proposed by the Euro-CTO club consensus document.6 The J-score was calculated for each lesion based on the length of the occlusion, morphology of the stump, calcification, tortuosity, and prior attempt to open the CTO.7
Continuous variables were expressed as mean ± standard deviation or median (interquartile range [IQR]), when appropriate. Categorical variables were expressed as percentages. The chi square test or Fisher’s exact test were used to compare the categorical variables. The Kolmogorov-Smirnov test was used to verify the normal distribution of continuous data. Continuous variables were compared according to their distributions using the Student t test or Mann-Whitney U test (success vs failed subgroups), and the ANOVA or Kruskal-Wallis test (comparison of 3 time periods). The estimates of cardiovascular death-and-MACE-free survival were shown by the Kaplan-Meier curves. Inter-groupt differences were assessed using the log-rank test. The logistic regression and Cox proportional hazard models were used to assess the independent contribution of variables to procedural success and mortality, respectively. Multivariate models included variables with P values < .2 in the univariate analysis. All statistical analyses were 2-tailed, and P values < .05 were considered statistically significant. The statistical analysis was performed using the statistical software package SPSS 15.0 (SPSS Inc., United States).
RESULTS
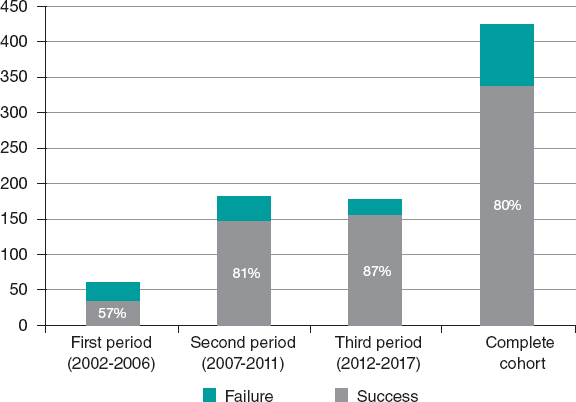
A total of 424 CTOs (408 patients) were included. In 339 patients (80%) procedural success was achieved. The number of procedures and the corresponding rate of success per period is shown on figure 1 .
Figure 1. Number of procedures and corresponding rate of success per period.
The baseline characteristics regarding the success or failure of the CTO procedure are featured on table 1 and table 2. Previous CABG and the ACS setting were more common among failed cases. Patients with successful procedures were more prone to left anterior descending coronary artery (LAD) involvement, microchannels, and Rentrop grade 3 collateral blood flow. Procedural success was higher in the LAD compared to other target vessels (87% vs 77%; P = .02). Procedural success in the circumflex artery was the lowest of all (76%). The complexity of the CTO according to the J-score was higher in failed cases.
Table 1. Baseline characteristics
| All (n = 424) | Success (n = 339) | Failure (n = 85) | P | |
|---|---|---|---|---|
| Age | 63 ± 12 | 63 ± 12 | 64 ± 13 | .48 |
| Male sex | 350 (83%) | 277 (82%) | 73 (86%) | .37 |
| Hypertension | 279 (66%) | 217 (64%) | 62 (73%) | .15 |
| Diabetes Mellitus | 120 (28%) | 95 (28%) | 25 (29%) | .91 |
| Dyslipidemia | 275 (65%) | 222 (65%) | 53 (62%) | .45 |
| Past/current smoker | 292 (69%) | 236 (70%) | 56 (66%) | .48 |
| Previous infarction | 147 (35%) | 111 (33%) | 36 (42%) | .72 |
| Previous CABG | 31 (7%) | 18 (5%) | 13 (15%) | .002 |
| Multivessel disease | 297 (70%) | 234 (69%) | 63 (74%) | .38 |
| Left ventricular ejection fraction | 55 ± 13 | 55 ± 13 | 57 ± 13 | .17 |
| Serum creatinine (mg/dL) | 1.03 ± 0.53 | 1.02 ± 0.49 | 1.04 ± 0.64 | .76 |
| Acute coronary syndrome | 103 (24%) | 74 (22%) | 29 (34%) | .021 |
|
CABG, coronary artery bypass graft. |
||||
Table 2. Angiographic characteristics of occlusive lesions
| All (n = 424) | Success (n = 339) | Failure (n = 85) | P | |
|---|---|---|---|---|
| Left anterior descending coronary artery | 129 (30%) | 112 (33%) | 17 (20%) | .02 |
| Right coronary artery | 211 (50%) | 163 (48%) | 48 (56%) | .17 |
| Left circumflex artery | 81 (19%) | 62 (18%) | 19 (22%) | .39 |
| Diameter (mm) | 3.15 ± 0.45 | 3.15 ± 0.46 | 3.16 ± 0.58 | .97 |
| Length (mm) | 23 ± 16 | 21 ± 13 | 29 ± 21 | .001 |
| Moderate-to-severe calcification | 303 (74%) | 232 (72%) | 71 (84%) | .028 |
| Moderate-to-severe tortuosity | 150 (35%) | 95 (28%) | 55 (65%) | .001 |
| Severe distal disease | 122 (29%) | 91 (27%) | 31 (36%) | .14 |
| Tandem occlusions | 53 (13%) | 31 (9%) | 22 (26%) | .001 |
| Microchannels | 86 (20%) | 75 (22%) | 11 (13%) | .04 |
| Ostial/side branch location | 163 (38%) | 120 (35%) | 43 (51%) | .033 |
| Tapered stump | 208 (49%) | 171 (50%) | 37 (44%) | .12 |
| Rentrop grade 3 collateral flow | 206 (48%) | 171 (50%) | 35 (41%) | .09 |
| J score > 3 | 192 (45%) | 129 (38%) | 63 (74%) | .001 |
|
Data are expressed as no. (%) or mean ± standard deviation. |
||||
Procedural details are shown on table 3. The use of 8-Fr catheters and dual injections was significantly higher among successful cases with a strong trend towards retrograde approach and intravascular ultrasound guidance. Drug-eluting stents were deployed in most of cases and limus-eluting stents were the most widely used by far (79%). PCIs were performed on at least 1 additional vessel in about two-thirds of the patients from the 2 groups. Independent predictors of failure were previous CABG, moderate-to-severe lesion tortuosity, tandem occlusions, lack of dual injection, and CTOs diagnosed in the ACS setting (table 4).
Table 3. Procedural characteristics
| All (n = 424) | Success (n = 339) | Failure (n = 85) | P | |
|---|---|---|---|---|
| Femoral access | 265 (63%) | 215 (63%) | 50 (59%) | .39 |
| 8-Fr catheter | 207 (49%) | 175 (52%) | 32 (38%) | .03 |
| Dual injection | 367 (87%) | 302 (89%) | 65 (76%) | .02 |
| Intervention | ||||
| Drug-eluting stent | 294 (87%) | NA | ||
| Bare metal stent | 20 (6%) | NA | ||
| Drug-eluting and bare-metal stent | 15 (4%) | NA | ||
| Balloon | 10 (3%) | 1 (1.2%) | ||
| Retrograde approach | 94 (22%) | 69 (20%) | 25 (29%) | .07 |
| IVUS | 61 (14%) | 56 (17%) | 5 (6%) | .06 |
| Duration (min) | 105 ± 41 | 106 ± 42 | 102 ± 39 | .43 |
| Fluoroscopy dose (cGy/m²) | 26 037 ± 2066 | 26 403 ± 2222 | 24 867 ± 13 019 | .57 |
| Contrast volume (mL) | 367 ± 175 | 377 ± 177 | 327 ± 158 | .002 |
|
IVUS, intravascular ultrasound. |
||||
Table 4. Multivariate predictors
| Failed procedure | HR | HR | P |
|---|---|---|---|
| Previous CABG | 7.51 | 2.83-19.90 | .0001 |
| Moderate-to-severe tortuosity | 3.78 | 2.02-7.08 | .0001 |
| ACS setting | 2.42 | 1.26-4.61 | .008 |
| Tandem occlusion | 2.32 | 1.11-4.87 | .027 |
| Lack of dual injection | 2.43 | 1.14-5.55 | .027 |
| Cardiovascular mortality | HR | HR | P |
| Renal failure (< 60 mL/min) | 5.67 | 1.95-16.48 | .002 |
| LAD occlusion | 3.30 | 1.12-9.74 | .032 |
| Failed procedure | 7.14 | 2.44-20.0 | .0001 |
|
ACS, acute coronary syndrome; CABG, coronary artery bypass graft; LAD, left anterior descending coronary artery. |
|||
Twenty-six coronary dissections (6.2%) and 21 femoral hematomas (5%) were the most common procedural complications of all. In the course of the attempts, perforations occurred in 5 successful cases (1.5%) and in 9 failed cases (10.8%). However, emergent pericardiocentesis due to cardiac tamponade was required in 1 patient only. Contrast-induced nephropathy occurred in 8 successful cases (2.5%) and in 1 failed case (3.1%). One patient died during hospitalization due to cardiogenic shock that occurred 24 hours after a failed CTO attempt.
The differences seen among the 3 time periods led us to think that procedural technical advances, the operator’s increasing skills, and the improvements made in the assessment of the patients’ profile and selection of the lesions, contributed to the 87% rate of success reported at the final time frame. The temporal trends shown on table 5 describe the techniques developed in each corresponding period, not that all procedures were performed with that technique. Since June 2013 numerous cases have been successfully completed using the dissection/re-entry technique.The median follow-up was 39.7 months [22–102]. Follow-up information was available in 407 patients (99.8%). Clinical outcomes during follow-up are shown on table 6.
Table 5. Temporal trends in baseline angiographic characteristics, procedural data, and results
| 1st period (2002-2007) | 2nd period (2007-2011) | 3rd period (2011-2017) | Total | P | |
|---|---|---|---|---|---|
| Age | 62 ± 16 | 63 ± 11 | 64 ± 11 | 63 ± 12 | NS |
| Multivessel disease | 61.7% | 60.1% | 82% | 70% | .0001 |
| ACS setting | 36.1% | 21.8% | 23.6% | 24.3% | .025 |
| Previous CABG | 9.8% | 10.0% | 3.9% | 7.3% | .020 |
| LAD | 27.9% | 33.3% | 28.5% | 30.4% | NS |
| Length (mm) | 23 ± 14 | 22 ± 13 | 21 ± 18 | 23 ± 16 | NS |
| J score > 3 | 45.0% | 44.8% | 45.8% | 45.2% | NS |
| Rentrop grade 3 cc. | 44.8% | 62.8% | 39.4% | 48.6% | .0001 |
| Femoral access | 49.2% | 68.0% | 62.6% | 62.5% | .016 |
| 8-Fr catheter | 11.7% | 58.3% | 54.3% | 48.8% | .0001 |
| Dual injection | 65.0% | 90.1% | 92.1% | 86.5% | .0001 |
| Retrograde approach | 1.6% | 23.3% | 28.8% | 22.1% | .0001 |
| IVUS | 21.2% | 18.3% | 11.1% | 14.4% | .033 |
| Fluoroscopy time (cGy/m²) | 33245 | 30310 | 19830 | 26037 | .0001 |
| Contrast volume (mL) | 453 ± 208 | 434 ± 178 | 281 ± 127 | 367 ± 175 | .0001 |
| Success rate | 57% | 81% | 87% | 80% | .001 |
| More widely used wires | Polymer coated wires (Whisper ES or MS, Pilot 50, 150 or 200, Abbott Vascular, United States) and tapered, stiff wires (Confianza Pro 12, Asahi Intecc., Japan). Used in 58%. | Runthrough wire (Terumo, Japan) and nontapered, stiff wires (Miracle 3 or 6 and Ultimate Bross 3, Asahi Intecc., Japan). Used in 62%. | Runthrough and nontapered, stiff wires widely used (59%). Sion and Gaia wires (Asahi Intecc., Japan) were used in 21%. | ||
| Specific devices and techniques | Antegrade approach only. Parallel and seesaw wiring techniques. | Retrograde approach, Corsair specific catheter (Asahi Intecc., Japan) and Guideliner (Vascular Solutions, United States). Kissing and reverse CART techniques. | Double lumen Nhancer catheter (Interventional Medical Device Solutions, The Netherlands). Hybrid techniques with the CrossBoss and Styngray catheters (Boston Scientific, United States). | ||
|
ACS, acute coronary syndrome; CABG, coronary artery bypass graft; ES, extra support; IVUS, intravascular ultrasound; LAD, left anterior descending coronary artery; MS, medium support. |
|||||
Table 6. Clinical outcomes at follow-up
| All (n = 424) | Success (n = 339) | Failure (n = 85) | P | |
|---|---|---|---|---|
| Overall mortality | 64 (15.1%) | 40 (11.8%) | 24 (28.2%) | .001 |
| Cardiovascular mortality | 24 (5.7%) | 12 (3.6%) | 12 (14.1%) | .001 |
| Myocardial infarction | 10 (2.4%) | 8 (2.4%) | 2 (2.4%) | .99 |
| Target vessel revascularization | 45 (10.6%) | 34 (10.1%) | 11 (12.9%) | .44 |
| Target lesion revascularization | 40 (9.5%) | 31 (9.2%) | 9 (10.6%) | .69 |
| CTO stent thrombosis | ||||
| Definite | 5 (1.5%) | NA | ||
| Probable | 1 (0.3%) | NA | ||
| MACE | 68 (16.1%) | 47 (13.9%) | 21 (24.7%) | .0015 |
|
CTO, chronic total coronary occlusion; MACE, major adverse cardiovascular events (cardiovascular death, myocardial infarction or need for surgical or percutaneous target vessel revascularization). |
||||
In the success group, 33 restenosis (9.7%) were angiographically diagnosed, 42% of which ended up being occlusive. Target lesion revascularization was achieved in 31 of these restenotic lesions (9.2%). Four of the 5 cases of definite thrombosis corresponded to a successfully opened right coronary artery.
One case of severe radiodermatitis was identified and it was successfully treated with local surgery 6 years after the intervention.
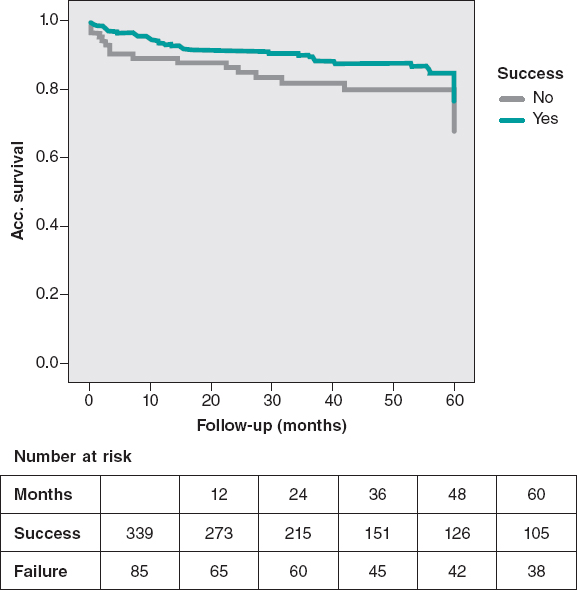
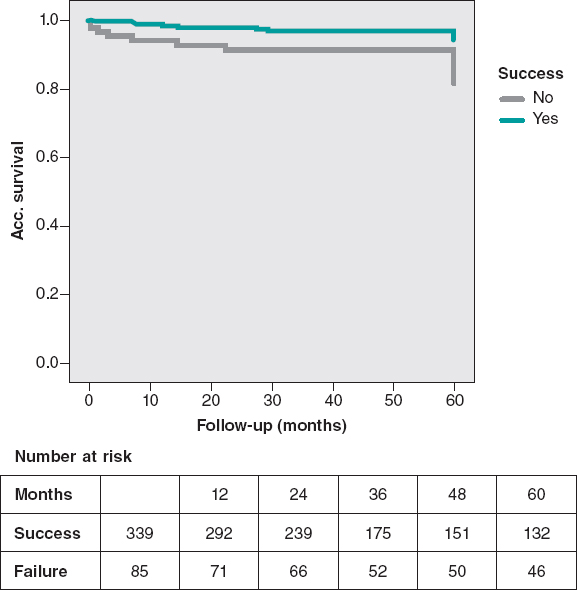
A remarkable difference in MACE was observed in favor of the success group, mainly driven by a lower rate of cardiovascular mortality. The cumulative cardiac survival and MACE survival curves associated with the success or failure of the PCI are shown on figure 2 and figure 3.
Figure 2. MACE-free at the 5-year follow-up. The rate of MACE (cardiovascular death, infarction, and surgical or percutaneous TVR) was lower in successful cases (P = .03).
MACE, major adverse cardiovascular events (cardiovascular death, myocardial infarction or need for surgical or percutaneous TVR); TVR, target vessel revascularization.
Figure 3. Cardiovascular mortality-free at the 5-year follow-up. Significant lower cardiovascular mortality in the success group (P = .005).
The multivariate analysis confirmed that a past medical history of chronic kidney disease with creatinine clearance < 60 mL/min, LAD occlusions, and procedural failure were independent predictors of cardiovascular mortality (table 4). Actually, increased mortality-related success rates were only seen in cases of failed LAD-CTO recanalization attempts compared to failed non-LAD CTO attempts (35% vs 9% P = .012).
DISCUSSION
These are the main results of this registry: a) the higher rates of success seen over the last 15 years confirm the improvements made in CTO recanalization devices and in the operator’s skills; b) the recanalization of CTOs shows high rates of success (80.0%) and low rates of complications; c) the rates of success were significantly lower in patients with previous CABG, moderate-to-severe lesion tortuosity, tandem occlusions, lack of dual injection, and patients with CTO treated in the ACS setting; d) successful procedures, especially in LAD occlusions, were associated with lower rates of cardiovascular mortality and MACE at the long-term follow-up.
The recanalization of the CTO is still uncertain and is not yet supported by randomized clinical trials. Several retrospective observational studies8-10 provide evidence that support this strategy. The results found in this analysis are consistent with previously published data, but disagree with others.11,12. In this sense, the more recent registries show better results regarding cardiovascular and overall mortality.13,14
Regarding randomized clinical trials, the EUROCTO trial revealed that the PCI of a CTO improves health status with improvements in angina frequency in patients with stable angina.15 However, the EXPLORE trial did not reveal any differences in the left ventricular function of patients with ST-segment elevation myocardial infarction. The DECISION-CTO showed similar inter-group rates of death, MI, stroke or TLR in patients with ACS or stable angina at the 3-year follow-up.16,17 The most recent clinical trial (REVASC) did not show an improved regional myocardial function. Although it was underpowered to measure clinical outcomes, it showed the advantage of performing the PCI of a CTO for clinically-driven repeat revascularization.18
Several characteristics of the current study should be emphasized to put the results into perspective. We believe this series of CTOs to be the big picture of interventional cardiology regarding CTOs since the start of the drug-eluting stent era until the arrival of contemporary new technologies. The study is based on a large cohort of consecutive patients from a single center. Most of them had multivessel disease and were treated in different time frames according to a specific dedicated CTO program.
Among the procedural characteristics that could explain the lower rates of success obtained with CTOs in the ACS setting we found the lowest use of retrograde approach and 8-Fr catheters in non-adequately staged procedures.
Regarding procedural features, the use of IVUS was limited to cases that required assessment of the distal vessel diameter and to optimize procedures with severe calcifications. It is very likely that more IVUS-guided procedures should have been performed.
Regarding variables related to procedural outcomes in the multivariable analysis, previous CABGs and more complicated CTOs were associated with failure as shown by other registries.13 However, intralesional tortuosity seems to us like the most consistent multivariable predictor with greater contribution to the model due to its narrow confidence interval. It might be possible that the inclusion of several angiographic variables in the regression model is responsible for the J-score not becoming an independent predictor. The high rate of retrograde procedures reveals the complexity of the CTOs in our series with J score > 3 in 45% of cases.
After dividing the series into 3 different periods of time, significant improvements in the rates of success were emphasized. As a result, we saw some interesting changes over time, such as the contribution of the retrograde approach to success. Considering that 73% of retrograde procedures were successful, it can be said that this technique led to a 19% increase in the rates of success in absolute terms. The rate of complications was quite similar to that from other studies.2-4,8-14
Our data provide additional evidence on the lower rate of cardiovascular mortality reported in patients with successful CTO recanalization in the long-term follow-up. As a matter of fact, the success of the PCI was a strong independent predictor of survival as several observational studies and 1 meta-analysis have consistently suggested.13,14,19
Possible explanations of the survival benefit from revascularizing a CTO may include a better left ventricular function and more tolerance for future acute coronary occlusive events.20 However, this cannot be confirmed as we didn’t measure the left ventricular ejection fraction systematically during follow-up. However, the trend showing a worst clinical profile in failed CTOs would validate this statement.
The role of LAD occlusions is decisive, as it seems an independent predictor of mortality. It should be mentioned that this effect of LAD occlusions on cardiovascular mortality was basically due to the higher mortality rate of failed cases compared to LAD recanalization attempts. The fact that LAD CTOs are much easier to open than CTOs located in other vessels makes LAD attempts not only feasible but also mandatory.
In conclusion, we think that this study —performed in a contemporary single cardiac catheterization laboratory for a long period of time practice in the drug eluting stent era— features new information on procedural results and long-term outcomes on CTO recanalizations.
This study was a prospective analysis and is subject to the limitations inherent to this type of research. The study does not allow us to draw any comparisons with other therapeutic strategies like medical therapy or CABG. Patients with failed procedures had different clinical and angiographic characteristics, which may have impacted prognosis.
Angiographic characteristics were not analyzed in a core lab but provided by a local investigator. There was no adjudication of clinical outcomes by a clinical events committee.
CONCLUSIONS
The implementation of a specific PCI program for CTOs has been associated with higher rates of success over time thanks to growing expertise and new technical advances. The rate of procedural success was lower when there was a history of previous CABG, moderate-to-severe lesion tortuosity, tandem occlusions, lack of dual injection, and in CTOs diagnosed in the ACS setting. Preserved renal function and successful recanalization —especially of the LAD— were associated with a lower rate of cardiovascular mortality in the long-term follow-up.
CONFLICTS OF INTEREST
J.M. de la Torre is the Editor-in-chief of REC: Interventional Cardiology; the editorial procedure established by REC: Publications was followed to guarantee the fair and unbiased handling of the manuscript.
WHAT IS KNOWN ABOUT THE TOPIC?
- CTOs are the most complex lesions to treat, and the prognostic benefit associated with their recanalization has not been properly established and if so, it could be selective.
- Most registries are limited in size and feature results in restricted time frames, often focused on specific devices, and not on long-term outcomes.
- The results of specific CTO programs in the long run have not been reported.
WHAT DOES THIS STUDY ADD?
- Our study describes the very long-term evolution of a PCI program for CTOs including the management and outcomes of PCI attempts on CTOs from 2002-2017.
- Our data, collected since the start of the drug-eluting stent era, confirm that implementing a program leads to higher rates of success over time. Independent predictors of PCI failure were identified in this large cohort.
- Lower rates of cardiovascular mortality were found in patients with successful recanalizations in the long-term follow-up.
- Also, the study provided new insights on the role played by LAD-CTO recanalizations on better outcomes.
REFERENCES
1. Anderson HV, Shaw RE, Brindis RG, et al. A contemporary overview of percutaneous coronary interventions. The American College of Cardiology-National Cardiovascular Data Registry (ACC-NCDR). J Am Coll Cardiol. 2002;39:1096-1103.
2. Suero JA, Marso SP, Jones PJ, et al. Procedural outcomes and long-term survival among patients undergoing percutaneous coronary intervention of chronic total occlusion in native coronary arteries:a 20-year experience. J Am Coll Cardiol. 2001;38:409-414.
3. Hoye A, van Domburg RT, Sonnenschein K, et al. Percutaneous coronary intervention for chronic total occlusions:the Thoraxcenter experience 1992-2002. Eur Heart J. 2005;26:2630-2636.
4. Noguchi T, Miyazaki S, Morii I, et al. Percutaneous transluminal coronary angioplasty of chronic total occlusions. Determinants of primary success and long-term clinical outcome.Cathet Cardiovasc Interv.2000;49:258-264.
5. Di Mario C, Sorini Dini C MD, Werner GS. Thousand Registries Are Not Worth a Randomized Trial. Also True for Chronic Total Occlusions?J Am Coll Cardiol Intv. 2017;10:1535-1537.
6. Di Mario C, Werner GS, Sianos G, et al. European perspective in the recanalisation of Chronic Total Occlusions:consensus document from the EuroCTO Club. Eurointervention. 2007;3:30-43.
7. Morino Y, Kimura T, Hayashi Y, et al. In-hospital outcomes of contemporary percutaneous coronary intervention in patients with chronic total occlusion insights from the J-CTO Registry (Multicenter CTO Registry in Japan). J Am Coll Cardiol Intv. 2010;3:143-151.
8. Olivari Z, Rubartelli P, Piscione F, et al.;TOAST-GISE Investigators. Immediate results and one-year clinical outcome after percutaneous coronary interventions in chronic total occlusions:data from a multicenter, prospective, observational study (TOAST-GISE). J Am Coll Cardiol. 2003;41:1672-1678.
9. Aziz S, Stables RH, Grayson AD, et al. Percutaneous coronary intervention for chronic total occlusions:improved survival for patients with successful revascularization compared to a failed procedure. Catheter Cardiovasc Interv. 2007;70:15-20.
10. Valenti R, Migliorini A, Signorini U, et al. Impact of complete revascularization with percutaneous coronary intervention on survival in patients with at least one chronic total occlusion. Eur Heart J. 2008;29:2336-2342.
11. Prasad A, Rihal CS, Lennon RJ, et al. Trends in outcomes after percutaneous coronary intervention for chronic total occlusions. A 25-year experience from the Mayo Clinic. J Am Coll Cardiol. 2007;49:1611-1618.
12. Labriolle A, Bonello B, Roy P, et al. Comparison of Safety, Efficacy, and Outcome of Successful Versus Unsuccessful Percutaneous Coronary Intervention in “True“Chronic Total Occlusions. Am J Cardiol.2008;102:1175-1181.
13. Borgia F,Viceconte N, Ali O, et al. Improved cardiac survival, freedom from MACE and angina-related quality of life after successful percutaneous recanalization of coronary artery chronic total occlusions. Int J Cardiol. 2012;161:31-38.
14. Tsai TT, Stanislawski MA, Shunk KA, et al. Contemporary Incidence, Management, and Long-Term Outcomes of Percutaneous Coronary Interventions for Chronic Coronary Artery Total Occlusions Insights From the VA CART Program. J Am Coll Cardiol Intv. 2017;10:866-875.
15. Werner GS. A Randomized Multicentre Trial to Evaluate the Utilization of Revascularization or Optimal Medical Therapy for the Treatment of Chronic Total Coronary Occlusions (EuroCTO) trial. Presented at;the PCR Congress Scientific Session 2017:Paris. Available online:https://www.pcronline.com/Cases-resources-images/Resources/Course-videos-slides/2017/Late-breaking-trials-and-trial-updates2?auth =true. Accessed 19 Dec 2019.
16. Park SJ. Drug-Eluting Stent Implantation Versus Optimal Medical Treatment in Patients With Chronic Total Occlusion (DECISION-CTO) trial. Presented at:the American College of Cardiology (ACC) 2017 Scientific Session. Washington, DC. Available online: http://www.acc.org/latest-in-cardiology/clinical-trials/2017/03/17/08/40/decision-cto. Accessed 19 Dec 2019.
17. Henriques JP, Hoebers LP, Råmunddal T, et al. :EXPLORE Trial Investigators. Percutaneous Intervention for Concurrent Chronic Total Occlusions in Patients With STEMI:The EXPLORE Trial. J Am Coll Cardiol.2016;68:1622-1632.
18. Mashayekhi K. REVASC:a randomized trial to assess recovery of left ventricular function after PCI of coronary artery chronic total occlusions. Presented at;TCT 2017. October 31, 2017:Denver, CO). Available online: https://www.tctmd.com/news/revasc-cto-pci-does-not-improve-lv-function-seems-provide-symptom-relief. Accessed 19 Dec 2019.
19. Khan MF, Wendel CS, Thai HM, et al. Effects of percutaneous revascularization of chronic total occlusions on clinical outcomes:a meta-analysis comparing successful versus failed percutaneous intervention for chronic total occlusion. Catheter Cardiovasc Interv. 2013;82:95-107.
20. Silva JC, Rochitte CE, Junior JS, et al. Late coronary artery recanalization effect on left ventricular remodelling and contractility by magnetic resonance imaging. Eur Heart J. 2005;26:36-43.
ABSTRACT
Introduction and objectives: Complex calcified lesions can affect stent expansion and lead to stent failure and adverse outcomes. Intracoronary lithotripsy (ICL) has emerged as a new tool that enables calcium modification. The Disrupt CAD II clinical trial has recently evaluated the safety and feasibility of ICL in patients with stable coronary disease and calcified coronary lesions. Although its use has increased rapidly, the experience already reported with this new device is limited. We report the results in real-life complex patients with heavy coronary calcification.
Methods: From October 2018 to March 2019, 25 patients (37 calcified lesions) were treated in 2 Spanish centers, which accounted for 2.7% of the patients treated with percutaneous coronary intervention.
Results: The device and clinical success rates were 84% and 95%, respectively. No procedure-related complications were seen. The crossing rate of the ICL balloon was 100% and balloon rupture during inflation occurred in 8%. The ICL was performed in a subset of highly complex lesions like left main coronary artery lesions and chronic total coronary occlusions. Compared to the Disrupt CAD II trial, our patients were younger but their clinical scenario was worse with a higher prevalence of diabetes (68%), renal failure (22%), and up to 76% suffered from acute coronary syndrome. The ICL failed to reach proper expansion in 3 out of 4 cases of stent underexpansion. The procedure was performed safely, and clinical and device success were high with no in-hospital mortality. One patient died of non-cardiac causes at the 30-day follow-up.
Conclusions: The ICL-assisted percutaneous coronary intervention was performed safely and effectively in a real-life cohort of patients with calcified and highly complex lesions.
Keywords: Lithotripsy. Calcium. Shockwave.
RESUMEN
Introducción y objetivos: Las lesiones coronarias calcificadas pueden impedir una correcta expansión del stent que en ocasiones conduce a eventos adversos. La litotricia intracoronaria es una nueva herramienta de modificación de la placa, cuyas seguridad y viabilidad en pacientes con enfermedad coronaria estable han sido evaluadas en el ensayo Disrupt CAD II. Aunque su uso ha aumentado rápidamente, hasta el momento solo se han comunicado casos aislados en escenarios concretos. Se presentan los resultados en pacientes clínicamente complejos de la vida real con calcificación coronaria grave.
Métodos: Entre octubre de 2018 y marzo de 2019 se trató a 25 pacientes (37 lesiones) en 2 centros españoles, lo que representa el 2,7% de los pacientes tratados con intervención coronaria percutánea.
Resultados: Las tasas de éxito clínico y del dispositivo fueron del 84 y el 95%, y no se observaron complicaciones relacionadas con el procedimiento. En todos los casos se consiguió cruzar la lesión con el balón de litotricia intracoronaria, si bien en el 8% de los casos se rompió el balón durante el inflado. Se trataron con éxito lesiones complejas, como oclusiones coronarias totales y estenosis del tronco común. En comparación con el estudio Disrupt CAD II, nuestros pacientes eran más jóvenes, pero tenían peor escenario clínico, con mayor prevalencia de diabetes (68%) e insuficiencia renal (22%), y hasta el 76% se presentó como síndrome coronario agudo. En 3 de 4 pacientes con infraexpansión de stent tratados con litotricia intracoronaria no se consiguió una expansión adecuada tras el procedimiento. No hubo complicaciones ni mortalidad hospitalaria. Un paciente falleció por causa no cardiaca a los 30 días de seguimiento.
Conclusiones: La litotricia intracoronaria se ha demostrado efectiva y segura en una cohorte de pacientes complejos de la vida real con lesiones calcificadas.
Palabras clave: Litotricia intracoronaria. Calcio. Ondas de choque.
Abreviaturas CTO: chronic total coronary occlusion. ICL: intracoronary lithotripsy. OCT: optical coherence tomography. PCI: percutaneous coronary intervention.
INTRODUCTION
Percutaneous coronary intervention (PCI) in calcified coronary lesions is often challenging and may be associated with suboptimal stent expansion and apposition both related to stent failure due to stent thrombosis and in-stent restenosis.1-3 The balloon angioplasty used in calcified lesions increases the risk of dissection of non-calcified segments usually without significant modification of calcified plaques and often without a proper luminal gain.4 The management of this subset of lesions is complex and often requires complex techniques such as rotational atherectomy or excimer laser coronary atherectomy.
Intracoronary lithotripsy (ICL) (Shockwave Medical, Freemont, CA, United States) has emerged as a new tool to modify calcium by applying a diffuse acoustic pulse through a balloon inflated at 4 to 6 atmospheres without damage to endovascular soft tissues. The multicenter, prospective, single-arm Disrupt CAD II clinical trial5 has recently evaluated the safety and feasibility of the ICL system prior to stent implantation in 120 patients with coronary artery disease and calcified coronary lesions. This study showed that the ICL appeared feasible with favorable initial success and complication rates in selected patients.5 Although its use has grown rapidly among interventional cardiologists and there are many case reports on the medical literature available to us, the experience already reported on this new device is quite limited. We present the initial results of lithotripsy-assisted PCIs in a real-life cohort of high-risk patients with complex, calcified lesions.
METHODS
Patient population and data collection
Two-center, prospective, observational registry including all consecutive PCI cases that required ICL prior to stent implantation to the operator’s discretion from October 2018 to March 2019. The baseline characteristics and procedural and in-hospital outcomes were prospectively recorded.
Intracoronary lithotripsy procedure
The ICL system is a portable and rechargeable generator connected to the ICL catheter. The catheter consists of a rapid exchange semi-compliant 12-mm balloon with 2 radiopaque emitters mounted inside available in 2.5, 3.0, 3.5, and 4 mm diameters. The catheter is compatible with a 6-Fr guiding catheter with a crossing profile range of 0.042 in and it is placed across the calcified lesion through a 0.014 in guidewire. Once in position, the balloon is inflated at 4 atmospheres to make intimate contact with the vessel wall and facilitate an efficient the transfer of energy. An electrical discharge from the emitters vaporizes the fluid inside the balloon generating sonic pressure waves that create a localized field effect. The ICL catheter is connected to a generator preprogrammed to deliver 10 pulses at a rate of 1 pulse per second. Each catheter can administer a maximum of 80 pulses. The sonic pulses through the soft vascular tissue cause selective microfractures at the intimal and medial calcium level of the vessel wall. After the pulse emission and the corresponding modification of calcium, the balloon is inflated up to 6 atmospheres to maximize luminal gain.
Definitions and outcomes
The use of the ICL catheter was based on the presence of a significant and severely calcified lesion (70% stenosis in an epicardial coronary vessel) on the angiography or intravascular imaging.
Coronary calcified lesions were defined by: a) the presence of radiopacities prior to contrast injection often involving both sides of the arterial wall; b) the presence of ≥ 270 degrees of calcium on at least one single cross-section on the intravascular ultrasound or optical coherence tomography (OCT); or c) subsets of calcified lesions with previous failed revascularization attempts.
According to the Disrupt CAD II trial criteria,5 lithotripsy delivery was considered successful when it facilitated stent delivery with < 50% residual stenosis and without any serious angiographic complications like severe dissection, perforation, slow flow or persistent no-reflow. In addition, clinical success was defined as residual stenosis < 50% after stenting without any evidence of in-hospital adverse events. We also assessed procedural complications such as PCI-related myocardial infarction (type 4a myocardial infarction, defined according to the fourth universal definition of myocardial infarction),6 and in-hospital and 30-day outcomes.
Statistical analysis
Categorical variables were expressed as number (percentage) and continuous variables as mean ± standard deviation or median according to their distribution. We analyzed all data using the STATA statistical package version 15.0 (StataCorp LP, College Station, Texas, United States).
RESULTS
Patients
Between October 2018 and March 2019, 25 patients with 37 calcified lesions were treated, which amounted to 2.7% of the patients treated with PCIs in both centers. The baseline characteristics of the patients are shown on table 1. Mean age was 71 ± 9 years and 68% of the patients were males. The traditional cardiovascular risk factors were common and the vast majority of patients had undergone a previous revascularization (PCI or coronary artery bypass graft). The indication for PCI was acute coronary syndrome in most cases (76%), all of them non–ST-elevation myocardial infarctions.
Table 1. Baseline characteristics (per patient)
| Clinical characteristics | N = 25 |
|---|---|
| Age, years | 71 ± 9 |
| Male sex | 17 (68) |
| Diabetes | 17 (68) |
| Renal failure | 7 (28) |
| Peripheral vascular disease | 8 (32) |
| Previous PCI | 14 (56) |
| Previous CABG | 3 (12) |
| LVEF | 49 ± 17 |
| ACS on admission | 19 (76) |
|
ACS, acute coronary syndrome; CABG, coronary artery bypass graft; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention. Data are expressed as no. (%) or mean ± standard deviation. |
|
Procedural characteristics
Procedural characteristics are shown on table 2. The mean SYNTAX score (Synergy between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery) was 19.3 ± 2, and the left anterior descending artery was the most-treated vessel. A non-negligible proportion of complex coronary artery lesions like chronic total coronary occlusions (CTO), bifurcation and ostial lesions, and stent underexpansion were also treated.
Table 2. Procedural characteristics
| Lesion characteristics | N = 37 |
|---|---|
| Protected LMCA | 1 (3) |
| Unprotected LMCA | 4 (11) |
| LAD | 17 (46) |
| LCx | 3 (8) |
| RCA | 12 (32) |
| Syntax Score | 19.3 ± 2 |
| Stent underexpansion treatment | 4 (11) |
| Ostial lesions | 13 (35) |
| Bifurcation lesion | 13 (35) |
| CTO | 3 (8) |
| Lesion severity by QCA | N = 37 |
| Pre-PCI diameter stenosis | 81.6 ± 2.5 |
| Post-PCI diameter stenosis | 15.9 ± 3.4 |
| Pre-PCI area stenosis | 84.6 ± 3.8 |
| Post-PCI area stenosis | 21.6 ± 3.5 |
| Total lesion length, mm | 20.7 ± 3 |
| Mean luminal diameter, mm | 0.77 ± 0.1 |
| Procedural characteristics | N = 25 |
| Radial access | 15 (60) |
| Mechanical support Impella device | 5 (20) |
| Fluoroscopy time, min | 31.5 ± 4.8 |
| Contrast, mL | 212 ± 28 |
| Number of vessels treated (per patient) | 1.3 ± 0.5 |
| Number of lesions treated (per patient) | 1.7 ± 0.8 |
| PCI characteristics | N = 37 |
| Pre-ICL balloon pre-dilatation | 23 (62) |
| Pre-ICL rotational atherectomy | 10 (27) |
| Pre-ICL cutting balloon | 2 (5) |
| ICL number of pulses | 46 ± 19 |
| ICL balloon rupture | 3 (8%) |
| Number of stents implanted | 1.2 ± 0.6 |
| Stent diameter, mm | 3.3 ± 1 |
| Stent length, mm | 23.1 ± 10 |
| Angiographical success | 35 (95) |
|
CTO, chronic total coronary occlusion; ICL, intracoronary lithotripsy; LAD, left anterior descending coronary artery; LCx, left circumflex artery; LMCA, left main coronary artery; PCI, percutaneous coronary intervention; QCA, quantitative coronary angiography; RCA, right coronary artery. Data are expressed as no. (%) or mean ± standard deviation. The ICL was used after a failed attempt of balloon pre-dilatation in 62% of the lesions, of which 10% showed balloon rupture. Ten lesions (27%) had previously been treated with rotational atherectomy. Only one lithotripsy catheter per lesion was required, and the mean number of pulses was 46 ± 19. The crossing rate of the lithotripsy balloon was 100% in pre-dilated and non-pre-dilated lesions, and the ICL balloon rupture occurred in 3 cases (8%) with no associated complications. An OCT prior to the ICL therapy was performed in a small percentage of cases (10%) to the operator’s discretion. All stents implanted were drug-eluting stents and successful angiographic result according to the definition was achieved in 95% of cases. |
|
Intracoronary lithotripsy in complex lesions
A subset of complex lesions was also treated with lithotripsy balloon (table 2). Success rate was 100% for left main coronary artery revascularizations, 100% for CTOs, and 86% for bifurcations.
Left main coronary artery lesions
Five patients with left main coronary artery lesions were treated with the ICL balloon. Four were unprotected lesions and were treated under hemodynamic support using the Impella device (all of them showed a severely depressed ejection fraction and/or a right coronary artery chronic total occlusion). Device and clinical success were achieved in all cases.
Bifurcation lesions
Seven lesions treated involved bifurcations, 4 were treated using a provisional stenting technique, and 3 cases were treated using the 2-stent technique (V stenting).
Chronic total coronary occlusions
The CTOs of 3 patients were treated using the ICL with complete success in all of them. The first patient had a severely calcified aorto-ostial lesion in the right coronary artery, a Japanese chronic total coronary occlusion score (Japanese Multicenter CTO Registry) of 2 (presence of calcification ≥ 20 mm in length). After unsuccessful pre-dilation using 2 balloons (1 of them ruptured) the ICL was performed and good balloon expansion was achieved without need for post-dilatation prior to the stenting. The second successfully treated CTO case involved the mid portion of the left anterior descending coronary artery (bifurcation according to the Medina classification 1,1,1), Japanese CTO score of 2 (calcification ≥ 20 mm in length) that had been treated with rotational atherectomy prior to the ICL. The third case was the CTO of a distal right coronary artery involving bifurcation (according to the Medina classification 1,0,0) and a Japanese CTO score of 3 (blunt-tip entry, calcification ≥ 20 mm in length). The artery was dilated using 5 balloons, some of which ruptured before performing the ICL. Lesion expansion was completed with a cutting balloon after the ICL, which allwed proper stent implantation.
Stent underexpansion
Four cases of stent underexpansion were treated, but results after the ICL were only successful in one case. There was 1 case that needed additional very high-pressure balloon dilatation (up to 40 atmospheres) for proper expansion, and 2 cases that remained unexpanded even after very high-pressure balloon dilatation (up to 40 atmospheres), and in-stent rotational atherectomy with 1.75 and 2.00 mm burrs.
In-hospital and 30-day outcomes
The procedure was performed safely in all cases. Both the clinical and device success were high with no in-hospital mortality. One patient died of non-cardiac causes at the 30-day follow-up (sepsis due to spontaneous bacterial peritonitis in the presence of hepatic cirrhosis). Procedural, in-hospital, and 30-day outcomes are shown on table 3.
Table 3. In-hospital and 30-day outcomes
| Clinical outcomes | N = 25 |
|---|---|
| Procedural complications | |
| Dissection | 0 (0) |
| Perforation | 0 (0) |
| No-reflow | 0 (0) |
| Type 4a acute myocardial infarction | 3 (12) |
| In-hospital mortality | 0 (0) |
| 30-d myocardial infarction | 0 (0) |
| 30-d target-vessel revascularization | 0 (0) |
| 30-d stent thrombosis | 0 (0) |
| 30-d mortality | 0 (0) |
| Cardiac death | 0 (0) |
| Non-cardiac death | 1 (4) |
|
Data are expressed as no. (%) or mean ± standard deviation. |
|
DISCUSSION
We present our initial experience with ICL in a cohort of real-life clinically complex patients with heavy coronary artery calcification and showed that the ICL is feasible with favorable initial outcomes and low complication rates.
Debulking techniques like rotational atherectomy, orbital atherectomy or excimer laser coronary atherectomy are commonly used to treat calcified coronary lesions. Back in 2018, in Spain up to 1517 patients were treated with rotational atherectomy and 88 with excimer laser coronary atherectomy.7 Recently, the ICL has emerged as an attractive option for the management of patients with severely calcified coronary lesions. Nevertheless, the experience reported on this new technique is still limited. The recently published single-arm Disrupt CAD II clinical trial confirmed the safety and performance of ICL to treat calcified coronary lesions.5 However, the clinical characteristics of the patients enrolled in this trial show a relatively low-risk population. Complex calcified coronary lesions are a common thing and they amount to 25% to 30% of all PCIs performed.3 Among our population, 2.7% of patients were considered eligible to receive ICL therapy, indicative of a highly demanding indication criterion. Compared to the Disrupt CAD II clinical trial5 our patients were younger but had a worse clinical scenario with a higher prevalence of diabetes (68% vs 32%) and renal failure (22% vs 9%), and up to 76% had suffered an acute coronary syndrome (none in the Disrupt CAD II trial). Another recent report described the initial experience with ICL in a cohort of 26 patients with calcified coronary lesions with findings for the clinical characteristics and results similar to the Disrupt CAD II.8
Regarding the procedure, it should be noted that the crossing rate for the ICL balloon was 100% despite a high percentage of plaque preparation was required (62% balloon pre-dilatation, 27% rotational atherectomy). Recently, the combination of rotational atherectomy and ICL has been described as RotaTripsy, suggestive that these 2 calcium debulking techniques may be complementary, since rotational atherectomy facilitates the ICL balloon crossing, and the latter facilitates proper expansion in the presence of circumferential deep calcium plaques.9 The device success rate was 84% (100% in the Disrupt CAD II linical trial) and the clinical success rate was 95% (94% in the Disrupt CAD II trial). And most important of all, no major procedural complications were seen, which is consistent with the Disrupt CAD II trial results. The rupture of the ICL balloon during inflation occurred in 3 cases (12%) without associated complications, yet the rupture of the balloon has been described in a case report resulting in a type C coronary dissection; the interventional cardiologist needs to be aware of this lithotripsy-related potential complication.10 Intravascular imaging were performed in few cases probably because the operator thought it would be difficult to cross an especially severe and calcified lesion with the OCT or IVUS catheter. Consistent with the results of the Disrupt CAD I and II clinical trials and OCT substudy,5,11 it was confirmed that the modification of calcium and the presence of fractures lead to an acute area gain and favorable stent expansion in the lesions assessed through OCT in our series. Figure 1 shows the coronary angiography and OCT of one complex patient treated with ICL; the red arrows seen on figure 1D,E indicate calcium fractures after the ICL.
Figure 1. Intracoronary lithotripsy, angiography, and optical coherence tomography. Patient with severe coronary artery heart disease with severely depressed left ventricular ejection fraction previously treated with coronary artery bypass graft (venous graft-left anterior descending coronary artery, currently occluded). Treatment of left main coronary artery, left anterior descending artery, and diagonal branches. A: pre-intracoronary lithotripsy angiography. B: Impella-assisted PCI of left anterior descending coronary artery. Arrow indicates inflated lithotripsy balloon. C: successful final angiographic result after stenting. D, E and F: optical coherence tomography cross-sectional images of a post-lithotripsy calcified lesion. Red arrows indicate calcium microfractures after intracoronary lithotripsy.
We used the ICL in a subset of highly complex lesions like left main coronary artery stenosis, CTO, stent underexpansion, and bifurcation lesions.
Five complex patients with calcified left main coronary artery and severe stenosis were treated with ICL; mechanical support with the Impella device was needed in 4 patients due to a depressed left ventricular ejection fraction. Recently, a case report with 2 patients that were successfully treated with ICL in a left main coronary artery stenosis has also been published.12 The ICL seems like a safe treatment option to treat calcified left main coronary artery stenoses even in technically complex cases that require hemodynamic support, a clinical scenario where the use of rotational atherectomy or excimer laser coronary atherectomy is rare.
Three patients with CTO lesions were successfully treated with ICL. Treatment of CTO with ICL has been previously described in 2 case reports. The first one was a patient with a CTO in the proximal right coronary artery. In this case, the ICL allowed the reverse controlled antegrade/retrograde tracking at the location of heavy calcification at the site of the chronic occlusion.13 The second case was a patient with a proximal right coronary artery CTO due to heavily calcified in-stent restenosis. The ICL achieved good lesion expansion prior to stent implantation.14 If performed properly, the ICL can be an alternative to other debulking techniques in heavily calcified CTO lesions to guarantee proper lesion expansion.
Several case reports of stent underexpansion due to heavily calcified lesions successfully treated with ICL have been reported recently.15,16 Surprisingly, in our series the ICL failed to achieve proper stent expansion in 3 out of the 4 cases attempted. The management of stent underexpansion using ICL should be performed with extra caution because sound waves can damage the metallic structure of the stent.
The management of calcified bifurcation lesions is often complex due to the high risk of side branch occlusion when applying debulking techniques such as rotational atherectomy or excimer laser coronary atherectomy because the treatment cannot be performed using a guidewire for side branch protection purposes.17,18 The ICL allows us to treat the main branch bifurcation with a guidewire in the side branch to guarantee quick access in case of flow impairment just like conventional procedures do.
Compared to atherectomy or specialty balloons, the ICL is said to offer several potential advantages5 and requires no specific training as the device is delivered similar to the standard catheter-based PCI. ICL therapy is balloon based, and, therefore, the risk of atheromatous embolization may be lower compared to free debulking devices; according to the Disrupt CAD I (19) or Disrupt CAD II trial5 results, none of the patients from our series experienced no-reflow events and the rate of periprocedural myocardial infarction was relatively low. Whereas standard and specialty balloons are inflated at high atmospheric pressure to modify calcium, the ICL is typically performed at low atmospheric pressure balloon inflation, thus minimizing mechanical vascular trauma. Lastly, side-branch protection using a guidewire may be easily performed using ICL, without running the risk of wire entrapment or severing associated with rotational or orbital atherectomy. However, there is no evidence regarding stent restenosis of lesions treated with ICL therapy. New studies like the Disrupt CAD III trial that has just begun and will be recruiting up to 400 patients with a 2-year follow-up are needed to determine long-term outcomes.
Limitations
This 2-center experience using the ICL balloon has the limitations inherent to an observational study with a small sample size, which limits drawing conclusions especially in subgroups of high-risk lesions treated with ICL. However, in our opinion, it may contribute by adding more evidence supporting the use of ICL. This study did not have a comparison group either among existing plaque-modifying techniques.
CONCLUSIONS
In our own experience, the ICL-enhanced PCI was performed safely and effectively in a real-life cohort of complex patients with severely calcified and highly complex lesions.
CONFLICTS OF INTEREST
The authors of the manuscript declared no conflicts of interest.
WHAT IS KNOWN ABOUT THE TOPIC?
-
Calcified lesions continue to be a challenge for interventional cardiologists since poor plaque preparation prevents proper stent expansion, which leads to a higher rate of periprocedural complications and long-term adverse events.
-
The ICL balloon is a new plaque modification tool whose safety and efficacy in patients with stable coronary heart disease has recently been evaluated in a cohort of 120 patients in the Disrupt CAD II clinical trial.
-
The use of the ICL balloon has grown rapidly in the cardiac catheterization laboratories. However, to this day extensive series in real-life patients have not been reported yet.
WHAT DOES THIS STUDY ADD?
-
We present the results of the ICL balloon in real-life patients referred to undergo coronary angioplasty.
-
Although the size of the sample in our series was not big enough to draw any conclusions, the patients included were clinically complex and a high percentage of acute coronary syndromes and technically complex interventions (left main coronary artery lesions, bifurcation lesions, CTOs and stent underexpansion) was reported.
-
The procedure was performed safely and successfully in a large percentage of cases (95%). In-hospital mortality was zero and only one patient died at the 30-day follow-up (due to non-cardiac reasons).
REFERENCES
1. Bourantas CV, Zhang YJ, Garg S, et al. Prognostic implications of coronary calcification in patients with obstructive coronary artery disease treated by percutaneous coronary intervention:a patient-level pooled analysis of 7 contemporary stent trials. Heart. 2014;100:1158-1164.
2. Fitzgerald PJ, Ports TA, Yock PG. Contribution of localized calcium deposits to dissection after angioplasty. An observational study using intravascular ultrasound. Circulation. 1992;86:64-70.
3. Khattab AA, Otto A, Hochadel M, Toelg R, Geist V, Richardt G. Drug-eluting stents versus bare metal stents following rotational atherectomy for heavily calcified coronary lesions:late angiographic and clinical follow-up results. J Interv Cardiol. 2007;20:100-106.
4. Mehanna E, Bezerra HG, Prabhu D, et al. Volumetric characterization of human coronary calcification by frequency-domain optical coherence tomography. Circ J. 2013;77:2334-2340.
5. Ali ZA, Nef H, Escaned J, et al. Safety and effectiveness of coronary intravascular lithotripsy for treatment of severely calcified estenoses. The Disrupt CAD II Study. Circ Cardiovasc Interv. 2019;12:e008434.
6. Thygesen K, Alpert JS, Jaffe AF, et al. ESC Scientific Document Group;Fourth universal definition of myocardial infarction (2018). Eur Heart J. 2019;40:237-269.
7. Cid-Alvarez AB, Rodriguez-Leor O, Moreno R, Pérez de Prado A. Registro Español de Hemodinámica y Cardiología Intervencionista. XXVIII Informe Oficial de la Sección de Hemodinámica y Cardiología Intervencionista de la Sociedad Española de Cardiología (1990-2018). Rev Esp Cardiol. 2019. https://doi.org/10.1016/j.recesp.2019.07.023.
8. Wong B, El-Jack S, Newcombe R, Glenie T, Armstrong G, Khan A. Shockwave Intravascular Lithotripsy for Calcified Coronary Lesions:First Real-World Experience. J Invasive Cardiol. 2019;31:46-48.
9. Jurado-Román A, Gonzálvez A, Galeote G, Jiménez-Valero S, Moreno R. RotaTripsy:Combination of Rotational Atherectomy and Intravascular Lithotripsy for the Treatment of Severely Calcified Lesions. JACC Cardiovasc Interv. 2019;12:e127-e129.
10. López-Lluva MT, Jurado-Román A, Sánchez-Pérez I, Abellán-Huerta J, Lozano Ruíz-Poveda F. Shockwave:Useful But Potentially Dangerous. JACC Cardiovasc Interv. 2019;12:500-501.
11. Ali ZA, Brinton TJ, Hill JM, et al. Optical coherence tomography characterization of coronary lithoplasty for treatment of calcified lesions:first description. JACC Cardiovasc Imaging. 2017;10:897-906
12. Salazar CH, Travieso A, Gonzalo N, Escaned J. Intracoronary lithotripsy in percutaneous treatment of calcified left main coronary stenoses. JACC:Case Rep. 2019;1:46-49.
13. Yeoh J, Hill J, Spratt JC. Intravascular lithotripsy assisted chronic total occlusion revascularization with reverse controlled antegrade retrograde tracking. Catheter Cardiovasc Interv. 2019;93:1295-1297.
14. Azzalini L, Bellini B, Montorfano, Carlino M. Intravascular lithotripsy in chronic total occlusion percutaneous coronary intervention. EuroIntervention. 2019. https://doi.org/10.4244/EIJ-D-19-00175.
15. Ali ZA, McEntegart M, Hill JM, Spratt JC. Intravascular lithotripsy for treatment of stent under expansion secondary to severe coronary calcification. Eur Heart J. 2018. https://doi.org/10.1093/eurheartj/ehy747.
16. Watkins S, Good R, Hill J, Brinton TJ, Oldroyd KG. Intravascular lithotripsy to treat a severely under-expanded coronary stent. EuroIntervention. 2019;15:124-125.
17. Rawlins J, Din JN, Talwar S, O'Kane P. Coronary intervention with the Excimer Laser:review of the technology and outcome data. Interv Cardiol. 2016;11:27-32.
18. Nageh T, Kulkarni NM, Thomas MR. High-speed rotational atherectomy in the treatment of bifurcation-type coronary lesions. Cardiology. 2001;95:198-205.
19. Brinton TJ, Ali ZA, Hill JM, et al. Feasibility of shockwave coronary intravascular lithotripsy for the treatment of calcified coronary stenosis. Circulation. 2019;139:834-836.
Corresponding author: Departamento de Cardiología Intervencionista, Institut del Cor (ICOR), Hospital Germans Trias i Pujol, Ctra. de Canyet s/n, 08916 Badalona, Barcelona, Spain.
E-mail address: (O. Rodríguez-Leor).
ABSTRACT
Introduction and objectives: According to the recommendations of the latest clinical practice guidelines, non-ST-elevation acute myocardial infarction (NSTEMI) patients should undergo an invasive coronary angiography. However, the best moment to perform this coronary angiography has not been stablished yet. Our main objective was to see if performing an early angiography (within the first 24 h) in NSTEMI patients was associated with better prognosis compared to delayed angiography (beyond the first 24 h).
Methods: From January 2014 to June 2016, 447 consecutive patients were admitted to the acute cardiac care unit of a tertiary hospital with a diagnosis of NSTEMI. They all underwent catheterization. We classified them into 3 groups depending on the moment when the coronary angiography was performed (within the first 24 h after diagnosis, 24 h to 72 h later, and > 72 h after diagnosis).
Results: Coronary angiography was performed within the first 24 h in 285 patients (63.8%). There were no differences among the groups regarding gender, distribution of cardiovascular risk factors, past medical history of coronary disease or presence of other comorbidities. We found no differences among the 3 groups in variables with known prognostic impact. The cardiovascular events and 1-year mortality at follow-up were similar among the 3 groups.
Conclusions: In our study, in the whole spectrum of NSTEMI, early coronary angiography (within the first 24 h) did not show any clinical benefits regarding survival or fewer major adverse cardiovascular events.
Keywords: Acute coronary syndrome. GRACE score. Early angiography. Prognosis. Mortality.
RESUMEN
Introducción y objetivos: Las guías clínicas recomiendan la realización de una coronariografía en los pacientes con infarto agudo de miocardio sin elevación del segmento ST (IAMSEST). Sin embargo, no está claramente establecido el mejor momento para hacerla. Por ello, el objetivo del presente trabajo fue analizar si practicar un cateterismo precoz (durante las primeras 24 h) se relaciona con un mejor pronóstico, en comparación con hacerlo de manera diferida (más allá de las 24 h).
Métodos: De enero de 2014 a junio de 2016 ingresaron en la unidad de cuidados agudos cardiológicos de un hospital terciario 447 pacientes consecutivos con diagnóstico de IAMSEST a los que se hizo una coronariografía. Se clasificó de forma retrospectiva a los pacientes en 3 grupos en función del momento de realización del cateterismo: durante las primeras 24 h, entre las 24 y las 72 h tras el diagnóstico, y después de las primeras 72 h.
Resultados: El cateterismo se llevó a cabo en las primeras 24 h en 285 pacientes (63,8%). No se identificaron diferencias entre los grupos en cuanto a sexo, prevalencia de factores de riesgo cardiovascular ni presencia de comorbilidad. Tampoco se encontraron diferencias en las variables pronósticas analizadas ni en la mortalidad. En el seguimiento a los 12 meses, la incidencia de eventos cardiovasculares y la mortalidad fueron similares entre los grupos.
Conclusiones: En el presente estudio, la realización de una coronariografía precoz (en las primeras 24 h) a los pacientes ingresados por IAMSEST no mostró beneficio clínico en términos de supervivencia o reducción de eventos cardiovasculares.
Palabras clave: Síndrome coronario agudo. GRACE score. Cateterismo precoz. Pronóstico. Mortalidad.
Abbreviations: CA: coronary angiography. NSTEMI: non-ST-elevation acute myocardial infarction.
INTRODUCTION
Coronary angiography (CA) is a key step in treatment of patients with non-ST-elevation acute myocardial infarction (NSTEMI). CA reduces mortality and the rates of new cardiovascular adverse events compared to the conservative approach.1,2 Therefore, the current European clinical practice guidelines on the management of NSTEMI recommend an invasive strategy to treat these patients.1
The appropriate time to perform the CA in NSTEMI patients is still under discussion. Early CA (within the first 24 h after diagnosis) is still recommended in patients with high-risk NSTEMI defined as a GRACE score > 140. However, the potential benefit of this approach has not been completely established yet.3
The objective of our study was to assess the prognostic impact of an early CA (within the first 24 h after diagnosis) in patients NSTEMI compared to a delayed CA strategy (after 24 h).
METHODS
This is a retrospective, observational cohort study. From January 2014 to June 2016, data from 447 patients with NSTEMI admitted to a tertiary referral hospital who underwent an invasive coronary angiography were consecutively collected.
NSTEMI was defined according to the guidelines and all patients were treated following the recommendations established by these guidelines.1
Data from all the cases were included prospectively in a continuous multipurpose database. The collection of data included detailed past clinical histories, physical examinations, pulse oximetry measures, 12-lead electrocardiograms, continuous electrocardiogram monitoring, blood tests, echocardiographies, and CAs. The Global Registry of Acute Coronary Events (GRACE) and Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes with Early implementation of the ACC/AHA guidelines (CRUSADE) scores were calculated for each patient.1
Patients were classified into 3 groups according to the time to CA (figure 1): catheterization within the first 24 h after diagnosis (group 1, n = 285 patients), 24 h to 72 h later (group 2, n = 102 patients) and after 72 h (group 3, n = 60 patients). The decision on when to perform the CA was made by the treating physician in each case. After being discharged from the hospital, the 12-month follow-up of patients was performed in a dedicated clinic.
Figure 1. Flowchart. CA, coronary angiography; NSTEMI, non-ST-elevation acute myocardial infarction.
The primary endpoints of our study were mortality and major adverse cardiovascular events (stroke, new acute coronary syndrome, new revascularization) during hospitalization and depending on the time to CA in patients with NSTEMI. The secondary endpoints were mortality and the rate of major cardiovascular events at the 1-year follow-up, and bleeding events according to the BARC criteria.4 We also analyzed the antiplatelet treatment prescribed at discharge and its correlation with MACE at follow-up.
Statistical analysis
Continuous variables are described as mean and standard deviation or median and interquartile range [IQR] when appropriate. The Kolmogorov-Smirnov test was used to assess the variables normal distribution. Regarding quantitative variables, the groups were compared using the 2-tailed Student t test or the Mann-Whitney U test when necessary. Categorical variables were expressed as frequency and percentage, and compared using the chi-square test or Fisher’s exact test when appropriate. No variable had losses > 15%.
A multivariate logistic regression analysis was performed to assess the potential impact of the CA timing on in-hospital mortality. The model included all variables that were statistically significant in the univariate analysis regarding mortality and time to CA. Adjusted odds ratios (OR) with 95% confidence intervals (95%CI) were calculated for each variable. Regarding the secondary endpoint of 1-year mortality, a Cox regression analysis was performed to assess any potential prognostic factors.
All tests were 2-tailed and the differences were considered statistically significant with P values < .05. The statistical analysis was performed using the statistical software package IBM SPSS Statistics V 22.0.
RESULTS
Table 1 shows the baseline characteristics of the patient population. Patients in group 1 were younger (66.5 ± 13.5 years vs 71.1 ± 12.7 years in group 2, and 70.7 13.5) in group 3, P = .016). There were no gender differences among the groups (P = .565). The cardiovascular risk factors, previous coronary artery disease (P = .314), and presence of other comorbidities were similar among the groups (table 1).
Table 1. Baseline characteristics among the 3 study groups and antiplatelet therapy at discharge
| Group 1 (n = 285) | Group 2 (n = 102) | Group 3 (n = 60) | P | |
|---|---|---|---|---|
| Age (years) | 66.5 (13.5) | 70.7 (13.5) | 71.1 (12.7) | .016 |
| Sex (male) | 78.9% | 80.2% | 73.3% | .565 |
| Diabetes | 34.9% | 37.6% | 35.0% | .880 |
| Hypercholesterolemia | 63.4% | 54.5% | 55.9% | .195 |
| Hypertension | 71.1% | 71.3% | 73.3% | .942 |
| GRACE score | 157 (44.9) | 161 (45.7) | 170 (39.6) | .041 |
| CRUSADE score | 32.8 | 34.8 | 36.4 | .251 |
| GFR | 72 | 69.8 | 66 | .118 |
| Peak CK levels | 659.9 | 479.4 | 590 | .623 |
| LVEF at discharge | 49.6 | 54.2 | 52 | .229 |
| Killip class | .604 | |||
| I | 194 (71.3%) | 75 (72.7%) | 37 (62.7%) | |
| II | 32 (11.8%) | 15 (15.2%) | 9 (15.3%) | |
| III | 23 (8.5%) | 7 (7.1%) | 8 (13.6%) | |
| IV | 23 (8.5%) | 5 (5.1%) | 5 (8.5%) | |
| Mechanical ventilation | 29 (10.7%) | 6 (6.1%) | 5 (8.3%) | .636 |
| Number of vessels with severe stenosis | .488 | |||
| 1 | 133 (46.8%) | 45 (44.6%) | 23 (38.6%) | |
| 2 | 77 (27.1%) | 23 (22.8%) | 22 (36.7%) | |
| 3 | 68 (23.9%) | 29 (28.7%) | 14 (23.3%) | |
| Successful revascularization | 211 (89.8%) | 70 (92.1%) | 42 (91.3%) | .930 |
| Antiplatelet therapy at discharge | ||||
| Ticagrelor | 154 (54%) | 52 (50.9%) | 29 (48.3%) | .154 |
| Clopidogrel | 105 (36.8%) | 40 (39.2%) | 24 (40%) | .358 |
| Prasugrel | 26 (9.2%) | 10 (9.9%) | 7 (11.7%) | .469 |
|
CK, creatine kinase; GFR, glomerular filtration rate; LVEF, left ventricular ejection fraction. Group 1: coronary angiography within the first 24 h after diagnosis; group 2: 24 h to 72 h later; group 3: coronary angiography > 72 h after diagnosis. |
||||
A CA was performed within the first 24 h in 285 patients (63.8%). Surprisingly, we noticed that the patients from group 1 showed lower GRACE scores [157.67 (44.9) points vs 170 (39.5) points in group 3, (P = .041)] and similar CRUSADE scores compared to the other 2 groups (P = .251).
There were no significant differences among the groups in the Killip class at admission (table 1). The left ventricular ejection fraction and the peak values of cardiac biomarkers were similar among the groups. The presence of multivessel disease was similarly in the 3 study groups (table 1). There were no significant differences in the primary endpoint among the 3 study groups (table 2). During hospitalization, strokes and bleeding events occurred similarly in the 3 groups (table 2). It is important to emphasize here the low rate of bleeding events (5 patients with BARC 2 and 2 patients with BARC 3 events in group 1, and 3 patients with BARC 2 and 2 patients with BARC 3 events in groups 2 and 3, with no fatal events). At the 1-year follow-up, cardiovascular adverse events and 1-year mortality were similar among the 3 groups (table 2).
Table 2. In-hospital and follow-up rate of adverse events and mortality (expressed as percentage) among the 3 groups
| Group 1 (n = 285) | Group 2 (n = 102) | Group 3 (n = 60) | P | |
|---|---|---|---|---|
| In-hospital events | ||||
| Heart failure | 74 (25.9%) | 26 (25.4%) | 21 (36%) | .246 |
| Non-fatal AMI | 3 (1%) | 4 (3.9%) | 3 (6%) | .371 |
| Acute kidney injury | 47 (16.5%) | 18 (17.6%) | 15 (25%) | .334 |
| Stroke | 3 (1%) | 2 (1.9%) | 2 (3.3%) | .548 |
| Bleeding events | 20 (7%) | 6 (5.8%) | 6 (10%) | .213 |
| In-hospital mortality | 19 (6.6%) | 7 (6.8%) | 2 (3.4%) | .358 |
| Events at the 1-year follow-up | ||||
| Death | 17 (5.9%) | 5 (4.9%) | 5 (8.3%) | .114 |
| Stroke | 3 (1.05%) | 3 (2.9%) | 1 (1.6%) | .271 |
| Major bleeding | 7 (2.45%) | 6 (5.8%) | 4 (6.6%) | .427 |
| Myocardial infarction | 16 (5.6%) | 5 (4.9%) | 4 (6.6%) | .907 |
|
AMI, acute myocardial infarction. Group 1: coronary angiography within the first 24 h after diagnosis; group 2: 24 h to 72 h later; group 3: coronary angiography > 72 h after diagnosis. |
||||
Regarding medical treatment at discharge, a similar percentage of patients received clopidogrel, prasugrel and ticagrelor in the 3 study groups (table 1). In our cohort, antiplatelet therapy was not associated with differences in the rate of major adverse cardiovascular events and mortality at the 12-month follow-up.
The multivariate logistic regression analysis performed to predict mortality revealed that hypertension, Killip class IV at admission, left ventricular ejection fraction, and myocardial damage (defined as peak creatine kinase levels) were independently associated with higher in-hospital mortality rates. The time to CA was not an independent predictor of in-hospital mortality after the multivariate adjustment (table 3).
Table 3. Multivariate logistic regression analysis to predict in-hospital mortality
| Variable | Odds ratio (95%CI) | P |
|---|---|---|
| CA after 72 h | reference | |
| CA within the first 24 h | 0.98 (0.26-3.74) | .978 |
| CA 24 h to 72 h later | 1.33 (0.28-6.24) | .716 |
| Hypertension | 6.25 (1.09-33.3) | .04 |
| Age (per year) | 1.03 (0.98-1.08) | .292 |
| Successful revascularization | 0.51 (0.12-2.21) | .371 |
| Peak CK levels (per pg/mL) | 1.00 (1.00-1.01) | .010 |
| LVEF | 0.93 (0.90-0.97) | < .001 |
| Killip class at admission | ||
| I | reference | .026 |
| II | 3.39 (0.98-11.75) | .054 |
| III | 3.24 (0.92-11.36) | .067 |
| IV | 15.34 (2.19-107.58) | .006 |
|
95%CI, 95% confidence interval; CA, coronary angiography; CK, creatine kinase; LVEF, left ventricular ejection fraction. |
||
Regarding 1-year mortality, the Cox regression analysis showed similar results. The time to CA was non-significant in the multivariate analysis. Hypertension, age, left ventricular ejection fraction, and Killip class at admission were independently associated with higher mortality rates at 1 year (table 4).
Table 4. Multivariate Cox regression analysis to predict 1-year mortality
| Variable | Hazard ratio (95%CI) | P |
|---|---|---|
| CA after 72 h | reference | |
| CA within the first 24 h | 0.96 (0.46-2.03) | .919 |
| CA 24 h to 72 h later | 0.82 (0.33-2.07) | .677 |
| Hypertension | 3.64 (1.30-10.3) | .014 |
| Age (per year) | 1.04 (1.01-1.07) | .022 |
| Successful revascularization | 0.94 (0.41-2.13) | .876 |
| Peak CK levels (per pg/mL) | 1.00 (1.00-1.01) | .198 |
| LVEF | 0.96 (0.94-0.98) | < .001 |
| Killip class at admission | ||
| I | reference | |
| II | 2.83 (1.32-6.08) | .008 |
| III | 2.78 (1.27-6.09) | .010 |
| IV | 2.91 (0.83-10.2) | .096 |
|
95%CI, 95% confidence interval; CA, coronary angiography; CK, creatine kinase; LVEF, left ventricular ejection fraction. |
||
DISCUSSION
Our study included a large cohort of 447 consecutive patients with NSTEMI that were retrospectively analyzed. Our results showed that early CAs (defined as a CA performed within the first 24 h after diagnosis) in NSTEMI patients did not improve the prognosis of this cohort of patients compared to delayed CAs. No differences were seen among the 3 groups regarding the time to CA in the in-hospital cardiovascular adverse event rate, mortality rate or at the 12-month follow-up either.
Early CA, within the first 24 h after diagnosis, is currently recommended by the clinical practice guidelines for the management of patients with NSTEMI. However, this recommendation is based on the results of relatively old clinical trials and a meta-analysis.4-8 Several recent trials have explored the prognostic impact of the CA timing on NSTEMI patients in order to find stronger evidence in this clinical setting.9,10
The results of the TIMACS study (Timing of Intervention in Acute Coronary Syndromes) showed that an early CA was associated with a reduction in the composite endpoint of death, myocardial infarction or refractory ischemia compared to a delayed CA strategy.11
A retrospective cohort study that included 19 704 propensity scorematched patients hospitalized with a first acute coronary syndrome conducted between January 1, 2005 and December 31, 2011 showed that the use of an early invasive treatment strategy was associated with a lower risk for cardiovascular mortality and re-hospitalization due to myocardial infarction compared to a conservative invasive approach.12 However, it is important to emphasize the retrospective nature of this study and the fact that patients were followed for 60 days only.
However, a meta-analysis that combined data from 83 229 patients did not show any significant differences regarding mortality, myocardial infarction or major bleeding events between the 2 strategies.13
Another meta-analysis that included 8 randomized controlled trials (n = 5324 patients) with a median follow-up of 180 days [180-360] and compared an early invasive group of NSTEMI patients to a delayed strategy showed that the early invasive strategy did not reduce mortality in all NSTEMI patients including high risk patients with GRACE score > 140 points.14
Similarly, a recent meta-analysis that combined the results of 10 clinical trials did not find any differences in mortality, myocardial infarction or major bleedings among NSTEMI patients based on the CA timing. Nevertheless, the early CA strategy was associated with less recurrent angina and shorter hospital stays.15
The LIPSIA-NSTEMI study randomized patients with NSTEMI to undergo CA within the first 2 h after randomization (immediate CA strategy), 10 h to 48 h after randomization (early CA), and the so-called “selectively invasive” arm, in which patients initially received medical treatment without showing any differences in the infarct size among the 3 study groups.16
A recent randomized controlled trial conducted by a Kofoed et al., the VERDICT trial, included a total of 2147 patients of which 1075 were allocated to very early invasive evaluation (within the first 12 h after diagnosis), and 1072 to receive standard invasive care (CA 61.6 h after randomization).17 The primary endpoint was a composite of all-cause mortality, nonfatal recurrent myocardial infarction, refractory myocardial ischemia-related hospital admission or heart failure-related hospital admission. In this trial, the very early invasive coronary evaluation strategy did not improve overall the long-term clinical outcome compared to the invasive strategy performed within 2 to 3 days in patients with non-ST-segment elevation acute coronary syndrome. However, in patients with the highest risk, the very early invasive therapy improved long-term outcomes17 which is consistent with the results shown by the TIMACS trial.
Despite all these data, there is still controversy on what the best timing is to perform a CA in patients with NSTEMI.
An important limitation of previous studies is heterogeneity in the definition of early and late CA, and the differences seen in the primary endpoints.4-14 The lack of uniform criteria makes it difficult to compare the results. The definition of NSTEMI has changed over time. Thus, old clinical trials used a different criterion for the definition of NSTEMI and included different patients from those of current studies. We should try to identify what patients with the highest risk would benefit from an early invasive strategy. In this sense, previous studies did not use risk grading systems to classify patients. However, in our study we calculated the ischemic and bleeding risks of all patients. As our objective was to assess the potential benefit of an early invasive strategy among NSTEMI patients, the GRACE risk score was estimated in the entire study population. However, despite the high ischemic risk of our patients, no significant differences were found between the 2 strategies (early or delayed CA) regarding mortality or adverse events.
Limitations
Our study has several limitations that should be considered when interpreting the results. Although we included a large number of NSTEMI patients with a collection of high quality data, this is an observational, retrospective, single center study with the limitations of this type of study. Besides, the current clinical practice guidelines recommend the PRECISE-DAPT score to assess bleeding risk in this clinical setting. In our study bleeding risk at admission was classified according to CRUSADE score.
CONCLUSIONS
The results of our study show that the early CA strategy did not improve prognosis or reduce mortality in NSTEMI patients. However, larger studies are still needed to clarify which group of patients may benefit from early CA strategies.
CONFLICTS OF INTEREST
None declared.
WHAT IS KNOWN ABOUT THE TOPIC?
- Early CA is recommended by the current clinical practice guidelines in patients with a high-risk suffering from non-ST-elevation acute myocardial infarctions.
- To this day, clinical trials and meta-analyses show contradictory results without clear prognostic differences between the early CA strategy and delayed catheterization.
WHAT DOES THIS STUDY ADD?
- A large cohort of consecutive NSTEMI patients was retrospectively studied. We assessed in-hospital progression and cardiovascular events and mortality at the 1-year follow-up.
- The results of our study show that the early CA strategy did not imporive prognosis or reduced mortality in NSTEMI patients.
- No differences among the 3 groups were seen based on the CA timing regarding cardiovascular adverse events and mortality during the hospital stay or at the 12-month follow-up.
- No differences among the 3 groups were seen based on the CA timing regarding cardiovascular adverse events and mortality during the hospital stay and at the 12-month follow-up.
REFERENCES
1. Roffi M, Patrono C, Collet JP, et al. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation:Task Force for the Management of Acute Coronary Syndromes in Patients Presenting Without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:267-315.
2. Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes:executive summary:a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:2354-2394.
3. Reuter PG, Rouchy C, Cattan S, et al. Early invasive strategy in high-risk acute coronary syndrome without ST-segment elevation. The Sisca randomized trial. Int J Cardiol. 2015;182:414-418.
4. Vranckx P, White HD, Huang Z, et al. Validation of BARC Bleeding Criteria in Patients With Acute Coronary Syndromes:The TRACER Trial. J Am Coll Cardiol. 2016;67:2135-2144.
5. Montalescot G, Cayla G, Collet JP, et al. ABOARD Investigators. Immediate vs delayed intervention for acute coronary syndromes:a randomized clinical trial. JAMA.2009;302:947-954.
6. De Winter RJ, Windhausen F, Cornel JH, et al. Early invasive versus selectively invasive management for acute coronary syndromes. N Engl J Med.2005;353:1095-1104.
7. Hirsch A, Windhausen F, Tijssen JG, et al. Long-term outcome after an early invasive versus selective invasive treatment strategy in patients with non-ST-elevation acute coronary syndrome and elevated cardiac troponin T (the ICTUS trial):a follow-up study. Lancet.2007;369:827-835.
8. O'Donoghue M, Boden WE, Braunwald E, et al. Early invasive vs conservative treatment strategies in women and men with unstable angina and non- ST-segment elevation myocardial infarction:a meta-analysis. JAMA. 2008;300:71-80.
9. Badings EA, The SH, Dambrink JH, el al. Early or late intervention in high-risk non-ST-elevation acute coronary syndromes:results of the ELISA-3 trial. EuroIntervention.2013;9:54-61.
10. Milosevic A, Vasiljevic-Pokrajcic Z, Milasinovic D, et al. Immediate Versus Delayed Invasive Intervention for Non-STEMI Patients:The RIDDLE-NSTEMI Study. JACC Cardiovasc Interv.2016;9:541-549.
11. Mehta SR, Granger CB, Boden WE, et al. Early versus delayed invasive intervention in acute coronary syndromes. N Engl J Med. 2009;360:2165-2175.
12. Hansen KW, Sorensen R, Madsen M, et al. Effectiveness of an early versus a conservative invasive treatment strategy in acute coronary syndromes:a nationwide cohort study. Ann Intern Med. 2015;163:737-746.
13. Navarese EP, Gurbel PA, Andreotti F, et al. Optimal timing of coronary invasive strategy in non-ST-segment elevation acute coronary syndromes:a systematic review and meta-analysis. Ann Intern Med. 2013;158:261-270.
14. Jobs A, Mehta SR, Montalescot G, et al. Optimal timing of an invasive strategy in patients with non-ST-elevation acute coronary syndrome:a meta-analysis of randomized trials. Lancet.2017;390:737-746.
15. Bonello l, Laine M, Puymirat E, et al. Timing of Coronary Invasive Strategy in Non-ST-Segment Elevation Acute Coronary Syndromes and Clinical Outcomes:An Updated Meta-Analysis. JACC Cardiovasc Interv. 2016;9:2267-2276.
16. Thiele H, Rach J, Klein N, et al. Optimal timing of invasive angiography in stable non-ST-elevation myocardial infarction:the Leipzig Immediate versus early and late PercutaneouS coronary Intervention triAl in NSTEMI (LIPSIA-NSTEMI Trial). Eur Heart J. 2012;33:2035-2043.
17. Kofoed KF, Kelbæk H, Hansen PR, et al. Early Versus Standard Care Invasive Examination and Treatment of Patients With Non-ST-Segment Elevation Acute Coronary Syndrome. Circulation. 2018;138:2741-2750.
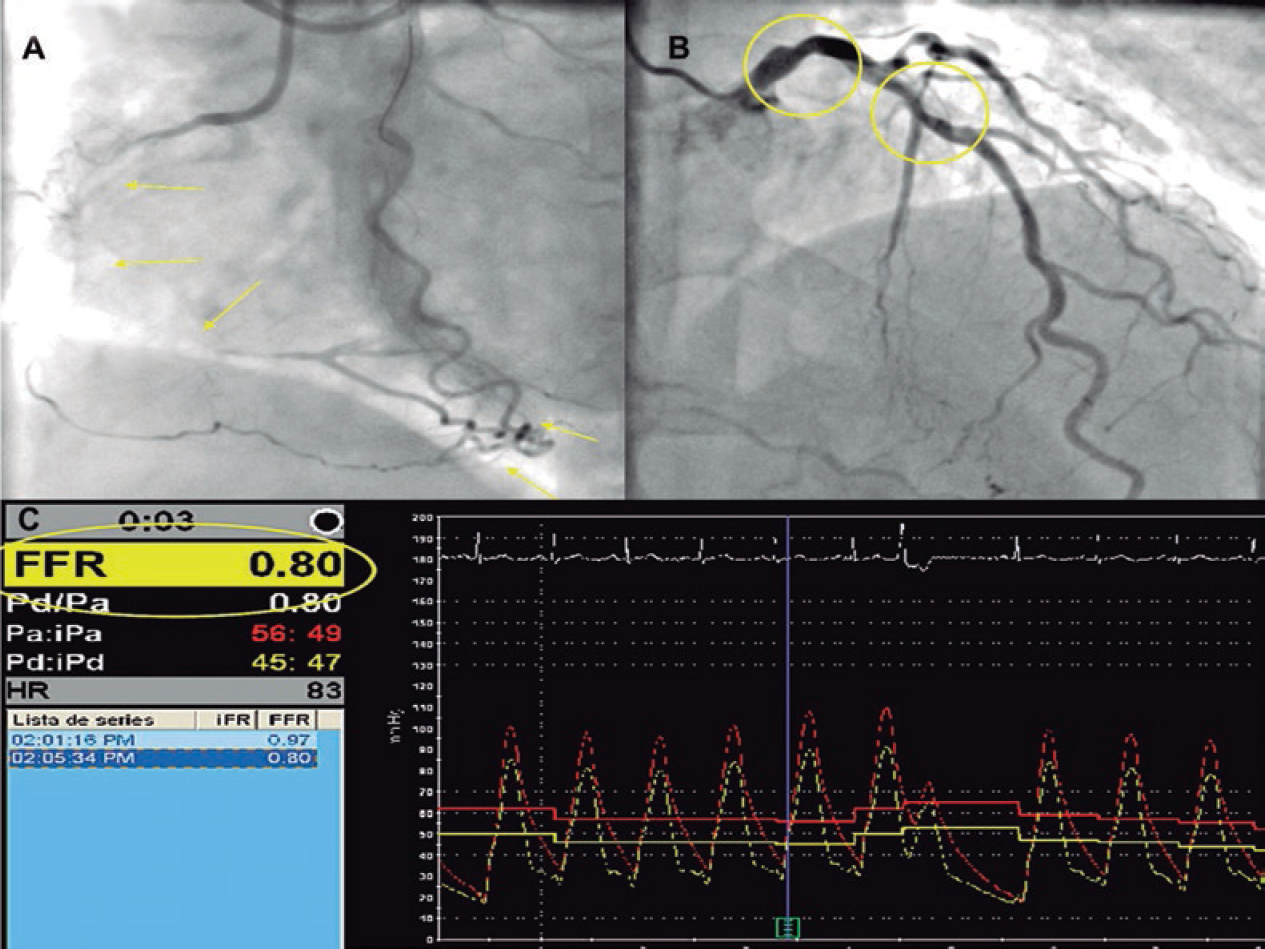
ABSTRACT
Introduction and objectives: The strategy of the percutaneous treatment of patients with multivessel disease associated with chronic total coronary occlusion (CTO) lesions is not well defined. Also, the functional significance of lesions located in the collateral donor artery has not been fully addressed. Using the fractional flow reserve (FFR) the objective was to evaluate the amount of ischemia related to the angiographically intermediate stenosis of collateral donor vessels before and immediately after successful percutaneous coronary intervention (PCI) of a CTO. Also, to assess any changes operated in the amount of ischemia using cardiovascular magnetic resonance imaging prior to the PCI and at 1-month follow-up.
Methods: Prospective pilot study including 14 patients with stable angina and a CTO receiving collateral circulation from a blood vessel with intermediate stenosis (50%-70% diameter stenosis measured using quantitative angiography). In order to indicate recanalization by PCI all patients were referred for magnetic resonance assessment of the presence of myocardial viability.
Results: Seven (50%) of the 14 patients included showed FFR values ≤ 0.80 before the PCI. FFR measures of the donor artery significantly increased after the revascularization of the CTO (0.75 [0.73-0.78] vs 0.83 [0.81-0.84]; P = .017). Eventually, only 3 patients showed hemodynamically significant FFR values after the recanalization of CTO requiring further revascularization. There was a tendency towards a reduction of the number of ischemic segments (2.5 [0-4] vs 0 [0-0.25]; P = .066) assessed using magnetic resonance imaging before and after the PCI. No major adverse cardiovascular events were reported at the 2-year follow-up.
Conclusions: Our data suggest that FFR measurements in intermediate stenoses of collateral donor vessels of a CTO may be misleading. Therefore, the strategy of focusing primarily on the revascularization of the CTO and then on the assessment of the intermediate lesion in a collateral donor vessel may be recommended.
Keywords: Chronic total coronary occlusion. Collateral donor vessel. Fractional flow reserve. Cardiovascular magnetic resonance imaging.
RESUMEN
Introducción y objetivos: La estrategia de tratamiento percutáneo de los pacientes con enfermedad multivaso y oclusión total crónica (OTC) no está bien definida. La importancia funcional de las lesiones localizadas en arterias donantes de colaterales no se ha abordado por completo. Nuestro objetivo fue evaluar mediante reserva fraccional de flujo (RFF) la cantidad de isquemia dependiente de una lesión angiográfica intermedia en un vaso donante de colaterales antes y después de la recanalización de la OTC, y valorar el cambio en la cantidad de isquemia por resonancia magnética cardiaca (RMC) antes y 1 mes después de la recanalización.
Métodos: Estudio piloto prospectivo en 14 pacientes con angina estable y una OTC que recibía circulación colateral de un vaso con una estenosis intermedia (50-70% por angiografía coronaria cuantitativa). Para indicar la revascularización, todos los pacientes presentaban viabilidad miocárdica por RMC.
Resultados: De los 14 pacientes, 7 (50%) evidenciaron una RFF ≤ 0,80 antes de la recanalización. Los valores medios de RFF de la arteria donante aumentaron significativamente tras la revascularización de la OTC (0,75 [0,73-0,78] frente a 0,83 [0,81-0,84]; p = 0,017). Solo 3 pacientes mostraron valores de RFF hemodinámicamente significativos después de la recanalización de una OTC que requirió revascularización adicional. Hubo una tendencia hacia una reducción del número de segmentos isquémicos (2,5 [0-4] frente a 0 [0-0,25]; p = 0,066) evaluados por RMC antes y después del intervencionismo. No se observaron eventos cardiacos adversos mayores durante el seguimiento de 2 años.
Conclusiones: Las mediciones de RFF en estenosis intermedias de vasos donantes de colaterales de una OTC pueden ser engañosas. En estos casos podría plantearse la estrategia de centrarse primero en la revascularización de la OTC y después en la evaluación de la lesión intermedia del vaso donante.
Palabras clave: Oclusión total crónica. Reserva fraccional de flujo. Resonancia magnética cardiaca. Vaso colateral donante.
Abreviaturas: CMR: cardiovascular magnetic resonance imaging. CTO: chronic total coronary occlusion. FFR: fractional flow reserve. PCI: percutaneous coronary intervention.
INTRODUCTION
The prevalence of chronic total coronary occlusions (CTO) is around 16% to 52% in patients with significant coronary artery disease on the angiography.1 In the presence of a CTO, collateral blood supply is often enought to maintain resting perfusion and contractility in the collateral-dependent myocardium.2 Restoration of antegrade flow by the percutaneous coronary intervention (PCI) of a CTO is associated with a rapid reduction in the collateral supply received in the treated vessel.3
Randomized trials support the use of fractional flow reserve (FFR) to guide the PCI with an established treatment threshold of ≤ 0.8.4-8 Although the FFR is reported to be independent of hemodynamic changes,9 it is intimately related to total coronary flow through a stenosis, which in turn is related to perfused myocardial mass.10 In keeping with this, there have been several reports of normalization of FFR values from collateral donor vessel after successful recanalization of a CTO.11 By removing nutrient flow to the collateralized territory by CTO recanalization, the collateral network almost immediately increased its resistance, thus favoring flow to the donor territory during maximal hyperemia.12
In patients with Rentrop grade-2 or grade-3 collateral flow, the FFR value of the donor artery increased at least 0.10 after revascularization of the recipient artery. However, the FFR value did not change significantly in patients with Rentrop grade-0 or grade-1 collateral flow following revascularization. This suggests that well-developed collateral circulation might overestimate the FFR value in the donor artery with mild stenosis.13
The assessment of myocardial-perfusion through cardiovascular magnetic resonance imaging (CMR) is a noninvasive imaging modality for the detection of coronary artery disease with a high degree of concordance with the FFR for ischemia detection.14-16 Also, the CMR has emerged as robust and reproducible method to assess the ischemia and viability of the myocardium related to the CTO.17-19 The MR-INFORM trial showed that in patients with stable angina and risk factors for coronary artery disease, the CMR of myocardial perfusion was associated with a lower incidence of coronary revascularization compared to the FFR and was noninferior to the FFR regarding major adverse cardiovascular events (all-cause mortality, non-fatal myocardial infarction or target-vessel revascularization) at 12 months.20 However, it is uncertain whether opening a CTO can modify the amount of ischemia related to an angiographically intermediate lesion of the collateral donor vessel. It could also be possible to diagnose microvascular dysfunction using CMR.21
Therefore, in this pilot study, using the FFR we assessed changes in the amount of ischemia related to the angiographically intermediate stenosis of collateral donor vessel before and immediately after the successful PCI of a CTO. We also tried to determine any changes in the amount of ischemia using the CMR prior to the PCI and 1 month after recanalization.
METHODS
In this prospective pilot study, we included patients with stable angina and CTO with collateralization of the distal vascular bed, and collateral donor vessel with a single angiographically intermediate lesion (50%-70% diameter stenosis by quantitative coronary angiography). In order to indicate recanalization through PCI all patients were referred for CMR evaluation to assess the presence of myocardial viability. During the procedure, the FFR of the donor vessel was measured before the PCI of the CTO (figure 1). Only with FFR values ≤ 0.80, the measure was reassessed after the procedure (figure 2). A second CMR was performed 1 month after the index PCI. All patients gave their informed consent, the local ethics committee approved the study, and all procedures were performed in accordance with the Helsinki Declaration. The study population was clinically followed for 2 years. The rate of major adverse cardiovascular events was established. This was defined as a composite of all-cause mortality, non-fatal acute myocardial infarction (AMI), clinically-driven target vessel revascularization or rehospitalization due to unstable or progressive angina according to Braunwald Unstable Angina Classification. The exclusion criteria were: prior IAM; failed recanalization of the CTO, inability to obtain signed written informed consents; severity of valvular heart disease; acutely decompensated chronic heart failure; asthma or obstructive sleep apnea; high risk of bleeding; known hypersensitivity or contraindication to aspirin; nursing subjects; patients with pacemakers/implantable cardioverter- defibrillators.
Figure 1. Example of chronic total coronary occlusion (CTO) of right coronary artery (panel A, yellow arrows) with collateralization of distal vascular bed, and left main and left anterior descendent artery (LAD) as the collateral donor vessel shows an angiographically intermediate lesion (panel B, yellow circles). During the procedure, the fractional flow reserve (FFR) of the donor vessel was measured before the percutaneous coronary intervention of the CTO (panel C).
Figure 2. Example of the recanalization of chronic total coronary occlusion (CTO) of the right coronary artery (panel A) with left anterior descendent artery (LAD) as the collateral donor vessel shows an angiographically intermediate lesion (panel B, yellow circles). Panel C: after the CTO repermeabilization, the fractional flow reserve (FFR) value of the LAD increased (FFR value = 0.91).
The percutaneous coronary intervention
The PCI was performed using bilateral femoral artery access and 7-Fr sheaths and guide catheters. Anticoagulation was achieved with 100 U/Kg of unfractionated heparin to maintain activated clotting times of 250-300 msec. All the procedures on the CTO were performed using the antegrade wire escalation technique. All patients were treated with drug-eluting stent implantation. The J-CTO score was calculated for each CTO lesion and assessed taking the following parameters into consideration: occlusion length, stump morphology, presence of calcification, presence of tortuosity and prior attempt to open the CTO.22 Collateral flow was graded in accordance with Rentrop collateral flow classification.23 Procedural success was defined as achievement of residual post-PCI stenosis < 30% in the target lesion associated with TIMI grade-3 flow without mortality, IAM or new lesion revascularization during the index hospitalization.
Assessment using fractional flow reserve
To measure FFR in the intermediate coronary lesions a 0.014-inch pressure-monitoring guidewire (Prime Wire Volcano Therapeutics, Inc, Rancho Cordova, CA, United States) was used. After calibration of both the aortic and wire pressures, the FFR wire was advanced until the tip of the guiding catheter. Equalization of both pressures was performed. Then, the wire was advanced and positioned distally at least 15 mm from the stenotic lesion followed by the administration of 0.2 mg of nitroglycerin to avoid any form of epicardial vasoconstriction. Maximal hyperemia was induced through the IV infusion of adenosine (180 µg/kg/min). After reaching the steady state we measured the FFR as the ratio between mean distal coronary pressure and mean aortic pressure. Values < 0.80 were considered significant from the hemodynamical standpoint. After FFR measurement and under maximal hyperemia, the pressure wire was pulled back until the sensor was close to the tip of the guiding catheter to make sure that no drift had occurred.
Cardiovascular magnetic resonance imaging
All CMR studies were performed using a General Electric Signa HDxt 1.5-T scanner equipped with an 8-channel coil and cardiac-dedicated software. Perfusion studies were conducted using a gradient-echo turbo-field sequence prescribed in the left ventricular short-axis orientation, at the basal, mid-ventricular and apical levels after 4 min of IV administration of adenosine (Atepo-din) at a dose of 180 µg/kg/min and simultaneous administration of 0.1 mmol/kg of gadobutrol (Gadovist, Bayer Hispania) at a 5 mL/s rate. The functional and volumetric assessment of the left ventricle (LV) was conducted using the conventional Steady State Free Precession (SSFP) cine sequence, prescribed in sequential short-axis slices, and encompassing the entire LV and the 2-, 3-, and 4-chamber views. The typical temporal and in-plane spatial resolution of these images was 40 ms and 1.4 × 1.4 mm, respectively. Rest perfusion images were obtained at least 10 min after the stress perfusion study using the same sequence, location, and contrast injection protocol. Ten minutes after administering the dose of gadolinium for the rest perfusion study, late gadolinium-enhanced images were obtained using a segmented inversion-recovery spoiled gradient echo sequence in the same location and identical spatial resolution as the cine images. To calculate left ventricular ejection fraction (LVEF), the LV mass and left ventricular end-systolic and end-diastolic volumes, the endocardial and epicardial borders were manually traced at end-systole and end-diastole in the cine short-axis images using a dedicated software package (ReportCard, GE). The regional wall motion analysis was performed by visual grading of the cine images according to the 17-segment model proposed by the American Heart Association.17 The pre- and post-PCI image analysis was conducted by 2 independent experienced operators masked to the patient’s coronary anatomy and the PCI results; the disparities in their evaluation were resolved by consensus with a third independent operator. The appropriate allocation between the involved myocardial segments and the correspondent coronary anatomy in each case was evaluated according to previously reported criteria.18
Statistical analysis
The distribution of continuous variables was assessed by visual inspection of frequency histograms and using the Shapiro–Wilk test. Continuous variables were expressed as mean ± standard deviation (SD) or median with interquartile range (IQR) when they followed a normal or non-normal distribution, respectively. The continuous variables were compared using the unpaired Student t test or Mann–Whitney U test and the categorical variables were compared using the chi-square test or Fisher’s exact test, as appropriate. Correlations between variables were conducted using the Pearson test. The software SPSS 17.0 (SPSS Italy, Florence, Italy) was used for statistical analyses.
RESULTS
We screened 23 patients with stable angina and CTO with collateralization of distal vascular bed, and collateral donor vessel with angiographically intermediate lesion. We excluded 9 patients who showed some exclusion criteria. Fourteen patients were finally included in the study (figure 3). The clinical characteristics and angiographic details are shown on table 1. Seven intermediate lesions (50%) of the collateral donor vessels showed FFR values ≤ 0.80 before the recanalization of the CTO. On average, FFR measures significantly increased after CTO revascularization (0.75 [0.73-0.78] vs 0.83 [0.81-0.84]; P = .017) (table 2 and figure 4). Four patients normalized their FFR values, while in the other 3 the FFR remained hemodynamically significant and required subsequent PCI. There was a tendency towards a reduction of the number of ischemic segments assessed through CMR before and after the recanalization of the CTO (2.5 [0-4] vs 0 [0-0.25]; P = .066). No differences were found in other parameters including the number of hypokinetic segments, left ventricular ejection fraction, left ventricular end-diastolic and end-systolic volumes; left ventricular mass; and necrotic mass before and after the PCI (table 2). In addition, the number of ischemic segments did not significantly correlate with the FFR values before or after PCI (R2 = -0.31, P = .328; R2 = -0.68, P = .20, respectively). Finally, no major adverse cardiovascular events were reported during the 2-year follow-up.
Figure 3. We screened 23 patients with stable angina and chronic total occlusion (CTO) with collateralization of distal vascular bed, and collateral donor vessel with angiographically intermediate lesion; 9 of them were excluded after meeting the exclusion criteria. In particular, 3 contraindications for dual antiplatelet therapy, 1 valvular heart disease requiring surgery, 3 refusals to sign the informed consent, and 3 pacemakers. CMR, cardiovascular magnetic resonance; PCI, percutaneous coronary intervention.
Table 1. Clinical and angiographic characteristics
| Clinical characteristics | Patients (n = 14) |
|---|---|
| Age, years | 67.44 ± 12.9 |
| Male | 12 (85) |
| Hypertension | 6 (42.8) |
| Smoking | 2 (14.3) |
| Hyperlipidemia | 10 (71.4) |
| Diabetes Mellitus | 5 (35.7) |
| Renal failure | 2 (14.3) |
| Prior CABG | 1 (7.1) |
| Medical treatment | |
| Beta-blockers | 5 (35.7) |
| Calcium antagonist | 2 (14.3) |
| ACE inhibitor | 4 (28.5) |
| Statins | 10 (71.4) |
| Angiographic characteristics | |
| CTO vessel | |
| LAD | 2 (14.3) |
| LCX | 1 (7.1) |
| RCA | 11 (78.6) |
| Calcification | 7 (50%) |
| Bending > 45 degrees | 2 (14.3) |
| Tapered | 8 (57.1) |
| Occlusion length, mm | 24.6 [6-43.3] |
| Rentrop > 1 | 13 (92.8) |
| J-CTO score > 2 | 3 (21.4) |
| Collateral donor vessel | |
| LAD | 7 (50) |
| LCX | 4 (28.6) |
| RCA | 3 (21.4) |
| Stenosis degree | 52 [50-55] |
|
Data are expressed as n (%), mean ± standard deviation or median [interquartile range]. ACE, angiotensin converting enzyme; CABG, coronary artery bypass grafting; CTO, chronic total occlusion; IQR, interquartile range; JCTO, Japanese CTO; LAD, left anterior descending artery; LCX, left circumflex artery; RCA, right coronary artery. |
|
Figure 4. Fractional flow reserve (FFR) values of 7 angiographically intermediate lesions in the collateral donor vessels before and after the percutaneous coronary intervention (PCI) of a chronic total coronary occlusion.
Table 2. FFR and CMR measures in the study population
| Before PCI (n = 7) | After PCI (n = 7) | P | |
|---|---|---|---|
| Pd/Pa | 0.93 (0.88-0.96) | 0.91 (0.89-0.93) | 1.00 |
| FFR | 0.75 (0.73-0.78) | 0.83 (0.81-0.84) | .017 |
| IS | 2.5 (0.0-4.0) | 0.0 (0.0-0.25) | .066 |
| HS | 1.0 (0.0-4.75) | 0.0 (0.0-0.50) | .15 |
| LVEF, % | 60.5 (55.0-63.25) | 63.5 (54.0-65.25) | .41 |
| LVEDV, ml | 111.3 (102.7-451.1) | 109.0 (100.6-139.2) | .50 |
| LVESV, ml | 41.1 (38.6-65.17) | 38.9 (35.2-81.4) | .49 |
| LV mass, gr | 83.4 (56.4-92.1) | 88.5 (69.1-110.2) | .50 |
| NM, gr | 0.83 (0.3-2.3) | 0.92 (0.4-1.5) | 1.0 |
|
CMR, cardiovascular magnetic resonance imaging; FFR, fractional flow reserve; HS, hypokinetic segments; IS, ischemic segments; LV, left ventricular; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume; NM, necrotic mass; Pd/Pa: resting distal coronary pressure to aortic pressure ratio; PCI, percutaneous coronary intervention. Data expressed as median (interquartile range). |
|||
DISCUSSION
These are the main findings of the study: a) functional assessment of intermediate lesions located in the collateral donor artery showed significantly lower FFR values than it would have in the absence of collateralized CTOs; b) after the recanalization of the CTO, the FFR values of the collateral donor artery normalized in most of patients; c) the amount of ischemia assessed through CMR used to decrease after successful CTO recanalization; d) no major adverse cardiovascular events were reported in our population at the long-term follow-up.
The FFR is a method used to assess the functional significance of coronary stenosis while taking in account the following parameters: severity of stenosis, myocardial territory and viability, and collateral perfusion.19 Results from the FAME trial showed that FFR-guided PCI was superior to the angiography-guided PCI at 1 and 2 years in terms of death or AMI and AMI alone.5,11 In the FAME 2 trial, the FFR-guided PCI reduced the rate of major adverse cardiovascular events compared to medical therapy alone.6 To this day, physiology has been proposed to outline which stenoses should be treated in the context of multivessel disease.24 However, there is uncertainty around what the waiting time is before performing an accurate pressure wire assessment of donor arteries after the successful recanalization of a CTO. Several studies have shown that full collateral regression does not happen immediately after the successful revascularization of a CTO.3 During embryonic development, collaterals derive either from capillary sprouting or pre-existing arteriolar connections.25 Collateral growth occurs through 2 major processes: arteriogenesis and angiogenesis. The former, stimulated by physical forces, consists of the growth, positive remodeling, and expansion of preexisting collateral vessels. The latter, induced by hypoxia, is the de novo growth of new capillaries by sprouting or intussusception from pre-existing vessels.26 Although once established, coronary collaterals are believed to persist and can be re-recruited, this process does not happen immediately. Well-developed collateral vessels close when the pressure gradient across the collateral network disappears. Also, the time needed to reopen the closed collaterals after reestablishing the pressure gradient seems to be directly related to the time interval between coronary occlusions.27 Recently, Mohdnazri et al. have showed that the successful recanalization of a right coronary artery CTO resulted in a modest but statistically significant and immediate increase of instantaneous wave-free ratio (iFR) in the predominant donor vessel following the recanalization of the CTO. At 4 months, both the FFR and the iFR showed significant improvement compared to pre-PCI values together with a concomitant reduction of collateral function.28 Ladwiniec et al. showed that the recanalization of a CTO resulted in a modest FFR increase of the predominant collateral donor vessel associated with a reduced coronary flow, of a similar magnitude at baseline and maximal hyperemia.29 Few patients of our study did not show this improvement. The persistence of non-angiographically visible collateral circulation, the presence of microcirculation dysfunction and type of prior collateral circulation grade,30 and distal embolization or myonecrosis following PCI recanalization may be potential causes of this lack of improvement. In this regard, in a recent study, measurements repeated shortly after the PCI of a CTO showed transient procedural-related changes like microvascular dysfunction secondary to distal embolization, catecholamine release, left ventricular stunning or hyperemic stimulus related to side-branch occlusion.29
Our data suggest that in the setting of CTOs and an angiographically intermediate lesion of the collateral donor vessel, it seems like the FFR measurement may be misleading. Therefore, it seems advisable to postpone the assessment of intermediate stenoses until achieving the successful recanalization of the associated CTO. This approach should avoid overtreating patients who only require the revascularization of their CTOs. On the contrary, if the recanalization of the CTO fails, treating the intermediate stenosis in the donor artery may be necesary to reduce ischemia in this territory. It also still is a good practice to try to re-open the CTO prior to performing any interventions on the donor vessel, due to the risk of extensive acute ischemia in case of troublesome PCIs.
Moreover, we did not find any correlations between the amount of ischemia assessed through CMR and the FFR values before or after the PCI. As far as we know, this is the first comparison between CMR and FFR assessment of an angiographically intermediate lesion in a collateral donor vessel related a CTO. Former studies have suggested that the CMR underestimates or that the FFR overestimates the number of ischemic segments in multi-vessel disease.31-32 This discrepancy seems to highlight the poor accuracy of the FFR method in the presence of collaterals involving territories that are from the target lesion to be assessed.
Finally, after treating the patients according to the FFR measures obtained after the PCI of a CTO, no major adverse cardiovascular events were detected at the 2-year follow-up.
Limitations
Several limitations should be acknowledged. First, due to the small size of the sample our findings should be, at best, hypothesis- generating findings. Secondly, we only used FFR as hyperemic index; other indices (eg. iFR, IMR, etc.) were not assessed. Similarly, we could not assess the influence of microcirculation through CMR or hyperemic microvascular resistance. Third, we did not assess whether collateral circulation originated from a segment proximal or distal to the target stenosis under study. Fourth, in patients with negative FFR before the recanalization of their CTO we did not repeat the FFR after the PCI. Finally, no follow-up CMRs were performed in patients with negative FFR prior to recanalization.
CONCLUSIONS
The FFR assessment of intermediate stenoses in a collateral donor vessel of a CTO may overestimate the severity of the lesion by increasing the territory at risk. Therefore, the strategy of first focusing on the revascularization of the CTO and then re-assess the intermediate lesion in a collateral donor vessel may be recommended to overcome this pitfall.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare.
WHAT IS KNOWN ABOUT THE TOPIC?
- In patients with CTOs, collateral circulation supplied by donor vessels is often seen.
- The progression of atherosclerosis in donor vessels may compromise the coronary circulation of several territories.
- Angiography is not a reliable technique to assess the hemodynamic compromise of an intermediate lesion located in a vessel that provides collateral circulation to a chronically-occluded vessel.
WHAT DOES THIS STUDY ADD?
- Patients with positive FFR of donor vessels before the recanalization of a CTO may show significant increases of FFR values (even normalization in most of them too) after successful revascularization of the CTO.
- Also, the revascularization of the CTO may lead to a reduction in the number of ischemic segments assessed through CMR before and after the PCI of the CTO.
- These findings support the strategy of recanalizing the CTO first and then performing the functional assessment of donor artery with intermediate lesions.
REFERENCES
1. Fefer P, Knudtson ML, Cheema AN, et al. Current perspectives on coronary chronic total occlusions:the Canadian Multicenter Chronic Total Occlusions Registry. J Am Coll Cardiol. 2012;59:991-997.
2. Hoebers L, Claessen B, Dangas G, et al. Contemporary overview and clinical perspectives of chronic total occlusions. Nat Rev Cardiol. 2014; 11:458-469.
3. Fujita M, Sasayama, S. Reappraisal of functional importance of coronary collateral circulation. Cardiology. 2010;117:246-252.
4. Bech GJ, De Bruyne B, Pijls NH, et al. Fractional flow reserve to determine the appropriateness of angioplasty in moderate coronary stenosis:a randomized trial. Circulation. 2001;103:2928-2934.
5. Tonino PA, De Bruyne B, Pijls NH, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360:213-224.
6. De Bruyne B, Pijls NH, Kalesan B, et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med. 2012; 367:991-1001.
7. Adjedj J, De Bruyne B, Flore V, et al. Significance of intermediate values of fractional flow reverse in patients with coronary artery disease. Circulation. 2016;133:502-508.
8. Pijls NH, Fearon WF, Tonino PA, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention in patients with multivessel coronary artery disease:2-year follow-up of the FAME (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation) study. J Am Coll Cardiol. 2010;56:177-184.
9. Pijls NH, van Son JA, Kirkeeide RL, De Bruyne B, Gould KL. Experimental basis of determining maximum coronary, myocardial, and collateral blood flow by pressure measurements for assessing functional stenosis severity before and after percutaneous transluminal coronary angioplasty. Circulation. 1993;87:1354-1367.
10. Christou MA, Siontis GC, Katritsis DG, Ioannidis JP. Meta-analysis of fractional flow reserve versus quantitative coronary angiography and noninvasive imaging for evaluation of myocardial ischemia. Am J Cardiol 2007;99:450-456.
11. Sachdeva R, Uretsky BF. The effect of CTO recanalization on FFR of the donor artery, Catheter Cardiovasc Interv. 2011;77:367-369.
12. Sachdeva R, Agrawal M, Flynn SE, et al. Reversal of Ischemia of Donor Artery Myocardium After Recanalization of a Chronic Total Occlusion. Catheter Cardiovasc Interv. 2013;82:E453-E458.
13. Tigen K, Durmus E, Sari I. Recanalization of a Total Occlusion With Marked Retrograde Collateral Supply:Impact of Collateral Circulation on Fractional Flow Reserve Measurements of Donor Artery. J Invasive Cardiol. 2014;26:E70-E75.
14. Greenwood JP, Maredia N, Younger JF, et al. Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CEMARC):a prospective trial. Lancet. 2012;379:453-460.
15. Watkins S, McGeoch R, Lyne J, et al. Validation of magnetic resonance myocardial perfusion imaging with fractional flow reserve for the detection of significant coronary heart disease. Circulation. 2009;120:2207-2213.
16. Takx RAP, Blomberg BA, El Aidi H, et al. Diagnostic accuracy of stress myocardial perfusion imaging compared to inva- sive coronary angiography with fractional flow reserve meta-analysis. Circ Cardiovasc Imaging. 2015;8:e002666-e6.
17. Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539-542.
18. Ortiz-Pe?rez JT, Rodri?guez J, Meyers SN, Lee DC, Davidson C, Wu E. Correspondence between the 17-segment model and coronary arterial anatomy using contrast-enhanced cardiac magnetic resonance imaging. J Am Coll Cardiol Img. 2008;1:282-293.
19. Christou MA, Siontis GC, Katritsis DG, Ioannidis JP. Meta-analysis of fractional flow reserve versus quantitative coronary angiography and noninvasive imaging for evaluation of myocardial ischemia. Am J Cardiol. 2007;99:450-456.
20. Nagel E, Greenwood JP, McCann GP, et al. Magnetic Resonance Perfusion or Fractional Flow Reserve in Coronary Disease. N Engl J Med. 2019;380:2418-2428.
21. Liu A, Wijesurendra RS, Liu JM, et al. Diagnosis of Microvascular An-gina Using Cardiac Magnetic Resonance. J Am Coll Cardiol. 2018;71:969-979.
22. Morino Y, Abe M, Morimoto T, et al. Predicting successful guidewire crossing through chronic total occlusion of native coronary lesions within 30 minutes:the J-CTO (Multicenter CTO Registry in Japan) score as a difficulty grading and time assessment tool. JACC Cardiovasc Interv. 2011; 4:213-221.
23. Rentrop KP, Cohen M, Blanke H, Phillips RA. Changes in collateral channel filling immediately after controlled coronary artery occlusion by an angioplasty balloon in human subjects. J Am Coll Cardiol. 1985;5:587-592.
24. Escaned J, Banning A, Farooq V, et al. Rationale and design of the SYNTAX II trial evaluating the short to long-term outcomes of state-of-the-art percutaneous coronary revascularisation in patients with de novo three-vessel disease. EuroIntervention. 2016;12:e224-e234.
25. Werner GS. The role of coronary collaterals in chronic total occlusions. Curr Cardiol Rev. 2014;10:57-64.
26. Zimarino M, D'Andreamatteo M, Waksman R, et al. The dynamics of the coronary collateral circulation . Nat Rev Cardiol. 2014;1:191-197.
27. Zimarino M, Ausiello A, Contegiacomo G, et al. Rapid decline of collateral circulation increases susceptibility to myocardial ischemia:the trade-off of successful percutaneous recanalization of chronic total occlusions. J Am Coll Cardiol. 2006;48:59-65.
28. Mohdnazri SR, Karamasis GV, Al-Janabi F, et al. The impact of coronary chronic total occlusion percutaneous coronary intervention upon donor vessel fractional flow reserve and instantaneous wave-free ratio:Implications for physiology-guided PCI in patients with CTO. Catheter Cardiovasc Interv. 2018;92:E139-148.
29. Ladwiniec A, Cunnington MS, Rossington J, et al. Collateral Donor Artery Physiology and the Influence of a Chronic Total Occlusion on Fractional Flow Reserve. Circ Cardiovasc Interv. 2015;8:e002219.
30. Brugaletta S, Martin-Yuste V, PadróT, et al. Endothelial and smooth muscle cells dysfunction distal to recanalized chronic total coronary occlusions and the relationship with the collateral connection grade. JACC Cardiovasc Interv. 2012;5:170-178.
31. Hussain ST, Chiribiri A, Morton G, et al. Perfusion cardiovascular magnetic resonance and fractional flow reserve in patients with angiographic multi-vessel coronary artery disease. J Cardiovasc Magn Reson. 2016;18:44.
32. Cardona M, Martín V, Prat-Gonzalez S, et al. Benefits of chronic total coronary occlusion percutaneous intervention in patients with heart failure and reduced ejection fraction:insights from a cardiovascular magnetic resonance study. J Cardiovasc Magn Reson. 2016;18:78.
- Procedural and clinical benefits of selective thrombus aspiration in primary PCI. Insights from the TAPER Registry
- Gender-related differences among patients with STEMI: a propensity score analysis
- Coronary artery calcium score with cardiac computed tomography to anticipate the need for rotational atherectomy
- Outcomes of percutaneous coronary intervention of chronic total occlusions in heart transplant recipients
Editorials
Expanding the role of drug-coated balloons in native large coronary artery disease
aDepartment of Biomedical Sciences, Humanitas University, Pieve Emanuele, Milan, Italy
bCardio Center, IRCCS Humanitas Research Hospital, Rozzano, Milan, Italy
cDivision of Cardiology, Montefiore Medical Center, Bronx, New York, United States
Original articles
Editorials
The role of angiography-derived physiological assessment techniques in the post-FAVOR III Europe era?
aServicio de Cardiología, Hospital General Universitario Gregorio Marañón, Instituto de Investigación Sanitaria Gregorio Marañón, Universidad Complutense de Madrid, Madrid, Spain
bCentro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), Instituto de Salud Carlos III, Madrid, Spain
Interviews
An interview with Camino Bañuelos
aServicio de Cardiología, Hospital Clínico San Carlos, Instituto de Investigación Sanitaria del Hospital Clínico San Carlos (IdISSC), Madrid, Spain
bServicio de Cardiología, Hospital Clínico Universitario de Santiago de Compostela, Santiago de Compostela, A Coruña, Spain