Article
Ischemic heart disease and acute cardiac care
REC Interv Cardiol. 2019;1:21-25
Access to side branches with a sharply angulated origin: usefulness of a specific wire for chronic occlusions
Acceso a ramas laterales con origen muy angulado: utilidad de una guía específica de oclusión crónica
Servicio de Cardiología, Hospital de Cabueñes, Gijón, Asturias, España
ABSTRACT
Introduction and objectives: Coronary bifurcation lesions are a common scenario in our interventional practice and can be challenging for our routine clinical practice. Yet despite the existence of well-defined techniques, side-branch compromise is still the most important problem. Currently, the standard strategy recommended is a 1-stent technique: balloon angioplasty and provisional stenting. Published non-randomized data reveal that in up to 26% of the cases the indication for rotational atherectomy was to preserve the side-branch. A randomized comparison between rotational atherectomy and provisional stenting (RAPS) and standard strategy (SS) for the management of bifurcation lesions is needed at this point.
Methods: We conducted a single center, prospective, randomized pilot study of consecutive patients from our center with bifurcation lesions. We compared the RAPS strategy to the SS. Lesions had to be located in the main vessel only. The bifurcation lesion angle was recorded. The primary endpoint was the need for side-branch therapy.
Results: 148 patients were included: 74 patients (95 rotational atherectomy) were enrolled in the RAPS group and 74 patients in the SS group. The bifurcation lesion most frequently treated was that of the proximal left anterior descending coronary artery. The primary endpoint was lower in the RAPS group compared to the SS group (1.1 vs 31.2%; P < .001). Target vessel failure (TVF) was 13.1% and 24.8% (P = .04) in RAPS and SS, respectively. Both the primary endpoint and TVF were higher with bifurcation lesion angles < 70º compared to bifurcation lesion angles ≥ 70º (P = .03 and P = .02) in both groups.
Conclusions: The need for side-branch therapy and TVF was lower when the RAPS strategy was used compared to the SS. Bifurcation lesion angles < 70º are associated with higher side-branch compromise and TVF rates. The SS was associated with a 4.92-fold higher risk of side-branch compromise compared to the RAPS strategy with bifurcation lesion angles < 70º. These data reinforce the idea of the overall clinical relevance of the RAPS strategy regarding the patency of the side-branch.
Keywords: Bifurcation lesion. Rotational atherectomy. Side-branch compromise. Coronary calcification. Bifurcation angle.
RESUMEN
Introducción y objetivos: Durante el intervencionismo coronario percutáneo es frecuente observar lesiones coronarias que afectan a las bifurcaciones. El compromiso de la rama lateral es la principal complicación observada con las diversas técnicas descritas para su tratamiento. La estrategia convencional (EC) recomendada en la actualidad es la colocación de un stent condicional. Los datos publicados de estudios no aleatorizados muestran que hasta en el 26% de los casos la indicación de la aterectomía rotacional fue el tratamiento de lesiones en las bifurcaciones. Es necesario el desarrollo de un estudio aleatorizado que compare la estrategia de aterectomía rotacional y stent condicional (ARSC) frente a la EC.
Métodos: Estudio piloto aleatorizado, prospectivo, de un solo centro, en pacientes con enfermedad coronaria en una bifurcación. Se comparó la estrategia de ARSC con la EC. Se prestó especial atención al ángulo de la bifurcación. El objetivo primario evalúa la necesidad de tratamiento de la rama lateral con ambas técnicas.
Resultados: Se incluyeron 148 pacientes: 74 (95 aterectomías rotacionales) en el grupo de ARSC y 74 en el grupo de EC. El objetivo primario fue menor con la ARSC que con la EC: 1,1% frente a 31,2% (p < 0,001). El objetivo de fallo del vaso tratado (FVT) fue del 13,1% en el grupo de ARSC y del 24,8% en el grupo de EC (p = 0,04). El objetivo primario y el FVT fueron mayores si la lesión era en una bifurcación < 70° en comparación con una bifurcación ≥ 70° en ambos grupos (p = 0,03 y p = 0,02).
Conclusiones: La necesidad de tratamiento de la rama lateral y el FVT fueron menores con la estrategia de ARSC que con la EC. Un ángulo < 70° en la bifurcación aumenta el riesgo de compromiso de la rama lateral y las tasas de FVT. La EC se asoció a un incremento del riesgo de compromiso de la rama lateral de 4,92 veces cuando el ángulo de la bifurcación era < 70°. Estos datos sugieren que el abordaje de lesiones en una bifurcación mediante aterectomía rotacional podría tener un beneficio clínico global.
Palabras clave: Lesión en bifurcación. Ángulo de la bifurcación. Aterectomía rotacional. Compromiso de rama lateral. Calcificación coronaria.
Abbreviations: CBL: coronary bifurcation lesion. PCI: percutaneous coronary intervention. RA: rotational atherectomy. RAPS: rotational atherectomy and provisional stenting. SS: standard strategy. SB: side-branch.
INTRODUCTION
Over the last few years, the profile of patients referred to undergo a coronary angiography has become worse. Similarly, angiographic findings have become worse as well. Recently, De María et al.1 published a study on the management of calcified lesions. They provided a nice contemporary overview on the management of calcified lesions in the catheterization laboratory focusing on the technologies available, intravascular imaging, and technical complexities. However, an important marker of procedural complexity was omitted: coronary bifurcation lesions. CBLs are often seen in interventional practice and can be challenging in our routine clinical practice. Yet spite the existence of several well-defined techniques to perform a percutaneous coronary intervention (PCI) on a CBL, side-branch compromise is still the most important problem.2,3 Currently, the standard strategy (SS) recommended for the management of CBL is a 1-stent technique2,4 (balloon angioplasty and provisional stenting) since it has proven to be non-inferior to the elective 2-stent technique.5 It is well-known that rotational atherectomy (RA) is underused during the PCI6 and no specific randomized data are available regarding its role in the management of CBL. The role of RA in this setting has been suggested in different studies not designed for that purpose. Data published reveal that in up to 26% of the cases the indication for RA was to preserve the side-branch.7-9 As far as we know, this extended use of RA is an off-label indication that has not been specifically tested in a randomized study. We report the procedural and long-term results of the rotational atherectomy and provisional stenting (RAPS) strategy compared to the SS (balloon angioplasty and provisional stenting) in a randomized pilot study.
METHODS
Study population
We conducted a single center, prospective, randomized pilot study of consecutive patients from our center with bifurcation lesions located only in main vessel (BLMV) and who were screened before being recruited. The angiographic criteria to define the CBLs that were eligible for the study were: a) lesions: > 70% located in a major bifurcation point regardless of the length, morphology, and angulation of the bifurcation lesion; b) thrombolysis in myocardial infarction (TIMI) flow grade > 2 on both the main vessel (MV) and the side-branch (SB); c) MV visual diameter: ≥ 2.5 mm; and 4.0. SB visual diameter: ≥ 2.0 mm. The presence of a heavily calcified lesion was not a prerequisite to enter the study.
The inclusion criteria were patients ≥ 18 years who signed their informed consent with Medina lesions type 1.0.0; 1.1.0 and 0.1.0 and who were eligible to undergo either one of the 2 strategies and with no confirmed or suspected contraindications for prolonged dual antiplatelet therapy.
The exclusion criteria were: a) SB < 2 mm; b) lesions with thrombus or dissection; c) vein graft lesions; d) cases of a single main vessel with severe left ventricle dysfunction (EF < 30%); e) hemodynamically unstable patients; f) contraindication for prolonged dual antiplatelet treatment; g) life expectancy < 1 year; and h) patient refusal.
Procedures
The random assignment of patients to the different treatment groups was done using the EPIDAT 4.0 software. After obtaining the patients’ informed consent they were randomized in a 1:1 ratio to the RAPS group, RA group or SS group. Patients were revascularized according to the current recommendations.1,10 In the SS group the strategy used was left to the operator’s discretion: 1 or 2 wires, previous BA or direct stenting, 1- or 2-stent technique, etc. Everything was decided in each case by the operator. In the RAPS group a single RotaWire was used in the main vessel and only in this vessel rotational atherectomy would be performed (videos 1-7 of the supplementary data).
The baseline clinical data collected include demographics and the patients’ cardiovascular past medical history and comorbid conditions. Both the angiographic and PCI data were recorded. The RA technique was performed following the current recommendations.6 CBLs were classified according to their angles: < 70º or ≥ 70º. Two different operators assessed each individual case.
Endpoints
The primary endpoint was defined as “need for side-branch therapy”. This “need for side-branch therapy” was considered in the presence of clinical, ECG or hemodynamic signs suggestive of TIMI flow ≤ 2 and/or ostial stenosis ≥ 70%.11 In contrast, “side-branch compromise” was considered when in the presence of impaired SB stenosis or TIMI flow whether severe or not. The secondary endpoints were: a) Target vessel failure (TVF): a composite of cardiac death, culprit vessel myocardial infarction, target vessel restenosis, and target bifurcation restenosis at the follow-up (appendix of the supplementary data); b) Angiographic outcomes: B.1. Procedural and annual assessment success rate and its correlation with the bifurcation angle. Procedural success was defined as TIMI flow grade-3 in both the MV and the SB and a visual residual stenosis < 20% in the MV; B.2. Angiographic complications rate including stent thrombosis, dissection, occlusion, perforation, no-reflow, target lesion restenosis (TLR), and target bifurcation restenosis at the FUP. c) The major adverse cardiovascular and cerebrovascular events (MACCE). Other relevant conditions such as hemorrhages, need for transfusion, and kidney disease were also recorded. All deaths were considered cardiac unless a definite non-cardiac cause was established. Both the bifurcation technique and stent used were left to the operator’s discretion.
The periprocedural drugs and laboratory test definitions are shown on in the appendix of the supplementary data. After discharge, the patients’ clinical follow-up was conducted through personal interviews or phone calls every 6 months. Patients underwent angiographic control clinically driven only. The monitoring of cardiovascular risk factors, drugs compliance, and blood test controls were left to the discretion of the referring physician.
The aforementioned study has been conducted in full compliance with The Code of Ethics of the World Medical Association Declaration of Helsinki. Also, it has been approved by the hospital local ethics committee. The patients’ written informed consent was obtained too.
Sample size
No randomized studies on this subset are available so we could not use the sample size formula. Instead, we used the ARCSIN approximation function and estimated that, at least, 60 subjects should be included in each group to find statistically significant differences (accepting an alpha risk of 0.05 and a beta risk of 0.2 in two-sided tests). A drop-out rate < 1% was anticipated.
Statistical analysis
Data were expressed as means ± standard deviation (SD) for the continuous variables and as frequencies and percentages for the categorical ones. The FUP period was expressed as the median with its interquartile range [IQR]. The chi-square or Fisher’s exact tests that assessed the effect and accuracy analyses with the prevalence ratio and 95% confidence interval, when necessary, were used to compare the continuous and categorical variables, respectively. The Mann-Whitney test was used to study the non-parametric variables. Cox regression models were used to perform univariate analyses to estimate the associated hazard-ratio of death and composite endpoints at the FUP. A multivariate analysis was performed as well. The Kaplan-Meier estimates were used to determine the time-to-event outcomes, overall survival rate, and MACCE-free survival rate. We tested the equality of the estimated survival curves using the stratified log-rank test. All analyses were performed using the Statistical Package for Social Scientists (SPSS Inc., 20.0 for Windows). P values < .05 were considered statistically significant in all of the tests.
RESULTS
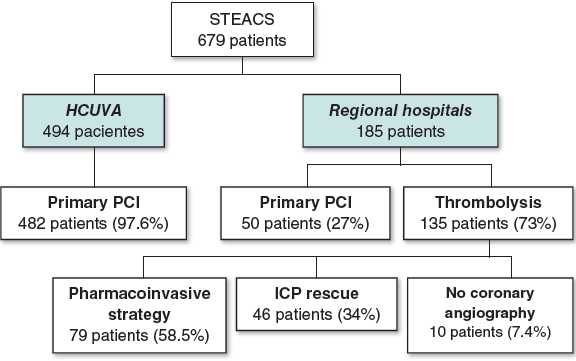
One-hundred and seventy-three out of 1028 patients who underwent a PCI between January 2015 and December 2018 were considered eligible to enter the study: 13 refused to participate, 8 patients dropped-out, and 4 patients withdrew their informed consent. Finally, 148 patients were included: 74 patients (95 RAs) were recruited in the RAPS group and 74 patients in the SS group. The inclusion/exclusion flowchart is shown on figure 1 of the supplementary data.
The baseline clinical, angiographic, and procedural data are shown on table 1 and table 2. No sex-based differences were seen. Only the prevalence of a left ventricular ejection fraction ≤ 45% was different between the groups: P = .03. No calcification, tortuosity or bifurcation angle differences were reported. The most common bifurcation was found at the first diagonal branch of the proximal left anterior descending coronary artery (D1-LAD) (51%) followed by the distal left main coronary artery (LMCA)/ostial LAD (22.5%) No inter-group differences in single vs staged revascularization were seen.
Table 1. Baseline characteristics
| Baseline clinical data | RAPS (N = 74) | SS (N = 74) | P |
|---|---|---|---|
| Age (mean; SD) | 78 (10) | 74 (7) | NS |
| Males (n; %) | 60 (81.2) | 58 (78.1) | NS |
| Weight (mean; SD) | 73.9 (11.9) | 75.4 (11.4) | NS |
| Height (m) (mean; SD) | 1.64 (0.7) | 1.66 (0.6) | NS |
| Body mass index (mean; SD) | 27.11 (3.4) | 29.24 (11.4) | NS |
| Current/Previous smoker (n; %) | 46 (62.1) | 53 (71.6) | NS |
| Hypertension (n; %) | 62 (92.2) | 74 (100) | NS |
| Diabetes mellitus (n; %) | 29 (39.1) | 30 (40.6) | NS |
| Dyslipidemia (n; %) | 69 (93.2) | 62 (83.7) | NS |
| Left ventricle ejection fraction ≤ 45 (%) | 28 (37.8) | 14 (18.7) | .03 |
| Previous myocardial infarction (n; %) | 42 (56.7) | 37 (50) | NS |
| Previous angioplasty | 42 (56.7) | 32 (43.2) | NS |
| Previous Stroke (n; %) | 10 (14.1) | 18 (24.3) | NS |
| Peripheral vascular disease (n; %) | 16 (21.6) | 23 (31) | NS |
| L-Euroscore (mean; DS) | 21.14 (22.15) | 13.7 (18.7) | NS |
| Syntax Score (mean; DS) | 34.05 (17.9) | 31.57 (17.9) | NS |
| Clinical onset (n; %) | |||
| Stable angina | 14 (19) | 20 (27) | NS |
| NSTEMI | 40 (54) | 47 (63.5) | NS |
| STEMI | 20 (27) | 7 (9.4) | NS |
| Discarded for cardiac surgery (n; %) | 24 (32.4) | 20 (27) | NS |
| NYHA Class ≥ III | 8 (9.3) | 9 (12.1) | NS |
| CCS I-II | 57 (77) | 41 (55.4) | NS |
| CCS III-IV | 17 (23) | 32 (44.6) | NS |
|
CCS, Canadian Class Classification angina score; NS, not significant; NSTEMI, non-ST-elevation acute myocardial infarction; NYHA: New York Heart Association; RAPS, rotational atherectomy and provisional stenting; SS, standard strategy; SD, standard deviation; STEMI, ST-segment elevation myocardial infarction. |
|||
Table 2. Angiographic and procedural data
| Angiographic/procedural data | RAPS (N = 74) | SS (N = 74) | P |
|---|---|---|---|
| 6-Fr sheath (n; %) | 62 (88) | 60 (81.2) | NS |
| Radial Approach (n; %) | 29 (39.1) | 30 (40.6) | |
| Femoral approach (n; %) | 45 (60.9) | 44 (59.3) | |
| Coadjuvant therapy (n; %) | |||
| Heparin | 21 (28.1) | 29 (40.6) | NS |
| Bivaluridin | 42 (56.3) | 23 (31.2) | .01 |
| Glycoprotein inhibitors | 11 (15.9) | 23 (31.2) | NS |
| Right Dominance (n; %) | 64 (87.5) | 64 (87.5) | NS |
| Vessel disease (n; %) | |||
| Left Main coronary artery | 15 (20.3) | 14 (18.7) | NS |
| Left anterior descending coronary artery | 72 (98.4) | 57 (78.1) | .02 |
| Left circumflex artery | 47 (64.1) | 55 (71.8) | NS |
| Right coronary artery | 54 (73.4) | 57 (78.1) | NS |
| Number of diseased vessels (n; %) | |||
| 1 vessel | 10 (13.5) | 13 (17.53) | NS |
| 2 vessels | 22 (29.7) | 24 (32.4) | NS |
| 3 vessels | 34 (45.9) | 31 (41.8) | NS |
| 4 vessels | 8 (10.8) | 6 (8.1) | NS |
| Multivessel (n; %) | 60 (81.2) | 53 (71.8) | NS |
| Coronary calcification (%) | |||
| Mild | 28 | 36 | NS |
| Moderate-severe | 72 | 64 | NS |
| B2C lesions (n; %) | 94 (98.4) | 60 (81.2) | .048 |
| Medina classification of bifurcation lesions (n; %) | |||
| 1.0.0 | 46 (48.4) | 17 (23) | .04 |
| 1.1.0 | 32 (33.6) | 22 (29.7) | NS |
| 0.1.0 | 17 (17.8) | 30 (40.1) | .03 |
| Bifurcation angle (n; %) | |||
| < 70º | 46 (62) | 50 (67.5) | NS |
| ≥ 70º | 28 (38) | 24 (32.5) | NS |
| Wire | |||
| Floppy [n (%)] | 88 (92.4) | N/A | NS |
| Directly advanced [n (%)] | 84 (88.5) | N/A | NS |
| Burr size ≤ 1.5 mm | 76 (80) | N/A | NS |
| Speed (rpm) (mean; SD) | 134650 (5670) | N/A | NS |
| Rotational atherectomies performed (% per patient) | 95 (1.28) | N/A | NS |
| Burr-to-artery ratio (mean; SD) | 0.55 (.04) | N/A | |
| Number of balloons per lesion | 1.3 | 4.6 | .02 |
| Stent (n) | |||
| Number of stents per lesion | 1.6 | 2.3 | .04 |
| Number of stents per patient | 2.7 | 2.33 | NS |
| Bare-metal stent [n (%)] | 24 (12.7) | 22 (23.2) | NS |
| Drug-eluting stent [n (%)] | 167 (86.9) | 72 (76.7) | NS |
| Stenting technique [n (%)] | |||
| Provisional stenting | 64 (100) | 41 (55.4) | .04 |
| Two-stent initial approach technique | 0 | 28 (37.8) | < .001 |
| Optimal treatment of the proximal LAD | 48 (64.8) | 24 (32.4) | < .05 |
| Final kissing balloon technique | 1 (1.5) | 59 (79.7) | < .001 |
| Final inflation pressure (atm) | 18 | 14 | .05 |
| Initial vessel diameter (Me; IQR) (mm) | 2.41 (0.34) | 2.89 (0.26) | .009 |
| Final vessel diameter (Me, IQR) (mm) | 3.1 (1.9) | 2.95 (0.37) | NS |
| Maximum length stented (Me; IQR) (mm) | 56 (48) | 44 (26.1) | .005 |
| Procedural time (min) (mean; SD) | 78.8 (30) | 98 (21) | .04 |
| Fluoroscopy time (min) (mean; SD) | 13 (7) | 29.2 (21) | .02 |
| Contrast media (ml) (mean; SD) | 179 (74) | 221 (73) | .05 |
| IVUS/OCT | 7 (9.4) | 11 (14.8) | NS |
|
IVUS, intravascular ultrasound; Me, median; NS, not significant; NYHA, New York Heart Association; OCT, optical coherence tomography; SD, standard deviation.s |
|||
Long-term follow-up
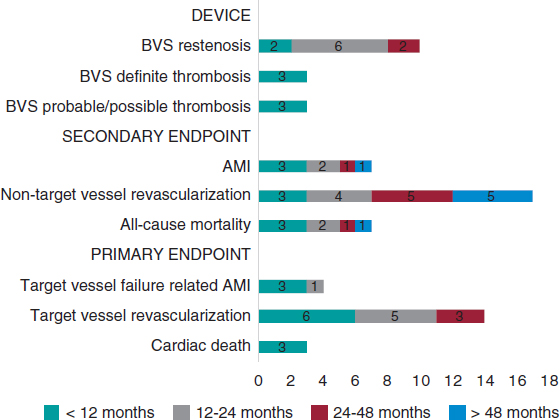
Both the clinical and angiographic success rates and outcomes were available for the entire population with a median FUP of 4.08 years [IQR: 3.18-4.78 years]. Both the all-cause and cardiovascular mortality rates were similar in both groups. The need for side-branch therapy was consistently lower in the RAPS strategy compared to the SS: 1.1% vs 27% (P < .001) (table 3). TVF was 12.1% and 24.8% (P =.04) in the RAPS strategy compared to the SS, respectively. Also, the statistical analysis confirmed that the use of the RA technique significantly reduced the risk of target vessel restenosis (P = .04), TLR (0.02), target bifurcation restenosis (P = .03), and major adverse cardiovascular events (P = .03). A positive correlation (r = 0.673, P = .03) was seen between the need for SB therapy and CBL angles < 70º. The strongest correlation was observed at the proximal D1-LAD: r = 0.79, P = .03. A weak but positive correlation was seen between the LMCA-LAD arteries angle (r = 0.412, P = .04) and the LMCA-LCx arteries angle (r = 0.342, P = .004). The sum of SS plus CBL angles < 70º was associated with a higher risk of SB compromise and TVF (OR, 4.92; 95%CI, 1.78-14.1; P = .03)
Table 3. Major adverse cardiovascular events at the follow-up
| RAPS (N = 74) | SS (N = 74) | P | |
|---|---|---|---|
| Clinical success (%) | 98.6 | 98 | NS |
| Associated cardiovascular mortality (hospitalizations) [n (%)] | 3 (4) | 2 (2.7) | NS |
| With procedure | 2 (2.7) | 2 (2.7) | |
| With rotational atherectomy | 1 (1.3) | N/A | |
| Angiographic success (%) | 96.5 | 97.5 | NS |
| Angiographic complications [n (%)] | |||
| Unable to advance the wire | 1 (1.3) | 2 (2.7) | NS |
| Burr entrapment | 0 | N/A | NS |
| Unable to deliver the stent | 1 (1.3) | 2 (2.7) | NS |
| Coronary dissection | 1 (1.3) | 6 (8,1) | .024 |
| Side-branch compromise* | 2 (2.7) | 23 (31) | < .001 |
| Need for side-branch therapy** | 1 (1.3) | 20 (27) | < .001 |
| Perforation | 0 | 0 | NS |
| Cardiac tamponade | 0 | 0 | NS |
| Stent thrombosis | 0 | 0 | NS |
| Need for pacemaker implantation | 0 | 0 | NS |
| Final flow compromise (TIMI ≤ 2) in SB | 0 | 2 (2.7) | NS |
| MACCE (4.08 years, ICA: 3.18-4.78) | |||
| GLOBAL: 27 (36.4%) | 18 (25%) | 30 (40.6%) | .03 |
| Overall death rate | 15 (20.3%) | 16 (21.8%) | NS |
| Hospitalization | 3 (4%) | 3 (4%) | NS |
| 30 days | 4 (5.4%) | 5 (6.7%) | NS |
| Cardiac Death | 5 (6.7%) | 7 (9.4%) | NS |
| Non-cardiac Death | 9 (12.1%) | 7 (9.4%) | NS |
| Stroke | 2 (2.7%) | 7 (9.4%) | .02 |
| TVF | 9 (12.1%) | 18 (24.8 %) | .04 |
| TLR | 2 (2.7%) | 11 (14.8%) | .02 |
| TVR | 3 (4%) | 7 (9.4%) | .03 |
| TBR | 2 (2.7%) | 7 (9.4%) | .03 |
| Stent thrombosis | 0 | 0 | NS |
|
ICA, interquartile amplitude; MACCE, major adverse cardiovascular and cerebrovascular events; NS, not significant; RAPS, rotational atherectomy and provisional stenting; SS, standard strategy; TBR, target bifurcation restenosis; TLR, target lesion restenosis; TVF, target vessel failure (composite of cardiac death, culprit vessel myocardial infarction); TVR, target vessel restenosis. * Shift plaque defined as ostial side-branch stenosis > 70% and/or TIMI flow < 3. ** Treatment included: a) angioplasty with conventional or drug-eluting balloon; b) bare-metal stent or drug-eluting stent. |
|||
DISCUSSION
Main findings
The main findings of this study are: a) the RAPS strategy for the management for CBLs minimizes the compromise of the SB, need for SB therapy, and TVF compared to the SS; b) There was a strong correlation between the compromise of the SB and acute CBL angles (< 70º); c) The SS was associated with a 4.92-fold higher risk of SB compromise compared to the RAPS strategy in CBL angles < 70º.
CBLs are a common thing in our interventional practice and can be challenging in our routine clinical practice. Side-branch compromise is still the most important problem. To our knowledge, this is the first randomized study that addressed this issue and described the role of RA in the management of CBLs. Former studies not specifically designed to address this specific question had already suggested this.8,9,12,13 We reported sustained short-term benefits of the RAPS strategy at the long-term follow up. Some differences had been previously reported,14 which is why differences in the primary endpoint could be expected, but still not so significant.
As a hypothesis-generating pilot study we defined a procedural primary endpoint.11 Selecting a “procedural” primary endpoint at this stage is a reasonable thing to do since the occlusion of large SBs is a serious complication that leads to adverse clinical outcomes.11,14 We studied whether the RAPS strategy could be as good as the SS for the management of CBL by comparing the compromise of the SB.15-17 Still, the current clinical practice guidelines minimize the indications for RA to heavily calcified lesions and rigid ostial lesions,10 although an expert consensus document recently published includes more extensive indications.6 The real-world use of RA for plaque modification in is nothing new.9 Actually, in the absence of plaque modification there are more chances of procedural failure, stent underexpansion, in-stent restenosis, and major clinical complications.2,5,18 Schwartz et al use it in up to 26% of their population.9
Percutaneous coronary intervention and bifurcation technique
Only BLMVs were included.19 Bifurcations are true bifurcations when a significant SB runs the risk of being compromised regardless of whether the disease reaches it or not. Thus, maybe we should rename them as “complex CBLs”, that is, those where the SB has baseline disease (1.1.1 in the Medina classification) and “simple CBLs”, those without baseline disease (again according to the Medina score). There is wide consensus that the main objective of complex PCIs in the management of CBLs is to keep the patency of both vessels regardless of the PCI technique used and the location of the lesion.2 For many years we have been focused on the optimization of SB, but clinical events such as TLR mostly occur in the main vessel.20 In up to 20% of the cases, the SB requires a stent, which means that the proper preparation of the CBL is essential.3,14,21
What the best bifurcation technique is for the management of CBL is still under discussion. Currently, the standard strategy recommended for the management of CBL is a 1-stent technique.2,4 Ideally, the technique selected should provide an easy access for a second stent in the SB even if conventional approach with a 1-stent technique is planned. In our cohort, the RA facilitated this approach. According to cumulative clinical trial data3 we reported a high rate of provisional stenting in the RAPS strategy that proved non-inferior to the elective 2-stent technique4,5 and ever better for the management of periprocedural myocardial infarction.22 The kissing balloon technique is being systematically used in cases of large territories supplied by the SB or when the SB exhibits flow impairment after MV stenting. Sometimes, in such situations a second stent is implanted in the SB.23 The differences reported in our population regarding the optimal treatment of the proximal LAD and final kissing balloon and 2-stent technique used are still under discussion. We saw a 4-fold higher rate of the balloon technique in the SS. Maybe these differences were due to the tight lesions described: in the SS there was a need of a step-up ballooning to cross and dilate the lesions and eventually for the final optimization of the stents. Eventually, at least 3 or 4 balloons were needed. Interestingly, as previously reported, when the final kissing balloon technique was used, the optimal treatment of the proximal LAD produced no benefit at all.24 Maybe this was the case because the stent located in the main vessel is properly expanded after using the kissing balloon technique. We saw a lower need for SB treatment and TVF rates7,18,25 in the RAPS strategy than previously reported.
Role of rotational atherectomy for the management of bifurcation lesions
The RAPS strategy facilitates the modification of the plaque without SB compromise by extending provisional stenting2,4 by a) minimizing plaque shift, b) optimizing plaque modification, c) reducing the need for 2 wires/stents and d) improving the stent expansion/apposition. Otherwise, certain maneuvers used in other strategies to avoid the occlusion of the SB may cause suboptimal stent expansion/apposition in the MV, which can be a major cause for stent thrombosis and restenosis.2,14 The bifurcation angle has been suggested as an important issue for the compromise of the SB.5,11,14 In our population, the LAD was the most commonly affected coronary artery. The LAD is particularly appealing given the angle of the origin of the diagonals. The crux is often at a right angle so it is less of a concern and the circumflex artery only matters when it is dominant. Acute CBL angles (< 70º) have shown to increase the compromise of the SB and, therefore, lead to worse outcomes. In our cohort, “SB compromise”, “need for SB therapy”, and TVF rates were lower in CBL angles < 70º both in the RAPS and the SS groups. The small size of the sample prevented us from drawing definitive conclusions, but these data were good enough to make us change our daily methodology: with CBL angles < 70º located in the main vessel with a large side-branch we use directly the RAPS technique. Maybe the explanation for the differences seen in the RAPS vs the standard strategy is the underlying mechanism of action of rotational atherectomy. As a matter of fact, this may explain the higher rates of SB compromise and need for SB therapy seen in the SS group: a more controlled plaque modification was achieved with RA that minimized the plaque shift. Unfortunately, our data did not include too many imaging modalities. In our cohort for events assignment, if during the PCI procedure any narrowing occurred adjacent to, and/or involving the origin of a significant SB it was allocated to the selected strategy used. The decision to use the 1-stent or 2-stent technique, the type of stent, etc. was left to the operator’s discretion. We should make a few comments on our study population: a) although most of the patients were unstable, this did not condition the results in any of the groups; b) in the RAPS strategy the use of the jailed wired technique is rare; c) CBL angles < 70º between branches facilitate the plaque shift.26 Thus, if the TIMI flow recorded after stent deployment was < 3 or residual stenosis was > 70% more bail-out balloons and stents were needed, which would explain the different outcomes seen when using the final kissing balloon technique; d) in a number of cases where the standard strategy was used it was complemented with the kissing balloon inflation technique at high-pressure balloon inflation instead of the final optimal treatment of the proximal LAD; and e) the differences seen in the coronary dissection rate on the angiographic study may be suggestive of micro-dissections due to inadequate balloon assessment through conventional angiography, which could be the underlying mechanism of the endpoint differences reported; performing more intravascular ultrasound/optical coherence tomography studies would provide better assessment here.
Patients were randomized in a 1:1 ratio so the differences seen in the left ventricular ejection fraction and LAD disease were absolutely due to the size of the study sample. Although we saw a lower cardiovascular mortality rate compared to the one published in the medical literature2,14,15,27 this study was not designed to compare the MACCE results between both groups. Interestingly, the rates of TVF were significantly lower in the RAPS strategy mainly due to fewer culprit vessel myocardial infarctions and target vessel restenoses. In any case, our data underscored the safety profile of the RAPS strategy in unstable patients and patients with left ventricular dysfunction (P = .03)
Limitations
We designed and conducted a single-center pilot study. Small sample sizes have inherent limitations. Our results should be interpreted with caution as a hypothesis-generating pilot study. Several confounding factors and biases could be present, which is why any assessments on this regard should be made with caution too. The study was extremely underpowered to show clinical outcome differences, which is why the clinical findings reported should be considered just exploratory. Our procedural endpoint and inclusion of BLMVs only could be discussed. There is wide consensus that the main objective of complex PCsI for the management of CBLs is to keep both vessels patent regardless of the PCI technique used.2
We thought it was the right thing to do to assess the data on the SB compromise by comparing both techniques used. Over thec years we have been focusing on optimizing the SB, but clinical events such as TLR mostly occur in the main vessel.20 Only BLMVs were included.19 A bifurcation should be considered as a true bifurcation when a significant SB you do not want to lose is compromised whether it shows coronary stenosis or not. We should mention that in the management of CBLs with the RAPS strategy a low rate of SB stenting is associated with a lower rate of major adverse events and clinically significant rates of restenosis. Therefore, very large numbers of patients are required for the proper assessment of the differences. Some baseline characteristics of coronary lesions vary depending on the interventional strategy used (as in the management of B2C lesions) to the point of impacting the final outcomes. The lack of differences seen in the stent thrombosis and stroke rates may be associated with the size of the study sample.
Although statistical significance was not observed, the percentage of bare metal stents used was numerically higher in the control group compared to the RAPS group. However, this study is not a comparison of drug-eluting stents versus bare-metal stents in bifurcation disease. These findings could be associated with the difference seen in TLR/target vessel restenosis, especially if we take into account that 31.2% of patients from the control group were treated using 2-stent techniques. We saw that RA followed by drug-eluting stents was associated with a low rate of MACCE compared to bare-metal stents. However, this study was not designed to make comparisons like this one. A higher percentage of bivaluridin was intentionally used in the RAPS group, but this did not produce any statistically significant differences. The use of more imaging modalities such as intravascular ultrasound or optical coherence tomography is desirable here. FUP was mostly conducted through phone calls and it may have underestimated the rate of MACCE. An off-label indication does not necessarily mean a contraindication of our promising, but support for the next step: a large randomized multicenter trial that is about to begin.
CONCLUSIONS
The RAPS strategy for the management of CBL preserves the SB ostium and minimizes the need for SB therapy compared to the SS. The rates of “SB compromise”, “need for SB therapy”, and TVF were higher with CBL angles < 70º for both the RAPS and the SS groups. Our data reinforce the idea of the overall clinical relevance of the RAPS strategy to keep the SB patent. Although no large clinical trials have taken this approach yet, the results published so far are promising.
CONFLICTS OF INTEREST
J. Palazuelos (corresponding author) is a consultant on the speaker’s bureau of Abbott, Boston Scientific, Biotronik, Innovative Health Technologies (IHT) and Medtronic. J. Palazuelos is a proctor for Rotational Atherectomy with a teaching contract with Boston Scientific that has funded this study with a grant. No other relation with the industry regarding this study was declared. He confirms he has had full access to all the study data and holds full responsibility for the decision to submit this manuscript for publication in Rec: Interventional Cardiology. The remaining authors have declared no conflicts of interest whatsoever regarding the contents of this manuscript.
WHAT IS KNOWN ABOUT THE TOPIC?
- Over the last few years, the profile of patients referred to undergo a coronary angiography has become worse. Similarly, angiographic findings have become worse as well. With the progressive ageing of the population and the arrival of better technologies, the balance between offer and demand in this field is in continuous expansion. Still, the management of such delicate situations requires profound knowledge of dedicated techniques and accurate clinical judgement. Calcified coronary lesions and bifurcated lesions are a common occurrence that accounts to between 25% and 30% of all PCIs. There are technologies available for the management of these lesions. The older one is rotational atherectomy. Currently, the objective is to modify the plaque since the lack of plaque modification is associated with more procedural failure, stent underexpansion, in-stent restenosis, and major clinical complications. Despite the existence of well-defined techniques for the use of PCI for the management of CBLs, side-branch compromise is still the most important complication.
WHAT DOES THIS STUDY ADD?
- The role of rotational atherectomy for the management of coronary bifurcation lesions has been suggested in different studies not specifically designed for that purpose. Our randomized data support the role of the RAPS strategy for the management of BLMV in a cohort of high-risk patients. The RAPS strategy provided higher SB patency and lower TVF. Still, larger studies are needed to shed light on this question.
SUPPLEMENTARY DATA
Video 1. Palazuelos J. H. DOI: 10.24875/RECICE.M20000138
Video 2. Palazuelos J. H. DOI: 10.24875/RECICE.M20000138
Video 3. Palazuelos J. H. DOI: 10.24875/RECICE.M20000138
Video 4. Palazuelos J. H. DOI: 10.24875/RECICE.M20000138
Video 5. Palazuelos J. H. DOI: 10.24875/RECICE.M20000138
Video 6. Palazuelos J. H. DOI: 10.24875/RECICE.M20000138
Video 7. Palazuelos J. H. DOI: 10.24875/RECICE.M20000138
REFERENCES
1. De Maria GL, Scarsini R, Banning AP. Management of Calcific Coronary Artery Lesions. Is it time to change our interventional therapeutic approach?JACC Cardiovasc Interv. 2019;12:1465-1478.
2. Lassen JF, Burzotta F, Banning AP, et al. Percutaneous coronary intervention for the left main stem and other bifurcation lesions:12th consensus document from the European Bifurcation Club. EuroIntervention. 2018;13:1540-1553.
3. Gao X-F, Zhang Y-J, Tian N, et al. Stenting strategy for coronary artery bifurcation with drug-eluting stents:a meta-analysis of nine randomized trials and systematic review. EuroIntervention. 2014;10:561-569.
4. Colombo A, Jabbour RJ. Bifurcation lesions:no need to implant two stents when one is sufficient!Eur Heart J. 2016;37:1929-1931.
5. Nairooz R, Saad M, Elgendy IY, et al. Long-term outcomes of provisional stenting compared with a two-stent strategy for bifurcation lesions:a meta-analysis of randomized trials. Heart. 2017;103:1427-1434.
6. Barbato E, CarriéD, Dardas P, et al. European expert consensus on rotational atherectomy. EuroIntervention. 2015;11:30-36.
7. Ito H, Piel S, Das P, et al. Long-term outcomesof plaque debulking with rotational atherectomy in side-branch ostial lesions to treat bifurcation coronary disease. J Invasive Cardiol. 2009;21:598-601.
8. Warth DC, Leon MB, O'Neill W, Zacca N, Polissar NL, Buchbinder M. Rotational atherectomy multicenter registry:acute results, complications and 6-month angiographic FUP in 709 patients. J Am Coll Cardiol. 1994;24:641-648.
9. Schwartz BG, Mayeda GS, Economides C, Kloner RA, Shavelle DM, Burstein S. Rotational atherectomy in the drug-eluting stent era:a single-center experience. J Invasive Cardiol. 2011;23:133-139.
10. Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87-165.
11. Burzotta F, Trani C, Todaro D, et al. Prospective Randomized Comparison of Sirolimus- or Everolimus-Eluting Stent to Treat Bifurcated Lesions by Provisional Approach. JACC Cardiovasc Interv. 2011;4:327-335.
12. Nageh T, Kulkarni NM, Thomas MR. High-speed rotational atherectomy in the treatment of bifurcation-type coronary lesions. Cardiology. 2001;95:198-205.
13. Dai Y, Takagi A, Konishi H, et al. Long-term outcomes of rotational atherectomy in coronary bifurcation lesions. Exp Ther Med. 2015;10:2375-2383.
14. Hahn JY, Chun WJ, Kim JH, et al. Predictors and outcomes of side-branch occlusion after main vessel stenting in coronary bifurcation lesions:results from the COBIS II Registry (COronary BIfurcation Stenting). J Am Coll Cardiol. 2013;62:1654-1659.
15. Tomey MI, Kini AS and Sharma SK. Current Status of Rotational Atherectomy. JACC Cardiovasc Interv. 2014;7:345-53.
16. Ellis SG, Popma JJ, Buchbinder M, et al. Relation of clinical presentation, stenosis morphology, and operator technique to the procedural results of rotational atherectomy and rotational atherectomy-facilitated angioplasty. Circulation. 1994;89:882-892.
17. Furuichi S, Sangiorgi GM, Godino C, et al. Rotational atherectomy followed by drug-eluting stent implantation in calcified coronary lesions. EuroIntervention. 2009;5:370-374.
18. Abdel-Wahab M, Baev R, Dieker P, et al. Long-Term Clinical Outcome of Rotational Atherectomy Followed by Drug-Eluting Stent implantation in Complex Calcified Coronary Lesions. Catheter Cardiovasc Interv. 2013;81:285-291.
19. Medina A, Suarez de Lezo J, Pan M. A new classification of coronary bifurcation lesions. Rev Esp Cardiol. 2006;59:183.
20. Gwon HC. Understanding the Coronary Bifurcation Stenting. Korean Circ J. 2018;48:481-491.
21. Vaquerizo B, Serra A, Miranda F, et al. Aggressive plaque modification with rotational atherectomy and/or cutting balloon before drug-eluting stent implantation for the treatment of calcified coronary lesions. J Interv Cardiol. 2010;23:240-248.
22. Song PS, Song YB, Yang JH, et al. Periprocedural myocardial infarction is not associated with an increased risk of long-term cardiac mortality after coronary bifurcation stenting. Int J Cardiol. 2013;167:1251-1256.
23. Burzotta F, Sgueglia GA, Trani C, et al. Provisional TAP-stenting strategy to treat bifurcated lesions with drug-eluting stents:one-year clinical results of a prospective registry. J Invasive Cardiol. 2009;21:532-537.
24. Kim MC1, Ahn Y, Sim DS, et al. Impact of Calcified Bifurcation Lesions in Patients Undergoing Percutaneous Coronary Intervention Using Drug-Eluting Stents:Results From the COronary BIfurcation Stent (COBIS) II Registry. EuroIntervention. 2017;13:338-344.
25. Benezet J, Diaz de la Llera LS, Cubero JM, Villa M, Fernandez-Quero M, Sanchez-Gonzalez A. Drug-eluting stents following rotational atherectomy for heavily calcified coronary lesions:long-term clinical outcomes. J Invasive Cardiol. 2011;23:28-32.
26. Koo BK, Waseda K, Kang HJ, et al. Anatomic and functional evaluation of bifurcation lesions undergoing percutaneous coronary intervention. Circ Cardiovasc Interv. 2010;3:113-119.
27. García de Lara J, Pinar E, Ramón Gimeno J, et al. Percutaneous coronary intervention in heavily calcified lesions using rotational atherectomy and paclitaxel-eluting stents:outcomes at one year. Rev Esp Cardiol. 2010;63:107-110.
ABSTRACT
Introduction and objectives: Between 10% and 25% of patients hospitalized due to an acute coronary syndrome develop acute kidney injury, a condition associated with higher morbidity and mortality rates. Scores have been developed to predict the occurrence of post-coronary angiography contrast-induced nephropathy (CIN) in patients with acute coronary syndrome. The objective of this study was to assess the association between microalbuminuria and post-coronary angiography CIN in patients with acute coronary syndrome.
Methods: Patients admitted with acute coronary syndrome in whom a coronary angiography was performed during their hospitalization and with urinary albumin-to-creatinine ratio (ACR) assessment within the first 24 hours were analyzed. The best ACR cutoff value for coronary angiography-induced CIN was determined using the C-statistic measure. The receiver operating characteristic (ROC) curves were built to compare between the predictive ability of the Mehran score alone and also in combination with the ACR.
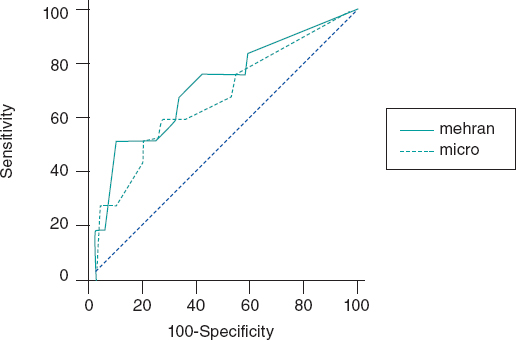
Results: A total of 148 patients were analyzed. Median age was 64 years (56-73), 35% were women, mean creatinine clearance rate at admission was 86 mL/min (66-107) and the ACR was 5 mg/g (0-14). The analysis showed that 9.6% of the patients developed post-coronary angiography CIN with ACR levels ≥ 20 mg/g compared to 1.6% when these levels were < 20 mg/g. The area under the ROC curve of the Mehran score to predict the development of post-coronary angiography CIN was 0.75 (95%CI, 0.68-0.81) and when the ACR was added it went up to 0.82 (95%CI, 0.76-0.87).
Conclusions: The ACR levels at admission were associated with the development of post-coronary angiography CIN and bring added value to an already validated predictive score. Therefore, the ACR should be used as a simple and accessible tool to detect and prevent this severe complication in patients with acute coronary syndrome.
Keywords: Contrast media. Coronary angiography. Microalbuminuria. Contrast-induced nephropathy. Urine albumin-to-creatinine ratio.
RESUMEN
Introducción y objetivos: Entre el 10 y el 25% de los pacientes hospitalizados por síndrome coronario agudo desarrollan insuficiencia renal aguda, lo que aumenta la morbimortalidad. Existen escalas para predecir la aparición de nefropatía inducida por contraste (NIC) tras la realización de una angiografía coronaria en pacientes con síndrome coronario agudo. El objetivo de este estudio fue evaluar la asociación entre el índice albúmina-creatinina (IAC) urinario y el desarrollo de NIC tras una angiografía coronaria en pacientes con síndrome coronario agudo.
Métodos: Se analizaron pacientes internados por síndrome coronario agudo a quienes se realizó angiografía coronaria durante el ingreso, con el cálculo del IAC en las primeras 24 horas. Se determinó el mejor valor de corte por curva ROC (Receiver Operating Characteristic)del IAC asociado a NIC. Se compararon las curvas ROC de la escala de Mehran sola y con el agregado de la variable de IAC.
Resultados: Se analizaron 148 pacientes. La mediana de la edad fue de 64 años (56-73), el 35% eran mujeres, el aclaramiento de creatinina fue de 86 ml/min (66-107) y el IAC de 5 mg/g (0-14). El 9,6% de los pacientes desarrollaron NIC tras la angiografía coronaria cuando su IAC fue ≥ 20 mg/g y el 1,6% cuando fue < 20 mg/g. El área bajo la curva ROC de la escala de Mehran para predecir el desarrollo de NIC tras la angiografía coronaria fue de 0,75 (intervalo de confianza del 95% [IC95%], 0,68-0,81); cuando se agregó la variable de IAC fue de 0,82 (IC95%, 0,76-0,87).
Conclusiones: El IAC basal se asoció con el desarrollo de NIC tras la angiografía coronaria. Al añadirlo a la escala de Mehran aumentó la capacidad discriminativa. El IAC podría ser una herramienta de simple uso, bajo costo y amplia disponibilidad para detectar pacientes en riesgo de desarrollar NIC y adoptar medidas preventivas apropiadas.
Palabras clave: Contraste intravenoso. Angiografía coronaria. Microalbuminuria. Nefropatía inducida por contraste. Índice albúmina-creatinina urinario.
Abbreviations:
ACR: Albumin-to-creatinine ratio. ACS: Acute coronary syndrome. AKI: Acute kidney injury. CIN: Contrast-induced nephropathy.
INTRODUCTION
Renal function impairment is associated with poor prognosis in patients with stable or acute coronary syndrome (ACS). One of the most common causes of acute kidney injury (AKI) in hospitalized patients is the nephropathy induced by the IV administration of contrast agents.1Its incidence varies between 1% and 6%, and increases considerably in high-risk conditions like in the ACS setting. The reported frequency of post-coronary angiography contrast-induced nephropathy (CIN) goes from 12% to 46% in patients with ACS.2,3
There are several potential causes that trigger CIN in patients without a past medical history of kidney failure such as hemodynamic instability, the IV administration of contrast agents, thromboembolic events, and adverse drug reactions, among others. Also, it is important to consider the type of contrast used, its osmolarity, the volume administered, and the lack of preventive measures.4-6
Because CIN is associated with poor prognosis in hospitalized patients, predictive scores have been designed to identify the most vulnerable patients who can develop this complication. The Mehran score is one of the most popular indices to estimate the chances of post-coronary angiography CIN.7
It is well-established that microalbuminuria is a predictor of kidney dysfunction mainly in diabetic and hypertensive patients.8-14Also, there is a correlation between high levels of microalbuminuria and the poor outcomes seen in patients with ACS.15-16 Currently, microalbuminuria is estimated through the dosage of the albumin-to-creatinine ratio (ACR) through a simple urine sample.17
The objective of this study is to calculate microalbuminuria using the ACR as a predictive variable of post-coronary angiography CIN in patients with ACS.
METHODS
Population
Patients with ACS consecutively admitted to the coronary care unit of a community hospital were analyzed. Those undergoing an in-hospital coronary angiography with non-ionic, hyperosmolar IV contrast agents such as iopamidol, optiray or xenetix, were included in the study. The volume of IV contrast for each angiographic study was calculated retrospectively. It was estimated that each injection of contrast material into the left coronary artery required an average 10 cc to 8 cc for the right coronary artery.
Patients with a past medical history of renal failure, macroalbuminuria, treatment with diuretics and patients with secondary angina were excluded from the study.
The urinary ACR was assessed in all patients included in the study using an immunoturbidimetric assay in simple urine samples within the first 24 hours after hospitalization.
Definitions
IV contrast-induced nephropathy(CIN) was defined as an increase in serum creatinine levels ≥ 25% 48 hours after performing the coronary angiography or an absolute increase of ≥ 0.5 mg/dL compared to levels at admission.
Microalbuminuria was defined as an abnormal urinary albumin excretion rate between 30 to 200 mg/min or 30 to 229 mg/day.
The study protocol was approved by the center review board and conducted in compliance with the Declaration of Helsinki, good clinical practice guidelines, and local regulatory requirements. Informed consents were obtained from all patients.
Biochemical considerations
A urine sample collected within the first 24 hours after admission (preferably during morning hours) was centrifuged at 3000 rpm and stored at -20° Celsius until biochemical analysis was conducted. The principle of the ACR test is immunoturbidimetry. This method is based on the reaction of human albumin antibodies to the antigen. Complexes are then measured after agglutination. The COBAS 6000 analyzer (ROCHE, Switzerland) was used to process the sample. The analytical detection limits of the assay were between 3 mg/g and 400 mg/g. The test variation coefficient was 3.8%.
Statistical analysis
The Kolmogorov-Smirnov test was used to analyze the distribution of continuous variables and their kurtosis-skewness measures. Data were expressed as mean and standard deviation or as median with interquartile range (25%-75%) and compared using Student’s t test or Mann-Whitney-Wilcoxon test for independent groups according to their parametric or non-parametric distribution, respectively.
Discrete variables were expressed as percentages and compared using the chi-square test. The cross-product ratio was expressed as odds ratio (OR) with its 95% confidence interval (95%CI). The C-statistic measure was used to detect the best ACR cutoff value associated with the primary endpoint and compare the discrimination capacity of the Mehran score alone and with the ACR combined.
A multivariable regression analysis will be built to predict CIN including ACR and adjusted using the Mehran score.
Both the IBM SPSS Statistics version 19 software and the MedCalc version 11.6.1 software (Mariakerke, Belgium) were used for statistical analysis and to calculate and compare the C-statistic measure. To test the additional predictive value of ACR, the C-statistic measurewas compared using the Mehran score alone and after adding the ACR information obtained.
RESULTS
Out of a total of 397 patients diagnosed with ACS, 148 (59.4%) underwent a coronary angiography during hospitalization and this was the study population. The mean age was 64 ± 12 years; 35% were women, 20% had diabetes, 54% dyslipidemia, 65% hypertension, and 42% were active smokers. The mean blood sugar levels on admission were 110 mg/dL (98-133 mg/dL), the median creatinine clearance rate (estimated using the MDRD) was 86 mL/min (66-107), and the ACR was 5 mg/g (0-14) (table 1). The patient comparison between these groups with or without CIN showed a higher rate of overweight and obesity, left bundle branch block, atrial fibrillation, and AMI Killip and Kimball class III-IV (table 2).
Table 1. Baseline characteristics of the patients
| Total number of patients | N = 148 |
|---|---|
| Age (years), median [25-75] | 64 [56-73] |
| Women | 35 |
| Hypertension | 65 |
| Diabetes mellitus | 20 |
| Dyslipidemia | 54 |
| Smoking | 42 |
| Previous AMI | 24.5 |
| STEAMI | 20.9 |
| NSTEACS | 79.1 |
| Fasting blood glucose levels, mg/dL | 110 [98-133] |
| Serum creatinine levels, mg/dL | 0.9 [0.8-1.0] |
| Creatinine clearance rate, mL/min | 86 [66-107] |
| Urinary albumin-to-creatinine ratio, mg/gr | 5 [0-14] |
| CPK, IU/L | 121 [73-264] |
| CK-MB, IU/L | 16 [12-34] |
| Troponin T levels, ng/mL | 0.01 [0.01-0.27] |
| Moderate to severe LVSF impairment (EF < 40%) | 5.79 |
|
Unless specified otherwise, data are expressed as % or mean and standard deviation. AMI, acute myocardial infarction; CK-MB, creatine kinase myocardial band; CPK, creatine phosphokinase; EF, ejection fraction; IQR, interquartile range; IU, international units; LVSF, left ventricular shortening fraction; NSTEACS, non-ST-segment elevation acute coronary syndrome; STEMI, ST-segment elevation myocardial infarction. |
|
Table 2. Comparison of patients with and without contrast-induced nephropathy
| CIN - (136) | CIN + (12) | P | |
|---|---|---|---|
| Age (years) | 63.7 [55-74] | 68 [61-76] | NS |
| Women | 25.3 | 16.7 | NS |
| Hypertensive | 67.7 | 58.3 | NS |
| Diabetic | 20 | 33.3 | NS |
| Body mass index | 26 [24-29] | 29 [25-31] | .05 |
| Creatinineclearence rate mg/dL | 85 [65-108] | 74 [50-98] | NS |
| Blood glucose levels at admission, mg/dL | 112 [100-142] | 143 [108-209] | NS |
| Previous AMI | 26 | 16 | NS |
| Previous PCI | 17 | 8.3 | NS |
| Previous stroke or TIA | 3.6 | 8.3 | NS |
| NSTEACS | 17.7 | 25 | NS |
| STEMI | 30.8 | 33.3 | NS |
| Left bundle branch block | 3.6 | 16.7 | .02 |
| Atrial fibrillation | 0.9 | 8.3 | .02 |
| Killip and Kimball III-IV | 4.1 | 22 | .001 |
|
Unless specified otherwise, data are expressed as % or mean and standard deviation 25%-75%. ACEI, angiotensin-converting enzyme inhibitors; AMI, acute myocardial infarction; ARA II, angiotensin II receptor antagonists; ASA, acetylsalicylic acid; CK-MB, creatine kinase myocardial band; CPK, creatine phosphokinase; EF, ejection fraction; HR, heart rate; IQR, interquartile range; IU, international units; LVSF, left ventricular shortening fraction; NS, not significant; NSTEACS, non-ST-segment elevation acute coronary syndrome; PCI, percutaneous coronary intervention; SBP, systolic blood pressure; STEMI, ST-segment elevation myocardial infarction; TIA, transient ischemic attack. |
|||
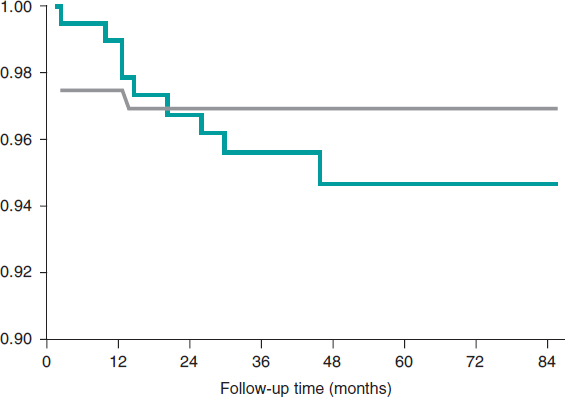
The C-statistic measure showed that the best CIN related ACR cutoff value was 20 mg/g. Twelve patients developed CIN (8.1%) and the ACR of 22% of the patients was >20 mg/g. The rate of ACR > 20 mg/g among patients without CIN was 2.9% and 11.3% (P = .01) among patients with CIN. Contrast-induced nephropathy was significantly higher when the ACR was ≥ 20 mg/g compared to when it was < 20 mg/g (≥ 9.6% vs 1.6%, res- pectively, P < .001). When the ACR was added to the Mehran score, its predictive power went up to 0.82 (95%CI, 0.76-0.87).(Figure 1).
Figure 1. Effect of albumin-to-creatinine ratio when added to the Mehran score. When the albumin-to-creatinine ratio was added to the Mehran score, its predictive power went up from 0.75 to 0.82 (95%CI, 0.76-0.87).
Using a multivariable regression analysis model the ACR > 20 mg/g turned out to be an independent predictor for CIN: OR, 3.2 (0.7-6.2); P = .01, adjusted by the Mehran score variables (age, women, body mass index, atrial fibrillation, Killip Class III-IV, and creatinine clearance rate).
DISCUSSION
Our study proved the association between the ACR and the development of CIN in patients admitted with ACS.
Acute kidney injury in the ACS setting predisposes to more complications such as in-hospital and long-term mortality; therefore, predicting it is of critical clinical importance. A recent study reported that the rate of AKI was close to 17% in the ACS setting with significant peaks of cardiovascular complications. In this study, the ACR was not used as an early marker of AKI. The development of CIN was not specifically analyzed either as a post-coronary angiography complication.18-22
Microalbuminuria calculated through the ACR obtained from a simple urine sample is also an established marker of endothelial dysfunction that has been validated to predict cardiovascular events and mortality in different clinical settings. A previous analysis of our group revealed that higher ACR levels are associated with significantly worse outcomes in patients with non-ST-segment elevation ACS, and with a higher rate of hard endpoints like mortality and/or non-fatal acute myocardial infarction at the long-term follow-up (12% vs 2.2%, P =/< .0001).23 Also, other authors proved its utility to assess the risk of developing AKI, mainly in the ACS setting or while being exposed to cardiac surgery.24Tziakas et al confirmed the significant correlation between AMI related higher ACR levels and the development of AKI after this event (area under the ROC curve 0.72; 95%CI, 0.67-0.77). However, the authors did not report on the clinical impact of this complication on the patient’s clinical course or its association with the use of contrast during coronary angiography.25
Special attention should be paid to patients with post-angiographic AKI in the ACS setting. Several studies have shown that CIN negatively impacts the prognosis of hospitalized and long-term patients. In our population, mortality in patients with CIN was significantly higher compared to those without this disease (33% vs 1.8%).
The use of urinary ACR has been less studied in this context. Meng et al. reported that high microalbuminuria levels (ACR in between 30 mg/g and 300 mg/g) were associated significantly with the development of post-contrast acute kidney injury in patients undergoing coronary catheterization (12.1% vs. 5.0%; P = .005). A key point here that distinguishes this study from ours is that they included patients with scheduled coronary angiographies only and out of the ACS setting.26Another relevant point is that the ACR cutoff value to develop CIN was determined from the analysis of the area under the ROC curve, and its value of 20 mg/g was even lower compared to the conventional standard threshold of 30 mg/g, a finding that was consistent with what other clinical studies reported.27
The rate of CIN and its impact on the clinical outcome of coronary patients triggered the development of predictive scores for this disease. One of the best known indices is the Mehran score that includes variables like age > 75 years, hypertension, functional class III/IV heart failure, diabetes mellitus, anemia, use of intra-aortic balloon pump, volume of contrast administered, and past medical history of renal dysfunction and is capable of identifying who the most vulnerable patients are to develop post-coronary angiography CIN (the area under the ROC curve was 0.75). Adding the ACR to this score showed an even greater discriminatory power to predict post-coronary angiography CIN in patients with ACS. This would prove the practical utility of adding this index as a variable to the Mehran score.
CIN, one of the most common causes for acute nephrotoxicity, is a multi-factor event. Among its causes we should mention the direct nephrotoxic effect of the contrast substances used during endovascular procedures on the renal endothelium and the development of acute tubular necrosis. It is estimated that the nephrotoxicity of hyperosmolar contrast enhanced by the hemodynamic alterations produced by the ongoing ACS could alter vascular resistance with changes in the regulation of the release and balance of vasoactive substances like adenosine, endothelin, and nitric oxide. The damage perpetuates the slowing down of renal perfusion, spinal hypoxemia, ischemic injury, and ultimately cell death. In addition to reducing the clearance of oxidative stress products, the lower glomerular filtration rate levels increase the concentration of inflammatory mediators triggering structural alterations at renal tubular epithelium level like edema, vacuolization, and death.28,29
We believe that these findings could help identify patients at high-risk of developing post-coronary angiography CIN in the ACS setting to promote preventive measures, behaviors, and strategies to avoid this complication.
Limitations
First, one of the main limitations of our work is its single center nature. However, we should mention that the population included was representative and covered the entire spectrum of patients with ACS admitted to our coronary care unit, which secures the internal validity and representativeness of our study. Secondly, the underpowered sample may have conditioned the appearance of false negative results due to its alpha error or lower power and stopped us from performing a proper multivariable analysis. Finally, certain data such as the volume of contrast used in each study was calculated retrospectively with the usual biases of this type of analysis.
CONCLUSIONS
The albumin-to-creatinine ratio, a recognized predictor of renal and endothelial dysfunction, was also a marker of CIN in patients with ACS with an added value when it was included in a widely validated clinical score. These results may be the beginning of a hypothesis-generating study to be confirmed prospectively at a multi-center level.
FUNDING
No funding or grants were received for this work.
CONFLICTS OF INTEREST
None declared.
ACKNOWLEDGEMENTS
We wish to thank the entire staff of the Hospital Alemán Coronary Care Unit, particularly the nursing staff who helped collect the urine samples that were crucial to conduct this study.
WHAT’S KNOWN ABOUT THE TOPIC?
- CIN is one of the most common causes for AKI in hospitalized patients. Microalbuminuria is an established marker of endothelial dysfunction and has been validated to predict cardiovascular events and mortality in different clinical settings. The ACR is useful to assess the risk of developing CIN basically in the ACS setting or while exposed to cardiac surgery.
WHAT DOES THIS STUDY ADD?
- Our study proved the association that exists between the ACR and the development of post-coronary angiography CIN in patients admitted with ACS. The C-statistic measure showed that the best CIN related ACR cutoff value was 20 mg/g. The ACR brings an added value when included in the Mehran score to assess the risk of developing post-coronary angiography CIN in the ACS setting.
REFERENCES
1. Hsiao PG, Hsieh CA, Yeh CF, et al. Early prediction of acute kidney injury in patients with acute myocardial injury. J Crit Care. 2012;27:525.e1-e7.
2. McCullough PA. Contrast-induced acute kidney injury. J Am Coll Cardiol. 2008;51:1419-1428.
3. Mehran R, Nikolski E. Contrast-induced nephropathy:Definition, epidemiology, and patients at risk. Kidney International. 2006;69:S11-S15.
4. Parikh CR, Coca SG, Wang Y et al. Long-term prognosis of acute kidney injury after acute myocardial infarction. Arch Intern Med. 2008;168:987-995
5. Persson PB, Hansell P, Liss P. Pathophysiology of contrast medium-induced nephropathy, Kidney Int. 2005;68:14-22.
6. Tsai TT, Patel UD, Chag TI, et al. Contemporary incidence, predictors and outcomes of acute kidney injury in patients undergoing percutaneous coronary interventions:insights from the NCDR Cath-PCI Registry. JACC Cardiovasc Interv. 2014;7:1-9.
7. Mehran R, Aymong ED, Nikolsky E, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention. J Am Coll Cardiol. 2004;44:1393-1399.
8. Mogensen CE, Christensen CK. Predicting diabetic nephropathy in insulin-dependent patients. N Engl J Med. 1984;31:89-93.
9. Valmadrid CT, Klein R, Moss SE, Klein BE. The risk of cardiovascular disease morbidity associated with microalbuminuria and gross proteinuria in persons with older-onset diabetes mellitus. Arch Intern Med. 2000;160:1093-1100.
10. Dogra G, Rich L, Stanton K, Watts Parving H. Microalbuminuria in essential hypertension and diabetes. J Hypertens. 1996;14:S89-S94.
11. Deckert T, Feldt-Rasmussen B, Borch-Johnsen K, Jensen T, Kofoed-Enevoldsen A. Albuminuria reflects under-spread vascular damage:the steno hypothesis. Diabetologia. 1989;32:219-226.
12. Estacio RO, Dale RA, Schrier R, Krantz MJ. Relation of reduction in urinary albumin excretion to ten-year cardiovascular mortality in patients with type 2 diabetes and systemic hypertension. Am J Cardiol. 2012;109:1743-1748.
13. Stehouwer CD, Smulders YM. Microalbuminuria and risk for cardiovascular disease:Analysis of potential mechanisms. J Am Soc Nephrol. 2006;17:2106-2111.
14. Bennett PH, Haffner S, Kasiske BL, et al. Screening and management of microalbuminuria in patients with diabetes mellitus:recommendations to the Scientific Advisory Board of the National Kidney Foundations from an ad hoc committee of the Council on Diabetes Mellitus of the National Kidney Foundation. Am J Kidney Dis. 1995;25:107-12.
15. Berton G, Cordiano R, Palmieri F, Cucchini R, De Toni R, Palatini P. Microalbuminuria during acute myocardial infarction. A strong predictor for 1-year morbidity. Eur Heart J. 2001;22:1466-1475.
16. Nazer B, Ray KK, Murphy SA, Gibson M, Cannon CP. Urinary albumin concentration and long-term cardiovascular risk in acute coronary syndrome patients:A PROVE IT-TIMI 22 sub-study. J Thromb Thrombolysis. 2013;36:233-239.
17. Jensen JS, Clausen P, Borch-Johnsen K, Jensen G, Feldt-Rasmussen B. Detecting microalbuminuria by urinary albumin/keratinize concentration ratio. Nephrol Dial Transplant. 1997;12S2:6-9.
18. Marenzi G, Assanelli E, Campodonico J, et al. Contrast volume during primary percutaneous coronary intervention and subsequent contrast-induced nephropathy and mortality. Ann Intern Med. 2009;150:170-177.
19. Rihal CS, Textor SC, Grill DE, et al. Incidence and prognostic importance of Acute Renal Failure after percutaneous coronary intervention. Circulation. 2002;105:2259-2264.
20. Garcia S, Ko B, Adabag S. Contrast-induced nephropathy and risk of acute kidney injury and mortality after cardiac operations. Ann Thorac Surg. 2012;94:772-777.
21. Bouzas-Mosquera A, Vázquez-Rodríguez JM, Calviño-Santos R, et al. Contrast-Induced Nephropathy and Acute Renal Failure Following Emergent Cardiac Catheterization:Incidence, Risk Factors and Prognosis. Rev Esp Cardiol. 2007;60:1026-1034.
22. Ueda J, Nygren A, Hansell P, Ulfendahl HR. Effect of intravenous contrast media on proximal and distal tubular hydrostatic pressure in the rat kidney. Acta Radiologica. 1993;34:83-87.
23. Higa CC, Novo FA, Nogues I, Ciambrone MG, Donato MS, Gambarte MJ, Rizzo N, Catalano MP, Korolov E, Comignani PD. Single spot albumin to creatinine ratio:A simple marker of long-term prognosis in non-ST segment elevation acute coronary syndromes. Cardiology J. 2016;23:236-241
24. Coca SG, Jammalamadaka D, Sint K, et al. Preoperative proteinuria predicts acute kidney injury in patients undergoing cardiac surgery. J Thorac Cardiovasc Surg. 2012;143:495-502.
25. Tziakas D, Chalikias G, Kareli D, et al. Spot urine albumin to creatinine ratio outperforms novel acute kidney injury biomarkers in patients with acute myocardial infarction. Int J Cardiol. 2015;197:48-55.
26. Meng H, Wu P, Zhao Y, et al. Microalbuminuria in patients with preserved renal function as a risk factor for contrast-Induced acute kidney injury following invasive coronary angiography. Eur J Radiol. 2016;85:1063-1067.
27. Heart Outcomes Prevention Evaluation Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus:Results of the HOPE study and MICRO HOPE sub-study. Lancet. 2000;355:253-259.
28. Maioli M, Toso A, Gallopin M, et al. Preprocedural score for risk of contrast-induced nephropathy in elective coronary angiography and intervention. J Cardiovasc Med (Hagerstown). 2010;11:444-449.
29. Goldenberg I, Matetzky S. Nephropathy induced by contrast media:pathogenesis, risk factors and preventive strategies. CMAJ. 2005;172:1461-1471.
ABSTRACT
Introductionand objectives: We assessed whether the routine use of subcutaneous nitroglycerin prior to a cannulation attempt improves transradial access significantly (the NiSAR study [subcutaneous nitroglycerin in radial access]).
Methods: Patients undergoing a coronary angiography were enrolled in a prospective, double-blind, multicenter, randomized trial in 2 groups (nitroglycerin group vs control group). The primary endpoints were the overall number of puncture attempts, access and procedural time, switch to transfemoral access, and local perceived discomfort score. The secondary endpoints were the pre- and post-anesthetic pulse score. A subgroup of patients underwent ultrasound scans performed through the radial artery.
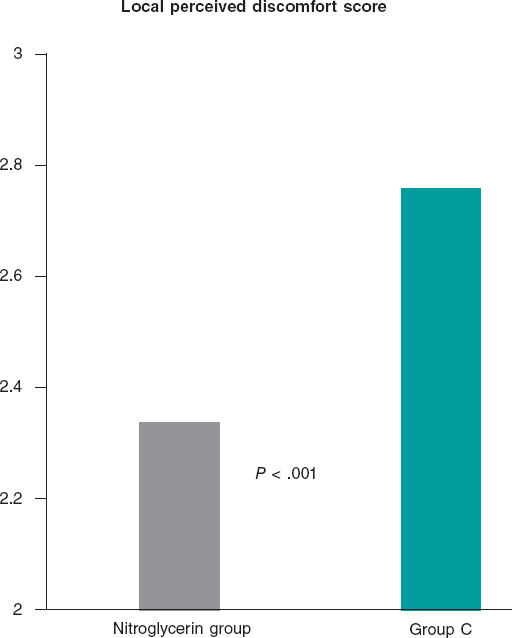
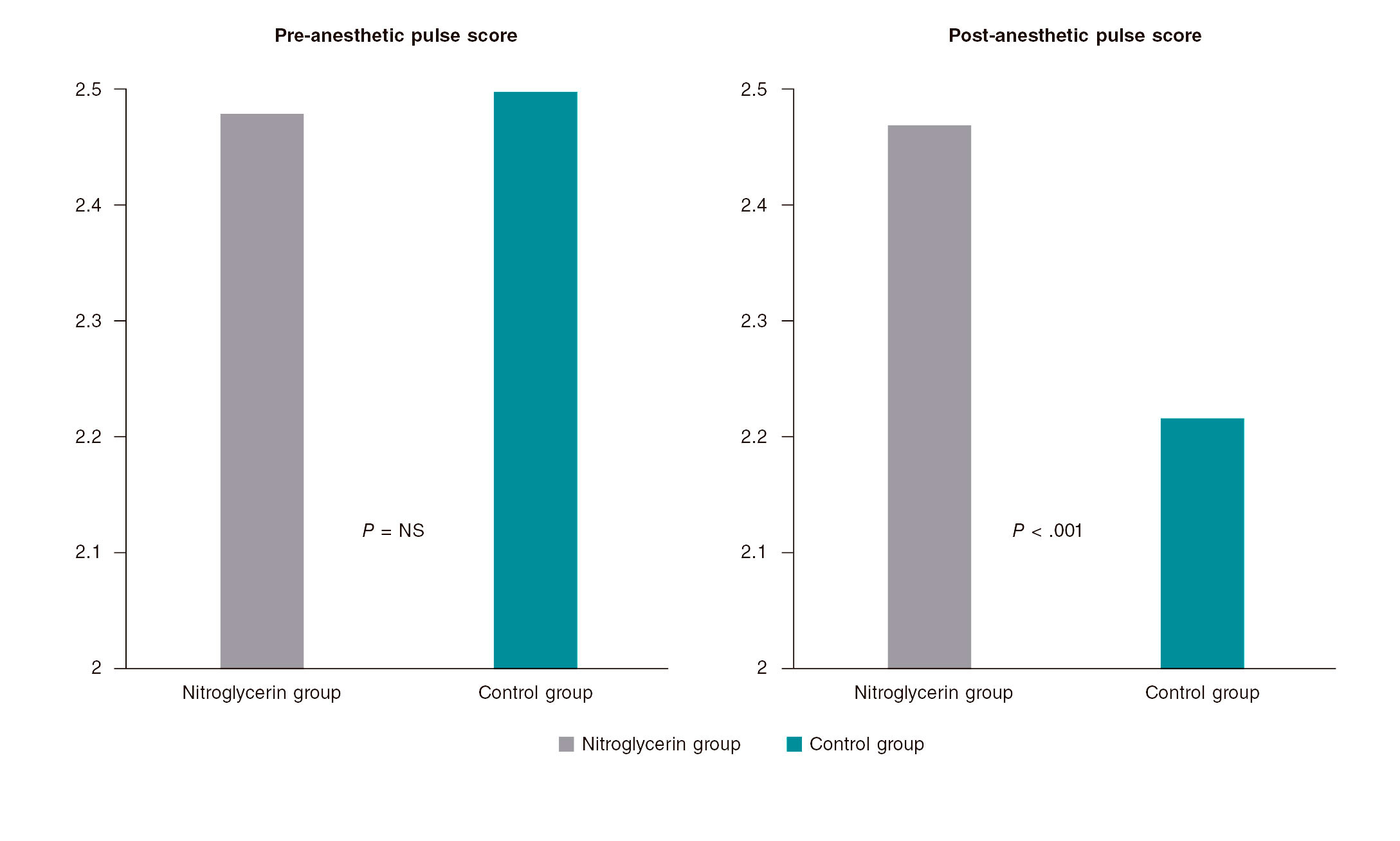
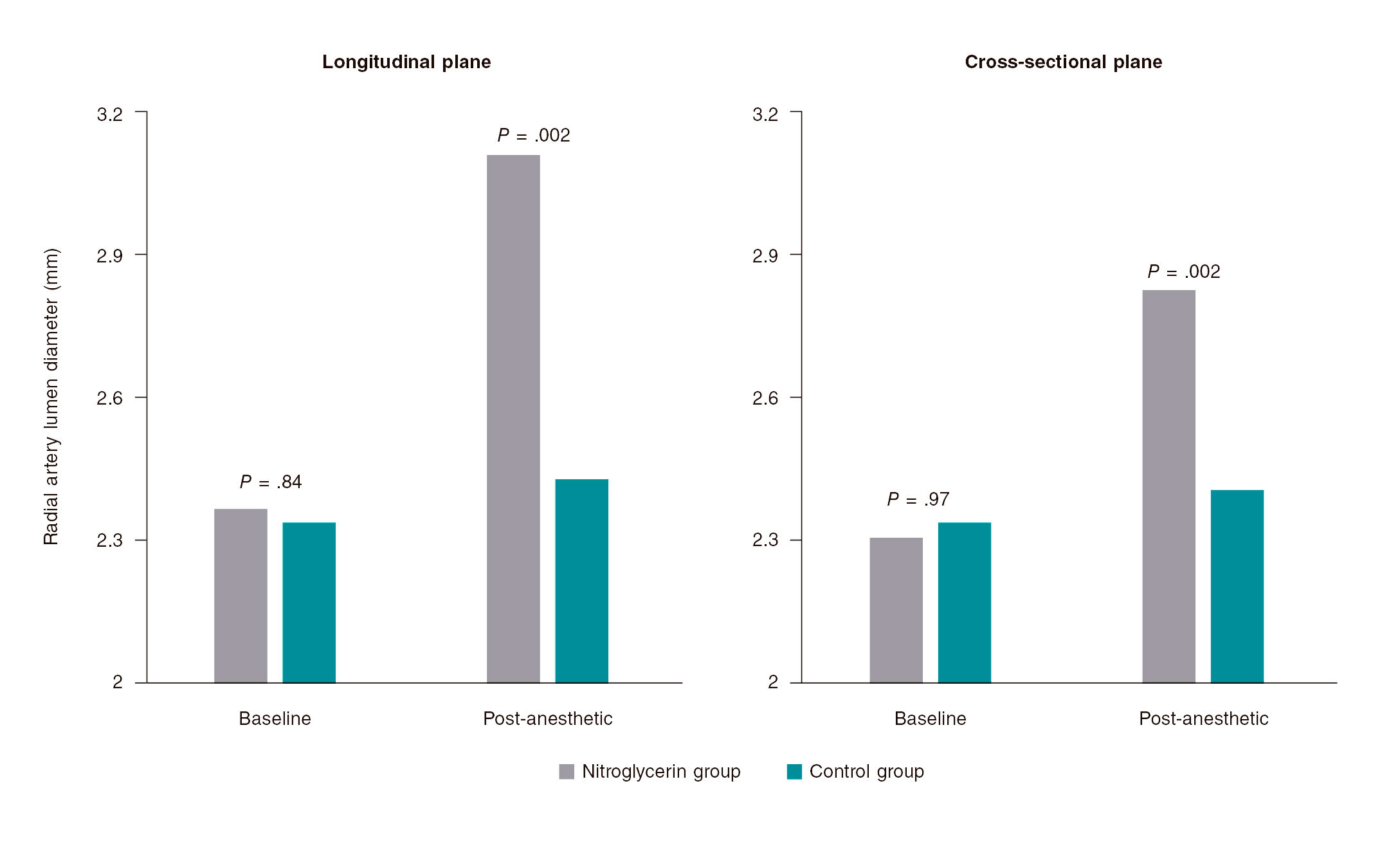
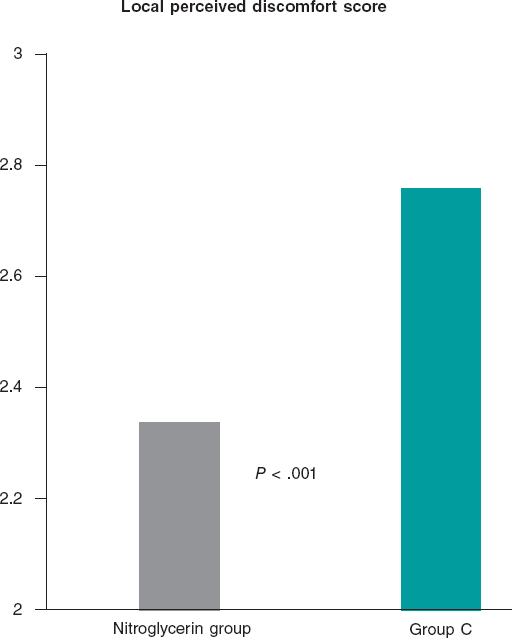
Results: 736 patients were enrolled in the trial: 379 in the nitroglycerin group and 357 in control group. The average number of puncture attempts was similar (1.70 vs 1.76; P = .42). Access and procedural time did not change significantly (61.1 s and 33.3 s vs 63 s and 33.4 s; P = .66 and P = .64, respectively). No significant differences were found either between the 2 groups in the number of switches to transfemoral access (7.1% vs 8.4%; P = .52). However, the average local perceived discomfort score and post-anesthetic pulse score were significantly better in the nitroglycerin group (2.34 vs 2.76; P< .001 and 2.47 vs 2.22; P< .001). The ultrasound scan performed through the radial artery showed post-anesthetic radial artery lumen diameters that were significantly higher in the nitroglycerin group in both the longitudinal (3.11 mm vs 2.43 mm; P = .002) and cross-sectional planes (2.83 mm vs 2.41 mm; P = .002). A trend towards fewer local hematomas in the nitroglycerin group was seen (6.1% vs 9.8%; P = .059). Headaches were more common in the nitroglycerin group (3.2% vs 0.6%; P = .021).
Conclusions: The routine use of subcutaneous nitroglycerin prior to radial puncture was not associated with fewer punctures or shorter access times. However, the lower local perceived discomfort and enlargement of the radial artery size would justify its daily use in the routine clinical practice to enhance the transradial experience for both patients and operators.
Keywords: Transradial access. Subcutaneous nitroglycerin. Radial spasm.
RESUMEN
Introducción y objetivos: Se evaluó si la utilización sistemática de nitroglicerina subcutánea previa a cualquier intento de canulación podía mejorar de forma significativa el acceso transradial (nitroglicerina subcutánea acceso radial [NiSAR]).
Métodos: Se incluyeron todos los pacientes sometidos a angiografía coronaria en un estudio prospectivo, multicéntrico, doble ciego y aleatorizado, y se dividió la población en 2 grupos: grupo de nitroglicerina y grupo control. Los objetivos primarios del estudio fueron el número total de punciones radiales, el tiempo total de acceso y de procedimiento, la necesidad de cambio a acceso femoral y la puntuación de disconfort local. El objetivo secundario fue la evaluación del pulso antes y tras la anestesia. Además, un subgrupo de pacientes fue evaluado con ecografía de la arteria radial.
Resultados: Se incluyeron736 pacientes: 379 en el grupo de nitroglicerina y 357 en el grupo C. El número promedio de intentos de punción radial fue similar en ambos (1,70 frente a 1,76; p = 0,42). No hubo diferencias significativas en los 2 grupos con respecto al tiempo total del acceso y del procedimiento (61,1 y 33,3 s frente a 63 y 33,4 s; p = 0,66 y p = 0,64, respectivamente). Tampoco se encontraron diferencias significativas entre los 2 grupos en la tasa de conversión a acceso femoral (7,1 en el grupo de nitroglicerina frente a 8,4% en el grupo C; p = 0,52). Sin embargo, el índice de malestar local y el de pulso tras la anestesia fueron significativamente mejores en el grupo de nitroglicerina (2,34 frente a 2,76, p < 0,001; 2,47 frente a 2,22, p < 0,001). La ecografía mostró un diámetro radial significativamente mayor en el grupo de nitroglicerina tanto en la vista longitudinal (3,11 frente a 2,43 mm; p = 0,002) como en la transversal (2,83 frente a 2,41 mm; p = 0,002). Hubo una menor incidencia de hematoma en el antebrazo en el grupo de nitroglicerina (6,1 frente a 9,8%; p = 0,059). La cefalea fue más frecuente en los pacientes del grupo de nitroglicerina (3,2 frente a 0,6%; p = 0,021).
Conclusiones: El uso sistemático de nitroglicerina subcutánea previo a la punción radial no estuvo asociado a una reducción en el número de punciones ni en el tiempo de acceso, pero el menor malestar local y el aumento del calibre de la arteria radial podrían justificar su uso en la práctica clínica para mejorar la experiencia del acceso transradial tanto en el paciente como en el operador.
Palabras clave: Espasmo radial. Nitroglicerina subcutánea. Acceso radial.
INTRODUCTION
Transradial access to perform coronary and peripheral procedures is becoming more successful compared to transfemoral access thanks to several advantages including more comfort as reported by the patients, early ambulation and discharge, less bleeding, and overall better outcomes.1-5However, the radial artery is more susceptible to spasm, which can stop the advance of the catheter, extend the duration of the procedure, and increase its difficulty.6Also, radial artery spasm has been identified as an independent predictor of radial access failure.7
When radial artery spasm occurs after an introducer sheath has been inserted, the intra-arterial administration of vasodilator drugs has proved to improve the conduit effectively.8Still, the subcutaneous administration of nitroglycerin relieves the spasm causing the reduction significantly and the eventual loss of pulse volume after several ineffective attempts to cannulate the radial artery.9Also, it enhances radial pulse palpation, and eventually makes the puncture of radial artery easier.10,11
Because the first puncture failure is a powerful predictor of radial artery spasm,12we conducted a double-blind, randomized, controlled trial in 4 Argentinian centers to see whether the routine subcutaneous administration of nitroglycerin prior to a cannulation attempt improved transradial access significantly (the NISAR study [subcutaneous nitroglycerin in radial access]).
Specifically, the primary endpoints of the study were to assess the number of radial artery puncture attempts, the time required to place the sheath introducer, the number of times that switching to transfemoral access was required, and the patients’ tolerance to the procedure. The secondary endpoints included the assessment of the radial artery pulse and diameter and local and systemic complications.
METHODS
Patients and procedures
Patients undergoing a coronary angiography with evidence of myocardial ischemia were enrolled in a prospective, multicenter, and randomized clinical trial conducted in 4 Argentinian centers into 2 different groups based on the periradial subcutaneous administration of nitroglycerin. In the nitroglycerin group, 2% xylocaine (1 mL) was used followed by 200 mcg of nitroglycerin (2 mL). In control group, 2% xylocaine (1 mL) was followed by the infusion of a normal saline solution (2 mL) used as placebo. Trained nurses from each center prepared the syringes following a 1:1 randomization scheme and making sure that their content was unknown to both the operators and the patients.
The coronary angiographies and revascularization procedures were performed using 5-Fr or 6-Fr diagnostic and guiding catheters as selected by the operators. In all cases a properly sized sheath introducer was inserted using the Seldinger or modified Seldinger technique. Five thousand units of heparin were consistently administered through a bolus injection with further additions to keep the activated clotting time between 250 and 300 seconds if a percutaneous coronary intervention was performed.
All procedures were performed after patients gave their informed consent by 8 skilled and experienced operators who had performed over 1500 transradial procedures. All operators used the right radial artery as the access of choice; the left radial artery was spared for cases with right radial artery occlusion and patients with left internal mammary artery graft. The Ethics Committe reviewed and approved this study. Patients' informed consent to publish was obtained.
Outcome measures
The primary outcome measures were the overall number of puncture attempts, access, and procedural time, switch to transfemoral access, and local perceived discomfort score.
Access time was defined as the time elapsed between the administration of local anesthesia and the insertion of the radial sheath introducer. When the initial radial access could not be completed, the contralateral radial access was never tried and access site changed to the femoral access. The local perceived discomfort score was assessed by the patient after undergoing the procedure and graded according to a radial-related pain score between 0 = no pain and 10 = unbearable pain.
The secondary outcome measures were the pre- and post-anesthetic pulse score assessed by the operator by palpating the radial pulse before and 1 minute after the administration of local anesthesia and graded as: 1 = weak pulse; 2 = easily palpable pulse; 3 = strong pulse. Also, local and systemic complications including forearm hematomas, radial artery spasm, headaches, and symptomatic hypotension were recorded. Also, a subgroup of patients underwent a radial artery ultrasound scan both at the baseline and after the administration of anesthesia. Patients were examined in the supine position using a commercially available ultrasound system. The radial artery lumen diameter was measured on M-mode imaging in both the longitudinal and cross-sectional planes and 1 cm proximal to the radius styloid process. Three measures were taken in each plane and their values averaged.
Statistics
Continuous variables were compared using the Student t test. Categorical variables were compared using Pearson chi-square test. Data were expressed as mean ± standard deviation or frequency (percentage). Two-tailed P values < .05 were considered statistically significant.
RESULTS
Characteristics of patients and procedural details
Overall, 736 patients (450 men, age 65 ± 10 years) were enrolled in the trial: 379 (51.5%) in the nitroglycerin group and 357 (48.5%) in control group. Table 1 shows their general characteristics. Active smoking and diabetes mellitus were reported by 292 (39.7%) and 168 (22.8%) of the patients, respectively and 240 (46.1%) showed an unstable presentation. The radial access was the first access attempted in 597 patients (81.1%).
Table 1. General characteristics of the patients
| Overall (N = 736) | Nitroglycerin group (N = 379) | Control group (N = 357) | P | |
|---|---|---|---|---|
| Age (years) | 64.9 ± 10.1 | 64.9 ± 10.1 | 65.1 ± 10.1 | .80 |
| Male gender | 450 (61.1%) | 230 (60.7%) | 220 (61.6%) | .79 |
| Body mass index | 28.5 ± 4.2 | 28.5 ± 4.2 | 28.4 ± 4.2 | .82 |
| Active smoking | 292 (39.7%) | 153 (40.3%) | 139 (38.9%) | .69 |
| Hypertension | 520 (70.6%) | 277 (73.1%) | 243 (68.1%) | .14 |
| High cholesterol | 365 (49.6%) | 189 (49.8%) | 176 (49.3%) | .88 |
| Diabetes mellitus | 168 (22.8%) | 97 (25.6%) | 71 (19.9%) | .07 |
| Clinical presentation | ||||
| ST-segment elevation myocardial infarction | 55 (7.5%) | 28 (7.4%) | 27 (7.6%) | .68 |
| Non-ST-elevation acute myocardial infarction | 285 (38.7%) | 139 (36.7%) | 146 (40.9%) | |
| Chronic stable angina | 90 (12.2%) | 51 (13.4%) | 39 (10.9%) | |
| Silent ischemia | 123 (16.7%) | 67 (17.7%) | 56 (15.7%) | |
| Preoperative assessment | 64 (8.7%) | 33 (8.7%) | 31 (8.7%) | |
| First transradial access attempt | 597 (81.1%) | 307 (81%) | 290 (81.2%) | .94 |
| Procedure | ||||
| Coronary angiography | 507 (68.9%) | 259 (68.3%) | 248 (69.5%) | .55 |
| Percutaneous coronary intervention | 24 (3.3%) | 15 (3.9%) | 9 (2.5%) | |
| Coronary angiography and ad hoc revascularization procedure | 205 (27.9%) | 105 (27.7%) | 100 (28%) |
Procedural details are shown on table 2. In most cases, the radial artery was punctured with a 20G IV catheter using the modified Seldinger technique and a plastic-jacked mini-guidewire advanced through the artery lumen. Small and short sheath introducers were used in less than half of the patients.
| Overall (N = 736) | Nitroglycerin group (N = 379) | Control group (N = 357) | P | |
|---|---|---|---|---|
| Allen test result | ||||
| Normal | 659 (89.5%) | 338 (89.2%) | 321 (89.9%) | .94 |
| Intermediate | 66 (9%) | 35 (9.2%) | 31 (8.7%) | |
| Abnormal | 11 (1.5%) | 6 (1.6%) | 5 (1.4%) | |
| Radial puncture and introducer placement | ||||
| 20G IV catheter | 719 (97.7%) | 371 (97.9%) | 348 (97.5%) | .71 |
| 0.021 in mini-guidewire | 701 (95.2%) | 364 (96%) | 337 (94.4%) | .29 |
| Plastic-jacketed mini-guidewire | 684 (92.9%) | 358 (94.4%) | 326 (91.3%) | .10 |
| Introducer length < 10 cm | 292 (39.7%) | 162 (42.7%) | 130 (36.4%) | .08 |
| Introducer size < 6-Fr | 318 (43.2%) | 166 (43.8%) | 152 (42.5%) | .74 |
| Radial artery angiography | 271 (36.8%) | 144 (38%) | 127 (35.6%) | .50 |
Outcomes
The average number of puncture attempts was similar in the nitroglycerin group compared to control group (1.70 vs 1.76; P = .42). Access and procedural times did not change significantly in either one of the 2 groups (61.1 s and 33.3 s vs 63 s and 33.4 s; P = .66 and P = .64, respectively). No significant inter-group differences were found either in the rate of switch to transfemoral access (7.1% in the nitroglycerin group vs 8.4% in control group, P = .52).
The main results of the patients and their local perceived discomfort score are shown on figure 1. The average local perceived discomfort score was significantly better in the nitroglycerin group (2.34 vs 2.76; P< .001) with a significantly higher rate of grade 0/1 (34.3% vs 25.2%; P = .088) and a lower rate of grade > 3 (33.5% vs 50.4%; P< .001).
Figure 1. Patients tolerance to the transradial procedure. The average local perceived discomfort score was significantly better in patients in whom nitroglycerin was administered subcutaneously compared to those in whom placebo was used (2.34 vs 2.76; P < .001).
figure 2 shows the results of pre- and post-anesthetic pulse score assessment. No significant differences were seen in the pre-anesthetic pulse score. However, the post-anesthetic pulse score was significantly higher in the nitroglycerin group (2.47 vs 2.22, P< .001). The rate of post-anesthetic pulse score < 3 was significantly lower in the nitroglycerin group compared to group C (41.7% vs 57.1%, P< .001).
Figure 2. Operator assessment of radial pulse. The left panel shows that no significant differences were found in the pre-anesthetic pulse score between patients in whom nitroglycerin was administered subcutaneously (nitroglycerin group) and those in whom placebo was used control group. The right panel shows that the post-anesthetic pulse score was significantly higher in the nitroglycerin group compared to control group (2.47 vs 2.22; P < .001).
Radial artery ultrasound scans were performed in 70 patients; the results are shown on figure 3. No significant inter-group differences were seen at the baseline between the longitudinal (2.37 mm vs 2.34 mm; P = .84) and cross-sectional planes (2.31 mm vs 2.34 mm; P = .97). However, the post-anesthetic radial artery lumen diameter was significantly higher in the nitroglycerin group in both the longitudinal (3.11 mm vs 2.43 mm; P = .002) and cross-sectional planes (2.83 mm vs 2.41 mm; P = .002).
Figure 3. Radial artery ultrasound scan. No significant inter-group differences were seen at the baseline between the longitudinal (2.37 mm vs 2.34 mm; P= .84) and the cross-sectional planes (2.31 mm vs 2.34 mm; P = .97). However, the post-anesthetic radial artery lumen diameter was significantly higher in the nitroglycerin group compared to control group in both the longitudinal (3.11 mm vs 2.43 mm; P = .002) and cross-sectional planes (2.83 mm vs 2.41 mm; P = .002).
As shown on table 3, no significant differences in local complications were seen, although a trend towards a lower rate of local hematomas was seen in the nitroglycerin group (6.1% vs 9.8% P = .059). Headaches were more common among patients from nitroglycerin groups (3.2% vs 0.6%, P = .021).
Table 3. Main local and systemic complications
| Overall (N = 736) | Nitroglycerin group (N = 379) | Control group (N = 357) | P | |
|---|---|---|---|---|
| Local complications | ||||
| Forearm hematoma | 58 (7.9%) | 23 (6.1%) | 35 (9.8%) | .059 |
| Radial artery spasm | 109 (14.8%) | 49 (12.9%) | 60 (16.8%) | .14 |
| Systemic complications | ||||
| Headache | 14 (1.9%) | 12 (3.2%) | 2 (0.6%) | .021 |
| Symptomatic hypotension | 16 (2.2%) | 11 (2.9%) | 5 (1.4%) | .25 |
DISCUSSION
The main findings of our study are that the subcutaneous administration of nitroglycerin plus the administration of a local anesthetic agent prior to radial artery puncture did not show any statistically significant differences in the number of punctures attempted, access and procedural time or switch to transfemoral access. However, it significantly improved: a) the patients’ perceived comfort during the procedure; b) the radial artery pulse; and c) the radial artery size. Also, our data suggest a possible reduction in the occurrence of local hematomas. Also consistent with former studies, the subcutaneous use of nitroglycerin significantly increased the diameter of the radial artery in patients in whom an ultrasound scan was performed.10,13
The radial artery spasm is the most common complication of transradial access in both coronary angiographies and procedures. It often holds up the regular course of the procedure impacting the patients’ compliance and interfering with the cath lab proceedings.6,9Also, the occurrence of radial artery spasm before radial artery cannulation is even more frustrating to treat and may anticipate that the cannulation of the vessel will be impossible.
Multiple puncture attempts are the leading cause for radial artery spasm and may be a specific issue in the teaching environment.14,15Also, the administration of local anesthetics such as lidocaine has vasoconstrictive properties16 and the radial artery has a relatively small diameter and a relatively thicker tunica media of smooth muscle cells, which leads to a high receptor-mediated vasomotion compared to other muscular arteries.17,18Conversely, the radial artery is particularly sensitive to nitroglycerin.19
Former studies have shown that nitroglycerin delivered through IV,20topical,21or intra-arterial16,22-24routes of administration determines the radial artery dilatation; current evidence with subcutaneous nitroglycerin to facilitate radial access suggests that it can be beneficial to increase the radial pulse and reduce the number of attempts. However, the evidence on this regard is scarce and based on small studies.10,11A review that assessed this issue also failed to find significant differences between both strategies.25Our study rigorously used a double-blind, randomized protocol to assess the role of the subcutaneous administration of nitroglycerin prior to radial artery puncture. It concluded that its systematic use can improve the patient’s perceived discomfort and make puncture easier for the operator but without reducing the number of punctures attempted or access time. Our findings are especially relevant in light of the improved safety associated with transradial access.26
The subcutaneous administration of nitroglycerin is a straightforward and inexpensive technique that allows a high concentration and long persistence of the vasoactive agent at the spasm site level without entering the bloodstream significantly.9As a matter of fact, in our study no significant differences were seen in the hemodynamic effect of patients who received subcutaneous nitroglycerin or placebo.
Also, the Doppler ultrasound scans performed on the radial artery pre- and post-nitroglycerin in a subgroup of patients triggered the new NISAR study (Eco nitroglicerina subcutánea acceso radial)—currently in its design phase—with echocardiographic evaluation of all the patients included.
Limitations
All the patients of this study were taking standard anti-ischemic drugs including nitrates. We did not study the confounding effect of the vasodilation caused by these drugs. The inter-observer and inter-operator variabilities were not studied either. The Doppler ultrasound scan was used in a small subgroup of patients.
CONCLUSIONS
The routine use of subcutaneous nitroglycerin prior to radial puncture was not associated with a lower number of punctures or shorter access times. However, the lower local perceived discomfort and improved radial artery size would justify its daily use in the routine clinical practice to enhance the transradial experience of both patients and operators.
FUNDING
No funding was received for this work.
CONFLICTS OF INTEREST
None declared.
WHAT IS KNOWN ABOUT THE TOPIC?
- Radial artery spasm is still an issue; intra-arterial nitroglycerin and calcium blockers are systematically used after achieving radial access to prevent it. However, the use of subcutaneous nitroglycerin plus the administration of a local anesthetic agent prior to radial puncture is still controversial. This is so because the studies conducted so far on this issue are mostly scarce, small, and not randomized. This was confirmed in a review published back in 2018.
WHAT DOES THIS STUDY ADD?
- The strength of our study is that it is the first prospective, randomized, multicenter, double-blind trial to assess this issue.
- Regarding the results from the trial and although some hard endpoints did not reach statistically significant differences, we believe that the fact that patients tolerated the procedure better, the increase seen in the pulse score and the radial artery diameter after the administration of subcutaneous nitroglycerin added to the simplicity, security and great availability of the procedure is indicative that this technique should be widely used.
REFERENCES
1. Rao SV, Cohen MG, Kandzari DE, Bertrand OF, Gilchrist IC. The transradial approach to percutaneous coronary intervention:historical perspective, current concepts, and future directions. J Am Coll Cardiol. 2010;55:2187-2195.
2. Cooper CJ, El-Shiekh RA, Cohen DJ, et al. Effect of transradial access on quality of life and cost of cardiac catheterization:A randomized comparison. Am Heart J. 1999;138:430-436.
3. Valgimigli M, Gagnor A, Calabro P, et al. Radial versus femoral access in patients with acute coronary syndromes undergoing invasive management:a randomised multicentre trial. Lancet. 2015;385:2465-2476.
4. Wiper A, Kumar S, MacDonald J, Roberts DH. Day case transradial coronary angioplasty:a four-year single-center experience. Catheter Cardiovasc Interv. 2006;68:549-553.
5. Bertrand OF, De Larochelliere R, Rodes-Cabau J, et al. A randomized study comparing same-day home discharge and abciximab bolus only to overnight hospitalization and abciximab bolus and infusion after transradial coronary stent implantation. Circulation. 2006;114:2636-2643.
6. Ruiz-Salmeron RJ, Mora R, Velez-Gimon M, et al. Radial artery spasm in transradial cardiac catheterization. Assessment of factors related to its occurrence, and of its consequences during follow-up. Rev Esp Cardiol. 2005;58:504-511.
7. Abdelaal E, Brousseau-Provencher C, Montminy S, et al. Risk score, causes, and clinical impact of failure of transradial approach for percutaneous coronary interventions. JACC Cardiovasc Interv. 2013;6:1129-1137.
8. Kiemeneij F, Vajifdar BU, Eccleshall SC, Laarman G, Slagboom T, van der Wieken R. Evaluation of a spasmolytic cocktail to prevent radial artery spasm during coronary procedures. Catheter Cardiovasc Interv. 2003;58:281-284.
9. Pancholy SB, Coppola J, Patel T. Subcutaneous administration of nitroglycerin to facilitate radial artery cannulation. Catheter Cardiovasc Interv. 2006;68:389-391.
10. Ezhumalai B, Satheesh S, Jayaraman B. Effects of subcutaneously infiltrated nitroglycerin on diameter, palpability, ease-of-puncture and pre-cannulation spasm of radial artery during transradial coronary angiography. Indian Heart J. 2014;66:593-597
11. Ouadhour A, Sideris G, Smida W, Logeart D, Stratiev V, Henry P. Usefulness of subcutaneous nitrate for radial access. Catheter Cardiovasc Interv. 2008;72:343-346.
12. Jia DA, Zhou YJ, Shi DM, et al. Incidence and predictors of radial artery spasm during transradial coronary angiography and intervention. Chin Med J. 2010;123:843-847.
13. Candemir B, Kumbasar D, Turhan S, Kilickap M, Ozdol C, Akyurek O,et al. Facilitation of radial artery cannulation by periradial subcuta-neous administration of nitroglycerin. J Vasc Interv Radiol. 2009;20:1151-1156.
14. Goldberg SL, Renslo R, Sinow R, French WJ. Learning curve in the use of the radial artery as vascular access in the performance of percutaneous transluminal coronary angioplasty. Cathet Cardiovasc Diagn. 1998;44:147-152.
15. Fukuda N, Iwahara S, Harada A, et al. Vasospasms of the radial artery after the transradial approach for coronary angiography and angioplasty. Jpn Heart J. 2004;45:723-731.
16. Abe S, Meguro T, Endoh N et al. Response of the radial artery to three vasodilatory agents. Catheter Cardiovasc Interv. 2000;49:253-256.
17. He GW, Yang CQ. Radial artery has higher receptor-mediated contractility but similar endothelial function compared with mammary artery. Ann Thorac Surg. 1997;63:1346-1352.
18. He GW, Yang CQ. Characteristics of adrenoceptors in the human radial artery:clinical implications. J Thorac Cardiovasc Surg. 1998;115:1136-1141.
19. Shapira OM, Xu A, Aldea GS, Vita JA, Shemin RJ, Keaney JF Jr. Enhanced nitric oxide-mediated vascular relaxation in radial artery compared with internal mammary artery or saphenous vein. Circulation. 1999;100:II322-7.
20. Zabeeda D, Medalion B, Jackobshvilli S, Ezra S, Schachner A, Cohen AJ. Comparison of systemic vasodilators:effects on flow in internal mammary and radial arteries. Ann Thorac Surg. 2001;71:138-141.
21. Beyer AT, Ng R, Singh A et al. Topical nitroglycerin and lidocaine to dilate the radial artery prior to transradial cardiac catheterization:a randomized, placebo-controlled, double-blind clinical trial:the PRE-DILATE Study. Int J Cardiol. 2013;168:2575-2578.
22. Boyer N, Beyer A, Gupta V, et al. The effects of intra-arterial vasodilators on radial artery size and spasm:implications for contemporary use of trans-radial access for coronary angiography and percutaneous coronary intervention. Cardiovasc Revasc Med. 2013;14:321-324.
23. Carrillo X, Fernandez-Nofrerias E, Ciompi F, et al. Changes in radial artery volume assessed using intravascular ultrasound:a comparison of two vasodilator regimens in transradial coronary interventions. J Invasive Cardiol. 2011;23:401-404.
24. Varenne O, Jegou A, Cohen R et al. Prevention of arterial spasm during percutaneous coronary interventions through radial artery:the SPASM study. Catheter Cardiovasc Interv. 2006;68:231-235.
25. Curtis E, Fernandez R, Lee A. The effect of topical medications on radial artery spasm in patients undergoing transradial coronary procedures:a systematic review. JBI Database System Rev Implement Rep. 2018;16:738-751.
26. Ferrante G, Rao SV, Juni P, et al. Radial Versus Femoral Access for Coronary Interventions Across the Entire Spectrum of Patients With Coronary Artery Disease:A Meta-Analysis of Randomized Trials. JACC Cardiovasc Interv. 2016;9:1419-1434.
ABSTRACT
Introduction and objectives: Reperfusion therapy during an ST-segment elevation acute coronary syndrome (STEACS) can be performed using fibrinolytic agents or primary percutaneous coronary intervention (pPCI). The pPCI is the reperfusion strategy of choice, but many patients with STEACS initially come to non-PCI capable hospitals. Regional networks have been launched with both reperfusion therapies using thrombolysis in indicated cases followed by routine angiographic studies (pharmacoinvasive strategy). Our objective was to analyze the results of treatment in patients with STEACS in the Region of Murcia, Spain based on the patient’s place of origin.
Methods: Retrospective study of a cohort of patients admitted due to STEACS to 3 health areas: pPCI-capable Area 1 (Hospital Clínico Universitario Virgen de la Arrixaca), and non-pPCI capable Areas IV and V (Hospital Comarcal del Noroeste, Caravaca de la Cruz, and Virgen del Castillo, Yecla).
Results: Six hundred and seventy-nine patients from health areas I, IV, and V of the Region of Murcia were treated of STEACS from 2006 through 2010. Out of the 494 patients from Area I, 97.6% (482 patients) were treated with pPCI while 2.4% (12 cases) received thrombolysis. In Areas IV and V, 73% (135) of patients were treated with pPCI and 27% (50) with thrombolysis. After thrombolysis, 46 patients (34%) required rescue angioplasty and 79 (58.5%) underwent a scheduled coronary angiography (pharmacoinvasive strategy). No statistically significant differences were reported in the overall mortality rate at 30-day (8.3% in Area I vs 6% in Areas IV and V; P = .31) or 1 year follow-up (11.3% vs 8.2%; P = .23) in Area I compared to Areas IV and V, nor for cardiac mortality.
Conclusions: Although immediate pPCIs are less accessible in remote health areas, the healthcare network from the Region of Murcia can achieve similar mortality results compared to populations with pPCI availability.
Keywords: ST-segment elevation acute coronary syndrome. Reperfusion therapy. Fibrinolysis. Primary percutaneous coronary intervention.
RESUMEN
Introducción y objetivos: El tratamiento de reperfusión en un síndrome coronario agudo con elevación del segmento ST (SCACEST) se puede realizar con agentes fibrinolíticos o con angioplastia primaria (ICPp). La ICPp es la estrategia de elección, pero muchos de los pacientes con SCACEST acuden inicialmente a hospitales sin ICPp. Se han desarrollado programas de asistencia al SCACEST en los que se integran ambos tratamientos, utilizando la trombolisis en casos indicados, seguida de un estudio angiográfico (estrategia farmacoinvasiva). El objetivo del estudio es analizar los resultados del tratamiento del SCACEST según sea diagnosticado en áreas de salud con o sin disponibilidad de ICPp inmediata.
Métodos: Estudio retrospectivo de una cohorte de pacientes diagnosticados de SCACEST en 3 áreas de salud de Murcia: área I con ICPp (Hospital Clínico Universitario Virgen de la Arrixaca) y áreas IV y V sin ICPp (Hospital Comarcal del Noroeste, Caravaca de la Cruz y Virgen del Castillo, Yecla).
Resultados: Entre 2006 y 2010 se atendió por SCACEST a 679 pacientes de las áreas I, IV y V de Murcia. De los 494 pacientes del área I, recibieron tratamiento con ICPp el 97,6% (482) y trombolisis el 2,4% (12). En los pacientes de las áreas sanitarias IV y V se realizó trombolisis al 73% (135) e ICPp al resto 27% (50). De los pacientes sometidos a trombolisis, el 34% (46) precisaron angioplastia de rescate y al 58,5% (79) se les realizó coronariografía programada (estrategia farmacoinvasiva). No hubo diferencias en la mortalidad total a 30 días (8,3% en el área I y 6% en las áreas IV y V; p = 0,31) ni al año (11,3 frente a 8,2%; p = 0,23); tampoco en la mortalidad por causa cardiaca.
Conclusiones: A pesar de la menor accesibilidad a la ICPp en las áreas sanitarias más alejadas, la red asistencial regional de Murcia permite unos resultados comparables a los de las áreas sanitarias con disponibilidad de ICPp.
Palabras clave: Síndrome coronario agudo con elevación del segmento ST. Reperfusión. Fibrinolisis. Angioplastia primaria.
Abbreviations: pPCI: primary percutaneous coronary intervention. STEACS: ST-segment elevation acute coronary syndrome.
INTRODUCTION
The management of ST-segment elevation acute coronary syndrome (STEACS) is based on the quick opening of the culprit artery through the use of fibrinolytic drugs or a percutaneous coronary intervention (PCI) that limits the size of the infarction and improves prognosis.1 Fibrinolytic drugs have proven capable of increasing survival,2 but they are more effective when administered within the first 3 hours after symptom onset. The primary percutaneous coronary intervention (pPCI) improves survival and reduces recurrent infarctions and strokes, which is why it is seen as the optimal therapy as long as it can be performed in a timely manner.3,4
The pPCI main limitation is the impossibility to use it in the entire population due to its limited geographic availability and the delays involved in the transfer of patients from non-pPCI centers to reference hospitals. Clinical practice guidelines recommend performing pPCI < 120 min. after the diagnosis of STEACS.1 Regional networks have been created to speed up these times and increase access to pPCI for patients with STEACS in non-pPCI hospitals. Yet despite this effort, many patients with STEACS are transferred late to pPCI centers which increases mortality and morbidity rates.
In order to improve results and administer reperfusion therapy as early as possible the so-called pharmacoinvasive strategy was implemented. It consists of the administration of fibrinolytic drugs in the pre-hospital or non-pPCI setting followed by the immediate transfer of the patient to a pPCI center capable of performing a bailout angioplasty if drug therapy fails or an early systematic angiography if it is successful.5,6
The experience gained over the years performing pPCIs at the Hospital Clínico Universitario Virgen de la Arrixaca (HCUVA) has been used for the optimal management of patients with STEACS. The recommendations established by the clinical guidelines have been followed and adapted to the geographic characteristics of the region, structure, and healthcare resources available. A protocol for the management of reperfusion in the acute phase that distinguished 2 groups has been established: the first group with patients treated in pPCI centers; the second one, with patients from regional hospitals who live in remote areas far from reference hospitals where the treatment recommended is fibrinolysis in the absence of contraindications.
METHODS
Retrospective study of a cohort of 679 patients diagnosed with STEACS from 2006 through 2010 in 2 groups of healthcare regions: region I, with pPCI capabilities at the HCUVA (El Palmar, Murcia), and non-pPCI regions assigned to the HCUVA intensive care unit. This second group includes region IV with the Hospital Comarcal del Noroeste (Caravaca de la Cruz) and region V with the Hospital Virgen del Castillo (Yecla).
Patients diagnosed with STEACS based on traditional criteria1 and symptoms of less than 24-hour duration were included. Selection was done by reviewing the HCUVA catheterization laboratory database on all ICU admissions, hospital urgent care provided, and 061 ambulance emergency transfer reports during the study period. The most adequate reperfusion therapy was administered following recommendations and the regional protocol.
Follow-up was conducted by reviewing the patients’ medical records by phone or through physical consultations.
The variables analyzed were past medical history, time elapsed since symptom onset until reperfusion therapy, electrocardiogram, echocardiographic and angiographic characteristics of angioplasty, patient progression, and treatment after hospital discharge. Major hemorrhages were defined as lethal or symptomatic in a critical area or organ (intracranial, intraspinal, intraocular, retroperitoneal, intraarticular, pericardial or intramuscular) causing compartmental syndrome or bleeding with reduced hemoglobin levels > 20 g/L (1.24 mmol/L) or need for 2 concentrate transfusions.
The short and long-term cardiovascular events were recorded at the 30-day and 1-year follow-up, respectively including the rates of overall mortality and cardiac mortality, acute myo- cardial reinfarction (re-AMI), stroke, and need for a new revascularization.
The study primary endpoint was to compare mortality and major cardiovascular events in patients treated of STEACS from the Region of Murcia based on the healthcare region they received care at. The study secondary endpoints were the analysis and comparison of the clinical characteristics of these populations and the identification of angiographic or PCI differences.
Statistical analysis
The results of continuous variables were expressed as mean ± standard deviation, and those of categorical variables as frequency or percentage. Categorical variables were compared using the chi-square test with Yates correction when necessary. Quantitative variables were compared using the Student t test based on the variables normal distribution. Event-free survival rates (overall and cardiac mortality, stroke, re-AMI, and restenosis) were calculated using the Kaplan-Meier method and their results were represented through survival curves. The log rank test was used to compare the event-free survival rate. The level of statistical significance used for hypothesis testing was P < .05. The Mac OS version of the SPSS statistical software (version 20) was used.
The study was conducted in full compliance with the Declaration of Helsinki and the good clinical practice guidelines approved by HCUVA Research Ethics Committee.
RESULTS
From January 2006 through December 2010, 679 patients from regions I, IV, and V of the Region of Murcia Healthcare System were treated of STEACS of less than 24-hour duration and received reperfusion therapy (figure 1). Ninety-seven-point-six per cent of the 494 patients from region I (HCUVA) underwent pPCI (482) while 2.4% received thrombolysis (12). Seventy-three percent (135) and 27% (50) of patients from regions IV and V (127 and 58, respectively) underwent thrombolysis and pPCI, respectively. Thirty-four percent (46) of those who received thrombolysis required a bailout angioplasty and 58.5% (79) a scheduled coronary angiography (pharmacoinvasive strategy) during their hospital stay. Only 10 patients (7.4%) did not undergo a coronary angiography.
Figure 1. Summary of the study patients and the reperfusion strategies used based on the patients’ healthcare regions. HCUVA, Hospital Clínico Universitario Virgen de la Arrixaca; PCI, percutaneous coronary intervention or angioplasty; STEACS, ST-segment elevation acute coronary syndrome.
Baseline characteristics of the populations
Baseline characteristics are shown on table 1. The HCUVA population was older and had more diabetic patients compared to the population from regional hospitals. On the contrary, the rate of atrial fibrillation was higher in the latter. No significant differences were seen based on sex or the remaining risk factors.
Table 1. Baseline characteristics of the population
| HCUVA (n = 494) | Regional hospitals (n = 185) | P | |
|---|---|---|---|
| Age (years) | 65.3 ± 13.7 | 62.9 ± 13 | .044 |
| Sex (women) | 111 (22.5) | 41 (22.2) | .93 |
| High blood pressure | 290 (58.7) | 111 (60) | .76 |
| Diabetes | 180 (36.4) | 52 (28.1) | .042 |
| Dyslipidemia | 200 (40.5) | 64 (34.6) | .16 |
| Smoking | 304 (61.5) | 112 (60.5) | .81 |
| Previous ischemic heart disease | 53 (10.7) | 21 (11.4) | .810 |
| Previous revascularization | 53 (10.7) | 18 (9.7) | .88 |
| Peripheral arterial disease | 25 (5.1) | 4 (2.2) | .096 |
| Previous stroke | 43 (8.7) | 12 (6.5) | .35 |
| Atrial fibrillation | 21 (4.3) | 16 (8.6) | .025 |
| Heart failure | 12 (2.4) | 2 (1.1) | .271 |
| Kidney disease | 39 (7.9) | 19 (10.3) | .324 |
| COPD | 43 (8.7) | 18 (9.7) | .677 |
| Valve disease | 9 (1.8) | 1 (0.5) | .217 |
| Previous angina | 122 (24.7) | 39 (21.1) | .324 |
|
COPD, chronic obstructive pulmonary disease; HCUVA, Hospital Clínico Universitario Virgen de la Arrixaca. Data are expressed as no. (%) or mean ± standard deviation. |
|||
No differences were seen in the time to reperfusion between both groups with a mean of 180 min. (interquartile range: [120-240]) in HCUVA vs 150 min. in regional hospitals (interquartile range: [90-240]; P = .4). Ischemia times < 3 hours were achieved in 59.6% of the HCUVA patients compared to 68.9% of patients from regional hospitals (table 2). Forty-nine patients (9.9%) from the first group had cardiogenic shock vs 17 patients (9.2%) from the second one (not statistically significant differences).
Table 2. Progression time (from symptom onset to reperfusion) and angiographic and electrocardiographic characteristics
| HCUVA (n = 494) | Regional hospitals (n = 185) | P | |
|---|---|---|---|
| Progression time(median,min.) | 180 | 150 | .4 |
| < 3 h | 295 (59.7) | 128 (69.1) | |
| 3-6 h | 141 (28.5) | 33 (17.7) | |
| 6-9 h | 32 (6.4) | 10 (5.6) | |
| 9h -12 h | 15 (3.1) | 7 (4) | |
| > 12 h | 11 (2.2) | 7 (4) | |
| STEACS location | .298 | ||
| Anterior | 205 (41.6) | 89 (48.1) | |
| Inferior | 236 (47.7) | 75 (40.5) | |
| Lateral | 49 (9.9) | 18 (9.7) | |
| Indeterminate | 4 (0.8) | 3 (1.6) | |
| Culprit vessel | .022 | ||
| Left anterior descending coronary artery | 205 (41.5) | 83 (44.9) | .429 |
| Circumflex artery | 62 (12.6) | 25 (13.5) | .738 |
| Right coronary artery | 204 (41.3) | 64 (34.6) | .111 |
| Left main coronary artery/graft | 9 (1.8) | 0 | .065 |
| Unidentified | 14 (2.8) | 13 (7) | .013 |
| Previous stent thrombosis | 24 (4.8) | 3 (1.6) | .075 |
| Number of injured vessels | .001 | ||
| 0 | 5 (1) | 15 (8) | .001 |
| 1 | 274 (55.4) | 109 (58.9) | .416 |
| 2 | 133 (27) | 38 (20.6) | .093 |
| 3 | 82 (16.6) | 23 (12.6) | .227 |
| Initial TIMI flow | .001 | ||
| 0 | 351 (71.1) | 65 (34.9) | .001 |
| 1 | 21 (4.2) | 4 (2.4) | .281 |
| 2 | 13 (2.7) | 9 (4.7) | .206 |
| 3 | 109 (22) | 107 (58) | .001 |
| Final TIMI flow grade 3 | 464 (93.9) | 171 (92.3) | .845 |
| Second revascularization | 95 (19.2) | 30 (16.2) | .322 |
| Complete revascularization | 347 (70.2) | 138 (74.6) | |
|
HCUVA, Hospital Clínico Universitario Virgen de la Arrixaca; STEACS, ST-segment elevation acute coronary syndrome; TIMI, Thrombolysis in Myocardial Infarction. Data are expressed as no. (%) |
|||
Regarding the coronary angiography, the percentage of radial access was similar: 45% and 48%, respectively. No significant differences were found either in the location of the STEACS (table 2). However, significant differences were seen in the culprit artery since it was a common thing to not be able to identify the vessel in patients from regional hospitals because the coronary arteries were patent. Differences were seen too in the initial TIMI flow (Thrombolysis in Myocardial Infarction) between both groups (P = .001) at the expense of a worse initial flow in HCUVA patients. After reperfusion therapy, TIMI flow grade-3 was achieved in the culprit artery in 93.9% of HCUVA patients and 92.3% of patients from regional hospitals. Revascularization was complete in 70.2% of the patients from region I and 74.6% of the patients from regions IV and V.
Analytic and echocardiographic characteristics and clinical progression
No differences were seen in the highest levels of cardiac necrosis markers between the different regions (table 3). On average the left ventricular ejection fraction was 52.15% in HCUVA patients and 52.29% in patients from regional hospitals without any significant differences in the systolic or diastolic function (table 3).
Table 3. Analytic, echocardiographic and disease progression characteristics at the hospital floor
| HCUVA (n = 494) | Regional hospitals (n = 185) | P | |
|---|---|---|---|
| Peak creatine kinase levels (µg/dL) | 1864.4 ± 1917.3 | 1938.3 ± 1834.4 | .671 |
| Peak creatine kinase-MB levels | 175.39 ± 132.34 | 182.26 ± 159.86 | .668 |
| Peak troponin T levels | 5.79 ± 9.4 | 9.38 ± 27.5 | .118 |
| Ejection fraction (%) | 52.15 ± 10.93 | 52.29 ± 11.46 | .886 |
| Normal | 255 (50.6) | 95 (51.5) | |
| Mild dysfunction | 152 (30.7) | 52 (28.1) | |
| Moderate dysfunction | 63 (12.8) | 32 (17.3) | |
| Severe dysfunction | 29 (5.9) | 6 (3.5) | |
| Diastolic pattern | .056 | ||
| Restrictive pattern | 19 (3.9) | 10 (5.3) | |
| Pseudo-normal pattern | 125 (25.3) | 33 (18) | |
| Prolonged relaxation | 307 (62.2) | 113 (61.3) | |
| Normal | 37 (7.6) | 23 (12.4) | |
| Atrial fibrillation | 5 (1.1) | 6 (3.3) | |
| Hospital stay (days) | 9.04 ± 5.72 | 9.81 ± 7.94 | .259 |
| Major hemorrhage | 11 (2.2) | 7 (3.8) | .261 |
| STEACS related complications | 6 (1.2) | 3 (1.6) | .71 |
| Killip Class I | 357 (72.3) | 154 (83.3) | .012 |
|
HCUVA, Hospital Clínico Universitario Virgen de la Arrixaca; STEACS, ST-segment elevation acute coronary syndrome; Data are expressed as no. (%) or median ± standard deviation. |
|||
No differences were seen in the rates of major bleeding and complications (cardiac ruptures: 2 and 2; intraventricular communication: 1 in regional hospitals, 2 in the HCUVA; papillary muscle rupture: 1 and 1). Patients from region I had more heart failure during their hospital stay (28.7% in the HCUVA vs 16.7% in regional hospitals).
30-day and 1-year follow-up results
Mean follow-up was 962 days in HCUVA patients and 1062 days in patients from regional hospitals. No differences were seen in the overall mortality or cardiac mortality rates at the 30-day or 1-year follow-up. No differences were seen either in the rates of AMI, stroke, and revascularization at the follow-up (table 4). Kaplan-Meier survival curves (figure 2) did not show any significant differences regarding mortality, cardiac death, AMI, and stroke.
Figure 2. Survival curves. Mortality, cardiac death, stroke, and AMI at the follow-up. AMI, acute myocardial infarction; HCUVA, Hospital Clínico Universitario Virgen de la Arrixaca.
Table 4. Mortality and major cardiovascular events
| Results (%) | HCUVA (n = 494) | Regional hospitals (n = 185) | P |
|---|---|---|---|
| Mortality | |||
| 30 days | 41 (8.3) | 11 (6) | .312 |
| 1 year | 56 (11.3) | 15 (8,2) | .229 |
| Cardiac death | |||
| 30 days | 35 (7.1) | 8 (4.3) | .19 |
| 1 year | 43 (8.7) | 9 (4.9) | .095 |
| Reinfarction | |||
| 30 days | 7 (1.4) | 2 (1.1) | .735 |
| 1 year | 20 (4) | 5 (2.7) | .409 |
| Stroke | |||
| 30 days | 8 (1.6) | 3 (1.6) | .996 |
| 1 year | 15 (3) | 3 (1.6) | .309 |
| Revascularization | |||
| 30 days | 7 (1.4) | 4 (2.2) | .494 |
| 1 year | 35 (7.1) | 9 (4.9) | .294 |
|
HCUVA: Hospital Clínico Universitario Virgen de la Arrixaca. Data are expressed as no. (%). |
|||
DISCUSSION
This study assessed the results of the management of STEACS from a population perspective and analyzed the consequences of the different care provided in each patient’s healthcare region. This was an observational and retrospective study conducted in 3 population areas from the Region of Murcia that share the same interventional cardiology unit and the same intensive care unit. A 5-year period was analyzed with an mean annual rate of 140 patients with STEACS who were admitted to the ER with symptoms of < 24-hour duration. To make the analysis more consistent and thorough, the past medical histories of patients admitted to their respective hospitals and the out-of-hospital ER system and 061 emergency service reports were reviewed to detect prehospital deaths.
The regional plan for the management of STEACS7 is part of the recommendation of designing regional networks beyond the idea of isolated hospital healthcare towards more comprehensive community healthcare systems including scientific recommendations, geographical peculiarities, resources and infrastructures available, and the characteristics of healthcare organization. This plan suggests initiating reperfusion therapy as early as possible whether mechanical with pPCI o pharmacological with fibrinolysis.
The pPCI is considered the treatment of choice for patients admitted to the ER within 60 min. since symptom onset.1,8,9 This is how patients diagnosed with STEACS in the metropolitan area of Murcia and nearby municipalities are treated.7 For remote areas such as healthcare regions IV and V, fibrinolytic therapy is recommended in the absence of contraindications followed by transfer to the HCUVA ICU plus urgent coronary angiography in the absence of reperfusion signs (bailout PCI) or elective coronary angiography within the first 24 hours to 48 hours (pharmacoinvasive strategy).7 The hospitals from such areas are 75 km and 110 km away (figure 3) respectively from the pPCI reference hospital.
Figure 3. Healthcare regions within the Region of Murcia, Spain.
Populations from pPCI-capable regions (494 patients) and those from remote regions (185 patients) are rather similar: 78% males, many diabetic patients (> 28%), and over 60% smokers. The only differences between both groups are that patients from region I are older and have a higher prevalence of diabetes (36.4% vs 28.1%). The percentage of diabetics in this series is higher compared to that of international studies like the STREAM trial (12.1% to 13.1%) and other national studies like those conducted by Rodríguez-Leor et al.10 (24.8%), and Hernández-Pérez et al.11 (19.1%), and similar to the EUROASPIRE-IV registry (27%).12
The studies conducted until 2006 in patients with STEACS admitted to the ER in a timely manner showed that up to 25% to 30% did not receive reperfusion therapy.13,14 This has improved with the implementation of STEACS care networks. Proof of this are the results from several networks in Europe and the United States with percentages from 100% (the Mayo Clinic network)15 to 84% (the Alberta network, Canada).16 Our data are indicative of a high percentage of reperfusion therapy in the studied regions.
In region I the pPCI was performed in almost all of the cases (97.6%) while in the remaining 2 regions 27% of the 185 patients were referred to other centers for mechanical reperfusion. The existence of contraindications for thrombolysis, the long progression time or the possibility of agile hospital transfers to the interventional cardiology unit facilitated the performance of pPCI in 1 out of every 4 patients with STEACS from these regions; the rest (73%) received fibrinolysis. These data are indicative of a greater use of fibrinolytic therapy compared to the one reported by other studies. Thus, a Belgium registry17 reported that fibrinolytic therapy was prescribed to 28.7% of the population from regional hospitals over the first few years (2007-2008). However, this percentage dropped to 12.6% over the last few years (2009-2010). The higher percentage of thrombolytic therapy seen in our study is associated with a longer distance between regional hospitals and the reference pPCI hospital. Even so, over the last few years, a higher percentage of patients with STEACS referred to pPCI centers has been reported in our region. At the program early stages,18 in healthcare regions IV and V, the percentage of pPCIs performed was between 1% and 2% of all reperfusion therapies. In our study, this percentage grew to 27% after reducing patient transfer times between hospitals.
Coronary angiography was performed in 95% of the patients who received fibrinolytic therapy, a similar percentage compared to that reported by other registries (96% in the FAST-MI,19 and 97% in the Mayo Clinic Care Network registry15) and higher to the one reported by the Belgium registry (69%).17
Reperfusion mean times are also similar to those reported by the registries mentioned above. Time delay until reperfusion therapy was < 3 hours in 59.6% of the patients from region I and 68.9% of the patients from regions IV and V. These are similar rates to those from the Belgium trial17 in which the time elapsed since symptom onset until reperfusion therapy was < 4 hours in 67% of the patients from pPCI hospitals and 63% of the patients from regional hospitals and to those from the Mayo Clinic Care Network AMI protocol.15 This protocol establishes a pharmacoinvasive strategy where total ischemia times were 103 min. in patients who received thrombolysis and 278 min. in those referred to undergo pPCI (with a mean time until reperfusion in regional hospitals of 181 min.).
No differences were seen in the location of the infarction between both groups. Patients referred from regional hospitals had more coronary arteries without lesions and a higher preprocedural rate of TIMI flow grade-3 compared to a higher rate of occluded infarct related culprit arteries in those referred for pPCI. Upon arrival to the catheterization laboratory, the initial TIMI flow grade was 0-1 in 75.6% of the patients referred for pPCI and 37.3% in those who received thrombolysis. Different studies show that when the coronary angiography is performed there is a higher percentage of patients with TIMI flow grade-3 among patients who received thrombolysis.20
Clinical progression was similar with no differences regarding major bleeding complications (2.2% vs 3.8%), stroke (1.6% vs 1.6% at 30 days), re-AMI (1.4% vs 1.1% at 30 days), and need for revascularization (1.4% vs 2.2% at 30 days, 7.1% vs 4.9% at 1 year). However, the rate of heart failure during the hospital stay was higher in HCUVA patients (27.3% vs 16.7%). This result may be explained by a tendency towards a greater grade of advanced diastolic dysfunction in these patients (25.3% vs 18%). However, despite the longer ischemia time there were no significant differences in the AMI size due to systolic dysfunction or peak creatinine kinase-MB levels with peak values of 175 vs 182 µg/dL.
The mortality of patients looked after in regions assigned to non-pPCI regional hospitals is similar to that of patients looked after in the reference pPCI hospital. At 1-month, the overall mortality rate was 8.3% in region I with pPCI capabilities and 6% in the most remote areas assigned to regional hospitals; cardiovascular mortality rate was 7.1% and 4.3%, respectively. These rates are similar to those reported by other studies conducted in our setting like the 7.5% from the RESCATE II,21 7.26% from the RECALCAR trial,22 11% from the PRIAMHO-II trial,23 and 7.6% from the MASCARA trial.24 They are also similar to those from the Belgium infarction care network17 where the mortality rates of regional and pPCI hospitals were 7% and 6.7%, respectively or the Mayo Clinic AMI Care Network where the mortality rates of patients from regional hospitals and pPCI hospitals were 5.2% and 7.2%, respectively.15
Based on these findings a reflection is to be made on some of the things that worry healthcare providers, Administration, and patients such as accessibility and equity in the healthcare system. In the STEACS setting there is an ongoing debate on how to make pPCI available for the entire population. Data from this and other studies,19,25 show that even if pPCI is the preferred reperfusion strategy, it is not the only one. In patients looked after in remote areas far from hospitals with experienced heart teams a pharmacoinvasive strategy with fibrinolytic treatment in the absence of complications is a good alternative.
Limitations
The scarce population from regions IV and V brings down the annual number of patients with STEACS, which is why the timeframe studied had to be a large one in order to study a representative sample. This was a retrospective analysis with the limitations of this type of studies. Basically, this shows how difficult it was to obtain certain data like those regarding different timeframes. The findings from this study where patients were always transferred to the reference hospital intensive care unit may vary from those of other regions where delays could occur if fibrinolysis was not successful. Another possible limitation would be that only patients treated with reperfusion therapy were studied. As already discussed, patients who may have died during the transfer or at the ER were searched for to discard differences in the results obtained from patients assigned to a reperfusion strategy and those finally treated. However, patients with STEACS who did not receive reperfusion therapy were not studied (cases with long symptom duration, etc.). The study compared the results based on the patients’ healthcare region, which may be decisive when assessing the management of STEACS in different healthcare regions, and the different ways of administering various types of reperfusion therapy. This does not seem to be a problem at the moment since reperfusion therapy is administered to over 80% of the cases without significant regional differences.
CONCLUSIONS
Patients diagnosed with STEACS from the most remote healthcare regions of the Region of Murcia (regions IV and V) show similar clinical characteristics compared to patients from region I. However, they are younger patients with not so much diabetes. Yet despite the lower accessibility to immediate pPCI for populations from these healthcare regions, the regional network gives results that are similar to those of populations from pPCI-capable healthcare regions. Pharmacoinvasive strategy is a valid reperfusion therapy for populations from non-pPCI healthcare regions within the times recommended, with similar survival rates to those of pPCI regions, without a higher rate of complications, and with similar short and long-term results.
CONFLICTS OF INTEREST
The authors declared no conflicts of interest whatsoever.
WHAT IS KNOWN ABOUT THE TOPIC?
- Fibrinolysis and pPCI are reperfusion therapies for the management of STEACS. The latter is superior to the former if performed in a timely manner and under the right conditions.
- The pPCI main limitation is that it is impossible to offer it to the entire population due to time delays and availability issues.
- Regional networks have been created to reduce time to reperfusion and increase the availability of pPCI.
- Yet despite this effort, some patients with STEACS do not make it on time to the ER to be treated with pPCI. This delay is associated with higher mortality and morbidity rates.
WHAT DOES THIS STUDY ADD?
- Accessibility to pPCI for patients diagnosed with STEACS from remote areas is much lower.
- Being part of a healthcare regional network gives results that are similar to those of populations from pPCI-capable regions.
- This study shows that in an infarction care regional network system, reperfusion therapy can be performed by combining pharmacoinvasive strategy and pPCI.
- That is the way to achieve survival rates similar to those of patients who live close to pPCI-capable hospitals without a higher rate of complications.
REFERENCES
1. Steg PG, James SK, Atar D, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:2569-2619.
2. Fibrinolytic Therapy Trialists'(FTT) Collaborative Group. Indications for fibrinolytic therapy in suspected acute myocardial infarction:collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients. Lancet. 1994;343:311-322.
3. Andersen HR, Nielsen TT, Rasmussen K, et al. A comparison of coronary angioplasty with fibrinolytic therapy in acute myocardial infarction. N Engl J Med. 2003;349:733-742.
4. Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction:a quantitative review of 23 randomised trials. Lancet. 2003;361:13-20.
5. Borgia F, Goodman SG, Halvorsen S, et al. Early routine percutaneous coronary intervention after fibrinolysis vs. standard therapy in ST-segment elevation myocardial infarction:a meta-analysis. Eur Heart J. 2010;31:2156-2169.
6. Armstrong PW, Gershlick AH, Goldstein P, et al. Fibrinolysis or primary PCI in ST-segment elevation myocardial infarction. N Engl J Med. 2013;368:1379-1387.
7. Servicio Murciano de Salud, Consejería de Sanidad y Consumo. Programa Integral de Atención a La Cardiopatía Isquémica 2010-2013. 2010. Disponible en:https://www.murciasalud.es/publicaciones.php?op=mostrar_publicacion&id=1771&idsec=88. Consultado 20 Dic 2020.
8. O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction:a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:e78-140.
9. Windecker S, Kolh P, Alfonso F, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization:The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2014;35:2541-2619.
10. Rodriguez-Leor O, Fernandez-Nofrerias E, Mauri F, et al. Analysis of reperfusion delay in patients with acute myocardial infarction treated with primary angioplasty based on first medical contact and time of presentation. Rev Esp Cardiol. 2011;64:476-483.
11. Hernandez-Perez FJ, Blasco-Lobo A, Goicolea L, et al. Use of the radial approach in primary angioplasty:results in 1029 consecutive patients and analyses in unfavorable subgroups. Rev Esp Cardiol. 2014;67:45-51.
12. Kotseva K, Wood D, De Bacquer D, et al. EUROASPIRE IV:a European Society of Cardiology survey on the lifestyle, risk factor and therapeutic management of coronary patients from 24 European countries. Eur J Prev Cardiol. 2016;23:636-648.
13. Eagle KA, Nallamothu BK, Mehta RH, et al. Trends in acute reperfusion therapy for ST-segment elevation myocardial infarction from 1999 to 2006we are getting better but we have got a long way to go. Eur Heart J. 2008;29:609-617.
14. Gibson CM, Pride YB, Frederick PD, et al. Trends in reperfusion strategies, door-to-needle and door-to-balloon times, and in-hospital mortality among patients with ST-segment elevation myocardial infarction enrolled in the National Registry of Myocardial Infarction from 1990 to 2006. Am Heart J. 2008;156:1035-1044.
15. Ting HH, Rihal CS, Gersh BJ, et al. Regional systems of care to optimize timeliness of reperfusion therapy for ST-elevation myocardial infarction:the Mayo Clinic STEMI Protocol. Circulation. 2007;116:729-736.
16. Shavadia J, Ibrahim Q, Sookram S, Brass N, Knapp D, Welsh RC. Bridging the gap for nonmetropolitan STEMI patients through implementation of a pharmacoinvasive reperfusion strategy. Can J Cardiol. 2013;29:951-959.
17. Claeys MJ, Sinnaeve PR, Convens C, et al. STEMI mortality in community hospitals versus PCI-capable hospitals:results from a nationwide STEMI network programme. Eur Heart J Acute Cardiovasc Care. 2012;1:40-47.
18. Valdés Chávarri M, Pinar Bermúdez E, Lacunza Ruiz J, et al. The primary percutaneous coronary intervention program in Murcia. Rev Esp Cardiol Supl. 2011;11(C):28-34.
19. Danchin N, Coste P, Ferrieres J, et al. Comparison of thrombolysis followed by broad use of percutaneous coronary intervention with primary percutaneous coronary intervention for ST-segment-elevation acute myocardial infarction:data from the french registry on acute ST-elevation myocardial inf. Circulation. 2008;118:268-276.
20. Giannopoulos G, Pappas L, Synetos A, et al. Association of virtual histology characteristics of the culprit plaque with post-fibrinolysis flow restoration in ST-elevation myocardial infarction. Int J Cardiol. 2014;174:678-682.
21. Garcia-Garcia C, Sanz G, Valle V, et al. Trends in in-hospital mortality and six-month outcomes in patients with a first acute myocardial infarction. Change over the last decade. Rev Esp Cardiol. 2010;63:1136-1144.
22. Bertomeu V, Cequier A, Bernal JL, et al. In-hospital mortality due to acute myocardial infarction. Relevance of type of hospital and care provided. RECALCAR study. Rev Esp Cardiol. 2013;66:935-942.
23. Aros F, Loma-Osorio A, Vila J, et al. Effect of combined beta-blocker and angiotensin-converting enzyme inhibitor treatment on 1-year survival after acute myocardial infarction:findings of the PRIAMHO-II registry. Rev Esp Cardiol. 2006;59:313-320.
24. Ferreira-Gonzalez I, Permanyer-Miralda G, Marrugat J, et al. MASCARA (Manejo del Sindrome Coronario Agudo. Registro Actualizado) study. General findings. Rev Esp Cardiol. 2008;61:803-816.
25. Larson DM, Duval S, Sharkey SW, et al. Safety and efficacy of a pharmaco-invasive reperfusion strategy in rural ST-elevation myocardial infarction patients with expected delays due to long-distance transfers. Eur Heart J. 2012;33:1232-1240.
Abstract
Introduction and objectives: Recent publications suggest that bioresorbable vascular scaffolds (BVS) are associated with an excess of thrombotic complications. We present the real-world, long-term results of a series of patients who received the Absorb BVS (Abbott Vascular, United States).
Methods: A total of 213 consecutive patients who received at least 1 BVS between May 2012 and December 2016 were analyzed. The main objective of the study was the rate of target vessel failure, a composite endpoint of infarction or target vessel revascularization and cardiac death.
Results: Seventy-five per cent of the patients were men (mean age, 61.4 years). The most common cause for admission was non-ST-elevation myocardial infarction (53.52%). The median follow-up was 44 months [28 months], the rate of the primary endpoint was 6.57% for the first 24 months and 7.98% at the end of the follow-up. Regarding the device, there were 6 cases (2.81%) of thrombosis (definitive, probable or possible) and 10 cases (4.69%) of restenosis. Patients with a past medical history of diabetes mellitus (HR, 1.72; 95%CI, 1.01-2.95; P = .05) and/or chronic oral anticoagulation (HR, 5.71; 95%CI, 1.12-28.94; P = .04) had a higher risk of target vessel failure.
Conclusions: In this series of patients, the rate of target vessel failure was similar to the one previously described by randomized clinical trials. Events were more common during the first 2 years of follow-up and in the presence of greater cardiovascular comorbidity.
Keywords: Absorb. Bioresorbable scaffolds. Coronary angioplasty.
RESUMEN
Introducción y objetivos: Las publicaciones sugieren que los armazones vasculares bioabsorbibles (AVB) conllevan un exceso de complicaciones trombóticas. Se describen los resultados en la vida real y a largo plazo de una serie de pacientes a los que se implantó un AVB Absorb (Abbott Vascular, EE.UU.).
Métodos: Se analizaron 213 pacientes consecutivos que recibieron al menos un AVB entre mayo de 2012 y diciembre de 2016. El objetivo principal del estudio fue la incidencia de fracaso del vaso diana, un evento compuesto que incluye infarto de miocardio, revascularización del vaso diana y muerte cardiaca.
Resultados: El 75% de los pacientes eran varones (edad media, 61,4 años). La causa más común de ingreso fue el infarto sin elevación del ST (53,52%). La mediana de seguimiento fue de 44 meses [28 meses]. La incidencia del evento primario fue del 6,57% durante los primeros 24 meses y del 7,98% al final del seguimiento. Respecto al dispositivo, hubo 6 casos (2,81%) de trombosis (definitiva, probable o posible) y 10 casos (4,69%) de reestenosis. Los pacientes con antecedentes de diabetes mellitus (HR = 1,72; IC95%, 1,01-2,95; p = 0,05) o con anticoagulación oral crónica (HR = 5,71; IC95%, 1,12-28,94; p = 0,04) tuvieron mayor riesgo de fracaso del vaso diana.
Conclusiones: En esta serie de pacientes, la incidencia de fracaso del vaso diana fue comparable a la descrita previamente en ensayos clínicos aleatorizados. Los eventos adversos fueron más frecuentes en los primeros 2 años de seguimiento y en presencia de mayor comorbilidad cardiovascular.
Palabras clave: Absorb. Armazón vascular bioabsorbible. Angioplastia coronaria.
Abbreviations BVS: bioresorbable vascular scaffold. AMI: acute myocardial infarction. DES: drug-eluting stent.
INTRODUCTION
Drug-eluting bioresorbable vascular scaffolds (BVS) were initially presented as a technological breakthrough to overcome the limitations and adverse events associated with permanent bare-metal stents, especially the development of neoatherosclerosis that is associated with a risk of thrombosis (0.2% per year) and secondary revascularization (2% to 3% per year).1-3
At the time, the implantation of a BVS was an innovative approach to treat coronary atherosclerosis by releasing the artery from a permanent metal jail and restoring the flow architecture. Also, it preserved parietal motility and its response to stimuli generated by coronary flow (shear stress). The Absorb (Abbott Vascular, United States)—a polymer everolimus-eluting scaffold with 157 µm-thick struts—was one of the first ones to be available in Spain and several clinical trials were conducted.4-8 The excellent initial results led to the widespread use of this device for several clinical indications.9-10 The Absorb BVS was approved by the U.S. Food and Drug Administration and obtained the CE marking certification in January 2011.11
However, the mid- and long-term data of the AIDA research group12,13 on the Absorb were disappointing. They showed a higher rate of late scaffold thrombosis compared to the XIENCE (Abbott Vascular, United States) (3.5% vs 0.9%; hazard ratio [HR], 3.87; 95% confidence interval [95%CI], 1.78-8.42; P < .001), an everolimus-eluting stent (EES).14,15 Therefore, the manufacturer stopped making the Absorb BVS and removed it from the market according to the European regulatory agency; however, some of these devices remain approved and are still available in Europe.16
Since the Absorb BVS was widely used in different clinical settings during market launch more than 7 years ago, the long-term follow-up results are available today. The objective of this study is to describe the incidence of long-term adverse events in a series of patients implanted with the Absorb BVS in different clinical settings of our multicenter registry.17
METHODS
Population, design, and definitions
The cases treated with percutaneous transluminal coronary angioplasty with at least 1 Absorb BVS in 3 hospitals between May 2012 and December 2016 were studied.17 Implantation was performed to the discretion of the operator in charge.
The study primary composite endpoint was the target vessel failure rate, a composite event of target vessel revascularization, target vessel related acute myocardial infarction (AMI), and cardiac death. The study secondary endpoint was the rate of the overall clinical endpoint including these adverse events: all-cause mortality, myocardial infarction, and all the new coronary revascularizations (including those of the non-target vessel).
The registry of the interventional cardiology unit of our hospital network was periodically reviewed every 6 to 12 months at the follow-up consultation at the interventional cardiology unit by a cardiologist. Also, it was completed through follow-up phone calls.
Statistical analysis
Data regarding quantitative variables are expressed as mean ± standard deviation and qualitative variables are expressed as percentages. Patients were grouped according to whether they had target vessel failure or not; inter-group averages were compared using the Student t test. Percentages were compared using the chi-square test. Kaplan-Meier analysis was conducted to estimate the likelihood of target vessel failure-free survival and BVS thrombosis and restenosis. Finally, the multivariate Cox regression analysis was conducted to study the survival function adjusted by different predefined variables: sex, age, cardiovascular risk factors, past medical history, clinical signs, size and length of the BVS implanted, overlapping of, at least, 2 BVSs, and use of intracoronary imaging modalities (optical coherence tomography [OCT] or intravascular ultrasound [IVUS]). Two-tailed P ≤ values .05 were considered statistically significant in all tests. Data were analyzed using the statistical software package Stata IC 14 (StataCorp, United States).
RESULTS
Study population
Two hundred and thirteen consecutive patients implanted with, at least, 1 Absorb BVS between May 2012 and December 2016 were included. Table 1 shows the baseline clinical characteristics of these patients. Most of the participants were males (75.12%) with a mean age of 61.40 ± 12.74 years, and a high prevalence of dyslipidemia (62.44%) and smoking (65.26%). Diabetes mellitus was present in 23.94% and 21.60% had been previously treated with a percutaneous coronary intervention. The most common clinical presentation during recruitment was non-ST-segment elevation acute coronary syndrome (53.52%).
Table 1. Baseline clinical characteristics of patients and differences based on the primary endpoint
| Characteristics | Patients who received BVS (n = 213) | Patients with BVS and target vessel failure (n = 17) | Patients with BVS without target vessel failure (n = 196) | P |
|---|---|---|---|---|
| Age (years) | 61.40 ± 12.74 | 66.71 ± 9.62 | 61.14 ± 12.98 | .07 |
| Sex (male) | 160 (75.12) | 12 (70.59) | 148 (75.51) | .65 |
| Risk factors | ||||
| Diabetes mellitus | 51 (23.94) | 7 (41.18) | 44 (22.45) | .06 |
| Hypertension | 118 (55.40) | 11 (64.71) | 107 (54.59) | .42 |
| Dyslipidemia | 133 (62.44) | 13 (76.47) | 120 (61.22) | .21 |
| Active smoking | 139 (65.26) | 10 (58.82) | 129 (65.82) | .56 |
| Past medical history | ||||
| Chronic kidney disease | 8 (3.76) | 1 (5.88) | 7 (3.57) | .63 |
| LVEF < 30% | 5 (4.5) | 1 (5.88) | 4 (2.04) | .55 |
| Previous stroke or TIA | 9 (4.2) | 3 (17.65) | 6 (3.06) | .01 |
| Chronic oral anticoagulation | 10 (4.69) | 3 (17.65) | 7 (3.57) | .01 |
| Peripheral vascular disease | 13 (6.10) | 1 (5.88) | 12 (6.12) | .96 |
| Previous myocardial infarction | 31 (14.55) | 1 (5.88) | 30 (15.31) | .29 |
| Previous PCI | 46 (21.60) | 4 (23.53) | 42 (21.43) | .84 |
| Previous coronary artery bypass surgery | 7 (3.29) | 2 (11.76) | 5 (2.55) | .04 |
| Clinical presentation | ||||
| STEACS | 31 (14.55) | 4 (23.53) | 27 (13.78) | .25 |
| Non-Q-wave AMI type of NSTEACS | 77 (36.15) | 6 (35.29) | 71 (36.22) | .66 |
| Unstable angina type of SCASEST | 37 (17.37) | 3 (17.65) | 34 (17.35) | .88 |
| Stable angina or documented ischemia | 68 (31.4) | 4 (23.53) | 64 (32.65) | .52 |
|
AMI, acute myocardial infarction; BVS, bioresorbable vascular scaffold; LVEF, left ventricular ejection fraction; NSTEACS, non-ST-segment elevation acute coronary syndrome; PCI, percutaneous coronary intervention; STEACS, ST-segment elevation acute coronary syndrome; TIA, transient ischemic attack. Data are expressed as no. (%) or mean ± standard deviation. |
||||
Index procedure of the bioresorbable vascular scaffold implantation
Table 2 shows the characteristics of the patients’ index procedure. Two hundred and thirty-three coronary lesions were treated with an average 1.3 ± 0.3 lesions per patient. Implantation was successful in 99.5% of the cases but failed in 1 patient due to the difficulty advancing the device across the lesions. The patient required the implantation of a DES, which is why he was excluded from the analysis. Predilatation occurred in 89.3% of the cases and postdilatation in 33.5% of the cases. Intracoronary imaging modalities (OCT or IVUS) were used to optimize the BVS implantation in 86 patients (40.38%).
Table 2. Characteristics of the index procedure and treatment
| Characteristics | Patients who received BVS (n = 213) |
|---|---|
| Lesions treated per patient | 1.3 ± 0.3 |
| Number of devices per patient | 1.2 ± 0.4 |
| Total length of the device per patient (mm) | 21.5 ± 13.5 |
| Minimum device diameter per patient (mm) | 2.75 ± 0.25 |
| Device implantation | |
| At least 1 BVS | 212 (99.5) |
| BVS only | 204 (95.8) |
| Overlapping with at least 2 AVBs | 20 (9.39) |
| Any DES | 8 (3.8) |
| After BVS implantation failure | 1 (0.5) |
| Procedural time (min.) | 44 ± 23 |
| Iodinated contrast used per procedure (mL) | 161 ± 72 |
| Predilatation of the first lesion treated | 189 (88.7) |
| Procedural success | 212 (99.5) |
| Lesions treated | |
| Total number | 233 |
| Predilatation | 208 (89.3) |
| Postdilatation | 78 (33.5) |
| 0.5 mm postdilatation balloon plus BVS | 21 (9.86) |
| Overall number of devices implanted | 261 |
| Overall number of devices per lesion | 1.12 ± 0.4 |
| Intracoronary imaging modality during implantation | |
| OCT or IVUS | 86 (40.38) |
|
BVS, bioresorbable vascular scaffold; DES, drug-eluting stent; IVUS, intravascular ultrasound; OCT, optical coherence tomography. Data are expressed as no. (%) or mean ± standard deviation. |
|
Clinical follow-up
The median follow-up was 44 months [28 months] with minimum times < 1 month. The primary composite endpoint of target vessel failure rate was 6.57% at the 24-month follow-up (table 3) and 7.98% at the end of the follow-up. Figure 1 shows the target vessel failure-free survival curve; at the 48-month follow-up it was 0.92 (95%CI, 0.87-0.95; P = .02). Regarding the secondary endpoint, the overall rate was 11.74% at the 24-month follow-up (table 3) and 17.84% at the end of the follow-up.
Table 3. Adverse events at the 2-year follow-up
| Adverse event | Patients who received BVS 2-year follow-up (n = 213) |
|---|---|
| Clinical events | |
| All-cause mortality | 5 (2.34) |
| Cardiac | 3 (1.41) |
| Non-cardiac | 2 (0.94) |
| All myocardial infarctions | 6 (2.82) |
| During index procedure | 2 (0.94) |
| Not during index procedure | 4 (1.88) |
| Target vessel | 3 (1.41) |
| Non-target vessel | 1 (0.47) |
| Death or myocardial infarction | 11 (5.16) |
| Any revascularization | 18 (8.46) |
| Target vessel | 11 (5.16) |
| Target lesion | 11 (5.16) |
| Device thrombosis | 3 (1.41) |
| Device restenosis | 8 (3.76) |
| Any other vessel | 7 (3.29) |
| Composite endpoint | |
| Target vessel failure | 14 (6.57) |
| Overall clinical endpoint | 25 (11.74) |
| Device thrombosis | |
| Definite | 3 (1.41) |
| Probable | 2 (0.94) |
| Possible | 1 (0.47) |
|
BVS, bioresorbable vascular scaffold. Data are expressed as no. (%). |
|
Figure 1. Kaplan-Meier survival curve for target vessel failure.
Figure 2 shows the rate of all adverse events depending on the time of clinical presentation. Regarding the primary endpoint, there were 3 (1.41%) cases of cardiac death, 4 (1.87%) cases of target vessel related AMI, and 14 (6.57%) cases of target vessel revascularization. Regarding the secondary endpoint, there were 7 (3.29%) cases of all-cause mortality, 7 (3,29%) cases of AMI, and 31 (14.56%) cases of any coronary revascularizations. Finally, regarding the device, there were 6 (2.81%) cases of thrombosis (definite, probable, and possible) all reported within the first 12 months. Dual antiplatelet therapy was kept, at least, for 12 months in 157 (73.7%) patients and 1 patient with late definite thrombosis received dual antithrombotic therapy (acenocoumarol and clopidogrel). Similarly, there were 10 (4.69%) cases of BVS restenosis within the first 48 months of follow-up (figure 3).
Figure 2. Chart of adverse events based on the time of presentation after the index procedure. AMI, acute myocardial infarction; BVS, bioresorbable vascular scaffold.
Figure 3. Kaplan-Meier survival curves for bioresorbable vascular scaffold restenosis and thrombosis.
Patients with target vessel failure had a higher prevalence of cerebrovascular disease (17.65% vs 3.06%; P = 0.01), chronic oral anticoagulation (17.65% vs 3.57%; P = .01), and previous coronary artery bypass graft surgery (11.76% vs 2.55%; P = .04). Similarly, there was a tendency towards a higher prevalence of diabetes mellitus in this group (41.18 vs 22.45%; P = .06) (table 1).
In the multivariate Cox regression analysis, a prior history of diabetes mellitus (HR, 1.72; 95%CI, 1.01-2.95; P = .05) and chronic oral anticoagulation (HR, 5.71; 95%CI, 1.12-28.94; P = .04) were identified as risk factors to develop target vessel failure at the follow-up. On the other hand, the use of intracoronary imaging modalities (OCT or IVUS) during BVS implantation showed a clear tendency towards significance as a protective factor (HR, 0.33; 95%CI, 0.10-1.07; P = .06) (table 4).
Table 4. Factors associated with target vessel failure: Cox regression analysis
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P | HR | 95%CI | P | |
| Past medical history | ||||||
| Diabetes mellitus | 1.72 | 1.04-2.86 | .04 | 1.72 | 1.01-2.95 | .05 |
| Previous stroke or TIA | 6.28 | 1.76-22.31 | .01 | 1.94 | 0.40-9.23 | .40 |
| Chronic oral anticoagulation | 5.34 | 1.51-18.97 | .01 | 5.71 | 1.12-28.95 | .04 |
| Use of intracoronary imaging modalities during implantation | ||||||
| OCT or IVUS | 0.32 | 0.11-1.03 | .06 | 0.33 | 0.10-1.06 | .06 |
|
95%CI, 95% confidence interval; HR, hazard ratio; IVUS, intravascular ultrasound; OCT, optical coherence tomography; TIA, transient ischemic attack. |
||||||
DISCUSSION
This study analyzed a consecutive series of patients who were implanted with, at least, 1 BVS in a high-volume setting and in real-life conditions. The primary composite endpoint of target vessel failure and the overall secondary composite clinical endpoint were similar to what had been reported by other previous randomized clinical trials on percutaneous coronary interventions.18-22
The AIDA clinical trial20 confirmed the lower rate of target vessel failure related AMI from our series. In our study, the patients’ baseline clinical characteristics and clinical presentation were similar to those of the population of the AIDA clinical trial. However, regarding the index procedure, the use of postdilatation was lower in our series. It has been reported that postdilatation does not bring any additional benefits to the implantation of a BVS in the ST-segment elevation acute coronary syndrome clinical setting. If elevation is excessive it could even have deleterious effects when destructuring or tearing the nonmetallic structure of the scaffold.23 The GHOST-EU registry24 proved that the PSP strategy (predilatation, scaffold sizing, and postdilatation) was a predictor of cardiovascular events.
The right selection of the lesion plays a crucial role in the clinical performance of BVS. Most of the patients of this series showed acute coronary syndrome. It is feasible that patients with AMI may benefit the most from BVS treatments.18 First, patients with acute coronary syndrome (with or without ST-segment elevation) often show a visible thrombus in the proximal segments and a less complex morphology with thin-cap fibroatheroma plaques and fewer calcified lesions. Secondly, aggressive antithrombotic therapy after an acute coronary syndrome may mitigate the rate of thrombotic complications.
Bioresorbable vascular scaffold thrombosis
A few studies have reported on a higher rate of BVS thrombosis associated with next-generation DESs,25,26 especially all in off-label uses.27 In our series, the definite or probable device thrombosis occurred in a similar percentage of the patients to that previously reported.12 Several mechanisms that may explain BVS thrombosis have been suggested including edge dissection, strut fracture, malapposition, and inadequate BVS sizing.28 In our series there were 2 cases of subacute definite thrombosis. In the coronary angiography, the OCT performed confirmed the presence of some structural mechanism (underexpansion or malapposition) that favored it. Early presentation at the follow-up is consistent with what has already been reported.29
Similarly, we identified that the use of intracoronary imaging modalities (OCT or IVUS) during BVS implantation showed a clear tendency towards significance as a protective factor of target vessel failure as Caixeta et al.30 had already confirmed in an international registry of 1933 patients. The recommendation here is to use intracoronary imaging modalities to optimize implantation and secure the correct apposition of the BVS, lack of underexpansion, and proper cover of the lesion.31
The main setback of the Absorb BVS is probably strut thickness and width (157 x 190.5 µm in 2.5 mm and 3.0 mm BVSs, and 157 µm x 216 µm in 3.5 mm BVSs), which can make the device more thrombogenic, especially when apposition is not the right one or expansion is incomplete. Today, ultra-thin drug-eluting stents (strut thickness < 70 µm) have lowered the risk of target lesion failure to just 1 year compared to modern second-generation DESs thanks to fewer AMIs and stent thrombosis.32 On this issue, the sirolimus-eluting MeRes100 BVS (Meril Life Sciences Pvt. Ltd., India) with thinner strut thickness (100 µm) confirmed the sustained efficacy and safety profile at the 2- and 3-year follow-up.33
Resistance to antiplatelet therapy can also be an important cause for BVS thrombosis.34 Both acetylsalicylic acid and clopidogrel are effective antiplatelet drugs for the secondary prevention of cardiovascular events. Still their clinical efficacy varies from one individual to the next.35 In our series, most of the patients remained on dual antiplatelet therapy for, at least, 12 months and there was 1 case of late thrombosis with dual antithrombotic therapy (acenocoumarol and clopidogrel). Due to his high bleeding risk, this last patient received dual antiplatelet therapy for the first 3 months; we do not know the international normalized ratio when the complication occurred, which is why the possibility of antiplatelet drug resistance cannot be discarded. However, the potential association between the BVS thrombosis and oral antiplatelet therapy had already been described.36 We know that the selection of duration of antiplatelet therapy following the implantation of the Absorb BVS was difficult,37 especially in anticoagulated patients because they are a population with comorbidities and high cardiovascular risk. Our data show that the implantation of the Absorb BVS in patients at high bleeding risk (including anticoagulated patients) shouldn’t probably be recommended according to the consensus document reached by the European Society of Cardiology and the European Association for Cardio-Thoracic Surgery. This document does not recommend the use of the Absorb BVS in patients intolerant to prolonged dual antiplatelet therapy or who require oral anticoagulation.16
Bioresorbable vascular scaffold restenosis
The most common cause for target lesion revascularization was stent restenosis within the first 48 months of follow-up. The mechanisms involved in bioresorbable vascular scaffold restenosis that may occur in the same patient are varied.38,39 The less intrinsic radial strength and its possible destructuring with an aggressive implantation may explain some of the early recurrences. In this study, aggressive implantation was less common since postdilatation with an up to 0.5 mm balloon combined with BVS implantation occurred in 9.86% of the cases. Also, postdilatation was not associated with restenosis at the follow-up. Also, it has been suggested that the slow resorption of the study device may have been associated with a significant spatial abnormality with loss of alignment of its structural elements, which favors restenosis.40,41 The complete disappearance of the BVS from the vascular wall won’t happen for another 3 years6 and most cases of scaffold restenosis occurred within the first 2 years of follow-up.
Our study results show that there is a correlation between the history of diabetes mellitus and chronic oral anticoagulation and the development of target vessel failure. It is well-known that this past medical history elevates cardiovascular morbimortality and that the CHADS2 and CHA2DS2-VASc scores can be used to estimate the risk of adverse clinical events in patients with acute coronary syndrome.42 In this sense, patients with a past medical history of diabetes mellitus, chronic oral anticoagulation, and coronary artery disease start with CHA2DS2-VASc scores of 4, that is, high risk of adverse clinical events.
Limitations
Selection bias was inevitable because, according to the operator’s criterion, the clinical assessment that may have influenced the decision to implant a BVS maybe did not come from the database, which is a common problem with observational studies like this one. However, the study shows a pragmatic approach to the use of this device in the real world.
CONCLUSIONS
In this series of patients implanted with the Absorb BVS, the composite endpoint of target vessel failure and the overall clinical composite endpoint were similar to what had already been reported by randomized clinical trials. Adverse events were more common within the first 2 years of follow-up in case of greater cardiovascular comorbidity and without intracoronary imaging modalities (OCT or IVUS) during implantation. Although the BVS studied is not available anymore there other bioresorbable devices are in the pipeline.16
FUNDING
R. Mori-Junco received the 2018 training grant from the European Society of Cardiology (APP000019660). L. Furuya-Kanamori received funding from the Australian National Health and Medical Research Council Early Career Fellowships (APP1158469).
CONFLICTS OF INTEREST
The authors declared no conflicts of interest whatsoever.
WHAT IS KNOWN ABOUT THE TOPIC?
- The implantation of a BVS is an innovative approach for the management of coronary atherosclerosis because it releases the coronary artery from a permanent metallic jail and restores the vessel architecture.
- However, the Absorb BVS has a higher rate of thrombotic complications compared to modern DESs, which is why it was removed.
WHAT DOES THIS STUDY ADD?
- In our interventional cardiology network, the implantation of the Absorb BVS showed rates of target vessel failure that were similar to those previously described by randomized clinical trials.
- Target vessel failure occurred basically within the first 24 months in patients with diabetes mellitus or chronic oral anticoagulation. The use of intracoronary imaging modalities during implantation showed a tendency towards becoming a protective factor.
- Our results will contribute to the proper selection of patients eligible for BVS implantation and to the implantation technique as well.
REFERENCES
1. Smits PC, Vlachojannis GJ, McFadden EP, et al. Final 5-Year Follow-Up of a Randomized Controlled Trial of Everolimus- and Paclitaxel-Eluting Stents for Coronary Revascularization in Daily Practice:The COMPARE Trial (A Trial of Everolimus-Eluting Stents and Paclitaxel Stents for Coronary Revas.cularization in Daily Practice). JACC Cardiovasc Interv. 2015;8:1157-1165.
2. Byrne RA, Stone GW, Ormiston J, Kastrati A. Coronary balloon angioplasty, stents, and scaffolds. Lancet. 2017;390:781-792.
3. Ellis SG, Riaz H. Bioresorbable stents:The future of interventional cardiology?Cleve Clin J Med. 2016;83:S18-S23.
4. Ellis SG, Kereiakes DJ, Metzger DC, et al. Everolimus-Eluting Bioresorbable Scaffolds for Coronary Artery Disease. N Engl J Med. 2015;373:1905-1915.
5. Ormiston JA, Serruys PW, Regar E, et al. A bioabsorbable everolimus-eluting coronary stent system for patients with single de-novo coronary artery lesions (ABSORB):a prospective open-label trial. Lancet. 2008;371:899-907.
6. Serruys PW, Garcia-Garcia HM, Onuma Y. From metallic cages to transient bioresorbable scaffolds:change in paradigm of coronary revascularization in the upcoming decade?Eur Heart J. 2012;33:16-25.
7. Serruys PW, Katagiri Y, Sotomi Y, et al. Arterial Remodeling After Biore.sorbable Scaffolds and Metallic Stents. J Am Coll Cardiol. 2017;70:60-74.
8. Serruys PW, Chevalier B, Sotomi Y, et al. Comparison of an everolim.us-eluting bioresorbable scaffold with an everolimus-eluting metallic stent for the treatment of coronary artery stenosis (ABSORB II):a 3 year, randomised, controlled, single-blind, multicentre clinical trial. Lancet. 2016;388:2479-2491.
9. Rampat R, Mayo T, Hildick-Smith D, Cockburn J. A randomized trial comparing two stent sizing strategies in coronary bifurcation treatment with bioresorbable vascular scaffolds –The Absorb Bifurcation Coronary (ABC) trial. Cardiovasc Revascularization Med. 2019;20:43-49.
10. Mitomo S, Naganuma T, Fujino Y, et al. Bioresorbable Vascular Scaffolds for the Treatment of Chronic Total Occlusions. Circ Cardiovasc Interv. 2017;10:e004265.
11. Abbott Laboratories. Abbott Receives CE Mark Approval for World's First Drug Eluting Bioresorbable Vascular Scaffold for Treatment of Coronary Artery Disease. Available online:https://www.prnewswire.com/news-releases/abbott-receives-ce-mark-approval-for-worlds-first-drug-eluting-bioresorbable-vascular-scaffold-for-treatment-of-coronary-artery-disease-113197364.html. Accessed 25 Aug 2019.
12. Wykrzykowska JJ, Kraak RP, Hofma SH, et al. Bioresorbable Scaffolds versus Metallic Stents in Routine PCI. N Engl J Med. 2017;376:2319-2328.
13. Kerkmeijer LSM, Tijssen RYG, Hofma SH, et al. Comparison of an evero.limus-eluting bioresorbable scaffold with an everolimus-eluting metallic stent in routine PCI:three-year clinical outcomes from the AIDA trial. EuroIntervention. 2019;15:603-606.
14. Stone GW, Gao R, Kimura T, et al. 1-year outcomes with the Absorb bioresorbable scaffold in patients with coronary artery disease:a patient-level, pooled meta-analysis. Lancet. 2016;387:1277-1289.
15. Katsikis A, Serruys PW. Bioresorbable scaffolds versus metallic stents in routine PCI:the plot thickens. J Thorac Dis. 2017;9:2296-2300.
16. Byrne RA, Stefanini GG, Capodanno D, et al. Report of an ESC-EAPCI Task Force on the evaluation and use of bioresorbable scaffolds for percutaneous coronary intervention:executive summary. Eur Heart J. 2018;39:1591-1601.
17. Nuñez Gil IJ, Bas M, Fernández-Ortiz A, et al. Long term experience with a novel interventional cardiology network model:Learned lessons. J Hosp Adm. 2016;5:87-94.
18. Byrne RA, Alfonso F, Schneider S, et al. Prospective, randomized trial of bioresorbable scaffolds vs. everolimus-eluting stents in patients undergoing coronary stenting for myocardial infarction:The Intracoronary Scaffold Assessment a Randomized evaluation of Absorb in Myocardial Infarction (ISAR-Absorb MI) trial. Eur Heart J. 2019;40:167-176.
19. Capodanno D, Gori T, Nef H, et al. Percutaneous coronary intervention with everolimus-eluting bioresorbable vascular scaffolds in routine clinical practice:Early and midterm outcomes from the European multicentre GHOST-EU registry. EuroIntervention. 2015;10:1144-1153.
20. Tijssen RYG, Kraak RP, Hofma SH, et al. Complete two-year follow-up with formal non-inferiority testing on primary outcomes of the AIDA trial comparing the Absorb bioresorbable scaffold with the XIENCE drug-eluting metallic stent in routine PCI. EuroIntervention. 2018;14:e426-e433.
21. Chevalier B, Onuma Y, Boven AJ Van. Randomised comparison of a bioresorbable everolimus- eluting scaffold with a metallic everolimus-eluting stent for ischaemic heart disease caused by de novo native coronary artery lesions:the 2-year clinical outcomes of the ABSORB II trial. EuroIntervention. 2016;12:1102-1107.
22. Alvarez M, Applegate RJ. Early and Late Bioresorbable Vascular Scaffold Thrombosis:Size Matters. JACC Cardiovasc Interv. 2017;10:2372-2374.
23. Yamaji K, Brugaletta S, SabatéM, et al. Effect of Post-Dilatation Following Primary PCI With Everolimus-Eluting Bioresorbable Scaffold Versus Everolimus-Eluting Metallic Stent Implantation. JACC Cardiovasc Interv. 2017;10:1867-1877.
24. Ortega-Paz L, Capodanno D, Gori T, et al. Predilation, sizing and post-dilation scoring in patients undergoing everolimus-eluting bioresorbable scaffold implantation for prediction of cardiac adverse events:Development and internal validation of the PSP score. EuroIntervention. 2017;12:2110-2117.
25. Lipinski MJ, Escarcega RO, Baker NC, et al. Scaffold Thrombosis After Percutaneous Coronary Intervention With ABSORB Bioresorbable Vascular Scaffold. JACC Cardiovasc Interv. 2016;9:12-24.
26. Alfonso F, Cuesta J. Very Late Bioresorbable Vascular Scaffold Thrombosis:Smoke or Fire?JACC Cardiovasc Interv. 2017;10:38-41.
27. Miyazaki T, Ruparelia N, Kawamoto H, Figini F, Latib A, Colombo A. Clinical outcomes following “off-label“versus “established“indications of bioresorbable scaffolds for the treatment of coronary artery disease in a real-world population. EuroIntervention. 2016;11:1475-1478.
28. Puricel S, Cuculi F, Weissner M, et al. Bioresorbable Coronary Scaffold Thrombosis. J Am Coll Cardiol. 2016;67:921-931.
29. Brugaletta S, Gori T, Low AF, et al. Absorb bioresorbable vascular scaffold versus everolimus-eluting metallic stent in ST-segment elevation myocardial infarction:1-year results of a propensity score matching comparison:the BVS-EXAMINATION Study (bioresorbable vascular scaffold - a clinical evaluation of everolimus eluting coronary stents in the treatment of patients with ST-segment elevation myocardial infarction). JACC Cardiovasc Interv. 2015;8:189-197.
30. Caixeta A, Campos CM, Felix C, et al. Predictors of long-term adverse events after Absorb bioresorbable vascular scaffold implantation:a 1,933.patient pooled analysis from international registries. EuroIntervention. 2019;15:623-630.
31. IJsselmuiden AJJ, Zwaan EM, Oemrawsingh RM, et al. Appropriate use criteria for optical coherence tomography guidance in percutaneous coronary interventions:Recommendations of the working group of interven.tional cardiology of the Netherlands Society of Cardiology. Neth Heart J. 2018;26:473-483.
32. Bangalore S, Toklu B, Patel N, Feit F, Stone GW. Newer-Generation Ultra-thin Strut Drug-Eluting Stents Versus Older Second-Generation Thicker Strut Drug-Eluting Stents for Coronary Artery Disease. Circulation. 2018;138:2216-2226.
33. Seth A, Onuma Y, Chandra P, et al. Three-year clinical and two-year multimodality imaging outcomes of a thin-strut sirolimus-eluting bioresorb.able vascular scaffold:MeRes-1 trial. EuroIntervention. 2019;15:607-614.
34. Fernández-Rodríguez D, Brugaletta S, Otsuki S, SabatéM. Acute Absorb bioresorbable vascular scaffold thrombosis in ST-segment elevation myocardial infarction:to stent or not to stent?EuroIntervention. 2014;10:600;discussion 600.
35. Tantry US, Navarese EP, Bliden KP, Gurbel PA. Acetylsalicylic acid and clopidogrel hyporesponsiveness following acute coronary syndromes. Kardiol Pol. 2018;76:1312-1319.
36. Cayla G, Koning R, Fajadet J, et al. Percutaneous coronary interventions with the Absorb Bioresorbable vascular scaffold in real life:1-year results from the FRANCE ABSORB registry. Arch Cardiovasc Dis. 2019;112:113-123.
37. Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS. Eur Heart J. 2018;39:213-260.
38. Núñez-Gil IJ, Echavarría M, Escaned J, Biagioni C, Feltes G, Fernández-Ortiz A. Bioresorbable stent restenosis:new devices, novel situations. J Invasive Cardiol. 2014;26:E164-6.
39. Longo G, Granata F, Capodanno D, et al. Anatomical features and manageµment of bioresorbable vascular scaffolds failure:A case series from the GHOST registry. Catheter Cardiovasc Interv. 2015;85:1150-1161.
40. Nakatani S, Onuma Y, Ishibashi Y, et al. Early (before 6 months), late (6-12 months) and very late (after 12 months) angiographic scaffold restenosis in the ABSORB Cohort B trial. EuroIntervention. 2015;10:1288-1298.
41. Räber L, Brugaletta S, Yamaji K, et al. Very Late Scaffold Thrombosis. J Am Coll Cardiol. 2015;66:1901-1914.
42. Chua S-K, Lo H-M, Chiu C-Z, Shyu K-G. Use of CHADS2 and CHA2DS2.VASc scores to predict subsequent myocardial infarction, stroke, and death in patients with acute coronary syndrome:data from Taiwan acute coro.nary syndrome full spectrum registry. PLoS One. 2014;9:e111167.
- Contemporary management of spontaneous coronary dissection
- Fifteen years of percutaneous coronary interventions for chronic total coronary occlusions. Experience, results, and clinical outcomes
- Intracoronary lithotripsy in a high-risk real-world population. First experience in severely calcified, complex coronary lesions
- Prognostic impact of early coronary angiography in patients with non-ST-elevation acute myocardial infarction
Editorials
Expanding the role of drug-coated balloons in native large coronary artery disease
aDepartment of Biomedical Sciences, Humanitas University, Pieve Emanuele, Milan, Italy
bCardio Center, IRCCS Humanitas Research Hospital, Rozzano, Milan, Italy
cDivision of Cardiology, Montefiore Medical Center, Bronx, New York, United States
Original articles
Editorials
The role of angiography-derived physiological assessment techniques in the post-FAVOR III Europe era?
aServicio de Cardiología, Hospital General Universitario Gregorio Marañón, Instituto de Investigación Sanitaria Gregorio Marañón, Universidad Complutense de Madrid, Madrid, Spain
bCentro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), Instituto de Salud Carlos III, Madrid, Spain
Interviews
An interview with Camino Bañuelos
aServicio de Cardiología, Hospital Clínico San Carlos, Instituto de Investigación Sanitaria del Hospital Clínico San Carlos (IdISSC), Madrid, Spain
bServicio de Cardiología, Hospital Clínico Universitario de Santiago de Compostela, Santiago de Compostela, A Coruña, Spain