Article
Ischemic heart disease and acute cardiac care
REC Interv Cardiol. 2019;1:21-25
Access to side branches with a sharply angulated origin: usefulness of a specific wire for chronic occlusions
Acceso a ramas laterales con origen muy angulado: utilidad de una guía específica de oclusión crónica
Servicio de Cardiología, Hospital de Cabueñes, Gijón, Asturias, España
ABSTRACT
Introduction and objectives: Although drug-eluting stents are the main treatment in percutaneous coronary interventions (PCI), drug-coated balloons (DCB) represent an appealing alternative as they eliminate the risk of stent thrombosis and avoid leaving any metal structure in the vessel wall. However, limited evidence has been published to date on the vessel wall healing processes, plaque remodeling, plaque composition, and the impact on the coronary microcirculation after percutaneous coronary intervention with DCB (DCB-PCI).
Methods: This is investigator-initiated, single-center, single-arm, open-label, pilot study of 30 patients with native vessel disease undergoing DCB-PCI. Intravascular ultrasound and angiography-derived index of microvascular resistance (IMRangio) will be performed before and immediately after PCI, and at 3 months of follow-up.
Conclusions: The study aims to provide new evidence on the modification of atherosclerotic plaque in patients with de novo lesions undergoing PCI with DCB. This will be assessed by examining the change in the percentage of atheroma volume and late lumen enlargement using intravascular ultrasound and by evaluating changes in the microcirculation using IMRangio.
Registered at Clinicaltrials.gov (NCT06080919).
Keywords: Drug-coated balloon. Intravascular ultrasound. Angiography-derived index of microvascular resistance.
RESUMEN
Introducción y objetivos: Pese a que los stents farmacoactivos son el tratamiento principal en las angioplastias coronarias, los balones farmacoactivos representan una alternativa interesante dado que eliminan el riesgo de trombosis del stent sin dejar ningún tipo de estructura metálica en la pared del vaso. No obstante, la evidencia en cuanto a los procesos de cicatrización de la pared del vaso, el remodelado, los cambios en la composición de la placa ateroesclerótica y el impacto en la microcirculación coronaria tras el intervencionismo coronario percutáneo (ICP) con balón farmacoactivo aún no se ha esclarecido.
Métodos: Estudio piloto abierto, de un solo grupo, iniciado por el investigador, de 30 pacientes con enfermedad de vaso nativo sometidos a ICP con balón farmacoactivo. Se realizará ecografía intravascular y se determinará el índice de resistencia microvascular derivado de la angiografía (angio-IRM) antes, inmediatamente después y a los 3 meses de seguimiento de la angioplastia.
Conclusiones: Se aportará nueva evidencia sobre la modificación de la placa en pacientes con enfermedad de vaso nativo tratados con balón farmacoactivo, evaluando el cambio en el porcentaje del volumen de ateroma y el aumento luminal tardío, así como los cambios en la microcirculación mediante angio-IRM.
Registrado en Clinicaltrials.gov (NCT06080919).
Palabras clave: Balón farmacoactivo. Ecografía intravascular. Índice de resistencia microvascular derivado de la angiografía.
Abbreviations
DCB: drug-coated balloon. EEM: external elastic membrane. IMRangio: angiography-derived index of microcirculatory resistance. IVUS: intravascular ultrasound. PCI: Percutaneous coronary intervention.
INTRODUCTION
Coronary artery disease is the leading single cause of mortality worldwide, accounting for more than 7 million deaths annually1 and its prevalence has been increasing in the last 20 years.2 Percutaneous coronary intervention (PCI) has been crucial in the treatment of coronary artery disease.3,4 The advent of drug-eluting stents (DES) has substantially reduced restenosis rates through the deposition of antiproliferative drugs in the vessel wall. DES have evolved over the years and have become the gold standard in PCI.5 Drug-coated balloons (DCB) represent an alternative in the setting of PCI. DCB consist of a balloon coated with antiproliferative agents encapsulated in a polymer matrix.6 Upon inflation, the balloon brings the antiproliferative drug into contact with the vessel wall. The main goal of DCB is to eliminate the risk of stent thrombosis and achieve lower restenosis rates by not leaving any type of metal structure in the treated segment.6
The safety and efficacy of DCB have been extensively studied in de novo coronary artery disease.6 In small vessel disease, DCB have demonstrated noninferiority to DES in several randomized clinical trials.7 A recent meta-analysis has shown that the use of DCB, compared with that of DES, is associated with a lower risk of vessel thrombosis and a trend toward a lower risk of acute myocardial infarction.8 In large vessel de novo lesions, current data do not support the widespread use of DCB over DES, although DCB appear to be safe and effective.9,10 Nevertheless, there is a need to elucidate the elution on the vessel wall, healing processes, plaque remodeling, plaque composition and the impact on the coronary microcirculation following PCI with DCB.
The present report describes the design and rationale for a study of plaque modification and impact on the microcirculation after PCI with DCB (the PLAMI study).
METHODS
The study will be an investigator-initiated, single-center, single-arm, open-label, pilot study in patients undergoing PCI with DCB for de novo lesions. The study has been approved by the hospital ethics committee on research involving medical products. The study has been registered in ClinicalTrials.gov (NCT06080919).
Procedure
Eligible patients will be informed about the study and will be required to provide signed informed consent prior to inclusion. Patients will undergo DCB-PCI under intravascular ultrasound (IVUS) guidance. Angiography-derived coronary physiology will be assessed after the procedure using Angio Plus software (Pulse Medical Imaging Technology, China). The angiography images will be used to obtain the angiography-derived index of microcirculatory resistance (IMRangio) values, before and after DCB-PCI. All procedures will be performed according to current European guidelines5: the target lesion will be predilated with semicompliant balloons or noncompliant balloons, with a diameter equal to the reference vessel diameter and with an appropriate length. Multiple predilations will be accepted. The DCB will be the paclitaxel-coated balloon Pantera Lux (BIOTRONIK AG, Switzerland).
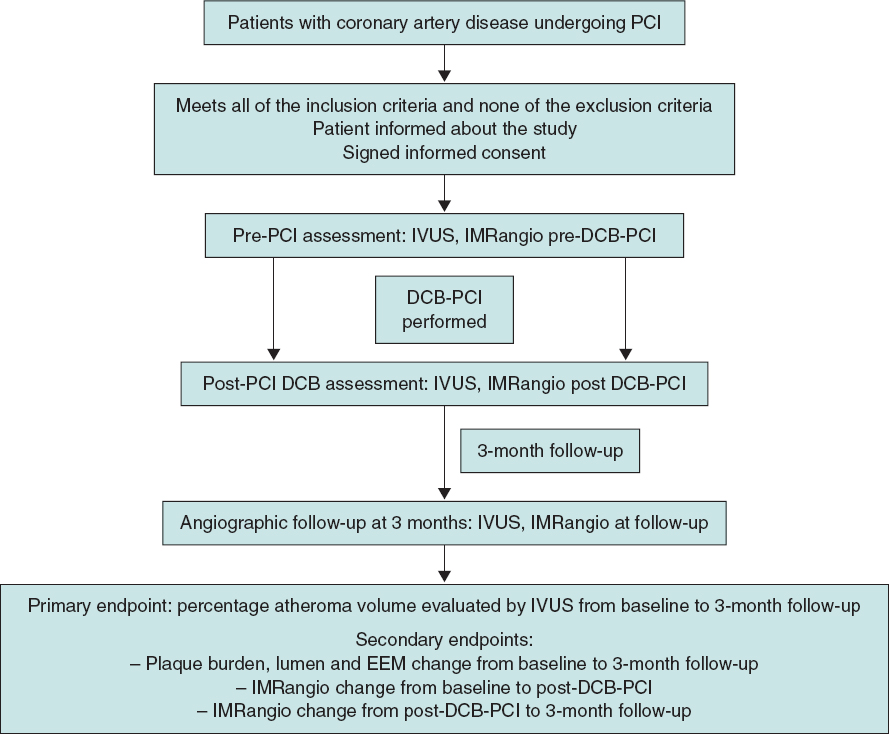
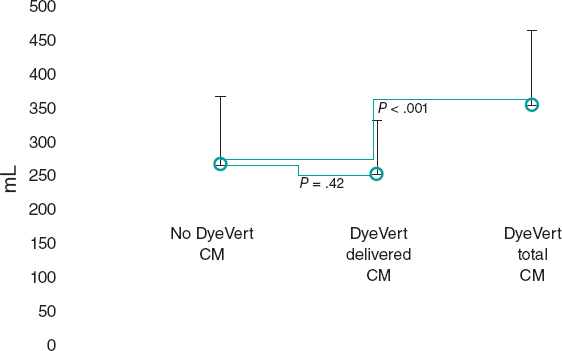
The lesion will then be treated with a DCB with a reference vessel diameter/balloon diameter ratio of 1:1. DCB length will be equal to lesion length + 5 mm. DCB inflation time will be set at 45 to 60 seconds to guarantee correct and complete drug elution. The prespecified reasons for DES implantation after DCB-PCI will be residual stenosis > 30%, dissections > type B and TIMI flow < 3.6 Angiographic follow-up with IVUS and IMRangio evaluation will be performed 3 months after the index procedure. The study timeline is summarized in figure 1.
Figure 1. Timeline of the PLAMI study. DCB, drug-coated balloon; IMRangio, angiography-based index of microcirculatory resistance; IVUS, intravascular ultrasound; PCI, percutaneous coronary intervention.
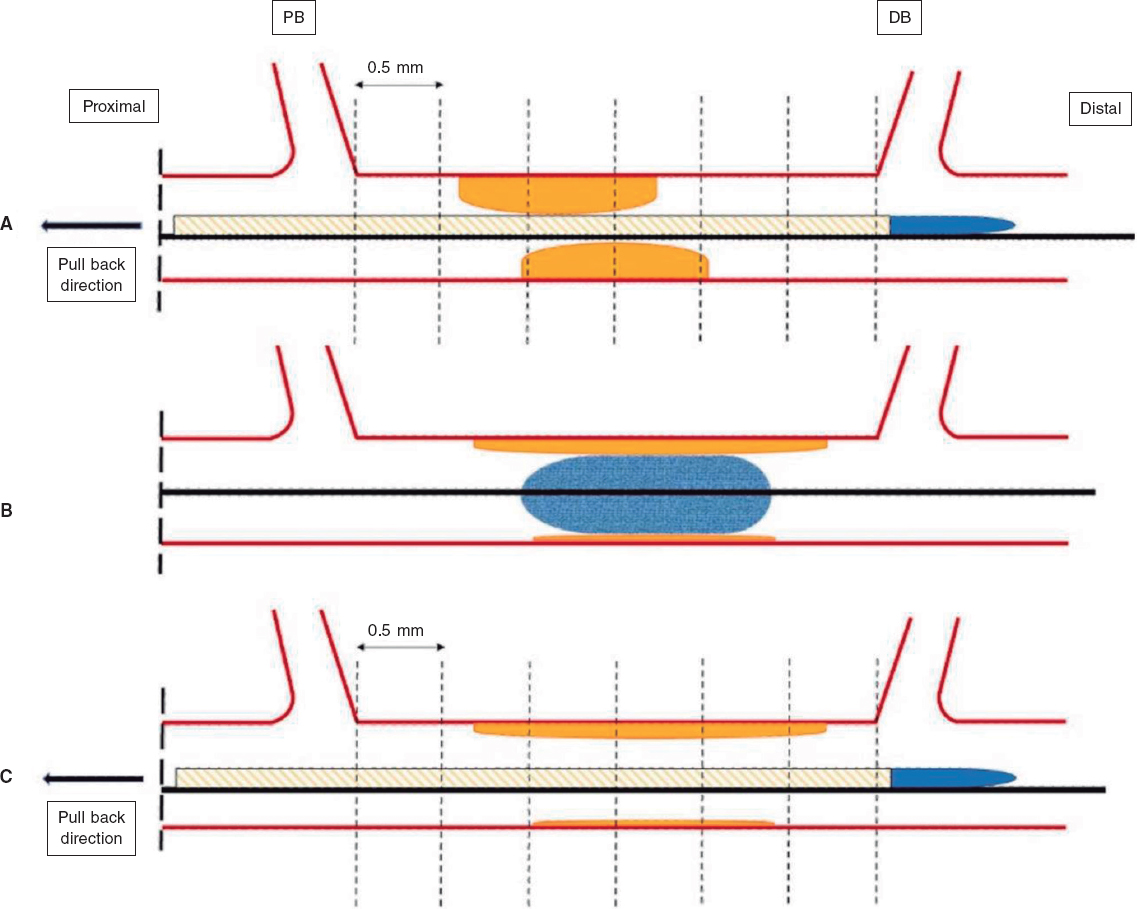
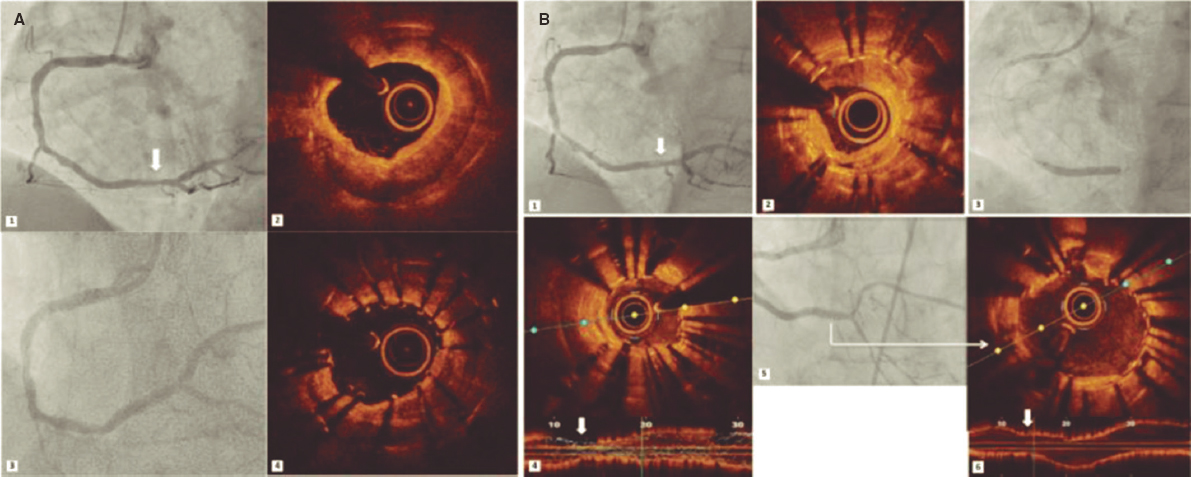
IVUS images will be taken before the DCB-PCI, immediately after, and at 3 months of follow-up using the Opticross HD 60 MHz (Boston Scientific Corp, United States) system. All IVUS studies will be performed after intracoronary administration of 200 μg of nitroglycerin. The IVUS images will be acquired at 30 frames per second with an automatic transducer pull back (at 0.5 mm/second) to the proximal reference vessel lesion. As there will be no stents to take as a reference, the proximal and distal side branches adjacent to the treated lesion will serve as references, matching the coronary angiographic images (figure 2). All IVUS images will be analyzed by an independent core lab.
Figure 2. Schematic representation of IVUS acquisition. The IVUS images will be acquired before (A) and after (C) DCB-PCI (B) at 30 frames per second with an automatic transducer pull back (at 0.5 mm/second) to the proximal reference vessel lesion. The same anatomic slice will be analyzed before, after, and at 3 months of follow-up after the PCI by using reproducible landmarks (side branches). The first frame analyzed will be the distal point of the treated vessel before the exit of the DB (represented by the rightmost dotted line), and the last frame analyzed will be the proximal point of the vessel before the split of the proximal branch. DB, distal branch; DCB-PCI, drug-coated balloon percutaneous coronary intervention; IVUS, intravascular ultrasound; PB, proximal branch.
Angiography-derived assessment of coronary physiology will be performed with Angio Plus software (Pulse Medical Imaging Technology, China). For the evaluation of each lesion, at least 2 projections with a difference of > 25° will be selected. The operator will manually mark the points proximal and distal to the lesion, and the system automatically outlines the contours of the detected vessel. If the traced vessel trajectory deviates from the normal lumen, the necessary manual modifications will be performed. The artificial intelligence-assisted software combines the intravascular imaging information with the estimated vessel flow to obtain the IMRangio. All the angiography images will be analyzed by an independent core lab to obtain the IMRangio.
Study population and enrolment criteria
Patients will be screened to ensure they meet the inclusion criteria and none of the exclusion criteria prior to study enrolment. Inclusion criteria consist of an indication to undergo PCI for a de novo lesion according to current guidelines (with no restrictions regarding vessel size).5 Inclusion and exclusion criteria are summarized in table 1.
Table 1. Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| Patient with CAD undergoing PCI with DCB with no limitation to vessel size | Age < 18 years |
| Cardiogenic shock | |
| ST-segment elevation myocardial infarction | |
| Use of mechanical circulatory support | |
| Complex coronary lesions* including chronic total occlusions, bifurcation lesions, left main coronary artery disease, severe calcified lesions, graft interventions and in-stent restenosis | |
| Inability to provide informed consent | |
| Unable to understand and follow study-related instructions or unable to comply with study protocol | |
| Currently participating in another trial | |
| Pregnant women | |
|
CAD, coronary artery disease; DCB, drug-coated balloon; PCI, percutaneous coronary intervention. |
|
Sample size
Because of the exploratory nature of this study, no formal sample size calculation is required. Based on previous pilot studies with similar designs,12 a sample of 30 lesions is planned to evaluate the impact of DCB on coronary healing and the microcirculatory territory.
Study endpoints
The primary endpoint is the change in percentage atheroma volume evaluated by IVUS from baseline to 3 months of follow-up. Secondary endpoints will include a) lumen change from baseline to 3 months of follow-up (minimum, maximum, average areas), b) the percentage of progressors and percentage of regressors, c) external elastic membrane (EEM) change from baseline to post DCB-PCI (average), d) EEM change post-DCB-PCI to the 3-month follow-up (average), e) the percentage of remodeling types (neutral, negative, and positive), f) IMRangio change from baseline to post- DCB-PCI, g) IMRangio change from post-DCB-PCI to the 3-month follow-up.
An independent clinical event committee, consisting of cardiologists not participating in the trial, will review and adjudicate all major adverse cardiac events according to the study protocol.
Considering the luminal area as the area delimited by the luminal border, the minimal luminal area is defined as the smallest lumen area within the length of the treated lesion.13,14 The atheroma or plaque burden is defined as the ratio of atheroma area to the vessel EEM and is calculated by dividing the sum of plaque and media cross-sectional area (CSA) by the EEM CSA.13,14 As the atheroma area can be calculated in each frame, the total atheroma volume is obtained by taking the sum of the differences between the EEM CSA area and the luminal CSA for all available images.15 The percent of the volume of the EEM occupied by atheroma is called the percentage atheroma volume.15,16
Serial arterial remodeling types will be classified as usual: neutral if there is no change in EEM, negative if there is a decrease in the EEM and positive if vice versa.
Statistical considerations
Continuous variables will be described as mean ± standard deviation or median [interquartile range]. Categorical variables will be described as percentages. The paired t-test will be used to compare continuous variables measured before and after treatment in the same patient, and differences in proportions will be tested with the chi-square or Fisher exact test. A P value less than .05 (typically ≤ .05) will be considered statistically significant. Statistical analyses will be performed using Stata software version 13.1 (StataCorp LP, United States).
DISCUSSION
Although the use of DES remains predominant in the performance of PCI, complications such as stent thrombosis and in-stent restenosis led to the development of DCB. DCB have the theoretical benefit of not leaving metallic material in the vascular lumen, thereby reducing the possibility of mechanical complications such as malapposition, stent fracture, and stent thrombosis. This could potentially reduce neointimal proliferation and shorten the duration of dual antiplatelet therapy.6 Current guidelines assign a level IA recommendation to the treatment of in-stent restenosis.5 While the use of DCB in de novo lesions seems promising, it is not yet widespread. In addition, PCI is not without risks, as it involves a certain degree of injury to the artery wall from balloon inflations and stent struts.17,19 The vascular response to endothelial cell and smooth muscle cell injury represents a complex network of biochemical responses that involve the immune system. All these factors regulate the processes of neointimal hyperplasia, vascular remodeling, and normal reendothelialization of the arterial wall.17
The pathophysiology of restenosis and lumen loss after angioplasty is a complex process involving various factors and is not limited to neointimal hyperplasia.19 Acutely, plain old balloon angioplasty (POBA) generates an increase in luminal area that is mainly due to an expansion of the EEM, mainly attributed to the elastic properties of the vessel rather than to plaque compression or removal.20 Subsequently, within the first few minutes after PCI, there is an “acute recoil” due to the elastic properties of the arterial wall. In the chronic phase, IVUS data indicate that luminal loss is mainly due to a progressive reduction in EEM rather than an increase in atherosclerotic plaque volume. Unlike the acute phase where loss of area is solely due to elastic properties, “chronic recoil” leading to the loss of area also involves a combination factors such as fibrosis, apoptosis, and changes in the extracellular matrix.19,21 Interestingly, not all patients show negative remodeling with a decrease in EEM; around 25% show a persistent increase in EEM, which is correlated with a reduced restenosis rate. Consequently, restenosis appears to be primarily due to the direction and magnitude of changes in arterial remodeling,19 although neointimal hyperplasia also plays a role .
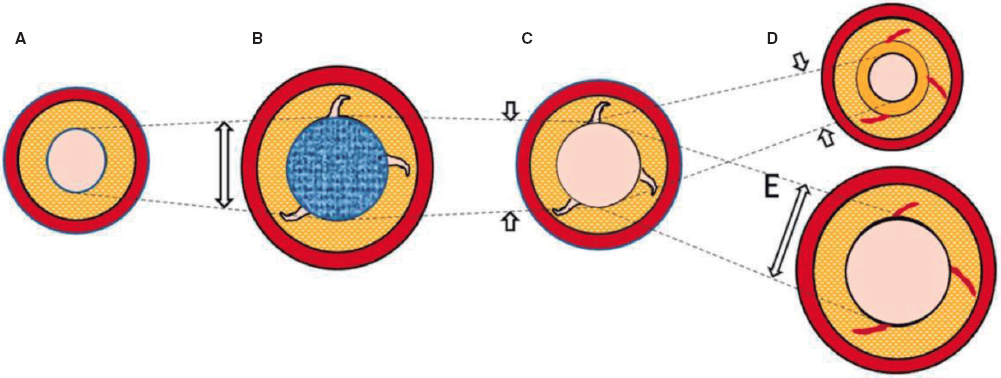
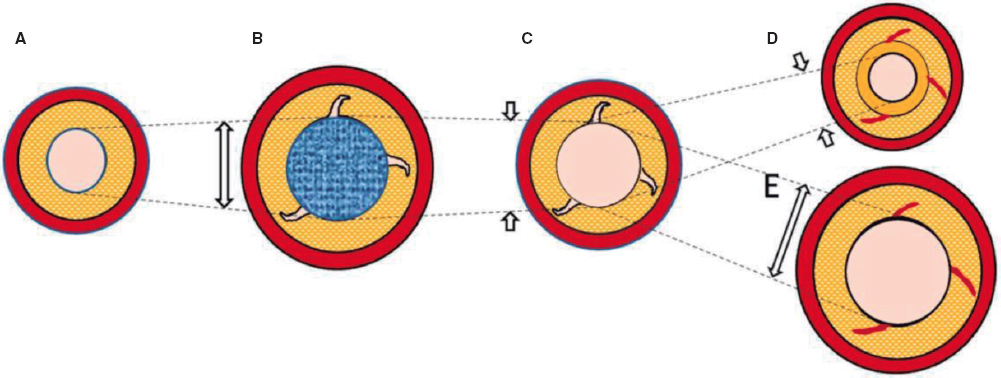
Nevertheless, the existing evidence is based on analysis after the use of traditional balloons. With DCB-PCI, late lumen enlargement has been observed compared with POBA.20 Although this finding has been partly attributed to the inhibition of neointimal proliferation by antiproliferative drugs,22 the role of plaque modification or vessel healing phenomena in influencing this process cannot be excluded. A previous study showed that late lumen enlargement was higher in areas with the highest plaque burden; however, that study was a retrospective assessment and used a quantitative coronary angiography protocol.23 It could be hypothesized that, by inducing controlled damage to the artery wall, together with the antiproliferative effect of DCB, positive vessel remodeling might be achieved, reducing restenosis rates without the need for DES. Therefore, with DCB-PCI, we are able to treat coronary stenosis not only from a mechanical point of view, but can also change the natural history of the disease and restenosis. In this regard, IVUS analysis will be essential to evaluate the reasons behind the gain or loss of luminal area. The dynamic changes produced after PCI are depicted in figure 3.
Figure 3. Central illustration. Schematic representation of timeline of DCB-PCI and lumen variation. A: pre-DCB-PCI de novo lesion. B: DCB-PCI (blue), generating injury to the vessel wall and an increase in lumen and EEM CSA. C: acute recoil. D, chronic recoil with decrease in EEM and neointimal hyperplasia. E. LLE due to maintenance of EEM area and no neointimal hyperplasia. The dotted lines represent the variations of the luminal area throughout the process. The image exemplifies how changes in luminal area, as well as plaque burden, are mainly due to variations in EEM rather than plaque compression. CSA, cross-sectional area; DCB-PCI, drug-coated balloon percutaneous coronary intervention; EEM, external elastic membrane; LLE, late lumen enlargement.
The DCB that will be used in our study, paclitaxel, has been extensively analyzed as a balloon-coating drug due to its lipophilic properties and its ability to elute into the vessel wall.24 Moreover, the available paclitaxel-DCB have shown good results in patients undergoing PCI for native vessel disease.25 In contrast, because of the hydrophobic characteristics of sirolimus, maintaining an adequate percentage in the wall over the mid-term poses technical challenges. However, advances in the formulation of the new generation of sirolimus DCB are anticipated to address this issue by facilitating adequate drug release into the vessel wall.24
As previously mentioned, the coronary microcirculation is closely related to proper coronary functioning and the pathophysiology of coronary artery disease. While it is believed that the performance of PCI, as well as the injury and healing of the coronary artery, may affect the coronary microcirculation, the evidence regarding DCB-PCI is scarce. Moreover, the plaque rupture, intimal dissections and thrombus formation that occur during balloon angioplasty are a potential source of embolism to the microvascular bed.
Since direct visualization of the microcirculation is not feasible in clinical practice,26 its assessment relies on parameters reflecting its functional status, usually coronary flow reserve and the IMR. Coronary flow reserve is defined as the ratio between hyperemic flow in response to nonendothelial vasodilation and resting blood flow. It is crucial to exclude epicardial stenosis before using coronary flow reserve, as it provides an integrated measurement of both epicardial and coronary microcirculation.26 IMR is calculated as the product of distal coronary pressure at maximal hyperemia multiplied by the hyperemic mean transit time.
In our study, we will perform a noninvasive, nonhyperemic assessment of the coronary microcirculation using IMRangio. This approach aims to characterize the baseline status of the microcirculation and assess the microvasculature changes induced by PCI and their variation over a 3-month period.
By monitoring IMRangio before and after treating the stenotic epicardial lesion, we will be able to assess the effects of acute fracture of the atherosclerotic plaque and injury to the arterial wall in the microvascular bed. We also aim to investigate whether these collateral harmful changes provoked during angioplasty remain consistent or vary significantly at 3 months of follow-up. In this same context, the analysis of IVUS during follow-up will allow us to correlate the changes in the arterial wall and atherosclerotic plaque after DCB-PCI with microcirculation physiology. To date, no insights into the anatomical and physiological process of healing of the injured arterial wall after DCB-PCI have been available in the published literature.
CONCLUSIONS
The PLAMI study is a first-in-man pilot study that aims to provide new information on the modification of atherosclerotic plaque assessed by intracoronary imaging in patients with de novo lesions undergoing PCI with DCB.
FUNDING
None reported.
ETHICAL CONSIDERATIONS
The study has been approved by the hospital ethics committee on research involving medical products. Eligible patients will be informed about the study and must provide written informed consent prior to inclusion in the study. Possible gender/sex biases have been considered.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
No artificial intelligence has been used in the preparation of this article.
AUTHORS’ CONTRIBUTIONS
J.A Sorolla Romero, A.Teira Calderón, J. Sanz Sánchez and H.M. Garcia-Garcia contributed to the conception, design, drafting and revision of the article. J.P. Vílchez Tschischke, P. Aguar Carrascosa, F.Ten Morro, L. Andrés Lalaguna, L. Martínez Dolz and J.L. Díez Gil contributed to the critical revision of the intellectual content.
CONFLICTS OF INTEREST
None declared.
WHAT IS KNOWN ABOUT THE TOPIC?
- DCB have proven clinical effectiveness in cases of in-stent restenosis and de novo lesions involving small vessel coronary artery disease.
- Several studies in small vessel coronary artery disease have shown a benefit of DCB in the vessel wall, with late lumen enlargement during follow-up.
- However, there is little evidence of their use in larger vessels.
- In addition, the impact of DCB on the coronary microcirculation has not been evaluated to date.
WHAT DOES THIS STUDY ADD?
- The PLAMI study aims to characterize vessel healing using IVUS after DCB-PCI in patients with native vessel disease and to correlate these findings with the impact on microcirculation.
REFERENCES
1. Ralapanawa U, Sivakanesan R. Epidemiology and the Magnitude of Coronary Artery Disease and Acute Coronary Syndrome:A Narrative Review. J Epidemiol Glob Health. 2021;11:169-177.
2. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease:A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596-e646.
3. Canfield J, Totary-Jain H. 40 Years of Percutaneous Coronary Intervention:History and Future Directions. J Pers Med. 2018;8:33.
4. Stefanini GG, Alfonso F, Barbato E, et al. Management of myocardial revascularisation failure:an expert consensus document of the EAPCI. EuroIntervention. 2020;16:e875-e890.
5. Neumann F, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87-165.
6. Jeger RV, Eccleshall S, Wan Ahmad WA, et al. Drug-Coated Balloons for Coronary Artery Disease:Third Report of the International DCB Consensus Group. JACC Cardiovasc Interv. 2020;13:1391-1402.
7. Tang Y, Qiao S, Su X, et al. Drug-Coated Balloon Versus Drug-Eluting Stent for Small-Vessel Disease:The RESTORE SVD China Randomized Trial. JACC Cardiovasc Interv. 2018;11:2381-2392.
8. Sanz Sánchez J, Chiarito M, Cortese B, et al. Drug-Coated balloons vs drug-eluting stents for the treatment of small coronary artery disease:A meta-analysis of randomized trials. Catheter Cardiovasc Interv. 2021;98:66-75.
9. Yerasi C, Case BC, Forrestal BJ, et al. Drug-Coated Balloon for de Novo Coronary Artery Disease:JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75:1061-1073.
10. Nishiyama N, Komatsu T, Kuroyanagi T, et al. Clinical value of drug-coated balloon angioplasty for de novo lesions in patients with coronary artery disease. Int J Cardiol. 2016;222:113-118.
11. Lawton JS, Tamis-Holland JE, Bangalore S, et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization. J Am Coll Cardiol. 2022;79:e21-e129.
12. Joner M, Finn AV, Farb A, et al. Pathology of drug-eluting stents in humans:delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006;48:193-202.
13. Xu J, Lo S. Fundamentals and role of intravascular ultrasound in percutaneous coronary intervention. Cardiovasc Diagn Ther. 2020;10:1358-1370.
14. Mintz GS, Nissen SE, Anderson WD, et al. American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS). A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2001;37:1478-1492.
15. Gogas BD, Farooq V, Serruys PW, Garcia-Garcia HM. Assessment of coronary atherosclerosis by IVUS and IVUS-based imaging modalities:progression and regression studies, tissue composition and beyond. Int J Cardiovasc Imaging. 2011;27:225-237.
16. Tobis JM, Perlowski A. Atheroma Volume by Intravascular Ultrasound as a Surrogate for Clinical End Points. J Am Coll Cardiol. 2009;53:1116-1118.
17. Feinberg MW. Healing the injured vessel wall using microRNA-facilitated gene delivery. J Clin Invest. 2014;124:3694-3697.
18. Inoue T, Croce K, Morooka T, Sakuma M, Node K, Simon DI. Vascular Inflammation and Repair:Implications for Reendothelialization, Restenosis, and Stent Thrombosis. JACC Cardiovasc Interv. 2011;4:1057-1066.
19. Mintz GS, Popma JJ, Pichard AD, et al. Arterial Remodeling After Coronary Angioplasty. Circulation. 1996;94:35-43.
20. Her AY, Ann SH, Singh GB, et al. Comparison of Paclitaxel-Coated Balloon Treatment and Plain Old Balloon Angioplasty for De Novo Coronary Lesions. Yonsei Med J. 2016;57:337-341.
21. Geary RL, Nikkari ST, Wagner WD, Williams JK, Adams MR, Dean RH. Wound healing:A paradigm for lumen narrowing after arterial reconstruction. J Vasc Surg. 1998;27:96-108.
22. Sogabe K, Koide M, Fukui K, et al. Optical coherence tomography analysis of late lumen enlargement after paclitaxel-coated balloon angioplasty for de-novo coronary artery disease. Catheter Cardiovasc Interv. 2021;98:E35-E42.
23. Kleber FX, Schulz A, Waliszewski M, et al. Local paclitaxel induces late lumen enlargement in coronary arteries after balloon angioplasty. Clin Res Cardiol. 2015;104:217-225.
24. Yerasi C, Case BC, Forrestal BJ, et al. Drug-Coated Balloon for De Novo Coronary Artery Disease:JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75:1061-1073.
25. Venetsanos D, Omerovic E, Sarno G, et al. Long term outcome after treatment of de novo coronary artery lesions using three different drug coated balloons. Int J Cardiol. 2021;325:30-36.
26. Kunadian V, Chieffo A, Camici PG, et al. An EAPCI Expert Consensus Document on Ischaemia with Non-Obstructive Coronary Arteries in Collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology &Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. Eur Heart J. 2020;41:3504-3520.
ABSTRACT
Introduction and objectives: In patients with ST-segment elevation myocardial infarction (STEMI) treatment delay significantly affects outcomes. The effect of admission time in STEMI patients is unknown when percutaneous coronary intervention (PCI) is the preferred reperfusion strategy. This study aimed to determine the association between STEMI outcomes and the timing of admission in a PCI center in south-western Europe.
Methods: This retrospective cohort study analyzed the local electronic data from 1222 consecutive STEMI patients treated with PCI. On-hours were defined as admission from Monday to Friday between 8:00 AM and 6:00 PM on non-national holidays.
Results: A total of 439 patients (36%) were admitted on-hours and 783 patients (64%) were admitted off-hours. Baseline characteristics were well-balanced between the 2 groups, including the percentage of patients admitted in cardiogenic shock (on-hours 5% vs off-hours 4%; P = .62). The median time from first medical contact to reperfusion did not differ between the 2 groups (on-hours 120 minutes vs off-hours 123 minutes, P = .54) and no association was observed between admission time and in-hospital mortality (on-hours 5% vs off-hours 5%, P = .90) or 1-year mortality (on-hours 10% vs off-hours 10%, P = .97). Survival analysis showed no differences in on-hours PCI vs off-hours PCI (HR, 1.1; 95%CI, 0.74-1.64; P = .64).
Conclusions: In a contemporary emergency network, the timing of STEMI patients’ admission to the PCI center was not associated with reperfusion delays or increased mortality.
Keywords: ST-segment elevation myocardial infarction. Admisison time. Percutaneous coronary intervention. Emergency medical services. Mortality.
RESUMEN
Introducción y objetivos: En pacientes con infarto agudo de miocardio con elevación del segmento ST (IAMCEST), el retraso en el tratamiento afecta de manera importante los resultados. El efecto del horario de atención en los pacientes con IAMCEST es dudoso cuando la intervención coronaria percutánea (ICP) es la estrategia de reperfusión preferida. Este estudio tuvo como objetivo determinar la asociación entre los resultados del IAMCEST y el momento de la admisión en un centro con ICP del suroeste de Europa.
Métodos: Estudio de cohorte retrospectivo en el que se analizaron los datos electrónicos locales de 1.222 pacientes consecutivos con IAMCEST tratados con ICP. El horario de atención laboral se definió como la admisión de lunes a viernes de 8 a 18 horas, en días no festivos.
Resultados: Un total de 439 pacientes (36%) ingresaron en horario laboral y 783 (64%) se admitieron fuera del horario. Las características iniciales estaban bien equilibradas entre los grupos, incluyendo el porcentaje de pacientes ingresados en shock cardiogénico (en horario laboral el 5% y fuera del horario laboral el 4%; p = 0,62). La mediana de tiempo desde el primer contacto médico hasta la reperfusión no fue diferente entre los 2 grupos (dentro del horario laboral 120 min y fuera del horario laboral 123 min; p = 0,54). No se observó asociación entre el tiempo de admisión y la mortalidad hospitalaria (dentro del horario laboral el 5% y fuera del horario laboral el 5%; p = 0,90) ni la mortalidad a 1 año (en horario laboral el 10% y fuera del horario el 10%; p = 0,97). El análisis de supervivencia no mostró diferencias entre la admisión dentro del horario laboral y la admisión fuera del horario laboral (HR = 1,1; IC95%, 0,74-1,64; p = 0,64).
Conclusiones: En una red de código infarto contemporáneo, el horario de admisión de pacientes con IAMCEST no se asoció con retrasos en la reperfusión ni con un aumento de la mortalidad.
Palabras clave: Infarto agudo de miocardio con elevación del segmento ST. Horario de ingreso. Intervención coronaria percutánea. Emergencia médica. Mortalidad.
Abbreviations
PCI: percutaneous coronary intervention. STEMI: ST-segment elevation myocardial infarction.
INTRODUCTION
Ischemic heart disease is the leading cause of death worldwide. In Europe, despite the decline in incidence and mortality between 1990 and 2009, these trends have slowed in recent years. Moreover, Mediterranean countries showed lower rate of decline during this period.1
ST-segment elevation myocardial infarction (STEMI) is a particularly important presentation, associated with high mortality in young individuals.2,3 Primary percutaneous coronary intervention (PCI) is recommended as the first-line therapy to lower mortality and morbidity in STEMI patients.4-6 The timing of treatment is crucial for positive outcomes, and minimization of the time from symptom onset to revascularization is essencial.7,8 While several factors affect treatment timing, emergency system delays play a crucial role as they can be more easily altered by organizational measures and are often used as a quality measurement in STEMI networks.4,9-13
To ensure timely treatment, primary PCI centers included in STEMI networks are recommended to have a 24/7 service.4 However, the impact of admission time (on- vs off-hours) on treatment delay and patient outcomes remains a matter of debate. Some studies and a large meta-analysis have shown that off-hours admission is associated with worse outcomes, partially explained by longer system delays, less guideline-directed management, and less revascularization.14-16 Conversely, studies in high-volume PCI centers integrated in STEMI networks, demonstrated no differences in outcomes according to admission time.17-20 Overall, these results are heterogeneous and include populations from different health care systems.
In Europe, efforts have been made to improve STEMI care through public awareness, emergency medical system operations, and the implementation of a full national coverage 24/7 PCI network.21
The aim of this study was to determine the association between timing of admission in a PCI center and STEMI patients’ outcomes, within a STEMI network in south-western Europe.
METHODS
Study design and population
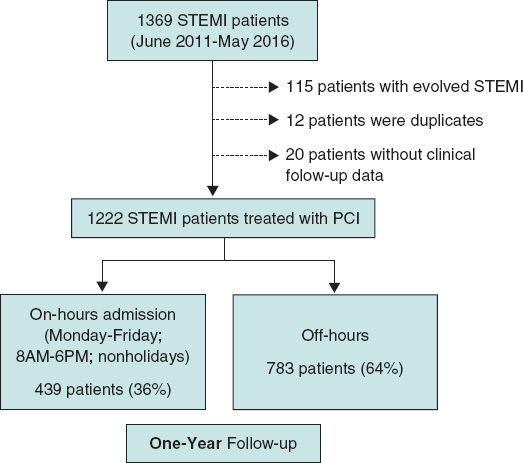
This retrospective observational cohort study identified 1369 consecutive patients treated with primary PCI at the catheterization laboratory of the Hospital de Braga (Portugal) between June 2011 and May 2016, through the local database that systematically includes all patients undergoing invasive coronary procedures. After an initial analysis, 115 patients were found to have evolved STEMI (> 12 hours since symptom onset) and were therefore excluded. To avoid duplication of results, we excluded 12 records of a repeat episode of STEMI in a patient previously identified in the selected time frame. Lastly, clinical follow-up data were not available for 20 patients, resulting in a final sample of 1222 patients (figure 1). These patients were divided into 2 groups according to admission time (on-hours and off-hours admission), and the main outcome measures evaluated were time delays, in-hospital mortality, and 1-year mortality.
Figure 1. Study flow-chart. PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
Definitions
STEMI was defined as the presence of symptoms of myocardial ischemia, associated with electrocardiographic criteria for ST-segment elevation.4
Admission time was based on arrival at the catheterization laboratory. On-hours were defined as admission from Monday to Friday between 8:00 AM and 6:00 PM on non-national holidays.
The first medical contact was defined as the first contact with a health service (hospital or primary care clinic). In patients primarily attended by the emergency medical system, the moment when the emergency vehicle carrying a trained physician arrived at the location of the patient was recorded. The reperfusion time was considered as the moment when the angioplasty guidewire crossed the culprit lesion. Time delays from symptom onset to first medical contact (patient-dependent time), from first medical contact to reperfusion (system-dependent time) and from symptom onset to reperfusion (total ischemic time) were characterized.
Patient stratification according to the Killip classification was based on physical examination and the development of heart failure. A Killip class IV classification was assigned to patients in cardiogenic shock.22
STEMI network organization
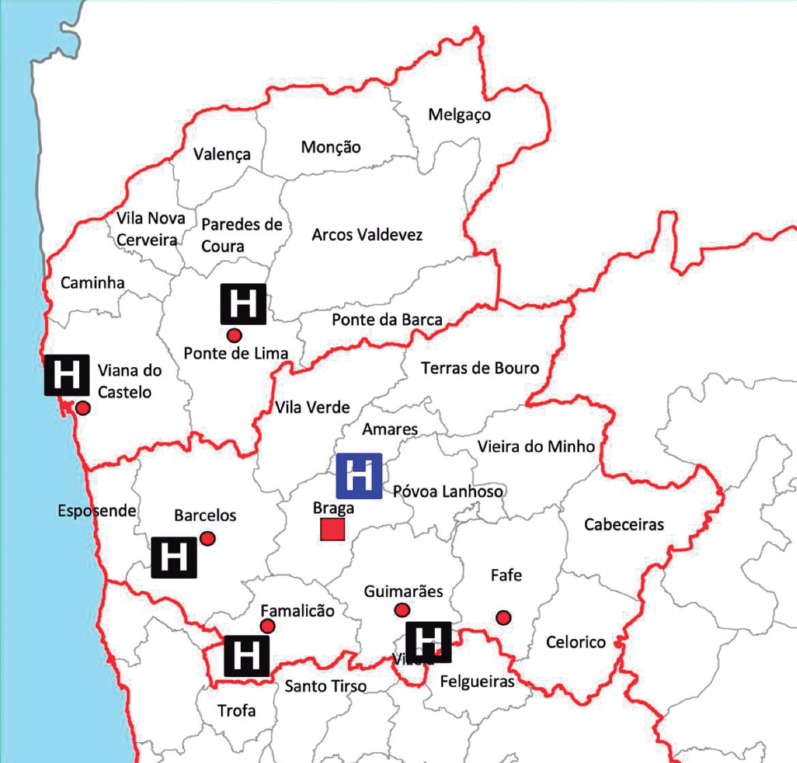
Hospital de Braga has a 24/7 catheterization laboratory service for primary PCI, performed by senior interventional cardiologists (on-call during off-hours). The hospital is the only primary PCI-capable hospital in the Minho region in the north of Portugal and serves approximately 1.1 million people (figure 2). First medical contact can be made by the emergency medical system or in non-PCI-capable hospitals and clinics, which decide whether to transfer the patient to the PCI-center after consulting the on-call clinical cardiologist. First medical contact can also be made in Hospital de Braga, with rapid triage to primary PCI.
Figure 2. Referral network of the catheterization laboratory of Hospital de Braga.
Data collection and statistical analysis
The data for the present study were obtained from the local database of the patient undergoing PCI, the patient’s clinical record, and the electronic health registry of Portugal. Clinical and demographic variables were collected.
The IBM Statistical Package for the Social Sciences (IBM SPSS) version 28.0 was used for data treatment. The variables studied to characterize the patients were divided into continuous variables and categorical variables. For the analysis of continuous variables, the distribution was first evaluated. If the variables showed symmetrical normal distribution, the results are presented as mean ± standard deviation, while for variables without normal distribution, the results are reported as median [interquartile range]. To compare continuous variables between the 2 groups of patients, parametric tests were applied for variables with normal distribution and nonparametric tests for the remainder. The Student t test for independent samples was used as the parametric test, after evaluation of the homogeneity of variances using the Levene test. The Mann-Whitney U test was the nonparametric test applied. For the description of categorical variables, absolute (No.) and relative (%) frequencies were calculated. The comparison of proportions between the study groups was made using the chi-square test or Fisher exact test when the percentage of cells in the table with an expected frequency less than 5 was greater than 20%. The 1-year survival analysis was performed using the Kaplan-Meier method, comparing the groups using the log-rank test. A multivariate analysis with Cox regression was performed, and was adjusted for confounding variables that were statistically significant in the univariate analysis (age, sex, smoking, diabetes mellitus, hypertension, cardiogenic shock, and total ischemia time), to determine if the timing of patient admission was an independent predictor of 1-year mortality. The adjusted hazard ratio (HR) and 95% confidence interval (95%CI) were analyzed to determine the significance of the predictor. In all analyses, results with probability values of P < .05 were considered statistically significant.
Confidentiality and ethical considerations
Informed consent for the procedure was obtained in all patients. The confidentiality and anonymity of all collected data were ensured during all phases of the study. This study was approved by the local ethics committee and complies with the provisions of the Helsinki Declaration. Informed consent for the present analysis was waived by the ethics committee due to the retrospective nature of the study.
RESULTS
Baseline characteristics
Between June 2011 and May 2016, of 1222 consecutive patients with confirmed STEMI, a total of 439 (36%) were admitted on-hours and 783 (64%) were admitted off-hours. Baseline characteristics were well-balanced between groups, including the percentage of patients admitted in cardiogenic shock (on-hours 5% vs off-hours 4%; P = .62) (table 1).
Table 1. Baseline characteristics
| Total (N = 1222) | On-hours (N = 439) | Off-hours (N = 783) | P | |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age, y | 61 ± 13 | 62 ± 13 | 61 ± 14 | .40 |
| Female | 269 (22) | 102 (23) | 167 (21) | .44 |
| Smoking (active or previous) | 625 (54) | 218 (51) | 407 (55) | .18 |
| Dyslipidemia | 553 (46) | 201 (46) | 352 (45) | .72 |
| Diabetes | 250 (22) | 104 (25) | 146 (20) | .04 |
| Hypertension | 622 (51) | 224 (52) | 398 (51) | .89 |
| Previous history | ||||
| ACS | 84 (7) | 28 (6) | 56 (7) | .63 |
| PCI | 62 (5) | 43 (4) | 19 (6) | .38 |
| CABG | 11 (1) | 5 (1) | 6 (1) | .50 |
| Presentation | ||||
| Direct admission | 452 (36) | 159 (37) | 293 (37) | .68 |
| Anterior MI | 642 (53) | 229 (52) | 413 (53) | .85 |
| Cardiogenic shock | 51 (4) | 20 (5) | 31 (4) | .62 |
| Angiography | ||||
| Multivessel disease | 583 (48) | 215 (49) | 368 (47) | .51 |
| Echocardiography | ||||
| LVEF | 44 ± 10 | 45 ± 10 | 44 ± 10 | .41 |
|
ACS, acute coronary syndrome; CABG, coronary artery bypass graft; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PCI, percutaneous coronary intervention. Data are expressed as No. (%) or mean ± standard deviation. |
||||
Comparison of treatment delays
The statistical analysis revealed no significant differences between groups for system-related, patient-related, and total ischemia time (table 2). Similarly, when examining patients directly admitted to the PCI-center, no significant differences were observed in terms of system-related, patient-related, and total ischemia time (table 2).
| Irrespective of place of FMC | Total (N = 1222) | On-hours (N = 439) | Off-hours (N = 783) | P |
|---|---|---|---|---|
| Patient-related SO-FMC, min | 87 [45-165] | 82 [45-160] | 89 [48-166] | .30 |
| Emergency system-related FMC-reperfusion, min | 123 [92-172] | 120 [91-169] | 123 [92-173] | .54 |
| Total ischemic time SO-reperfusion | 225 [164-354] | 220 [159-343] | 228 [165-360] | .39 |
| Admitted directly to the PCI-center | Subtotal (N1 = 452) | On-hours (N1 = 159) | Off-hours (N1 = 293) | P |
| Patient-related SO-FMC, min | 77 [40-150] | 75 [45-155] | 78 [40-150] | .96 |
| Emergency system-related FMC-reperfusion, min | 88 [68-115] | 87 [68-115] | 88 [70-115] | .54 |
| Total ischemic time SO-revascularization, min | 177 [125-265] | 175 [127-254] | 177 [124-267] | .92 |
|
FMC, first medical contact; PCI, percutaneous coronary intervention; SO, symptom onset. Values are expressed as median [interquartile range]. |
||||
Association between admission time and outcomes
A 1-year follow-up was completed for all patients included in the analysis. There was no association between on- and off-hours admission time and in-hospital (5% vs 5%; P = .90) or 1-year mortality (10% vs 10%; P = .97). Equally, in patients admitted on- and off-hours directly to the PCI center, in-hospital (4% vs 7%; P = .30) and 1-year mortality (9% vs 13%; P = .27) was similar.
Patients who experienced cardiogenic shock had significantly higher rates of both in-hospital (55% vs 3%; P < .01) and 1-year mortality (71% vs 7%; P < .01) compared with stable patients. However, the time of admission to the hospital did not show a significant impact on the in-hospital (on-hours 50% vs off-hours 58%; P = .57) or 1-year mortality (on-hours 65% vs off-hours 74%; P = .48) for those with cardiogenic shock.
Hospital admissions for heart failure did not differ in patients admitted on- and off-hours (3% vs 3%; P = .60).
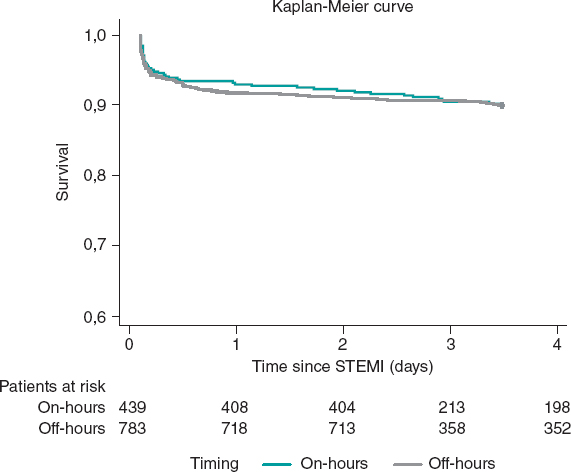
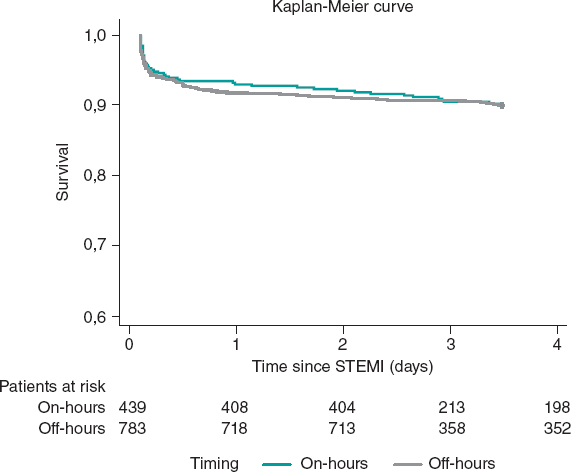
Kaplan-Meier curves showed no differences between timings in survival terms (log-rank P = .95) (figure 3). The timing of admission was not a predictor of 1-year mortality after adjustment (HR, 1.1; 95%CI, 0.74-1.64; P = .64). Independent predictors of mortality at 1-year are depicted in table 3, with cardiogenic shock emerging as the only strong predictor of 1-year mortality.
Figure 3. Kaplan-Meier curves for 1-year survival. STEMI, ST-segment elevation myocardial infarction.
Table 3. Predictive factors of 1-year mortality
| Adjusted HR* | 95%CI | P | |
|---|---|---|---|
| Age | 1.08 | 1.06-1.10 | < .01 |
| Cardiogenic shock | 12.64 | 7.60-19.47 | < .01 |
| Diabetes mellitus | 1.49 | 0.98-2.26 | .06 |
| Hypertension | 1.11 | 0.72-1.73 | .63 |
| Sex | 1.29 | 0.78-1.88 | .43 |
| Smoking | 1.06 | 0.65-1.74 | .81 |
| Total ischemic time | 1.00 | 1.00-1.01 | .06 |
|
95%CI, 95% confidence interval; HR, hazard ratio. * Multivariate analysis with Cox regression adjusted for confounding variables that were statistically significant in the univariate analysis (age, cardiogenic shock, diabetes mellitus, hypertension, sex, smoking, and total ischemia time). Admission time was not associated with 1-year mortality in univariate analysis (P = .95). |
|||
DISCUSSION
This study suggests that there is no association between the timing of admission in the PCI center and adverse outcomes, in a structured STEMI network that offers PCI as the standard of care 24/7. Patients admitted off-hours had the same characteristics and were offered the same quality of care as those admitted on-hours, reflected by the similarity in treatment delays. Previous studies, in networks that provided the same quality of care whatever the admission time, reported no differences in outcomes.17-20
On the other hand, studies that report worst outcomes in patients admitted off-hours, mainly reflect differences in care during this period, with increased delay before revascularization, lower delivery of primary PCI, different procedural characteristics, and fewer available staff during off-hours.16,23-25 Additionally, several studies found that patients tended to have worse clinical status on admission during off-hours, which adversely impacted outcomes.16,26 A finding that supports the outmost importance of presentation status is the fact that cardiogenic shock at admission was found to be an independent predictor of 1-year mortality in this study. However, we did not find significant differences in presentation status according to admission time.
This analysis emphasizes that good organization of STEMI networks, with fast-track 24/7 primary PCI, is key to improve patient outcomes and to obviate the adverse impact of off-hours. However, time delays can still be optimized. Public awareness is key to reduce patient-dependent delays, and efforts should be made to improve recognition of symptoms and activation of emergency medical systems. System delays are quality of care indexes, and in this study, they are in the upper margin for benefit of PCI over fibrinolysis (120 minutes).4,27 This group previously analyzed the impact of interhospital transfer in time from first medical contact to reperfusion, and suggested improvements in chest pain work-up in emergency rooms and prompt transfer protocols after STEMI detection.28
Mortality rates in STEMI differ widely among analyses according to the geographical area, time frame analyzed, patient inclusion criteria, and patient management protocols.29,30 Nonetheless, in this analysis, mortality rates (5% in-hospital and 10% 1-year mortality) were in line with those reported in contemporary registries.2,31
To the best of our knowledge, this is the first study in a STEMI network in south-western Europe ensuring the feasibility and safety of on-call off-hours primary PCI in a contemporary STEMI network. This provides substantial reassurance to the usual organization of cath labs with on-call professionals, essential for workload management and organization of the laboratory workforce.
Study limitations
First, this is a single-center study and may not reflect regional differences in STEMI network organization. Moreover, the results of this study reflect those of a high-volume PC center with a long-standing 24/7 primary PCI program, which may differ from others due to diverse organizational features and available resources. This could be tackled by a future study analyzing national registry data.
Second, the retrospective nature of this study has the limitations inherent to this type of design.
Third, the definition of off-hours admission time is heterogeneous across the literature. In this study, it was defined according to the organizational features of the cath lab, which may not reflect off-hours in other centers/networks.
Additionally, overall mortality in this study may be underestimated, as the group of patients diagnosed in hospitals other than the PCI center and who died before or during transfer were not included in this analysis.
Another limitation of this study is the focus on the management of the patient exclusively until the performance of the primary PCI. Other factors that affect outcomes in these patients, most importantly the delivery of guideline directed medical therapies immediately after revascularization, were not analyzed.
Our findings, based on procedures conducted between 2011 and 2016, may not fully reflect the most current health care trends, given the continuous development of clinical guidelines and treatment approaches. For instance, the reduced use of thrombus aspiration, in line with updated guidelines, highlights the imperative for ongoing research to capture the latest developments in the field.
CONCLUSIONS
In a contemporary emergency network, STEMI patients’ admission time in the PCI-center was not associated with reperfusion delays or increased in-hospital and 1-year mortality. Mortality in efficient STEMI networks is primarily affected by the severity of clinical presentation.
FUNDING
None.
ETHICAL CONSIDERATIONS
Informed consent for the procedure was obtained in all cases. The confidentiality and anonymity of all collected data were ensured during all phases of the study. This study was approved by the local ethics committee and complied with the provisions of the Helsinki Declaration. Informed consent for the present analysis was waived by the ethics committee due to the retrospective nature of the study.
Possible sex/gender biases were taken into account and avoided.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
The authors did not use artificial intelligence tools during the preparation of this study.
AUTHORS’ CONTRIBUTIONS
All the authors contributed to the study design, performed a critical review of the manuscript, gave their final approval, and are fully responsible for all aspects of the study guaranteeing both its integrity and accuracy.
CONFLICTS OF INTEREST
None.
WHAT IS KNOWN ABOUT THE TOPIC?
- The impact of admission time (on- vs off-hours) on treatment delay and patient outcomes remains a matter of debate. Some studies have shown that off-hours admission is associated with worse outcomes, while others disprove these findings.
- Previous analyses are heterogeneous and include populations from different health care systems.
WHAT DOES THIS STUDY ADD?
- Real-world clinical evidence that STEMI patients’ admission time to the PCI-center is not associated with reperfusion delays or increased in-hospital and 1-year mortality.
- Mortality in a STEMI network is primarily affected by the severity of clinical presentation.
REFERENCES
1. Vancheri F, Tate AR, Henein M, et al. Time trends in ischaemic heart disease incidence and mortality over three decades (1990–2019) in 20 Western European countries:systematic analysis of the Global Burden of Disease Study 2019. Eur J Prev Cardiol. 2022;29:396-403.
2. Zeymer U, Ludman P, Danchin N, et al. Reperfusion therapies and in-hospital outcomes for ST-elevation myocardial infarction in Europe:the ACVC-EAPCI EORP STEMI Registry of the European Society of Cardiology. Eur Heart J. 2021;42:4536-4549.
3. Fokkema ML, James SK, Albertsson P, et al. Population Trends in Percutaneous Coronary Intervention:20-Year Results From the SCAAR (Swedish Coronary Angiography and Angioplasty Registry). J Am Coll Cardiol. 2013;61:1222-1230.
4. Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2018;39:119-177.
5. Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction:A quantitative review of 23 randomised trials. Lancet. 2003;361:13-20.
6. Nielsen PH, Maeng M, Busk M, et al. Primary angioplasty versus fibrinolysis in acute myocardial infarction:Long-term follow-up in the danish acute myocardial infarction 2 trial. Circulation. 2010;121:1484-1491.
7. Cannon CP, Gibson CM, Lambrew CT, et al. Relationship of Symptom-Onset-to-Balloon Time and Door-to-Balloon Time With Mortality in Patients Undergoing Angioplasty for Acute Myocardial Infarction. JAMA. 2000;283:2941-2947.
8. Koul S, Andell P, Martinsson A, et al. Delay from first medical contact to primary PCI and all-cause mortality:a nationwide study of patients with ST-elevation myocardial infarction. J Am Heart Assoc. 2014;3:e000486.
9. Terkelsen CJ, Sørensen JT, Maeng M, et al. System Delay and Mortality Among Patients With STEMI Treated With Primary Percutaneous Coronary Intervention. JAMA. 2010;304:763-771.
10. Peterson MC, Syndergaard T, Bowler J, Doxey R. A systematic review of factors predicting door to balloon time in ST-segment elevation myocardial infarction treated with percutaneous intervention. Int J Cardiol. 2012;157:8-23.
11. Pereira H, CaléR, Pinto FJ, et al. Factors influencing patient delay before primary percutaneous coronary intervention in ST-segment elevation myocardial infarction:The Stent for life initiative in Portugal. Rev Port Cardiol. 2018;37:409-421.
12. Pereira H, CaléR, Pereira E, et al. Five years of Stent for Life in Portugal. Rev Port Cardiol. 2021;40:81-90.
13. Wein B, Bashkireva A, Au-Yeung A, et al. Systematic investment in the delivery of guideline-coherent therapy reduces mortality and overall costs in patients with ST-elevation myocardial infarction:Results from the Stent for Life economic model for Romania, Portugal, Basque Country and Kemerovo region. Eur Heart J Acute Cardiovasc Care. 2020;9:902-910.
14. Kostis WJ, Demissie K, Marcella SW, Shao YH, Wilson AC, Moreyra AE. Weekend versus weekday admission and mortality from myocardial infarction. N Engl J Med. 2007;356:1099-1109.
15. Magid DJ, Wang Y, Herrin J, et al. Relationship Between Time of Day, Day of Week, Timeliness of Reperfusion, and In-Hospital Mortality for Patients With Acute ST-Segment Elevation Myocardial Infarction. JAMA. 2005;294:803-812.
16. Sorita A, Ahmed A, Starr SR, et al. Off-hour presentation and outcomes in patients with acute myocardial infarction:systematic review and meta-analysis. BMJ. 2014;348:f7393.
17. de Boer SPM, Oemrawsingh RM, Lenzen MJ, et al. Primary PCI during off-hours is not related to increased mortality. Eur Heart J Acute Cardiovasc Care. 2012;1:33-39.
18. Rathod KS, Jones DA, Gallagher SM, et al. Out-of-hours primary percutaneous coronary intervention for ST-elevation myocardial infarction is not associated with excess mortality:a study of 3347 patients treated in an integrated cardiac network. BMJ Open. 2013;3:e003063.
19. Lattuca B, Kerneis M, Saib A, et al. On- Versus Off-Hours Presentation and Mortality of ST-Segment Elevation Myocardial Infarction Patients Treated With Primary Percutaneous Coronary Intervention. Cardiovascular Interventions. 2019;12:2260-2268.
20. Casella G, Ottani F, Ortolani P, et al. Off-hour primary percutaneous coronary angioplasty does not affect outcome of patients with ST-segment elevation acute myocardial infarction treated within a regional network for reperfusion:The REAL (Registro Regionale Angioplastiche dell'Emilia-Romagna) registry. JACC Cardiovasc Interv. 2011;4:270-278.
21. Wein B, Bashkireva A, Au-Yeung A, et al. Systematic investment in the delivery of guideline-coherent therapy reduces mortality and overall costs in patients with ST-elevation myocardial infarction:Results from the Stent for Life economic model for Romania, Portugal, Basque Country and Kemerovo region. Eur Heart J Acute Cardiovasc Care. 2020;9:902-910.
22. Killip T, Kimball JT. Treatment of myocardial infarction in a coronary care unit:A Two year experience with 250 patients. Am J Cardiol. 1967;20:457-464.
23. Magid DJ, Wang Y, Herrin J, et al. Relationship Between Time of Day, Day of Week, Timeliness of Reperfusion, and In-Hospital Mortality for Patients With Acute ST-Segment Elevation Myocardial Infarction. JAMA. 2005;294:803-812.
24. Barnett Pathak E, Strom JA. Disparities in Use of Same-Day Percutaneous Coronary Intervention for Patients With ST-Elevation Myocardial Infarction in Florida, 2001–2005. Am J Cardiol. 2008;102:802-808.
25. Cavallazzi R, Marik PE, Hirani A, Pachinburavan M, Vasu TS, Leiby BE. Association Between Time of Admission to the ICU and Mortality:A Systematic Review and Metaanalysis. Chest. 2010;138:68-75.
26. Glaser R, Naidu SS, Selzer F, et al. Factors Associated With Poorer Prognosis for Patients Undergoing Primary Percutaneous Coronary Intervention During Off-Hours. Biology or Systems Failure?JACC Cardiovasc Interv. 2008;1:681-688.
27. Pinto DS, Frederick PD, Chakrabarti AK, et al. Benefit of transferring ST-segment-elevation myocardial infarction patients for percutaneous coronary intervention compared with administration of onsite fibrinolytic declines as delays increase. Circulation. 2011;124:2512-2521.
28. Ferreira AS, Costa J, Braga CG, Marques J. Impacto na mortalidade da admissão direta versus transferência inter?hospitalar nos doentes com enfarte agudo do miocárdio com elevação do segmento ST submetidos a intervenção coronária percutânea primária. Rev Port Cardiol. 2019;38:621-631.
29. Williams C, Fordyce CB, Cairns JA, et al. Temporal Trends in Reperfusion Delivery and Clinical Outcomes Following Implementation of a Regional STEMI Protocol:A 12-Year Perspective. CJC Open. 2023;5:181-190.
30. Landon BE, Hatfield LA, Bakx P, et al. Differences in Treatment Patterns and Outcomes of Acute Myocardial Infarction for Low- and High-Income Patients in 6 Countries. JAMA. 2023;329:1088-1097.
31. Szummer K, Wallentin L, Lindhagen L, et al. Improved outcomes in patients with ST-elevation myocardial infarction during the last 20?years are related to implementation of evidence-based treatments:experiences from the SWEDEHEART registry 1995–2014. Eur Heart J. 2017;38:3056-3065.
ABSTRACT
Introduction and objectives: A systematic approach to patients with angina with no obstructed coronary arteries (ANOCA) or ischemia with no obstructed coronary arteries (INOCA) patients is not routinely implemented.
Methods: All consecutive patients diagnosed with ANOCA/INOCA were referred to a designated outpatient clinic for a screening visit to assess their eligibility for a NOCA program. If eligible, patients underwent scheduled coronary angiograms with coronary function testing and intracoronary acetylcholine provocation testing. Medical therapy was optimized accordingly. All patients were then followed up at 1, 3, 6, and 12 months. Baseline and 3-month follow-up assessments included the Seattle Angina Questionnaire (SAQ) and EuroQol-5D questionnaire.
Results: Of 77 patients screened, 23 (29.9%) were excluded and 54 (70.1%) were included (29 [53.7%] with INOCA and 25 [46.3%] with ANOCA). Microvascular angina was diagnosed in 19 (35.2%) patients, vasospastic angina in 12 (22.2%), both microvascular angina and vasospastic angina in 18 (33.3%), and noncoronary chest pain in 5 (9.3%). There was a notable increase in the use of beta-blockers, calcium channel blockers and nitrates. Complications occurred in 3 (5.5%) patients. Compared with baseline, there was no difference in the mean EQ-5D score at the 3-month follow-up, but there was a significant improvement in the SAQ score related to physical limitations, angina stability, and disease perception, with no differences in angina frequency or treatment satisfaction. No events were recorded at the 1-year follow-up.
Conclusions: A specific diagnostic and therapeutic protocol can be easily and safely implemented in routine clinical practice, leading to improvement in patients’ quality of life.
Keywords: INOCA. ANOCA. Diagnosis. Therapy. Protocol.
RESUMEN
Introducción y objetivos: El abordaje sistemático en pacientes con angina con arterias coronarias no obstruidas (ANOCA) o con isquemia con arterias coronarias no obstruidas (INOCA) no está bien protocolizado.
Métodos: Todos los pacientes con diagnóstico de INOCA o ANOCA se trasladaron a una clínica ambulatoria específica para evaluar su elegibilidad para el programa NOCA. Si eran elegibles, se sometían a una angiografía coronaria programada con pruebas de función coronaria y provocación intracoronaria con acetilcolina. La terapia médica se optimizó en consecuencia. Todos los pacientes tuvieron un seguimiento a 1, 3, 6 y 12 meses. Al inicio y a los 3 meses se aplicaron los cuestionarios SAQ y EuroQol-5D.
Resultados: De 77 pacientes se excluyeron 23 (29,9%) y se incluyeron 54 (70,1%) (29 [53,7%] con INOCA y 25 [46,3%] con ANOCA). Se diagnosticó angina microvascular a 19 (35,2 %) pacientes, angina vasoespástica a 12 (22,2 %), angina microvascular y angina vasoespástica a 18 (33,3 %), y dolor torácico no coronario a 5 (9,3 %). Hubo un aumento significativo en el uso de bloqueadores beta, bloqueadores del calcio y nitratos. Se presentaron complicaciones en 3 (5,5%) pacientes. No hubo diferencias en la puntuación media del EQ-5D a los 3 meses y se observó una mejora significativa en la puntuación SAQ respecto a la limitación física, la estabilidad de la angina y la percepción de enfermedad, sin diferencias en la frecuencia de angina y la satisfacción con el tratamiento. No se registraron eventos al año.
Conclusiones: Un protocolo diagnóstico y terapéutico específico podría implementarse de manera fácil y segura en la práctica clínica diaria, y con ello mejoraría la calidad de vida de los pacientes.
Palabras clave: INOCA. ANOCA. Diagnóstico. Terapia. Protocolo.
Abbreviations
ANOCA: angina with no obstructed coronary arteries. INOCA: ischemia with no obstructed coronary arteries.
INTRODUCTION
Ischemic heart disease is the leading cause of disability and mortality worldwide and is commonly characterized by the presence of obstructive coronary artery disease (CAD) (defined as any coronary artery stenosis ≥ 50% in diameter).1 However, up to 60% to 70% of patients with angina and/or documented myocardial ischemia do not have angiographic evidence of CAD.2 This condition is defined as angina with no obstructed coronary arteries (ANOCA) or ischemia with no obstructed coronary arteries (INOCA) when associated with evidence of myocardial ischemia.3 Of note, despite the absence of CAD, these patients are at an increased risk of future cardiovascular events such as acute coronary syndrome, heart failure hospitalization, stroke, and repeat cardiovascular procedures compared with healthy individuals.4,5 Therefore, appropriate management in terms of diagnosis and treatment is of the utmost importance to improve patients’ prognosis and outcomes.6 The Coronary Microvascular Angina (CorMicA) trial demonstrated that a strategy of adjunctive invasive testing for disorders of coronary function together with stratified medical therapy can improve outcomes (ie, reduction in angina severity and enhanced quality of life).7,8 However, there are still concerns about the implementation in real-world practice of a systematic diagnostic and therapeutic approach in INOCA and ANOCA patients, potentially impacting outcomes and quality of life.
We report our single-center experience of the implementation in clinical practice of a specific diagnostic and therapeutic protocol (no obstructed coronary arteries [NOCA] program) in INOCA and ANOCA patients.
METHODS
Eligibility criteria for the NOCA program
All consecutive patients diagnosed either at our hospital or at our referral centers with angina or ischemia with nonobstructive CAD on coronary angiography were referred to a specific outpatient clinic (the NOCA clinic at Hospital Clínic, Barcelona, Spain) for a screening visit. Nonobstructive CAD was defined as angiographic evidence of normal coronary arteries or diffuse atherosclerosis with stenosis < 50% and/or fractional flow reserve (FFR) > 0.80 if there was stenosis between 50% and 70%. During the screening visit, a team of expert cardiologists confirmed patients’ eligibility for the NOCA program based on the following criteria: a) diagnosis of ANOCA, defined as stable, chronic typical angina symptoms (eg, chest pain precipitated by physical exertion or emotional stress and relieved by rest or nitroglycerine); b) diagnosis of INOCA, defined as the demonstration of myocardial ischemia identified by a noninvasive test with pharmacologic or exercise stress tests such as cardiac single photon emission computed tomography, cardiac magnetic resonance, stress electrocardiography, or echocardiography.3 The exclusion criteria were: a) atypical angina symptoms, and b) clearly identifiable noncoronary causes of chest pain (figure 1). The study protocol adhered to the Declaration of Helsinki and the study was approved by our institutional review committee. All patients provided written informed consent to be included in this program and study. The clinical ethics committee gave their approval for a retrospective analysis of the collected data.
Figure 1. Central illustration. Flowchart for the inclusion of patients in the NOCA program. Cath lab, catheterization laboratory; INOCA: ischemia with no obstructed coronary arteries; NOCA, no obstructed coronary arteries; NOCAD, nonobstructive coronary artery disease.
NOCA program: diagnostic approach
After patient inclusion in the NOCA program, specialized counseling was provided by expert cardiologists and nurses. All patients were thoroughly informed about their disease, the importance of reaching a specific diagnosis, and the importance implementing tailored therapy. During the counseling sessions, the predicted benefits and low associated risks of an invasive procedure to specifically study coronary microcirculation and vasospasm were explained in detail. All patients provided written informed consent to undergo coronary angiography and intracoronary provocation testing with acetylcholine (ACh).
Subsequently, all patients underwent a scheduled coronary angiogram with a comprehensive diagnostic work-up consisting of the following: a) coronary function testing to assess coronary flow reserve (CFR) and the index of microvascular resistance (IMR); b) intracoronary ACh provocation testing to assess the presence of coronary vasomotion disorders (eg, epicardial or microvascular spasm).
Coronary function testing was performed using a pressure-temperature sensor guidewire (PressureWire X Guidewire and Coroventis CoroFlow Cardiovascular System, Abbott Vascular, United States) placed in the left anterior descending artery (LAD) as the prespecified target vessel, reflecting its subtended myocardial mass and coronary dominance. Steady-state hyperemia was induced using intravenous adenosine (140 µg/kg/min). If there was severe tortuosity of the LAD or evidence of myocardial ischemia in a region other than the territory of the LAD, the wire was placed in the right coronary artery or the left circumflex, as per the operator’s decision. CFR was calculated using thermodilution, defined as resting mean transit time divided by hyperemic mean transit time (abnormal CFR was defined as ≤ 2.5). IMR was calculated as the product of distal coronary pressure at maximal hyperemia multiplied by the hyperemic mean transit time (normal value < 25).6,9
Intracoronary ACh provocation testing was performed with a standardized protocol involving serial ACh infusions for 20 seconds at increasing concentrations (2-20-100 µg in the left coronary artery with an interval of 2-3 minutes between each injection) with concomitant assessment of the patient’s symptoms, electrocardiogram documentation, and angiographic scans. Patients taking vasoactive drugs (eg, calcium channel blockers and nitrates) underwent a wash-out period of at least 48 hours before the provocative test.10,11,12 Epicardial coronary spasm was defined as the reproduction of chest pain and ischemic electrocardiogram changes in association with a reduction in coronary diameter ≥ 90% from baseline in any epicardial coronary artery segment.13 Microvascular spasm was diagnosed when typical ischemic ST-segment changes (deviation ≥ 1 mm) and angina developed in the absence of epicardial coronary constriction (< 90% diameter reduction).14
Subsequently, patients were stratified into 4 endotypes: a) microvascular angina (MVA) (evidence of coronary microvascular dysfunction [CMD] defined as any abnormal CFR [< 2.5], IMR [≥ 25], or microvascular spasm); b) vasospastic angina (VSA) (CFR ≥ 2.5, IMR < 25 and epicardial spasm); c) both MVA and VSA (evidence of CMD and epicardial spasm); and d) noncoronary chest pain (CFR ≥ 2.5 and IMR < 25, with neither microvascular nor epicardial spasm).6
Any complications occurring during the invasive diagnostic work-up were documented, including bradyarrhythmias, atrial fibrillation, ventricular tachycardia or fibrillation, coronary perforations, death from any cause, and any other complications.
NOCA program: pharmacological and psychological therapeutic approach
Once the endotype was identified, medical treatment for each patient was optimized accordingly (table 1). In patients with MVA, treatment with beta-blockers and calcium channel blockers (CCBs) was started or up-titrated. Ranolazine was added if angina symptoms were not fully controlled by beta-blockers and CCBs. In patients with VSA, treatment with nondihydropyridine CCBs and long-acting nitrates was started or up-titrated. In patients with both MVA and VSA, treatment with nondihydropyridine CCBs or beta-blockers was started or up-titrated. In patients with noncoronary chest pain, vasoactive drugs were discontinued unless clinically indicated for other reasons. Additionally, treatment with angiotensin-converting enzyme inhibitors/angiotensin receptor blockers and statins was started or up-titrated in all patients. If a patient showed intolerance or had contraindications to a specific medication (eg, asthma for beta-blockers, perimalleolar edema for CCBs, severe bradycardia for both beta-blockers and CCBs), the treatment was tailored and modified accordingly.
Because stress is an important trigger factor for angina symptoms, all patients were also referred to a team of expert psychologists for psychological support.15
NOCA program: clinical outcome and quality of life evaluation
All patients were followed up at 1, 3, 6, and 12-months for treatment titration and assessment of clinical outcomes. At the time of coronary angiography (ie, baseline) and at the 3-month follow-up, all patients were administered the Seattle Angina Questionnaire (SAQ) and quality of life questionnaire (EuroQol-5D [EQ-5D]). The SAQ is a validated 19-item self-administered questionnaire that measures 5 dimensions of CAD: physical limitation, angina stability, angina frequency, treatment satisfaction, and disease perception.16 The EQ-5D is a standardized, nondisease-specific questionnaire used to describe and evaluate patients’ health status and was intended to complement other quality-of life measures.17 Figure 2 provides a visual representation of all the steps involved for patients included in the NOCA program.
Figure 2. Visual representation of the NOCA program. EQ-5D, EuroQol-5D; NOCA, no obstructed coronary arteries; SAQ, Seattle Angina Questionnaire. * See text for more details.
Statistical analysis
Data distribution was assessed according to the Kolgormonov-Smirnov test. Continuous variables were compared using the unpaired Student t-test or the Mann–Whitney U test, as appropriate. The data are expressed as mean ± standard deviation (SD) or as median and interquartile range [IQR]. Categorical data are expressed as numbers and percentages and were evaluated using the chi-square test or Fisher exact test, as appropriate. A 2-sided P value < .05 was considered significant. All analyses were performed using SPSS version 21 (SPSS, United States).
RESULTS
Baseline characteristics of the study population
From January 2021 to December 2021, a total of 77 patients were screened at the NOCA clinic for inclusion in the NOCA program. Following the screening visit, 23 (29.9%) patients were excluded from the NOCA program: 12 due to atypical angina symptoms and 11 due to a clearly identifiable noncoronary cause. Consequently, 54 patients were included in the NOCA program (mean age 64.4 ± 9.4 years, 39 [63.9%] women). A total of 29 (53.7%) patients had INOCA and 25 (46.3%) had ANOCA. All clinical and angiographic characteristics of the study population are shown in table 1.
Table 1. Medical therapy according to the specific endotype of ANOCA/INOCA
| Pathogenic mechanism of MINOCA | Therapeutic implications |
|---|---|
| MVA | Beta-blockers (Nebivolol 2.5–10 mg daily) |
| CCBs (amlodipine 10 mg daily, or verapamil 240 mg daily, or diltiazem 90 mg twice daily) | |
| Ranolazine (375-750 mg twice daily) | |
| VSA | Nondihydropyridine CCBs (verapamil 240 mg, or ciltiazem 90 mg twice daily) |
| Long-acting nitrates (isosorbide mononitrate 30 mg) | |
| MVA and VSA | CCBs (verapamil or diltiazem) or beta-blockers |
| Noncoronary chest pain | Beta-blockers or dihydropyridine CCBs if clinically indicated (eg, hypertension) |
| ACEi or ARB if clinically indicated | |
| Statins if clinically indicated | |
|
ACEi, angiotensin-converting enzyme inhibitors; ANOCA, angina with no obstructed coronary arteries; ARB, angiotensin receptor blockers; CCBs, calcium channel blockers; INOCA, ischemia with no obstructed coronary arteries; MINOCA, myocardial infarction with non-obstructive coronary artery disease; MVA, microvascular angina; VSA, vasospastic angina. |
|
NOCA program: diagnosis of the specific endotype and complications
The results of the invasive functional assessment are presented in table 2. The mean IMR and CFR values were 21.2 ± 10.6 and 2.3 ± 1.4, respectively. MVA was diagnosed in 19 (35.2%) patients, VSA in 12 (22.2%), and both MVA and VSA in 18 (33.3%). Finally, 5 (9.3%) patients were diagnosed with noncoronary chest pain.
Table 2. Clinical and angiographic characteristics of patients included in the NOCA program
| Characteristics | Study population (n = 54) |
|---|---|
| Clinical characteristics | |
| Age | 64.4 ± 9.4 |
| Female sex | 39 (72.2) |
| Clinical presentation | |
| ANOCA | 25 (46.3) |
| INOCA | 29 (53.7) |
| Diabetes mellitus | 12 (22.2) |
| Hypertension | 35 (64.8) |
| Dyslipidaemia | 28 (51.9) |
| Former smokers | 3 (5.7) |
| Current smoker | 14 (25.9) |
| Familiar history of CV disease | 5 (9.3) |
| Previous CV history | |
| Prior MI | 7 (13.0) |
| Prior PCI | 8 (14.8) |
| Prior CABG | 0 (0.0) |
| COPD | 1 (1.9) |
| CKD (eGFR < 60 mL/min/m2) | 4 (7.4) |
| Depression | 15 (27.8) |
| Anxiety | 19 (35.2) |
| Invasive functional evaluation | |
| Vessel explored | |
| LDA | 48 (88.9) |
| LCx | 3 (5.6) |
| RCA | 3 (5.6) |
| IMR | 21.2 ± 10.6 |
| Increased IMR (≥ 25) | 18 (33.3) |
| CFR | 2.3 ± 1.4 |
| Reduced CFR (< 2.5) | 33 (61.1) |
| Increased IMR (≥ 25) and reduced CFR (< 2.5) | 13 (24.1) |
| Diagnosis (endotype) | |
| MVA | 19 (35.2) |
| VSA | 12 (22.2) |
| MVA and VSA | 18 (33.3) |
| Noncoronary chest pain | 5 (9.3) |
|
ANOCA, angina with no obstructed coronary arteries; CABG, coronary artery bypass graft surgery; CFR, coronary flow reserve; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CV, cardiovascular; eGFR, estimated glomerular filtration rate; IMR, index of microcirculatory resistance; INOCA, ischemia with no obstructed coronary arteries; MI, myocardial infarction; MVA, microvascular angina; LAD, left anterior descending; LCx, left circumflex; PCI, percutaneous coronary intervention; RCA, right coronary artery; VSA, vasospastic angina. Values are expressed as No. (%), mean ± standard deviation or median [interquartile range]. |
|
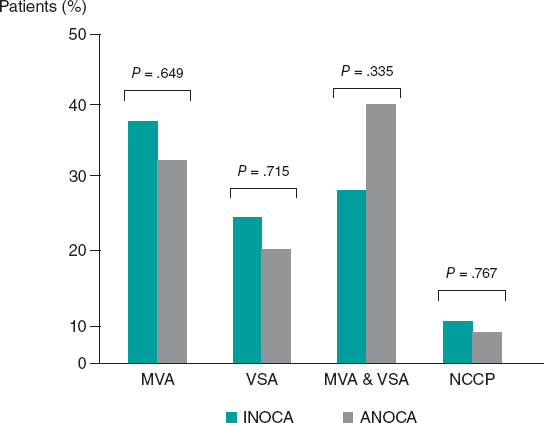
Among INOCA patients, MVA was diagnosed in 11 (37.9%) patients, VSA in 7 (24.1%), both MVA and VSA in 8 (27.6%), and noncoronary chest pain in 3 (10.3%). Among ANOCA patients, MVA was diagnosed in 8 (32.0%) patients, VSA in 5 (20.0%), both MVA and VSA in 10 (40.0%), and noncoronary chest pain in 2 (8.0%). There were no statistically significant differences in the prevalence of any endotype between INOCA and ANOCA patients (all P > .05, figure 3).
Figure 3. Prevalence of the different endotypes among INOCA and ANOCA patients. ANOCA, angina with no obstructed coronary arteries; INOCA, ischemia with no obstructed coronary arteries; MVA, microvascular angina; NCCP, noncoronary chest pain; VSA, vasospastic angina.
Complications occurred in 3 (5.5%) patients during intracoronary ACh provocation testing: 2 (3.7%) patients had transient bradyarrhythmias and 1 (1.8%) patient had paroxysmal atrial fibrillation that spontaneously reverted to sinus rhythm.
NOCA program: treatment optimization according to the specific endotype
Inclusion in the NOCA program led to statistically significant changes in medications after diagnosis of the specific endotype. There was a significant increase in the use of beta-blockers (33.3% before vs 57.4% after, P = .008), nondihydropyridine CCBs (9.3% before vs 37.0% after, P < .001), and long-acting nitrates (46.3% before vs 63.0% after, P = .012). There were no statistically significant differences in any other medications before and after the invasive assessment (all P > .05, figure 4). All changes in medications according to the specific endotype of ANOCA/INOCA are shown in figure 5.
Figure 4. Differences in medical treatment before and after patient inclusion in the NOCA program. ACEi, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blockers; DP-CCBs, dihydropyridine calcium channel blockers; LA, long-acting; ND-CCBs, nondihydropyridine calcium channel blockers.
Figure 5. Changes in medications according to the specific endotype of ANOCA/INOCA. A: patients with MVA. B: patients with VSA. C: Patients with MVA and VSA. D: patients with noncoronary chest pain. DP-CCBs, dihydropyridine calcium channel blockers; LA, long-acting; MVA, microvascular angina; ND-CCBs, nondihydropyridine calcium channel blockers; VSA, vasospastic angina.
NOCA program: clinical outcome evaluation
At 3 months of follow-up, there was no statistically significant difference in the mean EQ-5D score compared with baseline (64.8 ± 18.1 at baseline vs 66.1 ± 17.1 at 3 months of follow-up, P = .302) (figure 6). However, there was a statistically significant improvement in the SAQ score in terms of physical limitations (59.7 ± 19.3 at baseline vs 66.2 ± 16.9 at 3 months of follow-up, P = .037), angina stability (57.1 ± 28.1 at baseline vs 75.8 ± 22.3 at 3 months of follow-up, P = .010), and disease perception (42.5 ± 13.9 at baseline vs 50.8 ± 16.3 at 3 months follow-up, P = .015). No statistically significant difference was found in angina frequency (74.3 ± 20.4 at baseline vs 80.7 ± 19.8 at 3 months of follow-up, P = .193) or treatment satisfaction (68.1 ± 12.6 at baseline vs 70.5 ± 12.5 at 3 months of follow-up, P = .950) (figure 7). No events were recorded at the 1-year follow-up.
Figure 6. Differences in EuroQol-5D (EQ-5D) score at baseline and at 3 months of follow-up.
Figure 7. Differences in SAQ score at baseline and at 3 months of follow-up. AF, angina frequency; AS, angina stability; DP, disease perception; EQ-5D, EuroQol-5D; PL, physical limitations; SAQ, Seattle Angina Questionnaire; TS, treatment satisfaction.
DISCUSSION
The main results of our experience can be summarized as follows: a) the implementation of a specific diagnostic and therapeutic protocol (NOCA program) in patients with diagnosed with nonobstructive CAD is feasible and allowed a parsimonious use of medical resources; b) a comprehensive diagnostic work-up in INOCA and ANOCA patients is safe, with a low rate of mild and transient complications (5.5%); c) the inclusion of patients in the NOCA program led to significant changes in medications and a significant improvement in their angina symptoms at the 3-month follow-up with no adverse events at 1 year.
Although accumulating evidence has demonstrated that an approach consisting of a comprehensive diagnostic assessment and stratified medical therapy in INOCA and ANOCA is crucial to improve patients’ prognosis, such an approach is far from routinely implemented in clinical practice.7,8 There are still concerns mainly related to the cost-benefit ratio, the associated prolonged procedural time, increased costs, and the risk of possible associated complications. Furthermore, in the most recent European Society of Cardiology guidelines, invasive coronary function testing is assigned a class IIa (“should be considered”) recommendation, while ACh provocation testing is supported by a class IIb recommendation (“may be considered”) to assess microvascular spasm and class IIa in patients under consideration for VSA.3 As a result, the management of these patients is commonly left to physicians’ discretion or relies on the experience of each center. Consequently, diagnosis of a specific NOCA endotype is frequently missed, and medical therapy is not optimized. This, in turn, has a significant negative impact on patients’ quality of life and clinical outcomes, as well as on health care costs, due to the need for repeat hospitalization or invasive procedures.18
In reporting our experience, we demonstrate that a specific diagnostic and therapeutic protocol (ie, the NOCA program) in patients with a previous diagnosis of nonobstructive CAD can be easily implemented in clinical practice. A key innovation of our study, compared with prior publications, is the creation and implementation of a specific protocol for the INOCA/ANOCA population. Additionally, our approach involves a screening visit with assessment by a team of expert cardiologists for patients with a suspected diagnosis of INOCA/ANOCA. This approach improves identification of such patients, and, in our experience, led to the exclusion of almost one third of patients (29.9%) due to atypical angina symptoms or no clearly identifiable coronary causes of chest pain. This is another novelty of our study that could be extremely relevant in the management of these patients. Indeed, the selection of patients to be included in the program may allow clinical resources to be directed to patients who are most likely to benefit, while avoiding repeat invasive procedures and related risks in patients with unclear indications. Additionally, the specialized counseling provided by cardiologists and nurses during the screening visit, together with psychological support, are likely to be vital components of the management of INOCA/ANOCA patients. Indeed, recent studies have demonstrated how psychological factors, such as chronic stress, anxiety, depression, and social stressors are involved in the pathogenesis of MVA and VSA.19-23 Mental stress has been demonstrated to determine CMD mainly through endothelium-dependent mechanisms and endothelial dysfunction.24 Similarly, by activating brain areas involved in regulation of neuroendocrine and autonomic nervous systems, mental stress can lead to hyperreactivity of vascular smooth muscle cells, autonomic nervous system dysfunction, oxidative stress, vascular inflammation, and endothelial dysfunction, resulting in an increased propensity to coronary vasospasm.25-27
Furthermore, in line with previous studies,28-30 our experience demonstrates that performing a comprehensive invasive diagnostic assessment for the diagnosis of the specific endotype in INOCA and ANOCA patients is safe and is associated with a low rate of mild and transient complications. For all these reasons, patients and clinicians should be reassured about the lack of serious complications and cardiologists should be strongly encouraged to implement a specific diagnostic and therapeutic program in these patients. Indeed, the availability of such a program for INOCA and ANOCA patients may have significant clinical and therapeutic implications, as, in our experience, it resulted in substantial changes in medications and a marked improvement at the 3-month follow-up of the SAQ questionnaire regarding physical limitations, angina frequency, and disease perception. The lower and nonsignificant improvement in the other parameters (eg, angina frequency and treatment satisfaction) could be attributed to the already high baseline values (74.5 ± 19.9 and 69.6 ± 11.9, respectively). Similarly, the absence of a significant improvement in the EQ-5D questionnaire at 3 months might be due to the short follow-up period or the fact that it is a nondisease-specific questionnaire designed to describe and assess patients’ health status and is intended to complement other quality-of-life measures.31
Study limitations
Some limitations of this study should be acknowledged. First, this is a single-center study with a relatively small sample size and short follow-up. Second, we did not perform a cost-analysis and therefore we cannot speculate on the impact of the NOCA program on health care-related costs. Further studies in larger ANOCA and INOCA populations are warranted. Finally, the absence of a control group precluded a thorough assessment of the improvement in the quality of life among these patients.
CONCLUSIONS
Our experience demonstrates that a specific diagnostic and therapeutic protocol (NOCA program) can be easily and safely implemented in routine clinical practice. Such a protocol could ensure the best care for INOCA and ANOCA patients, as well as improve their quality of life and avoid inappropriate treatments and incomplete investigations. Future evidence from randomized clinical trials or recommendations from international clinical guidelines supporting the implementation of a specific protocol in these patients are strongly warranted.
FUNDING
This study received no funding.
ETHICAL CONSIDERATIONS
The study protocol complied with the Declaration of Helsinki and the study was approved by our Institutional Review Committee. All patients gave written informed consent to be included in this program and study. The clinical ethics committee gave their approval for a retrospective analysis of the data collected. In this work, the possible variables of sex and gender have been taken into account.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
No artificial intelligence tools were used during the preparation of this work.
AUTHORS’ CONTRIBUTIONS
R. Rinaldi, F. Spione, F.M. Verardi: data extraction and analysis and manuscript drafting; R. Rinaldi, F. Spione, S. Brugaletta: design and manuscript revision; P. Vidal Calés, V. Arévalos, R. Gabani, D. Cánovas, M. Gutiérrez, M. Pardo, R. Domínguez, L. Pintor, X. Torres, X. Freixa, A. Regueiro, O. Abdul-Jawad Altisent, M. Sabaté: manuscript revision. All authors have read and agreed to the published version of the manuscript.
CONFLICTS OF INTEREST
The authors have nothing to disclose.
ACKNOWLEDGMENTS
F. Spione has been supported by a research grant provided by the Cardiopath PhD program.z
WHAT IS KNOWN ABOUT THE TOPIC?
- Up to 60% to 70% of patients with angina and/or documented myocardial ischemia do not have angiographic evidence of obstructive coronary artery disease. This condition is defined as angina with no obstructed coronary arteries (ANOCA) or ischemia with no obstructed coronary arteries (INOCA) when associated with evidence of myocardial ischaemia. There are still concerns about the implementation in real practice of a systematic diagnostic and therapeutic approach in INOCA and ANOCA patients, potentially impacting outcomes and quality of life.
WHAT DOES THIS STUDY ADD?
- The implementation of a specific protocol (NOCA program) in patients with a diagnosis of nonobstructive CAD is feasible and allowed parsimonious use of medical resources. A comprehensive invasive diagnostic assessment in INOCA or ANOCA patients is safe and is associated with a low rate of mild and transient complications. The availability of a specific diagnostic and therapeutic program for INOCA and ANOCA patients may have important clinical and therapeutic implications, as, in our experience, it led to significant changes in medications and a notable improvement at 3 months of follow-up in the SAQ questionnaire regarding physical limitations, angina frequency, and perception of the disease.
REFERENCES
1. Roth GA, Mensah GA, Johnson CO, et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019:Update From the GBD 2019 Study. J Am Coll Cardiol. 2020;76:2982-3021.
2. Patel MR, Peterson ED, Dai D, et al. Low diagnostic yield of elective coronary angiography. N Engl J Med. 2010;362:886-895.
3. Neumann FJ, Sechtem U, Banning AP, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407-477.
4. Jespersen L, Hvelplund A, Abildstrøm SZ, et al. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J. 2012;33:734-744.
5. Taqueti VR, Solomon SD, Shah AM, et al. Coronary microvascular dysfunction and future risk of heart failure with preserved ejection fraction. Eur Heart J. 2018;39:840-849.
6. Kunadian V, Chieffo A, Camici PG, et al. An EAPCI Expert Consensus Document on Ischaemia with Non-Obstructive Coronary Arteries in Collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology &Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. Eur Heart J. 2020;41:3504-3520.
7. Ford TJ, Stanley B, Good R, et al. Stratified Medical Therapy Using Invasive Coronary Function Testing in Angina:The CorMicA Trial. J Am Coll Cardiol. 2018;72:2841-2855.
8. Ford TJ, Stanley B, Sidik N, et al. 1-Year Outcomes of Angina Management Guided by Invasive Coronary Function Testing (CorMicA). JACC Cardiovasc Interv. 2020;13:33-45.
9. Candreva A, Gallinoro E, van 't Veer M, et al. Basics of Coronary Thermodilution. JACC Cardiovasc Interv. 2021;14:595-605.
10. Montone RA, Meucci MC, De Vita A, Lanza GA, Niccoli G. Coronary provocative tests in the catheterization laboratory:Pathophysiological bases, methodological considerations and clinical implications. Atherosclerosis. 2021;318:14-21.
11. Ford TJ, Ong P, Sechtem U, et al. Assessment of Vascular Dysfunction in Patients Without Obstructive Coronary Artery Disease:Why, How, and When. JACC Cardiovasc Interv. 2020;13:1847-1864.
12. Gutiérrez E, Gómez-Lara J, Escaned J, et al. Assessment of the endothelial function and spasm provocation test performed by intracoronary infusion of acetylcholine. Technical report from the ACI-SEC;REC Interv Cardiol. 2021;3:286-296.
13. Beltrame JF, Crea F, Kaski JC, et al. International standardization of diagnostic criteria for vasospastic angina. Eur Heart J. 2017;38:2565-2568.
14. Ong P, Camici PG, Beltrame JF, et al. International standardization of diagnostic criteria for microvascular angina. Int J Cardiol. 2018;250:16-20.
15. Jespersen L, Abildstrøm SZ, Hvelplund A, Prescott E. Persistent angina:highly prevalent and associated with long-term anxiety, depression, low physical functioning, and quality of life in stable angina pectoris. Clin Res Cardiol. 2013;102:571-581.
16. Chan PS, Jones PG, Arnold SA, Spertus JA. Development and validation of a short version of the Seattle angina questionnaire. Circ Cardiovasc Qual Outcomes. 2014;7:640-647.
17. Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20:1727-1736.
18. Jespersen L, Abildstrom SZ, Hvelplund A, et al. Burden of hospital admission and repeat angiography in angina pectoris patients with and without coronary artery disease:a registry-based cohort study. PLoS One. 2014;9:e93170.
19. Smaardijk VR, Lodder P, Kop WJ, van Gennep B, Maas AHEM, Mommersteeg PMC. Sex- and Gender-Stratified Risks of Psychological Factors for Incident Ischemic Heart Disease:Systematic Review and Meta-Analysis. J Am Heart Assoc. 2019;8:e010859.
20. Mehta PK, Hermel M, Nelson MD, et al. Mental stress peripheral vascular reactivity is elevated in women with coronary vascular dysfunction:Results from the NHLBI-sponsored Cardiac Autonomic Nervous System (CANS) study. Int J Cardiol. 2018;251:8-13.
21. Konst RE, Elias-Smale SE, Lier A, Bode C, Maas AHEM. Different cardiovascular risk factors and psychosocial burden in symptomatic women with and without obstructive coronary artery disease. Eur J Prev Cardiol. 2019;26:657-659.
22. Gomez MA, Merz NB, Eastwood JA, et al. Psychological stress, cardiac symptoms, and cardiovascular risk in women with suspected ischaemia but no obstructive coronary disease. Stress Health. 2020;36:264-273.
23. Bekendam MT, Vermeltfoort IAC, Kop WJ, Widdershoven JW, Mommersteeg PMC. Psychological factors of suspect coronary microvascular dysfunction in patients undergoing SPECT imaging. J Nucl Cardiol. 2022;29:768-778.
24. Hammadah M, Kim JH, Al Mheid I, et al. Coronary and Peripheral Vasomotor Responses to Mental Stress. J Am Heart Assoc. 2018;7:e008532.
25. Shah A, Chen C, Campanella C, et al. Brain correlates of stress-induced peripheral vasoconstriction in patients with cardiovascular disease. Psychophysiology. 2019;56:e13291.
26. Hung MY, Mao CT, Hung MJ, et al. Coronary Artery Spasm as Related to Anxiety and Depression:A Nationwide Population-Based Study. Psychosom Med. 2019;81:237-245.
27. Crea F, Montone RA, Rinaldi R. Pathophysiology of Coronary Microvascular Dysfunction. Circ J. 2022;86:1319-1328.
28. Probst S, Seitz A, Martínez Pereyra V, et al. Safety assessment and results of coronary spasm provocation testing in patients with myocardial infarction with unobstructed coronary arteries compared with patients with stable angina and unobstructed coronary arteries. Eur Heart J Acute Cardiovasc Care.2020:2048∖20932422.
29. Montone RA, Rinaldi R, Del Buono MG, et al. Safety and prognostic relevance of acetylcholine testing in patients with stable myocardial ischaemia or myocardial infarction and nonobstructive coronary arteries. EuroIntervention 2022;18:e666-e676.
30. Rinaldi R, Salzillo C, CaffèA, Montone RA. Invasive Functional Coronary Assessment in Myocardial Ischemia with Non-Obstructive Coronary Arteries:from Pathophysiological Mechanisms to Clinical Implications. Rev Cardiovasc Med. 2022;23:371.
31. EuroQol--a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199-208.

ABSTRACT
Introduction and objectives: No previous studies have established the contemporary use and outcomes of Excimer laser coronary atherectomy (ELCA) in percutaneous coronary intervention (PCI) of severely calcified coronary lesions. The aim of this study was to assess the safety, efficacy, and 1-year outcomes of ELCA in this setting.
Methods: We retrospectively examined the clinical and angiographic characteristics and procedural outcomes of severely calcified lesions treated with ELCA-assisted PCI in our institution between 2016 and 2022.
Results: Seventy-eight consecutive patients (80 procedures) were included (mean age 71.2 ± 8.6 years, 80.5% men). Clinical presentation was stable coronary artery disease in 45 patients (56.2%) and acute coronary syndromes in 33 (43.8%). All the lesions were severely calcified. In addition, 40% were uncrossable lesions, 28.75% were undilatable lesions, 2.5% showed in-stent restenosis, 6.25% showed stent underexpansion, and 7.5% were chronic total occlusions. The combination of ≥ 2 of the above anatomic settings was found in 12.5% of the procedures. The maximum fluence was 73 ± 9.6 mJ/mm2, and the maximum frequency was 72.7 ± 10.4 Hz. The saline flushing technique was initially used in all the procedures, while contrast was used in 2 procedures. The ELCA success and technical success rates were both 91.25%. Adjuvant plaque modification therapies were required in 4 patients. The clinical success rate was 87.5%. ELCA-related complications occurred in 2 procedures (2.5%). After a median follow-up of 15.5 months [IQR, 5.0-29.3], major adverse cardiac events (MACE) (target lesion revascularization, myocardial infarction or cardiac death) occurred in 9 patients (11.25%).
Conclusions: Despite the complexity of PCI in severely calcified lesions, ELCA was effective with a relatively low incidence of ELCA-related complications and MACE during follow-up.
Keywords: Complex PCI. Excimer laser coronary atherectomy. Calcified coronary lesions.
RESUMEN
Introducción y objetivos: El uso contemporáneo y los resultados de la aterectomía coronaria con láser Excímer (ELCA) en el intervencionismo coronario percutáneo (ICP) de lesiones coronarias gravemente calcificadas no están establecidos. El objetivo de este estudio fue evaluar la eficacia, seguridad y resultados a 1 año de ELCA en este escenario.
Métodos: Se revisaron de forma retrospectiva las características clínicas y angiográficas, y los resultados de los procedimientos de revascularización de lesiones gravemente calcificadas tratadas con ICP asistido por ELCA en nuestro centro entre 2016 y 2022.
Resultados: Se incluyeron 78 pacientes consecutivos (80 procedimientos) (edad media 71,2 ± 8,6 años, 80,5% varones). La presentación clínica fue enfermedad arterial coronaria estable en 45 (56,2%) pacientes y síndromes coronarios agudos en 33 (43,8%). Todas las lesiones presentaban calcificación grave. Además, el 40% eran lesiones incruzables, el 28,75% lesiones indilatables, el 2,5% reestenosis intrastent, el 6,25% infraexpansión del stent y el 7,5% oclusiones crónicas. La combinación de ≥ 2 de los escenarios anatómicos anteriores existió en el 12,5% de los procedimientos. La fluencia máxima fue de 73 ± 9,6 mJ/mm2 y la frecuencia máxima de 72,7 ± 10,4 Hz. ELCA con lavado con solución salina se utilizó inicialmente en todos los procedimientos y se utilizó contraste en 2 procedimientos. La tasa de éxito de ELCA y de éxito técnico fueron del 91,25 %. Fueron necesarias terapias adyuvantes de modificación de placa en 4 casos. La tasa de éxito clínico fue del 87,5%. Ocurrieron complicaciones relacionadas con ELCA en 2 (2,5%) procedimientos. Tras una mediana de seguimiento de 15,5 meses (IQR, 5,0-29,3), se produjeron eventos cardiovasculares adversos mayores (MACE) (nueva revascularización de la lesión diana, infarto de miocardio o muerte cardiaca) en 9 pacientes (11,25%).
Conclusiones: A pesar de la complejidad de la ICP en lesiones gravemente calcificadas, ELCA demostró ser efectivo con una incidencia relativamente baja de complicaciones relacionadas con ELCA y MACE en el seguimiento.
Palabras clave: ICP compleja. Láser coronario. Lesiones coronarias calcificadas.
Abbreviations
CTO: chronic total occlusion. ELCA: excimer laser coronary atherectomy. ISR: in-stent restenosis. MACE: major adverse cardiovascular events. PCI: percutaneous coronary intervention. RA: rotational atherectomy.
INTRODUCTION
Moderate or severe coronary artery calcification is relatively common in patients undergoing percutaneous coronary interventions (PCI).1 This is closely related to advancing age and the high prevalence of comorbidities such as diabetes or chronic kidney disease. Coronary artery calcification is associated with a lower rate of successful PCI and complete revascularization, increased procedural-related complications, and a higher rate of major adverse cardiovascular events (MACE).2
Despite the availability of several plaque modification techniques, severely calcified lesions continue to pose a challenge to the successful performance of PCI.
Excimer laser coronary atherectomy (ELCA) is a plaque modification technique that has proved to be useful in various scenarios such as balloon failure (uncrossable or undilatable lesions), chronic total occlusions (CTO), stent underexpansion, in-stent restenosis (ISR), and thrombotic lesions. In recent years, incremental operator experience along with the standardization of the laser technique, has expanded its indications and decreased its complication rates.3,4
However, its effectiveness in calcified lesions is controversial. On one hand, some ELCA series have described a relationship between severe calcification and laser failure.5-8 On the other hand, moderate-to-severe calcification is found in more than 60% of cases in some ELCA series with a high success rate,9 suggesting that it could be useful in this setting.10
Due to the lack of evidence in this specific scenario, the aim of our study was to assess the safety and efficacy of ELCA in severely calcified coronary lesions, as well as the mid-term follow-up outcomes in a single center registry.
METHODS
Patient population
This single center retrospective observational study included all consecutive patients undergoing ELCA-assisted PCI for severely calcified lesions. From March 2016 to August 2022.
We excluded procedures using ELCA for any indication other than severe calcification. In all patients, PCI was indicated based on the presence of symptoms consistent with angina, demonstrated ischemia, or both. The study followed the international recommendations of clinical investigation (Declaration of Helsinki). All participants gave written informed consent and approval was obtained from the ethics committee of the center. The study took into consideration sex and gender variables according to SAGER guidelines. Patients were followed up in cardiology clinics at their referral center 3 to 6 months after the procedure, and thereafter at time intervals established at the discretion of their treating physician.
We analyzed data on clinical and angiographic characteristics, technical aspects of the procedure, and cardiovascular events during hospitalization and after discharge.
Procedure
All procedures were carried out by 5 different operators experienced in the use of ELCA. The decision to use ELCA was based on the presence of angiographically severe calcification.
Radial access was use by default. All cases were performed with the CVX-300 Excimer Laser System (Philips, Netherlands) using the 0.9 mm or 1.4 mm catheters. Saline infusion technique was used by default from the beginning, with fluence (mJ/mm2), frequency or repetition rate (Hertz), and the possibility to use ELCA without saline infusion or even with contrast left to the operator’s discretion. Additional dilatation with noncompliant balloons was performed in all procedures. Patients in which another plaque modification technique was used in combination with ELCA were included. All PCIs were performed following current recommendations.11
Definitions
Severely calcified lesions were angiographically defined as radiopacities observed on fluoroscopy without cardiac motion before contrast injection compromising 1 or both sides of the lumen.12 Balloon-uncrossable lesions were defined as lesions that could not be crossed with the lowest-profile balloon available or a microcatheter despite successful advancement of the guidewire distal to the lesion, having good guide catheter support with a guide extension catheter when required. Balloon-undilatable lesions were defined as those lesions in which a noncompliant balloon (diameter 1:1 according to the vessel diameter) failed to achieve adequate expansion. Anterograde flow was assessed by the Thrombolysis In Myocardial Infarction (TIMI) scale.
ELCA success was defined as the ability to cross the lesion completely with the laser catheter or, if crossing was not feasible, to allow the subsequent passage and expansion of a balloon sized 1:1 with the vessel diameter, after laser application. Technical success was defined as residual stenosis < 30% and anterograde TIMI 3 flow in the target vessel. Clinical success was defined as technical success and the absence of MACE during the current hospitalization (target lesion revascularization, procedure-related myocardial infarction [MI], or cardiovascular death). Procedural-related complications included coronary artery perforation leading to cardiac tamponade and requiring pericardial drainage, flow-limiting dissection, no-reflow, hemodynamic instability, MI type 4a according to the fourth universal definition of MI,13 ventricular arrhythmias, and major bleeding (bleeding requiring transfusion and/or surgical or percutaneous intervention). MACE occurring during follow-up were defined as a composite of target lesion revascularization, MI, or cardiac death.
Statistical analysis and data collection
All data were collected through the patients’ electronic medical records and were introduced in a local database. Angiograms were evaluated using local quantitative coronary analysis software and visual operators’ assessment. Categorical variables are reported as absolute values and percentages. Continuous variables are presented as the mean ± standard deviation (SD) or median (interquartile range [IQR] 25-75), depending on their normal or nonnormal distribution. All analyses were performed with StatIC 16.1 statistical software package.
RESULTS
Clinical characteristics
During the study period, a total of 78 patients with severely calcified coronary lesions underwent 80 ELCA-assisted PCIs and were included in the analysis. Patients undergoing ELCA for an indication other than severe calcification were excluded from the analysis. The distribution of the number of procedures per year, between March 2016 and May 2022, is shown in figure 1. A flowchart of patients in the present study is summarized in figure 2. Mean age was 71.2 ± 8.6 years, 62 (80.5%) were men, and there was a high prevalence of cardiovascular risk factors. Mean left ventricle ejection fraction was 52.9% ± 12.5%. Thirty-nine patients (50%) had a previous PCI. Clinical presentation was stable coronary artery disease in 45 procedures (56.2%), non–ST-segment elevation MI (NSTEMI) in 28 (35%), and ST-segment elevation MI (STEMI) in 7 (8.8%). Baseline clinical characteristics are summarized in table 1.
Figure 1. Distribution of the number of procedures per year (March 2016-May 2022).
Figure 2. Flowchart of patients in the present study. ELCA, excimer laser coronary atherectomy; PCI, percutaneous coronary intervention; RA, rotational atherectomy.
Table 1. Baseline clinical characteristics
| Age | 71.2 ± 8.6 |
| Male sex | 62 (80.5%) |
| Body mass index (kg/m2) | 28.7 ± 4.2 |
| Hypertension | 70 (89.7%) |
| Dyslipidemia | 61 (78.2%) |
| Diabetes mellitus | 46 (59.0%) |
| Current smoker | 19 (24.4%) |
| Prior PCI | 39 (50.0%) |
| Prior CABG | 8 (10.3%) |
| Hb (g/dL) | 13.5 ± 5.3 |
| Serum creatinine (mg/dL) | 1.42 ± 1.8 |
| Ejection fraction (%) | 52.9% ± 12.5 |
| Clinical presentation (n = 98) | |
| Stable coronary artery disease | 45 (56.2%) |
| NSTEMI | 28 (35.0%) |
| STEMI | 7 (8.8%) |
|
CABG, coronary artery bypass graft surgery; NSTEMI, non–ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction. Data are expressed as no. (%) or mean ± standard deviation. |
|
Angiographic characteristics
Severe multivessel disease was present in 56 patients (71.8%). The most common target vessel was the left anterior descending artery (38.75%). In 7 procedures (8.75%), more than 1 target vessel were identified. The anatomical settings in the target vessel included uncrossable lesions in 32 (40%), undilatable lesions in 23 (28.75%), ISR in 2 (2.5%), and stent underexpansion related to calcified plaque in 5 (6.25%). In 6 (7.5%) procedures, the main indication for ELCA was CTO combined with any of the previous settings. In 10 procedures (12.5%), the indication for ELCA resulted from the combination of 2 or more of the above. ELCA was used with the sole indication of severely calcified lesion, not included in any of the previous anatomical settings, in 2 procedures (2.5%).
Procedural characteristics
The radial approach was performed in 44 (55%) cases. There was no need for access conversion when the radial approach was attempted.
Dual antiplatelet treatment consisted of pretreatment with aspirin and oral P2Y12 receptor blockers in 58 patients (72.5%). Selection of P2Y12 inhibitor was left to the physician’s discretion. Cangrelor was used in the patients without prior dual antiplatelet treatment. After the procedure and during follow-up, dual antiplatelet treatment was prescribed as follows: in stable coronary artery disease (n = 45) clopidogrel was used in 21 patients, ticagrelor in 10 and prasugrel in 3 patients. In acute settings (n = 35), ticagrelor was administered in 16 patients, prasugrel in 10, and clopidogrel in 7. GPIIB/IIIA inhibitors were used in 6 procedures (7.5%) (tirofiban in all cases).
Intracoronary imaging was used in 58 procedures (72.5%). Optical coherence tomography (OCT) was used in 48 procedures (60%) and intravascular ultrasound in 10 (12.5%).
Circulatory support with intra-aortic balloon counterpulsation was required in only 1 patient in the context of left-main revascularization.
Regarding the ELCA technique, most lesions were treated with 0.9 mm laser catheters (97.5%). In 2 patients, larger catheters (1.4 mm) were used (1 case of ISR in the left anterior descending artery and 1 calcified lesion in a saphenous vein graft). Flushing saline was used in all the procedures, and contrast was required in 2 procedures (figure 3). Maximum fluence was 73 ± 9.6 mJ/mm2 and the maximum frequency 72.7 ± 10.4 Hz. The highest fluence of 80 mJ/mm2 was required in 48 (60%) procedures and the highest frequency of 80 Hz in 48 (60%). A mean of 5103 ± 3120 pulses was delivered, and the median lasing time was 62 seconds [IQR 40-91].
Figure 3. In-stent restenosis and stent underexpansion treated by excimer laser coronary atherectomy (ELCA). Severe in-stent restenosis (ISR) (A1) (arrow) of drug-eluting stent previously implanted in the right coronary artery. Optical coherence tomography (OCT) revealed calcified neoatherosclerosis with a minimum luminal area (MLA) of 1.25 mm2 (A2). An everolimus-eluting stent (2.75 × 20 mm) was implanted, and despite postdilatation with a 3-mm noncompliant (NC) balloon (A3), subsequent OCT confirmed stent underexpansion (MLA: 2.1 mm2) (A4). Sixteen months later, critical ISR of the previous stent (B1) (arrow) was noted with heterogeneous neointimal proliferation (B2). Laser atherectomy was performed, followed by dilation with 3- and 3.5-mm NC balloons up to 24 atm, and a 3-mm sirolimus-eluting stent was implanted with acceptable angiographic expansion (B3) but underexpansion on OCT (MLA: 1.5 mm2) (B4) (arrow). Laser application with contrast injection was repeated and was dilated with a 4 mm NC balloon, achieving adequate stent expansion (MLA: 4.5 mm2) (B5, B6) (arrow).
At least 1 new-generation drug-eluting stent was implanted in 70 procedures (87.5%). In the remaining procedures, stents were not delivered because of the presence of previous stents (6 ISR and 2 cases of stent underexpansion), which were treated with noncompliant and/or drug-eluting balloons, or due to ELCA failure (2 cases).
Angiographic and procedural characteristics and procedural strategy data are summarized in table 2.
Table 2. Angiographic and procedural characteristics
| Angiographic characteristics | |
|---|---|
| Target vessel | |
| Left anterior descending coronary artery | 31 (38.75%) |
| Right coronary artery | 28 (35.0%) |
| Circumflex artery | 10 (12.5%) |
| Left main coronary artery | 4 (5.0%) |
| Multivessel disease | 56 (71.8%) |
| Indication for ELCA | |
| Balloon-uncrossable lesion | 32 (40%) |
| Balloon-undilatable lesion | 23 (28.75%) |
| In-stent restenosis | 2 (2.5%) |
| Stent Underexpansion | 5 (6.25) |
| Chronic total occlusion | 6 (7.75%) |
| Combination of > 2 of the above | 10 (12.5%) |
| Severe calcification as sole indication | 2 (2.5%) |
| Bifurcation | 14 (17.7%) |
| Aorto-ostial | 2 (2.5%) |
| Procedural characteristics | |
| Access site | |
| Radial | 44 (55.0%) |
| Femoral | 33 (41.2%) |
| Femoral-radial | 3 (3.8%) |
| Guiding catheter French | |
| 6-Fr | 40 (50.0%) |
| 7-Fr | 34 (42.5%) |
| Intracoronary imaging | 58 (72.5%) |
| OCT | 48 (60.0%) |
| IVUS | 10 (12.5%) |
| Laser catheter | |
| 1.4 mm rapid-exchange catheter | 2 (2.5%) |
| 0.9 mm rapid-exchange catheter | 78 (97.5%) |
| Maximum fluence (mJ/mm2) | 72.97 ± 9.6 |
| Maximum frequency (Hz) | 72.7 ±10.4 |
| Number of pulses | 5 103 ± 3 120 |
| Total lasing time (sec) | 62 [40-91] |
| Contrast volume (mL) | 211 ± 68.0 |
| Fluoroscopy time (min) | 30 [22-39] |
| Radiation dose (Gy/cm2) | 103 [79-185] |
| Procedural time (min) | 72 [55-100] |
| Stent implantation | 70 [87.5%] |
| Stent diameter (mm | 3.04 ± 0.50 |
| Stents per procedure | 1.8 ± 1.14 |
| Total stent length (mm) | 43.7 ± 25.7 |
| Left ventricle assist device used | 1 (1.25%) |
| Timing of PCI (n = 98) | |
| Ad hoc | 22 (27.5%) |
| Deferred | 58 (72.5%) |
|
ELCA, excimer laser coronary atherectomy; OCT, optical coherence tomography; IVUS, intravascular ultrasound; PCI, percutaneous coronary intervention. Data are expressed as no. (%), mean ± standard deviation or median [interquartile range]. |
|
Procedural outcomes
The ELCA success rate was 91.25%. The success rate was 78.1% in uncrossable lesions and 100% in the other anatomical settings (P < .001). The ELCA success rate in the different anatomical settings is shown in figure 4.
Figure 4. ELCA success rate in the different anatomical settings. ISR, in-stent restenosis, CTO, chronic total occlusion.
Among intracoronary imaging-guided procedures, the ELCA success rate was 98.3%, and dropped to 72.7% in non-coronary imaging-guided PCI (P < .001). Final stent expansion was analyzed with intracoronary imaging in 32 procedures. The median stent expansion was 80.3% [IQR, 68.2%-95.2%].
Despite ELCA success, adjuvant plaque modification therapies (other than noncompliant [NC] balloon inflation after ELCA) were used in 4 procedures, including rotational atherectomy (RA) in 2 procedures, lithotripsy in 1 procedure and scoring balloon in 1 procedure. The procedures in which ELCA allowed subsequent successful RA (RASER technique14) or successful lithotripsy (ELCA-tripsy technique15) were considered ELCA success.
In 7 procedures (8.75%), ELCA failed. In 2 of them, RA was successfully performed. In 1 procedure, intravascular lithotripsy was attempted, but failed. In 1 case, the procedure was prematurely interrupted at the request of the patient. In the remaining 2 patients, no bailout therapy was attempted, and they were managed conservatively. Cases in which ELCA did not facilitate the passage of RA or intravascular lithotripsy were not classified as RASER or ELCA-tripsy techniques. The overall technical success rate was 91.25%.
In-hospital and follow-up outcomes
ELCA-related complications occurred in 2 procedures (2.5%) due to coronary artery perforation after ELCA application, with immediate sealing after stent implantation (although pericardiocentesis was necessary in 2 of them). A third perforation was observed, not immediately after ELCA application, but after dilatation with NC balloons. In 2 of the perforations, the target lesion was a severely calcified and undilatable lesion located in the left anterior descending artery. The third perforation was observed in an uncrossable lesion at the right coronary artery. In all of them, the 0.9 mm catheter was used, and ELCA was applied with maximum fluency and repetition rate during saline infusion. Intracoronary imaging prior to ELCA application was not performed in any of these patients: the OCT catheter could not cross the lesion in 2 of them and crossing was not attempted in the third. After the application of coronary laser and stent implantation, OCT was performed in 2 of the procedures, which confirmed the good final result.
Other procedural complications not related to ELCA occurred in 4 patients. One patient developed a vascular access complication with retroperitoneal hemorrhage and severe bleeding requiring transfusion and transarterial embolization of a deep femoral artery branch, although his clinical course was favorable. One patient with severe aortic stenosis and impaired left ventricular function showed hemodynamic instability requiring support with inotropes and orotracheal intubation. In 1 patient, no-reflow phenomenon occurred after stent implantation but resolved after intracoronary adenosine infusion.
In the remaining patient, coronary dissection occurred during the guidewire advancement before ELCA application and was complicated with occlusive intracoronary hematoma, which resolved after emergent PCI with successful revascularization. No patient died during the procedure. Three patients died during admission despite successful revascularization due to cardiac causes not related to the procedure (mostly advanced heart failure) and 1 patient died from respiratory sepsis. There were no other in-hospital complications. Overall, the clinical success rate was 87.5%.
After a median follow-up of 15.5 months [IQR, 5.02-29.3], MACE occurred in 9 patients (11.25%). Target lesion revascularization occurred in 7 patients (8.9%), in all patients due to ISR. The median time to target lesion revascularization among patients with successful revascularization was 11.4 [IQR, 8.1-22.6] months. Cardiorespiratory arrest secondary to acute stent thrombosis occurred in 1 patient with successful revascularization, whose family reported poor antiplatelet therapy adherence. One patient died from advanced heart failure after 3 years of follow-up, despite successful revascularization. Three patients died from noncardiac causes.
The procedural outcomes, clinical outcomes, and major complications are summarized in table 3. No significant differences were observed in the results between male and female patients.
Table 3. Procedural and clinical outcomes
| Procedural and clinical success | n (%) |
|---|---|
| ELCA success | 73 (91.25%) |
| Balloon-uncrossable lesion | 25 (78.13%) |
| Balloon-undilatable lesion | 23 (100%) |
| In-stent restenosis | 2 (100%) |
| Stent underexpansion | 5 (100%) |
| Chronic total occlusion | 6 (100%) |
| Combination of > 2 of the above | 10 (100%) |
| Severe calcification as sole indication | 2 (100%) |
| Technical success | 73 (91.25%) |
| Clinical success | 70 (87.5%) |
| Procedural complications | |
| ELCA-related complications | |
| Coronary artery perforation | 2 (2.5%) |
| Complications not related to ELCA | |
| Vascular access complication with major bleeding | 1 (1.25%) |
| Coronary perforation | 1 (1.25%) |
| Flow-limiting dissection | 1 (1.25%) |
| Hemodynamic instability | 1 (1.25%) |
| No-reflow | 1 (1.25%) |
| Ventricular arrhythmia | 0 (0%) |
| In-hospital MACE | |
| Recurrent angina requiring TLR | 0 (0%) |
| Procedure-related myocardial infarction | 1 (1.25%) |
| New-onset heart failure | 0 (0%) |
| Stroke | 0 (0%) |
| Cardiovascular death | 3 (3.75%) |
| All-cause death | 4 (5.0%) |
| MACE after discharge | |
| TLR | 7 (8.75%) |
| MI due to stent thrombosis | 1 (1.25%) |
| Death from cardiovascular causes | 2 (2.5%) |
| Non-cardiovascular related death | 3 (3.75%) |
|
ELCA, excimer laser coronary atherectomy; MACE, major adverse cardiovascular events; MI, myocardial infarction; TLR, target lesion revascularization. |
|
DISCUSSION
The main findings of our study are as follows: a) ELCA was associated with a high rate of technical success in severely calcified coronary lesions, whether isolated or combined with other plaque modification techniques, with an acceptable ELCA-related complications rate. b) The success rate was higher in undilatable than in uncrossable lesions and was 100% in peri-stent lesions (stent underexpansion or ISR).
As described in previous series, calcified lesions are associated with higher rates of PCI failure, complications, morbidity, and mortality.2,16 Although ELCA is known to have no direct effect on calcium, calcified atheromatous plaques have a mixed composition, including lipids, collagen, and other protein fibers.1,17 The interaction of ELCA with these components, due to its photochemical, photothermal and photokinetic properties, modifies the plaque structure, thus facilitating angioplasty in lesions with severe calcification.17 Moreover, in some cases, as occurs in our series, ELCA is complementary to other plaque modification techniques, allowing the passage of the microcatheter to introduce specific atherectomy guidewires, or even to allow the passage of the lithotripsy balloon.14,15 The RASER technique was used in 2 patients and the ELCA-tripsy technique in another patient with technical success in all 3 of them.
There is a lack of contemporary specific series on the use of ELCA in lesions with severe calcification, and data available in the medical literature are contradictory. Bilodeau et al.18 reported high procedural (93%) and clinical (86%) success in a series of 95 patients with complex coronary lesions, of which 57 had significant calcification. The Laser Veterans Affairs (LAVA) Multicenter Registry7 evaluated the use of ELCA in 131 target complex coronary lesions, of which 62% were moderately or severely calcified lesions, globally reporting 90% technical and 88.8% procedural success rate, which is consistent with our results. In the LEONARDO study,19 in which 75% of lesions were calcified, high laser energy levels were shown to be safe and effective (success rate 93.7%). In our series, the highest fluence and frequency were required in 60% of the procedures, with a similar success rate.
Nowadays, the main indication of ELCA is treatment of uncrossable and undilatable lesions. In uncrossable lesions, the laser catheter can be advanced over any 0.014¨angioplasty guidewire that crosses the lesion, unlike other plaque modification techniques. In a multicenter US registry, the success rate for laser-assisted PCI in uncrossable balloon CTO was 95%, which was higher than that for RA (89%) in this setting.20 In a retrospective study by Karacsonyi et al.,21 laser use in balloon-uncrossable and balloon-undilatable CTO was associated with higher technical (91.5% vs 83.1%) and procedural (88.9% vs 81.6%) success rates compared with cases without the use of laser. Ojeda et al.9 conducted a multicenter registry of 126 uncrossable lesions and reported ELCA success of 81.8%. In that registry, severe calcification was independently associated with ELCA failure, a finding already described in a previous study.22 In our series (with severe calcification in 100% of patients), the overall ELCA success rate was 91.25%, but the ELCA success in uncrossable lesions was lower than in undilatable lesions (78.1% vs 100%) and similar to that in the series by Ojeda et al.9 The lower success rate in uncrossable and severely calcified lesions can probably be explained by the different plaque composition and calcium distribution. Furthermore, the higher rate of use of intracoronary imaging could also be associated with better results (72.5% in our series compared with 22.5% reported by Ojeda et al.9). Of note, an ELCA success of 78.1% in uncrossable lesions with severe calcification could be a reasonable result, considering that, if even a microcatheter cannot cross the lesion, ELCA may be the only alternative for revascularization.
In other scenarios, the ELCA success rate of our series was high and similar to that of other series. An ELCA success rate of 86% to 93% has been reported in CTOs.8,23 RA in CTO has been associated with similar success rates (89%-95.6%)24,25 but with a high rate of slow/no flow phenomena.24 In patients with stent underexpansion and ISR, ELCA is feasible and effective,26,27 with 100% ELCA success in our series.
Intravascular imaging is useful to guide calcified coronary stenosis PCI.28,29 Contemporary rates of intravascular imaging for complex PCI remain low.30 In our study, intracoronary imaging was used in 72 procedures (73.4%), and intracoronary imaging-guided procedures resulted in a higher success rate. Its lower use in uncrossable lesions can probably be explained by the fact that the intravascular ultrasound/OCT catheter cannot cross the lesion, rather than necessarily being the reason for the lower success rate in this setting.
Limitations
Our study has some limitations. First, it is an observational study with a small sample size. However, to the best of our knowledge, our study represents the largest series of ELCA specifically performed in severely calcified lesions in contemporary PCI. Second, the severity of lesion calcification was initially assessed by conventional coronary angiography, which has only low to moderate sensitivity compared with intravascular ultrasound or OCT. In addition, sometimes the calcium observed by conventional angiogra- phy is adventitious, thus not affecting balloon dilation or stent expansion with conventional techniques. However, the use of intracoronary imaging techniques was higher than in previous series and confirmed the severity of calcification in all patients. In addition, a significant number of cases consisted of uncrossable lesions, limiting the use of intracoronary imaging to define the calcification from the beginning of the procedure. Finally, the operators involved in this study were experienced ELCA operators. This may limit the generalizability of our results since ELCA is not available in most centers and requires a learning curve.
CONCLUSIONS
ELCA is a useful tool in severe calcification lesions, with a high success rate, especially in the setting of undilatable or peri-stent lesions. The technique is also reasonably safe, given that it is used in highly complex procedures. Future randomized studies will shed light on its role in the management of severe calcified coronary lesions.
FUNDING
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ETHICAL CONSIDERATIONS
All patients signed an informed consent form and approval was obtained from the ethics committee of the center. The study has taken into consideration sex and gender variables according to SAGER guidelines.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
No artificial intelligence tool has been used during the preparation of this work.
AUTHORS’ CONTRIBUTIONS
A. Jurado-Román conceived and designed the study. L. Cobarro and A. Jurado-Román performed the analysis and wrote the initial draft. L. Cobarro, A. Jurado-Román, D. Tébar-Márquez, S. Vera-Vera, A. García-Escobar, C. Ugueto, C. Contreras, B. Rivero, S. Jiménez-Valero, G. Galeote, and R. Moreno collected the data and reviewed the final version of the manuscript.
CONFLICTS OF INTEREST
R. Moreno is associate editor of REC: Interventional Cardiology; the editorial procedure established in the journal has been followed to ensure impartial handling of the manuscript.
A. Jurado-Román is proctor of Philips-Biomenco, Boston Scientific, CSI-World Medica and Medtronic Inc and has received speaker fees from Boston Scientific, Abbott Vascular, World Medica, Biotronik, Philips-Biomenco, and Inari. R. Moreno has received speaker fees from Medtronic Inc, Boston Scientific, Abbott vascular, Biosensors, Biotronik, Edwards Lifesciences, AMGEN, AstraZeneca, and Daiichi Sankyo New Vascular Therapies and Biosensors.
WHAT IS KNOWN ABOUT THE TOPIC?
- Excimer laser coronary atherectomy (ELCA) is a plaque modification technique that has proved to be useful in several scenarios, such as balloon failure (uncrossable or undilatable lesions), chronic total occlusions (CTO), stent underexpansion, in-stent restenosis (ISR) and thrombotic lesions.
- In recent years, incremental operator experience along with the standardization of laser technique has expanded its indications and decreased its complication rates.
- The effectiveness of ELCA in calcified lesions is controversial. On one hand, some ELCA series have described a relationship between severe calcification and laser failure. In contrast, moderate-to-severe calcification is found in more than 60% of cases in some ELCA series with a high success rate, indicating that this technique could be useful in this setting.
- Due to the lack of evidence in this specific scenario, our study aimed to assess the contemporary safety and efficacy of ELCA in severely calcified coronary lesions.
WHAT DOES THIS STUDY ADD?
- ELCA is associated with a high rate of technical success in severely calcified coronary lesions, whether isolated or combined with other plaque modification techniques, with an acceptable ELCA-related complications rate.
- The success rate is higher in undilatable than in uncrossable lesions and was 100% in peri-stent lesions (stent underexpansion or restenosis). However, in uncrossable lesions, ELCA may be the only alternative for percutaneous revascularization.
- Clinical results after a median follow-up of 15.5 months were favorable, taking into account the complexity of this scenario.
REFERENCES
1. Mori H, Torii S, Kutyna M, Sakamoto A, Finn AV, Virmani R. Coronary Artery Calcification and its Progression:What Does it Really Mean?JACC Cardiovasc Imaging. 2018;11:127-142.
2. Huisman J, van der Heijden LC, Kok MM, et al. Impact of severe lesion calcification on clinical outcome of patients with stable angina, treated with newer generation permanent polymer-coated drug-eluting stents:A patient-level pooled analysis from TWENTE and DUTCH PEERS (TWENTE II). Am Heart J. 2016;175:121-129.
3. Jawad-Ul-Qamar M, Sharma H, Vetrugno V, et al. Contemporary use of excimer laser in percutaneous coronary intervention with indications, procedural characteristics, complications and outcomes in a university teaching hospital. Open Heart. 2021;8:e001522.
4. Sintek M, Coverstone E, Bach R, et al. Excimer Laser Coronary Angioplasty in Coronary Lesions:Use and Safety From the NCDR/CATH PCI Registry. Circ Cardiovasc Interv. 2021;14:e010061.
5. Stone GW, de Marchena E, Dageforde D, et al. Prospective, Randomized, Multicenter Comparison of Laser-Facilitated Balloon Angioplasty Versus Stand-Alone Balloon Angioplasty in Patients With Obstructive Coronary Artery Disease fn1fn1Funding for this study was provided in part by Eclipse Surgical Technologies, Inc., Sunnyvale, California. J Am Coll Cardiol. 1997;30:1714-1721.
6. Ocaranza-Sánchez R, Abellás-Sequeiros RA, Galvão-Braga C, Trillo-Nouche R, González-Juanatey JR. Uso de aterectomía coronaria con LASER Excimer como terapia coadyuvante en intervencionismo coronario percutáneo. Rev Esp Cardiol.2016;69:867-868.
7. Karacsonyi J, Armstrong EJ, Truong HTD, et al. Contemporary Use of Laser During Percutaneous Coronary Interventions:Insights from the Laser Veterans Affairs (LAVA) Multicenter Registry. J Invasive Cardiol. 2018;30:195-201.
8. Mohandes M, Rojas S, Moreno C, Fernández F, Fuertes M, Guarinos J. Excimer Laser in Percutaneous Coronary Intervention of Device Uncrossable Chronic Total and Functional Occlusions. Cardiovasc Revasc Med. 2020;21:657-660.
9. Ojeda S, Azzalini L, Suárez de Lezo J, et al. Excimer laser coronary atherectomy for uncrossable coronary lesions. A multicenter registry. Catheter Cardiovasc Interv. 2021;98:1241-1249.
10. Jurado-Román A, Gómez-Menchero A, Gonzalo N, et al. Plaque modification techniques to treat calcified coronary lesions. Position paper from the ACI-SEC. REC Interv Cardiol. 2023;5:46-61
11. Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87-165.
12. Mintz GS, Popma JJ, Pichard AD, et al. Patterns of Calcification in Coronary Artery Disease. Circulation. 1995;91:1959-1965.
13. Thygesen K. 'Ten Commandments'for the Fourth Universal Definition of Myocardial Infarction 2018. Eur Heart J. 2019;40:226-226.
14. Egred M. RASER angioplasty. Catheter Cardiovasc Interv. 2012;79:1009-1012.
15. Jurado-Román A, García A, Moreno R. ELCA-Tripsy:Combination of Laser and Lithotripsy for Severely Calcified Lesions. J Invasive Cardiol.2021;33:E754-E755.
16. Copeland-Halperin RS, Baber U, Aquino M, et al. Prevalence, correlates, and impact of coronary calcification on adverse events following PCI with newer-generation DES:Findings from a large multiethnic registry. Catheter Cardiovasc Interv. 2018;91:859-866.
17. Tsutsui RS, Sammour Y, Kalra A, et al. Excimer Laser Atherectomy in Percutaneous Coronary Intervention:A Contemporary Review. Cardiovasc Revasc Med. 2021;25:75-85.
18. Bilodeau L, Fretz EB, Taeymans Y, Koolen J, Taylor K, Hilton DJ. Novel use of a high-energy excimer laser catheter for calcified and complex coronary artery lesions. Catheter Cardiovasc Interv. 2004;62:155-161.
19. Ambrosini V, Sorropago G, Laurenzano E, et al. Early outcome of high energy Laser (Excimer) facilitated coronary angioplasty ON hARD and complex calcified and balloOn-resistant coronary lesions:LEONARDO Study. Cardiovasc Revasc Med. 2015;16:141-146.
20. Karacsonyi J, Karmpaliotis D, Alaswad K, et al. Prevalence, indications and management of balloon uncrossable chronic total occlusions:Insights from a contemporary multicenter US registry. Catheter Cardiovasc Interv. 2017;90:12-20.
21. Karacsonyi J, Alaswad K, Choi JW, et al. Laser for balloon uncrossable and undilatable chronic total occlusion interventions. Int J Cardiol. 2021;336:33-37.
22. Bittl JA. Clinical results with excimer laser coronary angioplasty. Semin Interv Cardiol. 1996;1:129-134.
23. Rawlins J, Din JN, Suneel T, O´Kane P. Coronary Intervention with the Excimer Laser:Review of the Technology and Outcome Data. Interv Cardiol. 2016;1:27-32.
24. Azzalini L, Dautov R, Ojeda S, et al. Long-term outcomes of rotational atherectomy for the percutaneous treatment of chronic total occlusions. Catheter Cardiovasc Interv. 2017;89:820-828.
25. Pagnotta P, Briguori C, Mango R, et al. Rotational atherectomy in resistant chronic total occlusions. Catheter Cardiovasc Interv. 2010;76:366-371.
26. Latib A, Takagi K, Chizzola G, et al. Excimer Laser LEsion Modification to Expand Non-dilatable sTents:The ELLEMENT Registry. Cardiovasc Revasc Med. 2014;15:8-12.
27. Lee C, Shlofmitz R, Song L, et al. The effectiveness of excimer laser angioplasty to treat coronary in-stent restenosis with peri-stent calcium as assessed by optical coherence tomography. J Am Coll Cardiol.2017;69:1086-1086.
28. Fujino A, Mintz GS, Matsumura M, et al. A new optical coherence tomography-based calcium scoring system to predict stent underexpansion. EuroIntervention. 2018;13:e2182-e2189.
29. Zhang M, Matsumura M, Usui E, et al. Intravascular Ultrasound–Derived Calcium Score to Predict Stent Expansion in Severely Calcified Lesions. Circ Cardiovasc Interv. 2021;14:e010296.
30. Hannan EL, Zhong Y, Reddy P, et al. Percutaneous Coronary Intervention With and Without Intravascular Ultrasound for Patients With Complex Lesions:Utilization, Mortality, and Target Vessel Revascularization. Circ Cardiovasc Interv. 2022;15:e011687.

ABSTRACT
Introduction and objectives: Contrast-induced-acute kidney injury (CI-AKI) is a potential complication of angiographic procedures. The DyeVert Contrast Reduction system (Osprey Medical, United States) is a device to reduce the concentration of contrast medium (CM) in the kidneys by decreasing the amount of CM delivered to patients. Unlike manual systems, few data are available on the DyeVert Power XT system, which is used in conjunction with automated contrast injection. The main aim of our study was to evaluate its effectiveness during percutaneous coronary interventions (PCI).
Methods: Between 2020 and 2022, 101 patients who underwent PCI with the DyeVert Power XT system (case group) were enrolled to evaluate the amount of CM saved through the use of this device, as well as the rate, severity, and predictors of CI-AKI. Patients who underwent PCI without the use of the device (control group) were enrolled to create a matched group allowing assessment of differences in CM and the CI-AKI rate.
Results: : In the case group, the amount of CM saved was 114 ± 42 mL, representing an average of 32% of the total CM. Fourteen patients (13.9%) developed CI-AKI. The only independent predictors of CI-AKI were hematocrit (OR, 0.86; 95%CI, 0.74-0.99; P = .04) and ejection fraction (OR, 0.88; 95%CI, 0.82-0.95; P = .001). As a result of diversion by the device, the amount of CM delivered was lower in the case group than in controls (252 vs 267 mL; P = .42), but this difference was nonsignificant. Equally, the reduction in CI-AKI (14.3% vs 16.3%) was nonsignificant.
Conclusions: Hematocrit and ejection fraction may be more important predictors of CI-AKI than the CM volume normally used during PCI in the general population. The net practical benefit of DyeVert Power XT was low.
Keywords: Acute kidney injury. Contrast media. Percutaneous coronary intervention. DyeVert.
RESUMEN
Introducción y objetivos: La nefropatía inducida por contraste (NIC) es una potencial complicación de los procedimientos angiográficos. El sistema DyeVert Power (Osprey Medical, Estados Unidos) permite reducir la concentración renal del medio de contraste al disminuir la cantidad administrada a los pacientes. Al contrario que sobre los sistemas manuales, existen pocos datos disponibles sobre el sistema DyeVert, que se utiliza junto a la inyección automática de contraste. El objetivo principal de este estudio fue evaluar su eficacia en procedimientos de intervencionismo coronario percutáneo (ICP).
Métodos: Entre 2020 y 2022 se incluyó a 101 pacientes a quienes se realizó ICP utilizando el sistema DyeVert Power XT (grupo de casos) para evaluar la cantidad ahorrada de medio de contraste, así como la tasa, la gravedad y los predictores de NIC. Además, se seleccionó un grupo control de pacientes a los que se había realizado ICP sin utilizar el sistema DyeVert para comparar la cantidad de medio de contraste administrado y la tasa de NIC.
Resultados: En el grupo de casos se redujo la administración de medio de contraste en 114 ± 42 ml (una media del 32% del total). Desarrollaron NIC 14 pacientes (13,9%). Los predictores de NIC fueron el hematocrito (OR = 0,86; IC95%: 0,74-0,99; p = 0,04) y la fracción de eyección (OR = 0,88; IC95%: 0,82-0,95; p = 0,001). Como resultado de la utilización del sistema DyeVert, la cantidad administrada de medio de contraste fue menor, pero sin diferencias estadísticamente significativas (252 frente a 267 ml; p = 0,42). La tasa de NIC fue menor con el sistema DyeVert, pero sin alcanzar la significación estadística (14,3 frente a 16,3%; p = 1,0).
Conclusiones: El hematocrito y la fracción de eyección, más que la cantidad de contraste administrada, pueden ser predictores de NIC en los pacientes que reciben ICP. El beneficio del sistema DyeVert fue bajo.
Palabras clave: Insuficiencia renal aguda. Medios de contraste. Intervención coronaria percutánea. DyeVert.
Abbreviations
CI-AKI: contrast induced-acute kidney injury. CM/CMV: contrast medium/contrast medium volume. PCI: percutaneous coronary intervention.
INTRODUCTION
Contrast induced-acute kidney injury (CI-AKI) is a dreaded complication after diagnostic and interventional angiographic procedures and is linked to increased morbidity and mortality. In a large recent meta-analysis, the pooled incidence of CI-AKI after coronary angiography was 12.8%, with 95% confidence interval (95%CI) 11.7%-13.9%, and the associated mortality was 20.2% (95%CI, 10.7%-29.7%).1 Multiple risk factors have been identified: contrast medium volume (CMV), advanced age (> 75 years), diabetes, anemia, conditions associated with hypotension, and ejection fraction (EF) < 40%.2,3 Many of these risk factors are included in the Mehran score,2 which identifies 4 risk classes of contrast-induced nephropathy (CIN) after PCI: low (≤ 5 points), moderate (6-10 points), high (11-15 points), and very high (≥ 16 points). The Mehran score and the recent Mehran 2 score4 assign 1 point for each 100 mL of CMV up to a dose of 299 mL. Because volume depletion increases the CM concentration in renal tubules, the main preprocedural measure to reduce the occurrence of CI-AKI is intravenous administration of normal saline before and after the procedure, because other solutions provide no benefits5; hydration should be started 12 hours before and continued for 24 hours after the procedure at 1 mL/kg/h or 0.5 mL/kg/h if EF < 35% or New York Heart Association (NYHA) class > 2.6 Another means of decreasing CM concentration in the kidneys is the DyeVert Contrast Reduction system (Osprey Medical Inc, United States), which reduces the amount of CMV delivered to patients during angiographic procedures, with noninferior image quality as attested by independent reviewers.7,8 The DyeVert, DyeVert Plus and DyeVert Plus EZ are used in conjunction with manual contrast injection, and the DyeVert Power XT is used with automated contrast injection; the latter system has been little studied. The main aim of our study was to evaluate the effectiveness of the DyeVert Power XT system in reducing CM delivery during PCI.
METHODS
Study population
This single center, observational study was performed in patients who underwent PCI between September 2020 and December 2022 with the DyeVert Power XT system (case group) and in patients who underwent PCI during a similar period without the use of the device (control group).
Inclusion criteria for both groups were as follows: chronic kidney disease (CKD) [estimated glomerular filtration rate (eGFR) < 60 mL/min/m2] and/or need for a complex PCI with the likelihood of receiving a large amount of CM; previous coronary artery bypass graft (CABG); chronic total occlusion (CTO) (complete blockage of a coronary artery lasting at least 3 months); bifurcation; and left main and/or multivessel disease (at least 2 vessels involved).
The exclusion criterion for both groups was the presence of end-stage kidney failure on dialysis treatment. We collected laboratory, instrumental, clinical, and procedural variables in the case and control groups. Definitions of all these variables are reported in table 1, table 2, table 3, and table 4. For the variables included in the Mehran score, we used the same descriptions as those used in the score. eGFR was calculated by the Modification of Diet in Renal Disease (MDRD) 4-variable equation, left ventricular EF by 2-dimensional echocardiography during hospitalization and before arrival in the catheterization laboratory, and the risk of any post-PCI CIN by the Mehran score. Bifurcation/left main treatment (with single/double stent) consisted of the proximal optimization technique (POT) with kissing balloon inflation and eventually re-POT in all cases. Total CMV represents the volume that would have been delivered if DyeVert had not been used, ie, the sum of CMV delivered to patients and the CMV saved by DyeVert. CM injection flow was 4 and 3 mL/sec for the left and right coronary artery, respectively.
Table 1. Laboratory, instrumental, clinical characteristics, and Mehran score in the overall population and according to incidence of CI-AKI in the case group
| Characteristics | Overall population (n = 101) | No CI-AKI (n = 87) | CI-AKI (n = 14) | P |
|---|---|---|---|---|
| Laboratory and istrumental characteristics | ||||
| eGFR, mL/min | 51 ± 18 | 52 ± 19 | 45 ± 16 | .18 |
| HCT | 38.6 ± 4.9 | 39.1 ± 4.8 | 35.5 ± 4.8 | .01* |
| EF | 50 [35-55] | 50 [40-55] | 30 [28-36] | < .001* |
| CKD [eGFR < 60 mL/min/ 1.73 m2] | 73 (72.3) | 63 (72.4) | 10 (71.4) | 1 |
| Anemia [male HCT < 39%, female HCT < 36%] | 48 (47.5) | 38 (43.7) | 10 (71.4) | .10 |
| Clinical characteristics | ||||
| Age, years | 74 (68-80) | 73 (67-80) | 75 (74-81) | .09 |
| Age > 75 years | 39 (38.6) | 32 (36.8) | 7 (50) | .52 |
| Male sex | 80 (79.2) | 68 (78.2) | 12 (85.7) | .73 |
| Overweight [body mass index ≥ 25] | 52 (51.5) | 46 (52.9) | 6 (42.9) | .68 |
| Hypertension | 78 (77.2) | 70 (80.5) | 8 (57.1) | .08 |
| Diabetes | 48 (47.5) | 40 (46) | 8 (57.1) | .62 |
| Dyslipidemia | 68 (67.3) | 57 (66) | 11 (79) | .51 |
| Current smoker | 24 (23.8) | 20 (23) | 4 (28.6) | .74 |
| Former smoker | 35 (34.7) | 32 (36.8) | 3 (21.4) | .37 |
| CHF [NYHA class ≥ 3 and/or history of pulmonary edema] | 37 (36.6) | 25 (28.7) | 12 (85.7) | < .001* |
| Acute coronary syndrome presentation | 38 (37.6) | 31 (35.6) | 7 (50) | .46 |
| Hypotension [Systolic arterial pressure < 80 mmHg for ≥ 1 h requiring inotrope] | 4 (4) | 2 (2.3) | 2 (14.3) | .09 |
| Mehran score | ||||
| Mehran CI-AKI risk class: | ||||
| Low | 24 (23.8) | 24 (27.6) | 0 (0) | .04* |
| Moderate | 26 (25.7) | 24 (27.6) | 2 (14.3) | .51 |
| High | 34 (33.7) | 29 (33.3) | 5 (35.7) | 1 |
| Very high | 17 (16.8) | 10 (11.5) | 7 (50) | .002* |
| Mehran score, points | 11 ± 5 | 10 ± 5 | 15 ± 4 | < .001* |
|
Values are expressed as No. (%), mean ± standard deviation, or median [first quartile-third quartile]. *Statistically significant P-value (P < .05). CHF, congestive heart failure; CI-AKI, contrast induced-acute kidney injury; CKD, chronic kidney disease; EF, ejection fraction; eGFR, estimated glomerular filtration rate; HCT, hematocrit; NYHA, New York Heart Association. |
||||
Table 2. Procedural characteristics in the overall population and according to incidence of CI-AKI in the case group
| Characteristics | Overall population (n = 101) | No CI-AKI (n = 87) | CI-AKI (n = 14) | P |
|---|---|---|---|---|
| Procedural characteristics (angiography/PCI complexity/complications) | ||||
| Previous CABG | 20 (19.8) | 18 (20.7) | 2 (14.3) | .73 |
| CTO [complete blockage of a coronary artery lasting at least 3 months] | 12 (11.9) | 11 (12.6) | 1 (7.1) | 1 |
| No. vessels treated in the same procedure: | ||||
| 1 | 57 (56.4) | 52 (59.8) | 5 (35.7) | .09 |
| 2 | 40 (39.6) | 32 (36.8) | 8 (57.1) | .15 |
| 3 | 4 (4) | 3 (3.4) | 1 (7.1) | .45 |
| No. bifurcations treated in the same procedure: | ||||
| 0 | 67 (66.3) | 58 (66.7) | 9 (64.3) | 1 |
| 1 | 31 (30.7) | 27 (31) | 4 (28.6) | 1 |
| 2 | 3 (3) | 2 (2.3) | 1 (7.1) | .36 |
| Left main treatment | 25 (24.8) | 20 (23) | 5 (35.7) | .33 |
| Stent, number | 2 [1-3] | 2 [1-3] | 2 [1-3] | .75 |
| Stent lenght, mm | 52 [31-88] | 51 [30-91] | 57 [36-73] | .95 |
| Perforation | 3 (3) | 3 (3.4) | 0 (0) | 1 |
| IABP use | 1 (1) | 0 (0) | 1 (7.1) | .14 |
| Rotablator use | 3 (3) | 1 (1.1) | 2 (14.3) | .05 |
| Procedural characteristics (others) | ||||
| Radial access | 88 (87.1) | 75 (86.2) | 13 (92.9) | .69 |
| Femoral access | 27 (26.7) | 21 (24.1) | 6 (42.9) | .19 |
| Operator | ||||
| L | 52 (51.5) | 47 (54) | 5 (35.7) | .20 |
| A | 30 (29.7) | 26 (29.9) | 4 (28.6) | 1 |
| B | 4 (4) | 3 (3.4) | 1 (7.1) | .46 |
| V | 13 (12.9) | 10 (11.5) | 3 (21.4) | .38 |
| S | 2 (1.9) | 1 (1.1) | 1 (7.1) | .26 |
| Contrast medium type: | ||||
| Iomeprol 350 | 9 (8.9) | 7 (8) | 2 (14.3) | .61 |
| Iohexol 350 | 13 (12.9) | 11 (12.6) | 2 (14.3) | 1 |
| Iodixanol 320 | 79 (78.2) | 69 (79.3) | 10 (71.4) | .50 |
| Contrast medium dose delivered, mL | 242 [189-300] | 240 [188-306] | 258 [195-277] | .95 |
| Total contrast medium dose [delivered plus saved], mL | 355 ± 110 | 354 ± 79 | 356 ± 106 | .95 |
| IVUS use | 24 (23.8) | 23 (26.4) | 1 (7.1) | .18 |
|
Data are expressed as No. (%), mean ± standard deviation, or median [first quartile-third quartile]. CABG, coronary artery bypass graft; CI-AKI, contrast induced-acute kidney injury; CTO, chronic total occlusion; IABP, intra-aortic balloon pump; IVUS, intravascular ultrasound. |
||||
Table 3. Laboratory, instrumental, clinical characteristics, and Mehran score of cases and controls in the matched group
| Characteristics | No DyeVert (n = 49) | DyeVert (n = 49) | P | Standardized mean difference |
|---|---|---|---|---|
| Laboratory and istrumental characteristics | ||||
| eGFR, mL/min | 53 ± 18 | 51 ± 18 | .70 | 0.11 |
| HCT | 37.8 ± 4.1 | 38.2 ± 4.9 | .68 | 0.08 |
| EF | 50 [40-55] | 50 [35-55] | .68 | 0.13 |
| CKD [eGFR < 60 mL/min/1.73 m2] | 36 (73.5) | 34 (69.4) | .82 | 0.09 |
| Anemia [male HCT < 39, Female HCT < 36] | 27 (55.1) | 24 (49) | .69 | 0.12 |
| Clinical characteristics | ||||
| Age, years | 75 ± 9 | 75 ± 9 | .96 | 0.01 |
| Age > 75 years | 26 (53.1) | 24 (49) | .84 | 0.08 |
| Male sex | 38 (77.6) | 41 (83.7) | .61 | 0.15 |
| Overweight [body mass index ≥ 25] | 22 (44.9) | 24 (49) | .84 | 0.08 |
| Hypertension | 33 (67.3) | 37 (75.5) | .50 | 0.19 |
| Diabetes | 19 (38.8) | 20 (40.8) | 1 | 0.04 |
| Dyslipidemia | 28 (57.1) | 32 (65.3) | .53 | 0.17 |
| Current smoker | 11 (22.4) | 10 (20.4) | 1 | 0.05 |
| Former smoker | 16 (32.7) | 18 (36.7) | .83 | 0.09 |
| CHF [NYHA class ≥ 3 and/or history of pulmonary edema] | 15 (30.6) | 15 (30.6) | 1 | < 0.01 |
| Acute coronary syndrome presentation | 27 (55.1) | 25 (51) | .84 | 0.08 |
| Hypotension [systolic pressure < 80 mmHg for ≥ 1 h requiring inotrope] | 2 (4.1) | 2 (4.1) | 1 | < 0.01 |
| Mehran score | ||||
| Mehran CI-AKI risk class: | ||||
| Low | 12 (24.5) | 10 (20.4) | .63 | 0.09 |
| Moderate | 12 (24.5) | 15 (30.6) | .50 | 0.14 |
| High | 13 (26.5) | 15 (30.6) | .65 | 0.09 |
| Very high | 12 (24.5) | 9 (18.4) | .46 | 0.16 |
| Mehran score, points | 11 ± 6 | 11 ± 6 | .86 | 0.04 |
|
Data are expressed as No. (%), mean ± standard deviation, or median [first quartile-third quartile]. CHF, congestive heart failure; CI-AKI, contrast induced-acute kidney injury; CKD, chronic kidney disease; EF, ejection fraction; eGFR, estimated glomerular filtration rate; HCT, hematocrit; NYHA, New York Heart Association. |
||||
Table 4. Procedural characteristics of cases and controls in the matched group
| Characteristics | No DyeVert (n = 49) | DyeVert (n = 49) | P | Standardized mean difference |
|---|---|---|---|---|
| Procedural characteristics (angiography/PCI complexity/complications) | ||||
| Previous CABG | 8 (16.3) | 6 (12.2) | .56 | 0.10 |
| CTO [complete blockage of a coronary artery lasting at least 3 months] | 6 (12.2) | 8 (16.3) | .77 | 0.13 |
| No. vessels treated in the same procedure: | ||||
| 1 | 32 (65.3) | 29 (59.2) | .53 | 0.12 |
| 2 | 15 (30.6) | 17 (34.7) | .67 | 0.08 |
| 3 | 2 (4.1) | 3 (6.1) | 1 | 0.10 |
| No. bifurcations treated in the same procedure: | ||||
| 0 | 33 (67.3) | 31 (63.3) | .67 | 0.09 |
| 1 | 15 (30.6) | 16 (32.7) | .83 | 0.04 |
| 2 | 1 (2.1) | 2 (4) | 1 | 0.12 |
| Left main treatment | 12 (24.5) | 13 (26.5) | 1 | 0.05 |
| Stent, number | 2 [1-3] | 2 [1-3] | .30 | 0.15 |
| Stent lenght, mm | 46 [30-85] | 52 [33-97] | .41 | 0.13 |
| Perforation | 2 (4.1) | 1 (2) | 1 | 0.12 |
| IABP use | 0 (0) | 1 (2) | 1 | 0.20 |
| Rotablator use | 0 (0) | 2 (4.1) | .49 | 0.24 |
| Procedural characteristics (others) | ||||
| Radial access | 41 (83.7) | 45 (91.8) | .35 | 0.24 |
| Femoral access | 11 (22.4) | 15 (30.6) | .49 | 0.18 |
| Operator: | ||||
| L | 24 (49) | 24 (49) | 1 | < 0.01 |
| A | 17 (34.7) | 17 (34.7) | 1 | < 0.01 |
| B | 5 (10.2) | 3 (6.1) | .71 | 0.20 |
| V | 3 (6.1) | 5 (10.2) | .71 | 0.12 |
| Contrast medium type: | ||||
| Iomeprol 350 | 7 (14.3) | 4 (8.2) | .34 | 0.21 |
| Iohexol 350 | 9 (18.4) | 10 (20.4) | .80 | 0.06 |
| Iodixanol 320 | 33 (67.3) | 35 (71.4) | .66 | 0.10 |
| IVUS use | 10 (20.4) | 11 (22.4) | 1 | 0.05 |
|
Data are expressed as No. (%), mean ± standard deviation, or median [first quartile-third quartile]. CABG, coronary artery bypass graft; CI-AKI, contrast induced-acute kidney injury; CTO, chronic total occlusion; IABP, intra-aortic balloon pump; IVUS, intravascular ultrasound. |
||||
Image quality was evaluated by operators during the procedures. When quality was inadequate, exclusion of the device from the CM line was allowed for the shortest possible time.
AKI was defined as a rise in the concentration of serum creatinine ≥ 0.3 mg/dL within 48 hours after CM administration from the baseline value obtained before CM injection; further measurements after 48 hours were collected in patients with worsening kidney function; for its prevention, all patients received hydration with sodium chloride 0.9% intravenous solution at a rate of 1 or 0.5 mL/kg/h, as appropriate. The severity of AKI was defined according to Kidney Disease Improving Global Outcome (KDIGO) stages.
The research reported was performed in accordance with recommendations for clinical investigation (Declaration of Helsinki of the World Medical Association, October 2013) and was approved by an ethics committee. We declare that relevant informed consent was obtained from all participants and is available.
Objectives
In the case group, we evaluated the following: a) the amount of CMV saved using DyeVert and image quality; b) the rate and severity of CI-AKI and the rate of in-hospital all-cause death; c) laboratory, instrumental, clinical, and procedural differences in the 2 subgroups defined on the basis of the incidence of AKI; and d) independent predictors of CI-AKI.
In the overall population of the case and control groups, we performed propensity score matching (PSM) to obtain a group of patients with a sufficiently good balance (matched group), in which we evaluated the following: a) differences in CMV, and b) rate and severity of CI-AKI.
Statistical analysis
Categorical variables are expressed as the number and percentage of patients. Continuous parametric data are reported as the mean ± standard deviation and continuous nonparametric data as the median [lower and upper quartile]; for assessment of normality, the Kolmogorov test was used. Patients’ categorical variables were compared using the chi-squared test (with Yates’ correction for continuity in the case of variables with only 2 categories) or the Fisher exact test, as appropriate. The unpaired t-test was used for continuous parametric variables and the Mann-Whitney U-test for continuous nonparametric variables; the same tests were used in the matched group. On univariate analysis, significance was defined as P < .05. To establish the independent predictors of AKI, we performed multivariable logistic regression analysis. Variables were selected according to significance in the univariate analysis. The chosen method was stepwise backward regression with a maximum of 20 iterations. Multicollinearity was assessed with tolerance and variance inflation factor (VIF) values. Receiver operating characteristic (ROC) curves were used to establish the optimal cutoffs of independent predictors for the diagnosis of AKI. To perform PSM, the algorithm used was nearest neighbor matching 1:1 with a caliper size of ± 0.2. Statistical analyses were performed using SPSS for Windows, release 29, with R 4.2 implementation to perform PSM.
RESULTS
Analysis in the case group
A total of 101 patients (median age 74 [68-80] years, male sex 79.2%, CKD 72.3%) underwent PCI with the use of the DyeVert Power XT system.
In the overall population of the case group, mean hematocrit (HCT) was 38.6 ± 4.9 %, median EF was 50% [35%-55%], and mean Mehran score was 11 ± 5 points.
Congestive heart failure (CHF) was present in 37 patients (36.6%), Mehran CI-AKI very high-risk class was present in 17 patients (16.8%) and Mehran CI-AKI low-risk class was present in 24 patients (23.8%) (table 1).
We enrolled 20 patients (19.8%) with previous CABG, 12 (11.9%) with CTO, 34 (33.7%) with bifurcations, 25 (24.8%) with left main coronary artery disease, and 44 (43.6%) with multivessel disease. Delivered CM was 242 (189-300) mL, total CM was 355 ± 110 mL, and saved CM was 114 ± 42 mL, with an average of 32% of the total CMV (table 2). In almost all patients (n = 96, 95% of patients), image quality was adequate, while the device was excluded to make it adequate for the shortest possible time in 5 patients. Without these exclusions, saved CMV would have been slightly higher and with trivial changes with regard to the comparison with controls: 33% of the total, a value derived from patients without exclusions (n = 96).
A total of 14 (13.9%) patients developed CI-AKI (AKI-KDIGO 1, 2, 3: 6.9%, 3%, and 4%, respectively). The results of the univariate analysis for the overall population and according to the incidence of CI-AKI in the case group are reported in table 1, table 2, and figure 1.
Figure 1. In-hospital all-cause mortality rate according to onset of CI-AKI in the case group. CI-AKI, contrast induced-acute kidney injury.
Compared with patients not developing CI-AKI, those in the CI-AKI subgroup had lower HCT values (35.5 ± 4.8 vs 39.1 ± 4.8; P = .01), lower EF values (30 [28-36] vs 50 [40-55]; P < .001) and higher Mehran score values (15 ± 4 vs 10 ± 5; P < .001).
In addition, the first patients more frequently had CHF [12 (85.7%) vs 25 (28.7%); P <.001] and Mehran CI-AKI very high-risk class (7 [50%] vs 10 [11.5%]; P =.002) and less frequently had Mehran CI-AKI low-risk class (0 [0%] vs 24 [27.6%]; P = .04).
No significant differences were found in the remaining laboratory, instrumental, or clinical features or the procedural variables between the 2 subgroups; in particular, CM was slightly higher in CI-AKI patients: 258 [195-277] vs 240 [188-306] mL, total 356 ± 106 vs 354 ± 79 mL; P = .95 for both variables delivered. In the multivariate analyses, independent predictors of CI-AKI were HCT (OR, 0.86, 95%CI, 0.74-0.99; P = .04) and EF (OR, 0.88, 95%CI, 0.82-0.95; P = .001); the percentage accuracy in classification of the model was 88%, while tolerance and VIF values (0.99 and 1.01, respectively) showed no multicollinearity. The HCT ROC curve showed the following values: area under curve (AUC) 0.71 with 95%CI 0.56-0.87; P = .01; a cutoff of 36.3% had the best sensitivity (72%) and specificity (71%) for the outcome (figure 2). The EF ROC curve showed the following values: AUC 0.83 with 95%CI 0.72-0.94; P = .001; a cutoff of 37% had the best sensitivity (82%) and specificity (79%) (figure 2); therefore, our best predictor was EF < 40%.
Figure 2. Receiver operating characteristic curves showing the diagnostic ability of HCT and EF for the diagnosis of CI-AKI in the case group. CI-AKI, contrast induced-acute kidney injury; EF, ejection fraction; HCT, hematocrit.
There were 4 in-hospital all-cause deaths overall, 2 deaths in each subgroup (CI-AKI and no–CI-AKI subgroups), as shown in figure 1.
Analysis in the matched group
After the matching process, 49 patients remained in the control (no DyeVert) and case (DyeVert) groups with no significant imbalance (ie, standardized mean differences < ± 0.25), as reported in table 3 and table 4. As shown in figure 3, delivered CM was slightly lower in the DyeVert group than in the no-DyeVert group, with no significant difference (252 ± 80 vs 267 ± 101 mL; P = .42), while total CM was significantly higher in the DyeVert group (354 ± 110 vs 267 ± 101 mL; P < .001). The CI-AKI rate was slightly lower in the case group than in the control group (14.3% vs 16.3%; P = .99) with slightly more advanced stages of AKI in controls (table 1 of the supplementary data), without significance.
Figure 3. Contrast medium in cases and controls in the matched group. CM, contrast medium; blue dots represent the median values; vertical black lines represent the standard deviations.
DISCUSSION
In the case group, the DyeVert Power XT system saved 32% of CM and image quality was adequate in almost all cases; the only independent predictors of CI-AKI were HCT and EF.
In the matched group, total CM was higher in cases than in controls. After diversion by the device, delivered CM was slightly lower in cases than in controls, but without significance. The reduction in CI-AKI was also nonsignificant.
The DyeVert system is a second-generation device to reduce the amount of CM delivered to patients during angiographic procedures. The first generation was the AVERT system (Osprey Medical Inc), which showed a relative reduction of approximately 23% in CMV among PCI patients compared with controls; the use of the device did not reduce the AKI rate.9 DyeVert Power XT is used in combination with automatic injection; few data are available in this context, being limited to 2 studies that investigated 2610 and 9 patients,11 without a control group. There are more data on manual injection (1696 patients, 15 studies); all these 17 studies were collectively analyzed in the meta-analysis by Tarantini et al.12
In that meta-analysis, the mean saved CMV in the DyeVert group was reported by 7 observational studies and ranged from 34% to 47% of total CMV; the pooled estimate value was approximately 39.5% using manual CM injection systems; of note, the lowest value (34%) was achieved using DyeVert Power XT. We found a similar value in the DyeVert (case) group. These reduced values compared with manual systems may be related to different pressures generated during automatic contrast injection.
In our case group analysis, CMV was not significantly correlated with the occurrence of CI-AKI, which instead was independently predicted by lower HCT and EF values, which are known risk factors, as shown by Mehran scores.2,4 EF was also an independent predictor in the study by Briguori et al.13 Our findings confirm the importance of first identifying the variables (eg, those in the Mehran or Mehran 2 scores)2,4 that classify patients at higher risk of CI-AKI to apply appropriate preventive strategies. In the present study, these patients were identified by HCT and EF and consequently the latter variables (especially EF) may be more important predictors than CMV, which is normally used during PCI in the general population. In the above-mentioned scores, CMV was also an independent predictor of CI-AKI and, consequently, using the smallest possible value of CMV is still important, especially in higher risk patients. DyeVert thus has the potential benefit of reducing CIN, depending on its efficacy compared with controls, which was evaluated in the above-mentioned meta-analysis and in the present study.
In the meta-analysis, approximately half of the studies included controls for comparison. Delivered CM was usually lower in DyeVert patients than in controls. In these cases, the difference ranged from 22 to 50 mL,12 with the highest differences being reported in the studies by Tajti et al. (200 [153-256] vs 250 [170-303] mL; P = .04) and Briguori et al. (99 ± 50 vs 130 ± 50 mL; P <.001).13,14 Delivered CMV was slightly higher (difference of 2 mL) in the DyeVert group only in the study by Bunney et al.15 The pooled analysis showed a significant decrease in delivered CMV with DyeVert use relative to the control group. Of note, details about prior CABG, CTO and left main treatment were reported only in 1 work14 and the number of vessels treated was reported only in another work.13 The treatment of bifurcations and differences in operators were not reported. All these procedural characteristics, which may influence the amount of CM delivered during PCI, were included in our study and we used a matched group with a sufficiently good balance in the studied characteristics.
In our matched group, delivered CM was lower in the case group than in the control group, but the difference was slight and nonsignificant, while total CM (also called attempted in the meta-analysis) was significantly higher in the case group than in the control group. Consequently, the net practical benefit of the device in terms of spared CM was low. In our work, procedural characteristics (eg, procedural complexity), which could cause discrepancies in CM injections, were balanced in the matched group. Based on these findings, we believe that the control group required more prolonged and/or a greater number of contrast injections (and consequently more total CM) to achieve adequate image quality. In previous studies, adequate image quality was achieved with DyeVert in 98% of cases,12 a value similar to ours, but those studies did not discuss the need for prolonged injections and more total CM compared with controls to maintain image quality when DyeVert is used. Few data are available on total CM, but previous studies reporting this information indicate that total CM was higher in DyeVert patients than in controls (Briguori et al., P-value almost significant; Kutschman et al., P-value not reported).12,13,16
The reduction in CI-AKI in the present study was not statistically significant. In the meta-analysis, the pooled relative risk for CI-AKI associated with DyeVert system use was 0.60 (95%CI, 0.40-0.90; P = .01), which was a result derived from 5 studies. Moreover, in a recent abstract not included in the meta-analysis, postprocedure eGFR values among patients undergoing coronary and/or peripheral angiography were significantly more stable in the DyeVert group than in controls.17
Analysis of the 5 above-mentioned studies separately revealed that our results are mainly in agreement; indeed, the relative risk was significantly lower in only 1 study in the nonpooled analysis.13
The type of CM was not associated with the occurrence of CI-AKI; as recommended,18 we used iso-osmolar (Iodixanol 320) or low-osmolar (Iomeprol 350 or Iohexol 350) contrast agents to prevent CIN. Given the presence of more favorable evidence,19 we preferred to use the iso-osmolar agent and reserved the other agents to low-risk patients.
Study limitations
Our study has some limitations. First, the sample size was relatively small. Second, the study design was single center, observational and retrospective, although we performed PSM to reduce potential confounding bias. Third, we excluded patients not meeting the inclusion criteria, as they were usually at low risk of CI-AKI. Therefore, our results should be generalized with caution, since the analyzed patients may be not representative of the general population. In this work the variable of sex has not been taken into account in accordance with the SAGER guidelines.
CONCLUSIONS
The DyeVert Power XT system saved 32% of CM, but only HCT and EF were independent predictors of CI-AKI and the main predictor was EF < 40%. Therefore, these variables (especially EF) may be more important than CMV, which is normally used during PCI in the general population.
PCI with this system required more total CM compared with that in controls to achieve adequate image quality. Consequently, after CM saving by the device, delivered CM was only slightly lower than CM in controls (mean difference of 15 mL) and this difference was nonsignificant. Therefore, the net practical benefit of the system was low. Equally, the reduction in CI-AKI (14.3% vs 16.3%) was not statistically significant.
Future studies are needed to confirm these results.
FUNDING
The authors did not receive any grants for this research.
ETHICAL CONSIDERATIONS
The work has been approved by an Ethics Committee/institution. Informed consent of patients was obtained and archived for the publication of their cases. In this work the variable of sex has not been taken into account in accordance with the SAGER guidelines.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
We didn’t use artificial intelligence for the development of our work.
AUTHORS’ CONTRIBUTIONS
F. Vergni, M.Arioti, and M.Leoncini contributed to the design of the work. F. Vergni, M. Arioti, V. Boasi, F.A. Sánchez, M.Leoncini, and F. Ferrari contributed to the acquisition of data. F. Vergni analyzed the data. F. Vergni, M.Arioti, V. Boasi, F.A. Sánchez, M. Leoncini, and F. Ferrari contributed to the interpretation of the data. F. Vergni and M.Arioti contributed to the drafting of the work. F. Vergni, M. Arioti, V. Boasi, F.A. Sánchez, M.Leoncini, and F. Ferrari revised the work and approved the final version to be published.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare.
What is known about the topic?
- The DyeVert Power XT system (which is used in conjunction with automated contrast injection) has been assessed in only 2 studies, which included a total of 35 patients investigated without a control group and mainly not during PCI.
What does this study add?
- Our study investigated the device in a larger population (n = 101) and during PCI. Moreover, we included a control group and performed propensity score matching to obtain a group of patients with a sufficiently good balance regarding laboratory, instrumental, clinical and procedural characteristics; in addition, among the latter features, we included the treatment of coronary bifurcations and differences between operators, which were not reported in previous studies. The above-mentioned characteristics may influence the outcome (ie, CI-AKI occurrence) and/or the volume of CM used and therefore their inclusion is important when assessing a device to spare CM.
REFERENCES
1. Lun Z, Liu L, Chen G, et al. The global incidence and mortality of contrast-associated acute kidney injury following coronary angiography:a meta-analysis of 1.2 million patients. J Nephrol. 2021;34:1479-1489.
2. Mehran R, Aymong ED, Nikolsky E, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention:development and initial validation. J Am Coll Cardiol. 2004;44:1393-1399.
3. Azzalini L, Spagnoli V, Ly HQ. Contrast-induced nephropathy:from pathophysiology to preventive strategies. Can J Cardiol. 2016;32:247-255.
4. Mehran R, Owen R, Chiarito M, et al. A contemporary simple risk score for prediction of contrast-associated acute kidney injury after percutaneous coronary intervention:derivation and validation from an observational registry. Lancet. 2021;398:1974-1983.
5. Almendarez M, Gurm HS, Mariani J Jr, et al. Procedural strategies to reduce the incidence of contrast-induced acute kidney injury during percutaneous coronary intervention. JACC Cardiovasc Interv. 2019;12:1877-1888.
6. Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87-165.
7. Desch S, Fuernau G, Pöss J, et al. Impact of a novel contrast reduction system on contrast savings in coronary angiography –the DyeVert randomised controlled trial. Int J Cardiol. 2018;257:50-53.
8. Zimin VN, Jones MR, Richmond IT, et al. A feasibility study of the DyeVerttm plus contrast reduction system to reduce contrast media volumes in percutaneous coronary procedures using optical coherence tomography. Cardiovasc Revasc Med. 2021;30:40-46.
9. Mehran R, Faggioni M, Chandrasekhar J, et al. Effect of a contrast modulation system on contrast media use and the rate of acute kidney injury after coronary angiography. JACC Cardiovasc Interv. 2018;11:1601-1610.
10. Amoroso G, Christian J, Christopher A. First European experience using a novel contrast reduction system during coronary angiography with automated contrast injection. [Abstract]. Eurointervention. 2020;16(Suppl. AC):Euro20A-POS426.
11. Bruno RR, Nia AM, Wolff G, et al. Early clinical experiences with a novel contrast volume reduction system during invasive coronary angiography. Int J Cardiol Heart Vasc. 2019;23:100377.
12. Tarantini G, Prasad A, Rathore S, et al. DyeVert Contrast Reduction System Use in Patients Undergoing Coronary and/or Peripheral Angiography:A Systematic Literature Review and Meta-Analysis. Front Med (Lausanne). 2022;9:841876.
13. Briguori C, Golino M, Porchetta N, et al. Impact of a contrast media volume control device on acute kidney injury rate in patients with acute coronary syndrome. Catheter Cardiovasc Interv. 2021;98:76-84.
14. Tajti P, Xenogiannis I, Hall A, et al. Use of the DyeVert system in chronic total occlusion percutaneous coronary intervention. J Invasive Cardiol. 2019;31:253-299.
15. Bunney R, Saenger E, Shah C, et al. Contemporary use of contrast dye reduction technology in a tertiary academic hospital:patient characteristics and acute kidney injury outcomes following percutaneous coronary interventions. In:Acc 2019. 1st Quality Summit;2019 March 13-15;New Orleans, United States.
16. Kutschman R, Davison L, Beyer J. Comprehensive clinical quality initiative for reducing acute kidney injury in at-risk patients undergoing diagnostic coronary angiogram and/or percutaneous coronary interventions. In:Scai 2019. 42nd Scientific Sessions;2019 May 19-22;Las Vegas, United States.
17. Olubowale O, Ur Rahman E, U Okoro K, et al. The DyeVert contrast reduction system and contrast induced nephropathy:is it any better?J Am Coll Cardiol. 2022;79(Suppl 9):S903.
18. Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87-165.
19. Zhao F, Lei R, Yang SK, et al. Comparative effect of iso-osmolar versus low-osmolar contrast media on the incidence of contrast-induced acute kidney injury in diabetic patients:a systematic review and meta-analysis. Cancer Imaging. 2019;19:38.
- Thermodilution assessment of vasoreactivity and microvascular function in the absence of obstructive coronary artery disease
- Design of the ROLLERCOASTR trial: rotational atherectomy, lithotripsy or laser for the management of calcified coronary stenosis
- Efficacy of virtual reality reducing anxiety during CTO revascularization: the ReViCTO trial design
- Regional differences in STEMI care in Spain. Data from the ACI-SEC Infarction Code Registry
Editorials
Expanding the role of drug-coated balloons in native large coronary artery disease
aDepartment of Biomedical Sciences, Humanitas University, Pieve Emanuele, Milan, Italy
bCardio Center, IRCCS Humanitas Research Hospital, Rozzano, Milan, Italy
cDivision of Cardiology, Montefiore Medical Center, Bronx, New York, United States
Original articles
Editorials
The role of angiography-derived physiological assessment techniques in the post-FAVOR III Europe era?
aServicio de Cardiología, Hospital General Universitario Gregorio Marañón, Instituto de Investigación Sanitaria Gregorio Marañón, Universidad Complutense de Madrid, Madrid, Spain
bCentro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), Instituto de Salud Carlos III, Madrid, Spain
Interviews
An interview with Camino Bañuelos
aServicio de Cardiología, Hospital Clínico San Carlos, Instituto de Investigación Sanitaria del Hospital Clínico San Carlos (IdISSC), Madrid, Spain
bServicio de Cardiología, Hospital Clínico Universitario de Santiago de Compostela, Santiago de Compostela, A Coruña, Spain