ABSTRACT
Introduction and objectives: This study aims to investigate if the non-invasive assessment of coronary calcium score using multislice cardiac computerized tomography (MSCT) may anticipate the need for elective rotational atherectomy (RA) during percutaneous coronary intervention.
Methods: Patients were considered eligible for the study after receiving a diagnosis of severe coronary stenosis with moderate or severely calcified plaques during index coronary angiography. Those patients underwent the Agatston coronary artery calcium (CAC) score quantification using the MSCT and then underwent percutaneous intervention. Only those lesions considered non-crossable or non-dilatable according to a pre-specified revascularization protocol were treated with RA. All operators were blinded to the MSCT results. According to the study protocol, clinical, angiographic and Agatston-related variables were included in the statistical analysis. Short and long-term outcomes were investigated in both treatment groups during follow-up.
Results: A total of 40 patients were included in the analysis: 20 underwent RA and 20 conventional percutaneous coronary interventions. Most patients were included after suffering from an acute coronary syndrome and had complex coronary anatomy (mean Syntax score, 25 points). The logistic regression analysis showed that creatinine levels and the per-lesion Agatston score were the only predictors of RA. No significant differences were observed regarding in-hospital or long-term procedural outcomes. A novel parameter, the CAC-Cre index, was found to be useful to anticipate the need for RA.
Conclusion: Coronary artery calcification analysis using the Agatston score is a simple technique that improves the non-invasive assessment of complex coronary plaques prior to percutaneous coronary intervention. The per-lesion Agatston score, serum creatinine levels, and the CAC-Cre index may become useful parameters to anticipate the need for elective RAs during percutaneous coronary intervention.
Keywords: Rotational atherectomy. Cardiac computerized tomography. Agatston. Calcium score. Calcified coronary lesions.
RESUMEN
Introducción y objetivos: El objetivo del estudio fue investigar si la evaluación no invasiva del índice de calcificación coronaria mediante tomografía computarizada cardiaca multidetector (TCMD) puede predecir la necesidad de una aterectomía rotacional (AR) electiva durante la intervención coronaria percutánea.
Métodos: Se incluyeron pacientes diagnosticados de estenosis coronaria grave con placas moderadamente o gravemente calcificadas durante la angiografía coronaria. Esos pacientes se sometieron a la cuantificación del índice de calcificación coronaria con la escala de Agatston utilizando TCMD y posteriormente a intervención percutánea. Solo fueron tratadas con AR las lesiones que se consideraba que no era posible cruzar ni dilatar, según un protocolo de revascularización prediseñado. Ninguno de los operadores conocía de antemano los resultados de la TCMD. Según el protocolo del estudio, en el análisis estadístico se incluyeron variables clínicas, angiográficas y relacionadas con la puntuación Agatston. Durante el seguimiento se estudiaron los resultados a corto y largo plazo en ambos grupos.
Resultados: Se analizaron 40 pacientes: 20 que recibieron AR y 20 con intervención coronaria percutánea convencional. La mayoría se incluyó después de un síndrome coronario agudo y tenían una anatomía coronaria compleja (puntuación media de la escala Syntax de 25 puntos). La creatinina y la puntuación de Agatston por lesión fueron los únicos factores predictivos de la AR. No se observaron diferencias significativas en el pronóstico dentro del hospital o a largo plazo. Un nuevo parámetro, el índice CAC-Cre, fue útil para predecir la necesidad de AR.
Conclusion: El análisis de la calcificación de las arterias coronarias mediante la puntuación de Agatston mejora la evaluación no invasiva de las placas coronarias complejas antes de la intervención coronaria percutánea. La puntuación de Agatston por lesión, la creatinina sérica y el índice CAC-Cre son parámetros útiles para predecir la necesidad de una AR electiva durante la intervención coronaria percutánea.
Palabras clave: Aterectomía rotacional. Tomografía computarizada cardiaca. Agatston. Índice de calcificación coronaria. Lesiones coronarias calcificadas.
Abbreviations: CAC: coronary artery calcium. MSCT: multislice cardiac computerized tomography. PCI: percutaneous coronary intervention. RA: rotational atherectomy.
INTRODUCTION
Coronary artery calcium (CAC) is a key marker of coronary artery disease and one of the most robust predictors of cardiovascular adverse events in different populations. Its prevalence increases with age and affects a large percentage of patients over 60 years of age.1
Increased life expectancy in developed countries has led interventional cardiologists to frequently face complex calcified lesions in patients undergoing percutaneous coronary interventions (PCI). This situation remains a challenging scenario due to lower success rate, higher risk of periprocedural complications and need for repeated revascularizations. Occasionally, plaque modification techniques such as rotational atherectomy (RA) are needed to obtain adequate stent expansion and apposition in severely calcified plaques, and they may improve angiographic and clinical outcomes in selected patients. However, the use of RA as a bailout technique may increase procedural time, the amount of contrast media and the incidence of procedural complications. Besides, the assessment of calcification using fluoroscopy only during the coronary angiography has significant limitations and cannot make reliable predictions on what lesions require RA during the intervention.
On the other hand, multislice cardiac computerized tomography (MSCT) improves the non-invasive assessment of coronary calcified lesions. The coronary artery calcium score analysis using MSCT has been related not only to the extension, complexity and severity of the obstructive coronary artery disease, but also to the risk of periprocedural complications after PCI.2,3
The primary objective of this study was to investigate whether accurate quantifications of CAC using MSCT may be useful to anticipate the need for RA during PCI due to calcified coronary lesions. Secondary objectives included the analysis of in-hospital and long-term outcomes.
METHODS
Study patients
Prospective, non-randomized, single-center study at a tertiary cardiac center that performs over 1100 PCI and 10-15 RA procedures per year. Between January 2011 and December 2013, patients undergoing coronary angiography who showed calcified obstructive coronary disease and were considered suitable to undergo PCI were screened to enter the study. All patients who had undergone coronary computerized tomography (CT) scans in the past, with all the inclusion criteria and without any exclusion criteria were enrolled in the present study (table 1 of the supplementary data). The exclusion criteria were: ST-segment elevation acute coronary syndrome within 7 days, previous PCI within 2 months, hemodynamic instability and total coronary occlusions. All the patients included gave their written informed consent, and the protocol was approved by the local ethics committee.
Table 1. Baseline clinical and angiographic characteristics
| RA Group | PCI Group | P | |
|---|---|---|---|
| Age | 72.4 ± 10.6 | 72.8 ± 10.2 | .91 |
| Men | 16 (80%) | 15 (75%) | .70 |
| BMI | 26.7 ± 4.8 | 26.6 ± 4.3 | .96 |
| Hypertension | 14 (70%) | 16 (80%) | .46 |
| Dyslipidemia | 12 (60%) | 17 (85%) | .07 |
| DM | 9 (45%) | 8 (40%) | .93 |
| Current smoker | 12 (60%) | 11 (55%) | .74 |
| Creatinine levels (mg/dL) | 1.64 ± 1.48 | 0.96 ± 0.23 | .05 |
| STEMI | 3 (15%) | 1 (5%) | .5 |
| NSTEMI | 10 (50%) | 10 (50%) | .5 |
| Stable angina | 7 (35%) | 9 (45%) | .5 |
| Previous MI | 4 (20%) | 1 (5%) | .25 |
| Previous PCI | 4 (20%) | 2 (10%) | .25 |
| Previous CABG | 0 (0%) | 1 (5%) | .25 |
| LM disease | 1 (5%) | 2 (10%) | .5 |
| Multivessel disease | 16 (80%) | 19 (95%) | .26 |
| EF < 50% | 2 (10%) | 6 (30%) | .28 |
| Multi-lesion PCI | 15 (75%) | 19 (95%) | .077 |
| New oral antiplatelet agents | 3 (15%) | 1 (5%) | .48 |
| SYNTAX observer A | 25.8 ± 15.7 | 24.4 ± 9.3 | .73 |
| SYNTAX observer B | 26.8 ± 17.2 | 24.4 ± 11.8 | .61 |
|
BMI, body mass index; CABG, coronary artery bypass graft surgery; DM, diabetes mellitus; EF, ejection fraction; LM, left main coronary artery; MI, myocardial infarction; NSTEMI, non-ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction. |
|||
Coronary angiography
Coronary angiography was performed based on our institutional protocol and the indication for PCI was based on clinical criteria. Both the SYNTAX score4 and coronary calcification were independently evaluated by 2 different experienced interventional cardiologists using at least 2 orthogonal fluoroscopic projections. Calcification was defined as an evident density within the arterial wall, visualized in fluoroscopy as a more radiopaque area. The degree of calcification was as follows: 1) moderate: radiopacities noted only during the cardiac cycle before contrast injection; 2) severe: radiopacities noted without cardiac motion before contrast injection usually involving both sides of the arterial lumen.5
Multislice cardiac computerized tomography
The MSCT was performed after the index coronary angiography and prior to the PCI procedure. All operators performing PCI were blinded to the MSCT results. CAC and non-contrast-enhanced coronary CT angiography data sets were acquired using a 64-slice single-source CT system (Aquilion-Toshiba, Medical systems corporation, Otawa, Japan). In order to quantify coronary calcification, the Agatston score was determined using the Vitrea 2 workstation (Vital Images Inc, Plymouth, MN, United States). Collimation was 4 x 3 mm; rotation time was 250 mseg; tube voltage 120 Kv; effective tube current 300 mA. Raw data from the CT scan were reconstructed using algorithms optimized for retrospectively ECG-gated segmental reconstruction with 2 mm slices thickness and at an increment of 2 mm.
Agatston score
The extent of calcification was measured individually for each patient (total calcium score), vessel (per-vessel calcium score), and segment lesion (per-lesion calcium score). Coronary calcium was defined as any plaque of at least 3 contiguous pixels with a density > 130 Hounsfield units. Per-lesion calcium scores were estimated by multiplying the target lesion area by a density factor derived from the maximal Hounsfield units within this area, and as described by Agatston.6 The Bypass Angioplasty Revascularization Investigation (BARI) nomenclature endorsed by 2018 ESC/EACTS Guidelines on myocardial revascularization was used to describe the specific anatomical location of a given coronary lesion.7,8
PCI
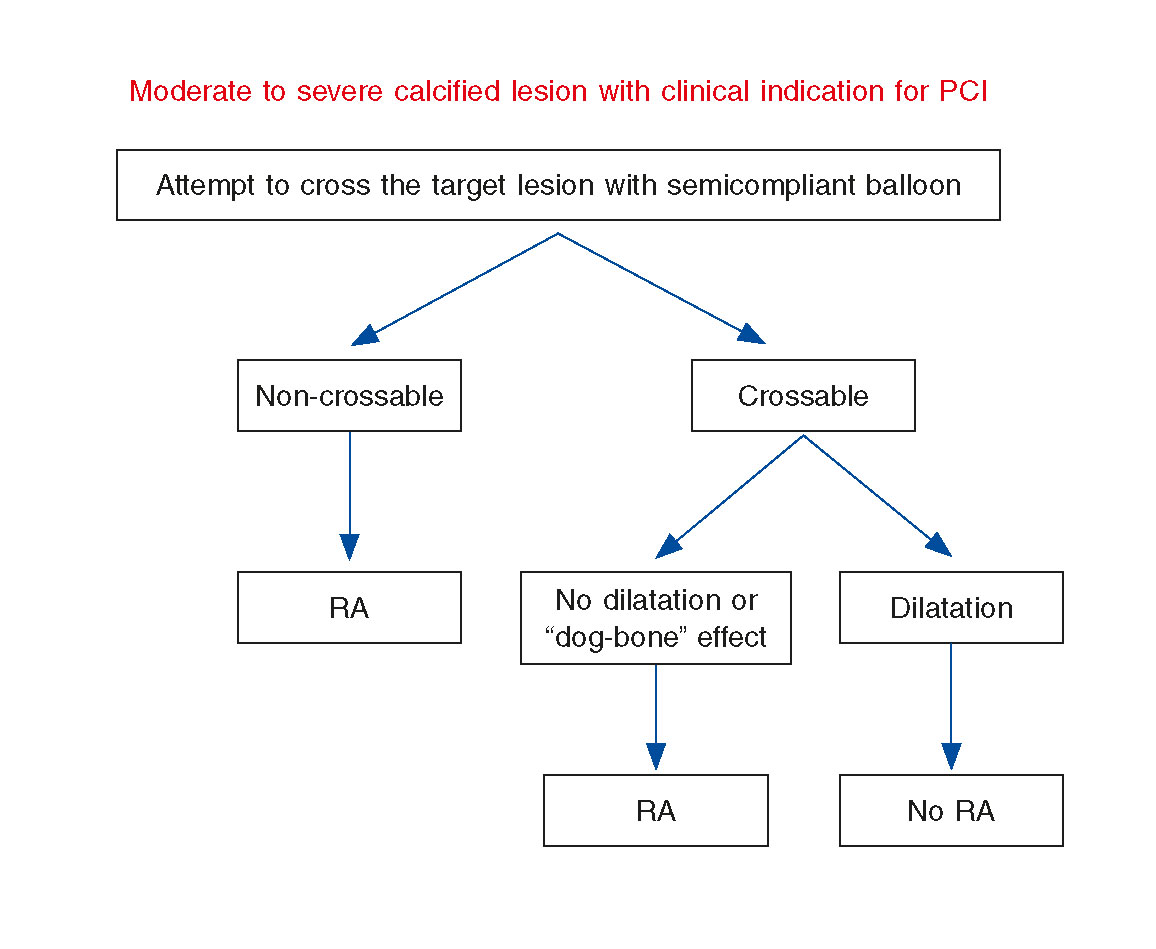
According to the previously designed protocol, PCI was performed after MSCT, and all operators were blinded to the study results. Femoral access was considered the preferred artery approach, 7-8 F guiding catheters were used, and intravenous heparin was administered to maintain an activated clotting time ≥ 250 ms. All patients received dual antiplatelet therapy with aspirin and a P2Y12 inhibitor (clopidogrel, ticagrelor, or prasugrel) according to the recommendations established by the clinical practice guidelines. Hydrophilic wires were used to cross the target lesion and predilation with a semi-compliant balloon up to 16 atm (burst rupture pressure) was performed. Non-compliant balloons were not used as most of them had worse crossing profile than semi-compliant at the time of our study. A specific balloon catheter of 15-20 mm in length was selected to meet a ratio of 0.8-1 with the reference diameter vessel by visual estimation. When the balloon did not cross the lesion, it was considered non-crossable. If the ratio between the minimal balloon diameter at 16 atm and the nominal balloon diameter was less than 80%, then the lesion was considered non-dilatable. It is important to explain that currently there is not such a thing as a clear-cut definition of non-dilatable coronary lesions. All non-crossable and non-dilatable lesions underwent RA, and the remaining ones were treated with conventional PCI. Therefore, 2 groups were established, and pre-specified variables were compared (figure 1). Routine use of intravascular ultrasound (IVUS) was not performed in this study due to the difficulties experienced when crossing these types of lesions.
Figure 1. Study revascularization protocol. Lesions were considered non-crossable if a semicompliant dilatation balloon could not pass through them. If the ratio between the minimal balloon diameter at 16 atm and the nominal balloon diameter was less than 80%, the lesion was considered non-dilatable. All non-crossable and non-dilatable lesions underwent rotational atherectomy, and the remaining ones were treated through conventional percutaneous coronary intervention. RA, rotational atherectomy.
Rotablation was performed using the Rotablator (Boston Scientific Corporation; Natick, MA, United States) and burr sizes from 1.25-2.5 mm. The burr size was selected to reach a burr/vessel ratio of 0.7. The recommended burr speed was 165 000-200 000 rpm with each sequence being less than 15 seconds, and care was taken to prevent any drops in the rotational speed > 5000 rpm. Tem- porary pacing was used in cases of rotablation of right coronary artery and dominant left circumflex.
Angiographic success was defined as adequate release and expansion of the stent with a residual stenosis of less than 20% of the target lesion in the presence of Thrombolysis in Myocardial Infarction (TIMI) flow grade 3; non-success was defined as an non-crossable injury or loss of the stent and other complications like dissection, perforation or no reflow.
Follow-up and endpoints
Troponin I levels were obtained 8-10 hours after the intervention and an ECG was performed in all patients the day after the procedure. The primary endpoint was defined as the need to perform RA during the revascularization of target lesions. Secondary endpoints included the incidence of cardiac and non-cardiac events during the index hospitalization and long-term follow up. Follow-up data were collected by phone or using central databases. Death was defined as all-cause mortality. Myocardial infarction was defined as chest pain or other clinical data consistent with myocardial ischemia, new pathologic Q waves in 2 or more contiguous leads or elevated troponin levels 5 times the normal values after the procedure. Target vessel revascularization was defined as either repeated percutaneous or surgical revascularization of the treated vessel, and target lesion revascularization as any reintervention anywhere within the stent implanted during the index procedure, or on the 5 mm proximal or distal edges of the stents. Stent thrombosis was defined following the criteria developed by the Academic Research Consortium.9
Statistical analysis
Categorical variables were expressed as absolute and relative frequencies and compared using the Chi-square test or Fisher’s exact test. Continuous variables were expressed as mean ± standard deviation or, when not normally distributed, as median and interquartile range. The differences among the continuous variables were analyzed using the Student t-test or the Kruskal-Wallis method, respectively. The level of inter-observer agreement was assessed using the Kappa and Phi coefficients. Forward stepwise logistic regression analysis was used to select candidate variables that improved the prediction of RA during PCI, with a statistically significant P value of .05. Receiver operating characteristic (ROC) curve analysis was performed to estimate the sensibility and specificity of the different cut-off points provided by the variables obtained through logistic regression.
RESULTS
Baseline clinical and angiographic characteristics
A total of 40 patients (77.5 % male, 72 ± 10.3 years) were included in the study. The most common indications for index coronary angiography were non-ST-segment elevation acute coronary syndromes (50%) and stable angina with a positive stress test (40%).
The baseline clinical characteristics are shown in table 1. There were no significant differences in the demographic characteristics or antithrombotic regimens between both arms, although there was a trend towards a higher rate of dyslipidemia in the PCI group (P = .077) and worse renal function in the RA group (P = .05).
The Syntax score was high in both treatment groups, without any significant differences between them and a good correlation between the 2 observers (Phi coefficient 0.83, P = .001).
Multislice cardiac computerized tomography
Agatston score was over 3000 in both arms, with no statistically significant differences (P = .24). However, the per-vessel and per-lesion Agatston scores were significantly higher in the RA group (table 2).
Table 2. Coronary artery calcium analysis using Agatston score
| RA Group | PCI Group | P | |
|---|---|---|---|
| Total Agatston score | 3772.0 ± 2154.7 | 3040.4 ± 1693.8 | .240 |
| Per-vessel Agatston score | 1628.5 ± 1142.8 | 833.2 ± 466.0 | .008 |
| Per-lesion Agatston score | 864.1 ± 471.0 | 458.4 ± 360.3 | .004 |
|
PCI, percutaneous coronary intervention; RA, rotational atherectomy. |
|||
Regarding the anatomical distribution of calcium, the vessel with a higher Agatston score was the right coronary artery, showing homogeneous calcification between the proximal and distal segments. The left anterior descending artery was the second most calcified blood vessel, especially at its proximal and middle segments. The circumflex artery had the lowest Agatston score.
Procedural details and outcomes
Procedural details are shown in table 3. Percutaneous access occurred through the femoral artery in 37 patients (92.5%). In 29 patients (72.5%), the target lesion could be crossed by the dilation balloon. Among these 29 crossable lesions, 9 (31%) showed the “dog bone” effect during balloon inflation and could not be dilated. Therefore, according to the study protocol, 20 patients (50%) underwent conventional PCI and the other 20 (50%) RA.
Table 3. Angiographic and procedural characteristics
| RA Group | PCI Group | P | |
|---|---|---|---|
| Location | .17 | ||
| LMCA | 0 | 1 | |
| Proximal LAD | 10 | 6 | |
| Mid LAD | 2 | 8 | |
| Proximal LCx | 3 | 4 | |
| Proximal RCA | 2 | 1 | |
| Mid RCA | 2 | 0 | |
| Ramus Intermedius | 1 | 0 | |
| RVD (mm) | 2.96 ± 0.43 | 2.91 ± 0.26 | .68 |
| Lesion length (mm) | 44.85 ± 17.84 | 41.25 ± 24.13 | .59 |
| Diameter stenosis, % | 79.2 ± 7.9 | 73.0 ± 9.2 | .028 |
| Bifurcation | 6 (46.2%) | 7 (53.8%) | .73 |
| Maximum burr size (mm) | 1.45 ± 0.15 | ||
| No. of stents/lesion | 1.84 ± 0.60 | 2.05 ± 0.89 | .40 |
| Contrast media (mL) | 312.0 ± 96.7 | 239.0 ± 66.5 | .018 |
| Contrast-induced nephropathy | 4 | 0 | .035 |
| Dissections | 2 | 2 | .36 |
| Perforations | 1 | 0 | .56 |
| Intraprocedural major complications | 0 | 0 | > .99 |
| Angiographic success | 90% | 100% | .14 |
| Death | 0 | 0 | > .99 |
| Target vessel re-PCI | 1 | 1 | > .90 |
| Myocardial infarction | 1 | 0 | .31 |
| Access site complications | 2 | 0 | .34 |
|
LAD, left anterior descending artery; LCx, left circumflex artery; LMCA, left main coronary artery; PCI, percutaneous coronary intervention; RA, rotational atherectomy; RCA, right coronary artery; RVD, reference vessel diameter. |
|||
The mean balloon size and length was similar in both arms. Among patients undergoing RA, a single burr was used in most lesions (95%) with a mean burr size of 1.45 ± 0.15 mm. In the entire study population, the most frequently treated artery was the left anterior descending artery (65%); 32.5% of the target lesions were bifurcations; 35 patients (87.5%) had multivessel disease, and 34 (85%) required intervention in more than one major coronary artery. No differences were seen on the target lesion treated no in the number of bifurcation lesions between both groups.
Angiographic success rate was 90% in the RA arm and 100% in the PCI arm, without any significant differences between groups. Coronary dissections, perforations, and no-/slow-flow phenomena were rare and occurred equally in both groups. A significantly larger contrast volume was used in the RA group compared to conventional PCI group.
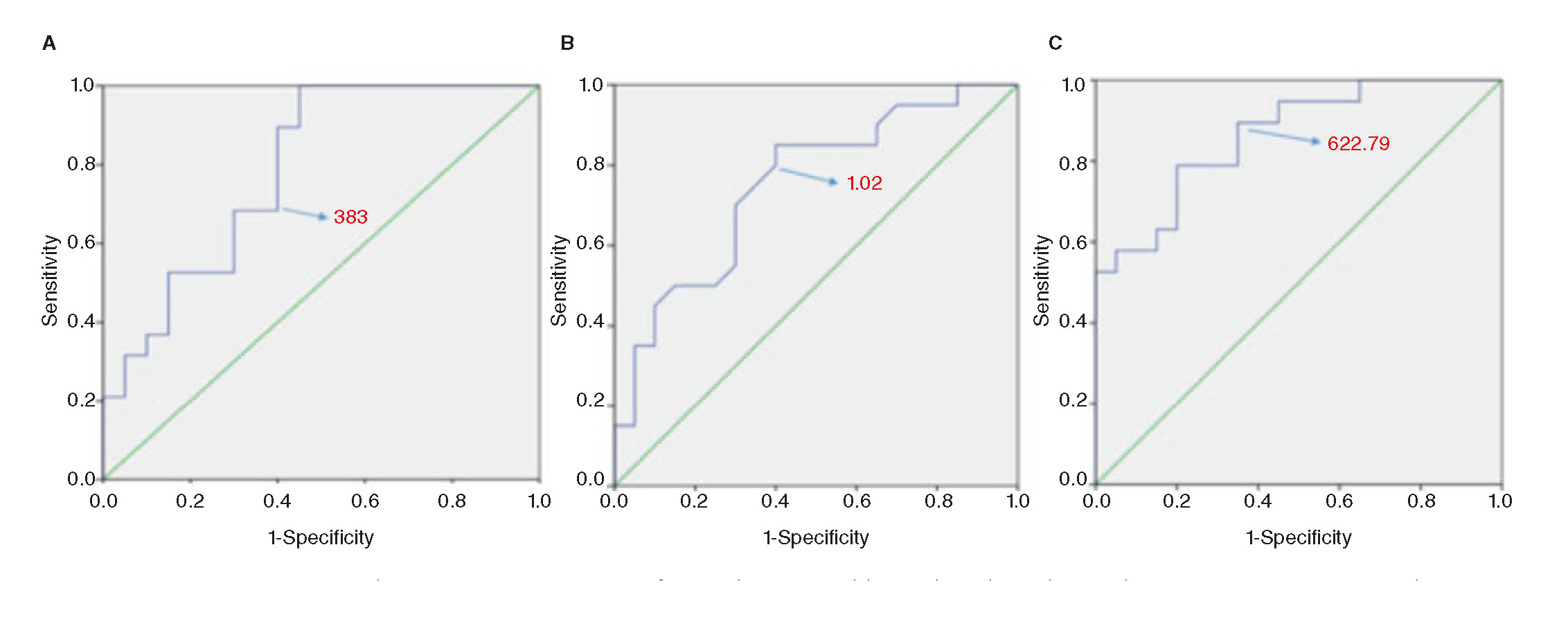
Stepwise logistic regression analysis showed that creatinine and per-lesion Agatston were the only predictors of RA. For every 0.1mg/dL increase in creatinine level, the probability of RA increased 48%. On the other hand, every 100 point increment in per-lesion Agatston score increased the probability of RA in 22%. Using the optimal cut-off value from ROC analysis (figure 2A), a per-lesion Agatston score of 383 resulted in a sensitivity of 89.5% and specificity of 60% (area under the curve, 0.79). ROC curve for serum creatinine level (figure 2B) showed a sensitivity of 75% and a specificity of 65% for an optimal cut-off point of 1.02 mg/dL (area under the curve, 0.75). Given the association of both variables with the use of RA, we created a combined index of creatinine (Cre) and per-lesion Agatston score (CAC-Cre index), obtained by multiplying the creatinine levels and the per-lesion Agatston score. The ROC analysis of CAC-Cre index (figure 2C) demonstrated better area under the curve (0.86), being 622.79 the value with the best sensitivity (78.9%) and specificity (80%).
Figure 2. Receiver operating characteristic curve (ROC) for predicting rotablation based on the per-lesion Agatston score (A), the serum creatinine levels (B) and the CAC-Cre index (C). The optimal thresholds for predicting rotablation were 383 per-lesion Agatston points, creatinine levels of 1.02 mg/dL, and a 622.79 CAC-Cre index, respectively.
In-hospital outcomes and long-term follow-up
There were no deaths during hospitalization. Two patients (1 in the RA group and 1 in the PCI group) underwent target vessel revascularization during the index admission. Only 1 patient, included in the RA group, experienced a protocol-defined MI. Complications in the access site were numerically higher in the RA group, without any significant differences. One patient of the PCI group required one intra-aortic balloon pump. On the other hand, the RA group showed a higher incidence of contrast-induced nephropathy (P = .035), possibly due to worse baseline renal function.
There were no differences between the 2 treatment groups regarding major cardiac events at the end of the follow up (4.1 ± 2.2 years) (table 4); with an overall mortality of 12 patients (30%) and 7 cardiovascular deaths (58.3%). Other cardiovascular events such as myocardial infarctions, target-vessel revascularizations and non-target vessel revascularizations, were statistically not-significant. None of the baseline clinical or angiographic variables included in the analysis was associated with the occurrence of major events at follow-up.
Table 4. Major adverse cardiac events during follow-up
| RA Group | PCI Group | P | |
|---|---|---|---|
| Death | 6 (30%) | 6 (30%) | .59 |
| MI | 3 (15%) | 5 (25%) | .34 |
| TLR | 1 (5%) | 3 (15%) | .3 |
| NTLR | 4 (20%) | 5 (25%) | .5 |
|
MI, myocardial infarction; NTLR, non-target lession revascularization; PCI, percutaneous coronary intervention; RA, rotational atherectomy; TLR, target lesion revascularization. |
|||
Regarding the analysis of coronary calcium using the MSCT, a statistically significant correlation was observed between long-term mortality and the total Agatston score (P = .005).
DISCUSSION
Severe coronary artery calcification remains a major challenge in contemporary interventional cardiology. It reduces the chances of angiographic success, and significantly increases the rate of procedural complications.10 The stent underexpansion or asymmetric expansion and malapposition are frequently observed in very calcified plaques; this results in a significantly greater incidence of restenosis and stent thrombosis.11 It is in this context that RA may be useful.12,13
In order to implement the most appropriate revascularization strategy, there is a growing interest in the non-invasive assessment of complex coronary lesions that may benefit from plaque modification techniques.15,16 If we were able to anticipate what patients will require elective RA, we would not have to use this technique as a bailout strategy, thus reducing procedural time, use of contrast media, and the number of ischemic complications.
Fluoroscopy is not useful to adequately quantify coronary calcium, because of its limited sensitivity and significant intra- and interobserver variability, and it has not proven useful either to anticipate the need for RA. Intravascular ultrasound improves the assessment of coronary plaques, providing an accurate evaluation of the amount of calcium in the arterial wall.17 However, it cannot adequately characterize calcium itself, limiting its ability to anticipate the response of a given plaque to balloon catheter dilatation. Another limitation of intravascular ultrasound is the inability to cross many complex lesions with the ultrasound catheter. Although some operators use this situation as a criterion for using RA, there is no clinical evidence that supports such a practice.
In this study we have seen that the Agatston score improves the identification of patients who would benefit from a plaque modification strategy with elective RA. The Agatston score analysis is a sensitive, reproducible and widely available technique that may improve the interventional management of patients with complex coronary lesions. As far as we know, there is only another study that has tested this hypothesis, although with a different methodology. Sekimoto et al.18 studied patients with chronic stable angina who underwent non-invasive angiography and coronary calcium quantification by CT prior to cardiac catheterization. In this study, the decision to perform RA was entirely left to the discretion of the interventional cardiologist.
Our work tried to investigate the use of these parameters both in stable ischemic heart disease and patients after an acute coronary syndrome, selecting a population with significant coronary calcification and high pre-test chances to have calcified circumferential lesions,17 that may perhaps be better treated with RA as the first-line proactive therapy.
Also, our study was designed in such a way that the decision to perform RA was not left at the discretion of the operator, but dictated by the formal prospective protocol. Only non-crossable or non-dilatable lesions with a balloon catheter were treated with RA, which is strictly in accordance with the clinical practice guidelines. This strategy also limits the disparity of criteria among different operators, providing a greater consistency to the study results.
We decided not to perform CT angiography because of its limitations in adequately characterizing the degree of stenosis in patients with significant calcification and also to avoid the use of unnecessary radiation and contrast. Regarding the Agatston calcium score, we selected 3 parameters: global, per-treated vessel and per-lesion or segment. In our study that included patients with complex coronary anatomy, global Agatston values were above 3000 in both treatment arms, with no significant differences between the 2 groups. As in the Japanese study,18 significantly higher values of per-vessel and per-lesion Agatston score were observed in patients who underwent rotational atherectomy. After logistic regression analysis, only the per-lesion score turned out to be an independent predictor of the need for RA. A per-lesion Agatstson score of 383 was the optimal cut-off value determined by the ROC analysis, relatively close to that described by Sekimoto et al.18 An analytical variable, serum creatinine, also turned out to be an independent predictor of RA (chronic renal failure was also significantly higher in the AR group, indicative of a clear clinical association between chronic renal failure and the percentage of intracoronary calcium; something already confirmed in the past). However, ROC curve analysis showed that its use resulted in an optimal classification of the patients. Additionally, we combined both predictors (per-lesion Agatston and serum creatinine) to create an index that would improve the prediction of RA in our patients, being 622.79 the value with the best sensitivity and specificity rate. Therefore, the CAC-Cre index may become useful in the decision-making process at the the cath lab.
Lesion length is one of the characteristics included in the Syntax score that increases complexity during PCI. Sekimoto et al. found that the length of the lesion was significantly associated with the use of RA during the revascularization procedure. In our series, in which the decision to perform rotablation was made following a strict protocol, we found no significant correlations between lesion length and the need for RA. These results are consistent with the findings of Dill et al., who did not observe a significant benefit from routine RA compared to simple angioplasty in patients with complex coronary disease and longer lesions.19
Baseline characteristics were well balanced between both treatment arms, both clinically and in terms of the complexity reflected in the Syntax score. The population included is representative of a subset of patients with severe coronary artery disease who are eligible for PCI in contemporary cardiology centers. In this group, RA showed good angiographic results, with no significant differences between groups during follow-up. Long term incidence of major adverse cardiovascular events was high, but similar to that described by other groups in patients with a similar risk profile.20,21
LIMITATIONS
This is an observational, non-randomized protocol with the corres-ponding limitations of its specific study design. The number of patients included was small, but provided useful information to plan interventions of complex lesions. Defining a lesion as non-dilatable or non-crossable may have a significant component of operator-dependency, and currently there is not such a thing as a clear-cut definition of non-dilatable coronary lesions. Thus, our protocol was designed to be straightforward in order to follow the actual clinical practice guidelines and reduce the inter-operator variability. On the other hand, although the Agatston calcium score is a common technique used in cardiac imaging units all over the world, the per-vessel and per-lesion assessment may require additional time and experienced staff. Selection bias may occur in patients without a CT study during the recruitment period. Also, the fact that this technique is not an indication after performing a diagnostic angiography involves a low use of this technique in the routine clinical practice.
CONCLUSIONS
Coronary artery calcification analysis using Agatston coronary calcium score is a simple technique that improves the non-invasive assessment of complex coronary plaques prior to PCI. The per-lesion Agatston score and the serum creatinine levels may be useful indicators to anticipate the need for elective rotational atherectomy during PCI. A new parameter created by combining both variables, the CAC-Cre index, improved even more the prediction of RA during PCI. A prospective study is needed to validate this index.
CONFLICTS OF INTEREST
R. Moreno is associate editor of REC: Interventional Cardiology. No other conflicts of interest were declared by the authors. The journal’s editorial procedure to ensure impartial handling of the manuscript has been followed.
WHAT IS KNOWN ABOUT THE TOPIC?
- The percutaneous treatment of moderate-to-severely calcified coronary lesions remains a challenge for contemporary interventional cardiologists since choosing the wrong management strategy may lead to severe complications.
- RA is a useful technique that may improve outcomes in non-crossable or non-dilatable coronary lesions, yet its results are not optimal when used as a bailout strategy
- The MSCT-determined Agatston score is the most useful technique for the quantitative assessment of coronary calcium. There is limited information on its role as a predictive tool for the assessment of a particular coronary plaque as non-crossable or non-dilatable.
- To our knowledge, the combination of a clinical variable such as creatinine levels with a CAC variable to create an index to anticipate the need of RA has not previously been reported.
WHAT DOES THIS STUDY ADD?
- In this study we have seen that the Agatston score improves the identification of patients who would benefit from a plaque modification strategy with elective RA.
- A per-lesion Agatstson score of 383 and the serum creatinine levels are independent predictors of RA. We combined both predictors to create an index that improved the prediction of RA in our patients (CAC-Crex index), being 622.7 the value with the best sensitivity and specificity rate..
REFERENCES
1. Wexler L, Brundage B, Crouse J, et al. Coronary Artery Calcification:Pathophysiology, Epidemiology, Imaging Methods, and Clinical Implications. Circulation. 1996;94:1175-1192.
2. Wang X, Liu X, Ge H, et al. Positive association of coronary calcium detected by computed tomography coronary angiography with periprocedural myocardial infarction. PLoS One. 2013;8:8-13.
3. Généreux P, Madhavan MV, Mintz GS, et al. Relation between coronary calcium and major bleeding after percutaneous coronary intervention in acute coronary syndromes (from the Acute Catheterization and Urgent Intervention Triage Strategy and Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction Trials). Am J Cardiol. 2014;113:930-935.
4. Sianos G, Morel MA, Kappetein AP, et al. The SYNTAX score:an angiographic tool grading the complexity of coronary artery disease. EuroIntervention. 2005;1:219-27.
5. Abdel-Wahab M, Richardt G, Joachim Büttner H, et al. High-speed rotational atherectomy before paclitaxel-eluting stent implantation in complex calcified coronary lesions:The randomized ROTAXUS (Rotational Atherectomy Prior to Taxus Stent Treatment for Complex Native Coronary Artery Disease) trial. JACC Cardiovasc Interv. 2013;6:10-19.
6. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827-832.
7. Alderman EL, Stadius M. The angiographic definitions of the Bypass Angioplasty Revascularization Investigation study (BARI). Coron Artery Dis. 1992;3:1189–1207.
8. Nuemann FJ, Sousa Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines of myocardial revascularization. Eur Heart J. 2019;40:86-165.
9. Mauri L, Hsieh W, Massaro JM, Ho KKL, D'Agostino R, Cutlip DE. Stent thrombosis in randomized clinical trials of drug-eluting stents. N Engl J Med. 2007;356:1020-1029.
10. Savage MP, Goldberg S, Hirshfeld JW, et al. Clinical and angiographic determinants of primary coronary angioplasty success. J Am Coll Cardiol. 1991;17:22-28.
11. Liu X, Doi H, Maehara A, et al. A Volumetric Intravascular Ultrasound Comparison of Early Drug-Eluting Stent Thrombosis Versus Restenosis. JACC Cardiovasc Interv. 2009;2:428-434.
12. Moussa I, Di Mario C, Moses J, et al. Coronary Stenting After Rotational Atherectomy in Calcified and Complex Lesions. Circulation. 1997;96:128-136.
13. Vaquerizo B, Serra A, Miranda F, et al. Aggressive plaque modification with rotational atherectomy and/or cutting balloon before drug-eluting stent implantation for the treatment of calcified coronary lesions. J Interv Cardiol. 2010;23:240-248.
14. Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011;124:e574-651.
15. Hoffmann U, Moselewski F, Nieman K, et al. Noninvasive Assessment of Plaque Morphology and Composition in Culprit and Stable Lesions in Acute Coronary Syndrome and Stable Lesions in Stable Angina by Multidetector Computed Tomography. J Am Coll Cardiol. 2006;47:1655-1662.
16. Estevez-Loureiro R, Ghione M, Kilickesmez K, Agudo P, Lindsay A, Di Mario C. The Role for Adjunctive Image in Pre-procedural Assessment and Peri-Procedural Management in Chronic Total Occlusion Recanalisation. Curr Cardiol Rev. 2014;10:120-126.
17. Tuzcu EM, Berkalp B, De Franco AC, et al. The dilemma of diagnosing coronary calcification:Angiography versus intravascular ultrasound. J Am Coll Cardiol. 1996;27:832-838.
18. Sekimoto T, Akutsu Y, Hamazaki Y, et al. Regional calcified plaque score evaluated by multidetector computed tomography for predicting the addition of rotational atherectomy during percutaneous coronary intervention. J Cardiovasc Comput Tomogr. 2016;10:221-8.
19. Dill T, Dietz U, Hamm CW, et al. A randomized comparison of balloon angioplasty versus rotational atherectomy in complex coronary lesions (COBRA study). Eur Heart J. 2000;21:1759-1766.
20. Édes IF, Ruzsa Z, SzabóG, et al. Clinical predictors of mortality following rotational atherectomy and stent implantation in high-risk patients:A single center experience. Catheter Cardiovasc Interv. 2015;86:634-641.
21. Tohamy A, Klomp M, Putter H, et al. Very Long-Term Follow-Up After Coronary Rotational Atherectomy:A Single-Center Experience. Angiology. 2016;68:519-527.