ABSTRACT
Introduction and objectives: Coronary in-stent restenosis (ISR) is associated with a high target lesion revascularization rate, while the drug-eluting balloon (DEB) presents IA class level of evidence for its treatment. Nevertheless, very long-term outcomes of DEB for ISR in non-selected populations of patients are unknown. Our goal is to evaluate the very long-term (5 year) effectiveness of DEBs in a real-world registry.
Methods: Retrospective registry from an ISR cohort treated with DEB. The primary outcome was the rate of target lesion revascularization (TLR) at 5 years. Secondary outcomes were evaluated according to the ARC-2 criteria.
Results: From January 2010 through December 2013, 53 ISRs were treated using DEBs in 48 patients. Patients were old (69.3 ± 11.8 years-old) and 55.8% had diabetes. The rate of TLR at 1 year was 9.4%, and 20.8% at 3 and 5 years, respectively. The rate of late TLR (after the first year) was 11.4%, only after DEB for bare metal ISR. The 5-year TLR was not associated with diabetes (22.7% vs 19.2%; P = .76) and was not significantly lower after cutting-balloon (12.5% vs 24.3%; P = .47) or in bare-metal stent ISR (20.6% vs 21.1%; P = .96). There was no definite/probable stent thrombosis of the lesions treated with DEB at follow-up.
Conclusions: In a real-world cohort, the 5-year TLR rate after DEB for ISR was 20.8%. Late TLR accounted for half of the TLR at follow-up (after DEB for bare metal ISR), while the rate of TLR seemed to stabilize at 3 years. There was no stent thrombosis of the lesions treated with DEB.
Keywords: Drug-eluting balloon. In-stent restenosis. Target lesion revascularization.
RESUMEN
Introducción y objetivos: La reestenosis de stents coronarios (RS) presentan altas tasas de necesidad de revascularización, y el balón farmacoactivo (BFA) presenta clase I (nivel de evidencia A) en su tratamiento. La eficacia de esta estrategia a muy largo plazo en pacientes no seleccionados es desconocida. Se pretende evaluar la eficacia del BFA en un registro de pacientes de la práctica clínica a muy largo plazo de seguimiento (5 años).
Métodos: Registro retrospectivo de una cohorte formada por pacientes con ISR tratados con BFA. El evento primario fue la tasa de revascularización de la lesión tratada (RLT) con BFA a 5 años. Se valoraron eventos secundarios según los criterios Academic Research Consortium-2.
Resultados: Entre enero de 2010 y diciembre de 2013 se usó BFA de forma eficaz en 53 RS de 48 pacientes. Los pacientes presentaban edad avanzada (69,3 ± 11,8 años) y alta prevalencia de diabetes (55,8%). La tasa de RLT a 1 año fue del 9,4%, y del 20,8% a los 3 y 5 años. La tasa de RLT tardía (más allá del año de seguimiento) fue del 11,4%, tan solo en reestenosis de stent convencional. La RLT a 5 años no se asoció a diabetes (22,7 frente a 19,2%; p = 0,76) ni fue significativamente menor con el uso de balón de corte (12,5 frente a 24,3%; p = 0,47) o en reestenosis de stent convencional (20,6 frente a 21,1%; p = 0,96). No hubo casos de trombosis de stent definitiva/probable de la lesión tratada con BFA.
Conclusiones: En una cohorte de la práctica clínica, el BFA para RS presenta una RLT a 5 años del 20,8%. La RLT tardía supone la mitad de los casos a lo largo del seguimiento, y se produce en RS convencional. La tasa de TLR parece estabilizarse a partir del tercer año de seguimiento. No se evidenció trombosis de stent de la lesión tratada con BFA.
Palabras clave: Balón farmacoactivo. Reestenosis de stent. Revascularización de lesión tratada.
Abbreviations: BMS: bare-metal stent. DAPT: dual antiplatelet therapy. DEB: drug-eluting balloon. DES: drug-eluting stent. ISR: in-stent restenosis. TLR: target lesion revascularization.
INTRODUCTION
In-stent restenosis (ISR) is still a common problem and a therapeutic challenge due to the high rates of culprit lesion revascularization. The treatment of choice for the management of ISR is still to be established.1 According to several randomized trials, drug-eluting balloon (DEB) angioplasty has better results for the management of ISR compared to conventional angioplasty and similar results compared to in-stent implantation of first-generation drug- eluting stents (DES),2-13 although it is inferior to second-generation DESs (especially in the ISR of DESs).14,15 This strategy has a class I indication (level of evidence A) for the management of ISR both in bare-metal stents (BMS) and DES.16
The effectiveness of this long-term strategy (over one year of follow-up) has already been established by the medical literature,17-22 yet it is largely unknown in the very long run (over 3 years of follow-up).23 Similarly, the studies conducted so far claim that high comorbidity is an exclusion criterion because the effect it has on real-world unselected patients is still unknown.
Our aim was to assess the effectiveness of DEBs in a registry of real world patients in a long-term follow-up period (5 years).
METHODS
Retrospective registry of a cohort of patients with ISR treated with DEBs at a high-volume center experienced in performing these procedures (> 1500/year) and percutaneous coronary interventions (> 800/year). The ISR was defined as > 50% angiographic stenosis as seen in 2 radiographic in-stent orthogonal projections or less than 5 mm away from its edges accompanied by symptoms of angina or objective ischemia. All lesions were treated with the same DEB (SeQuent Please, B. Braun Surgical, Melsungen, Germany). No clinical exclusion or angiographic criteria were established in the registry.
Both the clinical and the procedural characteristics were gathered from the center and cath. lab databases. Quantitative coronary angiography of the lesions was carried out using the Philips Xcelera system. Mehran’s classification for coronary restenosis was used to characterize the lesions.24 The procedural strategy and predilation with cutting-balloon or non-compliant high-pressure balloon was left at the discretion of the operator. Dilation with DEB was attempted for at least, 60 seconds at nominal pressure.
A 5-year follow-up period was established. The study was approved by the Clinical Trials Committee. All follow-up periods occurred were done in accordance to clinical criteria consulting the regional healthcare system electronic database, which keeps a comprehensive record of all patient-system communications.
All events were defined in a standard way following the Academic Research Consortium-2 (ARC-2) consensus.25 The primary endpoint was the need for target lesion revascularization (TLR) with the DEB and the total number of lesions treated. Secondary endpoints were any revascularization of acute coronary syndromes/acute myocardial infarctions according to the universal definition established by the European Society of Cardiology (the same or any location),26 all-cause mortality and cardiovascular mortality, and hemorrhage according to the Bleeding Academic Research Consortium (BARC) ≥ 3.27 Also, the following device-oriented composite endpoints (DOCE): TLR + acute coronary syndrome/acute myocardial infarction of the culprit vessel + cardiovascular mortality or patient-oriented composite endpoints (POCE): any revascularization + acute coronary syndrome/acute myocardial infarction + stroke + overall mortality were estimated on the total number of patients. Stent thrombosis was also defined following ARC-2 criteria and was also estimated on the total number of lesions.
For data analysis, the IBM SPSS 19.0 statistics software package was used. Quantitative variables were expressed as mean ± standard deviation, and qualitative variables was relative percentage. The cumulative incidence of events at follow-up was measured too. A bivariate analysis was conducted using the chi-square test or Fisher’s exact test, and the multinomial logistics regression analysis was used to estimate primary endpoint predictors (statistically significant value: P < .05). The Kaplan-Meier method was used to build the cumulative incidence curve during the follow-up of the primary endpoint.
RESULTS
A total of 53 ISRs in 48 patients were efficiently treated with DEBs from January 2010 through December 2013. In one patient, the DEB did not cross the lesion, so the patient was not included in the study (98.2% success rate of the procedure using the DEB). The baseline characteristics of the lesions and the coronary interventions are shown on table 1 and table 2; 49.1% (n = 26) of the lesions showed a Mehran I pattern of ISR and 24.5% (n = 13) of the lesions were located at the edges of the stent. Good angiographic results were obtained in all the patients (residual stenosis < 30%) and Thrombolysis in Myocardial Infarction grade 3 flow. The mean follow-up was 5.6 years (range: 0.2-8.2 years). There were no losses during follow-up.
Table 1. Baseline characteristic of patients
| Characteristics of patients | n = 48 |
|---|---|
| Age (years) | 69.3 ± 11.8 (44-93) |
| Male | 77.1% (37) |
| AHT | 75% (36) |
| Dyslipidemia | 56,3% (27) |
| Smoking | 35,4% (17) |
| Diabetes | |
| DM + OAD | 33.3% (16) |
| IDDM | 12.5% (6) |
| Atrial fibrillation (OAC) | 22.4% (11) |
| Prior AMI | 43.8% (21) |
| Prior indication for PCI | |
| Stable IHD | 37.5% (18) |
| ACS | 62.5% (30) |
| Prior CABG | 6.3% (3) |
| CRF (GFR < 60 mL/min) | 41.3% (19) |
| LVEF (%) | 55 ± 10.2 (65-29) |
| Multivessel disease | 77.1% (37) |
| Incomplete revascularization | 18.9% (9) |
| P2Y12 inhibitors | |
| Clopidogrel | 95.8% (46) |
| Ticagrelor/prasugrel | 4.2% (2) |
| Duration of DAPT | |
| 3 months | 22.4% (11) |
| 6 months | 33.3% (16) |
| 12 months | 43.8% (21) |
| ACEI/ARA-II | 87.5% (42) |
| Beta-blockers | 91.7% (44) |
| Statins | 100% (48) |
|
ACEI, angiotensin converting enzyme inhibitors; ACS, acute coronary syndrome; AHT, arterial hypertension; AMI, acute myocardial infarction; ARA-II, angiotensin II receptor antagonists; CABG, coronary artery bypass grafting; CRF, chronic renal failure; DAPT, dual anti-platelet therapy; DM, diabetes mellitus; GFR, glomerular filtration rate; IDDM, insulin dependent diabetes mellitus; IHD, ischemic heart disease; LVEF, left ventricular ejection fraction; OAC, oral anticoagulants; OAD, oral antidiabetics; PCI, percutaneous coronary intervention. |
Table 2. Characteristics of the lesions and the procedure
| Characteristics of the lesion/PCI | n = 53 |
|---|---|
| Location | |
| Anterior descending artery | 50.9% (27) |
| Circumflex artery | 15.1% (8) |
| Right coronary artery | 26.4% (14) |
| Left main stem | 5.7% (3) |
| Aortocoronary vein graft | 1.9% (1) |
| Bifurcation | 26.4% (14) |
| Diffuse ISR (Mehran II, III, IV) | 50.9% (27) |
| ISR in the edges of the stent | 24.5% (13) |
| Type of stent with ISR | |
| BMS | 64.2% (34) |
| DES | 22.6% (12) |
| DES over previous BMS | 7.5% (4) |
| DES over previous DES | 5.7% (3) |
| Second generation DES/total DES | 84.2% (16) |
| Stent ≥ 3 mm | 66% (35) |
| Diameter of the stent (mm) | 2.9 ± 0.4 (2-4) |
| Length of the stent (mm) | 22.8 ± 7.1 (8-38) |
| Diameter of the DEB (mm) | 3.1 ± 0.3 (2-3.5) |
| Length of the DEB (mm) | 20 ± 5.3 (15-30) |
| QCA prior to the PCI | |
| Reference diameter (mm) | 3.08 ± 0.39 |
| Minimal lumen diameter (mm) | 0.9 ± 0.43 |
| Stenosis (% diameter) | 62 ± 15 |
| Length | 13 ± 5.3 |
| QCA post-PCI | |
| Reference diameter (mm) | 3.1 ± 0.36 |
| Minimal lumen diameter (mm) | 2.27 ± 0.35 |
| Stenosis (% diameter) | 15 ± 6 |
| Cutting balloon | 30.2% (16) |
| Non-compliant balloon | 62.3% (33) |
| Radial access | 55.1% (27) |
| Successful PCI (residual stenosis < 30%) | 100% (53) |
|
BMS, bare-metal stent; DEB, drug-eluting balloon; DES, drug-eluting stent; ISR, in-stent restenosis; PCI, percutaneous coronary intervention; QCA, quantitative coronary angiography. |
Most patients (95.8%. n = 46) received clopidogrel as a P2Y12 receptor inhibitor as part of their dual anti-platelet therapy (DAPT) and 22.4% (n = 11) oral anticoagulants and short courses of DAPT (3 months).
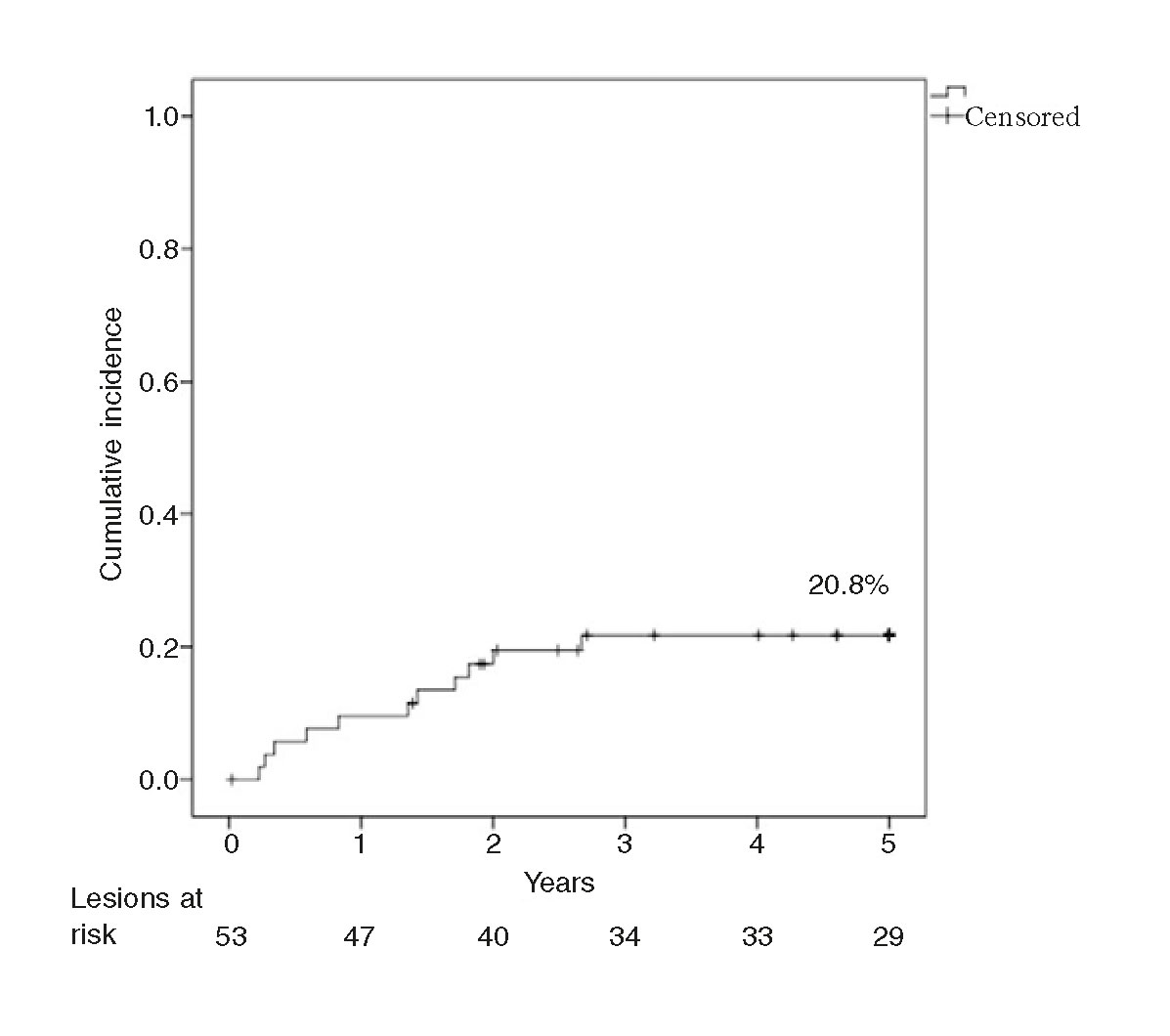
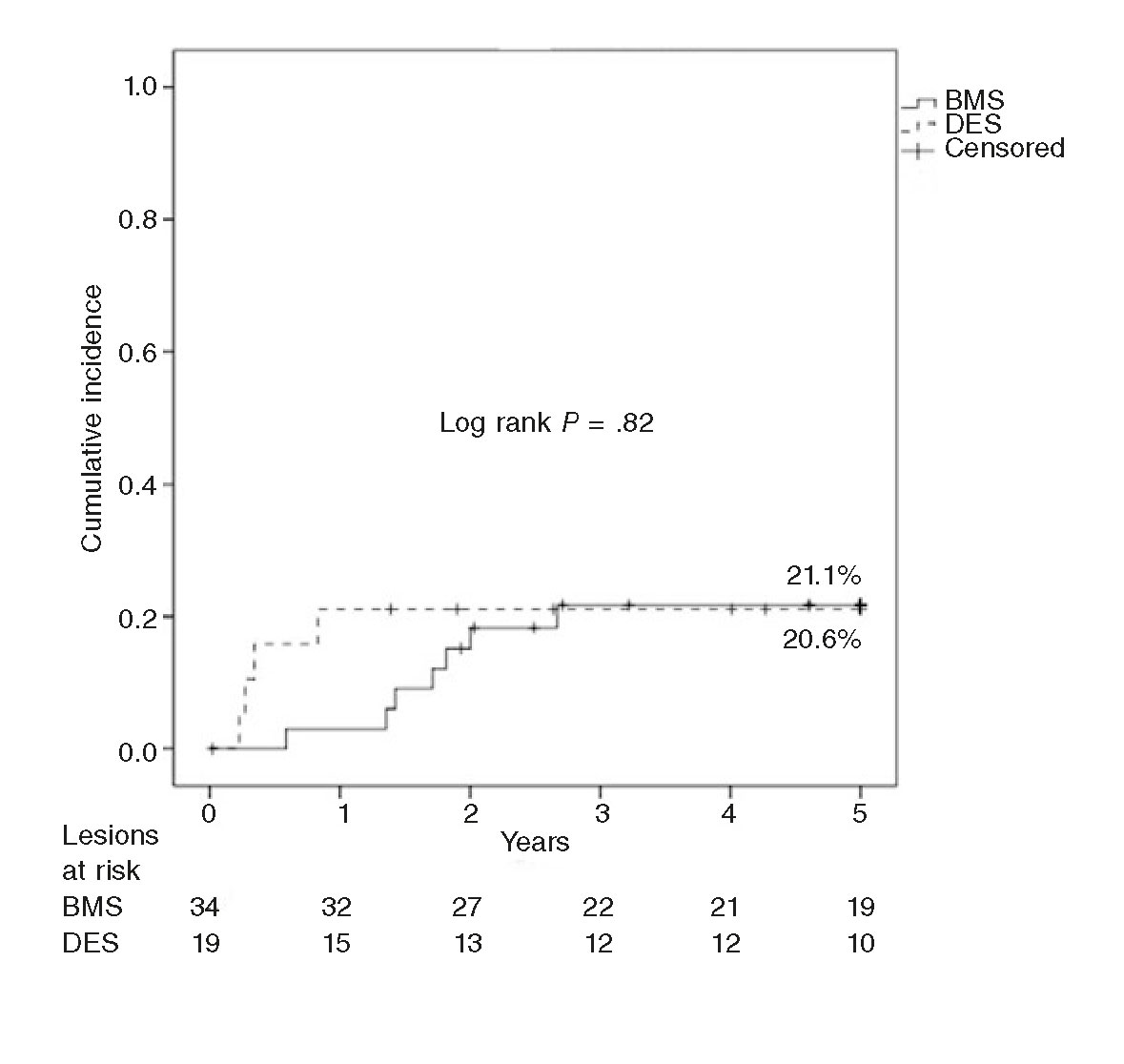
The rate of TLR at 1 year was 9.4%, and 20.8% at 3 and 5 years. The rate of late TLR (after the follow-up year) was 11.4%. The Kaplan-Meier analysis conducted (figure 1) shows that events accumulated during the first 3 years. The rate of TLR at 1 year was significantly slower in the ISR of BMSs (2.9% vs 21.1%; P = .05), and no late TLR was seen in the ISR of DESs. The rate of 5-year TLR was not associated with diabetes (22.7% vs 19.2%; P = .76) and it was not significantly lower with the use of the cutting balloon (12.5% vs 24.3%; P = .47) in ≥ 3 mm stents (25.7% vs 11.1%; P = .29) or in the ISR of BMSs (20.6% vs 21.1%; P = .96). Neither the bivariate analysis nor the logistics regression analysis identified any variables that would act as independent predictors of TLR.
Figure 1. Kaplan-Meier analysis of the cumulative incidence of target lesion revascularization (TLR) at a 5-year follow-up.
There were no cases of stent thrombosis in the target lesion. Two cases of acute coronary syndrome/acute myocardial infarction on the target vessel were reported: 1 case due to another lesion proximal to the lesion treated with DEB (with good results), and another case of acute coronary syndrome/non-ST-segment elevation acute myocardial infarction due to new onset ISR of the lesion treated with DEB.
At follow-up, a new coronary angiography was performed in 50% of the patients (n = 24). In 29.2% of the patients (n = 14) there was angiographic confirmation of lack of recurrent ISR in the stent treated with DEB. One patient underwent 2 TLRs in 2 different lesions at follow-up.
The remaining secondary and combined endpoints are shown on table 3. The overall mortality rate at 5 years was 33.3% (n = 16), and neoplasms were the most common cause (n = 5). According to the ARC-2 criteria, the cardiovascular mortality rate was up to 10.4% (n = 5): 2 sudden deaths at the 3-year follow-up (both with previous coronary angiography and good results of the lesions treated with DEB) and 3 strokes at the 2-year follow-up (one hemorrhagic stroke on oral anticoagulants).
Table 3. Secondary and composite outcomes at the 5-year follow-up
| Secondary outcomes at 5 years | n = 48 |
|---|---|
| Any revascularization | 29.2% (14) |
| ACS-AMI in the target vessel | 4.2% (2) |
| ACS-AMI in a different location | 10.4% (5) |
| Overall mortality | 33.3% (16) |
| Cardiovascular mortality | 10.4% (5) |
| Stroke | 12.5% (6) |
| DOCE | 31.3% (15) |
| POCE | 54.2% (26) |
| BARC ≥ 3 hemorrhages | 6.3% (3) |
|
ACS, acute coronary syndrome; AMI, acute myocardial infarction; BARC, Bleeding Academic Research Consortium; DOCE, device-oriented composite endpoints; POCE, patient-oriented composite endpoints. |
A total of 13 (27.1%) patients suffered hemorrhages at follow-up, although only 6.3% (n = 3) were on DAPT (one of them on oral anticoagulant medication). Three patients had BARC ≥ 3 hemorrhages: one patient, a BARC 3a GI hemorrhage, and 2 patients had BARC 5b hemorrhages (one due to a GI hemorrhage on DAPT and the other one due to an intracranial hemorrhage on oral anticoagulant medication).
DISCUSSION
As far as we know, this study is the first of its kind to describe the long-term progression of ISR lesions treated with DEBs in unselected (outside clinical trials) real-world patients (old and with high cardiovascular risk). The conclusions we can draw from our series are: a) although the per-year rate of TLR is similar to that of other studies already published, in the long run, it seems to be higher than the one reported in selected patients; b) late TLR (after 1 year of follow-up) represents half of the cases that require new revascularizations at follow-up; c) in the long term, TLR is similar in the ISR of both BMSs and DESs, yet late TLR occurs only in the ISR of BMSs; d) the rate of TLR seems to stabilize after 3 years; and e) the use of DEBs for the management of ISRs is a safe strategy from the standpoint of stent thrombosis, even in patients who receive short courses of DAPT.
The mid-term results (6-12 months) of the use of DEBs for the management of ISR have been widely described in randomized studies comparing this strategy with simple angioplasty or DES implantation in populations with clinical and angiographic exclusion criteria. Scheller et al.3 initially reported angiographic 6-month TLR rates of 0% and clinical 12-month-TLR rates of 4% in the PACCOCATH - ISR study (Treatment of in-Stent Restenosis by Paclitaxel Coated PTCA Balloons). Further studies show data more adjusted to actual results, with mid-term TLR rates of 6.6% to 8.8%,2,7,8,10,13-15 with differences based on whether we were dealing with the ISR of BMSs (6% to 8.7%)2,5,14 or DESs (4.3% to 22.1%).4,6-8,13,15,28 In the RIBS studies (Restenosis Intra-stent of Bare Metal Stents: Paclitaxel-eluting Balloon vs Everolimus-eluting Stent) V and IV (populations with a geographic location similar to that of the sample), Alfonso et al.14,15 reported a 1-year TLR rate of 6% and 13% in the ISR of BMSs and DESs, respectively. Also, these two studies showed 26 TLRs in 249 patients (95 with BMSs and 154 with DESs), that is, a 10.4% rate very similar to the 9.4% rate from our series.
Several studies have published their 3-year follow-up results: PEPCAD21 and RIBS V19 in BMSs, and RIBS IV,20 PEPCAD-DES,22 and ISAR-DESIRE 318 in DESs, with TLR rates of 6.2%, 8%, 15.6%, 19.4%, and 33,3%, respectively. These studies reported a total of 94 TLRs in 524 lesions amounting to a 3-year TLR rate of 17.9% lower than the 20.8% rate from our series. Although in our sample diabetes was not associated with TLR, it is a powerful predictor of ISR.29 This finding may be explained by a much greater prevalence of diabetes in our sample (55.5%) compared to the aforementioned studies (32% to 40%).
Late TLR is defined as a TLR occurring after one year of follow-up. The studies published indicate that TLR usually occurs over the first year of follow-up, not being very relevant thereafter, with rates between 0% and 4.1% (0%-25% of total cases). In our series, late TLR rate was 11.4% in over half of the cases (54% of all TLRs). Only the ISAR-DESIRE18 study and the study conducted by Habara et al.30 reported rates of late TLR close to the rates shown by our study (14.5% and 7.2%, respectively; 43% and 39% of all TLRs). The differences seen between those randomized studies and our series where TLR is clinical may be explained by the fact that most patients were angiographically followed during the first year, which in turn may have shown prematurely, in asymptomatic patients, a new angiographically significant ISR (> 50%), but not significant enough to cause any symptoms.
In our series there were only cases of late TLR in BMSs, since all TLRs in DESs occurred within the first year of follow-up. Initially, the effectiveness of DEBs was greater in the ISR of BMSs, yet the incidence of TLR went up at follow-up to the point of matching the incidence of TLR in the DES group (figure 2). These findings may be explained by the greater initial effect of the DEB over the characteristic pattern of ISR in BMSs (smooth muscle cells) vs the pattern of neoatherosclerosis, more common in the ISR of DESs.28 Thus, this neoatherosclerosis may be causing the late events reported in BMSs as described by Nakazawa et al.31 Our findings are different from those published by Habara et al.30 in a long series (550 lesions in 468 patients), where late TLR occurs predominantly in the ISR of DESs (odds ratio = 6; P = .002). Although the authors say that there were no differences in the rates of TLR reported between first- and second-generation DESs, 70% of these devices were first-generation DESs (14% were paclitaxel-eluting stents), vs 15% in our series (3 sirolimus-eluting stents and 0 paclitaxel-eluting stents). This study follow-up ends after 2 years, so the question of whether these findings stand in the long run still remains.
Figure 2. Kaplan-Meier analysis of target lesion revascularization (TLR) at 5 years after the use of drug-eluting balloon for the management of in-stent restenosis of bare-metal stents (BMS) vs drug-eluting stents (DES).
Scheller et al.23 published the 5-year follow-up of the PACCOCATH-ISR study, with a 5-year TLR rate of 9.3% and a prevalence of diabetes of 17%. Miura et al.32 reported 5-year TLR rates of 34% in 216 ISRs of DESs, a much higher rate compared to the rate from our sample, with a similar prevalence of diabetes. Both studies suggest that TLR rate increases in the long term. Curiously, in our series, TLR rate stabilized after the third year. In these studies, cases of 2-to-5-year TLR rates have been described, but the exact moment when the TLR occurred was never reported. It is possible that clinical TLR post-DEB occurs after one year follow-up (1-3 years) and then stabilizes over time.
In the ISAR-DESIRE 4 study, the use of cutting-balloon for the management of ISR with DEBs obtained better angiographic results (binary restenosis) and a significant reduction of the 1-year TLR (16.2% vs 21.8%; P = .26).33 In our series, patients treated with cutting-balloon showed a numerically lower rate of TLR (12.5% vs 24.3%), but with no statistical significance due to the size of the sample. Only 30% of the lesions were treated using this technique since when the study was conducted, the benefits of such technique had not yet been established.33
Our sample had 2 characteristics of which there is scarce information in the medical literature: old age and use of oral anticoagulants. The average age was almost 70 years with data from patients who were extremely old (up to 93 years of age) and over 20% were on oral anticoagulants. Even though this is not an exclusion criterion per se, the studies published so far do not provide information on the percentage of patients on oral anticoagulants or the rate of hemorrhages at follow-up.18-22 In these studies, DAPT was extended between 3 and 6 months. In our series, over 50% of the patients received DAPT over a course of 3-6 months and those patients on oral anticoagulants (22.4%) followed a 3-month strategy. The use of DEB with these courses was safe. The overall rate of hemorrhages was moderate, which is consistent with patients’ age, and only one patient had a BARC ≥ 3 hemorrhage while on DAPT. Yet despite the high percentage of patients on short courses of DAPT, no cases of definitive/probable target lesion thrombosis were reported. Except for the PEPCAD study, that did not provide information either on stent thrombosis of the lesion treated with DEB, the remaining studies reported long-term rates of stent thrombosis between 0.8% (ISAR-DESIRE study) and 2.6% (RIBS IV study).18-22
ARC-2 criteria are consensus criteria to make results uniform, although in elderly populations with high ischemic risk they can diminish the effectiveness of the procedure. The high 3-yar rate of DOCE reported (31.3%) was this big due to cardiovascular mortality (10.4%), that according to the ARC-2 criteria should also include stroke-induced mortality. As we have already mentioned, based on chronology and causality only, it is unlikely that the cardiovascular mortality rate seen in our sample can be attributed to DEBs. Even though they are combined outcomes with different definitions, DOCEs may look like traditional MACE (major adverse cardiac events), with a similar incidence to the one reported by the studies published, somewhere around 20%-38% at 3 years.18-22 Our patients’ high ischemic risk is evident on the high rates of acute coronary syndrome/acute myocardial infarction of the non-target lesions reported (10.2%) and the rates of stroke (12.5%) at follow-up. Both events together with age-related non-cardiovascular mortality (neoplasms) penalized the POCEs by doubling the DOCEs from our series (54.2%). Thus, approximately half of the composite outcomes according to the ARC-2 criteria at 5-year follow-up would not be attributable to the use of DEBs.
Limitations
Our study has some limitations. This was a retrospective, single-center study with limited cases that did not allow to obtain solid scientific evidence. In line with the results published by former studies and reported in the medical literature, our results support the use of DEBs for the management of ISR. Consequently, in the long run, around 80% of patients from unselected populations could avoid having to undergo the implantation of an additional layer of BMS, thus saving this strategy for DEB failure.
CONCLUSIONS
In a clinical practice cohort, DEBs for the management of ISR have a 5-year TLR rate of 20.8%. Late TLR accounts for half the cases at follow-up and occurs in the ISR of BMSs. TLR rate seems to stabilize after three years. No stent thrombosis was reported in lesions treated with DEBs.
CONFLICTS OF INTEREST
None declared.
WHAT IS KNOWN ABOUT THE TOPIC?
- The use of DEBs for the management of ISR is a strategy validated by randomized studies with 1-3-year follow-up in selected populations and TLR rates around 8%-10%. The 5-year effectiveness of this strategy has been reported in the literature in a merely formal way.
- Late TLR (after 1-year follow-up) is not very relevant in those studies.
WHAT DOES THIS STUDY ADD?
- The effectiveness and very long-term follow-up (5 years) of DEBs for the management of ISR in an unselected high cardiovascular risk population. Although the 1-year TLR is similar to that of randomized studies, in a real-world cohort late TLR may be more significant compared to the one described by the studies (above all in conventional stents), and even though it seems to stabilize after three years, it is higher in the very long-term follow-up (5 years).
- In patients with high risk of bleeding, short courses of DAPT were not associated with stent thrombosis of the lesions treated with DEB
REFERENCES
1. Alfonso F, Byrne RA, Rivero F, Kastrati A. Current treatment of in-stent restenosis. J Am Coll Cardiol.2014;63:2659-2673.
2. Pleva L, Kukla P, Kusnierova P, Zapletalova J, Hlinomaz O. CompaRSon of the Efficacy of Paclitaxel-Eluting Balloon Catheters and Everolimus-Eluting Stents in the Treatment of Coronary In-Stent Restenosis:The Treatment of In-Stent Restenosis Study. Circ Cardiovasc Interv. 2016;9:e003316.
3. Scheller B, Hehrlein C, Bocksch W, et al. Treatment of coronary in-stent restenosis with a paclitaxel-coated balloon catheter. N Engl J Med. 2006;355:2113-2124.
4. Habara S, Iwabuchi M, Inoue N, et al. A multicenter randomized comparison of paclitaxel-coated balloon catheter with conventional balloon angioplasty in patients with bare-metal stent restenosis and drug-eluting stent restenosis. Am Heart J. 2013;166:527-533.
5. Unverdorben M, Vallbracht C, Cremers B, et al. Paclitaxel-coated balloon catheter versus paclitaxel-coated stent for the treatment of coronary in-stent restenosis. Circulation. 2009;119:2986-2994.
6. Rittger H, Brachmann J, Sinha AM, et al. A randomized, multicenter, single-blinded trial comparing paclitaxel-coated balloon angioplasty with plain balloon angioplasty in drug-eluting stent restenosis:the PEPCAD-DES study. J Am Coll Cardiol. 2012;59:1377-1382.
7. Habara S, Mitsudo K, Kadota K, et al. Effectiveness of paclitaxel-eluting balloon catheter in patients with sirolimus-eluting stent restenosis. JACC Cardiovasc Interv. 2011;4:149-154.
8. Byrne RA, Neumann FJ, Mehilli J, et al. Paclitaxel-eluting balloons, paclitaxel-eluting stents, and balloon angioplasty in patients with restenosis after implantation of a drug-eluting stent (ISAR-DESIRE 3):a randomised, open- label trial. Lancet. 2013;381:461-467.
9. Indermuehle A, Bahl R, Lansky AJ, et al. Drug-eluting balloon angioplasty for in-stent restenosis:a systematic review and meta-analysis of randomised controlled trials. Heart. 2013;99:327-333.
10. Baan J, Jr., Claessen BE, Dijk KB, et al. A Randomized Comparison of Paclitaxel-Eluting Balloon Versus Everolimus-Eluting Stent for the Treatment of Any In-Stent Restenosis:The DARE Trial. JACC Cardiovasc Interv. 2018;11:275-283.
11. Siontis GC, Stefanini GG, Mavridis D, et al. Percutaneous coronary interventional strategies for treatment of in-stent restenosis:a network meta-analysis. Lancet. 2015;386:655-664.
12. Giacoppo D, Gargiulo G, Aruta P, Capranzano P, Tamburino C, Capodanno D. Treatment strategies for coronary in-stent restenosis:systematic review and hierarchical Bayesian network meta-analysis of 24 randomised trials and 4880 patients. BMJ. 2015;351:h5392.
13. Xu B, Gao R, Wang J, et al. A prospective, multicenter, randomized trial of paclitaxel-coated balloon versus paclitaxel-eluting stent for the treatment of drug-eluting stent in-stent restenosis:results from the PEPCAD China ISR trial. JACC Cardiovasc Interv. 2014;7:204-211.
14. Alfonso F, Perez-Vizcayno MJ, Cardenas A, et al. A randomized comparison of drug-eluting balloon versus everolimus-eluting stent in patients with bare-metal stent-in-stent restenosis:the RIBS V Clinical Trial (Restenosis Intra-stent of Bare Metal Stents:paclitaxel-eluting balloon vs. everolimus-eluting stent). J Am Coll Cardiol. 2014;63:1378-1386.
15. Alfonso F, Perez-Vizcayno MJ, Cardenas A, et al. A Prospective Randomized Trial of Drug-Eluting Balloons Versus Everolimus-Eluting Stents in Patients With In-Stent Restenosis of Drug-Eluting Stents:The RIBS IV Randomized Clinical Trial. J Am Coll Cardiol. 2015;66:23-33.
16. Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2018;40:87-165.
17. Xu B, Qian J, Ge J, et al. Two-year results and subgroup analyses of the PEPCAD China in-stent restenosis trial:A prospective, multicenter, randomized trial for the treatment of drug-eluting stent in-stent restenosis. Catheter Cardiovasc Interv. 2016;87(Suppl 1):624-629.
18. Kufner S, Cassese S, Valeskini M, et al. Long-Term Efficacy and Safety of Paclitaxel-Eluting Balloon for the Treatment of Drug-Eluting Stent Restenosis:3-Year Results of a Randomized Controlled Trial. JACC Cardiovasc Interv. 2015;8:877-884.
19. Alfonso F, Perez-Vizcayno MJ, Garcia Del Blanco B, et al. Long-Term Results of Everolimus-Eluting Stents Versus Drug-Eluting Balloons in Patients With Bare-Metal In-Stent Restenosis:3-Year Follow-Up of the RIBS V Clinical Trial. JACC Cardiovasc Interv. 2016;9:1246-1255.
20. Alfonso F, Perez-Vizcayno MJ, Cuesta J, et al. 3-Year Clinical Follow-Up of the RIBS IV Clinical Trial:A Prospective Randomized Study of Drug-Eluting Balloons Versus Everolimus-Eluting Stents in Patients With In-Stent Restenosis in Coronary Arteries Previously Treated With Drug-Eluting Stents. JACC Cardiovasc Interv. 2018;11:981-991.
21. Unverdorben M, Vallbracht C, Cremers B, et al. Paclitaxel-coated balloon catheter versus paclitaxel-coated stent for the treatment of coronary in-stent restenosis:the three-year results of the PEPCAD II ISR study. EuroIntervention. 2015;11:926-934.
22. Rittger H, Waliszewski M, Brachmann J, et al. Long-Term Outcomes After Treatment With a Paclitaxel-Coated Balloon Versus Balloon Angioplasty:Insights From the PEPCAD-DES Study (Treatment of Drug-eluting Stent [DES] In-Stent Restenosis With SeQuent Please Paclitaxel-Coated Percutaneous Transluminal Coronary Angioplasty [PTCA] Catheter). JACC Cardiovasc Interv. 2015;8:1695-1700.
23. Scheller B, Clever YP, Kelsch B, et al. Long-term follow-up after treatment of coronary in-stent restenosis with a paclitaxel-coated balloon catheter. JACC Cardiovasc Interv. 2012;5:323-330.
24. Mehran R, Dangas G, Abizaid AS, et al. Angiographic patterns of in-stent restenosis:classification and implications for long-term outcome. Circulation.1999;100:1872-1878.
25. Garcia-Garcia HM, McFadden EP, Farb A, et al. Standardized End Point Definitions for Coronary Intervention Trials:The Academic Research Consortium-2 Consensus Document. Circulation. 2018;137:2635-2650.
26. Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction (2018). Eur Heart J. 2019;40:237-269.
27. Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials:a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736-2747.
28. Alfonso F, Perez-Vizcayno MJ, Garcia Del Blanco B, et al. Usefulness of Drug-Eluting Balloons for Bare-Metal and Drug-Eluting In-Stent Restenosis (from the RIBS IV and V Randomized Trials). Am J Cardiol. 2017;119: 983-990.
29. Cassese S, Byrne RA, Tada T, et al. Incidence and predictors of restenosis after coronary stenting in 10 004 patients with surveillance angiography. Heart. 2014;100:153-159.
30. Habara S, Kadota K, Shimada T, et al. Late Restenosis After Paclitaxel-Coated Balloon Angioplasty Occurs in Patients With Drug-Eluting Stent Restenosis. J Am Coll Cardiol. 2015;66:14-22.
31. Nakazawa G, Otsuka F, Nakano M, et al. The pathology of neoatherosclerosis in human coronary implants bare-metal and drug-eluting stents. J Am Coll Cardiol. 2011;57:1314-1322.
32. Miura K, Kadota K, Habara S, et al. Five-Year Outcomes After Paclitaxel-Coated Balloon Angioplasty for Drug-Eluting Stent Restenosis. Am J Cardiol. 2017;119:365-371.
33. Kufner S, Joner M, Schneider S, et al. Neointimal Modification With Scoring Balloon and Efficacy of Drug-Coated Balloon Therapy in Patients With Restenosis in Drug-Eluting Coronary Stents:A Randomized Controlled Trial. JACC Cardiovasc Interv. 2017;10:1332-1340.