ABSTRACT
Introduction and objectives: The use of transradial access for percutaneous coronary procedures has increased due to its advantages over the femoral approach. However, this benefit comes at the expense of a higher rate of radial artery occlusion (RAO). Our objective was to assess the incidence and predictors of RAO following transradial catheterization. Additionally, we studied anatomic variations of the radial artery (RA).
Methods: This prospective study enrolled 427 patients who underwent coronary angiography or angioplasty via transradial access. The forearm arteries were evaluated by ultrasound. If RAO was present, follow-up ultrasound examinations were performed at 1 and 3 months postprocedure.
Results: Our study population included 288 men (67.4%) and 139 women (32.6%). The mean age was 61.9 ± 11.1 years. RAO occurred in 48 patients (11.24%), and spontaneous recanalization was observed within 3 months in 15 patients (32.6%). On multivariate analysis, independent predictors of RAO were younger age (OR, 0.642; 95%CI, 0.480-0.858; P = .031), low periprocedural systolic blood pressure (OR, 0.598; 95%CI, 0.415-0.862; P = .007), a small radial diameter (OR, 0.371; 95%CI, 0.323-0.618; P = .031), insufficient anticoagulation (OR, 0.287; 95%CI, 0.163-0.505; P < .001), occlusive hemostasis (OR, 0.128; 95%CI, 0.047-0.353; P < .001), and long duration of hemostasis. The overall incidence of RA anatomic variations was 14.8% (n = 63). Among these, 40 patients (63.5%) had a high radial origin, 18 (28.6%) had extreme RA tortuosity, and 5 (7.9%) had a complete radioulnar loop.
Conclusions: The main modifiable predictors of RAO are insufficient heparinization and occlusive hemostasis. Preventive strategies should focus primarily on these 2 predictive factors to reduce the risk of RAO.
Keywords: Anatomic variations. Cardiac catheterization. Doppler ultrasound. Percutaneous coronary intervention. Predictors. Radial artery occlusion. Transradial access.
RESUMEN
Introducción y objetivos: El acceso transradial para procedimientos coronarios percutáneos ha crecido en popularidad debido a sus ventajas sobre el abordaje femoral. Sin embargo, este beneficio se ve ensombrecido por una mayor tasa de oclusión de la arteria radial (OAR). Nuestro objetivo fue evaluar la incidencia y los factores predictivos de OAR tras el cateterismo transradial. También se estudiaron las variaciones anatómicas de la arteria radial (AR).
Métodos: En este estudio prospectivo participaron 427 pacientes a los que se había realizado angiografía coronaria o angioplastia mediante acceso transradial. Se realizó una evaluación ecográfica de las arterias del antebrazo. En caso de OAR, se llevó a cabo otro control ecográfico al mes y a los 3 meses de la intervención.
Resultados: La población de estudio incluyó a 288 varones (67,4%) y 139 mujeres (32,6%). La edad media fue de 61,9 ± 11,1 años. La OAR se produjo en 48 pacientes (11,24%), de los cuales en 15 (32,6%) se produjo recanalización espontánea en el plazo de 3 meses. En el análisis multivariante, la edad más joven (OR = 0,642; IC95%, 0,480-0,858; p = 0,031), la presión arterial sistólica periprocedimiento baja (OR = 0,598; IC95%, 0,415-0,862; p = 0,007), el diámetro radial pequeño (OR = 0,371; IC95%, 0,323-0,618; p = 0,031), la anticoagulación insuficiente (OR = 0,287; IC95%, 0,163-0,505; p < 0,001), la hemostasia oclusiva (OR = 0,128; IC95%, 0,047-0,353; p < 0,001) y la larga duración de la hemostasia aparecieron como predictores independientes de OAR. La incidencia global de variaciones anatómicas de la AR fue del 14,8% (n = 63). Entre estos pacientes, 40 (63,5%) tenían un origen radial alto, 18 (28,6%) presentaban una tortuosidad extrema de la AR y 5 (7,9%) tenían un asa radiocubital completa.
Conclusiones: La heparinización insuficiente y la hemostasia oclusiva son los principales predictores de OAR modificables. La estrategia preventiva debe centrarse principalmente en estos 2 factores predictivos.
Palabras clave: Variaciones anatómicas. Cateterismo cardiaco. Ecografía Doppler. Intervención coronaria percutánea. Predictores. Oclusión de la arteria radial. Acceso transradial.
Abbreviations
RA: radial artery. RAO: radial artery occlusion.
INTRODUCTION
The use of the transradial approach for coronary interventions has become increasingly widespread in interventional cardiology due to its numerous advantages.1 As a result, current guidelines recommend it as the first-line approach.2
However, the benefits of this technique are tempered by the risk of radial artery occlusion (RAO), with reported rates ranging from 5% to 30%.3,4 The aim of this study was to assess the incidence and predictors of RAO following transradial catheterization using Doppler ultrasound for evaluation.
METHODS
Patient population
This longitudinal, single-center prospective study was conducted in the cardiology department of the Military Central Hospital in Algiers. After applying exclusion criteria (hemodynamic instability and ST-segment elevation myocardial infarction), we included 427 consecutive patients undergoing transradial coronary procedures between January 2019 and March 2020. The study adhered to the principles of the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practices and was approved by the local ethics committee. All patients provided written informed consent.
Radial artery cannulation and retrograde radial arteriography
After radial artery (RA) puncture, a radial hydrophilic sheath (Radiofocus II, TERUMO Medical, Japan, or Prelude, MERIT Medical, United States) was introduced. An antispastic cocktail was then administered into the RA through the sheath, consisting of a saline solution, a vasodilator (1 mL of nicardipine), and a bolus of unfractionated heparin, which was administered either intravenously or directly into the RA as part of the spasmolytic cocktail, depending on the operator’s preference. In patients on vitamin K antagonists, these medications were not discontinued prior to the procedure.
Retrograde radial arteriography was performed by injecting a mixture of 4 mL of contrast and 4 mL of isotonic saline through the sheath. Radiographic images were then obtained in an anteroposterior projection.
Transradial coronary procedure
The standard approach was conventional right radial access. For coronary angiography, 5-French (Fr) hydrophilic sheaths and catheters were usually used. If the patient required revascularization, an ad hoc percutaneous coronary intervention was performed, using 6-Fr guiding catheters after exchanging the sheath from 5-Fr to 6-Fr. The usual dose of heparin is 5000 IU (2500 IU for oral anticoagulation with a vitamin K antagonist).
Hemostasis procedure
At the end of the procedure, the sheath was removed, and hemostasis was achieved using a hemostatic compression device (TR BAND, TERUMO Medical, Japan). A reverse Barbeau test5 was systematically performed. The hemostasis device was removed by nurses in the hospitalization unit. No standardized protocol for the duration of hemostasis was followed.
Assessment of postprocedural radial artery patency
Radial Doppler assessments were conducted before and after each transradial procedure. To evaluate RAO, pulsed Doppler was performed bilaterally on the radial and ulnar arteries. Normal arterial flow was indicated by a biphasic or triphasic signal, reflecting good perfusion. In cases of RAO, 2 additional ultrasonographic examinations were performed at 1 and 3 months, following the same protocol. Artery patency was assessed by an independent operator.
Classifications and definitions
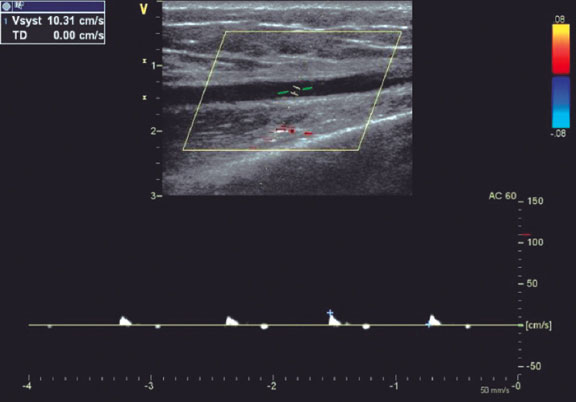
RAO was defined as the absence of anterograde flow in the RA on ultrasound (figure 1). The location of the radial occlusion was identified using color and pulsed Doppler. We delineated 3 anatomical territories: the distal third, extending from the radial styloid to approximately 7 to 10 cm proximally; the proximal third, from the elbow folds to approximately 7 to 10 cm distally; and the middle third, located between the previous 2 regions (middle part of the forearm).
Figure 1. Radial artery with occlusion in the distal third. Pulsed Doppler flow targets a stop flow indicating radial occlusion.
The type of hemostasis, whether occlusive or patent, was assessed: patent hemostasis was indicated by the presence of a plethysmographic signal in the RA during the reverse Barbeau test,5 which involves compression of the ulnar artery. The operator did not intervene during this process but simply recorded whether the artery remained patent or not.
The internal luminal diameter of the RA was defined as the distance between the leading edges of the intima-lumen interface on the superficial wall and the lumen-intima interface on the deep wall.6
The R/S ratio (radial/sheath) was calculated by dividing the luminal diameter of the RA by the external diameter of the sheath (Radiofocus II: 5-Fr = 2.29 mm, 6-Fr = 2.62 mm, 7-Fr = 2.97 mm; Prelude: 5-Fr = 2.52 mm, 6-Fr = 2.83 mm). This ratio was categorized qualitatively as < 1 or ≥ 1.
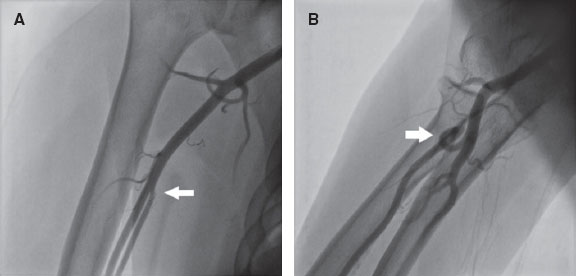
RA anatomical variations of clinical relevance were classified according to definitions provided in the literature.7,8 A high origin (high bifurcation) of the RA (figure 2) was defined with reference to the intercondylar line of the humerus. A radioulnar loop was characterized by the presence of a complete 360° loop of the RA, while radial tortuosity was identified by a curvature greater than 45°.
Figure 2. Anatomic variations of the radial artery. A: high origin of the radial artery. The radial and ulnar arteries separate at the level of the middle third of the humerus (arrow). B: radioulnar loop was defined as a complete 360° loop of the radial artery distal to the bifurcation of the brachial artery (arrow).
A blood pressure profile was obtained on the same side as the radial access. Forearm hematomas were classified according to the “EASY” study9: type I: < 5 cm in diameter; type II: < 10 cm; type III: > 10 cm but not extending to the elbow; type IV: extending beyond the elbow; type V: resulting in an ischemic lesion.
Statistical analysis
The statistical analysis was performed using IBM SPSS Software version 25. Parameters of interest are reported with their 95% confidence intervals (95%CI). For all tests, a significance threshold of 5% was retained. All tests were performed bilaterally. The following tests were used to compare groups: the chi-square test was used to compare 2 qualitative variables; the Student t-test or analysis of variance was used to compare a quantitative variable with a qualitative variable, with the Fisher test being applied when variances were unequal; and logistic regression was used to identify predictors of RAO.
RESULTS
Clinical and procedural characteristics of the study population
During the study period, 441 patients were screened. Of these, transradial access failed in 14 patients, who were excluded from the study, resulting in an eligible sample of 427 patients (mean age 61.9 ± 11.1 years, 67.4% male). Among the patients, 260 had hypertension (60.9%), and nearly half had diabetes (48.9%).
Table 1 summarizes the procedural data. The sheaths used were mainly 6-Fr (83.6%), and heparin was injected intra-arterially in 63.5% of patients. The mean heparin dose was 5669 ± 1394 IU, with a higher dose given when percutaneous coronary intervention was performed (4940 ± 339 IU vs 7491 ± 1368 IU; P < .001).
| Procedural characteristics | Patients N (%) |
|---|---|
| Indication | |
| CCS | 227 (53.2%) |
| ACS (NSTEMI) | 200 (46.8%) |
| Type of procedure | |
| Diagnostic angiography | 305 (71.4%) |
| PCI | 122 (28.6%) |
| Previous radial procedures | 68 (15.9%) |
| Right radial access | 410 (96.0%) |
| Puncture attempts | |
| 1 attempt | 258 (60.4%) |
| 2 attempts | 99 (23.2%) |
| ≥ 3 attempts | 70 (16.4%) |
| Sheath size | |
| 5-Fr | 68 (15.9%) |
| 6-Fr | 357 (83.6%) |
| 7-Fr | 2 (0.5%) |
| Heparin administration | |
| Intra-arterial | 271(63.5%) |
| Intravenous | 156 (36.5%) |
| Heparin dose (IU) | 5669 ± 1394 |
| Angiography | 4940 ± 339 |
| PCI | 7491 ± 1368 |
| Catheter diameter | |
| 5-Fr | 300 (70.3%) |
| 6-Fr | 125 (29.3%) |
| 7-Fr | 2 (0.5%) |
| Number of catheters used | |
| 1 | 43 (10.1%) |
| 2 | 271 (63.5%) |
| ≥ 3 | 113 (26.4%) |
| Fluoroscopy time (min) | 11.22 ± 12.09 |
| Radiation dose (mGy) | 564 ± 538 |
| Contrast amount (mL) | 98.97 ± 54.09 |
| Procedure time (min) | 39.16 ± 34.6 |
| Angiography | 21.63 ± 9.98 |
| PCI | 82.99 ± 35.39 |
| Coronary lesions | |
| Normal coronaries | 134 (31.4%) |
| 1 vessel disease | 131 (30.7%) |
| 2 vessel disease | 87 (20.4%) |
| 3 vessel disease | 75 (17.6%) |
|
ACS, acute coronary syndrome; CCS, chronic coronary syndrome; Fr, French; IU, international unit; NSTEMI, non–ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention. |
|
Incidence and characteristics of radial artery occlusion
RAO occurred in 48 patients (11.24%). Of these, 89.6% were asymptomatic, and the radial pulse remained palpable in 14 patients (29.2%). At 1 month, 2 patients were lost to follow-up. Among the remaining 46 patients, spontaneous recanalization occurred in 13 patients (28.3%). At the 3-month follow-up, the recanalization rate increased to 32.6% (15 cases).
The site of RAO was the distal third in 7 patients (14.6%), the middle third in 21 patients (43.8%), and the proximal third in 20 patients (41.7%).
Predictors of radial artery occlusion
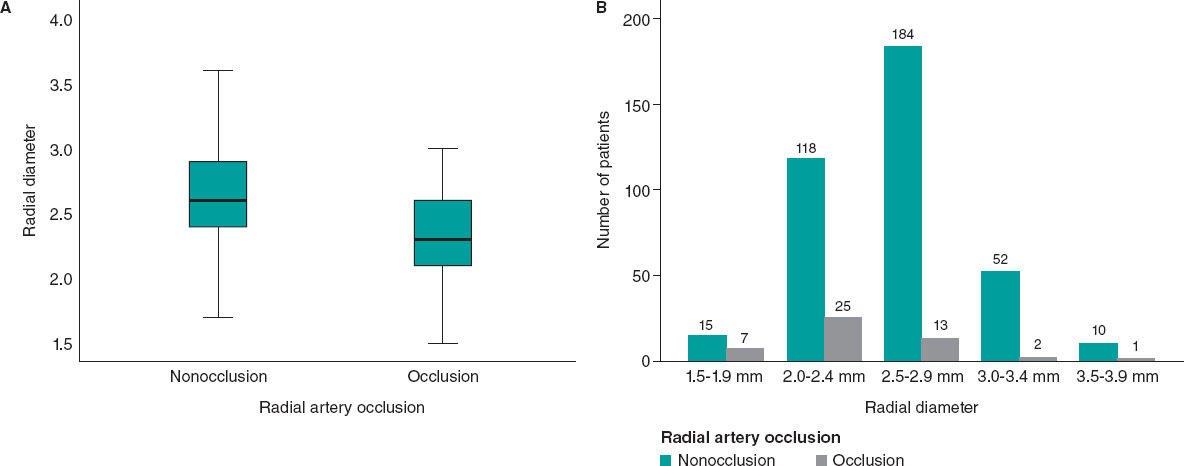
Patients with RAO were significantly younger (table 2). The mean periprocedural systolic blood pressure in the RAO group was significantly lower (138.04 mmHg ± 21.92, vs 145.84 mmHg ± 21.10; P = .017). Type A Barbeau test was associated with a higher risk of RAO compared with types B and C, and patients with occlusion had a smaller RA diameter (2.34 mm ± 0.40 vs 2.61 mm ± 0.37; P < .001) (figure 3).
Table 2. Comparison of patients with and without RAO
| Clinical data | Procedural data | ||||||
|---|---|---|---|---|---|---|---|
| Non-RAO (n= 379) | RAO (n= 48) | P | Non-RAO (n= 379) | RAO (n= 48) | P | ||
| Age | 62.6 ± 10.6 | 56.4 ± 14.0 | < .001* | Previous TRA | 61 (16.0%) | 7 (14.5) | .63 |
| Female sex | 122 (32.1%) | 17 (35.4%) | .65 | Diagnostic angiography | 266 (70.1%) | 39 (81.2%) | .11 |
| Hypertension | 237 (62.5%) | 23 (47%) | .051 | ≥ 2 puncture attempts | 147 (38.7%) | 22 (45.8%) | .41 |
| Diabetes | 182 (48%) | 27 (56%) | .28 | IV heparin | 136 (35.8%) | 20 (41.6%) | .43 |
| Dyslipidemia | 44 (11.6%) | 6 (12.5%) | .85 | Heparin dose (IU) | 5754 ± 1378 | 5007 ± 1352 | < .001* |
| Smoking | 75 (19.7%) | 9 (18.7%) | .86 | Spasm | 60 (15.8%) | 12 (25.0%) | .11 |
| BMI ≥ 30 kg/m2 | 123 (32.4%) | 16 (33.3%) | .90 | Procedure time (min) | 39.85 ± 34.56 | 33.73 ± 34.78 | .249 |
| Mean PSBP (mm Hg) | 145.84 ± 21.10 | 138.04 ± 21.92 | .017* | Number of catheters | 2.30 ± 0.88 | 2.21 ± 0.92 | .75 |
| Barbeau test type A | 99 (26.1%) | 20 (41.6%) | .044* | Occlusive hemostasis | 241 (63.5%) | 42 (87.5%) | .001* |
| RAD (mm) | 2.61 ± 0.37 | 2.34 ± 0.40 | < .001* | Hemostasis duration (h) | 4.29 ± 1.22 | 5.15 ± 1.41 | .006* |
| APT | 336 (88%) | 41 (85%) | .51 | ||||
| VKA (INR ≥ 2) | 16 (4.2%) | 5 (10.4%) | .061 | ||||
| MVCD | 149 (39.3%) | 13 (27.1%) | .20 | ||||
|
APT, antiplatelet therapy; BMI, body mass index; INR, international normalized ratio; IV, intravenous; IU, international unit; MVCD, multivessel coronary disease = ≥ 2 lesions ; PSBP, periprocedural systolic blood pressure; RAD , radial artery diameter; RAO, radial artery occlusion; TRA , transradial access; VKA, vitamin K antagonist. * Statistically significant. |
|||||||
Figure 3. Radial artery diameter as a predictor of occlusion. A: the radial diameter is significantly smaller if there is RAO. B: less than 2.5 mm, the risk of occlusion becomes greater.
RAO procedural factors are listed in table 2. An R/S ratio < 1 was found in 35 patients in the RAO group vs 153 patients in the non-RAO group (72.9% vs 40.3%, P < .001). The mean heparin dose was significantly lower in patients with RAO (5007 ± 1352IU vs 5754 ± 1378 IU; P < .001), and the dose adjusted to weight was also significantly lower in the RAO group (62.31 ± 17.82 IU/kg vs 75.73 ± 22.57 IU/kg; P < .001). In addition, the RAO rate decreased significantly when the heparin dose exceeded 70 IU/kg.
Forty-two patients in the RAO group had occlusive hemostasis vs 241 in the non-RAO group (87.5% vs 63.5%; P = .001). Surprisingly, two-thirds of our patients (283 [66.3%]) had occlusive hemostasis. The mean duration of hemostasis was longer if there was RAO (5.15 h ± 1.41 vs 4.29 h ± 1.22; P < .001).
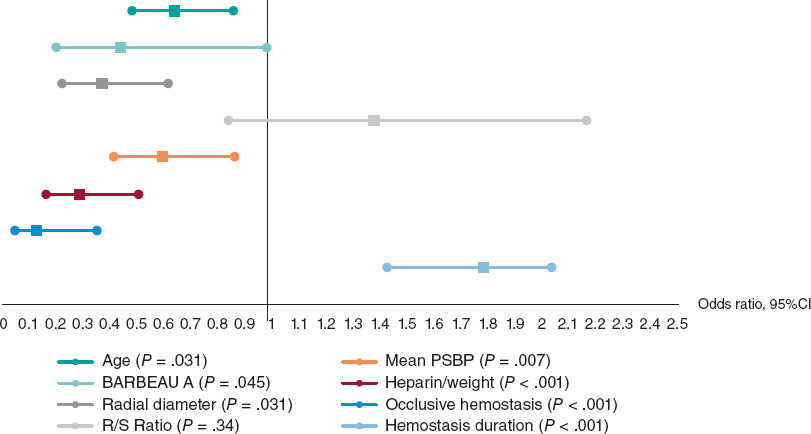
On multivariate logistic regression analysis (figure 4), the following factors were independent predictors of RAO: young age (odds ratio [OR], 0.642; 95%CI, 0.480-0.858; P = .031), low periprocedural systolic blood pressure (OR, 0.598; 95%CI, 0.415-0.862; P = .007), type A Barbeau test (OR, 0.441; 95%CI, 0.198-0.981; P = .045), small RA diameter (OR, 0.371; 95%CI, 0.323-0.618; P = .031), insufficient anticoagulation (OR, 0.287; 95%CI, 0.163-0.505; P < .001), occlusive hemostasis (OR, 0.128; 95%CI, 0.047-0.353; P < .001), and a long hemostasis duration (OR, 1.786; 95%CI, 1.428-2.039; P < .001).
Figure 4. Independent factors predictive of radial artery occlusion. Multiple logistic regression analysis revealed that the independent factors predictive of radial occlusion were young age, low periprocedural systolic blood pressure, type A Barbeau test, small radial artery diameter, insufficient anticoagulation, occlusive hemostasis, and long hemostasis duration. 95%CI, 95% confidence interval; PSBP, periprocedural systolic blood pressure; RAO, radial artery occlusion.
Anatomic variations of the radial artery
The mean radial diameter was 2.58 mm ± 0.39, and the diameter was larger in men (2.69 mm ± 0.37 vs 2.36 mm ± 0.31; P < .001) and smaller in patients with diabetes (2.53 mm ± 0.38 vs 2.64 mm ± 0.38; P = .003). The mean radial diameter was significantly larger than the mean ulnar diameter (2.58 mm ± 0.39 vs 2.22 mm ± 0.43; P < .001).
Radial anatomical variations affected 63 patients (14.8%). The most common variation was a high origin of the RA, observed in 63.5% of cases (40 patients), followed by radial tortuosity in 28.6% (18 patients), radioulnar loop in 7.9% (5 patients). Anatomical variations were more frequent in women (23% vs 10.8%; P = .001) and in older patients, with a mean age of 66.3 years ± 10.2 vs 61.2 years ± 11.2 in those without variations (P = .001).
Periprocedural complications
Radial spasm occurred in 72 patients (16.9%). This complication was more frequent in women (29% vs 10.1%; P < .001), patients with diabetes (22.5% vs 11.5%; P = .002), and when 6-Fr catheters were used (14% vs 24%; P = .035). Forearm hematoma occurred in 25 patients (5.85%). According to the EASY classification,9 most hematomas were type I (17 patients, 68%), followed by type II (6 patients, 24%), with type III occurring in only 2 patients (8%).
DISCUSSION
The rate of RAO remains relatively high in some institutions.10,11 In the PROPHET study, the acute incidence of RAO (12%) was almost halved in 28 days (7%).3 Recanalization occurs as a result of activation of primary fibrinolysis.12 In the present study, the rate of radial recanalization at 3 months was 32.6%. The only predictor of recanalization was radial diameter: the larger the diameter, the higher the rate of spontaneous recanalization.
Zankl et al.13 found that RAO was located in the distal third of the forearm in 49% of patients, in the distal and middle third in 13.7%, and in the entire forearm (proximal third) in 37.3%. Dissections of the media also occur in the proximal RA, likely due to catheter progression or manipulation without protection of the sheath.14 In our opinion, this would explain the location of RAO in the proximal part of the artery.
Among our patients with RAO, 29.2% had a radial pulse. According to Uhlemann et al.,4 in 19.5% of patients with RAO on Doppler, the RA pulse was still palpable. This was likely due to retrograde filling of the RA by collaterals. Therefore, the diagnosis of RAO should be confirmed using a more objective method, such as Doppler ultrasound.
Young age is a predictor of RAO, possibly due to higher sympathetic reactivity in younger individuals, which increases their risk of spasm. However, this characteristic does not influence the rate of recanalization, likely because prolonged radial spasm leads to the formation of a permanent intra-arterial thrombus.
Low mean systolic blood pressure was also a predictor of radial occlusion. We speculate that hypertension and arterial stiffness may prevent complete interruption of flow during compression, thereby helping to maintain radial patency.15
There was a higher incidence of occlusion with type A Barbeau test. We believe that in cases with well-developed ulnar circulation, the ulnar artery generates a competitive retrograde flow that opposes the radial flow, promoting occlusion and hindering recanalization.
The likelihood of developing RAO is related to the size of the sheath,16 or more precisely, the R/S ratio.17 A prospective registry showed that 5-Fr sheaths reduced the rate of RAO by up to 55% compared with 6-Fr.4
A study by Pancholy et al.,18 demonstrated that intravenous heparin is as effective as intra-arterial heparin in reducing the incidence of RAO, suggesting that the systemic effect of heparin is more important than its local effect. A recently published meta-analysis identified higher heparin doses as the most significant measure for decreasing RAO.12 This results is in line with our finding that a dose of less than 70 IU/kg seems to promote the occurrence of RAO. The high prevalence of RAO and the benefit of higher doses of unfractionated heparin (≥ 50 IU/kg) in this setting were also highlighted by a meta-analysis of 112 studies.19 In a randomized superiority trial comparing high-dose (100 IU/kg) and standard-dose (50 IU/kg) heparin, the RAO rate was significantly lower in the high-dose group.20 Recent evidence suggests that a small dose of rivaroxaban, given orally after a transradial procedure, may decrease the occurrence of RAO at 1 month.21,22
Using the reverse Barbeau test, Sanmartin et al.23 found that 60% of patients had an absence of radial flow during compression. These observations led to the concept of nonocclusive hemostasis (patent hemostasis). In the PROPHET study,3 RAO was significantly less frequent in the group that underwent nonocclusive hemostasis than in the control group.
The duration of hemostatic compression has been studied in large, randomized trials.24-26 The authors concluded that compression duration was a strong predictor of RAO.
In a meta-analysis by Rashid et al.,27 the incidence of RAO after diagnostic coronary angiography was notably higher compared with percutaneous coronary intervention, possibly due to the use of higher anticoagulation doses during interventions.12 However, opposite findings have been reported by other studies.
In our sample, the mean radial diameter was 2.58 mm ± 0.39 and was significantly larger in men. Velasco et al.28 reported a mean arterial diameter of 2.22 ± 0.35 mm, while a Polish study found a mean diameter of 2.17 ± 0.53 mm for the right RA and 2.25 ± 0.43 mm for the left RA.29 The ulnar artery is also used in interventional cardiology,30 although there is no consensus on its size compared with the RA.
Autopsy studies of arterial anatomic variations of the upper extremity have reported frequencies between 4% and 18.5%.8 In the literature, the most frequent anatomic variation of the RA is high bifurcation. Yoo et al.31 reported a 2.4% incidence of high radial origin in 1191 Korean patients. Tortuosity of the RA frequently affects patients with high radial origin, possibly due to the elongated course of the RA predisposing it to tortuosity, which is considered one of the most common causes of procedural failure, along with radial spasm.32
Radioulnar loop is the most common cause of procedural failure with experienced operators.33 Angiographic evaluation of the radioulnar anastomosis is mandatory in such cases, as there is often a negotiable anastomosis between the radial and ulnar arteries.
In our study, radial spasm was the leading cause of procedural failure, occurring in 50% of the 14 patients who experienced such failures. Ruiz-Salmerón et al.34 found that RA anatomic variations were strongly associated with radial spasm in a multivariate analysis. The relationship between radial spasm and anatomic variations is mainly explained by the strong correlation with high radial origin and the radioulnar loop.
Study limitations
Since this study is a prospective registry and not a randomized trial, selection bias cannot be excluded. Our study represents a single-center experience with a limited number of patients, despite being one of the largest prospective registries of vascular ultrasound in radial catheterization to date. Among the other limitations of the study, we note the lack of standardized protocols for both heparin use and compression.
CONCLUSIONS
With the increasing number of transradial procedures and the greater age of patients undergoing these interventions, leading to more complex procedures, it is essential to maintain the patency of the RA for future access. Although predictors of RAO after cardiac catheterization have been identified, implementing preventive measures in practice remains a challenge. The main modifiable predictors associated with the risk of RAO are insufficient heparinization and occlusive hemostasis. Therefore, preventive strategies should primarily focus on addressing these 2 factors.
FUNDING
None.
ETHICAL CONSIDERATIONS
The study was conducted in accordance with the provisions of the Declaration of Helsinki and with the International Conference on Harmonization Good Clinical Practices and was approved by the local ethics committee. All patients included in the study provided written informed consent, which is archived and available. Our study population included both sexes. Gender had no influence on the occurrence of radial occlusion.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
No artificial intelligence software was used in the preparation of this study.
AUTHORS’ CONTRIBUTIONS
All authors meet the criteria for authorship as defined by the International Committee of Medical Journal Editors. M.S. Lounes, A. Meftah, C. Belhadi, K. Allal, H. Boulaam, A. Sayah, I. Hafidi, and E. Tebache contributed to the acquisition and analysis of data for this article. M.S. Lounes, A. Bedjaoui, A. Allali, and S. Benkhedda were responsible for the study design and the writing of the article. M.S. Lounes, A. Allali, and S. Benkhedda contributed to writing and critical revision of the content. All authors have read and approved the final version of the article and agree to be accountable for all aspects of the work, including the accuracy and integrity of all its parts.
CONFLICTS OF INTEREST
None.
WHAT IS KNOWN ABOUT THE TOPIC?
- Despite recommendations on the prevention of RAO in interventional cardiology, its incidence remains relatively high in some centers.
- Spontaneous recanalization of the artery may occur during follow-up.
- Permanent occlusion of the radial artery prevents any possibility of its further use (interventional procedures, dialysis, etc.)
WHAT DOES THIS STUDY ADD?
- RAO is not limited to the distal part of the artery and can affect the entire length of the vessel.
- Diagnosis of RAO should be confirmed using Doppler ultrasound, which remains the gold standard.
- The 2 independent modifiable predictors of RAO are the anticoagulation protocol and hemostasis technique.
- Anatomic variations of the RA may impact the procedure. A high origin of the RA is the most frequent, followed by radial tortuosities. After radial spasm, the radioulnar loop is the most common cause of procedural failure with experienced operators.
REFERENCES
1. Cruden NL, Teh CH, Starkey IR, Newby DE. Reduced vascular complications and length of stay with transradial rescue angioplasty for acute myocardial infarction. Catheter Cardiovasc Interv. 2007;70:670-675.
2. Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87-165.
3. Pancholy SB, Coppola J, Patel T, Roke-Thomas M. Prevention of radial artery occlusion-patent hemostasis evaluation trial (PROPHET study):a randomized comparison of traditional versus patency documented hemostasis after transradial catheterization. Catheter Cardiovasc Interv. 2008;72:335-340.
4. Uhlemann M, Möbius-Winkler S, Mende M, et al. The Leipzig prospective vascular ultrasound registry in radial artery catheterization:impact of sheath size on vascular complications. JACC Cardiovasc Interv.2012;5:36-43.
5. Da Silva RL, Britto PF, Joaquim RM, et al. Clinical accuracy of reverse Barbeau test in the diagnosis of radial artery occlusion after transradial catheterization. J Transcat Intervent. 2021;29:eA20200037.
6. Costa F, van Leeuwen MA, Daemen J, et al. The Rotterdam Radial Access Research:Ultrasound-Based Radial Artery Evaluation for Diagnostic and Therapeutic Coronary Procedures. Circ Cardiovasc Interv. 2016;9:003129.
7. Uglietta JP, Kadir S. Arteriographic study of variant arterial anatomy of the upper extremities. Cardiovasc Intervent Radiol. 1989;12:145-148.
8. Rodríguez-Niedenführ M, Vázquez T, Nearn L, et al. Variations of the arterial pattern in the upper limb revisited:a morphological and statistical study, with a review of the literature. J Anat. 2001;199:547-566.
9. Bertrand OF. Acute forearm muscle swelling post transradial catheterization and compartment syndrome:prevention is better than treatment!Catheter Cardiovasc Interv. 2010;75:366-368.
10. Sadaka MA, Etman W, Ahmed W, et al. Incidence and predictors of radial artery occlusion after transradial coronary catheterization. Egypt Heart J. 2019;71:12.
11. Dousi M, Sotirakou K, Fatsi A. The Use of Acetylsalicylic Acid As A Measure of Prevention of Radial Artery Occlusion in Patients Who Perform Coronary Angiography with Tra Technique. J Radiol Clin Imaging. 2020;3:13-21.
12. Rashid M, Kwok CS, Pancholy S, et al. Radial Artery Occlusion After Transradial Interventions:A Systematic Review and Meta-Analysis. J Am Heart Assoc. 2016;5:002686.
13. Zankl AR, Andrassy M, Volz C, et al. Radial artery thrombosis following transradial coronary angiography:incidence and rationale for treatment of symptomatic patients with low-molecular-weight heparins. Clin Res Cardiol. 2010;99:841-847.
14. Bi XL, Fu XH, Gu XS, et al. Influence of Puncture Site on Radial Artery Occlusion After Transradial Coronary Intervention. Chin Med J (Engl). 2016;129:898-902.
15. Buturak A, Gorgulu S, Norgaz T, et al. The long-term incidence and predictors of radial artery occlusion following a transradial coronary procedure. Cardiol J. 2014;21:350-356.
16. Dahm JB, Vogelgesang D, Hummel A, et al. A randomized trial of 5 vs. 6 French transradial percutaneous coronary interventions. Catheter Cardiovasc Interv. 2002;57:172-176.
17. Saito S, Ikei H, Hosokawa G, Tanaka S. Influence of the ratio between radial artery inner diameter and sheath outer diameter on radial artery flow after transradial coronary intervention. Catheter Cardiovasc Interv.1999;46:173-178.
18. Pancholy SB. Comparison of the effect of intra-arterial versus intravenous heparin on radial artery occlusion after transradial catheterization. Am J Cardiol. 2009;104:1083-1085.
19. Hahalis GN, Aznaouridis K, Tsigkas G, et al. Radial Artery and Ulnar Artery Occlusions Following Coronary Procedures and the Impact of Anticoagulation:ARTEMIS Systematic Review and Meta-Analysis. J Am Heart Assoc. 2017;6:005430.
20. Hahalis GN, Leopoulou M, Tsigkas G, et al. Multicenter Randomized Evaluation of High Versus Standard Heparin Dose on Incident Radial Arterial Occlusion After Transradial Coronary Angiography:The SPIRIT OF ARTEMIS Study. JACC Cardiovasc Interv. 2018;11:2241-2250.
21. Liang D, Lin Q, Zhu Q, et al. Short-Term Postoperative Use of Rivaroxaban to Prevent Radial Artery Occlusion After Transradial Coronary Procedure:The RESTORE Randomized Trial. Circ Cardiovasc Interv. 2022;15:011555.
22. Hammami R, Abid S, Jihen J, et al. Prevention of radial artery occlusion with rivaroxaban after trans-radial access coronary procedures:The RIVARAD multicentric randomized trial. Front Cardiovasc Med. 2023;10:1160459.
23. Sanmartin M, Gomez M, Rumoroso JR, et al. Interruption of blood flow during compression and radial artery occlusion after transradial catheterization. Catheter Cardiovasc Interv. 2007;70:185-189.
24. Politi L, Aprile A, Paganelli C, et al. Randomized clinical trial on short-time compression with Kaolin-filled pad:a new strategy to avoid early bleeding and subacute radial artery occlusion after percutaneous coronary intervention. J Interv Cardiol. 2011;24:65-72.
25. Dharma S, Kedev S, Patel T, et al. A novel approach to reduce radial artery occlusion after transradial catheterization:postprocedural/prehemostasis intra-arterial nitroglycerin. Catheter Cardiovasc Interv.2015;85:818-825.
26. Aminian A, Saito S, Takahashi A, et al. Impact of sheath size and hemostasis time on radial artery patency after transradial coronary angiography and intervention in Japanese and non-Japanese patients:A substudy from RAP and BEAT randomized multicenter trial. Catheter Cardiovasc Interv. 2018;92:844-851.
27. Sinha SK, Jha MJ, Mishra V, et al. Radial Artery Occlusion. Incidence, Predictors and Long-term outcome after TRAnsradial Catheterization:clinico-Doppler ultrasound-based study (RAIL-TRAC study). Acta Cardiol. 2017;72:318-327.
28. Velasco A, Ono C, Nugent K, et al. Ultrasonic evaluation of the radial artery diameter in a local population from Texas. J Invasive Cardiol. 2012;24:339-341.
29. Peruga JP, Peruga JZ, Kasprzak JD, et al. Ultrasound evaluation of forearm arteries in patients undergoing percutaneous coronary intervention via radial artery access:results of one-year follow-up. Kardiologia Polska. 2015;73:502-510.
30. Knebel AV, Cardoso CO, Correa Rodrigues LH, et al. Safety and feasibility of transulnar cardiac catheterization. Tex Heart Inst J. 2008;35:268-272.
31. Yoo BS, Yoon J, Ko JY, et al. Anatomical consideration of the radial artery for transradial coronary procedures:arterial diameter, branching anomaly and vessel tortuosity. Int J Cardiol. 2005;101:421-427.
32. Pristipino C, Roncella A, Trani C, et al. Identifying factors that predict the choice and success rate of radial artery catheterisation in contemporary real world cardiology practice:a sub-analysis of the PREVAIL study data. EuroIntervention. 2010;6:240-246.
33. Louvard Y, Lefèvre T. Loops and transradial approach in coronary diagnosis and intervention. Catheter Cardiovasc Interv. 2000;51:250-252.
34. Ruiz-Salmerón RJ, Mora R, Vélez-Gimón M, et al. Radial artery spasm in transradial cardiac catheterization. Assessment of factors related to its occurrence, and of its consequences during follow-up. Rev Esp Cardiol. 2005;58:504-511.