Article
Ischemic heart disease and acute cardiac care
REC Interv Cardiol. 2019;1:21-25
Access to side branches with a sharply angulated origin: usefulness of a specific wire for chronic occlusions
Acceso a ramas laterales con origen muy angulado: utilidad de una guía específica de oclusión crónica
Servicio de Cardiología, Hospital de Cabueñes, Gijón, Asturias, España
ABSTRACT
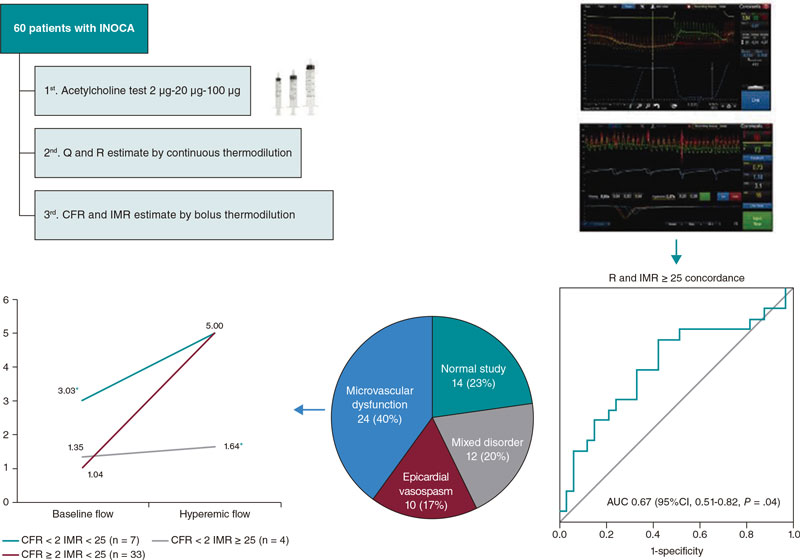
Introduction and objectives: Invasive diagnosis of vasoreactivity and microvascular function may be useful to optimize the management of patients with signs and/or symptoms of myocardial ischemia in the absence of significant coronary stenosis (INOCA). We analyzed the prevalence of the different endotypes, as well as the concordance between 2 diagnostic methods based on thermodilution assessment.
Methods: We prospectively included 60 patients with INOCA who underwent a vasoreactivity test with intracoronary acetylcholine, and measurement of absolute coronary blood flow (Q) and minimum microvascular resistance (R) using continuous thermodilution assessment. Finally, calculations of the coronary flow reserve (CFR) and index of microcirculatory resistance index (IMR) were made using the bolus thermodilution method considering CFR < 2 and MRI ≥ 25 as established pathological cut-off values.
Results: The invasive functional diagnostic procedure allowed patients to be categorized into 4 subgroups: microvascular dysfunction (40%), epicardial vasospasm (17%), mixed disorder (20%), and normal study (23%). No correlation was seen between the Q and the CFR. Using ROC curves, an R > 435 UW was estimated as the optimal cut-off value to identify patients with IMR ≥ 25 with an area under the curve of 0.67 (95%CI, 0.51-0.82; P = .04).
Conclusions: The invasive study of vasoreactivity and microcirculation was feasible and safe. Prevalence of vasospasm and microvascular dysfunction in patients with INOCA was high. The CFR/MRI/Q combined study allowed us to unmask a subtype of microvascular dysfunction characterized by an abnormally high coronary flow at baseline. The concordance seen between the microvascular resistance obtained by continuous thermodilution measurements and the reference method was low so future studies are justified to determine the usefulness of this technique.
Keywords: Microvascular dysfunction. Vasospasm. Acetylcholine. Continuous thermodilution measurements. Microvascular resistance. INOCA.
RESUMEN
Introducción y objetivos: El diagnóstico invasivo de la vasorreactividad y la función microvascular puede resultar de utilidad para optimizar el manejo de los pacientes con signos o síntomas de isquemia miocárdica en ausencia de estenosis coronarias significativas (INOCA). Se analizó la prevalencia de los distintos endotipos y la concordancia entre 2 métodos diagnósticos basados en la termodilución.
Métodos: Se incluyeron de forma prospectiva 60 pacientes con INOCA a quienes se realizó un test de vasorreactividad con acetilcolina intracoronaria, medida del flujo absoluto (Q) y la resistencia microvascular mínima (R) por termodilución continua y, por último, se calcularon la reserva de flujo coronario (RFC) y el índice de resistencia microvascular (IRM) por termodilución con bolos. Se consideraron como patológicos los puntos de corte establecidos de RFC < 2 e IRM ≥ 25.
Resultados: El procedimiento diagnóstico funcional invasivo permitió clasificar a los pacientes en 4 subgrupos: disfunción microvascular (40%), vasoespasmo epicárdico (17%), trastorno mixto (20%) y estudio normal (23%). No se observó correlación entre Q y RFC. Mediante curvas ROC se estimó una R > 435 UW como el punto de corte óptimo para identificar pacientes con IRM ≥ 25, con un área bajo la curva de 0,67 (IC95%, 0,51-0,82; p = 0,04).
Conclusiones: El estudio invasivo de la vasorreactividad y la microcirculación fue factible y seguro. La prevalencia de vasoespasmo y de disfunción microvascular en pacientes con INOCA fue elevada. El análisis conjunto de RFC, IRM y Q permitió desenmascarar un subtipo de disfunción microvascular caracterizado por un flujo coronario basal anormalmente elevado. La concordancia entre la resistencia microvascular obtenida por termodilución continua respecto al método de referencia fue baja, por lo que se requieren futuros estudios para determinar la utilidad de esta técnica.
Palabras clave: Disfunción microvascular. Vasoespasmo. Acetilcolina. Termodilución continua. Resistencia microvascular. INOCA.
Abbreviations
CFR: coronary flow reserve; INOCA: ischemia with nonobstructive coronary artery disease; IMR: index of microcirculatory resistance; Q: absolute coronary blood flow; R: coronary microvascular resistance.
INTRODUCTION
Over the past few years, the term INOCA (ischemia with nonobstructive coronary arteries) has established to define patients with signs or symptoms of ischemic heart disease without angiographically significant obstructive coronary artery disease.1 In these patients, coronary microvascular or epicardial vessel dysfunction could be the pathophysiological mechanism triggering the symptoms and ischemic impairment.2
Currently, the invasive study of microvascular function in patients with INOCA is a recommendation IIa according to the clinical practice guidelines of the European Society of Cardiology.3 What it does is measure the parameters that show its functional or structural status like coronary flow reserve (CFR) or index of microcirculatory resistance (IMR).4
Recently, the possibility of measuring absolute coronary blood flow (Q) and microvascular resistance (R) by continuous thermodilution with the infusion of a physiological saline solution through a specific coronary microcatheter has been described. This technique has potential advantages like its independence from the operator or not needing pharmacologically induced hyperemia.5
The objective of this study is to estimate the prevalence of the different endotypes of patients with INOCA and analyze the correlation between the measurements obtained by continuous thermodilution and the traditional method of intracoronary boluses of physiological saline solutions.
METHODS
This was a prospective and consecutive study of 60 referred patients due to symptoms or signs of myocardial ischemia without angiographically significant coronary artery stenosis on the visual estimate (< 50%) or after functional assessment (resting full-cycle ratio [RFR] > 0.89 or fractional flow reserve [FFR] > 0.80). Severe valvular heart disease, acute coronary syndrome, decompensated heart failure, and any clinical or anatomical condition where the study of microcirculation and vasoreactivity would be considered unnecessary were excluded.
All microcirculation and vasoreactivity studies were scheduled and second-staged. Nitrates and calcium antagonists were withdrawn prior to conducting the tests.
The coronary angiography was performed based on the routine clinical practice via radial access. A spasmolytic cocktail of 200 µg of nitroglycerin was administered. The target artery was the left main coronary artery.
The study was approved by the center ethics research committee and the patients’ written informed consent was obtained.
Vasoreactivity test
First, the vasoreactivity test was performed. Patient monitoring included precordial leads, and baseline angiograms were performed using 2 different projections. The sequential administration of acetylcholine was followed by increasing doses of 2 µg, 20 µg, and 100 µg in intracoronary bolus for 2 min. In the presence of significant bradycardia, the injection was interrupted, and if considered appropriate, it was re-administered at a slower rate. A follow-up angiogram was performed after every dose. In the presence of severe symptoms, changes to the echocardiogram or epicardial spasm 200 µg of intracoronary nitroglycerin were administered.
The test was considered positive based on the criteria established by the COVADIS (Coronary vasomotor disorders international study) group: epicardial spasm in the presence of chest pain, changes to the echocardiogram, and constriction ≥ 90%, and microvascular spasm in the presence of chest pain, and changes to the echocardiogram without epicardial spasm ≥ 90%.6
Indices obtained with continuous thermodilution
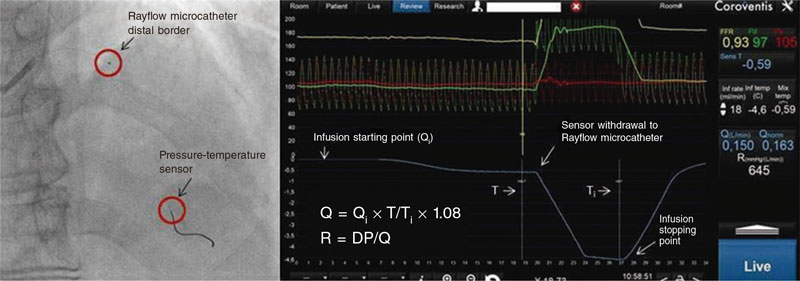
After the administration of unfractionated heparin (70 IU/kg), a pressure-temperature sensor guidewire Pressure Wire X (Abbott, United States) was inserted and pressures at the catheter distal border were equalized. The guidewire was advanced until it reached the left anterior descending coronary artery distal segment.
Resting full-cycle ratio was registered to confirm the lack of hemodynamically significant epicardial stenoses (RFR > 0.89).
Afterwards, a specific Rayflow (Hexacath, France) microcatheter for intracoronary infusion was placed in the left anterior descending coronary artery proximal segment. After confirmation that the guidewire sensor was, at least, 3 cm distal to the tip of the microcatheter, the intracoronary infusion of a physiological saline solution at room temperature and at a dose of 20 mL/min was started using an injector pump to induce hyperemia.
Pressure-temperatures curves were registered using Coroventis software (Abbott, United States). When the distal temperature drop was stabilized, the sensor was withdrawn up to the tip of the microcatheter to determine the infusion temperature.
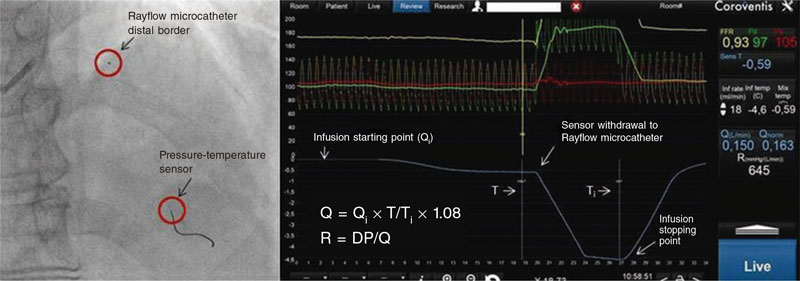
Afterwards, the injection of the physiological saline solution stopped, and Q (L/min) and R (Wood units) values were obtained automatically (figure 1).
Figure 1. Measurements obtained by continuous thermodilution: DP, distal pressure; Q, absolute coronary blood flow; Qi, infusion flow (mL/min); R, microvascular resistance; T, distal temperature; Ti, infusion temperature.
Indices obtained with bolus thermodilution of a physiological saline solution
After completion of the continuous thermodilution study, and once the Rayflow microcatheter was removed, the pressure-temperature guidewire was repositioned in its previous location, and thermodilution curves were registered using the Coroventis software after the vigorous manual injection of 3 intracoronary boluses of 3 mL of a physiological saline solution. Measurements were taken at rest and after inducing hyperemia with a peripheral intravenous bolus of regadenoson (400 µg) resulting in the calculation of CFR and IMR.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation or median [interquartile range]. The categorical ones were expressed as absolute value or percentage. ROC (Receiver operating characteristic) curves were used to estimate the optimal cut-off values for the continuous variables Q and R. The cut-off values established as pathological for CFR < 2 and IMR ≥ 25 were used as the reference framework. Once dichotomized, the variables Q and R were compared to the CFR and IMR values using chi-square tests. One-way ANOVA was used to compare the different quantitative variables. The statistical analysis was performed using the SPSS v 20 statistical software package (IBM, United States).P values < .05 were considered statistically significant.
RESULTS
Study patients
Table 1 shows the baseline characteristics of the 60 patients included in the study. Women (55%) were predominant. Also, there was a high prevalence of cardiovascular risk factors. Most showed typical angina-like clinical signs (76%) and had tested positive to an ischemia test performed before the coronary angiography (60%).
Table 1. Clinical and angiographic characteristics (N = 60)
| Age (years) | 63 ± 10 |
| Women | 33 (55%) |
| Hypertension | 39 (65%) |
| Diabetes | 21 (35%) |
| Dyslipidemia | 35 (58%) |
| Smoking (current or past) | 28 (47%) |
| Previous percutaneous revascularization | 4 (7%) |
| Previous myocardial infarction | 3 (5%) |
| Left ventricular systolic dysfunction | 4 (7%) |
| Ejection fraction (%) | 63 ± 8 |
| Clinical presentation | |
| Exertional angina | 19 (32%) |
| Resting angina | 13 (22%) |
| Mixed angina | 14 (23%) |
| Other | 14 (24%) |
| Ischemia test | |
| Ergometry | 19 (32%) |
| Isotopic scintigraphy | 18 (30%) |
| Dobutamine stress echocardiography | 3 (5%) |
| None | 20 (33%) |
| Coronary angiography | |
| Atheromatous disease | 22 (37%) |
| Slow flow | 13 (22%) |
|
Data are expressed as no. (%) or mean ± standard deviation. |
|
The baseline coronary angiography confirmed that 37% of the patients showed parietal irregularities consistent with atheromatous disease, and 22% had slow coronary flow. The FFR and RFR values were normal in all the cases studied.
Coronary vasoreactivity
As shown on table 2, 60% of the cases (36/60) had a positive response to acetylcholine in the vasoreactivity test. A total of 32% of the cases (19/60) showed severe epicardial vasoconstriction, and 23% (14/60) met the criteria for microvascular spasm. In 3 patients (5%), microvascular spasm was observed concomitantly with the medium dose (20 µg), and epicardial spasm with the high dose (100 µg), which added to the impaired indices of microvascular function was consistent with a mixed endotype.
| Pathological vasoreactivity testing | 36 (60%) |
| Epicardial vasospasm | 19 (32%) |
| Microvascular vasospasm | 14 (23%) |
| Combined vasospasm | 3 (5%) |
| Structural microvascular dysfunction (IMR ≥ 25) | 20 (33%) |
| Isolated | 5 (8%) |
| Associated with epicardial spasm | 8 (13%) |
| Associated with microvascular spasm | 4 (7%) |
| Associated with combined spasm | 3 (5%) |
| CFR < 2 | 11 (18%) |
| CFR < 2.5 | 17 (28%) |
| RFR | 0.93 [0.91-0.94] |
| FFR | 0.90 [0.87-0.93] |
| Q (mL/min) | 170 ([138-219] |
| R (WU) | 496 [381-654] |
| CFR | 3.0 [2.3-4.2] |
| IMR | 20 [12-28] |
|
Data are expressed as no. (%) or median [interquartile range]. |
|
Indices of microvascular function
Both studies—bolus thermodilution and continuous infusion thermodilution—were performed uneventfully in all of the patients. Table 2 shows the values of the measurements of microvascular function obtained with both techniques.
In the continuous infusion study, a median of absolute flow in the left anterior descending coronary artery of 170 mL/min [138-219 mL/min] was described while the median of microvascular resistance was 496 WU [381-654 WU].
A total of 18% of the patients (11/60) had a reduced CFR (CFR < 2) while 33% (20/60) showed elevated resistances (IMR ≥ 25).
The group of patients with microvascular dysfunction due to low CFR with normal IMR (7/60, 12%) with respect to cases with microvascular dysfunction due to high IMR with normal CFR (16/60, 27%) had a clinical profile with a lower mean age (61 ± 11 vs 66 ± 8), and a higher predominance of women (86% vs 58%) although this tendency was not statistically significant.
Table 3 shows the mean transit times (MTT) of bolus thermodilution tests. The cases with low CFR showed significantly shorter baseline MTT (0.48 ± 0.45 vs 1.13 ± 0.70), especially the subgroup of patients with low CFR and high Q (0.31 ± 0.15 vs 0.77 ± 0.68).
Table 3. Mean transit times obtained by bolus thermodilution
| Overall (N = 60) |
CFR < 2 (N = 11) |
CFR < 2 Q > 170 (N = 7) |
CFR < 2 Q < 170 (N = 4) |
|
|---|---|---|---|---|
| Baseline MTT | 1.13 ± 0.70 | 0.48 ± 0.45* | 0.31 ± 0.15* | 0.77 ± 0.68 |
| Hyperemic MTT | 0.36 ± 0.25 | 0.35 ± 0.28 | 0.25 ± 0.14 | 0.51 ± 0.41 |
|
Values (in seconds) are expressed as mean ± standard deviation. |
||||
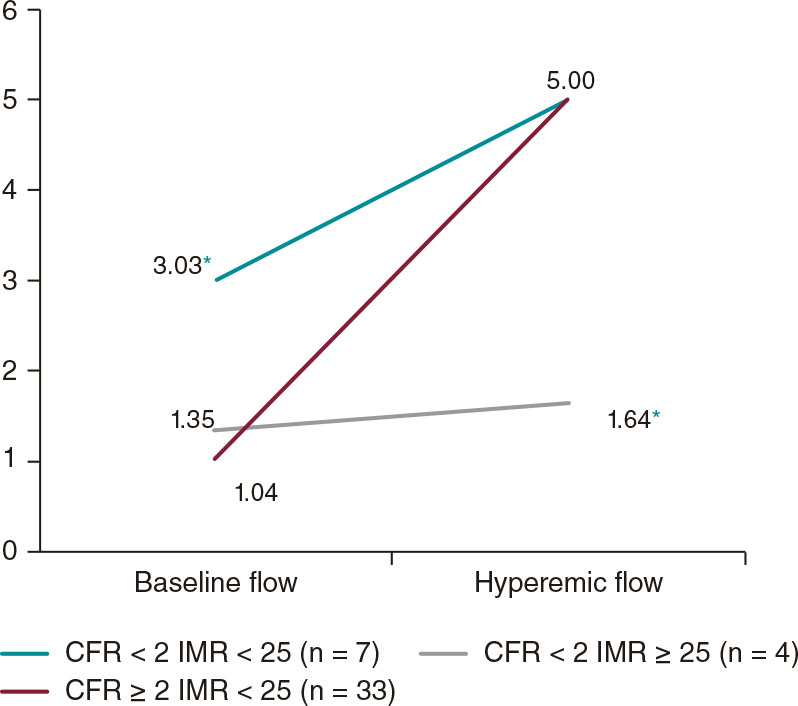
Figure 2 shows data of coronary flow estimated by MTT measurement divided into 3 groups based on CFR and IMR results. We should mention that patients with low CFR without elevated resistances had significantly high resting flows and hyperemic flows without significant differences compared to the rest while in patients with low CFR and elevated resistances, the opposite phenomenon was described.
Figure 2. Baseline and hyperemic mean flow estimated based on the MTT (1/MTT) and grouped based on the CFR and IMR results. Values are expressed as s–1. CFR, coronary flow reserve; IMR, index of microvascular resistance; MTT, mean transit time.
* P < .05.
Endotypes
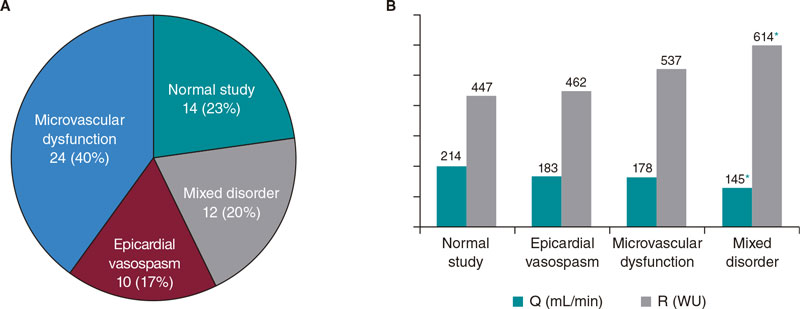
Figure 3A shows the percentages of endotypes based on the result of the acetylcholine test and the measurements of CFR and IMR. The most common pattern was microvascular dysfunction (24/60, 40%) followed by the normal study (14/60, 23%). In 20% of the patients (12/60), microvascular dysfunction overlapped with epicardial vasospasm while in 17% of the patients (10/60) isolated epicardial vasospasms were seen.
Figure 3. A: endotype-based classification. Values are expressed as absolute number and percentage.B: mean values of absolute flow and microvascular resistance grouped by endotypes. Q, absolute coronary blood flow; R, microvascular resistance.
* P < .05 with respect to normal study.
Table 4 shows how the mechanisms of vasomotor and microvascular dysfunction overlap in many cases.
Table 4.Results of the acetylcholine test and bolus thermodilution study (N = 60)
| Epicardial spasm | Microvascular spasm | IMR ≥ 25 | CFR < 2 | Endotype | Cases |
|---|---|---|---|---|---|
| − | − | − | − | Normal | 14 (23.3%) |
| + | − | − | − | Epicardial vasospasm | 10 (16.7%) |
| − | + | − | − | Microvascular dysfunction | 9 (15.0%) |
| − | − | + | − | Microvascular dysfunction | 5 (8.3%) |
| − | − | − | + | Microvascular dysfunction | 5 (8.3%) |
| − | + | + | − | Microvascular dysfunction | 3 (5.0%) |
| − | + | − | + | Microvascular dysfunction | 1 (1.6%) |
| − | + | + | + | Microvascular dysfunction | 1 (1.6%) |
| + | − | + | − | Mixed disorder | 6 (10.0%) |
| + | + | + | − | Mixed disorder | 2 (3.3%) |
| + | − | + | + | Mixed disorder | 2 (3.3%) |
| + | − | − | + | Mixed disorder | 1 (1.6%) |
| + | + | + | + | Mixed disorder | 1 (1.6%) |
|
Data are expressed as no. (%) |
|||||
The association between epicardial vasospasm and structural microvascular dysfunction (IMR ≥ 25) was the most prevalent combination in cases of mixed disorder (11/12). In turn, this endotype, in continuous thermodilution measurements, showed significant differences compared to the normal pattern, with reduced absolute flow values and elevated resistances (figure 3B) indicative of more serious structural and functional damage.
Concordance among the different indices of microvascular function
The ROC curve analysis of absolute coronary blood flow (Q) with respect to CFR < 2 determined an optimal cut-off value of 170 mL/min (a 64% sensitivity, and a 52% specificity) with an area underthe curve of 0.50 (95% confidence interval [95%CI], 0.33-0.66;P = .97), therefore showing no diagnostic utility.
Given the recent proposal to consider the cut-off value of CFR < 2.57,7 the analysis was performed using this threshold as the reference. In addition, no significant concordance was seen (area under the curve of 0.45 [95%CI, 0.30-0.61;P = .56]).
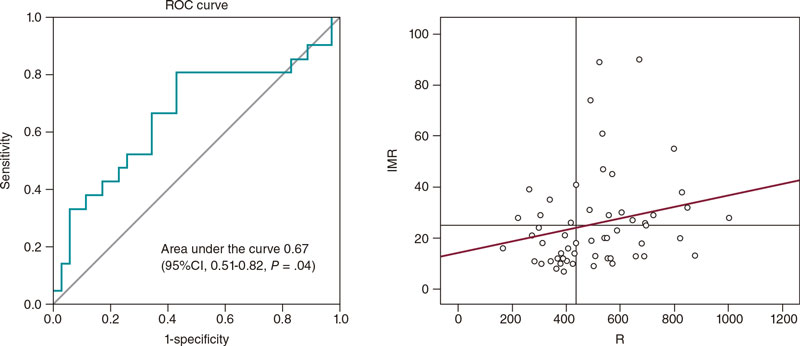
Regarding R with respect to IMR, an area under the curve of 0.67 (95%CI, 0.51-0.82;P = .04) was obtained, which was indicative of a weak yet significant diagnostic concordance (figure 4). The estimated optimal cut-off value was 435 WU, which was consistent with an 81% sensitivity and a 57% specificity. A total of 66% of cases with IMR ≥ 25 were categorized correctly using this index.
Figure 4. Analysis of the R cut-off value > 435 WU to predict IMR ≥ 25, and scatter plot showing the correlation between IMR and R. IMR, index of microvascular resistance; R, microvascular resistance.
The absence of an association between Q and CFR was confirmed in correlation tests (Spearman’s rho correlation coefficient= -0.02; 95%CI, -0.24-0.25;P = .99). However, a weak yet significant correlation was seen between Q and hyperemic MTT (Spearman’s rho= -0.28; 95%CI, -0.01-0.51;P = .04), and between R and IMR (Spearman’s rho= 0.28; 95%CI, 0.04-0.51;P = .03).
Complications
While the vasoreactivity test was being performed, 3 cases of transient bradycardia (5%) without clinical repercussions and 2 episodes of atrial fibrillation (4%) were reported, 1 of them self-limited while the other required sedation and electrical cardioversion. After the administration of regadenoson, most patients experienced some degree of discomfort, which was well-tolerated and reversed with the administration of 100 mg of intravenous theophylline. No other complications or adverse effects were reported.
DISCUSSION
This study confirms that a high percentage of patients with symptoms or signs of INOCA show microvascular dysfunction or vasospasm in invasive functional testing, and that it is feasible and safe to perform (figure 5).
Figure 5. Study design, endotype-based classification, and analysis using the ROC curve. AUC, area under the curve; CFR, coronary flow reserve; IMR, index of microvascular resistance; INOCA, ischemia with nonobstructive coronary artery disease; Q, absolute coronary blood flow; R, microvascular resistance.
* P < .05.
The percentage of patients with microcirculation or vasomotility alterations found in our study (77%) is consistent with former studies of patients with angina without obstructive coronary artery disease (64% to 89%8-11).
Vasoreactivity test
Some groups systematically use a dose of 200 µg of intracoronary acetylcholine to perform the vasoreactivity test; in our study, the high dose was established at 100 µg according to the COVADIS group, the CorMicA protocol, and the technical document of the Spanish Society of Cardiology Working Group on Cardiac Catheterization and Interventional Cardiology, which highlights its high sensitivity and specificity rates (90% and 99%, respectively).12 As a matter of fact, the high prevalence of positive results seen in our study in the acetylcholine test (60%) is similar to that reported in other series (57% to 71%13-15). In a recent study of 110 patients, Feenstra et al.11 revealed that 62% of the patients had a pathological acetylcholine test that confirmed the presence of epicardial vasospasm and microvascular spasm (36% and 26%, respectively).
In our study, the complications associated with the vasoreactivity test in our study are not very many: 2 cases of atrial fibrillation (4%), which is consistent with the incidence rate reported by the CorMIcA trial (5%).9
Prevalence of endotypes
The most common endotype in our patients was isolated microvascular dysfunction (40%), but not as much as in the CorMicA trial (52%). These differences could be explained by the discrepancy seen in the percentage of completely normal angiographies (22% in the CorMicA vs 63% in our study) due to the possible association between non-obstructive atheromatous disease and microvascular dysfunction.16,17
The prevalence of the remaining endotypes is similar to that reported in the CorMicA trial: isolated epicardial vasospasm (17% vs 17%), and mixed disorder (20% vs 21%). A recent meta-analysis that included 14 427 patients with INOCA also shows similar percentages.18
Indices of microvascular function obtained through bolus thermodilution
The analysis of the MTT obtained with this technique (figure 2), a parameter that correlates inversely with the direct measurement of coronary flow,19 reveals an interesting finding that is consistent with the data published by Nardone et al.20: patients with low CFR have 2 differentiated phenotypes based on the IMR. On the one hand, cases with reduced CFR and elevated resistances have normal baseline flow and low hyperemic flow, which would be indicative of an insufficient vasodilation response. However, in patients with normal resistances, a reduced CFR would be indicative of an abnormally elevated resting flow with hyperemic flow in the normal range. This phenomenon can also be observed in the analysis of patients with high Q (table 3) in whom a reduced CFR can be attributed to elevated baseline flow instead of an insufficient hyperemic response.
Therefore, this subgroup probably shows inefficient or dysregulated baseline myocardial flows. This characteristic, of indeterminate cause, could have important therapeutic implications like a lack of response to vasodilator drugs.
Indices of microvascular function obtained by continuous thermodilution
The continuous thermodilution technique has evolved to the point of quantifying Q and R with a microcatheter and specific software in a simple and precise fashion. The main advantages of this method are its independence from an operator, reproducibility, and induction of hyperemia with a physiological saline solution without the need for pharmacological agents.21-24 However, its main limitation is the lack of normal reference values.
In our study, the lack of a correlation between Q and CFR could be justified by the variations described of baseline myocardial flow. Estimating the CFR requires estimating the baseline coronary flow while Q is a measurement that is representative of hyperemic flow.
The weak concordance seen in this study between Q and hyperemic MTT and between R and IMR shows how difficult it is to establish valid cut-off values for patient comparison with these indices.
With an optimal cut-off value of R in our study of 435 WU (an 81% sensitivity, and a 57% specificity), a total of 66% of cases with IMR ≥ 25 were properly categorized with this index. This value is somewhat lower compared to the one shown by Rivero et al.,25 who analyzed 120 patients and found that an R > 500 WU properly categorized 80% of the cases with IMR ≥ 25.Konst et al.26 studied 84 patients with INOCA using both thermodilution techniques only to find no correlation between the Q-R combo and IMR.
The differences seen may be explained by the fact that the quantitative variability of Q and R values among individuals mostly depends on myocardial mass. However, in positron emission tomography studies, considerable ranges were seen even after adjusting for flow and resistance values for myocardial mass. Therefore, it has been speculated that the most plausible hypothesis is the natural variation of hyperemic myocardial perfusion among individuals.27
Therefore, indices like CFR estimated by continuous thermodilution and microvascular resistance reserve are currently in the pipeline. They correlate the absolute values of flow and resistance seen during hyperemia with those obtained at rest. Nonetheless,these new parameters will still need validation in futurestudies.28,29
Limitations
The data presented here should be interpreted while understanding that this is an observational, single-center study with a small sample size. Therefore, results may be biased by confounding factors associated with a study of this nature.
The left anterior anterior descending coronary artery was considered as the pre-specified target vessel. However, in the routine clinical practice, it may be appropriate to assess other arteries in the presence of negative tests and high clinical suspicion.1
The optimal sequence in invasive functional studies has not been established yet.1 In our case, we chose to perform the acetylcholine test first to minimize the instrumentation of the artery and avoid further guidewire-induced vasoreactivity. However, the spasm and symptoms seen during the provocation test, although transient, could interfere with subsequent measurements of microvascular function. The possibility of determining CFR by continuous thermodilution was established at the beginning of our study, and it was assumed that a comparison of the CFRs obtained with both techniques would have been more appropriate.
In most bolus thermodilution studies, intravenous adenosine is used to induce hyperemia. However, we chose regadenoson because it is easy to use, following our previous experience, and because evidence says it is equivalent to adenosine.30,31
Finally, we should not overlook that this is an invasive study so potential risks associated with the examination should be weighed in. To this date, however, conducting this study has not impacted prognosis.
CONCLUSIONS
The invasive study of coronary vasoreactivity and microcirculation is feasible and safe. These studies allow us to easily recognize different endotypes of patients with INOCA and help us optimize their treatment.
The analysis of CFR, IMR, and Q combined can unmask a subtype of microvascular dysfunction characterized by an abnormally high baseline coronary flow.
The new indices obtained by continuous thermodilution show low concordance with respect to the reference indices. Therefore, future studies will be required to determine the utility of this technique.
FUNDING
None whatsoever.
AUTHORS’ CONTRIBUTIONS
All the authors contributed substantially to the study idea, design, and data mining process. In addition, all approved the manuscript final version for publication.
CONFLICTS OF INTEREST
None reported.
WHAT IS KNOWN ABOUT THE TOPIC?
- The invasive diagnosis of microvascular dysfunction and coronary vasospasm have proven useful to improve the quality of life of patients without obstructive coronary artery disease on the coronary angiography.
- Indices of microvascular dysfunction obtained by continuous thermodilution offer potential advantages since are they are independent from the operator, reproducible, and do not require pharmacologically induced hyperemia.
WHAT DOES THIS STUDY ADD?
- Invasive coronary functional diagnosis is feasible and safe and highlights the high prevalence of microcirculation and vasomotility alterations in patients without obstructive coronary artery disease.
- The combined analysis of the different indices may be useful to characterize cases with decreased CFR.
- Future studies are needed to establish the utility of microvascular function measurements obtained by continuous thermodilution.
REFERENCES
1. Kunadian V, Chieffo A, Camici PG, et al. An EAPCI Expert Consensus Document on Ischaemia with Non-Obstructive Coronary Arteries in Collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. Eur Heart J. 2020;41:3504-3520.
2. Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007;356:830-840.
3. Knuuti J, Wijns W, Saraste A, et al. ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407-477.
4. Ford TJ, Ong P, Sechtem U, et al. COVADIS Study Group. Assessment of Vascular Dysfunction in Patients Without Obstructive Coronary Artery Disease: Why, How, and When. JACC Cardiovasc Interv. 2020;13:1847-1864.
5. Xaplanteris P, Fournier S, Keulards DCJ, et al. Catheter-Based Measurements of Absolute Coronary Blood Flow and Microvascular Resistance: Feasibility, Safety, and Reproducibility in Humans. Circ Cardiovasc Interv. 2018;11:e006194.
6. Beltrame JF, Crea F, Kaski JC, et al., On Behalf of the Coronary Vasomotion Disorders International Study Group (COVADIS). International standardization of diagnostic criteria for vasospastic angina. Eur Heart J. 2015;38:
2565-2568.
7. Demir OM, Boerhout CKM, de Waard GA, et al. Comparison of Doppler Flow Velocity and Thermodilution Derived Indexes of Coronary Physiology. JACC Cardiovasc Interv. 2022;15:1060-1070.
8. Suda A, Takahashi J, Hao K, et al. Coronary functional abnormalities in patients with angina and nonobstructive coronary artery disease. J Am Coll Cardiol. 2019;74:2350-2360.
9. Ford TJ, Stanley B, Good R, et al. Stratified medical therapy using invasive coronary function testing in angina: the CorMicAtrial. J Am Coll Cardiol. 2018;72:2841-2855.
10. Sara JD, Widmer RJ, Matsuzawa Y, Lennon RJ, Lerman LO, Lerman A. Prevalence of Coronary Microvascular Dysfunction Among Patients With Chest Pain and Nonobstructive Coronary Artery Disease. JACC Cardiovasc Interv. 2015;8:1445-1453.
11. Feenstra RGT, Boerhout CKM, Woudstra J, et al. Presence of Coronary Endothelial Dysfunction, Coronary Vasospasm, and Adenosine-Mediated Vasodilatory Disorders in Patients With Ischemia and Non obstructive Coronary Arteries. Circ Cardiovasc Interv. 2022;15:e012017.
12. Gutiérrez E, Gómez-Lara J, Escaned J, et al. Valoración de la función endotelial y provocación de vasoespasmo coronario mediante infusión intracoronaria de acetilcolina. Documento técnico de la ACI-SEC. REC Interv Cardiol. 2021;3:286-296.
13. Aziz A, Hansen HS, Sechtem U, Prescott E, Ong P. Sex-Related Differences in Vasomotor Function in Patients With Angina and Unobstructed Coronary Arteries. J Am Coll Cardiol. 2017;70:2349-2358.
14. Seitz A, Gardezy J, Pirozzolo G, et al. Long-Term Follow-Up in Patients With Stable Angina and Unobstructed Coronary Arteries Undergoing Intracoronary Acetylcholine Testing. J Am Coll Cardiol Interv. 2020;13:1865-1876.
15. Ong P, Athanasiadis A, Borgulya G, et al. Clinical usefulness, angiographic characteristics, and safety evaluation of intracoronary acetylcholine provocation testing among 921 consecutive white patients with unobstructed coronary arteries. Circulation. 2014;129:1723-1730.
16. Melikian N, Vercauteren S, Fearon WF, et al. Quantitative assessment of coronary microvascular function in patients with and without epicardial atherosclerosis. EuroIntervention. 2010;5:939-945.
17. Sharaf B, Wood T, Shaw L, et al. Adverse outcomes among women presenting with signs and symptoms of ischemia and no obstructive coronary artery disease: findings from the National Heart, Lung, and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation (WISE) angiographic corelaboratory. Am Heart J. 2013;166:134-141.
18. Mileva N, Nagumo S, Mizukami T, et al. Prevalence of Coronary Microvascular Disease and Coronary Vasospasm in Patients With Nonobstructive Coronary Artery Disease: Systematic Review and Meta-Analysis. J Am Heart Assoc. 2022;11:e023207.
19. De Bruyne B, Pijls NH, Smith L, et al. Coronary thermodilution to assess flow reserve: experimental validation. Circulation. 2001;104:2003-2006.
20. Nardone M, McCarthy M, Ardern CI, et al. Concurrently Low Coronary Flow Reserve and Low Index of Microvascular Resistance Are Associated With Elevated Resting Coronary Flow in Patients With Chest Pain and Nonobstructive Coronary Arteries. Circ Cardiovasc Interv. 2022;15:e011323.
21. Van’t Veer M, Adjedj J, Wijnbergen I, et al. Novel monorail infusion catheter for volumetric coronary blood flow measurement in humans: invitro validation. EuroIntervention. 2016;12:701-707.
22. Rivero F, Bastante T, Cuesta J, García-Guimaraes M, Maruri-Sánchez R, Alfonso F. Volumetric Quantification of Coronary Flow by Using a Monorail Infusion Catheter: Initial Experience. Rev Esp Cardiol. 2018;71:1082-1084.
23. Everaars H, de Waard GA, Schumacher SP, et al. Continuous thermodilution to assess absolute flow and microvascular resistance: validation in humans using [15O] H2O positron emission tomography. Eur Heart J. 2019;40:2350-2359.
24. Keulards DCJ, Van’t Veer M, Zelis JM, et al. Safety of absolute coronary flow and microvascular resistance measurements by thermodilution. EuroIntervention. 2021;17:229-232.
25. Rivero F, Gutiérrez-Barrios A, Gomez-Lara J, et al. Coronary microvascular dysfunction assessed by continuous intracoronary thermodilution: A comparative study with index of microvascular resistance. Int J Cardiol. 2021;333:1-7.
26. Konst RE, Elias-Smale SE, Pellegrini D, et al. Absolute Coronary Blood Flow Measured by Continuous Thermodilution in Patients With Ischemia and Nonobstructive Disease. J Am Coll Cardiol. 2021;77:728-741.
27. Fournier S, Keulards DCJ, van’t Veer M, et al. Normal values of thermodilution-derived absolute coronary blood flow and microvascular resistance in humans. EuroIntervention. 2021;17:e309-e316.
28. Gutiérrez-Barrios A, Izaga-Torralba E, Rivero Crespo F, et al. Continuous Thermodilution Method to Assess Coronary Flow Reserve. Am J Cardiol. 2021;141:31-37.
29. De Bruyne B, Pijls NHJ, Gallinoro E, et al. Microvascular Resistance Reserve for Assessment of Coronary Microvascular Function: JACC Technology Corner. J Am Coll Cardiol. 2021;78:1541-1549.
30. Federico P, Martínez L, Castelló T, Pomar F, Peris E. Regadenoson intravenoso frente a adenosina intracoronaria para la medida de la reserva fraccional de flujo. REC Interv Cardiol. 2019;1:77-82.
31. Gill GS, Gadre A, Kanmanthareddy A. Comparative efficacy and safety of adenosine and regadenoson for assessment of fractional flow reserve: A systematic review and meta-analysis. World J Cardiol. 2022;14:
319-328.
ABSTRACT
Introduction and objectives: Coronary calcification is one of the leading factors that affect negatively the safety and effectiveness of percutaneous coronary intervention. Several calcium modification techniques exist. However, there is a lack of randomized evidence on the therapy of choice in this scenario.
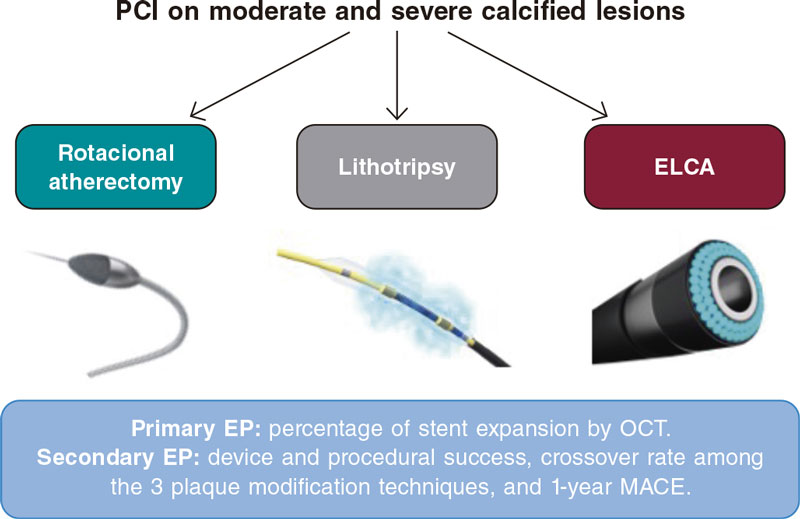
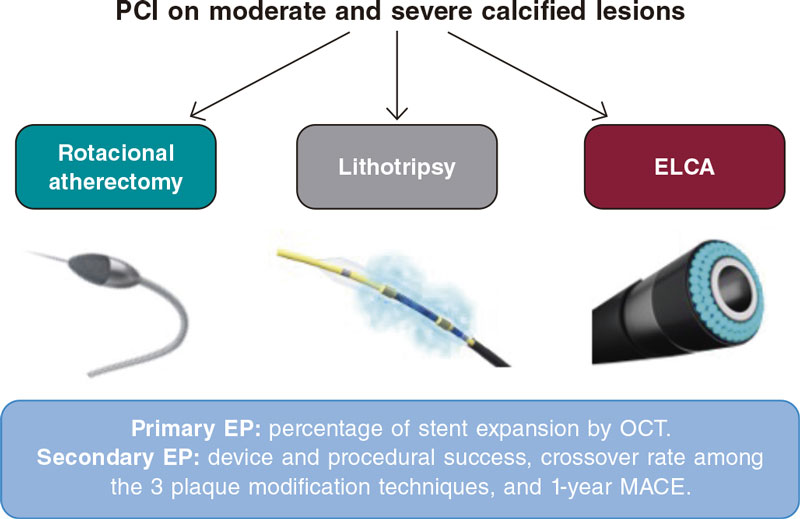
Methods: The ROLLERCOASTR is a prospective, multicenter, randomized clinical trial designed to compare the safety and efficacy profile of 3 plaque modification techniques in the moderate-to-severe coronary calcification setting: rotational atherectomy (RA), excimer laser coronary angioplasty (ELCA), and intravascular lithotripsy (IVL). The study primary endpoint is stent expansion evaluated by optical coherence tomography. An intention-to-treat analysis will be conducted with an alpha coefficient of 0.05 between the reference group (RA) and the remaining 2 groups (ELCA and IVL). An analysis of the study primary endpoint per protocol will be conducted for consistency purposes. If the non-inferiority hypothesis is confirmed, a superiority 2-sided analysis will be conducted. Both the clinical events committee and the independent core laboratory will be blinded to the treatment arm. Assuming an α error of 0.05, an β error of 0.2 (80% power), a margin of irrelevance (ε) of 7, and losses of 10% due to measurement difficulty or impossibility to complete the intervention, we estimate a sample size of 56 cases per group. The study secondary endpoints are device success, procedural success, crossover rate among the different techniques used, and the occurrence of major adverse cardiovascular events at 1-year follow-up.
Conclusions: The ROLLERCOASTR trial will evaluate and compare the safety and effectiveness of 3 plaque modification techniques: RA, ELCA, and IVL in patients with calcified coronary stenosis. This trial was registered at clinicaltrials.gov with identifier NCT04181268.
Keywords: Percutaneous coronary intervention. Calcified plaques. Laser. Lithotripsy. Rotational atherectomy. Optical coherence tomography.
RESUMEN
Introducción y objetivos: La calcificación coronaria es uno de los principales factores que inciden negativamente en la seguridad y la eficacia del intervencionismo coronario percutáneo. Existen varias técnicas de modificación del calcio, pero falta evidencia de estudios aleatorizados sobre la terapia de elección en este escenario.
Métodos: El ROLLERCOASTR es un estudio prospectivo, multicéntrico y aleatorizado, diseñado para comparar la seguridad y la eficacia de 3 técnicas de modificación de la placa en el contexto de calcificación coronaria moderada o grave: aterectomía rotacional (AR), aterectomía coronaria con láser láser excimer (ACLE) y litotricia intracoronaria (LIC). El objetivo primario es la expansión del stent evaluada mediante tomografía de coherencia óptica. Su análisis se hará por intención de tratar, con un α de 0,05 entre el grupo de referencia (AR) y cada uno de los otros grupos (ACLE y LIC). Se realizará también un análisis del objetivo primario por protocolo para mantener la coherencia. Si se confirma la hipótesis de no inferioridad, se realizará un análisis bilateral de superioridad. El comité de eventos clínicos y el laboratorio central independiente no conocerán la rama de tratamiento. Asumiendo un error α de 0,05, un error β de 0,2 (80% de potencia), un margen de irrelevancia (ε) del 7% y un 10% de pérdidas por dificultad de medición o imposibilidad de completar la intervención, se estima un tamaño de muestra de 56 casos en cada grupo. Los objetivos secundarios son el éxito del dispositivo, el éxito del procedimiento, la tasa de cruce entre técnicas y la presentación de eventos cardiovasculares adversos importantes al año de seguimiento.
Conclusiones: El estudio ROLLERCOASTR evaluará y comparará la seguridad y la eficacia, en pacientes con estenosis coronaria calcificada, de 3 técnicas de modificación de placa: AR, ACLE y LIC. Este ensayo se ha registrado en Clinicaltrials.gov: NCT04181268.
Palabras clave: Intervencionismo coronario percutáneo. Placas calcificadas. Láser. Litotricia. Aterectomía rotacional. Tomografía de coherencia óptica.
Abbreviations
DES: drug-eluting stent. ELCA: excimer laser coronary angioplasty. IVL: intravascular lithotripsy. OCT: optical coherence tomography. PCI: percutaneous coronary intervention. RA: rotational atherectomy.
INTRODUCTION
Percutaneous coronary intervention (PCI) with drug-eluting stent (DES) implantation is the most frequent mode of coronary revascularization.
Calcified coronary lesions pose a challenge to perform successful PCI.1 Coronary calcification impedes PCI by multiple mechanisms like limiting DES lesion crossing, altering the drug elution kinetics, and interfering with optimal stent expansion. In addition, inadequate stent expansion is a powerful predictor of stent thrombosis and restenosis.2-6 Coronary calcification also increases PCI-related procedural complications (dissection, perforation, myocardial infarction), and late adverse clinical outcomes like restenosis, repeat revascularization, stent fracture, and thrombosis.1 The optimal approach for the management of calcified stenosis requires taking into account the characteristics of the lesion, calcium distribution, and the mechanism of action of every plaque-modification device. In this regard, intracoronary imaging techniques such as intravascular ultrasound and optical coherence tomography (OCT) are essential not only to evaluate the severity of calcification and its pattern, but also to optimize stenting.7
Currently, plaque-modification techniques can be categorized into a) balloon-based technologies (cutting/scoring balloons, non-compliant and super high-pressure balloons, and intravascular lithotripsy (IVL), and b) non-balloon-based technologies (rotational atherectomy [RA], orbital atherectomy, and excimer laser coronary angioplasty [ELCA]).8,9
The widespread use of these techniques and devices has been limited due to the risk of complications, the operator’s experience, and the corresponding use of health resources. Over the past few decades, RA has been the therapy of choice for resistant calcified lesions. However, the development of new technologies such as IVL or the improvement of classical therapies such as ELCA has generated uncertainty on the optimal tool to modify calcified plaques as non-randomized comparisons between these techniques have been drawn.
The objective of this randomized trial is to assess the efficacy and safety profile of intensive plaque modification with RA, IVL or ELCA before DES implantation.
METHODS
Patients and study design
The ROLLERCOASTR (Rotational atherectomy, lithotripsy or laser for the treatment of calcified stenosis) is an investigator-initiated, multicenter, prospective, and randomized clinical trial that includes 6 large volume sites. Also, it includes men and women aged ≥ 18 years with a clinical indication for PCI (stable or unstable ischemic heart disease) in vessels with reference diameters ≥ 2.5 and ≤ 4.0 mm and moderate-to-severe calcification estimated by coronary angiography. The main study exclusion criteria are ST-segment elevation acute coronary syndrome as clinical presentation, cardiogenic shock, inability to tolerate dual antiplatelet therapy for, at least, 6 months for those who are not on oral anticoagulation, impossibility to obtain informed consent from the patient or conduct, at least, a 1-year follow-up.
Patients who meet all the inclusion criteria and none of the exclusion ones will be randomized on a 1:1:1 ratio to either lesion preparation with RA, ELCA or IVL. Randomization will on a web-based platform. The complete inclusion and exclusion criteria are shown on table 1 while the study flowchart is described on figure 1.
Table 1. Study inclusion and exclusion criteria
| Inclusion criteria |
|---|
| ≥ 18 years old |
| Diameter stenosis ≥ 70% or fractional flow reserve < 0.8/non-hyperemic indexes < 0.89 |
| Reference vessel diameter ≥ 2.5 and ≤ 4 mm |
| Moderate or severe calcification estimated by coronary angiography |
| Patients with stable coronary artery disease or non-ST-segment elevation acute coronary syndrome |
| Culprit lesions at native vessels or coronary bypasses |
| Exclusion criteria |
| Inability to tolerate a 6-month course of dual antiplatelet therapy in patients naïve to oral anticoagulation |
| ST-segment elevation acute coronary syndrome |
| Cardiogenic shock |
| Impossibility to obtain informed consent from the patient or his legal representative |
| Impossibility to conduct, at least, a 1-year follow-up |
Figure 1. Study flowchart. ELCA, excimer laser coronary angioplasty; EP, endpoint; MACE, major adverse cardiovascular events; OCT, optimal coherence tomography; PCI, percutaneous coronary intervention.
Study primary and secondary endpoints
The objective of this study is to evaluate and compare the results of RA, IVL, and ELCA for the management of calcified coronary lesions. This comparison will be made by assessing the angiographic and OCT findings after the implementation of these plaque modification techniques, and DES implantation and optimization.
The primary endpoint is the comparison between RA (reference group) vs ELCA and RA vs IVL in the percentage of stent expansion measured using OCT. As secondary endpoints we’ll be analyzing the device success (successful stent implantation with minimum stent area ≥ 5.5 mm2, final TIMI grade-3 flow, and no need for another plaque preparation strategy), procedural success (device success and no severe procedural complications like cardiovascular death, perioperative target vessel myocardial infarction, need for new target lesion revascularization, stent thrombosis, stroke or vessel perforation with extravasation [types II or III]), crossover from the assigned plaque modification technique to a different one, and occurrence of major adverse cardiovascular events at 1-year follow-up (cardiovascular death, target vessel myocardial infarction, target lesion revascularization or stent thrombosis). We’ll also be analyzing device success regarding the type of calcified plaque (concentric, eccentric, calcium nodule). The study primary and secondary endpoints are shown on table 2.
| Primary endpoint |
|---|
| Percentage of stent expansion measured by OCT |
| Key secondary endpoints |
| Device success (successful stent implantation with minimum stent area ≥ 5.5 mm2, final TIMI grade-3 flow, and no need for another plaque preparation strategy) |
| Device success depending on the type of the calcific plaque: concentric, eccentric or nodular |
| Procedural success (device success in the absence of procedural severe complications) |
| Crossover from the assigned plaque modification technique to a different one |
| 1 year-MACE (CD, TVMI, TLR or ST) |
|
CD, cardiac death; MACE, major adverse cardiovascular events; OCT, optical coherence tomography; ST, stent thrombosis; TLR, target lesion revascularization; TVMI, target vessel myocardial infarction. |
Devices
- – RA: Rotablator or RotaPro System (Boston Scientific, Unites States).
- – Coronary laser: Coronary laser-emitting device (CVX-300 ELCA System, Spectranetics Inc., United States).
- – Intracoronary lithotripsy: Shockwave System, (Shockwave Medical, United States).
- – OCT system: OCT Imaging system (Abbott Vascular, United States)
- – Stents: new-generation DES are mandatory (those currently being used in participant centers during the inclusion period).
Procedure
The angioplasty will be performed following the recommendations established by the current clinical practice guidelines on the management of coronary revascularization.10 After crossing the lesion with the angioplasty guidewire, a first OCT assessment should be performed. If necessary, balloon dilatation is allowed to cross the OCT catheter. After this first OCT pullback, the use of a plaque modification technique will be required (RA, laser or lithotripsy) on a randomized basis. Afterwards, a second OCT assessment is advised to analyze the effects of the therapy. Finally, the angioplasty will be completed with the implantation of a new-generation DES. Pre or postdilatation will be left to the operator’s criterion. After stenting (in the absence of postdilatation) or after the last postdilatation (if performed), a final OCT pullback will be performed to assess the final stent expansion.
Rotational atherectomy technique
The lesion will be crossed using the RotaWire (Boston Scientific, Unites States) directly or microcatheters or coaxial balloons. The RotaWire type (RotaWire Extra Support and RotaWire Floppy) will be used based on the characteristics of the plaque, the support required, and the operator’s preferences. Afterwards, the rotational atherectomy technique will be used based on the current recommendations.11 A 0.5:0.6 ratio between the burr and the vessel is advised. The rotational speed recommended is between 135 000 rpm and 180 000 rpm. Decelerations > 5000 rpm should be avoided. The burr should be advanced gradually with easy back-and-forth moves. Rotablation time should be < 20 seconds with pauses in between each cycle. Once rotablation has been performed, the burr should be removed with the Dynaglide mode on.
Intracoronary lithotripsy technique
The Shockwave balloon (Shockwave Medical, Inc., United States) is a 12 mm-long angioplasty balloon with 2.5 mm to 4 mm diameters. It can be mounted over a 0.014 in guidewire. Mechanical energy is transmitted to the lesion when the Shockwave balloon contacts the artery intima layer and cracks superficial and deep calcium layers. Therefore, the Shockwave balloon/reference vessel diameter ratio should be 1:1.12 Performing an OCT assessment prior to selecting the size of the balloon is also advised. Predilatation with balloons of smaller diameters is allowed to facilitate the passage of the lithotripsy balloon.
Once the Shockwave balloon is on the lesion, it is inflated at a pressure of 4 atm . Up to 80 pulses per balloon can be administrated (8 runs of 10 pulses). After every run (≤ 10 pulses), the Shockwave balloon is inflated at 6 atm and, after deflation, a new cycle can be applied if necessary. A minimum of 20 pulses per lesion is advised.
Laser technique
The size of the ELCA catheter will be selected considering the diameter of the target vessel on a 0.5-0.6 ratio with respect to its diameter.13 However, 0.9 mm catheters will be prioritized because of their greater crossing capabilities and capacity to emit laser energy with greater fluence (80 mJ/mm2) at the maximum pulse repetition rate (80 Hz). Regarding the device settings, it is recommended to start by applying a 60 mJ/mm2 fluence and a 60 Hz pulse repetition frequency that can go up to 80 mJ/mm2 and 80 Hz based on the operator’s criterion. Energy pulses will be released while the catheter slowly moves forward through the lesion at a rate of 0.5 mm/s, thus allowing proper energy absorption and plaque modification. Retrograde application is also feasible, especially in severe lesions with antegrade resistance. Saline-infusion technique is advised. Both blood and iodinated contrast contain non-aqueous cellular macromolecules like proteins that absorb most of the energy released by the laser creating microbubbles that increase the chances of traumatic dissection.14 On the contrary, the saline solution facilitates the passage of light from the tip of the catheter to the tissue without interferences or microbubbles at that level. Therefore, the saline solution infusion technique is used to safely control the energy that is being released, and minimize the risk of dissection.15 In order to wash out the blood from the catheter-based tissue interface the catheter needs to be properly intubated and the saline solution properly infused during laser application. The application of laser to blood or contrast is allowed in selected cases of uncrossable or undilatable lesions and left to the operator’s criterion.16 At the end of the procedure, parameters like the number of pulses administered, the time of therapy, fluence, and repetition rate will need to be collected.
Crossover
Combination of several plaque modification techniques is permitted as they have shown to be complementary in some cases.17,18 If a different plaque preparation technique is required, the technique should be changed based on why the first technique failed (table 3). This switch is consistent with the routine clinical practice. All the material and techniques used will be registered for further analysis.
Table 3. Crossover of plaque modification techniques
| Failed early technique | Reason for failure | 2nd technique |
|---|---|---|
| Rotational atherectomy | Uncrossable lesion with the rotablation olive-shaped burr | ELCA |
| Undilatable lesion (suboptimal balloon expansion after rotablation) | Lithotripsy | |
| Lithotripsy | Uncrossable lesion with Shockwave balloon (despite predilatation, if necessary) | Rotational atherectomy |
| Undilatable lesion (suboptimal balloon expansion after lithotripsy) | ELCA | |
| ELCA | Uncrossable lesion with ELCA | Rotational atherectomy |
| Undilatable lesion (suboptimal balloon expansion after ELCA) | Lithotripsy | |
|
ELCA, excimer laser coronary angioplasty. |
||
Optical coherence tomography image acquisition and stent optimization protocol
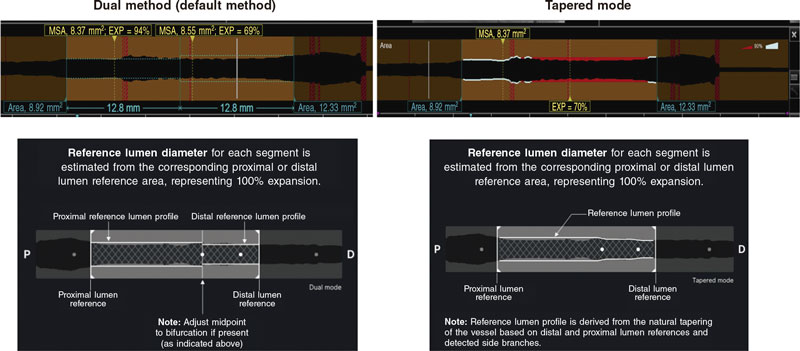
Intravascular OCT is performed using a commercially available system (the ILUMIEN OPTIS, OPTIS Integrated, OPTIS Mobile systems, OPTISIntegrated Next, OPTISMobile Next Abbott Vascular) that incorporates a rapid exchange catheter (Dragonfly OPTIS, Dragonfly OpStar Imaging Catheter; Abbott Vascular) and an integrated pullback system (18-36 mm/s). It acquires images at high axial resolution (~15 μm) with blood displacement. A total of 3 pullbacks are advised before and after using the plaque modification technique (to describe the calcified lesion and the effects of each modality over it, respectively), and optimizing the DES implanted. The automated OCT-angiography co-registration (where available) will be used, and recommendations for PCI guidance with OCT19 will be left to the operator’s criterion. Stent expansion can be estimated using 2 methods (figure 2): 1) dual method: it identifies the stented region and splits it in half. Minimum lumen expansion in the stented area (EXP) is estimated for each half (minimum stent area in each segment divided by the proximal or distal reference area x 100). The center point can be moved by the user (both the minimum stent area and the EXP recalculate automatically); 2) tapered mode: reference lumen profile is estimated based on the distal and proximal reference frame mean diameter and side branch mean diameter in between. The software automatically displays the minimum stent area and identifies the frame with the minimum lumen expansion in the stented area (EXP). A colored expansion indicator automatically pops up when a stent is detected. Automatic detection: minimum stent area frame/Automatic detection of minimum expansion frame (EXP).
Figure 2. Stent expansion estimate by optical coherence tomography. EXP, stented area. MSA, minimum stent area. Modified with permission from Abbott Vascular from User Manual of Ultreon 1.0, and User Instructions of AptiVue Software.
With stent lengths > 50 mm, the dual method is preferred. With stent lengths < 50 mm the tapered method is often used. If the dual method is used, the stent expansion percentage of both segments is recorded being considered for analysis the lowest of the 2.
Follow-up and clinical definitions
In-hospital and follow-up outcomes were prespecified in the online database, complied with the requirements set forth by the Spanish Data Protection Act, and were only accessible to participant operators and study coordinators.
After each PCI, electrocardiographic and cardiac biomarker seriation will be performed. Clinical assessment will be conducted 1, 6, 12 months after PCI. Angiographic follow-up will be only clinically driven in patients with new symptoms, ventricular function worsening or new ischemia in non-invasive tests.
Calcification is defined as moderate if radiopacities are noted only during the cardiac cycle before contrast injection, and severe if radiopacities are noted without cardiac motion before contrast injection often compromising both sides of the arterial lumen.
Device success is defined as successful stent implantation with minimum stent areas ≥ 5.5 mm2 by OCT, final TIMI grade-3 flow, and no need for another plaque preparation strategy.
Procedural success is defined as device success and no severe procedural complications: cardiovascular death, perioperative target vessel myocardial infarction, need for new target lesion revascularization, stent thrombosis, stroke or vessel perforation with extravasation [types II or III]).
Other procedural complications included ventricular arrhythmias or hemodynamic instability during PCI, major bleeding (bleeding requiring transfusion, vasopressors, surgery or percutaneous intervention), and flow limiting dissection.
Major cardiovascular adverse events include cardiovascular death, target vessel myocardial infarction, stent thrombosis or target lesion revascularization. All deaths were considered cardiac unless other specific causes were documented. Myocardial infarction was defined according to the current recommendations made,20 and only those associated with the targer lesion, perioperative or at follow-up were considered. Target lesion revascularization or stent thrombosis were defined according to the criteria established by the Academic Research Consortium.21
Primary outcome assessment will be conducted in a central core laboratory by looking at the OCT imaging after stenting. All medical data will be codified anonymously and stored, and confidentiality will be protected at any time in observance of the current legislation. Both the clinical events committee and the independent core laboratory will be blinded to the treatment arm.
Secondary outcome assessment will be performed by assessing both the angiography and the OCT in a central core laboratory and through on-site or phone clinical follow-up sessions with the patients.
Statistical considerations
Sample size determination
This is a non-inferiority study. We expect to obtain similar outcomes regarding stent expansion using rotational atherectomy, laser, and intracoronary lithotripsy. The sample size was estimated based on the design of the trial and the results of former studies.22-24 There are no standard criteria to define stent expansion in the routine clinical practice. In a recent expert consensus document, stent expansion > 80%19 was considered appropriate. However, most former studies did not reach this threshold. In the ILUMIEN II trial, the mean stent expansion measured by OCT was 72.8% with a standard deviation of 12.6.24 To calculate the size of the sample, we assume an α error of 0.05 and a β error of 0.2 (80% power), a margin of irrelevance (ε) of 7, and losses of 10% due to measurement difficulty or impossibility to complete the intervention. With these parameters we estimate a sample size of 56 cases per group.
Statistical analysis
The study primary endpoint analysis will be conducted by lesion and intention-to-treat with a 1-sided Student t test and an alpha coefficient of 0.05 between the reference group and the other groups (ELCA, and IVL). An analysis of the primary endpoint per protocol will be conducted and presented for consistency purposes. If the hypothesis of non-inferiority is confirmed, a 2-sided superiority analysis will be conducted. Clinical endpoints will be analyzed by patient.
Quantitative variables following a normal distribution will be expressed as median ± standard deviation. Those not following such distribution will be expressed as median and minimum and maximum values. Qualitative variables will be expressed as absolute values and frequencies.
P values < .05 will be considered statistically significant, and the 95% confidence interval of the study variables will be estimated. The Kolgomorov-Smirnov test will be used to confirm the adjustment of variables to normal distribution. Regarding mean comparisons, the Student t test or the non-parametric Mann-Whitney U test (in case of qualitative dichotomous variables), and the ANOVA test or the non-parametric Kruskal Wallis test (in case of qualitative non-dichotomous variables) will be used. Regarding the bivariate analysis of qualitative variables, the chi-square test or Fisher’s exact test will be used. If necessary, the linear correlation among the different quantitative variables will be performed using Pearson correlation coefficient or Spearman’s correlation.
Regarding the multivariate analysis, the Cox regression analysis with forward, stepwise selection will be used drawing event-free survival curves using the Kaplan-Meier estimator. Variables will be considered potential predictors of risk in the multivariate model in the presence of a statistically significant correlation in the univariate analysis or a trend towards significance. The SPSS statistical software (version 20.0, SPSS Inc) will be used for all the estimates.
Organization and ethical concerns
The study protocol has been approved at each participant center by its internal ethics committee. All patients will have to give their informed written consent prior to their participation. The study is an investigator-initiated trial and follows the good clinical practice guidelines applicable to epidemiological studies. The rights and integrity of participants shall be guaranteed at all time while data confidentiality shall be safeguarded in observance of EU directives, the Declaration of Helsinki, as well as local rules and regulations. The ROLLERCOASTR trial is registered at clinicaltrials.gov wit identifier NCT04181268. The study promoter is Fundación EPIC. The study is supported by unrestricted grants from Fundación EPIC. The steering committee is the trial main decision-making committee and has final word on the medical and scientific approach to the trial. The clinical events committee includes interventional cardiologists who don’t participate in the trial and are blinded to the randomized therapy. The clinical events committee will be responsible for developing specific criteria for the adjudication of the study clinical events and endpoints as per protocol. All members of the clinical events committee will be blinded to the study primary outcomes.
DISCUSSION
At least a third of all coronary lesions requiring PCI show significant calcification.9 As a matter of fact, this is probably one of the greatest challenges interventional cardiologists face to this date. Different tools are available to prepare calcified plaques. These techniques are increasingly used in the routine clinical context based on the operator’s experience or availability25 since there are barely any comparative studies on this regard.
The role of rotational atherectomy is to facilitate stenting in calcified non-dilatable lesions. The technology has evolved for over 20 years now, and lots of patients have been treated with it. The setback is that it has a longer learning curve compared to other plaque modification techniques and requires a specific guidewire. The evidence available on RA in the calcified lesion setting shows higher procedural success rates compared to conventional or modified balloons with almost the same clinical outcomes. However, even the most recent trials have important limitations as a limited use of intracoronary imaging techniques and new-generation DES.22,23
The arrival of laser to treat atherosclerosis goes back to the 1980s to treat lower limb ischemia at the beginning, and then coronary artery disease.26 However, both catheters and the techniques were rudimentary, and complications were a common thing. The early randomized clinical trials that compared ELCA to RA or balloon angioplasty (before the stent era) did not show favorable outcomes.27 The refinement of this technology followed by the introduction of safe laser-based techniques has improved its results. However, no direct comparisons have been drawn over the past few years. Although, traditionally, severe calcification has been a non-favorable scenario for ELCA, this technique has repeatedly obtained good results in settings in which calcium is a common finding: balloon failure (uncrossable or undilatable lesions), in-stent restenosis, underexpanded stents or chronic total coronary occlusions.13 Excimer laser releases energy in the UV range in very short pulses (nanoseconds). Billions of molecules per pulse are broken. Absorption depth is 50 µm, thus reducing the risk of collateral tissue damage (compared to previous infrared lasers). Laser ablates the atherosclerotic material mediated by 3 different mechanisms: photochemical (fracture of molecular bonds): the UV light pulse hits the plaque and is highly absorbed with each photon generated carrying sufficient energy to break molecular bonds; photothermal (tissue vaporization): molecular bonds also vibrate during the absorption process resulting in heat. Intracellular water is vaporized leading to cell rupture and the creation of a vapor bubble, and photokinetic (clearance of byproducts): the rapid expansion and collapse of the vapor bubble further breaks down the plaque, but it also helps clear byproducts of ablation like water, gases, and small particles. Laser effect is amplified especially when it acts directly on blood or a contrast agent. Therefore, to reduce the risk of coronary artery dissection, laser ablation is often performed during the continuous infusion of saline solution.13 One advantage of laser is its short learning curve. It can be used through conventional 0.014 in guidewires in a rapid-exchange fashion and conventional 6-Fr guiding catheters. In addition, most of these particles are small enough to be cleared by the reticuloendothelial system, thus minimizing the risk of distal microembolization (1 more advantage compared to other plaque modification techniques).13
Lithotripsy is the latest technology that has become available to treat heavily calcified lesions. It emits pulsatile mechanical waves through emitters integrated in a semi-compliant balloon that is initially inflated at 4 atm. Afterwards, energy pulses are applied, and the vibrations produced interact with the atherosclerotic plaque breaking down both the superficial and deep calcium deposits.9 This effect on deep calcium deposits is one of the greatest advantages of lithotripsy over other techniques. Also, this technique learning curve is short since it’s based on a well-known coronary balloon technology. The DISRUPT CAD trials12 have demonstrated the safety and efficacy profile of this technique treating heavily calcified lesions and its use has grown exponentially ever since. The main limitation of this technique is that, as it is a balloon-based technology with a smaller diameter of 2.5 mm, extremely tight stenoses can hamper its use as a first-line therapy, thus needing predilatation with lower profile balloons, and even with RA17 or laser18 combined to overcome this problem.
Intracoronary imaging modalities allow more accurate assessments of coronary artery disease compared to conventional angiography and give us essential information for PCI planning. This is particularly relevant during the management of calcified and complex lesions impacting the results of the angioplasty and the patient’s prognosis28 by optimizing DES implantation, thus leading to better stent expansion, vessel wall apposition, and eventually a greater luminal area. The OCT has greater spatial resolution9 compared to the intracoronary ultrasound and has proven useful showing the effect of plaque modification therapies and stent optimization. All these reasons and the lack of use of intracoronary imaging techniques in previous plaque modification techniques has led us to using OCT to assess the study primary endpoint: percentage of stent expansion.
The ROLLERCOASTR trial will compare the 3 strategies most used in the routine clinical practice to treat lesions with moderate-to-severe calcifications. In addition, it will provide us with information on the effect of each of these strategies and the specific settings where they can be more useful. To this end, an intracoronary imaging study with an OCT will be performed to know the specific substrate of calcification and the type of plaque on which the therapy is performed as well as the effects this therapy will have. The study hypothesis is that the 3 modalities complement each other and have different effects depending on the characteristics of the lesion. At manuscript submission, a total of 135 patients have been included.
CONCLUSIONS
The ROLLERCOASTR is a prospective, multicenter, randomized clinical trial designed to compare the safety and efficacy profile of 3 plaque modification techniques in the moderate-to-severe coronary calcification setting: RA, ELCA, and IVL. The study primary endpoint is stent expansion evaluated by OCT. The secondary endpoints are device success, procedural success, crossover rate among techniques, and the occurrence of major adverse cardiovascular events at 1-year follow-up (cardiac death, target vessel myocardial infarction, need for new target lesion revascularization or stent thrombosis). We will also be describing the effects of the 3 imaging modalities in calcified lesions with OCT. Enrollment will end in 2023.
FUNDING
The study is supported by unrestricted grants from Fundación EPIC.
AUTHORS’ CONTRIBUTIONS
A. Jurado-Román: conceptualization, original draft, review, and editing. A. Gómez-Menchero, I.J. Amat-Santos, J. Caballero-Borrego, S. Ojeda, and R. Ocaranza-Sánchez: drafting, review, and editing. S. Jiménez-Valero, G. Galeote, and R. Moreno: conceptualization, drafting, review, and editing.
CONFLICTS OF INTEREST
S. Ojeda and R. Moreno are associate editors of REC: Interventional Cardiology. The journal’s editorial procedure to ensure impartial handling of the manuscript has been followed. S. Ojeda has received consulting fees and participated on Medtronic and Edwards Lifesciences Data Safety Monitoring Board or Advisory Boards, and payment or honoraria for lectures, presentations, speakers bureaus, manuscript drafting or educational events organized by Philips, Biomenco, and World Medica. R. Moreno has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript drafting or educational events organized by Medtronic Inc, Boston scientific, Abbott vascular, Biosensors, Biotronik, Edwards Lifesciences, AMGEN, Astra Zeneca, Daiichi Sankyo New Vascular Therapies, and Biosensors. A. Jurado-Román has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript drafting or educational events organized by Boston Scientific, Shockwave, Philips, Biotronik, Biomenco, Abbott, and Medtronic. A. Gómez-Menchero, J. Caballero-Borrego, R. Ocaranza, G. Galeote, and S. Jiménez-Valero declared no conflicts of interest whatsoever. I. Amat-Santos has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript drafting or educational events organized by Boston Scientific.
WHAT IS KNOWN ABOUT THE TOPIC?
- Coronary calcification worsens the safety and efficacy of percutaneous coronary intervention.
- Several calcium modification techniques are currently available. However, there is a lack of randomized evidence on the therapy of choice in this scenario.
WHAT DOES THIS STUDY ADD?
- The ROLLERCOASTR is a multicenter randomized study that compared 3 advanced plaque modification techniques in the coronary calcification setting: rotational atherectomy, excimer laser, and lithotripsy.
- The study primary endpoint is stent expansion evaluated by optical coherence tomography.
- Secondary endpoints are device success (overall and depending on the type of calcific plaque), procedural success, crossover rate, and the occurrence of major adverse cardiovascular events at 1-year follow-up.
REFERENCES
1. Bourantas CV, Zhang YJ, Garg S, et al. Prognostic implications of coronary calcification in patients with obstructive coronary artery disease treated by percutaneous coronary intervention: a patient-level pooled analysis of 7 contemporary stent trials. Heart Br. 2014;100:1158-1164.
2. Mori S, Yasuda S, Kataoka Y, Morii I, Kawamura A, Miyazaki S. Significant association of coronary artery calcification in stent delivery route with restenosis after sirolimus-eluting stent implantation. Circ J. 2009;73:1856-1863.
3. Tzafriri AR, Garcia-Polite F, Zani B, et al. Calcified plaque modification alters local drug delivery in the treatment of peripheral atherosclerosis. J Control Release. 2017;264:203-210.
4. Wiemer M, Butz T, Schmidt W, Schmitz KP, Horstkotte D, Langer C. Scanning electron microscopic analysis of different drug eluting stents after failed implantation: from nearly undamaged to major damaged polymers. Catheter Cardiovasc Interv. 2010;75:905-911.
5. Kobayashi Y, Okura H, Kume T, et al. Impact of target lesion coronary calcification on stent expansion. Circ J. 2014;78:2209-2214.
6. Lee MS, Shah N. The Impact and Pathophysiologic Consequences of Coronary Artery Calcium Deposition in Percutaneous Coronary Interventions. J Invasive Cardiol. 2016;28:160-167.
7. di Mario C, Koskinas KC, Räber L. Clinical Benefit of IVUS Guidance for Coronary Stenting: The ULTIMATE Step Toward Definitive Evidence? J Am Coll Cardiol. 2018;72:3138-1341.
8. Barbato E, Shlofmitz E, Milkas A, Shlofmitz R, Azzalini L, Colombo A. State of the art: evolving concepts in the treatment of heavily calcified and undilatable coronary stenoses - from debulking to plaque modification, a 40-year-long journey. EuroIntervention. 2017;13:696-705.
9. De Maria GL, Scarsini R, Banning AP. Management of Calcific Coronary Artery Lesions: Is it Time to Change Our Interventional Therapeutic Approach? JACC Cardiovasc Interv. 2019;12:1465-1478.
10. Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87-165.
11. Barbato E, Carrié D, Dardas P, et al. European expert consensus on rotational atherectomy. EuroIntervention. 2015;11:30-36.
12. Kereiakes DJ, Di Mario C, Riley RF, et al. Intravascular Lithotripsy for Treatment of Calcified Coronary Lesions: Patient-Level Pooled Analysis of the Disrupt CAD Studies. JACC Cardiovasc Interv. 2021;14:1337-1348.
13. Rawlins J, Din JN, Talwar S, O’Kane P. Coronary Intervention with the Excimer Laser: Review of the Technology and Outcome Data. Interv Cardiol. 2016;11:27-32.
14. Baumbach A, Haase KK, Rose C, Oberhoff M, Hanke H, Karsch KR. Formation of pressure waves during in vitro excimer laser irradiation in whole blood and the effect of dilution with contrast media and saline. Lasers Surg Med. 1994;14:3-6.
15. Tcheng JE. Saline infusion in excimer laser coronary angioplasty. Semin Interv Cardiol SIIC. 1996;1:135-41.
16. Latib A, Takagi K, Chizzola G, et al. Excimer Laser LEsion modification to expand non-dilatable stents: the ELLEMENT registry. Cardiovasc Revasc Med. 2014;15:8-12.
17. Jurado-Román A, Gonzálvez A, Galeote G, Jiménez-Valero S, Moreno R. RotaTripsy: Combination of Rotational Atherectomy and Intravascular Lithotripsy for the Treatment of Severely Calcified Lesions. JACC Cardiovasc Interv. 2019;12:e127-129.
18. Jurado-Román A, García A, Moreno R. ELCA-Tripsy: Combination of Laser and Lithotripsy for Severely Calcified Lesions. J Invasive Cardiol. 2021;33:E754-755.
19. Räber L, Mintz GS, Koskinas KC, et al. Clinical use of intracoronary imaging. Part 1: guidance and optimization of coronary interventions. An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. EuroIntervention. 2018;14:656-677.
20. Thygesen K, Alpert JS, Jaffe AS, et al. Fourth Universal Definition of Myocardial Infarction (2018). J Am Coll Cardiol. 2018;72:2231-2264.
21. Cutlip DE, Windecker S, Mehran R, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344-2351.
22. de Waha S, Allali A, Büttner HJ, et al. Rotational atherectomy before paclitaxel-eluting stent implantation in complex calcified coronary lesions: Two-year clinical outcome of the randomized ROTAXUS trial. Catheter Cardiovasc Interv. 2016;87:691-700.
23. Abdel-Wahab M, Toelg R, Byrne RA, et al. High-Speed Rotational Atherectomy Versus Modified Balloons Prior to Drug-Eluting Stent Implantation in Severely Calcified Coronary Lesions. Circ Cardiovasc Interv. 2018;11:e007415.
24. Maehara A, Ben-Yehuda O, Ali Z, et al. Comparison of Stent Expansion Guided by Optical Coherence Tomography Versus Intravascular Ultrasound: The ILUMIEN II Study (Observational Study of Optical Coherence Tomography [OCT] in Patients Undergoing Fractional Flow Reserve [FFR] and Percutaneous Coronary Intervention). JACC Cardiovasc Interv. 2015;8:1704-1714.
25. Romaguera R, Ojeda S, Cruz-González I, Moreno R. Spanish Cardiac Catheterization and Coronary Intervention Registry. 30th Official Report of the Interventional Cardiology Association of the Spanish Society of Cardiology (1990-2020) in the year of the COVID-19 pandemic. Rev Esp Cardiol. 2021;74:1095-1105.
26. Bittl JA, Sanborn TA, Tcheng JE, Siegel RM, Ellis SG. Clinical success, complications, and restenosis rates with excimer laser coronary angioplasty. The Percutaneous Excimer Laser Coronary Angioplasty Registry. Am J Cardiol. 1992;70:1533-1539.
27. Appelman YE, Piek JJ, Strikwerda S, et al. Randomised trial of excimer laser angioplasty versus balloon angioplasty for treatment of obstructive coronary artery disease. Lancet. 1996;347:79-84.
28. Ahn JM, Kang SJ, Yoon SH, et al. Meta-analysis of outcomes after intravascular ultrasound-guided versus angiography-guided drug-eluting stent implantation in 26,503 patients enrolled in three randomized trials and 14 observational studies. Am J Cardiol. 2014;113:1338-1347.
ABSTRACT
Introduction and objectives: Percutaneous coronary interventions (PCI) of chronic total occlusions (CTO) are long procedures where many patients suffer moderate-to-high level anxiety and pain. Virtual reality (VR) has proven capable of reducing procedural pain and anxiety in many medical procedures. The objective of this study is to demonstrate that the use of VR during CTO PCI reduces anxiety and pain compared to conventional routine clinical practice.
Methods: Randomized, controlled, open-label, superiority trial clinical trial with 2 parallel arms including 58 patients with a scheduled CTO PCI randomized on a 1:1 ratio to VR during the procedure or conventional management. In both arms, the administration of anxiolytic drugs will be left to the lead operator’s discretion and based on the degree of anxiety o pain perceived. The remaining actions for the management of pre- and perioperative anxiety will be identical in both arms. The primary endpoint will be the maximum level of anxiety perceived by the patient. Secondary endpoints will be the level of patient-perceived pain, the need for intraoperative anxiolytic drug therapy, dose of drug administered, and satisfaction with the VR goggles.
Results: The results of this study will add significant knowledge on the utility of VR regarding anxiety reduction in CTO PCIs.
Conclusions: The ReViCTO trial is the first randomized clinical trial to use VR during a PCI CTO. Its results will show the utility of this technology to reduce anxiety and pain in PCIs performed on CTOs.
Diseño del ensayo registrado en ClinicalTrials.gov (identificador: NCT05458999).
Keywords: Chronic total coronary occlusion Virtual reality Anxiety
RESUMEN
Introducción y objetivos: Las intervenciones coronarias percutáneas (ICP) sobre oclusiones totales crónicas (OTC) son procedimientos largos en los que muchos pacientes sufren ansiedad y dolor. La realidad virtual ha demostrado reducir el dolor y la ansiedad en muchos procedimientos médicos. Nuestro objetivo es demostrar que el uso de la realidad virtual durante la ICP de OTC reduce la ansiedad y el dolor en comparación con la práctica convencional.
Métodos: Ensayo clínico aleatorizado, controlado, abierto y de superioridad con 2 grupos paralelos en el que 58 pacientes con una ICP de OTC programada serán aleatorizados 1:1 al uso de realidad virtual frente al tratamiento convencional. La administración de fármacos ansiolíticos será a criterio del operador principal y en función del grado de ansiedad o dolor percibido. El resto de las acciones para el tratamiento de la ansiedad serán idénticas en ambos grupos. El objetivo primario será el nivel máximo de ansiedad percibido por el paciente. Los objetivos secundarios serán el nivel de dolor percibido por el paciente, la necesidad de tratamiento farmacológico ansiolítico, la dosis de fármaco administrada y la satisfacción con la realidad virtual.
Resultados: Los resultados de este estudio añadirán conocimientos importantes sobre la utilidad de la realidad virtual en la reducción de la ansiedad en los procedimientos de ICP de OTC.
Conclusiones: El ensayo ReViCTO es el primer ensayo clínico aleatorizado que utiliza la realidad virtual durante la ICP en OTC. Sus resultados mostrarán la utilidad de esta tecnología para reducir la ansiedad y el dolor en esta intervención.
Diseño del ensayo registrado en ClinicalTrials.gov (identificador: NCT05458999).
Palabras clave: Oclusión total crónica Realidad virtual Ansiedad
Abbreviations
CTO: chronic total coronary occlusion. PCI: percutaneous coronary intervention. VAS: visual analogue scale. VASA: visual analogue scale of anxiety. VASP: visual analogue scale of pain. VR: virtual reality.
INTRODUCTION
Chronic total coronary occlusions (CTO) are diagnosed in up to 15% of patients with coronary artery disease undergoing coronary angiography.1 Percutaneous coronary interventions (PCI) of CTOs are one of the greatest challenges we face in interventional cardiology due to the complexity of these procedures and the increased risk of complications.2 Over the past few decades, advances in techniques and devices have made it possible to obtain better results while reducing the associated complications.3-5 Anxiety and pain during these procedures are often treated with oral benzodiazepines plus opioids or IV benzodiazepines upon request during the procedure. The possibility of performing these procedures without anesthesia or sedation avoids the risks associated with these therapies. On the contrary, it submits the patient to pain and anxiety during the procedure. Several factors such as long procedures, patient immobility (especially in biradial access), and monotonous and hostile environments (operating rooms or cath labs) influence patient anxiety. Virtual reality (VR) has been successfully used in several clinical settings such as transcatheter aortic valve implantation6 or atrial fibrillation ablation7 to reduce intraoperative anxiety. There is no evidence on the use of VR reducing perioperative patient anxiety during PCI, specifically in CTO PCI. Compared to standard PCI, this procedure could benefit even further from VR due to its longer duration, use of double arterial access, and possibility of triggering ischemia and chest pain.
The objective of this study is to determine whether the use of a VR system in PCIs on CTOs decreases the level of anxiety and pain during CTO procedures compared to conventional management.
METHODS
Overall study design
The Decreasing patient anxiety during revascularization of chronic total coronary occlusions using virtual reality glasses (ReViCTO) trial (ClinicalTrials.gov Identifier: NCT05458999) was designed as a randomized, controlled, open-label, superiority clinical trial with 2 parallel arms (procedural use of VR goggles vs conventional management) with a primary endpoint of maximum level of anxiety perceived by the patient measured through the visual analogue scale of anxiety (VASA).
The study will be conducted in full compliance with the principles set forth in the Declaration of Helsinki (1996) and the International Conference on Harmonization Good Clinical Practice Guideline. The study protocol was approved by the Clinical Research Ethics Committee (CREC) of Hospital Clínico Universitario de Valencia, Spain. All patients signed an informed consent form. The study is registered at clinicaltrials.gov (NCT05458999). The World Health Organization minimum standard list of items for clinical trials are listed in table 1 of the supplementary data. This protocol follows the SPIRIT 2013 Statement: Defining Standard Protocol Items for Clinical Trials.8
Study setting and eligibility criteria
The trial will be conducted at Hospital Clínico Universitario de Valencia, Spain, a reference teaching hospital on interventional cardiology that treats nearly 800 000 patients both from rural and metropolitan areas. Since this is a preliminary study it is designed as a single-center trial. All procedures will be performed by a team of 2 interventional cardiologists experienced in CTO revascularization. Patient enrolment started back in December 2021. On Dec. 25th 2022, 25 patients had already been enrolled in the study (43% of the target population). Patients with visual impairment, dementia, language barriers or any situations that would prevent the use of VR glasses will be excluded. Inclusion and exclusion criteria are listed in table 1. All patients must meet all the inclusion criteria and none of the exclusion criteria.
Table 1. Inclusion and exclusion criteria
| Inclusion criteria |
| Age > 18 years |
| Elective percutaneous coronary intervention on chronic total coronary occlusion |
| Physical and mental ability to wear virtual reality glasses |
| Exclusion criteria |
| Unable to or unwilling to give informed consent |
| Visual impairment |
| Dementia |
| Language barrier (unable to communicate fluently in Spanish or English) |
| Any other situations that would prevent the use of virtual reality glasses |
Assignment of interventions
Each patient will be randomized on a 1:1 ratio to the intervention (use of VR goggles during the CTO procedure) or the control arm (routine clinical practice). Given the small sample size estimated, permuted block randomization was used to guarantee an equal number of participants per arm.9 Random sequence was computer generated using blocks with a size unknown to the investigators until the end of recruitment. Patient enrolment and arm assignment will be performed by the investigators. Allocation concealment will be ensured using a web application that assigns a unique identification number and the assigned arm once the patient has been recruited for the trial. This system prevents changes to the identification number or arm deleting patients after randomization. Because of the nature of the trial no masking or blinding will be applied at any level.
Participant timeline
Since there is no follow-up, this study has a very simple timeline. Upon arrival to the cath lab, all patients scheduled for elective CTO PCI will be screened and checked to see if they meet all the inclusion criteria and none of the exclusion criteria. If they don’t meet these criteria, they will be considered a screening failure and will not participate in the study. If all criteria are met by the patients, the investigators will need to obtain their written informed consent right before patients arrive at the cath lab. The functioning of the trial will be explained orally and reading of the informed consent will be offered allowing enough time, if necessary. Patients will be randomized to wear VR goggles or to the control arm. During the PCI, all measures regarding anxiety will be applied regardless of the allocation arm. Also, all drug therapies administered will be registered by the study nurse. After the procedure, the patient’s perceived anxiety and pain will be assessed by the study nurse, this being the end of the trial for the patient (figure 1).
Figure 1. Central illustration. Trial flowchart.
Data collection
Demographics, the past medical history, preoperative (indication for revascularization, blood tests, left ventricular ejection fraction…), and perioperative variables [arterial access, radiation dose, maximum level of anxiety (VASA), and visual analogue scale of pain (VASP) perceived by the patient measured through visual analogue scale, nausea, and dizziness during the procedure] will be collected (table 2 of the supplementary data).
Clinical variables will be collected from the local and regional electronic clinical data system and asked directly to the patient when lacking. Blood test results will be collected from the local laboratory system using the last available determination. Echocardiographic and magnetic resonance imaging data will be collected from the local electronic clinical data system. The Seattle Angina Questionnaire, VASA, VASP, the presence of nausea or dizziness, overall satisfaction with the procedure, and overall satisfaction will be assessed by the study nurse and included in a dedicated form (table 3 of the supplementary data). All these data will be transferred to a dedicated database in 1 single local computer. This database is designed with range check for numerical variables to prevent erroneous data entry. Also, the database will check for duplicates when entering the hospital identification number. All data will be stored in a database kept in a dedicated computer with no Internet connection to avoid unwanted leaks or stole information. The investigators will have access to this database only.
Trial intervention
Eligible patients will be randomized to the intervention (VR goggles) or the control arm (routine clinical practice).
Virtual reality goggles
A commercial Oculus Quest 2 VR goggle system (Meta Platforms, Inc., United States) will be used. The viewing consisted of using the capabilities of the VR goggles to recreate a 2D playback that simulates the size of a large-format movie screen. Using Netflix video streaming system (Netflix Inc. United States), the documentary series “Our Planet” [Silverback Films, United Kingdom]10 will be played for all patients starting with chapter 1, and sequentially and automatically playing the following chapters. Before the procedure, the patient will be informed on the VR goggle system-based operation, possible side effects (nausea or dizziness), and the possibility to remove it at any time. Before the arterial puncture, the VR goggle system will be put on and checked for proper functioning. It will be removed before removing arterial introducers, when the patient wishes to do so or if serious complications occur. During the procedure, the patient’s general condition will be checked every 30 min.
The system will be prepared following these steps: 1) drawing the security perimeter with the controller; 2) starting the Netflix application; 3) selecting the “Void Theater” option; 4) searching for the documentary series “Our Planet” and playing the first episode; 5) adjusting the screen size with the controller; 6) selecting travel mode; 7) putting the VR goggles on the patient (figure 2); 8) asking the patient if he can watch and hear correctly. If not, the VR goggles should be repositioned.
Figure 2. Real patient wearing virtual reality goggles during a chronic total coronary occlusion revascularization procedure using double radial artery access.
Control arm
The comparator chosen is the current clinical practice with no VR goggles. A possible comparator using a VR goggle with no content was discarded because of the high chances of claustrophobia or mental discomfort.
Both arms will receive drugs upon request to reduce perceived pain and anxiety. In both arms, anxiolytic drugs (morphine chloride or midazolam at 1 mg boluses) will be administered by the circulating nurse if the patient explicitly expresses the need for such treatment or if external signs of anxiety or pain (agitation, complaints...) are observed. The last decision on treatment administration will be left to the lead operator. The remaining actions for the management of pre- and perioperative anxiety will be identical in both arms. Preoperative anxiolytic treatment was not routinely administered to all patients, only upon the patient’s request.
Endpoints
The primary endpoint will be to assess changes to the maximum level of anxiety perceived by the patient during the procedure. Secondary endpoints will be to assess a) changes to the maximum level of pain perceived by the patient during the procedure; b) differences in the need for intraoperative anxiolytic drug therapy or doses of anxiolytic drugs (midazolam or morphine chloride) administered during the procedure; and c) the overall satisfaction experienced with the VR goggles.
Both the primary (anxiety) and secondary endpoints of pain will be measured through the VASA11 and pain (VASP)12 that go from 1 to 10. Both VASA and VASP will be collected by a specialized nurse right after the end of the procedure before leaving the room through a specific survey on the maximum level of anxiety or pain perceived during the procedure (table 3 of the supplementary data).
The VAS will be used to quantify the patients’ responses objectively. The VAS eliminates the examiner influence or bias that often comes with verbal questioning and is a more appealing method of evaluation for participants. Although this method is not perfect, it remains a common way to assess anxiety and pain.13,14 The patient will also be asked if he’d like VR to be used in other similar settings. Total doses of benzodiazepines (midazolam) or opioids (morphine) administered during the procedure will be registered in total milligrams.
Sample size estimate
The sample size was estimated based on the primary endpoint. In former studies that assessed anxiety during catheterization the standard deviation of VASA was 2.715,16 (σ). VASA > 2 (μ1 – μ2) was descriptive of clinically significant differences. To detect differences ≥ 2 in VASA assuming a normal distribution, alpha and beta risks of 0.05 (α) and 0.2 (β) in bilateral contrast in a sample size of 58 patients (29 in each arm) were estimated.
Statistical analysis
Quantitative variables will be expressed as mean ± standard deviation when they follow a normal distribution and as median [interquartile range] if they don’t. Qualitative ones will be expressed as percentages (absolute value). Fisher’s exact test or the chi-square test will be used to compare qualitative variables. Also, the Student t test or the Mann-Whitney U test will be used if quantitative variables don’t follow a normal distribution.
The primary endpoint (VASA), VASP, and dosage of drugs will be compared in both arms using the Student t test. The use or non-use of drugs during the procedure, the presence or absence of dizziness or nausea will be compared using Fisher’s exact test or the chi-square test. Subgroup analyses will be performed based on sex, age, and previous experience with new technologies.
All statistical tests will be bilateral and considered significant if P < .05. Statistical analyses will be performed with R Core Team (2020) statistical software package (R Foundation for Statistical Computing, Austria).
DISCUSSION
CTOs are present in up to 20% of the patients with coronary artery disease. These numbers increase parallel to age (up to 40% in diabetics or patients with heart failure).17,18 In the past, most of these patients were referred for revascularization surgery due to poorly successful PCIs in this kind of lesions. Over the past few decades, several advances have been made regarding devices and technical materials, organization, and concentration of complex procedures in reference centers. Also, increased operator experience has led to a high success rate of 90%, and a very low rate of severe complications19,20 with the corresponding increase in the number indications for PCI CTO.
Although PCIs are a common and relatively low risk procedure, many patients undergoing these treatments experience anxiety (up to 37% in some populations).21-23 Anxiety involves feelings of fear, tension or panic or the prospect that something unpleasant is about to happen. State anxiety may be more clinically relevant for patients undergoing PCI because it is transient in nature and amenable to clinical procedures. Patients undergoing PCI have multiple sources of anxiety including their own concerns. These concerns can include fear of discomfort, uncertainty, and fear associated with survival that can be more distressing than chest pain itself.24
PCI CTO creates more anxiety for the patients compared to other interventional procedures for several reasons. In the first place, double access with high-calibre sheaths is frequently used, even biradial. Repeated access punctures with consequently an increased pain and limited patient mobility contributes to more discomfort and higher anxiety levels. Secondly, the duration of the procedure is long, and can be up to 3 to 4 hours or more in some special scenarios. Being exposed to immobility in a monotone and hostile scenario for such a long time is a reasonable cause for anxiety. Thirdly, cath labs are often strange environments for the patient with machinery and equipment that may be frightening for him at first. Furthermore, discussion with the treating team, the use of terms unfamiliar to the patient or the existence of beeps and alarms can make the patient think that something bad might happen to him, thus increasing the levels of anxiety. Fourthly, patients undergoing elective PCI CTO usually undergo, at best, at least, 1 invasive coronary angiography, and commonly up to several coronary interventions including failed CTO revascularization attempts. Previous procedures could be remembered as painful or stressful and anticipation anxiety could appear. Stress and anxiety associated with needle-related procedures may lead to needle phobia,25 which could also contribute to a high level of anxiety. Fifthly, chest pain is an important factor of procedural anxiety during CTO PCI, and it occurs in a large number of patients. For example, with retrograde approaches, the flow of collateral branches on which the CTO-related myocardial territory is completely dependent is interrupted due to their occupation by the guidewire or microcatheter, thus causing ischemia and pain). Therefore, there is a potential high anxiety level in patients undergoing CTO PCI that depends on multiple factors and mechanisms that feed from one another.
At the end of the 20th century, it was noted that both behavioral and pharmaceutical interventions should be used to manage pain during medical procedures.26 Distraction techniques may be effective reducing the patients’ pain during various invasive procedures because pain involves both physical stimuli and emotional responses. Studies have shown that various distraction techniques like music, massage, breathing exercises, and behavioral therapy can effectively reduce the feeling of pain and stress symptoms during painful procedures.27,28
VR is a computer-generated simulation of the physical world that allows people to experience it in a realistic way. VR goggles achieve visual and auditory semi-isolation that, together with the images projected, evade the patient while act on environmental and emotional factors of anxiety. VR has proven superior to other distraction methods such as television, listening to music or playing games.29,30 VR has been used to relieve anxiety and pain in patients undergoing several kinds of procedures as needle-related interventions,31 burn wound debridement,32,33 physical therapy,34 dental procedures,35 colonoscopy,36 minor surgical procedures,37 nasal endoscopy38 or chemotherapy.39 A total of 4 randomized clinical trials have been conducted to study the level of anxiety experienced by adults undergoing different medical procedures like hysteroscopy,40 labor,41 and colonoscopy.42 The studies used various measurement tools such as the VAS scale from 0 to 10, the 5-point Likert scale 0-5, and the State-Trait Anxiety Inventor.
Experiences with VR in interventional cardiology during procedures are scarce. Back In 2020, Bruno et al.6 used a randomized clinical trial to prove the that the use of a VR-based system was safe and feasible during TAVI and that VAS score was reduced by 3 points with the use of VR without impacting nausea or vomiting. Almost all patients said they would use this technology in a similar setting. It is remarkable that this study population was an old population (mean age, 83 years) without previous experience with VR and limited experience with new technologies. This shows that even in a population not used to new technologies that could be expected to reject or not tolerate VR goggles, its use was tolerated and effective. Moreover, this study found that it was important not only that patients accepted the new technology, but also that interventional cardiologists approved it. At first, their reaction went from full support to slight rejection. Those who hesitated to use this new approach thought that their interaction with the patient during the procedure might be limited. However, over time, when they saw that this was not the case, acceptance increased. Similarly, Roxburgh et al.7 tested the utility of VR in patients undergoing atrial fibrillation ablation in an observational study of 48 patients. They showed that VR reduced perceived pain during the procedure and that VR can be easily incorporated into the standard procedure workflow. As far as we know, no studies have ever been conducted on the use of VR during PCI CTO.
Most studies on VR technology have been observational and not standardized, which complicates comparing results and drawing solid conclusions. Additionally, currently, no guidelines or consensus have ever been published on how to incorporate VR technology to cardiac procedures.43 To address these challenges, an expert taskforce from the international scientific community may be useful to identify evidence gaps, set priorities, standardize research protocols, and create guidelines to implement VR technology in heart procedures.
Limitations
Some limitations should be taken in account in this clinical trial. First, the open-label nature of the trial could lead to bias favorable to the RV group due to the lack of blinding and potential for patients and investigators to influence the outcomes. The entire staff will be warned on this possible bias and advised to prevent it before each procedure. Second, some uncontrolled confounding factors may play a role in the differences seen in the administration of drug therapy between RV and the control groups. We will try to prevent or, at least, mitigate this treatment only if the patient explicitly wishes to do so or if outward signs of anxiety or pain are observed. Third, the results of this trial should be interpreted and applied with caution to other scenarios due to the single-center nature of this trial. Fourth, the primary and secondary endpoints of anxiolytic treatment needed could be closely correlated. Our objective is to determine whether the use of VR goggles decreases anxiety. However, it could happen that both groups have similar levels of anxiety and a greater need for anxiolytic treatment (which is a surrogate of increased anxiety, on the one hand, that could also expose patients to a higher risk of adverse effects, on the other). The choice of the primary and secondary endpoints of anxiety and need for anxiolytic treatment allows us to explore this possibility. Finally, the drug therapy of anxiety was not protocolized but left to the operator’s discretion.
CONCLUSIONS
The ReViCTO trial is the first randomized clinical trial ever designed to evaluate the use of VR during CTO PCI. Results will show the utility of this technology reducing anxiety and pain in PCI CTO.
FUNDING
None whatsoever.
AUTHORS’ CONTRIBUTIONS
A. Fernández-Cisnal, and G. Miñana: study idea or design, data curation, analysis, and interpretation, drafting, and final approval of the version submitted for publication. B. Silla, J.M. Ramón, E. Valero, and S. García-Blas: data curation, analysis, and interpretation, revision of the manuscript regarding significant intellectual content, and final approval of the version submitted for publication. J. Núñez, V. Bodí, and J. Sanchis: data analysis and interpretation, revision of the manuscript regarding significant intellectual content, and final approval of the version submitted for publication. A. Fernández-Cisnal, and G. Miñana agree to take full responsibility for all aspects of the manuscript, and investigate and resolve all questions regarding the accuracy and truthfulness of the study as a whole.
CONFLICTS OF INTEREST
J. Núñez received fees for participating in the advisory boards and educational activities from Astra Zeneca, Boehringer-Ingelheim, NovoNordisk, Bayer, and Novartis. J. Sanchis received speaker fees from Abbott Vascular, and Prosmedica. G. Miñana received speaker fees from Abbott Vascular, and Teleflex, and support for attending meetings from Medtronic and World Medical. The remaining authors declared no other conflicts of interest whatsoever.
WHAT IS KNOWN ABOUT THE TOPIC?
- CTO PCI is one of the greatest challenges for interventional cardiology due to the complexity of these procedures and the increased risk of complications.
- Several factors like long procedures, patient immobility, and the presence of a monotonous and hostile environment influence patient anxiety, which is usually treated with benzodiazepines and opioids upon request during the procedure.
- Virtual reality has been successfully used in several clinical settings reducing intraoperative anxiety. There is no evidence that the use of VR reduces perioperative patient anxiety during CTO PCI.
WHAT DOES THIS STUDY ADD?
- The ReViCTO trial is the first randomized clinical trial ever conducted to use VR during PCI CTO. Its results will show the utility of this technology reducing anxiety and pain in PCI CTO.
- Primary endpoint will be to assess changes to the maximum level of anxiety perceived by the patient.
- Secondary endpoints will be a) changes to the maximum level of pain during the procedure; b) differences in the need for intraoperative anxiolytic drug therapy; and c) overall satisfaction with the VR goggles.
REFERENCES
1. Tajti P, Burke MN, Karmpaliotis D, et al. Update in the Percutaneous Management of Coronary Chronic Total Occlusions. JACC Cardiovasc Interv. 2018;11:615-625.
2. Tajti P, Brilakis ES. Chronic Total Occlusion Percutaneous Coronary Intervention: Evidence and Controversies. J Am Heart Assoc. 2018;7:e006732.
3. Patel VG, Brayton KM, Tamayo A, et al. Angiographic success and procedural complications in patients undergoing percutaneous coronary chronic total occlusion interventions: a weighted meta-analysis of 18,061 patients from 65 studies. JACC Cardiovasc Interv. 2013;6:128-136.
4. Brilakis ES, Banerjee S, Karmpaliotis D, et al. Procedural outcomes of chronic total occlusion percutaneous coronary intervention: a report from the NCDR (National Cardiovascular Data Registry). JACC Cardiovasc Interv. 2015;8:245-253.
5. Azzalini L, Karmpaliotis D, Santiago R, et al. Contemporary Issues in Chronic Total Occlusion Percutaneous Coronary Intervention. JACC Cardiovasc Interv. 2022;15:1-21.
6. Bruno RR, Lin Y, Wolff G, et al. Virtual reality-assisted conscious sedation during transcatheter aortic valve implantation: a randomised pilot study. EuroIntervention. 2020;16:e1014-e1020.
7. Roxburgh T, Li A, Guenancia C, et al. Virtual Reality for Sedation During Atrial Fibrillation Ablation in Clinical Practice: Observational Study. J Med Internet Res. 2021;23:e26349.
8. Chan AW, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 Statement: Defining Standard Protocol Items for Clinical Trials. Ann Intern Med. 2013;158:200.
9. Schulz KF, Grimes DA. Unequal group sizes in randomised trials: guarding against guessing. Lancet. 2002;359:966-970.
10. Our Planet. Silverback Films, UK. Available at: https://www.netflix.com/es/title/80049832. Accessed 02 Feb 2023.
11. Williams VS, Morlock RJ, Feltner D. Psychometric evaluation of a visual analog scale for the assessment of anxiety. Health Qual Life Out. 2010;8:57.
12. Carlsson AM. Assessment of chronic pain. I. Aspects of the reliability and validity of the visual analogue scale. Pain. 1983;16:87-101.
13. Aubrun F, Paqueron X, Langeron O, Coriat P, Riou B. What pain scales do nurses use in the postanaesthesia care unit? Eur J Anaesth. 2003;20:745-749.
14. Kindler CH, Harms C, Amsler F, Ihde-Scholl T, Scheidegger D. The Visual Analog Scale Allows Effective Measurement of Preoperative Anxiety and Detection of Patients’ Anesthetic Concerns. Anesth Analg. 2000;90:706-712.
15. Delewi R, Vlastra W, Rohling WJ, et al. Anxiety levels of patients undergoing coronary procedures in the catheterization laboratory. Int J Cardiol. 2017;228:926-930.
16. Vlastra W, Delewi R, Rohling WJ, et al. Premedication to reduce anxiety in patients undergoing coronary angiography and percutaneous coronary intervention. Open Hear. 2018;5:e000833.
17. Damluji AA, Pomenti SF, Ramireddy A, et al. Influence of Total Coronary Occlusion on Clinical Outcomes (from the Bypass Angioplasty Revascularization Investigation 2 DiabetesTrial). Am J Cardiol. 2016;117:1031-1038.
18. Tajstra M, Pyka Ł, Gorol J, et al. Impact of Chronic Total Occlusion of the Coronary Artery on Long-Term Prognosis in Patients With Ischemic Systolic Heart Failure Insights From the COMMIT-HF Registry. JACC Cardiovasc Interv. 2016;9:1790-1797.
19. Christopoulos G, Karmpaliotis D, Alaswad K, et al. Application and outcomes of a hybrid approach to chronic total occlusion percutaneous coronary intervention in a contemporary multicenter US registry. Int J Cardiol. 2015;198:222-228.
20. Amat-Santos IJ, Martin-Yuste V, Fernández-Díaz JA, et al. Resultados inmediatos e impacto funcional y pronóstico tras la recanalización de oclusiones coronarias crónicas. Resultados del Registro Ibérico. Rev Esp Cardiol. 2019;72:373-382.
21. Astin F, Jones K, Thompson DR. Prevalence and patterns of anxiety and depression in patients undergoing elective percutaneous transluminal coronary angioplasty. Hear Lung J Acute Critical Care. 2005;34:393-401.
22. Lenzen MJ, Gamel CJ, Immink AW. Anxiety and Well-Being in First-Time Coronary Angioplasty Patients and Repeaters. Eur J Cardiovasc Nur. 2002;1:195-201.
23. Trotter R, Gallagher R, Donoghue J. Anxiety in patients undergoing percutaneous coronary interventions. Hear Lung J Acute Critical Care. 2011;40:185-192.
24. Heikkilä J, Paunonen M, Virtanen V, Laippala P. Fear of patients related to coronary arteriography. J Adv Nurs. 1998;28:54-62.
25. McLenon J, Rogers MAM. The fear of needles: A systematic review and meta-analysis. J Adv Nurs. 2019;75:30-42.
26. Zeltzer LK, Altman A, Cohen D, LeBaron S, Munuksela EL, Schechter NL. American Academy of Pediatrics Report of the Subcommittee on the Management of Pain Associated with Procedures in Children with Cancer. Pediatrics. 1990;86(5 Pt 2):826-831.
27. Manne SL, Bakeman R, Jacobsen PB, Gorfinkle K, Redd WH. An analysis of a behavioral intervention for children undergoing venipuncture. Health Psychol. 1994;13:556-566.
28. Burns-Nader S, Joe L, Pinion K. Computer tablet distraction reduces pain and anxiety in pediatric burn patients undergoing hydrotherapy: A randomized trial. Burns. 2017;43:1203-1211.
29. Hoffman HG, Chambers GT, Meyer WJ, et al. Virtual Reality as an Adjunctive Non-pharmacologic Analgesic for Acute Burn Pain During Medical Procedures. Ann Behav Med. 2011;41:183-191.
30. Malloy KM, Milling LS. The effectiveness of virtual reality distraction for pain reduction: A systematic review. Clin Psychol Rev. 2010;30:1011-1018.
31. Wang Y, Guo L, Xiong X. Effects of Virtual Reality-Based Distraction of Pain, Fear, and Anxiety During Needle-Related Procedures in Children and Adolescents. Front Psychol. 2022;13:842847.
32. Kipping B, Rodger S, Miller K, Kimble RM. Virtual reality for acute pain reduction in adolescents undergoing burn wound care: A prospective randomized controlled trial. Burns. 2012;38:650-657.
33. Khadra C, Ballard A, Déry J, et al. Projector-based virtual reality dome environment for procedural pain and anxiety in young children with burn injuries: a pilot study. J Pain Res. 2018;11:343-353.
34. Soltani M, Drever SA, Hoffman HG, et al. Virtual Reality Analgesia for Burn Joint Flexibility: A Randomized Controlled Trial. Rehabil Psychol. 2018;63:487-494.
35. Lahti S, Suominen A, Freeman R, Lähteenoja T, Humphris G. Virtual Reality Relaxation to Decrease Dental Anxiety: Immediate Effect Randomized Clinical Trial. Jdr Clin Transl Res. 2020;5:312-318.
36. Veldhuijzen G, Klaassen NJM, Wezel RJAV, Drenth JPH, Esch AAV. Virtual reality distraction for patients to relieve pain and discomfort during colonoscopy. Endosc Int Open. 2020;08:E959-E966.
37. Clerc PGB, Arneja JS, Zwimpfer CM, Behboudi A, Goldman RD. A Randomized Controlled Trial of Virtual Reality in Awake Minor Pediatric Plastic Surgery Procedures. Plast Reconstr Surg. 2021;148:400-408.
38. Liu KY, Ninan SJ, Laitman BM, Goldrich DY, Iloreta AM, Londino AV. Virtual Reality as Distraction Analgesia and Anxiolysis for Pediatric Otolaryngology Procedures. Laryngoscope. 2021;131:E1714-E1721.
39. Fabi A, Fotia L, Giuseppini F, et al. The immersive experience of virtual reality during chemotherapy in patients with early breast and ovarian cancers: The patient’s dream study. Frontiers Oncol. 2022;12:960387.
40. Deo N, Khan K, Mak J, et al. Virtual reality for acute pain in outpatient hysteroscopy: a randomised controlled trial. Bjog Int J Obstetrics Gynaecol. 2021;128:87-95
41. Xu N, Chen S, Liu Y, Jing Y, Gu P. The Effects of Virtual Reality in Maternal Delivery: Systematic Review and Meta-analysis. Jmir Serious Games. 2022;10:e36695.
42. Cakir SK, Evirgen S. The Effect of Virtual Reality on Pain and Anxiety During Colonoscopy: A Randomized Controlled Trial. Turk J Gastroenterol. 2021;32:451-547.
43. Mahtab EAF, Egorova AD. Current and future applications of virtual reality technology for cardiac interventions. Nat Rev Cardiol. 2022;19:779-780.
ABSTRACT
Introduction and objectives: Geographical and organizational differences between different autonomous communities (AC) can generate differences in care for ST-segment elevation myocardial infarction (STEMI). A total of 17 heart attack code programs have been compared in terms of incidence rate, clinical characteristics, reperfusion therapy, delay to reperfusion, and 30-day mortality.
Methods: National prospective observational study (83 centers included in 17 infarction networks). The recruitment period was 3 months (April 1 to June 30, 2019) with clinical follow-up at 30 days.
Results: 4366 patients with STEMI were included. The incidence rate was variable between different AC (P < .0001), as was gender (P = .003) and the prevalence of cardiovascular risk factors (P < .0001). Reperfusion treatment was primary angioplasty (range 77.5%-97.8%), fibrinolysis (range 0%-12.9%) or no treatment (range 2.2%- 13.5%). The analysis of the delay to reperfusion showed significant differences (P < .001) for all the intervals analyzed. There were significant differences in 30-days mortality that disappeared after adjusting for clinical and healthcare network characteristics.
Conclusions: Large differences in STEMI care have been detected between the different AC, in terms of incidence rate, clinical characteristics, reperfusion treatment, delay until reperfusion, and 30-day mortality. The differences in mortality disappeared after adjusting for the characteristics of the patient and the care network.
Keywords: STEMI. Population characteristics. Angioplasty.
RESUMEN
Introducción y objetivos: Las diferencias geográficas y organizativas entre distintas comunidades autónomas (CCAA) pueden generar diferencias en la atención al infarto agudo de miocardio con elevación del segmento ST (IAMCEST). Se han comparado 17 programas de Código Infarto en términos de incidencia, características clínicas, tratamiento de reperfusión, retraso hasta la reperfusión y mortalidad a 30 días.
Métodos: Estudio observacional prospectivo nacional (83 centros en 17 redes de infarto). El periodo de selección fue de 3 meses (1 de abril a 30 de junio de 2019), con seguimiento clínico a 30 días.
Resultados: Se incluyeron 4.366 pacientes con IAMCEST. La tasa de incidencia fue variable entre las CCAA (p < 0,0001), igual que el sexo (p = 0,003) y la prevalencia de factores de riesgo cardiovascular (p < 0,0001). El tratamiento de reperfusión fue angioplastia primaria (rango 77,5-97,8%), fibrinolisis (rango 0- 12,9%) o ninguno (rango 2,2-13,5%). El análisis del retraso hasta la reperfusión mostró diferencias significativas (p < 0,001) para todos los intervalos analizados. Hubo diferencias significativas en la mortalidad cruda a 30 días que desaparecieron tras ajustar por las características clínicas y dependientes de la red asistencial (primer contacto, tiempo hasta la reperfusión y abordaje de críticos).
Conclusiones: Se han detectado diferencias en la atención al IAMCEST entre las distintas CCAA, en términos de incidencia, características clínicas, tratamiento de reperfusión, retraso hasta la reperfusión y mortalidad a 30 días. Las diferencias en mortalidad desaparecen tras ajustar por las características del paciente y de la red asistencial.
Palabras clave: IAMCEST. Características de la población. Angioplastia.
Abbreviations
ACI-SEC: Interventional Cardiology Association at the Spanish Society of Cardiology. AC: autonomous communities. pPCI: primary percutaneous coronary intervention. STEMI: ST-segment elevation myocardial infarction.
INTRODUCTION
Infarction Code networks are key to treat ST-segment elevation myocardial infarction (STEMI) in the shortest time possible while optimizing reperfusion therapy.1 In Spain we have 17 different public regional STEMI networks, 1 in each autonomous community (AC) for a total of 83 pPCI-capable hospitals in programs on a 24/7/365 basis.2 According to data from the Annual Activity Registry of the Interventional Cardiology Association of the Spanish Society of Cardiology (ACI-SEC), back in 2019, a total of 22 529 interventional procedures were performed in patients with infarction.3 Recently, an analysis of the ACI-SEC Infarction Code Registry revealed the characteristics of infarction care in Spain with 87.5%, 4.4%, and 8.1% of the patients with STEMI being treated with pPCI, fibrinolysis, and without reperfusion, respectively. The 30-day mortality rate of STEMI was 7.9% dropping down to 6.8% in patients treated with pPCI.4
The geographical differences and heterogeneity of the organizational infrastructure among the different Infarction Code programs available can lead to regional differences as a survey conducted among health professionals involved in these programs revealed recently.5 These organizational differences can have an impact on the management of patients with STEMI. Their analysis and AC-based comparison facilitates finding matters where there is room for improvement to optimize treatment.
This analysis compared the incidence rate, clinical characteristics, type and time to reperfusion, the characteristics of pPCI, and the 30-day mortality rate of 17 different regional programs of the Infarction Code in Spain.
METHODS
Study design
The Registry design has already been introduced4. In conclusion, this was a national, observational, and prospective study of 83 centers from 17 different regional STEMI networks. The patients’ recruitment period was 3 months—from April 1 through June 30, 2019—with a 30-day clinical follow-up.
Registry protocol was approved by the reference central ethics committee that did not deem the obtention of the informed consent necessary since data anonymity was guaranteed at any time.
Inclusion criteria
All consecutive patients who, during the study period, triggered the activation of different regional infarction care networks with a final diagnosis of STEMI and met the following criteria were included in the study: a) diagnosis of ST-segment elevation acute coronary syndrome with symptoms consistent with acute coronary syndrome, electrocardiogram showing ST-segment elevation or new-onset left bundle branch block or suspected posterior infarction of, at least, 24-hour evolution since symptom onset or b) recovered cardiac arrest with suspected coronary etiology or c) cardiogenic shock with suspected coronary etiology.
Definition and collection of variables
Clinical variables were registered in an online form and previously published.4 The definitions of the different time intervals since symptom onset until reperfusion were given based on the recommendations established by the European clinical practice guidelines on the management of STEMI.1 Subjective judgment from a local investigator was requested on the delay sustained by the patient since his first medical contact (existence of unjustified delay—yes/no—and reason why). To estimate the incidence rate (number of cases per million inhabitants) population data from the National Statistics Institute from 2019 were used.6 Regarding the mortality adjusted analysis, the following characteristics of the care network were defined: the individual responsible for the first medical contact (emergency medical services, health center, non-pPCI-capable hospital, pPCI-capable hospital), time to reperfusion, and location where critical care was administered (intensive care unit or cardiac surgery intensive care unit).
Statistical analysis
Continuous variables were expressed as mean ± standard deviation. The categorical ones were expressed as frequencies and percentages. Inter-group comparisons of baseline variables were conducted using the chi-square test or the Student t test, when appropriate. Times to reperfusion were expressed as median and interquartile range and compared using the Mann-Whitney U test.
Poisson regression coefficient was used to estimate the 30-day mortality rate of each AC including patient-dependent factors (the confounding factors included were age, sex, hypertension, diabetes, dyslipidemia, smoking, previous ischemic heart disease, Killip classification, and anterior location of STEMI), and the healthcare network involved (location of the first medical contact, time between the onset of pain and reperfusion, and location where critically ill patients were treated).
The variable AC was introduced in the model in a second step, and a test of ratio of verisimilitude was performed to verify its statistical significance. When the AC variable was added, adjusted associations were obtained between AC and mortality. The Poisson regression coefficients became incidence rates using the marginal effect function. The estimated 30-day mortality rate for each AC was obtained from a mean distribution of confounding factors, which facilitated comparing mortality rate across the different AC. This method had been previously used in the acute myocardial infarction setting.7-9 Since there could be a selection bias across the different AC in patients without reperfusion therapy, these were not included in the adjusted mortality analysis.
P values < .05 were considered statistically significant. The STATA statistical software package version 15 SE (Stata Corp, College Station, United States) was used.
RESULTS
Patients
The registry included a total of 5401 patients, 4366 (81.2%) of whom had a final diagnosis of STEMI. The 888 patients (16.4%) with a diagnosis different from STEMI and the 147 (2.7%) without a final diagnosis were excluded from the analysis. Figure 1 shows the flow of patients and the AC-based distribution. Figure 2 shows the number of patients treated across the different AC plus the final diagnosis achieved adjusted by million inhabitants.6 Table 1 shows the clinical characteristics of patients with STEMI across the different AC.
Figure 1. Flow of patients and distribution across the different autonomous communities (AC) based on participant centers, number of codes activated, and number of patients with ST-segment elevation myocardial infarction (STEMI) as final diagnosis.
Figure 2. Patients treated across the different autonomous communities (AC) adjusted for million inhabitants. AC were arranged from largest to smallest number of patients treated per million inhabitants. Regarding the population estimate per million inhabitants, population data from the National Statistics Institute were used.6 STEMI, ST-segment elevation myocardial infarction.
Table 1. Clinical characteristics of patients with ST-segment elevation myocardial infarction treated in the Infarction Code networks per autonomous community
| Age, years | Sex, women | AHT | Diabetes | Dyslipidemia | Active smoking | Previous IHD | Previous PCI | Previous stroke | Early Killip I | Early Killip IV | Anterior location | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Andalusia | 63 ± 13 | 110/563 (19.5) | 297/560 (53.0) | 159/558 (28.5) | 252/559 (45.1) | 264/557 (47.4) | 60/561 (10.7) | 59/559 (10.6) | 31/556 (5.6) | 423/541 (78.2) | 31/541 (5.7) | 223/521 (42.8) |
| Aragon | 65 ± 14 | 30/127 (23.6) | 62/127 (48.8) | 28/125 (22.4) | 56/127 (44.1) | 59/124 (47.6) | 13/124 (10.5) | 17/126 (13.5) | 7/122 (5.7) | 99/124 (79.8) | 13/124 (10.5) | 56/120 (46.7) |
| Principality of Asturias | 66 ± 13 | 40/124 (32.3) | 61/124 (49.2) | 34/122 (27.9) | 54/124 (43.6) | 41/123 (33.3) | 20/123 (16.3) | 19/123 (15.4) | 7/123 (5.6) | 96/123 (78.1) | 11/123 (8.9) | 57/122 (46.7) |
| Balearic Islands | 63 ± 12 | 28/97 (28.9) | 44/94 (46.8) | 21/94 (22.3) | 49/93 (52.7) | 49/93 (52.7) | 14/93 (15.1) | 14/94 (14.9) | 4/92 (4.4) | 71/96 (74.0) | 5/96 (5.2) | 30/92 (32.6) |
| Canary Islands | 60 ± 12 | 40/178 (22.5) | 99/178 (55.6) | 52/178 (29.2) | 102/177 (57.6) | 93/178 (52.3) | 22/178 (12.4) | 18/178 (10.1) | 8/176 (4.6) | 146/168 (86.9) | 14/168 (8.3) | 65/163 (39.9) |
| Cantabria | 62 ± 13 | 15/59 (25.4) | 31/59 (52.5) | 21/58 (36.2) | 27/58 (46.6) | 31/57 (54.4) | 10/58 (17.2) | 10/59 (17.0) | 3/57 (5.3) | 46/56 (83.9) | 2/56 (3.6) | 25/58 (43.1) |
| Castile and Leon | 64 ± 13 | 56/296 (18.9) | 146/293 (49.8) | 73/291 (25.1) | 126/292 (43.2) | 117/292 (40.1) | 31/293 (10.6) | 31/294 (10.5) | 12/176 (4.1) | 236/287 (82.2) | 17/287 (5.9) | 138/280 (49.3) |
| Castile-La Mancha | 64 ± 13 | 26/197 (13.2) | 108/194 (55.7) | 58/192 (30.2) | 99/196 (50.5) | 92/193 (47.7) | 19/192 (9.9) | 18/194 (9.3) | 9/194 (4.6) | 157/196 (80.1) | 12/196 (6.1) | 89/194 (45.9) |
| Catalonia | 63 ± 13 | 195/854 (22.8) | 393/854 (46.0) | 198/854 (23.2) | 340/854 (39.8) | 354/854 (41.4) | 60/854 (7.0) | 62/854 (7.3) | 30/854 (3.5) | 683/826 (82.7) | 67/826 (8.1) | 351/767 (45.8) |
| Extremadura | 63 ± 13 | 18/127 (14.2) | 74/127 (58.3) | 26/126 (20.6) | 52/126 (41.3) | 48/127 (37.8) | 17/126 (13.5) | 14/126 (11.1) | 4/127 (3.2) | 91/122 (74.6) | 11/122 (9.0) | 56/121 (46.3) |
| Galicia | 63 ± 13 | 63/264 (23.9) | 130/262 (49.6) | 48/259 (18.5) | 138/261 (52.9) | 100/215 (46.5) | 18/261 (6.9) | 25/262 (9.5) | 12/263 (4.6) | 195/251 (77.7) | 31/251 (12.4) | 103/233 (44.2) |
| La Rioja | 59 ± 12 | 8/34 (23.5) | 14/34 (41.2) | 3/34 (8.8) | 16/34 (46.1) | 20/34 (58.8) | 1/34 (3.0) | 2/34 (5.9) | 0/34 (0) | 30/34 (88.2) | 3/34 (8.8) | 11/34 (32.4) |
| Community of Madrid | 63 ± 13 | 105/436 (24.1) | 212/432 (49.1) | 88/430 (20.5) | 208/431 (48.3) | 177/428 (41.4) | 41/429 (9.6) | 43/429 (10.0) | 11/429 (2.6) | 347/424 (81.8) | 35/424 (8.3) | 174/419 (41.5) |
| Region of Murcia | 64 ± 13 | 43/238 (18.1) | 127/237 (53.6) | 71/237 (30.0) | 100/237 (42.4) | 110/237 (46.4) | 41/237 (17.3) | 24/151 (15.9) | 3/151 (2.0) | 196/237 (82.7) | 18/237 (7.6) | 101/231 (43.7) |
| Chartered Community of Navarre | 65 ± 14 | 14/45 (31.1) | 18/44 (40.9) | 9/45 (20.0) | 29/45 (64.4) | 16/45 (35.6) | 3/45 (6.7) | 4/44 (9.1) | 3/45 (6.7) | 31/43 (72.1) | 4/43 (9.3) | 16/44 (36.4) |
| Basque Country | 64 ± 14 | 52/200 (26.0) | 101/197 (51.3) | 39/197 (19.8) | 101/198 (51.0) | 89/197 (45.2) | 26/195 (13.3) | 32/196 (16.3) | 11/193 (5.7) | 169/200 (84.5) | 12/200 (6.0) | 83/199 (41.7) |
| Valencian Community | 63 ± 13 | 119/526 (22.6) | 293/519 (56.5) | 163/514 (31.7) | 212/514 (41.3) | 235/514 (45.7) | 56/515 (10.9) | 53/511 (10.4) | 21/513 (4.1) | 445/520 (85.6) | 34/520 (6.5) | 217/503 (43.1) |
| P | .054 | .003 | .038 | < .0001 | < .0001 | .007 | < .0001 | .011 | .61 | .016 | .25 | .44 |
| Total | 63 ± 13 | 962/4365 (22.0) | 2210/4335 (51.0) | 1091/4314 (25.3) | 1961/4326 (45.3) | 1895/4268 (44.4) | 452/4318 (10.5) | 445/4234 (10.5) | 176/4222 (4.2) | 3462/4248 (81.5) | 320/4248 (7.5) | 1795/4101 (43.8) |
|
AHT, arterial hypertension; IHD, ischemic heart disease; PCI, percutaneous coronary intervention. |
||||||||||||
Reperfusion therapy used in patients with ST-segment elevation myocardial infarction
Out of the 4366 patients with STEMI, 3792 (86.9%) received pPCI, 189 (4.3%) fibrinolysis, and 353 (8.1%) no reperfusion therapy whatsoever. No reperfusion therapy was reported in 32 patients (0.7%). Figure 3 shows treatment distribution based on AC. Table 2 shows, across different AC and patients treated with cardiac catheterization, the angiographic findings and characteristics of interventional therapy had this procedure been performed.
Figure 3. Distribution of reperfusion therapy in patients with ST-segment elevation myocardial infarction by autonomous communities. pPCI, primary percutaneous coronary intervention.
Table 2. Angiographic findings and characteristics of interventional procedures in patients with ST-segment elevation myocardial infarction treated with cardiac catheterization per autonomous community
| Radial access | No. of diseased vessels | Early TIMI grade-0/1 flow | Final TIMI grade-3 flow | Need for hemodynamic support | Thrombus aspiration in IRA | BMS implantation in IRA | DES implantation in IRA | pPCI | Bailout PCI | Elective PCI after fibrinolysis | Coronary angiography without PCI | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Andalusia | 456/534 (85.4) | 1.49 ± 0.69 | 416/535 (77.8) | 502/536 (93.7) | 15/563 (2.7) | 76/563 (13.5) | 48/563 (8.5) | 456/563 (81.0) | 471/557 (84.6) | 36/557 (6.5) | 27/557 (4.9) | 23/557 (4.1) |
| Aragon | 111/122 (91.0) | 1.62 ± 0.78 | 90/120 (75.0) | 114/122 (93.4) | 5/127 (3.9) | 41/127 (32.3) | 0/127 (0) | 103/127 (81.1) | 108/124 (87.1) | 6/124 (4.8) | 1/124 (0.8) | 9/124 (7.3) |
| Principality of Asturias | 99/121 (81.8) | 1.54 ± 0.77 | 106/121 (87.6) | 111/121 (91.7) | 5/124 (4.0) | 39/124 (31.5) | 10/124 (8.1) | 98/124 (79.0) | 118/123 (95.9) | 0/123 (0) | 0/123 (0) | 5/123 (4.1) |
| Balearic Islands | 79/92 (85.9) | 1.46 ± 0.67 | 67/92 (72.8) | 85/92 (92.4) | 0/124 (0) | 27/97 (27.8) | 4/97 (4.1) | 80/97 (82.5) | 89/96 (92.7) | 4/96 (4.2) | 0/96 (0) | 3/96 (3.1) |
| Canary Islands | 138/169 (81.7) | 1.54 ± 0.76 | 131/170 (77.1) | 155/169 (91.7) | 6/179 (3.6) | 29/179 (16.2) | 3/179 (1.7) | 150/179 (83.8) | 145/176 (82.4) | 6/176 (3.4) | 15/176 (8.5) | 10/176 (5.7) |
| Cantabria | 17/56 (30.4) | 1.50 ± 0.68 | 51/57 (89.5) | 55/56 (98.2) | 1/59 (1.7) | 31/59 (52.5) | 0/59 (0) | 51/59 (86.4) | 57/59 (96.6) | 0/59 (0) | 1/59 (1.7) | 1/59 (1.7) |
| Castile and Leon | 263/281 (93.6) | 1.55 ± 0.74 | 192/241 (79.7) | 225/247 (91.1) | 15/296 (5.1) | 27/296 (9.1) | 9/296 (3.0) | 249/296 (84.1) | 255/291 (96.6) | 12/291 (4.1) | 16/291 (5.5) | 8/291 (2.8) |
| Castile-La Mancha | 164/191 (85.9) | 1.68 ± 0.73 | 164/192 (85.4) | 186/190 (97.9) | 9/197 (4.6) | 75/197 (38.1) | 10/197 (5.1) | 172/197 (97.3) | 185/196 (94.4) | 2/196 (1.0) | 4/196 (2.0) | 5/196 (2.6) |
| Catalonia | 727/781 (93.1) | 1.48 ± 0.70 | 594/844 (70.4) | 787/827 (95.2) | ND | 259/854 (30.3) | 117/854 (13.7) | 653/854 (76.5) | 807/849 (95.1) | 8/849 (0.9) | 3/849 (0.4) | 31/849 (3.7) |
| Extremadura | 119/121 (98.4) | 1.65 ± 0.79 | 104/122 (85.3) | 104/122 (85.3) | 6/127 (4.7) | 18/127 (14.2) | 12/127 (11.0) | 98/127 (77.2) | 112/126 (88.9) | 8/126 (6.4) | 2/126 (1.6) | 4/126 (3.2) |
| Galicia | 228/242 (94.2) | 1.53 ± 0.84 | 182/229 (79.5) | 214/229 (93.5) | 20/264 (7.6) | 77/264 (29.2) | 4/264 (1.5) | 215/264 (81.4) | 246/264 (93.2) | 0/264 (0) | 0/264 (0) | 18/264 (6.8) |
| La Rioja | 29/34 (85.3) | 1.15 ± 0.36 | 30/34 (88.2) | 31/34 (91.2) | 0/24 (0) | 10/34 (29.4) | 3/34 (8.8) | 27/34 (79.4) | 33/34 (97.1) | 0/34 (0) | 0/34 (0) | 1/34 (2.9) |
| Community of Madrid | 395/421 (93.8) | 1.48 ± 0.69 | 329/402 (81.8) | 392/425 (92.2) | 23/436 (5.3) | 80/436 (18.4) | 15/436 (3.4) | 352/436 (80.5) | 421/434 (97.0) | 3/434 (0.7) | 0/434 (0) | 10/434 (2.3) |
| Region of Murcia | 213/237 (89.9) | 1.48 ± 0.64 | 175/234 (74.8) | 223/236 (94.5) | 4/238 (1.7) | 56/238 (23.5) | 5/238 (2.1) | 209/238 (87.2) | 226/238 (95.0) | 7/238 (2.9) | 0/238 (0) | 5/238 (2.1) |
| Chartered Community of Navarre | 31/36 (86.1) | 2.00 ± 0.86 | 34/43 (79.1) | 39/45 (86.7) | 6/45 (13.3) | 22/45 (48.9) | 2/45 (4.4) | 39/45 (86.7) | 44/45 (97.8) | 0/45 (0) | 0/45 (0) | 1/45 (2.2) |
| Basque Country | 179/198 (90.4) | 1.51 ± 0.67 | 153/198 (77.3) | 191/199 (96.0) | 7/200 (3.5) | 100/200 (50.0) | 3/200 (1.5) | 174/200 (87.0) | 194/199 (97.5) | 4/199 (2.0) | 1/199 (0.5) | 0/199 (0) |
| Valencian Community | 484/514 (94.2) | 1.59 ± 0.76 | 390/496 (78.6) | 461/497 (92.8) | 8/256 (1.5) | 145/526 (27.6) | 34/526 (6.5) | 423/526 (80.4) | 482/518 (93.1) | 10/518 (1.9) | 4/518 (0.8) | 22/518 (4.3) |
| P | < .0001 | .84 | < .0001 | .002 | < .0001 | < .0001 | < .0001 | .004 | < .0001 | |||
| Total | 3732/4150 (89.9) | 1.50 ± 0.71 | 3208/4130 (77.7) | 3875/4147 (93.4) | 110/4366 (2.5) | 1112/4366 (25.5) | 281/4366 (6.4) | 3548/4366 (81.3) | 3992/4329 (92.2) | 106/4329 (2.5) | 74/4329 (1.7) | 157/4329 (3.6) |
|
BMS, bare metal stent; CL, cath lab; DES, drug-eluting stent; ECG, electrocardiogram; EMS, emergency medical services; FMC, first medical contact; IRA, infarct-related artery; PCI, percutaneous coronary intervention; pPCI, primary percutaneous coronary intervention. |
||||||||||||
Time intervals between symptom onset and reperfusion in patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention
Table 3 shows time intervals between symptom onset and reperfusion. Figure 4 shows the different time intervals analyzed for every AC with significant differences in all of them. Figure 5 summarizes the causes of unjustified delays between the first medical contact and reperfusion for every AC.
Table 3. Location of the first medical contact and time intervals between the first medical contact and reperfusion per autonomous community
| First EMS care | First care provided at the health center | First non-pPCI-capable center care | First pPCI-capable center care | Transfer without going to the CL right away* | Time of onset of pain to FMC | Time of FMC to ECG | Time of FMC to pPCI-capable center in transferred patients | Time from FMC to reperfusion | Time from onset of pain to reperfusion | |
|---|---|---|---|---|---|---|---|---|---|---|
| Andalusia | 206/537 (38.4) | 138/537 (25.7) | 93/537 (17.3) | 100/537 (18.6) | 188/427 (44.0) | 60 [30-123] | 5 [3-10] | 80 [50-120] | 113 [70-170] | 195 [135-330] |
| Aragon | 46/123 (37.4) | 23/123 (18.7) | 42/123 (34.1) | 12/123 (9.8) | 23/110 (20.9) | 62.5 [18.5-170] | 7 [4-12.5] | 84.5 [45-145] | 116.5 [70.5-177.5] | 229 [126-345] |
| Principality of Asturias | 32/123 (26.0) | 18/123 (14.6) | 36/123 (29.3) | 37/123 (30.1) | 4/86 (4.7) | 80 [32-210] | 10 [5-22] | 85 [60-119] | 108 [73-137] | 215 [134.5-351] |
| Balearic Islands | 33/95 (34.7) | 26/95 (27.4) | 27/95 (28.4) | 9/95 (9.5) | 3/85 (3.5) | 70 [30-164] | 6 [5-10] | 100 [55-139] | 124 [85-169] | 197.5 [143.5-391] |
| Canary Islands | 28/178 (15.7) | 103/178 (57.9) | 22/178 (12.4) | 25/178 (14.0) | 77/152 (50.7) | 75 [37.5-150] | 9 [5-15] | 85 [55-133] | 122 [95-172] | 220 [159-385] |
| Cantabria | 15/58 (25.9) | 19/58 (32.8) | 13/58 (22.4) | 11/58 (19.0) | 26/46 (56.5) | 53 [25-145] | 5 [4.5-10] | 60 [35-93] | 110 [81-188] | 210 [134-303.5] |
| Castile and Leon | 97/290 (33.5) | 70/290 (27.2) | 68/290 (23.5) | 46/290 (15.9) | 70/237 (29.5) | 90 [35-221] | 8 [4-15] | 115 [70-165] | 135 [85-197] | 242.5 [163-432.5] |
| Castile-La Mancha | 69/196 (35.2) | 61/196 (31.1) | 30/196 (17.3) | 36/196 (18.4) | 49/160 (30.6) | 68 [30-160] | 10 [5-15] | 86.5 [58-114] | 109 [80-155] | 205 [150-322] |
| Catalonia | 332/847 (39.2) | 161/847 (19.0) | 256/847 (30.2) | 98/847 (11.6) | 115/730 (15.8) | 63 [30-160] | 6 [3-14] | 75 [55-105] | 104 [80-138] | 180 [127-288] |
| Extremadura | 43/126 (34.1) | 36/126 (28.6) | 22/126 (17.5) | 25/126 (19.8) | 27/93 (29.0) | 81.5 [44-135] | 10 [5-12] | 91.5 [60-143] | 121 [90-178] | 240 [160-360] |
| Galicia | 84/264 (31.8) | 111/264 (42.1) | 28/264 (10.6) | 41/264 (15.5) | ND | 60 [26-179] | 9 [5-19] | 95 [70-140] | 115 [88.5-163] | 194 [134-353] |
| La Rioja | 10/34 (29.4) | 9/34 (26.5) | 6/34 (17.7) | 9/34 (26.5) | 3/25 (12.0) | 76.5 [35-110] | 4.5 [1-10] | 70 [46-86] | 90.5 [67-114] | 159.5 [118.5-212.5] |
| Community of Madrid | 196/429 (45.7) | 37/429 (8.6) | 80/429 (18.7) | 116/429 (27.0) | 142/309 (45.6) | 63 [35-140] | 6 [3-12] | 60 [42-85] | 95 [75-130] | 178.5 [135-257.5] |
| Region of Murcia | 102/238 (42.9) | 36/238 (15.1) | 74/238 (31.1) | 26/238 (10.9) | 25/212 (11.8) | 56.5 [24-131] | 5 [5-10] | 80 [60-120] | 103 [79-160] | 175 [130-305] |
| Chartered Community of Navarre | 22/45 (48.9) | 7/45 (15.6) | 3/45 (6.7) | 13/45 (28.9) | 12/32 (37.5) | 63.5 [29.5-124.5] | 1 [0-5] | 50 [35-91] | 90 [69-140] | 175 [128-262] |
| Basque Country | 76/199 (38.2) | 28/199 (14.1) | 37/199 (18.6) | 58/199 (29.2) | 61/138 (44.2) | 80 [32-184] | 6.5 [3-11] | 61 [49-77] | 97 [75-135] | 210 [134-345] |
| Valencian Community | 128/521 (24.6) | 146/521 (28.0) | 128/521 (24.6) | 119/521 (22.8) | 98/398 (24.6) | 82 [35-180] | 5 [0-10] | 94 [65-135] | 120 [93-165] | 220 [146-348] |
| P | < .0001 | < .0001 | < .0001 | < .0001 | < .001 | .001 | .0001 | .0001 | .0001 | .0001 |
| Total | 1519/4303 (35.3) | 1038/4303 (24.1) | 965/4303 (22.4) | 781/4303 (18.2) | 923/3240 (28.5) | 67 [30-165] | 7 [4-15] | 80 [55-120] | 110 [80-154] | 197 [135-330] |
|
CL, cath lab; ECG, electrocardiogram; EMS, emergency medical services; FMC, first medical contact; pPCI, primary percutaneous coronary intervention. * Patients treated early in a non-pPCI-capable center requiring immediate transfer to a pPCI-capable center. Data are expressed as no. (%) or mean [interquartile range]. Times are expressed in minutes. |
||||||||||
Figure 4. Time intervals between symptom onset and reperfusion in patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention (pPCI) for every autonomous community. A: time in min from the onset of pain to the first medical contact. B: time in min from the first medical contact to the electrocardiogram (ECG). C: time in min from the first medical contact to reperfusion. D: time in min from the onset of pain to reperfusion. E: time in min from the first medical contact to the arrival at the pPCI-capable center in patients requiring transfer from a non-pPCI-capable center.
Figure 5. Causes of unjustified time delays between the first medical contact and reperfusion. Unjustified time delays did not imply, necessarily, that the time between the first medical contact and reperfusion was > 120 min. As a matter of fact, overall, in 53.2% of the cases the time between the first medical contact and reperfusion was < 120 min, and, among these, excessive time delays were reported in 21.5%. EMS, emergency medical services; pPCI, primary percutaneous coronary intervention.
Mortality analysis in patients with ST-segment elevation myocardial infarction
Table 4 includes unadjusted mortality data at hospital admission and 30 days, and mortality for the adjusted model.
Table 4. Mortality analysis in patients treated with primary percutaneous coronary intervention per autonomous community
| Unadjusted hospital mortality | Unadjusted 30-day mortality | Adjusted 30-day mortality | |
|---|---|---|---|
| Andalusia | 30/563 (5.3) | 37/523 (7.1) | 6.0 [5.3-6-7] |
| Aragon | 8/127 (6.3) | 8/124 (6.5) | 5.5 [4.0-6.9] |
| Principality of Asturias | 9/124 (7.3) | 10/118 (8.5) | 6.7 [5.4-8.0] |
| Balearic Islands | 6/97 (6.2) | 6/88 (6.8) | 5.0 [3.3-6.7] |
| Canary Islands | 15/179 (8.4) | 15/155 (9.7) | 7.0 [5.5-8.6] |
| Cantabria | 0/59 (0) | 0/59 (0) | 0 |
| Castile and Leon | 18/296 (6.1) | 23/270 (8.5) | 8.4 [7.1-9.8] |
| Castile-La Mancha | 9/197 (4.6) | 10/191 (5.2) | 3.1 [2.3-3.8] |
| Catalonia | 29/854 (3.4) | 58/801 (7.2) | 6.0 [5.4-6.6] |
| Extremadura | 12/127 (9.5) | 16/125 (12.8) | 8.1 [6.6-9.5] |
| Galicia | 22/264 (8.3) | 28/260 (10.8) | 6.8 [5.6-7.9] |
| La Rioja | 1/34 (2.9) | 1/33 (3.0) | 5.6 [2.3-8.9] |
| Community of Madrid | 14/436 (3.2) | 21/421 (5.0) | 3.9 [3.3-4.6] |
| Region of Murcia | 21/237 (8.9) | 24/226 (10.6) | 9.2 [8.0-10.5] |
| Chartered Community of Navarre | 5/45 (11.1) | 5/45 (11.1) | 9.5 [6.7-12.3] |
| Basque Country | 12/200 (6.0) | 16/197 (8.1) | 8.9 [7.4-10.4] |
| Valencian Community | 47/526 (8.9) | 55/499 (11.0) | 10.2 [9.2-11.2] |
| P | < .001 | < .001 | .19 |
| Total | 258/4365 (5.9) | 337/4166 (8.1) | – |
|
Data are expressed as no. (%) or mean [interquartile range]. |
|||
30-day mortality rate was different across different AC (P < .001). When the analysis was adjusted for patient-dependent factors and the healthcare network, mortality difference across the AC lost its statistical significance (P = .19).
DISCUSSION
This study is a comparative of how the different STEMI care programs work in Spain. Results show differences in the incidence rate, the patients’ clinical profile, revascularization therapy, the characteristics of the interventional procedure performed, infarction care times, and the 30-day unadjusted mortality rate. Although mortality differences reduce, they’re still significantly different after adjusting for the patients’ risk and clinical characteristics. Also, they disappear after adjusting for whoever is responsible for the first medical contact, time to reperfusion, and location where critical care is administered, all of them factors associated with the way each network is organized.
Both functioning and results of infarction care networks are highly influenced by different factors like geography, the number of capable centers, transfer times, the availability of the right resources, infrastructure, and the characteristics of each healthcare system.2 In Spain, the plan of each AC has been designed independently. Also, the services rendered by the different AC is not homogeneous since resource allocation by the different administrations of the 17 Spanish AC is decentralized2 in such a way that there are inequalities in the ways these networks are organized.2,5,10,11 A recent consensus document on the requirements and sustainability of pPCI programs in Spain proposed measures to homogenize and secure their sustainability.2,12 Our study data reinforce the need for taking measures like the proposals made in the said consensus document.
Differences in the patients’ clinical profile
Registry data demonstrated a difference in the number of codes activated per million inhabitants. Also, in the number of patients with STEMI per million inhabitants across the different AC. These differences are multifactorial and can be seen, historically, in the ACI-SEC annual activity registry reports.3 Some AC have older populations and more cardiovascular risk factors, which could account for the higher rate of infarction reported.6 However, the lack of a unified criterion on the indication for Infarction Code activation could also account for these differences seen.5
Differences in reperfusion therapy
pPCI is the treatment of choice for the management of STEMI.1 The geographical (populations far from pPCI-capable centers) and organizational characteristics (availability of medical service transport with ECG monitorization) across the different AC lead to a variable number of patients be treated with fibrinolysis. A previous analysis of data on the Codi Infart in Catalonia revealed that patients treated with fibrinolysis in non- pPCI-capable centers had worse disease progression compared to those transferred to pPCI-capable centers within the first 140 min after diagnosis.13
Different time delays to reperfusion
Patient-dependent time delays (from symptom onset to first medical contact) were highly variable. Although the geographic distribution of the population could partially account for these differences, public campaigns should be run to increase awareness on STEMI symptoms and the need for calling out-of-hospital emergency care.1
System-dependent time delays (from first medical contact to reperfusion) is much easier to change with organizational measures. Also, it determines prognosis.14 Time delays to reperfusion depend on whoever is involved in the first medical contact. Therefore, patients treated by emergency medical services—those with the shortest times—showed high variability across the different programs. Better access to these systems for the population would also improve time delays to reperfusion.15
European clinical practice guidelines on the management of STEMI describe quality indicators that should be observed by the infarction networks to reduce the time to reperfusion, among these, a single coordination centralized center, interpreting the ECG before arriving at the hospital to achieve diagnosis and activate the system early, the direct transfer of patients to the cath lab without ER or ICU admissions or the follow-up of infarction care times, among other.1 Our study demonstrated that not all programs meet these recommendations meaning that, in many cases, there is a huge room for improvement. For example, currently, it does not seem reasonable that a significant number of patients who need to be transferred to the pPCI (up to 50% in some cases) wouldn’t end up at the cath lab right away. This simple measure can reduce time to reperfusion in 20 min and have a direct impact on prognosis.16,17
The presence of unjustified delayed reperfusion times was highly variable across the different AC, as well as the causes for these delays, which is indicative of the characteristics of each AC.
Mortality differences
A study conducted by Cequier et al.18 analyzed standardized mortality based on the risk of patients with STEMI across different AC from 2003 through 2012 and detected significant differences. However, across this period, not all regions had implemented Infarction Code programs and the rate of pPCI was highly variable. Our study demonstrated that there are still differences in crude mortality that disappear after adjusting for the clinical variables and care network-related variables (location of first medical contact, delay to reperfusion, and management of critically ill patients). We have already mentioned the importance that the first medical contact should be performed by emergency medical services and the measures used to reduce time to reperfusion. Regarding the management of critically ill patients, a study conducted by Sánchez-Salado et al.19 of 20 000 patients with cardiogenic shock demonstrated that the availability of cardiac surgery intensive care units was associated with a lower mortality rate. Data from this study added to the finding of our registry support the need for expanding the availability of cardiac surgery intensive care units in large volume centers of patients with acute coronary syndrome. In conclusion, the results of mortality study suggest that the organization of the different networks would increase the crude mortality rate seen in some AC.
Limitations
This study has some limitations. In the first place, it is based on self-reported data without external auditing. However, data on interventional cardiology are rather standardized across the world, and the electronic form for data curation was designed to be applied both intuitively and universally. Also, data from Catalonia and Galicia were collected from their official registries, reviewed, and then audited.
Secondly, the profile of patients may have been different across the different AC. To address this limitation and its possible impact on the different crude mortality rates reported, a mortality study was conducted across different AC after adjusting for different clinical variables and care networks. Therefore, some models may be over-adjusted, which is why formal statistical comparisons across AC should be interpreted as cautious as the associations described in any observational trial. The model did not include patients lacking some of the variables included in the model. Table 1 of the supplementary data shows patients discarded from the study for every AC.
Thirdly, patients with STEMI treated outside the infarction networks were not included in this study, although this is probably indicative of a mild selection bias due to its reduced number. Therefore, the greater bias occurs in patients without reperfusion therapy, who, at times, are not covered by these networks. For this reason, these patients were not considered in the mortality analysis. Similarly, patients with myocardial infarction and subacute presentation without emergency reperfusion criteria were not included in the study.
Fourthly, the way of collecting times may have presented some differences between centers and AC. However, since this was a prospective study with previously established definitions, we believe that these differences may have been minimized.
In the fifth place, the data presented date back to 2019. Since then, no big organizational changes have occurred to justify changes in the dynamics of functioning or relevant changes have been made in the European guidelines on the management of STEMI (published back in 2017). Also, in a study conducted during the first wave of the COVID-19 pandemic no differences were seen regarding the type of reperfusion therapy used or time between the first medical contact and reperfusion. However, an increased mortality rate was seen attributed, among other causes, to longer ischemia times.20
Finally, this study only included patients for a period of 3 months. However, we think these data can be generalized to what happens in a much larger period.
CONCLUSIONS
This registry showed significant differences in STEMI care across the different Spanish AC regarding incidence rate, the patients’ clinical characteristics, reperfusion therapy, time delays to reperfusion, and 30-day crude mortality rate. After adjusting for the clinical characteristics and variables associated with the care network, no differences mortality differences were reported across the different AC.
Standardizing the organization and functioning of Infarction Code networks could correct some of the differences seen in the management of STEMI.
FUNDING
None whatsoever.
AUTHORS’ CONTRIBUTIONS
Drafting of the manuscript: O. Rodríguez-Leor, A.B. Cid-Álvarez, A. Pérez de Prado, and X Rosselló. Process of manuscript revision: all the authors. Statistical analysis: O. Rodríguez-Leor, and X. Rosselló. Database review: O. Rodríguez-Leor, A.B. Cid-Álvarez, and A. Pérez de Prado. Data coordination across the different regional network: all the authors.
CONFLICTS OF INTEREST
A. Pérez de Prado received numerous personal fees from iVascular, Boston Scientific, Terumo, Bbraun, and Abbott Vascular. Á. Cequier received personal fees from Ferrer International, Terumo, Astra Zeneca, and Biotronik. R. Moreno, S. Ojeda, R. Romaguera, and A. Pérez de Prado are associate editors of REC: Interventional Cardiology. The journal’s editorial procedure to ensure impartial handling of the manuscript has been followed. The remaining authors did not declare any conflicts of interest associated with the content of this manuscript.
ACKNOWLEDGEMENTS
The authors wish to thank all health professionals involved in STEMI care programs for their not always rewarded work, effort, and dedication. Also, they wish to thank Meia Faixedas, and Josepa Mauri from the Departament de Salut de la Generalitat de Catalunya for granting us access to data from the Catalonian Registre de Codi Infart, and the entire personnel from Servicio Gallego de Salud (SERGAS) involved in the coordination of the REGALIAM registry for facilitating access to its data.
REFERENCES
1. Ibañez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology. Eur Heart J. 2018;39:39:119-177.
2. Cequier A, Pérez de Prado A, Cid-Álvarez AB, et al. Requisitos y sostenibilidad de los programas de ICP primaria en España en el IAMCEST. Documento de consenso de SEC, AEEC y SEMES. REC Interv Cardiol. 2019;1:108-119.
3. Ojeda S, Romaguera R, Cruz-González I, Moreno R. Registro español de hemodinámica y cardiología intervencionista. XXIX Informe Oficial de la Asociación de Cardiología Intervencionista de la Sociedad Española de Cardiología (1990-2019). Rev Esp Cardiol. 2020;73:927-936.
4. Rodríguez-Leor O, Cid-Álvarez AB, Pérez de Prado A, et al. Análisis de la atención al infarto con elevación del segmento ST en España. Resultados del Registro de Código Infarto de la ACI-SEC. Rev Esp Cardiol. 2022;75:669-680.
5. Rodríguez-Leor O, Cid-Álvarez AB, Moreno R, et al. Encuesta sobre las necesidades de los programas de angioplastia primaria en España. REC Interv Cardiol. 2020;1:8-14.
6. Instituto Nacional de Estadística. Datos de población de comunidades autónomas. Available online: https://www.ine.es/jaxiT3/Datos.htm?t=2915#!tabs-tabla. Accessed 24 Jan 2022.
7. Bueno H, Rosselló X, Pocock SJ, et al. In-hospital coronary revascularization rates and post-discharge mortality risk in non-ST-segment elevation acute coronary syndrome. J Am Coll Cardiol. 2019;74:1454-1461.
8. Rosselló X, Huo Y, Pocock S, et al. Global geographical variations in ST-segment elevation myocardial infarction management and post-discharge mortality. Int J Cardiol. 2017;245:27-34.
9. Bueno H, Rosselló X, Pocock S, et al. Regional variations in hospital management and post-discharge mortality in patients with non-ST-segment elevation acute coronary syndrome. Clin Res Cardiol. 2018;107:836-844.
10. Alter DA, Austin PC, Tu JV, et al. Canadian cardiovascular outcomes research. Community factors, hospital characteristics and inter-regional outcome variations following acute myocardial infarction in Canada. Can J Cardiol. 2005;21:247-255.
11. Bertomeu V, Cequier A, Bernal JL, et al. In-hospital mortality due to acute myocardial infarction. Relevance of type of hospital and care provided. RECALCAR study. Rev Esp Cardiol. 2013:66:935-942.
12. Moreno R, Ojeda S, Romaguera R, et al. Actualización de las recomendaciones sobre requisitos y equipamiento en cardiología intervencionista. Documento de consenso de la Asociación de Cardiología Intervencionista y la Asociación de Cardiopatía Isquémica y Cuidados Agudos Cardiovasculares de la Sociedad Española de Cardiología y la Asociación Española de Enfermería en Cardiología. REC Interv Cardiol. 2021;3:33-44.
13. Carrillo X, Fernandez-Nofrerias E, Rodriguez-Leor O, et al. Early ST elevation myocardial infarction in on-capable percutaneous coronary intervention centres: in situ fibrinolysis vs. percutaneous coronary intervention transfer. Eur Heart J. 2016;37:1034-1040.
14. Terkelsen CJ, Sorensen JT, Maeng M, et al. System delay and mortality among patients with STEMI treated with primary percutaneous coronary intervention. JAMA. 2010;304:763-771.
15. Rodríguez-Leor O, Fernández-Nofrerías E, Mauri F, et al. Analysis of reperfusion delay in patients with acute myocardial infarction treated with primary angioplasty based on first medical contact and time of presentation. Rev Esp Cardiol. 2011;64:476-483.
16. Rodríguez-Leor O, Fernández-Nofrerías E, Mauri J, et al. Integration of a local into regional primary angioplasty action plan (the Catalan Codi Infart network) reduces time to reperfusion. Int J Cardiol. 2013;168:4354-4357.
17. Bagai A, Jollis JG, Dauerman HL, et al. Emergency department bypass for ST-segment-elevation myocardial infarction patients identified with a prehospital electrocardiogram: a report from the American Heart Association Mission: Lifeline program. Circulation. 2013;128:352-359.
18. Cequier A, Ariza-Sole A, Elola FJ, et al. Impacto en la mortalidad de diferentes sistemas de asistencia en red para el tratamiento del infarto agudo de miocardio con elevación del segmento ST. La experiencia de España. Rev Esp Cardiol. 2017;70:155-161.
19. Sánchez-Salado JC, Burgos V, Ariza-Solé A, et al. Trends in cardiogenic shock management and prognostic impact of type of treating center. Rev Esp Cardiol. 2020;73:546-553.
20. Rodríguez-Leor O, Cid-Álvarez AB, Pérez de Prado A, et al. Impact of COVID-19 on ST-segment elevation myocardial infarction care. The Spanish experience. Rev Esp Cardiol. 2020;73:994-1002.
ABSTRACT
Introduction and objectives: The role of inflammation in the pathogenesis of coronary artery disease, and that resulting from percutaneous coronary intervention (PCI) is increasingly recognized, yet the effect of colchicine in attenuating peri-PCI inflammation remains unknown. This meta-analysis investigated the efficacy of colchicine in patients undergoing PCI for secondary prevention of coronary artery disease.
Methods: The Web of Science, PubMed, Ovid MEDLINE, Embase, Cochrane Central Register of Controlled Trials and ClinicalTrials.gov databases were searched. Data on studies assessing the efficacy profile of colchicine in patients undergoing PCI were pooled using a random-effects model.
Results: In 13 studies of 7414 patients, no differences were observed between patients treated with colchicine compared to those without for all-cause mortality (OR, 1.1; 95%CI, 0.72-1.56; I2 = 0%), cardiovascular mortality (OR, 0.98; 95%CI, 0.42-2.28; I2 = 14.2%), myocardial infarction (OR, 0.84; 95%CI, 0.65-1.08; I2 = 1.4%) or coronary revascularization (OR, 0.64; 95%CI, 0.28-1.42; I2 = 49.3%). However, patients treated with colchicine had a lower risk of stroke (OR, 0.33; 95%CI, 0.15-0.72; I2 = 0%).
Conclusions: Adding colchicine to standard medical therapy in patients undergoing PCI did not decrease all-cause mortality, cardiovascular mortality or urgent revascularization. However, it showed a trend towards a lower risk of myocardial infarction and a significantly lower risk of stroke.
Keywords: Coronary artery disease. Percutaneous coronary intervention. Inflammation. Colchicine.
RESUMEN
Introducción y objetivos: La importancia de la inflamación en la patogénesis de la enfermedad coronaria, así como tras la angioplastia percutánea, es un fenómeno reconocido. Sin embargo, el efecto de la colchicina para atenuar la inflamación tras la intervención coronaria percutánea se desconoce. Este metanálisis investigó la eficacia de la colchicina en pacientes que se sometieron a intervención coronaria percutánea con el objetivo de prevención secundaria
Métodos: Se revisaron las bases de datos Web of Science, PubMed, OVID MEDLINE, Embase, Cochrane Central Register of Controlled Trials y ClinicalTrials.gov, y se analizaron los datos de los estudios que investigaban la eficacia de la colchicina en pacientes que se sometieron a angioplastia coronaria percutánea, usando un modelo de efectos aleatorios.
Resultados: En 13 estudios, que incluyeron un total de 7.414 pacientes, no se observó ninguna diferencia entre los tratados con colchicina y los no tratados con colchicina en cuanto a mortalidad por cualquier causa (OR = 1,1; IC95%, 0,72-1,56; I2 = 0%), mortalidad por causa cardiovascular (OR = 0,98; IC95%, 0,42-2,28; I2 = 14,2%), infarto de miocardio (OR = 0,84; IC95%, 0,65-1,08; I2 = 1,4%) y revascularización coronaria (OR = 0,64; IC95%, 0,28-1,42; I2 = 49,3%). Sin embargo, los pacientes tratados con colchicina mostraron un menor riesgo de accidente vascular cerebral (OR = 0,33; IC95%, 0,15-0,72; I2 = 0%).
Conclusiones: Agregar colchicina a la terapia medica estándar en pacientes sometidos a angioplastia coronaria percutánea no modificó la mortalidad por cualquier causa, la mortalidad por causa cardiovascular ni la revascularización coronaria, pero si mostró una tendencia a un menor riesgo de infarto de miocardio y un menor riesgo significativo de accidente vascular cerebral.
Palabras clave: Enfermedad coronaria. Angioplastia percutánea. Inflamación. Colchicina.
Abbreviations
ACS: acute coronary syndrome. MI: myocardial infarction. NSTEMI: non-ST-elevation acute myocardial infarction. PCI: percutaneous coronary intervention. RCT: randomized controlled trial.
INTRODUCTION
Despite increasingly effective primary and secondary preventive treatments, coronary artery-related events continue to be the leading cause of morbidity and mortality worldwide.1,2 Lifestyle changes (eg, weight loss, low-salt diet, smoking cessation), medical therapy (eg, anti-hypertensive, lipid-lowering, glucose-lowering, and antithrombotic regimens) in addition to coronary revascularization via percutaneous coronary intervention (PCI) and coronary artery bypass graft (CABG) constitute the multifaceted approach of this disease. Yet despite the advances made in this multimodality approach, cardiovascular morbidity and mortality remain high.
More recently, the central role played by inflammation in the pathogenesis of coronary artery disease from atherosclerotic plaque formation to acute coronary syndrome (ACS), and PCI itself have gained important recognition. Colchicine, an anti-inflammatory agent indicated for multiple inflammatory conditions including pericarditis, gout, and familial Mediterranean fever, has gained attention as a potential attenuator of atherosclerotic inflammation. Acting via the inhibition of tubulin polymerization and eventually blunting immune cell activation and inflammatory response,3,4 recent evidence suggests a benefit of colchicine in the management of the cardiovascular events of patients with clinical signs of coronary artery disease.5 However, its impact among patients in the peri-PCI period remain controversial.
Recent trials have begun exploring the effects of colchicine in the PCI setting, albeit with mixed results. In the Colchicine-PCI trial of patients with non-ST-segment elevation acute coronary syndrome, the administration of colchicine immediately before and after PCI resulted in lower interleukin-6 and high-sensitivity C-reactive protein (hsCRP) levels at 24 hours, but did not show fewer PCI-related myocardial injuries.6 This trial was followed by COPE-PCI that found that when administered 6-to-24 hours before the PCI, colchicine did in fact reduce PCI-related myocardial injuries in a population of patients with stable angina and non-ST-elevation acute myocardial infarction (NSTEMI).7 Nevertheless, the more recent COVERT-MI trial8 found no difference in infarct size or left ventricular remodeling on the cardiac magnetic resonance imaging in patients treated with colchicine compared to those untreated with this agent.
These individual studies may not provide properly powered analyses, particularly in low-rate events such as strokes, on the impact of colchicine regarding secondary prevention in patients in the peri-PCI period, thus prompting the need for a systematic appraisal and meta-analysis of the quality of evidence and treatment effects on major adverse cardiovascular events.
METHODS
Protocol
The search process of this meta-analysis was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and is registered with PROSPERO (CRD42021247704). The meta-analysis did not require specific institutional review board approval since it utilized results published in former studies. All relevant information can be found in the trials included. The corresponding author had full access to all the data and final responsibility on the decision to submit the manuscript for publication. The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Search strategy
We performed a comprehensive literature search of all published studies—retrospective, observational, and randomized controlled trials—available on Web of Science, Embase, PubMed, Ovid MEDLINE, Cochrane Central Register of Controlled Trials (CENTRAL), and ClinicalTrials.gov (inception through August 23, 2021, without language restrictions. Case reports, letters to the editor, reviews, and book chapters were not included in this meta-analysis. The keywords used in the search were ‘colchicine,’ ‘coronary artery disease,’ ‘coronary heart disease,’ ‘angina,’ ‘myocardial infarction,’ ‘acute myocardial infarction,’ ‘myocardial ischemia,’ ‘acute coronary syndrome,’ ‘ischemic heart disease,’ ‘percutaneous coronary intervention,’ ‘percutaneous transluminal angioplasty,’ ‘percutaneous coronary revascularization,’ and ‘myocardial revascularization’ including their subheadings, MeSH terms, and all synonyms. References for each of the studies se lected were also screened (the detailed search strategy can be found on the supplementary data). The search process was reported according to the Preferred Reported Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Selection criteria
Studies were eligible if they included any the following criteria: a) compared the efficacy of colchicine treatment, at any dose and for any duration, to standard medical treatment with or without placebo; b) included populations of patients treated with PCI regardless of the indication; and c) reported, at least, 1 of the following cardiovascular outcomes: all-cause mortality, cardiovascular mortality, myocardial infarction (MI), stroke or urgent coronary revascularization. Study selection was conducted by 2 independent reviewers (C.E. Soria Jiménez, and J. Chang) first by screening titles and abstracts and then by reviewing full texts and their corresponding references. In case of disagreement over eligibility, a third reviewer (H.M. García-García) assessed discrepancy, and decisions were reached by consensus.
Data collection and study endpoints
Data on study characteristics, patient characteristics, and endpoint event rates were independently drawn and organized into a structured dataset by 2 reviewers (C.E. Soria Jiménez, and F. Hayat), and then compared. All discrepancies resulted in the re-evaluation of primary data and involvement of a third reviewer (H.M. García García). Disagreements were resolved by consensus.
Endpoints
The prespecified primary endpoint was all-cause mortality. Secondary clinical endpoints were cardiovascular mortality, MI, stroke, and any revascularization. Each endpoint was assessed according to the definitions reported in the original study protocols (summarized on table 1 of the supplementary data).
Risk of bias
The risk of bias in each study was assessed using the revised Cochrane Risk of Bias tool (RoB 2.0) for randomized controlled trials (RCTs), and the Risk of Bias in Non-randomized Studies of Interventions assessment Tool from the Cochrane handbook (ROBINS-I) for observational studies. Two investigators (C.E. Soria Jiménez, and J. Sanz Sánchez) independently assessed 5 domains of bias in RCTs: (1) randomization process, (2) deviations from intended procedures, (3) missing outcome data, (4) outcome measurement, and (5) selection of results reported. The same investigators independently assessed 7 domains of bias in observational studies: (1) confounding, (2) selection of participants, (3) classification of procedures, (4) deviations from intended interventions, (5) missing outcome data, (6) outcome measurement, and (7) selection of results reported (table 2 and 3 of the supplementary data).
Statistical analysis
Odds ratios (OR) and 95% confidence intervals (95%CI) were estimated using the DerSimonian and Laird random-effects model with the estimate of heterogeneity taken from the Mantel-Haenszel method. The presence of heterogeneity among the studies was evaluated using the Cochran Q test referred to chi-square distribution (P ≤ .10 was considered statistically significant) plus the I2 test to assess inconsistencies. Values of 0% indicated no observed heterogeneity, and values ≤ 25%, ≤ 50%, and > 50% indicated low, moderate, and high heterogeneity, respectively. The presence of publication bias was investigated using Harbord test and visual estimation with funnel plots. We conducted a leave-one-out sensitivity analysis for all outcomes by iteratively removing 1 study at a time to confirm that our findings were not driven by any single study. To account for the different follow-up durations across the studies, another sensitivity analysis was conducted using a Poisson regression model with random intervention effects to calculate the means of inverse-variance weighting of trial-specific log stratified incidence rate ratios. Results were shown as incidence rate ratios, which are exponential coefficients of the regression model.
A meta-regression analysis was conducted using the empirical Bayesian method to estimate the between-study variance tau-squared to assess the effect of colchicine dosage, follow-up duration, percentage of patients with ACS, and percentage of those with diabetes mellitus on treatment effects on the primary endpoint.
Two-tailed P values < .05 were considered statistically significant. Statistical analyses were conducted using the Stata software version 13.1 (StataCorp LP, College Station, United States).
RESULTS
Search results
Figure 1 shows the PRISMA study search and selection process. Out of a total of 1239 unique reports, 12 RCTs5-16 and 1 observational study17 were identified and included in this analysis. The corresponding author of the COOL trial15 was contacted regarding data from a number of patients treated with PCI; 58 out of a total of 80 patients evaluated (72.5%) underwent PCI. The study ultimately met the inclusion criteria and was included in our analysis. The main features of the studies included are shown on table 1. Data on the outcomes, mortality, MI, stroke, and urgent revascularization were reported in 12, 9, 5, and 6 trials, respectively. A total of 3741 and 3673 patients treated with and without colchicine were included (for a total of 7414 patients). Time elapsed from the PCI to the start of colchicine went from immediately before PCI to 13.5 days later as shown on table 1.
Figure 1. Preferred Reported Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart of database search results and study selection.
Table 1. Characteristics of trials selected
| Trial/Author | Year | Study design | Multicenter | Patients (n) | Population | Colchicine dose and duration | Time elapsed from PCI to start of colchicine | Follow-up |
|---|---|---|---|---|---|---|---|---|
| COVERT-MI8 | 2021 | RCT | Yes | 192 | Adults with a first-time STEMI referred for primary or bailout PCI | 2 mg oral loading dose followed by daily oral 0.5 mg twice daily for 5 days | Loading dose immediately before PCI; if not possible, immediately after PCI | 3 months |
| COPE-PCI7 | 2021 | RCT | No | 75 | Adults with stable angina or NSTEMI undergoing angiography and PCI | 1 mg followed by 0.5 mg 1 h later, 6 hrs to 24 hrs pre-PCI | 6 hrs to 24 hrs before coronary angiogram | 1 day |
| Colchicine-PCI6 | 2020 | RCT | No | 400 | Adults with suspected ischemic heart disease or ACS referred for angiography with possible PCI | 1.2 mg 1 h to 2 h pre-angiography, 0.6 mg 1 h later or immediately after the procedure if rushed for emergency angiography | 1 h to 2 h before coronary angiography | 1 month |
| COPS9 | 2020 | RCT | Yes | 795 | Adults presenting with ACS and evidence of CAD treated with angiography and managed with PCI or medical therapy | 0.5 mg twice daily for 1 month, then 0.5 mg daily for 11 months | Immediately after PCI and randomization | 13.2 months |
| LoDoCo-MI10 | 2019 | RCT | No | 237 | Adults who sustained a type 1 MI within the past 7 days | 0.5 mg daily for 30 days | 1.5 days following the index MI | 1 month |
| Talasaz11 | 2019 | RCT | No | 196 | Adults presenting with STEMI undergoing PCI | NA | NA | 1 month |
| COLCOT I5 | 2019 | RCT | Yes | 4745 | Adults with MI within the past 30 days who had completed some percutaneous revascularization | 0.5 mg once daily for, at least, 2 years | 13.5 days | 42 months |
| Vaidya17 | 2018 | Observational | No | 80 | Adults who presented with ACS < 1 month prior and underwent invasive coronary angiography and revascularization if indicated | 0.5 mg once daily for 1 year | NA (< 1 month from ACS per inclusion criteria) | 12.6 months |
| COLIN12 | 2017 | RCT (Open-label) | No | 44 | Adults admitted for STEMI with occlusion of 1 of the main coronary arteries treated with PCI | 1 mg once daily for 1 month | On the first day of the AMI | 1 month |
| Deftereos 201513 | 2015 | RCT (Pilot) | Yes | 151 | Adults presenting with STEMI of ≤ 12-hour evolution from pain onset treated with PCI | 2 mg loading dose, 0.5 mg twice daily for 5 days | Immediately after completion of diagnostic coronary angiography | 5 days |
| Deftereos 201314 | 2013 | RCT | No | 222 | Adults with diabetes, aged 40-80 treated with PCI with bare metal stent | 0.5 mg twice daily for 6 months | Within 24 hrs of index PCI | 6 months |
| COOL15 | 2012 | RCT | No | 80 | Adults with ACS or acute ischemic stroke | 1 mg once daily for 30 days | Immediately after randomization | 1 month |
| O’Keefe16 | 1992 | RCT | No | 197 | Adults who underwent elective angioplasty (single or multivessel, new or restenosed lesions) for silent, stable or unstable angina; CABG | 0.6 mg twice daily for 6 months | Somewhere between 12 hrs before and 24 hrs after balloon angioplasty | 6 months |
|
ACS, acute coronary intervention; CABG, coronary artery bypass graft; CAD, coronary artery disease; MI, myocardial infarction; NA, not available; NSTEMI, non-ST-elevation acute myocardial infarction; PCI, percutaneous coronary intervention; RCT, randomized controlled trial; STEMI, ST-segment elevation myocardial infarction. |
||||||||
Baseline characteristics
Main baseline characteristics of the patients included are shown on table 2. Most patients were men with a mean age of 60 years, had ACS, and underwent revascularization with drug-eluting stents.
Table 2. Baseline characteristics of patients from each trial
| Trial/Author | Mean Age | Men (%) | ACS (%) | DES (%) | HTN (%) | DM2 (%) | HLD (%) | Previous MI (%) | Previous PCI (%) | Previous CABG (%) | Underwent PCI (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| COVERT-MI8 | 60 | 80.3 | 100 | 95.7 | 30.8 | 13.1 | 33.1 | 0 | 0 | 0 | 93 |
| COPE-PCI7 | 64.7 | 71.5 | 58.7 | 97.0 | 54.5 | 22.9 | 63.5 | 17.5 | 16.0 | NA | 100 |
| Colchicine-PCI6 | 66.3 | 93.5 | 49.5 | NA | 91.7 | 57.8 | 88.8 | 25.8 | 37.6 | NA | 100 |
| COPS9 | 59.9 | 79.5 | 100.0 | NA | 50.5 | 19.0 | 46.0 | 15.0 | 13.0 | 4.5 | 88 |
| LoDoCo-MI10 | 61.0 | 77.0 | 100.0 | NA | 47.5 | 22.0 | NA | 15.0 | 11.5 | NA | 90 |
| Talasaz11 | NA | NA | 100.0 | NA | NA | NA | NA | NA | NA | NA | 100 |
| COLCOT I5 | 60.6 | 80.9 | 100.0 | NA | 51.1 | 20.2 | NA | 16.2 | 16.9 | 3.2 | 93 |
| Vaidya17 | 57.4 | 77.5 | 100.0 | NA | 53.8 | 31.3 | 85.0 | 51.3 | 63.8 | NA | 77.5 |
| COLIN12 | 59.9 | 79.4 | 100.0 | NA | 43.4 | 13.7 | 36.5 | NA | 4.6 | 2.4 | 100 |
| Deftereos 201513 | 58.0 | 69.0 | 100.0 | NA | 39.5 | 21.5 | 52.0 | 0.0 | NA | NA | 100 |
| Deftereos 201314 | 63.6 | 65.5 | 31.0 | 0 | 48.5 | 100.0 | NA | NA | NA | NA | 100 |
| COOL15 | 57.2 | 88.8 | 91.3 | NA | 42.5 | 16.3 | 47.5 | 17.5 | 0 | NA | 73 |
| O’Keefe16 | 60.5 | 86.0 | 39.5 | 0 | NA | 12.0 | NA | NA | NA | 25.5 | 100 |
|
ACS, acute coronary syndrome; CABG, coronary artery bypass graft; DES, drug-eluting stent; DM2, diabetes mellitus type 2; HLD, hyperlipidemia; HTN, hypertension; MI, myocardial infarction; NA, not available; PCI, percutaneous intervention. |
|||||||||||
Publication bias and asymmetry
Funnel-plot distributions of pre-specified outcomes indicate absence of publication bias for all the outcomes (figures 1 to 5 of the supplementary data).
Risk of bias assessment
Table 2 and table 3 of the supplementary data summarize the results of the risk of bias assessment. A total of 11 trials were ranked as trials with a low overall risk of bias, 1 presented some concerns while another one was ranked as a trial with a high overall risk of bias.
Outcomes
No differences were seen between patients treated with colchicine and those treated without it or placebo regarding all-cause mortality (OR, 1.06; 95%CI, 0.72-1.55; I2 = 0%), cardiovascular mortality (OR, 0.98; 95%CI, 0.42-2.28; I2 = 14.2%) or coronary revascularization (OR, 0.64; 95%CI, 0.29-1.42; I2 = 49.3%). However, patients treated with colchicine had a lower risk of stroke (OR, 0.38; 95%CI, 0.18-0.81; I2 = 0%), and a trend towards a lower risk of MI (OR, 0.84; 95%CI, 0.66-1.07; I2 = 0%) (figure 2).
Figure 2. Forrest plot analyses for the main outcomes of death (A), myocardial infarction (B), stroke (C), and revascularization (D). 95%CI, confidence interval; OR, odds ratio.
Sensitivity analyses
In the leave-one-out sensitivity analysis, results were consistent with the primary analysis (tables 4 to 8 of the supplementary data). Similarly, in a sensitivity analysis on the use of estimated incidence rate ratios to account for different lengths of follow-up, findings remained unchanged (table 9 of the supplementary data).
When the risk ratios with random-effects models were estimated, findings remained consistent with the main analysis for all endpoints (table 10 of the supplementary data). Random effect meta-regression analyses found no significant impact of colchicine dosage (P = .33), follow-up duration (P = .88), percentage of patients with ACS (P = .37) or percentage of patients with diabetes mellitus (P = .96) on treatment effect regarding the primary endpoint (table 11 of the supplementary data).
DISCUSSION
This meta-analysis included 7414 patients across 12 RCTs and 1 observational study. It showed some clinical benefits on cardiovascular events with the addition of colchicine to standard medical therapy in patients undergoing PCI. Specifically, we found that the addition of colchicine compared to no colchicine or placebo reduced the risk of stroke showing a trend towards a lower risk of MI both with no observed heterogeneity. Additionally, we observed no differences in all-cause mortality, cardiovascular mortality or coronary revascularization. Significantly, colchicine dosage, follow-up duration, percentage of patients with ACS or diabetes mellitus showed no impact on treatment effect (see PRISMA checklist on table 12 of the supplementary data).
Our outcomes regarding all-cause and cardiovascular mortality are consistent with a prior meta-analysis of 5 RCTs conducted by Fu et al.,18 that also found no significant reduction of mortality, MI, serious adverse events, and restenosis. One explanation for the lack of mortality benefit of both trials may be that although mortality rate was generally low and differences were largely not statistically significant in many of these trials, follow-up duration was generally short (< 30 days) in most studies, and it is possible that higher event rates may be seen with longer follow-up data. We should mention that the meta-analysis conducted by Fu et al.18 included 1 RCT of patients treated with CABG, not PCI. It is possible that the inflammatory profiles of this cohort of patients differ from those treated with PCI (eg, multivessel coronary artery disease, longer postoperative recovery, and higher risk of postoperative complications). As a matter of fact, this mixed population may have led to the lack of reduction seen in the overall rate of MI, serious adverse events, and restenosis. Similarly, a prior meta-analysis conducted by Fiolet et al.19 demonstrated that the addition of colchicine to standard medical therapy in patients with acute and chronic coronary syndromes reduced the risk of the primary endpoint significantly (a composite of MI, stroke, and cardiovascular mortality), and the individual endpoint of MI, stroke, and coronary revascularization with no differences whatsoever on all-cause or cardiovascular mortality. Our results demonstrating a lower risk of stroke and a trend towards a lower risk of MI are more consistent with this meta-analysis. A key difference among the different meta-analyses is the population of patients. Fiolet et al.19 included the LoDoCo20 and LoDoCo221 trials whose inclusion criteria were patients with chronic coronary disease and clinical stability for over 6 months. This amounted to > 50% of patients analyzed who were not in the peri-PCI period and likely had a different inflammatory profile at the time of colchicine administration. These 2 trials also had longer follow-ups (36 and 29 months, respectively) potentially allowing for more time to capture outcome differences like MI and urgent revascularization between the different treatment groups. In contrast, our meta-analysis only focused on patients in the peri-PCI as conducted by Fu et al.18 and expanded the total number of studies analyzed to 12 RCTs and 1 observational study. As far as we know, our study is the largest meta-analysis ever conducted to this date to assess the effects of colchicine on the clinical outcomes of patients in the peri-PCI period.
Alkouli et al.22 reported that the adjusted rate of ischemic stroke increased for patients treated with PCI due to ST-segment elevation myocardial infarction (STEMI) (0.6% to 0.96%), NSTEMI (0.5% to 0.6%), and unstable angina or stable ischemic heart disease (UA/SIHD, 0.3% to 0.72%). In turn, in-hospital mortality was higher (23.5% vs 11.0%, 9.5% vs 2.8%, and 11.5% vs 2.4% for STEMI, NSTEMI, and UA/SIHD cohorts, respectively), and post-PCI stroke was associated with a > 2-fold increase in LoS, a > 3-fold increase in non-home discharges, and a > 60% increase in cost. Given the increasing complexity of patients treated as well as the PCI techniques utilized over the past decade, effective preventive strategies and treatments are needed, and herein lies the opportunity for other anti-inflammatory drugs such as colchicine to further mitigate the morbidity and mortality of patients with post-PCI stroke. In the acute phase of MI, activated inflammasomes mount an intense inflammatory response.23 There is also endothelial damage after PCI, which may result in atherosclerotic plaque destabilization with subsequent thromboembolism causing cerebrovascular events.24 Colchicine may play a role preventing stroke by helping stabilize atherosclerotic plaques in patients undergoing PCI, though this effect may not be robust enough to overcome the direct endothelial injury present at the time of PCI.
Colchicine is a widely available drug with known anti-inflammatory properties. Its mechanism of action is yet to be fully elucidated but has been shown to work partly via the inhibition of NLRP3 (nucleotide-binding oligomerization domain-, leucine-rich repeat- and pyrin domain-containing protein 3) inflammasome, which ultimately downregulates interleukin-1B and interleukin-6, 2 known inflammatory mediators.23-27 It also causes microtubule disruption and decreased neutrophil activation and extravasation. Since elevated levels of inflammatory biomarkers are an independent predictor of major adverse cardiovascular events28-31 our results show that colchicine joining the current medical therapy is a potential addition to further attenuate inflammation regarding the secondary prevention of cardiovascular disease in patients undergoing PCI.
Some limitations of our study include the use of aggregate study-level data as opposed to patient-level data. While this limits subgroup analyses, the overall conclusions would remain the same. There was also a small percentage of patients in each of the studies analyzed who did not undergo PCI, which poses some limitations on the overall effects on a PCI population. However, in all studies, the vast majority of patients eventually underwent this procedure. Similarly, the LoDoCo221 trial enrolled patients who underwent PCI but was ultimately excluded from this analysis as patients required a period of clinical stability 6 months after PCI before starting colchicine therapy. A 6-month gap from PCI to colchicine initiation did not fit in with our period of interest (the peri-PCI period). The study conducted by O’keefe16 was completed in an era of balloon angioplasty, and colchicine treatment in this setting may not be comparable to patients who underwent PCI in the era of statins, modern stents, and antiplatelet agents. Additionally, most patients from our study underwent PCI due to the presentation of ACS, yet there were other clinical presentations including stable ischemic heart disease and unstable angina, and yet others that specifically excluded patients with acute MI. Given the different clinical status at presentation for PCI, it’s likely that the inflammatory profile of these different populations of patients also varied resulting in different clinical outcomes. Nevertheless, despite variation in the inclusion and exclusion criteria, outcome definitions, and colchicine dose and duration, this did not introduce heterogeneity into our results.
CONCLUSIONS
In patients undergoing PCI, the addition of colchicine to optimal medical therapy resulted in a significant reduction of strokes, and a trend towards a lower risk of MI. However, this did not result in lower all-cause and cardiovascular mortality rates, and urgent revascularization.
FUNDING
This research did not receive any specific grants from public, private or non-profit sectors.
AUTHORS’ CONTRIBUTIONS
M.B. Levine was involved in data curation and research. F. Hayat, and J. Chang were involved in data curation and research, as well as in drafting, editing, and reviewing the early draft of the manuscript. C.E. Soria Jiménez, J. Sanz Sánchez, and H. García-García were involved in project conceptualization, data curation, formal data analysis and investigation, methodology, project administration, resources, validation, visualization, as well as drafting, editing, and reviewing all manuscript drafts and its final version.
CONFLICTS OF INTEREST
H.M. García-García declared institutional grant support from Biotronik, Boston Scientific, Medtronic, Abbott, Neovasc, Shockwave, Phillips, and Corflow. The remaining authors declared no conflicts of interest whatsoever.
WHAT IS KNOWN ABOUT THE TOPIC?
- Inflammation plays a central role in the pathogenesis of coronary artery disease, and it’s involved in percutaneous coronary interventions. Colchicine is a powerful anti-inflammatory drug. Its effect, however, attenuating peri-PCI inflammation remains unknown.
WHAT DOES THIS STUDY ADD?
- In this meta-analysis of 12 RCTs and 1 observational study, the addition of colchicine to patients undergoing PCI resulted in a lower risk of stroke. Other major adverse cardiovascular events did not show any significant differences.
REFERENCES
1. Fox KA, Poole-Wilson P, Clayton TC, et al. 5-year outcome of an interventional strategy in non-ST-elevation acute coronary syndrome: the British Heart Foundation RITA 3 randomised trial. Lancet. 2005;366:914-920.
2. Boden WE, O’Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503-1516.
3. Deftereos S, Giannopoulos G, Papoutsidakis N, et al. Colchicine and the heart: pushing the envelope. J Am Coll Cardiol. 2013;62:1817-1825.
4. Nidorf SM, Eikelboom JW, Thompson PL. Colchicine for secondary prevention of cardiovascular disease. Curr Atheroscler Rep. 2014;16:391.
5. Tardif JC, Kouz S, Waters DD, et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N Engl J Med. 2019;381:2497-2505.
6. Shah B, Pillinger M, Zhong H, et al. Effects of Acute Colchicine Administration Prior to Percutaneous Coronary Intervention: COLCHICINE-PCI Randomized Trial. Circ Cardiovasc Interv. 2020;13:e008717.
7. Cole J, Htun N, Lew R, Freilich M, Quinn S, Layland J. Colchicine to Prevent Periprocedural Myocardial Injury in Percutaneous Coronary Intervention: The COPE-PCI Pilot Trial. Circ Cardiovasc Interv. 2021;14:e009992.
8. Mewton N, Roubille F, Bresson D, et al. Effect of Colchicine on Myocardial Injury in Acute Myocardial Infarction. Circulation. 2021;144:859-869.
9. Tong DC, Quinn S, Nasis A, et al. Colchicine in Patients With Acute Coronary Syndrome: The Australian COPS Randomized Clinical Trial. Circulation. 2020;142:1890-1900.
10. Hennessy T, Soh L, Bowman M, et al. The Low Dose Colchicine after Myocardial Infarction (LoDoCo-MI) study: A pilot randomized placebo controlled trial of colchicine following acute myocardial infarction. Am Heart J. 2019;215:62-69.
11. Talasaz AH, Jenab Y, Hosseini SH. P461. Colchicine before percutaneous coronary intervention in acute myocardial infarction. Eur Heart J. 2019(40):Suppl 1;ehz745.0994.
12. Akodad M, Lattuca B, Nagot N, et al. COLIN trial: Value of colchicine in the treatment of patients with acute myocardial infarction and inflammatory response. Arch Cardiovasc Dis. 2017;110:395-402.
13. Deftereos S, Giannopoulos G, Angelidis C, et al. Anti-Inflammatory Treatment With Colchicine in Acute Myocardial Infarction: A Pilot Study. Circulation. 2015;132:1395-1403.
14. Deftereos S, Giannopoulos G, Raisakis K, et al. Colchicine treatment for the prevention of bare-metal stent restenosis in diabetic patients. J Am Coll Cardiol. 2013;61:1679-1685.
15. Raju NC, Yi Q, Nidorf M, Fagel ND, Hiralal R, Eikelboom JW. Effect of colchicine compared with placebo on high sensitivity C-reactive protein in patients with acute coronary syndrome or acute stroke: a pilot randomized controlled trial. J Thromb Thrombolysis. 2012;33:88-94.
16. O’Keefe JH Jr, McCallister BD, Bateman TM, Kuhnlein DL, Ligon RW, Hartzler GO. Ineffectiveness of colchicine for the prevention of restenosis after coronary angioplasty. J Am Coll Cardiol. 1992;19:1597-1600.
17. Vaidya K, Arnott C, Martínez GJ, et al. Colchicine Therapy and Plaque Stabilization in Patients With Acute Coronary Syndrome: A CT Coronary Angiography Study. JACC Cardiovasc Imaging. 2018;11:305-316.
18. Fu C, Wang B. Colchicine administration for percutaneous coronary intervention: A meta-analysis of randomized controlled trials. Am J Emerg Med. 2021;46:121-125.
19. Fiolet A, Opstal T, Mosterd A, et al. Efficacy and Safety of Low-Dose Colchicine in Patients with Coronary Disease: A Systematic Review and Meta-Analysis of Randomized Trials. Eur Heart J. 2021;00:1-11.
20. Nidorf SM, Eikelboom JW, Budgeon CA, Thompson PL. Low-dose colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol. 2013;61:404-410.
21. Nidorf SM, Fiolet ATL, Mosterd A, et al. Colchicine in Patients with Chronic Coronary Disease. N Engl J Med. 2020;383(19):1838-1847.
22. Alkhouli M, Alqahtani F, Tarabishy A, Sandhu G, Rihal CS. Incidence, Predictors, and Outcomes of Acute Ischemic Stroke Following Percutaneous Coronary Intervention. JACC Cardiovasc Interv. 2019;12:1497-1506.
23. Chen B, Frangogiannis NG. Immune cells in repair of the infarcted myocardium. Microcirculation. 2017;e12305.
24. de Winter RJ, Heyde GS, Koch KT, et al. The prognostic value of pre-procedural plasma C-reactive protein in patients undergoing elective coronary angioplasty. Eur Heart J. 2002;23:960-966.
25. Rajamäki K, Lappalainen J, Oörni K, et al. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS One. 2010;5:e11765.
26. Paschke S, Weidner AF, Paust T, Marti O, Beil M, Ben-Chetrit E. Technical advance: Inhibition of neutrophil chemotaxis by colchicine is modulated through viscoelastic properties of subcellular compartments. J Leukoc Biol. 2013;94:1091-1096.
27. Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237-241.
28. Buffon A, Liuzzo G, Biasucci LM, et al. Preprocedural serum levels of C-reactive protein predict early complications and late restenosis after coronary angioplasty. J Am Coll Cardiol. 1999;34:1512-1521.
29. Kwaijtaal M, van Diest R, Bär FW, et al. Inflammatory markers predict late cardiac events in patients who are exhausted after percutaneous coronary intervention. Atherosclerosis. 2005;182:341-348.
30. Patti G, Di Sciascio G, D’Ambrosio A, Dicuonzo G, Abbate A, Dobrina A. Prognostic value of interleukin-1 receptor antagonist in patients undergoing percutaneous coronary intervention. Am J Cardiol. 2002;89:372-376.
31. Walter DH, Fichtlscherer S, Sellwig M, Auch-Schwelk W, Schächinger V, Zeiher AM. Preprocedural C-reactive protein levels and cardiovascular events after coronary stent implantation. J Am Coll Cardiol. 2001;37:839-846.
- High rate of uncovered struts in latest generation drug-eluting stents with durable, biodegradable polymer or lack of it 1 month after implantation
- Impact of time of intervention in patients with NSTEMI. The IMPACT-TIMING-GO trial design
- Long-term effectiveness of drug-coated balloon in the side branch treatment of bifurcation lesions
- EPIC03-BIOSS observational prospective study. Performance analysis of the BIOSS LIM C dedicated stent in coronary bifurcation lesion angioplasty
Editorials
Expanding the role of drug-coated balloons in native large coronary artery disease
aDepartment of Biomedical Sciences, Humanitas University, Pieve Emanuele, Milan, Italy
bCardio Center, IRCCS Humanitas Research Hospital, Rozzano, Milan, Italy
cDivision of Cardiology, Montefiore Medical Center, Bronx, New York, United States
Original articles
Editorials
The role of angiography-derived physiological assessment techniques in the post-FAVOR III Europe era?
aServicio de Cardiología, Hospital General Universitario Gregorio Marañón, Instituto de Investigación Sanitaria Gregorio Marañón, Universidad Complutense de Madrid, Madrid, Spain
bCentro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), Instituto de Salud Carlos III, Madrid, Spain
Interviews
An interview with Camino Bañuelos
aServicio de Cardiología, Hospital Clínico San Carlos, Instituto de Investigación Sanitaria del Hospital Clínico San Carlos (IdISSC), Madrid, Spain
bServicio de Cardiología, Hospital Clínico Universitario de Santiago de Compostela, Santiago de Compostela, A Coruña, Spain