ABSTRACT
Introduction and objectives: The results of Magmaris implantation in the acute coronary syndrome setting is uncertain and more studies will be needed to assess the long-term safety profile of these devices. The objective of this work was to conduct an observational study to analyze the clinical safety profile of Magmaris implanted in a single hospital center in the acute coronary syndrome setting beyond 12 months.
Methods: Registry of 36 patients with Magmaris devices implanted between November 2016 through November 2018 with a diagnosis of acute coronary syndrome included consecutively. The primary endpoint was considered the device-oriented composite endpoint of target vessel myocardial infarction, target lesion failure, and cardiac death. Secondary endpoints included Magmaris related thrombosis.
Results: Regarding the device-oriented combination, no target vessel myocardial infarction was observed, 0 cases (0%), while target lesion failure was seen in 2 cases (5.6%). There were no cases of Magmaris thrombosis at the follow-up and only 1 case of cardiac death (2.8%) was found 36 months after Magmaris implantation. The cause of death could not be determined since no autopsy was performed.
Conclusions: Our results with long-term follow-up confirm that Magmaris has a favorable clinical profile in the acute coronary syndrome complex setting.
Keywords: Magmaris. Acute coronary syndrome. Bioresorbable scaffold thrombosis.
RESUMEN
Introducción y objetivos: Los resultados del Magmaris en el síndrome coronario agudo son controvertidos y se necesitan más estudios para evaluar su seguridad a largo plazo. El objetivo del trabajo fue analizar mediante un estudio observacional la seguridad clínica más allá de 12 meses de los Magmaris implantados en un único centro hospitalario en pacientes con síndrome coronario agudo.
Métodos: Se registraron de manera consecutiva 36 pacientes con Magmaris implantados entre noviembre de 2016 y noviembre de 2018 con diagnóstico de síndrome coronario agudo. Para el objetivo primario se consideró el combinado orientado al dispositivo de infarto de miocardio del vaso diana, fracaso de la lesión diana y muerte de causa cardiovascular. Como objetivo secundario se incluyó la trombosis del dispositivo.
Resultados: En cuanto al combinado orientado al dispositivo no se observó infarto de miocardio del vaso diana (0%), en 2 casos (5,6%) se observó fracaso de la lesión diana y se constató 1 caso de muerte cardiaca (2,8%) a los 36 meses del implante del Magmaris, sin poder conocer la causa por no disponer de autopsia. Con respecto a los objetivos secundarios, no hubo casos de trombosis del Magmaris durante el seguimiento.
Conclusiones: Nuestros resultados, con un seguimiento a largo plazo, apoyan que los Magmaris presentan un perfil clínico favorable en el escenario complejo del síndrome coronario agudo.
Palabras clave: Magmaris. Sindrome coronario agudo. Trombosis armazon bioabsorbible.
INTRODUCTION
Magnesium-based bioresorbable scaffolds (Magmaris) are safe devices with good results in the long run like the BIOSOLVE II1 and BIOSOLVE III2 clinical trials show where no device thrombosis was seen at the long-term 12- to 24-month follow-up. Despite this fact, the device own limitations (ill-advised in cases of calcified complex coronary anatomy or in long lesions) have reduced its use significantly in the routine clinical practice to the point that only 224 procedures with bioresorbable devices were performed in Spain in 2019 (0.2% of the total number of devices implanted).3
As already mentioned, the good results reported in long-term follow-ups have turned the Magmaris (Biotronik, Germany) into the only bioresorbable metal scaffold to receive the CE marking (Conformité Européenne).4
The role Magmaris plays in the acute coronary syndrome setting is not widely known and further studies will be needed before its safety profile can be assessed. The objective of this work was to analyze—through an observational study in the routine clinical practice—the long-term (> 12 months) clinical safety of Magmaris scaffolds implanted in patients with acute coronary syndrome in the cath lab of a single center.
METHODS
Consecutive observational registry of patients diagnosed with acute coronary syndrome implanted with magnesium-based bioresorbable scaffolds between November 2016 and November 2018. The study was approved by the hospital ethics committee and all patients gave their signed written informed consent to participate in the study. The study primary endpoint was a composite of target vessel myocardial infarction, target lesion failure, and cardiovascular death. The study secondary endpoint included the device thrombosis. The PSP strategy (predilation, sizing, and postdilation) derived from the GHOSTEU registry was used in all the cases.5 In 100% of the patients the optical coherence tomography was used for the right characterization of the lesion and size of the vessel.
RESULTS
A total of 36 patients (29 males, 80%) were included with a median age of 59.61 ± 9.74 years. The follow-up period was 1001 days with an interquartile range of 342 days. Table 1 summarizes the baseline clinical characteristics of the sample as well as the main angiographic characteristics.
Table 1. Baseline clinical characteristics and angiographic parameters of the patients
| N (%) | |||
|---|---|---|---|
| Family history of ischemic heart disease | 11 (30.6) | ||
| Arterial hypertension | 19 (52.8) | ||
| Diabetes mellitus | 7 (19.4) | ||
| Dyslipidemia | 23 (63.9) | ||
| Smoker | 23 (63.9) | ||
| Type of acute coronary syndrome: | |||
| NSTEACS | 23 (63.9) | ||
| STEACS | 8 (22.2) | ||
| Unstable angina | 5 (13.9) | ||
| Number of diseased vessels: | |||
| 1 vessel | 16 (44.4) | ||
| 2 vessels | 15 (41.7) | ||
| 3 vessels | 5 (13.9) | ||
| Location of the lesion treated with Magmaris: | |||
| LAD | 27 (75%) | ||
| RCA | 10 (27.8%) | ||
| LCX | 4 (11.1%) | ||
| AHA classification of coronary lesions: | |||
| Type A | 16 (44.5%) | ||
| Type B | 12 (33.3%) | ||
| Type C | 8 (22.2%) | ||
| Immediate success after device implantation | 36 (100%) | ||
| Drug-eluting stent implantation | 12 (36%) | ||
| Normal LVEF | 26 (72.2%) | ||
| Antiplatelet therapy at discharge: | |||
| Acetylsalicylic acid | 36 (100%) | ||
| Ticagrelor | 29 (80.6%) | ||
| Clopidogrel | 6 (16.7%) | ||
| Prasugrel | 1 (2.8%) | ||
| Prolonged DAPT > 12 months | 14 (38.9%) | ||
| Statins | 36 (100%) | ||
| Beta-blockers | 31 (86.1%) | ||
| Angiographic parameters | Length (mm) | Diameter (mm) | Peak inflation pressure (atm) |
| Target lesion | 29.2 ± 13.4 | 3.4 ± 0.2 | |
| Predilation (noncompliant balloon) | 16.8 ± 2.9 | 3.2 ± 0.4 | 20.2 ± 1.2 |
| Magmaris | 22.5 ± 3.05 | 3.4 ± 0.2 | 15.9 ± 0.9 |
| Postdilation (noncompliant balloon) | 21.4 ± 1.5 | 3.7 ± 0.3 | 21.4 ± 1.5 |
|
AHA, American Heart Association; DAPT, dual antiplatelet therapy; LAD, left anterior descending coronary artery; LCX, left circumflex artery; LVEF, left ventricular ejection fraction; NSTEACS, non-ST-segment elevation acute coronary syndrome; RCA, right coronary artery; STEACS, ST-segment elevation acute coronary syndrome. |
|||
A total of 100% of the patients received a Magmaris device in the target lesion causing the study acute coronary event. Drug-eluting stents were implanted at the discretion of the operator in 12 of the 36 patients (33.3%), and in 1 patient only (2.8%) the implantation of the stent and the Magmaris scaffold overlapped. However, in the remaining patients they were not implanted in the target vessel.
Only 1 Magmaris scaffold was used in 15 patients (41.7%), 2 in 12 cases (33.3%), 3 in 2 cases (5.6%), 4 in 3 cases (8.3%), 5 in 3 cases (8.3%), and a maximum of 6 Magmaris devices in 1 single patient (2.8%). In 20 patients (55.6%) the stent and the Magmaris device implantation overlapped.
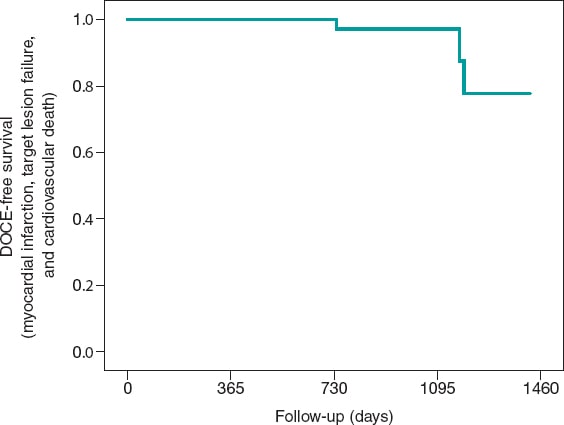
Regarding the device-oriented composite endpoint, 0 cases of target vessel myocardial infarction occurred (0%). However, 2 cases of target lesion failure were confirmed (5.6%), and 1 noncardiovascular death (2.8%) was reported at the 36 months following Magmaris implantation. However, the cause remained elusive for the lack of an autopsy (figure 1). Regarding the secondary endpoints, no Magmaris thrombosis was reported at the follow-up (table 2).
Figure 1. Kaplan-Meier survival curve with respect to the study primary endpoint: a device-oriented composite endpoint of target vessel myocardial infarction, target lesion failure, and cardiovascular death. DOCE, device-oriented composite endpoint.
Table 2. Primary (device-oriented composite endpoint) and secondary clinical events of the patients at the follow-up (N = 36, 100%)
| Event | Patients | Percentage |
|---|---|---|
| Target lesion failure | 2 | 5.6 % |
| Target vessel myocardial infarction | 0 | 0 % |
| Cardiovascular death* | 1 | 2.8 % |
| Magmaris device thrombosis | 0 | 0 % |
|
* 1084 days after Magmaris implantation. |
||
A total of 11 admissions were reported at the follow-up (30.6%). Among these, 8 were due to recurrent angina (22.2%), 2 to heart failure or de novo atrial fibrillation (5.6%), and 1 to atrioventricular block that required pacemaker implantation (2.8%).
The coronary angiography was repeated in 10 cases (27.8%), the absence of lesions was confirmed in 3 patients (8,3%), stent restenosis was reported in 1 patient 2.8%), Magmaris restenosis in 2 (5.6%), and de novo lesions in 4 patients (11.1%).
When the cases of Magmaris restenosis were studied across time it was found that all of these occurred after the 24-months follow-up (1 case after 737 days and the other after 1189 days). The cardiovascular death reported in 1 patient occurred 1084 days after implantation.
DISCUSSION
The magnesium-based bioresorbable scaffold (Magmaris) is a highly successful device when implanted following the recommendations made by the manufacturer6 including the right predilation and optimization of the lesions using intracoronary imaging modalities. A study proved that an optimal PSP technique was not associated with a lower rate of the device-oriented composite endpoint. However, patients with optimal PSP-3 had numerically fewer episodes compared to patients without optimal PSP-3 (0.5% vs 2.9%, P = .085, and 0.5% vs 1.8%, P = .248, respectively).7
The real-world 12-month follow-up results of a cohort registry with Magmaris have recently been published. These results have confirmed the Magmaris safety profile and its low rate of events (target lesion revascularization in 4.7%) and lack of thrombosis.8
Also, the use of magnesium-based bioresorbable scaffolds has been studied in a group of 50 patients with non-ST-segment elevation acute coronary syndrome. This device reached angiographic success in 100% of the cases. One case of failed target vessel revascularization was reported the day after the procedure that required the implantation of a bare-metal stent. No device-related events were reported at the 6-month follow-up.9
The Magmaris scaffold and the sirolimus-eluting stent were compared in a controlled, randomized, blinded, multicenter study of patients with ST-segment elevation acute coronary syndrome. This study proved that in 150 patients the primary endpoint of a greater vasomotor response to medication was better in the Magmaris group at the 1-year follow-up. However, the Magmaris scaffold was associated with a worse angiographic progression and a greater late luminal loss compared to the bare-metal stent. It was also associated with a higher rate of target lesion revascularization without a significantly higher number of thrombotic events being reported.10
In our registry, the immediate rate of successful device implantation was 100%. Device overlapping occurred in 55.6% of the cases with a high frequency of treatment of 2 and 3 vessels (55.4%). Despite the complexity of the lesions, device restenosis was only reported in 2 cases (5.6%) at the > 3-year follow-up. The rate of thrombosis at the follow-up was 0 cases and the rate of cardiovascular death—considering 1 case with the devices implanted 36 months beforehand (and without necropsy or previous clinical assessment)—was 2.8%. In our study of real-world clinical practice this confirms the device safety profile in the short and mid-term in the acute coronary syndrome setting. The results of the BIOSOLVE IV registry reported a target lesion failure rate of 4.3%,11 similar to the one seen in our registry—5.5%—despite our patients’ profile of higher ischemic risk (all of them with acute coronary syndrome and an average lesion length of 29.17 mm ± 13.39 mm with Magmaris overlapping in 55.6% of the cases).
Limitations
Our study main limitation is its small sample size, which shows the low penetration of resorbable scaffolds in our hospital setting. Another limitation is the registry observational nature with an inherent selection bias, without defined inclusion and exclusion criteria, and the use of second-generation drug-eluting stents in 33.3% of the patients outside de target vessel except for 1 case where the stent and the Magmaris device overlapped (2.8%).
Another limitation was the lack of an independent clinical event adjudication committee. However, 100% of the patients were followed-up (through electronic health records and phone calls) by the research working team.
CONCLUSIONS
In our registry of patients with acute coronary syndrome who received the Magmaris scaffold the primary endpoint of target lesion failure or target vessel myocardial infarction did not increase compared to registries previously published. No cases of scaffold definitive thrombosis were reported at the follow-up, and only 1 cardiovascular death was reported 36 months after implantation without knowing the definitive cause. Considering the aforementioned limitations, our results confirm that Magmaris scaffolds could have a favorable clinical profile in the complex setting of acute coronary syndrome.
FUNDING
No external funding has been received.
AUTHORS’ CONTRIBUTIONS
All authors contributed equally during the collection of clinical data and the performance of the interventional procedures including the follow-up of all of the patients.
CONFLICTS OF INTEREST
None reported.
WHAT IS KNOWN ABOUT THE TOPIC?
- Magnesium-based bioresorbable scaffolds (Magmaris) have proven safe and effective in former studies and registries.
- Despite the recent studies published on the acute coronary syndrome setting, the long-term safety of these devices has still not been confirmed yet.
WHAT DOES THIS STUDY ADD?
- A real-world registry with a very long-term follow-up showing a low rate of device-related events.
- Further multicenter registries with a high number of patients will be needed before solid conclusions can be drawn.
REFERENCES
1. Haude M, Ince H, Abizaid A, et al. Safety and performance of the second? generation drug?eluting absorbable metal scaffold in patients with de?novo coronary artery lesions (BIOSOLVE?II):6 month results of a prospective, multicentre, non?randomised, first?in?man trial. Lancet. 2016;387:31-39.
2. Haude M, Ince H, Kische S, et al. Sustained safety and clinical performance of a drug?eluting absorbable metal scaffold up to 24 months:pooled outcomes of BIOSOLVE?II and BIOSOLVE?III. EuroIntervention. 2017;13:432-439.
3. Datos de la Sección de Hemodinámica 2019. Available online: https://www.hemodinamica.com/wp-content/uploads/2020/12/Presentacion-Registro.pdf. Accessed 15 Nov 2021.
4. Sotomi Y, Onuma Y, Collet C, et al. Bioresorbable scaffold:the emerging reality and future directions. Circ Res. 201;120:1341-1352.
5. Ortega-Paz L, Capodanno D, Gori T, et al. Predilation, sizing, and post-dilation scoring in patients undergoing everolimus-eluting bioresorbable scaffold implantation for prediction of cardiac adverse events:development and internal validation of the PSP score. EuroIntervention. 2017;12:2110-2117.
6. Fajadet J, Haude M, Joner M, et al. Magmaris preliminary recommendation upon commercial launch:a consensus from the expert panel on 14 April 2016. Eurointervention. 2016;18:828-833.
7. Ortega-Paz L, Bruggaleta S, Capodanno D, et al. Efecto de la técnica de implantación en los resultados en pacientes tratados con armazón bioabsorbible en diferentes escenarios clínicos. REC Interv Cardiol. 2019;1:83-91.
8. Abellas-Sequeiros RA, Ocaranza-Sánchez R, Bayon-Lorenzo J, et al. 12-month clinical outcomes after Magmaris percutaneous coronary intervention in a real-world cohort of patients:Results from CardioHULA registry. Rev Port Cardiol. 2020;39:421-425.
9. Wlodarczak A, Lanocha M, Jastrzebski A, et al. Early outcome of magnesium bioresorbable scaffold implantation in acute coronary syndrome-the initial report from the Magmaris-ACS registry. Catheter Cardiovasc Interv. 2019;93:E287-E292.
10. SabatéM, Alfonso F, Cequier A, et al. Magnesium-Based Resorbable Scaffold Versus Permanent Metallic Sirolimus-Eluting Stent in Patients With ST-Segment Elevation Myocardial Infarction:The MAGSTEMI Randomized Clinical Trial. Circulation. 2019;140:1904-1916.
11. Verheye S, Wlodarczak A, Montorsi P, et al. Safety and performance of a reservable magnesium scaffold under real-world conditions:12 month outcomes of the first 400 patients enrolled in the BIOSOLVEIV registry. Eurointervention. 2020;15:e1383-e1386.