ABSTRACT
Introduction and objectives: The objective of this study was to describe our experience with coronary physiology assessment using the instantaneous wave-free ratio (iFR) and/or a Syncvision-guided iFR-pullback study [Syncvision version 4.1.0.5, Philips Volcano, Belgium] in all-comer patients.
Methods: Consecutive patients undergoing coronary physiology assessment with the iFR (and/or a Syncvision-guided iFR-pullback study) at our center between January 2017 and December 2019 were included. The iFR cut-off value was 0.89. The primary endpoint was a composite of cardiac death, myocardial infarction, probable or definitive stent thrombosis, and target lesion revascularization.
Results: A total of 277 patients with 433 lesions evaluated were included. The mean age was 65 ± 10 years and 74% were men. Personal history of diabetes mellitus was present in 41% of patients. Clinical presentation was stable angina in 160 patients (58%), and acute coronary syndrome in 117 patients (42%). iFRs > 0.89 were obtained in 266 lesions (61.4%) on which the PCI was postponed. The remaining lesions were revascularized. The Syncvision software was used to guide the iFR-pullback study in 155 lesions (36%) and the decision-making process, mainly in long, diffuse or sequential lesions (91 lesions, 58.7%), and intermediate lesions (52 lesions, 33.5%). After a median follow-up of 18 months, the primary endpoint occurred in 17 patients (6.1%) without differences regarding the baseline iFR (≤ 0.89 or > 0.89) (4.2% vs 3.8%; P = .9) or the clinical presentation (stable angina or acute coronary syndrome) (4.4% vs 8.5%; P = .1)
Conclusions: The use of coronary physiology assessment with the iFR and the Syncvision-guided iFR-pullback study in the routine daily practice and in all-comer patients seems safe with a low percentage of major adverse cardiovascular events at the mid-term follow-up.
Keywords: Physiological assessment. All-comer patients. Syncvision-guided iFR-pullback study.
RESUMEN
Introducción y objetivos: El propósito del estudio fue describir nuestra experiencia con el uso del índice diastólico instantáneo sin ondas (iFR) para la evaluación fisiológica coronaria o el uso del software Syncvision/iFR (Syncvision versión 4.1.0.5, Philips Volcano, Bélgica) en todo tipo de pacientes.
Métodos: Se incluyeron todos los pacientes consecutivos a quienes, entre enero de 2017 y diciembre de 2019, se realizó en nuestro centro una evaluación fisiológica coronaria con iFR o con Syncvision/iFR. El valor de corte establecido para el iFR fue 0,89. El objetivo primario fue un compuesto de muerte cardiaca, infarto de miocardio, trombosis de stent probable o definitiva y nueva revascularización de la lesión evaluada.
Resultados: Se incluyeron 277 pacientes con 433 lesiones evaluadas. La edad media fue de 65 ± 10 años y el 74% eran varones. El 41% tenía antecedente de diabetes mellitus. La presentación clínica fue angina estable en 160 pacientes (58%) y síndrome coronario agudo en 117 pacientes (42%). Se obtuvo un iFR > 0,89 en 266 lesiones (61,4%), en las cuales la intervención coronaria percutánea fue diferida. Las lesiones restantes se revascularizaron. El software Syncvision/iFR se usó en 155 lesiones (36%) para guiar la toma de decisiones, principalmente lesiones largas, difusas o secuenciales (91 lesiones, 58,7%) y lesiones intermedias (52 lesiones, 33,5%). Tras un periodo de seguimiento de 18 meses, el objetivo primario se observó en 17 pacientes (6,1%), sin diferencias en función del iFR basal (≤ 0,89 o > 0,89) (4,2 frente a 3,8%; p = 0,9) ni de la presentación clínica (angina estable o síndrome coronario agudo) (4,4 frente a 8,5%; p = 0,1).
Conclusiones: La evaluación fisiológica coronaria con iFR y el software Syncvision/iFR en la práctica diaria y en todo tipo de pacientes parece ser segura, con un bajo porcentaje de eventos cardiacos adversos mayores a medio plazo.
Palabras clave: Evaluacion fisiologica. Todo tipo de pacientes. Software Syncvision/iFR.
Abbreviations
iFR: instantaneous wave-free ratio. PCI: percutaneous coronary intervention. MACE: major adverse cardiovascular events.
INTRODUCTION
Physiological assessment using the fractional flow reserve (FFR) or the instantaneous wave-free ratio (iFR) is strongly recommended by the European guidelines to the guide percutaneous coronary intervention (PCI) decision-making process to treat intermediate coronary stenosis (indication I, level of evidence A) and multivessel disease (indication IIa, level of evidence B).1-7
The established cut-off values based on landmark trials to safely postpone treatment of a coronary lesion are FFRs > 0.80 and iFRs > 0.89.2-7 Unlike the FFR, the new iFR resting index allows us to analyze the physiological significance of each segment in the presence of coronary arteries with several lesions. Syncvision is a new software that analyzes the specific contribution of each coronary segment allowing us to predict physiological improvement after percutaneous treatment.8,9 It’s not necessary to use any vasodilators either, thus reducing any potential side effects.3,4
However, the evidence supporting the use of coronary physiology assessment with both indices and the use of the Syncvision software in other type of lesions and other clinical scenarios is scarce.8-10 For this reason, it is not quite clear whether the same cut-off value established in the landmark trials should be used; or if safety, utility, and efficacy will be the same.
The objective of this study is to describe our experience with coronary physiology assessment using the iFR (and/or the Syncvision- guided iFR-pullback study) in all-comer patients undergoing invasive coronary angiography.
METHODS
We performed a single-center retrospective study including all patients who underwent functional assessments (using the iFR) and/or the Syncvision software at our center between January 2017 and December 2019 on a PCI decision-making process. The cut-off value to consider the need for revascularization was the same one established by the landmark clinical trials (iFR ≤ 0.89).3,4 The pressure guidewires used for the functional assessment were the Volcano Verrata, and the Volcano Verrata Plus (Philips Volcano, Belgium). The use of the Syncvision software to guide the iFR study as well as the lesions assessed were left to the operator’s discretion.
All subjects included in the study gave their informed consent to undergo the procedure and for data analysis and publication. Additionally, the study received the proper ethical oversight and was approved by our center ethics committee.
Inclusion and exclusion criteria
Patients with the following criteria were included: a) consecutive patients in whom an invasive coronary angiography was performed due to stable or unstable symptoms or silent ischemia; b) presence of, at least, a lesion or vessel physiologically assessed with the iFR during the index procedure. The following exclusion criteria were stablished: a) impossibility to understand the informed consent during the index procedure; b) written informed consent to use data for research purposes not provided.
Lesion classification
The lesions physiologically assessed were classified based on their angiographic characteristics and/or clinical setting: a) intermediate lesions: lesions with a 40% to 80% angiographic stenosis as seen on the quantitative coronary angiography (QCA); b) sequential or diffuse coronary lesions: presence of, at least, 2 sequential lesions or a coronary segment with diffuse disease (coronary vessel with multiple plaques in most of the epicardial territory) with a total length of 25 mm; c) bifurcation lesions: presence of a coronary stenosis at bifurcation level with a side branch size large enough to be protected; d) in-stent restenosis: presence of focal or diffuse in-stent restenosis with a a 40% to 80% angiographic stenosis as seen on the QCA; e) coronary bypass lesion, defined as, at least, a lesion in the coronary artery bypass grafting or native vessel presenting with proximal total occlusion.
Endpoints
The primary endpoint of the study was the rate of major adverse cardiovascular events (MACE) at the follow-up. The MACE were defined as a composite of cardiac death, myocardial infarction (MI), definitive or probable stent thrombosis, and new target lesion revascularization (TLR). All deaths were considered cardiovascular unless unequivocal non-cardiac causes would be established. Myocardial infarction included spontaneous ST-segment elevation MI or non-ST-segment elevation acute myocardial infarction. The TLR was defined as a new revascularization of a baseline physiologically negative lesion at the follow-up or as a repeat revascularization of a baseline physiologically positive lesion percutaneously treated during the index procedure.
The secondary endpoints established were: a) analysis of the primary endpoint components separately; b) rate of MACE based on the clinical setting (stable angina or acute coronary syndrome), non-ST-segment elevation acute myocardial infarction (NSTEMI), and ST segment elevation myocardial infarction (STEMI); c) rate of MACE based on the baseline iFR; d) to determine the type of lesions where the Syncvision software was used for the iFR-pullback study.
Follow-up
The patients’ follow-up was performed through phone calls, hospital record reviews or outpatient visits.
Quantitative coronary measurements
Quantitative coronary measurements were performed using a validated system (CAAS system, Pied Medica Imaging, The Netherlands). These were the measurements analyzed: reference vessel diameter, minimum lumen diameter, percent diameter stenosis, and lesion length. All measurements were performed at baseline and after the PCI.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation, and the Student t test was used to establish comparisons. The categorical variables were expressed as frequency and percentage, and compared using the chi-square test. The univariate analysis was performed with the following covariates: age, male sex, current smoking status, dyslipidemia, left ventricular ejection fraction, acute coronary syndrome, multivessel disease, clopidogrel, ticagrelor, right coronary artery as the study vessel, other vessels analyzed, and baseline iFRs ≤ 0.89. Results were reported using odds ratios (OR), and two-sided 95% confidence intervals. In all the cases, P values < .05 were considered statistically significant. The statistical analysis was performed using the IBM-SPSS statistical software package (version 24.0 for Macintosh, SPSS Corp., United States).
RESULTS
The study flowchart is shown on figure 1. During the study period, a total of 2951 patients underwent coronary angiography at our center. The iFR-based physiological assessment was performed in 277 patients (9.4%) with 433 lesions. The baseline clinical data are shown on table 1. The mean age was 65 ± 10 years, and 74% of the patients (204) were men. The prevalence of comorbidities was high (diabetes mellitus, 41%; previous MI, 32%; peripheral arterial disease, 4%; cerebrovascular disease, 6%; chronic kidney disease, 13%). The clinical presentation included stable angina in 160 patients (58%), NSTEMI in 91 patients (33%), and STEMI in 26 patients (9%).
Table 1. Baseline clinical data
| Patients | Total (N = 277) | Stable angina (N = 160) | ACS (N = 117) | P |
|---|---|---|---|---|
| Age, years | 65 ± 10 | 65 ± 10 | 64 ± 11 | .071 |
| Sex, male, N (%) | 204 (74) | 116 (72) | 94 (80) | .112 |
| Hypertension, N (%) | 175 (63) | 101 (63) | 77 (66) | .645 |
| Diabetes mellitus, N (%) | 114 (41) | 58 (36) | 52 (44) | .169 |
| Dyslipidemia, N (%) | 157 (57) | 101 (63) | 58 (50) | .024 |
| Current smoker, N (%) | 72 (26) | 29 (18) | 42 (36) | .001 |
| Previous myocardial infarction, N (%) | 89 (32) | 53 (33) | 37 (32) | .792 |
| Previous revascularization, N (%) | 94 (34) | 50 (31) | 32 (27) | .518 |
| Percutaneous, N (%) | 80 (85) | 50 (31) | 30 (26) | .336 |
| Surgical, N (%) | 14 (15) | 8 (16) | 6 (19) | .095 |
| Atrial fibrillation, N (%) | 39 (14) | 19 (12) | 13 (11) | .844 |
| Heart failure, N (%) | 8 (3) | 7 (4) | 2 (2) | .216 |
| Prior ACE, N (%) | 17 (6) | 11 (7) | 9 (8) | .795 |
| Peripheral arterial disease, N (%) | 11 (4) | 7 (4) | 5 (4) | .967 |
| Previous bleeding, N (%) | 3 (1) | 2 (1) | 2 (2) | .752 |
| Chronic kidney disease, N (%) | 36 (13) | 19 (12) | 17 (15) | .486 |
| Hemoglobin, g/dL | 13.96 ± 1.7 | 13.87 ± 1.8 | 14.13 ± 1.8 | .365 |
| Creatinine, g/dL | 0.98 ± 0.47 | 1 ± 0.63 | 1 ± 0.37 | .584 |
| Left fentricular ejection fraction, % | 59 ± 15 | 57 ± 16 | 60 ± 13 | .098 |
|
ACE, acute cerebrovascular event; ACS, acute coronary syndrome. Data are expressed as number (N) and percentage (%). |
||||
Angiographic and procedural data
Angiographic and procedural data are shown on table 2. Radial access was the access of choice in most of the cases (392 lesions, 91%). A total of 186 patients (67%) showed angiographic multivessel disease. Regarding the angiographic Syntax I score, 232 patients (84%) had Syntax scores < 22, 41 patients (15%) between 22 and 32, and only 4 patients (1%) > 32 without any differences being reported between stable and unstable patients. The vessel most frequently analyzed was the left anterior descending coronary artery (180, 42%) followed by the right coronary artery (99, 23%). The left main coronary artery was evaluated in 23 patients (5%).
Table 2. Angiographic and procedural data
| Patients | Total (N = 277) | Stable angina (N = 160) | ACS (N = 117) | P |
|---|---|---|---|---|
| Radial access, N (%) | 251 (90) | 147 (92) | 104 (89) | .329 |
| Multivessel disease, N (%) | 165 (59) | 84 (52) | 81 (69) | .004 |
| Syntax score | 11 ± 8 | 10 ± 8 | 12 ± 8 | .885 |
| Low risk (< 22) | 45 (16) | 25 (16) | 20 (17) | .184 |
| Intermediate risk (22-32) | 6 (2) | 1 (1) | 5 (4) | .066 |
| High risk (> 32) | 1 (1) | 1 (1) | 0 | .331 |
| Acetylsalicylic acid, N (%) | 245 (88) | 142 (88) | 103 (88) | .740 |
| P2Y12 inhibitor, N (%) | 195 (71) | 98 (61) | 97 (83) | |
| Clopidogrel | 63 (23) | 40 (25) | 23 (20) | .011 |
| Ticagrelor | 127 (46) | 56 (35) | 71 (61) | .019 |
| Prasugrel | 65 (2) | 2 (1) | 3 (3) | .642 |
| Vessel analyzed, N (%) | ||||
| LAD | 121 (44) | 66 (41) | 55 (47) | .318 |
| LCx | 40 (14) | 26 (16) | 14 (12) | .327 |
| RCA | 75 (27) | 50 (31) | 25 (21) | .072 |
| LMCA | 15 (5) | 8 (5) | 7 (6) | .712 |
| Other | 27 (10) | 11 (7) | 16 (14) | .057 |
| Reference vessel diameter (mm) | 3.3 ± 3 | 3.3 ± 3 | 3.3 ± 3 | .971 |
| Vessel stenosis (%) | 49 ± 16 | 49 ± 17 | 49 ± 16 | .816 |
| Vessel minimal lumen diameter (mm) | 1.6 ± 0.6 | 1.5 ± 0.6 | 1.5 ± 0.5 | .203 |
| Vessel lesion length (mm) | 21 ± 12 | 21 ± 13 | 20 ± 11 | .174 |
| Vessel stent diameter (mm) | 2.8 ± 0.4 | 2.8 ± 0.4 | 2.8 ± 0.4 | .581 |
| Type of stent implanted (%) | ||||
| DES | 100 | |||
| BMS | 0 | |||
| Other | 0 | |||
| Immediate angiographic optimal result (%) | 100 | |||
| Contrast used (mL) | 142 ± 91 | 151 ± 110 | 164 ± 72 | .166 |
| Intracoronary imaging, N (%) | 6 (2) | 6 (4) | 0 | .034 |
| Procedural complications, N (%) | 3 (1) | 2 (1) | 1 (1) | .754 |
| Baseline iFR | 0.88 ± 0.12 | 0.89 ± 0.12 | 0.86 ± 0.14 | .097 |
| Final iFR | 0.93 ± 0.04 | 0.93 ± 0.04 | 0.93 ± 0.04 | .951 |
| Syncvision-guided iFR-pullback study, N (%) | 155 lesions (36) | 94 lesions (36) | 61 lesions (35) | .4 |
| Lesions evaluated | Total (N = 433) | Stable angina (N = 258) | ACS (N = 175) | P |
| Angiographically moderate lesions, N (%) | 244 (56.4) | 149 (58) | 95 (54) | .475 |
| Sequential/diffuse coronary lesions, N (%) | 118 (27.3) | 64 (25) | 53 (30) | .208 |
| Bifurcation lesions, N (%) | 51 (11.8) | 31 (12) | 20 (11) | .853 |
| In-stent restenosis, N (%) | 15 (3.5) | 11 (4.3) | 4 (2.3) | .269 |
| Coronary artery bypass grafting, N (%) | 2 (0.5) | 0 (0) | 2 (1.1) | .085 |
| Other lesions, N (%) | 3 (0.75) | 2 (0.8) | 1 (0.6) | .802 |
|
ACS, acute coronary syndrome; BMS, bare metal stent; DES, drug-eluting stent; iFR, instantaneous wave-free ratio; LAD, left anterior descending coronary artery; LCx, left circumflex artery; LMCA, left main coronary artery; RCA, right coronary artery. Data are expressed as number (N) and percentage (%). |
||||
The mean reference diameter was 3.3 mm ± 3 mm with a mean vessel stenosis of 49% ± 16%, and a mean lesion length of 21 mm ± 12 mm. The mean diameter of the stent implanted was 2.8 ± 0.4. All the stents implanted were drug-eluting stents (100%). Intracoronary imaging was used in 14 patients (3%).
The instantaneous wave-free ratio was obtained in 433 lesions, with a baseline value of 0.89 ± 0.12. The physiological assessment results after the PCI were obtained in 129 lesions (29.8%) with a final iFR of 0.93 ± 0.04.
The lesions physiologically assessed are shown on table 2. The most common type of lesions undergoing physiological assessment were angiographically moderate lesions (244, 56.4%) followed by sequential and diffuse lesions (118, 27.3%). Physiological assessment was used in 51 bifurcation lesions (11.8%) basically to guide the intervention over the side branch while using a provisional stenting strategy.
The Syncvision software for the iFR-pullback study was used in 155 lesions to guide the decision-making process (35.8%). Sequential and diffuse coronary lesions were the most common lesions analyzed by the iFR-pullback study (91 vessels, 58.7%, figure 2) followed by angiographically moderate lesions (52 vessels, 33.5%). This software was used in 5 bifurcation lesions (3.2%) to establish a baseline physiological classification or confirm an optimal physiological result after the PCI in both branches. The remaining lesions assessed by the iFR-pullback study were 6 focal or diffuse in-stent restenoses (3.9%) and 1 saphenous vein bypass graft with diffuse disease (0.6%).
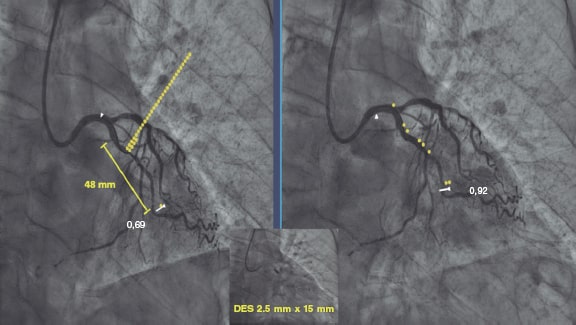
Figure 2. Images of iFR-coregistration with the Syncvision software from a left circumflex artery with diffuse disease in its middle segment (48 mm of lesion length). The baseline distal iFR was 0.69. The Syncvision-guided iFR-pullback study demonstrated physiological significance only in the proximal segment. Direct implantation of a 2.5 mm × 15 mm DES was performed with a final iFR of 0.92. The stent length reduction regarding the angiographic lesion was 33 mm.
Follow-up
Follow-up data were available for 274 out of 277 patients (99%). After a mean 18 ± 10-month follow-up, 17 patients (6.1 %) presented with a major adverse cardiovascular events (table 3), 7 patients (2.5 %) with TLR, 2 of them over a lesion treated during the index procedure (0.7%) and 5 (1.8%) due to disease progression of a baseline physiologically negative lesion; 6 patients (2.2 %) suffered from acute myocardial infarction (1 patient due to acute stent thrombosis, another to a new lesion not evaluated at the index procedure, another to a baseline physiologically non-significant lesion, and the remaining 3 patients due to failed previously revascularized lesions); also, 4 patients (1.4%) presented with unclear or cardiac death. There were no differences regarding MACE between baseline physiologically negative and positive lesions (table 3).
Table 3. Rate of major adverse cardiovascular events at the follow-up based on the clinical presentation
| MACE (277 patients, 433 lesions) | iFR ≤ 0.89 (N = 167 lesions) | iFR > 0.89 (N = 266 lesions) | P | Stable angina (N = 160) | ACS (N = 117) | P | |
|---|---|---|---|---|---|---|---|
| Overall, N (%) | 17 (6.1) | 7 (4.2) | 10 (3.8) | .9 | 7 (4.4) | 10 (8.5) | .1 |
| Unclear or cardiac death, N (%) | 4 (1.4) | 2 (1.2) | 2 (0.8) | .2 | 3 (1.9) | 1 (0.8) | .9 |
| Myocardial infarction, N (%) | 6 (2.2) | 1 (0.6) | 5 (1.9) | .46 | 1 (0.6) | 5 (4.3) | < .05 |
| Target lesion revascularization, N (%) | 7 (2.5) | 4 (2.4) | 3 (1.1) | .09 | 3 (1.9) | 4 (3.4) | .2 |
|
ACS, acute coronary syndrome; iFR, instantaneous wave-free ratio; MACE, major adverse cardiovascular events. Data are expressed as number (N) and percentage (%). |
|||||||
Based on their clinical signs, patients who presented with ACS had an increased rate of new myocardial infarction at the follow-up (5.3% vs 0.6%; P < .05), although no differences were found regarding unclear or cardiac death (0.9% vs 1.8%; P = .9) and the overall MACE (8.5% vs 4.4%; OR, 2.056, 0.759-5.572; P = .156 (table 3).
Finally, we performed a univariate analysis and found no risk or protective factors for MACE in this cohort of patients (table 4).
Table 4. Univariate analysis of the different variables with potential impact in the rate of major adverse cardiovascular events between groups
| Variable | Univariate analysis | |
|---|---|---|
| OR (95%CI) | P | |
| Age | 1.01 (0.97-1.06) | .608 |
| Male | 2.54 (0.57-11.40) | .224 |
| Current smoker | 1.23 (0.42-3.60) | .713 |
| Dyslipidemia | 1.39 (0.50-3.87) | .531 |
| Left ventricular ejection fraction (%) | 0.99 (0.95-1.04) | .684 |
| Acute coronary syndrome | 2.06 (0.76-5.57) | .156 |
| Multivessel disease | 0.90 (0.33-2.45) | .842 |
| Clopidogrel | 0.75 (0.23-2.44) | .623 |
| Ticagrelor | 1.52 (0.46-4.96) | .490 |
| Right coronary artery as examined vessel | 1.52 (0.54-4.26) | .428 |
| Other vessel analyzed | 1.26 (0.27-5.82) | .769 |
| Baseline iFR ≤ 0.89 | 1.43 (0.88-2.32) | .152 |
|
95%CI, confidence interval; iFR, instantaneous wave-free ratio; OR, odds ratio. |
||
DISCUSSION
This study tried to describe our experience using the physiological assessment and the Syncvision software in all-comer patients who underwent percutaneous coronary evaluations. The main findings of our study are: a) the use of the iFR in lesions of all-comer patients with the same cut-off values than established in the main trials showed a low percentage of MACE at the mid-term follow-up (6.1%); b) patients who presented with acute coronary syndrome showed an increased rate of myocardial infarction at the mid-term follow-up, and a trend towards a higher rate of MACE (OR, 2.056, 0.759-5.572; P = .156); c) The Syncvision-guided iFR-pullback study provided additional information to guide the PCI decision-making process, especially in complex lesions like sequential lesions and diffuse coronary artery disease.
The fractional flow reserve was the first physiological index that demonstrated its utility, safety, and efficacy guiding the revascularization decision-making process.2,5-7 To obtain it, the use of a hyperemic agent to reduce vascular resistance is mandatory. Adenosine is the most commonly used drug, but it presents a series of side effects and contraindications.3,4,11,12 The more recent resting index (the instantaneous wave-free ratio) has demonstrated similar utility, safety, and efficacy to the FFR.3,4 Furthermore, it has 2 main advantages: first, it is not necessary to use vasodilators, thus reducing side effects, contraindications for use, and procedural time; secondly, it allows us to assess the contribution of each lesion when the vessel presents several lesions, with the specific Syncvision-guided iFR-pullback study.8,9
For these reasons, the coronary physiology assessment is already the routine practice at the cath lab for the assessment of intermediate lesions,2-5 and multivessel disease.6,7 The main clinical setting included in these studies was stable angina. Patients with NSTEMI could be included if the lesion evaluated was identified as a non-culprit lesion. However, patients with STEMI, left main coronary artery lesions, and coronary artery bypass grafting lesions were not represented in the trials; also, the percentage of bifurcation lesions and sequential or diffuse coronary lesions is tiny. The cut-off value for the FFR and the iFR is well defined in those trials, being safe to postpone a lesion with a FFR > 0.80 or an iFR > 0.89. However, information is scarce on the utility and efficacy of physiological assessment and the same cut-off values in other types of lesions and clinical presentations.13 A multicenter registry that used the iFR to guide revascularization in patients with left main coronary artery stenosis has just been published. Using a cut-off value of 0.89, the authors conclude that postponing a left main coronary artery lesion with a iFR > 0.89 seems to be safe.10
Our study results suggest that the use of physiological assessment and the Syncvision software to guide the PCI decision-making process in all-comer patients with the same cut-off values as established by the landmark trials seems useful and safe regardless of the lesion and clinical presentation undergoing evaluation. Also, the MACE rates are similar to those reported by the landmark trials with selected lesions and patients.3,4 The iFR was the index used more often. The reasons are the faster and more comfortable use,3,4 and the possibility of lesion assessment with the Syncvision software.8,9
An important point of the study was to evaluate the rate of MACE based on the clinical presentation. Although no significant differences in the overall rate of MACE were found, patients who presented with acute coronary syndrome showed a significantly higher rate of MI at the follow-up, and a trend towards a higher rate of overall MACE. We think that this absence of statistical significance could be associated with a lack of statistical power.
A type of lesion included in the study was bifurcation lesions. Physiological assessment was used mainly to guide the side branch results during a provisional stenting strategy, thus keeping the pressure wire jailed as previously described.14,15 However, another interesting use of the iFR-pullback study with the Syncvision software was to stablish the baseline physiological contribution of every segment included in the most accepted classification.16
Finally, the Syncvision-guided iFR-pullback study was used in 155 lesions (36%). The main type of lesions where this software was used were diffuse and tandem lesions. This software can predict the physiological contribution of each lesion or coronary segment, which is why we believe that it is a very useful tool to avoid treating lesions without any physiological contribution and probably without clinical benefits. That is why this software seems to reduce the total stent length implanted regarding angiographically-guided revascularization with potential benefits at long-term follow-up.17,18 A clinical trial is currently in the recruitment phase to demonstrate the efficacy of this software reducing the length of the stent implanted in this type of lesions without detriment to the adverse events.19
In our experience, the key aspects to properly perform this technique are: a) a perfect aortic pressure curve allows the accurate detection of diastole through the software; b) passing the pressure sensor as distally as possible; c) finding a projection where the artery can be seen completely and with the least foreshortening possible; d) withdrawing the pressure guidewire very slowly so that the software can perfectly recognize the length of each arterial segment; e) checking that there is not drift when the pressure guidewire reaches the coronary ostium (iFR different to 1 ± 0.02) to avoid erroneous results; f) performing the coronary angiography in the same position as the guidewire withdrawal without any modifications to the height of the table or the C-arm, and with a higher flow and volume of contrast to facilitate the software recognition of all the lesions. The main problem when using this technique is the presence of lesions with complicated wiring. The pressure wire has a hydrophilic non-polymeric coating that is useful in most lesions. However, it may be very challenging to reach the distal part of the artery in very complex lesions (calcified, angled lesions…), and our experience with previous normalization, wire disconnection, the microcatheter exchange technique, and reconnection is very limited, but still there is a significant level of drift.
Limitations
The study presents several limitations. It is a retrospective, single-center analysis with a low number of patients and lesions. Therefore, the results should be interpreted with caution, although it could be a hypothesis-generating study for future larger scale randomized clinical trials.
CONCLUSIONS
The use of coronary physiology assessment using the iFR and the Syncvision-guided iFR-pullback study in the routine daily practice and in all-comer patients seems safe with a low percentage of MACE at the mid-term follow-up. The Syncvision-guided iFR-pullback study provides additional information to guide the PCI decision-making process.
FUNDING
The study has not had funding.
AUTHORS’ CONTRIBUTION
F.J. Hidalgo-Lesmes prepared the main draft of the manuscript. S. Ojeda-Pineda participated in the drafting of the manuscript. C. Pericet-Rodríguez, R. González-Manzanares, A. Fernández-Ruiz, and M.G. Flores-Vergara all contributed to the analysis and interpretation of data. A. Luque-Moreno, J. Suárez de Lezo, and F. Mazuelos-Bellido participated in the conception and design of the study. M.A. Romero-Moreno, and J.M. Segura Saint-Gerons revised the manuscript critically for important intellectual content. M. Pan Álvarez-Ossorio approved the final version of the manuscript.
CONFLICTS OF INTEREST
F.J. Hidalgo-Lesmes received minor fees from Philips Volcano Europe unrelated to the manuscript; S. Ojeda-Pineda received minor fees from Terumo and Philips Volcano Europe unrelated to the manuscript; M. Pan Álvarez-Ossorio received minor fees from Terumo, Abbott Vascular, and Philips Volcano Europe unrelated to the manuscript. The remaining authors declared no conflicts of interest.
WHAT IS KNOWN ABOUT THE TOPIC?
- Physiological assessments with the iFR are strongly recommended by the European guidelines on coronary revascularization to guide the PCI decision-making process in intermediate coronary stenosis.
- However, the evidence supporting the use of coronary physiology assessment, and the new Syncvision-iFR software in other type of lesions and clinical settings is scarce.
WHAT DOES THIS STUDY ADD?
- This study describes our experience with the iFR and the Syncvision-iFR software in all-comer patients and demonstrates an acceptable percentage of MACE at the mid-term follow-up.
- Furthermore, the study shows that the Syncvision-guided iFR-pullback study provides additional information to guide the PCI decision-making process, particularly in complex lesions like sequential lesions and diffuse coronary artery disease.
REFERENCES
1. Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87-165.
2. Pijls NHJ, van Schaardenburgh P, Manoharan G, et al. Percutaneous Coronary Intervention of Functionally Nonsignificant Stenosis. 5-Year Follow-Up of the DEFER Study. J Am Coll Cardiol. 2007;49:2105-2111.
3. Davies JE, Sen S, Dehbi H-M, et al. Use of the Instantaneous Wave-free Ratio or Fractional Flow Reserve in PCI. N Engl J Med. 2017;376:1824-1834.
4. Götberg M, Christiansen EH, Gudmundsdottir IJ, et al. Instantaneous Wave-free Ratio versus Fractional Flow Reserve to Guide PCI. N Engl J Med. 2017;376:1813-1823.
5. Pijls NHJ, de Bruyne B, Peels K, et al. Measurement of Fractional Flow Reserve to Assess the Functional Severity of Coronary-Artery Stenoses. N Engl J Med. 1996;334:1703-1708.
6. Tonino AL, Bruyne B De, Pijls NHJ, et al. Fractional Flow Reserve versus Angiography for Guiding Percutaneous Coronary Intervention Pim. N Engl J Med. 2015:687-696.
7. Van Nunen LX, Zimmermann FM, Tonino PAL, et al. Fractional flow reserve versus angiography for guidance of PCI in patients with multivessel coronary artery disease (FAME):5-year follow-up of a randomised controlled trial. Lancet. 2015;386:1853-1860.
8. Nijjer SS, Sen S, Petraco R, Mayet J, Francis DP, Davies JER. The Instantaneous wave-Free Ratio (iFR) pullback:A novel innovation using baseline physiology to optimise coronary angioplasty in tandem lesions. Cardiovasc Revasc Med. 2015;16:167-171.
9. Nijjer SS, Sen S, Petraco R, et al. Pre-angioplasty instantaneous wave-free ratio pullback provides virtual intervention and predicts hemodynamic outcome for serial lesions and diffuse coronary artery disease. JACC Cardiovasc Interv. 2014;7:1386-1396.
10. Warisawa T, Cook CM, Rajkumar C, et al. Safety of Revascularization Deferral of Left Main Stenosis Based on Instantaneous Wave-Free Ratio Evaluation. JACC Cardiovasc Interv. 2020;13:1655-1664.
11. Gili S, Barbero U, Errigo D, et al. Intracoronary versus intravenous adenosine to assess fractional flow reserve:A systematic review and meta-analysis. J Cardiovasc Med. 2018;19:274-283.
12. Patel HR, Shah P, Bajaj S, Virk H, Bikkina M, Shamoon F. Intracoronary adenosine-induced ventricular arrhythmias during fractional flow reserve (FFR) measurement:case series and literature review. Cardiovasc Interv Ther. 2017;32:374-380.
13. Ihdayhid AR, Koh JS, Ramzy J, et al. The Role of Fractional Flow Reserve and Instantaneous Wave-Free Ratio Measurements in Patients with Acute Coronary Syndrome. Curr Cardiol Rep. 2019;21.
14. Burzotta F, Lassen JF, Banning AP, et al. Percutaneous coronary intervention in left main coronary artery disease:The 13th consensus document from the European Bifurcation Club. EuroIntervention. 2018;14:112-120.
15. Hidalgo F, Pan M, Ojeda S, et al. Feasibility and Efficacy of the Jailed Pressure Wire Technique for Coronary Bifurcation Lesions. JACC Cardiovasc Interv. 2019;12:109-111.
16. Medina A, Suárez de Lezo J, Pan M. A New Classification of Coronary Bifurcation Lesions. Rev Esp Cardiol. 2006;59:183.
17. Mauri L, O'Malley AJ, Popma JJ, et al. Comparison of thrombosis and restenosis risk from stent length of sirolimus-eluting stents versus bare metal stents. Am J Cardiol. 2005;95:1140-1145.
18. Kikuta Y, Cook CM, Sharp ASP, et al. Pre-Angioplasty Instantaneous Wave-Free Ratio Pullback Predicts Hemodynamic Outcome In Humans With Coronary Artery Disease:Primary Results of the International Multicenter iFR GRADIENT Registry. JACC Cardiovasc Interv. 2018;11:757-767.
19. Hidalgo F, Ojeda S, de Lezo JS, et al. Usefulness of a co-registration strategy with iFR in long and/or diffuse coronary lesions (iLARDI):study protocol. REC Interv Cardiol. 2021;3:190-195.