Available online: 09/04/2019
Editorial
REC Interv Cardiol. 2020;2:310-312
The future of interventional cardiology
El futuro de la cardiología intervencionista
Emory University School of Medicine, Atlanta, Georgia, United States
While on his first hospital duty, a second-year cardiology resident receives a message in his pager about a patient who has just been admitted to the emergency room with chest pain. Specifically, he is asked to discard that the pain is of coronary origin. The resident questions the patient on his symptoms, examines his risk factors, and analyzes the electrocardiogram (ECG). With the ECG data, the qualitative information of pain provided by the patient, together with his past medical history, the presence of coronary pain is eventually discarded. The patient’s past medical history reads «the symptoms described by the patient, the presence of hypertension as the only risk factor, and the lack of specific changes on the ECG suggest that the chances of coronary pain are extremely low». Intuitively, the resident considers that the chances the pain is due to coronary artery disease (CAD) with the data available (qualitative data of pain, risk factors, ECG) is lower than, let’s say, 5%. In other words, the resident instinctively concludes that:
p(CAD |qualitative data, risk factors, ECG) < .05,
that is, the probability of having CAD according to all the abovementioned information (qualitative data, risk factors, and ECG) is < 5%.
However, before the patient is discharged, the resident decides to consult with the fifth-year cardiology resident who is at the Coronary Care Unit. He arrives to the emergency room and asks the patient about his symptoms, examines his medical history, and crosschecks his ECG once again. The much more experienced fifth-year resident considers that, although there are no risk factors other than hypertension, certain characteristics of the pain could have a coronary origin. And not only that, the analysis of the ECG reveals minimal repolarization alterations that appear as a mild—almost unnoticeable—rectification of the ST-segment. Intuitively, the resident considers that the chances that the pain is due to coronary artery disease (CAD) is undoubtedly > 5%, and possibly > 20%. In other words, the fifth-year resident intuitively concludes that:
p(CAD |qualitative data, risk factors, ECG) > .02
that is, the probability of having CAD according to all the information provided above (qualitative data, risk factors, and ECG) is > 20%.
With this early assessment, the fifth-year resident decides to perform a transthoracic echocardiogram (TTE) that reveals alterations in segmental contractility. With this new information available, the resident believes that the chances that the pain is of coronary origin have increased significantly:
p(CAD |qualitative data, risk factors, ECG, TTE) > .05
The intuition of a moderate-high probability of the coronary origin of the pain plus the information provided by the TTE suggest that high-sensitivity troponin (Tp) level should be tested, as Tp appears slightly elevated. Therefore, the intuitive probability that the patient’s chest pain is of coronary origin increases even more:
p(EC |qualitative data, risk factors, ECG, TTE, Tp) > .08
The previous example—though rather simplistic—illustrates several factors associated with conditioned probability and, more specifically, with traditional Bayes’ theorem.
Firstly, it illustrates how Bayes’ theorem is possibly a very appropriate mathematical approach to update our natural and intuitive decision making in medicine: we take an effect—which is what we find at the patient’s bedside—(chest pain), and make a decision on its cause with diagnostic, prognostic or treatment purposes, and then intuitively assign a probability to the cause under consideration (coronary artery disease). We do this with the information available we believe to be the effects of this cause while considering other variables closely associated with the cause such as the characteristics of pain, the ECG, the risk factors, etc.
Secondly, it illustrates how different sources of information added sequentially provide a more accurate definition on the probability of a given cause, always intuitively. And not only that, in the routine clinical practice, interpreting different sources of information varies from observer to observer, which will determine that the probability assigned to a given cause will vary significantly among observers. Here the clinician’s experience undoubtedly plays a key role.
This example also indirectly illustrates how difficult it is to obtain numerical—though approximate—estimates of the actual probability of the real cause for the effect (CAD) from the effects seen and other variables associated with this cause. As Armero et al.1 state in an article recently published in REC: Interventional Cardiology, the accurate probability estimate of the example would require solving the equation of Bayes’ theorem:
p(CAD |information available) = 
where information available, in the example, would be the qualitative data of pain, the risk factors, the ECG, etc. But here is where problems begin. Like Armero et al.1 say, the first problem is to obtain the prior distribution to estimate probabilities. Although estimates can be made on the probability of CAD in a population based on its prevalence or on the probabilities of hypertensive patients or on the probabilities of having an abnormal ECG, estimating the probability of the qualitative data of pain doesn’t have a clear distribution on which to lean on. Like Armero et al.1 say the second problem is implementing an analytical expression for the posterior distribution of parameters and estimating the function of verisimilitude. Again, although the probability of knowing that a hypertensive patient has CAD or that an ECG shows certain characteristics of coronary artery disease is feasible, the problem becomes more complicated when several sources of information are combined; what are the chances of having chest pain with certain characteristics in a hypertensive patient with a given ECG knowing that he’s got CAD? Although it is true that distribution samples can be simulated, the analytical method becomes complicated and—as Armero et al.1 say—interpretation probably stops being intuitive.
We should also add the problem of «expert knowledge» to the mix for the definition of prior distribution. Like the example illustrates, knowledge can depend on the expert’s interpretation. But also, to a great extent, prior knowledge can significantly be affected by publication bias or it can be erroneous, which is why the associated informative distribution will give rise to biased probability estimates.
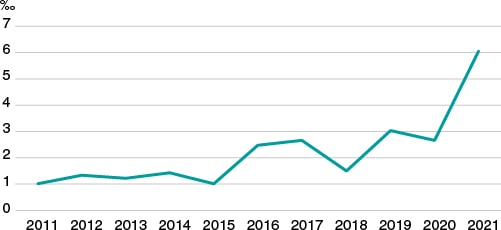
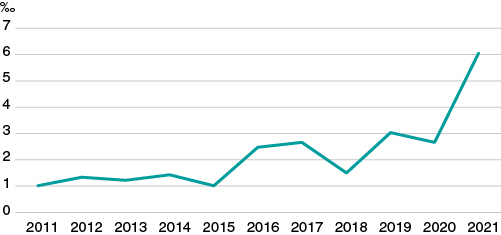
Due to all of this, although it is very possible that doctors can use Bayes’ theorem naturally for his decision-making process regarding diagnosis, prognosis, and treatment, this does not seem to have translated into a significantly wider use of this methodology in research. In an exercise of indirect approach to corroborate the this, we used PubMed to analyze «clinical trial» or «randomized clinical trial» publications in the cardiovascular field over the last 10 years. Then, we selected those where the term «Bayesian» was included somewhere in the text field. Like figure 1 shows—although it has increased significantly over the last few years—the overall rate of possible use of Bayesian methodology in clinical trials in the cardiovascular field is well under 7‰.
Figure 1. Clinical trials published over the last 10 years in the cardiovascular field with possible Bayesian methodology (for every 1000 cardiovascular publications).
If, like Armero et al.1 say, the Bayesian protocol is easy-to-use, robust, and conceptually powerful, why is it used so marginally compared to frequentist statistics? We believe there are several reasons for this. In the first place, frequentist statistics was the first ever used to answer research questions in medicine possibly because right from the start the most common probability distributions had already been perfectly defined, and it was easy to apply the inferential method for decision-making based on such distributions with perfectly defined parameters. However, analytical and computer problems associated with the use of Bayes’ theorem to estimate probabilities were not initially resolved. Secondly, because of the simplicity of being able to make decisions on whether a treatment, diagnostic method, procedure, etc. is effective or not based on the selection of P value < .05 so popular in the frequentist method also referred to by Armero et al. Finally, because the Bayesian conception of probability allocation to parameters and being able to establish direct probabilistic assessments means that the parameter is not an immovable fixed reference anymore. From the analytical standpoint this is a change of paradigm we are not used to. But maybe this loss also generates certain anxiety: parameters are not unchangeable anymore.
FUNDING
None reported.
CONFLICTS OF INTEREST
The authors did not declare any conflicts of interest in relation to this manuscript.
REFERENCES
Over the last 3 decades, the percutaneous treatment of congenital heart diseases has made significant progress. Currently, it is the therapy of choice to treat many of these diseases like atrioventricular septal defects, pulmonary valve stenosis or coarctation of the aorta. Multiple procedures throughout the life of a patient are required to treat complex heart disease like stenting and, more recently, percutaneous valves, which have become additional alternatives to surgery.
Since 1990, cath lab activity is regulated in the registries published by the Interventional Cardiology Association of the Spanish Society of Cardiology (ACI-SEC).1 In the annual publications of these registries there is a small section dedicated to congenital heart disease in the adult population with details on the activity performed, but without an in-depth analysis of the outcomes, complications or mortality.
The ACI-SEC and the Spanish Society of Pediatric Cardiology and Congenital Heart Disease Working Group on Hemodynamics have joined forces—for the first time in our country—to conduct a registry of the procedures performed in patients of all ages with congenital heart disease since the fetal stage until the adult age with the collaboration of pediatric and adult cardiologists.2 The Spanish Society of Cardiovascular and Endovascular Surgery has recently published a registry of surgical procedures performed in patients with congenital heart disease from 2019, and retrospectively, of the past 8 years.3 Therefore, we should mention that, to this date, in Spain, we have surgical and percutaneous activity registries in the specific field of congenital heart disease.
In the first official report from the Spanish Cardiac Catheterization in Congenital Heart Diseases Registry—recently published in REC: Interventional Cardiology—Ballesteros Tejerizo et al.2 reported the activity of 16 public centers, 7 of which have exclusive dedication to pediatric patients in an unaudited voluntary registry through an online database. The number of participant centers can be representative of pediatric activity. However, this seems like a very low number of hospitals to be representative enough of the activity developed in adult congenital heart disease. As a matter of fact, by 2014, there were already 24 PCI-capable centers with specialized consultations on the management of congenital heart diseases.4 Also, other centers perform interventional procedures to treat simple congenital heart disease like interatrial shunt and patent foramen ovale closures without specialized consultations. These 2 aspects can explain the huge difference seen between the ACI-SEC national registry that reported a total of 1341 interventional procedures performed in the adult population compared to the 367 procedures reported in this registry.2,5 This low representativity of interventional procedures in adult patients should be included in future registries to get an actual snapshot of the activity performed in this field.
The higher rate of almost 5% reported in the activity performed in the management of congenital heart disease in 2000—the pandemic year—compared to 2019 is not easy to explain either even though fewer hospitals (2) participated that year. This contrasts with other registries—like the Italian one—where 6 out of the 11 participant centers saw how their activity dropped over 50%, especially among adults and teenagers.6 Also, during the pandemic, patients with congenital heart disease were a susceptible population with a higher morbidity and mortality risk due to appointment cancellations, and delays in the diagnosis and treatment of complications.7
Another significant and debatable aspect is the definition of congenital heart disease included in the registry. The patent foramen ovale can be present in over 25% of the general population and has become part of the interventional procedures performed to treat congenital heart disease. In fact, it is the most prevalent one among the adult population. However, interventional procedures to treat bicuspid aortic valve— considered the most common congenital heart disease and present in 1% to 2% of the general population—was not included in this registry. Although valve disease following calcification often occurs at a younger age compared to the tricuspid aortic valve—at around 50 to 60 years old—current registries show that transcatheter aortic valve implantation is performed in 4% to 5% of the patients with bicuspid aortic valve.8 Also, bearing in mind that 4241 percutaneous aortic valves were implanted in Spain, it somehow seems logical to agree that nearly 190 were implanted in the bicuspid aortic valve, but this figure was never reported in this registry.
The pulmonary angioplasty section includes interventional procedures on pulmonary branches, the native right ventricular outflow tract, and prosthetic valve conduits. These are different procedures regarding complexity and potential complication, which is why they should be included in separate sections.
We should mention the low rates of serious complications (2%), and mortality (0.1%) reported, which are similar to the ones reported by the best international registries. The exception to this was interventricular shunt closure with a high rate of complications reported—over 10%—which emphasizes the technical difficulty of this procedure.
The availability of a national registry on pediatric percutaneous procedures and adult congenital procedures with a prospective database is essential for patients, and their families. Also, for doctors who treat and advice patients on the risks and outcomes of a given procedure, and for interventional cardiologists to improve their clinical practice. It allows us to analyze results and draw comparisons among hospitals, autonomous communities, and even countries to eventually implement process management upgrades.
Also, this is closely associated with the need for establishing the optimal conditions to perform percutaneous procedures to treat congenital heart disease, and with the accreditation of both centers and interventional cardiologists. A consensus document was drafted on the need to establish the infrastructure standards and experience that both centers and interventional cardiologists should have by the different working groups and associations on pediatric and adult congenital heart disease, and the European Society of Cardiology.9 Two different levels of centers were established based on the number of procedures performed each year. Therefore, in level 1 centers, the lead operator should perform, at least, 70 procedures with, at least, 10 percutaneous valve implantations, 10 angioplasties, and stenting to treat coarctations of the aorta, pulmonary arteries, surgical conduits or baffles (for a total of 10 in any of these areas). Also, the second operator should perform ≥ 30 procedures (over 100 in 1 year). Level 2 centers would need to perform over 60 procedures each year. Although these figures can seem arbitrary, it is essential to perform a high volume of procedures per center, and have onsite cardiac surgery teams expert in the management of congenital heart disease to solve potential and emergency complications that may arise. Heart teams including several specialties and experience in this field are required too. Therefore, this type of procedures should only focus on interventional cardiologists and experienced centers to obtain optimal results.
The future of structural and congenital heart procedures is guaranteed and looks bright thanks to the technological advances made in this field that will invariably improve the quality of life and increase the survival rate of patients with heart disease. Although there is large room for improvement in the quality of data curation for future registries, the first Spanish registry on interventional procedures for the management of congenital heart disease is a major first leap that should be interpreted as a snapshot of the state of interventional procedures to treat congenital heart disease, the activity, and results obtained nationwide. This will help standardize procedures and obtain excellent results.
FUNDING
None whatsoever.
CONFLICTS OF INTEREST
None reported.
REFERENCES
1. Romaguera R, Ojeda S, Cruz-González I, Moreno R. Registro español de hemodinámica y cardiología intervencionista. XXX informe oficial de la Asociación de Cardiología Intervencionista de la Sociedad Española de Cardiología (1990-2020) en el año de la pandemia de la COVID-19. Rev Esp Cardiol. 2021;74:1096-1106.
2. Ballesteros Tejerizo F, Coserría Sánchez F, Romaguera R, et al. Registro Español de Intervencionismo en Cardiopatías Congénitas. Primer Informe Oficial de la ACI-SEC y el GTH-SECPCC (2020). REC Interv Cardiol. 2022;4(3):173-180.
3. Polo López L, Centella Hernández T, Cuerpo Caballero G, et al. Registro de intervenciones en pacientes con cardiopatía congénita de la Sociedad Española de Cirugía Cardiovascular y Endovascular:2019 y retrospectiva de los últimos 8 años. Cir Cardiov. 2021;28:151-161.
4. Oliver Ruiz JM, Dos SubiráL, González-García A, Rueda Soriano J, Ávila Alonso P, Gallego P. Cardiopatías congénitas del adulto en España:estructura, actividad y características clínicas. Rev Esp Cardiol. 2020;73:804-811.
5. Sociedad Española de Cardiología, Asociación de Cardiología Intervencionista. Romaguera R, Ojeda S, Cruz I, Moreno R. Registro Nacional de Actividad en Cardiología Intervencionista 2020 año de la pandemia COVID-19. 2021. Available online: https://www.hemodinamica.com/cientifico/registro-de-actividad/. Accessed 3 Feb 2022.
6. Castaldi B, Sirico D, Meliota G, et al. Impact of hard lockdown on interventional cardiology procedures in congenital heart disease:a survey on behalf of the Italian Society of Congenital Heart Disease. J Cardiovasc Med. 2021;22:701-705.
7. Gallego P, Ruperti-Repilado FJ, Schwerzmann M. Adultos con cardiopatía congénita durante la pandemia de COVID-19:¿población de riesgo?Rev Esp Cardiol. 2020;73:795-798.
8. Makkar R, Yoon S-H, Chakravarty T, et al. Replacement for bicuspid vs tricuspid aortic stenosis and mortality or stroke among patients at low surgical risk. JAMA. 2021;326:1034-1044.
9. Chessa M, Baumgartner H, Michel-Behnke I, et al. ESC Working Group Position Paper:Transcatheter adult congenital heart disease interventions:organization of care –recommendations from a Joint Working Group of the European Society of Cardiology (ESC), European Association of Pediatric and Congenital Cardiology (AEPC), and the European Association of Percutaneous Cardiac Intervention (EAPCI). Eur Heart J. 2019;40:1043-1048.
Severe tricuspid regurgitation (TR) is known to be independently associated with adverse prognosis.1 Its importance is further emphasized by the prevalence of this entity, particularly in the aging population.2 Until the past few years, surgical management was the only available effective treatment for isolated severe TR. Operative mortality, nonetheless, remains high.3 Moreover, surgery is associated with a 45% recurrence rate after 5 years,4 and recent data suggest that surgery may not improve the survival rate in isolated severe cases of TR.5 Accordingly, guidelines recommend TR surgery as a class 1 indication only with concomitant left-sided valvular surgery.6 Conversely, patients with prohibitive surgical risk managed conservatively were shown to have dismal outcomes.7 Fortunately, the emergence of percutaneous devices expanded the horizon of TR treatment.
TACKLING THE MULTI-MECHANISTIC PATHOLOGY
TR is the product of various pathophysiological mechanisms including right ventricular (RV) dilatation with consequential leaflet tethering, tricuspid annular dilatation with subsequent mal-coaptation, atrial fibrillation causing further annular dilation through atrial enlargement, and abnormalities in the tricuspid valve leaflets and apparatus. At a certain point, regurgitation itself becomes an etiology through a vicious circle of RV and atrial remodeling. Accordingly, numerous percutaneous devices are being developed for transcatheter tricuspid valve repair (TTVr) and replacement (TTVR). They can be classified according to their mechanism—leaflet approximation, direct suture or ring annuloplasty and valve implantation that can be orthotopic—implantation in the anatomic tricuspid location or heterotopic implantation in the cavoatrial junction. The long-term stability of the orthotopic valve may be compromised due to the structural changes of the valvular apparatus over time that faces increased postoperative afterload. Some valves minimize this effect by not entirely relying on radial forces such as the Lux-Valve (Ningbo Jenscare Biotechnology, China) that attaches to the septum. Of note, a shortcoming of the Lux-valve is the thoracotomy requirement. An approach to relief RV pressure due to increased afterload is the Trisol valve (TriSol Medical, Israel) that induces leaflet coaptation by using a dome-shaped structure that enables larger RV closing volume. Additional orthotopic valves like the Intrepid (Medtronic, United States)—that received the Food and Drug Administration Breakthrough Device designation—and the Evoque (Edwards Lifesciences, United States) are showing encouraging results. Heterotopic valve implantation had been reported as double-valve implantation (inferior and superior vena cava), as well as single valve solely into the inferior vena cava. Nevertheless, the vena cavae can show substantial dynamic variations in size as well, particularly in self-expanding valves applying radial force on the compliant vessel, whereas right atrial enlargement can generate a funnel-shaped cavoatrial junction, thus increasing the risk of valve migration. Since the procedure results in the ventricularization of the right atrium, persistent overload can cause morphological changes and adversely impact the cardiac function.
Additional anatomical considerations play a role in device selection including the course of the right coronary artery, proximity to the atrioventricular node, and or/bundle of His (in this regard, the NaviGate valve—NaviGate Cardiac Structures, United States—has short graspers, and termed atrial winglets to prevent compression of the conduction system8), the dimensions of the inferior vena cava and its orientation relative to the right atrium (that can impact device stability and introduce difficulties achieving coaxiality), the size of the tricuspid annulus (large annuli may be modified as with the TriCinch—4Tech Cardio, Ireland—,9 that mimics the Kay procedure), the distance between the tricuspid annulus and the RV apex, the size of the iliac vein (eg, the Cardiovalve—Cardiovalve, Israel—and the Evoque utilize a relatively low profile), the presence of an intracardiac shunt, short leaflet length (with an insufficient grasping area), thickened nonpliable leaflets, coaptation gap and depth (usually over ~7mm is a predictor of leaflet approximation failure), tethering severity, the tenting area and height, the location of the regurgitant jet, the number of leaflets (eg, 3 or 4), a proper annular shelf, and the juxtaposed noncoronary sinus relative to the device anchoring to the anterior and septal leaflet commissures (that may be compromised by the anchoring mechanism).
Multiple factors must, therefore, be taken into observed when considering a patient for TTVr/TTVR, and a thorough evaluation is warranted. In advanced stages of the disease manifesting as severe RV enlargement, considerable tethering or very large annuli, TTVr and TTVR may not be technically feasible. The anatomical characteristics can exceed the device capabilities, eg, the maximum length of Cardioband (Edwards Lifesciences, United States) tricuspid system is 120 mm. Even if the intervention is technically possible with advanced disease, irreversible damage to the RV structure and function may impede clinical improvement. Nevertheless, the point of no return is not clear, and patients with RV dysfunction and pulmonary hypertension have the potential to improve.10
Finally, the patient’s characteristics must be reviewed as certain trials excluded patients with specific comorbidities like chronic kidney disease or systolic pulmonary artery pressure > 70 mmHg.
OUTCOMES OF PERCUTANEOUS TRICUSPID VALVE INTERVENTIONS
TriValve is the largest TTVr registry available predominantly describing edge-to-edge repair with MitraClip (Abbott Vascular, United States) in the tricuspid position. Use of MitraClip was described as bicuspidization of the tricuspid valve,11 by approximating the posterior or anterior leaflet to the septal leaflet (while anterior to the posterior leaflet clipping may distort the valvular apparatus). Devices operating with similar mechanisms are the TriClip (Abbott Structural Heart, United States), and the PASCAL (Edwards Lifesciences, United States).
Patients from studies describing the various devices were heterogenous and vary significantly from one to the other. Also, the 30-day mortality rate ranges from 0% to 13% with significant improvement in TR grade, and in the quality of life according to published data (table 1). Notable longer-term outcomes include a 20% mortality rate at 1-year follow-up in the MitraClip cohort,12 and a 26% mortality rate after 2 years in the Cardioband cohort.18 Currently, prospective efficacy data mostly rely on quality-of-life scores and TR grade. However, it was shown that patients with procedural failure have a significantly higher mortality rate,30 suggestive that patients would fare worse without the procedure. Moreover, a registry-based propensity-matched study that compared TTVr to conservative treatment showed symptomatic and survival benefits.31 Currently, 3 TTVr devices have received the CE marking: Cardioband, TriClip, and PASCAL system for the management of TR, but none have been approved by the U.S. Food and Drug Administration. Consequently, most percutaneous tricuspid interventions are applicable in trial settings or as compassionate use.
Table 1. Outcome data with transcatheter tricuspid valve devices
| Leaflet approximation devices | Annuloplasty devices | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Device (company) or procedure | MitraClip (Abbott Vascular, United States)12 | TriClip (Abbott Structural Heart, United States)13 | PASCAL (Edwards Lifesciences, United States)14 | TriCinch (4Tech Cardio, Ireland)9 | Mistral (Mitralix, Israel)15 | FORMA (Edwards Lifesciences, United States)16 | Millipede (Boston Scientific, United States)17 | Cardioband (Edwards Lifesciences, United States)18,19 | Trialign (Mitralign, United States)20 | PASTA21 | |
| No. | 249 | 85 | 34 | 1 | 7 | 29 | 2 | 30 | 15 | 1 | |
| TR grade improvement after 30 days | Yes | Yes | Yes | Yes | Yes | Yes | |||||
| Significant improvement in QoL measures after 30 days | Yes | Yes | Yes | No | Yes | ||||||
| Mortality after 30 days | 0% | 0% | 0% | 6.7% | 0% | ||||||
| TR grade improvement after 6 months | Yes | Yes | Yes | ||||||||
| Significant improvement in QoL measures after 6 months | Yes | Yes | Yes | No | |||||||
| Mortality after 6 months | 5% | 0% | 10% | 0% | |||||||
| TR grade improvement after 1 year | Yes | Yes | Yes (N = 16) | ||||||||
| Significant improvement in QoL measures after 1 year | Yes | Yes | Yes (N = 16) | ||||||||
| Mortality after 1 year | 20% | 7.1% | 0% (N = 16) | ||||||||
| TR grade improvement after 2 years | Yes | ||||||||||
| Significant improvement in QoL measures after 2 years | Yes | ||||||||||
| Mortality after 2 years | 26.7% | ||||||||||
| Orthotopic valve implantation | Annuloplasty devices | ||||||||||
| Device (company) | NaviGate (NaviGate Cardiac Structures, United States)22 | Trisol (TriSol Medical, Israel)23,* | Lux-Valve (Ningbo Jenscare Biotechnology, China)24,25 | Evoque (Edwards Lifesciences, United States)26 | Cardiovalve (Cardiovalve, Israel)27 | Intrepid (Medtronic, United States), NCT04433065* | TricValve (P+F Products + Features, Austria)28 | Sapien in ring (Edwards Lifesciences, United States)28 | Tricento (New Valve Technology, Switzerland)29 | ||
| No. | 5 | 12 | 25 | 1 | 7 | 14 | 1 | ||||
| TR grade improvement after 30 days | Yes | Yes | |||||||||
| Significant improvement in QoL measures after 30 days | Yes | Yes | |||||||||
| Mortality after 30 days | 8.3% | 0% | |||||||||
| TR grade improvement after 6 months | Yes | ||||||||||
| Significant improvement in QoL measures after 6 months | Yes | Yes | |||||||||
| Mortality after 6 months | 0% | 0% | |||||||||
| TR grade improvement after 1 year | Yes | No | |||||||||
| Significant improvement in QoL measures after 1 year | Yes | No | |||||||||
| Mortality after 1 year | 16.6% | 57% | |||||||||
| TR grade improvement after 2 years | Yes | ||||||||||
| Significant improvement in QoL measures after 2 years | Yes | ||||||||||
| Mortality after 2 years | 0% | ||||||||||
|
* No clinical follow-up data available yet. QoL, quality of life; TR, tricuspid regurgitation. |
|||||||||||
In conclusion, TTVR/TTVr devices are becoming considerably more viable options. Surgical, and most certainly, conservative treatment of patients with severe TR are not ideal choices and expanding treatment possibilities towards percutaneous approaches is, therefore, an obvious choice. Results from ongoing trials have been anticipated, which will hopefully be followed by the approval of additional devices.
FUNDING
None whatsoever.
CONFLICTS OF INTEREST
A. Latib has served on advisory boards or as consultant for Medtronic, Boston Scientific, Edwards Lifesciences, Abbott, and VDYNE. The remaining authors have no relations to disclose.
REFERENCES
1. Chorin E, Rozenbaum Z, Topilsky Y, et al. Tricuspid regurgitation and long-term clinical outcomes. Eur Heart J Cardiovasc Imaging. 2020;21:157-165.
2. Vassileva CM, Shabosky J, Boley T, Markwell S, Hazelrigg S. Tricuspid valve surgery:the past 10 years from the Nationwide Inpatient Sample (NIS) database. J Thorac Cardiovasc Surg. 2012;143:1043-1049.
3. Zack CJ, Fender EA, Chandrashekar P, et al. National Trends and Outcomes in Isolated Tricuspid Valve Surgery. J Am Coll Cardiol. 2017;70:2953-2960.
4. McCarthy PM, Bhudia SK, Rajeswaran J, et al. Tricuspid valve repair:durability and risk factors for failure. J Thorac Cardiovasc Surg. 2004;127:674-685.
5. Axtell AL, Bhambhani V, Moonsamy P, et al. Surgery Does Not Improve Survival in Patients With Isolated Severe Tricuspid Regurgitation. J Am Coll Cardiol. 2019;74:715-725.
6. Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease:A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143:e72-e227.
7. Pagnesi M, Montalto C, Mangieri A, et al. Tricuspid annuloplasty versus a conservative approach in patients with functional tricuspid regurgitation undergoing left-sided heart valve surgery:A study-level meta-analysis. Int J Cardiol. 2017;240:138-144.
8. Hahn RT, Kodali S, Fam N, et al. Early Multinational Experience of Transcatheter Tricuspid Valve Replacement for Treating Severe Tricuspid Regurgitation. JACC Cardiovasc Interv. 2020;13:2482-2493.
9. Latib A, Agricola E, Pozzoli A, et al. First-in-Man Implantation of a Tricuspid Annular Remodeling Device for Functional Tricuspid Regurgitation. JACC Cardiovasc Interv. 2015;8:e211-214.
10. Muntane-Carol G, Taramasso M, Miura M, et al. Transcatheter Tricuspid Valve Intervention in Patients With Right Ventricular Dysfunction or Pulmonary Hypertension:Insights From the TriValve Registry. Circ Cardiovasc Interv. 2021;14:e009685.
11. Latib A, Ancona MB, Agricola E, et al. Percutaneous Bicuspidization of the Tricuspid Valve. JACC Cardiovasc Imaging. 2017;10:488-489.
12. Mehr M, Taramasso M, Besler C, et al. 1-Year Outcomes After Edge-to-Edge Valve Repair for Symptomatic Tricuspid Regurgitation:Results From the TriValve Registry. JACC Cardiovasc Interv. 2019;12:1451-1461.
13. Lurz P, Stephan von Bardeleben R, Weber M, et al. Transcatheter Edge-to-Edge Repair for Treatment of Tricuspid Regurgitation. J Am Coll Cardiol. 2021;77:229-239.
14. Kodali S, Hahn RT, Eleid MF, et al. Feasibility Study of the Transcatheter Valve Repair System for Severe Tricuspid Regurgitation. J Am Coll Cardiol. 2021;77:345-356.
15. Planer D, Beeri R, Danenberg HD. First-in-Human Transcatheter Tricuspid Valve Repair:30-Day Follow-Up Experience With the Mistral Device. JACC Cardiovasc Interv. 2020;13:2091-2096.
16. Muntane-Carol G, Del Val D, Bedard E, Philippon F, Rodes-Cabau J. Transcatheter innovations in tricuspid regurgitation:FORMA device. Prog Cardiovasc Dis. 2019;62:496-499.
17. Rogers JH, Boyd WD, Bolling SF. Tricuspid annuloplasty with the Millipede ring. Prog Cardiovasc Dis. 2019;62:486-487.
18. Nickenig G, Weber M, Schuler R, et al. Tricuspid valve repair with the Cardioband system:two-year outcomes of the multicentre, prospective TRI-REPAIR study. EuroIntervention. 2021;16:e1264-e1271.
19. Davidson CJ, Lim DS, Smith RL, et al. Early Feasibility Study of Cardioband Tricuspid System for Functional Tricuspid Regurgitation:30-Day Outcomes. JACC Cardiovasc Interv. 2021;14:41-50.
20. Hahn RT, Meduri CU, Davidson CJ, et al. Early Feasibility Study of a Transcatheter Tricuspid Valve Annuloplasty:SCOUT Trial 30-Day Results. J Am Coll Cardiol. 2017;69:1795-1806.
21. Greenbaum AB, Khan JM, Rogers T, et al. First-in-human transcatheter pledget-assisted suture tricuspid annuloplasty for severe tricuspid insufficiency. Catheter Cardiovasc Interv. 2021;97:E130-E134.
22. Hahn RT, George I, Kodali SK, et al. Early Single-Site Experience With Transcatheter Tricuspid Valve Replacement. JACC Cardiovasc Imaging. 2019;12:416-429.
23. Vaturi M, Vaknin-Assa H, Shapira Y, et al. First-in-Human Percutaneous Transcatheter Tricuspid Valve Replacement With a Novel Valve. JACC Case Rep. 2021;3:1281-1286.
24. Ning XP, An Z, Qiao F, et al. [Safety and efficacy of transcatheter tricuspid valve replacement with LuX-Valve in patients with severe tricuspid regurgitation]. Zhonghua Xin Xue Guan Bing Za Zhi. 2021;49:455-460.
25. Sun Z, Zhang Z, Li H, et al. Twelve-month Outcomes of the LuX-Valve for Transcatheter Treatment of Severe Tricuspid Regurgitation. EuroIntervention. 2021;17:818-826.
26. Fam NP, von Bardeleben RS, Hensey M, et al. Transfemoral Transcatheter Tricuspid Valve Replacement With the EVOQUE System:A Multicenter, Observational, First-in-Human Experience. JACC Cardiovasc Interv. 2021;14:501-511.
27. Aoi S, Wiley J, Ho E, Goldberg Y, Chau M, Latib A. Transcatheter tricuspid valve implantation with the Cardiovalve system. Future Cardiol. 2021;17:963-969.
28. Lauten A, Figulla HR, Unbehaun A, et al. Interventional Treatment of Severe Tricuspid Regurgitation:Early Clinical Experience in a Multicenter, Observational, First-in-Man Study. Circ Cardiovasc Interv. 2018;11:e006061.
29. Montorfano M, Beneduce A, Ancona MB, et al. Tricento Transcatheter Heart Valve for Severe Tricuspid Regurgitation:Procedural Planning and Technical Aspects. JACC Cardiovasc Interv. 2019;12:e189-e191.
30. Taramasso M, Alessandrini H, Latib A, et al. Outcomes After Current Transcatheter Tricuspid Valve Intervention:Mid-Term Results From the International TriValve Registry. JACC Cardiovasc Interv. 2019;12:155-165.
31. Taramasso M, Benfari G, van der Bijl P, et al. Transcatheter Versus Medical Treatment of Patients With Symptomatic Severe Tricuspid Regurgitation. J Am Coll Cardiol. 2019;74:2998-3008.
Although mechanical reperfusion has been shown to achieve epicardial recanalization in almost all acutely occluded arteries, the optimal myocardial reperfusion still remains a major issue, and it is only achieved in barely 50% to 70% of the patients with ST- segment elevation myocardial infraction (STEMI).1 Several factors have been demonstrated to have an impact on myocardial reperfusion including preoperative Thrombolysis in Myocardial Infarction (TIMI) flow, ischemia time, ageing, diabetes, thrombus burden, and vessel size.1-3 Therefore, over the last few decades, several studies have been conducted on adjunctive therapies and devices to improve reperfusion such as antithrombotic therapies,4-5 and thrombectomy.6
The use of coronary stents, in particular drug-eluting stents, currently represents the standard of care,7 and considerable attention has been paid over the last decade on stenting techniques,8 and their impact on procedural results and outcomes.
In a paper recently published in REC: Interventional Cardiology, Vega et al.9 conducted a randomized trial to address the impact of the delivery system speed deflation on myocardial perfusion and the outcomes of patients with STEMI treated with direct stenting.
In fact, fast balloon deflation has been suggested to cause abrupt changes in coronary flow that may trigger the detachment of thrombotic material, and plaque fragments, disrupted by the stent strut coverage.10 Also, variations of intravascular pressure may increase the wall shear stress, which has been shown to promote plaque destabilization, endothelial dysfunction,11 and also the hydrostatic pressure inside the interstitial space favoring myocardial oedema, and cellular damage.12
Indeed, in post-conditioning strategies, balloon inflation has been proposed as a mechanism of protection against ischemic damage by inducing repeated sequences of ischemia-reperfusion that have been proven to reduce the infarct size.13
In a previous randomized study that recruited 211 patients, Gu et al.14 reported an improvement of coronary flow with the stent delivery system slow deflation strategy, yet with a not significant reduction of the no-reflow phenomenon, and null effects on the long-term outcomes.
However, this study primary endpoint was the corrected TIMI frame count, an index of coronary flow, whereas Vega et al.9 assessed the myocardial blush grade (MBG) and the ST-resolution, both parameters of myocardial perfusion that may be conditioned by several other factors.
In fact, the Spanish study9 was consistent with previous larger reports,8 and diabetes, hypertension, kidney disease, hemodynamic parameters, and lesion location emerged as independent predictors of MBG. Therefore, it may be argued that slow balloon deflation may have facilitated a successful epicardial reperfusion, although this did not translate into microcirculation differences or myocardial salvage.
Furthermore, although the extensive use of thrombectomy and glycoprotein IIb/IIIa inhibitors in the overall cohort of patients, as the authors very well pointed out, is not representative of the current guidelines-indicated strategies regarding primary percutaneous coronary intervention (PCI), it may have prevented such complications and minimized any potential benefits with the delivery system slow deflation strategy. Indeed, former studies and meta-analyses have demonstrated that the administration of these potent antiplatelet agents during a primary PCI, mainly as a downstream strategy,15 could translate into better myocardial perfusion, and reduce mortality.
In addition, the recruitment restriction to those lesions eligible for direct stenting in the study conducted by Vega et al.9 may have led to the selection of a very low-risk population where the occurrence of an impaired MBG was extremely low (observed in about 25% of the study population).
Finally, since the study was stopped after the recruitment of 50% of the predefined sample size for futility, we cannot discard that, with a larger population and different endpoints, any differences would have emerged. Future larger studies with a more heterogeneous and higher-risk population of patients with STEMI, more complex lesions, a higher rate of comorbidities, less extensive use of glycoprotein IIb/IIIa inhibitors, are certainly justified to better define the potential role of slow balloon deflation during primary PCI in terms of periprocedural complications, myocardial reperfusion, short- and long-term outcomes.
FUNDING
None whatsoever.
CONFLICTS OF INTEREST
None declared.
REFERENCES
1. De Luca G, van 't Hof AW, Ottervanger JP, et al. Unsuccessful reperfusion in patients with ST-segment elevation myocardial infarction treated by primary angioplasty. Am Heart J. 2005;150:557-562.
2. De Luca G, van 't Hof AW, Ottervanger JP, et al. Ageing, impaired myocardial perfusion, and mortality in patients with ST-segment elevation myocardial infarction treated by primary angioplasty. Eur Heart J. 2005;26:662-666.
3. Timmer JR, van der Horst IC, de Luca G, et al.;Zwolle Myocardial Infarction Study Group. Comparison of myocardial perfusion after successful primary percutaneous coronary intervention in patients with ST-elevation myocardial infarction with versus without diabetes mellitus. Am J Cardiol. 2005;95:1375-1377.
4. De Luca G, Navarese EP, Cassetti E, Verdoia M, Suryapranata H. Meta-analysis of randomized trials of glycoprotein IIb/IIIa inhibitors in high-risk acute coronary syndromes patients undergoing invasive strategy. Am J Cardiol. 2011;107:198-203.
5. Verdoia M, Schaffer A, Barbieri L, et al. Benefits from new ADP antagonists as compared with clopidogrel in patients with stable angina or acute coronary syndrome undergoing invasive management:a meta-analysis of randomized trials. J Cardiovasc Pharmacol. 2014;63:339-350.
6. De Luca G, Navarese EP, Suryapranata H. A meta-analytic overview of thrombectomy during primary angioplasty. Int J Cardiol. 2013;166:606-612.
7. Di Lorenzo E, Sauro R, Varricchio A, et al. Randomized comparison of everolimus-eluting stents and sirolimus-eluting stents in patients with ST elevation myocardial infarction:RACES-MI trial. JACC Cardiovasc Interv. 2014;7:849-856.
8. Capozzolo C, Piscione F, De Luca G, et al. Direct coronary stenting:effect on coronary blood flow, immediate and late clinical results. Catheter Cardiovasc Interv. 2001;53:464-473.
9. Vega B, Pérez de Prado A, Rondán J, et al. Deflation speed of the stent delivery system and primary angioplasty results:a randomized study. REC Interv Cardiol. 2022;4(2):91-98.
10. Hong YJ, Jeong MH, Choi YH, et al. Impact of plaque components on no-reflow phenomenon after stent deployment in patients with acute coronary syndrome:a virtual histology-intravascular ultrasound analysis. Eur Heart J. 2011;32:2059-2066.
11. Han D, Starikov A, ÓHartaigh B, et al. Relationship Between Endothelial Wall Shear Stress and High-Risk Atherosclerotic Plaque Characteristics for Identification of Coronary Lesions That Cause Ischemia:A Direct Comparison With Fractional Flow Reserve. J Am Heart Assoc. 2016;5:e004186.
12. Garcia-Dorado D, Oliveras J. Myocardial oedema:a preventable cause of reperfusion injury?Cardiovasc Res. 1993;27:1555-1563.
13. Xing Z, Tang L, Huang J, Peng X, Hu X. Effects of ischaemic postconditioning on outcomes of patients with ST-segment elevation myocardial infarction who underwent primary percutaneous coronary intervention:a meta-analysis. BMJ Open. 2019;9:e022509.
14. Gu J, Zhuo Y, Liu TJ, et al. Balloon Deflation Strategy during Primary Percutaneous Coronary Intervention in Acute ST-Segment Elevation Myocardial Infarction:A Randomized Controlled Clinical Trial and Numerical Simulation-Based Analysis. Cardiol Res Pract. 2020;2020:4826073.
15. De Luca G, Smit JJ, Ernst N, et al. Impact of adjunctive tirofiban administration on myocardial perfusion and mortality in patients undergoing primary angioplasty for ST-segment elevation myocardial infarction. Thromb Haemost. 2005;93:820-823.
Blood pressure indices like fractional flow reserve (FFR) and the instantaneous wave-free ratio (iFR) measure the distal-to-aortic pressure ratio of a lesion in a similar way using a pressure guidewire. FFR requires the induction of maximal hyperemia while the iFR is an index based on the assessment of pressure curves at rest. There is solid confirmation of the validity and efficacy of such indices from multiple studies while their use is widely backed by the clinical practice guidelines.1
However, the efficacy of blood pressure indices is based on 2 fundamental objectives. The first one is to prove that they are useful to indicate the revascularization of a lesion when the resulting value is below the cut-off point (≤ 0.80 for the FFR, and ≤ 0.89 for the iFR). The second one is to demonstrate that lesions with values above the cut-off point (> 0.80 or > 0.89, respectively) have a low risk of requiring revascularization, and lack of ischemic events at follow-up. Conceptually, the first indication is clear: a lesion with pathological values is an obstructive lesion that causes ischemia now. Therefore, if clinically indicated (and technically feasible) the best thing to do is to revascularize such a lesion. The second indication has a higher degree of uncertainty because a lesion that does not cause ischemia (now) does not necessarily mean that it will not cause it at some point in the future. This is so because the capacity of blood pressure indices (with normal results) to detect vulnerable plaques is limited, and they show a greater tendency towards progression or destabilization. Therefore, the negative predictive value of pressure indices is supposed to be worse when used in patients with a higher risk of progression into atherosclerotic cardiovascular disease.
Proof of this is that recent studies conducted in patients with acute myocardial infarction (AMI) and multivessel disease have demonstrated that the FFR of non-culprit lesions does not provide any clinical benefits regarding the revascularization of such lesions estimated visually and, therefore, under angiographic guidance.2,3 The prevalence of vulnerable plaques causing visual stenosis between 50% and 69% in non-culprit vessels of patients with AMI is estimated at around 30%. Actually this rate is probably higher when lesions with a greater degree of angiographic obstruction are studied.4 The COMBINE OCT-FFR trial also reported a similar rate of vulnerable plaques (25%) on the optical coherence tomography performed in diabetic patients with angiographic stenoses between 40% and 80% and normal FFR values.5 In this study, angiographic lesions with normal FFR values associated with vulnerable plaques had more adverse events (cardiac death, target vessel myocardial infarction, and clinically driven target lesion revascularization) compared to those not associated with vulnerable plaques (13% vs 3%; P < .001).
The article by Castro-Mejía et al.6 recently published in REC: Interventional Cardiology provides the mid-term follow-up (3.5 years) of a large single-center registry of patients with intermediate angiographic lesions assessed using blood pressure indices and with normal results. This study compared the adverse events between diabetic and nondiabetic patients with the necessary statistical adjustments to compensate for the inherent differences of the baseline characteristics of both groups. The authors conclude that blood pressure indices (FFR and iFR) had a similar efficacy in diabetic and nondiabetic patients for not predicting future target vessel myocardial infarctions or the need for target lesion revascularization. However, we should mention that diabetic patients had higher rates of all-cause mortality, AMI, and need for revascularization compared to nondiabetic patients.6 We should specifically mention that there was a 2.6-fold higher risk of infarction in any vessels at the follow-up in diabetic compared to nondiabetic patients (P < .063 after adjustments). However, only 13% of the AMIs registered were adjudicated to the study vessel in diabetic vs 38% in nondiabetic patients.6 This difference can be due to chance alone or to the fact that the lesions that cause AMIs at the follow-up are scarcely susceptible to be studied with a pressure guidewire at the index procedure in diabetic patients. It is well known that these patients have more diffuse atherosclerotic cardiovascular disease, which complicates the correct angiographic assessment of the lesions (and, also, prevents the use of a pressure guidewire in such vessel for assessment purposes). It is highly likely that a strategy based on intravascular imaging modalities alone or in combination like in the COMBINE OCT-FFR trial can be of greater utility to detect this type of lesions.
Three recent studies have reported contradictory outcomes regarding the negative predictive value of blood pressure indices in diabetic patients. Kennedy et al.7 found more AMIs and a greater need for target lesion revascularization with normal FFR values in diabetic patients at the 3-year follow-up. On the contrary, Van Belle et al.8 found no differences between diabetic and nondiabetic patients who were deferred (for having normal FFR values) in 2 large registries conducted in Portugal, and France at the 1-year follow-up. No significant differences were seen either in the DEFINE-FLAIR substudy that analyzed adverse events between diabetic and nondiabetic patients in deferred patients at 1-year follow-up.9 It should be interesting to assess the long-term follow-up of these last 2 studies to see if the negative predictive value of blood pressure indices stays the same in diabetic and nondiabetic patients.
Finally, Castro-Mejía et al.6 propose that resting physiological indices with nonpathological findings may have a better negative predictive value compared to the FFR values of diabetic patients. This would be explained by the fact that one of the causes of discrepancy between the FFR and the iFR is microvascular dysfunction (that is more common in diabetic patients). Patients with microvascular dysfunction can have smaller drops of the pressure index during maximal hyperemia since not enough hyperemia is induced. However, resting physiological indices are not hyperemia-dependent, meaning that they should be more sensitive to detect significant epicardial lesions. However, this hypothesis was not confirmed by the DEFINE-FLAIR substudy on deferred diabetic patients.9
In conclusion, diabetic patients still remain as one of the challenges of interventional cardiology because they have more adverse events compared to nondiabetic patients (despite the proper optimal medical therapy). A more aggressive strategy to assess intermediate coronary lesions using pressure guidewires is advisable to guide complete revascularization in diabetic patients. Future studies should also assess whether a mixed strategy with intravascular imaging modalities of nonfunctionally significant arteries can be useful to prevent future events in this group of patients.
FUNDING
None whatsoever.
CONFLICTS OF INTEREST
J. Gómez-Lara has received speaking fees for presentations on behalf of Abbott Vascular, Boston Scientific, and Terumo. R. Romaguera declared no conflicts of interests whatsoever.
REFERENCES
1. Knuuti J, Wijns W, Saraste A, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407-477.
2. Puymirat E, Cayla G, Simon T, et al. Multivessel PCI Guided by FFR or Angiography for Myocardial Infarction. N Engl J Med. 2021;385:297-308.
3. Denormandie P, Simon T, Cayla G, et al. Compared Outcomes of ST-Elevation Myocardial Infarction Patients with Multivessel Disease Treated with Primary Percutaneous Coronary Intervention and Preserved Fractional Flow Reserve of Non-Culprit Lesions Treated Conservatively and of Those with Low Fractional Flow Reserve Managed Invasively:Insights from the FLOWER MI trial. Circ Cardiovasc Interv. 2021. https://doi.org/10.1161/CIRCINTERVENTIONS.121.011314.
4. Pinilla-Echeverri N, Mehta SR, Wang J, et al. Nonculprit Lesion Plaque Morphology in Patients With ST-Segment-Elevation Myocardial Infarction:Results From the COMPLETE Trial Optical Coherence Tomography Substudys. Circ Cardiovasc Interv. 2020;13:e00≀.
5. Kedhi E, Berta B, Roleder T, et al. Thin-cap fibroatheroma predicts clinical events in diabetic patients with normal fractional flow reserve:the COMBINE OCT-FFR trial. Eur Heart J. 2021;42(45):4671-4679.
6. Castro-Mejía AF, Travieso-González A, Núñez-Gil IJ, et al. Diabetes mellitus and long-term safety of FFR and iFR-based coronary revascularization deferral. REC Interv Cardiol. 2022;4(2):99-106.
7. Kennedy MW, Kaplan E, Hermanides RS, et al. Clinical outcomes of deferred revascularisation using fractional flow reserve in patients with and without diabetes mellitus. Cardiovasc Diabetol. 2016;15:100.
8. Van Belle E, Cosenza A, Baptista SB, et al. Usefulness of Routine Fractional Flow Reserve for Clinical Management of Coronary Artery Disease in Patients With Diabetes. JAMA Cardiol. 2020;5:272-281.
9. DEFINE-FLAIR Trial Investigators, Lee JM, Choi KH, et al. Comparison of Major Adverse Cardiac Events Between Instantaneous Wave-Free Ratio and Fractional Flow Reserve-Guided Strategy in Patients With or Without Type 2 Diabetes:A Secondary Analysis of a Randomized Clinical Trial. JAMA Cardiol. 2019;4:857-864.
- Small margins and big gains: evidence for angioplasty with cutting or scoring balloons in patients with in-stent restenosis
- Limitations of angiography in the detection of target lesion calcium. No significant differences today compared to 1995
- New drug-eluting stents: technological refinement continues
- TAVI in nonagenarians, what do we know so far?
Subcategories
Special articles
Original articles
Editorials
Original articles
Editorials
Post-TAVI management of frail patients: outcomes beyond implantation
Unidad de Hemodinámica y Cardiología Intervencionista, Servicio de Cardiología, Hospital General Universitario de Elche, Elche, Alicante, Spain
Original articles
Debate
Debate: Does the distal radial approach offer added value over the conventional radial approach?
Yes, it does
Servicio de Cardiología, Hospital Universitario Sant Joan d’Alacant, Alicante, Spain
No, it does not
Unidad de Cardiología Intervencionista, Servicio de Cardiología, Hospital Universitario Galdakao, Galdakao, Vizcaya, España