Available online: 09/04/2019
Editorial
REC Interv Cardiol. 2020;2:310-312
The future of interventional cardiology
El futuro de la cardiología intervencionista
Emory University School of Medicine, Atlanta, Georgia, United States
Ischemic postconditioning (iPost) was first described in 2003 as a strategy capable of reducing the size of infarction after prolonged coronary occlusion in dogs through the immediate application of reperfusion after 3 cycles of 30 seconds of coronary reocclusion followed by 30 seconds of reperfusion.1 These results were soon confirmed independently, and the potential mechanisms involved described including, among others, a delayed normalization of pH levels, less accumulation of intracellular calcium, inhibition of the mitochondrial permeability transition pore, and less oxidative stress.2 Compared to the robust protective effect of ischemic preconditioning, it was confirmed that iPost was only beneficial if the procedure started right after reperfusion. However, it was attenuated in elderly subjects or in the presence of comorbidities or certain drug therapies.2,3
Despite these limitations, iPost soon called the attention of interventional cardiologists because it was easy to apply during primary percutaneous coronary intervention. Back in 2005 the very first study ever conducted in humans was published. In this study, iPost reduced the size of creatine kinase release compared to the control group in patients with ST-segment elevation myocardial infarction (STEMI).4 However, successive trials that estimated the size of infarction using similar methods or was more reliably measured by contrast-enhanced cardiac magnetic resonance imaging showed contradictory results. Some of these confirmed iPost protective effect while others revealed the opposite or even less myocardial salvage in patients treated with iPost compared to those who were iPost-naive.5-7 So far, no clinical trial has been able to demonstrate that iPost reduces clinical events. The largest trial ever conducted is the DANAMI-3–iPOST that included 1234 patients with STEMI treated with primary percutaneous coronary intervention within the first 12 hours of disease progression and with the culprit artery occluded at the beginning of the procedure. These patients were randomized to receive iPost or a conventional percutaneous coronary intervention.8 After a median of 38 months of follow-up, the rate of the primary endpoint (death or hospitalization due to heart failure) was similar in both the iPost and the control group (10.5% vs 11.2%, respectively; non-significant P value) with no differences being reported in their individual components, other events, ST-segment elevation resolution or in the size of infarction measured by cardiac magnetic resonance imaging in a subgroup. A follow-up meta-analysis confirmed the lack of tangible clinical benefits in iPost in an aggregate population of 3619 patients with STEMI.9
Given these results, clinicians have consequently lost interest in this strategy. Therefore, iPost has not joined the therapeutic arsenal for the management of patients with STEMI. However, the reason behind the contradictory results of the mentioned trials is worth analyzing to identify, if any, subgroups of patients who could benefit from the protective effect of iPost. A possible explanation could be that the benefit of iPost depends on the duration of previous ischemia.10
In an article recently published in REC: Interventional Cardiology, Nuche et al.11 put this hypothesis to the test by comparing the effect of iPost on the size of infarction in a series of pigs undergoing left anterior descending coronary artery occlusion through 30-min balloon inflation (N = 19) to a different series from a previous report12 where occlusion went on for 40 min (N = 10). Except for the duration of ischemia, the experimental protocol was identical. iPost consisted of 4 cycles of balloon reinflation and deflation (1 min each) started 1 min after reperfusion. The area at risk was measured on the contrast-enhanced multidetector computed tomography scan with contrast during ischemia while the size of infarction was measured on the contrast-enhanced cardiac magnetic resonance imaging at 7 days.
iPost did not reduce the size of infarction in animals with 30-min coronary occlusion (0.3% [0.0-3.9] vs 0.9 [0.0-2.6] of left ventricular mass in animals treated with iPost or in the control group, respectively) or 40-min coronary occlusion (31.1% [27.3-32.8] vs 27.3 [25.1-27.5], respectively; both with non-significant P values]). Overall, T1 relaxation times were longer in animals treated with iPost. Authors conclude that iPost did not reduce the size of infarction in any of the 2 series, which goes against the possible interaction between its effect and the duration of previous ischemia. Also, longer T1 relaxation times—a marker of interstitial fibrosis—in animals with iPost suggests potential damage associated with the procedure.
The trial11 comes from a group of researchers with solid experience in the area, it is technically demanding, and has been conducted following a highly sophisticated methodology, for which the authors should be credited. Results go against an interaction between the benefit of iPost and the duration of previous ischemia. However, before this becomes the definitive conclusion, some methodological considerations should be made. In the first place, to assess the effect of any protective procedures, the size of infarction in the control group should have certain variability and, on average, should not be too large or too small.13 However, in this trial, after 30 min of ischemia barely any infarction was reported (3.8% [0.0-8.5] of the area at risk) while after 40 min infarctions were massive (98.2% [70.7-98.8] of the area at risk). Although these ischemia times were selected because they had caused medium-sized infarctions in former trials,14 homogeneity of the infarction size seen in both series and the almost non-existent infarctions in the 30 min series complicate discarding a possible beneficial effect of iPost in the results reported. Secondly, and on this regard too, it was surprising to see that by increasing ischemia time in just 10 min we went from almost non-existent infarctions to infarctions that occupy the entire area at risk. Although the experimental protocol was the same, as both series were conducted in different moments in time, variations in the conditions of the experiment such as animal breed, room temperature, materials used, etc, may have impacted the results and, therefore, cannot be ruled out. In this sense, results stress out the possible setback associated with the use of historic series. Finally, for the lack of a targeted anatomopathological study, a possible explanation for the massive infarctions reported in the 40 min series is that maybe some animals had coronary reocclusions between the end of the experiment and when the size of infarction was estimated 7 days later. Reocclusion is a common occurrence in this experimental model, especially when ischemia has been prolonged, and although the risk of ischemia drops with antiplatelet therapy (3 doses of clopidogrel were used in this trial) it does not go away completely.15
Despite these considerations, the truth is that the results of this study11 do not offer any signs of a potential cardioprotective effect of iPost by changing the ischemia times in this experimental model. This, added to the lack of clinical benefits reported in the previously mentioned trials confirms that, currently, iPost should not be used in patients with STEMI. This anticipates that it will be difficult to find a population of target patients in whom this procedure might be beneficial.
FUNDING
J.A. Barrabés received research funds from Instituto de Salud Carlos III (PI20/01681 project) and Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV) co-financed by the European Regional Development Fund.
CONFLICTS OF INTEREST
None reported.
REFERENCES
1. Zhao ZQ, Corvera JS, Halkos ME, et al. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285:H579-H588.
2. Hausenloy DJ, Barrabes JA, Bøtker HE, et al. Ischaemic conditioning and targeting reperfusion injury: a 30 year voyage of discovery. Basic Res Cardiol. 2016;111:70.
3. Kin H, Zhao ZQ, Sun HY, et al. Postconditioning attenuates myocardial ischemia-reperfusion injury by inhibiting events in the early minutes of reperfusion. Cardiovasc Res. 2004;62:74-85.
4. Staat P, Rioufol G, Piot C, et al. Postconditioning the human heart. Circulation. 2005;112:2143-2148.
5. Khan AR, Binabdulhak AA, Alastal Y, et al. Cardioprotective role of ischemic postconditioning in acute myocardial infarction: a systematic review and meta-analysis. Am Heart J. 2014;168:512-521.e4.
6. Khalili H, Patel VG, Mayo HG, et al. Surrogate and clinical outcomes following ischemic postconditioning during primary percutaneous coronary intervention of ST-segment elevation myocardial infarction: a meta-analysis of 15 randomized trials. Catheter Cardiovasc Interv. 2014;84:978-986.
7. Freixa X, Bellera N, Ortiz-Pérez JT, et al. Ischaemic postconditioning revisited: lack of effects on infarct size following primary percutaneous coronary intervention. Eur Heart J. 2012;33:103-112.
8. Engstrøm T, Kelbæk H, Helqvist S, et al. Effect of ischemic postconditioning during primary percutaneous coronary intervention for patients with ST-segment elevation myocardial infarction: a randomized clinical trial. JAMA Cardiol. 2017;2:490-497.
9. Mentias A, Mahmoud AN, Elgendy IY, et al. Ischemic postconditioning during primary percutaneous coronary intervention. Catheter Cardiovasc Interv. 2017;90:1059-1067.
10. Manintveld OC, Te Lintel Hekkert M, van den Bos EJ, et al. Cardiac effects of postconditioning depend critically on the duration of index ischemia. Am J Physiol Heart Circ Physiol. 2007;292:H1551-H1560.
11. Nuche J, Galán-Arriola C, Fernández-Jiménez R, et al. Ischemic postconditioning fails to reduce infarct size in pig models of intermediate and prolonged ischemia. REC Interv Cardiol. 2022. https://doi.org/10.24875/RECICE.M22000333.
12. Fernández-Jiménez R, Galán-Arriola C, Sánchez-González J, et al. Effect of ischemia duration and protective interventions on the temporal dynamics of tissue composition after myocardial infarction. Circ Res. 2017;121:439-450.
13. Garcia-Dorado D, Théroux P, Elizaga J, et al. Myocardial reperfusion in the pig heart model: infarct size and duration of coronary occlusion. Cardiovasc Res. 1987;21:537-544.
14. Lobo-Gonzalez M, Galán-Arriola C, Rossello X, et al. Metoprolol blunts the time-dependent progression of infarct size. Basic Res Cardiol. 2020;115:55.
15. Barrabés JA, Garcia-Dorado D, Oliveras J, et al. Intimal injury in a transiently occluded coronary artery increases myocardial necrosis. Effect of aspirin. Pflugers Arch. 1996;432:663-670.
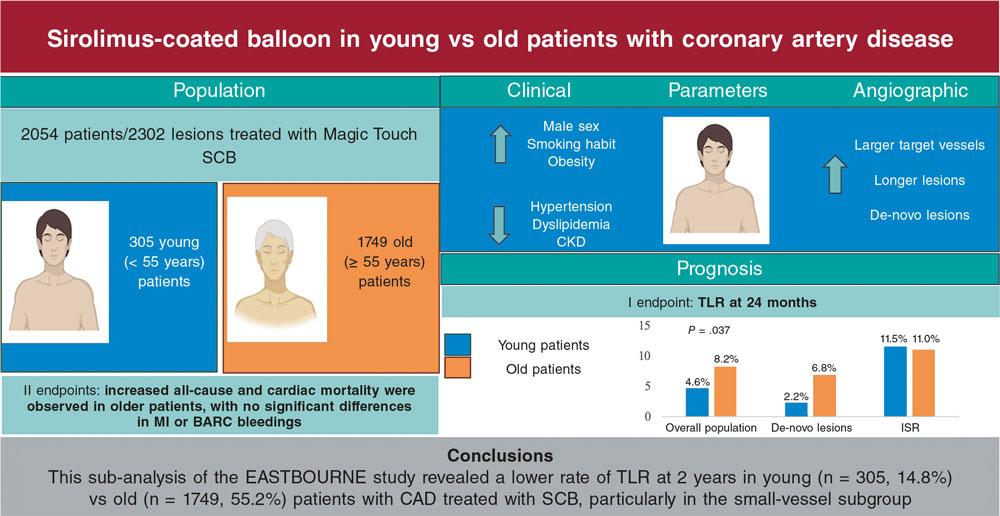
The use of drug-coated balloons (DCB) to treat stenotic coronary artery lesions is a treatment strategy whose main asset is to avoid leaving a permanent intracoronary stent device. Although highly effective in the percutaneous coronary intervention setting, it is associated with a risk of acute thrombosis, future events like restenosis, and late thrombosis following processes known as neointimal proliferation, neoatherosclerosis or fractures of material. This could be even more relevant in younger patients with a long trajectory of possible coronary events ahead of them.
The use of DCB is widely accepted to treat in-stent restenosis and de novo lesions in small vessels1, and it is considered an interesting option in patients with high risk of bleeding. Another possible indication currently under scrutiny due to its possible potential is the management of bifurcations—one of the most interesting indications of all. However, clearly defined recommendations have not been established yet.2
Over the last few years, small clinical trials have been published on the use of DCB in this indication; although they have not proven definitive for a strong guideline recommendation, they provide valuable data. In general, trials have grouped into those looking into the safety and efficacy profile of DCB—without comparison group—and trials that compared strategies with DCBs or conventional balloons (CB).
PROSPECTIVE NON-RANDOMIZED TRIALS WITHOUT COMPARISON CONTROL GROUP
Table 1 shows 5 small trials (between 28 and 50 patients) including this type of different strategies with acceptable results regarding late lumen loss and safety.3-9
Table 1. Non-randomized, prospective clinical trials without comparison control group
| Trial | Name or author and DCB | No. of patients | LLL TLR events, and restenosis | Restenosis, and MACE |
|---|---|---|---|---|
| DCB into both branches and BMS into the main branch | PEPCAD-V4 (Sequent Please B. Braun, Germany) |
28 | 0.21 ± 0.48 in the SB 0.38 ± 0.46 mm in the MB Only 1 TLR (3.57%) and 3 restenoses (10.7%) |
2 patients (7.14%) had late thrombosis at 6 and 8 months |
| Paclitaxel DES into the MB, and DCB into the SB | DEBSIDE (NCT01485081) (Danubio, France) |
50 | LLL in the SB: -0.04 ± 0.34 mm and in the MB: 0.54 ± 0.60 mm TLR in 1 patient (2%) Restenosis, 7.5. |
1 AMI (2%) without cardiac deaths |
| -limus DES into the MB, and DCB into the SB | BIOLUX-A (www.anzctr.org.au, ID 335843) (Pantera Lux, Biotronik AG, SwitzeSBand) |
35 | LLL in the SB: 0.1 ± 0.43 mm 1 TLR (2.85%) No restenosis |
1 patient died, and 3 AMIs were reported in different vessels |
| SARPEDON5 (Pantera Lux, BIOTRONIK AG, Bülach, Switzerland) |
50 | TLR, 5.2% at 1 year 4% of restenosis in the MB, and 6% in the SB |
Stent thrombosis, 0% | |
| Estudio de Valencia et al.6 (Sequent Please) | 54 | TLR, 3.6% | Overall mortality, 3.7% | |
| DCB alone into both branches | Schulz et al.7 (Sequent Please) |
39 | 10% restenosis, and all in the left main coronary artery bifurcation | |
| Bruch et al.8 (Sequent Please) |
127 | TLR, 4.5 | MACE, 6.1% Use of bailout stent in 45% |
|
| DCB alone into 1 branch | Her et al.9 (Sequent Please) (Only in the MB) |
16 | There was a significant increase in the SB luminal area at 9 months, 0.37 mm2 ± 0.64 mm2; (P = .013), with a similar increase in the MB luminal area | The use of DCB alone in the MB also had a favorable impact on an area gain of 52% in the SB ostium |
| Vaquerizo et al. (NCT01375465) (Eurocor GmbH, Germany) (Only in the SB and 001 lesions) |
31 | LLL in the SB, 0.32 mm2 ± 0.73 mm2, and binary restenosis, and TLR of 22.5% | High need for bailout BMS (14%) 1 AMI (3.2%) |
|
|
AMI, acute myocardial infarction; BMS, bare metal stent; CB, conventional balloon; DCB, drug-coated balloon; DES, drug-eluting stent; LLL, late lumen loss; MACE, major adverse cardiovascular events; MB, main branch; SB, side branch; TLR, target lesion revascularization. |
||||
TRIALS COMPARING THE RESULTS TO DIFFERENT STRATEGIES AND 2 COMPARISON GROUPS, MOST OF THEM RANDOMIZED
Table 2 shows the 6 landmark trials comparing different strategies, 5 of them randomized,10-14 and 1 non-randomized.15
Table 2. Trials that compared the results with different strategies in 2 randomized comparison groups (except for the one conducted by Li et al.15)
| Trial | Name and no. of patients | LLL | Restenosis and MACE, TLR events | Takeaway |
|---|---|---|---|---|
| DCB alone vs CB as a first-line therapy in lesions without damage to the proximal segment | PEPCAD-BIF10 (Sequent Please) 64 patients |
LLL in the DCB group, 0.08 mm ± 0.31 mm vs 0.47 ± 0.61 mm in the CB group (P = .006). | Rates of restenosis of 26% vs 6% Rates of TLR of 9% vs 3% Favorable to DCB |
In this type of lesions, stents were required in < 10% of the cases only |
| DCB vs CB in the SB with the use of BMS in the MB | DEBIUT11 (Dior-I, Eurocor GmbH, Germany) 117 patients A) DCB in both branches and BMS in the MB B) BMS in the MB, and CB in the SB C) Paclitaxel DES in the MB, and CB in the SB |
LLL in the SB was 0.19 mm ± 0.66 mm in group A, 0.21 mm ± 0.57 mm in group B, and 0.11 mm ± 0.43 mm in group C (P = .001) LLL in the MB, 0.31 mm ± 0.48 mm in group A vs 0.16 mm ± 0.38 mm in group B (P = .15) |
The rates of binary restenosis were 24.2%, 28,6%, and 15%; (P = .45), and the rates of MACE were 20%, 29.7%, and 17.5%; (P = .40) in groups A, B, and C, respectively | With this strategy, pretreatment of both branches with DCB was not superior to conventional BMS with the provisional stenting technique. Also, the use of DES was superior to DCB plus BMS |
| BABILON12 (Sequent Please) 108 patients A) DCB in both branches, and BMS in the MB B) Everolimus DES in the MB, and CB in the SB |
LLL in the SB, –0.04 mm ± 0.76 mm in group A vs -0.03 mm ± 0.51 mm in group B (P = .983) | The rates of MACE and TLR were higher in group A in the MB (17.3% vs 7.1% [P = .10], and 15.4% vs 3.6%; [P = .045]) due to more restenosis in the MB (13.5% vs 1.8%; P = .027) | Bifurcation pretreatment with DCB with BMS in the MB had more LLL and higher rates of MACE vs DES in the MB and CB in the SB Also, both strategies gave similar and very good results in the SB |
|
| Paclitaxel DES in the MB with CB vs DCB in the SB | Herrador et al.13 (Sequent Please) 50 patients |
LLL, 0.40 mm ± 0.50 mm vs 0.09 mm ± 0.40 mm, (P = .01) favorable to the DCB group | The rates of SB restenosis were 20% vs 7%, (P = .08), and the rates of TLR, 22% vs 12% (P = .16) | The rates of MACE at 12 months were 24% vs 11% (P = .11) |
| -limus DES in the MB with CB vs DCB in the SB | BEYOND14, (Bingo, Yinyi Biotech, China) 222 patients with coronary bifurcation lesions excluding the left main coronary artery |
Significantly lower LLL in the DCB compared to the CB group (–0.06 mm ± 0.32 mm vs 0.18 mm ± 0.34 mm; P < .0001) | The rates of restenosis were 28.7% vs 40% (P < .0001) | No differences regarding MACE (0.9% vs 3.7%, P = .16) or non-fatal AMI were found (0% vs 0.9%, P = .49) |
| Li et al.15 (Sequent Please) NON-randomized |
LLL of SB in the DCB group was lower compared to the CB group (0.11 mm ± 0.18 mm vs 0.19 mm ± 0.25 mm; P = .024) at 12-month follow-up | Multivariate COX analysis indicated that the DCB group had less MACE (23.9% vs 12.8%; P = .03) | Better results in the SB with DCB and fewer composite endpoints, but basically at the expense of unstable angina | |
|
AMI, acute myocardial infarction; BMS, bare metal stent; CB, conventional balloon; DCB, drug-coated balloon; DES, drug-eluting stent; LLL, late lumen loss; MACE, major adverse cardiovascular events; MB, main branch; SB, side branch; TLR, target lesion revascularization. |
||||
CONCLUSIONS FROM TRIAL RESULTS
- 1. The use of bare metal stents (now in disuse) neutralizes all positive effects from the DCB in the main or side branch (DEBIUT11 and BABILON trials.12)
- 2. In lesions without proximal damage to the bifurcation, an early strategy of DCB can only be considered in 1 or in both branches (PEPCAD-BIF.10) Also, non-flow-limiting dissections have good prognosis at follow-up.
- 3. The use of DCB alone into the main branch can also have positive effects on the side branch ostium. Even using a limus-eluting stent in the main branch can only have a positive remodeling effect on the side branch ostium (aside from the study conducted by Her et al.,9 the BABILON trial already suggested it.12). In any case, the use of a DCB as a single stent-less strategy (unless results are poor or in the presence of flow-limiting dissections) seems like a reasonable option with a favorable long-term remodeling both in the main and side branches.
- 4. The use of a limus-eluting stent in the main branch with a DCB implanted in the side branch (currently the most widely used strategy) can improve angiographic intraluminal parameters like late lumen loss or minimum lumen diameter without any significant clinical repercussions on the long-term events (the BEYOND trial.14). This is probably so because, in the other group, late lumen loss in the side branch is also small since events are more conditioned by the main compared to the side branch (BABILON12), and also because there are barely any myocardial infarctions or target lesion revascularizations associated with the side branch in any of the 2 groups.
- 5. The results obtained with different balloons could also be different.
However, we should mention other aspects like vessel length, and not only vessel diameter since some studies demonstrate that length—and not diameter—can be a more important predictor of the impact side branch occlusion. Moreover, almost all these trials included side branch lesions < 10 mm in length, which is a well-known favorable predictor for the provisional stenting technique. Side branch lesions > 10 mm plus other signs of complexity like calcium, etc. can require the double stenting strategy, especially in left main coronary artery bifurcation lesions.16
Its role in more complex settings like left main coronary artery bifurcations or in-stent restenosis in bifurcations has also been studied, with reasonably good results.17,18
The article by Valencia et al.6 recently published in REC: Interventional Cardiology falls within the category of observational studies without control group that do not include angiographic measurements to allow, at least, a rough result comparison with other studies. This article combines treatment strategies like drug-eluting stent implantation into the main vessel in 71% of the cases or DCB alone into the main branch in 29% of the cases followed by DCB implantation into the side branch or DCB alone into the side branch, since 18% of the lesions were Medina 0,0,1 while, overall, 37.5% had no proximal damage.
According to the authors, this article contribution is the presentation of the clinical results of a small series of 54 patients with 55 lesions and the authors’ management of this type of lesions without excluding patients with higher risk of restenosis, as 32.1% of the patients with in-stent restenosis in the bifurcation and 8.9% with left main coronary artery lesions showed. Nevertheless the clinical outcomes are good with a median follow-up of 12 months. The rates of all-cause mortality, lesion thrombosis or infarction, and target lesion revascularization were 3.7%, 0%, and 3.6%, respectively, precisely in the most unfavorable cases of all, patients with in-stent restenosis.
The study limitations are obvious and well-established by the authors in the corresponding section. In brief, a small number of patients, no control group or angiographic follow-up, and the assumption that asymptomatic patients had no side branch restenosis. Also, since follow-up was not conducted on-site, possible developments of new Q waves associated with the side branch segment could not be detected. However, the study shows what many interventional cardiologists currently do in their cath labs and maintains interest for this strategy that should undoubtedly be taken into consideration when treating bifurcations. The most recent trials on drug-eluting stent and DCB implantation into the main and side branch, respectively, show good results in both branches, though with small differences in the repercussion of clinical events. Randomized clinical trials with a large cohort of patients are needed so that all possible trends favorable to the side branch become significant. Despite the presence of complex patients, the results from the trial conducted by Valencia et al.6 are good, promising, and their data welcome.
FUNDING
None whatsoever.
CONFLICTS OF INTEREST
None reported.
REFERENCES
1. Jeger RV, Eccleshall S, Wan Ahmad WA, et al. Drug-Coated Balloons for Coronary Artery Disease: Third Report of the International DCB Consensus Group. JACC Cardiovasc Interv. 2020;13:1391-1402.
2. Hildick-Smith D, Arunothayaraj S, Stankovic G, Chen SL. Percutaneous coronary intervention of bifurcation lesions. EuroIntervention. 2022;18:e273-e291.
3. Corballis NH, Paddock S, Gunawardena T, Merinopoulos I, Vassiliou VS, Eccleshall SC. Drug coated balloons for coronary artery bifurcation lesions: A systematic review and focused meta-analysis. PLoS One. 2021;16: e0251986.
4. Mathey DG, Wendig I, Boxberger M, Bonaventura K, Kleber FX. Treatment of bifurcation lesions with a drug-eluting balloon: the PEPCAD V (Paclitaxel Eluting PTCA Balloon in Coronary Artery Disease) trial. EuroIntervention. 2011;7 Suppl K:K61-65.
5. Jim MH, Lee MK, Fung RC, Chan AK, Chan KT, Yiu KH. Six month angiographic result of supplementary paclitaxel-eluting balloon deployment to treat side branch ostium narrowing (SARPEDON). Int J Cardiol. 2015;187:594-597.
6. Valencia J, Torres-Mezcua F, Herrero-Brocal M, et al. Efectividad a largo plazo del balón farmacoactivo en el tratamiento de la rama lateral de lesiones en bifurcación. REC Interv Cardiol. 2022. https://doi.org/10.24875/RECIC.M22000317.
7. Schulz A, Hauschild T, Kleber FX. Treatment of coronary de novo bifurcation lesions with DCB only strategy. Clin Res Cardiol. 2014;103:451-456.
8. Bruch L, Zadura M, Waliszewski M, et al. Results From the International Drug Coated Balloon Registry for the Treatment of Bifurcations. Can a Bifurcation Be Treated Without Stents? J Interv Cardiol. 2016;29(4):348-56.
9. Her AY, Ann SH, Singh GB, et al. Serial Morphological Changes of Side-Branch Ostium after Paclitaxel-Coated Balloon Treatment of De Novo Coronary Lesions of Main Vessels. Yonsei Med J. 2016;57:606-613.
10. Kleber FX, Rittger H, Ludwig J, et al. Drug eluting balloons as stand alone procedure for coronary bifurcational lesions: results of the randomized multicenter PEPCAD-BIF trial. Clin Res Cardiol. 2016;105:613-621.
11. Stella PR, Belkacemi A, Dubois C, et al. A multicenter randomized comparison of drug-eluting balloon plus bare-metal stent versus bare-metal stent versus drug-eluting stent in bifurcation lesions treated with a single-stenting technique: six-month angiographic and 12-month clinical results of the drug eluting balloon in bifurcations trial. Catheter Cardiovasc Interv. 2012;80:1138-1146.
12. López Mínguez JR, Nogales Asensio JM, Doncel Vecino LJ, et al. A prospective randomised study of the paclitaxel-coated balloon catheter in bifurcated coronary lesions (BABILON trial): 24-month clinical and angiographic results. EuroIntervention. 2014;10:50-57.
13. Herrador JA, Fernandez JC, Guzman M, Aragon V. Drug-eluting vs. conventional balloon for side branch dilation in coronary bifurcations treated by provisional T stenting. J Interv Cardiol. 2013;26:454-462.
14. Jing QM, Zhao X, Han YL, et al. A drug-eluting Balloon for the trEatment of coronarY bifurcation lesions in the side branch: a prospective multicenter ranDomized (BEYOND) clinical trial in China. Chin Med J. 2020;133:899-908.
15. Li Y, Mao Q, Liu H, Zhou D, Zhao J. Effect of Paclitaxel-Coated Balloon Angioplasty on Side Branch Lesion and Cardiovascular Outcomes in Patients with De Novo True Coronary Bifurcation Lesions Undergoing Percutaneous Coronary Intervention. Cardiovasc Drugs Ther. 2022;36:859–866.
16. Zhang JJ, Ye F, Xu K, et al. Multicentre, randomized comparison of two-stent and provisional stenting techniques in patients with complex coronary bifurcation lesions: the DEFINITION II trial. Eur Heart J. 2020;41:2523-2536.
17. Liu H, Tao H, Han X, et al. Improved Outcomes of Combined Main Branch Stenting and Side Branch Drug-Coated Balloon versus Two-Stent Strategy in Patients with Left Main Bifurcation Lesions. J Interv Cardiol. 2022. https://doi.org/10.1155/2022/8250057.
18. Harada Y, Colleran R, Pinieck S, et al. Angiographic and clinical outcomes of patients treated with drug-coated balloon angioplasty for in-stent restenosis after coronary bifurcation stenting with a two stent technique. EuroIntervention. 2017;12:2132-2139.
Endorsed by the current clinical practice guidelines, the indication to perform percutaneous coronary intervention (PCI) of intermediate coronary stenosis should be guided by either fractional flow reserve (FFR) or instantaneous wave-free ratio (iFR) if evidence of ischemia is lacking.1 Despite these clear recommendations, the uptake of physiology in clinical practice remains low supporting the development of new non-invasive tools that no longer mandate the need for dedicated coronary guidewires or microcatheters along with the need to administer hyperemic agents in case of FFR.1
Advances in computational power and three-dimensional quantitative coronary angiography has facilitated the development of angiography-based-FFR indices, thus allowing easy, online physiological lesion assessments. Besides anatomical and angiographic exclusion criteria like severe tortuosity, aorto-ostial lesions or overlapping vessels, pivotal studies demonstrated that with angiography-based-FFR indices the need for invasive coronary artery instrumentation and hyperemic agents can, in most cases, be avoided.2
Currently, 4 angiography-based-FFR indices have emerged and are currently commercially available.1 Despite workflow differences and embedded simplified computational fluid dynamics models, these indices demonstrated to have a good diagnostic performance with pressure guidewire based FFR as a reference.1
Among these, vessel fractional flow reserve (vFFR, CAAS Workstation 8.5 Pie Medical Imaging, Netherlands) uses a computational fluid dynamic approach based on simplified Navier–Stokes equations and 2 angiographic views separated, at least, 30 degrees to generate a 3D reconstruction of the coronary artery. Using aortic pressure as inlet boundary condition, the algorithm applies automated and harmonized optimal end-diastolic frame selection in the 2 views by electrocardiogram triggering, thus allowing physiological lesion assessment without the need for full cardiac tree assessment or manual frame counting.3
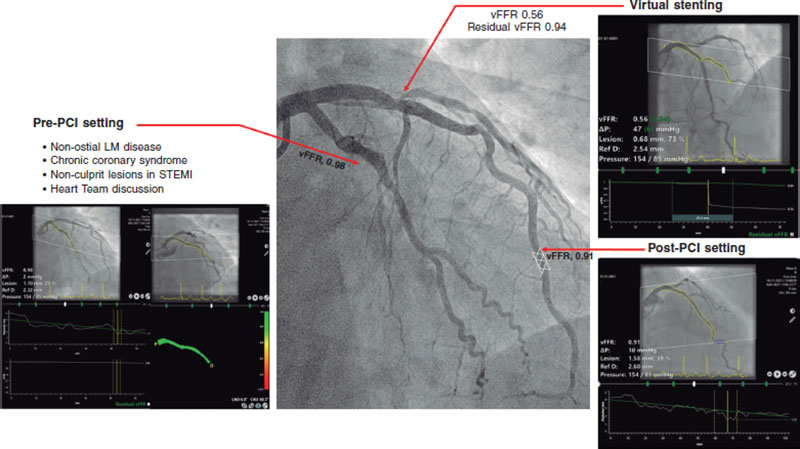
This review provides an overview of the currently available clinical evidence on the use of vFFR (table 1 and figure 1).
Vessel fractional flow reserve was first validated in 2 retrospective, single-center studies where the technology demonstrated an excellent diagnostic performance in intermediate coronary artery lesions compared to FFR, which was consistent among different anatomical and patient subsets including tandem lesions, and patients presenting with non-ST-segment elevation acute coronary syndrome.3,4 These findings were later confirmed in the multicenter, prospective FAST II study, in which vFFR computed offline by local site personnel and a blinded core lab showed excellent diagnostic accuracy in identifying lesions with invasive guidewire-based FFR ≤ 0.80 (area under the curve [AUC], 0.93; P < .001). Positive and negative predictive values, sensitivity and specificity of vFFR were 90%, 90%, 81% and 95%, respectively.5 The system allows accurate automated vessel contour detection with manual correction required in merely 9.3% of vessel contours.5 Regarding reproducibility, vFFR showed a low inter-observer variability when computed offline by blinded academic operators (r = 0.95; P < .001) or local personnel vs a blinded core lab (r = 0.87; P < .001). Additionally, a low coefficient of variation (3.92%) was observed when vFFR was analyzed at 2 different timeframes by an independent core lab.6
Following these promising data, we explored the potential value of vFFR in a variety of clinical and procedural settings (table 1 and figure 1).
Table 1. Major studies trials investigating the diagnostic performance of vessel fractional flow reserve (vFFR)
| Study/Author | Year | Study design | Number of vessel (patient) | Primary endpoint |
|---|---|---|---|---|
| Pre-PCI setting | ||||
| FAST study | 2019 | Retrospective | 100 (100) | AUC = 0.93 (95%CI, 0.88-0.97) |
| FAST EXTEND | 2020 | Retrospective | 294 (294) | AUC = 0.94 (95%CI, 0.92-0.97) |
| FAST II | 2021 | Prospective | 334 (334) | AUC = 0.93 (95%CI, 0.90-0.96) |
| FAST Heart Team | 2022 | Retrospective | 1248 (416) | Mismatch between vFFR and revascularization = 29.8% |
| FAST III | Ongoing | Prospective | ||
| Imaging | ||||
| Tomaniak et al. (Left main coronary artery disease) |
2022 | Retrospective | 63 (63) | AUC = 0.95 (95%CI, 0.89-1.0) |
| FAST OCT | Ongoing | Prospective | ||
| Post-PCI setting | ||||
| FAST POST | 2021 | Retrospective | 100 (100) | AUC = 0.98 (95%CI, 0.96-1.0) |
| FAST OUTCOME | 2022 | Retrospective | 832 (748) | vFFR tertiles = TVF 24.6%, 21.5% vs 17.1% |
| STEMI and multivessel disease | ||||
| FAST STEMI II | Ongoing | Prospective | ||
|
95%CI, 95% confidence interval; AUC, area under the curve; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction; TVF, target vessel failure. |
||||
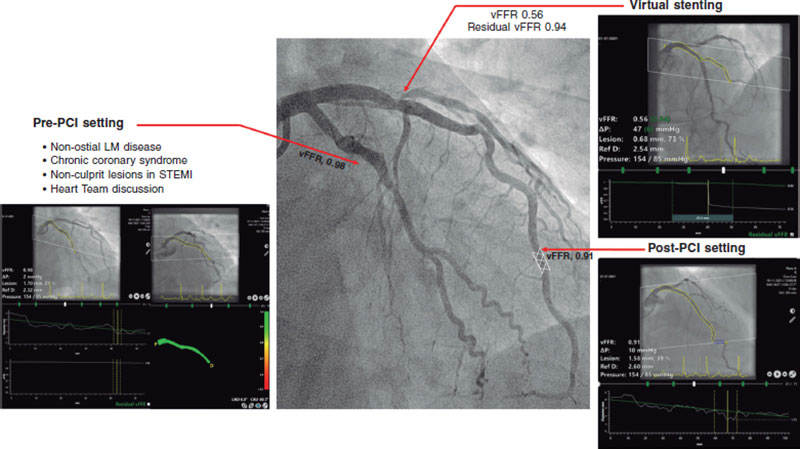
Figure 1. Clinical application of vessel fractional flow reserve (vFFR). LM, left main; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
First, the evaluation of left main coronary artery (LMCA) lesions remains challenging and often warrants a multimodality approach, including physiological assessment and intravascular imaging. Since patients with LMCA disease are often under-represented in the studies, a dedicated analysis comparing vFFR to intravascular ultrasound in patients with non-ostial LMCA disease was performed. vFFR was shown to correlate well to the LMCA minimum lumen area (MLA) as assessed by intravascular ultrasound (r = 0.79; P = .001) and to have excellent diagnostic accuracy identifying LMCA lesions with MLA < 6.0 mm2 (AUC = 0.95; P = .001).7
Second, the use of physiology in the ACS setting has been topic of disussion as the benefit of physiology-guided-PCI has been mainly demonstrated in patients with stable disease.1 The latter is an important limitation since most patients present with ACS, which in up to 31% of cases occurs in the context of plaque rupture/erosion or calcium nodules located in intermediate coronary artery lesions. Conversely, a thrombotic component was identified in 602/695 of the culprit lesions (87%), which may affect the validity of both the pressure guidewire and the angiography-based-FFR assessments (TACTIS Registry, TCT 2022). In that perspective, the FAST OCT study (NCT04683133) will assess the agreement between vFFR and optical coherence tomography detected causes of luminal obstruction in intermediate lesions of patients presenting with ACS.
Whether the use of vFFR can be extended to patients with ST-segment elevation acute coronary syndrome and multivessel disease will be explored in the ongoing FAST STEMI program.
Next to the potential for online use, the concept of angiography-based-FFR caries significant potential in an offline setting where this technology could be used for clinical decision-making in patients with multivessel disease or those referred for heart team discussion. In a recent retrospective analysis, 3-vessel vFFR screening demonstrated a discordance between lesion significance and revascularization in 30% of the cases.8
Third, post-PCI physiological assessment has gained attention since several studies demonstrated that low post-PCI FFR values are detectable in up to 58% of vessels.9 Although the relevance of low post-PCI FFR was demonstrated by a significantly increased risk for future adverse cardiovascular events, the uptake of post-PCI FFR in the routine clinical practice is still limited.9 Hypothetically, the concept of having a wire-free method to detect suboptimal stent deployment, residual disease, and additional procedural optimization is promising. In the retrospective, single-center FAST POST study, vFFR demonstrated a good correlation with conventional invasive post-PCI FFR (r = 0.88), and a higher accuracy in the identification of patients with FFR values < 0.90 (AUC = 0.98) compared to three-dimensional quantitative coronary angiography (AUC = 0.62).10 In the light of these results, the hypothesis that post-PCI vFFR may predict future adverse cardiac events was proven in the FAST OUTCOME study.11
Fourth, the ability to predict functional outcomes of PCI may entail another step forward in the identification of patients who could benefit the most from PCI and thereby avoid the risk of a futile invasive procedure. Recent developments in vFFR software have allowed to simulate the effects of a ‘virtual’ PCI and estimate post-PCI FFR (residual vFFR). Using pre-PCI virtual pullbacks, residual vFFR showed a good correlation with invasive post-PCI FFR and post-PCI vFFR values (r = 0.84, and r = 0.77, respectively), and good discriminative ability to identify post-PCI FFR < 0.90 (AUC = 0.93).12 Of note, the current algorithm assumes an almost perfect PCI result, and thus, cannot account for heavy calcifications or stent underexpansion suggesting a potential need for future hybrid technologies combining multimodality invasive and non-invasive imaging modalities and physiology tools.
Finally, following the positive data from the FAVOR III outcome trial that proved the superiority of quantitative flow ratio (QFR, Pulse Medical Imaging Technology, China) vs angiography-guided-PCI in a Chinese population, the results of, at least, 5 currently ongoing angiography based FFR outcome trials (FAVOR III Europe Japan trial [NCT03729739], PIONEER IV [NCT04923191], FAST III [NCT04931771], LIPSIA STRATEGY [NCT03497637], FLASH FFR II [NCT04575207]) are eagerly awaited and may enhance guideline adoption.2 Specific to vFFR, the ongoing multicenter, randomized FAST III trial will assess whether a vFFR-based diagnostic strategy yields non-inferior clinical outcomes compared to an FFR-based strategy.
Up until the results of these studies will be released, angiography-based-FFR indices, including vFFR, remains an appealing alternative to conventional physiological indices in a broad selection of anatomical and clinical scenarios with the potential to increase the use of physiology and improve patient outcome.
FUNDING
None whatsoever.
AUTHOR’S CONTRIBUTIONS
A. Scoccia contributed to the drafting of this manuscript, and made a critical review of its intellectual content. J. Daemen also contributed to the drafting of this manuscript, made a critical review of its intellectual content, and gave his final approval to the version that would eventually be published.
CONFLICTS OF INTEREST
J. Daemen received institutional grant/research support from Astra Zeneca, Abbott Vascular, Boston Scientific, ACIST Medical, Medtronic, Microport, Pie Medical, and ReCor medical; and consultancy and speaker fees from Abiomed, ACIST medical, Boston Scientific, ReCor Medical, PulseCath, Pie Medical, Siemens Health Care and Medtronic. A. Scoccia declared no conflicts of interest.
REFERENCES
1. Kogame N, Ono M, Kawashima H, et al. The Impact of Coronary Physiology on Contemporary Clinical Decision Making. JACC Cardiovasc Interv. 2020;13:1617-1638.
2. Xu B, Tu S, Song L, et al. Angiographic quantitative flow ratio-guided coronary intervention (FAVOR III China): a multicentre, randomised, sham-controlled trial. Lancet. 2021;398:2149-2159.
3. Masdjedi K, van Zandvoort LJC, Balbi MM, et al. Validation of a three-dimensional quantitative coronary angiography-based software to calculate fractional flow reserve: the FAST study. EuroIntervention. 2020;16:591-599.
4. Neleman T, Masdjedi K, Van Zandvoort LJC, et al. Extended Validation of Novel 3D Quantitative Coronary Angiography-Based Software to Calculate vFFR: The FAST EXTEND Study. JACC Cardiovasc Imaging. 2021;14:504-506.
5. Masdjedi K, Tanaka N, Van Belle E, et al. Vessel fractional flow reserve (vFFR) for the assessment of stenosis severity: the FAST II study. EuroIntervention. 2022;17:1498-1505.
6. Scoccia A, Neleman T, Kardys I, et al. Reproducibility of 3D vessel Fractional Flow Reserve (vFFR): A core laboratory variability analysis of FAST II study. Cardiovasc Revasc Med. 2022;44:101-102.
7. Tomaniak M, Masdjedi K, van Zandvoort LJ, et al. Correlation between 3D-QCA based FFR and quantitative lumen assessment by IVUS for left main coronary artery stenoses. Catheter Cardiovasc Interv. 2021;97:E495-E501.
8. Tomaniak M, Masdjedi K, Neleman T, et al. Three-dimensional QCA-based vessel fractional flow reserve (vFFR) in Heart Team decision-making: a multicentre, retrospective, cohort study. BMJ Open. 2022;12:e054202.
9. Hwang D, Koo BK, Zhang J, et al. Prognostic Implications of Fractional Flow Reserve After Coronary Stenting: A Systematic Review and Meta-analysis. JAMA Netw Open. 2022;5(9):e2232842.
10. Masdjedi K, van Zandvoort LJ, Balbi MM, et al. Validation of novel 3-dimensional quantitative coronary angiography based software to calculate fractional flow reserve post stenting. Catheter Cardiovasc Interv. 2021;98:671-677.
11. Neleman T, Scoccia A, Masdjedi K, et al. The prognostic value of angiography-based vessel fractional flow reserve after percutaneous coronary intervention: The FAST Outcome study. Int J Cardiol. 2022;359:14-19.
12. Tomaniak M, Neleman T, Ziedses des Plantes A, et al. Diagnostic Accuracy of Coronary Angiography-Based Vessel Fractional Flow Reserve (vFFR) Virtual Stenting. J Clin Med. 2022;11:1397.
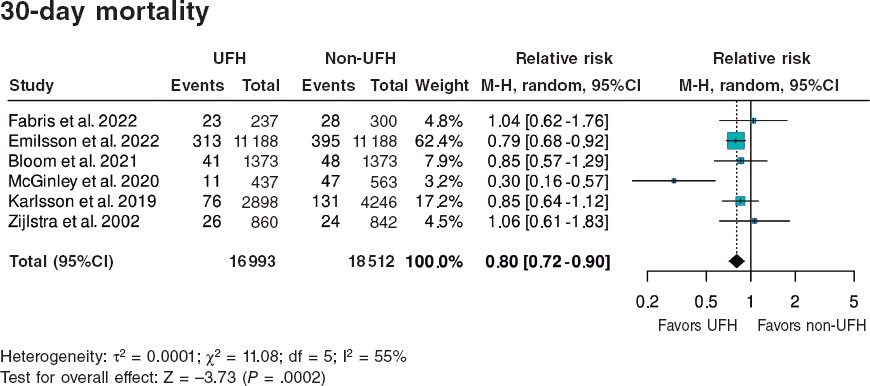
Diabetes mellitus is a comorbidity that is present in 20% to 30% of the patients with coronary artery disease and an indication for revascularization. Also, it poses a scenario of greater complexity for several reasons. The presence of diabetes is associated with more extensive, diffuse, calcified coronary artery disease, and graft and stent failure. All of it is associated with a higher risk of repeat revascularizations and worse prognosis for the patients, which is why diabetes is a differential element here since it establishes the revascularization method in patients with multivessel disease based on the clinical practice guidelines.1 Currently, the recommendation of coronary artery bypass graft (CABG) is superior to percutaneous coronary intervention (PCI) in diabetic patients. This indication comes from numerous studies being the FREEDOM trial2 one of the most important of all. However, are patients from the routine clinical practice or real-world patients similar to those included in these clinical trials?
In this sense, the study conducted by Puyol-Ruiz et al.3 recently published in REC: Interventional Cardiology provides valuable observational information on the results of coronary revascularization in diabetic patients in the routine clinical practice. This study shows the results from a historical cohort (2012-2014) of 733 patients with diabetes and multivessel coronary artery disease with a clinical indication for coronary angiography. Authors divide the study population based on the degree of revascularization (complete or incomplete) and the clinical profile consistent, or not, with the inclusion criteria of the FREEDOM clinical trial.2 In this cohort, 80.8% and 14.5% of the patients were revascularized percutaneously and surgically, respectively compared to 4.8% who received medical therapy only. Authors found a tendency towards a lower rate of clinical events at 35-month follow-up in patients with complete revascularization. Also, both the risk profile and the rate of events of the FREEDOM study population (41%) was significantly lower compared to the non-FREEDOM study population (59%): lower rate of death (5.5% vs 38.4%; P = .006), cardiac death (3.2% vs 31.2%; P = .002), and major adverse cardiovascular events (6.5% vs 40.0%; P = .012). Therefore, we can deduce that patients from the FREEDOM trial are a selected subpopulation of lower risk representative of less than half of the real-world patients with diabetes and multivessel disease. Other studies that have tried to identify, in a population from the real-world clinical practice, the group of patients potentially eligible for clinical trial show similar prevalences (around 50%) of selection criteria for clinical trials on coronary revascularization, a population that also shows a significantly lower rate of cardiovascular adverse events.4
On the other hand, regarding the interpretation of these data, we should remember that over 10 years have passed since the FREEDOM trial, and the recruitment phase into the cohort of the study conducted by Puyol-Ruiz et al.3. Let’s see what elements have changed in the revascularization of diabetic patients through all this time.
Modern PCI is not similar to the one described in the FREEDOM trial that used first-generation drug-eluting stents (sirolimus in 51%, and paclitaxel in 43%). Current platforms have exceeded paclitaxel-eluting stents in multiple clinical settings including diabetic patients.5 Sirolimus-eluting stents had higher rates of thrombosis and stent failure compared to current stents in relation to the mechanisms of hypersensitivity to polymer.6 Also, ultrathin strut drug-eluting stents have proven to be associated with a lower rate of adverse events compared to first-generation stents (> 120 μm).7 As a matter of fact, more recent studies with all-comers design have demonstrated that state-of-the-art stents like the polymer-free amphilimus-eluting stent improves results even more (target lesion failure) compared to second-generation reference stents.8 This huge improvement in stent technology seen over the last few years, the calcified plaque modification techniques used, and the intracoronary imaging modality-guided PCI performed or with pressure guidewires lead us to think that the current PCI results improve significantly those reported both in the FREEDOM trial and in this cohort of patients. Such statement can be confirmed in the comparison between the SYNTAX II cohort and the SYNTAX PCI group that used paclitaxel-eluting stents.9,10
However, CABG results have also improved, at least, in the clinical trials. For example, the 1-year rate of adverse events (death, myocardial infarction, stroke or repeat revascularization) has dropped from 12.4% in the CABG group of the SYNTAX trial10 down to 6.9% in the CABG group of the FAME 3 trial.11 This reduction is probably due to better perioperative care and the optimal medical therapy since no major changes in the surgical technique have been reported.
Regarding the impact diabetes has on the results of complete revascularization, the results from the study conducted by Puyol-Ruiz et al.3 are consistent with a meta-analysis of 28 studies and 83 695 patients published by Zimarino et al. This analysis revealed that complete revascularization produced similar benefits in diabetic and non-diabetic patients in terms of mortality and adverse events reporting, in the former, significantly lower rates of new myocardial infarctions.12 Despite this benefit, the numbers of residual coronary artery disease are still high both in the present study (CABG 49/106 [46.2%], PCI 396/592 [66.9%]), and in other PCI cohort studies (28.6%)13 or CABG (33.1% with residual SYNTAX score > 18.514). Therefore, there is this doubt on whether incomplete revascularization is just a technical problem or else a risk marker associated with a more advanced stage of the disease.
In conclusion, the therapeutic management of multivessel coronary artery disease in diabetic patients is still challenging for cardiology today. While clinical trials keep being conducted in selected low-risk populations it’ll be of paramount importance to complement them with information from the results of the actual clinical practice as this article did. In future studies we should make all the necessary efforts to use pragmatic designs where exclusion criteria are minimized to encourage their immediate applicability to the routine clinical practice.
FUNDING
None whatsoever.
AUTHORS’ CONTRIBUTIONS
P. Salinas designed, supervised, reviewed, and drafted the manuscript. A. Travieso drafted and reviewed the original manuscript version.
CONFLICTS OF INTEREST
None reported.
REFERENCES
1. Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87-165.
2. Farkouh ME, Domanski M, Sleeper LA, et al. Strategies for Multivessel Revascularization in Patients with Diabetes. N Engl J Med. 2012;367:2375-2384.
3. Puyol-Ruiz F, Chueca-González EM, Carrasco-Chinchilla F, et al. Clinical impact of complete revascularization on real-life diabetic patients. REC Interv Cardiol. 2022;4:343-350.
4. Hordijk-Trion M, Lenzen M, Wijns W, et al. Patients enrolled in coronary intervention trials are not representative of patients in clinical practice: Results from the Euro Heart Survey on Coronary Revascularization. Eur Heart J. 2006;27:671-678.
5. Kaul U, Bangalore S, Seth A, et al. Paclitaxel-Eluting versus Everolimus-Eluting Coronary Stents in Diabetes. N Engl J Med. 2015;373:1709-1719.
6. Virmani R, Guagliumi G, Farb A, et al. Localized Hypersensitivity and Late Coronary Thrombosis Secondary to a Sirolimus-Eluting Stent: Should We Be Cautious? Circulation. 2004;109:701-705.
7. Madhavan MV, Howard JP, Naqvi A, et al. Long-term follow-up after ultrathin vs. conventional 2nd-generation drug-eluting stents: a systematic review and meta-analysis of randomized controlled trials. Eur Heart J. 2021;42:2643-2654.
8. Romaguera R, Salinas P, Gomez-Lara J, et al. Amphilimus- vs. zotarolimus-eluting stents in patients with diabetes mellitus and coronary artery disease: the SUGAR trial. Eur Heart J. 2022;43:1320-1330.
9. Banning AP, Serruys P, De Maria GL, et al. Five-year outcomes after state-of-the-art percutaneous coronary revascularization in patients with de novo three-vessel disease: final results of the SYNTAX II study. Eur Heart J. 2022;43:1307-1316.
10. Serruys PW, Morice M-C, Kappetein AP, et al. Percutaneous Coronary Intervention versus Coronary-Artery Bypass Grafting for Severe Coronary Artery Disease. N Engl J Med. 2009;360:961-972.
11. Fearon WF, Zimmermann FM, De Bruyne B, et al. Fractional Flow Reserve–Guided PCI as Compared with Coronary Bypass Surgery. N Engl J Med. 2022;386:128-137.
12. Zimarino M, Ricci F, Romanello M, Di Nicola M, Corazzini A, De Caterina R. Complete myocardial revascularization confers a larger clinical benefit when performed with state-of-the-art techniques in high-risk patients with multivessel coronary artery disease: A meta-analysis of randomized and observational studies. Catheter Cardiovasc Interv. 2016;87:3-12.
13. Park TK, Hahn JY, Yang JH, et al. Modified residual SYNTAX score and clinical outcomes in patients with multivessel disease undergoing percutaneous coronary intervention. EuroIntervention. 2017;13:87-96.
14. Melina G, Angeloni E, Refice S, et al. Clinical SYNTAX score predicts outcomes of patients undergoing coronary artery bypass grafting. Am Heart J. 2017;188:118-126.
Coronary artery perforation is one of the most feared complications of chronic total occlusion (CTO) percutaneous coronary intervention (PCI), as it can lead to pericardial effusion, tamponade, hemodynamic deterioration, need for emergency pericardiocentesis or surgery, or death.1 The incidence of perforation is higher in CTO PCI compared with non-CTO PCI, likely due to higher anatomic complexity of CTOs and the use of advanced wiring techniques, such as antegrade dissection and re-entry and retrograde crossing.2
Coronary perforations have traditionally been classified according to severity using the Ellis classification.3 Because perforation location has important implications for management, another key classification of coronary perforations is according to location, as follows: a) large vessel perforation; b) distal vessel perforation; and c) collateral vessel perforation, in either a septal or an epicardial collateral.4
The first step in perforation management is immediate balloon inflation proximal to or at the site of perforation to prevent accumulation of blood in the pericardial space and tamponade. The balloon should be the same size as the perforated vessel and the inflation often last for several minutes unless the patient develops severe ischemic symptoms.5
Large vessel perforations are usually treated with covered stents, such as the PK Papyrus (Biotronik, United States), and the Graftmaster Rx (Abbott Vascular, United States).6 Delivery of the covered stent can be achieved using either a single guide catheter (“block and deliver” technique)7 or 2 guide catheters (“ping pong”, also called “dueling guide catheter” technique)8. Both techniques are used to minimize bleeding into the pericardium while preparing for delivery and deployment of the covered stent. Covered stents require excellent guide catheter support for delivery and should be post-dilated aggressively after deployment to achieve good expansion. Large vessel perforations of CTO vessels can be sealed by coil deployment proximal to the perforation. Another option for treating large vessel perforations is through extraplaque crossing of the CTO segment (either antegrade or retrograde) followed by stenting: the tissue flap created can successfully seal the perforation.9,10
The most widely used treatment for distal vessel perforations is coil11 and autologous fat embolization12. Sometimes both fat and coil embolization are needed.12 Thrombin injection13 and embolization of microparticles, or other materials, such as gelfoam14 are also sometimes used.
In most cases embolization can be achieved through a single guide catheter using the “block and deliver” technique.7 The starting point for fat or coil delivery is advancing a microcatheter just proximal to the perforation site. Fat can be delivered through any microcatheter, but many coils are not compatible with the microcatheters typically used for PCI, such as the Corsair, Corsair XS, Caravel (Asahi Intecc, Japan), Turnpike, Turnpike LP, Mamba (Boston Scientific, United States) and Teleport (OrbusNeich, China) and instead require larger 0.035 inch lumen microcatheters (such as the Progreat, Terumo, Japan). Use of 0.014 inch coils (typically used for neurovascular applications, such as the Axium coils [Medtronic, United States) are compatible with all coronary microcatheters, facilitating use in the cardiac catheterization laboratory. Based on the coil mechanism of release, coils are classified as pushable and detachable. Pushable coils are inserted into the microcatheter and pushed with a coil pusher until they exit the microcatheter. Pushable coil delivery is unpredictable and irreversible. In contrast, detachable coils can be delivered to the desired location and then retracted and repositioned until optimal positioning is achieved, followed by release using a dedicated release device that connects with the back end of the coil.
Septal collateral perforations are unlikely to have adverse consequences and usually no specific treatment is required. In contrast, perforation of epicardial collaterals branch can rapidly lead to tamponade and may be difficult to control. Embolization of epicardial perforations may need to be performed from both sides of the perforation.15
In cases of pericardial effusion and tamponade, emergency pericardiocentesis should be promptly performed.5 Although hemodynamic instability requires immediate pericardiocentesis, smaller size pericardial infusions can often be managed conservatively, as the accumulated blood increases the pressure in the pericardial space potentially preventing further bleeding.
Prevention is critical to decrease the incidence of perforation during CTO PCI. Key preventive strategies include: a) confirmation of guidewire position within the vessel architecture in multiple angiographic projections before balloon dilation and/or microcatheter advancement, usually through injection of the donor vessel to opacity the distal portion of the CTO vessel; b) use of intravascular imaging to determine the need for lesion preparation, and to guide balloon and stent size; c) outlining the anatomy of the collateral channels before and during crossing.5
Meticulous CTO PCI technique, continuous surveillance of the patient and availability and knowledge of how to treat coronary perforations can reduce the morbidity and mortality associated with this complication during CTO PCI.
FUNDING
No funding.
CONFLICTS OF INTEREST
S. Kostantinis has no conflicts of interest to disclose. E.S. Brilakis declares consulting/speaker honoraria from Abbott Vascular, American Heart Association (associate editor of Circulation), Amgen, Asahi Intecc, Biotronik, Boston Scientific, Cardiovascular Innovations Foundation (Board of Directors), ControlRad, CSI, Elsevier, GE Healthcare, IMDS, InfraRedx, Medicure, Medtronic, Opsens, Siemens, and Teleflex; research support from Boston Scientific, GE Healthcare; owner, Hippocrates LLC; shareholder of MHI Ventures, Cleerly Health, Stallion Medical.
REFERENCES
1. Moroni F, Brilakis ES, Azzalini L. Chronic total occlusion percutaneous coronary intervention: managing perforation complications. Expert Rev Cardiovasc Ther. 2021;19:71-87.
2. Karmpaliotis D, Karatasakis A, Alaswad K, et al. Outcomes With the Use of the Retrograde Approach for Coronary Chronic Total Occlusion Interventions in a Contemporary Multicenter US Registry. Circ Cardiovasc Interv. 2016;9:e003434
3. Ellis SG, Ajluni S, Arnold AZ, et al. Increased coronary perforation in the new device era. Incidence, classification, management, and outcome. Circulation. 1994;90:2725-2730.
4. Ybarra LF, Rinfret S, Brilakis ES, et al. Definitions and Clinical Trial Design Principles for Coronary Artery Chronic Total Occlusion Therapies: CTO-ARC Consensus Recommendations. Circulation. 2021;143:479-500.
5. Brilakis ES. Manual of percutaneous coronary interventions: a step-by-step approach. Amsterdam: Elsevier; 2021.
6. Sandoval Y, Lobo AS, Brilakis ES. Covered stent implantation through a single 8-french guide catheter for the management of a distal coronary perforation. Catheter Cardiovasc Interv. 2017;90:584-588.
7. Tarar MN, Christakopoulos GE, Brilakis ES. Successful management of a distal vessel perforation through a single 8-French guide catheter: Combining balloon inflation for bleeding control with coil embolization. Catheter Cardiovasc Interv. 2015;86:412-416.
8. Ben-Gal Y, Weisz G, Collins MB, et al. Dual catheter technique for the treatment of severe coronary artery perforations. Catheter Cardiovasc Interv. 2010;75:708-712.
9. Xenogiannis I, Tajti P, Nicholas Burke M, Brilakis ES. An alternative treatment strategy for large vessel coronary perforations. Catheter Cardiovasc Interv. 2019;93:635-638.
10. Kartas A, Karagiannidis E, Sofidis G, Stalikas N, Barmpas A, Sianos G. Retrograde Access to Seal a Large Coronary Vessel Balloon Perforation Without Covered Stent Implantation. JACC Case Rep. 2021;3:542-545.
11. Kostantinis S, Brilakis ES. When and how to close vessels in the cardiac catheterization laboratory. Catheter Cardiovasc Interv. 2021;98:1332-1334.
12. Guddeti RR, Kostantinis ST, Karacsonyi J, Brilakis ES. Distal coronary perforation sealing with combined coil and fat embolization. Cardiovasc Revasc Med. 2021;40:222-224.
13. Kotsia AP, Brilakis ES, Karmpaliotis D. Thrombin injection for sealing epicardial collateral perforation during chronic total occlusion percutaneous coronary interventions. J Invasive Cardiol. 2014;26:E124-E126.
14. Dixon SR, Webster MW, Ormiston JA, Wattie WJ, Hammett CJ. Gelfoam embolization of a distal coronary artery guidewire perforation. Catheter Cardiovasc Interv. 2000;49:214-217.
15. Boukhris M, Tomasello SD, Azzarelli S, Elhadj ZI, Marza F, Galassi AR. Coronary perforation with tamponade successfully managed by retrograde and antegrade coil embolization. J Saudi Heart Assoc. 2015;27:216-221.
- Bayesian vs frequentist statistics: afraid of losing the reference?
- The need for a Spanish registry of interventional procedures to treat congenital heart disease and standards for center accreditation
- The role of percutaneous tricuspid regurgitation interventions in the current clinical practice: tackling a heterogenous disease
- Stent delivery during primary angioplasty: speed doesn’t matter
Subcategories
Special articles
Original articles
Editorials
Original articles
Editorials
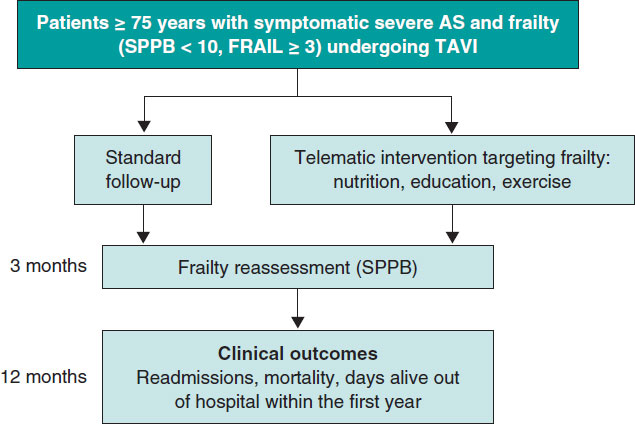
Post-TAVI management of frail patients: outcomes beyond implantation
Unidad de Hemodinámica y Cardiología Intervencionista, Servicio de Cardiología, Hospital General Universitario de Elche, Elche, Alicante, Spain
Original articles
Debate
Debate: Does the distal radial approach offer added value over the conventional radial approach?
Yes, it does
Servicio de Cardiología, Hospital Universitario Sant Joan d’Alacant, Alicante, Spain
No, it does not
Unidad de Cardiología Intervencionista, Servicio de Cardiología, Hospital Universitario Galdakao, Galdakao, Vizcaya, España