ABSTRACT
Introduction and objectives: Spontaneous coronary artery dissection (SCAD) is a rare but increasingly recognized cause for acute coronary syndrome. The optimal management and treatment of SCAD is still unknown.
Methods: Data analysis of a prospective protocol including centralized care management of a consecutive series of patients with SCAD diagnosed between January 2010 and December 2018. Major adverse cardiovascular events included all-cause mortality, new myocardial infarction, coronary revascularization, ventricular arrhythmia, heart failure or stroke.
Results: A total of 33 consecutive patients were included (41 lesions). Intravascular imaging modalities were used to confirm the diagnosis in 42% patients. None of the patient showed images of thrombus formation in the true lumen. Conservative treatment was the initial approach in most of the cases (82%). No deaths were reported during the index admission, but 15% experienced major adverse cardiovascular events. The coronary computed tomography angiography performed in 58% of patients during the admission identified SCADs in 79% of the patients. Most of the patients managed with conservative treatment received only 1 antiplatelet agent for a limited period of time (17 months [9-35]). During a median clinical follow-up of 33 months [13-49], 82% of patients did not have any adverse events. The angiographic surveillance obtained in 48% of patients at the 6-month follow-up confirmed the complete healing of the SCAD image in 86% of the patients. The screening for extracoronary vascular findings (97% of patients) resulted in a high prevalence of abnormalities (59%).
Conclusions: The unrestricted use of intravascular imaging modalities showed no thrombus in the true lumen of patients with SCAD. In patients managed with conservative treatment, a limited course of antiplatelet monotherapy is safe and provides good clinical outcomes. Performing a coronary computed tomography angiography in the acute phase of SCAD is useful at the follow-up. The screening for extracoronary vascular findings confirmed a high prevalence of abnormalities.
Keywords: Spontaneous coronary artery dissection. Coronary artery disease. Acute coronary syndrome. Optical coherence tomography. Fibromuscular dysplasia.
RESUMEN
Introducción y objetivos: La disección coronaria espontánea (DCE) constituye una causa infrecuente, pero cada vez más reconocida, de síndrome coronario agudo. La actitud diagnóstico-terapéutica idónea sigue sin esclarecerse.
Métodos: Análisis del seguimiento prospectivo y centralizado de una serie de pacientes consecutivos diagnosticados de DCE desde enero de 2010 hasta diciembre de 2018. Se definió evento cardiovascular adverso mayor como la aparición de muerte de cualquier causa, reinfarto no mortal, revascularización no planificada, arritmia ventricular, insuficiencia cardiaca o ictus.
Resultados: Se incluyó a 33 pacientes con DCE (41 lesiones). En el 42% se realizó un estudio con imagen intracoronaria para confirmar el diagnóstico, sin identificar trombo en la luz verdadera en ninguno de ellos. En la mayoría de los casos (82%) se eligió un tratamiento médico conservador. Ningún paciente falleció durante el ingreso, pero el 15% presentó algún evento mayor. En el momento agudo se realizó tomografía computarizada coronaria al 58% de los pacientes y se identificó la DCE en el 79% de los casos. La mayoría de los pacientes con tratamiento conservador recibieron antiagregación simple un tiempo limitado (17 meses [9-35]). Con una mediana de seguimiento de 33 meses [13-49], el 82% no sufrió ningún evento adverso. Al 48% se les realizó control angiográfico a los 6 meses, que mostró resolución en el 86% de los casos. El cribado de anomalías vasculares extracoronarias se realizó en el 97% de los pacientes y se hallaron alteraciones en el 59%, incluyendo 3 pacientes con aneurisma intracraneal.
Conclusiones: En esta serie, con una amplia utilización de imagen intracoronaria, no se ha identificado trombo en la luz verdadera en ningún caso de DCE. En los pacientes tratados de forma conservadora, la monoterapia antiagregante es segura y se asocia a buenos resultados clínicos. La tomografía computarizada coronaria durante el ingreso es útil en el seguimiento. El cribado sistemático de anomalías vasculares extracoronarias revela una alta prevalencia de alteraciones.
Palabras clave: Disección coronaria espontánea. Enfermedad coronaria. Síndrome coronario agudo. Tomografía de coherencia óptica. Displasia fibromuscular.
Abbreviations ACS: acute coronary syndrome. EVA: extracoronary vascular abnormalities. FMD: fibromuscular dysplasia. PCI: percutaneous coronary intervention. SCAD: spontaneous coronary artery dissection.
INTRODUCTION
Spontaneous coronary artery dissection (SCAD) is a rare cause of acute coronary syndrome (SCA). However, especially in women, it has been identified as the underlying pathophysiological mechanism in a growing percentage of cases. SCAD is defined as the separation of the coronary artery wall layers not associated with trauma, iatrogenesis, atherosclerosis or extension of an aortic dissection.1 Clinical signs are myocardial ischemia and are due to the coronary flow limitation that alters the arterial parietal structures.
The first description ever reported by Pretty2 back in 1931 was followed by the description of isolated cases and small series for years. However, we have recently seen a significant increase of information on SCADs lately. Nowadays, clinical profile, diagnostic and therapeutic approach, and prognosis can be found in the SCAD and they vary significantly compared to atherosclerosis—the most common cause of ACS.3,4 Even the European Society of Cardiology5 and the American Heart Association6 have recently published 2 consensus documents on this disease.
In light of the growing evidence and in an attempt to enrich it, back in 2010 our center started a specific program of diagnosis and follow-up of patients with SCAD. The results and conclusions are presented here.
METHODS
All cases of SCAD were collected prospectively since 2010. Diagnosis, treatment, and follow-up were centralized and unified according to the scientific evidence available at the time. Given the length of the study period (9 years) and the extensive medical literature available on this issue over the years, new aspects in the assessment of patients (such as fibromuscular dysplasia [FMD]) have been introduced gradually. This protocol and the data collection book were approved by our center ethics committee and registered in a validated repository (NCT03607981). The patients’ informed consents were obtained in all cases.
Clinical information and follow-up
The demographic characteristics, the patients’ personal past medical histories, data at admission, and disease progression were collected in the clinical history at admission and follow-up in a SCAD monographic review (T. Bastante). The coronary angiography and intravascular imaging studies were analyzed by 3 expert interventional cardiologists (T. Bastante, M. García-Guimaraes, and F. Alfonso) and the final diagnosis of SCAD was only established if they all agreed unanimously. The use of intracoronary imaging modalities (intravascular ultrasound [IVUS] or optical coherence tomography [OCT]) was left to the operator’s discretion. However, it was recommended in cases of suspicious diagnosis (especially type 2 and 3 SCADs according to Saw angiographic classification7) or need to perform PCI as long as the segment under study was accessible and in a not overly tortuous artery. When the OCT was used, the intracoronary image was classified as double lumen when the separation of the arterial layers originated true and false lumens, both with lack of refraction due to complete contrast washout. Intramural hematoma was defined as the separation of arterial layers occupied by moderately refracting material with an attenuation consistent with intraparietal bleeding without complete contrast washout. Both the IVUS and the OCT tried to identify the communication between the false and the true lumen and the presence of thrombotic material in the latter (figure 1 shows typical examples). The initial recommended treatment was a wait-and-see conservative approach and the PCI was only performed in cases of clinical instability or symptom persistence. During admission, and as long as it was possible, a coronary computed tomography (CT) scan was performed for a better characterization of coronary lesions. This information was used during follow-up as a comparative pattern in a new coronary CT scan to confirm the healing of the SCAD or in the reappearance of symptoms for reevaluation purposes. Patients with a diagnosis suggestive, but not definitive, of SCAD were scheduled to receive a control coronary angiography within the following months.
Figure 1. Images of angiography (A-C) and optical coherence tomography (OCT) (D-I). A: type 1 spontaneous coronary dissection (SCAD) in the medial portion of the left anterior descending coronary artery. The arrows point to the characteristic imaging of double lumen with linear intraluminal filling defect outlined by contrast (video 1 of the supplementary data). B: type 2 SCAD in distal left anterior descending coronary artery and diagonal branch. The arrows point to the sudden loss of vessel caliber with length > 20 mm (video 2 of the supplementary data). C: type 3 SCAD in obtuse marginal artery. The arrows point to focal stenosis with length < 20 mm similar to an atherosclerotic lesion (video 3 of the supplementary data). D-F: OCT images showing the double lumen morphology (TL, true lumen; FL, false lumen). Note the unusual image of subintimal calcium displaced with the flap (++). G-H: morphology of intramural hematoma (IMH). I: entry (arrow) with partially thrombosed false lumen. The asterisk (*) shows guidewire artifact.
Definitions
In order to classify the angiographic patterns of SCAD, the aforementioned specific classification developed by Saw et al.7 was used (figure 1 shows examples of this). Two different criteria of success were established for cases where a PCI was required. In the first place, conventional procedural success was defined as a final TIMI flow grade 2-3 (Thrombolysis in Myocardial Infarction) with residual stenosis < 30% after stent/scaffold implantation or < 50% after simple balloon angioplasty. Secondly, the PCI-SCAD was considered successful with flow improvements ≥ 1 grade in the TIMI score and a final TIMI flow grade of 2-3.8 Major cardiovascular adverse events (MACE) at the follow-up included all-cause mortality, reinfarction, unscheduled revascularization, ventricular arrhythmia, heart failure, and stroke.
Screening of extracoronary vascular abnormalities
Since 2013 and as long as it was possible, a selective angiography of both renal and iliac arteries during the diagnostic coronary angiography was performed. Also, 3 to 6 months after the event, the study was completed using the angio-CT scan to examine the floor of the middle cranial fossa up to the femoral arteries (modification of the protocol published by Liang et al.9) including intracranial vessels, supra-aortic trunks, the aorta, and mesenteric, renal, and iliac branches. FMD was defined as the presence of focal narrowing separated by dilatation areas with the traditional «pearl necklace» appearance (multifocal shape) or the presence of tubular focal lesions (unifocal shape). Aneurysms were defined as dilatations > 50% with respect to the caliber of the normal, adjacent arterial segment. Dissection was defined as a double lumen morphology in the arterial segment. The screening of extracoronary vascular abnormalities (EVA) was considered complete when the intracranial territories, supra-aortic trunks, the aorta, and the splanchnic, renal, and iliac territories all had been examined (using angiography, angio-CT scan or both).
Statistical analysis
Quantitative variables were expressed as mean ± standard deviation or median [interquartile range] according to their distribution. Categorical variables were expressed as numbers (percentage). The analysis was conducted using the STATA 12 statistical software package (StataCorp LLC, United States).
RESULTS
Between January 2010 and December 2018 our center performed 12 951 diagnostic coronary angiographies that identified 37 SCADs (41 lesions) in 33 patients (0.28%). Prevalence among the coronary angiographies performed due to ACS (4185) was 1%, although prevalence among women in this context rose to 3%. If the percentage of patients with a final diagnosis of SCAD in the group of women with ACS under 50 is analyzed, prevalence rose to 12.5%. There are more diagnoses over the years from 1 or 2 patients per year initially to 5-7 annual patients over the last period (figure 1 of the supplementary data).
The baseline characteristics of the patients included in the study are shown on table 1. Most (97%) were middle-aged women (56 ± 12 years). Only 7 women (21%) had no traditional cardiovascular risk factors. Five patients (15%) had a personal past medical history of ischemic heart disease, 2 of them with a confirmed diagnosis of SCAD. A study conducted a posteriori confirmed that the remaining 3 patients showed clinical signs consistent with an initially misdiagnosed SCAD (ACS with coronary arteries interpreted as normal, 1 of them in the peripartum).
Table 1. Baseline characteristics of the patients
| n = 33 | |
|---|---|
| Women | 32 (97) |
| Age (years) | 56 ± 12 |
| Race | |
| Caucasian | 28 (85) |
| Other | 5 (15) |
| Cardiovascular risk factors | |
| Smoking habit | |
| Current smoker | 9 (27) |
| Former smoker | 7 (21) |
| Hypertension | 12 (36) |
| Hypercholesterolemia | 14 (42) |
| Diabetes | 2 (6) |
| Family history of ischemic heart disease | 4 (12) |
| Family history of SCAD | 2 (6) |
| Relevant findings | |
| Previous diagnosis of ischemic heart disease | 5 (15) |
| Confirmed diagnosis of previous SCAD | 2 (6) |
| Chronic inflammatory disease | 3 (9) |
| Depressive disorder | 5 (15) |
| Anxiety disorder | 9 (27) |
| History of hypothyroidism | 11 (33) |
| Gynecological/obstetric past medical history | n = 32 |
| Menopause | 24 (75) |
| Menopause age (years) | 49 ± 4 |
| Hormone replacement therapy | 2 (7) |
| Oral hormonal contraceptive | 1 (3) |
| Intrauterine device | 1 (3) |
| Nulliparous | 3 (9) |
| Multiparous | 18 (44) |
| History of miscarriage | 3 (9) |
|
SCAD, spontaneous coronary artery dissection. Data are expressed as no. (%) or mean ± standard deviation. |
|
Table 2 shows the characteristics at hospital admission and during the angiographic assessment. All patients presented with myocardial infarction, most of them (73%) with non-ST-elevation acute myocardial infarction. There was a trigger factor in one third of the cases; the most common was emotional stress (21%) followed by intense physical exercise (9%). Presentation at the peripartum was rare (1 patient only). The artery most frequently compromised was the left anterior descending coronary artery (51%). Eighteen percent of the patients had multivessel disease. Intracoronary imaging modalities (IVUS or OCT) were used in 42% of the cases, mostly OCT (33%). Sixteen lesions in 14 patients were assessed. Those assessed through the OCT confirmed the presence of fenestration between the false and the true lumen in 7 lesions (58%). There were no images consistent with thrombi in the true lumen in any of the cases assessed using intracoronary imaging modalities.
Table 2. Characteristics of the patients at hospital admission and in the angiographic assessment
| n = 33 | |
|---|---|
| Clinical diagnosis at admission | |
| STEMI | 9 (27) |
| NSTEMI | 24 (73) |
| Event-triggering factors | 11 (33) |
| Intense physical exercise | 3 (9) |
| Emotional stress | 7 (21) |
| Peripartum | 1 (3) |
| Angiographic characteristics | n = 33 (41 lesions) |
| Access | |
| Radial | 29 (88) |
| Femoral | 4 (12) |
| Diseased vessel | |
| Left anterior descending coronary artery | 21 (51) |
| Circumflex artery | 10 (24) |
| Right coronary artery | 10 (24) |
| Diseased segment | |
| Proximal | 10 (24) |
| Medial | 11 (27) |
| Distal | 20 (49) |
| Secondary branches | 18 (44) |
| Multivessel disease | 6 (18) |
| Multi-segment disease | 13 (32) |
| Saw et al. angiographic classification7 | |
| Type 1 | 6 (15) |
| Type 2 | 32 (78) |
| Type 3 | 3 (7) |
| Percentage of stenosis (visual estimate) | 77 ± 24 |
| Length of the lesion (mm) | 41 ± 28 |
| Initial TIMI flow grade | |
| 0 | 5 (12) |
| 1 | 5 (12) |
| 2 | 1 (2) |
| 3 | 3 (73) |
| Intracoronary imaging modality | n = 14 (16 lesions) |
| IVUS | 4 lesiones |
| Fenestration | 0 |
| Thrombus | 0 |
| OCT | 12 lesions |
| Double lumen | 7 (58) |
| Intramural hematoma | 2 (16) |
| Both | 3 (25) |
| Fenestration | 7 (58) |
| Thrombus | 0 |
|
CT, computed tomography; IVUS, intravascular ultrasound; NSTEMI, non-ST-elevation acute myocardial infarction; OCT, optical coherence tomography; STEMI, ST-segment elevation myocardial infarction; TIMI, Thrombolysis in Myocardial Infarction. Data are expressed as no. (%) or mean ± standard deviation. |
|
Table 3 shows treatment and the in-hospital disease progression. Initial conservative treatment was the first option in most cases (82%). Only 6 patients were treated with PCI as the initial strategy, 4 of them due to progressive flow worsening with the injections of contrast. The PCI conventional success was reported in 50% of the cases, and the PCI-SCAD success in 67% of the cases. One iatrogenic dissection was reported in the left main coronary artery.
Table 3. Management and in-hospital disease progression of patients
| n = 33 | |
|---|---|
| Initial treatment | |
| Conservative | 27 (82) |
| PCI | 6 (18) |
| PTCA-balloon | 2 (6) |
| Bare-metal stent | 2 (6) |
| Drug-eluting stent | 1 (3) |
| Bioresorbable vascular scaffold device | 1 (3) |
| Results from the PCI group | n = 6 |
| Conventional success | 3 (50) |
| PCI-SCAD success | 4 (67) |
| In-hospital disease progression | n = 33 |
| Peak troponin T levels (ng/mL) | 378 [132-1705] |
| Peak creatine kinase levels (U/L) | 403 [169-1181] |
| Left ventricular dysfunction [LVEF < 50%] | 5 (17) |
| Segmental abnormalities on the TTE | 17 (52) |
| MACE | 5 (15) |
| Death | 0 |
| Reinfarction | 0 |
| New coronary angiography | 4 (12) |
| Unplanned revascularization | 3 (9) |
| PCI group (n = 6) | 2 (33) |
| Conservative management group (n = 27) | 1 (4) |
| Ventricular tachycardia/fibrillation | 2 (6) |
| Heart failure | 1 (3) |
| Hospital stay (days) | 4 [3-7] |
| Coronary CT scan at admission | n = 19 (58) |
| SCAD visible on the coronary CT scan | 15 (79) |
| Treatment at hospital discharge | n = 33 |
| ASA | 31 (94) |
| Clopidogrel | 9 (27) |
| Ticagrelor | 5 (15) |
| Prasugrel | 0 |
| Dual antiplatelet therapy | 14 (42) |
| Anticoagulation | 2 (6) |
| Beta-blockers | 28 (85) |
| ACEI/ARA II | 21 (64) |
| Statins | 25 (76) |
| Nitrates | 3 (9) |
| Calcium antagonists | 3 (9) |
|
ACEI, angiotensin-converting enzyme inhibitors; ARA-II, angiontensin II receptor antagonist; ASA, acetylsalicylic acid; CT, coronary tomography; LVEF, left ventricular ejection fraction; MACE, major cardiovascular adverse events; PCI, percutaneous coronary intervention; PTCA, percutaneous transluminal coronary angioplasty; SCAD, spontaneous coronary artery dissection; TTE, transthoracic echocardiography. Data are expressed as no. (%) or mean ± standard deviation or median [interquartile range]. |
|
During in-hospital disease progression no patient died or suffered any reinfarctions. However, a new coronary angiography was required in 4 patients with symptoms. Except for the patient with a left main coronary artery iatrogenic dissection initially treated with conservative treatment no case was due to failed initial conservative treatment. The remaining 3 patients had acute stent thrombosis, SCAD of a vessel other than the index, and progression of the SCAD adjacent to the segment treated with the stent. Overall, the rate of in-hospital MACE was 15% and events focused on patients who required PCI. Acetylsalicylic acid (ASA) was prescribed to 94% of the patients at hospital discharge and dual antiplatelet therapy to 14 patients only (42%) of whom 7 required PCI. Fifty-eight percent of the patients received coronary CT scans during admission and images consistent with SCAD were found in 79% of the cases.
Table 4 shows out-of-hospital disease progression. Median follow-up was 33 months [13-49], the overall rate of events was 18%. Two deaths were reported, 1 due to cardiovascular causes (sudden death 6 years after the SCAD) and the other due to non-cardiovascular causes (sepsis in the abdominal postoperative). Only 1 patient required a new revascularization due to restenosis of the stent implanted to treat the SCAD. Three out of the 4 patients (12%) with SCAD relapse had suffered events prior to the index event that were compatible with SCAD; that is, each one of them had presented with, at least, 3 events. Except for 1 recurrence at the 7-month follow-up, most events occurred more than 2 years after the index event (figure 2). Regarding pharmacological treatment, ASA was kept for a median 17 months [9-35] after the event and the second antiplatelet drug was withdrawn early in most of the patients. Of the patients who received conservative treatment, only 25% were still on dual antiplatelet therapy 6 months after the event (median 0 months [0-6]). In those patients who required PCI, dual antiplatelet therapy was keep for a median 5 months [1-7].
Table 4. Out-of-hospital disease progression and follow-up of the patients
| n = 33 | |
|---|---|
| Follow-up time (months) | 33 [13-49] |
| MACE | 6 (18) |
| Death | 2 (6) |
| New AMI | 3 (9) |
| Recurrence | 4 (12) |
| New revascularization | 1 (3) |
| Heart failure | 1 (3) |
| Stroke | 1 (3) |
| Time on ASA (months) | 17 [9-35] |
| Time on dual antiplatelet therapy (months) | |
| Conservative treatment group | 0 [0-6] |
| PCI group | 5 [1-7] |
| Control SCAD | n = 16 (48) |
| Coronary CT scan | 9 |
| Planned | 6 |
| Due to symptoms | 3 |
| Invasive coronary angiography | 11 |
| Planned | 3 |
| Due to symptoms | 8 |
| Screening of EVA | N = 32 (97) |
| Type of screening | |
| CT scan | 18 (56) |
| Angiography | 5 (16) |
| Angiography + CT scan | 9 (28) |
| Complete screening | 28 (88) |
| EVA data | 19 (59) |
| Type of EVA | |
| Fibromuscular dysplasia | 15 (47) |
| Aneurysm | 5 (15) |
| Other | 1 (3) |
| Location of EVA | |
| Renal arteries | 9 (28) |
| Iliac arteries | 7 (22) |
| Supra-aortic trunks | 5 (16) |
| Intracranial | 3 (9) |
| Other | 5 (16) |
|
AMI, acute myocardial infarction; ASA, acetylsalicylic acid; CT, computed tomography; EVA, extracoronary vascular abnormalities; MACE, major cardiovascular adverse events; PCI, percutaneous coronary intervention; SCAD, spontaneous coronary artery dissection. Data are expressed as no. (%) or mean ± standard deviation or median [interquartile range]. |
|
Angiography control was performed in 48% of the patients, in 9 of them using coronary CT scan and invasive coronary angiography in 11 patients. The coronary CT scan was performed in 3 patients in the context of a new episode of chest pain. After comparing it with the previous CT scan performed at the index event, the new SCAD was discarded (figure 3). However, most coronary angiographies were performed in the context of a new cardiac event; only 3 patients received a planned control coronary angiography. Out of the 16 patients on angiographic control, imaging improved with restitutio ad integrum in 75% of them. Six months after the SCAD, the documented rate of resolution rose to 86%.
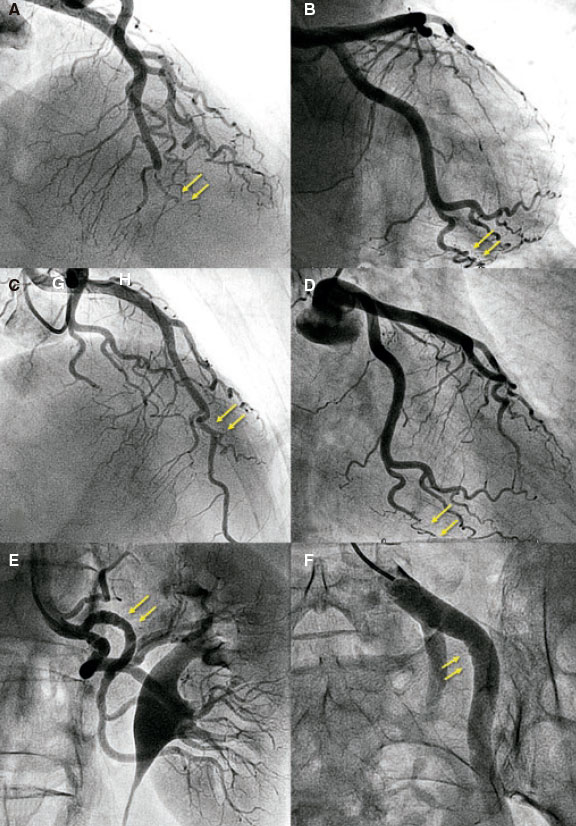
Figure 2. Fifty-five-year old woman with non-ST-elevation acute myocardial infarction (NSTEMI). She had experienced 2 previous acute myocardial infarctions with «normal coronary arteries» according to the coronary angiography. A: sudden caliber loss with tapering until the occlusion in the medial-distal portion of the left anterior descending coronary artery compatible with spontaneous coronary artery dissection (SCAD); conservative treatment. B: tortuous obtuse marginal artery without evident abnormalities. Two years later new hospitalization due to NSTEMI. C: caliber and flow recovery in the anterior descending coronary artery. D: obtuse marginal artery with sudden caliber loss and tapering (compare to B) compatible with SCAD; conservative treatment. E-F: selective angiographies of left renal and left iliac arteries with fibromuscular dysplasia.
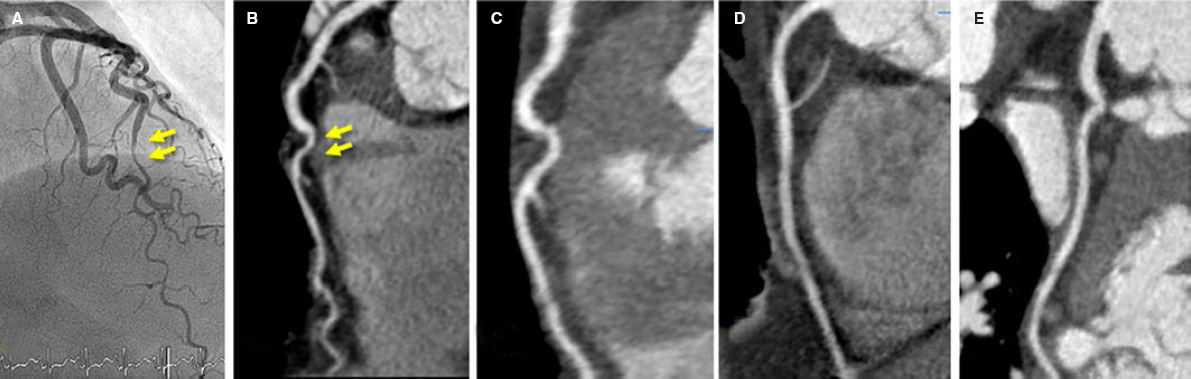
Figure 3. Fifty-three-year old woman with non-ST-elevation acute myocardial infarction. After a tortuous segment, the sudden caliber loss of the left anterior descending coronary artery with vessel tapering can be seen (arrows) on the coronary angiography (A) and coronary computed tomography (CT) scan (B) compatible with a spontaneous coronary artery dissection (SCAD) at the medial-distal portion of the left anterior descending coronary artery. Four years later, the patient presents to the ER with prolonged chest pain without alterations on the ECG or high markers of myocardial damage. The coronary CT scan shows the coronary arteries without images indicative of SCAD. C: left anterior descending coronary artery. D: right coronary artery. E: circumflex artery.
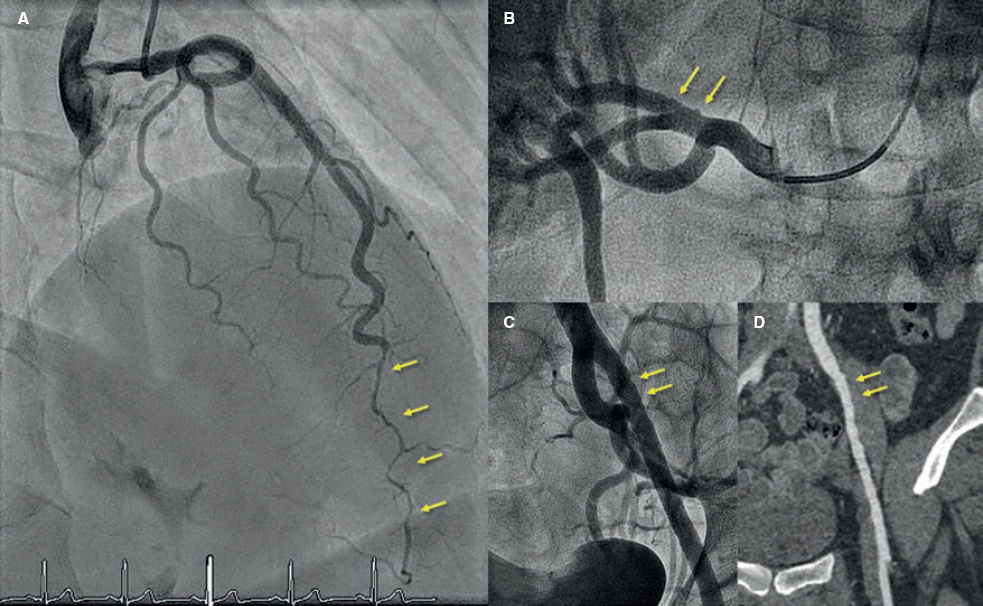
Figure 4. Forty-five-year old woman with non-ST-elevation acute myocardial infarction. The coronary angiography (A) shows a spontaneous coronary artery dissection at the medial-distal portion of the left anterior descending coronary artery (arrows). During catheterization the selective coronary angiography performed on the right renal (B) and left iliac arteries (C) shows wall irregularities compatibles with fibromuscular dysplasia (arrows). D: coronary computed tomography angiography at the follow-up with findings compatible with fibromuscular dysplasia on the left external iliac artery (arrows); note the greater sensitivity of coronary angiography (C) for the detection of subtle parietal abnormalities compared to coronary computed tomography angiography (D).
The screening of EVA was performed in 97% of the patients (full screening in 88%). Fifty-nine percent of the patients showed abnormalities that went up to 61% when the screening of EVA was complete. The abnormality most commonly found was FMD (47%) followed by arterial aneurysms (in 5 patients, 3 of which were intracranial aneurysms). The renal and iliac arteries were the most commonly compromised arteries of all: half of the patients studied showed abnormalities in either one of these arteries (examples in figure 2 and figure 4). After the study, the stroke team indicated the closure of the 3 intracranial aneurysms.
DISCUSSION
This study prospectively reports on the results of a current series of patients with SCAD with an updated and systematized diagnostic-therapeutic process and prolonged clinical follow-up. The clinical profile is consistent with what it is known about this disease:3,8,10 middle-aged woman with risk factors and low concomitance of chronic inflammatory disorders, autoimmune diseases or collagen diseases. Both the presentation and the angiographic characteristics were consistent with what has already been described: non-ST-elevation acute myocardial infarction that damaged the medial-distal segments and secondary branches predominantly with a higher incidence reported on the left anterior descending coronary artery. The most common Saw angiographic classification was type 2. Comparatively, in this series, the use of intracoronary imaging modalities was superior to other larger and recent series (42% vs 7.6% and 13% in the series of Saw et al.10 and Tweet et al.,8 respectively); this brings high reliability in the inequivocal diagnosis of SCAD. The most important conclusions of intracoronary imaging are: a) when OCT was the imaging modality used, the fenestration of both lumens could be identified in half of the lesions; b) the presence of mixed patterns (double lumen and intramural hematoma) within the same lesion is not an uncommon finding, which supports the evolutionary theory between both patterns; and c) lastly and probably the most important conclusion of all, intraluminal thrombi were not found in any of the lesions studied.
As it has already been described, an initial wait-and-see conservative approach with no interventions seems to bring good results to patients with SCAD.8,10,11 The rate of in-hospital MACE was low (15%). No deaths were reported, and bailout revascularizations were not necessary in any of the patients who received conservative treatment, except for 1 case due to iatrogenic dissection of left main coronary artery during the initial catheterization. Also, during the patients’ initial disease progression, they already showed preserved left ventricular ejection fraction. Similarly, out-of-hospital disease progression was good: 2 deaths were reported (1 due to non-cardiovascular causes) at the 2.7-year median follow-up, and 12% had a new episode of SCAD. These are similar data to those described in a Canadian series12 (10.4% at the 3.1-year median follow-up) and significantly lower to the rate of recurrence of 27% at the 2.3-years of median follow-up reported by Mayo Clinic.8
Unlike the atherosclerosis related ACS, in SCAD the ideal antithrombotic therapy has not been totally established. It seems logical to avoid aggressiveness, especially when 1 of the most plausible etiopathogenic theories is intraparietal bleeding of vasa vasorum as the initial event.13,14 Therefore, given the lack of intraluminal thrombus in a high percentage of patients studied with IVUS and OCT in this series a low-intensity antithrombotic therapy was used. ASA was kept for an average 1.5 years and the second antiplatelet drug was only indicated at hospital discharge in patients who required PCI and for the shortest period of time possible. The satisfactory disease progression reported with rates of out-of-hospital events consistent with those reported in other large series (from 10% to 20%)1 and the low rate of recurrence suggest that low-intensity antithrombotic therapy can be an excellent option for these patients.
There is very little information on the value of coronary CT scan during SCAD related hospitalizations. It was performed in 58% of patients from this series and SCAD was identified in three fourths of the cases. A more extensive analysis of these findings has been recently published by our group.15 The current study shows that this information was very useful in the follow-up of 3 patients to discard new episodes of SCAD and avoid the coronary angiography and associated risks for the patients (3.4% of iatrogenic dissections in patients with SCAD).16 However, in one fourth of the patients the SCAD could not be identified in the acute phase not even with the previous coronary angiography as guidance. Therefore, the value of coronary CT scan as an early diagnostic imaging modality is limited in this context.
Back in 2012 the association between SCAD and FMD17 was described for the first time, and later studies only not confirmed the high prevalence of this association but also of other EVA (aneurysms, dissections, and thrombosis).18-20 In the European consensus document recently published the screening of EVA is recommended in patients with SCAD.5 To our knowledge and up to this day this study shows the results of the most complete screening of FMD and other EVA. With a study in 97% of the patients—complete in 88%—the great presence of EVA (60%) confirms this interesting association. The need to conduct these studies may be put into question since most findings are associated with discrete and typical parietal abnormalities of FMD that do not lead necessarily to significant functional disorders. As a matter of fact, after the long follow-up of patients and despite the high prevalence of EVA, the extracardiac arterial events reported were only 1 stroke. However, there are 3 reasons to support the screening: a) in case of suspicious diagnosis, it may be the key to confirm the diagnosis of SCAD;21 b) knowing arterial parietal structural alterations can be useful for the diagnosis and treatment of future extracardiac events; and c) the finding of intracranial aneurysms is not negligible (9% in our series, but up to 14% in the Canadian series16) and it is relevant due to the risk of intracranial bleeding and secondary morbimortality. As a matter of fact, in 3 of our patients a percutaneous coronary intervention was indicated to seal the intracranial aneurysm.
Limitations
The main limitations of this study are the small size of the sample and the fact that it focused on a single center only. However, this study has a long follow-up with a unified treatment given the centralization of the patients.
CONCLUSIONS
In our center the centralization and protocolization of patients with SCAD systematized both treatment and the performance of additional tests. Intracoronary imaging allows us to confirm diagnosis in angiographically suspicious cases without showing any thrombi in the true lumen whatsoever. A low-intensity antithrombotic strategy with ASA only and for a limited period of time seems to give good results in the management of SCADs with conservative treatment. The high rate of spontaneous resolution of SCAD was confirmed in the 6-month images. Over half of the patients with SCADs show some EVA. Performing a coronary CT scan in the acute phase was useful, comparatively speaking, in new events and scheduled controls.
CONFLICTS OF INTEREST
F. Alfonso is an associate editor of REC: Interventional Cardiology; the editorial protocol of the journal was observed to guarantee an impartial manuscript handling.
WHAT IS KNOWN ABOUT THE TOPIC?
- SCAD is a rare disease more commonly regarded as the cause of ACS, especially in women.
- The pathophysiological substrate and prognosis are different from common atherosclerosis as well as the management recommended.
- To this day, the information on SCADs comes from many retrospective series since no randomized, controlled clinical trials have been conducted yet.
WHAT DOES THIS STUDY ADD?
- This was a prospective study with a fairly long follow-up that collected data on a specific diagnostic, therapeutic, centralized, and updated approach based on the new scientific evidence available on the management of SCAD.
- The study presented the results of an almost universal screening of ECA with a high percentage of patients with unequivocal diagnosis of SCAD (thanks to the common use of intravascular imaging modalities) and angiographic control during disease progression.
- Treatment with a very low-intensity antithrombotic strategy (antiplatelet therapy with ASA only and not indefinitely) is safe with excellent results during disease progression.
SUPPLEMENTARY DATA
Video 1. Bastante T. DOI: 10.24875/RECICE.M20000096
Video 2. Bastante T. DOI: 10.24875/RECICE.M20000096
Video 3. Bastante T. DOI: 10.24875/RECICE.M20000096
REFERENCES
1. Saw J, Mancini GBJ, Humphries KH. Contemporary Review on Spontaneous Coronary Artery Dissection. J Am Coll Cardiol. 2016;68:297-312.
2. Pretty H. Dissecting aneurysms of coronary artery in woman aged 42:rupture. BMJ. 1931;1:667.
3. Alfonso F, Bastante T. Spontaneous coronary artery dissection novel diagnostic insights from large series of patients. Circ Cardiovasc Interv. 2014;7:638-641.
4. Bastante T, Cuesta J, García-Guimaraes M, et al. Current management of spontaneous coronary artery dissection. Expert Rev Cardiovasc Ther. 2017;15:619-628.
5. Adlam D, Alfonso F, Maas A, et al. European Society of Cardiology, acute cardiovascular care association, SCAD study group:a position paper on spontaneous coronary artery dissection. Eur Heart J. 2018;39:3353-3368.
6. Hayes SN, Kim CESH, Saw J, et al. Spontaneous Coronary Artery Dissection:Current State of the Science:A Scientific Statement from the American Heart Association. Circulation. 2018;137:e523-e557.
7. Saw J. Coronary angiogram classification of spontaneous coronary artery dissection. Catheter Cardiovasc Interv. 2014;84:1115-1122.
8. Tweet MS, Eleid MF, Best PJM, et al. Spontaneous coronary artery dissection:Revascularization versus conservative therapy. Circ Cardiovasc Interv. 2014;7:777-786.
9. Liang JJ, Prasad M, Tweet MS, et al. A novel application of CT angiography to detect extracoronary vascular abnormalities in patients with spontaneous coronary artery dissection. J Cardiovasc Comput Tomogr. 2014;8:189-197.
10. Saw J, Starovoytov A, Humphries K, et al. Canadian spontaneous coronary artery dissection cohort study:in-hospital and 30-day outcomes. Eur Heart J. 2019;40:1188-1197.
11. Alfonso F, Paulo M, Lennie V, et al. Spontaneous coronary artery dissection:long-term follow-up of a large series of patients prospectively managed with a “conservative“ therapeutic strategy. JACC Cardiol Intv. 2012;5:1062-1070.
12. Saw J, Humphries K, Aymong E, et al. Spontaneous coronary artery dissection:Clinical outcomes and risk of recurrence. J Am Coll Cardiol. 2017;70:1148-1158.
13. Waterbury TM, Tweet MS, Hayes SN, et al. Early natural history of spontaneous coronary artery dissection. Circ Cardiovasc Interv. 2018;11:e006772.
14. Jackson R, Al-Hussaini A, Joseph S, et al. Spontaneous coronary artery dissection. Patophisiological insights from optical coherence tomography. JACC Cardiovasc Imaging. 2019;12:2475-2488.
15. Pozo-Osinalde E, García-Guimaraes M, Bastante T, et al. Characteristic findings of acute spontaneous coronary artery dissection by cardiac computed tomography. Coron Artery Dis. 2019. https://doi.org/10.1097/MCA.0000000000000819
16. Prakash R, Starovoytov A, Heydari M, et al. Catheter-Induced iatrogenic coronary artery dissection in patients with spontaneous coronary artery dissection. J Am Coll Cardiol Intv. 2016;9:1851-1852.
17. Saw J, Poulter R, Fung A, et al. Spontaneous coronary artery dissection in patients with fibromuscular dysplasia:a case series. Circ Cardiovasc Interv. 2012;5:134-137.
18. Saw J, Aymong E, Sedlak T, et al. Spontaneous coronary artery dissection:association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv. 2014;7:645-655.
19. Bastante T, Rivero F, Cuesta J, et al. Association of spontaneous coronary artery dissection with fibromuscular dysplasia. Rev Esp Cardiol. 2015;68:719-720.
20. Prasad M, Tweet MS, Hayes SN, et al. Prevalence of extracoronary vascular abnormalities and fibromuscular dysplasia in patients with spontaneous coronary artery dissection. Am J Cardiol. 2015;115:1672-1677.
21. Bastante T, García-Guimaraes M, Rivero F, et al. Isolated septal branch lesion as the only diagnostic clue for spontaneous coronary artery dissection. Coron Artery Dis. 2020;31:98-99.