ABSTRACT
Introduction and objectives: Systemic coronary artery embolism is one of the mechanisms of acute myocardial infarction of nonatherosclerotic origin. However, the epidemiological, clinical, and angiographic profile of this entity has not been properly established yet. Our objective was to describe the clinical characteristics, angiographic features, and prognosis of acute coronary syndromes (ACS) due to systemic embolism (ACS-E), compare them to those due to coronary atherosclerosis (ACS-A), and identify predictive clinical factors of ACS-E.
Methods: All consecutive patients with ACS—admitted to a tertiary hospital from 2003 through 2018—were classified as ACS-E (n = 40) or ACS-A (n = 4989), and prospectively recruited on a multipurpose database.
Results: Patients with ACS-E were younger (27.5% vs 9.6% were < 45 years old, P < .001), more often women (42.5% vs 22.5%, P = .003), and had higher rates of atrial fibrillation (AF) (40.0% vs 5.3%, P < .001), previous stroke (15.0% vs 3.6%, P < .001), active neoplasms (17.5% vs 6.9%, P =.009), and previous valvular surgery (12.5% vs 0.5%, P < .001). Also, a higher proportion of them were on warfarin (27.5% vs 2.9%, P < .001). The most frequent culprit vessel was the left anterior descending coronary artery in both groups. A percutaneous coronary intervention was attempted in all patients with ACS-A, and in 75.0% of those with ACS-E (P < .001) being successful in 99.1% and 80.0%, respectively. The in-hospital all-cause mortality rate was 15.0% regarding ACS-E, and 4.0% in the control group (P < .001). A multivariate analysis was performed to study the independent predictors of ACS-E, identify AF, previous valvular surgery, and active neoplasms, younger age, and female sex.
Conclusions: ACS-E and ACS-A have different clinical and angiographic characteristics. Atrial fibrillation, previous valvular surgery, active neoplasms, younger age, and female sex were all independent predictors of ACS-E.
Keywords: Coronary artery embolism. Atrial fibrillation. Acute coronary syndrome. Myocardial infarction.
RESUMEN
Introducción y objetivos: La embolia coronaria de origen sistémico representa uno de los mecanismos de infarto agudo de miocardio de causa no aterosclerótica. Sin embargo, el perfil epidemiológico, clínico y angiográfico de esta entidad no ha sido aún bien definido. Nuestro objetivo fue describir las características clínicas y angiográficas y el pronóstico de los síndromes coronarios agudos (SCA) de origen embólico (SCA-E), compararlos con aquellos debidos a aterosclerosis (SCA-A) e identificar predictores clínicos de SCA-E.
Métodos: Todos los pacientes con SCA atendidos en un hospital terciario entre 2003 y 2018 se clasificaron en SCA-E (n = 40) o SCA-A (n = 4.989) e incluidos de forma prospectiva en un registro multipropósito.
Resultados: Entre los pacientes con SCA-E existía mayor proporción de jóvenes (27,5 frente a 9,6% tenían menos de 45 años, p < 0,001), mujeres (42,5 frente a 22,5%, p = 0,003), fibrilación auricular (FA) (40,0 frente a 5,3%, p < 0,001), neoplasias activas (17,5 frente a 6,9%, p = 0,009), cirugía valvular previa (12,5 frente a 0,5%, p < 0,001) y una mayor proporción de los mismos se encontraba en tratamiento con warfarina (27,5 frente a 2,9%, p < 0,001). El vaso responsable con mayor frecuencia fue la descendente anterior en ambos grupos. En todos los pacientes con SCA-A se llevó a cabo una intervención coronaria percutánea, frente al 75,0% de los pacientes con SCA-E (p < 0,001), la cual se completó con éxito en el 99,1% y el 80,0% de los casos, respectivamente. La mortalidad por todas las causas en el grupo de SCA-E fue del 15,0% frente al 4,0% en el grupo control (p < 0,001). Se llevó a cabo un análisis multivariante para estudiar predictores independientes de SCA-E, identificando la FA, la cirugía valvular previa, la presencia de una neoplasia activa, una menor edad y el sexo femenino.
Conclusiones: Los SCA-E y los SCA-A presentan características clínicas y angiográficas diferentes. La FA, la cirugía valvular previa, la presencia de una neoplasia activa, ser más joven y el sexo femenino son predictores independientes de SCA-E.
Palabras clave: Embolia coronaria. Fibrilacion auricular. Sindrome coronario agudo. Infarto de miocardio.
Abbreviations
ACS: acute coronary syndrome. ACS-A: acute coronary syndrome due to atherosclerosis. ACS-E: acute coronary syndrome due to systemic embolism. AF: atrial fibrillation. AMI: acute myocardial infarction. STEMI: ST-segment elevation myocardial infarction.
INTRODUCTION
Systemic coronary artery embolism is one of the mechanisms of acute myocardial infarction (AMI) of non-atherosclerotic origin and represents 3% to 14% of all acute coronary syndromes (ACS) reported, according to angiographic and autopsy studies. However, the real prevalence of this entity remains unknown due to the uncertainty of its diagnosis in the acute setting.1,2
Atrial fibrillation (AF), cardiomyopathies, valvular heart disease, malignancies, and infective endocarditis have previously been associated with ACS due to systemic embolism (ACS-E).1,3 Nevertheless, the epidemiological, clinical, and angiographic profile of this entity has not been properly established yet.
Our objective was to describe the clinical characteristics, angiographic features, therapeutic management, and prognosis of ACS-E, compare it to ACS due to coronary atherosclerosis (ACS-A), and identify predictive clinical factors of ACS-E.
METHODS
Study population
All consecutive patients with ACS—admitted to a tertiary hospital from January 2003 through December 2018—were evaluated, classified as ACS-E or ACS-A, and prospectively recruited on a multipurpose database. The protocol was approved by the local ethics committee (internal code 22/137-E), and patients’ informed consent was waived because it involved only the analysis of data obtained during standard clinical practice.
AMI was defined as elevated cardiac troponin levels (myocardial injury) with clinical evidence of acute myocardial ischemia including symptoms, new ischemic electrocardiographic changes, development of pathological Q waves on the electrocardiogram, new regional wall motion abnormalities in a pattern consistent with ischemic aetiology, and/or angiographic identification of a coronary thrombus.4 All patients underwent a thorough diagnostic work-up including detailed clinical histories and physical examinations, serial electrocardiograms, blood tests, transthoracic echocardiographies, and invasive coronary angiographies. Intracoronary imaging techniques like optical coherence tomography or intravascular ultrasound were left to the operator’s discretion.
Diagnosis of ACS-E was achieved according to the angiographic evidence of coronary artery thrombosis without atherosclerotic components, concomitant multi-site coronary artery embolism or concomitant systemic embolization excluding left ventricular thrombus due to AMI.1 Only emboli of principal coronary arteries were considered. Patients with the following angiographic findings were systematically excluded: a) presence of atherosclerosis at culprit lesion level, b) evidence of > 25% coronary artery stenosis outside the culprit lesion, c) plaque rupture or coronary erosion at culprit lesion level found on the intravascular imaging, d) coronary artery ectasia, and e) other causes of non-atherosclerotic AMI (vasospasm, spontaneous coronary artery dissection).
Angiographic evaluation of the culprit site was performed by 2 expert operators with an intention to rule out a) the presence of thrombus (defined as noncalcified filling defect outlined by contrast media), b) presence of angiographic stenosis, and c) signs of atherosclerosis (eg, vessel wall calcification). The rest of the angiogram was assessed looking for angiographic stenosis or atherosclerosis.
Clinical events
Epidemiological data, clinical features, angiographic characteristics, management, and outcomes were prospectively collected as patients were recruited and retrospectively analysed. The long-term follow-up of ACS-E was performed by monitoring any recurrences of systemic emboli (including cardiogenic stroke), and the occurrence of major adverse cardiovascular and cerebrovascular events including cardiac death, myocardial infarction, new percutaneous coronary intervention (PCI), hospitalization due to heart failure or stroke more than 30 days after admission due to ACS-E.
In the present study, we first performed a detailed description of the episodes of ACS-E followed by a comparison to ACS-A to identify clinical peculiarities, and predictors.
Statistical analysis
Quantitative variables were expressed as median and interquartile range [IQR] or mean and standard deviation. The assessment of normality and equality of variances for continuous data was performed using the Shapiro-Wilk test and the Levene test, respectively. Thereafter, continuous variables were compared using the Student t test, the Fisher-Pittman permutation test or the median test when appropriate. Categorical variables were expressed as frequencies and percentages.
Variables in which statistically significant differences were seen in the univariate model and those clinically relevant were introduced in a multivariate analysis using stepwise logistic regression to identify clinical predictors of ACS-E.
All tests were 2-sided, and differences were considered statistically significant with P values < .05. Statistical analyses were performed using Stata/IC12.1 statistical software package (StataCorp, College Station, Texas, United States).
RESULTS
During the study period, a total of 5029 patients with ACS were included. After applying the previously described diagnostic criteria, 40 patients (0.8%) were classified as ACS-E and 4989 (99.2%) as ACS-A.
Acute coronary syndrome due to systemic embolism population
Regarding patients with ACS-E, 17 were women (42.5%), and the population’s mean age was 60.3 years old. A total of 2 patients (5.0%) had a past medical history of exertional angina, 4 (10.0%) carried a prosthetic valve, and 2 (5.0%) and 1 (2.5%) had non-corrected severe mitral regurgitation, and severe aortic stenosis, respectively. The mean left ventricular ejection fraction was 55.0% ± 12.3%, and 16 patients (40.0%) had any form of AF. Also, 1 patient (2.5%) was diagnosed with infective endocarditis in the aortic valve right after being admitted due to ACS. Regarding other medical conditions, 7 patients (17.5%) had active neoplasms, and 3 (7.5%) chronic kidney disease. Information associated with other baseline characteristics is shown on table 1 of the supplementary data.
Table 1. Baseline epidemiological and clinical characteristics
| ACS-A N = 4989 | ACS-E N = 40 | P | |
|---|---|---|---|
| Age (years) | 63.0 ± 13.4 | 60.3 ± 18.7 | .129 |
| Age < 45 years | 480 (9.6) | 11 (27.5) | < .001 |
| Age > 80 years | 559 (11.2) | 9 (22.5) | .025 |
| Female sex | 1120 (22.5) | 17 (42.5) | .003 |
| Diabetes | 1087 (21.8) | 4 (10.0) | .070 |
| Hypertension | 2632 (52.8) | 16 (40.0) | .108 |
| Dyslipidemia | 2192 (43.9) | 11 (27.5) | .037 |
| Smoking | 3101 (62.2) | 22 (55.0) | .353 |
| BMI | 27.6 ± 4.1 | 27.1 ± 4.2 | .424 |
| Chronic kidney failure | 239 (4.8) | 3 (7.5) | .425 |
| Peripheral vascular disease | 241 (4.8) | 1 (2.5) | .493 |
| Stroke | 181 (3.6) | 6 (15.0) | < .001 |
| Active neoplasm | 343 (6.9) | 7 (17.5) | .009 |
| AF | 262 (5.3) | 16 (40.0) | < .001 |
| Treatment with warfarin | 143 (2.9) | 11 (27.5) | < .001 |
| Previous valvular surgery | 25 (0.5) | 5 (12.5) | < .001 |
| Past medical history of angina | 1698 (34.0) | 2 (5.0) | < .001 |
|
ACS-A, acute coronary syndrome due to atherosclerosis; ACS-E, acute coronary syndrome due to systemic embolism; AF, atrial fibrillation; BMI, body mass index; NSTEMI, non-ST-elevation acute myocardial infarction; STEMI, ST-segment elevation myocardial infarction. |
|||
A total of 32 patients (80.0%) had ST-segment elevation myocardial infarction (STEMI) 3 of whom received fibrinolytic therapy, undergoing bailout PCI in 2 of the cases. A total of 28 patients (70.0%) underwent a primary PCI and the remaining 12 (30.0%) were catheterized in another scenario. The most frequent culprit vessel was the left anterior descending coronary artery (LAD) that accounted for 13 (32.5%) of the cases followed by the right coronary artery (n = 10; 25.0%), and the left circumflex artery (n = 9; 22.5%). Besides, the proximal (n = 12; 30.0%) and medium (n = 12; 30.0%) segments of the vessels were the ones most often compromised (table 2 of the supplementary data).
| ACS-A N = 4989 | ACS-E N = 40 | P | |
|---|---|---|---|
| Culprit lesions | |||
| LMCA | 113 (2.3%) | 1 (2.5%) | .921 |
| LAD | 2274 (45.6%) | 15 (37.5%) | .108 |
| Cx | 1064 (21.3%) | 11 (27.5%) | .344 |
| RCA | 1902 (38.1%) | 10 (25.0%) | .125 |
| Number of vessels with moderate lesions (> 50%) | 1.6 ± 0.0 | 0.8 ± 0.1 | < .001 |
| Number of vessels with severe lesions (> 70%) | 1.3 ± 0.0 | 0.8 ± 0.1 | < .001 |
|
ACS-A, acute coronary syndrome due to atherosclerosis; ACS-E, acute coronary syndrome due to systemic embolism; Cx, circumflex coronary artery; LAD, left anterior descending coronary artery; LMCA, left main coronary artery; PCI, percutaneous coronary intervention; RCA, right coronary artery. |
|||
On the coronary angiography, 25 patients (62.5%) showed TIMI grade-0 flow (Thrombolysis in Myocardial Infarction) before crossing the wire. Twenty-nine cases (72.5%) received thrombus aspiration therapy and 7 underwent balloon angioplasty. None of the patients were treated with stenting, but TIMI grade-3 flow was observed in 32 cases (77.5%) after the PCI (table 3 of the supplementary data). Regarding intracoronary imaging during the PCI, pre- and postoperative optical coherence tomography and intravascular ultrasound were performed in 3 (7.5%) and 1 (2.5%) patients, respectively. Antithrombotic treatment at presentation and after PCI is shown on table 4 of the supplementary data.
Table 3. Complications during PCI, hospitalization, and follow-up in patients with ACS-E
| During PCI | |
| Cardiac arrest | 3 (7.5) |
| Slow flow/no reflow | 8 (20.0) |
| Perforation | 1 (2.5) |
| Embolizationa | 15 (37.5) |
| Coronary dissection | 0 (0) |
| Coronary perforation | 1 (2.5) |
| Cardiac tamponade | 0 (0) |
| During admission | |
| Vascular complicationsb | 2 (5.0) |
| Heart failure | 12 (30.0) |
| Arrhythmic complicationsc | 7 (17.5) |
| Extracardiac complicationsd | 9 (22.5) |
| Death | 6 (15.0) |
| At the follow-up | |
| MACCE | 13 (38.2) |
| AMI | 4 (11.8) |
| New PCI | 2 (5.9) |
| Stroke | 2 (5.9) |
| Hospitalization | 11 (32.4) |
| Heart failure | 11 (32.4) |
| NYHA | |
| I | 24 (70.6) |
| II | 6 (17.6) |
| III | 1 (2.9) |
| IV | 4 (11.8) |
| Systemic embolism | 0 (0) |
| Pulmonary embolism | 1 (2.9) |
| Deathe | 12 (35.3) |
|
AMI, acute myocardial infarction; PCI, Percutaneous coronary intervention; MACCE, major adverse cardiovascular and cerebrovascular events; NYHA, New York Heart Association Functional Classification. a In 2 cases, embolization of thrombotic material reached a different vessel from the culprit one. b 1 case of femoral pseudoaneurysm and radial pseudoaneurysm, respectively were treated with conservative measures. c 4 cases of bradyarrhythmia and 3 cases of tachyarrhythmia. d 8 cases of infection and 1 case of stroke coexisting with subarachnoid haemorrhage. e Due to heart failure in 5 cases, ventricular arrythmia in the AMI setting in 1 case and multiorgan failure due to advanced pulmonary neoplasm in a different case. In the remaining the patients, the cause of death could not be identified. |
|
Table 4. Multivariate analysis to identify clinical predictors of acute coronary syndrome due to systemic embolism
| Variables* | Adjusted OR (95%CI) | P |
|---|---|---|
| Age (years) | 0.95 (0.92-0.97) | < .001 |
| Female sex | 2.80 (1.37-5.65) | .007 |
| Dyslipidemia | 0.45 (0.22-0.93) | .024 |
| Active neoplasm | 3.37 (1.33-8.54) | .019 |
| Previous valvular surgery | 4.28 (1.19-15.5) | .038 |
| Past medical history of angina | 0.17 (0.05-0.55) | < .001 |
| AF | 16.10 (7.23-35.9) | < .001 |
|
95%CI, 95% confidence interval; AF, atrial fibrillation; OR, odds ratio. * Variables from the univariate model introduced in the analysis included: age, female sex, diabetes, dyslipidemia, stroke, active neoplasm, previous valvular surgery, past medical history of angina, AF, and chronic treatment with oral anticoagulants. |
||
Comparison between acute coronary syndrome due to systemic embolism and acute coronary syndrome due to atherosclerosis
Baseline characteristics
Compared to ACS-A, there was a significantly higher proportion of patients under 45 and over 80 years old in the ACS-E group. Besides, a higher proportion of women was observed (42.5% vs 22.5%; P = .003). Among these patients, cardiovascular risk factors were less prevalent compared to those with ACS-A, although statistically significant differences were only seen regarding dyslipidemia. A significantly higher proportion of patients with ACS-E had active neoplasms, AF, previous strokes, and had undergone heart valve surgery. Also, 27.5% of the patients from the ACS-E group were on warfarin (P < .001) at presentation whereas the patients with ACS-A often had a past medical history of angina (34.0% vs 5.0%; P < .001). The inter-group differences regarding other medical conditions are also shown on table 1.
Clinical and angiographic characteristics and outcomes
Regarding the episode of ACS, no differences were seen regarding the presentation as STEMI between both groups (ACS-E, 80.0% vs ACS-A, 67.0%; P = .082). However, patients with ACS-A showed significantly longer times between the diagnosis of ACS and the performance of a coronary angiography (1.16 ± 0.8 hours vs 0.81 ± 0.5 hours; P = .003). No differences were seen regarding the rate of cardiogenic shock.
The presence of other moderate or severe stenoses, apart from the culprit one, was significantly more frequent among patients with ACS-A (table 2). PCI was attempted in all the patients with ACS-A and in 75.0% of those with ACS-E (P < .001) being successful in 99.1% and 80.0%, respectively. Conversely, adjuvant treatment with GP IIb/IIIa inhibitors was used in 55.0% of the patients with ACS-E and 36.0% of the patients from the ACS-A group (P = .020).
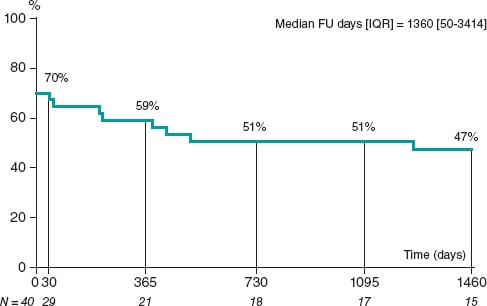
Complications during PCI and hospitalization in the ACS-E group are shown on table 3 including death that occurred in 5 patients (12.5%) due to heart failure/cardiogenic shock and anoxic encephalopathy after cardiac arrest in another case. A control coronary angiography was performed in 14 cases (40.0%) with persistence of culprit vessel compromise in 2 (14.3%). The median follow-up after the episode was 5.8 ± 4.8 years. Three days after emergency thrombus aspiration due to acute LAD occlusion, a 51-year-old woman with acute myeloid leukemia presented a recurrent ACS-E with new compromise of both the LAD and a marginal branch. No recurrent emboli in other systemic territories were identified in any of the cases. However, major adverse cardiovascular and cerebrovascular events at the follow-up occurred in 13 patients with ACS-E (38.2%) while death occurred in 12 patients (35.3%) being attributed to cardiac causes in 6 cases (50.0%) (table 3). The overall major adverse cardiovascular and cerebrovascular events-free survival during hospitalization and at the follow-up was estimated using Kaplan-Meier curves (figure 1).
Figure 1. MACCE-free survival during admission and at the 4-year follow-up in patients with ACS-E. ACS-E, acute coronary syndrome due to systemic embolism, FU, follow-up; IQR, interquartile range; MACCE, major adverse cardiovascular and cerebrovascular events.
The in-hospital all-cause mortality rate was 15.0% in the ACS-E group and 4.0% in the control group (P < .001).
Predictors of acute coronary syndrome due to systemic embolism
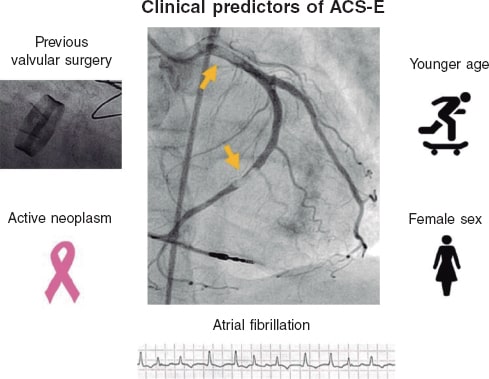
To determine the clinical predictors of ACS-E, a multivariate analysis was performed including those variables with statistically significant differences in the univariate model and those considered clinically relevant. Therefore, younger age, female sex, an active neoplasm, previous heart valve surgery, and a past medical history of AF were identified as independent predictive factors for ACS-E (table 4, figure 2).
Figure 2. Independent clinical predictors of ACS-E. ACS-E, acute coronary syndrome due to systemic embolism.
DISCUSSION
The main findings of our study include a) the prevalence of ACS-E in patients admitted due to AMI was low (0.8%); b) the in-hospital mortality rate was higher among patients with ACS-E as compared to ACS of atherosclerotic origin; and c) being younger, female sex, an active neoplasm, previous heart valve surgery, and AF were identified as ACS-E predictors.
Systemic coronary artery embolism is one of the underlying mechanisms of AMI of non-atherosclerotic cause.4 First autopsy studies reported a prevalence of coronary emboli in patients with AMI of 13%5 although subsequent studies conducted at the clinical setting described a frequency of around 3%.1 The low prevalence seen in our series (0.8%) may be associated with strict diagnostic criteria excluding patients with coronary artery stenosis > 25% outside the culprit lesion, and emboli due to secondary coronary arteries. However, the real occurrence of ACS-E remains unknown since the early presentation can be indistinguishable from an ACS-A.6
Also, the limited rate of coronary artery emboli reported compared to other vascular territories may also be associated with intrinsic anatomical and physiological characteristics like aortic caliber differences, the acute angle at which the coronary arteries originate at the sinus of Valsalva,7 and the position of the coronary ostia behind the valve cusps during systole.3,8
Although some series comparing ACS-A and ACS-E have not described gender differences when focusing on STEMI,2 in our study, the proportion of women was significantly higher among ACS-E (43% vs 22%; P = .003). Similarly, Shibata et al. reported rates of 40% vs 29% (P = .087).1 Besides, according to the aforementioned authors, a lower prevalence of traditional cardiovascular risk factors was seen within the embolic group of our cohort, although statistically significant differences were only noticed regarding dyslipidemia (27.5% vs 43.9%; P = .037).
Regarding the compromise of coronary arteries, the LAD was the most commonly affected vessel in both the ACS-E and the ACS-A (table 3). Similarly, a previous autopsy study had shown that coronary emboli are up to 4 times more common in the LAD compared to the right coronary artery, and in the LAD compared to the the left circumflex artery.5 Also, in a recent systematic review including 129 case reports and case series of coronary emboli, Lacey et al. described that the LAD was the most frequently affected vessel (45.3%).6 However, such differences in the distribution of culprit coronary vessels may be explained by bias associated with the fact that arteries with larger territories are more likely to be involved in autopsies1 and case reports.
On the interventional treatment used, in our study, 30 patients (75.0%) from the ACS-E group underwent thrombus aspiration followed by balloon angioplasty in 8 cases. None of the patients from this group were treated with stenting. Similarly, Shibata et al. performed initial thrombus aspiration in 96.6% of embolic patients undergoing PCI followed by balloon angioplasty in 14.3% of the cases and stenting in 17.9%.1 Thrombus aspiration has proven to be a feasible option to treat AMI with angiographic evidence of thrombus including cases associated with coronary emboli.9 However, these devices may be less useful to aspirate large thrombi due to the smaller diameter of the lumen of the inner catheter.10 Besides, in specific situations like small arteries or distal coronary occlusions, simple wire manipulation added to antithrombotic drugs (including glycoprotein IIb/IIIa inhibitors, which were more frequently used in the ACS-E group) may be the preferred option to achieve reperfusion.2
At the follow-up after an episode of ACS-E (5.8 ± 4.8 years) in our series, the major adverse cardiovascular and cerebrovascular events occurred in 37.1% of the patients. However, no recurrences of systemic emboli were documented in accordance with other previous series.2 The in-hospital all-cause mortality rate was significantly higher among patients with ACS-E (15% vs 4%; P < .001) mainly due to cardiovascular causes. Shibata et al. reported no differences in the 30-day mortality rate, but significantly higher cardiovascular and all-cause mortality rates in ACS-E compared to ACS-A.1 Similarly, Popovic et al. observed that 64% of all deaths reported at the follow-up after an episode of STEMI due to coronary embolism were due to cardiac causes.2
Finally, after multivariate analysis, AF, previous heart valve surgery, active neoplasm, female sex, and younger age were identified as clinical predictors of ACS-E. AF has been described as the most frequent condition predisposing to coronary artery embolism being present in 40.0% of ACS-E in our study and in up to 73% in other current series.1,3 However, early studies reported that valvular heart disease, especially rheumatic, and infective endocarditis represented the most common causes of coronary artery embolism.5,11 This disparity may be associated with the advances made in antibiotic therapy implementation over the last few decades, and the remarkable increase of AF prevalence parallel to the gradual aging of the population.1,2,12,13 Furthermore, it has been reported that the risk of AMI associated with AF is significantly higher in women14,15 and patients without coronary artery disease.15-18
On the other hand, it is fully recognized that patients with active neoplasms are at a significantly higher risk of developing thrombotic events, both venous and arterial.19 The pathogenesis of cancer-associated coagulopathy is complex including a multifactorial interaction among the patient’s comorbidities, the specific malignancy, and treatment with several chemotherapeutic agents or immunomodulatory drugs that often lead to hypercoagulability, platelet activation, and endothelial injury.20 Besides, it has also been described that malignancy is associated with a higher risk of developing AF following interactions at the pathophysiological level.21,22 In our series, 17.5% of the patients presented active neoplasms in accordance with Popovic et al.2 who reported a prevalence of 15.1% notably higher than the one reported by Shibata et al.1 and Lacey et al.6 of 10% and 1.4%, respectively.
Limitations
The present study presents several limitations. First, being a retrospective study may have resulted in a certain degree of bias. Secondly, applying strict diagnostic criteria which excluded patients with ≥ 25% coronary artery stenosis outside the culprit lesion may have omitted cases of ACS-E in patients with concomitant coronary artery disease. Thirdly, in contrast with all previous reports on this matter, only emboli of major coronary arteries were considered, which possibly resulted in a lower number of ACS-E being diagnosed. Finally, including patients over a long period of time may explain some differences in treatment modalities, and the low use of intracoronary imaging seen in our series.
CONCLUSIONS
ACS-E and ACS-A have different clinical and angiographic characteristics. Female sex, younger age, past medical history of active neoplasms, previous valvular surgery, and AF were all independent predictors of ACS-E. Patients with ACS-E had a higher in-hospital mortality rate mainly due to cardiovascular causes.
FUNDING
None whatsoever.
AUTHORS’ CONTRIBUTIONS
All authors contributed to the study conception and design. Material preparation and data collection were prepared by A. Jerónimo, A. Travieso, A. McInerney, B. Hennessey, and L. Marroquín. Statistical analysis was conducted by A. Jerónimo, M.J. Pérez- Vyzcaino, and N. Gonzalo. The manuscript first draft was written by A. Jerónimo, and N. Gonzalo, and all authors commented on previous versions of the manuscript. All authors read and approved the manuscript final version.
CONFLICTS OF INTEREST
None reported.
WHAT IS KNOWN ABOUT THE TOPIC?
- According to angiographic studies and autopsies, systemic coronary artery embolism is representative of 3% to 14% of all ACSs. However, the real prevalence of this entity remains unknown due to uncertainty of its diagnosis in the acute setting. AF, infective endocarditis, valvular heart disease, and malignancies have been associated with ACS-E, but the clinical and angiographic profile of this entity has not been properly established to this date.
WHAT DOES THIS STUDY ADD?
- Our study describes the epidemiological, clinical, and angiographic characteristics of patients with ACS-E comparing them to ACS-A and admitted to a single centre during the same period of time. On this regard, patients with ACS-E were younger compared to those with ACS-A, female in a higher proportion, and more often had AF, previous stroke, previous valvular surgery, and active neoplasms. The left anterior descending coronary artery was the most common culprit vessel in both groups, but patients with ACS-A presented with a significantly higher proportion of other significant stenoses. On the therapeutic approach regarding the PCI, thrombus aspiration was the most frequent strategy in ACS-E without stenting in any of the cases. Besides, the in-hospital all-cause mortality rate was significantly higher among patients with ACS-E mainly due to cardiovascular causes. A younger age, female sex, active neoplasms, previous valvular surgery, and a past medical history of AF were identified as independent clinical predictors of ACS-E.
REFERENCES
1. Shibata T, Kawakami S, Noguchi T, et al. Prevalence, Clinical Features, and Prognosis of Acute Myocardial Infarction Attributable to Coronary Artery Embolism. Circulation. 2015;132:241–250.
2. Popovic B, Agrinier N, Bouchahda N, et al. Coronary Embolism Among ST-Segment-Elevation Myocardial Infarction Patients:Mechanisms and Manegement. Circ Cardiovasc Interv. 2018;11:e005587.
3. Kolodgie FD, Virmani R, Finn A V, Romero ME. Embolic Myocardial Infarction as a Consequence of Atrial Fibrillation:A Prevailing Disease of the Future. Circulation. 2015;132:223–226.
4. Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction (2018). Eur Heart J. 2019;40:237-269.
5. Prizel KR, Hutchins GM, Bulkley BH. Coronary artery embolism and myocardial infarction. Ann Intern Med. 1978;88:155-161.
6. Lacey MJ, Raza S, Rehman H, Puri R, Bhatt DL, Kalra A. Coronary embolism:A systematic review. Cardiovasc Revasc Med. 2020;21:367-374.
7. Cheng JT, Cahill WJ, Foley EF. Coronary embolism. J Am Med Assoc. 1953;153:211-213.
8. Cheng TO. Coronary embolism. Int J Cardiol. 2009;136:1-3.
9. Kotooka N, Otsuka Y, Yasuda S, Morii I, Kawamura A, Miyazaki S. Three cases of acute myocardial infarction due to coronary embolism:treatment using a thrombus aspiration device. Jpn Heart J. 2004;45:861-866.
10. Stoel MG, von Birgelen C, Zijlstra F. Aspiration of embolized thrombus during primary percutaneous coronary intervention. Catheter Cardiovasc Interv. 2009;73:781-786.
11. Charles RG, Epstein EJ. Diagnosis of coronary embolism:A review. J R Soc Med. 1983;76:863-869.
12. Roxas CJ, Weekes AJ. Acute myocardial infarction caused by coronary embolism from infective endocarditis. J Emerg Med. 2011;40:509-514.
13. Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS):The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373-498.
14. Soliman EZ, Safford MM, Muntner P, et al. Atrial fibrillation and the risk of myocardial infarction. JAMA Intern Med. 2014;174:107-114.
15. Emdin CA, Wong CX, Hsiao AJ, et al. Atrial fibrillation as risk factor for cardiovascular disease and death in women compared with men:systematic review and meta-analysis of cohort studies. BMJ. 2016;532:h7013.
16. Ruddox V, Sandven I, Munkhaugen J et al. Atrial fibrillation and the risk for myocardial infarction, all-cause mortality and heart failure:A systematic review and meta-analysis. Eur J Prev Cardiol. 2017;24:1555-1566.
17. Guo XY, Li N, Du X, et al. Atrial fibrillation is associated with an increased risk of myocardial infarction:insights from a meta-analysis. Atherosclerosis. 2016;254:1–7.
18. Bayturan O, Puri R, Tuzcu EM, et al. Atrial fibrillation, progression of coronary atherosclerosis and myocardial infarction. Eur J Prev Cardiol. 2017;24:373–381.
19. Falanga A, Schieppati F, Russo D. Cancer Tissue Procoagulant Mechanisms and the Hypercoagulable State of Patients with Cancer. Semin Thromb Hemost. 2015;41:756-764.
20. Aronson D, Brenner B. Arterial thrombosis and cancer. Thromb Res. 2018;164 Suppl 1:S23-S28.
21. Liu F, Xu Z, Luo J, et al. Effectiveness and Safety of DOACs vs. VKAs in AF Patients With Cancer:Evidence From Randomized Clinical Trials and Observational Studies. Front Cardiovasc Med. 20215;8:766377.
22. Chu G, Versteeg HH, Verschoor AJ, et al. Atrial fibrillation and cancer - An unexplored field in cardiovascular oncology. Blood Rev. 2019;35:59-67.