Coronary artery disease (CAD) is one of the leading causes of death worldwide. These rates are only likely to be buoyed in the future by the rising prevalence of obesity, diabetes, and metabolic syndrome.
The pathogenesis of atherosclerosis, the main cause of CAD, has been a topic of great interest.1 As a brief review, dyslipidemia plays a key role. Beginning with the incorporation of serum low-density lipoprotein into the endothelium tunica intima, chemokines and endothelial adhesion molecules attract macrophages into the arterial wall. Eventually, cholesterol droplets are incorporated into the macrophage cytosol, become oxidized, and form the so-called ‘foam cell’. In a reciprocal exchange, inflammatory mediators released by foam cells trigger ongoing endothelial damage, fibrosis, and intimal hyperplasia. Eventually, coronary artery stenosis occurs; a condition at the basis of CAD and for which inflammation is a key component.
Inflammation is likely to play a role in both stable and unstable CAD. Many patients may present small atheromas, but still suffer from acute coronary thromboses–the acute coronary syndrome. Pathological studies have shown that T-cells, macrophages, and mast cells congregate to plaque rupture sites where they upregulate matrix metalloproteases, degrade collagen, and weaken the fibrous cap that supports the plaques. Additionally, once the endothelium is exposed to the plaque following a rupture, these inflammatory cells facilitate thrombosis, and platelet plug formation. As clues to their damage, such patients show high levels of interleukin-6, and C-reactive protein in their blood.1
ANTI-INFLAMMATORY THERAPIES FOR CAD
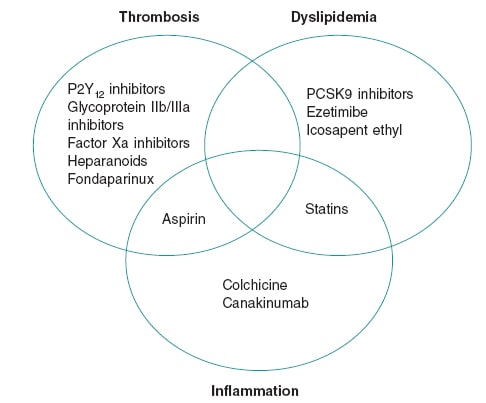
The centerpiece role of inflammation in CAD led to descriptions of atherosclerosis as a “chronic inflammation of the arteries,” a fact borne out by science as early as the 1980s.2 Yet, decades later no routinely used CAD therapies target any inflammatory pathways (figure 1). Many extant anti-inflammatory therapies have tried so but have proven unsuccessful. For instance, corticosteroids exhibit a broad range of anti-inflammatory properties, but their promotion of dyslipidemia and hypertension ultimately cause atherogenesis. Non-steroidal anti-inflammatory drugs other than aspirin inhibit prostacyclin, which increases vascular tone and platelet aggregation in the coronary arteries. Interest in methotrexate arose from observational studies that showed cardioprotective properties in patients with rheumatoid arthritis. However, a randomized clinical trial that analyzed its effect in patients with atherosclerosis showed that it does not lower the serum inflammatory markers or prevent myocardial infarction.3
Figure 1. Therapeutic foundations for the management of coronary artery disease. The figure shows agents with proven mortality benefits in the management of coronary artery disease. Although thrombosis, dyslipidemia, and inflammation are all key in the pathogenesis of coronary artery disease, the scarcity of effective anti-inflammatory agents is evident.
An interleukin-1b inhibitor, canakinumab, was the first agent to successfully prevent cardiovascular events in patients with a recent myocardial infarction. In the CANTOS trial, canakinumab 150 mg once daily lowered the rates of nonfatal myocardial infarction, stroke, and death (hazard ratio [HR], 0.85; 95% confidence interval [95%CI], 0.74-0.98) at the cost of increasing the rate of fatal sepsis, and infection (0.31 vs 0.18 per 100 person-years; P = .02). This trial was an important scientific breakthrough that showed that the inflammatory hypothesis is a therapeutic option for CAD. However, the costs of therapy and a modest effect size have both limited its use.4
Like methotrexate, interest in colchicine was borne out by observational data. In patients suffering gout flares, the use of colchicine was associated with fewer cardiovascular events compared to non-use.5 These observations led to a non-blinded randomized analysis of colchicine (the Low-dose colchicine for secondary prevention of cardiovascular disease [LoDoCo] trial) that proved a reduction in cardiovascular events in patients with stable CAD.6 Although there were some methodological limitations, the trial served as the niche for more upcoming robust clinical data on colchicine.
Colchicine is an inhibitor of microtubules and may prevent leukocyte migration into sites of plaque formation and rupture. Moreover, colchicine helps inhibit the formation of the NLRP3 inflammasome –a structure recently involved in cytokine mediated cell death. These pro-inflammatory protein complexes are activated by cholesterol crystals in macrophages and secrete interleukin (IL)-1b, the cytokine target of canakinumab. Colchicine has also been shown to reduce C-reactive protein, IL-1, and IL-6.7
A larger follow up trial to the initial LoDoCo trial, the LoDoCo2, enrolled 5522 patients with chronic CAD, and randomized them to receive colchicine or placebo for a median of 28.6 months after an open label run-in period to ensure colchicine tolerability. Colchicine was associated with a 31% reduction (HR, 0.69; 95%CI, 0.57-0.83; P < .001) of cardiovascular death, infarction, ischemic stroke, and revascularization. Adversely, patients who received colchicine showed higher rates of non-cardiovascular death, although the rate of events was low (HR, 1.51; 95%CI, 0.99-2.31; P = .06).8 Other adverse occurrences and intolerances were rare.
COLCHICINE IN ACS
Another 2 trials examined colchicine in a recent myocardial infarction setting: the COLCOT trial (efficacy and safety profile of low doses of colchicine after myocardial infarction), and the COPS trial (colchicine in patients with acute coronary syndrome). The first and larger of the two, the COLCOT trial, randomized 4745 patients who had a myocardial infarction within 30 days into colchicine therapy or placebo and followed them for a median of 22.6 months. The patients who received colchicine enjoyed a 23% reduction (HR, 0.77; 95%CI, 0.61-0.97; P = .02) in the composite endpoint of cardiovascular death, cardiac arrest, ischemic stroke, infarction, and angina requiring urgent revascularization. Though a positive outcome, it was driven primary by fewer revascularizations in the colchicine arm. Additionally, patients randomized to colchicine experienced more pneumonia (0.9% vs 0.4%; P = .03) compared to placebo.9 An early administration of colchicine was associated with greater benefits (within 3 days) in the COLCOT trial (HR, 0.52; 95%CI, 0.32-0.84 for initiation within 3 days vs HR, 0.98; 95%CI, 0.53-1.75 for initiation between days 4 and 7).10
The COPS trial enrolled 795 patients and randomized them to receive colchicine or placebo for 12 months. At the 1-year follow-up, no statistical differences were seen in the composite endpoint of cardiovascular death, infarction, ischemic stroke, and cardiac arrest with colchicine compared to placebo (HR, 0.65; 95%CI, 0.38-1.09; P = .10), likely due to a lower rate of events from a shorter follow-up period, and a smaller sample size. Moreover, 8 patients from the colchicine arm and 1 from the placebo group died, an effect that reached statistical significance and was driven by non-cardiovascular mortality (P = .047).11 Although this trial enrolled relatively few patients, it reproduced a signal towards mortality as seen on the larger LoDoCo2 trial.
The largest trial of colchicine post-myocardial infarction, the Colchicine and spironolactone in acute MI (CLEAR SYNGERY, NCT03048825), is underway. It will randomize 7000 patients and help solve the issue of whether colchicine increases non-cardiovascular mortality. The findings of the COPS and LoDoCo2 trials may be spurious findings, similar to what was found in earlier statin trials, and eventually disproved. On the other hand, there could be an important impact of colchicine due to a reduction of host defenses that went previously unnoticed.
Colchicine may be the first widely available, inexpensive anti-inflammatory therapy for the management of CAD. However, the issue of non-cardiovascular mortality should be resolved before it is widely adopted.
FUNDING
The authors received no specific funding for this work.
CONFLICTS OF INTEREST
S.S. Jolly reports grant support from Boston Scientific and the Canadian Institutes of Health Research. W. Hijazi declared no conflicts of interest.
REFERENCES
1. Bouabdallaoui N, Tardif JC, Waters DD, et al. Time-to-treatment initiation of colchicine and cardiovascular outcomes after myocardial infarction in the Colchicine Cardiovascular Outcomes Trial (COLCOT). Eur Heart J. 2020;41:4092-4099.
2. Butt AK, Cave B, Maturana M, Towers WF, Khouzam RN. The Role of Colchicine in Coronary Artery Disease. Curr Probl Cardiol. 2021;46:100690.
3. Hansson GK. Inflammation, Atherosclerosis, and Coronary Artery Disease. N Engl J Med. 2005;352:1685-1695.
4. Nabel EG, Braunwald E., 2012. A Tale of Coronary Artery Disease and Myocardial Infarction. N Engl J Med. 2012;366:54-63.
5. Nidorf SM, Eikelboom JW, Budgeon CA, Thompson PL. Low-Dose Colchicine for Secondary Prevention of Cardiovascular Disease. J Am Coll Cardiol. 2013;61:404-410.
6. Nidorf SM, Fiolet ATL, Mosterd A, et al.;LoDoCo2 Trial Investigators. Colchicine in Patients with Chronic Coronary Disease. N Engl J Med. 2020;383:1838-1847.
7. Ridker PM, Everett BM, Pradhan A, et al.;CIRT Investigators. Low-Dose Methotrexate for the Prevention of Atherosclerotic Events. N Engl J Med. 2019;380:752-762.
8. Ridker PM, Everett BM, Thuren T, et al.;CANTOS Trial Group Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017;377:1119-1131.
9. Solomon D, Liu CC, Kuo IH, Zak A, Kim SC. Effects of colchicine on risk of cardiovascular events and mortality among patients with gout:a cohort study using electronic medical records linked with Medicare claims. Ann Rheum Dis. 2016;75:1674-1679.
10. Tardif JC, Kouz S, Waters DD, et al. 2019. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N Engl J Med. 2019;381:2497-2505.
11. Tong DC, Quinn S, Nasis A, et al. 2020. Colchicine in Patients With Acute Coronary Syndrome. Circulation. 2020;142:1890-1900.