INTRODUCTION
The randomized clinical trial (RCT) has become the gold standard for evaluating clinical treatments thanks to its low selection bias and unknown confounders. However, good clinical practice guidelines and demands from regulatory agencies have become so elaborate over time that, basically, only big pharmaceutical companies have the resources to conduct large RCTs. Therefore, important questions raised by academic scientists could be impossible to test in clinical trials.
One way to circumvent these problems is to use the registry-based randomized clinical trial (RRCT) design. A RRCT uses the platform of an already-existing high-quality observational health registry as a case-report form for randomization and follow-up purposes. This design facilitates the randomization of a large number of patients over a short period of time, reduces costs to a fraction of the cost of conventional randomized clinical trials, and facilitates the follow-up of all eligible patients not enrolled in the study (table 1).1-4
Table 1. Major functions for trial conduction provided by the registry
| Major functions for trial conduction provided by the registry |
| Identification of eligible patients |
| Alert investigator of an eligible patient |
| Link to randomization module |
| Randomization |
| Collection of baseline and procedural characteristics from a registry (eCRF) |
| Presentation of additional trial-specific questions for eCRF |
| Identification of clinical endpoints (endpoint detection) |
| Clinical outcomes reporting |
| Reporting of characteristics of enrolled and non-enrolled patients from the overall population |
|
eCRF, electronic case report form. |
RRCT-SUITABLE REGISTRIES
Nearly all healthcare data are stored digitally today, which poses an excellent opportunity to use these data in a RRCT. However, healthcare records are often not structured in a way that allows useful data extraction. Today, disease-specific quality registries with full nationwide coverage are the most suitable ones as the basis for RRCTs, but this may change in the future. Our experience comes from using the Swedish Web-system for the Enhancement and Development of Evidence-based care in Heart disease Evaluated According to Recommended Therapies (SWEDEHEART) and its Swedish Coronary Angiography and Angioplasty Registry (SCAAR)5 through which a large number of RRCTs have been conducted or are in ongoing phases (table 2).6-10 The validation of registry data vs health records has an overall percent agreement of 96%.11 The first pure RRCT was the TASTE trial where thrombus aspiration in patients with ST-segment elevation myocardial infarction (STEMI) was studied with mortality as the primary endpoint.1 A large number of patients were rapidly included and with a limited budget by only using registries of baseline demographics, randomization, and endpoint collection in a prospective and randomized fashion. In this trial of a simple device intervention and a solid endpoint, the SWEDEHEART registry provided all the necessary steps to conduct a RCT (table 1). First, identify eligible patients and “flag” them with a pop-up window to the investigator appointed prior to the procedure. Secondly, open up a randomization window with 2 questions: Have the inclusion/exclusion criteria been met? Has the patient given his consent to enter the study? If the answers to both these questions were positive, the patient was randomized and the result shown on the screen momentarily. Thirdly, both the baseline characteristics and the follow-up endpoints were collected from the registry. Furthermore, data on all of the unrecruited patients with complete baseline characteristics are collected. It is interesting to compare the TASTE to the TOTAL trial that examined thrombus aspiration using the traditional RCT design.12 While the cost of the TOTAL trial was approximately €15 000 000 with 87 centers enrolling patients for 48 months on a 6-month follow-up, the cost of the TASTE trial was €500 000 (3%!) with 30 centers enrolling patients for 33 months on a 42-month follow-up being the results nearly identical. In these circumstances of low complexity in both treatment and endpoints an RRCT is superior, in almost every aspect, to a traditional RCT.
Table 2. Registry-based randomized clinical trials in the SWEDEHEART registry: completed, ongoing or in the pipeline
| RRCT | Patients, N | Question | Dates |
|---|---|---|---|
| TASTE, Fröbert et al.1 (2013) | 7200 | Thrombus aspiration in primary PCI | 2013 + 2014 |
| IFR-SWEDEHEART, Gotberg et al.6 (2017) | 2018 | iFR vs FFR in stable angina or ACS | 2017 |
| VALIDATE-SWEDEHEART Erlinge et al.7 (2017) | 6006 | Bivalirudin vs UFH for PCI in ACS | 2017 |
| DETO2X-AMI, Hofmann et al.8 (2017) | 6629 | Oxygen therapy in MI | 2017 |
| FULL-REVASC, NCT02862119 | 4052 | FFR-guidance in MI | Enrollment stopped after 1545 patients |
| PROSPECT-II, NCT02171065 | 900 | Near-infrared spectroscopy in PCI | Presented, TCT 2020 |
| IAMI, Fröbert ret al.9 (2017) | 4400 | Influenza vaccination after MI | Completed enrollment |
| SPIRRIT, NCT02901184 | 3200 | Spironolactone for HFpEF | Ongoing |
| REDUCE, NCT03278509 | 6600 | Beta-blocker post MI in patients with ejection fraction > 50% | Ongoing |
| ABC-AF, NCT03753490 | 6500 | Biomarker score-based treatment vs standard care | Ongoing |
| MINOCA-BAT, NCT03686696 | 2048 | ACEi/beta-blockers after MI with non-obstructive CAD | Ongoing |
| TACSI, NCT03560310 | 2200 | Post-CABG ACS, ticagrelor | Ongoing |
| SWEDEGRAFT, NCT03501303 | 902 | CABG grafting | Completed enrollment |
| Infinity-Swedeheart, NCT04562805 | 2400 | Disengaging DES vs DES | Ongoing |
| DAPA-MI, NCT04564742 | 6400 | SGLT2 inhibitor post-AMI | Ongoing |
| HELP-SWEDEHEARTa | 20 000 | Helicobacter pylori screening after AMI to prevent upper gastrointestinal bleeding (cluster-randomization) | Q2, 2021 |
| SWITCHb | 20 000 | Prasugrel or ticagrelor post-MI (cluster-randomization) | In the pipeline |
| BROKEN-Swedeheart, NCT04666454 | 1000 | Optimal medical treatment for Tako-tsubo syndrome | In the pipeline |
|
a Still unregistered; pilot study: NCT04289012. b Still unregistered. ACEi, angiotensin-converting enzyme inhibitor; AMI, acute myocardial infarction; ACS, acute coronary syndromes; CABG, coronary artery bypass graft; CAD, coronary artery disease; DES, drug-eluting stent; FFR, fractional flow reserve; HFpEF, heart failure with preserved ejection fraction; iFR, instantaneous wave-free ratio; MI, myocardial infarction; EFPCI cabg SGLT2, sodium-glucose cotransporter-2; PCI percutaneous coronary intervention; TCT, Transcatheter Cardiovascular Therapeutics conference; UFH, unfractionated heparin. |
|||
ADVANTAGES AND LIMITATIONS OF PURE RRCTs COMPARED TO TRADITIONAL RCTs
The major advantages of the RRCT design are: a) a broader and more representative population to clinical reality; in the TASTE and VALIDATE-SWEDEHEART trials 70% of all eligible patients were included1,7; b) clinically significant endpoints were included, and not multiple composite weak or surrogate endpoints; c) long-term follow-up periods, actually life-long follow-ups, if applicable; d) thanks to random selection, bias and confounding factors are reduced to a minimum; e) significantly lower costs; f) rapid inclusion of a large number of patients; and g) initiated and conducted by independent academic researchers with no links to the industry.
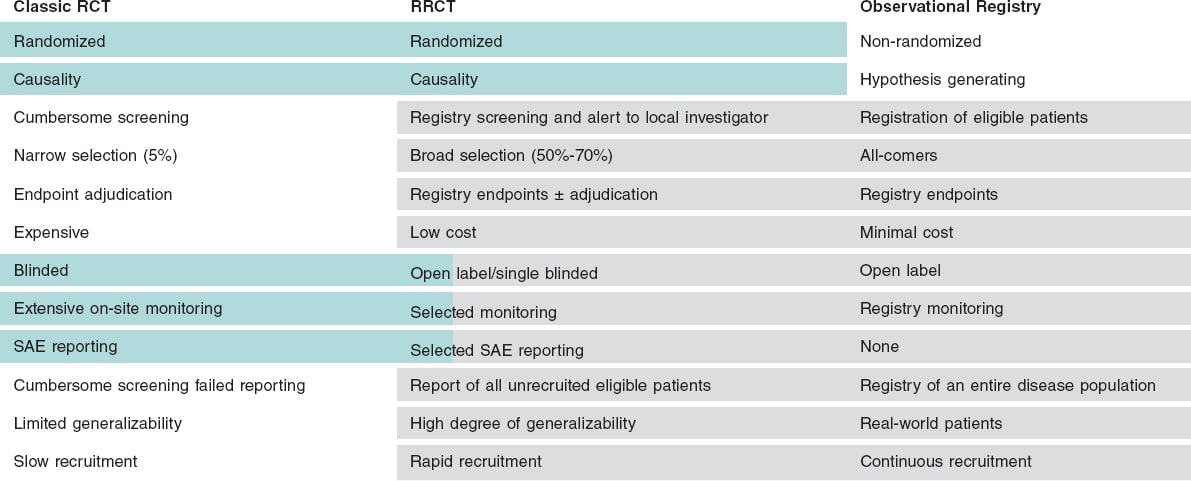
The limitations are: a) open label design with a risk of biased endpoint reporting; b) rare, unexpected events may be missing, and serious adverse event reporting may be difficult; c) events are, for the most part, not adjudicated, which may result in variable data quality; d) difficulties having central chemical analysis and biobanking; e) long-term oral drugs can be difficult to distribute and follow; and f) lack of or limited site monitoring (figure 1).
Figure 1. Comparison of traditional randomized clinical trials (RCTs), registry studies, and registry-based randomized clinical trials (RRCT). Classical RCTs are the gold standard of clinical research, but they have limitations because they are very expensive, selective, and a cumbersome process. Retrospective registry studies can be conducted much cheaper, and may be more representative of the real world, yet they are always hampered by unknown confounders. An RRCT can profit from the best parts of these modalities. SAE, serious adverse event.
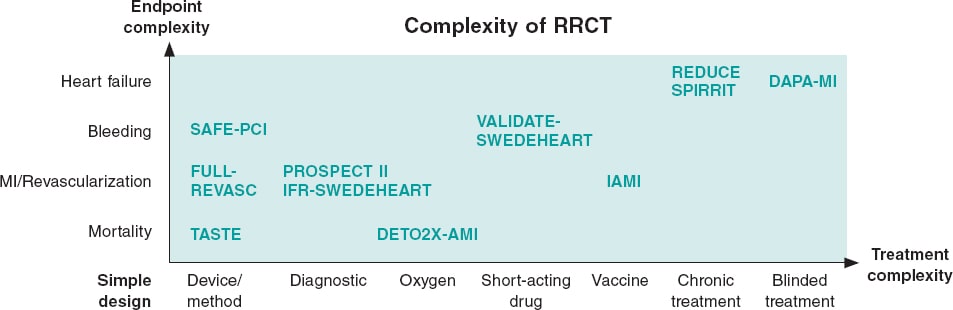
Depending on the limitations of the registry used, need for treatment escalation or endpoint complexity the RRCT can be complemented with different traditional trial elements resulting in a hybrid RRCT (figure 2).
Figure 2. Simple versus complex registry-based randomized clinical trials (RRCT). The purest RRCT examines a simple therapy like a thrombus aspiration device and has a robust endpoint like mortality. When the complexity increases for either treatment or endpoint, then additions to the RRCT design have to be made. These additions could be phone calls, central adjudication, or even blinded treatment with placebo. This increases complexity and costs, but the registry may still be the basis for the trial and facilitate performance. MI, myocardial infarction.
DEVELOPMENT OF RRCTs
In the SORT OUT series of coronary stent trials, baseline demographics and endpoint screening were conducted using a registry approach. However, randomization took place using different approaches (telephone allocation service, internet-based randomization systems), and endpoints were centrally adjudicated.13 In the SAFE-PCI study, randomization was performed outside the registry obtaining additional clinical information and adjudication to the registry data.14
In the VALIDATE-SWEDEHEART trial, 2 short-acting IV antithrombotic agents (bivalirudin and heparin) were assessed using the RRCT approach. As far as we know, this was the first pharmaceutical RRCT ever conducted.7 In a pharmaceutical trial the requirements from the medical regulatory authorities are more demanding even if the drugs have been approved and used for decades. Furthermore, we realized that our registry did not capture bleeding complications satisfactorily. Therefore, we added phone calls after 7 to 180 days followed by the central adjudication of bleeding complications and MI, limited serious event reporting, and data on the entire index hospitalization. Thanks to the simplicity of the trial, 25 centers were able to enroll over 6000 patients with MI over 2 years. Some large centers enrolled more than 1000 patients (figure 2, table 2).
In the IFR-SWEDEHEART trial the instantaneous wave-free ratio diagnostic modality was evaluated. The complexity of the intervention was low, but the composite endpoint included MI and unplanned revascularization.6 Although the endpoints were found in the registries, data were collected from medical records from the centers and adjudicated by a central committee.
In the DETO2X-AMI trial the endpoint was mortality, which does not need adjudication; however, the oxygen of the procedure had to be administered to the patient in a single blinded fashion adding some extra complexity to the study8 (figure 2, table 2). Similarly, the influenza vaccine study conducted post-MI (IAMI trial) needed blinded treatment.9 Furthermore, other countries without the SWEDEHEART registry structure were needed to get a sufficient number of patients, which resulted in a parallel randomization module and electronic case report forms (figure 2, table 2).9
There are 2 ongoing RRCTs with chronic oral treatment and a composite endpoint of death and hospitalizations due to heart failure: the REDUCE (beta-blocker post-MI, NCT03278509) and the SPIRRIT (Spironolactone for Heart Failure with Preserved Ejection Fraction, NCT02901184). However, despite the complex treatment and endpoint of both of them, they rely nearly only on registries. The treatment is randomized in the registry, prescribed, and then followed by the Swedish Prescribed Drug Register. Hospitalizations due to heart failure are collected from the National Patient Register where this diagnosis has proven to have a high validity in previous studies.
FUTURE POSSIBILITIES OF THE RRCT CONCEPT
So far, the RRCT technology has mostly been used for the assessment of devices or generic drugs that have been used for decades often with results that the treatment examined has been redundant, as it has been the case with the TASTE and VALIDATE-SWEDEHEART trials. Sometimes, as it occurred with the IFR-SWEDEHEART study, a new diagnostic procedure proves to be non-inferior to the current standard.6 This resulted in an IA recommendation for the instantaneous wave-free ratio in the clinical practice guidelines. In general, RRCTs have been deemed unsuitable by the medical regulatory authorities for first approval, but this is about to change. The INFINITY trial (NCT04562805) is examining a new type of stent capable of disengaging its metal struts after half a year. This is a currently ongoing RRCT whose objective is to support an approval given by the US Food and Drug Administration (FDA). Demographics, randomization, and endpoint follow-up are already taken care of by the SWEDEHEART registry, still a phone call at 1 month and 1 year combined with central adjudication was added.
The first study, that has been analyzing an expanded use for an oral drug, is the DAPA-MI study (NCT04564742) where dapagliflozin is being tested for post-MI patients with reduced ejection fraction but without diabetes. The registry is the basis of the study, yet visits have been added to dispense the blinded medication. The study is sponsored by AstraZeneca and intends to develop new more cost-efficient ways to conduct phase III trials. The study profits from 2 countries with nationwide MI registries, the UK and Sweden, with their MINAP16 and SWEDEHEART5 registries, respectively.
In Europe, the European Society of Cardiology has mostly relied on surveys to register different heart conditions. These are valuable, but they only give us a snapshot of a short timeframe and the selection of patients is unclear and may not be representative of the real world. However, a new initiative called EuroHeart17 has been trying to establish a common basic structure for continuous cardiac registries that could be used by any countries. One of its objectives is to facilitate conducting RRCTs in several European countries making the results more representative and allowing larger studies being conducted more rapidly.
CLUSTER-RANDOMIZED RRCTs
Cluster randomization design simplifies enrollment and does not often require signed informed consent forms, only general information about the ongoing study. It facilitates the recruitment of nearly all patients from a region during a certain period of time and it basicaly uses a cross-over design. In the HELP-SWEDEHEART trial—still not registered—20 000 patients diagnosed with MI in the SWEDEHEART registry will, based on hospital data, be cluster-randomized in a crossover design to receive Helicobacter pylori screening and, if they test positive, be recommended eradication therapy. The primary endpoint is upper gastrointestinal bleeding, which is collected from the National Patient Register. The still unregistered SWITCH trial is planning to investigate prasugrel compared to ticagrelor for the treatment of patients hospitalized due to MI with the composite endpoint of death, MI or stroke collected from the National Patient Register and the National Cause of Death Registry. A total of 4 Swedish regions will be randomized in blocks to standard use of either prasugrel or ticagrelor over 2 years.
In conclusion, RRCTs combine some of the best parts of the classical RCT design and traditional registries when conducting large, randomized, real-world, representative, and cost-effective clinical studies. They give academic researchers an opportunity to obtain important clinical answers that would have never been funded by industry.
FUNDING
The study is supported by the Swedish Heart and Lung Foundation, the Swedish Scientific Research Council, and the Knut and Alice Wallenberg Foundation. The author is solely responsible for the content of this manuscript.
CONFLICTS OF INTEREST
D. Erlinge declares having received speaker or advisory board fees from AstraZeneca, Bayer, Sanofi, and Chiesi.
REFERENCES
1. Fröbert O, Lagerqvist B, Olivecrona GK, et al. Thrombus aspiration during ST-segment elevation myocardial infarction. N Engl J Med. 2013;369:1587-1597.
2. James S, Frobert O, Lagerqvist B. Cardiovascular registries:a novel platform for randomised clinical trials. Heart. 2012;98:1329-1331.
3. Yndigegn T, Hofmann R, Jernberg T, Gale CP. Registry-based randomised clinical trial:efficient evaluation of generic pharmacotherapies in the contemporary era. Heart. 2018;104:1562-1567.
4. Lauer MS, D'Agostino RB, Sr. The randomized registry trial--the next disruptive technology in clinical research?N Engl J Med. 2013;369:1579-1581.
5. Start - SWEDEHEART (uu.se). Available at: https://www.ucr.uu.se/swedeheart/start-scaar. Accessed 8 Feb 2021.
6. Gotberg M, Christiansen EH, Gudmundsdottir IJ, et al. Instantaneous Wave-free Ratio versus Fractional Flow Reserve to Guide PCI. N Engl J Med. 2017;376:1813-1823.
7. Erlinge D, Omerovic E, Frobert O, et al. Bivalirudin versus Heparin Monotherapy in Myocardial Infarction. N Engl J Med. 2017;377:1132-1142.
8. Hofmann R, James SK, Jernberg T, et al. Oxygen Therapy in Suspected Acute Myocardial Infarction. N Engl J Med. 2017;377:1240-1249.
9. Fröbert O, Gotberg M, Angeras O, et al. Design and rationale for the Influenza vaccination After Myocardial Infarction (IAMI) trial. A registry-based randomized clinical trial. Am Heart J. 2017;189:94-102.
10. Hambraeus K, Held C, Johansson P, et al. SWEDEHEART Annual report 2012. Scan Cardiovasc J. 2014;48(Suppl 63):2-133.
11. Jernberg T, Attebring MF, Hambraeus K, et al. The Swedish Web-system for enhancement and development of evidence-based care in heart disease evaluated according to recommended therapies (SWEDEHEART). Heart. 2010;96:1617-1621.
12. Jolly SS, Cairns JA, Dzavik V. Primary PCI with or without Thrombectomy. N Engl J Med. 2015;373:682-683.
13. Raungaard B, Jensen LO, Tilsted HH, et al. Zotarolimus-eluting durable-polymer-coated stent versus a biolimus-eluting biodegradable-polymer-coated stent in unselected patients undergoing percutaneous coronary intervention (SORT OUT VI):a randomised non-inferiority trial. Lancet. 2015;385:1527-1535.
14. Rao SV, Hess CN, Barham B, et al. A registry-based randomized trial comparing radial and femoral approaches in women undergoing percutaneous coronary intervention:the SAFE-PCI for Women (Study of Access Site for Enhancement of PCI for Women) trial. JACC Cardiovasc Interv. 2014;7:857-867.
15. Neumann FJ, Sousa-Uva M, Ahlsson A, et al.;ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87-165.
16. NICOR. Myocardial Ischaemia/MINAP (Heart Attack audit). Available at: https://www.nicor.org.uk/national-cardiac-audit-programme/heart-attack-audit-minap/. Accessed 8 Feb 2021.
17. European Society of Cardiology. EuroHeart. Available at: https://www.escardio.org/Research/euroheart. Accessed 8 Feb 2021.