ABSTRACT
Introduction and objectives: Transcatheter aortic valve implantation (TAVI) is an established treatment option for patients with symptomatic severe aortic stenosis often performed via transfemoral access route (TF-TAVI). Therefore, successful closure of large-bore access sites is essential. This study aims to investigate the safety and effectiveness of the MANTA (Teleflex/Essential Medical, United States) vascular closure device (VCD) in patients undergoing TF-TAVI in an unselected and consecutive cohort of patients.
Methods: We conducted a single-center, observational study of 245 consecutive patients undergoing TF-TAVI in whom the arterial large-bore femoral access was closed with a MANTA device from March 2020 through February 2022. The primary efficacy outcome measure was the rate of VCD failure according to the VARC-3 definition.
Results: Successful closure of the large-bore access site occurred in 92.2% of the patients (n = 226). According to the VARC-3 definition, no major vascular or bleeding complications related to the plug-based VCD were reported. Patients with failed VCDs (7.8%) had significantly smaller minimal femoral artery diameters (6.6 ± 1.1 mm vs 7.6 ± 1.4 mm; P = .005) and consequently, significant higher sheath-to-femoral artery diameter ratios (0.78 ± 0.16 vs 0.69 ± 0.15; P = .019). No other inter-group differences were found.
Conclusions: In this single-center, real-world, unselected large cohort of consecutive patients treated with TF-TAVI, a plug-based VCD for large-bore arteriotomy closure turned out effective and safe, and enabled arterial access-site management with a low rate of complications.
Keywords: Aged. Aortic valve stenosis. Transcatheter aortic valve implantation. Vascular closure devices.
RESUMEN
Introducción y objetivos: El implante percutáneo de válvula aórtica (TAVI) es una opción de tratamiento establecida para pacientes con estenosis aórtica grave sintomática, generalmente realizado por acceso transfemoral (TAVI-TF). Por lo tanto, el cierre exitoso de los sitios de acceso de gran calibre es esencial. Este estudio tiene como objetivo investigar la seguridad y la eficacia del dispositivo de cierre vascular (DCV) MANTA (Teleflex/Essential Medical, Estados Unidos) en pacientes tratados con TAVI-TF en una cohorte consecutiva y no seleccionada.
Métodos: Se realizó un estudio observacional de un solo centro, con 245 pacientes consecutivos tratados con TAVI-TF en quienes el acceso femoral arterial de gran calibre se cerró con MANTA, entre marzo de 2020 y febrero de 2022. La medida de resultado de eficacia primaria fue la incidencia de fallo del DCV usando la definición VARC-3.
Resultados: En el 92,2% (n = 226) de los pacientes se logró el cierre exitoso del sitio de acceso de gran calibre. De acuerdo con la definición VARC-3, no se informaron complicaciones vasculares ni hemorrágicas importantes relacionadas con el DCV basado en tapón. Los pacientes con fallo del DCV (7,8%) tenían un diámetro mínimo de la arteria femoral significativamente más pequeño (6,6 ±1,1 frente a 7,6 ±1,4 mm; p =0,005) y, en consecuencia, una relación significativamente mayor entre el diámetro de la vaina y la arteria femoral (0,78 ±0,16 frente a 0,69 ±0,15; p = 0,019). No se encontraron otras diferencias entre los grupos.
Conclusiones: En esta gran cohorte no seleccionada de un solo centro, del mundo real, de pacientes con TAVI-TF consecutivos, un DCV basado en tapón para el cierre de la arteriotomía de gran calibre fue eficaz y seguro, lo que permitió el manejo del sitio de acceso arterial con una baja tasa de complicaciones.
Palabras clave: Edad avanzada. Estenosis de válvula aórtica. Reemplazo de válvula aórtica transcatéter. Dispositivos de cierre vascular.
Abbreviations
TAVI: transcatheter aortic valve implantation. TF-TAVI: transfemoral transcatheter aortic valve implantation. VARC-3: Valve Academic Research Consortium 3. VCD: vascular closure device.
INTRODUCTION
Transcatheter aortic valve implantation (TAVI) is an established treatment option for patients with severe symptomatic aortic stenosis,1 especially those at high surgical risk2 (also those at low and intermediate risk), and is often performed via transfemoral access route (TF-TAVI).1 However, TF-TAVI access requires large-bore catheters (5 mm to 7 mm) and their management is responsible for a significant number of TAVI-related adverse events. Complications can affect between 5% and 20% of the patients,3 and impact short- and long-term clinical outcomes.3-5 Therefore, successful closure of large-bore access sites remains essential.6,7
During the early years of TF-TAVI, the femoral artery was closed surgically.2 More recently, this method has been replaced by the use of suture-based vascular closure devices (VCD)1 like the Perclose ProGlide VCD (Abbott Vascular, United States).1,8 Although this closure technique proved to be a safe and effective first-line strategy for large-bore arterial access,1 major vascular complications occurred in up to 5% of patients mainly due to failed arteriotomy closure devices.
MANTA (Teleflex/Essential Medical, United States) is a plug-based VCD that has shown promising results in TF-TAVI.29-14 This device is dedicated to large-bore vessel closure and built on a proven concept of an intra-arterial toggle and extra-arterial collagen plug.5,15 Early feasibility trials have reported encouraging safety and efficacy outcomes in a variety of procedures requiring large-bore vascular access.1,9,11,16 Also, registry-based studies have demonstrated equivalence to suture-based techniques.1,17 However, data regarding vascular complications and device failure rates compared to the well-known suture-based VCDs are scarce.2 Recent studies, including small, selected patient cohorts concluded that compared to suture-based VCDs, plug-based VCDs were associated with significantly shorter lengths of stay following the procedure,1,7 and less VCD failure.1,5,7 No significant differences regarding mortality, bleeding or vascular complications were ever reported.7
This single-center study aims to investigate the safety and effectiveness of the MANTA VCD in patients undergoing TF-TAVI in an unselected and consecutive cohort of patients. We hypothesize that arterial closure with MANTA is associated with a high feasibility and low risk of major vascular and bleeding complications.
METHODS
Study design
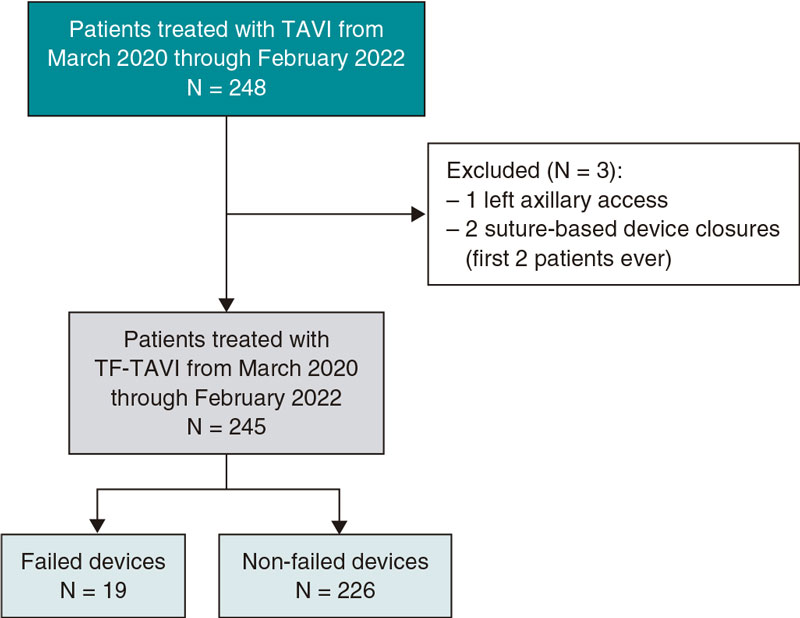
This single-center observational, and retrospective study included a total of 245 consecutive patients who underwent TF-TAVI and in whom the MANTA device was used to close the arterial large-bore femoral access from March 2020 through February 2022 (figure 1). All patients had symptomatic severe aortic stenosis. Eligibility for TF-TAVI procedure was assessed by the heart team. The study was approved by the regional Human Research Ethics Committee (HREC) at Coimbra, Portugal. Informed consent was waived. Demographics, preoperative, intraoperative, and postoperative data were collected from medical records.
Figure 1. Central illustration. Flowchart of the patients incuded. TAVI, transcatheter aortic valve implantation; TF-TAVI, transfemoral transcatheter aortic valve implantation.
Study outcomes
The study primary efficacy outcome measure was the rate of device failure according to the Valve Academic Research Consortium 3 (VARC-3) definition.18 Device failure was defined as failure to successfully achieve hemostasis at the access site, thus leading to alternative treatment (surgery, balloon or covered stent).
The study secondary outcome was safety. Here we assessed the rate of VARC-3 major vascular and bleeding complications associated with the access site. Major vascular complications are lesions (perforations, ruptures, dissections, stenoses, ischemias, arterial thromboses, arteriovenous fistulas, pseudoaneurysms, hematomas, retroperitoneal hematomas, infections) resulting in death, VARC type ≥ 2 bleeding, limb or visceral ischemia or irreversible neurological impairment; distal embolization (noncerebral) from a vascular source resulting in death, amputation, limb or visceral ischemia or irreversible end-organ damage; unplanned endovascular or surgical intervention resulting in death, VARC type ≥ 2 bleeding, limb or visceral ischemia or irreversible neurological impairment. A major bleeding is considered type 2 if an overt bleeding requires the transfusion of 2–4 units of whole blood/red blood cells or else in the presence of an overt bleeding associated with a drop of hemoglobin levels from 3 g/dL to 5 g/dL; type 3 if bleeding is life-threatening, and type 4 if it leads to death.
As exploratory endpoints, we also evaluated cardiovascular and non-cardiac mortality during the index hospitalization and at 30-day follow-up.
Procedural details
Before TF-TAVI, all patients underwent an echocardiogram, blood tests, and a multi-detector computed tomography according to the TAVI protocol using a dedicated software (3mensio, Maastricht, The Netherlands). Multi-detector computed tomography was performed to assess iliofemoral arteries for vascular access prior to the procedure. The size of the vessel, tortuosity, amount of calcification, and minimal lumen diameter were all analyzed. Calcification and tortuosity were separately scored by consensus visual analysis of 2 observers who were blind to the remaining patient data. The sheath-to-femoral artery diameter ratios were analyzed too. Anticoagulant and antiplatelet therapies according to the guidelines: if the patient was on anticoagulation, he didn’t use the anticoagulant the day before and resumed it on the day of the procedure (in the absence of bleeding complications); if on antiplatelet therapy, single antiplatelet therapy was used; if not on anticoagulant or antiplatelet agent, an aspirin loading dose was used before the procedure followed by a single antiplatelet therapy agent. All procedures were performed under conscious sedation. Puncture of the femoral artery was performed with ultrasound guidance. During the procedure, heparin was administered and an activated clotting time between 250 s and 300 s was targeted. The bioprosthetic valves implanted were the CoreValve Evolut R/Pro (Medtronic, United States), ACURATE neo (Boston Scientific, United States), SAPIEN (Edwards Lifesciences, United States), and Navitor (Abbott, United States). After valve implantation, in cases where the activated clotting time was > 200 s, protamine sulfate was given before vascular closure.
MANTA device
The MANTA VCD is a collagen-based technology available in 2 sizes: 14-Fr and 18-Fr. It can be used for sheath sizes that go from 10-Fr to 14-Fr, and 15-Fr to 22-Fr, respectively. In brief, arteriotomy depth is determined with a centimeter-marked sizing tool before large-bore sheath insertion. At the end of the procedure, the large-bore sheath is exchanged for the dedicated MANTA sheath to accomodate the toggle–plug assembly. Afterwards, the anchor is opened 1.5 cm more than the femoral arterial wall-skin distance. The sheath is then removed, and the puncture site sandwiched between toggle and collagen that remain connected by a stainless-steel lock. Hemostasis success after the use of the MANTA VCD was evaluated through an angiography after vascular closure. As a standard practice, we typically use the contralateral femoral artery as a backup access, but do not routinely use a protection guide. In situations where access to the main site is necessary, we use a 0.035 in Glidewire Advantage guidewire that is advanced through the pigtail situated in the contralateral femoral artery and placed under the balloon. In the presence of bleeding complications that are not resolved by prolonged balloon inflation, a covered stent is often implanted.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation if normally distributed or as median [interquartile range] if not. Normality was checked using the Shapiro–Wilk test and histogram observation. Categorical data were expressed as numbers and percentages and compared using Pearson’s chi-square test or Fisher’s exact test, when appropriate. Continuous variables were compared using the Student t test. Formal tests for interaction were performed using logistic regression. Statistical significance was always set at a 2-tailed probability level of < 0.05. Statistics were performed using SPSS version 28 (IBM, United States).
RESULTS
Baseline characteristics
A total of 245 of consecutive patients treated with TF-TAVI whose arterial large-bore femoral access was closed with the MANTA 18-Fr VCD were included in our study. The baseline characteristics are shown on table 1. The population mean age was 81 ± 6 years with a median EuroSCORE II of 3.15% [2.07%-4.75%]. Most patients were women (53.9%). The Evolut R/Pro valve was implanted in > 50% of the cases (52.7%).
Table 1. Baseline characteristics
| General characteristics | N = 245 |
|---|---|
| Age, years | 81 ± 6 |
| Female sex | 132 (52.7) |
| BMI, kg/m2 | 27 ± 4.8 |
| NYHA | |
| II | 134 (54.7) |
| III-IV | 111 (45.3) |
| NT-proBNP (pg/mL) | 2197 (884-5545) |
| LVEF | 53 ± 11 |
| EuroSCORE II, % | 3.15 (2.07-4.75) |
| Aortic valve MG, mmHg | 48 ± 15 |
| AVA, cm2 | 0.67 ± 0.17 |
| Antiplatelet therapy | 78 (31.8) |
| Anticoagulant therapy | 87 (35.5) |
| Femoral artery characteristics (MANTA side) | |
| Minimal diameter – mm | 7.5 ± 1.4 |
| Calcification | |
| None | 24 (10.4) |
| Mild | 143 (61.9) |
| Moderate | 44 (19.0) |
| Severe | 20 (8.7) |
| Tortuosity | |
| None | 137 (59.1) |
| Mild | 70 (30.2) |
| Moderate | 17 (7.3) |
| Severe | 8 (3.4) |
|
AVA, aortic valve area; BMI, body mass index; LVEF, left ventricular ejection fraction; MG, mean gradient; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association. |
|
Primary outcome: efficacy of the MANTA plug-based vascular closure device
Successful closure of the large-bore access site occurred in 92.2% of the patients (n = 226). The main access artery was the right common femoral one in 90.6% of the patients (n = 222). The mean minimal femoral arterial diameter at the access site was 7.5 ± 1.4 mm (table 1). The mean sheath-to-femoral artery diameter ratio was 0.69 ± 0.15 (table 2). Only 1 out of the 19 patients with device failure (7.8%) required a secondary surgical approach (due to occlusion of the femoral artery unresolved with balloon dilatation) (table 3).
Table 2. Procedural characteristics
| Procedural characteristics | N = 245 |
|---|---|
| Valves | |
| ACURATE neo2, Boston Scientific, United States | 36 (14.7) |
| Evolut PRO, Medtronic, United States | 129 (52.7) |
| Portico (Abbott, United States) | 43 (17.5) |
| SAPIEN, Edwards Lifesciences, United States | 37 (15.1) |
| Femoral sheaths (MANTA side) | |
| 14-Fr | 135 (55.1) |
| 16-Fr | 75 (30.6) |
| 18-Fr | 33 (13.5) |
| 20-Fr | 2 (0.8) |
| Sheath-to-femoral artery diameter ratio | 0.69 ± 0.15 |
|
Data are expressed as no. (%) or mean ± standard deviation. |
|
Table 3. Primary endpoint – efficacy
| N = 245 | |
|---|---|
| Device failure | 19 (7.8) |
| Balloon | 16 (6.5) |
| Balloon + covered stent | 2 (0.8) |
| Surgical correction | 1 (0.4) |
|
Data are expressed as no. (%). |
|
Secondary outcome: safety of the MANTA plug-based vascular closure device
According to the VARC-3 definition, no major vascular or bleeding complications associated with the plug-based device were reported. A total of 8.6% of all the patients included (n = 21) had a minor VARC-3 vascular complication (table 4). Two-thirds (n = 14) showed femoral artery stenoses, 23.8% (n = 5) hematomas, and 9.5% (n = 2) pseudoaneurysms associated with the common femoral artery. The rate of minor bleeding complications associated with the main access site (closed with MANTA) was 2.9% only (n = 7).
Table 4. Secondary outcome: safety
| N = 245 | |
|---|---|
| Bleeding complications: access site-related | 7 (2.9) |
| Minor (VARC-1 type) | 7 (2.9) |
| Major (VARC-2, -3 or -4 types) | 0 (0.0) |
| Vascular complications: access site-related | 21 (8.6) |
| Minor | 21 (8.6) |
| Major | 0 (0.0) |
|
VARC, Valve Academic Research Consortium 3. |
|
Predictors of device failure
Patients with device failure (7.8%) had a significant smaller minimal femoral artery diameter (6.6 ± 1.1 mm vs 7.6 ± 1.4 mm; P = .005) and consequently, significant higher sheath-to-femoral artery diameter ratios (0.78 ± 0.16 vs 0.69 ± 0.15; P = .019) (table 5>). Patients with failed VCDs had more tortuous and calcified arterial accesses as shown on table 5. No other differences were found.
Table 5. Failed device vs non-failed device
| Failed device (N = 19) |
Non-failed device (N = 226) |
Odds ratio | 95%CI | P | |
|---|---|---|---|---|---|
| Minimal femoral artery diameter, mm | 6.6 ± 1.1 | 7.6 ± 1.4 | 0.56 | 0.37-0.84 | .005 |
| Sheath-to-femoral artery ratio | 0.78 ± 0.16 | 0.69 ± 0.15 | 32 | 1.78-579.48 | .019 |
| Body mass index, kg/m2 | 27 ± 6 | 27 ± 5 | 0.99 | 0.89-1.09 | .769 |
| Postoperative length of stay, days | 4 [3-5] | 4 [3-5] | — | — | .728 |
| Moderate-to-severe tortuosity | 57.9 | 13.1 | 9.1 | 3.35-24.90 | < .001 |
| Moderate-to-severe calcification | 52.6 | 22.7 | 2.9 | 1.12-7.49 | .028 |
| Anticoagulation therapy at baseline | 31.6 | 35.8 | 0.83 | 0.30-2.26 | .71 |
| Antiplatelet therapy at baseline | 31.6 | 31.9 | 0.99 | 0.36-2.70 | .98 |
|
Data are expressed as %, mean ± standard deviation or median [interquartile range]. |
|||||
In-hospital and short-term outcomes
Duringt the index hospitalization, 2 patients (0.8%) died of cardiovascular causes not associated with the VCD closure. One patient died due to aortic dissection and left ventricular rupture during the procedure despite conversion to open heart surgery. A second patient died following an ischemic stroke. The 30-day mortality rate was 3/245 (1.2%) because another patient died of urosepsis 14 days after discharge. Device failure did not extend the postoperative length of stay (median time to discharge, 4 days [3-5]).
DISCUSSION
This single-center study shows the real-world experience with the MANTA VCD for large caliber arteriotomy closure in an unselected consecutive cohort of patients referred for TF-TAVI. Our results, in an older and more frail population compared to RCT1,5 [with a EuroSCORE II of 3.15% (2.07-4.75), higher than the 2.6% (1.9-3.6) of the MASH trial, but lower than the 4.5 ± 4.8% of the CHOICE-CLOSURE] demonstrate a low MANTA device failure rate of 7.8% with no major device-related bleeding or vascular complications.
Compared to the most recent RCTs (MASH5 and CHOISE-CLOSURE1 trials) that included 216 and 510 patients, respectively, our results showed a lower rate of device failure with the MANTA device compared to the MASH trial (7.8% vs 20%), but > 4.7% compared to the CHOISE-CLOSURE,1,5 and 5.2% in a recent metanalysis.19 However, in the latter trial, authors did not consider balloon dilatation bailout as device failure, which may explain the lower failure rate of the plug-based VCD while we used balloon dilatation in most devices failures (16 out of 19 failures).
The high success rate of the MANTA device (92.2%) is probably due to our extensive experience with this type of device. Since the beginning of the TAVI program, this has been the most widely used VCD, which differentiates us from former studies. Furthermore, all TF-TAVIs were performed by a small group of 3 experienced operators with a large volume of vascular closure cases using this device. Conversely, in the MASH trial, MANTA was introduced later in the clinical practice and, consequently, experience was more limited.5 Compared to suture-based large-bore arteriotomy closure, the MANTA device showed less device failure and shorter time to hemostasis in both trials.1,5
We did not report any major vascular or bleeding complications associated with the MANTA VCD unlike RCTs1,5 and the most recent observational study. Still, this last study used VARC-2 criteria to define major complications.20 Halim et al.2 showed rates of minor vascular and bleeding complications of 13.7% and 5.5%, respectively, almost doubling those of our cohort (8.6% and 2.9%, respectively). As previously mentioned, this may be explained by our experience with this VCD. Another possible reason was that we were used to smaller plug-based devices like Angioseal.1,9,16 Therefore, familiarity with this technique seems to facilitate short learning curves with the MANTA device.1,13,14
In the MASH and CHOISE-CLOSURE trials, patients whose large-bore arterial access site were closed with the MANTA device had more minor vascular and hemorrhagic complications compared to those whose access sites were closed with suture-based devices. However, they were not significatively different and no differences in major complications were ever reported. This fact can be explained by the greater knowledge and experience using the ProGlide VCD by these teams.1,5 In a meta-analysis that compared MANTA vs suture-based VCD7 the authors conclude that plug-based vascular closure with MANTA was associated with fewer chances of device failure following large-bore arteriotomy procedures without significant differences being reported in bleeding or vascular complications compared to suture-based closure devices.
Although recommendations21 tell us that we should not use the MANTA device in vessels with minimum diameters < 6 mm, some of our patients had lower values. The main reason observed for VCD-related adverse vascular events was common femoral artery stenosis due to poor toggle positioning in smaller femoral arteries. This has been previously reported in the medical literature.22 Therefore, the implantation of MANTA in smaller femoral arteries should be avoided. Thus, as expected, those with device failure had a significantly smaller minimal femoral diameter, higher sheath-to-femoral artery diameter ratios, and more tortuous and calcified arterial accesses.
A recent study23 showed that when ultrasound was used to guide MANTA implantation, fewer vascular complications and device failure were reported perhaps because ultrasound helps clinicians choose the best site, thus avoiding calcium plaques and confirming the anchor in the proper position and the collagen pad delivered to the vessel wall through subcutaneous tissue. We had already used ultrasound to guide femoral access, which may explain our low rate of vascular complications. However, if in the future we also guide the deployment of MANTA, it can be possible to further reduce device failure not associated with postoperative length of stay or mortality as in former studies.1,5,7 We had a lower rate of in-hospital mortality (0.8%) compared to the 1.5% of the CHOICE-CLOSURE trial. No deaths were associated with the MANTA device.1,5
As far as we know, this is the largest real-world study ever conducted using MANTA to close large-bore femoral arterial accesses after TF-TAVI whose primary outcome was device efficacy using the VARC-3 criteria. The MANTA device shows promising results with low rates of vascular/bleeding complications and device failure without compromising the length of stay or the in-hospital/short-term mortality in the real-world setting. The shorter learning curve compared to suture-based VCDs is another plus. Therefore, the MANTA device could become the preferred option for large-bore vascular closure in TF-TAVI. The ultrasound-guided implantation of MANTA seems to be a solution for the complications and device failure seen in these patients. Thus, futures studies must compare the efficacy and safety profile of suture-based VCDs and ultrasound-guided plug-based VCDs in the real-world setting.
Limitations
This was a retrospective study and a single-center experience where we did not compare the plug-based VCD to another closure system. Therefore, it may be subject to different biases. Finally, our study did not perform postoperative ultrasounds in all patients. Therefore, we could have left out the asymptomatic vascular complications despite the angiographies performed after the procedure. However, no clinical impact was seen at follow-up of the putative asymptomatic vascular complications.
CONCLUSIONS
In this single-center, real-world, unselected and consecutive large cohort of patients treated with TF-TAVI, a plug-based VCD for large-bore arteriotomy closure turned out effective and safe, and enabled arterial access-site management with a low rate of complications. Smaller minimal femoral artery diameters and higher sheath-to-femoral artery diameter ratios were associated with a higher risk of failed VCDs.
FUNDING
None whatsoever.
ETHICAL CONSIDERATIONS
The study was approved by the regional Human Research Ethics Committee (HREC) at Coimbra, Portugal. Informed consent was waived. The authors confirm that variables such as sex and gender were taken into account in accordance with SAGER guidelines.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
Artificial intelligence was not used for the development of this work.
AUTHORS’ CONTRIBUTIONS
Study design, data collection and review, statistical analysis, and manuscript preparation: S. Martinho, E. Jorge, A. Vera Marinho, R. Baptista, M. Costa, and L. Gonçalves; Coordination and document submission: S. Martinho. All authors reviewed and approved the final version of the manuscript.
CONFLICTS OF INTEREST
None reported.
WHAT IS KNOWN ABOUT THE TOPIC?
- TF-TAVI access requires large-bore catheters. Therefore, successful closure of large-bore access sites is essential. Previously, MANTA showed promising results in TF-TAVI.
- Data regarding the rates of vascular complications and device failure with MANTA compared to the well-known suture based VCDs are scarce.
WHAT DOES THIS STUDY ADD?
- This is the largest real-world study of MANTA devices ever conducted to close the large-bore femoral arterial access after TF-TAVI. The study primary outcome was device efficacy using the VARC-3 criteria.
- The MANTA device shows promising results with low rates of vascular/bleeding complications and device failure without compromising the length of stay or the in-hospital/short-term mortality in a real-world setting. It proved to be easy to use.
- Our results may encourage the use of this VCD as a first option to close large caliber arterial vessels given its consistent efficacy and safety profile.
REFERENCES
1. Abdel-Wahab M, Hartung P, Dumpies O, et al. Comparison of a Pure Plug-Based Vs a Primary Suture-Based Vascular Closure Device Strategy for Transfemoral Transcatheter Aortic Valve Replacement: The CHOICE-CLOSURE Randomized Clinical Trial. Circulation. 2022;145:170-183.
2. Halim J, Missault L, Lycke M, van der Heyden J. Assessment of the MANTA closure device in transfemoral transcatheter aortic valve replacement: a single-centre observational study. Neth Heart J. 2020;28:639-644.
3. Généreux P, Webb JG, Svensson LG, et al. Vascular Complications After Transcatheter Aortic Valve Replacement. J Am Coll Cardiol. 2012;60:1043-1052.
4. van Mieghem NM, Tchetche D, Chieffo A, et al. Incidence, Predictors, and Implications of Access Site Complications With Transfemoral Transcatheter Aortic Valve Implantation. Am J Cardiol. 2012;110:1361-1367.
5. van Wiechen MP, Tchétché D, Ooms JF, et al. Suture- or Plug-Based Large-Bore Arteriotomy Closure: A Pilot Randomized Controlled Trial. JACC Cardiovasc Interv. 2021;14:149-157.
6. Hoffmann P, Al-Ani A, von Lueder T, et al. Access site complications after transfemoral aortic valve implantation - a comparison of Manta and ProGlide. CVIR Endovasc. 2018;1:20.
7. Al-Abdouh A, Abusnina W, Mhanna M, et al. MANTA Vs Suture-based Closure Devices Following Transcatheter Aortic Valve Replacement: An Updated Meta-Analysis. JSCAI. 2022;1:100397.
8. Toggweiler S, Leipsic J, Binder RK, et al. Management of Vascular Access in Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv. 2013;6:643-653.
9. van Mieghem NM, Latib A, van der Heyden J, et al. Percutaneous Plug-Based Arteriotomy Closure Device for Large-Bore Access. JACC Cardiovasc Interv. 2017;10:613-619.
10. de Palma R, Settergren M, Rück A, Linder R, Saleh N. Impact of percutaneous femoral arteriotomy closure using the MANTA TM device on vascular and bleeding complications after transcatheter aortic valve replacement. Catheter Cardiovasc Interv. 2018;92:954-961.
11. Wood DA, Krajcer Z, Sathananthan J, et al. Pivotal Clinical Study to Evaluate the Safety and Effectiveness of the MANTA Percutaneous Vascular Closure Device. Circ Cardiovasc Interv. 2019;12:e007258.
12. Biancari F, Romppanen H, Savontaus M, et al. MANTA vs ProGlide vascular closure devices in transfemoral transcatheter aortic valve implantation. Int J Cardiol. 2018;263:29-31.
13. Moriyama N, Lindström L, Laine M. Propensity-matched comparison of vascular closure devices after transcatheter aortic valve replacement using MANTA vs ProGlide. EuroIntervention. 2019;14:e1558-e1565.
14. Moccetti F, Brinkert M, Seelos R, et al. Insights From a Multidisciplinary Introduction of the MANTA Vascular Closure Device. JACC Cardiovasc Interv. 2019;12:1730-1736.
15. van Gils L, de Jaegere PPT, Roubin G, van Mieghem NicolasM. The MANTA Vascular Closure Device. JACC Cardiovasc Interv. 2016;9:1195-1196.
16. van Gils L, Daemen J, Walters G, et al. MANTA, a novel plug-based vascular closure device for large bore arteriotomies: technical report. EuroIntervention. 2016;12:896-900.
17. Dumpies O, Kitamura M, Majunke N, et al. Manta vs Perclose ProGlide vascular closure device after transcatheter aortic valve implantation: Initial experience from a large European center. Cardiovasc Revasc Med. 2022;37:34-40.
18. Généreux P, Piazza N, Alu MC, et al. Valve Academic Research Consortium 3: Updated endpoint definitions for aortic valve clinical research. Eur Heart J. 2021;42:1825-1857.
19. Sedhom R, Dang AT, Elwagdy A, et al. Outcomes with plug‐based vs suture‐based vascular closure device after transfemoral transcatheter aortic valve replacement: A systematic review and meta‐analysis. Catheter Cardiov Inter. 2023;101:817-827.
20. Kastengren M, Settergren M, Rück A, et al. Percutaneous plug-based vascular closure device in 1000 consecutive transfemoral transcatheter aortic valve implantations. Int J Cardiol. 2022;359:7-13.
21. Essential Medical Inc. MANTATM Vascular Closure Device INSTRUCTIONS FOR USE 14F 18F.2019. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf18/p180025c.pdf. Accessed 20 Dec 2022.
22. Masiero G, D’Angelo L, Fovino LN, et al. Real-World Experience With a Large Bore Vascular Closure Device During TAVI Procedure: Features and Predictors of Access-Site Vascular Complications. Front Cardiovasc Med. 2022;9:832242.
23. Miyashita H, Moriyama N, Dahlbacka S, et al. Ultrasound-Guided vs Conventional MANTA Vascular Closure Device Deployment After Transcatheter Aortic Valve Implantation. Am J Cardiol. 2022;180:116-123.