ABSTRACT
Introduction and objectives: De-escalation from prasugrel and ticagrelor to clopidogrel in patients undergoing percutaneous coronary intervention after acute coronary syndrome (ACS) is a strategy aimed at reducing bleeding. This study evaluates whether VerifyNow (Werfen, Spain)–guided de-escalation, based on platelet aggregation measurement, provides a therapeutic benefit in ACS management.
Methods: This ongoing multicenter, prospective, randomized 1:1 trial will enroll 634 patients with ACS who underwent revascularization with a sirolimus-eluting stent and were discharged on dual antiplatelet therapy with ticagrelor or prasugrel. Only those patients with a very low platelet reactivity level (platelet reactivity units ≤ 30) based on VerifyNow 1 month after discharge will be included. The primary endpoint is a composite of cardiovascular death, nonfatal acute myocardial infarction, nonfatal stroke, and bleeding at 1-year follow-up.
Results: The EPIC17-VERONICA study (NCT04654052) will reveal the efficacy profile of the de-escalation strategy, based on the VerifyNow platelet aggregation test, and determine the role of this device in the selection of patients who are eligible to benefit from this strategy.
Conclusions: This study will determine whether platelet function testing provide clinical benefit in the management of patients with ACS.
Keywords: Acute coronary syndrome. Antiplatelet therapy. Platelet function test. Bleeding.
RESUMEN
Introducción y objetivos: La desescalada desde prasugrel y ticagrelor a clopidogrel en pacientes tras intervencionismo coronario percutáneo por síndrome coronario agudo (SCA) constituye una de las estrategias para intentar disminuir las hemorragias. El objetivo de este estudio es averiguar si dicha desescalada guiada por la prueba de agregación plaquetaria VerifyNow (Werfen, España) tiene un efecto beneficioso en el tratamiento del SCA.
Métodos: Estudio multicéntrico, prospectivo y aleatorizado 1:1, en curso. Se incluirán 634 pacientes con SCA y revascularización con stent de sirolimus que sean dados de alta con doble terapia antiagregante con ticagrelor o prasugrel. Solo se incluirán aquellos con un nivel de reactividad plaquetaria muy bajo (unidades de reactividad plaquetaria ≤ 30) basado en VerifyNow al mes del alta. El objetivo primario es un combinado de muerte por causa cardiovascular, infarto agudo de miocardio no fatal, accidente cerebrovascular no fatal y sangrado en un seguimiento a 1 año.
Resultados: El estudio EPIC17-VERONICA (NCT04654052) permitirá averiguar la eficacia de la estrategia de desescalada basada en la prueba de agregación plaquetaria VerifyNow, además de conocer el papel de este dispositivo en la selección de los pacientes candidatos a beneficiarse de esta estrategia.
Conclusiones: Este estudio determinará si las pruebas de función plaquetaria aportan beneficio en el tratamiento tras el SCA.
Palabras clave: Síndrome coronario agudo. Terapia antiagregante. Prueba de función plaquetaria. Sangrado.
Abbreviations
ACS: acute coronary syndrome. PCI: percutaneous coronary intervention. PRU: platelet reactivity units.
INTRODUCTION
Following percutaneous coronary intervention (PCI) in patients with acute coronary syndrome (ACS), a 12-month regimen of dual antiplatelet therapy with a P2Y12 receptor inhibitor and acetylsalicylic acid is recommended, regardless of the type of stent implanted, except when contraindicated.1 Although prasugrel and ticagrelor are preferred over clopidogrel in this setting, there is ongoing debate regarding the potency and duration of dual antiplatelet therapy. This controversy stems from the fact that most patients concurrently face 2 opposing and potentially fatal risks—ischemic and hemorrhagic—which must be carefully balanced on an individual basis.
The introduction of stents with reduced thrombogenicity, together with evidence that thrombotic risk is highest during the first few months after PCI while hemorrhagic risk remains relatively constant throughout time, has led to research efforts focused on minimizing bleeding complications. These strategies include shortening dual antiplatelet therapy, using P2Y12 inhibitors as monotherapy, and implementing de-escalation strategies.2,3
De-escalation consists of switching from prasugrel or ticagrelor to clopidogrel and can be guided (using genetic or platelet function testing) or unguided. Because this strategy may increase ischemic events, it is not recommended within the first month after PCI.1
In the TOPIC trial,4 the unguided de-escalation strategy initiated 1 month after ACS significantly reduced hemorrhagic events (Bleeding Academic Research Consortium [BARC] grade ≥ 2 bleeding events) at 1 year without increasing the ischemic ones. In the TROPICAL-ACS trial,5 the platelet function testing–guided de-escalation from prasugrel to clopidogrel 2 weeks after revascularization was noninferior to standard therapy, showing a trend toward fewer hemorrhages at 12 months and a similar rate of thrombotic events.1,2,6 In the TALOS-AMI trial,7 12-month event rates were lower, primarily because of fewer hemorrhagic events among patients who underwent unguided de-escalation 1 month after ACS. Table 1 summarizes these studies.
Table 1. De-escalation clinical trials in patients with acute coronary syndrome
| TOPIC (2017)4 | TROPICAL-ACS (2018)5 | TALOS-AMI (2021)12 | |
|---|---|---|---|
| Population | n = 645 | n = 2610 | n = 2697 |
| Design | Open-label, single-center, randomized, superiority trial | Open-label, multicenter, randomized, noninferiority trial | Open-label, multicenter, randomized, noninferiority trial |
| Strategy | Standard therapy vs unguided de-escalation | Standard therapy vs platelet function testing–guided therapy (Multiplate device) | Standard therapy vs unguided de-escalation |
| Control group | Continued dual antiplatelet therapy with acetylsalicylic acid and ticagrelor or prasugrel | Continued dual antiplatelet therapy with acetylsalicylic acid and prasugrel | Continued dual antiplatelet therapy with acetylsalicylic acid and ticagrelor |
| Experimental group | De-escalation to acetylsalicylic acid and clopidogrel | 1-week regimen of prasugrel, followed by 1-week regimen of clopidogrel and either prasugrel or clopidogrel from day 14 onward, according to platelet function testing results | De-escalation to acetylsalicylic acid and clopidogrel |
| Time from revascularization to de-escalation | 1 month | 2 weeks | 1 month |
| Follow-up | 1 year | 1 year | 1 year |
| Primary endpoint | Cardiac death, emergency revascularization, stroke, or BARC ≥ 2 bleeding events | Cardiac death, myocardial infarction, stroke, or BARC ≥ 2 bleeding events | Cardiac death, myocardial infarction, stroke, or BARC ≥ 2 bleeding events |
| Results | 13.4% in experimental group vs 26.3% in control group (HR, 0.48; 95%CI, 0.34–0.68; P < .01) | 7.3% in experimental group vs 9.0% in control group (HR, 0.81; 95%CI, 0.62–1.06; P = .0004) | 4.6% in experimental group vs 8.2% in control group (HR, 0.55; 95%CI, 0.42–0.76; P < .0001) |
|
95%CI, 95% confidence interval; BARC, Bleeding Academic Research Consortium; HR, hazard ratio. |
|||
After the positive results of the TOPIC trial, the VerifyNow to optimise platelet inhibition in coronary acute syndrome (EPIC17-VERONICA) trial (ClinicalTrials.gov: NCT04654052) aims to further refine this strategy by only applying de-escalation to patients with excessive antiplatelet effects from prasugrel or ticagrelor after the first month who are at theoretical risk of hemorrhage based on the VerifyNow platelet aggregation test (Werfen, Spain). Thus, patients demonstrating an adequate pharmacologic response will continue prasugrel or ticagrelor therapy for 1 year, whereas those with very low platelet reactivity after a 1-month regimen of dual antiplatelet therapy with these agents constitute the target population of this study.
METHODS
Design
We are conducting a multicenter, prospective, randomized clinical trial at 16 Spanish centers. Based on the results of the platelet aggregation test for P2Y12 inhibition (platelet reactivity units [PRU]) using the VerifyNow system, patients with very low platelet reactivity (PRU ≤ 30) are randomized in a 1:1 ratio to either continue treatment with ticagrelor or prasugrel, or to de-escalate to clopidogrel. Patients with PRU > 30 are not randomized. The study flowchart is shown in figure 1.
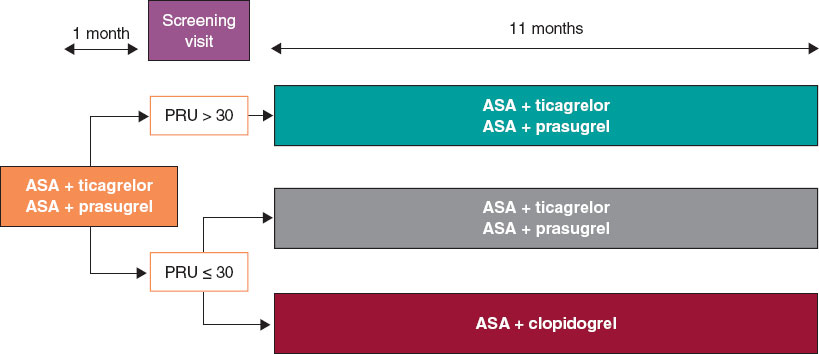
Figure 1. Study flowchart. AAS, acetylsalicylic acid; PRU, platelet reactivity units.
The study is being conducted in full compliance with the principles outlined in the Declaration of Helsinki and has been approved by the central ethics committee (Comité del Bierzo, León, Spain) and endorsed by the ethics committees of all participant centers. The appendix lists the participant centers and principal investigators.
The study sponsor (Fundación para la Educación en Procedimientos de Intervencionismo en Cardiología [EPIC]) is fully responsible, together with the principal investigators, for data management and confidentiality.
Population
Inclusion and exclusion criteria
Table 2 summarizes the inclusion and exclusion criteria. Briefly, all patients with ACS undergoing PCI with a sirolimus-eluting stent and a bioresorbable polymer during hospitalization and discharged on dual antiplatelet therapy with acetylsalicylic acid and ticagrelor or prasugrel are eligible for inclusion.
Table 2. Inclusion and exclusion criteria
| Inclusion criteria |
|---|
| Patients > 18 years |
| Patients with acute coronary syndrome undergoing percutaneous revascularization with a sirolimus-eluting stent with a bioresorbable polymer and discharged on dual antiplatelet therapy with acetylsalicylic acid and ticagrelor or prasugrel |
| Signed informed consent |
| Exclusion criteria |
| History of intracranial hemorrhage |
| Contraindication to acetylsalicylic acid, clopidogrel, prasugrel, or ticagrelor |
| Major ischemic or bleeding events during the first month of antiplatelet therapy |
| Thrombocytopenia < 50 000/µL |
| Permanent oral anticoagulation |
| Pregnancy or breastfeeding |
| Inability to complete the 1-year follow-up |
| Life expectancy < 24 months |
Written informed consent must be obtained before the platelet aggregation tes is performed.
Study protocol and randomization
Eligible patients are scheduled for P2Y12 receptor inhibition testing with the VerifyNow system between 30 and 40 days after hospital discharge. Measurements are obtained at least 6 hours after the administration of the last P2Y12 inhibitor dose. Patients with PRU ≤ 30 (very low platelet reactivity) are randomized in a 1:1 ratio using an electronic system to either continue their current treatment or de-escalate to clopidogrel, 75 mg once daily. De-escalation is preceded by a loading dose of 600 mg administered 24 hours after the last dose of ticagrelor or 75 mg 24 hours after the last dose of prasugrel, in accordance with the 2017 European Society of Cardiology clinical practice guidelines.8
The remaining patients with PRU > 30 are not randomized, and their dual antiplatelet therapy remains unchanged from discharge.
Clinical follow-up
Patients in the 2 randomized groups undergo telephone follow-up to monitor clinical events at 2, 5, 8, and 11 months after enrollment, corresponding to 3, 6, 9, and 12 months after hospital discharge.
For patients with PRU > 30 on the 1-month VerifyNow platelet aggregation test who are not randomized, only baseline characteristics are recorded, and no further follow-up is conducted.
Protocol of the VerifyNow platelet aggregation test
The VerifyNow system determines platelet activity by measuring in vitro aggregation in a blood sample exposed to specific agonists. This optical detection instrument (figure 2), which operates on a turbidimetric principle, uses single-use cartridges. In this study, PRUTest-specific kits are employed. (Werfen, Spain) to assess platelet aggregation while on P2Y12 receptor inhibitor therapy (ticagrelor, prasugrel, and clopidogrel). Each PRUTest kit contains lyophilized microbeads coated with fibrinogen, platelet activators, and a buffered solution. The test is based on the ability of activated platelets to bind fibrinogen-coated microbeads. Light transmission increases as activated platelets bind to and aggregate with the fibrinogen-coated microspheres. The kit measures this change in the optical signal and reports the results in PRU units (figure 3).

Figure 2. VerifyNow system. Reproduced with permission from Werfen.
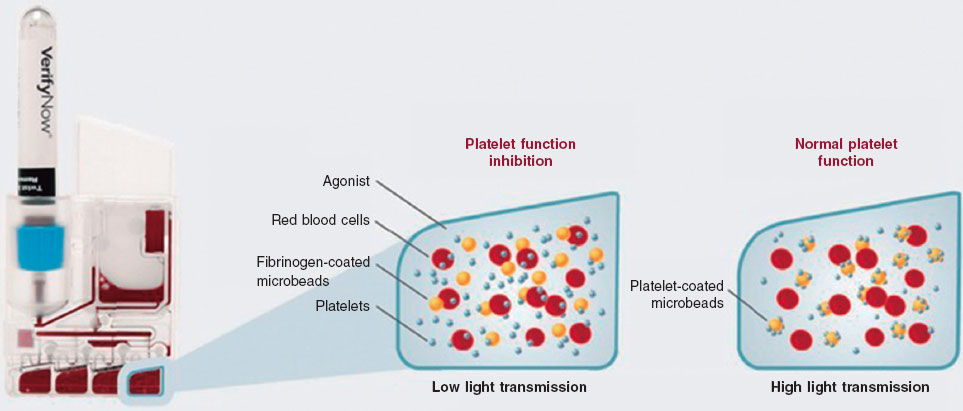
Figure 3. Performance of the VerifyNow system based on light transmission aggregometry. Light transmission increases as activated platelets bind and aggregate to the fibrinogen-coated microbeads in the kit. Therefore, high light transmission (corresponding to elevated platelet reactivity unit [PRU] values) indicates normal platelet function, whereas low light transmission (decreased PRU values) reflects platelet inhibition induced by the tested drugs.
An antiplatelet effect of the drug is considered present with PRU ≤ 180 (figure 4). Only patients with PRU ≤ 30 are randomized, as these are considered to have very low platelet reactivity while on antiplatelet therapy.
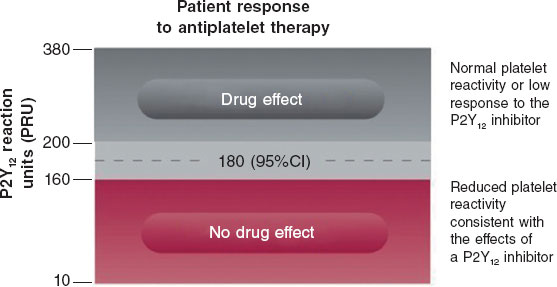
Figure 4. Reference levels for platelet reactivity units (PRU). 95%CI, 95% confidence interval.
Endpoints
The primary endpoint of the study is to compare the efficacy of de-escalation from ticagrelor or prasugrel to clopidogrel in patients undergoing PCI in the ACS setting, using the VerifyNow platelet aggregation test vs standard dual antiplatelet therapy at the 1-year follow-up. The rate of net adverse cardiovascular events is the primary endpoint of the study, defined as a composite of cardiac death, nonfatal myocardial infarction, nonfatal stroke, and hemorrhage (defined as Bleeding Academic Research Consortium [BARC] grade ≥ 2 bleeding events). The BARC scale is shown in table S1.
Furthermore, the study aims to compare several secondary endpoints (table 3), such as the occurrence of ischemic events during follow-up: cardiac death and all-cause mortality, acute myocardial infarction, stroke, stent thrombosis, and need for emergency revascularization. Moreover, the hemorrhage rate (defined as BARC grade ≥ 2 bleeding events) will be compared. The definitions of all study endpoints are shown in table S2.
Table 3. Endpoints of the study
| Primary endpoint |
|---|
| To compare the percentage of net adverse cardiovascular events between the 2 subgroups of patients with low platelet reactivity (PRU ≤ 30) who were randomized to de-escalation to clopidogrel vs standard therapy |
| Secondary endpoints |
| To compare the rate of cardiac death between the 2 randomized patient subgroups |
| To compare the rate of all-cause mortality between the 2 randomized patient subgroups |
| To compare the rate of acute myocardial infarction between the 2 randomized patient subgroups |
| To compare the rate of stroke between the 2 randomized patient subgroups |
| To compare the rate of stent thrombosis between the 2 randomized patient subgroups |
| To compare the rate of emergency revascularization between the 2 randomized patient subgroups |
| To compare the rate of bleeding events (defined as BARC ≥ 2) between the 2 randomized patient subgroups |
|
BARC, Bleeding Academic Research Consortium; PRU, platelet reactivity units. |
Statistics
Sample size calculation
Sample size was calculated for the randomized clinical trial cohort. The total number of patients (including those not randomized with PRU > 30) will depend on the total required to reach the estimated sample size for the randomized clinical trial.
We estimate a smaller difference in event rates across the groups than that observed in the TOPIC trial,4 specifically, 14% in the de-escalation group vs 22% in the standard therapy group. Assuming a significance level of 0.05, a power of 80%, a 2-tailed P-value and a 10% loss to follow-up, a total of 634 randomized patients (317 per group) will be required.
Statistical analysis plan
Quantitative variables will be expressed as mean and standard deviation if normally distributed, or as median and interquartile range otherwise. Categorical variables will be expressed as absolute values and percentages. Study data will be analyzed using one-way analysis of variance (ANOVA) for continuous variables, and Fisher’s exact or chi-square tests for categorical variables, as appropriate. Nonparametric tests will be used for variables that are not normally distributed or cannot be normalized. For the main outcome measure, Kaplan-Meier survival curves with log-rank statistics will be presented for prespecified criteria, and multivariable Cox regression will be performed to adjust for known risk factors and potential confounders. Hazard ratios and 95% confidence intervals will be reported for all statistically significant variables.
Intention-to-treat (according to randomization assignment) and per-protocol analyses (in case of crossover) will be conducted. The former will serve as the study primary analysis.
DISCUSSION
The EPIC17-VERONICA trial aims to demonstrate the efficacy of a VerifyNow platelet aggregation test-guided de-escalation strategy in reducing hemorrhagic events without increasing ischemic events in patients with ACS who have undergone percutaneous revascularization and exhibit very low platelet reactivity after the first month of treatment with prasugrel or ticagrelor.
The initial lack of expected results from platelet function testing to identify patients at risk for thrombotic events while on clopidogrel in the GRAVITAS,9 TRIGGER-PCI,10 and ARCTIC11 trials relegated its use to a class IIb recommendation in the European Society of Cardiology antiplatelet guidelines for determining the optimal timing of cardiac surgery after ACS.8 However, the 1-year results of the large-scale multicenter ADAPT-DES trial12 with 8500 PCI patients demonstrated that platelet reactivity assessed with the VerifyNow platelet aggregation test is an independent predictor of bleeding events.
In the TOPIC4 and TALOS-AMI7 trials, the unguided de-escalation strategy significantly reduced bleeding events without increasing ischemic events. In the TROPICAL-ACS5 trial, this platelet aggregation test–guided de-escalation strategy showed a trend toward fewer hemorrhages, with a similar rate of thrombotic complications.
The EPIC17-VERONICA study further seeks to improve the application of this de-escalation strategy by using the VerifyNow platelet aggregation test to identify patients with very low platelet reactivity (PRU ≤ 30) as those most likely to benefit from de-escalation.
CONCLUSIONS
The EPIC17-VERONICA trial has been designed to investigate the efficacy of de-escalating from the most potent antiplatelet agents (ticagrelor and prasugrel) to clopidogrel after the first month of therapy in patients with ACS and very low platelet reactivity, aiming to reduce bleeding events without increasing ischemic complications. Furthermore, it will provide evidence on the clinical utility of the VerifyNow platelet aggregation test for patient selection.
FUNDING
None declared.
ETHICAL CONSIDERATIONS
The study is being conducted in full compliance with the principles outlined in the Declaration of Helsinki on clinical research and has been approved by the central ethics committee (Comité del Bierzo, León, Spain) and endorsed by the ethics committees of all participant centers. Written informed consent is required prior to performing ant platelet aggregation measurements. Sex and gender bias considerations have been addressed.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
No artificial intelligence was used in the preparation of this manuscript.
AUTHORS’ CONTRIBUTIONS
C. Garilleti Cámara and I. Lozano Martínez-Luengas drafted the manuscript; the remaining authors critically revised the document and approved the final version.
CONFLICTS OF INTEREST
J.M. de la Torre Hernández is Editor-in-Chief of REC: Interventional Cardiology; A. Pérez de Prado is Associate Editor of REC: Interventional Cardiology. In both cases, the journal’s editorial procedure to ensure impartial handling of the manuscript has been followed. The remaining authors declared no conflicts of interest whatsoever.
WHAT IS KNOWN ABOUT THE TOPIC?
- De-escalation from the most potent antiplatelet agents to clopidogrel is one of the strategies used to reduce hemorrhage after percutaneous revascularization in acute coronary syndrome. This de-escalation can be performed guided or unguided by genetic or platelet function testing.
WHAT DOES THIS STUDY ADD?
- The VERONICA trial is the first to use the VerifyNow platelet aggregation test to select patients eligible for de-escalation.
REFERENCES
1. Byrne RA, Rossello X, Coughlan JJ, et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J. 2023;44:3720-3826.
2. Angiolillo DA, Galli M, Collet JP, Kastrati A, O’Donoghue MO. Antiplatelet therapy after percutaneous coronary intervention. EuroIntervention. 2022; 17:e1371-e1396.
3. Angiolillo DJ. The Evolution of Antiplatelet Therapy in the Treatment of Acute Coronary Syndromes. Drugs. 2012;72:2087-2116.
4. Cuisset T, Deharo P, Quilici J, et al. Benefit of switching dual antiplatelet therapy after acute coronary syndrome: the TOPIC (timing of platelet inhibition after acute coronary syndrome) randomized study. Eur Heart J. 2017;38:3070-3078.
5. Sibbing D, Aradi D, Jacobshagen C, et al. Guided de-escalation of antiplatelet treatment in patients with acute coronary syndrome undergoing percutaneous coronary intervention (TROPICAL-ACS): a randomised, open-label, multicentre trial. Lancet. 2017;390:1747-1757.
6. Gorog DA, Ferreiro JL, Ahrens I, et al. De-escalation or abbreviation of dual antiplatelet therapy in acute coronary syndromes and percutaneous coronary intervention: a Consensus Statement from an international expert panel on coronary thrombosis. Nat Rev Cardiol. 2023;20:830-844.
7. Kim CJ, Park MW, Kim MC, et al. Unguided de-escalation from ticagrelor to clopidogrel in stabilised patients with acute myocardial infarction undergoing percutaneous coronary intervention (TALOS-AMI): an investigator-initiated, open-label, multicentre, non-inferiority, randomised trial. Lancet. 2021;398:1305-1316.
8. Valgimigli A del G de TM, Bueno H, Byrne RA, et al. Actualización ESC 2017 sobre el tratamiento antiagregante plaquetario doble en la enfermedad coronaria, desarrollada en colaboración con la EACTS. Rev Esp Cardiol. 2018;71:42.e1-42.e58.
9. Price MJ, Berger PB, Teirstein PS, et al. Standard- vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial. JAMA. 2011;305:1097-105. Erratum in: JAMA. 2011;305;2174. Stillablower, Michael E [corrected to Stillabower, Michael E]. PMID: 21406646.
10. Trenk D, Stone GW, Gawaz M, et al. A Randomized Trial of Prasugrel Versus Clopidogrel in Patients With High Platelet Reactivity on Clopidogrel After Elective Percutaneous Coronary Intervention With Implantation of Drug-Eluting Stents. J Am Coll Cardiol. 2012;59:2159-2164.
11. Collet JP, Cuisset T, Rangé G, et al. Bedside Monitoring to Adjust Antiplatelet Therapy for Coronary Stenting. N Engl J Med. 2012;367:2100-2109.
12. Sibbing D, Schulz S, Braun S, et al. Antiplatelet effects of clopidogrel and bleeding in patients undergoing coronary stent placement. J Thromb Haemost. 2010;8:250-256.










