ABSTRACT
Introduction and objectives: No previous studies have established the contemporary use and outcomes of Excimer laser coronary atherectomy (ELCA) in percutaneous coronary intervention (PCI) of severely calcified coronary lesions. The aim of this study was to assess the safety, efficacy, and 1-year outcomes of ELCA in this setting.
Methods: We retrospectively examined the clinical and angiographic characteristics and procedural outcomes of severely calcified lesions treated with ELCA-assisted PCI in our institution between 2016 and 2022.
Results: Seventy-eight consecutive patients (80 procedures) were included (mean age 71.2 ± 8.6 years, 80.5% men). Clinical presentation was stable coronary artery disease in 45 patients (56.2%) and acute coronary syndromes in 33 (43.8%). All the lesions were severely calcified. In addition, 40% were uncrossable lesions, 28.75% were undilatable lesions, 2.5% showed in-stent restenosis, 6.25% showed stent underexpansion, and 7.5% were chronic total occlusions. The combination of ≥ 2 of the above anatomic settings was found in 12.5% of the procedures. The maximum fluence was 73 ± 9.6 mJ/mm2, and the maximum frequency was 72.7 ± 10.4 Hz. The saline flushing technique was initially used in all the procedures, while contrast was used in 2 procedures. The ELCA success and technical success rates were both 91.25%. Adjuvant plaque modification therapies were required in 4 patients. The clinical success rate was 87.5%. ELCA-related complications occurred in 2 procedures (2.5%). After a median follow-up of 15.5 months [IQR, 5.0-29.3], major adverse cardiac events (MACE) (target lesion revascularization, myocardial infarction or cardiac death) occurred in 9 patients (11.25%).
Conclusions: Despite the complexity of PCI in severely calcified lesions, ELCA was effective with a relatively low incidence of ELCA-related complications and MACE during follow-up.
Keywords: Complex PCI. Excimer laser coronary atherectomy. Calcified coronary lesions.
RESUMEN
Introducción y objetivos: El uso contemporáneo y los resultados de la aterectomía coronaria con láser Excímer (ELCA) en el intervencionismo coronario percutáneo (ICP) de lesiones coronarias gravemente calcificadas no están establecidos. El objetivo de este estudio fue evaluar la eficacia, seguridad y resultados a 1 año de ELCA en este escenario.
Métodos: Se revisaron de forma retrospectiva las características clínicas y angiográficas, y los resultados de los procedimientos de revascularización de lesiones gravemente calcificadas tratadas con ICP asistido por ELCA en nuestro centro entre 2016 y 2022.
Resultados: Se incluyeron 78 pacientes consecutivos (80 procedimientos) (edad media 71,2 ± 8,6 años, 80,5% varones). La presentación clínica fue enfermedad arterial coronaria estable en 45 (56,2%) pacientes y síndromes coronarios agudos en 33 (43,8%). Todas las lesiones presentaban calcificación grave. Además, el 40% eran lesiones incruzables, el 28,75% lesiones indilatables, el 2,5% reestenosis intrastent, el 6,25% infraexpansión del stent y el 7,5% oclusiones crónicas. La combinación de ≥ 2 de los escenarios anatómicos anteriores existió en el 12,5% de los procedimientos. La fluencia máxima fue de 73 ± 9,6 mJ/mm2 y la frecuencia máxima de 72,7 ± 10,4 Hz. ELCA con lavado con solución salina se utilizó inicialmente en todos los procedimientos y se utilizó contraste en 2 procedimientos. La tasa de éxito de ELCA y de éxito técnico fueron del 91,25 %. Fueron necesarias terapias adyuvantes de modificación de placa en 4 casos. La tasa de éxito clínico fue del 87,5%. Ocurrieron complicaciones relacionadas con ELCA en 2 (2,5%) procedimientos. Tras una mediana de seguimiento de 15,5 meses (IQR, 5,0-29,3), se produjeron eventos cardiovasculares adversos mayores (MACE) (nueva revascularización de la lesión diana, infarto de miocardio o muerte cardiaca) en 9 pacientes (11,25%).
Conclusiones: A pesar de la complejidad de la ICP en lesiones gravemente calcificadas, ELCA demostró ser efectivo con una incidencia relativamente baja de complicaciones relacionadas con ELCA y MACE en el seguimiento.
Palabras clave: ICP compleja. Láser coronario. Lesiones coronarias calcificadas.
Abbreviations
CTO: chronic total occlusion. ELCA: excimer laser coronary atherectomy. ISR: in-stent restenosis. MACE: major adverse cardiovascular events. PCI: percutaneous coronary intervention. RA: rotational atherectomy.
INTRODUCTION
Moderate or severe coronary artery calcification is relatively common in patients undergoing percutaneous coronary interventions (PCI).1 This is closely related to advancing age and the high prevalence of comorbidities such as diabetes or chronic kidney disease. Coronary artery calcification is associated with a lower rate of successful PCI and complete revascularization, increased procedural-related complications, and a higher rate of major adverse cardiovascular events (MACE).2
Despite the availability of several plaque modification techniques, severely calcified lesions continue to pose a challenge to the successful performance of PCI.
Excimer laser coronary atherectomy (ELCA) is a plaque modification technique that has proved to be useful in various scenarios such as balloon failure (uncrossable or undilatable lesions), chronic total occlusions (CTO), stent underexpansion, in-stent restenosis (ISR), and thrombotic lesions. In recent years, incremental operator experience along with the standardization of the laser technique, has expanded its indications and decreased its complication rates.3,4
However, its effectiveness in calcified lesions is controversial. On one hand, some ELCA series have described a relationship between severe calcification and laser failure.5-8 On the other hand, moderate-to-severe calcification is found in more than 60% of cases in some ELCA series with a high success rate,9 suggesting that it could be useful in this setting.10
Due to the lack of evidence in this specific scenario, the aim of our study was to assess the safety and efficacy of ELCA in severely calcified coronary lesions, as well as the mid-term follow-up outcomes in a single center registry.
METHODS
Patient population
This single center retrospective observational study included all consecutive patients undergoing ELCA-assisted PCI for severely calcified lesions. From March 2016 to August 2022.
We excluded procedures using ELCA for any indication other than severe calcification. In all patients, PCI was indicated based on the presence of symptoms consistent with angina, demonstrated ischemia, or both. The study followed the international recommendations of clinical investigation (Declaration of Helsinki). All participants gave written informed consent and approval was obtained from the ethics committee of the center. The study took into consideration sex and gender variables according to SAGER guidelines. Patients were followed up in cardiology clinics at their referral center 3 to 6 months after the procedure, and thereafter at time intervals established at the discretion of their treating physician.
We analyzed data on clinical and angiographic characteristics, technical aspects of the procedure, and cardiovascular events during hospitalization and after discharge.
Procedure
All procedures were carried out by 5 different operators experienced in the use of ELCA. The decision to use ELCA was based on the presence of angiographically severe calcification.
Radial access was use by default. All cases were performed with the CVX-300 Excimer Laser System (Philips, Netherlands) using the 0.9 mm or 1.4 mm catheters. Saline infusion technique was used by default from the beginning, with fluence (mJ/mm2), frequency or repetition rate (Hertz), and the possibility to use ELCA without saline infusion or even with contrast left to the operator’s discretion. Additional dilatation with noncompliant balloons was performed in all procedures. Patients in which another plaque modification technique was used in combination with ELCA were included. All PCIs were performed following current recommendations.11
Definitions
Severely calcified lesions were angiographically defined as radiopacities observed on fluoroscopy without cardiac motion before contrast injection compromising 1 or both sides of the lumen.12 Balloon-uncrossable lesions were defined as lesions that could not be crossed with the lowest-profile balloon available or a microcatheter despite successful advancement of the guidewire distal to the lesion, having good guide catheter support with a guide extension catheter when required. Balloon-undilatable lesions were defined as those lesions in which a noncompliant balloon (diameter 1:1 according to the vessel diameter) failed to achieve adequate expansion. Anterograde flow was assessed by the Thrombolysis In Myocardial Infarction (TIMI) scale.
ELCA success was defined as the ability to cross the lesion completely with the laser catheter or, if crossing was not feasible, to allow the subsequent passage and expansion of a balloon sized 1:1 with the vessel diameter, after laser application. Technical success was defined as residual stenosis < 30% and anterograde TIMI 3 flow in the target vessel. Clinical success was defined as technical success and the absence of MACE during the current hospitalization (target lesion revascularization, procedure-related myocardial infarction [MI], or cardiovascular death). Procedural-related complications included coronary artery perforation leading to cardiac tamponade and requiring pericardial drainage, flow-limiting dissection, no-reflow, hemodynamic instability, MI type 4a according to the fourth universal definition of MI,13 ventricular arrhythmias, and major bleeding (bleeding requiring transfusion and/or surgical or percutaneous intervention). MACE occurring during follow-up were defined as a composite of target lesion revascularization, MI, or cardiac death.
Statistical analysis and data collection
All data were collected through the patients’ electronic medical records and were introduced in a local database. Angiograms were evaluated using local quantitative coronary analysis software and visual operators’ assessment. Categorical variables are reported as absolute values and percentages. Continuous variables are presented as the mean ± standard deviation (SD) or median (interquartile range [IQR] 25-75), depending on their normal or nonnormal distribution. All analyses were performed with StatIC 16.1 statistical software package.
RESULTS
Clinical characteristics
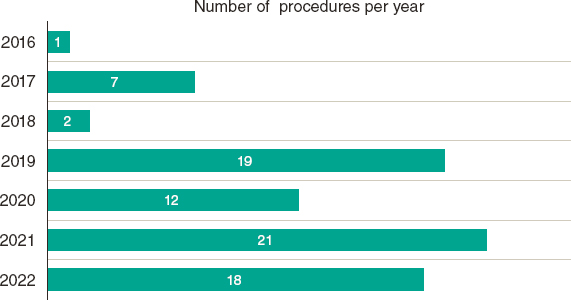
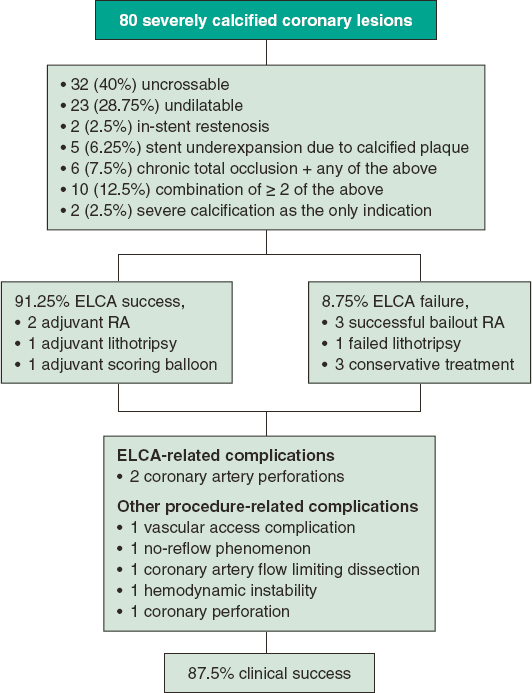
During the study period, a total of 78 patients with severely calcified coronary lesions underwent 80 ELCA-assisted PCIs and were included in the analysis. Patients undergoing ELCA for an indication other than severe calcification were excluded from the analysis. The distribution of the number of procedures per year, between March 2016 and May 2022, is shown in figure 1. A flowchart of patients in the present study is summarized in figure 2. Mean age was 71.2 ± 8.6 years, 62 (80.5%) were men, and there was a high prevalence of cardiovascular risk factors. Mean left ventricle ejection fraction was 52.9% ± 12.5%. Thirty-nine patients (50%) had a previous PCI. Clinical presentation was stable coronary artery disease in 45 procedures (56.2%), non–ST-segment elevation MI (NSTEMI) in 28 (35%), and ST-segment elevation MI (STEMI) in 7 (8.8%). Baseline clinical characteristics are summarized in table 1.
Figure 1. Distribution of the number of procedures per year (March 2016-May 2022).
Figure 2. Flowchart of patients in the present study. ELCA, excimer laser coronary atherectomy; PCI, percutaneous coronary intervention; RA, rotational atherectomy.
Table 1. Baseline clinical characteristics
| Age | 71.2 ± 8.6 |
| Male sex | 62 (80.5%) |
| Body mass index (kg/m2) | 28.7 ± 4.2 |
| Hypertension | 70 (89.7%) |
| Dyslipidemia | 61 (78.2%) |
| Diabetes mellitus | 46 (59.0%) |
| Current smoker | 19 (24.4%) |
| Prior PCI | 39 (50.0%) |
| Prior CABG | 8 (10.3%) |
| Hb (g/dL) | 13.5 ± 5.3 |
| Serum creatinine (mg/dL) | 1.42 ± 1.8 |
| Ejection fraction (%) | 52.9% ± 12.5 |
| Clinical presentation (n = 98) | |
| Stable coronary artery disease | 45 (56.2%) |
| NSTEMI | 28 (35.0%) |
| STEMI | 7 (8.8%) |
|
CABG, coronary artery bypass graft surgery; NSTEMI, non–ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction. Data are expressed as no. (%) or mean ± standard deviation. |
|
Angiographic characteristics
Severe multivessel disease was present in 56 patients (71.8%). The most common target vessel was the left anterior descending artery (38.75%). In 7 procedures (8.75%), more than 1 target vessel were identified. The anatomical settings in the target vessel included uncrossable lesions in 32 (40%), undilatable lesions in 23 (28.75%), ISR in 2 (2.5%), and stent underexpansion related to calcified plaque in 5 (6.25%). In 6 (7.5%) procedures, the main indication for ELCA was CTO combined with any of the previous settings. In 10 procedures (12.5%), the indication for ELCA resulted from the combination of 2 or more of the above. ELCA was used with the sole indication of severely calcified lesion, not included in any of the previous anatomical settings, in 2 procedures (2.5%).
Procedural characteristics
The radial approach was performed in 44 (55%) cases. There was no need for access conversion when the radial approach was attempted.
Dual antiplatelet treatment consisted of pretreatment with aspirin and oral P2Y12 receptor blockers in 58 patients (72.5%). Selection of P2Y12 inhibitor was left to the physician’s discretion. Cangrelor was used in the patients without prior dual antiplatelet treatment. After the procedure and during follow-up, dual antiplatelet treatment was prescribed as follows: in stable coronary artery disease (n = 45) clopidogrel was used in 21 patients, ticagrelor in 10 and prasugrel in 3 patients. In acute settings (n = 35), ticagrelor was administered in 16 patients, prasugrel in 10, and clopidogrel in 7. GPIIB/IIIA inhibitors were used in 6 procedures (7.5%) (tirofiban in all cases).
Intracoronary imaging was used in 58 procedures (72.5%). Optical coherence tomography (OCT) was used in 48 procedures (60%) and intravascular ultrasound in 10 (12.5%).
Circulatory support with intra-aortic balloon counterpulsation was required in only 1 patient in the context of left-main revascularization.
Regarding the ELCA technique, most lesions were treated with 0.9 mm laser catheters (97.5%). In 2 patients, larger catheters (1.4 mm) were used (1 case of ISR in the left anterior descending artery and 1 calcified lesion in a saphenous vein graft). Flushing saline was used in all the procedures, and contrast was required in 2 procedures (figure 3). Maximum fluence was 73 ± 9.6 mJ/mm2 and the maximum frequency 72.7 ± 10.4 Hz. The highest fluence of 80 mJ/mm2 was required in 48 (60%) procedures and the highest frequency of 80 Hz in 48 (60%). A mean of 5103 ± 3120 pulses was delivered, and the median lasing time was 62 seconds [IQR 40-91].
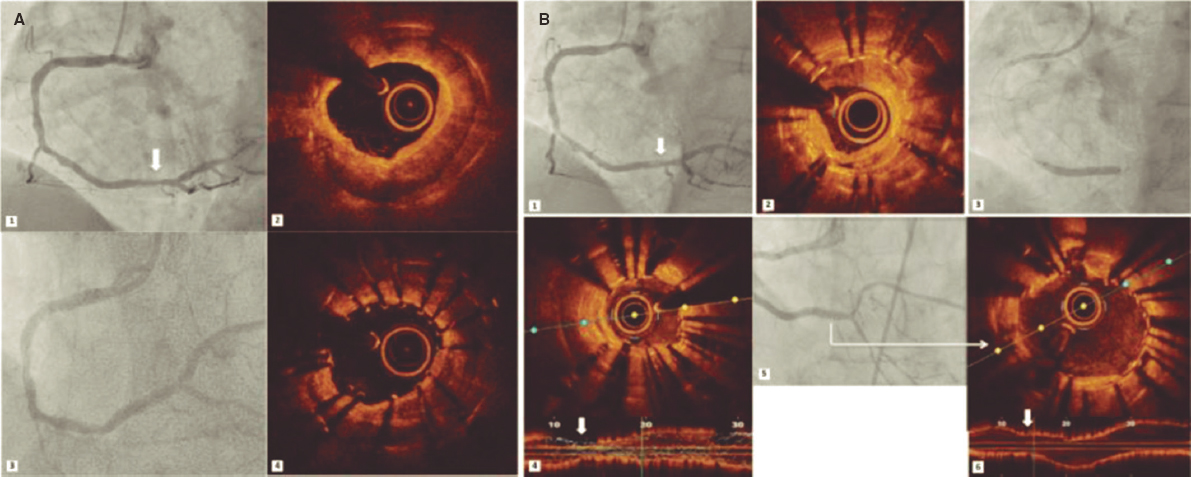
Figure 3. In-stent restenosis and stent underexpansion treated by excimer laser coronary atherectomy (ELCA). Severe in-stent restenosis (ISR) (A1) (arrow) of drug-eluting stent previously implanted in the right coronary artery. Optical coherence tomography (OCT) revealed calcified neoatherosclerosis with a minimum luminal area (MLA) of 1.25 mm2 (A2). An everolimus-eluting stent (2.75 × 20 mm) was implanted, and despite postdilatation with a 3-mm noncompliant (NC) balloon (A3), subsequent OCT confirmed stent underexpansion (MLA: 2.1 mm2) (A4). Sixteen months later, critical ISR of the previous stent (B1) (arrow) was noted with heterogeneous neointimal proliferation (B2). Laser atherectomy was performed, followed by dilation with 3- and 3.5-mm NC balloons up to 24 atm, and a 3-mm sirolimus-eluting stent was implanted with acceptable angiographic expansion (B3) but underexpansion on OCT (MLA: 1.5 mm2) (B4) (arrow). Laser application with contrast injection was repeated and was dilated with a 4 mm NC balloon, achieving adequate stent expansion (MLA: 4.5 mm2) (B5, B6) (arrow).
At least 1 new-generation drug-eluting stent was implanted in 70 procedures (87.5%). In the remaining procedures, stents were not delivered because of the presence of previous stents (6 ISR and 2 cases of stent underexpansion), which were treated with noncompliant and/or drug-eluting balloons, or due to ELCA failure (2 cases).
Angiographic and procedural characteristics and procedural strategy data are summarized in table 2.
Table 2. Angiographic and procedural characteristics
| Angiographic characteristics | |
|---|---|
| Target vessel | |
| Left anterior descending coronary artery | 31 (38.75%) |
| Right coronary artery | 28 (35.0%) |
| Circumflex artery | 10 (12.5%) |
| Left main coronary artery | 4 (5.0%) |
| Multivessel disease | 56 (71.8%) |
| Indication for ELCA | |
| Balloon-uncrossable lesion | 32 (40%) |
| Balloon-undilatable lesion | 23 (28.75%) |
| In-stent restenosis | 2 (2.5%) |
| Stent Underexpansion | 5 (6.25) |
| Chronic total occlusion | 6 (7.75%) |
| Combination of > 2 of the above | 10 (12.5%) |
| Severe calcification as sole indication | 2 (2.5%) |
| Bifurcation | 14 (17.7%) |
| Aorto-ostial | 2 (2.5%) |
| Procedural characteristics | |
| Access site | |
| Radial | 44 (55.0%) |
| Femoral | 33 (41.2%) |
| Femoral-radial | 3 (3.8%) |
| Guiding catheter French | |
| 6-Fr | 40 (50.0%) |
| 7-Fr | 34 (42.5%) |
| Intracoronary imaging | 58 (72.5%) |
| OCT | 48 (60.0%) |
| IVUS | 10 (12.5%) |
| Laser catheter | |
| 1.4 mm rapid-exchange catheter | 2 (2.5%) |
| 0.9 mm rapid-exchange catheter | 78 (97.5%) |
| Maximum fluence (mJ/mm2) | 72.97 ± 9.6 |
| Maximum frequency (Hz) | 72.7 ±10.4 |
| Number of pulses | 5 103 ± 3 120 |
| Total lasing time (sec) | 62 [40-91] |
| Contrast volume (mL) | 211 ± 68.0 |
| Fluoroscopy time (min) | 30 [22-39] |
| Radiation dose (Gy/cm2) | 103 [79-185] |
| Procedural time (min) | 72 [55-100] |
| Stent implantation | 70 [87.5%] |
| Stent diameter (mm | 3.04 ± 0.50 |
| Stents per procedure | 1.8 ± 1.14 |
| Total stent length (mm) | 43.7 ± 25.7 |
| Left ventricle assist device used | 1 (1.25%) |
| Timing of PCI (n = 98) | |
| Ad hoc | 22 (27.5%) |
| Deferred | 58 (72.5%) |
|
ELCA, excimer laser coronary atherectomy; OCT, optical coherence tomography; IVUS, intravascular ultrasound; PCI, percutaneous coronary intervention. Data are expressed as no. (%), mean ± standard deviation or median [interquartile range]. |
|
Procedural outcomes
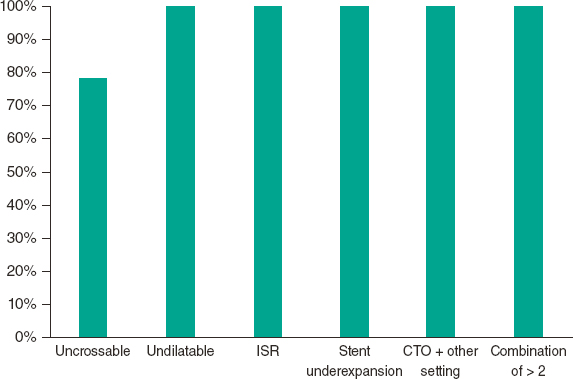
The ELCA success rate was 91.25%. The success rate was 78.1% in uncrossable lesions and 100% in the other anatomical settings (P < .001). The ELCA success rate in the different anatomical settings is shown in figure 4.
Figure 4. ELCA success rate in the different anatomical settings. ISR, in-stent restenosis, CTO, chronic total occlusion.
Among intracoronary imaging-guided procedures, the ELCA success rate was 98.3%, and dropped to 72.7% in non-coronary imaging-guided PCI (P < .001). Final stent expansion was analyzed with intracoronary imaging in 32 procedures. The median stent expansion was 80.3% [IQR, 68.2%-95.2%].
Despite ELCA success, adjuvant plaque modification therapies (other than noncompliant [NC] balloon inflation after ELCA) were used in 4 procedures, including rotational atherectomy (RA) in 2 procedures, lithotripsy in 1 procedure and scoring balloon in 1 procedure. The procedures in which ELCA allowed subsequent successful RA (RASER technique14) or successful lithotripsy (ELCA-tripsy technique15) were considered ELCA success.
In 7 procedures (8.75%), ELCA failed. In 2 of them, RA was successfully performed. In 1 procedure, intravascular lithotripsy was attempted, but failed. In 1 case, the procedure was prematurely interrupted at the request of the patient. In the remaining 2 patients, no bailout therapy was attempted, and they were managed conservatively. Cases in which ELCA did not facilitate the passage of RA or intravascular lithotripsy were not classified as RASER or ELCA-tripsy techniques. The overall technical success rate was 91.25%.
In-hospital and follow-up outcomes
ELCA-related complications occurred in 2 procedures (2.5%) due to coronary artery perforation after ELCA application, with immediate sealing after stent implantation (although pericardiocentesis was necessary in 2 of them). A third perforation was observed, not immediately after ELCA application, but after dilatation with NC balloons. In 2 of the perforations, the target lesion was a severely calcified and undilatable lesion located in the left anterior descending artery. The third perforation was observed in an uncrossable lesion at the right coronary artery. In all of them, the 0.9 mm catheter was used, and ELCA was applied with maximum fluency and repetition rate during saline infusion. Intracoronary imaging prior to ELCA application was not performed in any of these patients: the OCT catheter could not cross the lesion in 2 of them and crossing was not attempted in the third. After the application of coronary laser and stent implantation, OCT was performed in 2 of the procedures, which confirmed the good final result.
Other procedural complications not related to ELCA occurred in 4 patients. One patient developed a vascular access complication with retroperitoneal hemorrhage and severe bleeding requiring transfusion and transarterial embolization of a deep femoral artery branch, although his clinical course was favorable. One patient with severe aortic stenosis and impaired left ventricular function showed hemodynamic instability requiring support with inotropes and orotracheal intubation. In 1 patient, no-reflow phenomenon occurred after stent implantation but resolved after intracoronary adenosine infusion.
In the remaining patient, coronary dissection occurred during the guidewire advancement before ELCA application and was complicated with occlusive intracoronary hematoma, which resolved after emergent PCI with successful revascularization. No patient died during the procedure. Three patients died during admission despite successful revascularization due to cardiac causes not related to the procedure (mostly advanced heart failure) and 1 patient died from respiratory sepsis. There were no other in-hospital complications. Overall, the clinical success rate was 87.5%.
After a median follow-up of 15.5 months [IQR, 5.02-29.3], MACE occurred in 9 patients (11.25%). Target lesion revascularization occurred in 7 patients (8.9%), in all patients due to ISR. The median time to target lesion revascularization among patients with successful revascularization was 11.4 [IQR, 8.1-22.6] months. Cardiorespiratory arrest secondary to acute stent thrombosis occurred in 1 patient with successful revascularization, whose family reported poor antiplatelet therapy adherence. One patient died from advanced heart failure after 3 years of follow-up, despite successful revascularization. Three patients died from noncardiac causes.
The procedural outcomes, clinical outcomes, and major complications are summarized in table 3. No significant differences were observed in the results between male and female patients.
Table 3. Procedural and clinical outcomes
| Procedural and clinical success | n (%) |
|---|---|
| ELCA success | 73 (91.25%) |
| Balloon-uncrossable lesion | 25 (78.13%) |
| Balloon-undilatable lesion | 23 (100%) |
| In-stent restenosis | 2 (100%) |
| Stent underexpansion | 5 (100%) |
| Chronic total occlusion | 6 (100%) |
| Combination of > 2 of the above | 10 (100%) |
| Severe calcification as sole indication | 2 (100%) |
| Technical success | 73 (91.25%) |
| Clinical success | 70 (87.5%) |
| Procedural complications | |
| ELCA-related complications | |
| Coronary artery perforation | 2 (2.5%) |
| Complications not related to ELCA | |
| Vascular access complication with major bleeding | 1 (1.25%) |
| Coronary perforation | 1 (1.25%) |
| Flow-limiting dissection | 1 (1.25%) |
| Hemodynamic instability | 1 (1.25%) |
| No-reflow | 1 (1.25%) |
| Ventricular arrhythmia | 0 (0%) |
| In-hospital MACE | |
| Recurrent angina requiring TLR | 0 (0%) |
| Procedure-related myocardial infarction | 1 (1.25%) |
| New-onset heart failure | 0 (0%) |
| Stroke | 0 (0%) |
| Cardiovascular death | 3 (3.75%) |
| All-cause death | 4 (5.0%) |
| MACE after discharge | |
| TLR | 7 (8.75%) |
| MI due to stent thrombosis | 1 (1.25%) |
| Death from cardiovascular causes | 2 (2.5%) |
| Non-cardiovascular related death | 3 (3.75%) |
|
ELCA, excimer laser coronary atherectomy; MACE, major adverse cardiovascular events; MI, myocardial infarction; TLR, target lesion revascularization. |
|
DISCUSSION
The main findings of our study are as follows: a) ELCA was associated with a high rate of technical success in severely calcified coronary lesions, whether isolated or combined with other plaque modification techniques, with an acceptable ELCA-related complications rate. b) The success rate was higher in undilatable than in uncrossable lesions and was 100% in peri-stent lesions (stent underexpansion or ISR).
As described in previous series, calcified lesions are associated with higher rates of PCI failure, complications, morbidity, and mortality.2,16 Although ELCA is known to have no direct effect on calcium, calcified atheromatous plaques have a mixed composition, including lipids, collagen, and other protein fibers.1,17 The interaction of ELCA with these components, due to its photochemical, photothermal and photokinetic properties, modifies the plaque structure, thus facilitating angioplasty in lesions with severe calcification.17 Moreover, in some cases, as occurs in our series, ELCA is complementary to other plaque modification techniques, allowing the passage of the microcatheter to introduce specific atherectomy guidewires, or even to allow the passage of the lithotripsy balloon.14,15 The RASER technique was used in 2 patients and the ELCA-tripsy technique in another patient with technical success in all 3 of them.
There is a lack of contemporary specific series on the use of ELCA in lesions with severe calcification, and data available in the medical literature are contradictory. Bilodeau et al.18 reported high procedural (93%) and clinical (86%) success in a series of 95 patients with complex coronary lesions, of which 57 had significant calcification. The Laser Veterans Affairs (LAVA) Multicenter Registry7 evaluated the use of ELCA in 131 target complex coronary lesions, of which 62% were moderately or severely calcified lesions, globally reporting 90% technical and 88.8% procedural success rate, which is consistent with our results. In the LEONARDO study,19 in which 75% of lesions were calcified, high laser energy levels were shown to be safe and effective (success rate 93.7%). In our series, the highest fluence and frequency were required in 60% of the procedures, with a similar success rate.
Nowadays, the main indication of ELCA is treatment of uncrossable and undilatable lesions. In uncrossable lesions, the laser catheter can be advanced over any 0.014¨angioplasty guidewire that crosses the lesion, unlike other plaque modification techniques. In a multicenter US registry, the success rate for laser-assisted PCI in uncrossable balloon CTO was 95%, which was higher than that for RA (89%) in this setting.20 In a retrospective study by Karacsonyi et al.,21 laser use in balloon-uncrossable and balloon-undilatable CTO was associated with higher technical (91.5% vs 83.1%) and procedural (88.9% vs 81.6%) success rates compared with cases without the use of laser. Ojeda et al.9 conducted a multicenter registry of 126 uncrossable lesions and reported ELCA success of 81.8%. In that registry, severe calcification was independently associated with ELCA failure, a finding already described in a previous study.22 In our series (with severe calcification in 100% of patients), the overall ELCA success rate was 91.25%, but the ELCA success in uncrossable lesions was lower than in undilatable lesions (78.1% vs 100%) and similar to that in the series by Ojeda et al.9 The lower success rate in uncrossable and severely calcified lesions can probably be explained by the different plaque composition and calcium distribution. Furthermore, the higher rate of use of intracoronary imaging could also be associated with better results (72.5% in our series compared with 22.5% reported by Ojeda et al.9). Of note, an ELCA success of 78.1% in uncrossable lesions with severe calcification could be a reasonable result, considering that, if even a microcatheter cannot cross the lesion, ELCA may be the only alternative for revascularization.
In other scenarios, the ELCA success rate of our series was high and similar to that of other series. An ELCA success rate of 86% to 93% has been reported in CTOs.8,23 RA in CTO has been associated with similar success rates (89%-95.6%)24,25 but with a high rate of slow/no flow phenomena.24 In patients with stent underexpansion and ISR, ELCA is feasible and effective,26,27 with 100% ELCA success in our series.
Intravascular imaging is useful to guide calcified coronary stenosis PCI.28,29 Contemporary rates of intravascular imaging for complex PCI remain low.30 In our study, intracoronary imaging was used in 72 procedures (73.4%), and intracoronary imaging-guided procedures resulted in a higher success rate. Its lower use in uncrossable lesions can probably be explained by the fact that the intravascular ultrasound/OCT catheter cannot cross the lesion, rather than necessarily being the reason for the lower success rate in this setting.
Limitations
Our study has some limitations. First, it is an observational study with a small sample size. However, to the best of our knowledge, our study represents the largest series of ELCA specifically performed in severely calcified lesions in contemporary PCI. Second, the severity of lesion calcification was initially assessed by conventional coronary angiography, which has only low to moderate sensitivity compared with intravascular ultrasound or OCT. In addition, sometimes the calcium observed by conventional angiogra- phy is adventitious, thus not affecting balloon dilation or stent expansion with conventional techniques. However, the use of intracoronary imaging techniques was higher than in previous series and confirmed the severity of calcification in all patients. In addition, a significant number of cases consisted of uncrossable lesions, limiting the use of intracoronary imaging to define the calcification from the beginning of the procedure. Finally, the operators involved in this study were experienced ELCA operators. This may limit the generalizability of our results since ELCA is not available in most centers and requires a learning curve.
CONCLUSIONS
ELCA is a useful tool in severe calcification lesions, with a high success rate, especially in the setting of undilatable or peri-stent lesions. The technique is also reasonably safe, given that it is used in highly complex procedures. Future randomized studies will shed light on its role in the management of severe calcified coronary lesions.
FUNDING
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ETHICAL CONSIDERATIONS
All patients signed an informed consent form and approval was obtained from the ethics committee of the center. The study has taken into consideration sex and gender variables according to SAGER guidelines.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
No artificial intelligence tool has been used during the preparation of this work.
AUTHORS’ CONTRIBUTIONS
A. Jurado-Román conceived and designed the study. L. Cobarro and A. Jurado-Román performed the analysis and wrote the initial draft. L. Cobarro, A. Jurado-Román, D. Tébar-Márquez, S. Vera-Vera, A. García-Escobar, C. Ugueto, C. Contreras, B. Rivero, S. Jiménez-Valero, G. Galeote, and R. Moreno collected the data and reviewed the final version of the manuscript.
CONFLICTS OF INTEREST
R. Moreno is associate editor of REC: Interventional Cardiology; the editorial procedure established in the journal has been followed to ensure impartial handling of the manuscript.
A. Jurado-Román is proctor of Philips-Biomenco, Boston Scientific, CSI-World Medica and Medtronic Inc and has received speaker fees from Boston Scientific, Abbott Vascular, World Medica, Biotronik, Philips-Biomenco, and Inari. R. Moreno has received speaker fees from Medtronic Inc, Boston Scientific, Abbott vascular, Biosensors, Biotronik, Edwards Lifesciences, AMGEN, AstraZeneca, and Daiichi Sankyo New Vascular Therapies and Biosensors.
WHAT IS KNOWN ABOUT THE TOPIC?
- Excimer laser coronary atherectomy (ELCA) is a plaque modification technique that has proved to be useful in several scenarios, such as balloon failure (uncrossable or undilatable lesions), chronic total occlusions (CTO), stent underexpansion, in-stent restenosis (ISR) and thrombotic lesions.
- In recent years, incremental operator experience along with the standardization of laser technique has expanded its indications and decreased its complication rates.
- The effectiveness of ELCA in calcified lesions is controversial. On one hand, some ELCA series have described a relationship between severe calcification and laser failure. In contrast, moderate-to-severe calcification is found in more than 60% of cases in some ELCA series with a high success rate, indicating that this technique could be useful in this setting.
- Due to the lack of evidence in this specific scenario, our study aimed to assess the contemporary safety and efficacy of ELCA in severely calcified coronary lesions.
WHAT DOES THIS STUDY ADD?
- ELCA is associated with a high rate of technical success in severely calcified coronary lesions, whether isolated or combined with other plaque modification techniques, with an acceptable ELCA-related complications rate.
- The success rate is higher in undilatable than in uncrossable lesions and was 100% in peri-stent lesions (stent underexpansion or restenosis). However, in uncrossable lesions, ELCA may be the only alternative for percutaneous revascularization.
- Clinical results after a median follow-up of 15.5 months were favorable, taking into account the complexity of this scenario.
REFERENCES
1. Mori H, Torii S, Kutyna M, Sakamoto A, Finn AV, Virmani R. Coronary Artery Calcification and its Progression:What Does it Really Mean?JACC Cardiovasc Imaging. 2018;11:127-142.
2. Huisman J, van der Heijden LC, Kok MM, et al. Impact of severe lesion calcification on clinical outcome of patients with stable angina, treated with newer generation permanent polymer-coated drug-eluting stents:A patient-level pooled analysis from TWENTE and DUTCH PEERS (TWENTE II). Am Heart J. 2016;175:121-129.
3. Jawad-Ul-Qamar M, Sharma H, Vetrugno V, et al. Contemporary use of excimer laser in percutaneous coronary intervention with indications, procedural characteristics, complications and outcomes in a university teaching hospital. Open Heart. 2021;8:e001522.
4. Sintek M, Coverstone E, Bach R, et al. Excimer Laser Coronary Angioplasty in Coronary Lesions:Use and Safety From the NCDR/CATH PCI Registry. Circ Cardiovasc Interv. 2021;14:e010061.
5. Stone GW, de Marchena E, Dageforde D, et al. Prospective, Randomized, Multicenter Comparison of Laser-Facilitated Balloon Angioplasty Versus Stand-Alone Balloon Angioplasty in Patients With Obstructive Coronary Artery Disease fn1fn1Funding for this study was provided in part by Eclipse Surgical Technologies, Inc., Sunnyvale, California. J Am Coll Cardiol. 1997;30:1714-1721.
6. Ocaranza-Sánchez R, Abellás-Sequeiros RA, Galvão-Braga C, Trillo-Nouche R, González-Juanatey JR. Uso de aterectomía coronaria con LASER Excimer como terapia coadyuvante en intervencionismo coronario percutáneo. Rev Esp Cardiol.2016;69:867-868.
7. Karacsonyi J, Armstrong EJ, Truong HTD, et al. Contemporary Use of Laser During Percutaneous Coronary Interventions:Insights from the Laser Veterans Affairs (LAVA) Multicenter Registry. J Invasive Cardiol. 2018;30:195-201.
8. Mohandes M, Rojas S, Moreno C, Fernández F, Fuertes M, Guarinos J. Excimer Laser in Percutaneous Coronary Intervention of Device Uncrossable Chronic Total and Functional Occlusions. Cardiovasc Revasc Med. 2020;21:657-660.
9. Ojeda S, Azzalini L, Suárez de Lezo J, et al. Excimer laser coronary atherectomy for uncrossable coronary lesions. A multicenter registry. Catheter Cardiovasc Interv. 2021;98:1241-1249.
10. Jurado-Román A, Gómez-Menchero A, Gonzalo N, et al. Plaque modification techniques to treat calcified coronary lesions. Position paper from the ACI-SEC. REC Interv Cardiol. 2023;5:46-61
11. Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87-165.
12. Mintz GS, Popma JJ, Pichard AD, et al. Patterns of Calcification in Coronary Artery Disease. Circulation. 1995;91:1959-1965.
13. Thygesen K. 'Ten Commandments'for the Fourth Universal Definition of Myocardial Infarction 2018. Eur Heart J. 2019;40:226-226.
14. Egred M. RASER angioplasty. Catheter Cardiovasc Interv. 2012;79:1009-1012.
15. Jurado-Román A, García A, Moreno R. ELCA-Tripsy:Combination of Laser and Lithotripsy for Severely Calcified Lesions. J Invasive Cardiol.2021;33:E754-E755.
16. Copeland-Halperin RS, Baber U, Aquino M, et al. Prevalence, correlates, and impact of coronary calcification on adverse events following PCI with newer-generation DES:Findings from a large multiethnic registry. Catheter Cardiovasc Interv. 2018;91:859-866.
17. Tsutsui RS, Sammour Y, Kalra A, et al. Excimer Laser Atherectomy in Percutaneous Coronary Intervention:A Contemporary Review. Cardiovasc Revasc Med. 2021;25:75-85.
18. Bilodeau L, Fretz EB, Taeymans Y, Koolen J, Taylor K, Hilton DJ. Novel use of a high-energy excimer laser catheter for calcified and complex coronary artery lesions. Catheter Cardiovasc Interv. 2004;62:155-161.
19. Ambrosini V, Sorropago G, Laurenzano E, et al. Early outcome of high energy Laser (Excimer) facilitated coronary angioplasty ON hARD and complex calcified and balloOn-resistant coronary lesions:LEONARDO Study. Cardiovasc Revasc Med. 2015;16:141-146.
20. Karacsonyi J, Karmpaliotis D, Alaswad K, et al. Prevalence, indications and management of balloon uncrossable chronic total occlusions:Insights from a contemporary multicenter US registry. Catheter Cardiovasc Interv. 2017;90:12-20.
21. Karacsonyi J, Alaswad K, Choi JW, et al. Laser for balloon uncrossable and undilatable chronic total occlusion interventions. Int J Cardiol. 2021;336:33-37.
22. Bittl JA. Clinical results with excimer laser coronary angioplasty. Semin Interv Cardiol. 1996;1:129-134.
23. Rawlins J, Din JN, Suneel T, O´Kane P. Coronary Intervention with the Excimer Laser:Review of the Technology and Outcome Data. Interv Cardiol. 2016;1:27-32.
24. Azzalini L, Dautov R, Ojeda S, et al. Long-term outcomes of rotational atherectomy for the percutaneous treatment of chronic total occlusions. Catheter Cardiovasc Interv. 2017;89:820-828.
25. Pagnotta P, Briguori C, Mango R, et al. Rotational atherectomy in resistant chronic total occlusions. Catheter Cardiovasc Interv. 2010;76:366-371.
26. Latib A, Takagi K, Chizzola G, et al. Excimer Laser LEsion Modification to Expand Non-dilatable sTents:The ELLEMENT Registry. Cardiovasc Revasc Med. 2014;15:8-12.
27. Lee C, Shlofmitz R, Song L, et al. The effectiveness of excimer laser angioplasty to treat coronary in-stent restenosis with peri-stent calcium as assessed by optical coherence tomography. J Am Coll Cardiol.2017;69:1086-1086.
28. Fujino A, Mintz GS, Matsumura M, et al. A new optical coherence tomography-based calcium scoring system to predict stent underexpansion. EuroIntervention. 2018;13:e2182-e2189.
29. Zhang M, Matsumura M, Usui E, et al. Intravascular Ultrasound–Derived Calcium Score to Predict Stent Expansion in Severely Calcified Lesions. Circ Cardiovasc Interv. 2021;14:e010296.
30. Hannan EL, Zhong Y, Reddy P, et al. Percutaneous Coronary Intervention With and Without Intravascular Ultrasound for Patients With Complex Lesions:Utilization, Mortality, and Target Vessel Revascularization. Circ Cardiovasc Interv. 2022;15:e011687.