INTRODUCTION
Transcatheter aortic valve implantation (TAVI) is a well-established procedure for severe symptomatic aortic stenosis. Previously, this procedure was used exclusively to treat inoperable and high-risk patients but is now a common approach for intermediate and low-risk populations. In parallel, there has been growing global experience in several off-label scenarios, as in bicuspid, valve-in-valve, and noncalcified aortic regurgitation (NCAR).1
AORTIC REGURGITATION
Due to abnormalities in the aortic leaflets or their supporting structures (ie, aortic root and annulus), or both, aortic regurgitation (AR) causes diastolic reflux of blood from the aorta into the left ventricle (LV). This leads to LV volume overload and dilatation, allowing the ejection of a larger stroke volume. However, over time, it results in a decline in systolic function causing symptoms conferring a poor prognosis.2 Current European and American guidelines recommend surgical intervention when significant AR is accompanied by symptoms, reduced LV ejection fraction, or severe LV dilatation.3-4 Although moderate or severe AR affects around 2.2% of the population aged 70 years or older, up to 30% of these individuals are deemed inoperable due to advanced age or comorbidities, with percutaneous options—including dedicated and nondedicated devices—gaining increased interest due to the absence of safe surgical alternatives.5
CHALLENGES OF TAVI IN NCAR
Specific challenges for transcatheter devices are due to the characteristics of patients with NCAR, including larger aortic annular dimensions, aortic root dilatation, insufficient annular calcification for anchoring, and larger stroke volume with backflow to the LV causing a “suction effect”. All of these factors create difficulties in selecting the appropriate device and positioning and deploying it correctly, and consequently increase the risk of embolization or malpositioning of the prosthesis, requiring a second valve implantation. Ultimately, these challenges are associated with increased mortality. In particular, the primary concern is valve embolization. To reduce the risk of this complication, prosthesis oversizing is routinely performed, but the exact degree of oversizing with each device has not been standardized. Furthermore, oversizing is associated with a high rate of conduction system disturbances and may carry a higher risk of annular rupture.
DEVICES AND EVIDENCE
There are 2 dedicated-devices for NCAR: The Trilogy system (JenaValve Technology Inc, California, United States) and the J-Valve (JC Medical Inc, California, United States). The most recent experience with these dedicated devices has been reported by Adam et al.6 and García et al.7 The Trilogy system was associated with a 30-day mortality rate of 1.7% (vs 3.7% with J-Valve) and both had 0% ≥ moderate residual AR. However, 7.4% required conversion to surgery with the J-Valve, leading to some technical changes in the technology. Of note, the pacemaker rate with both systems was above 10% (19.6% and 13%, respectively). These promising dedicated technologies still have certain shortcomings, the main one being the lack of sizes covering large annuli; indeed, for 10% oversizing, the proportion of AR patients whose aortic structures were above the recommended indications was up to ~50%.8
The first-in-human compassionate use of SAPIEN (Edwards Lifesciences, California, United States) was reported in 2012,9 followed by multiple case series and registries reporting the feasibility of TAVI in NCAR with new-generation nondedicated devices. The most recent summary of the experience was reported in a meta-analysis by Takagi et al.,10 and the pooled analysis at 30 days demonstrated 80.4% device success, 9.5% all-cause mortality, 7.4% ≥ moderate residual AR, and a pacemaker rate of 11.6%. Although these results were promising and significantly better than with early-generation devices in all aspects, there was still a substantial gap to achieve similar outcomes to TAVI in aortic stenosis and, indeed, the rate of valve embolization was high (above 9% with all the technologies, even the dedicated technologies).10
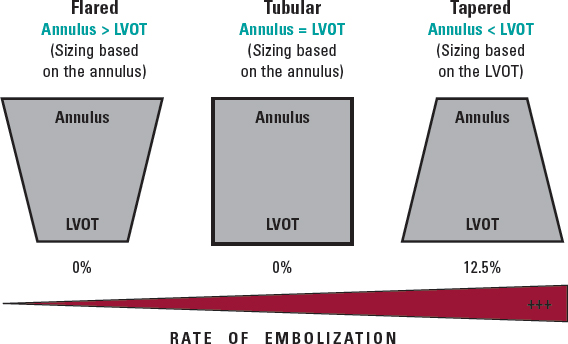
More recently, the new Myval balloon-expandable valve (Meril Lifesciences Ltd, Vapi, India) has demonstrated better outcomes than those reported by Takagi et al. likely due to the availability of extra-large sizes (30.5 and 32 mm), allowing a greater degree of oversizing and covering annuli up to 100.5 mm perimeter and 840 mm2 area at its nominal volume, increasing the proportion of inoperable patients who can be treated percutaneously. Because of the high procedural success rate (94.7%), and the absence of severe anatomical complications (ie, annular rupture, aortic dissection, or coronary obstruction), the Myval balloon-expandable valve is a promising new approach to this condition until dedicated devices for these annular sizes become available. Nevertheless, prosthesis embolization occurs in 3.5% of patients, leaving room for improvement; according to this research, the interplay of the aortic annulus and the LV outflow tract could help to predict the risk of valve embolization, suggesting that, in borderline annular sizes, when 20% oversizing cannot be achieved and adverse (tapered) morphology is detected, the intervention should be avoided or might be carried out under mechanical circulatory support (figure 1).11
Figure 1. Anatomical predictor of valve embolization during transcatheter aortic valve implantation for noncalcified aortic regurgitation. LVOT, left ventricular outflow tract.
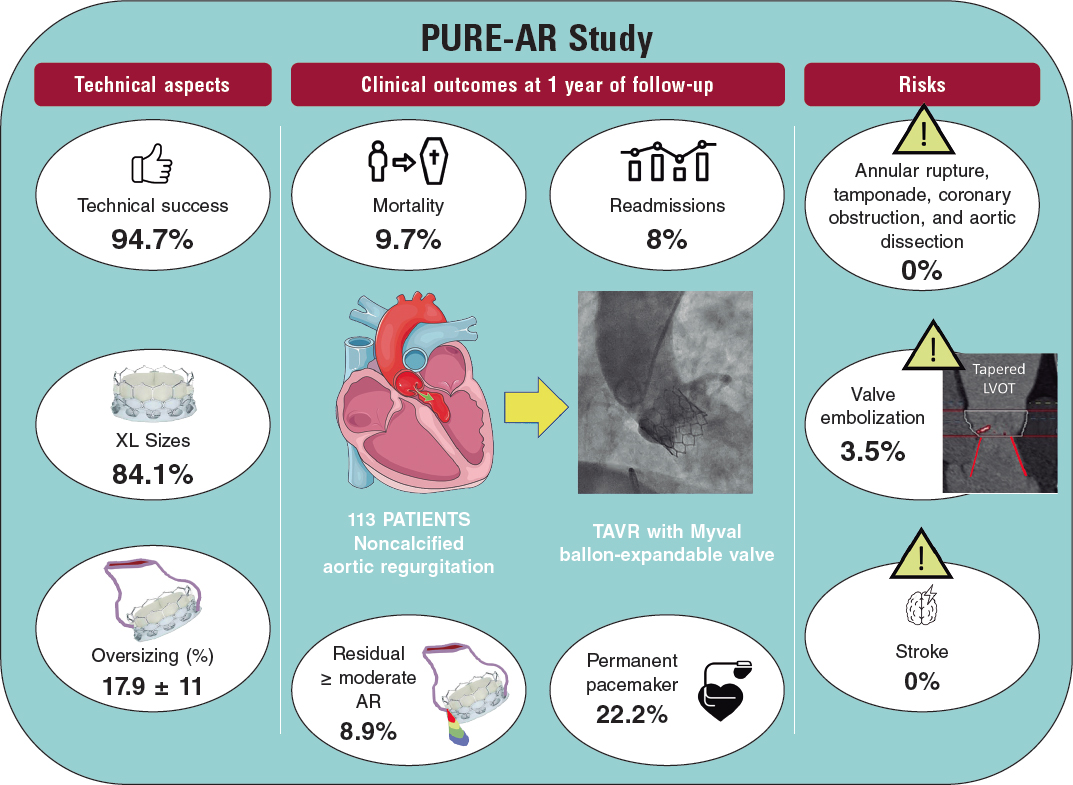
Poletti et al.12 recently compared latest-iteration nondedicated devices and suggested that Myval might provide the best outcomes compared with other devices; indeed, the subanalysis (not published yet) comparing the 2 balloon-expandable platforms suggested that, despite being used in patients with significantly smaller aortic annuli, SAPIEN-3 had a lower device success rate (72% vs 90%) and a higher rate of prosthesis migration/embolization (SAPIEN 17% vs MyVal 5%).12 A summary of the PURE-AR study11 with the Myval device is shown in figure 2. The current evidence on different devices is summarized in table 1.
Figure 2. Main outcomes reported in the Myval registry for treatment of noncalcified aortic annuli with large annuli. AR, aortic regurgitation; LVOT, left ventricular outflow tract; TAVR, transcatheter aortic valve regurgitation.
Table 1. Comparison of outcomes between the international registries
| Registry | No. of patients | Type of device | Device success | All-cause mortality | ≥ Moderate residual aortic regurgitation | Permanent pacemaker implantation rate |
|---|---|---|---|---|---|---|
| Yoon et al.13 (2017) | 331 patients with NCAR and FSHV | Nondedicated devices | Overall: 74.3% | Overall: 10.9% | Overall: 9.6% | Overall: 18.2% |
| Early-generation devices (CoreValve, SAPIEN XT) | Early-generation devices: 61.3% | Early-generation devices: 13.4% | Early-generation devices: 18.8% | Early-generation devices: 17.5% | ||
| New-generation devices (Evolut R, JenaValve, Engager, Portico, ACURATE, Lotus, Direct Flow, SAPIEN 3) | New-generation devices: 81.1% | New-generation devices: 9.4% | New-generation devices: 4.2% | New-generation devices: 18.6% | ||
| De Backer et al.14 (2018) | 254 patients with NCAR | Nondedicated devices Early-generation devices (CoreValve, SAPIEN XT) | Early-generation devices: 47% | Early-generation devices: 17% | Early-generation THV’s: 26% | Not reported |
| New-generation devices (Evolut R, JenaValve, Engager, Portico, ACURATE, Lotus, Direct Flow, SAPIEN 3) | Newer-generation devices: 82% | Newer-generation devices: 8% | Newer-generation THV’s: 5% | |||
| Sawaya et al.15 (2018) | 146 patients with NCAR and failing surgical heart valves (FSHV) | Early-generation devices (CoreValve SAPIEN XT) | NCAR: 72% | NCAR: 13% | NCAR: 13% | NCAR: 18% |
| New-generation devices (Evolut R, JenaValve, Lotus, Direct Flow, SAPIEN 3) | FSHV: 71% | FSHV: 6% | FSHV: 6% | FSHV: 5% | ||
| Early-generation devices: 54% | Early-generation devices: 22% | Early-generation devices: 27% | ||||
| New-generation devices: 85% | New-generation devices: 8% | New-generation devices: 3% | ||||
| Sánchez-Luna et al.11 2023 | 113 patients with NCAR | New-generation nondedicated device: Myval | 94.7% | 9.7% | 8.9% | 22.2% |
| Poletti et al.12 2023 | 201 patients with NCAR | New-generation devices: SEV (Evolut R/Pro, ACURATE Neo/Neo2, Jena Valve, Navitor/Portico) | Overall: 76.1% | Overall: 5% | Overall: 9.5% | Overall: 22.3% |
| SEV: 75.8% | SEV: 5.3% | SEV: 9.2% | SEV: 22.6% | |||
| BEV (SAPIEN, Myval) | BEV: 76.8% | BEV: 4.4% | BEV: 10.1% | BEV: 21.8% | ||
| Adam et al.6 (2023) | 58 patients with NCAR | Dedicated device: Trilogy system (JenaValve) | 98% | 1.7% | 0% | 19.6% |
| Garcia S et al.7 (2023) | 27 patients with NCAR | Dedicated device: J-Valve | 81% | 3.7% | 0% | 13% |
|
BEB, balloon-expandable valve; FSHV, failing surgical heart valve; NCAR, noncalcified aortic regurgitation; SEV, self-expandable valve; THV, transcatheter heart valve. Medtronic, United States: CoreValve, Engager, and Evolut R. Edwards Lifesciences, United States: SAPIEN XT, SAPIEN 3. Abbott Vascular, United States: Navitor/Portico. Meril Life, India: Myval. Boston Scientific, United States: ACURATE Neo/Neo2, Lotus. Trilogy, Germany: JenaValve. Direct Flow Medical, United States: Direct Flow. JC Medical, United States: J-Valve. |
||||||
In conclusion, although the surgical approach remains the standard-of-care in NCAR patients, the outcomes of new-generation dedicated and nondedicated TAVI devices are rapidly improving. The main caveats include the lack of large-sized dedicated devices and the risk of valve embolization with nondedicated devices, although this risk can be minimized by extra-large sizes and morphological analysis of the LV outflow tract. The rate of conduction disturbances is still higher than for TAVI in aortic stenosis and its decrease will require specific technology and strategies, given its potentially negative impact in LV remodelling. Clinical trials comparing surgery and TAVI in this setting for high-risk patients are an urgent need.
FUNDING
None.
AUTHORS’ CONTRIBUTIONS
I.J. Amat-Santos and J.P. Sánchez-Luna equally contributed in the design, data gathering, analysis, and final approval of the manuscript.
CONFLICTS OF INTEREST
I.J. Amat-Santos is proctor for Boston Scientific, Medtronic, and Meril Life. There are no other potential conflicts of interest related to this work.
REFERENCES
1. Santos-Martínez S, Amat-Santos IJ. New Challenging Scenarios in Transcatheter Aortic Valve Implantation: Valve-in-valve, Bicuspid and Native Aortic Regurgitation. Eur Cardiol. 2021;16:e29.
2. Maurer G. Aortic regurgitation. Heart. 2006;92:994-1000.
3. Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. EuroIntervention. 2022;17:e1126-e1196.
4. Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143:e35-e71.
5. Singh JP, Evans JC, Levy D, et al. Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham Heart Study). Am J Cardiol. 1999;83:897-902.
6. Adam M, Tamm AR, Wienemann H, et al. Transcatheter Aortic Valve Replacement for Isolated Aortic Regurgitation Using a New Self-Expanding TAVR System. JACC Cardiovasc Interv. 2023;16:1965-1973.
7. Garcia S, Ye J, Webb J, et al. Transcatheter treatment of native aortic valve regurgitation: The North American Experience with a Novel Device. JACC Cardiovasc Interv. 2023;16:1953-1960.
8. Chen Y, Zhao J, Liu Q, et al. Computed tomography anatomical characteristics based on transcatheter aortic valve replacement in aortic regurgitation. Int J Cardiovasc Imaging. 2023;39:2063–2071.
9. D'Ancona G, Pasic M, Buz, S, et al. TAVI for pure aortic valve insufficiency in a patient with a left ventricular assist device. Ann Thorac Surg. 2012;93:e89-e91.
10. Takagi H, Hari Y, Kawai N, et al. Meta-analysis and meta-regression of transcatheter aortic valve implantation for pure native aortic regurgitation. Heart Lung Circ. 2020;29:729-741.
11. Sánchez-Luna JP, Martin P, Dager AE, et al. Clinical outcomes of TAVI with the Myval balloon-expandable valve for non-calcified aortic regurgitation. EuroIntervention. 2023;19:580-588.
12. Poletti E, De Backer O, Scotti A, et al. Transcatheter Aortic Valve Replacement for Pure Native Aortic Valve Regurgitation: The PANTHEON International Project. JACC Cardiovasc Interv. 2023;16:1974-1985
13. Yoon SH, Schmidt T, Bleiziffer S, et al. Transcatheter Aortic Valve Replacement in Pure Native Aortic Valve Regurgitation. J Am Coll Cardiol. 2017;70:2752-2763.
14. De Backer O, Pilgrim T, Simonato M, et al. Usefulness of Transcatheter Aortic Valve Implantation for Treatment of Pure Native Aortic Valve Regurgitation. Am J Cardiol. 2018;122:1028-1035.
15. Sawaya FJ, Deutsch MA, Seiffert M, et al. Safety and Efficacy of Transcatheter Aortic Valve Replacement in the Treatment of Pure Aortic Regurgitation in Native Valves and Failing Surgical Bioprostheses: Results From an International Registry Study. JACC Cardiovasc Interv. 2017;10:1048-1056.