To the Editor:
Transcatheter aortic valve implantation (TAVI) is a well-established technique for the treatment of severe symptomatic aortic stenosis.
Frailty is a multidimensional clinical syndrome that reflects a patient’s vulnerability to adverse events. Its evaluation is complex, with multiple scales available: the Fried scale, which includes parameters such as weight loss or grip strength; the Short Physical Performance Battery (SPPB), which evaluates balance, gait speed, and the ability to rise from a chair; and others such as the Essential Frailty Toolset, the Clinical Frailty Scale, etc.1 In addition to classical features such as age and comorbidity, it is essential to assess frailty before deciding on valve intervention, as it correlates with prognosis after TAVI.2
In patients with mild or severe frailty, the decision-making process regarding the procedure is clearer. However, patients with moderate frailty—defined as the presence of 1 or 2 frailty criteria—represent an intermediate group between robustness and advanced frailty. These patients have a moderate risk of falls, disability, hospitalization, and death, and are more likely to progress to severe frailty in the coming years.3
We evaluated the characteristics and prognosis of patients with moderate frailty treated with TAVI. For this purpose, we conducted a single-center retrospective study of patients with severe symptomatic aortic stenosis and moderate frailty who underwent TAVI between 2016 and 2023. The outcomes analyzed were overall mortality, all-cause readmissions, and heart failure (HF) decompensation. The study was approved by our center ethics committee (No. 2019/8735/I).
The geriatrics department performs a comprehensive evaluation, classifying frailty as mild, moderate, or severe. To assess frailty in patients with severe aortic stenosis who are eligible for TAVI, we used the SPPB scale4 due to its high ability to discriminate between robust and frail patients in a rapid and objective manner, allowing identification of irreversible frailty. The SPPB provides greater precision than other scales in predicting adverse events and readmissions, and it is simple to apply in clinical practice. Moderate frailty was defined as a score < 10 on the SPPB, along with dependence for instrumental activities and mild dependence for basic activities of daily living, mild-to-moderate cognitive impairment, and risk of malnutrition.
Of the 306 patients evaluated, those with severe frailty, major comorbidity, or who declined the procedure were excluded and received conservative treatment. A total of 236 patients underwent TAVI, 54 of whom exhibited moderate frailty and constituted the study cohort.
Clinical, functional, and laboratory data were collected at the time of the procedure. Variables were analyzed using the chi-square or Fisher’s exact tests for categorical variables, and the Student’s t test or nonparametric tests for continuous variables. The Kaplan-Meier method was used to analyze the association between previous HF and events of mortality, HF decompensation, and hospital readmission. Survival curves were compared using the log-rank test. Previous HF was analyzed as a potential independent prognostic factor using a Cox regression model, adjusted for clinically relevant variables: diabetes mellitus, left ventricular ejection fraction prior to TAVI, chronic kidney disease, and ischemic heart disease.
The patients’ mean age was 83.4 ± 4 years, with a predominance of women (81.5%) and a mean Barthel index of 89. The most common comorbidities were hypertension (94.5%), chronic kidney disease stage ≥ 3 (50%), diabetes mellitus (22.2%), atrial fibrillation (44.4%), and ischemic heart disease (31.5%). The mean left ventricular ejection fraction was 60 ± 10%. A total of 59.3% had a prior diagnosis of congestive HF requiring IV diuretic therapy.
Regarding the clinical presentation that led to the indication for TAVI, 48.2% exhibited congestive HF; 40.7%, dyspnea without signs of congestion; 7.4%, angina pectoris; and 3.7%, syncope.
During a mean follow-up of 25.5 months, 26 deaths (48.1%) were reported, with cardiovascular causes being the most frequent (51.9%). The median time to death was 24 months [10.5–41.5]. A total of 57.4% of patients were readmitted, most commonly for infection (24.1%); 42.6% presented with HF decompensation and required IV diuretics.
In the analysis of variables, only prior HF was significantly associated with higher overall mortality, all-cause readmissions, and higher risk of HF decompensation during follow-up (table 1). The Kaplan-Meier curve showed a higher proportion of decompensations in patients with previous HF during follow-up (figure 1). Statistical significance was not reached for mortality or readmission. In multivariate analysis, prior HF was the only variable significantly associated with HF decompensation (adjusted hazard ratio, 5.4; 95%CI, 1.2-24.1; P < .026).
Table 1. Clinical characteristics associated with events after TAVI in patients with moderate frailty (n = 54)
| Variable | Composite endpoint* (n = 39) | No composite endpoint (n = 15) | P |
|---|---|---|---|
| Female sex, n (%) | 32 (82.1) | 12 (80) | 1 |
| Cardiovascular comorbidity, n (%) | |||
| Hypertension | 37 (94.9) | 14 (93.3) | 1 |
| Diabetes mellitus | 11 (28.2) | 1 (6.7) | .145 |
| Dyslipidemia | 19 (48.7) | 11 (73.3) | .133 |
| Ischemic heart disease | 12 (70.6) | 6 (29.4) | 1 |
| Atrial fibrillation | 19 (48.7) | 5 (33.3) | .28 |
| Previous heart failure | 28 (71.8) | 4 (26.7) | .003 |
| Previous valvuloplasty | 9 (23.1) | 6 (40) | .309 |
| Other comorbidity, n (%) | |||
| COPD | 8 (20.5) | 3 (20) | 1 |
| OSAHS | 4 (10.3) | 1 (6.7) | 1 |
| CKD (eGFR < 60) | 19 (48.7) | 8 (53.3) | .761 |
| Stroke | 6 (15.4) | 2 (13.3) | 1 |
| Peripheral vascular disease | 4 (10.3) | 1 (6.7) | 1 |
| Neoplasm | 3 (7.7) | 3 (20) | .331 |
| Functional and cognitive aspects, n (%) | |||
| Lives alone | 14 (35.9) | 2 (13.3) | .182 |
| Cognitive impairment | 5 (12.8) | 6 (40) | .054 |
| TAVI, n (%) | |||
| Intraoperative complications | 21 (53.8) | 6 (40) | .362 |
| Post-TAVI pacemaker implantation | 7 (17.9) | 2 (13.3) | 1 |
|
CKD, chronic kidney disease (estimated glomerular filtration rate < 60 mL/min/1.73 m2); COPD, chronic obstructive pulmonary disease; OSAHS, obstructive sleep apnea-hypopnea syndrome; TAVI: transcatheter aortic valve implantation. * Composite endpoint: hospital admission, heart failure decompensation, or death. |
|||
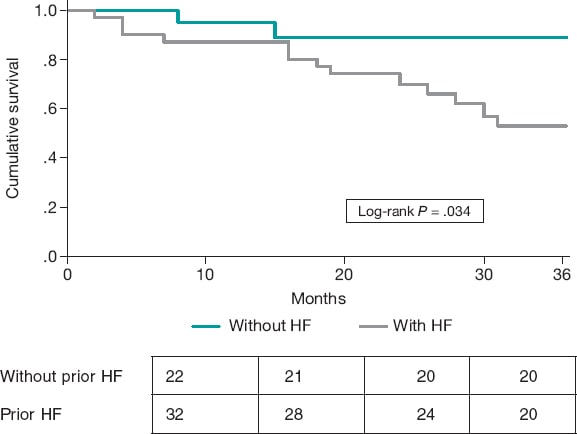
Figure 1. Kaplan-Meier curve for the event of heart failure (HF) decompensation during follow-up based on the presence of prior HF.
HF is associated with higher mortality and readmission rates after TAVI.5 The risk scores proposed by the European Society of Cardiology clinical practice guidelines for pre-TAVI assessment (FRANCE-2 Risk Score and PARTNER Risk Score) do not include previous HF as a risk factor, although FRANCE-2 considers New York Heart Association functional class IV as a futility factor after TAVI. Even the CAPRI risk score,6 which predicts the risk of HF after TAVI based on comorbidities and echocardiographic parameters, does not take it into account.
In the group of patients with moderate frailty, in whom clinical decision-making is more complex, identifying the factors that predict unfavorable outcomes after TAVI is of paramount importance. However, the available evidence on this issue is limited. Our results are consistent with former studies, showing that a history of congestive HF is significantly associated with a higher risk of HF readmission during follow-up after TAVI.
Identification of this high-risk subgroup for new decompensations would allow individualized follow-up and optimization of postoperative management to prevent adverse events and improve prognosis. Larger prospective studies are needed to confirm these findings and strengthen the available evidence. Despite these limitations, our study underscores the importance of prior congestive HF as a key predictor of HF development after TAVI in frail patients.
FUNDING
None declared.
ETHICAL CONSIDERATIONS
This study is in full compliance with relevant ethical considerations and was reviewed and approved by Hospital del Mar Ethics Committee (Barcelona, Spain) in December 2019 (Research Project 2019/8735/I). We confirm that informed consent for publication of patient cases was obtained and is archived. Similarly, we confirm that the SAGER guidelines were appropriately followed. Sex/gender of participants was considered as a potentially relevant variable, and this information was reported transparently. Differences according to sex/gender were also analyzed and reported when pertinent.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
No artificial intelligence tools were used in the preparation of this text.
AUTHOR’S CONTRIBUTIONS
C. Belmonte Herrera: data collection and management, manuscript drafting. D.M. Rojas Aguirre: data collection and management. L.C. Belarte Tornero: statistical analysis. S. Ruiz Bustillo: validation and review. B. Vaquerizo Montilla: validation and review. S. Valdivielso Moré: study design, validation and review.
CONFLICTS OF INTEREST
None declared.
ACKNOWLEDGMENTS
To the entire cardiology team of Hospital del Mar, especially the heart failure unit of the cardiology department.
REFERENCES
1. Chung KJNC, Wilkinson C, Veerasamy M, Kunadian V. Frailty Scores and Their Utility in Older Patients with Cardiovascular Disease. Interv Cardiol. 2021;16:e05.
2. Li Z, Dawson E, Moodie J, et al. Measurement and prognosis of frail patients undergoing transcatheter aortic valve implantation:a systematic review and meta-analysis. BMJ Open. 2021;11:e040459.
3. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults:evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146-56.
4. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function:association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85-M94.
5. Nilsson K, Buccheri S, Christersson C, et al. Causes, pattern, predictors, and prognostic implications of new hospitalizations after transcatheter aortic valve implantation:a long-term nationwide observational study. Eur Heart J Qual Care Clin Outcomes. 2022;8:150-160.
6. Harbaoui B, Durand E, DupréM, et al. Significance of the CAPRI risk score to predict heart failure hospitalization post-TAVI:The CAPRI-HF study. Int J Cardiol. 2019;296:98-102.










