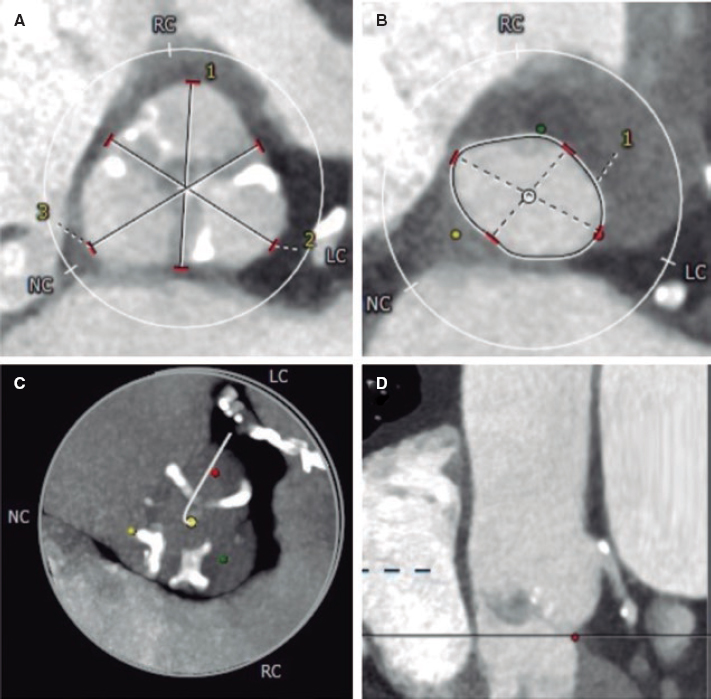
A 78-year-old man with a past medical history of hypertension, pulmonary thromboembolism, atrial fibrillation, and prostate cancer presented with dyspnea. The patient was diagnosed with severe aortic stenosis (mean gradient, 49 mmHg; area of 0.7 cm2), and severe ventricular hypertrophy. Heart function was preserved. The heart team decided to perform transcatheter aortic valve implantation (TAVI). Computed tomography revealed the presence of a scarcely calcified annulus with greater calcium distribution at the level of leaflet commissures and a 73.5-mm perimeter (figure 1).
Figure 1.
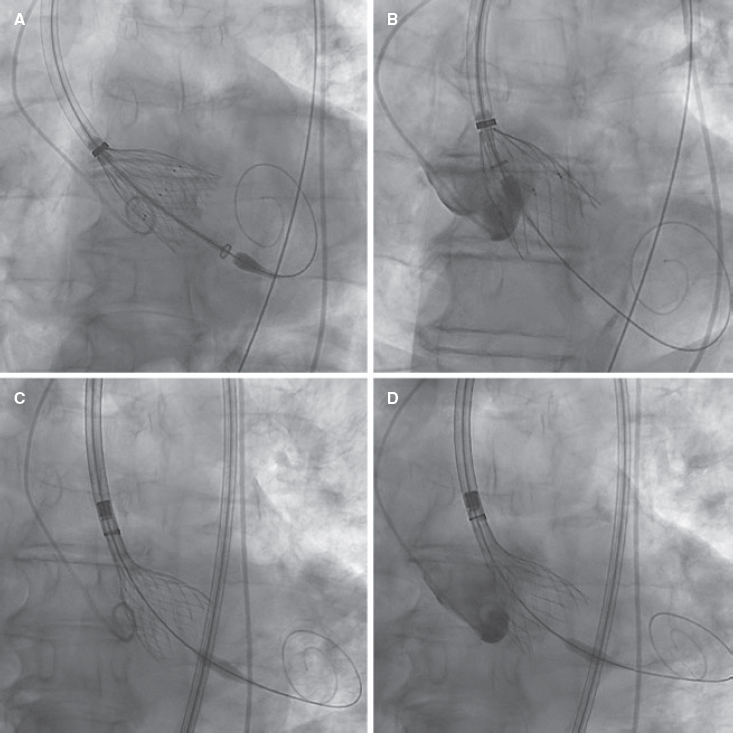
We decided to implant a 23-mm self-expandable, supra-annular, fully recapturable, and nonrepositionable ALLEGRA valve (New-Valve-Technology, Switzerland). The valve was predilated using a 20-mm balloon but showed pop-up and distal migration towards the outflow tract despite pacing (figure 2A,B). The same complication occurred with a 27-mm ALLEGRA device. We then attempted to use a 29-mm CoreValve Evolut PRO+ valve (Medtronic, United States), because this device is fully repositionable, but the same complication recurred even with pacing (figure 2C,D).
Figure 2.
Due to severe aortic regurgitation after the failed implantations, the patient became unstable (video 1 of the supplementary data). Considering the calcium distribution, we used the repositionable but not recapturable ACCURATE neo2 L valve (Boston Scientific, United States), which is equipped with stabilizing arches on the upper crown for the ascending aorta. The valve was then released and implanted under pacing, with an excellent final result. The patient’s condition improved (video 1 of the supplementary data) and he was discharged uneventfully 5 days later, requiring a permanent pacemaker.
This case illustrates the advantages of familiarity with various valves and their distinct implantation mechanisms to achieve the necessary stability for a proper anatomical match. We believe that proficiency in assembling and using multiple valves is key to addressing technically challenging and clinically complex situations.
We obtained the patient’s written informed consent for publication.
FUNDING
None.
ETHICAL CONSIDERATIONS
We obtained the patient’s written informed consent for publication. As this is a unique case, the variables of sex and gender of SAGER guidelines do not apply.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
No type of artificial intelligence or related technology was used in the writing of this article.
AUTHORS’ CONTRIBUTIONS
S. López Tejero and P. Antúnez Muiños drafted the manuscript. A. Diego-Nieto and G. Barreira-de Sousa reviewed the medical literature available and revised the manuscript. I. Cruz-González and J. Martín-Moreiras conceived the study design, analyzed its topic specifically, revised the manuscript, and were its main supervisors.
CONFLICTS OF INTEREST
I. Cruz-González is a proctor for Medtronic, Boston-Scientific, and New Valve Technology. The remaining authors report no conflicts of interest.
SUPPLEMENTARY DATA
Vídeo 1. López-Tejero S. DOI: 10.24875/RECICE.M23000398