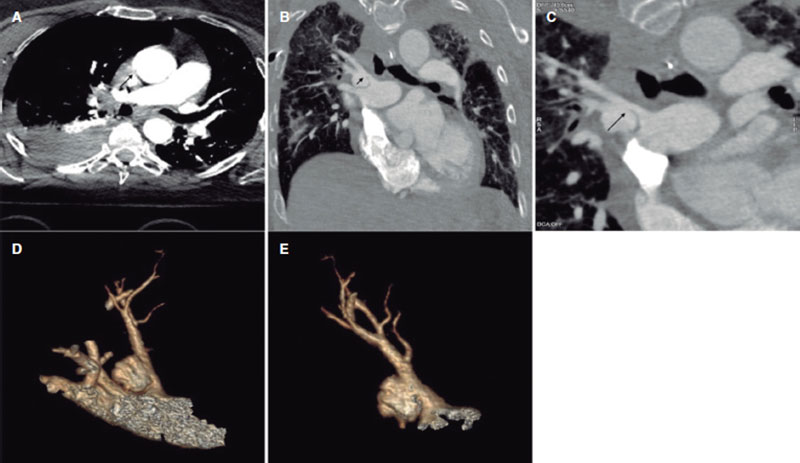
This is the case of a 68-year-old woman admitted due to polytrauma following a fall from a great height. While on mechanical ventilation she shows signs of self-limited hemoptysis without hemodynamic impairment. Several computed tomography (CT) scans reveal the presence of a 20 mm × 15 mm × 15 mm pseudoaneurysm at right upper lobe branch level without any data of active bleeding or erosion, but presence of progressive growth (5 mm) in 3 successive CT scans performed within 5 days (figure 1, arrows). Given the risk of rupture, percutaneous coronary intervention is attempted to seal the pseudoaneurysm. All the corresponding informed consents were obtained.
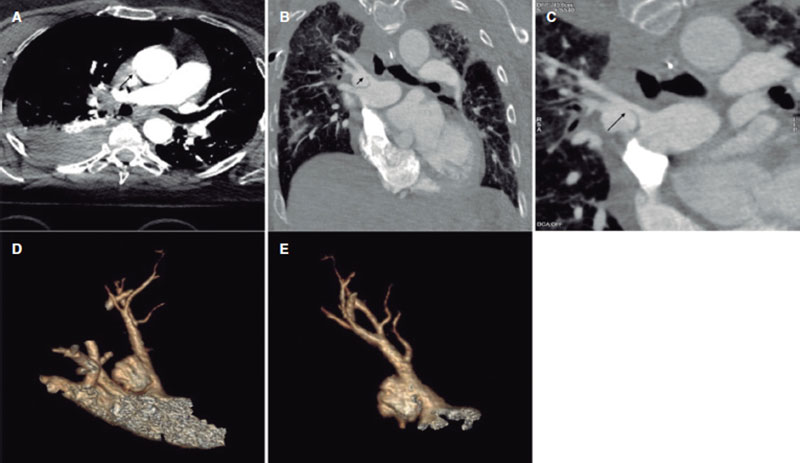
Figure 1.
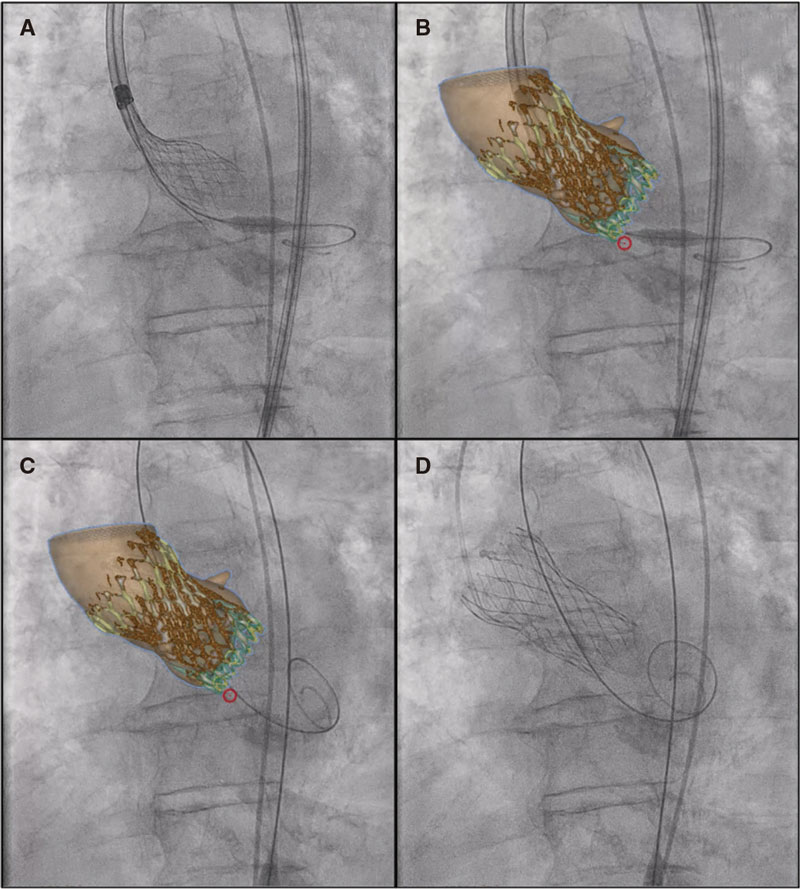
The angiography confirms the presence of the pseudoaneurysm including the bifurcation of 2 lobar branches (figure 2A, arrow; video 1 of the supplementary data) unsuitable for sealing with coils or intravascular plug and without a clear proper landing zone for stenting, which is why it is decided to implant a covered stent towards the upper subdivision to isolate it. Using a Judkins right 4 catheter (Launcher, Medtronic, United States) selective catheterization is achieved by advancing a 0.035 in guidewire. Afterwards, a 7-Fr Destination sheath (Terumo, Japan) is advanced through which a 6 mm × 28 mm Begraft expanded polytetrafluoroethylene (ePTFE)-covered stent (Bentley InnoMed, Germany) is implanted. The stent proximal region is postdilated with a 10 mm × 30 mm semicompliant Crystal Balloon (Balt, France). The pseudoaneurysm total exclusion is confirmed on the angiographic follow-up (figure 2B-F, arrow; video 2 of the supplementary data). The patient’s clinical progression is good, and she currently remains asymptomatic without clinical or radiographic data of pulmonary infarction at 6-month follow-up.
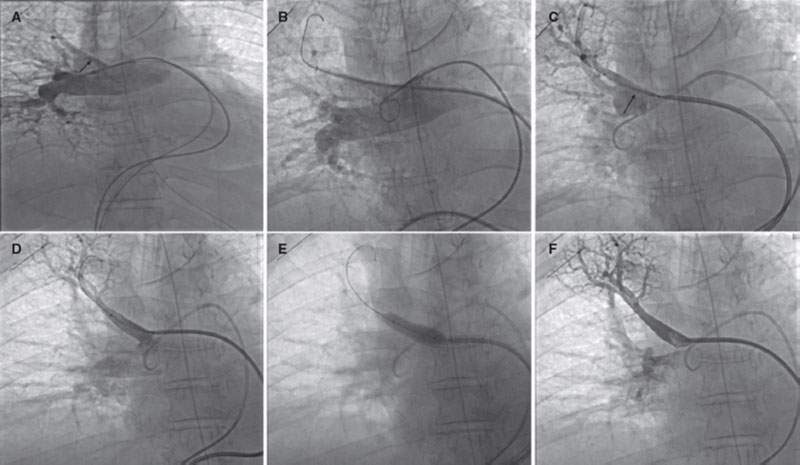
Figure 2.
FUNDING
None reported.
AUTHORS’ CONTRIBUTIONS
All the authors contributed equally to the drafting of this manuscript.
CONFLICTS OF INTEREST
None whatsoever.
SUPPLEMENTARY DATA
Vídeo 1. Fernández González L. DOI: 10.24875/RECICE.M22000350
Vídeo 2. Fernández González L. DOI: 10.24875/RECICE.M22000350
* Corresponding author.
E-mail address: luisfg82@hotmail.com (L. Fernández González).
Transcatheter aortic valve implantation (TAVI) success rate is very high and has a low rate of complications. Therefore, the number of TAVIs has been increasing worldwide. However, complications such as paravalvular leak (PVL) or permanent pacemaker implantation (PPMI) need still to be resolved, particularly in younger patients.
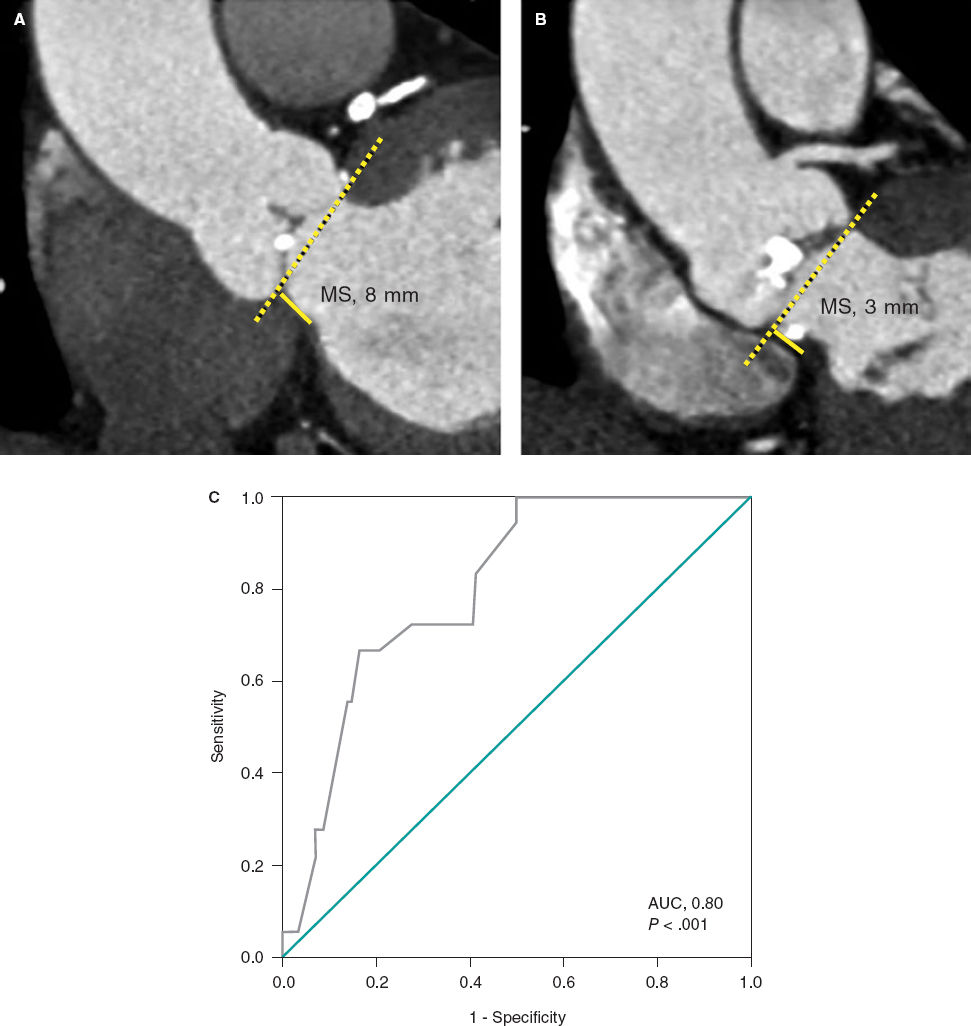
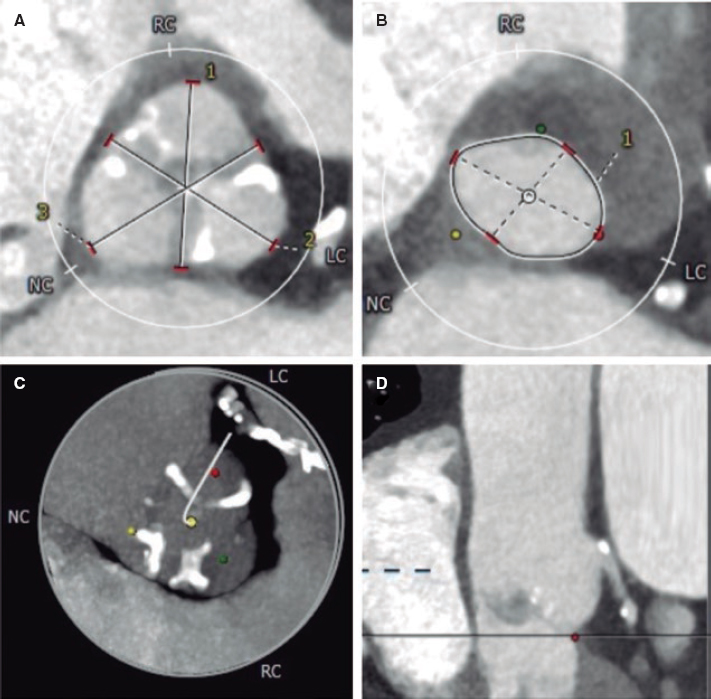
At this point, new technologies may help solve these problems. The FEops HEARTguide is a software that simulates the interaction between the device and patient’s anatomy (figure 1A,B). FEops provides the operator with different options and device sizes in a higher or deeper position (EVOLUT no. 26 and no. 29, Medtronic, United States), predicts the theoretical membranous septum, and the device contact pressure by analyzing the tissue characteristics of patient´s anatomy in the computed tomography (CT) images or risk of residual PVL or PPMI (figure 1C-H). Therefore, preoperative planning with FEops can be used to choose the most suitable size and device position for each patient.
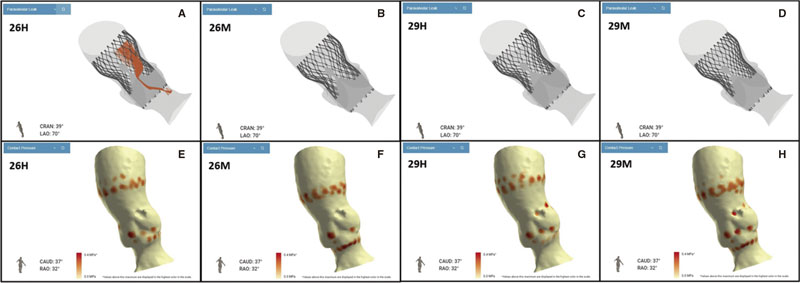
Figure 1.
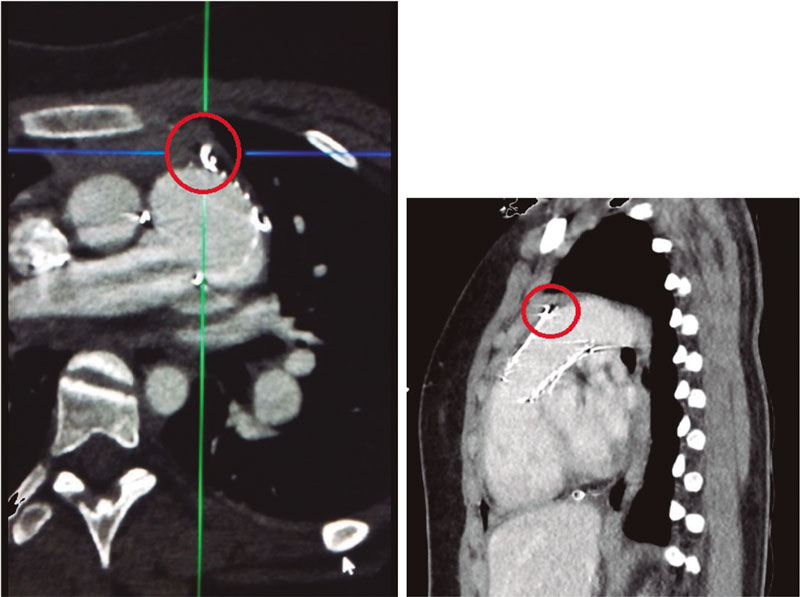
On the other hand, synchronized co-registration CT-fluoro has proven useful during TAVI. This is the first case ever performed worldwide using FEops image co-registration with live fluoroscopy in a TAVI procedure (figure 2, video 1 of the supplementary data; red circle: marks the membranous septum). However, no live correlation with heart and lung movements is its main limitation.
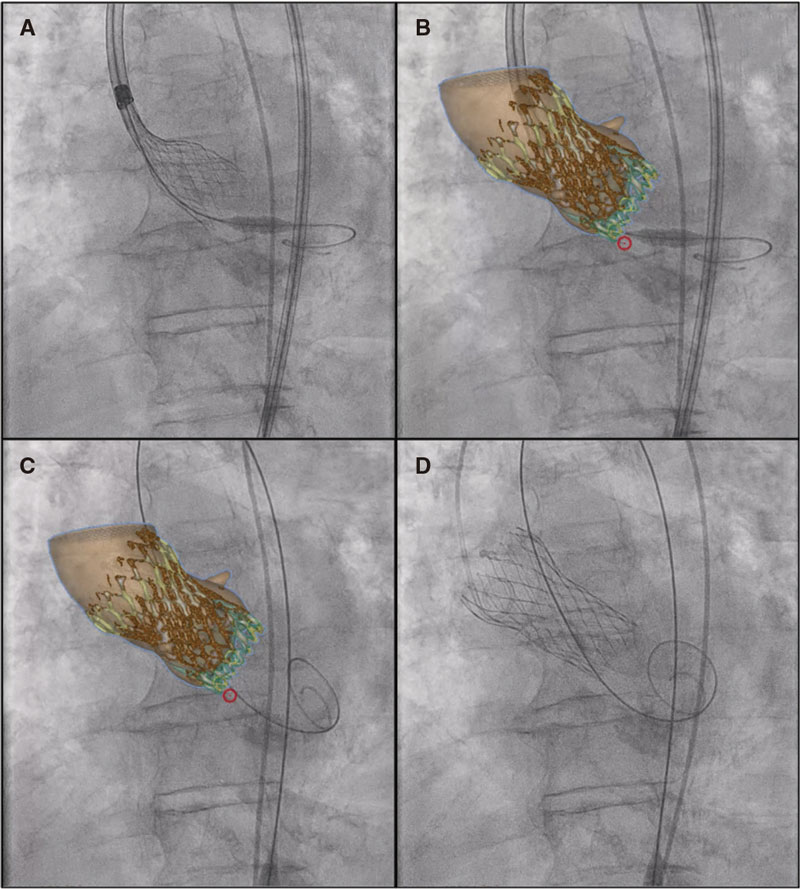
Figure 2.
In conclusion, FEops is potentially useful in TAVI not only for preoperative planning but also co-registration with fluoroscopy imaging during the procedure may reduce the complications associated with TAVI, especially in complex anatomies. Also, it can reduce the contrast used and the learning curve regarding difficult anatomies.
Written and oral informed consent were obtained before performing the procedure and for publication purposes.
FUNDING
None whatsoever.
AUTHORS’ CONTRIBUTIONS
All the authors participated in this manuscript, reviewed, and fully agreed on its content.
CONFLICTS OF INTEREST
I. Cruz Gonzalez is a proctor and consultor for Medtronic.
SUPPLEMENTARY DATA
Vídeo 1. Antúnez-Muiños PJ. DOI: 10.24875/RECICE.M22000349
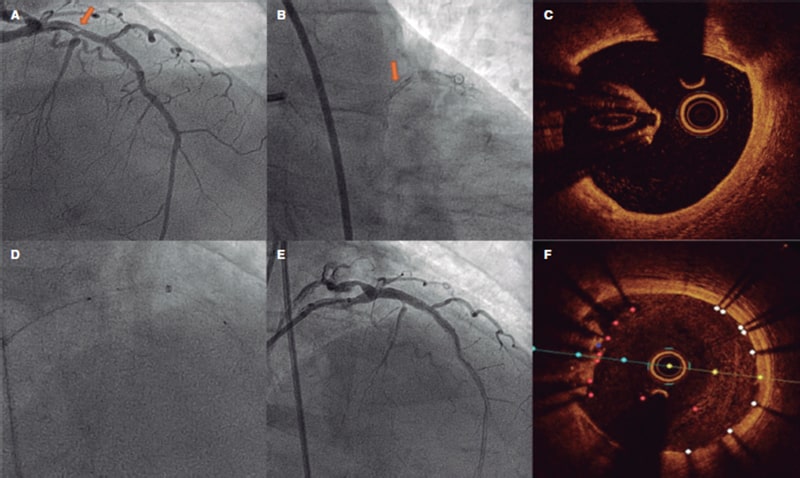
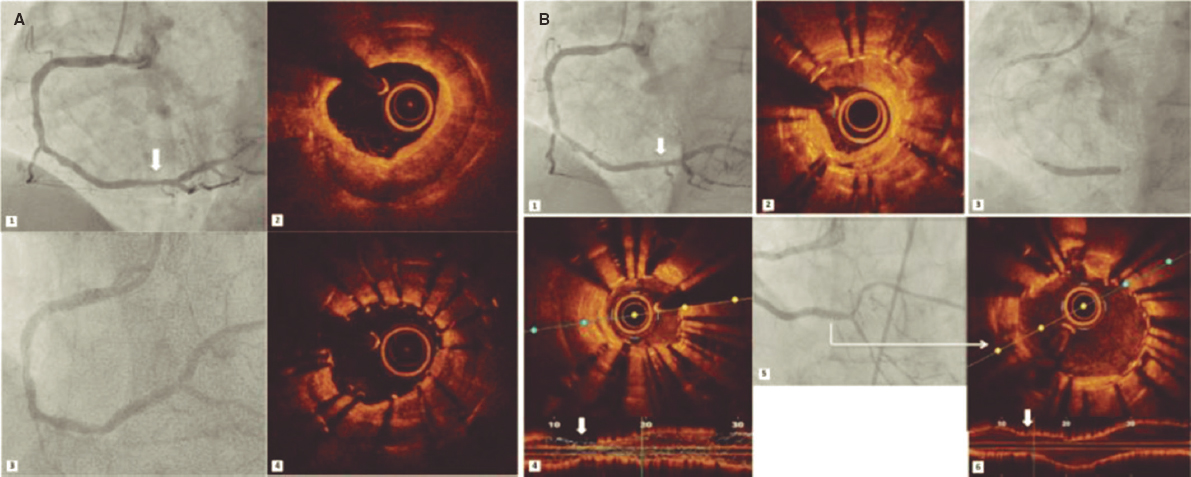
This is the case of a 78-year-old man with revascularized coronary artery disease 10 years ago (left anterior descending coronary artery and left circumflex artery) who was admitted to the hospital with signs of ST-segment elevation acute coronary syndrome. The angiography shows an unusual image on the proximal left anterior descending coronary artery that seems to be causing an angiographically significant stenosis (figure 1A,B). To confirm diagnosis, a catheter is unsuccessfully advanced with optical coherence tomography (OCT) guidance through a polymeric guidewire while trying to cross the most stenotic region. Two attempts are made after predilatation (with balloons of 1.5 mm and 2.5 mm in diameter) that prove unsuccessful. Afterwards, a guide catheter extension system is advanced (figure 1D) that successfully crosses the lesion facilitating the OCT that reveals the presence of an underexpanded coronary stent with complete endothelization, and a possible thrombus attached to it (figure 1C). Upon suspicion that this is the culprit lesion, decision is made to treat it. To crush the underexpanded stent against the lumen of the vessel, it is first effortlessly predilated using a 3.5 mm x 12 mm balloon. Afterwards, a 3.5 mm x 15 mm drug-eluting stent is implanted with good angiographic results (figure 1E). A new OCT confirms the excellent expansion of the new stent including the entire forgotten stent that is crushed between the new stent and the vessel endothelium (figure 1F).
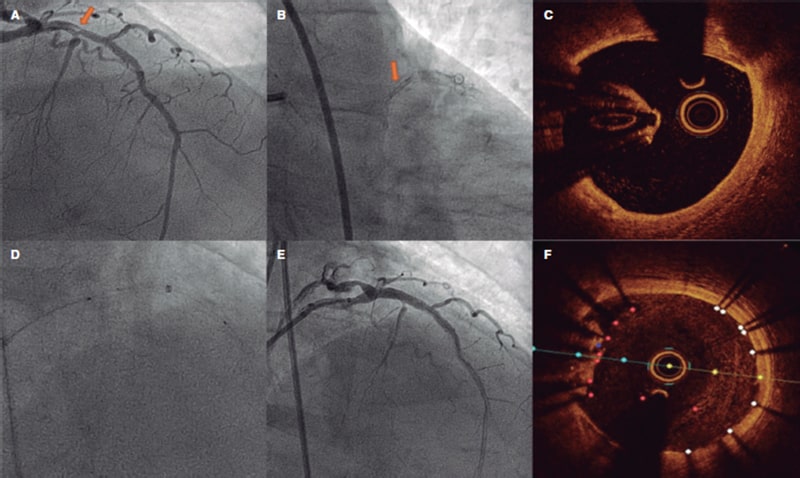
Figure 1.
Stent loss inside the coronary tree is a rare complication that can, however, be solved if removed during the procedure. However, when forgotten for years, the stent endothelizes and its extraction becomes complicated and is no stranger to complications. In these cases, the most efficient option is to exclude it by implanting a new drug-eluting stent.
The patient’s written informed consent was requested before publishing this article.
FUNDING
None whatsoever.
AUTHORS’ CONTRIBUTIONS
All the authors were involved in the procedure. They also reviewed the images and drafted the manuscript.
CONFLICTS OF INTEREST
R. Moreno in an associate editor of REC: Interventional Cardiology; the journal’s editorial procedure to ensure impartial handling of the manuscript has been followed.
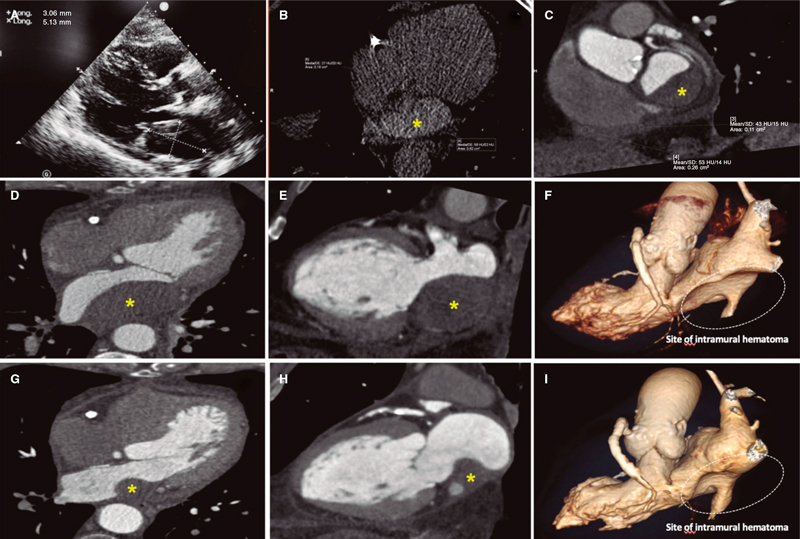
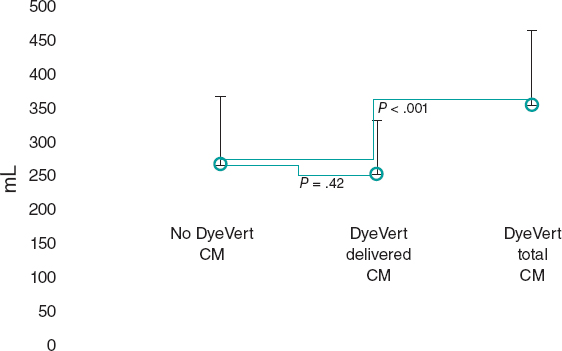
This is the case of a 75-year-old man who underwent coronary angiography due to new-onset dyspnea and left bundle branch block with a long, diffuse, and heavily calcified lesion with a maximum stenosis of 90% in his dominant right coronary artery. Patient was treated with complex percutaneous coronary intervention (PCI) (double-guidewire technique—both hydrophilic wires—guide catheter extension system, and compliant and non- compliant balloon dilatations), which eventually led to the successful distal-to-proximal implantation of 3 drug-eluting stents. A few hours later, he complained of pleuritic chest pain while remaining hemodynamically stable, and with a normal physical examination. Lab tests showed troponin I levels of 8 ng/mL (reference < 0.012 ng/mL). The echocardiogram showed no regional motion abnormalities, but revealed the presence of a 55.3 mm x 29 mm left atrial mass emerging from the posterior atrial wall almost occluding the complete atrial cavity without conditioning significant mitral valve dysfunction or an impaired transmitral flow. Pericardial effusion suggestive of hemopericardium was also described (figure 1A; video 1 of the supplementary data). Left atrial intramural hematoma (LAIH) was suspected and CCTA confirmed the lesion high attenuation (56 Hounsfield Units), which was suggestive of hematic component (*, figure 1B-F). The patient remained hospitalized until the stability of the lesion was confirmed (discharge size, 48 mm x 28 mm) while on dual antiplatelet therapy. After monthly clinical follow-ups, the control CCTA performed at 3 months confirmed significant reduction (30 mm x 20 mm) (*, figure 1G-I). LAIH is a rare complication associated with complex PCI procedures (probably caused while positioning the guidewires, penetrating distal vasculature, and causing the bleeding) being a potential cause for conduction disorders and hemodynamic instability. The patient’s verbal consent was obtained.
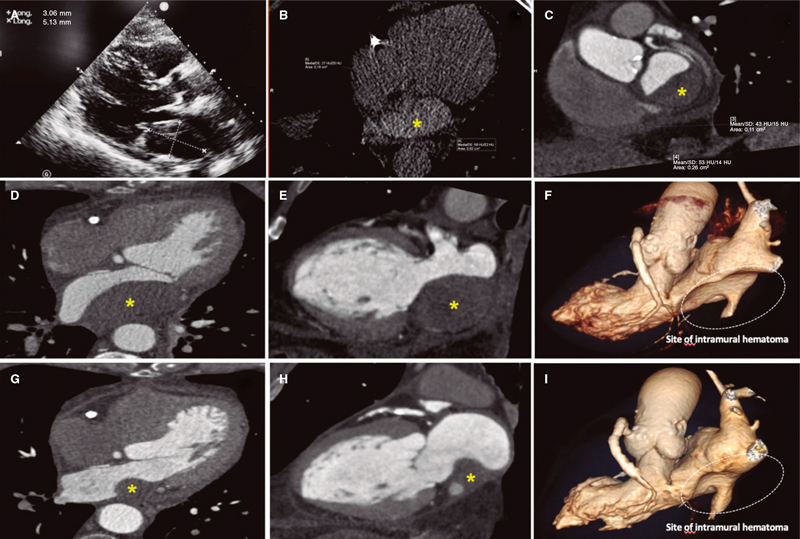
Figura 1.
FUNDING
None whatsoever.
AUTHORS’ CONTRIBUTIONS
L. Nieto-Roca, M. Tomás-Mallebrera, and R. Carda Barrio: contributed substantially to the drafting of this case, obtained the patient’s informed consent, and compiled all the images. They gave their approval to the manuscript final version. They take full responsibility for all aspects related to the article and commit themselves to investigating and solving all questions regarding the accuracy and truthfulness of any part of the work. J.A. Esteban-Chapel, and M.L. Martín-Mariscal contributed to the interpretation of the case, and the corresponding images. They gave their approval to the manuscript final version. They take full responsibility for all aspects related to the article and commit themselves to investigating and solving all questions regarding the accuracy and truthfulness of any part of the work.
CONFLICTS OF INTEREST
None reported.
SUPPLEMENTARY DATA
Vídeo 1. Nieto-Roca L. DOI: 10.24875/RECICE.M22000329
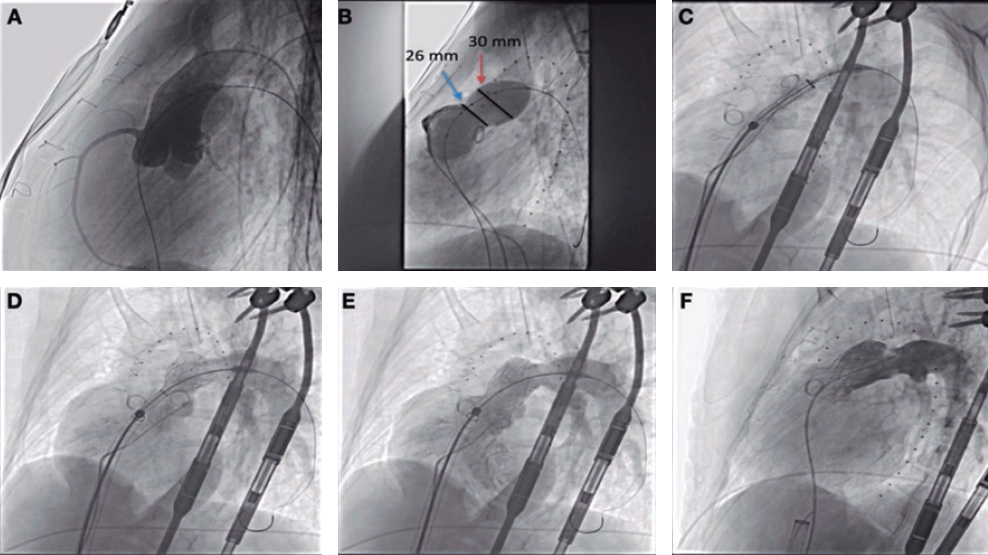
This is the case of 13-year-old teenage girl diagnosed with pulmonary atresia with intact ventricular septum treated in the neonatal period with valvulotomy with radiofrequency and percutaneous pulmonary valvuloplasty. Since then, the patient has developed severe pulmonary regurgitation and moderate tricuspid regurgitation. Valve implantation into the right ventricular outflow tract (RVOT) is decided due to worsening functional class with restrictive behavior of the right ventricle (without anticipated dilatation), and hepatic congestion. Cardiac catheterization reveals the presence of a dilated and pulsatile (pulmonary annulus: 29 mm) RVOT with supravalvular stenosis (minimum diameter: 21 mm), and a 34 mm post-stenotic dilatation (figure 1). A second-staged stent is implanted for percutaneous valve implantation. Given the absence of specific material for RVOTs so dilated, a 30 mm x 40 mm self-expandable Sinus-XL stent (Optimed, Germany) (off-label) is selected for being long enough, easy to implant, having enough navigability for the patient’s age (10-Fr sheath), and requiring less radial strength (favorable for dilated RVOTs).
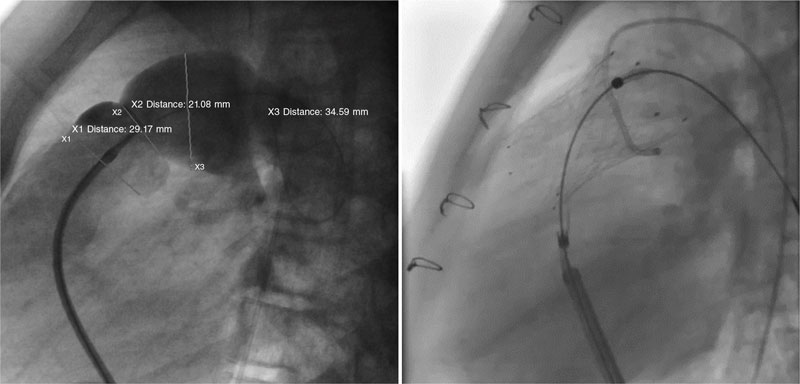
Figure 1.
A 14-Fr sheath was used to perform position angiographies (figure 1). A few hours later, the patient showed hemodynamic instability with transthoracic echocardiography findings compatible with cardiac tamponade. An emergency computed tomography scan (figure 2) confirmed the perforation of the pulmonary trunk at the operating room (figure 3; RA, right atrium; PA, pulmonary artery; RV, right ventricle). The stent was removed, and a pulmonary valve was implanted with favorable progression.
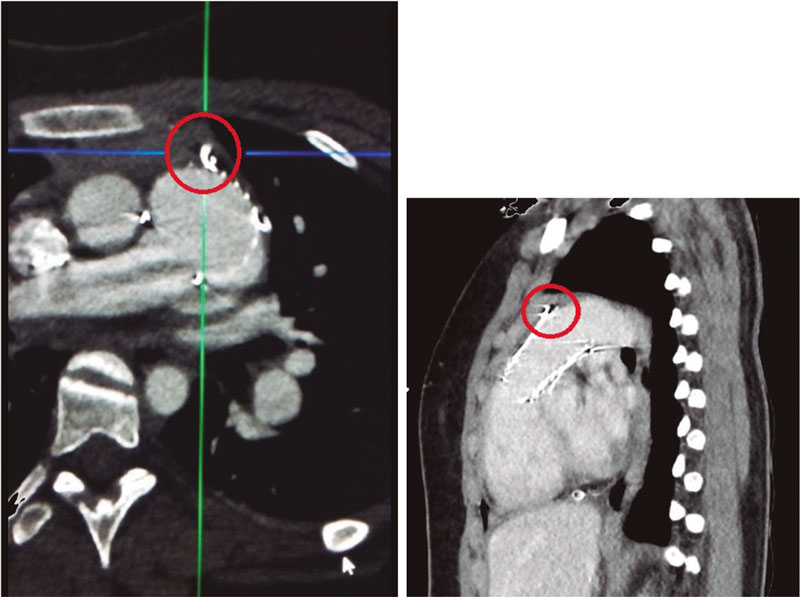
Figure 2.
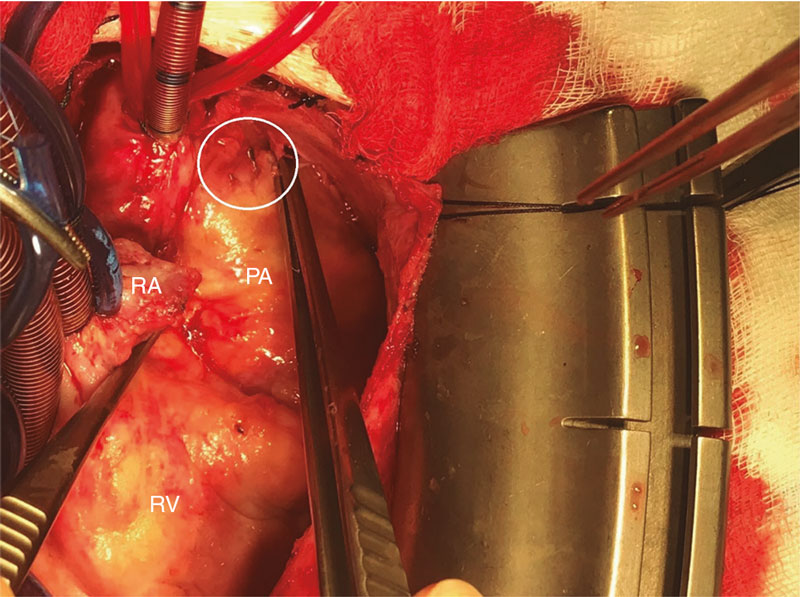
Figure 3.
The design of the stent is similar to that used during hybrid procedures in certain neonatal heart diseases, which means that similar complications can be expected.
The patient’s parents’ informed consent was obtained to be able to publish her case.
FUNDING
None whatsoever.
AUTHORS’ CONTRIBUTIONS
A. Rasines Rodríguez: drafted the case. C. Abelleira Pardeiro: critical review and image selection. Direct patient care. E.J. Balbacid Domingo: critical review and image selection. Direct patient care. All authors: contributed to the study idea and design, data curation or its analysis and interpretation and final approval of the version that would eventually be published.
CONFLICTS OF INTEREST
None reported.
* Corresponding author.
E-mail address: alejandro.rasines@gmail.com (A. Rasines-Rodríguez).
Editorials
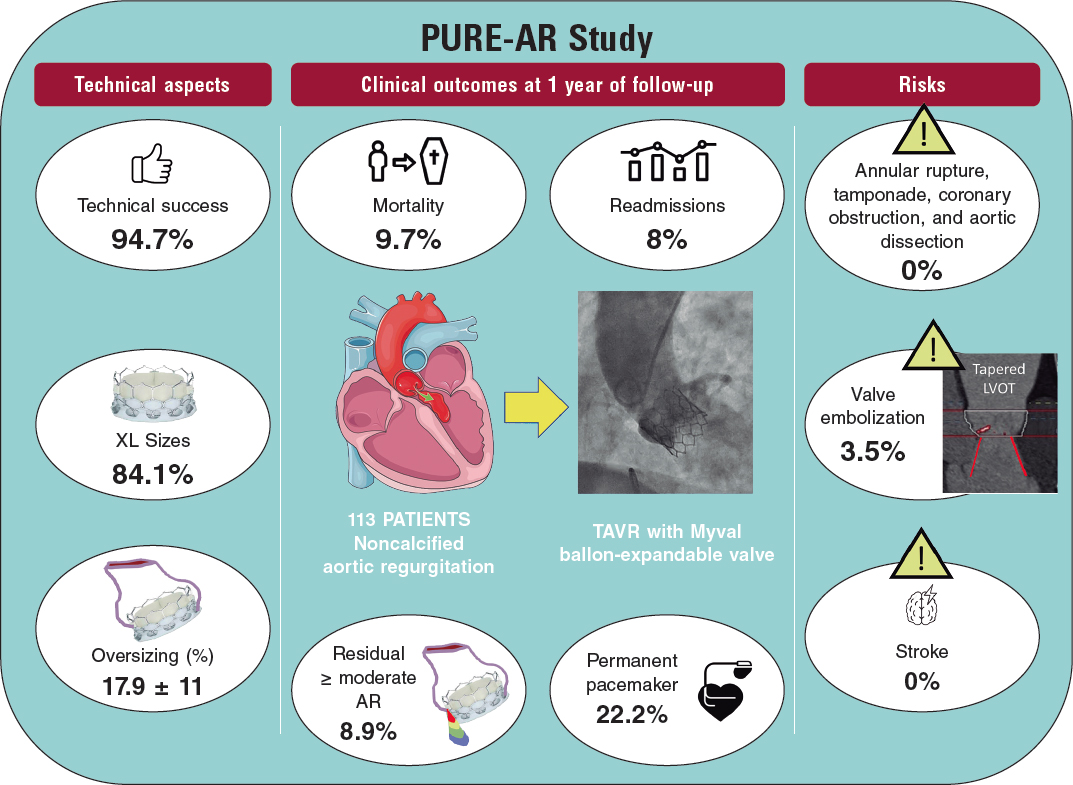
Transcatheter aortic valve replacement for noncalcified aortic regurgitation. Where are we now?
aServicio de Cardiología, Hospital Clínico Universitario, Valladolid, Spain
bCentro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), Spain
Original articles
Editorials
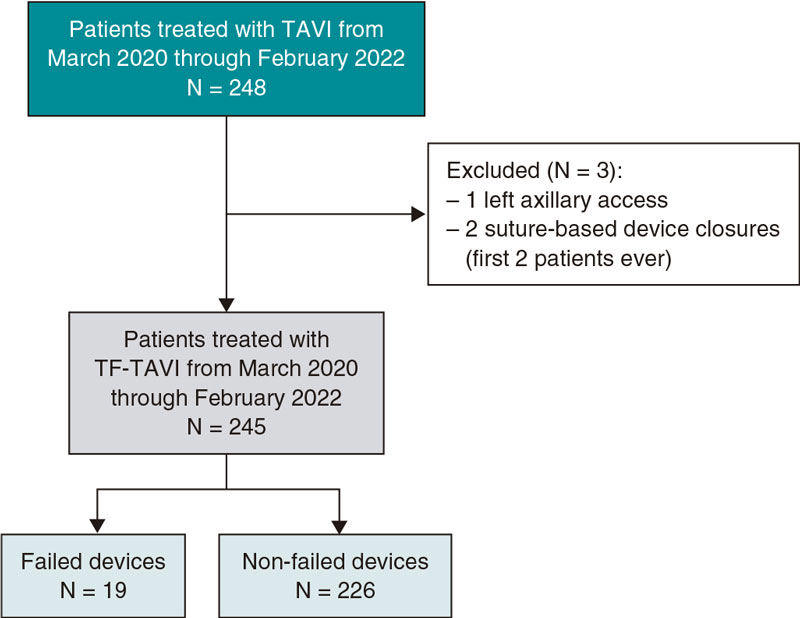
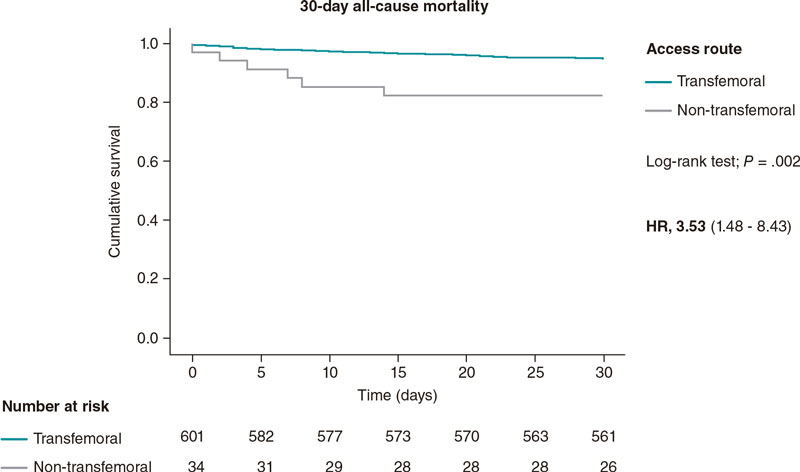
Vascular closure devices: the jury is still out
aUnidad de Hemodinámica, Servicio de Cardiología, Hospital General Universitario Dr. Balmis, Instituto de Investigación Sanitaria y Biomédica de Alicante (ISABIAL), Alicante, Spain
bDepartamento de Medicina Clínica, Universidad Miguel Hernández, Alicante, Spain
Original articles
Debate
Debate: Asymptomatic severe aortic stenosis: when should we intervene?
The clinician’s perspective
Servicio de Cardiología, Hospital Ramón y Cajal, Madrid, Spain
The interventional cardiologist’s perspective
Unidad de Cardiología Intervencionista, Hospital Álvaro Cunqueiro, Complejo Hospitalario Universitario de Vigo, Vigo, Pontevedra, Spain