Pre-stenting of the right ventricular outflow tract (RVOT) is often performed before percutaneous pulmonary valve implantation to guarantee a stable landing zone. One common off-label indication is the presence of a large native RVOT, which requires the anchoring of an extra-large stent.
This is the case of a 17-year-old woman with a repaired Fallot´s tetralogy admitted for the assessment of severe pulmonary regurgitation with RV dilatation (video 1 of the supplementary data). The patient was ranked as NYHA functional class III. The cardiac magnetic resonance imaging revealed the presence of a large RVOT with diameters of 27 mm x 26 mm (video 2 of the supplementary data).
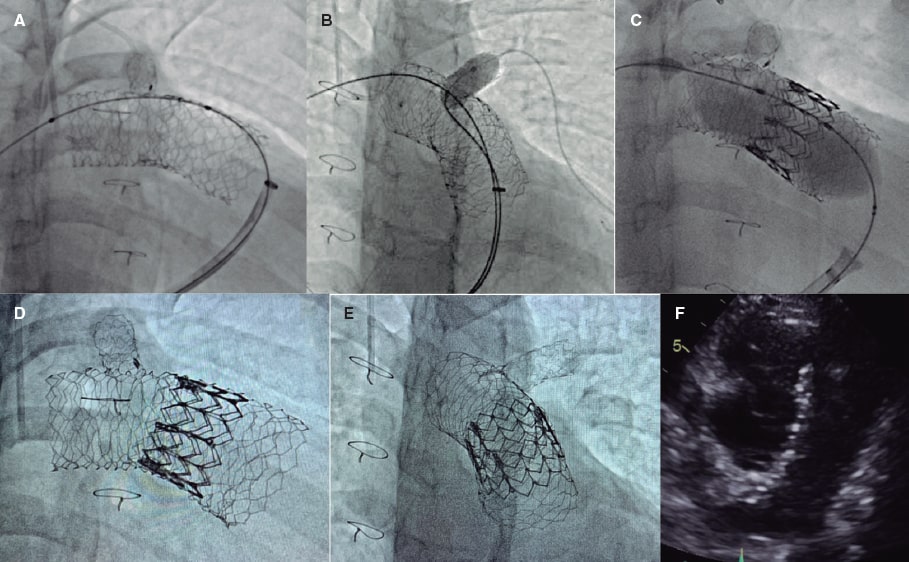
For SAPIEN XT valve implantation (Edwards Lifesciences, United States) and to prevent stent migration, the “double-stent” technique was used. A 57 mm AndraStent XXL stent (Andramed, Germany) was pre-mounted on a 26 mm balloon followed by distal-to-proximal implantation from right pulmonary artery to pulmonary trunk. Afterwards, a second 48 mm AndraStent XXL stent was pre-mounted on a 30 mm balloon and partially implanted overlapping the former stent (figure 1A). A stent that remained in the left pulmonary branch that was implanted at a younger age was dilated with a 10 mm balloon through the new stents (figure 1B). Then, a 29 mm SAPIEN XT valve with 2 extra mL for balloon inflation was advanced and implanted successfully (figure 1C,F). No residual gradient or significant regurgitation were reported. The patient was discharged 2 days later and remains in NYHA class I after 8 months. The father’s patient has given his verbal consent for the publication of this case report.
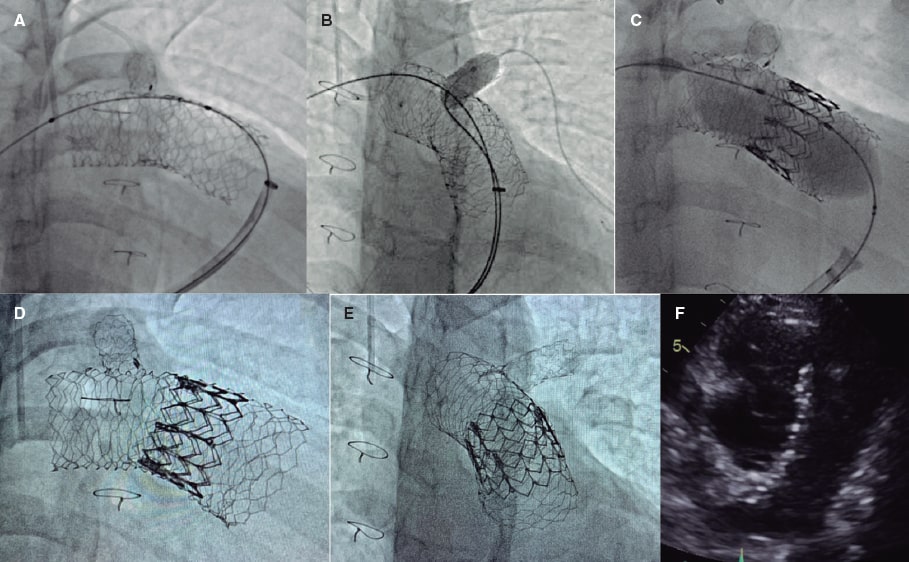
Figure 1.
Double overlapping pre-stenting with the AndraStent XXL stent provides valid anchoring support for percutaneous pulmonary valve implantation in large native RVOTs.
FUNDING
None reported.
AUTHORS’ CONTRIBUTIONS
All the authors drafted this manuscript, read and approved its final version.
CONFLICTS OF INTEREST
None whatsoever.
SUPPLEMENTARY DATA
Vídeo 1. Hernández F. DOI: 10.24875/5/RECICE.M22000275
Vídeo 2. Hernández F. DOI: 10.24875/5/RECICE.M22000275
* Corresponding author.
E-mail address: felipeivus@hotmail.com (F. Hernández Hernández).