Transcatheter aortic valve implantation (TAVI) success rate is very high and has a low rate of complications. Therefore, the number of TAVIs has been increasing worldwide. However, complications such as paravalvular leak (PVL) or permanent pacemaker implantation (PPMI) need still to be resolved, particularly in younger patients.
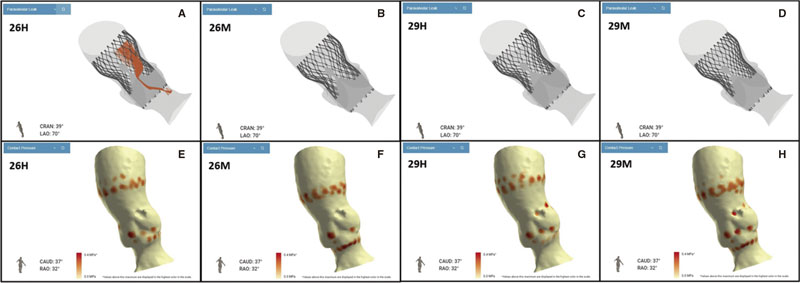
At this point, new technologies may help solve these problems. The FEops HEARTguide is a software that simulates the interaction between the device and patient’s anatomy (figure 1A,B). FEops provides the operator with different options and device sizes in a higher or deeper position (EVOLUT no. 26 and no. 29, Medtronic, United States), predicts the theoretical membranous septum, and the device contact pressure by analyzing the tissue characteristics of patient´s anatomy in the computed tomography (CT) images or risk of residual PVL or PPMI (figure 1C-H). Therefore, preoperative planning with FEops can be used to choose the most suitable size and device position for each patient.
Figure 1.
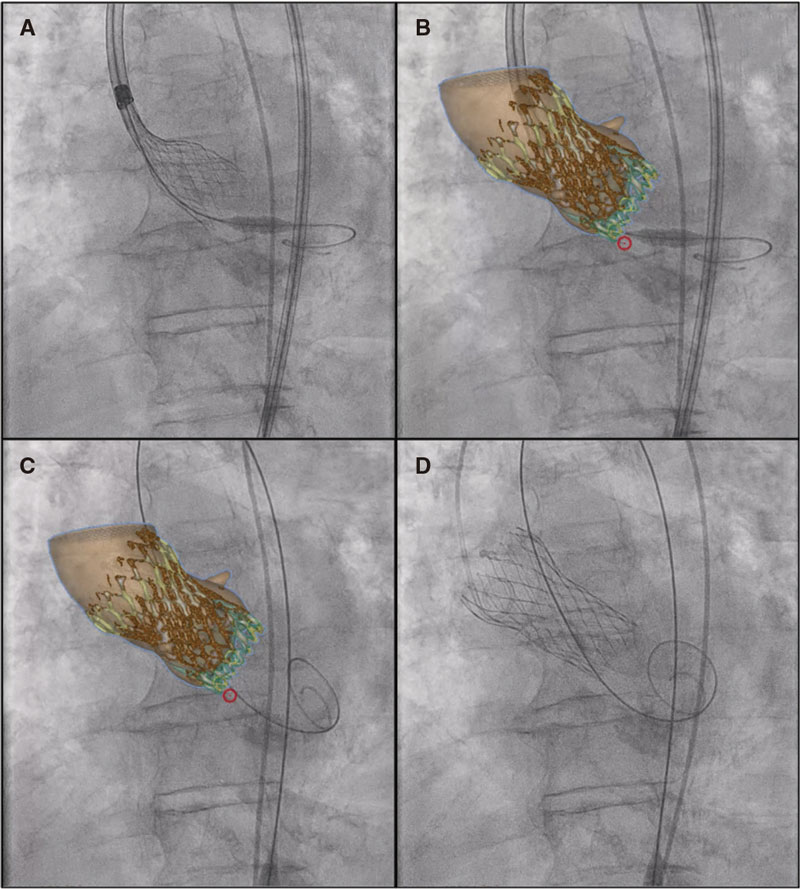
On the other hand, synchronized co-registration CT-fluoro has proven useful during TAVI. This is the first case ever performed worldwide using FEops image co-registration with live fluoroscopy in a TAVI procedure (figure 2, video 1 of the supplementary data; red circle: marks the membranous septum). However, no live correlation with heart and lung movements is its main limitation.
Figure 2.
In conclusion, FEops is potentially useful in TAVI not only for preoperative planning but also co-registration with fluoroscopy imaging during the procedure may reduce the complications associated with TAVI, especially in complex anatomies. Also, it can reduce the contrast used and the learning curve regarding difficult anatomies.
Written and oral informed consent were obtained before performing the procedure and for publication purposes.
FUNDING
None whatsoever.
AUTHORS’ CONTRIBUTIONS
All the authors participated in this manuscript, reviewed, and fully agreed on its content.
CONFLICTS OF INTEREST
I. Cruz Gonzalez is a proctor and consultor for Medtronic.
SUPPLEMENTARY DATA
Vídeo 1. Antúnez-Muiños PJ. DOI: 10.24875/RECICE.M22000349