We present the case of a 16-year-old girl with a prenatal diagnosis of Ebstein’s anomaly and an atrial septal defect with severe tricuspid regurgitation.
In 2020, the patient was referred due to functional deterioration. Surgical repair included the implantation of a 26-mm Contour 3D tricuspid annuloplasty ring (Medtronic, United States) with the cone reconstruction technique and closure of the atrial septal defect. During the postoperative follow-up, the patient developed moderate paravalvular leak lateral to the ring that progressed to severe regurgitation with moderate right ventricular dilatation without dysfunction a year and a half later. The patient experienced no episodes of heart failure until 2 years after surgery.
Cardiac catheterization was performed via transjugular and right femoral access under general anesthesia and transesophageal echocardiography guidance, revealing the presence of a 13 mm × 10 mm leak posterolateral to the tricuspid annuloplasty ring that appeared as a 15 mm leak on ventriculography (figure 1). The first closure attempt with a 14-mm Konar MF device (Lifetech, China) did not achieve complete closure or attachment at the defect. Therefore, a second attempt was made with an 18-mm Amplatzer Muscular VSD device (Abbott, United States), which achieved proper attachment and almost complete occlusion (figure 2). After release, minimal residual regurgitation was confirmed by transesophageal echocardiography (figure 3 and video of the supplementary data). There were no irregular heart rhythms or repolarization abnormalities.
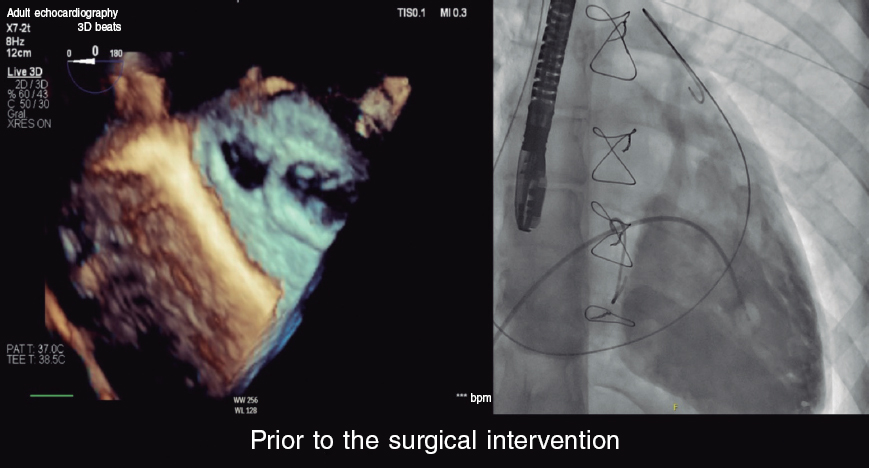
Figure 1.
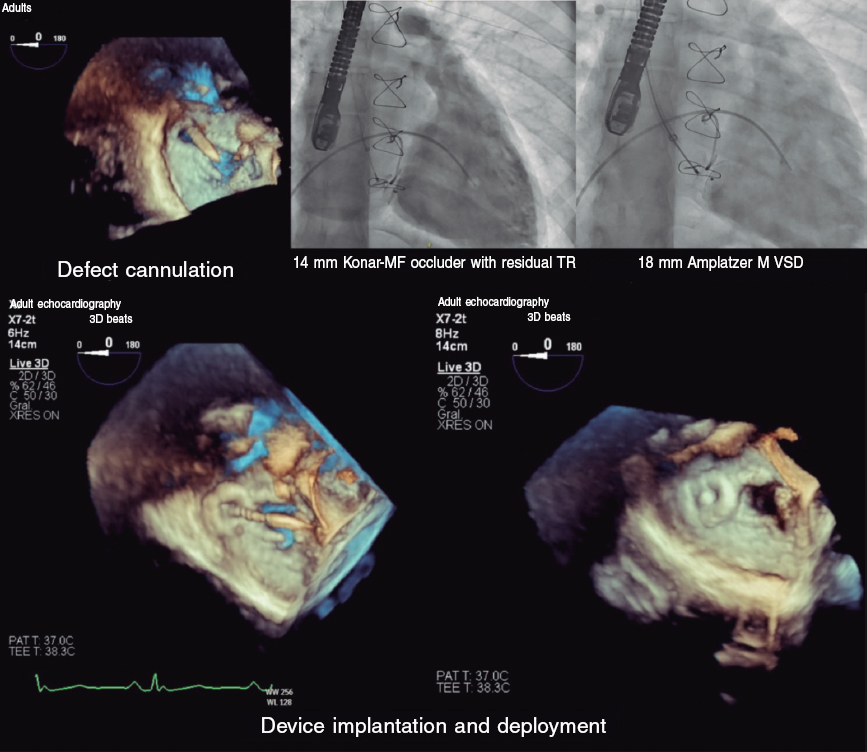
Figure 2.
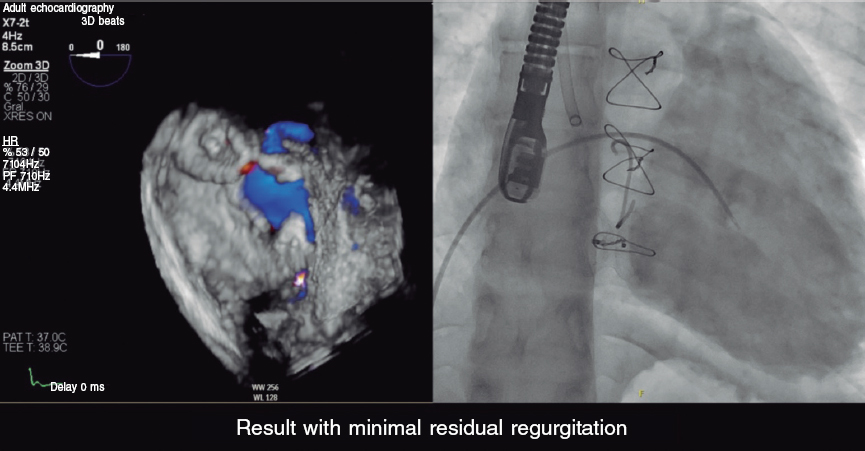
Figure 3.
The patient was discharged 24 hours after the procedure, with the initiation of aspirin therapy. Follow-up revealed a normal electrocardiogram, with minimal residual regurgitation, and no hemolysis. The patient reported improvement during exertion.
FUNDING
None declared.
ETHICAL DISCLOSURES
This case was approved for publication by the pediatric cardiology unit, and did not require further evaluations by the research ethics committee. The parents of the minor and the mature minor herself gave their prior written informed consent. Because this is a presentation of an isolated case, the SAGER guidelines did not need to be followed.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
No artificial intelligence tool has been used during the preparation of this work.
AUTHORS’ CONTRIBUTIONS
All authors participated in the procedure, image review, and manuscript drafting.
CONFLICTS OF INTEREST
None declared.
ACKNOWLEDGEMENTS
We wish to thank the pediatric cardiology unit at Hospital Universitario La Paz for their professionalism and attention to publication details. Final results are a direct consequence of their excellent patient care.
SUPPLEMENTARY DATA
Vídeo 1. Guillén Mendoza NB. DOI: 10.24875/RECICE.M23000411
* Corresponding author.
E-mail address: noelia.guillen.m@gmail.com (N.B. Guillén Mendoza).
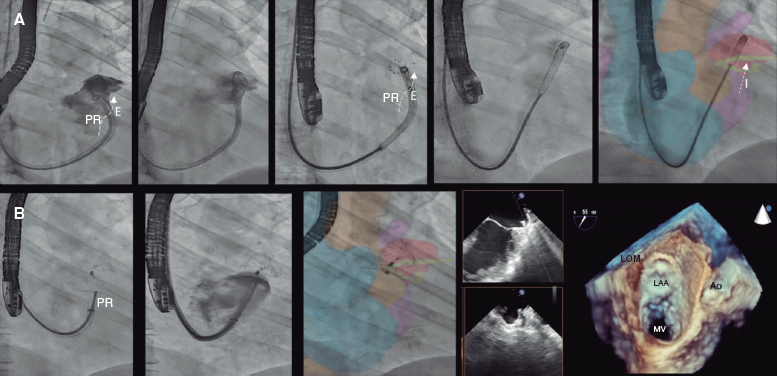
A 52-year-old woman with atrial fibrillation, thrombocytopenia, and severe gastrointestinal bleeding while on several anticoagulants was referred for left atrial appendage closure. A year earlier, pulmonary vein ablation had been attempted but femoral access proved impossible due to a previously undetected congenital interruption of the inferior vena cava (figure 1A; asterisk). A right transjugular procedure was planned under general anesthesia and guided by transesophageal echocardiography and computed tomography-fluoroscopy fusion imaging (video 1 of the supplementary data). The left atrial appendage had a windsock morphology, with a mean diameter of 20 mm at the landing zone and 30 mm at the ostium (figure 1B,C). Consequently, a 24 mm x 30 mm LAmbre LAA Occluder system (LifeTech Scientific, China) was used, because its secure anchorage and closure mainly through the disk could facilitate the procedure. Transseptal puncture was performed using an SL1 sheath and a BRK-1 XS needle (Abbott, United States) by pre-shaping a secondary curve, followed by the insertion of a SafeSept guidewire (Pressure Products, United States) specifically designed for greater accuracy of transseptal puncture (figure 2A,B; the asterisk indicates the posterior and mid-puncture. Ao, aorta; LAA, left atrial appendage; SVC, superior vena cava). The device was implanted through a 10-Fr Fustar steerable sheath (LifeTech Scientific, China), which improved reach and coaxiality (figure 3A,B. E, extension; I, impulse; LOM, ligament of Marshall; MV, mitral valve; PR, posterior rotation). The patient was discharged at 24 hours without complications and on apixaban therapy (2.5 mg for 45 days).
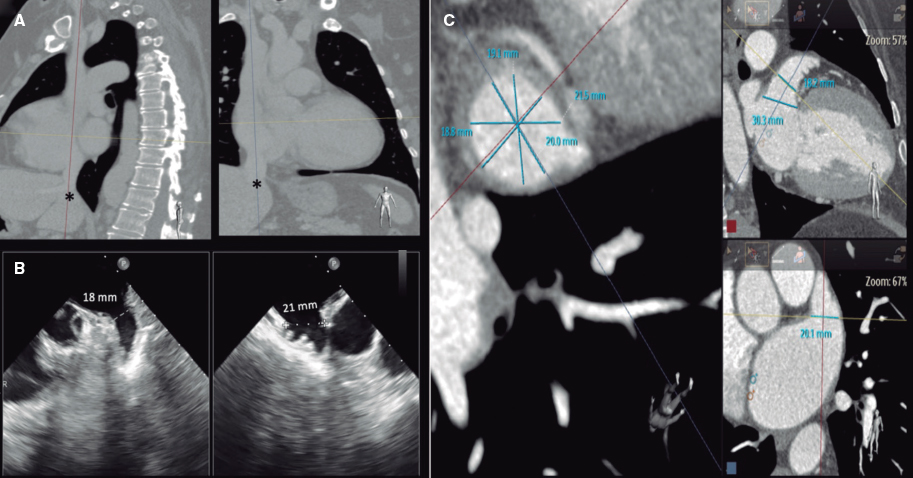
Figure 1.
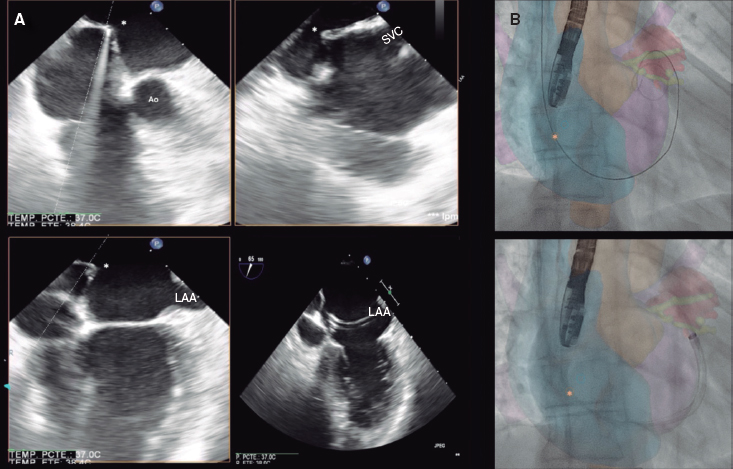
Figure 2.
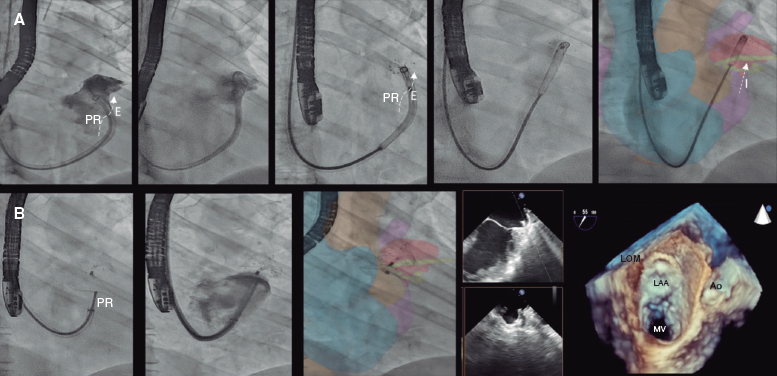
Figure 3.
Multimodal imaging, tools that facilitate precise transseptal puncture, and steerable sheaths can simplify the performance of procedures via upper access with efficiency and safety.
FUNDING
None declared.
ETHICAL CONSIDERATIONS
This work was published after approval was obtained from the research ethics committee of Complejo Hospitalario Universitario de Albacete, Spain. The patient gave her prior written informed consent to the intervention and publication of her case. Sex and gender variables were taken into consideration based on the SAGER guidelines.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
No artificial intelligence tool has been used during the preparation of this work.
AUTHORS’ CONTRIBUTIONS
All the authors participated in the drafting of this manuscript: J.G. Córdoba-Soriano was involved in drafting and design tasks, J.C. García-López in image selection and manuscript revision, and J. Jiménez-Mazuecos in manuscript revision and proofreading.
CONFLICTS OF INTEREST
None declared.
SUPPLEMENTARY DATA
Vídeo 1. Córdoba-Soriano JG. DOI: 10.24875/RECICE.M23000410
We present the case of a 19-year-old woman with DiGeorge syndrome associated with psychomotor retardation, tetralogy of Fallot, and right pulmonary artery agenesis, treated with right ventricular outflow tract (RVOT) transannular patch augmentation during childhood, with severe pulmonary regurgitation and progressive right ventricular enlargement. As a result, pulmonary valve replacement was indicated. Cardiac computed tomography (CT) revealed the presence of severe scoliosis, right sternal deviation, and an elongated RVOT with a minimum diameter of 26 mm at the annular level and 30 mm at the supravalvular level (figure 1A-D, arrows). Because of the clinical and biomechanical characteristics, the anatomy of the RVOT, and the presence of a single pulmonary artery, we performed transcatheter implantation of a self-expanding bioprosthetic Venus valve (Medtech, China). Other valves suitable for large-caliber RVOTs, such as the Myval (Meril, India) have not been granted CE marking for pulmonary implantation.
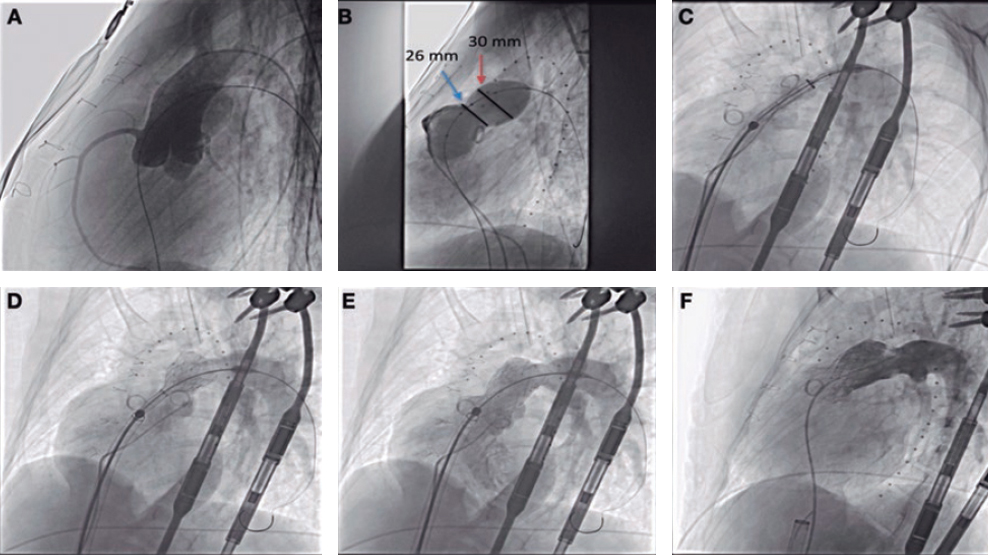
Figure 1.
Prior to implantation, the RVOT was sized, and coronary compression was ruled out after occlusive inflation with a 35-mm PTS-X sizing balloon catheter (NuMED, United States). The measurements obtained were consistent with the CT scan results. Consequently, a 30-mm to 25-mm valve was selected (figure 2A,B). A 24-Fr GORE DrySeal introducer sheath and an extra stiff Lunderquist wire guide (Cook Medical, United States) were used to access the left pulmonary artery and progressively deploy the valve initially from the distal segment at the origin of the pulmonary artery and subsequently the proximal segment. Withdrawal of the introducer sheath revealed optimal apposition to the RVOT (figure 2C-F; videos 1 to 4 of the supplementary data). The patient was discharged from hospital 24 hours later, and the valve has remained fully functional ever since with no signs of residual valvular regurgitation.
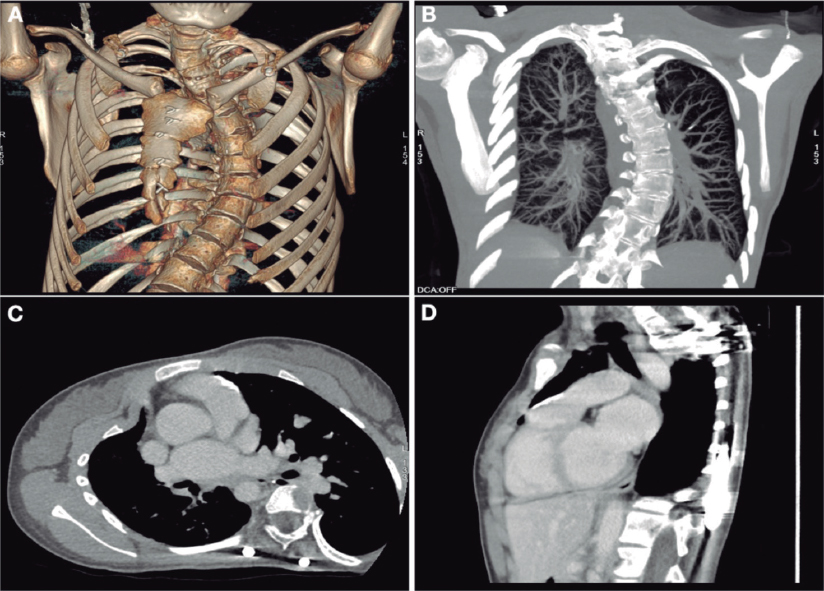
Figure 2.
The patient and her family gave their written informed consent for the publication of this article.
FUNDING
None declared.
ETHICAL CONSIDERATIONS
The work has been approved by the ethics committee of our centre. Informed consent was obtained from the patient and family. Our work has not taken into account possible sex and gender variables in accordance with SAGER guidelines.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
No artificial intelligence was used.
AUTHORS’ CONTRIBUTIONS
All the authors contributed equally to this work.
CONFLICTS OF INTEREST
None declared.
SUPPLEMENTARY DATA
Vídeo 1. Fernández González L. DOI: 10.24875/RECICE.M23000407
Vídeo 2. Fernández González L. DOI: 10.24875/RECICE.M23000407
Vídeo 3. Fernández González L. DOI: 10.24875/RECICE.M23000407
Vídeo 4. Fernández González L. DOI: 10.24875/RECICE.M23000407
* Corresponding author.
E-mail address: luisfg82@hotmail.com (L. Fernández González).
Finalist case in the ACCIS 2023 Madrid course
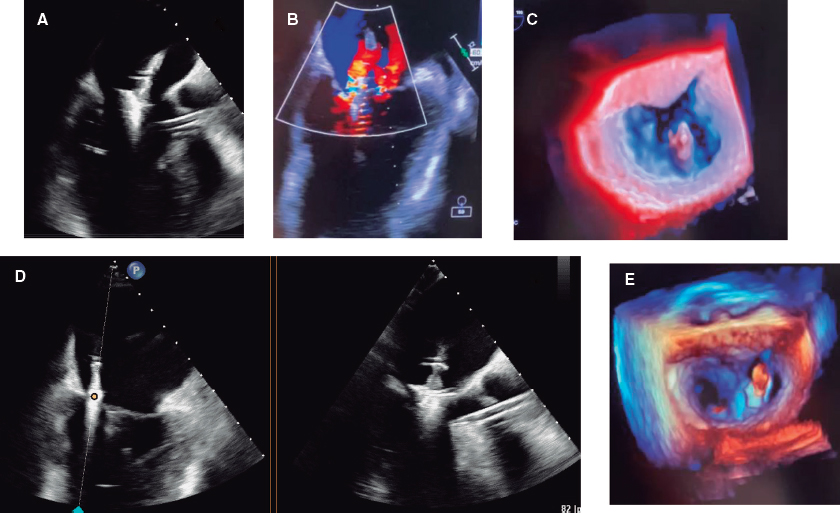
A 59-year-old man was admitted to the cardiac intensive care unit (CICU) due to evolved inferior-posterior ST-segment elevation myocardial infarction complicated by cardiogenic shock. Upon arrival, transesophageal echocardiography revealed severe mitral regurgitation (MR) secondary to posterior leaflet restriction (figure 1A,B). After rupture of papillary muscles was ruled out, the patient was transferred to the cath lab, where a 100% thrombotic lesion was observed in the proximal left circumflex artery (figure 1D-E). Due to hypotension, we decided to support the angioplasty with the Impella CP device (Abiomed, United States) (figure 1C). Flow was finally restored (figure 1F).
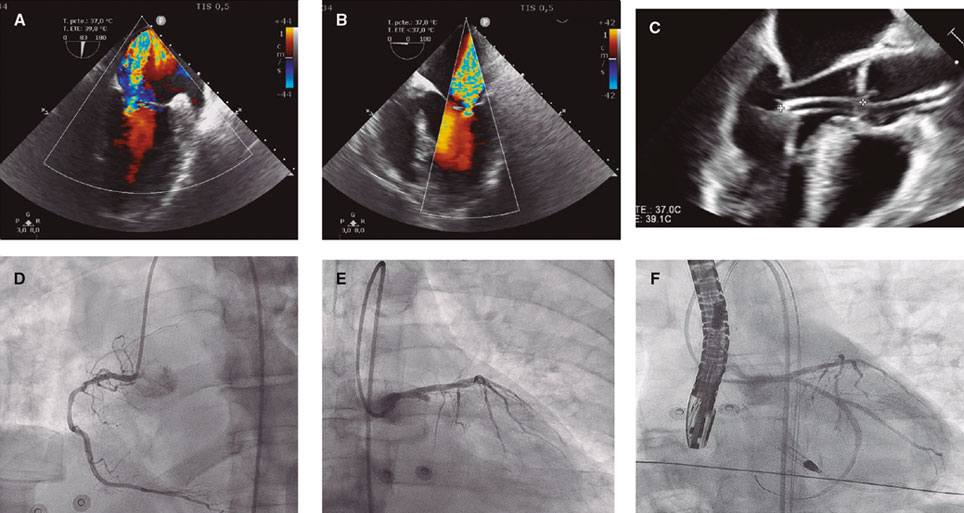
Figure 1.
Five days later, the patient was hemodynamically stable with Impella at P6, but developed multiple complications, including acute kidney failure, significant bleeding, and hemolysis. Three-dimensional echocardiography showed MR without changes. At this point, spontaneous improvement of MR seemed unlikely, and the risk of heart transplant or surgery was unacceptable. Finally, we decided to implant a MitraClip (Abbott, United States) supported by Impella.
The first MitraClip NTW (Abbott, United States) was placed between P2 and A2, with significant posterior-medial regurgitation (figure 2A-C). The second MitraClip NT (Abbott, United States) was implanted nearby. Residual MR was mild (figure 2D,E, video 1 of the supplementary data).
Figure 2.
The patient was extubated after the procedure and the Impella device was removed the following day. He left the CICU 10 days later.
This case is in line with other case reports suggesting that MitraClip, supported by Impella CP, could be an effective strategy in patients with severe functional MR. In this case, hemodynamic support by Impella CP was used to complete the primary percutaneous coronary intervention during CICU admission and during edge-to-edge mitral valve repair. Informed consent was obtained from the patient to publish this manuscript.
FUNDING
None.
ETHICAL CONSIDERATIONS
Informed consent was obtained from the patient to publish this properly anonymized manuscript. Because this is a single case report, approval from ethics committee was not required and gender considerations were not applicable.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
No artificial intelligence software was used to write this manuscript.
AUTHORS’ CONTRIBUTIONS
All authors contributed to data collection, drafting, review, and approval of the manuscript.
CONFLICTS OF INTEREST
I. Pascual Calleja: payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events for Abbot Vascular. C. Garrote Coloma: proctor for MitraClip implant, Abbot. J.M. de la Torre-Hernández: grants or contracts from Abbot, Amgen, Boston SCI; consulting fees from Medtronic, Boston SCI, Abbot; support for attending meetings from Medtronic, Abbot, Boston SCI.
J.M. de la Torre-Hernández is also editor-in-chief of REC: Interventional Cardiology. The journal’s editorial procedure to ensure impartial handling of the manuscript has been followed.
The remaining authors have no conflicts of interest.
SUPPLEMENTARY DATA
Vídeo 1. Coroas Pascual C. DOI: 10.24875/RECICE.M23000414
* Corresponding author.
E-mail address: mikearri@gmail.com (M. Arrizabalaga Gil).
A 78-year-old man with a past medical history of hypertension, pulmonary thromboembolism, atrial fibrillation, and prostate cancer presented with dyspnea. The patient was diagnosed with severe aortic stenosis (mean gradient, 49 mmHg; area of 0.7 cm2), and severe ventricular hypertrophy. Heart function was preserved. The heart team decided to perform transcatheter aortic valve implantation (TAVI). Computed tomography revealed the presence of a scarcely calcified annulus with greater calcium distribution at the level of leaflet commissures and a 73.5-mm perimeter (figure 1).
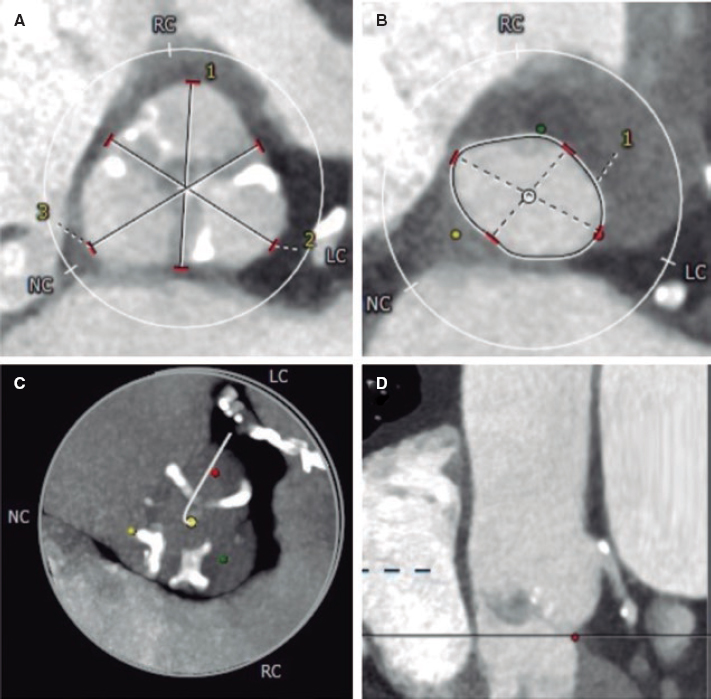
Figure 1.
We decided to implant a 23-mm self-expandable, supra-annular, fully recapturable, and nonrepositionable ALLEGRA valve (New-Valve-Technology, Switzerland). The valve was predilated using a 20-mm balloon but showed pop-up and distal migration towards the outflow tract despite pacing (figure 2A,B). The same complication occurred with a 27-mm ALLEGRA device. We then attempted to use a 29-mm CoreValve Evolut PRO+ valve (Medtronic, United States), because this device is fully repositionable, but the same complication recurred even with pacing (figure 2C,D).
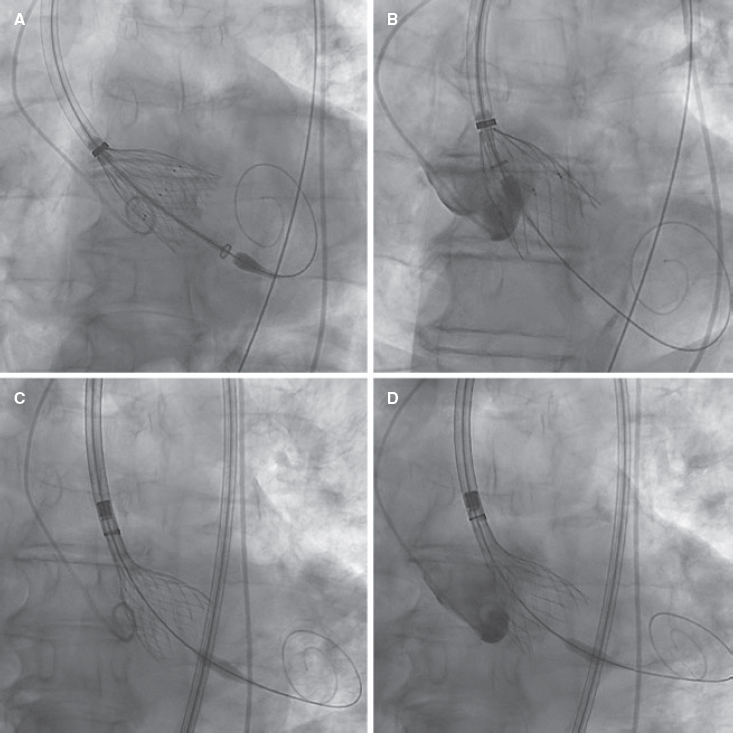
Figure 2.
Due to severe aortic regurgitation after the failed implantations, the patient became unstable (video 1 of the supplementary data). Considering the calcium distribution, we used the repositionable but not recapturable ACCURATE neo2 L valve (Boston Scientific, United States), which is equipped with stabilizing arches on the upper crown for the ascending aorta. The valve was then released and implanted under pacing, with an excellent final result. The patient’s condition improved (video 1 of the supplementary data) and he was discharged uneventfully 5 days later, requiring a permanent pacemaker.
This case illustrates the advantages of familiarity with various valves and their distinct implantation mechanisms to achieve the necessary stability for a proper anatomical match. We believe that proficiency in assembling and using multiple valves is key to addressing technically challenging and clinically complex situations.
We obtained the patient’s written informed consent for publication.
FUNDING
None.
ETHICAL CONSIDERATIONS
We obtained the patient’s written informed consent for publication. As this is a unique case, the variables of sex and gender of SAGER guidelines do not apply.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
No type of artificial intelligence or related technology was used in the writing of this article.
AUTHORS’ CONTRIBUTIONS
S. López Tejero and P. Antúnez Muiños drafted the manuscript. A. Diego-Nieto and G. Barreira-de Sousa reviewed the medical literature available and revised the manuscript. I. Cruz-González and J. Martín-Moreiras conceived the study design, analyzed its topic specifically, revised the manuscript, and were its main supervisors.
CONFLICTS OF INTEREST
I. Cruz-González is a proctor for Medtronic, Boston-Scientific, and New Valve Technology. The remaining authors report no conflicts of interest.
SUPPLEMENTARY DATA
Vídeo 1. López-Tejero S. DOI: 10.24875/RECICE.M23000398
Editorials
Transcatheter aortic valve replacement for noncalcified aortic regurgitation. Where are we now?
aServicio de Cardiología, Hospital Clínico Universitario, Valladolid, Spain
bCentro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), Spain
Original articles
Editorials
Vascular closure devices: the jury is still out
aUnidad de Hemodinámica, Servicio de Cardiología, Hospital General Universitario Dr. Balmis, Instituto de Investigación Sanitaria y Biomédica de Alicante (ISABIAL), Alicante, Spain
bDepartamento de Medicina Clínica, Universidad Miguel Hernández, Alicante, Spain
Original articles
Debate
Debate: Asymptomatic severe aortic stenosis: when should we intervene?
The clinician’s perspective
Servicio de Cardiología, Hospital Ramón y Cajal, Madrid, Spain
The interventional cardiologist’s perspective
Unidad de Cardiología Intervencionista, Hospital Álvaro Cunqueiro, Complejo Hospitalario Universitario de Vigo, Vigo, Pontevedra, Spain