To the Editor,
Transcatheter paravalvular leak (PVL) closure through the implantation of occlusion devices has become an alternative to surgery.1 The closure of aortic PVLs is usually performed through retrograde approach (via femoral artery and with echocardiographic and fluoroscopic guidance).2 There are times that the antegrade approach is necessary (via femoral vein through transseptal puncture, rarely transapical approach). However, certain cases require additional support using an arteriovenous loop. This is often done through the snaring of a wire introduced into the left atrium with a catheter that is advanced through the interatrial septum (transseptal).3-4 We describe a non-reported approach so far: the transcatheter closure of paravalvular leak using an arterio-arterial loop by passing the wire through the bioprosthesis and using radial access to snare the wire.
An 89 year old male was examined in our hospital for dyspnea on exertion. He had a past medical history of surgical aortic valve replacement with a Hancock-II bioprosthesis (Medtronic, United States) back in 2013. The transthoracic echocardiography performed revealed the presence of severe aortic regurgitation. The transesophageal echocardiography (TEE) performed assessed the mechanism of regurgitation. The TEE confirmed the presence of a very eccentric, severely calcified and crescent shaped 6-7mm PVL located underneath the right coronary sinus (figure 1A,B).
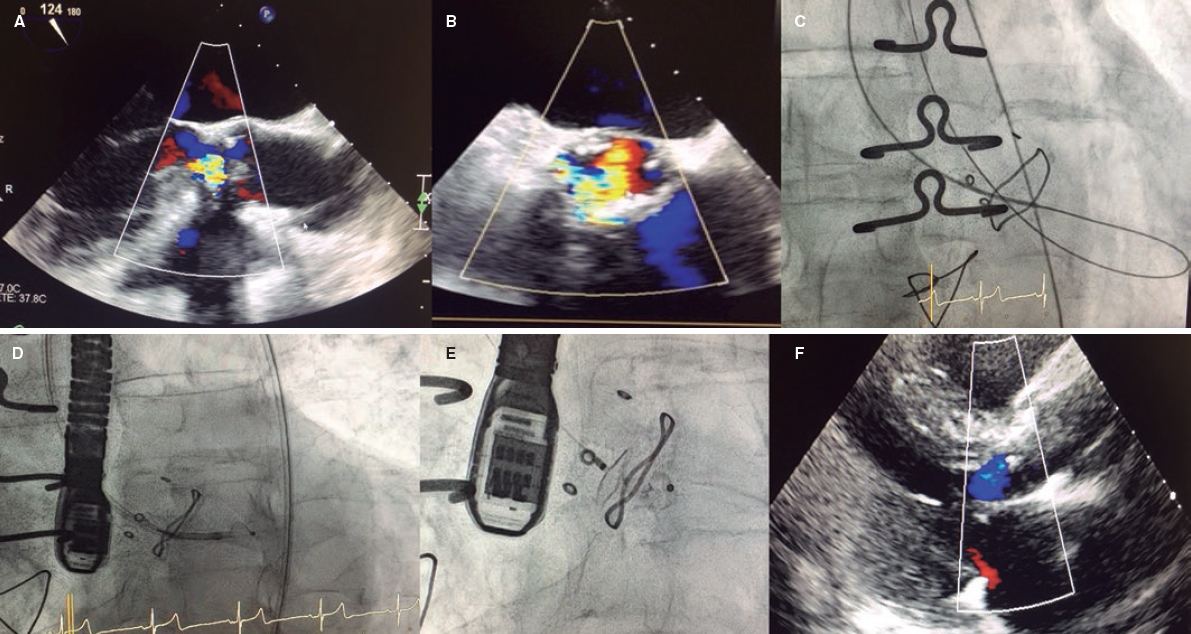
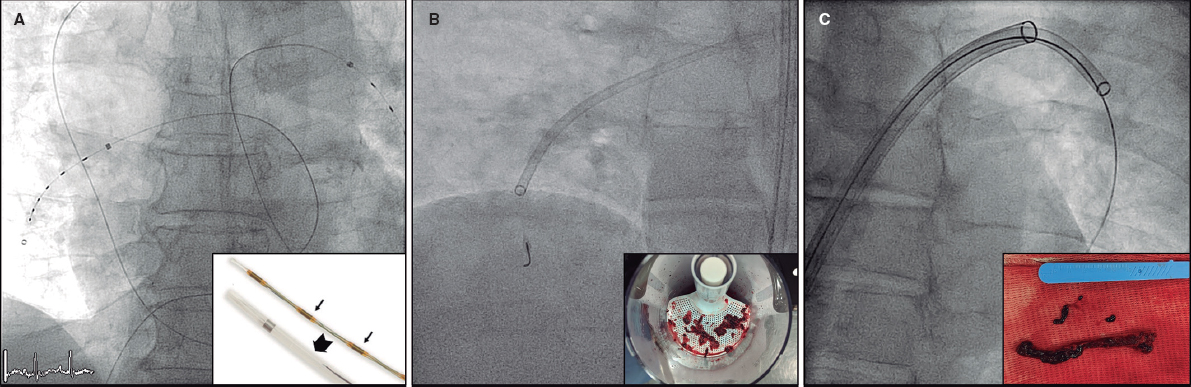
Figure 1. A and B: the tansesophageal echocardiography X-plane view revealed severe aortic regurgitation from a crescent-shaped PVL located underneath the sinus of the right coronary artery in the anterior position and surrounded by mild aortic root calcification. Also, see video 1 of the supplementary data. C: arterio-arterial loop in the left ventricle using the radial access to create the loop. D: 5-Fr 90 cm sheath crossing the leak. E: 10 × 5 mm Amplatzer Vascular Plug III delivered. F: 2-month follow-up transthoracic echocardiography showing mild aortic regurgitation.
According to the Society of Thoracic Surgeons risk calculator the estimated 30-day mortality surgical risk was 5.6% and the patient was considered mildly frail. The case was discussed by the heart team and a decision was made to use the transcatheter closure approach.
The closure of paravalvular leak was performed under general anesthesia and TEE guidance. The right femoral artery approach was used and the leak was crossed through the retrograde approach using a 5-Fr vertebral catheter and a hydrophilic guidewire. However, none of the catheters used (Judkins right 5-Fr, Multipurpose 5-Fr, Glidecath 4-Fr) could be advanced through the leak over the hydrophilic wire. It was decided to advance the hydrophilic guidewire from the left ventricle, cross the biological prosthesis, and place it in the ascending aorta. The wire was snared using an 18-30 mm snare (EN Snare Endovascular Snare Systems, Inc., United States) advanced through the right radial artery. Thus, the arterio-arterial loop was created from the femoral artery and through the aortic leak, aortic bioprosthesis, and radial artery (figure 1C).
This extra support allowed the retrograde 5-Fr Torquevue sheath progression (figure 1D). A 10 × 5 mm Amplatzer Vascular Plug III device (Abbott Medical, United States) was successfully deployed. The TEE confirmed the good positioning and effective closure of the PVL (figure 1E and video 1 of the supplementary data). The patient was discharged 4 days later after treatment with dual antiplatelet therapy (aspirin and clopidogrel). The patient improved significantly at the 2-month follow-up and the transthoracic echocardiography performed revealed the presence of mild aortic regurgitation (figure 1F).
The aortic closure of PVL has been routinely performed using the retrograde approach and femoral access. When additional support is required, an arterio-venous loop can be performed. This requires femoral venous access, transseptal puncture, and snaring of the wire placed in the left atrium, which increases time and procedural complexity. However, in our case the arterio-arterial loop was created easy and fast through the radial access after the hydrophilic guidewire was placed in the ascending aorta. When the loop is created the support for the advancement of large sheaths grows significantly as our case confirmed and the occlusion device can be successfully deployed. It should be noted that these Amplatzer vascular plug III devices can be delivered through smaller sheaths (5-or 6-Fr), which is an important advantage of this occlusion device.
The percutaneous closure of aortic PVL using radial access to release the occlusion device has been reported here.5 In our case, due to the presence of a very eccentric, severely calcified, and crescent shaped leak, we decided to create the loop to increase support and advance the catheter to release the occlusion device. Another case of closure of aortic PVL using an arterio-arterial loop has been reported. Unlike our case, Estevez-Loureiro et al.6 performed the closure of an aortic PVL in a CoreValve transcatheter aortic valve implantation using an arterio-arterial loop through femoral access to create the loop and add extra support. While reducing vascular complications, radial access also provides a better angle for loop traction.
The use of an arteriovenous rail is well known among practitioners of complex structural interventions. This case illustrates a novel approach to create an arterio-arterial loop easy and fast through the radial access. This technique could be useful in other difficult structural heart interventions.
SUPPLEMENTARY DATA
Video 1. Jiménez-Brítez G. DOI: 10.24875/RECICE.M20000101
REFERENCES
1. Millán X, Li CH, Arzamendi D. Percutaneous management of paravalvular leaks:an alternative to surgery or first-line therapy. Rev Esp Cardiol. 2020; 73:110-113.
2. Rihal CS, Sorajja P, Booker JD, Hagler DJ, Cabalka AK. Principles of percutaneous paravalvular leak closure. JACC Cardiovasc Interv. 2012;5:121-130.
3. Cruz-Gonzalez I, Rama-Merchan JC, Calvert PA, et al. Percutaneous Closure of Paravalvular Leaks:A Systematic Review. J Interv Cardiol. 2016;29:382-392.
4. Cruz-Gonzalez I, Rama-Merchan JC, Martín-Moreiras J, Rodríguez-Collado J, Arribas-Jimenez A. Percutaneous retrograde closure of mitral paravalvular leak in patients with mechanical aortic valve prostheses. Can J Cardiol. 2013;29:1531.e15-16.
5. Giacchi G, Freixa X, Hernández-Enríquez M, et al. Minimally Invasive Transradial Percutaneous Closure of Aortic Paravalvular Leaks:Following the Steps of Percutaneous Coronary Intervention. Can J Cardiol. 2016;32:1575.
6. Estévez-Loureiro R, Benito-González T, Gualis J, Pérez de Prado A, Cuellas C, Fernandez-Vazquez F. Percutaneous paravalvular leak closure after CoreValve transcatheter aortic valve implantation using an arterio-arterial loop. J Thorac Dis. 2017;9:E103-E108.
Corresponding author:Instituto del Corazón, Centro Médico Teknon - Quironsalud. Vilana 12 - 08022, Barcelona, Spain.
E-mail address: cardio.jimenezb@gmail.com (G. Jiménez-Brítez).