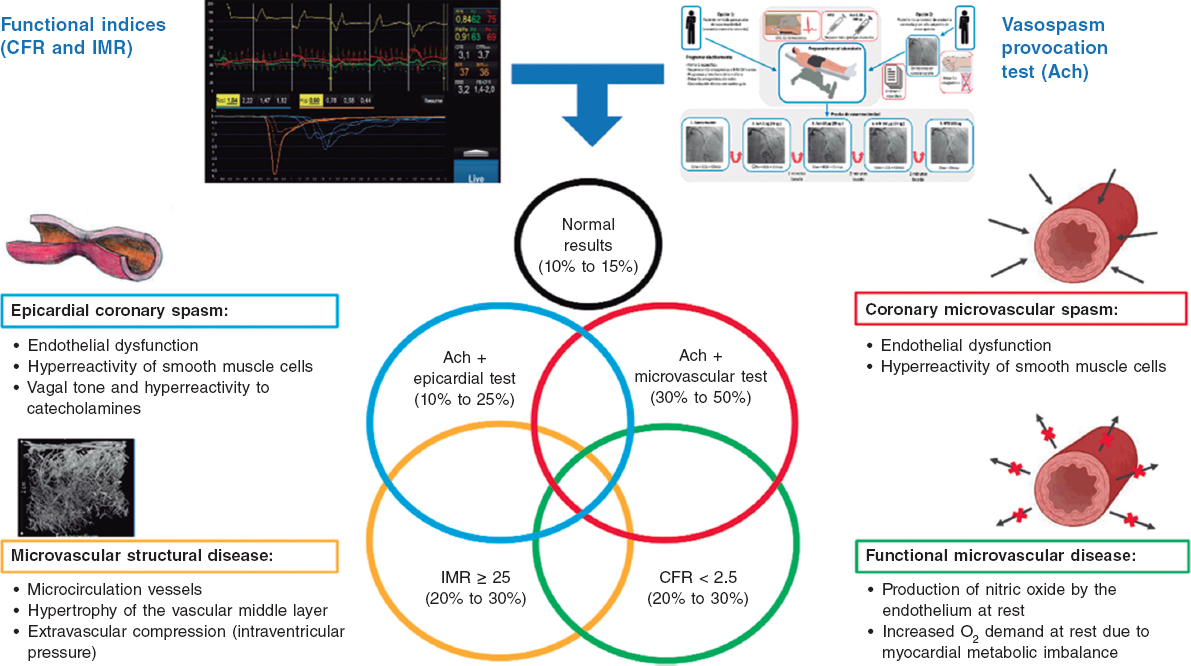
QUESTION: In your opinion, what conclusions can be drawn from the 2 ORBITA trials?1,2
ANSWER: The ORBITA trials focus on a specific aspect of the management of patients with acute coronary syndrome: the benefit in terms of symptom relief of angina.1,2 The first ORBITA trial1 is a double-blind, randomized, multicenter clinical trial, with 230 patients with severe single-vessel disease and ischemic symptoms that analyzed whether percutaneous coronary intervention (PCI) was associated with an improvement in angina-free exercise time compared with a placebo procedure.1 There were no statistically significant differences in the primary endpoint (differences in exercise increment on the stress test) at the 6-week follow-up between the 2 groups. The second ORBITA trial2, a double-blind, multicenter clinical trial, randomized 301 patients with exertional angina to undergo PCI or a placebo procedure.2 The methodology differs from that of ORBITA trial: all patients discontinued antianginal medication 2 weeks before randomization and were only included if they experienced angina throughout this period (assessed by a complex scoring system through a mobile application).3 Only patients with at least 1 severe coronary stenosis confirmed through physiological assessment were included; additionally, the 2 groups underwent the intervention (which was simulated in the group treated with the placebo procedure), and all patients received dual antiplatelet therapy. In total, 80% of patients had single-vessel disease, mostly involving the left anterior descending coronary artery, and complete revascularization was achieved in approximately 100% (using intracoronary imaging in 70% of PCIs). At the 12-week follow-up, patients treated with PCI experienced statistically significant greater angina relief, as well as improved exercise tolerance and quality of life than those in the placebo group.
Q.: What would be the key features of these 2 studies?
A.: Despite introducing the novel concept of simulating a PCI in the placebo group (thus avoiding the effect of attributing clinical improvement to the procedure per se), the main limitations of the first ORBITA trial were its small sample size and limited follow-up time. Moreover, the use of exercise tolerance with the stress test as the main study endpoint has been criticized due to its heterogeneity. Of note, 29% of patients had a negative functional flow reserve study (> 0.80), suggesting that there was no symptom improvement after PCI. Indeed, a prespecified substudy determined that, unlike angina (assessed by scores or exercise time), functional flow reserve did predict an improvement in ischemia (assessed by dobutamine stress echocardiography).4 All in all, the possible impact of this study on clinical practice seems limited.
Unlike the first trial, the main criticism of ORBITA-2—which evaluated patients with lesions in more than 1 vessel—is the discontinuation of antianginal treatment (ie, it compared PCI with patients without pharmacological treatment, unlike ORBITA, in which patients remained on optimal medical therapy), against the recommendation of clinical practice guidelines.5 Although the effect of PCI is expected to be immediate and sustained, the 12-week follow-up remains limited. Indeed, the main criticism that can be made of the study is its methodology: using a placebo procedure—not optimal medical therapy—for comparison may limit its clinical applicability. Nonetheless, the double-blind design of the study helps provide further evidence on PCI treatment in patients with coronary ischemia (both anatomical and functional) by improving the pathophysiology of the imbalance between oxygen supply and demand.
Q.: What do you think these 2 studies contribute compared with the much larger ISCHEMIA trial?
A.: In the context of chronic coronary syndrome, revascularization aims to provide 2 benefits: prognostic or symptomatic. In summary, the prognostic benefit of revascularization is well established in patients with severe left main or multivessel disease and left ventricular ejection fraction < 35%.5,6 However, there is more uncertainty surrounding the prognostic benefit in patients with extensive ischemic territory (a topic of discussion in the ISCHEMIA trial) and in evaluating the symptomatic benefit of the intervention regarding angina. The ISCHEMIA trial, with a larger sample size than the ORBITA trials, randomized a total of 5179 patients with stable coronary artery disease and moderate-to-severe ischemia on stress testing to an initial invasive or conservative strategy.7 After a median follow-up of 3.2 years, there were no significant differences between the 2 strategies in the primary endpoint (cardiovascular death, myocardial infarction, unstable angina hospitalization, heart failure, or resuscitated cardiac arrest). Although the multiple limitations of the study may affect the interpretation of its results (a high crossover rate between the 2 groups, up to 14% of the patients included in the study had mild or no ischemia, and the inclusion of perioperative infarctions which could bias the primary endpoint—more numerous in the invasive treatment group), patients randomized to the invasive treatment group showed greater symptomatic relief than those in the conservative treatment group. This benefit was greater in patients with more frequent episodes of angina at baseline and was less significant in asymptomatic patients, even with inducible ischemia.8
In my opinion, the main difference between the ORBITA and ISCHEMIA trials, beyond the sample size and limitations of the methodology of the former, is the blinding of patients undergoing invasive treatment in the ORBITA trials. Of note, symptoms are subjective and evaluating any intervention on cardiovascular events can have both a physiological component and a placebo effect. Therefore, we should welcome invasive studies to simulate the procedure in the control group because they allow testing the direct effect of the intervention on subjective endpoints, such as angina relief.
Q.: Based on all this evidence, what are the benefits, if any, of the invasive approach over the conservative approach?
A.: Current clinical practice guidelines (while awaiting the 2024 update from the European Society of Cardiology on the management of chronic coronary syndrome) state that the PCI should be reserved for patients who, despite being on optimal medical therapy, exhibit refractory symptoms,5,6 and the aforementioned evidence does not indicate the need to change this indication. The ORBITA trials have demonstrated that the relationship between epicardial coronary stenosis, ischemia, and symptoms is more complex than we had initially thought, while the ISCHEMIA trial has revealed the questionable impact of relieving ischemia on the incidence of events. Indeed, the severity of ischemia is a reflection of the burden of atherosclerotic disease, which is why only revascularizing the identified blockages will not have any clinical impact, as the intervention cannot change the underlying process.9 Moreover, an important point that should be made is that up to one-third of patients still experience angina symptoms despite successful revascularization.10 In this scenario, even the cost-effectiveness of the invasive approach vs optimal medical therapy remains to be elucidated.11 Therefore, beyond revascularization per se, an invasive hemodynamic study can provide valuable information to confirm the mechanism of ischemia (microcirculation abnormalities, vasomotor dysfunction, etc) and help optimize pharmacological treatment.
Q.: What indications do you take into consideration in your routine clinical practice to decide which invasive approach you should use in patients with stable angina?
A.: Setting aside scenarios where revascularization has previously shown prognostic improvement, as mentioned earlier, it seems reasonable to believe that the gold standard for stable angina should be pharmacological therapy. However, the fact that stable angina is a chronic disease, and the patient requires long-term antianginal drugs can complicate proper symptom control. Additionally, factors such as poor medication tolerability, suboptimal adherence, or the patient’s own preference must be considered. In all these situations, the invasive approach may be the preferred option.
FUNDING
None declared.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
Not used.
CONFLICTS OF INTEREST
None declared.
REFERENCES
1. Al-Lamee R, Thompson D, Dehbi H-M, et al. Percutaneous coronary intervention in stable angina (ORBITA):a double-blind, randomised controlled trial. Lancet. 2018;391:31-40.
2. Rajkumar CA, Foley MJ, Ahmed-Jushuf F, et al. A Placebo-Controlled Trial of Percutaneous Coronary Intervention for Stable Angina. N Engl J Med. 2023;389:2319-2330.
3. Nowbar AN, Rajkumar C, Foley M, et al. A double-blind randomised placebo-controlled trial of percutaneous coronary intervention for the relief of stable angina without antianginal medications:design and rationale of the ORBITA-2 trial. EuroIntervention. 2022;17:1490-1497.
4. Al-Lamee R, Howard JP, Shun-Shin MJ, et al. Fractional Flow Reserve and Instantaneous Wave-Free Ratio as Predictors of the Placebo-Controlled Response to Percutaneous Coronary Intervention in Stable Single-Vessel Coronary Artery Disease. Circulation. 2018;138:1780-1792.
5. Knuuti J, Wijns W, Saraste A, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407-477.
6. Lawton JS, Tamis-Holland JE, Bangalore S, et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization:A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e18-e114.
7. Maron DJ, Hochman JS, Reynolds HR, et al. Initial Invasive or Conservative Strategy for Stable Coronary Disease. N Engl J Med. 2020;382:1395-1407.
8. Spertus JA, Jones PG, Maron DJ, et al. Health-Status Outcomes with Invasive or Conservative Care in Coronary Disease. N Engl J Med. 2020;382:1408-1419.
9. Teoh Z, Al-Lamee RK. COURAGE, ORBITA, and ISCHEMIA. Percutaneous Coronary Intervention for Stable Coronary Artery Disease. Intervent Cardiol Clin. 2020;9:469-482.
10. Arnold SV, Jang J-S, Tang F, Graham G, Cohen DJ, Spertus JA. Prediction of residual angina after percutaneous coronary intervention. Eur Heart J Qual Care Clin Oucomes. 2015;1:23-30.
11. McCreanor V, Nowbar A, Rajkumar C, et al. Cost-effectiveness analysis of percutaneous coronary intervention for single-vessel coronary artery disease:an economic evaluation of the ORBITA trial. BMJ Open. 2021;11:e044054.