ABSTRACT
On May 26, 2021, the European Medical Device Regulation (EU-MDR) entered into effect resulting in a major shift in the requirements for assessment of medical devices in Europe. The EU-MDR Cardiovascular Collaboratory (EU-MCVC) was founded to contribute to the development of faster, more efficient, and more effective pathways for innovation of cardiac medical devices. A registry is an organized system that collects uniform data and evaluates specified outcomes in a population defined by a disease, condition, or exposure. Most registries have been created to improve the quality of care and provide feedback to physicians, hospitals, and health providers. Clinical registries represent an ideal construct for scientific, clinical, and policy-making collaboration. We describe diverse experiences from 5 European countries and address the traditional quality components in clinical trials. Continued collaboration is expected among academics, clinical trialists, patient representatives, regulatory experts, research organizations, registry platforms, regulatory bodies, and industry partners. Data quality is a primary concern and registry leaders need to optimize data quality to become regulatory compliant. A collaborative approach among medical device stakeholders may improve quality of care, reduce costs, and provide faster access to innovative technologies, with the common objective of improving cardiovascular care and outcomes.
Keywords: Regulatory science. Clinical registries. Clinical trials.
RESUMEN
El 26 de mayo de 2021 entró en vigor el Reglamento Europeo de Productos Sanitarios (EU-MDR), que supuso un importante cambio en los requisitos de evaluación de los productos sanitarios en Europa. El EU-MDR Cardiovascular Collaboratory (EU-MCVC) se fundó con el fin de contribuir al desarrollo de vías más rápidas, eficientes y eficaces para la innovación de productos sanitarios cardiacos. Un registro es un sistema organizado que recoge datos uniformes y evalúa resultados específicos en una población definida por una enfermedad, afección o exposición. La mayoría de los registros se han desarrollado para mejorar la calidad de la atención y proporcionar información a médicos, hospitales y proveedores de servicios sanitarios. Los registros clínicos representan una construcción ideal para la colaboración científica, clínica y política. Describimos diversas experiencias de 5 países europeos y abordamos los componentes de calidad tradicionales en los ensayos clínicos. Se espera una colaboración continua entre académicos, especialistas en ensayos clínicos, representantes de pacientes, expertos en regulación, organizaciones de investigación, plataformas de registros, organismos reguladores y socios de la industria. La calidad de los datos es una preocupación primordial y los responsables de los registros deben optimizarla para cumplir con la normativa. Un enfoque colaborativo entre las partes interesadas en los dispositivos médicos puede mejorar la calidad de la atención, reducir los costes y proporcionar un acceso más rápido a tecnologías innovadoras, con el objetivo común de mejorar la atención y los resultados cardiovasculares.
Palabras clave: Ciencia reguladora. Registros clínicos. Ensayos clínicos.
Abbreviations EMA: European Medicines Agency. EU-MCVC: European Medical Device Regulation Cardiovascular Collaboratory. EU-MDR: European Medical Device Regulation. PMCF: Post-marketing clinical follow-up. RCT: Randomized controlled trial.
INTRODUCTION
On May 26, 2021, the European Medical Device Regulation (EU-MDR) was enacted and the European Union underwent a major shift in the requirements for research and development of medical devices.1 This coordinated regulatory upgrade, however, allowed each European country to adopt the regulation as understood locally, which introduced steep learning curves. Ethics committees, competent authorities, notified bodies, academic and nonacademic health institutions, as well as contract research organizations experienced delays, longer waiting times, increased workload, and loss of effectiveness. This resulted in some cases in manufacturers deciding to deprioritize Europe as a potential location for the development of new therapies. Three years after the implementation of EU-MDR, the learning curves have been overcome and Europe has been reprioritized. Nonetheless, increased requirements and higher costs call for alternative pathways for generating regulatory data.
A pertinent upgrade in this regulation is the need for manufacturers to conduct postmarket clinical follow-up (PMCF) activities requiring the collection of clinical data on the use of devices that are already commercially available. The purpose reflects the desire to confirm the safety and performance requirements under normal conditions of the intended use of the device, the evaluation of potentially rare adverse effects and the assurance that the risk-benefit, specific for each device, remains favorable.1 Although postmarketing studies were common under the previous directive, the EU-MDR makes them mandatory. Beyond the financial consequences, these requirements inevitably result in an increased workload in the hospitals where the devices are used and/or implemented. This additional workload could potentially be mitigated by the establishment of public-private partnerships for efficient, effective, and high-quality data collection and reporting.
The successful management of cardiac conditions requires the use or implementation of medical devices, and the EU-MDR has had a fundamental impact on access to research, innovation, and improved therapies in European cardiology. In May 2023, the EU-MDR Cardiovascular Collaboratory (EU-MCVC) was initiated by Cardialysis (Rotterdam, The Netherlands) and established as an informal, voluntary, pro-bono international expert network bringing together European academics, clinical trialists, and regulatory experts to collaborate with clinical research stakeholders, both regionally and globally.2 The purpose of EU-MCVC is to create a dynamic and open conversation to facilitate, in real time, effective implementation of clinical research in Europe with an emphasis on navigating the EU-MDR. Relevant stakeholders in this collaboration are cardiovascular research organizations, registry platforms, regulatory bodies, and industry partners.
The priority focus in 2023 to 2024 is the definition, requirements, and establishment of efficient, effective, and high-quality cardiovascular clinical registries as a valuable pathway for PMCF data collection. This article addresses the following 4 topics: a) the definition of registries and considerations related to informed consent; b) perspectives from 5 European leaders on the establishment and performance of clinical registries; c) the interplay between traditional clinical trial quality processes and clinical registries; d) registry data requirements and their potential and current use.
This perspectives document was drafted on the basis of voluntary contributions from all authors. The manuscript generation process had 2 components: a) a hybrid think-tank organized by EU-MCVC and Cardialysis, with faculty members attending primarily in-person (11/13), on September 8, 2023 in Rotterdam, The Netherlands; and b) the compilation of presentations, discussions, and conclusions, in a draft document that was critically reviewed and expanded by each of the authors.
Definition of clinical registries
The European Medicines Agency (EMA), the United States of America Food and Drug and the International Medical Device Regulators Forum provide guidance on defining clinical registries (table 1).3-6 The common components of these definitions describe a registry as an organized system that collects uniform data and evaluates specified outcomes in a population defined by a disease, condition, or exposure. The International Medical Device Regulators Forum definition has a focus on quality of patient care, and thus requires a reasonably generalizable size, which would be most useful for informing policy decision-making. The United States of America Food and Drug Administration definition, however, adapts the goals to either scientific, clinical, or policy purposes. The EMA definition emphasizes the need to center the definition on the patient level, highlighting the focus of the registry on health information.
Table 1. Defining clinical registries
| International Medical Device Regulators Forum (IMDRF) Definition |
| A registry is an organized system that continuously and consistently collects relevant data in conjunction with routine clinical care, evaluates meaningful outcomes, and comprehensively covers the population defined by exposure to particular device(s) at a reasonably generalizable scale (eg, international, regional, health system) with the primary aim of improving the quality of patient care |
| United States of America Food and Drug Administration (US FDA) Definition |
| A registry is an organized system that uses observational study methods to collect uniform data (clinical and other) to evaluate specified outcomes for a population defined by a particular disease, condition, or exposure, and that services one or more predetermined scientific, clinical, or policy purposes |
| European Medicines Agency (EMA) Definition “Patient Registry” |
| Organized system that collects uniform data (clinical and other) to identify specified outcomes for a population defined by a particular disease, condition, or exposure. The term ‘patient’ highlights the focus of the registry on health information. It is broadly defined and may include patients with a certain disease, pregnant or lactating women or individuals presenting with another condition such as a birth defect or a molecular or genomic feature |
| EU-MDR Cardiovascular Collaboratory (EU-MCVC) elements of a common clinical registry definition |
| Organized system |
| Collects uniform (continuously and consistently) data (clinical and other) |
| Evaluates specified (meaningful) outcomes |
| Population defined by a disease, condition, or exposure |
| EU-MCVC perspectives on clinical registry sizes |
| Single-center vs multicenter clinical registry |
| Exhaustive (all centers) vs nonexhaustive (selected centers) national clinical registry |
| Exhaustive (all centers) vs nonexhaustive (selected centers) international clinical registry |
| Clinical registry networks (multiple registries merging independent databases, either at patient-level or at registry-level) |
When targeting EU-MDR requirements, a population is defined by exposure to a specific device, which has important consequences for the setting up of registry platforms in Europe. EMA defines at least 3 registry categories that, in ideal circumstances, could be interconnected. First, the EMA defines a disease registry as a patient registry whose participants are defined by a particular disease or disease-related patient characteristics, regardless of their exposure to therapies. A disease registry is purely observational. Second, the EMA defines a registry-based study as an investigation of a research question using a patient population within a patient registry. The interpretation of the EU-MCVC is that this refers either to investigational interventions or when the clinical investigation requires additional invasive or burdensome procedures or follow-up rules. A purely observational or descriptive analysis should ideally be defined within the umbrella of a disease registry. Third, the EMA refers to product or device registries, which generally apply to PMCF studies. PMCF studies are required to follow the regulations that apply to traditional clinical trials (eg, single-arm study) under MDR, unless no additional invasive or burdensome procedures are incorporated in the registry protocol.
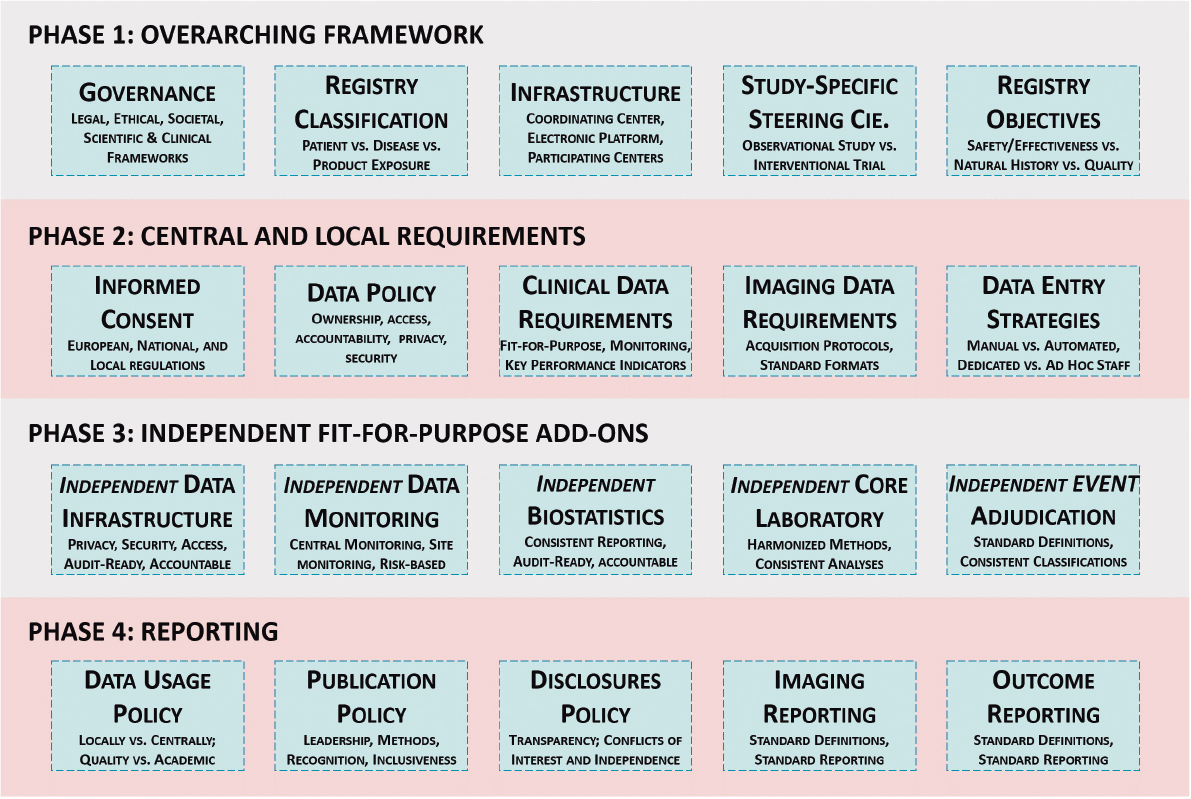
The development of sustainable clinical registries may improve the quality of care, reduce costs, and provide faster access to better therapies. Figure 1 presents the conceptual framework for the implementation of a clinical registry.
Figure 1. Conceptual framework for the implementation of a clinical registry. Phase 1 requires building a legal and scientific framework, as well as setting up agreements and designing the overall distribution of tasks among collaborators. Phase 2 touches upon the design and implementation of the registry, where most attention is paid to data requirements, and data quality should be a common denominator. Phase 3 presents optional activities to be provided by independent parties to increase consistency, quality, and long-term reliability. Phase 4 must be readily documented and available when results are expected. All phases shall be discussed and implemented simultaneously as the final product requires having assessed these 20 components. Detailed written documentation of agreements are to be held by the executive committee of the registry. All components of phases 1, 2 and 4 are required. Phase 3 components are optional.
Background of the utopian all-comers design and how registries may be the answer
Randomized controlled trials (RCTs) are the gold standard for evaluating the safety and efficacy of medical interventions, as, by design, they eliminate confounding factors as much as possible. RCTs are the standard for premarket evaluation, and typically select a narrow population by means of carefully selected eligibility criteria, which has 4 consequences: a) most confounders will be avoided and the purest possible estimates of “therapy effects” will be achieved; b) strict eligibility criteria may make it difficult to enroll patients, requiring more centers and more time to complete enrollment, although typically these studies require smaller sample sizes; c) the limited external validity of the intervention effect estimate, due to the highly selected population will require subsequent studies in larger populations, usually in the postmarket setting; and d) the design allows only limited information on potential rare adverse effects.
As a possible solution to challenges 2 and 3 above, the “all-comers design” was introduced, characterized by having simple eligibility criteria. This approach facilitates the enrollment of a more representative patient population. However, in a trial evaluating coronary stents,7 at least 50% of eligible patients were still not enrolled after screening. The main reasons were related to the informed consent process (33% inability to provide informed consent, 19% refused to provide consent) and 27% did not meet the eligibility criteria. Furthermore, those who were not enrolled had poorer outcomes. Such observations have been replicated in many subsequent publications. Other potential issues to consider in the all-comers approach are: a) the addition of uncontrolled confounding factors that may lead to a ‘dilution’ of the therapy effect (initially designed for a specific and selected population) and an observed null-effect in a randomized comparison; and b) investigators tend to exclude the most severe presentations (eg, heart failure, cardiogenic shock). Thus, the all-comers approach still remains selective. The advent of registry-based research offers a unique opportunity to collect data on all patients, especially in purely observational studies, and to better understand outcomes in all subpopulations, particularly those traditionally excluded from clinical trials. The view of the EU-MCVC is that unselected populations should not be considered for early randomized comparisons unless a device is expected to benefit an unselected population. In contrast, if the effect of an intervention is primarily expected in a subgroup of patients, these subgroups of patients should be investigated first instead of launching an all-comers approach as the initial approach.
Informed consent
Patients who are admitted to or registered in a health care institution are not automatically aware that their clinical data may be used in multiple manners. However, they will generally presume that the most important function of health care data, as it relates to them personally, is to enable health care professionals to offer them the best care possible, to improve their well-being, quality of life, and life expectancy. However, depending on local health care frameworks, other users of their data can be insurance companies, national databases, partner organizations (eg, hospital networks), and quality-of-care databases.
When invited to participate in traditional clinical trials, patients should be informed in detail of the objectives of the study, the potential associated risks and benefits, extra burdens or commitments, and any other potentially relevant information. Under the EU General Data Protection Regulation, patients not only need to voluntarily provide their informed consent to enroll in a clinical investigation, but also need to voluntarily provide permission for each specific use of their data and may withdraw their consent at any time.1 For the purposes of clinical trials and patient registries, patient data are typically coded or pseudonymized, which ensures that their personal identifiers will not be shared outside their treating health care institution. The use of coded personal data complies with the privacy requirements of the EU General Data Protection Regulation.
The question of whether informed consent is required for enrolling patients in a patient registry hinges on whether registry data collection is part of the standard of care (eg, quality registry) and defined in the terms and conditions of the institution, or whether the registry is beyond the scope of the standard of care. In the former, the registry may be part of the patient health care records, and institutional and regulatory national conditions may not require a registry-specific consent. In the latter, patients should be consented before entering a patient registry. EU-MCVC recommends always liaising with the local ethics committee to define the need for informed consent in the setting of a clinical registry.
Patient registries are expected to be purely observational. In the case of interventional registry studies (ie, with an experimental intervention) or in observational studies with additional invasive or burdensome procedures or follow-up rules, it is generally accepted that patients must be invited to participate and sign an informed consent form. Purely observational registries may also require the informed consent process depending on its objectives, and national and local requirements. An informed consent form for observational registries should clearly state that all coded data being collected might be used for multiple observational data analyses (either locally or in the full registry database), for which the patient will not be reconsented. Patients always retain the right to withdraw their informed consent.
Cardiovascular clinical registries: The Spanish experience
Spain has a long tradition of registry-based data collection in interventional cardiology under the motto “unity makes strength” in a population of more than 47 million people.8 With one of the oldest interventional cardiology quality registries, the community has delivered impactful data based on registries. A nationwide registry in interventional cardiology has been published without interruption since 1990, collecting data through extensive surveys that are completed annually and reflect the volume of activity rather than patient-level data.9,10 Consequently, from a research perspective, its value is highly limited.
Patient-level registries started as academic collaboration among colleagues who voluntarily and without external funding developed common databases to collect interventional cardiology procedural data and clinical outcomes at follow-up. Efforts started in 2004 and evolved from single-center registries to a multimodal academic interactive network. These voluntary contributions meant countless hours of structured data entry and follow-up plans. A salient example is the seminal article published in 2008 on stent thrombosis that included 23 500 patients enrolled in 20 Spanish centers.11 Additional registries conducted in subsequent years led to more than 10 publications. From observational registries, this network expanded into randomized studies and interventional registries, with growing international collaborations.
In recognition of a clear trend toward collaborative research in the setting of complex disease and complex therapeutic approaches, the Spanish multimodal network has evolved into the EPIC Foundation (Education and Promotion of Investigation in Cardiovascular disease). EPIC was founded in 2016 and is currently engaged in academic research, industry-sponsored research, and observational studies with a track record of 47 projects, including 11 PMCF and Post-market surveillance under MDR. Currently, each registry is set up as a clinical study, following the traditional rules of ISO14155:2020, and including informed consent from patients. EPIC is expanding its capabilities in regulatory research in collaboration with local and international partners. Registries are funded by national grants or by industry. As an independent organization overseen by interventional cardiologists, EPIC is the only such platform in Spain as there is no national registry platform funded by the health authorities.
Cardiovascular clinical registries: The Belgian experience
Belgium has highly favorable conditions for registry-based research in a population of more than 11 million people. Notably, it is one of the rare examples where registry data collection is mandatory and funded according to national standard-of-care requirements. However, it also exemplifies the challenges related to governance in order to address the 3 main areas of interest as defined by the United States of America Food and Drug Administration: scientific, clinical, and policy-making. The Belgian interventional cardiology registry started as a physician-driven initiative that aimed to assess the effectiveness of therapies, regional disparities, and adherence to guidelines in order to improve patient outcomes and to advance scientific research.
In 1996 the Belgian working group of interventional cardiology started collecting a limited set of clinical and procedural data (by fax), which did not meet scientific rigor, as occurs with other quality-of-care registries. Since 2006, data entry shifted to a database hosted by the European Society of Cardiology, but owned and managed by the Belgian working group of interventional cardiology. In 2012, the Quality Electronic Registration of Medical acts, Implants and Devices (Qermid) database, hosted by health authorities, came into effect.12 Data completion is mandatory for reimbursement of procedures and devices. This allows collection of ~100% of procedures but adds a new administrative burden.
Qermid collects data for policy-making and quality-of-care, but currently the registry is not led or managed by the scientific community. This creates a paradox, where the ideal situation (full data collection) exists, but insufficient scientific advantage is taken from such a valuable infrastructure. Moreover, there are no dedicated resources for on-site data entry (currently performed by physicians or assistants) and data are not sufficiently monitored. Nevertheless, through the Belgian working group of interventional cardiology and Qermid, Belgium has been able to publish reliable metrics for more than 27 years, and provide nationwide real-world data on complex procedures,13 and to elegantly describe the effect of the COVID-19 pandemic.14
Cardiovascular clinical registries: The Swedish experience
The Swedish Coronary Angiography and Angioplasty Registry (SCAAR), within the Swedish Web-system for Enhancement and Development of Evidence-based care in Heart disease Evaluated According to Recommended Therapies (SWEDEHEART) platform, is seen as a role model among coronary registries.15 The registry was founded voluntarily by physicians to improve quality of care. Data collection is nationwide, data entry is required for all procedures, and the health care system now supports the infrastructure. Moreover, Sweden mesmerized the cardiovascular community with the very first investigator-initiated, registry-based RCTs, published in top tier journals.16,17 With a population of more than 10 million people, the SWEDEHEART quality registries capture over 80 000 procedures on a yearly basis. Data collection includes baseline, procedural, and outcome data, amounting to more than 300 variables on average. SWEDEHEART offers high compliance with > 95% data agreement when monitoring activities are performed.15
SWEDEHEART has certain characteristics that create ideal conditions. First, all patients who are treated at a hospital are included in the registry. Informed consent to enter the registry is not needed, but if the patient decides to leave the registry, it is still possible to opt out. Second, a common patient identifier is used that allows the merging of multiple national databases, making the scope of data availability much wider than in a hospital-based registry (table 2). This allows for indefinite follow-up unless a patient leaves the country; the governance infrastructure takes into consideration the 3 elements (ie, scientific, clinical, or policy); more specifically, the process allows for feedback to the staff, the leadership, the patients, and the public.
Table 2. Connected databases that build up SWEDEHEART
| Disease specific databases (eg, SCAAR for PCI) |
| Registries at the National Board of Health and Welfare |
| The national registry of causes of death |
| The national patient register (all ICD codes, all admissions since 1987) |
| The Swedish prescribed drug register (all dispensed drugs since 2005) |
| Central Bureau of Statistics (eg, marital status, country of birth, income, educational level) |
| The Swedish Social Insurance Agency (sick leave) |
| Other National Quality Registers (about 100 at present) |
|
ICD, International Classification of Diseases; PCI, percutaneous coronary intervention; SCAAR, Swedish Coronary Angiography and Angioplasty Registry. |
Since 2018, SCAAR/SWEDEHEART have been approached by the medical device industry to support regulatory reports in the context of ongoing clinical follow-up and completeness of data. Since then, SCAAR/SWEDEHEART have been able to support most of the major PCI device companies with MDR reports. The existing experience allows for predefined reports, either at patient-level or device-level, as well as in patient subgroups (eg, older adults, diabetes) or lesion subgroups (eg, small vessels, long lesions). This platform is especially interesting for devices that are not commonly used (eg, left main devices, small stents). Currently the SWEDEHEART model is expanding in Europe through the European Society of Cardiology (ESC)-driven EuroHeart program. Some countries already had similar registries on different platforms, which is why one of the first important steps was to establish common data standards.18
Cardiovascular clinical registries: the Icelandic experience
Iceland has a population of around 400 000 people, and has an interventional cardiology center at Landspitali University Hospital (Reykjavik). This center has collaborated in SCAAR/SWEDEHEART since 2008, with prospective data collection. As a single-center experience and outside Sweden, Dr Guðmundsdóttir confirms that data collection in the SCAAR database is time-efficient and viewed as part of patients’ essential health records. Patient and procedural data are entered by the treating physician and cath lab nurses immediately at the time of the procedure, which is accurate and time-efficient, given the simplified approach for data collection. The registry includes all treated patients, allows quality-of-care assessments, and represents a pathway for participation in multicenter registry-based clinical trials. The registry enables easy access to all Icelandic data for local research and quality control. However, a couple of the challenges observed are the following: a) Icelandic databases are not integrated as in the case of Sweden (table 2), and b) data aggregation and data sharing can be complex. Since routine data entry into the registry is seen as a part of patient care, it does not require informed consent. However, in the case of registry-based studies, ethics committee approval is a requirement and signed informed consent must be obtained from each participant. Iceland is collaborating in the EuroHeart program by providing data but is not currently using the EuroHeart platform.
Cardiovascular clinical registries: the Leiden experience on a noninvasive imaging databank
Leiden University was founded in 1575 and is the oldest university in The Netherlands. Leiden University Medical Center is highly involved with innovation and development, collaborating with organizations locally and globally. Its cardiology department is no exception and collaborates with 18 countries in research, PhD programs, and postgraduate training. In this environment, and due to adequate infrastructure and leadership, a powerful noninvasive imaging databank was established prospectively in 2000 and has collected retrospective data since 1990. By using standardized acquisition protocols according to care tracks (eg, patient disease or condition) and dedicated analysis efforts (powered by extensive work by research fellows and faculty), Leiden has offered the global scientific community a better understanding of disease natural history, identified populations at higher risk, and informed the design of clinical trials.
The Leiden experience offers 5 important lessons: a) individual centers collect a wealth of data that, if used properly, can change our understanding of disease and its management; b) consistent acquisition and analysis methodologies are required to compare data over time and, by spending time on a good acquisition, facilitate all future efforts; c) to adequately analyze these enormous amounts of data, countless hours are needed, which is facilitated by well-organized PhD programs; and d) collaboration among international imaging registries is especially powerful for less prevalent conditions and is most productive when good and high-volume centers are selected, standardized evaluation is in place, databases are well-organized, there are engaging professionals ready to grow in their academic career, and integration of multi-modality imaging techniques creates better possibilities to answer clinically relevant questions. Some examples include Leiden’s experience of moderate aortic stenosis, bicuspid aortic valve disease, and acute myocardial infarction.19-21
Cardiovascular clinical registries: the European Cardiovascular Research Institute-Cardialysis Perspective
The European Cardiovascular Research Institute is a foundation bringing together a community of top clinical researchers and private/public partners in order to perform clinical investigations that improve cardiovascular health care. Since 2012, the European Cardiovascular Research Institute has performed some of the most ambitious European interventional cardiology trials, enrolling almost 30 000 participants and providing high-quality data that have impacted clinical guidelines around the globe. As an academic research organization, the European Cardiovascular Research Institute partners with Cardialysis, which is a quality-oriented, independent cardiac imaging core laboratory and a cardiology-focused research organization. Cardialysis has the largest track record on the conduct of interventional cardiology trials in Europe with more than 400 studies completed in 40 years with a total enrollment of more than 200 000 patients.2 In this context, Cardialysis has experienced increased demand for both industry-initiated and investigator-initiated registries since 2021, in which it is pivotal to develop awareness and common acceptance of the quality required for various purposes (eg, premarket approval, postmarket follow-up, scientific research, guidelines). It has become a priority to define how registry platforms may be supported externally with specific quality components. The term ‘Externally-Supported Clinical Registries’ was introduced at the EU-MCVC’s first think tank and refers to registry networks that use independent providers to boost the quality of the registry, depending on the objectives.
A call for quality and multi-stakeholder engagement
Cardiology is characterized by its very high standards in clinical research. Most research questions in cardiology are simple, binary comparisons. However, the wealth of information required to plan an adequate binary comparison leads to high complexity and requires the involvement of experts from different disciplines and backgrounds. Due to the latter, the establishment of standards has become an effective catalyst for innovation. These standards start with requirements from regulatory agencies,3-6 definitions and trial design principles,22 standard data elements, standard methodologies, and standard reporting. Failed adequately powered clinical trials continue to be a regular feature of the clinical trial landscape, as devices or strategies that held promise in initial trials with a limited number of patients sometimes have contradicting confirmatory data in subsequent larger trials. It is the view of EU-MCVC that confirmatory trials should be performed using high-quality standards. Methodological components that add quality to a clinical investigation are summarized in table 3.
Table 3. Methodological components that add quality to cardiovascular clinical investigations
| Trial design and protocol development according to international standards (eg, ISO 14155) |
| Use of standard definitions (eg, ARC definitions) |
| Independent and nonconflicted expert committees (eg, steering committees, clinical events committees, data and safety monitoring boards) |
| Independent and nonconflicted cardiac imaging core laboratories |
| Adequate site selection (eg, optimal research infrastructure) |
| Independent and nonconflicted site monitoring including data verification (eg, completeness, accuracy) |
| Consistent coding of adverse events (eg, MedDRA) |
| Regulatory-compliant electronic data capture system |
| Statistical analysis plan and predefined publication strategy |
| Independent statistical reporting or independent statistical validation |
| Timely use of public databases (eg, ClinicalTrials.gov) |
| Consistent quality assurance, regulatory compliance, and site audits |
| Clinical study reports according to international standards (eg, ISO 14155) |
|
ARC, Academic Research Consortium; ISO, International Organization for Standardization; MedDRA, Medical Dictionary for Regulatory Activities. |
In a recent systematic review, CORE-MD (Coordinating Research and Evidence for Medical Devices), published the results of their assessment of the 11 currently running European registries for coronary stents and transcatheter valve therapies.23 They concluded that there is wide heterogeneity and incomplete public transparency to structure and methods, and a need to create a minimum set of quality criteria. They reported that on average, data quality and completeness criteria were met in less than 20% of the registries and that data on safety and performance was adequately addressed in less than 30%. This information confirms that the priority remains to improve the quality of data collection and that broadly accepted metrics need to be developed.
A consideration requiring further investigation is the need and relative value of on-site monitoring activities and on-site audits in the context of clinical registries. Automated and centralized mechanisms of data monitoring may offer efficiency; however, the effect of site monitoring visits on data completeness and quality is unknown. In general terms, on-site monitoring has been used in sponsor-driven device registries, but has generally not been used in academically-driven patient registries.
Quality add-ons to traditional clinical registries
Independent core laboratory analyses
Establishing an independent core laboratory for a clinical trial increases quality by addressing the following quality requirements: a) optimizing image quality by developing a uniform image acquisition protocol for all participating centers. Adherence to the acquisition protocol may require confirmation that the image acquisition protocol was studied (or training received) and that a test-run is provided to confirm protocol adherence; b) ensuring that data are handled consistently (eg, pseudonymized, adequate privacy and security standards, adequate format, consistent analysis software); c) ensuring that data are analyzed consistently (eg, standard methodology, reproducible assessments, adequately trained personnel); d) ensuring that data adjudication is performed consistently (eg, regurgitation severity); and e) central availability of original datasets for regulatory audits.24
Imaging measurements and assessments obtained from real-world data (eg, site-reported) will not comply with the quality requirements mentioned in the prior paragraph and will be associated with increased variability of assessments and increased risk of investigator bias. For academic research, items 1 and 4 in the prior paragraph may be addressed, and the absence of the remainder may be acceptable as long as imaging data are not transferred outside the treating institution. For regulatory trials, however, all 5 are necessary, especially if imaging endpoints are part of the primary endpoint or main mechanism of action of the investigational device. In the setting of postmarket clinical registries, given the potential large number of patients, intermediate solutions need to be designed. Table 4 highlights the differences between a regulatory-compliant core laboratory and a local academic core laboratory.
Table 4. Requirements for academic vs regulatory-compliant imaging core laboratories
| Regulatory-compliant imaging studya | Local academic imaging research | |
|---|---|---|
| Image acquisition | ||
| Study-specific imaging manual/protocol | Y | N/Yb |
| Study-specific personnel training/qualification | Y | N/Yb |
| Dedicated resources for image acquisition | Y | N/Yb |
| Imaging data management | ||
| Anonymization of Protected Health Information | Y | Nc |
| Secure image e-Transfer system | Y | Nc |
| Adequate material handling and filing | Y | Nc |
| Quality feedback and queries handling | Y | N |
| Image analysis | ||
| Standardized analysis methodology (conforming to guidelines, accepted definitions, ensuring feasibility) | Y | N/Yb |
| Dedicated Image Workstation | Y | N/Yb |
| Primary reader – sonographer/imaging analyst | Y | N/Yb |
| Overread – imaging expert/supervisor | Y | N/Yb |
| Personnel training/qualification | Y | N/Yb |
| Reproducibility testing | Y | N |
| Imaging database | ||
| Validated, study-specific electronic case report form | Y | N |
| High data entry requirements (automatic worksheet upload and queries, Part 11 compliant, audit trail) | Y | N |
| Data source verification and quality control | Y | N |
| Data release procedure after data base lock | Y | N |
|
a Pamela Douglas – JASE. |
||
Independent endpoint adjudication
Establishing an independent clinical events committee (CEC) increases quality by addressing the following quality requirements: a) adherence to standard definitions to ascertain and classify adverse events that potentially meet the definition of an endpoint for a given study. Having an expert committee for a given trial also offers consistency in the classification of complex events, such as periprocedural myocardial infarction and heart failure events; b) ensuring that assessments are performed utilizing a similar amount of information (eg, consistent checklist of documents and imaging materials required for adjudication); c) central availability of original source documents for regulatory audits; and d) importantly, given that device indications are largely based on primary endpoints that are clinical, the CEC must be shown to be independent from the manufacturer of a given device and have no perceived conflicts of interest to perform the tasks.25
In the context of premarket approval, it is the view of EU-MCVC that an independent CEC committee should be in place for the 4 reasons explained in the previous paragraph. In the context of a cardiovascular registry, however, it appears that site-reported data, especially when reporting is complete and uses standard definitions, might be sufficient from a quality perspective. Scientifically, it remains to be proven whether clinical outcome data from registries are sufficient without a CEC in place. Furthermore, in the context of registry-based randomized studies currently being set-up for regulatory purposes, it is the opinion of the EU-MCVC that a CEC should be in place, and its use and validity explored prospectively.
All-cause mortality, however, does not usually need endpoint adjudication. Especially if the specific registry or study has access to national mortality databases. It is not known whether subclassifications of death can be reliably documented using site-reported data or whether a CEC will provide additional value. Other endpoints for which there are ongoing efforts to clarify whether adjudication is or is not beneficial are myocardial infarction and revascularization, when coded as binary (yes/no). In the view of the EU-MCVC, most other endpoints (eg, heart failure, bleeding, subtypes of myocardial infarction, stent thrombosis, stroke, unstable angina, unplanned revascularization) do exhibit an advantage when undergoing adjudication, although this needs to be proven prospectively.
An example of adjudication of clinical events in a registry-based RCT is the Bivalirudin vs Heparin Monotherapy in Myocardial Infarction (VALIDATE SWEDEHEART) trial, which was a proof-of-concept of this methodology.26 Furthermore, innovative adjudication approaches are being designed and tested with the aim of maintaining quality but lowering costs. For example, in the DAPA-MI trial, only death and heart failure hospitalization were adjudicated, while myocardial infarction, revascularization, and stroke were site-reported.27 Additional examples are presented in table 5.
Table 5. Use of endpoint adjudication in registry-based randomized clinical trials
| Study | Endpoint | Adjudication | Registry endpoints | Event trigger | Data collection | Other info |
|---|---|---|---|---|---|---|
| TASTE | All-cause death | No | Yes | N.A. | No | - |
| VALIDATE | MACE and major bleeding | Yes | N.A. | Site-reported | Yes, eCRF and hospital notes | - |
| DETOX | All-cause death | No | Yes | N.A. | No | - |
| iFR | MACE and major bleeding | Yes | N.A. | Site-reported | Yes, eCRF and hospital notes | Core Lab |
| HELP | Bleeding events | N.A. | Yes | N.A. | No | - |
| REDUCE | All-cause death and MI | N.A. | Yes | N.A. | No | - |
| Full REVASC | MI and unplanned revascularization | Yes | N.A. | SCAAR/Riks-HIA | Yes, eCRF and hospital notes | - |
| SPIRRIT | All-cause death and HF hospitalization | Yes | ICD codes | ICD codes and mortality register | Yes, eCRF and hospital notes | Simplified adjudication process |
| DAPA-MI | All-cause death and HF hospitalization | Yes | N.A. | Site-reported | Yes, eCRF and hospital notes | |
| INFINITY | Device-oriented composite endpoint | Yes | N.A. | Site-reported | Yes, eCRF and hospital notes | Core Lab |
|
eCRF, electronic case report form; HF, heart failure; ICD, International Classification of Diseases; MACE, major adverse cardiac events; MI, myocardial infarction; Riks-HIA, Register of Information and Knowledge About Swedish Heart Intensive Care Admissions; SCAAR, Swedish Coronary Angiography and Angioplasty Registry. |
||||||
Independent statistical analysis or validation
The assessment of appropriate trial databases goes beyond the locked statistical analysis database and includes a thorough assessment of a trial or registry design. From the perspective of a statistician, one must consider the study design, patient selection, choice of the comparator, regulatory compliance, description of the statistical methods, and finally both the assessment of the outcomes per se and the consistency among findings. The data quality gap between the evidence-based medicine paradigm and the real-world data paradigm is currently strikingly evident, and this is conceptually correct by design, given that real-world data refer to routinely collected data, which are by design of lower granularity and precision than clinical trial data. If real-world data are to be considered for use in regulatory pathways, they must comply with the following requirements: a) data sources are of demonstrated good quality; b) internal and external validity is expected; c) there is consistency across data sources; and d) data are adequate and precise. Regulatory documents using real-world data should also report on adjustment for potential confounders, identify potential for selection bias and information bias, describe how missing data are managed, and offer a robust data analysis.
Adequately designed and supported clinical registries offer multiple advantages from a clinical perspective, such as better insights into the natural history of diseases, better characterization of target populations, and the identification of new targets of therapies. In addition, registries offer the potential to introduce novel statistical approaches to pool and analyze data. When patient-level data are available within a single registry, traditional statistical approaches should be used, taking into account data limitations. When only registry-level data are available, meta-analytical methods can be implemented and may be used for policy decision-making or public health decision-making, but not for assessment of the safety, efficacy, or effectiveness or a device, which require the deepest granularity, which is not provided by registry-level meta-analytical data.
Role of clinical registries in European guidelines committees
The process of evidence generation that leads to the recommendations of the European clinical guidelines is well established and follows most robust standards, where adequately powered randomized controlled clinical trials represent the best source of information for decision-making. Ideally, a class IA recommendation should have more than one confirmatory, adequately powered clinical trial or at least one properly executed meta-analysis. In the absence of RCTs, however, other sources of data are used and ultimately contribute to the decision-making process of a committee.
With the aim of optimizing cardiovascular care and outcomes, the ESC has proposed a methodical approach for the development of quality indicators and, in collaboration with the European Unified Registries for Heart Care Evaluation and Randomized Trials (EuroHeart), has proposed data standards for acute coronary syndrome and PCI, transcatheter aortic valve implantation, heart failure, and atrial fibrillation/flutter catheter ablation.18 There has been rapid adoption with more than 40 000 cases of aggregated individual participant data collected in 2022 in 7 participating countries. This novel ecosystem is rapidly evolving and promises to have a high-impact on policy decision-making and public health priorities.
The existing strengths of adequately planned and supported clinical registries include being resource effective, offering a high representativeness, integration with clinical routine, and unselected consecutive recruitment. This setting, especially as implemented by SWEDEHEART, has opened pathways for registry-based RCTs that reduce workload, minimize selected bias, and provide better access for investigator-driven research and, recently, the opportunity for multinational trials. Continued research may better inform the scientific community and guideline committees on the use of registry-based RCTs results for decision-making, and their ultimate role in evidence-based medicine. A comparison between traditional registries, registry-based RCTs, and RCTs is available in table 6.
Table 6. Comparative table on the role of clinical registries in evidence-based medicine
| Registries | Registry-based randomized controlled trials | Traditional randomized controlled trials |
|---|---|---|
| Purely observational Not adequate to support a conclusion on efficacy |
Pragmatic Open-label evaluation of commonly used therapeutic alternatives in settings with existing registries |
Highest level of scientific evidence Gold standard for comparative studies |
| True all-comers – representativeness Provide data on power calculation Clinically meaningful results Low risk populations Low frequency events |
True all-comers – representativeness Provides information on characteristics and follow-up of patients randomized and noneligible individuals |
Select eligible patients Attainment of patient consent Random assignment for treatment Control for confounders Detection and adjudication of clinical endpoints |
| Hypothesis generating | Causal inference To evaluate treatments, strategies, and devices or acute-phase pharmacological agents Evaluation of pharmaceutical agents for new indications |
Causal inference |
| Resource effective | Low cost | Resource intense |
Limitations
The information presented and the organizations represented in this manuscript are limited to the experience of the participants of the Think Tank that took place in Rotterdam (September 8, 2023) and describes the perspectives of the coauthors. This is neither a consensus document nor a systematic review. Information on other organizations or countries involved in developing or currently running interventional cardiology registries was not captured or represented. The EU-MCVC welcomes voluntary participation of other established cardiovascular research organizations and/or cardiovascular societies, and may be contacted through the corresponding author.
CONCLUSIONS
The EU-MDR has increased requirements for postmarket follow-up activities to be performed by all device manufacturers that market medical devices in Europe. Adequately planned and supported clinical registries have the potential to address the additional requirements by creating collaborative frameworks. Data quality is a primary concern and current registries and future registry platforms need to consider strategies to optimize data quality to become regulatory compliant. This collaborative approach may improve the quality of care, reduce costs, and provide faster access to innovative technologies. Existing registries, networks, standards, and procedures should be adopted and used consistently. The multiple potential uses of registry-based data collection make it an area that deserves continued and increased attention by all medical device industry stakeholders, with the joint objective of improving cardiovascular care and outcomes.
FUNDING
None.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
Not used.
AUTHORS’ CONTRIBUTIONS
E. Spitzer and J.G.P. Tijssen contributed to the initiation, design and planning. E. Spitzer, J.M. de la Torre Hernández, I.J. Guðmundsdóttir, E. McFadden, C. Held, C. Hanet, E. Boersma, C.B. Ren, V. Delgado, D. Erlinge, A. Pérez de Prado, J. Bax, and J.G.P. Tijssen contributed with data/presentations. E. Spitzer compiled and drafted the manuscript, which was critically reviewed and revised by J.M. de la Torre Hernández, I.J. Guðmundsdóttir, E. McFadden, C. Held, C. Hanet, E. Boersma, C.B. Ren, V. Delgado, D. Erlinge, A. Pérez de Prado, J. Bax, and J.G.P. Tijssen. All authors approved the final manuscript.
CONFLICTS OF INTEREST
E. Spitzer reports institutional contracts for which he receives no direct compensation with Boston Scientific, Cardiawave, Edwards Lifesciences, Medtronic, Shanghai Microport Medical Co Ltd, NVT GmBH, Pie Medical Imaging, and Siemens Healthcare GmBH. C.B. Ren reports institutional contracts for echocardiography core laboratory analyses with Boston Scientific, Cardiawave, Edwards Lifesciences, NVT GmBH/Biosensors, for which she has received no personal compensation; and has received speaker fee from Abbott. V. Delgado received speaker fees from Edwards Lifesciences, GE Healthcare, Novartis, and Philips; and consulting fees from Edwards Lifesciences, Novo Nordisk and MSD. A. Pérez de Prado is President of the EPIC Foundation. J.G.P. Tijssen is Executive Board member at the European Cardiovascular Research Institute. Other authors have nothing to disclose.
A. Pérez de Prado is associate editor of REC: Interventional Cardiology. The journal’s editorial procedure to ensure impartial processing of the manuscript has been followed.
J. M. de la Torre Hernández is editor-in-chief of REC: Interventional Cardiology. The journal’s editorial procedure to ensure impartial processing of the manuscript has been followed.
REFERENCES
1. European Parliament and the Council of the European Union. Regulation (EU) 2017/745 of 5 April 2017 on medical devices, amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and repealing Council Directives 90/385/EEC and 93/42/EEC. Available at https://eur-lex.europa.eu/eli/reg/2017/745/oj. Accessed 9 Jan 2024.
2. Spitzer E. Forty years of Cardialysis:a leading European cardiovascular research organization. REC:Interv Cardiol. 2023;5:250-253.
3. US Food &Drud Administration (FDA). Use of Real-World Evidence to Support Regulatory Decision-Making for Medical Devices:Guidance for Industry and Food and Drug Administration Staff. 2017. Available at:https://www.fda.gov/regulatory-information/search-fda-guidance-documents/use-real-world-evidence-support-regulatory-decision-making-medical-devices. Accessed 9 Jan 2024.
4. European Medicines Agency (EMA). Patient Registry Initiative - Strategy and Mandate of the Cross-Committee Task Force. 2017. Available at:https://www.ema.europa.eu/en/documents/other/patient-registry-initiative-strategy-mandate-cross-committee-task-force_en.pdf. Accessed 9 Jan 2024.
5. European Medicines Agency (EMA). Guideline on Registry-based Studies. 2021. Avaiable at:https://www.ema.europa.eu/en/guideline-registry-based-studies-scientific-guideline. Accessed 9 Jan 2024.
6. International Medical Device Regulators Forum (IMDRF). Tools for Assessing the Usability of Registries in Support of Regulatory Decision-Making. 2018. Available at:https://www.imdrf.org/documents/tools-assessing-usability-registries-support-regulatory-decision-making. Accessed 9 Jan 2024.
7. de Boer SP, Lenzen MJ, Oemrawsingh RM, et al. Evaluating the 'all-comers'design:a comparison of participants in two 'all-comers'PCI trials with non-participants. Eur Heart J. 2011;32:2161-2167.
8. Jurado-Roman A, Freixa X, Cid B, et al. Spanish cardiac catheterization and coronary intervention registry. 32nd official report of the Interventional Cardiology Association of the Spanish Society of Cardiology (1990-2022). Rev Esp Cardiol. 2023;76:1021-1031.
9. Biswas S, Lefkovits J, Liew D, Gale CP, Reid CM, Stub D. Characteristics of national and major regional percutaneous coronary intervention registries:a structured literature review. EuroIntervention. 2018;14:1112-1120.
10. Freixa X, Jurado-Roman A, Cid B, Cruz-Gonzalez I. Spanish cardiac catheterization and coronary intervention registry. 31st official report of the Interventional Cardiology Association of the Spanish Society of Cardiology (1990-2021). Rev Esp Cardiol. 2022;75:1040-1049.
11. de la Torre-Hernández JM, Alfonso F, Hernandez F, et al. Drug-eluting stent thrombosis:results from the multicenter Spanish registry ESTROFA (Estudio ESpanol sobre TROmbosis de stents FArmacoactivos). J Am Coll Cardiol. 2008;51:986-90.
12. Hanet C, Claeys MJ, Carlier M, Desmet W. Quality assessment in percutaneous coronary interventions:the QERMID Belgian PCI registry. Acta Cardiol. 2018;73:388-391.
13. Kayaert P, Coeman M, Demolder A, et al. Mortality in STEMI Patients With Cardiogenic Shock:Results From a Nationwide PCI Registry and Focus on Left Main PCI. J Invasive Cardiol. 2022;34:E142-E148.
14. Claeys MJ, Argacha JF, Collart P, et al. Impact of COVID-19-related public containment measures on the ST elevation myocardial infarction epidemic in Belgium:a nationwide, serial, cross-sectional study. Acta Cardiol. 2021;76:863-869.
15. Jernberg T, Attebring MF, Hambraeus K, et al. The Swedish Web-system for enhancement and development of evidence-based care in heart disease evaluated according to recommended therapies (SWEDEHEART). Heart. 2010;96:1617-21.
16. Erlinge D, Omerovic E, Frobert O, et al. Bivalirudin versus Heparin Monotherapy in Myocardial Infarction. N Engl J Med. 2017;377:1132-1142.
17. Frobert O, Lagerqvist B, Olivecrona GK, et al. Thrombus aspiration during ST-segment elevation myocardial infarction. N Engl J Med. 2013;369:1587-1597.
18. Batra G, Aktaa S, Wallentin L, et al. Methodology for the development of international clinical data standards for common cardiovascular conditions:European Unified Registries for Heart Care Evaluation and Randomised Trials (EuroHeart). Eur Heart J Qual Care Clin Outcomes. 2023;9:161-168.
19. Butcher SC, Prevedello F, Fortuni F, et al. Prevalence and Prognostic Implications of Moderate or Severe Mitral Regurgitation in Patients with Bicuspid Aortic Valve. J Am Soc Echocardiogr. 2023;36:402-410.
20. Laenens D, Stassen J, Galloo X, et al. The impact of atrial fibrillation on prognosis in aortic stenosis. Eur Heart J Qual Care Clin Outcomes. 2023;9:778-784.
21. Nabeta T, Meucci MC, Westenberg JJM, et al. Prognostic implications of left ventricular inward displacement assessed by cardiac magnetic resonance imaging in patients with myocardial infarction. Int J Cardiovasc Imaging. 2023;39:1525-1533.
22. Spitzer E, McFadden E, Vranckx P, et al. Critical Appraisal of Contemporary Clinical Endpoint Definitions in Coronary Intervention Trials:A Guidance Document. JACC Cardiovasc Interv. 2019;12:805-819.
23. Hoogervorst LA, Geurkink TH, Lubbeke A, et al. Quality and Utility of European Cardiovascular and Orthopaedic Registries for the Regulatory Evaluation of Medical Device Safety and Performance Across the Implant Lifecycle:A Systematic Review. Int J Health Policy Manag. 2023;12:7648.
24. Douglas PS, DeCara JM, Devereux RB, et al. Echocardiographic imaging in clinical trials:American Society of Echocardiography Standards for echocardiography core laboratories:endorsed by the American College of Cardiology Foundation. J Am Soc Echocardiogr. 2009;22:755-765.
25. Spitzer E, Fanaroff AC, Gibson CM, et al. Independence of clinical events committees:A consensus statement from clinical research organizations. Am Heart J. 2022;248:120-129.
26. Erlinge D, Koul S, Eriksson P, et al. Bivalirudin versus heparin in non-ST and ST-segment elevation myocardial infarction-a registry-based randomized clinical trial in the SWEDEHEART registry (the VALIDATE-SWEDEHEART trial). Am Heart J. 2016;175:36-46.
27. James S, Erlinge D, Storey RF, et al. Rationale and design of the DAPA-MI trial:Dapagliflozin in patients without diabetes mellitus with acute myocardial infarction. Am Heart J. 2023;266:188-197.