ABSTRACT
Computed tomography is a noninvasive imaging technique with high spatial resolution, providing excellent definition of calcium and intravascular space through the use of contrast media. This imaging modality allows both highly accurate measurements and virtual simulations for preprocedural planning in coronary and structural heart disease interventions. Computed tomography is currently the gold standard technique for patient selection and preprocedural planning in numerous scenarios, such as transcatheter aortic valve implantation, left atrial appendage occlusion, transcatheter mitral valve repair, and transcatheter tricuspid valve repair. This article reviews the role of computed tomography in transcatheter coronary and structural heart disease interventions.
Keywords: Computed tomography. Structural heart disease interventions. TAVR. LAAO. TMVR.
RESUMEN
La tomografía computarizada es una técnica no invasiva, de gran resolución espacial, con excelente definición del calcio y del espacio intravascular al emplear medios de contraste, que brinda la posibilidad de realizar tanto mediciones como simulaciones virtuales de intervencionismo coronario y estructural. Se ha establecido como la técnica de referencia en la selección de pacientes y la planificación de procedimientos de intervencionismo transcatéter coronario y estructural en diferentes escenarios (implante percutáneo de válvula aórtica, cierre percutáneo de orejuela izquierda, reemplazo de válvula mitral transcatéter y reemplazo de válvula tricúspide transcatéter). El presente trabajo revisa el papel de la tomografía computarizada en el intervencionismo cardiaco coronario y estructural.
Palabras clave: Tomografía computarizada. Intervencionismo estructural. TAVI. LAAO. TMVR.
Abbreviations CT: computed tomography. ECG: electrocardiogram. LAAO: left atrial appendage occlusion. LVOT: left ventricular outflow tract. TAVI: transcatheter aortic valve implantation. TMVR: transcatheter mitral valve replacement.
INTRODUCTION
Coronary and structural heart disease interventions have traditionally relied on fluoroscopy and transesophageal echocardiography as the imaging modalities of choice, especially for intraprocedural monitoring. Imaging-based patient selection has also usually relied on echocardiography. However, the technological and knowledge advancements made in recent years have led to the incorporation of new imaging modalities—particularly computed tomography (CT) and, to a lesser extent, magnetic resonance—into the field of structural heart interventions.
Currently, CT is the imaging modality of choice before structural heart interventions in a wide range of procedures, as well as the screening technique for coronary artery disease, and even for planning coronary interventions.
This review examines the applications and indications of cardiac CT in transcatheter coronary and structural heart disease interventions.
GENERAL FEATURES OF CARDIAC COMPUTED TOMOGRAPHY
Cardiac CT is an optimal technique for evaluating patients prior to a structural heart intervention. This modality offers contrast-enhanced noninvasive imaging with excellent definition of calcium and intravascular space, submillimeter isotropic spatial resolution, and acceptable temporal resolution.
Like invasive coronary angiography, cardiac CT uses an X-ray source to create the image. Modern machines feature an O-shaped gantry ring with the X-ray tube positioned opposite a ring of detectors. The emitted radiation beam is attenuated and absorbed depending on tissue densities, with the captured energy reconstructed to form a medical image.
When acquiring tomographic images of heart structures and coronary arteries, it is important to consider their small-caliber, with each structure moving independently in all 3 spatial axes. Therefore, the equipment must be technically capable of producing conclusive studies. Table 1 outlines key technical parameters of CT generated images.
Table 1. Main basic concepts of computed tomography
| Concept | Definition |
|---|---|
| Spatial resolution | The ability to visualize 2 separate points that are very close together. Depends on the size of the detectors; in modern CT scanners, it is < 1 mm. |
| Isotropism | Image composed of voxels with a similar size in all 3 spatial planes. Allows for image reformatting while minimizing the loss of resolution. |
| Temporal resolution | The shortest time required by the CT scanner to acquire an image. Depends on the gantry rotation speed and the acquisition method. |
A cardiac CT scan should employ the ECG-gated technique to compensate for cardiac motion, with the study conducted during breath-holding to minimize respiratory movements. Acquisitions can cover the entire cardiac cycle or a preselected phase. Acquisition of the entire cardiac cycle (called “retrospective” in scanners with < 16 cm z-axis coverage) offers the advantage of allowing reconstruction of all phases, as well as functional assessments (volumes, ejection fraction, leaflet motion) and 4D reconstructions. However, this method requires higher radiation doses. This can be partially mitigated through retrospective acquisitions with dose modulation, acquiring high-quality images in 1 or more predefined phases while capturing the rest at lower quality, thereby reducing radiation exposure.1
Technological advances and the wider availability of CT scanners with cardiac acquisition software have allowed this imaging modality to be established as a standard in various structural interventional procedures. While it is widely acknowledged that the minimum equipment required includes an ECG-gated 64-slice CT scanner, the latest models offer superior image quality, decreased radiation exposure, and reduced contrast use. The latest generation of CT scanners follow various development paths: a) wide-detector CT scanners increase the scanned distance per heartbeat by incorporating more detectors; some scanners have more than 300 detectors, enabling cardiac coverage in a single heartbeat; b) high-pitch dual-source CT scanners use 2 radiation sources at a 90° offset and a high speed table to markedly enhance temporal resolution); c) spectral CT scanners use detectors with differing sensitivities or various energy levels from the emitter to capture images at different energy spectra, allowing a certain degree of tissue characterization; and d) photon-counting CT scanners eliminate the need for intermediate photoluminescent detectors, thus enhancing spatial resolution to 0.2 mm.
In addition to the CT scanner, an at least dual-phase injector is required to allow high flow (4-7 mL/s), a contrast agent with an iodine concentration around 350 mg/mL (ideally iso-osmolar), and a digital processing and image storage system in DICOM format (Digital Imaging and Communication in Medicine).
Preparing patients for a cardiac CT is essential to ensure high-quality diagnostic tests. Prior to the procedure, patients must provide informed consent and undergo an assessment to rule out any contraindications. A peripheral venous line is usually established in the right antecubital fossa (18-20 G). Patients are usually placed in the supine position with their arms raised above their heads. ECG electrodes are applied, ensuring excellent trace quality. It is important to explain and practice the breath-holding technique required during the scan with the patient, as well as to monitor ECG-quality during the breath-hold.
Depending on the indication of the study, if the patient’s heart rate is high or the rhythm is irregular, premedication may be necessary, with the most common choice being IV beta-blockers. In studies that require assessing the coronary lumen, sublingual nitroglycerin is usually also administered. When performing a cardiac CT prior to structural intervention, it is important to remember that severe symptomatic aortic or mitral stenosis is a contraindication for nitroglycerin use. Beta-blockers should be administered with caution, under the supervision of qualified personnel, ensuring that advanced cardiopulmonary resuscitation can be performed if necessary.
APPLICATION TO STRUCTURAL HEART INTERVENTIONS
Coronary computed tomography angiography (CCTA) provides a detailed anatomical assessment of the coronary tree, including its origin and course, detects the presence of atherosclerotic lesions, quantifies affected segments, and determines the severity of stenosis and atherosclerotic burden. CCTA is the standard imaging modality to assess symptomatic patients and can be considered in selected high-risk asymptomatic patients. It has a sensitivity of 97% and a specificity of 78% when taking invasive coronary angiography in a population with a pretest probability of 56% as a reference. While CCTA has the highest sensitivity compared with other invasive imaging modalities, functional imaging techniques such as stress magnetic resonance (80%), stress echocardiography (82%), and positron emission tomography (85%) have superior specificity.2 Despite its lower specificity, the CT-based anatomical strategy has been proven to be noninferior in terms of prognosis compared with the ischemia test-based functional strategy (PROMISE trial).3
Due to its high negative predictive value, CT is recommended by clinical practice guidelines as a first-line imaging modality to rule out obstructive coronary artery disease in low-to-intermediate risk symptomatic patients.4 Table 2 outlines the main indications for CCTA in various clinical scenarios.
Table 2. Current indications for computed tomography of coronary arteries and measurement of coronary artery calcium based on the European Society of Cardiology clinical practice guidelines
| Acute symptoms | Degree of recommendation | Level of evidence | Year | Ref. |
|---|---|---|---|---|
| Suspected acute coronary syndrome, normal or uncertain range troponins, normal electrocardiogram, and no recurrence of pain; may be considered as part of the initial diagnostic evaluation | IIA | A | 2023 | 5 |
| Systematic use in patients with suspected acute coronary syndrome | III | B | 2023 | 5 |
| Stable symptoms | Degree of recommendation | Level of evidence | Year | Ref. |
| Symptomatic patient with suspected coronary artery disease that cannot be clinically ruled out | I | B | 2019 | 4 |
| Risk stratification in patients with suspected or newly diagnosed coronary artery disease | I | B | 2019 | 4 |
| Patients with suspected vasospastic angina to study underlying coronary artery disease | I | C | 2019 | 4 |
| Screening for coronary artery disease in hemodynamically stable patients with aortic vegetations requiring cardiac surgery | I | B | 2023 | 6 |
| Patients with a low-to-intermediate probability of coronary artery disease and a previous equivocal noninvasive stress test | IIA | C | 2021 | 7 |
| Alternative to invasive coronary angiography prior to valvular cardiac surgery in patients with a low probability of coronary artery disease | IIA | C | 2021 | 8 |
| Patients with suspected cardiomyopathy for screening of coronary artery disease, or coronary anomalies that may be causing the cardiomyopathy | IIA | C | 2023 | 9 |
| Intermediate-to-high risk patients with prior nonemergency, noncardiac surgery: a) low-to-intermediate probability of coronary artery disease and suspected chronic or acute coronary syndrome without enzyme mobilization; b) patients ineligible for noninvasive functional tests | IIA | C | 2022 | 10 |
| Coronary computed tomography angiography is not recommended for the routine follow-up of patients with established coronary artery disease | III | C | 2019 | 4 |
| Asymptomatic | Degree of recommendation | Level of evidence | Year | Ref. |
| Calcium scoring as a risk modifier in asymptomatic patients with moderate cardiovascular risk | IIB | B | 2019 | 4 |
| Selected individuals with no history of coronary artery disease, high cardiovascular risk (SCORE > 10%, strong family history, familial hypercholesterolemia) and desire to start an intensive exercise program | IIB | B | 2021 | 11 |
| High cardiovascular risk (diabetes mellitus, family history, or previous test suggesting coronary artery disease) | IIB | C | 2019 | 4 |
| Asymptomatic adults (> 40 years) with diabetes mellitus | IIB | B | 2019 | 4 |
| Asymptomatic nondiabetic low-risk adults | III | C | 2019 | 4 |
Technological advances and the incorporation of new imaging modalities, such as stress CT perfusion and fractional flow reserve CT (FFRCT) have increased specificity rates to 85% to 87%.12 This enhances the positive predictive value of the imaging modality and allows meticulous evaluation of intermediate-to-high risk patients.
Landmark studies have been published on the prognosis of patients evaluated using CT. The SCOT-HEART trial13 demonstrated a reduction in cardiovascular deaths and nonfatal myocardial infarctions at the 5-year follow-up with a CT-guided strategy with outcome-based treatment adjustment compared with a conventional management strategy. On the other hand, the DISCHARGE trial14 showed a similar risk of major cardiovascular events during follow-up in patients with intermediate probability and stable chest pain randomized to CT vs invasive coronary angiography, with a lower rate of complications in the noninvasive imaging modality group. These studies support CT as a first-line imaging modality to rule out coronary artery disease, establish preventive treatment in patients with nonobstructive coronary artery disease, stratify patients with obstructive coronary artery disease, and offer an alternative to invasive coronary angiography in a wide range of patients.
In patients with a history of coronary artery disease, CCTA can be used to assess coronary artery bypass graft surgery, verify the patency of coronary stents in specific cases (proximal segments and stents > 3.0 mm), and assess chronic total occlusions prior to percutaneous coronary revascularization. In the BYPASS-CTCA trial,15 which randomized patients with prior surgical coronary revascularization to undergo CT-based anatomical assessment and invasive coronary angiography, or isolated invasive coronary angiography, shorter procedures and fewer episodes of contrast-induced nephropathy were observed in patients with noninvasive assessment of coronary artery bypass grafts.
CCTA should adhere to the recommendations established by the Society of Cardiovascular Computed Tomography.16 There are different image representation formats (axial, multiplanar reformatting, maximum intensity projection, curved multiplanar reformatting, or volumetric reconstruction), each with complementary uses. CCTA reading begins by assessing its quality, identifying potential artifacts, and visualizing the origin, course, and coronary dominance. The following are general principles for interpretation: a) cross-sectional systematic review of each coronary segment from multiple planes; b) vigilance for possible artifacts; c) evaluation of lesion morphology and composition; and d) grading lesion severity using high-resolution images in longitudinal and cross-sectional views of the vessel lumen. Following the modified distribution of the American Heart Association, coronary arteries are divided into 18 coronary segments. Identified lesions are listed based on the affected segment, the nature of the lesion (noncalcified, partially calcified, or calcified), and degree of resulting stenosis: normal (no lesion or stenosis), minimal (< 25% lumen reduction), mild (25%-49%), moderate (50%-69%), severe (70%-99%), or occlusion (> 99%).
Detailed analysis of the CT image enables the selection of a plan for transcatheter intervention and the materials to be used, and potentially reduces procedural length and complexity. This can be particularly useful when optimizing the fluoroscopy angle based on CT analysis in complex or bifurcated coronary artery lesions, as well as when performing complex cardiac catheterizations in patients with percutaneous aortic valve prostheses.17
The overall complexity of coronary artery disease can be represented by indices such as the coronary calcium score, or the number of segments with some degree of coronary artery disease, but several specific scales are available. Among these, the most widely used are the CAD-RADSTM (Coronary Artery Disease Reporting and Data System)18 and its updated version, the CAD-RADSTM 2.0,19 which incorporates parameters of perfusion and plaque complexity. Other more specific scales include the CT-SYNTAX20 scale, which combines CT-based anatomical information with clinical data from the SYNTAX scale, and the Functional CT-SYNTAX21 and Functional FFRCT22 scales, which add incorporate FFRCT-based functional information. These scales help refine the decision between surgical and percutaneous revascularization strategies, with promising initial results.23 Their prognostic validation in different scenarios, and their implementation in clinical practice, may represent a paradigm shift in the performance of invasive diagnostic imaging studies in stable patients.
In patients with chronic total coronary occlusions, preprocedural CT analysis allows estimation of the probability of success of percutaneous coronary revascularization; several prognostic scales have been developed for this purpose, such as the J-CTO,24 the CT-RECTOR,25 and the KCCT26 (table 3). The parameters analyzed include the extent of calcification, vascular tortuosity, the morphology of the occlusion stump, the presence of multiple occlusions, and the length of the lesion.
Table 3. Prediction scales for the success and complications associated with the revascularization of chronic total occlusions by computed tomography
| Score | Variables (points) | Classification |
|---|---|---|
| J-CTO | Tapered (0) vs blunt end (1) | Easy (0) Intermediate (1) Difficult (2) Very difficult (≥ 3) |
| No calcification (0) vs some calcification (1) | ||
| Occlusion angle ≤ 45° (0) vs > 45° (1) | ||
| Occlusion length < 20 mm (0) vs ≥ 20 mm (1) | ||
| No previous failed revascularization attempts (0) vs with previous attempts (1) | ||
| CT-RECTOR | < 2 occlusions (0) vs ≥ 2 complete interruptions (1) | Easy (0) Intermediate (1) Difficult (2) Very difficult (≥ 3) |
| Tapered (0) vs blunt end (1) | ||
| < 50% calcification of vessel perimeter on short axis (0) vs ≥ 50% calcification at some point of the occlusion (1) | ||
| Occlusion angle ≤ 45° (0) vs > 45° (1) | ||
| No previous failed revascularization attempts (0) vs with previous attempts (1) | ||
| Duration of chronic total coronary occlusion < 12 months (0) vs ≥ 12 months (1) | ||
| KCCT | Tapered (0) vs blunt end (1) | Easy (0) Intermediate (1) Difficult (2) Very difficult (3) Extremely difficult (≥ 4) |
| No adjacent collateral branches (0) vs with collateral branches (1) | ||
| Occlusion length < 15 mm (0) vs ≥ 15 mm (1) | ||
| Occlusion angle ≤ 45° (0) vs > 45° (1) | ||
| Vessel calcification on the short axis < 180° of perimeter or < 50% of area (0) vs ≥ 180° of perimeter and ≥ 50% of area (1) vs complete central calcification of 360° of perimeter and 100% of area (2) | ||
| No previous failed revascularization attempts (0) vs with previous attempts (1) | ||
| Duration of chronic total coronary occlusion < 12 months (0) vs ≥ 12 months (1) |
APPLICATION TO STRUCTURAL HEART INTERVENTIONS
Transcatheter aortic valve implantation
After echocardiographic diagnosis of severe aortic stenosis, CT is the imaging modality of choice for a comprehensive assessment of patients eligible for transcatheter aortic valve implantation (TAVI).27 In a single scan, CT can evaluate vascular access, verify the degree of aortic stenosis and valve morphology, measure the aortic annulus, assess the risk of coronary occlusion, and determine the optimal fluoroscopy angles, among other aspects. In addition, in a high percentage of cases, CT facilitates the screening of proximal obstructive coronary artery disease and assessment of extracardiac findings.28
Preprocedural assessment for TAVI includes: a) an optional noncontrast acquisition to quantify aortic valve calcium; b) ECG-gated acquisition in the systolic phase, at least in the region of the aortic valve complex; and c) depending on the speed and coverage of the equipment used, 1 or more acquisitions for iliofemoral access, without the need for ECG-gated synchronization in this region. The study requires the injection of contrast medium (50-90 mL, with a flow rate of 3-5 mL/s, subject to variations based on the equipment used and the patient’s body surface area).28
The main aspects that should appear in the CT report prior to performing TAVI are listed in table 4.
Table 4. Main features that need to be included in the computed tomography report prior to transcatheter aortic valve implantation or percutaneous left atrial appendage occlusion
| Transcatheter aortic valve implantation | |
|---|---|
| Aortic annulus | Measurement in systolic phase |
| Area and perimeter | |
| Major and minor diameters, | |
| Optimal fluoroscopy view | |
| Calcium and valve | Presence, morphology, and extent of calcium |
| Valvular morphology | |
| Aorta and accesses | Height of the origin of coronary arteries |
| Minimum luminal diameter of each vascular segment | |
| Description of calcifications and vascular disease | |
| Others | Coronary anatomy |
| Extracardiac findings | |
| Percutaneous left atrial appendage occlusion | |
| Thrombus | Screening for arterial/venous filling defect |
| Morphology and landing zone | Describe the morphology and presence of proximal lobes |
| Measure the landing zone, maximum diameter | |
| Measure the depth and length of the appendage | |
| Optimal fluoroscopy view | |
| Others | Anatomy of the interatrial septum |
| Anatomy of the pulmonary veins | |
| Describe if there is pericardial effusion | |
Currently, there are 2 general designs of transcatheter aortic valve prostheses: balloon-expandable and self-expanding. Balloon-expandable TAVIs use radial force along with balloon inflation to fit their circular design to the oval shape of the aortic annulus. In contrast, self-expanding TAVIs expand on their own, due to nitinol memory, to fit over the annulus. In addition to technical and design differences, it is important to note that the sizing algorithms for these devices are not interchangeable. Sizing of balloon-expandable prostheses is based on the area of the aortic annulus, while that of self-expandig valves is based on the perimeter.
All the assessments necessary before TAVI are illustrated in Figure 2.
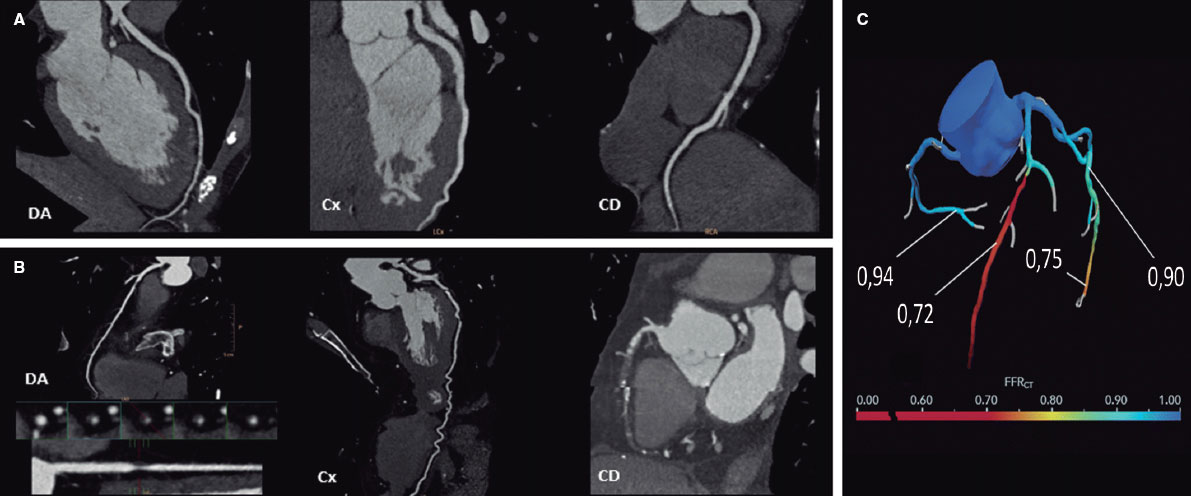
Figure 1. Computed tomography allows the study of coronary arteries to rule out the presence of coronary artery disease (A, normal coronary arteries), or to establish the severity and location of obstructive coronary disease (B, severe lesion in the proximal left anterior descending coronary artery [LAD] and chronic total occlusion in the mid and distal regions of the right coronary artery [RCA]). The functionality of the lesions can be assessed using computer simulation (C, fractional flow reserve computed tomography [FFRCT], severe lesion in the mid LAD and distal left circumflex artery [LCx]).
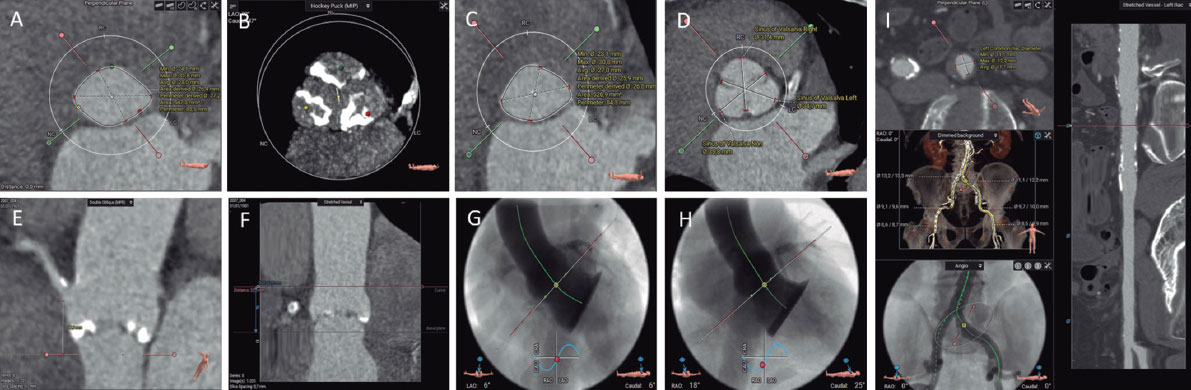
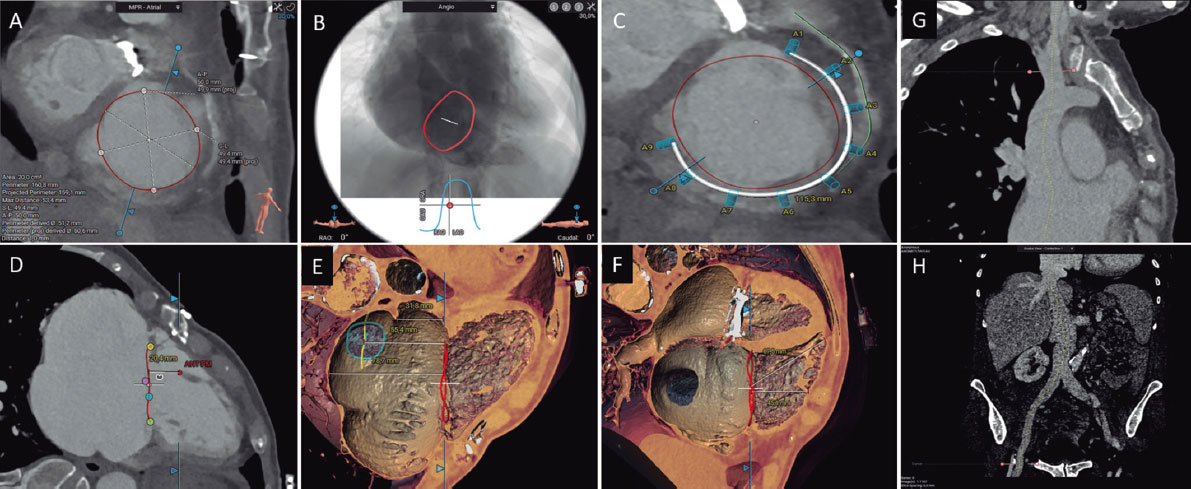
Figure 2. Preassessment for transcatheter aortic valve implantation using computed tomography and 3mensio CT analysis software: aortic annulus (A), aortic valvular calcium (B), left ventricular outflow tract (C), diameters of the Valsalva sinuses (D), height of the right coronary artery origin (E), height of the sinotubular junction (F), 3-cusp coplanar view (G), cusp-overlap view (H), and transfemoral accesses (I).
It is important to understand and analyze the anatomy of the aortic valve complex, which comprises the left ventricular outflow tract (LVOT), the Valsalva sinuses, the fibrous triangles between the aortic leaflets, and the leaflets themselves. A key measurement is the correct assessment of the plane of the aortic annulus, defined as the virtual plane aligned with the lowest insertion point of each aortic cusp or nadir. This involves determining the major and minor diameters, area, and perimeter of the aortic annulus. These measurements guide the selection of TAVI size. The aortic annulus undergoes changes in size and shape throughout the cardiac cycle, with mesosystole (30-35% R-R) often being the optimal time for measurement (larger size and reduced ellipticity).29 Specialized software is available to automate these measurements and simulate the implant procedure, streamlining workflow and reducing inter- and intra-observer variability.
The landing zone for the prosthesis includes the aortic cusps, the aortic annulus, and the LVOT. Severe calcification in the LVOT and aortic valve increases the risk of subsequent periprosthetic regurgitation, while large nodular calcifications may pose a higher risk of aortic annulus rupture, especially with balloon-expandable prostheses.30 It is essential to describe the location and extent of calcification in the aortic valve and the first 5 to 7 mm of the LVOT, as this area serves as the sealing zone for most available TAVIs. The morphology and degree of calcification of the aortic valve should be systematically reported, with particular attention to the presence of bulky calcification or partial fusion of the aortic commissures.28
The perpendicular height from the plane of the aortic annulus to the origin of the coronary arteries must be evaluated. Although absolute cutoff values have not been established, a coronary artery origin height of < 12 mm and sinuses of Valsalva < 30 mm are associated with a higher risk of TAVI-related coronary occlusion.31
The report should also include the optimal CT projections for valve deployment. Identifying these projections reduces radiation dose, contrast, and procedure duration.29 Angulation should be reported to obtain a coplanar projection (3 cusps), aligning the cusps, and the angulation for obtaining an overlapping projection (cusp-overlap), with the left and right cusps overlapped. This plane deploys the LVOT and allows better control of implant depth during valve deployment, especially with self-expanding valves.32
CT allows assessment of vascular access in a single study, providing excellent resolution and detailed delineation of the presence and extent of calcifications. Vascular complications increase the morbidity and mortality associated with TAVI. Factors associated with the occurrence of vascular complications include the sheath-to-femoral artery ratio, the presence of moderate to severe calcification, and vascular tortuosity.33 The report should include details on the minimum luminal diameters, the extent, distribution, and severity of calcification, as well as the presence or absence of vascular disease in all vascular segments between the aortic valve and the left and right common femoral arteries at the level of the femoral head.28 If femoral accesses are deemed unsuitable, alternative accesses can be considered, with the most common being axillary/subclavian, carotid, transcaval, and transapical accesses.
Special attention should be paid to the bicuspid aortic valve, given its lower success rate in procedures and higher rates of periprosthetic regurgitation, albeit with similar clinical outcomes.34 It is essential to determine the type of bicuspid valve (whether sinus fusion, 2 sinuses, or forme fruste),35 presence of a raphe, calcium distribution, annulus size and eccentricity, as well as the origin and height of the coronary arteries. Measuring the aortic annulus can be particularly complex in 2-sinus bicuspid valves, requiring specific methodology.28 The aortic annulus is defined as the virtual plane aligned with the lowest insertion point of the anterior/lateral cusp. Starting from this point, counterclockwise rotation to the lowest insertion point of the posterior/medial cusp is performed. Measurements should be taken at the line perpendicular to these 2 points, centered at the point where the smallest cross-sectional area is reached (as improper angulation can lead to inaccurate size estimation). The major and minor diameters, area, and perimeter of the aortic annulus are then determined. Algorithms have been developed for prosthesis size selection based on aortic annulus size, considering raphe length, calcium volume, and distribution (CASPER, calcium algorithm sizing for bicuspid evaluation with raphe).36 Additionally, a method (LIRA, level of implantation at the raphe) has been proposed by delineating the perimeter of the bicuspid valve opening,37 although its superiority over conventional measurements remains unclear.38
A variant of TAVI is the valve-in-valve implant, in which a percutaneous prosthesis is placed over a dysfunctional bioprosthesis. CT plays a key role in prosthesis size selection, especially when the model or size of the implanted prosthesis is unknown, but also in stratifying the risk of coronary occlusion. Among the main parameters for determining the risk of coronary obstruction are the level reached by the prosthesis cusps relative to the origin of the coronary arteries and the sinotubular junction, risk associated with the proximity of the valve to the sinotubular junction, < 2 mm distance from the virtual TAVI to the sinotubular junction, < 4 mm distance from the virtual TAVI to the origin of the coronary arteries, a prior supra-annular or supracoronary prosthesis, a surgical prosthesis with leaflets implanted outside the annulus (Mitroflow or Trifecta type), a prior implant in a high position, and the presence of moderate or severe commissural misalignment.39,40
After the TAVI procedure, CT allows assessment of the position and geometry of the prosthesis, as well as the thickness and mobility of the prosthetic leaflets. Following TAVI, a CT scan may be performed if prosthetic dysfunction or degeneration is identified by echocardiography, suspected thrombosis, infectious endocarditis, or periprosthetic regurgitation requiring anatomical assessment. The phenomenon of thickening with hypoattenuation and reduced mobility in the prosthetic leaflets has been described, which is associated with subclinical thrombosis and resolves with anticoagulation therapy. This finding has been associated with a higher but nonsignificant tendency for embolic events, and consequently there is no consensus or established indication for systematic performance of CT after TAVI. Its occurrence is more common in valve-in-valve, balloon-expandable prostheses, and larger prostheses, as well as those with eccentric expansion due to bicuspid valves, for example.41
Lastly, there is the option of using CT scans to resolve diagnostic uncertainties regarding the severity of aortic stenosis. Assessing aortic valve calcium can be especially helpful in patients with low-flow, low-gradient aortic stenosis and preserved ejection fraction. Agatston scores ≥ 2000 in men and ≥ 1200 in women indicate severe degenerative aortic stenosis, while scores < 1600 in men and < 800 in women suggest the absence of severe degenerative stenosis.8
Percutaneous left atrial appendage occlusion
Percutaneous closure of the left atrial appendage (LAAO) is an alternative to oral anticoagulation in patients with atrial fibrillation and a contraindication to oral anticoagulation. The traditional technique used for patient selection is transesophageal echocardiography (TEE) to rule out the presence of thrombus in the appendage and to take measurements for device selection. Three-dimensional measurements (3D-TEE, CT) have consistently been shown to be more accurate in selecting device size than 2D-TEE. Therefore, CT is an alternative technique in patient selection, as it allows visualization of the presence of thrombus and evaluation of the anatomy and size of the appendage, as well as the interatrial septum.42
CT evaluation of LAAO should be performed with ECG-gated acquisition, ideally in the telesystolic phase (when the left atrial appendage is maximally expanded), and a second acquisition should be performed in the venous phase, 60 to 90 seconds after contrast administration, to assess the presence or absence of thrombus in the left atrial appendage.43 The main features that should be included in a CT report for LAAO are listed in table 4. If the quality allows, it is advisable to perform an assessment of coronary anatomy.
The morphology of the left atrial appendage is highly variable and complex. Several devices for LAAO have been marketed, with the most commonly used being lobe and disc devices. Measurement of the landing zone is performed using multiplanar reformatting from 2-chamber and coronal planes. In the case of lobe devices, the landing zone extends from the circumflex artery to a point located 10 to 20 mm inside the ligament of Marshall.
The morphology of the left atrial appendage is highly variable and complex. Different devices for LAAO have been commercialized, with the most commonly used being lobe and disc devices. Measurement of the deployment zone is performed using multiplanar reformatting from two-chamber and coronal planes. In the case of lobe devices, the deployment zone extends from the circumflex artery to a point located 10-20 mm inside the ligament of Marshall. The depth is determined from the landing zone to the most distal end of the appendage. With disc devices, the landing zone is located 10 to 12 mm inside the ostium of the appendage, covering the course of the circumflex artery at its lower end. The depth in this type of device is defined from the ostium to the opposite wall of the appendage.43 It is also important to assess the anatomy of adjacent structures, especially the ligament of Marshall, to assess the feasibility of fully covering it with a disc device and to avoid thrombus formation during follow-up,44 as well as the anatomical characteristics of the pulmonary artery in relation to the left atrial appendage.45
Specific software has been designed to automate these measurements and simulate the implantation process (figure 3). Utilizing simulation software through computing enhances device selection and procedural outcomes.46
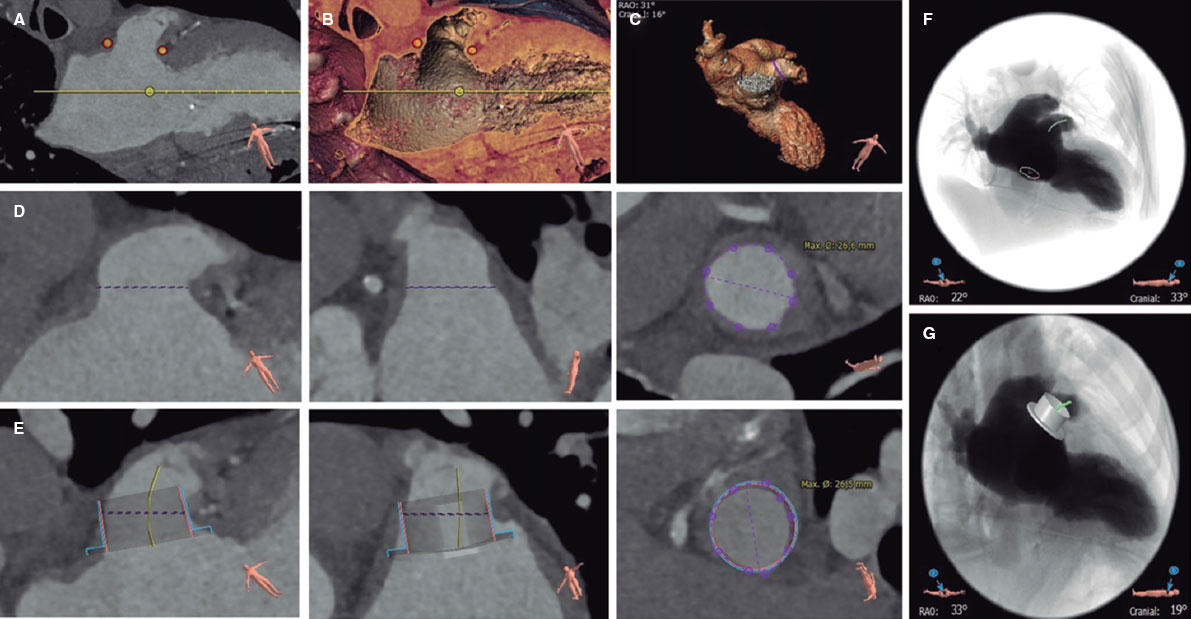
Figure 3. Planning for percutaneous left atrial appendage occlusion using computed tomography and 3mensio CT analysis software: identification of the left atrial appendage ostium (A and B), left atrial appendage morphology (C), measurement of the landing zone (D, longitudinal and cross-sectional views), simulation of the occluder device (E, longitudinal and cross-sectional views), simulation of the fluoroscopy view and position of the transseptal puncture (F), and simulation of the occluder device in fluoroscopy (G).
After LAAO, it is recommended to perform an imaging test 45 to 60 days postimplantation to verify the stability and positioning of the device, to search for residual leaks, and to rule out the presence of device-related thrombus. The most commonly used techniques are TEE and CT. CT allows better visualization of the position and deployment of the device, has equal thrombus detection capability, and has higher sensitivity in detecting residual contrast passage. The latter may be due to device malapposition, the presence of a peridevice leak, or the patency of the covering tissue.47 The clinical relevance of residual leaks, as well as the importance of their size, are not entirely clear.48
Transcatheter mitral valve replacement
Within transcatheter mitral valve intervention, there are options for repair and replacement. Edge-to-edge repair techniques are clinically established, with patient selection and procedural monitoring conducted via TEE. In contrast, for various valve replacement techniques, CT is indispensable. CT with ECG-gated acquisition is required to cover and reconstruct the entire cardiac cycle after contrast administration with adequate opacification of at least the left chambers, and ideally the right chambers, as well as to enhance visualization of the anatomy and its relationships. Detailed recommendations for acquisition and optimization have been published.49 CT allows evaluation of mitral annulus size and shape, selection of prosthesis type and size for implantation, virtual simulation of implantation, assessment of resulting neo-TSVI, selection of optimal fluoroscopy angles, and planning of vascular access (transseptal or transapical).49 (figure 4). Specific measurements for each device are determined by the manufacturer.
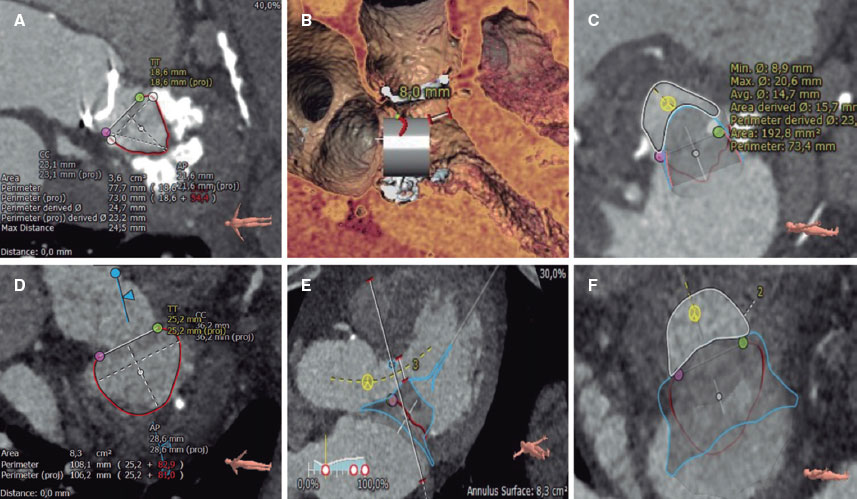
Figure 4. Several steps in the planning of transcatheter mitral valve replacement using computed tomography and 3mensio CT analysis software in 2 patients with valve-in-MAC (A-C) and native valve (D-F): delineation and measurement of the mitral annulus (A and D), evaluation of the distance from the virtual valve to the interventricular septum (D and E), and measurement of the neo-left ventricular outflow tract (C and F).
Transcatheter mitral valve replacement (TMVR) has been described for native valve, prior surgical annuloplasty (valve-in-ring), dysfunctional bioprosthetic valve (valve-in-valve), and severely calcified native mitral annulus (valve-in-MAC).50 CT is particularly useful to select prosthesis size and assess embolic risk in valve-in-MAC procedures by evaluating the thickness of the mitral annular calcium, its extension around the posterior perimeter or mitral trigones, and the damage to the mitral leaflets.51
The main complication to avoid during TMVR planning is LVOT obstruction after the procedure. The neo-LVOT refers to the distance or area between the lower edge of the virtual implant and the interventricular septum. The main predictors of neo-LVOT obstruction are detailed in table 5.52 The neo-LVOT area should be assessed in meso-telesystole (40%-50% R-R; the smallest area during the cardiac cycle), with obstruction risk increasing as the neo-LVOT area decreases: < 170 mm² indicates very high risk, 170 to 190 mm² indicates high risk, 190 to 220 mm² indicates acceptable risk, and > 220 mm² indicates low risk. In selected high-risk cases, techniques such as laceration of the anterior mitral leaflet (LAMPOON) or interventricular septal ablation (alcohol septal ablation) can be employed to enlarge the neo-LVOT area.53
Table 5. Predictors of left ventricular outflow tract obstruction in transcatheter mitral valve replacement
| Obstruction predictors | Obstruction risk limit |
|---|---|
| Area of the neo-LVOT | < 1.9 cm² |
| Area of the neo-LVOT skirt | < 1.5 cm² |
| Sizes of the anterior mitral leaflet | > 25 mm |
| Protruding interventricular septum | Thickness > 15 mm |
| Distance between the mitral annulus and the interventricular septum | < 17.8 mm |
| Acute aortomitral angle | < 110° |
| Small left ventricle | End-diastolic diameter < 48 mm |
| Left ventricular hypertrophy | Indexed myocardial mass > 105 g/m² |
|
LVOT, left ventricular outflow tract. |
|
Transcatheter tricuspid valve replacement
Transcatheter procedures for the tricuspid valve mainly include edge-to-edge repair, annuloplasty, and both orthotopic and heterotopic valve replacement (valve prostheses in the venae cavae).
The acquisition process is similar to that of pre-TMVR CT (ECG-gated covering and reconstructing the entire cardiac cycle following contrast administration). However, it is optimized for contrast in the right heart chambers using triphasic injection protocols (a mixture of contrast and saline at different concentrations). Detailed recommendations for acquisition and optimization have been published.49 CT imaging allows assessment of the tricuspid annulus geometry and size throughout the cardiac cycle, the morphology and mobility of the tricuspid leaflets, the position and relationship of the right coronary artery to the tricuspid annulus, right ventricular volume and ejection fraction, the optimal fluoroscopy angle, and vascular access54 (figure 5).
Figure 5. Evaluation of the tricuspid valve using computed tomography and 3mensio CT analysis software: measurement of the tricuspid annulus (A), simulation of the fluoroscopy view (B), simulation of the percutaneous annuloplasty anchors relative to the right coronary artery (C), distance from the tricuspid annulus to the anterior papillary muscle (D), distance from the tricuspid annulus to the roof of the coronary sinus, the inferior vena cava, and the roof of the right atrium (E), distance from the tricuspid annulus to the right ventricular free wall and apex (F), curved multiplanar reconstruction of the superior (G) and inferior venae cavae, and femoral accesses (H).
CT imaging can also aid in assessing the position and relationship of pacing leads with the tricuspid leaflets in selected cases of edge-to-edge repair. However, its main role lies in patient selection and planning of annuloplasty and valve replacement procedures, in which it is the imaging modality of choice. In annuloplasty, CT imaging facilitates device sizing, allows certain possibilities to be ruled out via simulation of the interaction of anchoring systems and the course of the right coronary artery, and evaluates tricuspid leaflet tenting to assess potential residual regurgitation postprocedure.54 In heterotopic replacement, CT enables sizing of the superior and inferior vena cava at different levels, assesses the anatomy and location of the suprahepatic veins, and determines the size of the right atrium, all of which determine the type and size of the device to be implanted.55 Finally, in orthotopic replacement, the selection criteria largely depend on the chosen device; however, it is generally necessary to evaluate the annulus size, distance to the anterior papillary muscle or free wall of the right ventricle, the confluence position of the vena cavae, and the angles between these and the tricuspid annulus, as well as the access route.56
Other procedures
Paravalvular leak closure
CT has shown good diagnostic performance in detecting aortic and mitral paravalvular leaks, allowing definition of the number, location, shape, and size of the defects.57 CT is especially useful in assessing infective endocarditis-related complications,58 as well as for planning and supporting the closure of paravalvular leaks in the aortic position.59 In addition, CT-based simulation prior to procedures can predict the occurrence of paravalvular leaks.60
Congenital heart diseases
Magnetic resonance imaging is the technique of choice in the diagnosis, evaluation, and follow-up of congenital heart diseases due to its ability to acquire any imaging geometry and perform anatomical and functional assessment, tissue characterization, and flow analysis, as well as the absence of radiation in a generally young population. CT is reserved for selected patients and cases.
Either CT or magnetic resonance can be used for patient selection, device choice, and sizing prior to intervention in congenital heart diseases. CT offers higher spatial resolution, enabling more precise delineation of calcification areas and proper sizing of prostheses. The use of CT or magnetic resonance is essential before transcatheter pulmonary valve replacement and percutaneous treatment of aortic coarctation. CT may also prove useful in cases of patent ductus arteriosus and complex fistulas. However, CT has lower added value in the closure of septal defects, such as atrial or ventricular septal defects.61 Nevertheless, in postmyocardial infarction ventricular septal defects, CT can be highly useful for sizing the defect and assessing their morphology, extent, and borders, given the often intricate and complex nature of these defects, which hampers accurate evaluation by echocardiography.62
CT-fluoroscopy image fusion during structural heart interventions
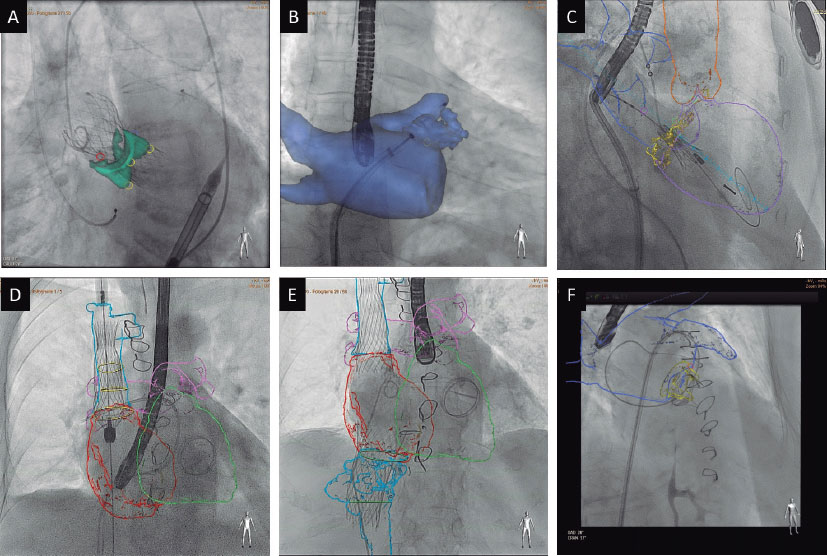
The anatomical information and preprocedural planning can be integrated into procedural monitoring. Using specific software and a workstation, cardiac structures are semiautomatically segmented and coregistered with the patient’s anatomy on the cath lab treatment table from 2 fluoroscopy projections. After coregistration, all CT information can be integrated into the procedure, allowing for expanded visibility, improved understanding of anatomical relationships, placement of markers or trajectories, and planning of optimal fluoroscopy angles.63 However, these are static non-ECG- or respiratory-gated images (figure 6).
Figure 6. Examples of computed tomography and fluoroscopy image fusion in various procedures using Heart Navigator (Philips): transcatheter aortic valve replacement (A), left atrial appendage occlusion (B), transcatheter valve-in-MAC mitral valve replacement (C), valve implantation in the superior (D) and inferior venae cavae (E), and closure of mitral paravalvular leak (F).
CT-fluoroscopy image fusion has been shown to reduce procedural length, contrast volume, and radiation exposure in TAVI and LAAO procedures, as well as a decreased need for intraprocedural device size adjustments in LAAO. The application and utility of CT- fluoroscopy image fusion have been reported in various procedures and have been shown to be particularly advantageous in complex interventions such as TMVR, transcatheter tricuspid valve replacement, transcaval TAVI, and paravalvular leak closure.64
CONCLUSIONS
CT is a high spatial resolution noninvasive imaging modality, providing excellent delineation of calcium and intravascular space using contrast media. The technique offers the possibility of performing measurements and virtual simulations for both coronary and structural interventions. CT has been established as the gold standard for patient selection and procedural planning in various scenarios of transcatheter coronary and structural interventions (such as TAVI, LAAO, TMVR, and transcatheter tricuspid valve replacement).
FUNDING
None declared.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
No artificial intelligence was used in the preparation of this work.
AUTHORS’ CONTRIBUTIONS
The manuscript was drafted by M. Barreiro-Pérez and I. Cruz-González and thoroughly reviewed and approved by all authors. Berenice Caneiro Queija and M. Barreiro-Pérez made corrections and editorial changes, and responded the reviewers.
CONFLICTS OF INTEREST
M. Barreiro-Pérez has received payments for presentations or educational activities from Abbott Vascular, Edwards Lifesciences, Venus MedTech, Lifetech, and Cardiovalve. I. Cruz-González has received payments for presentations or educational activities from Abbott Vascular and Boston Scientific. R. Estévez Loureiro has received payments for presentations or educational activities from Abbott Vascular, Boston Scientific, Edwards Lifesciences, and Venus MedTech.
REFERENCES
1. Abbara S, Blanke P, Maroules CD, et al. SCCT guidelines for the performance and acquisition of coronary computed tomographic angiography:A report of the society of Cardiovascular Computed Tomography Guidelines Committee:Endorsed by the North American Society for Cardiovascular Imaging (NASCI). J Cardiovasc Comput Tomogr. 2016;10:435-449.
2. Knuuti J, Ballo H, Juarez-Orozco LE, et al. The performance of non-invasive tests to rule-in and rule-out significant coronary artery stenosis in patients with stable angina:a meta-analysis focused on post-test disease probability. Eur Heart J. 2018;39:3322-3330.
3. Douglas PS, Hoffmann U, Patel MR, et al. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med. 2015;372:1291-1300.
4. Knuuti J, Wijns W, Saraste A, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407-477.
5. Byrne RA, Rossello X, Coughlan JJ, et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J. 2023;44:3720-3826.
6. Delgado V, Ajmone Marsan N, De Waha S, et al. 2023 ESC Guidelines for the management of endocarditis. Eur Heart J. 2023;44:3948-4042.
7. McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599-3726.
8. Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2022;43:561-632.
9. Arbelo E, Protonotarios A, Gimeno JR, et al. 2023 ESC Guidelines for the management of cardiomyopathies. Eur Heart J. 2023;44:3503-3626.
10. Halvorsen S, Mehilli J, Cassese S, et al. 2022 ESC Guidelines on cardiovascular assessment and management of patients undergoing non-cardiac surgery. Eur Heart J. 2022;43:3826-3924.
11. Pelliccia A, Sharma S, Gati S, et al. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur Heart J. 2021;42:17-96.
12. Pontone G, Baggiano A, Andreini D, et al. Stress Computed Tomography Perfusion Versus Fractional Flow Reserve CT Derived in Suspected Coronary Artery Disease:The PERFECTION Study. JACC Cardiovasc Imaging. 2019;12(8 Pt 1):1487-1497.
13. Newby DE, Adamson PD, Berry C, et al. Coronary CT Angiography and 5-Year Risk of Myocardial Infarction. N Engl J Med. 2018;379:924-933.
14. Maurovich-Horvat P, Bosserdt M, Kofoed KF, et al. CT or Invasive Coronary Angiography in Stable Chest Pain. N Engl J Med. 2022;386:1591-1602.
15. Jones DA, Beirne AM, Kelham M, et al. Computed Tomography Cardiac Angiography Before Invasive Coronary Angiography in Patients With Previous Bypass Surgery:The BYPASS-CTCA Trial. Circulation. 2023;148:1371-1380.
16. Leipsic J, Abbara S, Achenbach S, et al. SCCT guidelines for the interpretation and reporting of coronary CT angiography:A report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr. 2014;8:342-358.
17. Andreini D, Collet C, Leipsic J, et al. Pre-procedural planning of coronary revascularization by cardiac computed tomography:An expert consensus document of the Society of Cardiovascular Computed Tomography. J Cardiovasc Comput Tomogr. 2022;16:558-572.
18. Cury RC, Abbara S, Achenbach S, et al. CAD-RADSTM Coronary Artery Disease –Reporting and Data System. An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Radiology (ACR) and the North American Society for Cardiovascular Imaging (NASCI). Endorsed by the American College of Cardiology. J Cardiovasc Comput Tomogr. 2016;10:269-281.
19. Cury RC, Leipsic J, Abbara S, et al. CAD-RADSTM 2.0 –2022 Coronary Artery Disease-Reporting and Data System:An Expert Consensus Document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Cardiology (ACC), the American College of Radiology (ACR), and the North America Society of Cardiovascular Imaging (NASCI). J Cardiovasc Comput Tomogr. 2022;16:536-557.
20. Papadopoulou SL, Girasis C, Dharampal A, et al. CT-SYNTAX score:a feasibility and reproducibility study. JACC Cardiovasc Imaging. 2013;6:413-415.
21. Collet C, Miyazaki Y, Ryan N, et al. Fractional Flow Reserve Derived From Computed Tomographic Angiography in Patients With Multivessel CAD. J Am Coll Cardiol. 2018;71:2756-2769.
22. Gabara L, Hinton J, Kira M, et al. Derivation and validation of a novel functional FFRCT score incorporating the burden of coronary stenosis severity and flow impairment to predict clinical events. J Cardiovasc Comput Tomogr. 2024;18:33-42.
23. Kageyama S, Serruys PW, Kotoku N, et al. Coronary computed tomography angiography-based SYNTAX score for comprehensive assessment of advanced coronary artery disease. J Cardiovasc Comput Tomogr. 2024;18:120-136.
24. Morino Y, Abe M, Morimoto T, et al. Predicting successful guidewire crossing through chronic total occlusion of native coronary lesions within 30 minutes:the J-CTO (Multicenter CTO Registry in Japan) score as a difficulty grading and time assessment tool. JACC Cardiovasc Interv. 2011;4:213-221.
25. Opolski MP, Achenbach S, Schuhbäck A, et al. Coronary computed tomographic prediction rule for time-efficient guidewire crossing through chronic total occlusion:insights from the CT-RECTOR multicenter registry (Computed Tomography Registry of Chronic Total Occlusion Revascularization). JACC Cardiovasc Interv. 2015;8:257-267.
26. Yu CW, Lee HJ, Suh J, et al. Coronary Computed Tomography Angiography Predicts Guidewire Crossing and Success of Percutaneous Intervention for Chronic Total Occlusion:Korean Multicenter CTO CT Registry Score as a Tool for Assessing Difficulty in Chronic Total Occlusion Percutaneous Coronary Intervention. Circ Cardiovasc Imaging. 2017;10:e005800.
27. Otto CM, Kumbhani DJ, Alexander KP, et al. 2017 ACC Expert Consensus Decision Pathway for Transcatheter Aortic Valve Replacement in the Management of Adults With Aortic Stenosis:A Report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2017;69:1313-1346.
28. Blanke P, Weir-McCall JR, Achenbach S, et al. Computed Tomography Imaging in the Context of Transcatheter Aortic Valve Implantation (TAVI)/Transcatheter Aortic Valve Replacement (TAVR):An Expert Consensus Document of the Society of Cardiovascular Computed Tomography. JACC Cardiovasc Imaging. 2019;12:1-24.
29. Francone M, Budde RPJ, Bremerich J, et al. CT and MR imaging prior to transcatheter aortic valve implantation:standardisation of scanning protocols, measurements and reporting —a consensus document by the European Society of Cardiovascular Radiology (ESCR). Eur Radiol. 2020;30:2627-2650.
30. Hahn RT, Kodali S, Tuzcu EM, et al. Echocardiographic imaging of procedural complications during balloon-expandable transcatheter aortic valve replacement. JACC Cardiovasc Imaging. 2015;8:288-318.
31. Ribeiro HB, Webb JG, Makkar RR, et al. Predictive factors, management, and clinical outcomes of coronary obstruction following transcatheter aortic valve implantation:insights from a large multicenter registry. J Am Coll Cardiol. 2013;62:1552-1562.
32. Kim WK, Toggweiler S, Renker M, et al. Comparison of 3-Cusp Coplanar and 2-Cusp Overlap Views for the Implantation of a Self-Expanding Transcatheter Heart Valve. JACC Cardiovasc Interv. 2023;16:1422-1424.
33. Toggweiler S, Gurvitch R, Leipsic J, et al. Percutaneous aortic valve replacement:vascular outcomes with a fully percutaneous procedure. J Am Coll Cardiol. 2012;59:113-118.
34. Jilaihawi H, Chen M, Webb J, et al. A Bicuspid Aortic Valve Imaging Classification for the TAVR Era. JACC Cardiovasc Imaging. 2016;9:1145-1158.
35. Michelena HI, Della Corte A, Evangelista A, et al. International consensus statement on nomenclature and classification of the congenital bicuspid aortic valve and its aortopathy, for clinical, surgical, interventional and research purposes. Eur J Cardiothorac Surg. 2021;60:448-476.
36. Petronio AS, Angelillis M, De Backer O, et al. Bicuspid aortic valve sizing for transcatheter aortic valve implantation:Development and validation of an algorithm based on multi-slice computed tomography. J Cardiovasc Comput Tomogr. 2020;14:452-461.
37. Iannopollo G, Romano V, Buzzatti N, et al. Supra-annular sizing of transcatheter aortic valve prostheses in raphe-type bicuspid aortic valve disease:the LIRA method. Int J Cardiol. 2020;317:144-151.
38. Weir-McCall JR, Attinger-Toller A, Blanke P, et al. Annular versus supra-annular sizing for transcatheter aortic valve replacement in bicuspid aortic valve disease. J Cardiovasc Comput Tomogr. 2020;14:407-413.
39. Tang GHL, Komatsu I, Tzemach L, et al. Risk of coronary obstruction and the need to perform BASILICA:the VIVID classification. EuroIntervention. 2020;16:E757-E759.
40. Tarantini G, Sathananthan J, Fabris T, et al. Transcatheter Aortic Valve Replacement in Failed Transcatheter Bioprosthetic Valves. JACC Cardiovasc Interv. 2022;15:1777-1793.
41. Jilaihawi H, Asch FM, Manasse E, et al. Systematic CT Methodology for the Evaluation of Subclinical Leaflet Thrombosis. JACC Cardiovasc Imaging. 2017;10:461-470.
42. Eng MH, Wang DD, Greenbaum AB, et al. Prospective, randomized comparison of 3-dimensional computed tomography guidance versus TEE data for left atrial appendage occlusion (PRO3DLAAO). Catheter Cardiovasc Interv. 2018;92:401-407.
43. Korsholm K, Berti S, Iriart X, et al. Expert Recommendations on Cardiac Computed Tomography for Planning Transcatheter Left Atrial Appendage Occlusion. JACC Cardiovasc Interv. 2020;13:277-292.
44. Cepas-Guillén P, Flores-Umanzor E, Leduc N, et al. Impact of Device Implant Depth After Left Atrial Appendage Occlusion. JACC Cardiovasc Interv. 2023;16:2139-2149.
45. Halkin A, Cohen C, Rosso R, et al. Left atrial appendage and pulmonary artery anatomic relationship by cardiac-gated computed tomography:Implications for late pulmonary artery perforation by left atrial appendage closure devices. Heart Rhythm. 2016;13:2064-2069.
46. De Backer O, Iriart X, Kefer J, et al. Impact of Computational Modeling on Transcatheter Left Atrial Appendage Closure Efficiency and Outcomes. JACC Cardiovasc Interv. 2023;16:655-666.
47. Agudelo V, Millán X, Li CH, et al. Prevalence, mechanisms and impact of residual patency and device-related thrombosis following left atrial appendage occlusion:a computed tomography analysis. EuroIntervention. 2021;17:E944-E952.
48. Saw J, Fahmy P, DeJong P, et al. Cardiac CT angiography for device surveillance after endovascular left atrial appendage closure. Eur Heart J Cardiovasc Imaging. 2015;16:1198-1206.
49. Pulerwitz TC, Khalique OK, Leb J, et al. Optimizing Cardiac CT Protocols for Comprehensive Acquisition Prior to Percutaneous MV and TV Repair/Replacement. JACC Cardiovasc Imaging. 2020;13:836-850.
50. Babaliaros VC, Lederman RJ, Gleason PT, et al. The Art of SAPIEN 3 Transcatheter Mitral Valve Replacement in Valve-in-Ring and Valve-in-Mitral-Annular-Calcification Procedures. JACC Cardiovasc Interv. 2021;14:2195-2214.
51. Guerrero M, Wang DD, Pursnani A, et al. A Cardiac Computed Tomography-Based Score to Categorize Mitral Annular Calcification Severity and Predict Valve Embolization. JACC Cardiovasc Imaging. 2020;13:1945-1957.
52. Yoon SH, Bleiziffer S, Latib A, et al. Predictors of Left Ventricular Outflow Tract Obstruction After Transcatheter Mitral Valve Replacement. JACC Cardiovasc Interv. 2019;12:182-193.
53. Barreiro-Perez M, Caneiro-Queija B, Puga L, et al. Imaging in Transcatheter Mitral Valve Replacement:State-of-Art Review. J Clin Med. 2021;10:5973.
54. Hell MM, Emrich T, Kreidel F, et al. Computed tomography imaging needs for novel transcatheter tricuspid valve repair and replacement therapies. Eur Heart J Cardiovasc Imaging. 2021;22:601-610.
55. Lopes BBC, Hashimoto G, Bapat VN, Sorajja P, Scherer MD, Cavalcante JL. Cardiac Computed Tomography and Magnetic Resonance Imaging of the Tricuspid Valve:Preprocedural Planning and Postprocedural Follow-up. Interv Cardiol Clin. 2022;11:27-40.
56. Barreiro-Pérez M, González-Ferreiro R, Caneiro-Queija B, et al. Transcatheter Tricuspid Valve Replacement:Illustrative Case Reports and Review of State-of-Art. J Clin Med. 2023;12:1371.
57. Koo HJ, Lee JY, Kim GH, et al. Paravalvular leakage in patients with prosthetic heart valves:cardiac computed tomography findings and clinical features. Eur Heart J Cardiovasc Imaging. 2018;19:1419-1427.
58. Entrikin DW, Gupta P, Kon ND, Carr JJ. Imaging of infective endocarditis with cardiac CT angiography. J Cardiovasc Comput Tomogr. 2012;6:399-405.
59. Lesser JR, Han BK, Newell M, Schwartz RS, Pedersen W, Sorajja P. Use of cardiac CT angiography to assist in the diagnosis and treatment of aortic prosthetic paravalvular leak:a practical guide. J Cardiovasc Comput Tomogr. 2015;9:159-164.
60. Morris MF, Pena A, Kalya A, et al. Predicting paravalvular leak after transcatheter mitral valve replacement using commercially available software modeling. J Cardiovasc Comput Tomogr. 2020;14:495-499.
61. Siripornpitak S, Goo HW. CT and MRI for Repaired Complex Adult Congenital Heart Diseases. Korean J Radiol. 2021;22:308-323.
62. Arzamendi D, Li CH, Serra A. Cardiac Computed Tomography-guided Closure of Ventricular Septal Defect Secondary to Myocardial Infarction. Rev Esp Cardiol. 2015;68:626.
63. Hussain MA, Nabi F. Complex Structural Interventions:The Role of Computed Tomography, Fluoroscopy, and Fusion Imaging. Methodist Debakey Cardiovasc J. 2017;13:98-105.
64. Barreiro-Perez M, Cruz-Gonzalez I, Moreno-Samos JC, Barahona MF, Sanchez PL. Cardiovascular Structural Interventions —Echo/Computed Tomography-Fluoroscopy Fusion Imaging Atlas. Circ J. 2018;82:2206-2207.
* Corresponding author.
E-mail address: (M. Barreiro-Pérez).
@manuelbarreirop; @icruzgonzalez; @RodrigoEstvez1; @CHPedroLi; @che_parada; @lvaroRodriperez; @b_caneiro