Aortic valve stenosis is one of the most acute and chronic cardiovascular disease conditions. Bicuspid aortic valve is the most common congenital heart abnormality and affected individuals have a 50% chance of developing severe aortic valve stenosis during their lifetime. In aortic valve disease (both aortic valve stenosis and bicuspid aortic valve), the heart valves are damaged and do not work properly. This condition can rapidly affect the pumping action of the heart and can progress to heart failure. Heart failure is a deadly disease affecting at least 26 million people worldwide and its prevalence is increasing with high mortality and morbidity.1 Most importantly, aortic valve disease commonly coexists with other cardiovascular diseases, giving rise to the most general yet fundamentally challenging scenario: complex valvular, ventricular, and vascular diseases (C3VD). In C3VD, multiple valvular, ventricular, and vascular pathologies interact with one another, while the physical phenomena associated with each pathology amplify the effects of others on the cardiovascular system.
Left ventricle (LV) pressure-volume (P-V) loop analysis is a powerful tool to assess cardiac mechanics. This analysis can reveal the pathophysiological mechanisms of heart failure, including heart failure with preserved ejection fraction and myocardial and valvular diseases. It is, therefore, instrumental in the evaluation and management of heart failure and valvular heart disease and can also help explain the short- and long-term effects of valvular and other interventions with cardiac implications. In addition, ventricular P-V loop analysis can be used to monitor the cardiac effects of related medical devices, mechanical heart support, therapeutic interventions, and medications.2-5 Indeed, ventricular P-V loop analysis has exceptional potential for integration into current clinical practice to advance the standard of care.
Aortic valvular disease increases LV pressure, LV end-diastolic pressure, LV workload, the stiffness of the systemic arterial system, and LV afterload, contributing to LV systolic and diastolic dysfunction,6 an important cause of heart failure in such patients. While transcatheter aortic valve implantation (TAVI) provides positive outcomes and has markedly reduced the mortality rate, TAVI fails in nearly 25% to 35% of patients (patients either die or do not recover a reasonable quality of life after the procedure).7 Indeed, the immediate intraprocedural as well the longitudinal hemodynamic changes affecting the aortic-left ventricular system after aortic valve replacement are poorly understood. While TAVI universally reduces the transvalvular pressure gradient, it is anticipated to improve systolic and diastolic LV function in the long-term. Despite the benefits, invasive LV P-V loop analysis revealed impaired LV systolic and diastolic function in the early phase following TAVI.6,8 LV P-V loop analysis also revealed exacerbated heart failure despite successful TAVI procedures in many patients.5,9 Indeed, LV P-V loop analysis elucidated that reductions in transvalvular pressure gradient post-TAVI were not always accompanied by improvements in LV workload. TAVI has been shown to have no effect on LV workload in many patients, while LV workload post-TAVI significantly rose in many others.2,5,10
In clinical settings, cardiac catheterization is the gold standard for evaluating pressure and flow through the heart to perform ventricular P-V loop analysis. However, due to its invasiveness, cost, and high risk, it is impractical for diagnosis in routine daily clinical applications or serial follow-up examinations. Most importantly, cardiac catheterization only provides access to the blood pressure in very limited regions rather than details of physiological flow and pressures throughout the heart and circulatory system. In addition, there is no method to invasively or non-invasively quantify the heart workload that can provide a contribution breakdown of each component of the cardiovascular diseases. This is especially crucial in the presence of TAVI and C3VD, in which quantification of left ventricular workload and its breakdown are important to guide the priority of interventions. Moreover, there is no noninvasive method for determining LV end-diastolic pressure, instantaneous LV pressure, and contractility. All these parameters provide valuable information about the patient’s cardiac deterioration and heart recovery.
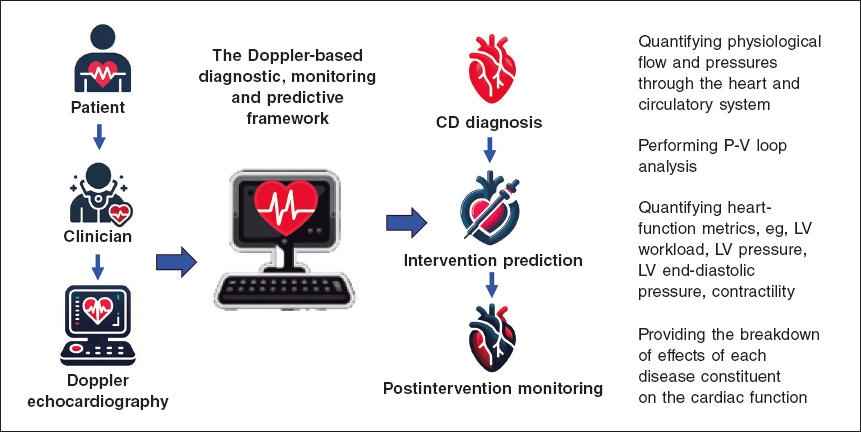
Keshavarz-Motamed11,12 developed the first and the only Doppler-based noninvasive patient-specific diagnostic, monitoring, and predictive tool that can investigate and quantify the effects of interventions, medications, and C3VD constituents on the function of the heart and circulatory system at no risk to the patient (figure 1).
Figure 1. Doppler-based patient-specific diagnostic, monitoring, and predictive tool flowchart. The tool uses a few input parameters that can all be measured using Doppler echocardiography simply and reliably. This novel tool4,10–15 was validated against cardiac catheterization and 4D flow MRI in patients with C3VD (so far ~ n = 600) with substantial inter- and intra-patient variability with a wide range of (adult and congenital) cardiovascular diseases. 4D, 4-dimensional; C3VD, complex valvular, ventricular, and vascular diseases; CD, cardiovascular disease; LV, left ventricular; MRI, magnetic resonance imaging.
This novel method4,10-15 offers several key capabilities (figure 2, sample results): a) quantifying details of physiological pulsatile flow and pressures through the heart and circulatory system; b) tracking cardiac and vascular states based on accurate time-varying models that reproduce physiological responses; c) performing LV P-V loop analysis and quantifying heart function metrics, specifically in terms of the heart workload; d) providing a breakdown of the effects of disease constituents on global heart function (eg, heart workload) to help predict the effects of interventions and plan the sequence of interventions in C3VD; e) quantifying other heart-function metrics, including LV end-diastolic pressure, instantaneous LV pressure, and contractility. None of the above metrics can be obtained non-invasively in patients, and when invasive cardiac catheterization is undertaken, the collected metrics cannot be as complete as what the novel method can provide. While such information is vitally needed for the effective use of advanced therapies to improve clinical outcomes and to guide interventions in patients, they are not currently accessible in clinical settings.
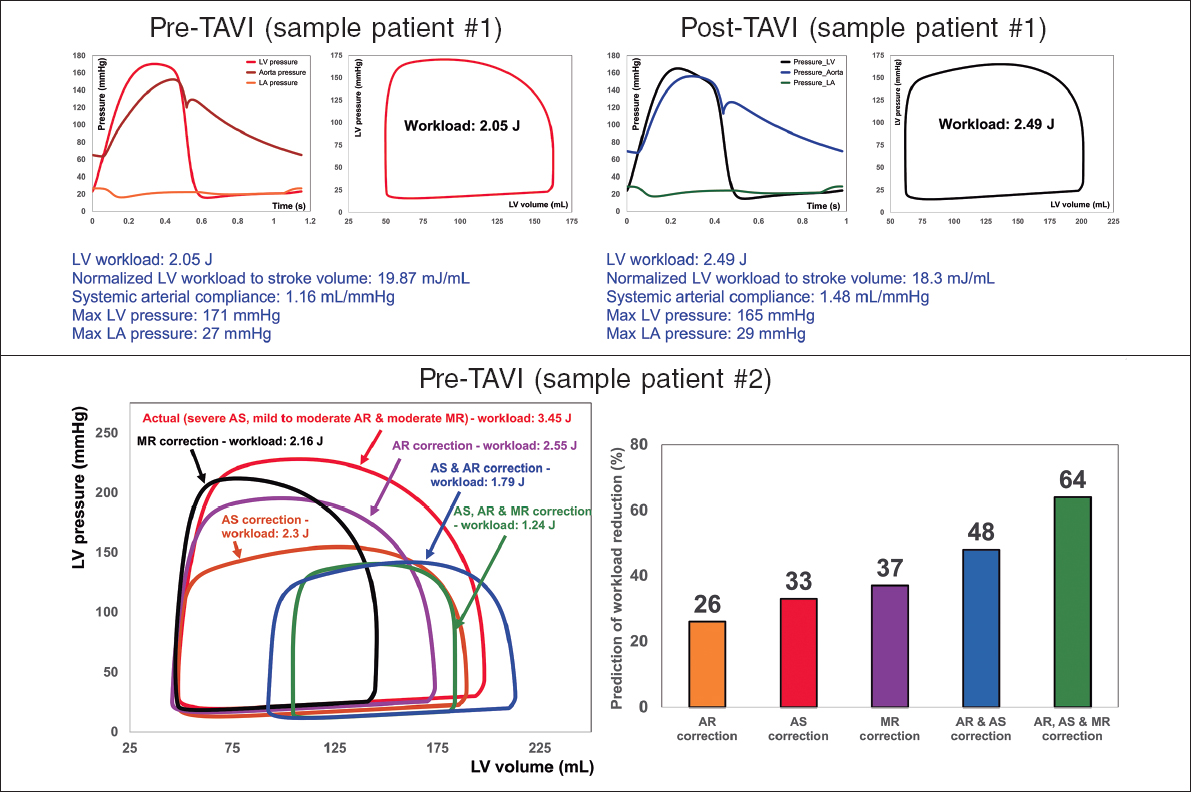
Figure 2. Diagnosis and monitoring in sample patient 1 from baseline to 90 days: this patient did not fully benefit form transcatheter aortic valve implantation (TAVI). Instead of improving the patient’s heart condition by reducing LV workload, TAVI caused an increase in LV workload. Example of workload breakdown analysis and prediction for effects of interventions in sample patient 2: right: P-V loops of the actual disease condition and prediction of several valve interventions. Left: predicted percent decrease in LV workload following valve interventions. Both mitral valve regurgitation (38% increase) and aortic valve stenosis and regurgitation (48% increase) substantially contributed to increasing the workload. This patient only underwent TAVI. However, considering this calculation, the decision to also perform mitral intervention at the time of aortic valve intervention should have been evaluated. AS, aortic stenosis; AR, aortic regurgitation; MR, mitral regurgitation; LV, left ventricle; LA, left atrium. We retrospectively received patients’ data from multicentre in which waiver of informed consent and data transfer were approved by their Institutional Review Boards.
CONCLUSION
The novel method, developed and verified by Keshavarz-Motamed,11,12 purposefully uses reliable and noninvasive input parameters measured by Doppler echocardiography to continuously calculate patient-specific hemodynamics to be used for diagnosis, monitoring, and prediction of cardiac function and circulatory status. This innovative method holds potential applications: a) in clinical trials, enabling the noninvasive analysis of cardiac and circulatory function metrics; b) as a diagnostic tool to noninvasively analyze cardiac function metrics for routine care, ambulatory care, or intensive and critical care units; c) as a patient monitoring tool, potentially integrated into personal wearable devices; and d) as a module incorporated into the software of Doppler echocardiography machines for enhanced diagnosis and prediction.
FUNDING
None.
CONFLICTS OF INTEREST
None.
REFERENCES
1. Savarese G, Lund LH. Global Public Health Burden of Heart Failure. Card Fail Rev. 2017;3:7-11.
2. Keshavarz-Motamed Z, Khodaei S, Rikhtegar Nezami F, et al. Mixed Valvular Disease Following Transcatheter Aortic Valve Replacement:Quantification and Systematic Differentiation Using Clinical Measurements and Image-Based Patient-Specific In Silico Modeling. J Am Heart Assoc. 2020;9:e015063.
3. Bastos MB, Burkhoff D, Maly J, et al. Invasive left ventricle pressure-volume analysis:overview and practical clinical implications. Eur Heart J. 2020;41:1286-1297.
4. Khodaei S, Abdelkhalek M, Maftoon N, Emadi A, Keshavarz-Motamed Z. Early Detection of Risk of Neo-Sinus Blood Stasis Post-Transcatheter Aortic Valve Replacement Using Personalized Hemodynamic Analysis. Struct Heart. 2023;7:100180.
5. Ben-Assa E, Brown J, Keshavarz-Motamed Z, et al. Ventricular stroke work and vascular impedance refine the characterization of patients with aortic stenosis. Sci Transl Med. 2019;11:eaaw0181.
6. Seppelt PC, De Rosa R, Mas-Peiro S, Zeiher AM, Vasa-Nicotera M. Early hemodynamic changes after transcatheter aortic valve implantation in patients with severe aortic stenosis measured by invasive pressure volume loop analysis. Cardiovasc Interv Ther. 2022;37:191-201.
7. Arnold SV. Calculating Risk for Poor Outcomes After Transcatheter Aortic Valve Replacement. J Clin Outcomes Manag. 2019;26:125-129.
8. Sarraf M, Burkhoff D, Brener MI. First-in-Man 4-Chamber Pressure-Volume Analysis During Transcatheter Aortic Valve Replacement for Bicuspid Aortic Valve Disease. JACC Case Rep. 2021;3:77-81.
9. Fischer-Rasokat U, Renker M, Liebetrau C, et al. Outcome of patients with heart failure after transcatheter aortic valve implantation. PLoS One. 2019;14:e0225473.
10. Khodaei S, Garber L, Abdelkhalek M, Maftoon N, Emadi A, Keshavarz-Motamed Z. Reducing Long-Term Mortality Post Transcatheter Aortic Valve Replacement Requires Systemic Differentiation of Patient-Specific Coronary Hemodynamics. Journal of the American Heart Association. 2023;12:e029310.
11. Keshavarz-Motamed Z. A diagnostic, monitoring, and predictive tool for patients with complex valvular, vascular and ventricular diseases. Sci Rep. 2020;10:1-19.
12. Keshavarz Motamed Z. Diagnostic, monitoring, and predictive tool for subjects with complex valvular, vascular and ventricular diseases. US &Canada patent US11844645B2, granted, 2023.
13. Bahadormanesh N, Tomka B, Kadem M, Khodaei S, Keshavarz-Motamed Z. An ultrasound-exclusive non-invasive computational diagnostic framework for personalized cardiology of aortic valve stenosis. Med Image Anal. 2023;87:102795.
14. Sadeghi R, Tomka B, Khodaei S, Garcia J, Ganame J, Keshavarz-Motamed Z. Reducing Morbidity and Mortality in Patients With Coarctation Requires Systematic Differentiation of Impacts of Mixed Valvular Disease on Coarctation Hemodynamics. J Am Heart Assoc.2022;11:e022664.
15. Sadeghi R, Tomka B, Khodaei S, et al. Impact of extra-anatomical bypass on coarctation fluid dynamics using patient-specific lumped parameter and Lattice Boltzmann modeling. Sci Rep. 2022;12:9718.